Abstract
Background
Previous studies have shown systemic lupus erythematosus (SLE) patients had a significantly higher prevalence of thyroid diseases and hypothyroidism than matched controls, and some case reports showed SLE may occur after Hashimoto’s thyroiditis (HT).
Objective
This study aimed to investigate the subsequent risk of SLE in patients with HT.
Methods
In this retrospective cohort study done by the Taiwan National Health Insurance Research Database, the HT group (exposure group) and the non-HT group (comparator group) were propensity score matched at a ratio of 1:2 by demographic data, comorbidities, medications, and the index date. We used Cox proportional hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Several sensitivity analyses were done for cross-validation of our findings.
Results
We identified 15,512 HT patients and matched 31,024 individuals. The incidence rate ratio of SLE was 3.58 (95% CI, 2.43–5.28; p < 0.01). Several sensitivity analyses show adjusted hazard ratio (aHR) (CIs) of 4.35 (3.28–5.76), 4.39 (3.31–5.82), 5.11 (3.75–6.98), and 4.70 (3.46–6.38), consistent with the results of the main model.
Conclusion
Our study showed an increased risk of SLE in the HT group after adjustment for baseline characteristics, comorbidities, and medical confounders compared with the reference group.
Keywords: Hashimoto’s thyroiditis, Systemic lupus erythematosus, Retrospective cohort study
Introduction
Systemic lupus erythematosus (SLE) is a systematic autoimmune disease affecting almost every organ, in which the immune system attacks tissues and cells leading to inflammation and damage [1]. Previous studies have shown that both the loss of B cell self-tolerance due to genetic factor and Th1 lymphocyte response are necessary for the development of SLE [2–4]. However, some studies indicated that genetic susceptibility alone is not sufficient to account for all SLE patients, with only 24% concordance within monozygotic twins with SLE, which is much lower than previous estimate [5]. Another study revealed that environmental exposures are more related to SLE. In this study, a higher concordance of autoimmune disease, including SLE, was presented among monozygotic twins than among dizygotic twins, which was explained as environment factors that monozygotic twins usually share are more similar than dizygotic do [6], rather than just considered as a genetic similarity. The environment factors for SLE include but not limited to ultraviolet radiation, infection, and hormone [7]. Besides, having a history of other autoimmune diseases is also a risk factor for another one, according to some studies about multiple autoimmune syndrome (MAS) [8, 9].
Studies also revealed that T3 and T4 act as modulators in the immune system [10, 11]. Thyroid hormone not only stimulates T cells, B cells [12, 13], neutrophils, and macrophage chemotaxis [14, 15] but also enhances the generation of ROS and IL-18 [16]. Despite that abundant studies about T3 and T4 alternating the immune status, we now only noticed that SLE patients are more likely to develop autoimmune thyroiditis or even hypothyroidism in some cohort study [2, 17], and there is no definite evidence that make sure whether hypothyroidism will cause SLE so far in spite of two case reports about two young girls and two women respectively evolving SLE after being diagnosed as hypothyroidism and Hashimoto’s thyroiditis (HT) [18, 19].
Based on the concept mentioned above, we hypothesized that a history of HT increases the risk of subsequent SLE, and, as the epidemiological correlation between HT and SLE has remained unclear, we designed a retrospective cohort study to investigate this issue.
Materials and methods
Method
Study design
In this national wide, retrospective cohort study with propensity score matched (PSM), the data was extracted from the National Health Insurance Research Database (NHIRD), which is a database constructed by 95% of residents in Taiwan since 1995 through the National health care insurance (NHI) system. Disease profiles are based on the International Classification of Diseases, 9th Revision, and Clinical Modification (ICD-9-CM) systems; the NHIRD provides information including demographics, outpatient visits, and hospitalizations with dates, prescriptions codes, diagnostic codes, laboratory tests and interventional procedure codes, and medical costs. De-identification was done for the protection of personal privacy.
We also extract the major illness registry data, also called the catastrophic illness registry, which is a certification for patients who were diagnosed with some severe, chronic, or fatal disease including cancer, diabetes mellitus, major injury, and SLE. The certification provides a discount on admission and medical charge. The Longitudinal Health Insurance Database 2000 (LHID 2000) was also used in the comparator group without HT of this study; LHID 2000 has collected a population of 1 million which was randomly sampled from the beneficiaries’ registration files within the year 2000. The representative of gender and age distribution has been statistically confirmed between the LHID 2000 and the origin NHIRD data.
Study population and propensity score match
We identified our population as patients who have had at least three outpatient visits or one hospital admission for autoimmune thyroiditis (Hashimoto’s thyroiditis) (ICD9 code: 245.2) between 2003 and 2012. The index date for the corresponding matching was defined as the date of the first diagnosis of autoimmune thyroiditis, outpatient, or admission.
We also constructed a comparator group without HT sampled from the LHID 2000 data, which includes patients ever visited outpatient departments between 2005 and 2012. The index date of the comparator group without HT was defined as the first visit to the outpatient department each year. Those who ever had at least one outpatient visit plus one hospitalization under the diagnosis of disorders of the thyroid gland (ICD9 codes 240–246: 240 for simple and unspecified goiter, 241 for nontoxic nodular goiter, 242 for thyrotoxicosis with or without goiter, 243 for congenital hypothyroidism, 244 for acquired hypothyroidism, 245 for thyroiditis, 246 for other disorders of the thyroid) between 1997 and 2013 were excluded.
We excluded the data of index date which were unmatched among the study and comparator group without HT (2003–2004), SLE (ICD9 code: 710.0) diagnosed before the index date and death during follow-up.
Overall, we extracted a total population of 17,978 cases with HT between the years 2005 and 2012 as our study group; and the comparator group without HT contained 783,345 patients without any disorders of the thyroid gland. To reduce the confounding bias, propensity score matching (PSM) was used, which was estimated by logistic regression modeling. Predictors involved index date, gender, and selected co-morbidities. The 1:2 matched comparator shares the same propensity score as the exposure group.
Outcomes and comorbidities
The primary outcome of the study was SLE occurrence, which was defined as patients who were diagnosed with SLE (ICD9 code: 710.0) and were identified as having “major illness” according to the NHI document for ensuring only correctly diagnosed patients were included. The follow-up started on the respective index date for different individuals until SLE was diagnosed or withdrawn from NHI due to any cause such as death, leaving, loss of data, or end of the study (December 2013), whichever occurred first. Relevant data of background variation including gender, age, urbanization, low income, length of hospital stays, times of outpatient department visits, medication control, and co-morbidities were also extracted and listed in Table 1.
Table 1.
Baseline characteristics among the Hashimoto’s thyroiditis group and non-Hashimoto’s thyroiditis group
| Before PSM (1:20 age matching) | 1:2 PSM | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-Hashimoto’s thyroiditis, n = 315,020 | Hashimoto’s thyroiditis, n = 15,751 | p value | ASD | Non-Hashimoto’s thyroiditis, n = 31,024 | Hashimoto’s thyroiditis, n = 15,512 | p value | ASD | |
| Before, any time | ||||||||
| Hyperthyroidism, hypothyroidism | < 0.001 | < 0.001 | ||||||
| No hyperthyroidism and no hypothyroidism | 314,531 (99.8) | 6044 (38.4) | 30,969 (99.8) | 5910 (38.1) | ||||
| Hyperthyroidism only | 294 (0.1) | 2392 (15.2) | 36 (0.1) | 2367 (15.3) | ||||
| Hypothyroidism only | 182 (0.1) | 5604 (35.6) | 18 (0.1) | 5549 (35.8) | ||||
| Hyperthyroidism and hypothyroidism | 13 (0.004) | 1711 (10.9) | 1 (0.003) | 1686 (10.9) | ||||
| Sex | 1.000 | 0.000 | 1.000 | 0.000 | ||||
| Female | 271,700 (86.2) | 13,585 (86.2) | 26,750 (86.2) | 13,375 (86.2) | ||||
| Male | 43,320 (13.8) | 2166 (13.8) | 4274 (13.8) | 2137 (13.8) | ||||
| Age | 43.4 ± 16.0 | 43.4 ± 16.0 | 1.000 | 0.000 | 43.4 ± 16.0 | 43.4 ± 16.0 | 0.951 | 0.001 |
| Urbanization | < 0.001 | 0.240 | 0.758 | 0.001 | ||||
| Urban | 100,057 (31.8) | 6638 (42.1) | 13,003 (41.9) | 6535 (42.1) | ||||
| Suburban | 151,327 (48.0) | 6944 (44.1) | 13,798 (44.5) | 6844 (44.1) | ||||
| Rural | 63,636 (20.2) | 2169 (13.8) | 4223 (13.6) | 2133 (13.8) | ||||
| Low incomec | 159,152 (50.5) | 7078 (44.9) | < 0.001 | 0.112 | 13,957 (45.0) | 6994 (45.1) | 0.838 | 0.002 |
| 3 months before the index date | ||||||||
| Times of visiting the outpatient department | 2.8 ± 4.1 | 6.6 ± 5.6 | < 0.001 | 3.2 ± 4.4 | 6.5 ± 5.6 | < 0.001 | ||
| Number of patients visited the outpatient department | 182,102 (57.8) | 14,898 (94.6) | < 0.001 | 19,243 (62) | 14,661 (94.5) | < 0.001 | ||
| Length of hospital stays | 0.2 ± 2.2 | 0.4 ± 3.1 | < 0.001 | 0.3 ± 2.7 | 0.4 ± 3.0 | 0.002 | ||
| 0 days | 308,464 (97.9) | 15,034 (95.4) | < 0.001 | 30,056 (96.9) | 14,826 (95.6) | < 0.001 | ||
| 1–6 days | 3954 (1.3) | 439 (2.8) | 574 (1.9) | 427 (2.8) | ||||
| ≥ 7 days | 2602 (0.8) | 278 (1.8) | 394 (1.3) | 259 (1.7) | ||||
| Follow up for 3 months after the index date | ||||||||
| Times of visiting the outpatient department | 5.2 ± 4.3 | 8.1 ± 5.5 | < 0.001 | 5.5 ± 4.6 | 8.1 ± 5.5 | < 0.001 | ||
| Number of patients visited the outpatient department | 315,020 (100) | 15,748 (100) | < 0.001 | 31,024 (100) | 15,509 (100) | 0.014 | ||
| Length of hospital stays | 0.3 ± 2.8 | 0.7 ± 3.9 | < 0.001 | 0.4 ± 3.1 | 0.6 ± 3.7 | < 0.001 | ||
