Abstract
BACKGROUND
In a previously reported randomized trial of standard and intensive systolic blood-pressure control, data on some outcome events had yet to be adjudicated and post-trial follow-up data had not yet been collected.
METHODS
We randomly assigned 9361 participants who were at increased risk for cardiovascular disease but did not have diabetes or previous stroke to adhere to an intensive treatment target (systolic blood pressure, <120 mm Hg) or a standard treatment target (systolic blood pressure, <140 mm Hg). The primary outcome was a composite of myocardial infarction, other acute coronary syndromes, stroke, acute decompensated heart failure, or death from cardiovascular causes. Additional primary outcome events occurring through the end of the intervention period (August 20, 2015) were adjudicated after data lock for the primary analysis. We also analyzed post-trial observational follow-up data through July 29, 2016.
RESULTS
At a median of 3.33 years of follow-up, the rate of the primary outcome and all-cause mortality during the trial were significantly lower in the intensive-treatment group than in the standard-treatment group (rate of the primary outcome, 1.77% per year vs. 2.40% per year; hazard ratio, 0.73; 95% confidence interval [CI], 0.63 to 0.86; all-cause mortality, 1.06% per year vs. 1.41% per year; hazard ratio, 0.75; 95% CI, 0.61 to 0.92). Serious adverse events of hypotension, electrolyte abnormalities, acute kidney injury or failure, and syncope were significantly more frequent in the intensive-treatment group. When trial and post-trial follow-up data were combined (3.88 years in total), similar patterns were found for treatment benefit and adverse events; however, rates of heart failure no longer differed between the groups.
CONCLUSIONS
Among patients who were at increased cardiovascular risk, targeting a systolic blood pressure of less than 120 mm Hg resulted in lower rates of major adverse cardiovascular events and lower all-cause mortality than targeting a systolic blood pressure of less than 140 mm Hg, both during receipt of the randomly assigned therapy and after the trial. Rates of some adverse events were higher in the intensive-treatment group. (Funded by the National Institutes of Health; SPRINT ClinicalTrials.gov number, NCT01206062.)
The Systolic Blood Pressure Intervention Trial (SPRINT) was designed to determine whether a systolic blood-pressure target of less than 120 mm Hg (intensive treatment) would be associated with a lower rate of clinical events than a systolic blood-pressure target of less than 140 mm Hg (standard treatment). We previously reported primary results of the trial through August 20, 2015,1 when the sponsor halted the intervention because of benefit in the intensive-treatment group. The report included data available in October 2015. We continued to adjudicate potential trial events and collected data during an observational postintervention period. We report here an updated analysis of all events occurring through August 20, 2015, as well as an analysis of data through July 29, 2016, including data from post-trial close-out visits.
METHODS
TRIAL DESIGN AND OVERSIGHT
We previously reported the trial rationale and design,2 and the protocol is available with the full text of this article at NEJM.org.3 The methods are those reported in 20151 with the addition of post-trial follow-up. The steering committee (see Section 1 in the Supplementary Appendix, available at NEJM.org) designed the trial, gathered data with the collaborating investigators, decided to submit the manuscript for publication, and vouches for the fidelity of the trial to the protocol. The trial was approved by the institutional review board at each participating site. The coordinating center analyzed the data, and the writing committee interpreted the analyses, wrote the manuscript, and vouches for the completeness and accuracy of the data. All aspects of manuscript writing and revision were performed by the writing committee. In accordance with the policy of the National Institutes of Health (the sponsor), data are shared through the National Heart, Lung, and Blood Institute repository (https://biolincc.nhlbi.nih.gov/home/).
TRIAL POPULATION
We enrolled participants who were 50 years of age or older and had a systolic blood pressure of 130 to 180 mm Hg with or without antihypertensive drug treatment (Section 2 of the Supplementary Appendix) along with at least one additional indicator of cardiovascular risk: clinical or subclinical cardiovascular disease, chronic kidney disease (defined as an estimated glomerular filtration rate [eGFR] of 20 to 59 ml per minute per 1.73 m2 of body-surface area), a 15% or greater 10-year risk of cardiovascular disease as determined on the basis of the Framingham cardiovascular risk score, or an age of 75 years or older. We excluded persons with diabetes mellitus, previous stroke, or dementia. All participants provided written informed consent. We enrolled participants from November 2010 through March 2013 at 102 clinical sites.
RANDOMIZATION, INTERVENTIONS, AND FOLLOW-UP
Participants were randomly assigned in a 1:1 ratio to a systolic blood-pressure goal below 120 mm Hg (intensive treatment) or below 140 mm Hg (standard treatment). During the trial, blood-pressure medications were adjusted on the basis of treatment group–specific algorithms (Figs. S1 and S2 and Table S1 in the Supplementary Appendix).2
Clinical and laboratory data were obtained at quarterly follow-up visits. At each visit, trained staff measured blood pressure with an automated device (HEM-907XL, Omron Healthcare) while participants were seated,2,4 in accordance with a prespecified research protocol. Identical structured interviews were used quarterly in both groups to obtain data on participant-reported clinical cardiovascular and end-stage renal disease outcomes.1,2
Data on serious adverse events were collected at all trial visits. Hypotension, syncope, bradycardia, electrolyte abnormalities, and injurious falls were reported as adverse events if the participant underwent evaluation in the emergency department. Acute kidney injury and acute renal failure were monitored and recorded if the event was noted on admission or occurred during a hospitalization and was reported in the hospital discharge summary as a primary or main secondary diagnosis. Periodic National Death Index searches were used to ascertain vital status.
