Abstract
Unlike typical chorismate mutases, the enzyme from Mycobacterium tuberculosis (MtCM) has only low activity on its own. Remarkably, its catalytic efficiency kcat/Km can be boosted more than 100-fold by complex formation with a partner enzyme. Recently, an autonomously fully active MtCM variant was generated using directed evolution, and its structure was solved by X-ray crystallography. However, key residues were involved in crystal contacts, challenging the functional interpretation of the structural changes. Here, we address these challenges by microsecond molecular dynamics simulations, followed up by additional kinetic and structural analyses of selected sets of specifically engineered enzyme variants. A comparison of wild-type MtCM with naturally and artificially activated MtCMs revealed the overall dynamic profiles of these enzymes as well as key interactions between the C-terminus and the active site loop. In the artificially evolved variant of this model enzyme, this loop is preorganized and stabilized by Pro52 and Asp55, two highly conserved residues in typical, highly active chorismate mutases. Asp55 stretches across the active site and helps to appropriately position active site residues Arg18 and Arg46 for catalysis. The role of Asp55 can be taken over by another acidic residue, if introduced at position 88 close to the C-terminus of MtCM, as suggested by molecular dynamics simulations and confirmed by kinetic investigations of engineered variants.
Introduction
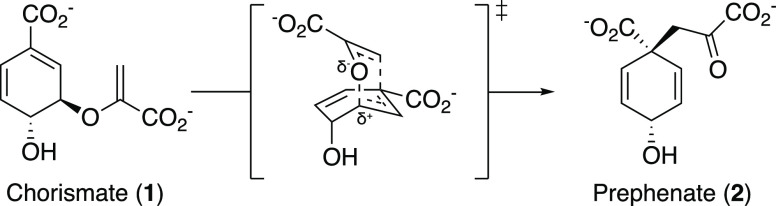
Pericyclic reactions are common in industrial processes, but very rare in biology.1−4 Chorismate mutase (CM) catalyzes the only known pericyclic process in primary metabolism, the Claisen rearrangement of chorismate (1) to prephenate (2), via a chair-like transition state (Scheme 1).5 This catalytic step at the branch point of the shikimate pathway funnels the key metabolite chorismate toward the synthesis of tyrosine and phenylalanine, as opposed to tryptophan and several aromatic vitamins.6,7 The CM reaction is a concerted unimolecular transformation that is well studied by both experimental and computational means.8 It proceeds ostensibly via the same transition state in both solution and enzyme catalysis.9,10 Due to these factors, CM has long been a model enzyme for computational chemists.11
Scheme 1. Chorismate Mutase Reaction.
The Claisen rearrangement catalyzed by chorismate mutase converts chorismate (1) to prephenate (2) and proceeds via a highly polarized chair-like transition state carrying partial charges at the C–O bond that is broken during the reaction.
Natural CMs belong to two main classes with two distinct folds AroH and AroQ, which are equally efficient, with typical kcat/Km values in the range of (1–5) × 105 M–1 s–1.12 The AroH fold, exemplified by the Bacillus subtilis CM, has a trimeric pseudo-α/β-barrel structure,13,14 whereas the structures of AroQ enzymes have all-α-helical folds.15−21 The AroQ family is further divided into four subfamilies, α–δ.20,21 The AroQδ subfamily shows abnormally low catalytic activity compared to prototypical CM enzymes. In fact, the first discovered AroQδ enzyme, the intracellular CM from Mycobacterium tuberculosis (MtCM),20,21 is on its own only a poor catalyst (kcat/Km = 1.8 × 103 M–1 s–1),21 despite its crucial role for producing the aromatic amino acids Tyr and Phe. However, this low activity can be boosted more than 100-fold to a kcat/Km of 2.4 × 105 M–1 s–1 through formation of a noncovalent complex with the first enzyme of the shikimate pathway, 3-deoxy-d-arabino-heptulosonate 7-phosphate (DAHP) synthase (MtDS) (Figure 1A).21
Figure 1.
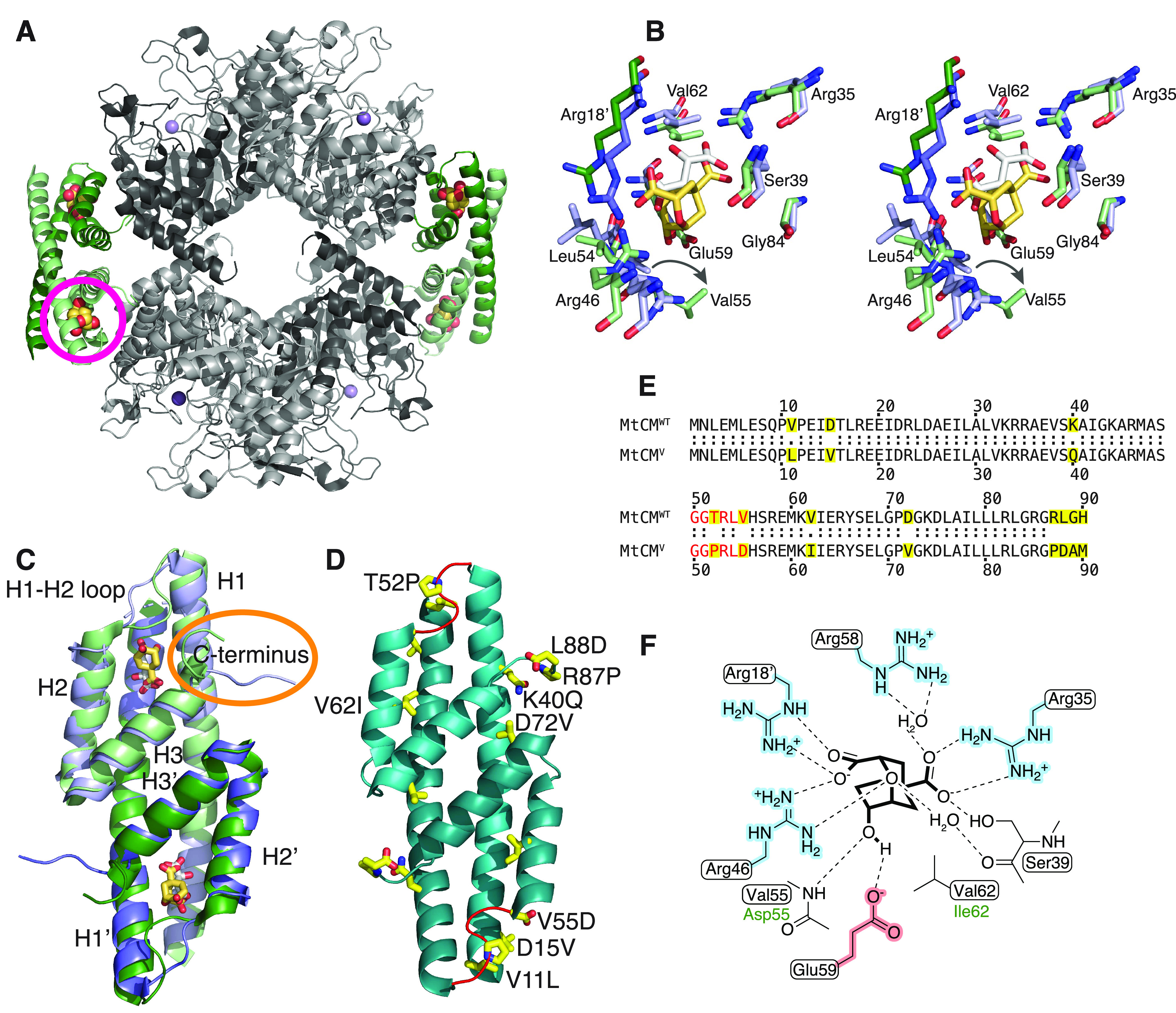
Structural information on M. tuberculosis chorismate mutase. (A) Cartoon illustration of the heterooctameric complex of MtCM with DAHP synthase (MtDS) (Protein Data Bank (PDB) ID: 2W1A).21 MtCM is colored in shades of green and MtDS in shades of gray to emphasize individual subunits; Bartlett’s transition state analogue (TSA)51 is shown with golden spheres. The location of one of the four active sites of MtCM is marked with a circle. (B) Stereo superimposition of CM active sites of MtCM (shades of violet, with malate bound, white sticks) (PDB ID: 2VKL)21 and of the MtCM–MtDS complex (with TSA bound, golden sticks) (PDB ID: 2W1A),21 showing several active site residues as sticks. Shades of violet/green (and prime notation for Arg18′) illustrate separate protomers of MtCM/MtCM–MtDS structures, respectively. An arrow shows the shift in the position of Val55 upon MtDS binding, allowing H-bond formation of its backbone to TSA. (C) Cartoon superimposition of MtCM (PDB ID: 2VKL;21 violet, with white sticks for malate ligand) and activated MtCM (MtCMDS) from the MtCM–MtDS complex (PDB ID: 2W1A;21 green, with golden sticks for TSA). The biggest structural changes upon activation are a kink observed in the H1–H2 loop and interaction of the C-terminus (circled in orange) with the active site of MtCM. (D) Cartoon representation of the artificially evolved MtDS-independent super-active MtCM variant N-s4.15 (PDB ID: 5MPV;12 cyan), dubbed MtCMV in this work, having a kcat/Km typical for the most efficient CMs known to date.12 Amino acid replacements accumulated after four cycles of directed evolution are emphasized as yellow side-chain sticks (A89 and M90 are not resolved) and labeled for one of the protomers. The H1–H2 loop (shown in red) adopts a kinked conformation similar to that observed for the MtDS-activated MtCMDS shown in (C). (E) Sequence alignment of wild-type MtCM (MtCMWT) and the highly active variant N-s4.15 (MtCMV).12 Substituted residues are highlighted in yellow, and the H1–H2 loop is colored red. (F) Schematic representation of the active site of MtCM with bound TSA. Boxed residues refer to the wild-type enzyme, and green font color (Asp55, Ile62) refers to those substituted in MtCMV. Charged residues are highlighted in red and blue.
The active site of AroQ CMs is dominated by positive charges, contributed by four arginine residues (Figure 1F). In MtCM, these are Arg18′, Arg35, Arg46, and Arg58 (with the prime denoting a different MtCM protomer). Of particular importance for catalysis is Arg46,21 or its corresponding cationic residues in other CMs (of both AroH and AroQ families).22 However, high catalytic prowess is only achieved when this cationic residue is optimally positioned such that it can stabilize the developing negative charge at the ether oxygen in the transition state (Scheme 1).11,14,21,23−25 In MtCM, this is not the case unless MtCM is activated by MtDS.21 The MtDS partner repositions residues of the C-terminus of MtCM for interaction with the H1–H2 loop of MtCM that covers its active site, thereby inducing a characteristic kink in this loop (orange circle in Figure 1C). This interaction leads to a rearrangement of active site residues to catalytically more favorable conformations (Figure 1B)21 and is likely a key contributing factor for the increase in CM activity, as shown by randomizing mutagenesis of the C-terminal region followed by selection for functional variants.26 Complex formation also endows MtCM with feedback regulation by Tyr and Phe through binding of these effectors to the MtDS partner.21,27,28 Such inter-enzyme allosteric regulation28 allows for dynamic adjustment of the CM activity to meet the changing needs of the cell.
