Abstract
Background
The optimal treatment duration of community-acquired pneumonia (CAP) in children has been controversial in high-income countries. We conducted a meta-analysis to compare short antibiotic treatment (3–5 days) with longer treatment (7–10 days) among children aged ≥6 months.
Methods
On 31 January 2022, we searched PubMed, Scopus, and Web of Science databases for studies published in English from 2003 to 2022. We included randomized controlled trials focusing on antibiotic treatment duration in children with CAP treated as outpatients. We calculated risk differences (RDs) with 95% confidence intervals and used the fixed-effect model (low heterogeneity). Our main outcome was treatment failure, defined as need for retreatment or hospitalization within 1 month. Our secondary outcome was presence of antibiotic-related harms.
Results
A total of 541 studies were screened, and 4 studies with 1541 children were included in the review. Three studies had low risk of bias, and one had some concerns. All 4 studies assessed treatment failures, and the RD was 0.1% (95% confidence interval, −3.0% to 2.0%) with high quality of evidence. Two studies (1194 children) assessed adverse events related to antibiotic treatment, and the RD was 0.0% (−5.0% to 5.0%) with moderate quality of evidence. The diagnostic criteria varied between the included studies.
Conclusions
A short antibiotic treatment duration of 3–5 days was equally effective and safe compared with the longer (current) recommendation of 7–10 days in children aged ≥6 months with CAP. We suggest that short antibiotic courses can be implemented in treatment of pediatric CAP.
Keywords: pneumonia, antibiotic, meta-analysis, children
Based on the meta-analysis of 4 randomized controlled studies with 1541 children, short antibiotic treatment (3–5 days) was as effective as the longer treatment (7–10 days) for community-acquired pneumonia in children in high-income countries.
Community-acquired pneumonia (CAP) affects 0.3%–1.5% of children yearly in Western countries [1, 2]. The current standard treatment strategy in international guidelines for CAP is 7–10 days of oral amoxicillin, regardless of etiology [2, 3]. In children <5 years of age, viruses alone or in mixed infections with bacteria are the most common cause of CAP. Evidence shows that Mycoplasma pneumoniae is involved in more than half of the cases after 10 years of age, but the usefulness of antibiotics remains obscure [4]. At all ages the most important bacterial pathogen is Streptococcus pneumoniae [3, 5]. Complications are rare, but when they are present, they usually follow pneumococcal CAP [6].
The optimal treatment duration for CAP in high-income countries has been controversial. A systematic review published in 2014, including randomized controlled trials (RCTs), addressed the efficacy of the shorter treatment duration for CAP. Only 1 of the 8 RCTs was from a high-income country and most of the RCTs did not compare shorter antibiotic treatment with the current treatment recommendation [7]. The most recent systematic review without meta-analysis included 11 trials (of which 8 were from developing countries) and concluded that more studies from high-income countries were needed to determine the safety and effectiveness of shorter antibiotic treatment in children [8].
Shorter antibiotic treatments for pediatric CAP cases have, at least in theory, several potential benefits over current treatment strategies. Shorter courses may prevent development of antibiotic resistance by minimizing exposure of both pathogenic and nonpathogenic microbes of normal flora to antibiotics, which diminishes the selection pressure for emergence of resistant strains [1]. In addition, unnecessary long courses increase the risk of adverse events, such as diarrhea, which is common in young children treated with antibiotics [9].
This meta-analysis comprising RCTs focused on antibiotic treatment duration of CAP in children treated as outpatients in high-income countries. Our primary aim was to determine whether a short antibiotic treatment duration of 3–5 days was as effective and safe as the longer treatment duration of 7–10 days. In addition, we evaluated antibiotic-related adverse events as a secondary objective of the study.
METHODS
Search Strategy
We searched PubMed (MEDLINE), Scopus, and Web of Science databases on 31 January 2022. The full search strategy is shown in Supplement 1. We used language restriction criteria and only included studies published in English. We also used a time restriction filter and restricted the search only to studies published within the last 20 years (2003–2022). The results were then uploaded to Covidence software (Covidence).
