Preface
Haematological malignancies were previously thought to be driven solely by genetic or epigenetic lesions within haematopoietic cells. However, the niches which maintain and regulate daily production of blood and immune cells are now increasingly being recognised as having an important role in the pathogenesis and chemoresistance of haematological malignancies. Within haematopoietic cells, the accumulation of a small number of recurrent mutations initiates malignancy. Concomitantly, specific alterations of the niches, which support haematopoietic stem cells and their progeny, can act as predisposition events, facilitating mutant haematopoietic cell survival and expansion as well as contributing to malignancy progression and providing protection of malignant cells from chemotherapy, ultimately leading to relapse. In this Perspective article, we summarise our current understanding of the composition and function of the specialised haematopoietic niches of the bone marrow (BM) during health and disease. We discuss disease mechanisms (rather than malignancy subtypes) to provide a comprehensive description of key niche-associated pathways that are shared across multiple haematological malignancies. These mechanisms include primary driver mutations in bone marrow niche cells, changes associated with increased hypoxia, angiogenesis and inflammation as well as metabolic reprogramming by stromal niche cells. Consequently, remodelling of bone marrow niches can facilitate immune evasion and activation of survival pathways favouring malignant haematopoietic cell maintenance, defence against excessive reactive oxygen species (ROS) and protection from chemotherapy. Lastly, we suggest guidelines for the handling and biobanking of patient samples and analysis of the niche to ensure that basic research identifying therapeutic targets can be more efficiently translated to the clinic. The hope is that integrating knowledge of how bone marrow niches contribute to haematological disease predisposition, initiation, progression and response to therapy into future clinical practice will likely improve the treatment of these disorders.
Table of contents summary
This Perspective outlines our current understanding of how the bone marrow niche contributes to both the initiation and progression of haematological malignancies and suggests guidelines for the field which might help to overcome existing research challenges.
Introduction
Haematological malignancies represent a heterogeneous group of blood neoplasias commonly characterised by abnormal production of blood cells (haematopoiesis). The World Health Organization (WHO) broadly categorises the haematological malignancies based on the lineage of origin of the neoplastic cell (either myeloid or lymphoid), their derivation from precursor or stem or differentiated or committed cells, their acute or chronic development, and more specifically based on their clinical, morphological and genetic features1,2. In some cases, the maintenance of a haematological malignancy depends upon transformed haematopoietic stem cells (HSCs), which can be refractory to chemotherapy (chemoresistant) and therefore represent a major cause of disease progression and relapse3.
Normal haematopoietic cells rely on local interactions in the microenvironment with stromal cells and other haematopoietic cells that facilitate their survival and regulate their functions. These specialised microenvironments are broadly referred to as ‘niches’. Whilst HSCs, positioned at the top of the haematopoietic hierarchy, can primarily self-renew in bone marrow (BM) niches, other haematopoietic cells find their niches in secondary lymphoid organs, such as the spleen or the lymph nodes. Leukaemia stem cells (LSCs) share many features with healthy HSCs, including a dependence on BM niches for their survival and resistance to chemotherapy, which can cause relapse3.
Although the clinical management of haematological malignancies has improved significantly over the last years, its socio-economical cost is a concern and key challenges remain, such as relapse, refractory disease and malignancy-associated morbidity and mortality4. Recent discoveries of the underlying genetic and epigenetic alterations offer some hope for personalised medicine; however, this is hampered by the high molecular diversity and complexity found in leukaemia, its variable evolution during disease and therapy and the acquisition of mechanisms of immune evasion and chemoresistance, partly dependent on the niche5,6. Therefore, the niche may represent a therapeutic target to improve treatment outcome. In this Perspective article we will briefly summarise our current understanding of the haematopoietic niches in health to understand key changes occurring during malignancy development, maintenance, therapy and relapse. Ultimately, a better knowledge of the key pathophysiological mechanisms and their similarities and differences across different haematological malignancies will likely provide alternative or adjuvant therapies targeting the niche to ameliorate or refine current treatments.
Bone marrow niches
In normal haematopoiesis
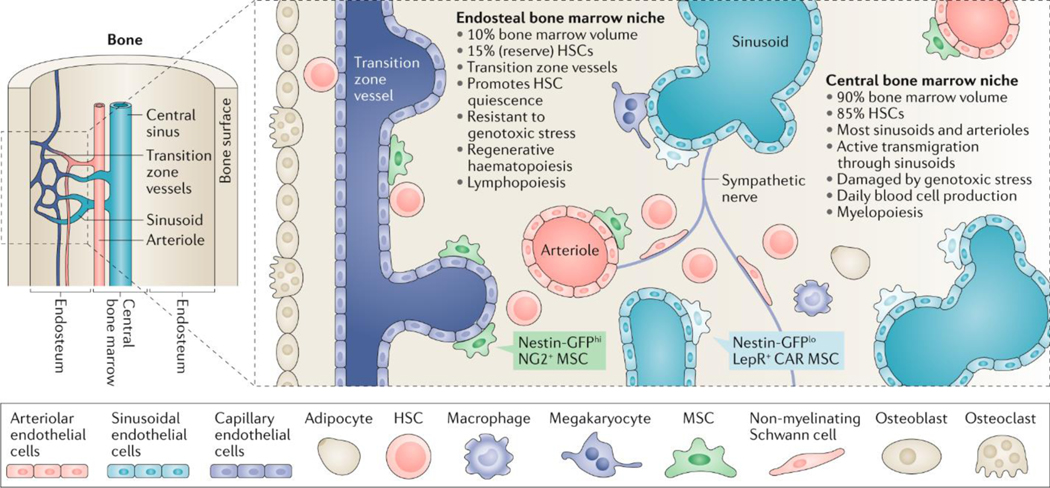
HSCs reside in a unique BM microenvironment referred to as an ‘HSC niche’. Several types of HSCs with various differentiation biases (platelet and myeloid versus lymphoid or balanced) or different states (dormant versus quiescent versus activated) have been defined7 and it is possible that spatially distinct niches exist for each of them8–10. Currently, mouse HSC niches are defined based on their anatomical location in the BM and the type of blood vessel they contain: sinusoids11, arterioles12 or transition zone vessels, which connect arterioles with sinusoids in the BM, and are close to the bone surface13. At least two anatomically-different HSC niches exist in the BM (Figure 1): the central niche, which is located in the inner BM, and the endosteal niche, in close proximity to the bone surface (both in the diaphysis (the central part of a long bone) and at the epiphysis (the end part), which contains the trabecular BM). The central niche contains the majority of sinusoids and arterioles (which are located throughout the BM), comprises >90% of the BM volume and harbours 85% of the HSCs11. The specific functions of arteriolar versus sinusoidal niches remain a subject of controversy with different laboratories obtaining opposing results depending on the Cre lines used12,14–16. Since these Cre lines actually recombine in overlapping cell populations17,18, it is likely that disagreement among different groups in the recombination pattern and penetrance of the Cre lines used explains these divergent interpretations19. Being a much smaller niche (<10% of the BM volume), the endosteal niche is relatively enriched in HSCs (15% of all HSCs)19 and contains all transition zone vessels13. It is possible that both niches are functionally different. In support of this idea, active HSCs transmigrate through the sinusoidal niche20, which contains the bulk of active BM responsible for daily blood production, and is sensitive to genotoxic stress induced by irradiation or myeloablation (suppression of the ability of the BM to produce blood cells)21. HSC migration through the sinusoids is regulated by sympathetic nerve fibres22,23. In contrast, the endosteal niche is preserved after these insults and seems important for subsequent haematopoietic regeneration24.
Fig. 1: Main features of anatomically-defined haematopoietic stem cell niches in the mouse bone marrow.
Schematic summarizing the key cell types and functional features of the central and endosteal bone marrow (BM) niches of haematopoietic stem cells (HSCs). Mesenchymal stem cells (MSCs; also known as mesenchymal stem and progenitor cells) give rise to bone-forming cells (osteoblasts) and fat cells (adipocytes), whereas bone-resorbing cells (osteoclasts) share a monocytic origin with macrophages. Nestin-GFPhi neural–glial antigen 2 (NG2)+ MSCs are associated with endosteal transition zone vessels and arterioles located throughout the BM, whereas Nestin-GFPlo leptin receptor (LEPR)+ CXC-chemokine ligand 12 (CXCL12)-abundant reticular (CAR) MSCs are associated with sinusoids in the central BM. Sympathetic nerve fibres regulate the migration of HSCs through the sinusoids. Different MSC subpopulations, endothelial cells, non-myelinating Schwann cells and megakaryocytes might contribute to regulate the balance between HSC proliferation and quiescence during daily or regenerative haematopoiesis. Changes in specialized BM niches might directly affect myeloid versus lymphoid output, and the imbalanced production of mature haematopoietic cells at specific niches might, in turn, remodel the local microenvironment for these cells. During ageing, the landscape of the mouse HSC niche changes substantially. Specifically, the central niche expands with morphological and functional changes including an increase in noradrenergic fibres, capillaries with Nestin-GFP+ MSCs, HSC proliferation and myelopoiesis. By contrast, the endosteal niche and its associated components and functions are reduced with concomitant decreases in transition zone vessels and arterioles, HSC quiescence and self-renewal, resistance to genotoxic stress, regenerative haematopoiesis and lymphopoiesis.
In both the endosteum and central BM, HSCs reside in perivascular niches25, where endothelial cells26 and their associated perivascular mesenchymal stem cells (MSCs; also known as mesenchymal stem and progenitor cells) critically maintain and regulate HSCs both in mice27 and humans28. Among these HSC niche-forming MSCs, Nestin-GFPbright and/or neural–glial antigen 2 (NG2)+ cells are associated with arterioles throughout the BM12 and with transition zone vessels near the bone surface29, whereas Nestin-GFPdim–CXC-chemokine ligand 12 (CXCL12)-abundant reticular (CAR)30–leptin receptor (LEPR)+ MSCs16 are associated with sinusoids in the central BM16 (Figure 1). In adult human BM, MSCs can be enriched for the lin⁻CD45⁻CD271⁺CD140a-/dim population31, where CD146 expression appears to discriminate perisinusoidal (CD146+) from endosteal (CD146-/dim) MSCs32.
In the endosteal niche, several signals have been described to promote HSC quiescence24,25,33–39, which is essential to preserve HSC self-renewal capacity. However, quiescent HSCs can be also found near sinusoids11, where other niche cells may restrict HSC proliferation. For instance, megakaryocytes are mostly adjacent to sinusoids and can promote HSC quiescence through secretion of transforming growth factor β (TGFβ), thrombopoietin (TPO) or platelet factor 4 (PF4)40–43. Additionally, non-myelinating Schwann cells associated with nerve fibres can promote HSC quiescence by activating latent TGFβ in endosteal or central BM niches44. Therefore, it is possible that HSC quiescence is regulated differently in various locations to accommodate the requirements of steady-state or emergency haematopoiesis. This heterogeneity and complexity of HSC niches has recently been appreciated through single-cell studies45–47. Combined single-cell RNA sequencing and spatially-resolved transcriptomics has revealed two different subsets of CAR cells: a more abundant Adipo-CAR cell population expressing adipocyte-related genes located adjacent to sinusoids, and a less abundant Osteo-CAR cell population (enriched in osteolineage-related genes) located in avascular BM regions or associated with arteriole-like vessels47 (presumably also including transition zone vessels).
Haematopoietic lineage commitment also appears to be differentially regulated in endosteal and perisinusoidal niches in mice. Specific deletion of Cxcl12 in osteoblasts (bone-forming cells located adjacent to the bone surface) affects the maintenance of early lymphoid progenitors8 and BM niches for lymphoid cells have been previously described near the bone surface8,48–50. However, perivascular MSCs can provide key signals for B cell lymphopoiesis (such as CXCL12 and interleukin-7 (IL-7)) throughout the BM9,51–53. In contrast, the maturation of some myeloid cells (such as megakaryocytes) occurs in perisinusoidal niches54,55. Furthermore, microenvironmental regulation of lineage commitment might also underlie changes to haematopoiesis during aging56. For instance, a reduction of endosteal niches and a concurrent expansion of central BM niches might facilitate age-associated lymphoid-to-myeloid skewing29,57 (Figure 1). This could potentially be relevant to age-related myeloid malignancies, where niche remodelling with age might facilitate the expansion of mutant clones driving these neoplasias.
In malignancy
The possible role of the niche as an oncogenic driver or facilitator of malignancy was suggested over two decades ago58. Niche alterations were hypothesized to be potentially genotoxic or oncogenic. However, an alternative explanation also put forward was that the malignant cells might transform the normal niche or create their own niche for expansion. Since these initial suggestions, experimental models have highlighted different, yet non-mutually-exclusive possibilities of crosstalk between malignant and/or premalignant cells and their niches as a key contributor to disease initiation, progression and resistance to therapy. The following sections will describe in detail the alterations that can occur to haematopoietic niches.
The transformed niche
The identification of alterations in both neoplastic cells and niche cells is reminiscent of ‘the chicken or the egg’ conundrum whereby it is unclear if the main role of the niche is in the initiation or the progression of some malignancies such as myelodysplastic syndrome (MDS) and acute myeloid leukaemia (AML)58–61. Two non-mutually exclusive contributions for haematopoietic niches to leukaemogenesis have been proposed: 1) the acquisition of mutations or functional alterations by niche cells that predispose for malignancy development and 2) niche remodelling by transformed haematopoietic cells that facilitates disease manifestation and/or progression. Herein, we present evidence for both possibilities.
