Abstract
Background
Reports of mortality-associated risk factors in patients with the novel coronavirus disease 2019 (COVID-19) are limited.
Methods
We evaluated the clinical features that were associated with mortality among patients who died during hospitalization (n = 158) and those who were alive at discharge (n = 2,736) from the large-scale, multicenter, retrospective, observational cohort CLOT-COVID study, which enrolled consecutively hospitalized COVID-19 patients from 16 centers in Japan from April to September 2021. Data from 2,894 hospitalized COVID-19 participants of the CLOT-COVID study were analyzed in this study.
Results
Patients who died were older (71.1 years vs 51.6 years, P < 0.001), had higher median D-dimer values on admission (1.7 µg/mL vs 0.8 µg/mL, P < 0.001), and had more comorbidities. On admission, the patients who died had more severe COVID-19 than did those who survived (mild: 16% vs 63%, moderate: 47% vs 31%, and severe: 37% vs 6.2%, P < 0.001). In patients who died, the incidence of thrombosis and major bleeding during hospitalization was significantly higher than that in those who survived (thrombosis: 8.2% vs 1.5%, P < 0.001; major bleeding: 12.7% vs 1.4%, P < 0.001). Multivariable logistic regression analysis revealed that age >70 years, high D-dimer values on admission, heart disease, active cancer, higher COVID-19 severity on admission, and development of major bleeding during hospitalization were independently associated with a higher mortality risk.
Conclusion
This large-scale observational study in Japan identified several independent risk factors for mortality in hospitalized patients with COVID-19 that could facilitate appropriate risk stratification of patients with COVID-19.
Key words: COVID-19, mortality, risk factors, thrombosis
INTRODUCTION
The novel coronavirus disease 2019 (COVID-19) is an enormous global threat1,2 that has affected more than 517 million people and has caused more than 6.2 million deaths as of May 2022.3 Appropriate mortality risk stratification is essential for the clinical identification of patients who require close monitoring and intensive treatment. Several clinical studies have reported mortality-associated risk factors in COVID-19 patients worldwide, including in North America, Europe, and China.3–13 Recent meta-analyses have shown that advanced age; male sex; current smoking; and comorbidities, such as diabetes, hypertension, asthma, heart disease, obesity, and active cancer are independent risk factors for COVID-19 mortality.14,15
Nonetheless, the clinical features of COVID-19 patients and the mortality risk could vary by race or ethnicity, clinical practice, and accessibility of medical resources in each country and region. A meta-analysis from overseas showed that the mortality rate of hospitalized patients with COVID-19 was 17.6%,15 while the corresponding rate in Japan was 5.7%.16 A few studies have reported the risk factors for COVID-19 “severity” in Japanese patients; these include a study that analyzed data in a nationwide registry.17 However, there have been no reports on the risk factors for COVID-19-associated mortality in Japanese patients hospitalized for the disease; therefore, our large-scale, multicenter, observational study aimed to investigate such risk factors.
METHODS
Study population
This physician-initiated, observational cohort study involved the analysis of data accumulated in the nationwide “The Thrombosis and Anticoagulation Therapy in patients with COVID-19 in Japan Study” (CLOT-COVID Study; UMIN000045800), which enrolled consecutive hospitalized COVID-19 patients from April to September 2021 in 16 centers in Japan.18–22 The primary objective of the CLOT-COVID study was to reveal the current status of thrombosis and anticoagulation therapy in patients with COVID-19 in Japan.18 Patients with COVID-19 confirmed by polymerase chain reaction were eligible for inclusion. The CLOT-COVID Study enrolled 2,894 hospitalized COVID-19 patients. The study population was divided into patients who died during hospitalization and those who survived to discharge. We compared the patient characteristics during hospitalization and the clinical outcomes between the two groups and investigated the risk factors for mortality.
We performed all study-related procedures in accordance with the code of medical ethics specified in the Declaration of Helsinki. All participating centers’ ethics committees or relevant review boards approved the research protocol. The Fukushima Daiichi Hospital’s ethical committee served as the primary ethics committee (Approval number: 2021-11-2). The requirement of written informed consent was waived because we used clinical data obtained in routine clinical practice and none of the patients refused to participate when contacted for follow-up. By posting detailed information about the studies on each institution’s website, the patients could request to opt out of the study at any time between enrollment and follow-up. This method is concordant with the guidelines for epidemiological studies issued by the Ministry of Health, Labour, and Welfare in Japan.
Data collection and definition of participant characteristics
Based on prespecified definitions, we searched the hospital databases and accumulated data regarding the participants’ clinicodemographic characteristics, pharmacological thromboprophylaxis management, and clinical outcomes. These data were entered into an electronic case report form. At the general office, the data were checked for missing inputs and values that were outside the anticipated range.
We classified the severity of COVID-19 as mild, moderate, and severe if the patients did not need oxygen, needed oxygen, or needed mechanical ventilation or extracorporeal membrane oxygenation, respectively.23,24 We defined pharmacological thromboprophylaxis as the use of anticoagulants during hospitalization, not including use for the treatment of thrombosis. We included patients who continued anticoagulant therapy from prior to hospitalization. Therapeutic unfractionated heparin use was definded as the use of unfractionated heparin while aming for a therapeutic range based on the activated partial thromboplastin time (APTT). We defined prophylactic unfractionated heparin use as the use of unfractionated heparin without consideration of APTT. eMaterial 1 contains detailed definitions of the other participant characteristics.
