Abstract
The kidneys are responsible for maintaining physiologic homeostasis. The kidneys clear a variety of drugs and other substances through passive (filtration) and active processes that utilize transport proteins. Renal clearance is comprised of the processes of glomerular filtration, tubular secretion, and tubular reabsorption. Endogenous biomarkers, such as creatinine and cystatin C, are routinely used to estimate renal clearance. Understanding the contributing components of renal function and clearance, through the use of biomarkers, is necessary in elucidating the renal pharmacology of drugs and other substances. While exogenous markers of kidney function have been known for decades, several complexities have limited their usage. Several endogenous markers are being evaluated and hold promise to elucidate the individual components of kidney function that represent filtration, secretion, and reabsorption.
Keywords: Kidney, Biomarker, Drug clearance, Filtration, Secretion, Reabsorption
Introduction
The kidneys are responsible for maintaining water, electrolyte, and acid-base homeostasis. They are effective at removing metabolic waste products, xenobiotics (drugs and toxins) and maintaining physiologic osmolality (Fig. 1). Renal clearance is characterized as the composite of three processes – glomerular filtration, tubular secretion, and tubular reabsorption (Equation (1)) [1]. While filtration and secretion add substances to the urinary ultrafiltrate, reabsorption removes compounds from the ultrafiltrate. The expanded renal clearance equation includes a term for fraction reabsorbed to account for the negative contribution to clearance (Equation (2)).
| Equation 1 |
| Equation 2 |
where ClR is renal clearance, Fu is the fraction unbound, Q is the renal blood flow, Cli is the intrinsic renal clearance, and Fr is fraction of the compound reabsorbed from the tubule lumen.
Figure. 1.
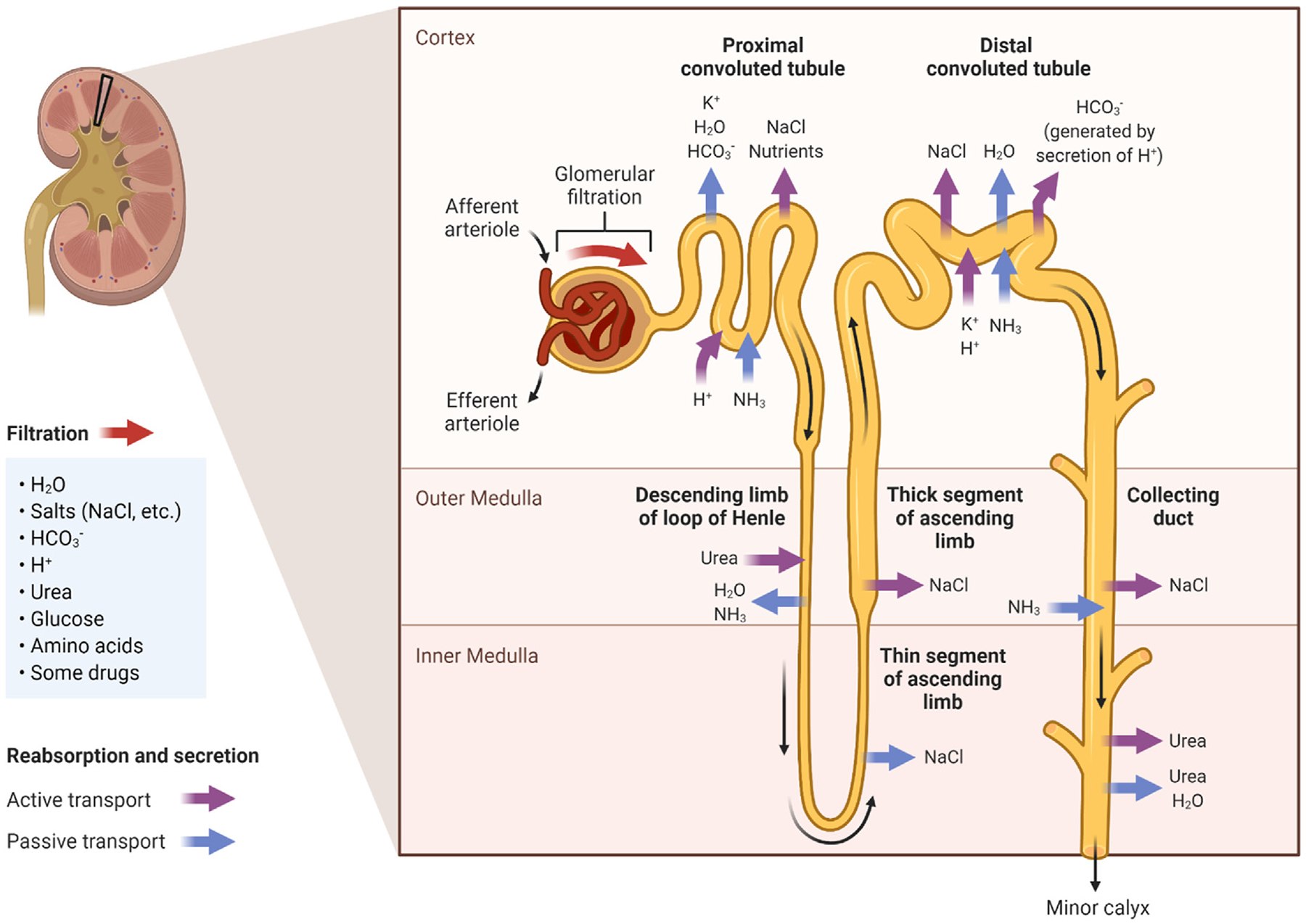
The kidneys maintain physiologic osmolality and remove waste products, drugs, and toxins from the blood through the processes of filtration, secretion, and reabsorption. Compounds must fit within size and charge exclusion properties to be filtered across the glomerulus and into the urinary ultrafiltrate. Transporters in the proximal and distal convoluted tubules secrete compounds from the blood into the ultrafiltrate and reabsorb substances from the ultrafiltrate back into the blood. Adapted from “Kidney Reabsorption and Secretion”, by BioRender.com (2022).
While some small molecular weight drugs are excreted unchanged through the kidneys, metabolism leads to the addition of a functional group which increases the charge and molecular weight, requiring renal transporters for the urinary excretion of most drugs. Secretion and reabsorption of drugs in the kidneys are facilitated by transport proteins in the tubules, leading to unidirectional or bidirectional movement of both organic anions and cations (Fig. 2).
Figure. 2.
