Abstract
Adaptation of the brain to the presence of a drug predicts withdrawal on cessation. The outcome of adaptation is often referred to as ‘physical dependence’ in pharmacology, as distinct from addiction, although these terms have unfortunately become conflated in some diagnostic guides. Physical dependence to antidepressants may occur in some patients, consistent with the fact that some patients experience withdrawal effects from these medications. It is thought that longer duration of use, higher dose and specific antidepressants affect the risk of antidepressant withdrawal effects as they might cause greater adaptation of the brain. We searched PubMed for relevant systematic reviews and other relevant analyses to summarise existing data on determinants of antidepressant withdrawal incidence, severity and duration. Overall, data were limited. From survey data, increased duration of use was associated with an increased incidence and severity of withdrawal effects, consistent with some evidence from data provided by drug manufacturers. Duration of use may be related to duration of withdrawal effects but data are heterogenous and sparse. Serotonin and noradrenaline reuptake inhibitors and paroxetine are associated with higher risks than other antidepressants, though data for some antidepressants are lacking. Higher doses of antidepressant has some weak association with an increased risk of withdrawal, with some ceiling effects, perhaps reflecting receptor occupancy relationships. Past experience of withdrawal effects is known to predict future risk. Based on these data, we outline a preliminary rubric for determining the risk of withdrawal symptoms for a particular patient, which may have relevance for determining tapering rates. Given the limited scope of the current research, future research should aim to clarify prediction of antidepressant withdrawal risk, especially by examining the risk of withdrawal in long-term users of medication, as well as the severity and duration of effects, to improve the preliminary tool for predictive purposes. Further research into the precise adaptations in long-term antidepressant use may improve the ability to predict withdrawal effects for a particular patient.
Key Points
| Physical dependence to antidepressants may occur in some patients, caused by adaptation of the brain to long-term use of the medication. As pharmacologically defined, this physical dependence is a distinct phenomenon from addiction, and is manifested by a drug withdrawal syndrome. |
| Longer duration of treatment with antidepressants may increase the incidence and severity, and perhaps duration, of antidepressant withdrawal. |
| Serotonin and noradrenaline reuptake inhibitors and paroxetine are associated with a higher risk of withdrawal effects compared with other antidepressants. |
| Based on characteristics of antidepressant use, such as type, duration, dose and past experience, we have developed a preliminary tool to aid estimation of the risk of withdrawal effects on stopping. Further research is required to validate this tool. |
Introduction
In 2019/2020, one in six adults in England were given at least one prescription for an antidepressant, representing 7.8 million people [1], with approximately half of those on antidepressants estimated to be taking them for more than 2 years [2], and at least 930,000 people taking them for at least 3 years [3].
Although there is uncertainty about the precise number, perhaps up to one-half of those taking antidepressants will experience withdrawal symptoms when they stop them, with some of those effects being severe and long lasting [4–7]. Severe withdrawal effects can lead to misdiagnosis of other medical conditions or a misdiagnosis of relapse [8, 9], presentations to the emergency department [10] and suicide attempts [11]. Some people will find withdrawal effects so aversive that they will recommence their antidepressant, leading to long-term unwarranted use and unnecessary exposure to adverse effects [10, 12]. The estimated cost of antidepressant withdrawal syndrome has not yet been evaluated, but costs to the health system and social costs may be substantial [8, 13].
There has been widespread debate on how commonly withdrawal symptoms from antidepressants occur, as well as their severity and duration [4, 5, 14]. It has also been suggested that various aspects of antidepressant use are likely to affect the risk of withdrawal, including dosage, duration of use and characteristics of the antidepressant [10, 15, 16]. In this paper, we briefly review the neurobiological causes of withdrawal symptoms before examining what is known about the determinants of antidepressant withdrawal from the existing literature on the subject. From this review, we develop a preliminary risk calculator to estimate the risk of withdrawal symptoms in a given patient.
Neurobiology of Physical Dependence and Withdrawal Symptoms from Antidepressants
Physiological Dependence Distinguished from Addiction
The term ‘dependence’ has recently come to be used interchangeably with ‘addiction’ (to mean uncontrolled drug-seeking behaviour), for example in the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) [17]. This choice was made in the Diagnostic and Statistical Manual of Mental Disorders (Third Edition) because the term ‘addiction’ was thought to be pejorative while the word ‘dependence’ was thought more neutral [17]. However, the original usage of the word ‘dependence’ referred to “physiological adaptation that occurs when medications acting on the central nervous system are ingested with rebound when the medication is abruptly discontinued” [17]. This usage is referred to by many expert groups: according to a consensus statement from the American Academy of Pain Medicine, the American Pain Society and the American Society of Addiction Medicine, “Physical dependence is a state of adaptation that is manifested by a drug class specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist” [18]. Similarly, the National Institute on Drug Abuse states “Dependence means that when a person stops using a drug, their body goes through ‘withdrawal’: a group of physical and mental symptoms that can range from mild (if the drug is caffeine) to life threatening (such as alcohol or opioids, including heroin and prescription pain relievers). Many people who take a prescription medicine every day over a long period of time can become dependent; when they go off the drug, they need to do it gradually, to avoid withdrawal discomfort. But people who are dependent on a drug or medicine aren’t necessarily addicted” [19]. Indeed, Goodman and Gilman’s textbook of pharmacology points out “The appearance of a withdrawal syndrome when administration of the drug is terminated is the only actual evidence of physical dependence” [20].
Addiction “is characterized by behaviours that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving” [18]. Therefore, “For drugs not associated with abuse potential, an individual may still develop dependence; but again, this would not be classified as an addiction” [21]. Additionally, according to the National Institute on Drug Abuse, “a person can be dependent on a drug, or have a high tolerance to it, without being addicted to it” (emphasis in original) [19]. Physical dependence is therefore an important concept to retain as without this term it becomes difficult to communicate how a non-addictive substance like caffeine can cause withdrawal effects, equally applicable to antidepressants (for which there is no evidence of addiction). The process of physical dependence also allows us to understand why certain characteristics of medication (half-life, receptor targets, dose, duration of use) might predict an increased risk of withdrawal as all these characteristics may affect the degree of adaptation to the drugs.
Antidepressants and Physiological Dependence
All major classes of antidepressants [selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors, monoamine oxidase inhibitors, tricyclic antidepressants, noradrenaline and specific serotonergic antidepressants] can be associated with withdrawal symptoms on cessation or a dose reduction in a substantial proportion of patients, likely as a result of physical dependence (a normal neurobiological response to drugs that act on the central nervous system) in these patients [3, 10, 17, 21–23]. Physical dependence arises because the body and brain undergo adaptations to the presence of a drug, countering its effect in order to maintain homeostasis [17, 24, 25]. The only evidence of a state of physical dependence is the appearance of withdrawal symptoms on reducing or stopping the drug [20]. It is also clear that vast majority of antidepressants—with the exception of tranylcypromine and amineptine—do not cause addiction, as they do not induce compulsion, craving and other symptoms of addiction [4, 26].
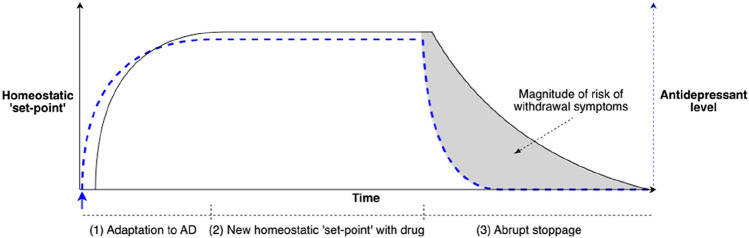
During ongoing administration of antidepressants, as for other drugs acting on the central nervous system, neuroadaptation establishes a new homeostatic equilibrium, in which the system accommodates to alterations produced by the drug. When the medication is reduced or stopped, the homeostasis is perturbed, resulting in withdrawal symptoms [24, 25, 27]. Adaptations to the presence of the drug predict withdrawal effects because these adaptations do not resolve instantaneously upon stopping the drugs but will persist for some period: the ‘mis-match’ between the level of drug action to which the body has been adapted to and the lesser amount of drug action it receives (upon dose reduction or cessation) gives rise to withdrawal effects [27]. As a Professor of Pharmacology, Prof. Reidenberg puts it “For drugs given chronically, one can look for laboratory evidence of adaptation to the drug. If such adaptation occurs it is likely that, after discontinuation, the body will eliminate the drug more rapidly than the adaptation will subside. Any laboratory evidence of adaptation … should be interpreted as presenting a potential discontinuation syndrome” [27]. Further, Reidenberg said that “drug discontinuation effects are part of the pharmacology of a drug” when the body eliminates a drug faster than adaptations to the presence of the drug can subside (Fig. 1) [27].
Fig. 1.