Clinical outcomes
We evaluated the occurrence of thrombotic events, major bleeding, and all-cause mortality during hospitalization as clinical outcomes. The primary endpoint was all-cause mortality, and the secondary endpoints were thrombosis and major bleeding. In this study, we defined venous thromboembolism (VTE), ischemic stroke, myocardial infarction, and systemic arterial thromboembolism as thrombosis. VTE included deep vein thrombosis or pulmonary embolism diagnosed by ultrasonography, contrast-enhanced computed tomography, pulmonary angiography, ventilation-perfusion lung scintigraphy, contrast venography, or autopsy. Ischemic stroke was defined as a stroke that lasted longer than 24 hours and necessitated or extended hospitalization. We defined myocardial infarction based on the universal myocardial infarction guidelines.25 Finally, major bleeding was defined by a minimum 2 g/dL reduction in the hemoglobin level, transfusion of at least two units of blood, or hemorrhage at a critical site according to the International Society of Thrombosis and Hemostasis guidelines.26
Statistical analysis
Categorical variables are summarized as the number and percentage and were compared using Fisher’s exact test or the chi-square test as appropriate. Continuous variables are expressed, based on their distribution, as the mean and standard deviation or the median and interquartile range, and they were compared using Student’s t-test or the Wilcoxon rank-sum test. The number of events and the percentages of clinical outcomes are presented. Furthermore, using stratified analysis, we evaluated the clinical outcomes based on COVID-19 severity at the time of admission. To investigate the mortality-associated risk factors during hospitalization, we performed multivariable logistic regression analysis to estimate the odds ratios (ORs) and 95% confidence intervals (Cis) of the potential risk factors. In accordance with previous reports14,15,27–29 and with regard to clinical relevance, we selected 13 clinically relevant variables from among the baseline characteristics (age >70 years, male sex, body mass index >30 kg/m2, D-dimer values on admission >1.0 µg/mL, hypertension, diabetes mellitus, heart disease, respiratory disease, active cancer, history of major bleeding, history of VTE, and moderate and high COVID-19 severity at admission), the use of pharmacological thromboprophylaxis, and the development of thrombosis and major bleeding during hospitalization. Patients were considered to have missing data if data regarding the D-dimer values quantified at the time of admission were missing. We performed a multivariable logistic regression analysis after excluding the missing data. R version 4.1.2 was used for all statistical analyses (R Foundation for Statistical Computing, Vienna, Austria).30 All tests were two-tailed, and statistical significance was set at P < 0.05.
RESULTS
Participant characteristics
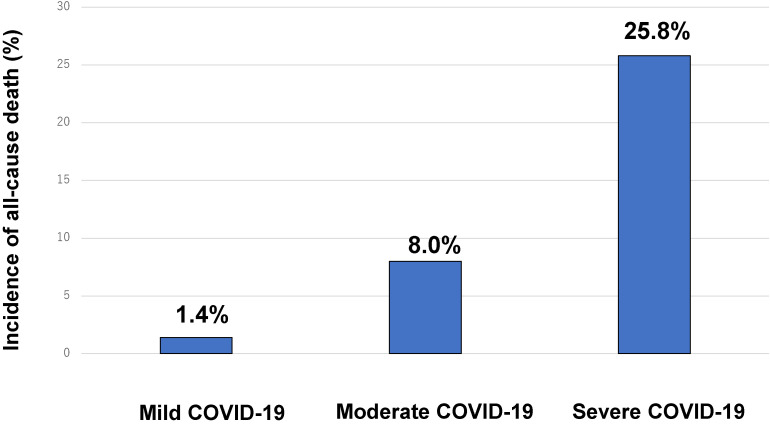
Among the 2,894 hospitalized COVID-19 patients, 158 died during hospitalization (hospital mortality 5.5%). A higher COVID-19 severity on admission was associated with an increase in the in-hospital mortality (mild 1.4%; moderate 8.0%; and severe 25.8%; Figure 1). Patients who died during hospitalization were older (71.1 years vs 51.6 years, P < 0.001), had higher median D-dimer values on admission (1.7 µg/mL vs 0.8 µg/mL, P < 0.001), and had more comorbidities, including hypertension (56% vs 29%, P < 0.001), diabetes mellitus (30% vs 20%, P = 0.006), heart disease (25% vs 7.9%, P < 0.001), active cancer (8.2% vs 1.7%, P < 0.001), and history of VTE (1.9% vs 0.4%, P = 0.045; Table 1). In patients who died, the median hospitalization duration was longer than that in those who survived (16 vs 9 days, P < 0.001). In the group of patients who died, a higher proportion of patients used pharmacological thromboprophylaxis than in the group of those who survived (82% vs 41%, P < 0.001).
Figure 1. Incidence of all-cause mortality according to the COVID-19 severity on admission. Patients with mild, moderate, and severe COVID-19 were defined as those who did not require oxygen, required oxygen, and required mechanical ventilation or extracorporeal membrane oxygenation. COVID-19, novel coronavirus disease 2019.
Table 1. Patient characteristics and pharmacological thromboprophylaxis managements.
