Abstract
Background:
Antipsychotic medications are the mainstay of treatment for schizophrenia and are associated with a reduction in psychiatric hospitalization and overall mortality. Some evidence suggest that antipsychotic medications might have a varying effect on the improvement of comorbid substance use disorders (SUDs), with clozapine showing more favorable outcomes.
Aim:
We systematically reviewed all available evidence on effects of clozapine on the improvement of SUDs other than nicotine.
Methods:
Electronic searches of MEDLINE, Embase, PsycINFO, and CINHAL were conducted up to March 1, 2022. Studies of any methodological design involving two concepts: (1) clozapine and (2) SUD terms (excluding nicotine) were included. For SUD outcomes with three or more comparative studies with available raw data meta-analysis was performed. SUD outcomes not meeting criteria for meta-analysis were described qualitatively. Risk of bias was examined using “Downs and Black,” and “Q-Coh” instruments.
Results:
The majority of individuals in the included 31 studies were male and of European ancestry. Abstinence was the most common outcome. Most of the studies were of low-to-moderate quality, and none of the studies met all the quality criteria. Pooled findings from four observational studies in samples of patients with predominantly comorbid alcohol use disorder showed that clozapine treatment is associated with significantly higher odds of remaining abstinent. In addition clozapine was associated with decreased odds of psychiatric hospitalization in all but one observational study.
Conclusions:
Our systematic review and meta-analysis builds upon previous reviews, and it suggests the association of clozapine treatment with significantly higher odds of remaining abstinent from substance use and decreased likelihood of psychiatric hospitalization, compared with continuing treatment with other antipsychotic medications. Still, the validity of this association needs greater exploration and providing recommendations for the utility of clozapine in individuals without treatment-resistant psychosis and comorbid SUDs would be premature.
Keywords: Clozapine, substance use disorder, schizophrenia, dual diagnosis, concurrent disorders
Introduction
Chronic persistent psychosis is mainly caused by schizophrenia with median incidence of 287 (uncertainty interval 246-331)/100,000 (GBD 2019 Mental Disorders Collaborators, 2022). Over the past three decades, high prevalence of comorbid (non-nicotine) substance use disorders (SUD) was found in individuals with schizophrenia (0.417, 95% CI 0.393, 0.441), with trends for increased substance use over time (Hunt et al., 2018). Moderate or severe substance use in this population results in poorer outcomes in domains of psychosis, symptoms of depression, and quality of life compared with mild users or abstinent individuals (Kerfoot et al., 2011). Global high rates of comorbid SUDs and schizophrenia spectrum disorders (SSD) suggest that this population has risk factors that make them vulnerable to developing SUDs (Hunt et al., 2018).
Antipsychotic medications are the mainstay of treatment for schizophrenia and are associated with a reduction in psychiatric hospitalization (Tiihonen et al., 2017) and overall mortality (Taipale et al., 2018). However, there is sparse research looking at the effectiveness of antipsychotics on SUD outcomes, such as: maintaining abstinence; intensity of substance use and cravings; rate of substance use-related psychiatric hospitalization; rate of developing SUD; or time to onset of SUD. Some preliminary evidence in individuals with SSD and a comorbid SUD suggest that antipsychotic medications, despite treating psychosis, might have a varying effect on the improvement of SUDs, with clozapine showing more favorable outcomes (Khokhar et al., 2017).
A systematic review and meta-analysis of prospective randomized controlled trials (RCT) reported the effects of antipsychotics on SUD improvement in schizophrenia (Krause et al., 2019). Clozapine was superior to other antipsychotic drugs in terms of mean cannabis use, driven by one small study. In another systematic review, clozapine was inferred to be superior to first-generation antipsychotics (FGAs) in improving SUD and remission (Arranz et al., 2017). The World Federation of Societies of Biological Psychiatry guidelines (Hasan et al., 2015) recommends clozapine for schizophrenia and comorbid alcohol use disorder (Category of evidence B, level of recommendation 3). More recently, the Canadian (Crockford and Addington, 2017) and Spanish (Arranz et al., 2022) guidelines for schizophrenia did not recommend using one antipsychotic over another for individuals with comorbid SUDs due to limited evidence from randomized prospective trials.
In the highly complex area of concurrent disorders, where there is a lack of prospective RCTs, analysis of observational studies and clinical cases often informs clinical practice. A previous review had gathered available literature, including observational studies and clinical cases, regarding clozapine efficacy in improving comorbid SUDs (Arranz et al., 2017). Our current review builds upon this work, and it is the first to conduct a meta-analysis of cohort studies that examined clozapine association with substance use abstinence. Given the previously available work (Arranz et al., 2017), we excluded nicotine use disorder from our review.
Materials and methods
Search strategy and selection criteria
This systematic review was prospectively registered with PROSPERO (registration number: CRD42022307739) and followed the 2020 PRISMA (Preferred Reporting Items for Systematic Reviews Meta-Analyses) reporting recommendations (Page et al., 2021). Systematic searches of the peer-reviewed literature were conducted following PRESS guidelines (McGowan et al., 2015) in consultation with a medical librarian in four electronic databases (i.e., MEDLINE, PsycINFO, Embase, and CINAHL) from inception to March 1, 2022. The keywords included two concepts: (1) clozapine and (2) substance use or SUD terms (excluding nicotine). Database searches and an exhaustive list of key terms are provided in the Supplemental Table S2. Two reviewers performed title/abstract screening and full-text article screening (RR and MD) for eligibility for inclusion. Articles for which a consensus between the two reviewers was not obtained were evaluated by a third reviewer (CS). In addition, a web-based citation chaser (Haddaway et al., 2022) was used for backward and forward citation chasing for additional references not identified in our primary searches. Literature in human subjects published in English was included according to the following criteria: (1) research including participants treated with clozapine and (2) research providing information on SUD parameters other than nicotine. Prospective clinical trials with or without randomization, prospective and retrospective observational studies, cross-sectional studies, and case reports were included. Abstracts of presentations and conference posters were also considered for inclusion.