Conceptual model of the neurobiology of antidepressant withdrawal. In this diagram, the homeostatic ‘set-point’ is shown in black and antidepressant (AD) drug concentrations are shown in blue dotted lines [77]. (1) The system is at baseline. At the blue arrow, an antidepressant is administered; drug plasma concentrations increase. Physiological adaptations of the system to the presence of the drug begin (which may be the period for which ‘start-up side effects’ are most pronounced). (2) At the plateau, drug plasma concentrations (and target receptor activation) have reached a steady state with a new homeostatic set-point of the system established (‘start-up side effects’ may reduce). (3) The antidepressant is abruptly ceased and plasma drug concentrations drop to zero (exponentially, according to the elimination half-life of the drug). This difference between the homeostatic set point (the ‘expectations’ of the system) and the concentration of drug in the system (dotted blue line) is experienced as withdrawal symptoms. The duration of withdrawal symptoms is largely determined by the time required for adaptations to the drug to resolve. Hence, withdrawal symptoms may worsen or peak even long after the drug has been eliminated from the system. The shaded area under the curve, representing the difference between the homeostatic set-point and the concentration of the drug, indicates the degree of risk of withdrawal symptoms: the larger the area the greater the risk. The greater the departure of drug concentration from the homeostatic set-point, the greater the risk.
Adapted, with permission, from [77]
The exact nature of neurobiological adaptation to antidepressants has received relatively little study, but it is thought to involve down-regulation of serotonergic receptors in response to higher levels of synaptic serotonin arising as a consequence of serotonin transporter (SERT) antagonism, the primary target of antidepressants [16, 28]. There is evidence that adaptation to antidepressants occurs in humans as measured by positron emission tomography binding studies: short-term SSRI use reduces the sensitivity of cortical 5-HT2A receptors [29] in patients with depression, 5-HT4 receptor in healthy controls [30] as well as down-regulates 5-HT1A receptors in patients with depression [31]. Additionally, there is evidence that 5-HT1A down-regulation can persist for months and years after antidepressants are ceased [31]. In one neuroimaging study, patients who had been previously treated with antidepressants showed 5-HT1A down-regulation in 38 out of 40 brain regions analysed a mean of 29 months after antidepressants were ceased (range 8–60 months) [32]. The relationship of these persistent receptor changes and withdrawal symptoms has not been investigated empirically, but suggests a mechanism whereby a ‘mis-match’ between the brain’s expectations and inputs may give rise to (potentially long-lasting) withdrawal symptoms [27]. Indeed, there is some indirect evidence connecting this receptor to antidepressant withdrawal symptoms, in that patients with the -1019C allele of the 5-HT1A receptor gene more commonly experienced withdrawal symptoms than those with the -1019G homozygote [33].
Animal studies of antidepressant discontinuation have found a number of alterations to the serotonergic system that persist after SSRI cessation (resolving over varying time periods from 7 to 60 days) following weeks of treatment with antidepressants, in conjunction with the detection of withdrawal effects [16]. The following changes have been detected after antidepressants were stopped: lowered serotonin and serotonin metabolites in the hippocampus and frontal cortex, reduced SERT binding, reduced SERT mRNA in the raphe nucleus, reduced 5-HT1B mRNA in the raphe nucleus and reduced 5-HT2C mRNA in the frontal cortex, reduced oxytocin response (in response to a 5-HT1A receptor agonist) and reduced 5-HT1A sensitivity [16]. Notably, many of these changes persisted for up to 2 weeks in rodent models [16], with 17 days in rats equivalent to a human year [34]. Consistent with this, in animals, long-term treatment with antidepressants produces a reduction in endogenously synthesised levels of serotonin detected [35] after an initial increase [36], although this phenomenon has not been studied in humans. In one study that measured changes for longer, 14 days of fluoxetine treatment in rats produced a reduced oxytocin response that was still present 60 days after drug cessation—four times longer than the period of treatment [16, 37]. Using a widely cited means of drawing equivalencies between rat time and human time, 60 days is equivalent to 3 human years, although this equivalence has not been specifically verified in regard to the duration of adaptations to antidepressant treatment [34, 37]. This is consistent with the time period demonstrated in neuro-imaging of patients who had previously used antidepressants.
Several authors have argued withdrawal symptoms are unlikely to be mediated simply through serotonergic receptors as there are many other downstream effects including effects on norepinephrine, dopamine, glutamate and GABA-ergic pathways, which may also adapt to long-term administration of antidepressants [16, 25]. For example, some authors suggest that the rapid reversal of the inhibition of noradrenergic function in the locus coeruleus caused by the inhibitory influence of increased serotonin levels during antidepressant treatment observed in animal studies when antidepressants are stopped may explain some symptoms of antidepressant withdrawal [38]. The anti-cholinergic effects of paroxetine may lead to cholinergic rebound on cessation, partly accounting for the severity of its withdrawal syndrome [38]. There has been no empirical study of these effects in human subjects.
The pathophysiological principle of adaptation makes it clear why the major determinant of how long withdrawal symptoms persist is not a drug characteristic such as a half-life, but how long it takes neurobiological adaptations to the drug to resolve to a pre-drug state [27]. An analogy might be made to the experience of walking out of a loud concert (more signal as with the increased synaptic serotonin during antidepressant treatment) into a quiet street (physiological levels of serotonin after drug removal), where sounds appear muted because of adaptation of tympanic sensitivity (as with serotonergic sensitivity) for a few minutes while your tympanic membrane re-accommodates to a different average amount of sound (an analogous delay for the serotonergic system to re-adapt to less signal, although it seems to take much longer than a few minutes). The time for the sound to dissipate (or for synaptic serotonin levels to normalise on removal of the drug) is trivial; it is the time taken for tympanic re-accommodation that determines the duration of the withdrawal effects (as for re-accommodation of the processes affected by long-term antidepressant use). Because of wide-ranging adaptations in the brain and body to antidepressants, withdrawal symptoms can manifest in both physical and psychological symptoms [10, 39].
Notably, the understanding that the degree of adaptation to the drug predicts withdrawal effects suggests lines of research that could be undertaken to further explore this phenomenon. The degree of adaptation to the drug for an individual could be quantified to predict the risk of withdrawal. Drawing from animal studies and human neuro-imaging studies, adaptation may be found in the reduced sensitivity and number of serotonergic receptors [29, 30], lowered serotonin and serotonin metabolites, reduced SERT binding, reduced oxytocin response, or reduced 5-HT1A sensitivity [16]. For example, further studies evaluating the hypothesis that patients with a greater degree of 5-HT1A down-regulation caused by antidepressant administration are more likely to have withdrawal symptoms on cessation would be informative. Currently, the adaptations to antidepressants demonstrated in neuro-imaging of clinical subjects and in animal studies provide a plausible mechanism for withdrawal but we cannot draw definitive conclusions that this represents the underlying pathophysiology of withdrawal effects.
Literature Search
In order to investigate the aspects of antidepressant use that influence withdrawal incidence, severity and duration, we performed a narrative review following principles outlined in the Scale for the Assessment of Narrative Review Articles (SANRA) [40].
A search on PubMed for systematic reviews published from 2000 until 13 May, 2022 was performed using the search string “antidepressants” AND (“withdrawal OR discontinuation”), restricting the search to systematic reviews. This yielded 325 results. Further analyses were added as suggested by experts in the field, reviewers of this article or by a search of the references of included articles, including an examination performed by the Committee on the Safety of Medicines (CSM) on antidepressant withdrawal, bringing the total to 331 results. We selected studies that were in English, dealt with human adults and which included some examination of a hypothesised determinant of withdrawal risk such as varying dose of antidepressant, different types of antidepressants or varying duration of antidepressant treatment. The full text of 11 studies was screened and after screening, nine reviews or other analyses were included. The intention of this review was to glean evidence about the determinants of antidepressant withdrawal symptoms from these existing reviews or analyses.
Antidepressant Withdrawal Symptoms
Incidence of Antidepressant Withdrawal Symptoms
A 2019 systematic review identified 14 relevant studies from which to calculate the incidence of antidepressant withdrawal symptoms [5]. The incidence rates ranged from 27 to 86%, with a median of 55% and a weighted average of 56.4% [5]. Restricting the analysis only to double-blind randomised controlled trials (RCTs) from this review, the incidence of withdrawal syndrome was 53.9% (six RCTs, 731 participants) [Table 1] (where the majority of studies used an increase in discontinuation emergent signs and symptoms [DESS] [41] of ≥ 4 to define a withdrawal syndrome).
Table 1.
Incidence of withdrawal in double-blind RCTs captured in Davies and Read [5]
| Double-blind RCTs (year) | Period of treatment before cessation | Period of observation | Definition of withdrawal syndrome | People with withdrawal syndromes | Total stopped from medication | Proportion with withdrawal (%) |
|---|---|---|---|---|---|---|
| Oehrberg [78] (1995) | 12 weeks | 2 weeks | ‘Any adverse effect on discontinuation’ | 19 | 55 | 34.6 |
| Rosenbaum [41] (1998) | 11.4 months | 5–8 days | DESS ≥ 4 | 86 | 185 | 46.5 |
| Zajecka [45] (1998) | 12 weeks | 6 weeks | ‘New or worsened events’ | 64 | 95 | 67.4 |
| Hindmarch [43] (2000) | ‘At least 3 months’ | 4–7 days | DESS ≥ 4 | 66 | 86 | 76.7 |
| Montgomery [79] (2005) | 12 weeks | 2 weeks | DESS ≥ 4 | 49 | 181 | 27.1 |
| Sir [52] (2005) | 8 weeks | 2 weeks | Any discontinuation-emergent symptom | 110 | 129 | 85.3 |
| Total | 394 | 731 | 53.9 |
DESS discontinuation-emergent signs and symptoms, RCTs randomised controlled trials
A potential limitation of this review was that, in addition to RCTs and observational studies, it included three online surveys; critics point out it is possible that surveys may capture a skewed sample of patients motivated to answer the survey because of their experience with more severe withdrawal symptoms than average, and withdrawal symptoms were reported by patients rather than using objective withdrawal questionnaires [4, 14]. However, the weighted average incidence of withdrawal symptoms was similar in the six RCTs (53.9%) to the five observational studies (52.5%) and the three online surveys (57.1%) [5]. Restricting analysis to studies of SSRIs, the most widely used class of antidepressants, discontinuation syndromes occurred with a median rate of 53.6%, and a weighted average of 50.5% [5]. The double-blind, placebo-controlled, staggered discontinuation studies are the most reliable of the studies conducted and are highlighted in Table 1.