| Total (N = 2,894) |
Patients who died during hospitalization (N = 158) |
Patients alive at discharge (N = 2,736) |
P-value | |
| Baseline characteristics | ||||
| Age, years | 52.7 (17.9) | 71.1 (11.8) | 51.6 (17.6) | <0.001 |
| Age >70 years | 535 (19%) | 99 (63%) | 436 (16%) | <0.001 |
| Men | 1,885 (65%) | 109 (69%) | 1,776 (65%) | 0.35 |
| Body weight, kg | 68.9 (18.5) | 66.0 (16.1) | 69.0 (18.6) | 0.049 |
| Height, cm | 164.4 (12.4) | 163.1 (9.8) | 164.4 (12.5) | 0.19 |
| Body mass index, kg/m2 | 25.3 (5.4) | 24.7 (4.9) | 25.3 (5.4) | 0.18 |
| Body mass index ≥30 kg/m2 | 459 (16%) | 20 (13%) | 439 (16%) | 0.31 |
| D-dimer level on admission, µg/mL (N = 2,771) | 0.8 (0.5–1.3) | 1.7 (1.1–3.5) | 0.8 (0.5–1.2) | <0.001 |
| D-dimer level on admission >1.0 µg/mL (N = 2,771) | 974 (35%) | 120 (80%) | 854 (32%) | <0.001 |
| Length of hospitalization, days | 9 (6–14) | 16 (10–24) | 9 (6–13) | <0.001 |
| Comorbidities | ||||
| Hypertension | 874 (30%) | 89 (56%) | 785 (29%) | <0.001 |
| Diabetes mellitus | 597 (21%) | 47 (30%) | 550 (20%) | 0.006 |
| Heart disease | 255 (8.8%) | 40 (25%) | 215 (7.9%) | <0.001 |
| Respiratory disease | 298 (10%) | 21 (13%) | 277 (10%) | 0.22 |
| Active cancer | 60 (2.1%) | 13 (8.2%) | 47 (1.7%) | <0.001 |
| History of major bleeding | 28 (1.0%) | 4 (2.5%) | 24 (0.9%) | 0.06 |
| History of VTE | 15 (0.5%) | 3 (1.9%) | 12 (0.4%) | 0.045 |
| Severity of COVID-19 on admission | ||||
| Mild | 1,738 (60%) | 25 (16%) | 1,713 (63%) | <0.001 |
| Moderate (Need oxygen) | 927 (32%) | 74 (47%) | 853 (31%) | |
| Severe (Need mechanical ventilation/ECMO) | 229 (7.9%) | 59 (37%) | 170 (6.2%) | |
| Pharmacological thromboprophylaxis managements | ||||
| Anticoagulants | 1,245 (43%) | 130 (82%) | 1,115 (41%) | <0.001 |
| Unfractionated heparin of prophylactic dose | 685/1,245 (55%) | 44/130 (34%) | 641/1,115 (58%) | <0.001 |
| Unfractionated heparin of therapeutic dose | 161/1,245 (13%) | 55/130 (42%) | 106/1,115 (9.5%) | |
| Low-molecular-weight heparin of prophylactic dose | 204/1,245 (16%) | 16/130 (12%) | 188/1,115 (17%) | |
| Low-molecular-weight heparin of therapeutic dose | 0/1,245 (0%) | 0/130 (0%) | 0/1,115 (0%) | |
| Direct oral anticoagulants | 164/1,245 (13%) | 14/130 (11%) | 150/1,115 (14%) | |
| Warfarin | 19/1,245 (1.5%) | 1/130 (0.8%) | 18/1,115 (1.6%) | |
| Others | 12/1,245 (1.0%) | 0/130 (0%) | 7/1,115 (0.6%) | |
APTT, activated partial thromboplastin time; COVID-19, novel coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; VTE, venous thromboembolism.
Categorical variables are presented as numbers and percentages, and continuous variables are presented as the mean and standard deviation or the median and interquartile range based on their distributions. Categorical variables were compared using the chi-squared test when appropriate; otherwise, Fisher’s exact test was used. Continuous variables were compared using the Student’s t test or Wilcoxon’s rank sum test based on distribution. Unfractionated heparin of a therapeutic dose was defined as the administration of unfractionated heparin targeting a therapeutic range referencing the APTT. Unfractionated heparin of a prophylactic dose was defined as the administration of unfractionated heparin of a fixed dose without a referencing the APTT.
Clinical outcomes during hospitalization
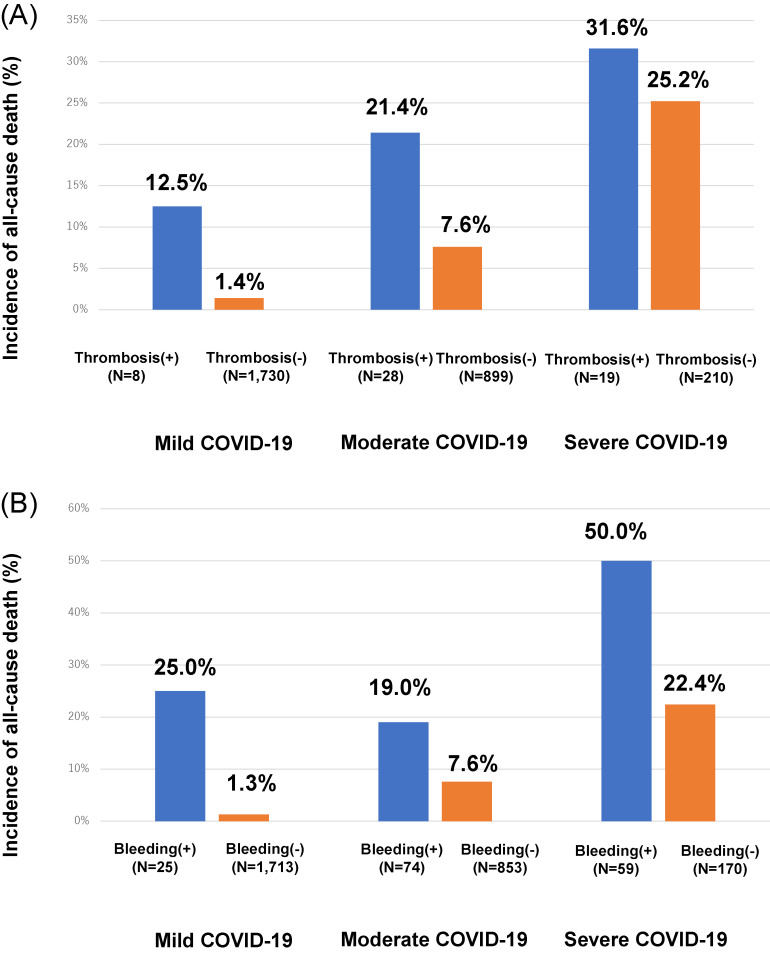
In patients who died, the incidence of thrombosis was higher than in those who survived (8.2% vs 1.5%, P < 0.001; Table 2). Similarly, in patients who developed thrombosis, the mortality rate was numerically higher than that in those who did not develop thrombosis, regardless of the COVID-19 severity on admission (mild, 12.5% vs 1.4%; moderate, 21.4% vs 7.6%; severe, 31.6% vs 25.2%; Figure 2A).
