Abstract
Simple Summary
Pain associated with cancer diagnoses is a serious concern and one of the most common symptoms reported by cancer patients. The insufficient relief of cancer pain can have a major impact on patients’ quality of life. Recent developments in oncology such as new pain management guidelines, drugs and treatment strategies may have had a positive effect on the prevalence and severity of pain. Therefore, the aim of this systematic literature review was to assess the prevalence of pain and pain severity in cancer patients throughout all phases of treatment in the 2014–2021 literature period. Our results show a decline in both the prevalence and severity of cancer pain, compared to previous research. Nevertheless, with 44.5% of cancer patients still experiencing pain, the prevalence remains high, emphasizing the need for ongoing attention regarding the management of cancer pain.
Abstract
Experiencing pain and insufficient relief can be devastating and negatively affect a patient’s quality of life. Developments in oncology such as new treatments and adjusted pain management guidelines may have influenced the prevalence of cancer pain and severity in patients. This review aims to provide an overview of the prevalence and severity of pain in cancer patients in the 2014–2021 literature period. A systematic literature search was performed using the databases PubMed, Embase, CINAHL, and Cochrane. Titles and abstracts were screened, and full texts were evaluated and assessed on methodological quality. A meta-analysis was performed on the pooled prevalence and severity rates. A meta-regression analysis was used to explore differences between treatment groups. We identified 10,637 studies, of which 444 studies were included. The overall prevalence of pain was 44.5%. Moderate to severe pain was experienced by 30.6% of the patients, a lower proportion compared to previous research. Pain experienced by cancer survivors was significantly lower compared to most treatment groups. Our results imply that both the prevalence of pain and pain severity declined in the past decade. Increased attention to the assessment and management of pain might have fostered the decline in the prevalence and severity of pain.
Keywords: cancer pain, prevalence, systematic review, meta-analysis, meta-regression
1. Introduction
Each year, more than ten million people worldwide are diagnosed with cancer [1]. Pain associated with cancer diagnoses is a serious concern and one of the most common symptoms reported by cancer patients. Experiencing pain and insufficient relief can be devastating and negatively affect a patient’s performance status and emotional well-being, leading to increased anxiety, anger, feelings of depression and even cognitive dysfunction, reducing a patient’s quality of life [1,2,3,4,5,6].
Despite increased attention to the assessment and management of pain in cancer patients, previous research concluded that no major advances have been made in the management of cancer pain in 50 years [7,8]. A recent systematic literature review on the prevalence of pain during cancer treatment by Evenepoel et al. (2022) showed that pain during cancer treatment remains high during and up to three months after curative cancer treatment [9].
Literature indicates several reasons for the lack of improvement in the prevalence of cancer pain. Common professional barriers include a lack of knowledge and skills, clinicians’ reluctance to prescribe opioids, and poor pain assessment [10]. Patient-related barriers include patients’ reluctance to discuss pain with clinicians, patients’ reluctance to receive treatment for their pain, adherence to analgesic prescriptions, limited knowledge on the assessment of undertreatment, and cognitive and psychological factors of patients such as depression [11,12]. A systematic literature review by Makhlouf and colleagues (2020) found similar attitudinal barriers to effective cancer pain management among professionals and patients, including fear of medication addiction, tolerance of medication, and side effects of opioids [13].
Global trends may have influenced the prevalence of cancer pain. People worldwide are living longer, and ageing populations are associated with increased multimorbidity [14,15,16]. Developments in oncology, including new drugs and treatment strategies, may also have influenced the prevalence of cancer pain and pain severity [17,18,19,20]. Global opioid analgesics use increased between 2015 and 2019, but regional variations exist [21]. This was probably influenced by the response to the opioid epidemic [22,23]. Chen et al. (2022) performed a study on opioid use among cancer patients in the United States and showed a declining trend between 2013 and 2018 [24]. This could potentially lead to further undertreatment of cancer pain. On the other hand, the publication of new pain therapy guidelines may have improved the management of cancer pain [25,26,27].
Many new studies have been published on the prevalence of cancer pain since the 2016 systematic literature review of van den Beuken-van Everdingen et al. The review of Evenepoel et al. (2022) was limited to cancer patients receiving curative treatment, excluding other phases of the disease. Therefore, the aim of this systematic literature review was to assess the prevalence of cancer pain and pain severity in cancer patients throughout all phases of the disease.
2. Material and Methods
A systematic literature review was performed in accordance with the recommendations of the “Preferred Reporting Items for Systematic Reviews and Meta-analyses” (PRISMA) guidelines [28].
2.1. Search Strategy
A systematic electronic search of the literature published from January 2014 until December 2021 was performed on the 19th of January 2022 using the databases PubMed, Embase, CINAHL, and Cochrane. The search string was inspired by the search strings of van den Beuken-van Everdingen et al. (2016), Evenepoel et al. (2022) and Rietjens et al. (2019) [7,9,29]. Important search terms were “pain”, “symptom”, “prevalence”, and “cancer”. The search string was initially developed for PubMed and later adapted for the other databases: Embase, CINAHL, and Cochrane. More details of the search strategy can be found in Supplemental S1.
2.2. Study Selection
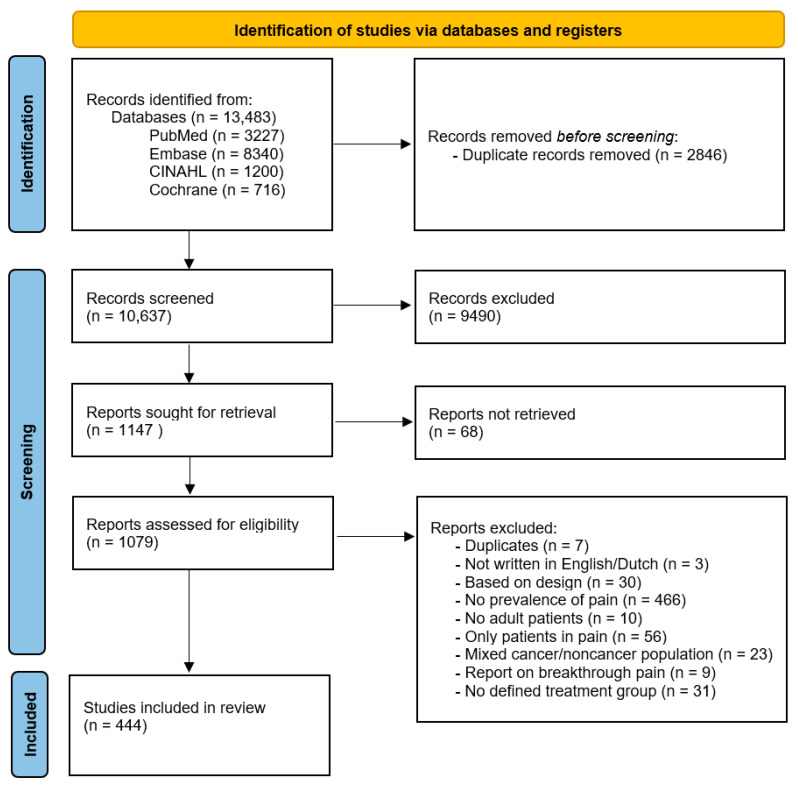
After identification and exclusion of duplicates, one author (R.A.H.S.) screened the titles and abstracts. Full texts of selected studies were examined by all four authors (R.A.H.S., L.B., M.T., and M.H.J.v.d.B.-v.E.) regarding the eligibility criteria. Studies that reported on the prevalence of cancer pain were eligible for inclusion. Inclusion of studies was based on the design, including original studies and secondary analyses of studies in which the patient group had not been included yet, population (studies that included patients ≥18 years, studies reporting on pain, and differentiating between cancer and noncancer patients), setting (including inpatient, outpatient, and palliative care facility (e.g., hospice, palliative care unit, and referred to a palliative care service)), while studies performed in pain clinics/including only patients with pain were excluded (Figure 1). In case of doubt about whether or not to include a study, the study in question was discussed among the authors until consensus was reached.
Figure 1.
PRISMA flow diagram of study selection.
Corresponding authors of studies that met the inclusion criteria were contacted by e-mail to obtain missing information on the prevalence of pain. If this information remained unclear after a term of two weeks, the respective percentages of pain were estimated based on study figures.
2.3. Data Extraction
A standard extraction form was developed for consistent data extraction across the authors. General characteristics were listed for each study and included the author(s), year of publication, continent of origin, study design, method of data collection, aim of the study, and the sample size. Study population characteristics included: ethnicity, gender, age, setting (inpatient, outpatient, patient in palliative care facility), Eastern Cooperative Oncology Group performance status (ECOG), and the type of cancer (head and neck, oesophagus, bronchus/lung, breast, pancreatic, other gastro-intestinal, prostate, other urological, ovary, uterus, other gynaecological, haematological, and other). Studies that included >3 types of cancer were registered as such.
Phases of the disease trajectory were registered and used to categorise studies. Nine groups were defined. Group 1—treatment-naïve cancer patients; Group 2—including cancer patients receiving curative treatment; Group 3—including cancer patients receiving palliative treatment; Group 4—including patients with either curative or palliative treatment, or treatment intent not specified; Group 5—including patients after curative cancer treatment; Group 6—including patients for whom anti-cancer treatment is no longer feasible or wanted; Group 7—including patients in different phases of treatment; Group 8—including patients on anti-cancer treatment (Group 2, Group 3 and Group 4); Group 9—including patients with advanced, metastatic or terminal disease (Group 3 and Group 6).
With regard to the experience of pain, the main percentage of pain (%) in a study was extracted. In cases where a study reported on different types of pain (e.g., headache, abdominal pain, and joint pain) and did not report an overall percentage of pain, the highest percentage was registered. In addition, pain severity was extracted and registered as prevalence (%) of mild, moderate, moderate–severe, and severe pain. In case the severity of pain was not specified in the study and presented with Visual Analogue Scale (VAS) or Numeric Rating Scale (NRS), the rating of Serlin et al. (1995) was applied: none (0), mild (1–4), moderate (5–6), and severe (≥7) [30]. Finally, the pain recall period was extracted (point prevalent, week(s), month(s), year(s)).
2.4. Methodological Quality
The quality of the included studies was assessed by all four authors (R.A.H.S., L.B., M.T., M.H.J.v.d.B.-v.E.) using the scoring criteria presented in Table 1. These criteria were also used in the systematic literature review of van den Beuken-van Everdingen et al. (2016) and based on LeBoeuf-Yde and Lauritsen (1995), including criteria developed to assess the methodological quality for prevalence studies [31]. The criteria relate to the representativeness of the study sample (three items); quality of the data (three items); description of the methods and results (three items); and a definition of the prevalence of pain (one item), resulting in a methodological quality score ranging from 0–20 points. Studies were included in this systematic literature review regardless of the methodological quality score.
Table 1.
Methodological quality criteria for prevalence studies.
| A. Representatives of population | 1. At least one of the following should apply for the study: an entire target population, randomly selected sample, or sample stated to represent the target population (two points) |
| 2. At least one of the following: reasons for nonresponse described, non-responders described, comparison of responders and non-responders, or comparison of sample and target population (two points) | |
| 3. Response rate ≥90% (two points) Response rate 70–90% (one point) Response rate ˂70% (zero points) |
|
| B. Quality of data | 4. Were the primary data from a prevalence study (two points) or were they taken from a survey not specifically designed for the purpose (one point)? |
| 5. The same mode of data collection should be used for all subjects (two points), if not (zero points). | |
| 6. The data have been collected directly from the patient by means of a validated questionnaire/interview (three points), no validated questionnaire/interview (two points), data have been collected from proxies or retrospectively from medical record (zero points) | |
| C. General description of method and results | 7. Description of the target population and setting where patients were found (two points) |
| 8. Description of stage of disease (one point) | |
| 9. Description of type of cancer, gender, and age: all (two points), 2 of 3 (one point), 1 of 3 (zero points) | |
| 10. Final sample size (one point) | |
| D. Definition of pain prevalence | 11. Prevalence recall periods should be stated (one point) |
2.5. Data Analyses
Descriptive statistics are presented for both studies with <15 points, and studies with ≥15 points on the methodological quality assessment. Details of the studies scoring <15 points on the methodological quality assessment can be found in Supplemental S2.
A meta-analysis was performed on the included studies using STATA (version 17.0, StataCorp Texas). The meta-analysis included studies with ≥15 points, a defined sample size, and pain prevalence or proportion of moderate to severe pain reported. Prevalence rates were pooled for each defined patient group with reference to pain, and pain severity (moderate–severe pain). The reciprocal of variance was chosen as a weighting factor to reflect the amount of information that each study contained as it is closely related to the study’s sample size [32]. The 95% Confidence Interval (CI) of each prevalence rate was calculated with the formula: p ± z. √((p(1-p)/n) with p being the pain prevalence rate (p = x/n), with a z-value of 1.96 for a 95% CI. To analyse if the variation in prevalence rates between studies was due to more than chance alone, the grade of heterogeneity was assessed by performing a chi-square test (I2). If the heterogeneity test was significant (p < 0.05), extra variation was incorporated into the analysis by use of the random-effects model. If the prevalence of pain was 100%, the percentage was set as 99.9% in the analysis to avert statistical issues.
A meta-regression analysis was performed to explore if the pooled pain prevalence rates differed significantly between the patient groups. In addition, meta-regression analyses were also used to explore the association between the prevalence of pain and the type of cancer, ECOG score, age of the population, ethnicity, continent of origin, recall period, setting and method of data collection. The coefficient in the meta-regression analysis indicates how each subgroup differs from the nominated reference group; a negative coefficient in each subgroup suggests a lower prevalence of pain compared to the reference. A number of cancer types were regrouped for the analyses; pancreatic and other gastro-intestinal were regrouped as gastro-intestinal; ovary, uterus and other gynaecological were regrouped as gynaecological cancers.
3. Results
3.1. Study Selection
The electronic literature search identified 13,483 studies; PubMed yielded 3227 studies, Embase 8340 studies, CINAHL 1200 studies, and Cochrane 716 studies. After the exclusion of duplicates, titles and abstracts of 10,637 studies were screened, of which 1147 were selected for a full text evaluation. The full text assessment resulted in the inclusion of 444 studies. An overview of the reasons for exclusion is presented in Figure 1. Authors (n = 6) were e-mailed to obtain missing prevalence data, of which pain prevalence was estimated in five studies because they did not respond.
3.2. Study Characteristics
This systematic literature review includes 444 studies, of which 160 studies had <15 points on the methodological quality assessment. Of the 160 studies that scored <15 points on the methodological quality assessment, most were performed in North America (n = 46), and the least in Australia/New Zealand (n = 6). In 24 studies, the primary objective was to evaluate the prevalence of pain in cancer patients. Most studies included solely gastro-intestinal cancer patients (n = 20) and 54 studies included patients of more than three types of cancer (Supplemental S2).
This systematic literature review included 284 studies with a quality score of ≥15 points (Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 and Table 8). Of the studies with a quality score of ≥15 points, most were performed in Europe (n = 97), and the least in Africa (n = 7). In 99 studies, the primary objective was to evaluate the prevalence of pain in cancer patients; 185 studies focused on another primary study objective (e.g., quality of life, predictors of depression, fatigue or sleeping problems). Most studies included solely breast cancer patients (n = 72), and 91 studies included patients of more than three types of cancer. Two hundred seventy-seven studies of the 284 with a quality score of ≥15 points were included in the meta-analysis.
Table 2.
Pain prevalence in treatment-naïve cancer patients (Group 1) (n = 20).
| % Pain | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Quality | Continent a | Setting b | Cancer c | Mean Age | Sample | None | Mild | Moderate | ModSev d | Severe | Overall |
| Alt-Epping, 2016 [33] | 16 | 2 | 2 | 60.6 | 22 | 22.7 | 18.2 | 45.5 | 59.1 | 13.6 | 77.3 | |
| Andersen, 2014 [34] | 16 | 2 | 2 | 5 | 133 | 60 | 7 | 37 | ||||
| Bibby, 2019 [35] | 15 | 2 | 2 | 11 | 64 | 229 | 43.7 | |||||
| Efficace, 2015 [36] | 16 | 1,2,3 | 2 | 10 | 70.02 | 280 | 45 | 43.2 | 11.8 | 55 | ||
| Esser, 2017 [37] | 15 | 2 | 2 | 10 | 50.4 | 239 | 15 | |||||
| Gjeilo, 2020 [38] | 18 | 2 | 1,2 | 4 | 65.8 | 264 | 60 | 40 | ||||
| Godby–a, 2021 [39] Godby–b, 2021 [39] |
18 18 |
1 1 |
2 2 |
6 6 |
70 70 |
46 309 |
75.6 40.7 |
|||||
| Hong, 2015 [40] | 15 | 3 | 1 | 6 | 62.18 | 165 | 62.4 | |||||
| Kirchheiner, 2015 [41] | 17 | 2 | 2 | 9 | 50 | 6.0 | ||||||
| Kuon, 2019 [42] | 17 | 2 | 2 | 4 | 63.6 | 208 | 34.6 | |||||
| Lunde, 2019 [43] | 18 | 2 | 2 | 9 | 67.2 | 207 | 16.9 | |||||
| Ravn Munkvold, 2018 [44] | 18 | 2 | 1 | 2 | 507 | 48 | 30 | 12 | 22 | 10 | 52 | |
| Roy, 2016 [45] | 16 | 3 | 2 | 4 | 56.4 | 36 | 47.2 | |||||
| Russo, 2018 [46] | 17 | 2 | 2 | 2 | 52.7 | 527 | 12.5 | |||||
| Salwey, 2020 [47] | 19 | 2 | 2 | 60 | 26.7 | 20 | 53.3 | 73.3 | ||||
| Tang, 2015 [48] | 18 | 6 | 1 | 11 | 55.1 | 91 | 46.8 | 21.1 | 63.2 | |||
| Thomas, 2017 [49] | 15 | 1 | 2 | 1 | 66 | 105 | 63 | |||||
| Wang, 2021 [50] | 16 | 3 | 2 | 5 | 50.99 | 88 | 4.5 | |||||
| Williams, 2021 [51] | 15 | 1 | 2 | 6 | 70.1 | 364 | 45.1 | |||||
| Yao, 2020 [52] | 15 | 1 | 2 | 7 | 83 | 69.2 | 23.1 | 5.8 | 7.7 | 1.9 | 30.8 | |
a 1 = North America; 2 = Europe; 3 = Asia; 4 = South America; 5 = Africa; 6 = Australia/New Zealand; b 1 = inpatient; 2 = outpatient; 3 = patient in a palliative care setting; 4 = all; 5 = other; c 1 = >3 types of cancer; 2 = head and neck; 3 = oesophagus, 4 = bronchus/lung; 5 = breast; 6 = gastro-intestinal; 7 = prostate; 8 = other, urological; 9 = gynaecological; 10 = haematological; 11 = other; d moderate–severe pain.
Table 3.
Pain prevalence in patients with curative treatment (Group 2) (n = 34).
| % Pain | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Quality | Continent a | Setting b | Cancer c | Mean Age | Sample | None | Mild | Moderate | ModSev d | Severe | Overall |
| Andersen, 2014 [34] | 16 | 2 | 2 | 5 | 133 | 20 | 26 | 78 | ||||
| Berliere–a, 2021 [53] Berliere–b, 2021 [53] |
16 16 |
2 2 |
1,2 1,2 |
5 5 |
53 | 32 31 |
46.0 29.0 |
|||||
| Bretschneider, 2016 [54] | 16 | 1 | 2 | 9 | 58.1 | 152 | 53.4 | 29.7 | 8.8 | 16.9 | 8.1 | 46.6 |
| Browall, 2017 [55] | 17 | 2 | 2 | 5 | 59 | 124 | 67 | |||||
| Calderon, 2019 [56] | 15 | 2 | 2 | 5 | 53.2 | 240 | 4.2 | |||||
| Choo, 2019 [57] | 17 | 3 | 2 | 5 | 60.35 | 192 | 59.9 | |||||
| de Menezes Couceiro, 2014 [58] | 16 | 4 | 2 | 5 | 54 | 250 | 44.4 | |||||
| Dylke, 2015 [59] | 15 | 6 | 2 | 5 | 55 | 157 | 83 | |||||
| El-Aqoul, 2018 [60] | 17 | 3 | 1 | 1 | 47.6 | 800 | 43.6 e | 56.4 | ||||
| Fenlon, 2014 [61] | 16 | 2 | 2 | 5 | 57 | 455 | 53 | |||||
| Fjell, 2020 [62] | 16 | 2 | 2 | 5 | 49 | 150 | 42.5 | |||||
| Hagiwara–a, 2018 [63] Hagiwara–b, 2018 [63] |
19 19 |
3 3 |
2 2 |
6 6 |
178 176 |
42.0 41.0 |
||||||
| Haryani, 2018 [64] | 16 | 3 | 2 | 1 | 50.51 | 207 | 38.2 | 17.9 | 22.7 | 4.8 | 60.87 | |
| Ho, 2015 [65] | 15 | 3 | 2 | 5 | 49 | 133 | 24 | |||||
| Hong, 2014 [66] | 16 | 3 | 1 | 1 | 51.24 | 1217 | 88 | 12 | ||||
| Jarden, 2021 [67] | 16 | 2 | 1 | 10 | 53.1 | 70 | 55.2 | |||||
| Jensen, 2018 [68] | 17 | 2 | 2 | 9 | 49 | 1176 | 60.6 | 26.8 | 9.1 | 12.6 | 3.5 | 39.4 |
| Khan–a, 2017 [69] Khan–b, 2017 [69] |
15 15 |
1 1 |
2 2 |
5 5 |
62 60.5 |
80 80 |
36 50 |
30 20 |
30 26 |
30 27 |
0 1 |
60 47 |
| Kim, 2016 [70] | 16 | 3 | 1 | 5 | 50.3 | 1499 | 8.8 e | 91.2 | ||||
| Kirchheiner, 2015 [41] | 17 | 2 | 2 | 9 | 50 | 42.0 | ||||||
| Kirkham, 2018 [71] | 15 | 1 | 1 | 5 | 50 | 24 | 54 | |||||
| Lewis, 2015 [72] | 16 | 6 | 2 | 1 | 61 | 276 | 55.0 | |||||
| Lewis, 2021 [73] | 18 | 3 | 2 | 2 | 600 | 45.2 | ||||||
| McFarland, 2018 [74] | 15 | 1 | 2 | 5 | 55.4 | 125 | 19.5 | |||||
| Moloney, 2016 [75] | 16 | 6 | 1 | 5 | 54.2 | 121 | 24 | 46.2 | ||||
| Nogueira de Oliveira Martins–a, 2017 [76] Nogueira de Oliveira Martins–b, 2017 [76] |
17 17 |
4 4 |
2 2 |
5 5 |
49.6 49.6 |
11 11 |
90.9 72.2 |
|||||
| Okamoto, 2018 [77] | 17 | 3 | 2 | 5 | 59 | 123 | 48.8 | 42.3 | 8.9 | 14 | 5.1 | 51.2 |
| Ribas, 2020 [78] | 15 | 2 | 2 | 9 | 65 | 109 | 20.1 | |||||
| Røhrl, 2016 [79] | 17 | 2 | 2 | 6 | 60.7 | 68 | 44.1 | |||||
| Shaulov, 2019 [80] | 16 | 1 | 1,2 | 10 | 49.1 | 318 | 51.3 | 8.3 | 22.9 | 40.4 | 17.5 | 49.2 |
| Tran, 2020 [81] | 16 | 3 | 1 | 6 | 57.7 | 197 | 87 | |||||
| Wang, 2021 [50] | 16 | 3 | 2 | 5 | 80 | 12.5 | ||||||
| Xu–a, 2020 [82] Xu–b, 2020 [82] Xu–c, 2020 [82] |
15 15 15 |
3 3 3 |
2 2 2 |
5 5 5 |
51 51 51 |
235 210 227 |
66.1 70.5 72.2 |
20.9 19.5 19.4 |
11.5 9.5 8.4 |
12.35 10 8.4 |
0.85 0.5 0 |
33.25 29.5 27.8 |
| Yi, 2018 [83] | 16 | 3 | 2 | 5 | 53.56 | 110 | 13.6 | 61.8 | 20 | 24.5 | 4.5 | 86.4 |
a 1 = North America; 2 = Europe; 3 = Asia; 4 = South America; 5 = Africa; 6 = Australia/New Zealand; b 1 = inpatient; 2 = outpatient; 3 = patient in a palliative care setting; 4 = all; 5 = other; c 1 = >3 types of cancer; 2 = head and neck; 3 = oesophagus, 4 = bronchus/lung; 5 = breast; 6 = gastro-intestinal; 7 = prostate; 8 = other, urological; 9 = gynaecological; 10 = haematological; 11 = other; d moderate–severe pain; e none–mild pain.
Table 4.
Pain prevalence in patients with palliative treatment (Group 3) (n = 22).
| % Pain | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Quality | Continent a | Setting b | Cancer c | Mean Age | Sample | None | Mild | Moderate | ModSev d | Severe | Overall |
| Agarwal, 2020 [84] | 15 | 3 | 3 | 1 | 46.8 | 110 | 4.5 | 24.5 | 38.2 | 70.9 | 32.7 | 95.5 |
| Al-Zahrani, 2014 [85] | 18 | 3 | 3 | 1 | 56 | 124 | 14.5 | 43.5 | 85.5 | |||
| Blank, 2018 [86] | 15 | 1 | 2 | 1 | 13 | 0 | 7.7 | 46.2 | 92.4 | 46.2 | 100 * | |
| Bouché, 2018 [87] | 17 | 2 | 2 | 6 | 151 | 22.5 | ||||||
| Bullock, 2017 [88] | 16 | 1 | 2 | 6 | 40 | 80 | ||||||
| Green, 2015 [89] | 17 | 1 | 2 | 7 | 396 | 6 | 8 | 2 | ||||
| Iwase, 2015 [90] | 16 | 3 | 2 | 1 | 63.5 | 183 | 10.5 | 25.4 | 27.6 | 64.1 | 36.5 | 89.5 |
| Jespersen, 2021 [91] | 16 | 2 | 2 | 10 | 92 | 13.0 | 23.9 | 56.5 | 63.0 | 6.5 | 86.9 | |
| King, 2018 [92] | 15 | 1,2,3,6 | 2 | 9 | 62.5 | 903 | 20.5 | 38.5 | 23.1 | 41 | 17.9 | 79.5 |
| Koldenhof–a, 2014 [93] Koldenhof–b, 2014 [93] |
16 16 |
2 2 |
2 2 |
6 6 |
62 66 |
26 16 |
23 17 |
|||||
| Lavdaniti, 2018 [94] | 17 | 2 | 1 | 1 | 63.8 | 123 | 41.5 | 0 | 17.1 | 58.5 | 41.4 | 58.5 |
| LeBlanc, 2015 [95] | 16 | 1 | 2 | 4 | 63 | 97 | 22.7 | 32 | 9.3 | |||
| Lechner, 2016 [96] | 16 | 1 | 1,2 | 1 | 62 | 25.8 | ||||||
| Madsen, 2017 [97] | 17 | 2 | 2 | 11 | 63 | 112 | 45 | |||||
| McFarland, 2017 [98] | 15 | 1 | 2 | 10 | 57.7 | 117 | 21.5 | |||||
| Mercadante–a, 2016 [99] Mercadante–b, 2016 [99] Mercadante–c, 2016 [99] Mercadante–d, 2016 [99] |
15 15 15 15 |
2 2 2 2 |
4 4 4 4 |
1 1 1 1 |
54.5 69.6 79.7 88.8 |
103 89 116 104 |
35.0 38.2 42.2 44.2 |
45.6 34.8 31.9 31.7 |
15.5 20.2 22.4 20.2 |
19.4 26.9 25.8 24.0 |
3.9 6.7 3.4 3.8 |
65.0 61.7 57.7 55.7 |
| Røhrl, 2016 [79] | 17 | 2 | 2 | 6 | 65.5 | 52 | 59.6 | |||||
| Sampogna–a, 2019 [100] Sampogna–b, 2019 [100] |
17 17 |
2 2 |
2 2 |
11 11 |
63.9 63.9 |
368 65 |
24 5 |
|||||
| Selvy, 2021 [101] | 17 | 2 | 2 | 10 | 66.7 | 67 | 59.7 | |||||
| Steel, 2016 [102] | 15 | 1 | 1 | 61 | 261 | 31.7 | ||||||
| Walling, 2015 [103] | 15 | 1 | 4,6 | 5422 | 15 | 48.2 | ||||||
| Zhou–a, 2017 [104] | 17 | 1 | 2 | 1 | 51.4 | 119 | 19.3 | |||||
| Zhou–b, 2017 [104] | 17 | 1 | 2 | 1 | 49.6 | 42 | 19.0 | |||||
| Zhou–c, 2017 [104] | 17 | 1 | 2 | 1 | 50.2 | 93 | 43.0 | |||||
| Zhou–d, 2017 [104] | 17 | 1 | 2 | 1 | 48.7 | 52 | 50.0 | |||||
a 1 = North America; 2 = Europe; 3 = Asia; 4 = South America; 5 = Africa; 6 = Australia/New Zealand; b 1 = inpatient; 2 = outpatient; 3 = patient in a palliative care setting; 4 = all; 5 = other; c 1 = >3 types of cancer; 2 = head and neck; 3 = oesophagus, 4 = bronchus/lung; 5 = breast; 6 = gastro-intestinal; 7 = prostate; 8 = other, urological; 9 = gynaecological; 10 = haematological; 11 = other; d moderate–severe pain; * 99.9% in meta-analysis.
Table 5.
