Abstract
SARS-CoV-2-specific T cell response has been proven essential for viral clearance, COVID-19 outcome and long-term memory. Impaired early T cell-driven immunity leads to a severe form of the disease associated with lymphopenia, hyperinflammation and imbalanced humoral response. Analyses of acute SARS-CoV-2 infection have revealed that mild COVID-19 course is characterized by an early induction of specific T cells within the first 7 days of symptoms, coordinately followed by antibody production for an effective control of viral infection. In contrast, patients who do not develop an early specific cellular response and initiate a humoral immune response with subsequent production of high levels of antibodies, develop severe symptoms. Yet, delayed and persistent bystander CD8+ T cell activation has been also reported in hospitalized patients and could be a driver of lung pathology. Literature supports that long-term maintenance of T cell response appears more stable than antibody titters. Up to date, virus-specific T cell memory has been detected 22 months post-symptom onset, with a predominant IL-2 memory response compared to IFN-γ. Furthermore, T cell responses are conserved against the emerging variants of concern (VoCs) while these variants are mostly able to evade humoral responses. This could be partly explained by the high HLA polymorphism whereby the viral epitope repertoire recognized could differ among individuals, greatly decreasing the likelihood of immune escape. Current COVID-19-vaccination has been shown to elicit Th1-driven spike-specific T cell response, as does natural infection, which provides substantial protection against severe COVID-19 and death. In addition, mucosal vaccination has been reported to induce strong adaptive responses both locally and systemically and to protect against VoCs in animal models. The optimization of vaccine formulations by including a variety of viral regions, innovative adjuvants or diverse administration routes could result in a desirable enhanced cellular response and memory, and help to prevent breakthrough infections. In summary, the increasing evidence highlights the relevance of monitoring SARS-CoV-2-specific cellular immune response, and not only antibody levels, as a correlate for protection after infection and/or vaccination. Moreover, it may help to better identify target populations that could benefit most from booster doses and to personalize vaccination strategies.
Keywords: T cell, SARS-CoV-2, vaccination, adaptive immunity, hybrid immunity
Graphical Abstract
Introduction
The pandemic caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) has resulted in more than 634 million cases of coronavirus disease-19 (COVID-19) and 6.5 million deaths reported worldwide (1). Although the landscape of COVID-19 has evolved substantially since the pandemic onset, understanding how adaptive immune responses develop after infection and vaccination, how this immunity translates into protection against infection and severe forms of the disease, and how we can improve vaccination to reduce transmission remains a major challenge.
Studies on the development of adaptive immunity during the acute infection with other highly transmissible and pathogenic coronaviruses, such as severe acute respiratory syndrome-coronavirus (SARS-CoV) and Middle East respiratory syndrome-coronavirus (MERS-CoV) showed similar dynamics to those reported during the current COVID-19 pandemic (2), which will be discussed in depth later in this review. During SARS-CoV infection, specific serum antibodies are detected 4-7 days after symptom onset (3, 4), and most patients seroconvert within 21 days of onset (5). Similarly, in MERS-CoV infection, the seroconversion process is observed during 15–21 days after infection (6), however, it has been reported that this antibody response is not likely to be correlated with the viral clearance (7, 8). Regarding the T cell response, a dominant Th1 response has been described during SARS-CoV infection (9), whereas in MERS-CoV infection the early increase of CD8+ T cells correlates with disease severity and a dominant Th1 response is observed in the early convalescent stages (10). In addition, a rapid and significant CD4+ and CD8+ T lymphopenia was associated with adverse disease outcome during SARS-CoV (9, 11) and MERS-CoV infection (12), as it has been widely reported during the severe course of COVID-19 (13, 14).
Additionally, studies on the duration of immunity against these coronaviruses report long-lasting SARS-CoV-specific memory T cells up to 17 years after the outbreak of SARS-CoV in 2003 (15) even though antibodies and peripheral memory B cell responses are not detected beyond 3 years after the onset of infection (16, 17). In the same way, T cell responses to MERS-CoV have been detected in patients with undetectable antibody responses up to 2 years after infection (18).
Along these lines, this review summarizes data on the role of SARS-CoV-2-specific T cells in the different phases of natural infection, their relationship to disease outcome and their long-term maintenance. In addition, the impact of vaccine-triggered cell-mediated immunity and the effect of emerging new variants of concern (VoCs) on the ability of T cells to protect from infection and severe COVID-19 are discussed.
SARS-CoV-2-specific cellular response during acute infection
A coordinated response between innate and adaptive immunity is necessary to control and eliminate SARS-CoV-2 infection. A highly impaired interferon (IFN) type I response, characterized by the absence of IFN-β and low IFN-α production and activity, has been described in severe and critical patients. This defect is associated with persistent blood viral load and exacerbated inflammatory response (19, 20) accompanied by a plasma cytokine signature of elevated CXCL10, interleukin (IL)-6, and IL-8 (21–23). Bastard et al. identified that at least 10% of patients with life threatening COVID-19 pneumonia have neutralizing autoantibodies against type I IFNs (24), while Zhang et al. reported that inborn errors of TLR3- and IRF7-dependent type I immunity underlie critical COVID-19 (25).
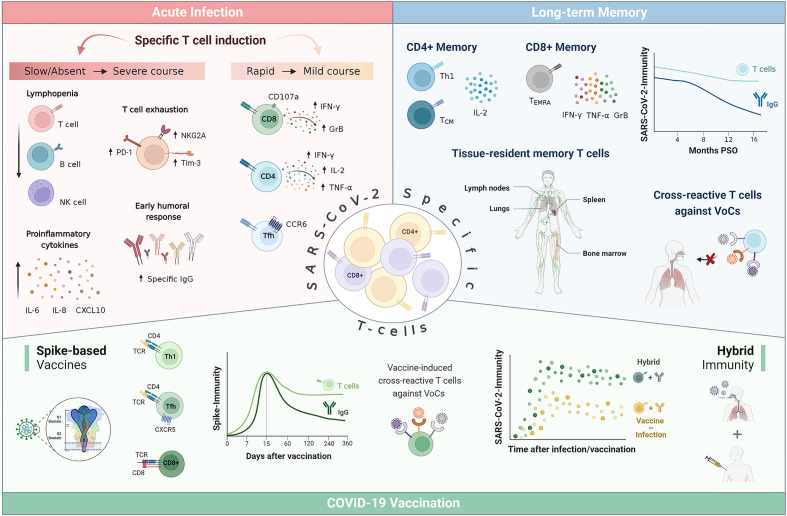
Regarding the adaptive immunity, in the context of acute SARS-CoV-2 infection, virus-specific CD8+ T cells with cytolytic capacity against infected cells can be detected as early as day 1 post symptom onset (PSO) (26). Their rapid induction normally occurred within the first 7 days and peaked at 2 weeks PSO (27, 28). This virus-specific CD8+ T cell dynamic has been associated with better COVID-19 outcomes (28–30), as has their capacity to produce high levels of cytotoxic effector molecules, such as IFN-γ, granzyme B and perforin (26, 29, 31). A CD8+ T cell depletion study in non-human primates resulted in a loss of protection in the upper respiratory tract against rechallenge with SARS-CoV-2, which suggests that CD8+ T cells contribute to virological control (32). In addition, Liao et al. demonstrated that a larger proportion of CD8+ T cell effectors with tissue-resident and highly expanded features were present in bronchoalveolar lavage fluid (BALF) from patients with moderate COVID-19 compared to patients with severe/critical infection (33). However, at a local level, lymphocytes with the capacity to kill SARS-CoV-2-infected cells have also been related to further tissue damage and pathogenicity, even late after acute COVID-19. Increased cytotoxic T cells in BALF have been linked to epithelial damage and airway dysfunction (34), and a marked interstitial CD8+ T cell infiltration has been observed in cryotransbronchial biopsies of patients with persistent SARS-CoV-2 pneumonitis (35).
SARS-CoV-2-specific CD4+ T cells can also normally be detected as early as day 2–4 PSO (29, 36) and are more prominent than CD8+ T cell responses (31, 37). Their rapid induction, magnitude and breadth have been associated with accelerated viral clearance and mild COVID-19 (36, 38), whilst their absence within the 22 days PSO has been linked with severe or fatal COVID-19 (39). SARS-CoV-2 infection mainly supports the expansion of Th1 and T follicular helper (Tfh) CD4+ T cells (39–41). Th1 cells have antiviral activity via production of IFN-γ and related cytokines and support cellular and innate immunity against pathogens (42), while Tfh cells are specialized in helping B cells to differentiate into plasmablasts and produce class-switched antibodies within secondary lymphoid organs (43). The majority of SARS-CoV-2-specific CD4+ T cells from COVID-19 patients show a clear IFN-γ, tumor necrosis factor (TNF) and IL-2 protein signature characteristic of canonical Th1 cells (30, 31, 39). While, some studies have reported an incorrect Th polarization with an underrepresentation of Th1 subset in severe COVID-19 patients (44, 45), other authors have informed that a prolonged Th1 cytokine profile was maintained in patients with severe COVID-19 (46). Of note, more recent work has highlighted the importance of Th1-polarized CD4+ T cells to switch towards an IFN-γ and IL-10 functional profile to limit the process of tissue inflammation (47, 48). SARS-CoV-2-specific circulating Tfh (cTfh) cells are generated during acute infection (41, 49) and their frequency has been associated with reduced disease severity (29). A substantial proportion of SARS-CoV-2-specific cTfh are CXCR3-CCR6+ (cTfh17), which has also been observed for the common cold coronavirus, potentially indicative of mucosal airway homing (50). The other two cTfh subtypes, CCR6-CXCR3+ (cTfh1) and CCR6-CXCR3- (cTfh2) have been found to positively correlate with neutralizing activity (50, 51).
The vast majority of SARS-CoV-2 infected individuals seroconvert within 5–15 days PSO (52, 53). Neutralizing antibodies also develop rapidly, on the same time frame as seroconversion (54), reaching maximum levels within 3–5 weeks after infection (55). However, a strong humoral response has been reported in severe COVID-19 patients that is not associated with reduced disease severity (56–59), which had also been observed in MERS-CoV infected patients (60).
SARS-CoV-2-specific cellular response protects against severe COVID-19
Several studies have revealed that severe COVID-19 patients fail to mount an early, robust T cell response against SARS-CoV-2 and instead initiate a humoral immune response with subsequent production of high antibodies levels unable to efficiently clear the virus (36, 61, 62). Interestingly, results from our group showed that severe patients who subsequently died had a complete lack of specific cellular response while severe patients who survived had a low but detectable number of specific T cells (61). Moreover, we have demonstrated that the specific cellular response against the domain 1 of spike (S1), measured at emergency room is a protective factor against severity (OR per 100 IFN-γ sfu/106 PBMC increment: 0.47, 95%CI 0.20–0.87, p<0.05), independently of age and sex (61). These data suggest that for a total effective control of SARS-CoV-2 infection, an early induction of specific T cells within the first 7 days which peaks at 2 weeks PSO, followed by coordinated antibody production, is necessary (29, 36, 38, 61, 63). The disconnection between humoral and cellular immunity observed in severe patients (29, 36, 62) could be due to a delay in the innate immune response that may lead to a failure in T cell priming (19, 20). Furthermore, other remarkable studies have shown increased frequency of SARS-CoV-2-specific T cells in highly exposed individuals who remain seronegative and PCR negative for SARS-CoV-2 (31, 64–66), suggesting that a rapid deployment of virus-specific cellular immunity suppresses viral replication and successfully aborts infection. In addition, Nelde et al. have shown that the diversity of T cell responses to SARS-CoV-2, not just their magnitude, was associated with mild COVID-19 symptoms, demonstrating that immunity requires recognition of multiple epitopes to control the disease severity (65).