Table 2. Clinical outcomes during hospitalization.
| Total (N = 2,894) |
Patients who died during hospitalization (N = 158) |
Patients alive at discharge (N = 2,736) |
P-value | |
| Thrombosis | 55 (1.9%) | 13 (8.2%) | 42 (1.5%) | <0.001 |
| VTE | 39 (1.3%) | 8 (5.1%) | 31 (1.1%) | 0.001 |
| Arterial thrombotic events | 12 (0.4%) | 3 (1.9%) | 9 (0.3%) | <0.001 |
| Ischemic stroke | 9 (0.3%) | 2/3 (67%) | 7/9 (78%) | — |
| Myocardial infarction | 2 (0.07%) | 1/3 (33%) | 1/9 (11%) | — |
| Systemic arterial thromboembolism | 1 (0.04%) | 0/3 (0%) | 1/9 (11%) | — |
| Other thrombosis | 7 (0.2%) | 3 (1.9%) | 4 (0.1%) | <0.001 |
| Major bleeding | 57 (2.0%) | 20 (12.7%) | 37 (1.4%) | <0.001 |
VTE, venous thromboembolism.
The clinical outcomes are presented as numbers of events and percentages, which were compared using the chi-squared test when appropriate; otherwise, Fisher’s exact test was used as categorial variables.
Figure 2. Comparative incidences of thrombosis (A) and major bleeding (B) between the groups of patients who died and those who survived in analyses that were stratified by the COVID-19 severity on admission. COVID-19, novel coronavirus disease 2019.
In patients who died, the incidence of major bleeding was higher than that in those who survived (12.7% vs 1.4%, P < 0.001; Table 2). Similarly, the mortality was higher in patients who developed bleeding than in those who did not develop thrombosis, regardless of the COVID-19 severity on admission (mild, 25.0% vs 1.3%; moderate, 19.0% vs 7.6%; severe, 50.0% vs 22.4%; Figure 2B).
Risk factors of all-cause mortality during hospitalization
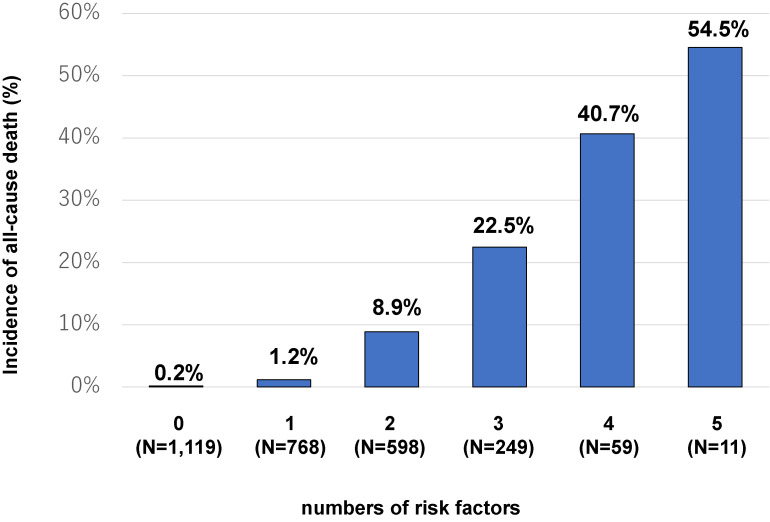
We performed multivariate logistic regression analysis and revealed that age >70 years; D-dimer >1.0 µg/mL on admission; heart disease, active cancer, or more severe COVID-19 on admission; and major bleeding during hospitalization were independently associated with a higher mortality risk (Table 3). Among the potential risk factors for mortality, severe COVID-19 on admission had the strongest influence on mortality (adjusted OR 13.20; 95% CI, 6.72–26.10), followed by age >70 years (adjusted OR 7.96; 95% CI, 5.05–12.60). A higher number of potential risk factors for mortality was linked to a higher incidence of all-cause mortality (Figure 3).
Table 3. Multivariable analysis for the risk factors for all-cause mortality during hospitalization.
| Univariate | Multivariable | |||
|
|
|
|||
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age >70 years | 8.85 (6.31–12.40) | <0.001 | 7.96 (5.05–12.60) | <0.001 |
| Men | 1.20 (0.85–1.70) | 0.30 | 1.02 (0.67–1.55) | 0.92 |
| Body mass index ≥30 kg/m2 | 0.76 (0.47–1.23) | 0.26 | 1.18 (0.65–2.14) | 0.59 |
| D-dimer levels on admission >1.0 µg/mL | 8.41 (5.59–12.70) | <0.001 | 2.90 (1.83–4.59) | <0.001 |
| Hypertension | 3.21 (2.32–4.44) | <0.001 | 1.28 (0.84–1.92) | 0.25 |
| Diabetes mellitus | 1.68 (1.18–2.40) | 0.003 | 0.95 (0.61–1.46) | 0.80 |
| Heart disease | 3.97 (2.71–5.84) | <0.001 | 1.69 (1.03–2.76) | 0.04 |
| Respiratory disease | 1.36 (0.85–2.19) | 0.20 | 0.76 (0.42–1.38) | 0.37 |
| Active cancer | 5.13 (2.71–9.69) | <0.001 | 3.20 (1.44–7.09) | 0.004 |
| History of major bleeding | 2.94 (1.01–8.56) | 0.049 | 1.30 (0.38–4.45) | 0.67 |
| History of VTE | 4.39 (1.23–15.70) | 0.02 | 1.39 (0.25–7.75) | 0.71 |
| Severity of COVID-19 on admission | ||||
| Mild (Reference) | — | — | — | — |
| Moderate (versus Mild) | 5.94 (3.75–9.42) | <0.001 | 3.02 (1.74–5.25) | <0.001 |
| Severe (versus Mild) | 23.80 (14.50–39.00) | <0.001 | 13.20 (6.72–26.10) | <0.001 |
| Pharmacological thromboprophylaxis | 6.75 (4.46–10.20) | <0.001 | 1.46 (0.86–2.50) | 0.16 |
| Development of thrombosis during hospitalization | 5.75 (3.02–11.00) | <0.001 | 1.77 (0.78–3.99) | 0.17 |
| Development of major bleeding during hospitalization | 10.60 (5.98–18.70) | <0.001 | 2.44 (1.17–5.05) | 0.02 |
CI, confidence interval; COVID-19, novel coronavirus disease 2019; OR, odds ratio; VTE, venous thromboembolism.