Niche as a predisposition factor
Several studies in mice have suggested that certain haematological malignancies can originate in the niche itself or, at least, that concomitant mutations and functional alterations in niche cells contribute to disease initiation (Table 1). In fact, studies performed over two decades ago demonstrated the presence of genetic alterations in MSCs from patients with MDS and AML, albeit alterations that were different from the oncogenic drivers present in the leukaemic blasts58,59,62,63. However, in most cases the possible functional consequences of these mutations remain to be elucidated. Further evidence for the niche as a predisposing factor comes from clinical cases of allogeneic HSC transplantation. There are rare examples whereby leukaemias arise in the transplanted recipient, which can be either donor-derived (carrying the same mutation later found in the donor cells) or result from transformation of healthy haematopoietic cells into a malignant clone under the selective pressure of the recipient’s BM microenvironment64.
Table 1:
Niche alterations in mouse models predispose to haematological malignancies
| Genetic alteration | Cell Population | Mechanism | Disease | References |
|---|---|---|---|---|
| Ikba deletion | Non-haematopoietic cells | Notch activation in HSCs by JAGGED1 | MPN-like | 65 |
| Rarg deletion | Non-haematopoietic cells | Increased TNF from multiple cell sources | MPN-like | 67 |
| Rb1 deletion | Non-haematopoietic cells; synergistic effect with deletion in myeloid cells |
Unclear; myeloproliferation secondary to Rb1 deletion in non-haematopoietic cells | MPN-like | 68 |
| Mib1 deletion | Non-haematopoietic cells | Defective Notch activation in HSCs | MPN-like | 69 |
| Crebbp haploinsufficiency | Non-haematopoietic cells | Decreased MMP9 and KITL, increased ESAM1 and CDH5 in Crebbp+/− endothelial cells | MPN-like | 70 |
| Dicer1 or Sbds deletion | BM MSCs (Osterix-Cre), but not mature osteoblasts (Osteocalcin-Cre) | Genotoxic stress (through p53, S100A8 and S100A9) | MDS-like, sporadic AML (in Dicer1 deletion) | 71, 73 |
| Constitutive β-catenin activation | Osteoblasts (Col2.3-Cre) | Notch activation in HSCs by JAGGED1 | AML-like | 72 |
| Activating Ptpn11 | BM MSCs (Nestin-Cre) | Inflammatory environment through monocyte recruitment owing to high levels of CCL3 | MPN-like (JMML) | 76 |
AML, acute myeloid leukaemia; BM MSC, bone marrow mesenchymal stem cells; CCL3, CC chemokine ligand 3; CDH5, cadherin 5; Crebbp, CREB-binding protein; ESAM1, endothelial cell-selective adhesion molecule 1; Ikba, nuclear factor-κB (NF-κB) inhibitor α; JMML, juvenile myelomonocytic leukaemia; KITL, KIT ligand; Mib1, Mindbomb 1; MDS, myelodysplastic syndrome; MMP9, matrix metalloproteinase 9; MPN, myeloproliferative neoplasm; Ptpn11, protein tyrosine phosphatase non-receptor type 11; Rarg, retinoic acid receptor γ; TNF, tumour necrosis factor.
Niche alterations as potential oncogenic events have been proposed to occur more frequently in the case of myeloid malignancies. Different experimental genetic alterations of niche components in mice can cause MDS- or myeloproliferative neoplasm (MPN)-like diseases, which are considered pre-leukaemic disorders owing to their high risk of transformation to secondary leukaemia. These possible niche initiating events were first demonstrated experimentally in newborn mice lacking nuclear factor-κB (NF-κB) inhibitor α (IκBα) specifically in non-haematopoietic cells65. IκBα-deficient mice developed MPN-like disease65. Deletion of the gene encoding this particular protein was tested after the discovery that IκBα inhibits NF-κB-dependent transcription of genes regulating haematopoietic cell proliferation and differentiation66. The possibility that a dysfunctional niche can initiate MPN received further experimental support in mice carrying other, different genetic alterations that were not present in the haematopoietic system. For instance, deletion of retinoic acid receptor γ (Rarg)67 in mice carrying wildtype haematopoietic cells, or concomitant deletion of the tumour suppressor gene Rb1 from myeloid-derived cells (granulocytes, macrophages and osteoclasts (bone-resorbing cells)) and the BM microenvironment (whilst retaining Rb1 expression in the HSC compartment)68 similarly causes MPN-like disease. Inhibition of Notch activation69 or haploinsufficiency of CREB-binding protein (Crebbp)70 in non-haematopoietic cells similarly causes myeloproliferation.
Subsequent studies have identified specific niche cell populations that can initiate a haematological malignancy when tested in experimental models. First, deletion of the RNA processing enzyme Dicer1 in MSCs (but not in mature osteoblasts) causes MDS-like disease with sporadic transformation to AML71. Second, enforced expression of a constitutively activated form of β-catenin in osteoblasts has been reported to induce AML in mice72. However, the relevance of this observation for human disease is not yet clear and remains to be elucidated. A subsequent study of mice which develop an MDS-like disease following deletion of Sbds −a DICER1 target− from MSCs proposed a mechanism to explain this myelodysplasia: MSCs lacking Sbds activate p53 and release the damage-associated molecular pattern (DAMP) molecules, S100A8 and S100A9, which cause genotoxic stress in haematopoietic stem and progenitor cells (HSPCs), potentially favouring leukaemic transformation73. However, Sbds deficiency in HSPCs in mice causes neutropenia (a low level of neutrophils)73. Therefore, the disease arising in patients harbouring a germline mutation in SBDS might result from both niche and haematopoietic cell-intrinsic alterations, particularly affecting ribosomal assembly74. SBDS, DICER1 and other genes encoding RNA processing components show abnormal expression in BM MSCs from patients with MDS75; although a definitive demonstration that these niche alterations can cause human MDS is warranted.
Other germline mutations can also trigger or aggravate myeloproliferative diseases through their effects on BM MSCs. One example is mutations in protein tyrosine phosphatase non-receptor type 11 (PTPN11), which are present in 50% of patients with Noonan Syndrome and predispose to the childhood MPN juvenile myelomonocytic leukaemia (JMML)28.Although the Ptpn11 mutation in HSCs can cause a cell-autonomous MPN resembling JMML, an activating mutation of the Ptpn11 gene in BM MSCs (but not in differentiated osteoblasts or endothelial cells) promotes the development and progression of JMML76.
A common feature of all of these studies is the pro-inflammatory environment. For instance, the MPN generated by deletion of Rarg is partly dependent on increased levels of tumour necrosis factor (TNF), a key molecule in systemic inflammation67. In JMML, CC-chemokine ligand 3 (CCL3) secreted by BM MSCs expressing mutated Ptpn11 recruits inflammatory monocytes, which amplify the inflammation through other cytokines, such as IL-1β76. In turn, IL-1β can trigger pro-inflammatory damage of the microenvironment and contribute to MPN progression77. Inflammation is a hallmark of ageing, and as mentioned above, myeloid malignancies are more prevalent in the elderly, suggesting that the inflamed microenvironment might be an important predisposition factor for myeloid malignancies as we age. In line with this possibility, recent studies suggest that HSCs can only be maintained in apposition to Nestin-GFP+/lo cells in the central BM sinusoidal niches of the aged mouse78. Notably, the presence of endosteal BM niches is severely reduced, whereas central BM capillaries increase in the aged mouse, associated with impaired lymphopoiesis and excessive myelopoiesis29. Furthermore, alterations in β-adrenergic signalling through sympathetic nerves in the BM can influence the balance between lymphoid and myeloid lineages during both premature or physiological aging29,79. Overall, niche remodelling promotes myeloid cell expansion both in normal ageing and in premature haematopoietic ageing, which has been observed in mouse models of Hutchinson-Gilford Progeria Syndrome29. Therefore, by stimulating myeloid cell expansion, ageing of the microenvironment might facilitate the growth of premalignant clones and the development of myeloid malignancies.
Indeed, myeloid malignancies may develop over many years through a continuous process spanning the initial acquisition of clonal-haematopoiesis-related somatic mutations to the development of clonal haematopoiesis, MPN or MDS and secondary leukaemia80. Clonal haematopoiesis resulting from somatic mutations of HSCs commonly arises with ageing81–83 and selective pressures, such as cytotoxic chemotherapy, can lead to clonal expansion favouring cells with certain mutations, such as protein phosphatase 1D (PPM1D) or TP5384–86. The most commonly acquired genetic variants include DNA methyltransferase 3A (DNMT3A), tet methylcytosine dioxygenase 2 (TET2), additional sex combs-like 1 (ASXL1), Janus kinase 2 (JAK2) and TP53, but clonal haematopoiesis can occur in the absence of a recognised leukaemic driver mutation and still correlates with reduced survival87. Clonal haematopoiesis is a risk factor for cardiovascular disease and increased mortality, owing to the interaction of circulating clonally-derived monocytes with endothelial cells, which promotes inflammation and atherogenesis (the formation of fatty deposits in the arteries)88–90, and for the development of myeloid neoplasms, including MDS and AML91,92. In fact, mutations in DNMT3A have been detected early during AML evolution, when a clonally expanded pool of pre-leukaemic HSCs might initiate leukemogenesis, and later on cause relapse93.
Although a preprint study suggests that the competitive fitness advantage of HSCs in the BM conferred by specific mutations influences clonal emergence and correlates with the variant allele frequency94 (VAF; the incidence of a gene variant in a population), it is unclear why mutant haematopoietic cells often persist in the BM for many years as small, indolent mutant clones if the acquired mutations truly confer a strong growth or survival advantage95–97. This stability may be owing to the presence of regions of the BM niche that are not able to be occupied by mutant clones unless a stronger secondary leukemic driver mutation is acquired, or other factors such as endogenous immunity, which may keep emergent clones in a stable state and prohibit further expansion.
Clonal dynamics are not exclusively a consequence of cell-intrinsic behaviours and must be understood in the context of the microenvironment98. For example, mutations that provide no advantage in a healthy microenvironment may confer an advantage in the setting of a dysregulated microenvironment. Using error-corrected sequencing techniques that are sensitive to the presence of mutations below 1% VAF, clonal mutations in haematopoietic cells can be detected in almost all individuals in their 50s96,99. Since small clones driven by somatic mutations are universal with ageing, whereas haematological neoplasia is relatively rare, it seems unlikely that the acquisition of mutations in haematopoietic progenitors is the rate-limiting step in the development of haematological malignancy. More broadly, it remains unclear why a subset of individuals carrying clonal-haematopoiesis-related somatic mutations exhibit clonal dominance as a prerequisite for the development of myeloid malignancies or cardiovascular diseases81–83,87,88,100–102. As discussed above, alterations to the BM niche might facilitate disease initiation. For example, age-related remodelling of BM HSC niches, which promotes myeloid cell proliferation29, might facilitate the expansion of certain clones carrying clonal-haematopoiesis-related somatic mutations.
Niche remodelling
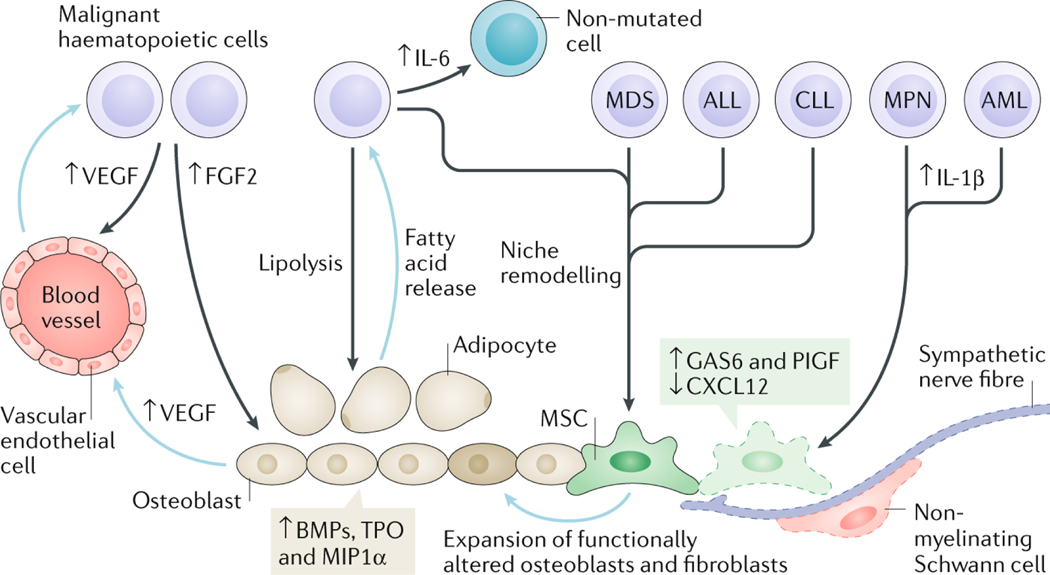
Whereas the clinical evidence for niche lesions as initiating events in haematological malignancies requires future work and validation, it is now clear that malignant cells transform the niche to promote their own survival. Specific niche stromal cells influenced by malignant cells are MSCs, osteoblasts, endothelial cells, adipocytes (fat cells) and peripheral neurons and their associated Schwann cells. The underlying mechanisms by which the remodelled niche favours malignancy represent an area of intense research. Consequently, a picture is emerging whereby key alterations of the microenvironment contribute to malignancy progression over a long time period, concomitantly with the slow accumulation of driver mutations in haematopoietic cells (Figure 2).
Fig. 2: Bone marrow niche remodelling favours disease progression in haematological malignancies.
