Abstract
From the very beginning of their life, human beings are immersed in a social and interactive environment that contributes to shaping their social and cognitive development under typical and at-risk conditions. In order to understand human development in its bidirectional relationship with the social environment, we need to develop a ‘complexity-sensitive’ approach in neuroscience. Recent advances have started to do so with the application of hyperscanning techniques which involve recording adult and child neural activity simultaneously and highlighting the presence of similar patterns of brain activity in the dyad. Numerous studies focused on typically developing children have been published in recent years with the application of this technique to different fields of developmental research. However, hyperscanning techniques could also be extremely beneficial and effective in studying development in atypical and clinical populations. Such application, namely translational hyperscanning, should foster the transition toward a two-brain translational neuroscience. In this paper, we envision how the application of hyperscanning to atypical and clinical child populations can inform family-centered care for children and their parents.
Keywords: children, hyperscanning, neuroscience, parenting, translational science
In 2020, researchers found a set of footprints in the White Sands National Park (New Mexico) which were those of an adult moving along with a child (Bennett et al., 2021). The footprints date back to the Pleistocene, and they represent the most ancient proof of our inner nature of humans as living beings who are inherently socially wired and care for the most fragile ones. In developmental and social neuroscience, the notion of humans as living beings who cannot be separated from their social environment is clearly established. This has been highlighted by the well-known research on newborns’ imitative skills (Simpson et al., 2014) and infants’ sensitivity to transient interactive ruptures and violations of expected contingencies (Provenzi et al., 2016; Perone et al., 2020). While the inherently relational nature of humans is widely recognized, its translation to consistent and effective clinical practices and policies in health-care settings is still partial. In pediatric settings, barriers to proper family-centered care include institutional culture and policies, absence of care coordination, insufficient training and family-related factors (Lotze et al., 2010; Hamilton et al., 2020). A recent survey on the status of family-centered care for preterm newborns in Europe revealed large variations among countries (Aija et al., 2019), suggesting that the road to translating developmental and neuroscientific evidence into consistent clinical programs is still not within walking distance.
In the present paper, we take advantage of newly available research opportunities in developmental and social neurosciences—namely, hyperscanning techniques—to help bridge the gap. Hyperscanning research allows us to assess and quantify the real-time brain-to-brain attunement between two or more interactive partners (Nguyen et al., 2020). With the concept of attunement, we refer here to a general form of dyadic co-variance in the activity rhythms of the brain of the interactive partners. From a developmental viewpoint, the possibility of obtaining direct information about how parents and children co-regulate their brain activity while engaging in reciprocal exchanges is opening new frontiers for scientists (Marriott Haresign et al., 2022; Turk et al., 2022). At the same time, hyperscanning applications to pediatric clinical populations are yet to be explored. Here, we highlight the potential applications of parent–child hyperscanning in preventive, therapeutic and rehabilitative approaches to child health care. We do so starting from a well-established epistemic framework that values the nature of humans as socially wired living beings—i.e. the non-linear dynamic system theory (Camras and Witherington, 2005)—and by integrating principles from translational neuroscience (Roos et al., 2018) and neuro-constructivism (Karmiloff-Smith, 2009). The emergent vision of what we call translational hyperscanning outlines not only a promising novel area of investigation but also a programmatic manifesto that can promote a more effective culture of translational research in pediatric neurosciences.
Wearing complexity-sensitive glasses: translational neuroscience and development
Translational neuroscience includes both basic experimental science and clinical research (Wiggins and Monk, 2013; Roos et al., 2018). The application of translational neuroscience in pediatric settings presents many challenges. First and foremost, the model needs to be tested in rapidly evolving phenotypes shaped by the interaction between genetic predispositions and environmental exposures (Wiggins and Monk, 2013). The non-linear dynamic system theory describes the apparent messiness of developmental processes as a never-stabilized equilibrium between constraints and degrees of freedom (Smith and Thelen, 2003). When exposed to limited environmental perturbations, a living system presents fluctuations of different parameters—from brain activity to behavior regulation and from respiration to alertness—within specific thresholds or constraints. Within these thresholds, the system features specific degrees of freedom that determine all plausible outcomes. Nonetheless, degrees of freedom might expand or reduce to face more intense or repeated environmental perturbations that require a systematic adaptation and a recalibration of constraints and thresholds, a process known as allostatic load (McEwen, 2012; Doan, 2021).
The application of translational neuroscience to human development is further challenged by two other systemic characteristics: multifinality and equifinality. Multifinality means that two individuals who share the same constraints—e.g. biological predispositions to stress reactivity—may exhibit different phenotypes and risk gradients for psychopathology in later childhood or adult life (Del Giudice, 2012; Ellis et al., 2022b). Equifinality suggests that two different living beings may start from very different conditions—in terms of rearing, quality of care, available resources and social context—and still show comparable phenotypes (Cicchetti and Rogosch, 1996). It should be clear that the action theory needed to build effective translational neuroscience models in human development is not immediate, as it needs to incorporate some degrees of such untidy complexity.
Finally, translational neuroscience in pediatric settings needs to recognize the crucial role of experience as the primary driver of central nervous system development (Karmiloff-Smith, 1998). And for humans, experience is primarily social. This is true during the first months of life when innate intersubjectivity makes infants sensitive and ready to interact face-to-face (Trevarthen and Aitken, 2001). This is also evident from studies describing how children co-create meanings about the physical and social environment during interactions with their caregivers (Yoshida and Smith, 2008). In typical and atypical conditions, the possibility to experience contingencies within the interaction with the caregiver—while exploring social or physical environments—steers infants’ learning and contributes to reliable self and other mental representations (Beeghly and Tronick, 2011; Montirosso and McGlone, 2020). As such, it is not surprising that even small deviations, mismatches, asynchronies or missed contingencies in the two-person environment of a child can accumulate alongside the developmental experience contributing to exerting large and cascade effects on the later phenotype (Karmiloff-Smith, 1998).
In sum, understanding development—and developmental psychopathology—requires opting for a multifaceted perspective on humans that recognizes and embeds complexity into the action models that will inform translational neuroscience approaches. Such perspective should not neglect the importance of assessing the equilibrium through space and time between constraints and degrees of freedom (allostatic load), the non-linear pathways of change that originates from similar and diverse starting points (equifinality and multifinality) and the key role of two-person experiences for children self-development (interpersonal contingency).