POSTINTERVENTION PERIOD
An observational postintervention period began after August 20, 2015, when participants’ blood-pressure control and medication management were returned to their usual health care providers. Trial investigators were instructed to change participants’ trial medications only if warranted for safety. Participants and their health care providers were free to change or continue blood-pressure medications or treatment goals.
Quarterly visits and safety visits (if needed) continued until the close-out visit period began. Each participant had one close-out visit during the period from December 1, 2015, through July 29, 2016. The close-out visit included ascertainment of clinical outcomes and collection of blood-pressure measurements, data on medication use, and other data. Before and by the end of the close-out period, sites were required to search available sources for participants who had been lost to follow-up, withdrawn, or missed their last visit. A final National Death Index search was completed in December 2016.
TRIAL OUTCOMES
The primary outcome was a composite of myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, or death from cardiovascular causes (Section 3 of the Supplementary Appendix).1,2 Secondary outcomes included individual components of the primary outcome, all-cause mortality, and a composite of the primary outcome plus all-cause mortality. We also included post hoc analyses of the primary outcome with nonfatal heart failure excluded. In participants who had chronic kidney disease at baseline (eGFR 20 to 59 ml per minute per 1.73 m2), the main composite renal outcome was end-stage renal disease or a 50% or greater decline in eGFR from baseline.1,2 In participants who did not have chronic kidney disease at baseline, the renal outcome was defined as a 30% or greater decline in eGFR to a value less than 60 ml per 1.73 m2. The same ascertainment and adjudication protocols were used during the intervention period and during the postintervention (observational) period. Outcome-adjudication committee members were unaware of treatment assignments.
STATISTICAL ANALYSIS
The published primary results of the trial1 were based on all events that had occurred through August 20, 2015, and had been ascertained and adjudicated at the time of publication (median follow-up, 3.26 years). We compared these results with updated results reflecting complete outcome adjudication of all events that had occurred through August 20, 2015 (median follow-up, 3.33 years). We also examined outcomes from randomization through July 29, 2016, including data from close-out visits (median follow-up, 3.88 years). These updated analyses followed the same analysis plan that had been used previously,1 but with complete ascertainment and adjudication of events. In addition, errors in the Framingham cardiovascular risk score5 and safety coding and algorithmic errors were corrected and data on safety and clinical events were updated.6
For the primary analysis, we used Cox proportional-hazards regression with two-sided tests at the 5% level of significance. Follow-up time was censored at the date of last ascertainment for most participants. For participants who had not had an event by August 20, 2015, but had an event after that date, we censored follow-up time at August 20, 2015, for updated trial analyses. Clinical site was used as a stratification factor for efficacy analyses but not for safety analyses because of the small numbers of serious adverse events. Tests of secondary outcomes were conducted with models similar to those used in the primary analysis. Nominal P values without adjustment for multiple comparisons were prespecified to show a pattern of effects closely related to the primary outcome; inferences drawn may not be reproducible since they were not adjusted for multiplicity. For tests of interactions between treatment and prespecified subgroups, we used likelihood-ratio tests with Hommel adjustment for multiplicity.7 Hazard ratios before and after August 20, 2015, were compared in a time-varying–covariate model with the use of an indicator of whether each event occurred on or before that date or after that date. We compared all primary outcome events (first and recurrent events) between the groups with Andersen–Gill models8 using data through July 29, 2016, and site stratification. We used multiple imputation in sensitivity analyses to assess the influence of missing data (Section 4 of the Supplementary Appendix). Early termination of the trial intervention had not been anticipated; therefore, analyses that include data after August 20, 2015, were not prespecified.
RESULTS
PARTICIPANTS AND BLOOD PRESSURE
From November 2010 through March 2013, a total of 9361 participants underwent randomization (Fig. S3). At baseline, participants had a mean age of 67.9 years, 28.2% were at least 75 years of age, 28.3% had chronic kidney disease, and 20.0% had previous cardiovascular disease (Table S2). Thiazide diuretics were being used at the time of screening by 38.2% of participants who had been assigned to intensive treatment and by 40.0% of those who had been assigned to standard treatment and were being used after randomization by 56.2% and 43.3%, respectively.
Differences in systolic blood pressure between the groups developed rapidly and were sustained during the intervention period (Fig. S4). The mean systolic blood pressure at the last intervention-period visit was 120.0 mm Hg in the intensive-treatment group and 133.9 mm Hg in the standard-treatment group. In the comparison of the postintervention close-out visit with the final intervention-period visit, the mean systolic blood pressure was 6.9 mm Hg higher in the intensive-treatment group and 2.6 mm Hg higher in the standard-treatment group at the close-out visit than at the last intervention-period visit (Fig. S5).
