Abstract
Background
Critically ill patients routinely receive antibiotics with activity against anaerobic gut bacteria. However, in other disease states and animal models, gut anaerobes are protective against pneumonia, organ failure and mortality. We therefore designed a translational series of analyses and experiments to determine the effects of anti-anaerobic antibiotics on the risk of adverse clinical outcomes among critically ill patients.
Methods
We conducted a retrospective single-centre cohort study of 3032 critically ill patients, comparing patients who did and did not receive early anti-anaerobic antibiotics. We compared intensive care unit outcomes (ventilator-associated pneumonia (VAP)-free survival, infection-free survival and overall survival) in all patients and changes in gut microbiota in a subcohort of 116 patients. In murine models, we studied the effects of anaerobe depletion in infectious (Klebsiella pneumoniae and Staphylococcus aureus pneumonia) and noninfectious (hyperoxia) injury models.
Results
Early administration of anti-anaerobic antibiotics was associated with decreased VAP-free survival (hazard ratio (HR) 1.24, 95% CI 1.06–1.45), infection-free survival (HR 1.22, 95% CI 1.09–1.38) and overall survival (HR 1.14, 95% CI 1.02–1.28). Patients who received anti-anaerobic antibiotics had decreased initial gut bacterial density (p=0.00038), increased microbiome expansion during hospitalisation (p=0.011) and domination by Enterobacteriaceae spp. (p=0.045). Enterobacteriaceae were also enriched among respiratory pathogens in anti-anaerobic-treated patients (p<2.2×10−16). In murine models, treatment with anti-anaerobic antibiotics increased susceptibility to Enterobacteriaceae pneumonia (p<0.05) and increased the lethality of hyperoxia (p=0.0002).
Conclusions
In critically ill patients, early treatment with anti-anaerobic antibiotics is associated with increased mortality. Mechanisms may include enrichment of the gut with respiratory pathogens, but increased mortality is incompletely explained by infections alone. Given consistent clinical and experimental evidence of harm, the widespread use of anti-anaerobic antibiotics should be reconsidered.
Short abstract
In an observational study of 3032 mechanically ventilated patients, early anti-anaerobic antibiotics increased mortality risk. In secondary studies, anti-anaerobic antibiotics disrupted patients' gut microbiota and worsened outcomes in mouse models. https://bit.ly/3BLbpvz
Introduction
Decades of observational and experimental data have established that gastrointestinal microbiota play an important role in the pathogenesis of ventilator-associated pneumonia (VAP), multi-organ failure and other outcomes in critically ill patients [1–6]. In health, the gut microbiome provides resistance against colonisation by exogenous pathogens, whereas in critical illness, antibiotics disrupt native intestinal microbiota and allow proliferation of potential pathogens [7–9]. This phenomenon is the theoretical basis of selective decontamination of the digestive tract (SDD), a regimen of topical enteric and parenteral antibiotics tailored to preserve gut anaerobes while suppressing overgrowth of potential pathogens [10]. A recent Cochrane review concluded that SDD decreases VAP incidence and improves mortality [11]. Furthermore, across diverse experimental models (pneumonia, lung injury and shock), depletion of anaerobic gut microbiota (either via enteric antibiotics or use of germ-free animals) alters animal susceptibility to bacterial pneumonia, lung injury and mortality [12–16]. Thus, the clinical and biological importance of anaerobic gut microbiota in critical illness is robustly established across human studies and experimental inquiry.
Yet despite this well-established importance of gut anaerobes in critical illness, clinicians routinely prescribe empiric antibiotics with potent anti-anaerobic activity [17–19]. This widespread practice has recently been called into question given the infrequency of anaerobic pathogens among hospitalised patients (even including patients with aspiration pneumonia) [19]. Among other at-risk populations (i.e. haematopoietic stem cell transplant recipients), exposure to anti-anaerobic antibiotics is strongly predictive of adverse clinical outcomes [20]. To the best of our knowledge, no study has evaluated the effects of gut anaerobe depletion on clinical outcomes in critically ill patients.
To address this gap, we conducted a retrospective single-centre cohort study of critically ill patients receiving mechanical ventilation. We compared clinical outcomes and respiratory microbiology in patients who did and did not receive anti-anaerobic antibiotics early in their hospital stay. To explore potential mechanisms, we performed a longitudinal study of gut microbiota in a subcohort, and modelled the effects of anaerobe depletion in murine models of infectious and noninfectious lung pathology.
Methods
Additional details are available in the supplementary material.
Study population, primary exposure, and primary and secondary outcomes
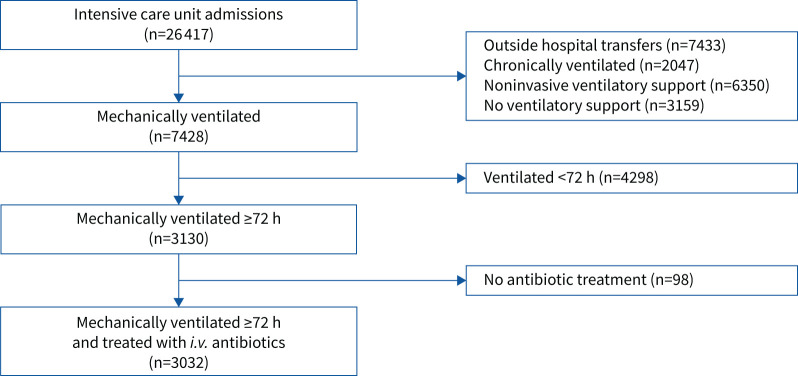
We performed a retrospective cohort study of patients at the University of Michigan Hospital (Ann Arbor, MI, USA) between 2016 and 2019 who received mechanical ventilation for at least 72 h and were treated with intravenous antibiotics. VAP, one of our primary outcomes of interest, occurs after 48 h of mechanical ventilation and thus the 72-h time frame was chosen to identify a population with nonzero probability of developing one of the primary outcomes of interest. Our primary exposure of interest was administration of anti-anaerobic antibiotic therapy. We defined antibiotic exposure as receipt of at least one dose of i.v. antibiotic prior to the first 72 h of mechanical ventilation. We excluded patients who were mechanically ventilated for <72 h and patients transferred from an outside medical facility. For patients with multiple hospital admissions during the study period, we recorded data for the first hospitalisation only (figure 1).
FIGURE 1.
Retrospective cohort overview. We identified a cohort of 3032 mechanically ventilated patients treated with i.v. antibiotics admitted to the University of Michigan Hospital in 2016–2019. We excluded patients transferred from an outside facility, patients with chronic ventilator dependence and those who received mechanical ventilation for a period <72 h.
Classification of anti-anaerobic antibiotics
Antibiotics were classified as “anti-anaerobic” if they were recommended for treatment of anaerobic pathogens based on guidelines from the Infectious Diseases Society of America [21–26]. A complete listing of anti-anaerobic antibiotic classification and a description of antimicrobial use are presented in the supplementary material (supplementary tables S3 and S5, and supplementary figure S1).
Primary outcome: VAP-free survival
Our primary outcome was composite “VAP-free survival”: the time from the initiation of mechanical ventilation to the time of VAP onset or death. We chose this end-point because 1) it is a validated measure used in randomised clinical trials for VAP prevention [27, 28] and 2) it addresses threats to validity introduced by competing risks (e.g. patients may die before they have the opportunity to develop VAP or may die due to VAP before it can be diagnosed). Patients were censored from survival analysis at the time of hospital discharge if they were discharged prior to 30 days. VAP was diagnosed using a streamlined version of the Centers for Disease Control and Prevention (CDC) surveillance criteria for VAP (table 1) [29–31].
TABLE 1.
Ventilator-associated pneumonia (VAP) diagnostic criteria
| Sustained increase in oxygen requirement (at least one) |
| 2 days of increasing daily minimum FIO2 ≥0.15 points |
| 2 days of increasing PEEP >2.5 mmH2O |
| Evidence of systemic inflammation (at least one) |
| Fever (temperature >38°C) |
| Hypothermia (temperature <35°C) |
| Leukocytosis (white blood cell count >12 000 mm−3) |
| Leukopenia |
| Purulent sputum production (at least one) |
| >26 neutrophils per high power field on Gram stain of endotracheal aspirate |
| >500 leukocytes on cell count of bronchoalveolar lavage fluid |
| Pathogen growth on respiratory culture |
| Confirmed VAP: pathogen growth |
| Probable VAP: no pathogen growth |
FIO2: inspiratory oxygen fraction; PEEP: positive end-expiratory pressure.
Secondary outcomes: infection-free survival and mortality
We defined nosocomial infection as a culture-confirmed infection that was not present on admission meeting clinical criteria set by major medical societies [25, 32–35]. We used the time of culture acquisition, death or discharge to calculate “infection-free survival”. We determined patient vital status at 30 days via review of the electronic medical record as well as local [36] and national [37] death indices. Death notes were processed using the CDC Instructions for Classifying the Underlying Cause of Death [38, 39] to assign a cause of death to decedents in the cohort and compare relative frequency of the cause of death in each antibiotic treatment group.
Statistical analysis of clinical data
All statistical analyses were performed using R version 4.1.2 [40]. We compared age, race, gender, frequency of medical comorbidities, weighted Charlson Comorbidity Index [41–43], Acute Physiology and Chronic Health Evaluation (APACHE) IV score [44] within 24 h of initiation of mechanical ventilation and the proportion of patients admitted to each hospital unit between treatment groups with the two-sample independent t-test.
We constructed Kaplan–Meier curves to determine median VAP-free, infection-free and all-cause survival. We used a stratified log-rank statistic to determine the statistical significance of differences in survival between groups. We built Cox proportional hazards models incorporating early treatment with anti-anaerobic antibiotics, hospital unit of admission, age, gender, race, APACHE IV score within 24 h of initiation of mechanical ventilation and weighted Charlson Comorbidity Index. All survival analysis was done with the survival (version 3.1-8) package in R [45]. We then built a logistic regression model using 30-day VAP-free survival as a binary variable (alive and VAP-free at 30 days or not) with the same covariates as our survival model. We used this model to calculate the average marginal effects of all covariates with the margins (version 0.3-26) package in R [46]. The neurological intensive care unit (ICU) was the unit with a rate of VAP or death closest to the overall rate in the cohort, and thus was chosen as the standard for comparison with other units in our survival and logistic regression models.