Humans: two-brained living beings
As we conceptualize humans as living beings who are inherently socially wired—we may say ‘born to be wired’—we need to develop a concept of translational neuroscience that is not focused on the individual’s central nervous system; rather, that incorporates and takes advantage from a dual unit of analysis (Hoehl and Markova, 2018). Innovative studies suggest that, from very early in life, we can coordinate with others during interactions and calibrate the timing and intensity of our behavioral outputs accordingly (Dumas et al., 2014). Starting from fetal life, the movements of twin fetuses are not random or accidental. Since the 14th week of gestation, each twin fetus executes movements specifically aimed at their co-twin and themself (Castiello et al., 2010). After birth, newborns actively contribute to regulating the breast temperature during kangaroo care and skin-to-skin contact, suggesting that biological predispositions to thermal synchrony exist since the early hours of life and are key to mother–infant adaptation (Ludington-Hoe et al., 2005). Focusing on the emerging field of social touch, Montirosso and McGlone (2020) have recently further suggested that the dyad should be the minimum unit of analysis for developmental and social neuroscience. They propose that the child’s body image is co-constructed within the moment-by-moment interaction with the caregiver and shaped by interpersonal tactile stimulations primarily vehiculated by c-tactile fibers and elaborated in the anterior insula (Olausson et al., 2010). In line with Dumas (2011), here we propose to consider humans as inherently bi-cerebral living beings.
For translational neuroscience to be effectively applied in both typical and atypical conditions, its principles should be made explicit to guide methodological choices and research questions. First, the early social experience with the caregiver is the privileged locus of infants’ learning and meaning-making (Beeghly and Tronick, 2011; Provenzi et al., 2020), emotion regulation development (Norona and Baker, 2014), social (Sharp and Fonagy, 2008; Tamis-LeMonda et al., 2014) and cognitive skills acquisition (Yoshida and Smith, 2008; Lee et al., 2017). Second, theoretical models of human development and brain functioning should be framed by a transactional viewpoint characterized by non-linear causality and bidirectional influences between the human system and the environment (Wiggins and Monk, 2013). Third, interactions should be the primary targets of early preventive, therapeutic and rehabilitative interventions, as protective and adaptive changes promoted in the immediate surrounding environment are warranted to initiate cascade effects for all the engaged individuals. Applying a two-brain perspective in developmental science could allow us to embed into action-theory models elements of the physical and social environment experienced by infants in their everyday life. Consistently, the translational hyperscanning field will inform interventions that shape the optimal growth and well-being of both infants and their caregivers.
Hyperscanning as a privileged observation point
Hyperscanning is a synchronous recording of multiple brain activities. It can be performed with different techniques such as functional magnetic resonance imaging (fMRI) (Montague, 2002; Misaki et al., 2021), functional near-infrared spectroscopy (fNIRS) (Miller et al., 2019), electroencephalography (EEG) (Toppi et al., 2016) and magnetoencephalography (Hirata et al., 2014; Czeszumski et al., 2020). Such simultaneous recordings allow us to obtain neurophysiological measures of human dyadic coordination, namely inter-brain synchrony (IBS) (Czeszumski et al., 2020). The focus can be directed on processes underlying many fundamental social phenomena, such as collaborative behaviors in lovers (Pan et al., 2017), interpersonal learning (Pan et al., 2020), cooperation and competition (Balconi and Fronda, 2020), joint attention (Lachat et al., 2012), communication (Kawasaki et al., 2013) and behavioral co-regulation (Dumas et al., 2014). Another advantage of hyperscanning techniques is that we might explore IBS in a wide variety of settings, from laboratory tasks to ecological conditions (Hoehl and Markova, 2018; Czeszumski et al., 2020). For example, laboratory settings often conducted hyperscanning research to observe the inter-brain underpinnings of behavioral coordination during experimentally designed collaborative tasks (e.g. two individuals were asked to press a key simultaneously or to imitate the partner’s hand movements) (Hu et al., 2017). Examples of more ecological applications are the study of brain-to-brain attunement in musicians playing together (Acquadro et al., 2016) or in pilots and co-pilots during the takeoff, flight and landing of an airplane (Astolfi et al., 2011).
Synchrony is a key dimension of the early parent–child relationship as it is involved in a wide set of interactive formats that characterize the precocious exchange between caregivers and children, from imitation to face-to-face encounters and from joint attention to daily routines such as feeding and bathing (Striano et al., 2003; Feldman, 2012; Provenzi et al., 2018a). By applying hyperscanning approaches to the study of early parent–child interaction, we may be able to understand and describe the neural underpinnings that allow caregivers and children to achieve and regulate dyadic states of interactive synchrony. There are two ways to use inter-brain EEG effects as neuromarkers (Koike et al., 2015): first, as indicators of social interactions’ quality in daily life through coherence or synchronization (e.g. a measure of successful learning processes in educational environments; Nguyen et al., 2020); second, they may signal bidirectional influences between two brains, which could be a better marker of interaction quality when there is an asymmetry in the interaction (e.g. verbal communication between individuals on different teams in a competitive relationship; Astolfi et al., 2011). Among the different techniques that can be used for hyperscanning studies, both EEG and fNIRS are suitable for pediatric populations and have the particular advantage of allowing ecological investigations. Such techniques are not invasive and allow freedom of movement, opening the possibility of studying, for instance, mother–infant interactions in an ecological approach from the first year of life (Nguyen et al., 2020).
