Abstract
Background
Although many clinical parameters have been identified as predictors for cardiovascular disease (CVD) development in the general population, the accurate predictor for CVD in patients with obesity is still unknown.
Objective
The study aimed to explore an additional risk factor and predictor for CVD in patients with overweight/obesity considering the interaction of obesity‐related pathophysiology.
Methods
The Japan Obesity and Metabolic Syndrome study, a multicenter prospective study, enrolled 787 outpatients, of which 318 eligible patients were analyzed. Patients with fasting plasma glucose (FPG) levels ≥6.11 and < 6.11 mmol/L were considered to have high FPG (HFPG) and normal FPG (NFPG), respectively. Thirty‐six patients who developed CVD during the 5 years follow‐up were assigned to the CVD group.
Results
Cox's proportional hazards model revealed no significant association between CVD and cystatin C‐based estimated glomerular filtration rate (eGFRcys) or creatinine‐based eGFR (eGFRcr) in the NFPG group. In the HFPG group, lower eGFRcys, but not eGFRcr, was significantly associated with CVD development. A generalized linear mixed model demonstrated greater reduction in eGFRcys levels over time with HFPG than with NFPG. Although the CVD group showed gradual reduction in eGFRcys levels, the non‐CVD group—matched using propensity scores—did not show a decline in eGFRcys levels.
Conclusions
Lower eGFRcys levels may be more accurate than eGFRcr in predicting CVD development in patients with overweight/obesity and hyperglycemia. Furthermore, eGFRcys reduction over time is associated with CVD development.
Clinical Trial Registry Number
UMIN000000559
Keywords: cardiovascular disease, hyperglycemia, kidney, obesity
1. INTRODUCTION
The prevalence of overweight and obesity is increasing in Western countries and also in Japan and other Asian countries, albeit to a lesser extent. 1 A Japanese cohort study showed that the prevalence of a body mass index (BMI) ≥25 increased from 7% to almost 30% in the last 40 years, and obesity, that is, BMI ≥30 reached approximately 4%. 2 , 3 Obesity is an independent risk factor for cardiovascular disease (CVD), 4 , 5 , 6 which is the leading cause of death 7 and a serious economic burden for both individuals and the nation. 8 , 9 Therefore, surrogate markers that accurately predict CVD risk are needed for effectively treating individuals at a high risk of obesity.
The Japan Obesity and Metabolic Syndrome (JOMS) study is a multicenter prospective study conducted by several hospitals in the Japanese National Hospital Organization. The JOMS study aimed to identify predictive markers for CVD and investigate its pathophysiology in Japanese patients with obesity. Previous studies reported that the cardio‐ankle vascular index (CAVI), an index of arterial stiffness, urinary cystatin C, and serum amyloid A‐oxidized low‐density lipoprotein (LDL) are valuable indices for assessing and managing CVD risk in patients with obesity. 10 , 11 , 12
Hypertension, dyslipidemia, and impaired glucose metabolism are general risk factors for CVD 13 , 14 , 15 and are often complicated with obesity. 15 , 16 , 17 , 18 These risk factors for CVD may interact with each other. For example, insulin resistance induced by several adipokines elevates the risk for CVD through Endothelial dysfunction (ED) and dyslipidemia. 19 , 20 Understanding such interactions may improve the preventive medicine for CVD in people with obesity. However, such interactions among CVD risks have not been fully understood. Therefore, the present study explored the clinical risk factors and their interactions in predicting CVD development in Japanese patients with overweight/obesity.
2. METHODS
2.1. Participants
The JOMS study, a multicenter prospective cohort study, enrolled 787 Japanese outpatients with obesity aged >20 years between September 2005 and August 2010. Five National Hospital Organization hospitals (Kyoto, Tokyo, Nagoya, Kokura Medical Centers, and Mie Hospital) and the Oishi Clinic (Kyoto) were involved. The JOMS study was registered on the University Hospital Medical Information Network system (UMIN000000559; registration date, 25 December 2006).
Participants with missing data for baseline measurements, those who underwent baseline measurements without fasting, those who did not undergo follow‐up measurements, and those with a history of CVD at baseline were excluded from the analysis. The number of patients included in the final analysis was 318.
The present study was approved by the ethics committee of National Hospital Organization Kyoto Medical Center (approval number: 14–034) and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent for inclusion prior to participation.
2.2. Baseline measurements
BMI, systolic blood pressure (SBP), and diastolic BP (DBP) were measured. 10 Blood samples were drawn from the antecubital vein in the morning after fasting for 12 h without medication intake. Hemoglobin A1c, fasting plasma glucose (FPG), gamma‐glutamyl transferase, aspartate aminotransferase, alanine aminotransferase, serum total cholesterol, high‐density lipoprotein‐cholesterol, LDL‐cholesterol, and triglyceride levels were determined according to standard procedures. 10 High‐sensitivity C‐reactive protein levels were assessed using an enzyme‐linked immunosorbent assay (AssayPro LLC, St. Charles, USA). Immunoreactive insulin levels were measured using an enzyme immunoassay (Tosoh, Tokyo, Japan). Serum creatinine levels were measured using the enzymatic method. Serum cystatin C levels were determined using a latex particle‐enhanced immunoturbidimetric assay (Ikagaku, Kyoto, Japan). Estimated glomerular filtration rates were calculated based on serum cystatin C (eGFRcys) or creatinine (eGFRcr) levels using the following equations modified for the Japanese population 21 , 22 :
eGFRcr for men: 194 × (serum creatinine)−1.094 × (age)−0.287
eGFRcr for women: 194 × (serum creatinine)−1.094 × (age)−0.287 × 0.739
eGFRcys for men: [104 × (serum cystatin C)−1.019 × 0.996age] − 8
eGFRcys for women: [104 × (serum cystatin C)−1.019 × 0.996age × 0.929] − 8
Patients were categorized as non‐, ex‐, and current smokers according to their habits. Information regarding intake of anti‐diabetic, lipid‐lowering, anti‐hypertensive, and anti‐obesity medications was collected from the participants' medical records.
The cut point of FPG was set at 6.11 mmol/L in this study based on the criteria proposed by the World Health Organization 23 and Japan Diabetes Society 24 defining FPG of 6.11–6.94 mmol/L as impaired fasting glucose. Therefore, patients with a baseline FPG ≥6.11 mmol/L were assigned to the high FPG (HFPG) group, and those with an FPG below this value were assigned to the normal FPG (NFPG) group.
2.3. Follow‐up and outcomes
Patients were followed up at 3 and 6 months and 1, 2, 3, 4, and 5 years after the baseline measurement. At each follow‐up visit, the same measurements were performed as at baseline. The physicians of each hospital diagnosed CVD development and wrote the case report form including CVD onset date for the present study. Patients who developed CVD during the follow‐up period were categorized into the CVD group, while those who did not were categorized into the non‐CVD group. Patients assigned to the CVD group developed one of the following diseases: coronary heart disease, heart failure, cerebrovascular disease, aortic aneurysm, cardiomyopathy, or peripheral arterial disease.