Adjusted for age >70 years, male sex, body mass index >30 kg/m2, D-dimer values on admission >1.0 µg/mL, hypertension, diabetes mellitus, heart disease, respiratory disease, active cancer, history of major bleeding, history of VTE, and moderate and high COVID-19 severity at admission.
Figure 3. Incidences of all-cause mortality according to the number of potential mortality-associated risk factors, including age >70 years, high D-dimer values on admission, heart disease, active cancer, more severe COVID-19 on admission, and onset of major bleeding during hospitalization. COVID-19, novel coronavirus disease 2019.
DISCUSSION
The study’s main findings are that, compared to those who survived to discharge, patients who died: i) were older, had higher D-dimer values on admission, and had more comorbidities; ii) had higher incidences of thrombosis and major bleeding during hospitalization; and iii) had independent risk factors for mortality, including age >70 years, D-dimer >1.0 µg/mL on admission, heart disease, active cancer, more severe status of COVID-19 on admission, and major bleeding following hospitalization.
Several previous reports of the risk factors for the disease severity and progression of COVID-19 in Japan have been published.31,32 A recent large-scale Japanese registry-based study that included 3,829 COVID-19 patients reported that male sex, higher age, hypertension, obesity, cardiovascular disease, and diabetes were independent risk factors for disease progression.17 However, the mortality-associated risk factors for COVID-19 patients in Japan have not been reported. A large-scale United Kingdom cohort study that examined the risk factors for mortality in more than 17 million COVID-19 patients showed that higher age, poverty, male sex, liver disease, diabetes, asthma, and kidney disorders were independent risk factors for mortality.33 Another population-based large observational study that evaluated data from 1.8 million people in Stockholm, Sweden, reported that heart failure, ischemic heart disease, obesity, kidney failure, and diabetes were independent risk factors for mortality.28 A recent meta-analysis of 42 studies in 423,117 COVID-19 patients revealed the following independent risk factors for mortality: higher age, male sex, current smoking, chronic obstructive pulmonary disease, acute kidney injury, active cancer, diabetes, heart disease, obesity, hypertension, and a high D-dimer value on admission.15 Our study revealed that, in Japan, old age, heart disease, active cancer, and high D-dimer values on admission were mortality-related risk factors in COVID-19 patients. Furthermore, this study showed that severe COVID-19 on admission had the most substantial influence on mortality, which is in line with previous studies that reported severe COVID-19 as a strong risk factor for severe respiratory conditions and the use of invasive mechanical ventilation.14
Several risk factors for mortality are in line with those reported previously, while some are not. COVID-19 mortality risk differs among races; therefore, the risk factors for mortality might vary depending on the race.6,34–36 The overall mortality rate for COVID-19 in Japan was 0.34% as of June 2022,37 which is approximately one fourth the global mortality rate (1.2%).38 A meta-analysis of data from more than 423,000 patients in countries other than Japan showed an in-hospital mortality rate of 17.6%.15 In this study, the in-hospital mortality rate was 5.5%, which is consistent with that reported in another Japanese registry-based study of 5,194 patients with COVID-19 (5.7%).39 The reasons for the differences in mortality and the risk factors for mortality in COVID-19 patients remain unclear; however, some possible mechanisms have been speculated. First, COVID-19 mortality is affected by not only patient characteristics but also the socioeconomic status and quality of clinical care and medical service.36 Second, different patient characteristics, including the prevalence of obesity and comorbidities, depending on the country, could have had a specific influence on the mortality risk.40 Third, different genetic properties, such as human leukocyte antigen,41–44 angiotensin-converting enzyme 1 polymorphism,45 Neanderthal haplotypes,46 and vitamin D47 could explain the racial differences in mortality. Fourth, depending on each country, various hospitalization options could have influenced the in-hospital mortality. Sixth, the hospitalization criteria in each facility could vary according to the community and affect the in-hospital mortality. Seventh, virus variants could affect mortality; however, the mortality rate was 5.5% in the present study, which was similar to that of a previous study that was conducted during the first and second waves (5.7%).48
Notably, thrombosis was not identified as an independent risk factor for COVID-19-associated mortality in this study, contrary to the findings reported previously.49–51 Several studies have shown that thrombosis during hospitalization influences COVID-19-related mortality. Compared to those without VTE, patients with VTE have a higher mortality risk.49 The present study revealed that the development of thrombosis during hospitalization had a numerically high OR of 1.77 for mortality, although this was not statistically significant. This association can be partly explained as follows. The lower incidence of thrombosis in Japan relative to other countries could explain the reduced statistical power for detecting significant differences. The incidence of thrombosis in this study was 1.9%, whereas the incidence in another study that analyzed data from Europe, the United States, and China showed an incidence of 21%, although the absolute OR of thrombosis for mortality was 1.74,50 which might be consistent with the result obtained in the present study.
