Abstract
Radical cure of Plasmodium vivax malaria must include elimination of quiescent ‘hypnozoite’ forms in the liver; however, the only FDA-approved treatments are contraindicated in many vulnerable populations. To identify new drugs and drug targets, we screened the Repurposing, Focused Rescue, and Accelerated Medchem library against P. vivax liver stages and identified the DNA methyltransferase inhibitors hydralazine and cadralazine as active against hypnozoites. We then used bisulfite sequencing and immunostaining to identify cytosine modifications in the infectious stage (sporozoites) and liver stages, respectively. A subsequent screen of epigenetic inhibitors revealed hypnozoites are broadly sensitive to histone acetyltransferase and methyltransferase inhibitors, indicating that several epigenetic mechanisms are likely modulating hypnozoite persistence. Our data present an avenue for the discovery and development of improved radical cure antimalarials.
One-Sentence Summary:
A drug repurposing screen reveals antihypertension drugs are active against P. vivax hypnozoites and epigenetic mechanisms play a role in hypnozoite quiescence.
Introduction
Controlling malaria caused by Plasmodium vivax is complicated by the ability of P. vivax sporozoites, the infectious stage inoculated by mosquitoes, to invade hepatocytes and become quiescent (1). These quiescent ‘hypnozoites’ persist, undetectable, for months or even years before resuming growth and initiating a ‘relapse’ blood stage infection, leading to subsequent transmission back to mosquitoes (2). Hypnozoites are refractory to all antimalarials except the 8-aminoquinoline class, which cannot be administered to pregnant women or glucose-6-phosphate dehydrogenase-deficient individuals and are ineffective in persons with specific cytochrome P450 genotypes (3). New antimalarial drug discovery and development with a target chemo-profile for killing hypnozoites, termed radical cure, has only recently been made possible with the introduction of cell-based phenotypic screening platforms featuring a monolayer of hepatocytes infected with sporozoites, a portion of which go on to form hypnozoites (4). Protein target-based approaches for hypnozonticidal drug discovery are in their infancy as the transcriptome of hypnozoites has only recently been reported and robust methods for routine in vitro propagation and genetic manipulation of blood stages of P. vivax are still underdeveloped (5, 6).
To address the lack of radical cure drug leads and targets, we used our advanced P. vivax liver stage platform to screen the Repurposing, Focused Rescue, and Accelerated Medchem (ReFRAME) library (7). This library consists of approximately 12,000 developmental, approved, and discontinued drugs with the expectation that the repurposing of compounds with some optimization or regulatory success could expedite the decade-long path drugs typically progress through from discovery to licensure (8). To accomplish this screen, we assembled an international collaboration with laboratories in malaria-endemic countries whereby vivax-malaria patient blood was collected and fed to mosquitoes to produce sporozoites for infecting primary human hepatocytes (PHH) in screening assays performed on-site. As expected, few hits with anti-hypnozoite activity were detected since discovery of inhibitors for non-proliferating cells is notoriously difficult (9, 10). Interestingly, two structurally related compounds used for treating hypertension, hydralazine and cadralazine, were found effective at killing hypnozoites. As these inhibitors have been shown to modulate DNA methylation (11), we next pursued and confirmed the existence of cytosine modifications in P. vivax sporozoite and liver stages. To further investigate the role of epigenetics in hypnozoites, we screened an epigenetic inhibitor library using an improved version of the screening platform. Hypnozoites were found to be susceptible to several classes of epigenetic inhibitors, including six different histone deacetylase inhibitors and two inhibitors with targets regulating histone methylation. In lieu of traditional molecular biology methods for understanding this pathogen, our chemical biology approach reveals multiple, druggable pathways in P. vivax hypnozoites and sheds light on some of the processes underpinning their quiescence.
Results
ReFRAME library screening cascade, hit identification, and confirmation
Hypnozoites become insensitive to most antimalarials after five days in culture, indicating they mature following hepatocyte infection (12, 13). Importantly, development of hits with radical cure activity in vivo requires the screening against mature hypnozoites in vitro (14). While our 8-day P. vivax liver stage platform has been used for screening smaller libraries against mature hypnozoites (13), the size of the ReFRAME library presented a logistical challenge. We anticipated dozens of P. vivax cases, each with a unique genetic background, would be needed to produce the sporozoites required to screen the 40 microtiter plates containing the library, thus the library was split such that plates were screened at Shoklo Malaria Research Unit (SMRU) in Thailand and the Institute Pasteur of Cambodia (IPC). Ultimately, 36 P. vivax cases from either site were needed to complete the primary screen over the course of 18 months (Fig. 1A and fig. S1).