Apart from the lack of early T cell response, severe COVID-19 is also characterized by a marked lymphopenia (13, 14, 67), particularly of CD8+ T cells (68, 69) which resolution correlates with recovery (70). This lymphopenia could partly be due to the robust activated CD8+ T cell recruitment into the lung and brain tissue in critically ill patients (34, 71, 72). However, Szabo et al. have proposed that higher T cell frequencies in the lung correlated positively with survival, whereas higher lung infiltrative myeloid cells correlated with mortality and older age in severe COVID-19 patients (73). Other mechanisms underlying lymphopenia could be an increased T cell apoptosis in patients with COVID-19 (27, 74) or an impaired lymphocyte proliferation (75, 76). However, other studies have described intense T cell proliferation concurring with the lymphopenia (77, 78). Similarly, several studies have reported an exhausted phenotype of CD8+ T cells in severe COVID-19, with an increased expression of inhibitory receptors such as PD-1 and Tim-3 (78–80) and related to the overexpression of the natural killer group 2 member A (NKG2A) in CD8+ T cells (81, 82). On the contrary, recent studies have reported that the expression of exhaustion markers (CTLA-4, PD-1, TIGIT, and Tox) in some SARS-CoV-2-specific CD8+ T cells was not associated with T cell dysfunction (83, 84). An overaggressive CD8+ T cell response or a hyperactive state have also been linked to severe COVID-19 (61, 85, 86).
Aging has also been associated with poor disease outcome, with increased rates of severe and fatal COVID-19 among individuals older than 65 years (29, 87, 88). Age-related remodelling of the immune system, or immunosenescence, is considered the main cause of increased susceptibility to infections, particularly respiratory infections such as influenza, and reduced vaccine effectiveness among the elderly (89, 90). In addition, studies in animal models have reported a decrease in the number of professional antigen presenting cells in the lungs with advanced age (91, 92), which together with the age-associated weaker type I IFN responses may lead to poorer T cell responses to viral infection (93, 94). SARS-CoV-2 capacity to early evade innate immunity (23, 95) may further limit T cell priming in the elderly. Scarcity of naïve T cells in older individuals has also been highlighted as a specific risk factor for severe COVID-19 (96, 97). Finally, the overall disruption of coordinated SARS-CoV-2-specific adaptive responses in older individuals, with a loss of coordination between CD4+ T cell and antibody responses, may contribute to a failure of infection control (29).
In addition, SARS-CoV-2 T cell cross-reactivity, i.e., the existence of memory T cells induced by previous pathogens, could play an important role in the COVID-19 outcome (98). It has been reported that recently seasonal coronavirus infected individuals develop less severe COVID-19 (99). Similarly, pre-existing cross-reactive T cells facilitated the expansion of SARS-CoV-2-specific CD8+ and CD4+ T cell responses during infection (100, 101) and were associated with control of viral replication and abortive infection (66, 102). Although some studies have reported the presence of SARS-CoV-2 cross-reactive T cells in 20% to 80% of unexposed individuals (15, 37, 103, 104), in other series no cross-reactive T cells have been found (30, 105). However, these disparities may be due to the different technical approaches performed (106). T cell cross-reactivity has been described to be more frequent among children and young adults (107, 108), and to be mainly directed against the domain 2 of the SARS-CoV-2 spike protein due to its moderate amino acid conservation (107, 109).
Duration of T cell immunity and its potential role in protection against re-infection
Numerous studies have assessed the duration and characteristics of both cellular and humoral immunity following SARS-CoV-2 infection. To date, the presence of SARS-CoV-2-specific memory T cells in blood has been demonstrated 10 (110), 12 (111) and up to 22 months (112) post-infection. Likewise, SARS-CoV-2-specific antibodies have been detected 12 (113), 13 (114) or 15 months (115) after the onset of infection. Nevertheless, the kinetics of both branches of adaptive immunity exhibit different patterns, as cellular response tends to remain stable (61, 116–118) while antibodies decay rapidly in early convalescence (119–121).
It has been described that SARS-CoV-2 memory response is skewed towards more CD4+ helper T cells than CD8+ cytotoxic T cells (37, 116). More specifically, Cohen et al. have described that the median frequency of SARS-CoV-2-specific memory CD4+ T cells was 0.51% of the T cell repertoire, while the median frequency of SARS-CoV-2-specific CD8+ T cells was 0.2% (122). Nevertheless, a wide range in magnitude of specific T cells among the analysed patients has been observed probably due to the differential kinetics of expansion and contraction of the two cell populations in convalescent individuals (123). Most of the virus-specific memory CD8+ T cells are terminally differentiated effector memory cells (TEMRA) (116, 124), antigen-experienced cells that re-express the naïve cell marker CD45RA (125), and which in the context of influenza have been associated with protection against severe disease (126). These SARS-CoV-2-specific CD8+ memory T cells secrete mainly IFN-γ, TNF-α, and granzyme B (122). CD4+ T cell memory is predominantly T central memory (TCM) with phenotypic features characteristic of Th1 polarization, lymph node homing, robust helper function and longevity (116, 118, 124). The dominant cytokine response in CD4+ T cell subsets is IL-2, indicating proliferative potential, which may suggest a robust long-term immune memory (61, 118).
Apart from circulating memory T cells, tissue-resident SARS-CoV-2-specific memory T cells have also been reported particularly in the airway and associated lymph nodes, but also in oropharyngeal tonsils, bone marrow and spleen, and their frequency correlated with that of circulating specific T cells (127, 128). A study in convalescent patients found tissue-resident SARS-CoV-2-specific CD8+ T cells in nasal mucosa, which had overlapping T cell receptors with circulating T cells within each patient, and suggested that these local memory cells could rapidly attenuate re-infection by SARS-CoV-2 (129).
In addition to the long-term maintenance of specific T cell responses and their capacity to prevent severe disease (29, 36, 38, 61, 63), an increasing number of studies have demonstrated that infection-induced SARS-CoV-2-specific T cells largely tolerate the amino acid mutations of the different VoCs. The impact of the different mutations in the early VoCs (Alpha, Beta, Gamma, and Epsilon) is limited therefore the majority of CD4+ and CD8+ T cell responses are preserved (130–132). Studies on the impact of newer variants such as Omicron, which holds more than 50 mutations, 37 of them in the spike protein (133), have reported that 70% to 90% of the CD4+ and CD8+ T cell response to spike was maintained (134, 135). The reduced likelihood of cellular immune escape of new SARS-CoV-2 variants is probably partly related to the high polymorphism of the HLA system. Therefore, the set of viral epitopes recognized by each individual´s lymphocytes may be different, and overall, at the population level, be broader (136–138). The progressive emergence of VoCs that mainly accumulate mutations in the spike protein (139, 140) and thus have an increased ability to evade neutralizing antibodies (141–144) has led new analyses to focus on determining correlates of protection against severe disease rather than infection (98). Epidemiological observations have reported that despite the increasing infection rate related with the successive waves of VoCs (145, 146), protection against hospitalization and death due to re-infection have remained extremely high (147, 148). Given the waning humoral immunity after infection (119, 121) and the VoCs ability to evade neutralizing antibodies (141, 142, 144), it may be suggested that T cells play an important role in this protection against severe COVID-19, also in the context of VoC infection.
T cell response after vaccination
Studies on the immunodominant regions of SARS-CoV-2 have been particularly important for vaccine development. Griffoni et al. have reported that T cells can recognize up to 10 different SARS-CoV-2 proteins, with spike being the most immunodominant, followed by nucleocapsid, membrane, and other non-structural proteins (149, 150). These data, together with the fact that the receptor binding domain (RBD) of the SARS-CoV-2 spike protein is the main target of neutralizing antibodies (53, 151), led to the development of different spike-based vaccine candidates.
Up to date, ten COVID-19 vaccines have been approved by the World Health Organization (WHO) for global use. Among them, four different vaccination platforms can be distinguished: messenger RNA (mRNA) vaccines (Moderna’s Spikevax mRNA-1273 and Pfizer-BioNTech’s Comirnaty BNT162b2), adenovirus vector-based vaccines (AstraZeneca’s Vaxzevria, Covishield ChAdOx1 and Johnson & Johnson-Janssen’s Ad26.COV2.S), inactivated virus vaccines (Sinopharm’s Covilo, Sinovac’s CoronaVac, and Bharat Biotech’s Covaxin) and adjuvanted protein vaccines (Novavax’s Nuvaxovid and Covovax NVX-CoV2373) (139, 152). All have demonstrated high rates of efficacy in preventing COVID-19, especially protecting against severe disease and death (153–158). Since vaccination protocols started in December 2020 with BNT162b2 vaccine, it has been estimated that global anti-SARS-CoV-2 vaccination saved nearly 20 million lives in its first year of application (159).
Several studies have shown that mRNA vaccines elicit an early and potent humoral immune response (160, 161) that declines sharply beyond 3 months post-vaccination (162–165). Neutralizing antibody titers developed after mRNA SARS-CoV-2 vaccination exhibit similar dynamics (112, 166, 167), with a half-life of approximately 60 days (168). In contrast, it has been reported that the Ad26.COV2.S vaccine induces lower initial levels of neutralizing antibodies but these remain stable up to 8 months after vaccination (166, 169).
It has also been proven that COVID-19 vaccination elicits a robust T cell response. As expected, mRNA- and adenovirus vector-based spike vaccines induce only spike-specific T cells (112, 161, 170–172), whereas inactivated virus vaccines induce T cells capable of recognizing several SARS-CoV-2 antigens (173, 174). As in natural infection, the phenotypic profile of mRNA vaccine-induced T cells is predominantly Th1 (61, 172, 175–177). In addition, induction of follicular helper CD4+ T cells along with cytotoxic CD8+ T cells has been described (132, 178, 179). Early induction of these specific CD8+ T cells may explain the vaccine-mediated protection against severe disease that has been described as early as ten days after prime vaccination (180), when neutralizing antibodies are barely detectable (176, 181). Similarly, studies in non-human primates highlight the potential of vaccine-induced CD8+ T cell responses to contribute to viral load reduction and COVID-19 containment (182–184). In addition, it has also been demonstrated that vaccine-induced T cells are able to cross-recognize VoCs (185–189), while VoCs partially evade neutralizing antibodies elicited by vaccination (190–192), as it happens in natural infection. In particular, vaccine-induced T cells show high cross-reactivity (over 80%) against Omicron (135, 193, 194), even in the absence of high neutralizing antibodies titers (195, 196). Vaccine induced T cell memory has generally been described as long-lasting as and more stable than humoral response even though it usually shows some degree of contraction within the first 3 months after vaccination (112, 139, 185, 197).
Comparisons of T cell immunogenicity and memory elicited by the different COVID-19 vaccines platforms have been challenging and limited, mainly due to the lack of standardized cellular assays, unlike the standardization of binding and neutralizing antibodies provided by WHO (198). Comparative studies on the development and maintenance of immunity elicited by different vaccine platforms highlight that mRNA vaccines induced the highest CD4+ T cell responses (199, 200). In addition, both the CD4+ T cell peak and memory responses developed by the BNT162b2 vaccine were lower than those generated by the mRNA-1273 vaccine (200, 201). The acute and memory responses of CD8+ T cells generated by BNT162b2 were comparable to those of mRNA-1273, but slightly lower in frequency and multifunctionality (200). Timing and vaccine dosing regimens, as mRNA-1273 contains 100 μg mRNA, while BNT162b2 contains 30 μg (200), may be behind these differences. CD8+ T cell responses were particularly high after Ad26.COV2.S vaccination (166, 199) and low, but detectable, after NVX-CoV2373 immunization (200). This variable immunogenicity among the COVID-19 vaccines could explain the differences in the efficacy and effectiveness that have been reported (157, 180, 202, 203). In all cases, vaccine efficacy against hospitalization remains stable over time, unlike efficacy against infection (204, 205). This suggests that, as in natural infection, vaccine-induced T cells are contributing to the control of COVID-19, preventing the development of severe disease, in a context of waning humoral immunity, independently of the SARS-CoV-2 variant evolution.