| 0 days | 304,835 (96.8) | 14,602 (92.7) | < 0.001 | 29,976 (96.6) | 14,456 (93.2) | < 0.001 | ||
| 1–6 days | 6269 (2.0) | 676 (4.3) | 636 (2.1) | 632 (4.1) | ||||
| ≥ 7 days | 3916 (1.2) | 473 (3.0) | 412 (1.3) | 424 (2.7) | ||||
| Follow up for 6 months after the index date | ||||||||
| Times of visiting the outpatient department | 8.9 ± 7.8 | 14.4 ± 9.8 | < 0.001 | 9.6 ± 8.2 | 14.3 ± 9.6 | < 0.001 | ||
| Number of patients visited the outpatient department | 315,020 (100) | 15,749 (100) | < 0.001 | 31,024 (100) | 15,510 (100) | 0.045 | ||
| Length of hospital staysa | 0.6 ± 4.9 | 1.0 ± 5.9 | < 0.001 | 0.7 ± 5.3 | 0.9 ± 5.6 | < 0.001 | ||
| 0 days | 298,484 (94.8) | 14,170 (90.0) | < 0.001 | 29,253 (94.3) | 14,029 (90.4) | < 0.001 | ||
| 1–6 days | 9996 (3.2) | 933 (5.9) | 1058 (3.4) | 892 (5.8) | ||||
| ≥ 7 days | 6540 (2.1) | 648 (4.1) | 713 (2.3) | 591 (3.8) | ||||
| Co-morbidity | ||||||||
| 1 previous year before the index date: outpatient department visits × 3, admission × 1 | ||||||||
| RA | 819 (0.3) | 167 (1.1) | < 0.001 | 0.099 | 203 (0.7) | 134 (0.9) | 0.012 | 0.024 |
| SS | 459 (0.1) | 355 (2.3) | < 0.001 | 0.195 | 262 (0.8) | 151 (1.0) | 0.162 | 0.014 |
| SSc | 24 (0.01) | 8 (0.1) | < 0.001 | 0.025 | 8 (0.03) | 5 (0.03) | 0.695 | 0.004 |
| Vasculitis | 33 (0.01) | 17 (0.1) | < 0.001 | 0.040 | 17 (0.1) | 8 (0.1) | 0.888 | 0.001 |
| Hypertension | 34,921 (11.1) | 2093 (13.3) | < 0.001 | 0.067 | 4294 (13.8) | 2060 (13.3) | 0.097 | 0.016 |
| Diabetes mellitus | 16,681 (5.3) | 1090 (6.9) | < 0.001 | 0.068 | 2205 (7.1) | 1073 (6.9) | 0.450 | 0.007 |
| Hyperlipidemia | 13,795 (4.4) | 1402 (8.9) | < 0.001 | 0.182 | 2795 (9.0) | 1369 (8.8) | 0.513 | 0.006 |
| Coronary artery disease | 7348 (2.3) | 603 (3.8) | < 0.001 | 0.087 | 1110 (3.6) | 586 (3.8) | 0.278 | 0.011 |
| Osteoporosis | 2771 (0.9) | 248 (1.6) | < 0.001 | 0.063 | 418 (1.3) | 241 (1.6) | 0.076 | 0.017 |
| Cerebral vascular accident | 4937 (1.6) | 306 (1.9) | < 0.001 | 0.029 | 524 (1.7) | 296 (1.9) | 0.090 | 0.016 |
| COPD/asthma | 6898 (2.2) | 541 (3.4) | < 0.001 | 0.075 | 1076 (3.5) | 527 (3.4) | 0.693 | 0.004 |
| Chronic kidney disease | 1632 (0.5) | 91 (0.6) | 0.310 | 0.008 | 147 (0.5) | 91 (0.6) | 0.108 | 0.016 |
| Chronic liver diseases | 6248 (2.0) | 633 (4.0) | < 0.001 | 0.120 | 1250 (4.0) | 601 (3.9) | 0.421 | 0.008 |
| Pancreatitis | 286 (0.1) | 27 (0.2) | 0.001 | 0.022 | 42 (0.1) | 24 (0.2) | 0.601 | 0.005 |
| Affective psychosis | 1421 (0.5) | 259 (1.6) | < 0.001 | 0.117 | 508 (1.6) | 239 (1.5) | 0.434 | 0.008 |
| Ankylosing spondylitis | 185 (0.1) | 43 (0.3) | < 0.001 | 0.053 | 62 (0.2) | 33 (0.2) | 0.771 | 0.003 |
| IBD | 254 (0.1) | 18 (0.1) | 0.151 | 0.011 | 32 (0.1) | 18 (0.1) | 0.689 | 0.004 |
| HIV infection | 43 (0.01) | 1 (0.01) | 0.438 | 0.007 | 3 (0.01) | 1 (0.01) | 0.724 | 0.004 |
| AIHA | 3 (0.001) | 5 (0.03) | < 0.001 | 0.024 | 1 (0.003) | 1 (0.01) | 0.617 | 0.005 |
| ITP | 36 (0.01) | 15 (0.1) | < 0.001 | 0.036 | 19 (0.1) | 9 (0.1) | 0.894 | 0.001 |
| Hashimoto’s thyroiditis treatment at baselineb | ||||||||
| No drug admiration | 4139 (26.3) | 4115 (26.5) | ||||||
| Anti-thyroid medication (carbimazole, propylthiouracil, methimazole)/eltroxin only | 8564 (54.4) | 8538 (55.0) | ||||||
| HCQ/corticosteroid+/− anti-thyroid medication/eltroxin | 3048 (19.4) | 2859 (18.4) | ||||||
aLength of hospital stay was identified within 2 years before the index date
bHashimoto’s thyroiditis treatment was identified within 6 months after diagnosis with Hashimoto’s thyroiditis
cInsured income lower than median income (21,000 New Taiwan dollars/month)
To minimize surveillance bias, patients who were diagnosed with SLE during the period of 2 years before the index date were excluded. Besides, due to the chronic and latent nature of SLE, patients who were diagnosed with SLE whose follow-up time was less than 3 months and 6 months were excluded, respectively, in 2 scenarios, which were conducted to increase the accuracy, and in the background variations, groups of 3 months before the index date and 3 and 6 months after the index date were also corrected with length of hospital stays and times of outpatient department visits (Table 1).
Comorbidities were captured by tracing all the ambulatory care and admission records in the NHI database within 1 previous year of the index date and have had at least three outpatient visits or one hospital admission. We analyzed autoimmune disorders including rheumatoid arthritis (RA, ICD9 code: 714.0), Sjögren’s syndrome (SS, ICD9 code: 710.2), systemic sclerosis (SSc, ICD9 code: 710.1), and vasculitis (ICD9 code: 433.0) that not rarely occur with SLE. Other common comorbidities such as hypertension (ICD9 codes: 401–405), diabetes mellitus (ICD9 code: 250), hyperlipidemia (ICD9 codes: 272.0–272.4), coronary artery disease (ICD9 codes: 410–414), osteoporosis (ICD9 code: 733), cerebral vascular accident (ICD9 codes: 430–438), chronic obstructive pulmonary disease (COPD)/asthma (ICD9 codes: 490–496), chronic kidney disease (CKD, ICD9 code: 585), chronic liver diseases (ICD9 codes: 571, 573), pancreatitis (ICD9 codes: 577.0, 577.1), affective psychosis (ICD9 code: 296), ankylosing spondylitis (ICD9 code: 720.0), inflammatory bowel disease (ICD9 codes: 555–556), HIV infection (ICD9 codes: 042–044, V08), autoimmune hemolytic anemia (AIHA) (ICD9 code: 283.0), and idiopathic thrombocytopenic purpura (ITP) (ICD9 code: 287.3) were also included in the study. Baseline treatment of HT including (1) no drug admiration, (2) anti-thyroid medication (carbimazole, propylthiouracil, methimazole)/eltroxin only, and (3) HCQ/corticosteroid+/− anti-thyroid medication/eltroxin was also analyzed, and all treatments were given within 6 months after diagnosis. Besides, hyperthyroidism (ICD9 code: 242) and hypothyroidism (ICD9 codes: 243, 244) diagnosed before the index date were separately analyzed by multivariable statistical analysis, which was listed in Tables 5, 6, and 7.
Table 5.
Cox proportional hazard regressions for estimation of adjusted HRs
| 1:20 age-matched and sex-matched population | 1:2 PSM population | ||||||
|---|---|---|---|---|---|---|---|
| Model 1: Hashimoto’s thyroiditis exposure alone | Model 2a: Hashimoto’s thyroiditis exposure + demographic variables | Model 3a: model 2 + medical utilization and comorbidities at baseline | Model 2b: Hashimoto’s thyroiditis exposure + demographic variables | Model 3b: model 2 + medical utilization and comorbidities at baseline | Model A: conditional Cox model with Hashimoto’s thyroiditis exposure alone | Model B: conditional Cox model with Hashimoto’s thyroiditis exposure, hyperthyroidism, and hypothyroidism | |
| Hashimoto’s thyroiditis | 6.79 (5.32–8.66) | 5.83 (4.50–7.56) | 4.35 (3.28–5.76) | 3.54 (2.40–5.22) | |||
| Hyperthyroidism, hypothyroidism | |||||||
| No Hashimoto’s thyroiditis | Ref. | Ref. | Ref. | Ref. | |||
| Hashimoto’s thyroiditis, no hyperthyroidism, and no hypothyroidism | 7.46 (5.26–10.57) | 6.52 (4.55–9.34) | 4.53 (3.08–6.66) | 3.47 (2.12–5.69) | |||
| Hashimoto’s thyroiditis and hyperthyroidism only | 3.21 (1.43–7.21) | 2.88 (1.27–6.50) | 2.63 (1.16–5.96) | 1.72 (0.68–4.35) | |||
| Hashimoto’s thyroiditis and hypothyroidism only | 7.59 (5.34–10.81) | 6.40 (4.45–9.21) | 4.99 (3.41–7.32) | 4.27 (2.67–6.83) | |||
| Hashimoto’s thyroiditis, hyperthyroidism, and hypothyroidism | 6.59 (3.39–12.81) | 5.29 (2.70–10.36) | 3.67 (1.83–7.37) | 3.77 (1.76–8.05) | |||
| Sex—male | 0.19 (0.10–0.36) | 0.18 (0.09–0.35) | 0.20 (0.10–0.39) | 0.18 (0.10–0.36) | 0.20 (0.10–0.39) | ||
| Age | 1.00 (0.99–1.01) | 1.00 (0.99–1.004) | 0.99 (0.98–0.99) | 1.00 (0.99–1.004) | 0.99 (0.98–0.99) | ||
| Urbanization | |||||||
| Urban | Ref. | Ref. | Ref. | Ref. | |||
| Suburban | 0.91 (0.72–1.16) | 0.98 (0.77–1.25) | 0.96 (0.76–1.23) | 0.99 (0.77–1.26) | 0.97 (0.76–1.24) | ||
| Rural | 1.04 (0.78–1.40) | 1.17 (0.87–1.57) | 1.11 (0.83–1.49) | 1.17 (0.87–1.57) | 1.11 (0.83–1.50) | ||
| Low income (≤ median 21,000 NTD/month) | 1.09 (0.88–1.35) | 1.10 (0.89–1.36) | 1.04 (0.84–1.29) | 1.09 (0.88–1.35) | 1.04 (0.84–1.29) | ||
| 3 months before the index date | |||||||
| Times of visiting the outpatient department | 1.06 (1.05–1.08) | 1.04 (1.02–1.06) | 1.01 (0.99–1.03) | 1.04 (1.02–1.06) | 1.01 (0.99–1.03) | ||
| Length of hospital stays* | |||||||
| 0 days | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| 1–6 days | 2.41 (1.28–4.52) | 1.71 (0.90–3.23) | 1.39 (0.72–2.67) | 1.74 (0.92–3.29) | 1.43 (0.74–2.76) | ||
| ≥ 7 days | 5.30 (2.90–9.66) | 3.85 (2.08–7.13) | 2.72 (1.42–5.20) | 3.88 (2.09–7.18) | 2.71 (1.41–5.19) | ||
| Co-morbidity | |||||||
| 1 previous year of the index date: OPD visits × 3, admission × 1 | |||||||
| RA | 22.12 (14.23–34.40) | 3.82 (2.20–6.65) | 3.74 (2.14–6.52) | ||||
| SS | 50.39 (35.35–71.82) | 11.40 (7.13–18.22) | 11.29 (7.03–18.15) | ||||
| SSc | 133.44 (49.86–357.09) | 6.73 (2.24–20.18) | 6.88 (2.26–20.95) | ||||
| Vasculitis | 103.27 (42.71–249.70) | 7.24 (2.66–19.69) | 6.99 (2.55–19.16) | ||||
| Hypertension | 1.31 (0.96–1.79) | 1.46 (1.01–2.13) | 1.46 (1.01–2.13) | ||||
| Diabetes mellitus | 0.64 (0.35–1.16) | 0.49 (0.26–0.93) | 0.49 (0.26–0.94) | ||||
| Hyperlipidemia | 1.02 (0.61–1.71) | 0.79 (0.45–1.37) | 0.77 (0.44–1.34) | ||||
| Coronary artery disease | 1.05 (0.52–2.11) | 0.67 (0.32–1.42) | 0.68 (0.32–1.44) | ||||
| Osteoporosis | 5.01 (2.99–8.40) | 2.89 (1.66–5.05) | 2.88 (1.65–5.03) | ||||
| Cerebral vascular accident | 1.75 (0.87–3.53) | 1.27 (0.60–2.69) | 1.25 (0.59–2.65) | ||||
| COPD/asthma | 2.22 (1.35–3.67) | 1.44 (0.84–2.47) | 1.44 (0.84–2.47) | ||||
| Chronic kidney disease | 2.22 (0.71–6.91) | 2.00 (0.63–6.38) | 1.95 (0.61–6.23) | ||||
| Chronic liver diseases | 3.23 (2.08–5.03) | 2.28 (1.42–3.65) | 2.27 (1.42–3.65) | ||||
| Pancreatitis | 3.67 (0.52–26.11) | 1.18 (0.16–8.91) | 1.15 (0.15–8.77) | ||||
| Affective psychosis | 2.37 (0.88–6.35) | 1.27 (0.45–3.58) | 1.29 (0.46–3.63) | ||||
| Ankylosing spondylitis | 4.25 (0.60–30.28) | 0.73 (0.08–6.36) | 0.79 (0.09–6.74) | ||||
| IBD | 0.00 (0.00–6.17E135) | 0.00 (0.00–1.85E178) | 0.00 (0.00–5.59E177) | ||||
| HIV infection | 0.00 (0.00–2.35E139) | 0.00 (0.00–9.99E999) | 0.00 (0.00–9.99E999) | ||||
| AIHA | 322.78 (45.35–2297.39) | 133.96 (18.18–986.89) | 127.00 (17.22–936.73) | ||||
| ITP | 81.51 (30.42–218.43) | 36.62 (13.18–101.76) | 37.20 (13.32–103.90) | ||||
Table 6.
