Abstract
Cannabinoids and the endocannabinoid system have been well established to play a crucial role in the regulation of the immune response. Also, emerging data from numerous investigations unravel the imperative role of gut microbiota and their metabolites in the maintenance of immune homeostasis and gut barrier integrity. In this review, we concisely report the immunosuppressive mechanisms triggered by cannabinoids, and how they are closely associated with the alterations in the gut microbiome and metabolome following exposure to endogenous or exogenous cannabinoids. We discuss how cannabinoid-mediated induction of microbial secondary bile acids, short chain fatty acids, and indole metabolites, produced in the gut, can suppress inflammation even in distal organs. While clearly, more clinical studies are necessary to establish the cross talk between exo- or endocannabinoid system with the gut microbiome and the immune system, the current evidence opens a new avenue of cannabinoid-gut-microbiota-based therapeutics to regulate immunological disorders.
Keywords: inflammation, cannabinoids, microbiota, drug abuse, endocannabinoids
Introduction
Cannabis sativa, otherwise known as marijuana, has a rich history of being used for medical and recreational purposes. The complex biochemical metabolism of cannabis leads to the production of over 550 chemical constituents of which over 100 are identified as phytocannabinoids (1). The chemical structure of the non-psychoactive cannabinoid compound, cannabidiol (CBD) was first deduced in 1963 followed by the identification of the psychoactive cannabinoid, δ9-tertrahydrocannabinol (THC), in 1964 (2). Cannabinoids accomplish their physiological and behavioral consequences via their binding to the cannabinoid G-protein-coupled receptors (GPCRs), CB1 and CB2 (CBRs). The existence of these receptors and their endogenous ligands, named endocannabinoids (eCBs), in the human system was discovered in the 1990s thus revealing the functions of eCB system in neuronal and immunomodulatory functions. eCBs such as anandamide (AEA) and 2-arachydonoylglycerol (2-AG), which are native lipid-based retrograde neurotransmitters, as well as exogenous cannabinoids such as THC, act as strong agonists of CBRs. CBD, unlike THC, is not psychoactive and considered to be a negative allosteric modulator of the CB1 receptors (3). Fatty acid amide hydrolase (FAAH) and monoacylglycerol acid lipase that break down AEA and 2–AG, respectively, are the two other important participants of the eCB signaling system (4-6). The CB1 and CB2 receptors are expressed primarily in the brain and immune cells respectively and mediate nearly all the effects of both endogenous and exogenous cannabinoids (6, 7).
The downstream signaling initiated by CBRs involves the inactivation of protein kinase A following inhibition of adenyl cyclase activity and a decrease in cyclic adenosine monophosphate (cAMP) levels. In addition, CBRs trigger an array of various mechanisms in the wake of activation. This includes multiple effector protein kinase signaling cascades related to cell proliferation and survival such as phosphoinositide-3-kinase–protein kinase B/Akt (PI3K-PKB/AKT), p38 mitogen-activated protein (p38 MAP) kinases, extracellular signal-regulated kinase (ERK) as well as focal adhesion kinase (FAK) (8). Coupling to ion channels, phospholipase-cb activation, and ceramide biosynthesis are certain other pathways activated by CBRs (7–11). It is demonstrated that eCBs can also bind to non-CBRs, of which the most investigated are transient receptor potential vanilloid 1 (TRPV1) channel along with peroxisome proliferator-activated receptor and orphan GPCRs (9, 12). Most of these pathways mentioned above are entwined with maintenance of immune homeostasis. For example, certain subsets of effector CD4+ T cells depend on the PI3K signaling activation for their differentiation and steering of effector functions. Additionally, PI3K and p38MAPK signaling are involved in the production of inflammatory cytokines (13, 14), ERK signaling plays a role in resistance to immunomodulatory drugs (15), and nuclear translocation of FAK is important to regulate inflammatory gene expression of chemokines and cytokines (16). Such studies demonstrate that the downstream signaling pathways initiated by CBRs can lead to immunomodulation.
It is well documented that the gut microbiome plays a crucial role in host metabolism as well as the balance between pro- and anti-inflammatory responses, thereby controlling disease pathogenesis (17). Short-chain fatty acids (SCFAs), lipopolysaccharides (LPS), and other biologically active metabolites generated by various microbial species contribute toward immune regulation, activation or suppression (18). Thus, dietary and medical interventions that manipulate the composition of the microbiome have been shown to cause pro- or anti-inflammatory milieu (19). There is emerging evidence that the gut microbiome and eCB system communicate via signaling pathways involved in nutrient processing and energy metabolism (17). In this mini-review, we briefly recite an account of the effects and mechanisms of endogenous and exogenous cannabinoids on immunosuppression via microbiome-mediated activities.
Mechanisms and Nature of Immunomodulation Caused by Cannabinoids
Cannabinoids are well established as anti-inflammatory agents with a significant and wide range of immunosuppressive properties that have been meticulously reviewed before (20–25). CBD, the nonpsychotic cannabinoid, was shown to induce myeloid-derived suppressor cells (MDSCs) which suppressed T cell proliferation in vitro and in vivo (26). MDSCs mostly express CD11b and Gr-1 and represent a heterogeneous population of immature myeloid cells which produce arginase 1 and inducible nitric oxide synthase that enables them to suppress T cell proliferation (27). Cannabinoid-induced MDSCs upon adoptive transfer were shown to attenuate LPS-induced acute inflammation in vivo (26, 28). The psychoactive THC was also shown to induce MDSCs independent of TLR4. THC mobilized MDSCs from bone marrow and caused their expansion in the periphery (29). In addition to the generation of MDSCs, cannabinoids have also been shown to induce regulatory T cells (Tregs) (30–33). Such T cells express FoxP3, a transcription factor that plays a critical role in their differentiation and functions, and secrete immunosuppressive cytokines such as interleukin (IL)-10 and transforming growth factor β (TGF-β) (34). Additionally, cannabinoids can also induce apoptosis of immune cells such as T and B lymphocytes, macrophages, and dendritic cells (DCs) leading to immunosuppression (35). THC triggered DC apoptosis via reduction of mitochondrial membrane permeability, cleavage of Bid, activation of caspase cascade, and release of cytochrome-c (36). THC treatment caused phosphorylation of IkappaB-alpha and augmented apoptotic gene transcription regulated by NF-kappaB (35–38). Moreover, agonists of CBRs can disrupt the balance of pro and anti-inflammatory cytokines. THC exposure restrained the production of IL2, IL-12, and interferon-gamma (IFN-γ), and altered the equilibrium of T helper 1 (Th1)/T helper 2 (Th2) cytokines in a CB2R dependent manner (39, 40). Also, THC and AEA were shown to suppress inflammatory Th1 and Th17 response during delayed-type hypersensitivity response (41, 42).
Epigenetic modulations are additional mechanisms of immunosuppression triggered by cannabinoids (43). The eCB system undergoes epigenetic modifications and such variations are observed in pathological disorders such as Diabetes, Parkinson’s, Alzheimer’s, and colorectal cancer. The main targets of these modifications are the genes CNR1 and CNR2 that encode for CB1R and CB2R along with FAAH (44–46). Recent investigations provide insights into cannabinoid-mediated epigenome modifications and their impact on the suppression of the immune system. THC treatment increased methylation of the promoter region of DNA methyl transferases, DNMT3a and DNMT3b in MDSCs leading to subsequent reduction in DNMT3a and DNMT3b expressions in C57BL/6 mice. Moreover, a decrease in the methylation of Arg1 and STAT3 promoter regions was observed that led to over-expression of Arg1 and STAT3 (47). THC was shown to activate or suppress the expression of genes via histone modifications. THC treatment led to histone modifications that led to increases in Th2 cytokine genes while suppressing Th1 cytokine genes, thereby switching the immune response from Th1 to Th2 (48).