Fig. 1. Hypnozonticidal hit detection and confirmation.
(A) Index chart depicting the primary screen of the ReFRAME library against P. vivax hypnozoites in an 8-day assay. Hypnozoite counts were normalized by mean quantity per well for each plate (Z factor). Teal: library, black: DMSO, red: 1 μM monensin. (B) Primary screen hits were confirmed by dose-response in 8-day P. vivax liver stage assays and counterscreened against P. berghei liver schizonts, P. falciparum asexual blood stages (strain Dd2), HEK293T, and HepG2. Values represent pEC50 or pCC50 ± SD of all independent experiments (n=2–6) for which a pEC50 or pCC50 was obtained. (C) Dose-response curves for cadralazine against P. vivax and P. cynomolgi liver forms in 8-day assays at the IPC, UGA, and NITD. (B,C) Heat maps represent red as more potent and blue as inactive at highest dose tested. Asterisk (*) indicates only one independent experiment resulted in a calculated pEC50 or pCC50. (C) All replicate wells were plotted together from all independent experiments (n=3 for P. vivax at IPC, n=1 for P. vivax at NITD, n=2 for P. cynomolgi at UGA, and n=4 for P. cynomolgi at NITD), bars represent SEM.
From our analysis of primary screen activity, we noted several hydrazinophthalazine vasodilators were potentially active (fig. S1). We then selected 72 compounds, including 10 hydrazinophthalazine analogs, for confirmation of activity and potency determination in a dose-response format, as well as counterscreening for additional antimalarial activity and cytotoxicity. Of the twelve hits which exhibited selective hypnozonticidal activity, cadralazine displayed the best combination of potency (pEC50 = 6.33 ± 0.33) and maximal inhibition near 100% (Fig. 1, B and C, and fig. S2). To ensure hits were not merely specific to our platform, select hits were communicated to the Novartis Institute for Tropical Diseases (NITD), where the activities of hydralazine and cadralazine were independently confirmed using a P. vivax case from southern Thailand and separate batches of compounds (Fig. 1C and fig. S3).
Currently, the gold-standard for in vivo anti-relapse efficacy is rhesus macaques infected with Plasmodium cynomolgi, a zoonotic, relapsing species closely related to P. vivax (15). To further pursue drug repurposing of hydrazinophthalazines, we sought to confirm and measure the potency of cadralazine and other ReFRAME hits against P. cynomolgi B strain hypnozoites in vitro using an 8-day assay featuring primary simian hepatocytes (PSH) at NITD. Surprisingly, hydralazine and cadralazine were found inactive when tested in three different PSH donors (Fig. 1C and fig. S3). This negative result was later confirmed in an 8-day, simianized version of the platform at the University of Georgia (UGA) using the P. cynomolgi Rossan strain infected into two different PSH lots (Fig. 1C). Furthermore, hydralazine and cadralazine were not identified as hits in any of the 112 screens of the ReFRAME published to date (ReFRAMEdb.org), suggesting these compounds are specific to the P. vivax liver stage assay.
Immunofluorescent detection of DNA methylation in P. vivax and P. cynomolgi liver stages
Hydrazinophthalazines have been shown to involve direct inhibition of DNA methyltransferases (16) and hydralazine has also been used to study DNA methylation in the Plasmodium falciparum asexual blood stages (17). Investigations into blood-stage parasites methylation have identified the presence of low levels of 5-methylcytosine (5mC), 5-hydroxmethylcystosine (5hmC), and under-characterized 5hmC-like marks throughout the genome (17, 18). To confirm the possible mechanism of hydrazinophthalazines on hypnozoites, we first used immunofluorescence staining with commercial anti-5mC and anti-5hmC monoclonal antibodies to identify methylation patterns in P. vivax liver schizonts and hypnozoites at 6 days post-infection. We found clear evidence of 5mC, but not 5hmC, in both schizonts and hypnozoites, morphologically consistent with the presence of 5mC in the parasite’s nucleus (7) (Fig. 2A and figs. S4–S6). Due to expected 5mC and 5hmC signals from host hepatic nuclei, we opted to use high-content imaging (HCI) as an unbiased approach for quantifying 5mC signal within parasites. Image masks were generated to quantify the area of 5mC or 5hmC stain within each parasite (fig. S7), the values of which were then plotted as stain area per hypnozoite or per schizont (Fig. 2B). While some evidence of 5hmC-positive forms did appear from this analysis, the net area per parasite was found significantly lower than that quantified for 5mC-stained forms, indicating the relative level of 5mC marks predominate that of 5hmC.