Cox proportional hazard regressions for estimation of adjusted HRs. Follow-up duration of samples≧3 months
| 1:20 age-matched and sex-matched population | 1:2 PSM population | ||||||
|---|---|---|---|---|---|---|---|
| Model 1: Hashimoto’s thyroiditis exposure alone | Model 2a: Hashimoto’s thyroiditis exposure + demographic variables | Model 3a: model 2 + medical utilization and comorbidities at baseline | Model 2b: Hashimoto’s thyroiditis exposure + demographic variables | Model 3b: model 2 + medical utilization and comorbidities at baseline | Model A: conditional Cox model with Hashimoto’s thyroiditis exposure alone | Model B: conditional Cox model with Hashimoto’s thyroiditis exposure, hyperthyroidism, and hypothyroidism | |
| Hashimoto’s thyroiditis | 6.44 (4.97–8.33) | 5.05 (3.84–6.65) | 3.84 (2.84–5.19) | 3.41 (2.28–5.10) | |||
| Hyperthyroidism, hypothyroidism | |||||||
| No Hashimoto’s thyroiditis | Ref. | Ref. | Ref. | Ref. | |||
| Hashimoto’s thyroiditis, no hyperthyroidism, and no hypothyroidism | 6.89 (4.74–10.02) | 5.36 (3.65–7.89) | 3.81 (2.51–5.79) | 3.10 (1.83–5.24) | |||
| Hashimoto’s thyroiditis and hyperthyroidism only | 3.45 (1.54–7.76) | 2.75 (1.21–6.23) | 2.66 (1.17–6.05) | 1.82 (0.71–4.61) | |||
| Hashimoto’s thyroiditis and hypothyroidism only | 7.20 (4.96–10.47) | 5.74 (3.91–8.43) | 4.43 (2.94–6.67) | 4.21 (2.60–6.82) | |||
| Hashimoto’s thyroiditis, hyperthyroidism, and hypothyroidism | 6.29 (3.11–12.73) | 4.67 (2.29–9.51) | 3.43 (1.63–7.23) | 3.97 (1.85–8.52) | |||
| Sex—male | 0.18 (0.09–0.37) | 0.17 (0.08–0.34) | 0.19 (0.10–0.39) | 0.17 (0.09–0.35) | 0.19 (0.10–0.39) | ||
| Age | 1.00 (0.99–1.01) | 0.99 (0.99–1.001) | 0.99 (0.98–0.99) | 0.99 (0.99–1.001) | 0.99 (0.98–0.99) | ||
| Urbanization | |||||||
| Urban | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| Suburban | 0.93 (0.72–1.20) | 0.99 (0.77–1.27) | 0.98 (0.76–1.26) | 0.99 (0.77–1.27) | 0.98 (0.76–1.27) | ||
| Rural | 1.09 (0.81–1.48) | 1.19 (0.88–1.62) | 1.16 (0.85–1.58) | 1.19 (0.88–1.61) | 1.16 (0.85–1.58) | ||
| Low income (≤ median 21,000 NTD/month) | 1.08 (0.87–1.34) | 1.05 (0.84–1.31) | 1.02 (0.81–1.28) | 1.04 (0.84–1.30) | 1.02 (0.81–1.27) | ||
| 3 months before the index date | |||||||
| Times of visiting the outpatient department | 1.06 (1.05–1.08) | 0.99 (0.97–1.02) | 0.97 (0.95–1.000) | 0.99 (0.97–1.02) | 0.97 (0.95–1.001) | ||
| Length of hospital stays* | |||||||
| 0 days | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| 1–6 days | 2.09 (1.04–4.22) | 1.35 (0.66–2.74) | 1.09 (0.52–2.30) | 1.36 (0.67–2.78) | 1.12 (0.53–2.37) | ||
| ≥ 7 days | 5.34 (2.84–10.02) | 2.30 (1.18–4.49) | 2.08 (1.04–4.16) | 2.30 (1.18–4.50) | 2.07 (1.03–4.16) | ||
| 3 months after the index date | |||||||
| Times of visiting the outpatient department | 1.07 (1.06–1.08) | 1.06 (1.03–1.08) | 1.06 (1.04–1.09) | 1.06 (1.03–1.08) | 1.06 (1.04–1.09) | ||
| Length of hospital stays* | |||||||
| 0 days | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| 1–6 days | 2.93 (1.77–4.85) | 2.00 (1.20–3.33) | 1.36 (0.78–2.36) | 2.03 (1.22–3.39) | 1.36 (0.78–2.36) | ||
| ≥7 days | 10.17 (6.75–15.32) | 5.99 (3.84–9.35) | 3.77 (2.33–6.11) | 6.06 (3.88–9.46) | 3.80 (2.35–6.16) | ||
| Co-morbidity | |||||||
| 1 previous year of the index date: OPD visits × 3, admission × 1 | |||||||
| RA | 21.73 (13.67–34.55) | 4.21 (2.36–7.52) | 4.18 (2.33–7.48) | ||||
| SS | 46.80 (31.94–68.59) | 8.12 (4.86–13.58) | 8.01 (4.77–13.46) | ||||
| SSc | 109.05 (35.03–339.49) | 3.28 (0.91–11.88) | 3.46 (0.94–12.72) | ||||
| Vasculitis | 89.68 (33.46–240.33) | 6.77 (2.19–20.88) | 6.48 (2.09–20.13) | ||||
| Hypertension | 1.27 (0.92–1.76) | 1.30 (0.88–1.94) | 1.30 (0.87–1.93) | ||||
| Diabetes mellitus | 0.63 (0.34–1.19) | 0.48 (0.25–0.95) | 0.49 (0.25–0.96) | ||||
| Hyperlipidemia | 1.04 (0.61–1.78) | 0.83 (0.47–1.48) | 0.82 (0.46–1.45) | ||||
| Coronary artery disease | 1.15 (0.57–2.31) | 0.71 (0.33–1.52) | 0.71 (0.33–1.53) | ||||
| Osteoporosis | 5.08 (2.97–8.68) | 2.75 (1.54–4.92) | 2.74 (1.54–4.91) | ||||
| Cerebral vascular accident | 1.93 (0.96–3.90) | 1.19 (0.55–2.57) | 1.17 (0.54–2.53) | ||||
| COPD/asthma | 2.27 (1.35–3.81) | 1.48 (0.85–2.56) | 1.47 (0.85–2.55) | ||||
| Chronic kidney disease | 1.64 (0.41–6.59) | 1.42 (0.35–5.82) | 1.40 (0.34–5.76) | ||||
| Chronic liver diseases | 3.37 (2.14–5.29) | 2.24 (1.37–3.65) | 2.22 (1.36–3.62) | ||||
| Pancreatitis | 4.04 (0.57–28.78) | 0.78 (0.10–6.22) | 0.78 (0.10–6.29) | ||||
| Affective psychosis | 1.28 (0.32–5.15) | 0.47 (0.10–2.16) | 0.48 (0.11–2.18) | ||||
| Ankylosing spondylitis | 4.63 (0.65–32.92) | 0.76 (0.08–7.10) | 0.81 (0.09–7.33) | ||||
| IBD | 0.00 (0.00–1.97E141) | 0.00 (0.00–4.81E157) | 0.00 (0.00–3.17E156) | ||||
| HIV infection | 0.00 (0.00–2.91E146) | 0.00 (0.00–9.99E999) | 0.00 (0.00–9.99E999) | ||||
| AIHA | 408.92 (57.36–2915.47) | 133.16 (17.51–1012.64) | 125.86 (16.48–961.45) | ||||
| ITP | 44.25 (11.02–177.67) | 15.18 (3.63–63.38) | 15.42 (3.68–64.63) | ||||
Table 7.