Inclusion and Exclusion Criteria
We included RCTs focusing on antibiotic treatment duration in CAP among children at age ≥6 months treated as outpatients, regardless of blinding. We excluded nonrandomized trials and observational studies. We excluded studies conducted in middle- and low-income countries owing to the different definitions of pneumonia and different healthcare facilities and organizations in these countries. Income rankings were based on the list of Organization for Economic Co-operation and Development for the year 2020. Furthermore, we excluded studies focusing on atypical pathogens, such as M. pneumoniae and Chlamydia pneumoniae. Finally, we excluded studies focusing on pneumonia treatment duration in children aged ≥6 months.
Review Process
Two authors (I. K. and J. J.) individually screened the abstracts, and conflicts were resolved by a third author (M. R.). Full texts were then assessed by 2 authors (I. K. and J.J.), and data were extracted into an Excel spreadsheet. We used the Cochrane risk of bias 2.0 tool to assess risk of bias in the included studies [10]. The risk of bias plot was generated with the robvis package in R software, version 4.0.3 [11]. We assessed reporting quality using Grading of Recommendations Assessment, Development and Evaluation (GRADE) methods [12].
Outcome Measures
Our main outcomes were the need for antibiotic retreatment, hospitalization, or treatment failure (including either need for retreatment or hospitalization) within 1 month after the randomization. Our secondary outcomes were antibiotic-related adverse effects.
Statistical Analysis
Review Manager software, version 5.4, was used for the meta-analysis. Data analyses were performed according to the Cochrane Handbook of Systematic Review Guidelines. We calculated risk differences (RDs) with 95% confidence intervals (CI) for dichotomous outcomes. Forest plots were presented for all outcomes. We analyzed inconsistency index (I2) statistics for heterogeneity, and because the heterogeneity was low (<40%) in all analyses, we used the fixed-effect model.
Because one of the studies (Greenberg et al [13]) ended one of its study arms prematurely (3-day treatment duration), we decided to exclude this arm from our analysis. We reported our systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [14] (Supplement 2).
Protocol Registration
We registered our protocol in Prospero (registration no. 308618).
RESULTS
The initial search retrieved 779 records, and after the removal of duplicates (238 records) we assessed 541 abstracts. We excluded 530 studies based on the abstracts, and 11 full texts were evaluated; finally, 4 studies [13, 15–17] were included in the analysis (Supplementary Figure 1).
All of the included studies were double blinded. Three studies were designed as noninferiority trials [13, 15, 16] and one as a superiority trial [17]. In 3 of 4 studies, the only antibiotic used was amoxicillin, and the fourth study treated patients with amoxicillin, amoxicillin and clavulanate (5.3%), or cefdinir (3.9%) (Supplementary Table 1).
Patients included in the studies were treated as outpatients. In every study, patients with suspected complicated pneumonia were excluded. The definition of pneumonia varied between the studies. Two studies [13, 15] required chest radiographs consistent with CAP to be included, while one [16] made the diagnosis based on symptoms and signs. One study [17] used a different approach: the patients were diagnosed with pneumonia before their inclusion in the study. They were then randomized to further antibiotics or placebo on day 5. The initial diagnoses did not have any specific diagnostic criteria for CAP and the randomization on the fifth day was done for those with early clinical improvement; therefore, it may have included milder pneumonia cases than the other included studies (Supplementary Table 2).
A total of 1573 children were included in the studies, of whom 784 belonged to the short-course and 789 to the long-course arms. The mean ages of children were between 28 and 36.8 months. Only a single study tested patients for respiratory viruses, and 104 children (83.9%) in the short-course group and 101 children (80.1%) in the long-course group tested positive for viruses (Supplementary Table 3).
The risk of bias was considered low in 3 of 4 studies (Supplementary Figure 2). One study [13] had some concerns about deviations from an intended intervention, as it replaced the initial 3-day intervention with a 5-day intervention in the early phase.
