Abstract
Cancer cells undergo metabolic reprogramming and switch to a ‘glycolysis-dominant’ metabolic profile to promote their survival and meet their requirements for energy and macromolecules. This phenomenon, also known as the ‘Warburg effect,’ provides a survival advantage to the cancer cells and make the tumor environment more pro-cancerous. Additionally, the increased glycolytic dependence also promotes chemo/radio resistance. A similar switch to a glycolytic metabolic profile is also shown by the immune cells in the tumor microenvironment, inducing a competition between the cancer cells and the tumor-infiltrating cells over nutrients. Several recent studies have shown that targeting the enhanced glycolysis in cancer cells is a promising strategy to make them more susceptible to treatment with other conventional treatment modalities, including chemotherapy, radiotherapy, hormonal therapy, immunotherapy, and photodynamic therapy. Although several targeting strategies have been developed and several of them are in different stages of pre-clinical and clinical evaluation, there is still a lack of effective strategies to specifically target cancer cell glycolysis to improve treatment efficacy. Herein, we have reviewed our current understanding of the role of metabolic reprogramming in cancer cells and how targeting this phenomenon could be a potential strategy to improve the efficacy of conventional cancer therapy.
Keywords: glycolysis, cancer metabolism, combination therapy
1. Introduction
Cancer cells reprogram their metabolism to promote growth, metastasis, and survival. They exhibit an increased glycolytic dependency and show an elevated glucose uptake and fermentation of glucose to lactate to meet the heightened anabolic needs for cancer cell proliferation [1]. Increased glycolysis is not only important for meeting the energy needs of the cells but is also crucial for the generation of metabolic intermediates necessary for macromolecule synthesis in cancer cells [2,3]. This phenomenon, often referred to as the ‘Warburg effect,’ is observed even in the presence of completely functional mitochondria [4]. The Warburg effect has been studied for over 90 years, and several studies have explored the mechanisms governing the increased glycolytic dependency of cancer cells. Several oncogenic proteins and tumor suppressors, including the hypoxia-inducible factor (HIF-1), Myc, p53, and PI3K/Akt/mTOR pathway, have been implicated in regulating this cancer cell-specific metabolic reprogramming.
The altered metabolism in the cancer cells provides an avenue for developing cancer cell-specific therapeutic targets and anti-cancer agents. Indeed, therapeutic strategies that target glycolysis and cancer cell-specific biosynthetic pathways are a major focus area in cancer research. Although the increased glycolytic dependency of neoplastic cells suggests the potential therapeutic efficacy of glycolytic inhibitors in cancer treatment, glycolytic inhibition alone is not effective in a clinical setting [5]. Targeting metabolism, especially in combination with chemotherapy, is expected to improve therapy responses and may help overcome drug resistance [6]. Elevated glycolysis in cancer cells and the resulting lactic acidosis modulate the tumor stroma to a pro-tumorigenic microenvironment. Targeting glycolytic changes in the tumor microenvironment has been shown to be a safe and effective strategy to enhance therapeutic efficacy [7,8]. In this review, we explore and discuss glycolytic modulation in cancer cells and how it could aid as a therapeutic strategy in combination therapies with chemotherapy and radiotherapy, immunotherapy, hormonal therapy, and photodynamic therapy (PDT).
2. Modulating Glycolysis to Improve Chemotherapy and Radiotherapy
Metabolic modulation has been shown to sensitize cancer cells toward chemotherapy and radiotherapy. Increased glycolysis, facilitated by an increased glucose uptake, is the major energy source in cancer cells apart from being the major source of macromolecules for cell proliferation and survival [9]. Recently it was demonstrated that glycolysis-addicted cancer cells show metabolic rewiring via mTORC1 activation [10,11]. Sustained mTORC1 activation bypasses glycolysis by directing the glucose flux toward the pentose phosphate pathway. Metabolic rewiring, including dysregulated glycolysis, elevated ATP production, and cell-death escape mechanisms, are the major culprits for therapeutic resistance in cancer cells. The intracellular ATP level in cancer cells is also associated with metastasis and stemness. Thus, targeting glycolysis or intracellular/extracellular ATP levels [12,13,14] is a promising strategy to sensitize cancer cells toward chemotherapy. Several studies have reported that glycolytic inhibitors improve the efficacy of cancer treatment and that glycolytic inhibition is a promising strategy when used as a combination therapy with other treatment modalities. In line with this, inhibiting glycolytic enzymes, hexokinase (HK) [15], pyruvate kinase (PK) [16], and lactate dehydrogenase (LDH) [17], have shown sensitizing effects with several chemotherapeutic agents [5].
2.1. Targeting Glucose Transporters and Glucose Uptake to Improve Chemotherapy
The inhibition of glucose transporters (GLUTs), a critical rate-limiting step in glucose metabolism, modulates the therapeutic efficacy of several drugs. GLUT expression is elevated in several types of cancers and is associated with a poor prognosis suggesting their key role in cancer cell metabolism [18,19,20,21,22]. Glycolysis inhibition using inhibitors of GLUT1, when combined with routine cancer therapy, has proven to be relevant in potentiating their effects in a synergistic manner in pre-clinical studies for several cancers [19,23,24,25,26]. GLUT1 inhibition curbs the self-renewing capacity and tumor-initiating potential of cancer stem cells and has a substantial significance from a therapeutic perspective [27].