In sum, hyperscanning is a privileged standpoint to observe humans as two-brained living beings. To date, many studies have been conducted with this methodology. Here, we will lay out some relevant findings from a subset of these studies that give some notion of how parent–child interactions can be a fundamental experience in shaping neurodevelopment (Norton et al., 2022). This happens from the most basic face-to-face interactions to language development and more complex social tasks, such as joint actions (Hasson et al., 2012). For instance, dual-EEG recordings of 8-month-old infants and an adult show changes in neural network connectivity depending on the adults’ changes in gaze orienting: both the adults and the infants had a greater influence on the interactive partner’s neural activity during direct rather than indirect gaze (Leong et al., 2017). Moreover, it has been shown that the real-time temporal alignment of brains during social interactions is facilitated by ostensive social signals (Wu et al., 2014). Another study showed that parent–infant IBS is modulated by dyadic social interactions’ emotional quality and tone (Santamaria et al., 2020). Mothers were asked here to show positive and negative emotions toward objects while interacting with their 10-month-old infants. The stronger integration of the dyads’ neural processes was observed during maternal demonstrations of positive than negative emotions, whereas infants had a stronger influence on the inter-brain connectivity during negative emotional states. Again, other dual-EEG recordings performed from 12-month-old infants and their primary caregivers measured the neural correlates of attention shared during social interactions, i.e. while playing with an object together (joint play) or alone (solo play). When infants were engaged in solo play, fluctuations in neural activation—specifically in the theta power—predicted visual attention in both infants and adults (Wass et al., 2018a). Interestingly, during interactive social play, this predictive relationship within individuals was reduced, but caregivers’ brain activation was more attuned to infants’ brain activation, successfully tracking their attention. The study of IBS can be informative for older children as well. For instance, a recent study involving dyads of 24- to 42-month-old toddlers and their mothers examined the neurobiological correlates of dialogic reading (DR) and mobile phone–interrupted DR. Mother–child neural synchrony decreased in the DR-interrupted condition compared to the uninterrupted DR condition (Zivan et al., 2022). Evidence such as this shows how environmental factors can interfere with IBS in caregiver–child dyads in several ways during developmental phases in which language, cognitive and socio-emotional domains are undergoing fundamental changes.
It is important to note that various aspects are still debated. For example, for the most part, linear associations between IBS and cognitive or behavioral measures have been tested. Yet, it is also possible that more complex and non-linear associations are present; thus, a model based on the assumption that ‘more synchrony is better’ could be merely simplistic (Mitsven et al., 2022). In real life, most social interactions are asymmetric and imply different roles and turns in taking action. Even when hyperscanning paradigms consider these behavioral mismatches, it is not clear if coherence analysis can capture this aspect (Hamilton, 2021). Furthermore, we mentioned here only examples of interactive settings between two partners. Crucially, triadic (e.g. with both parents) and group contexts (e.g. with peers) are interesting observational settings, too. Examples of hyperscanning with triads or groups of adults are present in the literature (for a review, see Nam et al., 2020), while fewer studies have done the same with pediatric populations. While focusing on the dyad limits the complexity of human social interactions, it provides a first observational tool with the advantage of starting to build knowledge on the parent–infant brain during interactions, which are uniquely informative on development (Norton et al., 2022). Moreover, hyperscanning alone cannot address a fundamental issue, namely whether the observed synchronizations in neural activity are something that individuals achieve to better function in their environment or whether they are a mere consequence of sharing the same sensory input or motor activity. The only way to disentangle this matter would be to exogenously manipulate IBS through multi-brain stimulation and assess its causal effects on social interactions (Novembre and Iannetti, 2021). Despite these limitations, the potential of this developing field of investigation remains vast. Having real-time neurophysiological and behavioral indexes of adult–child social interactions has been suggested to be a promising framework for developing new social brain health tools (Leong, 2022). This is what we call translational hyperscanning.
Envisioning translational hyperscanning
Using hyperscanning techniques in clinical settings may improve our ability to construct development models consistent with the principles of the dynamic systems framework and capable of embedding complexity levels that maximize their exploitation in ecological environments and clinical settings. The accumulating knowledge arising from parent–child hyperscanning research sets the stage to integrate findings into a theory of action that might inform smarter care strategies in pediatric health-care settings in multiple ways, for instance, obtaining a deeper knowledge of inter-brain dynamics as they unfold in daily life in at-risk conditions, acquiring potentially new indexes of diagnostic or prognostic paths in atypical development, understanding how environmental manipulation might affect and improve brain-to-brain coupling and providing tailored neurofeedback for developmental care and cognitive training. Such a translational approach to hyperscanning can be successfully applied to a variety of developmental risk conditions and pediatric health-care settings. Here, we highlight a few exemplificative areas of relevant preventive, therapeutic and rehabilitative applications for translational hyperscanning (Figure 1). Importantly, not all three applications will be possible in every scenario, and these exemplifications are not meant to be exhaustive.
Fig. 1.
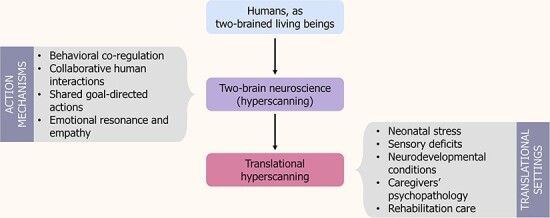
Summary of the proposed model. Starting from the accumulating knowledge arising from IBS parent–child hyperscanning research, we envision implications of an innovative field of translational neuroscience—namely, translational hyperscanning—as applied to different at-risk and clinical conditions of child development and care contexts.
Developmental psychopathology
The application of hyperscanning techniques to study inter-brain co-regulation in children and parents with psychopathology may reveal primary dyadic pathways that are involved in setting a higher risk for behavioral and affective disturbances later in life. Depressed mothers may show altered brain responses to their own infants’ signals (e.g. cry acoustics) (Laurent and Ablow, 2012), and children of depressed mothers might present structural brain alterations (e.g. cortical thickness in frontal and temporal regions; Lebel et al., 2016). Additionally, differences have been shown in functional brain activity. For example, during the second year of life, infants of depressed mothers showed reduced left frontal brain EEG activity, which was significantly associated with measures of maternal affectionate engagement. In contrast, increased generalized frontal EEG activity was linked with the reports of more negative temperament (Dawson et al., 1999). Interestingly, Foland-Ross et al. (2016) showed that depressed mothers and their children might exhibit concordant alterations in regional gray matter thickness. By investigating the two-brain regulatory correlates of parental and child psychopathology, we can integrate our knowledge with innovative and valid evidence to inform preventive interventions focused on the parent–child relationship. From this point of view, it may be proposed that a pragmatic application of translational hyperscanning may be the development of double-brain neurophysiological feedback interventions paired with well-known video-feedback approaches to support the quality of early parent–child relationships (Fukkink, 2008). In future applications, it is possible to speculate that by receiving immediate graphical feedback on their brain-to-brain coupling while interacting with their children, parents may be supported in providing contingent responses to their children’s needs and empowered in their caregiving role.