Nocebo/Psychosomatic Effects
Another limitation to the Davies and Read review is reporting of single-arm frequencies, that is, withdrawal effects from stopping antidepressants are not compared to withdrawal effects from stopping placebo or continuing antidepressants as a control. Some have suggested that withdrawal symptoms may be a psychosomatic response rather than genuine physiological symptoms [4]. These authors have hypothesised that patients have negative expectations of the consequence of stopping their antidepressants, leading to nocebo withdrawal effects (the opposite of the placebo effect) [4]. The presence of antidepressant withdrawal symptoms in both animals [16] and neonates of antidepressant-using mothers [42] suggests that the process is primarily physiological rather than psychosomatic. Randomised controlled trials conducted to detect withdrawal symptoms used double-blind placebo-controlled designs so that the patient and doctor were unaware whether the patient was receiving a continuation of their antidepressant or identical placebo pills for several days [41, 43]. This design minimises the role of psychological expectation or nocebo effects and therefore suggests that withdrawal effects are physiological consequences of stopping the medication [41]. In one carefully conducted study, the average number of new symptoms recorded on the DESS scale was 5.7 (standard deviation 6.96) for sertraline patients and 7.8 (standard deviation 8.55) for paroxetine-treated patients, suggesting a large number of symptoms, including physical symptoms (such as dizziness, and headache) with onset at the same time, and resolution upon re-commencing the antidepressants (unbeknownst to the participants) consistent with a physiological syndrome [41]. Furthermore, cessation of fluoxetine (whose half-life of 7–15 days makes withdrawal symptoms unlikely in the 5–8 days of the study) produced a non-significant increase of 0.2 symptoms, serving as a useful negative control group [41].
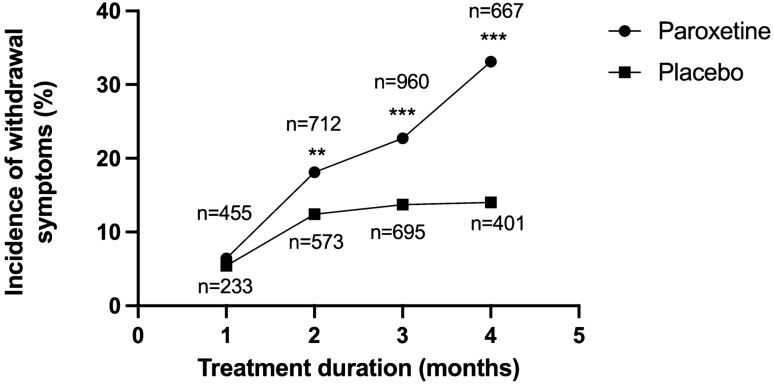
The rate of such nocebo withdrawal effects has been explored in six double-blind RCTs in two ways: either by cessation of placebo or continuation of antidepressants unbeknownst to the patient. These six studies were reviewed previously [44] and results are presented in Table 2 [44]. Excluding the outlier of 75% (defined as experiencing any symptom of withdrawal) [45] yields a weighted average of nocebo withdrawal effects of 11.8%. A limitation to this analysis is that the severity of withdrawal effects is not measured in these studies, and it is not clear that ‘dizziness’ or ‘nausea’ reported in the nocebo group is the same as such symptoms in the withdrawal group (there are case reports of such symptoms being severe enough to prompt an investigation of stroke) [46]. Furthermore, withdrawal effects from placebo show no relationship with duration of treatment, in the same way that withdrawal from paroxetine does, with marked divergence over time (Fig. 2, with Fisher’s exact tests showing significant differences between the two groups after 2 months [p = 0.005], 3 months [p < 0.001] and more than 4 months of treatment [p < 0.001]) [47]. Therefore, as with all health symptoms, there may be a psychological component to withdrawal effects but this is likely to be minor. For example, the data in Fig. 2 appear to be consistent with a ceiling value similar to that calculated above for nocebo withdrawal. Adjusting the reported single-arm frequencies for antidepressant withdrawal for the rate of withdrawal in the ‘nocebo’ condition yields an incidence of 42.1% (53.9–11.8%) for antidepressants in double-blind RCTs and 38.7% (50.5–11.8%) for trials looking specifically at SSRIs [44].
Table 2.
Summary of studies that have estimated the role of nocebo effects in the incidence of withdrawal syndrome
| Study (year) | Method | Proportion withdrawal syndrome (%) | Number of patients | Criteria used for detection of withdrawal syndrome (comment) |
|---|---|---|---|---|
| Baldwin [80] (2006)a | Placebo discontinuation | 12.2 | 123 | DESS increase ≥ 4 |
| Lader (2004) [81] | Placebo discontinuation | 1.9 | 116 | DESS increase ≥ 4 |
| Oehrberg et al. [78] (1995) | Placebo discontinuation | 13.5 | 52 | Any adverse event on discontinuation |
| Montgomery [79] (2005) | Antidepressant continuation (blinded) | 9.2 | 125 | DESS increase ≥ 4 |
| Zajecka et al. [45] (1998) | Antidepressant continuation (blinded) | 75 | 299 | Any adverse event on discontinuation |
| Rosenbaum et al. [41] (1998) | Antidepressant continuation (blinded) | 14 | 81 | DESS increase ≥ 4 (fluoxetine stopped for 5–8 days is equivalent to continuation given the long half-life) |
Fig. 2.
Relationship between duration of treatment (before stopping) and proportion of patients who experienced withdrawal effects on stopping either paroxetine or placebo (overall trend p value <0.001) [47]. Significance for Fisher exact tests for by-month group comparisons. *p < 0.05; **p < 0.01; ***p < 0.001
Severity of Antidepressant Withdrawal Symptoms
The severity of the withdrawal syndrome from SSRIs varies widely, with a range from mild short-lasting cases that can be managed with education and reassurance, to severe cases that cause significant disruptions to normal functioning [5, 10]. This variability presumably relates to differing degrees of neurobiological adaptation to antidepressants amongst individuals. In its severe form, the SSRI withdrawal syndrome has been reported to be associated with ataxia leading to falls, electric shock sensations that impair walking and driving [10], and urgent consultations at emergency departments [46, 48], in published case studies. The discontinuation period is also associated with a 60% relative increase in suicide attempts, compared with previous users of antidepressants [11]. It is improbable that these suicide attempts arose as a consequence of relapse, as relapse is generally thought to be delayed in onset for more than 2 weeks after stopping antidepressants [49].
The systematic review also identified five studies that evaluated the severity of withdrawal effects [5], with nearly half of participants who had experienced withdrawal effects choosing the most extreme option in the scale offered to them to describe the severity of those effects [5]. For example, in response to a question ‘How severely do you feel withdrawal has affected your life?’ on a scale of 0–10 given to 580 people who had attempted withdrawal from antidepressants, mostly SSRIs, 43% (249) of participants chose 10, the highest level of the scale [50]. As above, it is possible that the online survey method employed by four of these studies may be biased by patients with more negative experiences; however, it is notable that somewhat more than half of the participants surveyed in these studies had used antidepressants for more than 3 years [51], similar to the wider English population (where about half of antidepressant users have been taking them for more than 2 years) [2]. However, the self-selected nature of this population of respondents limits the ability to extrapolate to the wider population of people taking antidepressants.
The remaining study, conducted by Pfizer, found that 34.3% of patients treated with sertraline for 8 weeks experienced moderately severe symptoms (as rated by an investigator on a global assessment), 23.9% of them experienced a mild withdrawal reaction, while 23.9% reported a minimal reaction [52]. For venlafaxine, after only 8 weeks of use, 38.7% of patients were rated by study researchers as experiencing moderately severe withdrawal symptoms, with 3.2% as ‘severe’ and 1.6% as ‘very severe’ [52]. As a longer duration of treatment appears to be associated with a greater incidence and severity of withdrawal symptoms (see below) [47, 51], patients who are taking antidepressants for longer than 8 weeks are more likely to experience more severe withdrawal symptoms.
Other double-blind staggered RCTs of discontinuation of antidepressants do not measure the severity of withdrawal per se but the mean number of symptoms recorded on the DESS, for each antidepressant provides a proxy measure of severity, albeit imperfect, because a patient could have many minimal symptoms or one very severe symptom-aspects that would not be captured by such a method. Nevertheless, as shown in Table 3, withdrawal symptoms from paroxetine are numerically greater than for citalopram and sertraline, which are in turn numerically greater than fluoxetine (albeit measured only over short periods of time), consistent with their risk of withdrawal (see below).
Table 3.