To compare rates of VAP independently (rather than within a composite outcome), we compared the cumulative incidence of VAP in 30 days for each treatment group and determined the estimated marginal probability of VAP in 30 days. We compared VAP-specific cumulative incidence functions with Gray's test [47]. Competing risk analysis was performed with the cmprsk (version 2.2-6) package in R [48].
We compared distributions of bacterial pathogens between groups with Chi-squared testing and compared the frequency of individual bacterial pathogens between treatment groups with two-sample independent t-testing. All statistical tests used p=0.05 as a threshold for significance.
Microbiome analysis of rectal swab specimens
We performed a secondary analysis of bacterial community data generated for a previously published study [49, 50]. We characterised the bacterial density and community composition of bacteria on rectal swabs collected from 116 hospitalised patients, all of whom were within our larger retrospective cohort. We analysed bacteria in rectal swabs collected at the time of admission and a repeat swab acquired later during hospitalisation. Although all patients in our subcohort were eventually intubated and treated with antibiotics prior to hour 72 of mechanical ventilation, some patients did not receive antibiotics prior to admission rectal swab and some had rectal swabs acquired on medical floors before intubation (and were later transferred to the ICU). Patient characteristics and microbiome analysis have been previously reported [49, 50].
16S rRNA gene sequencing and bacterial density quantification
The V4 region of the 16S rRNA gene was amplified using published primers and the dual-indexing sequencing strategy described in a previously published protocol [51]. Sequencing was performed using the MiSeq platform and MiSeq reagent kit V2 (500 cycles) according to the manufacturer's instructions (Illumina, San Diego, CA, USA) with minor modifications described previously [51, 52]. Bacterial DNA was quantified using a QX200 Droplet Digital PCR System (Bio-Rad, Hercules, CA, USA) with primers and cycling conditions performed according to previously published protocols [53].
Statistical analysis of microbiome data
We built a mixed effects multivariable linear regression model using, age, gender, race, weighted Charlson Comorbidity Index, APACHE IV score at admission and treatment with anti-anaerobic antibiotics prior to admission rectal swab to predict log-transformed bacterial density, relative abundance and absolute abundance of Enterobacteriaceae over time. We included an interaction term of anti-anaerobic antibiotic treatment and time to compare the daily change in bacterial density, the relative abundance of Enterobacteriaceae and the absolute abundance of Enterobacteriaceae between anti-anaerobic-treated patients and untreated patients. We built the mixed effects models with the lme4 (version 1.1-5) package in R [54].
Murine modelling
Mice (8–10 weeks old, female C57BL/6) were obtained from Jackson Laboratories (Bar Harbor, ME, USA). All experiments were conducted with approval from the University of Michigan Institutional Animal Care and Use Committee. To control for cage effect, mice in each treatment arm were co-housed and in all experiments each treatment group had 15 mice per arm.
Infectious model of pneumonia/lung injury
Mice were pre-treated with either sham, cefepime or piperacillin–tazobactam daily for 3 days, allowed a washout period of 24 h and subsequently inoculated with 107 CFU methicillin-resistant S. aureus (MRSA USA300, strain NRS384) or 107 CFU Klebsiella pneumoniae (strain KPPR1 [55]), instilled intratracheally under anaesthesia. Mock-infected control mice were challenged with PBS instead of bacterial inoculation. Mice were harvested at 24 h to collect bronchoalveolar lavage fluid (BALF) or whole lung tissue for culture and lung injury assessment. We measured bacterial clearance with change in CFU after an initial infectious challenge. Lung injury was assessed with total protein and IgM in BALF.
Noninfectious model of lung injury: hyperoxia exposure
Mice were pre-treated with either sham, cefepime or piperacillin–tazobactam daily for 3 days, and subsequently exposed to either 100% oxygen or room air (controls) on days 0, 1 and 2. Mice were harvested on day 3 to collect BALF. Lung injury was again assessed with total protein and IgM in BALF.
Murine tissue collection and processing
Lung samples collected for this study were harvested and processed according to previously published protocols [16, 56, 57]. Lung bacterial culture was performed using six 10-fold serial dilutions of lung homogenate from mice inoculated with S. aureus and K. pneumoniae along with planted control samples to identify sources of contamination.
Results
Cohort characteristics
We identified 3032 consecutive patients admitted to the University of Michigan Hospital between 2016 and 2019 who received mechanical ventilation for at least 72 h and were treated with at least one dose of i.v. antibiotics within the first 72 h of mechanical ventilation (figure 1). The baseline characteristics of both groups are shown in table 2. Compared with patients who did not receive anti-anaerobic antibiotics, patients who received anti-anaerobic antibiotics were younger (mean age 55.4 years for anti-anaerobic group versus 59.1 years for no anti-anaerobic group; p=6.0×10−9) and less likely to have peripheral vascular disease, coronary artery disease or cerebrovascular disease. Most patients in the anti-anaerobic group were admitted to the medical and surgical ICUs (1249 out of 1942 patients (64%)). In contrast, patients who did not receive anti-anaerobic treatment were more evenly distributed among ICUs, and were more frequently admitted to the neurological, cardiac and cardiothoracic ICUs. Groups were similar in terms of severity of illness (mean APACHE IV score 89.9 for anti-anaerobic group versus 90.1 for no anti-anaerobic group; p=0.89) and burden of medical comorbidities (median Charlson Comorbidity Index 4.6 for anti-anaerobic group versus 4.5 for no anti-anaerobic group; p=0.63).
TABLE 2.
Cohort demographics and comorbidities
| Anti-anaerobic coverage | No anti-anaerobic coverage | p-value | |
| Subjects | 1942 | 1090 | |
| Age, years | 55.4±16.9 | 59.1±17.0 | <0.001 |
| Female | 757 (39) | 462 (42) | 0.67 |
| Non-Caucasian | 377 (20) | 218 (20) | 0.70 |
| APACHE IV score | 90.1±26.0 | 89.9±25.7 | 0.89 |
| Charlson Comorbidity Index (median±sd) | 4.6±3.2 | 4.5±3.0 | 0.63 |
| Medical comorbidities | |||
| Coronary artery disease | 424 (22) | 223 (21) | 0.37 |
| Congestive heart failure | 714 (37) | 505 (46) | <0.001 |
| Peripheral vascular disease | 501 (26) | 385 (35) | <0.001 |
| History of prior stroke | 417 (22) | 389 (36) | <0.001 |
| Dementia | 61 (3.1) | 33 (3.0) | 0.86 |
| COPD | 622 (32) | 360 (33) | 0.57 |
| Connective tissue disorder | 98 (5.0) | 55 (5.0) | 1.00 |
| Peptic ulcer disease | 132 (6.8) | 56 (5.1) | 0.059 |
| Cirrhosis | 438 (23) | 126 (12) | <0.001 |
| Diabetes | 562 (29) | 312 (29) | 0.85 |
| End-stage renal disease | 700 (36) | 415 (38) | 0.27 |
| Active malignancy | 370 (19) | 180 (17) | 0.077 |
| ICU of admission | |||
| Medical | 819 (42) | 223 (20) | <0.001 |
| Surgical | 430 (22) | 114 (10) | <0.001 |
| Cardiothoracic-surgical | 202 (10) | 310 (28) | <0.001 |
| Neurological | 140 (7.0) | 212 (19) | <0.001 |
| Cardiac | 172 (9.0) | 122 (11) | 0.043 |
| Trauma-burn | 179 (9.0) | 109 (10) | 0.051 |
Data are presented as n, mean±sd or n (%), unless otherwise stated. ICU: intensive care unit; APACHE: Acute Physiology and Chronic Health Evaluation.
Cohort antibiotic use
Having identified our cohort, we next characterised their antibiotic use (table 3). We characterised individual anti-anaerobic exposure as a binary variable (i.e. patients either did or did not receive antibiotics with anti-anaerobic activity within the first 72 h of mechanical ventilation). The antibiotics reported in table 3 represent >97% of all administered antibiotic doses in the study cohort. Vancomycin was the most frequently administered antibiotic overall, with 2305 out of 3032 patients (76% of the total cohort) receiving treatment, evenly distributed across treatment groups. Piperacillin–tazobactam and metronidazole were the most frequently administered anti-anaerobic antibiotics (57% and 27% of the anti-anaerobic-treated population, respectively). The most administered antibiotics without anti-anaerobic activity were cefepime (27% of anti-anaerobic group and 55% of no anti-anaerobic group), azithromycin (23% of anti-anaerobic group and 46% of no anti-anaerobic group) and cefazolin (8% of anti-anaerobic group and 23% of no anti-anaerobic group). A comprehensive list of antibiotic exposures in the cohort can be found in supplementary table S5 and supplementary figure S1.
TABLE 3.
Summary of the most frequently administered antibiotics in the study cohort
| Antibiotic | Anti-anaerobic coverage (n=1942) | No anaerobic coverage (n=1090) | p-value |
| Vancomycin | 1477 (76) | 828 (76) | 0.95 |
| Cefepime | 529 (27) | 596 (55) | <0.001 |
| Azithromycin | 442 (23) | 504 (46) | <0.001 |
| Cefazolin | 170 (8.0) | 254 (23) | <0.001 |
| Piperacillin–tazobactam | 1107 (57) | 0 | |
| Metronidazole | 530 (27) | 0 | |
| Ampicillin–sulbactam | 262 (14) | 0 | |
| Ceftriaxone | 224 (12) | 0 | |
| Clindamycin | 136 (7) | 0 | |
| Meropenem | 90 (5) | 0 |
Data are presented as n (%), unless otherwise stated.
Early exposure to anti-anaerobic antibiotic therapy is associated with decreased VAP-free survival, infection-free survival and overall survival
We next sought to determine if anti-anaerobic antibiotic treatment was associated with decreased VAP-free survival. We selected this commonly used composite outcome (VAP or death) to mitigate bias introduced by competing risks (i.e. patients may die early due to undetected VAP or early mortality from other causes may preclude the opportunity for patients to develop VAP) [27, 28, 58, 59].
