Abstract
This proceedings article presents the scope of pediatric coma and disorders of consciousness based on presentations and discussions at the First Pediatric Disorders of Consciousness Care and Research symposium held on September 14th, 2021. Herein we review the current state of pediatric coma care and research opportunities as well as shared experiences from seasoned researchers and clinicians. Salient current challenges and opportunities in pediatric and neonatal coma care and research were identified through the contributions of the presenters, who were Jose I. Suarez, MD, Nina F. Schor, MD, PhD, Beth S. Slomine, PhD Erika Molteni, PhD, and Jan-Marino Ramirez, PhD, and moderated by Varina L. Boerwinkle, MD, with overview by Mark Wainwright, MD, and subsequent audience discussion. The program, executively planned by Varina L. Boerwinkle, MD, Mark Wainwright, MD, and Michelle Elena Schober, MD, drove the identification and development of priorities for the pediatric neurocritical care community.
Keywords: Coma, Pediatric, Neurocritical care, Disorders of consciousness, Resting state fMRI, Covert consciousness, Withdrawal of care, Neonatal
Introduction
The Neurocritical Care Society’s Curing Coma Campaign has ignited the advancement of research and care for patients with disorders of consciousness (DoC), fueling a new DoC focus group within the Pediatric Neurocritical Care Research Group (PNCRG). Given the relative lack of well validated technological and examination-based diagnostic and prognostic assessment tools for children with DoC, the initial initiative of the PNCRG DoC Focus Group was to host the First Pediatric Disorders of Consciousness Care and Research symposium. This event took place virtually on September 14th, 2021, to discuss opportunities for collaborative clinical and bench research, learn from the experience of researchers in the field, and provide information about future efforts. The event was attended by ~ 75 people, who were pediatric neurocritical care intensivists, neurologists, advance practice providers, medical students, residents, fellows, and research coordinators.
Thus, because the symposium was designed to inform and inspire our research and care community, five different interested parties presented, one representing the Neurocritical Care Research Central, one representing the National Institute of Neurological Disorders and Stroke (NINDS), and three DoC investigators. The Neurocritical Care Research Central representative overviewed the Curing Coma Campaign (CCC) mission, committee structure, and current progress toward identified goals. The NINDS representative discussed the current state of funded pediatric DoC studies relative to the proportion of other works for prospective and pointed to relevant viable funding opportunities. Of the three DoC investigators, the first explained current pediatric DoC examination-based standardized neurobehavioral measures, the second reviewed advanced technological DoC biomarkers, and the third explored bench side work on DoC network mechanisms.
These parties’ contributions summarized here highlight the committees tasked with developing various aspects of research and clinical care innovation needed for pediatric patients with a DoC. This is the beginning phase of this project, with great potential for promoting pediatric DoC care advancement.
Background
Recent technological advancements enable detection of states of covert consciousness when a patient appears to be in coma but has higher awareness [1] and prediction of DoC outcomes. This progress for adults with DoC has inspired the pediatric coma caregiving community to launch similar efforts so that children and neonates may come to similarly benefit from these and future discoveries of cure of coma.
Severe acquired catastrophic brain injury, congenital, and other forms of relatively pediatric-centric brain dysfunction, when leading to DoC, including coma, unleashes disastrous consequences for the life of the patients and their families [2]. DoC encompass a spectrum of impairments that include alteration in arousal and/or awareness of self and the environment. The spectrum of DoC is currently classified into five clinical states, as defined for the general population by consensus-based guidelines [3]. The DoC are defined by a combination of bedside-exam-based behavioral markers and technological instrumental assessments employed in coma and beyond (Table 1, Fig. 1). Each state has different prognostic implications: coma, unresponsiveness wakefulness syndrome/vegetative state (UWS/VS), minimally conscious state (MCS), and emergence from minimally conscious state (eMCS) [4–6]. In addition to covert consciousness, the often confused functional locked-in syndrome with clear signs of awareness is further defined in Table 1.
Table 1.
Definitions and assessment of states of DoC
| DoC | Behavioral features used to classify states of disorders of consciousness |
|---|---|
| Coma | Complete absence of arousal and awareness; No periods of wakefulness; eyes are closed [7] |
| UWS/VS | Clear periods of sleep and wakefulness; responses are reflexive [8] |
| MCS |
Periods of sleep and wakefulness with evidence of inconsistent, sustained, reproducible purposeful or voluntary behavior [3]; MCS has been divided into MCS− and MCS+ [9] MCS- low-level behavior without language (visual fixation, visual tracking, localization of noxious stimulation, appropriate smiling or crying to emotional stimuli) MCS+ high-level behavior with language (e.g., command following; intelligible verbalizations; intentional communication) [3] |
| eMCS | Clear and consistent functional object use and/or functional communication (e.g., consistent, accurate yes/no responses) |
| Covert consciousness | |
| A state of MCS+ or eMCS identified when volitional brain activities is detected by task-based fMRI or EEG in individuals who display behavioral features of coma, VS/UWS, or MCS-, and thereby do not show command following at the bedside [10]. Synonymous terms are covert cognition and cognitive motor dissociation (CMD) [1, 11, 12]. A subtle but key distinction among these terms centers around the possibility of covert awareness and falsely negative task-based test. The limitation of the task-based command designed to illicit activation of the putative supporting network(s) may not be among the remaining network supporting covert consciousness. Thus, CMD is defined by network activation to command. Whereas covert consciousness is broader term encompassing CMD and awareness that is not necessarily detected by an applied task-paradigm, such as in those with language deficits precluding command following but have intact alternate higher-level networks. Less specific is the term covert processing, which is defined by network processing of internal or external stimuli that may or may not be occurring in a state of awareness. Examples include night terrors or severe brain injury with only an isolated set of networks with processing capacity not linked to potential for awareness | |
| LIS | |
| eMCS defined by the process of recovery from brainstem injury with quadriplegia and aphonia with full awareness. In this process there is recovery of cognitive abilities, arousal regulation impairment, and eye movements (classically vertical and blinking) [13] | |
| AM | |
| Not a DoC, but highlighted here to demonstrate this entity’s deficits that bear some overlap with eMCS typically after cerebellar surgery in which there is command following but with significant response delays, functional use of objects but inconsistently, sustained visual pursuit, purposeful but reduced or absent spontaneous movement, present sleep–wake, and partial awareness of the self in this condition [13] | |
VS/UWS vegetative state/unresponsive wakefulness syndrome, MCS minimally conscious state, fMRI functional magnetic resonance imaging, EEG electroencephalogram, CMD cognitive motor dissociation, LIS Locked-in Syndrome, AM akinetic mutism
Fig. 1.
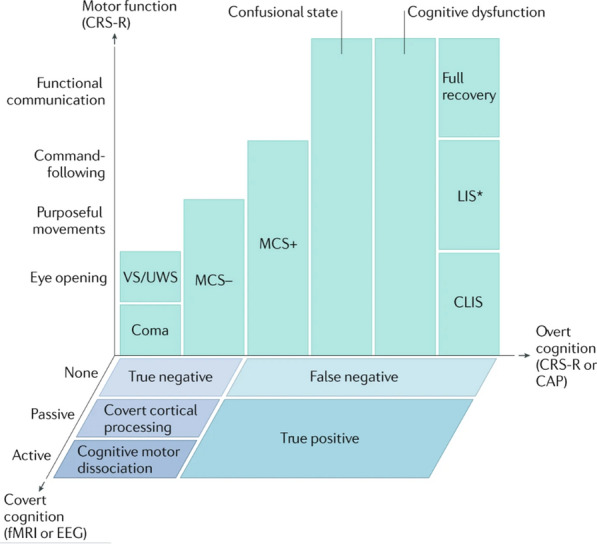
Multidimensional assessment of consciousness with pediatric consideration. Patients in coma are evaluated for overt cognition and motor function using the Coma Recovery Scale—Pediatrics (CRS-P), among other available measures, which classifies the patient’s DoC. In those who emerge from a minimally conscious state with language (MCS+) to a confusional state, the Confusion Assessment Protocol differentiates between a confusional state, cognitive dysfunction, and full recovery in adults and older children. When there is no behavioral evidence of language function (MCS-), task-functional MRI (fMRI) or EEG evidence of command-following indicates cognitive motor dissociation, fMRI or EEG responses within an association cortex during passive stimuli indicate covert cortical processing, and an absence of fMRI or EEG responses indicates a true negative fMRI/EEG classification. In pediatrics, the feasibility hurdles of task-fMRI/EEG (requirement of current awake state, developmental level of adequate cognition for test instruction comprehension, trained bedside technician, and extra equipment) may be overcome by resting state fMRI-based identification of DoC, possibly covert cognition, treatable pathological networks, and stratification of both recovery and epilepsy potential [19–25]. Patients with behavioral evidence of language or higher cognition are classified as false negatives if there are no fMRI or EEG responses, and as true positives if there are fMRI and EEG responses. CLIS, complete locked-in syndrome; LIS, locked-in syndrome; MCS–, minimally conscious state without language; VS/UWS, vegetative state/unresponsive wakefulness syndrome. *Patients with LIS are identified by the presence of consistent purposeful movements, typically vertical eye movements, and a reliable movement-based communication system. Patients with LIS who demonstrate inconsistent movements would not be distinguishable by behavioral measures from patients with CLIS, cognitive dysfunction, confusional state or MCS. Some patients with LIS are able to communicate via assistive communication devices. Adapted with permission from Edlow et al. [6]
A key practice gap stems from the lack of definition of the neural correlates and developmental-behavioral milestones of consciousness corresponding to respective states and developmental stages of consciousness in younger children and neonates; consequently, the DoC diagnoses have higher uncertainty, and the subsequent treatment and outcomes for these children remain unclear [2].
Tools that can determine DoC in adults with absolute certainty do not exist. Recent guidelines recommend that any adult with DoC should be diagnosed with the highest level of consciousness ascertainable based on a multimodal approach that may include comprehensive behavioral assessment, electroencephalography (EEG), or task [14] and resting state fMRI (tb-fMRI and rs-fMRI) [14–16]. The most recent of the professional society guidelines (European Academy of Neurology, 2020) acknowledges the relative paucity of pediatric data and posits that it is reasonable to consider application of the advanced measures [15]. Given the alternative of lack of checking for covert consciousness, or any of the otherwise defined neural correlates of good DoC recovery, in the context of relatively early withdrawal of life-sustaining therapies (WoLST), removal of treatments prolonging life that are no longer desired or do not provide comfort to the patient as determined by the patient or patient proxy [17], in DoC [18], such implementation may be the most ethical option, if available.
In pediatric populations, since the time of the most recent guidelines, advanced magnetic resonance imaging (MRI) and EEG technologies have been clinically applied to the diagnosis or outcomes of DoC [19–25]. Clinical application of tb-fMRI and rs-fMRI after acute brain insult, when early WoLST may be impacted, has been reported [19–22, 24]. These findings indicate the potential in children for diagnosis of covert consciousness, for detection of seizure onset zones not detectable by EEG (thought to be due to deeply located pathological networks), and for studying their association with long-term cognitive, motor, and epilepsy outcomes [21–24, 26, 27]. In chronic pediatric DoC, fMRI and diffusion tensor imaging also show associations with outcomes [28, 29]. Prior to these studies, certain extensively studied prognostication tools have shown relatively high rates of false negatives in children; these tools include somatosensory evoked potentials [30], brainstem auditory evoked response [31], standard electroencephalogram EEG [32], structural MRI [33], and Transcranial Doppler Ultrasound [34].
The current approach to DoC outcome prognostication for children does not incorporate technologically advanced solutions, that query for covert consciousness or covert processing, or developmentally—and outcome—validated behavioral bedside examinations measures; rather prognostication currently depends on clinician expertise, and therefore relies heavily on serial clinical examination. These clinical examinations are largely centered on phenotypically expressed function and serial assessments, to determine whether changes in behavioral responses are unequivocal, durable, and significant. A commonly used pediatric coma scale is the pediatric Glasgow Coma Score [35] and another, less commonly applied, is the pediatric Full Outline of Unresponsiveness score [7, 36, 37]. Each have significant limitations: for example, Glasgow Coma Score is limited in intubated and nonverbal children. The validated scale includes children younger than 2 years old. However, of those younger than 2 years, the age distribution and number with DoC is unknown from the only study available (range 10 days after birth to 17.9 years old, standard deviation 5.3 years, mean 8.3 years; 327 study participants were less than 2 years old, of which 15 had traumatic brain injury [TBI]) [38, 39]. In addition, neither of the tests have been validated to determine DoC severity nor the desired accuracy and resolution at clinically relevant difference in outcome prognosis in children. Comparatively, the Coma Recovery Scale- Revised (CRS-R) is the gold standard behavioral assessment for adults [40]. A modified version of the CRS-R for pediatrics was developed, with promising results; the CRS-Pediatric (CRS-P) differentiated between responses compatible with arousal in UWS and eMCS in patients and healthy children as young as 12 months [41]. Compared with current assessments, new ones incorporate the impact in quality of life from different perspectives. Two outcome scales by Bedell et al. [42] and Soo et al. [43] were developed for children and include quality of life components utilizing the World Health Organization International Classification of Functioning and Disability.