The naturally low activity of MtCM in the absence of its MtDS partner enzyme also provided a unique opportunity for laboratory evolution studies aimed at increasing MtCM efficiency. After four major rounds of directed evolution, the top-performing MtCM variant N-s4.15 emerged,12 which is abbreviated as MtCMV in this manuscript. This variant showed autonomous CM activity (kcat/Km = 4.7 × 105 M–1 s–1) twice exceeding that of wild-type MtCM in the MtCM–MtDS complex, and can no longer be activated further through the addition of MtDS.12 The biggest gains in catalytic activity were due to replacements T52P and V55D in the H1–H2 loop and R87P, L88D, G89A, and H90M at the C-terminus (Figure 1C–E). Of these residues, Pro52 and Asp55 are conserved in the H1–H2 loop of naturally highly active CMs, such as the prototypic CMs from the α- and γ-AroQ subclasses, i.e., EcCM from Escherichia coli(16) and *MtCM, the secreted CM from M. tuberculosis,20 respectively.12 The single amino acid exchange that had the largest beneficial effect on activity was V55D (12-fold enhancement of kcat/Km), followed by T52P (6-fold gain).12 Combined, these two changes, discussed in detail in a previous publication, gave a kcat/Km that was 22 times higher compared to wild-type MtCM.12 The four C-terminal amino acid replacements together increased the activity more modestly (by a factor of 4), and the five exchanges introduced in the two final evolutionary rounds yielded an additional factor of 5. The resulting combination of large-impact and more subtle residue substitutions in MtCMV (Figure 1D,E) gave a kcat/Km about 500 times greater than that of the parental starting point, thereby reaching the values of the most efficient CMs known to date.12
The crystal structure of MtCMV revealed a strongly kinked conformation of the H1–H2 loop. This is reminiscent of the conformation adopted by MtCM when in the complex with MtDS (the crystal structure of MtDS-bound MtCM is in the following referred to as MtCMDS) and differs considerably from that observed in free MtCM (Figure 1C,D).12 However, in the crystal structures of free wild-type and top-evolved MtCMV, both the H1–H2 loop and the C-terminus are involved in extensive crystal contacts, making an unbiased structural evaluation of the sequence alterations in these parts of the enzyme impossible. In solution, these regions are assumed to be more flexible compared to the α-helical segments of MtCM.
Here, we used molecular dynamics (MD) simulations to investigate the behavior of MtCM in the absence or presence of ligands and to analyze whether the protein is able to interconvert between activated and nonactivated conformations in the absence of the MtDS partner enzyme. We also compared the wild-type MtCM with the evolved MtCMV, to see if the acquired amino acid substitutions introduced any new interactions or if they altered the probabilities of existing ones, with potential impact on catalytic activity. From an assessment of the dynamic properties of MtCM and MtCMV, we proposed a set of single, double, and triple C-terminal variants of the enzyme and subsequently tested these experimentally.
Materials and Methods
Construction of Untagged MtCM Variants
General cloning was carried out in E. coli DH5α or XL1-Blue (both Stratagene, La Jolla, California). All cloning techniques and bacterial culturing were performed according to standard procedures.29 Oligonucleotide synthesis and DNA sequencing were performed by Microsynth AG (Balgach, Switzerland).
For the construction of expression plasmids pKTCMM-H-V55D and pKTCMM-H-T52P for the native MtCM single variants, the individual site-directed mutants were first constructed in the pKTNTET background (providing an N-terminal His6 tag, first 5 residues missing). Parts of the MtCM gene (Gene Rv0948c) were amplified using oligonucleotides 412-MtCM-N-V55D (5′-GTTCGCTAGCGGAGGTACACGTTTGGATCATAGTCGGGAGATGAAGGTCATCGAAC) or 413-MtCM-N-T52P (5′-GTTCGCTAGCGGAGGTCCGCGTTTGGTCCATAGTCGGGAGATGAAGGTCATCGAAC) together with oligonucleotides 386-LpLib-N2 (5′-GGTTAAAGCTTCCGCAGCCACTAGTTATTAGTGACCGAGGCGGCCACGGCCCAAT) on template pMG24812 to create a 163 bp PCR product. The PCR products were restriction digested with NheI and HindIII and the resulting 148 bp fragments were individually ligated to the accordingly cut 2873 bp fragment from acceptor vector pKTNTET-0.12 The ligation was performed with T4 DNA ligase (New England Biolabs, Ipswich, Massachusetts) overnight at 16 °C. The ligation products were transformed into chemically competent E. coli XL1-Blue cells. The cloned PCR’ed DNA fragments were confirmed by Sanger sequencing. Subsequently, the genes for MtCM-T52P and MtCM-V55D were isolated by restriction digestion using enzymes XhoI and SpeI followed by a preparative agarose gel, yielding corresponding 260 bp fragments. pKTCMM-H21 was used as acceptor vector and was accordingly restriction digested with XhoI and SpeI, yielding a 4547 bp acceptor fragment. The fragments were ligated overnight at 16 °C, using T4 DNA ligase. The ligation products were transformed into chemically competent E. coli KA12 cells23 and the inserts were analyzed by Sanger sequencing. The gene for variant PHS10-3p3,12 carrying an N-terminal His6-tag and missing the first five residues, was recloned into the native format provided by plasmid pKTCMM-H. Acceptor vector pKTCMM-H and pKTNTET-PHS10-3p3 were restriction digested with XhoI and SpeI, and the fragments were isolated from preparative agarose gels. The 4547 bp and 260 bp fragments were ligated overnight at 16 °C with T4 DNA ligase and transformed into chemically competent XL1-Blue cells. The relevant gene sequence was confirmed by Sanger sequencing.
Different C-terminal variants of the MtCM gene were generated by PCR mutagenesis. DNA fragments were amplified with the same forward primer (containing an NdeI site, underlined) and different reverse primers (containing an SpeI site, underlined) on different DNA templates. The gene encoding MtCM L88D was produced by PCR with primers LB5 (5′-TCCGCACATATGAACCTGGAAATG) and LB4 (5′-TAAGCAACTAGTTATTAGTGACCGTCGCG) on the template plasmid pKTCMM-H carrying the wild-type gene.21 The gene for the triple variant MtCM (T52P V55D L88D) was assembled with primers LB5 and LB4 on a pKTCMM-H derivative containing MtCM variant 3p3 (T52P V55D).12 The gene for MtCM variant PNAM (D88N) was generated with primers LB5 and LB6 (5′-TAAGCAACTAGTTATTACATAGCATTCGGA), and for the MtCM variant PLAM (D88L) with primers LB5 and LB7 (5′-TAAGCAACTAGTTATTAGTGACCAAGCGGA), in both cases using a version of the template plasmid pKTCMM-H, into which the gene for the top-evolved s4.15 variant had been inserted.12 The resulting 296 bp PCR fragments containing NdeI and SpeI restriction sites at the 5′ and 3′ ends of the MtCM gene, respectively, were digested with the corresponding enzymes to yield 278 bp fragments. These fragments were ligated to the 4529 bp NdeI–SpeI fragment of pKTCMM-H yielding the final 4807 bp plasmids.
Protein Production and Purification
E. coli strain KA1318,30 carrying an endogenous UV5 Plac-expressed T7 RNA polymerase gene was used to overproduce the (untagged) MtCM variants. KA13 cells were transformed by electroporation with the appropriate pKTCMM-H plasmid derivative that carries the desired MtCM gene variant.
For the two crystallized MtCM variants T52P (MtCMT52P) and V55D (MtCMT52P), the transformed cells were grown in baffled flasks at 30 °C in LB medium containing 100 μg/mL sodium ampicillin until the OD600 reached 0.5. Gene expression was induced through the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 0.5 mM, and incubation was continued overnight. The cells were harvested by centrifugation (6500g for 20 min at 4 °C) and frozen at −80 °C before being resuspended in a buffer suitable for ion exchange chromatography, supplemented with DNase I (Sigma), 150 μM phenylmethanesulfonyl fluoride (PMSF) and cOmplete protease inhibitor cocktail (Roche). The cells were lysed using BeadBeater (BioSpec BSP 74340, Techtum Lab AB), with four times 30 s pulses with a 60 s wait between each pulse. Insoluble debris was removed by centrifugation (48,000g for 30 min at 4 °C).
The resuspension buffer was selected based on the theoretical isoelectric point (pI) of the protein. MtCM T52P has a pI of 8.14, so the pellet was resuspended in 50 mM 2-(N-morpholino)ethanesulfonic acid (MES), pH 6.5. MtCM V55D has a pI of 6.74; therefore, the pellet was resuspended in 50 mM acetic acid, pH 5.25. After lysis and centrifugation, the soluble lysate was loaded onto a HiTrap XL SP column (GE Healthcare) for cation exchange chromatography and eluted with a 0–0.5 M NaCl gradient. The purity of the eluted fractions was gauged by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis and sufficiently pure fractions were pooled and concentrated using concentrator tubes with a 5 kDa molecular mass cutoff (Vivaspin MWCO 5K). The proteins were then further purified by size-exclusion chromatography using a Superdex 75 300/10 column (GE Healthcare) with running buffer 20 mM 1,3-bis[tris(hydroxymethyl)methylamino]propane (BTP), pH 7.5, 150 mM NaCl. Finally, the proteins were concentrated (Vivaspin MWCO 5K), frozen, and stored at −80 °C.
For the sets of MtCM variants probed for the catalytic impact of particular C-terminal amino acid exchanges, 500 mL LB medium cultures containing 150 μg mL–1 sodium ampicillin were inoculated with 5 mL overnight culture of the desired transformant and grown at 37 °C and 220 rpm shaking to an OD600 nm of 0.3–0.5. Protein production was induced by the addition of IPTG to 0.5 mM, and culture growth was continued overnight at 30 °C.
The cells were harvested by centrifugation (17,000g for 10 min at 4 °C) and washed once with 100 mM tris(hydroxymethyl)aminomethane (Tris)–HCl, pH 7.5. The cells were pelleted again, and the cell pellet was either frozen for storage at −20 °C or directly resuspended in 80 mL of sonication buffer (50 mM sodium phosphate, 0.3 M NaCl, pH 7.0). The cells were disrupted by sonication on ice (15 min total pulse time with 45 s pulse/30 s pause cycles at 50% amplitude; Q700 sonicator, QSonica). The crude lysate was cleared by centrifugation (20,000g for 20 min at 4 °C). The supernatant was supplemented with sonication buffer to 100 mL, 42 g of ammonium sulfate was added, and the solution was stirred at 4 °C for 1.5 h. The precipitate was pelleted by centrifugation (10,000g for 30 min at 4 °C), dissolved in 8 mL of low-salt buffer (20 mM piperazine, pH 9.0), and dialyzed against 1 L of low-salt buffer overnight. Dialysis was repeated against another 1 L of low-salt buffer for 3 h before application to a MonoQ (MonoQ HR 10/10, Pharmacia) FPLC column (Biologic Duoflow system, Bio-Rad). The sample was eluted over 80 mL in 20 mM piperazine by applying a gradient from 0 to 30% of a high-salt buffer (20 mM piperazine, 1 M NaCl, pH 9.0).
The MonoQ fractions containing the protein of interest were pooled and concentrated to less than 1 mL. The concentrated sample was directly applied to a gel-filtration column (Superdex Increase 75 10/300 GL, GE Healthcare) and eluted in 20 mM BTP, 150 mM NaCl, pH 7.5. Protein identity was confirmed by liquid chromatography–mass spectrometry (LC-MS) (MoBiAS facility, Laboratory of Organic Chemistry, ETH Zurich), with the observed mass being within 1 Da of the calculated mass. Protein purity was assessed by SDS-PAGE (PhastGel Homogenous 20 precast gels, GE Healthcare) and the enzyme concentration ([E]) was determined using the Bradford assay.31
X-ray Crystallography
MtCM variants T52P (MtCMT52P) and V55D (MtCMT52P) were crystallized in 96-well two-drop MRC crystallization plates (SWISSCI) by the sitting drop vapor diffusion technique. Diffraction-quality crystals of MtCMT52P grew at 20 °C from a 1:1 (375 nL + 375 nL) mixture of protein (28 mg mL–1 in 20 mM BTP, pH 7.5) and reservoir solution containing 0.2 M sodium malonate, 20% PEG 3350 (w/v), and 0.1 M Bis Tris propane buffer, pH 8.5 (PACT premier crystallization screen, condition H12; Molecular Dimensions Ltd.). Crystals of MtCMV55D were obtained from a 1:1 (375 nL + 375 nL) mixture of protein (44 mg mL–1 in 20 mM Bis Tris propane, pH 7.5, 150 mM NaCl) and reservoir solution containing 0.2 M zinc acetate dihydrate, 10% w/v PEG 3000, and 0.1 M sodium acetate, pH 4.5 (JCSG-plus crystallization screen, condition C7; Molecular Dimensions Ltd.) at 20 °C.