Fig. 2. Cytosine modifications in P. vivax liver forms.
(A) Immunofluorescent imaging of a 5mC-positive (left) or 5hmC-negative (right) P. vivax hypnozoite (top) and schizont (bottom) at day 6 post-infection. White arrows indicate hepatocyte nuclei positive for 5mC or 5hmC. Bars represent 10 μm. (B) High-content quantification of 5mC or 5hmC stain area within hypnozoites or schizonts from sporozoites generated from three different P. vivax cases. Significance determined using Kurskal-Wallis tests, for hypnozoites H(7) = 194.3, p <.0001, for schizonts H(7) = 88.66, p <.0001, with Dunn’s multiple comparisons, *p <.05, ***p <.001, ****p <.0001, ns = not significant. Line, box and whiskers represent median, upper and lower quartiles, and minimum-to-maximum values, respectively, of all hypnozoites (177 ≤ n ≤ 257) or all schizonts (30 ≤ n ≤ 142) in culture for each case, 2’ indicates a secondary stain only control.
Given the different susceptibility of P. cynomolgi hypnozoites to hydrazinophthalazines as compared to P. vivax, we performed HCI analysis of 5mC- and 5hmC-stained P. cynomolgi M/B-strain liver schizonts and hypnozoites at 8 and 12 days post-infection. Like P. vivax, we found both P. cynomolgi liver schizonts and hypnozoites are positive for 5mC, but not 5hmC. However, the 5mC stain morphology and intensity were relatively lower in P. cynomolgi hypnozoites versus P. vivax hypnozoites, indicating the possibility of different roles of methylation in the epigenetic network of these two species (fig. S8).
Detection of cytosine modifications in P. vivax and P. cynomolgi sporozoites using liquid chromatography-tandem mass spectrometry and bisulfite sequencing
We next sought to confirm the presence of cytosine methylation in the P. vivax and P. cynomolgi genomes using mass spectrometry and bisulfite sequencing. Initially, we assessed if these methodologies were plausible on P. vivax liver stages, but concluded that, without an available single live-cell approach, sequencing coverage of the parasite’s genome would be overwhelmed by the genomic material from the host cell and neighboring uninfected hepatocytes following the harvesting of liver stage cultures (19). We therefore collected sufficient genomic material from P. vivax and P. cynomolgi sporozoites to analyze the nucleoside mixture arising from the enzymatic digestion of genomic DNA by liquid chromatography-tandem mass spectrometry as well as for detection of DNA methyltransferase (DNMT) activity using a commercial in vitro DNA methylation assay (17). While we detected 5mC and DNMT activity in Plasmodium-enriched samples with these approaches, possible contamination by the mosquito’s microbiota could not be excluded (fig. S9). We next analyzed DNA methylation loci at single-nucleotide resolution using bisulfite sequencing of 3×107 P. vivax sporozoites, generated from three different cases, as well as 3×107 P. cynomolgi sporozoites (Fig. 3, A and B). A total of 161 and 147 million high-quality reads were sequenced for P. vivax and P. cynomolgi samples, respectively (fig. S9). The average 5mC level detected across all cytosines was 0.49% and 0.39% for P. vivax and P. cynomolgi, respectively. These percentages are comparable to the 0.58% methylation level detected in P. falciparum blood stages (17), but likely underestimate methylated loci considering the coverage we achieved (see methods).
Fig. 3. Density of cytosine and methylated cytosine (5mC) in sporozoites.
(A) CG content of chromosome 1 for P. vivax and P. cynomolgi. The total number of cytosines was quantified on each strand using 1 kbp long non-overlapping windows. (B) The total number of methylated cytosines was quantified on each strand using 1 kbp long non-overlapping windows. (C) The number of 5mC present in all possible contexts (CG, CHG, and CHH) quantified throughout the genome of P. vivax and P. cynomolgi. (D) Repartitioned 5mC quantity within different compartments of the genome in P. vivax and P. cynomolgi. (E) Strand-specificity of 5mC for all genes in P. vivax and P. cynomolgi. Flanking regions and gene bodies were divided into five bins and the methylation level of each bin was averaged among all genes. Red: template strand, blue: non-template strand. (F) The previously reported mRNA abundance of P. vivax sporozoites was retrieved (20) and genes ranked. The 5mC levels in 5’ flanking regions, gene bodies, and 3’ flanking regions were placed into five bins and are shown for highly expressed (90th percentile, left) and weakly expressed (10th percentile, right) genes. Red: template strand, blue: non template strand.