Two independent reviewers (RR and MD) used a custom data extraction template to summarize the selected articles. Extraction information included author names, year, study design, sample size, country, medications used, patient characteristics (i.e., age, sex, and ancestry), diagnosis, and funding. When information was missing or incomplete for an eligible study, a request for additional information was made to the corresponding author of the eligible study.
Risk-of-bias assessment
An assessment of comparative study quality was conducted independently by the two reviewers (RR and MD). We used Downs and Black instrument (Downs and Black, 1998) for RCTs, which contains 27 items for randomized and non-randomized comparative studies, providing a total score of 28 for each study. Quality levels are reported as excellent (26–28); good (20–25); fair (15–19); and poor (⩽14). For cohort studies, we used the Quality of Cohort Studies (Q-Coh), which is a tool used to assess methodology and identify studies that would be a source of bias in the meta-analysis (Jarde et al., 2013). It is divided into the following domains—representativeness, comparability of groups, quality of exposure measures, maintenance of comparability, quality of outcome measures, and attrition. For each domain, a positive or a negative inference is made. Three categories—good (none or one domain was assessed negatively), adequate (two domains were judged negatively), or low (more than two domains were judged negatively)—are used to summarize an overall quality assessment. Discussion was used to resolve any disagreements among reviewers until an agreement was reached.
Data analysis
For SUD outcomes with three or more comparative studies with available raw data meta-analysis was performed. Data were analyzed using the Cochrane Review Manager, RevMan 5. The pooled odds ratio (OR) was calculated using a random-effects model for dichotomous data that applied the Mantel–Haenszel method. Heterogeneity in effect sizes between studies was tested using the chi-square statistic (with p < 0.10 indicating significant heterogeneity), and its magnitude was quantified using the I-squared statistic, which is an index that describes the proportion of the total variation in the study effect size estimates that is due to heterogeneity and is independent of the number of studies included in the meta-analysis and the metric of the effect size. SUD outcomes not meeting criteria for meta-analysis were described qualitatively.
Results
Search results and study characteristics
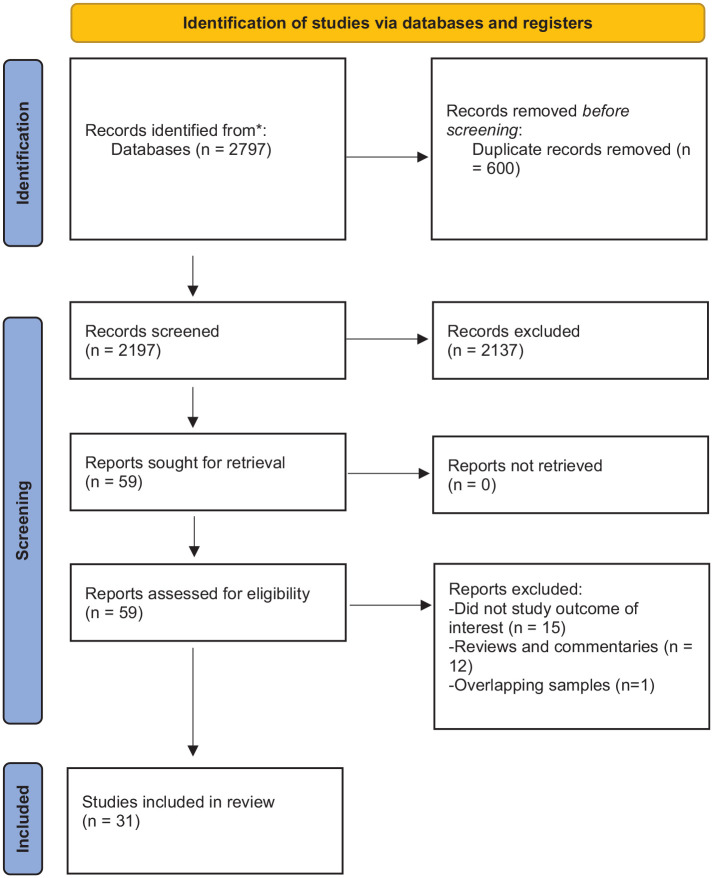
Our systematic search yielded 2797 studies. A summary of the article selection process is in Figure 1. After reviewing titles and abstracts, 2737 studies were excluded because they were duplicates or did not meet the study eligibility criteria. After the full-text screening of the remaining 59 articles, 28 articles were excluded. Summary characteristics of the remaining 31 articles are presented in Table 1. A detailed summary of each article can be found in Supplemental Table S1. The majority of individuals studied were male and of European ancestry. Most studies included multiple SUDs; abstinence was the most common outcome. Most of the studies were of low-to-moderate quality, and none of the studies met all the quality criteria (Supplemental Table S1).
Figure 1.
PRISMA flow chart detailing the article selection process.
Table 1.