Diffraction data of MtCMT52P and MtCMV55D crystals were collected at the European Synchrotron Radiation Facility (ESRF, Grenoble, France) at the ID30A-3/MASSIF-3 (Dectris Eiger X 4M detector) and ID29 (Pilatus detector) beamlines, respectively, covering 120° with 0.1° oscillation. Diffraction images were integrated and scaled using the XDS software package;32 merging and truncation were performed with AIMLESS(33) from the CCP4 program suite.34 Since data collection statistics of both crystals suggested the presence of anisotropy, the XDS output was reprocessed for anisotropy correction and truncation using the STARANISO server.35 The “aniso-merged” output files (merged MTZ file with an anisotropic diffraction cutoff) were subsequently used for structure solution and refinement (Table S1).
The crystal structures of MtCMT52P and MtCMV55D were solved by molecular replacement with the program Phaser.36 The structure of the top-evolved MtCM variant MtCMV (PDB ID: 5MPV)12 was used as a search model for solving the structure of MtCMT52P since it was expected to be a better match at the Pro52-containing H1–H2 loop compared to wild-type MtCM. For MtCMV55D, we used the MtCM structure from the MtCM–MtDS complex (PDB ID: 2W1A)21 as a search model, after truncation of the termini and the H1–H2 loop, and removal of the ligand.
The two structures were subsequently refined, alternating between real-space refinement cycles using Coot(37) and maximum-likelihood refinement with REFMAC5.38 The models were improved stepwise by first removing ill-defined side chains, and subsequently adding missing structural elements as the quality of the electron density map improved. Water molecules and alternative side-chain conformations were added to the MtCMT52P model toward the end of the refinement process, where positive peaks in the σA-weighted Fo−Fc difference map and the chemical surroundings allowed for their unambiguous identification. As a last step, occupancy refinement was carried out with phenix.refine, a tool of the PHENIX software suite.39 The final structure of MtCMT52P was deposited in the Protein Data Bank (PDB)40 with deposition code 6YGT. Data collection and refinement statistics are summarized in Supporting Table S1.
Determination of Enzyme Kinetic Parameters
Michaelis–Menten kinetics of the untagged purified MtCM variants were determined by a continuous spectroscopic chorismate depletion assay (Lambda 20 UV/VIS spectrophotometer, PerkinElmer). The purified enzymes were diluted into 20 mM potassium phosphate, pH 7.5, containing 0.01 mg mL–1 bovine serum albumin to obtain suitable working concentrations for starting the reactions, depending on the activity of individual variants. The assays were performed at 30 °C in either 50 mM potassium phosphate, pH 7.5, or 50 mM BTP, pH 7.5. Different chorismate concentrations ([S]) ranging from 10 to 1500 μM were used at 274 nm (ε274 = 2630 M–1 cm–1) or 310 nm (ε310 = 370 M–1 cm–1). Chorismate disappearance upon enzyme addition was monitored to determine the initial reaction velocity (v0). The obtained data were fitted to the Michaelis–Menten equation with the program KaleidaGraph (Synergy Software, Reading, Pennsylvania) to obtain the catalytic parameters kcat and Km.
Molecular Dynamics Simulations
Molecular dynamics (MD) simulations were carried out on a number of representative structures for CM. They included two independent sets of simulations for apo MtCM, starting either from the X-ray crystal structure of MtCM in complex with malate (after removing malate) (PDB ID: 2VKL)21 or from the structure of the CM polypeptide in the apo MtCM–MtDS complex (PDB ID: 2W19,21 chain D). The malate complex was chosen over ligand-free MtCM (PDB ID: 2QBV)41 due to its higher resolution and better refinement statistics. Both simulations gave essentially the same result; therefore, we will not refer to the second data set any further. For the highly active evolved MtCM variant (MtCMV), we used the recent crystal structure (PDB ID: 5MPV).12 The MtCM–ligand complex (MtCMLC) was taken from PDB ID: 2W1A,21 excluding the MtDS partner protein, where MtCM was co-crystallized with a transition state analog (TSA) in its active site (Figure 1). Finally, the V55D variant was modeled based on a partially refined experimental structure (Table S1). Residues that were not fully defined were added to the models using (often weak) electron density maps as reference in Coot.37 When no interpretable density was visible, geometric restraints (and α-helical restraints for residues in helix H1) were applied during model building, to ensure stable starting geometries. The N-termini of all of the models were set at Glu13, corresponding to the first defined residue in almost all of the resolved structures available. Glu13 was capped with an acetyl group to imply the continuation of the H1 helix. CM dimers were generated by 2-fold crystallographic symmetry.
Missing H-atoms were added to the model and the systems were solvated in a periodic box filled with explicit water molecules, retaining neighboring crystallographic waters, and keeping the protein at least 12 Å from the box boundaries. The systems were neutralized through the addition of Cl– ions at a minimum distance of 7 Å from the protein and each other. Additional buffering moieties like glycerol or sulfate ions found in the crystals were not considered. MD simulations were run using the Gromacs 5.1.4 package42,43 using the AMBER 12 force fields for the protein moieties44,45 and the TIP3P model for water.46 The ligand was modeled using the GAFF force field.47 The smooth particle mesh Ewald method was used to compute long-range electrostatic interactions,48 while a cutoff of 11 Å was used to treat the Lennard–Jones potential.
The systems were minimized using the steepest descent/conjugate gradients algorithms for 500/1500 steps until the maximum force was less than 1000 kJ mol–1 nm–1. To equilibrate and heat the systems, first we ran 100 ps MD in the NVT ensemble starting from a temperature of 10 K, using the canonical velocity rescaling thermostat49 followed by 100 ps in the NpT ensemble with a Parrinello–Rahman barostat50 targeting a final temperature of 310 K and a pressure of 1 atm. After initial equilibration, 1 μs of MD simulation was performed for each system. In all MD simulations, the time step size was set to 2 fs.
Results
The fact that MtCM exhibits only low natural catalytic activity provided us with a perfect opportunity to probe features that optimize CM catalysis by directed evolution.12 Since the biggest gains in catalytic activity were contributed by exchanging the H1–H2 loop residues 52 (T52P) and 55 (V55D), we set out to determine the crystal structures of these two enzyme variants. Together, these two substitutions led to an increase in kcat/Km by 22-fold compared to the parent enzyme.12
Crystal Structures of MtCMT52P and MtCMV55D
Whereas MtCMT52P crystals had the same space group (P43212) and similar cell parameters as the wild-type enzyme (PDB IDs: 2VKL(21) and 2QBV(41)), with one protomer in the asymmetric unit, MtCMV55D crystallized in a different space group (P22121), where the asymmetric unit contained the biological dimer. The MtCMT52P structure was refined to 1.6 Å and Rwork/Rfree values of 24.0/26.5% (Table S1 and Figure S1B), whereas MtCMV55D diffraction data yielded lower-quality electron density, particularly for the H1–H2 loop (Figure S1C,D). Consistent with this, the Wilson B-factor of MtCMV55D is high (57.8 Å2), indicating structural disorder. Refinement of the 2.1 Å MtCMV55D model stalled at Rwork/Rfree values of 27.6/34.9%, with very high B-factors for H1–H2 loop residues, especially for protomer B. For both structures, residues preceding residue Glu13 and C-terminal to Leu88 showed poorly defined electron density. Therefore, the terminal residues were not included in the final model.
Overall, the crystal structures of both MtCMT52P (PDB ID: 6YGT) and MtCMV55D are very similar to the structure of substrate-free wild-type MtCM (PDB ID: 2QBV),41 with RMSD = 0.3 and 0.4 Å, respectively. However, the H1–H2 loops (47MASGGPRLDHS57) of both protomers of MtCMV55D adopt a different conformation (RMSD = 2.3 Å compared to PDB ID: 2QBV), which most closely resembles the kinked conformation in the MtCM–MtDS complex (PDB ID: 2W19;21 RMSD = 0.8 Å) (Figure 2A). In the crystal structure of MtCMV55D, Asp55 in the H1–H2 loop forms a salt bridge with Arg46, similar to the one in MtCMV (compare Figure S1E,G,H). This interaction preorganizes the active sites of both MtCM variants for catalytic activity, mimicking MtCM in the complex with MtDS (Figure 2B). However, the overall conformation of the active site loop, which is involved in extensive crystal contacts that are highly distinct for the different crystal forms (Figure S2), differs significantly between the structures (Figures S1 and 2A).
Figure 2.
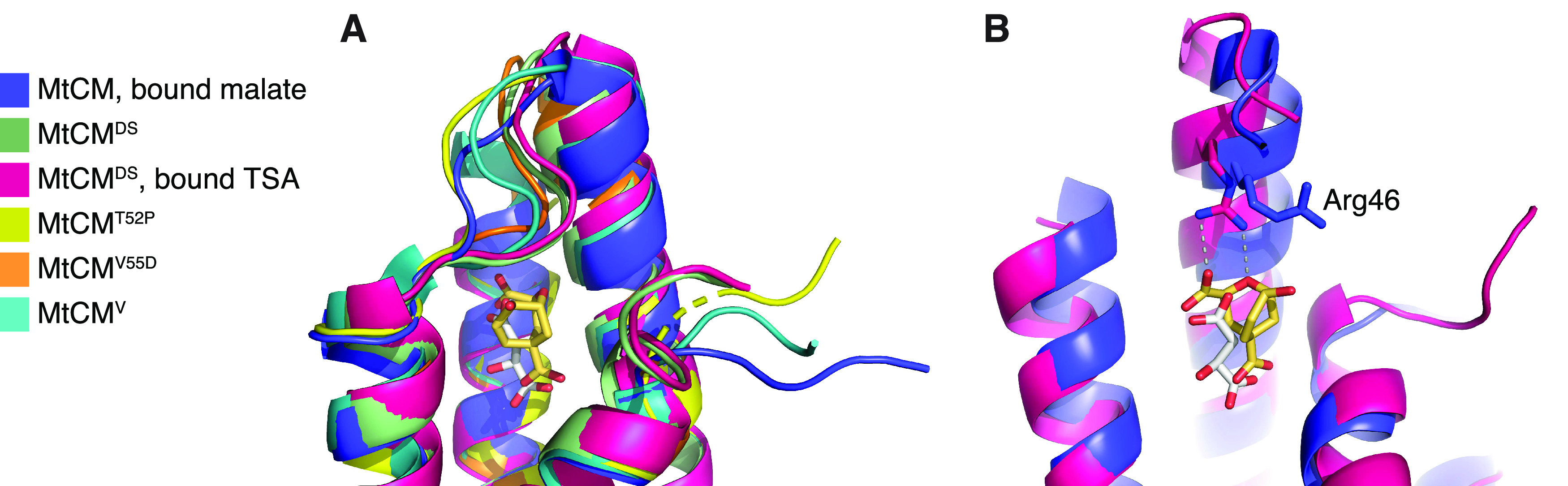
Comparison of MtCM crystal structures, with focus on Arg46 and H1–H2 loop. (A) Superimposition of the active site of MtCM (PDB ID: 2VKL;21 violet), MtCMDS (PDB ID: 2W19;21 green), MtCMDS–TSA complex (PDB ID: 2W1A;21 pink), MtCMT52P (PDB ID: 6YGT, this work; yellow), MtCMV55D (this work; orange), and top-evolved MtCMV (PDB ID: 5MPV;12 cyan); cartoon representation featuring the H1–H2 loop, with the ligands depicted as sticks. (B) Superimposition of MtCM (PDB ID: 2VKL;21 violet, with bound malate in gray sticks) and MtCM in the MtCM–MtDS complex (PDB ID: 2W1A;21 pink, with TSA in golden sticks, corresponding to MtCMLC) in cartoon representation, with the catalytically important Arg46 depicted as sticks. MtDS binding promotes the catalytically competent conformation of Arg46. Helix H2 was removed for clarity.
MD Simulations
To evaluate the behavior of MtCM in the absence of crystal contacts, we probed the MtCM structures by MD simulations. We used four model systems: low-activity apo wild-type MtCM, MtCMLC (“ligand complex”: wild-type MtCM from the MtCM–MtDS structure in complex with TSA, the transition state analog of the CM reaction;51Scheme 1 and Figure 1), MtCMV, corresponding to the highly active evolved variant N-s4.15,12 and MtCMV55D, which shows the highest catalytic activity among the single-substitution MtCM variants.12 We compared the overall dynamic profiles of these models and inspected the interactions formed between the C-termini and the H1–H2 loops covering the active sites, to find general features that could be associated with increased catalytic competence.
