Key Features.
The LIFE-Adult-Study is a population-based cohort study investigating the prevalence and incidence of common diseases and subclinical disease phenotypes, the complex interactions between genetic and lifestyle factors regarding the co-occurrence and development of subclinical phenotypes and diseases, and the role of biomarkers to predict disease initiation and progression.
The study comprises an age-stratified and sex-stratified random sample of 10 000 adult individuals (aged 18–79 years) from Leipzig, Germany. The baseline assessment was conducted from August 2011 to November 2014 and the first follow-up was conducted from October 2017 to August 2021. A total of 5512 individuals completed postal follow-up questionnaires and 1799 individuals participated in a physical examination follow-up programme.
The study focuses on cardiovascular and metabolic disorders, cognition and brain function, depression, sleep disorders and electroencephalography-vigilance regulation, eye diseases, voice and allergies. The assessment programme comprises physical and medical examinations, personal interviews, self-administered questionnaires, psychometric tests, and clinical chemistry from blood and urine samples (including biobank asservation).
Data usage requests can be submitted to: Dr Christoph Engel, Institute for Medical Informatics, Statistics and Epidemiology, Leipzig University, Haertelstrasse 16–18, 04107 Leipzig, Germany, e-mail: christoph.engel@imise.uni-leipzig.de.
Why was the cohort set up?
Population-based cohort studies are an essential foundation for investigating associations of genetic and non-genetic risk factors with the occurrence of diseases. Several such studies have been initiated in Germany over the past decades focusing on specific phenotypes and diseases.1–5 In 2009, the Leipzig Research Centre for Civilization Diseases (LIFE) was established to set up population-based and patient-based cohorts with comprehensive molecular characterization and deep disease phenotyping programmes for a broad spectrum of common diseases. LIFE focuses on the characterization of subclinical disease phenotypes and the identification of determinants predicting their progression to clinically manifest disease. It is part of the Medical Faculty of Leipzig University, enabling the participation of clinicians, epidemiologists and methodologists researchers experienced in molecular and genetic profiling, biostatistics and bioinformatics. LIFE is funded by the European Union, the European Regional Development Fund and funds of the Free State of Saxony, Germany.
One of the main cohort studies of LIFE is the ‘LIFE-Adult-Study’—a population-based study of 10 000 randomly selected adult citizens of Leipzig, a city with currently ∼600 000 inhabitants. The LIFE-Adult-Study pursues three major goals: (i) to assess the prevalence and incidence of common diseases and subclinical disease phenotypes, (ii) to investigate the complex interactions between genetic and lifestyle factors regarding co-occurrence and development of subclinical phenotypes and diseases and (iii) to investigate longitudinally the role of biomarkers to predict risks for disease initiation and progression. The study focuses on primarily age-related clinical phenotypes: cardiovascular disorders, metabolic disorders, cognition and brain function, depression, sleep disorders and electroencephalography-vigilance regulation, eye diseases with a focus on retinal degeneration, voice and allergies. Other major LIFE cohort studies are the ‘LIFE-Child-Study’ and the ‘LIFE-Heart-Study’.6,7 An up-to-date list of all publications of LIFE is available at https://www.uniklinikum-leipzig.de/einrichtungen/life/life-forschungszentrum/publikationen.
Who is in the cohort?
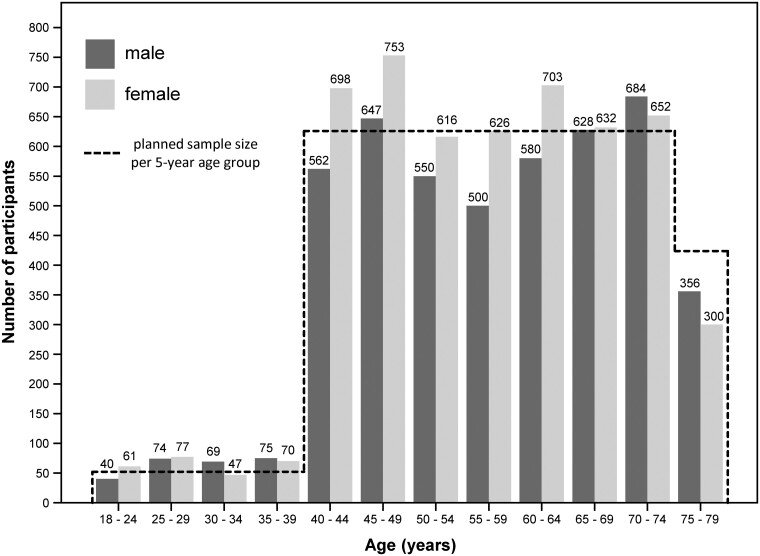
Details on the design of the LIFE-Adult-Study are described elsewhere.8 Briefly, the study comprises an age-stratified and sex-stratified random sample of 10 000 residents of the city of Leipzig, who were mostly of central European descent. Participants are 18–79 years of age, with a focus on individuals aged between 40 and 79 years. Address lists of randomly sampled citizens were provided by the resident’s registration office of the city of Leipzig. In the age group of 40–79 years, a total of 29 535 citizens were invited to participate in the study, of whom 31.0% took part, 29.0% refused participation and 36.3% did not respond. The remaining individuals (3.7%) could not be successfully contacted or did not participate despite declaring their willingness. Among 2386 individuals aged 18–39 years, 16.6% were willing to participate in the study, 22.0% refused to take part and 61.4% did not respond. Figure 1 shows the planned and achieved sample sizes. The baseline recruitment and examination of the 10 000 study participants were conducted from August 2011 until November 2014. A comparison of study participants with both the Leipzig population and non-participants using official statistics and short questionnaire data showed that study participants were less often elderly women and more often married, highly educated, employed, healthier and current non-smokers compared with both the Leipzig population and non-participants.9
Figure 1.
Planned and achieved sample size (baseline recruitment)
How often have they been followed up?
The baseline data collection (questionnaires and physical examinations) was performed at the study centre of the LIFE-Adult-Study. All study participants underwent a core assessment programme with an average duration of 5–6 h within 1 day (‘core programme’). Participants aged ≥65 years (or ≥60 years since March 2013) were invited to take part in two additional assessment programmes, particularly with magnetic resonance imaging (MRI), which were scheduled shortly after the core programme visit on 2 separate days with an average duration of 3–4 h each.
The first follow-up phase started in October 2017 with a questionnaire-based postal interview of all participants of the baseline round. In addition, participants who underwent MRI at baseline were reinvited for physical examinations at the study centre. The first follow-up ended in August 2021. A total of 6115 individuals joined the first follow-up phase, of whom 1799 also joined the examination programme at the study centre. Non-participation (3885 individuals) was due to death (n = 450), active refusal (n = 788), loss to follow-up (n = 61) or non-response (n = 2586).
Follow-up participants had a mean age of 64 years at invitation and 53% were female. Individuals who actively refused participation were older on average (69 years) and more often female (57%). Non-responders were younger (59 years) and only slightly more often female (54%) compared with participants.
What has been measured?
Baseline assessment (August 2011–November 2014)
The baseline assessment programme comprised physical and medical examinations (Table 1), computer-assisted personal interviews, computer-based or paper-based self-administered questionnaires, psychometric tests (Table 2) and clinical chemistry from blood and urine samples (Table 3). The first additional programme for participants aged ≥65 years (or ≥60 years since March 2013) focused on cognitive function and included a brain MRI. The second additional programme comprised detailed assessments of depressive symptomatology and vigilance, including multi-paradigm electroencephalography.
Table 1.
Physical examinations and assessments of adults in the LIFE-Adult-Study cohort at baseline and follow-up
| Examination | Baseline | Follow-up |
|---|---|---|
| Bio-specimen asservation | ||
| Serum, plasma, dried blood spot cards, whole blood | A | S |
| Peripheral blood mononuclear cells (PBMC) | A | – |
| DNA, RNA | A | – |
| Urine | A | – |
| Anthropometry | ||
| Classical: body weight, body height, circumference measures | A | S |
| 3D laser-based optical body surface scan | A | S |
| MRI-based abdominal fat tissue volumetry | S | – |
| Bioelectrical impedance analysis (BIA) | – | S |
| Cardiovascular system | ||
| Blood pressure | A | S |
| Electrocardiogram (10 s, 12 leads) | A | S |
| Echocardiography | A | – |
| Carotid ultrasound | A | S |
| Ankle-brachial index (ABI), pulse wave velocity (PWV) | – | S |
| Ultrasound of leg arteries (A. fem. comm., A. fem. superf., A. popl.) | – | S |
| 7-day electrocardiogram | – | S |
| Diabetes | ||
| Oral glucose tolerance test (OGTT) | S | – |
| Physical activity and fitness | ||
| Hand grip strength | A | – |
| 7-day actimetry | A | – |
| Eye | ||
| Optical coherence tomography and fundus photography | A | S |
| Visual acuity measurement | – | S |
| Ocular biometry | – | S |
| Autorefractometry | – | S |
| Brain | ||
| MRI | S | S |
| Electroencephalography | S | S |
| Liver | ||
| Transient liver elastography | – | S |
| Miscellaneous | ||
| Voice profile | S | – |
| Olfactory test (Sniffin' sticks 12) | S | S |
| Skin prick test (six inhalative allergens) | A | – |
| Measurement of skin ageing | – | S |
A, all participants; S, subgroup; MRI, magnetic resonance imaging; A. fem. comm., femoral artery; A. fem. superf., superficial femoral artery; A. popl., popliteal artery.