We then monitored the distribution of detected 5mC along the P. vivax and P. cynomolgi chromosomes (figs. S10 and S11) and observed a stable methylation level throughout, including in telomeric and sub-telomeric regions. We further examined the context of genome-wide methylations and, similar to what we previously observed in P. falciparum (17), methylation was detected as asymmetrical, with CHH (where H can be any nucleotide but G) at 69.5% and 70.5%, CG at 16% and 15.7%, and CHG at 14.3% and 13.64%, for P. vivax and P. cynomolgi, respectively (Fig. 3C). We then measured the proportion of 5mC in the various compartments of gene bodies (exons, the introns, promoters, and terminators) as well as strand-specificity (Fig. 3, D and E). We observed a slightly increased distribution of 5mC in promoters and exons compared to the intronic region, as well as in the template versus non-template strand, in P. vivax and P. cynomolgi. These results were consistent with previous data obtained in P. falciparum and in plants (17). Such a strand specificity of DNA methylation patterns can affect the affinity of the RNA polymerase II and impact transcription. We therefore investigated the relationship between DNA methylation and transcription and examined the methylation level in the various compartments of gene bodies and compared it to the mRNA levels measured by RNA-seq in P. vivax sporozoites (20). We found a trend between methylation and mRNA abundance in the proximal promoter regions and the beginning of the gene bodies, with highly-expressed genes appearing hypomethylated and weakly-expressed genes hypermethylated (Fig. 3F). These results suggest that methylation level in proximal promoter regions as well as in the first exon of the genes may affect, at least partially, gene expression in malaria parasites. While these data will need to be further validated and linked to hypnozoite formation at a single-cell level, we have determined that 5mC is present at a low level in P. vivax and P. cynomolgi sporozoites and could control liver stage development and hypnozoite quiescence.
Assay improvements and epigenetic inhibitor library screen
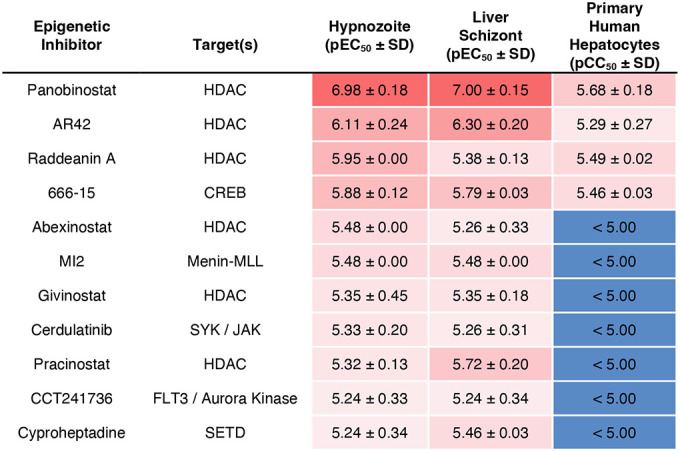
Upon completion of the ReFRAME and previously reported screens (13), we retroactively analyzed the screening platform’s performance and developed an improved protocol to address two shortcomings. First, we hypothesized that false negatives could arise when a true-positive compound results in nonviable forms which persist in culture and are counted during HCI; and that extending the assay endpoint by four days would allow more time for attenuated forms to be cleared from the culture (13). Second, because the ReFRAME primary screen was completed using two lots of PHH, we noted a few lot-specific results, such as differences in potency of the monensin control (fig. S12). We therefore added the phase I metabolism inhibitor 1-aminobenzotriazole to culture media on treatment days to minimize the effect of lot-specific hepatic metabolism on assay results (21) (fig. S13). Following development of the improved in vitro assay, this 12-day protocol was used to reconfirm twelve ReFRAME hits (fig. S14), resulting in confirmation of poziotinib--a primary screen hit that was previously confirmed in the NITD P. vivax assay only (Fig. 1B and fig. S3). Given the apparent importance of epigenetic mechanisms in hypnozoites, we obtained a commercially-available epigenetic inhibitor library and screened it against P. vivax liver stages at SMRU and IPC using the improved protocol (fig. S15). Confirmation of hits in dose-response assays resulted in selective potency for 11 epigenetic inhibitors targeting five different epigenetic mechanisms (Table 1).