Sensory impairment
Translational hyperscanning can benefit families of infants with sensory impairment, such as low-vision or blind infants. For these infants and their parents, much of what we know about the role of face-to-face contingency in early interactions is only partially applicable. A recent review of the literature highlighted that reading their infants’ signals can be particularly challenging for these parents and that the rhythm of dyadic coordination can be largely disrupted or at least altered in these dyads (Grumi et al., 2021). By applying a hyperscanning approach to the study of the interaction between parents and their low-vision or blind infants, we may collect relevant information that may be beneficial for researchers and clinicians. Consider, for instance, the case of a caregiver–infant dyad where the infant has a severe visual impairment. Interactive dynamics may not be functional, as the lack of shared visual attention can be reflected in the parents’ strategies for interpreting and providing contingent responses to children’s communication signals (Tadic̈ et al., 2013; Nagayoshi et al., 2017). By applying hyperscanning in these dyadic populations, we might detect how sensory processes other than sight are implied in building synchrony and co-regulation patterns. We could then develop potential interventions built on these same processes and action mechanisms, providing families of children with low vision with evidence-based and targeted family-centered support. For instance, parents can be assisted in discovering and investing in different strategies (e.g. auditory and/or tactile channels) to stimulate, catch and sustain the social attention of their visually impaired infant (Adamson et al., 2019). This knowledge can be further applied and explored in dyads of sighted infants, enriching our comprehension of the sensory processes involved in the early establishment of parent–child attunement and co-regulation.
Preterm birth
Another context of a beneficial translational hyperscanning application is preterm birth and the neonatal intensive care unit (NICU) environment. During the NICU stay, infants born before term are exposed to life-saving yet stress-inducing procedures that feature intense sensory stimulations (lights and sounds), painful skin-breaking maneuvers and partial access to parent regulatory contact and emotional bonding (Axelin and Salanterä, 2008; Flacking et al., 2012; Aita et al., 2013). These precocious adverse exposures may contribute to the long-lasting developmental trajectories of preterm infants, even for those who do not present severe neonatal comorbidities (Montirosso and Provenzi, 2015). These include adrenocortical stress dysregulation (Grunau, 2013), increased risk for behavioral problems (Vinall and Grunau, 2014), altered brain structure and neurophysiological activity (Brummelte et al., 2015; Ranger et al., 2015) and epigenetic alterations of stress-related genes (Provenzi et al., 2018b). NICU stress may also impact parental psychological well-being (Caporali et al., 2020), increasing the risk of depression and anxiety (Treyvaud et al., 2019), and later parenting stress and bonding challenges after discharge (Ionio et al., 2016; Mäkelä et al., 2018). At the same time, it has been shown that by investing in early developmental care interventions that maximize physical and emotional closeness, the NICU staff may achieve outcomes of neuroprotection, promoting better outcomes for both preterm infants and their parents (Feeley et al., 2016; Browne, 2021; He et al., 2021). Hyperscanning approaches to the study of the interaction between parents and their preterm infants may provide markers of risk related to precocious stress and separation exposures, highlighting how early adversity in the caregiving environment may leave dual-brain neurophysiological footprints. Moreover, these same markers can be used to guide the development of interaction-focused early interventions that stem from investing in parental engagement and aim to promote better behavioral, neurological and cognitive outcomes for infants while also protecting parental psychological well-being.
Cerebral palsy
Families of infants with cerebral palsy can also benefit from translational hyperscanning. Cerebral palsy results from prenatal, perinatal or postnatal brain damage, and it entails chronic motor and postural difficulties (Rosenbaum et al., 2007). In this scenario, neuroscience has already provided clinicians with instruments to improve the developmental outcomes of children with cerebral palsy. Numerous studies have shown how the motor and premotor cortexes involved in producing movement are the same recruited during the observation of finalized gestures and movements, and based on this knowledge, innovative treatments—e.g. the well-known action observation therapy (AOT)—have been developed (Kirkpatrick et al., 2016; Buccino et al., 2018). Several randomized clinical trials have been launched in recent years, and they witnessed the efficacy of AOT in treating children with hemiplegic (e.g. upper limb) (Sgandurra et al., 2011; Buccino et al., 2012) and unilateral cerebral palsy (Simon-Martinez et al., 2020). AOT appears to be a promising rehabilitation tool to favor motor recovery in this clinical population by affecting cortical plasticity in driving the reorganization of sensorimotor areas (Quadrelli et al., 2019). Translational hyperscanning can further improve this field by collecting data on dyadic interactions during this type of intervention. While existing studies have so far focused on actions targeting objects, interactive exchanges are particularly salient during development. Expanding this field of investigation and moving from one-person sensorimotor networks to two-person inter-brain networks can lead to the discovery of new strategies for enhancing cortical reorganization.
Neurodevelopmental disorders
A two-brained perspective would also be beneficial when considering the study and treatment of children with neurodevelopmental disorders (NDDs). Most of what we currently know on neural mechanisms underlying NDDs is based on studies that apply highly controlled experimental paradigms observing neural processes through a single brain perspective (Licari et al., 2020). As previously highlighted, child neural development and learning happen during social interactions; however, currently, no studies are available focusing on adult–child neural dynamics in these clinical populations. This is unfortunate as, in the typically developing population, studies showed that measures of neural synchrony during interactions could be seen as an index of optimal attention (Santamaria et al., 2020) and have been linked to more effective learning (; Piazza et al., 2021). An approach that integrates social interactions in the study of neural development would be extremely relevant in clinical populations not only where social functioning deficits characterize the clinical phenotype (e.g. autism spectrum disorder) but also where interactive abilities are considered as a strength. As previously highlighted, development is a dynamic and probabilistic process, and thus, classically neuropsychological approaches identifying ‘impaired’ and ‘spared’ functions are oversimplistic (Karmiloff-Smith, 1998). First, we should consider that, for the most part, interventions and treatments developed for children with social interaction difficulties are based on interactions between therapist and child or parent and child (with the guidance of a therapist). In this context, a two-brained perspective could highlight specific behaviors in the parent and/or therapist that can lead to more effective learning for the child, potentially obtaining more effective and individualized treatments. Second, even in NDD populations with secondary consequences for the quality of social interactions (e.g. attention deficit and hyperactivity disorders), the assessment of interpersonal brain synchronization dynamics could be a relevant tool to highlight which intervention strategies (often based not only on adult–child but also on child–child interactions) better support adaptive outcomes. As previously highlighted, social interactions can have a strong impact on both adult and child’s attentional dynamics from an early age (Wass et al., 2018b). Thus, collecting information on the associations among interaction strategies, adult–child brain synchronization and attention dynamics during real-life interactions, in both child-to-adult and adult-to-child directions, could be beneficial to shaping and sustaining child attention and learning. To understand child neural and cognitive development and hope to positively impact them, we should strive more and more to study neural development in the same context where it normally happens.