Apo Structures of MtCM Are Characterized by Significant Flexibility
We anticipated that the model systems would more or less retain the same fold as observed in the crystal structures, but that regions associated with crystal contacts, like the C-termini and the H1–H2 loop, would rapidly move away from their starting positions. Instead, the MD simulations revealed large changes from the initial crystal geometries in the apo protein structures, causing a rather high root-mean-square deviation (RMSD) from the original crystal structure geometry for the CM core regions (RMSD = 2.8 ± 1.2 Å (MtCM) or 3.4 ± 1.5 Å (MtCMV)). In particular, helix H2 showed a tendency to unravel (Figure 3). Due to the large flexibility observed, the two protomers making up the biological dimer instantaneously broke their symmetry, independently exploring different conformations in two chains. In contrast, the ligand-bound structure MtCMLC retained the secondary structure throughout the 1 μs simulation (Figure 3), with a lower RMSD (1.7 ± 0.6 Å) than the two apo structures. Intriguingly, a similar stabilization was observed for the unliganded variant MtCMV55D (Figure 3).
Figure 3.
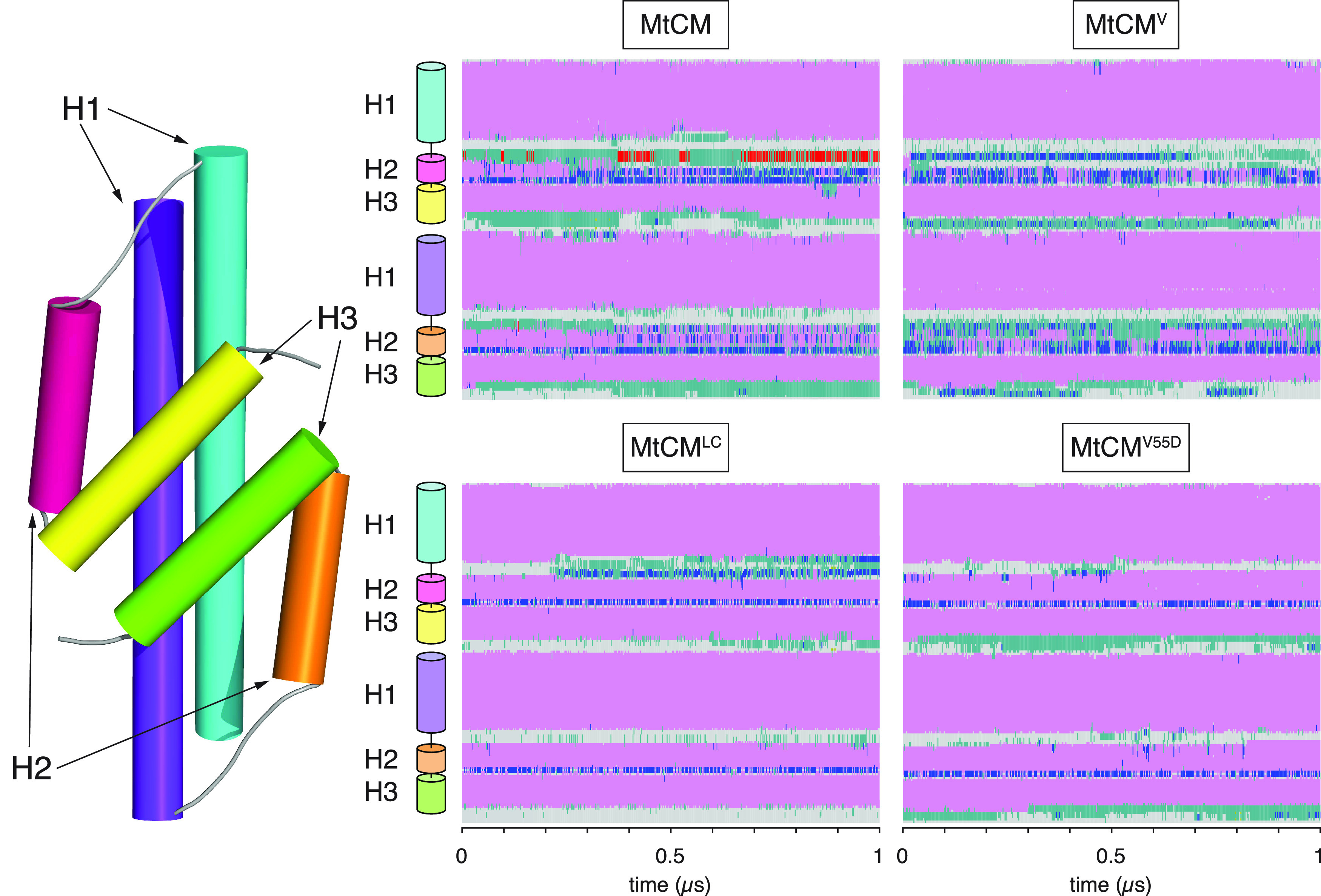
Secondary structure changes of MtCM during MD simulations. Estimated secondary structure of MtCM over 1 μs of MD simulation. Color code: α-helical structure (magenta), 310 helix (blue), π-helix (red), turn (green), coil (gray). The top panels report data for apo MtCM and MtCMV, showing clear instability of H2. The bottom panels present the data for the holo-MtCMLC system and for the single V55D variant (apo structure), which in contrast retained all secondary structure elements within the simulation time.
Kinked Conformation of the H1–H2 Loop
One of the biggest conformational changes in the crystal structure upon formation of the MtCM–MtDS complex occurs in the H1–H2 loop (Figures 1C and 2A).21 Whereas in the X-ray structure of the MtDS-activated MtCM, the H1–H2 loop is strongly kinked, this is not the case in nonactivated MtCM. We investigated the conformational landscape of this loop by simulations, using Arg53 from the loop as reporter residue. As shown in Figure 4, in one of the two protomers of MtCM, Arg53 remained in an extended conformation for the entire 1 μs MD simulation. In contrast, the same amino acid in the other protomer oscillated between the extended and the helical region of the Ramachandran plot (Figure 4), the latter being characteristic of the catalytically active conformation of the loop. Statistically averaging the two distributions, it appears that the apo form of MtCM is preferentially found in its inactive conformation, whereas in MtCMV both protomers assumed the kinked active loop conformation, and retained it for the whole length of the simulation. However, TSA binding promoted the active conformation also in wild-type MtCM (represented by MtCMLC). The fact that the fluctuations of the MtCMV H1–H2 loop are contained within the conformational basin of the catalytically competent geometry (Figure 4 and Table 1) is an indication that MtCMV has an intrinsically preorganized loop, a condition that helps to minimize the entropy loss during substrate binding and consequently favors catalysis.
Figure 4.
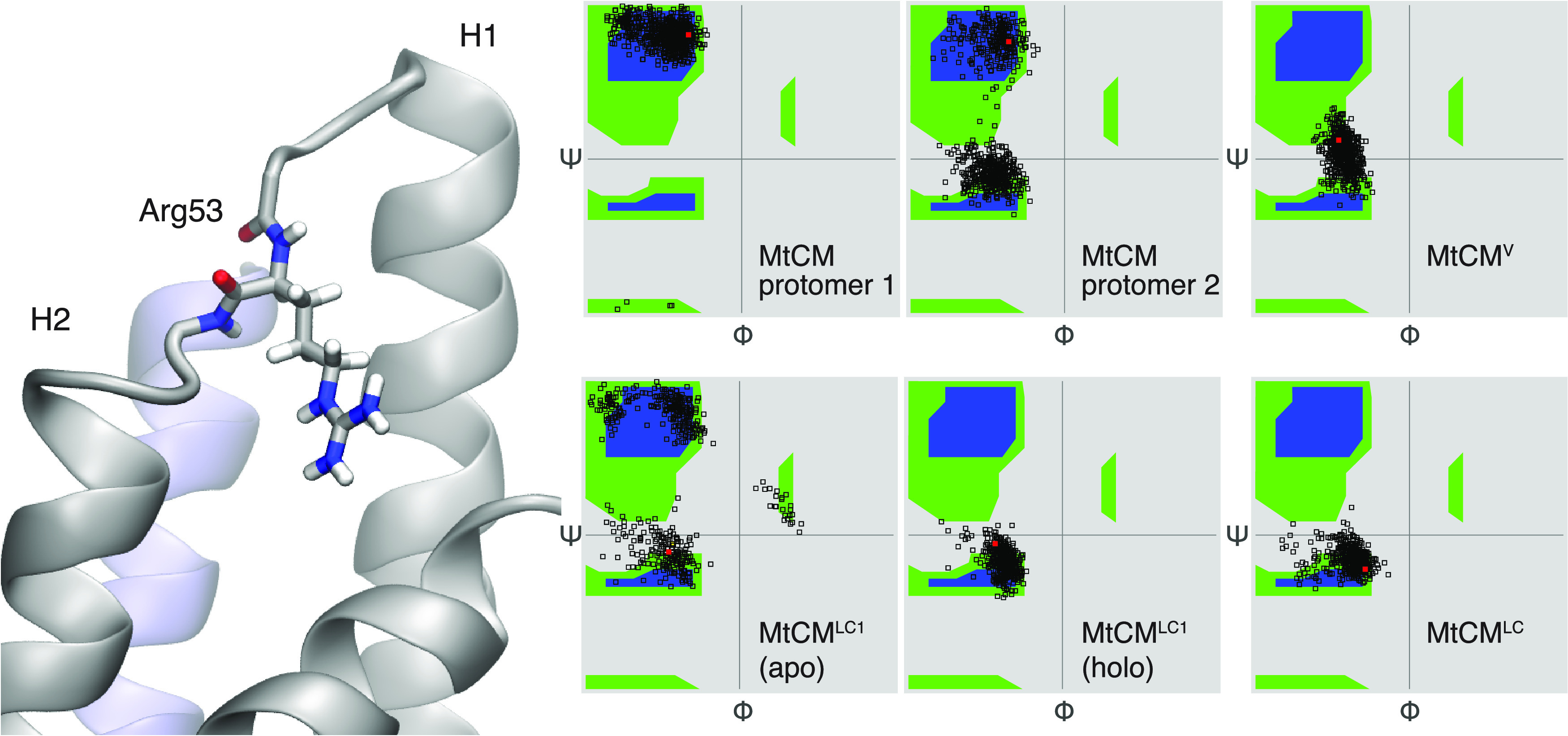
Conformation of Arg53 in the H1–H2 loop. Ramachandran plot showing backbone dihedral angles Φ and ψ for Arg53. Red dots mark starting conformations. For MtCM, the H1–H2 loops from the two protomers (left and middle plots in the top row) assume different ensembles of conformations; overall, the catalytically favored conformation (Ψ ∼ 0) is observed less frequently than the nonproductive one. In contrast, in MtCMV, both loops retained the active conformation during the entire course of the simulation, similarly to that observed for MtCMLC. When only one ligand was bound (MtCMLC1), the TSA-loaded site (holo) retained the active conformation, while the loop in the other protomer (apo) remained flexible.
Table 1. Root-Mean-Square Fluctuation (RMSF) Values of Selected Active Site Residuesa.
| RMSF
(Å) |
||||
|---|---|---|---|---|
| MtCMWT | MtCMV | MtCMLC | MtCMV55D | |
| Arg18′ | 2.2 ± 0.5 | 1.5 ± 1.0 | 0.7 ± 0.2 | 1.1 ± 0.5 |
| Arg35 | 0.8 ± 0.4 | 0.7 ± 0.4 | 0.4 ± 0.1 | 0.6 ± 0.2 |
| Arg46 | 2.0 ± 0.6 | 1.7 ± 0.7 | 0.7 ± 0.5 | 0.9 ± 0.5 |
| Arg58 | 2.3 ± 1.0 | 1.9 ± 2.0 | 2.0 ± 0.9 | 1.5 ± 0.6 |
RMSF values were calculated as an average over all nonhydrogen atoms for each residue compared to the average structure of the simulation. The reported σ values reflect the different relative fluctuations of the individual atoms composing the residues in the two symmetric protomers.
