Abstract
Age-related changes in the immune system increase susceptibility to infectious diseases. Vaccines are an important tool to prevent infection or boost immunological memory; however, vaccines are less effective in aged individuals. In order to protect our aging population from the threat of infectious diseases, we must gain a better understanding of age-related alterations in the immune response at the site of infection. The lung is one site of frequent infection in older individuals. In this study, we expanded on our previous work to study vaccine-induced immune responses in the local lung environment in a pilot study of aged rhesus macaques. To do this, we developed an in vivo model to probe recall responses to tuberculin challenge in the lungs 8 weeks and 16 weeks post-Mycobacterium bovis BCG vaccination by performing targeted bronchoalveolar lavages. In parallel, we determined peripheral blood responses in vaccinated animals to compare systemic and local tissue responses to tuberculin challenge. We found that following lung tuberculin challenge 8 weeks post-vaccination, aged animals had reduced T cell responses, particularly within the CD8+ T cell compartment. Aged animals had decreased CD8+ effector and memory T cell recall responses and less activated CD8+ T cells. This diminished lung CD8+ T cell response in aged animals was maintained over time. Despite changes in the CD8+ T cell compartment, lung CD4+ T cell responses were similar between age groups. In the peripheral blood, we observed age-related changes in immune cell populations and plasma levels of immune mediators that were present prior to vaccination. Lastly, we found that peripheral blood mononuclear cells from aged BCG-vaccinated animals were functional in their response to antigen stimulation, behaving in a similar manner to those from their adult counterparts. These systemic observations were similar to those found in our previous study of BCG-vaccinated baboons, supporting the notion that tissue immune responses, and not systemic responses, to vaccination and challenge are impaired with age. These findings expand on our previous work to show that in addition to the skin, age-related changes in the lung environment impact recall immune responses to vaccination and challenge. The impact of age on local tissue responses to infectious challenge should be accounted for in the development of therapeutics or medical interventions aimed at boosting immune recall responses of aged individuals.
Keywords: Aging, rhesus macaques, lung, tuberculin, immune response, BCG vaccination
1. INTRODUCTION
The population of individuals 65 years of age and older is predicted to nearly double by the year 2060 (1). This shift in demographics will have significant public health and economic consequences. As we age, we experience health-related changes, both physical and physiological, that impact quality of life and health outcomes (2). Age-related changes in our immune system contribute to the enhanced risk of our aging population to infection-related morbidity and mortality (3). The COVID-19 pandemic highlighted this susceptibility linking disease severity and death with increasing age (4). Vaccination is the most effective method to prevent infection and requires development of an adaptive immune system that is sustained to provide immunologic memory; however, many studies have shown that vaccines are less effective in the aging population (5). Reasons for this can include delayed immune responses, immune-senescence, and impaired local tissue responses (5). In this regard, studies have shown that tissue and peripheral responses differ in the elderly (6;7). In our previously published study, we observed reduced early immune responses to tuberculin challenge in the skin of BCG-vaccinated aged baboons relative to their adult counterparts (8). Despite these differences in the skin, we did not find any vaccine-induced changes in peripheral blood, including peripheral blood mononuclear (PBMC) composition, functional responses, or migration capabilities, in adult and aged animals suggesting that local tissue responses, and not systemic responses, to vaccination and challenge are impaired with age (8). Like the skin, the lung is a site of frequent infection. Ninety percent of deaths due to pneumonia and influenza occur in those aged 65 and older (9). The lung contains resident and recruited cells of both the innate and adaptive arms of the immune system (10-12). A better understanding of recall responses specific to the lung would allow for therapeutic intervention in the elderly to improve health outcomes. Lung fluid samples are, however, difficult to obtain from elderly humans due to ethical and safety reasons. New models are therefore needed to evaluate immune responses to infection in the aging lung.
Here we expand on our previous work (8) to study immune responses to BCG vaccination with a model of tuberculin challenge in the lung in BCG-vaccinated adult and aged rhesus macaques. We took advantage of the high homology between humans and rhesus macaques to establish an in vivo model of vaccination and lung challenge followed by bronchoalveolar lavage (BAL) to evaluate recall responses in the local lung environment. In this pilot study, three adult and three aged macaques were vaccinated with Mycobacterium bovis BCG (BCG) and the classical delayed-type hypersensitivity (DTH) reaction of the tuberculin skin test (TST) was adapted to the lung using targeted tuberculin instillation to evaluate antigen-specific recall responses in BAL fluid (BALF) (13;14). We tested recall responses at 8 weeks and 16 weeks post-vaccination to evaluate the impact of age on vaccine-induced tissue immunity over time. In parallel, peripheral responses were evaluated throughout the duration of the pilot study.
2. MATERIALS AND METHODS
2.1. Animal procedures
Studies were conducted in six (three adult and three aged) rhesus macaques at The Oregon National Primate Research Center (ONPRC) and Oregon Health and Science University (OHSU). All animals were SIV/HIV and TST negative and paired indoor housed in ABSL1 throughout the study duration. All procedures were approved by the University Institutional Animal Care and Use Committee at OHSU (Protocol #IP00003551) and by the OHSU Biosafety Committee (PROTO202100001). Animals were vaccinated with Mycobacterium bovis BCG (Pasteur strain, ATCC, Manassas, VA) via the intradermal route in the upper arm at a dose of 5 × 105 CFUs. Skin tests were performed on animals 8 and 16 weeks post-BCG vaccination by injecting 100 μL of tuberculin (Colorado Serum Company, Denver, CO) containing 5 tuberculin units of purified protein derivative (PPD) (15). Saline injections (100 μL of 0.9% NaCl, Baxter International Inc., Deerfield, IL) were performed in animals to serve as negative controls. All skin tests were performed in the chest skin and resulted in positive TST responses as determined by visual observation. The bronchoscopic challenge was adapted from a published study (14). On the day of and just prior to lung instillation of tuberculin (challenge) or 0.9% NaCl (control), a non-targeted BAL was performed of the right middle lobe for baseline measurements using up to 4 aliquots of a volume of 20mL pre-warmed 0.9% NaCl. Next, the challenge was performed by instillation of 0.5 tuberculin units (50 μL of the commercial preparation) in 10 ml of 0.9% NaCl into the right middle lobe. Last, the control was administered by instilling 10mL of 0.9% NaCl into the lower portion of the lung superior lobe. At 48- and 72-hours post-lung challenge, targeted BALs were performed to test for tuberculin-specific recall responses. Briefly, BAL was performed first on the right middle lobe followed by a second BAL of the lower left superior. BAL fluid (BALF) was collected in individual 50mL conical tubes and stored separately on ice. Each targeted BAL was collected using up to 4 aliquots of a volume of 20mL pre-warmed 0.9%. Blood was collected in sodium heparin-vacutainers according to the timeline in Fig. 1 and shipped overnight to Texas Biomed for processing. All procedures were performed under anesthesia (10 mg/kg Ketamine and 0.015 mg/kg Dexmedetomidine; Covetrus, Portland, ME), and included monitoring of weight, body temperature, heart rate, respiration rate and capillary refill time.
