Abstract
The bed nucleus of the stria terminalis (BNST) is a sexually dimorphic, neuropeptide-rich node of the extended amygdala that has been implicated in responses to stress, drugs of abuse, and natural rewards. Its function is dysregulated in neuropsychiatric disorders that are characterized by stress- or drug-induced alterations in mood, arousal, motivation, and social behavior. However, compared to the BNST’s role in mood, arousal, and motivation, its role in social behavior has remained relatively understudied. Moreover, the precise cell types and circuits underlying the BNST’s role in social behavior have only recently begun to be explored using modern neuroscience techniques. Here, we systematically review the existing literature investigating the neurobiological substrates within the BNST that contribute to the coordination of various sex-dependent and sex-independent social behavioral repertoires, focusing largely on pharmacological and circuit-based behavioral studies in rodents. We suggest that the BNST coordinates social behavior by promoting appropriate assessment of social contexts to select relevant behavioral outputs and that disruption of socially-relevant BNST systems by stress and drugs of abuse may be an important factor in the development of social dysfunction in neuropsychiatric disorders.
Keywords: neural circuits, neuropeptides, social behavior, extended amygdala
Introduction
The bed nucleus of the stria terminalis (BNST) is a neurochemically diverse hub of the extended amygdala that integrates sensory, stress, and reward-related information to mediate responses to threats, natural reinforcers, and drugs of abuse. It has been strongly implicated in human psychopathologies such as generalized anxiety disorder (GAD), post-traumatic stress disorder (PTSD), and substance use disorder (SUD), all of which can be characterized by alterations in mood, arousal, sleep, appetite, and social behavior (Avery et al., 2016). Animal studies modeling these disorders have subsequently identified important roles for specific BNST cell types and circuits in mediating a number of the associated symptoms, particularly disordered arousal and mood. Though the BNST largely promotes negative affect and aversion-related behaviors, distinct BNST cell types and circuits can in fact drive reward-related behaviors (Jennings et al., 2013). In an attempt to unify these seemingly dichotomous functions, it has been suggested that the BNST performs what has been termed “valence surveillance” (Lebow & Chen, 2016). This proposes that the BNST’s role in behavior is to assess the positive or negative valence of a given context or stimulus and act appropriately by integrating incoming information about mood, arousal, relevant sensory stimuli, and motivational state.
Anatomically, the BNST acts as a relay station between cortical limbic regions, arousal centers, and reward centers, and displays dense reciprocal connectivity with the amygdala, extended amygdala, and hypothalamus. Though often overlooked as such, the BNST is a critical node in the social behavioral network and interfaces with regions that are integral for social decision making and the processing of multimodal social stimuli (O’Connell & Hofmann, 2011; Lebow & Chen, 2016). However, despite this pattern of anatomical connectivity, the role of the BNST in many social behaviors remains relatively understudied and thus poorly understood at the circuit level. Following a brief overview of BNST anatomy and neurochemical architecture, this review will synthesize what is known about the specific BNST cells and circuits implicated in social behavior in healthy states, stressed states, and states of drug withdrawal and intoxication. In addition, it will cover emerging data demonstrating BNST dysfunction in humans with social anxiety. We note that unless otherwise stated, all rodent studies discussed in this review were performed in commonly used inbred laboratory mouse strains, primarily the C57BL6/J strain. While decades of research suggest that the BNST plays an important role in in a multitude of different social behaviors, including but not limited to maternal care and aggression, intermale aggression, prosocial behaviors, sexual behaviors, and social dysfunction associated with stress and drug exposure, few articles have systematically reviewed these studies. With the idea of “valence surveillance” in mind, we hypothesize that a central function of the BNST in social behavior is to integrate both internal and external evaluations of the conditions of a given social interaction to inform behavior. More specifically, we suggest that the BNST assesses whether a given social target should be ignored, avoided, or engaged with by assessing the identity of the social target and the context of the interaction, the internal motivational state and stress history of the animals, the physical or emotional threat posed by the social interaction, and the reward value of the social interaction.
Anatomy of the BNST
Connectivity
The BNST is a sexually dimorphic nucleus comprised of up to 18 different sub-nuclei, each expressing a distinct combination of receptors, neurotransmitters, and transcription factors as well as sending and receiving projections to and from distinct regions. While extensive efforts have been made to characterize the makeup, connectivity, and function of these sub-regions (reviewed thoroughly in Lebow and Chen 2016), it is not known how they individually integrate information from multiple intra- and extra-BNST sources to drive behavior. This may be due, at least in part, to the technical difficulties associated with targeting and manipulating anatomically small sites with available stereotaxic, viral, and pharmacological methods. Due to these limitations, studies investigating the functional role of BNST sub-regions in behavior or sub-region-specific connectivity patterns often distinguish only between dorsal and ventral or anterior and posterior BNST, although the oval nucleus may also be targeted. While all of these BNST sub-regions interface with regions that are important for social behavior, they do so in distinct ways. The ventral portion of the BNST, for example, displays extensive connectivity with the medial amygdala (MeA) and ventromedial hypothalamus (VMH) and expresses a high density of steroid hormone receptors (Laflamme et al., 1998; Dong & Swanson, 2004a; 2006a; Ni et al., 2016). It also communicates heavily with regions of the mesocorticolimbic reward pathway like the ventral tegmental area (VTA), nucleus accumbens (NAc), and medial prefrontal cortex (mPFC), as well as the dorsal raphe nucleus (DRN) (Dong & Swanson, 2004a; 2006a; Ni et al., 2016). In addition, the ventral BNST receives the densest brainstem-derived noradrenergic inputs of any site in the brain (Forray & Gysling, 2004). While the dorsal BNST also expresses steroid hormone receptors and communicates with the DRN, VTA, NAc, and mPFC, it is also strongly connected with neuroendocrine centers like the paraventricular hypothalamus (PVN) and nodes of the extended amygdala like the central amygdala (CeA) (Dong & Swanson, 2006b; Ni et al., 2016). It is indeed possible that the ventral and dorsal BNST modulate unique aspects of social behavior based on their relatively distinct patterns of connectivity. For example, communication between the ventral BNST and the VMH may specifically modulate aggressive and sexual behavior, while communication between the dorsal BNST and oxytocin (OXT) neurons in the PVN may specifically modulate prosocial approach and avoidance. The anterior BNST is reciprocally connected with the amygdala and hypothalamus and receives notable inputs from the periaqueductal gray (PAG), DRN, VTA, and mPFC (Peyron et al., 1998; Forray & Gysling, 2004; Vertes, 2004; Shin et al., 2008; Herr et al., 2012; Ni et al., 2016). The posterior BNST is the most sexually dimorphic sub-region (Laflamme et al., 1998; Campi et al., 2013) and receives dense inputs from the olfactory bulb (OB), accessory olfactory bulb (AOB), and MeA while sending outputs to the VTA, hypothalamus, and lateral septum (LS) (Dong & Swanson, 2004b; Shin et al., 2008; Ni et al., 2016). Based on these patterns of connectivity, it is likely that the posterior BNST is particularly important for modulating innate sex-specific social behaviors like aggression, mating, and parental care, whereas the anterior BNST plays a role in mediating responses to social threats and stress-induced impairments in social behavior. While this idea has been investigated to some extent, future research is required to elucidate the precise inputs, outputs, sub-regions, and cell populations of the BNST that modulate particular aspects of social behavior.
Neurochemical architecture
The vast majority of BNST neurons release the inhibitory neurotransmitter GABA, form symmetric synapses, and express the vesicular GABA transporter (vGAT) (Kudo et al., 2012; Mazzone et al., 2018; Moffitt et al., 2018; Welch et al., 2019; Rodriguez-Romaguera et al., 2020). Thus, BNST outputs generally inhibit their target cells. However, roughly one-tenth of BNST neurons release glutamate, form asymmetric synapses, and express the vesicular glutamate transporter 2 (vGlut2) (Kudo et al., 2012; Moffitt et al., 2018; Welch et al., 2019; Rodriguez-Romaguera et al., 2020). Although there is also a small population of vesicular glutamate transporter 3 (vGlut3)-expressing neurons in the BNST, these neurons form symmetric synapses, express enzymes necessary for GABA production, and release GABA (Kudo et al., 2012; Welch et al., 2019). BNST neurons are also known to express a large variety of neuropeptides, which include but are not limited to dynorphin (Dyn), enkephalin (Enk), OXT, arginine vasopressin (AVP), corticotropin-releasing factor (CRF), cholecystokinin (CCK), neuropeptide Y (NPY), somatostatin (SOM), pituitary adenylate cyclase-activating polypeptide (PACAP), neurotensin (NTS), and nociceptin (NC) (Kash et al., 2015). Single BNST neurons can express widely varying combinations of the aforementioned neurotransmitters, neuropeptides, and their receptors; thus the identification of transcriptionally-defined “cell types” in the BNST has proven challenging. For example, one recent study that performed single-cell transcriptional profiling of BNST neurons reported that they can be divided into as many as 41 different transcriptional clusters (Welch et al., 2019). Another study suggested that BNST neurons can be divided into 11 distinct transcriptional clusters, and that expression of pre-pro-nociceptin, the precursor for the NC neuropeptide, is distributed across 4 of these 11 clusters (Rodriguez-Romaguera et al., 2020). These two studies likely identified differing numbers of BNST cell types because of the clustering analyses used. Welch et al. employed integrative non-negative matrix factorization to identify both shared and dataset-specific transcripts across datasets. Rodriguez-Romaguera, on the other hand, used a more traditional principal component analysis to cluster cells and did not include transcripts if they were not present in all datasets. Regardless of how many transcriptionally-defined BNST “cell types” there may be, there does appear to be a spatial and functional relationship between transcriptionally similar neurons in the BNST (Moffitt et al., 2018). The BNST is also a major site of release for monoamines, catecholamines, hypothalamic-derived neuropeptides, and steroid hormones (Kash et al., 2015), all of which may dynamically modulate the output activity of BNST neurons in cell type and circuit-specific manners. Furthermore, male and female rodents display marked differences in BNST steroid hormone responsiveness and neuropeptide synthesis (Laflamme et al., 1998; Toufexis, 2007; Campi et al., 2013; Gungor & Paré, 2016). This complexity of sex-specific neurochemical signaling within the BNST combined with its intricate sub-region-specific connectivity may ultimately underlie its capacity to orchestrate a broad range of social behavioral repertoires.