Up-regulation or down-regulation of microRNAs (miRNAs) are another major route of epigenetic alterations prompted by cannabinoids. Treatment of C57BL/6 mice with THC elevated miR-21 while lowered miR-29b expression that was associated with a corresponding increase in SMAD7 and decrease in IFN-γ expressions. This in turn inhibited Th1/Th17 activation in delayed-type hypersensitivity reaction (47). The eCB, AEA mitigated Staphylococcal enterotoxin B (SEB)-induced acute respiratory distress syndrome (ARDS) in mice via down-regulation of miRNA-23a-3p, which up-regulated arginase and TGF-β2, and miRNA-34a-5p that prompted FoxP3 induction. A reduction in pro-inflammatory cytokines such as IL-2, TNF-α, and IFN-γ, while an increase in MDSCs was detected following AEA treatment (49). THC administration into SEB-injected C3H/HeJ mice was reported to down-regulate miR-17/92 and miR-374b/421 clusters while up-regulating miR146a leading to the release of PTEN thus acting as an AKT inhibitor leading to a reduction in IFN-γ production (31). Another study reported that THC altered expressions of the members of miR-17-92 cluster, particularly miR-18a that directed the release of PTEN (50). A detailed description of cannabinoid-mediated epigenetic modulations pertaining to immune suppression has been recently published (43). It is interesting to note that the intestinal microbiota and their metabolites have been shown to regulate several epigenetic pathways (51). This raises the question of whether the cannabinoid-mediated changes in the epigenetic pathways are linked to the gut microbiota.
Gut Microbiota, eCB System, and Gut-Brain Axis
The diverse intestinal microbial population found in the gut shares a mutual symbiotic relationship with the host. The microbiota benefits the host by modulation of gut motility, intestinal barrier function, and nutrient absorption. Moreover, gut microbiota plays a major role in host metabolism and is associated with regulation of the inflammatory status of the host not only in the gut but also in other organs such as the brain (52, 53). Thus, alterations in the microbiota, called dysbiosis, caused by nutrition, stress, environmental factors, and drugs, can have either beneficial or deleterious effects on the inflammatory status of the host. Gut-brain axis, which is the bidirectional crosstalk between the central and enteric nervous systems is influenced by gut microbiota via neural, endocrine, and immune networks (54). Emerging data establishes the influence of gut microbiota in anxiety and depression-like behavior (55). Clinical studies denote the abundance of pro-inflammatory and reduced SCFA producing bacterial species in these disease conditions, and this pathophysiology relates to the transmission of peripheral inflammation to the brain (56). There are recent reviews which have discussed the effects of cannabinoids, including CBD and alcohol in the microbiota-gut-brain axis (57) and therefore not further discussed this topic in this review.
It has been widely recognized that eCB is dynamically involved in the regulation of glucose and energy metabolism. It is also important to note that the immunomodulatory effects of eCBs are not always mediated via CBRs. Metabolism of 2-AG and AEA generates lipid components and hence acts as a source of arachidonic acid in the biosynthesis of additional pro-inflammatory lipids (58). Advanced research in the field of eCB system and immune modulation indicates the contribution of bio lipid members of eCB system in the onset or progression of various diseases such as obesity, diabetes, inflammatory bowel disease (IBD), and multiple sclerosis (MS) which are also reported to be augmented by alterations in the microbiota (59). Elevated eCBs level impedes excitatory and inhibitory neurotransmitters release which affects immune homeostasis and energy balance while increasing gut permeability (59, 60). Direct evidence of intestinal microbe-mediated eCB system manipulation comes from a recent study where Candida albicans manifestation altered the levels of lipid and eCBs in the brain and gastrointestinal (GI) tract leading to increased anxiety-like behavior in mice (61). Considering the relevance of eCB system and gut microbiota in the manipulation of the immune system, it is inevitable to explore the possible relationships and mechanisms between both systems from the perspective of inflammatory diseases.
Alteration of the Gut Microbiota by Cannabinoids
Various lines of ongoing research have connected the gut microbiota with metabolic and neurological disorders (52). Dietary interventions with specific fatty acids have been reported to increase the level of eCBs in human observational studies. These changes in eCBs have been attributed to variations in Peptostreptococcaceae, Veillonellaceae, and Akkermansiaceae (62). Cannabis consumption has been demonstrated to alter eCB tone and induce mucosal healing in ulcerative colitis (UC) patients in addition to improving quality of life (63). Modulation of eCB system using cannabinoids has been demonstrated to favor immune suppression in vivo (64). Present-day research targets to unravel the role of exogenous as well as endogenous cannabinoids in gut microbiota modulations and their impact on neurological and inflammatory conditions. Our lab has published multiple research articles on the alterations of microbiome and inflammation employing endogenous and exogenous cannabinoids (64, 65). A recent study demonstrated that the eCB, AEA, reversed the adverse microbiota perturbations instigated by SEB-mediated ARDS in mice. AEA treatment increased the abundance of beneficial bacteria producing SCFAs such as butyrate. In addition, AEA treatment curbed inflammation in the lungs and in the gut-associated mesenteric lymph node (MLN). Production of antimicrobial peptides (AMPs) and tight junction proteins (TJPs) which are key molecules sustaining epithelial barrier integrity in lung epithelial cells, as validated by single-cell RNA (Sc-RNA) sequencing were reported to attenuate the inflammation. Also, in this study, pathogenic Enterobacteriaceae and Pseudomonas were seen in the lungs of mice with ARDS while treatment with AEA led to their disappearance. Furthermore, the relative abundance of butyrate producing Lachnospiraceae and Clostridia were enhanced with AEA treatment (64). Emphasizing this observation, the abundance of butyrate-producing Firmicutes compared to Bacteroides was discovered following THC treatment of mice with diet-induced obesity (DIO) (66). In a similar line of study, the efficacy of THC to ease SEB-induced ARDS was examined. THC treatment improved the abundance of beneficial bacteria, Ruminococcus gnavus, while reducing pathogenic Akkermansia muciniphila in the lungs and gut. THC administration enriched SCFAs, specifically propionic acid, which attenuated the inflammatory response and protected mice from fatality. This study concluded that THC-induced reversal of microbial dysbiosis played a central role in the diminution of SEB-induced ARDS (65).
Colitis is another noteworthy disease model where the influence of cannabinoids on microbiota has been effectively demonstrated (67, 68). One study from our lab explored the effects of CBR activation following administration of THC and CBD either alone or in combination, in a chemically-induced murine colitis model. THC improved colonic barrier integrity as a result of higher mucus, AMPs, and TJPs production. Albeit alteration of the gut microbiota towards gram-negative bacteria was observed, the authors noted that the favorable effects of THC were not associated with microbiome modulation (67). A recent study presented the synergistic effect of fish oil and CBD treatment in the murine model of colitis. Co-administration of fish oil and CBD reduced inflammatory markers and ameliorated intestinal permeability in dextran sulfate sodium (DSS) model of mouse colitis. However, independent treatment with either of these failed to generate a favorable effect. The colonic inflammation was alleviated independent of the increased abundance of A. muciniphila. Of note is that the combination therapy reduced the abundance of Marinifilaceae, Desulfovibrionaceae, and Ruminococcaceae. Interestingly, Desulfovibrionaceae abundance has been reported in IBD and UC patients suggesting the functional role these microbial families play in GI diseases (68, 69). Another study investigated the role of gut microbiota in tempering clinical symptoms of paralysis and inflammation following cannabinoids treatment in an experimental autoimmune encephalomyelitis (EAE), a mouse model of MS. A combination of THC and CBD alleviated the symptoms of EAE and decreased pro-inflammatory cytokines while enhancing anti-inflammatory cytokine production. The EAE disease model showed abundant mucin degrading A. muciniphila which was considerably decreased following treatment with THC and CBD. A higher level of LPS was found in the brains of EAE mice while this scenario was reversed with cannabinoids treatment (70). The potential of cannabis extract to improve gut barrier function was investigated in the poultry industry where necrotic enteritis caused by Clostridium perfringens caused mortality in birds leading to economic loss along with the potential hazard of pathogen transmission to the consumer via the food chain. A combination of cannabis extract and selenium nanoparticles altered the response of chickens towards C. perfringens. This treatment upregulated the expression of genes involved in gut barrier function and improved collagenase activity. However, the extract alone could not generate significant beneficial effects (71).