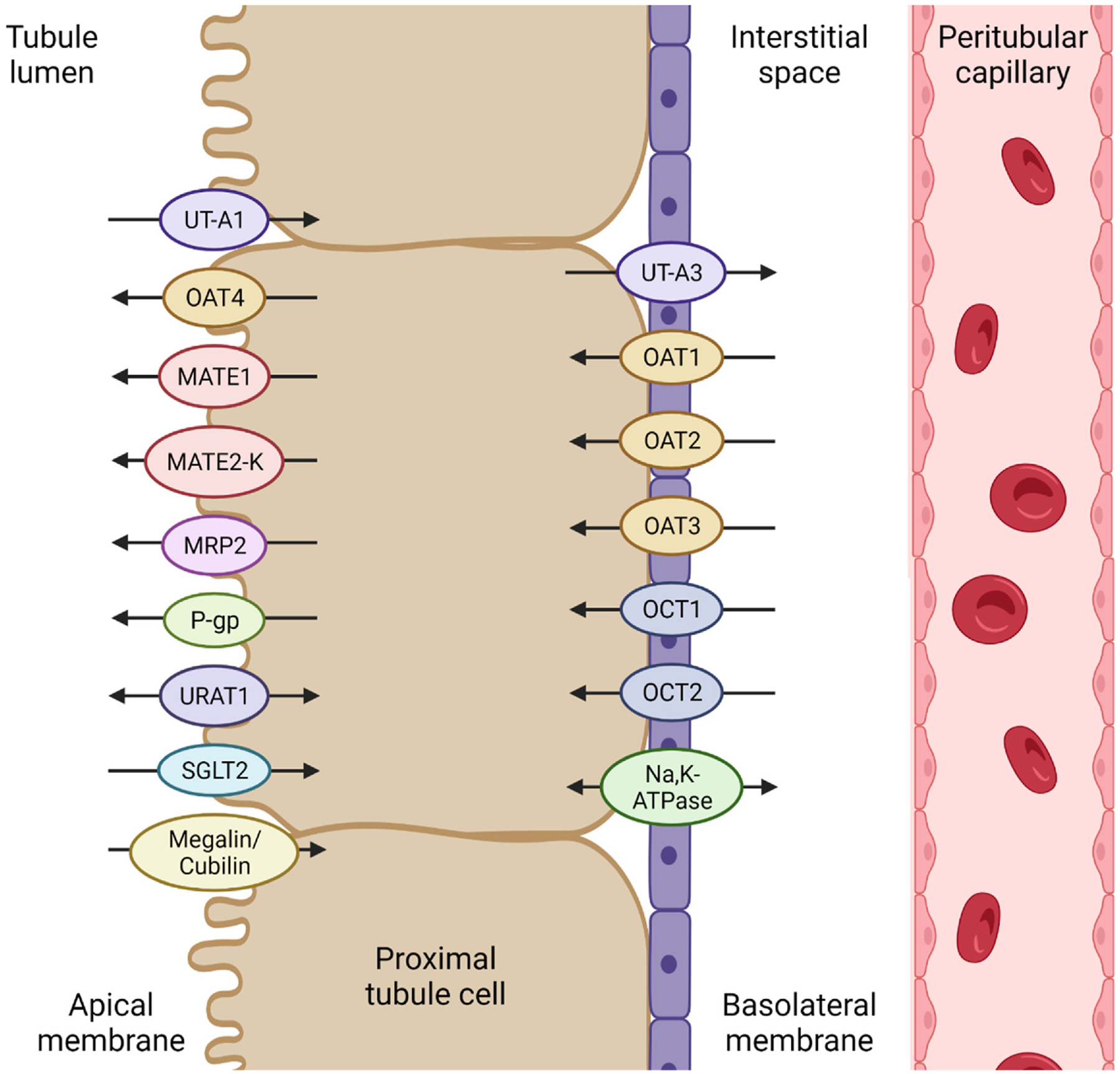
Representative renal transporters that are responsible for secretion and reabsorption in proximal tubule cells. Transporters on the basolateral membrane transport molecules from the blood into the proximal tubule cells. Apical transporters are responsible for the efflux of molecules from the proximal tubule cells into the urinary ultrafiltrate. Some transporters work bi-directionally and reabsorb certain molecules from the ultrafiltrate back into the blood. UT: urea transporter; OAT: organic anion transporter; MATE: multidrug and toxin extrusion protein; MRP: multidrug resistance-associated protein; P-gp: P-glycoprotein; URAT: urate transporter; SGLT: sodium-glucose co-transporter; OCT: organic cation transporter.
Glomerular filtration rate (GFR) and creatinine clearance (CrCl) are used for evaluating kidney function and deciding drug dosing requirements (Table 1) [2–4]. Similar to GFR and CrCl equations is the use of serum creatinine as the endogenous marker. Numerous publications have reported on the limitations of serum creatinine for determination of kidney function [5–8]. The use of creatinine to estimate GFR is limited secondary to several factors including muscle mass differences, age, sex, drugs, disease states, diets, and physical activity levels [5,6]. As creatinine is generally assumed to be 85% filtered and 15% secreted, it is primarily used to represent glomerular filtration. Equations to estimate GFR (referred to in the nephrology community as eGFR, representing an estimate) include age, sex, and serum creatinine (Table 1) [9]. Race was removed as a variable from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula in 2021 following a scientific debate [10].
Table 1.
Equations for estimating kidney function using endogenous markers.
| Name | Biomarkers | Equation | References | |||
|---|---|---|---|---|---|---|
| CKD-EPI Creatinine GFR Equation (2021) | Creatinine | GFR = 142 * (SCr/A)B * 0.9938Age * (1.012 if female) | [10] | |||
| Male | Female | |||||
| SCr ≤0.9 | A = 0.9 B = −0.302 | SCr ≤0.7 | A = 0.7 B = −0.241 | |||
| SCr >0.9 | A = 0.9 B = −1.2 | SCr >0.7 | A = 0.7 B = −1.2 | |||
| CKD-EPI Creatinine GFR Equation (2009) | Creatinine | GFR = A * (SCr/B)C * 0.993Age * (1.159 if Black) | [9] | |||
| Male | Female | |||||
| SCr ≤0.9 | A = 141 B = 0.9 C = −0.411 | SCr ≤0.7 | A = 144 B = 0.7 C = −0.329 | |||
| SCr >0.9 | A = 141 B = 0.9 C = −1.209 | SCr >0.7 | A = 144 B = 0.7 C = −1.209 | |||
| MDRD GFR Equation (2006) | Creatinine | GFR = 175 * (SCr)−1.154 * Age−0.203 * (1.212 if Black) * (0.742 if female) | [89] | |||
| CrCl GFR Equation | Creatinine | GFR ≈ CrCl = (UCr * V)/SCr | [90] | |||
| Cockcroft-Gault CrCl Equation (1976) | Creatinine | CrCl = (140 – Age) * (Weight) * (0.85 if female)/(72 * SCr) | [30] | |||
| CKD-EPI Cystatin C GFR Equation (2012) | Cystatin C | GFR = 133 * (Scys/A)B * 0.9938Age * (1.012 if female) | [37] | |||
| Male | Female | |||||
| A = −0.499 B = 1 | A = −0.499 B = 0.932 | |||||
| CKD-EPI Creatinine-Cystatin C GFR Equation (2021) | Creatinine, Cystatin C | GFR = 135 * (SCr/A)B * (Scys/C)D * 0.9961Age * (0.963 if female) | [10] | |||
| Male: | ||||||
| SCr ≤0.9 | SCr >0.9 | |||||
| Scys ≤0.8 | A = 0.9 B = −0.144 C = 0.8 D = −0.323 | A = 0.9 B = −0.544 C = 0.8 D = −0.323 | ||||
| Scys >0.8 | A = 0.9 B = −0.144 C = 0.8 D = −0.778 | A = 0.9 B = −0.544 C = 0.8 D = −0.778 | ||||
| Female: | ||||||
| SCr ≤0.7 | SCr >0.7 | |||||
| Scys ≤0.8 | A = 0.7 B = −0.219 C = 0.8 D = −0.323 | A = 0.7 B = −0.544 C = 0.8 D = −0.323 | ||||
| Scys >0.8 | A = 0.7 B = −0.219 C = 0.8 D = −0.778 | A = 0.7 B = −0.544 C = 0.8 D = −0.778 | ||||
| CKD-EPI Creatinine-Cystatin C GFR Equation (2012) | Creatinine, Cystatin C | GFR = A * (SCr/B)C * (Scys/0.8)D * 0.995Age * (1.08 if Black) | [37] | |||
| Male: | ||||||
| SCr ≤0.9 | SCr >0.9 | |||||
| Scys ≤0.8 | A = 135 B = 0.9 C = −0.207 D = −0.375 | A = 135 B = 0.9 C = −0.601 D = −0.375 | ||||
| Scys >0.8 | A = 135 B = 0.9 C = −0.207 D = −0.711 | A = 135 B = 0.9 C = −0.601 D = −0.711 | ||||
| Female: | ||||||
| SCr ≤0.7 | SCr >0.7 | |||||
| Scys ≤0.8 | A = 130 B = 0.7 C = −0.248 D = −0.375 | A = 130 B = 0.7 C = −0.601 D = −0.375 | ||||
| Scys >0.8 | A = 130 B = 0.7 C = −0.248 D = −0.711 | A = 130 B = 0.7 C = −0.601 D = −0.711 | ||||
| CKD-EPI B2M GFR Equation (2016) | B2M | GFR = 133 * B2M−0.852 | [40] | |||
| CKD-EPI BTP GFR Equation (2016) | BTP | GFR = 55 * BTP−0.695 * 0.998Age * (0.899 if female) | [40] | |||
| CKD-EPI BTP-B2M GFR Equation (2016) | B2M, BTP | GFR = 96 * B2M−0.588 * BTP−0.278 | [40] | |||
| CKiD Pediatric GFR Equation (2012) | Creatinine, Cystatin C, Urea | GFR = 39.8 * (Height/SCr)0.456 * (1.8/Scys)0.418 * (30/BUN)0.079 * (Height/1.4)0.179 * (1.076 if male) | [62] | |||
| Hippurate RPF Equation | Hippurate | RPF = (UH * V)/PH | [67] | |||
| Renal Clearance Equation | Secretory solutes | ClR = (UEx from 0 to 24 h)/(AUC Px from 0 to 24 h) | [71,75] | |||
| Net Renal Secretion Clearance Equation | Secretory solutes | Clsec = ClR − Fu * GFR | [74] | |||
| Fractional Excretion Equation | Secretory solutes | FEx = (Ux * PCr)/(Px * UCr) | [72] | |||
| Tubular Reabsorption Equation | Reabsorbed solutes | FEx = (Ux/Px)/(UCr/PCr) | [77] | |||