The BNST and aggressive social behaviors
Aggression is a social behavior that evolved to promote the protection of self, resources, progeny, and territory. Though it may be adaptive or maladaptive in animals depending on its severity, aggression in humans is largely maladaptive and is a hallmark of neuropsychiatric disorders like SUD and PTSD. In rodents, aggression is strongly influenced by the sex, sexual history, and stress history of the experimental animal and the social target (Miczek et al., 2001; Miczek et al., 2013). As female rodents primarily engage in aggression during the postpartum period and direct attacks towards males, interfemale aggression has been historically understudied. However, recent work has found that nulliparous female mice co-housed with castrated males will reliably exhibit aggression towards female social targets, which opens up new avenues for studying interfemale aggression (Newman et al., 2019). On the other hand, nearly all male rodents are capable of exhibiting aggression towards other males, and even toward juveniles, throughout their adult lives and in a variety of social contexts (Miczek et al., 2001; Miczek et al., 2013). Prior winning experience increases subsequent intermale aggression, as does social isolation, copulation, and brief exposure to a physically inaccessible male conspecific prior to aggression testing (Valzelli, 1973; Flannelly et al., 1982; Potegal, 1992; Miczek et al., 2001; Hsu et al., 2006). Specific offensive behaviors exhibited during intermale aggression include biting, chasing, tail rattling, and making lateral threats (Blanchard et al., 1984; Miczek et al., 2001). Most aggressive contests also require both animals to engage in defensive behaviors such as crouching and darting (Blanchard et al., 1984). In addition, because aggression requires that animals properly identify male versus female, novel versus familiar, and adult versus juvenile social targets, pheromone signaling plays a large role. Recent studies also suggest that there is a strong motivational component to aggressive behavior, as aggressive male rodents will operantly self-administer for the opportunity of aggression as reinforcement—e.g. they will lever press to gain access to submissive social targets they can attack (Falkner et al., 2016; Golden et al., 2017; Golden et al., 2019). Similarly, aggressive mice form conditioned-place preferences for contexts associated with winning aggressive contests (Golden et al., 2016; Aleyasin et al., 2018; Flanigan et al., 2020). Furthermore, both stress and drugs of abuse modulate the expression of aggressive behavior. For example, social isolation stress as well as withdrawal from alcohol or morphine increases aggression in male rodents (Miczek et al., 2013; Miczek et al., 2015; Piccin & Contarino, 2020). Therefore, because the BNST is a hub between pheromone signaling centers like the AOB, reward centers like the VTA, and aggression initiation centers like the MeA, LS, and VMH, it is well positioned to influence multiple aspects of aggressive behavior.
BNST neurons are broadly activated by aggression-related stimuli as well as aggressive social interactions themselves. For example, male rats that are presented with both a female cage mate and an unfamiliar male while in the bore of an fMRI magnet, which would generally provoke aggressive behavior towards the unfamiliar male, display increased BNST activation (Ferris et al., 2008). This level of activation is higher than that induced by presentation of the female cage mate alone. Male mice also display increased BNST c-Fos expression (a marker of neural activation) following an aggressive encounter (Haller et al., 2006; Lin et al., 2011; Bayless et al., 2019). Interestingly, female California mice exhibiting aggression towards female intruders also display robust increases in BNST c-Fos following aggression, suggesting that the BNST’s role in female aggression may extend beyond that of the maternal context, depending on the mouse strain investigated (Davis & Marler, 2004). This is an important area of research to pursue, as human females engage in aggression outside of the postpartum period yet we know very little about the underlying mechanisms.
Manipulations of GABA, glutamate, CRF, and AVP signaling in the BNST all have effects on aggressive behavior in male rodents. For example, infusion of a metabotropic glutamate receptor 7 (mGluR7) antagonist into the BNST reduces aggressive behavior, and mGluR7 knockout mice display reductions in aggression and reduced BNST activation in response to sex-specific olfactory cues (Masugi-Tokita et al., 2016). Though a GABA-A receptor agonist infused into the BNST of male Syrian hamsters prior to an aggressive contest does not alter defensive responses, if this treatment is applied following a social defeat defensive behaviors are reduced in subsequent contests (Markham et al., 2009). This implies that the BNST regulates experience-dependent flexibility in the expression of aggressive behaviors. The BNST CRF system is likely involved in this process, as natural increases in submissive behaviors following social defeat in male Syrian hamsters are blocked by CRF receptor 1 (CRFR1) or CRF receptor 2 (CRFR2) antagonist infusion into the BNST (Jasnow et al., 2004; Cooper & Huhman, 2010). The role of BNST AVP signaling in aggressive behavior appears to depend on the species tested and the stress history of the experimental animals. For example, highly aggressive male rats display reduced AVP release during aggression, and AVP infusion into the BNST of these male rats functionally reduces aggression (Veenema et al., 2010). Interestingly, antagonism of AVP receptor 1a (AVPR1a) in the BNST in this study did not affect rat intermale aggression. The results of this study are somewhat surprising, as AVP release in other brain regions has been shown to promote aggression in male rats (Dumais & Veenema, 2016; Terranova et al., 2017), and aggressive behaviors in Syrian hamsters like flank marking are associated with increased activity in AVPR1a-containing regions of the BNST (Bamshad et al., 1996). Furthermore, aggression in unstressed, but not stressed, California mice is positively correlated with the number of BNST AVP cell bodies (Steinman et al., 2015). Thus, further research is required to untangle the precise conditions in which BNST AVP neurons drive versus dampen aggression in various rodent species.
OXT neurons in the PVN (which project to the BNST) can drive prosocial or aggressive social behaviors in a manner that is highly dependent on the context of the social interaction. For example, optogenetic stimulation of PVN OXT cell bodies in the resident intruder (RI) test, whereby an experimental mouse (“resident”) is exposed to an unfamiliar conspecific (“intruder”) in the experimental mouse’s home cage, increases the incidence of aggressive behaviors (Miczek et al., 2013; Anpilov et al., 2020). Interestingly, stimulation of these neurons in a group social setting with familiar cage mates increases the incidence of prosocial interactions on the first day of testing but increases aggressive chases in the following days of testing (Anpilov et al., 2020). While there is some evidence that OXT release in the BNST positively modulates intermale aggression, it is unclear which BNST OXT sources (PVN or BNST) and which targets of BNST OXT- and oxytocin receptor (OXTR)-containing neurons are involved. Male mice who have engaged in pup-directed aggression display increased c-Fos expression in BNST OXTR-expressing neurons (Moffitt et al., 2018), and OXTR binding is increased in the BNST of highly aggressive rats (Calcagnoli et al., 2014). PVN OXT mRNA is simultaneously reduced in these highly aggressive rats, suggesting that aggressive behavior is controlled by brain site-specific alterations in OXT signaling (Calcagnoli et al., 2014). It should be tested whether optogenetic stimulation of PVN OXT axons in the BNST modulates aggressive behavior in a similar manner to that of broad PVN OXT stimulation.
Steroid hormone signaling in the BNST has also been implicated in aggressive behavior. Androgen receptors (AR) are activated by circulating testosterone and are expressed in a sexually dimorphic manner in the posterior BNST (Wu et al., 2009). Mice who have won aggressive contests display increased AR mRNA and protein in the BNST (Fuxjager et al., 2010), and increased dominance behavior is associated with estrogen receptor alpha 1a (ESR1a) mRNA expression in the BNST (Greenberg et al., 2014b). Interestingly, BNST AR neurons are not activated during attack (as measured using the in-vivo calcium imaging technique fiber photometry (Gunaydin et al., 2014)), but their activity is increased upon presentation of an unfamiliar male social target (Bayless et al., 2019). While functional manipulation of BNST AR-expressing neurons does not alter maternal aggression in female mice, chemogenetic inhibition or caspase-mediated ablation of BNST AR neurons reduces attacks between males (Bayless et al., 2019). Remarkably, if these neurons are optogenetically stimulated either persistently during a male-male interaction or only upon the start of the interaction, aggression is reduced and mating behavior is initiated towards the male social targets (Bayless et al., 2019). This indicates that BNST AR neurons play a role in aggressive behavior largely via their influence on processes of sex discrimination in a manner that is dependent on the time course of BNST AR neuron activation.
Recent studies have begun to disentangle the roles of specific BNST inputs and outputs in aggressive behavior, particularly the MeA and the VMH. The VMH was originally discovered to be a crucial locus for the initiation of indiscriminate attack behavior in cats and has since been found to play a similar role in rodents (Hess, 1927; Lin et al., 2011). Its activity is tightly controlled by the LS, which suppresses aggression through GABAergic inhibition of the VMH (Brayley & Albert, 1977). In fact, lesions of the LS produce what is known as “septal rage” as a result of VMH disinhibition (Maeda, 1977). The BNST communicates bidirectionally with both the VMH and LS (Dong & Swanson, 2004b; a; 2006a; Shin et al., 2008). When the VMH is stimulated simultaneously with the BNST, there is an increase in active defense behaviors compared to VMH stimulation alone (Shaikh et al., 1986). Infusion of acetylcholine (ACh) into the VMH increases the stimulation threshold necessary for attack, but this does not occur if the BNST is lesioned prior to stimulation (Kono et al., 1986). This suggests that the BNST may play a role in balancing offensive with defensive aggressive behaviors through its connections with the VMH. The dynamic circuit interactions between the BNST and the VMH have recently been characterized and may partly explain the complexity of the BNST’s role in aggression. The output of VMH projection neurons in the VMH core are inhibited by the VMH shell, which receive inhibitory inputs from BNST neurons (Yamamoto et al., 2018). Though BNST neurons also project to the VMH core, BNST-evoked IPSCs are greater in VMH shell neurons. Depending on the firing rate of shell neurons, inputs from the BNST to the VMH can either result in net inhibition or net excitation of the VMH output neurons in the core. Thus, the BNST may modulate aggression via context-dependent interactions with various VMH neuronal populations. The MeA projects to both the VMH and the BNST, and optogenetic stimulation of multiple genetically-defined MeA cell types can influence aggression in opposing ways (Canteras et al., 1995; Pardo-Bellver et al., 2012; Hong et al., 2014; Unger et al., 2015; Padilla et al., 2016). A very recent study found that both aggressive experience and traumatic stress, which produce experience-dependent increases in aggressive behavior, potentiate connections between the MeA and the BNST in an N-methyl-D-aspartate receptor (NMDAR)-dependent manner to promote the initiation, but not the maintenance, of intermale aggressive behaviors (Nordman et al., 2020). The MeA-VMH projection, on the other hand, undergoes these same experience-dependent adaptations to promote the maintenance, but not initiation, of aggression. Remarkably, functional manipulation of either of these circuits does not alter non-aggressive social behaviors. Thus, the MeA-BNST and MeA-VMH circuits contribute to unique aspects of aggressive social interactions. Future studies should investigate whether these BNST circuits are dysregulated in response to stress or drug exposure to drive changes in aggressive behavior.