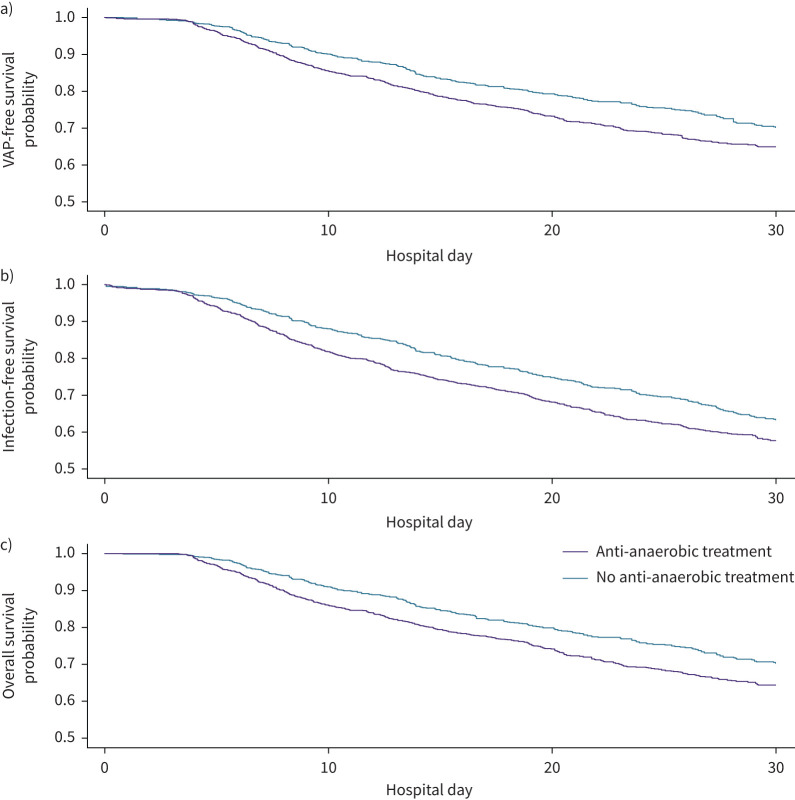
We first performed a single-variable analysis by constructing Kaplan–Meier survival curves to estimate the 30-day VAP-free survival of each antibiotic treatment group (figure 2a). We found that a total of 931 patients (30.7%) reached our composite end-point of VAP or death, with 89 cases of culture-confirmed VAP and 842 deaths. Patients not treated with anti-anaerobic antibiotics had a 72% probability of VAP-free survival at 30 days (at risk n=472), whereas patients treated with anti-anaerobic antibiotics had a 65% probability of VAP-free survival at 30 days (at risk n=574), with a significant difference noted between the survival functions of each group (hazard ratio (HR) 1.26, 95% CI 1.10–1.44; p=0.00074). We concluded that in single-variable analysis, early exposure to anti-anaerobic antibiotics was associated with a 7% absolute decrease in 30-day VAP-free survival.
FIGURE 2.
Anti-anaerobic antibiotic therapy predicts decreased ventilator-associated pneumonia (VAP)-free survival, infection-free survival and overall survival in critically ill patients. In a cohort of 3032 mechanically ventilated patients treated with i.v. antibiotics, the use of anti-anaerobic antibiotic therapy prior to day 3 of mechanical ventilation was associated with a) decreased VAP-free survival at 30 days (p=0.00074 by log-rank test), b) infection-free survival at 30 days (p=0.00044 by log-rank test) and c) overall survival at 30 days (p=0.00064 by log-rank test). There were 842 deaths, 89 cases of VAP and 472 cases of nosocomial infection in the cohort.
We next sought to determine if exposure to anti-anaerobic antibiotics was independently associated with decreased VAP-free survival. To accomplish this, we built a multivariable Cox proportional hazards regression model (table 4 and figure 3). Covariates were specified a priori based on known risk factors for VAP and ICU mortality and sources of variation in antibiotic prescribing practices. We included age, sex, race, severity of acute illness as measured by APACHE IV score calculated during the first 24 h of invasive mechanical ventilation, Charlson Comorbidity Index and unit of admission. We found that anti-anaerobic antibiotic treatment was independently associated with an increased hazard of VAP or death (HR 1.24, 95% CI 1.06–1.45; p=0.0078). We then built a multivariable logistic regression model to compare the 30-day event rate of VAP or death between treatment groups and found that anti-anaerobic antibiotic treatment was associated with an average marginal effect of 3.9% (95% CI 0.40–7.5%; p=0.029) (table 5 and supplementary figure S2). We concluded that early exposure to anti-anaerobic antibiotics was independently associated with a 3.9% increased risk of VAP or death at 30 days.
TABLE 4.
Hazard ratios (HRs) from Cox proportional hazards models of ventilator-associated pneumonia (VAP)-free, infection-free and overall survival
| VAP-free survival | Infection-free survival | Overall survival | ||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Anaerobic coverage | 1.24 (1.06–1.45) | 0.0078 | 1.22 (1.09–1.38) | 0.00072 | 1.14 (1.02–1.28) | 0.028 |
| Demographics and comorbidities | ||||||
| Age | 1.00 (0.99–1.01) | 0.62 | 1.00 (0.99–1.01) | 0.93 | 1.01 (1.00–1.01) | 0.039 |
| Non-Caucasian | 1.03 (0.86–1.23) | 0.74 | 0.96 (0.84–1.10) | 0.58 | 1.02 (0.90–1.16) | 0.79 |
| Male | 0.93 (0.81–1.07) | 0.3 | 0.94 (0.84–1.04) | 0.23 | 0.93 (0.81–1.07) | 0.13 |
| APACHE IV | 1.01 (1.01–1.02) | <0.001 | 1.01 (1.006–1.014) | <0.001 | 1.01 (1.01–1.01) | <0.001 |
| Charlson Comorbidity Index | 0.99 (0.97–1.02) | 0.66 | 1.01 (0.99–1.03) | 0.28 | 1.02 (1.00–1.04) | 0.019 |
| ICU of admission | ||||||
| Cardiac | 1.08 (0.82–1.42) | 0.57 | 0.75 (0.61–0.94) | 0.012 | 0.89 (0.71–1.10) | 0.27 |
| Medical | 1.09 (0.87–1.37) | 0.43 | 1.09 (0.87–1.37) | 0.43 | 1.08 (0.91–1.29) | 0.37 |
| Trauma-burn | 0.78 (0.57–1.07) | 0.12 | 0.79 (0.62–1.00) | 0.49 | 0.59 (0.45–0.76) | <0.001 |
| Cardiothoracic-surgical | 0.70 (0.54–0.92) | 0.01 | 0.56 (0.46–0.69) | <0.001 | 0.72 (0.59–0.87) | <0.001 |
| Surgical | 0.70 (0.54–0.91) | 0.01 | 0.58 (0.47–0.70) | <0.001 | 0.64 (0.52–0.78) | <0.001 |
| Neurological | 1 | Reference | 1 | Reference | 1 | Reference |
APACHE: Acute Physiology and Chronic Health Evaluation; ICU: intensive care unit.
FIGURE 3.
Anti-anaerobic antibiotic therapy is independently associated with decreased ventilator-associated pneumonia (VAP)-free survival. We constructed a multivariable Cox proportional hazards regression model including Acute Physiology and Chronic Health Evaluation (APACHE) IV score, Charlson Comorbidity Index, demographics, intensive care unit (ICU) of admission and anti-anaerobic antibiotic treatment to predict VAP-free survival. The model was highly significant (concordance of 0.63; p<2.2×10−6 for both Wald test and likelihood ratio test). Anti-anaerobic antibiotic treatment was independently associated with increased hazard of VAP or death (HR 1.24, 95% CI 1.06–1.45).
TABLE 5.
Risk ratios from a logistic regression model of ventilator-associated pneumonia or death at 30 days
| Risk ratio (95% CI) | p-value | |
| Anaerobic coverage | 1.23 (1.02–1.49) | 0.030 |
| Demographics and comorbidities | ||
| Age | 1.01 (1.00–1.02) | 0.36 |
| Non-Caucasian | 1.01 (0.82–1.25) | 0.91 |
| Male | 0.92 (0.78–1.09) | 0.36 |
| APACHE IV | 1.02 (1.01–1.02) | <0.001 |
| Charlson Comorbidity Index | 1.02 (0.99–1.05) | 0.14 |
| ICU of admission | ||
| Cardiac | 1.28 (0.90–1.82) | 0.17 |
| Medical | 1.28 (0.96–1.71) | 0.090 |
| Trauma-burn | 0.94 (0.64–1.38) | 0.74 |
| Cardiothoracic-surgical | 0.72 (0.52–0.99) | 0.045 |
| Surgical | 0.76 (0.55–1.06) | 0.11 |
| Neurological (reference) | 1 | Reference |
APACHE: Acute Physiology and Chronic Health Evaluation; ICU: intensive care unit.
Although VAP comprised a relatively small component of our composite outcome (89 out of 931 (9.5%)), we sought to determine if we could detect differences in the rate of VAP between treatment groups independent of mortality. To account for competing risks, we compared the cumulative incidence of VAP for each treatment group and estimated the marginal probability of VAP at 30 days. Patients not treated with anti-anaerobic antibiotics had an estimated marginal probability of VAP of 3.1% at 30 days (n=21 cases of culture-confirmed VAP), whereas patients treated with anti-anaerobic antibiotics had an estimated marginal probability of VAP of 3.5% at 30 days (n=68 cases of culture-confirmed VAP) (supplementary figure S3), with no significant difference noted between treatment groups (χ2=0.28, p=0.60). Given the small number of VAP cases in the cohort, we concluded that we were inadequately powered to detect differences in the rate of VAP as a single outcome in this cohort.
Given experimental and clinical evidence that gut anaerobes play a protective role in nonpneumonia outcomes both in animal models [15, 16, 60–62] and clinical trials [11, 63, 64], we next asked if anti-anaerobic antibiotic treatment was associated with other clinically important ICU outcomes. We asked if anti-anaerobic antibiotic treatment was associated with decreased infection-free survival and overall survival. To accomplish this, we constructed Kaplan–Meier survival curves to estimate the 30-day infection-free survival and overall survival of each antibiotic treatment group (figures 2b and c). In multivariable Cox models, anti-anaerobic antibiotic therapy remained independently associated with decreased infection-free survival (HR 1.22, 95% CI 1.09–1.38; p=0.00072) and overall survival (HR 1.14, 95% CI 1.02–1.28; p=0.028) (table 4).