| Tubular Reabsorption of Phosphate Equation | Phosphate | TRP (%) = 1 − [(UP/PP) * (PCr/UCr)] | [88] | |||
CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; MDRD: Modification of Diet in Renal Disease; CrCl: creatinine clearance; GFR: glomerular filtration rate; B2M: beta-2 microglobulin; BTP: beta-trace protein; CKiD: Chronic Kidney Disease in Children; RPF: renal plasma flow; SCr: serum creatinine; UCr: urinary creatinine; V: urine volume; SCys: serum cystatin C; BUN: blood urea nitrogen; UH: urinary hippurate; PH: plasma hippurate; ClR: renal clearance; UEx: urinary excretion amount of solute x; AUC: area under the curve; Px: plasma concentration of x; Clsec: net renal secretion clearance; Fu: fraction unbound; FEx: fractional excretion of x; Ux: urine concentration of x; PCr: plasma creatinine; TRP: tubular reabsorption of phosphate; UP: urinary phosphate; PP: plasma phosphate.
There has been a renewed interest within the nephrology community to find improved markers of kidney function and more precisely differentiate the individual renal clearance components. There is increasing recognition of the limitations of the markers currently in use [3,4,11]. This review will briefly discuss exogenous markers of renal filtration and then highlight the most contemporary literature related to endogenous markers of glomerular filtration, tubular secretion, and tubular reabsorption (Table 2).
Table 2.
Endogenous markers of kidney function.
| Biomarker | Matrix | Usage | Normal reference range | Comments |
|---|---|---|---|---|
| Creatinine | Serum | Glomerular filtration | M: 0.74–1.35 mg/dL F: 0.59–1.04 mg/dL |
Most frequently used clinical marker to estimate GFR but heavily influenced by muscle mass; increases only after significant reductions in GFR |
| Urine | Glomerular filtration and tubular secretion | M: 20–320 mg/dL F: 20–275 mg/dL |
CrCl overestimates GFR due to tubular secretion of creatinine | |
| Cystatin C | Serum | Glomerular filtration | 0.62–1.15 mg/L | Outperforms SCr in calculating eGFR; less variation across populations than SCr |
| Urine | Tubular damage | <0.1 mg/dL | Catabolized in proximal tubular cells, presence in urine indicates kidney injury | |
| B2M | Plasma | Glomerular filtration | 0.8–2.5 mg/L | Elevated concentrations associated with decreased kidney function |
| Urine | Tubular reabsorption | <300 μg/L | Catabolized in proximal tubular cells, presence in urine indicates impaired tubular reabsorption; unstable in acidic urine | |
| BTP | Serum | Glomerular filtration | M: 0.37–0.77 mg/L F: 0.40–0.70 mg/L |
Elevated concentrations associated with reduced GFR |
| Urine | Glomerular filtration | M: < 7.79 mg/L F: < 3.13 mg/L |
Urinary excretion increases as GFR decreases | |
| SDMA | Serum | Glomerular filtration | M: 61–136 μg/L F: 55–127 μg/L |
Elevated concentrations associated with reduced GFR; consistent across species, often used in veterinary medicine |
| Albumin | Serum | Glomerular filtration | 3.4–5.4 g/dL | Low concentrations associated with decreased eGFR and increased creatinine tubular secretion |
| Urine | Glomerular filtration and tubular reabsorption | 0.2–1.9 mg/dL | Not excreted by healthy kidneys, high specificity for renal injury | |
| Urea | Serum | Glomerular filtration and tubular reabsorption | 6–24 mg/dL | BUN increases as GFR decreases |
| Urine | Glomerular filtration and tubular reabsorption | 12–20 g/day | Low concentrations indicate kidney injury while high concentrations can indicate high protein intake | |
| Hippurate | Urine: plasma ratio | Tubular secretion | P: < 0.5 mg/dL U: < 1070 mg/g Cr FE: > 3.19 |
Fractional excretion is decreased in patients with kidney injury |
| Cinnamoylglycine | Urine: plasma ratio | Tubular secretion | P: < 8.1 μg/L U: < 4 mg/g Cr FE: > 0.89 |
Fractional excretion is decreased in patients with kidney injury |
| NMN | Plasma | Tubular secretion | 6–40 μg/L | Used to evaluate OCT2 and MATE1/2K secretory transporters in drug–drug interactions |
| Urine | Tubular secretion | 4.3–7.8 mg/day | Decreased excretion associated with impaired kidney function | |
| A1M | Serum | Tubular reabsorption | 20–42 mg/L | Elevated concentrations associated with decreased kidney function; concentrations correlated with creatinine concentrations |
| Urine | Tubular reabsorption | <8 mg/L | Catabolized in proximal tubule cells, presence in urine indicates impaired tubular reabsorption | |
| FABP1 | Serum | Tubular reabsorption | 9–17 μg/L | Concentration increases in parallel with worsening diabetic nephropathy |
| Urine | Tubular reabsorption | 1.7–9.3 μg/g Cr | Increased excretion associated with impaired tubular reabsorption | |
| Phosphate | Plasma | Tubular reabsorption | 3–4.5 mg/dL | Elevated concentrations associated with reduced kidney function |
| Urine | Tubular reabsorption | 0.4–1.31 g/day TRP >85% |
TRP calculated using plasma and urine concentrations over 24 h |
B2M: beta-2 microglobulin; BTP: beta-trace protein; SDMA: symmetric dimethylarginine; NMN: N1-methylnicotinamide; A1M: alpha-1-microglobulin; FABP1: fatty acid binding protein 1; M: for males; F: for females; P: plasma; U: urine; Cr: creatinine; FE: fractional excretion relative to creatinine; TRP: tubular reabsorption of phosphate; CrCl: creatinine clearance; SCr: serum creatinine; BUN: blood urea nitrogen.