Need for Antibiotic Re-treatment
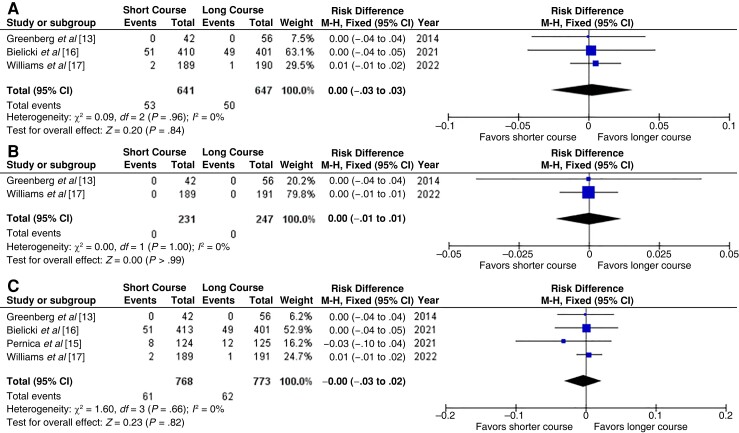
Three studies [13, 16, 17] with 1288 children assessed the need for antibiotic retreatment within 1 month of starting the initial treatment. The need for retreatment was 8.3% in the short-course and 7.7% in the long-course group (RD, 0.00 [95% CI −.03 to .03]; I2 = 0%) (Figure 1A). We ranked the quality of evidence as high and the risk of bias as low (Table 1).
Figure 1.
Need for antibiotic retreatment (A), need for hospitalization (B), and treatment failure (C) (includes need for antibiotic retreatment of need for hospitalization) within 1 month after randomization [13, 15–17]. Abbreviations: CI, confidence interval; df, degrees of freedom; I2, inconsistency index; M-H, Mantel–Haenszel test.
Table 1.
Body of Evidence for Outcomes Assessed Using Grading of Recommendations Assessment, Development and Evaluation (GRADE) Methods
| Outcome | Quality Assessment | Summary of Findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients, No. With Outcome/Total | Effect (95% CI) | Quality of Evidence | ||||||||||
| Studies, No. | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Intervention Group | Control Group | Relative Risk | Absolute RD, % | ||
| Need for antibiotic retreatment | 3 | RCT | Low | No serious limitations | Not present | Serious limitations: CI includes 0 | Not present | 53/641 | 50/647 | 1.07 (.74–1.55) | 0.5 (−2.4 to 3.5) | High |
| Need for hospitalization | 2 | RCT | Low | No serious limitations | Not present | Serious limitations: CI includes 0 | Not present | 0/231 | 0/247 | NA | 0.0 (−.8 to .8) | High |
| Treatment failure | 4 | RCT | Low | No serious limitations | Not present | Serious limitations: CI includes 0 | Not present | 61/768 | 62/773 | 0.99 (.71–1.39) | −0.1 (−2.8 to 2.6) | High |
| Any antibiotic-related adverse event | 2 | RCT | Some concerns (1 study did not report any adverse effects and 1 reported only severe effects) | No serious limitations | Not present | Serious limitations: CI includes 0 | Not present | 374/602 | 366/592 | 1.00 (.92–1.09) | 0.3 (−5.2 to 5.8) | Moderate |
| Severe antibiotic-related adverse event | 2 | RCT | Some concerns (1 study did not report any adverse effects and 1 did not report severity) | No serious limitations | Not present | Serious limitations: CI includes 0 | Not present | 1/329 | 2/332 | 0.50 (.05–5.52) | −0.3 (−1.3 to .7) | Moderate |
Abbreviations: CI, confidence interval; RCT, randomized controlled trial; RD, risk difference.
Need for Hospitalization
Two studies [13, 17] with 478 children assessed the need for hospitalization. No hospitalizations occurred in either of the studies (RD, 0.00 [95% CI −.01 to .01]; I2 = 0%) (Figure 1B). We ranked the quality of evidence as high and the risk of bias as low (Table 1).