Hybrid immunity against SARS-CoV-2
Despite protection against severe COVID-19 and death has been achieved, an increase in infection rates has been observed (145, 146, 206), probably due to the combination of the successive emerging VoCs and a decreasing immunity landscape. Seroprevalence surveys suggest that more than half of the global population has been infected with SARS-CoV-2 (148). This, together with the massive application of vaccines, has triggered a great interest in the characterization of the so-called hybrid immunity against SARS-CoV-2. The term hybrid immunity applies to individuals with a previous SARS-CoV-2 infection who were subsequently vaccinated against SARS-CoV-2 or vice versa, thus exhibiting a combination of infection- and vaccine-induced immunity (96, 207). Numerous studies have revealed that individuals with previous SARS-CoV-2 infection develop unusually strong immune responses to COVID-19 vaccines (208, 209). Specifically, the neutralizing antibody titers were dramatically higher in previously infected individuals receiving at least one dose of COVID-19 vaccine (161, 207, 210) and their memory B cells were increased 5- to 10-fold compared with natural infection or vaccination alone (161, 211). Similarly, multiple studies have observed an overall increase in T cell response in hybrid immunity compared to either infection or vaccination (112, 212, 213). It has been shown that COVID-19 vaccines can substantially boost spike-specific CD4+ T cell responses, and have a modest effect boosting spike-specific CD8+ T cells, in previously infected individuals after immunization (98, 185, 211). Likewise, hybrid immunity resulting from vaccination plus subsequent infection (breakthrough infection) also results in equally robust adaptive immune responses (214, 215).
It should be noted that most COVID-19 vaccines in use consist of a single antigen, the spike protein, whereas 29 different viral proteins are present in SARS-CoV-2 (216). Therefore, the breadth of the resulting immune response following COVID-19 vaccination is more restricted than in natural infection (207, 217). One of the advantages of hybrid immunity is that it consists of both spike and non-spike memory, resulting in a broader repertoire of virus-specific antibodies (218, 219), B cells (217, 220) and T cells (83, 217). Comparative analyses have reported that hybrid immunity provides greater and more durable protection against re-infection than immunity triggered by two or three doses of COVID-19 vaccine in uninfected individuals (221, 222) and this superior protection conferred by hybrid immunity has been maintained even against VoCs, including Omicron (218, 219, 223, 224). However, even though additional antigen exposure from natural infection substantially boosts the quantity and breadth of immune response, recent studies have shown no differences between T cell cross-reactivity against Omicron in vaccinated- compared to hybrid immune-subjects (188, 225).
It has also been shown that infection following spike-based vaccination does not prevent the development of a non-spike-specific T cell response, but on the contrary, it gives rise to a broad repertoire of T cells specific against other SARS-CoV-2 antigens (83, 112). This indicates that individuals with breakthrough infection do not have a preferential bias toward spike responses, which is especially important given the continued emergence of VoCs and the current increase in the rate of breakthrough infection (131, 226).
One of the most recently discussed concerns has been the possible deleterious effect of the repeated SARS-CoV-2 antigen exposure on the protective capacity of memory T cells. Repeated flu vaccination has been shown to result in a ‘vaccine exhaustion’ with significantly reduced protection rate (227). However, and regarding cellular immunity in particular, recent work by Minervina et al. has shown that repeated antigen exposure did not result in SARS-CoV-2-specific T cell dysfunction (83). Despite the expression of T cell exhaustion markers (CTLA-4, PD-1, TIGIT and Tox), SARS-CoV-2-specific CD8+ T cells retained their proliferative capacity. Based on these evidences future immunization boosters with vaccine platforms including a variety of SARS-CoV-2 antigens seems to be recommendable.
New COVID-19 vaccines are being developed to improve efficacy and protection against breakthrough infections, especially relevant in the current situation with periodic emergence of new VoCs. In this regard, new mRNA vaccine platforms that contain equal amounts of mRNAs encoding the ancestral SARS-CoV-2 and Beta or Omicron variant spike proteins have been developed (228–230). These bivalent boosters enhance the humoral immune response, eliciting superior and more durable neutralizing antibody responses against VoCs compared to monovalent vaccine formulations (229, 231–233). However, the effect of bivalent vaccines on T cell immunity remains less understood and further studies are still required. A recent study by Tan et al. has shown that bivalent booster vaccination leads to a robust recall of memory T cell responses within the first 7 days after boost, that exhibit cross-reactivity against Omicron BA.1 and BA.5 variants regardless of the priming regime administered (234).
Another interesting new strategy for the vaccination against COVID-19 is the application of intranasal booster vaccination, as mucosal immunity has been shown to protect against breakthrough infections (128, 129). Currently approved intramuscular COVID-19 vaccines induce variable levels of mucosal humoral immunity (235–237), and while a recent study reported the presence of high frequency of spike-specific T cells in the airways of mRNA-vaccinated individuals (238), others detected spike-specific T cells only in the BALF of SARS-CoV-2 convalescent patients but not in the BALF of vaccinated-only individuals (239, 240). On the contrary, mucosal vaccination can elicit strong adaptive responses both locally and systemically as reported in animal models. It has been shown in mice that intranasal vaccination against SARS-CoV-2 led to the induction of broadly neutralizing antibodies and polyfunctional central memory T cells locally, in the draining lymphoid organs, and systemically in the spleen (241). Mao et al. propose the use of intranasal booster administration which can induce robust tissue-resident memory B and T cell responses, as well as boost systemic immunity (242). Importantly, an intranasal vaccine with non-spike antigens in Cynomolgus macaques reduced the viral load two days after being challenged by intranasal SARS-CoV-2. The viral control was observed in the absence of neutralizing antibodies, and it was linked to the induction of specific CD8+ T cell responses (183). Finally, intranasal immunization has been shown to provide protection against VoCs in animal models (243, 244). These data highlight both the protective value of SARS-CoV-2-specific T cells resident in the upper respiratory tract and the effective next-generation COVID-19 intranasal vaccine approach to induce mucosal immunity against current and future VoCs, which could be a promising strategy to reduce breakthrough infections and curb SARS-CoV-2 transmission.
Concluding remarks
Much information has been generated since the onset of the COVID-19 pandemic about the role of T cell-mediated immunity in SARS-CoV-2 infection and its association with disease control. It has been shown that, although neutralizing antibodies play a dominant role in the prevention of infection, an adequate disease control depends on the synchronized function of the two branches of the adaptive immunity, where the coordinated development of an early T cell response followed by a controlled humoral response is essential.
Although the development and massive application of COVID-19 vaccines has led to a dramatic decrease in the frequency of severe disease and death, it is still necessary to establish accurate correlates of protection. Thus, increasing evidence supports that since antibody titers are not a surrogate indicator of the magnitude of memory T cells, the use of antibody serodiagnostic tests alone will not be a robust indicator of protective immunity against SARS-CoV-2. In addition, it has been shown that T cells remain largely stable over time while antibodies and their neutralizing activity sharply decrease. This fact, together with the robust evidence that the T cell response is preserved against VoCs, as opposed to neutralization, further highlights the association between the cellular immunity and the decreased rates of severe disease, regardless of SARS-CoV-2 variant evolution. Along these lines, it would be essential to implement a cellular response surveillance program to fully understand the extent of immunity among the population and to be able to identify vulnerable groups.
In parallel, comparative studies between protection conferred by natural infection, vaccination or hybrid immunity have highlighted the value of developing a broad immune repertoire against SARS-CoV-2. Therefore, the inclusion of viral antigens other than spike in new vaccine designs could result in further benefit.
Author contributions
All authors contributed to conception, writing, and editing of the final manuscript. PA-V and RL-G contributed equally to literature review and first draft. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank all the patients and clinical colleagues that have contributed to our COVID-19 research. We would also like to thank all the members in GIDIT and our Immunology Department for their helpful discussion.
Funding Statement
This work was supported by grants to E. P-A from the Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation, cofounded by the European Development Regional Fund (FEDER), A way to achieve Europe (PI22/00586), and from the Comunidad de Madrid (Ayuda REACT-UE Inmunovacter 2022/0093). R.L-G holds a research contract “Rio Hortega” (CM19/00120) from the Instituto de Salud Carlos III.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. WHO . Who coronavirus (Covid-19) dashboard (2020). Available at: https://covid19.who.int/.
- 2. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol (2019) 17(3):181–92. doi: 10.1038/s41579-018-0118-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen J, Subbarao K. The immunobiology of sars*. Annu Rev Immunol (2007) 25:443–72. doi: 10.1146/annurev.immunol.25.022106.141706 [DOI] [PubMed] [Google Scholar]
- 4. Li G, Chen X, Xu A. Profile of specific antibodies to the sars-associated coronavirus. N Engl J Med (2003) 349(5):508–9. doi: 10.1056/NEJM200307313490520 [DOI] [PubMed] [Google Scholar]
- 5. Hsueh PR, Huang LM, Chen PJ, Kao CL, Yang PC. Chronological evolution of igm, iga, igg and neutralisation antibodies after infection with sars-associated coronavirus. Clin Microbiol Infect (2004) 10(12):1062–6. doi: 10.1111/j.1469-0691.2004.01009.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prompetchara E, Ketloy C, Palaga T. Immune responses in covid-19 and potential vaccines: Lessons learned from sars and mers epidemic. Asian Pac J Allergy Immunol (2020) 38(1):1–9. doi: 10.12932/AP-200220-0772 [DOI] [PubMed] [Google Scholar]
- 7. Corman VM, Albarrak AM, Omrani AS, Albarrak MM, Farah ME, Almasri M, et al. Viral shedding and antibody response in 37 patients with middle East respiratory syndrome coronavirus infection. Clin Infect Dis (2016) 62(4):477–83. doi: 10.1093/cid/civ951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park WB, Perera RA, Choe PG, Lau EH, Choi SJ, Chun JY, et al. Kinetics of serologic responses to mers coronavirus infection in humans, south Korea. Emerg Infect Dis (2015) 21(12):2186–9. doi: 10.3201/eid2112.151421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu M. Sars immunity and vaccination. Cell Mol Immunol (2004) 1(3):193–8. [PubMed] [Google Scholar]