Synthetic cannabinoids have also been extensively studied for their anti-inflammatory properties and their ability to alter the gut microbiota. Treatment with the CB2R agonist, JWH133 alleviated overgrowth of bacteria, bacterial translocation, and bacterial peritonitis, up-regulated intestinal TJPs, and reduced intestinal oxidative stress in cirrhotic rats. Furthermore, the treatment considerably diminished the levels of TNFα and inflammatory facilitators, intestinal mucosal impairment, and infection (72). Blockade of CB1R using the antagonist, Rimonabant reduced DIO and inflammatory cytokines. Trafficking of M1 macrophages and decreased intestinal permeability were also observed with CB1R blocking. Further metagenomics analysis demonstrated an elevated relative abundance of A. muciniphila and reduced abundance of Lanchnospiraceae and Erysipelotrichaceae in the gut (73). Nabilone, a CB1R agonist, was found useful in the treatment of post-traumatic stress disorder (PTSD), nausea, and vomiting associated with chemotherapy and pain management (74, 75). Administration of nabilone for 3 months improved health and alleviated diarrheal symptoms in patients. Although microbial dysbiosis was not investigated in this study, it encourages further clinically-oriented investigations on the effect of cannabinoids on such disease models as related to microbial dysbiosis (76).
Immunomodulatory Mechanisms of Gut Microbiota
Endogenous, as well as exogenous cannabinoids, have been widely recognized to regulate inflammation and mucosal permeability of the GI tract where they possibly interact with the gut microbiome. In this section, we have tried to pull together the known mechanisms through which cannabinoids control microbial dysbiosis and accompanying inflammation. The indispensable role of gut microbiota on immune regulation has been excellently validated by multiple investigations. For example, one such study demonstrated the ability of commensal segmented filamentous bacterium (SFB) to induce CD4+ T cells to produce IL-17 and IL-22 in the lamina propria of mice. SFB adhered to the Th17 cells and induced inflammation and production of antimicrobial defensins (77). In the gut, under normal circumstances, eCB system is regulated by CB1R. However, both CB1R and CB2R get activated during inflammation leading to anti-inflammatory cytokine production that suppresses inflammation and intestinal damage (78).
The crosstalk between eCB system and gut microbiota has been established with murine models of obesity where low-grade inflammation and increased eCB system tone are reported. Obese mice exhibited higher colonic CB1R colonic mRNA and the modulation of gut microbiota with the use of prebiotics reduced this scenario. Moreover, prebiotic treatment alleviated CB1R mRNA and concentration of AEA in genetically obese mice explaining the involvement of the gut microbial community on CB1R and eCB expression. The same study disclosed the maintenance of intestinal barrier integrity by eCB system (79). Reduction in the number of TJPs increases the space between epithelial cells promoting paracellular translocation of microbial metabolites from the intestinal lumen to circulation and other organs, and elevated LPS levels impair adipogenesis and promote inflammation (79). THC administration has been reported to reduce LPS levels in mice while increasing TJPs. THC mediated CBR modulation reduced plasma LPS levels by altering the distribution and localization of TJPs which led to improved gut barrier function (65, 70).
Microbial-derived SCFAs, neurotransmitters, and amino acids take part in the immune, endocrinal, and neuronal signaling pathways via binding to host receptors (80, 81). Multiple investigations have validated the potential of AEA and THC to enhance the levels of SCFAs and AMPs in murine models of inflammation (64–67). SCFAs are produced in the colon by fermentation and subsequent degradation of undigested dietary fibers by gut harboring bacteria and they contribute to the regulation of both innate and adaptive immunity of the host. Acetate and propionate, produced by Bacteroidetes, and butyrate, produced by Firmicutes are the major SCFAs involved in host-bacterial communications (82). Blocking of histone deacetylases (HDAC) and activation of GPCRs are two main signaling pathways modulated by microbial SCFAs (80–82). Interestingly, GPCRs such as GPR43 and GPR109A are expressed by adipose tissue macrophages and dendritic cells (DCs). The binding of SCFAs to these receptors induces K+ efflux and membrane hyperpolarization which in turn stimulates NLRP3 inflammasome in primed macrophages to produce IL-18 (83, 84). Butyrate-dependent activation of GPR109A induces apoptosis of colon cancer cells. Furthermore, these receptor/ligand complexes inhibit nuclear factor-kappaB (NF-κB) activation in the colon of mice (85). Butyrate enhanced the function of human TGFβ1 in the intestinal epithelial cells (IECs) which in turn directed the accumulation of Treg cells in the lumen, and the study suggested inhibition of HDAC as the major mechanism behind this activity. Butyrate-induced HDAC inhibition down-regulated the generation of LPS-triggered pro-inflammatory cytokines such as IL-6 and IL-12 (86, 87). Another study demonstrated that butyrate enhanced the expression of AMPs, LL-37, and CAP-18 by IECs in rabbits (88). In a similar manner, activation of genes encoding host defense peptides in HD11 macrophages and monocytes has been observed in chickens following butyrate consumption (89). Succinate, another SCFA produced by the gut bacteria, Prevotella copri was shown to be involved in gut gluconeogenesis and improved glucose homeostasis (90). Once transported into circulation, SCFAs exert their effect on distant organs as well. For example, circulating propionate modified bone marrow hematopoiesis by increasing levels of macrophages and DCs precursors. The phagocytic DCs invaded the lung but lacked Th2 effector cell differentiation ability and controlled inflammation (91). SCFAs, as evident from numerous studies, represent the most important connecting link between gut microbiome and host immune homeostasis.