CLINICAL OUTCOMES: INTERVENTION PERIOD
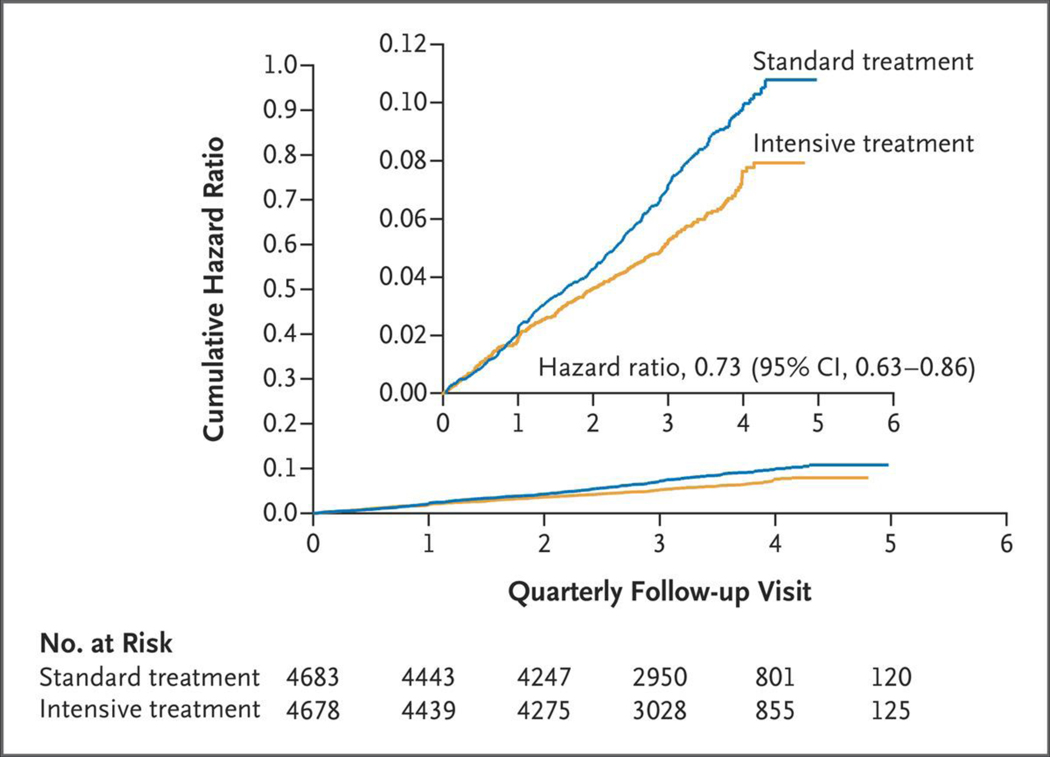
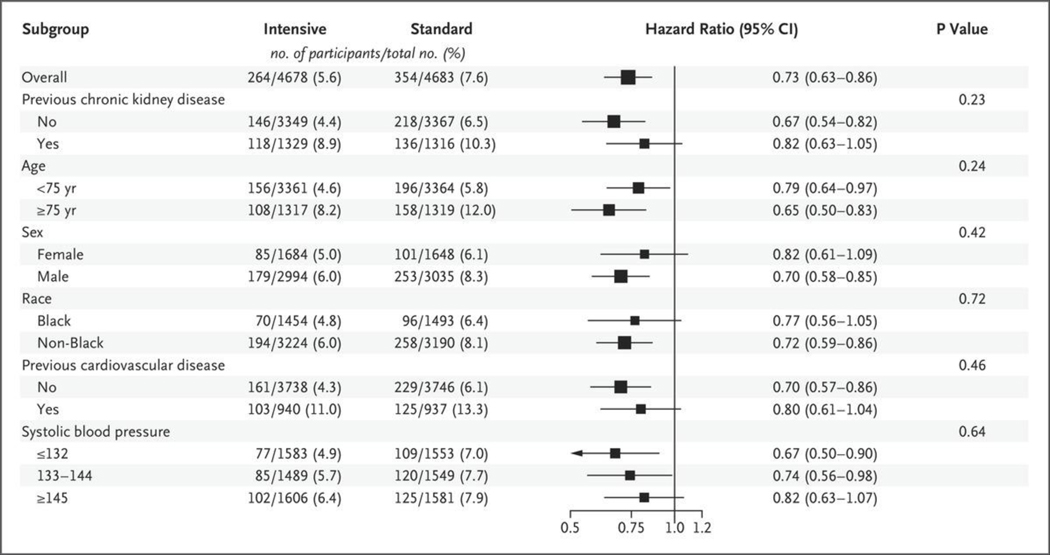
Numbers of outcome events during the intervention period that were included in the previous analysis, along with additional numbers of events in the current analysis, are shown in Table S3. The rate of the primary outcome was 1.77% per year in the intensive-treatment group and 2.40% per year in the standard-treatment group (hazard ratio, 0.73; 95% confidence interval [CI], 0.63 to 0.86; P<0.001) (Fig. 1 and Table 1), similar to the previously published findings (1.65% per year and 2.19% per year, respectively; hazard ratio, 0.75; 95% CI, 0.64 to 0.89; P<0.001).1 Results were similar for the post hoc analysis of the primary outcome in which nonfatal heart failure was excluded (hazard ratio, 0.75; 95% CI, 0.63 to 0.89; P = 0.001). All-cause mortality was 1.06% per year in the intensive-treatment group and 1.41% per year in the standard-treatment group (hazard ratio, 0.75; 95% CI, 0.61 to 0.92; P = 0.006) (Fig. S6), similar to the previously published findings (1.03% per year and 1.40% per year, respectively; hazard ratio, 0.73; 95% CI, 0.60 to 0.90; P = 0.003). Rates of myocardial infarction, heart failure, and death from cardiovascular causes were significantly lower in the intensive-treatment group than in the standard-treatment group. There was no evidence of heterogeneity of the treatment effect across prespecified subgroups for the primary outcome or for all-cause mortality (Figs. 2 and S7).