- 10. Shin HS, Kim Y, Kim G, Lee JY, Jeong I, Joh JS, et al. Immune responses to middle East respiratory syndrome coronavirus during the acute and convalescent phases of human infection. Clin Infect Dis (2019) 68(6):984–92. doi: 10.1093/cid/ciy595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong RS, Wu A, To KF, Lee N, Lam CW, Wong CK, et al. Haematological manifestations in patients with severe acute respiratory syndrome: Retrospective analysis. BMJ (2003) 326(7403):1358–62. doi: 10.1136/bmj.326.7403.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet (2015) 386(9997):995–1007. doi: 10.1016/s0140-6736(15)60454-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mazzoni A, Salvati L, Maggi L, Capone M, Vanni A, Spinicci M, et al. Impaired immune cell cytotoxicity in severe covid-19 is il-6 dependent. J Clin Invest (2020) 130(9):4694–703. doi: 10.1172/JCI138554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest (2020) 130(5):2620–9. doi: 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, et al. Sars-Cov-2-Specific T cell immunity in cases of covid-19 and sars, and uninfected controls. Nature (2020) 584(7821):457–62. doi: 10.1038/s41586-020-2550-z [DOI] [PubMed] [Google Scholar]
- 16. Tang F, Quan Y, Xin ZT, Wrammert J, Ma MJ, Lv H, et al. Lack of peripheral memory b cell responses in recovered patients with severe acute respiratory syndrome: A six-year follow-up study. J Immunol (2011) 186(12):7264–8. doi: 10.4049/jimmunol.0903490 [DOI] [PubMed] [Google Scholar]
- 17. Wu LP, Wang NC, Chang YH, Tian XY, Na DY, Zhang LY, et al. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis (2007) 13(10):1562–4. doi: 10.3201/eid1310.070576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao J, Alshukairi AN, Baharoon SA, Ahmed WA, Bokhari AA, Nehdi AM, et al. Recovery from the middle East respiratory syndrome is associated with antibody and T-cell responses. Sci Immunol (2017) 2(14). doi: 10.1126/sciimmunol.aan5393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe covid-19 patients. Science (2020) 369(6504):718–24. doi: 10.1126/science.abc6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arunachalam PS, Wimmers F, Mok CKP, Perera R, Scott M, Hagan T, et al. Systems biological assessment of immunity to mild versus severe covid-19 infection in humans. Science (2020) 369(6508):1210–20. doi: 10.1126/science.abc6261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuri-Cervantes L, Pampena MB, Meng W, Rosenfeld AM, Ittner CAG, Weisman AR, et al. Comprehensive mapping of immune perturbations associated with severe covid-19. Sci Immunol (2020) 5(49). doi: 10.1126/sciimmunol.abd7114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li S, Jiang L, Li X, Lin F, Wang Y, Li B, et al. Clinical and pathological investigation of patients with severe covid-19. JCI Insight (2020) 5(12). doi: 10.1172/jci.insight.138070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Moller R, et al. Imbalanced host response to sars-Cov-2 drives development of covid-19. Cell (2020) 181(5):1036–45 e9. doi: 10.1016/j.cell.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Autoantibodies against type I ifns in patients with life-threatening covid-19. Science (2020) 370(6515). doi: 10.1126/science.abd4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, et al. Inborn errors of type I ifn immunity in patients with life-threatening covid-19. Science (2020) 370(6515). doi: 10.1126/science.abd4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schulien I, Kemming J, Oberhardt V, Wild K, Seidel LM, Killmer S, et al. Characterization of pre-existing and induced sars-Cov-2-Specific Cd8(+) T cells. Nat Med (2021) 27(1):78–85. doi: 10.1038/s41591-020-01143-2 [DOI] [PubMed] [Google Scholar]
- 27. Notarbartolo S, Ranzani V, Bandera A, Gruarin P, Bevilacqua V, Putignano AR, et al. Integrated longitudinal immunophenotypic, transcriptional and repertoire analyses delineate immune responses in covid-19 patients. Sci Immunol (2021) 6(62). doi: 10.1126/sciimmunol.abg5021 [DOI] [PubMed] [Google Scholar]
- 28. Bergamaschi L, Mescia F, Turner L, Hanson AL, Kotagiri P, Dunmore BJ, et al. Longitudinal analysis reveals that delayed bystander Cd8+ T cell activation and early immune pathology distinguish severe covid-19 from mild disease. Immunity (2021) 54(6):1257–75 e8. doi: 10.1016/j.immuni.2021.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to sars-Cov-2 in acute covid-19 and associations with age and disease severity. Cell (2020) 183(4):996–1012 e19. doi: 10.1016/j.cell.2020.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peng Y, Mentzer AJ, Liu G, Yao X, Yin Z, Dong D, et al. Broad and strong memory Cd4(+) and Cd8(+) T cells induced by sars-Cov-2 in uk convalescent individuals following covid-19. Nat Immunol (2020) 21(11):1336–45. doi: 10.1038/s41590-020-0782-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sekine T, Perez-Potti A, Rivera-Ballesteros O, Stralin K, Gorin JB, Olsson A, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild covid-19. Cell (2020) 183(1):158–68 e14. doi: 10.1016/j.cell.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, et al. Correlates of protection against sars-Cov-2 in rhesus macaques. Nature (2021) 590(7847):630–4. doi: 10.1038/s41586-020-03041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with covid-19. Nat Med (2020) 26(6):842–4. doi: 10.1038/s41591-020-0901-9 [DOI] [PubMed] [Google Scholar]
- 34. Vijayakumar B, Boustani K, Ogger PP, Papadaki A, Tonkin J, Orton CM, et al. Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-Covid-19 respiratory disease. Immunity (2022) 55(3):542–56 e5. doi: 10.1016/j.immuni.2022.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Puzyrenko A, Felix JC, Ledeboer NA, Sun Y, Rui H, Sheinin Y. Cytotoxic Cd8-positive T-lymphocyte infiltration in the lungs as a histological pattern of sars-Cov-2 pneumonitis. Pathology (2022) 54(4):404–8. doi: 10.1016/j.pathol.2021.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tan AT, Linster M, Tan CW, Le Bert N, Chia WN, Kunasegaran K, et al. Early induction of functional sars-Cov-2-Specific T cells associates with rapid viral clearance and mild disease in covid-19 patients. Cell Rep (2021) 34(6):108728. doi: 10.1016/j.celrep.2021.108728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T cell responses to sars-Cov-2 coronavirus in humans with covid-19 disease and unexposed individuals. Cell (2020) 181(7):1489–501 e15. doi: 10.1016/j.cell.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tarke A, Potesta M, Varchetta S, Fenoglio D, Iannetta M, Sarmati L, et al. Early and polyantigenic Cd4 T cell responses correlate with mild disease in acute covid-19 donors. Int J Mol Sci (2022) 23(13). doi: 10.3390/ijms23137155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Braun J, Loyal L, Frentsch M, Wendisch D, Georg P, Kurth F, et al. Sars-Cov-2-Reactive T cells in healthy donors and patients with covid-19. Nature (2020) 587(7833):270–4. doi: 10.1038/s41586-020-2598-9 [DOI] [PubMed] [Google Scholar]
- 40. Weiskopf D, Schmitz KS, Raadsen MP, Grifoni A, Okba NMA, Endeman H, et al. Phenotype and kinetics of sars-Cov-2-Specific T cells in covid-19 patients with acute respiratory distress syndrome. Sci Immunol (2020) 5(48). doi: 10.1126/sciimmunol.abd2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thevarajan I, Nguyen THO, Koutsakos M, Druce J, Caly L, van de Sandt CE, et al. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe covid-19. Nat Med (2020) 26(4):453–5. doi: 10.1038/s41591-020-0819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Strutt TM, McKinstry KK, Dibble JP, Winchell C, Kuang Y, Curtis JD, et al. Memory Cd4+ T cells induce innate responses independently of pathogen. Nat Med (2010) 16(5):558–64. doi: 10.1038/nm.2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular helper T cells. Annu Rev Immunol (2016) 34:335–68. doi: 10.1146/annurev-immunol-041015-055605 [DOI] [PubMed] [Google Scholar]
- 44. Gil-Etayo FJ, Garcinuno S, Utrero-Rico A, Cabrera-Marante O, Arroyo-Sanchez D, Mancebo E, et al. An early Th1 response is a key factor for a favorable covid-19 evolution. Biomedicines (2022) 10(2). doi: 10.3390/biomedicines10020296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gutierrez-Bautista JF, Rodriguez-Nicolas A, Rosales-Castillo A, Jimenez P, Garrido F, Anderson P, et al. Negative clinical evolution in covid-19 patients is frequently accompanied with an increased proportion of undifferentiated Th cells and a strong underrepresentation of the Th1 subset. Front Immunol (2020) 11:596553. doi: 10.3389/fimmu.2020.596553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, et al. Longitudinal analyses reveal immunological misfiring in severe covid-19. Nature (2020) 584(7821):463–9. doi: 10.1038/s41586-020-2588-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cardone J, Le Friec G, Vantourout P, Roberts A, Fuchs A, Jackson I, et al. Complement regulator Cd46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol (2010) 11(9):862–71. doi: 10.1038/ni.1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chauss D, Freiwald T, McGregor R, Yan B, Wang L, Nova-Lamperti E, et al. Autocrine vitamin d signaling switches off pro-inflammatory programs of Th1 cells. Nat Immunol (2022) 23(1):62–74. doi: 10.1038/s41590-021-01080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meckiff BJ, Ramirez-Suastegui C, Fajardo V, Chee SJ, Kusnadi A, Simon H, et al. Imbalance of regulatory and cytotoxic sars-Cov-2-Reactive Cd4(+) T cells in covid-19. Cell (2020) 183(5):1340–53 e16. doi: 10.1016/j.cell.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Juno JA, Tan HX, Lee WS, Reynaldi A, Kelly HG, Wragg K, et al. Humoral and circulating follicular helper T cell responses in recovered patients with covid-19. Nat Med (2020) 26(9):1428–34. doi: 10.1038/s41591-020-0995-0 [DOI] [PubMed] [Google Scholar]
- 51. Zhang J, Wu Q, Liu Z, Wang Q, Wu J, Hu Y, et al. Spike-specific circulating T follicular helper cell and cross-neutralizing antibody responses in covid-19-Convalescent individuals. Nat Microbiol (2021) 6(1):51–8. doi: 10.1038/s41564-020-00824-5 [DOI] [PubMed] [Google Scholar]
- 52. Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. Antibody responses to sars-Cov-2 in patients with covid-19. Nat Med (2020) 26(6):845–8. doi: 10.1038/s41591-020-0897-1 [DOI] [PubMed] [Google Scholar]
- 53. Premkumar L, Segovia-Chumbez B, Jadi R, Martinez DR, Raut R, Markmann A, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in sars-Cov-2 patients. Sci Immunol (2020) 5(48). doi: 10.1126/sciimmunol.abc8413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Suthar MS, Zimmerman MG, Kauffman RC, Mantus G, Linderman SL, Hudson WH, et al. Rapid generation of neutralizing antibody responses in covid-19 patients. Cell Rep Med (2020) 1(3):100040. doi: 10.1016/j.xcrm.2020.100040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Roltgen K, Powell AE, Wirz OF, Stevens BA, Hogan CA, Najeeb J, et al. Defining the features and duration of antibody responses to sars-Cov-2 infection associated with disease severity and outcome. Sci Immunol (2020) 5(54). doi: 10.1126/sciimmunol.abe0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cervia C, Nilsson J, Zurbuchen Y, Valaperti A, Schreiner J, Wolfensberger A, et al. Systemic and mucosal antibody responses specific to sars-Cov-2 during mild versus severe covid-19. J Allergy Clin Immunol (2021) 147(2):545–57 e9. doi: 10.1016/j.jaci.2020.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hansen CB, Jarlhelt I, Perez-Alos L, Hummelshoj Landsy L, Loftager M, Rosbjerg A, et al. Sars-Cov-2 antibody responses are correlated to disease severity in covid-19 convalescent individuals. J Immunol (2021) 206(1):109–17. doi: 10.4049/jimmunol.2000898 [DOI] [PubMed] [Google Scholar]