The effect of underlying cause of coma and many other factors when prognosticating or evaluation of coma in children is understudied. Supportively, the practice guidelines for diagnosis and management of DoC in children are lacking [44, 45]. Of the 18 recommendations in the 2018 practice guideline update, three of them refer to pediatrics only to state that established therapies and prognostication tools for children are not established [14]. Pediatric outcomes after acquired brain injury differ across a multitude of factors that increase the complexity and reduce reliability of the forecast. The exception of a few specific subgroups such as pediatric in-hospital cardiac arrest [46, 47]. Outcomes after TBI, as in adults, varies as a function of severity of injury and secondary insults. In children under one year of age, prognostication is even more challenging in part because coma assessments are less reliable particularly in the neonate [48]. In one retrospective study of children with catastrophic brain injury, 70% of the population died after withdrawal of life-sustaining support, whereas the majority of survivors improved significantly after months to years after injury [49]. In children under one year of age, prognostication is even more challenging in part because coma assessments are less reliable particularly in the neonate [48]. In one retrospective study of children with catastrophic brain injury, 70% of the population died after WoLST, while most survivors improved significantly after months to years after injury [49].
Thus, age-developmental level groups and broader array of etiology than adults, to name a few, precluding confident prognostication in many patients. Thus, the developmental stage and pediatric-specific neuroplasticity dramatically increases the variability of behavioral expression of consciousness impacting recovery, and cloud capacity of biomarkers lacking incorporation of these factors. Steering away from less specific behavioral based or gross measures of brain function toward biomarkers more intimately measuring correlates of covert consciousness stands to circumvent the insurmountable degree of variability in pediatric acute brain injury for more accurate neuroprognostication, as it has already done so in adults. For example, the neonatal acute brain injury study with acute phase rs-fMRI had more associations with outcomes than other standard tests, event-related potentials, and quantitative EEG also show promise across developmental capacities [22, 44].
Families who live this experience and navigate their child’s a state of severe impairment, are tasked in being a proxy to patient values while participating in care and legal determinations based on multiple other considerations including fulfilling resources for possibly a lifetime of care, as well as cultural and religious concerns. Parents reported that often a deeper level of family–community support was not present due to the need for enduring along with the child’s prolonged course, and the parent burden of advocating gave them a feeling of being at odds with the care and community members [50]. It appears these sentiments may follow a three phased process with the first characterized by the urge to be protective of life, the second is being protective from suffering, and the final is being protective of what remains to rebuild life [51].
Despite advancements in current family support in surrogate decisions, these provider-family communications have yet to impact the long-term psychological distress of the family, nor do they well address racial disparities in care experiences [52–54]. Thus, there is room for improvement in the care team approach in this environment. Families have expressed a desire for direct and clear information about condition and prognosis, as well as uncertainties, which have impacted recent care recommendations [54].
There is scant information to inform therapy during the acute phase of pediatric DoC. Amantadine, thus far, is the only well validated therapy, though other medication-based neuromodulating and neurostimulants have suggested benefit [2, 15, 18, 55, 56]. As the current guidelines and future pathways described by the CCC experts, advanced therapies in various stages of validation of adults include neuromodulation through stimulation from sources such as deep brain stimulator, vagal nerve stimulation, and transcranial magnetic stimulation. These may also be shown to improve outcomes in children.
Early initiation of inpatient rehabilitation after TBI indicated acute care improves outcomes [57, 58]. However, only 27% of children in the US transfer to inpatient rehabilitation [59], pointing toward limited access. The limited access by all supportive data is financially driven. Total health care costs after moderate and severe TBI in children are 88% higher than for mild TBI despite accounting for ~ 3% and 97% of this patient population, respectively [60]. In the United States, White children are more likely to receive inpatient rehabilitation compared with Black children, despite higher incidence of TBI-acute care in the later [61]. Age, insurance status, and geographical location are associated with inpatient rehabilitation in children after TBI-acute hospitalization [59]. Children without insurance coverage were 68% less likely to transfer to inpatient rehabilitation, compared with those with Medicaid coverage.
Socioeconomic status can influence the recovery in terms of behavioral, social, and academic outcomes [62, 63] after severe TBI in children. More behavioral problems, and worse adaptive ability or academic performance following severe TBI are found in the lower economic rank [64–66]. Family dysfunction and caregiver burden negatively impact the long-term recovery [67–69], and worsen social outcomes [70].
In making WoLST determinations, families of children reported the most to least important factors were based on suffering (64%), quality of life (51%), influence of physician-delivered prognosis (43%), and financial burden (7%) [71].
The pediatric arm of the Neurocritical Care Society and the PNCRG are aware of the relative lack of well validated technological and examination-based diagnostic and prognostic assessment tools for children with DoC and the validated means of well integrating them into care of children taking into consideration family perspectives. For this purpose, this symposium took place, and this article intends to summarize the symposium and leaderships’ contributions, discussed in Parts I-VI below. From these discussions, Table 2 describes the summary of symposium-driven prioritized research needs and suggested approaches to advance the pediatric DoC research and clinical practice.
Table 2.
Prioritized research needs and suggested approaches to address them and advance in pediatric DoC research and practice
| Prioritized research needs | Suggested approach | Impact |
|---|---|---|
| Current definitions of consciousness use behavioral measures | Phenotype DoC using new technologies (task and resting state fMRI, EEG, other neurophysiology) and behavioral biomarkers |
Identify children who may be eligible for emerging therapies to promote recovery after brain injury Reduction in reliance solely on clinician expertise effecting neuroprognostication and withdrawal of life-sustaining therapies Reduce the rate of misdiagnosis of DoC in children |
| Technology-intensive measures of covert consciousness |
Validate emerging measures of consciousness Expand education on pediatric DoC Link technologies to improved outcome measures |
Phenotyping of children with DoC using behavioral and neuromonitoring biomarkers |
| Outcome measures not well defined for pediatrics | Increase details in outcome measures specific to pediatric DoC | Precise measurement of outcomes across all age groups |
| Age-dependent changes in reliable behavioral measures |
Develop behavioral tools for measures of consciousness including language, motor and visual function for different age groups Longitudinal follow-up |
Reliable assessment of recovery in children with DoC prior to language development |
| Relative lack of research funding for disease areas which cause DoC in children |
Increase pediatric funding opportunities in cardiac-respiratory arrest, CNS infections, autoimmune disorders Increase awareness of numerous current NIH (NINDS) funding opportunities relevant to DoC |
Greater advocacy on behalf of children and families affected by DoC Mechanism-based understanding of pediatric DoC is supported by high quality science New therapies for DoC have scientific validity |
| Ontogeny of thalamocortical, reticular activating, and other supportive circuits mediating consciousness is not well understood | Promote basic neuroscience research into developmental aspects of consciousness | Combine age-dependent phenotyping of pediatric DOC with targeted therapeutic interventions to promote meaningful neurologic recovery |
| Understanding of the basic mechanisms of consciousness is primarily derived from adult model systems | Identify tractable mechanisms to promote recovery after neurologic injury | |
| Heterogeneity of causes and locations of injury leading to DoC |
Standardize definitions of pediatric DoC Combine clinical, EEG, imaging and other data into large datasets across institutions |
Precise phenotyping of DoC using clinical and technological assessments Enable a global approach to the treatment of pediatric DoC |
CCC and International Efforts: Update by Jose I. Suarez, MD.
The Neurocritical Care Research Central and subcommittees have worked efficiently to address expert opinions and identify knowledge gaps to improve the standard of care in patients with DoC. For this purpose, the CCC was officially launched at the 17th Neurocritical Care Society Annual Meeting in Vancouver in October 2019 [18]. The principal objective for the CCC is to tackle the goal of coordinating a global public health and research effort around the unifying concept that coma is a treatable medical entity. To achieve this objective, the CCC identified three principal elements: coma and DoC endotyping, biomarkers, and proof-of-concept clinical trials. The CCC seeks to break down barriers to global research, advocacy, and awareness by increasing knowledge about patients with DoC, enhance scientific efforts to understand coma, describe current clinical management, and to define knowledge gaps. The first CCC and Scientific Advisory Council meeting served to frame the initial scientific challenges. The second meeting was virtual due to the coronavirus disease of 2019 pandemic. The proceedings from the meetings detailing the expert determinations and discussions on future research for common DoC were published [18, 72].
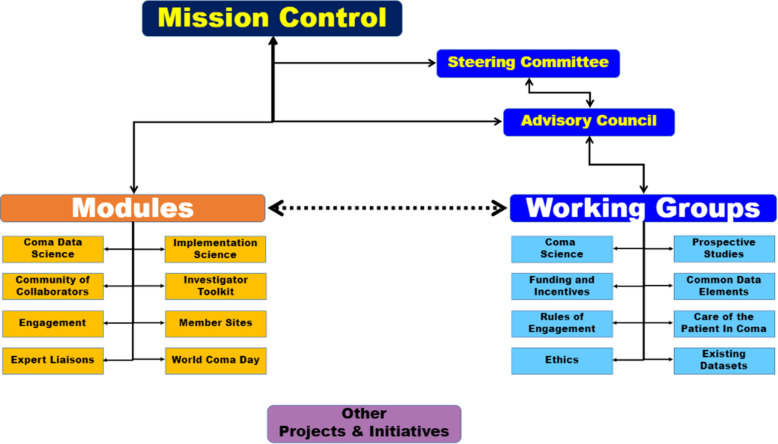
The process involves three major steps. The first step is creating the CCC community, in which pediatric representation has been a success. The second is designing and employing the CCC infrastructure (Fig. 2), and finally, the third is tackling the “grand challenge” of improving consciousness recovery [73]. The CCC’s Scientific Advisory Council is a small group of individuals who direct the campaign’s mission and promote interactions between all the modules and working groups.
Fig. 2.
Organizational structure of the Curing Coma Campaign. Reprinted with permission [73]
Eight major workgroups were created to develop the scientific roadmap of the CCC across several domains: (1) Coma Science/Biology of Coma, (2) Coma Database, (3) Funding and Incentives, (4) Care of Comatose Patients, (5) Rules of Engagement, (6) Ethics, (7) Prospective Studies, and (8) Common Data Elements (CDE). In addition, eight modules were established to focus on the execution of the CCC priorities and outreach: (1) Coma Data Science, (2) Implementation Science, (3) Community of Collaborators, (4) Investigators Toolkit, (5) Engagement, (6) Member Sites, (7) Expert Liaisons, and (8) World Coma Day.
The DoC CDE project was developed in collaboration with the NINDS (Program Officer: Carolina Mendoza-Puccini), which aims to improve DoC clinical research using content standards that allow clinical researchers to systematically collect, analyze, and share data across the research community. The final DoC CDE project is expected to be finalized by the end of 2022. The impact of the project is to reduce time and subsequent cost of developing data collection instruments, reduce study start-up time, promote consistent data collection, improve data quality, and encourage collaboration between studies.
The Prospective Studies working group developed World Coma Day, a virtual symposium spanning 24 h including people from around the world. The World Coma Day was designed to inform and share expert experiences related to coma care and research. The idea is not only to identify what is known, but also to inform on how to gain access to the data, learn from each other’s experience, avoid replication of mistakes, and learn from successes. For example, World Coma Day 2022 featured interviews with the first pediatric patients discovered to be in covert consciousness with their families [19, 20, 74, 75] which emphasized the potential for the advanced brain-network based markers in children with coma.
The most recent addition is the Care of Comatose Patients working group headed by Daiwai Olson, a registered nurse from University of Texas Southwestern, and Gisele Sampaio Silva from the University of Sao Paulo in Brazil. The objective of this working group is to focus on the science of current care and investigate evidence-based best practices of bedside care in coma, especially in relation to nursing practices. One main area of investigation would be the determination of practice variability with the aim to standardize such heterogeneity of care and its impact on patient outcome.
There is also a governance group who establish the campaigns policies of scientific review and rules of engagement for the working groups and with this, the development of principles of scientific collaboration and participation to set clear expectations about data use and sharing and publication authorship.
In addition, the CCC has been working directly with Dr. Jeremy Brown, Director of the Office of Emergency Care Research, at NINDS, thereby demonstrating to the community the importance of this effort. Moreover, the Neurocritical Care Foundation (NCCF) was recently approved by the Neurocritical Care Society Board of Directors with the idea of encouraging philanthropic funding. Since its inception in early 2022, the NCCF’s main goal has been to foster collaboration between individuals and entities that support and perform neurocritical care research projects including those related to the CCC.
The Prospective Studies and the Existing Database work groups continue exploring other research funding opportunities. The campaign is focusing on NINDS based funding, but other federal agencies are being explored such as other areas within the National Institutes of Health (NIH), Patient-Centered Outcomes Research Institute, and philanthropic funding.
The Mission Control is the centralized manager, interacting with all the operational modules and working groups, while promoting their independence. For example, the Engagement Module led by Theresa Human and Stephan Mayer, have been very active at overseeing the investigator toolkit and management of social media, including the World Coma Day.
Since the CCC was launched, the common message has been to provide hope for those patients with DoC, including children, and advocate to improve health, and making research a top priority given the lack of cohesion and direction across neurocritical care. To reach the goal, the Neurocritical Care Society encourages involvement from primarily researchers, clinicians, and trainees (Fig. 2).
Funding for CCC Initiatives: A Focus on Children, as reviewed by Dr. Nina F. Schor, MD, PhD.