Figure 1. Cumulative Hazard Ratios for the Primary Outcome during the Intervention Period (through August 20, 2015).
The inset shows the same data on an enlarged y axis.
Table 1.
Outcomes during the Intervention Period (through August 20, 2015).*
| Outcome | Intensive Treatment | Standard Treatment | Hazard Ratio (95% Cl) | P Value † | ||
|---|---|---|---|---|---|---|
| no. of participants | % per year | no. of participants | % per year | |||
| All participants | (N = 4678) | (N = 4683) | ||||
| Primary outcome‡ | 264 | 1.77 | 354 | 2.40 | 0.73 (0.63–0.86) | <0.001 |
| Primary outcome without nonfatal heart failure | 222 | 1.48 | 293 | 1.97 | 0.75 (0.63–0.89) | 0.001 |
| Secondary outcomes‡ | ||||||
| Myocardial infarction | 102 | 0.68 | 140 | 0.93 | 0.72 (0.56–0.93) | 0.01 |
| Acute coronary syndrome | 42 | 0.28 | 41 | 0.27 | 1.02 (0.66–1.57) | 0.93 |
| Stroke | 69 | 0.45 | 78 | 0.52 | 0.89 (0.64–1.23) | 0.48 |
| Heart failure | 68 | 0.45 | 105 | 0.70 | 0.63 (0.46–0.86) | 0.003 |
| Nonfatal heart failure | 66 | 0.43 | 101 | 0.67 | 0.64 (0.47–0.87) | 0.004 |
| Death from cardiovascular causes | 41 | 0.27 | 71 | 0.47 | 0.58 (0.39–0.84) | 0.004 |
| Death from any cause | 163 | 1.06 | 215 | 1.41 | 0.75 (0.61–0.92) | 0.006 |
| Primary outcome or death from any cause | 370 | 2.47 | 474 | 3.20 | 0.77 (0.67–0.88) | <0.001 |
| Participants with CKD at baseline | (N = 1330) | (N = 1316) | ||||
| Composite renal outcome§ | 17 | 0.39 | 16 | 0.37 | 1.03 (0.52–2.06) | 0.93 |
| >50% Reduction in eGFR¶ | 12 | 0.28 | 12 | 0.28 | 0.98 (0.43–2.22) | 0.97 |
| Long-term dialysis | 7 | 0.16 | 10 | 0.23 | 0.66 (0.24–1.72) | 0.39 |
| Kidney transplantation | 0 | 0 | — | — | ||
| Incident albuminuria║ | 64 | 3.93 | 85 | 5.61 | 0.71 (0.50–1.00) | 0.05 |
| Participants without CKD at baseline | (N = 3332) | (N = 3345) | ||||
| ≥30% Reduction in eGFR¶ | 148 | 1.39 | 41 | 0.38 | 3.67 (2.62–5.26) | <0.001 |
| Long-term dialysis | 0 | 0 | - | - | ||
| Kidney transplantation | 0 | 0 | — | — | ||
| Incident albuminuria║ | 142 | 2.54 | 184 | 3.25 | 0.77 (0.62–0.96) | 0.02 |
Additional events not previously reported1 are included. Cl denotes confidence interval, CKD chronic kidney disease, and eGFR estimated glomerular filtration rate.
P values for between-group differences are shown; inferences drawn from these tests may not be reproducible, since none of the P values or 95% confidence intervals were adjusted for multiplicity.
The primary outcome was the first occurrence of myocardial infarction, acute coronary syndrome not resulting in infarction, stroke, acute decompensated heart failure, or death from cardiovascular causes. Components of the primary outcome are shown with fatal and nonfatal cases included, except where noted for nonfatal heart failure.
The composite renal outcome for participants with CKD at baseline was the first occurrence of a reduction in eGFR of 50% or more, long-term dialysis, or kidney transplantation.
Reductions in eGFR were confirmed by a second laboratory test at least 90 days later.
Incident albuminuria was defined by a doubling of the ratio of urinary albumin (in milligrams) to creatinine (in grams) from less than 10 at baseline to greater than 10 during follow-up. The numbers of participants represent those without albuminuria at baseline.
Figure 2. Subgroup Analysis of Hazard Ratios for the Primary Outcome during the Intervention Period (through August 20, 2015).