- 58. Piccoli L, Park YJ, Tortorici MA, Czudnochowski N, Walls AC, Beltramello M, et al. Mapping neutralizing and immunodominant sites on the sars-Cov-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell (2020) 183(4):1024–42 e21. doi: 10.1016/j.cell.2020.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zheng J, Deng Y, Zhao Z, Mao B, Lu M, Lin Y, et al. Characterization of sars-Cov-2-Specific humoral immunity and its potential applications and therapeutic prospects. Cell Mol Immunol (2022) 19(2):150–7. doi: 10.1038/s41423-021-00774-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sariol A, Perlman S. Lessons for covid-19 immunity from other coronavirus infections. Immunity (2020) 53(2):248–63. doi: 10.1016/j.immuni.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Almendro-Vazquez P, Laguna-Goya R, Ruiz-Ruigomez M, Utrero-Rico A, Lalueza A, Maestro de la Calle G, et al. Longitudinal dynamics of sars-Cov-2-Specific cellular and humoral immunity after natural infection or Bnt162b2 vaccination. PloS Pathog (2021) 17(12):e1010211. doi: 10.1371/journal.ppat.1010211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Oja AE, Saris A, Ghandour CA, Kragten NAM, Hogema BM, Nossent EJ, et al. Divergent sars-Cov-2-Specific T- and b-cell responses in severe but not mild covid-19 patients. Eur J Immunol (2020) 50(12):1998–2012. doi: 10.1002/eji.202048908 [DOI] [PubMed] [Google Scholar]
- 63. Chandran A, Rosenheim J, Nageswaran G, Swadling L, Pollara G, Gupta RK, et al. Rapid synchronous type 1 ifn and virus-specific T cell responses characterize first wave non-severe sars-Cov-2 infections. Cell Rep Med (2022) 3(3):100557. doi: 10.1016/j.xcrm.2022.100557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. da Silva Antunes R, Pallikkuth S, Williams E, Dawen Yu E, Mateus J, Quiambao L, et al. Differential T-cell reactivity to endemic coronaviruses and sars-Cov-2 in community and health care workers. J Infect Dis (2021) 224(1):70–80. doi: 10.1093/infdis/jiab176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nelde A, Bilich T, Heitmann JS, Maringer Y, Salih HR, Roerden M, et al. Sars-Cov-2-Derived peptides define heterologous and covid-19-Induced T cell recognition. Nat Immunol (2021) 22(1):74–85. doi: 10.1038/s41590-020-00808-x [DOI] [PubMed] [Google Scholar]
- 66. Swadling L, Diniz MO, Schmidt NM, Amin OE, Chandran A, Shaw E, et al. Pre-existing polymerase-specific T cells expand in abortive seronegative sars-Cov-2. Nature (2022) 601(7891):110–7. doi: 10.1038/s41586-021-04186-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Laguna-Goya R, Utrero-Rico A, Talayero P, Lasa-Lazaro M, Ramirez-Fernandez A, Naranjo L, et al. Il-6-Based mortality risk model for hospitalized patients with covid-19. J Allergy Clin Immunol (2020) 146(4):799–807 e9. doi: 10.1016/j.jaci.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, et al. Predictors of mortality for patients with covid-19 pneumonia caused by sars-Cov-2: A prospective cohort study. Eur Respir J (2020) 55(5). doi: 10.1183/13993003.00524-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Urra JM, Cabrera CM, Porras L, Rodenas I. Selective Cd8 cell reduction by sars-Cov-2 is associated with a worse prognosis and systemic inflammation in covid-19 patients. Clin Immunol (2020) 217:108486. doi: 10.1016/j.clim.2020.108486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (Covid-19). Front Immunol (2020) 11:827. doi: 10.3389/fimmu.2020.00827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kreutmair S, Unger S, Nunez NG, Ingelfinger F, Alberti C, De Feo D, et al. Distinct immunological signatures discriminate severe covid-19 from non-Sars-Cov-2-Driven critical pneumonia. Immunity (2021) 54(7):1578–93 e5. doi: 10.1016/j.immuni.2021.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schwabenland M, Salie H, Tanevski J, Killmer S, Lago MS, Schlaak AE, et al. Deep spatial profiling of human covid-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-Cell interactions. Immunity (2021) 54(7):1594–610 e11. doi: 10.1016/j.immuni.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Szabo PA, Dogra P, Gray JI, Wells SB, Connors TJ, Weisberg SP, et al. Longitudinal profiling of respiratory and systemic immune responses reveals myeloid cell-driven lung inflammation in severe covid-19. Immunity (2021) 54(4):797–814 e6. doi: 10.1016/j.immuni.2021.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhu L, Yang P, Zhao Y, Zhuang Z, Wang Z, Song R, et al. Single-cell sequencing of peripheral mononuclear cells reveals distinct immune response landscapes of covid-19 and influenza patients. Immunity (2020) 53(3):685–96 e3. doi: 10.1016/j.immuni.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Moss P. The T cell immune response against sars-Cov-2. Nat Immunol (2022) 23(2):186–93. doi: 10.1038/s41590-021-01122-w [DOI] [PubMed] [Google Scholar]
- 76. Popescu I, Snyder ME, Iasella CJ, Hannan SJ, Koshy R, Burke R, et al. Cd4(+) T-cell dysfunction in severe covid-19 disease is tumor necrosis factor-Alpha/Tumor necrosis factor receptor 1-dependent. Am J Respir Crit Care Med (2022) 205(12):1403–18. doi: 10.1164/rccm.202111-2493OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen Z, John Wherry E. T Cell responses in patients with covid-19. Nat Rev Immunol (2020) 20(9):529–36. doi: 10.1038/s41577-020-0402-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, et al. Deep immune profiling of covid-19 patients reveals distinct immunotypes with therapeutic implications. Science (2020) 369(6508). doi: 10.1126/science.abc8511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, Fidanza L, et al. Marked T cell activation, senescence, exhaustion and skewing towards Th17 in patients with covid-19 pneumonia. Nat Commun (2020) 11(1):3434. doi: 10.1038/s41467-020-17292-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Song JW, Zhang C, Fan X, Meng FP, Xu Z, Xia P, et al. Immunological and inflammatory profiles in mild and severe cases of covid-19. Nat Commun (2020) 11(1):3410. doi: 10.1038/s41467-020-17240-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mohammed RN, Tamjidifar R, Rahman HS, Adili A, Ghoreishizadeh S, Saeedi H, et al. A comprehensive review about immune responses and exhaustion during coronavirus disease (Covid-19). Cell Commun Signal (2022) 20(1):79. doi: 10.1186/s12964-022-00856-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in covid-19 patients. Cell Mol Immunol (2020) 17(5):533–5. doi: 10.1038/s41423-020-0402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Minervina AA, Pogorelyy MV, Kirk AM, Crawford JC, Allen EK, Chou CH, et al. Sars-Cov-2 antigen exposure history shapes phenotypes and specificity of memory Cd8(+) T cells. Nat Immunol (2022) 23(5):781–90. doi: 10.1038/s41590-022-01184-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rha MS, Jeong HW, Ko JH, Choi SJ, Seo IH, Lee JS, et al. Pd-1-Expressing sars-Cov-2-Specific Cd8(+) T cells are not exhausted, but functional in patients with covid-19. Immunity (2021) 54(1):44–52 e3. doi: 10.1016/j.immuni.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Stephenson E, Reynolds G, Botting RA, Calero-Nieto FJ, Morgan MD, Tuong ZK, et al. Single-cell multi-omics analysis of the immune response in covid-19. Nat Med (2021) 27(5):904–16. doi: 10.1038/s41591-021-01329-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yu K, Wu Y, He J, Liu X, Wei B, Wen W, et al. Thymosin alpha-1 protected T cells from excessive activation in severe covid-19. Res Square (2020). doi: 10.21203/rs.3.rs-25869/v2 [DOI] [Google Scholar]
- 87. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 uk patients in hospital with covid-19 using the isaric who clinical characterisation protocol: Prospective observational cohort study. BMJ (2020) 369:m1985. doi: 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with sars-Cov-2 admitted to icus of the Lombardy region, Italy. JAMA (2020) 323(16):1574–81. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li H, Manwani B, Leng SX. Frailty, inflammation, and immunity. Aging Dis (2011) 2(6):466–73. [PMC free article] [PubMed] [Google Scholar]
- 90. Pinti M, Appay V, Campisi J, Frasca D, Fulop T, Sauce D, et al. Aging of the immune system: Focus on inflammation and vaccination. Eur J Immunol (2016) 46(10):2286–301. doi: 10.1002/eji.201546178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chen J, Lau YF, Lamirande EW, Paddock CD, Bartlett JH, Zaki SR, et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (Sars-cov) infection in senescent Balb/C mice: Cd4+ T cells are important in control of sars-cov infection. J Virol (2010) 84(3):1289–301. doi: 10.1128/JVI.01281-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhao J, Zhao J, Legge K, Perlman S. Age-related increases in Pgd(2) expression impair respiratory dc migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest (2011) 121(12):4921–30. doi: 10.1172/JCI59777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Qian F, Wang X, Zhang L, Lin A, Zhao H, Fikrig E, et al. Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile virus. J Infect Dis (2011) 203(10):1415–24. doi: 10.1093/infdis/jir048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Welsh RM, Bahl K, Marshall HD, Urban SL. Type 1 interferons and antiviral Cd8 T-cell responses. PloS Pathog (2012) 8(1):e1002352. doi: 10.1371/journal.ppat.1002352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of covid-19: Current state of the science. Immunity (2020) 52(6):910–41. doi: 10.1016/j.immuni.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sette A, Crotty S. Immunological memory to sars-Cov-2 infection and covid-19 vaccines. Immunol Rev (2022) 310(1):27–46. doi: 10.1111/imr.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ. Sars-Cov-2 and covid-19 in older adults: What we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience (2020) 42(2):505–14. doi: 10.1007/s11357-020-00186-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bertoletti A, Le Bert N, Tan AT. Sars-Cov-2-Specific T cells in the changing landscape of the covid-19 pandemic. Immunity (2022) 55(10):1764–78. doi: 10.1016/j.immuni.2022.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sagar M, Reifler K, Rossi M, Miller NS, Sinha P, White LF, et al. Recent endemic coronavirus infection is associated with less-severe covid-19. J Clin Invest (2021) 131(1). doi: 10.1172/JCI143380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lineburg KE, Grant EJ, Swaminathan S, Chatzileontiadou DSM, Szeto C, Sloane H, et al. Cd8(+) T cells specific for an immunodominant sars-Cov-2 nucleocapsid epitope cross-react with selective seasonal coronaviruses. Immunity (2021) 54(5):1055–65 e5. doi: 10.1016/j.immuni.2021.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Low JS, Vaqueirinho D, Mele F, Foglierini M, Jerak J, Perotti M, et al. Clonal analysis of immunodominance and cross-reactivity of the Cd4 T cell response to sars-Cov-2. Science (2021) 372(6548):1336–41. doi: 10.1126/science.abg8985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kundu R, Narean JS, Wang L, Fenn J, Pillay T, Fernandez ND, et al. Cross-reactive memory T cells associate with protection against sars-Cov-2 infection in covid-19 contacts. Nat Commun (2022) 13(1):80. doi: 10.1038/s41467-021-27674-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mateus J, Grifoni A, Tarke A, Sidney J, Ramirez SI, Dan JM, et al. Selective and cross-reactive sars-Cov-2 T cell epitopes in unexposed humans. Science (2020) 370(6512):89–94. doi: 10.1126/science.abd3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Stoddard CI, Galloway J, Chu HY, Shipley MM, Sung K, Itell HL, et al. Epitope profiling reveals binding signatures of sars-Cov-2 immune response in natural infection and cross-reactivity with endemic human covs. Cell Rep (2021) 35(8):109164. doi: 10.1016/j.celrep.2021.109164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bacher P, Rosati E, Esser D, Martini GR, Saggau C, Schiminsky E, et al. Low-avidity Cd4(+) T cell responses to sars-Cov-2 in unexposed individuals and humans with severe covid-19. Immunity (2020) 53(6):1258–71 e5. doi: 10.1016/j.immuni.2020.