Treatment Failure
All 4 studies [13, 15–17] assessed the composite outcome of treatment failure (including need for antibiotic retreatment or hospitalization), and 1541 children were included. The treatment failure rates were 7.9% in the short-course and 8.0% in the long-course group (RD, −0.00 [95% CI, −.03 to .02]; I2 = 0%) (Figure 1C). We ranked the quality of the evidence as high and the risk of bias as low (Table 1).
Adverse Events Related to Antibiotic Treatment
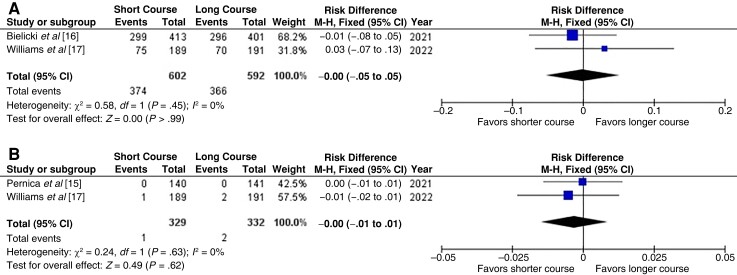
Two studies [16, 17] with 1194 children assessed all antibiotic-related adverse events. There were no differences between the groups (RD, −0.00 [CI −.05 to .05]; 12 = 0%) (Figure 2A). Two studies [15, 17] with 661 children assessed only severe antibiotic-related adverse events, there were 1 event in the short-course and 2 in the long-course group (RD, −0.00 [95% CI, −.01 to .01]; I2 = 0%) (Figure 2B). Because one of the studies [13] did not report any information related to adverse events, we downgraded the quality of the evidence to moderate, and the risk of bias was assessed as being of some concern (Table 1).
Figure 2.
Any antibiotic-related adverse event (A) and severe antibiotic-related adverse events (B) within 1 month after randomization [13, 15–17]. Abbreviations: CI, confidence interval; df, degrees of freedom; I2, inconsistency index; M-H, Mantel–Haenszel test.
DISCUSSION
The main result of this meta-analysis on the duration of antibiotic courses in children treated for CAP as outpatients was that a short antibiotic course of 3–5 days was not inferior to a long course of 7–10 days (the recommendation in the currently available guidelines) [1, 2]. The analysis included 4 RCTs from high-income countries with a total of 1451 children aged ≥6 months [13, 15–17]. Treatment failure was defined as the need for retreatment or hospitalization within 1 month, and the rates were surprisingly similar: 7.9% and 8.0%, respectively. The RD between the arms was 0.1%, and the 95% CI was very narrow, confirming that the result for the primary outcome was reliable. In addition, there was no substantial heterogeneity between the 4 studies for reporting retreatments and hospitalizations within 1 month after starting of primary antibiotics [13, 15–17].
Adverse events, which was the secondary outcome of the analyses, were reported in 2 studies [16, 17] and they did not differ between the short-course and long-course treatment arms. Adverse events rates were 62.1% and 61.8%, respectively. These adverse events, such as diarrhea and rash, were mild and were often seen in children during antibiotic treatment. Two studies [15, 17] reported severe adverse events, and such events were seen in 0.3% of the cases, which means that the short-course antibiotic therapy was safe. A previous meta-analysis stated that each additional day of antibiotic treatment increases the rates of antibiotic-related adverse effects [9]. Two of the included studies [16, 17] also analyzed the effects of treatment duration on antibiotic resistance and found the shorter course to be more beneficial, but we did not include this as an outcome in the meta-analysis.
High-dose amoxicillin (80–100 mg/kg/d) was the prescribed antibiotic in 3 of the 4 studies [13, 15, 16], and in the fourth study 5% received amoxicillin-clavulanic acid [17]. A study from England compared short and long amoxicillin courses with low (35–50 mg/kg/d) and high amoxicillin doses separately. It found that short courses were not inferior to long courses with either dose, and low doses were not inferior to high doses [16].
High doses of amoxicillin have been needed in areas where penicillin or multiple antibiotic resistance is common in pneumococcal strains, but a double amoxicillin dose equates to double antibiotic exposure. Therefore, countries with low resistance rates have used low-dose amoxicillin as the first choice for children’s CAP [18, 19], and the efficacy of low-dose in shorter duration should be confirmed in further studies.