Inadequate coverage of a causative bacterial pathogen in patients admitted to the ICU with sepsis can be fatal [65]. To determine if differences in mortality between treatment groups were confounded by adequacy of initial antimicrobial treatment, we analysed the clinical microbiology of patients admitted to the ICU to determine 1) if a patient had a culture-confirmed bacterial infection on presentation to the ICU and 2) if the antibiotic treatment a patient received covered the pathogen isolated. We found that although all patients in our cohort were treated with antibiotics, only 522 out of 1942 (26.8%) anti-anaerobic-treated patients and 119 out of 1090 (10.9%) patients who did not receive anti-anaerobic coverage had evidence of infection preceding the initiation of antibiotic treatment. Of those patients with a confirmed bacterial infection on ICU admission, five patients who were not treated with anti-anaerobic antibiotics and 18 patients treated with anti-anaerobic antibiotics received treatment inadequate to cover the causative pathogen. When we included adequacy of antibiotic treatment in our multivariable models, we found that anti-anaerobic antibiotic treatment remained independently associated with decreased VAP-free survival (HR 1.25, 95% CI 1.10–1.44; p=0.00075), infection-free survival (HR 1.21, 95% CI 1.08–1.37; p=0.0082) and overall survival (HR 1.14, 95% CI 1.02–1.28; p=0.031). We concluded that it was unlikely that the observed association between anti-anaerobic antibiotic treatment and survival was confounded by adequacy of antibiotic treatment.
Administration of anti-anaerobic antibiotic therapy is associated with increased frequency of Enterobacteriaceae VAP
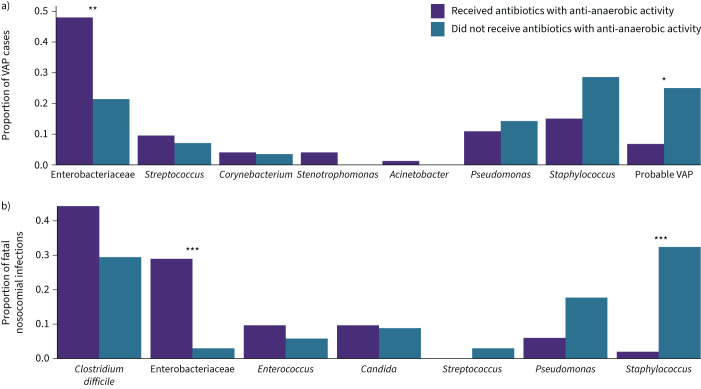
Having established that anti-anaerobic antibiotic therapy is associated with decreased VAP-free survival, we next asked if anti-anaerobic antibiotic therapy was associated with changes in the clinical microbiology of VAP cases. To accomplish this, we compared the identity of culture-identified respiratory pathogens among cases of VAP in each antibiotic treatment group. We included in our analysis cases classified as “probable VAP” (cases meeting all criteria other than identification of a pathogen via culture) that we had previously excluded in our survival analyses. We found that the distribution of pathogens differed significantly between groups (p<2.2×10−16, Chi-squared test) (figure 4a). This difference was driven primarily by the increased identification of Enterobacteriaceae spp. among patients treated with anti-anaerobic antibiotics (n=35 (48%) cases of VAP in anti-anaerobic-treated versus n=6 (21%) cases of VAP among untreated; p=0.0090) and secondarily by a decrease in the frequency of “probable VAP” among anti-anaerobic-treated patients (n=5 (7%) cases of VAP in anti-anaerobic-treated versus n=7 (25%) cases of VAP among untreated: p=0.049). We noted that overall, S. aureus was the most common organism in patients that did not receive anaerobic coverage (n=8 (29%)) and Enterobacteriaceae spp. were the most common organisms in the anti-anaerobic group (n=35 (48%), as already noted). A complete listing of the pathogens causing VAP in the cohort can be found in supplementary tables S6 and S7.
FIGURE 4.
Administration of anti-anaerobic antibiotics is associated with increased identification of gut-associated bacteria in respiratory cultures. a) We compared the distribution of pathogens causing ventilator-associated pneumonia (VAP) among treatment groups and found an increased identification of Enterobacteriaceae spp. in patients treated with anti-anaerobic antibiotics (48% of cases of VAP in anti-anaerobic-treated versus 21% of cases of VAP without anaerobic treatment; p=0.0090 by t-test). b) We compared the distribution of pathogens causing fatal nosocomial infection among treatment groups and again found an increased identification of Enterobacteriaceae in patients treated with anti-anaerobic antibiotics (29% of cases in anti-anaerobic-treated versus 3% of cases without anaerobic treatment; p=0.0004 by t-test). *: p<0.05; **: p<0.01; ***: p<0.001.
Among mechanically ventilated patients who die, those treated with anti-anaerobic antibiotics are more likely to die of infection-related causes
Having observed that the difference in our composite outcome (VAP-free survival) was largely driven by differences in mortality rather than VAP, we next asked if causes of death varied across antibiotic treatment groups. We reviewed the electronic medical records of the 842 decedents in our cohort to determine cause of death, categorising the causes of death into 10 groups as guided by the CDC National Vital Statistics instructions for classifying underlying cause of death [38]. Cause of death was determined without knowledge of anti-anaerobic antibiotic exposure. We found that the distribution of causes of death varied significantly between groups (table 6), driven primarily by increased infection-related deaths in anti-anaerobic-treated patients, increased hepatic failure in anti-anaerobic-treated patients and decreased deaths from a neurological process in patients with anti-anaerobic therapy. Among infection-related deaths, fatal infections were equally likely to be caused by the primary infectious process that led to admission (88 out of 177 (49% of infections)) as by subsequent nosocomial infections (89 out of 177 (51% of infections)). We concluded that patients treated with anti-anaerobic therapy were more likely to die of infection-related causes, although this difference does not entirely explain the mortality difference observed across treatment groups.
TABLE 6.
Causes of death in study cohort
| Anti-anaerobic coverage (n=1942) | No anaerobic coverage (n=1090) | p-value | |
| Cardiovascular | 102 (18) | 69 (26) | 0.01 |
| Pulmonary | 125 (22) | 54 (20) | 0.56 |
| Renal | 9 (2.0) | 2 (1.0) | 0.26 |
| Gastrointestinal | 47 (8.0) | 13 (5.0) | 0.05 |
| Infection | 138 (24) | 39 (14) | <0.001 |
| Hepatic | 38 (7.0) | 4 (1.0) | <0.001 |
| Neurological | 63 (11) | 64 (24) | <0.001 |
| Poisoning or overdose | 9 (2.0) | 4 (1.0) | 0.93 |
| Malignancy | 22 (4.0) | 9 (3.0) | 0.72 |
| Unknown | 20 (3.0) | 11 (4.0) | 0.68 |
| Total | 573 (30) | 269 (24) | 0.009 |
Data are presented as n (%), unless otherwise stated.
Anti-anaerobic antibiotic therapy with increased frequency of Enterobacteriaceae spp. in fatal nosocomial infection
Having observed an increased prevalence of Enterobacteriaceae spp. in anti-anaerobic-treated patients, we next asked if this same pattern was present in fatal nosocomial infections, including infections other than pneumonia. To accomplish this, we compared the distribution of pathogens and sites of infection among patients with culture-confirmed nosocomial infection that led to death (figure 4b). We found that patients treated with anti-anaerobic therapy were more likely to die of an infection caused by Enterobacteriaceae spp. (29% of cases of fatal infection in anti-anaerobic-treated versus 3.0% of cases of fatal infection without anaerobic treatment; p=0.026) and less likely to die of an infection caused by Staphylococcus (2.0% of cases of fatal infection in anti-anaerobic-treated versus 32% of cases of fatal infection no anaerobic treatment; p=0.00078), mirroring the changes we observed in VAP. We concluded that anti-anaerobic antibiotic treatment was associated with an increased frequency of fatal Enterobacteriaceae infections relative to other pathogens (including Staphylococcus spp.). A complete comparison of the pathogens causing fatal nosocomial infection can be found in supplementary tables S8 and S9.
Early anti-anaerobic antibiotic therapy is associated with reduced gut bacterial density and subsequent enrichment with Enterobacteriaceae spp. during hospitalisation
Given our observation that anti-anaerobic therapy was associated with an increased frequency of infection with enteric pathogens (specifically Enterobacteriaceae spp.), we speculated that this difference could be attributable to differential effects of these antibiotic regimens on gut microbiota. We thus sought to determine the impact of anti-anaerobic therapy on gut microbiota of hospitalised patients. To accomplish this, we characterised the bacterial density and community composition of gut microbiota of 116 hospitalised patients, all of whom were within our larger retrospective cohort. We analysed microbiota detected in rectal swabs collected at the time of admission (after administration of antibiotics in the emergency department) and a repeat swab acquired later during hospitalisation. Details regarding this subcohort have previously been published [50].
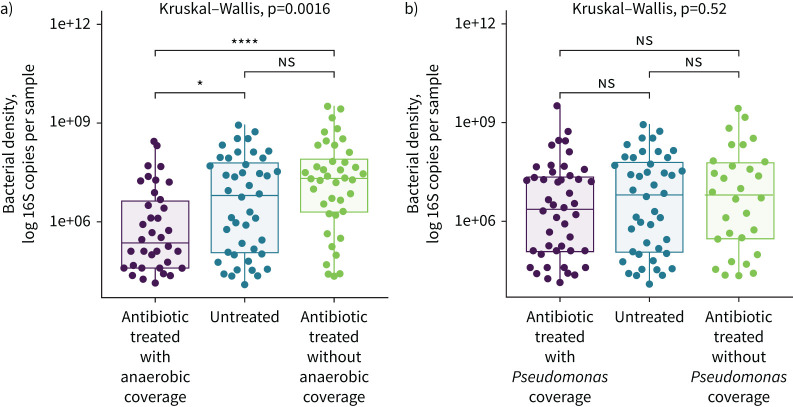
We first analysed the bacterial density of rectal swab specimens collected at admission using droplet digital PCR [50]. Although all 116 patients in the cohort eventually went on to receive antibiotic treatment, not all patients received antibiotics prior to their initial rectal swab collection at the time of admission. Therefore, we compared three groups: patients that did not receive antibiotics prior to their first swab collection (n=32), patients that received antibiotics with anti-anaerobic activity (n=44) and patients that received antibiotics without anaerobic activity (n=40). We found that bacterial density varied significantly across groups (p=0.0016). Patients treated with anti-anaerobic antibiotics had a lower admission bacterial density compared both with antibiotic-untreated patients (1.34 log 16S copies per sample; 95% CI for difference −1.39– −0.13 log 16S copies per sample; p=0.010) and with patients treated with antibiotics without anaerobic activity (1.82 log 16S copies per sample; 95% CI for difference −1.97– −0.78 log 16S copies per sample; p=1.5×10−8) (figure 5a). We concluded that anti-anaerobic antibiotic therapy is associated with a rapid and significant (1.34 log fold) decrease in the density of gut microbiota among hospitalised patients.