The box sizes are proportional to the precision of the estimates, with larger boxes indicating a greater degree of precision. The subgroup of participants with no previous chronic kidney disease includes some participants with unknown status with respect to chronic kidney disease at baseline. Black race includes Hispanic Black and Black as part of a multiracial identification. P values were adjusted for multiple subgroups tested.
Among the participants who had chronic kidney disease at baseline, there was no significant between-group difference in the renal composite outcome of a 50% or more reduction in eGFR, dialysis, or kidney transplantation; however, there were few events (Table 1). Among the participants who did not have chronic kidney disease at baseline, a 30% reduction in eGFR to less than 60 ml per minute per 1.73 m2 was significantly more common in the intensive-treatment group than in the standard-treatment group (hazard ratio, 3.67; 95% CI, 2.62 to 5.26; P<0.001).
CLINICAL OUTCOMES: OBSERVATIONAL POSTINTERVENTION PERIOD
After the intervention period, 108 primary outcome events and 110 deaths occurred (Table 2). Outcomes during the intervention and postintervention periods were compared with the use of a time-varying–covariate model including an interaction term (Table S4). There was no evidence of a differential effect of treatment group on the primary outcome (P = 0.44) or on all-cause mortality (P = 0.16) between the intervention and postintervention periods. For the outcome of acute decompensated heart failure, the lower event rate in the intensive-treatment group than in the standard-treatment group during the intervention period (hazard ratio, 0.68; 95% CI, 0.50 to 0.92) was followed by a higher event rate in the intensive-treatment group during the postintervention period (hazard ratio, 1.63; 95% CI, 1.02 to 2.57; P = 0.001 for interaction). Rates of the composite renal outcome among participants without chronic kidney disease at baseline also differed according to period; during the postintervention period, between-group differences were attenuated and were not significant (hazard ratio, 1.37 [95% CI, 0.62 to 2.79] vs. 3.71 [95% CI, 2.68 to 5.22] during the intervention period; P = 0.004 for interaction). There were no other significant differences between the intervention and postintervention periods.
Table 2.
Outcomes during the Observational Postintervention Period (through July 29, 2016).*
| Outcome | Intensive Treatment | Standard Treatment | Hazard Ratio (95% Cl) | P Value† | ||
|---|---|---|---|---|---|---|
| no. of participants | % per year | no. of participants | % per year | |||
| All participants | (N = 4515) | (N = 4468) | ||||
| Primary outcome ‡ | 51 | 2.35 | 57 | 2.70 | 0.87 (0.59–1.27) | 0.46 |
| Primary outcome without nonfatal heart failure | 36 | 1.64 | 49 | 2.29 | 0.72 (0.46–1.10) | 0.13 |
| Secondary outcomes‡ | ||||||
| Myocardial infarction | 13 | 0.58 | 21 | 0.96 | 0.61 (0.30–1.22) | 0.16 |
| Acute coronary syndrome | 3 | 0.13 | 6 | 0.27 | 0.51 (0.11–1.94) | 0.33 |
| Stroke | 10 | 0.44 | 18 | 0.81 | 0.51 (0.23–1.10) | 0.09 |
| Heart failure | 32 | 1.42 | 13 | 0.59 | 2.34 (1.25–4.64) | 0.007 |
| Nonfatal heart failure | 32 | 1.42 | 13 | 0.59 | 2.34 (1.25–4.64) | 0.007 |
| Death from cardiovascular causes | 15 | 0.66 | 16 | 0.71 | 0.95 (0.46–1.94) | 0.89 |
| Death from any cause | 53 | 2.31 | 57 | 2.51 | 0.90 (0.61–1.31) | 0.57 |
| Primary outcome or death from any cause | 85 | 3.90 | 89 | 4.20 | 0.91 (0.68–1.23) | 0.55 |
| Participants with CKD at baseline | (N = 1148) | (N = 1125) | ||||
| Composite renal outcome§ | 1 | 0.16 | 3 | 0.50 | 0.31 (0.02–2.42) | 0.27 |
| ≥50% Reduction in eGFR¶ | 1 | 0.16 | 1 | 0.17 | 0.79 (0.03–20.00) | 0.87 |
| Long-term dialysis | 1 | 0.16 | 2 | 0.33 | 0.33 (0.03–5.82) | 0.62 |
| Kidney transplantation | 0 | 1 | 0.17 | 0.00 | 0.23 | |
| Incident albuminuria║ | 5 | 2.30 | 9 | 4.71 | 0.55 (0.16–1.67) | 0.29 |
| Participants without CKD at baseline | (N = 2887) | (N = 2967) | ||||
| ≥30% Reduction in eGFR¶ | 10 | 0.64 | 7 | 0.43 | 1.49 (0.57–4.10) | 0.42 |
| Long-term dialysis | 0 | 0 | — | — | ||
| Kidney transplantation | 0 | 0 | — | — | ||
| Incident albuminuria║ | 8 | 0.99 | 14 | 1.76 | 0.55 (0.22–1.30) | 0.18 |
Included in the table are data from participants who were not known to be dead at the end of the trial intervention period.
P values for between-group differences are shown; inferences drawn from these tests may not be reproducible, since none of the P values or 95% confidence intervals were adjusted for multiplicity.