To test the effect of ligand binding, we repeated simulations of MtCM loaded with only one TSA ligand (MtCMLC1). Interestingly, ligand presence in one of the two binding pockets was sufficient to stabilize the structure of the whole dimer. Nonetheless, the H1–H2 active site loop of the apo protomer retained its intrinsic flexibility (Figure 4). The fact that the active site loop of the unloaded protomer behaved like the apo MtCM system suggests that the two active sites in MtCM retain considerable independence.
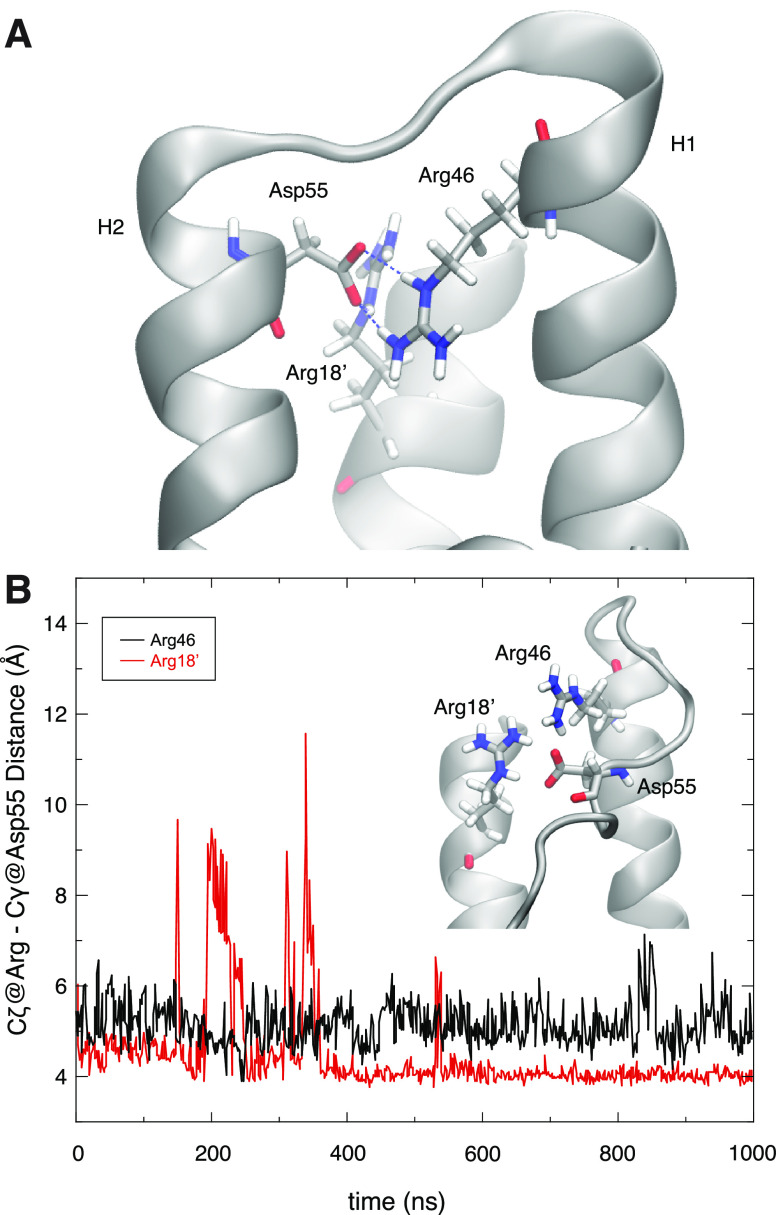
Contrary to MtCM and MtCMV (Figure 1C,D), the presence of the additional carboxylate group in MtCMV55D promoted the elongation of helix H2, resulting in a significant shortening of the active H1–H2 loop (Figure 5A). This structural rearrangement is associated with the formation of persistent salt bridges between Asp55, now localized in the first turn of H2, and active site residues Arg18′ and Arg46 that were retained for the entire length of the simulation. Noticeably, in MtCMWT, where such stabilizing electrostatic interactions are absent, no such contacts were observed, with the side chain of Val55 keeping a distance of more than 10 Å from the side chains of both Arg18′ and Arg46 for the whole duration of the simulation.
Figure 5.
Role of MtCMV residue Asp55 in positioning active site residues. (A) Extension of H2 and stabilization of the H1–H2 loop by residue Asp55. Substitution of Val55 by Asp stabilizes helix H2 through interactions with Arg18′ and Arg46 across the active site (the image shows the structure of V55D after 1 μs of MD simulations). Note that Arg46 is a catalytically essential residue for MtCM and its correct orientation is critical for catalytic proficiency. (B) Distance plotted between MtCMV Arg46 (black, chain A) or Arg18′ (red, from chain B), and Asp55 (chain A) observed during the simulation. In both cases, the distance measured is between Asp Cγ and Arg Cζ, using PDB nomenclature.
Overall, MtCMWT shows a noisier RMSF profile over the whole amino acid sequence compared to MtCMV and to the ligand complex MtCMLC (Figure S3). This result reflects the expected rigidification occurring upon substrate binding due to additional protein–ligand interactions in MtCMLC. MtCMV accomplishes rigidification as a direct consequence of its evolved sequence. Interestingly, also MtCMV55D shows generally dampened fluctuations, possibly due to the extended helical motif observed in that structure.
Positioning of Active Site Residues
The MtCM active site contains four arginine residues (Figure 1F), among them the key catalytic residue Arg46. In contrast to the observation in the two MtCM–MtDS crystal structures, the conformation of Arg46 was not strictly maintained during MD simulations. In the absence of a ligand, Arg18′, Arg46, and Arg58 repelled each other, and at least one of the residues was pushed out of the active site in the majority of the simulations. Only one of the four arginine residues (Arg35) maintained its position (Table 1), appropriately placed for substrate binding by wild-type MtCM, with an RMSF below 1 Å, while RMSF values >2 Å for the other Arg residues signal a substantial increase in the conformational freedom. This changes upon complex formation with MtDS, guiding also the important Arg46 into a catalytically competent conformation.
In contrast to MtCMWT, the two variants MtCMV and MtCMV55D exhibited lower RMSF values for all active site Arg residues (Table 1 and Figure 1F) and maintained their catalytically competent conformation during the MD simulations even in the apo forms (Figure 5). The more stable positioning of Arg18′ and Arg46 appears to be a direct consequence of the replacement of Val55 with Asp, which introduces a negative charge, mitigating the surplus positive charges in the active site.
Interactions between C-Terminal Residues and H1–H2 Loop
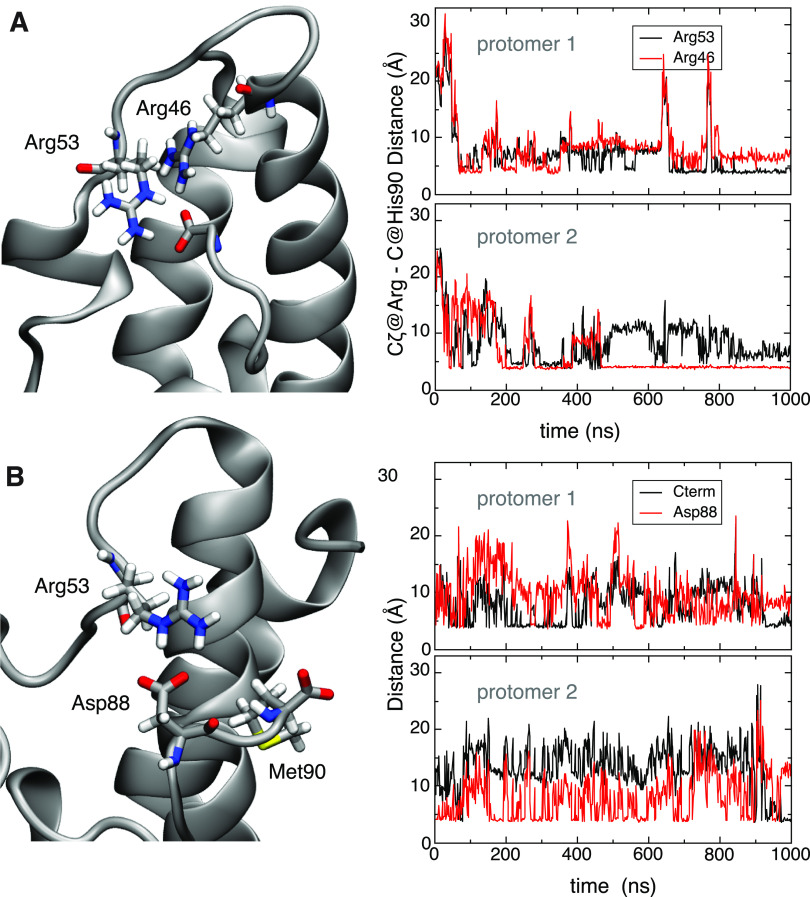
A crucial factor for the enhanced activity of MtCM in the MtCM–MtDS complex is an MtDS-induced interaction between MtCM’s H1–H2 loop and its C-terminus.21 The interaction can be divided into two contributions: a salt bridge between the C-terminal carboxylate and the side chain of Arg53, and a hydrophobic contact between Leu54 and Leu88 (Figure S4A).
Our 1 μs-long simulations detected persistent, multiple interactions involving the C-terminal carboxylate. In contrast, the hydrophobic contacts between Leu54 and Leu88 were disrupted in the first nanoseconds, and almost never observed again during the rest of the simulation time (Figure S4B,C).
Salt Bridges with C-Terminus
In our MD simulations, the C-terminal carboxylate formed interchangeable contacts with Arg53 and the catalytically important Arg46,21 which is located in the last turn of helix H1 (Figure 6A). Notably, the presence of a salt bridge between Arg46 and the C-terminus correlated with the apparently active conformation of the H1–H2 loop (Figure 6A).
Figure 6.
Interaction between C-terminal carboxylate of MtCM and H1–H2 loop. (A) Right panels show the distance between the Cζ carbon of arginine residues 53 or 46 and the carboxylate carbon of the C-terminus of MtCM in the two protomers (top and bottom panels). Formation of a steady contact (<5 Å) with Arg46 (bottom panel) corresponds to stabilization of the catalytically productive H1–H2 loop conformation, which allows for stabilization of the transition state of the chorismate to prephenate rearrangement (Figure 1B,F). (B) Interaction between C-terminal residues and the H1–H2 loop in MtCMV. Salt bridge contacts between Arg53 and the carboxyl groups of Asp88 (red line) and Met90 (C-terminus; black line) in MtCMV in the two protomers. The top and bottom panels on the right show the evolution of the distances over time between Arg53′s Cζ and the corresponding carboxylate carbons for each of the two protomers of MtCMV.
The observed fluctuations suggest that the catalytically competent conformation of the binding site is malleable in wild-type MtCM and that additional interactions, i.e., with the substrate, are required to stabilize it. This is in line with studies of a topologically redesigned monomeric CM from Methanococcus jannaschii. This artificial enzyme was found to be catalytically active in the presence of the substrate despite showing extensive structural disorder without a ligand, reminiscent of a molten globule.52
MtCMV Exhibits Strengthened Interactions between C-Terminus and H1–H2 Loop
In MtCMV, the four C-terminal residues Arg–Leu–Gly–His (RLGH) are substituted with Pro–Asp–Ala–Met (PDAM) at positions 87–90, which include another carboxylate, introduced through Asp88. Our MD simulations show that the Asp88 carboxylate in the evolved variant MtCMV offers an alternative mode of interaction with Arg53 of the H1–H2 loop (Figures 6B and S5), which is not possible for wild-type MtCM. This allows for a persistent interaction of C-terminal residues with the H1–H2 loop throughout the simulation, while maintaining a highly flexible C-terminus. Moreover, in MtCMV, Arg46 is topologically displaced from its original position with respect to the loop and no longer able to engage in a catalytically unproductive salt bridge with the C-terminus.
Another interesting substitution, which emerged within the four C-terminal residues during the laboratory evolution toward variant MtCMV, is a proline residue (RLGH to PDAM).12 However, in contrast to Pro52, Pro87 did not appear to have a major influence on the simulations. While Pro52 is likely contributing to H1–H2 loop rigidity, with an average RMSF of 1.6 Å in MtCMV compared to 2.5 Å (MtCM) for this region, the C-termini showed similarly high RMSF values in the two models (>3 Å). Although Pro87 induced a kink at the C-terminus, this did not appear to affect the flexibility of the three terminal residues Asp88–Ala89–Met90.