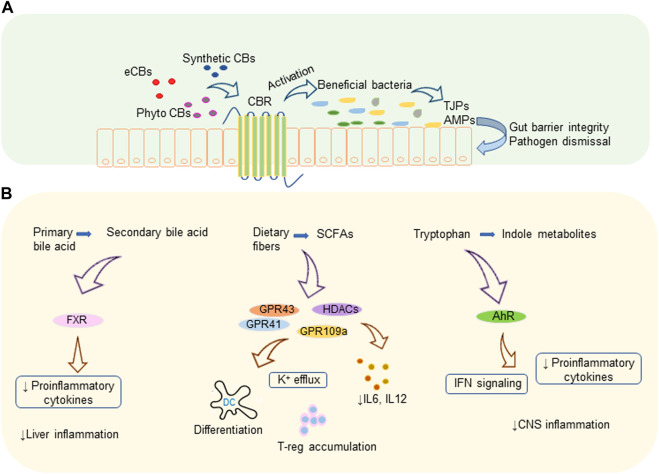
Bile acid metabolism is another activity implemented by a variety of gut microbes harboring the gut. Microbes convert primary bile acid to secondary and tertiary bile acids via various mechanisms that include deconjugation of glycine and taurine by bile salt hydrolase, de-hydroxylation as well as dehydrogenation and epimerization of cholesterol core (92). Members of the genera Bifidobacterium, Clostridium, and Lactobacillus are reported to efficiently metabolize primary bile acids (92, 93). Secondary bile acid metabolism and prevention of bile acid production in the liver by activating nuclear receptor farnesoid X receptor (FXR) in the ileum by gut microbiota controls liver inflammation. Also, intestinal microbiota decreases the levels of pro-inflammatory cytokines which are involved in reducing the transcription of FXR target genes (94). Proteins and peptides in the diets are digested to free amino acids as a result of microbial fermentation and major amino acid of such kind is tryptophan. Tryptophan metabolites are another set of biologically active metabolites generated by intestinal bacteria that affect intestinal epithelial barrier integrity as well as the organogenesis of intestinal lymphoid follicles. Members of the phylum Firmicutes convert tryptophan to tryptamine and other indole derivatives (95, 96). A recent study showed that tryptamine can attenuate neuroinflammation in the murine model of MS (97). Lactobacillus strains were found to efficiently metabolize Tryptophan to its derivatives which act as aryl hydrocarbon receptor (AhR) ligands in the colitis mouse model (98). These metabolites, mostly indoles, act as AhR agonists and regulate type-1 IFN signaling in astrocytes leading to suppression of central nervous system (CNS) inflammation (99). AhR signaling mediates IL-22 production in the gut by activating innate lymphoid cell 3 (ILC3) (100). In addition, AhR has been shown to play a vital role in the development of ILC and intraepithelial lymphocytes (100, 101). How AhR activation leads to suppression of inflammation has been the topic of recent reviews (102, 103). Figure 1 illustrates a summary of cannabinoid mediated microbiome modulation and the immunomodulatory mechanism of microbial metabolites.
FIGURE 1.
Cannabinoids and gut microbiota. (A) Cannabinoid mediated microbiome modulation: endogenous or exogenous cannabinoids increase the beneficial bacteria which produce TJPs that improve gut barrier integrity and AMPs that eliminate pathogens. (B) Immunomodulatory mechanisms of microbial metabolites: microbiota generated secondary bile acids, SCFAs, and indole metabolites modulate various receptors leading to decreased pro-inflammatory cytokines and immune suppression. AhR, aryl hydrocarbon receptor; AMP, antimicrobial protein; CBR, cannabinoid receptor; CBs, cannabinoids; CNS, central nervous system; eCBs, endocannabinoids; FXR, farnesoid X receptor; GPR, G-protein-coupled receptors; HDACs, histone deacetylases; IFN, interferon; IL, interleukin; K, potassium; TJP, tight junction proteins; T-reg, regulatory T cell.
While most of the studies that we have reviewed above have shown an association between the administration of cannabinoids and suppression of inflammation to changes in the microbiota, one can question whether these studies merely indicate a relationship between these events or whether the cannabinoid-mediated alterations in the microbiota are actually responsible for inducing attenuation of inflammation. The association between microbial changes seen following exposure to cannabinoids and the consequent impact of such changes in immunomodulation can only be proven through fecal microbiota transplants (FMT).
There is evidence to suggest the role of microbiota on eCB signaling through use of FMT. Multiple studies reported that FMT-mediated microbial dysbiosis can modify eCB signaling (104, 105). One study clearly investigated the impact of FMT from conventionally raised mice to germ-free mice. Endocannabinoidome gene expression and lipidomics were analyzed by transcriptomics and LC-MS/MS before and after FMT. Age-dependent endocannabinoidome gene expression and lipid variations in the germ-free mice were reversed following FMT from age-matched conventionally raised donor mice (106). In another study, FMT from murine models of EAE disease treated with THC and CBD, into antibiotics treated, microbe depleted mice demonstrated that the recipient mice showed decreased EAE disease severity (70). A similar kind of study was conducted in a murine model of SEB-mediated ARDS. The microbiota transplanted from THC-treated ARDS mice into antibiotic-treated, microbiome-depleted recipient mice showed better survival from ARDS than those that received FMT from the control group. FMT from THC-treated groups caused a decrease in inflammatory CD4+ and CD8+ T cells and an increase in immune suppressive MDSCs and Tregs in the lungs (65). Such studies clearly demonstrate that endogenous and exogenous cannabinoids can promote beneficial microbiota in the gut that can attenuate inflammatory diseases even in distal organs.
Conclusion
The communications among eCB system, immune regulation, and gut microbiota are intricately interconnected. CBRs agonists/antagonists have been pre-clinically validated to be useful in the treatment of metabolic conditions, such as obesity and diabetes as well as in disease models of colitis and cardiometabolic malfunctions. Also, well-established is the role of intestinal microbial community in the onset or progression of these disorders. The numerous groups of microbial clusters and the myriad of biologically active metabolites produced by them along with their receptors trigger extensive signaling pathways that affect the energy balance and immune homeostasis of the host. The microbiome-eCB signaling modulation exploiting exo- or endogenous cannabinoids opens a new avenue of cannabinoid-gut microbiota-based therapeutics to curb metabolic and immune-oriented conditions. However, more clinical investigations are essential to validate this concept.
Funding Statement
This work was supported in part by NIH grants: R01ES019313, R01MH094755, R01AI123947, R01AI129788, R01AT006888, P01AT003961, and P20GM103641.