Background: Current Status
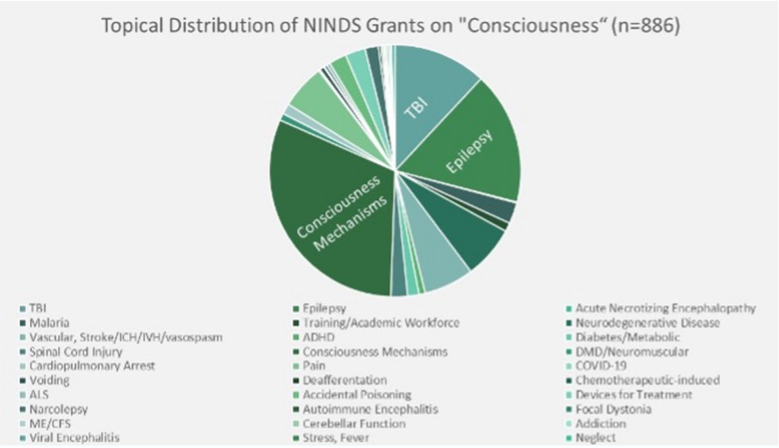
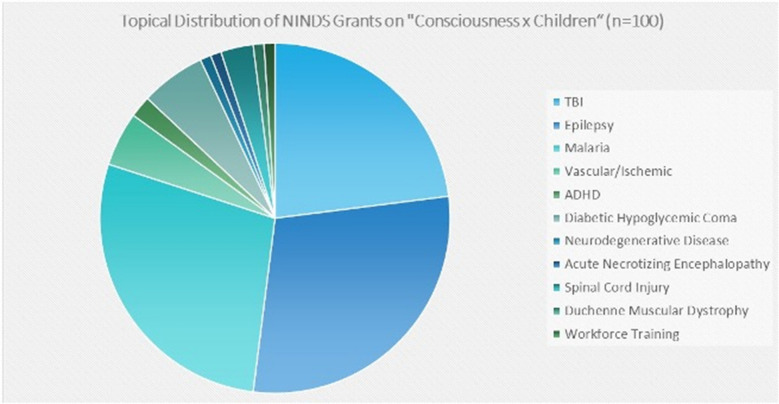
Currently NIH-funded DoC research, as determined using a query of publicly available NIH database, Reporter [76], revealed that, as of July 2021, 886 active grants to study research related to the keyword “consciousness” were funded by the USA’s NINDS, the “neurological institute.” Fig. 3 breaks these grants down by topical focus and demonstrates the prominence among funded grants of research related to TBI, epilepsy, and basic mechanisms of consciousness. An analogous search for active grants related to the intersection of the keywords “consciousness” and “children” revealed 100 grants were funded by NINDS. Figure 4 demonstrates the prominence of TBI, epilepsy, and malaria. Related to suppressed consciousness, a focused initiative on childhood cerebral malaria was ongoing through the Fogarty International Center at NIH.
Fig. 3.
Distribution of active grants (July 2021) awarded by NINDS by topical area [81], Results of a search using the keyword “Consciousness” (n = 886)
Fig. 4.
Results of a search using the intersection of the keywords “Consciousness” and “Children” (n = 100)
These data underscore several important points. First, in this temporal snapshot of NINDS-funded grants, only 11% of grants related to consciousness involve the study of consciousness in childhood. Second, TBI and epilepsy account for a higher percentage of studies of consciousness in children than in adults. Third, studies of children predominate among studies of infectious causes of abnormalities of consciousness. Fourth, studies of children account for half of studies of metabolic causes of abnormalities of consciousness.
NINDS-funded studies of basic mechanisms of consciousness predominantly involve adult model systems, whereas mechanistic studies of developmental aspects of consciousness appear to be uncommon. In addition, less commonly studied aspects of consciousness in childhood than in adults include vascular disorders, ingestions, cardiopulmonary arrest, and health and health care disparities. It is not possible from these data to distinguish among possible reasons for this discrepancy. However, pediatric critical care medicine is a very young research discipline relative to its adult-focused counterpart [77], making it likely that a lower frequency of submission of proposals to study developmental aspects of consciousness is a contributor to this discrepancy.
Objectives: Future Aims
Over approximately 1.5 years, NINDS worked and partnered with patients, grantees, trainees, and colleagues in the industrial and advocacy communities to create the 2021–2026 NINDS Strategic Plan (https://bit.ly/NINDSStrategicPlan) [78]. Many of the critically important but understudied aspects of consciousness in childhood are reflected in the priorities of the NINDS Strategic Plan.
Eleven cross-cutting themes (https://bit.ly/NINDSCross-cuttingStrategies) [79] overarch the Strategic Plan. They include equity, diversity, and inclusion; rigor and transparency; investigator-initiated research; innovative team science; neuro-ethics; patient engagement; access to technology; valuing the entire spectrum of model systems for research; data sharing and data science; collaboration and partnership; and leveraging the unique NINDS intramural research program to understand biological function and improve human health.
Three of these themes, namely: equity, diversity, and inclusion; neuro-ethics; and patient engagement in study design and operationalization, will facilitate our understanding of the cultural, philosophical, ethical, and socioeconomic determinants and significance of consciousness and disorders thereof in childhood. Both investigator-initiated and team science will be critical contributors to the science of the future. Innovation in study design and analysis is necessary to ensure that the complexities of developmental modulation of the manifestations and outcomes of DoC are understood. Rigor, transparency, and data sharing are essential elements of the generation of any credible, useful body of scientific knowledge, especially one that impacts children and families so robustly. Democratization of access to technology; valuing the whole spectrum of experimental models, from in vitro and ex vivo assays to cellular studies like those using induced pluripotent stem cells to organoids to animal and human studies; and collaboration and partnership, including that between the extramural scientific community and the unique resources and capabilities of the intramural NIH workforce, will bring everything we can to bear on the science and medicine of childhood coma.
The objectives of the Science Taskforce of the NINDS Strategic Planning Initiative underscored the fact that NINDS feels strongly that the scientific community itself should define what is important and what is topically current and that NINDS should fund the very best science in whatever topical areas are the subjects of submitted applications.
Consequently, the scientific objectives (https://bit.ly/NINDSNeuroscienceResearch) [80] of the Strategic Plan do not refer to a specific disease or part of the nervous system. Rather, they aim to understand the basic science of the healthy nervous system and develop and validate biomarkers for disease, resilience, susceptibility, and recovery in the nervous system; accelerate development of individualized treatments for disease that improve quality of life, resilience, and recovery in the nervous system; optimize use of developed treatments; develop individualized testing and monitoring of disease in the nervous system; develop individualized preventive strategies for disease in the nervous system; and advance health equity, including ensuring the accessibility, availability, and acceptability of developed diagnostic strategies, therapies, tests, monitoring, and preventive strategies to all people.
In short, NINDS hopes to get grant application submissions for studies of, for example, consciousness and disorders thereof in childhood from the most basic science to the most directly translational studies.
In its decisions about which grant applications to fund, NINDS funds first those applications judged by study sections, most of which are assembled and run by the NIH Center for Scientific Review and not by any of the NIH institutes, to be the most meritorious science. The priority score assigned to a proposal by a study section is the most important determinant of whether it will be funded by NINDS. In addition, applications from high priority investigators, including early-stage investigators, investigators whose funding will run out and who need a one-year “bridge” to submitting their revised application, and investigators who bring a unique background and perspective to the science of their work; and applications that focus on high priority issues, recently including the coronavirus disease of 2019 pandemic, may receive special consideration from NINDS.
Finally, special, congressionally mandated initiatives, including Helping to End Addiction Long-term, Brain Research through Advancing Innovative Neurotechnologies, and Alzheimer’s disease related dementia, are considered in the context of funds allocated solely for use in these initiatives (https://bit.ly/NINDSFunding) [81].
Future directions: Advancements from pediatric neurocritical care and the CCC
NINDS believes the future is yours to create and ours to enable and fuel! As you envision your research endeavors, you should ask yourself the following critical questions:
What do those in the field think are the most important questions and issues?
What do the patients and families affected by DoC think are the most important questions and issues?
What new tools are needed or need to be validated (particularly for use in children) to answer those questions and solve those issues?
What populations have not optimally benefited from currently available answers and solutions?
NINDS wants to fund the very best science, launch new investigators, sustain current excellent investigators, and train and build a diverse workforce in service of its mission: to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease for all people (https://bit.ly/NINDSMission) [82]. We at NINDS are grateful for your partnership and look forward to enabling the future you imagine and fueling, with you, the health and well-being of children and families!
Validity of Exam-Based (Neurobehavioral) Biomarkers of DoC in the Pediatric Age Spectrum (Infant, Child, Adolescence) and Pediatric Outcome Assessments, as reviewed by Beth Slomine, PhD.
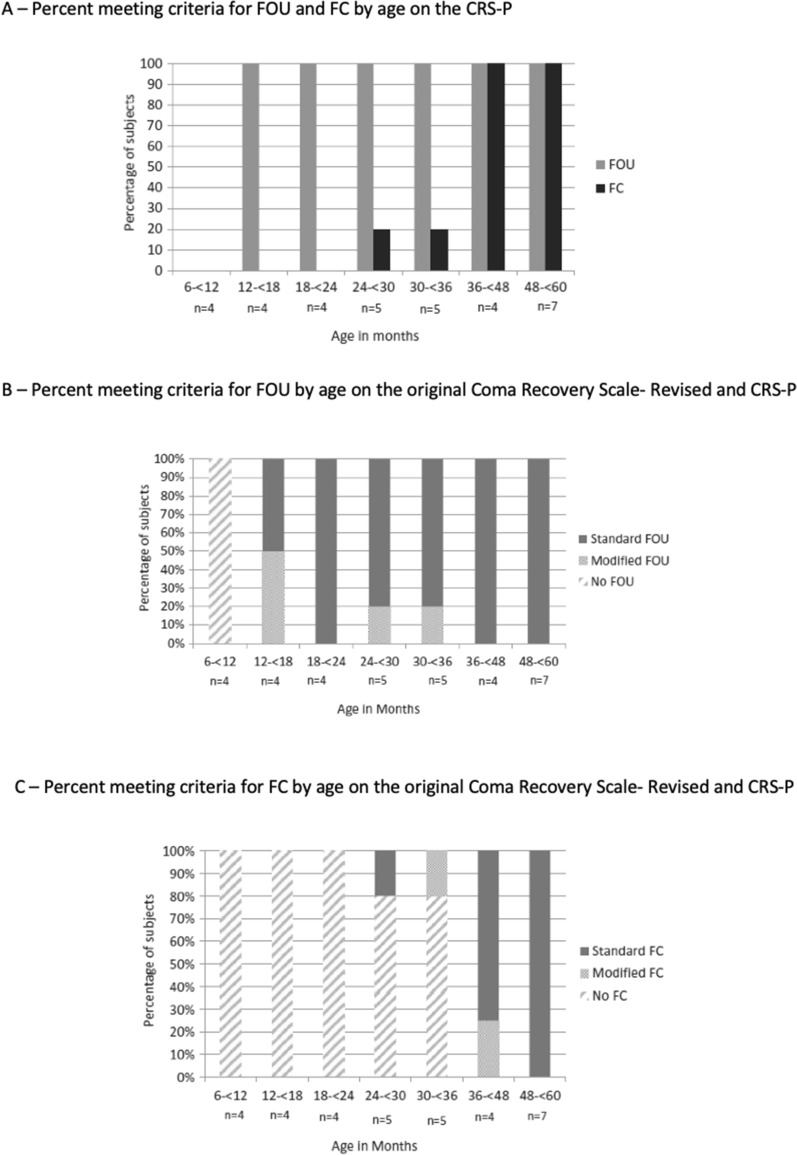
This section focuses on neurobehavioral assessment for diagnosing states of DoC in children, especially very young children, and describing methods for assessment of long-term neurobehavioral outcomes (Fig. 5).
Fig. 5.
Functional Object Use (FOU) and Functional Communication (FC) on the Coma Recovery Scale – Pediatrics (CRS-P) in a Typically Developing Sample of Young Children. A Percent of sample meeting criteria for FOU and FC by age on CRP-S. Twenty-nine (88%) of the 33 participants displayed at least one of the two behaviors that signal emergence into a conscious state using either the original or modified CRS-P criteria. All 29 exhibited FOU, while 13 (45%) also showed FC. There were no cases in which FC occurred in the absence of FOU. Two of the participants who demonstrated FC were < 3 years of age (29 and 32 months). The remainder of the sample were at least 3 years old. B Percent meeting criteria for FOU by age on the original Coma Recovery Scale- Revised and CRS-P. FOU, defined by CRS-R as movements generally compatible with a specific function of object, was observed in all children over 12 months of age. A total of four (13%) of the 29 children who did not exhibit FOU based on the original CRS-R scoring instructions met the modified criteria for FOU. C Percent meeting criteria for FC by age on the original Coma Recovery Scale- Revised and CRS-P. Thirteen children, all at least 29 months of age, demonstrated functional communication (defined in the CRS-R as clearly discernible and accurate verbal or gestural “yes” or “no” responses to six consecutive visual or aurally based situational orientation questions or by the modified CRS-P criteria, which require clearly discernible and accurate verbal or gestural “yes” or “no” responses to six consecutive questions about images in a picture book). Among these 13 children, two participants (ages 32 and 37 months) who did not display FC utilizing the original CRS-R met criteria using the picture book question set.