2.4. Statistical analysis
Continuous variables were summarized as means and standard deviations and categorical variables were summarized as numbers (n) and percentages (%). Student's t‐test and chi‐squared test were used to compare differences in mean levels of continuous and categorical variables, respectively, at baseline between the non‐CVD and CVD groups. A two‐way analysis of covariance (ANCOVA) was used to examine the interaction between CVD development and FPG levels on eGFRcys/eGFRcr at baseline after adjusting for sex, age, BMI, and SBP. The association of eGFRcr and eGFRcys at baseline with CVD development was examined using Cox's proportional hazards model after adjusting for sex, age, BMI, SBP, and FPG. A generalized linear mixed model (GLMM) was used to assess differences in changes in eGFRcys over time between the non‐CVD and CVD groups after adjusting for sex, age, BMI, SBP, and FPG, with participants as a variable factor. Additionally, the effect of baseline FPG levels on the change in eGFRcys over time was examined using a GLMM after adjusting for sex, age, BMI, and SBP, with participants as a variable factor.
Patients in the CVD group were matched with those in the non‐CVD group using propensity score matching (PSM; caliper width 0.1). The PSM ratio was 1:1. Age and eGFRcys at baseline were included in the PSM. To compare changes in eGFRcys levels over time between the CVD and non‐CVD groups—which had similar eGFRcys levels at baseline—a GLMM was used after adjusting for sex, age, BMI, SBP, and FPG, with participants as a variable factor.
All statistical analyses were performed using the Japanese version of IBM SPSS Statistics version 27.0 (IBM Japan, Tokyo, Japan). Statistical significance was set at a two‐sided p‐value <0.05.
3. RESULTS
3.1. Patient characteristics at baseline
Of 318 patients, 36 developed CVD for the first time during the follow‐up period. The patients were followed up for a mean duration of 3.1 ± 1.8 years, and the incidence of CVD was 35.8 events per 1000 person‐years. Patient characteristics at baseline are shown in Table 1. The mean age of patients in the CVD group was significantly higher than that of patients in the non‐CVD group (58 ± 12 years vs. 51 ± 14 years, p = 0.003). The CVD group showed significantly lower eGFRcr and eGFRcys than the non‐CVD group (74 ± 24 ml/min/1.73 m2 vs. 84 ± 24 ml/min/1.73 m2, p = 0.021; and 88 ± 30 ml/min/1.73 m2 vs. 104 ± 27 ml/min/1.73 m2, p = 0.001, respectively). However, no significant difference in other clinical parameters was observed between the non‐CVD and CVD groups. The ratio of patients receiving lipid‐lowering or anti‐hypertensive medications at baseline was significantly higher in the CVD group than in the non‐CVD group (69% vs. 47%, p = 0.009; and 69% vs. 47%, p = 0.012, respectively).
TABLE 1.
Baseline characteristics of the patients
| Total (n = 318) | Non‐CVD (n = 282) | CVD (n = 36) | p‐value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Female (n, %) | 184 | 58 | 168 | 60 | 16 | 44 | 0.083 |
| Age (y) | 52 | 14 | 51 | 14 | 58 | 12 | 0.003 |
| BMI (kg/m2) | 31.2 | 5.5 | 31.2 | 5.5 | 31.0 | 6.0 | 0.83 |
| SBP (mmHg) | 141.5 | 18.3 | 141.4 | 17.9 | 142.3 | 21.6 | 0.80 |
| DBP (mmHg) | 83.8 | 11.7 | 84.0 | 11.7 | 82.6 | 11.4 | 0.50 |
| FPG (mmol/L) | 7.0 | 3.0 | 6.9 | 2.9 | 7.3 | 3.6 | 0.47 |
| HbA1c (mmol/mol) | 52.1 | 16.7 | 52.1 | 16.8 | 52.6 | 16.2 | 0.87 |
| HbA1c (%) | 6.9 | 1.5 | 6.9 | 1.5 | 7.0 | 1.5 | 0.87 |
| IRI (pmol/L) | 129 | 151 | 124 | 126 | 170 | 271 | 0.32 |
| GGT (μkat/L) | 0.79 | 0.67 | 0.79 | 0.67 | 0.76 | 0.69 | 0.78 |
| AST (μkat/L) | 0.46 | 0.22 | 0.46 | 0.23 | 0.42 | 0.16 | 0.32 |
| ALT (μkat/L) | 0.60 | 0.43 | 0.61 | 0.45 | 0.54 | 0.34 | 0.40 |
| TC (mmol/L) | 5.4 | 0.9 | 5.4 | 0.9 | 5.3 | 1.0 | 0.33 |
| HDL‐C (mmol/L) | 1.4 | 0.4 | 1.4 | 0.3 | 1.3 | 0.5 | 0.10 |
| LDL‐C (mmol/L) | 3.3 | 0.8 | 3.4 | 0.8 | 3.1 | 0.8 | 0.64 |
| Triglyceride (mmol/L) | 2.1 | 1.3 | 2.0 | 1.3 | 2.1 | 1.0 | 0.91 |
| hsCRP (mg/L) | 1.95 | 5.22 | 1.70 | 2.79 | 3.87 | 13.41 | 0.34 |
| eGFRcr (mL min−1 [1.73 m]−2) | 82 | 24 | 84 | 24 | 74 | 24 | 0.021 |
| eGFRcys (mL min−1 [1.73 m]−2) | 103 | 28 | 104 | 27 | 88 | 30 | 0.001 |
| Smoking status (n, %) | 0.32 | ||||||
| Ex‐smoker | 34 | 11 | 29 | 10 | 5 | 14 | |
| Current smoker | 56 | 18 | 47 | 17 | 9 | 25 | |
| Anti‐diabetic agents (n, %) | 118 | 37 | 104 | 37 | 14 | 39 | 0.81 |
| Lipid‐lowering agents (n, %) | 156 | 49 | 131 | 47 | 25 | 69 | 0.009 |
| Antihypertensive agents (n, %) | 158 | 50 | 133 | 47 | 25 | 69 | 0.012 |
| Antiobesity agents (n, %) | 16 | 5 | 15 | 5 | 1 | 3 | 0.51 |
| HFPG (n, %) | 155 | 49 | 136 | 48 | 19 | 53 | 0.61 |
Note: p‐values were obtained using Student's t‐test for continuous variables and the chi‐squared test for categorical variables. p‐values <0.05 are in bold.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFRcr, estimated glomerular filtration rate calculated using serum creatinine; eGFRcys, estimated glomerular filtration rate calculated using serum cystatin C; FPG, fasting plasma glucose; GGT, gamma‐glutamyl transferase; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein‐cholesterol; HFPG, high fasting plasma glucose; hsCRP, high‐sensitivity C‐reactive protein; IRI, immunoreactive insulin; LDL‐C, low‐density lipoprotein‐cholesterol; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol.