Author Contributions
KKV prepared the draft, PN and MN provided the concept and edited the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
- 2-AG
2-arachydonoylglycerol
- AEA
anandamide
- AhR
aryl hydrocarbon receptor
- AMPs
antimicrobial peptides
- ARDS
acute respiratory distress syndrome
- cAMP
cyclic adenosine monophosphate
- CBD
cannabidiol
- CNS
central nervous system
- CBR
cannabinoid receptors
- DCs
dendritic cells
- DIO
diet-induced obesity
- DNMT
DNA methyl transferases
- DSS
dextran sulfate sodium
- EAE
experimental autoimmune encephalomyelitis
- ERK
extracellular signal-regulated kinase
- FAAH
fatty acid amide hydrolase
- FAK
focal adhesion kinase
- FMT
fecal microbiota transplants
- FXR
farnesoid X receptor
- GPCRs
G-protein-coupled receptors
- GI
gastrointestinal
- HDAC
histone deacetylase
- IBD
inflammatory bowel disease
- IECs
intestinal epithelial cells
- IFN-γ
interferon-gamma
- IL
interleukin
- ILC3
innate lymphoid cell 3
- LPS
lipopolysaccharides
- MDSCs
myeloid-derived suppressor cells
- miRNAs
microRNAs
- MLN
mesenteric lymph node
- MS
multiple sclerosis
- PI3K-PKB/AKT
phosphoinositide-3-kinase–protein kinase B/Akt
- p38 MAP
p38 mitogen-activated protein
- PTSD
post-traumatic stress disorder
- SCFAs
short-chain fatty acids
- Sc-RNA
single-cell RNA
- SEB
staphylococcal enterotoxin B
- SFB
segmented filamentous bacterium
- TGF-β
transforming growth factor β
- Th1
T helper 1
- Th2
T helper 2
- THC
δ9-tertrahydrocannabinol
- TJPs
tight junction proteins
- Tregs
regulatory T cells
- TRPV1
transient receptor potential vanilloid 1
- UC
ulcerative colitis
References
- 1. Boggs DL, Nguyen JD, Morgenson D, Taffe MA, Ranganathan M. Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Δ9-Tetrahydrocannabinol. Neuropsychopharmacol. (2018) 43(1):142–54. 10.1038/npp.2017.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pertwee RG. Cannabinoid Pharmacology: the First 66 Years. Br J Pharmacol (2006) 147(Suppl. 1):S163–71. 10.1038/sj.bjp.0706406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tham M, Yilmaz O, Alaverdashvili M, Kelly MEM, Denovan-Wright EM, Laprairie RB. Allosteric and Orthosteric Pharmacology of Cannabidiol and Cannabidiol-Dimethylheptyl at the Type 1 and Type 2 Cannabinoid Receptors. Br J Pharmacol (2019) 176(10):1455–69. 10.1111/bph.14440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Munro S, Thomas KL, Abu-Shaar M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature (1993) 365(6441):61–5. 10.1038/365061a0 [DOI] [PubMed] [Google Scholar]
- 5. Devane WA, Hanuš L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and Structure of a Brain Constituent that Binds to the Cannabinoid Receptor. Science (1992) 258(5090):1946–9. 10.1126/science.1470919 [DOI] [PubMed] [Google Scholar]
- 6. Marzo VD, Bifulco M, Petrocellis LD. The Endocannabinoid System and its Therapeutic Exploitation. Nat Rev Drug Discov (2004) 3(9):771–84. 10.1038/nrd1495 [DOI] [PubMed] [Google Scholar]
- 7. Demuth DG, Molleman A. Cannabinoid Signalling. Life Sci (2006) 78(6):549–63. 10.1016/j.lfs.2005.05.055 [DOI] [PubMed] [Google Scholar]
- 8. Derkinderen P, Toutant M, Burgaya F, Le Bert M, Siciliano JC, de Franciscis V, et al. Regulation of a Neuronal Form of Focal Adhesion Kinase by Anandamide. Science (1996) 273(5282):1719–22. 10.1126/science.273.5282.1719 [DOI] [PubMed] [Google Scholar]
- 9. McAllister SD, Glass M. CB(1) and CB(2) Receptor-Mediated Signalling: a Focus on Endocannabinoids. Prostaglandins Leukot Essent Fatty Acids (2002) 66(2-3):161–71. 10.1054/plef.2001.0344 [DOI] [PubMed] [Google Scholar]
- 10. Piomelli D. The Molecular Logic of Endocannabinoid Signalling. Nat Rev Neurosci (2003) 4(11):873–84. 10.1038/nrn1247 [DOI] [PubMed] [Google Scholar]
- 11. Derkinderen P, Valjent E, Toutant M, Corvol J-C, Enslen H, Ledent C, et al. Regulation of Extracellular Signal-Regulated Kinase by Cannabinoids in hippocampus. J Neurosci (2003) 23(6):2371–82. 10.1523/jneurosci.23-06-02371.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pertwee RG, Ross RA. Cannabinoid Receptors and Their Ligands. Prostaglandins Leukot Essent Fatty Acids (2002) 66(2):101–21. 10.1054/plef.2001.0341 [DOI] [PubMed] [Google Scholar]
- 13. Cuenda A, Rousseau S. p38 MAP-Kinases Pathway Regulation, Function and Role in Human Diseases. Biochim Biophys Acta (Bba) - Mol Cell Res (2007) 1773(8):1358–75. 10.1016/j.bbamcr.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 14. Han JM, Patterson SJ, Levings MK. The Role of the PI3K Signaling Pathway in CD4+ T Cell Differentiation and Function. Front Immun (2012) 3:245. 10.3389/fimmu.2012.00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu J, Hideshima T, Xing L, Wang S, Zhou W, Samur MK, et al. ERK Signaling Mediates Resistance to Immunomodulatory Drugs in the Bone Marrow Microenvironment. Sci Adv (2021) 7(23). 10.1126/sciadv.abg2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Canel M, Taggart D, Sims AH, Lonergan DW, Waizenegger IC, Serrels A. T-cell Co-stimulation in Combination with Targeting FAK Drives Enhanced Anti-tumor Immunity. eLife (2020) 9. 10.7554/eLife.48092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iannotti FA, Di Marzo V. The Gut Microbiome, Endocannabinoids and Metabolic Disorders. J Endocrinol (2021) 248(2):R83–r97. 10.1530/joe-20-0444 [DOI] [PubMed] [Google Scholar]
- 18. Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The Gut Microbiota and Inflammation: An Overview. Int J Environ Res Public Health (2020) 17(20). 10.3390/ijerph17207618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bolte LA, Vich Vila A, Imhann F, Collij V, Gacesa R, Peters V, et al. Long-term Dietary Patterns Are Associated with Pro-inflammatory and Anti-inflammatory Features of the Gut Microbiome. Gut (2021) 70(7):1287–98. 10.1136/gutjnl-2020-322670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M. Cannabinoids as Novel Anti-inflammatory Drugs. Future Med Chem (2009) 1(7):1333–49. 10.4155/fmc.09.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giacobbe J, Marrocu A, Di Benedetto MG, Pariante CM, Borsini A. A Systematic, Integrative Review of the Effects of the Endocannabinoid System on Inflammation and Neurogenesis in Animal Models of Affective Disorders. Brain Behav Immun (2021) 93:353–67. 10.1016/j.bbi.2020.12.024 [DOI] [PubMed] [Google Scholar]
- 22. Dong C, Chen J, Harrington A, Vinod KY, Hegde ML, Hegde VL. Cannabinoid Exposure during Pregnancy and its Impact on Immune Function. Cell Mol Life Sci (2019) 76(4):729–43. 10.1007/s00018-018-2955-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joshi N, Onaivi ES. Endocannabinoid System Components: Overview and Tissue Distribution. Adv Exp Med Biol (2019) 1162:1–12. 10.1007/978-3-030-21737-2_1 [DOI] [PubMed] [Google Scholar]