Conclusions
With the rapid emergence of the hyperscanning research field, we are facing an ongoing neuroscientific revolution that facilitates the integration between the epistemic complexity principles of the dynamic system theory and innovative approaches to the study of humans as two-brained living beings (Marriott Haresign et al., 2022). Such a revolution is still beginning, and many limitations must be carefully addressed. In the introduction paragraph, for instance, we have mentioned some of these challenges: the non-linear associations between neural activities, the asymmetric nature of most social interactions and the scarce investigation of triadic patterns. Yet, it holds the promise to change the way we think about typical and atypical child development. For example, it is possible that recalibrations of IBS may be not only a negative outcome but rather an adaptive response of the dyad to specific developmental challenges and risk factors (Young et al., 2022; Ellis et al., 2022a). Similarly, while building on our understanding of the importance of parent–infant two-brain co-regulation, the future findings might also suggest the benefits of moving beyond a simple model that more synchrony is better (Mitsven et al., 2022). At the same time, this is a potential revolution that needs to be steered together by researchers and clinicians to maximize the application potential and bridge the gap between neuroscientific research and health care in pediatric settings.
The translational hyperscanning arena we envision in this paper aims to provide a common viewpoint for researchers and clinicians to partner in designing, conducting, disseminating and exploiting the intriguing data obtained from hyperscanning research and translate them into smarter and effective care strategies for at-risk children and their parents. Of course, many procedural and methodological challenges come along with the seductive allure of hyperscanning research. Nonetheless, as we become more and more capable of nurturing the field of hyperscanning research according to a complex and transactional view of human development (Karmiloff-Smith, 2009; Wiggins and Monk, 2013), the translational hyperscanning field will provide us with greater and smarter opportunities to improve the quality of family-centered care in pediatric settings.
Contributor Information
Livio Provenzi, Department of Brain and Behavioral Sciences, University of Pavia, Pavia 27100, Italy; Developmental Psychobiology Lab, IRCCS Mondino Foundation, Pavia 27100, Italy.
Elisa Roberti, Developmental Psychobiology Lab, IRCCS Mondino Foundation, Pavia 27100, Italy.
Elena Capelli, Developmental Psychobiology Lab, IRCCS Mondino Foundation, Pavia 27100, Italy.
Funding
This study is partially funded by the Italian Ministry of Health under the grant Ricerca Finalizzata Giovani Ricercatori 2021 to author LP (grant number GR-2021-12375213) and under Ricerca Corrente 2022.
Conflict of interest
The authors declared that they had no conflict of interest with respect to their authorship or the publication of this article.
References
- Acquadro M.A.S., Congedo M., de Riddeer D. (2016). Music performance as an experimental approach to hyperscanning studies. Frontiers in Human Neuroscience, 10, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson L.B., Bakeman R., Suma K., Robins D.L. (2019). Sharing sounds: the development of auditory joint engagement during early parent–child interaction. Developmental Psychology, 55(12), 2491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aija A., Toome L., Axelin A., Raiskila S., Lehtonen L. (2019). Parents’ presence and participation in medical rounds in 11 European neonatal units. Early Human Development, 130, 10–6. [DOI] [PubMed] [Google Scholar]
- Aita M., Johnston C., Goulet C., Oberlander T.F., Snider L. (2013). Intervention minimizing preterm infants’ exposure to NICU light and noise. Clinical Nursing Research, 22(3), 337–58. [DOI] [PubMed] [Google Scholar]
- Astolfi L., Toppi J., Borghini G., et al. (2011). Study of the functional hyperconnectivity between couples of pilots during flight simulation: an EEG hyperscanning study. In: Annual International Conference of the IEEE Engineering in Medicine and Biology SocietyBoston, MA, 2338–41. [DOI] [PubMed] [Google Scholar]
- Axelin A., Salanterä S. (2008). Ethics in neonatal pain research. Nursing Ethics, 15(4), 492–9. [DOI] [PubMed] [Google Scholar]
- Balconi M., Fronda G. (2020). The dialogue between two or more brains: the “hyperscanning” for organization. Frontiers in Psychology, 11, 598332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeghly M., Tronick E. (2011). Early resilience in the context of parent infant relationships: a social developmental perspective. Current Problems in Pediatric and Adolescent Health Care, 41(7), 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M.R., Bustos D., Pigati J.S., et al. (2021). Evidence of humans in North America during the Last Glacial Maximum. Science (New York, N.Y.), 373(6562), 1528–31. [DOI] [PubMed] [Google Scholar]
- Browne J.V. (2021). Infant mental health in intensive care: laying a foundation for social, emotional and mental health outcomes through regulation, relationships and reflection. Journal of Neonatal Nursing, 27(1), 33–9. [Google Scholar]
- Brummelte S., Chau C.M.Y., Cepeda I.L., et al. (2015). Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology, 51, 151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G., Arisi D., Gough P., et al. (2012). Improving upper limb motor functions through action observation treatment: a pilot study in children with cerebral palsy. Developmental Medicine and Child Neurology, 54(9), 822–8. [DOI] [PubMed] [Google Scholar]
- Buccino G., Molinaro A., Ambrosi C., et al. (2018). Action observation treatment improves upper limb motor functions in children with cerebral palsy: a combined clinical and brain imaging study. Neural Plasticity, 2018, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camras L.A., Witherington D.C. (2005). Dynamical systems approaches to emotional development. Developmental Review, 25(3–4), 328–50. [Google Scholar]
- Caporali C., Pisoni C., Gasparini L., et al. (2020). A global perspective on parental stress in the neonatal intensive care unit: a meta-analytic study. Journal of Perinatology, 40(12), 1739–52. [DOI] [PubMed] [Google Scholar]
