Supplemental Digital Content is Available in the Text.
Keywords: Randomised controlled trials, Placebos, Placebo effect, Control groups, Systematic review, Physical therapy modalities, Rehabilitation, Psychotherapy
Abstract
Blinding is challenging in randomised controlled trials of physical, psychological, and self-management therapies for pain, mainly because of their complex and participatory nature. To develop standards for the design, implementation, and reporting of control interventions in efficacy and mechanistic trials, a systematic overview of currently used sham interventions and other blinding methods was required. Twelve databases were searched for placebo or sham-controlled randomised clinical trials of physical, psychological, and self-management treatments in a clinical pain population. Screening and data extraction were performed in duplicate, and trial features, description of control methods, and their similarity to the active intervention under investigation were extracted (protocol registration ID: CRD42020206590). The review included 198 unique control interventions, published between 2008 and December 2021. Most trials studied people with chronic pain, and more than half were manual therapy trials. The described control interventions ranged from clearly modelled based on the active treatment to largely dissimilar control interventions. Similarity between control and active interventions was more frequent for certain aspects (eg, duration and frequency of treatments) than others (eg, physical treatment procedures and patient sensory experiences). We also provide an overview of additional, potentially useful methods to enhance blinding, as well as the reporting of processes involved in developing control interventions. A comprehensive picture of prevalent blinding methods is provided, including a detailed assessment of the resemblance between active and control interventions. These findings can inform future developments of control interventions in efficacy and mechanistic trials and best-practice recommendations.
1. Introduction
The opioid crisis and the insufficiency of many widely used pain treatments highlight the need for nonpharmacological and nonsurgical pain therapies.1,95,98,119 Such therapies include cognitive-behavioural approaches, exercise and rehabilitation, manual therapies, acupuncture, mind–body techniques such as yoga, devices such as ultrasound and light therapy, electrical therapies, and education; referred to as physical, psychological, and self-management therapies (PPS) from here on. Current guidelines recommend various nondrug therapies as a first-line treatment for low back and chronic musculoskeletal pain.24,98,126 However, most recommendations are based on low-quality or moderate-quality evidence,139 a widespread concern in PPS interventions.7,46,49,64,67,101,137 A lack of high-quality research means that the role of many of these therapies in the prevention, treatment, and management of pain is unclear. This lack of high-quality data is partly because of methodological difficulties specific to efficacy and mechanistic trials of PPS for pain, mainly centred around issues of placebo control and blinding.32,33,103
Placebo interventions in clinical trials are conceptualised as “a control intervention with similar appearance as the experimental treatment, but void of the components in the experimental intervention whose effects the trial is designed to evaluate.”88 Recognising that, in nonpharmacological trials, such control interventions are not usually “inert,” the term “sham intervention” is used in this context.85,113 Sham-controlling a trial is desirable when specific and context-related treatment effects are to be distinguished (efficacy trials), to test the effects of particular treatment components (mechanistic trials) and to reduce bias by allowing for blinding of participants and ideally researchers and clinical personnel.78,133 Blinding or masking refers to the attempt to conceal group allocation or study hypotheses from study participants, therapists, or researchers58 so that expectation effects and manipulation of trial procedures do not undermine internal validity.159 Notably, the prominent role of blinding in clinical trials is debated.9,59,117,162 Irrespectively, there are many scenarios in which controlling for placebo effects is considered important, including pain research because of the arguable susceptibility of subjective symptoms to placebo154,161,168 and to address the question of whether treatments are efficacious beyond context-dependent effects.9,60,93,131
In nonpharmacological randomised controlled trials (RCTs), sham-controlling is more challenging than in drug studies and blinding is more difficult7,33 because care providers are often an integral part of the treatment and cannot be blinded. The complex participatory nature of these interventions often precludes the design of control conditions that feel authentic to patients. Notable exceptions are device-delivered therapies, where the sham simply involves detuned devices31; surgery where much work on sham controls is conducted and which benefits from general anaesthesia for blinding22,53,71,160; and acupuncture, using needling in nonacupuncture points or non- or low-level penetrating sham needles, resulting in reasonable opportunity for participant blinding.34,36,149 These therapies are therefore not discussed here.
In all other areas of PPS interventions, however, unifying criteria for the development, implementation, and reporting of dedicated control interventions for efficacy and mechanistic trials are lacking. Instead, trials of cognitive-behavioural interventions, rehabilitation, exercise, mind–body therapies, and physical and manual therapies often resort to waitlist controls as comparators or different therapeutic modalities, arguing that “blinding is not possible.”33 However, comparisons with no-treatment arms lead to exaggerated effect sizes,61,115 and comparative effectiveness designs commonly address different research questions than efficacy and mechanistic trials.56,65,171 In 2007, it was found that sham interventions in nonpharmacological RCTs did not frequently resemble the experimental treatment,31 arguably increasing unblinding risk. In particular, nonmatching controls do not reliably distinguish specific treatment effects from context-dependent effects.31,125,149 The concept of “structural equivalence” was proposed to enhance matching between control and experimental treatments.21 Furthermore, a range of features for which conditions should be similar or even “indistinguishable” was introduced, from the number of treatments, to procedural steps in the application of interventions, to the personal interactions with therapists and staff.16,35,36,62,77,125 Recently, reporting guidelines for sham interventions were published, encompassing many of these features.86 There is, however, no evidence-based and unifying framework that specifies which theoretical, practical, and ethical considerations should guide researchers in the development, implementation, and evaluation of control interventions in efficacy and mechanistic trials.
To inform such guidance applicable across PPS interventions, a comprehensive overview of currently used sham interventions and other methods to enhance blinding is needed. This systematic review of methods aimed to identify common and less common control intervention designs in RCTs of PPS for a clinical population of patients with pain. Furthermore, we provide a detailed similarity assessment across 25 features for which matching between control and experimental treatments has been said to be important, allowing for comparisons between therapy types. In addition, we identify studies that report on blinding effectiveness and control intervention validation studies. In a parallel publication,81 the potential impact of these control methods on trial results are formally examined.
2. Methods
A systematic review of methods was conducted and is reported according to the PRISMA 2020 statement.121
2.1. Protocol and registration
The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO, registration ID: CRD42020206590). The material here presented is the first part of this protocol (including the results of the following analyses: descriptives and subgroups, trial reporting, degree of similarity between control intervention and treatment, blinding indices); a second article includes the meta-analysis.81
2.2. Eligibility criteria
This review included RCTs of PPS interventions for adults living with pain, irrespective of sex, underlying pathology or pain severity and duration. At least 1 pain-related primary outcome measure had to be reported. Physical, psychological, and self-management included all forms of manual and physical therapy; exercise and rehabilitation therapy; conversation-based and psychological therapies; body–mind, spiritual, religious, and other nonmaterial healing practices; web-based therapies; relaxation; and educational interventions (the latter 2 were classified as “self-management” here). To be eligible, trials had to use a sham control intervention (or “attention” or “placebo control” Table 1).
Table 1.
Eligibility criteria for inclusion into the systematic review.
| Population | Interventions | Comparator | Outcomes | Design | Timeframe |
|---|---|---|---|---|---|
| Included: | |||||
| Any pain Adults |
Physical therapies Psychological interventions Self-management |
Placebo Sham Attention controls |
Pain-related primary | RCTs | January 2008-24 November 2021 |
| Excluded: | |||||
| Experimental pain |
Surgery Devices Acupuncture Meridian therapy |
Other designated intervention groups (comparative effectiveness) Waitlist or no treatment Treatment as usual |
Pilot and feasibility RCTs (as defined by the primary study authors) |
RCT, randomised controlled trial.
Excluded were studies where pharmacological or drug interventions formed the mainstay of treatment and studies of surgical or otherwise invasive interventions. Furthermore, all therapies relying on the permanent introduction of some form of matter into the body were excluded. Owing to specific considerations and solutions to the sham-control problem in device-based and needle-based therapies,31,34,36,149 studies from these categories were also not eligible. Implanted and externally applied devices, all acupuncture modalities, and therapies based on assumed reflex points or energy meridians were excluded.
We excluded nonrandomised studies, observational studies, cross-sectional studies, case-control, case-series, and case-report studies. Pilot or feasibility RCTs were excluded, except for validation studies assessing the sham interventions in an adult population of patients with pain, irrespective of using pain-related outcomes.
For included studies, trial protocols were consulted where available and required for additional method information. The first reporting guideline for nonpharmacological therapy trials was published in February 2008.32 Therefore, this review systematically assessed studies published from 2008 onwards.
2.3. Data sources
The following databases were searched from 2008 to November 2021 (initial search conducted on June 23, 2020, then updated; latest search: November 24, 2021): MEDLINE, Embase, PsychInfo, the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials (CENTRAL), NIH ClinicalTrials.gov, AMED (Allied and Complementary Medicine), CINAHL (nursing and allied health), the Physiotherapy Evidence Database (pedro.org.au), ostmed.dr (ostmed-dr.oclc.org), Osteopathic Research Web (osteopathic-research.com), and the Index to Chiropractic Literature (chiroindex.org).
2.4. Search strategy
The search strategy was built around the following keywords, developed based on existing literature and with database experts, and is provided in full for each database in the digital supplemental materials (supplemental digital content 1, spreadsheet including search results, available at http://links.lww.com/PAIN/B671).
Pain OR painful conditions AND Physical, Psychological, Self-management therapies (specific therapy and technique names) AND placebo control OR sham control OR attention control AND controlled clinical trials. Limit: 2008 to present.
2.5. Study selection
Eligibility screening was performed in duplicate by 2 independent reviewers drawn from a pool of specifically trained research contributors. Disagreements were resolved by a third reviewer. The screening was first performed based on study title and abstract. Full-text eligibility was assessed in a second step.
2.6. Data extraction
The data extraction process also required a minimum of 2 independent reviewers. Discrepancies were resolved through discussion or by a third independent reviewer.
Publications reporting multiple sham controls were extracted independently for each pair of intervention and sham, with data from an active intervention arm used twice for comparisons with control interventions if required. Where a single placebo control group acted as a comparator for multiple active interventions, data were extracted from the active intervention that most resembled the control intervention.
Data extraction was trialled using a sample of potentially eligible studies. Data extraction was performed by volunteer reviewers with at least a Masters-level qualification in a biomedical subject and a minimum time commitment of 3 hours per week on the project. Training in systematic review methods, trial design, and the use of online platforms was provided by the lead investigator (D.H.-S.) before starting data extraction. The results of the pilot testing informed the final approach to data extraction, with detailed annotations for extraction items available to reviewers and reliability monitored throughout.79
Data extraction domains were bibliographic data, general study design, trial reporting, sham control and blinding methods, trial results, and risk of bias (the latter 2 are reported in a separate publication81).
2.7. Data analysis
2.7.1. Descriptive analysis and subgroups
This publication reports the qualitative part of the data synthesis, providing an overview of blinding methods used in the field of PPS therapies for pain, including basic description of sham interventions, their development and reported rationale, the similarity between control and active interventions, compliance with relevant reporting guidelines (notably the intervention description and blinding items of the Consolidated Standards of Reporting Trials (CONSORT), extension for nonpharmacological trials,30 and reports of blinding effectiveness. Apart from providing these data for the entire sample, data were subgrouped by therapy type where appropriate. Given the size and complexity of this review, the results of a formal risk of bias assessment138 and analyses including pain-related and other outcome data are reported in a parallel article81 to ensure sufficient interpretation.
2.8. Meta-analysis: similarity index and ratings
A high degree of similarity between control and test intervention is commonly assumed to be a desirable feature of controlled efficacy and mechanistic trial designs.16,21,36,62,77,108,125,146 While some authors have used concepts of “indistinguishability” and “structural equivalence” to denote different levels of similarity,21,108,125 we drew on such work to define 25 features across which control and treatment interventions may be compared. Assessed features are listed in Figures 1 and 2 and were based on a review of the following pertinent literature:16,21,30,35,36,41,48,62,77,87,125,128,143,146
Figure 1.