Table 2.
Computer-assisted personal interviews (I), self-administered questionnaires (Q) and cognitive tests (T)
| Baseline |
Follow-up |
||
|---|---|---|---|
| Assessment | (physical) | (written) | (physical) |
| Socio-demographics and medical history | |||
| Socio-demographics and socio-economic status | I | Q | I |
| Medical history | I | Q | Q |
| Family medical history | I | Q | – |
| Medication (last 7 days) | I | Q | I |
| Allergies | I | Q | – |
| Male and female gender questions | Q | – | Q |
| Immune competence | Q | – | – |
| Oral health | Q | Q | – |
| Pneumonia | – | Q | – |
| Language skills | – | – | I |
| Place of residence biography | – | Q | Q |
| Life style | |||
| Smoking | I/Q | Q | – |
| Food frequency and alcohol consumption | Q | Q | – |
| International Physical Activity Questionnaire (IPAQ) | Q | Q | – |
| Three-Factor-Eating Questionnaire (TFEQ) | Q | – | – |
| Yale Food Scale | Q | – | Q |
| E-cigarette use | – | Q | – |
| Eating behaviour | – | – | Q |
| Sun exposition of the skin | – | – | I |
| Use of green areas | – | Q | – |
| Housing situation and noise | – | Q | – |
| Psychosocial aspects | |||
| Satisfaction with Life Scale (SWLS) | Q | Q | – |
| Generalized Anxiety Disorder (GAD-7) | Q | Q | – |
| Patient Health Questionnaire-15 (PHQ-15) | Q | Q | – |
| SF-8 Health Survey | Q | Q | – |
| Life Orientation Test Revised (LOT-R) | Q | Q | – |
| ENRICHD Social Support Instrument (ESSI) | Q | Q | – |
| Personality Adjective List (16 AM) | Q | – | – |
| Lubben Social Network Scale (LSNS) | Q | Q | – |
| EuroQol Visual Analogue Scale (EQ VAS) | Q | – | – |
| Sense of Coherence Leipzig Short Scale (SOC-L9) | Q | – | Q |
| Childhood Trauma Screener (CTS) | Q | – | – |
| Use of healthcare services | Q | – | – |
| Barratt's Impulsivity Scale Version 11 (BIS-11) | Q | – | Q |
| Behavioural inhibition system/behavioural activation system (BIS/BAS) | Q | – | Q |
| NEO-Five-Factor Inventory (NEO-FFI-30) | Q | – | Q |
| Need Inventory of Sensation Seeking (NISS) | Qa | – | – |
| Hypomanic Personality Scale (HPS) | Qa | – | – |
| State-Trait Anger Expression Inventory (STAXI) | Qa | – | Q |
| The Trier Inventory for Chronic Stress (TICS) | Qa | – | Q |
| Loneliness Scale | – | Q | – |
| Perceived Stress Questionnaire (PSQ) | – | Q | – |
| Copenhagen Psychosocial Questionnaire (COPSOQ) B3, B8, B9 | – | – | Q |
| Occupational cognitive demands (O*NET) | – | – | Q |
| Employee–work requirement misfit | – | – | Q |
| Sleep | |||
| Pittsburgh Sleep Quality Index (PSQI) | Q | – | Q |
| Multidimensional Fatigue Inventory (MFI-20) | Q | – | – |
| Epworth Sleepiness Scale (ESS) | Q | – | Q |
| Morningness-Eveningness-Questionnaire (MEQ) | Q | – | – |
| Sleep Questionnaire (SF-A) | Qa | – | – |
| Cognition | |||
| Stroop test | T | – | – |
| Trail-Making Test A&B | T | – | T |
| Subjective Memory Impairment | I | – | I |
| Verbal Fluency Test ‘Animals’ | T | – | T |
| Behavioural Assessment of the dysexecutive syndrome (DEX) | Q | – | Q |
| Triangle test | Ta | – | – |
| Reading the mind in the eyes test | Ta | – | T |
| SIDAM | I/Ta | – | I/T |
| Barthel-Index for basic Activities of Daily Living (BI ADL) | Ia | – | I |
| Instrumental Activities of Daily Living Scales (IADL) | Ia | – | I |
| CERADplus neuropsychological test battery | Ta | – | T |
| Wechsler Memory Scale (WMS) | Ta | – | – |
| Iowa Gambling Task (IGT) | T | – | – |
| n-back task | T | – | – |
| Reversal learning task | T | – | – |
| Mnemonic Similarity Task (MST) | – | – | T |
| Depression | |||
| Centre of Epidemiologic Studies—Depression Scale (CES-D) | Q | – | Q |
| Childhood Trauma Questionnaire (CTQ) | Q | – | – |
| CIDI DIA-X Screener | Q | – | – |
| Structured Clinical Interview for DSM Disorders (SCID) | Ia | – | – |
| Geriatric Depression Scale (15-item version, GDS-15) | Qa | – | Qb |
| Inventory of Complicated Grief (ICG) | Qa | – | – |
| Inventory of Depressive Symptoms (IDS-SR) | Qa | – | Qb |
| Leipzig Life Event List | Qa | – | Q |
| Stress Appraisal Measure (SAM) | – | – | Q |
| Copenhagen Burnout Inventory (CBI) | – | – | Q |
Only aged 60–79 years.
Only participants who underwent the electroencephalography assessment.
SIDAM, Structured Interview for the diagnosis of Dementia of the Alzheimer type, Multi-infarct dementia and dementias of other etiology according to ICD-10, DSM-III-R and DSM-IV; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; CIDI DIA-X, Composite International Diagnostic Interview Diagnostic Expert System.
Table 3.
Laboratory analyses and biomaterials
| Parameter |
|---|
| Electrolytes: Sodium, Potassium, Chloride, Magnesium |
| Liver and pancreas: Alanine transaminase, Aspartate transaminase, Choline esterase, Gamma-glutamyltransferase, Bilirubin (total and direct), Lipase, Total protein, Albumin, Urea |
| Kidney: Creatinine, Cystatin C |
| Cardiac markers: Creatine kinase, Creatine kinase MB, Myoglobin, Troponine T high sensitive, N-terminal prohormone of brain natriuretic peptide |
| Lipid metabolism: Cholesterol, High density lipoprotein cholesterol, Low density lipoprotein cholesterol, Apolipoprotein B, Apolipoprotein A1, Trigycerides, Lipoprotein (a) |
| Glucose metabolism: Glucose, Insulin, C-peptide, Glycated haemoglobin (HbA1c) |
| Iron metabolism: Transferrin, Ferritin |
| Vitamins: Folic acid, Vitamin B12 |
| Bone metabolism: Alkaline phosphatase, Phosphate, Calcium, Osteocalcine, Beta-CrossLaps, Propeptide of type I collagen, Parathormone, 25-Hydroxy vitamin D3 |
| Endocrine function/hormones: Cortisol, Luteinizing hormone, Follicle stimulating hormone, Estradiol, Testosterone, Sex hormone-binding globulin, Dehydroepiandrosterone sulphate |
| Thyroid: Thyrotropin (TSH), Free triiodothyronine, Free thyroxine, TSH receptor antibodies, thyroglobulin antibodies, Thyreoperoxidase antibodies |
| Inflammatory mediators: Interleukin 6, C-reactive protein high sensitive |
| Allergy diagnostics: Specific immunoglobulin E sx1 (timothy grass, rye, birch, mugwort, Cladosporium herbarum, Dermatophagoides pteronyssinus, cat, dog), fx5 (hen’s egg, cow’s milk, fish, wheat, peanut, soy), total immunoglobulin E |
| Hematology: Complete blood cell count with differential, Reticulocytes |
| Urine: Albumin, Creatinine, Immunoglobulin G |
| Targeted metabolomics: Amino acids/acylcarnitines, Plant sterols and cholesterol precursors, Trimethylaminoxid and precursors, Oxysterols and bile acids |
| Targeted proteomics: Apolipoprotein profile |
First follow-up assessment (October 2017–August 2021)
The follow-up programme consisted of two parts: (i) a set of paper-based self-administered questionnaires sent out to all 10 000 baseline participants and (ii) a 2- to 3-day programme of different physical examinations at the study centre. Tables 1–3 provide an overview of the physical examinations, interviews, questionnaires, tests and laboratory analyses conducted during the follow-up. A number of assessments was newly introduced, e.g. bioelectrical impedance analysis, ultrasound of lower limb arteries, 7-day electrocardiogram, transient liver elastography, visual acuity measurement, ocular biometry and autorefractometry.