The BNST and maternal social behaviors
Maternal care and aggression represent two female-specific social behaviors that are integral for the survival and fitness of offspring. Rodent maternal care behaviors include pup licking and grooming, nest building, nursing, and retrieval of scattered pups (also referred to maternal motivation), which support healthy physical and behavioral development of the offspring (Champagne et al., 2001). Maternal aggression occurs in a short (7-10d) window in the early postpartum period and is necessary for pup defense against hostile adult males (Lonstein & Gammie, 2002; Bosch, 2011; 2013). Exposure to stress or drugs of abuse profoundly disrupts maternal behaviors in both humans and rodents. For example, human mothers using cocaine during pregnancy display reduced sensitivity and responsivity to infant-related cues than drug-free mothers (Gottwald & Thurman, 1994). In rodents, chronic cocaine treatment during the postpartum period reduces nursing behaviors, pup licking, and nest building but increases maternal aggression (Johns et al., 1997; Nelson et al., 1998). Current research suggests that the appropriate expression of maternal behaviors requires reductions in innate anxiety in the lactating mother that are driven by adaptations in BNST neuropeptide systems (Lonstein & Gammie, 2002; Bosch, 2011; 2013). While numerous studies indicate that the dysregulation of these BNST neuropeptide systems by stress has detrimental effects on maternal care, maternal motivation, and maternal aggression, few studies have investigated whether dysregulation of these maternal behaviors by drugs of abuse also involves neural adaptations in the BNST.
The expression of several neuropeptide receptors in the BNST varies throughout pregnancy, parturition, and lactation to regulate BNST activity and the expression of subsequent maternal behaviors. These primarily include receptors for the neuropeptides AVP and OXT, which have been widely implicated in maternal behavior in other brain regions like the mPOA (Bosch, 2013). Sources of BNST AVP and OXT include both the PVN and the BNST itself (Steinman et al., 2016; Janeček & Dabrowska, 2019; Kompier et al., 2019). Dams display increased expression of AVP receptor 1a (AVPR1a) and OXT receptor (OXTR) mRNA, as well as increased AVPR1a and OXT receptor binding, during parturition and lactation (Meddle et al., 2007; Naik & de Jong, 2017). During these periods, AVP peptide production in the BNST is stimulated by estrogen on a massive scale, with target regions like the LS and the lateral habenula (LHb) seeing particularly large increases in BNST-derived AVP (Fink et al., 1996). Parturition and lactation also increase c-Fos in the dorsal BNST (Hasen & Gammie, 2005) as well as in BNST neurons expressing OXTR in the posterior BNST (Meddle et al., 2007). Gestational cocaine treatment, which enhances maternal aggression during lactation, increases OXTR binding in the BNST (Jarrett et al., 2006). While antagonism of AVPR1a in the BNST does not alter measures of maternal care like pup retrieval or nursing, it reduces maternal aggression as well as anxiety-like behavior in lactating females, suggesting that BNST AVPR1a signaling is important for promoting maternal aggression but not maternal motivation (Bosch et al., 2010). However, these results are counter to the idea that maternal aggression requires reductions in anxiety, as anxiety and aggression were reduced by the same mechanism. Consistent with the fact that BNST OXTR expression, binding, and neural activation increase during the postpartum period, antagonism of OXTRs in the posterior BNST reduces pup retrieval behavior, suggesting that OXT in the BNST functionally drives maternal care, particularly maternal motivation (Klampfl & Bosch, 2019). On the other hand, administration of OXT directly into the BNST dose-dependently reduces maternal aggression towards unfamiliar males (Consiglio et al., 2005). This highlights a potentially divergent role for BNST OXT signaling in maternal care versus maternal aggression. Alternatively, the different effects of BNST OXT signaling on maternal care versus maternal aggression may be related to the contexts in which these experiments were performed—while pup retrieval experiments were conducted in a novel cage, maternal aggression experiments were conducted in the home cage. Future studies should investigate the anatomical targets of OXT, OXTR, AVP, and AVP1aR-containing neurons in the BNST and determine how they each contribute to various maternal behaviors in projection-specific manners. Moreover, it will be important to determine how extra-BNST sources of OXT and AVP modulate maternal care and aggression in various contexts.
As mentioned above, exposure to stressors or drugs of abuse reduces maternal care and increases maternal aggression. In turn, neglected offspring display increased mortality rates and greater incidence of maladaptive behaviors associated with human neuropsychiatric diseases like GAD, SUD, and depression (Orso et al., 2019). Existing research suggests that the BNST CRF system plays a central role in mediating the effects of stress on maternal behavior. Like AVP and OXT, sources of BNST CRF include both the PVN and the BNST itself (Klampfl & Bosch, 2019). BNST CRF mRNA is decreased during lactation, though the expression of CRFR1 and CRFR2 does not differ between lactating and virgin females (Klampfl et al., 2014; 2016a; Klampfl et al., 2016b). However, signaling via CRFR1 and CRFR2 in the BNST during the postpartum period affects both maternal care and aggression in a sub-nuclei and receptor-specific manner (Klampfl et al., 2014; 2016a; Klampfl et al., 2016b). Direct administration of CRFR1 or CRFR2 agonists into the posterior BNST reduces nursing behavior, while antagonism of these receptors prevents stress-induced impairment of nursing behavior and reduces anxiety-like behavior (Klampfl et al., 2014). Interestingly, maternal aggression is increased by CRFR2, but not CRFR1, agonist infusion into the posterior BNST (Klampfl et al., 2014). While administration of a CRFR1 agonist into the anterior BNST reduces nursing behavior, CRFR2 agonists cause a brief decrease in nursing followed by a long-term increase in nursing; neither manipulation affects maternal motivation, maternal aggression, or anxiety-like behavior (Klampfl et al., 2016a). CRF-binding protein (CRF-BP) is a critical regulator of the CRF system that primarily sequesters the endogenous ligands of CRFR1, CRF and urocortin-1 (UCN1), to decrease CRFR1’s activation (Haass-Koffler 2018). Pharmacological inhibition of CRF-BP in the posterior BNST has no effect on maternal care under baseline conditions, but impairs the reinstatement of maternal care following stress exposure (Klampfl et al., 2016b). This manipulation also enhances maternal aggression under baseline conditions while concurrently increasing anxiety-like behavior. Interestingly, CRF-BP inhibition in the anterior BNST following stress has no effects on maternal care or aggression (Klampfl et al., 2016b). Though these studies suggest that downregulation of CRF signaling in the BNST, particularly the posterior BNST, is critical for the expression of appropriate maternal social behaviors, the mechanisms driving this downregulation remain unknown. Similarly, it is unknown how communication between BNST CRF neurons and their downstream targets is altered by stressors that impact maternal behaviors. There is evidence that the CRF and OXT systems interact during the postpartum period to influence maternal behaviors, but the mechanisms of these interactions have only begun to be untangled. In males, both central and intra-BNST inhibition of CRFR2 increases local OXT release, but in lactating females this effect is only observed with central inhibition of CRFR2 (Martinon & Dabrowska, 2018; Klampfl & Bosch, 2019). This suggests that increased activation of CRFR2 in lactating dams during stress may mediate impairments in maternal behavior via reduction of PVN-derived OXT release in the BNST. As OXT neurons express CRFR2 and CRF neurons express OXTR (Dabrowska et al., 2011), the interactions between these peptide systems within the BNST are likely complex and bidirectional. Future studies should investigate whether these CRF/OXT interactions occur in the context of other social behaviors such as sexual and prosocial behaviors.
The BNST and sexual social behaviors
Sexually reproducing animals display sex-specific mating behaviors that enhance their fitness and reproductive success. In order for these mating interactions to occur appropriately, animals must correctly differentiate between social targets of different sexes as well as display motivation to engage in the sexual encounter. In both humans and rodents, sexual behaviors are profoundly suppressed by both stress and pain (Monga et al., 1998; Hamilton & Meston, 2013; Manzano Nieves et al., 2019; Pitcher et al., 2019). In addition, sexual dysfunction and lack of sexual desire is prevalent among human patients with stress-related neuropsychiatric disorders like depression and GAD, and the degree of dysfunction is associated with the severity of the disorder (Williams & Reynolds, 2006). Interestingly, drugs of abuse may impact sexual behaviors in distinct ways depending on the subject’s sex, the type of drug, and whether the subject is acutely intoxicated or in withdrawal. For example, male mice acutely intoxicated with cocaine display increased sniffing of female social targets but reduced consummatory sexual behaviors (Kohtz et al., 2019b), whereas female mice acutely intoxicated with cocaine display increased avoidance of male sexual targets and reduced sexual receptivity (Guarraci & Clark, 2003; Kohtz et al., 2019a). Withdrawal from chronic cocaine exposure, on the other hand, vastly reduces the motivation to seek out sexual encounters in male and female mice (Guarraci & Bolton, 2014; Barnea-Ygael et al., 2016). While some studies suggest an important role for the BNST in the recognition of potential sexual partners and motivation for/initiation of consummatory sexual behaviors, whether the effects of stress and drugs of abuse on sexual behavior also involve the BNST remains to be tested.
In rodents, BNST neurons are innervated by AOB neurons, which provide contextual information about a given social interaction such as sex and sexual history via pheromone-related signals that arise in the vomeronasal organ (VNO), which is a direct input to the AOB (Davis et al., 1978; Ni et al., 2016). A short (90s) social interaction between an intact male mouse and a ovariectomized female mouse (in which copulatory behaviors do not occur) induces activation of posterior and ventral BNST as well as inputs to the BNST from the AOB in the male mouse (Kim et al., 2015). Moreover, activation of the posterior BNST in this context is positively correlated with the time the male mouse spent engaged in ano-genital sniffing and close following of the female mouse. The level of posterior BNST activation in males interacting with females was also greater than that of males interacting with males, female scents, and novel objects. Consistent with these findings, lesions of the posterior BNST markedly reduce male ano-genital sniffing of female social targets and induce deficits in sexual behavior (Powers et al., 1987; Claro et al., 1995; Liu et al., 1997). In females, posterior BNST lesions reduce urine marking for male odors but not for female odors and increase ultra-sonic vocalizations in the presence of males, but do not affect lordosis behavior (Kirn & Floody, 1985; Martinez & Petrulis, 2011). Though these studies suggest that the posterior BNST might be important for the discrimination of opposite-sex olfactory cues and engagement in sexual behaviors, they unfortunately do not provide information about the specific BNST cell types and circuits that are important for these processes.