PRISMA flow diagram of the systematic search and selection process. The total number of included studies differs from the number of test treatment or control comparisons because of trials with multiple sham controls or single sham controls used as comparators for multiple active arms. In total, 198 unique sham interventions were included, one of which used twice for a comparison with an active arm and reported in 194 publications. A complete search strings per database and a list of all studies excluded at the full-text screening stage are provided in the supplementary digital content 1, available at http://links.lww.com/PAIN/B671. RCT, randomised controlled trial.
Figure 2.
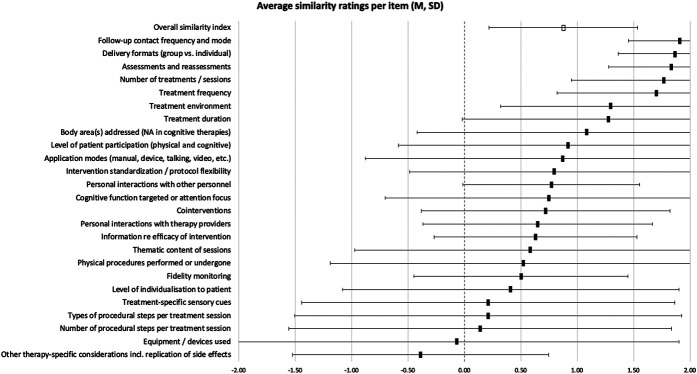
Similarity between tested intervention and placebo control intervention for all included studies. Items were rated if applicable to individual studies (N provided in supplemental digital content 3, available at http://links.lww.com/PAIN/B673), with the following possible ratings and corresponding numerical values: yes = 2, probably yes = 1, not reported = 0, probably no = −1, no = −2. Full squares indicate mean and SDs are provided as error bars. The empty square represents the overall mean across all items and studies. N = 198.
Similarity ratings were based on the reviewers' evaluation of how similar individual items were between active and sham interventions. Specifically, “yes” (similar) and “no” (dissimilar) evaluations were rated as 2 and −2, respectively. “Probably yes” and “probably no” were awarded 1 and −1 points, and 0 points were given for each item that could not be rated because of insufficient information. Nonapplicable items were not rated. In addition, each trial's total ratings were divided by the number of rated items to produce a single value, encompassing similarity across all applicable items. This is for illustrative purposes only because it is unclear whether all items can be weighted equally. Values of the item-specific group averages and the overall similarity average range from −2 (dissimilar across all studies or rated items) to 2 (similar). Data for individual items and the overall index were synthesised as means and standard deviations for each therapy group.
2.9. Meta-analysis: reports of blinding success and blinding indices
During data extraction, we identified studies indicating the effectiveness of the used blinding methods, for example, by having patients guess their group allocation or rate the treatment credibility. Methodological detail and self-reported blinding effectiveness of these studies are reported descriptively. Where group guesses were reported in a manner that allowed for the calculation of Bang's blinding index, the index was calculated for active and control groups individually.20 Specifically, absolute numbers or the percentages of participants per group guessing their allocation correctly, incorrectly, or being unsure were extracted. A ratio of Bang's blinding index was calculated as Hedges g for each comparison between test and control group.50
3. Results
3.1. Sample description
The flowchart in Figure 1 provides an overview of the study selection process and Table 2 of the reviewed trials' characteristics. Data were extracted from 194 publications (plus protocols where available), reporting 198 sham control interventions. Manual therapy trials dominated, followed by psychological and rehabilitation trials. Most commonly, patients with musculoskeletal pain were treated.
Table 2.
Overview of included studies.
| n of studies | % | |
|---|---|---|
| Therapy types | ||
| Manual therapy with spinal manipulation | 48 | 24.2 |
| Craniosacral therapy and gentle myofascial release | 22 | 11.1 |
| Other manual therapy | 64 | 32.3 |
| Rehabilitation or physiotherapy | 22 | 11.1 |
| Self-management | 5 | 2.5 |
| Cognitive-behavioural and other psychotherapy | 27 | 13.6 |
| Spiritual or energetic or esoteric healing | 8 | 4.0 |
| Other | 2 | 1.0 |
| Intervention complexity† | ||
| Simple | 112 | 56.6 |
| Complex | 86 | 43.4 |
| Pain descriptor | ||
| Musculoskeletal pain | 121 | 61.1 |
| Diffuse chronic pain | 18 | 9.1 |
| Cancer-related pain | 6 | 3.0 |
| Visceral pain | 5 | 2.5 |
| Neuropathic pain | 5 | 2.5 |
| Pregnancy-related pain | 1 | 0.5 |
| Not specified | 1 | 0.5 |
| Median | Q1/Q3 | |
|---|---|---|
| Sample size at randomization | ||
| Overall sample size (all trial arms combined) | 64 | 40/101 |
| Sample size per trial arm (only groups included in review) | 27 | 19.5/46 |
| n | % | |
|---|---|---|
| Registered trial protocol available | ||
| Registered | 114 | 57.9 |
| Group design | ||
| Parallel group | 163 | 92.9 |
| Cross-over | 14 | 7.1 |
| No. of study conditions per trial* | ||
| 2 | 139 | 71.6 |
| 3 | 46 | 23.7 |
| 4 | 8 | 4.1 |
| 5 | 1 | 0.5 |
| 6 | 1 | 0.5 |
| Additional nonsham comparators included | ||
| Active comparator (comparative effectiveness) ± | 29 | 14.9 |
| No treatment or waitlist | 20 | 10.3 |
| Usual care/treatment as usual | 13 | 6.7 |
The types of therapies, intervention complexity, and pain population are provided for the entire sample. Special cases: In 1 trial, data from the active intervention group were used twice to compare it with 2 different sham controls: Bialosky et al. (2014) used a “standard” and an “enhanced” sham control. Three publications reported more than 1 trial: D'Souza et al. (2008) studied 2 groups with different types of headaches, and the publication of Assefi et al. (2008) included 2 active interventions and a matching sham control each. Finally, Sharpe et al. (2012) reported 2 trials in a single publication, which were treated entirely independently here. In general, only patients who informed the present analyses are counted in this table; patients were not counted twice, and analyses of reporting refer to individual trials.
Each intervention or sham intervention was counted, irrespective of whether the trial was a single-arm cross-over trial. ± So-called attention controls were not counted as active comparator, only experimental conditions that were clearly assessed because they were deemed potentially effective alternatives (comparative effectiveness intention).
Intervention complexity: Single-step or single-technique interventions were judged as “simple,” irrespective of how often these were applied, and others as complex. N = 194 publications with 198 comparisons between treatment and a sham control.
3.2. Validation studies
We included 8 validation studies of sham control interventions tested in patients with pain.37,48,55,73,76,94,112,150
3.3. Placebo and sham control intervention designs
The CONSORT statement asks researchers to describe “[t]he interventions for each group with sufficient details to allow replication, including how and when they were actually administered.”30,133 In our sample of 198 sham control interventions, 67% complied with this reporting item and provided a description of the control intervention while 77% did for the experimental treatment.
Table 3 provides an overview of the main features of all reviewed sham interventions, categorised by therapy type (see supplemental digital content 2 for table providing classification at study level, available at http://links.lww.com/PAIN/B672).
Table 3.
Overview of used placebo control interventions per therapy type.
| Therapy type | Total N | Placebo control or sham interventions | N | % |
|---|---|---|---|---|
| Manual therapy with spinal manipulation | 49 | Manual, simulated manoeuvre | 30 | 61.2 |
| Manual, soft touch | 9 | 18.4 | ||
| Manual, technique to different area | 1 | 2.0 | ||
| Disabled device | 6 | 12.2 | ||
| Rest time-control | 1 | 2.0 | ||
| Other (therapist attention, general anaesthesia) | 2 | 4.1 | ||
| Craniosacral therapy and gentle myofascial release | 22 | Manual, simulated manoeuvre | 2 | 9.1 |
| Manual, soft touch | 8 | 36.4 | ||
| Disabled device | 10 | 45.5 | ||
| Other (low-strength static magnets, active joint movement) | 2 | 9.1 | ||
| Other manual therapy | 63 | Manual, simulated manoeuvre | 21 | 33.3 |
| Manual, soft touch | 19 | 30.2 | ||
| Manual, technique to different area | 1 | 1.6 | ||
| Manual, simulated manoeuvre and to different area | 1 | 1.6 | ||
| Disabled device | 16 | 25.4 | ||
| Other (time-attention control, therapist attention, active joint movement, 2x low-pressure algometry) | 5 | 7.9 | ||
| Rehabilitation or physiotherapy | 22 | Exercise, nonspecific | 8 | 36.4 |
| Exercise, key components altered | 1 | 4.5 | ||
| Disabled device | 5 | 22.7 | ||
| Manual, simulated manoeuvre | 1 | 4.5 | ||
| Manual, soft touch | 1 | 4.5 | ||
| Educational attention control | 5 | 22.7 | ||
| Other (nonspecific visualisation, rest + therapist attention) | 2 | 9.1 | ||
| Cognitive-behavioural and other psychotherapy | 27 | Multicomponent therapist interaction | 11 | 40.7 |
| Educational attention control | 7 | 25.9 | ||
| Cognitive task, nonspecific | 2 | 7.4 | ||
| Writing attention control | 2 | 7.4 | ||
| Other (relaxing music, nonspecific visualisation, open-label saline injection, nonhypnotic relaxation suggestions and white noise, nonspecific video and sound) | 5 | 18.5 | ||
| Spiritual, energetic, or esoteric healing | 8 | Simulated hands-off manoeuvre (actor) | 7 | 87.5 |
| Simulated hands-on manoeuvre (actor) | 1 | 12.5 | ||
| Self-management | 5 | Rest time control | 2 | 40.0 |
| Educational attention control | 1 | 20.0 | ||
| Web site, nonspecific | 1 | 20.0 | ||
| Other (white noise) | 1 | 20.0 | ||
| Other | 2 | Other (white noise, headphones without sound) | 2 | 100.0 |
N = 198.
3.4. Similarity between experimental and sham interventions
Conceptually, 29% of all studies explicitly reported matching or controlling for certain intervention components, but the degree to which sham control interventions resembled the tested intervention varied widely.
The average similarity between experimental and sham intervention per trial was 0.88 (SD ± 0.66) across all rated features. Assessment of individual features showed that some items were frequently designed to match the active intervention, while this was rare for others (Fig. 2, table with statistical detail provided in supplemental digital content 3, available at http://links.lww.com/PAIN/B673). For most items, however, confidence intervals were large. Overall ratings were different between simple and complex intervention trials (t(1,195) = 4.67, P < 0.0001), with comparisons between simple interventions and their shams being on average 0.4 points more similar (0.24-0.6 95% confidence interval).