- 24. Young AP, Denovan-Wright EM. The Dynamic Role of Microglia and the Endocannabinoid System in Neuroinflammation. Front Pharmacol (2021) 12:806417. 10.3389/fphar.2021.806417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu D, Gao F, Chen C. Endocannabinoid Metabolism and Traumatic Brain Injury. Cells (2021) 10(11). 10.3390/cells10112979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elliott DM, Singh N, Nagarkatti M, Nagarkatti PS. Cannabidiol Attenuates Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis through Induction of Myeloid-Derived Suppressor Cells. Front Immunol (2018) 9:1782. 10.3389/fimmu.2018.01782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karin N. The Development and Homing of Myeloid-Derived Suppressor Cells: From a Two-Stage Model to a Multistep Narrative. Front Immunol (2020) 11:557586. 10.3389/fimmu.2020.557586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hegde VL, Singh UP, Nagarkatti PS, Nagarkatti M. Critical Role of Mast Cells and Peroxisome Proliferator-Activated Receptor γ in the Induction of Myeloid-Derived Suppressor Cells by Marijuana Cannabidiol In Vivo . J.I. (2015) 194(11):5211–22. 10.4049/jimmunol.1401844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hegde VL, Nagarkatti M, Nagarkatti PS. Cannabinoid Receptor Activation Leads to Massive Mobilization of Myeloid-Derived Suppressor Cells with Potent Immunosuppressive Properties. Eur J Immunol (2010) 40(12):3358–71. 10.1002/eji.201040667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Becker W, Alrafas HR, Wilson K, Miranda K, Culpepper C, Chatzistamou I, et al. Activation of Cannabinoid Receptor 2 Prevents Colitis-Associated Colon Cancer through Myeloid Cell De-activation Upstream of IL-22 Production. iScience (2020) 23(9):101504. 10.1016/j.isci.2020.101504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rao R, Nagarkatti PS, Nagarkatti M. Δ9Tetrahydrocannabinol Attenuates Staphylococcal Enterotoxin B-Induced Inflammatory Lung Injury and Prevents Mortality in Mice by Modulation of miR-17-92 Cluster and Induction of T-Regulatory Cells. Br J Pharmacol (2015) 172(7):1792–806. 10.1111/bph.13026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berg BB, Soares JS, Paiva IR, Rezende BM, Rachid MA, Cau SBd A, et al. Cannabidiol Enhances Intestinal Cannabinoid Receptor Type 2 Receptor Expression and Activation Increasing Regulatory T Cells and Reduces Murine Acute Graft-Versus-Host Disease without Interfering with the Graft-Versus-Leukemia Response. J Pharmacol Exp Ther (2021) 377(2):273–83. 10.1124/jpet.120.000479 [DOI] [PubMed] [Google Scholar]
- 33. Angelina A, Pérez‐Diego M, Maldonado A, Rückert B, Akdis M, Martín‐Fontecha M, et al. The Cannabinoid WIN55212‐2 Suppresses Effector T‐cell Responses and Promotes Regulatory T Cells in Human Tonsils. Allergy (2022) 77(3):1029–32. 10.1111/all.15160 [DOI] [PubMed] [Google Scholar]
- 34. Schmidt A, Oberle N, Krammer PH. Molecular Mechanisms of Treg-Mediated T Cell Suppression. Front Immun (2012) 3:51. 10.3389/fimmu.2012.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lombard C, Nagarkatti M, Nagarkatti P. CB2 Cannabinoid Receptor Agonist, JWH-015, Triggers Apoptosis in Immune Cells: Potential Role for CB2-Selective Ligands as Immunosuppressive Agents. Clin Immunol (2007) 122(3):259–70. 10.1016/j.clim.2006.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mohammed A, F K Alghetaa H, Miranda K, Wilson K, Cai G, P Singh N, et al. Δ9-Tetrahydrocannabinol Prevents Mortality from Acute Respiratory Distress Syndrome through the Induction of Apoptosis in Immune Cells, Leading to Cytokine Storm Suppression. Int J Mol Sci (2020) 21(17). 10.3390/ijms21176244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Do Y, McKallip RJ, Nagarkatti M, Nagarkatti PS. Activation through Cannabinoid Receptors 1 and 2 on Dendritic Cells Triggers NF-kappaB-dependent Apoptosis: Novel Role for Endogenous and Exogenous Cannabinoids in Immunoregulation. J Immunol (2004) 173(4):2373–82. 10.4049/jimmunol.173.4.2373 [DOI] [PubMed] [Google Scholar]
- 38. Singh UP, Singh NP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Cannabinoid Receptor-2 (CB2) Agonist Ameliorates Colitis in IL-10−/− Mice by Attenuating the Activation of T Cells and Promoting Their Apoptosis. Toxicol Appl Pharmacol (2012) 258(2):256–67. 10.1016/j.taap.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yuan M, Kiertscher SM, Cheng Q, Zoumalan R, Tashkin DP, Roth MD. Delta 9-Tetrahydrocannabinol Regulates Th1/Th2 Cytokine Balance in Activated Human T Cells. J Neuroimmunol (2002) 133(1-2):124–31. 10.1016/s0165-5728(02)00370-3 [DOI] [PubMed] [Google Scholar]
- 40. Klein TW, Newton CA, Nakachi N, Friedman H. Delta 9-tetrahydrocannabinol Treatment Suppresses Immunity and Early IFN-Gamma, IL-12, and IL-12 Receptor Beta 2 Responses to Legionella pneumophila Infection. J Immunol (2000) 164(12):6461–6. 10.4049/jimmunol.164.12.6461 [DOI] [PubMed] [Google Scholar]
- 41. Sido JM, Jackson AR, Nagarkatti PS, Nagarkatti M. Marijuana-derived Δ-9-tetrahydrocannabinol Suppresses Th1/Th17 Cell-Mediated Delayed-type Hypersensitivity through microRNA Regulation. J Mol Med (2016) 94(9):1039–51. 10.1007/s00109-016-1404-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jackson AR, Nagarkatti P, Nagarkatti M. Anandamide Attenuates Th-17 Cell-Mediated Delayed-type Hypersensitivity Response by Triggering IL-10 Production and Consequent microRNA Induction. PloS one (2014) 9(4):e93954. 10.1371/journal.pone.0093954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holloman BL, Nagarkatti M, Nagarkatti P. Epigenetic Regulation of Cannabinoid-Mediated Attenuation of Inflammation and its Impact on the Use of Cannabinoids to Treat Autoimmune Diseases. Int J Mol Sci (2021) 22(14). 10.3390/ijms22147302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. D'Addario C, Di Francesco A, Arosio B, Gussago C, Dell'Osso B, Bari M, et al. Epigenetic Regulation of Fatty Acid Amide Hydrolase in Alzheimer Disease. PloS one (2012) 7(6):e39186. 10.1371/journal.pone.0039186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meccariello R, Santoro A, D'Angelo S, Morrone R, Fasano S, Viggiano A, et al. The Epigenetics of the Endocannabinoid System. Int J Mol Sci (2020) 21(3). 10.3390/ijms21031113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gomes TM, Dias da Silva D, Carmo H, Carvalho F, Silva JP. Epigenetics and the Endocannabinoid System Signaling: An Intricate Interplay Modulating Neurodevelopment. Pharmacol Res (2020) 162:105237. 10.1016/j.phrs.2020.105237 [DOI] [PubMed] [Google Scholar]
- 47. Sido JM, Yang X, Nagarkatti PS, Nagarkatti M. Δ9 -Tetrahydrocannabinol-Mediated Epigenetic Modifications Elicit Myeloid-Derived Suppressor Cell Activation via STAT3/S100A8. J Leukoc Biol (2015) 97(4):677–88. 10.1189/jlb.1a1014-479r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang X, Hegde VL, Rao R, Zhang J, Nagarkatti PS, Nagarkatti M. Histone Modifications Are Associated with Δ9-Tetrahydrocannabinol-mediated Alterations in Antigen-specific T Cell Responses. J Biol Chem (2014) 289(27):18707–18. 10.1074/jbc.m113.545210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sultan M, Alghetaa H, Mohammed A, Abdulla OA, Wisniewski PJ, Singh N, et al. The Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome by Downregulating miRNA that Target Inflammatory Pathways. Front Pharmacol (2021) 12:644281. 10.3389/fphar.2021.644281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang X, Bam M, Nagarkatti PS, Nagarkatti M. RNA-seq Analysis of δ9-Tetrahydrocannabinol-treated T Cells Reveals Altered Gene Expression Profiles that Regulate Immune Response and Cell Proliferation. J Biol Chem (2016) 291(30):15460–72. 10.1074/jbc.m116.719179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu Y, Wang CZ, Wan JY, Yao H, Yuan CS. Dissecting the Interplay Mechanism between Epigenetics and Gut Microbiota: Health Maintenance and Disease Prevention. Int J Mol Sci (2021) 22(13). 10.3390/ijms22136933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu H-J, Wu E. The Role of Gut Microbiota in Immune Homeostasis and Autoimmunity. Gut microbes (2012) 3(1):4–14. 10.4161/gmic.19320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dopkins N, Nagarkatti PS, Nagarkatti M. The Role of Gut Microbiome and Associated Metabolome in the Regulation of Neuroinflammation in Multiple Sclerosis and its Implications in Attenuating Chronic Inflammation in Other Inflammatory and Autoimmune Disorders. Immunology (2018) 154(2):178–85. 10.1111/imm.12903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carabotti M, Scirocco A, Maselli MA, Severi C. The Gut-Brain axis: Interactions between Enteric Microbiota, central and Enteric Nervous Systems. Ann Gastroenterol (2015) 28(2):203–9. [PMC free article] [PubMed] [Google Scholar]