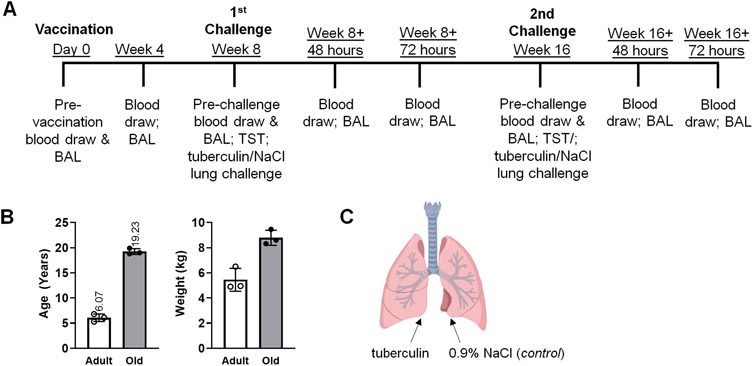
Figure 1. Establishment of an in vivo model of lung recall response to tuberculin in Mycobacterium bovis BCG (BCG)-vaccinated rhesus macaques.
(A) Study timeline: adult and aged macaques were BCG-vaccinated and challenged by lung instillation with tuberculin or saline (NaCl, control) for determination of recall responses over time. BALs were performed 48hr days and 72hr post-challenge. Peripheral blood was collected at the times indicated for determination of systemic responses in vaccinated macaques. (B) Age and weight of 3 adult and 3 aged macaques included in the study, shown as averages. (C) Schematic of tuberculin and NaCl (control) lung challenge. Saline instillation serves as the control for antigen-specific responses. Created with BioRender.com.
2.2. BALF processing for flow cytometric analysis
After collection on ice, BALF was centrifuged for 5 minutes, 4°C, 300g, using a low brake. Cell pellets were washed 2X in cold 1X PBS and counted. Cells were adjusted to a final concentration of 10 million cells/mL and 100μL of cell suspension was added to wells of a 96-well V-bottom plate. Cells were washed 1X with PBS and stained with Live/Dead Stain (Aqua; Biolegend; 1:1000 dilution) in 1X PBS for 15 minutes at RT in the dark. At the end of the Live/Dead incubation, 75μL of dRPMI+10% heat inactivated fetal bovine serum (FBS) (flow staining buffer) was added and cells were washed 1X with flow staining buffer. Following the wash, cells were stained with antibody cocktails (panels, isotypes/FMOs, or single-color compensations; Antibody List in Table S3) for 20 minutes in the dark at 4°C. At the end of the antibody incubation, 75μL of flow staining buffer was added to dilute the antibodies and then centrifuge for 5 minutes, 4°C, 300g, low brake. Cells were washed 1X with flow staining buffer and fixed in 2% PFA in 1x PBS for 15 minutes at RT in the dark. Cells were then washed 2X with flow staining buffer and re-suspended in a final volume of 200μL. All samples were analyzed using a BD LSRII flow cytometer. At least 50,000 events were counted and analyzed using FlowJo Version 10.7.0 and 10.8.0 Software.
2.3. PBMC isolation from whole blood
Blood was processed for whole blood staining as we published previously (8). Briefly, overnight-shipped blood samples were diluted with 1X PBS and Lymphocyte Separation Media (Corning Life Sciences, Tewksbury, MA), followed by centrifugation at 950 x g for 20 min at room temperature with no brake. After centrifugation, the PBMC interface was collected, washed once with 1X PBS, and red blood cells were lysed with incubation in freshly prepared lysis solution (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2-EDTA). Cells were washed to remove lysis solution and re-suspended in complete cell medium: 1X RPMI 1640 supplemented with 25 mM HEPES (MilliporeSigma), 10% heat-inactivated FBS (Atlas Biologicals, Fort Collins, CO), 1% HyClone, 1% L-glutamine, and 1% MEM Non-Essential Amino Acids (all from ThermoFisher Scientific).
2.4. PBMC stimulation
PBMCs were stimulated as we previously described (8). Briefly, PBMCs were plated in 96-well plates at a final concentration of 250,000 cells/well. Cells were stimulated for 5 days in the presence of media or Mycobacterium tuberculosis culture filtrate protein (CFP, 10 μg/mL), which contains BCG cross-reactive antigens (BEI Resources, Manassas, VA). Supernatants were collected at the end of the incubation and stored at −80°C.
2.5. Collection of plasma from whole blood
Blood in sodium-heparin vacutainers was centrifuged at 200 x g for 15 minutes at room temperature. Plasma was collected and stored at −80°C.
2.6. Whole blood staining for flow cytometric analysis
Flow cytometric analysis was performed on whole blood by staining 150μL whole blood with antibody cocktails (Table S3) prepared in deficient RPMI (dRPMI; RPMI-1640 supplemented with HEPES and 1g/L sodium azide [ThermoFisher Scientific]) with 10% heat-inactivated FBS (dRPMI+FBS) for 15 minutes at room temperature. After antibody incubation, red blood cells were lysed and cells were washed once with 1X PBS. Cells were then washed twice with dRPMI+FBS. After the final wash, cells were fixed with 2% paraformaldehyde (ThermoFisher Scientific) for 15 minutes at room temperature in the dark. All stained and fixed cells were suspended in dRPMI+FBS and stored at 4°C until analyzed using a BD FACS Symphony flow cytometer. At least 100,000 events were counted and analyzed using FlowJo Version 10.7.0 and 10.8.0 Software.
2.7. Luminex assay for measurement of immune mediators
Immune mediators were measured in cell supernatants and plasma using the NHP XL Cytokine 13plex for detection of CCL5, CCL20, CD40L, CXCL10, GM-CSF, IFNγ, IL1®, IL2, IL6, IL-10, PD-L1, TNFα, and VEGF (R&D Systems, Minneapolis, MN) according to kit instructions.