Obesity is reported as a mortality-related risk factor in several studies that included patients with COVID-19 worldwide. A meta-analysis that examined obesity as a risk factor for mortality in patients with COVID-19 overseas reported a risk ratio of 1.42 (95% CI, 1.24–1.63),51 while a national study in Japan revealed that Japanese COVID-19 patients with a high BMI showed a higher risk for progression of disease severity, although this was not associated with a higher risk for mortality.52 In line with a previous study, we showed that obesity in individuals with a BMI ≥30 kg/m2 was not an independent risk factor associated with lower mortality risk. Specifically, Japanese patients commonly have lower body weights than those of other ethnicities, and low body weight is reportedly associated with mortality risk53; the body weights in Japan versus overseas could affect this difference in these results.
There are several limitations of this study. First, the observational study design inherently confers various biases. In particular, the attending physicians made all treatment-related decisions, which could have influenced the clinical results. Second, only the clinical outcomes during hospitalization were analyzed in this study; therefore, the risk of postdischarge mortality could not be ascertained. Third, although we tried to comprehensively explore the potential risk factors for mortality, there could be other unknown risk factors that were not considered in the present study. Fourth, there were no data regarding the treatment during hospitalization and vaccination status, which might have affected mortality.
In conclusion, this large-scale observational study in Japan identified several independent risk factors for mortality in hospitalized COVID-19 patients that could facilitate appropriate risk stratification of COVID-19 patients.
ACKNOWLEDGMENTS
This study was conducted with the support and collaboration of the Japanese Society of Pulmonary Embolism Research and the Japanese Society of Phlebology. We appreciate Ms Emi Kuroki’s technical support of the Japanese Society of Phlebology. We also thank editing of Editage and assessment of this manuscript for English language (http://www.editage.com).
Data availability: Data cannot be shared due to ethical or privacy concerns.
Funding: This work was supported by the Foundation Kyoto Health Care Society and the Fujiwara Memorial Foundation in Kyoto, Japan. The sponsors were not involved in design and execution of the study; collection, management, analysis, and interpretation of data; or preparation, review, or approval of the manuscript.
Conflicts of interest: None declared.
SUPPLEMENTARY MATERIAL
The following is the supplementary data related to this article:
eMaterial 1. Definitions of patient characteristics
REFERENCES
- 1.Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Gerwen M, Alsen M, Little C, et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93:907–915. 10.1002/jmv.26337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soares RCM, Mattos LR, Raposo LM. Risk factors for hospitalization and mortality due to COVID-19 in Espírito Santo State, Brazil. Am J Trop Med Hyg. 2020;103:1184–1190. 10.4269/ajtmh.20-0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenguer J, Ryan P, Rodríguez-Baño J, et al. ; COVID-19@Spain Study Group; Fundación SEIMC-GESIDA; Hospital General Universitario Gregorio Marañón; Hospital Universitario La Paz; Hospital Infanta Leonor; Complejo Hospitalario Virgen de la Salud; Hospital Universitario Rafael Méndez; Hospital Universitario de Cruces; Hospital de Melilla; Hospital San Eloy de Barakaldo; Hospital Universitario Central de Asturias; Hospital General Universitario de Alicante; Hospital Virgen de la Victoria; Hospital Universitario Puerto Real; EOXI Pontevedra e Salnés; Hospital de Figueres; Hospital Sant Jaume de Calella; Hospital del Mar; Hospital Virgen de la Arrixaca; Hospital de Can Misses; Hospital de Sagunto; Hospital Clínico San Cecilio; Hospital Universitario Príncipe de Asturias; Parc Sanitari Sant Joan de Déu; Hospital Nuestra Señora de Gracia; HC Marbella Internacional Hospital; Hospital La Princesa; Hospital Josep Trueta; Hospital Dos de Maig; Hospital Arnau de Vilanova-Lliria; Hospital General Universitario de Elche; Hospital Clínico Universitario de Valencia; Complejo Asistencial de Ávila; Hospital Comarcal de Alcañiz; Hospital Universitario Marqués de Valdecilla; Hospital Quiron-Salud de Torrevieja; Hospital Universitario Miguel Servet; SCIAS, Hospital de Barcelona; Fundación Hospital Universitario Alcorcón; Hospital Álvaro Cunqueiro; Complejo Asistencial Universitario de Salamanca; Hospital Universitario Severo Ochoa; Hospital CIMA-Sanitas; Hospital HLA Inmaculada; Hospital Universitario Rio Hortega; Hospital de Guadalajara; Hospital Universitario Infanta Sofía; Hospital Comarcal de Blanes; Hospital Universitari de Tarragona Joan XXIII; Hospital Universitario Basurto; Hospital Universitario de Canarias; Hospital Universitario de Gran Canaria Dr Negrín; Hospital Son Espases; Hospital Universitario de Móstoles; Complejo Hospitalario Universitario A Coruña; Hospital Costa del Sol; Hospital Clínico Universitario Lozano Blesa; Hospital Mutua de Terrassa; Hospital de la Plana; Hospital Virgen de la Concha–Complejo Asistencial de Zamora; Complejo Hospitalario Universitario Insular Materno-Infantil; Hospital de la Marina Baixa; Hospital Universitario Virgen Macarena; Hospital Universitari de Bellvitge; Hospital Universitario y Politécnico la Fe; Hospital Universitario del Vinalopó; Hospital de Sabadell (Parc Tauli); Hospital Clinic de Barcelona; Hospital Universitario de la Ribera; Fundación Jiménez Díaz; Hospital Clínico Universitario de Valladolid; Hospital Clínico San Carlos; Hospital Santa Creu i Sant Pau; Clínica Universitaria de Navarra–Campus Madrid; Hospital Son Llatzer; Hospital General de la Defensa Gómez Ulla; Hospital Universitario de Álava; Hospital Santos Reyes; Hospital Dr José Molina Orosa; Hospital Vall d’Hebrón; Hospital Universitario Rey Juan Carlos; Complejo Hospitalario Universitario Santa Lucía; Hospital Santa Bárbara; Complejo Hospitalario Universitario de Ferrol; Hospital de l’Esperit Sant; Hospital Universitario los Arcos del Mar Menor; Hospital HLA Universitario Moncloa; Hospital Virgen del Puerto; Hospital Marina Salud de Dénia; Hospital Universitario de Jerez; Hospital Reina Sofía de Tudela; Hospital Clínico Universitario de Santiago de Compostela; Hospital Universitario del Henares; Hospital Universitario Lucus Augusti; Hospital de Donostia; Hospital de Urduliz Alfredo Espinosa; Hospital de