Pain prevalence in patients with either curative or palliative treatment, or treatment intent not specified (Group 4) (n = 36).
| % Pain | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Quality | Continent a | Setting b | Cancer c | Mean Age | Sample | None | Mild | Moderate | ModSev d | Severe | Overall |
| Abu-Saad Huijer, 2015 [105] | 16 | 3 | 2 | 1 | 54.32 | 190 | 45.3 | |||||
| Bernardes, 2019 [106] | 15 | 6 | 1,2 | 1 | 125 | 81 | 10 | 10 | 20 | |||
| Chan, 2015 [107] | 16 | 3 | 11 | 57.3 | 79 | 25.3 | ||||||
| Chen, 2021 [108] | 15 | 3 | 2 | 10 | 41.7 | 132 | 57.6 | |||||
| Cheng, 2021 [109] | 15 | 3 | 2 | 5 | 127 | 21.3 | ||||||
| Damm, 2020 [110] | 16 | 2 | 1,2 | 6 | 139 | 43.9 | 17.9 | 18.6 | 0.7 | 63 | ||
| Fujii, 2017 [111] | 19 | 3 | 2 | 1 | 60.8 | 524 | 46.2 | 39.9 | 7.6 | 13.9 | 6.3 | 53.8 |
| Gosselin, 2016 [112] | 15 | 1 | 2 | 6 | 275 | 48 | 24 | 28 | 28 | 0 | 52 | |
| Han, 2019 [113] | 15 | 1 | 2 | 1 | 57.9 | 399 | 59.4 | |||||
| Joseph, 2021 [114] | 15 | 5 | 2 | 1 | 347 | 14.1 | 21.9 | 48.7 | 64 | 15.3 | 85.9 | |
| Kim, 2015 [115] | 15 | 3 | 2 | 1 | 60.02 | 167 | 25.7 | 92.8 | ||||
| Kuperus, 2021 [116] | 15 | 1 | 2 | 8 | 113 | 74 | 14.7 | 8.8 | 11.3 | 2.5 | 26 | |
| Lee–a, 2016 [117] Lee–b, 2016 [117] |
20 20 |
1 1 |
2 2 |
5 5 |
56.0 56.0 |
358 335 |
16 f 31 f |
|||||
| Li, 2017 [118] | 15 | 3 | 1 | 55.36 | 317 | 60.25 | 29.02 | 5.36 | 10.41 | 5.05 | 39.43 | |
| Llamas Ramos, 2016 [119] | 15 | 2 | 2 | 1 | 59.98 | 246 | 13.4 | 24.4 | 37 | 12.6 | 50.4 | |
| Matsumoto, 2020 [120] | 19 | 3 | 2 | 6 | 45 | 55.6 | ||||||
| Molassiotis, 2019 [121] | 16 | 2,3 | 1,2 | 1 | 55.15 | 343 | 14.2 | |||||
| Moye, 2014 [122] | 16 | 1 | 2,3,6 | 64.66 | 170 | 21.6 | 32.4 | 10.8 | ||||
| Pearce–a, 2017 [123] Pearce–b, 2017 [123] Pearce–c, 2017 [123] |
15 15 15 |
6 6 6 |
2 2 2 |
5 6 4 |
243 142 56 |
74 77 77 |
||||||
| Pérez, 2015 [124] | 17 | 2 | 2 | 1 | 60.7 | 358 | 45.8 | 26.3 | 54.2 | |||
| Pettersson, 2014 [125] | 16 | 2 | 2 | 6 | 65 | 104 | 41 | |||||
| Raj, 2014 [126] | 19 | 2 | 2 | 1 | 60 | 305 | 48.2 | |||||
| Ritchie–a, 2014 [127] Ritchie–b, 2014 [127] Ritchie–c, 2014 [127] Ritchie–d, 2014 [127] |
16 16 16 16 |
6 6 6 6 |
2 2 2 2 |
1 1 1 1 |
62.1 67.3 72.4 78.8 |
78 94 76 82 |
66.7 50.0 59.2 53.7 |
|||||
| Salvetti, 2020 [128] | 15 | 4 | 1 | 55 | 107 | 42.1 | ||||||
| Schumacher–a, 2021 [129] Schumacher–b, 2021 [129] |
15 15 |
6 6 |
2 2 |
7 7 |
67.9 67.8 |
72 43 |
18.1 23.3 |
|||||
| Seven, 2016 [130] | 15 | 2 | 1 | 9 | 59 | 134 | 57.3 | 15.7 | 20.2 | 26.9 | 6.7 | 42.6 |
| Spoelstra, 2015 [131] | 16 | 1 | 2 | 1 | 65.1 | 30 | 63.3 | |||||
| Stamm, 2021 [132] | 15 | 2 | 2 | 10 | 61 | 47 | 69 | |||||
| Steffen McLouth, 2020 [133] | 16 | 1 | 4 | 62.5 | 60 | 41.7 | ||||||
| Thiagarajan, 2016 [134] | 16 | 3 | 1,2 | 1 | 52.7 | 303 | 15.6 | 13.8 | 19.4 | 23.8 | 4.4 | 37.6 |
| Turner, 2014 [135] | 15 | 6 | 2 | 1 | 76.7 | 385 | 26 | |||||
| Unseld, 2021 [136] | 15 | 2 | 2 | 1 | 57.4 | 846 | 36.5 | 43.5 | 13.6 | 20 | 6.4 | 63.5 |
| van der Baan, 2020 [137] | 15 | 2 | 1,2 | 1 | 58.2 | 1919 | 24 | |||||
| Wang, 2014 [138] | 16 | 3 | 4 | 58.25 | 183 | 71 | ||||||
| Yahaya, 2015 [139] | 16 | 3 | 1 | 53.4 | 268 | 51.1 | ||||||
| Zhong, 2017 [140] | 17 | 3 | 1 | 1 | 59.7 | 517 | 70.2 e | 29.8 | ||||
a 1 = North America; 2 = Europe; 3 = Asia; 4 = South America; 5 = Africa; 6 = Australia/New Zealand; b 1 = inpatient; 2 = outpatient; 3 = patient in a palliative care setting; 4 = all; 5 = other; c 1 = >3 types of cancer; 2 = head and neck; 3 = oesophagus, 4 = bronchus/lung; 5 = breast; 6 = gastro-intestinal; 7 = prostate; 8 = other, urological; 9 = gynaecological; 10 = haematological; 11 = other; d moderate–severe pain; e none–mild pain; f mild–moderate pain.
Table 6.
Pain prevalence in patients after curative treatment (Group 5) (n = 88).
| % Pain | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Quality | Continent a | Setting b | Cancer c | Mean Age | Sample | None | Mild | Moderate | ModSev d | Severe | Overall |
| Adams, 2014 [141] | 17 | 2 | 2 | 1 | 70.6 | 418 | 15.5 | |||||
| Ahmed, 2014 [142] | 17 | 3 | 2 | 5 | 49.33 | 73 | 5.4 | |||||
| Al Maqbali, 2021 [143] | 15 | 3 | 2 | 5 | 133 | 45.1 | ||||||
| Andersen, 2015 [144] | 19 | 2 | 2 | 5 | 475 | 86 | 14 | |||||
| Asplund, 2015 [145] | 18 | 2 | 2 | 6 | 545 | 79 | 7 | 21 | ||||
| Baden, 2020 [146] | 18 | 2 | 2 | 7 | 3348 | 24 | ||||||
| Bao, 2018 [147] | 18 | 1 | 2 | 5 | 1280 | 33.3 | ||||||
| Bennedsgaard–a, 2020 [148] Bennedsgaard–b, 2020 [148] |
17 17 |
2 2 |
2 2 |
5 6 |
56.6 68.0 |
80 52 |
47.5 45.1 |
|||||
| Boehmer–a, 2021 [149] Boehmer–b, 2021 [149] |
17 17 |
1 1 |
2 2 |
6 6 |
116 302 |
6.1 6.6 |
||||||
| Bøhn, 2019 [150] | 16 | 2 | 2 | 1 | 49.1 | 1088 | 10 | |||||
| Bonhof, 2020 [151] | 17 | 2 | 2 | 6 | 477 | 9 | ||||||
| Bovbjerg, 2019 [152] | 15 | 1 | 2 | 5 | 59.4 | 417 | 50.6 | |||||
| Bulley, 2014 [153] | 15 | 2 | 2 | 5 | 62.3 | 595 | 28.8 | |||||
| Cameron, 2018 [154] | 16 | 1 | 2 | 1 | 26.9 | 124 | 54.5 | |||||
| Capelan, 2017 [155] | 15 | 2 | 2 | 5 | 214 | 29 | ||||||
| Chiang, 2019 [156] | 18 | 6 | 2 | 5 | 60.2 | 201 | 23 | 4 | 55 | |||
| Cramer, 2018 [2] | 18 | 1 | 2 | 2 | 175 | 54.9 | 22 | 11.6 | 23.1 | 11.5 | 45.1 | |
| Cui, 2018 [157] | 15 | 3 | 2 | 5 | 52.4 | 420 | 36.2 | |||||
| de Groef, 2017 [158] | 16 | 2 | 2 | 5 | 54.1 | 147 | 50 | |||||
| Dieterich–a, 2021 [159] Dieterich–b, 2021 [159] |
15 15 |
2 2 |
2 2 |
5 5 |
60.9 64.4 |
120 55 |
60.8 62.3 |
27.5 20.8 |
8.4 15 |
11.7 16.9 |
3.3 1.9 |
39.2 37.7 |
| Drury, 2017 [160] | 17 | 2 | 2 | 6 | 66.4 | 252 | 64 | 26 | 8 | 10 | 2 | 36 |
| Dualé, 2014 [161] | 18 | 2 | 2 | 5 | 442 | 37.1 | ||||||
| Efficace, 2019 [162] | 15 | 2 | 2 | 10 | 52.5 | 244 | 51 | 27 | 22 | 49 | ||
| Engvall, 2021 [163] | 16 | 2 | 2 | 5 | 60.7 | 646 | 46.3 | |||||
| Esser, 2017 [37] | 15 | 2 | 2 | 10 | 47.7 | 102 | 8 | |||||
| Ezendam, 2014 [164] | 17 | 2 | 2 | 9 | 65 | 180 | 31.7 | |||||
| Farrukh, 2020 [165] | 17 | 1 | 2 | 10 | 736 | 39.4 | ||||||
| Feddern, 2015 [166] | 19 | 2 | 2 | 6 | 1369 | 13 | 13 | 18 | 5 | 31 | ||
| Ferreira, 2019 [167] | 18 | 2 | 2 | 5 | 4262 | 51 | ||||||
| Gallaway, 2020 [168] | 17 | 1 | 2 | 1 | 12,019 | 9.5 | ||||||
| Gjeilo, 2020 [38] | 18 | 2 | 2 | 4 | 194 | 55 | ||||||
| Gong, 2020 [169] | 16 | 3 | 2 | 5 | 49.3 | 1983 | 28.2 | |||||
| Götze–a, 2018 [170] Götze–b, 2018 [170] |
16 16 |
2 2 |
2 2 |
1 1 |
66.3 67.3 |
660 342 |
51.2 50.8 |
|||||
| Hammer, 2014 [171] | 15 | 1 | 2 | 9 | 63.5 | 213 | 22.5 | |||||
| Hamood–a, 2016 [172] Hamood–b, 2016 [172] |
15 15 |
3 3 |
2 2 |
5 5 |
57 50 |
54 29 |
25.9 10.3 |
9.3 10.3 |
48.2 34.5 |
48.2 55.2 |
0 20.7 |
57.4 65.5 |
| Hamood, 2018 [173] | 17 | 3 | 2 | 5 | 410 | 64 | 74.4 | |||||
| Haviland, 2017 [174] | 17 | 2 | 2 | 5 | 55.1 | 864 | 32.3 | |||||
| Henderson–a, 2014 [175] Henderson–b, 2014 [175] |
16 16 |
2 2 |
2 2 |
5 5 |
63 49 |
138 134 |
73.2 61.9 |
17.4 26.1 |
9.4 10.4 |
9.4 11.9 |
0 1.5 |
26.8 38 |
| Henry, 2020 [176] | 16 | 1 | 2 | 63.3 | 145 | 16.6 | 15.9 | 21.4 | 5.5 | 37.9 | ||
| Hope-Stone, 2015 [177] | 15 | 2 | 2 | 11 | 66.7 | 179 | 77 | 23 | ||||
| Huang, 2020 [178] | 15 | 1 | 2 | 1 | 33.6 | 1208 | 18.7 | |||||
| Janah, 2020 [179] | 16 | 2 | 2 | 1 | 4093 | 36.5 | 63.5 | |||||
| Jansen, 2018 [180] | 15 | 2 | 2 | 2 | 70 | 283 | 8.8 | |||||
| Jardim, 2020 [181] | 16 | 4 | 2 | 5 | 55 | 151 | 18.5 | |||||
| Jariwala, 2021 [182] | 19 | 3 | 2 | 5 | 50 | 212 | 12 | |||||
| Johannsdottir, 2017 [183] | 15 | 2 | 2 | 11 | 124 | 56.1 | 33.3 | 10.6 | 10.6 | 0 | 43.9 | |
| Johannsen, 2015 [184] | 17 | 2 | 2 | 5 | 1905 | 72.7 | ||||||
| Juhl, 2016 [185] | 19 | 2 | 2 | 5 | 63.6 | 261 | 13 | 19.2 | 25.3 | 6.1 | 38.3 | |
| Karlson, 2020 [186] | 15 | 1 | 2 | 1 | 10,012 | 71.4 e | 20.5 | 28.6 | 8.1 | |||
| Kaur, 2018 [187] | 15 | 3 | 2 | 5 | 49.76 | 230 | 63.5 | |||||
| Kelada, 2019 [188] | 16 | 6 | 2 | 10,11 | 26.27 | 404 | 28.7 | |||||
| Kibar, 2017 [189] | 16 | 2 | 2 | 5 | 52.5 | 201 | 68.2 | 31.8 | ||||
| Kjaer, 2016 [190] | 15 | 2 | 2 | 2 | 64 | 369 | 16.0 | |||||
| Knowlton, 2020 [191] | 15 | 1 | 2 | 1 | 294 | 24.2 | ||||||
| Koehler, 2018 [192] | 19 | 1 | 2 | 5 | 56 | 36 | 44.0 | |||||
| Kramer, 2019 [193] | 15 | 5 | 2 | 5 | 60.05 | 349 | 26 | 46 | 14 | 28 | 14 | 74 |
| Lou, 2021 [194] | 16 | 1 | 2 | 2 | 59.3 | 77 | 40 f | |||||
| Lunde, 2019 [43] | 18 | 2 | 2 | 9 | 67.2 | 207 | 14.9 | |||||
| Lunde, 2020 [195] | 19 | 2 | 2 | 9 | 66.1 | 140 | 13.6 | |||||
| Madsen, 2017 [97] | 17 | 2 | 2 | 11 | 63 | 95 | 37 | |||||
| Mao, 2018 [196] | 15 | 1 | 2 | 5 | 1103 | 26.2 | ||||||
| Mertz, 2017 [197] | 19 | 2 | 2 | 5 | 473 | 67 | 21 | 12 | 33 | |||
| Miaskowski, 2014 [198] | 18 | 1 | 2 | 5 | 54.98 | 394 | 41.6 | 58.4 | ||||
| Min, 2021 [199] | 15 | 3 | 2 | 7 | 64 | 111 | 12.6 | |||||
| Mulrooney, 2019 [200] | 19 | 1 | 2 | 10 | 980 | 70 | 23 | 5 | 7 | 2 | 30 | |
| Paek, 2019 [201] | 18 | 3 | 2 | 1 | 62.2 | 1037 | 71.9 | 28.1 | 28.1 | |||
| Park, 2018 [202] | 17 | 3 | 2 | 11 | 36.43 | 144 | 75 | 25 | ||||
| Poço Conçalves, 2021 [203] | 17 | 2 | 2 | 1 | 65.33 | 85 | 77.6 | 0 | 20 | 22.4 | 2.4 | 22.4 |
| Ravn Munkvold, 2018 [44] | 18 | 2 | 2 | 2 | 357 | 70 | 19 | 7 | 11 | 4 | 30 | |
| Reilly, 2016 [204] | 17 | 1 | 2 | 10 | 47.6 | 31 | 68 | |||||
| Ren, 2021 [205] | 17 | 1 | 2 | 2 | 61.5 | 505 | 55 | 17.7 | 19.5 | 26.9 | 7.4 | 45 |
| Rogers, 2021 [206] | 15 | 1 | 2 | 11 | 47 | 248 | 49 | |||||
| Rosenberg, 2015 [207] | 15 | 1 | 2 | 5 | 55.8 | 2086 | 48 | |||||
| Sanchez-Birkhead, 2017 [208] | 15 | 1 | 2 | 5 | 52.6 | 35 | 38.3 | 79 | ||||
| Sanford, 2019 [209] | 15 | 1 | 2 | 1 | 7565 | 67.5 | 32.5 | |||||
| Schou Bredal, 2014 [210] | 16 | 2 | 2 | 5 | 56 | 834 | 21 | 17 | 20 | 3 | 41 | |
| Selvy, 2020 [211] | 16 | 2 | 2 | 6 | 66.3 | 406 | 11.5 | |||||
| Steyaert, 2016 [212] | 15 | 2 | 2 | 5 | 56.5 | 128 | 43.8 | |||||
| Tang, 2015 [48] | 18 | 6 | 1 | 11 | 55.1 | 76 | 53.9 | 19.7 | 46.1 | |||
| Terkawi, 2017 [213] | 16 | 3 | 2 | 2 | 49.6 | 102 | 30 | |||||
| Tonning Olsson, 2021 [214] | 16 | 1 | 2 | 1 | 32.2 | 2836 | 61.1 | 38.9 | 38.9 | |||
| Tung, 2019 [215] | 18 | 1 | 2 | 6 | 615 | 44.87 | ||||||
| van de Luijtgaarden, 2014 [216] | 17 | 2 | 2 | 11 | 45.7 | 24 | 93 | |||||
| van Eck, 2020 [217] | 15 | 2 | 2 | 11 | 60 | 752 | 14 | |||||
| Variawa, 2016 [218] | 18 | 5 | 2 | 5 | 58.54 | 92 | 38.04 | |||||
| Vuksanovic, 2021 [219] | 16 | 6 | 2 | 5 | 130 | 34.4 | ||||||
| Wang, 2021 [50] | 16 | 3 | 2 | 5 | 75 | 4.5 | ||||||
| Wilson, 2020 [220] | 17 | 1 | 1 | 11 | 136 | 16.3 | ||||||
a 1 = North America; 2 = Europe; 3 = Asia; 4 = South America; 5 = Africa; 6 = Australia/New Zealand; b 1 = inpatient; 2 = outpatient; 3 = patient in a palliative care setting; 4 = all; 5 = other; c 1 = >3 types of cancer; 2 = head and neck; 3 = oesophagus, 4 = bronchus/lung; 5 = breast; 6 = gastro-intestinal; 7 = prostate; 8 = other, urological; 9 = gynaecological; 10 = haematological; 11 = other; d moderate–severe pain; e none–mild pain; f mild–moderate pain.
Table 7.
Pain prevalence in patients without feasible anti-cancer treatment (Group 6) (n = 17).
| % Pain | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Quality | Continent a | Setting b | Cancer c | Mean Age | Sample | None | Mild | Moderate | ModSev d | Severe | Overall |
| Aktas, 2014 [221] | 17 | 1 | 4 | 1 | 1000 | 84 | ||||||
| Corli, 2020 [222] | 19 | 2 | 1,2,4 | 1 | 74.2 | 865 | 60.5 | |||||
| de la Cruz, 2015 [223] | 15 | 1 | 4 | 1 | 78 | 51 | ||||||
| Drat-Gzubicka, 2021 [224] | 18 | 2 | 3 | 76 | 29 | |||||||
| Gupta, 2016 [225] | 16 | 3 | 1 | 1 | 52.49 | 110 | 32.73 | 11.82 | 22.73 | 55.46 | 32.73 | 67.27 |
| Guthrie, 2019 [226] | 15 | 1 | 2 | 1 | 21,119 | 48.3 | ||||||
| Hui, 2020 [227] | 18 | 1 | 4 | 1 | 60 | 200 | 67 | |||||
| Mejin, 2019 [228] | 15 | 3 | 3 | 1 | 57,1 | 151 | 18.5 | 14.6 | 19.2 | 66.9 | 47.7 | 81.5 |
| Mercadante, 2018 [229] | 15 | 2 | 3 | 1 | 72.1 | 263 | 6.9 | |||||
| Morita–a, 2014 [230] Morita–b, 2014 [230] |
16 16 |
3 3 |
4 5 |
1 1 |
67 68 |
859 857 |
42 43 |
41 40 |
8.9 8.4 |
17.2 16 |
8.3 7.6 |
58 59 |
| Rojas-Concha, 2020 [231] | 17 | 2 | 3 | 1 | 5449 | 95.8 | 0.4 | 1.8 | 3.9 | 2.1 | 4.2 | |
| Seow–a, 2021 [232] Seow–b, 2021 [232] |
15 15 |
1 1 |
2 1 |
11,407 15,888 |
64.9 61.4 |
|||||||
| Silvia, 2021 [233] | 17 | 4 | 3 | 1 | 490 | 49.6 | 26.5 | 12.9 | 23.9 | 11 | 50.4 | |
| Tofthagen, 2019 [234] | 16 | 1 | 4 | 1 | 72.7 | 717 | 68.9 | |||||
| van der Baan, 2020 [137] | 15 | 2 | 1,2 | 1 | 63.4 | 224 | 45 | |||||
| Yamagishi, 2014 [235] | 16 | 3 | 1 | 1 | 67 | 859 | 84 | 16 | ||||
| Yanaizumi, 2021 [236] | 17 | 3 | 2 | 1 | 108 | 6.5 | 12.9 | 80.6 | 93.5 | |||
a 1 = North America; 2 = Europe; 3 = Asia; 4 = South America; 5 = Africa; 6 = Australia/New Zealand; b 1 = inpatient; 2 = outpatient; 3 = patient in a palliative care setting; 4 = all; 5 = other; c 1 = >3 types of cancer; 2 = head and neck; 3 = oesophagus, 4 = bronchus/lung; 5 = breast; 6 = gastro-intestinal; 7 = prostate; 8 = other, urological; 9 = gynaecological; 10 = haematological; 11 = other; d moderate–severe pain.
Table 8.
Pain prevalence including patients in different phases of treatment (Group 7) (n = 79).
| % Pain | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Quality | Continent a | Setting b | Cancer c | Mean Age | Sample | None | Mild | Moderate | ModSev d | Severe | Overall |
| Aguilar, 2017 [237] | 16 | 1 | 2 | 2 | 61.7 | 23 | 20.3 | |||||
| Alemayehu, 2018 [238] | 18 | 5 | 1,2 | 1 | 43.5 | 390 | 31 e | 69 | ||||
| Armstrong, 2016 [239] | 15 | 1 | 2 | 11 | 621 | 15.6 | ||||||
| Bacorro, 2017 [240] | 15 | 3 | 1,2 | 2 | 51.8 | 100 | 53 | 26 | 9 | 21 | 12 | 47 |
| Batalini, 2017 [241] | 16 | 4 | 2 | 1 | 59 | 277 | 40 | |||||
| Bauml, 2015 [242] | 16 | 1 | 2 | 5 | 60.5 | 203 | 78.7 e | 21.3 | ||||
| Beesley, 2016 [243] | 15 | 6 | 2 | 6 | 67 | 116 | 32 | |||||
| Bhattacharya, 2019 [244] | 16 | 2 | 2 | 5 | 957 | 10.5 | ||||||
| Bonhof, 2018 [245] | 16 | 2 | 2 | 9 | 64.8 | 98 | 37.5 | |||||
| Bouhassira, 2017 [246] | 18 | 2 | 2 | 1 | 61.4 | 509 | 28.2 | |||||
| Boyes, 2015 [247] | 17 | 6 | 2 | 10 | 311 | 12 | ||||||
| Braamse–a, 2014 [248] Braamse–b, 2014 [248] |
16 16 |
2 2 |
2 2 |
10 10 |
55.4 57.8 |
123 125 |
34.8 32.2 |
|||||
| Braga-Neto, 2018 [249] | 16 | 4 | 2 | 6 | 207 | 83 | ||||||
| Bubis, 2020 [250] | 15 | 1 | 2,4 | 1 | 22,650 | 33 | ||||||
| Buckley, 2018 [251] | 16 | 1 | 1,2 | 10 | 82 | 60 | ||||||
| Cho–a, 2019 [252] Cho–b, 2019 [252] |
16 16 |
3 3 |
2 1 |
53.7 58.02 |
708 2581 |
60.6 44 |
||||||
| Clover, 2017 [253] | 15 | 6 | 2 | 1 | 62 | 9133 | 17 | |||||
| Daly, 2020 [254] | 15 | 2 | 5 | 1 | 1027 | 25.3 | ||||||
| Davies, 2021 [255] | 17 | 2 | 3 | 1 | 250 | 6.0 | 11.6 | 24.5 | 12.9 | 30.5 | ||
| Davis, 2018 [256] | 15 | 1 | 2 | 5 | 59.7 | 23,840 | 35.5 | 13.2 | ||||
| de Groef, 2016 [257] | 18 | 2 | 2 | 5 | 60.5 | 100 | 50 | |||||
| de Mello, 2020 [258] | 16 | 4 | 1 | 6 | 348 | 55.61 | ||||||
| de Oliveira, 2014 [259] | 18 | 1 | 2 | 5 | 300 | 36.7 | ||||||
| Deshields, 2014 [260] | 16 | 1 | 2 | 1 | 60.6 | 513 | 44 | |||||
| Deshields, 2017 [261] | 16 | 1 | 2 | 1 | 60.63 | 558 | 45 | |||||
| Dhingra, 2015 [262] | 17 | 1 | 2 | 1 | 62.3 | 1436 | 44.9 | |||||
| Donovan, 2014 [263] | 18 | 1 | 2 | 9 | 56 | 104 | 31.7 | |||||
| Doubova–a, 2021 [264] Doubova–b, 2021 [264] |
19 19 |
4 4 |
2 2 |
5 9 |
247 165 |
75.3 e 67.3 e |
24.7 32.7 |
|||||
| Feiten, 2014 [265] | 16 | 2 | 2 | 5 | 730 | 34 | ||||||
| Fokdal, 2018 [266] | 18 | 1,2,3 | 2 | 9 | 914 | 13.4 | ||||||
| Giuliani, 2016 [267] | 15 | 1 | 2 | 4 | 89 | 21 | ||||||
| Götze, 2019 [268] | 16 | 2 | 2 | 10 | 75.6 | 200 | 34 | |||||
| Gough, 2017 [269] | 16 | 2 | 11 | 59 | 113 | 24 | 77 | |||||
| Guedes, 2018 [270] | 16 | 4 | 2 | 5 | 55.97 | 103 | 44.7 | 55.3 | ||||
| Honkanen, 2021 [271] | 16 | 2 | 2 | 5 | 56.9 | 121 | 35.5 | 14 | 49.5 | |||
| Horick, 2018 [272] | 18 | 1 | 2 | 1 | 309 | 45 | ||||||
| Huang, 2018 [273] | 17 | 3 | 1 | 6 | 59 | 300 | 39.7 | 25 | 25.3 | 35.5 | 10 | 60.3 |
| Hunnicutt–a, 2017 [274] Hunnicutt–b, 2017 [274] |
15 15 |
1 1 |
4 1 |
1 1 |
27,790 31,271 |
40.0 50.0 |
38.2 31.6 |
21.7 18.4 |
59.9 50.0 |
|||
| Jesdale–a, 2020 [275] Jesdale–b, 2020 [275] Jesdale–c, 2020 [275] |
15 15 15 |
1 1 1 |
2 2 2 |
329,150 42,317 12,290 |
77.8 74.6 75.4 |
|||||||
| Jewett, 2020 [276] | 17 | 1 | 2 | 9 | 59.9 | 355 | 25.1 | |||||
| Karawekpanyawong–a, 2021 [277] Karawekpanyawong–b, 2021 [277] |
16 16 |
3 3 |
2 2 |
9 9 |
52.7 55.8 |
27 173 |
23.51 46.3 |
|||||
| Khan, 2021 [278] | 15 | 3 | 2 | 5 | 97 | 75.3 | 24.7 | |||||
| Kim, 2015 [279] | 15 | 3 | 2 | 5 | 52.1 | 827 | 56.5 | 1.1 | 43.5 | |||
| Kim–a, 2017 [280] Kim–b, 2017 [280] |
15 15 |
3 3 |
2 2 |
2 2 |
53.2 43.6 |
77 81 |
37.7 27.0 |
|||||
| Lefkowits, 2014 [281] | 15 | 1 | 2 | 9 | 305 | 32 | 60.1 | |||||
| Liu, 2015 [282] | 16 | 1 | 5 | 5 | 51.7 | 97 | 53.6 | |||||
| Loh, 2018 [283] | 15 | 1 | 2 | 1 | 389 | 49.6 | ||||||
| Lu, 2017 [284] | 15 | 3 | 2 | 1 | 52.9 | 919 | 49.4 | |||||
| Mavros, 2020 [285] | 16 | 1 | 5 | 6 | 2168 | 39 | ||||||
| Mejdahl, 2015 [286] | 15 | 2 | 2 | 5 | 286 | 28 | ||||||
| Nakanotani, 2014 [287] | 15 | 3 | 2 | 1 | 57.6 | 807 | 17.6 | |||||
| Nappi, 2021 [288] | 15 | 2 | 2 | 5 | 58.4 | 175 | 65.9 | |||||
| Oosterling, 2016 [289] | 17 | 2 | 2 | 1 | 892 | 77 e | 23 | |||||
| Pandya, 2019 [290] | 16 | 1 | 1 | 1 | 81 | 359 | 56 | |||||
| Pinto, 2021 [291] | 15 | 5 | 1,3 | 1 | 46.1 | 294 | 33.3 | 62.6 | 29.3 | 83.7 | ||
| Ramsenthaler, 2016 [292] | 15 | 2 | 1,2 | 10 | 68.4 | 557 | 24 | 34 | 47.8 | 13.8 | 71.5 | |
| Rau, 2015 [293] | 19 | 3 | 2 | 1 | 57.47 | 2075 | 50.7 | |||||
| Reuter, 2019 [294] | 16 | 1 | 2 | 1 | 61.62 | 817 | 35.4 | |||||
| Rosario, 2019 [295] | 17 | 1 | 1 | 1 | 75 | 33 | 60 | |||||
| Saarelainen, 2014 [296] | 15 | 6 | 2 | 1 | 76.7 | 385 | 26 | |||||
| Sager, 2020 [297] | 18 | 1 | 2 | 1 | 65.31 | 123 | 26.8 | |||||
| Sakurai, 2019 [298] | 17 | 3 | 2,3 | 1 | 64.4 | 142 | 66.2 | |||||
| Sampogna, 2020 [299] | 16 | 2 | 2 | 11 | 44.3 | |||||||
| Sánchez Sánchez, 2020 [300] | 15 | 2 | 2 | 7 | 70.71 | 184 | 60.9 | 39.1 | 39.1 | |||
| Schreiber, 2021 [301] | 15 | 1 | 2 | 5 | 55.5 | 201 | 32 | |||||
| Shen, 2017 [302] | 16 | 3 | 2 | 1 | 57 | 2652 | 37.4 | 29.8 | 23.2 | 32.8 | 9.6 | 62.6 |
| Sloan–a, 2016 [303] Sloan–b, 2016 [303] |
16 16 |
1 1 |
2 2 |
4 4 |
66 66 |
296 1013 |
49.0 33.5 |
|||||
| Smith, 2019 [304] | 15 | 1 | 2 | 5,6 | 59.7 | 2257 | 61.2 | |||||
| Tadele, 2015 [305] | 18 | 5 | 2 | 1 | 388 | 77.6 | ||||||
| Tegegn, 2017 [306] | 15 | 5 | 1 | 1 | 83 | 8.4 | 25.3 | 57.8 | 66.2 | 8.4 | 91.6 | |
| Tjong, 2021 [307] | 17 | 1 | 2 | 4 | 13,289 | 62 | ||||||
| Tung, 2019 [308] | 18 | 1 | 2 | 6 | 2623 | 61.8 | ||||||
| Valery, 2017 [309] | 15 | 6 | 1 | 82 | 10 | |||||||
| van Arsdale, 2016 [310] | 16 | 1 | 2 | 9 | 59 | 165 | 18.8 | |||||
| van den Brekel, 2020 [311] | 15 | 2 | 1 | 1 | 60 | 2144 | 28 | |||||
| Vistad, 2018 [312] | 16 | 2 | 9 | 61.9 | 82 | 56 | ||||||
| von Gruenigen, 2018 [313] | 17 | 1 | 2 | 9 | 63.6 | 65 | 69 | |||||
| Williams, 2020 [314] | 17 | 1 | 1 | 6 | 70.1 | 336 | 20.9 | |||||
| Xu, 2017 [315] | 15 | 3 | 2 | 11 | 43.5 | 142 | 30 | |||||
a 1 = North America; 2 = Europe; 3 = Asia; 4 = South America; 5 = Africa; 6 = Australia/New Zealand; b 1 = inpatient; 2 = outpatient; 3 = patient in a palliative care setting; 4 = all; 5 = other; c 1 = >3 types of cancer; 2 = head and neck; 3 = oesophagus, 4 = bronchus/lung; 5 = breast; 6 = gastro-intestinal; 7 = prostate; 8 = other, urological; 9 = gynaecological; 10 = haematological; 11 = other; d moderate–severe pain; e none–mild pain.