2.8. Statistical analyses
Data analysis, graphing, and statistical analysis was performed using GraphPad Prism version 8&9 (La Jolla, CA). Statistical analysis was performed as described in the figure legends: for multiple testing we used One-way ANOVA and Tukey’s post hoc correction and for testing the means between two groups we used Student’s t test. Statistical differences between groups were reported significant when the p-value is less than or equal to 0.05. The data are presented in mean ± SEM for n = 3 animals per age group. If values are missing from an animal or sample due to sample handling or collection issues, one two values were be shown.
3. RESULTS
3.1. Establishment of an in vivo model to test lung recall responses in BCG-vaccinated macaques
To determine the impact of age on vaccine-induced recall immunity in the local tissue environment, we vaccinated 3 adult (average of 6.07 years +/− 0.75) and 3 aged (average of 19.23 years +/− 0.59) female rhesus macaques with BCG via the intradermal route at a dose of 5-8 × 105 CFUs (timeline - Fig 1A; animal information - Fig. 1B and Table S1). Four weeks following BCG vaccination, the vaccine injection site was evaluated and aged animals had little to no noticeable changes in the skin at the injection site. In contrast, adult animals had raised lesions at the injection site suggesting localized post-vaccine immune responses (Table S2).
At 8 weeks post-vaccination and prior to the first challenge timepoint, TST tests were administered in all animals to ensure the presence of positive skin reactions to tuberculin. All animals had positive TST reactions at the first and second challenge time points (timeline, Fig 1A; data not shown). Baseline BALs were collected prior to lung instillation (Week 8 & 16, timeline, Fig. 1A). We then targeted instillations of tuberculin, or 0.9% saline (control), in the right middle lobe or lower portion of the left superior lobe of the lung, respectively, followed by BAL of the same lobes at specific times post TST instillation (schematic show in Fig. 1C). The first tuberculin challenge was performed 8 weeks post-vaccination, followed by BALs at 48- and 72-hours post-challenge (time chosen to correspond with the development of a skin DTH response). A second targeted instillation of tuberculin or control was performed 16 weeks post-vaccination, again followed by BALs, to determine recall responses long-term. Collected BALF was analyzed to evaluate the impact of age of cellular recall responses to tuberculin in the local lung environment (gating strategy, Figure S1).
3.2. Aged animals have a reduced lung T cell recall response to tuberculin challenge
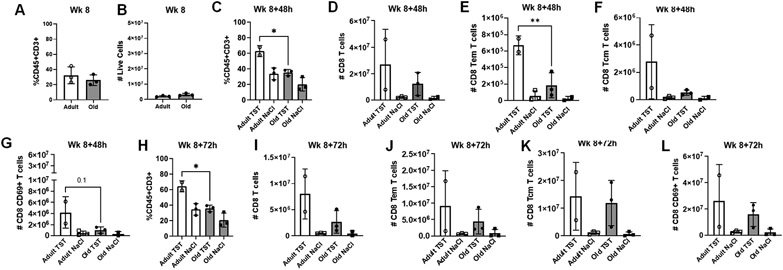
Prior to the first tuberculin challenge (8 weeks), no differences were observed in the BAL for the proportion of CD45+CD3+ (Fig. 2A) or total live cells (Fig. 2B) for adult or aged animals. At 48hrs post tuberculin challenge more CD45+CD3+ T cells accumulated in the BAL of adult relative to aged animals after 48 hours (Fig. 2C).
Figure 2. Adult macaques have increased T cells present in BALF following tuberculin instillation.
(A) Proportions of T cells (CD45+CD3+) in BALF of adult and aged macaques prior to tuberculin lung instillation (Week 8). (B) Number of live cells present in BALF of adult and aged macaques prior to tuberculin lung instillation (Week 8). (C) Proportions of T cells (CD45+CD3+) in BALF of adult and aged macaques 48 hours post-tuberculin challenge. (D) Number of CD8+ T cells, (E) CD8+ effector memory T cells, (F) CD8+ central memory T cells, and (G) CD69+CD8+T cells in BALF of adult and aged macaques 48 hours post-tuberculin challenge. (H) Proportions of T cells (CD45+CD3+) in BALF of adult and aged macaques 72 hours post-tuberculin challenge. (I) Number of CD8+ T cells, (J) CD8+ effector memory T cells, (K) CD8+ central memory T cells, and (L) CD69+CD8+ T cells in BALF of adult and aged macaques 72 hours post-tuberculin challenge. One-way ANOVA post-Tukey analyses comparing means between groups as indicated; *p<0.05, **p<0.01. TST = tuberculin instillation; NaCl = saline (control) instillation.
Prior to tuberculin challenge, CD8+ T cell numbers did not differ between adult and aged animals (Fig. S2A-D). At 48hrs post tuberculin challenge, the total numbers of CD8+ T cells trended higher in the BAL of adult animals compared to aged animals (Fig. 2D) and adult macaques had higher numbers of CD8+ effector memory T cells (Fig. 2E) and CD8+ central memory T cells post-challenge relative to aged animals (Fig. 2F). CD8+ CD69+ T cells also trended higher in the BAL of adult macaques (Fig. 2G) and a trend towards increased numbers of CD8+ T cell expressing the chemokine receptors CCR5 and CCR6 was observed for adult animals (Fig. S2E). These data suggest age-related differences to antigen recall in the memory compartment and activation state of CD8+ T cells.
At 72hrs post tuberculin challenge, a higher percentage of CD45+CD3+ T cells were maintained in adult animals (Fig. 2H) and we observed trend increases for the total numbers of CD8+ T cells (Fig. 2I), CD8+ effector (Fig. 2J) and central memory (Fig. 2K), and CD8+ CD69+ T cells (Fig. 2L) were maintained. A continued trend towards increased numbers of CD8+ T cell expressing the chemokine receptors CCR5 and CCR6 was observed for adult animals (Fig. S2F).
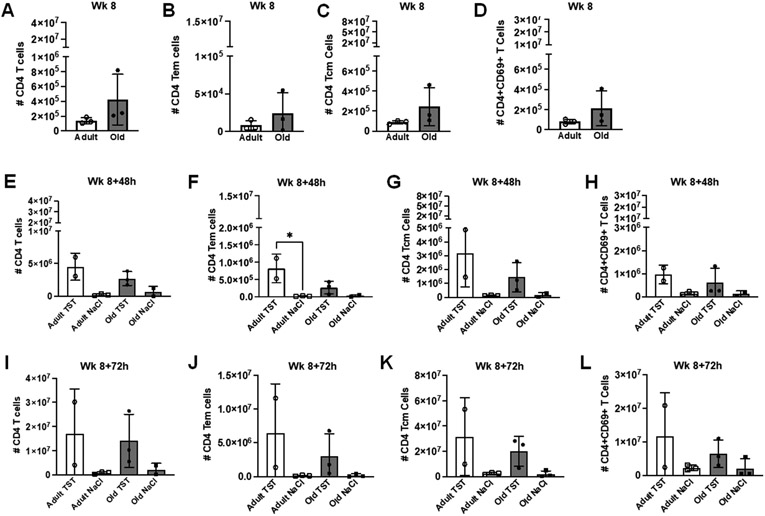
Prior to first tuberculin challenge, total CD4+ T cells numbers trended higher in the BAL of aged animals (Fig. 3A), along with CD4+ effector memory (Fig. 3B), central memory T cells (Fig. 3C), and CD4+ CD69+ T cells (Fig. 3D). At 48hrs (Fig. 3 E-H) and 72hrs (Fig. 3I-L) post tuberculin challenge, these modest differences were lost.