Representative features of the remodelled bone marrow (BM) niche contributing to the progression of different haematological malignancies. These diseases can arise through genetic and/or epigenetic alterations in haematopoietic cells causing remodelling of the niche that supports their own growth and survival at the expense of normal haematopoiesis. Myeloproliferative neoplasm (MPN) and acute myeloid leukaemia (AML) cells cause neuroglial damage (affecting Schwann cells and sympathetic nerve fibres) in the BM and reduced expression of CXC-chemokine ligand 12 (CXCL12) in mesenchymal stem cells (MSCs). In MPN, the neuroglial damage is caused by increased production of cytokines, like interleukin-1β (IL-1β), leading to apoptosis of Nestin-GFP+ MSCs, which can be rescued through chronic treatment with sympathicomimetic drugs that indirectly improve reticulin fibrosis in mice and humans. In AML, a reduction in BM sympathetic innervation has been correlated with proliferation of Nestin-GFP+ MSCs primed for osteoblastic differentiation at the expense of neural–glial antigen 2 (NG2)+ periarteriolar niche cells, although the potential relevance of these alterations in human AML is unclear. Increased growth arrest-specific 6 (GAS6) and placental growth factor (PlGF) expression by BM MSCs fosters leukaemia cell survival, proliferation and therapy resistance. Vascular endothelial growth factor (VEGF) secreted by both malignant haematopoietic cells and osteoblasts stimulates angiogenesis. In addition, other factors produced by endothelial cells (such as granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-6 and stem cell factor (SCF)) promote survival and proliferation of malignant haematopoietic cells. IL-6 produced by chronic myeloid leukaemia (CML) cells renders non-mutated haematopoietic and stromal cells pro-inflammatory. Malignant haematopoietic cells stimulate expansion of stromal cells through secretion of growth factors, such as bone morphogenetic proteins (BMPs). A reciprocal relationship occurs between malignant haematopoietic cells and adipocytes wherein malignant cells induce lipolysis from adipocytes and, in turn, adipocytes release fatty acids, which are used as an energy source by malignant haematopoietic cells. Blue arrows in this figure indicate processes or factors secreted by niche cells and black arrows indicate processes or factors released by malignant haematopoietic cells. ALL, acute lymphoblastic leukaemia; CLL, chronic lymphocytic leukaemia; FGF2, fibroblast growth factor 2; MDS, myelodysplastic syndrome; MIP1α, macrophage inhibitory protein 1α; TPO, thrombopoietin.
Niche stromal cell reprogramming
Several studies have highlighted how malignant cells change the transcriptome, proteome and function of niche stromal cells. BM MSCs from patients with MDS show increased apoptosis and production of inflammatory cytokines, such as IL-1β, IL-6 and TNF103,104. One study has suggested that human MDS cells reprogramme BM MSCs to enhance their expansion105. In vitro co-culture of patient-derived MDS cells triggers overproduction of certain cytokines by BM MSCs, which enables the engraftment and propagation of MDS in vivo105. Similarly, phenotypic, transcriptomic and epigenetic changes associated with BM MSCs from patients with MDS can impair the growth and function of healthy HSPCs, which can be rescued by treatment with hypomethylating agents106. A different exosomal content has also been found in BM MSCs from patients with MDS and therefore, might mediate their abnormal crosstalk with healthy HSPCs107. However, another study was unable to demonstrate increased engraftment into immunodeficient mice of patient-derived MDS cells in the presence of patient-derived MSCs108. Hence, it is possible that technical differences explain these divergent results.
In chronic myeloid leukaemia (CML), BM MSCs activated through soluble factors such as thrombopoietin (TPO) and macrophage inflammatory protein 1α (MIP1α; also known as CCL3), and also through direct interaction with CML cells, overproduce functionally altered, inflammatory osteoblasts that poorly support normal HSCs109. T cell acute lymphoblastic leukaemia (T-ALL) cells also impair normal haematopoiesis, which is associated with a dramatic loss of osteoblasts and relative preservation of perivascular stromal cells110. In chronic lymphocytic leukaemia (CLL) and AML, extracellular vesicles derived from the neoplastic clones can contribute to reshaping the niche111–114. These extracellular vesicles modulate stromal cells leading to decreased production of HSC-supporting factors111, altered natural killer (NK) cell function114 and accelerated progression in AML112 and transition towards a cancer-associated-fibroblast (CAF) phenotype in CLL113. Interestingly, exosomes from AML cells can transfer endoplasmic reticulum (ER) stress to MSCs and induce their osteogenic commitment partly through bone morphogenetic protein 2 (BMP2)115. Therefore, both secreted factors and exosomes derived from the malignant haematopoeitic cells can remodel MSC function in the haematological malignancies.
Inflammation
Abnormal myeloid cell proliferation typically causes overproduction of pro-inflammatory cytokines. These are produced by both mutated and non-mutated haematopoietic cells, which are reprogrammed to become proinflammatory116,117. The order in which inflammatory cytokines are overproduced during malignant transformation does not appear to be random and might cause specific changes to the BM niche. For example, IL-1β released by premalignant cells is one of the first proinflammatory cytokines abnormally increased during MPN and CML development77, whereas IL-6 appears to increase later during disease progression118,119. Both IL-1β and IL-6 critically contribute to the osteolytic lesions and pathogenesis of multiple myeloma120,121, a disease which involves a multistep transformation process of plasma cells122 paralleled by a progressive remodelling of the BM microenvironment123. Under other conditions, IL-1β stimulates HSC proliferation and myeloid expansion124, and can damage sensitive niche components, such as neural terminals, Schwann cells and Nestin+ MSCs77. In fact, chronic administration of β3-adrenergic agonists to compensate for the defective innervation caused by IL-1β can rescue Nestin+ niche cells and improve myelofibrosis (BM scarring disrupting normal haematopoiesis) both in mice77 and humans125. Similarly, a reduction in BM sympathetic innervation has been proposed to be pathogenic in experimental AML126; although the potential relevance for human AML is unclear.
One early inflammation-driven BM MSC alteration shared among MPN77, AML126 and CLL127 is reduced expression of CXCL12, which favours malignant HSCs over normal HSCs. Following progression of MPN and CML, IL-6 expression by mutated haematopoietic cells increases and renders normal haematopoietic cells proinflammatory116. Other cytokines and growth factors, such as BMPs, TPO, platelet-derived growth factor (PDGF) and CCL3 produced by mutated haematopoietic cells and/or reprogrammed niche cells render the BM microenvironment pro-inflammatory and cause the expansion of MSCs, osteoblastic cells and/or myelofibrotic cells in MPN, CML and multiple myeloma109,128–131. Of note, myelofibrotic cells can be derived from multiple inflamed cell types, including LEPR+ MSCs130, GLI1+ MSCs131 and monocyte-derived fibrocytes132,133. Deregulated signalling downstream of receptor activator of NF-κB ligand (RANKL; also known as TNFSF11; an essential factor for osteoclast maturation) and its decoy receptor osteoprotegerin underlie the abnormal bone remodelling in myelofibrosis and multiple myeloma134,135. In contrast, terminal osteogenic MSC differentiation appears to be impaired in AML46,126; although the reasons for this differentiation blockade remain unclear.
Hypoxia and Angiogenesis
Hypoxia and angiogenesis are two common features in many haematological malignancies. Malignant haematopoietic cells highly consume oxygen, secrete pro-angiogenic molecules, such as vascular endothelial growth factor A (VEGFA)136 and interleukins, and induce placental growth factor (PlGF) secretion by BM MSC137. Neovascularisation not only provides increased nutrients and oxygen, but is also a source of factors that promote the survival, proliferation and chemoresistance of malignant cells. For example, malignant haematopoietic cell-derived VEGFA stimulates angiogenesis but also signals in an autocrine manner to malignant cells to promote their own survival and proliferation136,138–140. Similarly, endothelial VEGF signalling not only stimulates angiogenesis, but it also induces the secretion of cytokines (such as granulocyte macrophage-colony stimulating factor (GM-CSF), macrophage-CSF (M-CSF), granulocyte-CSF (G-CSF), IL-6 and stem cell factor (SCF) produced by different cell types) which promote malignant haematopoietic cell proliferation141–143.
Increased BM angiogenesis has been noted in different malignancies, such as AML, MPN and MDS, correlating with increased levels of angiogenic factors including VEGF, fibroblast growth factor 2 (FGF2), and hepatocyte growth factor (HGF)144–148. Whether angiogenesis occurs equally throughout the BM or whether it specifically affects endosteal or central niches should be investigated with more detail. These neovessels exhibit abnormal morphology both in MPN and AML126,146. Moreover, the impaired vessel function caused by the leukaemia cells, which manifests as increased vascular permeability owing to excessive ROS, hypoxia and nitric oxide (NO) in endothelial cells, is thought to compromise the delivery of chemotherapeutic drugs149,150. In patient-derived xenograft (PDX) models of human AML, BM endothelial cells specifically show increased activation of nitric oxide synthase 3 (NOS3) causing NO-mediated increases in vascular permeability; however, NO inhibitors can restore vessel function and improve chemotherapy response149.
Hypoxia causes stabilisation of hypoxia-inducible factors (HIFs) that have important established roles in solid tumours, whilst their expression in leukaemia cells has been shown to either impair151,152 or promote153–155 leukaemogenesis. Some studies suggest that HIF1α and HIF2α suppress AML development in mouse models151,152, whereas other studies have concluded that HIF1α and HIF2α promote leukaemic cell survival in a mouse model of CML and in a PDX model of AML153–155. Whether these differences are species-, stage- or disease-specific remains to be established.
Niche mechanisms favour malignancy
Activation of survival pathways
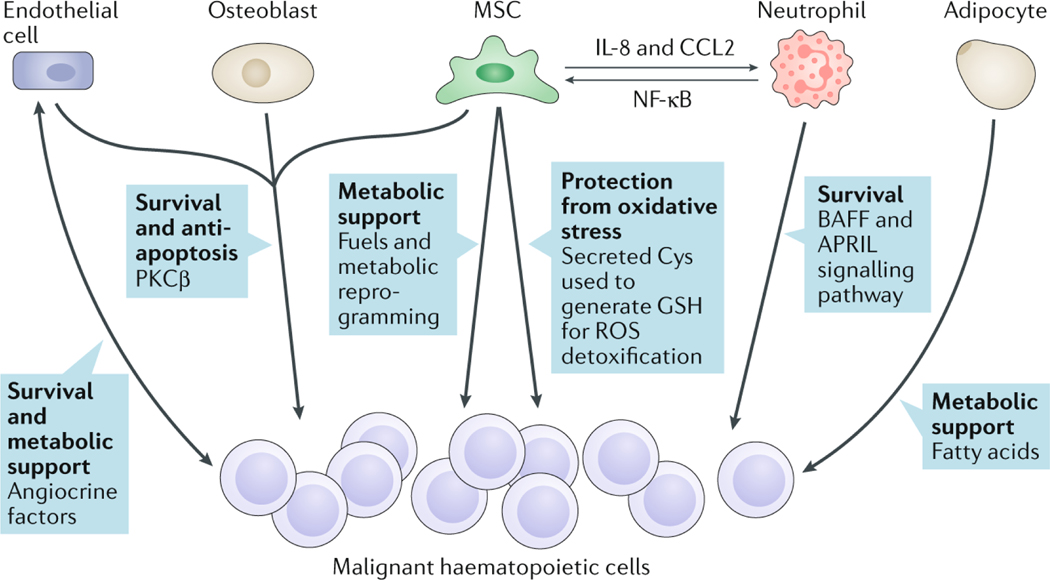
Survival signals are directly linked to pro-inflammatory pathways, like NF-κB. Stromal cells activate the NF-κB pathway in CLL cells as a requisite for survival156. The mechanism involves the initial induction of protein kinase C-β (PKCβ) in stromal cells by the CLL cells. This effect is cell-contact-dependent and is mediated by IL-1α and IL-15. In fact, PKCβ knockout mice transplanted with CLL cells do not develop the disease156. This pathway can be induced in different cell types, including human vascular endothelial cells (HUVECs) and osteoblasts156. Since PKCβ is upregulated in the human BM in CLL157, ALL158 and mantle-cell lymphoma159, this pathway might represent a common survival mechanism in lymphoid malignancies (Figure 3). Indeed, a recent study has shown that PKCβ inhibition in MSCs using small molecules enhances chemosensitivity and overcomes drug resistance in B cell malignancies160.
Fig. 3: Contributions of the bone marrow niche to survival and chemoresistance of malignant haematopoietic cells.
Schematic showing the diverse mechanisms by which reprogrammed niche cells promote the survival, metabolism and chemoresistance of malignant haematopoietic cells. Different stromal cells in the bone marrow (BM), such as endothelial cells, mesenchymal stem cells (MSCs) and adipocytes, provide metabolic support to malignant haematopoietic cells, which in turn produce angiocrine factors promoting neovascularization in the BM. Induction of protein kinase C-β (PKCβ) in niche cells or B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL) signalling from neutrophils can stimulate malignant haematopoietic cell survival. Interleukin-8 (IL-8) and CC-chemokine ligand 2 (CCL2) expression by MSCs recruits and activates neutrophils, which render MSCs pro-inflammatory through nuclear factor-κB (NF-κB) activation, which is also induced in other niche cells and in malignant haematopoietic cells. Cysteine (Cys) secreted by niche stromal cells can be utilized by cancer cells to synthesize glutathione (GSH) and reduce levels of reactive oxygen species (ROS).
Malignant haematopoietic cells can also instruct BM MSCs to provide other survival signals. For instance, both AML and multiple myeloma cells trigger expression of growth arrest-specific protein 6 (GAS6) in BM MSCs to foster leukaemic cell survival, proliferation and therapy resistance161,162. GAS6 can bind different receptors, including the tyrosine kinases AXL and MER, dependent on the disease. In this way, AXL plays an essential role in AML161, whereas MER is more important in multiple myeloma162. Another niche-dependent survival signal in AML are WNT ligands produced by osteoblasts, which bind to Frizzled receptors expressed by AML cells163.
An interesting 3-way crosstalk between tumour-associated neutrophils, CAFs and malignant B cells has been revealed to occur in the lymphoid niches of secondary lymphoid organs in germinal centre B-cell lymphomas and CLL. Whereas neutrophils can directly support the survival of diffuse large B-cell lymphoma (DLBCL) cells through B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL) signalling, they can also activate stromal cells to recruit additional neutrophils through CCL2 and IL-8 secretion, which in turn promote neutrophil survival and formation of neutrophil extracellular traps (NETs)164. NETs are composed of fibril matrix normally released by neutrophils to kill extracellular pathogens165. However, NETs have also been proposed to activate the NF-κB pathway in DLBCL164 and CLL166. Therefore, complex feed-forward loops among stromal cells, immune cells and leukaemia cells sustain chronic inflammation.