Kinetic Analysis to Probe Predicted Key Interactions of Engineered MtCM Variants
In the course of the directed evolution of MtCMV, the L88D replacement was only acquired after the H1–H2 loop-stabilizing substitutions T52P and V55D were already introduced. Guided by the outcome of the MD simulations, we therefore probed the kinetic impact of the innocuous single L88D exchange in the context of three different sets of MtCM variants to experimentally assess the benefit of the introduced negative charge for fine-tuning and optimizing catalytic efficiency. We looked at (i) changing Asp88 in the MtCMV sequence 87PDAM90 into Asn88 or Leu88, (ii) directly introducing Asp88 into the MtCM wild-type sequence, and (iii) the triple variant T52P V55D L88D (MtCM Triple). All variants were obtained in their native format, i.e., with their native N-terminus and without a His-tag, to allow for optimal comparison with the structural and computational results. The variants were purified by ion-exchange and size-exclusion chromatography from the E. coli host strain KA13, which is devoid of CM genes to rule out contamination by endogenous CMs.18,30 Subsequently, the enzymes’ kinetic parameters were characterized by a spectrophotometric chorismate depletion assay.
As shown in Table 2, removing the negative charge at residue 88 by replacing Asp with Asn in the top-evolved variant MtCMV leads to a 2.5-fold drop in the catalytic efficiency kcat/Km to 1.7 × 105 M–1 s–1. This decrease is due both to a slightly lower catalytic rate constant (kcat) as well as a reduced substrate affinity (doubled Km). When residue 88 is further changed to the similarly sized but nonpolar wild-type residue Leu88 in variant MtCM PLAM, the catalytic parameters essentially remain the same as for the Asn88 variant (Table 2), independently confirming the catalytic advantage of the negative charge introduced through Asp88.
Table 2. Catalytic CM Activities of Purified MtCM Variants to Experimentally Address the Importance of the Leu88 to Asp88 Substitution that Emerged during the Directed Evolution of MtCMV.
| Variant | Residue changes | kcat (s–1)a | Km (μM)a | kcat/Km (M–1 s–1)a |
|---|---|---|---|---|
| MtCMV (PDAM) | PD/PDAMb, V62I, D72V, V11L, D15V, K40Q | 9.4 ± 1.3 | 22 ± 2 | 430,000 ± 30,000 |
| MtCM PNAM | MtCMV, D88N | 7.6 ± 0.2 | 45 ± 7 | 170,000 ± 20,000 |
| MtCM PLAM | MtCMV, D88L | 6.0 ± 0.4 | 38 ± 1 | 160,000 ± 10,000 |
| MtCMWTc | 1.7 ± 0.2 | 980 ± 80 | 1700 ± 300 | |
| MtCM L88Dc | L88D | 3.0 ± 0.1 | 1110 ± 70 | 2700 ± 300 |
| MtCM 3p3 | T52P V55D | 8.5 ± 0.0 | 450 ± 70 | 19,000 ± 3000 |
| MtCM Triple | T52P V55D L88D | 11 ± 2 | 510 ± 130 | 22,000 ± 1000 |
All values are experimental means from assays performed with at least two independently produced and purified protein batches with their calculated standard deviations (σn–1). The kcat/Km parameters were obtained as the mean from averaging kcat/Km values derived directly from individually fitted independent Michaelis–Menten plots with the calculated error of the corresponding average.
PD/PDAM indicates amino acid substitutions T52P, V55D, R87P, L88D, G89A, and H90M.
The default for measuring kinetics involved assays performed in 50 mM K-phosphate, pH 7.5, at 274 nm, whereas the kinetic parameters of these low-performing variants were determined in 50 mM BTP, pH 7.5, at 310 nm. Measuring these variants in 50 mM K-phosphate, pH 7.5, resulted in ∼40% reduction in kcat, as was already observed previously for wild-type MtCM.21
For the second set of variants that directly started out from the sluggish MtCM wild-type enzyme (MtCMWT), a trend for an increase in catalytic activity upon replacing Leu88 by Asp88 was observed (1.6-fold higher kcat/Km, reaching 2.7 × 103 M–1 s–1; Table 2). This is mainly caused by an increase in kcat rather than an altered substrate affinity. Interestingly, the L88D exchange together with T52P and V55D in the MtCM triple variant does not lead to a significant increase in kcat/Km compared to MtCM 3p3,12 which just carries the two loop substitutions T52P and V55D.
Thus, the substitution of Leu88 with Asp88 indeed results in a beneficial effect on the performance of MtCM. However, this effect is only prominent in combination with other selected exchanges, such as those present in MtCMV. As a single amino acid replacement in the wild-type enzyme or on top of the two substitutions in the H1–H2 loop, the effect of L88D is less noticeable, if present at all.
In summary, a comparison of the dynamic behavior of wild-type MtCM in its apo and ligand-bound states with MtCMV and MtCMV55D revealed that the catalytically favorable conformation of the active site is achieved by the interplay of several interactions, which balance charges and entropic disorder of the H1–H2 loop. Structuring is promoted, in particular, by increasing the number of the negatively charged carboxylate groups that can both shield the electrostatic charge of the various arginine side chains within or next to the active site and orient catalytically important residues by hydrogen bonding and salt bridge formation. Simulations of MtCMV revealed the special importance of Asp55 in the V55D variant for coordinating Arg18′ and Arg46, thus promoting the preorganization of the active site region. These results echo the conclusions from directed evolution, which also identified the V55D substitution as the most important contributor for catalytic enhancement, causing a 12-fold increase in kcat/Km.12 At the same time, we determined and rationalized the more subtle and context-dependent effect of the L88D replacement that introduced an additional negative charge for electrostatic preorganization of the active site. Overall, the high catalytic activity of MtCMV clearly results from many individual larger and smaller contributions mediated by substitutions at diverse locations within the enzyme structure.
Discussion
Important Activating Factors in MtCMDS and MtCMV
MtCM has intrinsically low activity but can be activated to rival the performance of the best CMs known to date12 through the formation of a heterooctameric complex with MtDS,21 which aligns crucial active site residues to catalytically competent conformations. Most importantly, binding to MtDS induces preorganization of Arg46 into a catalytically favorable conformation (Figure 2B), via H-bonding to the carbonyl oxygens of Thr52 and Arg53.21 Arg46 is the crucial catalytic residue interacting with the ether oxygen of Bartlett’s transition state analogue (TSA)51 in the complex with MtDS (PDB ID: 2W1A)21 (Figures 1B,F and 2B); upon replacing Arg with Lys, the enzyme’s efficiency drops 50-fold.21
Both MtCMDS and MtCMV exhibit a kinked H1–H2 loop conformation (Figures 1C,D and 2A), which was hypothesized to be important for increased catalytic efficiency.12 However, in MtCMV and MtCMV55D, the kink is exacerbated by crystal contacts, which are different in the two crystal forms (Figure S2). This kink is much less prominent in wild-type MtCM, or even MtCMT52P (Figures 2A and S1B), and completely lost during the simulations of MtCMWT (we did not carry out simulations on the single variant MtCMT52P). Thus, this conformation may well be a crystallization artifact rather than a prerequisite for an active MtCM.
Nevertheless, preorganization and prestabilization appear to be of crucial importance for the catalytic prowess of MtCM. The largest boost in catalytic efficiency (12-fold enhancement) by a single substitution was observed for the V55D replacement found in the evolved MtCMV.12 This residue is located on the C-terminal side of the H1–H2 loop (Figure 1D,E) and forms a salt bridge to the catalytically important Arg46 at the top of helix H1 (Figure 5A), an interaction that is also observed in the crystal structure of MtCMV55D (Figure S1G,H). During the MD simulations of MtCMV and the single variant MtCMV55D, the presence of Asp55 reduced the mobility of active site residues. By interacting with Arg18′ and Arg46, this residue helps to preorganize the active site for catalysis and reduce unfavorable conformational fluctuations caused by electrostatic repulsion in the absence of a substrate. This is supported by the lower RMSF values of MtCMV compared to uncomplexed wild-type MtCM (Table 1) and by a slightly higher melting temperature of MtCMV55D (ΔT = 3 °C from differential scanning fluorimetry (DSF) measurements; preliminary data). By decreasing thermal fluctuations in the active site, Asp55 likely also reduces the entropic penalty associated with substrate binding. Pro52 appears to exert a similar stabilizing effect on the protein, despite the rather small structural changes, as suggested by a 2 °C increase in melting temperature of MtCMT52P in DSF experiments compared to MtCM (preliminary data). This single substitution alone raises the kcat/Km value of the enzyme by a factor of six.12 It is worth noting that the simultaneous substitution of T52P and V55D increased the melting temperature by 6 °C (monitored by circular dichroism spectroscopy) and boosted kcat/Km by 22-fold.12 The top-evolved MtCMV even showed a melting temperature of 83 °C compared to 74 °C for the parent MtCM.12
Importance of the C-Terminus
MtCM activation by MtDS involves a change in conformation of the C-terminus of MtCM and its active site H1–H2 loop.21 Specifically, a salt bridge is formed between the C-terminal carboxylate of MtCM (which is repositioned upon MtDS binding) and loop residue Arg53, possibly bolstered by a newly formed hydrophobic interaction between Leu88 and Leu54 (Figure S4A). The 1 μs simulations suggest that salt bridge formation with Arg53 occurs in solution in all tested cases, whereas the hydrophobic contact is less important.
Directed evolution experiments carried out by randomizing the final four C-terminal positions 87–90 of MtCM had previously revealed that a great variety of residues with quite distinct physico-chemical properties are compatible with a functional catalytic machinery.26 Conserved positions emerged only when probing for an intact activation mechanism by MtDS.26 Still, when residues 87–90 of MtCMV were evolved from Arg–Leu–Gly–His to Pro–Asp–Ala–Met (Figure 1E), an increase in kcat/Km by roughly a factor of four was achieved.12 Here, we resolved this apparent paradox by investigating C-terminal factors important for the fine-tuned optimization of CM function. Even though the replacement R87P induced a kink in the structure, the presence of the proline did not appear to have a major influence in the simulations. Notably, the C-terminal substitutions together result in a change in net charge from +1 to −2, including the terminal carboxylate, providing the basis for more extensive electrostatic interactions with the positively charged Arg53 than is possible for wild-type MtCM. Indeed, our kinetic analysis of Asp88-containing MtCM variants demonstrates that this residue increases CM’s catalytic efficiency (Table 2). The fact that Asp88 did not significantly augment kcat/Km in the context of the MtCM double variant T52P V55D (i.e., MtCM Triple; Table 2) suggests that the extent of catalytic improvement by L88D depends on the particular structural context.
Our simulations indicate that in free wild-type MtCM, an interaction of the C-terminal carboxylate with the key active site residue Arg46 is possible but infrequent due to fluctuations (Figure 6A and Table 1). In contrast, in MtCMV and MtCMDS the side chain of Arg46 points toward the catalytic pocket (Figures 5 and 2B), and any unproductive reorientation of Arg46 toward the C-terminus would easily result in a clash with the H1–H2 loop. Thus, an additional feature of this loop may be to act as a conditional shield (illustrated for MtCMV in Figure 7). In the conformation assumed in MtCMV and MtCMDS, this loop blocks the reorientation of Arg46 toward the C-terminus and hence prevents an unproductive conformation accessible for free wild-type MtCM. MtCMDS and MtCMV use different means to correctly position active site residues, which correlates with a bent H1–H2 loop in both cases. This is either achieved through conformational changes imposed upon MtCMDS by MtDS binding, or by establishing a salt bridge across the active site, between Arg46 and Asp55, as seen for MtCMV and also for the single variant MtCMV55D (Figures 5 and S1E,G,H).