Neurobehavioral Assessment
Several neurobehavioral measures have been developed to detect behavioral biomarkers of DoC and eMCS in adults. The Coma Recovery Scale – Revised (CRS-R) has the highest evidence for adequate reliability and validity in adults compared to others available [40]. The CRS-R, however, has not been validated in children. Understanding the bedside exam-based behavioral biomarkers of DoC is particularly challenging in the youngest children due to their limited repertoire of developmentally appropriate skills that are consistent with conscious behavior as described in adults.
To better assess the behavioral features of DoC in young children, the CRS-Pediatrics (CRS-P) was developed and c preliminary validation of this tool has been reported in a typically developing cohort of very young children [41]. Investigation of behavioral responses to CRS-P items across distinct age levels is an initial step toward clinical application of this tool. When developing the CRS-P, modifications were made to make the measure more pediatric-friendly.
Two of the main modifications of the CRS-P include changes to the two behavioral indicators of eMCS [functional communication (FC) and functional object use (FOU)]. On the original CRS-R, FC is defined as accurate yes/no responses to 6/6 yes or no questions; FOU is defined as demonstrating use of two different objects functionally on command to 4/4 trials. For the FC item of CRS-P, a picture book modality was added to promote age-appropriate engagement in very young children, and for the FOU item of the CRS-P, scoring criteria were changed to allow spontaneous occurrences to be credited if required response criteria were met (instead of only scoring responses to commands to use the objects).
The CRS-P was administered to 33 children ages 8–59 months (49% men, 85% White). Interrater reliability for the CRS-P subscale scores was strong, suggesting that the CRS-P can be reliably administered in very young children. All children who were at least 12 months of age showed FOU (n = 29) and no child displayed FC without FOU. Of those 29 children who displayed FOU, 13 (45%) also displayed FC. Of those who demonstrated FC, the youngest was 29 months of age. Importantly, 4/29 children (13.7%) displayed FOU only with the CRS-P modifications, and 2/13 (15%) displayed FC only with the CRS-P modifications [40].
Findings demonstrate that these modifications to the FC and FOU items of the CRS-R were useful in capturing behavioral biomarkers consistent with eMCS in typically developing children. Moreover, FOU can be seen in children as young as 12 months of age whereas FC was not observed until at least age 2 years. Results suggest that the CRS-P is appropriate for clinical use in children who are at least 12 months of age and had normal development prior to brain injury. Given that language-based behavioral biomarkers of eMCS are not consistently present in typically developing children under the age of 3 years, even with the CRS-P modifications, the CRS-P should be used cautiously with young children who may not yet have developed the necessary language skills to demonstrate FC and who have injury-related or preexisting visual and motor impairments that may impact their ability to demonstrate FOU.
In another study, in parallel to the development and preliminary validation of the CRS-P, behavioral features of very young children (n = 54; 6-months through 5 years) who were admitted to an inpatient rehabilitation facility following TBI or acquired brain injury were explored [83]. At admission to inpatient rehabilitation, on average 38 days after injury, 15 children displayed characteristics consistent with VS (28%), 15 demonstrated characteristics consistent with MCS (28%), and 24 demonstrated characteristics consistent with eMCS (44%). The most common feature of MCS was demonstration of contingent affect (an emotional reaction consistent with the situation that suggests awareness of the environment); however, only two children were classified as MCS due to demonstrating contingent affect only. Other common features of MCS included visual fixation or pursuit, automatic motor behavior, and contingent communicative intent. No children in MCS showed command-following or intelligible verbalizations. Of those in VS at admission, 9/15 children (60%) emerged to MCS and none to eMCS by discharge. Of those in MCS at admission, 5/15 children (33%) emerged from MCS by discharge, and none were classified as UWS/VS at discharge.
Similar to the CRS-P development and validation study [41], FOU (based on spontaneous behavior) was observed in all children in eMCS, and FC was observed in a subset. Also, the youngest child showing FOU at admission was 12-months of age, and the youngest showing FC was older (20-months-old). Taken together, results of both studies highlight the need for pediatric-specific behavioral biomarkers of DoC, especially in very young children, as visual and motor skills may be most applicable in diagnosis states of DoC, and language-based skills may be less applicable. Moreover, using pediatric-specific behavioral biomarkers, identification of states of DoC at admission to inpatient rehabilitation may have important prognostic implications.
Long-Term Outcomes Assessment
Research exploring long-term neurobehavioral outcomes after pediatric DoC is limited. As a result, little is known about the natural history of DoC and predictors of outcome [14]. There are many challenges to studying long-term outcomes after pediatric DoC, including the small number of patients with prolonged DoC at individual centers, lack of coordinated care for children pediatric DoC which results in loss to clinical and research follow-up, and limited set of measures available to capture the full range of outcomes across the entire pediatric age range. Moreover, studies requiring onsite follow-up at an academic medical center to collect outcomes place a high burden of time and resources on professionals and families. Using clinical data from follow-up appointments at a medical center and obtaining information via telephone follow-up are two methodologies that have potential to successfully address these challenges. Below are two examples of studies of long-term neurobehavioral outcomes in children with a period of pediatric DoC. In one study, clinical follow-up data were used and, in the other, caregiver-report of child functioning obtained via telephone was utilized.
In one study, outcomes were obtained via chart review in 37 children ages 2–18 years who were admitted to a pediatric inpatient facility with DoC following TBI. Mean time from injury to rehabilitation admission was 28 days. Those included had follow-up at 1-year and either another follow-up appointment (> 2 years after injury) or died after their 1-year visit [84]. Clinical notes were reviewed at 1-year and most recent follow-up (2 to 12 years after injury) to rate the Glasgow Outcome Scale-Extended for Pediatrics (GOS-E peds) and determine state of DoC. By 1-year follow-up, 3 (8.1%) were in VS, 7 (18.9%) were in MCS, and 27 (73.0%) were eMCS. By most recent follow-up, four patients died and two more emerged (one in MCS + at 1-year emerged by 1.3 years post injury; one in MCS- at 1-year emerged by 2.2 years post injury). Among the 33 survivors, 69.2% (9/13) in VS at admission emerged, whereas 95% (19/20) in MCS at admission emerged (χ2 [1] = 4.07, p = 0.04). None of the three individuals still in VS at 1 year showed improvement by the most recent follow-up. Of the 33 surviving to the most recent follow-up, 6 (18.1%) improved on the GOS-E Peds between 1-year and most recent follow-up.
Results highlight that most children in DoC at admission for rehabilitation after TBI are at high risk for long-term disability, although most emerged from MCS. A minority showed gains in functioning more than 1-year post-injury. Limitations include the small sample size from one institution with data collected over 14 years.
In another study, feasibility and utility of measures of long-term outcomes collected via telephone interview was examined in a convenience sample of 41 caregivers of children from two different hospitals who were admitted to inpatient rehabilitation in a state of DoC following either traumatic or acquired brain injury, using the Vineland-3, a detailed measure of neurobehavioral functioning, and the Glasgow Outcome Scale – Extended for Pediatrics (GOS-E Peds), a much shorter measure of global neurological functioning [85, 86]. All those who consented completed the interview. Time from injury to follow-up ranged from 1 to 17 years since injury (mean = 5 years). Administration time of the GOS-E Peds ranged from 2 to 10 min (m = 3.14) and the Vineland-3 ranged from 13 to 101 min (m = 50.38). While less than 10% of patients were rated as vegetative on the GOS-E Peds (the lowest score in survivors), 19.5% earned the lowest possible score on the Vineland-3.
Findings from this convenience sample suggest that telephone administration of the GOS-E Peds and Vineland-3 are feasible and complementary in capturing long-term outcomes in children with a history of DoC after brain injury for traumatic or acquired etiologies; if both measures are used together, range and variability are maximized. Neither measure captures the behaviors necessary to classify a state of DoC and other measures may be needed to supplement, should this be an outcome of interest. As such, caution is needed when using these measures when state of DoC is an outcome of interest.
Importantly, while improvement can occur after pediatric DoC, significant long-term disability and a high level of ongoing needs is common. In both the TBI and mixed brain injury samples described above, the largest proportion of children (43% and 54% respectively) fell in the “lower severe disability” category on the GOS-E Peds (requiring assistance for activities of daily living in the home every day) [61, 62]. Moreover, the caregivers who participated in the telephone outcomes study [62] reported that 80% of the children required a wheelchair, 51% had a feeding tube, and 51% had unmet healthcare needs at the time of follow-up [86].
Taken together, our understanding of pediatric DoC is in its infancy. Future research exploring neurobehavioral tools for assessment of states of DoC and describing long-term outcomes after pediatric DoC require larger sample sizes that will only be achieved through multicenter collaboration and use of CDE to allow for comparison across centers.
2.4. Use of Medical Devices and Technological Instrumentation for Assessing the Neurorehabilitation Path and Outcome after Acquired Brain Injury in the Pediatric Population, as reviewed by Erika Molteni, PhD.
DoC misdiagnosis rates may be as high as 20% for adults using neurobehavioral tools [87]. In children the rate of misdiagnosis can only be higher, due to incomplete development of some assessed abilities. In addition, inaccurate or erroneous diagnosis affects the formulation of a precise long-term prognosis, which can impact short-term decisions, such as the treatments offered, and the WoLST.
WoLST is inhomogeneous across countries, as different legal contexts make interruption of therapy for persons in VS a variegated practice. For example, in Europe, sharp differences have been estimated for WoLST between Italy (8% of cases), Spain (34%), France (50%) and England (85%) [88]. Three percent of all deaths among pediatric intensive care unit admissions are related to poor neurological status leading to the WoLST [89]. In the neonatal population, 2–6 per 1000 live births have acute brain injury, of which 1.5 per 1000 is from neonatal hypoxic ischemic encephalopathy [90, 91]. These patients have 20% moderate to severe morbidity and 25% mortality, with 91% of deaths due to withdrawal of life-sustaining therapy (WLST) [92]. In most, WoLST is informed by the DoC examination linked to brain imaging and electrophysiology [18]. However, because there are no consensus definitions of neonatal and young children consciousness or DoC [2], the link between exam and imaging-electrophysiology is inherently unknown. Since consciousness cannot be clearly defined nor thereby determined or, at best, encompass less specific DOC categorizations than older children and adults, then reliance on imaging-electrophysiology in WoLST determinations will remain higher in neonates and young children. The combination of average DoC misdiagnosis rates (e.g., ~ 40%) and high WoLST rates (e.g., ~ 85%) determines high rates of inappropriate interruption of treatment in patients with a DoC (in this example, ~ 8.5% of interruptions in patients who had better condition than the one diagnosed, if assuming equal chances of optimistic and pessimistic misdiagnosis) [93]. The scientific community, together with the clinical practitioners, has the responsibility to decrease this rate as much as possible.
Assessment through medical devices or instrumentation can assist in decreasing the diagnostic and prognostic errors. The two main techniques used in pediatric DoC are neurophysiology recordings and brain imaging through computed tomography (CT) and MRI.
Neurophysiology methods, particularly electroencephalography, are not new to the field. EEG is applied in various DoC etiologies, including TBI and encephalitis. For decades it has been assisting the characterization of factors that hamper recovery or mask signs of consciousness such as status epilepticus, epilepsy, jerks, myoclonus, and paroxysm, including the identification of seizure foci [94]. Nowadays, EEG is also used to identify the cortical reactivity to cognitive or emotional stimuli; It helps exclude cases of “locked-in” syndrome and covert cognition; and it is deemed to have some prognostic value [6, 95, 96]. For these reasons, its use is extended beyond the acute brain injury, in the post-acute and chronic phase of DoC.
Emergence from a coma toward UWS/VS and then higher levels of consciousness corresponds to the progressive restructuring of the circadian rhythm, including the alternation of sleep and wake periods. Children’s sleep is sensibly different from adults’ sleep, and it generally consists in fewer sleep stages, faster cycles, and higher total number of rapid eye movement periods. Consequently, sleep scoring needs specific criteria in DoC, which are not the same for adults and children. Polysomnography is the method used to record the sleep patterns, identify the sleep stages, and build hypnograms (i.e., diagrams of sleep stages and duration). A specific scale for the visual classification of sleep stages in children with DoC is available [97]. Also, the presence of “figures” of sleep (e.g., spindle, slow wave) is considered a good prognostic indicator, as it entails the integrity of the underlying generative brain networks [98, 99]. The regulation of parietal slow wave activity build-up has also been put forward as prognostic indicator for DoC, which needs further confirmation [100].
Evoked potentials are also used to determine prognosis. During the acute phase of disease after acquired brain injury, the preservation of brainstem activity has prognostic value to determine survival. Brainstem auditory evoked response are applied to assess the presence of residual brainstem activity and/or the extent of brainstem damage, despite high false negative rate. This assessment is often performed when the patient is still in a coma, in combination with other tests, and once transitioned to MCS, there is less need for this assessment [101]. Furthermore, sensory pathways are often damaged or disrupted in pediatric patients with DoC: they are assessed through somatosensory evoked potentials, which provide indication on the integrity of the sensory pathways to the brain, from peripheral nerves to the spinal cord and brain, and through visual or auditory potentials to a lesser extent. Event Related Potential (ERPs) evaluation is applied for cognitive evaluation, most often during the post-acute phase of disease.