3.3. Prevalence of Pain
The meta-analysis of the pooled pain prevalence rates including all groups (Group 1–Group 7) resulted in an overall pain prevalence of 44.5% (95% CI 41.1–47.9). The pooled pain prevalence rates from the group analyses are presented in Table 9. Forest plots of the meta-analysis on the pooled pain prevalence rates (effect size (ES)) can be found in Supplemental S3.
Table 9.
Pooled pain prevalence, and severity rates by treatment group.
| N Studies | N cancer Patients | Overall Pain | |
|---|---|---|---|
| Group 1 | 18 | 3294 | 40.3% (95% CI 30.2–50.4) |
| Group 2 | 31 | 7995 | 47.8% (95% CI 39.4–56.1) |
| Group 3 | 19 | 8719 | 54.3% (95% CI 42.0–66.6) |
| Group 4 | 32 | 9118 | 50.3% (95% CI 42.5–58.0) |
| Group 5 | 77 | 59,766 | 35.8% (95% CI 31.5–40.1) |
| Group 6 | 15 | 33,162 | 55.2% (95% CI 39.2–71.3) |
| Group 7 | 66 | 508,827 | 45.0% (95% CI 39.8–50.1) |
| Group 8 | 81 | 25,832 | 50.3% (95% CI 44.6–56.1) |
| Group 9 | 34 | 41,881 | 54.6% (95% CI 43.8–65.5) |
| Moderate–Severe Pain | |||
| Group 1 | 9 | 1895 | 33.1% (95% CI 22.0–44.2) |
| Group 2 | 12 | 5483 | 27.8% (95% CI 6.7–48.8) |
| Group 3 | 11 | 8057 | 39.1% (95% CI 30.0–48.1) |
| Group 4 | 16 | 4966 | 25.2% (95% CI 19.0–31.4) |
| Group 5 | 30 | 24,490 | 22.8% (95% CI 19.0–26.7) |
| Group 6 | 7 | 35,319 | 43.3% (95% CI 19.7–66.9) |
| Group 7 | 22 | 107,128 | 34.2% (95% CI 27.6–40.8) |
| Group 8 | 39 | 18,506 | 31.0% (95% CI 22.7–39.3) |
| Group 9 | 18 | 43,376 | 40.7% (95% CI 28.5–52.9) |
Group 1: treatment-naïve patients; Group 2: patients receiving curative treatment; Group 3: patients receiving palliative treatment; Group 4: patients receiving curative or palliative treatment, or treatment intent not specified; Group 5: after curative treatment; Group 6: patients without feasible anti-cancer treatment; Group 7: including patients in different phases of treatment; Group 8: patients on anti-cancer treatment; Group 9: patients with advanced, metastatic or terminal disease.
Patients in both the group after curative treatment (Group 5) and the treatment-naïve group (Group 1) experienced less pain compared with patients in the palliative treatment group and the group without feasible anti-cancer treatment, respectively Group 3, p = 0.000, and p = 0.031; and Group 6, p = 0.001, and p = 0.038. Furthermore, the prevalence of pain was significantly lower in the group after curative treatment (Group 5) compared with the group receiving curative treatment (Group 2, p = 0.005), the group receiving all kinds of treatment (Group 4, p = 0.001), and the group including patients in different phases of treatment (Group 7, p = 0.006).
3.4. Pain Severity
The meta-analysis of the pooled pain severity including all treatment groups (Group 1–Group 7) resulted in a prevalence of moderate to severe pain of 30.6% (95% CI 26.9–34.4). The results of the group analyses on the pooled moderate to severe pain prevalence rates are presented in Table 9.
Patients in both the palliative treatment group (Group 3) and the group without feasible anti-cancer treatment (Group 6) experienced more moderate to severe pain compared with patients included in the all-treatments group and the group after curative treatment, respectively; Group 4, p = 0.035 and, p = 0.023; and Group 5, p = 0.006, and p = 0.005. In addition, the prevalence of moderate to severe pain was significantly higher in the group including patients in different phases of treatment (Group 7) compared with the group including patients after curative treatment (Group 5, p = 0.030).
3.5. Determinants of Pain Prevalence
The results of the meta-regression analyses that included all treatment groups (Group 1–Group 7) are presented in Table 10. The prevalence of pain in patients with prostate cancer was significantly lower than in patients with haematological, lung, and breast cancer (p = 0.034, p = 0.039, and p = 0.036 respectively). Age, ethnicity, and performance status (ECOG) were not associated with the overall prevalence of pain (p > 0.05). Recall periods of a week(s) (p = 0.013) or a month(s) (p = 0.001) did result in lower pain rates compared with using point prevalence. Studies that used medical records for data collection showed significantly higher pain rates than studies that used a patient questionnaire (p = 0.007). The prevalence of pain in patients who stayed in a palliative care setting was significantly higher compared to the prevalence of pain in outpatients (p = 0.040). Studies from South America, Asia and Africa showed significantly higher pain rates compared to studies from Europe (p = 0.033, p = 0.016 and p = 0.000 respectively). Moreover, studies from Africa showed a significantly higher prevalence of pain compared to studies from all other continents, North America (p = 0.000), Asia (p = 0.000), South America (p = 0.014) and Australia/New Zealand (p = 0.000).
Table 10.
Meta-regression to explore determinants of pain prevalence.
| Determinant | β (95% CI) | p-Value |
|---|---|---|
| Age (years) | ||
| 18–49 | 0.45 (0.37 to 0.54) | ref. |
| 50–59 | 0.01 (–0.09 to 0.11) | 0.787 |
| 60–69 | –0.03 (–0.13 to 0.07) | 0.570 |
| 70+ | 0.01 (–0.12 to 0.15) | 0.843 |
| Type of cancer | ||
| Prostate | 0.25 (0.08 to 0.41) | ref. |
| Head and neck | 0.16 (–0.04 to 0.35) | 0.116 |
| Lung | 0.22 (0.01 to 0.43) | 0.039 |
| Breast | 0.19 (0.01 to 0.36) | 0.036 |
| Gastro-intestinal | 0.18 (–0.01 to 0.36) | 0.061 |
| Gynaecological | 0.11 (–0.08 to 0.30) | 0.253 |
| Haematological | 0.21 (0.02 to 0.40) | 0.034 |
| Ethnicity | ||
| Caucasian | 0.38 (0.18 to 0.59) | ref. |
| Black | 0.36 (–0.15 to 0.87) | 0.144 |
| Asian | 0.17 (–0.12 to 0.45) | 0.219 |
| Hispanic | 0.39 (–0.01 to 0.78) | 0.054 |
| Performance status | ||
| ECOG 2 | 0.68 (0.43 to 0.94) | ref. |
| ECOG 0 | –0.28 (–0.74 to 0.17) | 0.198 |
| ECOG 1 | –0.05 (–0.35 to 0.25) | 0.733 |
| Data collection method | ||
| Questionnaire patient | 0.43 (0.40 to 0.45) | ref. |
| Interview patient | 0.07 (–0.02 to 0.16) | 0.106 |
| Medical record | 0.19 (0.05 to 0.32) | 0.007 |
| Recall period | ||
| Point | 0.50 (0.45 to 0.55) | ref. |
| Week(s) | –0.08 (–0.14 to –0.02) | 0.013 |
| Month(s) | –0.18 (–0.28 to –0.07) | 0.001 |
| Setting | ||
| Outpatient | 0.42 (0.39 to 0.45) | ref. |
| Inpatient | 0.08 (–0.02 to 0.18) | 0.115 |
| Palliative care setting | 0.16 (0.01 to 0.32) | 0.040 |
| Continent of origin | ||
| Europe | 0.39 (0.36 to 0.43) | ref. |
| North America | 0.06 (–0.00 to 0.12) | 0.068 |
| South America | 0.15 (0.01 to 0.29) | 0.033 |
| Asia | 0.08 (0.01 to 0.14) | 0.016 |
| Africa | 0.43 (0.25 to 0.62) | 0.000 |
| Australia/New Zealand | 0.06 (–0.03 to 0.16) | 0.193 |
ECOG = Eastern Cooperative Oncology Group. Bold indicates significant. The item first mentioned is the reference.
4. Discussion
The current literature on the prevalence of pain and pain severity in patients with cancer shows that both the prevalence of pain and pain severity have declined over the past decade. Pooled pain prevalence rates resulted in an overall prevalence of 44.5%. The treatment group with the lowest prevalence of pain was the group after curative treatment (35.8%). Despite the decline found, the results of this systematic literature review show that the prevalence of pain remains high, especially in advanced, metastatic and terminal cancer patients (54.6%).
The meta-analysis of moderate to severe pain resulted in an overall prevalence of 30.6%. The treatment group with the lowest proportion of moderate to severe pain was the group after curative treatment (22.8%). Moderate to severe pain was most frequently reported in the group including patients without feasible anti-cancer treatment (43.3%).
Some findings deserve attention. First, a slight decrease in the prevalence of pain was found compared with the 2016 systematic literature review by van den Beuken-van Everdingen et al. for the group after curative treatment (35.8% < 39.3%), and for the group during anti-cancer treatment (50.3% < 55.0%) [7]. A more substantial decrease was found for the advanced, metastatic or terminal disease group (54.6% < 66.4%). Our systematic review resulted in a pooled prevalence of 47.8% during curative treatment, which is higher than the 40% found by Evenepoel et al. (2022) [9]. A possible explanation can be found in different inclusion criteria. In contrast to Evenepoel et al. (2022), we did include unsolid tumours and studies conducted in nursing homes. The proportion of cancer types may also have been influential, as Evenepoel et al. (2022) included 12 studies of which ten included breast cancer patients (83%). The proportion of studies that included breast cancer patients in our review was high, but lower compared to the review of Evenepoel et al. (2022).
Second, the prevalence of moderate to severe pain decreased (30.6% < 38.0%) compared with the rate presented in the study by van den Beuken-van Everdingen and colleagues (2016) [7]. In contrast to the 2016 systematic literature review, our meta-analysis did include treatment-naïve patients, and patients after finishing curative treatment. This affects the overall prevalence of moderate to severe pain as this is lower in patients after curative treatment [7].
Several factors may have played a role in the decrease in the prevalence of pain and pain severity in cancer patients. In the literature review of Kwon (2014), the suggestion is made that a multidirectional interdisciplinary approach might be the best way to improve cancer pain management [10]. Therefore, more, and better collaboration between healthcare professionals from different disciplines involved in the patients’ treatment may have led to better management of cancer pain. Shrestha et al. (2022) conducted a systematic literature review and meta-analysis on the effect of pharmacists’ involvement in cancer pain management. Their results showed that pharmacists significantly improved cancer patients’ clinical outcomes related to pain. The direct involvement of pharmacists, or in collaboration with a multidisciplinary oncology team, is highly beneficial for patients [316].
The observed decrease in cancer pain prevalence and pain severity may also be positively impacted by raised awareness and knowledge of healthcare professionals about cancer pain treatment and the publication of new pain therapy guidelines [26,27]. However, there is still room for improvement as the literature indicates that there is a difference in knowledge between healthcare professionals and among professionals from different countries [317,318]. Our results showed that the prevalence of cancer pain was significantly higher in South America, Asia and Africa than in Europe. Silbermann and colleagues have shown that the majority of patients with cancer in low-income countries are undertreated for their pain, partly due to a lack of appropriate education [318]. Furthermore, compared with nurses, physicians have more knowledge about pain management, while both lack knowledge regarding the side effects and pharmacology of opioids [317]. Moreover, a systematic literature review on cancer pain management showed that most oncology nurses have poor knowledge about cancer pain management [319].
Furthermore, in line with our results, previous research on the prevalence of undertreatment of pain in cancer patients indicate that undertreatment of pain decreased over time [320,321,322]. In 2008 and 2014, two systematic literature reviews were published in which the prevalence of undertreatment of pain in cancer patients was 43% and 32%, respectively [320,321]. In 2022, an updated review was published including studies published from 2014 to 2020 that showed a mean weighted prevalence score for undertreatment of 40.2% [322].
Developments in oncology, including new drugs and treatment strategies, may also have had a positive impact on the prevalence of cancer pain and pain severity. Targeted therapy and immunotherapy changed the paradigm of cancer treatment. Compared to conventional chemotherapy, these treatment strategies more subtly reduce and control tumour growth, achieving better survival outcomes and improving patients’ quality of life [323,324]. The literature indicates that patients treated with immunotherapy have less cancer pain and reduced pain severity. For example, the study of Zhou and colleagues showed reduced opioid use and cancer pain scores after adoptive immunotherapy with autologous T-cell infusions [325]. They recommend controlled clinical studies to clarify the relationship between immune cell-mediated immunotherapy and pain relief.
Strengths and Limitations
A strength of this systematic literature review is its scope, including cancer patients at all stages of the disease. This allowed us to examine differences in the prevalence of pain and pain severity between treatment groups.
However, this systematic literature review has some limitations that should be taken into account when interpreting the results. First, not every study used the criteria of Serlin et al. (1995) for defining mild, moderate or severe pain [30]. This has led to differences in pain severity as the cut-off score for mild and moderate pain differed between these studies. Furthermore, some studies did not describe the severity of pain, or gave an overall prevalence of pain but described multiple types of pain (e.g., headache, abdominal pain, and joint pain). In these cases, the highest percentage of pain was used. Almost no studies mentioned the type of pain (nociceptive, neuropathic, mixed). Another methodological consideration relates to the meta-regression analyses of the determinants of pain. To obtain a more robust statistical analysis with more data, we chose to perform the meta-regression analyses on all studies regardless of treatment group. If a cancer type is over-represented in a treatment group with respectively low or high pain prevalence, this affects the overall prevalence of pain in that cancer type.
Although our analyses did not allow us to draw conclusions about detailed subgroups, this review provides a valuable overview of cancer pain prevalence and pain severity over the past decade.
5. Conclusions
In conclusion, this systematic literature review shows that both the prevalence of pain and pain severity have declined in the past decade. The development of new treatment strategies and increased attention on assessment and management of pain may have facilitated the decreases in the prevalence and severity of pain. However, with more than half of the patients experiencing pain, the prevalence in advanced, metastatic and terminal patients remains high. To further decrease the prevalence of pain and pain severity, ongoing attention and improved educational programs on cancer pain management are needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15030591/s1, Supplemental S1: Search strategy.; Supplemental S2: Studies that scored <15 point on the methodological quality assessment [326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355,356,357,358,359,360,361,362,363,364,365,366,367,368,369,370,371,372,373,374,375,376,377,378,379,380,381,382,383,384,385,386,387,388,389,390,391,392,393,394,395,396,397,398,399,400,401,402,403,404,405,406,407,408,409,410,411,412,413,414,415,416,417,418,419,420,421,422,423,424,425,426,427,428,429,430,431,432,433,434,435,436,437,438,439,440,441,442,443,444,445,446,447,448,449,450,451,452,453,454,455,456,457,458,459,460,461,462,463,464,465,466,467,468,469,470,471,472,473,474,475,476,477,478,479,480,481,482,483,484,485].; Supplemental S3: Forest plots [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,52].
Author Contributions
Conceptualization, R.A.H.S., L.B., M.T. and M.H.J.v.d.B.-v.E.; methodology, R.A.H.S., L.B., M.T. and M.H.J.v.d.B.-v.E.; software, R.A.H.S.; validation, R.A.H.S., L.B., M.T. and M.H.J.v.d.B.-v.E.; formal analysis, R.A.H.S.; investigation, R.A.H.S., L.B., M.T. and M.H.J.v.d.B.-v.E.; data curation, R.A.H.S.; writing—original draft preparation, R.A.H.S.; writing—review and editing, R.A.H.S., L.B., M.T. and M.H.J.v.d.B.-v.E.; visualization, R.A.H.S.; supervision, L.B. and M.H.J.v.d.B.-v.E.; project administration, R.A.H.S. All authors have read and agreed to the published version of the manuscript.”
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.IASP [(accessed on 15 June 2022)]. Available online: https://www.iasp-pain.org/advocacy/global-year/cancer-pain/
- 2.Cramer J.D., Johnson J.T., Nilsen M.L. Pain in Head and Neck Cancer Survivors: Prevalence, Predictors, and Quality-of-Life Impact. Otolaryngol. Head Neck Surg. 2018;159:853–858. doi: 10.1177/0194599818783964. [DOI] [PubMed] [Google Scholar]
- 3.Kroenke K., Theobald D., Wu J., Loza J.K., Carpenter J.S., Tu W. The association of depression and pain with health-related quality of life, disability, and health care use in cancer patients. J. Pain Symptom Manag. 2010;40:327–341. doi: 10.1016/j.jpainsymman.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ośmiałowska E., Misiąg W., Chabowski M., Jankowska-Polańska B. Coping Strategies, Pain, and Quality of Life in Patients with Breast Cancer. J. Clin. Med. 2021;10:4469. doi: 10.3390/jcm10194469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin C.C., Lai Y.L., Ward S.E. Effect of cancer pain on performance status, mood states, and level of hope among Taiwanese cancer patients. J. Pain Symptom Manag. 2003;25:29–37. doi: 10.1016/S0885-3924(02)00542-0. [DOI] [PubMed] [Google Scholar]
- 6.Strang P. Cancer pain-a provoker of emotional, social and existential distress. Acta Oncol. 1998;37:641–644. doi: 10.1080/028418698429973. [DOI] [PubMed] [Google Scholar]
- 7.van den Beuken-van Everdingen M.H., Hochstenbach L.M., Joosten E.A., Tjan-Heijnen V.C., Janssen D.J. Update on Prevalence of Pain in Patients with Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2016;51:1070–1090.e9. doi: 10.1016/j.jpainsymman.2015.12.340. [DOI] [PubMed] [Google Scholar]
- 8.van den Beuken-van Everdingen M.H., de Rijke J.M., Kessels A.G., Schouten H.C., van Kleef M., Patijn J. Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann. Oncol. 2007;18:1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- 9.Evenepoel M., Haenen V., De Baerdemaecker T., Meeus M., Devoogdt N., Dams L., Van Dijck S., Van der Gucht E., De Groef A. Pain Prevalence During Cancer Treatment: A Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2022;63:e317–e335. doi: 10.1016/j.jpainsymman.2021.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Kwon J.H. Overcoming barriers in cancer pain management. J. Clin. Oncol. 2014;32:1727–1733. doi: 10.1200/JCO.2013.52.4827. [DOI] [PubMed] [Google Scholar]
- 11.Mercadante S., Adile C., Tirelli W., Ferrera P., Penco I., Casuccio A. Barriers and Adherence to Pain Management in Advanced Cancer Patients. Pain Pract. 2021;21:388–393. doi: 10.1111/papr.12965. [DOI] [PubMed] [Google Scholar]
- 12.Stoorvogel H., van Haastregt J., Theunissen M., Schoenmaekers J., Hoeben A., van den Beuken-van Everdingen M.H. Unacceptable pain in oncology: The patients’ perspective on reasons for absence of pain interventions. Eur. J. Cancer Care. 2022;31:e13628. doi: 10.1111/ecc.13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makhlouf S.M., Pini S., Ahmed S., Bennett M.I. Managing Pain in People with Cancer-a Systematic Review of the Attitudes and Knowledge of Professionals, Patients, Caregivers and Public. J. Cancer Educ. 2020;35:214–240. doi: 10.1007/s13187-019-01548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UN [(accessed on 20 July 2022)]. Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf.
- 15.WHO [(accessed on 20 July 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
- 16.Divo M.J., Martinez C.H., Mannino D.M. Ageing and the epidemiology of multimorbidity. Eur. Respir. J. 2014;44:1055–1068. doi: 10.1183/09031936.00059814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braunlin M., Belani R., Buchanan J., Wheeling T., Kim C. Trends in the multiple myeloma treatment landscape and survival: A U.S. analysis using 2011–2019 oncology clinic electronic health record data. Leuk. Lymphoma. 2021;62:377–386. doi: 10.1080/10428194.2020.1827253. [DOI] [PubMed] [Google Scholar]
- 18.Douglas S.R., Lizarraga I.M., Boughey J.C., Weiss A., Hunt K.K., Dickson-Witmer D., Subhedar P.D., Park K.U., Zhao B., Blair S.L. National Cancer Database trends in surgical resection of the breast primary for stage IV breast cancer. Surg. Oncol. 2022;42:101778. doi: 10.1016/j.suronc.2022.101778. [DOI] [PubMed] [Google Scholar]
- 19.Evers J., de Jaeger K., Hendriks L.E.L., van der Sangen M., Terhaard C., Siesling S., De Ruysscher D., Struikmans H., Aarts M.J. Trends and variations in treatment of stage I-III non-small cell lung cancer from 2008 to 2018: A nationwide population-based study from the Netherlands. Lung Cancer. 2021;155:103–113. doi: 10.1016/j.lungcan.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Lorenzen S., Lordick F., Loosen S.H., Tacke F., Trautwein C., Roderburg C., Ettrich T.J., Perkhofer L., Reinacher-Schick A., Stein A. Current status of immunotherapy in gastrointestinal malignancies. Z. Gastroenterol. 2020;58:542–555. doi: 10.1055/a-1071-8322. [DOI] [PubMed] [Google Scholar]
- 21.Ju C., Wei L., Man K.K.C., Wang Z., Ma T.T., Chan A.Y.L., Brauer R., Chui C.S.L., Chan E.W., Jani Y.H., et al. Global, regional, and national trends in opioid analgesic consumption from 2015 to 2019: A longitudinal study. Lancet Public Health. 2022;7:e335–e346. doi: 10.1016/S2468-2667(22)00013-5. [DOI] [PubMed] [Google Scholar]
- 22.Bernard S.A., Chelminski P.R., Ives T.J., Ranapurwala S.I. Management of Pain in the United States-A Brief History and Implications for the Opioid Epidemic. Health Serv. Insights. 2018;11:1–6. doi: 10.1177/1178632918819440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks J.V., Poague C., Formagini T., Roberts A.W., Sinclair C.T., Keirns C.C. Palliative Care’s Role Managing Cancer Pain During the Opioid Crisis: A Qualitative Study of Patients, Caregivers, and Clinicians. J. Pain Symptom Manag. 2020;60:1127–1135.e2. doi: 10.1016/j.jpainsymman.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y., Spillane S., Shiels M.S., Young L., Quach D., Berrington de González A., Freedman N.D. Trends in Opioid Use among Cancer Patients in the United States: 2013–2018. JNCI Cancer Spectr. 2022;6:pkab095. doi: 10.1093/jncics/pkab095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pain, Dutch Guideline of Cancer. [(accessed on 4 July 2022)]. Available online: https://www.pallialine.nl/pijn-bij-patienten-met-kanker.