Summary of characteristics of included studies in the systematic review, excluding case series/reports (N = 31).
| Type of SUD assessed (N) studies | |
| Cannabis | 6 |
| Alcohol | 5 |
| Opioids | — |
| Cocaine | 2 |
| Methamphetamine | 1 |
| Poly-drug use | 16 |
| Not specified | 1 |
| Study design (N) studies | |
| Case reports/series | 13 |
| Phase 1 | 1 |
| Pharmacovigilance case analysis | 1 |
| Retrospective observational cohort | 6 |
| Prospective observational cohort | 5 |
| RCT | 3 |
| National Survey Study | 1 |
| Observational registry-based study | 1 |
| Antipsychotic comparison to clozapine excluding case reports and series, (N) studies | |
| FGA only | 3 |
| Second-generation antipsychotic (SGA) only | 5 |
| FGA + SGA | 8 |
| Outcome reported (N) studies | |
| Abstinence | 15 |
| Reduction in use | 10 |
| Reduction in craving | 3 |
| Reduction in psychiatric hospitalization | 3 |
| Odds of developing SUD | 1 |
| Country of origin of the studies | |
| USA | 15 |
| Europe | 11 |
| Canada | 1 |
| Australia | 1 |
| Malaysia | 1 |
| Iran | 1 |
| S. Korea | 1 |
Effects of clozapine on maintaining abstinence or decrease in substance use
Case reports/series
All published case reports and case-series (n = 13) reported on maintaining abstinence and/or reducing use (Supplemental Table S1). Participants were predominantly poly-drug users. The most common substances used were alcohol, cannabis, and cocaine. There were four published cases on augmentation strategies of clozapine and achieving abstinence/or reduction in use in comorbid alcohol use disorder, lamotrigine n = 3 (Kalyoncu et al., 2005), and amisulpride n = 1 (Dervaux and Cazali, 2007). There was one published case (Pang et al., 2017) of clozapine in comorbid opioid/amphetamine-type-substance co-use disorder, maintaining abstinence with daily witnessed ingestion of methadone and clozapine. In two cases, patients with amphetamine-type-SUD achieved remission from resistant psychosis and maintained abstinence while on clozapine (Seddigh et al., 2014). All the case reports/series except one (Marcus and Snyder, 1995) documented presence of treatment-resistant SSD before initiation of clozapine.
Observational studies
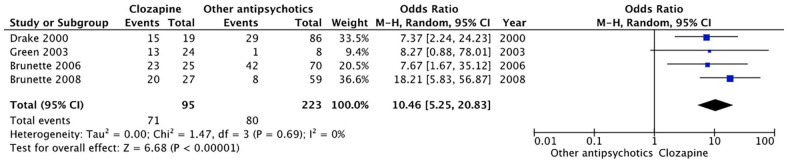
Four observational studies (Brunette et al., 2006, 2008; Drake et al., 2000; Green et al., 2003) with available raw data reported the proportion of individuals with concurrent SSD and SUDs (predominantly alcohol) who remained abstinent (defined as either complete cessation of use or use without impairment) on clozapine versus other antipsychotics for the duration of follow-up. Characteristics of these studies are shown in Table 2. Pooled results from these studies are shown with the random-effect model (Figure 2). Clozapine treatment was associated with greater odds for remaining abstinent (OR = 10.46, 95% CI = 5.83–56.87, p < 0.00001). Antipsychotics compared with clozapine were predominantly FGAs with some inclusion of second-generation antipsychotics (SGAs) in the more recent ones. None of the observational studies clearly defined that the criteria for treatment-resistant SSD were met before initiation of clozapine.
Table 2.
Characteristics of studies based on available raw data (n = 4) included in the meta-analysis of abstinence in patients with SSDs and comorbid SUDs.
| Study (Author et al.) | Study design | N | Age [mean, years] | Sex [male %] | Ancestry | Diagnosis | Antipsychotic (s) exposure [mean dose] | Substance use outcomes/assessment of substance use | Type of substance assessed |
|---|---|---|---|---|---|---|---|---|---|
| Drake et al. (2000) | Prospective Cohort Study 3 years follow up | 105 | 32.3 | 77.5 | Predominantly European (96.7%) | Schz/SAD + SUD | 1. Clozapine (n = 19) 2. FGA (n = 86) |
Remission defined as AUS < 3 i.e., abstinence or use without impairment/TLBF, ASI, UDS, AUS, DUS, SATS | Alcohol |
| Green et al. (2003) | Retrospective Cohort Study 1 year follow-up | 41 | 44.7 | 76 | NS | Schz/SAD + SUD | 1. Clozapine (n = 24)
439 mg/day 2. Risperidone (n = 8) 3.9 mg/day |
Abstinence defined as cessation of all alcohol and cannabis use/Chart review as per clinician notes | Alcohol, cannabis |
| Brunette et al. (2006) | Prospective Cohort Study 1 year follow-up following “remission” | 95 | 34 | 74.4 | Predominantly European (96.4%) | Schz/SAD + SUD | 1. Clozapine (n = 25)
484 mg/day 2. Other Aps (n = 70) |
Remission defined as AUS/DUS < 3 i.e., abstinence or use without impairment /TLFB, ASI, UDS, AUS, DUS, SATS | Alcohol, cannabis, cocaine |
| Brunette et al. (2008) | Retrospective Cohort Study 6 to 12 months follow-up | 86 | 40.3 | 79.1 | NS | Schz/SAD + SUD | 1. Clozapine 448.2 mg/day
(n = 27) 2. Other Aps (n = 59) FGA = 35 405 mg/day CPZ equivalent Olanzapine = 24 18.2 mg/day |
Abstinence defined as cessation of all alcohol use/Chart review as per clinician notes/ blood or urine test results, and recordings of Breathalyzer results | Alcohol |
Aps: Antipsychotics; ASI: Addiction Severity Index; AUS: Alcohol Use Scale; BP: Bipolar Disorder; CPZ: chlorpromazine; DUS: Drug Use Scale; NS: Not Stated; FGA: First generation antipsychotics; SAD: Schizoaffective Disorder; SATS: Substance Abuse Treatment Scale; Schz: Schizophrenia; SUD: Substance Use Disorder; TLBF: Time-Line Follow-Back; UDS: Urine Drug Screen.