Table 1. Confirmed hits from an epigenetic inhibitor library screened against P. vivax liver stages.
Mean pEC50 or pCC50 and SD from two independent experiments. HDAC: histone deacetylase. CREB: cAMP response element-binding protein. FLT3: fms-like tyrosine kinase 3. SYK: spleen tyrosine kinase. JAK: Janus kinase. SETD: SET domain containing histone lysine methyltransferase.
|
Discussion
Herein we demonstrate several significant advances that are prerequisites for radical cure antimalarial drug discovery and development, including the first report of screening a medium-sized compound library against mature hypnozoites as well as detection of novel hits with mechanisms unrelated to that of 8-aminoquinolines. Despite the success of the original screening platform protocol, the improvements described herein should be considered for other platforms grappling with the biological complexity of metabolically-active primary hepatocytes or using a phenotypic readout without a validated hypnozoite viability marker. This report also provides an opportunity to compare radical cure hits assayed against both P. vivax and P. cynomolgi hypnozoites. While the P. cynomolgi-infected rhesus macaque system has long been used as a model system for studying malaria relapse, the only class of compounds available to assess the predictive value of this model for chemotherapeutics are the 8-aminoquinolines, which lack a distinct parasite target (22–25). We found mixed results, with hydrazinophthalazines negative, and poziotinib positive, against P. cynomolgi hypnozoites. While further studies are needed to confirm if the target of any hit in this report is parasite- or host-directed, this would indicate there is sufficient diversity in gene expression, structural biology, or mechanisms of hepatic quiescence between P. cynomolgi and P. vivax hypnozoites that some hits will be species-specific. However, this result might also be attributed to differential metabolism in human and monkey hepatocytes (26). Regardless, these results highlight that in vivo P. vivax relapse models should be further developed and validated, as those hits lacking activity in P. cynomolgi might be abandoned for no other reason than the inability to demonstrate in vivo efficacy prior to first-in-human studies (27).
This report also adds to the broader discussion surrounding the successes and challenges of drug repurposing (28). Of the hits we identified, colforsin daropate, rhodamine 123, and poziotinib are used for cancer indications and, like the hydrazinophthalazines, have known human targets, implying that selectivity and off-target toxicity are hurdles to be addressed if repurposing for radical cure is to be successful. While direct repositioning of a known drug as a safe treatment for a new indication is the ideal outcome, it is also challenging. However, the identification of hits that can serve as advanced starting points for further optimization has potential for reducing the time and cost involved in developing an efficacious therapy. Alternatively, identification of tool compounds that delve into and reveal novel aspects of the biology of a disease can also promote further drug discovery approaches. As such, this report provides significant chemical biology leads into the essential nature of hypnozoite quiescence. Epigenetic control of pathogenic dormancy via DNA methylation has been described for cancer cells (29) and tuberculosis (30); and is a critical process in plants, which share evolutionary traits with Plasmodium (31). DNA methylation in the genus Plasmodium was first described in P. falciparum blood stages (17), and one type of modification has been demonstrated as positively associated with continuous gene expression (18). As technologies improve, it will be critical to validate the importance of such modifications in liver stages, including hypnozoites.
Several additional chemical biology leads were revealed in our screens. Five hydroxamic acid-containing inhibitors (panobinostat, AR42, abexinostat, givinostat and practinostat) and one natural product (raddeanin A) targeting histone deacetylase were found to selectively kill hypnozoites. Furthermore, the hit MI2, targeting the interaction between menin, a global regulator of gene expression, and MLL, a DNA-binding protein that methylates histone H3 lysine 4 (32), and the hit cyproheptadine, targeting SET-domain-containing lysine methyltransferase (33), highlight the interplay between DNA methylation and histone modifications (34) and are further evidence histone methylation regulates hypnozoites (20, 35). Other hits, including 666–15, targeting the transcription factor cAMP response element-binding protein (36), and the kinase inhibitors cerdulatinib and CCT241736, suggest transcription factors and kinase signaling may also play a role in establishing, maintaining, or exiting quiescence. Similarly, the cancer drug poziotinib inhibits HER2, a tyrosine protein kinase associated with the downregulation of apoptosis and metastasis (37). As we recently reported that host apoptotic pathways are downregulated in P. vivax-infected hepatocytes, poziotinib could act by increasing apoptotic pathways in infected host cells (19). The hit MS-0735, an analog of our previously reported hypnozonticidal hit MMV018983 (13), is a ribonucleotide-reductase (RNR) inhibitor used as an antiviral. Needed for producing deoxyribonucleosides for DNA synthesis, RNR is a peculiar mechanism for nonreplicating hypnozoites, however, it has been reported that RNR is critical for DNA damage repair (38) and is expressed in P. vivax liver schizonts and hypnozoites (19). Ongoing efforts to make available disruptive methods for studying P. vivax, including genetic manipulation and in vivo models for relapse, will enable further validation of these leads and a more comprehensive understanding of the mechanisms of hypnozoite quiescence.