- 26.Fallon M., Giusti R., Aielli F., Hoskin P., Rolke R., Sharma M., Ripamonti C.I. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018;29:iv166–iv191. doi: 10.1093/annonc/mdy152. [DOI] [PubMed] [Google Scholar]
- 27.Swarm R.A., Paice J.A., Anghelescu D.L., Are M., Bruce J.Y., Buga S., Chwistek M., Cleeland C., Craig D., Gafford E., et al. Adult Cancer Pain, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019;17:977–1007. doi: 10.6004/jnccn.2019.0038. [DOI] [PubMed] [Google Scholar]
- 28.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 29.Rietjens J.A., Bramer W.M., Geijteman E.C., van der Heide A., Oldenmenger W.H. Development and validation of search filters to find articles on palliative care in bibliographic databases. Palliat. Med. 2019;33:470–474. doi: 10.1177/0269216318824275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serlin R.C., Mendoza T.R., Nakamura Y., Edwards K.R., Cleeland C.S. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 31.Leboeuf-Yde C., Lauritsen J.M. The prevalence of low back pain in the literature. A structured review of 26 Nordic studies from 1954 to 1993. Spine (Phila. Pa. 1976) 1995;20:2112–2118. doi: 10.1097/00007632-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Egger M. Systematic Reviews. 2nd ed. BMJ Publishing Group; London, UK: 2001. [Google Scholar]
- 33.Alt-Epping B., Seidel W., Vogt J., Mehnert A., Thomas M., van Oorschot B., Wolff H., Schliephake H., Canis M., Dröge L.H., et al. Symptoms and Needs of Head and Neck Cancer Patients at Diagnosis of Incurability-Prevalences, Clinical Implications, and Feasibility of a Prospective Longitudinal Multicenter Cohort Study. Oncol. Res. Treat. 2016;39:186–192. doi: 10.1159/000445307. [DOI] [PubMed] [Google Scholar]
- 34.Andersen K.G., Aasvang E.K., Kroman N., Kehlet H. Intercostobrachial nerve handling and pain after axillary lymph node dissection for breast cancer. Acta. Anaesthesiol. Scand. 2014;58:1240–1248. doi: 10.1111/aas.12393. [DOI] [PubMed] [Google Scholar]
- 35.Bibby A.C., Halford P., De Fonseka D., Morley A.J., Smith S., Maskell N.A. The Prevalence and Clinical Relevance of Nonexpandable Lung in Malignant Pleural Mesothelioma. A Prospective, Single-Center Cohort Study of 229 Patients. Ann. Am. Thorac. Soc. 2019;16:1273–1279. doi: 10.1513/AnnalsATS.201811-786OC. [DOI] [PubMed] [Google Scholar]
- 36.Efficace F., Gaidano G., Breccia M., Criscuolo M., Cottone F., Caocci G., Bowen D., Lübbert M., Angelucci E., Stauder R., et al. Prevalence, severity and correlates of fatigue in newly diagnosed patients with myelodysplastic syndromes. Br. J. Haematol. 2015;168:361–370. doi: 10.1111/bjh.13138. [DOI] [PubMed] [Google Scholar]
- 37.Esser P., Kuba K., Scherwath A., Schirmer L., Schulz-Kindermann F., Dinkel A., Balck F., Koch U., Kröger N., Götze H., et al. Posttraumatic stress disorder symptomatology in the course of allogeneic HSCT: A prospective study. J. Cancer Surviv. 2017;11:203–210. doi: 10.1007/s11764-016-0579-7. [DOI] [PubMed] [Google Scholar]
- 38.Gjeilo K.H., Oksholm T., Follestad T., Wahba A., Rustøen T. Trajectories of Pain in Patients Undergoing Lung Cancer Surgery: A Longitudinal Prospective Study. J. Pain Symptom Manag. 2020;59:818–828.e1. doi: 10.1016/j.jpainsymman.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Godby R.C., Dai C., Al-Obaidi M., Giri S., Young-Smith C., Kenzik K., McDonald A.M., Paluri R.K., Gbolahan O.B., Bhatia S., et al. Depression among older adults with gastrointestinal malignancies. J. Geriatr. Oncol. 2021;12:599–604. doi: 10.1016/j.jgo.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong J., Wei Z., Wang W. Preoperative psychological distress, coping and quality of life in Chinese patients with newly diagnosed gastric cancer. J. Clin. Nurs. 2015;24:2439–2447. doi: 10.1111/jocn.12816. [DOI] [PubMed] [Google Scholar]
- 41.Kirchheiner K., Nout R.A., Czajka-Pepl A., Ponocny-Seliger E., Sturdza A.E., Dimopoulos J.C., Dörr W., Pötter R. Health related quality of life and patient reported symptoms before and during definitive radio(chemo)therapy using image-guided adaptive brachytherapy for locally advanced cervical cancer and early recovery—A mono-institutional prospective study. Gynecol. Oncol. 2015;136:415–423. doi: 10.1016/j.ygyno.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 42.Kuon J., Vogt J., Mehnert A., Alt-Epping B., van Oorschot B., Sistermanns J., Ahlborn M., Ritterbusch U., Stevens S., Kahl C., et al. Symptoms and Needs of Patients with Advanced Lung Cancer: Early Prevalence Assessment. Oncol. Res. Treat. 2019;42:650–659. doi: 10.1159/000502751. [DOI] [PubMed] [Google Scholar]
- 43.Lunde S., Petersen K.K., Kugathasan P., Arendt-Nielsen L., Søgaard-Andersen E. Chronic Postoperative Pain after Robot-Assisted Laparoscopic Hysterectomy for Endometrial Cancer. J. Gynecol. Surg. 2019;35:140–146. doi: 10.1089/gyn.2018.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ravn Munkvold B.K., Sagberg L.M., Jakola A.S., Solheim O. Preoperative and Postoperative Headache in Patients with Intracranial Tumors. World Neurosurg. 2018;115:e322–e330. doi: 10.1016/j.wneu.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 45.Roy S., Pathy S., Mohanti B.K., Raina V., Jaiswal A., Kumar R., Kalaivani M. Accelerated hypofractionated radiotherapy with concomitant chemotherapy in locally advanced squamous cell carcinoma of lung: Evaluation of response, survival, toxicity and quality of life from a Phase II randomized study. Br. J. Radiol. 2016;89:20150966. doi: 10.1259/bjr.20150966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russo M., Villani V., Taga A., Genovese A., Terrenato I., Manzoni G.C., Servadei F., Torelli P., Pace A. Headache as a presenting symptom of glioma: A cross-sectional study. Cephalalgia. 2018;38:730–735. doi: 10.1177/0333102417710020. [DOI] [PubMed] [Google Scholar]
- 47.Salwey L., L’Huillier V., Zaid M., Vené Y., Tavernier L., Mauvais O. Neuropathic pain at diagnosis of head and neck squamous cell carcinoma. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020;137:377–380. doi: 10.1016/j.anorl.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Tang M.H., Castle D.J., Choong P.F.M. Identifying the prevalence, trajectory, and determinants of psychological distress in extremity sarcoma. Sarcoma. 2015;2015:745163. doi: 10.1155/2015/745163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas S., Walsh D., Shrotriya S., Aktas A., Hullihen B., Estfan B., Budd G.T., Hjermstad M.J., O’Connor B. Symptoms, Quality of Life, and Daily Activities in People with Newly Diagnosed Solid Tumors Presenting to a Medical Oncologist. Am. J. Hosp. Care. 2017;34:611–621. doi: 10.1177/1049909116649948. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y.J., Chan Y.N., Jheng Y.W., Wu C.J., Lin M.W., Tseng L.M., Tsai Y.F., Liu L.C. Chemotherapy-induced peripheral neuropathy in newly diagnosed breast cancer survivors treated with taxane: A prospective longitudinal study. Support. Care Cancer. 2021;29:2959–2971. doi: 10.1007/s00520-020-05796-0. [DOI] [PubMed] [Google Scholar]
- 51.Williams G.R., Al-Obaidi M., Dai C., Harmon C., Buford T.W., Gbolahan O., Pergolotti M., Bhatia S., Giri S. Fatigue is independently associated with functional status limitations in older adults with gastrointestinal malignancies—Results from the CARE registry. Support. Care Cancer. 2021;29:6793–6800. doi: 10.1007/s00520-021-06273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao H.H.I., Crump R.T., Charbonneau C., Khan A., Barton C., Brotherhood H., Jiang J., Carlson K.V., Baverstock R.J. Baseline patient reported outcomes data shows high prevalence of overactive bladder, sexual dysfunction, depression and anxiety in Canadian men with newly diagnosed localized prostate cancer. Transl. Androl. Urol. 2020;9:2046–2053. doi: 10.21037/tau-20-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berliere M., Piette N., Bernard M., Lacroix C., Gerday A., Samartzi V., Coyette M., Roelants F., Docquier M.A., Touil N., et al. Hypnosis sedation reduces the duration of different side effects of cancer treatments in breast cancer patients receiving neoadjuvant chemotherapy. Cancers. 2021;13:4147. doi: 10.3390/cancers13164147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bretschneider C.E., Doll K.M., Bensen J.T., Gehrig P.A., Wu J.M., Geller E.J. Prevalence of pelvic floor disorders in women with suspected gynecological malignancy: A survey-based study. Int. Urogynecol. J. 2016;27:1409–1414. doi: 10.1007/s00192-016-2962-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Browall M., Brandberg Y., Nasic S., Rydberg P., Bergh J., Rydén A., Xie H., Eriksson I., Wengström Y., Rydén A., et al. A prospective exploration of symptom burden clusters in women with breast cancer during chemotherapy treatment. Support. Care Cancer. 2017;25:1423–1429. doi: 10.1007/s00520-016-3527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calderon C., Carmona-Bayonas A., Hernández R., Ghanem I., Castelo B., Martinez de Castro E., Ferreira E., Ciria L., Muñiz M., Jimenez-Fonseca P. Effects of pessimism, depression, fatigue, and pain on functional health-related quality of life in patients with resected non-advanced breast cancer. Breast. 2019;44:108–112. doi: 10.1016/j.breast.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 57.Choo S.B., Saifulbahri A., Zullkifli S.N., Fadzil M.L., Redzuan A.M., Abdullah N., Bustamam R.S.A., Ahmad H.Z., Shah N.M. Adjuvant endocrine therapy side-effects among postmenopausal breast cancer patients in Malaysia. Climacteric. 2019;22:175–181. doi: 10.1080/13697137.2018.1540563. [DOI] [PubMed] [Google Scholar]
- 58.de Menezes Couceiro T.C., Valença M.M., Raposo M.C.F., de Orange F.A., Amorim M.M.R. Prevalence of Post-Mastectomy Pain Syndrome and Associated Risk Factors: A Cross-Sectional Cohort Study. Pain Manag. Nurs. 2014;15:731–737. doi: 10.1016/j.pmn.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 59.Dylke E.S., Kilbreath S. Current rehabilitation processes do not prevent long-term impairments after treatment for breast cancer in Australia. Aust. Fam. Physician. 2015;44:405–409. [PubMed] [Google Scholar]
- 60.El-Aqoul A., Obaid A., Yacoub E., Al-Najar M., Ramadan M., Darawad M. Factors Associated with Inadequate Pain Control among Postoperative Patients with Cancer. Pain Manag. Nurs. 2018;19:130–138. doi: 10.1016/j.pmn.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Fenlon D., Powers C., Simmonds P., Clough J., Addington-Hall J. The JACS prospective cohort study of newly diagnosed women with breast cancer investigating joint and muscle pain, aches, and stiffness: Pain and quality of life after primary surgery and before adjuvant treatment. BMC Cancer. 2014;14:467. doi: 10.1186/1471-2407-14-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fjell M., Langius-Eklöf A., Nilsson M., Wengström Y., Sundberg K. Reduced symptom burden with the support of an interactive app during neoadjuvant chemotherapy for breast cancer—A randomized controlled trial. Breast. 2020;51:85–93. doi: 10.1016/j.breast.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hagiwara Y., Ohashi Y., Uesaka K., Boku N., Fukutomi A., Okamura Y., Konishi M., Matsumoto I., Kaneoka Y., Shimizu Y., et al. Health-related quality of life of adjuvant chemotherapy with S-1 versus gemcitabine for resected pancreatic cancer: Results from a randomised phase III trial (JASPAC 01) Eur. J. Cancer. 2018;93:79–88. doi: 10.1016/j.ejca.2018.01.081. [DOI] [PubMed] [Google Scholar]
- 64.Haryani H., Hsu Y.Y., Warsini S., Wang S.T. Measuring the Symptom Experience of Patients with Cancer in Indonesia: Cross-Cultural Adaptation and Validation of the Memorial Symptom Assessment Scale—Indonesian Version. J. Pain Symptom Manag. 2018;56:920–927. doi: 10.1016/j.jpainsymman.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 65.Ho R.T.H., Kwan T.T.C., Cheung I.K.M., Chan C.K.P., Lo P.H.Y., Yip P.S.F., Luk M.Y., Chan C.L.W. Association of fatigue with perceived stress in Chinese women with early stage breast cancer awaiting adjuvant radiotherapy. Stress Health. 2015;31:214–221. doi: 10.1002/smi.2548. [DOI] [PubMed] [Google Scholar]
- 66.Hong J.S., Tian J. Prevalence of anxiety and depression and their risk factors in Chinese cancer patients. Support. Care Cancer. 2014;22:453–459. doi: 10.1007/s00520-013-1997-y. [DOI] [PubMed] [Google Scholar]
- 67.Jarden M., Møller T., Christensen K.B., Buchardt A.S., Kjeldsen L., Adamsen L. Longitudinal symptom burden in adult patients with acute leukaemia participating in the PACE-AL randomised controlled exercise trial—An explorative analysis. Eur. J. Cancer Care. 2021;30:e13462. doi: 10.1111/ecc.13462. [DOI] [PubMed] [Google Scholar]
- 68.Jensen N.B.K., Pötter R., Kirchheiner K., Fokdal L., Lindegaard J.C., Kirisits C., Mazeron R., Mahantshetty U., Jürgenliemk-Schulz I.M., Segedin B., et al. Bowel morbidity following radiochemotherapy and image-guided adaptive brachytherapy for cervical cancer: Physician- and patient reported outcome from the EMBRACE study. Radiother. Oncol. 2018;127:431–439. doi: 10.1016/j.radonc.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 69.Khan Q.J., Kimler B.F., Reddy P.S., Sharma P., Klemp J.R., Nydegger J.L., Yeh H.W., Fabian C.J. Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms in women with breast cancer receiving adjuvant letrozole. The VITAL trial. Breast Cancer Res. Treat. 2017;166:491–500. doi: 10.1007/s10549-017-4429-8. [DOI] [PubMed] [Google Scholar]
- 70.Kim Y.S., Do H., Lee J.W., Jeong J., Shin Y.W., Yi K., Kim J., Lee S.B., Sohn G., Yang N., et al. Patient reporting pain intensity immediately after surgery can be associated with underlying depression in women with breast cancer. Psychooncology. 2016;25:308–315. doi: 10.1002/pon.3919. [DOI] [PubMed] [Google Scholar]
- 71.Kirkham A.A., Eves N.D., Shave R.E., Bland K.A., Bovard J., Gelmon K.A., Virani S.A., McKenzie D.C., Stöhr E.J., Waburton D.E.R., et al. The effect of an aerobic exercise bout 24 h prior to each doxorubicin treatment for breast cancer on markers of cardiotoxicity and treatment symptoms: A RCT. Breast Cancer Res. Treat. 2018;167:719–729. doi: 10.1007/s10549-017-4554-4. [DOI] [PubMed] [Google Scholar]
- 72.Lewis L., Williams A.M., Athifa M., Brown D., Budgeon C.A., Bremner A.P. Evidence-Based Self-care Guidelines for People Receiving Chemotherapy: Do They Reduce Symptom Burden and Psychological Distress? Cancer Nurs. 2015;38:e1–e8. doi: 10.1097/NCC.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 73.Lewis S., Pandey S., Salins N., Deodhar J., Patil V., Gupta T., Laskar S.G., Budrukkar A., Murthy V., Joshi A., et al. Distress Screening in Head and Neck Cancer Patients Planned for Cancer-Directed Radiotherapy. Laryngoscope. 2021;131:2023–2029. doi: 10.1002/lary.29491. [DOI] [PubMed] [Google Scholar]
- 74.McFarland D.C., Shaffer K.M., Tiersten A., Holland J. Prevalence of physical problems detected by the distress thermometer and problem list in patients with breast cancer. Psychooncology. 2018;27:1394–1403. doi: 10.1002/pon.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moloney N., Sung J.M.W., Kilbreath S., Dylke E. Prevalence and risk factors associated with pain 21 months following surgery for breast cancer. Support. Care Cancer. 2016;24:4533–4539. doi: 10.1007/s00520-016-3292-1. [DOI] [PubMed] [Google Scholar]
- 76.Nogueira de Oliveira Martins T., dos Santos L.F., do Nascimento Petter G., da Silva Ethur J.N., Medeiros Braz M., Foletto Pivetta H.M. Immediate breast reconstruction versus non-reconstruction after mastectomy: A study on quality of life, pain and functionality. Fisioter. Pesqui. 2017;24:412–419. [Google Scholar]
- 77.Okamoto A., Yamasaki M., Yokota I., Mori M., Matsuda M., Yamaguchi Y., Yamakita S., Ueno H., Sawa T., Taguchi T., et al. Classification of acute pain trajectory after breast cancer surgery identifies patients at risk for persistent pain: A prospective observational study. J. Pain Res. 2018;11:2197–2206. doi: 10.2147/JPR.S171680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ribas Y., Bonet M., Torres L., Núñez M., Esther Jovell-Fernández E., Aranda E., Andreyev H.J. Bowel dysfunction in survivors of gynaecologic malignancies. Support. Care Cancer. 2020;28:5501–5510. doi: 10.1007/s00520-020-05402-3. [DOI] [PubMed] [Google Scholar]
- 79.Røhrl K., Guren M.G., Miaskowski C., Cooper B.A., Diep L.M., Rustøen T. No Differences in Symptom Burden Between Colorectal Cancer Patients Receiving Curative Versus Palliative Chemotherapy. J. Pain Symptom Manag. 2016;52:539–547. doi: 10.1016/j.jpainsymman.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 80.Shaulov A., Rodin G., Popovic G., Caraiscos V.B., Le L.W., Rydall A., Schimmer A.D., Zimmermann C. Pain in patients with newly diagnosed or relapsed acute leukemia. Support. Care Cancer. 2019;27:2789–2797. doi: 10.1007/s00520-018-4583-5. [DOI] [PubMed] [Google Scholar]
- 81.Tran B.T., Pham N.H., Nguyen T.X., Choi K.S., Sohn D.K., Kim S.Y., Suh J.K., Nguyen T.D., Phan V.S., Tran D.T., et al. Measurement of health-related quality of life among colorectal cancer patients using the vietnamese value set of the eq-5d-5l. Patient Prefer. Adherence. 2020;14:2427–2437. doi: 10.2147/PPA.S281500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu Y., Hu X., Zhou L., Zhao Y. Effect of sequencing of preoperative systemic therapy on patients with locally advanced breast cancer. Breast J. 2020;26:1987–1994. doi: 10.1111/tbj.14017. [DOI] [PubMed] [Google Scholar]
- 83.Yi M., Hwang E. Pain and Menopause Symptoms of Breast Cancer Patients with Adjuvant Hormonal Therapy in Korea: Secondary Analysis. Asia Pac. J. Oncol. Nurs. 2018;5:262–269. doi: 10.4103/apjon.apjon_45_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Agarwal S., Garg R., Minhas V., Bhatnagar S., Mishra S., Kumar V., Bharati S.J., Gupta N., Khan M.A. To assess the Prevalence and Predictors of Cancer-related Fatigue and its Impact on Quality of Life in Advanced Cancer Patients Receiving Palliative Care in a Tertiary Care Hospital: A Cross-sectional Descriptive Study. Indian J. Palliat. Care. 2020;26:523–527. doi: 10.4103/IJPC.IJPC_223_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Al-Zahrani O., Eldali A., Al-Shahri M.Z. Prevalence and severity of pain in cancer patients in an outpatient palliative care setting in Saudi Arabia. Qatar Med. J. 2014;1:38–45. doi: 10.5339/qmj.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blank A.T., Lerman D.M., Shaw S., Dadrass F., Zhang Y., Liu W., Hung M., Jones K.B., Randall R.L. PROMIS(®) scores in operative metastatic bone disease patients: A multicenter, prospective study. J. Surg. Oncol. 2018;118:532–535. doi: 10.1002/jso.25159. [DOI] [PubMed] [Google Scholar]
- 87.Bouché O., Le Cesne A., Rios M., Chaigneau L., Italiano A., Duffaud F., Lecomte T., Arsène D., Manfredi S., Aparicio T., et al. EPigist: An observational real-life study on patients with metastatic gastrointestinal stromal tumors receiving imatinib. PLoS ONE. 2018;13:e0204117. doi: 10.1371/journal.pone.0204117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bullock A., Stuart K., Jacobus S., Abrams T., Wadlow R., Goldstein M., Miksad R. Capecitabine and oxaliplatin as first and second line treatment for locally advanced and metastatic pancreatic ductal adenocarcinoma. J. Gastrointest. Oncol. 2017;8:945–952. doi: 10.21037/jgo.2017.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Green A.K., Corty R.W., Wood W.A., Meeneghan M., Reeder-Hayes K.E., Basch E., Milowsky M.I., Dusetzina S.B. Comparative effectiveness of mitoxantrone plus prednisone versus prednisone alone in metastatic castrate-resistant prostate cancer after docetaxel failure. Oncologist. 2015;20:516–522. doi: 10.1634/theoncologist.2014-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iwase S., Kawaguchi T., Tokoro A., Yamada K., Kanai Y., Matsuda Y., Kashiwaya Y., Okuma K., Inada S., Ariyoshi K., et al. Assessment of cancer-related fatigue, pain, and quality of life in cancer patients at palliative care team referral: A multicenter observational study (JORTC PAL-09) PLoS ONE. 2015;10:e0134022. doi: 10.1371/journal.pone.0134022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jespersen E., Nielsen L.K., Larsen R.F., Möller S., Jarlbæk L. Everyday living with pain-reported by patients with multiple myeloma. Scand. J. Pain. 2021;21:127–134. doi: 10.1515/sjpain-2020-0087. [DOI] [PubMed] [Google Scholar]
- 92.King M.T., Stockler M.R., O’Connell R.L., Buizen L., Joly F., Lanceley A., Hilpert F., Okamoto A., Aotani E., Bryce J., et al. Measuring what matters MOST: Validation of the Measure of Ovarian Symptoms and Treatment, a patient-reported outcome measure of symptom burden and impact of chemotherapy in recurrent ovarian cancer. Qual. Life Res. 2018;27:59–74. doi: 10.1007/s11136-017-1729-8. [DOI] [PubMed] [Google Scholar]
- 93.Koldenhof J.J., Witteveen P.O., De Vos R., Walraven M., Tillier C.N., Verheul H.M.W., Teunissen S.C.C.M. Symptoms from treatment with sunitinib or sorafenib: A multicenter explorative cohort study to explore the influence of patient-reported outcomes on therapy decisions. Support. Care Cancer. 2014;22:2371–2380. doi: 10.1007/s00520-014-2223-2. [DOI] [PubMed] [Google Scholar]
- 94.Lavdaniti M., Fradelos E.C., Troxoutsou K., Zioga E., Mitsi D., Alikari V., Zyga S. Symptoms in Advanced Cancer Patients in a Greek Hospital: A Descriptive Study. Asian Pac. J. Cancer Prev. 2018;19:1047–1052. doi: 10.22034/APJCP.2018.19.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.LeBlanc T.W., Nickolich M., Rushing C.N., Samsa G.P., Locke S.C., Abernethy A.P. What bothers lung cancer patients the most? A prospective, longitudinal electronic patient-reported outcomes study in advanced non-small cell lung cancer. Support. Care Cancer. 2015;23:3455–3463. doi: 10.1007/s00520-015-2699-4. [DOI] [PubMed] [Google Scholar]
- 96.Lechner B., Chow S., Chow R., Zhang L., Tsao M., Danjoux C., Barnes E., DeAngelis C., Vuong S., Ganesh V., et al. The incidence of neuropathic pain in bone metastases patients referred for palliative radiotherapy. Radiother. Oncol. 2016;118:557–561. doi: 10.1016/j.radonc.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 97.Madsen M., Grønbæk H., Finnerup N.B., Dam G. A descriptive cross-sectional study of pain in patients with neuroendocrine tumors. Scand. J. Gastroenterol. 2017;52:431–436. doi: 10.1080/00365521.2016.1261937. [DOI] [PubMed] [Google Scholar]
- 98.McFarland D.C., Shaffer K.M., Polizzi H., Mascarenhas J., Kremyanskaya M., Holland J., Hoffman R. Prevalence of Physical Problems Detected by the Distress Thermometer and Problem List in Patients with Myeloproliferative Disorders. J. Natl. Compr. Cancer Netw. 2017;15:1503–1508. doi: 10.6004/jnccn.2017.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mercadante S., Aielli F., Masedu F., Valenti M., Verna L., Porzio G. Age differences in the last week of life in advanced cancer patients followed at home. Support. Care Cancer. 2016;24:1889–1895. doi: 10.1007/s00520-015-2988-y. [DOI] [PubMed] [Google Scholar]
- 100.Sampogna F., Paradisi A., Iemboli M.L., Ricci F., Sonego G., Abeni D. Comparison of quality of life between melanoma and non-melanoma skin cancer patients. Eur. J. Dermatol. 2019;29:185–191. doi: 10.1684/ejd.2019.3523. [DOI] [PubMed] [Google Scholar]
- 101.Selvy M., Kerckhove N., Pereira B., Barreau F., Nguyen D., Busserolles J., Giraudet F., Cabrespine A., Chaleteix C., Soubrier M., et al. Prevalence of Chemotherapy-Induced Peripheral Neuropathy in Multiple Myeloma Patients and its Impact on Quality of Life: A Single Center Cross-Sectional Study. Front. Pharmacol. 2021;12:637593. doi: 10.3389/fphar.2021.637593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Steel J.L., Geller D.A., Kim K.H., Butterfield L.H., Spring M., Grady J., Sun W., Marsh W., Antoni M., Dew M.A., et al. Web-based collaborative care intervention to manage cancer-related symptoms in the palliative care setting. Cancer. 2016;122:1270–1282. doi: 10.1002/cncr.29906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Walling A.M., Weeks J.C., Kahn K.L., Tisnado D., Keating N.L., Dy S.M., Arora N.K., Mack J.W., Pantoja P.M., Malin J.L. Symptom prevalence in lung and colorectal cancer patients. J. Pain Symptom Manag. 2015;49:192–202. doi: 10.1016/j.jpainsymman.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou T., Yang K., Thapa S., Liu H., Wang B., Yu S. Differences in Symptom Burden among Cancer Patients with Different Stages of Cachexia. J. Pain Symptom Manag. 2017;53:919–926. doi: 10.1016/j.jpainsymman.2016.12.325. [DOI] [PubMed] [Google Scholar]
- 105.Abu-Saad Huijer H., Sagherian K., Tamim H. Validation of the Arabic Version of the Memorial Symptom Assessment Scale among Lebanese Cancer Patients. J. Pain Symptom Manag. 2015;50:559–565. doi: 10.1016/j.jpainsymman.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 106.Bernardes C.M., Beesley V., Martin J., Sabesan S., Baade P., Meiklejohn J.A., Garvey G., Valery P.C. Unmet supportive care needs among people with cancer: A cross-cultural comparison between Indigenous and Non-Indigenous Australians. Eur. J. Cancer Care. 2019;28:e13080. doi: 10.1111/ecc.13080. [DOI] [PubMed] [Google Scholar]
- 107.Chan A., Lim E., Ng T., Shih V., Quek R., Cheung Y.T. Symptom burden and medication use in adult sarcoma patients. Support. Care Cancer. 2015;23:1709–1717. doi: 10.1007/s00520-014-2533-4. [DOI] [PubMed] [Google Scholar]
- 108.Chen F., Leng Y., Zhang L., Xu J., Zhang D., Qin Y., Li J., Zheng Y. The Correlation of Symptom Clusters and Functional Performance in Adult Acute Leukemia Patients under Chemotherapy. Cancer Nurs. 2021;44:E287–E295. doi: 10.1097/NCC.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 109.Cheng H.L., Molassiotis A., Leung A.K.T., Wong K.H. Docetaxel-Induced Peripheral Neuropathy in Breast Cancer Patients Treated with Adjuvant or Neo-Adjuvant Chemotherapy. Breast Care. 2021;16:269–275. doi: 10.1159/000507843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Damm M., Weniger M., Kölsch A.K., Lampert C., Ceyhan G.O., Beer S., Schorn S., Moir J., Michl P., Rosendahl J. The quality of pain management in pancreatic cancer: A prospective multi-center study. Pancreatology. 2020;20:1511–1518. doi: 10.1016/j.pan.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 111.Fujii A., Yamada Y., Takayama K., Nakano T., Kishimoto J., Morita T., Nakanishi Y. Longitudinal assessment of pain management with the pain management index in cancer outpatients receiving chemotherapy. Support. Care Cancer. 2017;25:925–932. doi: 10.1007/s00520-016-3482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gosselin T.K., Beck S., Abbot D.H., Grambow S.C., Provenzale D., Berry P., Kahn K.L., Malin J.L. The Symptom Experience in Rectal Cancer Survivors. J. Pain Symptom Manag. 2016;52:709–718. doi: 10.1016/j.jpainsymman.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 113.Han C.J., Reding K., Cooper B.A., Paul S.M., Conley Y.P., Hammer M., Kober K.M., Levine J.D., Miaskowski C. Stability of Symptom Clusters in Patients with Gastrointestinal Cancers Receiving Chemotherapy. J. Pain Symptom Manag. 2019;58:989–1001.e10. doi: 10.1016/j.jpainsymman.2019.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Joseph A.O., Salako O., Alabi A., Habeebu M., Balogun O., Ayodele O., Awofeso O.M., Adenipekun A. Cancer pain control in a Nigerian oncology clinic: Treating the disease and not the patient. Pan Afr. Med. J. 2021;40:104. doi: 10.11604/pamj.2021.40.104.25225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim H.S., Oh E.G., Lee H., Kim S.H., Kim H.K. Predictors of symptoms experience in Korean patients with cancer undergoing chemotherapy. Eur. J. Oncol. Nurs. 2015;19:644–653. doi: 10.1016/j.ejon.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 116.Kuperus J.M., Busman R.D., Kuipers S.K., Broekhuizen H.T., Noyes S.L., Brede C.M., Tobert C.M., Lane B.R. Comparison of Side Effects and Tolerability Between Intravesical Bacillus Calmette-Guerin, Reduced-Dose BCG and Gemcitabine for Non-Muscle Invasive Bladder Cancer. Urology. 2021;156:191–198. doi: 10.1016/j.urology.2021.04.062. [DOI] [PubMed] [Google Scholar]