Figure 3. Age-related changes in CD4+ T cell compartment in BALF following tuberculin challenge.
(A) Number of CD4+ T cells in BALF of adult and aged macaques prior to tuberculin lung instillation (Week 8). Number of CD4+ effector memory T cells (B), CD4+ central memory T cells (C), and CD69+CD4+ T cells (D) in BALF of adult and aged macaques prior to tuberculin lung instillation (Week 8). (E) Number of CD4+ T cells in BALF of adult and aged macaques 48 hours post-tuberculin challenge. Number of CD4+ effector memory T cells (F), CD4+ central memory T cells (G), and CD69+CD4+ T cells (H) in BALF of adult and aged macaques 48 hours post-tuberculin challenge. (I) Number of CD4+ T cells in BALF of adult and aged macaques 72 hours post-tuberculin challenge. Number of CD4+ effector memory T cells (J), CD4+ central memory T cells (K), and CD69+CD4+ T cells (L) in BALF of adult and aged macaques 72 hours post-tuberculin challenge. One-way ANOVA post-Tukey analyses comparing means between groups as indicated; *p<0.05. TST = tuberculin instillation; NaCl = saline (control) instillation.
3.3. Reduced lung T cell responses to challenge in aged animals are maintained over time
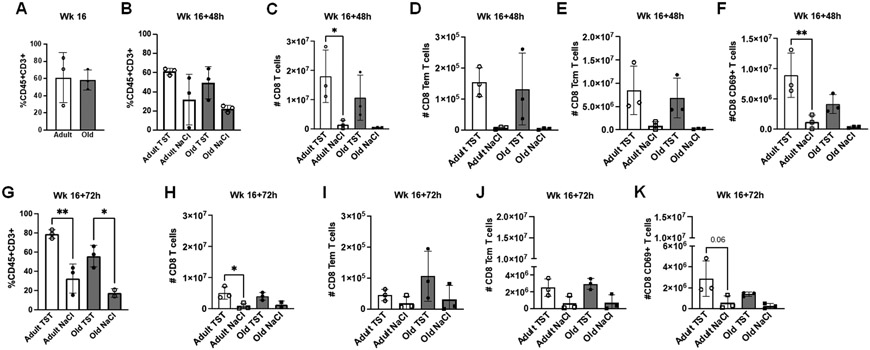
At 16 weeks post-vaccination the total cells in the BAL of adult and aged animals (Fig. 4A) were similar to those observed at 8 weeks post-vaccination (Fig. 2A). At 48hrs post tuberculin challenge both age groups had an increased proportion of CD45+CD3+ cells in BAL (versus control), although there were no tuberculin-induced differences between adult and aged animals (Fig. 4B). At 48hrs post tuberculin challenge, adult animals had more CD8+ T cells that aged animals (Fig. 4C), but no differences in CD8+ effector (Fig. 4D) or central memory T cells (Fig. 4E) were observed; however, adult animals had more CD8+ CD69+ T cells in response to tuberculin relative to aged animals (Fig. 4F). Increased trends were observed in adult animals at 72hrs post-challenge in the proportion of CD45+CD3+ T cells (Fig. 4G), total CD8 T cells (Fig. 4H), and CD8+ CD69+ T cells (Fig. 4K), with no differences observed between age groups in effector (Fig. 4I) and central memory CD8+ T cells (Fig. 4J).
Figure 4. Adult macaques have increased T cells present in BALF maintained over time following tuberculin instillation.
(A) Proportions of T cells (CD45+CD3+) in BALF of adult and aged macaques prior to tuberculin lung instillation (Week 16). (B) Proportions of T cells (CD45+CD3+) in BALF of adult and aged macaques 48 hours post-tuberculin challenge. Number of CD8+ T cells (C), CD8+ effector memory T cells (D), CD8+ central memory T cells (E), and CD69+CD8+ T cells (F) in BALF of adult and aged macaques 48 hours post-tuberculin challenge. (G) Proportions of T cells (CD45+CD3+) in BALF of adult and aged macaques 72 hours post-tuberculin challenge. Number of CD8+ T cells (H), CD8+ effector memory T cells (I), CD8+ central memory T cells (J), and CD69+CD8+ T cells (K) in BALF of adult and aged macaques 72 hours post-tuberculin challenge. One-way ANOVA post-Tukey analyses comparing means between groups as indicated; *p<0.05, **p<0.01. TST = tuberculin instillation; NaCl = saline (control) instillation.
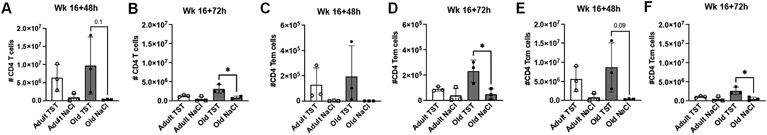
At both 48hrs (Fig. 5A) and 72hrs (Fig. 5B) post second tuberculin challenge, aged BCG-vaccinated animals had more CD4+ T cells in the BAL. The same trend was observed for effector memory (Fig. 5 C, D) and central memory (Fig. 5 E, F) CD4+ T cells relative to aged animals receiving saline. These increases in the CD4+ T cell compartment of aged animals were present prior to challenge (Fig. S3A - C, Week 16) suggesting that baseline increases were maintained post-challenge. No difference in responses to tuberculin was observed between CD4+ T cells from aged and adult animals. At 48hrs (Fig. S3D) and 72hrs (Fig. S3E) post-challenge, there were trends towards more CD4+ T cell expressing CCR5 and CCR6 in the BAL of adult macaques.
Figure 5. Age-related changes in CD4+ T cell compartment in BALF following the 2nd tuberculin challenge.