3.2. Comparison of baseline characteristics between the non‐CVD and CVD groups according to FPG levels
Patients were stratified according to their FPG levels to investigate the effect of FPG levels on patient characteristics. Table 2 compares the baseline characteristics of the non‐CVD and CVD groups according to FPG levels. The CVD patients were significantly older than the non‐CVD patients in the NFPG group (57 ± 10 years vs. 49 ± 14 years, p = 0.005), while no significant difference in age was observed between the two subgroups in the HFPG group (59 ± 14 years vs. 54 ± 14 years, p = 0.14). In the HFPG group, the CVD patients showed significantly lower eGFRcr and eGFRcys levels than the non‐CVD patients (69 ± 26 ml/min/1.73 m2 vs. 82 ± 23 ml/min/1.73 m2, p = 0.022; and 79 ± 26 ml/min/1.73 m2 vs. 103 ± 27 ml/min/1.73 m2, p < 0.001, respectively), whereas no significant difference was observed in the eGFR in the NFPG group (79 ± 22 ml/min/1.73 m2 vs. 85 ± 25 ml/min/1.73 m2, p = 0.36; and 97 ± 32 ml/min/1.73 m2 vs. 106 ± 27 ml/min/1.73 m2, p = 0.22, respectively). In the NFPG group, the proportion of patients with smoking habits and receiving lipid‐lowering medications was significantly higher among patients with CVD compared with non‐CVD group (current smoker, 29% vs. 15%, p = 0.027; lipid‐lowering medications, 76% vs. 46%, p = 0.017, respectively).
TABLE 2.
Comparison of baseline characteristics between the non‐CVD and CVD groups according to fasting plasma glucose (FPG) levels
| NFPG | HFPG | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non‐CVD (n = 146) | CVD (n = 17) | p‐value | Non‐CVD (n = 136) | CVD (n = 19) | p‐value | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Female (n, %) | 85 | 58 | 7 | 41 | 0.18 | 83 | 61 | 9 | 47 | 0.26 |
| Age (y) | 49 | 14 | 57 | 10 | 0.005 | 54 | 14 | 59 | 14 | 0.14 |
| BMI (kg/m2) | 31.6 | 6.0 | 31.6 | 5.9 | 0.99 | 30.8 | 4.9 | 30.5 | 6.2 | 0.79 |
| SBP (mmHg) | 141 | 18 | 136 | 18 | 0.35 | 142 | 18 | 148 | 24 | 0.25 |
| DBP (mmHg) | 84 | 12 | 82 | 12 | 0.52 | 84 | 12 | 83 | 11 | 0.76 |
| FPG (mmol/L) | 5.2 | 0.6 | 5.1 | 0.5 | 0.84 | 8.9 | 3.3 | 9.3 | 4.0 | 0.60 |
| HbA1c (mmol/mol) | 43.2 | 10.4 | 43.9 | 11.1 | 0.78 | 61.6 | 17.2 | 60.3 | 16.3 | 0.76 |
| HbA1c (%) | 6.1 | 1.0 | 6.2 | 1.0 | 0.78 | 7.8 | 1.6 | 7.7 | 1.5 | 0.76 |
| IRI (pmol/L) | 129 | 151 | 124 | 126 | 0.48 | 140 | 138 | 241 | 359 | 0.24 |
| GGT (μkat/L) | 0.76 | 0.66 | 0.76 | 0.56 | 0.99 | 0.83 | 0.69 | 0.76 | 0.80 | 0.70 |
| AST (μkat/L) | 0.45 | 0.21 | 0.43 | 0.13 | 0.64 | 0.47 | 0.26 | 0.41 | 0.19 | 0.37 |
| ALT (μkat/L) | 0.62 | 0.46 | 0.54 | 0.26 | 0.45 | 0.59 | 0.43 | 0.55 | 0.41 | 0.68 |
| TC (mmol/L) | 5.6 | 1.0 | 5.5 | 0.7 | 0.62 | 5.3 | 0.9 | 5.1 | 1.2 | 0.44 |
| HDL‐C (mmol/L) | 1.5 | 0.4 | 1.4 | 0.5 | 0.42 | 1.4 | 0.3 | 1.3 | 0.4 | 0.14 |
| LDL‐C (mmol/L) | 3.4 | 0.9 | 3.4 | 0.8 | 0.89 | 3.1 | 0.8 | 3.1 | 0.9 | 0.68 |
| Triglyceride (mmol/L) | 2.0 | 1.5 | 2.2 | 0.9 | 0.72 | 2.1 | 1.2 | 2.0 | 1.1 | 0.82 |
| hsCRP (mg/L) | 1.63 | 3.16 | 1.96 | 2.81 | 0.68 | 1.78 | 2.33 | 5.57 | 18.34 | 0.38 |
| eGFRcr (mL min−1 [1.73 m]−2) | 85 | 25 | 79 | 22 | 0.36 | 82 | 23 | 69 | 26 | 0.022 |
| eGFRcys (mL min−1 [1.73 m]−2) | 106 | 27 | 97 | 32 | 0.22 | 103 | 27 | 79 | 26 | <0.001 |
| Smoking status (n, %) | 0.027 | 0.44 | ||||||||
| Ex‐smoker | 18 | 12 | 5 | 29 | 11 | 8 | 0 | 0 | ||
| Current smoker | 22 | 15 | 5 | 29 | 25 | 18 | 4 | 21 | ||
| Antidiabetic agents (n, %) | 23 | 16 | 2 | 12 | 0.67 | 81 | 60 | 12 | 63 | 0.76 |
| Lipid‐lowering agents (n, %) | 67 | 46 | 13 | 76 | 0.017 | 64 | 47 | 12 | 63 | 0.19 |
| Antihypertensive agents (n, %) | 60 | 41 | 11 | 65 | 0.063 | 73 | 54 | 14 | 74 | 0.10 |
| Antiobesity agents (n, %) | 10 | 7 | 1 | 6 | 0.88 | 5 | 4 | 0 | 0 | 0.40 |
Note: p‐values were obtained using Student's t‐test for continuous variables and the chi‐squared test for categorical variables. p‐values <0.05 are in bold.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFRcr, estimated glomerular filtration rate calculated using serum creatinine; eGFRcys, estimated glomerular filtration rate calculated using serum cystatin C; FPG, fasting plasma glucose; GGT, gamma‐glutamyl transferase; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein‐cholesterol; HFPG, high fasting plasma glucose; hsCRP, high‐sensitivity C‐reactive protein; IRI, immunoreactive insulin; LDL‐C, low‐density lipoprotein‐cholesterol; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol.
3.3. Interaction between the development of CVD and FPG levels on eGFR at baseline
Two‐way ANCOVA was performed to assess the interaction between CVD development and FPG levels on eGFR at baseline. Table 3 shows the results of the two‐way ANCOVA. There were no significant main effects of CVD development and FPG levels on eGFRcys (p = 0.067 and 0.067, respectively) or eGFRcr levels (p = 0.42 and 0.26, respectively). However, a significant interaction was observed between CVD development and FPG levels on eGFRcys (p = 0.017), but not on eGFRcr levels (p = 0.22).