- 117.Lee E., Takita C., Wright J.L., Reis I.M., Zhao W., Nelson O.L., Hu J.J. Characterization of risk factors for adjuvant radiotherapy-associated pain in a tri-racial/ethnic breast cancer population. Pain. 2016;157:1122–1131. doi: 10.1097/j.pain.0000000000000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Z., Shi Q., Liu M., Jia L., He B., Yang Y., Liu J., Lin H., Lin H.K., Li P., et al. Validation and Application of the MD Anderson Symptom Inventory for Traditional Chinese Medicine (MDASI-TCM) J. Natl. Cancer Inst. Monogr. 2017;52:lgx010. doi: 10.1093/jncimonographs/lgx010. [DOI] [PubMed] [Google Scholar]
- 119.Llamas Ramos I., Llamas Ramos R., Martín Nogueras A.M., Alvarado Omenat J.J., Calvo Arenillas J.I., Fonseca Sánchez E., Cortés Rodríguez M. Reliability and Validity of the Spanish Version of the Memorial Symptom Assessment Scale in Oncology Patients. J. Pain Symptom Manag. 2016;52:884–891. doi: 10.1016/j.jpainsymman.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 120.Matsumoto Y., Yoshida Y., Kiba S., Yamashiro S., Nogami H., Ohashi N., Kajitani R., Munechika T., Nagano H., Komono A., et al. Acute chemotherapy-induced peripheral neuropathy due to oxaliplatin administration without cold stimulation. Support. Care Cancer. 2020;28:5405–5410. doi: 10.1007/s00520-020-05387-z. [DOI] [PubMed] [Google Scholar]
- 121.Molassiotis A., Cheng H.L., Lopez V., Au J.S.K., Chan A., Bandla A., Leung K.T., Li Y.C., Wong K.H., Suen L.K.P., et al. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer. 2019;19:132. doi: 10.1186/s12885-019-5302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moye J., June A., Martin L.A., Gosian J., Herman L.I., Naik A.D. Pain is prevalent and persisting in cancer survivors: Differential factors across age groups. J. Geriatr. Oncol. 2014;5:190–196. doi: 10.1016/j.jgo.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pearce A., Haas M., Viney R., Pearson S.A., Haywood P., Brown C., Ward R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS ONE. 2017;12:e0184360. doi: 10.1371/journal.pone.0184360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pérez C., Sánchez-Martínez N., Ballesteros A., Blanco T., Collazo A., González F., Villoria J. Prevalence of pain and relative diagnostic performance of screening tools for neuropathic pain in cancer patients: A cross-sectional study. Eur. J. Pain. 2015;19:752–761. doi: 10.1002/ejp.598. [DOI] [PubMed] [Google Scholar]
- 125.Pettersson G., Berterö C., Unosson M., Börjeson S. Symptom prevalence, frequency, severity, and distress during chemotherapy for patients with colorectal cancer. Support. Care Cancer. 2014;22:1171–1179. doi: 10.1007/s00520-013-2069-z. [DOI] [PubMed] [Google Scholar]
- 126.Raj S.X., Thronaes M., Brunelli C., Hjermstad M.J., Klepstad P., Kaasa S. A cross-sectional study on prevalence of pain and breakthrough pain among an unselected group of outpatients in a tertiary cancer clinic. Support. Care Cancer. 2014;22:1965–1971. doi: 10.1007/s00520-014-2178-3. [DOI] [PubMed] [Google Scholar]
- 127.Ritchie C., Dunn L.B., Paul S.M., Cooper B.A., Skerman H., Merriman J.D., Aouizerat B., Alexander K., Yates P., Cataldo J., et al. Differences in the symptom experience of older oncology outpatients. J. Pain Symptom Manag. 2014;47:697–709. doi: 10.1016/j.jpainsymman.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salvetti M.G., Machado C.S.P., Donato S.C.T., Silva A.M.D. Prevalence of symptoms and quality of life of cancer patients. Rev. Bras. Enferm. 2020;73:e20180287. doi: 10.1590/0034-7167-2018-0287. [DOI] [PubMed] [Google Scholar]
- 129.Schumacher O., Galvao D.A., Taaffe D.R., Spry N., Joseph D., Tang C., Chee R., Newton R.U. Effect of Exercise Adjunct to Radiation and Androgen Deprivation Therapy on Patient-Reported Treatment Toxicity in Men with Prostate Cancer: A Secondary Analysis of 2 Randomized Controlled Trials. Pract. Radiat. Oncol. 2021;11:215–225. doi: 10.1016/j.prro.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 130.Seven M., Sahin E., Yilmaz S., Akyuz A. Palliative care needs of patients with gynaecologic cancer. J. Clin. Nurs. 2016;25:3152–3159. doi: 10.1111/jocn.13280. [DOI] [PubMed] [Google Scholar]
- 131.Spoelstra S.L., Given C.W., Sikorskii A., Majumder A., Schueller M., Given B.A. Treatment with oral anticancer agents: Symptom severity and attribution, and interference with comorbidity management. Oncol. Nurs. Forum. 2015;42:80–88. doi: 10.1188/15.ONF.42-01P. [DOI] [PubMed] [Google Scholar]
- 132.Stamm S.L., Spichiger E., Pabst T., Bachnick S., Jeitziner M.M. Symptom prevalence and health-related quality of life in patients undergoing autologous stem cell transplantation—A longitudinal observational study. Eur. J. Oncol. Nurs. 2021;53:101997. doi: 10.1016/j.ejon.2021.101997. [DOI] [PubMed] [Google Scholar]
- 133.Steffen McLouth L.E., Lycan T.W., Levine B.J., Gabbard J., Ruiz J., Farris M., Grant S.C., Pajewski N.M., Weaver K.E., Petty W.J. Patient-Reported Outcomes From Patients Receiving Immunotherapy or Chemoimmunotherapy for Metastatic Non–Small-Cell Lung Cancer in Clinical Practice. Clin. Lung Cancer. 2020;21:255–263.e4. doi: 10.1016/j.cllc.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thiagarajan M., Chan C.M., Fuang H.G., Beng T.S., Atiliyana M.A., Yahaya N.A. Symptom Prevalence and Related Distress in Cancer Patients Undergoing Chemotherapy. Asian Pac. J. Cancer Prev. 2016;17:171–176. doi: 10.7314/APJCP.2016.17.1.171. [DOI] [PubMed] [Google Scholar]
- 135.Turner J.P., Shakib S., Singhal N., Hogan-Doran J., Prowse R., Johns S., Thynne T., Bell J.S. Statin use and pain in older people with cancer: A cross-sectional study. J. Am. Geriatr. Soc. 2014;62:1900–1905. doi: 10.1111/jgs.13051. [DOI] [PubMed] [Google Scholar]
- 136.Unseld M., Zeilinger E.L., Fellinger M., Lubowitzki S., Krammer K., Nader I.W., Hafner M., Kitta A., Adamidis F., Masel E.K., et al. Prevalence of pain and its association with symptoms of post-traumatic stress disorder, depression, anxiety and distress in 846 cancer patients: A cross sectional study. Psychooncology. 2021;30:504–510. doi: 10.1002/pon.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van der Baan F.H., Koldenhof J.J., de Nijs E.J., Echteld M.A., Zweers D., Hesselmann G.M., Vervoort S.C., Vos J.B., de Graaf E., Witteveen P.O., et al. Validation of the Dutch version of the Edmonton Symptom Assessment System. Cancer Med. 2020;9:6111–6121. doi: 10.1002/cam4.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang D., Fu J. Symptom clusters and quality of life in China patients with lung cancer undergoing chemotherapy. Afr. Health Sci. 2014;14:49–55. doi: 10.4314/ahs.v14i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yahaya N.A., Subramanian P., Bustam A.Z., Taib N.A. Symptom experiences and coping strategies among multi- ethnic solid tumor patients undergoing chemotherapy in Malaysia. Asian Pac. J. Cancer Prev. 2015;16:723–730. doi: 10.7314/APJCP.2015.16.2.723. [DOI] [PubMed] [Google Scholar]
- 140.Zhong B.L., Li S.H., Lv S.Y., Tian S.L., Liu Z.D., Li X.B., Zhuang H.Q., Tao R., Zhang W., Zhuo C.J. Suicidal ideation among Chinese cancer inpatients of general hospitals: Prevalence and correlates. Oncotarget. 2017;8:25141–25150. doi: 10.18632/oncotarget.15350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Adams E., Boulton M.G., Horne A., Rose P.W., Durrant L., Collingwood M., Oskrochi R., Davidson S.E., Watson E.K. The effects of pelvic radiotherapy on cancer survivors: Symptom profile, psychological morbidity and quality of life. Clin. Oncol. (R. Coll. Radiol.) 2014;26:10–17. doi: 10.1016/j.clon.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 142.Ahmed A., Bhatnagar S., Rana S.P.S., Ahmad S.M., Joshi S., Mishra S. Prevalence of phantom breast pain and sensation among postmastectomy patients suffering from breast cancer: A prospective study. Pain Pract. 2014;14:E17–E28. doi: 10.1111/papr.12089. [DOI] [PubMed] [Google Scholar]
- 143.Al Maqbali M. Sleep disturbance among Arabic breast cancer survivors. Support. Care Cancer. 2021;29:5179–5186. doi: 10.1007/s00520-021-06088-x. [DOI] [PubMed] [Google Scholar]
- 144.Andersen K.G., Duriaud H.M., Jensen H.E., Kroman N., Kehlet H. Predictive factors for the development of persistent pain after breast cancer surgery. Pain. 2015;156:2413–2422. doi: 10.1097/j.pain.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 145.Asplund D., Prytz M., Bock D., Haglind E., Angenete E. Persistent perineal morbidity is common following abdominoperineal excision for rectal cancer. Int. J. Color. Dis. 2015;30:1563–1570. doi: 10.1007/s00384-015-2328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baden M., Lu L., Drummond F.J., Gavin A., Sharp L. Pain, fatigue and depression symptom cluster in survivors of prostate cancer. Support. Care Cancer. 2020;28:4813–4824. doi: 10.1007/s00520-019-05268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bao T., Seidman A., Li Q., Seluzicki C., Blinder V., Meghani S.H., Farrar J.T., Mao J.J. Living with chronic pain: Perceptions of breast cancer survivors. Breast Cancer Res. Treat. 2018;169:133–140. doi: 10.1007/s10549-018-4670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bennedsgaard K., Ventzel L., Themistocleous A.C., Bennett D.L., Jensen A.B., Jensen A.R., Andersen N.T., Jensen T.S., Tankisi H., Finnerup N.B. Long-term symptoms of polyneuropathy in breast and colorectal cancer patients treated with and without adjuvant chemotherapy. Cancer Med. 2020;9:5114–5123. doi: 10.1002/cam4.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boehmer U., Potter J., Clark M.A., Ozonoff A., Winter M., Berklein F., Ward K.C., Hartshorn K. Assessing the relationship between symptoms and health care utilization in colorectal cancer survivors of different sexual orientations. Support. Care Cancer. 2021;29:5821–5830. doi: 10.1007/s00520-021-06157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bøhn S.K.H., Thorsen L., Kiserud C.E., Fosså S.D., Lie H.C., Loge J.H., Wisløff T., Haugnes H.S., Reinertsen K.V. Chronic fatigue and associated factors among long-term survivors of cancers in young adulthood. Acta Oncol. 2019;58:753–762. doi: 10.1080/0284186X.2018.1557344. [DOI] [PubMed] [Google Scholar]
- 151.Bonhof C.S., Trompetter H.R., Vreugdenhil G., van de Poll-Franse L.V., Mols F. Painful and non-painful chemotherapy-induced peripheral neuropathy and quality of life in colorectal cancer survivors: Results from the population-based PROFILES registry. Support. Care Cancer. 2020;28:5933–5941. doi: 10.1007/s00520-020-05438-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bovbjerg D.H., Keefe F.J., Soo M.S., Manculich J., Van Denburg A., Zuley M.L., Ahrendt G.M., Skinner C.S., Edmond S.N., Shelby R.A. Persistent breast pain in post-surgery breast cancer survivors and women with no history of breast surgery or cancer: Associations with pain catastrophizing, perceived breast cancer risk, breast cancer worry, and emotional distress. Acta Oncol. 2019;58:763–768. doi: 10.1080/0284186X.2019.1574023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bulley C., Coutts F., Blyth C., Jack W., Chetty U., Barber M., Tan C.W. A morbidity Screening Tool for identifying fatigue, pain, upper limb dysfunction and lymphedema after breast cancer treatment: A validity study. Eur. J. Oncol. Nurs. 2014;18:218–227. doi: 10.1016/j.ejon.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 154.Cameron K.E., Kole M.B., Sammel M.D., Ginsberg J.P., Gosiengfiao Y., Mersereau J.E., Su H.I., Gracia C.R. Acute Menopausal Symptoms in Young Cancer Survivors Immediately following Chemotherapy. Oncology. 2018;94:200–206. doi: 10.1159/000485917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Capelan M., Battisti N.M.L., McLoughlin A., Maidens V., Snuggs N., Slyk P., Peckitt C., Ring A., Battisti N.M.L. The prevalence of unmet needs in 625 women living beyond a diagnosis of early breast cancer. Br. J. Cancer. 2017;117:1113–1120. doi: 10.1038/bjc.2017.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chiang D.L.C., Rice D.A., Helsby N.A., Somogyi A.A., Kluger M.T. The Prevalence, Impact, and Risk Factors for Persistent Pain After Breast Cancer Surgery in a New Zealand Population. Pain Med. 2019;20:1803–1814. doi: 10.1093/pm/pnz049. [DOI] [PubMed] [Google Scholar]
- 157.Cui L., Fan P., Qiu C., Hong Y. Single institution analysis of incidence and risk factors for post-mastectomy pain syndrome. Sci. Rep. 2018;8:11494. doi: 10.1038/s41598-018-29946-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.De Groef A., Van Kampen M., Vervloesem N., De Geyter S., Christiaens M.R., Neven P., Vos L., De Vrieze T., Geraerts I., Devoogdt N. Myofascial techniques have no additional beneficial effects to a standard physical therapy programme for upper limb pain after breast cancer surgery: A randomized controlled trial. Clin. Rehabil. 2017;31:1625–1635. doi: 10.1177/0269215517708605. [DOI] [PubMed] [Google Scholar]
- 159.Dieterich M., Allmendinger S., Gerber B., Reimer T., Hartmann S., Stachs A., Stubert J. Prevalence, Clinical Significance and Risk Factors for Developing Scar Pain and Sensibility Disorders in Breast Cancer Patients after Breast-Conserving Therapy and Mastectomy. Breast Care. 2021;16:507–515. doi: 10.1159/000513241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Drury A., Payne S., Brady A.M. The cost of survival: An exploration of colorectal cancer survivors’ experiences of pain. Acta Oncol. 2017;56:205–211. doi: 10.1080/0284186X.2016.1266084. [DOI] [PubMed] [Google Scholar]
- 161.Dualé C., Ouchchane L., Schoeffler P., Dubray C. Neuropathic aspects of persistent postsurgical pain: A French multicenter survey with a 6-month prospective follow-up. J. Pain. 2014;15:e1–e24. doi: 10.1016/j.jpain.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 162.Efficace F., Breccia M., Avvisati G., Cottone F., Intermesoli T., Borlenghi E., Carluccio P., Rodeghiero F., Fabbiano F., Luppi M., et al. Health-related quality of life, symptom burden, and comorbidity in long-term survivors of acute promyelocytic leukemia. Leukemia. 2019;33:1598–1607. doi: 10.1038/s41375-018-0325-4. [DOI] [PubMed] [Google Scholar]
- 163.Engvall K., Gréen H., Fredriksson M., Åvall-Lundqvist E. Persistent neuropathy among early-stage breast cancer survivors in a population-based cohort. Br. J. Cancer. 2021;125:445–457. doi: 10.1038/s41416-021-01429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ezendam N.P.M., Pijlman B., Bhugwandass C., Pruijt J.F.M., Mols F., Vos M.C., Pijnenborg J.M.A., Van De Poll-Franse L.V. Chemotherapy-induced peripheral neuropathy and its impact on health-related quality of life among ovarian cancer survivors: Results from the population-based PROFILES registry. Gynecol. Oncol. 2014;135:510–517. doi: 10.1016/j.ygyno.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 165.Farrukh N., Hageman L., Chen Y., Wu J., Ness E., Kung M., Francisco L., Parman M., Landier W., Arora M., et al. Pain in older survivors of hematologic malignancies after blood or marrow transplantation: A BMTSS report. Cancer. 2020;126:2003–2012. doi: 10.1002/cncr.32736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Feddern M.L., Jensen T.S., Laurberg S. Chronic pain in the pelvic area or lower extremities after rectal cancer treatment and its impact on quality of life: A population-based cross-sectional study. Pain. 2015;156:1765–1771. doi: 10.1097/j.pain.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 167.Ferreira A.R., Di Meglio A., Pistilli B., Gbenou A.S., El-Mouhebb M., Dauchy S., Charles C., Joly F., Everhard S., Lambertini M., et al. Differential impact of endocrine therapy and chemotherapy on quality of life of breast cancer survivors: A prospective patient-reported outcomes analysis. Ann. Oncol. 2019;30:1784–1795. doi: 10.1093/annonc/mdz298. [DOI] [PubMed] [Google Scholar]
- 168.Gallaway M.S., Townsend J.S., Shelby D., Puckett M.C. Pain among Cancer Survivors. Prev. Chronic Dis. 2020;17:E54. doi: 10.5888/pcd17.190367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gong Y., Tan Q., Qin Q., Wei C. Prevalence of postmastectomy pain syndrome and associated risk factors: A large single-institution cohort study. Medicine. 2020;99:e19834. doi: 10.1097/MD.0000000000019834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Götze H., Taubenheim S., Dietz A., Lordick F., Mehnert A. Comorbid conditions and health-related quality of life in long-term cancer survivors-associations with demographic and medical characteristics. J. Cancer Surviv. 2018;12:712–720. doi: 10.1007/s11764-018-0708-6. [DOI] [PubMed] [Google Scholar]
- 171.Hammer S.M., Brown J.C., Segal S., Chu C.S., Schmitz K.H. Cancer-related impairments influence physical activity in uterine cancer survivors. Med. Sci. Sports Exerc. 2014;46:2195–2201. doi: 10.1249/MSS.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hamood R., Hamood H., Merhasin I., Keinan-Boker L.A. Feasibility study to assess the validity of administrative data sources and self-reported information of breast cancer survivors. Isr. J. Health Policy Res. 2016;5:50. doi: 10.1186/s13584-016-0111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hamood R., Hamood H., Merhasin I., Keinan-Boker L. Chronic pain and other symptoms among breast cancer survivors: Prevalence, predictors, and effects on quality of life. Breast Cancer Res. Treat. 2018;167:157–169. doi: 10.1007/s10549-017-4485-0. [DOI] [PubMed] [Google Scholar]
- 174.Haviland J.S., Mannino M., Griffin C., Porta N., Sydenham M., Bliss J.M., Yarnold J.R. Late normal tissue effects in the arm and shoulder following lymphatic radiotherapy: Results from the UK START (Standardisation of Breast Radiotherapy) trials. Radiother. Oncol. 2017;126:155–162. doi: 10.1016/j.radonc.2017.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Henderson J.R., Tao A., Kirwan C.C., Barr L. Immediate breast reconstruction does not increase postmastectomy pain. Ann. Surg. Oncol. 2014;21:113–117. doi: 10.1245/s10434-013-3293-y. [DOI] [PubMed] [Google Scholar]
- 176.Henry M., Alias A., Cherba M., Woronko C., Rosberger Z., Hier M., Zeitouni A., Kost K., Mlynarek A., Richardson K., et al. Immediate post-treatment supportive care needs of patients newly diagnosed with head and neck cancer. Support. Care Cancer. 2020;28:5557–5567. doi: 10.1007/s00520-020-05368-2. [DOI] [PubMed] [Google Scholar]
- 177.Hope-Stone L., Brown S.L., Heimann H., Damato B., Salmon P. Phantom Eye Syndrome: Patient Experiences after Enucleation for Uveal Melanoma. Ophthalmology. 2015;122:1585–1590. doi: 10.1016/j.ophtha.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 178.Huang I.C., Alberts N.M., Buckley M.G., Li Z., Ehrhardt M.J., Brinkman T.M., Allen J., Krull K.R., Klosky J.L., Greene W.L., et al. Change in pain status and subsequent opioid and marijuana use among long-term adult survivors of childhood cancer. JNCI Cancer Spectr. 2020;4:pkaa070. doi: 10.1093/jncics/pkaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Janah A., Bouhnik A.D., Touzani R., Bendiane M.K., Peretti-Watel P. Underprescription of Step III Opioids in French Cancer Survivors with Chronic Pain: A Call for Integrated Early Palliative Care in Oncology. J. Pain Symptom Manag. 2020;59:836–847. doi: 10.1016/j.jpainsymman.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 180.Jansen F., Eerenstein S.E.J., Lissenberg-Witte B.I., van Uden-Kraan C.F., Leemans C.R., Leeuw I.M.V.D. Unmet supportive care needs in patients treated with total laryngectomy and its associated factors. Head Neck. 2018;40:2633–2641. doi: 10.1002/hed.25358. [DOI] [PubMed] [Google Scholar]
- 181.Jardim L.C., Flores P.T., do Carmo dos Santos Araújo M., Chiesa J., de Moraes C.M.B., Antoniazzi R.P. Oral health–related quality of life in breast cancer survivors. Support. Care Cancer. 2020;28:65–71. doi: 10.1007/s00520-019-04792-3. [DOI] [PubMed] [Google Scholar]
- 182.Jariwala P., Kaur N.A. descriptive study on prevalence of arm/shoulder problems and its impact on quality of life in breast cancer survivors. Indian J. Cancer. 2021;58:201–206. doi: 10.4103/ijc.IJC_22_19. [DOI] [PubMed] [Google Scholar]
- 183.Johannsdottir I.M.R., Hamre H., Fosså S.D., Loge J.H., Drolsum L., Lund M.B., Nordsletten L., Kiserud C. Adverse Health Outcomes and Associations with Self-Reported General Health in Childhood Lymphoma Survivors. J. Adolesc. Young Adult Oncol. 2017;6:470–476. doi: 10.1089/jayao.2017.0018. [DOI] [PubMed] [Google Scholar]
- 184.Johannsen M., Christensen S., Zachariae R., Jensen A.B. Socio-demographic, treatment-related, and health behavioral predictors of persistent pain 15 months and 7–9 years after surgery: A nationwide prospective study of women treated for primary breast cancer. Breast Cancer Res. Treat. 2015;152:645–658. doi: 10.1007/s10549-015-3497-x. [DOI] [PubMed] [Google Scholar]
- 185.Juhl A.A., Christiansen P., Damsgaard T.E. Persistent pain after breast cancer treatment: A questionnaire-based study on the prevalence, associated treatment variables, and pain type. J. Breast Cancer. 2016;19:447–454. doi: 10.4048/jbc.2016.19.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Karlson C.W., Alberts N.M., Liu W., Brinkman T.M., Annett R.D., Mulrooney D.A., Schulte F., Leisenring W.M., Gibson T.M., Howell R.M., et al. Longitudinal pain and pain interference in long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2020;126:2915–2923. doi: 10.1002/cncr.32853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kaur N., Gupta A., Sharma A.K., Jain A. Survivorship issues as determinants of quality of life after breast cancer treatment: Report from a limited resource setting. Breast. 2018;41:120–126. doi: 10.1016/j.breast.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 188.Kelada L., Wakefield C.E., Heathcote L.C., Jaaniste T., Signorelli C., Fardell J.E., Donoghoe M., McCarthy M.C., Gabriel M., Cohn R.J. Perceived cancer-related pain and fatigue, information needs, and fear of cancer recurrence among adult survivors of childhood cancer. Patient Educ. Couns. 2019;102:2270–2278. doi: 10.1016/j.pec.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 189.Kibar S., Dalyan Aras M., Ünsal Delialioğlu S. The risk factors and prevalence of upper extremity impairments and an analysis of effects of lymphoedema and other impairments on the quality of life of breast cancer patients. Eur. J. Cancer Care. 2017;26:e12433. doi: 10.1111/ecc.12433. [DOI] [PubMed] [Google Scholar]
- 190.Kjær T.K., Johansen C., Andersen E., Karlsen R., Nielsen A.L., Frederiksen K., Rørth M., Ibfelt E., Dalton S.O. Influence of social factors on patient-reported late symptoms: Report from a controlled trial among long-term head and neck cancer survivors in Denmark. Head Neck. 2016;38:e1713–e1721. doi: 10.1002/hed.24306. [DOI] [PubMed] [Google Scholar]
- 191.Knowlton S.E., O’Donnell E.K., Horick N., Perez G.K., Park E., Rabin J., Quain K.M., Garton J., Peppercorn J.M. Moving forward on all fronts: Impact, patterns, and barriers to exercise in cancer survivors and patients living with advanced disease. Support. Care Cancer. 2020;28:4979–4988. doi: 10.1007/s00520-020-05344-w. [DOI] [PubMed] [Google Scholar]
- 192.Koehler L.A., Hunter D.W., Blaes A.H., Haddad T.C. Function, Shoulder Motion, Pain, and Lymphedema in Breast Cancer with and Without Axillary Web Syndrome: An 18-Month Follow-Up. Phys. Ther. 2018;98:518–527. doi: 10.1093/ptj/pzy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kramer N., Shamley D., Ramjith J. Prevalence of shoulder morbidity after treatment for breast Cancer in South Africa. Support. Care Cancer. 2019;27:2591–2598. doi: 10.1007/s00520-018-4540-3. [DOI] [PubMed] [Google Scholar]
- 194.Lou D.I., Dietrich M.S., Deng J., Murphy B.A. Mechanisms of pain and their manifestations in head and neck cancer: Importance of classifying pain subtypes. Head Neck. 2021;43:3720–3729. doi: 10.1002/hed.26859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lunde S., Petersen K.K., Søgaard-Andersen E., Arendt-Nielsen L. Preoperative quantitative sensory testing and robot-assisted laparoscopic hysterectomy for endometrial cancer: Can chronic postoperative pain be predicted? Scand. J. Pain. 2020;20:693–705. doi: 10.1515/sjpain-2020-0030. [DOI] [PubMed] [Google Scholar]
- 196.Mao H., Bao T., Shen X., Li Q., Seluzicki C., Im E.O., Mao J.J. Prevalence and risk factors for fatigue among breast cancer survivors on aromatase inhibitors. Eur. J. Cancer. 2018;101:47–54. doi: 10.1016/j.ejca.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mertz B.G., Duriaud H.M., Kroman N., Andersen K.G. Pain, sensory disturbances and psychological distress are common sequelae after treatment of ductal carcinoma in situ: A cross-sectional study. Acta Oncol. 2017;56:724–729. doi: 10.1080/0284186X.2017.1295167. [DOI] [PubMed] [Google Scholar]
- 198.Miaskowski C., Paul S.M., Cooper B., West C., Levine J.D., Elboim C., Hamolsky D., Abrams G., Luce J., Dhruva A., et al. Identification of patient subgroups and risk factors for persistent arm/shoulder pain following breast cancer surgery. Eur. J. Oncol. Nurs. 2014;18:242–253. doi: 10.1016/j.ejon.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Min J., Yoo S., Kim M.J., Yang E., Hwang S., Kang M., Yu M.S., Yoon C., Heo J.E., Choi Y., et al. Exercise participation, barriers, and preferences in Korean prostate cancer survivors. Ethn. Health. 2021;26:1130–1142. doi: 10.1080/13557858.2019.1634184. [DOI] [PubMed] [Google Scholar]
- 200.Mulrooney D.A., Hyun G., Ness K.K., Bhakta N., Pui C.H., Ehrhardt M.J., Krull K.R., Crom D.B., Chemaitilly W., Srivastava D.K., et al. The changing burden of long-term health outcomes in survivors of childhood acute lymphoblastic leukaemia: A retrospective analysis of the St Jude Lifetime Cohort Study. Lancet Haematol. 2019;6:e306–e316. doi: 10.1016/S2352-3026(19)30050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Paek J., Choi Y.J. Association between hand grip strength and impaired health-related quality of life in Korean cancer survivors: A cross-sectional study. BMJ Open. 2019;9:e030938. doi: 10.1136/bmjopen-2019-030938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Park J.W., Kim H.S., Yun J.Y., Han I. Neuropathic pain after sarcoma surgery: Prevalence and predisposing factors. Medicine. 2018;97:e10852. doi: 10.1097/MD.0000000000010852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Poço Gonçalves J., Veiga D., Araújo A. Chronic pain, functionality and quality of life in cancer survivors. Br. J. Pain. 2021;15:401–410. doi: 10.1177/2049463720972730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Reilly C.M., Esiashvili N., Parashar S., Higgins M. Subclinical Cardiovascular Disease in Lymphoma Survivors by Sex. J. Obstet. Gynecol. Neonatal Nurs. 2016;45:438–453. doi: 10.1016/j.jogn.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 205.Ren J.L., Rojo R.D., Perez J.V.D., Yeung S.C.J., Hanna E.Y., Reyes-Gibby C.C. Variations in pain prevalence, severity, and analgesic use by duration of survivorship: A cross-sectional study of 505 post-treatment head and neck cancer survivors. BMC Cancer. 2021;21:1304. doi: 10.1186/s12885-021-09024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rogers J.L., Vera E., Acquaye A., Briceno N., Jammula V., King A.L., Leeper H., Quezado M.M., Gonzalez Alarcon J., Boris L., et al. Living with a central nervous system (CNS) tumor: Findings on long-term survivorship from the NIH Natural History Study. Neurooncol. Pract. 2021;8:460–474. doi: 10.1093/nop/npab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rosenberg S.M., Stanton A.L., Petrie K.J., Partridge A.H. Symptoms and symptom attribution among women on endocrine therapy for breast cancer. Oncologist. 2015;20:598–604. doi: 10.1634/theoncologist.2015-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sanchez-Birkhead A.C., Carbajal-Salisbury S., Larreta J.A., Lovlien L., Hendricks H., Dingley C., Beck S.L. A Community-Based Approach to Assessing the Physical, Emotional, and Health Status of Hispanic Breast Cancer Survivors. Hisp. Health Care Int. 2017;15:166–172. doi: 10.1177/1540415317738016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sanford N.N., Sher D.J., Butler S.S., Xu X., Ahn C., Aizer A.A., Mahal B.A. Prevalence of chronic pain among cancer survivors in the United States, 2010–2017. Cancer. 2019;125:4310–4318. doi: 10.1002/cncr.32450. [DOI] [PubMed] [Google Scholar]