Supplementary Material
Acknowledgments:
We thank the malaria patients of Thailand and Cambodia for participation in this study. We thank the Sporocore at UGA for generating P. berghei-infected mosquitoes. We are grateful to Calibr’s Compound Management and High Throughput Screening Groups for their assistance with this project. HCI data from drug studies was produced by the Biomedical Microscopy Core at UGA, supported by the Georgia Research Alliance. SMRU is part of the Mahidol Oxford Research Unit, supported by the Wellcome Trust of Great Britain (#220211). Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. This publication includes data generated at the University of California, San Diego IGM Genomics Center utilizing an Illumina NovaSeq 6000 that was purchased with funding from a National Institutes of Health SIG grant (#S10 OD026929).
Funding:
Funding support was provided by the Bill & Melinda Gates Foundation (#OPP1107194 to Calibr, INV-031788 to CJJ, and #OPP1023601 to DEK), Medicines for Malaria Venture (RD/17/0042 and RD/15/0022 to BW and AV and RD/15/0022 to SPM and DEK), the National Institutes of Allergy and Infectious Diseases of the National Institutes of Health (#HHSN272201200031C to MRG and #1R01 AI136511 to KGLR) and the University of California, Riverside (#NIFA-Hatch-225935 to KGLR)
Funding Statement
Funding support was provided by the Bill & Melinda Gates Foundation (#OPP1107194 to Calibr, INV-031788 to CJJ, and #OPP1023601 to DEK), Medicines for Malaria Venture (RD/17/0042 and RD/15/0022 to BW and AV and RD/15/0022 to SPM and DEK), the National Institutes of Allergy and Infectious Diseases of the National Institutes of Health (#HHSN272201200031C to MRG and #1R01 AI136511 to KGLR) and the University of California, Riverside (#NIFA-Hatch-225935 to KGLR)
Footnotes
Competing interests: TM and KC are employees of BioIVT. AH-C, ELF, and SAM are employees of the Novartis Institute for Tropical Disease, BC is an employee of MMV. All other authors have no competing interests.
Data and materials availability:
For the purpose of Open Access, the authors have applied a CC BY public copyright license to any Author Accept Manuscript version arising from this submission. All bisulfite sequencing data generated in this study can be found in the Sequence Read Archive (SRA) at the NCBI National Library of Medicine (https://www.ncbi.nlm.nih.gov/sra) under the BioProject code PRJNA925570.
References and Notes
- 1.Wells T. N., Burrows J. N., Baird J. K., Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol 26, 145–151 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Adams J. H., Mueller I., The Biology of Plasmodium vivax. Cold Spring Harb Perspect Med 7, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird J. K., 8-Aminoquinoline Therapy for Latent Malaria. Clin Microbiol Rev 32, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valenciano A. L., Gomez-Lorenzo M. G., Vega-Rodríguez J., Adams J. H., Roth A., In vitro models for human malaria: targeting the liver stage. Trends Parasitol 38, 758–774 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruberto A. A. et al. , Single-cell RNA profiling of Plasmodium vivax-infected hepatocytes reveals parasite- and host- specific transcriptomic signatures and therapeutic targets. Front Cell Infect Microbiol 12, 986314 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermúdez M., Moreno-Pérez D. A., Arévalo-Pinzón G., Curtidor H., Patarroyo M. A., Plasmodium vivax in vitro continuous culture: the spoke in the wheel. Malar J 17, 301 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roth A. et al. , A comprehensive model for assessment of liver stage therapies targeting Plasmodium vivax and Plasmodium falciparum. Nat Commun 9, 1837 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janes J. et al. , The ReFRAME library as a comprehensive drug repurposing library and its application to the treatment of cryptosporidiosis. Proc Natl Acad Sci U S A 115, 10750–10755 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Rycker M., Baragaña B., Duce S. L., Gilbert I. H., Challenges and recent progress in drug discovery for tropical diseases. Nature 559, 498–506 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonova-Koch Y. et al. , Open-source discovery of chemical leads for next-generation chemoprotective antimalarials. Science 362, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornacchia E. et al. , Hydralazine and procainamide inhibit T cell DNA methylation and induce autoreactivity. J Immunol 140, 2197–2200 (1988). [PubMed] [Google Scholar]