- 55. Tian P, Wang G, Zhao J, Zhang H, Chen W. Bifidobacterium with the Role of 5-hydroxytryptophan Synthesis Regulation Alleviates the Symptom of Depression and Related Microbiota Dysbiosis. J Nutr Biochem (2019) 66:43–51. 10.1016/j.jnutbio.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 56. Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM. The Gut Microbiota in Anxiety and Depression - A Systematic Review. Clin Psychol Rev (2021) 83:101943. 10.1016/j.cpr.2020.101943 [DOI] [PubMed] [Google Scholar]
- 57. Karoly HC, Mueller RL, Bidwell LC, Hutchison KE. Cannabinoids and the Microbiota-Gut-Brain Axis: Emerging Effects of Cannabidiol and Potential Applications to Alcohol Use Disorders. Alcohol Clin Exp Res (2020) 44(2):340–53. 10.1111/acer.14256 [DOI] [PubMed] [Google Scholar]
- 58. Khan RN, Maner-Smith K, A Owens J, Barbian ME, M Jones R, R Naudin C. At the Heart of Microbial Conversations: Endocannabinoids and the Microbiome in Cardiometabolic Risk. Gut microbes (2021) 13(1):1–21. 10.1080/19490976.2021.1911572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mestre L, Carrillo-Salinas FJ, Mecha M, Feliú A, Guaza C. Gut Microbiota, Cannabinoid System and Neuroimmune Interactions: New Perspectives in Multiple Sclerosis. Biochem Pharmacol (2018) 157:51–66. 10.1016/j.bcp.2018.08.037 [DOI] [PubMed] [Google Scholar]
- 60. Cani PD, Plovier H, Van Hul M, Geurts L, Delzenne NM, Druart C, et al. Endocannabinoids--at the Crossroads between the Gut Microbiota and Host Metabolism. Nat Rev Endocrinol (2016) 12(3):133–43. 10.1038/nrendo.2015.211 [DOI] [PubMed] [Google Scholar]
- 61. Markey L, Hooper A, Melon LC, Baglot S, Hill MN, Maguire J, et al. Colonization with the Commensal Fungus Candida Albicans Perturbs the Gut-Brain axis through Dysregulation of Endocannabinoid Signaling. Psychoneuroendocrinology (2020) 121:104808. 10.1016/j.psyneuen.2020.104808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Castonguay-Paradis S, Lacroix S, Rochefort G, Parent L, Perron J, Martin C, et al. Dietary Fatty Acid Intake and Gut Microbiota Determine Circulating Endocannabinoidome Signaling beyond the Effect of Body Fat. Scientific Rep (2020) 10(1):15975. 10.1038/s41598-020-72861-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tartakover Matalon S, Azar S, Meiri D, Hadar R, Nemirovski A, Abu Jabal N, et al. Endocannabinoid Levels in Ulcerative Colitis Patients Correlate with Clinical Parameters and Are Affected by Cannabis Consumption. Front Endocrinol (2021) 12:685289. 10.3389/fendo.2021.685289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sultan M, Wilson K, Abdulla OA, Busbee PB, Hall A, Carter T, et al. Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome through Modulation of Microbiome in the Gut-Lung Axis. Cells (2021) 10(12). 10.3390/cells10123305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mohammed A, Alghetaa HK, Zhou J, Chatterjee S, Nagarkatti P, Nagarkatti M. Protective Effects of Δ(9) -tetrahydrocannabinol against Enterotoxin-Induced Acute Respiratory Distress Syndrome Are Mediated by Modulation of Microbiota. Br J Pharmacol (2020) 177(22):5078–95. 10.1111/bph.15226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cluny NL, Keenan CM, Reimer RA, Le Foll B, Sharkey KA. Prevention of Diet-Induced Obesity Effects on Body Weight and Gut Microbiota in Mice Treated Chronically with Δ9-Tetrahydrocannabinol. PloS one (2015) 10(12):e0144270. 10.1371/journal.pone.0144270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Becker W, Alrafas HR, Busbee PB, Walla MD, Wilson K, Miranda K, et al. Cannabinoid Receptor Activation on Haematopoietic Cells and Enterocytes Protects against Colitis. J Crohn's colitis (2021) 15(6):1032–48. 10.1093/ecco-jcc/jjaa253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Silvestri C, Pagano E, Lacroix S, Venneri T, Cristiano C, Calignano A, et al. Fish Oil, Cannabidiol and the Gut Microbiota: An Investigation in a Murine Model of Colitis. Front Pharmacol (2020) 11:585096. 10.3389/fphar.2020.585096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rowan F, Docherty NG, Murphy M, Murphy B, Calvin Coffey J, O'Connell PR. Desulfovibrio Bacterial Species Are Increased in Ulcerative Colitis. Dis colon rectum (2010) 53(11):1530–6. 10.1007/dcr.0b013e3181f1e620 [DOI] [PubMed] [Google Scholar]
- 70. Al-Ghezi ZZ, Busbee PB, Alghetaa H, Nagarkatti PS, Nagarkatti M. Combination of Cannabinoids, delta-9-tetrahydrocannabinol (THC) and Cannabidiol (CBD), Mitigates Experimental Autoimmune Encephalomyelitis (EAE) by Altering the Gut Microbiome. Brain Behav Immun (2019) 82:25–35. 10.1016/j.bbi.2019.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Konieczka P, Szkopek D, Kinsner M, Fotschki B, Juśkiewicz J, Banach J. Cannabis-derived Cannabidiol and Nanoselenium Improve Gut Barrier Function and Affect Bacterial Enzyme Activity in Chickens Subjected to C. perfringens challenge. Vet Res (2020) 51(1):141. 10.1186/s13567-020-00863-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yang YY, Hsieh SL, Lee PC, Yeh YC, Lee KC, Hsieh YC, et al. Long-term Cannabinoid Type 2 Receptor Agonist Therapy Decreases Bacterial Translocation in Rats with Cirrhosis and Ascites. J Hepatol (2014) 61(5):1004–13. 10.1016/j.jhep.2014.05.049 [DOI] [PubMed] [Google Scholar]
- 73. Mehrpouya-Bahrami P, Chitrala KN, Ganewatta MS, Tang C, Murphy EA, Enos RT, et al. Blockade of CB1 Cannabinoid Receptor Alters Gut Microbiota and Attenuates Inflammation and Diet-Induced Obesity. Scientific Rep (2017) 7(1):15645. 10.1038/s41598-017-15154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cameron C, Watson D, Robinson J. Use of a Synthetic Cannabinoid in a Correctional Population for Posttraumatic Stress Disorder-Related Insomnia and Nightmares, Chronic Pain, Harm Reduction, and Other Indications: a Retrospective Evaluation. J Clin Psychopharmacol (2014) 34(5):559–64. 10.1097/jcp.0000000000000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tsang CC, Giudice MG. Nabilone for the Management of Pain. Pharmacotherapy (2016) 36(3):273–86. 10.1002/phar.1709 [DOI] [PubMed] [Google Scholar]
- 76. Pellesi L, Verga MC, De Maria N, Villa E, Pini LA, Guerzoni S. Nabilone Administration in Refractory Chronic Diarrhea: a Case Series. BMC Gastroenterol (2019) 19(1):105. 10.1186/s12876-019-1024-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ivanov, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria. Cell (2009) 139(3):485–98. 10.1016/j.cell.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jansma J, Brinkman F, van Hemert S, El Aidy S. Targeting the Endocannabinoid System with Microbial Interventions to Improve Gut Integrity. Prog neuro-psychopharmacology Biol Psychiatry (2021) 106:110169. 10.1016/j.pnpbp.2020.110169 [DOI] [PubMed] [Google Scholar]