Mendaro; Hospital Juan Ramón Jiménez; Hospital de Tortosa Virgen de la Cinta; Hospital Riotinto; Hospital Vega Baja; Hospital Puerta de Hierro; Hospital Universitario de Getafe; Hospital General de la Palma; Hospital El Bierzo; Fundación Hospital de Calahorra; Hospital Alto Deba; Hospital Universitario San Juan de Alicante; Hospital de Guadarrama; Hospital Universitario de Jaén; Hospital de Mataró; Hospital de Palamós; Hospital Universitario de Valme; Clínica Universitaria de Navarra–Campus Navarra; Hospital Clínica Benidorm; Hospital Doce de Octubre; Hospital Universitario Virgen del Rocío; Hospital Universitario Ramón y Cajal; Hospital Universitario San Pedro; Hospital Quirón A Coruña; HM Sanchinarro; Hospital Francesc de Borja; Complejo Hospitalario Universitario Nuestra Señora de La Candelaria; Hospital Universitario HM Montepríncipe; Hospital Universitario HM Puerta del Sur; Hospital Universitario HM Torrelodones; Hospital Universitario HM Madrid; Hospital Don Benito-Villanueva de la Serena; Hospital de Viladecans; Centro Nacional de Epidemiología . Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin Microbiol Infect. 2020;26(11):1525–1536. 10.1016/j.cmi.2020.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382:2534–2543. 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parra-Bracamonte GM, Lopez-Villalobos N, Parra-Bracamonte FE. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Ann Epidemiol. 2020;52:93–98.e2. 10.1016/j.annepidem.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikami T, Miyashita H, Yamada T, et al. Risk factors for mortality in patients with COVID-19 in New York City. J Gen Intern Med. 2021;36:17–26. 10.1007/s11606-020-05983-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Severe obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring). 2020;28:1595–1599. 10.1002/oby.22913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grasselli G, Greco M, Zanella A, et al. ; COVID-19 Lombardy ICU Network . Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. 10.1001/jamainternmed.2020.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Bai H, Liu J, et al. Distinct clinical characteristics and risk factors for mortality in female inpatients with coronavirus disease 2019 (COVID-19): a sex-stratified, large-scale cohort study in Wuhan, China. Clin Infect Dis. 2020;71:3188–3195. 10.1093/cid/ciaa920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernández-Galdamez DR, González-Block MÁ, Romo-Dueñas DK, et al. Increased risk of hospitalization and death in patients with COVID-19 and pre-existing noncommunicable diseases and modifiable risk factors in Mexico. Arch Med Res. 2020;51:683–689. 10.1016/j.arcmed.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi C, Wang L, Ye J, et al. Predictors of mortality in patients with coronavirus disease 2019: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:663. 10.1186/s12879-021-06369-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21:855. 10.1186/s12879-021-06536-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinoshita R, Jung SM, Kobayashi T, Akhmetzhanov AR, Nishiura H. Epidemiology of coronavirus disease 2019 (COVID-19) in Japan during the first and second waves. Math Biosci Eng. 2022;19:6088–6101. 10.3934/mbe.2022284 [DOI] [PubMed] [Google Scholar]
- 17.Terada M, Ohtsu H, Saito S, et al. Risk factors for severity on admission and the disease progression during hospitalisation in a large cohort of patients with COVID-19 in Japan. BMJ Open. 2021;11:e047007. 10.1136/bmjopen-2020-047007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimoto Y, Yachi S, Takeyama M, et al. ; CLOT-COVID Study Investigators . The current status of thrombosis and anticoagulation therapy in patients with COVID-19 in Japan: From the CLOT-COVID study. J Cardiol. 2022;80(4):285–291. 10.1016/j.jjcc.2022.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda N, Yachi S, Takeyama M, et al. ; CLOT-COVID Study Investigators . D-dimer values and venous thromboembolism in patients with COVID-19 in Japan - From the CLOT-COVID Study. Circ Rep. 2022;4(5):215–221. 10.1253/circrep.CR-22-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita Y, Yachi S, Takeyama M, et al. ; CLOT-COVID Study Investigators . Influence of sex on development of thrombosis in patients with COVID-19: from the CLOT-COVID study. Thromb Res. 2022;213:173–178. 10.1016/j.thromres.2022.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashita Y, Yachi S, Takeyama M, et al. Therapeutic-Dose vs. Prophylactic-dose anticoagulation therapy for critically ill patients with COVID-19 in a practice-based observational study. Circ J. 2022;86:1137–1142. 10.1253/circj.CJ-22-0209 [DOI] [PubMed] [Google Scholar]
- 22.Ikeda S, Ueno Y, Maemura K, et al. ; CLOT-COVID Study Investigators . Association between the development of thrombosis and worsening of disease severity in patients with moderate COVID-19 on admission - From the CLOT-COVID Study. Circ J. 2022. doi:10.1253/circj.CJ-22-0252. 10.1253/circj.CJ-22-0252 [DOI] [PubMed] [Google Scholar]
- 23.Yamashita Y, Yamada N, Mo M. The primary prevention of venous thromboembolism in patients with COVID-19 in Japan: current status and future perspective. Ann Vasc Dis. 2021;14:1–4. 10.3400/avd.ra.20-00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita Y, Maruyama Y, Satokawa H, et al. ; Taskforce of VTE and COVID-19 in Japan Study . Incidence and clinical features of venous thromboembolism in hospitalized patients with coronavirus disease 2019 (COVID-19) in Japan. Circ J. 2021;85(12):2208–2214. 10.1253/circj.CJ-21-0169 [DOI] [PubMed] [Google Scholar]
- 25.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 26.Schulman S, Angerås U, Bergqvist D, Eriksson B, Lassen MR, Fisher W; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8(1):202–204. 10.1111/j.1538-7836.2009.03678.x [DOI] [PubMed] [Google Scholar]
- 27.Pijls BG, Jolani S, Atherley A, et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open. 2021;11:e044640. 10.1136/bmjopen-2020-044640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hergens MP, Bell M, Haglund P, et al. Risk factors for COVID-19-related death, hospitalization and intensive care: a population-wide study of all inhabitants in Stockholm. Eur J Epidemiol. 2022;37:157–165. 10.1007/s10654-021-00840-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang JJ, Dong X, Liu GH, Gao YD. Risk and protective factors for COVID-19 morbidity, severity, and mortality. Clin Rev Allergy Immunol. 2022. doi:10.1007/s12016-022-08921-5. 10.1007/s12016-022-08921-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Available at https://www.R-project.org/.