A main goal of the follow-up was to obtain additional health-related data from external sources such as health insurances, cancer registries, general practitioners and specialized physicians. Therefore, participants were asked to provide their specific consent for record linkage. Up to March 2021, health insurance data for 1800 participants were obtained. Self-reported changes of health status are validated through a process of requesting confirmations by the treating physicians. In addition, the vital status of 98.6% of the participants was updated with the public population register.
What has it found? Key findings and publications
Tables 4–8 summarize selected characteristics of the 10 000 participants by sex and age group. Data from the baseline assessments served as the basis of a large number of published data analyses and research projects (see Supplementary File 1, available as Supplementary data at IJE online, for a full list of publications). Selected published results are briefly presented in the following paragraphs. Additional selected results are described in Supplementary File 2 (available as Supplementary data at IJE online).
Table 4.
Basic characteristics of study participants at baseline examination
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| 18–39 | 40–59 | 60–79 | 18–39 | 40–59 | 60–79 | |
| n = 258 | n = 2259 | n = 2248 | n = 255 | n = 2693 | n = 2287 | |
| CASMIN classification | n = 258 | n = 2254 | n = 2240 | n = 254 | n = 2688 | n = 2278 |
| Low education | 14 (5.4) | 111 (4.9) | 321 (14.3) | 12 (4.7) | 76 (2.8) | 393 (17.3) |
| Medium education | 149 (57.8) | 1552 (68.9) | 1204 (53.8) | 146 (57.5) | 1911 (71.1) | 1474 (64.7) |
| High education | 95 (36.8) | 591 (26.2) | 715 (31.9) | 96 (37.8) | 701 (26.1) | 411 (18.0) |
| BMI WHO category | n = 258 | n = 2253 | n = 2231 | n = 254 | n = 2690 | n = 2278 |
| BMI <25 (normal weight, including underweight) | 154 (59.7) | 712 (31.6) | 476 (21.3) | 198 (78.0) | 1286 (47.8) | 645 (28.3) |
| BMI 25 to <30 (pre-obesity) | 80 (31.0) | 1030 (45.7) | 1110 (49.8) | 42 (16.5) | 835 (31.0) | 923 (40.5) |
| BMI ≥30 (obesity) | 24 (9.3) | 511 (22.7) | 645 (28.9) | 14 (5.5) | 569 (21.2) | 710 (31.2) |
| Waist circumference (cm), mean ± SD | n = 258 | n = 2249 | n = 2230 | n = 254 | n = 2687 | n = 2275 |
| 89.1 ± 11.0 | 99.3 ± 12.3 | 104.1 ± 11.0 | 80.0 ± 9.9 | 90.1 ± 13.4 | 95.6 ± 12.1 | |
| Blood pressure | n = 256 | n = 2246 | n = 2224 | n = 254 | n = 2663 | n = 2259 |
| Systolic (mmHg), mean ± SD | 125.2 ± 10.4 | 129.7 ± 13.8 | 135.1 ± 17.5 | 113.4 ± 9.4 | 121.2 ± 14.9 | 132.1 ± 18.4 |
| Diastolic (mmHg), mean ± SD | 72.3 ± 8.7 | 79.0 ± 9.6 | 75.1 ± 10.0 | 68.9 ± 7.7 | 75.0 ± 9.5 | 73.6 ± 9.7 |
| Hypertension prevalence | n = 252 | n = 2233 | n = 2193 | n = 247 | n = 2650 | n = 2216 |
| 35 (13.9) | 934 (41.8) | 1628 (74.2) | 8 (3.2) | 775 (29.2) | 1534 (69.2) | |
| Maximum handgrip strength, mean ± SD | n = 193 | n = 2027 | n = 2056 | n = 202 | n = 2439 | n = 2089 |
| 48.2 ± 9.6 | 47.3 ± 9.0 | 39.3 ± 8.4 | 30.2 ± 5.1 | 29.3 ± 6.1 | 24.6 ± 5.7 | |
| Smoking | n = 258 | n = 2225 | n = 2110 | n = 250 | n = 2640 | n = 2107 |
| Never smoker | 127 (50.4) | 835 (38.3) | 828 (38.8) | 144 (59.0) | 1252 (48.2) | 1494 (70.3) |
| Former smoker | 34 (13.5) | 670 (30.7) | 1011 (47.4) | 41 (16.8) | 642 (24.7) | 407 (19.2) |
| Current smoker | 97 (38.5) | 720 (33.0) | 271 (12.7) | 65 (26.6) | 746 (28.7) | 206 (9.7) |
| Medication: number of drugs taken within last 7 days | n = 252 | n = 2256 | n = 2246 | n = 249 | n = 2688 | n = 2283 |
| 0 | 143 (56.7) | 875 (38.8) | 258 (11.5) | 58 (23.3) | 491 (18.3) | 185 (8.1) |
| 1 | 60 (23.8) | 492 (21.8) | 253 (11.3) | 82 (32.9) | 703 (26.2) | 280 (12.3) |
| 2–4 | 46 (18.3) | 671 (29.7) | 862 (38.4) | 97 (39.0) | 1112 (41.4) | 935 (41.0) |
| 5+ | 3 (1.2) | 218 (9.7) | 873 (38.9) | 12 (4.8) | 382 (14.2) | 883 (38.7) |
Numbers represent absolute counts and numbers in brackets represent percentages, unless otherwise specified. CASMIN, Comparative Analysis of Social Mobility in Industrial Nations; BMI, body mass index; WHO, World Health Organization.
Table 5.
Sleep quality at baseline examination
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| 18–39 | 40–59 | 60–79 | 18–39 | 40–59 | 60–79 | |
| n = 258 | n = 2259 | n = 2248 | n = 255 | n = 2693 | n = 2287 | |
| Diurnal preference (D-MEQ) | n = 252 | n = 2146 | n = 1886 | n = 240 | n = 2484 | n = 1789 |
| Definitive evening type | 4 (1.6) | 15 (0.7) | 0 (0.0) | 5 (2.1) | 11 (0.4) | 3 (0.2) |
| Moderate evening type | 43 (17.1) | 121 (5.6) | 22 (1.2) | 27 (11.3) | 134 (5.4) | 42 (2.3) |
| Neutral type | 157 (62.3) | 989 (46.1) | 561 (29.7) | 149 (62.1) | 1260 (50.7) | 663 (37.1) |
| Moderate morning type | 46 (18.3) | 900 (41.9) | 1072 (56.8) | 56 (23.3) | 952 (38.3) | 884 (49.4) |
| Definitive morning type | 2 (0.8) | 121 (5.6) | 231 (12.2) | 3 (1.3) | 127 (5.1) | 197 (11.0) |
| Actigraphy-based sleep duration (night–day cyclesa) | n = 57 | n = 501 | n = 604 | n = 58 | n = 709 | n = 634 |
| Short sleep duration (<6 h) | 27 (47.4) | 184 (36.7) | 144 (23.8) | 17 (29.3) | 140 (19.7) | 98 (15.5) |
| Medium sleep duration (6–8 h) | 30 (52.6) | 296 (59.1) | 382 (63.2) | 36 (62.1) | 503 (70.9) | 430 (67.8) |
| Long sleep duration (>8 h) | 0 (0.0) | 21 (4.2) | 78 (12.9) | 5 (8.6) | 66 (9.3) | 106 (16.7) |
| Actigraphy-based sleep efficiency (night–day cyclesa) | n = 57 | n = 501 | n = 604 | n = 58 | n = 709 | n = 634 |
| Low sleep efficiency (<70%) | 6 (10.5) | 65 (13.0) | 86 (14.2) | 9 (15.5) | 43 (6.1) | 40 (6.3) |
| Medium efficiency (70–85%) | 40 (70.2) | 280 (55.9) | 327 (54.1) | 38 (65.5) | 353 (49.8) | 317 (50.0) |
| High sleep efficiency (>85%) | 11 (19.3) | 156 (31.1) | 191 (31.6) | 11 (19.0) | 313 (44.1) | 277 (43.7) |
Numbers represent absolute counts and numbers in brackets represent percentages, unless otherwise specified.
Night–day cycles were defined as duration from individual bedtime on Day 1 and individual bedtime on Day 2 (for further details on the actigraphic assessment, see37). The analysis included all individuals with at least five evaluable night–day cycles in the 1-week actigraphy.
D-MEQ, German Morningness-Eveningness Questionnaire.
Table 6.