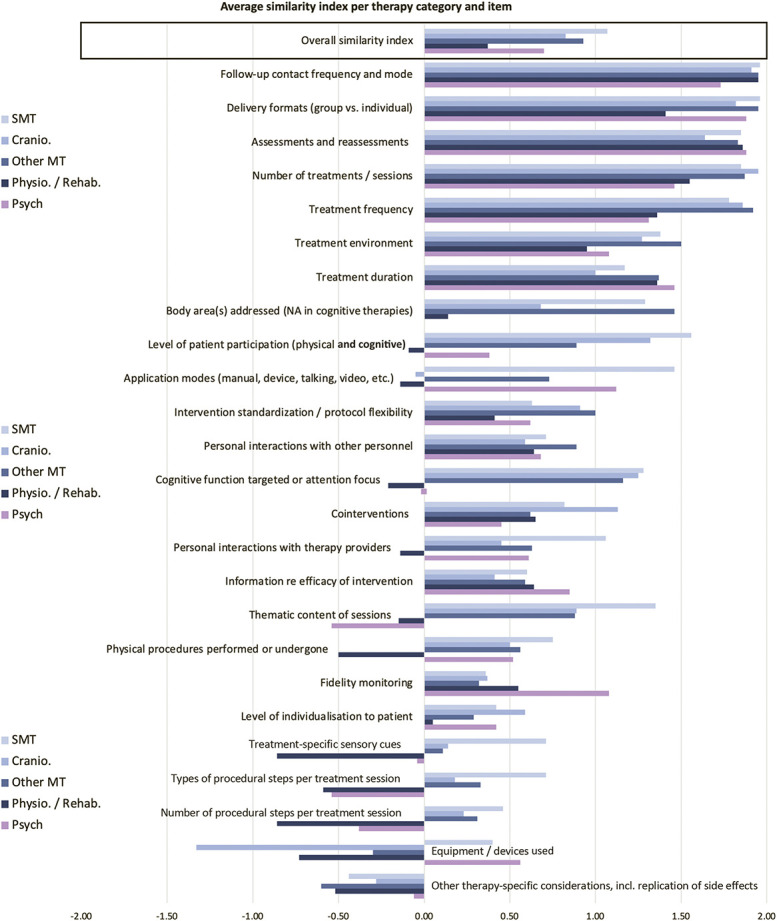
Notable therapy-specific differences existed for overall similarity ratings (F(7,190) = 5.28, P < 0.001), with physiotherapy or rehabilitation trials having significantly lower average ratings (0.37 ± 0.77) than spinal manipulation trials (1.07 ± 0.54, P < 0.001), other manual therapies (excluding craniosacral therapy) (0.93 ± 0.6, P = 0.008), and trials of spiritual or energetic therapies (1.52 ± 0.42, P < 0.001). Figure 3 provides therapy-specific similarity ratings across all assessed features. A table with statistical detail is provided as supplement (supplemental digital content 3, available at http://links.lww.com/PAIN/B673).
Figure 3.
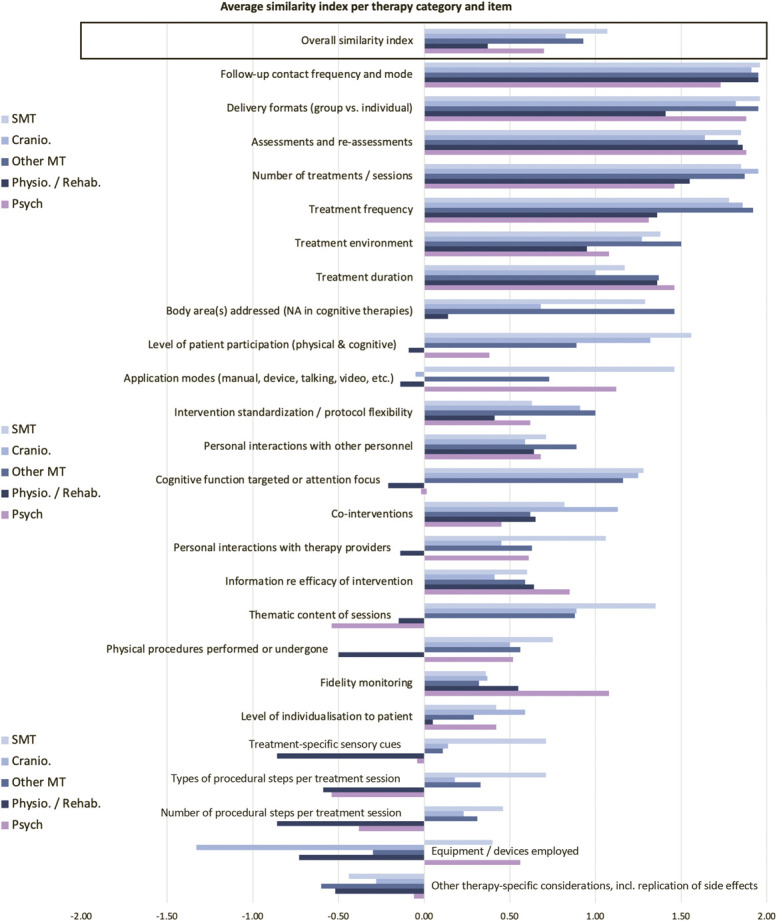
Average similarity ratings categorised by therapy type, comparing active and control interventions across 25 features. The overall mean across all items is provided in the top row. The end of each bar indicates the average rating. Measures of variability are provided in a supplementary table (supplemental digital content 3, available at http://links.lww.com/PAIN/B673) and the number of trials per group in the overview table 3.
3.5. Provider-related similarity
Interventions in the test and control groups were delivered by the same (set of) providers in at least 120 (59%) of all trials (clearly reported). Different providers were used in at least 32 trials (16%), and this could not be ascertained or was not relevant because of treatment automation in a further 46 (23%).
In trials where it was clearly reported that different providers were used, we further assessed whether these were matched for expertise (eg, educational background), experience (eg, years in practice), behaviour, and if trial-specific training had been similar between groups (Table 4).
Table 4.
Matching of provider-related factors.
| Provider expertise | Provider experience | Provider behaviour | Trial-specific provider training | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Matched | 7 | 21.9 | 3 | 9.4 | 12 | 37.5 | 9 | 28.1 |
| Not matched | 18 | 56.3 | 16 | 50.0 | 5 | 15.6 | 9 | 28.1 |
| Not reported | 7 | 21.9 | 13 | 40.6 | 14 | 43.8 | 11 | 34.4 |
Assessed in trials for which it could be ascertained that different providers were used for active and control interventions. N = 32.
3.6. Additional features of placebo control interventions
Within the trials, methods of enhancing patients' expectations of a therapeutic effect included describing potential benefits of the sham intervention. Relatedly, some trials informed patients that only effective treatments were studied, either directly or by naming the sham control intervention differently (eg, a “physical modality”105). Providers' positive expectations were modified, for example, by not informing them that the sham device was disabled or by telling them that simple touch could have beneficial effects (Table 5).
Table 5.
Additional features of placebo control design and implementation.
| Feature | Reported in | ||
|---|---|---|---|
| n | % | ||
| (Mis)informing patients that only efficacious treatments are investigated | 32 | 16.2 | |
| Deliberately enhancing patients' expectations of therapeutic benefit (other means) | 16 | 8.1 | |
| Only enrolling treatment-naïve patients | 30 | 15.2 | |
| Enhancing control provider expectations of therapeutic benefit | 9 | 4.8 | Not applicable in 10 automated interventions (n = 189) |
| Providing specific training to the therapists to deliver the control intervention | 43 | 22.8 | Not applicable in 10 automated interventions (n = 189) |
Total n = 178, unless otherwise indicated. Note that the provided assessments are dependent on author reporting.
3.7. Sham control intervention development and theory
We examined the reporting of processes and theoretical considerations underpinning the development of each sham control intervention. Where reported, information on development processes or theoretical considerations was brief, often no more than a half sentence. Theoretical considerations included justifying why certain elements of a sham intervention were chosen or omitted. Overall, many studies provided no indication how the design of the control intervention was informed (Table 6).
Table 6.
Reporting of placebo control development processes and theoretical considerations.
| Sham control development | Reported in | Comment | |
|---|---|---|---|
| n | % | ||
| Processes involved in the development of the sham control intervention | 90 | 45.5 | 29 (14.6%) in the text and with some explanation |
| 61 (30.8%) provided a reference to previous work | |||
| Specific testing of the control intervention (including feasibility or validation phase, either as part of the trial or externally) | 22 | 11.6 | Not applicable in 8 validation studies (n = 190) |
| Consulting with patient, practitioners, or public involvement groups | 4 | 2.0 | |
| Consideration of ethical aspects of respective sham intervention | 14 | 7.1 | |
| Theory | Reported in | ||
|---|---|---|---|
| n | % | ||
| Theoretical considerations in the design of the sham control intervention | 59 | 29.8 | |
| Any hypothesized active (or specific) components of the treatment under investigation | 160 | 80.8 | |
| Any hypothesized nonspecific (or common) components of the treatment under investigation | 58 | 32.6 | |
| Matching or controlling of the nonspecific (or common) factors between therapy and sham | 58 | 29.3 | |
Total n = 178, unless otherwise indicated.
3.8. Blinding
Assessing compliance with a relevant CONSORT reporting item, 53% of all included studies reported the blinding status of all involved stakeholders (patients, providers, outcome assessors, and statisticians). An additional 36% reported the blinding status for some of the above. Although trials were designed to blind patients to group allocation in 75% of cases, information on patient blinding was not provided in 13% of reports, and 12% of sham-controlled RCTs reported that the trial was not designed to blind participants to the nature of the intervention received or the group allocated to. Although trial reports were often ambiguous on the specific circumstances, it seems that in these instances, patients may have been aware of the group they had been allocated to, often because the 2 interventions were very dissimilar. They were, however, in most instances likely not aware that the control intervention was a sham control with no supposed effect on outcomes.5,10,11,40,44,45,92,102,105,111,134 Consequently, these trials were sham-controlled but used deception as to the nature of the comparator intervention. In other instances,6,13,57,70,90,96,132,153,157,158 control interventions were used that were not believed to be entirely inert but have circumstantial effects on outcomes. Almost exclusively, these latter were so-called attention controls for cognitive-behavioural interventions.
Providers were blinded in a minority of 3% of trials, but the methods to achieve double-blinding are noteworthy: Ajimsha et al.3 and Moraska et al.116 did not inform practitioners that the used ultrasound machine was nonfunctional. In another case, the control intervention was provided by family members who read to the patients, essentially providing an attention control without knowing about its rationale in the trial context.51 A similar strategy was applied to practitioners by Vitiello et al.,152 with providers delivering an educational attention control not knowing that it was the trial's control condition. In a further 7% of trials, provider blinding was of no concern because, for example, automated or prerecorded interventions were studied.
Blinded outcome assessment was reported for 58% of studies. A further 24% exclusively used patient-reported outcome measures, thus ensuring blinded outcome reporting where patient blinding was successful. Unblinded outcome assessment was reported in 6% of trials, and information on blinding status of outcome assessors was not reported in 11% of trials. The separation of treatment provider and outcome assessor roles was another common method to enhance internal trial validity, reported in 67% of trials (not performed in 6% and not reported in 28%).
Whether the statistical analysis was blinded was rarely reported (69% not reported), with 21% of trials reporting blinded, and 10% unblinded, statistical analysis.
3.9. Reports of blinding effectiveness and patient expectancy
Of 198 control interventions, 150 (76%) were most likely designed to blind participants to the received intervention. Only in 35 (23%) of these cases did researchers evaluate whether participants blinding had been successful, which included all but one of the 8 sham control validation studies. Blinding was mainly assessed by patients guessing their group allocation and occasionally through treatment credibility as proxy. The methods to analyse and interpret blinding success were highly variable.
In 19 reports, blinding indices were provided or data were reported in a manner that allowed for calculating Bang's index. Only 4 studies reported unsuccessful participant blinding as per their own criteria; all others reported successful blinding or provided descriptive data without judgement. Details and results are reported in a supplementary table (supplemental digital content 4, available at http://links.lww.com/PAIN/B674).
Two small cross-over studies assessed blinding effectiveness.76,142 In cross-over designs, patients can directly compare experimental and sham treatments, arguably making it easier to correctly guess group allocation. However, there was no indication of less successful blinding in the second phase of the trial by Hall et al.76 Teys et al.142 indicated successful blinding but did not provide useful data for independent assessment.
The time points of blinding assessment differed, with most trials obtaining ratings after the first session or after the end of the treatment. Few studies monitored blinding throughout the course of a longer trial.47,104 Notably, however, 22 other studies (11%) reported that their sham intervention had been tested previously.
Apart from reporting on blinding success, 29 studies (14.6%) assessed the patients' expectation of treatment benefit, albeit in a very heterogeneous manner, or their satisfaction with the received interventions (9 studies overlapping with those reporting on blinding success). Occasionally, this was reported as a proxy for successful blinding but more commonly to study potential influences of patient expectancy on clinical outcomes. Further detail is provided in supplementary digital content 2, available at http://links.lww.com/PAIN/B672.