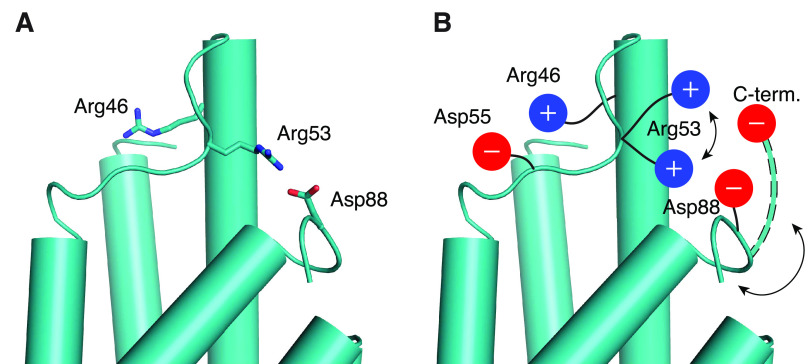
Figure 7.
Shielding interaction mediated by the H1–H2 loop. (A) Conformation of important Arg residues in chain A of MtCMV (cyan) after 31.7 ns of MD simulations. The key active site residue Arg46 is positioned on the opposite side of the H1–H2 loop, which in turn is bolted to the C-terminus by a salt bridge between Arg53 and Asp88 (cartoon representation, with side chains shown as sticks). (B) Cartoon summarizing the important stabilizing interactions in the top-evolved variant MtCMV depicted in (A) that properly position Arg46 for catalysis. Asp55 stabilizes the stretched-out conformation of Arg46, whereas alternating salt bridges accessible for Arg53 with the negatively charged groups present in the C-terminal region hinder Arg46 from adopting an unfavorable interaction with the C-terminal carboxylate. One example of an alternative backbone conformation that allows for interactions between the C-terminal carboxylate and Arg53 is depicted with dashed outlines.
General Implications for CM Catalysis
It is obviously impossible to directly transfer our findings of critical detailed molecular contacts from the AroQδ subclass CM of M. tuberculosis to the evolutionary distinct AroH class CMs, or even to the structurally and functionally divergent AroQα, AroQβ, and AroQγ subclasses.53 Neither of those groups of CMs have evolved to be deliberately poor catalysts that become proficient upon regulatory interaction with a partner protein such as MtDS.21 To be amenable to ‘inter-enzyme allosteric’ regulation,28 the H1–H2 loop in MtCM must be malleable and allow for conformational switching between a poorly and a highly active form. In contrast, this region is rigidified in a catalytically competent conformation in the overwhelming majority of CMs from other subclasses. This is exemplified by the prototypic EcCM (AroQα subclass) and the secreted *MtCM (AroQγ), which possess the sequence 45PVRD48 and 66PIED69, respectively, at the position corresponding to the malleable H1–H2 loop sequence 52TRLV55 of wild-type MtCM.12 Remarkably, the two most impactful substitutions T52P and V55D occurring during the evolution of MtCMV have led to the tetrapeptide sequence 52PRLD55, with both Pro and Asp being conserved in naturally highly active CMs.12
The AroQδ subclass CM from Corynebacterium glutamicum is another structurally well-characterized poorly active CM (kcat/Km = 110 M–1 s–1) that requires complex formation with its cognate DAHP synthase for an impressive 180-fold boost in catalytic efficiency.54 In that case, inter-enzyme allosteric regulation involves a conformational change of a different malleable segment between helices H1 and H2. Thus, while the molecular details important for the activation of a particular AroQδ CM cannot be transferred directly from one system to another, our findings suggest as a general regulatory principle the deliberate and reversible destabilization of a catalytically critical loop conformation.
In both the M. tuberculosis(12) and the C. glutamicum systems,54 crystal contacts in the H1–H2 loop region impede the structural interpretation of the activity switching. The MD simulations shown here represent an interesting alternative approach to dynamic high-resolution structure determination methods for sampling the conformational space adopted by malleable peptide segments with and without ligands.
Conclusions
MD greatly aided the analysis of crystal structures that were compromised or biased by extensive crystal contacts at the most interesting structural sites. Our aim was to obtain insight into the crucial factors underlying CM activity by comparing the structure and dynamics of the poorly active wild-type MtCM (kcat/Km = 1.7 × 103 M–1 s–1) with the top-performing MtCM variant MtCMV (kcat/Km = 4.3 × 105 M–1 s–1), which emerged from directed evolution experiments. Both in MtDS-activated wild-type MtCM and in MtCMV, high activity correlated with a kinked H1–H2 loop conformation and an interaction of this region with the C-terminus of MtCM. The autonomously fully active variant MtCMV had amino acid changes in both of these regions that augment these structural features. In this report, we focussed on substitutions T52P, V55D, and L88D.
The active site of all natural CMs contains a high density of positive charges. In MtCM, four arginine residues (Arg18′, Arg35, Arg46, and Arg58, of which Arg18′ is contributed by a different MtCM protomer) are responsible for binding and rearranging the doubly negatively charged substrate chorismate. Only one of these residues (Arg35) is firmly in position before the substrate enters the active site. Of critical importance for catalysis is Arg46. During the MD simulations, Arg46 competes with another arginine residue (Arg53) for binding to the C-terminal carboxylate (Figure 6A) and adopts a catalytically unproductive conformation unless an aspartate residue (Asp55 or Asp88) comes to its rescue. As shown here, Asp55 not only properly orients Arg46 for catalysis but additionally stabilizes the active site. Together with T52P, which preorders the H1–H2 loop, the V55D exchange results in reduced mobility of residues in the active site through stabilizing interactions, thereby preorganizing it for efficient catalysis and lowering the entropic cost of substrate binding. Another aspartate residue (Asp88), also acquired in the top-evolved MtCMV,12 helps to balance charges, and—by interacting with Arg53—imposes a steric block that prevents nonoptimal positioning of Arg46 (Figure 7), explaining why the L88D exchange can increase kcat/Km by about 2- to 3-fold.
In summary, we tested our hypotheses on the specific importance of critical substitutions acquired during the directed evolution of MtCMV, namely, T52P, V55D, and L88D by investigating single variants as well as combinations with other residue replacements that were found to augment catalysis. The variants were characterized by crystallography, MD simulations, and enzyme kinetics. The two residues Pro52 and Asp55 exert a major impact by prestabilization and preorganization of catalytically competent conformations of active site residues, while Asp88 contributes to fine-tuning and optimizing the catalytic process. By expanding on the previous directed evolution studies, we have shown here how the accumulated set of amino acid substitutions found in MtCMV has resulted in an activity level matching that of the most active CMs known to date.12
Acknowledgments
The authors thank Regula Grüninger-Stössel for help with the construction of the T52P and V55D variants of MtCM, and Joel B. Heim for collecting X-ray data for MtCMT52P. The experiments were performed on beamlines ID30A-3/MASSIF-3 and ID29 at the European Synchrotron Radiation Facility (ESRF), Grenoble, France. We are grateful to Montserrat Soler Lopez and Daniele De Sanctis at the ESRF for providing assistance in using beamlines ID30A-3/MASSIF-3 and ID29, respectively. Finally, they acknowledge services provided by the MoBiAS facility, Laboratory of Organic Chemistry, ETH Zurich, and by the UiO Structural Biology Core Facilities.
Glossary
Abbreviations
- BTP
1,3-bis[tris(hydroxymethyl)methylamino]propane
- CM
chorismate mutase
- DAHP
3-deoxy-d-arabino-heptulosonate 7-phosphate
- IPTG
isopropyl-β-d-thiogalactopyranoside
- LC
ligand complex (i.e., complex with TSA)
- MD
molecular dynamics
- MES
2-(N-morpholino)ethanesulfonic acid
- MtCM
chorismate mutase from M. tuberculosis
- MtCMDS
MtCM from MtCM–MtDS complex
- MtCMLC
TSA-bound MtCM from MtCM–MtDS complex (LC1 refers to only one of the protomers containing TSA)
- MtCMT52P
MtCM variant T52P
- MtCMV
top-performing MtCM variant N-s4.15 from directed evolution study
- MtCM Triple
MtCM variant with the three substitutions T52P, V55D, and L88D
- MtCMV55D
MtCM variant V55D
- MtCMWT
wild-type MtCM
- MtDS
DAHP synthase from M. tuberculosis
- MWCO
molecular weight cutoff
- pI
isoelectric point
- PMSF
phenylmethanesulfonyl fluoride
- RMSD
root-mean-square deviation (or root-mean-square difference, if concerning structural comparisons)
- RMSF
root-mean-square fluctuation
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- Tris
tris(hydroxymethyl)aminomethane
- TSA
transition state analogue
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.biochem.2c00635.
Crystal structures and electron density maps shown for catalytically important regions (Figure S1); crystal contacts of the H1–H2 loop (Figure S2); root-mean-square fluctuations of MtCM during MD simulations (Figure S3); interactions in MtCM between its C-terminus and H1–H2 loop (Figure S4); MD snapshots of interactions between C-terminus and H1-H2 loop of MtCMV (Figure S5); and data collection and refinement statistics (Table S1) (PDF)
Accession Codes
Author Contributions
∥ H.V.T., L.B., and T.K. contributed equally to this work.
Author Contributions
U.K. conceived the study. H.V.T., P.K., and Mi.C. were additionally involved in the planning of the experiments. H.V.T. performed most of the calculations, transformed, produced, purified, and crystallized the two single MtCM variants, and solved the crystal structure of MtCMV55D, supervised by Mi.C. and U.K., respectively. Ma.C. contributed with additional simulations, supervised by Mi.C. T.K. solved the crystal structure of MtCMT52P and refined the crystal structures of both MtCM variants, supervised by G.C. and U.K., who also validated the structures. L.B. constructed, produced, and purified additional sets of MtCM variants and characterized their kinetic parameters to validate computational results, and K.W.-R. designed and constructed the MtCM variants T52P and V55D and prepared the final figures; both were supervised by P.K. The initial version of the manuscript was written by H.V.T. and U.K., which was complemented with contributions from all authors and revised by P.K., Mi.C., and U.K.
This work was funded by grants from the Swiss National Science Foundation to P.K. (grant 310030M_182648), the Norwegian Research Council (grants 247730 and 245828), and CoE Hylleraas Centre for Quantum Molecular Sciences (grant 262695), and through the Norwegian Supercomputing Program (NOTUR) (grant NN4654K). The work was additionally supported through funds from the University of Oslo (position of H.V.T.).
The authors declare no competing financial interest.