TABLE 3.
Interaction between CVD development and fasting plasma glucose (FPG) levels on eGFR at baseline
| Groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| Non‐CVD | CVD | p for FPG levels a | p for CVD groups b | p for interaction c | ||||
| Dependent variables | FPG levels | Mean | SD | Mean | SD | |||
| eGFRcys (mL min−1 [1.73 m]−2) | NFPG | 106 | 27 | 97 | 32 | 0.067 | 0.067 | 0.017 |
| HFPG | 103 | 27 | 79 | 26 | ||||
| eGFRcr (mL min−1 [1.73 m]−2) | NFPG | 85 | 25 | 79 | 22 | 0.42 | 0.26 | 0.22 |
| HFPG | 82 | 23 | 69 | 26 | ||||
Note: Analyses were adjusted for sex, age, body mass index, and systolic blood pressure.
Abbreviations: CVD, cardiovascular disease; eGFRcys, estimated glomerular filtration rate calculated using serum cystatin C; eGFRcr, estimated glomerular filtration rate calculated using serum creatinine; FPG, fasting plasma glucose; HFPG, high fasting plasma glucose; NFPG, normal fasting plasma glucose; SD, standard deviation.
a: p‐value of the main effect of FPG levels on estimated glomerular filtration rate;
b: p‐value of the main effect of CVD development on estimated glomerular filtration rate;
c: p‐value of the interaction between FPG levels and CVD development on estimated glomerular filtration rate at baseline. p‐values <0.05 are in bold.
3.4. Cox's proportional hazards model of CVD risk according to FPG levels
Cox's proportional hazards model was used to examine the association between eGFR at baseline and CVD development after adjusting for covariates (Table 4). eGFRcys and eGFRcr were included as independent variables in models 1 and 2, respectively.
TABLE 4.
Cox's proportional hazards model of cardiovascular disease development as a dependent variable
| Total | NFPG | HFPG | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | |||||||||||
| Variables | HR | Lower | Upper | p‐value | HR | Lower | Upper | p‐value | HR | Lower | Upper | p‐value | |
| Model 1 | Female (vs. male) | 0.50 | 0.25 | 1.00 | 0.049 | 0.43 | 0.15 | 1.21 | 0.11 | 0.38 | 0.14 | 1.07 | 0.067 |
| Age (per 1 year) | 1.02 | 0.99 | 1.06 | 0.19 | 1.07 | 1.01 | 1.13 | 0.015 | 0.99 | 0.95 | 1.05 | 0.82 | |
| BMI (per 1 kg/m2) | 1.04 | 0.97 | 1.11 | 0.27 | 1.06 | 0.97 | 1.15 | 0.18 | 1.01 | 0.91 | 1.13 | 0.86 | |
| SBP (per 1 mmHg) | 1.00 | 0.98 | 1.02 | 0.72 | 0.99 | 0.96 | 1.02 | 0.36 | 1.00 | 0.97 | 1.02 | 0.90 | |
| FPG (per 1 mmol/L) | 1.05 | 0.94 | 1.16 | 0.39 | 0.93 | 0.40 | 2.16 | 0.87 | 1.09 | 0.95 | 1.25 | 0.20 | |
| eGFRcys (per 1 ml min−1 [1.73 m]−2) | 0.99 | 0.97 | 1.00 | 0.059 | 1.01 | 0.99 | 1.03 | 0.45 | 0.97 | 0.94 | 0.99 | 0.003 | |
| Model 2 | Female (vs. male) | 0.50 | 0.25 | 0.99 | 0.048 | 0.43 | 0.15 | 1.23 | 0.12 | 0.37 | 0.13 | 1.04 | 0.058 |
| Age (per 1 year) | 1.03 | 1.00 | 1.07 | 0.032 | 1.06 | 1.01 | 1.12 | 0.013 | 1.02 | 0.97 | 1.07 | 0.40 | |
| BMI (per 1 kg/m2) | 1.06 | 0.99 | 1.13 | 0.098 | 1.05 | 0.96 | 1.14 | 0.27 | 1.05 | 0.94 | 1.18 | 0.39 | |
| SBP (per 1 mmHg) | 1.00 | 0.98 | 1.02 | 0.76 | 0.98 | 0.96 | 1.01 | 0.28 | 1.00 | 0.98 | 1.03 | 0.70 | |
| FPG (per 1 mmol/L) | 1.04 | 0.94 | 1.15 | 0.47 | 0.99 | 0.45 | 2.21 | 0.98 | 1.08 | 0.95 | 1.24 | 0.25 | |
| eGFRcr (per 1 ml min−1 [1.73 m]−2) | 0.99 | 0.97 | 1.01 | 0.23 | 1.01 | 0.98 | 1.03 | 0.58 | 0.98 | 0.95 | 1.00 | 0.070 | |
Note: HR indicates the hazard ratio for cardiovascular disease development. Models 1 and 2 include the same independent variables, except for eGFR. p‐values <0.05 are in bold.
Abbreviations: BMI, body mass index; CI, confidence interval; eGFRcys, estimated glomerular filtration rate calculated using serum cystatin C; eGFRcr, estimated glomerular filtration rate calculated using serum creatinine; FPG, fasting plasma glucose; HR, hazard ratio; HFPG, high fasting plasma glucose; NFPG, normal fasting plasma glucose; SBP, systolic blood pressure.
Among all patients, neither eGFRcys nor eGFRcr showed a significant association with CVD development (hazard ratio (HR) = 0.99, 95% confidence interval (CI) = 0.97–1.00, p = 0.059; and HR = 0.99, 95% CI = 0.97–1.01, p = 0.23, respectively). Moreover, a significant association between age and CVD was observed only in model 2.
In the NFPG group, neither eGFRcys nor eGFRcr were significantly associated with CVD (HR = 1.01, 95% CI = 0.99–1.03, p = 0.45; and HR = 1.01, 95% CI = 0.98–1.03, p = 0.58, respectively). Nevertheless, older patients had significantly higher CVD risk in both models 1 and 2 (HR = 1.07, 95% CI = 1.01–1.13, p = 0.015; and HR = 1.06, 95% CI = 1.01–1.12, p = 0.013, respectively).
In the HFPG group, patients with lower eGFRcys levels showed a higher risk of CVD development after adjusting for covariates (HR = 0.97, 95% CI = 0.94–0.99, p = 0.003). However, the association between eGFRcr and CVD development was not significant (HR = 0.98, 95% CI = 0.95–1.00, p = 0.070).