4. Discussion
We analysed 198 sham control interventions and compared them with respective experimental treatments, identifying a range of common control intervention designs. We found notable gaps in reporting important information about the development, rationale, and validation of used sham controls, complicating the assessment of control intervention quality as well as the replication of methods by future researchers. Blinding effectiveness was also rarely reported and, if so, was performed in a variety of ways. The large and heterogenous sample studied here allows for a nuanced discussion of control and blinding methods in PPS trials for pain.
Based on the concepts of “structural equivalence” and “indistinguishability,”21,108,125 we provided a detailed assessment of the similarity between control and experimental interventions. In our sample, similarity was prioritised for features concerned with the extent and timing of treatments and outcome assessments and the delivery format. The environments in which control and experimental interventions took place were also similar on average, but the variability was larger and nonreporting contributed to lower ratings. Many other compared features were less commonly matched between groups. These concerned the patient experience (eg, treatment-specific sensory cues such as touch or sound, attention focus during interventions, personal interactions with providers and staff), procedural aspects of interventions (individualisation to patients, similarity and complexity of physical procedures performed, devices used in the application of control but not experimental treatments, use of cointerventions), and research-related aspects (eg, differences in fidelity monitoring). Furthermore, developing closely matched control interventions is less common for complex intervention studies.
4.1. Challenges of control intervention design
The findings illustrate the intricacies of designing adequate control interventions in efficacy and mechanistic trials. For example, the closer control interventions are matched to experimental treatments, the more challenging the necessary mechanistic considerations become. In manual therapy trials, concerns regarding the supposed inherent benefits of human touch110 may lead authors to consider nontouch control interventions. Interestingly, while massage-based or mobilisation-based treatments and craniosacral therapies are often compared with detuned ultrasound or other devices,46 the field of spinal manipulation research has opted against such an approach.48,112,125,150 Mechanistic studies of spinal manipulation have focussed on the “click” phenomenon and thrust forces.75,123,124 Contrastingly, in nonthrust techniques, the supposed mechanism is less clear-cut or more subtle, leaving more room for the potential role of touch.26,27 The use of actors was the preferred control intervention in RCTs of energetic or spiritual healing practices,14,19,29,42,129,130,135 likely again explained by mechanistic considerations where the healer themselves is the mechanism or medium through which healing occurs.141
Relatedly, in a trial of guided imagery for pain relief,18 the patient's attention focus on the breath and away from the pain experience is an integral part of the treatment and will thus not be matched. As such, it is unclear whether an optimal control should direct attention to something else non–pain-related (as would be the case in a general health education programme used as attention control) or not manipulate attention at all (as in the given example, using “rest” as sham control). The question of attention focus also applies to physical and manual therapy trials. Some control interventions involved treatment of or exercises for nonaffected body parts,54,73,91,114,122,144 producing a mismatch with the experimental treatment where patients were likely to focus on painful body areas.
In psychological intervention research, the complexity of treatment mechanisms has probably contributed to a relative sparsity of controlled efficacy or mechanistic trials. Instead, psychological interventions such as cognitive-behavioural therapy are often compared with treatment-as-usual or no-treatment controls,61 against which they show small to moderate effects.166 Existing studies with active comparators, few of which qualified as sham or attention controls in our review, only show very small effects on pain and disability.166 Indeed, “specific” (eg, behaviour change) and “common” (eg, the therapeutic relationship) treatment mechanisms are often linked and difficult to isolate in psychological interventions156 and elsewhere.97,148 As an alternative approach, mediation analyses have been used within trials of active psychological treatments to advance understanding of purported mechanisms of change.118,145
Although the challenges for sham-controlled psychological intervention trials are certainly immense, there are mechanistic theories that could guide control intervention development.4,38,39,109 Furthermore, our review demonstrates that high-similarity control interventions are feasible,70,90,153 likely providing more insight into treatment efficacy and mechanism than unmatched active comparator treatments such as education, relaxation, or exercise.165 In addition, many manual therapy2,15,17,25,28,37,63,73,74,89,100,106,120,136,140,142,151,167,170 and some exercise trials69,84 found promising solutions to the sham control problem, creating largely similar control interventions through the consideration of mechanistic treatment rationales and the mimicking of main contextual treatment aspects. This approach may in turn inspire development in other therapy fields, including psychological interventions.
The above examples of touch, attention focus, and active comparator treatments also illustrate another challenge of controlled efficacy RCTs in PPS research: It is unclear what the implications for a trial are if the used control intervention is considered a treatment in its own right under different circumstances, such as cognitive distraction, nonspecific exercise, generic education, provider support, or touch. Calling control interventions “sham” rather than “placebo control” acknowledges that these may not be as clearly “inert” as a sugar pill. Nevertheless, the question remains whether the effect sizes expected in pharmacotherapy research can realistically be demanded from sham-controlled RCTs of PPS interventions, given the potentially considerable effects produced by complex sham comparators.61
What constitutes an appropriate control intervention can be informed by placebo research147 and may depend on the trial's objectives. If the aim is to create similar levels of patient expectations of benefit, then studies need to explore whether this can be achieved with very dissimilar controls68,127,169 or even unblinded designs.164 If the aim is to control for context effects or study treatment mechanisms, then a careful matching is likely beneficial.125 Blinding to sham allocation alone may also be achieved with very dissimilar but equally credible interventions, as illustrated by 2 reviewed trials that assessed blinding success.23,52 However, this approach is unreliable,155 and blinding is likely helped by intervention similarity.35 Measuring potential outcome mediators such as expectancy and blinding status is laudable but uncommon and so is the testing of control interventions in pilot studies. In the absence of such information, readers of a trial can only put themselves into the patients' shoes and ask whether this control intervention would feel credible and effective to them.83 In our parallel publication, we further assess the impact of matched or nonmatched controls on trial outcomes and discuss potential “giveaways” that may undermine the blinding success of even well-designed control interventions.
For further inspiration for control interventions and examples from a given group of therapies, the reader is referred to the comprehensive supplementary table (supplemental digital content 2, available at http://links.lww.com/PAIN/B672) where each trial and its control design is categorised and to the supplementary table on reported blinding effectiveness (supplemental digital content 4, available at http://links.lww.com/PAIN/B674).
4.2. Additional blinding considerations
Blinding of treatment providers was very rare in the included trials (reported in 3%). Arguably, however, the potential for unblinded therapists to undermine participant and staff blinding is considerable and so is their capacity for producing different contextual effects between groups.99 Especially in studies where providers spend substantial time with patients, it seems reasonable to suspect that providers might “compensate” for providing control treatment by changes in behaviour and possibly additional advice or other contraventions of trial protocols. It is inherently challenging to achieve provider blinding, especially when a trial is delivered in a real-world clinical setting. However, unless nonblinded providers are prepared for situations in which their natural inclination to help might contravene trial requirements, a trial's internal validity is at risk.99,107,165
Where patient blinding to group allocation is an objective of the control intervention, the assessment of whether blinding was successful seems reasonable. In our sample, 25% of the relevant studies did examine this, some of which, however, were validation studies of new control interventions. Many of the recent arguments against such assessments and against blinding overall9,162 may not apply to the studied patient population and group of therapies. For example, unblinding because of dramatic treatment efficacy is unlikely in musculoskeletal pain and PPS interventions, and adverse effects are less common.43 The practical argument against blinding, however, namely that it may simply not be possible in such complex interventions,162 does warrant some consideration: This review has clearly shown that trial researchers and funders in pain research perceive there to be a need for sham-controlled and blinded trials, especially across the manual therapies. As Anand et al.9 rightly point out, there are research areas in which placebo effects are likely and where the case for the superiority of an intervention over a sham control has not yet been fully examined. On the other hand, emerging conflicting evidence regarding the impact of blinding status and blinding success warrants further scientific attention.12,66,117
The diversity and sometimes sophistication of used control interventions, plus the existence of multiple successfully blinded trials, demonstrates that patient blinding is a feasible, if challenging task. The complexity of the task, however, does lead to considerable research expense and, in the absence of best-practice standards for control interventions in efficacy and mechanistic trials, likely also research waste because of noncredible control interventions.83 Comparative effectiveness studies are the obvious alternative to sham-controlled RCTs in complex interventions, but their adequacy needs to be considered in the light of the research question, existing evidence of efficacy, and the availability of suitable active comparator treatments.165 Given the need for larger sample sizes in such trials, it further seems questionable whether these designs are always more economical than a well-designed explanatory RCT.16,82
4.3. Reporting
Insufficient reporting of blinding methods has been identified as a problem before and has not seemed to improve.8,72,163 Recently, a checklist specific to the reporting of placebo controls was published (Template for Intervention Description and Replication [TIDieR]—Placebo).86 Although not formally assessed in our review because data extraction was completed before the publication of TIDieR-Placebo, we suspect that most procedural items of the reporting checklist are complied within PPS trials (what was provided as part of the control intervention, through which delivery modes, when and how much; items 1, 3, 4, 6, 8, and 9). However, we showed the reporting of provider characteristics to be deficient in trials where control and active interventions were not delivered by the same set of providers. Notably, TIDieR-Placebo requires little information on provider behaviour (only expertise, but not potential behaviour matching), an element for which we identified a large need for improved reporting. As for the theoretical background and rationale of the control intervention, TIDieR-Placebo asks researchers to “[d]escribe any rationale, theory, or goal of the elements essential to the placebo/sham intervention.” We were able to ascertain that information to this effect was only provided in about a third of the studies. Even so, this was rarely sufficient to understand the relevant theoretical considerations regarding the control intervention design, including the purpose of using a sham control in this specific trial (blinding to group allocation, controlling for contextual effects, both), or to isolate the specific treatment components of the experimental treatment. Knowing the trial authors' reasoning allows readers to assess the appropriateness of the control intervention.165
While reporting guidance for intervention components only became available in 2014,80 reporting guidelines for general trial features have been available longer. Specifically, the 2008 publication of the first CONSORT statement for nonpharmacological intervention RCTs30,32 is the reason why we included studies published from then onwards. Irrespectively, reporting of the 2 major items relevant to this review's objectives—the detailed description of the control intervention (66%) and reporting of the blinding status of all involved stakeholders (51%)—requires some improvement.
5. Conclusions
Overall, our findings call attention to the need for more guidance on the design of control interventions and blinding methods in mechanistic and efficacy trials, informed by current practice and common challenges in the field of psychological, physical, and self-management intervention research. Currently, sham controls range from closely resembling the test treatment to highly dissimilar, with differences between therapy groups. Especially, physiotherapy and certain kinds of manual therapies use dissimilar controls. Despite being a primary objective of most sham control interventions, it is infrequently reported whether participant blinding was effective.
Future recommendations for sham control interventions need to begin with a consideration of whether a sham-controlled RCT is the adequate design for a given research question and, if so, what the phenomena to be controlled for are. Control intervention development is likely improved by being theory-driven. In this context, insights from placebo research may be useful and we examine the link between sham similarity and trial outcomes in a second publication of this review.81 Feasibility testing may be helpful to ascertain whether a control intervention can achieve its objectives. To be useful for end users, the reporting standard of control procedures needs to be enhanced.
While the complexity of the task may mean that research efforts cannot be directly compared with pharmacological RCTs and that alternative designs may have to be considered, our review clearly demonstrated the feasibility of successful blinding by means of dedicated complex control interventions in large-scale RCTs of PPS therapies.