Exogenous markers of filtration
Exogenous markers that focus on filtration and effective renal plasma flow (eRPF) have been used for several decades and inulin and para-aminohippurate (PAH), respectively, are considered the gold standards [12,13]. There are well-established methods for using these markers [14–18]. Inulin is not routinely used clinically as it requires a continuous infusion, frequent timed serum and urine collections, and an assay that is not convenient for clinical practice [12]. PAH is unbound, has a high clearance, and undergoes both filtration and tubular secretion. At low plasma concentrations (10–20 mg/L), about 90% of PAH is cleared by the kidneys in a single pass [19]. To measure eRPF, PAH is administered as an intravenous infusion, to sustain a plasma concentration of 20 mg/L, and frequent timed plasma and urine samples are collected [16–19]. However, these studies are arduous due to the long infusion needed to achieve steady-state and the need for timed collections of plasma and urine [18]. High doses of PAH at plasma levels 400–600 mg/L, saturate tubular secretion of PAH and can be used to parse out the tubular secretion component [19,20].
Although inulin and PAH are considered gold standards for assessment of kidney function, they are expensive, invasive, and cumbersome procedures that are not conducive to routine use. The search for an ideal exogenous filtration marker should be centered on the characteristics of 1) excreted solely by the kidneys, 2) no protein binding, 3) no secretion or reabsorption, 4) easy to use, and 5) low distribution volume, indicating a relatively short redistribution phase and therefore a shorter test. Newer exogenous markers, such as iothalamate, iohexol, and radioactive compounds, have been evaluated. Common methods for measuring GFR are the urinary clearance of non-radioactive iothalamate and the plasma clearance of iohexol [12]. For these tests, the exogenous compound is administered as either a sub-cutaneous injection (for urinary clearance) or infusion (for plasma clearance) and timed blood and/or urine samples are collected over the clearance period [21]. Measuring plasma clearance is often advantageous in populations with bladder impairment as it does not require urine collection [22]. However, when calculating GFR by plasma clearance using a limited number of samples, an equation to correct for the absence of the early compartment must be used to account for the redistribution phase [23]. Iohexol is not metabolized or transported in the kidneys and is excreted predominantly by glomerular filtration, making it an excellent candidate marker of GFR [24]. Radioactive compounds such as I-125 iothalamate, chromium-51 labelled ethylene diamine tetra-acetic acid (51Cr-EDTA), and technetium-99 m diethylenetriaminepentaacetic acid (99mTc-DPTA) can also be used for measuring GFR. Urinary and plasma clearance methods following a single injection accurately measure GFR and produce similar results [25,26]. The CKD-EPI 2009 and 2012 eGFR equations were validated using urinary non-radioactive iothalamate clearance while the CKD-EPI 2021 equations were validated using the plasma clearance of iohexol and 51Cr-EDTA (Table 1) [10,27].
Numerous limitations with exogenous assessment methods exist including long infusions to achieve equilibration, bias due to methodology or sample timing, and in individuals with low GFR, the elimination of the marker may not be fully elucidated during the sample collection period [12,13]. Given these limitations, there has been interest in evaluating endogenous compounds.
Endogenous markers of filtration
An ideal endogenous marker of GFR has been defined to have a constant rate of endogenous production, free passage through the glomerulus, no-to-limited protein binding, excretion exclusively by glomerular filtration, and measurement that is simple, accurate, and cost-effective [28]. This section will review conventional and contemporary endogenous markers of filtration and Table 2 provides concise data usages and normal ranges.
1. Creatinine
Serum creatinine (SCr) is the oldest and most commonly used endogenous marker to estimate GFR (Tables 1 and 2). Since creatinine undergoes filtration and secretion, patients with reduced kidney function can exhibit a reduction in creatinine filtration and an increase in the secretion contribution [29]. There can be confusion around the degree of kidney function with SCr, as observations of elevated concentrations may be delayed until after GFR has been reduced by over 50% [7,8]. Creatinine is a relatively insensitive endogenous biomarker for early detection of kidney injury.
Endogenous CrCl has traditionally been used to guide drug dosing and uses the Cockcroft–Gault equation (Table 1) [30]. CrCl can also be calculated by collecting urine and blood and using SCr and urinary creatinine concentrations (Table 1). CrCl can overestimate actual GFR due to the tubular secretion of creatinine [31].
2. Cystatin C
Cystatin C is a protein that is eliminated exclusively by glomerular filtration but subsequently undergoes catabolism in the lysosomes of tubular cells, resulting in limited appearance in the urine of healthy individuals [32,33]. Cystatin C in the blood is not a product of muscle mass and is instead produced by all nucleated cells, meaning its production is more uniform across populations than creatinine [34]. However, increased age, smoking, obesity, hyperthyroidism, and the use of corticosteroids are all associated with increased concentrations [34,35]. Many studies have demonstrated that eGFR determination using both SCr and serum cystatin C concentrations results in improved accuracy over eGFR determined from either SCr or serum cystatin C alone (Tables 1 and 2) [5,10,36,37].
3. Beta-2 microglobulin (B2M)
Beta-2 microglobulin (B2M) is generated by all nucleated cells in the body. B2M is freely filtered by the glomerulus and concentrations (plasma and urinary) can increase early after a kidney insult [38,39]. Studies have shown that the CKD-EPI B2M equation for estimating GFR, which is independent of age and sex, performs similarly to the CKD-EPI creatinine-cystatin C equation (Table 1) [40–42].
4. Beta-trace protein (BTP)
Beta-trace protein (BTP), also known as lipocalin-type-prostaglandin-d-synthase, is filtered by the glomerulus with limited tubular reabsorption [43]. Elevated serum and urine BTP concentrations have been associated with renal and cardiovascular diseases, as well as mortality [44]. However, the CKD-EPI BTP equation does not outperform either the CKD-EPI creatinine or cystatin C equations in estimating GFR (Table 1) [40,45].
5. Symmetric dimethylarginine (SDMA)
Symmetric dimethylarginine (SDMA) is the endogenous catabolic product of methylated arginine-containing proteins and is mainly excreted by the kidneys [46]. SDMA is not influenced by the non-renal factors that impact creatinine and/or cystatin C, such as diet, muscle mass, etc., and is only minimally influenced by obesity, gender, and age [47]. SDMA levels are increased in the plasma of patients with kidney disease and correlate with GFR in patients with CKD [48]. Additionally, the use of SDMA as a biomarker of renal function is consistent across species (human, cat, dog) and is often used in veterinary medicine [47,49–51].
6. Albumin
Albumin is filtered by the glomerulus and then largely reabsorbed in the tubules (~97%) [52]. The presence of albumin in the urine (albuminuria) signifies structural damage to the kidney glomerulus, due to diseases such as glomerulonephritis or diabetes mellitus. This damage results in the “filtering” of large molecular weight compounds. There can also be a reduced ability of the tubules to reabsorb albumin due to either saturation of reabsorption capacity or injury leading to decreases in the function of uptake pathways [53,54]. Urinary albumin excretion is used in CKD staging and is an independent risk factor of mortality [55]. After a stage of hyperfiltration (high GFR), individuals with diabetes or obesity develop albuminuria, and this is associated with the development of CKD, cardiovascular disease, and death [56]. Increased GFR is associated with albuminuria and an increased urinary albumin-to-creatinine ratio [57]. Decreased serum albumin (hypoalbuminemia) is associated with decreased eGFR [58].