Protection from excessive ROS
Several in vitro studies have suggested that stromal cells enable malignant haematopoietic cells to control levels of reactive oxygen species (ROS) for survival and chemoresistance. For example, in vitro co-culture experiments have shown that stromal cells provide CLL cells with cysteine to generate glutathione (GSH) for ROS detoxification127. This mechanism has additionally been implicated in B cell ALL (B-ALL)167 as well as solid tumours, such as ovarian cancer168. Chemotherapy efficacy often relies on ROS-dependent DNA damage of cancer cells. Therefore, increased GSH availability might provide a general mechanism for chemoresistance of neoplastic cells. However, an alternative mechanism has been identified whereby stromal cells through inducing mitochondrial calcium influx into ALL cells, can increase intracellular ROS levels triggering redox adaptation and ultimately, chemoresistance169. Nevertheless, these studies highlight how elucidating the mechanisms of niche-dependent chemoresistance represent an important area of future research.
Metabolic reprogramming
The regulation of leukaemic cell metabolism by stromal cells has been proposed to promote leukaemia cell survival and chemoresistance. Chemoresistant AML cells heavily rely on oxidative metabolism and frequently use fatty acids as biofuel170. This is achieved by reduced expression of prolyl hydroxylase 3 (PHD3; also known as EGLN3), which inhibits fatty acid oxidation171, and by increased expression of fatty acid binding protein 4 (FABP4)172, which facilitates usage of this energy source.
To meet their high bioenergetic demands, leukaemia cells can invade other tissues and hijack additional energy sources. One example is CML, which can trigger lipolysis in gonadal adipose tissue; the released fatty acids are taken up and oxidised as biofuel by the leukaemia cells173. Curiously, a subset of LSCs in CML can reside in the unique niche provided by the gonadal adipose tissue, where their fatty acid demands are fulfilled enabling survival, increased quiescence and chemoresistance173. One explanation for these features could be the continuous generation of NADPH from fatty acid oxidation, which could potentially reduce mitochondrial oxidative phosphorylation and associated ROS, thus facilitating chemotherapy resistance173.
Metabolic reprogramming not only involves increased bioavailability of nutrients, but in some cases the direct transfer of key machinery or organelles between cells. For example, tunneling nanotubes (TNTs) allow intracellular exchange between BM MSCs and malignant cells174–176. In B-ALL, TNTs mediate the transfer of prosurvival cytokines from BM MSCs to B-ALL precursors and contribute to B-ALL resistance to the steroid prednisolone174. Through endocytic pathways or TNTs, stromal cells can transfer mitochondria to AML cells, a process boosted by chemotherapy and associated with increased ATP production in the recipient cells175,177. Similarly, mitochondria transferred from BM MSCs through TNTs can trigger metabolic reprogramming in multiple myeloma178. Yet, the fate of the transferred mitochondria and the clinical relevance of this process in these and other haematological malignancies remains to be clearly established.
Immunosuppression
MSCs in the BM niche can promote an immunosuppressive microenvironment via decreased activation of effector lymphocytes mediated by secretion of indoleamine 2,3 dioxygenase 1 (IDO1), heme oxygenase 1 (HO1), arginase 1 and arginase 2, NOS2, HGF, TGFβ, IL-10 and prostaglandin E2 (PGE2) (reviewed in179–181). Additionally, TGFβ secreted by MSCs reduces the expression of the natural killer group 2D (NKG2D) activating receptor on NK cells, CD8+ T cells and other T cell subpopulations, decreasing its capacity to interact with the NKG2D ligands expressed by malignant haematopoietic cells182.
To avoid immune cell attack, AML cells themselves can utilize other mechanisms of immune evasion such as downregulation of the expression of major histocompatibility complex (MHC) class I and class II molecules, induction of dendritic cell dysfunction and expansion of regulatory T (Treg) cells183 as well as upregulation of programmed cell death protein 1 ligand 1 (PDL1) and other inhibitory immune checkpoint molecules, such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) and lymphocyte activation gene 3 (LAG3)184. Expression of certain molecules such as CD200, which normally suppress macrophages and NK cells, or CD48, which binds to the activating NK cell receptor CD244, are directly altered by the presence of the t(8;21) or inv(16) aberrations, respectively in AML cells185. Another immunosuppressive mechanism involves IDO1 expression by AML cells, which correlates with poor outcome in childhood AML186.
In MDS, activation of the immune microenvironment might contribute to lower-risk MDS, and indeed it has been demonstrated that immunosuppressive treatments can be beneficial in some cases of lower-risk MDS187. In contrast, immune activation, for instance in the form of immune checkpoint inhibitors may impair disease progression in higher-risk MDS, which is also characterized by escape from immune surveillance188.
CLL and lymphoma cells in secondary lymphoid organs are also dependent on an immunosuppressive niche to ensure their survival. In this case, CLL cells or lymphoma cells impair the function of infiltrating immune cells by preventing the formation of the F-actin-rich immune synapse189. However, normal T cell function can be restored following treatment with the immunomodulatory drug lenalidomide189, which has recently been approved for lymphoma190. Expression of the inhibitory B7 family molecules CD200, PDL1, CD276 (also known as B7-H3) and CD270 (also known as HVEM) have been identified to be causative of the defective T-cell synapse191. Among those, the programmed cell death protein 1 (PD1)–PDL1 axis represents a highly valuable target in lymphoma and CLL192,193.
Therapeutic resistance
From initial observations of leukaemia cells engaging with specific BM niches enriched in expression of E-selectin and CXCL12194, the possibility arose that LSCs might be protected from the effects of chemotherapy through their anchorage in such niches. Some studies argue for the existence of specific anatomical locations of chemotherapy-resistant AML cells, frequently found to home and engraft in the endosteal BM195. Others have found that several adhesion molecules, such as the glycoprotein CD44196–198, the integrin α4β1 (also known as VLA4)199–201 and the receptor for CXCL12, CXC-chemokine receptor 4 (CXCR4)197,202–206 anchor LSCs to niches, increasing LSC survival as well as decreasing LSC proliferation and chemosensitivity.
Indeed, it has been shown that CD44, expressed on LSCs of the myeloid malignancies, CML196, multiple myeloma197 and AML198 is important for interactions with the BM microenvironment. In CML, the quiescence and therapy resistance of LSCs is maintained207 by CD44 binding to one of its receptors, E-selectin, which is exclusively expressed on endothelium and mediates the homing and engraftment of LSCs208. Similar interactions between other E-selectin ligands and E-selectin have been demonstrated to increase chemotherapy resistance in multiple myeloma209. β1 integrins, which interact with vascular cell adhesion molecule 1 (VCAM1) and intercellular adhesion molecule 1 (ICAM1) expressed on stromal cells, are involved in the adhesion of CML cells to the BM microenvironment199. In AML, the α4β1 integrin–VCAM1 axis has been shown to mediate chemoresistance via activation of the NF-κB pathway in stromal cells200 while in CLL chemoresistance may act through β1 and β2 integrin binding to endothelial cells201. As a consequence, studies using animal models have found that blockade of E-selectin, CD44, α4β1 or CXCR4 can release CML and AML cells from protective BM niches and sensitise them to conventional chemotherapy196,198,202,210,211.
Changing niche dependencies
Evidence indicates that malignant haematopoietic cells may compete with normal haematopoietic cells for niche occupancy212. This not only applies to haematological malignancies213 but also solid tumours that metastasise to the BM214. In some cases LSCs depend on canonical HSC niche pathways, such as the CXCL12–CXCR4 axis215. CXCL12 (mainly produced by BM MSCs) is required for the retention and quiescence of BM HSCs216. Antagonizing CXCR4 can displace LSCs from their niches, which can then be repopulated by normal HSCs202,210. Another example of niche competition has been demonstrated in a PDX mouse model where B-ALL cells, which overproduce SCF, change the interaction of normal and malignant haematopoietic cells with their niches217.
However, several studies have suggested that leukaemia cells become progressively more independent from niche control during disease development. For instance, upon transplantation into irradiated mice, LSCs home to endosteal BM regions (resembling normal HSCs) early in leukaemogenesis, but at more advanced stages in the progression of AML, LSCs engraft more centrally in the BM and show reduced sensitivity to WNT signals163. Similarly, multiple myeloma cells inhibit canonical WNT signalling and progressively suppress bone formation218. Alterations in hedgehog signalling have been found in the niche in MPN wherein excess hedgehog signalling and reduced expression of the receptor Patched 2 decrease numbers of BM MSCs and osteoblasts and their production of HSC maintenance factors (CXCL12, SCF and JAGGED1), which accelerates JAK2V617F-driven MPN and may cause leukaemic transformation219.
The progressive independence of leukaemia cells from normal niche signals and the alteration of the niche by advanced disease and/or in response to therapy might explain some discrepancies arising in the field, whereby different laboratories have reported opposite roles for a particular pathway in the same leukaemia type. For instance, HIF signalling has been reported to either impair151,152 or promote153–155 myeloid leukaemogenesis. In lymphoid leukaemias, such as T-ALL, some studies have suggested that T-ALL cells stably contact BM niche cells (MSCs and endothelial cells) and require the secretion of CXCL12 from these niche cells for survival220,221. Conversely, another study was unable to find evidence for this dependence of T-ALL cells110; although a subsequent study by the same group has confirmed that CXCR4 inhibition alters T-ALL (but not AML) distribution with the BM222. It is not yet clear whether these differences in T-ALL niche dependencies are owing to heterogeneous disease subtype and/or stage223. As mentioned before, in the myeloid malignancies LSCs exhibit stage-dependent sensitivities to niche signals. This is also the case across different myeloid malignancies. For instance, AML cells are comparatively less sensitive to TGFβ-induced quiescence than CML cells224. In a broader sense, similar to the clonal evolution of haematopoietic driver mutations, it is possible that the dependencies of leukaemia cells on their microenvironment also evolve during disease progression and in response to therapy.
The niche as a therapeutic target
The ultimate goal of research into the niche of haematological malignancies is the development of therapies targeting the BM microenvironment in conjunction with existing therapies. This could be accomplished by targeting of a) interactions between the malignant cells and their niche, b) malignant haematopoietic cell-intrinsic pathways that influence the niche or c) the BM microenvironment directly.
Induction of the mobilisation of malignant haematopoietic cells, for instance by using G-CSF225 or CXCR4-antagonists (reviewed in205) has been intensely investigated. CXCR4 inhibition is safe and modestly improves remission rates in patients with AML203 and multiple myeloma226. Plerixafor (AMD3100), the CXCR4 antagonist most frequently used in the clinic, has a low affinity for CXCR4. Consequently, a new generation of CXCR4 inhibitors with higher affinity and persistent receptor binding have been developed and demonstrated to block CXCR4 more efficiently227 as well as synergise with FMS-like tyrosine kinase 3 (FLT3) or BCL-2 inhibitors in AML treatment204. Hence, several clinical studies are currently evaluating disruption of CXCL12–CXCR4 signalling in haematological malignancies.
A more recent approach to cause detachment from the niche is the targeting of E-selectin which, as mentioned above, is expressed on endothelium. For instance, E-selectin inhibition via the administration of the E-selectin antagonist GMI-1271 in preclinical models, can increase the chemosensitivity of leukaemia cells. GMI-1271 dislocates CML cells from the BM endothelium, increasing the leukaemia cell cycle and expression of the transcription factor and protooncogene T cell acute lymphocytic leukaemia 1 (TAL1; also known as SCL). TAL1 itself can repress transcription of CD44 reinforcing the decreased adhesion of CML cells to BM endothelium207. Since E-selectin similarly contributes to chemotherapy resistance in multiple myeloma209 and AML198, GMI-1271 is currently under clinical evaluation for the treatment of multiple myeloma228 and AML229.
A recent clinical study attempting to restore functional noradrenergic innervation of the BM niche in patients with MPN with the use of a sympathicomimetic drug (the β3-adrenergic agonist mirabegron) has found that this treatment can rescue the loss of Nestin+ niche cells, which in turn correlated with improved reticulin fibrosis (myelofibrosis with reticulin deposition)125. Furthermore, in experimental MPN, myelofibrosis can be significantly improved by blocking the PDGF receptor-α (PDGFRα) with imatinib130, GLI1 and GLI2 with the inhibitor GANT61131 or conversion of monocytes to fibrocytes with the fibrocyte inhibitor serum amyloid P (SAP; also known as pentraxin-2)132. The latter is currently under clinical evaluation230. The female sex hormones, oestrogens regulate HSPCs directly231,232 and indirectly, through their effects on niches cells, such as osteolineage cells and osteoclasts (partly responsible for post-menopausal osteoporosis, characterised by weakened bones)233. The observation that the selective oestrogen receptor modulator tamoxifen can induce apoptosis of malignant HSPCs in preclinical models231 has led to its current clinical testing in patients with MPN234.
As mentioned above, TYRO3, AXL and MER which constitute the TAM subfamily of tyrosine kinases can bind to GAS6. The interaction between GAS6 and AXL provides a proliferative advantage and chemoprotective niche for AML cells161,162. As a result, targeting the GAS6–AXL interaction with AXL inhibitors is currently under clinical evaluation in CLL235, AML and MDS236.