Conflict of interest statement
Mr Hohenschurz-Schmidt reports support through a PhD Studentship from the Alan and Sheila Diamond Trust for this work and personal fees from Altern Health Ltd, outside the submitted work; Dr. Draper-Rodi reports grants from Alan and Sheila Diamond Charitable Trust, during the conduct of the study; Dr. Scott reports grants from Medical Research Council and Versus Arthritis, and from the National Institute for Health and Care Research, outside the submitted work; Dr. Vollert reports personal fees from Vertex Pharmaceuticals and personal fees from Embody Orthopaedic, outside the submitted work; Prof Rice reports personal fees from IMMPACT and grants from the Alan and Sheila Diamond Trust during the conduct of the study, and personal fees from Imperial Consultants, personal fees from MD Anderson Cancer Center, other from spinifex, other from Medicines and Healthcare products Regulatory Agency (MHRA), and Commission on Human Medicines - Neurology, Pain & Psychiatry Expert Advisory Group, all outside the submitted work; In addition, Dr. Rice has a patent WO 2005/079771 & a patent EP13702262.0/ WO2013 110945 pending. All other authors report that they have no conflicts of interest.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B671, http://links.lww.com/PAIN/B672, http://links.lww.com/PAIN/B673, and http://links.lww.com/PAIN/B674.
Acknowledgements
The authors thank all additional volunteers who did not complete the training or the screening and data extraction, the participants from a parallel Delphi process who were presented with the systematic review and provided feedback on methods, and Prof Ben Colagiuri's group for facilitating the blinding index calculation.
The PhD position supporting this work was funded by the Alan and Sheila Diamond Charitable Trust.
All listed authors have contributed substantially to the project and fulfil ICMJE criteria.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
Contributor Information
Jerry Draper-Rodi, Email: Jerry.Draper-Rodi@uco.ac.uk.
Lene Vase, Email: lenevase@psy.au.dk.
Whitney Scott, Email: whitney.scott@kcl.ac.uk.
Alison McGregor, Email: a.mcgregor@imperial.ac.uk.
Nadia Soliman, Email: n.soliman16@imperial.ac.uk.
Andrew MacMillan, Email: Andrew.MacMillan@uco.ac.uk.
Axel Olivier, Email: axel.olivier18@imperial.ac.uk.
Cybill Ann Cherian, Email: cybil_cherian@hotmail.com.
Daniel Corcoran, Email: dcorcoran.aus@gmail.com.
Hilary Abbey, Email: Hilary.Abbey@uco.ac.uk.
Sascha Freigang, Email: sascha.freigang@medunigraz.at.
Jessica Chan, Email: jessica.chan18@imperial.ac.uk.
Jules Phalip, Email: jules.phalip@gmail.com.
Lea Nørgaard Sørensen, Email: lea.soerensen@live.dk.
Maite Delafin, Email: maitedelafin@gmail.com.
Margarida Baptista, Email: mbaptista1997@gmail.com.
Naomi R. Medforth, Email: naomi.medforth@gmail.com.
Nuria Ruffini, Email: nuria.ruff@gmail.com.
Stephanie Skøtt Andresen, Email: skottandersen@gmail.com.
Sylvain Ytier, Email: sylvain.ytier@gmail.com.
Dorota Ali, Email: dorota.ali@kcl.ac.uk.
Harriet Hobday, Email: harriet.hobday@kcl.ac.uk.
Anak Agung Ngurah Agung Adhiyoga Santosa, Email: anak_agung.santosa@kcl.ac.uk.
Jan Vollert, Email: j.vollert@imperial.ac.uk.
Andrew S.C. Rice, Email: a.rice@imperial.ac.uk.
References
- [1].Robert Kerns AJ. Researching nondrug approaches to pain management. JAMA 2018;319:1535–7. [DOI] [PubMed] [Google Scholar]
- [2].Aghabati N, Mohammadi E, Pour Esmaiel Z. The effect of therapeutic touch on pain and fatigue of cancer patients undergoing chemotherapy. Evid Based Complement Altern Med 2010;7:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ajimsha MS, Binsu D, Chithra S. Effectiveness of myofascial release in the management of plantar heel pain: a randomized controlled trial. Foot (Edinb) 2014;24:66–71. [DOI] [PubMed] [Google Scholar]
- [4].Åkerblom S, Perrin S, Rivano Fischer M, McCracken LM. The mediating role of acceptance in multidisciplinary cognitive-behavioral therapy for chronic pain. The J pain 2015;16:606–15. [DOI] [PubMed] [Google Scholar]
- [5].Albert HB, Manniche C. The efficacy of systematic active conservative treatment for patients with severe sciatica: a single-blind, randomized, clinical, controlled trial. Spine 2012;37:531–42. [DOI] [PubMed] [Google Scholar]
- [6].Allen KD, Oddone EZ, Coffman CJ, Datta SK, Juntilla KA, Lindquist JH, Walker TA, Weinberger M, Bosworth HB. Telephone-based self-management of osteoarthritis: a randomized trial. Ann Intern Med 2010;153:570–9. [DOI] [PubMed] [Google Scholar]
- [7].Alvarez G, Núñez-Cortés R, Solà I, Sitjà-Rabert M, Fort-Vanmeerhaeghe A, Fernández C, Bonfill X, Urrutia G. Sample size, study length and inadequate controls were the most common self-acknowledged limitations in manual therapy trials: a methodological review. J Clin Epidemiol 2020;130:96–106. [DOI] [PubMed] [Google Scholar]
- [8].Alvarez G, Solà I, Sitjà-Rabert M, Fort-Vanmeerhaeghe A, Gich I, Fernández C, Bonfill X, Urrútia G. A methodological review revealed that reporting of trials in manual therapy has not improved over time. J Clin Epidemiol 2020;121:32–44. [DOI] [PubMed] [Google Scholar]
- [9].Anand R, Norrie J, Bradley JM, McAuley DF, Clarke M. Fool's gold? Why blinded trials are not always best. BMJ 2020;368:l6228. [DOI] [PubMed] [Google Scholar]
- [10].Andreae SJ, Andreae LJ, Richman JS, Cherrington AL, Safford MM. Peer-delivered cognitive behavioral training to improve functioning in patients with diabetes: a cluster-randomized trial. Ann Fam Med 2020;18:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Arcos-Carmona IM, Castro-Sanchez AM, Mataran-Penarrocha GA, Gutierrez-Rubio AB, Ramos-Gonzalez E, Moreno-Lorenzo C. Effects of aerobic exercise program and relaxation techniques on anxiety, quality of sleep, depression, and quality of life in patients with fibromyalgia: a randomized controlled trial [in Spanish]. Med Clin (Barc) 2011;137:398–401. [DOI] [PubMed] [Google Scholar]
- [12].Armijo-Olivo S, Dennett L, Arienti C, Dahchi M, Arokoski J, Heinemann AW, Malmivaara A. Blinding in rehabilitation research: empirical evidence on the association between blinding and treatment effect estimates. Am J Phys Med Rehabil 2020;99:198–209. [DOI] [PubMed] [Google Scholar]
- [13].Ashar YK, Gordon A, Schubiner H, Uipi C, Knight K, Anderson Z, Carlisle J, Polisky L, Geuter S, Flood TF, Kragel PA, Dimidjian S, Lumley MA, Wager TD. Effect of pain reprocessing therapy vs placebo and usual care for patients with chronic back pain: a randomized clinical trial. JAMA Psychiatry 2021;79:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Assefi N, Bogart A, Goldberg J, Buchwald D. Reiki for the treatment of fibromyalgia: a randomized controlled trial. J Altern Complement Med 2008;14:1115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Attali TV, Bouchoucha M, Benamouzig R. Treatment of refractory irritable bowel syndrome with visceral osteopathy: short-term and long-term results of a randomized trial. J Dig Dis 2013;14:654–61. [DOI] [PubMed] [Google Scholar]
- [16].Aycock DM, Hayat MJ, Helvig A, Dunbar SB, Clark PC. Essential considerations in developing attention control groups in behavioral research. Res Nurs Health 2018;41:320–8. [DOI] [PubMed] [Google Scholar]
- [17].Baconnier P, Vial B, Vaudaux G, Vaudaux G, Bosson JL. Evaluation of microkinesitherapy effectiveness in post-traumatic cervicalgia: a new approach applied to previous data. Man Ther Posturology Rehabil J 2019;17:1–4. [Google Scholar]
- [18].Baird CL, Murawski MM, Wu J. Efficacy of guided imagery with relaxation for osteoarthritis symptoms and medication intake. Pain Manag Nurs 2010;11:56–65. [DOI] [PubMed] [Google Scholar]
- [19].Baldwin AL, Vitale A, Brownell E, Kryak E, Rand W. Effects of reiki on pain, anxiety, and blood pressure in patients undergoing knee replacement: a pilot study. Holist Nurs Pract 2017;31:80–9. [DOI] [PubMed] [Google Scholar]
- [20].Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials. Controlled Clin Trials 2004;25:143–56. [DOI] [PubMed] [Google Scholar]
- [21].Baskin TW, Tierney SC, Minami T, Wampold BE. Establishing specificity in psychotherapy: a meta-analysis of structural equivalence of placebo controls. J consulting Clin Psychol 2003;71:973–9. [DOI] [PubMed] [Google Scholar]
- [22].Beard DJ, Campbell MK, Blazeby JM, Carr AJ, Weijer C, Cuthbertson BH, Buchbinder R, Pinkney T, Bishop FL, Pugh J, Cousins S, Harris IA, Lohmander LS, Blencowe N, Gillies K, Probst P, Brennan C, Cook A, Farrar-Hockley D, Savulescu J, Huxtable R, Rangan A, Tracey I, Brocklehurst P, Ferreira ML, Nicholl J, Reeves BC, Hamdy F, Rowley SC, Cook JA. Considerations and methods for placebo controls in surgical trials (ASPIRE guidelines). Lancet 2020;395:828–38. [DOI] [PubMed] [Google Scholar]
- [23].Bennell K, Wee E, Coburn S, Green S, Harris A, Staples M, Forbes A, Buchbinder R. Efficacy of standardised manual therapy and home exercise programme for chronic rotator cuff disease: randomised placebo controlled trial. BMJ 2010;340:c2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bernstein IA, Malik Q, Carville S, Ward S. Low back pain and sciatica: summary of NICE guidance. BMJ 2017;356:i6748. [DOI] [PubMed] [Google Scholar]
- [25].Beselga C, Neto F, Alburquerque-Sendín F, Hall T, Oliveira-Campelo N. Immediate effects of hip mobilization with movement in patients with hip osteoarthritis: a randomised controlled trial. Man Ther 2016;22:80–5. [DOI] [PubMed] [Google Scholar]
- [26].Bialosky JE, Beneciuk JM, Bishop MD, Coronado RA, Penza CW, Simon CB, George SZ. Unraveling the mechanisms of manual therapy: modeling an approach. J Orthop Sports Phys Ther 2018;48:8–18. [DOI] [PubMed] [Google Scholar]
- [27].Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther 2009;14:531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bialosky JE, Bishop MD, Price DD, Robinson ME, Vincent KR, George SZ. A randomized sham-controlled trial of a neurodynamic technique in the treatment of carpal tunnel syndrome. J orthopaedic Sports Phys Ther 2009;39:709–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bliddal H, Christensen R, Hojgaard L, Bartels EM, Ellegaard K, Zachariae R, Danneskiold-Samsoe B. Spiritual healing in the treatment of rheumatoid arthritis: an exploratory single centre, parallel-group, double-blind, three-arm, randomised, sham-controlled trial. Evid Based Complement Altern Med 2014;2014:269431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P; for the CONSORT NPT Group. CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med 2017;167:40–7. [DOI] [PubMed] [Google Scholar]
- [31].Boutron I, Guittet L, Estellat C, Moher D, Hróbjartsson A, Ravaud P. Reporting methods of blinding in randomized trials assessing nonpharmacological treatments. PLOS Med 2007;4:e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P; CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295–309. [DOI] [PubMed] [Google Scholar]
- [33].Boutron I, Tubach F, Giraudeau B, Ravaud P. Blinding was judged more difficult to achieve and maintain in nonpharmacologic than pharmacologic trials. J Clin Epidemiol 2004;57:543–50. [DOI] [PubMed] [Google Scholar]
- [34].Braithwaite FA, Walters JL, Li LSK, Moseley GL, Williams MT, McEvoy MP. Effectiveness and adequacy of blinding in the moderation of pain outcomes: systematic review and meta-analyses of dry needling trials. Peerj 2018;6:e5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Braithwaite FA, Walters JL, Moseley GL, Williams MT, McEvoy MP. Towards more credible shams for physical interventions: a Delphi survey. Clin Trials 2020;17:295–305. [DOI] [PubMed] [Google Scholar]