(A) Number of CD4+ T cells in BALF of adult and aged macaques 48 hours post-tuberculin lung instillation (Week 16+48h). (B) Number of CD4+ T cells in BALF of adult and aged macaques 72 hours post-tuberculin lung instillation (Week 16+72). Number of CD4+ effector memory T cells (C) and CD4+ central memory T cells (D) 48 hours post-tuberculin lung instillation. Number of CD4+ effector memory T cells (E) and CD4+ central memory T cells (F) 72 hours post-tuberculin lung instillation. One-way ANOVA post-Tukey analyses comparing means between groups as indicated; *p<0.05. TST = tuberculin instillation; NaCl = saline (control) instillation.
3.4. Aged-related differences in the periphery are independent of vaccination
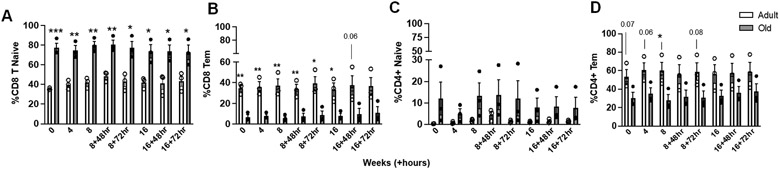
We previously found in a baboon model (8) that despite age-related changes in local tissue immune responses, aged animals responded similarly to their adult counterparts in the periphery. We analyzed immune cell populations in the peripheral blood of BCG-vaccinated macaques (gating strategy, FS4). Aged animals had higher proportions of CD8+ naïve T cells and lower levels of CD8+ effector memory T cells in the blood (Fig. 6A & B). Aged animals had trend increases in the proportions of CD4+ naïve T cells and lower CD4+ effector memory T cells (Fig. 6C & D). We also observed early increases in CD4+CD8+ T cells in the blood of aged animals, although these differences were lost over time (Fig. S5A). Lastly, aged animals had no differences in proportions of CD4+ T cells (Fig. S5B), but had increased proportions of CD4+ T cells expressing the chemokine receptors CCR5 and CXCR3 (Fig. S5C & D), although CXCR3 expression normalized following the first challenge timepoint. No differences between age groups were observed in other peripheral blood cell populations (Fig. S5; E - I). Because differences were present prior to BCG vaccination, these data suggest that age-related changes in peripheral cell populations are not dependent on vaccination.
Figure 6. Age-related changes in peripheral blood T cell populations, independent of BCG-vaccination, are observed in aged macaques in the memory compartment.
PBMCs from adult and aged vaccinated macaques from the time points indicated were stained for surface markers and analyzed by flow cytometry. (A) Percentage of naive CD8+ T cell and (B) CD8+ effector memory T cell populations throughout the study timeline. (C) Percentage of naive CD4+ T cell and (D) CD4+ effector memory T cell populations throughout the study timeline. Naïve T cells defined as CD95+CD28− and effector memory T cells defined as CD95−CD28+. Student t test Adult vs Old, *p<0.05, **p<0.01, ***p<0.001. Adult, white bars and open circles; old, grey bars and black circles.
Circulating immune mediators were observed in the plasma of aged animals at higher levels throughout the study. Elevated CCL20 and VEGF were present at baseline (Fig. S6A & B), and aged animals had an increase in plasma levels of CD40L at later time points (Fig. S6C). No differences in plasma levels were observed between age groups for the chemokines CXCL10 and CCL5 (Fig. S6D & E). Most immune mediators tested were higher in the plasma of aged animals prior to BCG vaccination, suggesting aged-related differences independent of vaccination status.
3.5. Aged animals have functional systemic responses to antigen stimulation
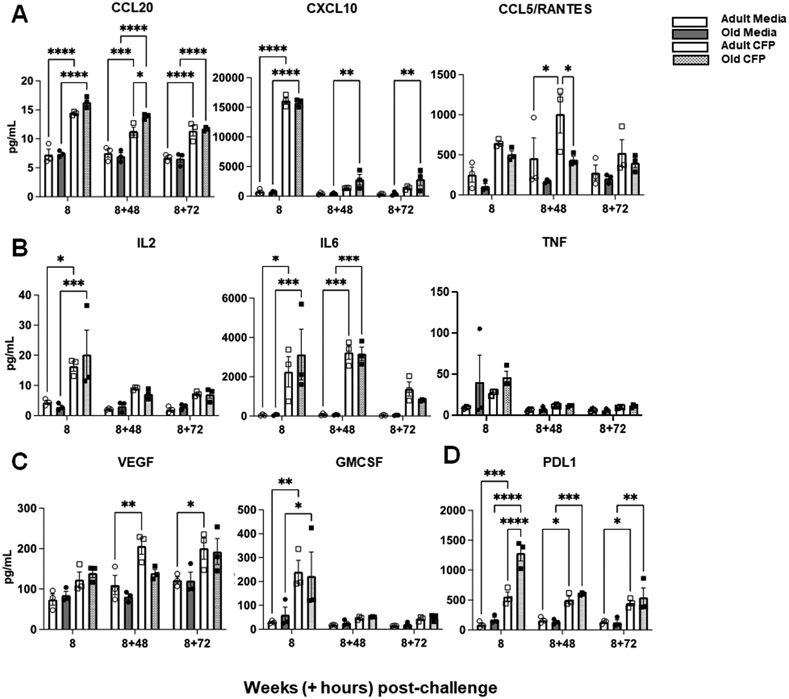
With our observations of few vaccine-induced differences in the peripheral blood of adult and aged macaques, we tested functional responses of PBMCs to antigen stimulation (mycobacterial culture filtrate protein, CFP) relative to media (control). We found that 8 weeks post-vaccination, PBMCs from aged animals were as functional as PBMCs from adults. Increased chemokine (CCL20, CXCL10, and CCL5; Fig. 7A), cytokine (IL2, IL6, and TNF; Fig. 7B), and growth factor (VEGF and GMCSF; Fig. 7C) production were observed in both age groups in response to antigen stimulation. These observations were maintained over time (16 weeks post-vaccination; Fig. S7A - D). PBMCs from aged animals also produced higher levels of soluble PD-L1 throughout the timepoints tested (Figure 7D; Fig. S7D). Overall, these data suggest that PBMCs from adult and aged animals could respond similarly to antigen-specific stimulation.
Figure 7. Antigen-stimulated PBMCs from adult and aged macaques are functionally similar.