The Bruton tyrosine kinase (BTK) inhibitor ibrutinib, which is approved for CLL treatment, has activities beyond BTK targeting. Ibrutinib modulates the immunosuppressive environment in CLL237, MPN238 and AML239 and inhibits CXCR4–CXCL12-signalling mediated migration of leukaemia cells, which in CLL and multiple myeloma is dependent on BTK signalling240,241. Finally, other innovative strategies to target the BM microenvironment are NOS inhibitors, which ‘normalise’ the abnormal BM vascular permeability associated with AML and improve chemotherapy efficacy149. An autocrine effect of FGF2 produced by MSCs stimulates the release of leukaemia-protecting exosomes from MSCs242. Therefore, targeting exosomes or administrating hypoxia-activated prodrugs, which could target hypoxic regions in the niche with the potential to augment the chemotherapeutic effect, may be exploited in the future243.
Research challenges and guidelines
The development of clinical trials aimed at targeting the niche in haematological malignancies is still in its infancy (see125,203,226,228–230,234–236). It will be essential that researchers, clinicians and drug companies, alike, appreciate the need for novel strategies such as those targeting the niche, to eradicate malignant haematopoietic cells in haematological malignancies. However, knowledge, analysis and targeting of the niche in haematological malignancies pose a unique and substantial challenge, warranting careful, coordinated, multidisciplinary investigation. Whilst our understanding of the BM microenvironment in haematological malignancies has made substantial progress of late, targeting the niche as an adjunct therapy is only starting to be developed. We have identified the following areas where improvements could be made to fortify and augment existing efforts in basic, translational and clinical research in this field.
Logistical aspects
BM biopsies
Although pathologists receive blood, BM, spleen and lymph node samples to diagnose haematological malignancies, the samples obtained are often insufficient for analysis of the niche, as the necessity has not been obvious in the past. Frequently, tissue biopsy samples are inadequate, too small or of poor quality. In some countries, BM biopsies are no longer routinely performed for diagnosis and have been largely replaced by BM aspirates. Thus, valuable information about the BM microenvironment, which cannot be obtained from BM aspirates alone, is lost. Moreover, valuable information about the morphological characteristics of the malignant niche assessed by haematoxylin & eosin staining or about the expression of cytokines, their receptors, intracellular signalling molecules and other proteins, is frequently not available for careful pathological evaluation. In addition, the possibility of obtaining cells from tissues for single-cell analysis, which is needed to resolve heterogeneity, is severely hampered. Therefore, to advance translational research, we advocate for the inclusion of BM biopsies in the diagnostic routine when sampling the BM for a possible haematological malignancy. Along with samples collected in the haematology laboratory, bone fragments collected in the orthopaedic or surgery department (for instance during hip prosthesis surgery or bone grafting) are an invaluable source of normal HSCs from elderly patients who may have clonal haematopoiesis, as well as BM stromal cells. Following informed consent by the patient, direct, prompt and efficient communication to the researcher about sample availability is required.
Biobanking of fresh, frozen bone and lymph node biopsies
Many centres have established biobanks for the preservation and storage of haematological samples for future use. However, the storage of stromal cells from BM aspirates, biopsies or lymph nodes would be an extremely valuable tool to address questions related to the niche. The biobanking of such stromal cells may require culture conditions which are different from the preservation of other haematological samples. These conditions will need to be developed, refined and standardised in the future.
Communication and collaboration
Translational research of the BM niche in haematological malignancies requires careful planning, detailed communication, coordination and extensive collaboration between clinicians, surgeons, pathologists and researchers. If research on human samples is desired, investigators are dependent on being informed about the availability of samples, their careful processing and delivery to the research facility. Due to the scarcity of material from patients with comparatively rare diseases, international collaboration with exchange of protocols and patient samples is recommended. This concerted effort could harmonise the processing and analysis of samples and facilitate consistency and cross-comparison of results.
Practical aspects
Immunophenotypic markers of stromal cells
A myriad of cluster of differentiation (CD) antigens and other markers are available for the discrimination of haematopoietic cells. However, reliable and specific markers for stromal cells, such as MSCs, osteolineage cells or endothelial cells in the human system are scarce. Standardizing the nomenclature across the field, particularly with respect to MSCs, will help to avoid confusion in the field and facilitate comparison across studies. In addition, MSCs are rare and frequently difficult to obtain from biopsy samples, sometimes requiring special digestion of the tissues to release them for analysis. However, different enzymatic treatments can variably cleave cell surface molecules used to identify these cells, leading to heterogeneous results. Therefore, standardizing isolation protocols will increase the uniformity of results. Conditioning regimes, such as irradiation or myeloablation with 5-flurouracil (5-FU), or malignancy progression may alter the expression of antigens on stromal cells, as reported previously for the haematopoietic system244. Therefore, careful examination of the stability of cellular antigens and the use of multiple markers are warranted to ensure consistency in the cell populations analysed and the results obtained.
Culture methods
One common problem is the proper in vitro expansion of stromal cells, which is often required to obtain sufficient cell numbers for certain applications. The development of single-cell technologies and reliable amplification methods is presently enabling the study of freshly-isolated cells and avoiding notorious variables, alterations or noise caused by the ex vivo expansion. However, when higher cell numbers are required or cell–cell interactions need to be modelled in vitro, preserving the original properties or features of cultured cells can be a real challenge. For example, BM MSCs are generally isolated by taking advantage of their high adherence to plastic but careful removal and/or exclusion of monocytes and macrophages is necessary to avoid the overgrowth of these cells. In addition, whilst culturing adherent BM MSCs with fetal bovine serum (FBS)-supplemented medium has been sufficient to unravel important immunomodulatory properties of these cells, it might not be sufficient to preserve stemness or other original properties of BM MSCs, such as their niche functions for HSCs. In that regard, non-adherent BM MSC cultures supplemented with growth factors, avoiding the differentiating effects of serum and attachment to plastic, better retain in vivo BM MSC self-renewal and HSC support in culture245,246. More generally, standardizing culture protocols (taking into account the specific purpose of the study) will help to enable correlation and validation of results across different laboratories.
Animal models
For certain haematological malignancies such as lymphomas or MDS, animal models truly reflective of the disease are lacking. Even if suitable animal models do exist, there are still challenges with using them for preclinical research. For example, once therapeutic targets in the niche have been identified, their testing in vivo is challenging, as these therapeutic efforts will likely influence not only the niche but also haematopoietic cells, making it almost impossible to distinguish the direct effects of the niche-targeting therapies. Combining improved inducible Cre lines targeting BM niche cell populations with lineage-tracing models and haematopoietic chimeras might facilitate this task in the future. In this regard, the use of conditional (instead of constitutive) Cre lines is recommended due to their higher specificity, and the recombination pattern, efficiency and penetrance of the Cre lines used should be carefully assessed to avoid divergent interpretations19 and clarify the relationships among niche cell populations17,18.
Imaging and sequencing technologies
Using mouse models, sophisticated imaging technologies such as in vivo 2-photon microscopy, confocal microscopy or quantitative 3-dimensional microscopy have enabled HSC–niche interactions to be dissected spatially and temporally20,27,110,150,247. However, for the analysis of the BM microenvironment of patient samples, microscopic imaging is only just beginning to be employed as an important modality. A step towards this is the application of niche scaffolds implanted with human malignant haematopoietic cells transplanted under the skin of immunosuppressed mice, which have been now been successfully imaged248. Continuous improvement of bone-clearing methods will likely advance imaging of patient samples as well.
Combined single-cell RNA sequencing and spatially-resolved transcriptomics47 could be very useful in the future to understand overall transcriptomic changes in BM niches of the haematological malignancies. The development of computational methods to infer potential ligand–receptor interactions17 could pave the way to understand how these interactions change in haematological malignancies. Whereas these RNA sequencing studies are extremely useful, proteome-based studies would be essential to investigate molecules that are often regulated by posttranscriptional mechanisms. In that regard, the application of multicolour quantitative confocal imaging cytometry249 and the development of Imaging Mass Cytometry will undoubtedly represent important technical advances.
Conclusion
In summary, through increasingly sophisticated technologies, our insight into the BM niche has progressed considerably over the last few years, revealing a complex entity which dynamically and specifically interacts with healthy haematopoietic cells and their malignant counterparts. Research has shown that, even two myeloid leukaemias arising from different genetic alterations, myeloid versus lymphoid leukaemias or various haematological malignancies specifically and differentially interact with their microenvironment and even the same niche cells. Importantly, continuous monitoring of the niche during therapy for a haematological malignancy is essential and would stretch beyond the snapshot analysis of the BM microenvironment at diagnosis. Our newly gained knowledge offers opportunities for therapeutic exploitation and targeted intervention of these pathways which may lead to the eradication of the malignant clones. We have reached a turning point at which basic and translational researchers, clinicians, pathologists and industry must come together to establish a new realm of research, diagnostics and targeted treatments, as targeting of BM niches in haematological malignancies, to be used in conjunction with existing therapies, may hold potential to follow in the footsteps of the successes of immunotherapy.
Acknowledgements.
The authors thank Dr. María García-Fernández for helping to structure and critically edit the manuscript. Original work discussed in this article was supported by core support grants from the Wellcome Trust and the MRC to the Cambridge Stem Cell Institute, National Health Service Blood and Transplant (United Kingdom), European Union’s Horizon 2020 research (ERC-2014-CoG-64765) and a Programme Foundation Award from Cancer Research UK to S.M.-F. The LOEWE Center for Cell and Gene Therapy Frankfurt (CGT, funded by HMWK reference number: III L 4-518/17.004; 2010) and the Georg-Speyer-Haus (funded jointly by the German Federal Ministry of Health (BMG) and the Ministry of Higher Education, Research and the Arts of the State of Hessen, HMWK) to D.S.K. The authors acknowledge the support of the European School of Hematology (ESH) and apologise for the omission of relevant literature due to space limitations.
Footnotes
Competing interests
The authors declare no relevant conflicts of interest.
Subject categories
Biological sciences/cancer/haematological cancer
[URI/631/67/1990];
Biological sciences/Stem cells/Cancer stem cells
[URI/631/532/71];
Biological sciences/Cancer/Cancer microenvironment
[URI/631/67/327];
Biological sciences/Developmental biology/Stem cells/Stem-cell niche [URI/631/136/532/2139]
References
- 1.Swerdlow SH et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127, 2375–2390, doi: 10.1182/blood-2016-01-643569 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arber DA et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127, 2391–2405, doi: 10.1182/blood-2016-03-643544 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Huntly BJ & Gilliland DG Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer 5, 311–321, doi: 10.1038/nrc1592 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Uyl-de Groot CA & Lowenberg B.Sustainability and affordability of cancer drugs: a novel pricing model. Nat Rev Clin Oncol 15, 405–406, doi: 10.1038/s41571-018-0027-x (2018). [DOI] [PubMed] [Google Scholar]