- [36].Braithwaite FA, Walters JL, Moseley GL, Williams MT, McEvoy MP. Towards more homogenous and rigorous methods in sham-controlled dry needling trials: two Delphi surveys. Physiotherapy 2020;106:12–23. [DOI] [PubMed] [Google Scholar]
- [37].Brose SW, Jennings DC, Kwok J, Stuart CL, O'Connell SM, Pauli HA, Liu B. Sham manual medicine protocol for cervical strain-counterstrain research. PM R 2013;5:400–7. [DOI] [PubMed] [Google Scholar]
- [38].Burns JW, Nielson WR, Jensen MP, Heapy A, Czlapinski R, Kerns RD. Specific and general therapeutic mechanisms in cognitive behavioral treatment of chronic pain. J Consult Clin Psychol 2015;83:1–11. [DOI] [PubMed] [Google Scholar]
- [39].Burns JW, Van Dyke BP, Newman AK, Morais CA, Thorn BE. Cognitive behavioral therapy (CBT) and pain education for people with chronic pain: tests of treatment mechanisms. J Consult Clin Psychol 2020;88:1008–18. [DOI] [PubMed] [Google Scholar]
- [40].Buttagat V, Narktro T, Onsrira K, Pobsamai C. Short-term effects of traditional Thai massage on electromyogram, muscle tension and pain among patients with upper back pain associated with myofascial trigger points. Complement Ther Med 2016;28:8–12. [DOI] [PubMed] [Google Scholar]
- [41].Campbell MK, Entwistle VA, Cuthbertson BH, Skea ZC, Sutherland AG, McDonald AM, Norrie JD, Carlson RV, Bridgman S; study group KORAL. Developing a placebo-controlled trial in surgery: issues of design, acceptability and feasibility. Trials 2011;12:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Carneiro EM, Barbosa LP, Bittencourt AC, Hernandez CG, Timoteo RP, Almeida CO, Borges MF. Effects of Spiritist “passe” (Spiritual healing) on stress hormone, pain, physiological parameters and length of stay in preterm newborns: a randomized, double-blind controlled trial. J Complement Integr Med 2018;15. [DOI] [PubMed] [Google Scholar]
- [43].Carnes D, Mars TS, Mullinger B, Froud R, Underwood M. Adverse events and manual therapy: a systematic review. Man Ther 2010;15:355–63. [DOI] [PubMed] [Google Scholar]
- [44].Castro-Sanchez AM, Mataran-Penarrocha GA, Arroyo-Morales M, Saavedra-Hernandez M, Fernandez-Sola C, Moreno-Lorenzo C. Effects of myofascial release techniques on pain, physical function, and postural stability in patients with fibromyalgia: a randomized controlled trial. Clin Rehabil 2011;25:800–13. [DOI] [PubMed] [Google Scholar]
- [45].Castro-Sanchez AM, Mataran-Penarrocha GA, Sanchez-Labraca N, Quesada-Rubio JM, Granero-Molina J, Moreno-Lorenzo C. A randomized controlled trial investigating the effects of craniosacral therapy on pain and heart rate variability in fibromyalgia patients. Clin Rehabil 2011;25:25–35. [DOI] [PubMed] [Google Scholar]
- [46].Cerritelli F, Verzella M, Cicchitti L, D'Alessandro G, Vanacore N. The paradox of sham therapy and placebo effect in osteopathy: a systematic review. Medicine (Baltimore) 2016;95:e4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].ChAibi A, Benth JŠ, Tuchin PJ, Russell MB, ChAibi A. Chiropractic spinal manipulative therapy for migraine: a three-armed, single-blinded, placebo, randomized controlled trial. Eur J Neurol 2017;24:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chaibi A, Šaltytė Benth J, Bjørn Russell M. Validation of placebo in a manual therapy randomized controlled trial. Sci Rep 2015;5:11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chen W, Yang GY, Liu B, Manheimer E, Liu JP. Manual acupuncture for treatment of diabetic peripheral neuropathy: a systematic review of randomized controlled trials. PLoS One 2013;8:e73764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Colagiuri B, Sharpe L, Scott A. The blind leading the not-so-blind: a meta-analysis of blinding in pharmacological trials for chronic pain. J Pain 2019;20:489–500. [DOI] [PubMed] [Google Scholar]
- [51].Collinge W, Kahn J, Walton T, Kozak L, Bauer-Wu S, Fletcher K, Yarnold P, Soltysik R. Touch, Caring, and Cancer: randomized controlled trial of a multimedia caregiver education program. Support Care Cancer 2013;21:1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Costa LOP, Maher CG, Latimer J, Hodges PW, Herbert RD, Refshauge KM, McAuley JH, Jennings MD. Motor control exercise for chronic low back pain: a randomized placebo-controlled trial. Phys Ther 2009;89:1275–86. [DOI] [PubMed] [Google Scholar]
- [53].Cousins S, Blencowe NS, Tsang C, Chalmers K, MArdAnpour A, Carr AJ, Campbell MK, Cook JA, Beard DJ, Blazeby JM, MArdAnpour A. Optimizing the design of invasive placebo interventions in randomized controlled trials. Br J Surg 2020;107:1114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cunali PA, Almeida FR, Santos CD, Valdrichi NY, Nascimento LS, Dal-Fabbro C, Tufik S, Bittencourt LRA. Mandibular exercises improve mandibular advancement device therapy for obstructive sleep apnea. Sleep Breath 2011;15:717–27. [DOI] [PubMed] [Google Scholar]
- [55].Curtis P, Gaylord SA, Park J, Faurot KR, Coble R, Suchindran C, Coeytaux RR, Wilkinson L, Mann JD. Credibility of low-strength static magnet therapy as an attention control intervention for a randomized controlled study of CranioSacral therapy for migraine headaches. J Altern Complement Med 2011;17:711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dal-Ré R, de Boer A, James SK. The design can limit PRECIS-2 retrospective assessment of the clinical trial explanatory/pragmatic features. J Clin Epidemiol 2020;126:193–201. [DOI] [PubMed] [Google Scholar]
- [57].Davis MC, Zautra AJ. An online mindfulness intervention targeting socioemotional regulation in fibromyalgia: results of a randomized controlled trial. Ann Behav Med 2013;46:273–84. [DOI] [PubMed] [Google Scholar]
- [58].Day SJ, Altman DG. Statistics notes: blinding in clinical trials and other studies. BMJ 2000;321:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Drucker AM, Chan A-W. Blindsided: challenging the dogma of masking in clinical trials. BMJ 2020;368:m229. [DOI] [PubMed] [Google Scholar]
- [60].Dworkin RH, Turk DC, Peirce-Sandner S, Burke LB, Farrar JT, Gilron I, Jensen MP, Katz NP, Raja SN, Rappaport BA, Rowbotham MC, Backonja M-M, Baron R, Bellamy N, Bhagwagar Z, Costello A, Cowan P, Fang WC, Hertz S, Jay GW, Junor R, Kerns RD, Kerwin R, Kopecky EA, Lissin D, Malamut R, Markman JD, McDermott MP, Munera C, Porter L, Rauschkolb C, Rice ASC, Sampaio C, Skljarevski V, Sommerville K, Stacey BR, Steigerwald I, Tobias J, Trentacosti AM, Wasan AD, Wells GA, Williams J, Witter J, Ziegler D. Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. PAIN 2012;153:1148–58. [DOI] [PubMed] [Google Scholar]
- [61].Faltinsen E, Todorovac A, Bruun LS, Hróbjartsson A, Gluud C, Kongerslev MT, Simonsen E, Storebø OJ. Control interventions in randomised trials among people with mental health disorders. Cochrane Database Syst Rev 2022;4:MR000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Felson DT, Redmond AC, Chapman GJ, Smith TO, Hamilton DF, Jones RK, Holt CA, Callaghan MJ, Mason DJ, Conaghan PG, Conaghan PG. Recommendations for the conduct of efficacy trials of treatment devices for osteoarthritis: a report from a working group of the Arthritis Research UK Osteoarthritis and Crystal Diseases Clinical Studies Group. Rheumatology (Oxford) 2016;55:320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fernandez-Carnero J, Sierra-Silvestre E, Beltran-Alacreu H, Gil-Martinez A, La Touche R. Neural tension technique improves immediate conditioned pain modulation in patients with chronic neck pain: a randomized clinical trial. Pain Med 2019;20:1227–35. [DOI] [PubMed] [Google Scholar]
- [64].Finnegan-John J, Molassiotis A, Richardson A, Ream E. A systematic review of complementary and alternative medicine interventions for the management of cancer-related fatigue. Integr Cancer Ther 2013;12:276–90. [DOI] [PubMed] [Google Scholar]
- [65].Ford I, Norrie J. Pragmatic trials. N Engl J Med 2016;375:454–63. [DOI] [PubMed] [Google Scholar]
- [66].Freed B, Williams B, Situ X, Landsman V, Kim J, Moroz A, Bang H, Park JJ. Blinding, sham, and treatment effects in randomized controlled trials for back pain in 2000–2019: a review and meta-analytic approach. Clin Trials 2021;18:361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Fregni F, Imamura M, Chien HF, Lew HL, Boggio P, Kaptchuk TJ, Riberto M, Hsing WT, Battistella LR, Furlan A. Challenges and recommendations for placebo controls in randomized trials in physical and rehabilitation medicine: a report of the international placebo symposium working group. Am J Phys Med Rehabil 2010;89:160–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Frisaldi E, Shaibani A, Benedetti F. Why we should assess patients' expectations in clinical trials. Pain Ther 2017;6:107–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ganderton C, Semciw A, Cook J, Moreira E, Pizzari T. Gluteal loading versus sham exercises to improve pain and dysfunction in postmenopausal women with greater trochanteric pain syndrome: a randomized controlled trial. J Womens Health (Larchmt) 2018;27:815–29. [DOI] [PubMed] [Google Scholar]
- [70].Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, Howard MO. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol 2014;82:448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].George A, Collett C, Carr A, Holm S, Bale C, Burton S, Campbell M, Coles A, Gottlieb G, Muir K, Parroy S, Price J, Rice A, Sinden J, Stephenson C, Wartolowska K, Whittall H. When should placebo surgery as a control in clinical trials be carried out? Bulletin 2016;98:75–9. [Google Scholar]
- [72].Golomb BA, Erickson LC, Koperski S, Sack D, Enkin M, Howick J. What's in placebos: who knows? Analysis of randomized, controlled trials. Ann Intern Med 2010;153:532–5. [DOI] [PubMed] [Google Scholar]
- [73].González ÁC, Berenguer SB, Luque Mañas JM, Martin-Pintado-Zugasti A. Validation of a sham novel neural mobilization technique in patients with non-specific low back pain: a randomized, placebo-controlled trial. Musculoskelet Sci Pract 2021;53:102378. [DOI] [PubMed] [Google Scholar]
- [74].Guimaraes JF, Salvini TF, Siqueira ALJ, Ribeiro IL, Camargo PR, Alburquerque-Sendin F. Immediate effects of mobilization with movement vs sham technique on range of motion, strength, and function in patients with shoulder impingement syndrome: randomized clinical trial. J Manipulative Physiol Ther 2016;39:605–15. [DOI] [PubMed] [Google Scholar]
- [75].Gyer G, Michael J, Inklebarger J, Tedla JS. Spinal manipulation therapy: is it all about the brain? A current review of the neurophysiological effects of manipulation. J Integr Med 2019;17:328–37. [DOI] [PubMed] [Google Scholar]
- [76].Hall S, Lewith G, Brien S, Little P. An exploratory pilot study to design and assess the credibility of a sham kinesiology treatment. Forsch Komplementmed 2008;15:321–6. [DOI] [PubMed] [Google Scholar]
- [77].Hart T, Bagiella E. Design and implementation of clinical trials in rehabilitation research. Arch Phys Med Rehabil 2012;93:S117–26. [DOI] [PubMed] [Google Scholar]
- [78].Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Higgins JPT, Thomas J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.0. 2nd ed. Chichester: John Wiley & Sons, 2019. Available at: www.training.cochrane.org/handbook. Accessed September 2, 2019. [Google Scholar]