3.5. GLMM for assessing changes in eGFRcys levels during follow‐up
The significance and effect of changes in eGFRcys on CVD development were examined using a GLMM. Figure 1 shows the changes in eGFRcys levels during follow‐up. In the CVD group, eGFRcys levels were significantly lower than in the non‐CVD group (p = 0.024), whereas no significant difference was observed in the changes in eGFRcys levels between the non‐CVD and CVD groups during follow‐up (p for interaction = 0.74) (Figure 1A). The HFPG group demonstrated significantly lower eGFRcys levels than the NFPG group (p = 0.020) during follow‐up (Figure 1B). Furthermore, the HFPG group showed a significantly greater decrease in eGFRcys levels during follow‐up than the NFPG group (p for interaction = 0.04). The GLMM results for the NFPG and HFPG groups are shown in Figure 1C,D, respectively. No significant difference (p = 0.99) or interaction (p for interaction = 0.65) in eGFRcys levels was observed between patients with CVD and non‐CVD in the NFPG group. In the HFPG group, CVD patients demonstrated significantly lower eGFRcys levels (p = 0.002), but no significant interaction was observed (p for interaction = 0.76).
FIGURE 1.
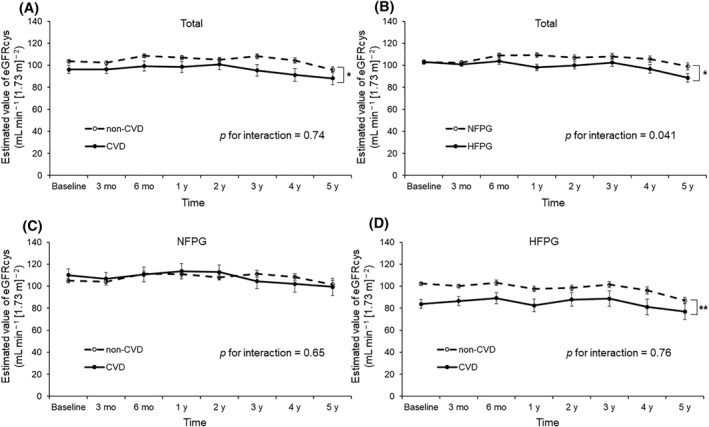
A generalized linear mixed model (GLMM) for the assessment of changes in eGFRcys during follow‐up. Panel A shows the changes in eGFRcys over time with adjustment for sex, age, body mass index (BMI), systolic blood pressure (SBP), and fasting plasma glucose (FPG) in all patients. Panel B shows the changes in eGFRcys with adjustment for sex, age, BMI, and SBP according to baseline FPG levels. Panels C and D show the changes in eGFRcys over time with adjustment for sex, age, BMI, SBP, and FPG in the normal FPG group (NFPG) and HFPG groups, respectively. Total population, n = 318; population with CVD, n = 36; NPFG group, n = 163; NFPG with CVD group, n = 17; HPFG group, n = 155; NFPG with CVD group, n = 19. *p < 0.05, **p < 0.01. CVD, cardiovascular disease; eGFRcys, estimated glomerular filtration rate calculated using serum cystatin C; FPG, fasting plasma glucose; HFPG, high fasting plasma glucose; NFPG, normal plasma blood glucose
3.6. Baseline characteristics of patients after PSM
PSM was performed to match patients with similar characteristics in the non‐CVD group with those in the CVD group, considering age and eGFRcys levels. In total, 36 pairs of patients were successfully matched using PSM. The propensity scores of the non‐CVD and CVD groups were 0.126 ± 0.064 and 0.153 ± 0.072, respectively. Table 5 shows the baseline characteristics of patients matched by PSM. No significant difference was observed between the non‐CVD and CVD groups in age and eGFRcys levels (52 ± 17 years vs. 58 ± 12 years, p = 0.090; and 95 ± 24 ml/min/1.73 m2 vs. 88 ± 30 ml/min/1.73 m2, p = 0.29, respectively). The proportion of female patients was significantly higher in the non‐CVD group (69% vs. 44%, p = 0.032). The ratio of patients receiving lipid‐lowering and anti‐hypertensive medications was significantly higher in the CVD group (69% vs. 44%, p = 0.032; and 69% vs. 36%, p = 0.005, respectively).
TABLE 5.
Baseline characteristics of patients matched by propensity scores
| Total (n = 72) | Non‐CVD (n = 36) | CVD (n = 36) | p‐value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Female (n, %) | 41 | 57 | 25 | 69 | 16 | 44 | 0.032 |
| Age (y) | 55 | 15 | 52 | 17 | 58 | 12 | 0.090 |
| BMI (kg/m2) | 31.4 | 5.6 | 31.9 | 5.3 | 31.0 | 6.0 | 0.52 |
| SBP (mmHg) | 142 | 20 | 143 | 18 | 142 | 22 | 0.93 |
| DBP (mmHg) | 82 | 11 | 82 | 11 | 83 | 11 | 0.70 |
| FPG (mmol/L) | 7.2 | 3.5 | 7.0 | 3.4 | 7.3 | 3.6 | 0.68 |
| HbA1c (mmol/mol) | 52.7 | 15.2 | 52.8 | 14.4 | 52.6 | 16.2 | 0.96 |
| HbA1c (%) | 7.0 | 1.4 | 7.0 | 1.3 | 7.0 | 1.5 | 0.96 |
| IRI (pmol/L) | 138 | 214 | 93 | 65 | 170 | 271 | 0.17 |
| GGT (μkat/L) | 0.80 | 0.76 | 0.85 | 0.83 | 0.76 | 0.69 | 0.63 |
| AST (μkat/L) | 0.46 | 0.21 | 0.50 | 0.24 | 0.42 | 0.16 | 0.096 |
| ALT (μkat/L) | 0.60 | 0.43 | 0.66 | 0.50 | 0.54 | 0.34 | 0.25 |
| TC (mmol/L) | 5.4 | 0.9 | 5.5 | 0.8 | 5.3 | 1.0 | 0.33 |
| HDL‐C (mmol/L) | 1.4 | 0.4 | 1.5 | 0.4 | 1.3 | 0.5 | 0.051 |
| LDL‐C (mmol/L) | 3.3 | 0.8 | 3.3 | 0.8 | 3.2 | 0.8 | 0.45 |
| Triglyceride (mmol/L) | 2.0 | 0.9 | 1.8 | 0.9 | 2.1 | 1.0 | 0.29 |
| hsCRP (mg/L) | 2.82 | 9.56 | 1.77 | 1.81 | 3.87 | 13.41 | 0.36 |
| eGFRcr (mL min−1 [1.73 m]−2) | 79 | 27 | 84 | 28 | 74 | 24 | 0.11 |
| eGFRcys (mL min−1 [1.73 m]−2) | 91 | 28 | 95 | 24 | 88 | 30 | 0.29 |
| Smoking status (n, %) | 0.41 | ||||||
| Ex‐smoker | 9 | 13 | 4 | 11 | 5 | 14 | |
| Current smoker | 14 | 19 | 5 | 14 | 9 | 25 | |
| Antidiabetic agents (n, %) | 29 | 40 | 15 | 42 | 14 | 39 | 0.81 |
| Lipid‐lowering agents (n, %) | 41 | 57 | 16 | 44 | 25 | 69 | 0.032 |
| Antihypertensive agents (n, %) | 38 | 53 | 13 | 36 | 25 | 69 | 0.005 |
| Antiobesity agents (n, %) | 1 | 1 | 0 | 0 | 1 | 3 | 0.31 |
| HFPG (n, %) | 34 | 47 | 15 | 42 | 19 | 53 | 0.35 |
Note: p‐values were obtained using Student's t‐test for continuous variables and the chi‐squared test for categorical variables. p‐values <0.05 are in bold.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFRcr, estimated glomerular filtration rate calculated using serum creatinine; eGFRcys, estimated glomerular filtration rate calculated using serum cystatin C; FPG, fasting plasma glucose; GGT, gamma‐glutamyl transferase; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein‐cholesterol; HFPG, high fasting plasma glucose; hsCRP, high‐sensitivity C‐reactive protein; IRI, immunoreactive insulin; LDL‐C, low‐density lipoprotein‐cholesterol; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol.