Incidence of withdrawal and measure of severity (by numerical count of DESS or investigator global assessment) for specific antidepressants in double-blind randomised controlled trials captured in Davies and Read [5]
| Antidepressant | Study (year) | Definition of withdrawal syndrome | People with withdrawal syndromes | Total stopped from medication | Proportion with withdrawal (%) | Average rate of withdrawal (%) | Severity or numerical score of withdrawal |
|---|---|---|---|---|---|---|---|
| Escitalopram | Montgomery (2005) | DESS ≥ 4 | 49 | 181 | 27.1 | 27.1 | N/A |
| Paroxetine | Oerhberg (1995) | Any D-E symptom | 19 | 55 | 35 | 58.9 | N/A |
| Rosenbaum (1998) | DESS ≥ 4 | 39 | 59 | 66 | 7.8 DESS | ||
| Hindmarch (2000) | DESS ≥ 4 | 22 | 22 | 100 | 10.1 DESS | ||
| Fluoxetine | Rosenbaum (1998) | DESS ≥ 4 | 9 | 53 | 14 | 50 | 0.2 DESS |
| Zajecka (1998) | Any D-E symptom | 64 | 95 | 67 | N/A | ||
| Hindmarch (2000) | DESS ≥ 4 | 17 | 22 | 77 | 1.3 DESS | ||
| Sertraline | Rosenbaum (1998) | DESS ≥ 4 | 38 | 63 | 60 | 59.2 | 5.7 DESS |
| Hindmarch (2000) | DESS ≥ 4 | 13 | 22 | 59 | 2.5 DESS | ||
| Sir (2005) | Any D-E symptom | 39 | 67 | 58 | 34.3% (moderate or worse)a | ||
| Citalopram | Hindmarch (2000) | DESS ≥ 4 | 14 | 20 | 70 | 70.0 | 3.0 DESS |
| Venlafaxine | Sir (2005) | Any D-E symptom | 55 | 62 | 88.7 | 88.7 | 43.5% (moderate or worse)a |
D-E discontinuation-emergent, DESS discontinuation-emergent signs and symptoms, N/A not available
aIn Sir [52], the AntiDepressant Discontinuation Scale was used to measure withdrawal symptoms. This is a clinician-rated checklist of 30 signs and symptoms that assess the intensity (0–3 scale) of adverse events and the putative relationship of adverse events to discontinuation (1–4 scale) developed for this study. The AntiDepressant Discontinuation Scale also included a global investigator assessment of severity of discontinuation symptoms on a 6-point Likert scale (from 0 = none to 5 = very severe). Proportions reported are those patients who were rated as having moderate, severe or very severe discontinuation symptoms, i.e. a score of 4 or greater out of 6 on this Likert severity scale
Duration of Withdrawal Symptoms
There is significant evidence that withdrawal symptoms can last for weeks, months or even years in some cases [13, 50], but a weighted average of the ten studies included in the recent systematic review was not possible, owing to methodological heterogeneity [5]. One study examining reports from doctors to the Medicines and Healthcare products Regulatory Agency in the UK described a duration of withdrawal symptoms from 1 to 52 days, with an average of 10.5 days, although this is likely to represent an underestimate as a number of patients taking paroxetine had to be re-started on the drug because their withdrawal symptoms were too severe [53]. A Royal College of Psychiatrists online survey found that for the 512 users who experienced withdrawal, the symptoms lasted for up to 6 weeks, and a quarter of the group reported anxiety lasting more than 12 weeks [5]. This is consistent with an earlier study that found withdrawal symptoms lasted at least 6 weeks in 40% of people [45]. In another online study of 580 people who had withdrawn from antidepressant medication, 86.7% responded that the syndrome had lasted at least 2 months, 58.6% at least 1 year and 16.2% for more than 3 years [50], although this study may have surveyed a population with a more severe experience of withdrawal than average. Other studies also report longer durations of withdrawal symptoms, in at least some cases, symptoms can persist for years [13, 50, 54]. It is difficult to establish to what extent these very long-lasting syndromes represent outliers, as it is not possible to establish that these are representative populations of antidepressant users, but withdrawal symptoms likely persist significantly longer than the 1-week or 2-week periods that have been previously ascribed to them, albeit in an unknown proportion of patients [55].
Determinants of Antidepressant Withdrawal Symptoms
As adaptations to the presence of the drug are thought to underlie withdrawal symptoms, a longer duration of use and a higher dosage would be expected to contribute to the degree of adaptation and thus to the incidence, severity and duration of withdrawal symptoms. Drugs with shorter half-lives may induce more severe withdrawal effects with an earlier onset than those drugs with longer half-lives because the mis-match between what the brain has accommodated to and what is provided is greater (Fig. 1). Alternatively, different antidepressants may cause greater effects on the brain, perhaps related to their different receptor targets and binding properties. Individual physiological differences [10, 27] may also affect the degree of adaptation to the drug and thus the risk of withdrawal symptoms. We explore evidence for all of these determinants.
Effect of Duration of Use on Incidence, Severity and Duration of Withdrawal Symptoms
Although the primary data are not publicly available, the CSM was granted access to unpublished manufacturer’s data for antidepressant withdrawal effects for a variety of antidepressants [47]. The Committee was provided with all clinical trial data (placebo-controlled or active-controlled studies) from which the manufacturers had evaluated the incidence and severity of withdrawal effects (calculated according to the manner that they had been evaluated in the trials) and their relationship to duration of use and tapering.
Duration of use of paroxetine was found to be related to the incidence of withdrawal symptoms on stopping, when adult clinical trial data were obtained from the manufacturer (Fig. 2) [47]. Although there was not enough information for the CSM to make a determination of the role of duration of treatment in citalopram withdrawal effects, there was some indication that a longer duration of treatment with escitalopram increased the risk of a withdrawal reaction [47]. There was limited evidence of an effect related to the duration of use for fluoxetine, fluvoxamine (although there was confusion between adverse effects that led to a drop out from trials and adverse effects arising from the withdrawal process itself), mirtazapine (pooled analyses not appropriate to evaluate this question) or sertraline. Venlafaxine demonstrated some evidence of a duration of treatment effect: 14% of patients after 8 weeks of treatment experienced dizziness, while this rose to 29% after 24 weeks of treatment [47].
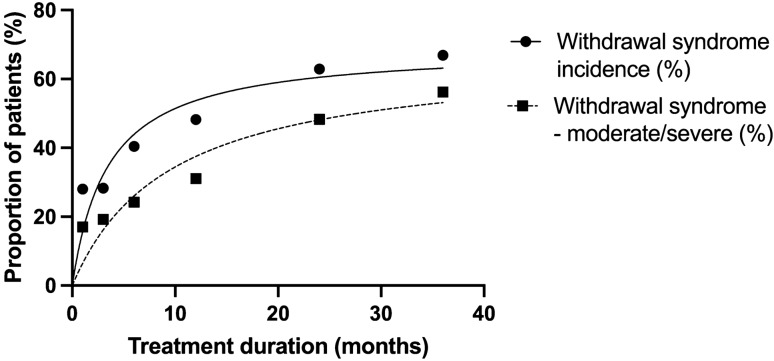
Although no RCTs examined the severity of withdrawal symptoms in association with treatment duration (rather, they only counted the number of symptoms), online surveys of patients did so [56, 57]. Although these surveys may have captured skewed samples, the line of best fit suggests a clear gradient between the duration of use and severity (as well as the incidence) of withdrawal syndrome (Fig. 3) [57]. These surveys included patients who stopped taking various antidepressants including paroxetine, venlafaxine, citalopram, fluoxetine, escitalopram, sertraline and tricyclic antidepressants [57]. Duration of antidepressant use is likely to lead to greater physiological adaptations increasing the risk and severity of withdrawal symptoms [57]. The relationship between duration of use and the risk of withdrawal effects also appears to have a second-order relationship (the gradient between the risk of withdrawal effects become less steep over time) perhaps consistent with ceiling effects for adaptation.
Fig. 3.
Relationship between duration of treatment and severity and duration of withdrawal symptoms from surveys of antidepressant users and observational studies. The relationship between duration of treatment of antidepressants and incidence of moderate or severe withdrawal symptoms. Graph is derived from data in Read et al. [57]
Effect of Dose on Withdrawal Symptoms
The CSM also examined the relationship between the dose of medication and the risk of withdrawal, and though it was not explicit about the nature of these studies, the implication is that these data were derived from studies mostly abruptly stopping the medications [47]. Although it is difficult to make comparisons between such studies analysed by the CSM, and other studies that looked at dose-dependent effects [58] because such data are susceptible to the ecological fallacy, there is evidence of dose-dependent effects within individual trials (Table 4). There was a higher incidence of withdrawal effects for higher dosages of paroxetine in the analysis by the CSM [47], although the effect reached a threshold at 20 mg (Table 4), probably because of the hyperbolic relationship between antidepressant dosage and effect on its target receptors [59–62]. There was a more pronounced dose-dependent relationship for venlafaxine withdrawal effects (Table 4) [47], with an increased incidence at higher dosages possibly related to greater noradrenergic effects at these dosages [63, 64]. Fluvoxamine and mirtazapine did not demonstrate clear dose-dependent effects; however, the CSM cautioned that the pooled analysis applied may not have been appropriate to detect these effects [47]. Overall, dosage does appear to have some relationship to the risk of withdrawal symptoms (where higher doses were associated with an increased risk of withdrawal), but its influence may not be as strong as the duration of use, perhaps because higher dosages have only small additional pharmacological effects over minimum clinically employed dosages because of the hyperbolic shape of their dose-response curves [60, 62, 65–67].
Table 4.