- 210.Schou Bredal I., Smeby N.A., Ottesen S., Warncke T., Schlichting E. Chronic pain in breast cancer survivors: Comparison of psychosocial, surgical, and medical characteristics between survivors with and without pain. J. Pain Symptom Manag. 2014;48:852–862. doi: 10.1016/j.jpainsymman.2013.12.239. [DOI] [PubMed] [Google Scholar]
- 211.Selvy M., Pereira B., Kerckhove N., Gonneau C., Feydel G., Pétorin C., Vimal-Baguet A., Melnikov S., Kullab S., Hebbar M., et al. Long-term prevalence of sensory chemotherapy-induced peripheral neuropathy for 5 years after adjuvant folfox chemotherapy to treat colorectal cancer: A multicenter cross-sectional study. J. Clin. Med. 2020;9:2400. doi: 10.3390/jcm9082400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Steyaert A., Forget P., Dubois V., Lavand’homme P., De Kock M., Lavand’homme P. Does the perioperative analgesic/anesthetic regimen influence the prevalence of long-term chronic pain after mastectomy? J. Clin. Anesth. 2016;33:20–25. doi: 10.1016/j.jclinane.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 213.Terkawi A.S., Tsang S., Alshehri A.S., Mulafikh D.S., Alghulikah A.A., AlDhahri S.F. The burden of chronic pain after major head and neck tumor therapy. Saudi J. Anaesth. 2017;11:s71–s79. doi: 10.4103/sja.SJA_162_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tonning Olsson I., Alberts N.M., Li C., Ehrhardt M.J., Mulrooney D.A., Liu W., Pappo A.S., Bishop M.W., Anghelescu D.L., Srivastava D., et al. Pain and functional outcomes in adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort study. Cancer. 2021;127:1679–1689. doi: 10.1002/cncr.33303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tung S., Davis L.E., Hallet J., Mavros M.N., Mahar A.L., Bubis L.D., Hammad A., Zhao H., Earle C.C., Barbera L., et al. Population-Level Symptom Assessment Following Pancreaticoduodenectomy for Adenocarcinoma. JAMA Surg. 2019;154:e193348. doi: 10.1001/jamasurg.2019.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.van de Luijtgaarden A.C.M., Kapusta L., Bellersen L., Bokkerink J.P.M., Kaal S.E.J., Versleijen-Jonkers Y.M.H., Schreuder H.W.B., van der Graaf W.T.A. High prevalence of late adverse events in malignant bone tumour survivors diagnosed at adult age. Neth. J. Med. 2014;72:516–522. [PubMed] [Google Scholar]
- 217.van Eck I., Den Hollander D., Desar I.M.E., Soomers V.L.M.N., van de Sande M.A.J., de Haan J.J., Verhoef C., Vriens I.J.H., Bonenkamp J.J., van der Graaf W.T.A., et al. Unraveling the heterogeneity of sarcoma survivors’ health-related quality of life regarding primary sarcoma location: Results from the Survsarc study. Cancers. 2020;12:3083. doi: 10.3390/cancers12113083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Variawa M.L., Scribante J., Perrie H., Chetty S. The prevalence of chronic postmastectomy pain syndrome in female breast cancer survivors. S. Afr. J. Anaesth. Analg. 2016;22:108–113. doi: 10.1080/22201181.2016.1191214. [DOI] [Google Scholar]
- 219.Vuksanovic D., Sanmugarajah J., Lunn D., Sawhney R., Eu K., Liang R. Unmet needs in breast cancer survivors are common, and multidisciplinary care is underutilised: The Survivorship Needs Assessment Project. Breast Cancer. 2021;28:289–297. doi: 10.1007/s12282-020-01156-2. [DOI] [PubMed] [Google Scholar]
- 220.Wilson C.L., Brinkman T.M., Cook C., Huang S., Hyun G., Green D.M., Furman W.L., Bhakta N., Ehrhardt M.J., Krasin M.J., et al. Clinically ascertained health outcomes, quality of life, and social attainment among adult survivors of neuroblastoma: A report from the St. Jude Lifetime Cohort. Cancer. 2020;126:1330–1338. doi: 10.1002/cncr.32678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Aktas A., Walsh D., Hu B. Cancer symptom clusters: An exploratory analysis of eight statistical techniques. J. Pain Symptom Manag. 2014;48:1254–1266. doi: 10.1016/j.jpainsymman.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 222.Corli O., Pellegrini G., Bosetti C., Riva L., Crippa M., Amodio E., Scaccabarozzi G. Impact of palliative care in evaluating and relieving symptoms in patients with advanced cancer. Results from the demetra study. Int. J. Environ. Res. Public Health. 2020;17:8429. doi: 10.3390/ijerph17228429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.de la Cruz M., Noguera A., San Miguel-Arregui M.T., Williams J., Chisholm G., Bruera E. Delirium, agitation, and symptom distress within the final seven days of life among cancer patients receiving hospice care. Palliat. Support. Care. 2015;13:211–216. doi: 10.1017/S1478951513001144. [DOI] [PubMed] [Google Scholar]
- 224.Drat-Gzubicka J., Pyszora A., Budzyński J., Currow D., Krajnik M. Is neuropathic pain a good marker of peripheral neuropathy in hospice patients with advanced cancer? The single center pilot study. Diagnostics. 2021;11:1377. doi: 10.3390/diagnostics11081377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gupta M., Sahi M.S., Bhargava A.K., Talwar V. A Prospective Evaluation of Symptom Prevalence and Overall Symptom Burden among Cohort of Critically Ill Cancer Patients. Indian J. Palliat. Care. 2016;22:118–124. doi: 10.4103/0973-1075.179601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Guthrie D.M., Harman L.E., Barbera L., Burge F., Lawson B., McGrail K., Sutradhar R., Seow H. Quality Indicator Rates for Seriously Ill Home Care Clients: Analysis of Resident Assessment Instrument for Home Care Data in Six Canadian Provinces. J. Palliat. Med. 2019;22:1346–1356. doi: 10.1089/jpm.2019.0022. [DOI] [PubMed] [Google Scholar]
- 227.Hui D., Abdelghani E., Chen J., Dibaj S., Zhukovsky D., Dev R., Tanco K., Haider A., Azhar A., Reddy A., et al. Chronic non-malignant pain in patients with cancer seen at a timely outpatient palliative care clinic. Cancers. 2020;12:214. doi: 10.3390/cancers12010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mejin M., Keowmani T., Abdul Rahman S., Liew J., Lai J., Chua M., Ilmiyah C.W. Prevalence of pain and treatment outcomes among cancer patients in a Malaysian palliative care unit. Pharm. Pract. 2019;17:1–9. doi: 10.18549/PharmPract.2019.1.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mercadante S., Masedu F., Balzani I., De Giovanni D., Montanari L., Pittureri C., Bertè R., Russo D., Ursini L., Marinangeli F., et al. Prevalence of delirium in advanced cancer patients in home care and hospice and outcomes after 1 week of palliative care. Support. Care Cancer. 2018;26:913–919. doi: 10.1007/s00520-017-3910-6. [DOI] [PubMed] [Google Scholar]
- 230.Morita T., Sato K., Miyashita M., Yamagishi A., Kizawa Y., Shima Y., Kinoshita H., Suzuki S., Shirahige Y., Yamaguchi T., et al. Does a regional comprehensive palliative care program improve pain in outpatient cancer patients? Support. Care Cancer. 2014;22:2445–2455. doi: 10.1007/s00520-014-2232-1. [DOI] [PubMed] [Google Scholar]
- 231.Rojas-Concha L., Hansen M.B., Petersen M.A., Groenvold M. Which symptoms and problems do advanced cancer patients admitted to specialized palliative care report in addition to those included in the EORTC QLQ-C15-PAL? A register-based national study. Support. Care Cancer. 2020;28:1725–1735. doi: 10.1007/s00520-019-04976-x. [DOI] [PubMed] [Google Scholar]
- 232.Seow H., Guthrie D.M., Stevens T., Barbera L.C., Burge F., McGrail K., Chan K.K.W., Peacock S.J., Sutradhar R. Trajectory of End-of-Life Pain and Other Physical Symptoms among Cancer Patients Receiving Home Care. Curr. Oncol. 2021;28:1641–1651. doi: 10.3390/curroncol28030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Silvia A.P., Adriana P.N., Laura G.G., Edith M.C., Emma V.A. Reality, Delays, and Challenges within Pain Prevalence and Treatment in Palliative Care Patients: A Survey of First-Time Patients at the National Cancer Institute in Mexico. J. Palliat. Care. 2021;36:181–187. doi: 10.1177/0825859719861946. [DOI] [PubMed] [Google Scholar]
- 234.Tofthagen C., Visovsky C., Dominic S., McMillan S. Neuropathic symptoms, physical and emotional well-being, and quality of life at the end of life. Support. Care Cancer. 2019;27:3357–3364. doi: 10.1007/s00520-018-4627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yamagishi A., Sato K., Miyashita M., Shima Y., Kizawa Y., Umeda M., Kinoshita H., Shirahige Y., Akiyama M., Yamaguchi T., et al. Changes in quality of care and quality of life of outpatients with advanced cancer after a regional palliative care intervention program. J. Pain Symptom Manag. 2014;48:602–610. doi: 10.1016/j.jpainsymman.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 236.Yanaizumi R., Nagamine Y., Harada S., Kojima K., Tazawa T., Goto T. Prevalence of neuropathic pain in terminally ill patients with cancer admitted to a general ward: A prospective observational study. J. Int. Med. Res. 2021;49:1–10. doi: 10.1177/0300060520987726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Aguilar M.L., Sandow P., Werning J.W., Brenneman L., Psoter W.J. The Head and Neck Cancer Patient Concern Inventory(©): Patient Concerns’ Prevalence, Dental Concerns’ Impact, and Relationships of Concerns with Quality of Life Measures. J. Prosthodont. 2017;26:186–195. doi: 10.1111/jopr.12496. [DOI] [PubMed] [Google Scholar]
- 238.Alemayehu M., Deyessa N., Medihin G., Fekadu A. A descriptive analysis of depression and pain complaints among patients with cancer in a low income country. PLoS ONE. 2018;13:e0193713. doi: 10.1371/journal.pone.0193713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Armstrong T.S., Vera-Bolanos E., Acquaye A.A., Gilbert M.R., Ladha H., Mendoza T. The symptom burden of primary brain tumors: Evidence for a core set of tumor- and treatment-related symptoms. Neuro-Oncology. 2016;18:252–260. doi: 10.1093/neuonc/nov166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bacorro W.R., Sy Ortin T.T., Suarez C.G., Mendoza T.R., Que J.C. Validation of the MD Anderson Symptom Inventory-Head-and-Neck-Filipino (MDASI-HN-F): Clinical utility of symptom screening among patients with head-and-neck cancer. BMJ Support. Palliat. Care. 2017;7:140–149. doi: 10.1136/bmjspcare-2014-000787. [DOI] [PubMed] [Google Scholar]
- 241.Batalini F., Gomes M., Fábio I., Kuwae F., Macanhan G., Pereira J.L.B. Cancer complaints: The profile of patients from the emergency department of a Brazilian oncology teaching hospital. F1000Research. 2017;6:1919.3. doi: 10.12688/f1000research.12632.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bauml J., Chen L., Chen J., Boyer J., Kalos M., Li S.Q., DeMichele A., Mao J.J. Arthralgia among women taking aromatase inhibitors: Is there a shared inflammatory mechanism with co-morbid fatigue and insomnia? Breast Cancer Res. 2015;17:89. doi: 10.1186/s13058-015-0599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Beesley V.L., Wockner L.F., O’Rourke P., Janda M., Goldstein D., Gooden H., Merrett N.D., O’Connell D.L., Rowlands I.J., Wyld D.K., et al. Risk factors for current and future unmet supportive care needs of people with pancreatic cancer. A longitudinal study. Support. Care Cancer. 2016;24:3589–3599. doi: 10.1007/s00520-016-3212-4. [DOI] [PubMed] [Google Scholar]
- 244.Bhattacharya I.S., Haviland J.S., Kirby A.M., Kirwan C.C., Hopwood P., Yarnold J.R., Bliss J.M., Coles C.E. Patient-Reported Outcomes Over 5 Years After Whole- or Partial-Breast Radiotherapy: Longitudinal Analysis of the IMPORT LOW (CRUK/06/003) Phase III Randomized Controlled Trial. J. Clin. Oncol. 2019;37:305–317. doi: 10.1200/JCO.18.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bonhof C.S., Mols F., Vos M.C., Pijnenborg J.M.A., Boll D., Vreugdenhil G., Ezendam N.P.M., van de Poll-Franse L.V. Course of chemotherapy-induced peripheral neuropathy and its impact on health-related quality of life among ovarian cancer patients: A longitudinal study. Gynecol. Oncol. 2018;149:455–463. doi: 10.1016/j.ygyno.2018.03.052. [DOI] [PubMed] [Google Scholar]
- 246.Bouhassira D., Luporsi E., Krakowski I. Prevalence and incidence of chronic pain with or without neuropathic characteristics in patients with cancer. Pain. 2017;158:1118–1125. doi: 10.1097/j.pain.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 247.Boyes A.W., Clinton-McHarg T., Waller A.E., Steele A., D’Este C.A., Sanson-Fisher R.W. Prevalence and correlates of the unmet supportive care needs of individuals diagnosed with a haematological malignancy. Acta Oncol. 2015;54:507–514. doi: 10.3109/0284186X.2014.958527. [DOI] [PubMed] [Google Scholar]
- 248.Braamse A.M.J., Van Meijel B., Visser O., Huijgens P.C., Beekman A.T.F., Dekker J. Distress, problems and supportive care needs of patients treated with auto-or allo-SCT. Bone Marrow Transplant. 2014;49:292–298. doi: 10.1038/bmt.2013.155. [DOI] [PubMed] [Google Scholar]
- 249.Braga-Neto M.B., Carneiro J.G., de Castro Barbosa A.M., Silva I.S., Maia D.C., Maciel F.S., de Alcântara R.J.A., Vasconscelos P.R.L., Braga L.L.B.C., de Alcântara R.J.A. Clinical characteristics of distal gastric cancer in young adults from Northeastern Brazil. BMC Cancer. 2018;18:131. doi: 10.1186/s12885-018-3995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bubis L.D., Davis L.E., Canaj H., Gupta V., Jeong Y., Barbera L., Li Q., Moody L., Karanicolas P.J., Sutradhar R., et al. Patient-Reported Symptom Severity Among 22,650 Cancer Outpatients in the Last Six Months of Life. J. Pain Symptom Manag. 2020;59:58–66.e4. doi: 10.1016/j.jpainsymman.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 251.Buckley S.A., Jimenez-Sahagun D., Othus M., Walter R.B., Lee S.J., Jimenez-Sahagun D. Quality of life from the perspective of the patient with acute myeloid leukemia. Cancer. 2018;124:145–152. doi: 10.1002/cncr.30982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Cho S.F., Rau K.M., Shao Y.Y., Yen C.J., Wu M.F., Chen J.S., Chang C.S., Yeh S.P., Chiou T.J., Hsieh R.K., et al. Patients with head and neck cancer may need more intensive pain management to maintain daily functioning: A multi-center study. Support. Care Cancer. 2019;27:1663–1672. doi: 10.1007/s00520-018-4404-x. [DOI] [PubMed] [Google Scholar]
- 253.Clover K.A., Rogers K.M., Britton B., Oldmeadow C., Attia J., Carter G.L. Reduced prevalence of pain and distress during 4 years of screening with QUICATOUCH in Australian oncology patients. Eur. J. Cancer Care. 2017;26:e12636. doi: 10.1111/ecc.12636. [DOI] [PubMed] [Google Scholar]
- 254.Daly L.E., Dolan R.D., Power D.G., Ní Bhuachalla É., Sim W., Cushen S.J., Fallon M., Simmons C., McMillan D.C., Laird B.J., et al. Determinants of quality of life in patients with incurable cancer. Cancer. 2020;126:2872–2882. doi: 10.1002/cncr.32824. [DOI] [PubMed] [Google Scholar]
- 255.Davies A., Buchanan A., Todd J., Gregory A., Batsari K.M. Oral symptoms in patients with advanced cancer: An observational study using a novel oral symptom assessment scale. Support. Care Cancer. 2021;29:4357–4364. doi: 10.1007/s00520-020-05903-1. [DOI] [PubMed] [Google Scholar]
- 256.Davis L.E., Bubis L.D., Mahar A.L., Li Q., Sussman J., Moody L., Barbera L., Holloway C.M.B., Coburn N.G. Patient-reported symptoms after breast cancer diagnosis and treatment: A retrospective cohort study. Eur. J. Cancer. 2018;101:1–11. doi: 10.1016/j.ejca.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 257.De Groef A., Van Kampen M., Tieto E., Schönweger P., Christiaens M.R., Neven P., Geraerts I., Gebruers N., Devoogdt N. Arm lymphoedema and upper limb impairments in sentinel node-negative breast cancer patients: A one year follow-up study. Breast. 2016;29:102–108. doi: 10.1016/j.breast.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 258.De Mello M.R.S.P., Moura S.F., Muzi C.D., Guimarães R.M. Clinical evaluation and pattern of symptoms in colorectal cancer patients. Arq. Gastroenterol. 2020;57:131–136. doi: 10.1590/s0004-2803.202000000-24. [DOI] [PubMed] [Google Scholar]
- 259.De Oliveira G.S., Jr., Chang R., Khan S.A., Hansen N.M., Khan J.H., McCarthy R.J., Apkarian A.V. Factors associated with the development of chronic pain after surgery for breast cancer: A prospective cohort from a tertiary center in the United States. Breast J. 2014;20:9–14. doi: 10.1111/tbj.12207. [DOI] [PubMed] [Google Scholar]
- 260.Deshields T.L., Potter P., Olsen S., Liu J. The persistence of symptom burden: Symptom experience and quality of life of cancer patients across one year. Support. Care Cancer. 2014;22:1089–1096. doi: 10.1007/s00520-013-2049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Deshields T.L., Penalba V., Liu J., Avery J. Comparing the symptom experience of cancer patients and non-cancer patients. Support. Care Cancer. 2017;25:1103–1109. doi: 10.1007/s00520-016-3498-2. [DOI] [PubMed] [Google Scholar]
- 262.Dhingra L.K., Lam K., Cheung W., Shao T., Li Z., Van de Maele S., Chang V.T., Chen J., Ye H., Wong R., et al. Variation in symptom distress in underserved Chinese American cancer patients. Cancer. 2015;121:3352–3359. doi: 10.1002/cncr.29497. [DOI] [PubMed] [Google Scholar]
- 263.Donovan K.A., Boyington A.R., Judson P.L., Wyman J.F. Bladder and bowel symptoms in cervical and endometrial cancer survivors. Psychooncology. 2014;23:672–678. doi: 10.1002/pon.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Doubova S.V., Pérez-Cuevas R. Association of supportive care needs and quality of patient-centered cancer care with depression in women with breast and cervical cancer in Mexico. Psychooncology. 2021;30:591–601. doi: 10.1002/pon.5608. [DOI] [PubMed] [Google Scholar]
- 265.Feiten S., Dünnebacke J., Heymanns J., Köppler H., Thomalla J., van Roye C., Wey D., Weide R. Breast cancer morbidity: Questionnaire survey of patients on the long term effects of disease and adjuvant therapy. Dtsch. Ärztebl. Int. 2014;111:537–544. doi: 10.3238/arztebl.2014.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Fokdal L., Pötter R., Kirchheiner K., Lindegaard J.C., Jensen N.B.K., Kirisits C., Chargari C., Mahantshetty U., Jürgenliemk-Schulz I.M., Segedin B., et al. Physician assessed and patient reported urinary morbidity after radio-chemotherapy and image guided adaptive brachytherapy for locally advanced cervical cancer. Radiother. Oncol. 2018;127:423–430. doi: 10.1016/j.radonc.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 267.Giuliani M.E., Milne R.A., Puts M., Sampson L.R., Kwan J.Y.Y., Le L.W., Alibhai S.M.H., Howell D., Abdelmutti N., Liu G., et al. The prevalence and nature of supportive care needs in lung cancer patients. Curr. Oncol. 2016;23:258–265. doi: 10.3747/co.23.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Götze H., Köhler N., Taubenheim S., Lordick F., Mehnert A. Polypharmacy, limited activity, fatigue and insomnia are the most frequent symptoms and impairments in older hematological cancer survivors (70+): Findings from a register-based study on physical and mental health. J. Geriatr. Oncol. 2019;10:55–59. doi: 10.1016/j.jgo.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 269.Gough N., Koffman J., Ross J.R., Riley J., Judson I. Symptom Burden in Advanced Soft-Tissue Sarcoma. J. Pain Symptom Manag. 2017;53:588–597. doi: 10.1016/j.jpainsymman.2016.10.357. [DOI] [PubMed] [Google Scholar]
- 270.Guedes T.S.R., Dantas de Oliveira N.P., Holanda A.M., Reis M.A., Silva C.P., Rocha e Silva B.L., Cancela M.C., de Souza D.L.B. Body Image of Women Submitted to Breast Cancer Treatment. Asian Pac. J. Cancer Prev. 2018;19:1487–1493. doi: 10.22034/APJCP.2018.19.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Honkanen N., Mustonen L., Kalso E., Meretoja T., Harno H. Breast reconstruction after breast cancer surgery—Persistent pain and quality of life 1–8 years after breast reconstruction. Scand. J. Pain. 2021;21:522–529. doi: 10.1515/sjpain-2021-0026. [DOI] [PubMed] [Google Scholar]
- 272.Horick N.K., Muzikansky A., Gutierrez H.L., Boyd K.L., Finkelstein D.M. Physical symptoms in long-term survivors of rare cancer. J. Cancer Surviv. 2018;12:835–842. doi: 10.1007/s11764-018-0721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Huang W., Yang J., Liu Y., Liu C., Zhang X., Fu W., Shi L., Liu G. Assessing health-related quality of life of patients with colorectal cancer using EQ-5D-5L: A cross-sectional study in Heilongjiang of China. BMJ Open. 2018;8:e022711. doi: 10.1136/bmjopen-2018-022711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Hunnicutt J.N., Tjia J., Lapane K.L. Hospice Use and Pain Management in Elderly Nursing Home Residents with Cancer. J. Pain Symptom Manag. 2017;53:561–570. doi: 10.1016/j.jpainsymman.2016.10.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jesdale B.M., Mack D.S., Forrester S.N., Lapane K.L. Cancer Pain in Relation to Metropolitan Area Segregation and Nursing Home Racial and Ethnic Composition. J. Am. Med. Dir. Assoc. 2020;21:1302–1308.e7. doi: 10.1016/j.jamda.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Jewett P.I., Teoh D., Petzel S., Lee H., Messelt A., Kendall J., Hatsukami D., Everson-Rose S.A., Blaes A.H., Vogel R.I. Cancer-Related Distress: Revisiting the Utility of the National Comprehensive Cancer Network Distress Thermometer Problem List in Women with Gynecologic Cancers. JCO Oncol. Pract. 2020;16:e649–e659. doi: 10.1200/JOP.19.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Karawekpanyawong N., Kaewkitikul K., Maneeton B., Maneeton N., Siriaree S. The prevalence of depressive disorder and its association in Thai cervical cancer patients. PLoS ONE. 2021;16:e0252779. doi: 10.1371/journal.pone.0252779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Khan S.Z., Nofil S., Arif A., Jan M.M.I.R., Riaz B., Sherwani N.Z.F.K. Frequency and Risk Factors for Post Mastectomy Pain Syndrome [PMPS] in Female Breast Cancer patients. Pak. J. Med. Health Sci. 2021;15:2530–2533. [Google Scholar]
- 279.Kim S.H., Jo M.W., Lee J.W., Lee H.J., Kim J.K. Validity and reliability of EQ-5D-3L for breast cancer patients in Korea. Health Qual. Life Outcomes. 2015;13:203. doi: 10.1186/s12955-015-0399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kim S.R., Shin Y.S., Kim J.H., Choi M., Yoo S.H. Differences in Type Composition of Symptom Clusters as Predictors of Quality of Life in Patients with Meningioma and Glioma. World Neurosurg. 2017;98:50–59. doi: 10.1016/j.wneu.2016.10.085. [DOI] [PubMed] [Google Scholar]
- 281.Lefkowits C., Rabow W.M., Sherman A.E., Kiet T.K., Ruskin R., Chan J.K., Chen L.M. Predictors of high symptom burden in gynecologic oncology outpatients: Who should be referred to outpatient palliative care? Gynecol. Oncol. 2014;132:698–702. doi: 10.1016/j.ygyno.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 282.Liu S., Sun Y., Louie W. Symptom Distress and Its Association with Traditional Chinese Medicine Use in Chinese American Women with Cancer. Oncol. Nurs. Forum. 2015;42:e24–e32. doi: 10.1188/15.ONF.E24-E32. [DOI] [PubMed] [Google Scholar]
- 283.Loh K.P., Zittel J., Kadambi S., Pandya C., Xu H., Flannery M., Magnuson A., Bautista J., McHugh C., Mustian K., et al. Elucidating the associations between sleep disturbance and depression, fatigue, and pain in older adults with cancer. J. Geriatr. Oncol. 2018;9:464–468. doi: 10.1016/j.jgo.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 284.Lu F., Song L., Xie T., Tian J., Fan Y., Liu H. Current Status of Malignant Neuropathic Pain in Chinese Patients with Cancer: Report of a Hospital-based Investigation of Prevalence, Etiology, Assessment, and Treatment. Pain Pract. 2017;17:88–98. doi: 10.1111/papr.12422. [DOI] [PubMed] [Google Scholar]
- 285.Mavros M.N., Davis L.E., Hallet J., Tung S., Mahar A.L., Bubis L.D., Hammad A., Zhao H., Earle C.C., Barbera L., et al. Symptom Burden of Nonresected Pancreatic Adenocarcinoma: An Analysis of 10,753 Patient-Reported Outcome Assessments. Pancreas. 2020;49:1083–1089. doi: 10.1097/MPA.0000000000001629. [DOI] [PubMed] [Google Scholar]
- 286.Mejdahl M.K., Mertz B.G., Bidstrup P.E., Andersen K.G. Preoperative distress predicts persistent pain after breast cancer treatment: A prospective cohort study. J. Natl. Compr. Cancer Netw. 2015;13:995–1003. doi: 10.6004/jnccn.2015.0120. [DOI] [PubMed] [Google Scholar]
- 287.Nakanotani T., Akechi T., Takayama T., Karato A., Kikuuchi Y., Okamoto N., Katayama K., Yokoo M., Ogawa A. Characteristics of elderly cancer patients’ concerns and their quality of life in Japan: A Web-based survey. Jpn J. Clin. Oncol. 2014;44:448–455. doi: 10.1093/jjco/hyu029. [DOI] [PubMed] [Google Scholar]
- 288.Nappi R.E., Palacios S., Bruyniks N., Particco M., Panay N. The European Vulvovaginal Epidemiological Survey (EVES). Impact of history of breast cancer on prevalence, symptoms, sexual function and quality of life related to vulvovaginal atrophy. Gynecol. Endocrinol. 2021;37:78–82. doi: 10.1080/09513590.2020.1813273. [DOI] [PubMed] [Google Scholar]
- 289.Oosterling A., te Boveldt N., Verhagen C., van der Graaf W.T., Van Ham M., Van der Drift M., Vissers K., Engels Y. Neuropathic Pain Components in Patients with Cancer: Prevalence, Treatment, and Interference with Daily Activities. Pain Pract. 2016;16:413–421. doi: 10.1111/papr.12291. [DOI] [PubMed] [Google Scholar]
- 290.Pandya C., Magnuson A., Flannery M., Zittel J., Duberstein P., Loh K.P., Ramsdale E., Gilmore N., Dale W., Mohile S.G. Association Between Symptom Burden and Physical Function in Older Patients with Cancer. J. Am. Geriatr. Soc. 2019;67:998–1004. doi: 10.1111/jgs.15864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Pinto E., Gonçalves F., Sacarlal J., Castro L., Rego G. Pain management in cancer patients in the main hospitals in Mozambique. Ann. Palliat. Med. 2021;10:4069–4079. doi: 10.21037/apm-20-2009. [DOI] [PubMed] [Google Scholar]
- 292.Ramsenthaler C., Osborne T.R., Wei G., Siegert R.J., Edmonds P.M., Schey S.A., Higginson I.J., Gao W. The impact of disease-related symptoms and palliative care concerns on health-related quality of life in multiple myeloma: A multi-centre study. BMC Cancer. 2016;16:1–16. doi: 10.1186/s12885-016-2410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Rau K.M., Chen J.S., Wu H.B., Lin S.F., Lai M.K., Chow J.M., Huang M.L., Wang C.J., Tai C.J., Hwang W.L., et al. The impact of pain control on physical and psychiatric functions of cancer patients: A nation-wide survey in Taiwan. Jpn J. Clin. Oncol. 2015;45:1042–1049. doi: 10.1093/jjco/hyv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Reuter Q., Marshall A., Zaidi H., Sista P., Powell E.S., McCarthy D.M., Dresden S.M. Emergency Department-Based Palliative Interventions: A Novel Approach to Palliative Care in the Emergency Department. J. Palliat. Med. 2019;22:649–655. doi: 10.1089/jpm.2018.0341. [DOI] [PubMed] [Google Scholar]
- 295.Rosario C.O., Puts M., Jang R., Bezjak A., Yokom D., Alibhai S.M.H. Exploring the geriatric needs of oncology inpatients at an academic cancer centre. J. Geriatr. Oncol. 2019;10:824–828. doi: 10.1016/j.jgo.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 296.Saarelainen L.K., Turner J.P., Shakib S., Singhal N., Hogan-Doran J., Prowse R., Johns S., Lees J., Bell J.S. Potentially inappropriate medication use in older people with cancer: Prevalence and correlates. J. Geriatr. Oncol. 2014;5:439–446. doi: 10.1016/j.jgo.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 297.Sager Z.S., Wachen J.S., Naik A.D., Moye J. Post-Traumatic Stress Disorder Symptoms from Multiple Stressors Predict Chronic Pain in Cancer Survivors. J. Palliat. Med. 2020;23:1191–1197. doi: 10.1089/jpm.2019.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Sakurai H., Miyashita M., Imai K., Miyamoto S., Otani H., Oishi A., Kizawa Y., Matsushima E. Validation of the Integrated Palliative care Outcome Scale (IPOS)—Japanese Version. Jpn. J. Clin. Oncol. 2019;49:257–262. doi: 10.1093/jjco/hyy203. [DOI] [PubMed] [Google Scholar]