Figure 2.
Forest plot for the association of clozapine treatment with odds of abstinence in comparison with other antipsychotics.
A retrospective cohort study (n = 204) reported on point prevalence of current diagnosis of substance abuse in a sample of patients with SSD and SUDs on clozapine versus “other antipsychotics” (Lee et al., 1998). Approximately half of each cohort had past diagnosis of substance abuse. There was a significant between-group difference in current diagnosis of substance abuse (0% in clozapine-treated versus 13% other antipsychotics).
A prospective cohort study (n = 223) found clozapine to be associated to “early” recovery in a sample of patients with SSD and SUDs (predominantly alcohol). Early-recovery clients engaged quickly in treatment, achieved abstinence within the first year, and remained abstinent for years. Use of clozapine was remarkably common (over one third) for this group (Xie et al., 2009). The analysis of clozapine treatment in this study was not hypothesized a priori.
The 2017 Australian National survey (Siskind et al., 2017) reported on lifetime and past year substance use in individuals with SSD (n = 1049) treated with clozapine versus other antipsychotics. They did not indicate whether the criteria for SUDs were met in these individuals at some point in their life; however, clozapine treatment was associated with significantly lower odds of past year alcohol, cannabis, amphetamine and other drug use despite similar lifetime odds (alcohol: OR = 0.516, 95% CI = 0.366–0.727, p < 0.001; cannabis: OR = 0.398, 95% CI = 0.282–0.563, p < 0.001; amphetamine: OR = 0.368, 95% CI = 0.219–0.620, p < 0.001; “other” substances: OR = 0.452, 95% CI = 0.245–0.835, p < 0.05).
Randomized controlled trials
Two small single-blind RCTs examined the amount of cannabis use in concurrent SSD and cannabis use disorder. The first RCT (n = 31) showed a non-significant decrease in self-reported cannabis use in clozapine-treated individuals compared with other antipsychotics (~4.5 joints/week, d = 0.60, p = 0.086) after 12 weeks of follow-up (Brunette et al., 2011). The second RCT (n = 30) showed no difference between clozapine-treated versus ziprasidone-treated individuals after 12 months of follow-up with high dropout rates (Schnell et al., 2014).
Special populations
The only published study in an adolescent sample (Tang et al., 2017) was a small retrospective cohort study (n = 27), with a non-significant association of a reduction in cannabis use in clozapine-treated patients in comparison with those treated with other antipsychotics (OR = 2.8, 95% CI = 0.97–7.9, p = 0.06)
Effects of clozapine on the intensity of cravings
One prospective cohort study (n = 123) reported reduction in craving for cannabis in clozapine-treated patients compared with risperidone-treated and olanzapine-treated patients (Machielsen et al., 2012). Clozapine and olanzapine treatments were associated with significant craving reduction compared with risperidone. However, no significant differences were found between the intensity of cravings in clozapine-treated and olanzapine-treated patients.
In another small open-label RCT (n = 28) with 4 weeks of follow-up, clozapine treatment showed significant reduction in craving for cannabis compared with risperidone (Machielsen et al., 2014).
A prospective case-series (n = 25) examined olfactory hedonic ratings between clozapine-treated participants with concurrent psychotic and SUDs compared to FGAs (Mesholam-Gately et al., 2014). Clozapine treatment was associated with a broader and stronger experience of rewarding olfactory stimuli, both pleasant and unpleasant, suggesting potential for ameliorating dysfunction within the brain’s reward system.
Effects of clozapine on psychiatric hospitalization
Maremmani et al. (2006) in a retrospective cohort study (n = 56) found that treatment of clozapine in patients with concurrent cannabis use disorder was associated with a shorter duration of hospitalization compared with those without concurrent cannabis use, suggesting specific effectiveness for clozapine in the former subgroup (Lee-desu statistic = 4.08; DF = 1; p = 0.043).
Kim et al. (2008) in a prospective cohort study of patients with SSD and comorbid alcohol use disorder (n = 61) with up to 2 years follow-up after a psychiatric hospitalization in South Korea found time to rehospitalization to be significantly longer in clozapine-treated patients compared with risperidone-treated patients (log-rank test, df = 1, p = 0.045).
In contrast, Yee et al. (2021) in a 10-year retrospective cohort study (n = 179) of individuals enrolled in an assertive community treatment program with primarily SSD and concurrent SUDs (not limited to alcohol), clozapine treatment was associated with increased odds of psychiatric hospitalization (OR 2.30, 95% CI 1.25–4.24, p = 0.021).
More recently, a large longitudinal observational study (n = 45,476) of two independent national Scandinavian cohorts (Lahteenvuo et al., 2022) reported clozapine treatment in individuals with both SSD and SUDs to be associated with less risk of psychiatric hospitalization (aHR 0.51, 95% CI 0.48–0.54 for Finland, aHR 0.51, 95% CI 0.44–0.58 for Sweden) and hospitalization due to SUD (Finland: aHR 0.59, 95% CI 0.53–0.66; Sweden aHR 0.71, 95% CI 0.54–0.94). However, both cohorts also observed similar ORs in “antipsychotic polytherapy” and “long-acting injectable.” In addition, in individuals without concurrent SUDs, clozapine treatment was associated with the lowest risk of developing an initial SUD (aHR 0.20, 95% CI 0.16–0.24, p < 0.001, in Finland; 0.35, 0.24–0.50, p < 0.001, in Sweden).