7. Urea
Urea is produced by the liver as a product of protein and amino acid catabolism. In the kidneys, it is freely filtered from the blood and allows the kidneys to create hyperosmotic urine, helping to prevent the loss of water [12]. The amount of urea reabsorbed in the collecting ducts (~50%) is dependent on the permeability and the tubular concentration of urea, which are both regulated by antidiuretic hormone (ADH), also known as vasopressin. ADH synthesis in the hypothalamus is triggered by increases in blood osmolarity, such as an increase in sodium concentration, and acts in the kidneys to increase water reabsorption to prevent dehydration [59]. ADH renders the medullary collecting ducts highly permeable to urea by increasing phosphorylation and apical plasma-membrane accumulation of urea transporters A1 (UT-A1) and A3 (UT-A3) (Fig. 2) [60]. UT-A1 (apical) and UT-A3 (basolateral, apical after ADH stimulation) are found in the inner medullary collecting duct. Additionally, in the presence of ADH, water is avidly reabsorbed in the distal tubule and urea becomes highly concentrated, driving urea reabsorption. Alternatively, the absence of ADH results in decreased collecting duct permeability and high levels of water in the collecting duct, diluting the concentration of tubular urea and decreasing urea reabsorption. While blood osmolarity and volume are the main factors that affect ADH synthesis, angiotensin, pain, nausea, hypoglycemia, nicotine, and certain medications can also promote ADH secretion [59]. ADH secretion is inhibited by ethanol, explaining the increased diuresis and free water loss during intoxication [59].
Blood urea nitrogen (BUN) concentrations are ~46% of blood urea concentrations [61]. BUN has an inverse relationship with GFR. Previous reports have demonstrated the usefulness of eGFR calculated by equations based on SCr, BUN, height, gender, and cystatin C serum levels, particularly in children with CKD (Table 1) [62,63]. Since the serum and urinary concentrations of urea depend on ADH production, urine flow rate, diet, and urea cycle enzymes, it is a poor marker of GFR [7].
Endogenous markers of secretion
Tubular secretion is the primary mechanism of drug excretion through the kidneys (Fig. 1) [3,4]. Tubular secretion removes drugs from circulation, including those that are protein-bound, via transporter proteins on the basolateral membrane of the proximal tubule, while proteins on the apical membrane further contribute by moving drugs from the proximal tubule to the urine ultrafiltrate (Fig. 2). Transporters can be bidirectional depending on pH. Transporters can also facilitate the movement of drugs back into the blood from the urinary ultrafiltrate. Unlike glomerular filtration, tubular secretion is a saturable process and can be altered by competitive binding interactions between medications and other circulating substances [2]. An ideal endogenous marker of secretion should be excreted into the urine without degradation in the body and be measured easily, accurately, and cost-effectively. Despite the importance of tubular secretion, it is difficult to quantify using endogenous markers and hence there are no established user-friendly equations analogous to GFR or CrCl for filtration [2,4].
1. Creatinine
As mentioned previously, creatinine is actively secreted by the tubules in addition to being filtered by the glomerulus. CrCl calculations result in an overestimation of kidney filtration function since creatinine is 15% cleared through tubular secretion [31]. Studies often compare the measured renal clearance of a drug to the CrCl to predict whether the drug undergoes a significant secretory component [64]. For example, if the renal clearance of a drug is greater than CrCl, secretion is presumed to be a contributing process. Drugs that are substrates of organic cation transporters may compete with creatinine renal secretion and result in an underestimation of GFR [65]. It is important to differentiate whether a reduction in GFR or CrCl is due to a transporter competition or due to drug-induced kidney injury [64].
2. Hippurate
Hippurate, an organic anion, is a glycine conjugate of benzoic acid formed by gut bacterial metabolism and by mitochondria in the liver and kidneys [66]. The primary excretion route is through renal tubular secretion via OAT1 and OAT3, located on the tubular basolateral membrane (Fig. 2). Endogenous hippurate is cleared on a single pass through the kidneys (50–90% extraction) and the clearance, measured using a timed urine collection and a single plasma sample, can provide an estimate of an individual’s eRPF (Table 1) [67]. Hippurate urinary excretion decreases with decreased tubular secretion and accumulates in the plasma of patients with renal failure [68,69]. Individual variability in the gut production of hippurate, influenced by diet and microbiome composition, may limit its ability to estimate eRPF [66].
3. Cinnamoylglycine
Cinnamoylglycine is a gut-derived metabolite with a renal clearance greater than CrCl, suggestive of a secretion component [69]. Clearance of cinnamoylglycine is only moderately correlated with eGFR (0.40), confirming that it is primarily secreted rather than filtered [70]. Like hippurate, cinnamoylglycine accumulates in the plasma of patients with renal failure [69]. Multiple studies have used cinnamoylglycine excretion to estimate tubular secretion (Table 1) [71,72].
4. N1-methylnicotinamide (NMN)
Nicotinamide undergoes liver metabolism to produce N1-methylnicotinamide (NMN), a substrate of OCT2 and the multidrug and toxin extrusion proteins MATE1 and MATE2-K, the latter of which are transporters on the tubular apical membrane (Fig. 2). NMN is unbound and renal clearance is higher than GFR, implying significant excretion via tubular secretion [73]. NMN clearance has been evaluated as a potential endogenous biomarker of OCT2/MATE transporter secretory function in drug–drug interaction studies (Table 1) [73–75].
Endogenous markers of reabsorption
The tubules reabsorb most of the urinary ultrafiltrate, including water, sodium, and other nutrients (Fig. 1). Most reabsorption of the filtrate occurs in the proximal tubules, which reabsorb ~70% of the filtered load. The distal tubules are responsible for the finer regulation of the water, electrolyte, and hydrogen-ion balance. With damage to the glomerulus and tubules, more proteins undergo filtration through the leaky glomerulus and the reabsorption mechanisms in the tubules are oversaturated (and damaged), leading to the presence of proteins in the urine ultrafiltrate. Megalin, also known as low-density lipoprotein receptor-related protein 2 (LRP2), is an endocytic receptor that works in cooperation with the receptor cubilin to drive the reabsorption of nearly all filtered plasma proteins (Fig. 2) [76]. Certain compounds that are filtered out of the blood by the glomerulus and subsequently reabsorbed by the tubules, such as those discussed in this section, can be quantified in urine samples to estimate tubular reabsorption. For example, if a drug undergoes significant reabsorption after filtration, the renal clearance would be predicted to be lower than CrCl. Tubular reabsorption of drugs is calculated by dividing the urine to plasma ratio of the drug by the urine to plasma ratio of creatinine (Table 1) [77].
1. Alpha-1-microglobulin (A1M)
Alpha-1-microglobulin (A1M) is produced by hepatocytes and freely filtered by the glomerulus. About 99% of free A1M in the urinary ultrafiltrate is reabsorbed by megalin/cubilin-mediated endocytosis and subsequently catabolized in the tubules [32,78]. An increase in urinary A1M concentration indicates impaired tubular reabsorption and an increased risk of AKI, rapid CKD progression, and higher mortality [79–81].