Recent literature indicates that protocols should include multimodal stimulation, to address different sensory pathways and provide stimulation to virtually all the sensory system and associated brain networks [14, 102]. In any case, the somatosensory pathways and the auditory evoked potentials provide the advantage that they do not require active participation by the patient. In addition, patients’ fatigability is coped with by delivering stimuli with high salience and emotional content [102] in short sessions. In case of event related designs, the protocol is kept simple, and cognitive requests basic [103]. Importantly, the reliability of ERPs in healthy children is low, which creates obstacles to the employment of ERPs as biomarkers.
Neuroimaging, and CT in particular, is largely used in the early diagnosis and monitoring of acquired brain injury. However, nonionizing neuroimaging is increasingly employed during the post-acute and chronic phases of disease to assess children with DoC. This includes the surveillance of hydrocephalus and atrophy through structural imaging; the observation of white matter inflammation, degeneration or change in microstructure, and tract disruption, typically through diffusion weighted MRI [104]; and the assessment of anatomical plasticity occurring in correspondence with treatment [105]. In this last case, however, cautious interpretation should be applied, not to infer causality beyond the study design.
Functional and metabolic imaging are the pillar of instrumental DoC assessment. Positron Emission Tomography (PET) enables the assessment of the metabolic consumption in the brain, which is known to be reduced in patients with DoC. In adults, it was observed that brain glucose consumption in the vegetative state can decrease by some measure until − 40% or − 50% of regular consumption during acute acquired brain injury, and by some measure until − 30% or − 40% of consumption during the subacute and chronic stages, thus including prolonged DoC [106]. In children with DoC, the PET is employed in acquired brain injury infrequently, probably due to long-term radiation risks, an historical lack of normative data, lack of consensus on the reference brain area(s) to be selected, and the absence of information on the theoretical threshold for the decrease in glucose consumption to transition from the VS to the MCS and above. Nowadays, reference tables are available for children, and some case studies employing PET in children with DoC have been published [96, 107, 108]. Overall, pediatric DoC PET studies showed marked global 18F-fluorodeoxyglucose uptake reduction in all cortical and subcortical regions in both brain hemispheres, including the basal ganglia, cerebellum, and brainstem, which suggests severe and diffuse glucose hypometabolism, in the same range of adults with UWS/VS. Single photon emission computed tomography (SPECT) similarly revealed mild or moderate hypoperfusion in the frontal and temporal cortices, as well as in the cerebellar hemispheres. Also, PET and SPECT have been successfully employed to assess metabolic modifications induced in the brain by pharmacological interventions such as zolpidem administration [96].
Functional MRI (fMRI) is employed to passively investigate the integrity of the default mode network and other brain networks involved in awareness through the resting state technique, as well as to detect covert cognition by recording brain activity probatory of active participation contingent to environmental stimulations or requests [6]. In children with DoC, fMRI has been applied in case studies and small case series only [21, 24, 28]. In neonates with acute brain injury, rs-fMRI has shown association with consciousness (as defined by this study related to degree of responsiveness to environmental stimuli), mortality, developmental outcomes, and epilepsy [22]. At term, the default mode network was recently shown to integrate information across diverse sensory and higher-order functional modules, which is interpreted as the generative mechanism building conscious awareness, and thus, may allow for future consciousness and developmental capacity determination after mild to severe acute brain injury [109]. In adults, cohort studies associating rs-fMRI findings with DoC outcomes are available; however, the intrapatient repeatability of recordings remains a requirement for assessment. Similarly, pediatric research should strive to obtain reproducibility of single-subject results and monitor the risk of false positives introduced by those who opt to employ voxel-wise analysis through general linear models combined with the conduction of multiple statistical tests.
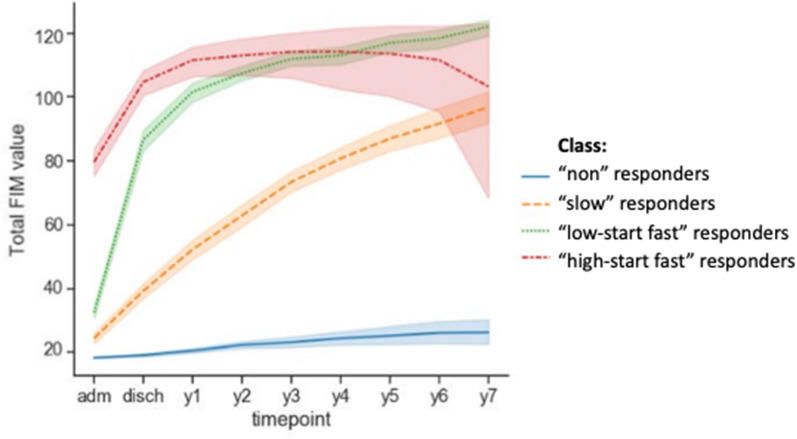
In DoC, due to the variety of different etiologies and multiplicity of brain lesions, the severity of acquired brain injury at initial presentation does not fully explain the trajectory of recovery, nor the outcome. Instrumental assessment is useful to obtain information at different stages of the disease course, and to reduce diagnostic and prognostic uncertainty. In particular, the modeling of the DoC trajectory over time can incorporate instrumental information and assist with diagnosis of DoC and prediction of DoC evolution. This field is understudied in pediatrics, and existing works only incorporate information from neurofunctional-behavioral scales in recovery trajectories after severe acquired brain injury [110–112]. However, trajectory modeling has the potential to assist precision medicine, being a flexible tool, which can account for virtually any factor affecting a patient’s personal recovery (e.g., treatments, infections, noninfectious complications, environmental modulators) through incorporation of a multiplicity of data types (scales, imaging, neurophysiology). Analogously, trajectory modeling can assist identifying endotypes, i.e., patients who have similar disease course, as demonstrated in Fig. 6 [112]. Such trajectory modeling is the statistical modeling of the time course of clinical measurements, scales, and quantitative measurements from images, neurophysiology examinations, or wet biomarkers. This is usually intended to capture the evolution of the patient’s condition over time. If a treatment is offered in the modeled timeframe, methods can be applied to study the effect of treatment on outcome. Alternatively, patient’s early data can be used to predict whether the patient will respond to treatment through the expected recovery or response trajectory, based on data from previous study participants. Trajectory modeling can also isolate the pure effect of treatment from the concurrent effects of other factors affecting recovery, thus contributing to reduce sample sizes in clinical trials [104].
Fig. 6.
Example of typological analysis with four classes clustering based on the Functional Independence Measurement (FIM) scale. The method separated between four endotypes: high-start fast (red), low-start fast (green), slow (orange) and nonresponders (blue). “adm” and “disch” indicate first admission and discharge, respectively, followed by yearly assessments (Color figure online).
Adapted from Molteni et al. [112], with permission from the authors. Of note, “nonresponder” status is conditional to the assessment tool used
Artificial intelligence can also be employed to identify which data are most informative to patients’ classification, as well as to the prediction of their future outcome or prognosis [113]. This includes the possibility to train algorithms on modeled trajectories of disease to infer the future course of patients, for whom early clinical and imaging data (e.g., at admission and in acute phase) are available only. In pediatric DoC, the limited dimensions of existing databases, due to frequent small size of the available databases of cohorts, make classical machine learning (i.e., nondeep learning methods) the first methodological choice. However, tasks for which large databases (i.e., big data) are available, such as the classification of brain lesions in CT scans of patients with TBI, can make use of deep neural networks; these grant automatic data driven feature extraction ahead of classification [114], allow the establishment of highly specific automated solutions, and have the potential to transfer learning from adult databases to pediatric data when appropriate network architectures and training designs are deployed.
In summary, neurophysiology, and imaging offer assessment opportunities in pediatric DoC. These techniques have largely been explored in regard of their feasibility, but more research is needed to identify reliable and repeatable instrumental biomarkers for pediatric DoC. Mathematics and statistics will provide tools for modeling the disease evolution. Also, improvement in study designs will increase the level of evidence in this field. Strategic and multicentric data acquisition will also assist in this task.
Developmental Neuroscience from a Bench Perspective with a Focus on Brain Circuits, as Reviewed by Jan-Marino Ramirez, PhD
The human brain is continuously active whether we are awake or asleep [115]. Many neurons in the cortex are rhythmically active [116], yet their activity is mostly desynchronized during the awake state. The generation of this baseline persistent activity consumes close to 95% of brain’s metabolic energy [117, 118]. Transitioning from awake into the sleep state, this desynchronized persistent activity is altered. The neurons become hyperpolarized in a synchronized manner, which leads to the generation of the rhythmic up states and down states. However, there are many different rhythmic states in the neocortex, which includes the delta rhythm, beta rhythm, and theta rhythm. These rhythmic activities are integrated with activities in hippocampus, amygdala, striatum, and cerebellum. Indeed, to generate brain functions, these different rhythms must interact. In doing so, neocortical activities interact with many brain regions including the hippocampus, thalamus, and basal ganglia [119–121]. The complex coordinated integration of rhythmic activities across the entire brain emerges from the activities of numerous distributed rhythmogenic microcircuits and their circuit interactions.
Most brain regions are capable of intrinsically generating rhythmic activities when isolated from the rest of the brain. Maintained in culture medium that mimics the extracellular ionic composition of the brain, thin slices obtained from the medulla will generate respiratory rhythmic activity (Fig. A) [122], while neocortex will generate rhythmic up and down states that are reminiscent of deep sleep (Fig. B) [123–127]. Modifications of the culture medium could keep these organotypic slices alive and rhythmically active for several weeks not only in mouse tissue [128], but also when these slices were obtained from resected human cortical tissue [129]. Connected in the intact brain, the respiratory rhythm controls breathing, while the up and down states are critical for memory consolidation. During sleep, up and down states are synchronized with the neocortex and hippocampus. Animal models for Alzheimer disease reveal that the synchronization between hippocampus and neocortex is lost [130]. Because of the loss of neuronal interactions, the animals are incapable of memory formation. Another example for the disturbance of functional interactions occurs in epilepsy. Synchronized rhythmic activity across different brain regions spreads during seizures.
Thus, most networks in the brain can intrinsically generate rhythmic activity, and brain functions emerge through the complex interactions between multiple intrinsically active networks. The ability of most brain areas to generate activity intrinsically may have clinical implications, as it raises the hope that functionally important circuit interactions can be potentially reactivated, in the case that the connectivity is lost or disturbed. A great example is spinal cord injury, for which brain computer interface technologies are being developed to bypass the site of injury, to reactivate spinal circuits that are still intact but disconnected. To what extent brain computer interface technologies or other approaches can be clinically used to reestablish brain-wide functional integration is still an open question.
These considerations are relevant for curing coma because a loss of connectivity renders the neocortex intrinsically active which results in the loss of functional integration. Depending on how much conductivity is still intact or lost there are cases of MCS and other cases of a total unconscious state. It will be important to understand which circuit interactions are critical to maintain conscious state and to leverage this understanding for restoring consciousness under the various coma conditions.
The loss of functional integration in coma conditions, however, is typically not only restricted to higher brain functions. Functional disintegration frequently also affects the control of important autonomic functions involved in cardiorespiratory coupling [131, 132]. Such disturbances are driven in part by reactive oxygen species that can be produced by inflammation, heavy metals such as lead, biotic stress, air pollutants or intermittent hypoxia [133, 134]. ROS production is known to have multiple effects on network interactions that control cardiorespiratory coupling, which has been studied in detail for an intrinsically active network that is critical for the generation of the respiratory rhythm: the Pre-Bötzinger complex, as detailed in Fig. 7 [135]. Considering dysautonomia and the disintegration of brainstem networks as one of the consequences of network disintegration as seen in coma and many other neurological conditions such as in Rett syndrome, and epilepsy, familial dysautonomia is clinically important [136–138].
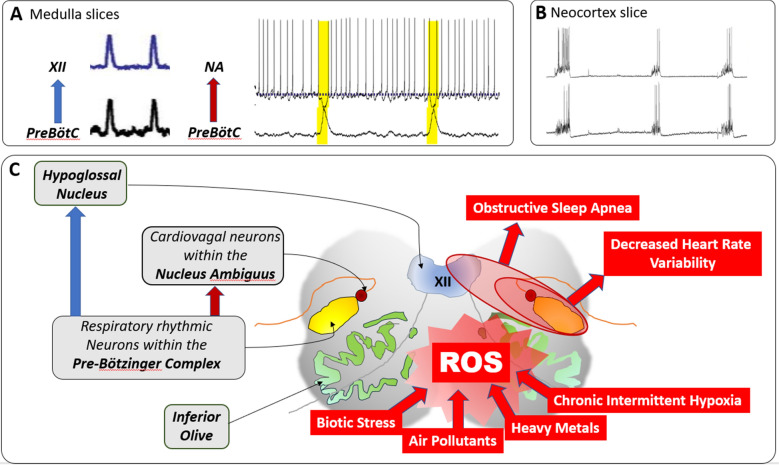
Fig. 7.