Cox proportional hazard regressions for the estimation of adjusted HRs. Follow-up duration of samples ≧ 6 months
| 1:20 age-matched and sex-matched population | 1:2 PSM population | ||||||
|---|---|---|---|---|---|---|---|
| Model 1: Hashimoto’s thyroiditis exposure alone | Model 2a: Hashimoto’s thyroiditis exposure + demographic variables | Model 3a: model 2 + medical utilization and comorbidities at baseline | Model 2b: Hashimoto’s thyroiditis exposure + demographic variables | Model 3b: model 2 + medical utilization and comorbidities at baseline | Model A: conditional Cox model with Hashimoto’s thyroiditis exposure alone | Model B: conditional Cox model with Hashimoto’s thyroiditis exposure, hyperthyroidism, and hypothyroidism | |
| Hashimoto’s thyroiditis | 6.58 (5.02–8.61) | 5.26 (3.95–7.01) | 4.13 (3.02–5.65) | 3.54 (2.33–5.36) | |||
| Hyperthyroidism, hypothyroidism | |||||||
| No Hashimoto’s thyroiditis | Ref. | Ref. | Ref. | Ref. | |||
| Hashimoto’s thyroiditis, no hyperthyroidism, and no hypothyroidism | 6.91 (4.67–10.25) | 5.58 (3.72–8.36) | 4.10 (2.65–6.36) | 3.22 (1.87–5.53) | |||
| Hashimoto’s thyroiditis and hyperthyroidism only | 3.85 (1.71–8.65) | 3.11 (1.37–7.05) | 2.97 (1.30–6.75) | 1.98 (0.78–5.05) | |||
| Hashimoto’s thyroiditis and hypothyroidism only | 7.21 (4.86–10.68) | 5.79 (3.86–8.68) | 4.65 (3.03–7.14) | 4.25 (2.57–7.03) | |||
| Hashimoto’s thyroiditis and hyperthyroidism and hypothyroidism | 6.99 (3.45–14.15) | 5.26 (2.57–10.76) | 3.94 (1.87–8.33) | 4.33 (2.01–9.32) | |||
| Sex—male | 0.20 (0.10–0.41) | 0.19 (0.10–0.39) | 0.21 (0.11–0.43) | 0.20 (0.10–0.40) | 0.21 (0.11–0.43) | ||
| Age | 1.00 (0.99–1.01) | 0.99 (0.99–1.001) | 0.99 (0.98–0.99) | 0.99 (0.99–1.001) | 0.99 (0.98–0.99) | ||
| Urbanization | |||||||
| Urban | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| Suburban | 0.98 (0.76–1.28) | 1.05 (0.80–1.37) | 1.03 (0.79–1.35) | 1.05 (0.80–1.37) | 1.04 (0.79–1.35) | ||
| Rural | 1.05 (0.76–1.45) | 1.14 (0.82–1.58) | 1.10 (0.79–1.53) | 1.14 (0.82–1.58) | 1.10 (0.79–1.53) | ||
| Low income (≤ median 21,000 NTD/month) | 1.06 (0.84–1.34) | 1.04 (0.82–1.31) | 1.02 (0.80–1.29) | 1.04 (0.82–1.31) | 1.01 (0.80–1.28) | ||
| 3 months before the index date | |||||||
| Times of visiting the outpatient department | 1.06 (1.05–1.08) | 0.99 (0.96–1.02) | 0.97 (0.94–1.01) | 0.99 (0.96–1.02) | 0.97 (0.94–1.01) | ||
| Length of hospital stays* | |||||||
| 0 days | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| 1–6 days | 1.72 (0.77–3.86) | 1.09 (0.48–2.48) | 0.93 (0.40–2.17) | 1.11 (0.49–2.51) | 0.95 (0.40–2.22) | ||
| ≥ 7 days | 4.79 (2.37–9.67) | 2.11 (1.01–4.41) | 1.90 (0.88–4.09) | 2.10 (1.004–4.41) | 1.90 (0.88–4.08) | ||
| 6 months after the index date | |||||||
| Times of visiting the outpatient department | 1.04 (1.03–1.04) | 1.03 (1.01–1.04) | 1.03 (1.01–1.04) | 1.03 (1.01–1.04) | 1.03 (1.01–1.04) | ||
| Length of hospital stays* | |||||||
| 0 days | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| 1–6 days | 3.35 (2.24–5.03) | 2.51 (1.66–3.80) | 2.05 (1.33–3.17) | 2.55 (1.69–3.85) | 2.06 (1.33–3.19) | ||
| ≥ 7 days | 7.08 (4.72–10.61) | 4.63 (2.99–7.19) | 3.46 (2.17–5.50) | 4.66 (3.00–7.23) | 3.47 (2.18–5.53) | ||
| Co-morbidity | |||||||
| 1 previous year of the index date: OPD visits × 3, admission × 1 | |||||||
| RA | 18.76 (11.16–31.55) | 3.37 (1.77–6.42) | 3.34 (1.75–6.38) | ||||
| SS | 42.89 (28.24–65.16) | 7.49 (4.33–12.96) | 7.45 (4.28–12.94) | ||||
| SSc | 120.88 (38.75–377.04) | 3.98 (1.08–14.59) | 4.12 (1.10–15.42) | ||||
| Vasculitis | 98.63 (36.76–264.64) | 6.66 (2.11–21.07) | 6.33 (1.98–20.28) | ||||
| Hypertension | 1.35 (0.96–1.89) | 1.42 (0.94–2.14) | 1.41 (0.94–2.13) | ||||
| Diabetes mellitus | 0.71 (0.38–1.33) | 0.53 (0.27–1.05) | 0.54 (0.27–1.05) | ||||
| Hyperlipidemia | 1.08 (0.62–1.88) | 0.83 (0.46–1.51) | 0.82 (0.45–1.50) | ||||
| Coronary artery disease | 1.28 (0.63–2.58) | 0.83 (0.39–1.78) | 0.83 (0.39–1.78) | ||||
| Osteoporosis | 5.20 (2.99–9.07) | 2.97 (1.62–5.42) | 2.95 (1.62–5.39) | ||||
| Cerebral vascular accident | 1.62 (0.72–3.63) | 1.04 (0.44–2.47) | 1.03 (0.44–2.44) | ||||
| COPD/asthma | 2.00 (1.12–3.56) | 1.25 (0.68–2.33) | 1.24 (0.67–2.32) | ||||
| Chronic kidney disease | 1.86 (0.46–7.48) | 1.47 (0.36–6.05) | 1.45 (0.35–5.98) | ||||
| Chronic liver diseases | 3.35 (2.08–5.40) | 2.34 (1.41–3.91) | 2.32 (1.39–3.87) | ||||
| Pancreatitis | 4.53 (0.64–32.28) | 1.13 (0.15–8.80) | 1.14 (0.15–8.91) | ||||
| Affective psychosis | 1.42 (0.35–5.69) | 0.52 (0.11–2.46) | 0.53 (0.11–2.47) | ||||
| Ankylosing spondylitis | 5.11 (0.72–36.31) | 0.76 (0.08–7.38) | 0.79 (0.08–7.57) | ||||
| IBD | 0.00 (0.00–1.05E148) | 0.00 (0.00–2.28E174) | 0.00 (0.00–1.49E174) | ||||
| HIV infection | 0.00 (0.00–2.85E155) | 0.00 (0.00–9.99E999) | 0.00 (0.00–9.99E999) | ||||
| AIHA | 0.02 (0.00–2.33E144) | 0.00 (0.00–9.99E999) | 0.00 (0.00–9.99E999) | ||||
| ITP | 24.38 (3.43–173.51) | 8.72 (1.19–63.99) | 8.79 (1.19–64.67) | ||||
Statistical analysis
To compare and increase the similarities between our exposure group of HT and the comparator group without HT, the chi-square (χ2) tests and the two-tailed T test was used for the baseline demographic characteristics such as gender, age, urbanization, income level, admission duration, and comorbidities. Time-to-event analysis was conducted based on the index date defined as the fixed time point (January 2005) for every participation. All participants were followed up from their respective index date until the occurrence of SLE, until withdrawal, or until the end of 2013, whichever occurred first.
We also constructed a multivariable Cox proportional hazard model to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the SLE incidence. In the 1:20 age- and gender-matched population, 3 models were conducted. The first would be the model of HT alone, which also analyzed other thyroid disorders. For the second model, hyperthyroid and hypothyroid disorders were excluded. Model 3 contains HT with demographic variables, medical utilization, and comorbidities, and for the 1:2 PSM population, models A and B were constructed by controlling variables such as HT, hyperthyroid, and hypothyroid disorders. All the data and statistics were processed and analyzed by the Statistics Analysis System (SAS) software version 9.3 (SAS Institute, Inc., Cary, NC), and a p-value less than 0.05 was considered to indicate statistical significance.
Sensitivity analysis
To test the reliability of our study results, we established 4 sensitivity analysis scenarios including SLE medication treatment and the exclusion of autoimmune thyroiditis with other autoimmune diseases. The SLE treatment was identified as systemic corticosteroids or disease-modifying anti-rheumatic drugs (DMARDs) (including hydroxychloroquine (HCQ) or azathioprine) within 6 months after the first diagnosis of SLE. In scenarios 1–3, adjusted hazard ratio (aHR) was analyzed based on a different definition of SLE event. The main finding without medication treatment analysis would be scenario 1, systemic corticosteroids or DMARDs treatment were brought into scenario 2, and systemic corticosteroids were excluded in scenario 3. Scenario 4 modified the exclusion criteria and exclusion of the patients with rheumatic arthritis (RA), Sjögren’s syndrome (SS), systematic sclerosis (SSc), vasculitis, ankylosing spondylitis (AS), and inflammatory bowel disease (IBD) at baseline; hence, the autoimmune thyroiditis accompanied with other autoimmune diseases could be ruled out. The sensitivity analysis scenarios were listed in Tables 8, 9, and 10.
Table 8.
Sensitivity analysis in the estimation of the SLE risk for Hashimoto’s thyroiditis exposure in the age-matched and sex-matched population
| Model 3, aHRa (95%CI) | ||
|---|---|---|
| Scenario 1 | Definition of SLE event: major illness registry (main finding) | 4.35 (3.28–5.76) |
| Scenario 2 | Definition of SLE event: scenario 1 + treated with systemic corticosteroids or DMARDs (including HCQ or azathioprine) | 4.39 (3.31–5.82) |
| Scenario 3 | Definition of SLE event: scenario 1 + treated with DMARDs (including HCQ or azathioprine)b | 5.11 (3.75–6.98) |
| Scenario 4 | Exclusion of patients with RA, SS, SSc, vasculitis, AS, and IBD at baseline (excluding autoimmune thyroiditis accompanied with other autoimmune diseases) | 4.70 (3.46–6.38) |
aHR Adjusted HR, HCQ Hydroxychloroquine, RA Rheumatoid arthritis, SS Sjögren’s syndrome, SSc Systemic sclerosis, AS Ankylosing spondylitis, IBD Inflammatory bowel disease, SLE Systemic lupus erythematosus
aaHR: the covariates including urbanization, low income, and comorbidities listed in Table 1
bThe treatment of SLE was identified within 6 months after the first diagnosis of SLE
Table 9.
Sensitivity analysis in the estimation of the SLE risk for Hashimoto’s thyroiditis exposure in the age-matched and sex-matched population. Follow-up duration of samples ≧ 3 months
| Model 3, aHRa (95%CI) | ||
|---|---|---|
| Scenario 1 | Definition of SLE event: major illness registry (main finding) | 3.84 (2.84–5.19) |
| Scenario 2 | Definition of SLE event: scenario 1 + treated with systemic corticosteroids or DMARDs (including HCQ or azathioprine) | 3.91 (2.89–5.30) |
| Scenario 3 | Definition of SLE event: scenario 1 + treated with DMARDs (including HCQ or azathioprine)b | 4.72 (3.41–6.55) |
| Scenario 4 | Exclusion of patients with RA, SS, SSc, vasculitis, AS, and IBD at baseline (excluding autoimmune thyroiditis accompanied with other autoimmune diseases) | 4.21 (3.06–5.79) |
aHR adjusted HR, HCQ Hydroxychloroquine, RA Rheumatoid arthritis, SS Sjögren’s syndrome, SSc Systemic sclerosis, AS Ankylosing spondylitis, IBD Inflammatory bowel disease, SLE Systemic lupus erythematosus
aaHR: the covariates including urbanization, low income, and comorbidities listed in Table 1
bThe treatment of SLE was identified within 6 months after the first diagnosis of SLE
Table 10.
Sensitivity analysis in the estimation of the SLE risk for Hashimoto’s thyroiditis exposure in the age-matched and sex-matched population. Follow-up duration of samples ≧ 6 months
| Model 3, aHRa (95%CI) | ||
|---|---|---|
| Scenario 1 | Definition of SLE event: major illness registry (main finding) | 4.13 (3.02–5.65) |
| Scenario 2 | Definition of SLE event: scenario 1 + treated with systemic corticosteroids or DMARDs (including HCQ or azathioprine) | 4.23 (3.09–5.79) |
| Scenario 3 | Definition of SLE event: scenario 1 + treated with DMARDs (including HCQ or azathioprine)b | 5.18 (3.70–7.26) |
| Scenario 4 | Exclusion of patients with RA, SS, SSc, vasculitis, AS, and IBD at baseline (excluding autoimmune thyroiditis accompanied with other autoimmune diseases) | 4.70 (3.39–6.51) |
aHR Adjusted HR, HCQ Hydroxychloroquine, RA Rheumatoid arthritis, SS Sjögren’s syndrome, SSc Systemic sclerosis, AS Ankylosing spondylitis, IBD Inflammatory bowel disease, SLE Systemic lupus erythematosus
aaHR: the covariates including urbanization, low income, and comorbidities listed in Table 1
bThe treatment of SLE was identified within 6 months after the first diagnosis of SLE
Results
After exclusion and 1:20 age match, we identified 15,751 patients among 25,018 HT patients as our study group; 315,020 patients were extracted for the comparator group without HT. Furthermore, the 1:2 PSM filtered out 15,512 cases for the study group with 31,024 cases for the comparator group without HT. The baseline demographic characteristics, medical utilizations, and comorbidities of both groups were listed in Table 1. There was a significant higher proportion of most listed comorbidities (p < 0.001) except for CKD, IBD, and HIV infection in the HT group. As for the baseline medical treatment, over half of the cases (54.4%) in this study were under anti-thyroid medication only, such as carbimazole, propylthiouracil, methimazole, and eltroxin; about 26.3% of cases without medication control; and 19.3% patients were taking HCQ or corticosteroids. Also, Table 1 contained the baseline proportion of thyroid function disorders, which leads to our further multivariable Cox regression analysis.