- 5.Korn C.& Mendez-Ferrer S.Myeloid malignancies and the microenvironment. Blood 129, 811–822, doi: 10.1182/blood-2016-09-670224 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forte D, Krause DS, Andreeff M, Bonnet D.& Mendez-Ferrer S.Updates on the hematological tumor microenvironment and its therapeutic targeting. Haematologica, doi: 10.3324/haematol.2018.195396 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laurenti E.& Gottgens B.From haematopoietic stem cells to complex differentiation landscapes. Nature 553, 418–426, doi: 10.1038/nature25022 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding L.& Morrison SJ Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235, doi: 10.1038/nature11885 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordeiro Gomes A.et al. Hematopoietic Stem Cell Niches Produce Lineage-Instructive Signals to Control Multipotent Progenitor Differentiation. Immunity 45, 1219–1231, doi: 10.1016/j.immuni.2016.11.004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinho S.et al. Lineage-Biased Hematopoietic Stem Cells Are Regulated by Distinct Niches. Dev Cell 44, 634–641 e634, doi: 10.1016/j.devcel.2018.01.016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acar M.et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526, 126–130, doi: 10.1038/nature15250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunisaki Y.et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643, doi: 10.1038/nature12612 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusumbe AP, Ramasamy SK & Adams RH Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328, doi: 10.1038/nature13145 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asada N.et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol 19, 214–223, doi: 10.1038/ncb3475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comazzetto S.et al. Restricted Hematopoietic Progenitors and Erythropoiesis Require SCF from Leptin Receptor+ Niche Cells in the Bone Marrow. Cell stem cell 24, 477–486 e476, doi: 10.1016/j.stem.2018.11.022 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding L, Saunders TL, Enikolopov G.& Morrison SJ Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462, doi: 10.1038/nature10783 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mende N.et al. Prospective isolation of non-hematopoietic cells of the niche and their differential molecular interactions with HSCs. Blood 134, 1214–1226, doi: 10.1182/blood.2019000176 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Mendez-Ferrer S.Molecular interactome between HSCs and their niches. Blood 134, 1197–1198, doi: 10.1182/blood.2019002615 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Beerman I, Luis TC, Singbrant S, Lo Celso C.& Mendez-Ferrer S.The evolving view of the hematopoietic stem cell niche. Exp Hematol 50, 22–26, doi: 10.1016/j.exphem.2017.01.008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itkin T.et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532, 323–328, doi: 10.1038/nature17624 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooper AT et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell stem cell 4, 263–274, doi: 10.1016/j.stem.2009.01.006 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendez-Ferrer S, Lucas D, Battista M.& Frenette PS Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442–447, doi: 10.1038/nature06685 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Garcia A.et al. Dual cholinergic signals regulate daily migration of hematopoietic stem cells and leukocytes. Blood 133, 224–236, doi: 10.1182/blood-2018-08-867648 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao M.et al. N-Cadherin-Expressing Bone and Marrow Stromal Progenitor Cells Maintain Reserve Hematopoietic Stem Cells. Cell Rep 26, 652–669 e656, doi: 10.1016/j.celrep.2018.12.093 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugiyama T, Kohara H, Noda M.& Nagasawa T.Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C.& Morrison SJ SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Mendez-Ferrer S.et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834, doi: 10.1038/nature09262 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacchetti B.et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Ho YH et al. Remodeling of Bone Marrow Hematopoietic Stem Cell Niches Promotes Myeloid Cell Expansion during Premature or Physiological Aging. Cell stem cell 25, 407–418, doi: 10.1016/j.stem.2019.06.007 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Omatsu Y.et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33, 387–399, doi: 10.1016/j.immuni.2010.08.017 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Li H.et al. Low/Negative Expression of PDGFR-alpha Identifies the Candidate Primary Mesenchymal Stromal Cells in Adult Human Bone Marrow. Stem cell reports 3, 965–974, doi: 10.1016/j.stemcr.2014.09.018 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tormin A.et al. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood 117, 5067–5077, doi: 10.1182/blood-2010-08-304287 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taichman RS & Emerson SG Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med 179, 1677–1682, doi: 10.1084/jem.179.5.1677 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J.et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Calvi LM et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Arai F.et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149–161 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Yoshihara H.et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell stem cell 1, 685–697, doi: 10.1016/j.stem.2007.10.020 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Adams GB et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599–603 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Kinashi T.& Springer TA Steel factor and c-kit regulate cell-matrix adhesion. Blood 83, 1033–1038 (1994). [PubMed] [Google Scholar]
- 40.Bruns I.et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med 20, 1315–1320, doi: 10.1038/nm.3707 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao M.et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med 20, 1321–1326, doi: 10.1038/nm.3706 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Nakamura-Ishizu A, Takubo K, Fujioka M.& Suda T.Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochem Biophys Res Commun 454, 353–357, doi: 10.1016/j.bbrc.2014.10.095 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Nakamura-Ishizu A, Takubo K, Kobayashi H, Suzuki-Inoue K.& Suda T.CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. J Exp Med 212, 2133–2146, doi: 10.1084/jem.20150057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamazaki S.et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147, 1146–1158, doi: 10.1016/j.cell.2011.09.053 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Tikhonova AN et al. The bone marrow microenvironment at single-cell resolution. Nature 569, 222–228, doi: 10.1038/s41586-019-1104-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baryawno N.et al. A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell 177, 1915–1932 e1916, doi: 10.1016/j.cell.2019.04.040 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baccin C.et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat Cell Biol 22, 38–48, doi: 10.1038/s41556-019-0439-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Visnjic D.et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103, 3258–3264 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Zhu J.et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood 109, 3706–3712 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Wu JY et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A 105, 16976–16981, doi: 10.1073/pnas.0802898105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zehentmeier S.& Pereira JP Cell circuits and niches controlling B cell development. Immunol Rev 289, 142–157, doi: 10.1111/imr.12749 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fistonich C.et al. Cell circuits between B cell progenitors and IL-7(+) mesenchymal progenitor cells control B cell development. J Exp Med 215, 2586–2599, doi: 10.1084/jem.20180778 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balzano M.et al. Nidogen-1 Contributes to the Interaction Network Involved in Pro-B Cell Retention in the Peri-sinusoidal Hematopoietic Stem Cell Niche. Cell Rep 26, 3257–3271 e3258, doi: 10.1016/j.celrep.2019.02.065 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Stegner D.et al. Thrombopoiesis is spatially regulated by the bone marrow vasculature. Nature communications 8, 127, doi: 10.1038/s41467-017-00201-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plo I.et al. Genetic Alterations of the Thrombopoietin/MPL/JAK2 Axis Impacting Megakaryopoiesis. Front Endocrinol (Lausanne) 8, 234, doi: 10.3389/fendo.2017.00234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kusumbe AP et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 532, 380–384, doi: 10.1038/nature17638 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho YH & Mendez-Ferrer S.Microenvironmental contributions to hematopoietic stem cell aging. Haematologica 105, 38–46, doi: 10.3324/haematol.2018.211334 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duhrsen U.& Hossfeld DK Stromal abnormalities in neoplastic bone marrow diseases. Annals of hematology 73, 53–70 (1996). [DOI] [PubMed] [Google Scholar]
- 59.Kim Y.et al. Genetic and epigenetic alterations of bone marrow stromal cells in myelodysplastic syndrome and acute myeloid leukemia patients. Stem Cell Res 14, 177–184, doi: 10.1016/j.scr.2015.01.004 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Kim JA et al. Microenvironmental remodeling as a parameter and prognostic factor of heterogeneous leukemogenesis in acute myelogenous leukemia. Cancer Res 75, 2222–2231, doi: 10.1158/0008-5472.CAN-14-3379 (2015). [DOI] [PubMed] [Google Scholar]
- 61.von der Heide EK et al. Molecular alterations in bone marrow mesenchymal stromal cells derived from acute myeloid leukemia patients. Leukemia 31, 1069–1078, doi: 10.1038/leu.2016.324 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Blau O.et al. Chromosomal aberrations in bone marrow mesenchymal stroma cells from patients with myelodysplastic syndrome and acute myeloblastic leukemia. Exp Hematol 35, 221–229, doi: 10.1016/j.exphem.2006.10.012 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Blau O.et al. Mesenchymal stromal cells of myelodysplastic syndrome and acute myeloid leukemia patients have distinct genetic abnormalities compared with leukemic blasts. Blood 118, 5583–5592, doi: 10.1182/blood-2011-03-343467 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sala-Torra O.et al. Evidence of donor-derived hematologic malignancies after hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 12, 511–517, doi: 10.1016/j.bbmt.2006.01.006 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Rupec RA et al. Stroma-mediated dysregulation of myelopoiesis in mice lacking I kappa B alpha. Immunity 22, 479–491, doi: 10.1016/j.immuni.2005.02.009 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Ghosh S.& Karin M.Missing pieces in the NF-kappaB puzzle. Cell 109 Suppl, S81–96, doi: 10.1016/s0092-8674(02)00703-1 (2002). [DOI] [PubMed] [Google Scholar]
- 67.Walkley CR et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell 129, 1097–1110, doi: 10.1016/j.cell.2007.05.014 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walkley CR, Shea JM, Sims NA, Purton LE & Orkin SH Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell 129, 1081–1095, doi: 10.1016/j.cell.2007.03.055 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim YW et al. Defective Notch activation in microenvironment leads to myeloproliferative disease. Blood 112, 4628–4638, doi: 10.1182/blood-2008-03-148999 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Zimmer SN et al. Crebbp haploinsufficiency in mice alters the bone marrow microenvironment, leading to loss of stem cells and excessive myelopoiesis. Blood 118, 69–79, doi: 10.1182/blood-2010-09-307942 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raaijmakers MH et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 464, 852–857, doi: 10.1038/nature08851 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kode A.et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature 506, 240–244, doi: 10.1038/nature12883 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zambetti NA et al. Mesenchymal Inflammation Drives Genotoxic Stress in Hematopoietic Stem Cells and Predicts Disease Evolution in Human Pre-leukemia. Cell stem cell 19, 613–627, doi: 10.1016/j.stem.2016.08.021 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Warren AJ Molecular basis of the human ribosomopathy Shwachman-Diamond syndrome. Adv Biol Regul 67, 109–127, doi: 10.1016/j.jbior.2017.09.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santamaria C.et al. Impaired expression of DICER, DROSHA, SBDS and some microRNAs in mesenchymal stromal cells from myelodysplastic syndrome patients. Haematologica 97, 1218–1224, doi: 10.3324/haematol.2011.054437 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong L.et al. Leukaemogenic effects of Ptpn11 activating mutations in the stem cell microenvironment. Nature 539, 304–308, doi: 10.1038/nature20131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arranz L.et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 512, 78–81, doi: 10.1038/nature13383 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Sacma M.et al. Haematopoietic stem cells in perisinusoidal niches are protected from ageing. Nat Cell Biol 21, 1309–1320, doi: 10.1038/s41556-019-0418-y (2019). [DOI] [PubMed] [Google Scholar]
- 79.Maryanovich M.et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med, doi: 10.1038/s41591-018-0030-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deininger MWN, Tyner JW & Solary E.Turning the tide in myelodysplastic/myeloproliferative neoplasms. Nat Rev Cancer 17, 425–440, doi: 10.1038/nrc.2017.40 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Genovese G.et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 371, 2477–2487, doi: 10.1056/NEJMoa1409405 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jaiswal S.et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 371, 2488–2498, doi: 10.1056/NEJMoa1408617 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xie M.et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med 20, 1472–1478, doi: 10.1038/nm.3733 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coombs CC et al. Therapy-Related Clonal Hematopoiesis in Patients with Non-hematologic Cancers Is Common and Associated with Adverse Clinical Outcomes. Cell stem cell 21, 374–382 e374, doi: 10.1016/j.stem.2017.07.010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kahn JD et al. PPM1D-truncating mutations confer resistance to chemotherapy and sensitivity to PPM1D inhibition in hematopoietic cells. Blood 132, 1095–1105, doi: 10.1182/blood-2018-05-850339 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wong TN et al. Cellular stressors contribute to the expansion of hematopoietic clones of varying leukemic potential. Nature communications 9, 455, doi: 10.1038/s41467-018-02858-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zink F.et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood 130, 742–752, doi: 10.1182/blood-2017-02-769869 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jaiswal S.et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med 377, 111–121, doi: 10.1056/NEJMoa1701719 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fuster JJ et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847, doi: 10.1126/science.aag1381 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sano S.et al. Tet2-Mediated Clonal Hematopoiesis Accelerates Heart Failure Through a Mechanism Involving the IL-1beta/NLRP3 Inflammasome. J Am Coll Cardiol 71, 875–886, doi: 10.1016/j.jacc.2017.12.037 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Steensma DP et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 126, 9–16, doi: 10.1182/blood-2015-03-631747 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abelson S.et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature 559, 400–404, doi: 10.1038/s41586-018-0317-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shlush LI et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 506, 328–333, doi: 10.1038/nature13038 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Watson CJ et al. The evolutionary dynamics and fitness landscape of clonal haematopoiesis. bioRxiv, 569566, doi: 10.1101/569566 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Yoshizato T.et al. Somatic Mutations and Clonal Hematopoiesis in Aplastic Anemia. N Engl J Med 373, 35–47, doi: 10.1056/NEJMoa1414799 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Young AL, Challen GA, Birmann BM & Druley TE Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nature communications 7, 12484, doi: 10.1038/ncomms12484 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walter MJ et al. Clonal architecture of secondary acute myeloid leukemia. N Engl J Med 366, 1090–1098, doi: 10.1056/NEJMoa1106968 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cooper JN & Young NS Clonality in context: hematopoietic clones in their marrow environment. Blood 130, 2363–2372, doi: 10.1182/blood-2017-07-794362 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schmitt MW et al. Detection of ultra-rare mutations by next-generation sequencing. Proc Natl Acad Sci U S A 109, 14508–14513, doi: 10.1073/pnas.1208715109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fuster JJ, Maclauchlan S, Zuriaga MA, Polackal MN & Walsh K.Clonal Hematopoiesis Associated with Tet2 Deficiency Accelerates Atherosclerosis in Hyperlipidemic Mice. Atherosclerosis 263, E14–E14, doi: 10.1016/j.atherosclerosis.2017.06.074 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Busque L.et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet 44, 1179–1181, doi: 10.1038/ng.2413 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McKerrell T.et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell Rep 10, 1239–1245, doi: 10.1016/j.celrep.2015.02.005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Flores-Figueroa E, Gutierrez-Espindola G, Montesinos JJ, Arana-Trejo RM & Mayani H.In vitro characterization of hematopoietic microenvironment cells from patients with myelodysplastic syndrome. Leukemia research 26, 677–686 (2002). [DOI] [PubMed] [Google Scholar]