- 299.Sampogna F., Abeni D., Gieler U., Tomas-Aragones L., Lien L., Poot F., Jemec G.B.E., Szabo C., Linder D., Van Middendorp H., et al. Exploring the EQ-5D dimension of pain/discomfort in dermatology outpatients from a multicentre study in 13 European countries. Acta Derm. Venereol. 2020;100:1–6. doi: 10.2340/00015555-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Sánchez Sánchez E., González Baena A.C., González Cáliz C., Caballero Paredes F., Moyano Calvo J.L., Castiñeiras Fernández J. Prevalence of Anxiety and Depression in Prostate Cancer Patients and Their Spouses: An Unaddressed Reality. Prostate Cancer. 2020;2020:4393175. doi: 10.1155/2020/4393175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Schreiber K.L., Zinboonyahgoon N., Flowers K.M., Hruschak V., Fields K.G., Patton M.E., Schwartz E., Azizoddin D., Soens M., King T., et al. Prediction of Persistent Pain Severity and Impact 12 Months After Breast Surgery Using Comprehensive Preoperative Assessment of Biopsychosocial Pain Modulators. Ann. Surg. Oncol. 2021;28:5015–5038. doi: 10.1245/s10434-020-09479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Shen W.C., Chen J.S., Shao Y.Y., Lee K.D., Chiou T.J., Sung Y.C., Rau K.M., Yen C.J., Liao Y.M., Liu T.C., et al. Impact of Undertreatment of Cancer Pain with Analgesic Drugs on Patient Outcomes: A Nationwide Survey of Outpatient Cancer Patient Care in Taiwan. J. Pain Symptom Manag. 2017;54:55–65.e1. doi: 10.1016/j.jpainsymman.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 303.Sloan J.A., Cheville A.L., Liu H., Novotny P.J., Wampfler J.A., Garces Y.I., Clark M.M., Yang P. Impact of self-reported physical activity and health promotion behaviors on lung cancer survivorship. Health Qual. Life Outcomes. 2016;14:66. doi: 10.1186/s12955-016-0461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Smith T.G., Troeschel A.N., Castro K.M., Arora N.K., Stein K., Lipscomb J., Brawley O.W., McCabe R.M., Clauser S.B., Ward E. Perceptions of Patients with Breast and Colon Cancer of the Management of Cancer-Related Pain, Fatigue, and Emotional Distress in Community Oncology. J. Clin. Oncol. 2019;37:1666–1676. doi: 10.1200/JCO.18.01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Tadele N. Evaluation of quality of life of adult cancer patients attending Tikur Anbessa specialized referral hospital, Addis Ababa Ethiopia. Ethiop. J. Health Sci. 2015;25:53–62. doi: 10.4314/ejhs.v25i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Tegegn H.G., Gebreyohannes E.A. Cancer Pain Management and Pain Interference with Daily Functioning among Cancer Patients in Gondar University Hospital. Pain Res. Manag. 2017;2017:5698640. doi: 10.1155/2017/5698640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tjong M.C., Doherty M., Tan H., Chan W.C., Zhao H., Hallet J., Darling G., Kidane B., Wright F.C., Mahar A., et al. Province-Wide Analysis of Patient-Reported Outcomes for Stage IV Non-Small Cell Lung Cancer. Oncologist. 2021;26:e1800–e1811. doi: 10.1002/onco.13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Tung S., Coburn N.G., Davis L.E., Mahar A.L., Myrehaug S., Zhao H., Earle C.C., Nathens A., Hallet J. Population-based study of the prevalence and management of self-reported high pain scores in patients with non-resected pancreatic adenocarcinoma. Br. J. Surg. 2019;106:1666–1675. doi: 10.1002/bjs.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Valery P.C., Bernardes C.M., Beesley V., Hawkes A.L., Baade P., Garvey G. Unmet supportive care needs of Australian Aboriginal and Torres Strait Islanders with cancer: A prospective, longitudinal study. Support. Care Cancer. 2017;25:869–877. doi: 10.1007/s00520-016-3475-9. [DOI] [PubMed] [Google Scholar]
- 310.Van Arsdale A., Rosenbaum D., Kaur G., Pinto P., Kuo D.Y.S., Barrera R., Goldberg G.L., Nevadunsky N.S. Prevalence and factors associated with cognitive deficit in women with gynecologic malignancies. Gynecol. Oncol. 2016;141:323–328. doi: 10.1016/j.ygyno.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 311.van den Brekel L., van der Baan F.H., Zweers D., Koldenhof J.J., Vos J.B.H., de Graeff A., Witteveen P.O., Teunissen S.C.C.M. Predicting Anxiety in Hospitalized Cancer Patients. J. Pain Symptom Manag. 2020;60:522–530.e1. doi: 10.1016/j.jpainsymman.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 312.Vistad I., Cvancarova M., Lindviksmoen Astrup G., Rustøen T., Liavaag A.H. Symptom Experience and Self-rated Physical Functioning in Patients with Ovarian Cancer Receiving Chemotherapy: A Longitudinal Study. Int. J. Gynecol. Cancer. 2018;28:1167–1175. doi: 10.1097/IGC.0000000000001287. [DOI] [PubMed] [Google Scholar]
- 313.von Gruenigen V.E., Huang H.Q., Cella D., Zevon M., LaChance J.A., Walker J.L., Salani R., Modesitt S.C., Morris R.T., Bradley W.H., et al. Quality of life, symptoms and care needs in patients with persistent or recurrent platinum-resistant ovarian cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2018;150:119–126. doi: 10.1016/j.ygyno.2018.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Williams G.R., Al-Obaidi M., Dai C., Mir N., Challa S.A., Daniel M., Patel H., Barlow B., Young-Smith C., Gbolahan O., et al. Association of malnutrition with geriatric assessment impairments and health-related quality of life among older adults with gastrointestinal malignancies. Cancer. 2020;126:5147–5155. doi: 10.1002/cncr.33122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Xu N., Li Z., Wei F., Liu X., Jiang L., Meng N., Jiang P., Yu M., Wu F., Dang L., et al. A Cross-sectional Study on the Symptom Burden of Patients with Spinal Tumor: Validation of the Chinese Version of the M.D. Anderson Symptom Inventory—Spine Tumor Module. J. Pain Symptom Manag. 2017;53:605–613. doi: 10.1016/j.jpainsymman.2016.10.360. [DOI] [PubMed] [Google Scholar]
- 316.Shrestha S., Kc B., Blebil A.Q., Teoh S.L. Pharmacist Involvement in Cancer Pain Management: A Systematic Review and Meta-Analysis. J. Pain. 2022;23:1123–1142. doi: 10.1016/j.jpain.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 317.Jho H.J., Kim Y., Kong K.A., Kim D.H., Choi J.Y., Nam E.J., Choi J.Y., Koh S., Hwang K.O., Baek S.K., et al. Knowledge, practices, and perceived barriers regarding cancer pain management among physicians and nurses in Korea: A nationwide multicenter survey. PLoS ONE. 2014;9:e105900. doi: 10.1371/journal.pone.0105900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Silbermann M., Calimag M.M., Eisenberg E., Futerman B., Fernandez-Ortega P., Oliver A., Yaeger Monje J.P., Guo P., Charalambous H., Nestoros S., et al. Evaluating Pain Management Practices for Cancer Patients among Health Professionals: A Global Survey. J. Palliat. Med. 2022;25:1243–1248. doi: 10.1089/jpm.2021.0596. [DOI] [PubMed] [Google Scholar]
- 319.Bouya S., Balouchi A., Maleknejad A., Koochakzai M., AlKhasawneh E., Abdollahimohammad A. Cancer Pain Management among Oncology Nurses: Knowledge, Attitude, Related Factors, and Clinical Recommendations: A Systematic Review. J. Cancer Educ. 2019;34:839–846. doi: 10.1007/s13187-018-1433-6. [DOI] [PubMed] [Google Scholar]
- 320.Deandrea S., Montanari M., Moja L., Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann. Oncol. 2008;19:1985–1991. doi: 10.1093/annonc/mdn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Greco M.T., Roberto A., Corli O., Deandrea S., Bandieri E., Cavuto S., Apolone G. Quality of cancer pain management: An update of a systematic review of undertreatment of patients with cancer. J. Clin. Oncol. 2014;32:4149–4154. doi: 10.1200/JCO.2014.56.0383. [DOI] [PubMed] [Google Scholar]
- 322.Roberto A., Greco M.T., Uggeri S., Cavuto S., Deandrea S., Corli O., Apolone G. Living systematic review to assess the analgesic undertreatment in cancer patients. Pain Pract. 2022;22:487–496. doi: 10.1111/papr.13098. [DOI] [PubMed] [Google Scholar]
- 323.Mielgo-Rubio X., Uribelarrea E.A., Cortés L.Q., Moyano M.S. Immunotherapy in non-small cell lung cancer: Update and new insights. J. Clin. Transl. Res. 2021;7:1–21. [PMC free article] [PubMed] [Google Scholar]
- 324.Valkema M.J., Mostert B., Lagarde S.M., Wijnhoven B.P.L., van Lanschot J.J.B. The effectivity of targeted therapy and immunotherapy in patients with advanced metastatic and non-metastatic cancer of the esophagus and esophago-gastric junction. Updates Surg. 2022 doi: 10.1007/s13304-022-01327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zhou X., Qiao G., Ren J., Wang X., Wang S., Zhu S., Yuan Y., Morse M.A., Hobeika A., Lyerly H.K. Adoptive immunotherapy with autologous T-cell infusions reduces opioid requirements in advanced cancer patients. Pain. 2020;161:127–134. doi: 10.1097/j.pain.0000000000001702. [DOI] [PubMed] [Google Scholar]
- 326.Abbas N.T., Sheikha A., Mjali A. Clinical outcomes of patients with plasma cell neoplasm in sulaymaniyah province of Iraq. Syst. Rev. Pharm. 2020;11:1142–1144. [Google Scholar]
- 327.Alkhayyat M., Saleh M.A., Coronado W., Abureesh M., Zmaili M., Qapaja T., Almomani A., Khoudari G., Mansoor E., Cooper G. Epidemiology of neuroendocrine tumors of the appendix in the USA: A population-based national study (2014–2019) Ann. Gastroenterol. 2021;34:713–720. doi: 10.20524/aog.2021.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Baburao A., Narayanswamy H. Clinico-Pathological Profile and Haematological Abnormalities Associated with Lung Cancer in Bangalore, India. Asian Pac. J. Cancer Prev. 2015;16:8235–8238. doi: 10.7314/APJCP.2015.16.18.8235. [DOI] [PubMed] [Google Scholar]
- 329.Bassey G.O., Osunde O.D., Anyanechi C.E. Maxillofacial tumors and tumor-like lesions in a Nigerian teaching hospital: An eleven year retrospective analysis. Afr. Health Sci. 2014;14:56–63. doi: 10.4314/ahs.v14i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Comelli I., Lippi G., Campana V., Servadei F., Cervellin G. Clinical presentation and epidemiology of brain tumors firstly diagnosed in adults in the Emergency Department: A 10-year, single center retrospective study. Ann. Transl. Med. 2017;5:269. doi: 10.21037/atm.2017.06.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Dai J., Liao N., Shi J., Tao J.Q. Study of prevalence and influencing factors of depression in tumor patients and the therapeutic effects of fluoxetine. Eur. Rev. Med. Pharmacol. Sci. 2017;21:4966–4974. [PubMed] [Google Scholar]
- 332.de Carvalho W.R.S., de Souza L.L., Pontes F.S.C., Uchôa D.C.C., Corrêa D.L., de Cáceres C.V.B.L., Lopes M.A., Santos-Silva A.R., Vargas P.A., de Andrade B.A.B., et al. A multicenter study of oral sarcomas in Brazil. Oral Dis. 2020;26:43–52. doi: 10.1111/odi.13211. [DOI] [PubMed] [Google Scholar]
- 333.Du Plessis D.E., Van Deventer H., Fernandez P., Van Der Merwe A. A prospective observational study of the epidemiology and pathological profile of RCC in a South African referral centre. Afr. J. Urol. 2020;26:15. doi: 10.1186/s12301-020-00022-z. [DOI] [Google Scholar]
- 334.Glover M., Mansoor E., Panhwar M., Parasa S., Cooper G.S. Epidemiology of Colorectal Cancer in Average Risk Adults 20–39 Years of Age: A Population-Based National Study. Dig. Dis. Sci. 2019;64:3602–3609. doi: 10.1007/s10620-019-05690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Hanna E.Y., Mendoza T.R., Rosenthal D.I., Gunn G.B., Sehra P., Yucel E., Cleeland C.S. The symptom burden of treatment-naive patients with head and neck cancer. Cancer. 2015;121:766–773. doi: 10.1002/cncr.29097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Hlapane R.S., Khumalo T.L., Makhathini B.S., Moodley J. Impact of a delayed diagnosis of vulvar cancer and its association with HIV infection: A 4-year review at a tertiary hospital in KwaZulu-Natal, South Africa. S. Afr. J. HIV Med. 2021;22:1272. doi: 10.4102/sajhivmed.v22i1.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Hu X.L., Xu S.T., Wang X.C., Hou D.N., Chen C.C., Song Y.L., Yang D. Prevalence of and risk factors for presenting initial respiratory symptoms in patients undergoing surgery for lung cancer. J. Cancer. 2018;9:3515–3521. doi: 10.7150/jca.26209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Iglesias P., Nocete I., Moure Rodríguez M.D., Venegas-Moreno E., Ares J., Biagetti B., Rodríguez Berrocal V., Guerrero-Pérez F., Vicente A., Villar-Taibo R., et al. Craniopharyngioma in the Elderly: A Multicenter and Nationwide Study in Spain. Neuroendocrinology. 2021;111:925–936. doi: 10.1159/000512161. [DOI] [PubMed] [Google Scholar]
- 339.Khawaja S.N., Jamshed A., Hussain R.T. Prevalence of pain in oral cancer: A retrospective study. Oral Dis. 2021;27:1806–1812. doi: 10.1111/odi.13701. [DOI] [PubMed] [Google Scholar]
- 340.Kkrishnappa P., Loh E.J., Mohamad I.B., Tata M.D., Akhilesh M., Palayan K. Histomorphology and Immunohistochemistry of Gastrointestinal Stromal Tumors in a Malaysian Population. Asian Pac. J. Cancer Prev. 2016;17:2795–2799. [PubMed] [Google Scholar]
- 341.Kono Y., Kanzaki H., Tsuzuki T., Takatani M., Nasu J., Kawai D., Takenaka R., Tanaka T., Iwamuro M., Kawano S., et al. A multicenter observational study on the clinicopathological features of gastric cancer in young patients. J. Gastroenterol. 2019;54:419–426. doi: 10.1007/s00535-018-1525-4. [DOI] [PubMed] [Google Scholar]
- 342.Kuguyo O., Misi F.D., Chibonda S., Matimba A., Nhachi C., Tsikai N. Pain management strategies among cervical cancer patients in Zimbabwe. Pain Manag. 2021;11:715–729. doi: 10.2217/pmt-2020-0108. [DOI] [PubMed] [Google Scholar]
- 343.Kurnatowski P., Moqbil S., Kaczmarczyk D. Signs, symptoms and the prevalence of fungi detected from the oral cavity and pharynx of radiotherapy subjects with head and neck tumors, and their susceptibility to chemotherapeutics. Ann. Parasitol. 2014;60:207–213. [PubMed] [Google Scholar]
- 344.Lal P., Saleh M.A., Khoudari G., Gad M.M., Mansoor E., Isenberg G., Cooper G.S. Epidemiology of Large Bowel Carcinoid Tumors in the USA: A Population-Based National Study. Dig. Dis. Sci. 2020;65:269–275. doi: 10.1007/s10620-019-05725-0. [DOI] [PubMed] [Google Scholar]
- 345.Marchegiani G., Andrianello S., Miatello C., Pollini T., Secchettin E., Tedesco G., D’Onofrio M., Malleo G., Bassi C., Salvia R. The Actual Prevalence of Symptoms in Pancreatic Cystic Neoplasms: A Prospective Propensity Matched Cohort Analysis. Dig. Surg. 2019;36:522–529. doi: 10.1159/000495039. [DOI] [PubMed] [Google Scholar]
- 346.Mohme M., Mende K.C., Krätzig T., Plaetke R., Beseoglu K., Hagedorn J., Steiger H.J., Floeth F.W., Eicker S.O. Impact of spinal cord compression from intradural and epidural spinal tumors on perioperative symptoms—Implications for surgical decision making. Neurosurg. Rev. 2017;40:377–387. doi: 10.1007/s10143-016-0790-z. [DOI] [PubMed] [Google Scholar]
- 347.Paganini Piazzolla L., Medeiros De Almeida R., Nóbrega Dos Santos A.C., Gonçalves De Oliveira P., Freitas Da Silva E., Batista De Sousa J. Does aging influence clinical presentation and pathological staging in colorectal cancer? Eur. Geriatr. Med. 2015;6:433–436. doi: 10.1016/j.eurger.2015.04.007. [DOI] [Google Scholar]
- 348.Pakish J.B., Lu K.H., Sun C.C., Burzawa J.K., Greisinger A., Smith F.A., Fellman B., Urbauer D.L., Soliman P.T. Endometrial Cancer Associated Symptoms: A Case-Control Study. J. Womens Health. 2016;25:1187–1192. doi: 10.1089/jwh.2015.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Philip E.J., Bergerot C.D., Clark K., Bergerot P., Loscalzo M. Obesity and psychosocial well-being among cancer patients and survivors. Psychooncology. 2019;28:2141–2148. doi: 10.1002/pon.5181. [DOI] [PubMed] [Google Scholar]
- 350.Poursadegh M., Poursadegh F., Esmaeili M., Bakhshaee M. Epidemiological survey of sinonasal malignancy in North-East Iran. Iran. J. Otorhinolaryngol. 2015;27:225–229. [PMC free article] [PubMed] [Google Scholar]
- 351.Rudd P., Gorman D., Meja S., Mtonga P., Jere Y., Chidothe I., Msusa A.T., Bates M.J., Brown E., Masamba L. Cervical cancer in southern Malawi: A prospective analysis of presentation, management, and outcomes. Malawi Med. J. 2017;29:124–129. doi: 10.4314/mmj.v29i2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Shen C., Dasari A., Xu Y., Zhou S., Gu D., Chu Y., Halperin D.M., Shih Y.T., Yao J.C. Pre-existing Symptoms and Healthcare Utilization Prior to Diagnosis of Neuroendocrine Tumors: A SEER-Medicare Database Study. Sci. Rep. 2018;8:16863. doi: 10.1038/s41598-018-35340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Antony A., Joel J.J., Shetty J., Umar N.F. Identification and analysis of adverse drug reactions associated with cancer chemotherapy in hospitalized patients. Int. J. Pharm. Pharm. Sci. 2016;8:448–451. [Google Scholar]
- 354.Berger A., Kumar G., LeVan T., Meza J. Symptom Clusters And Quality Of Life Over 1 Year In Breast Cancer Patients Receiving Adjuvant Chemotherapy. Asia Pac. J. Oncol. Nurs. 2020;7:134–140. doi: 10.4103/apjon.apjon_57_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Bianchini C., Malagò M., Crema L., Aimoni C., Matarazzo T., Bortolazzi S., Ciorba A., Pelucchi S., Pastore A. Post-operative pain management in head and neck cancer patients: Predictive factors and efficacy of therapy. Acta Otorhinolaryngol. Ital. 2016;36:91–96. doi: 10.14639/0392-100X-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Dervis E., Ayer M., Belli A.A., Barut S.G. Cutaneous adverse reactions of imatinib therapy in patients with chronic myeloid leukemia: A six-year follow up. Eur. J. Dermatol. 2016;26:133–137. doi: 10.1684/ejd.2015.2684. [DOI] [PubMed] [Google Scholar]
- 357.Fatiregun O., Sowunmi A.C., Habeebu M., Okediji P., Alabi A., Fatiregun O., Adeniji A., Awofeso O., Adegboyega B. Prevalence and Correlates of Unmet Supportive Needs of Nigerian Patients with Cancer. J. Glob. Oncol. 2019;5:1–9. doi: 10.1200/JGO.19.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Frowen J., Hughes R., Skeat J. The prevalence of patient-reported dysphagia and oral complications in cancer patients. Support. Care Cancer. 2020;28:1141–1150. doi: 10.1007/s00520-019-04921-y. [DOI] [PubMed] [Google Scholar]
- 359.Germano F., Melone P., Testi D., Arcuri L., Marmiroli L., Petrone A., Arcuri C. Oral complications of head and neck radiotherapy: Prevalence and management. Minerva Stomatol. 2015;64:189–202. [PubMed] [Google Scholar]
- 360.Kartin P.T., Tasci S., Soyuer S., Elmali F. Effect of an oral mucositis protocol on quality of life of patients with head and neck cancer treated with radiation therapy. Clin. J. Oncol. Nurs. 2014;18:e118–e125. doi: 10.1188/14.CJON.E118-E125. [DOI] [PubMed] [Google Scholar]
- 361.Rahnama M., Madej-Czerwonka B., Jastrzębska-Jamrogiewicz I., Jamrogiewicz R. Analysis of the influence of parenteral cancer chemotherapy on the health condition of oral mucosa. Contemp. Oncol. 2015;19:77–82. doi: 10.5114/wo.2014.45291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Sahora K., Crippa S., Zamboni G., Ferrone C., Warshaw A.L., Lillemoe K., Mino-Kenudson M., Falconi M., Fernandez-del Castillo C. Intraductal papillary mucinous neoplasms of the pancreas with concurrent pancreatic and periampullary neoplasms. Eur. J. Surg. Oncol. 2016;42:197–204. doi: 10.1016/j.ejso.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 363.Takayama Y., Kaneoka Y., Maeda A., Takahashi T., Kiriyama M., Seita K. Assessment of perioperative symptoms of patients with gastric cancer using the edmonton symptom assessment system revised japanese version (Esas-r-j) J. Med. Investig. 2021;68:90–95. doi: 10.2152/jmi.68.90. [DOI] [PubMed] [Google Scholar]
- 364.Wagland R., Richardson A., Ewings S., Armes J., Lennan E., Hankins M., Griffiths P. Prevalence of cancer chemotherapy-related problems, their relation to health-related quality of life and associated supportive care: A cross-sectional survey. Support. Care Cancer. 2016;24:4901–4911. doi: 10.1007/s00520-016-3346-4. [DOI] [PubMed] [Google Scholar]
- 365.Wang Y., Lu Q., Zhang L., Zhuang B., Zhang T., Jin S., Sun Y., Xiao S., Zheng B., Fang Y., et al. Nutrition Impact Symptom Clusters in Patients with Head and Neck Cancer Receiving Concurrent Chemoradiotherapy. J. Pain Symptom Manag. 2021;62:277–285. doi: 10.1016/j.jpainsymman.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 366.Yanque O. Uterine leiomyosarcoma: A 10-year review in a referral hospital in Peru, 2005–2014. S. Afr. J. Obstet. Gynaecol. 2018;24:87–89. doi: 10.7196/sajog.1355. [DOI] [Google Scholar]
- 367.Ahmadi Z., Wysham N.G., Lundström S., Janson C., Currow D.C., Ekström M. End-of-life care in oxygen-dependent ILD compared with lung cancer: A national population-based study. Thorax. 2016;71:510–516. doi: 10.1136/thoraxjnl-2015-207439. [DOI] [PubMed] [Google Scholar]
- 368.Alsirafy S.A., Abd El-Aal H.H., Farag D.E., Radwan R.H., El-Sherief W.A., Fawzy R. High Symptom Burden among Patients with Newly Diagnosed Incurable Cancer in a Developing Country. J. Pain. Symptom Manag. 2016;51:e1–e5. doi: 10.1016/j.jpainsymman.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 369.Bischoff K.E., O’Riordan D.L., Fazzalaro K., Kinderman A., Pantilat S.Z. Identifying Opportunities to Improve Pain among Patients with Serious Illness. J. Pain Symptom Manag. 2018;55:881–889. doi: 10.1016/j.jpainsymman.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 370.Cuyun Carter G., Kaltenboeck A., Ivanova J., Liepa A.M., San Roman A., Koh M., Rajan N., Cheng R., Birnbaum H., Chen J.S. Treatment patterns in patients with advanced gastric cancer in Taiwan. Asia Pac. J. Clin. Oncol. 2017;13:185–194. doi: 10.1111/ajco.12497. [DOI] [PubMed] [Google Scholar]
- 371.Eriksson H., Milberg A., Hjelm K., Friedrichsen M. End of Life Care for Patients Dying of Stroke: A Comparative Registry Study of Stroke and Cancer. PLoS ONE. 2016;11:e0147694. doi: 10.1371/journal.pone.0147694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Family L., Xu H., Cannavale K., Mehta B., Pourmoussa A., Xu L., Chao C. Symptom burdens related to chemotherapy-induced anemia in stage IV cancer. J. Community Support. Oncol. 2018;16:e260–e271. doi: 10.12788/jcso.0432. [DOI] [Google Scholar]
- 373.Hasegawa T., Goto N., Matsumoto N., Sasaki Y., Ishiguro T., Kuzuya N., Sugiyama Y. Prevalence of unmet needs and correlated factors in advanced-stage cancer patients receiving rehabilitation. Support. Care Cancer. 2016;24:4761–4767. doi: 10.1007/s00520-016-3327-7. [DOI] [PubMed] [Google Scholar]
- 374.Langlais B.T., Geyer H., Scherber R., Mesa R.A., Dueck A.C. Quality of life and symptom burden among myeloproliferative neoplasm patients: Do symptoms impact quality of life? Leuk. Lymphoma. 2019;60:402–408. doi: 10.1080/10428194.2018.1480768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Nnonyelum O.N., Anazoeze M.J., Eunice N.O., Emmanuel O.O., Stella A.T., Marcus A.I., Taiwo B.M., Olufela K.O., Chinawaeze A.J., Orkuma J.A., et al. Multiple myeloma in Nigeria: A multi-centre epidemiological and biomedical study. Pan Afr. Med. J. 2015;22:292. doi: 10.11604/pamj.2015.22.292.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Pimentel L.E., Yennurajalingam S., Chisholm G., Edwards T., Guerra-Sanchez M., De La Cruz M., Tanco K., Vidal M., Bruera E. The frequency and factors associated with the use of a dedicated Supportive Care Center Telephone Triaging Program in patients with advanced cancer at a comprehensive cancer center. J. Pain Symptom Manag. 2015;49:939–944. doi: 10.1016/j.jpainsymman.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 377.Richter J., Sanchez L., Biran N., Wang C.K., Tanenbaum K., DeVincenzo V., Grunman B., Vesole D.H., Siegel D.S., Pecora A., et al. Prevalence and Survival Impact of Self-Reported Symptom and Psychological Distress among Patients with Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2021;21:e284–e289. doi: 10.1016/j.clml.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 378.Sandgren A., Strang P. Palliative care needs in hospitalized cancer patients: A 5-year follow-up study. Support. Care Cancer. 2018;26:181–186. doi: 10.1007/s00520-017-3831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Scharf M., Petry V., Daniel H., Rinke A., Gress T.M. Bone Metastases in Patients with Neuroendocrine Neoplasm: Frequency and Clinical, Therapeutic, and Prognostic Relevance. Neuroendocrinology. 2018;106:30–37. doi: 10.1159/000457954. [DOI] [PubMed] [Google Scholar]
- 380.Spence W., Ghosh S., Palen M., Liska A., Ha V., Wong R., Huang F. Symptom burden among Northern Alberta radiotherapy patients with advanced cancer: Mapping needs and gaps. Support. Care Cancer. 2020;28:4963–4969. doi: 10.1007/s00520-020-05330-2. [DOI] [PubMed] [Google Scholar]
- 381.Unseld M., Fischöder S., Jachs M., Drimmel M., Siebenhüner A., Bianconi D., Kieler M., Puhr H., Minichsdorfer C., Winder T., et al. Different toxicity profiles predict third line treatment efficacy in metastatic colorectal cancer patients. J. Clin. Med. 2020;9:1772. doi: 10.3390/jcm9061772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Wangnamthip S., Euasobhon P., Siriussawakul A., Jirachaipitak S., Laurujisawat J., Vimolwattanasarn K. Effective Pain Management for Inpatients at Siriraj Hospital: A Retrospective Study. J. Med. Assoc. Thai. 2016;99:565–571. [PubMed] [Google Scholar]
- 383.Yanamandra U., Saini N., Chauhan P., Sharma T., Khadwal A., Prakash G., Varma N., Lad D., Varma S., Malhotra P. AYA-Myeloma: Real-World, Single-Center Experience Over Last 5 Years. J. Adolesc. Young Adult Oncol. 2018;7:120–124. doi: 10.1089/jayao.2017.0034. [DOI] [PubMed] [Google Scholar]
- 384.Yi Y.S., Ban W.H., Sohng K.Y. Effect of COPD on symptoms, quality of life and prognosis in patients with advanced non-small cell lung cancer. BMC Cancer. 2018;18:1053. doi: 10.1186/s12885-018-4976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Abid H., Kober K.M., Smoot B., Paul S.M., Hammer M., Levine J.D., Lee K., Wright F., Cooper B.A., Conley Y.P., et al. Common and Distinct Characteristics Associated with Trajectories of Morning and Evening Energy in Oncology Patients Receiving Chemotherapy. J. Pain Symptom Manag. 2017;53:887–900.e2. doi: 10.1016/j.jpainsymman.2016.12.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Agyemang-Yeboah F., Yorke J., Obirikorang C., Batu E.N., Acheampong E., Frempong E.A., Anto E.O., Amankwaa B. Patterns and presentations of colorectal cancer at Komfo-Anokye teaching hospital Kumasi, Ghana. Pan Afr. Med. J. 2017;28:121. doi: 10.11604/pamj.2017.28.121.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Allgar V.L., Chen H., Richfield E., Currow D., Macleod U., Johnson M.J. Psychometric Properties of the Needs Assessment Tool-Progressive Disease Cancer in U.K. Primary Care. J. Pain Symptom Manag. 2018;56:602–612. doi: 10.1016/j.jpainsymman.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 388.Alsughayer L.Y., Altamimi L.A., Alsaleh F.S., Alsaghan L., Alfurayh I., Abdel-Aziz N.M., Alsaleh K.A., Alosaimi F.D. Prevalence and determinants of distress among oncology patients at a tertiary care medical city in Riyadh, Saudi Arabia. Saudi Med. J. 2021;42:761–768. doi: 10.15537/smj.2021.42.7.20210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Bell S.G., Dalton L., McNeish B.L., Fang F., Henry N.L., Kidwell K.M., McLean K. Aromatase inhibitor use, side effects and discontinuation rates in gynecologic oncology patients. Gynecol. Oncol. 2020;159:509–514. doi: 10.1016/j.ygyno.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Crossnohere N.L., Richardson D.R., Reinhart C., O’Donoghue B., Love S.M., Smith B.D., Bridges J.F.P. Side effects from acute myeloid leukemia treatment: Results from a national survey. Curr. Med. Res. Opin. 2019;35:1965–1970. doi: 10.1080/03007995.2019.1631149. [DOI] [PubMed] [Google Scholar]
- 391.Hirpara D.H., Gupta V., Davis L.E., Zhao H., Hallet J., Mahar A.L., Sutradhar R., Doherty M., Louie A.V., Kidane B., et al. Severe symptoms persist for Up to one year after diagnosis of stage I-III lung cancer: An analysis of province-wide patient reported outcomes. Lung Cancer. 2020;142:80–89. doi: 10.1016/j.lungcan.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 392.Jazieh A.M., AlSumai T.S., Ali Y.Z., Sheblaq N.R., Alkaiyat M. The pattern of bone involvement, management, and outcomes in patients with nonsmall cell lung cancer: A retrospective study. Ann. Thorac. Med. 2018;13:150–155. doi: 10.4103/atm.ATM_385_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Kawazoe H., Mori N., Ido S., Uozumi R., Tsuneoka K., Takeuchi A., Matsuo M., Yamauchi M., Nakai M., Sumikawa S., et al. Liquid Formulation of Gemcitabine Increases Venous Pain in Patients with Cancer: A Retrospective Study. Clin. Ther. 2020;42:712–719. doi: 10.1016/j.clinthera.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 394.Kebebew T., Mavhandu-Mudzusi A.H., Mosalo A. A cross-sectional assessment of symptom burden among patients with advanced cervical cancer. BMC Palliat. Care. 2021;20:190. doi: 10.1186/s12904-021-00883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Kokkonen K., Tasmuth T., Lehto J.T., Kautiainen H., Elme A., Jaaskelainen A.S., Saarto T. Cancer patients’ symptom burden and health-related quality of life (HRQoL) at Tertiary Cancer Center from 2006 to 2013: A cross-sectional study. Anticancer Res. 2019;39:271–277. doi: 10.21873/anticanres.13107. [DOI] [PubMed] [Google Scholar]