Tables 2, 3, and 4 present the time-to-event analysis of the SLE incidence rate, including sensitivity analysis scenarios listed in Tables 8, 9, and 10, including data before and after PSM. Before PSM, the incidence rate ratio was similar in scenarios 1–3 (6.83, 95% CI: 5.35–8.72; 6.86, 95% CI: 5.37–8.77; 7.35 95% CI: 5.60–9.63, respectively) and slightly lower in scenario 4 (5.53, 95% CI: 4.16–7.35) which excluded other autoimmune disorders that might have a symptom of thyroiditis. However, after 1:2 PSM, the incidence rate ratio in scenario 4 became slightly higher than in scenarios 1–2 and still lower than in scenario 3. These results were also noted even though we set more barriers on following time, which are presented in Tables 3 and 4. Overall, patients with HT presented a significant increasing risk of SLE in all 4 scenarios (p, long rank p < 0.001). The cumulative probability of SLE incidence after PSM 1:2 was presented via Kaplan-Meier curves, and they were analyzed with the shortest follow-up period of 3 months and 6 months (Fig. 1a–c). In Fig. 2a–c, the factors of hyperthyroidism and hypothyroidism were discussed also. Their data were shown in Table 11.
Table 2.
Incidence rate. No restriction for follow-up duration
| Variable | Total | Event (%) | Total person-years | Incidence rate (/105 years) | IRR (95%CI) | p value | Log-rank p | Proportional hazards assumption |
|---|---|---|---|---|---|---|---|---|
| Before PSM (1:20 age, sex matching) | ||||||||
| Scenario 1 | ||||||||
| Non-Hashimoto’s thyroiditis | 315,020 | 258 (0.08) | 1,512,453 | 17.06 | Ref. | < 0.001 | 0.849 | |
| Hashimoto’s thyroiditis | 15,751 | 86 (0.55) | 73,793 | 116.54 | 6.83 (5.35–8.72) | < 0.001 | ||
| Scenario 2 | ||||||||
| Non-Hashimoto’s thyroiditis | 315,020 | 254 (0.08) | 1,512,465 | 16.79 | Ref. | < 0.001 | 0.790 | |
| Hashimoto’s thyroiditis | 15,751 | 85 (0.54) | 73,795 | 115.18 | 6.86 (5.37–8.77) | < 0.001 | ||
| Scenario 3 | ||||||||
| Non-Hashimoto’s thyroiditis | 315,020 | 198 (0.06) | 1,512,698 | 13.09 | Ref. | < 0.001 | 0.249 | |
| Hashimoto’s thyroiditis | 15,751 | 71 (0.45) | 73,835 | 96.16 | 7.35 (5.60–9.63) | < 0.001 | ||
| Scenario 4 | ||||||||
| Non-Hashimoto’s thyroiditis | 313,366 | 228 (0.07) | 1,504,643 | 15.15 | Ref. | < 0.001 | 0.253 | |
| Hashimoto’s thyroiditis | 15,201 | 60 (0.39) | 71,563 | 83.84 | 5.53 (4.16–7.35) | < 0.001 | ||
| 1:2 PSM | ||||||||
| Scenario 1 | ||||||||
| Non-Hashimoto’s thyroiditis | 31,024 | 40 (0.13) | 149,219 | 26.81 | Ref. | < 0.001 | 0.999 | |
| Hashimoto’s thyroiditis | 15,512 | 70 (0.45) | 72,894 | 96.03 | 3.58 (2.43–5.28) | < 0.001 | ||
| Scenario 2 | ||||||||
| Non-Hashimoto’s thyroiditis | 31,024 | 38 (0.12) | 149,227 | 25.46 | Ref. | < 0.001 | 0.922 | |
| Hashimoto’s thyroiditis | 15,512 | 70 (0.45) | 72,894 | 96.03 | 3.77 (2.54–5.60) | < 0.001 | ||
| Scenario 3 | ||||||||
| Non-Hashimoto’s thyroiditis | 31,024 | 23 (0.07) | 149,267 | 15.41 | Ref. | < 0.001 | 0.194 | |
| Hashimoto’s thyroiditis | 15,512 | 59 (0.38) | 72,926 | 80.90 | 5.25 (3.24–8.50) | < 0.001 | ||
| Scenario 4 | ||||||||
| Non-Hashimoto’s thyroiditis | 30,501 | 28 (0.09) | 146,799 | 19.07 | Ref. | < 0.001 | 0.832 | |
| Hashimoto’s thyroiditis | 15,180 | 58 (0.38) | 71,496 | 81.12 | 4.25 (2.71–6.68) | < 0.001 | ||
Table 3.
Incidence rate. Follow-up duration of samples ≧ 3 months
| Variable | Total | Event (%) | Total person-years | Incidence rate (/105 years) | IRR (95%CI) | p value | Log-rank p | Proportional hazards assumption |
|---|---|---|---|---|---|---|---|---|
| Before PSM (1:20 age, sex matching) | ||||||||
| Scenario 1 | ||||||||
| Non-Hashimoto’s thyroiditis | 310,704 | 241 (0.08) | 1,512,207 | 15.94 | Ref. | < 0.001 | 0.478 | |
| Hashimoto’s thyroiditis | 15,725 | 76 (0.48) | 73,789 | 103.00 | 6.46 (4.99–8.36) | < 0.001 | ||
| Scenario 2 | ||||||||
| Non-Hashimoto’s thyroiditis | 310,704 | 237 (0.08) | 1,512,219 | 15.67 | Ref. | < 0.001 | 0.510 | |
| Hashimoto’s thyroiditis | 15,726 | 76 (0.48) | 73,791 | 102.99 | 6.57 (5.08–8.51) | < 0.001 | ||
| Scenario 3 | ||||||||
| Non-Hashimoto’s thyroiditis | 310,709 | 186 (0.06) | 1,512,452 | 12.30 | Ref. | < 0.001 | 0.202 | |
| Hashimoto’s thyroiditis | 15,730 | 66 (0.42) | 73,832 | 89.39 | 7.27 (5.49–9.63) | < 0.001 | ||
| Scenario 4 | ||||||||
| Non-Hashimoto’s thyroiditis | 309,068 | 214 (0.07) | 1,504,399 | 14.22 | Ref. | < 0.001 | 0.125 | |
| Hashimoto’s thyroiditis | 15,180 | 54 (0.36) | 71,560 | 75.46 | 5.30 (3.94–7.15) | < 0.001 | ||
| 1:2 PSM | ||||||||
| Scenario 1 | ||||||||
| Non-Hashimoto’s thyroiditis | 30,680 | 38 (0.12) | 149,198 | 25.47 | Ref. | < 0.001 | 0.810 | |
| Hashimoto’s thyroiditis | 15,490 | 64 (0.41) | 72,891 | 87.80 | 3.45 (2.31–5.15) | < 0.001 | ||
| Scenario 2 | ||||||||
| Non-Hashimoto’s thyroiditis | 30,680 | 36 (0.12) | 149,206 | 24.13 | Ref. | < 0.001 | 0.910 | |
| Hashimoto’s thyroiditis | 15,490 | 64 (0.41) | 72,891 | 87.80 | 3.64 (2.42–5.47) | < 0.001 | ||
| Scenario 3 | ||||||||
| Non-Hashimoto’s thyroiditis | 30,680 | 21 (0.07) | 149,246 | 14.07 | Ref. | < 0.001 | 0.246 | |
| Hashimoto’s thyroiditis | 15,493 | 56 (0.36) | 72,923 | 76.79 | 5.46 (3.31–9.01) | < 0.001 | ||
| Scenario 4 | ||||||||
| Non-Hashimoto’s thyroiditis | 30,159 | 27 (0.09) | 146,778 | 18.40 | Ref. | < 0.001 | 0.603 | |
| Hashimoto’s thyroiditis | 15,160 | 53 (0.35) | 71,493 | 74.13 | 4.03 (2.54–6.41) | < 0.001 | ||
Table 4.
Incidence rate. Follow-up duration of samples ≧ 6 months
| Variable | Total | Event (%) | Total person-years | Incidence rate (/105 years) | IRR (95%CI) | p value | Log-rank p | Proportional hazards assumption |
|---|---|---|---|---|---|---|---|---|
| Before PSM (1:20 age, sex matching) | ||||||||
| Scenario 1 | ||||||||
| Non-Hashimoto’s thyroiditis | 309,065 | 218 (0.07) | 1,511,589 | 14.42 | Ref. | < 0.001 | 0.581 | |
| Hashimoto’s thyroiditis | 15,685 | 70 (0.45) | 73,774 | 94.88 | 6.58 (5.03–8.61) | < 0.001 | ||
| Scenario 2 | ||||||||
| Non-Hashimoto’s thyroiditis | 309,065 | 214 (0.07) | 1,511,601 | 14.16 | Ref. | < 0.001 | 0.631 | |
| Hashimoto’s thyroiditis | 15,686 | 70 (0.45) | 73,776 | 94.88 | 6.70 (5.12–8.78) | < 0.001 | ||
| Scenario 3 | ||||||||
| Non-Hashimoto’s thyroiditis | 309,075 | 168 (0.05) | 1,511,836 | 11.11 | Ref. | < 0.001 | 0.259 | |
| Hashimoto’s thyroiditis | 15,691 | 61 (0.39) | 73,817 | 82.64 | 7.44 (5.55–9.97) | < 0.001 | ||
| Scenario 4 | ||||||||
| Non-Hashimoto’s thyroiditis | 307,441 | 195 (0.06) | 1,503,786 | 12.97 | Ref. | < 0.001 | 0.259 | |
| Hashimoto’s thyroiditis | 15,147 | 52 (0.34) | 71,547 | 72.68 | 5.60 (4.13–7.61) | < 0.001 | ||
| 1:2 PSM | ||||||||
| Scenario 1 | ||||||||
| Non-Hashimoto’s thyroiditis | 30,524 | 35 (0.11) | 149,139 | 23.47 | Ref. | < 0.001 | 0.999 | |
| Hashimoto’s thyroiditis | 15,454 | 61 (0.39) | 72,877 | 83.70 | 3.57 (2.35–5.40) | < 0.001 | ||
| Scenario 2 | ||||||||
| Non-Hashimoto’s thyroiditis | 30,524 | 33 (0.11) | 149,147 | 22.13 | Ref. | < 0.001 | 0.871 | |
| Hashimoto’s thyroiditis | 15,454 | 61 (0.39) | 72,877 | 83.70 | 3.78 (2.48–5.78) | < 0.001 | ||
| Scenario 3 | ||||||||
| Non-Hashimoto’s thyroiditis | 30,524 | 18 (0.06) | 149,187 | 12.07 | Ref. | < 0.001 | 0.472 | |
| Hashimoto’s thyroiditis | 15,457 | 53 (0.34) | 72,909 | 72.69 | 6.02 (3.53–10.28) | < 0.001 | ||
| Scenario 4 | ||||||||
| Non-Hashimoto’s thyroiditis | 30,004 | 25 (0.08) | 146,719 | 17.04 | Ref. | < 0.001 | 0.844 | |
| Hashimoto’s thyroiditis | 15,129 | 52 (0.34) | 71,481 | 72.75 | 4.27 (2.65–6.88) | < 0.001 | ||
Fig. 1.