- 104.Flores-Figueroa E.et al. Functional analysis of myelodysplastic syndromes-derived mesenchymal stem cells. Leukemia research 32, 1407–1416, doi: 10.1016/j.leukres.2008.02.013 (2008). [DOI] [PubMed] [Google Scholar]
- 105.Medyouf H.et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell stem cell 14, 824–837, doi: 10.1016/j.stem.2014.02.014 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Poon Z.et al. Bone marrow MSCs in MDS: contribution towards dysfunctional hematopoiesis and potential targets for disease response to hypomethylating therapy. Leukemia 33, 1487–1500, doi: 10.1038/s41375-018-0310-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muntion S.et al. Microvesicles from Mesenchymal Stromal Cells Are Involved in HPC-Microenvironment Crosstalk in Myelodysplastic Patients. PloS one 11, e0146722, doi: 10.1371/journal.pone.0146722 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rouault-Pierre K.et al. Preclinical modeling of myelodysplastic syndromes. Leukemia 31, 2702–2708, doi: 10.1038/leu.2017.172 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schepers K.et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell stem cell 13, 285–299, doi: 10.1016/j.stem.2013.06.009 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hawkins ED et al. T-cell acute leukaemia exhibits dynamic interactions with bone marrow microenvironments. Nature 538, 518–522, doi: 10.1038/nature19801 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kumar B.et al. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia 32, 575–587, doi: 10.1038/leu.2017.259 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huan J.et al. RNA trafficking by acute myelogenous leukemia exosomes. Cancer Res 73, 918–929, doi: 10.1158/0008-5472.CAN-12-2184 (2013). [DOI] [PubMed] [Google Scholar]
- 113.Paggetti J.et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood 126, 1106–1117, doi: 10.1182/blood-2014-12-618025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL & Boyiadzis M.Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica 96, 1302–1309, doi: 10.3324/haematol.2010.039743 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Doron B.et al. Transmissible ER stress reconfigures the AML bone marrow compartment. Leukemia 33, 918–930, doi: 10.1038/s41375-018-0254-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Welner RS et al. Treatment of chronic myelogenous leukemia by blocking cytokine alterations found in normal stem and progenitor cells. Cancer Cell 27, 671–681, doi: 10.1016/j.ccell.2015.04.004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kleppe M.et al. JAK-STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response. Cancer Discov 5, 316–331, doi: 10.1158/2159-8290.CD-14-0736 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mendez-Ferrer S, Garcia-Fernandez M.& de Castillejo CL Convert and conquer: the strategy of chronic myelogenous leukemic cells. Cancer Cell 27, 611–613, doi: 10.1016/j.ccell.2015.04.012 (2015). [DOI] [PubMed] [Google Scholar]
- 119.Reynaud D.et al. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell 20, 661–673, doi: 10.1016/j.ccr.2011.10.012 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cozzolino F.et al. Production of interleukin-1 by bone marrow myeloma cells. Blood 74, 380–387 (1989). [PubMed] [Google Scholar]
- 121.Gaillard JP et al. Increased and highly stable levels of functional soluble interleukin-6 receptor in sera of patients with monoclonal gammopathy. European journal of immunology 23, 820–824, doi: 10.1002/eji.1830230408 (1993). [DOI] [PubMed] [Google Scholar]
- 122.Hallek M, Bergsagel PL & Anderson KC Multiple myeloma: increasing evidence for a multistep transformation process. Blood 91, 3–21 (1998). [PMC free article] [PubMed] [Google Scholar]
- 123.Mouhieddine TH, Weeks LD & Ghobrial IM Monoclonal gammopathy of undetermined significance. Blood 133, 2484–2494, doi: 10.1182/blood.2019846782 (2019). [DOI] [PubMed] [Google Scholar]
- 124.Pietras EM et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol 18, 607–618, doi: 10.1038/ncb3346 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Drexler B.et al. The sympathomimetic agonist mirabegron did not lower JAK2-V617F allele burden, but restored nestin-positive cells and reduced reticulin fibrosis in patients with myeloproliferative neoplasms: results of phase II study SAKK 33/14. Haematologica 104, 710–716, doi: 10.3324/haematol.2018.200014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hanoun M.et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell stem cell 15, 365–375, doi: 10.1016/j.stem.2014.06.020 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang W.et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat Cell Biol 14, 276–286, doi: 10.1038/ncb2432 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Laperrousaz B.et al. Primitive CML cell expansion relies on abnormal levels of BMPs provided by the niche and on BMPRIb overexpression. Blood 122, 3767–3777, doi: 10.1182/blood-2013-05-501460 (2013). [DOI] [PubMed] [Google Scholar]
- 129.Oyajobi BO et al. Dual effects of macrophage inflammatory protein-1alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood 102, 311–319, doi: 10.1182/blood-2002-12-3905 (2003). [DOI] [PubMed] [Google Scholar]
- 130.Decker M.et al. Leptin-receptor-expressing bone marrow stromal cells are myofibroblasts in primary myelofibrosis. Nat Cell Biol 19, 677–688, doi: 10.1038/ncb3530 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schneider RK et al. Gli1(+) Mesenchymal Stromal Cells Are a Key Driver of Bone Marrow Fibrosis and an Important Cellular Therapeutic Target. Cell stem cell 20, 785–800 e788, doi: 10.1016/j.stem.2017.03.008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Verstovsek S.et al. Role of neoplastic monocyte-derived fibrocytes in primary myelofibrosis. J Exp Med 213, 1723–1740, doi: 10.1084/jem.20160283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maekawa T.et al. Increased SLAMF7(high) monocytes in myelofibrosis patients harboring JAK2V617F provide a therapeutic target of elotuzumab. Blood 134, 814–825, doi: 10.1182/blood.2019000051 (2019). [DOI] [PubMed] [Google Scholar]
- 134.Sezer O, Heider U, Zavrski I, Kuhne CA & Hofbauer LC RANK ligand and osteoprotegerin in myeloma bone disease. Blood 101, 2094–2098, doi: 10.1182/blood-2002-09-2684 (2003). [DOI] [PubMed] [Google Scholar]
- 135.Bock O.et al. Osteosclerosis in advanced chronic idiopathic myelofibrosis is associated with endothelial overexpression of osteoprotegerin. Br J Haematol 130, 76–82, doi: 10.1111/j.1365-2141.2005.05573.x (2005). [DOI] [PubMed] [Google Scholar]
- 136.Dias S.et al. Inhibition of both paracrine and autocrine VEGF/ VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc Natl Acad Sci U S A 98, 10857–10862, doi: 10.1073/pnas.191117498 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schmidt T.et al. Loss or inhibition of stromal-derived PlGF prolongs survival of mice with imatinib-resistant Bcr-Abl1(+) leukemia. Cancer Cell 19, 740–753, doi: 10.1016/j.ccr.2011.05.007 (2011). [DOI] [PubMed] [Google Scholar]
- 138.Fiedler W.et al. Vascular endothelial growth factor, a possible paracrine growth factor in human acute myeloid leukemia. Blood 89, 1870–1875 (1997). [PubMed] [Google Scholar]
- 139.Dias S.et al. Autocrine stimulation of VEGFR-2 activates human leukemic cell growth and migration. J Clin Invest 106, 511–521, doi: 10.1172/JCI8978 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dias S, Choy M, Alitalo K.& Rafii S.Vascular endothelial growth factor (VEGF)-C signaling through FLT-4 (VEGFR-3) mediates leukemic cell proliferation, survival, and resistance to chemotherapy. Blood 99, 2179–2184 (2002). [DOI] [PubMed] [Google Scholar]
- 141.Bellamy WT, Richter L, Frutiger Y.& Grogan TM Expression of vascular endothelial growth factor and its receptors in hematopoietic malignancies. Cancer Res 59, 728–733 (1999). [PubMed] [Google Scholar]
- 142.Griffin JD Growth factors required for proliferation of clonogenic cells in acute myeloblastic leukemia (AML). Haematologica 72, 85–88 (1987). [PubMed] [Google Scholar]
- 143.Poulos MG et al. Activation of the vascular niche supports leukemic progression and resistance to chemotherapy. Exp Hematol 42, 976–986 e971–973, doi: 10.1016/j.exphem.2014.08.003 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hussong JW, Rodgers GM & Shami PJ Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood 95, 309–313 (2000). [PubMed] [Google Scholar]
- 145.Aguayo A.et al. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood 96, 2240–2245 (2000). [PubMed] [Google Scholar]
- 146.Lundberg LG et al. Bone marrow in polycythemia vera, chronic myelocytic leukemia, and myelofibrosis has an increased vascularity. Am J Pathol 157, 15–19, doi: 10.1016/S0002-9440(10)64511-7 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Medinger M.et al. Angiogenesis and vascular endothelial growth factor-/receptor expression in myeloproliferative neoplasms: correlation with clinical parameters and JAK2-V617F mutational status. Br J Haematol 146, 150–157, doi: 10.1111/j.1365-2141.2009.07726.x (2009). [DOI] [PubMed] [Google Scholar]
- 148.Pruneri G.et al. Angiogenesis in myelodysplastic syndromes. British journal of cancer 81, 1398–1401, doi: 10.1038/sj.bjc.6693515 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Passaro D.et al. Increased Vascular Permeability in the Bone Marrow Microenvironment Contributes to Disease Progression and Drug Response in Acute Myeloid Leukemia. Cancer Cell 32, 324–341 e326, doi: 10.1016/j.ccell.2017.08.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Duarte D.et al. Inhibition of Endosteal Vascular Niche Remodeling Rescues Hematopoietic Stem Cell Loss in AML. Cell stem cell 22, 64–77 e66, doi: 10.1016/j.stem.2017.11.006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Velasco-Hernandez T, Hyrenius-Wittsten A, Rehn M, Bryder D.& Cammenga J.HIF-1alpha can act as a tumor suppressor gene in murine acute myeloid leukemia. Blood 124, 3597–3607, doi: 10.1182/blood-2014-04-567065 (2014). [DOI] [PubMed] [Google Scholar]
- 152.Vukovic M.et al. Hif-1alpha and Hif-2alpha synergize to suppress AML development but are dispensable for disease maintenance. J Exp Med 212, 2223–2234, doi: 10.1084/jem.20150452 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Y, Liu Y, Malek SN, Zheng P.& Liu Y.Targeting HIF1alpha eliminates cancer stem cells in hematological malignancies. Cell stem cell 8, 399–411, doi: 10.1016/j.stem.2011.02.006 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang H, Li H, Xi HS & Li S.HIF1alpha is required for survival maintenance of chronic myeloid leukemia stem cells. Blood 119, 2595–2607, doi: 10.1182/blood-2011-10-387381 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rouault-Pierre K.et al. HIF-2alpha protects human hematopoietic stem/progenitors and acute myeloid leukemic cells from apoptosis induced by endoplasmic reticulum stress. Cell stem cell 13, 549–563, doi: 10.1016/j.stem.2013.08.011 (2013). [DOI] [PubMed] [Google Scholar]
- 156.Lutzny G.et al. Protein kinase c-beta-dependent activation of NF-kappaB in stromal cells is indispensable for the survival of chronic lymphocytic leukemia B cells in vivo. Cancer Cell 23, 77–92, doi: 10.1016/j.ccr.2012.12.003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.El-Gamal D.et al. PKC-beta as a therapeutic target in CLL: PKC inhibitor AEB071 demonstrates preclinical activity in CLL. Blood 124, 1481–1491, doi: 10.1182/blood-2014-05-574830 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Saba NS & Levy LS Apoptotic induction in B-cell acute lymphoblastic leukemia cell lines treated with a protein kinase Cbeta inhibitor. Leukemia & lymphoma 52, 877–886, doi: 10.3109/10428194.2011.552136 (2011). [DOI] [PubMed] [Google Scholar]
- 159.Morschhauser F.et al. Results of a phase I/II study of ocrelizumab, a fully humanized anti-CD20 mAb, in patients with relapsed/refractory follicular lymphoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 21, 1870–1876, doi: 10.1093/annonc/mdq027 (2010). [DOI] [PubMed] [Google Scholar]
- 160.Park E.et al. Stromal cell protein kinase C-beta inhibition enhances chemosensitivity in B cell malignancies and overcomes drug resistance. Science translational medicine 12, doi: 10.1126/scitranslmed.aax9340 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ben-Batalla I.et al. Axl, a prognostic and therapeutic target in acute myeloid leukemia mediates paracrine crosstalk of leukemia cells with bone marrow stroma. Blood 122, 2443–2452, doi: 10.1182/blood-2013-03-491431 (2013). [DOI] [PubMed] [Google Scholar]
- 162.Waizenegger JS et al. Role of Growth arrest-specific gene 6-Mer axis in multiple myeloma. Leukemia 29, 696–704, doi: 10.1038/leu.2014.236 (2015). [DOI] [PubMed] [Google Scholar]
- 163.Lane SW et al. Differential niche and Wnt requirements during acute myeloid leukemia progression. Blood 118, 2849–2856, doi: 10.1182/blood-2011-03-345165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gregoire M.et al. Neutrophils trigger a NF-kappaB dependent polarization of tumor-supportive stromal cells in germinal center B-cell lymphomas. Oncotarget 6, 16471–16487, doi: 10.18632/oncotarget.4106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brinkmann V.et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535, doi: 10.1126/science.1092385 (2004). [DOI] [PubMed] [Google Scholar]
- 166.Sangaletti S.et al. Defective stromal remodeling and neutrophil extracellular traps in lymphoid tissues favor the transition from autoimmunity to lymphoma. Cancer Discov 4, 110–129, doi: 10.1158/2159-8290.CD-13-0276 (2014). [DOI] [PubMed] [Google Scholar]
- 167.Boutter J.et al. Image-based RNA interference screening reveals an individual dependence of acute lymphoblastic leukemia on stromal cysteine support. Oncotarget 5, 11501–11512, doi: 10.18632/oncotarget.2572 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang W.et al. Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer. Cell 165, 1092–1105, doi: 10.1016/j.cell.2016.04.009 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu J.et al. Stromal cell-mediated mitochondrial redox adaptation regulates drug resistance in childhood acute lymphoblastic leukemia. Oncotarget 6, 43048–43064, doi: 10.18632/oncotarget.5528 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Farge T.et al. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discov 7, 716–735, doi: 10.1158/2159-8290.CD-16-0441 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.German NJ et al. PHD3 Loss in Cancer Enables Metabolic Reliance on Fatty Acid Oxidation via Deactivation of ACC2. Molecular cell 63, 1006–1020, doi: 10.1016/j.molcel.2016.08.014 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shafat MS et al. Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood 129, 1320–1332, doi: 10.1182/blood-2016-08-734798 (2017). [DOI] [PubMed] [Google Scholar]