3.7. GLMM after PSM
A GLMM was performed to compare the changes in eGFRcys over time between the non‐CVD and CVD groups matched using propensity scores. Figure 2 shows the changes in eGFRcys levels during follow‐up. The CVD group showed significantly lower eGFRcys than the non‐CVD group during follow‐up (p = 0.014). The GLMM revealed that the CVD group demonstrated a gradual reduction in eGFRcys levels, whereas the non‐CVD group maintained eGFRcys levels above the baseline value throughout the follow‐up period (p for interaction = 0.001).
FIGURE 2.
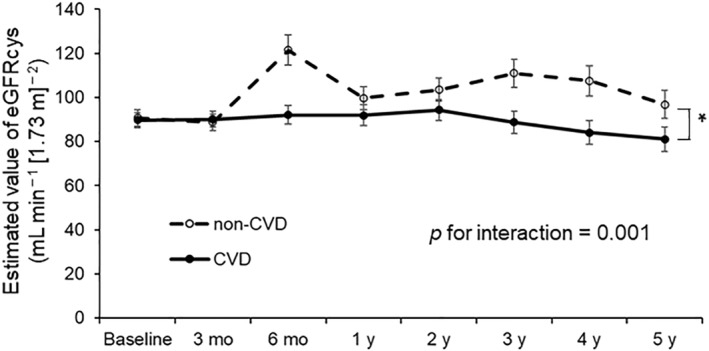
A generalized linear mixed model (GLMM) after propensity score matching. Changes in eGFRcys over time with adjustment for sex, age, body mass index (BMI), systolic blood pressure (SBP), and fasting plasma glucose (FPG) are shown. Non‐CVD group, n = 36; CVD group, n = 36. *p < 0.05. CVD, cardiovascular disease; eGFRcys, estimated glomerular filtration rate calculated using serum cystatin C
4. DISCUSSION
Our findings revealed that lower eGFRcys levels—and their further decline—were significantly associated with CVD development in the HFPG group but not in all patients or the NFPG group. The other parameter, eGFRcr, showed no significant association with CVD development in any group. The results of the GLMM showed that the slope of decline in eGFRcys levels was greater in the HFPG group than in the NFPG group, whereas no significant difference between the non‐CVD and CVD groups in the changes in eGFRcys levels was observed in all patients, the NFPG, and HFPG groups. Additionally, GLMM after PSM demonstrated that the CVD group showed a gradual decline in eGFRcys levels during follow‐up, while 36 patients in the non‐CVD group matched to those in the CVD group according to the baseline eGFRcys levels maintained their eGFRcys levels above the baseline value.
Several longitudinal studies reported that CVD development is associated with lower kidney function, as demonstrated by lower eGFRcr levels. 25 , 26 However, our findings revealed the lack of association between decreased kidney function and CVD development that may be attributed to the use of eGFRcr but not eGFRcys as a surrogate marker for kidney function and its interaction with FPG levels in previous studies. In the multivariate analysis, no significant association between eGFRcr and CVD development was observed in this study. Serum creatinine levels are influenced by skeletal muscle mass, diet, and physical activity. 27 , 28 The eGFRcr levels could overestimate kidney function in elderly people because aging‐associated sarcopenia reduces creatinine levels. Creatinine levels are also affected by absolute skeletal muscle mass, resulting in the underestimation of kidney function in people with overweight/obesity. However, such aging‐ and weight‐associated alterations in skeletal muscle mass do not affect cystatin C levels. Previous reports suggest the accuracy of cystatin C—rather than creatinine—in predicting CVD development in the elderly 29 , 30 , 31 and people with overweight/obesity. 32 , 33 The present study supports these previous reports that cystatin C predicts CVD development more accurately than creatinine.
Although the previous studies 32 , 33 showed a significant association between decreased eGFRcys and CVD risk in participants with overweight/obesity, there are differences from this study that should be considered in age and outcome. The mean age of the previous study 32 was around 65 years, which was considerably older than that of the present study (52 ± 14 years). Another study 33 used Framingham coronary heart disease (CHD) score as an index of CHD risk and did not assess CVD events. Therefore, the present study may provide valuable evidence to predict CVD development, particularly in patients with overweight/obesity aged around 50 years.
Furthermore, this is the first study to discover the interaction between eGFRcys and hyperglycemia in predicting CVD development in patients with overweight/obesity. An association of eGFRcys with CVD development was observed in patients with overweight/obesity and hyperglycemia but not in those without hyperglycemia. These findings are valuable in understanding the pathophysiology of obesity‐associated CVD and may be applied to generating a tailor‐made approach for preventing CVD in patients with overweight/obesity.
Interaction between the heart and kidneys is well‐recognized, and dysfunction in this relationship may cause cardiorenal syndrome (CRS). 34 ED, dysregulation of body fluids, and anemia are the underlying mechanisms of CRS. ED is promoted by asymmetric dimethylarginine (ADMA), which disturbs the activation of nitric oxide. 35 , 36 Furthermore, a prospective study reported an association between higher ADMA levels and CVD development. 37 Previous studies also showed that hyperglycemia elevates ADMA levels via the inactivation of dimethylarginine dimethylaminohydrolase—an enzyme involved in the metabolism of ADMA. 38 , 39 In the GLMM analysis, the HFPG group showed a significantly greater decline in eGFRcys levels over time than the NFPG group. CRS could be promoted through ED, accelerated by ADMA in the HFPG group, resulting in a significant association between lower eGFRcys levels at baseline and CVD development only in the HFPG group.
In the NFPG group, eGFRcys levels at baseline were not associated with CVD development, and the decline in eGFRcys levels over time was smaller in magnitude than in the HFPG group. Therefore, the involvement of eGFRcys in CVD development may not be remarkable in patients with overweight/obesity and normoglycemia. Visceral fat accumulation causes various CVD risk factors to develop, such as dyslipidemia, impaired glucose tolerance, insulin resistance, and hypertension. 6 , 40 However, no significant association of these factors with CVD development was observed. Therefore, the predictive value of surrogate markers for CVD in patients with overweight/obesity and normoglycemia remains unclear.