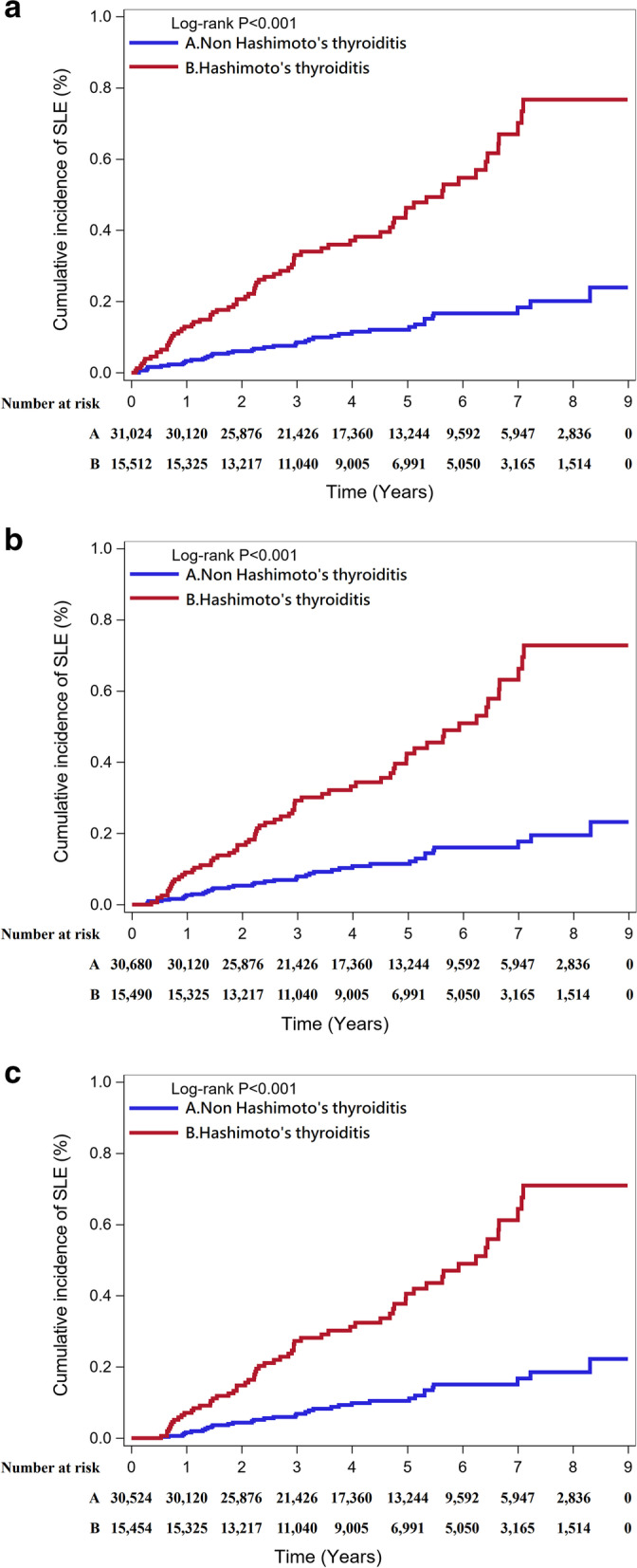
a The cumulative probability of SLE in non-HT and HT patients after PSM 1:2. b The cumulative probability of SLE in non-HT and HT patients after PSM 1:2. Follow-up duration of samples ≧ 3 months. c The cumulative probability of SLE in non-HT and HT patients after PSM 1:2. Follow-up duration of samples ≧ 6 months
Fig. 2.
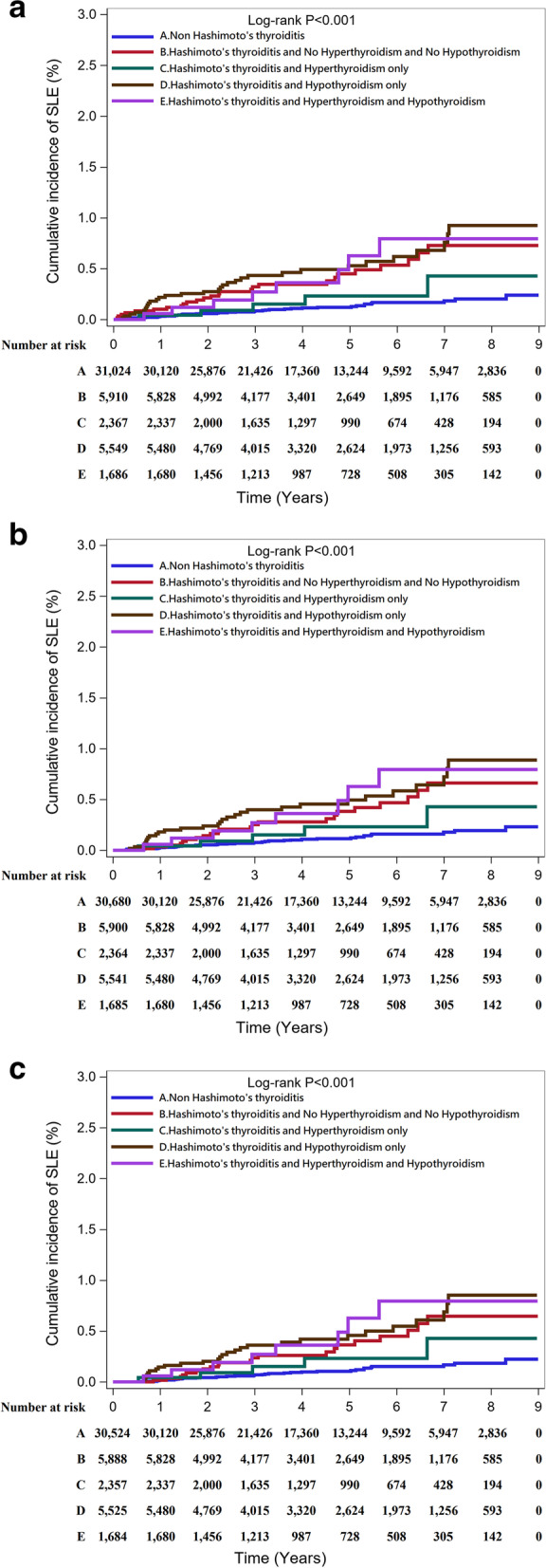
a The cumulative probability of SLE in non-HT and HT patients with hyperthyroidism or hypothyroidism after PSM 1:2. b The cumulative probability of SLE in non-HT and HT patients with hyperthyroidism or hypothyroidism after PSM 1:2. Follow-up duration of samples ≧ 3 months. c The cumulative probability of SLE in non-HT and HT patients with hyperthyroidism or hypothyroidism after PSM 1:2. Follow-up duration of samples ≧ 6 months
Table 11.
Number at risk after PSM 1:2
| Year after the beginning of this study | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| No restriction on follow-up duration | ||||||||||
| Non-Hashimoto’s thyroiditis | 31,024 | 30,120 | 25,876 | 21,426 | 17,360 | 13,244 | 9592 | 5947 | 2836 | 0 |
| Hashimoto’s thyroiditis | 15,512 | 15,325 | 13,217 | 11,040 | 9005 | 6991 | 5050 | 3165 | 1514 | 0 |
| Hashimoto’s thyroiditis, no hyperthyroidism, and no hypothyroidism | 5910 | 5828 | 4992 | 4177 | 3401 | 2649 | 1895 | 1176 | 585 | 0 |
| Hashimoto’s thyroiditis and hyperthyroidism only | 2367 | 2337 | 2000 | 1635 | 1297 | 990 | 674 | 428 | 194 | 0 |
| Hashimoto’s thyroiditis and hypothyroidism only | 5549 | 5480 | 4769 | 4015 | 3320 | 2624 | 1973 | 1256 | 593 | 0 |
| Hashimoto’s thyroiditis, hyperthyroidism, and hypothyroidism | 1686 | 1680 | 1456 | 1213 | 987 | 728 | 508 | 305 | 142 | 0 |
| Follow-up duration of samples ≧ 3 months | ||||||||||
| Non-Hashimoto’s thyroiditis | 30,680 | 30,120 | 25,876 | 21,426 | 17,360 | 13,244 | 9592 | 5947 | 2836 | 0 |
| Hashimoto’s thyroiditis | 15,490 | 15,325 | 13,217 | 11,040 | 9005 | 6991 | 5050 | 3165 | 1514 | 0 |
| Hashimoto’s thyroiditis, no hyperthyroidism, and no hypothyroidism | 5900 | 5828 | 4992 | 4177 | 3401 | 2649 | 1895 | 1176 | 585 | 0 |
| Hashimoto’s thyroiditis and hyperthyroidism only | 2364 | 2337 | 2000 | 1635 | 1297 | 990 | 674 | 428 | 194 | 0 |
| Hashimoto’s thyroiditis and hypothyroidism only | 5541 | 5480 | 4769 | 4015 | 3320 | 2624 | 1973 | 1256 | 593 | 0 |
| Hashimoto’s thyroiditis, hyperthyroidism, and hypothyroidism | 1685 | 1680 | 1456 | 1213 | 987 | 728 | 508 | 305 | 142 | 0 |
| Follow-up duration of samples ≧ 6 months | ||||||||||
| Non-Hashimoto’s thyroiditis | 30,524 | 30,120 | 25,876 | 21,426 | 17,360 | 13,244 | 9592 | 5947 | 2836 | 0 |
| Hashimoto’s thyroiditis | 15,454 | 15,325 | 13,217 | 11,040 | 9005 | 6991 | 5050 | 3165 | 1514 | 0 |
| Hashimoto’s thyroiditis, no hyperthyroidism, and no hypothyroidism | 5888 | 5828 | 4992 | 4177 | 3401 | 2649 | 1895 | 1176 | 585 | 0 |
| Hashimoto’s thyroiditis and hyperthyroidism only | 2357 | 2337 | 2000 | 1635 | 1297 | 990 | 674 | 428 | 194 | 0 |
| Hashimoto’s thyroiditis and hypothyroidism only | 5525 | 5480 | 4769 | 4015 | 3320 | 2624 | 1973 | 1256 | 593 | 0 |
| Hashimoto’s thyroiditis, hyperthyroidism, and hypothyroidism | 1684 | 1680 | 1456 | 1213 | 987 | 728 | 508 | 305 | 142 | 0 |
Tables 5, 6, and 7 show the results of univariable and multivariable Cox regression analyses. The adjusted hazard ratio in HT exposure alone (model 1) was 6.79 (95% CI: 5.32–8.66), which indicates that the increased risk of SLE in the HT exposure and other thyroid disorders was also analyzed, and shows that patients with either HT, hyperthyroidism, or hypothyroidism were all supposed to have an increased SLE incident risk. The aHR of model 2a, with demographic adjustment including sex, age, urbanization, low income, length of hospital stays at baseline, and times of outpatient department visits, was 5.83 (95% CI: 4.50–7.56) and showed increasing risk on long hospital stay patients. Model 3a shows that the aHR after adjustment of demographic variables, medical utilization, and comorbidities at baseline was 4.35 (95% CI: 3.28–5.76). Autoimmune diseases listed in the table including RA, SS, SSc, vasculitis, AIHA, and ITP as comorbidities also increased the incidence rate of SLE compared with the comparator group without HT. Models 2b and 3b included the variations of HT, hyperthyroidism, and hypothyroidism; these models still reveal similar results. HT, hyperthyroidism, and hypothyroidism were all supposed to increase the SLE incident risk, and the HT only group shows the highest aHR of 6.52 (4.55–9.34) in model 2b and second highest aHR of 4.53 (3.08–6.66) in model 3b; compared to HT combined with hyperthyroidism, those who have combined HT and hypothyroidism were more likely to develop SLE. Demographic variables were similar to the previous description. As for the comorbidities analysis, a high hazard ratio of SLE was also found in other autoimmune diseases such as RA, SS, SSc, and vasculitis.
After 1:2 PSM, the HR of the conditional Cox model with HT exposure alone (model A) was 3.54 (95% CI: 2.40–5.22). In model B, the group of HT and hypothyroidism only presented the highest aHR of 4.27 (95% CI: 2.67–6.83), followed by HT, no hyperthyroidism, and no hypothyroidism: 3.47 (95% CI: 2.12–5.69) and HT, hyperthyroidism, and hypothyroidism: 3.77 (95% CI: 1.76–8.05). The least risk was HT and hyperthyroidism only, aHR: 1.72 (95% CI: 0.68–4.35), with no significant difference compared with the result before PSM. The group of the 3 months barrier for following up time shares the same results, which are presented in Table 6, but the group of 6 months barrier presents the highest aHR of 4.33 (95% CI: 2.01–9.32), presented in Table 7.
We also conducted the sensitivity analysis in the estimation of the SLE risk for HT exposure in age- and sex-matched population. In the 4 scenarios, 2 different SLE treatment plan and the exclusion of other autoimmune diseases were listed in Tables 8, 9, and 10. Under the constructive of model 3, the aHR was 4.35 (95% CI: 3.28–5.76) in scenario 1, 4.39 (95% CI: 3.31–5.82) in scenario 2, 5.11 (95% CI: 3.75–6.98) in scenario 3, and 4.70 (95% CI: 3.46–6.38) in scenario 4. The sensitivity analysis was also performed on the groups of the 3 and 6 months barrier, and all 4 scenarios in the 3 groups have high enough aHRs to support the major result.