The primary outcome was the first occurrence of myocardial infarction, acute coronary syndrome not resulting in infarction, stroke, acute decompensated heart failure, or death from cardiovascular causes. Components of the primary outcome are shown with fatal and nonfatal cases included, except where noted for nonfatal heart failure.
The composite renal outcome for participants with CKD at baseline was the first occurrence of a reduction in eGFR of 50% or more, long-term dialysis, or kidney transplantation.
Reductions in eGFR were confirmed by a second laboratory test at least 90 days later.
Incident albuminuria was defined by a doubling of the ratio of urinary albumin (in milligrams) to creatinine (in grams) from less than 10 at baseline to greater than 10 during follow-up. The numbers of participants represent those without albuminuria at baseline.
We examined whether changes in blood-pressure medication in the postintervention period could explain the higher rate of heart-failure events during this period. We found that diuretics, angiotensin-converting–enzyme (ACE) inhibitors, and hydralazine were more frequently discontinued during the postintervention period in the intensive-treatment group and were more often added during the postintervention period in the standard-treatment group (test for trend, P<0.001 for diuretics, P<0.001 for ACE inhibitors, and P = 0.005 for hydralazine) (Tables S5 and S7). However, most participants with heart failure outcomes that occurred after August 20, 2015, in both the intensive-treatment group and in the standard-treatment group had no medication changes recorded at their first postintervention-period visit (Tables S6 and S7).
When intervention and postintervention results were combined, the primary outcome and death rates remained significantly lower in the intensive-treatment group than in the standard-treatment group (hazard ratio for the primary outcome, 0.76; 95% CI, 0.65 to 0.88; P<0.001; hazard ratio for death, 0.79; 95% CI, 0.66 to 0.94; P = 0.009) (Table S8). Rates of myocardial infarction remained significantly lower in the intensive-treatment group than in the standard-treatment group (hazard ratio, 0.71; 95% CI, 0.56 to 0.90; P = 0.005), as did rates of death from cardiovascular causes (hazard ratio, 0.65; 95% CI, 0.46 to 0.90; P = 0.01), whereas rates of heart-failure events did not differ significantly between the groups. Multiple-imputation analyses of the primary outcome to account for missing data showed similar results (Table S9). Finally, the Andersen–Gill recurrent-events model showed significantly fewer first and reoccurring primary outcome events in the intensive-treatment group than in the standard-treatment group (435 events and 552 events, respectively; hazard ratio, 0.78; 95% CI, 0.69 to 0.89; P<0.001) (Fig. S8).
SERIOUS ADVERSE EVENTS
In the intervention period, rates of serious adverse events overall did not differ significantly between the groups (Table 3). However, hypotension, electrolyte abnormalities, and acute kidney injury or renal failure occurred more often in the intensive-treatment group than in the standard-treatment group. Syncope was more common in the intensive-treatment group when serious adverse events and emergency department events were combined. Similar patterns were found when intervention and postintervention data were combined (Table S10).
Table 3.
Adverse Events during the Clinical Trial Intervention Period (through August 20, 2015).
| Event* | Intensive Treatment (N = 4678) | Standard Treatment (N =4683) | Hazard Ratio (95% Cl) | P Value† | ||
|---|---|---|---|---|---|---|
| no. of participants | % per year | no. of participants | % per year | |||
| Serious adverse event‡ | 1799 (38.5) | 14.8 | 1742 (37.2) | 14.2 | 1.04 (0.97–1.11) | 0.23 |
| Conditions of interest | ||||||
| Serious adverse event | ||||||
| Hypotension | 99 (2.1) | 0.7 | 58 (1.2) | 0.4 | 1.71 (1.24–2.38) | 0.001 |
| Syncope | 97 (2.1) | 0.6 | 73 (1.6) | 0.5 | 1.33 (0.98–1.81) | 0.07 |
| Bradycardia | 78 (1.7) | 0.5 | 68 (1.5) | 0.5 | 1.15 (0.83–1.59) | 0.41 |
| Electrolyte abnormality | 138 (2.9) | 0.9 | 104 (2.2) | 0.7 | 1.33 (1.03–1.72) | 0.03 |
| Injurious fall§ | 102 (2.2) | 0.7 | 101 (2.2) | 0.7 | 1.01 (0.76–1.33) | 0.97 |
| Acute kidney injury or acute renal failure¶ | 193 (4.1) | 1.3 | 115 (2.5) | 0.8 | 1.69 (1.34–2.13) | <0.001 |
| Emergency department visit or serious adverse event | ||||||
| Hypotension | 144 (3.1) | 1.0 | 79 (1.7) | 0.5 | 1.83 (1.40–2.42) | <0.001 |
| Syncope | 148 (3.2) | 1.0 | 100 (2.1) | 0.7 | 1.48 (1.15–1.92) | 0.002 |
| Bradycardia | 94 (2.0) | 0.6 | 76 (1.6) | 0.5 | 1.24 (0.91–1.68) | 0.17 |
| Electrolyte abnormality | 170 (3.6) | 1.1 | 127 (2.7) | 0.9 | 1.34 (1.07–1.69) | 0.01 |
| Injurious fall§ | 335 (7.2) | 2.3 | 317(6.8) | 2.2 | 1.06 (0.91–1.23) | 0.49 |
| Acute kidney injury or acute renal failure¶ | 201 (4.3) | 1.3 | 120 (2.6) | 0.8 | 1.69 (1.35–2.12) | <0.001 |
| Monitored clinical events: adverse laboratory measures║ | ||||||
| Serum sodium <130 mmol/liter | 189 (4.0) | 1.3 | 103 (2.2) | 0.7 | 1.85 (1.46–2.36) | <0.001 |
| Serum sodium >150 mmol/liter | 6 (0.1) | 0.04 | 0 | 0 | — | 0.004 |
| Serum potassium <3.0 mmol/liter | 117 (2.5) | 0.8 | 75 (1.6) | 0.5 | 1.56 (1.17–2.10) | 0.002 |
| Serum potassium >5.5 mmol/liter | 184 (3.9) | 1.2 | 173 (3.7) | 1.2 | 1.06 (0.86–1.31) | 0.56 |
Duplicate events of the same (i.e.,in the same row) in a given participant were counted only once, but the same participant could be included in more than one row for the same event.