Similar to aggressive behavior, AR-containing BNST neurons appear to exert their effects on sexual behavior largely via their involvement in sex discrimination. In sexually naïve males, BNST AR neurons are activated during multiple phases of a copulatory interaction with a female, as measured using the in-vivo cell-type specific bulk calcium imaging technique fiber photometry (Gunaydin et al., 2014; Bayless et al., 2019). Remarkably, these neurons actually appear to encode the sex of social targets, as they respond more strongly to female social targets than male social targets in a manner that is dependent on upstream pheromone signaling. Female BNST AR neuron activity, however, does not change during copulatory interactions with males. Selective ablation or chemogenetic inhibition of BNST AR neurons in males eliminates preferences for female over male urine and disrupts mating, but these manipulations have no effects in females. Together, these findings indicate that in male mice, AR-expressing BNST neurons are critical for both identifying the sex of social targets and engaging appropriately in sex-specific copulatory behaviors. Future studies should investigate whether BNST AR activation itself (as opposed to activation of AR-containing BNST neurons), is important for these behaviors, or whether it merely serves as a convenient transcriptional marker of the relevant neural population.
Recently, it was discovered that the juvenile mouse pheromone ESP22, which drives sexual suppression in adult female mice, drives robust activation of the entire BNST (Osakada et al., 2018). ESP22 is a protein secreted from lacrimal gland cells and is most highly expressed in male and female juvenile mice from birth until 4 weeks of age, after which ESP22 expression and secretion declines sharply (Ferrero et al. 2013). Moreover, activation of inhibitory BNST outputs to the ventrolateral subdivision of the VMH via ESP22 promotes sexual suppression in female mice. While ESP22 has also been shown to promote sexual suppression in male mice, whether this also requires activation of the BNST-VMH pathway has not been tested (Ferrero et al., 2013). Furthermore, it is unknown what neuropeptides are released from BNST-VMH neurons and whether they influence the expression of sexual rejection via downstream signaling in the VMH. BNST AVP neurons regulate interactions with opposite sex conspecifics in a sex-specific manner, but it is not clear if they project to the VMH. Ablation of BNST AVP neurons in males increases urine marking in the presence of a female, but does not alter ultra-sonic vocalizations, copulatory behavior, or investigation of the females or their odor (Rigney et al., 2019). However, in females, this manipulation reduces female receptivity to mounting but does not alter any other social behaviors. Thus, it is possible that AVP neurons in females represent a parallel population within the BNST separate from neurons activated by ESP22 that communicate with regions like the VMH to promote sexual receptivity rather than sexual rejection. To facilitate these investigations, the specific inputs and projection targets of BNST AVP neurons should be comprehensively mapped and compared between the sexes.
The BNST and prosocial behaviors
Same-sex prosocial interactions promote survival and fitness through the sharing of resources and are crucial for maintaining psychological wellbeing. Prosocial deprivation is a major stressor that induces a vast number of behavioral alterations in species from mice to humans, including anhedonia, social avoidance, aggression, and aberrant responses to non-social stressors (Matthews & Tye, 2019). Prosocial interactions are highly rewarding, as rodents will choose to self-administer access to social targets over drugs of abuse (Venniro et al., 2018; Venniro et al., 2020) and form conditioned place preferences for contexts associated with prosocial encounters (Dolen et al., 2013). Specific behaviors engaged in during prosocial interactions in same-sex individuals include sniffing, grooming, huddling, and approaching (Bartal et al., 2011). Like its role in opposite sex social interactions and same-sex aggressive social interactions, the BNST’s role in same-sex prosocial interactions appears to primarily involve its ability to mediate proper discrimination of individual social targets and engage with them appropriately. Furthermore, stressors and drugs of abuse promote adaptations in BNST circuitry that alter subsequent prosocial behaviors, including social vigilance, social recognition, and social preference.
To date, only a handful studies have investigated the role of the BNST in same-sex prosocial interactions between naïve (non-stressed) rodents. Despite this, there is evidence that a variety of BNST neuropeptide systems are involved in cell-type, circuit-, and sex-specific ways, including OXT, CRF, AVP, and orexin/hypocretin. Prosocial interactions with same-sex conspecifics increase both c-Fos and cocaine and amphetamine related transcript (CART) expression in the BNST (Perkins et al., 2017). Non-conditional chemogenetic activation of the BNST reduces same-sex social preference and social recognition in both males and females (Emmons et al., 2020), and pharmacological inhibition of BNST GABA synthesis reduces free prosocial interaction behaviors while increasing anxiety-like behavior (Sajdyk et al., 2008). Similarly, orexin-A infusion into the BNST reduces prosocial behaviors and increases anxiety-like behavior in male mice, an effect that is dependent on NMDAR-mediated depolarization of BNST target neurons (Lungwitz et al. 2010). Repeated sub-anxiogenic doses of UCN1, a ligand of CRFR1, in the BNST causes a long-term (4 week) suppression of prosocial behaviors, an effect that is blocked by pre-treatment with CRFR1 antagonists (Lee et al., 2008). Thus, even in unstressed animals, the BNST CRF system negatively modulates prosocial behavior. Antagonism of AVP1aR in the BNST reduces prosocial behavior in both male and female California mice but only increases anxiety in males (Duque-Wilckens et al., 2016). Ablation of BNST AVP neurons reduces social recognition of same-sex conspecifics in male C57BL6/J mice, but does not affect this behavior in females (Whylings et al., 2020). Hence, there may be a sex-specific and species-specific role for BNST AVP in recognizing same-sex conspecifics. However, it is possible that the lack of effect of BNST AVP neuron ablation on social recognition in females was related to the estrous cycle phase of the female animals at the time of testing, which was not monitored in the study. OXTR antagonist infusion into the posterior BNST reduces social recognition of same-sex conspecifics in both sexes, but OXT infusion only increases social recognition in males (Dumais et al., 2016). The reasons for this finding remain unclear, as there are no differences in social interaction-induced OT release between the sexes (Dumais et al., 2016). In contrast, a recent study found that both males and females display reductions in social approach behavior following infusion of OXT into the anterior BNST (Duque-Wilckens et al., 2020). The discrepancies between these studies may be due to the fact that experimenters did not specifically manipulate intra- versus extra-BNST OXT sources or that they targeted different sub-regions of the BNST (anterior versus posterior). As mentioned in the above sections on other types of social behavior, future studies investigating the role of BNST OXT signaling in prosocial interactions should utilize cell-type and circuit-specific tools that can perform in-vivo manipulations of BNST OXT sources individually and in concert.
Healthy prosocial behaviors are disrupted by a variety of environmental factors, most prominently stress and drugs of abuse. For example, social defeat stress, maternal separation stress, drug withdrawal, and chronic social isolation stress induce social avoidance of and promote social vigilance toward same-sex conspecifics (Overstreet et al., 2002; Blanco-Gandía et al., 2015; Becker et al., 2017; Duque-Wilckens et al., 2018; Matthews & Tye, 2019). Stress and/or drugs of abuse are also significant factors leading to the development of various psychiatric disorders that are characterized by impairments in prosocial behavior, including social anxiety disorder (SAD), substance use disorder (SUD), anti-social personality disorder, and depression (Jamieson et al., 2013; Cacioppo & Cacioppo, 2014; Blanco-Gandía et al., 2015; Castellano et al., 2015). In order to develop more effective treatments for psychiatric patients displaying social behavioral symptoms, a better understanding of the neurobiological substrates that drive these symptoms is required. Given its role in healthy prosocial behaviors (see above) and its responsivity to stress and drugs of abuse (Kash et al. 2015), the BNST likely underlies multiple aspects of stress and drug-induced impairments in prosocial behavior.
Similar to maternal behavior, impairment of same-sex prosocial behavior by stress appears to be mediated, at least in part, by dysregulation of BNST CRF and OXT systems. Intranasal OXT treatment reduces prosocial approach, increases social vigilance (orienting towards social target but not approaching it), and increases expression of the immediate-early gene early growth response 1 (Egr1) in the anterior BNST of female, but not male, California mice exposed to social defeat stress (Duque-Wilckens et al., 2018). Infusion of an OXTR antagonist into the anterior BNST of stressed female California mice increases social approach and reduces social vigilance (Duque-Wilckens et al., 2018). However, inhibition of OXT production in the BNST using a morpholino targeted to OXT mRNA prevents social defeat stress-induced impairments in social approach and social vigilance in both male and female California mice. Remarkably, vicarious social defeat stress in female C57BL6/J mice, whereby a female mouse observes social defeat of a familiar male by a male aggressor, increases social vigilance and the number of OXT-containing cell bodies in the ventral BNST (Duque-Wilckens et al., 2020). Thus, contrary to PVN OXT neurons projecting to the BNST, OXT-producing cells within the ventral and anterior BNST appear to negatively impact prosocial behaviors in both stressed and unstressed states. While the BNST CRF system also appears to negatively influence prosocial behaviors in the context of stress, this idea is informed by a relatively small number of studies. One found that intra-BNST delivery of a dual CRF receptor antagonist normalizes social avoidance following social defeat stress (Jasnow et al., 2004). Another found, somewhat surprisingly, that antagonism of CRF-BP in the BNST, which is generally thought to increase the availability of ligands for CRFR1, prevents reductions in social approach induced by social defeat stress (Vasconcelos et al., 2019). However, there have been some reports that CRF-BP can act in concert with CRFR2 to promote CRF’s effects (Ungless et al., 2003), which could better explain these results.
Some recent studies suggest that the BNST may differentially modulate social behavior depending on the stress history of the experimental animal. Cellular and synaptic adaptations occurring in BNST neurons as a result of stress exposure likely underlie these effects, but this has not been widely investigated. Social defeat stress, which induces prosocial avoidance, reduces GABA synthesis and increases brain derived neurotrophic factor (BDNF) protein and mRNA in the BNST (Greenberg et al., 2014a; Makinson et al., 2015). While intra-BNST infusion of a tyrosine receptor kinase-b (Trk-B) antagonist, which blocks BDNF’s activity, normalizes social avoidance induced by social defeat stress, it has no effect in unstressed mice (Greenberg et al., 2014a). Similarly, non-conditional inhibition of BNST neurons normalizes maternal separation stress-induced reductions in social preference and recognition, but does not have effects in control mice (Emmons et al., 2020). On the other hand, non-conditional chemogenetic activation of BNST neurons has no effect in mice that underwent juvenile maternal separation stress but is successful in reducing social preference in control mice (Emmons et al., 2020). This indicates that maternal separation stress induces such an intense state of BNST hyperactivity that exogenous activation is incapable of impairing social behavior further (a ceiling effect). Future studies should investigate whether cellular, molecular, and synaptic adaptations in particularly stress-responsive BNST neural populations, such as CRF neurons, underlie these effects of juvenile maternal separation stress on adult prosocial behavior.