The results of GLMM after PSM revealed that the non‐CVD group maintained its eGFRcys levels above the baseline value during follow‐up, whereas the CVD group showed a gradual decline in eGFRcys levels during follow‐up. Our results are supported by previous findings that deterioration of kidney function is a hallmark for CVD development. 41 , 42 , 43 , 44 , 45 Therefore, maintaining eGFRcys levels may be beneficial in preventing CVD development in patients with overweight/obesity, even if their eGFRcys levels were comparable with that of patients who developed CVD at baseline.
Our study had several limitations. First, due to unknown confounding factors, an observational study cannot definitively prove a causal link between eGFR levels at baseline and changes in eGFR with CVD development. Second, because the degree of obesity in the Japanese patients recruited in this study is different from that of Western patients, the application of our results to Western populations may be limited. Finally, alteration in medication regimens during follow‐up was not considered in the analysis.
In conclusion, lower eGFRcys, but not eGFRcr levels at baseline, is associated with CVD development in patients with overweight/obesity and hyperglycemia, but not in those without hyperglycemia. Furthermore, eGFRcys reduction over time is associated with CVD development. These findings deepen our understanding of the pathophysiology in overweight/obesity‐associated CVD. The eGFRcys combined with hyperglycemia may be a valuable surrogate marker for a tailor‐made approach to prevent CVD in patients with overweight/obesity.
AUTHOR CONTRIBUTIONS
Toshinari Takamura designed the study, interpreted the results, and edited the manuscript. Keita Suzuki analyzed the data, interpreted the results, and wrote the manuscript with inputs from all authors. Hiromasa Tsujiguchi, Akinori Hara, and Hiroyuki Nakamura analyzed and discussed the data. Noriko Satoh‐Asahara and Hajime Yamakage led the clinical experiments, collected the data, and reviewed the manuscript. Kazuhiko Kotani and Mitsuhiko Noda contributed to interpreting the results and reviewed the manuscript. All authors have given the final approval for the manuscript version to be published. Toshinari Takamura and Noriko Satoh‐Asahara are the guarantors of this study and, as such, had full access to all the data in the study and took responsibility for the integrity of the data and data analyses.
CONFLICT OF INTEREST
The authors declare no other conflict of interest.
ACKNOWLEDGMENTS
Special thanks go to Kazunori Koyama, Yasuhisa Kato, Tsutomu Yamada, Taiichiro Okajima, Makito Tanabe, Rika Aaraki, and Mariko Ooishi for their contribution to patient recruitment. This work was supported in part by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (JSPS KAKENHI Grant Numbers 24590719 and 15K08634); a grant from the Ministry of Health, Labor and Welfare of Japan (15ek0210029h0002); and grants from the National Hospital Organization for Collaborative Clinical Research (H26‐NHO‐02), the Smoking Research Foundation, the Research Award of Japanese Society of Anti‐Aging Medicine, the Mitsui Life Social Welfare Foundation, the Danone Institute of Japan Foundation (DIJF) for the financial support of the 2015 DIJF, and Grants from Japan Association for Diabetes Education and Care (2021‐FND‐023). The study funders were not involved in the study design, collection, analysis, and interpretation of data or writing the report and did not impose any restrictions regarding the publication of the report.
Suzuki K, Tsujiguchi H, Hara A, et al. Cystatin C‐based eGFR predicts cardiovascular disease in patients with overweight/obesity and hyperglycemia. Obes Sci Pract. 2023;9(1):4‐14. 10.1002/osp4.630
REFERENCES
- 1. OECD . The Heavy Burden of Obesity: The Economics of Prevention, OECD Health Policy Studies. OECD Publishing; 2019. [Google Scholar]
- 2. Hata J, Ninomiya T, Hirakawa Y, et al. Secular trends in cardiovascular disease and its risk factors in Japanese: half‐century data from the Hisayama Study (1961‐2009). Circulation. 2013;128(11):1198‐1205. 10.1161/circulationaha.113.002424 [DOI] [PubMed] [Google Scholar]
- 3. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341(6):427‐434. 10.1056/nejm199908053410607 [DOI] [PubMed] [Google Scholar]
- 5. Chen Y, Copeland WK, Vedanthan R, et al. Association between body mass index and cardiovascular disease mortality in east Asians and south Asians: pooled analysis of prospective data from the Asia Cohort Consortium. BMJ. 2013;347(oct01 1):f5446. 10.1136/bmj.f5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26‐year follow‐up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968‐977. 10.1161/01.cir.67.5.968 [DOI] [PubMed] [Google Scholar]
- 7. Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13‐27. 10.1056/nejmoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marquina C, Talic S, Vargas‐Torres S, et al. Future burden of cardiovascular disease in Australia: impact on health and economic outcomes between 2020 and 2029. Eur J Prev Cardiol. 2021. [DOI] [PubMed] [Google Scholar]
- 9. Slavin SD, Khera R, Zafar SY, Nasir K, Warraich HJ. Financial burden, distress, and toxicity in cardiovascular disease. Am Heart J. 2021;238:75‐84. 10.1016/j.ahj.2021.04.011 [DOI] [PubMed] [Google Scholar]
- 10. Satoh N, Shimatsu A, Kato Y, et al. Evaluation of the cardio‐ankle vascular index, a new indicator of arterial stiffness independent of blood pressure, in obesity and metabolic syndrome. Hypertens Res. 2008;31(10):1921‐1930. 10.1291/hypres.31.1921 [DOI] [PubMed] [Google Scholar]
- 11. Kotani K, Satoh N, Kato Y, et al. A novel oxidized low‐density lipoprotein marker, serum amyloid A‐LDL, is associated with obesity and the metabolic syndrome. Atherosclerosis. 2009;204(2):526‐531. 10.1016/j.atherosclerosis.2008.09.017 [DOI] [PubMed] [Google Scholar]
- 12. Satoh‐Asahara N, Suganami T, Majima T, et al. Urinary cystatin C as a potential risk marker for cardiovascular disease and chronic kidney disease in patients with obesity and metabolic syndrome. Clin J Am Soc Nephrol. 2011;6(2):265‐273. 10.2215/cjn.04830610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293‐302. 10.1038/nrendo.2014.29 [DOI] [PubMed] [Google Scholar]
- 14. Havlik RJ, Hubert HB, Fabsitz RR, Feinleib M. Weight and hypertension. Ann Intern Med. 1983;98(5_Part_2):855‐859. 10.7326/0003-4819-98-5-855 [DOI] [PubMed] [Google Scholar]
- 15. Jousilahti P, Tuomilehto J, Vartiainen E, Pekkanen J, Puska P. Body weight, cardiovascular risk factors, and coronary mortality. 15‐year follow‐up of middle‐aged men and women in eastern Finland. Circulation. 1996;93(7):1372‐1379. 10.1161/01.cir.93.7.1372 [DOI] [PubMed] [Google Scholar]
- 16. Björntorp P. Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10(4):493‐496. 10.1161/01.atv.10.4.493 [DOI] [PubMed] [Google Scholar]
- 17. Modan M, Halkin H, Almog S, et al. Hyperinsulinemia. A link between hypertension obesity and glucose intolerance. J Clin Invest. 1985;75(3):809‐817. 10.1172/jci111776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stamler R, Stamler J, Riedlinger WF, Algera G, Roberts RH. Weight and blood pressure. Findings in hypertension screening of 1 million Americans. JAMA. 1978;240(15):1607‐1610. 10.1001/jama.1978.03290150053024 [DOI] [PubMed] [Google Scholar]
- 19. Elagizi A, Kachur S, Carbone S, Lavie CJ, Blair SN. A review of obesity, physical activity, and cardiovascular disease. Curr Obes Rep. 2020;9(4):571‐581. 10.1007/s13679-020-00403-z [DOI] [PubMed] [Google Scholar]
- 20. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875‐880. 10.1038/nature05487 [DOI] [PubMed] [Google Scholar]
- 21. Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. GFR estimation using standardized serum cystatin C in Japan. Am J kidney Dis Off J Natl Kidney Found. 2013;61(2):197‐203. 10.1053/j.ajkd.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 22. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J kidney Dis Off J Natl Kidney Found. 2009;53(6):982‐992. 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 23. Organization WH, Federation ID. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; 2006.