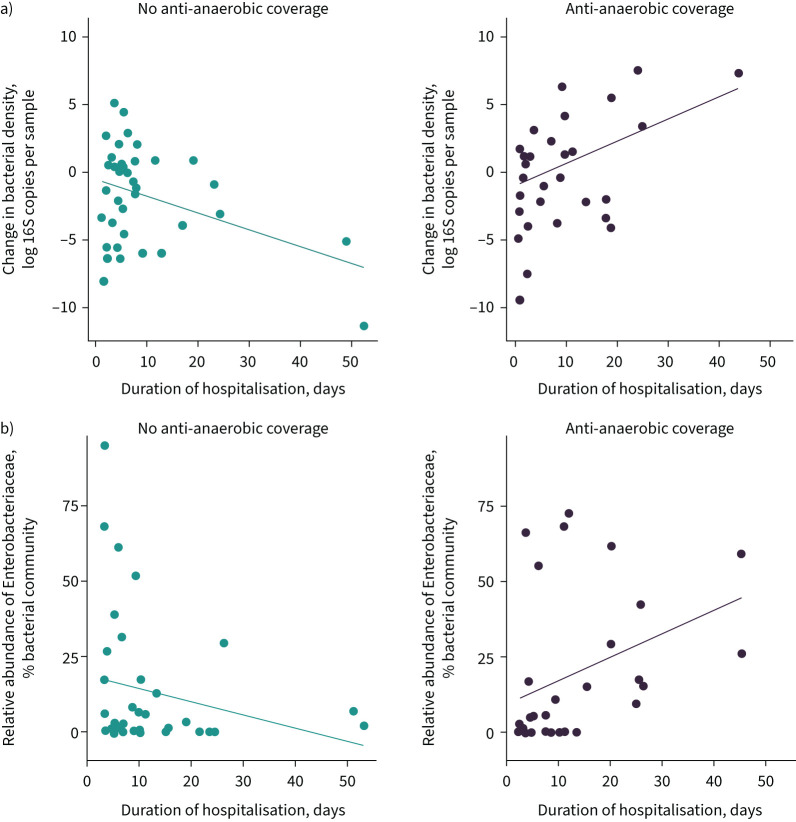
FIGURE 5.
Administration of antibiotics with anti-anaerobic activity is associated with decreased gut bacterial density among hospitalised patients. We compared the bacterial density of rectal swabs collected at the time of admission for a subset of 116 hospitalised patients and compared patients based on the spectrum of coverage of antibiotics administered in the emergency department. a) Patients who received antibiotics with anti-anaerobic activity had lower gut bacterial density than patients who did not receive antibiotics (95% CI for difference −1.39– −0.13 log 16S copies per sample; p=0.010 by t-test) or who received antibiotics without anaerobic activity (95% CI for difference −1.97– −0.78 log 16S copies per sample; p=1.5×10−8 by t-test). b) We found no significant association between spectrum of Pseudomonas coverage and bacterial density of admission rectal swab (Kruskal–Wallis test, p=0.52) nor any significant difference in mean bacterial density when comparing groups. ns: nonsignificant; *: p<0.05; ****: p<0.0001 after correcting for multiple comparisons with Tukey's method.
Piperacillin–tazobactam, an antibiotic with both anaerobic and broad-spectrum Gram-negative activity, was the most common antibiotic used as an anti-anaerobic treatment in our cohort. We therefore sought to determine if the effects of anti-anaerobic antibiotic therapy on gut microbiota were confounded by differences in broad-spectrum Gram-negative coverage. To accomplish this, we re-classified antibiotic exposure by spectrum of Gram-negative activity (figure 5b). Specifically, antibiotic exposure was classified by coverage of Pseudomonas aeruginosa, a common drug-resistant Gram-negative pathogen. We found no significant association between anti-pseudomonal coverage and bacterial density among hospitalised patients (p=0.52) nor any difference in density when comparing groups. We also found no association between anti-pseudomonal coverage and VAP-free survival, infection-free survival and overall survival. We thus concluded that anti-anaerobic coverage, and not Gram-negative coverage, is associated with reduction in gut bacterial density and adverse clinical outcomes.
We next sought to characterise the effects of early anti-anaerobic antibiotics on changes in gut microbiota during hospitalisation. To accomplish this, we measured the bacterial density and relative abundance of Enterobacteriaceae detected in rectal swabs at ICU admission and on a repeat rectal swab acquired later during hospitalisation. We focused on the relative abundance of Enterobacteriaceae spp. as these were the organisms that were enriched in VAP and fatal nosocomial infection. We built two mixed effects models, stratified by patient, to model the bacterial density and relative abundance of Enterobacteriaceae, respectively, over time. We included age, sex, race, treatment with anti-anaerobic antibiotics, Charlson Comorbidity Index and APACHE IV score as covariates in the model (figure 6 and table 7).
FIGURE 6.
Changes in gut microbiota during hospitalisation among patients with and without exposure to anti-anaerobic antibiotic treatment. We measured the bacterial density and relative abundance of Enterobacteriaceae spp. in a subset of 116 subjects with a rectal swab specimen collected at the time of intensive care unit (ICU) admission and ICU discharge. a) Patients treated with anti-anaerobic antibiotics exhibited an increase in gut bacterial density over time, while other antibiotic-treated patients exhibited a decrease in gut bacterial density over time (β for interaction 0.089 log 16S copies per day of hospitalisation; p=0.011). Mixed models were used for statistical analysis; change regression is depicted in this figure for ease of visualisation. b) Patients treated with anti-anaerobic antibiotics exhibited an increase in Enterobacteriaceae relative abundance over time (β for interaction 0.26% relative abundance per day of hospitalisation; p=0.042).
TABLE 7.
Coefficients for fixed effects in models of bacterial density, relative abundance of Enterobacteriaceae and absolute abundance of Enterobacteriaceae
| Coefficient (95% CI) | p-value | |
| Model: bacterial density# | ||
| Hospital day | −0.066 (−0.12–0.0059) | 0.029 |
| Anti-anaerobic treatment | −1.48 (−2.41– −0.59) | 0.0017 |
| Age | 0.026 (−0.0061–0.058) | 0.12 |
| Male | −0.38 (−1.28–0.51) | 0.42 |
| Non-Caucasian | −1.09 (−2.36–0.17) | 0.10 |
| APACHE IV score | −0.073 (−0.22–0.080) | 0.36 |
| Charlson Comorbidity Index | 0.22 (0.0010–0.44) | 0.058 |
| Hospital day×anti-anaerobic treatment | 0.089 (0.020–0.16) | 0.011 |
| Model: relative abundance of Enterobacteriaceae¶ | ||
| Hospital day | −0.19 (−0.41–0.032) | 0.099 |
| Anti-anaerobic treatment | −1.79 (−5.63–2.05) | 0.36 |
| Age | 0.043 (−0.12–0.21) | 0.62 |
| Male | 1.10 (−3.51–5.71) | 0.65 |
| Non-Caucasian | −0.19 (−6.73–6.36) | 0.96 |
| APACHE IV score | 0.36 (−0.43–1.14) | 0.39 |
| Charlson Comorbidity Index | 0.25 (−0.89–1.40) | 0.68 |
| Hospital day×anti-anaerobic treatment | 0.26 (0.0093–0.51) | 0.045 |
| Model: absolute abundance of Enterobacteriaceae+ | ||
| Hospital day | −0.17 (−0.28– −0.066) | 0.0018 |
| Anti-anaerobic treatment | −1.31 (−3.11–0.45) | 0.15 |
| Age | 0.085 (0.0030–0.17) | 0.048 |
| Male | 0.294 (−2.00–2.59) | 0.81 |
| Non-Caucasian | 0.597 (−2.63–3.81) | 0.72 |
| APACHE IV score | −0.033 (−0.44–0.37) | 0.88 |
| Charlson Comorbidity Index | −0.15 (−0.75–0.44) | 0.62 |
| Hospital day×anti-anaerobic treatment | 0.14 (0.0070–0.28) | 0.043 |
APACHE: Acute Physiology and Chronic Health Evaluation. #: the mixed effects regression modelled the bacterial density (log 16S copies per sample) by hospital day. The interaction term between hospital day and anti-anaerobic treatment represents the log change in bacterial density per hospital day in the anti-anaerobic-treated group compared with the nontreated group. ¶: the mixed effects regression modelled the relative abundance of Enterobacteriaceae (% of bacterial community) by hospital day. The interaction term between hospital day and anti-anaerobic treatment represents the relative abundance of Enterobacteriaceae per hospital day in the anti-anaerobic-treated group compared with the nontreated group. +: the mixed effects regression modelled the absolute abundance of Enterobacteriaceae (% of bacterial community) by hospital day. The interaction term between hospital day and anti-anaerobic treatment represents the absolute abundance of Enterobacteriaceae per hospital day in the anti-anaerobic-treated group compared with the nontreated group.
We first modelled bacterial density as a function of our covariates and discovered that overall, gut bacterial density decreased 0.066 log fold for every hospital day (95% CI −0.12– −0.00059; p=0.029). However, early administration of anti-anaerobic antibiotic therapy changed the relationship between time and bacterial density. Patients who received early anti-anaerobic antibiotics experienced increased bacterial density over time (0.089 for interaction, 95% CI 0.020–0.16; p=0.011) (table 7 and figure 6a). Similarly, while there was no strong association between time and relative abundance of Enterobacteriaceae in the overall patient population (−0.19, 95% CI −0.41–0.030; p=0.10), patients with early anti-anaerobic antibiotic treatment had increasing abundance of Enterobacteriaceae over time (0.26 for interaction, 95% CI 0.00931–0.51; p=0.045) (table 7 and figure 6b). We concluded that early anti-anaerobic antibiotic treatment was predictive both of increasing gut bacterial density during hospitalisation as well as an increased relative abundance of Enterobacteriaceae spp.