- [80].Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan A-W, Michie S. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- [81].Hohenschurz-Schmidt D, Draper-Rodi J, Vase L, Scott W, McGregor A, Soliman N, MacMillan A, Olivier A, Cherian CA, Corcoran D, Abbey H, Freigang S, Chan J, Phalip J, Sørensen LN, Delafin M, Baptista M, Medforth N, Ruffini N, Andresen SS, Ytier S, Ali D, Hobday H, Santosa AA, Vollert J, Rice AS. Blinding and sham control methods in trials of physical, psychological, and self-management interventions for pain (article II): a meta-analysis relating methods to trial results. PAIN 2023;164:509–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hohenschurz-Schmidt D, Kleykamp BA, Draper-Rodi J, Vollert J, Chan J, Ferguson M, McNicol E, Phalip J, Evans SR, Turk DC, Dworkin RH, Rice ASC. Pragmatic trials of pain therapies: a systematic review of methods. PAIN 2022;163:21–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hohenschurz-Schmidt D, Vollert J, Vogel S, Rice ASC, Draper-Rodi J. Performing and interpreting randomized clinical trials. J Osteopathic Med 2021;121:443–5. [DOI] [PubMed] [Google Scholar]
- [84].Holmgren T, Björnsson Hallgren H, Öberg B, Adolfsson L, Johansson K. Effect of specific exercise strategy on need for surgery in patients with subacromial impingement syndrome: randomised controlled study. BMJ 2012;344:e787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Howick J. The relativity of “placebos”: defending a modified version of Grünbaum's definition. Synthese 2017;194:1363–96. [Google Scholar]
- [86].Howick J, Webster RK, Rees JL, Turner R, Macdonald H, Price A, Evers AWM, Bishop F, Collins GS, Bokelmann K, Hopewell S, Knottnerus A, Lamb S, Madigan C, Napadow V, Papanikitas AN, Hoffmann T. TIDieR-Placebo: a guide and checklist for reporting placebo and sham controls. PLoS Med 2020;17:e1003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hróbjartsson A, Emanuelsson F, Skou Thomsen AS, Hilden J, Brorson S. Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub-studies. Int J Epidemiol 2014;43:1272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hróbjartsson A, Miller F. Chapter 2: blinding in nonpharmacological randomized controlled trials. In: Boutron I, Ravaud P, Moher D, eds. Randomized Clinical Trials of Nonpharmacologic Treatments. Boca Raton: Chapman and Hall/CRC, 2011. [Google Scholar]
- [89].Hudson R, Richmond A, Sanchez B, Stevenson V, Baker RT, May J, Nasypany A, Reordan D. Innovative treatment of clinically diagnosed meniscal tears: a randomized sham-controlled trial of the Mulligan concept “squeeze” technique. J Man Manip Ther 2018;26:254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ilgen MA, Bohnert ASB, Chermack S, Conran C, Jannausch M, Trafton J, Blow FC. A randomized trial of a pain management intervention for adults receiving substance use disorder treatment. Addiction 2016;111:1385–93. [DOI] [PubMed] [Google Scholar]
- [91].Ishiyama H, Inukai S, Nishiyama A, Hideshima M, Nakamura S, Tamaoka M, Miyazaki Y, Fueki K, Wakabayashi N. Effect of jaw-opening exercise on prevention of temporomandibular disorders pain associated with oral appliance therapy in obstructive sleep apnea patients: a randomized, double-blind, placebo-controlled trial. J Prosthodont Res 2017;61:259–67. [DOI] [PubMed] [Google Scholar]
- [92].Izgu N, Gok Metin Z, Karadas C, Ozdemir L, Metinarikan N, Corapcıoglu D. Progressive muscle relaxation and mindfulness meditation on neuropathic pain, fatigue, and quality of life in patients with type 2 diabetes: a randomized clinical trial. J Nurs Scholarsh 2020;52:476–87. [DOI] [PubMed] [Google Scholar]
- [93].Katz N, Dworkin RH, North R, Thomson S, Eldabe S, Hayek SM, Kopell BH, Markman J, Rezai A, Taylor RS, Turk DC, Buchser E, Fields H, Fiore G, Ferguson M, Gewandter J, Hilker C, Jain R, Leitner A, Loeser J, McNicol E, Nurmikko T, Shipley J, Singh R, Trescot A, van Dongen R, Venkatesan L. Research design considerations for randomized controlled trials of spinal cord stimulation for pain: IMMPACT/ION/INS recommendations. PAIN 2021;162:1935–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kawchuk GN, Haugen R, Fritz J. A true blind for subjects who receive spinal manipulation therapy. Arch Phys Med Rehabil 2009;90:366–8. [DOI] [PubMed] [Google Scholar]
- [95].Kerns RD, Brandt CA, Peduzzi P. NIH-DoD-VA pain management collaboratory. Pain Med 2019;20:2336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Khodneva Y, Richman J, Andreae S, Cherrington A, Safford MM. Peer support intervention improves pain-related outcomes among rural adults with diabetes and chronic pain at 12-month follow-up. J Rural Health 2021;37:394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kirsch I. Are drug and placebo effects in depression additive? Biol Psychiatry 2000;47:733–5. [DOI] [PubMed] [Google Scholar]
- [98].Kligler B, Bair MJ, Banerjea R, DeBar L, Ezeji-Okoye S, Lisi A, Murphy JL, Sandbrink F, Cherkin DC. Clinical policy recommendations from the VHA state-of-the-art conference on non-pharmacological approaches to chronic musculoskeletal pain. J Gen Intern Med 2018;33:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Koes BW. How to evaluate manual therapy: value and pitfalls of randomized clinical trials. Man Ther 2004;9:183–4. [DOI] [PubMed] [Google Scholar]
- [100].Kogure A, Kotani K, Katada S, Takagi H, Kamikozuru M, Isaji T, Hakata S. A randomized, single-blind, placebo-controlled study on the efficacy of the arthrokinematic approach-hakata method in patients with chronic nonspecific low back pain. PLoS One 2015;10:e0144325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kumar S, Beaton K, Hughes T. The effectiveness of massage therapy for the treatment of nonspecific low back pain: a systematic review of systematic reviews. Int J Gen Med 2013;6:733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kumar SP. Efficacy of segmental stabilization exercise for lumbar segmental instability in patients with mechanical low back pain: a randomized placebo controlled crossover study. North Am J Med Sci 2011;3:456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lavazza C, Galli M, Abenavoli A, Maggiani A. Sham treatment effects in manual therapy trials on back pain patients: a systematic review and pairwise meta-analysis. BMJ Open 2021;11:e045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Lee K, Lewis GN. Short term relief of multisite chronicpain with Bowen Therapy: a double-blind, randomized controlled trial. J Bodyw Mov Ther 2020;24:271–9. [DOI] [PubMed] [Google Scholar]
- [105].Lehtola V, Korhonen I, Airaksinen O. A randomised, placebo-controlled, clinical trial for the short-term effectiveness of manipulative therapy and acupuncture on pain caused by mechanical thoracic spine dysfunction. Int Musculoskelet Med 2010;32:25–32. [Google Scholar]
- [106].Lewis C, Khan A, Souvlis T, Sterling M. A randomised controlled study examining the short-term effects of Strain-Counterstrain treatment on quantitative sensory measures at digitally tender points in the low back. Man Ther 2010;15:536–41. [DOI] [PubMed] [Google Scholar]
- [107].London AJ. Equipoise in research: integrating ethics and science in human research. JAMA 2017;317:525–6. [DOI] [PubMed] [Google Scholar]
- [108].Machado LAC, Kamper SJ, Herbert RD, Maher CG, McAuley JH. Imperfect placebos are common in low back pain trials: a systematic review of the literature. Eur Spine J 2008;17:889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].McCracken LM, Morley S. The psychological flexibility model: a basis for integration and progress in psychological approaches to chronic pain management. J Pain 2014;15:221–34. [DOI] [PubMed] [Google Scholar]
- [110].McGlone F, Cerritelli F, Walker S, Esteves J. The role of gentle touch in perinatal osteopathic manual therapy. Neurosci Biobehav Rev 2017;72:1–9. [DOI] [PubMed] [Google Scholar]
- [111].Messier SP, Mihalko SL, Beavers DP, Nicklas BJ, DeVita P, Carr JJ, Hunter DJ, Lyles M, Guermazi A, Bennell KL, Loeser RF. Effect of high-intensity strength training on knee pain and knee joint compressive forces among adults with knee osteoarthritis: the START randomized clinical trial. JAMA 2021;325:646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Michener LA, Kardouni JR, Sousa CO, Ely JM. Validation of a sham comparator for thoracic spinal manipulation in patients with shoulder pain. Man Ther 2015;20:171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Moerman DE. Against the “placebo effect”: a personal point of view. Complement Therap Med 2013;21:125–30. [DOI] [PubMed] [Google Scholar]
- [114].Mohamed AA, Jan Y-K, El Sayed WH, Wanis MEA, Yamany AA. Dynamic scapular recognition exercise improves scapular upward rotation and shoulder pain and disability in patients with adhesive capsulitis: a randomized controlled trial. J Man Manip Ther 2020;28:146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Mohr DC, Ho J, Hart TL, Baron KG, Berendsen M, Beckner V, Cai X, Cuijpers P, Spring B, Kinsinger SW, Schroder KE, Duffecy J. Control condition design and implementation features in controlled trials: a meta-analysis of trials evaluating psychotherapy for depression. Transl Behav Med 2014;4:407–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Moraska AF, Schmiege SJ, Mann JD, Butryn N, Krutsch JP. Responsiveness of myofascial trigger points to single and multiple trigger point release massages: a randomized, placebo controlled trial. Am J Phys Med Rehabil 2017;96:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Moustgaard H, Clayton GL, Jones HE, Boutron I, Jørgensen L, Laursen DRT, Olsen MF, Paludan-Müller A, Ravaud P, Savović J, Sterne JAC, Higgins JPT, Hróbjartsson A. Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study. BMJ 2020;368:l6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Murillo C, Vo T-T, Vansteelandt S, Harrison LE, Cagnie B, Coppieters I, Chys M, Timmers I, Meeus M. How do psychologically based interventions for chronic musculoskeletal pain work? A systematic review and meta-analysis of specific moderators and mediators of treatment. Clin Psychol Rev 2022;94:102160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].National Center for Complementary, Integrative Health (NCCIH). Nonpharmacologic Management of Pain. NCCIH, 2016. Available at: https://nccih.nih.gov/about/strategic-plans/2016/Nonpharmacologic-Management-Pain. Accessed November 15, 2019. [Google Scholar]
- [120].Nguyen C, Boutron I, Zegarra-Parodi R, Baron G, Alami S, Sanchez K, Daste C, Boisson M, Fabre L, Krief P, Krief G, Lefèvre-Colau MM, Rannou F. Effect of osteopathic manipulative treatment vs sham treatment on activity limitations in patients with nonspecific subacute and chronic low back pain: a randomized clinical trial. JAMA Intern Med 2021;181:620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol 2021;134:178–89. [DOI] [PubMed] [Google Scholar]
- [122].Paungmali A, Joseph LH, Sitilertpisan P, Pirunsan U, Uthaikhup S. Lumbopelvic core stabilization exercise and pain modulation among individuals with chronic nonspecific low back pain. Pain Pract 2017;17:1008–14. [DOI] [PubMed] [Google Scholar]
- [123].Pickar JG. Neurophysiological effects of spinal manipulation. Spine J 2002;2:357–71. [DOI] [PubMed] [Google Scholar]
- [124].Potter L, McCarthy C, Oldham J. Physiological effects of spinal manipulation: a review of proposed theories. Phys Ther Rev 2005;10:163–70. [Google Scholar]