Allergy at baseline examination
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| 18–39 | 40–59 | 60–79 | 18–39 | 40–59 | 60–79 | |
| n = 258 | n = 2259 | n = 2248 | n = 255 | n = 2693 | n = 2287 | |
| Skin prick testa | n = 181 | n = 1524 | n = 1316 | n = 167 | n = 1689 | n = 1298 |
| Ambrosia | 11 (6.1) | 65 (4.3) | 36 (2.7) | 7 (4.2) | 60 (3.6) | 42 (3.2) |
| Birch | 64 (35.4) | 296 (19.4) | 135 (10.3) | 32 (19.2) | 234 (13.9) | 98 (7.6) |
| Timothy grass | 49 (27.1) | 268 (17.6) | 141 (10.7) | 28 (16.8) | 298 (17.6) | 174 (13.4) |
| Mugwort | 24 (13.3) | 86 (5.6) | 31 (2.4) | 9 (5.4) | 70 (4.1) | 15 (1.2) |
| Alternaria | 29 (16.0) | 179 (11.7) | 85 (6.5) | 19 (11.4) | 172 (10.2) | 90 (6.9) |
| House dust mite (Dermatophagoides pt.) | 49 (27.1) | 131 (8.6) | 95 (7.2) | 23 (13.8) | 126 (7.5) | 44 (3.4) |
| Allergy questionnairea | n = 233 | n = 2119 | n = 2096 | n = 224 | n = 2464 | n = 2124 |
| Urtikaria | 18 (7.7) | 104 (4.9) | 72 (3.4) | 16 (7.1) | 247 (10.0) | 161 (7.6) |
| Anaphylaxis | 6 (2.6) | 43 (2.0) | 43 (2.1) | 7 (3.1) | 98 (4.0) | 65 (3.1) |
| Food allergy | 42 (18.0) | 254 (12.0) | 164 (7.8) | 48 (21.4) | 466 (18.9) | 263 (12.4) |
| Insect allergy | 14 (6.0) | 151 (7.1) | 125 (6.0) | 26 (11.6) | 306 (12.4) | 270 (12.7) |
Numbers represent absolute counts and numbers in brackets represent percentages, unless otherwise specified.
Groups are not mutually exclusive.
Table 7.
Selected conditions and measurements at baseline examination
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| 18–39 | 40–59 | 60–79 | 18–39 | 40–59 | 60–79 | |
| n = 258 | n = 2259 | n = 2248 | n = 255 | n = 2693 | n = 2287 | |
| Diabetes | n = 252 | n = 2182 | n = 2135 | n = 244 | n = 2599 | n = 2125 |
| No | 225 (89.3) | 1271 (58.2) | 736 (34.5) | 235 (96.3) | 2037 (78.4) | 1053 (49.6) |
| Yes, by medical history and/or medication | 2 (0.8) | 144 (6.6) | 500 (23.4) | 3 (1.2) | 104 (4.0) | 364 (17.1) |
| Yes, yet undiagnosed pre-diabetes | 24 (9.5) | 714 (32.7) | 789 (37.0) | 6 (2.5) | 423 (16.3) | 631 (29.7) |
| Yes, yet undiagnosed diabetes | 1 (0.4) | 53 (2.4) | 110 (5.2) | 0 (0.0) | 35 (1.3) | 77 (3.6) |
| Carotid artery characteristics | ||||||
| Mean carotid intima media thickness (mm), median (IQR) | n = 247 | n = 2178 | n = 2141 | n = 245 | n = 2622 | n = 2212 |
| 0.54 (0.49 ‒ 0.60) | 0.68 (0.61 ‒ 0.78) | 0.83 (0.74 ‒ 0.93) | 0.52 (0.48 ‒ 0.58) | 0.65 (0.59 ‒ 0.73) | 0.81 (0.73 ‒ 0.90) | |
| Prevalence of plaque | 14/243 (5.8) | 751/2090 (35.9) | 1604/2070 (77.5) | 3/244 (1.2) | 593/2567 (23.1) | 1278/2154 (59.3) |
| Psychological and health variables | ||||||
| Anxiety (GAD-7), mean ± SD | n = 255 | n = 2226 | n = 2134 | n = 255 | n = 2669 | n = 2180 |
| 2.92 ± 3.03 | 3.19 ± 3.26 | 2.83 ± 2.97 | 3.90 ± 3.28 | 4.27 ± 3.74 | 3.85 ± 3.27 | |
| Bodily complaints (PHQ-15), mean ± SD | n = 255 | n = 2180 | n = 2000 | n = 255 | n = 2594 | n = 1966 |
| 3.71 ± 2.80 | 4.38 ± 3.39 | 5.00 ± 3.83 | 5.63 ± 3.74 | 6.18 ± 4.02 | 6.58 ± 4.12 | |
| Satisfaction with Life Scale (SWLS), mean ± SD | n = 256 | n = 2233 | n = 2123 | n = 255 | n = 2665 | n = 2177 |
| 26.50 ± 5.26 | 25.50 ± 5.90 | 27.20 ± 5.06 | 26.80 ± 5.35 | 26.30 ± 5.75 | 26.90 ± 5.31 | |
| Optimism (LOT-R, total score), mean ± SD | n = 255 | n = 2240 | n = 2133 | n = 255 | n = 2663 | n = 2163 |
| 16.80 ± 4.00 | 16.20 ± 3.89 | 15.90 ± 3.52 | 17.10 ± 3.96 | 16.70 ± 4.02 | 15.90 ± 3.57 | |
| Olfactory dysfunction | n = 202 | n = 1700 | n = 1576 | n = 184 | n = 1998 | n = 1607 |
| Anosmic | 1 (0.5) | 47 (2.8) | 177 (11.1) | 1 (0.5) | 33 (1.7) | 112 (7.0) |
| Dysosmic | 53 (26.2) | 851 (50.1) | 984 (62.4) | 61 (33.2) | 925 (46.3) | 934 (58.1) |
| Normosmic | 148 (73.3) | 802 (47.2) | 415 (26.3) | 122 (66.3) | 1040 (52.1) | 561 (34.9) |
| Retinal nerve fibre layer thickness (μm), mean ± SD | n = 194 | n = 1644 | n = 1194 | n = 191 | n = 1981 | n = 1267 |
| 97.6 ± 8.8 | 97.6 ± 9.1 | 95.2 ± 10.1 | 98.9 ± 8.6 | 98.4 ± 8.9 | 96.5 ± 9.4 | |
Numbers represent absolute counts and numbers in brackets represent percentages, unless otherwise specified. GAD-7, Generalized Anxiety Disorder (7 items); PHQ-15, Patient Health Questionnaire (15 items); SWLS, Satisfaction with Life Scale; LOT-R, Life Orientation Test Revised.
Table 8.
Omics measurements at baseline examination
| Omics layer | Technique | Sample size | References |
|---|---|---|---|
| Genetics | Affymetrix Axiom CEU1 SNP-array | 7669 | 38–53 |
| Transcriptomics | Illumina HT12-v4 | 3359 | 54 |
| Proteomics | Olink Cardiovascular III | 2016 | – |
| Metabolomics (amino acids and acylcarnitines) | LC–MS/MS | 9622 | 55 |
| Phytosterols/zoosterols | LC–MS/MS | 1689 | 56 |
| Steroid hormones | ECLIA, LC–MS/MS | 8329 | 50,57 |
| Adipokines | ELISA | 3111 | – |
| Cytomics | 10-color flow cytometry | 1365 | 58–61 |
LC–MS/MS, liquid chromatography—mass spectrometry and liquid chromatography—tandem mass spectrometry; ECLIA, electrochemiluminescence immunoassay.
Brain MRI studies
A major goal of the LIFE-Adult-Study is to investigate structural and functional brain alterations and their associations with a broad spectrum of phenotypes and disease susceptibility. Using high-resolution 3-Tesla MRI, we found that higher body mass index, higher systolic blood pressure, smoking and higher HbA1c blood levels were associated with altered grey and white matter (micro) structure, even in young to middle-aged adults.10–18 These changes translated into subtle changes in cognitive performance, such as verbal fluency and memory. Moreover, visceral fat accumulation was related to lesions in the deep white matter through higher levels of interleukin-6 in blood, indicative of low-grade systemic inflammation as a pathomechanistic link.19 Evidence integrating measures of neuroimaging and metabolic imaging, sex hormones and cognition supports differences in women and men in the association of visceral adipose tissue with structural brain networks important for memory.20 In women, higher estradiol levels were associated with increased structural brain network covariance and cognitive performance during mid-life. In sum, these findings highlight the need for early preventive and therapeutic strategies to reduce cardiovascular and metabolic risk factor-mediated brain damage.