Intermittent hypoxia multiple effects on network interactions controlling cardiorespiratory coupling. Among these networks is an intrinsically active network that is critical for the generation of the respiratory rhythm: the Pre-Bötzinger complex (panel A, C) [135]. This microcircuit is located within the ventrolateral medulla, in close vicinity to the Nucleus Ambiguus which includes the cardiovagal neurons that inhibit the heart (panel C) [139]. Exposure to intermittent hypoxia affects not only the central nervous system but leads also to the activation of the carotid body, a chemosensory organ that is critical for cardiorespiratory coupling. Activation of the carotid body by intermittent hypoxia leads to a system-wide upregulation of Hypoxia-Inducible Factor 1-alpha, a prooxidant gene regulator that activates a cascade of prooxidant genes [133, 134]. Neurons in the Central Nervous System are also affected by intermittent hypoxia produce hydrogen peroxide and reactive oxygen species (ROS). The effect of chronic intermittent hypoxia on that tissue is reflected in an increased lipid peroxidation. Chronic intermittent hypoxia also affects the pre-Bötzinger complex, as neurons in the respiratory network lose excitability resulting in desynchronization and disturbances in rhythmogenesis [140, 141]. The preBötzinger complex neurons activate the hypoglossal nucleus (panel A) and due to such synchronization failure in the rhythmogenic networks, hypoglossus-mediated apnea occurs, causing an obstruction and pharyngeal collapse (panel C). The pre-Bötzinger complex inhibits the cardiovagal neurons located in the Nucleus ambiguous (panel A). Thus, disturbances in the interactions between the Pre-Bötzinger complex and the cardiovagal neurons lead to a decrease in heart-rate variability. Interestingly, these desynchronizations are driven not by the hypoxia, but by the reactive oxygen species. By applying reactive oxygen species scavengers, the rhythmic activity can be restored, including activation of the hypoglossus [137]
In conclusion, there is an urgent need to understand how central neuronal networks generate intrinsic activity, how the interactions between these networks lead to a functional integration and the emergence of brain functions, and how injury, or hypoxia, disrupts these interactions. In the context of a loss of consciousness and coma, it will be interesting to learn how thalamocortical interactions contribute to consciousness. This may lead to a better understanding in which way during unconscious states thalamocortical control is lost. Determining the degree of loss of connectivity will be an important step in the diagnosis of minimally conscious states.
Conclusions
The expert perspectives were discussed during the First Pediatric Disorders of Consciousness and Research Symposium, and future efforts are promising. The pediatric critical care community is aware of the extensive lack of developmentally appropriate diagnostic assessments for children and reliable outcomes measurements. Through the CCC, the Neurocritical Care Society offers infrastructure to develop research and engage our community. Relative underrepresentation of pediatrics in this area at NIH and NINDS exists, but with the encouraging thoughts that there are many excellent opportunities for grant funding. The importance and need to explore advanced biomarkers and behavioral outcome measures across all pediatric age ranges is clear. The goals are to gain understanding of the mechanisms underlying coma and DoC, to develop safe interventions, and determine the impact of those interventions. For this purpose, global collaborations are needed not only to improve the science behind coma and DoC in the pediatric population, but to convert these findings into improvement of care.
Abbreviations
- ABI
Acute brain injury
- AM
Akinetic mutism
- ADRD
Alzheimer’s disease related dementia
- BAER
Brainstem auditory evoked response
- BRAIN
Brain Research Through Advancing Innovative Neurotechnologies
- CAP
Confusional assessment protocol
- CMD
Cognitive motor dissociation
- CBI
Catastrophic brain injury
- CLIS
Complete locked-in syndrome
- CRS-P
Coma recovery scale for pediatrics
- CRS-R
Coma recovery scale – revised
- CT
Computed tomography
- DoC
Disorder of consciousness
- DTI
Diffusion tensor imaging
- DWI
Diffusion weighted magnetic resonance imaging
- EEG
Electroencephalography
- eMCS
Emergence from a minimally conscious state
- EP
Evoked potentials
- FC
Functional communication
- FIM
Functional Independence Measurement
- fMRI
Functional magnetic resonance imaging
- FOU
Functional object use
- FOUR
Full outline of Unresponsiveness
- GCS
Glasgow coma score
- GOS-E Peds
Glasgow outcome scale-extended for pediatrics
- HEAL
Helping to end addiction long-term
- LIS
Locked-in syndrome
- MCS
Minimally conscious state
- MCS−
Minimally conscious state minus
- MCS+
Minimally conscious state plus
- MRI
Magnetic resonance imaging
- NIH
National Institutes of Health
- NINDS
National institute of neurological disorders and stroke
- PCORI
Patient-centered outcomes research institute
- PET
Positron emission tomography
- REM
Rapid eye movement
- ROS
Reactive oxygen species
- rs-fMRI
Resting state functional magnetic resonance imaging
- SPECT
Single photon emission computed tomography
- SSEP
Somatosensory evoked potentials
- TBI
Traumatic brain injury
- UWS/VS
Unresponsive wakefulness syndrome/vegetative state
- WoLST
Withdrawal of life sustaining therapies
Author contributions
Conception, design: VB, NS, BS, EM, LR, MS, MW, JS; Drafting the manuscript: VB, NS, BS, EM, JR, LR, MG, MS, MW, JS; Revising for intellectual content: VB, NS, BS, EM, JR, LR, SW, MG, KG, MS, MW, JS; Final approval of version to be published: VB, NS, BS, EM, JR, LR, SW, MG, KG, MS, MW, JS.
Source of support
No funding was obtained for the work.
Declarations
Conflicts of interest
Erika Molteni has received funds from the Medical Research Council UK (Skills Development Scheme) and from the National Institute for Health Research UK (grant n.134293).
Jose I. Suarez is member of the CEC for the REACT Study funded by Idorsia, Inc; Ex-Officio and member of the Board of Directors of the Neurocritical Care Society; Member Editorial Board Stroke.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schiff ND. Cognitive motor dissociation following severe brain injuries. JAMA Neurol. 2015;72(12):1413–1415. doi: 10.1001/jamaneurol.2015.2899. [DOI] [PubMed] [Google Scholar]
- 2.LaRovere KL, Tasker RC. Defining catastrophic brain injury in children leading to coma and disorders of consciousness and the scope of the problem. Curr Opin Pediatr. 2020;32(6):750–758. doi: 10.1097/MOP.0000000000000951. [DOI] [PubMed] [Google Scholar]
- 3.Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58(3):349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- 4.Plum F, Posner JB. The diagnosis of stupor and coma. Brain Nerve. 2015;67(3):344–345. [PubMed] [Google Scholar]
- 5.Bruno MA, Gosseries O, Ledoux D, Hustinx R, Laureys S. Assessment of consciousness with electrophysiological and neurological imaging techniques. Curr Opin Crit Care. 2011;17(2):146–151. doi: 10.1097/MCC.0b013e328343476d. [DOI] [PubMed] [Google Scholar]
- 6.Edlow BL, Claassen J, Schiff ND, Greer DM. Recovery from disorders of consciousness: mechanisms, prognosis and emerging therapies. Nat Rev Neurol. 2021;17(3):135–156. doi: 10.1038/s41582-020-00428-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. a practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 8.Laureys S, Celesia GG, Cohadon F, et al. Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med. 2010;8:68. doi: 10.1186/1741-7015-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruno MA, Vanhaudenhuyse A, Thibaut A, Moonen G, Laureys S. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: recent advances in our understanding of disorders of consciousness. J Neurol. 2011;258(7):1373–1384. doi: 10.1007/s00415-011-6114-x. [DOI] [PubMed] [Google Scholar]
- 10.Schnakers C, Giacino JT, Løvstad M, et al. Preserved covert cognition in noncommunicative patients with severe brain injury? Neurorehabil Neural Repair. 2015;29(4):308–317. doi: 10.1177/1545968314547767. [DOI] [PubMed] [Google Scholar]
- 11.Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Using functional magnetic resonance imaging to detect covert awareness in the vegetative state. Arch Neurol. 2007;64(8):1098–1102. doi: 10.1001/archneur.64.8.1098. [DOI] [PubMed] [Google Scholar]
- 12.Schnakers C, Bauer C, Formisano R, et al. What names for covert awareness? A systematic review. Front Hum Neurosci. 2022;16:971315. doi: 10.3389/fnhum.2022.971315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbaum AM, Giacino JT. Chapter 25 - clinical management of the minimally conscious state. In: Grafman J, Salazar AM, editors. Handbook of clinical neurology. Amsterdam: Elsevier; 2015. pp. 395–410. [DOI] [PubMed] [Google Scholar]
- 14.Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: disorders of consciousness: report of the guideline development, dissemination, and implementation subcommittee of the american academy of neurology; the american congress of rehabilitation medicine; and the national institute on disability, independent living, and rehabilitation research. Neurology. 2018;91(10):450–460. doi: 10.1212/WNL.0000000000005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kondziella D, Bender A, Diserens K, et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol. 2020;27(5):741–756. doi: 10.1111/ene.14151. [DOI] [PubMed] [Google Scholar]
- 16.Monti MM, Schnakers C. Flowchart for implementing advanced imaging and electrophysiology in patients with disorders of consciousness. Neurology. 2022;98(11):452. doi: 10.1212/WNL.0000000000200038. [DOI] [PubMed] [Google Scholar]
- 17.Taylor A. Chapter 4 - palliative care hospice care advance care planning and advance directives. In: Krishnan MS, Racsa M, Yu H-HM, editors. Handbook of supportive and palliative radiation oncology. Boston: Academic Press; 2017. pp. 39–56. [Google Scholar]
- 18.Mainali S, Aiyagari V, Alexander S, et al. Proceedings of the second curing coma campaign NIH symposium: challenging the future of research for coma and disorders of consciousness. Neurocrit Care. 2022 doi: 10.1007/s12028-022-01505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boerwinkle V. Patient Stories: Road to Recovery. Neurocritical Care Society. (https://bit.ly/Peds-CC1).
- 20.Boerwinkle V. Family Story: Struggles of family advocacy. March 22, 2022. Neurocritical Care Society. Access date August 11, 2022. (https://bit.ly/Peds-CC2).