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ogbe A, Kronsteiner B, Skelly DT, Pace M, Brown A, Adland E, et al. T Cell assays differentiate clinical and subclinical sars-Cov-2 infections from cross-reactive antiviral responses. Nat Commun (2021) 12(1):2055. doi: 10.1038/s41467-021-21856-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dowell AC, Butler MS, Jinks E, Tut G, Lancaster T, Sylla P, et al. Children develop robust and sustained cross-reactive spike-specific immune responses to sars-Cov-2 infection. Nat Immunol (2022) 23(1):40–9. doi: 10.1038/s41590-021-01089-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ng KW, Faulkner N, Cornish GH, Rosa A, Harvey R, Hussain S, et al. Preexisting and De novo humoral immunity to sars-Cov-2 in humans. Science (2020) 370(6522):1339–43. doi: 10.1126/science.abe1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Khan T, Rahman M, Ali FA, Huang SSY, Ata M, Zhang Q, et al. Distinct antibody repertoires against endemic human coronaviruses in children and adults. JCI Insight (2021) 6(4). doi: 10.1172/jci.insight.144499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nelson RW, Chen Y, Venezia OL, Majerus RM, Shin DS, Collection M--, et al. Sars-Cov-2 epitope-specific Cd4(+) memory T cell responses across covid-19 disease severity and antibody durability. Sci Immunol (2022) 7(73):eabl9464. doi: 10.1126/sciimmunol.abl9464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lu Z, Laing ED, Pena DaMata J, Pohida K, Tso MS, Samuels EC, et al. Durability of sars-Cov-2-Specific T-cell responses at 12 months postinfection. J Infect Dis (2021) 224(12):2010–9. doi: 10.1093/infdis/jiab543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Almendro-Vazquez P, Chivite-Lacaba M, Utrero-Rico A, Gonzalez-Cuadrado C, Laguna-Goya R, Moreno-Batanero M, et al. Cellular and humoral immune responses and breakthrough infections after three sars-Cov-2 mrna vaccine doses. Front Immunol (2022) 13:981350. doi: 10.3389/fimmu.2022.981350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Krutikov M, Palmer T, Tut G, Fuller C, Azmi B, Giddings R, et al. Prevalence and duration of detectable sars-Cov-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (Vivaldi study): Prospective cohort study in England. Lancet Healthy Longev (2022) 3(1):e13–21. doi: 10.1016/S2666-7568(21)00282-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gallais F, Gantner P, Bruel T, Velay A, Planas D, Wendling MJ, et al. Evolution of antibody responses up to 13 months after sars-Cov-2 infection and risk of reinfection. EBioMedicine (2021) 71:103561. doi: 10.1016/j.ebiom.2021.103561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Marcotte H, Piralla A, Zuo F, Du L, Cassaniti I, Wan H, et al. Immunity to sars-Cov-2 up to 15 months after infection. iScience (2022) 25(2):103743. doi: 10.1016/j.isci.2022.103743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to sars-Cov-2 assessed for up to 8 months after infection. Science (2021) 371(6529). doi: 10.1126/science.abf4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jung JH, Rha MS, Sa M, Choi HK, Jeon JH, Seok H, et al. Sars-Cov-2-Specific T cell memory is sustained in covid-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat Commun (2021) 12(1):4043. doi: 10.1038/s41467-021-24377-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zuo J, Dowell AC, Pearce H, Verma K, Long HM, Begum J, et al. Robust sars-Cov-2-Specific T cell immunity is maintained at 6 months following primary infection. Nat Immunol (2021) 22(5):620–6. doi: 10.1038/s41590-021-00902-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Crawford KHD, Dingens AS, Eguia R, Wolf CR, Wilcox N, Logue JK, et al. Dynamics of neutralizing antibody titers in the months after severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis (2021) 223(2):197–205. doi: 10.1093/infdis/jiaa618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, et al. Rapid decay of anti-Sars-Cov-2 antibodies in persons with mild covid-19. N Engl J Med (2020) 383(11):1085–7. doi: 10.1056/NEJMc2025179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, et al. Clinical and immunological assessment of asymptomatic sars-Cov-2 infections. Nat Med (2020) 26(8):1200–4. doi: 10.1038/s41591-020-0965-6 [DOI] [PubMed] [Google Scholar]
- 122. Cohen KW, Linderman SL, Moodie Z, Czartoski J, Lai L, Mantus G, et al. Longitudinal analysis shows durable and broad immune memory after sars-Cov-2 infection with persisting antibody responses and memory b and T cells. Cell Rep Med (2021) 2(7):100354. doi: 10.1016/j.xcrm.2021.100354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bertoletti A, Tan AT, Le Bert N. The T-cell response to sars-Cov-2: Kinetic and quantitative aspects and the case for their protective role. Oxford Open Immunol (2021) 2(1). doi: 10.1093/oxfimm/iqab006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Neidleman J, Luo X, Frouard J, Xie G, Gill G, Stein ES, et al. Sars-Cov-2-Specific T cells exhibit phenotypic features of helper function, lack of terminal differentiation, and high proliferation potential. Cell Rep Med (2020) 1(6):100081. doi: 10.1016/j.xcrm.2020.100081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tian Y, Babor M, Lane J, Schulten V, Patil VS, Seumois G, et al. Unique phenotypes and clonal expansions of human Cd4 effector memory T cells re-expressing Cd45ra. Nat Commun (2017) 8(1):1473. doi: 10.1038/s41467-017-01728-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med (2013) 19(10):1305–12. doi: 10.1038/nm.3350 [DOI] [PubMed] [Google Scholar]
- 127. Niessl J, Sekine T, Lange J, Konya V, Forkel M, Maric J, et al. Identification of resident memory Cd8(+) T cells with functional specificity for sars-Cov-2 in unexposed oropharyngeal lymphoid tissue. Sci Immunol (2021) 6(64):eabk0894. doi: 10.1126/sciimmunol.abk0894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Poon MML, Rybkina K, Kato Y, Kubota M, Matsumoto R, Bloom NI, et al. Sars-Cov-2 infection generates tissue-localized immunological memory in humans. Sci Immunol (2021) 6(65):eabl9105. doi: 10.1126/sciimmunol.abl9105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Roukens AHE, Pothast CR, Konig M, Huisman W, Dalebout T, Tak T, et al. Prolonged activation of nasal immune cell populations and development of tissue-resident sars-Cov-2-Specific Cd8(+) T cell responses following covid-19. Nat Immunol (2022) 23(1):23–32. doi: 10.1038/s41590-021-01095-w [DOI] [PubMed] [Google Scholar]
- 130. Riou C, Keeton R, Moyo-Gwete T, Hermanus T, Kgagudi P, Baguma R, et al. Escape from recognition of sars-Cov-2 variant spike epitopes but overall preservation of T cell immunity. Sci Transl Med (2022) 14(631):eabj6824. doi: 10.1126/scitranslmed.abj6824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Geers D, Shamier MC, Bogers S, den Hartog G, Gommers L, Nieuwkoop NN, et al. Sars-Cov-2 variants of concern partially escape humoral but not T-cell responses in covid-19 convalescent donors and vaccinees. Sci Immunol (2021) 6(59). doi: 10.1126/sciimmunol.abj1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tarke A, Sidney J, Methot N, Yu ED, Zhang Y, Dan JM, et al. Impact of sars-Cov-2 variants on the total Cd4(+) and Cd8(+) T cell reactivity in infected or vaccinated individuals. Cell Rep Med (2021) 2(7):100355. doi: 10.1016/j.xcrm.2021.100355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zhao Z, Zhou J, Tian M, Huang M, Liu S, Xie Y, et al. Omicron sars-Cov-2 mutations stabilize spike up-rbd conformation and lead to a non-Rbm-Binding monoclonal antibody escape. Nat Commun (2022) 13(1):4958. doi: 10.1038/s41467-022-32665-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Gao Y, Cai C, Grifoni A, Muller TR, Niessl J, Olofsson A, et al. Ancestral sars-Cov-2-Specific T cells cross-recognize the omicron variant. Nat Med (2022) 28(3):472–6. doi: 10.1038/s41591-022-01700-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Keeton R, Tincho MB, Ngomti A, Baguma R, Benede N, Suzuki A, et al. T Cell responses to sars-Cov-2 spike cross-recognize omicron. Nature (2022) 603(7901):488–92. doi: 10.1038/s41586-022-04460-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Foix A, Lopez D, Diez-Fuertes F, McConnell MJ, Martin-Galiano AJ. Predicted impact of the viral mutational landscape on the cytotoxic response against sars-Cov-2. PloS Comput Biol (2022) 18(2):e1009726. doi: 10.1371/journal.pcbi.1009726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lopez D. Predicted hla class I and class ii epitopes from licensed vaccines are largely conserved in new sars-Cov-2 omicron variant of concern. Front Immunol (2022) 13:832889. doi: 10.3389/fimmu.2022.832889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Dolton G, Rius C, Hasan MS, Wall A, Szomolay B, Behiry E, et al. Emergence of immune escape at dominant sars-Cov-2 killer T cell epitope. Cell (2022) 185(16):2936–51 e19. doi: 10.1016/j.cell.2022.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Barouch DH. Covid-19 vaccines - immunity, variants, boosters. N Engl J Med (2022) 387(11):1011–20. doi: 10.1056/NEJMra2206573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Escalera A, Gonzalez-Reiche AS, Aslam S, Mena I, Laporte M, Pearl RL, et al. Mutations in sars-Cov-2 variants of concern link to increased spike cleavage and virus transmission. Cell Host Microbe (2022) 30(3):373–87 e7. doi: 10.1016/j.chom.2022.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A, et al. Omicron escapes the majority of existing sars-Cov-2 neutralizing antibodies. Nature (2022) 602(7898):657–63. doi: 10.1038/s41586-021-04385-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Liu C, Ginn HM, Dejnirattisai W, Supasa P, Wang B, Tuekprakhon A, et al. Reduced neutralization of sars-Cov-2 B.1.617 by vaccine and convalescent serum. Cell (2021) 184(16):4220–36 e13. doi: 10.1016/j.cell.2021.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zhang J, Xiao T, Cai Y, Lavine CL, Peng H, Zhu H, et al. Membrane fusion and immune evasion by the spike protein of sars-Cov-2 delta variant. Science (2021) 374(6573):1353–60. doi: 10.1126/science.abl9463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wibmer CK, Ayres F, Hermanus T, Madzivhandila M, Kgagudi P, Oosthuysen B, et al. Sars-Cov-2 501y.V2 escapes neutralization by south African covid-19 donor plasma. Nat Med (2021) 27(4):622–5. doi: 10.1038/s41591-021-01285-x [DOI] [PubMed] [Google Scholar]
- 145. Altarawneh HN, Chemaitelly H, Hasan MR, Ayoub HH, Qassim S, AlMukdad S, et al. Protection against the omicron variant from previous sars-Cov-2 infection. N Engl J Med (2022) 386(13):1288–90. doi: 10.1056/NEJMc2200133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Pulliam JRC, van Schalkwyk C, Govender N, von Gottberg A, Cohen C, Groome MJ, et al. Increased risk of sars-Cov-2 reinfection associated with emergence of omicron in south Africa. Science (2022) 376(6593):eabn4947. doi: 10.1126/science.abn4947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Medic S, Anastassopoulou C, Lozanov-Crvenkovic Z, Vukovic V, Dragnic N, Petrovic V, et al. Risk and severity of sars-Cov-2 reinfections during 2020-2022 in vojvodina, Serbia: A population-level observational study. Lancet Reg Health Eur (2022) 20:100453. doi: 10.1016/j.lanepe.2022.100453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Pilz S, Theiler-Schwetz V, Trummer C, Krause R, Ioannidis JPA. Sars-Cov-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ Res (2022) 209:112911. doi: 10.1016/j.envres.2022.112911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Grifoni A, Sette A. From alpha to omicron: The response of T cells. Curr Res Immunol (2022) 3:146–50. doi: 10.1016/j.crimmu.2022.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Grifoni A, Sidney J, Vita R, Peters B, Crotty S, Weiskopf D, et al. Sars-Cov-2 human T cell epitopes: Adaptive immune response against covid-19. Cell Host Microbe (2021) 29(7):1076–92. doi: 10.1016/j.chom.2021.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Dickey TH, Tang WK, Butler B, Ouahes T, Orr-Gonzalez S, Salinas ND, et al. Design of the sars-Cov-2 rbd vaccine antigen improves neutralizing antibody response. Sci Adv (2022) 8(37):eabq8276. doi: 10.1126/sciadv.abq8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. WHO . Covid19 vaccine tracker (2022). Available at: https://covid19.trackvaccines.org/agency/who/.