- 31.Ninomiya T, Otsubo K, Hoshino T, et al. Risk factors for disease progression in Japanese patients with COVID-19 with no or mild symptoms on admission. BMC Infect Dis. 2021;21:850. 10.1186/s12879-021-06574-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada G, Hayakawa K, Matsunaga N, et al. Predicting respiratory failure for COVID-19 patients in Japan: a simple clinical score for evaluating the need for hospitalisation. Epidemiol Infect. 2021;149:e175. 10.1017/S0950268821001837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med. 2021;174:362–373. 10.7326/M20-6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yaya S, Yeboah H, Charles CH, Otu A, Labonte R. Ethnic and racial disparities in COVID-19-related deaths: counting the trees, hiding the forest. BMJ Glob Health. 2020;5:e002913. 10.1136/bmjgh-2020-002913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open. 2021;4:e2134147. 10.1001/jamanetworkopen.2021.34147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ministry of Health, Labour and Welfare, Japan. Visualizing the data: information on COVID-19 infections. https://covid19.mhlw.go.jp/en; Accessed June 9, 2022.
- 38.Rica C, Salvador E. ECDC. COVID-19 situation update worldwide, as of week 18 2022. 2022. Available at https://covid19-country-overviews.ecdc.europa.eu/. 2021.
- 39.Matsunaga N, Hayakawa K, Terada M, et al. Clinical epidemiology of hospitalized patients with coronavirus disease 2019 (COVID-19) in Japan: report of the COVID-19 registry Japan. Clin Infect Dis. 2021;73:e3677–e3689. 10.1093/cid/ciaa1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez-Guzman PN, Daunt A, Mukherjee S, et al. Clinical characteristics and predictors of outcomes of hospitalized patients with coronavirus disease 2019 in a Multiethnic London National Health Service Trust: a retrospective cohort study. Clin Infect Dis. 2021;73(11):e4047–e4057. 10.1093/cid/ciaa1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amoroso A, Magistroni P, Vespasiano F, et al. ; Italian Network of Regional Transplant Coordinating Centers . HLA and AB0 polymorphisms may influence SARS-CoV-2 infection and COVID-19 severity. Transplantation. 2021;105(1):193–200. 10.1097/TP.0000000000003507 [DOI] [PubMed] [Google Scholar]
- 42.Novelli A, Andreani M, Biancolella M, et al. HLA allele frequencies and susceptibility to COVID-19 in a group of 99 Italian patients. HLA. 2020;96:610–614. 10.1111/tan.14047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lorente L, Martín MM, Franco A, et al. ; Working Group on COVID-19 Canary ICU; Annex. Members of the BIOMEPOC group . [HLA genetic polymorphisms and prognosis of patients with COVID-19]. Med Intensiva (Engl Ed). 2021;45(2):96–103. 10.1016/j.medin.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W, Zhang W, Zhang J, He J, Zhu F. Distribution of HLA allele frequencies in 82 Chinese individuals with coronavirus disease-2019 (COVID-19). HLA. 2020;96:194–196. 10.1111/tan.13941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto N, Ariumi Y, Nishida N, et al. SARS-CoV-2 infections and COVID-19 mortalities strongly correlate with ACE1 I/D genotype. Gene. 2020;758:144944. 10.1016/j.gene.2020.144944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeberg H, Pääbo S. The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature. 2020;587:610–612. 10.1038/s41586-020-2818-3 [DOI] [PubMed] [Google Scholar]
- 47.Rhodes JM, Subramanian S, Laird E, Griffin G, Kenny RA. Perspective: Vitamin D deficiency and COVID-19 severity - plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J Intern Med. 2021;289:97–115. 10.1111/joim.13149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saito S, Asai Y, Matsunaga N, et al. First and second COVID-19 waves in Japan: a comparison of disease severity and characteristics. J Infect. 2021;82:84–123. 10.1016/j.jinf.2020.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kollias A, Kyriakoulis KG, Lagou S, Kontopantelis E, Stergiou GS, Syrigos K. Venous thromboembolism in COVID-19: a systematic review and meta-analysis. Vasc Med. 2021;26:415–425. 10.1177/1358863X21995566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100639. 10.1016/j.eclinm.2020.100639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poly TN, Islam MM, Yang HC, et al. Obesity and mortality among patients diagnosed with COVID-19: a systematic review and meta-analysis. Front Med (Lausanne). 2021;8:620044. 10.3389/fmed.2021.620044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee H, Chubachi S, Namkoong H, et al. ; Japan COVID-19 Task Force . Effects of mild obesity on outcomes in Japanese patients with COVID-19: a nationwide consortium to investigate COVID-19 host genetics. Nutr Diabetes. 2022;12(1):38. 10.1038/s41387-022-00217-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. 10.1016/j.jacc.2008.12.068 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.