- 21.Boerwinkle VL, Torrisi SJ, Foldes ST, et al. Resting-state fMRI in disorders of consciousness to facilitate early therapeutic intervention. Neurol Clin Pract. 2019 doi: 10.1212/CPJ.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boerwinkle VL, Sussman BL, Manjón I, et al. Association of network connectivity via resting state functional MRI with consciousness, mortality, and outcomes in neonatal acute brain injury. Neuroimage Clin. 2022;34:102962. doi: 10.1016/j.nicl.2022.102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boerwinkle VL, Sussman BL, Manjón I, et al. Resting state functional MRI connectivity association with consciousness, mortality, longitudinal and two-year outcomes in neonatal acute brain injury. medRxiv. 2022 doi: 10.1101/2022.06.07.22275838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boerwinkle VL, Agarwal S, Sussman B, Scher MS. Serial multimodal functional brain network evaluation in children with acute disorders of consciousness, covert consciousness, and brain death: a novel demonstrative case series. OSFPREPRINTS. 2022 doi: 10.31219/osf.io/p8bn7. [DOI] [Google Scholar]
- 25.Kirsch M, Guldenmund P, Ali Bahri M, et al. Sedation of patients with disorders of consciousness during neuroimaging: effects on resting state functional brain connectivity. Anesth Analg. 2017;124(2):588–598. doi: 10.1213/ANE.0000000000001721. [DOI] [PubMed] [Google Scholar]
- 26.Boerwinkle VL, Mohanty D, Foldes ST, et al. Correlating resting-state functional magnetic resonance imaging connectivity by independent component analysis-based epileptogenic zones with intracranial electroencephalogram localized seizure onset zones and surgical outcomes in prospective pediatric intractable epilepsy study. Brain Connect. 2017;7(7):424–442. doi: 10.1089/brain.2016.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boerwinkle VL, Cediel EG, Mirea L, et al. Network targeted approach and postoperative resting state functional MRI are associated with seizure outcome. Ann Neurol. 2019 doi: 10.1002/ana.25547. [DOI] [PubMed] [Google Scholar]
- 28.Nicholas CR, McLaren DG, Gawrysiak MJ, Rogers BP, Dougherty JH, Nash MR. Functional neuroimaging of personally-relevant stimuli in a paediatric case of impaired awareness. Brain Inj. 2014;28(8):1135–1138. doi: 10.3109/02699052.2014.890745. [DOI] [PubMed] [Google Scholar]
- 29.Ishaque M, Manning JH, Woolsey MD, et al. Functional integrity in children with anoxic brain injury from drowning. Hum Brain Mapp. 2017;38(10):4813–4831. doi: 10.1002/hbm.23745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomita Y, Fukuda C, Maegaki Y, Hanaki K, Kitagawa K, Sanpei M. Re-evaluation of short latency somatosensory evoked potentials (P13, P14 and N18) for brainstem function in children who once suffered from deep coma. Brain Dev. 2003;25(5):352–356. doi: 10.1016/s0387-7604(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 31.Goitein KJ, Amit Y, Fainmesser P, Sohmer H. Diagnostic and prognostic value of auditory nerve brainstem evoked responses in comatose children. Crit Care Med. 1983;11(2):91–94. doi: 10.1097/00003246-198302000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Mizrahi EM, Pollack MA, Kellaway P. Neocortical death in infants: behavioral, neurologic, and electroencephalographic characteristics. Pediatr Neurol. 1985;1(5):302–305. doi: 10.1016/0887-8994(85)90033-5. [DOI] [PubMed] [Google Scholar]
- 33.Dubowitz DJ, Bluml S, Arcinue E, Dietrich RB. MR of hypoxic encephalopathy in children after near drowning: correlation with quantitative proton MR spectroscopy and clinical outcome. AJNR Am J Neuroradiol. 1998;19(9):1617–1627. [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkham FJ, Levin SD, Padayachee TS, Kyme MC, Neville BG, Gosling RG. Transcranial pulsed Doppler ultrasound findings in brain stem death. J Neurol Neurosurg Psychiatry. 1987;50(11):1504–1513. doi: 10.1136/jnnp.50.11.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molteni E, Slomine BS, Castelli E, Zasler N, Schnakers C, Estraneo A. International survey on diagnostic and prognostic procedures in pediatric disorders of consciousness. Brain Inj. 2019;33(4):517–528. doi: 10.1080/02699052.2019.1565899. [DOI] [PubMed] [Google Scholar]
- 36.Wijdicks EF, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: the FOUR score. Ann Neurol. 2005;58(4):585–593. doi: 10.1002/ana.20611. [DOI] [PubMed] [Google Scholar]
- 37.Czaikowski BL, Liang H, Stewart CT. A pediatric FOUR score coma scale: interrater reliability and predictive validity. J Neurosci Nurs. 2014;46(2):79–87. doi: 10.1097/jnn.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 38.Holmes JF, Palchak MJ, MacFarlane T, Kuppermann N. Performance of the pediatric glasgow coma scale in children with blunt head trauma. Acad Emerg Med. 2005;12(9):814–819. doi: 10.1197/j.aem.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Palchak MJ, Holmes JF, Vance CW, et al. A decision rule for identifying children at low risk for brain injuries after blunt head trauma. Ann Emerg Med. 2003;42(4):492–506. doi: 10.1067/s0196-0644(03)00425-6. [DOI] [PubMed] [Google Scholar]
- 40.Seel RT, Sherer M, Whyte J, et al. Assessment scales for disorders of consciousness: evidence-based recommendations for clinical practice and research. Arch Phys Med Rehabil. 2010;91(12):1795–1813. doi: 10.1016/j.apmr.2010.07.218. [DOI] [PubMed] [Google Scholar]
- 41.Slomine BS, Suskauer SJ, Nicholson R, Giacino JT. Preliminary validation of the coma recovery scale for pediatrics in typically developing young children. Brain Inj. 2019;33(13–14):1640–1645. doi: 10.1080/02699052.2019.1658221. [DOI] [PubMed] [Google Scholar]
- 42.Bedell GM. Developing a follow-up survey focused on participation of children and youth with acquired brain injuries after discharge from inpatient rehabilitation. NeuroRehabilitation. 2004;19(3):191–205. doi: 10.3233/NRE-2004-19303. [DOI] [PubMed] [Google Scholar]
- 43.Soo C, Tate RL, Williams L, Waddingham S, Waugh MC. Development and validation of the paediatric care and needs scale (PCANS) for assessing support needs of children and youth with acquired brain injury. Dev Neurorehabil. 2008;11(3):204–214. doi: 10.1080/17518420802259498. [DOI] [PubMed] [Google Scholar]
- 44.Ismail FY, Saleem GT, Ljubisavljevic MR. Brain data in pediatric disorders of consciousness: special considerations. J Clin Neurophysiol. 2022;39(1):49–58. doi: 10.1097/WNP.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 45.Vitello MM, Szymkowicz E, Laureys S, Alnagger N, Gosseries O, Thibaut A. Neuroimaging and neurophysiological diagnosis and prognosis in paediatric disorders of consciousness. Dev Med Child Neurol. 2022;64(6):681–690. doi: 10.1111/dmcn.15150. [DOI] [PubMed] [Google Scholar]
- 46.Morgan RW, Kirschen MP, Kilbaugh TJ, Sutton RM, Topjian AA. Pediatric In-hospital cardiac arrest and cardiopulmonary resuscitation in the united states: a review. JAMA Pediatr. 2021;175(3):293–302. doi: 10.1001/jamapediatrics.2020.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Topjian AA, de Caen A, Wainwright MS, et al. Pediatric post-cardiac arrest care: a scientific statement from the american heart association. Circulation. 2019;140(6):e194–e233. doi: 10.1161/CIR.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 48.Natarajan N, Pardo AC. Challenges in neurologic prognostication after neonatal brain injury. Semin Perinatol. 2017;41(2):117–123. doi: 10.1053/j.semperi.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 49.LaRovere KL, De Souza BJ, Szuch E, et al. Clinical Characteristics and Outcomes of Children with Acute Catastrophic Brain Injury: A 13-Year Retrospective Cohort Study. Neurocrit Care. 2022;36(3):715–726. doi: 10.1007/s12028-021-01408-9. [DOI] [PubMed] [Google Scholar]
- 50.Roscigno CI, Swanson KM. Parents' experiences following children's moderate to severe traumatic brain injury: a clash of cultures. Qual Health Res. 2011;21(10):1413–1426. doi: 10.1177/1049732311410988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verhaeghe S, Zuuren FJ, Grypdonck M, Duijnstee M, Defloor T. The focus of family members' functioning in the acute phase of traumatic coma: Part One: The initial battle and protecting life. J Clin Nurs. 2010;19:574–582. doi: 10.1111/j.1365-2702.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 52.Cox CE, White DB, Hough CL, et al. Effects of a personalized web-based decision aid for surrogate decision makers of patients with prolonged mechanical ventilation: a randomized clinical trial. Ann Intern Med. 2019;170(5):285–297. doi: 10.7326/m18-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White DB, Angus DC, Shields AM, et al. A randomized trial of a family-support intervention in intensive care units. N Engl J Med. 2018;378(25):2365–2375. doi: 10.1056/NEJMoa1802637. [DOI] [PubMed] [Google Scholar]
- 54.Muehlschlegel S, Perman SM, Elmer J, et al. The Experiences and needs of families of comatose patients after cardiac arrest and severe neurotrauma: The perspectives of national key stakeholders during a national institutes of Health–Funded workshop. Crit Care Explor. 2022;4(3):e0648. doi: 10.1097/CCE.0000000000000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giacino JT, Katz DI, Schiff ND, et al. Comprehensive systematic review update summary: disorders of consciousness: report of the guideline development, dissemination, and implementation subcommittee of the american academy of neurology; the american congress of rehabilitation medicine; and the national institute on disability, independent living, and rehabilitation research. Arch Phys Med Rehabil. 2018;99(9):1710–1719. doi: 10.1016/j.apmr.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Seel RT, Douglas J, Dennison AC, Heaner S, Farris K, Rogers C. Specialized early treatment for persons with disorders of consciousness: program components and outcomes. Arch Phys Med Rehabil. 2013;94(10):1908–1923. doi: 10.1016/j.apmr.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 57.Sörbo A, Rydenhag B, Sunnerhagen KS, Blomqvist M, Svensson S, Emanuelson I. Outcome after severe brain damage, what makes the difference? Brain Inj. 2005;19(7):493–503. doi: 10.1080/02699050400013709. [DOI] [PubMed] [Google Scholar]
- 58.Godbolt AK, Stenberg M, Lindgren M, et al. Associations Between Care Pathways and Outcome 1 Year After Severe Traumatic Brain Injury. J Head Trauma Rehabil. 2015;30(3):E41–E51. doi: 10.1097/HTR.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 59.Greene NH, Kernic MA, Vavilala MS, Rivara FP. Variation in pediatric traumatic brain injury outcomes in the United States. Arch Phys Med Rehabil. 2014;95(6):1148–1155. doi: 10.1016/j.apmr.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Graves JM, Rivara FP, Vavilala MS. Health care costs 1 year after pediatric traumatic brain injury. Am J Public Health. 2015;105(10):e35–41. doi: 10.2105/ajph.2015.302744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the united states: differences by race. J Head Trauma Rehabil. 2005;20(3):229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Marr A, Coronado V. Central Nervous System Injury Surveillance Data Submission Standards-2002. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control 2004.
- 63.Marr A, Coronado V, editors. Central Nervous System Injury Surveillance Data Submission Standards—2002. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control2004.
- 64.Taylor HG, Alden J. Age-related differences in outcomes following childhood brain insults: an introduction and overview. J Int Neuropsychol Soc. 1997;3(6):555–567. doi: 10.1017/S1355617797005559. [DOI] [PubMed] [Google Scholar]
- 65.Taylor HG, Yeates KO, Wade SL, Drotar D, Klein SK, Stancin T. Influences on first-year recovery from traumatic brain injury in children. Neuropsychology. 1999;13(1):76–89. doi: 10.1037/0894-4105.13.1.76. [DOI] [PubMed] [Google Scholar]
- 66.Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term outcomes after traumatic brain injury in children: behavior and achievement. Neuropsychology. 2002;16(1):15–27. doi: 10.1037/0894-4105.16.1.15. [DOI] [PubMed] [Google Scholar]
- 67.Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Attentional and processing skills following traumatic brain injury in early childhood. Brain Inj. 2005;19(9):699–710. doi: 10.1080/02699050400025281. [DOI] [PubMed] [Google Scholar]
- 68.Anderson V, Godfrey C, Rosenfeld JV, Catroppa C. Predictors of cognitive function and recovery 10 years after traumatic brain injury in young children. Pediatrics. 2012;129(2):e254–e261. doi: 10.1542/peds.2011-0311. [DOI] [PubMed] [Google Scholar]
- 69.Nadebaum C, Anderson V, Catroppa C. Executive function outcomes following traumatic brain injury in young children: a five year follow-up. Dev Neuropsychol. 2007;32(2):703–728. doi: 10.1080/87565640701376086. [DOI] [PubMed] [Google Scholar]
- 70.Yeates KO, Bigler ED, Dennis M, et al. Social outcomes in childhood brain disorder: a heuristic integration of social neuroscience and developmental psychology. Psychol Bull. 2007;133(3):535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Michelson KN, Koogler T, Sullivan C, Ortega Mdel P, Hall E, Frader J. Parental views on withdrawing life-sustaining therapies in critically ill children. Arch Pediatr Adolesc Med. 2009;163(11):986–992. doi: 10.1001/archpediatrics.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Provencio JJ, Hemphill JC, Claassen J, et al. The curing coma campaign: framing initial scientific challenges-proceedings of the first curing coma campaign scientific advisory council meeting. Neurocrit Care. 2020;33(1):1–12. doi: 10.1007/s12028-020-01028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olson DM, Hemphill JC, Provencio JJ, et al. The curing coma campaign and the future of coma research. Semin Neurol. 2022 doi: 10.1055/a-1887-7104. [DOI] [PubMed] [Google Scholar]
- 74.Boerwinkle LV. Stories of Hope: Struggles of family advocacy. Neurocritical Care Society Podcast. Access Date August 2, 2022 (https://ncspodcast.libsyn.com/stories-of-hope-struggles-of-family-advocacy).
- 75.Boerwinkle LV. Stories of Hope: Road to Recovery. Neurocritical Care Society Podcast. Curing Coma Campaign. World Coma Day 2022. Access Date November 1, 2022. (https://ncspodcast.libsyn.com/stories-of-hope-eli).
- 76.Reporter. National Institutes of Health. Access Date March 4, 2022. (https://reporter.nih.gov/).
- 77.Levin DL, Downes JJ, Todres ID. History of pediatric critical care medicine. J Pediatr Intensive Care. 2013;2(4):147–167. doi: 10.3233/pic-13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.2021–2026 NINDS Strategic Plan: Investing in the future of neuroscience. National Institute of Neurological Disorders and Stroke (https://www.ninds.nih.gov/About-NINDS/Strategic-Plans-Evaluations/Strategic-Plans/NINDS-Strategic-Plan-and-Priorities).
- 79.2021–2026 NINDS Strategic Plan: Cross-cutting strategies. National Institute of Neurological Disorders and Stroke. (https://www.ninds.nih.gov/About-NINDS/Strategic-Plans-Evaluations/Strategic-Plans/NINDS-Strategic-Plan-and-Priorities/Cross-Cutting-Strategies).
- 80.2021–2026 NINDS Strategic Plan: Neuroscience research. National Institute of Neurological Disorder and Stroke. (https://www.ninds.nih.gov/About-NINDS/Strategic-Plans-Evaluations/Strategic-Plans/NINDS-Strategic-Plan-and-Priorities/Neuroscience-Research).
- 81.NINDS funding strategy 2021. National Institute of Neurological Disorders and Stroke. (https://www.ninds.nih.gov/Funding/About-Funding/NINDS-Funding-Strategy/NINDS-Funding-Strategy-FY-2021).
- 82.NINDS mission. National Institute of Neurological Disorders and Stroke. (https://www.ninds.nih.gov/About-NINDS/Who-We-Are/Mission).
- 83.Alvarez G, Suskauer SJ, Slomine B. Clinical features of disorders of consciousness in young children. Arch Phys Med Rehabil. 2019;100(4):687–694. doi: 10.1016/j.apmr.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 84.Rodgin S, Suskauer SJ, Chen J, Katz E, Davis KC, Slomine BS. Very Long-term outcomes in children admitted in a disorder of consciousness after severe traumatic brain injury. Arch Phys Med Rehabil. 2021;102(8):1507–1513. doi: 10.1016/j.apmr.2021.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ludwig S, Suskauer S, Rodgin S, et al. Outcome measurement in children with a history of disorders of consciousness after severe brain injury: Telephone administration of the Vineland Adaptive Behavior Scales, Third Edition, and Glasgow Outcome Scale-Extended Pediatric Revision. Pediatr Crit Care Med. 2023;24(2):e76–e83. doi: 10.1097/PCC.0000000000003121.. [DOI] [PubMed] [Google Scholar]
- 86.Jafri A, Borda A, Slomine B, Suskauer S. Service Utilization and Unmet needs of Children and Young Adults with a History of Disorder of Consciousness after Brain Injury. Poster presented at the American Academy of Physical Medicine and Rehabilitation Conference, Baltimore, MD2022.