Inferences drawn from these tests may not be reproducible, since none of the P values or 95% confidence intervals were adjusted for multiplicity.
A serious adverse event was defined as an event that was fatal or life-threatening, that resulted in significant or persistent disability, that led to or prolonged a hospitalization, or that was an important medical event that the investigator judged to be a substantial hazard or harm to the participant that may have led to medical or surgical intervention to prevent one of the other events listed above.
Injurious fall was defined as a fall that resulted in evaluation in an emergency department or that resulted in hospitalization.
Acute kidney injury or acute renal failure was coded if the diagnosis was listed in the hospital discharge summary and was thought to be one of the top three reasons for admission or continued hospitalization. Although acute kidney injury was not reportable as a condition of interest if it resulted only in evaluation in an emergency department, it was noted in a few cases (8 in the intensive-treatment group and 5 in the standard-treatment group) in which the participant presented to an emergency department for one of the other conditions of interest.
Adverse laboratory measures were detected on routine or unscheduled tests; routine laboratory tests were performed at 1 month, then quarterly during the first year, then every 6 months.
DISCUSSION
The updated findings from the intervention period in our trial confirm the significant benefits of intensive blood-pressure control for the primary composite outcome, the components of the primary outcome (myocardial infarction, heart failure, and death from cardiovascular causes), a post hoc composite outcome that excluded heart failure, and all-cause mortality. Some have suggested that the benefit with respect to the rate of heart-failure events was due to differential fluid shifts resulting from more frequent use of chlorthalidone in the intensive-treatment group.9 However, the difference in primary-outcome event rates was still significant when heart-failure events were excluded. Furthermore, we used rigorous, objective, validated criteria10 for confirmation of acute decompensated heart failure, which was adjudicated by a committee that was unaware of the treatment assignments. We have reported elsewhere that detailed analyses did not show evidence that differential use of diuretics contributed to these findings.11
As in the initial trial report,1 no difference was found in the incidence of the composite renal outcome — a decrease in eGFR of 50% or more or end-stage renal disease — between the two treatment groups among the participants who had chronic kidney disease at baseline. However, among participants who did not have kidney disease, a 30% decrease in eGFR to less than 60 ml per minute per 1.73 m2 remained more common in the intensive-treatment group than in the standard-treatment group. In addition, higher rates of serious adverse events related to acute kidney injury persisted in the intensive-treatment group.
As reported previously, most of the acute adverse events affecting the kidney were solitary, were mild (as determined on the basis of the modified Kidney Disease: Improving Global Outcomes [KDIGO] criteria), and were followed by nearly complete recovery of kidney function within 1 year.12 The decrease in eGFR occurred early among the patients without chronic kidney disease in the intensive-treatment group, and after 18 months the difference between treatment groups stabilized, a finding consistent with a hemodynamic reduction in glomerular pressure.13 Similar effects have been noted in other trials.14–17 Analyses of urine markers of tubule function in trial participants who had chronic kidney disease at baseline lend support for this hemodynamic-effect hypothesis.18 Longer-term effects are being examined in a separate SPRINT trial (Alzheimer’s, Seniors, and Kidney Study [SPRINT ASK]).19
Rates of serious adverse events due to hypotension or electrolyte abnormalities remained higher in the intensive-treatment group. Syncope was more common in the intensive-treatment group when serious adverse events and adverse events leading to emergency department visits were combined. We previously found that hypotension and syncope adverse events were more common among participants with chronic kidney disease, frailty, or older age; there was no age-by-treatment interaction for these events.6
During the postintervention period, blood pressure was managed by each participant’s usual health care provider. Systolic blood-pressure differences between the groups narrowed. During this period, the event rate for acute decompensated heart failure was significantly higher in the intensive-treatment group than in the standard-treatment group. Potential explanations include a lower intensity of blood-pressure treatment in the intensive-treatment group and random variation due to few cases having occurred during the postintervention period. We did observe decreases in the use of some blood pressure–lowering medications in the intensive-treatment group and increases in the use of such medications in the standard-treatment group in a minority of participants. Thus, although modest changes in therapeutic intensity may affect some benefits of intensive treatment, this speculation is based on few events in the postintervention period.