Social withdrawal in SUD patients negatively contributes to treatment outcomes and increases incidences of relapse to drug and alcohol taking (Wise & Koob, 2014; Heilig et al., 2016). Moreover, social exclusion is associated with higher rates of SUD (Todd et al., 2004), suggesting that the neurobiological systems mediating responses to drugs and social interaction may dynamically regulate each other. While acute drug and alcohol intoxication tends to increase prosocial behavior in rodents due to reductions in anxiety and altered reward sensitivity, withdrawal from chronic drug and alcohol use is associated with reductions in prosocial behaviors (Varlinskaya & Spear, 2015; Becker et al., 2017). While many BNST systems are affected by drug and alcohol exposure (Kash et al., 2015), few of them have been explored as potential mediators of substance-induced social disruption. Despite this, there is some evidence that alterations in BNST serotonin signaling induced by chronic alcohol exposure contribute to alcohol-induced impairments in prosocial behavior during withdrawal. Marcinkiewcz et al. found that systemic treatment with a serotonin 2c receptor (5HT2cR) antagonist normalizes deficits in prosocial behavior and reduces ventral BNST hyperactivity associated with alcohol withdrawal (Marcinkiewcz et al., 2015). Furthermore, alcohol withdrawal increases neuronal excitability in the ventral BNST in a 5HT2cR-dependent manner, and intra-BNST infusion of a 5HT2cR antagonist is sufficient to normalize alcohol-induced social dysfunction. These results suggest that the BNST serotonin system, and particularly 5HT2cRs, are important for mediating the negative effects of alcohol withdrawal on social behavior. Whether 5HT2cRs play a similar role in social dysfunction associated with other drugs of abuse remains to be tested. The reduction of drug-withdrawal-related stress by social interaction may involve CRF signaling in the BNST, as a very recent study found that prosocial encounters can reduce the expression of BNST CRF mRNA induced by chronic cocaine treatment in the conditioned place preference task (Lemos et al., 2020). This indicates that states of heightened stress induced by drug-related adaptations in the BNST may be buffered by social interactions.
Human studies implicating the BNST in social behavior
Despite the importance of social behavior in daily life and its disruption in human disease, very little is known about the BNST’s role in social behavior in humans. This is largely due to the technical limitations of non-invasive neuroimaging techniques like functional Magnetic Resonance Imaging (fMRI), which have historically not provided sufficient spatial resolution to parse the BNST specifically. However, with the recent development of novel tools and methods that improve this spatial resolution, researchers have begun to investigate BNST function in human patients. Indeed, dysregulation of BNST function is a common feature among patients with generalized anxiety disorder and is thought to contribute to hypervigilance and maladaptive responses to uncertain threats (Buff et al., 2017; Brinkmann et al., 2018). This finding was recently extended to patients displaying social anxiety disorder (SAD). For example, patients with SAD display increased phasic BNST responses to temporally unpredictable aversive non-social stimuli (Figel et al., 2019). In addition, SAD is associated with greater functional connectivity between the PFC and BNST, as well as the anterior cingulate cortex (ACC) and the BNST, during exposure to temporally unpredictable aversive non-social stimuli (Clauss et al., 2019). Unfortunately, whether the BNST is also important for mediating aversive responses to social stimuli in SAD patients has not been tested. Interestingly, a very recent study found an association between BNST activation and daily helping (opening doors for people, giving directions, taking photos, etc.) in both normal adults and adults who had engaged in acts of extraordinary altruism (Vekaria et al., 2020). BNST activation during an empathy task predicted acts of everyday helping over a subsequent 14 day period in both groups and was particularly strong for acts of helping strangers and proactive helping. These results may indicate that in healthy patients with relatively low baseline BNST activation, the BNST enhances vigilance for socio-affective environmental cues to promote engagement in prosocial behaviors.
Conclusions and future directions
While much work remains to be done, there is a clear body of evidence implicating the BNST in a wide variety of rodent social behaviors. So far, this evidence suggests that the BNST guides both sex-dependent and sex-independent social behaviors in relatively similar ways. Across social behaviors, the BNST facilitates accurate social recognition and social context assessment through the integration of relevant sensory information: What is the sex, sexual history, stress history, age, and novelty of a given conspecific? The BNST assesses the valence of a given social interaction: Does the conspecific pose a physical or emotional threat, or will the interaction be rewarding? This depends of course on the identities of the animals engaged in the social interaction, their internal motivational states, and the context of the social interaction. Unfortunately, this role of the BNST has been less widely explored than the role of the BNST in social recognition. To elucidate the precise role of the BNST in social reward and motivation, future studies should utilize a wider range of behavioral models, including social conditioned place preference and self-administration. Finally, once the BNST has processed this incoming information regarding internal and external stimuli, it guides appropriate behavioral responses through outputs to specific downstream regions: Should the conspecific be ignored, avoided, or approached? If the conspecific is approached, how should it be engaged with?
As mentioned above, exposure to stress or drugs of abuse induce myriad alterations in BNST neurons that drive negative behavioral states. As a result, BNST manipulations often have different effects on social behavior in stress/drug exposed animals versus control animals. It is likely that exposure to stress or drugs alters the baseline activity of many BNST cell populations such that further exacerbating these BNST alterations has no further impact on stress- or drug-induced social impairments. Conversely, driving these BNST alterations in control animals may have no effect or an entirely opposing effect, as the homeostatic starting point is much different. The mechanisms by which these divergent effects occur likely involve micro-circuit interactions between BNST systems controlling innate social behaviors and BNST systems mediating responses to stress and drugs. Elucidation of the specific BNST systems that are responsible for driving the negative effects of stress and drugs on social behaviors will help inform future treatment options for many neuropsychiatric patients suffering from social dysfunction.
Figure 1:
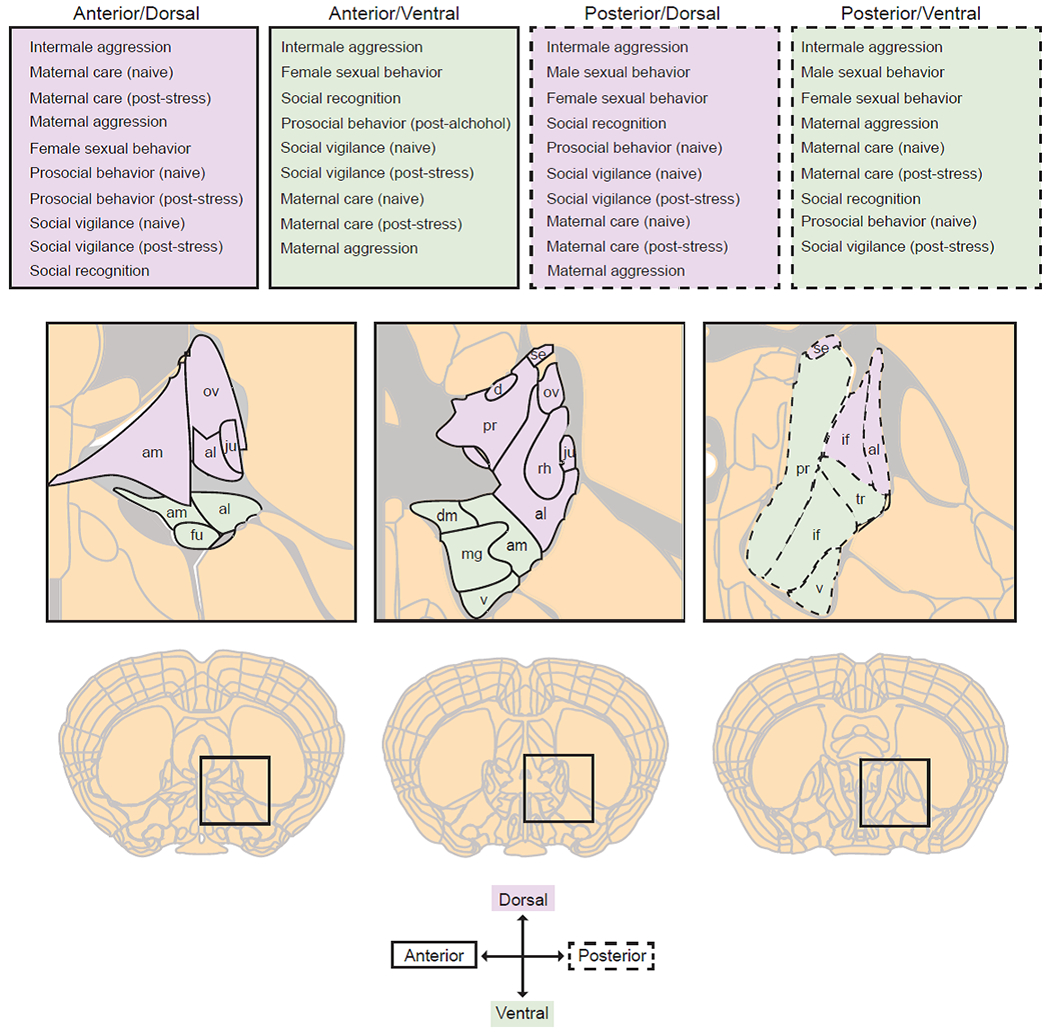
Diagram of BNST sub-regions according to the Allen Brain Adult Mouse Reference Atlas and list of social behaviors associated with each sub-region; purple regions with solid outline = anterior/dorsal BNST, green regions with solid outline = anterior/ventral, purple regions with dashed outline = posterior/dorsal, green regions with dashed outline = posterior/ventral. Sub-nuclei abbreviations: am = anteromedial, ov = oval, al = anterolateral, fu = fusiform, ju = juxtacapsular, dm = dorsomedial, mg = magnocellular, pr = principal, v = ventral, rh = rhomboid, se = strial extension, d = dorsal, if = intrafascicular, tr = transverse.
Acknowledgements
This work was supported by National Institute on Alcohol Abuse and Alcoholism grants T32AA007573 (M.F.), R01AA019454 (T.K.), U01AA020911 (T.K.), and R01AA025582 (T.K.).
List of abbreviations
- 5HT2cR
Serotonin 2c receptor
- ACC
Anterior cingulate cortex
- ACh
Acetylcholine
- AOB
Accessory olfactory bulb
- AR
Androgen receptor
- AVP
Arginine vasopressin
- AVPR1a
Arginine vasopressin receptor 1a
- BDNF
Brain-derived neurotrophic factor
- BNST
Bed nucleus of stria terminalis
- CART
Cocaine and amphetamine
- CCK
Cholecystokinin
- CeA
Central amygdala
- CRF
Corticotropin-releasing factor
- CRF-BP
Corticotropin-releasing factor binding protein
- CRFR1
Corticotropin-releasing factor receptor 1
- CRFR2
Corticotropin-releasing factor receptor 2
- DRN
Dorsal raphe
- Dyn
Dynorphin
- Enk
Enkephalin
- GAD
General anxiety disorder
- LHb
Lateral habenula
- LS
Lateral septum
- MeA
Medial amygdala
- mGluR7
Metabotropic glutamate receptor 7
- mPFC
Medial prefrontal cortext
- NAc
Nucleus accumbens
- NC
Nociceptin
- NMDAR
N-methyl-D-aspartate receptor
- NPY
Neuropeptide Y
- NTS
Neurotensin
- OB
Olfactory bulb
- OXT
Oxytocin
- OXTR
Oxytocin receptor
- PACAP
Pituitary-adenylate cyclase activating polypeptide
- PAG
Peri-aqueductal gray
- PTSD
Post-traumatic stress disorder
- PVN
Paraventricular hypothalamus
- RI
Resident intruder
- SAD
Social anxiety disorder
- SOM
Somatostatin
- SUD
Substance use disorder
- Trk-B
Tyrosine receptor kinase B
- UCN1
Urocortin 1
- vGAT
Vesicular GABA transporter
- vGlut2
Vesicular glutamate transporter 2
- vGlut3
Vesicular glutamate transporter 3
- VMH
Ventromedial hypothalamus
- VTA
Ventral tegmental area
Footnotes
Conflict of Interest Statement
The authors declare no conflicts of interest.