Cognition studies
Another major objective of the LIFE-Adult-Study is to characterize neurodegenerative diseases and their prodromal stages based on questionnaires and neuropsychological tests (Table 2). We obtained new empirical data on the prevalence of mild neurocognitive disorder (miNCD) and types of impaired neurocognitive domains. We found a substantial proportion (20%) of older adults having miNCD.21 We also found that memory-related subjective cognitive symptoms are very common and unspecific in the non-demented adult population aged 40–79 years.22 We provided new age-specific, sex-specific and education-specific reference values for the cognitive performance of older German-speaking adults.23 Another study revealed that lower executive functioning performance in cognitively intact older apolipoprotein E epsilon (APOE) 4 allele carriers might be related to an early Alzheimer's dementia prodrome.24
3D laser-based anthropometry
For the first time in epidemiology, we applied laser-based 3D whole-body scanning (3D-BS) for anthropometry. This method allows the determination of >150 anthropometric variables by a single scan with a duration of ∼10 s. Most of these variables showed good intra-observer and inter-observer reliability.25 Using self-organizing maps, we identified 15 clusters of human body shapes, most of which were sex-specific.26,27 3D-BS provides a more detailed description of the human body shape beyond traditional measures such as body mass index and waist-to-hip ratio. We also used 3D-BS to validate commonly used empirical formulae for calculating the body surface area, which is essential for many medical applications.28
Optical coherence tomography (OCT) of the retina
The retinal nerve fibre layer thickness (RNFLT) around the optic nerve head (circumpapillary RNFLT, cpRNFLT) is important to detect optic neuropathies like glaucoma and to monitor their progression. We applied OCT to provide a detailed quantitative description of cpRNFLT at a so-far unprecedented resolution of 768 angular locations.29 In addition to age-dependent differences, we determined estimates of the true scanning diameter, which is a measure of individual eye anatomy.29 We found a considerable sex-dependency of RNFLT, which is currently not considered in existing normative data sets.30 In addition, we described cpRNFLT differences between right and left eyes.31 These norms are accessible as part of the RNFLT(D)-Visualizer application via https://apps.health-atlas.de/rnflt-visualizer. We found markers of renal function and lipid metabolism to be independent predictors of sectoral cpRNFLT, which may improve early diagnosis of eye disease.32
Measurements of the speaking and singing voice
Voice range profiles are widely used in clinical practice to assess voice disorders. We employed this method in our epidemiological setting.33 Based on the currently largest sample of 2472 speaking and singing voice profiles worldwide, we established population-based reference values of different voice parameters. We found that the fundamental frequency of females was six to seven semi-tones lower than previously described.34 Moreover, current smokers had lower speaking voice frequencies compared with non-smokers and former smokers. An association study of the speaking voice with sex hormone levels and anthropometric parameters obtained from 3D-BS revealed that body mass index (BMI), body height, body weight, breast-to-abdomen-ratio, bioavailable testosterone and dehydroepiandrosterone sulphate were associated with the speaking voice in adults.35
What are the main strengths and weaknesses?
The LIFE-Adult-Study is a large population-based epidemiological study with uniquely deep phenotyping and a comprehensive assessment of psychosocial and lifestyle factors. A specific strength of the assessment programme is its particularly detailed characterization of subclinical phenotypes, with emphasis on anthropometry, cognition, depression, vascular diseases and retinal health. To the best of our knowledge, our study was the first to employ a 3D body scanner to collect >150 anthropometric parameters from each participant within a population-based epidemiological research setting. The LIFE-Adult-Study provides the largest population-based data set worldwide on voice range profiles for the singing and speaking voice to date. Moreover, we have initiated a structured process to validate self-reported medical events by contacting the attending physicians of the participants. In addition, we have initiated a record-linkage process with the participants' health insurance companies as well as with the registers providing mortality data.
The representativeness of our study was limited due to selective participation. A comparison of study participants aged 40–79 years with both the Leipzig population and non-participants using official statistics and short questionnaire data suggested that participation was associated with higher social status, healthier lifestyle and lower burden of disease.9 Even though analyses corrected for this bias by applying sampling weights, frequencies of major health conditions in the general population are likely to be underestimated. The external validity, i.e. generalizability, of our regional study must be considered limited with respect to the estimation of disease prevalences and incidences, as these are known to differ across different regions in Germany. However, this limitation should affect associative data analyses less.
Can I get hold of the data? Where can I find out more?
We welcome collaborations with external researchers and have already extensively shared data and biospecimens. Use and access of data comply with the FAIR criteria.36 A data portal has been established to search for available data. Selected data sets are available on a data-sharing portal (https://www.health-atlas.de/). For access to any data or biosamples, it is mandatory to submit a detailed written proposal describing the background, objectives, methods, timelines, names and affiliations of all researchers involved; the type and scope of requested data and biomaterial; the publication and exploitation strategy; and how results and newly generated data will be returned for further use. Upon review and approval by the Use-and-Access Committee, data and samples can be provided. Specific contracts and data or material transfer agreements may be necessary, particularly for researchers from countries not bound to the EU General Data Privacy Regulation. All enquiries should be directed via e-mail to Dr Christoph Engel (E-mail: christoph.engel@imise.uni-leipzig.de).
Notes
Members of the LIFE-Adult-Study working group:
Peter Ahnert,1,2 Yoon Ju Bae,3 Daniel Baier,4 Martin Berg,1,2 Thomas Berger,2,5 Frank Beutner,6 Frauke Beyer,7,8 Elmar Brähler,9,10 Petra Büttner,6 Ralph Burkhardt,2,11 Julia Dittrich,2,3 Ezgi Dogan-Sander,12 Tobias Elze,2,13 Michael Gaebler,2,8 Stephan Gielen,14 Heide Glaesmer,9 Ulrich Hegerl,15 Tilman Hensch,2,12,16 Anja Hilbert,17 Felix S. Hussenoeder,18 Daniela Husser,19 Philippe Jawinski,2,20 Lasse Jost,1,2 Jan Keil,21 Shahrzad Kharabian Masouleh,8,22 Alexander Kiel,2 Toralf Kirsten,1,2 Michael Kluge,12 Rüya-Daniela Kocalevent,23 Jelena Kornej,1,2,24,25 Andreas Kühnapfel,1,2 Deniz Kumral,8 Jana Kynast,8 Leonie Lampe,8 Franziskus Liem,26,27 Antje Löffler,28 Henry Loeffler-Wirth,2,29 Noah Lorenz,30 Tobias Luck,31 Daniel S. Margulies,26 Mila Massué,1,2 Susanne Melzer,26,32 Jeffrey Netto,2,3 Matthias Nüchter,2 Maryna Polyakova,2,8,33 Janne Pott,1,2 Madlen Reinicke,3 Nigar Reyes,1,2 Francisca S. Rodriguez,34 H. Lina Schaare,8 Peter Schönknecht,35,36 Jan C. Simon,37 Janek Spada,38 Ronald Speer,2 Daniela Stanikova,18,39,40 Andrej Teren,2,14 Christine Ulke,2,12 Gunnar Wichmann,2,41 Barbara Wicklein,1,2 Anja Willenberg,3 Dirk Alexander Wittekind,12 Maryam Yahiaoui-Doktor,1,2 Silke Zachariae,1,2 Rui Zhang,8 Rachel G. Zsido,8 and Andrea E. Zuelke18
1Institute for Medical Informatics, Statistics and Epidemiology, Leipzig University, Leipzig, Germany
2Leipzig Research Centre for Civilization Diseases, Leipzig University, Leipzig, Germany
3Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, University of Leipzig Medical Center, Leipzig, Germany
4Department of Paediatrics, University Hospital of Würzburg, Würzburg, Germany
5Division Otolaryngology, Head and Neck Surgery, Phoniatrics and Audiology, University of Leipzig Medical Center, Leipzig, Germany
6Department of Internal Medicine/Cardiology, Heart Center Leipzig at Leipzig University, Leipzig, Germany
7CRC Obesity Mechanisms, Subproject A1, Leipzig University, Leipzig, Germany
8Department of Neurology, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany
9Department of Medical Psychology and Medical Sociology, Leipzig University, Leipzig, Germany
10Department of Psychosomatic Medicine and Psychotherapy, University Medical Center Mainz, Mainz, Germany
11Institute of Clinical Chemistry and Laboratory Medicine, University Hospital Regensburg, Regensburg, Germany
12Department of Psychiatry and Psychotherapy, University of Leipzig Medical Center, Leipzig, Germany
13Schepens Eye Research Institute, Harvard Medical School, Boston, MA, USA
14Department of Cardiology, Angiology and Intensive Care Medicine, Klinikum Lippe Detmold, Detmold, Germany
15Department of Psychiatry, Psychosomatics and Psychotherapy, Goethe-Universität Frankfurt, Frankfurt a. M., Germany
16Department of Psychology, IU International University of Applied Sciences, Erfurt, Germany
17Integrated Research and Treatment Center AdiposityDiseases, Behavioral Medicine Research Unit, Department of Psychosomatic Medicine and Psychotherapy, University of Leipzig Medical Center, Leipzig, Germany
18Institute of Social Medicine, Occupational Health and Public Health, Leipzig University, Leipzig, Germany
19Department of Electrophysiology, Heart Center Leipzig at University of Leipzig, Leipzig, Germany
20Department of Psychology, Humboldt-Universität zu Berlin, Berlin, Germany
21Department of Child and Adolescent Psychiatry, Psychotherapy and Psychosomatics, Leipzig University, Leipzig, Germany
22Institute of Neuroscience and Medicine (INM-7: Brain and Behaviour), Research Centre Jülich, Jülich, Germany
23Department of General Practice/Primary Care, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
24Boston University Medical Campus, 72 E Concord St, Boston, MA 02118, USA
25Framingham Heart Study, 73 Mt Wayte Ave, Framingham, MA 01702, USA
26Research Group Neuroanatomy & Connectivity, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany
27University Research Priority Program Dynamics of Healthy Aging, University of Zurich, Zurich, Switzerland
28Department of Public Health, Brandenburg University of Technology, Senftenberg, Germany
29Interdisciplinary Centre for Bioinformatics, Leipzig University, Leipzig, Germany
30Medical Faculty, Department of Psychiatry and Psychotherapy, Leipzig University, Leipzig, Germany
31Faculty of Applied Social Sciences, University of Applied Sciences Erfurt, Erfurt, Germany
32The Clinical Trial Centre (ZKS) Leipzig, Leipzig University, Leipzig, Germany
33Cognitive Neurology, University of Leipzig Medical Center, Leipzig, Germany
34RG Psychosocial Epidemiology and Public Health, German Center for Neurodegenerative Diseases, Greifswald, Germany
35Outpatient Department, Prevention and Forensic Psychiatry, University of Leipzig Medical Center, Leipzig, Germany
36Academic Hospital Arnsdorf, Arnsdorf, Germany
37Dept. of Dermatology, Venerology and Allergology, University of Leipzig Medical Center, Leipzig, Germany
38Department of Psychology, Leipzig University, Leipzig, Germany
39Institute of Experimental Endocrinology, Biomedical Research Centre of Slovak Academy of Sciences, Bratislava, Slovak Republic
40Paediatric Clinic of Medical Faculty of Comenius University and National Institute of Children's Health, Bratislava, Slovak Republic
41Department of Otolaryngology, Head and Neck surgery, University of Leipzig Medical Center, Leipzig, Germany
Ethics approval
The LIFE-Adult-Study is conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Medical Faculty of Leipzig University (approval numbers 263–2009-14122009, 263/09-ff, 201/17-ek). Written informed consent was obtained from all participants.