When data from the intervention and postintervention periods were combined, the benefits of intensive treatment with respect to the primary outcome and all-cause mortality remained highly significant and similar in magnitude to effects seen during the trial. Finally, an Andersen–Gill model showed significantly fewer primary outcome events in the intensive-treatment group than in the standard-treatment group when both incident and recurrent events that occurred through July 29, 2016, were considered.
In this final report of the main outcomes of the SPRINT trial, involving patients at increased risk for cardiovascular events, intensive treatment to lower blood pressure was associated with lower rates of fatal and nonfatal cardiovascular events and death from any cause than standard treatment. However, some adverse events occurred more frequently with the lower blood-pressure target. During a post-trial observational period, the achieved blood-pressure differential between the treatment groups was attenuated, and more frequent heart failure was noted in the intensive-treatment group.
Supplementary Material
Acknowledgments
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the U.S. Government.
Supported by contracts (HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C) and an interagency agreement (A-HL-13-002-001) from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke. Several trial sites were supported by Clinical and Translational Science Awards funded by the National Center for Advancing Translational Sciences of the NIH (UL1TR000439 to Case Western Reserve University, UL1RR025755 to Ohio State University, UL1RR024134 and UL1TR000003 to the University of Pennsylvania, UL1RR025771 to Boston University, UL1TR000093 to Stanford University, UL1RR025752, UL1TR000073, and UL1TR001064 to Tufts University, UL1TR000050 to the University of Illinois, UL1TR000005 to the University of Pittsburgh, 9U54TR000017-06 to the University of Texas Southwestern, UL1TR000105-05 to the University of Utah, UL1TR000445 to Vanderbilt University, UL1TR000075 to George Washington University, UL1TR000002 to the University of California, Davis, UL1TR000064 to the University of Florida, UL1TR000433 to the University of Michigan, and P30GM103337 COBRE Award NIGMS to Tulane University). The trial was also supported in part with resources and use of facilities through the Department of Veterans Affairs. Azilsartan and azilsartan combined with chlorthalidone were donated by Takeda Pharmaceuticals International and Arbor Pharmaceuticals; neither company had any other role in the trial.
We thank Karen Potvin Klein, M.A. (Wake Forest University Health Sciences), for editorial assistance with an earlier version of the manuscript.
Footnotes
References
- 1.The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373: 2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014; 11:532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Systolic Blood Pressure Intervention Trial (SPRINT) protocol version 4.0. November 1, 2012. (https://www.sprinttrial.org/public/Protocol_Current.pdf).
- 4.Johnson KC, Whelton PK, Cushman WC, et al. Blood pressure measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension 2018; 71: 848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Correction to: A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2017;3 77: 2506. [DOI] [PubMed] [Google Scholar]
- 6.Sink KM, Evans GW, Shorr RI, et al. Syncope, hypotension, and falls in the treatment of hypertension: results from the randomized clinical Systolic Blood Pressure Intervention Trial. J Am Geriatr Soc 2018; 66: 679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika 1988;7 5: 383–6. [Google Scholar]
- 8.Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat 1982; 10: 1100–20. [Google Scholar]
- 9.Kjeldsen SE, Narkiewicz K, Hedner T, Mancia G. The SPRINT study: outcome may be driven by difference in diuretic treatment demasking heart failure and study design may support systolic blood pressure target below 140 mmHg rather than below 120 mmHg. Blood Press 2016; 25: 63–6. [DOI] [PubMed] [Google Scholar]
- 10.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail 2012; 5: 152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Upadhya B, Lovato LC, Rocco M, et al. Heart failure prevention in older patients using intensive blood pressure reduction: potential role of diuretics. JACC Heart Fail 2019; 7:1 032–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocco MV, Sink KM, Lovato LC, et al. Effects of intensive blood pressure treatment on acute kidney injury events in the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis 2018;7 1: 352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beddhu S, Rocco MV, Toto R, et al. Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease: a secondary analysis of a randomized trial. Ann Intern Med 2017; 167:375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtkamp FA, de Zeeuw D, Thomas MC, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 2011;8 0: 282–7. [DOI] [PubMed] [Google Scholar]
- 15.Peralta CA, McClure LA, Scherzer R, et al. Effect of intensive versus usual blood pressure control on kidney function among individuals with prior lacunar stroke: a post hoc analysis of the Secondary Prevention of Small Subcortical Strokes (SPS3) randomized trial. Circulation 2016; 133: 584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362: 1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appel LJ, Wright JT Jr, Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363:9 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malhotra R, Craven T, Ambrosius WT, et al. Effects of intensive blood pressure lowering on kidney tubule injury in CKD: a longitudinal subgroup analysis in SPRINT. Am J Kidney Dis 2019; 73: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Project details: the SPRINT Alzheimer’s, Seniors and Kidney Study (SPRINT ASK). NIH Research Portfolio Online Reporting Tools (RePORT) (https://reporter.nih.gov/project-details/9288712).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.