Relationship between dosage of antidepressants and incidence of withdrawal effects
| Study (year) | Medication | Dose | |||
|---|---|---|---|---|---|
| Proportion of patients with withdrawal effects/(number of patients in group) | |||||
| CSM [47] (2005) | Paroxetine | 10 mg | 20 mg | 30 mg | 40 mg |
| 9% (46) | 16% (55) | 18% (61) | 17% (60) | ||
| CSM [47] (2005) | Venlafaxine | 37.5 mg | 75 mg | 150 mg | |
| 13% (92) | 11% (92) | 24% (98) | |||
| Lader et al. [58, 81] (2004)a | Escitalopram | 5 mg | 10 mg | 20 mg | |
| 15.1% (124) | 17.1% (125) | 21.7% (111) | |||
| Baldwin et al. [58, 80] (2006)a | Escitalopram | 5 mg | 10 mg | ||
| 6.9% (116) | 12.2% (115) | ||||
Effect of Drug Type on Withdrawal Symptoms
It has been suggested that the risk of withdrawal symptoms varies between different antidepressants. This could be due to differing half-lives, with drugs with shorter half-lives being eliminated more quickly and therefore producing more precipitous drops in inputs ‘expected’ by the system (Fig. 1) [15]. This is supported by the finding that percentage reductions in plasma concentrations of fluoxetine, sertraline and paroxetine, following cessation, showed a significant correlation with the appearance of withdrawal symptoms [68]. Cessation of paroxetine for several days causes withdrawal symptoms in 66–100% of patients [41, 43], cessation of sertraline in 59–60% of patients [41, 43] and fluoxetine in 14–77% of patients [41, 43], (double-blind RCTs examining drugs are summarised in Table 3). In surveys, which may include a self-selected population, these differences among common SSRIs are roughly preserved: 69, 62 and 44% of patients stopping paroxetine, sertraline, and fluoxetine, respectively, report withdrawal symptoms [5].
However, withdrawal symptoms following the cessation of fluoxetine, the SSRI with the longest half-life (7–15 days for its active metabolite, nor-fluoxetine), has been observed to occur with a delay of onset of 4–6 weeks after discontinuation in one study [45], and 2 weeks after discontinuation in another [69]. In two studies that have examined withdrawal effects for fluoxetine for several days after stopping, one found a minor increase on average of 1.3 points (95% confidence interval − 1.8 to 4.3) on the DESS [43] and the other found a barely detectable mean increase of 0.2 (standard deviation 5.22) points on the DESS [41], which seems to suggest that the 77% incidence in Hindmarch et al. [43] may provide an exaggerated view of the likelihood of withdrawal symptoms from this medication in the short term. However, given the delayed onset of withdrawal effects predicted by the long elimination half-life of fluoxetine, it is unclear whether there would be a greater incidence of withdrawal effects with a longer follow-up; withdrawal may be rarer than for other antidepressants.
Interestingly, in the CSM analysis, mirtazapine appeared to cause less withdrawal effects than placebo [47], although reports to a helpline concerning withdrawal effects suggest that mirtazapine is associated with withdrawal problems as, or more, commonly as SSRIs [23]. Similarly, patients treated with fluvoxamine in drug company trials showed a similar rate of adverse effects on stopping as those patients treated with placebo (for some durations of treatment, withdrawal incidence was higher for the placebo group), although the CSM commented that the non-systematic manner in which these data were collected may underestimate the withdrawal effects [47]. This finding is in contrast to the data presented by an analysis of calls to a medication helpline in England for withdrawal effects (normalised to national prescription rates), which found that fluvoxamine was markedly over-represented with more calls than any other SSRIs, except paroxetine, and more calls than for venlafaxine [23], perhaps reflecting differences for the longer term use seen in clinical practice than in short-term manufacturer studies.
In general, this is a major limitation with studies examining withdrawal effects from antidepressants—most are conducted in patients enrolled in acute efficacy trials who are exposed to several weeks (or sometimes a short number of months) of treatment [4, 58]. As half of the people taking antidepressants in England are taking these medications for more than 2 years [2], and a longer duration predicts greater withdrawal effects, such studies are likely to under-estimate the withdrawal effects of medication, despite being otherwise well conducted. For example, in Rosenbaum et al. [41], 66% of patients treated for 11.6 months on average with paroxetine met the threshold for a withdrawal syndrome (four or more new-onset symptoms, as measured by the DESS), but in the analysis by Baldwin et al. [58] after 12 weeks of paroxetine treatment, only 28.4–31.5% of patients met the same criteria for a withdrawal syndrome (with 32.7% after 27 weeks of treatment).
Paroxetine and fluoxetine are both metabolised by cytochrome P450 2D6 (while the active metabolite of fluoxetine, norfluoxetine, is metabolised by cytochrome P450 3A4) and inhibit their own metabolism, resulting in non-linear kinetics [70]. This predicts disproportionate declines in plasma concentrations during dose reduction. While this effect may not be clinically significant for fluoxetine because of its long half-life, it is likely to be significant for paroxetine [28]. In addition, paroxetine may produce a more severe withdrawal syndrome than other SSRIs because it exhibits the highest known binding affinity for the central site of SERT [71], and demonstrates muscarinic antagonist effects and moderate norepinephrine transporter-inhibiting effects as well [16, 28].
One study each has examined withdrawal effects for agomelatine and vortioxetine. No withdrawal effects were detected for agomelatine after 12 weeks of treatment [72], consistent with the finding that agomelatine was the antidepressant least likely to be reported to the World Health Organization pharmacovigilance service [73]. No significant increase in withdrawal symptoms after 8 weeks of vortioxetine treatment was detected when compared to placebo, although the relatively long elimination half-life of vortioxetine (66 h) limits the conclusions that can be drawn from this study [74]. Henssler and colleagues designated vortioxetine a ‘moderate risk’ of withdrawal based on a systematic appraisal of studies, not restricted to RCTs.
Tiers of Risk Based on Drug Type
A recent structured analysis by Henssler et al. [15] attempted to quantify the relative risks of different antidepressants based on controlled trials, cohort studies, retrospective analyses and case reports [15]. We have supplemented this review with an analysis of calls to an English medication helpline for issues related to withdrawal, normalised to prescription numbers [23], as well as a comparative study of withdrawal effects from the World Health Organization pharmacovigilance database to provide a summary table of levels of risk for antidepressants (Table 5). We categorised antidepressants based on the metric of calls to a helpline for withdrawal, normalised to prescription numbers according to the following categories: high (≥ 15), moderate (6–14) and low (≤ 5) [23]. We also incorporated information from the review by Henssler et al., compressing ‘very high risk’ and ‘high risk’ into ‘high risk’. Last, we utilised the World Health Organization pharmacovigilance database to designate four antidepressants as ‘high risk’ based on a reporting odds ratio of greater than 2: duloxetine, paroxetine, venlafaxine and desvenlafaxine [73]. When there was contradictory categorisation, we chose the higher risk category on the precautionary principle. However, for some of the antidepressants outlined here only case reports were available [15], thus this summary can only be considered preliminary. Notably, the categories of risk generated from this procedure are consistent with findings of an earlier analysis that found that escitalopram had significantly lower rates of withdrawal effects than venlafaxine or paroxetine [58].
Table 5.
Common antidepressants stratified by risk of withdrawal symptoms, derived from Henssler et al. [15], calls to a withdrawal helpline, normalised to prescription numbers [23] and analysis of reports to a WHO pharmacovigilance service [73]
| Likelihood of withdrawal and severity of withdrawal | Henssler [15] | Taylor [23] | WHO [73] | Summary |
|---|---|---|---|---|
| Severe/frequent withdrawal |
Tranylcypromine Phenelzine Paroxetine Tricyclic antidepressants Venlafaxine Desvenlafaxine |
Tranylcypromine Moclobemide Isocarboxazid Phenelzine Fluvoxamine Mirtazapine Venlafaxine Reboxetine |
Desvenlafaxine Venlafaxine Duloxetine Paroxetine |
SNRIs Venlafaxine Desvenlafaxine Duloxetine Some SSRIs Paroxetine Fluvoxaminea MAOIs Tranylcypromine Phenelzine Isocarboxazida Moclobemidea Some TCAs Amitriptyline Imipramine Miscellaneous Mirtazapinea Reboxetinea |
| Moderately severe/moderately frequent withdrawal |
Citalopram Escitalopram Sertraline Duloxetine Vortioxetine |
Escitalopram Sertraline Citalopram Imipramine Clomipramine Lofepramine Nortriptyline |
Most SSRIs Citalopram Escitalopram Sertraline Some TCAs Nortriptyline Clomipraminea Lofepraminea |
|
| Less severe/less frequent withdrawal |
Fluoxetine Milnacipran |
Fluoxetine Trazadone Amitriptyline Mianserin Doxepin Trimipramine Dosulepin |
Fluoxetine Milnacipran Mianserin Doxepin Trimipramine Trazodone Dosulepin |
|
| Minimal/lowest withdrawal risk | Agomelatine | Agomelatine | ||
| Frequency and severity of withdrawal not known |
Mirtazapine Bupropion |
Bupropion Vortioxetineb |
MAOI monoamine oxidase inhibitor, SNRI serotonin and norepinephrine reuptake inhibitor, SSRI selective serotonin reuptake inhibitor, TCA tricyclic antidepressant, WHO World Health Organization
aThese medications have been upgraded on the basis of the Taylor et al. paper alone
bBased on feedback from a reviewer and a lack of evidence for vortioxetine captured in the Henssler review
Additionally, it is possible that formulations of antidepressants that convey sustained-release properties may be associated with less withdrawal effects than instant-release preparations, given the association observed between short-half and long half-life antidepressants [73], although these comparisons have not yet been studied.