Supplementary Material
Acknowledgements
We thank the external advisory board (Andreas Heinz, Annette Grüters-Kieslich, Peter Propping, Ernst Theodor Rietschel, Ulrich Walter, Heinz-Erich Wichmann, Henry Völzke) for their helpful support during the set-up and conduct of the study. We also wish to thank the city of Leipzig for its generous support and all citizens who were willing to take part in the study. We thank the Medical Faculty of the University of Leipzig for supporting LIFE and we thank the University of Leipzig Medical Center for its financial support to provide and reconstruct the LIFE study site. We acknowledge gratefully the support of Roche Diagnostics GmbH (Mannheim, Germany) for granting clinical chemical test kits (Cobas 8000 and E-modul). The tests were granted for the implementation of studies for new sex-linked and age-linked reference values of laboratory biomarkers. ALK-Abello (Hamburg, Germany) kindly provided skin prick test material. We thank the entire study team for their commitment to make this study a success: Diane Backsmann, Ute Bartz, Heike Bauch, Kristin Beier, Thomas Berger, Andrea Bergmann, József Bocsi, Holger Bogatsch, Andrea Böger, Maya Böhm, Verena Brendler, Anja Broda, Janina Brombosczc, Linda Brüll, Peter Buske, Mandy Claus, Margarita Denich, Rica Dietrich, Yvonne Dietz, Kati Drechsel, Felix Duczek, Melanie Eberl, Christine Fritsche, Christin Fuchs, Juliane Garbrecht, Ulrike Gessendorfer, Friederike Hanisch, Juliana Hantschick, Eike Hänsel, Gabriela Härtel, Alexander Heinzig, Carina Heise, Robert Hinze, Conny Höpfner, Jenny Kaftan, Michael Kleinert, Eric Knäschke, Carola Knigge, Kristin Kolbe, Florian Koschke, Susann Kunz, Uwe Lange, Constance Langheinrich, Ronald Lohan, Nicole Mauche, Anne Morgenstern, Maria Müller, Jeffrey Netto, Gabriela Pelz, Julia Pieruschka, Susann Riedel, Birgit Ronneberger, Carolin Rook, Britt Rosin, Mathias Rühle, Anja Schade, Ulrike Scharrer, Jana Schleinitz, Anne-Kathrin Schmidt, Simone Schrieber, Birgit Schulze, Madlen Siegemund, Nadine Sonnabend, Sina Stangneth, Hartmut Stollberg, Ina Strauch, Silvia Stübner, Sandra Tautenhahn, Alexander Uciteli, Diego Witt, Jeannette Witt, Hendrik Wötzel, Gerlind Zocher and Franziska Zwicker.
Conflict of interest
None declared.
Contributor Information
Christoph Engel, Institute for Medical Informatics, Statistics and Epidemiology, Leipzig University, Leipzig, Germany; Leipzig Research Centre for Civilization Diseases, Leipzig University, Leipzig, Germany.
Kerstin Wirkner, Institute for Medical Informatics, Statistics and Epidemiology, Leipzig University, Leipzig, Germany; Leipzig Research Centre for Civilization Diseases, Leipzig University, Leipzig, Germany.
Samira Zeynalova, Institute for Medical Informatics, Statistics and Epidemiology, Leipzig University, Leipzig, Germany; Leipzig Research Centre for Civilization Diseases, Leipzig University, Leipzig, Germany.
Ronny Baber, Leipzig Research Centre for Civilization Diseases, Leipzig University, Leipzig, Germany; Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, University of Leipzig Medical Center, Leipzig, Germany.
Hans Binder, Interdisciplinary Centre for Bioinformatics, Leipzig University, Leipzig, Germany.
Uta Ceglarek, Leipzig Research Centre for Civilization Diseases, Leipzig University, Leipzig, Germany; Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, University of Leipzig Medical Center, Leipzig, Germany.
Cornelia Enzenbach, Institute for Medical Informatics, Statistics and Epidemiology, Leipzig University, Leipzig, Germany; Leipzig Research Centre for Civilization Diseases, Leipzig University, Leipzig, Germany.
Michael Fuchs, Leipzig Research Centre for Civilization Diseases, Leipzig University, Leipzig, Germany; Division Otolaryngology, Head and Neck Surgery, Phoniatrics and Audiology, University of Leipzig Medical Center, Leipzig, Germany.
Andreas Hagendorff, Department of Cardiology, University of Leipzig Medical Center, Leipzig, Germany.
Sylvia Henger, Institute for Medical Informatics, Statistics and Epidemiology, Leipzig University, Leipzig, Germany; Leipzig Research Centre for Civilization Diseases, Leipzig University, Leipzig, Germany.
Andreas Hinz, Department of Medical Psychology and Medical Sociology, Leipzig University, Leipzig, Germany.
Franziska G Rauscher, Institute for Medical Informatics, Statistics and Epidemiology, Leipzig University, Leipzig, Germany; Leipzig Research Centre for Civilization Diseases, Leipzig University, Leipzig, Germany.
Matthias Reusche, Institute for Medical Informatics, Statistics and Epidemiology, Leipzig University, Leipzig, Germany; Leipzig Research Centre for Civilization Diseases, Leipzig University, Leipzig, Germany.
Steffi G Riedel-Heller, Institute of Social Medicine, Occupational Medicine and Public Health (ISAP), Leipzig University, Leipzig, Germany.
Susanne Röhr, Institute of Social Medicine, Occupational Medicine and Public Health (ISAP), Leipzig University, Leipzig, Germany; Global Brain Health Institute (GBHI), Trinity College Dublin, Dublin, Ireland.
Julia Sacher, Cognitive Neurology, University of Leipzig Medical Center, Leipzig, Germany; Department of Neurology, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany.
Christian Sander, Leipzig Research Centre for Civilization Diseases, Leipzig University, Leipzig, Germany; Department of Psychiatry and Psychotherapy, University of Leipzig Medical Center, Leipzig, Germany.
Matthias L Schroeter, Cognitive Neurology, University of Leipzig Medical Center, Leipzig, Germany; Department of Neurology, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany.
Attila Tarnok, Institute for Medical Informatics, Statistics and Epidemiology, Leipzig University, Leipzig, Germany; Department of Preclinical Development and Validation, Fraunhofer Institute for Cell Therapy and Immunology, Leipzig, Germany.
Regina Treudler, Department of Dermatology, Venerology and Allergology, University of Leipzig Medical Center, Leipzig, Germany; Leipzig Interdisciplinary Allergy Center (LICA)—Comprehensive Allergy Center, University of Leipzig Medical Center, Leipzig, Germany.
Arno Villringer, Cognitive Neurology, University of Leipzig Medical Center, Leipzig, Germany; Department of Neurology, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany.