- 153. Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, et al. Prevention and attenuation of covid-19 with the Bnt162b2 and mrna-1273 vaccines. N Engl J Med (2021) 385(4):320–9. doi: 10.1056/NEJMoa2107058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Bruxvoort KJ, Sy LS, Qian L, Ackerson BK, Luo Y, Lee GS, et al. Real-world effectiveness of the mrna-1273 vaccine against covid-19: Interim results from a prospective observational cohort study. Lancet Reg Health Am (2022) 6:100134. doi: 10.1016/j.lana.2021.100134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. Bnt162b2 mrna covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med (2021) 384(15):1412–23. doi: 10.1056/NEJMoa2101765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Nordstrom P, Ballin M, Nordstrom A. Effectiveness of heterologous Chadox1 ncov-19 and mrna prime-boost vaccination against symptomatic covid-19 infection in Sweden: A nationwide cohort study. Lancet Reg Health Eur (2021) 11:100249. doi: 10.1016/j.lanepe.2021.100249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sadoff J, Gray G, Vandebosch A, Cardenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.Cov2.S vaccine against covid-19. N Engl J Med (2021) 384(23):2187–201. doi: 10.1056/NEJMoa2101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Jara A, Undurraga EA, Gonzalez C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an inactivated sars-Cov-2 vaccine in Chile. N Engl J Med (2021) 385(10):875–84. doi: 10.1056/NEJMoa2107715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of covid-19 vaccination: A mathematical modelling study. Lancet Infect Dis (2022) 22(9):1293–302. doi: 10.1016/S1473-3099(22)00320-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Wang Z, Schmidt F, Weisblum Y, Muecksch F, Barnes CO, Finkin S, et al. Mrna vaccine-elicited antibodies to sars-Cov-2 and circulating variants. Nature (2021) 592(7855):616–22. doi: 10.1038/s41586-021-03324-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Goel RR, Apostolidis SA, Painter MM, Mathew D, Pattekar A, Kuthuru O, et al. Distinct antibody and memory b cell responses in sars-Cov-2 naive and recovered individuals following mrna vaccination. Sci Immunol (2021) 6(58). doi: 10.1126/sciimmunol.abi6950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Shrotri M, Navaratnam AMD, Nguyen V, Byrne T, Geismar C, Fragaszy E, et al. Spike-antibody waning after second dose of Bnt162b2 or Chadox1. Lancet (2021) 398(10298):385–7. doi: 10.1016/S0140-6736(21)01642-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Pegu A, O’Connell SE, Schmidt SD, O’Dell S, Talana CA, Lai L, et al. Durability of mrna-1273 vaccine-induced antibodies against sars-Cov-2 variants. Science (2021) 373(6561):1372–7. doi: 10.1126/science.abj4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, et al. Durability of responses after sars-Cov-2 mrna-1273 vaccination. N Engl J Med (2021) 384(1):80–2. doi: 10.1056/NEJMc2032195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to Bnt162b2 covid-19 vaccine over 6 months. N Engl J Med (2021) 385(24):e84. doi: 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Collier AY, Yu J, McMahan K, Liu J, Chandrashekar A, Maron JS, et al. Differential kinetics of immune responses elicited by covid-19 vaccines. N Engl J Med (2021) 385(21):2010–2. doi: 10.1056/NEJMc2115596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Evans JP, Zeng C, Carlin C, Lozanski G, Saif LJ, Oltz EM, et al. Neutralizing antibody responses elicited by sars-Cov-2 mrna vaccination wane over time and are boosted by breakthrough infection. Sci Transl Med (2022) 14(637):eabn8057. doi: 10.1126/scitranslmed.abn8057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Doria-Rose N, Suthar MS, Makowski M, O’Connell S, McDermott AB, Flach B, et al. Antibody persistence through 6 months after the second dose of mrna-1273 vaccine for covid-19. N Engl J Med (2021) 384(23):2259–61. doi: 10.1056/NEJMc2103916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Barouch DH, Stephenson KE, Sadoff J, Yu J, Chang A, Gebre M, et al. Durable humoral and cellular immune responses 8 months after Ad26.Cov2.S vaccination. N Engl J Med (2021) 385(10):951–3. doi: 10.1056/NEJMc2108829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Khoo NKH, Lim JME, Gill US, de Alwis R, Tan N, Toh JZN, et al. Differential immunogenicity of homologous versus heterologous boost in Ad26.Cov2.S vaccine recipients. Med (N Y) (2022) 3(2):104–18 e4. doi: 10.1016/j.medj.2021.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Stephenson KE, Le Gars M, Sadoff J, de Groot AM, Heerwegh D, Truyers C, et al. Immunogenicity of the Ad26.Cov2.S vaccine for covid-19. JAMA (2021) 325(15):1535–44. doi: 10.1001/jama.2021.3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Painter MM, Mathew D, Goel RR, Apostolidis SA, Pattekar A, Kuthuru O, et al. Rapid induction of antigen-specific Cd4(+) T cells is associated with coordinated humoral and cellular immunity to sars-Cov-2 mrna vaccination. Immunity (2021) 54(9):2133–42 e3. doi: 10.1016/j.immuni.2021.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Bueno SM, Abarca K, Gonzalez PA, Galvez NMS, Soto JA, Duarte LF, et al. Safety and immunogenicity of an inactivated severe acute respiratory syndrome coronavirus 2 vaccine in a subgroup of healthy adults in Chile. Clin Infect Dis (2022) 75(1):e792–804. doi: 10.1093/cid/ciab823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lim JME, Hang SK, Hariharaputran S, Chia A, Tan N, Lee ES, et al. Omicron reactive multi protein specific Cd4 T cells defines cellular immune response induced by inactivated virus vaccines. medRxiv (2022). doi: 10.1101/2022.05.25.22275616 [DOI] [Google Scholar]
- 175. Mateus J, Dan JM, Zhang Z, Rydyznski Moderbacher C, Lammers M, Goodwin B, et al. Low-dose mrna-1273 covid-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science (2021) 374(6566):eabj9853. doi: 10.1126/science.abj9853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. Covid-19 vaccine Bnt162b1 elicits human antibody and Th1 T cell responses. Nature (2020) 586(7830):594–9. doi: 10.1038/s41586-020-2814-7 [DOI] [PubMed] [Google Scholar]
- 177. Guerrera G, Picozza M, D’Orso S, Placido R, Pirronello M, Verdiani A, et al. Bnt162b2 vaccination induces durable sars-Cov-2-Specific T cells with a stem cell memory phenotype. Sci Immunol (2021) 6(66):eabl5344. doi: 10.1126/sciimmunol.abl5344 [DOI] [PubMed] [Google Scholar]
- 178. Lederer K, Castano D, Gomez Atria D, Oguin TH, 3rd, Wang S, Manzoni TB, et al. Sars-Cov-2 mrna vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity (2020) 53(6):1281–95 e5. doi: 10.1016/j.immuni.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Oberhardt V, Luxenburger H, Kemming J, Schulien I, Ciminski K, Giese S, et al. Rapid and stable mobilization of Cd8(+) T cells by sars-Cov-2 mrna vaccine. Nature (2021) 597(7875):268–73. doi: 10.1038/s41586-021-03841-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the Bnt162b2 mrna covid-19 vaccine. N Engl J Med (2020) 383(27):2603–15. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. Bnt162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature (2021) 595(7868):572–7. doi: 10.1038/s41586-021-03653-6 [DOI] [PubMed] [Google Scholar]
- 182. Liu J, Yu J, McMahan K, Jacob-Dolan C, He X, Giffin V, et al. Cd8 T cells contribute to vaccine protection against sars-Cov-2 in macaques. Sci Immunol (2022) 7(77):eabq7647. doi: 10.1126/sciimmunol.abq7647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Ishii H, Nomura T, Yamamoto H, Nishizawa M, Thu Hau TT, Harada S, et al. Neutralizing-Antibody-Independent sars-Cov-2 control correlated with intranasal-Vaccine-Induced Cd8(+) T cell responses. Cell Rep Med (2022) 3(2):100520. doi: 10.1016/j.xcrm.2022.100520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Routhu NK, Cheedarla N, Gangadhara S, Bollimpelli VS, Boddapati AK, Shiferaw A, et al. A modified vaccinia Ankara vector-based vaccine protects macaques from sars-Cov-2 infection, immune pathology, and dysfunction in the lungs. Immunity (2021) 54(3):542–56 e9. doi: 10.1016/j.immuni.2021.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM, et al. Mrna vaccines induce durable immune memory to sars-Cov-2 and variants of concern. Science (2021) 374(6572):abm0829. doi: 10.1126/science.abm0829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Abu-Raddad LJ, Chemaitelly H, Butt AA, National Study Group for C-V. Effectiveness of the Bnt162b2 covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med (2021) 385(2):187–9. doi: 10.1056/NEJMc2104974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Tarke A, Coelho CH, Zhang Z, Dan JM, Yu ED, Methot N, et al. Sars-Cov-2 vaccination induces immunological T cell memory able to cross-recognize variants from alpha to omicron. Cell (2022) 185(5):847–59 e11. doi: 10.1016/j.cell.2022.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. De Marco L, D’Orso S, Pirronello M, Verdiani A, Termine A, Fabrizio C, et al. Assessment of T-cell reactivity to the sars-Cov-2 omicron variant by immunized individuals. JAMA Netw Open (2022) 5(4):e2210871. doi: 10.1001/jamanetworkopen.2022.10871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Alter G, Yu J, Liu J, Chandrashekar A, Borducchi EN, Tostanoski LH, et al. Immunogenicity of Ad26.Cov2.S vaccine against sars-Cov-2 variants in humans. Nature (2021) 596(7871):268–72. doi: 10.1038/s41586-021-03681-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM, et al. Multiple sars-Cov-2 variants escape neutralization by vaccine-induced humoral immunity. Cell (2021) 184(9):2372–83 e9. doi: 10.1016/j.cell.2021.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Hachmann NP, Miller J, Collier AY, Ventura JD, Yu J, Rowe M, et al. Neutralization escape by sars-Cov-2 omicron subvariants Ba.2.12.1, Ba.4, and Ba.5. N Engl J Med (2022) 387(1):86–8. doi: 10.1056/NEJMc2206576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, et al. Omicron extensively but incompletely escapes pfizer Bnt162b2 neutralization. Nature (2022) 602(7898):654–6. doi: 10.1038/s41586-021-04387-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Jung MK, Jeong SD, Noh JY, Kim DU, Jung S, Song JY, et al. Bnt162b2-induced memory T cells respond to the omicron variant with preserved polyfunctionality. Nat Microbiol (2022) 7(6):909–17. doi: 10.1038/s41564-022-01123-x [DOI] [PubMed] [Google Scholar]