PBMCs from adult and aged BCG-vaccinated macaques from the time points indicated were stimulated with CFP for 5 days (adult CFP shown in white bars & open squares; old CFP shown in grey dotted bars & closed squares). Supernatants were collected post 1st tuberculin challenge and antigen-specific responses (in pg/mL) were detected by Luminex for production of (A) chemokines, (B) cytokines, (C) growth factors, and (D) the ligand PDL1. Media serves as the control for non-specific responses to stimulation and is shown for adult PBMCs in white bars & open circles and old PBMCs in grey bars and closed circles. Student t test Adult vs Old, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
4. DISCUSSION
Respiratory infections affect approximately 13 million individuals each year in the United States (16). Moreover, morbidity and mortality related to respiratory infections are significantly higher for those over the age of 65 (16). As our aging population increases in number, there is a growing need to better understand age-associated susceptibility and finds ways to intervene to improve health outcomes in elderly individuals. A primary method to combat infections is vaccination; however, vaccines are less effective in the elderly (5). Infection susceptibility and inability to mount protective vaccine-induced responses has been linked to reduced or impaired immunological responses with increased age (5). In order to improve lung responses to infection, we need to study the immune response in the lung following vaccination and infection. This is difficult to do in elderly humans, due to ethical and safety reasons. To overcome this challenge, we established an in vivo model to probe lung immune responses to vaccination and challenge in rhesus macaques, taking advantage of the high homology between non-human primates and adults and study feasibility (17). In addition, rhesus macaques are a well characterized aging model (18). We designed a pilot study in which we vaccinated 3 adult and 3 aged macaques with BCG, followed by tuberculin challenge in the lung. BCG vaccination and tuberculin administration in the skin is a standard model to evaluate delayed-type hypersensitivity. Our previous work established this model of the tuberculin skin test (TST) in NHPs, and here we have expanded our work to include tuberculin lung challenge to evaluate lung recall responses (8). This NHP model can be used to overcome the safety and ethical issues associated with these studies in humans.
Using a tuberculin challenge directly into the lung we were able to assess recall immunity in the lungs of aged or adult BCG vaccinated rhesus macaques. At 48 hours following tuberculin instillation to the lung, aged animals had fewer CD45+CD3+ cells in the BAL, suggesting an age-related decrease or delay in T cell recall responses to the pulmonary challenge site. Further evaluation of this cellular compartment found that aged animals had alterations in CD8+ and CD4+ T cell subsets, which was more prominent in the CD8+ T cell subsets with fewer CD8+ memory T cells in the BAL of aged animals, most prominent at 48 hours post-tuberculin administration. We observed more CD8 T cells in adult BAL that expressed CCR5 and CCR6 specifically at the early timepoint of tuberculin instillation (48hrs; Fig. S3A), perhaps suggestive of a higher influx of cells into the lung when challenged.
Similar trends in CD8+ and CD4+ T cell recall responses were observed between age groups at 16 weeks; however, differences observed at 16 weeks post-challenge were not as pronounced at those observed at 8 weeks post-challenge. This may suggest that recall responses waned over time regardless of age. Many studies have shown decreased BCG-induced protection over time and that use of a BCG booster may enhance protection long-term (19). The impact of boosting in this model on adult and aged animals is unknown; however, we would hypothesize that a BCG booster would enhance recall responses in both age groups at the second challenge (16 weeks) with adult animals having a higher magnitude of response as was observed at 8 weeks. Future studies can be performed to investigate this hypothesis and confirm our results using more animals to increase our sample size. Despite the small sample size in our pilot study, we found that animals within the same age group responded similarly to one another. Moreover, our findings observed in this study support our previous pilot study, strengthening the small samples sizes of our two pilot studies.
Evaluation of immune cell population in the peripheral blood found that differences were age-related and not dependent on BCG-vaccination. Throughout the study, old animals had a shift in CD4+ and CD8+ T cell populations to more naïve and less effector memory cell populations. Aging literature shows that naïve populations, specifically CD8+ T cells, decrease with age (20;21) and our observations are not consistent with these findings. We were unable to investigated in this difference in more detail, but other studies suggest that age may impact numbers or diversity of naïve T cells (20). Our peripheral data do suggest that systemic responses are not reflective of those occurring at the local tissue site. These data also suggest that evaluating systemic responses are not sufficient to gain a complete understanding of aged responses to infectious challenge. Studies should evaluate age-related alterations in immune responses at the site of infectious challenge (i.e. the tissue) to identify ways to improve elderly health outcomes.
At 4 weeks post BCG-vaccination, skin observations at the BCG injection site showed visual differences between the age groups, with aged animals having little to no noticeable changes at the vaccination site, despite all animals having positive TST reactions 8 weeks post-BCG vaccination. Despite the lack of reaction to BCG vaccination at the injection site, aged animals generated immunity as they had a positive TST response at the eight weeks challenge, and PBMC could also respond equally to mycobacterial antigenic stimulation at every collection timepoint. Therefore, a delayed primary response, or a response of lower magnitude, in the aged may explain the lack of response at the BCG vaccine site (22). It is established that elderly individuals have both delayed and decreased magnitude of responses to vaccination, supporting our observations in aged NHPs (23).
4.1. Conclusion
Our previous pilot study established an in vivo model to evaluate skin recall responses to tuberculin in BCG-vaccinated baboons (8). We built upon this model in our current pilot study to evaluate lung recall responses to tuberculin in BCG-vaccinated macaques. Elderly individuals have increased frequency of both skin and lung infections (24;25) making these tissues important to study to evaluate age-related alterations in the immune response. In both pilot studies, aged and adult animals had positive TST reactions, despite the subsequent age-related decreases in tissue responses. Studies in elderly humans show that TST responses wane with age (26;27); however, in our studies we found that old animals respond the same as their adult counterparts. Instead, we identified age-related changes at the site of challenge that may explain increased susceptibility of the elderly to infections. In both of our published studies as well as work by our group and others (6-7;28), we found that systemic responses do not reflect those occurring in the tissue. Therefore, designing studies based on peripheral blood collection and evaluation may fall short of informing tissue responses to infection. Overall, our findings suggest that aged BCG-vaccinated animals have decreased lung CD8+ and CD4+ T cell responses to tuberculin challenge, suggesting that finding ways to boost vaccine-induced recall immune responses in the aging lung to adult levels may provide greater protection and/or reduce susceptibility to infectious challenge.
Supplementary Material
Supplementary Table 1. Table of information related to study animals. Information for the 3 adult and 3 aged rhesus macaques included in the study with animal ID, sex, age (in years), and weight (in kilograms).
Supplementary Table 2. Table of observations at the BCG vaccination site. Observations at the vaccination site 4 weeks post-BCG injection for adult and aged rhesus macaques.
Supplementary Table 3. Table of antibodies used in the study for flow cytometric analyses. Antibody information as used in the study, including fluorochrome, marker, clone, vendor, and catalog number.