Clozapine safety in individuals with comorbid SUDs
A descriptive analysis of the European Medicines Agency Pharmacovigilance Database (Chiappini et al., 2020) explored cases reported due to adverse drug reactions (ADRs) related to clozapine. There were 326 cases out of 11,847 suspected clozapine-related ADRs reporting on “drug abuser,” “drug abuse,” “drug diversion,” “intentional product misuse,” “product use issue,” and “substance abuse.” Moreover, 258 cases described “withdrawal syndrome” involving possible severe and long-lasting symptoms due to abrupt clozapine discontinuation.
In a phase-1 study (Farren et al., 2000) of four oral challenges with ascending doses of clozapine (12.5, 25, and 50 mg) and placebo followed 2 h later by a 2 mg/kg dose of intranasal cocaine, one participant out of eight had to be removed from the challenge study due to syncope. Furthermore, clozapine challenge resulted in the elevation of serum cocaine levels.
One published case report (Sand and Soyka, 1997) described the ineffectiveness of clozapine in improving SUD and resulting in excess sedation and its eventual discontinuation. Another case report (Feeley et al., 2017) described clozapine resistance, lack of improvement in SUD, and the incidence of small bowel obstruction resulting in an eventual switch to aripiprazole with clinically meaningful therapeutic outcomes.
Discussion
The current systematic review and meta-analysis builds upon previous reviews, both suggesting the association of clozapine treatment with improvement in SUD outcomes in patients with SSD. The bulk of the support comes from observational studies. The present pooled findings from four observational studies in samples of patients with predominantly comorbid alcohol use disorder showed that clozapine treatment is associated with significantly higher odds of remaining abstinent. In addition, in terms of non-abstinence-based outcomes, clozapine was associated with decreased odds of psychiatric hospitalization in all but one observational study.
In SSD, dysfunction in dopamine-mediated brain reward pathways found in mesocorticolimbic tracts (“reward deficiency syndrome”) is postulated to underlie the high prevalence of comorbid SUDs (Green et al., 2008). These dopaminergic pathways serve as the site of reward-based effects of addictive substances and are hypothesized to mediate motivation, pleasure, and contentment in daily life (Khokhar et al., 2017). Although using substances might have transient amelioration of this reward dysfunction by potentiating dopamine activity, they can have a deteriorating impact on dopamine-mediated brain reward pathways in the long term. The in-vivo neuroimaging studies have shown downregulation of striatal dopaminergic function in substance users with clinical implications in triggering craving and relapse (Ashok et al., 2017; Kamp et al., 2019). Even though, these studies excluded patients with SSD, further dopaminergic downregulation due to substance use may explain the observation of higher incidence of antipsychotic associated extrapyramidal symptoms (EPS) in SSD-SUD population in comparison with SSD (Potvin et al., 2006). Furthermore, incidence of antipsychotic associated EPS may increase relapse to substance use (Potvin et al., 2006; Voruganti et al., 1997).
Although requiring further investigation, clozapine’s effectiveness in improving SUD outcomes may be due to its unique actions on multiple neurotransmitter systems. Firstly, clozapine’s selective and relatively low occupancy in striatal dopamine type-2 receptors (Pilowski et al., 1997) may not contribute to further striatal dopaminergic downregulation and results in a low liability to cause EPS. Secondly, clozapine’s propensity to increase γ-aminobutyric acid-B (GABA-B)-mediated inhibitory neurotransmission (Nair et al., 2020), N-desmethylclozapine’s muscarinic acetylcholine receptor M1 agonist activity (Weiner et al., 2004), and clozapine’s potent blockade of alpha-2 noradrenergic receptors coupled with an increase in norepinephrine levels (Green et al., 2008) are not only hypothesized to be part of the mechanism of treating resistant psychosis but may also have potential roles in treating comorbid SUDs (Cousins et al., 2002; Green et al., 2008; Walker et al., 2022). Lastly, clozapine’s propensity to decrease striatal glutamate levels (McQueen et al., 2021) has potential as an anti-glutamatergic agent to attenuate rewarding effects of substance use (D’Souza, 2015).
Still, the validity of this association needs greater exploration. Given the prescriber bias in observational studies, significant heterogeneity and limitations among included studies, providing recommendations for the utility of clozapine in individuals without treatment-resistant psychosis and comorbid SUDs would be premature. The preliminary findings summarized here do however, further demonstrate the importance of early identification of individuals with treatment-resistant SSD and SUDs for clozapine initiation while maintaining high standards of monitoring to ensure safety.
Study limitations
Certain limitations should be considered when interpreting our findings. First, we applied broad inclusion criteria to our search to capture all studies involving clozapine use in individuals with SSD and SUDs. However, limiting our studies to English language articles may have narrowed the number of eligible trials detected. Heterogeneity in the pool of included studies, both at the methodological level, outcome measured, and antipsychotic comparison, complicated interpretation of results. Furthermore, there was a lack of reporting on differences in antipsychotic discontinuation rates compared with clozapine. Due to major methodological differences between studies, we grouped similar study designs reporting on the same outcome together to weigh the evidence. Other factors that accounted for heterogeneity included duration of follow-up, dosage, and study sample characteristics such as stage of illness and type of SUD. The potential influence of these factors on the generalizability of the results is discussed below.
Antipsychotic comparison heterogeneity
For most observational studies, clozapine was compared to FGAs with little description of dosing. Given that incidence of EPS is a dose-related phenomenon and might occur with higher frequency in treatment with high potency antipsychotics, analysis of dosing and type of antipsychotics used would be an important consideration to strengthen results. Indeed, in the only registry-based observational study (Lahteenvuo et al., 2022), the odds of decreasing psychiatric and substance use-related hospitalization were similar between clozapine and any long-acting injectable antipsychotics.