- Castiello U., Becchio C., Zoia S., et al. (2010). Wired to be social: the ontogeny of human interaction. PLoS One, 5(10), e13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D., Rogosch F.A. (1996). Equifinality and multifinality in developmental psychopathology. Development and Psychopathology, 8(4), 597–600. [Google Scholar]
- Czeszumski A., Eustergerling S., Lang A., et al. (2020). Hyperscanning: a valid method to study neural inter-brain underpinnings of social interaction. Frontiers in Human Neuroscience, 14, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G., Frey K., Panagiotides H., Yamada E., Hessl D., Osterling J. (1999). Infants of depressed mothers exhibit atypical frontal electrical brain activity during interactions with mother and with a familiar, nondepressed adult. Child Development, 70(5), 1058–66. [DOI] [PubMed] [Google Scholar]
- Del Giudice M. (2012). Fetal programming by maternal stress: insights from a conflict perspective. Psychoneuroendocrinology, 37(10), 1614–29. [DOI] [PubMed] [Google Scholar]
- Doan S.N. (2021). Allostatic load: developmental and conceptual considerations in a multi-system physiological indicator of chronic stress exposure. Developmental Psychobiology, 63(5), 825–36. [DOI] [PubMed] [Google Scholar]
- Dumas G. (2011). Towards a two-body neuroscience. Communicative & Integrative Biology, 4(3), 349–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas G., Laroche J., Lehmann A. (2014). Your body, my body, our coupling moves our bodies. Frontiers in Human Neuroscience, 8, 1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis B.J., Abrams L.S., Masten A.S., Sternberg R.J., Tottenham N., Frankenhuis W.E. (2022a). Hidden talents in harsh environments. Development and Psychopathology, 34(1), 95–113. [DOI] [PubMed] [Google Scholar]
- Ellis B.J., Sheridan M.A., Belsky J., McLaughlin K.A. (2022b). Why and how does early adversity influence development? Toward an integrated model of dimensions of environmental experience. Development and Psychopathology, 34(2), 447–71. [DOI] [PubMed] [Google Scholar]
- Feeley N., Genest C., Niela-Vilén H., Charbonneau L., Axelin A. (2016). Parents and nurses balancing parent-infant closeness and separation: a qualitative study of NICU nurses’ perceptions. BMC Pediatrics, 16(1), 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. (2012). Bio-behavioral synchrony: a model for integrating biological and microsocial behavioral processes in the study of parenting. Parenting, 12(2–3), 154–64. [Google Scholar]
- Flacking R., Lehtonen L., Thomson G., et al. (2012). Closeness and separation in neonatal intensive care. Acta Paediatrica, International Journal of Paediatrics, 101(10), 1032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross L.C., Behzadian N., LeMoult J., Gotlib I.H. (2016). Concordant patterns of brain structure in mothers with recurrent depression and their never-depressed daughters. Developmental Neuroscience, 38(2), 115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukkink R.G. (2008). Video feedback in widescreen: a meta-analysis of family programs. Clinical Psychology Review, 28(6), 904–16. [DOI] [PubMed] [Google Scholar]
- Grumi S., Cappagli G., Aprile G., et al. (2021). Togetherness, beyond the eyes: a systematic review on the interaction between visually impaired children and their parents. Infant Behavior & Development, 64, 101590. [DOI] [PubMed] [Google Scholar]
- Grunau R.E. (2013). Neonatal pain in very preterm infants: long-term effects on brain, neurodevelopment and pain reactivity. Rambam Maimonides Medical Journal, 4(4), e0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A. (2021). Hyperscanning: beyond the hype. Neuron, 109(3), 404–7. [DOI] [PubMed] [Google Scholar]
- Hamilton R., Kleinpell R., Lipman J., Davidson J.E. (2020). International facilitators and barriers to family engagement in the ICU: results of a qualitative analysis. Journal of Critical Care, 58, 72–7. [DOI] [PubMed] [Google Scholar]
- Hasson U., Ghazanfar A.A., Galantucci B., Garrod S., Keysers C. (2012). Brain-to-brain coupling: a mechanism for creating and sharing a social world. Trends in Cognitive Sciences, 16(2), 114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F.B., Axelin A., Ahlqvist-Björkroth S., Raiskila S., Löyttyniemi E., Lehtonen L. (2021). Effectiveness of the Close Collaboration with Parents intervention on parent-infant closeness in NICU. BMC Pediatrics, 21(1), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Ikeda T., Kikuchi M., et al. (2014). Hyperscanning MEG for understanding mother–child cerebral interactions. Frontiers in Human Neuroscience, 8, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehl S., Markova G. (2018). Moving developmental social neuroscience toward a second-person approach. PLOS Biology, 16(12), e3000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Hu Y., Li X., Pan Y., Cheng X. (2017). Brain-to-brain synchronization across two persons predicts mutual prosociality. Social Cognitive and Affective Neuroscience, 12(12), 1835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionio C., Colombo C., Brazzoduro V., et al. (2016). Mothers and fathers in NICU: the impact of preterm birth on parental distress. Europe’s Journal of Psychology, 12(4), 604–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A. (1998). Development itself is the key to understanding developmental disorders. Trends in Cognitive Sciences, 2(10), 389–98. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. (2009). Nativism versus neuroconstructivism: rethinking the study of developmental disorders. Developmental Psychology, 45(1), 56–63. [DOI] [PubMed] [Google Scholar]
- Kawasaki M., Yamada Y., Ushiku Y., Miyauchi E., Yamaguchi Y. (2013). Inter-brain synchronization during coordination of speech rhythm in human-to-human social interaction. Scientific Reports, 3(1), 1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick E., Pearse J., James P., Basu A. (2016). Effect of parent-delivered action observation therapy on upper limb function in unilateral cerebral palsy: a randomized controlled trial. Developmental Medicine and Child Neurology, 58(10), 1049–56. [DOI] [PubMed] [Google Scholar]
- Koike T., Tanabe H.C., Sadato N. (2015). Hyperscanning neuroimaging technique to reveal the “two-in-one” system in social interactions. Neuroscience Research, 90, 25–32. [DOI] [PubMed] [Google Scholar]