Data Availability
There is no original data associated with this manuscript.
References
- Aleyasin H, Flanigan ME, Golden SA, Takahashi A, Menard C, Pfau ML, Multer J, Pina J, McCabe KA, Bhatti N, Hodes GE, Heshmati M, Neve RL, Nestler EJ, Heller EA & Russo SJ (2018) Cell-Type-Specific Role of ΔFosB in Nucleus Accumbens In Modulating Intermale Aggression. J Neurosci, 38, 5913–5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anpilov S, Shemesh Y, Eren N, Harony-Nicolas H, Benjamin A, Dine J, Oliveira VEM, Forkosh O, Karamihalev S, Hüttl RE, Feldman N, Berger R, Dagan A, Chen G, Neumann ID, Wagner S, Yizhar O & Chen A (2020) Wireless Optogenetic Stimulation of Oxytocin Neurons in a Semi-natural Setup Dynamically Elevates Both Pro-social and Agonistic Behaviors. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA & Blackford JU (2016) The Human BNST: Functional Role in Anxiety and Addiction. Neuropsychopharmacology, 41, 126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Cooper TT, Karom M & Albers HE (1996) Glutamate and vasopressin interact to control scent marking in Syrian hamsters (Mesocricetus auratus). Brain Res, 731, 213–216. [DOI] [PubMed] [Google Scholar]
- Barnea-Ygael N, Gal R & Zangen A (2016) Chronic cocaine administration induces long-term impairment in the drive to obtain natural reinforcers in high- but not low-demanding tasks. Addiction biology, 21, 294–303. [DOI] [PubMed] [Google Scholar]
- Bartal IB-A, Decety J & Mason P (2011) Empathy and pro-social behavior in rats. Science, 334, 1427–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless DW, Yang T, Mason MM, Susanto AAT, Lobdell A & Shah NM (2019) Limbic Neurons Shape Sex Recognition and Social Behavior in Sexually Naive Males. Cell, 176, 1190–1205.e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JAJ, Kieffer BL & Le Merrer J (2017) Differential behavioral and molecular alterations upon protracted abstinence from cocaine versus morphine, nicotine, THC and alcohol. Addiction biology, 22, 1205–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Fukunaga-Stinson C, Takahashi LK, Flannelly KJ & Blanchard RJ (1984) Dominance and aggression in social groups of male and female rats. Behav Processes, 9, 31–48. [DOI] [PubMed] [Google Scholar]
- Blanco-Gandía MC, Mateos-García A, García-Pardo MP, Montagud-Romero S, Rodríguez-Arias M, Miñarro J & Aguilar MA (2015) Effect of drugs of abuse on social behaviour: a review of animal models. Behav Pharmacol, 26, 541–570. [DOI] [PubMed] [Google Scholar]
- Bosch O, Pförtsch J, Beiderbeck D, Landgraf R & Neumann I (2010) Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. Journal of neuroendocrinology, 22, 420–429. [DOI] [PubMed] [Google Scholar]
- Bosch OJ (2011) Maternal nurturing is dependent on her innate anxiety: the behavioral roles of brain oxytocin and vasopressin. Horm Behav, 59, 202–212. [DOI] [PubMed] [Google Scholar]
- Bosch OJ (2013) Maternal aggression in rodents: brain oxytocin and vasopressin mediate pup defence. Philos Trans R Soc Lond B Biol Sci, 368, 20130085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayley KN & Albert DJ (1977) Suppression of VMH-lesion-induced reactivity and aggressiveness in the rat by stimulation of lateral septum, but not medial septum or cingulate cortex. J Comp Physiol Psych, 91, 290–299. [DOI] [PubMed] [Google Scholar]
- Brinkmann L, Buff C, Feldker K, Neumeister P, Heitmann CY, Hofmann D, Bruchmann M, Herrmann MJ & Straube T (2018) Inter-individual differences in trait anxiety shape the functional connectivity between the bed nucleus of the stria terminalis and the amygdala during brief threat processing. Neuroimage, 166, 110–116. [DOI] [PubMed] [Google Scholar]
- Buff C, Brinkmann L, Bruchmann M, Becker MPI, Tupak S, Herrmann MJ & Straube T (2017) Activity alterations in the bed nucleus of the stria terminalis and amygdala during threat anticipation in generalized anxiety disorder. Soc Cogn Affect Neurosci, 12, 1766–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT & Cacioppo S (2014) Social Relationships and Health: The Toxic Effects of Perceived Social Isolation. Soc Personal Psychol Compass, 8, 58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnoli F, de Boer SF, Beiderbeck DI, Althaus M, Koolhaas JM & Neumann ID (2014) Local oxytocin expression and oxytocin receptor binding in the male rat brain is associated with aggressiveness. Behav Brain Res, 261, 315–322. [DOI] [PubMed] [Google Scholar]
- Campi KL, Jameson CE & Trainor BC (2013) Sexual Dimorphism in the Brain of the Monogamous California Mouse (Peromyscus californicus). Brain Behav Evol, 81, 236–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB & Swanson LW (1995) Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol, 360, 213–245. [DOI] [PubMed] [Google Scholar]
- Castellano F, Bartoli F, Crocamo C, Gamba G, Tremolada M, Santambrogio J, Clerici M & Carrà G (2015) Facial emotion recognition in alcohol and substance use disorders: A meta-analysis. Neurosci Biobehav Rev, 59, 147–154. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S & Meaney MJ (2001) Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A, 98, 12736–12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claro F, Segovia S, Guilamón A & Del Abril A (1995) Lesions in the medial posterior region of the BST impair sexual behavior in sexually experienced and inexperienced male rats. Brain research bulletin, 36, 1–10. [DOI] [PubMed] [Google Scholar]
- Clauss JA, Avery SN, Benningfield MM & Blackford JU (2019) Social anxiety is associated with BNST response to unpredictability. Depression and Anxiety, 36, 666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consiglio AR, Borsoi A, Pereira GA & Lucion AB (2005) Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiol Behav, 85, 354–362. [DOI] [PubMed] [Google Scholar]
- Cooper MA & Huhman KL (2010) Blocking corticotropin-releasing factor-2 receptors, but not corticotropin-releasing factor-1 receptors or glucocorticoid feedback, disrupts the development of conditioned defeat. Physiol Behav, 101, 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, Muller JF, Young LJ & Rainnie DG (2011) Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology, 36, 1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BJ, Macrides F, Youngs WM, Schneider SP & Rosene DL (1978) Efferents and centrifugal afferents of the main and accessory olfactory bulbs in the hamster. Brain Research Bulletin, 3, 59–72. [DOI] [PubMed] [Google Scholar]
- Davis ES & Marler CA (2004) c-fos Changes following an aggressive encounter in female California mice: a synthesis of behavior, hormone changes and neural activity. Neuroscience, 127, 611–624. [DOI] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW & Malenka RC (2013) Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature, 501, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW & Swanson LW (2004a) Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol, 468, 277–298. [DOI] [PubMed] [Google Scholar]
- Dong HW & Swanson LW (2004b) Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol, 471, 396–433. [DOI] [PubMed] [Google Scholar]
- Dong HW & Swanson LW (2006a) Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol, 494, 142–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW & Swanson LW (2006b) Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol, 494, 75–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Alonso AG, Immormino MA, Bredewold R & Veenema AH (2016) Involvement of the oxytocin system in the bed nucleus of the stria terminalis in the sex-specific regulation of social recognition. Psychoneuroendocrinology, 64, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM & Veenema AH (2016) Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol, 40, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Steinman MQ, Busnelli M, Chini B, Yokoyama S, Pham M, Laredo SA, Hao R, Perkeybile AM, Minie VA, Tan PB, Bales KL & Trainor BC (2018) Oxytocin Receptors in the Anteromedial Bed Nucleus of the Stria Terminalis Promote Stress-Induced Social Avoidance in Female California Mice. Biol Psychiatry, 83, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Steinman MQ, Laredo SA, Hao R, Perkeybile AM, Bales KL & Trainor BC (2016) Inhibition of vasopressin V1a receptors in the medioventral bed nucleus of the stria terminalis has sex- and context-specific anxiogenic effects. Neuropharmacology, 110, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Torres LY, Yokoyama S, Minie VA, Tran AM, Petkova SP, Hao R, Ramos-Maciel S, Rios RA, Jackson K, Flores-Ramires FJ, Garcia-Carachure I, Pesavento PA, Iñiguez SD, Grinevich V & Trainor BC (2020) Extra-hypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. bioRxiv, 2020.2006.2002.129981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons R, Sadok T, Rovero NG, Belnap MA, Henderson HJM, Quan AJ, Del Toro NJ & Halladay LR (2020) Chemogenetic manipulation of the bed nucleus of the stria terminalis counteracts social behavioral deficits induced by early life stress in C57BL/6J mice. J Neurosci Res. [DOI] [PubMed] [Google Scholar]
- Falkner AL, Grosenick L, Davidson TJ, Deisseroth K & Lin D (2016) Hypothalamic control of male aggression-seeking behavior. Nat Neurosci, 19, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero DM, Moeller LM, Osakada T, Horio N, Li Q, Roy DS, Cichy A, Spehr M, Touhara K & Liberles SD (2013) A juvenile mouse pheromone inhibits sexual behaviour through the vomeronasal system. Nature, 502, 368–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Kulkarni P, Murugavel M, Blanchard R, Blanchard DC, Febo M, Brevard M & Simon NG (2008) Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neurosci, 9, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figel B, Brinkmann L, Buff C, Heitmann CY, Hofmann D, Bruchmann M, Becker MPI, Herrmann MJ & Straube T (2019) Phasic amygdala and BNST activation during the anticipation of temporally unpredictable social observation in social anxiety disorder patients. Neuroimage Clin, 22, 101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G, Sumner BE, Rosie R, Grace O & Quinn JP (1996) Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol, 16, 325–344. [DOI] [PubMed] [Google Scholar]
- Flanigan ME, Aleyasin H, Li L, Burnett CJ, Chan KL, LeClair KB, Lucas EK, Matikainen-Ankney B, Durand-de Cuttoli R, Takahashi A, Menard C, Pfau ML, Golden SA, Bouchard S, Calipari ES, Nestler EJ, DiLeone RJ, Yamanaka A, Huntley GW, Clem RL & Russo SJ (2020) Orexin signaling in GABAergic lateral habenula neurons modulates aggressive behavior in male mice. Nat Neurosci, 23, 638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannelly KJ, Blanchard RJ, Muraoka MY & Flannelly L (1982) Copulation increases offensive attack in male rats. Physiol Behav, 29, 381–385. [DOI] [PubMed] [Google Scholar]