Supplementary Figure 1. Flow gating strategy for BALF. Total events from BALF are gated by singlets (forward scatter height vs area). BAL cells are gated based on forward and side scatter, followed by live cells. Live BAL cells are further gated on by CD45+CD3+ to identify total T cells, followed by CD4+ and CD8+. Within CD4+ and CD8+ T cell gates, memory populations are defined by CD95+ and CD28+ expression and activation of T cells are defined by CD69 expression. FMOs were included as controls for gating on CD28, CD95, and CD69 (data not shown).
Supplementary Figure 2. CD8+ T cells in the BALF of adult and aged rhesus macaques. Prior to the first tuberculin challenge (Week 8), (A) Number of CD8+ T cells, (B) number of CD8+ effector memory T cells, (C) number of CD8+ central memory T cells, and (D) number of CD69+ CD8+ T cells in BALF of adult and aged macaques. Numbers of CD8+ T cells expressing CCR5 and CCR6 in BALF of adult and aged macaques 48 hours (E) and 72 hours (F) post-tuberculin challenge. TST = tuberculin instillation; NaCl = saline (control) instillation. Student t test Adult vs Old and One-way ANOVA post-Tukey analyses comparing means between groups.
Supplementary Figure 3. CD4+ T cells in the BALF of adult and aged rhesus macaques. Prior to the second tuberculin challenge (Week 16), (A) Number of CD4+ T cells, (B) CD4+ effector memory T cells, and (C) CD4+ central memory T cells in BALF of adult and aged macaques. Numbers of CD4+ T cells expressing CCR5 and CCR6 in BALF of adult and aged macaques 48 hours (D) and 72 hours (E) post second tuberculin challenge. TST = tuberculin instillation; NaCl = saline (control) instillation. Student t test Adult vs Old and One-way ANOVA post-Tukey analyses comparing means between groups.
Supplementary Figure 4. Flow gating strategy for peripheral blood. (A) Total events from whole blood-stained cells are gated by singlets (forward side height vs area). Lymphocytes are further gated based on forward and side scatter, followed by CD3 expression. CD3+ cells are further gated on by CD8, γδTCR, and CD4 to identify proportions of CD8+ T cells, γδTCR+ T cells, and CD4+ T cells, respectively. (B) Within CD4+ and CD8+ T cell gates, memory populations are defined by CD95 and CD28 expression. (C) Within CD4+ and CD8+ T cell gates, expression of the chemokine receptors CXCR3 and CCR5 are determined, using FMOs as controls to place gates (D).
Supplementary Figure 5. Immune cell populations present in the peripheral blood of aged and adult rhesus macaques throughout the study. Percentage of (A) CD4+CD8+ T cells, (B) CD4+ T cells, (C) CD4+CCR5+ T cells, (D) CD4+CXCR3+ T cells, (E) CD8+ T cells, (F) CD8+CCR5+ T cells, (G) CD8+CXCR3+ T cells, (H) γδTCR+ T cells, and (I) CD20+ B cells present in the blood of adult and aged macaques up until Week 16 + 72 hours post-tuberculin challenge. Student t test Adult (adult bars, open circles) vs Old (grey bars, closed circles); *p<0.05, **p<0.01.
ACKNOWLEDGEMENTS
We would like to acknowledge the research team led by Dr. Mark Slifka and the veterinary team at Oregon Health and Science University (OHSU) and the Oregon National Primate Research Center for performing the animal studies and flow cytometry studies. We would also like to acknowledge the Texas Biomed Biology Core for their assistance with data collection for Luminex assays and for running flow cytometry samples.
FUNDING
Research reported in this publication was supported by National Institute of Aging (NIA) of the National Institutes of Health under award number P01-AG051428 to JT, NIH/NIA NRSA T32-AG021890 to JMS, and by the Office Of The Director, National Institutes Of Health of the National Institutes of Health under Award Number S10OD028653. Start-up funds to JT supported components of this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
ETHICS DECLARATION
These studies were performed in compliance with and with approval from the University Institutional Animal Care and Use Committee at OHSU (Protocol #IP00003551) and by the OHSU Biosafety Committee (PROTO202100001).
COMPETING INTERESTS
The authors declare that they have no competing interests.
REFERENCES
- 1.World’s older population grows dramatically [press release]. NIH: NIH; 2016. [Google Scholar]
- 2.Bektas A, Schurman SH, Sen R, Ferrucci L. Aging, inflammation and the environment. Exp Gerontol. 2018;105:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haynes L Aging of the Immune System: Research Challenges to Enhance the Health Span of Older Adults. Frontiers in Aging. 2020;1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajaj V, Gadi N, Spihlman AP, Wu SC, Choi CH, Moulton VR. Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections? Frontiers in Physiology. 2021;11(1793). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinti M, Appay V, Campisi J, Frasca D, Fülöp T, Sauce D, et al. Aging of the immune system: Focus on inflammation and vaccination. European Journal of Immunology. 2016;46(10):2286–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vukmanovic-Stejic M, Sandhu D, Seidel JA, Patel N, Sobande TO, Agius E, et al. The Characterization of Varicella Zoster Virus–Specific T Cells in Skin and Blood during Aging. Journal of Investigative Dermatology. 2015;135(7):1752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brighenti S, Andersson J. Local Immune Responses in Human Tuberculosis: Learning From the Site of Infection. The Journal of Infectious Diseases. 2012;205(suppl_2):S316–S24. [DOI] [PubMed] [Google Scholar]
- 8.Scordo JM, Piergallini TJ, Reuter N, Headley CA, Hodara VL, Gonzalez O, et al. Local immune responses to tuberculin skin challenge in Mycobacterium bovis BCG-vaccinated baboons: a pilot study of younger and older animals. Immunity & Ageing. 2021;18(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mouton CP, Bazaldua OV, Pierce B, Espino DV. Common infections in older adults. Am Fam Physician. 2001;63(2):257–68. [PubMed] [Google Scholar]
- 10.Muruganandah V, Kupz A. Immune responses to bacterial lung infections and their implications for vaccination. International Immunology. 2021; 34(5):231–248. [DOI] [PubMed] [Google Scholar]
- 11.Mizgerd JP. Respiratory Infection and the Impact of Pulmonary Immunity on Lung Health and Disease. American Journal of Respiratory and Critical Care Medicine. 2012;186(9):824–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ardain A, Marakalala MJ, Leslie A. Tissue-resident innate immunity in the lung. Immunology. 2020;159(3):245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vukmanovic-Stejic M, Reed JR, Lacy KE, Rustin MHA, Akbar AN. Mantoux Test as a model for a secondary immune response in humans. Immunology Letters. 2006;107(2):93–101. [DOI] [PubMed] [Google Scholar]
- 14.Silver RF, Zukowski L, Kotake S, Li Q, Pozuelo F, Krywiak A, et al. Recruitment of Antigen-Specific Th1-Like Responses to the Human Lung following Bronchoscopic Segmental Challenge with Purified Protein Derivative of Mycobacterium tuberculosis. American Journal of Respiratory Cell and Molecular Biology. 2003;29(1):117–23. [DOI] [PubMed] [Google Scholar]
- 15.CDC. Tuberculin Skin Testing CDC 2020. [Available from: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.htm.