The original purpose of the study was to compare clinical cures between short-course and long-course arms, but the clinical parameters expressing cure were heterogenous between the included studies and could not be pooled to produce any summary estimates. Instead, the retreatments and hospitalizations within 1 month were constantly reported in all studies, and these failures were combined and used as the primary outcome in the analyses. Furthermore, owing to the low failure rates in the included studies, we decided to use RDs in the analyses instead of risk ratios. Three of the included studies were noninferiority-designed RCTs, but the pooling of these with a cenventional parallel superiority-designed RCT is feasible, and we aimed to seek differences between groups in our conventional meta-analysis instead of using a prespecified noninferiority margin in the analyses [20, 21].
Until now, most data on the length of antibiotic courses have come from low-income or middle-income countries. The main differences in relation to the high-income countries concern the definition of CAP by the World Health Organization criteria for developing countries [22], which highlight increased respiratory rate and rates of severe treatment failure >5% [8, 23], which are not acceptable in Western countries.
The main strength of our review is the high quality and low risk of bias of the included studies, which increases the validity of our results. Furthermore, we did not have any major protocol deviations. Moreover, the numbers of cases—1451 for the primary outcome and 1194 for the secondary outcome of antibiotic-related adverse events—allow reliable meta-analysis. The exclusion of studies focusing on treatment of atypical pneumonias increased the consistency of data, since the necessity to treat them remains open [4].
A minor limitation to our results is the fact that more than half of the patients were from a single study [16], but this should not cause bias in the estimates, given that the results were similar in all studies. The main limitation of our study is the differing definition of CAP in the included studies, because 2 of them required chest radiographs and 2 did not. The currently available guidelines recommend that the diagnosis of CAP treated at home is clinical, and imaging is needed only in complicated cases [1, 2]. Viral determinations were performed in only one study, but virus identification does not necessarily influence decision making in CAP, since mixed viral-bacterial infections are common and it is recommended that all pediatric CAP cases in children ≥6 months of age are treated with antibiotics. Therefore, future studies should focus more on optimal differential recognition of bacterial and viral pneumonia, and a double-blinded placebo design should be used to determine whether antibiotics are needed at all in mild pneumonia. As seen in a previous study from England, antibiotics did not improve outcomes in nonsevere lower respiratory tract infections [24].
In conclusion, our meta-analysis, including 4 high-level RCTs, showed that short treatment was equally safe and effective as longer treatment of 7–10 days for CAP in outpatient children aged ≥6 months in high-income countries. We suggest that this shorter treatment for 3–5 days can now be implemented into clinical practice.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Ilari Kuitunen, Department of Pediatrics, Mikkeli Central Hospital, Mikkeli, Finland; Institute of Clinical Medicine and Department of Pediatrics, University of Eastern Finland, Kuopio, Finland.
Johanna Jääskeläinen, Institute of Clinical Medicine and Department of Pediatrics, University of Eastern Finland, Kuopio, Finland.
Matti Korppi, Centre for Child Health Research, Faculty of Medicine and Life Sciences, University of Tampere and University Hospital, Tampere, Finland.
Marjo Renko, Institute of Clinical Medicine and Department of Pediatrics, University of Eastern Finland, Kuopio, Finland; Department of Pediatrics, Kuopio University Hospital, Kuopio, Finland.
Notes
Data availability . All analyses and materials used are provided in the supplements or can be requested directly from the corresponding author.