2. Beta-2 microglobulin (B2M)
B2M is removed from circulation primarily by glomerular filtration and more than 99.9% of the protein is reabsorbed from the urinary ultrafiltrate and catabolized by lysosomes in the tubules [41]. Elevated urinary B2M is indicative of decreased tubular reabsorption [39].
3. Fatty acid binding protein 1 (FABP1)
Fatty acid binding protein 1 (FABP1), also known as liver-type FABP (LFABP), is expressed mainly in the liver and kidneys, and is freely filtered by the glomerulus and reabsorbed in the tubules by megalin/cubilin. Both serum and urinary FABP1 concentrations have been proposed as biomarkers for early detection of AKI [82,83]. Urinary FABP1 concentrations are associated with serum FABP1 concentrations and the inverse of protein reabsorption capacity [84]. Excretion of FABP1 increases in both kidney and liver injury, meaning it can be used as a biomarker of both reduced tubular reabsorption and liver function [84,85].
4. Phosphate
The electrolyte phosphate is formed when phosphorous is combined with oxygen. Phosphate is the most abundant intracellular anion in the body and is involved in energy production. Elevated plasma concentrations (hyper-phosphatemia) are associated with AKI, CKD, cardiovascular events, and mortality [86]. Phosphate levels are highly dependent on dietary intake so CKD patients with hyper-phosphatemia are prescribed phosphate-restrictive diets and phosphate binding drugs to reduce intestinal absorption of phosphate [87]. When phosphate intake is excessive, circulating levels of fibroblast growth factor 23 (FGF23) increase. FGF23 suppresses phosphate reabsorption in renal tubules, raising the tubular phosphate concentration until eventually, calcium phosphate crystals are formed which damage tubule cells and lead to interstitial fibrosis and nephron loss [87]. Urinary and plasma concentrations measured over 24 h can be used to calculate the percent tubular reabsorption of phosphate (Table 1) [88].
5. Albumin
Albumin is filtered by the glomerulus and then largely reabsorbed in the tubules (~97%; 71% in proximal convoluted tubule, 23% in loop of Henle and distal tubule, 3% in collecting duct) by megalin/cubilin-mediated endocytosis [52]. Albuminuria, or albumin in the urine, can signify tubular stress/dysfunction and decreased reabsorption.
6. Urea
Urea is freely filtered in the glomerulus and the amount of urea reabsorbed in the collecting ducts (~50%) is regulated by antidiuretic hormone (ADH) [12,59]. BUN levels increase as a result of enhanced tubular reabsorption [64].
Conclusions
While exogenous markers of kidney function have been used for decades, limitations related to lengthy, cumbersome protocols and extensive sample collection have limited their utility. In the era of personalized medicine and to circumvent the observed limitations, there is interest in using endogenous markers as tools to assess an individual’s kidney functional capacity. Several endogenous markers are being evaluated and hold promise to more fully elucidate the individual components of filtration, secretion, and reabsorption of kidney function and clearance.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Figures created with BioRender.com.
Abbreviations:
- 51Cr-EDTA
chromium-51 labelled ethylene diamine tetra-acetic acid
- 99mTc-DTPA
technetium-99 m diethylenetriaminepentaacetic acid
- A1M
alpha-1-microglobulin
- ADH
antidiuretic hormone
- AKI
acute kidney injury
- B2M
beta-2 microglobulin
- BTP
beta-trace protein
- BUN
blood urea nitrogen
- CKD
chronic kidney disease
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- CrCl
creatinine clearance
- eGFR
estimated glomerular filtration rate
- eRPF
effective renal plasma flow
- FABP1
fatty acid binding protein 1
- FGF23
fibroblast growth factor 23
- GFR
glomerular filtration rate
- LFABP
liver-type fatty acid binding protein
- LRP2
low-density lipoprotein receptor-related protein 2
- MATE
multidrug and toxin extrusion
- NMN
N1-methylnicotinamide
- OAT
organic anion transporter
- OCT
organic cation transporter
- PAH
para-aminohippurate
- SCr
serum creatinine
- SDMA
symmetric dimethylarginine
- UT
urea transporter
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Tett SE, et al. : Principles and clinical application of assessing alterations in renal elimination pathways. Clin Pharmacokinet 2003, 42:1193–1211. [DOI] [PubMed] [Google Scholar]
- 2.Wang K, Kestenbaum B: Proximal tubular secretory clearance: a neglected partner of kidney function. Clin J Am Soc Nephrol 2018, 13:1291–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3**.Chen Y, et al. : Prediction of kidney drug clearance: a comparison of tubular secretory clearance and glomerular filtration rate. J Am Soc Nephrol 2021, 32:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study compared renal clearance of furosemide and famciclovir, plasma and urine concentrations of eight endogenous secretory solutes, and GFR measured by iohexol clearance (iGFR) in patients with varying renal function (eGFR 21–140 mL/min per 1.73 m2). The authors found that the clearance of secretory solutes, such as cinnamoylglycine, can be used to accurately predict renal drug clearance.
- 4.Suchy-Dicey AM, et al. : Tubular secretion in CKD. J Am Soc Nephrol 2016, 27:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Ebert N, et al. : Assessment of kidney function: clinical indications for measured GFR. Clin Kidney J 2021, 14: 1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review focused on the limitations of estimating GFR using serum creatinine, highlighting clinical scenarios and conditions where the use of SCr-based eGFR may be invalid. Including cystatin C increased the accuracy of the GFR estimate but the best way to overcome the limitations of creatinine and cystatin C was by measuring GFR using an exogenous marker.
- 6.Wen Y, Parikh CR: Current concepts and advances in biomarkers of acute kidney injury. Crit Rev Clin Lab Sci 2021, 58: 354–368. [DOI] [PubMed] [Google Scholar]
- 7.Luft FC: Biomarkers and predicting acute kidney injury. Acta Physiol 2021, 231:e13479. [DOI] [PubMed] [Google Scholar]
- 8.Porrini E, et al. : Estimated GFR: time for a critical appraisal. Nat Rev Nephrol 2019, 15:177–190. [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, et al. : A new equation to estimate glomerular filtration rate. Ann Intern Med 2009, 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Inker LA, et al. : New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021, 385: 1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the definitive study that removed race from the CKD-EPI creatinine- and cystatin C-based eGFR equations. The authors found that the old creatinine equation that included race as a variable over-estimated GFR in Blacks and in non-Blacks to a lesser degree. As compared to the old creatinine equation, the new creatinine equations result in increased population estimates of CKD prevalence among Blacks and similar or lower prevalence among non-Blacks.
- 11*.Chen Y, et al. : Association of tubular solute clearances with the glomerular filtration rate and complications of chronic kidney disease: the Chronic Renal Insufficiency Cohort study. Nephrol Dial Transplant 2020, 36:1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study quantified candidate tubular secretory solutes, including hippurate and cinnamoylglycine, in paired urine and plasma samples from 1240 CKD patients. Patients also had iGFR measured using 125I-iothalamate clearance. The authors found that tubular secretory clearances were modestly correlated with iGFR and lower net tubular secretory clearances were associated with metabolic complications independent of GFR and albuminuria.
- 12*.Inker LA, Titan S: Measurement and estimation of GFR for use in clinical practice: core curriculum 2021. Am J Kidney Dis 2021, 78:736–749. [DOI] [PubMed] [Google Scholar]; This review highlighted the assessment of GFR in the clinic to determine kidney function. The authors discussed the strengths and limitations of different methods for measuring GFR, including the use of endogenous biomarkers and exogenous compounds.