Sample heterogeneity
There was a lack of clear description of the study samples included in terms of severity/treatment resistance and severity of SUD. As the efficacy of non-clozapine antipsychotics in treating psychosis in treatment-resistant SSD is poor, characterization of cohorts being compared for SUD outcomes is vital. However, none of the observational studies characterized whether or not the included patients met the criteria for treatment-resistant SSD. Thus, it remains unclear whether clozapine improves SUD outcomes by directly impacting psychopathology or has additional “anti-craving” effects. Furthermore, as different non-prescribed substances vary in impact on the striatal dopamine system (Ashok et al., 2017; Kamp et al., 2019), comparing individuals without characterizing the type and severity of consequences of the substance(s) used may increase heterogeneity in observational studies.
Heterogeneity in outcome measures used
Studies in our review reported a range of outcome measures, including abstinence, reduction in use or craving, risk of psychiatric hospitalization and risk of developing SUDs post initiation of antipsychotic therapy. While pooling of the results from observational studies showed clozapine is associated with higher odds of maintaining abstinence, given that prescribing was based on clinical judgment rather than random assignment, bias towards prescribing clozapine to patients who were more likely to adhere to pharmacological and non-pharmacological treatments cannot be ruled out.
Safety concerns
Safety concerns include added CNS depression with clozapine in the presence of depressant substances such as opioids and alcohol, increased risk of syncope, risk of decreased metabolism of cocaine, and severe psychotic symptoms due to abrupt discontinuation of clozapine in a group that is more likely to be nonadherent due to substance use. These concerns need to be weighed against potential benefits of clozapine initiation in individuals with SSD and SUDs. Furthermore, the higher odds of comorbidities such as diabetes, obesity, and seizure disorder, as well as debilitating adverse effects such as constipation, excessive sedation, problem swallowing and sialorrhea, always require consideration at baseline before initiation of clozapine (Siskind et al., 2017). Many clozapine adverse effects can be managed by proper dosing, therapeutic drug monitoring and pharmacological/non-pharmacological interventions, and they need not be a barrier for individuals with treatment-resistant SSD and SUDs (Correll et al., 2022).
Conclusions
Our systematic review and meta-analysis builds upon previous reviews, and it suggests the association of clozapine treatment with significantly higher odds of remaining abstinent from substance use and decreased likelihood of psychiatric hospitalization, compared with continuing treatment with other antipsychotic medications. These findings must be interpreted with caution when considering the number of studies and the suboptimal quality of evidence from these studies. It is unclear whether clozapine improves these outcomes by better treating psychosis in a treatment-resistant population or has additional “anti-craving” effects compared with other antipsychotics. There is an urgent need to address the limitations of previous studies in the field with adequately powered, pragmatic randomized, double-blind, controlled clinical trials with larger samples, followed by open-label follow-up studies. The preliminary findings summarized here do however, further demonstrate the importance of early identification of individuals with treatment-resistant SSD and SUDs for clozapine initiation while maintaining high standards of monitoring to ensure safety.
Supplemental Material
Supplemental material, sj-docx-2-jop-10.1177_02698811221142575 for Effects of clozapine treatment on the improvement of substance use disorders other than nicotine in individuals with schizophrenia spectrum disorders: A systematic review and meta-analysis by Reza Rafizadeh, Marlon Danilewitz, Chad A Bousman, Nickie Mathew, Randall F White, Anees Bahji, William G Honer and Christian G Schütz in Journal of Psychopharmacology
Supplemental material, sj-xlsx-1-jop-10.1177_02698811221142575 for Effects of clozapine treatment on the improvement of substance use disorders other than nicotine in individuals with schizophrenia spectrum disorders: A systematic review and meta-analysis by Reza Rafizadeh, Marlon Danilewitz, Chad A Bousman, Nickie Mathew, Randall F White, Anees Bahji, William G Honer and Christian G Schütz in Journal of Psychopharmacology
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RR has none to declare; DM reports receiving personal fees from Eisai Ltd, Otsuka, Winterlight Labs and the Ontario Brain Institute; CAB is founder and CEO of Sequence2Script Inc.; NM has none to declare; RFW has received income from Canadian Agency for Drugs and Technologies in Health and Advisory Board Activities for HLS Therapeutics; AB has none to declare; WGH is a consultant to AbbVie; and Translational Life Sciences, CGS is a consultant to Clearmind Medicine.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Reza Rafizadeh  https://orcid.org/0000-0002-4163-8241
https://orcid.org/0000-0002-4163-8241
Supplemental material: Supplemental material for this article is available online.