- 16.Childs A, Zullo AR, Joyce NR, et al. The burden of respiratory infections among older adults in long-term care: a systematic review. BMC Geriatr. 2019;19(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang MC, Hild S, & Grieder F. Nonhuman primate models for SARS-CoV-2 research: Consider alternatives to macaques. Lab Animal. 2021;50:113–114. [DOI] [PubMed] [Google Scholar]
- 18.Colman RJ. Non-human primates as a model for aging, Biochimica et Biophysica Acta - Molecular Basis of Disease. 2018;1864(9A):2733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanner R, Villarreal-Ramos B, Vordermeier MH, and McShane H. The Humoral Immune Response to BCG Vaccination. Frontiers in Immunology. 2019;10:1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goronzy JJ, Fang F, Cavanagh MM, Qi Q, Weyand CM. Naive T cell maintenance and function in human aging. J Immunol. 2015;194(9):4073–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vladimir J, Ilhem M, & Nikolich-Žugich J. Phenotypic and functional T-cell aging in rhesus macaques (Macaca mulatta): differential behavior of CD4 and CD8 subsets. Blood. 2003;102(9):3244–3251. [DOI] [PubMed] [Google Scholar]
- 22.Gustafson CE, Kim C, Weyand CM, Goronzy JJ. Influence of immune aging on vaccine responses. J Allergy Clin Immunol. 2020;145(5):1309–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciabattini A, Nardini C, Santoro F, Garagnani P, Franceschi C, and Medaglini D. Vaccination in the elderly: The challenge of immune changes with aging. Seminars in Immunology. 2018;40:83–94. [DOI] [PubMed] [Google Scholar]
- 24.Meyer KC. Lung infections and aging. Ageing Res Rev. 2004;3(1):55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laube S, Farrell AM. Bacterial skin infections in the elderly: diagnosis and treatment. Drugs Aging. 2002;19(5):331–42. [DOI] [PubMed] [Google Scholar]
- 26.Chambers ES, Vukmanovic-Stejic M. Skin barrier immunity and ageing. Immunology. 2020;160(2):116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nayak S, Acharjya B. Mantoux test and its interpretation. Indian Dermatol Online J. 2012;3(1):2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ault R, Dwivedi V, Koivisto E, Nagy J, Miller K, Nagendran K, et al. Altered monocyte phenotypes but not impaired peripheral T cell immunity may explain susceptibility of the elderly to develop tuberculosis. Exp Gerontol. 2018;111:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Table of information related to study animals. Information for the 3 adult and 3 aged rhesus macaques included in the study with animal ID, sex, age (in years), and weight (in kilograms).
Supplementary Table 2. Table of observations at the BCG vaccination site. Observations at the vaccination site 4 weeks post-BCG injection for adult and aged rhesus macaques.
Supplementary Table 3. Table of antibodies used in the study for flow cytometric analyses. Antibody information as used in the study, including fluorochrome, marker, clone, vendor, and catalog number.
Supplementary Figure 1. Flow gating strategy for BALF. Total events from BALF are gated by singlets (forward scatter height vs area). BAL cells are gated based on forward and side scatter, followed by live cells. Live BAL cells are further gated on by CD45+CD3+ to identify total T cells, followed by CD4+ and CD8+. Within CD4+ and CD8+ T cell gates, memory populations are defined by CD95+ and CD28+ expression and activation of T cells are defined by CD69 expression. FMOs were included as controls for gating on CD28, CD95, and CD69 (data not shown).
Supplementary Figure 2. CD8+ T cells in the BALF of adult and aged rhesus macaques. Prior to the first tuberculin challenge (Week 8), (A) Number of CD8+ T cells, (B) number of CD8+ effector memory T cells, (C) number of CD8+ central memory T cells, and (D) number of CD69+ CD8+ T cells in BALF of adult and aged macaques. Numbers of CD8+ T cells expressing CCR5 and CCR6 in BALF of adult and aged macaques 48 hours (E) and 72 hours (F) post-tuberculin challenge. TST = tuberculin instillation; NaCl = saline (control) instillation. Student t test Adult vs Old and One-way ANOVA post-Tukey analyses comparing means between groups.
Supplementary Figure 3. CD4+ T cells in the BALF of adult and aged rhesus macaques. Prior to the second tuberculin challenge (Week 16), (A) Number of CD4+ T cells, (B) CD4+ effector memory T cells, and (C) CD4+ central memory T cells in BALF of adult and aged macaques. Numbers of CD4+ T cells expressing CCR5 and CCR6 in BALF of adult and aged macaques 48 hours (D) and 72 hours (E) post second tuberculin challenge. TST = tuberculin instillation; NaCl = saline (control) instillation. Student t test Adult vs Old and One-way ANOVA post-Tukey analyses comparing means between groups.
Supplementary Figure 4. Flow gating strategy for peripheral blood. (A) Total events from whole blood-stained cells are gated by singlets (forward side height vs area). Lymphocytes are further gated based on forward and side scatter, followed by CD3 expression. CD3+ cells are further gated on by CD8, γδTCR, and CD4 to identify proportions of CD8+ T cells, γδTCR+ T cells, and CD4+ T cells, respectively. (B) Within CD4+ and CD8+ T cell gates, memory populations are defined by CD95 and CD28 expression. (C) Within CD4+ and CD8+ T cell gates, expression of the chemokine receptors CXCR3 and CCR5 are determined, using FMOs as controls to place gates (D).
Supplementary Figure 5. Immune cell populations present in the peripheral blood of aged and adult rhesus macaques throughout the study. Percentage of (A) CD4+CD8+ T cells, (B) CD4+ T cells, (C) CD4+CCR5+ T cells, (D) CD4+CXCR3+ T cells, (E) CD8+ T cells, (F) CD8+CCR5+ T cells, (G) CD8+CXCR3+ T cells, (H) γδTCR+ T cells, and (I) CD20+ B cells present in the blood of adult and aged macaques up until Week 16 + 72 hours post-tuberculin challenge. Student t test Adult (adult bars, open circles) vs Old (grey bars, closed circles); *p<0.05, **p<0.01.