Other Determinants of Withdrawal Risk
Individual characteristics may influence the risk of antidepressant withdrawal symptoms, related to the metabolism of the SSRI, sensitivity of SERT to inhibition and psychological factors [10, 75]. It has been hypothesised that different rates of metabolism of drugs (determined by cytochrome P450 polymorphisms) may affect the risk of withdrawal [59] but no such studies have investigated this relationship. The likelihood of withdrawal symptoms has been be associated with the C(-1019)G polymorphism of the 5HT1A receptor gene, known to be affected by long-term antidepressant treatment [33]. One clinical indication of likelihood of withdrawal is past experience of withdrawal, either when accidentally forgetting medication (e.g. when going on holiday) or on previous attempts to stop. There are likely to be other factors that determine the incidence and severity of withdrawal but this has not been widely studied.
Although there has been limited research into the topic, analyses conducted so far indicate that there is no difference in the risk of withdrawal effects depending on the diagnosis for which the medication was commenced [58]. This is consistent with the understanding that it is the effect of the drug on the brain that determines withdrawal effects [25].
Stratifying Patients on Risk of Withdrawal Effects
Based on the above characteristics that influence the risk of withdrawal symptoms, we have derived a broad means of stratifying patients with regard to their risk of withdrawal symptoms via means of a preliminary tool. From clinical experience, the strongest predictor of withdrawal symptoms is past experience of withdrawal symptoms (in a previous attempt at discontinuation, a drug switch or after skipped doses), as recognised in similar efforts to determine risk [76], and so this is given strong weighting (3 points) [Table 6]. Duration of use appears to have a strong effect on the risk of withdrawal symptoms, including their severity and therefore this has been given strong emphasis (3 points). Antidepressant type (4 points) has been associated with varying risk, and higher doses (1 point) as well, though to a lesser extent. An assessment of these risk factors can help to estimate the risk of withdrawal for a particular individual (Table 6 legend), which may inform the rate of taper suggested, with lower risk patients more likely to be able to tolerate quicker tapers, and higher risk patients selected for slower approaches.
Table 6.
Preliminary tool for evaluation of risk of withdrawal for an individual patient
| Determinant of withdrawal risk | Weighting |
|---|---|
| Duration of usea | |
| Short term (1–6 months) | 0 points |
| Intermediate term (6–12 months) | 1 point |
| Long term (1–3 years) | 2 points |
| Very long-term use (> 3 years) | 3 points |
| Antidepressant type | |
| Lowest risk (e.g. agomelatine) | 0 points |
| Low risk (e.g. fluoxetine, milnacipran, trimipramine, doxepin, dosulepin) | 1 point |
| Moderate risk (SSRIs: citalopram, escitalopram, sertraline, vortioxetine; some TCAs: nortriptyline, clomipramine, lofepramine) | 2 points |
| High risk (e.g. SNRIs: desvenlafaxine, duloxetine, venlafaxine; paroxetine; MAOIs: phenelzine, moclobemide; some TCAs: amitriptyline, imipramine; mirtazapine) | 4 points |
| Dosage | |
| Minimum therapeutic dosage or lower | 0 points |
| Greater than the minimum therapeutic dosage | 1 point |
| Past experience of withdrawal symptoms | |
| Stopped antidepressant in past with no withdrawal symptoms/unknown | 0 points |
| Mild to moderate withdrawal symptoms | 1 point |
| Severe withdrawal symptoms | 2 points |
| Very severe withdrawal symptoms | 3 points |
Low risk = 0 points. Medium risk = 1–3 points. High risk = 4–6 points. Very high risk = or >7 points
MAOI monoamine oxidase inhibitor, SNRI serotonin and norepinephrine reuptake inhibitor, SSRI selective serotonin reuptake inhibitor, TCA tricyclic antidepressant
aNote that very short-term use (<4 weeks) is not normally associated with a significant risk of withdrawal
This approach to risk estimation can only be seen as preliminary, building on similar previous efforts [76]. This tool requires validation before it is widely adopted to guide the choice of discontinuation strategies in clinical practice. This validation could be performed by the aggregation of sociodemographic, clinical and medication usage characteristics from large numbers of patients discontinuing medication, and their rate of tapering and severity (and duration) of withdrawal effects or analysis of existing databases containing such information. This would allow development of the tool to iteratively reflect the risk of withdrawal effects, allowing better prediction for individual patients.
Conclusions
We have reviewed the existing literature on the incidence, severity and duration of withdrawal symptoms as well as examined the relationship between characteristics of use (such as dosage, duration of use and type of antidepressant) and withdrawal symptoms. Information in these domains is limited and more research is required to draw firmer conclusions about the determinants of withdrawal symptoms, particularly regarding severity and duration.
From existing data, there appears to be a relationship between the duration of antidepressant use and the risk of withdrawal symptoms, consistent with the idea that a greater duration of use will produce greater neurological adaptation. We also reviewed data suggesting some evidence of a weak relationship between a greater dosage of antidepressant and a greater chance of withdrawal symptoms, but that there may be ceiling effects, consistent with the hyperbolic relationship between the dose and effect of antidepressants resulting in target receptor saturation [59]. There appears also to be a wide variation of risk of withdrawal based on which antidepressant is taken. Past experience of withdrawal symptoms on reducing or stopping medication is a strong predictor of withdrawal symptoms on subsequent attempts to reduce or stop.
From these risk factors, we have derived a simple rubric for determining withdrawal risk for a given patient, which may be useful in clinical practice for stratifying people according to risk. We hope that future empirical work will be able to offer a refined version of this risk calculator.
Declarations
Funding
This paper was not supported by any funding. Mark Abie Horowitz is supported by a Clinical Research Fellowship through the North East London NHS Foundation Trust. This funding source had no role in the writing of the manuscript or the decision to submit it for publication. MAH is an honorary Clinical Research Fellow at UCL. The Psychiatry Research Trust funded the open access publication of this article. This funder was not involved in the planning or writing of the manuscript, or decision to submit for publication.
Conflict of interest
Anders Sørensen has no conflicts of interest. Mark Abie Horowitz declares that he is a co-founder of Outro Health, a company aiming to help people safely stop unnecessary antidepressants in Canada and North America. Adele Framer declares she is a co-founder of Outro Health. Michael P. Hengartner reports royalties from Palgrave Macmillan, London, UK for his book published in December, 2021, called “Evidence-biased Antidepressant Prescription.” David Taylor reports grants and personal fees from Janssen, Sunovion, Recordati and Mylan, and personal fees from Accord, outside the submitted work.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data in this study are available in the public domain.
Code availability
Not applicable.
Author contributions
MAH conceived the idea of the manuscript, conducted the searches and wrote the first draft. MPH performed the data analysis and contributed substantially to the manuscript. AF further developed the concepts of the paper and substantially contributed to the final form of the manuscript. AS and DT revised and contributed substantially to the final form of the manuscript. DT supervised the project. All authors have read and approve the final submitted manuscript and all agree to be accountable for the work.
References
- 1.NHS Business Services Authority. Medicines used in mental health England 2015/16 to 2019/20. 2020. Available from: https://nhsbsa-opendata.s3-eu-west-2.amazonaws.com/mh-annual-narrative-final.html. Accessed 4 May 2021.
- 2.Johnson CF, Macdonald HJ, Atkinson P, Buchanan AI, Downes N, Dougall N. Reviewing long-term antidepressants can reduce drug burden: a prospective observational cohort study. Br J Gen Pract. 2012;62:e773–e779. doi: 10.3399/bjgp12X658304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Public Health England. Dependence and withdrawal associated with some prescribed medicines: an evidence review. 2019. Available from: https://www.gov.uk/government/publications/prescribed-medicines-review-report. Accessed 25 May 2021.