Discussion
Although previous studies about thyroid and SLE showed that SLE patients are prone to develop hypothyroidism [2, 17], this study indeed told us that HT might also be associated with SLE (Table 11). In this population-based study in Taiwan, we found patients with a history of HT (aHR: 6.79, 95% CI: 5.32–8.66) or HT with hypothyroidism (aHR: 7.59, 95% CI: 5.34–10.81) were vulnerable to develop SLE compared to non-HT, no hyperthyroidism, and no hypothyroidism. Besides, hyperthyroidism was also a minor risk factor for SLE with a less ratio (aHR: 3.21, 95% CI: 1.43–7.21). More interestingly, in those HT patients who were combined with hyperthyroidism, the incidence of SLE decreased slightly but still higher than in the comparator group without HT. On the other hand, if HT patients once had hypothyroidism, then whether they had hyperthyroidism or not, the incidence of SLE is hardly affected compared to those with hypothyroidism only.
The reason that patients with HT are prone to develop SLE needs to be clarified. In our opinion, first, impairment of regulatory T cells (Treg) might be a key. Impaired Treg might cause the loss of self-toleration and increases the risk of autoimmune disease, including SLE [20], and according to previous studies, the loss of Treg function was found in both HT and SLE [21–23]. Second, interleukin-17 (IL-17) and Th17 which are known to participate in inflammation [24] also play important roles in autoimmune diseases, including HT and SLE [25, 26]. A study pointed out that the more IL-17 is produced by Th17, the more thyroid function is lost in HT patients [27]. As SLE shares a similar pathogenesis [26], elevated Th17 and IL-17 in HT might stimulate the progression of SLE. Third, the common presence of antinuclear antibodies (ANA) in HT patients might be a crucial factor to induce other autoimmune diseases, including SLE. A study evaluating HT patients showed that 47% of HT patients were ANA positive, and 72% of them have other autoimmunity parameters besides anti-thyroid peroxidase (anti-TPO) or antithyroglobulin (anti-Tg), and/or have an autoimmune disease besides HT [28], indicating that it is possible for HT patients to come out with other autoimmune diseases. ANA is also a highly sensitive (98%) screening marker for SLE [29]. Thus, the common presence of ANA in patients with HT might be a crucial factor to induce the development of SLE. Last, studies have shown that phagocytosis was stimulated by physiological concentrations of thyroid hormone [30], and it was decreased after thyroid suppression in an animal model [31]. It means HT patients with hypothyroidism might lose the ability to clean up the autoimmune complex and develop SLE [32], which might explain why patients with hypothyroidism have a higher risk of SLE than hyperthyroidism in our study.
The finding of HT being associated with subsequent SLE is important in clinical practice. HT is not a difficult disease to treat, however, its complications are usually forgotten, for example, thyroid lymphoma [33]. And now SLE might also be another potential complication of HT according to our study. Once SLE has been developed, it will affect the whole body from sleep disturbance [34] to pulmonary complications [35] and cardiovascular disease [36], deteriorating the health condition. Moreover, since genetic factors cannot be modified easily, it seems important to investigate the other risk factors of SLE. Early identification and intervention of SLE will minimize the deterioration and damage to the body [37].
Previous studies have told us some environmental factors causing SLE. Besides ultraviolet radiation [38], bacteria and virus infections have also been proven that they are linked to SLE by affecting the immune system [39, 40], including nontyphoidal– Salmonella [41] and Varicella zoster virus [42]. Hormones have also been investigated for their correlation with SLE. For example, estrogen can rescue autoreactive B cells from apoptosis [43], explaining why females are more prone to autoimmune disease. Progesterone can regulate CD4+ T cells [44] to prevent pregnant women from producing anti-fetal antibodies, so low levels of progesterone are considered as a predisposing factor for SLE [45]. According to many studies, thyroid hormone also can affect the immune system [10–16]. Acquiring any kind of autoimmune disease is another risk to developing another one with unclear reasons, and when there is more than three autoimmune coexistence, it is called as “multiple autoimmune syndrome (MAS)” [8, 9], and HT is classified as one of MAS [46], which might be a risk factor of developing SLE.
Despite that many studies indicated the function of thyroid hormone in the immune system [10–16] and the hypothesis about MAS increasing the risk of SLE, there are only two case reports about two young girls and two women, respectively, implying HT might be a risk factor of SLE [18, 19]. This time, we conducted a large-scale retrospective cohort study, which is more persuasive than a case report, attempting to prove this thought.
In our study, we not only surveyed the relation between HT and SLE, but also consider other factors. We have described above the thought of MAS being a factor in developing SLE, and now, our data supported it again with an extremely higher hazard ratio of SLE with other autoimmune diseases, including RA (aHR: 14.57, CI: 9.51–22.33), SS (aHR: 20.96, CI: 13.92–31.56), SSc (aHR: 61.44, CI: 19.78–190.79), vasculitis (aHR: 81.34, CI: 36.38–181.88), AIHA (aHR: 417.97, CI: 104.30–1674.87), and ITP (aHR: 67.09, CI: 27.81–161.85). On the other hand, we also found the possibility of HT alone increasing the risk of SLE by excluding other common autoimmune diseases (aHR: 4.70, CI: 3.46–6.38), which means SLE following HT might not be attributed to other autoimmune diseases.
In addition, we considered whether the status of thyroid function would affect the risk of SLE. The results showed a higher risk of SLE among patients with hypothyroidism. Despite the absolute rate being low, the increased hazard ratio of SLE gave clinical physicians a hint for caring for these HT patients. This outcome might be explained by the ability of phagocytosis of the autoimmune complex which we have mentioned above [30–32]. However, the detailed mechanism between HT and SLE still needs to be clarified.
Our study was validated enough to be a presentative of the general population by using the NHIRD of Taiwan, which has multiple advantages including a great sample size covering over 99% of nationals of Taiwan, and long-term comprehensive follow-up to assess the risk of new-onset SLE in patients with HT [47]. In addition, we performed a sensitivity analysis to confirm the conclusion of this study by using four different scenarios. In other subgroups, urbanization, low income, length of hospital stays, and other factors were examined. Gender, age, and other factors were adjusted appropriately in PSM to minimize the selection bias.
Despite the advantages mentioned above, there are still some limitations in our study. First, the use of a “major illness registry” might lead to an underestimated incidence of SLE because few patients might not get this identification. Second, smoking status, a confounder factor of SLE [48], was unavailable in the NHIRD. Hence, we used chronic obstructive pulmonary disease as a surrogate variable for cigarette smoking because of its close correlation with cigarettes [49]. Third, our study cannot explain why HT patients with hyperthyroidism had a decreased incidence of SLE despite the fact that excessive thyroid hormone can also impair Treg cells [50]. It cannot prove the pathogenesis of SLE following HT directly, either. Thus, the mechanism still needs to be investigated through more experiments. Fourth, although we have adjusted many confounders, we still missed genetic factors which are associated with both HT and SLE due to the difficulty of collecting genetic data. Last, because people recorded in NHIRD are usually Taiwanese, this study might not be applicable to non-Asian ethnic groups.
In conclusion, this population-based study suggested an increased risk of SLE in the HT group after adjustment for baseline characteristics, comorbidities, and medical confounders compared with the reference group. It could provide hints for further research to clarify the pathogenesis between HT, hypothyroidism, hyperthyroidism, and SLE.
Acknowledgements
This study was supported by Taichung Veterans General Hospital.
Patient and Public Involvement patients and/or the public were not involved in the design, conduct, reporting, or dissemination of plans of this research.
Patient consent for publication is not required because the data we used comprised a de-identified secondary data set which had been released for research purposes and analyzed anonymously.
Abbreviations
- SLE
Systemic lupus erythematosus
- HT
Hashimoto’s thyroiditis
- DMARDs
Disease-modifying anti-rheumatic drugs
- HCQ
Hydroxychloroquine
- RA
Rheumatic arthritis
- SS
Sjögren’s syndrome
- SSc
Systematic sclerosis
- AS
Ankylosing spondylitis
- IBD
Inflammatory bowel disease
- COPD
Chronic obstructive pulmonary disease
- CKD
Chronic kidney disease
- AIHA
Autoimmune hemolytic anemia
- ITP
Idiopathic thrombocytopenic purpura
- ANA
Antinuclear antibody
- MAS
Multiple autoimmune syndrome
Authors’ contributions
All authors were involved in the drafting of the article or revising it, and all authors approved the final version to be published. Study conception and design: H-HC and JC-CW. Acquisition of data: H-HC and JC-CW. Analysis and interpretation of the data: H-HC, H-CL, H-MC, and Y-MH. Writing (original draft preparation): H-CL and H-MC. Writing (review and editing): Y-MH and RC. The author(s) read and approved the final manuscript.
Funding
This study did not receive any funding support.
Availability of data and materials
Summarized individual data are available on request to the corresponding author. The data set used in this study is managed by the Taiwan Ministry of Health and Welfare and, thus, cannot be made available publicly. Researchers interested in accessing this data set can submit a formal application to the Ministry of Health and Welfare to request access (the postal address No. 488, Section 6, Zhongxiao E Rd, Nan-gang District, Taipei City 115, Taiwan; website: https://dep.mohw.gov.tw/ DOS/cp-2516-3591-113.html).
Declarations
Ethics approval and consent to participate
The Institutional Review Board of Taichung Veterans General Hospital (number: CE17100B) approved this study. The requirement for informed consent was waived as personal information was anonymized before data analyses.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hong-Ci Lin and Hsu-Min Chang contributed equally to this work.
Contributor Information
Hong-Ci Lin, Email: henry991216@gmail.com.
Hsu-Min Chang, Email: kevin970918@gmail.com.
Yao-Min Hung, Email: ymhung1@gmail.com.
Renin Chang, Email: rhapsody1881@gmail.com.
Hsin-Hua Chen, Email: shc5555@hotmail.com.
James Cheng-Chung Wei, Email: wei3228@gmail.com.