- 13.Speeckaert MM, et al. : Measured glomerular filtration rate: the query for a workable golden standard technique. J Personalized Med 2021, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterner G, et al. : Determining ‘true’ glomerular filtration rate in healthy adults using infusion of inulin and comparing it with values obtained using other clearance techniques or prediction equations. Scand J Urol Nephrol 2008, 42:278–285. [DOI] [PubMed] [Google Scholar]
- 15.Schnurr E, Lahme W, Kuppers H: Measurement of renal clearance of inulin and PAH in the steady state without urine collection. Clin Nephrol 1980, 13:26–29. [PubMed] [Google Scholar]
- 16.Carrara F, et al. : Glomerular resistances predict long-term GFR decline in type 2 diabetic patients without overt nephropathy: a longitudinal subgroup analysis of the DEMAND trial. Acta Diabetol 2021, 59:309–317. [DOI] [PubMed] [Google Scholar]
- 17.Stroobant L, et al. : Simultaneous measurement of glomerular filtration rate, effective renal plasma flow and tubular secretion in different poultry species by single intravenous bolus of iohexol and para-aminohippuric acid. Animals (Basel) 2020, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seegmiller JC, et al. : Tubular secretion markers, glomerular filtration rate, effective renal plasma flow, and filtration fraction in healthy adolescents. Kidney Med 2020, 2:670–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aminohippurate sodium “PAH” [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2011. [Google Scholar]
- 20.Dowling TC, et al. : Characterization of tubular functional capacity in humans using para-aminohippurate and famotidine. Kidney Int 2001, 59:295–303. [DOI] [PubMed] [Google Scholar]
- 21.Seegmiller JC, et al. : Discordance between iothalamate and iohexol urinary clearances. Am J Kidney Dis 2016, 67:49–55. [DOI] [PubMed] [Google Scholar]
- 22.Ronnhedh C, Jaquenod M, Mather LE: Urineless estimation of glomerular filtration rate and renal plasma flow in the rat. J Pharmacol Toxicol Methods 1996, 36:123–129. [DOI] [PubMed] [Google Scholar]
- 23.Delanaye P, et al. : Comparison of plasma clearance with early-compartment correction equations and urinary clearance in high GFR ranges. Kidney Int Rep 2021, 6:1622–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz GJ, Work DF: Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 2009, 4: 1832–1843. [DOI] [PubMed] [Google Scholar]
- 25.McMeekin H, et al. : 99mTc DTPA vs. 51Cr EDTA for glomerular filtration rate measurement: is there a systematic difference? Nucl Med Commun 2019, 40:1224–1229. [DOI] [PubMed] [Google Scholar]
- 26.Vidal-Petiot E, et al. : Comparison of (51)Cr-EDTA and (99m)Tc-DTPA for glomerular filtration rate measurement. J Nephrol 2021, 34:729–737. [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Inker LA: GFR as the “gold standard”: estimated, measured, and true. Am J Kidney Dis 2016, 67:9–12. [DOI] [PubMed] [Google Scholar]
- 28.den Bakker E, Gemke R, Bokenkamp A: Endogenous markers for kidney function in children: a review. Crit Rev Clin Lab Sci 2018, 55:163–183. [DOI] [PubMed] [Google Scholar]
- 29.Branten AJ, Vervoort G, Wetzels JF: Serum creatinine is a poor marker of GFR in nephrotic syndrome. Nephrol Dial Transplant 2005, 20:707–711. [DOI] [PubMed] [Google Scholar]
- 30.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16:31–41. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, et al. : Tubular secretion of creatinine and kidney function: an observational study. BMC Nephrol 2020, 21:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang WR, Parikh CR: Biomarkers of acute and chronic kidney disease. Annu Rev Physiol 2019, 81:309–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mussap M, Plebani M: Biochemistry and clinical role of human cystatin C. Crit Rev Clin Lab Sci 2004, 41:467–550. [DOI] [PubMed] [Google Scholar]
- 34.Shlipak MG, Mattes MD, Peralta CA: Update on cystatin C: incorporation into clinical practice. Am J Kidney Dis 2013, 62: 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knight EL, et al. : Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 2004, 65:1416–1421. [DOI] [PubMed] [Google Scholar]
- 36*.den Bakker E, et al. : Concordance between creatinine- and cystatin C-based eGFR in clinical practice. Scand J Clin Lab Invest 2021, 81:142–146. [DOI] [PubMed] [Google Scholar]; This study evaluated the difference between eGFR calculated using serum creatinine and cystatin C concentrations. The results demonstrated that in the majority of the population (87%), eGFRcrea and eGFRcys measurements are less than 40% different, indicating high agreement. The authors concluded that the mean of eGFRcrea and eGFRcys can be used to accurately estimate GFR.
- 37.Inker LA, et al. : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012, 367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuwata K, et al. : Comparison of changes in urinary and blood levels of biomarkers associated with proximal tubular injury in rat models. J Toxicol Pathol 2015, 28:151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Obert LA, et al. : A review of specific biomarkers of chronic renal injury and their potential application in nonclinical safety assessment studies. Toxicol Pathol 2021, 49:996–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review focused on novel biomarkers being used for the detection of both AKI and CKD. Early biomarkers of AKI included urinary KIM-1, NGAL, cystatin C, and B2M. Biomarkers of CKD included serum cystatin C, SDMA, and measured GFR.