- 4.Jauhar S, Hayes J, Goodwin GM, Baldwin DS, Cowen PJ, Nutt DJ. Antidepressants, withdrawal, and addiction; where are we now? J Psychopharmacol. 2019;33:655–659. doi: 10.1177/0269881119845799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies J, Read J. A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: are guidelines evidence-based? Addict Behav. 2019;97:111–121. doi: 10.1016/j.addbeh.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Hengartner MP. Commentary on Jauhar and Hayes. Addict Behav. 2019;97:131. doi: 10.1016/j.addbeh.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Davies J, Read J. Authors’ response to a critique by Jauhar and Hayes of “A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: are guideline evidence-based?”. Addict Behav. 2019;97:127–130. doi: 10.1016/j.addbeh.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Guy A, Brown M, Lewis S, Horowitz MA. The “Patient Voice”—patients who experience antidepressant withdrawal symptoms are often dismissed, or mis-diagnosed with relapse, or onset of a new medical condition. Ther Adv Psychopharmacol. 2020;10:204512532096718. doi: 10.1177/2045125320967183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hengartner MP. How effective are antidepressants for depression over the long term? A critical review of relapse prevention trials and the issue of withdrawal confounding. Ther Adv Psychopharmacol. 2020;10:2045125320921694. doi: 10.1177/2045125320921694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddad PM, Anderson IM. Recognising and managing antidepressant discontinuation symptoms. Adv Psychiatr Treat. 2007;13:447–457. [Google Scholar]
- 11.Valuck RJ, Orton HD, Libby AM. Antidepressant discontinuation and risk of suicide attempt. J Clin Psychiatry. 2009;70:1069–1077. doi: 10.4088/JCP.08m04943. [DOI] [PubMed] [Google Scholar]
- 12.Young A, Haddad P. Discontinuation symptoms and psychotropic drugs. Lancet. 2000;355:1184–1185. doi: 10.1016/S0140-6736(05)72262-5. [DOI] [PubMed] [Google Scholar]
- 13.Hengartner MP, Schulthess L, Sorensen A, Framer A. Protracted withdrawal syndrome after stopping antidepressants: a descriptive quantitative analysis of consumer narratives from a large internet forum. Ther Adv Psychopharmacol. 2020;10:2045125320980573. doi: 10.1177/2045125320980573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jauhar S, Hayes J. The war on antidepressants: what we can, and can’t conclude, from the systematic review of antidepressant withdrawal effects by Davies and Read. Addict Behav. 2019;97:122–125. doi: 10.1016/j.addbeh.2019.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henssler J, Heinz A, Brandt L, Bschor T. Antidepressant withdrawal and rebound phenomena. Dtsch Arztebl Int. 2019;116:355–361. doi: 10.3238/arztebl.2019.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renoir T. Selective serotonin reuptake inhibitor antidepressant treatment discontinuation syndrome: a review of the clinical evidence and the possible mechanisms involved. Front Pharmacol. 2013;4:45. doi: 10.3389/fphar.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien C. Addiction and dependence in DSM-V. Addiction. 2011;106:866–867. doi: 10.1111/j.1360-0443.2010.03144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Academy of Pain Medicine, the American Pain Society and the American Society of Addition Medicine Definitions related to the use of opioids for the treatment of pain. WMJ. 2001;100:28–29. [PubMed] [Google Scholar]
- 19.National Institute on Drug Abuse. Is there a difference between physical dependence and addiction?. National Institute on Drug Abuse. Available from: https://nida.nih.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/frequently-asked-questions/there-difference-between-physical-dependence-addiction. Accessed 31 May 2022.
- 20.Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman’s the pharmacological basis of therapeutics. 12. New York: McGraw-Hill Education; 2011. [Google Scholar]
- 21.Lerner A, Klein M. Dependence, withdrawal and rebound of CNS drugs: an update and regulatory considerations for new drugs development. Brain Commun. 2019;1:fcz025. doi: 10.1093/braincomms/fcz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howland RH. Potential adverse effects of discontinuing psychotropic drugs: part 2: antidepressant drugs. J Psychosoc Nurs Ment Health Serv. 2010;48:9–12. doi: 10.3928/02793695-20100527-98. [DOI] [PubMed] [Google Scholar]
- 23.Taylor D, Stewart S, Connolly A. Antidepressant withdrawal symptoms: telephone calls to a national medication helpline. J Affect Disord. 2006;95:129–133. doi: 10.1016/j.jad.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Turton S, Lingford-Hughes A. Neurobiology and principles of addiction and tolerance. Medicine. 2016;44(12):693–696. [Google Scholar]
- 25.Hyman SE, Nestler EJ. Initiation and adaptation: a paradigm for understanding psychotropic drug action. Am J Psychiatry. 1996;153:151–162. doi: 10.1176/ajp.153.2.151. [DOI] [PubMed] [Google Scholar]
- 26.Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13:300–307. doi: 10.1177/026988119901300321. [DOI] [PubMed] [Google Scholar]
- 27.Reidenberg MM. Drug discontinuation effects are part of the pharmacology of a drug. J Pharmacol Exp Ther. 2011;339:324–328. doi: 10.1124/jpet.111.183285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olver JS, Burrows GD, Norman TR. Discontinuation syndromes with selective serotonin reuptake inhibitors. CNS Drugs. 1999;12:171–177. [Google Scholar]
- 29.Meyer JH, Kapur S, Eisfeld B, Brown GM, Houle S, DaSilva J, et al. The effect of paroxetine on 5-HT2Areceptors in depression: an [18F]setoperone PET imaging study. Am J Psychiatry. 2001;158:78–85. doi: 10.1176/appi.ajp.158.1.78. [DOI] [PubMed] [Google Scholar]
- 30.Haahr ME, Fisher PM, Jensen CG, Frokjaer VG, McMahon B, Madsen K, et al. Central 5-HT4receptor binding as biomarker of serotonergic tonus in humans: a [11C]SB207145 PET study. Mol Psychiatry. 2014;19:427–432. doi: 10.1038/mp.2013.147. [DOI] [PubMed] [Google Scholar]
- 31.Gray NA, Milak MS, DeLorenzo C, Ogden RT, Huang Y-Y, Mann JJ, et al. Antidepressant treatment reduces serotonin-1A autoreceptor binding in major depressive disorder. Biol Psychiatry. 2013;74:26–31. doi: 10.1016/j.biopsych.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol Psychiatry. 2004;9:386–392. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- 33.Murata Y, Kobayashi D, Imuta N, Haraguchi K, Ieiri I, Nishimura R, et al. Effects of the serotonin 1A, 2A, 2C, 3A, and 3B and serotonin transporter gene polymorphisms on the occurrence of paroxetine discontinuation syndrome. J Clin Psychopharmacol. 2010;30:11–17. doi: 10.1097/JCP.0b013e3181c8ae80. [DOI] [PubMed] [Google Scholar]
- 34.Quinn R. Comparing rat’s to human’s age: how old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Bosker FJ, Tanke MAC, Jongsma ME, Cremers TIFH, Jagtman E, Pietersen CY, et al. Biochemical and behavioral effects of long-term citalopram administration and discontinuation in rats: role of serotonin synthesis. Neurochem Int. 2010;57:948–957. doi: 10.1016/j.neuint.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Kitaichi Y, Inoue T, Nakagawa S, Boku S, Kakuta A, Izumi T, et al. Sertraline increases extracellular levels not only of serotonin, but also of dopamine in the nucleus accumbens and striatum of rats. Eur J Pharmacol. 2010;647:90–96. doi: 10.1016/j.ejphar.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 37.Raap DK, Garcia F, Muma NA, Wolf WA, Battaglia G, van de Kar LD. Sustained desensitization of hypothalamic 5-Hydroxytryptamine1A receptors after discontinuation of fluoxetine: inhibited neuroendocrine responses to 8-hydroxy-2-(dipropylamino)tetralin in the absence of changes in Gi/o/z proteins. J Pharmacol Exp Ther. 1999;288:561–567. [PubMed] [Google Scholar]
- 38.Blier P, Tremblay P. Physiologic mechanisms underlying the antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(Suppl. 4):8–13. [PubMed] [Google Scholar]
- 39.Schatzberg AF, Haddad P, Kaplan EM, Lejoyeux M, Rosenbaum JF, Young AH, et al. Serotonin reuptake inhibitor discontinuation syndrome: a hypothetical definition. Discontinuation Consensus Panel. J Clin Psychiatry. 1997;58(Suppl. 7):5–10. [PubMed] [Google Scholar]
- 40.Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4:5. doi: 10.1186/s41073-019-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenbaum JF, Fava M, Hoog SL, Ascroft RC, Krebs WB. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44:77–87. doi: 10.1016/s0006-3223(98)00126-7. [DOI] [PubMed] [Google Scholar]
- 42.Levinson-Castiel R, Merlob P, Linder N, Sirota L, Klinger G. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Arch Pediatr Adolesc Med. 2006;160:173–176. doi: 10.1001/archpedi.160.2.173. [DOI] [PubMed] [Google Scholar]
- 43.Hindmarch I, Kimber S, Cockle SM. Abrupt and brief discontinuation of antidepressant treatment: effects on cognitive function and psychomotor performance. Int Clin Psychopharmacol. 2000;15:305–318. doi: 10.1097/00004850-200015060-00001. [DOI] [PubMed] [Google Scholar]
- 44.Horowitz MA, Taylor D. Distinguishing relapse from antidepressant withdrawal: clinical practice and antidepressant discontinuation studies. B J Psych Adv. 2022;28:297–311. [Google Scholar]
- 45.Zajecka J, Fawcett J, Amsterdam J, Quitkin F, Reimherr F, Rosenbaum J, et al. Safety of abrupt discontinuation of fluoxetine: a randomized, placebo-controlled study. J Clin Psychopharmacol. 1998;18:193–197. doi: 10.1097/00004714-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Haddad P, Devarajan S, Dursun S. Antidepressant discontinuation (withdrawal) symptoms presenting as “stroke”. J Psychopharmacol. 2001;15:139–141. doi: 10.1177/026988110101500210. [DOI] [PubMed] [Google Scholar]
- 47.Weller I, Ashby D, Chambers M, Chick J, Drummond C, Ebmeier K, et al. Report of the CSM Expert Working Group on the safety of selective serotonin reuptake inhibitors antidepressants. London: MHRA; 2005. [Google Scholar]
- 48.Pacheco L, Malo P, Aragues E, Etxebeste M. More cases of paroxetine withdrawal syndrome. Br J Psychiatry. 1996;169:384. doi: 10.1192/bjp.169.3.384a. [DOI] [PubMed] [Google Scholar]
- 49.Rosenbaum JF, Zajecka J. Clinical management of antidepressant discontinuation. J Clin Psychiatry. 1997;58(Suppl. 7):37–40. [PubMed] [Google Scholar]
- 50.Davies J, Regina P, Montagu L. Antidepressant withdrawal: a survey of patients’ experience by the All-Party Parliamentary Group for Prescribed Drug Dependence. All-Party Parliamentary Group for Prescribed Drug Dependence; 2018. Available from: http://prescribeddrug.org/wp-content/uploads/2018/10/APPG-PDD-Survey-of-antidepressant-withdrawal-experiences.pdf. Accessed 7 Oct 2022.