- Forray MI & Gysling K (2004) Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Brain Res Rev, 47, 145–160. [DOI] [PubMed] [Google Scholar]
- Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP & Marler CA (2010) Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc Natl Acad Sci U S A, 107, 12393–12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Heins C, Venniro M, Caprioli D, Zhang M, Epstein DH & Shaham Y (2017) Compulsive Addiction-like Aggressive Behavior in Mice. Biol Psychiatry, 82, 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, Aleyasin H, Menard C, Zhang H, Hodes GE, Bregman D, Khibnik L, Tai J, Rebusi N, Krawitz B, Chaudhury D, Walsh JJ, Han MH, Shapiro ML & Russo SJ (2016) Basal forebrain projections to the lateral habenula modulate aggression reward. Nature, 534, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Jin M, Heins C, Venniro M, Michaelides M & Shaham Y (2019) Nucleus Accumbens Drd1-Expressing Neurons Control Aggression Self-Administration and Aggression Seeking in Mice. J Neurosci, 39, 2482–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald SR & Thurman SK (1994) The Effects of Prenatal Cocaine Exposure on Mother--Infant Interaction and Infant Arousal in the Newborn Period. Topics in Early Childhood Special Education, 14, 217–231. [Google Scholar]
- Greenberg G, Laman-Maharg A, Campi K, Voigt H, Orr V, Schaal L & Trainor B (2014a) Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Frontiers in Behavioral Neuroscience, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg GD, Howerton CL & Trainor BC (2014b) Fighting in the home cage: Agonistic encounters and effects on neurobiological markers within the social decision-making network of house mice (Mus musculus). Neurosci Lett, 566, 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarraci FA & Bolton JL (2014) “Sexy stimulants”: The interaction between psychomotor stimulants and sexual behavior in the female brain. Pharmacology Biochemistry and Behavior, 121, 53–61. [DOI] [PubMed] [Google Scholar]
- Guarraci FA & Clark AS (2003) Amphetamine modulation of paced mating behavior. Pharmacology, biochemistry, and behavior, 76, 505–515. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC & Deisseroth K (2014) Natural neural projection dynamics underlying social behavior. Cell, 157, 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor NZ & Paré D (2016) Functional Heterogeneity in the Bed Nucleus of the Stria Terminalis. J Neurosci, 36, 8038–8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Tóth M, Halasz J & De Boer SF (2006) Patterns of violent aggression-induced brain c-fos expression in male mice selected for aggressiveness. Physiol Behav, 88, 173–182. [DOI] [PubMed] [Google Scholar]
- Hamilton LD & Meston CM (2013) Chronic stress and sexual function in women. The journal of sexual medicine, 10, 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasen NS & Gammie SC (2005) Differential fos activation in virgin and lactating mice in response to an intruder. Physiol Behav, 84, 681–695. [DOI] [PubMed] [Google Scholar]
- Heilig M, Epstein DH, Nader MA & Shaham Y (2016) Time to connect: bringing social context into addiction neuroscience. Nat Rev Neurosci, 17, 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr NR, Park J, McElligott ZA, Belle AM, Carelli RM & Wightman RM (2012) In vivo voltammetry monitoring of electrically evoked extracellular norepinephrine in subregions of the bed nucleus of the stria terminalis. J Neurophysiol, 107, 1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess WR (1927) Stammganglien-reizversuche. Verlag Julius Springer. [Google Scholar]
- Hong W, Kim DW & Anderson DJ (2014) Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell, 158, 1348–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y, Earley RL & Wolf LL (2006) Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol Rev Camb Philos Soc, 81, 33–74. [DOI] [PubMed] [Google Scholar]
- Jamieson JP, Nock MK & Mendes WB (2013) Changing the conceptualization of stress in social anxiety disorder: Affective and physiological consequences. Clinical psychological science, 1, 363–374. [Google Scholar]
- Janeček M & Dabrowska J (2019) Oxytocin facilitates adaptive fear and attenuates anxiety responses in animal models and human studies-potential interaction with the corticotropin-releasing factor (CRF) system in the bed nucleus of the stria terminalis (BNST). Cell Tissue Res, 375, 143–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett TM, McMurray MS, Walker CH & Johns JM (2006) Cocaine treatment alters oxytocin receptor binding but not mRNA production in postpartum rat dams. Neuropeptides, 40, 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Davis M & Huhman KL (2004) Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behav Neurosci, 118, 1052–1061. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL & Stuber GD (2013) Distinct extended amygdala circuits for divergent motivational states. Nature, 496, 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, Li L & Pedersen CA (1997) Effects of Short- and Long-Term Withdrawal from Gestational Cocaine Treatment on Maternal Behavior and Aggression in Sprague-Dawley Rats. Developmental Neuroscience, 19, 368–374. [DOI] [PubMed] [Google Scholar]
- Kash TL, Pleil KE, Marcinkiewcz CA, Lowery-Gionta EG, Crowley N, Mazzone C, Sugam J, Hardaway JA & McElligott ZA (2015) Neuropeptide regulation of signaling and behavior in the BNST. Mol Cells, 38, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Venkataraju KU, Pradhan K, Mende C, Taranda J, Turaga SC, Arganda-Carreras I, Ng L, Hawrylycz MJ & Rockland KS (2015) Mapping social behavior-induced brain activation at cellular resolution in the mouse. Cell reports, 10, 292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn J & Floody OR (1985) Differential effects of lesions in three limbic areas on ultrasound production and lordosis by female hamsters. Behav Neurosci, 99, 1142–1152. [DOI] [PubMed] [Google Scholar]
- Klampfl SM & Bosch OJ (2019) When mothers neglect their offspring: an activated CRF system in the BNST is detrimental for maternal behavior. Arch Womens Ment Health, 22, 409–415. [DOI] [PubMed] [Google Scholar]
- Klampfl SM, Brunton PJ, Bayerl DS & Bosch OJ (2014) Hypoactivation of CRF receptors, predominantly type 2, in the medial-posterior BNST is vital for adequate maternal behavior in lactating rats. J Neurosci, 34, 9665–9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klampfl SM, Brunton PJ, Bayerl DS & Bosch OJ (2016a) CRF-R1 activation in the anterior-dorsal BNST induces maternal neglect in lactating rats via an HPA axis-independent central mechanism. Psychoneuroendocrinology, 64, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klampfl SM, Schramm MM, Stinnett GS, Bayerl DS, Seasholtz AF & Bosch OJ (2016b) Brain CRF-binding protein modulates aspects of maternal behavior under stressful conditions and supports a hypo-anxious state in lactating rats. Horm Behav, 84, 136–144. [DOI] [PubMed] [Google Scholar]
- Kohtz AS, Walf AA & Frye CA (2019a) Effects of non-contingent cocaine on 3 alpha-androstanediol. II. Disruption of lordosis of proestrous rats. Physiol Behav, 203, 113–119. [DOI] [PubMed] [Google Scholar]
- Kohtz AS, Walf AA & Frye CA (2019b) Effects of non-contingent cocaine on 3alpha-androstanediol. I. Disruption of male sexual behavior. Physiol Behav, 203, 120–127. [DOI] [PubMed] [Google Scholar]
- Kompier NF, Keysers C, Gazzola V, Lucassen PJ & Krugers HJ (2019) Early Life Adversity and Adult Social Behavior: Focus on Arginine Vasopressin and Oxytocin as Potential Mediators. Front Behav Neurosci, 13, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono R, Tashiro N & Nakao H (1986) Inhibitory effects of acetylcholine on aggressive-defense reaction induced by electrical stimulation of the hypothalamus in cats. Brain research bulletin, 16, 491–495. [DOI] [PubMed] [Google Scholar]
- Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, Minami M & Watanabe M (2012) Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J Neurosci, 32, 18035–18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme N, Nappi RE, Drolet G, Labrie C & Rivest S (1998) Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol, 36, 357–378. [DOI] [PubMed] [Google Scholar]
- Lebow MA & Chen A (2016) Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry, 21, 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Fitz S, Johnson PL & Shekhar A (2008) Repeated stimulation of CRF receptors in the BNST of rats selectively induces social but not panic-like anxiety. Neuropsychopharmacology, 33, 2586–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos C, Salti A, Amaral IM, Fontebasso V, Singewald N, Dechant G, Hofer A & El Rawas R (2020) Social interaction reward in rats has anti-stress effects. Addiction biology, e12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P & Anderson DJ (2011) Functional identification of an aggression locus in the mouse hypothalamus. Nature, 470, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Salamone JD & Sachs BD (1997) Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J Neurosci, 17, 5245–5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS & Gammie SC (2002) Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev, 26, 869–888. [DOI] [PubMed] [Google Scholar]
- Maeda H (1977) Septum and aggression: A review. [Septum and aggression: A review.]. Kyushu Neuro-psychiatry, 23, 7–16. [Google Scholar]
- Makinson R, Lundgren KH, Seroogy KB & Herman JP (2015) Chronic social subordination stress modulates glutamic acid decarboxylase (GAD) 67 mRNA expression in central stress circuits. Physiology & behavior, 146, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano Nieves G, Schilit Nitenson A, Lee HI, Gallo M, Aguilar Z, Johnsen A, Bravo M & Bath KG (2019) Early Life Stress Delays Sexual Maturation in Female Mice. Front Mol Neurosci, 12, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Dorrier CE, Lopez AJ & Kash TL (2015) Ethanol induced adaptations in 5-HT2c receptor signaling in the bed nucleus of the stria terminalis: implications for anxiety during ethanol withdrawal. Neuropharmacology, 89, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Norvelle A & Huhman KL (2009) Role of the bed nucleus of the stria terminalis in the acquisition and expression of conditioned defeat in Syrian hamsters. Behav Brain Res, 198, 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA & Petrulis A (2011) The bed nucleus of the stria terminalis is critical for sexual solicitation, but not for opposite-sex odor preference, in female Syrian hamsters. Horm Behav, 60, 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon D & Dabrowska J (2018) Corticotropin-Releasing Factor Receptors Modulate Oxytocin Release in the Dorsolateral Bed Nucleus of the Stria Terminalis (BNST) in Male Rats. Front Neurosci, 12, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masugi-Tokita M, Flor PJ & Kawata M (2016) Metabotropic Glutamate Receptor Subtype 7 in the Bed Nucleus of the Stria Terminalis is Essential for Intermale Aggression. Neuropsychopharmacology, 41, 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews GA & Tye KM (2019) Neural mechanisms of social homeostasis. Ann N Y Acad Sci, 1457, 5–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone CM, Pati D, Michaelides M, DiBerto J, Fox JH, Tipton G, Anderson C, Duffy K, McKlveen JM, Hardaway JA, Magness ST, Falls WA, Hammack SE, McElligott ZA, Hurd YL & Kash TL (2018) Acute engagement of G(q)-mediated signaling in the bed nucleus of the stria terminalis induces anxiety-like behavior. Mol Psychiatry, 23, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meddle SL, Bishop VR, Gkoumassi E, van Leeuwen FW & Douglas AJ (2007) Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology, 148, 5095–5104. [DOI] [PubMed] [Google Scholar]