Rolf Wachter, Clinic and Policlinic for Cardiology, University of Leipzig Medical Center, Leipzig, Germany.
A Veronica Witte, Cognitive Neurology, University of Leipzig Medical Center, Leipzig, Germany; Department of Neurology, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany.
Joachim Thiery, Leipzig Research Centre for Civilization Diseases, Leipzig University, Leipzig, Germany; Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, University of Leipzig Medical Center, Leipzig, Germany.
Markus Scholz, Institute for Medical Informatics, Statistics and Epidemiology, Leipzig University, Leipzig, Germany; Leipzig Research Centre for Civilization Diseases, Leipzig University, Leipzig, Germany.
Markus Loeffler, Institute for Medical Informatics, Statistics and Epidemiology, Leipzig University, Leipzig, Germany; Leipzig Research Centre for Civilization Diseases, Leipzig University, Leipzig, Germany.
LIFE-Adult-Study working group:
Peter Ahnert, Yoon Ju Bae, Daniel Baier, Martin Berg, Thomas Berger, Frank Beutner, Frauke Beyer, Elmar Brähler, Petra Büttner, Ralph Burkhardt, Julia Dittrich, Ezgi Dogan-Sander, Tobias Elze, Michael Gaebler, Stephan Gielen, Heide Glaesmer, Ulrich Hegerl, Tilman Hensch, Anja Hilbert, Felix S Hussenoeder, Daniela Husser, Philippe Jawinski, Lasse Jost, Jan Keil, Shahrzad Kharabian Masouleh, Alexander Kiel, Toralf Kirsten, Michael Kluge, Rüya-Daniela Kocalevent, Jelena Kornej, Andreas Kühnapfel, Deniz Kumral, Jana Kynast, Leonie Lampe, Franziskus Liem, Antje Löffler, Henry Loeffler-Wirth, Noah Lorenz, Tobias Luck, Daniel S Margulies, Mila Massué, Susanne Melzer, Jeffrey Netto, Matthias Nüchter, Maryna Polyakova, Janne Pott, Madlen Reinicke, Nigar Reyes, Francisca S Rodriguez, H Lina Schaare, Peter Schönknecht, Jan C Simon, Janek Spada, Ronald Speer, Daniela Stanikova, Andrej Teren, Christine Ulke, Gunnar Wichmann, Barbara Wicklein, Anja Willenberg, Dirk Alexander Wittekind, Maryam Yahiaoui-Doktor, Silke Zachariae, Rui Zhang, Rachel G Zsido, and Andrea E Zuelke
Data availability
See ‘Can I get hold of the data?’ above.
Supplementary data
Supplementary data are available at IJE online.
Author contributions
C.Eng. wrote the manuscript with contributions from K.W., S.Z., R.B., U.C., C.Enz., M.F., A.H., F.G.R., M.R., S.R., J.S., C.S., R.T., A.V.W., M.S. and M.L. The study was designed by C.Eng., K.W., S.Z., J.T., M.S. and M.L., who act as guarantors for the paper. C.Eng., K.W., S.Z., R.B., H.B., U.C., C.Enz., M.F., A.Ha., A.Hi., F.G.R., S.G.R.H., J.S., C.S., M.L.S., A.T., R.T., A.V., R.W., A.V.W., J.T., M.S. and M.L. designed the examination and questionnaire programme of the study. K.W. was responsible for the study centre. R.B., U.C., A.T. and J.T. were responsible for the biobank and laboratory analyses. S.Z. was responsible for secondary data validation. C.Enz., S.H. and M.R. were responsible for data management and quality control. C.Eng., K.W., S.Z., C.Enz., A.Hi., F.G.R., M.R., S.R., C.S., R.T., A.V.W. and M.S. performed the data analyses shown in Tables 4–8. M.L. and J.T. obtained funding for the study. All authors read and approved the final manuscript.
Funding
The LIFE-Adult-Study is supported by LIFE—Leipzig Research Centre for Civilization Diseases, an organizational unit affiliated to the Medical Faculty of the University of Leipzig. LIFE is funded by means of the European Union, by the European Regional Development Fund (ERDF), by funds of the Free State of Saxony within the framework of the excellence initiative (project numbers 713–241202, 713–241202, 14505/2470, 14575/2470), by funds of the Medical Faculty of Leipzig University and by own funds of the participating institutions. Additional support was received from the Federal Ministry of Education and Research (BMBF) grant i: DSem—Integrative Data Semantics in Systems Medicine (031L0026). Furthermore, the ophthalmological imaging work was in part supported by the National Institutes of Health (R21 EY030142-01, R21 EY030631-01, R01 EY030575-01). Blood measurements were partly funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) (209933838 A01–SFB 1052).
References
- 1. Greiser KH, Kluttig A, Schumann B. et al. Cardiovascular disease, risk factors and heart rate variability in the elderly general population: design and objectives of the CARdiovascular disease, Living and Ageing in Halle (CARLA) Study. BMC Cardiovasc Disord 2005;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holle R, Happich M, Lowel H, Wichmann HE; MONICA/KORA Study Group. KORA--a research platform for population based health research. Gesundheitswesen 2005;67(Suppl 1):S19–25. [DOI] [PubMed] [Google Scholar]
- 3. Volzke H, Alte D, Schmidt CO. et al. Cohort profile: the study of health in Pomerania. Int J Epidemiol 2011;40:294–307. [DOI] [PubMed] [Google Scholar]
- 4. Schmermund A, Mohlenkamp S, Stang A. et al. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study: risk factors, evaluation of coronary calcium and lifestyle. Am Heart J 2002;144:212–18. [DOI] [PubMed] [Google Scholar]
- 5. Bellach BM, Knopf H, Thefeld W.. [The German Health Survey. 1997/98]. Gesundheitswesen 1998;60(Suppl 2):S59–68. [PubMed] [Google Scholar]
- 6. Scholz M, Henger S, Beutner F. et al. Cohort profile: the Leipzig Research Center for Civilization Diseases-Heart study (LIFE-Heart). Int J Epidemiol 2020;49:1439–40. [DOI] [PubMed] [Google Scholar]
- 7. Quante M, Hesse M, Dohnert M. et al. ; LIFE Child Study Investigators. The LIFE child study: a life course approach to disease and health. BMC Public Health 2012;12:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loeffler M, Engel C, Ahnert P. et al. The LIFE-Adult-Study: objectives and design of a population-based cohort study with 10,000 deeply phenotyped adults in Germany. BMC Public Health 2015;15:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Enzenbach C, Wicklein B, Wirkner K, Loeffler M.. Evaluating selection bias in a population-based cohort study with low baseline participation: the LIFE-Adult-Study. BMC Med Res Methodol 2019;19:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kharabian Masouleh S, Arelin K, Horstmann A. et al. Higher body mass index in older adults is associated with lower gray matter volume: implications for memory performance. Neurobiol Aging 2016;40:1–10. [DOI] [PubMed] [Google Scholar]
- 11. Schaare HL, Kharabian Masouleh S, Beyer F. et al. Association of peripheral blood pressure with gray matter volume in 19- to 40-year-old adults. Neurology 2019;92:e758–73. [DOI] [PubMed] [Google Scholar]
- 12. Beyer F, Kharabian Masouleh S, Kratzsch J. et al. A metabolic obesity profile is associated with decreased gray matter volume in cognitively healthy older adults. Front Aging Neurosci 2019;11:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang R, Beyer F, Lampe L. et al. White matter microstructural variability mediates the relation between obesity and cognition in healthy adults. Neuroimage 2018;172:239–49. [DOI] [PubMed] [Google Scholar]
- 14. Beyer F, Garcia-Garcia I, Heinrich M. et al. Neuroanatomical correlates of food addiction symptoms and body mass index in the general population. Hum Brain Mapp 2019;40:2747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beyer F, Kharabian Masouleh S, Huntenburg JM. et al. Higher body mass index is associated with reduced posterior default mode connectivity in older adults. Hum Brain Mapp 2017;38:3502–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beyer F, Zhang R, Scholz M. et al. Higher BMI, but not obesity-related genetic polymorphisms, correlates with lower structural connectivity of the reward network in a population-based study. Int J Obes (Lond) 2021;45:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kharabian Masouleh S, Beyer F, Lampe L. et al. Gray matter structural networks are associated with cardiovascular risk factors in healthy older adults. J Cereb Blood Flow Metab 2018;38:360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thomas K, Beyer F, Lewe G. et al. Higher body mass index is linked to altered hypothalamic microstructure. Sci Rep 2019;9:17373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lampe L, Zhang R, Beyer F. et al. Visceral obesity relates to deep white matter hyperintensities via inflammation. Ann Neurol 2019;85:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zsido RG, Heinrich M, Slavich GM. et al. Association of estradiol and visceral fat with structural brain networks and memory performance in adults. JAMA Netw Open 2019;2:e196126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luck T, Then FS, Schroeter ML. et al. Prevalence of DSM-5 mild neurocognitive disorder in dementia-free older adults: results of the population-based LIFE-Adult-Study. Am J Geriatr Psychiatry 2017;25:328–39. [DOI] [PubMed] [Google Scholar]