- 173.Ye H.et al. Leukemic Stem Cells Evade Chemotherapy by Metabolic Adaptation to an Adipose Tissue Niche. Cell stem cell 19, 23–37, doi: 10.1016/j.stem.2016.06.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Polak R, de Rooij B, Pieters R.& den Boer ML B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood 126, 2404–2414, doi: 10.1182/blood-2015-03-634238 (2015). [DOI] [PubMed] [Google Scholar]
- 175.Marlein CR et al. NADPH oxidase-2 derived superoxide drives mitochondrial transfer from bone marrow stromal cells to leukemic blasts. Blood 130, 1649–1660, doi: 10.1182/blood-2017-03-772939 (2017). [DOI] [PubMed] [Google Scholar]
- 176.de Rooij B, Polak R, Stalpers F, Pieters R.& den Boer ML Tunneling nanotubes facilitate autophagosome transfer in the leukemic niche. Leukemia 31, 1651–1654, doi: 10.1038/leu.2017.117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Moschoi R.et al. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood 128, 253–264, doi: 10.1182/blood-2015-07-655860 (2016). [DOI] [PubMed] [Google Scholar]
- 178.Marlein CR et al. CD38-Driven Mitochondrial Trafficking Promotes Bioenergetic Plasticity in Multiple Myeloma. Cancer Res 79, 2285–2297, doi: 10.1158/0008-5472.CAN-18-0773 (2019). [DOI] [PubMed] [Google Scholar]
- 179.Poggi A.& Giuliani M.Mesenchymal Stromal Cells Can Regulate the Immune Response in the Tumor Microenvironment. Vaccines (Basel) 4, doi: 10.3390/vaccines4040041 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kalluri R.The biology and function of fibroblasts in cancer. Nat Rev Cancer 16, 582–598, doi: 10.1038/nrc.2016.73 (2016). [DOI] [PubMed] [Google Scholar]
- 181.Turley SJ, Cremasco V.& Astarita JL Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol 15, 669–682, doi: 10.1038/nri3902 (2015). [DOI] [PubMed] [Google Scholar]
- 182.Zocchi MR et al. High ERp5/ADAM10 expression in lymph node microenvironment and impaired NKG2D ligands recognition in Hodgkin lymphomas. Blood 119, 1479–1489, doi: 10.1182/blood-2011-07-370841 (2012). [DOI] [PubMed] [Google Scholar]
- 183.Curti A.et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cells. Blood 109, 2871–2877, doi: 10.1182/blood-2006-07-036863 (2007). [DOI] [PubMed] [Google Scholar]
- 184.Zafeiris D.et al. Discovery and application of immune biomarkers for hematological malignancies. Expert review of molecular diagnostics 17, 983–1000, doi: 10.1080/14737159.2017.1381560 (2017). [DOI] [PubMed] [Google Scholar]
- 185.Tonks A.et al. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia 21, 566–568, doi: 10.1038/sj.leu.2404559 (2007). [DOI] [PubMed] [Google Scholar]
- 186.Folgiero V.et al. Indoleamine 2,3-dioxygenase 1 (IDO1) activity in leukemia blasts correlates with poor outcome in childhood acute myeloid leukemia. Oncotarget 5, 2052–2064, doi: 10.18632/oncotarget.1504 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sloand EM, Wu CO, Greenberg P, Young N.& Barrett J.Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 26, 2505–2511, doi: 10.1200/JCO.2007.11.9214 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Orskov AD et al. Hypomethylation and up-regulation of PD-1 in T cells by azacytidine in MDS/AML patients: A rationale for combined targeting of PD-1 and DNA methylation. Oncotarget 6, 9612–9626, doi: 10.18632/oncotarget.3324 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ramsay AG et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood 114, 4713–4720, doi: 10.1182/blood-2009-04-217687 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sindel A, Al-Juhaishi T.& Yazbeck V.Marginal Zone Lymphoma: State-of-the-Art Treatment. Curr Treat Options Oncol 20, 90, doi: 10.1007/s11864-019-0687-5 (2019). [DOI] [PubMed] [Google Scholar]
- 191.Ramsay AG, Clear AJ, Fatah R.& Gribben JG Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood 120, 1412–1421, doi: 10.1182/blood-2012-02-411678 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.McClanahan F.et al. Mechanisms of PD-L1/PD-1-mediated CD8 T-cell dysfunction in the context of aging-related immune defects in the Emicro-TCL1 CLL mouse model. Blood 126, 212–221, doi: 10.1182/blood-2015-02-626754 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.McClanahan F.et al. PD-L1 checkpoint blockade prevents immune dysfunction and leukemia development in a mouse model of chronic lymphocytic leukemia. Blood 126, 203–211, doi: 10.1182/blood-2015-01-622936 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sipkins DA et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435, 969–973 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ishikawa F.et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 25, 1315–1321, doi: 10.1038/nbt1350 (2007). [DOI] [PubMed] [Google Scholar]
- 196.Krause DS, Lazarides K, von Andrian UH & Van Etten RA Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med 12, 1175–1180, doi: 10.1038/nm1489 (2006). [DOI] [PubMed] [Google Scholar]
- 197.Azab AK et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood 113, 4341–4351, doi: 10.1182/blood-2008-10-186668 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F.& Dick JE Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med 12, 1167–1174, doi: 10.1038/nm1483 (2006). [DOI] [PubMed] [Google Scholar]
- 199.Lundell BI, McCarthy JB, Kovach NL & Verfaillie CM Activation of beta1 integrins on CML progenitors reveals cooperation between beta1 integrins and CD44 in the regulation of adhesion and proliferation. Leukemia 11, 822–829 (1997). [DOI] [PubMed] [Google Scholar]
- 200.Jacamo R.et al. Reciprocal leukemia-stroma VCAM-1/VLA-4-dependent activation of NF-kappaB mediates chemoresistance. Blood 123, 2691–2702, doi: 10.1182/blood-2013-06-511527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Maffei R.et al. Physical contact with endothelial cells through beta1- and beta2- integrins rescues chronic lymphocytic leukemia cells from spontaneous and drug-induced apoptosis and induces a peculiar gene expression profile in leukemic cells. Haematologica 97, 952–960, doi: 10.3324/haematol.2011.054924 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nervi B.et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood 113, 6206–6214, doi: 10.1182/blood-2008-06-162123 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Uy GL et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood 119, 3917–3924, doi: 10.1182/blood-2011-10-383406 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Abraham M.et al. The CXCR4 inhibitor BL-8040 induces the apoptosis of AML blasts by downregulating ERK, BCL-2, MCL-1 and cyclin-D1 via altered miR-15a/16–1 expression. Leukemia 31, 2336–2346, doi: 10.1038/leu.2017.82 (2017). [DOI] [PubMed] [Google Scholar]
- 205.Cho BS, Kim HJ & Konopleva M.Targeting the CXCL12/CXCR4 axis in acute myeloid leukemia: from bench to bedside. Korean J Intern Med 32, 248–257, doi: 10.3904/kjim.2016.244 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tavor S.et al. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res 64, 2817–2824 (2004). [DOI] [PubMed] [Google Scholar]
- 207.Godavarthy PS et al. The vascular bone marrow niche influences outcome in chronic myeloid leukemia via the E-selectin - SCL/TAL1 - CD44 axis. Haematologica, doi: 10.3324/haematol.2018.212365 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Krause DS, Lazarides K, Lewis JB, von Andrian UH & Van Etten RA Selectins and their ligands are required for homing and engraftment of BCR-ABL1+ leukemic stem cells in the bone marrow niche. Blood 123, 1361–1371, doi: 10.1182/blood-2013-11-538694 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Natoni A.et al. E-selectin ligands recognised by HECA452 induce drug resistance in myeloma, which is overcome by the E-selectin antagonist, GMI-1271. Leukemia 31, 2642–2651, doi: 10.1038/leu.2017.123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Boyd AL et al. Niche displacement of human leukemic stem cells uniquely allows their competitive replacement with healthy HSPCs. J Exp Med 211, 1925–1935, doi: 10.1084/jem.20140131 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Matsunaga T.et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med 9, 1158–1165, doi: 10.1038/nm909 (2003). [DOI] [PubMed] [Google Scholar]
- 212.Akinduro O.et al. Proliferation dynamics of acute myeloid leukaemia and haematopoietic progenitors competing for bone marrow space. Nature communications 9, 519, doi: 10.1038/s41467-017-02376-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Boyd AL et al. Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche. Nat Cell Biol 19, 1336–1347, doi: 10.1038/ncb3625 (2017). [DOI] [PubMed] [Google Scholar]
- 214.Shiozawa Y.et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest 121, 1298–1312, doi: 10.1172/JCI43414 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lapidot T.et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367, 645–648, doi: 10.1038/367645a0 (1994). [DOI] [PubMed] [Google Scholar]
- 216.Nagasawa T.et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382, 635–638 (1996). [DOI] [PubMed] [Google Scholar]
- 217.Colmone A.et al. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science 322, 1861–1865, doi: 10.1126/science.1164390 (2008). [DOI] [PubMed] [Google Scholar]
- 218.Oshima T.et al. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood 106, 3160–3165, doi: 10.1182/blood-2004-12-4940 (2005). [DOI] [PubMed] [Google Scholar]
- 219.Klein C.et al. Ptch2 loss drives myeloproliferation and myeloproliferative neoplasm progression. J Exp Med 213, 273–290, doi: 10.1084/jem.20150556 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Pitt LA et al. CXCL12-Producing Vascular Endothelial Niches Control Acute T Cell Leukemia Maintenance. Cancer Cell 27, 755–768, doi: 10.1016/j.ccell.2015.05.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Passaro D.et al. CXCR4 Is Required for Leukemia-Initiating Cell Activity in T Cell Acute Lymphoblastic Leukemia. Cancer Cell 27, 769–779, doi: 10.1016/j.ccell.2015.05.003 (2015). [DOI] [PubMed] [Google Scholar]
- 222.Duarte D.et al. Defining the in vivo characteristics of acute myeloid leukemia cells behavior by intravital imaging. Immunol Cell Biol 97, 229–235, doi: 10.1111/imcb.12216 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rak J.& Mendez-Ferrer S.T-ALL: several homes rather than homeless? Immunol Cell Biol 95, 1–2, doi: 10.1038/icb.2016.103 (2017). [DOI] [PubMed] [Google Scholar]
- 224.Krause DS et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat Med 19, 1513–1517, doi: 10.1038/nm.3364 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Saito Y.et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol 28, 275–280, doi: 10.1038/nbt.1607 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ghobrial IM et al. Phase I/II trial of the CXCR4 inhibitor plerixafor in combination with bortezomib as a chemosensitization strategy in relapsed/refractory multiple myeloma. American journal of hematology 94, 1244–1253, doi: 10.1002/ajh.25627 (2019). [DOI] [PubMed] [Google Scholar]
- 227.Abraham M.et al. Single Dose of the CXCR4 Antagonist BL-8040 Induces Rapid Mobilization for the Collection of Human CD34(+) Cells in Healthy Volunteers. Clinical cancer research : an official journal of the American Association for Cancer Research 23, 6790–6801, doi: 10.1158/1078-0432.CCR-16-2919 (2017). [DOI] [PubMed] [Google Scholar]
- 228.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02811822. (2016). [DOI] [PubMed]
- 229.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03616470. (2018). [DOI] [PubMed]
- 230.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01981850. (2013). [DOI] [PubMed]
- 231.Sánchez-Aguilera A.et al. Estrogen Signaling Selectively Induces Apoptosis of Hematopoietic Progenitors and Myeloid Neoplasms without Harming Steady-State Hematopoiesis. Cell stem cell 15, 1–14, doi: 10.1016/j.stem.2014.11.002 (2014). [DOI] [PubMed] [Google Scholar]
- 232.Nakada D.et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature 505, 555–558, doi: 10.1038/nature12932 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sanchez-Aguilera A.& Mendez-Ferrer S.Regulation of hematopoietic progenitors by estrogens as a basis for new antileukemic strategies. Mol Cell Oncol 3, e1009728, doi: 10.1080/23723556.2015.1009728 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.EU Clinical Trials Register, https://www.clinicaltrialsregister.eu/ctr-search/search?query=2015-005497–38. (2016).
- 235.US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT03572634. (2019). [DOI] [PubMed]
- 236.US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT02488408. (2014). [DOI] [PubMed]
- 237.Kondo K.et al. Ibrutinib modulates the immunosuppressive CLL microenvironment through STAT3-mediated suppression of regulatory B-cell function and inhibition of the PD-1/PD-L1 pathway. Leukemia 32, 960–970, doi: 10.1038/leu.2017.304 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Nimmagadda SC et al. SDF1alpha-induced chemotaxis of JAK2-V617F-positive cells is dependent on Bruton tyrosine kinase and its downstream targets PI3K/AKT, PLCgamma1 and RhoA. Haematologica 104, e288–e292, doi: 10.3324/haematol.2018.201921 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zaitseva L.et al. Ibrutinib inhibits SDF1/CXCR4 mediated migration in AML. Oncotarget 5, 9930–9938, doi: 10.18632/oncotarget.2479 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bam R.et al. Role of Bruton’s tyrosine kinase in myeloma cell migration and induction of bone disease. American journal of hematology 88, 463–471, doi: 10.1002/ajh.23433 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ponader S.et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 119, 1182–1189, doi: 10.1182/blood-2011-10-386417 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Javidi-Sharifi N.et al. FGF2-FGFR1 signaling regulates release of Leukemia-Protective exosomes from bone marrow stromal cells. eLife 8, doi: 10.7554/eLife.40033 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Baran N.& Konopleva M.Molecular Pathways: Hypoxia-Activated Prodrugs in Cancer Therapy. Clinical cancer research : an official journal of the American Association for Cancer Research 23, 2382–2390, doi: 10.1158/1078-0432.CCR-16-0895 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mendez-Ferrer S, Scadden DT & Sanchez-Aguilera A.Bone marrow stem cells: current and emerging concepts. Ann N Y Acad Sci 1335, 32–44, doi: 10.1111/nyas.12641 (2015). [DOI] [PubMed] [Google Scholar]
- 245.Isern J.et al. Self-renewing human bone marrow mesenspheres promote hematopoietic stem cell expansion. Cell Rep 3, 1714–1724, doi: 10.1016/j.celrep.2013.03.041 (2013). [DOI] [PubMed] [Google Scholar]
- 246.Ghazanfari R, Li H, Zacharaki D, Lim HC & Scheding S.Human Non-Hematopoietic CD271(pos)/CD140a(low/neg) Bone Marrow Stroma Cells Fulfill Stringent Stem Cell Criteria in Serial Transplantations. Stem Cells Dev 25, 1652–1658, doi: 10.1089/scd.2016.0169 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lo Celso C.et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 457, 92–96 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Abarrategi A.et al. Modeling the human bone marrow niche in mice: From host bone marrow engraftment to bioengineering approaches. J Exp Med 215, 729–743, doi: 10.1084/jem.20172139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Coutu DL, Kokkaliaris KD, Kunz L.& Schroeder T.Multicolor quantitative confocal imaging cytometry. Nat Methods 15, 39–46, doi: 10.1038/nmeth.4503 (2018). [DOI] [PubMed] [Google Scholar]