- 12.Posfai D. et al. , Plasmodium vivax Liver and Blood Stages Recruit the Druggable Host Membrane Channel Aquaporin-3. Cell Chem Biol 27, 719–727.e715 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maher S. P. et al. , Probing the distinct chemosensitivity of Plasmodium vivax liver stage parasites and demonstration of 8-aminoquinoline radical cure activity in vitro. Sci Rep 11, 19905 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeeman A. M. et al. , PI4 Kinase Is a Prophylactic but Not Radical Curative Target in Plasmodium vivax-Type Malaria Parasites. Antimicrob Agents Chemother 60, 2858–2863 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joyner C., Barnwell J. W., Galinski M. R., No more monkeying around: primate malaria model systems are key to understanding Plasmodium vivax liver-stage biology, hypnozoites, and relapses. Front Microbiol 6, 145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh N., Dueñas-González A., Lyko F., Medina-Franco J. L., Molecular modeling and molecular dynamics studies of hydralazine with human DNA methyltransferase 1. ChemMedChem 4, 792–799 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Ponts N. et al. , Genome-wide mapping of DNA methylation in the human malaria parasite Plasmodium falciparum. Cell Host Microbe 14, 696–706 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammam E. et al. , Discovery of a new predominant cytosine DNA modification that is linked to gene expression in malaria parasites. Nucleic Acids Res 48, 184–199 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruberto A. A. et al. , Single-cell RNA profiling of. Front Cell Infect Microbiol 12, 986314 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vivax Sporozoite Consortium, Transcriptome and histone epigenome of Plasmodium vivax salivary-gland sporozoites point to tight regulatory control and mechanisms for liver-stage differentiation in relapsing malaria. Int J Parasitol 49, 501–513 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortiz de Montellano P. R., Mathews J. M., Autocatalytic alkylation of the cytochrome P450 prosthetic haem group by 1-aminobenzotriazole. Isolation of an NN-bridged benzyne-protoporphyrin IX adduct. Biochem J 195, 761–764 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson D. E. et al. , New tissue schizontocidal antimalarial drugs. Bull World Health Organ 59, 463–479 (1981). [PMC free article] [PubMed] [Google Scholar]
- 23.Dong Y. et al. , Metabolic, Pharmacokinetic, and Activity Profile of the Liver Stage Antimalarial (RC-12). ACS Omega 7, 12401–12411 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson J. A. et al. , The clinical pharmacology of tafenoquine in the radical cure of Plasmodium vivax malaria: An individual patient data meta-analysis. Elife 11, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camarda G. et al. , Antimalarial activity of primaquine operates via a two-step biochemical relay. Nat Commun 10, 3226 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang X. et al. , In Vitro Hepatic Uptake in Human and Monkey Hepatocytes in the Presence and Absence of Serum Protein and Its In Vitro to In Vivo Extrapolation. Drug Metab Dispos 48, 1283–1292 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Flannery E. L. et al. , latent liver infection is characterized by persistent hypnozoites, hypnozoite-derived schizonts, and time-dependent efficacy of primaquine. Mol Ther Methods Clin Dev 26, 427–440 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnamurthy N., Grimshaw A. A., Axson S. A., Choe S. H., Miller J. E., Drug repurposing: a systematic review on root causes, barriers and facilitators. BMC Health Serv Res 22, 970 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrer A. I., Trinidad J. R., Sandiford O., Etchegaray J. P., Rameshwar P., Epigenetic dynamics in cancer stem cell dormancy. Cancer Metastasis Rev 39, 721–738 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Shell S. S. et al. , DNA methylation impacts gene expression and ensures hypoxic survival of Mycobacterium tuberculosis. PLoS Pathog 9, e1003419 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merrick C. J., Hypnozoites in Plasmodium: Do Parasites Parallel Plants? Trends Parasitol 37, 273–282 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Cierpicki T., Grembecka J., Challenges and opportunities in targeting the menin-MLL interaction. Future Med Chem 6, 447–462 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirano T. et al. , Development of Novel Inhibitors for Histone Methyltransferase SET7/9 based on Cyproheptadine. ChemMedChem 13, 1530–1540 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Vaissière T., Sawan C., Herceg Z., Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat Res 659, 40–48 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Dembele L. et al. , Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nat Med 20, 307–312 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Xie F. et al. , Identification of a Potent Inhibitor of CREB-Mediated Gene Transcription with Efficacious in Vivo Anticancer Activity. J Med Chem 58, 5075–5087 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kavarthapu R., Anbazhagan R., Dufau M. L., Crosstalk between PRLR and EGFR/HER2 Signaling Pathways in Breast Cancer. Cancers (Basel) 13, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elledge S. J., Zhou Z., Allen J. B., Ribonucleotide reductase: regulation, regulation, regulation. Trends Biochem Sci 17, 119–123 (1992). [DOI] [PubMed] [Google Scholar]