References
- Arranz B, Garriga M, García-Rizo C, et al. (2017) Clozapine use in patients with schizophrenia and a comorbid substance use disorder: A systematic review. Eur Neuropsychopharmacol 28: 227–242. [DOI] [PubMed] [Google Scholar]
- Arranz B, Garriga M, Bernardo M, et al. (2022) Clinical practice guideline on pharmacological and psychological management of adult patients with schizophrenia spectrum disorders and a comorbid substance use. Adicciones 34: 110–127. [DOI] [PubMed] [Google Scholar]
- Ashok AH, Mizuno Y, Volkow ND, et al. (2017) Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: A systematic review and meta-analysis. JAMA Psychiatry 74: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunette MF, Dawson R, O’Keefe CD, et al. (2011) Clozapine vs. other antipsychotics for schizophrenia and co-occurring cannabis use disorder. Schizophr Bull 37: 297. [Google Scholar]
- Brunette MF, Drake RE, Xie H, et al. (2006) Clozapine use and relapses of substance use disorder among patients with co-occurring schizophrenia and substance use disorders. Schizophr Bull 32: 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunette MF, O’Keefe C, Zimmet S, et al. (2008) Clozapine, olanzapine, or typical antipsychotics for alcohol use disorder in patients with schizophrenia. J Dual Diagn 4: 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappini S, Schifano F, Corkery JM, et al. (2020) Focus on clozapine withdrawal- and misuse-related cases as reported to the European medicines agency (EMA) pharmacovigilance database. Brain Sci 10: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Agid O, Crespo-Facorro B, et al. (2022) A guideline and checklist for initiating and managing clozapine treatment in patients with treatment-resistant schizophrenia. CNS Drugs 36: 659–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins M, Roberts D, de Wit H. (2002) GABA(B) receptor agonists for the treatment of drug addiction: A review of recent findings. Drug Alcohol Depend 65: 209–220. [DOI] [PubMed] [Google Scholar]
- Crockford D, Addington D. (2017) Canadian schizophrenia guidelines: Schizophrenia and other psychotic disorders with coexisting substance use disorders. Can J Psychiatry 62: 624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervaux A, Cazali J. (2007) Clozapine and amisulpride in refractory schizophrenia and alcohol dependence. J Clin Psychopharmacol 27: 514–516. [DOI] [PubMed] [Google Scholar]
- Downs SH, Black N. (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and nonrandomised studies of health care interventions. J Epidemiol Commun Health 52: 377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RE, Xie H, McHugo GJ, et al. (2000) The effects of Clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull 26: 441–449. [DOI] [PubMed] [Google Scholar]
- D’Souza MS. (2015) Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci 9: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farren CK, Hameedi FA, Rosen MA, et al. (2000) Significant interaction between Clozapine and cocaine in cocaine addicts. Drug Alcohol Depend 59: 153–163. [DOI] [PubMed] [Google Scholar]
- Feeley RJ, Arnaout B, Yoon G. (2017) Effective switch from clozapine to aripiprazole in treatment-resistant schizophrenia and comorbid alcohol use disorder. J Clin Psychopharmacol 37: 729–730. [DOI] [PubMed] [Google Scholar]
- GBD 2019. Mental Disorders Collaborators (2022) Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet Psychiatry 9: 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AI, Burgess E, Dawson R, et al. (2003) Alcohol and cannabis use in schizophrenia: Effects of Clozapine vs. risperidone. Schizophr Res 60: 81–85. [DOI] [PubMed] [Google Scholar]
- Green AI, Noordsy DL, Brunette MF, et al. (2008) Substance abuse and schizophrenia: Pharmacotherapeutic intervention. J Subst Abuse Treat 34: 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddaway N, Grainger M, Gray C. (2022) Citationchaser: A tool for transparent and efficient forward and backward citation chasing in systematic searching. Res Synth Methods 13: 533–545b. [DOI] [PubMed] [Google Scholar]
- Hasan A, Falkai P, Wobrock T, et al. (2015) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia. Part 3: update 2015 management of special circumstances: Depression, suicidality, substance use disorders and pregnancy and lactation. World J Biol Psychiatry 16: 142–170. [DOI] [PubMed] [Google Scholar]
- Hunt GE, Large MM, Cleary M, et al. (2018) Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990–2017: Systematic review and meta-analysis. Drug Alcohol Depend 191: 234–258. [DOI] [PubMed] [Google Scholar]
- Jarde A, Losilla JM, Vives J, et al. (2013) Q-Coh: A tool to screen the methodological quality of cohort studies in systematic reviews and meta-analysis. Int J Clin Health Psychol 13: 138–146. [Google Scholar]
- Kalyoncu A, Mirsal H, Pektas O, et al. (2005) Use of lamotrigine to augment Clozapine in patients with resistant schizophrenia and comorbid alcohol dependence: A potent anti-craving effect?. J Psychopharmacol 19: 301–305. [DOI] [PubMed] [Google Scholar]
- Kamp F, Proebstl L, Penzel N, et al. (2019) Effects of sedative drug use on the dopamine system: A systematic review and meta-analysis of in vivo neuroimaging studies. Neuropsychopharmacology 44: 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfoot KE, Rosenheck RA, Petrakis IL, et al. (2011) Substance use and schizophrenia: Adverse correlates in the CATIE study sample. Schizophr Res 132: 177–182. [DOI] [PubMed] [Google Scholar]
- Khokhar JY, Henricks AM, Sullivan EDK, et al. (2017) Unique effects of clozapine: A pharmacological perspective. Adv Pharmacol 82: 137–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim D, Marder SR. (2008) Time to rehospitalization of Clozapine versus risperidone in the naturalistic treatment of comorbid alcohol use disorder and schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 32: 984–988. [DOI] [PubMed] [Google Scholar]
- Krause M, Huhn M, Schneider-Thoma J, et al. (2019) Efficacy, acceptability and tolerability of antipsychotics in patients with schizophrenia and comorbid substance use. A systematic review and meta-analysis. Eur Neuropsychopharmacol 29: 32–45. [DOI] [PubMed] [Google Scholar]