- 51.Read J, Williams J. Adverse effects of antidepressants reported by a large international cohort: emotional blunting, suicidality, and withdrawal effects. Curr Drug Saf. 2018;13:176–186. doi: 10.2174/1574886313666180605095130. [DOI] [PubMed] [Google Scholar]
- 52.Sir A, D’Souza RF, Uguz S, George T, Vahip S, Hopwood M, et al. Randomized trial of sertraline versus venlafaxine XR in major depression: efficacy and discontinuation symptoms. J Clin Psychiatry. 2005;66:1312–1320. doi: 10.4088/jcp.v66n1015. [DOI] [PubMed] [Google Scholar]
- 53.Price JS, Waller PC, Wood SM, Mackay AVP. A comparison of the post-marketing safety of four selective serotonin re-uptake inhibitors including the investigation of symptoms occurring on withdrawal. Br J Clin Pharmacol. 1996;42:757–763. doi: 10.1046/j.1365-2125.1996.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhanji NH, Chouinard G, Kolivakis T, Margolese HC. Persistent tardive rebound panic disorder, rebound anxiety and insomnia following paroxetine withdrawal: a review of rebound-withdrawal phenomena. Can J Clin Pharmacol. 2006;13:e69–74. [PubMed] [Google Scholar]
- 55.Davies J, Read J, Hengartner MP, Cosci F, Fava G, Chouinard G, et al. Clinical guidelines on antidepressant withdrawal urgently need updating. BMJ. 2019;365:l2238. doi: 10.1136/bmj.l2238. [DOI] [PubMed] [Google Scholar]
- 56.Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216:67–73. doi: 10.1016/j.psychres.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 57.Read J, Cartwright C, Gibson K. How many of 1829 antidepressant users report withdrawal effects or addiction? Int J Ment Health Nurs. 2018;27:1805–1815. doi: 10.1111/inm.12488. [DOI] [PubMed] [Google Scholar]
- 58.Baldwin DS, Montgomery SA, Nil R, Lader M. Discontinuation symptoms in depression and anxiety disorders. Int J Neuropsychopharmacol. 2007;10:73–84. doi: 10.1017/S1461145705006358. [DOI] [PubMed] [Google Scholar]
- 59.Horowitz MA, Taylor D. Tapering of SSRI treatment to mitigate withdrawal symptoms. Lancet Psychiatry. 2019;6:538–546. doi: 10.1016/S2215-0366(19)30032-X. [DOI] [PubMed] [Google Scholar]
- 60.Holford N. Pharmacodynamic principles and the time course of delayed and cumulative drug effects. Transl Clin Pharmacol. 2018;26:56. doi: 10.12793/tcp.2018.26.2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161:826–835. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- 62.Furukawa TA, Cipriani A, Cowen PJ, Leucht S, Egger M, Salanti G. Optimal dose of selective serotonin reuptake inhibitors, venlafaxine, and mirtazapine in major depression: a systematic review and dose-response meta-analysis. Lancet Psychiatry. 2019;6:601–609. doi: 10.1016/S2215-0366(19)30217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Debonnel G, Saint-André É, Hébert C, De Montigny C, Lavoie N, Blier P. Differential physiological effects of a low dose and high doses of venlafaxine in major depression. Int J Neuropsychopharmacol. 2007;10:51–61. doi: 10.1017/S1461145705006413. [DOI] [PubMed] [Google Scholar]
- 64.Owens MJ, Krulewicz S, Simon JS, Sheehan DV, Thase ME, Carpenter DJ, et al. Estimates of serotonin and norepinephrine transporter inhibition in depressed patients treated with paroxetine or venlafaxine. Neuropsychopharmacology. 2008;33:3201–3212. doi: 10.1038/npp.2008.47. [DOI] [PubMed] [Google Scholar]
- 65.Horowitz MA, Taylor D. Tapering of SSRI treatment to mitigate withdrawal symptoms: authors’ reply. Lancet Psychiatry. 2019;6:562–563. doi: 10.1016/S2215-0366(19)30219-6. [DOI] [PubMed] [Google Scholar]
- 66.Moncrieff J, Gupta S, Horowitz MA. Barriers to stopping neuroleptic (antipsychotic) treatment in people with schizophrenia, psychosis or bipolar disorder. Ther Adv Psychopharmacol. 2020;10:2045125320937910. doi: 10.1177/2045125320937910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horowitz MA, Murray RM, Taylor D. Tapering antipsychotic treatment. JAMA. Psychiatry. 2021;78:125–126. doi: 10.1001/jamapsychiatry.2020.2166. [DOI] [PubMed] [Google Scholar]
- 68.Michelson D, Fava M, Amsterdam J, Apter J, Londborg P, Tamura R, et al. Interruption of selective serotonin reuptake inhibitor treatment: double-blind, placebo-controlled trial. Br J Psychiatry. 2000;176:363–368. doi: 10.1192/bjp.176.4.363. [DOI] [PubMed] [Google Scholar]
- 69.Fava GA, Gatti A, Belaise C, Guidi J, Offidani E. Withdrawal symptoms after selective serotonin reuptake inhibitor discontinuation: a systematic review. Psychother Psychosom. 2015;84:72–81. doi: 10.1159/000370338. [DOI] [PubMed] [Google Scholar]
- 70.Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors: an overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet. 1997;32(Suppl. 1):1–21. doi: 10.2165/00003088-199700321-00003. [DOI] [PubMed] [Google Scholar]
- 71.Coleman JA, Navratna V, Antermite D, et al. Chemical and structural investigation of the paroxetine-human serotonin transporter complex. Elife. 2020;9:e56427. doi: 10.7554/eLife.56427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montgomery SA, Kennedy SH, Burrows GD, Lejoyeux M, Hindmarch I. Absence of discontinuation symptoms with agomelatine and occurrence of discontinuation symptoms with paroxetine: a randomized, double-blind, placebo-controlled discontinuation study. Int Clin Psychopharmacol. 2004;19:271–280. doi: 10.1097/01.yic.0000137184.64610.c8. [DOI] [PubMed] [Google Scholar]
- 73.Quilichini J-B, Revet A, Garcia P, Bouquié R, Hamard J, Yrondi A, et al. Comparative effects of 15 antidepressants on the risk of withdrawal syndrome: a real-world study using the WHO pharmacovigilance database. J Affect Disord. 2021;297:189–193. doi: 10.1016/j.jad.2021.10.041. [DOI] [PubMed] [Google Scholar]
- 74.Baldwin DS, Chrones L, Florea I, Nielsen R, Nomikos GG, Palo W, et al. The safety and tolerability of vortioxetine: analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol. 2016;30:242–252. doi: 10.1177/0269881116628440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bitter I, Filipovits D, Czobor P. Adverse reactions to duloxetine in depression. Expert Opin Drug Saf. 2011;10:839–850. doi: 10.1517/14740338.2011.582037. [DOI] [PubMed] [Google Scholar]
- 76.Ruhe HG, Horikx A, van Avendonk MJP, Groeneweg BF, Woutersen-Koch H. Discontinuation of Antidepressants Taskforce. Tapering of SSRI treatment to mitigate withdrawal symptoms. Lancet Psychiatry. 2019;6:561–562. doi: 10.1016/S2215-0366(19)30182-8. [DOI] [PubMed] [Google Scholar]
- 77.Horowitz MA, Taylor D. How to reduce and stop psychiatric medication. Eur Neuropsychopharmacol. 2021;55:4–7. doi: 10.1016/j.euroneuro.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 78.Oehrberg S, Christiansen PE, Behnke K, Borup AL, Severin B, Soegaard J, et al. Paroxetine in the treatment of panic disorder: a randomised, double-blind, placebo-controlled study. Br J Psychiatry. 1995;167:36–42. doi: 10.1192/bjp.167.3.374. [DOI] [PubMed] [Google Scholar]
- 79.Montgomery SA, Nil R, Durr-Pal N, Loft H, Boulenger J-P. A 24-week randomized, double-blind, placebo-controlled study of escitalopram for the prevention of generalized social anxiety disorder. J Clin Psychiatry. 2005;66:1270–1278. doi: 10.4088/jcp.v66n1009. [DOI] [PubMed] [Google Scholar]
- 80.Baldwin DS, Huusom AKT, Maehlum E. Escitalopram and paroxetine compared to placebo in the treatment of generalised anxiety disorder (GAD) Br J Psychiatry. 2006;189:264–272. doi: 10.1192/bjp.bp.105.012799. [DOI] [PubMed] [Google Scholar]
- 81.Lader M, Stender K, Bürger V, Nil R. Efficacy and tolerability of escitalopram in 12- and 24-week treatment of social anxiety disorder: randomised, double-blind, placebo-controlled, fixed-dose study. Depress Anxiety. 2004;19:241–248. doi: 10.1002/da.20014. [DOI] [PubMed] [Google Scholar]