- 396.Kolb N.A., Smith A.G., Singleton J.R., Beck S.L., Howard D., Dittus K., Karafiath S., Mooney K. Chemotherapy-related neuropathic symptom management: A randomized trial of an automated symptom-monitoring system paired with nurse practitioner follow-up. Support. Care Cancer. 2018;26:1607–1615. doi: 10.1007/s00520-017-3970-7. [DOI] [PubMed] [Google Scholar]
- 397.Ladaninejad S., Ilali E., Mousavinasab N., Taraghi Z. The Relationship between Depressive Symptoms and Demographic-Medical Characteristics among Elder People with Cancer. Asia Pac. J. Oncol. Nurs. 2019;6:424–430. doi: 10.4103/apjon.apjon_13_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Lim J.S., Koh C.E., Liu H., Solomon M.J., Johnstone C.S.H. The Price We Pay for Radical Curative Pelvic Exenterations: Prevalence and Management of Pain. Dis. Colon Rectum. 2018;61:314–319. doi: 10.1097/DCR.0000000000001013. [DOI] [PubMed] [Google Scholar]
- 399.Molassiotis A., Cheng H.L., Leung K.T., Li Y.C., Wong K.H., Au J.S.K., Sundar R., Chan A., Ng T.R.D., Suen L.K.P., et al. Risk factors for chemotherapy-induced peripheral neuropathy in patients receiving taxane- and platinum-based chemotherapy. Brain Behav. 2019;9:e01312. doi: 10.1002/brb3.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Motah M., Gams Massi D., Fouda Bekolo F., Akweseh Nju N., Ndoumbe A., Moumi M., Sango A., Shu P., Eyenga V. Epidemiological profile of brain tumors in Cameroon: A retrospective study. Egypt. J. Neurol. Psychiatr. Neurosurg. 2021;57:126. doi: 10.1186/s41983-021-00381-6. [DOI] [Google Scholar]
- 401.Neves Duarte Lisboa I., Dantas De Sá Tinôco J., Dias Fernandes M.I.D.C., Da Silva R.R., Student N., Barbosa Da Silva J., Freire Delgado M., Oliveira Lopes M.V., Brandão De Carvalho Lira A.L. Constipation in Chemotherapy Patients: A Diagnostic Accuracy Study. Asian Pac. J. Cancer Prev. 2021;22:3017–3021. doi: 10.31557/APJCP.2021.22.9.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Parás-Bravo P., Paz-Zulueta M., Alonso-Blanco M.C., Salvadores-Fuentes P., Alconero-Camarero A.R., Santibañez M. Association among presence of cancer pain, inadequate pain control, and psychotropic drug use. PLoS ONE. 2017;12:e0178742. doi: 10.1371/journal.pone.0178742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Porta-Sales J., Nabal-Vicuna M., Vallano A., Espinosa J., Planas-Domingo J., Verger-Fransoy E., Julià-Torras J., Serna J., Pascual-López A., Rodríguez D., et al. Have We Improved Pain Control in Cancer Patients? A Multicenter Study of Ambulatory and Hospitalized Cancer Patients. J. Palliat. Med. 2015;18:923–932. doi: 10.1089/jpm.2015.29002.jps. [DOI] [PubMed] [Google Scholar]
- 404.Pozzar R.A., Hammer M.J., Paul S.M., Cooper B.A., Kober K.M., Conley Y.P., Levine J.D., Miaskowski C. Distinct sleep disturbance profiles among patients with gynecologic cancer receiving chemotherapy. Gynecol. Oncol. 2021;163:419–426. doi: 10.1016/j.ygyno.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 405.Santos I., Mendes L., Mansinho H., Santos C.A. Nutritional status and functional status of the pancreatic cancer patients and the impact of adjacent symptoms. Clin. Nutr. 2021;40:5486–5493. doi: 10.1016/j.clnu.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 406.Williams L.A., Bohac C., Hunter S., Cella D. Patient and health care provider perceptions of cancer-related fatigue and pain. Support. Care Cancer. 2016;24:4357–4363. doi: 10.1007/s00520-016-3275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Beyaz S.G., Ergönenç J.Ş., Ergönenç T., Sönmez Ö.U., Erkorkmaz Ü., Altintoprak F. Postmastectomy pain: A cross-sectional study of prevalence, pain characteristics, and effects on quality of life. Chin. Med. J. 2016;129:66–71. doi: 10.4103/0366-6999.172589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Cardoso L.R., Rizzo C.C., de Oliveira C.Z., dos Santos C.R., Carvalho A.L. Myofascial pain syndrome after head and neck cancer treatment: Prevalence, risk factors, and influence on quality of life. Head Neck. 2015;37:1733–1737. doi: 10.1002/hed.23825. [DOI] [PubMed] [Google Scholar]
- 409.Cox-Martin E., Anderson-Mellies A., Borges V., Bradley C. Chronic pain, health-related quality of life, and employment in working-age cancer survivors. J. Cancer Surviv. 2020;14:179–187. doi: 10.1007/s11764-019-00843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Fuchs H., Hölscher A.H., Leers J., Bludau M., Brinkmann S., Schröder W., Alakus H., Mönig S., Gutschow C.A. Long-term quality of life after surgery for adenocarcinoma of the esophagogastric junction: Extended gastrectomy or transthoracic esophagectomy? Gastric Cancer. 2016;19:312–317. doi: 10.1007/s10120-015-0466-3. [DOI] [PubMed] [Google Scholar]
- 411.Huang I.C., Hudson M.M., Robison L.L., Krull K.R. Differential Impact of Symptom Prevalence and Chronic Conditions on Quality of Life in Cancer Survivors and Non-Cancer Individuals: A Population Study. Cancer Epidemiol. Biomark. Prev. 2017;26:1124–1132. doi: 10.1158/1055-9965.EPI-16-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Jefford M., Ward A.C., Lisy K., Lacey K., Emery J.D., Glaser A.W., Cross H., Krishnasamy M., McLachlan S.A., Bishop J. Patient-reported outcomes in cancer survivors: A population-wide cross-sectional study. Support. Care Cancer. 2017;25:3171–3179. doi: 10.1007/s00520-017-3725-5. [DOI] [PubMed] [Google Scholar]
- 413.Jiang C., Wang H., Wang Q., Luo Y., Sidlow R., Han X. Prevalence of Chronic Pain and High-Impact Chronic Pain in Cancer Survivors in the United States. JAMA Oncol. 2019;5:1224–1226. doi: 10.1001/jamaoncol.2019.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Kimberg C.I., Klosky J.L., Zhang N., Brinkman T.M., Ness K.K., Srivastava D.K., Robison L.L., Hudson M.M., Krull K.R. Predictors of health care utilization in adult survivors of childhood cancer exposed to central nervous system-directed therapy. Cancer. 2015;121:774–782. doi: 10.1002/cncr.29121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Lang A.E., Murphy M., Dickerson C.R., Stavness I., Soo Y.K. Shoulder Dysfunction in Breast Cancer Survivors: Can Treatment Type or Musculoskeletal Factors Identify Those at Higher Risk? Rehabil. Oncol. 2021;39:143–151. doi: 10.1097/01.REO.0000000000000224. [DOI] [Google Scholar]
- 416.Maass S., Boerman L.M., Brandenbarg D., Verhaak P.F.M., Maduro J.H., de Bock G.H., Berendsen A.J. Symptoms in long-term breast cancer survivors: A cross-sectional study in primary care. Breast. 2020;54:133–138. doi: 10.1016/j.breast.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Maguire R., Drummond F.J., Hanly P., Gavin A., Sharp L. Problems sleeping with prostate cancer: Exploring possible risk factors for sleep disturbance in a population-based sample of survivors. Support. Care Cancer. 2019;27:3365–3373. doi: 10.1007/s00520-018-4633-z. [DOI] [PubMed] [Google Scholar]
- 418.Mahmood N.G., Mahmood R.A., Ibrahim R.H. The postmastectomy pain syndrome among women in city of mosul-iraq. Teikyo Med. J. 2021;44:1625–1630. [Google Scholar]
- 419.McDonough A.L., Lei Y., Kwak A.H., Haggett D.E., Jimenez R.B., Johnston K.T., Moy B., Spring L.M., Peppercorn J. Implementation of a Brief Screening Tool to Identify Needs of Breast Cancer Survivors. Clin. Breast Cancer. 2021;21:e88–e95. doi: 10.1016/j.clbc.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 420.Mehdizadeh O.B., Dhar S.I., Evangelista L., Nativ-Zeltzer N., Bewley A.F., Belafsky P.C. Prevalence of profound laryngeal sensory neuropathy in head and neck cancer survivors with feeding tube-dependent oropharyngeal dysphagia. Head Neck. 2020;42:898–904. doi: 10.1002/hed.26059. [DOI] [PubMed] [Google Scholar]
- 421.Moreno P.I., Ramirez A.G., San Miguel-Majors S.L., Castillo L., Fox R.S., Gallion K.J., Munoz E., Estabrook R., Perez A., Lad T., et al. Unmet supportive care needs in Hispanic/Latino cancer survivors: Prevalence and associations with patient-provider communication, satisfaction with cancer care, and symptom burden. Support. Care Cancer. 2019;27:1383–1394. doi: 10.1007/s00520-018-4426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Mustafa Ali M., Moeller M., Rybicki L., Moore H.C.F. Prevalence and correlates of patient-reported symptoms and comorbidities in breast cancer survivors at a tertiary center. J. Cancer Surviv. 2017;11:743–750. doi: 10.1007/s11764-017-0612-5. [DOI] [PubMed] [Google Scholar]
- 423.Oancea S.C., Brinkman T.M., Ness K.K., Krull K.R., Smith W.A., Srivastava D.K., Robison L.L., Hudson M.M., Gurney J.G. Emotional distress among adult survivors of childhood cancer. J. Cancer Surviv. 2014;8:293–303. doi: 10.1007/s11764-013-0336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Olsson I.T., Lubas M.M., Li C., Mandrell B.N., Banerjee P., Howell C.R., Ness K.K., Srivastava D., Robison L.L., Hudson M.M., et al. Insomnia and neurocognitive functioning in adult survivors of childhood cancer. JNCI Cancer Spectr. 2020;4:pkaa008. doi: 10.1093/jncics/pkaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Pezdirec M., Strojan P., Boltezar I.H. Swallowing disorders after treatment for head and neck cancer. Radiol. Oncol. 2019;53:225–230. doi: 10.2478/raon-2019-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Romero A., Torà-Rocamora I., Baré M., Barata T., Domingo L., Ferrer J., Torà N., Comas M., Merenciano C., Macià F., et al. Prevalence of persistent pain after breast cancer treatment by detection mode among participants in population-based screening programs. BMC Cancer. 2016;16:735. doi: 10.1186/s12885-016-2768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Salani R., Preston M.M., Hade E.M., Johns J., Fowler J.M., Paskett E.P., Katz M.L. Swelling among women who need education about leg lymphedema: A descriptive study of lymphedema in women undergoing surgery for endometrial cancer. Int. J. Gynecol. Cancer. 2014;24:1507–1512. doi: 10.1097/IGC.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Soares E.W.S., Nagai H.M., Bredt L.C., da Cunha Jr A.D., Andrade R.J., Soares G.V.S. Morbidity after conventional dissection of axillary lymph nodes in breast cancer patients. World J. Surg. Oncol. 2014;12:67. doi: 10.1186/1477-7819-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Wulff-Burchfield E., Dietrich M.S., Ridner S., Murphy B.A. Late systemic symptoms in head and neck cancer survivors. Support. Care Cancer. 2019;27:2893–2902. doi: 10.1007/s00520-018-4577-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Yen H.J., Eissa H.M., Bhatt N.S., Huang S., Ehrhardt M.J., Bhakta N., Ness K.K., Krull K.R., Srivastava D.K., Robison L.L., et al. Patient-reported outcomes in survivors of childhood hematologic malignancies with hematopoietic stem cell transplant. Blood. 2020;135:1847–1858. doi: 10.1182/blood.2019003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Bandeali S., des Ordons A.R., Sinnarajah A. Comparing the physical, psychological, social, and spiritual needs of patients with non-cancer and cancer diagnoses in a tertiary palliative care setting. Palliat. Support. Care. 2020;18:513–518. doi: 10.1017/S1478951519001020. [DOI] [PubMed] [Google Scholar]
- 432.Covarrubias-Gómez A., Hernández-Martínez E.E., Ruiz-Ramírez S., López Collada-Estrada M. Assessment of pain and other symptoms in Mexican patients with advanced illness. J. Pain Palliat. Care Pharmacother. 2014;28:394–398. doi: 10.3109/15360288.2014.959235. [DOI] [PubMed] [Google Scholar]
- 433.Díez-Manglano J., Isasi de Isasmendi Pérez S., García Fenoll R., Sánchez L.Á., Formiga F., Giner Galvañ V., Dueñas C., Roca B., Estrada Díaz C., Casariego Vales E. Palliative Sedation in Patients Hospitalized in Internal Medicine Departments. J. Pain Symptom Manag. 2020;59:302–309. doi: 10.1016/j.jpainsymman.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 434.Effendy C., Vissers K., Osse B.H.P., Tejawinata S., Vernooij-Dassen M., Engels Y. Comparison of Problems and Unmet Needs of Patients with Advanced Cancer in a European Country and an Asian Country. Pain Pract. 2015;15:433–440. doi: 10.1111/papr.12196. [DOI] [PubMed] [Google Scholar]
- 435.Goto H., Yoshikawa S., Otsuka M., Omodaka T., Yoshimi K., Yoshida Y., Yamamoto O., Kiyohara Y. Symptom prevalence in patients with advanced skin cancer. J. Dermatol. 2017;44:123–126. doi: 10.1111/1346-8138.13527. [DOI] [PubMed] [Google Scholar]
- 436.Jaime-Pérez J.C., Turrubiates-Hernández G.A., Nava-Obregón T., Coronado-Hernández B., Gutiérrez-Aguirre H., Cantú-Rodríguez O.G., Herrera-Garza J.L., Gómez-Almaguer D. Palliative Care for Patients with Hematologic Malignancies in a Low-Middle Income Country: Prevalence of Symptoms and the Need for Improving Quality of Attention at the End of Life. Am. J. Hosp. Palliat. Care. 2020;37:600–605. doi: 10.1177/1049909119887951. [DOI] [PubMed] [Google Scholar]
- 437.Jakobsen T.B.T., Pittureri C., Seganti P., Borissova E., Balzani I., Fabbri S., Amati P., Donigaglia S., Gallina S., Fabbri E. Incidence and prevalence of pressure ulcers in cancer patients admitted to hospice: A multicentre prospective cohort study. Int. Wound J. 2020;17:641–649. doi: 10.1111/iwj.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Janberidze E., Pereira S.M., Hjermstad M.J., Knudsen A.K., Kaasa S., van der Heide A., Onwuteaka-Philipsen B. Depressive symptoms in the last days of life of patients with cancer: A nationwide retrospective mortality study. BMJ Support. Palliat. Care. 2016;6:201–209. doi: 10.1136/bmjspcare-2014-000722. [DOI] [PubMed] [Google Scholar]
- 439.Mercadante S., Aielli F., Masedu F., Valenti M., Ficorella C., Porzio G. Pain characteristics and analgesic treatment in an aged adult population: A 4-week retrospective analysis of advanced cancer patients followed at home. Drugs Aging. 2015;32:315–320. doi: 10.1007/s40266-015-0253-1. [DOI] [PubMed] [Google Scholar]
- 440.Parra Palacio S., Giraldo Hoyos C.E., Arias Rodríguez C., Mejía Arrieta D., Vargas Gómez J.J., Krikorian A. Palliative sedation in advanced cancer patients hospitalized in a specialized palliative care unit. Support. Care Cancer. 2018;26:3173–3180. doi: 10.1007/s00520-018-4164-7. [DOI] [PubMed] [Google Scholar]
- 441.Prado B.L., Gomes D.B.D., Usón Júnior P.L.S., Taranto P., França M.S., Eiger D., Mariano R.C., Hui D., Del Giglio A. Continuous palliative sedation for patients with advanced cancer at a tertiary care cancer center. BMC Palliat. Care. 2018;17:13. doi: 10.1186/s12904-017-0264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Romem A., Tom S.E., Beauchene M., Babington L., Scharf S.M., Romem A. Pain management at the end of life: A comparative study of cancer, dementia, and chronic obstructive pulmonary disease patients. Palliat. Med. 2015;29:464–469. doi: 10.1177/0269216315570411. [DOI] [PubMed] [Google Scholar]
- 443.Seiler A., Schubert M., Hertler C., Schettle M., Blum D., Guckenberger M., Weller M., Ernst J., von Känel R., Boettger S. Predisposing and precipitating risk factors for delirium in palliative care patients. Palliat. Support. Care. 2020;18:437–446. doi: 10.1017/S1478951519000919. [DOI] [PubMed] [Google Scholar]
- 444.Soares L.G.L., Japiassu A.M., Gomes L.C., Pereira R., Peçanha C., Goldgaber T. Prevalence and intensity of dyspnea, pain, and agitation among people dying with late stage dementia compared with people dying with advanced cancer: A single-center preliminary study in Brazil. Ann. Palliat. Med. 2018;7:437–443. doi: 10.21037/apm.2018.05.06. [DOI] [PubMed] [Google Scholar]
- 445.Aboumrad M., Shiner B., Riblet N., Mills P.D., Watts B.V. Factors contributing to cancer-related suicide: A study of root-cause analysis reports. Psychooncology. 2018;27:2237–2244. doi: 10.1002/pon.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Aghdassi A., Christoph A., Dombrowski F., Döring P., Barth C., Christoph J., Lerch M.M., Simon P. Gastrointestinal Stromal Tumors: Clinical Symptoms, Location, Metastasis Formation, and Associated Malignancies in a Single Center Retrospective Study. Dig. Dis. 2018;36:337–345. doi: 10.1159/000489556. [DOI] [PubMed] [Google Scholar]
- 447.Aldossary M.Y., Alayed A.A., Amr S.S., Alqahtani S., Alnahawi M., Alqahtani M.S. Gallbladder cancer in Eastern Province of Saudi Arabia: A retrospective cohort study. Ann. Med. Surg. 2018;35:117–123. doi: 10.1016/j.amsu.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Aljumah A.A., Kuriry H., Alzunaitan M., Al Ghobain M., Al Muaikeel M., Al Olayan A., Azzumeea F., Almutairi B., Alalwan A., Alghamdi H. Clinical Presentation, Risk Factors, and Treatment Modalities of Hepatocellular Carcinoma: A Single Tertiary Care Center Experience. Gastroenterol. Res. Pract. 2016;2016:1989045. doi: 10.1155/2016/1989045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Alsalamah A.K., Maktabi A.M.Y., Alkatan H.M. Adult orbital lesions in Saudi Arabia: A multi-centered demographic study with clinicopathological correlation. J. Epidemiol. Glob. Health. 2020;10:359–366. doi: 10.2991/jegh.k.200720.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Alzghoul B.N., Zayed Y., Obeidat A., Alzghoul B., Naser A., Shilbayeh A.R., Innabi A., Al-Hakim T., Buchanan M., Mehrad B., et al. Clinical Characteristics of Sarcoidosis Patients with Self-Reported Lymphoma: A US Nationwide Registry Study. Lung. 2021;199:611–618. doi: 10.1007/s00408-021-00485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Anvari M.S., Naderan M., Eslami Shahr Babaki A., Shoar S., Boroumand M.A., Abbasi K. Clinicopathologic review of non-myxoma cardiac tumors: A 10-year single-center experience. Cardiology. 2014;129:199–202. doi: 10.1159/000365916. [DOI] [PubMed] [Google Scholar]
- 452.Bentley R., O’Cathail M., Aznar-Garcia L., Crosby V., Wilcock A., Christian J. Defining patterns of care in the management of patients with brain metastases in a large oncology centre: A single-centre retrospective audit of 236 cases. Eur. J. Cancer Care. 2019;28:e13059. doi: 10.1111/ecc.13059. [DOI] [PubMed] [Google Scholar]
- 453.Bubis L.D., Delibasic V., Davis L.E., Jeong Y., Chan K., Kosyachkova E., Mahar A., Karanicolas P., Coburn N.G. Patient-reported symptoms in metastatic gastric cancer patients in the last 6 months of life. Support. Care Cancer. 2021;29:515–524. doi: 10.1007/s00520-020-05501-1. [DOI] [PubMed] [Google Scholar]
- 454.Bunduc Ș., Iacob R., Costache R., Stoica B., Radu C., Gheorghe C. Very Early Onset Pancreatic Adenocarcinoma-Clinical Presentation, Risk Factors and Therapeutic Options. Chirurgia. 2018;113:405–411. doi: 10.21614/chirurgia.113.3.405. [DOI] [PubMed] [Google Scholar]
- 455.De Melo N.B., de Sousa V.M., Bernardino Í.M., de Melo D.P., Castro Gomes D.Q., Bento P.M. Oral health related quality of life and determinant factors in patients with head and neck cancer. Med. Oral Patol. Oral Cir. Bucal. 2019;24:e281–e289. doi: 10.4317/medoral.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.de Sire A., Losco L., Cisari C., Gennari A., Boldorini R., Fusco N., Cigna E., Invernizzi M. Axillary web syndrome in women after breast cancer surgery referred to an Oncological Rehabilitation Unit: Which are the main risk factors? A retrospective case-control study. Eur. Rev. Med. Pharmacol. Sci. 2020;24:8028–8035. doi: 10.26355/eurrev_202008_22486. [DOI] [PubMed] [Google Scholar]
- 457.Diallo I., Ndiaye B., Touré M., Sow A., Mbengue A., Diawara P.S., Gning S.B., Mbaye P.S., Fall F., Mbengue M. Hepatocellular carcinoma in senegal: Epidemiological, clinical and etiological aspects about 229 cases at hôpital principal de dakar. PAMJ One Health. 2021;38:99. doi: 10.11604/pamj.2021.38.99.25195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Dubé C.E., Mack D.S., Hunnicutt J.N., Lapane K.L. Cognitive Impairment and Pain among Nursing Home Residents with Cancer. J. Pain Symptom Manag. 2018;55:1509–1518. doi: 10.1016/j.jpainsymman.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Guérin A., Sasane M., Zhang J., Culver K.W., Dea K., Nitulescu R., Wu E.Q. Brain metastases in patients with ALK+ non-small cell lung cancer: Clinical symptoms, treatment patterns and economic burden. J. Med. Econ. 2015;18:312–322. doi: 10.3111/13696998.2014.1003644. [DOI] [PubMed] [Google Scholar]
- 460.Hallet J., Davis L.E., Mahar A.L., Law C.H.L., Isenberg-Grzeda E., Bubis L.D., Singh S., Myrehaug S., Zhao H., Beyfuss K., et al. Patterns of Symptoms Burden in Neuroendocrine Tumors: A Population-Based Analysis of Prospective Patient-Reported Outcomes. Oncologist. 2019;24:1384–1394. doi: 10.1634/theoncologist.2019-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Hamieh N.M., Akel R., Anouti B., Traboulsi C., Makki I., Hamieh L., Tfayli A. Cancer-Related Pain: Prevalence, Severity and Management in a Tertiary Care Center in the Middle East. Asian Pac. J. Cancer Prev. 2018;19:769–775. doi: 10.22034/APJCP.2018.19.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Haynes-Lewis H., Clayton M.F., Viswanathan S., Moadel-Robblee A., Clark L., Caserta M. Distress and Supportive Care Needs of Ethnically Diverse Older Adults with Advanced or Recurrent Cancer. Oncol. Nurs. Forum. 2018;45:496–507. doi: 10.1188/18.ONF.496-507. [DOI] [PubMed] [Google Scholar]
- 463.Iadeluca L., Mardekian J., Chander P., Hopps M., Makinson G.T. The burden of selected cancers in the US: Health behaviors and health care resource utilization. Cancer Manag. Res. 2017;9:721–730. doi: 10.2147/CMAR.S143148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Jeon M.S., Dhillon H.M., Koh E.S., Nowak A.K., Hovey E., Descallar J., Miller L., Marshall N.S., Agar M.R. Exploring sleep disturbance among adults with primary or secondary malignant brain tumors and their caregivers. Neurooncol. Pract. 2021;8:48–59. doi: 10.1093/nop/npaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Kamieniarz L., Armeni E., O’Mahony L.F., Leigh C., Miah L., Narayan A., Bhatt A., Cox N., Mandair D., Navalkissoor S., et al. Orbital metastases from neuroendocrine neoplasms: Clinical implications and outcomes. Endocrine. 2020;67:485–493. doi: 10.1007/s12020-019-02130-5. [DOI] [PubMed] [Google Scholar]
- 466.Miyashita M., Wada M., Morita T., Ishida M., Onishi H., Sasaki Y., Narabayashi M., Wada T., Matsubara M., Takigawa C., et al. Independent Validation of the Japanese Version of the EORTC QLQ-C15-PAL for Patients with Advanced Cancer. J. Pain Symptom Manag. 2015;49:953–959. doi: 10.1016/j.jpainsymman.2014.11.299. [DOI] [PubMed] [Google Scholar]
- 467.O’Connor J.M., Marmissolle F., Bestani C., Pesce V., Belli S., Dominichini E., Mendez G., Price P., Giacomi N., Pairola A., et al. Observational study of patients with gastroenteropancreatic and bronchial neuroendocrine tumors in argentina: Results from the large database of a multidisciplinary group clinical multicenter study. Mol. Clin. Oncol. 2014;2:673–684. doi: 10.3892/mco.2014.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Parrozzani R., Lombardi G., Midena E., Londei D., Padovan M., Marchione G., Caccese M., Midena G., Zagonel V., Frizziero L. Ocular Side Effects of EGFR-Inhibitor ABT-414 in Recurrent Glioblastoma: A Long-Term Safety Study. Front. Oncol. 2020;10:593461. doi: 10.3389/fonc.2020.593461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Phanphaisarn A., Patumanond J., Settakorn J., Chaiyawat P., Klangjorhor J., Pruksakorn D. Prevalence and Survival Patterns of Patients with Bone Metastasis from Common Cancers in Thailand. Asian Pac. J. Cancer Prev. 2016;17:4335–4340. [PubMed] [Google Scholar]
- 470.Pimentel C.B., Briesacher B.A., Gurwitz J.H., Rosen A.B., Pimentel M.T., Lapane K.L. Pain management in nursing home residents with cancer. J. Am. Geriatr. Soc. 2015;63:633–641. doi: 10.1111/jgs.13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Presley C.J., Canavan M., Wang S.Y., Feder S.L., Kapo J., Saphire M.L., Sheinfeld E., Kent E.E., Davidoff A.J. Severe functional limitation due to pain & emotional distress and subsequent receipt of prescription medications among older adults with cancer. J. Geriatr. Oncol. 2020;11:960–968. doi: 10.1016/j.jgo.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Roila F., Fumi G., Ruggeri B., Antonuzzo A., Ripamonti C., Fatigoni S., Cavanna L., Gori S., Fabi A., Marzano N., et al. Prevalence, characteristics, and treatment of fatigue in oncological cancer patients in Italy: A cross-sectional study of the Italian Network for Supportive Care in Cancer (NICSO) Support. Care Cancer. 2019;27:1041–1047. doi: 10.1007/s00520-018-4393-9. [DOI] [PubMed] [Google Scholar]
- 473.Sadik M., Ozlem K., Huseyin M., AliAyberk B., Ahmet S., Ozgur O. Attributes of cancer patients admitted to the emergency department in one year. World J. Emerg. Med. 2014;5:85–90. doi: 10.5847/wjem.j.issn.1920-8642.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Saito M., Kage H., Ando T., Sawada R., Amano Y., Goto Y., Shinoda Y., Nagase T. Prevalence of bone pain decreases as lymph node stage increases in nonsmall cell lung cancer patients. Curr. Probl. Cancer. 2019;43:86–91. doi: 10.1016/j.currproblcancer.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 475.Santos H.B.P., dos Santos T.K.G., Paz A.R., Cavalcanti Y.W., Nonaka C.F.W., Godoy G.P., Alves P.M. Clinical findings and risk factors to oral squamous cell carcinoma in young patients: A 12-year retrospective analysis. Med. Oral Patol. Oral Cir. Bucal. 2016;21:e151–e156. doi: 10.4317/medoral.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Somboon K., Siramolpiwat S., Vilaichone R.K. Epidemiology and survival of hepatocellular carcinoma in the central region of Thailand. Asian Pac. J. Cancer Prev. 2014;15:3567–3570. doi: 10.7314/APJCP.2014.15.8.3567. [DOI] [PubMed] [Google Scholar]
- 477.Song P.H., Beyhaghi H., Sommer J., Bennett A.V. Symptom burden and life challenges reported by adult chordoma patients and their caregivers. Qual. Life Res. 2017;26:2237–2244. doi: 10.1007/s11136-017-1544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Taghizadeh A., Pourali L., Vaziri Z., Saedi H.R., Behdani F., Amel R. Psychological distress in cancer patients. Middle East J. Cancer. 2018;9:143–149. [Google Scholar]
- 479.Teo I., Cheung Y.B., Lim T.Y.K., Namuduri R.P., Long V., Tewani K. The relationship between symptom prevalence, body image, and quality of life in Asian gynecologic cancer patients. Psychooncology. 2018;27:69–74. doi: 10.1002/pon.4457. [DOI] [PubMed] [Google Scholar]
- 480.van Londen G.J., Beckjord E.B., Dew M.A., Cooper K.L., Davidson N.E., Bovbjerg D.H., Donovan H.S., Thurston R.C., Morse J.Q., Nutt S., et al. Associations between adjuvant endocrine therapy and onset of physical and emotional concerns among breast cancer survivors. Support. Care Cancer. 2014;22:937–945. doi: 10.1007/s00520-013-2041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Wei-Yun W., Shung-Tai H., Shang-Liang W., Chi-Ming C., Chun-Sung S., Kwua-Yun W., Chun-Yu L., Wang W.Y., Ho S.T., Wu S.L., et al. Trends in Clinically Significant Pain Prevalence among Hospitalized Cancer Patients at an Academic Hospital in Taiwan: A Retrospective Cohort Study. Medicine. 2016;95:e2099. doi: 10.1097/MD.0000000000002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Yassin M.A., Taher A., Mathews V., Hou H.A., Shamsi T., Tuğlular T.F., Xiao Z., Kim S.J., Depei W., Li J., et al. MERGE: A Multinational, Multicenter Observational Registry for Myeloproliferative Neoplasms in Asia, including Middle East, Turkey, and Algeria. Cancer Med. 2020;9:4512–4526. doi: 10.1002/cam4.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Yates P., Miaskowski C., Cataldo J.K., Paul S.M., Cooper B.A., Alexander K., Aouizerat B., Dunn L., Ritchie C., McCarthy A., et al. Differences in Composition of Symptom Clusters Between Older and Younger Oncology Patients. J. Pain Symptom Manag. 2015;49:1025–1034. doi: 10.1016/j.jpainsymman.2014.11.296. [DOI] [PubMed] [Google Scholar]
- 484.Yenugadhati N., Albalawi A.N., Qureshey A.T., Qureshey E.T., Al-Jahdali H., Jazieh A.R., Ahmed A.E. Associated factors for oral health problems in a sample of saudi cancer patients. Cancer Manag. Res. 2018;10:1285–1293. doi: 10.2147/CMAR.S165310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Yung C.S.Y., Leung D.K.C., Cheung J.P.Y. The prevalence and impact of cervical spine pathologies in patients with nasopharyngeal carcinoma. Oral Oncol. 2019;90:48–53. doi: 10.1016/j.oraloncology.2019.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.