- Lachat F., Hugueville L., Lemaréchal J.-D., Conty L., George N. (2012). Oscillatory brain correlates of live joint attention: a dual-EEG study. Frontiers in Human Neuroscience, 6, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent H.K., Ablow J.C. (2012). A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Social Cognitive and Affective Neuroscience, 7(2), 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walton M., Letourneau N., Giesbrecht G.F., Kaplan B.J., Dewey D. (2016). Prepartum and postpartum maternal depressive symptoms are related to children’s brain structure in preschool. Biological Psychiatry, 80(11), 859–68. [DOI] [PubMed] [Google Scholar]
- Lee R., Skinner A., Bornstein M.H., et al. (2017). Through babies’ eyes: practical and theoretical considerations of using wearable technology to measure parent–infant behaviour from the mothers’ and infants’ view points. Infant Behavior & Development, 47, 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong V., Byrne E., Clackson K., Georgieva S., Lam S., Wass S. (2017). Speaker gaze increases information coupling between infant and adult brains. Proceedings of the National Academy of Sciences of the United States of America, 114(50), 13290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong V. (2022). Neural sociometrics: toward early screening of infant psychosocial and brain health to improve lifelong mental well-being. Policy Insights from the Behavioral and Brain Sciences, 9(1), 111–9. [Google Scholar]
- Licari M.K., Alvares G.A., Varcin K., et al. (2020). Prevalence of motor difficulties in autism spectrum disorder: analysis of a population-based cohort. Autism Research, 13(2), 298–306. [DOI] [PubMed] [Google Scholar]
- Lotze G.M., Bellin M.H., Oswald D.P. (2010). Family-centered care for children with special health care needs: are we moving forward? Journal of Family Social Work, 13(2), 100–13. [Google Scholar]
- Ludington-Hoe S.M., Hosseini R., Torowicz D.L. (2005). Skin-to-skin contact (Kangaroo Care) analgesia for preterm infant heel stick. AACN Clinical Issues: Advanced Practice in Acute and Critical Care, 16(3), 373–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä H., Axelin A., Feeley N., Niela-Vilén H. (2018). Clinging to closeness: the parental view on developing a close bond with their infants in a NICU. Midwifery, 62, 183–8. [DOI] [PubMed] [Google Scholar]
- Marriott Haresign I., Phillips E.A.M., Whitehorn M., et al. (2022). Measuring the temporal dynamics of inter-personal neural entrainment in continuous child-adult EEG hyperscanning data. Developmental Cognitive Neuroscience, 54, 101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. (2012). Brain on stress: how the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America, 109(Suppl 2), 17180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.G., Vrtička P., Cui X., et al. (2019). Inter-brain synchrony in mother-child dyads during cooperation: an fNIRS hyperscanning study. Neuropsychologia, 124, 117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M., Kerr K.L., Ratliff E.L., et al. (2021). Beyond synchrony: the capacity of fMRI hyperscanning for the study of human social interaction. Social Cognitive and Affective Neuroscience, 16(1–2), 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsven S.G., Prince E.B., Messinger D.S., et al. (2022). Testing the mid-range model: attachment in a high risk sample. Developmental Science, 25(3), e13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague P. (2002). Hyperscanning: simultaneous fMRI during linked social interactions. NeuroImage, 16(4), 1159–64. [DOI] [PubMed] [Google Scholar]
- Montirosso R., McGlone F. (2020). The body comes first. Embodied reparation and the co-creation of infant bodily-self. Neuroscience and Biobehavioral Reviews, 113, 77–87. [DOI] [PubMed] [Google Scholar]
- Montirosso R., Provenzi L. (2015). Implications of epigenetics and stress regulation on research and developmental care of preterm infants. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 44(2), 174–82. [DOI] [PubMed] [Google Scholar]
- Nagayoshi M., Hirose T., Toju K., et al. (2017). Related visual impairment to mother-infant interaction and development in infants with bilateral retinoblastoma. European Journal of Oncology Nursing, 28, 28–34. [DOI] [PubMed] [Google Scholar]
- Nam C.S., Choo S., Huang J., Park J. (2020). Brain-to-brain neural synchrony during social interactions: a systematic review on hyperscanning studies. Applied Sciences, 10(19), 6669. [Google Scholar]
- Nguyen T., Bánki A., Markova G., Hoehl S. (2020). Studying parent-child interaction with hyperscanning. Progress in Brain Research, 254, 1–24. [DOI] [PubMed] [Google Scholar]
- Norona A.N., Baker B.L. (2014). The transactional relationship between parenting and emotion regulation in children with or without developmental delays. Research in Developmental Disabilities, 35(12), 3209–16. [DOI] [PubMed] [Google Scholar]
- Norton E.S., Manning B.L., Harriott E.M., et al. (2022). Social EEG: a novel neurodevelopmental approach to studying brain-behavior links and brain-to-brain synchrony during naturalistic toddler–parent interactions. Developmental Psychobiology, 64(3), e22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novembre G., Iannetti G.D. (2021). Hyperscanning alone cannot prove causality. Multibrain stimulation can. Trends in Cognitive Sciences, 25(2), 96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H., Wessberg J., Morrison I., McGlone F., Vallbo A. (2010). The neurophysiology of unmyelinated tactile afferents. Neuroscience and Biobehavioral Reviews, 34(2), 185–91. [DOI] [PubMed] [Google Scholar]
- Pan Y., Cheng X., Zhang Z., Li X., Hu Y. (2017). Cooperation in lovers: an fNIRS-based hyperscanning study. Human Brain Mapping, 38(2), 831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Dikker S., Goldstein P., Zhu Y., Yang C., Hu Y. (2020). Instructor-learner brain coupling discriminates between instructional approaches and predicts learning. NeuroImage, 211, 116657. [DOI] [PubMed] [Google Scholar]
- Perone S., Gartstein M.A., Anderson A.J. (2020). Dynamics of frontal alpha asymmetry in mother-infant dyads: insights from the Still Face Paradigm. Infant Behavior & Development, 61, 101500. [DOI] [PubMed] [Google Scholar]
- Piazza E.A., Cohen A., Trach J., Lew-Williams C. (2021). Neural synchrony predicts children’s learning of novel words. Cognition, 214, 104752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzi L., Giusti L., Montirosso R. (2016). Do infants exhibit significant cortisol reactivity to the face-to-face still-face paradigm? A narrative review and meta-analysis. Developmental Review, 42, 34–55. [Google Scholar]