- [125].Puhl AA, Reinhart CJ, Doan JB, Vernon H. The quality of placebos used in randomized, controlled trials of lumbar and pelvic joint thrust manipulation-a systematic review. Spine J 2017;17:445–56. [DOI] [PubMed] [Google Scholar]
- [126].Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American college of physicians. Ann Intern Med 2017;166:514–30. [DOI] [PubMed] [Google Scholar]
- [127].Rief W, Nestoriuc Y, Mueller EM, Hermann C, Schmidt K, Bingel U. Generic rating scale for previous treatment experiences, treatment expectations, and treatment effects (GEEE). G-EEE 2021. doi: 10.23668/psycharchives.4717 [DOI] [Google Scholar]
- [128].Safer DL, Hugo EM. Designing a control for a behavioral group therapy. Behav Ther 2006;37:120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Sagkal Midilli T, Ciray Gunduzoglu N. Effects of reiki on pain and vital signs when applied to the incision area of the body after cesarean section surgery: a single-blinded, randomized, double-controlled study. Holist Nurs Pract 2016;30:368–78. [DOI] [PubMed] [Google Scholar]
- [130].Santana LC, Puerta PP, Gomez IdeB, Montoro AA, Marco PL, Espinos ID. REIKI efficacy of therapy in improving pain, fatigue, quality of life and its impact on activities's of women daily living suffering from fibromyalgia. Nure Investigación 2013;32:14p. [Google Scholar]
- [131].Saxena S, Weerasuriya C, Shanahan F. The Placebo Effect: is achieving trial success all in our minds? Atlania Food Clinical Trials. Available at: https://atlantiaclinicaltrials.com/white-papers/Placebo-Response.pdf Accessed 6 Aug 2022. [Google Scholar]
- [132].Schmidt S, Grossman P, Schwarzer B, Jena S, Naumann J, Walach H. Treating fibromyalgia with mindfulness-based stress reduction: results from a 3-armed randomized controlled trial. PAIN 2011;152:361–9. [DOI] [PubMed] [Google Scholar]
- [133].Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Schwerla F, Bischoff A, Nurnberger A, Genter P, Guillaume JP, Resch KL. Osteopathic treatment of patients with chronic non-specific neck pain: a randomised controlled trial of efficacy. Forsch Komplementmed 2008;15:138–45. [DOI] [PubMed] [Google Scholar]
- [135].Shaybak E, Abdollahimohammad A, Rahnama M, Masinaeinezhad N, Azadi-Ahmadabadi C, Firouzkohi M. The effect of reiki energy healing on CABG postoperative chest pain caused by coughing and deep breathing. Indian J Public Health Res Develop 2017;8:305–10. [Google Scholar]
- [136].Simoni G, Bozzolan M, Bonnini S, Grassi A, Zucchini A, Mazzanti C, Oliva D, Caterino F, Gallo A, Da Roit M. Effectiveness of standard cervical physiotherapy plus diaphragm manual therapy on pain in patients with chronic neck pain: a randomized controlled trial. J Bodyw Mov Therap 2021;26:481–91. [DOI] [PubMed] [Google Scholar]
- [137].Skelly AC, Chou R, Dettori JR, Turner JA, Friedly JL, Rundell SD, Fu R, Brodt ED, Wasson N, Kantner S, Ferguson AJR. Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review Update. Rockville, MD: Agency for Healthcare Research and Quality (AHRQ), 2020. [PubMed] [Google Scholar]
- [138].Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- [139].Stochkendahl MJ, Kjaer P, Hartvigsen J, Kongsted A, Aaboe J, Andersen M, Andersen MØ, Fournier G, Højgaard B, Jensen MB, Jensen LD, Karbo T, Kirkeskov L, Melbye M, Morsel-Carlsen L, Nordsteen J, Palsson TS, Rasti Z, Silbye PF, Steiness MZ, Tarp S, Vaagholt M. National Clinical Guidelines for non-surgical treatment of patients with recent onset low back pain or lumbar radiculopathy. Eur Spine J 2018;27:60–75. [DOI] [PubMed] [Google Scholar]
- [140].Surenkok O, Aytar A, Baltaci G. Acute effects of scapular mobilization in shoulder dysfunction: a double-blind randomized placebo-controlled trial. J Sport Rehabil 2009;18:493–501. [DOI] [PubMed] [Google Scholar]
- [141].Teut M, Stöckigt B, Holmberg C, Besch F, Witt CM, Jeserich F. Perceived outcomes of spiritual healing and explanations - a qualitative study on the perspectives of German healers and their clients. BMC Complement Altern Med 2014;14:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Teys P, Bisset L, Vicenzino B. The initial effects of a Mulligan's mobilization with movement technique on range of movement and pressure pain threshold in pain-limited shoulders. Man Ther 2008;13:37–42. [DOI] [PubMed] [Google Scholar]
- [143].Tough EA, White AR, Richards SH, Lord B, Campbell JL. Developing and validating a sham acupuncture needle. Acupunct Med 2009;27:118–22. [DOI] [PubMed] [Google Scholar]
- [144].Tramontano M, Pagnotta S, Lunghi C, Manzo C, Manzo F, Consolo S, Manzo V. Assessment and management of somatic dysfunctions in patients with patellofemoral pain syndrome. J Am Osteopath Assoc 2020;120:165–73. [DOI] [PubMed] [Google Scholar]
- [145].Turner JA, Anderson ML, Balderson BH, Cook AJ, Sherman KJ, Cherkin DC. Mindfulness-based stress reduction and cognitive-behavioral therapy for chronic low back pain: similar effects on mindfulness, catastrophizing, self-efficacy, and acceptance in a randomized controlled trial. PAIN 2016;157:2434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Turner JA, Deyo RA, Loeser JD, Von Korff M, Fordyce WE. The importance of placebo effects in pain treatment and research. JAMA 1994;271:1609–14. [PubMed] [Google Scholar]
- [147].Vase L. Can insights from placebo and nocebo mechanism studies help improve randomized controlled trials? Clin Pharmacol Ther 2019;106:1169–71. [DOI] [PubMed] [Google Scholar]
- [148].Vase L, Amanzio M, Price DD. Nocebo vs. Placebo: the challenges of trial design in analgesia research. Clin Pharmacol Ther 2015;97:143–50. [DOI] [PubMed] [Google Scholar]
- [149].Vase L, Baram S, Takakura N, Takayama M, Yajima H, Kawase A, Schuster L, Kaptchuk TJ, Schou S, Jensen TS, Zachariae R, Svensson P. Can acupuncture treatment Be double-blinded? An evaluation of double-blind acupuncture treatment of postoperative pain. PLoS One 2015;10:e0119612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Vernon HT, Triano JJ, Ross JK, Tran SK, Soave DM, Dinulos MD. Validation of a novel sham cervical manipulation procedure. Spine J 2012;12:1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Villalta Santos L, Lisboa Cordoba L, Benite Palma Lopes J, Santos Oliveira C, Andre Collange Grecco L, Bovi Nunes Andrade AC, Pasin Neto H. Active visceral manipulation associated with conventional physiotherapy in people with chronic low back pain and visceral dysfunction: a preliminary, randomized, controlled, double-blind clinical trial. J Chiropr Med 2019;18:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Vitiello MV, McCurry SM, Shortreed SM, Balderson BH, Baker LD, Keefe FJ, Rybarczyk BD, Von Korff M.VCognitive-Behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the lifestyles randomized controlled trial. J Am Geriatr Soc 2013;61:947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Vitiello MV Rybarczyk B Von Korff M,VStepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med 2009;5:355–62. [PMC free article] [PubMed] [Google Scholar]
- [154].Vollert J, Cook NR, Kaptchuk TJ, Sehra ST, Tobias DK, Hall KT.VAssessment of placebo response in objective and subjective outcome measures in rheumatoid arthritis clinical trials. JAMA Netw Open 2020;3:e2013196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Walker BF, Hebert JJ, Stomski NJ, Losco B, French SD. Short-term usual chiropractic care for spinal pain: a randomized controlled trial. Spine 2013;38:2071–8. [DOI] [PubMed] [Google Scholar]
- [156].Wampold BE. How important are the common factors in psychotherapy? An update. World Psychiatry 2015;14:270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Wang C, Schmid CH, Hibberd PL, Kalish R, Roubenoff R, Rones R, McAlindon T. Tai Chi is effective in treating knee osteoarthritis: a randomized controlled trial. Arthritis Rheum 2009;61:1545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Wang C, Schmid CH, Rones R, Kalish R, Yinh J, Goldenberg DL, Lee Y, McAlindon T. A randomized trial of tai chi for fibromyalgia. N Engl J Med 2010;363:743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Wartolowska K, Beard D, Carr A. Blinding in trials of interventional procedures is possible and worthwhile. F1000Res 2017;6:1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Wartolowska K, Judge A, Hopewell S, Collins GS, Dean BJF, Rombach I, Brindley D, Savulescu J, Beard DJ, Carr AJ. Use of placebo controls in the evaluation of surgery: systematic review. BMJ 2014;348:g3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Wartolowska KA, Gerry S, Feakins BG, Collins GS, Cook J, Judge A, Carr AJ. A meta-analysis of temporal changes of response in the placebo arm of surgical randomized controlled trials: an update. Trials 2017;18:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Webster RK, Bishop F, Collins GS, Evers AWM, Hoffmann T, Knottnerus JA, Lamb SE, Macdonald H, Madigan C, Napadow V, Price A, Rees JL, Howick J. Measuring the success of blinding in placebo-controlled trials: should we be so quick to dismiss it? J Clin Epidemiol 2021;135:176–81. [DOI] [PubMed] [Google Scholar]
- [163].Webster RK, Howick J, Hoffmann T, Macdonald H, Collins GS, Rees JL, Napadow V, Madigan C, Price A, Lamb SE, Bishop FL, Bokelmann K, Papanikitas A, Roberts N, Evers AWM. Inadequate description of placebo and sham controls in a systematic review of recent trials. Eur J Clin Invest 2019;49:e13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].von Wernsdorff M, Loef M, Tuschen-Caffier B, Schmidt S. Author Correction: effects of open-label placebos in clinical trials: a systematic review and meta-analysis. Sci Rep 2021;11:17436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Williams ACdC, Fisher E, Hearn L, Eccleston C. Evidence-based psychological interventions for adults with chronic pain: precision, control, quality, and equipoise. PAIN 2021;162:2149–53. [DOI] [PubMed] [Google Scholar]
- [166].Williams ACdeC, Fisher E, Hearn L, Eccleston C. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2020;8:CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Wolny T, Linek P. Neurodynamic techniques versus “sham” therapy in the treatment of carpal tunnel syndrome: a randomized placebo-controlled trial. Arch Phys Med Rehabil 2018;99:843–54. [DOI] [PubMed] [Google Scholar]
- [168].Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, Gluud C, Martin RM, Wood AJG, Sterne JAC. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336:601–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Younger J, Gandhi V, Hubbard E, Mackey S. Development of the Stanford Expectations of Treatment Scale (SETS): a tool for measuring patient outcome expectancy in clinical trials. Clin Trials 2012;9:767–76. [DOI] [PubMed] [Google Scholar]
- [170].Zemadanis K, Betsos T, Mandalidis D. The short and long-term effect of weight-bearing mobilization-with-movement (MWM) and automobilization-MWM techniques on pain and functional status in patients with hip osteoarthritis. Int J Physiother 2017;4:160–7. [Google Scholar]
- [171].Zwarenstein M, Treweek S, Loudon K. PRECIS-2 helps researchers design more applicable RCTs while CONSORT Extension for Pragmatic Trials helps knowledge users decide whether to apply them. J Clin Epidemiol 2017;84:27–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B671, http://links.lww.com/PAIN/B672, http://links.lww.com/PAIN/B673, and http://links.lww.com/PAIN/B674.