- 40.Inker LA, et al. : GFR estimation using beta-trace protein and beta2-microglobulin in CKD. Am J Kidney Dis 2016, 67:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Argyropoulos CP, et al. : Rediscovering beta-2 microglobulin as a biomarker across the spectrum of kidney diseases. Front Med 2017, 4:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yue L, et al. : Comparison between the beta-2 microglobulin-based equation and the CKD-EPI equation for estimating GFR in CKD patients in China: ES-CKD study. Kidney Dis 2020, 6: 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwab S, et al. : Beta-trace protein as a potential biomarker of residual renal function in patients undergoing peritoneal dialysis. BMC Nephrol 2021, 22:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bacci MR, et al. : The impact of lipocalin-type-prostaglandin-D-synthase as a predictor of kidney disease in patients with type 2 diabetes. Drug Des Devel Ther 2015, 9:3179–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wajda J, et al. : Does beta-trace protein (BTP) outperform cystatin C as a diagnostic marker of acute kidney injury complicating the early phase of acute pancreatitis? J Clin Med 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Khoury JM, et al. : Comparison of symmetric dimethylarginine with creatinine, cystatin C and their eGFR equations as markers of kidney function. Clin Biochem 2016, 49:1140–1143. [DOI] [PubMed] [Google Scholar]
- 47.Hokamp JA, Nabity MB: Renal biomarkers in domestic species. Vet Clin Pathol 2016, 45:28–56. [DOI] [PubMed] [Google Scholar]
- 48.Schwedhelm E, Boger RH: The role of asymmetric and symmetric dimethylarginines in renal disease. Nat Rev Nephrol 2011, 7:275–285. [DOI] [PubMed] [Google Scholar]
- 49.Relford R, Robertson J, Clements C: Symmetric dimethylarginine: improving the diagnosis and staging of chronic kidney disease in small animals. Vet Clin North Am Small Anim Pract 2016, 46:941–960. [DOI] [PubMed] [Google Scholar]
- 50.Michael HT, et al. : A longitudinal study of the persistence of increased creatinine and concordance between kidney biomarkers in cats and dogs. Vet J 2021, 276:105729. [DOI] [PubMed] [Google Scholar]
- 51.Ko HY, et al. : Cystatin C and neutrophil gelatinase-associated lipocalin as early biomarkers for chronic kidney disease in dogs. Top Companion Anim Med 2021, 45:100580. [DOI] [PubMed] [Google Scholar]
- 52.Tojo A, Kinugasa S: Mechanisms of glomerular albumin filtration and tubular reabsorption. Int J Nephrol 2012, 2012: 481520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner MC, et al. : Proximal tubules have the capacity to regulate uptake of albumin. J Am Soc Nephrol 2016, 27: 482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner MC, et al. : Mechanism of increased clearance of glycated albumin by proximal tubule cells. Am J Physiol Ren Physiol 2016, 310:F1089–F1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chronic Kidney Disease Prognosis C, et al. : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010, 375: 2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chagnac A, et al. : Consequences of glomerular hyperfiltration: the role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron 2019, 143:38–42. [DOI] [PubMed] [Google Scholar]
- 57.Melsom T, et al. : Association of increasing GFR with change in albuminuria in the general population. Clin J Am Soc Nephrol 2016, 11:2186–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lang J, et al. : Association of serum albumin levels with kidney function decline and incident chronic kidney disease in elders. Nephrol Dial Transplant 2018, 33:986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cuzzo B, Padala SA, Lappin SL: Physiology, vasopressin. In StatPearls; 2022. Treasure Island (FL). [PubMed] [Google Scholar]
- 60.Sands JM, Blount MA, Klein JD: Regulation of renal urea transport by vasopressin. Trans Am Clin Climatol Assoc 2011, 122:82–92. [PMC free article] [PubMed] [Google Scholar]
- 61.Hosten AO: BUN and creatinine. In Clinical methods: the history, physical, and laboratory examinations. Boston: Butterworths; 1990. [PubMed] [Google Scholar]
- 62.Schwartz GJ, et al. : Improved equations estimating GFR in children with chronic kidney disease using an immunone-phelometric determination of cystatin C. Kidney Int 2012, 82: 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Atkinson MA, et al. : The CKiD study: overview and summary of findings related to kidney disease progression. Pediatr Nephrol 2021, 36:527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brater DC: Measurement of renal function during drug development. Br J Clin Pharmacol 2002, 54:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ciarimboli G, et al. : Proximal tubular secretion of creatinine by organic cation transporter OCT2 in cancer patients. Clin Cancer Res 2012, 18:1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pallister T, et al. : Hippurate as a metabolomic marker of gut microbiome diversity: modulation by diet and relationship to metabolic syndrome. Sci Rep 2017, 7:13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar R, et al. : The renal transport of hippurate and protein-bound solutes. Phys Rep 2020, 8:e14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lees HJ, et al. : Hippurate: the natural history of a mammalian-microbial cometabolite. J Proteome Res 2013, 12:1527–1546. [DOI] [PubMed] [Google Scholar]
- 69.Sirich TL, et al. : Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int 2013, 84: 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y, et al. : Association between kidney clearance of secretory solutes and cardiovascular events: the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis 2021, 78:226–235 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71*.Bhatraju PK, et al. : Assessment of kidney proximal tubular secretion in critical illness. JCI Insight 2021, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study utilized urine-to-plasma ratios of endogenous secretory solutes to determine tubular secretion in both healthy and critically ill individuals. The authors found that composite secretion score was somewhat correlated with SCr and cystatin C and among critically ill patients, a reduction in tubular secretion was associated with adverse outcomes.
- 72.Wang K, et al. : Alterations of proximal tubular secretion in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2020, 15:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y, et al. : Endogenous biomarkers for SLC transporter-mediated drug-drug interaction evaluation. Molecules 2021, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bergagnini-Kolev MC, et al. : Pregnancy increases the renal secretion of N(1)-methylnicotinamide, an endogenous probe for renal cation transporters, in patients prescribed metformin. Drug Metab Dispos 2017, 45:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miyake T, et al. : Identification of appropriate endogenous biomarker for risk assessment of multidrug and toxin extrusion protein-mediated drug-drug interactions in healthy volunteers. Clin Pharmacol Ther 2021, 109:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duan S, et al. : Current challenges and future perspectives of renal tubular dysfunction in diabetic kidney disease. Front Endocrinol 2021, 12:661185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haschke M, et al. : Urinary excretion of carnitine as a marker of proximal tubular damage associated with platin-based anti-neoplastic drugs. Nephrol Dial Transplant 2010, 25:426–433. [DOI] [PubMed] [Google Scholar]
- 78.Andersson L, et al. : Methodological issues on the use of urinary alpha-1-microglobuline in epidemiological studies. Nephrol Dial Transplant 2008, 23:1252–1256. [DOI] [PubMed] [Google Scholar]
- 79.Robles NR, et al. : Alpha-1-microglobulin: prognostic value in chronic kidney disease. Med Clin 2021, 157:368–370. [DOI] [PubMed] [Google Scholar]
- 80.Amatruda JG, et al. : Urine alpha-1-microglobulin levels and acute kidney injury, mortality, and cardiovascular events following cardiac surgery. Am J Nephrol 2021, 52:673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bullen AL, et al. : Biomarkers of kidney tubule health, CKD progression, and acute kidney injury in SPRINT (systolic blood pressure intervention trial) participants. Am J Kidney Dis 2021, 78:361–368 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thi TND, et al. : Evaluation of urinary L-FABP as an early marker for diabetic nephropathy in type 2 diabetic patients. J Med Biochem 2020, 39:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsai IT, et al. : FABP1 and FABP2 as markers of diabetic nephropathy. Int J Med Sci 2020, 17:2338–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kawakami R, et al. : Urinary FABP1 is a biomarker for impaired proximal tubular protein reabsorption and is synergistically enhanced by concurrent liver injury. J Pathol 2021, 255:362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Juanola A, et al. : Urinary L-FABP is a promising prognostic biomarker of ACLF and mortality in patients with decompensated cirrhosis. J Hepatol 2022, 76:107–114. [DOI] [PubMed] [Google Scholar]
- 86.Jung SY, et al. : Phosphate is a potential biomarker of disease severity and predicts adverse outcomes in acute kidney injury patients undergoing continuous renal replacement therapy. PLoS One 2018, 13:e0191290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87*.Shiizaki K, et al. : Calcium phosphate microcrystals in the renal tubular fluid accelerate chronic kidney disease progression. J Clin Invest 2021, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used kidney cells, mice, and CKD patients to explore the negative effects of excess dietary phosphate intake. When phosphate intake was increased, circulating levels of fibroblast growth factor 23 (FGF23) were increased in order to maintain phosphate homeostasis. FGF23 suppressed phosphate reabsorption in renal tubules, raising the tubular phosphate concentration until eventually, calcium phosphate crystals were formed which damaged tubule cells and led to interstitial fibrosis and nephron loss. In humans, progression of CKD was associated with FGF23 serum levels exceeding 53 pg/mL. This study identified calcium phosphate particles in renal tubular fluid as a marker of tubular damage and potential target for treatment.
- 88.Hong YA, et al. : Assessment of tubular reabsorption of phosphate as a surrogate marker for phosphate regulation in chronic kidney disease. Clin Exp Nephrol 2015, 19:208–215. [DOI] [PubMed] [Google Scholar]
- 89.Levey AS, et al. : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006, 145: 247–254. [DOI] [PubMed] [Google Scholar]
- 90.van Acker BA, et al. : Creatinine clearance during cimetidine administration for measurement of glomerular filtration rate. Lancet 1992, 340:1326–1329. [DOI] [PubMed] [Google Scholar]