- 87.Edlow BL, Sanz LRD, Polizzotto L, et al. Therapies to restore consciousness in patients with severe brain injuries: a gap analysis and future directions. Neurocrit Care. 2021;35(Suppl 1):68–85. doi: 10.1007/s12028-021-01227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Tanti A, Saviola D, Basagni B, et al. Recovery of consciousness after 7 years in vegetative state of non-traumatic origin: a single case study. Brain Inj. 2016;30(8):1029–1034. doi: 10.3109/02699052.2016.1147078. [DOI] [PubMed] [Google Scholar]
- 89.Fink EL, Kochanek PM, Tasker RC, et al. International survey of critically ill children with acute neurologic insults: the prevalence of acute critical neurological disease in children: a global epidemiological assessment study. Pediatr Crit Care Med. 2017;18(4):330–342. doi: 10.1097/PCC.0000000000001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86(6):329–338. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 91.Russ JB, Simmons R, Glass HC. Neonatal encephalopathy: beyond hypoxic-ischemic encephalopathy. NeoReviews. 2021;22(3):e148–e162. doi: 10.1542/neo.22-3-e148. [DOI] [PubMed] [Google Scholar]
- 92.Lemmon ME, Boss RD, Bonifacio SL, Foster-Barber A, Barkovich AJ, Glass HC. Characterization of death in neonatal encephalopathy in the hypothermia era. J Child Neurol. 2017;32(4):360–365. doi: 10.1177/0883073816681904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schnakers C, Vanhaudenhuyse A, Giacino J, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009;9:35. doi: 10.1186/1471-2377-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ashwal S, Cranford R. The minimally conscious state in children. Semin Pediatr Neurol. 2002;9:19–34. doi: 10.1053/spen.2002.30334. [DOI] [PubMed] [Google Scholar]
- 95.Machado C, Korein J, Aubert E, et al. Recognizing a mother's voice in the persistent vegetative state. Clin EEG Neurosci. 2007;38(3):124–126. doi: 10.1177/155005940703800306. [DOI] [PubMed] [Google Scholar]
- 96.Snyman N, Egan JR, London K, et al. Zolpidem for persistent vegetative state–a placebo-controlled trial in pediatrics. Neuropediatrics. 2010;41(5):223–227. doi: 10.1055/s-0030-1269893. [DOI] [PubMed] [Google Scholar]
- 97.Avantaggiato P, Molteni E, Formica F, et al. Polysomnographic sleep patterns in children and adolescents in unresponsive wakefulness syndrome. J Head Trauma Rehabil. 2015;30(5):334–346. doi: 10.1097/htr.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 98.Chéliout-Heraut F, Rubinsztajn R, Ioos C, Estournet B. Prognostic value of evoked potentials and sleep recordings in the prolonged comatose state of children. Prelim Data Neurophysiol Clin. 2001;31(5):283–292. doi: 10.1016/s0987-7053(01)00270-2. [DOI] [PubMed] [Google Scholar]
- 99.Zieleniewska M, Duszyk A, Różański P, Pietrzak M, Bogotko M, Durka P. Parametric description of EEG profiles for assessment of sleep architecture in disorders of consciousness. Int J Neural Syst. 2019;29(3):1850049. doi: 10.1142/s0129065718500491. [DOI] [PubMed] [Google Scholar]
- 100.Mouthon AL, van Hedel HJA, Meyer-Heim A, et al. High-density electroencephalographic recordings during sleep in children with disorders of consciousness. Neuroimage Clin. 2016;11:468–475. doi: 10.1016/j.nicl.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parain D, Devaux AM, Proust B. Contribution of the EEG and evoked potentials in the prognosis of post-anoxic comas in children. Neurophysiol Clin. 1989;19(6):489–494. doi: 10.1016/s0987-7053(89)80005-x. [DOI] [PubMed] [Google Scholar]
- 102.Duszyk A, Dovgialo M, Pietrzak M, Zieleniewska M, Durka P. Event-related potentials in the odd-ball paradigm and behavioral scales for the assessment of children and adolescents with disorders of consciousness: A proof of concept study. Clin Neuropsychol. 2019;33(2):419–437. doi: 10.1080/13854046.2018.1555282. [DOI] [PubMed] [Google Scholar]
- 103.Steppacher I, Kaps M, Kissler J. Against the odds: a case study of recovery from coma after devastating prognosis. Ann Clin Transl Neurol. 2015;3(1):61–65. doi: 10.1002/can3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Molteni E, Rocca MA, Strazzer S, et al. A diffusion tensor magnetic resonance imaging study of paediatric patients with severe non-traumatic brain injury. Dev Med Child Neurol. 2017;59(2):199–206. doi: 10.1111/dmcn.13332. [DOI] [PubMed] [Google Scholar]
- 105.Errante A, Saviola D, Fasano F, et al. Application of an intensive rehabilitation program after very late recovery of consciousness: a single-case neurorehabilitation and neuroimaging study. J Cent Nerv Syst Dis. 2019;11:1179573519843492. doi: 10.1177/1179573519843492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stender J, Kupers R, Rodell A, et al. Quantitative rates of brain glucose metabolism distinguish minimally conscious from vegetative state patients. J Cereb Blood Flow Metab. 2015;35(1):58–65. doi: 10.1038/jcbfm.2014.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiaretti A, Conti G, Falsini B, et al. Intranasal Nerve Growth Factor administration improves cerebral functions in a child with severe traumatic brain injury: a case report. Brain Inj. 2017;31(11):1538–1547. doi: 10.1080/02699052.2017.1376760. [DOI] [PubMed] [Google Scholar]
- 108.Larsen PD, Gupta NC, Lefkowitz DM, Van Gundy JC, Hadford DJ. PET of infant in persistent vegetative state. Pediatr Neurol. 1993;9(4):323–326. doi: 10.1016/0887-8994(93)90074-m. [DOI] [PubMed] [Google Scholar]
- 109.Hu H, Cusack R, Naci L. Typical and disrupted brain circuitry for conscious awareness in full-term and preterm infants. Brain Commun. 2022;4(2):fcac071. doi: 10.1093/braincomms/fcac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Forsyth RJ, Salorio CF, Christensen JR. Modelling early recovery patterns after paediatric traumatic brain injury. Arch Dis Child. 2010;95(4):266–270. doi: 10.1136/adc.2008.147926. [DOI] [PubMed] [Google Scholar]
- 111.Kelly G, Mobbs S, Pritkin JN, et al. Gross motor function measure-66 trajectories in children recovering after severe acquired brain injury. Dev Med Child Neurol. 2015;57(3):241–247. doi: 10.1111/dmcn.12592. [DOI] [PubMed] [Google Scholar]
- 112.Molteni E, Ranzini MBM, Beretta E, Modat M, Strazzer S. Individualized prognostic prediction of the long-term functional trajectory in pediatric acquired brain injury. J Pers Med. 2021 doi: 10.3390/jpm11070675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Molteni E, Colombo K, Beretta E, et al. Comparison of multi-class machine learning methods for the identification of factors most predictive of prognosis in neurobehavioral assessment of pediatric severe disorder of consciousness through LOCFAS scale. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:269–272. doi: 10.1109/embc.2019.8856880. [DOI] [PubMed] [Google Scholar]
- 114.Monteiro M, Newcombe VFJ, Mathieu F, et al. Multiclass semantic segmentation and quantification of traumatic brain injury lesions on head CT using deep learning: an algorithm development and multicentre validation study. Lancet Digit Health. 2020;2(6):e314–e322. doi: 10.1016/s2589-7500(20)30085-6. [DOI] [PubMed] [Google Scholar]
- 115.Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14(4):180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 116.Roopun AK, Kramer MA, Carracedo LM, et al. Temporal interactions between cortical rhythms. Front Neurosci. 2008;2(2):145–154. doi: 10.3389/neuro.01.034.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cunningham MO, Pervouchine DD, Racca C, et al. Neuronal metabolism governs cortical network response state. Proc Natl Acad Sci. 2006;103(14):5597–5601. doi: 10.1073/pnas.0600604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang D, Raichle ME. Disease and the brain's dark energy. Nat Rev Neurol. 2010;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 119.Tort AB, Kramer MA, Thorn C, et al. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci U S A. 2008;105(51):20517–20522. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hahn TT, Sakmann B, Mehta MR. Phase-locking of hippocampal interneurons' membrane potential to neocortical up-down states. Nat Neurosci. 2006;9(11):1359–1361. doi: 10.1038/nn1788. [DOI] [PubMed] [Google Scholar]
- 121.Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009;8(12):982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Onimaru H, Arata A, Homma I, et al. Primary respiratory rhythm generator in the medulla of brainstem-spinal cord preparation from newborn rat. Brain Res. 1998;445(2):314–24. doi: 10.1016/0006-8993. [DOI] [PubMed] [Google Scholar]
- 123.Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423(6937):288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- 124.Reig R, Sanchez-Vives MV. Synaptic transmission and plasticity in an active cortical network. PLoS ONE. 2007;2(7):e670. doi: 10.1371/journal.pone.0000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beggs JM, Plenz D. Neuronal avalanches in neocortical circuits. J Neurosci. 2003;23(35):11167–11177. doi: 10.1523/jneurosci.23-35-11167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Giugliano M, Darbon P, Arsiero M, Lüscher HR, Streit J. Single-neuron discharge properties and network activity in dissociated cultures of neocortex. J Neurophysiol. 2004;92(2):977–996. doi: 10.1152/jn.00067.2004. [DOI] [PubMed] [Google Scholar]
- 127.Hoffman KL, Battaglia FP, Harris K, MacLean JN, Marshall L, Mehta MR. The upshot of up states in the neocortex: from slow oscillations to memory formation. J Neurosci. 2007;27(44):11838–11841. doi: 10.1523/jneurosci.3501-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Trasande CA, Ramirez JM. Activity deprivation leads to seizures in hippocampal slice cultures: is epilepsy the consequence of homeostatic plasticity? J Clin Neurophysiol. 2007;24(2):154–164. doi: 10.1097/WNP.0b013e318033787f. [DOI] [PubMed] [Google Scholar]
- 129.Wickham J, Corna A, Schwarz N, et al. Human cerebrospinal fluid induces neuronal excitability changes in resected human neocortical and hippocampal brain slices. Front Neurosci. 2020;14:283. doi: 10.3389/fnins.2020.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Busche MA, Kekuš M, Adelsberger H, et al. Rescue of long-range circuit dysfunction in Alzheimer's disease models. Nat Neurosci. 2015;18(11):1623–1630. doi: 10.1038/nn.4137. [DOI] [PubMed] [Google Scholar]
- 131.Benghanem S, Mazeraud A, Azabou E, et al. Brainstem dysfunction in critically ill patients. Crit Care. 2020;24(1):5. doi: 10.1186/s13054-019-2718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baguley IJ, Nicholls JL, Felmingham KL, Crooks J, Gurka JA, Wade LD. Dysautonomia after traumatic brain injury: a forgotten syndrome? J Neurol Neurosurg Psychiatry. 1999;67(1):39–43. doi: 10.1136/jnnp.67.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Semenza GL, Prabhakar NR. The role of hypoxia-inducible factors in carotid body (patho) physiology. J Physiol. 2018;596(15):2977–2983. doi: 10.1113/jp275696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Prabhakar NR, Semenza GL. Oxygen sensing and homeostasis. Physiology (Bethesda) 2015;30(5):340–348. doi: 10.1152/physiol.00022.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254(5032):726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ramirez JM, Karlen-Amarante M, Wang JJ, et al. The pathophysiology of rett syndrome with a focus on breathing dysfunctions. Physiology (Bethesda) 2020;35(6):375–390. doi: 10.1152/physiol.00008.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ramirez JM, Garcia AJ, 3rd, Anderson TM, et al. Central and peripheral factors contributing to obstructive sleep apneas. Respir Physiol Neurobiol. 2013;189(2):344–353. doi: 10.1016/j.resp.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Garcia AJ, 3rd, Koschnitzky JE, Dashevskiy T, Ramirez JM. Cardiorespiratory coupling in health and disease. Auton Neurosci. 2013;175(1–2):26–37. doi: 10.1016/j.autneu.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dyavanapalli J, Jameson H, Dergacheva O, Jain V, Alhusayyen M, Mendelowitz D. Chronic intermittent hypoxia-hypercapnia blunts heart rate responses and alters neurotransmission to cardiac vagal neurons. J Physiol. 2014;592(13):2799–2811. doi: 10.1113/jphysiol.2014.273482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Garcia AJ, 3rd, Dashevskiy T, Khuu MA, Ramirez JM. Chronic intermittent hypoxia differentially impacts different states of inspiratory activity at the level of the preBötzinger complex. Front Physiol. 2017;8:571. doi: 10.3389/fphys.2017.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Garcia AJ, 3rd, Zanella S, Dashevskiy T, et al. Chronic intermittent hypoxia alters local respiratory circuit function at the level of the preBötzinger complex. Front Neurosci. 2016;10:4. doi: 10.3389/fnins.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]