Supplementary Material
References
- Ohashi M.; Liu F.; Hai Y.; Chen M.; Tang M.-C.; Yang Z.; Sato M.; Watanabe K.; Houk K. N.; Tang Y. SAM-dependent enzyme-catalysed pericyclic reactions in natural product biosynthesis. Nature 2017, 549, 502–506. 10.1038/nature23882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami A.; Oikawa H. Recent advances of Diels-Alderases involved in natural product biosynthesis. J. Antibiot. 2016, 69, 500–506. 10.1038/ja.2016.67. [DOI] [PubMed] [Google Scholar]
- Tang M.-C.; Zou Y.; Watanabe K.; Walsh C. T.; Tang Y. Oxidative cyclization in natural product biosynthesis. Chem. Rev. 2017, 117, 5226–5333. 10.1021/acs.chemrev.6b00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.-I.; McCarty R. M.; Liu H.-W. The enzymology of organic transformations: A survey of name reactions in biological systems. Angew. Chem., Int. Ed. 2017, 56, 3446–3489. 10.1002/anie.201603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo S. G.; Widlanski T. S.; Hoare J. H.; Grimshaw C. E.; Berchtold G. A.; Knowles J. R. Stereochemistry of the rearrangement of chorismate to prephenate: Chorismate mutase involves a chair transition state. J. Am. Chem. Soc. 1984, 106, 2701–2703. 10.1021/ja00321a039. [DOI] [Google Scholar]
- Bentley R. The shikimate pathway - A metabolic tree with many branches. Crit. Rev. Biochem. Mol. Biol. 1990, 25, 307–384. 10.3109/10409239009090615. [DOI] [PubMed] [Google Scholar]
- Herrmann K. M.; Weaver L. M. The shikimate pathway. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 473–503. 10.1146/annurev.arplant.50.1.473. [DOI] [PubMed] [Google Scholar]
- Lamb A. L. Pericyclic reactions catalyzed by chorismate-utilizing enzymes. Biochemistry 2011, 50, 7476–7483. 10.1021/bi2009739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copley S. D.; Knowles J. R. The uncatalyzed Claisen rearrangement of chorismate to prephenate prefers a transition state of chairlike geometry. J. Am. Chem. Soc. 1985, 107, 5306–5308. 10.1021/ja00304a064. [DOI] [Google Scholar]
- Wiest O.; Houk K. N. Stabilization of the transition state of the chorismate-prephenate rearrangement: An ab initio study of enzyme and antibody catalysis. J. Am. Chem. Soc. 1995, 117, 11628–11639. 10.1021/ja00152a002. [DOI] [Google Scholar]
- Burschowsky D.; Krengel U.; Uggerud E.; Balcells D. Quantum chemical modeling of the reaction path of chorismate mutase based on the experimental substrate/product complex. FEBS Open Bio 2017, 7, 789–797. 10.1002/2211-5463.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrig-Kamarauskaitė J.; Würth-Roderer K.; Thorbjørnsrud H. V.; Mailand S.; Krengel U.; Kast P. Evolving the naturally compromised chorismate mutase from Mycobacterium tuberculosis to top performance. J. Biol. Chem. 2020, 295, 17514–17534. 10.1074/jbc.RA120.014924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chook Y. M.; Ke H.; Lipscomb W. N. Crystal structures of the monofunctional chorismate mutase from Bacillus subtilis and its complex with a transition state analog. Proc. Natl. Acad. Sci. U.S.A. 1993, 90, 8600–8603. 10.1073/pnas.90.18.8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chook Y. M.; Gray J. V.; Ke H.; Lipscomb W. N. The monofunctional chorismate mutase from Bacillus subtilis. Structure determination of chorismate mutase and its complexes with a transition state analog and prephenate, and implications for the mechanism of the enzymatic reaction. J. Mol. Biol. 1994, 240, 476–500. 10.1006/jmbi.1994.1462. [DOI] [PubMed] [Google Scholar]
- Xue Y.; Lipscomb W. N.; Graf R.; Schnappauf G.; Braus G. The crystal structure of allosteric chorismate mutase at 2.2-Å resolution. Proc. Natl. Acad. Sci. U.S.A. 1994, 91, 10814–10818. 10.1073/pnas.91.23.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. Y.; Karplus P. A.; Ganem B.; Clardy J. Atomic structure of the buried catalytic pocket of Escherichia coli chorismate mutase. J. Am. Chem. Soc. 1995, 117, 3627–3628. 10.1021/ja00117a038. [DOI] [Google Scholar]
- Sträter N.; Schnappauf G.; Braus G.; Lipscomb W. N. Mechanisms of catalysis and allosteric regulation of yeast chorismate mutase from crystal structures. Structure 1997, 5, 1437–1452. 10.1016/S0969-2126(97)00294-3. [DOI] [PubMed] [Google Scholar]
- MacBeath G.; Kast P.; Hilvert D. A small, thermostable, and monofunctional chorismate mutase from the archaeon Methanococcus jannaschii. Biochemistry 1998, 37, 10062–10073. 10.1021/bi980449t. [DOI] [PubMed] [Google Scholar]
- Calhoun D. H.; Bonner C. A.; Gu W.; Xie G.; Jensen R. A. The emerging periplasm-localized subclass of AroQ chorismate mutases, exemplified by those from Salmonella typhimurium and Pseudomonas aeruginosa. Genome Biol. 2001, 2, 1–16. 10.1186/gb-2001-2-8-research0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ökvist M.; Dey R.; Sasso S.; Grahn E.; Kast P.; Krengel U. 1.6 Å crystal structure of the secreted chorismate mutase from Mycobacterium tuberculosis: Novel fold topology revealed. J. Mol. Biol. 2006, 357, 1483–1499. 10.1016/j.jmb.2006.01.069. [DOI] [PubMed] [Google Scholar]
- Sasso S.; Ökvist M.; Roderer K.; Gamper M.; Codoni G.; Krengel U.; Kast P. Structure and function of a complex between chorismate mutase and DAHP synthase: Efficiency boost for the junior partner. EMBO J. 2009, 28, 2128–2142. 10.1038/emboj.2009.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. Y.; Stewart J. D.; Clardy J.; Ganem B. New insight into the catalytic mechanism of chorismate mutases from structural studies. Chem. Biol. 1995, 2, 195–203. 10.1016/1074-5521(95)90269-4. [DOI] [PubMed] [Google Scholar]
- Kast P.; Asif-Ullah M.; Jiang N.; Hilvert D. Exploring the active site of chorismate mutase by combinatorial mutagenesis and selection: The importance of electrostatic catalysis. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 5043–5048. 10.1073/pnas.93.10.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast P.; Grisostomi C.; Chen I. A.; Li S.; Krengel U.; Xue Y.; Hilvert D. A strategically positioned cation is crucial for efficient catalysis by chorismate mutase. J. Biol. Chem. 2000, 275, 36832–36838. 10.1074/jbc.M006351200. [DOI] [PubMed] [Google Scholar]
- Burschowsky D.; van Eerde A.; Ökvist M.; Kienhöfer A.; Kast P.; Hilvert D.; Krengel U. Electrostatic transition state stabilization rather than reactant destabilization provides the chemical basis for efficient chorismate mutase catalysis. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 17516–17521. 10.1073/pnas.1408512111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderer K.; Neuenschwander M.; Codoni G.; Sasso S.; Gamper M.; Kast P. Functional mapping of protein-protein interactions in an enzyme complex by directed evolution. PLoS One 2014, 9, e116234 10.1371/journal.pone.0116234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore N. J.; Nazmi A. R.; Hutton R. D.; Webby M. N.; Baker E. N.; Jameson G. B.; Parker E. J. Complex formation between two biosynthetic enzymes modifies the allosteric regulatory properties of both: An example of molecular symbiosis. J. Biol. Chem. 2015, 290, 18187–18198. 10.1074/jbc.M115.638700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munack S.; Roderer K.; Ökvist M.; Kamarauskaitė J.; Sasso S.; van Eerde A.; Kast P.; Krengel U. Remote control by inter-enzyme allostery: A novel paradigm for regulation of the shikimate pathway. J. Mol. Biol. 2016, 428, 1237–1255. 10.1016/j.jmb.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Sambrook J.; Russel D. W.. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, 2001. [Google Scholar]
- MacBeath G.; Kast P. UGA read-through artifacts—When popular gene expression systems need a pATCH. Biotechniques 1998, 24, 789–794. 10.2144/98245st02. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Kabsch W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 133–144. 10.1107/S0907444909047374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. R.; Murshudov G. N. How good are my data and what is the resolution?. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 1204–1214. 10.1107/S0907444913000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn M. D.; Ballard C. C.; Cowtan K. D.; Dodson E. J.; Emsley P.; Evans P. R.; Keegan R. M.; Krissinel E. B.; Leslie A. G. W.; McCoy A.; McNicholas S. J.; Murshudov G. N.; Pannu N. S.; Potterton E. A.; Powell H. R.; Read R. J.; Vagin A.; Wilson K. S. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 235–242. 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickle I. J.; Flensburg C.; Keller P.; Paciorek W.; Sharff A.; Vonrhein C.; Bricogne G.. STARANISO; Global Phasing Ltd.: Cambridge, United Kingdom, 2018. [Google Scholar]
- McCoy A. J.; Grosse-Kunstleve R. W.; Adams P. D.; Winn M. D.; Storoni L. C.; Read R. J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P.; Lohkamp B.; Scott W. G.; Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalevskiy O.; Nicholls R. A.; Long F.; Carlon A.; Murshudov G. N. Overview of refinement procedures within REFMAC5: Utilizing data from different sources. Acta Crystallogr. D Struct. Biol. 2018, 74, 215–227. 10.1107/S2059798318000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebschner D.; Afonine P. V.; Baker M. L.; Bunkóczi G.; Chen V. B.; Croll T. I.; Hintze B.; Hung L.-W.; Jain S.; McCoy A. J.; Moriarty N. W.; Oeffner R. D.; Poon B. K.; Prisant M. G.; Read R. J.; Richardson J. S.; Richardson D. C.; Sammito M. D.; Sobolev O. V.; Stockwell D. H.; Terwilliger T. C.; Urzhumtsev A. G.; Videau L. L.; Williams C. J.; Adams P. D. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 2019, 75, 861–877. 10.1107/S2059798319011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman H. M.; Westbrook J.; Feng Z.; Gilliland G.; Bhat T. N.; Weissig H.; Shindyalov I. N.; Bourne P. E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-K.; Reddy S. K.; Nelson B. C.; Robinson H.; Reddy P. T.; Ladner J. E. A comparative biochemical and structural analysis of the intracellular chorismate mutase (Rv0948c) from Mycobacterium tuberculosis H37Rv and the secreted chorismate mutase (y2828) from Yersinia pestis. FEBS J. 2008, 275, 4824–4835. 10.1111/j.1742-4658.2008.06621.x. [DOI] [PubMed] [Google Scholar]
- Berendsen H. J. C.; van der Spoel D.; van Drunen R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. 10.1016/0010-4655(95)00042-E. [DOI] [Google Scholar]
- Abraham M. J.; Murtola T.; Schulz R.; Páll S.; Smith J. C.; Hess B.; Lindahl E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. 10.1016/j.softx.2015.06.001. [DOI] [Google Scholar]
- Cornell W. D.; Cieplak P.; Bayly C. I.; Gould I. R.; Merz K. M. Jr.; Ferguson D. M.; Spellmeyer D. C.; Fox T.; Caldwell J. W.; Kollman P. A. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J. Am. Chem. Soc. 1995, 117, 5179–5197. 10.1021/ja00124a002. [DOI] [Google Scholar]
- Case D. A.; Darden T. A.; Cheatham T. E. III; Simmerling C. L.; Wang J.; Duke R. E.; Luo R.; Walker R. C.; Zhang W.; Merz K. M.; Roberts B.; Hayik S.; Roitberg A.; Seabra G.; Swails J.; Götz A. W.; Kolossváry I.; Wong K. F.; Paesani F.; Vanicek J.; Wolf R. M.; Liu J.; Wu X.; Brozell S. R.; Steinbrecher T.; Gohlke H.; Cai Q.; Ye X.; Wang J.; Hsieh M.-J.; Cui G.; Roe D. R.; Mathews D. H.; Seetin M. G.; Salomon-Ferrer R.; Sagui C.; Babin V.; Luchko T.; Gusarov S.; Kovalenko A.; Kollman P. A.. AMBER 12; University of California: San Fransisco, 2012. [Google Scholar]
- Jorgensen W. L.; Madura J. D. Solvation and conformation of methanol in water. J. Am. Chem. Soc. 1983, 105, 1407–1413. 10.1021/ja00344a001. [DOI] [Google Scholar]
- Wang J.; Wolf R. M.; Caldwell J. W.; Kollman P. A.; Case D. A. Development and testing of a general Amber force field. J. Comput. Chem. 2004, 25, 1157–1174. 10.1002/jcc.20035. [DOI] [PubMed] [Google Scholar]
- Essmann U.; Perera L.; Berkowitz M. L.; Darden T.; Lee H.; Pedersen L. G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. 10.1063/1.470117. [DOI] [Google Scholar]
- Bussi G.; Donadio D.; Parrinello M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- Parrinello M.; Rahman A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. 10.1063/1.328693. [DOI] [Google Scholar]
- Bartlett P. A.; Johnson C. R. An inhibitor of chorismate mutase resembling the transition-state conformation. J. Am. Chem. Soc. 1985, 107, 7792–7793. 10.1021/ja00311a111. [DOI] [Google Scholar]
- Vamvaca K.; Vögeli B.; Kast P.; Pervushin K.; Hilvert D. An enzymatic molten globule: Efficient coupling of folding and catalysis. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 12860–12864. 10.1073/pnas.0404109101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderer K.; Kast P. Evolutionary cycles for pericyclic reactions – Or why we keep mutating mutases. CHIMIA 2009, 63, 313–317. 10.2533/chimia.2009.313. [DOI] [Google Scholar]
- Burschowsky D.; Thorbjørnsrud H. V.; Heim J. B.; Fahrig-Kamarauskaitė J.; Würth-Roderer K.; Kast P.; Krengel U. Inter-enzyme allosteric regulation of chorismate mutase in Corynebacterium glutamicum: structural basis of feedback activation by Trp. Biochemistry 2018, 57, 557–573. 10.1021/acs.biochem.7b01018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.