- 39.World Medical Association General Assembly, World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Int Bioethique 15, 124–9 (2004). [PubMed] [Google Scholar]
- 40.National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. (National Academies Press, ed. 8, 2011). [Google Scholar]
- 41.Maher S. P. et al. , A Phenotypic Screen for the Liver Stages of. Bio Protoc 11, e4253 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schafer C. et al. , A recombinant antibody against Plasmodium vivax UIS4 for distinguishing replicating from dormant liver stages. Malar J 17, 370 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins W. E. et al. , Transmission of different strains of Plasmodium cynomolgi to Aotus nancymaae monkeys and relapse. J Parasitol 95, 349–352 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Mikolajczak S. A. et al. , Plasmodium vivax liver stage development and hypnozoite persistence in human liver-chimeric mice. Cell Host Microbe 17, 526–535 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plouffe D. M. et al. , High-Throughput Assay and Discovery of Small Molecules that Interrupt Malaria Transmission. Cell Host Microbe 19, 114–126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ekland E. H., Schneider J., Fidock D. A., Identifying apicoplast-targeting antimalarials using high-throughput compatible approaches. FASEB J 25, 3583–3593 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hott A. et al. , Artemisinin-resistant Plasmodium falciparum parasites exhibit altered patterns of development in infected erythrocytes. Antimicrob Agents Chemother 59, 3156–3167 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oduola A. M., Weatherly N. F., Bowdre J. H., Desjardins R. E., Plasmodium falciparum: cloning by single-erythrocyte micromanipulation and heterogeneity in vitro. Exp Parasitol 66, 86–95 (1988). [DOI] [PubMed] [Google Scholar]
- 49.Canfield C. J., Pudney M., Gutteridge W. E., Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp Parasitol 80, 373–381 (1995). [DOI] [PubMed] [Google Scholar]
- 50.Lambros C., Vanderberg J. P., Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65, 418–420 (1979). [PubMed] [Google Scholar]
- 51.Pathak A. K. et al. , Streamlining sporozoite isolation from mosquitoes by leveraging the dynamics of migration to the salivary glands. Malar J 21, 264 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swann J. et al. , High-Throughput Luciferase-Based Assay for the Discovery of Therapeutics That Prevent Malaria. ACS Infect Dis 2, 281–293 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joyner C. J. et al. , Humoral immunity prevents clinical malaria during Plasmodium relapses without eliminating gametocytes. PLoS Pathog 15, e1007974 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh N., Barnes S. J., Jenwithisuk R., Sattabongkot J., Adams J. H., A simple and efficient method for cryopreservation and recovery of viable Plasmodium vivax and P. falciparum sporozoites. Parasitol Int 65, 552–557 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Canier L. et al. , An innovative tool for moving malaria PCR detection of parasite reservoir into the field. Malar J 12, 405 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J. et al. , Further evaluation of the NWF filter for the purification of Plasmodium vivax-infected erythrocytes. Malar J 16, 201 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russell B. et al. , A reliable ex vivo invasion assay of human reticulocytes by Plasmodium vivax. Blood 118, e74–81 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rangel G. W. et al. , Enhanced Ex Vivo Plasmodium vivax Intraerythrocytic Enrichment and Maturation for Rapid and Sensitive Parasite Growth Assays. Antimicrob Agents Chemother 62, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For the purpose of Open Access, the authors have applied a CC BY public copyright license to any Author Accept Manuscript version arising from this submission. All bisulfite sequencing data generated in this study can be found in the Sequence Read Archive (SRA) at the NCBI National Library of Medicine (https://www.ncbi.nlm.nih.gov/sra) under the BioProject code PRJNA925570.