Having discovered that patients treated with anti-anaerobic antibiotics had an increase in relative abundance of Enterobacteriaceae throughout hospitalisation, we sought to determine if this was due to a depletion of other taxa or expansion of Enterobacteriaceae. To accomplish this, we modelled the absolute abundance of Enterobacteriaceae, calculated as total bacterial density multiplied by the relative abundance of Enterobacteriaceae. We discovered that overall, the absolute abundance of Enterobacteriaceae decreased 0.17 log fold for every hospital day (95% CI −0.28– −0.066; p=0.0018) (table 7). We noted, again, that early anti-anaerobic antibiotic treatment changed the relationship between time and absolute abundance of Enterobacteriaceae, with anti-anaerobic antibiotic treatment being associated with a 0.14 log increase in absolute abundance of Enterobacteriaceae for every hospital day (95% CI 0.007–0.279; p=0.043). We concluded that the increase in the relative abundance of Enterobacteriaceae was due to an expansion and bloom of Enterobacteriaceae, and not a depletion of commensal gut anaerobes.
In murine models, gut anaerobe depletion increases susceptibility to Enterobacteriaceae pneumonia and increases lethality of hyperoxia
Given that our human observational data were vulnerable to selection bias and confounding by indication, we next sought to confirm our findings and interrogate the biology underlying our observation that anti-anaerobic antibiotics increase the risk of VAP and mortality using experimental murine modelling. Informed by prior literature, we hypothesised that 1) anti-anaerobic antibiotics cause decreased bacterial clearance and altered alveolar immunity in an infectious model [14], and 2) anti-anaerobic antibiotics augment the pulmonary and systemic toxicity of a noninfectious model (hyperoxia) [16]. We compared mice treated with piperacillin–tazobactam or cefepime, as these antibiotics were common in our human cohort and have a similar spectrum of activity, but differ in their anaerobic coverage (present in piperacillin–tazobactam, absent in cefepime). As an infectious model, we used intratracheal instillation of K. pneumoniae (as Enterobacteriaceae were the most enriched family of pathogens in patients treated with anti-anaerobic antibiotics) and S. aureus (as it was the most enriched pathogen among patients not treated with anti-anaerobic antibiotics). As a noninfectious model, we used hyperoxia, which is a common ICU exposure [66], causes lung injury and multi-organ failure in animal models [67, 68], and is modulated in part by the microbiome [16].
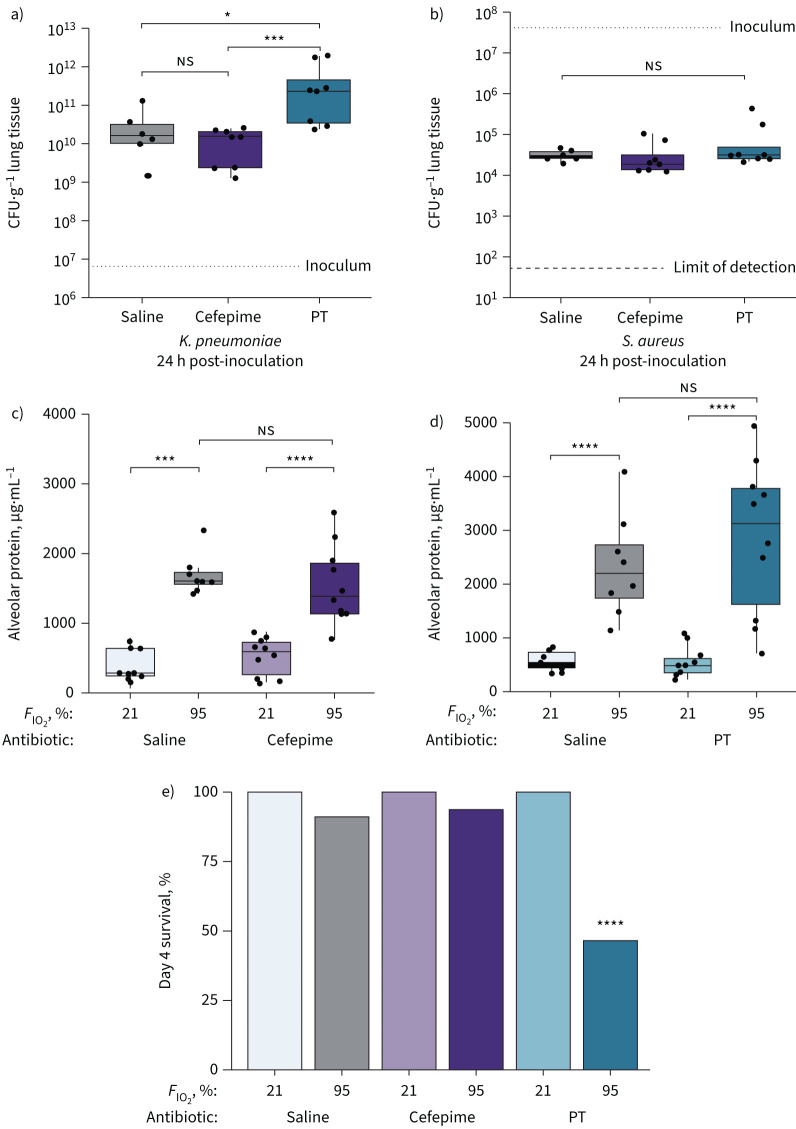
In our pneumonia model, we found that pre-treatment with anti-anaerobic antibiotics (piperacillin–tazobactam) resulted in decreased clearance of K. pneumoniae 24 h following inoculation, both compared with mice pre-treated with saline (control) and cefepime (which lacks anti-anaerobic activity) (figure 7). In contrast, pre-treatment with piperacillin–tazobactam had no effect on S. aureus clearance. We thus concluded that anti-anaerobic antibiotic therapy results in impaired bacterial clearance in a taxonomically specific manner that is aligned with our observational human data (figure 4).
FIGURE 7.
The effects of gut anaerobe depletion on infectious and noninfectious lung injury in mice. We experimentally modulated gut microbiota in female C57BL/6 mice using intraperitoneal piperacillin–tazobactam (PT) (anti-anaerobic), cefepime (not anti-anaerobic) and saline sham controls. We compared alveolar inflammation and bacterial clearance in a, b) two infectious models (intratracheal Klebsiella pneumoniae and Staphylococcus aureus) and c–e) a noninfectious model (hyperoxia). a, b) In the pneumonia models, gut anaerobe depletion decreased clearance of a) K. pneumoniae but not b) S. aureus. In the hyperoxia model, gut anaerobe depletion had no significant effect on c, d) day 3 lung injury but e) increased day 4 mortality. FIO2: inspiratory oxygen fraction. ns: nonsignificant; *: p<0.05; ***: p<0.001; ****: p<0.0001. Significance determined using Kruskal–Wallis rank sum test with Dunn's multiple comparisons test.
In our hyperoxia model, we measured lung injury and survival in mice exposed to 3–4 days of inspiratory oxygen fraction (FIO2) 95%. Following 3 days of hyperoxia, piperacillin–tazobactam-treated mice did not differ from cefepime- or saline-treated mice in alveolar protein or alveolar IgM (indices of capillary leak) (figure 7). However, following 4 days of hyperoxia (at which time-point we have previously demonstrated that ceftriaxone results in augmented lung injury [16]), we observed a marked increase in mortality among piperacillin–tazobactam-treated mice compared with cefepime- and saline-treated mice (p=0.00020).
Taken together, we concluded that while gut anaerobe depletion (via piperacillin–tazobactam) has no effect on bacterial clearance in S. aureus pneumonia or early oxygen-induced lung injury, it has deleterious effects both on experimental Enterobacteriaceae pneumonia and the lethality of hyperoxia.
Discussion
The core finding of this study is that among critically ill patients, early treatment with anti-anaerobic antibiotics is associated with decreased VAP-free, infection-free and overall survival. While anti-anaerobic antibiotics were associated with disruption of gut microbiota and alteration of respiratory microbiology, our human and animal findings both suggest that their mortality risk is incompletely explained by infection-related outcomes alone.
To the best of our knowledge, our study is the first to demonstrate that anti-anaerobic antibiotic therapy is associated with adverse outcomes in critically ill patients. While prior antibiotic exposure is a known risk factor for VAP [69–71], specific classes have not been implicated. Contemporary studies have suggested that the prevalence of anaerobic pathogens among hospitalised patients is far less than previously thought [17, 19]. We thus believe the widespread empiric use of anti-anaerobic antibiotics in hospitalised patients should be reconsidered.
While our core findings are novel, they are consistent with prior experimental and observational findings. Animal modelling has revealed that depletion of gut anaerobes increases susceptibility to pneumococcal pneumonia [14] and anti-anaerobic antibiotic therapy has previously been associated with expansion of enteric pathogens in hospitalised patients [72, 73]. Contemporary, culture-independent studies have demonstrated that piperacillin–tazobactam, the most prescribed anti-anaerobic antibiotic in our cohort, is particularly disruptive to gut microbiota [20, 50, 74, 75]. We have previously demonstrated that treatment with ceftriaxone (which depletes gut anaerobes [76–79]) increases the severity of hyperoxic lung injury [16]. Exposure to anti-anaerobic antibiotics is predictive of adverse clinical outcomes in haematopoietic stem cell transplant recipients [20] and ICU survivors [80]. Thus the experimental and observational evidence supporting a protective role of gut anaerobes across disease states and species is robust.
While anti-anaerobic antibiotic exposure was associated with increased risk of VAP and nosocomial infection, overall differences in survival could not be explained by increased infection rates alone. Most deaths were due to noninfectious causes in both treatment groups and the overall survival difference (7% unadjusted and 3.9% adjusted) exceeds the anticipated 1% attributable mortality of VAP [81]. Our animal experimentation similarly showed that anaerobe depletion had both a direct effect of increased susceptibility to Enterobacteriaceae pneumonia and markedly increased lethality of hyperoxia. These findings parallel results from the randomised clinical trials of anaerobe-sparing SDD, which has a mortality benefit that exceeds the attributable mortality of VAP [11, 63, 64]. These findings suggest that the protective effects of gut anaerobes are multifaceted and do not simply represent colonisation resistance against potential pathogens.
Patients treated with anti-anaerobic antibiotics exhibited both domination of gut microbiota by Enterobacteriaceae spp. during hospitalisation as well as enrichment of Enterobacteriaceae spp. among culture-confirmed VAP pathogens. In two recent studies, enrichment of Enterobacteriaceae within the lung microbiome of mechanically ventilated patients was associated with acute respiratory distress syndrome and predictive of adverse clinical outcomes [82–84]. These findings, considered with recent experimental and human evidence [85], suggest that translocation of gut bacteria is one mechanism by which anaerobe depletion may contribute to VAP, lung injury and extrapulmonary organ failure in critical illness.