- 194. Liu J, Chandrashekar A, Sellers D, Barrett J, Jacob-Dolan C, Lifton M, et al. Vaccines elicit highly conserved cellular immunity to sars-Cov-2 omicron. Nature (2022) 603(7901):493–6. doi: 10.1038/s41586-022-04465-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Bekker LG, Garrett N, Goga A, Fairall L, Reddy T, Yende-Zuma N, et al. Effectiveness of the Ad26.Cov2.S vaccine in health-care workers in south Africa (the sisonke study): Results from a single-arm, open-label, phase 3b, implementation study. Lancet (2022) 399(10330):1141–53. doi: 10.1016/S0140-6736(22)00007-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Gray G, Collie S, Goga A, Garrett N, Champion J, Seocharan I, et al. Effectiveness of Ad26.Cov2.S and Bnt162b2 vaccines against omicron variant in south Africa. N Engl J Med (2022) 386(23):2243–5. doi: 10.1056/NEJMc2202061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Kedzierska K, Thomas PG. Count on us: T cells in sars-Cov-2 infection and vaccination. Cell Rep Med (2022) 3(3):100562. doi: 10.1016/j.xcrm.2022.100562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kristiansen PA, Page M, Bernasconi V, Mattiuzzo G, Dull P, Makar K, et al. Who international standard for anti-Sars-Cov-2 immunoglobulin. Lancet (2021) 397(10282):1347–8. doi: 10.1016/S0140-6736(21)00527-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, et al. Homologous and heterologous covid-19 booster vaccinations. N Engl J Med (2022) 386(11):1046–57. doi: 10.1056/NEJMoa2116414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Zhang Z, Mateus J, Coelho CH, Dan JM, Moderbacher CR, Galvez RI, et al. Humoral and cellular immune memory to four covid-19 vaccines. Cell (2022) 185(14):2434–51 e17. doi: 10.1016/j.cell.2022.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Barbeau DJ, Martin JM, Carney E, Dougherty E, Doyle JD, Dermody TS, et al. Comparative analysis of human immune responses following sars-Cov-2 vaccination with Bnt162b2, mrna-1273, or Ad26.Cov2.S. NPJ Vaccines (2022) 7(1):77. doi: 10.1038/s41541-022-00504-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Naranbhai V, Garcia-Beltran WF, Chang CC, Berrios Mairena C, Thierauf JC, Kirkpatrick G, et al. Comparative immunogenicity and effectiveness of mrna-1273, Bnt162b2, and Ad26.Cov2.S covid-19 vaccines. J Infect Dis (2022) 225(7):1141–50. doi: 10.1093/infdis/jiab593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mrna-1273 sars-Cov-2 vaccine. N Engl J Med (2021) 384(5):403–16. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Nordstrom P, Ballin M, Nordstrom A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of covid-19 vaccine: A retrospective, total population cohort study in Sweden. Lancet (2022) 399(10327):814–23. doi: 10.1016/S0140-6736(22)00089-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mrna Bnt162b2 covid-19 vaccine up to 6 months in a Large integrated health system in the USA: A retrospective cohort study. Lancet (2021) 398(10309):1407–16. doi: 10.1016/S0140-6736(21)02183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Faria NR, Mellan TA, Whittaker C, Claro IM, Candido DDS, Mishra S, et al. Genomics and epidemiology of the P.1 sars-Cov-2 lineage in manaus, Brazil. Science (2021) 372(6544):815–21. doi: 10.1126/science.abh2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Crotty S. Hybrid immunity. Science (2021) 372(6549):1392–3. doi: 10.1126/science.abj2258 [DOI] [Google Scholar]
- 208. Leon TM, Dorabawila V, Nelson L, Lutterloh E, Bauer UE, Backenson B, et al. Covid-19 cases and hospitalizations by covid-19 vaccination status and previous covid-19 diagnosis - California and new York, may-November 2021. MMWR Morb Mortal Wkly Rep (2022) 71(4):125–31. doi: 10.15585/mmwr.mm7104e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Frieman M, Harris AD, Herati RS, Krammer F, Mantovani A, Rescigno M, et al. Sars-Cov-2 vaccines for all but a single dose for covid-19 survivors. EBioMedicine (2021) 68:103401. doi: 10.1016/j.ebiom.2021.103401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Luczkowiak J, Labiod N, Rivas G, Rolo M, Lasala F, Lora-Tamayo J, et al. Neutralizing response against sars-Cov-2 variants 8 months after Bnt162b2 vaccination in naive and covid-19-Convalescent individuals. J Infect Dis (2022) 225(11):1905–8. doi: 10.1093/infdis/jiab634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Tauzin A, Nayrac M, Benlarbi M, Gong SY, Gasser R, Beaudoin-Bussieres G, et al. A single dose of the sars-Cov-2 vaccine Bnt162b2 elicits fc-mediated antibody effector functions and T cell responses. Cell Host Microbe (2021) 29(7):1137–50 e6. doi: 10.1016/j.chom.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Lozano-Ojalvo D, Camara C, Lopez-Granados E, Nozal P, Del Pino-Molina L, Bravo-Gallego LY, et al. Differential effects of the second sars-Cov-2 mrna vaccine dose on T cell immunity in naive and covid-19 recovered individuals. Cell Rep (2021) 36(8):109570. doi: 10.1016/j.celrep.2021.109570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Payne RP, Longet S, Austin JA, Skelly DT, Dejnirattisai W, Adele S, et al. Immunogenicity of standard and extended dosing intervals of Bnt162b2 mrna vaccine. Cell (2021) 184(23):5699–714 e11. doi: 10.1016/j.cell.2021.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Walls AC, Sprouse KR, Bowen JE, Joshi A, Franko N, Navarro MJ, et al. Sars-Cov-2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses. Cell (2022) 185(5):872–80 e3. doi: 10.1016/j.cell.2022.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Wratil PR, Stern M, Priller A, Willmann A, Almanzar G, Vogel E, et al. Three exposures to the spike protein of sars-Cov-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat Med (2022) 28(3):496–503. doi: 10.1038/s41591-022-01715-4 [DOI] [PubMed] [Google Scholar]
- 216. Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A sars-Cov-2 protein interaction map reveals targets for drug repurposing. Nature (2020) 583(7816):459–68. doi: 10.1038/s41586-020-2286-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Rodda LB, Morawski PA, Pruner KB, Fahning ML, Howard CA, Franko N, et al. Imprinted sars-Cov-2-Specific memory lymphocytes define hybrid immunity. Cell (2022) 185(9):1588–601 e14. doi: 10.1016/j.cell.2022.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Reynolds CJ, Pade C, Gibbons JM, Butler DK, Otter AD, Menacho K, et al. Prior sars-Cov-2 infection rescues b and T cell responses to variants after first vaccine dose. Science (2021). doi: 10.1126/science.abh1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Stamatatos L, Czartoski J, Wan YH, Homad LJ, Rubin V, Glantz H, et al. Mrna vaccination boosts cross-variant neutralizing antibodies elicited by sars-Cov-2 infection. Science (2021). doi: 10.1126/science.abg9175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Terreri S, Piano Mortari E, Vinci MR, Russo C, Alteri C, Albano C, et al. Persistent b cell memory after sars-Cov-2 vaccination is functional during breakthrough infections. Cell Host Microbe (2022) 30(3):400–8 e4. doi: 10.1016/j.chom.2022.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, Yassine HM, Benslimane FM, Al Khatib HA, et al. Association of prior sars-Cov-2 infection with risk of breakthrough infection following mrna vaccination in Qatar. JAMA (2021) 326(19):1930–9. doi: 10.1001/jama.2021.19623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman LS, Ash N, et al. Protection and waning of natural and hybrid immunity to sars-Cov-2. N Engl J Med (2022) 386(23):2201–12. doi: 10.1056/NEJMoa2118946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med (2022) 387(1):21–34. doi: 10.1056/NEJMoa2203965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Marking U, Havervall S, Norin NG, Christ W, Gordon M, Ng H, et al. High rate of Ba.1, Ba.1.1 and Ba.2 infection in triple vaccinated. medRxiv (2022). doi: 10.1101/2022.04.02.22273333 [DOI] [Google Scholar]
- 225. Naranbhai V, Nathan A, Kaseke C, Berrios C, Khatri A, Choi S, et al. T Cell reactivity to the sars-Cov-2 omicron variant is preserved in most but not all individuals. Cell (2022) 185(6):1041–51 e6. doi: 10.1016/j.cell.2022.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Altmann DM, Boyton RJ, Beale R. Immunity to sars-Cov-2 variants of concern. Science (2021) 371(6534):1103–4. doi: 10.1126/science.abg7404 [DOI] [PubMed] [Google Scholar]
- 227. Azim Majumder MA, Razzaque MS. Repeated vaccination and ‘Vaccine exhaustion’: Relevance to the covid-19 crisis. Expert Rev Vaccines (2022) 21(8):1011–4. doi: 10.1080/14760584.2022.2071705 [DOI] [PubMed] [Google Scholar]
- 228. Administration FaD. Food and Drug Administration . Coronavirus (Covid-19) update: Fda authorizes moderna, pfizer-biontech bivalent covid-19 vaccines for use as a booster dose (2022). Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use.
- 229. Chalkias S, Harper C, Vrbicky K, Walsh SR, Essink B, Brosz A, et al. A bivalent omicron-containing booster vaccine against covid-19. N Engl J Med (2022) 387(14):1279–91. doi: 10.1056/NEJMoa2208343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Choi A, Koch M, Wu K, Chu L, Ma L, Hill A, et al. Safety and immunogenicity of sars-Cov-2 variant mrna vaccine boosters in healthy adults: An interim analysis. Nat Med (2021) 27(11):2025–31. doi: 10.1038/s41591-021-01527-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Alsoussi WB, Malladi SK, Zhou JQ, Liu Z, Ying B, Kim W, et al. Sars-Cov-2 omicron boosting induces De novo b cell response in humans. bioRxiv (2022). doi: 10.1101/2022.09.22.509040 [DOI] [PubMed] [Google Scholar]
- 232. Branche AR, Rouphael NG, Diemert DJ, Falsey AR, Losada C, Baden LR, et al. Sars-Cov-2 variant vaccine boosters trial: Preliminary analyses. medRxiv (2022). doi: 10.1101/2022.07.12.22277336 [DOI] [Google Scholar]
- 233. Chalkias S, Eder F, Essink B, Khetan S, Nestorova B, Feng J, et al. Safety, immunogenicity and antibody persistence of a bivalent beta-containing booster vaccine against covid-19: A phase 2/3 trial. Nat Med (2022) 28(11):2388–97. doi: 10.1038/s41591-022-02031-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Tan NH, Geers D, Sablerolles RSG, Rietdijk WJR, Goorhuis A, Postma DF, et al. Evaluation of bivalent omicron Ba.1 booster vaccination after different priming regimens in healthcare workers (Switch on): A randomized controlled trial. medRxiv (2022). doi: 10.1101/2022.12.18.22283593 [DOI] [Google Scholar]
- 235. Sano K, Bhavsar D, Singh G, Floda D, Srivastava K, Gleason C, et al. Sars-Cov-2 vaccination induces mucosal antibody responses in previously infected individuals. Nat Commun (2022) 13(1):5135. doi: 10.1038/s41467-022-32389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Guerrieri M, Francavilla B, Fiorelli D, Nuccetelli M, Passali FM, Coppeta L, et al. Nasal and salivary mucosal humoral immune response elicited by mrna Bnt162b2 covid-19 vaccine compared to sars-Cov-2 natural infection. Vaccines (Basel) (2021) 9(12). doi: 10.3390/vaccines9121499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Ketas TJ, Chaturbhuj D, Portillo VMC, Francomano E, Golden E, Chandrasekhar S, et al. Antibody responses to sars-Cov-2 mrna vaccines are detectable in saliva. Pathog Immun (2021) 6(1):116–34. doi: 10.20411/pai.v6i1.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Ssemaganda A, Nguyen HM, Nuhu F, Jahan N, Card CM, Kiazyk S, et al. Expansion of cytotoxic tissue-resident Cd8(+) T cells and Ccr6(+)Cd161(+) Cd4(+) T cells in the nasal mucosa following mrna covid-19 vaccination. Nat Commun (2022) 13(1):3357. doi: 10.1038/s41467-022-30913-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Lim JME, Tan AT, Le Bert N, Hang SK, Low JGH, Bertoletti A. Sars-Cov-2 breakthrough infection in vaccinees induces virus-specific nasal-resident Cd8+ and Cd4+ T cells of broad specificity. J Exp Med (2022) 219(10). doi: 10.1084/jem.20220780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Tang J, Zeng C, Cox TM, Li C, Son YM, Cheon IS, et al. Respiratory mucosal immunity against sars-Cov-2 after mrna vaccination. Sci Immunol (2022) 7(76):eadd4853. doi: 10.1126/sciimmunol.add4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. O’Neill A, Kala MP, Wah TC, Saron WAA, Mantri CK, Rathore APS, et al. Mucosal vaccination for sars-Cov-2 elicits superior systemic T central memory function and cross-neutralizing antibodies against variants of concern. bioRxiv (2022). doi: 10.1101/2022.09.09.507250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Mao T, Israelow B, Pena-Hernandez MA, Suberi A, Zhou L, Luyten S, et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science (2022):eabo2523. doi: 10.1126/science.abo2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Afkhami S, D’Agostino MR, Zhang A, Stacey HD, Marzok A, Kang A, et al. Respiratory mucosal delivery of next-generation covid-19 vaccine provides robust protection against both ancestral and variant strains of sars-Cov-2. Cell (2022) 185(5):896–915 e19. doi: 10.1016/j.cell.2022.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Perez P, Astorgano D, Albericio G, Flores S, Sanchez-Cordon PJ, Luczkowiak J, et al. Intranasal administration of a single dose of mva-based vaccine candidates against covid-19 induced local and systemic immune responses and protects mice from a lethal sars-Cov-2 infection. Front Immunol (2022) 13:995235. doi: 10.3389/fimmu.2022.995235 [DOI] [PMC free article] [PubMed] [Google Scholar]