- 24. Seino Y, Nanjo K, Tajima N, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Diabetol Int. 2010;1(5):2‐20. 10.1111/j.2040-1124.2010.00074.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nagata M, Ninomiya T, Kiyohara Y, et al. Prediction of cardiovascular disease mortality by proteinuria and reduced kidney function: pooled analysis of 39, 000 individuals from 7 cohort studies in Japan. Am J Epidemiol. 2013;178:1‐11. 10.1093/aje/kws447 [DOI] [PubMed] [Google Scholar]
- 26. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296‐1305. 10.1056/nejmoa041031 [DOI] [PubMed] [Google Scholar]
- 27. Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans ROB, Bakker SJL. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534‐540. 10.1016/j.atherosclerosis.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 28. Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011;80(1):17‐28. 10.1038/ki.2010.483 [DOI] [PubMed] [Google Scholar]
- 29. Kühn A, van der Giet M, Kuhlmann MK, et al. Kidney function as risk factor and predictor of cardiovascular outcomes and mortality among older adults. Am J Kidney Dis. 2021;77(3):386‐396.e1. 10.1053/j.ajkd.2020.09.015 [DOI] [PubMed] [Google Scholar]
- 30. Barr EL, Reutens A, Magliano DJ, et al. Cystatin C estimated glomerular filtration rate and all‐cause and cardiovascular disease mortality risk in the general population: AusDiab study. Nephrology. 2017;22(3):243‐250. 10.1111/nep.12759 [DOI] [PubMed] [Google Scholar]
- 31. Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352(20):2049‐2060. 10.1056/nejmoa043161 [DOI] [PubMed] [Google Scholar]
- 32. Peralta CA, Katz R, Sarnak MJ, et al. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol. 2011;22(1):147‐155. 10.1681/asn.2010050483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ito R, Yamakage H, Kotani K, et al. Comparison of cystatin C‐ and creatinine‐based estimated glomerular filtration rate to predict coronary heart disease risk in Japanese patients with obesity and diabetes. Endocr J. 2015;62(2):201‐207. 10.1507/endocrj.ej14-0352 [DOI] [PubMed] [Google Scholar]
- 34. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(19):1527‐1539. 10.1016/j.jacc.2008.07.051 [DOI] [PubMed] [Google Scholar]
- 35. Kajimoto H, Kai H, Aoki H, et al. Inhibition of eNOS phosphorylation mediates endothelial dysfunction in renal failure: new effect of asymmetric dimethylarginine. Kidney Int 2012;81(8):762‐768. 10.1038/ki.2011.476 [DOI] [PubMed] [Google Scholar]
- 36. Ueda S, Yamagishi S.‐I, Kaida Y, Okuda S. Asymmetric dimethylarginine may be a missing link between cardiovascular disease and chronic kidney disease. Nephrology. 2007;12(6):582‐590. 10.1111/j.1440-1797.2007.00840.x [DOI] [PubMed] [Google Scholar]
- 37. Zoccali C, Bode‐Böger S, Mallamaci F, et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end‐stage renal disease: a prospective study. Lancet. 2001;358(9299):2113‐2117. 10.1016/s0140-6736(01)07217-8 [DOI] [PubMed] [Google Scholar]
- 38. Miyazaki H, Matsuoka H, Cooke JP, et al. Endogenous nitric oxide synthase inhibitor: a novel marker of atherosclerosis. Circulation. 1999;99(9):1141‐1146. 10.1161/01.cir.99.9.1141 [DOI] [PubMed] [Google Scholar]
- 39. Ueda S, Yamagishi S.‐I, Yokoro M, Okuda S. Role of asymmetric dimethylarginine in cardiorenal syndrome. Curr Pharmaceut Des. 2014;20(14):2448‐2455. 10.2174/13816128113199990480 [DOI] [PubMed] [Google Scholar]
- 40. Matsuzawa Y, Nakamura T, Shimomura I, Kotani K. Visceral fat accumulation and cardiovascular disease. Obes Res. 1995;3 (Suppl 5):645S‐647S. 10.1002/j.1550-8528.1995.tb00481.x [DOI] [PubMed] [Google Scholar]
- 41. Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol. 2009;20(12):2617‐2624. 10.1681/asn.2009010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheng T.‐YD, Wen S.‐F, Astor BC, Tao XG, Samet JM, Wen CP. Mortality risks for all causes and cardiovascular diseases and reduced GFR in a middle‐aged working population in Taiwan. Am J Kidney Dis. 2008;52(6):1051‐1060. 10.1053/j.ajkd.2008.05.030 [DOI] [PubMed] [Google Scholar]
- 43. Naimark DMJ, Grams ME, Matsushita K, et al. Past decline versus current eGFR and subsequent mortality risk. J Am Soc Nephrol. 2016;27(8):2456‐2466. 10.1681/asn.2015060688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Flack JM, Neaton JD, Daniels B, Esunge P. Ethnicity and renal disease: lessons from the multiple risk factor intervention trial and the treatment of mild hypertension study. Am J Kidney Dis. 1993;21(4):31‐40. 10.1016/s0272-6386(12)80859-6 [DOI] [PubMed] [Google Scholar]
- 45. Guo Y, Cui L, Ye P, Li J, Wu S, Luo Y. Change of kidney function is associated with all‐cause mortality and cardiovascular diseases: results from the kailuan study. J Am Heart Assoc. 2018;7(21):e010596. 10.1161/jaha.118.010596 [DOI] [PMC free article] [PubMed] [Google Scholar]