Our retrospective, observational human study was vulnerable to selection bias and confounding by indication. However, our murine experimentation, with its controlled experimental design, was not vulnerable to clinical confounding. We believe that our murine modelling recapitulated both the added mortality effect of anti-anaerobic antibiotics in addition to the increased vulnerability to Enterobacteriaceae infection. Our results are also consistent with numerous observational and experimental studies. Despite this, we believe that prospective, randomised controlled trials would be necessary to definitively establish the harm of gut anaerobe depletion among critically ill patients, and additional experimental and translational inquiry will be necessary to determine the mechanisms by which gut anaerobes provide protection against organ failure and mortality in critically ill patients.
Conclusions
In critically ill patients, the use of anti-anaerobic antibiotics is associated with decreased VAP-free, infection-free and overall survival. Both observational clinical data and animal modelling suggest that this mortality risk is not solely attributable to increased rates of infection. Further inquiry is needed to interrogate the mechanisms underlying this mortality risk. Clinically, the widespread use of anti-anaerobic antibiotics in hospitalised patients should be reconsidered given clinical and experimental evidence of harm.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00910-2022.Supplement (879.8KB, pdf)
Shareable PDF
Footnotes
Author contributions: R. Chanderraj, J.M. Baker, N.R. Falkowski, M.W. Sjoding and R.P. Dickson designed the analyses and experiments. R. Chanderraj and S.G. Kay performed clinical data abstraction, adjudication and model building. R. Chanderraj, N.R. Falkowski and K.J. Hinkle performed DNA extraction, 16S rRNA gene amplicon sequencing and droplet digital PCR quantification of rectal swab specimens. R. Chanderraj and C.A. Brown performed 16S rRNA analysis of rectal swab specimens. J.M. Baker, N.R. Falkowski, D.J. Fergle, R.A. McDonald and J.D. Metcalf performed the murine experiments, and J.M. Baker analysed the murine data. R. Chanderraj wrote the first draft and all authors participated in revision of the manuscript. All authors approved the final version of the manuscript.
Data availability: The dataset supporting the results of this article has been posted to the NIH Sequence Read Archive (accession number PRJNA633879). Operational taxonomic unit tables, taxonomy classification tables and metadata tables are available at https://github.com/rishichanderraj/RectalSwab_BacterialDensity.
Conflict of interest: All authors have nothing to disclose.
Support statement: This work was supported by the National Institutes of Health (R01HL144599 to R.P. Dickson, T32HL007749 to R. Chanderraj and R.P. Dickson, 1F31HL158033 to J.M. Baker, R01LM013325 and K01HL136687 to M.W. Sjoding, and R01AI143852 to R.J. Woods). This manuscript does not represent the views of the Department of Veterans Affairs or the US Government. This material is the result of work supported with resources and use of facilities at the Ann Arbor VA Medical Center (Ann Arbor, MI, USA). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Bonten MJ. Ventilator-associated pneumonia and the gastropulmonary route of infection: a pendulum. Am J Respir Crit Care Med 2011; 184: 991–993. doi: 10.1164/rccm.201108-1537ED [DOI] [PubMed] [Google Scholar]
- 2.Bonten MJ, Gaillard CA, de Leeuw PW, et al. Role of colonization of the upper intestinal tract in the pathogenesis of ventilator-associated pneumonia. Clin Infect Dis 1997; 24: 309–319. doi: 10.1093/clinids/24.3.309 [DOI] [PubMed] [Google Scholar]
- 3.Ewig S, Torres A, El-Ebiary M, et al. Bacterial colonization patterns in mechanically ventilated patients with traumatic and medical head injury. Incidence, risk factors, and association with ventilator-associated pneumonia. Am J Respir Crit Care Med 1999; 159: 188–198. doi: 10.1164/ajrccm.159.1.9803097 [DOI] [PubMed] [Google Scholar]
- 4.Papadomichelakis E, Kontopidou F, Antoniadou A, et al. Screening for resistant gram-negative microorganisms to guide empiric therapy of subsequent infection. Intensive Care Med 2008; 34: 2169–2175. doi: 10.1007/s00134-008-1247-9 [DOI] [PubMed] [Google Scholar]
- 5.Gorrie CL, Mirceta M, Wick RR, et al. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin Infect Dis 2017; 65: 208–215. doi: 10.1093/cid/cix270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickson RP. The microbiome and critical illness. Lancet Respir Med 2016; 4: 59–72. doi: 10.1016/S2213-2600(15)00427-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clasener HA, Vollaard EJ, van Saene HK. Long-term prophylaxis of infection by selective decontamination in leukopenia and in mechanical ventilation. Rev Infect Dis 1987; 9: 295–328. doi: 10.1093/clinids/9.2.295 [DOI] [PubMed] [Google Scholar]
- 8.van der Waaij D. History of recognition and measurement of colonization resistance of the digestive tract as an introduction to selective gastrointestinal decontamination. Epidemiol Infect 1992; 109: 315–326. doi: 10.1017/S0950268800050317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Waaij D, Berghuis-de Vries JM, Lekkerkerk L-V. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg 1971; 69: 405–411. doi: 10.1017/S0022172400021653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wittekamp BHJ, Oostdijk EAN, Cuthbertson BH, et al. Selective decontamination of the digestive tract (SDD) in critically ill patients: a narrative review. Intensive Care Med 2020; 46: 343–349. doi: 10.1007/s00134-019-05883-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minozzi S, Pifferi S, Brazzi L, et al. Topical antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving mechanical ventilation. Cochrane Database Syst Rev 2021; 1: CD000022. doi: 10.1002/14651858.CD000022.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuevas P, De la Maza LM, Gilbert J, et al. The lung lesion in four different types of shock in rabbits. Arch Surg 1972; 104: 319–322. doi: 10.1001/archsurg.1972.04180030067015 [DOI] [PubMed] [Google Scholar]
- 13.Souza DG, Vieira AT, Soares AC, et al. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol 2004; 173: 4137–4146. doi: 10.4049/jimmunol.173.6.4137 [DOI] [PubMed] [Google Scholar]
- 14.Schuijt TJ, Lankelma JM, Scicluna BP, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 2016; 65: 575–583. doi: 10.1136/gutjnl-2015-309728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Dwyer DN, Ashley SL, Gurczynski SJ, et al. Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am J Respir Crit Care Med 2019; 199: 1127–1138. doi: 10.1164/rccm.201809-1650OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashley SL, Sjoding MW, Popova AP, et al. Lung and gut microbiota are altered by hyperoxia and contribute to oxygen-induced lung injury in mice. Sci Transl Med 2020; 12: eaau9959. doi: 10.1126/scitranslmed.aau9959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kioka MJ, DiGiovine B, Rezik M, et al. Anaerobic antibiotic usage for pneumonia in the medical intensive care unit. Respirology 2017; 22: 1656–1661. doi: 10.1111/resp.13111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vedamurthy A, Rajendran I, Manian F. Things we do for no reason: routine coverage of anaerobes in aspiration pneumonia. J Hosp Med 2020; 15: 754–756. doi: 10.12788/jhm.3506 [DOI] [PubMed] [Google Scholar]
- 19.Marin-Corral J, Pascual-Guardia S, Amati F, et al. Aspiration risk factors, microbiology, and empiric antibiotics for patients hospitalized with community-acquired pneumonia. Chest 2021; 159: 58–72. doi: 10.1016/j.chest.2020.06.079 [DOI] [PubMed] [Google Scholar]
- 20.Shono Y, Docampo MD, Peled JU, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med 2016; 8: 339ra371. doi: 10.1126/scitranslmed.aaf2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. J Am Dent Assoc 2008; 139: Suppl. 1, 3S–24S. doi: 10.14219/jada.archive.2008.0346 [DOI] [PubMed] [Google Scholar]
- 22.Smiley CJ, Tracy SL, Abt E, et al. Evidence-based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc 2015; 146: 525–535. doi: 10.1016/j.adaj.2015.01.026 [DOI] [PubMed] [Google Scholar]
- 23.Brook I. Microbiology and principles of antimicrobial therapy for head and neck infections. Infect Dis Clin North Am 2007; 21: 355–391. doi: 10.1016/j.idc.2007.03.014 [DOI] [PubMed] [Google Scholar]
- 24.Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012; 54: e132–e173. doi: 10.1093/cid/cis346 [DOI] [PubMed] [Google Scholar]
- 25.Stevens DL, Bisno AL, Chambers HF, et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America. New York, Oxford University Press, 2014; pp. e10–e52. [DOI] [PubMed] [Google Scholar]
- 26.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 2010: 50: 133–164. doi: 10.1086/649554 [DOI] [PubMed] [Google Scholar]
- 27.Nseir S, Zerimech F, Fournier C, et al. Continuous control of tracheal cuff pressure and microaspiration of gastric contents in critically ill patients. Am J Respir Crit Care Med 2011; 184: 1041–1047. doi: 10.1164/rccm.201104-0630OC [DOI] [PubMed] [Google Scholar]
- 28.Nseir S, Lorente L, Ferrer M, et al. Continuous control of tracheal cuff pressure for VAP prevention: a collaborative meta-analysis of individual participant data. Ann Intensive Care 2015: 5: 43. doi: 10.1186/s13613-015-0087-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klompas M, Khan Y, Kleinman K, et al. Multicenter evaluation of a novel surveillance paradigm for complications of mechanical ventilation. PLoS One 2011; 6: e18062. doi: 10.1371/journal.pone.0018062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klompas M, Kleinman K, Khan Y, et al. Rapid and reproducible surveillance for ventilator-associated pneumonia. Clin Infect Dis 2012; 54: 370–377. doi: 10.1093/cid/cir832 [DOI] [PubMed] [Google Scholar]
- 31.Magill SS, Klompas M, Balk R, et al. Developing a new, national approach to surveillance for ventilator-associated events. Am J Crit Care 2013; 22: 469–473. doi: 10.4037/ajcc2013893 [DOI] [PubMed] [Google Scholar]
- 32.Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology 2013; 57: 1651–1653. doi: 10.1002/hep.26359 [DOI] [PubMed] [Google Scholar]
- 33.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52: e103–e120. doi: 10.1093/cid/ciq257 [DOI] [PubMed] [Google Scholar]
- 34.Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010; 50: 625–663. doi: 10.1086/650482 [DOI] [PubMed] [Google Scholar]
- 35.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36: 309–332. doi: 10.1016/j.ajic.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 36.Michigan Department of Health and Human Services . Genealogical Death Indexing System. 2019. https://vitalstats.michigan.gov/osr/gendisx/index.asp Date last accessed: 1 October 2022.