- Miczek KA, de Boer SF & Haller J (2013) Excessive aggression as model of violence: a critical evaluation of current preclinical methods. Psychopharmacology (Berl), 226, 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, DeBold JF, Hwa LS, Newman EL & de Almeida RM (2015) Alcohol and violence: neuropeptidergic modulation of monoamine systems. Ann N Y Acad Sci, 1349, 96–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW & Faccidomo S (2001) Aggressive behavioral phenotypes in mice. Behav Brain Res, 125, 167–181. [DOI] [PubMed] [Google Scholar]
- Moffitt JR, Bambah-Mukku D, Eichhorn SW, Vaughn E, Shekhar K, Perez JD, Rubinstein ND, Hao J, Regev A, Dulac C & Zhuang X (2018) Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga TN, Tan G, Ostermann HJ, Monga U & Grabois M (1998) Sexuality and sexual adjustment of patients with chronic pain. Disabil Rehabil, 20, 317–329. [DOI] [PubMed] [Google Scholar]
- Naik RR & de Jong TR (2017) Transient and persistent behavioral and molecular changes in primiparous female Wistar rats. Eur J Neurosci, 45, 797–804. [DOI] [PubMed] [Google Scholar]
- Nelson CJ, Meter KE, Walker CH, Ayers AA & Johns JM (1998) A Dose–Response Study of Chronic Cocaine on Maternal Behavior in Rats. Neurotoxicology and Teratology, 20, 657–660. [DOI] [PubMed] [Google Scholar]
- Newman EL, Covington HE 3rd, Suh J, Bicakci MB, Ressler KJ, DeBold JF & Miczek KA (2019) Fighting Females: Neural and Behavioral Consequences of Social Defeat Stress in Female Mice. Biol Psychiatry, 86, 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni RJ, Luo PH, Shu YM, Chen JT & Zhou JN (2016) Whole-brain mapping of afferent projections to the bed nucleus of the stria terminalis in tree shrews. Neuroscience, 333, 162–180. [DOI] [PubMed] [Google Scholar]
- Nordman JC, Ma X, Gu Q, Potegal M, Li H, Kravitz AV & Li Z (2020) Potentiation of Divergent Medial Amygdala Pathways Drives Experience-Dependent Aggression Escalation. J Neurosci, 40, 4858–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell LA & Hofmann HA (2011) The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol, 519, 3599–3639. [DOI] [PubMed] [Google Scholar]
- Orso R, Creutzberg KC, Wearick-Silva LE, Wendt Viola T, Tractenberg SG, Benetti F & Grassi-Oliveira R (2019) How Early Life Stress Impact Maternal Care: A Systematic Review of Rodent Studies. Frontiers in Behavioral Neuroscience, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada T, Ishii KK, Mori H, Eguchi R, Ferrero DM, Yoshihara Y, Liberles SD, Miyamichi K & Touhara K (2018) Sexual rejection via a vomeronasal receptor-triggered limbic circuit. Nature Communications, 9, 4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ & Breese GR (2002) Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res, 26, 1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla SL, Qiu J, Soden ME, Sanz E, Nestor CC, Barker FD, Quintana A, Zweifel LS, Rønnekleiv OK, Kelly MJ & Palmiter RD (2016) Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat Neurosci, 19, 734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Bellver C, Cádiz-Moretti B, Novejarque A, Martínez-García F & Lanuza E (2012) Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice. Front Neuroanat, 6, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins AE, Woodruff ER, Chun LE, Spencer RL, Varlinskaya E & Deak T (2017) Analysis of c-Fos induction in response to social interaction in male and female Fisher 344 rats. Brain Res, 1672, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M & Luppi PH (1998) Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience, 82, 443–468. [DOI] [PubMed] [Google Scholar]
- Piccin A & Contarino A (2020) Long-lasting pseudo-social aggressive behavior in opiate-withdrawn mice. Prog Neuropsychopharmacol Biol Psychiatry, 97, 109780. [DOI] [PubMed] [Google Scholar]
- Pitcher MH, Tarum F, Lehmann M & Bushnell MC (2019) Persistent inflammatory pain alters sexually-motivated behavior in male rats. Behav Brain Res, 356, 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potegal M (1992) Time course of aggressive arousal in female hamsters and male rats. Behav Neural Biol, 58, 120–124. [DOI] [PubMed] [Google Scholar]
- Powers JB, Newman SW & Bergondy ML (1987) MPOA and BNST lesions in male Syrian hamsters: differential effects on copulatory and chemoinvestigatory behaviors. Behav Brain Res, 23, 181–195. [DOI] [PubMed] [Google Scholar]
- Rigney N, Whylings J, Mieda M, de Vries G & Petrulis A (2019) Sexually Dimorphic Vasopressin Cells Modulate Social Investigation and Communication in Sex-Specific Ways. eNeuro, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Ung RL, Nomura H, Otis JM, Basiri ML, Namboodiri VM, Zhu X, Robinson JE, McHenry JA, Kosyk O, Jhou TC, Kash TL, Bruchas MR & Stuber GD (2020) Prepronociceptin expressing neurons in the extended amygdala encode and promote rapid arousal responses to motivationally salient stimuli. bioRxiv, 2020.2001.2021.914341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk T, Johnson P, Fitz S & Shekhar A (2008) Chronic inhibition of GABA synthesis in the bed nucleus of the stria terminalis elicits anxiety-like behavior. J Psychopharmacol, 22, 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh MB, Brutus M, Siegel HE & Siegel A (1986) Regulation of feline aggression by the bed nucleus of stria terminalis. Brain Res Bull, 16, 179–182. [DOI] [PubMed] [Google Scholar]
- Shin JW, Geerling JC & Loewy AD (2008) Inputs to the ventrolateral bed nucleus of the stria terminalis. J Comp Neurol, 511, 628–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA, Laman-Maharg A, Manning CE, Doig IE, Lopez EM, Walch K, Bales KL & Trainor BC (2016) Sex-Specific Effects of Stress on Oxytocin Neurons Correspond With Responses to Intranasal Oxytocin. Biol Psychiatry, 80, 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Laredo SA, Lopez EM, Manning CE, Hao RC, Doig IE, Campi KL, Flowers AE, Knight JK & Trainor BC (2015) Hypothalamic vasopressin systems are more sensitive to the long term effects of social defeat in males versus females. Psychoneuroendocrinology, 51, 122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova JI, Ferris CF & Albers HE (2017) Sex Differences in the Regulation of Offensive Aggression and Dominance by Arginine-Vasopressin. Front Endocrinol (Lausanne), 8, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J, Green G, Harrison M, Ikuesan B, Self C, Pevalin DJ & Baldacchino A (2004) Social exclusion in clients with comorbid mental health and substance misuse problems. Social psychiatry and psychiatric epidemiology, 39, 581–587. [DOI] [PubMed] [Google Scholar]
- Toufexis D (2007) Region- and sex-specific modulation of anxiety behaviours in the rat. J Neuroendocrinol, 19, 461–473. [DOI] [PubMed] [Google Scholar]
- Unger EK, Burke KJ Jr., Yang CF, Bender KJ, Fuller PM & Shah NM (2015) Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep, 10, 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D & Bonci A (2003) Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron, 39, 401–407. [DOI] [PubMed] [Google Scholar]
- Valzelli L (1973) The “isolation syndrome” in mice. Psychopharmacologia, 31, 305–320. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI & Spear LP (2015) Social consequences of ethanol: Impact of age, stress, and prior history of ethanol exposure. Physiol Behav, 148, 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos M, Stein DJ, Albrechet-Souza L, Miczek KA & de Almeida RMM (2019) Recovery of stress-impaired social behavior by an antagonist of the CRF binding protein, CRF(6-33,) in the bed nucleus of the stria terminalis of male rats. Behav Brain Res, 357-358, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Beiderbeck DI, Lukas M & Neumann ID (2010) Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm Behav, 58, 273–281. [DOI] [PubMed] [Google Scholar]
- Vekaria KM, O’Connell K, Rhoads SA, Brethel-Haurwitz KM, Cardinale EM, Robertson EL, Walitt B, VanMeter JW & Marsh AA (2020) Activation in bed nucleus of the stria terminalis (BNST) corresponds to everyday helping. Cortex, 127, 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Russell TI, Ramsey LA, Richie CT, Lesscher HMB, Giovanetti SM, Messing RO & Shaham Y (2020) Abstinence-dependent dissociable central amygdala microcircuits control drug craving. Proc Natl Acad Sci U S A, 117, 8126–8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, Morales M, Epstein DH & Shaham Y (2018) Volitional social interaction prevents drug addiction in rat models. Nat Neurosci, 21, 1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse, 51, 32–58. [DOI] [PubMed] [Google Scholar]
- Welch JD, Kozareva V, Ferreira A, Vanderburg C, Martin C & Macosko EZ (2019) Single-Cell Multi-omic Integration Compares and Contrasts Features of Brain Cell Identity. Cell, 177, 1873–1887.e1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whylings J, Rigney N, Peters NV, de Vries GJ & Petrulis A (2020) Sexually dimorphic role of BNST vasopressin cells in sickness and social behavior in male and female mice. Brain Behav Immun, 83, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K & Reynolds MF (2006) Sexual dysfunction in major depression. CNS spectrums, 11, 19–23. [DOI] [PubMed] [Google Scholar]
- Wise RA & Koob GF (2014) The development and maintenance of drug addiction. Neuropsychopharmacology, 39, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N & Shah NM (2009) Estrogen masculinizes neural pathways and sex-specific behaviors. Cell, 139, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Ahmed N, Ito T, Gungor NZ & Pare D (2018) Optogenetic Study of Anterior BNST and Basomedial Amygdala Projections to the Ventromedial Hypothalamus. eNeuro, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no original data associated with this manuscript.