References
- 1. Bradley JS, Byington CL, Shah SS, et al. . Executive summary: the management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011; 53:617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harris M, Clark J, Coote N, et al. . British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 2011; 66:ii1–23. [DOI] [PubMed] [Google Scholar]
- 3. Leung AKC, Wong AHC, Hon KL. Community-acquired pneumonia in children. Recent Pat Inflamm Allergy Drug Discov 2018; 12:136–44. [DOI] [PubMed] [Google Scholar]
- 4. Biondi E, McCulloh R, Alverson B, Klein A, Dixon A, Ralston S. Treatment of Mycoplasma pneumonia: a systematic review. Pediatrics 2014; 133:1081–90. [DOI] [PubMed] [Google Scholar]
- 5. Don M, Canciani M, Korppi M. Community-acquired pneumonia in children: what's old? what’s new? Acta Paediatr 2010; 99:1602–8. [DOI] [PubMed] [Google Scholar]
- 6. de Benedictis FM, Kerem E, Chang AB, Colin AA, Zar HJ, Bush A. Complicated pneumonia in children. Lancet 2020; 396:786–98. [DOI] [PubMed] [Google Scholar]
- 7. Ben-Shimol S, Levy-Litan V, Falup-Pecurariu O, Greenberg D. Evidence for short duration of antibiotic treatment for non-severe community acquired pneumonia (CAP) in children—are we there yet? a systematic review of randomised controlled trials. Pneumonia (Nathan) 2014; 4:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Korppi M. Antibiotic therapy in children with community-acquired pneumonia. Acta Paediatr 2021; 110:3246–50. [DOI] [PubMed] [Google Scholar]
- 9. Curran J, Lo J, Leung V, et al. . Estimating daily antibiotic harms: an umbrella review with individual study meta-analysis. Clin Microbiol Infect 2022; 28:479––90.. [DOI] [PubMed] [Google Scholar]
- 10. Higgins JP, Altma Gotzsche PC, et al. . The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 2021; 12:55–61. [DOI] [PubMed] [Google Scholar]
- 12. Guyatt GH, Oxman AD, Vist GE, et al. . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J 2014; 33:136–42. [DOI] [PubMed] [Google Scholar]
- 14. Page MJ, McKenzie JE, Bossuyt PM, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pernica JM, Harman S, Kam AJ, et al. . Short-course antimicrobial therapy for pediatric community-acquired pneumonia: the SAFER randomized clinical trial. JAMA Pediatrics 2021; 175:475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bielicki JA, Stöhr W, Barratt S, et al. . Effect of amoxicillin dose and treatment duration on the need for antibiotic re-treatment in children with community-acquired pneumonia: the CAP-IT randomized clinical trial. JAMA 2021; 326:1713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Williams DJ, Creech CB, Walter EB, et al. . Short- vs standard-course outpatient antibiotic therapy for community-acquired pneumonia in children: the SCOUT-CAP randomized clinical trial. JAMA Pediatr 2022; 176:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tapiainen T, Aittoniemi J, Immonen J, et al. . Finnish guidelines for the treatment of community-acquired pneumonia and pertussis in children. Acta Paediatr 2016; 105:39–43. [DOI] [PubMed] [Google Scholar]
- 19. Andrejko K, Ratnasiri B, Hausdorff WP, Laxminarayan R, Lewnard JA. Antimicrobial resistance in paediatric Streptococcus pneumoniae isolates amid global implementation of pneumococcal conjugate vaccines: a systematic review and meta-regression analysis. Lancet Microbe 2021; 2:e450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Z, Nayfeh T, Sofiyeva N, et al. . Including non-inferiority trials in contemporary meta-analyses of chronic medical conditions: a meta-epidemiological study. J General Internal Med 2020; 35:2162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Acuna SA, Dossa F, Baxter N. Meta-analysis of noninferiority and equivalence trials: ignoring trial design leads to differing and possibly misleading conclusions. J Clin Epidemiol 2020; 127:134–41. [DOI] [PubMed] [Google Scholar]
- 22. Revised WHO classification and treatment of childhood pneumonia at health facilities: evidence summaries. https://www.who.int/publications/i/item/9789241507813. Assessed February 14, 2022.
- 23. Ginsburg A, Mvalo T, Nkwopara E, et al. . Amoxicillin for 3 or 5 days for chest-indrawing pneumonia in Malawian children. New Engl J Med 2020; 383:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Little P, Francis NA, Stuart B, et al. . Antibiotics for lower respiratory tract infection in children presenting in primary care in England (ARTIC PC): a double-blind, randomised, placebo-controlled trial. Lancet 2021; 398:1417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.