- 37.National Center for Health Statistics . Data Access – National Death Index. 2019. www.cdc.gov/nchs/ndi/index.htm Date last accessed: 1 October 2022.
- 38.National Center for Health Statistics . Section I – Instructions for Classifying the Underlying Cause of Death. 2017. www.cdc.gov/nchs/nvss/index.htm Date last accessed: 1 October 2022.
- 39.Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959–2017. JAMA 2019; 322: 1996–2016. doi: 10.1001/jama.2019.16932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, R Foundation for Statistical Computing, 2019. [Google Scholar]
- 41.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 42.Radovanovic D, Seifert B, Urban P, et al. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002–2012. Heart 2014; 100: 288–294. doi: 10.1136/heartjnl-2013-304588 [DOI] [PubMed] [Google Scholar]
- 43.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173: 676–682. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 44.Zimmerman JE, Kramer AA, McNair DS, et al. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med 2006; 34: 1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0 [DOI] [PubMed] [Google Scholar]
- 45.Therneau T. A package for survival analysis in R. 2015. https://CRAN.R-project.org/package=survival Date last accessed: 1 October 2022.
- 46.Leeper TJ. Margins: marginal effects for model objects. R package version 0.3.26. 2021. https://github.com/leeper/margins Date last accessed: 1 October 2022.
- 47.Gray RJ. A class of $k$-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154. [Google Scholar]
- 48.Gray R. cmprsk. 2.2-11 ed. 2021. https://cran.r-project.org/web/packages/cmprsk/index.html Date last accessed: 1 October 2022.
- 49.Chanderraj R, Brown CA, Hinkle K, et al. Gut microbiota predict enterococcus expansion but not vancomycin-resistant Enterococcus acquisition. mSphere 2020; 5: e00537-20. doi: 10.1128/mSphere.00537-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chanderraj R, Brown CA, Hinkle K, et al. The bacterial density of clinical rectal swabs is highly variable, correlates with sequencing contamination, and predicts patient risk of extraintestinal infection. Microbiome 2022; 10: 2. doi: 10.1186/s40168-021-01190-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kozich JJ, Westcott SL, Baxter NT, et al. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 2013; 79: 5112–5120. doi: 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schloss PD. MiSeq SOP:mothur. 2019. https://mothur.org/wiki/miseq_sop Date last accessed: 1 October 2022.
- 53.Sze MA, Abbasi M, Hogg JC, et al. A comparison between droplet digital and quantitative PCR in the analysis of bacterial 16S load in lung tissue samples from control and COPD GOLD 2. PLoS One 2014; 9: e110351. doi: 10.1371/journal.pone.0110351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bates D, Machler M, Bolker BM, et al. Fitting linear mixed-effects models using lme4. J Stat Softw 2015; 67: 1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 55.Bachman MA, Breen P, Deornellas V, et al. Genome-wide identification of Klebsiella pneumoniae fitness genes during lung infection. mBio 2015; 6: e00775-15. doi: 10.1128/mBio.00775-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dickson RP, Erb-Downward JR, Falkowski NR, et al. The lung microbiota of healthy mice are highly variable, cluster by environment, and reflect variation in baseline lung innate immunity. Am J Respir Crit Care Med 2018; 198: 497–508. doi: 10.1164/rccm.201711-2180OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baker JM, Hinkle KJ, McDonald RA, et al. Whole lung tissue is the preferred sampling method for amplicon-based characterization of murine lung microbiota. Microbiome 2021; 9: 99. doi: 10.1186/s40168-021-01055-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kollef M, Pittet D, Sanchez Garcia M, et al. A randomized double-blind trial of iseganan in prevention of ventilator-associated pneumonia. Am J Respir Crit Care Med 2006; 173: 91–97. doi: 10.1164/rccm.200504-656OC [DOI] [PubMed] [Google Scholar]
- 59.Weng H, Li JG, Mao Z, et al. Probiotics for preventing ventilator-associated pneumonia in mechanically ventilated patients: a meta-analysis with trial sequential analysis. Front Pharmacol 2017; 8: 717. doi: 10.3389/fphar.2017.00717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fine J, Frank H, Schweinburg F, et al. The bacterial factor in traumatic shock. Ann NY Acad Sci 1952; 55: 429–445. doi: 10.1111/j.1749-6632.1952.tb26558.x [DOI] [PubMed] [Google Scholar]
- 61.Rush BF Jr, Redan JA, Flanagan JJ Jr, et al. Does the bacteremia observed in hemorrhagic shock have clinical significance? A study in germ-free animals. Ann Surg 1989; 210: 342–345. doi: 10.1097/00000658-198909000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang D, Chen G, Manwani D, et al. Neutrophil ageing is regulated by the microbiome. Nature 2015; 525: 528–532. doi: 10.1038/nature15367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oostdijk EAN, Kesecioglu J, Schultz MJ, et al. Effects of decontamination of the oropharynx and intestinal tract on antibiotic resistance in ICUs: a randomized clinical trial. JAMA 2014; 312: 1429–1437. doi: 10.1001/jama.2014.7247 [DOI] [PubMed] [Google Scholar]
- 64.Roquilly A, Marret E, Abraham E, et al. Pneumonia prevention to decrease mortality in intensive care unit: a systematic review and meta-analysis. Clin Infect Dis 2015; 60: 64–75. doi: 10.1093/cid/ciu740 [DOI] [PubMed] [Google Scholar]
- 65.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; 43: 304–377. doi: 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 66.Tyagi S, Brown CA, Dickson RP, et al. Outcomes and predictors of severe hyperoxemia in patients receiving mechanical ventilation: a single-center cohort study. Ann Am Thoracic Soc 2022; 19: 1338–1345. doi: 10.1513/AnnalsATS.202107-804OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frank L, Bucher JR, Roberts RJ. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol Respir Environ Exerc Physiol 1978; 45: 699–704. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez-Gonzalez R, Martin-Barrasa JL, Ramos-Nuez A, et al. Multiple system organ response induced by hyperoxia in a clinically relevant animal model of sepsis. Shock 2014; 42: 148–153. doi: 10.1097/SHK.0000000000000189 [DOI] [PubMed] [Google Scholar]
- 69.Kollef MH. Ventilator-associated pneumonia. A multivariate analysis. JAMA 1993; 270: 1965–1970. doi: 10.1001/jama.1993.03510160083034 [DOI] [PubMed] [Google Scholar]
- 70.Trouillet JL, Chastre J, Vuagnat A, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med 1998; 157: 531–539. doi: 10.1164/ajrccm.157.2.9705064 [DOI] [PubMed] [Google Scholar]
- 71.Ranjan N, Chaudhary U, Chaudhry D, et al. Ventilator-associated pneumonia in a tertiary care intensive care unit: analysis of incidence, risk factors and mortality. Indian J Crit Care Med 2014; 18: 200–204. doi: 10.4103/0972-5229.130570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nord CE, Heimdahl A, Kager L, et al. The impact of different antimicrobial agents on the normal gastrointestinal microflora of humans. Rev Infect Dis 1984; 6: Suppl. 1, S270–S275. doi: 10.1093/clinids/6.Supplement_1.S270 [DOI] [PubMed] [Google Scholar]
- 73.Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000; 343: 1925–1932. doi: 10.1056/NEJM200012283432604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haak BW, Argelaguet R, Kinsella CM, et al. Integrative transkingdom analysis of the gut microbiome in antibiotic perturbation and critical illness. mSystems 2021; 6: e01148-20. doi: 10.1128/mSystems.01148-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pettigrew MM, Gent JF, Kong Y, et al. Gastrointestinal microbiota disruption and risk of colonization with carbapenem-resistant Pseudomonas aeruginosa in intensive care unit patients. Clin Infect Dis 2019; 69: 604–613. doi: 10.1093/cid/ciy936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cleeland R, Squires E. Antimicrobial activity of ceftriaxone: a review. Am J Med 1984; 77: 3–11. [PubMed] [Google Scholar]
- 77.Roberts SA, Shore KP, Paviour SD, et al. Antimicrobial susceptibility of anaerobic bacteria in New Zealand: 1999–2003. J Antimicrob Chemother 2006; 57: 992–998. doi: 10.1093/jac/dkl052 [DOI] [PubMed] [Google Scholar]
- 78.van Ogtrop ML, Guiot HF, Mattie H, et al. Modulation of the intestinal flora of mice by parenteral treatment with broad-spectrum cephalosporins. Antimicrob Agents Chemother 1991; 35: 976–982. doi: 10.1128/AAC.35.5.976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sullivan A, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis 2001; 1: 101–114. doi: 10.1016/S1473-3099(01)00066-4 [DOI] [PubMed] [Google Scholar]
- 80.Baggs J, Jernigan JA, Halpin AL, et al. Risk of subsequent sepsis within 90 days after a hospital stay by type of antibiotic exposure. Clin Infect Dis 2018; 66: 1004–1012. doi: 10.1093/cid/cix947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bekaert M, Timsit JF, Vansteelandt S, et al. Attributable mortality of ventilator-associated pneumonia: a reappraisal using causal analysis. Am J Respir Crit Care Med 2011; 184: 1133–1139. doi: 10.1164/rccm.201105-0867OC [DOI] [PubMed] [Google Scholar]
- 82.Dickson RP, Schultz MJ, van der Poll T, et al. Lung microbiota predict clinical outcomes in critically ill patients. Am J Respir Crit Care Med 2020; 201: 555–563. doi: 10.1164/rccm.201907-1487OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Panzer AR, Lynch SV, Langelier C, et al. Lung microbiota is related to smoking status and to development of acute respiratory distress syndrome in critically ill trauma patients. Am J Respir Crit Care Med 2018; 197: 621–631. doi: 10.1164/rccm.201702-0441OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitsios GD, Yang H, Yang L, et al. Respiratory tract dysbiosis is associated with worse outcomes in mechanically-ventilated patients. Am J Respir Crit Care Med 2020; 202: 1666–1677. doi: 10.1164/rccm.201912-2441OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singer BH, Dickson RP, Denstaedt SJ, et al. Bacterial dissemination to the brain in sepsis. Am J Respir Crit Care Med 2018; 197: 747–756. doi: 10.1164/rccm.201708-1559OC [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00910-2022.Supplement (879.8KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00910-2022.Shareable (640.1KB, pdf)