References
- 1.Fava A, Petri M. Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun. 2019;96:1–13. doi: 10.1016/j.jaut.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari SM, Elia G, Virili C, Centanni M, Antonelli A, Fallahi P. Systemic lupus erythematosus and thyroid autoimmunity. Front Endocrinol (Lausanne) 2017;8:138. doi: 10.3389/fendo.2017.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gravano DM, Hoyer KK. Promotion and prevention of autoimmune disease by CD8+ T cells. J Autoimmun. 2013;45:68–79. doi: 10.1016/j.jaut.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201(5):703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deapen D, Escalante A, Weinrib L, Horwitz D, Bachman B, Roy-Burman P, Walker A, Mack TM. A revised estimate of twin concordance in systemic lupus erythematosus. Arthritis Rheum. 1992;35(3):311–318. doi: 10.1002/art.1780350310. [DOI] [PubMed] [Google Scholar]
- 6.Cooper GS, Miller FW, Pandey JP. The role of genetic factors in autoimmune disease: implications for environmental research. Environ Health Perspect. 1999;107(Suppl 5):693–700. doi: 10.1289/ehp.99107s5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Parks CG, Gilkeson GS. Hormonal, environmental, and infectious risk factors for developing systemic lupus erythematosus. Arthritis Rheum. 1998;41(10):1714–1724. doi: 10.1002/1529-0131(199810)41:10<1714::AID-ART3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 8.Cojocaru M, Cojocaru IM, Silosi I. Multiple autoimmune syndrome. Maedica (Bucur) 2010;5(2):132–134. [PMC free article] [PubMed] [Google Scholar]
- 9.Sloka S. Observations on recent studies showing increased co-occurrence of autoimmune diseases. J Autoimmun. 2002;18(3):251–257. doi: 10.1006/jaut.2002.0588. [DOI] [PubMed] [Google Scholar]
- 10.De Vito P, Incerpi S, Pedersen JZ, Luly P, Davis FB, Davis PJ. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. 2011;21(8):879–890. doi: 10.1089/thy.2010.0429. [DOI] [PubMed] [Google Scholar]
- 11.Rubingh J, van der Spek A, Fliers E, Boelen A. The role of thyroid hormone in the innate and adaptive immune response during infection. Compr Physiol. 2020;10(4):1277–1287. doi: 10.1002/cphy.c200003. [DOI] [PubMed] [Google Scholar]
- 12.Robinson MV, Obut TA, Melnikova EV, Trufakin VA. Parameters of cellular and humoral immunity in experimental hyperthyroidism and its correction. Bull Exp Biol Med. 2014;156(4):473–475. doi: 10.1007/s10517-014-2377-4. [DOI] [PubMed] [Google Scholar]
- 13.Robinson MV, Obut TA, Mel’nikova EV, Trufakin VA. Effects of thyroxin and mercazolyl on immunological parameters of blood lymphocytes and lymphoid organs. Bull Exp Biol Med. 2013;156(2):236–238. doi: 10.1007/s10517-013-2319-6. [DOI] [PubMed] [Google Scholar]
- 14.Marino F, Guasti L, Cosentino M, De Piazza D, Simoni C, Piantanida E, Cimpanelli M, Klersy C, Bartalena L, Venco A, et al. Thyroid hormone regulation of cell migration and oxidative metabolism in polymorphonuclear leukocytes: clinical evidence in thyroidectomized subjects on thyroxine replacement therapy. Life Sci. 2006;78(10):1071–1077. doi: 10.1016/j.lfs.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Ortega E, Forner MA, Garcia JJ, Rodriguez AB, Barriga C. Enhanced chemotaxis of macrophages by strenuous exercise in trained mice: thyroid hormones as possible mediators. Mol Cell Biochem. 1999;201(1-2):41–47. doi: 10.1023/a:1007020804138. [DOI] [PubMed] [Google Scholar]
- 16.Magsino CH, Jr, Hamouda W, Ghanim H, Browne R, Aljada A, Dandona P. Effect of triiodothyronine on reactive oxygen species generation by leukocytes, indices of oxidative damage, and antioxidant reserve. Metabolism. 2000;49(6):799–803. doi: 10.1053/meta.2000.6263. [DOI] [PubMed] [Google Scholar]
- 17.Watad A, Mahroum N, Whitby A, Gertel S, Comaneshter D, Cohen AD, Amital H. Hypothyroidism among SLE patients: case-control study. Autoimmun Rev. 2016;15(5):484–486. doi: 10.1016/j.autrev.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Bakr A, Laimon W, El-Ziny MA, Hammad A, El-Hawary AK, Elsharkawy AA, El-Refaey AM, Salem NA, El-Mougy A, Zedan MM, et al. The emergence of systemic lupus erythematosus in hypothyroid patients: two case reports and mini review. Lupus. 2014;23(8):825–828. doi: 10.1177/0961203314525866. [DOI] [PubMed] [Google Scholar]
- 19.Dhir R, Ahluwalia AI, Sridhar J, Mani H, Pruthi HS, Shah KM. Autoimmune thyroiditis perdating the presentation of systemic lupus erythematosus: two cases and a review of literature. Indian J Dermatol Venereol Leprol. 2002;68(5):292–294. [PubMed] [Google Scholar]
- 20.Li W, Deng C, Yang H, Wang G. The regulatory T cell in active systemic lupus erythematosus patients: a systemic review and meta-analysis. Front Immunol. 2019;10:159. doi: 10.3389/fimmu.2019.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glick AB, Wodzinski A, Fu P, Levine AD, Wald DN. Impairment of regulatory T-cell function in autoimmune thyroid disease. Thyroid. 2013;23(7):871–878. doi: 10.1089/thy.2012.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheinecker C, Bonelli M, Smolen JS. Pathogenetic aspects of systemic lupus erythematosus with an emphasis on regulatory T cells. J Autoimmun. 2010;35(3):269–275. doi: 10.1016/j.jaut.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Hu Y, Zhang L, Chen H, Liu X, Zheng X, Shi H, Jiang L, Cui D. Analysis of regulatory T cell subsets and their expression of Helios and PD-1 in patients with hashimoto thyroiditis. Int J Endocrinol. 2019;2019:5368473. doi: 10.1155/2019/5368473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 25.Tabarkiewicz J, Pogoda K, Karczmarczyk A, Pozarowski P, Giannopoulos K. The role of IL-17 and Th17 lymphocytes in autoimmune diseases. Arch Immunol Ther Exp (Warsz) 2015;63(6):435–449. doi: 10.1007/s00005-015-0344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pernis AB. Th17 cells in rheumatoid arthritis and systemic lupus erythematosus. J Intern Med. 2009;265(6):644–652. doi: 10.1111/j.1365-2796.2009.02099.x. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Cai W, Gu R, Zhang Y, Zhang H, Tang K, Xu P, Katirai F, Shi W, Wang L, et al. Th17 cell plays a role in the pathogenesis of Hashimoto’s thyroiditis in patients. Clin Immunol. 2013;149(3):411–420. doi: 10.1016/j.clim.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Fiducia M, Lauretta R, Lunghi R, Kyanvash S, Pallotti S. Hashimoto’s thyroiditis and autoimmunity parameters: descriptive study. Minerva Med. 2007;98(2):95–99. [PubMed] [Google Scholar]
- 29.Wichainun R, Kasitanon N, Wangkaew S, Hongsongkiat S, Sukitawut W, Louthrenoo W. Sensitivity and specificity of ANA and anti-dsDNA in the diagnosis of systemic lupus erythematosus: a comparison using control sera obtained from healthy individuals and patients with multiple medical problems. Asian Pac J Allergy Immunol. 2013;31(4):292–298. doi: 10.12932/AP0272.31.4.2013. [DOI] [PubMed] [Google Scholar]
- 30.Perrotta C, Buldorini M, Assi E, Cazzato D, De Palma C, Clementi E, Cervia D. The thyroid hormone triiodothyronine controls macrophage maturation and functions: protective role during inflammation. Am J Pathol. 2014;184(1):230–247. doi: 10.1016/j.ajpath.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Hampton LMT, Finch MG, Martyniuk CJ, Venables BJ, Jeffries MKS. Developmental thyroid disruption causes long-term impacts on immune cell function and transcriptional responses to pathogen in a small fish model. Sci Rep. 2021;11(1):14496. doi: 10.1038/s41598-021-93929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma C, Xia Y, Yang Q, Zhao Y. The contribution of macrophages to systemic lupus erythematosus. Clin Immunol. 2019;207:1–9. doi: 10.1016/j.clim.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Dündar HZ, Sarkut P, Kırdak T, Korun N. Primary thyroid lymphoma. Ulus Cerrahi Derg. 2016;32(1):75–77. doi: 10.5152/UCD.2015.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Q, Deng N, Chen S, Cui Y, Du X, Gu Z. Systemic lupus erythematosus is associated with negatively variable impacts on domains of sleep disturbances: a systematic review and meta-analysis. Psychol Health Med. 2018;23(6):685–697. doi: 10.1080/13548506.2018.1442011. [DOI] [PubMed] [Google Scholar]
- 35.Hannah JR, D’Cruz DP. Pulmonary complications of systemic lupus erythematosus. Semin Respir Crit Care Med. 2019;40(2):227–234. doi: 10.1055/s-0039-1685537. [DOI] [PubMed] [Google Scholar]
- 36.Torres A, Askari AD, Malemud CJ. Cardiovascular disease complications in systemic lupus erythematosus. Biomark Med. 2009;3(3):239–252. doi: 10.2217/bmm.09.14. [DOI] [PubMed] [Google Scholar]
- 37.Samnaliev M, Barut V, Weir S, Langham J, Langham S, Wang X, Desta B, Hammond E. Health-care utilization and costs in adults with systemic lupus erythematosus in the United Kingdom: a real-world observational retrospective cohort analysis. Rheumatol Adv Pract. 2021;5(3):rkab071. doi: 10.1093/rap/rkab071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bijl M, Kallenberg CG. Ultraviolet light and cutaneous lupus. Lupus. 2006;15(11):724–727. doi: 10.1177/0961203306071705. [DOI] [PubMed] [Google Scholar]
- 39.Zandman-Goddard G, Shoenfeld Y. Infections and SLE. Autoimmunity. 2005;38(7):473–485. doi: 10.1080/08916930500285352. [DOI] [PubMed] [Google Scholar]
- 40.Navarra SV, Leynes MS. Infections in systemic lupus erythematosus. Lupus. 2010;19(12):1419–1424. doi: 10.1177/0961203310374486. [DOI] [PubMed] [Google Scholar]
- 41.Tu TY, Yeh CY, Hung YM, Chang R, Chen HH, Wei JC. Association between a history of nontyphoidal Salmonella and the risk of systemic lupus erythematosus: a population-based, case-control study. Front Immunol. 2021;12:725996. doi: 10.3389/fimmu.2021.725996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun F, Chen Y, Wu W, Guo L, Xu W, Chen J, Sun S, Li J, Chen Z, Gu L, et al. Varicella zoster virus infections increase the risk of disease flares in patients with SLE: a matched cohort study. Lupus Sci Med. 2019;6(1):e000339. doi: 10.1136/lupus-2019-000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen-Solal JF, Jeganathan V, Hill L, Kawabata D, Rodriguez-Pinto D, Grimaldi C, Diamond B. Hormonal regulation of B-cell function and systemic lupus erythematosus. Lupus. 2008;17(6):528–532. doi: 10.1177/0961203308089402. [DOI] [PubMed] [Google Scholar]
- 44.Hughes GC, Clark EA, Wong AH. The intracellular progesterone receptor regulates CD4+ T cells and T cell-dependent antibody responses. J Leukoc Biol. 2013;93(3):369–375. doi: 10.1189/jlb.1012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arnalich F, Benito-Urbina S, Gonzalez-Gancedo P, Iglesias E, de Miguel E, Gijon-Baños J. Inadequate production of progesterone in women with systemic lupus erythematosus. Br J Rheumatol. 1992;31(4):247–251. doi: 10.1093/rheumatology/31.4.247. [DOI] [PubMed] [Google Scholar]
- 46.Shahbaz A, Aziz K, Umair M, Sachmechi I. Prolonged duration of hashitoxicosis in a patient with Hashimoto’s thyroiditis: a case report and review of literature. Cureus. 2018;10(6):e2804. doi: 10.7759/cureus.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med. 2015;175(9):1527–1529. doi: 10.1001/jamainternmed.2015.3540. [DOI] [PubMed] [Google Scholar]
- 48.Barbhaiya M, Tedeschi SK, Lu B, Malspeis S, Kreps D, Sparks JA, Karlson EW, Costenbader KH. Cigarette smoking and the risk of systemic lupus erythematosus, overall and by anti-double stranded DNA antibody subtype, in the Nurses’ Health Study cohorts. Ann Rheum Dis. 2018;77(2):196–202. doi: 10.1136/annrheumdis-2017-211675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung WS, Kung PT, Chang HY, Tsai WC. Demographics and medical disorders associated with smoking: a population-based study. BMC Public Health. 2020;20(1):702. doi: 10.1186/s12889-020-08858-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong Y, Lu TT, Liu XM, Liu BL, Hu Y, Liu S, Wang J, Li GQ, Mao XM. High levels of thyroid hormone impair regulatory T cell function via reduced PD-1 expression. J Clin Endocrinol Metab. 2021;106(9):2738–2753. doi: 10.1210/clinem/dgab191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Summarized individual data are available on request to the corresponding author. The data set used in this study is managed by the Taiwan Ministry of Health and Welfare and, thus, cannot be made available publicly. Researchers interested in accessing this data set can submit a formal application to the Ministry of Health and Welfare to request access (the postal address No. 488, Section 6, Zhongxiao E Rd, Nan-gang District, Taipei City 115, Taiwan; website: https://dep.mohw.gov.tw/ DOS/cp-2516-3591-113.html).