- 79. Muccioli GG, Naslain D, Bäckhed F, Reigstad CS, Lambert DM, Delzenne NM, et al. The Endocannabinoid System Links Gut Microbiota to Adipogenesis. Mol Syst Biol (2010) 6:392. 10.1038/msb.2010.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The Orphan G Protein-Coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J Biol Chem (2003) 278(13):11312–9. 10.1074/jbc.m211609200 [DOI] [PubMed] [Google Scholar]
- 81. Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, et al. Short-chain Fatty Acids Induce Both Effector and Regulatory T Cells by Suppression of Histone Deacetylases and Regulation of the mTOR–S6k Pathway. Mucosal Immunol (2015) 8(1):80–93. 10.1038/mi.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hosseinkhani F, Heinken A, Thiele I, Lindenburg PW, Harms AC, Hankemeier T. The Contribution of Gut Bacterial Metabolites in the Human Immune Signaling Pathway of Non-communicable Diseases. Gut microbes (2021) 13(1):1–22. 10.1080/19490976.2021.1882927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, et al. Metabolite-sensing Receptors GPR43 and GPR109A Facilitate Dietary Fibre-Induced Gut Homeostasis through Regulation of the Inflammasome. Nat Commun (2015) 6(1):6734. 10.1038/ncomms7734 [DOI] [PubMed] [Google Scholar]
- 84. Nakajima A, Nakatani A, Hasegawa S, Irie J, Ozawa K, Tsujimoto G, et al. The Short Chain Fatty Acid Receptor GPR43 Regulates Inflammatory Signals in Adipose Tissue M2-type Macrophages. PloS one (2017) 12(7):e0179696. 10.1371/journal.pone.0179696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, et al. GPR109A Is a G-Protein-Coupled Receptor for the Bacterial Fermentation Product Butyrate and Functions as a Tumor Suppressor in colon. Cancer Res (2009) 69(7):2826–32. 10.1158/0008-5472.can-08-4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chang PV, Hao L, Offermanns S, Medzhitov R. The Microbial Metabolite Butyrate Regulates Intestinal Macrophage Function via Histone Deacetylase Inhibition. Proc Natl Acad Sci United States America (2014) 111(6):2247–52. 10.1073/pnas.1322269111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Martin-Gallausiaux C, Béguet-Crespel F, Marinelli L, Jamet A, Ledue F, Blottière HM, et al. Butyrate Produced by Gut Commensal Bacteria Activates TGF-Beta1 Expression through the Transcription Factor SP1 in Human Intestinal Epithelial Cells. Scientific Rep (2018) 8(1):9742. 10.1038/s41598-018-28048-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Raqib R, Sarker P, Bergman P, Ara G, Lindh M, Sack DA, et al. Improved Outcome in Shigellosis Associated with Butyrate Induction of an Endogenous Peptide Antibiotic. Proc Natl Acad Sci United States America (2006) 103(24):9178–83. 10.1073/pnas.0602888103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sunkara LT, Achanta M, Schreiber NB, Bommineni YR, Dai G, Jiang W, et al. Butyrate Enhances Disease Resistance of Chickens by Inducing Antimicrobial Host Defense Peptide Gene Expression. PloS one (2011) 6(11):e27225–e. 10.1371/journal.pone.0027225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab (2016) 24(1):151–7. 10.1016/j.cmet.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 91. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut Microbiota Metabolism of Dietary Fiber Influences Allergic Airway Disease and Hematopoiesis. Nat Med (2014) 20(2):159–66. 10.1038/nm.3444 [DOI] [PubMed] [Google Scholar]
- 92. Guzior DV, Quinn RA. Review: Microbial Transformations of Human Bile Acids. Microbiome (2021) 9(1):140. 10.1186/s40168-021-01101-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lucas LN, Barrett K, Kerby RL, Zhang Q, Cattaneo LE, Stevenson D, et al. Dominant Bacterial Phyla from the Human Gut Show Widespread Ability to Transform and Conjugate Bile Acids. mSystems (2021) 2021:e0080521. 10.1128/msystems.00805-21 [DOI] [PubMed] [Google Scholar]
- 94. Jia W, Xie G, Jia W. Bile Acid–Microbiota Crosstalk in Gastrointestinal Inflammation and Carcinogenesis. Nat Rev Gastroenterol Hepatol (2018) 15(2):111–28. 10.1038/nrgastro.2017.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Taleb S. Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases. Front Immunol (2019) 10:2113. 10.3389/fimmu.2019.02113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, et al. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front Cell Infect Microbiol (2018) 8:13. 10.3389/fcimb.2018.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dopkins N, Becker W, Miranda K, Walla M, Nagarkatti P, Nagarkatti M. Tryptamine Attenuates Experimental Multiple Sclerosis through Activation of Aryl Hydrocarbon Receptor. Front Pharmacol (2020) 11:619265. 10.3389/fphar.2020.619265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, et al. CARD9 Impacts Colitis by Altering Gut Microbiota Metabolism of Tryptophan into Aryl Hydrocarbon Receptor Ligands. Nat Med (2016) 22(6):598–605. 10.1038/nm.4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I Interferons and Microbial Metabolites of Tryptophan Modulate Astrocyte Activity and central Nervous System Inflammation via the Aryl Hydrocarbon Receptor. Nat Med (2016) 22(6):586–97. 10.1038/nm.4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Abdulla OA, Neamah W, Sultan M, Alghetaa HK, Singh N, Busbee PB, et al. The Ability of AhR Ligands to Attenuate Delayed Type Hypersensitivity Reaction Is Associated with Alterations in the Gut Microbiota. Front Immunol (2021) 12:684727. 10.3389/fimmu.2021.684727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, et al. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front Cell Infect Microbiol (2018) 8:13. 10.3389/fcimb.2018.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cannon AS, Nagarkatti PS, Nagarkatti M. Targeting AhR as a Novel Therapeutic Modality against Inflammatory Diseases. Int J Mol Sci (2021) 23(1). 10.3390/ijms23010288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Puccetti M, Pariano M, Costantini C, Giovagnoli S, Ricci M. Pharmaceutically Active Microbial AhR Agonists as Innovative Biodrugs in Inflammation. Pharmaceuticals (Basel, Switzerland) (2022) 15(3). 10.3390/ph15030336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hua D, Li S, Li S, Wang X, Wang Y, Xie Z, et al. Gut Microbiome and Plasma Metabolome Signatures in Middle-Aged Mice with Cognitive Dysfunction Induced by Chronic Neuropathic Pain. Front Mol Neurosci (2021) 14:806700. 10.3389/fnmol.2021.806700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chevalier G, Siopi E, Guenin-Macé L, Pascal M, Laval T, Rifflet A, et al. Effect of Gut Microbiota on Depressive-like Behaviors in Mice Is Mediated by the Endocannabinoid System. Nat Commun (2020) 11(1):6363. 10.1038/s41467-020-19931-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Manca C, Boubertakh B, Leblanc N, Deschênes T, Lacroix S, Martin C, et al. Germ-free Mice Exhibit Profound Gut Microbiota-dependent Alterations of Intestinal Endocannabinoidome Signaling. J lipid Res (2020) 61(1):70–85. 10.1194/jlr.ra119000424 [DOI] [PMC free article] [PubMed] [Google Scholar]