- Lahteenvuo M, Luykx J, Taipale H, et al. (2022) Associations between antipsychotic use, substance use and relapse risks in patients with schizophrenia: Real-world evidence from two national cohorts. Br J Psychiatry 221: 758–765. [DOI] [PubMed] [Google Scholar]
- Lee ML, Dickson RA, Campbell M, et al. (1998) Clozapine and substance abuse in patients with schizophrenia. Can J Psychiatry 43: 855–856. [PubMed] [Google Scholar]
- Machielsen MW, Beduin AS, Dekker N, et al. (2012) Differences in craving for cannabis between schizophrenia patients using risperidone, olanzapine or clozapine. J Psychopharmacol 26: 189–195. [DOI] [PubMed] [Google Scholar]
- Machielsen MW, Veltman DJ, van den Brink W, et al. (2014) The effect of Clozapine and risperidone on attentional bias in patients with schizophrenia and a cannabis use disorder: An fMRI study. J Psychopharmacol 28: 633–634. [DOI] [PubMed] [Google Scholar]
- Marcus P, Snyder R. (1995) Reduction of comorbid substance abuse with Clozapine. Am J Psychiatry 152: 959. [DOI] [PubMed] [Google Scholar]
- Maremmani I, Pacini M, Lazzeri A, et al. (2006) Concurrent abuse of cannabis is associated with a shorter duration of hospitalization in treatment-resistant psychotic bipolar inpatients treated with Clozapine. Addict Disord Their Treat 5: 1–7. [Google Scholar]
- McQueen G, Sendt K, Gillespie A, et al. (2021) Changes in brain glutamate on switching to clozapine in treatment-resistant schizophrenia. Schizophr Bull 47: 662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J, Sampson M, Salzwedel DM, et al. (2015) PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 75: 40–45. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately R, Gibson LE, Seidman LJ, et al. (2014) Schizophrenia and co-occurring substance use disorder: Reward, olfaction and Clozapine. Schizophr Res 155: 45–51. [DOI] [PubMed] [Google Scholar]
- Nair P, Mckinnon R, Miners J, et al. (2020) Binding of clozapine to the GABAB receptor: Clinical and structural insights. Mol Psychiatry 25: 1920–1919. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, et al. (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang NTP, Mohamad Isa M, Singh Suarn, et al. (2017) Directly observed therapy for Clozapine with concomitant methadone prescription: A method for improving adherence and outcome. BMJ Case Rep 2017: bcr2017221048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowski L, Mulligan R, Acton PD, et al. (1997) Limbic selectivity of clozapine. Lancet 350: 490–491. [DOI] [PubMed] [Google Scholar]
- Potvin S, Pampoulova T, Mancini-Marië A, et al. (2006) Increased extrapyramidal symptoms in patients with schizophrenia and a comorbid substance use disorder. J Neurol Neurosurg Psychiatry 77: 796–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand PG, Soyka M. (1997) Substance abuse in clozapine-treated schizophrenic patients. J Neuropsychiatry Clin Neurosci 9: 626–627. [PubMed] [Google Scholar]
- Schnell T, Koethe D, Krasnianski A, et al. (2014) Ziprasidone versus Clozapine in the treatment of dually diagnosed (DD) patients with schizophrenia and cannabis use disorders: A randomized study. Am J Addict 23: 308–312. [DOI] [PubMed] [Google Scholar]
- Seddigh R, Keshavarz-Akhlaghi AB, Shariati B. (2014) Treating methamphetamine-induced resistant psychosis with Clozapine. Case Rep Psychiatry 2014: 845145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind DJ, Harris M, Phillipou A, et al. (2017) Clozapine users in Australia: Their characteristics and experiences of care based on data from the 2010 National Survey of High Impact Psychosis. Epidemiol Psychiatr Sci 26: 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. (2018) Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res 197: 274–280. [DOI] [PubMed] [Google Scholar]
- Tang SM, Ansarian A, Courtney DB. (2017) Clozapine treatment and cannabis use in adolescents with psychotic disorders - A retrospective cohort chart review. J Can Acad Child Adolesc Psychiatry 26: 51–58. [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. (2017) Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry 74: 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voruganti LNP, Heslegrave RJ, Awad AG. (1997) Neuroleptic dysphoria may Be the missing link between schizophrenia and substance abuse. J Nerv Ment Dis 185: 463–465. [DOI] [PubMed] [Google Scholar]
- Walker L, Campbell E, Huckstep K, et al. (2022) M1 muscarinic receptor activation decreases alcohol consumption via a reduction in consummatory behavior. Pharmacol Res Perspect 10: e00907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner D, Meltzer H, Veinbergs I, et al. (2004) The role of M1 muscarinic receptor agonism of N-desmethylclozapine in the unique clinical effects of clozapine. Psychopharmacology 177: 207–216. [DOI] [PubMed] [Google Scholar]
- Xie H, McHugo G, Drake RE. (2009) Subtypes of clients with serious mental illness and co-occurring disorders: Latent-class trajectory analysis. Psychiatr Serv 60: 804–811. [DOI] [PubMed] [Google Scholar]
- Yee MR, Espiridon E, Oladunjoye AO, et al. (2021) The use of clozapine in the serious mental illness patients enrolled in an assertive community treatment program. Cureus 13: e15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-2-jop-10.1177_02698811221142575 for Effects of clozapine treatment on the improvement of substance use disorders other than nicotine in individuals with schizophrenia spectrum disorders: A systematic review and meta-analysis by Reza Rafizadeh, Marlon Danilewitz, Chad A Bousman, Nickie Mathew, Randall F White, Anees Bahji, William G Honer and Christian G Schütz in Journal of Psychopharmacology
Supplemental material, sj-xlsx-1-jop-10.1177_02698811221142575 for Effects of clozapine treatment on the improvement of substance use disorders other than nicotine in individuals with schizophrenia spectrum disorders: A systematic review and meta-analysis by Reza Rafizadeh, Marlon Danilewitz, Chad A Bousman, Nickie Mathew, Randall F White, Anees Bahji, William G Honer and Christian G Schütz in Journal of Psychopharmacology