- 22. Luck T, Roehr S, Rodriguez FS. et al. Memory-related subjective cognitive symptoms in the adult population: prevalence and associated factors: results of the LIFE-Adult-Study. BMC Psychol 2018;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luck T, Pabst A, Rodriguez FS. et al. Age-, sex-, and education-specific norms for an extended CERAD Neuropsychological Assessment Battery: results from the population-based LIFE-Adult-Study. Neuropsychology 2018;32:461–75. [DOI] [PubMed] [Google Scholar]
- 24. Luck T, Then FS, Luppa M. et al. Association of the apolipoprotein E genotype with memory performance and executive functioning in cognitively intact elderly. Neuropsychology 2015;29:382–87. [DOI] [PubMed] [Google Scholar]
- 25. Kuehnapfel A, Ahnert P, Loeffler M, Broda A, Scholz M.. Reliability of 3D laser-based anthropometry and comparison with classical anthropometry. Sci Rep 2016;6:26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loffler-Wirth H, Willscher E, Ahnert P. et al. Novel anthropometry based on 3D-bodyscans applied to a large population based cohort. PLoS One 2016;11:e0159887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frenzel A, Binder H, Walter N, Wirkner K, Loeffler M, Loeffler-Wirth H.. The aging human body shape. NPJ Aging Mech Dis 2020;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuehnapfel A, Ahnert P, Loeffler M, Scholz M.. Body surface assessment with 3D laser-based anthropometry: reliability, validation, and improvement of empirical surface formulae. Eur J Appl Physiol 2017;117:371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang M, Elze T, Li D. et al. Age, ocular magnification, and circumpapillary retinal nerve fiber layer thickness. J Biomed Opt 2017;22:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li D, Rauscher FG, Choi EY. et al. Sex-specific differences in circumpapillary retinal nerve fiber layer thickness. Ophthalmology 2020;127:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baniasadi N, Rauscher FG, Li D. et al. Norms of interocular circumpapillary retinal nerve fiber layer thickness differences at 768 retinal locations. Trans Vis Sci Tech 2020;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rauscher FG, Wang M, Francke M. et al. Renal function and lipid metabolism are major predictors of circumpapillary retinal nerve fiber layer thickness-the LIFE-Adult Study. BMC Med 2021;19:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berger T, Fuchs M, Dippold S. et al. Standardization and feasibility of voice range profile measurements in epidemiological studies. J Voice 2022;36:142 e9–20. [DOI] [PubMed] [Google Scholar]
- 34. Berg M, Fuchs M, Wirkner K, Loeffler M, Engel C, Berger T.. The speaking voice in the general population: normative data and associations to sociodemographic and lifestyle factors. J Voice 2017;31:257.e13–24. [DOI] [PubMed] [Google Scholar]
- 35. Jost L, Fuchs M, Loeffler M. et al. Associations of sex hormones and anthropometry with the speaking voice profile in the adult general population. J Voice 2018;32:261–72. [DOI] [PubMed] [Google Scholar]
- 36. Wilkinson MD, Dumontier M, Aalbersberg IJ. et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data 2016;3:160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dogan-Sander E, Willenberg A, Batmaz I. et al. Association of serum 25-hydroxyvitamin D concentrations with sleep phenotypes in a German community sample. PLoS One 2019;14:e0219318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spada J, Sander C, Burkhardt R. et al. Genetic association of objective sleep phenotypes with a functional polymorphism in the neuropeptide S receptor gene. PLoS One 2014;9:e98789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jawinski P, Sander C, Mauche N. et al. Brain arousal regulation in carriers of bipolar disorder risk alleles. Neuropsychobiology 2015;72:65–73. [DOI] [PubMed] [Google Scholar]
- 40. Jawinski P, Tegelkamp S, Sander C. et al. Time to wake up: No impact of COMT Val158Met gene variation on circadian preferences, arousal regulation and sleep. Chronobiol Int 2016;33:893–905. [DOI] [PubMed] [Google Scholar]
- 41. Jawinski P, Kirsten H, Sander C. et al. Human brain arousal in the resting state: a genome-wide association study. Mol Psychiatry 2019;24:1599–609. [DOI] [PubMed] [Google Scholar]
- 42. Spada J, Scholz M, Kirsten H. et al. Genome-wide association analysis of actigraphic sleep phenotypes in the LIFE Adult Study. J Sleep Res 2016;25:690–701. [DOI] [PubMed] [Google Scholar]
- 43. Pott J, Burkhardt R, Beutner F. et al. Genome-wide meta-analysis identifies novel loci of plaque burden in carotid artery. Atherosclerosis 2017;259:32–40. [DOI] [PubMed] [Google Scholar]
- 44. Buchmann N, Scholz M, Lill CM. et al. Association between lipoprotein(a) level and type 2 diabetes: no evidence for a causal role of lipoprotein(a) and insulin. Acta Diabetol 2017;54:1031–38. [DOI] [PubMed] [Google Scholar]
- 45. Davies G, Lam M, Harris SE. et al. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat Commun 2018;9:2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Franceschini N, Giambartolomei C, de Vries PS. et al. ; MEGASTROKE Consortium. GWAS and colocalization analyses implicate carotid intima-media thickness and carotid plaque loci in cardiovascular outcomes. Nat Commun 2018;9:5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vojinovic D, Adams HH, Jian X. et al. Genome-wide association study of 23,500 individuals identifies 7 loci associated with brain ventricular volume. Nat Commun 2018;9:3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walker CJ, Oakes CC, Genutis LK. et al. Genome-wide association study identifies an acute myeloid leukemia susceptibility locus near BICRA. Leukemia 2019;33:771–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wuttke M, Li Y, Li M. et al. ; V. A. Million Veteran Program. A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet 2019;51:957–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pott J, Bae YJ, Horn K. et al. Genetic association study of eight steroid hormones and implications for sexual dimorphism of coronary artery disease. J Clin Endocrinol Metab 2019;104:5008–23. [DOI] [PubMed] [Google Scholar]
- 51. Pott J, Schlegel V, Teren A. et al. Genetic regulation of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) plasma levels and its impact on atherosclerotic vascular disease phenotypes. Circ Genom Precis Med 2018;11:e001992. [DOI] [PubMed] [Google Scholar]
- 52. Pott J, Beutner F, Horn K. et al. Genome-wide analysis of carotid plaque burden suggests a role of IL5 in men. PLoS One 2020;15:e0233728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schmidt M, Hopp L, Arakelyan A. et al. The human blood transcriptome in a large population cohort and its relation to aging and health. Front Big Data 2020;3:548873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vosa U, Claringbould A, Westra HJ. et al. ; i2QTL Consortium. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat Genet 2021;53:1300–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dittrich J, Adam M, Maas H. et al. Targeted on-line SPE-LC-MS/MS assay for the quantitation of 12 apolipoproteins from human blood. Proteomics 2018;18:1700279. [DOI] [PubMed] [Google Scholar]
- 56. Reinicke M, Schroter J, Muller-Klieser D, Helmschrodt C, Ceglarek U.. Free oxysterols and bile acids including conjugates: simultaneous quantification in human plasma and cerebrospinal fluid by liquid chromatography-tandem mass spectrometry. Anal Chim Acta 2018;1037:245–55. [DOI] [PubMed] [Google Scholar]
- 57. Bae YJ, Zeidler R, Baber R. et al. Reference intervals of nine steroid hormones over the life-span analyzed by LC-MS/MS: effect of age, gender, puberty, and oral contraceptives. J Steroid Biochem Mol Biol 2019;193:105409. [DOI] [PubMed] [Google Scholar]
- 58. Melzer S, Zachariae S, Bocsi J, Engel C, Löffler M, Tárnok A.. Reference intervals for leukocyte subsets in adults: results from a population-based study using 10-color flow cytometry. Cytometry B Cytometry 2015;88:270–81. [DOI] [PubMed] [Google Scholar]
- 59. Wichmann G, Gaede C, Melzer S. et al. Discrimination of head and neck squamous cell carcinoma patients and healthy adults by 10-color flow cytometry: development of a score based on leukocyte subsets. Cancers (Basel) 2019;11:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zeynalova S, Bucksch K, Scholz M. et al. Monocyte subtype counts are associated with 10-year cardiovascular disease risk as determined by the Framingham Risk Score among subjects of the LIFE-Adult study. PLoS One 2021;16:e0247480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bocsi J, Melzer S, Dahnert I, Tarnok A.. OMIP-023: 10-color, 13 antibody panel for in-depth phenotyping of human peripheral blood leukocytes. Cytometry A 2014;85:781–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
See ‘Can I get hold of the data?’ above.