- Provenzi L., Di Minico G.S., Giusti L., Guida E., Müller M. (2018a). Disentangling the dyadic dance: theoretical, methodological and outcomes systematic review of mother-infant dyadic processes. Frontiers in Psychology, 9(Mar), 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzi L., Guida E., Montirosso R. (2018b). Preterm behavioral epigenetics: a systematic review. Neuroscience and Biobehavioral Reviews, 84, 262–71. [DOI] [PubMed] [Google Scholar]
- Provenzi L., Rosa E., Visintin E., et al. (2020). Understanding the role and function of maternal touch in children with neurodevelopmental disabilities. Infant Behavior & Development, 58, 101420. [DOI] [PubMed] [Google Scholar]
- Quadrelli E., Anzani A., Ferri M., et al. (2019). Electrophysiological correlates of action observation treatment in children with cerebral palsy: a pilot study. Developmental Neurobiology, 79(11–12), 934–48. [DOI] [PubMed] [Google Scholar]
- Ranger M., Zwicker J.G., Chau C.M.Y., et al. (2015). Neonatal pain and infection relate to smaller cerebellum in very preterm children at school age. Journal of Pediatrics, 167(2), 292–298.e1. [DOI] [PubMed] [Google Scholar]
- Roos L.E., Horn S., Berkman E.T., Pears K., Fisher P.A. (2018). Leveraging translational neuroscience to inform early intervention and addiction prevention for children exposed to early life stress. Neurobiology of Stress, 9, 231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum P., Paneth N., Leviton A., et al. (2007). A report: the definition and classification of cerebral palsy April 2006. Developmental Medicine and Child Neurology. Supplement, 109, 8–14. [PubMed] [Google Scholar]
- Santamaria L., Noreika V., Georgieva S., Clackson K., Wass S., Leong V. (2020). Emotional valence modulates the topology of the parent-infant inter-brain network. NeuroImage, 207, 116341. [DOI] [PubMed] [Google Scholar]
- Sgandurra G., Ferrari A., Cossu G., et al. (2011). Upper limb children action-observation training (UP-CAT): a randomised controlled trial in hemiplegic cerebral palsy. BMC Neurology, 11, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp C., Fonagy P. (2008). The parent’s capacity to treat the child as a psychological agent: constructs, measures and implications for developmental psychopathology. Social Development, 17(3), 737–54. [Google Scholar]
- Simon-Martinez C., Mailleux L., Jaspers E., et al. (2020). Effects of combining constraint-induced movement therapy and action-observation training on upper limb kinematics in children with unilateral cerebral palsy: a randomized controlled trial. Scientific Reports, 10(1), 10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E.A., Murray L., Paukner A., Ferrari P.F. (2014). The mirror neuron system as revealed through neonatal imitation: presence from birth, predictive power and evidence of plasticity. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1644), 20130289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L.B., Thelen E. (2003). Development as a dynamic system. Trends in Cognitive Sciences, 7(8), 343–8. [DOI] [PubMed] [Google Scholar]
- Striano T., Rochat P., Legerstee M. (2003). The role of modelling and request type on symbolic comprehension of objects and gestures in young children. Journal of Child Language, 30(1), 27–45. [DOI] [PubMed] [Google Scholar]
- Tadic̈ V., Pring L., Dale N. (2013). Story discourse and use of mental state language between mothers and school-aged children with and without visual impairment. International Journal of Language and Communication Disorders, 48(6), 679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamis-LeMonda C.S., Kuchirko Y., Song L. (2014). Why is infant language learning facilitated by parental responsiveness? Current Directions in Psychological Science, 23(2), 121–6. [Google Scholar]
- Toppi J., Borghini G., Petti M., et al. (2016). Investigating cooperative behavior in ecological settings: an EEG hyperscanning study. PLoS One, 11(4), e0154236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarthen C., Aitken K.J. (2001). Infant intersubjectivity: research, theory, and clinical applications. Journal of Child Psychology and Psychiatry, 42(1), 3–48. [PubMed] [Google Scholar]
- Treyvaud K., Spittle A., Anderson P.J., O’Brien K. (2019). A multilayered approach is needed in the NICU to support parents after the preterm birth of their infant. Early Human Development, 139, 104838. [DOI] [PubMed] [Google Scholar]
- Turk E., Vroomen J., Fonken Y., Levy J., Heuvel M.I. (2022). In sync with your child: the potential of parent–child electroencephalography in developmental research. Developmental Psychobiology, 64(3), e22221. [DOI] [PubMed] [Google Scholar]
- Vinall J., Grunau R.E. (2014). Impact of repeated procedural pain-related stress in infants born very preterm. Pediatric Research, 75(5), 584–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass S.V., Noreika V., Georgieva S., et al. (2018a). Parental neural responsivity to infants’ visual attention: how mature brains influence immature brains during social interaction. PLoS Biology, 16(12), e2006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass S.V., Noreika V., Georgieva S., et al. (2018b). Parental neural responsivity to infants’ visual attention: how mature brains influence immature brains during social interaction. PLoS Biology, 16(12), e2006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins J.L., Monk C.S. (2013). A translational neuroscience framework for the development of socioemotional functioning in health and psychopathology. Development and Psychopathology, 25(4pt2), 1293–309. [DOI] [PubMed] [Google Scholar]
- Wu R., Tummeltshammer K.S., Gliga T., Kirkham N.Z. (2014). Ostensive signals support learning from novel attention cues during infancy. Frontiers in Psychology, 5, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Smith L.B. (2008). What’s in view for toddlers? Using a head camera to study visual experience. Infancy, 13(3), 229–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E.S., Frankenhuis W.E., DelPriore D.J., Ellis B.J. (2022). Hidden talents in context: cognitive performance with abstract versus ecological stimuli among adversity-exposed youth. Child Development, 93, 1493–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivan M., Gashri C., Habuba N., Horowitz-Kraus T. (2022). Reduced mother-child brain-to-brain synchrony during joint storytelling interaction interrupted by a media usage. Child Neuropsychology, 28(7), 918–37. [DOI] [PubMed] [Google Scholar]