A widely studied small molecule inhibitor of GLUT1, WZB117 synergistically inhibits breast cancer cells by inducing DNA damage when treated in combination with an allosteric AKT inhibitor [24,28]. WZB117 also sensitizes breast cancer cells toward treatment with adriamycin [29] and radioresistant breast cancer cells to radiotherapy [27,30]. Previous studies have reported the synergistic effects of GLUT1 inhibitor #43 in melanoma cells and have shown that GLUT1 inhibition induces apoptosis, intracellular reactive oxygen species (ROS) generation, and the loss of mitochondrial membrane potential. Combination therapy with GLUT1 inhibitor #43 enhances the DNA-damaging effects of cisplatin by regulating the AKT/mTOR pathways [24]. Another GLUT1 inhibitor, BAY-876, enhances the cisplatin-mediated inhibition of esophageal squamous cell carcinoma [23]. siRNA-mediated GLUT1 inhibition also showed similar results and improved the efficacy of low-dose cisplatin treatment [23]. In vivo studies in uterine cancer, patient-derived models have shown that glycolytic activation contributes to the stemness of uterine endometrial cancer, and BAY-876-mediated GLUT inhibition synergistically suppressed endometrial cancer cell proliferation when used in combination with paclitaxel [31].
Combination therapy with GLUT modulators can also improve the bioavailability of chemotherapeutic drugs. The co-treatment of paclitaxel with silybin (a GLUT modulator) significantly improved the oral bioavailability of the drug in several in vitro and in vivo studies and overcame the major drawback of the limited oral bioavailability of paclitaxel [32,33]. A nanomedicine-based combination therapy using GLUT1 inhibitor and chemotherapeutic agent, curcumin, deprived cancer cells of glucose and sensitized cancer cells to chemotherapy, induced apoptosis, improved anti-tumor effects, and alleviated side-effects in vitro and in vivo [34]. Thus, combination therapy with GLUT1 inhibitors might be a rational therapeutic strategy and could also allow for low-dose treatment with chemotherapy drugs providing a paradigm for high-efficacy, low-toxicity therapeutic options [35].
Another straightforward and interesting strategy to improve the effectiveness of chemotherapy is to deprive cancer cells of glucose [36]. Icard et al. proposed that the modulation of glucose intake in combination with chemotherapy could improve the efficacy of the drug via the deprivation of ATP to cancer cells. A recent study showed that intermittent fasting throughout chemotherapy was well tolerated in patients and reduced chemotherapy-induced toxicity as measured by hematologic, metabolic, and inflammatory parameters [37]. Contrastingly, an opposite effect was reported in a pre-clinical model of pancreatic ductal adenocarcinoma (PDAC), wherein a relative glucose abundance sensitized PDAC cells to chemotherapy. Hyperglycemic patients with stage IV PDAC showed an enhanced response to chemotherapy, possibly via impaired glutathione biosynthesis [38]. A case report on non-small cell lung cancer (NSCLC) with bone and brain metastasis reported the efficacy of glucose uptake inhibition in combination with chemotherapy as a palliative treatment strategy. Fasting-induced hypoglycemia or insulin-induced hypoglycemia combined with low-dose chemotherapy could benefit cancer patients, particularly those who do not tolerate the conventional dosage of drugs [39].
2.2. Targeting Glycolysis Enzymes to Improve Chemotherapy and Radiotherapy
The enhanced glycolysis in the cancer cells correlates with an upregulation and activation of critical glycolytic enzymes. Targeting the key glycolytic enzymes is a promising strategy to rewire the altered tumor metabolism, to sensitize (or re-sensitize, when resistance develops) cancers to chemotherapy.
HK catalyzes the first step in glucose metabolism and converts glucose to glucose-6-phosphate. Four HK isoforms, HK1-4, with different cellular distributions and glucose affinity have been identified. HK1 and HK2 are located on the outer mitochondrial membrane and are associated with AKT-mediated cell survival [40,41]. Further, HK2 is associated with the recurrence and poor prognosis of breast cancer (BC) [42]. HK2 expression is also elevated in lung cancer, and shows significant association with the tumor stage. The deletion of the Hk2 gene in lung cancer cells ameliorated glucose-derived ribonucleotides and glutamine-derived carbon utilization in anaplerosis [43]. Targeting HK2 inhibits cell proliferation and shifts the metabolic profile of cancer cells from glycolytic to oxidative phosphorylation (OXPHOS) [43,44].
2-Deoxy glucose (2-DG), an HK inhibitor, has been shown to sensitize cancer cells to chemotherapy and radiotherapy and is being investigated in clinical trials. 2-DG is a glucose analog that triggers the intracellular accumulation of 2-deoxy-d-glucose-6-phosphate (2-DG6P), inhibiting the function of HK and glucose-6-phosphate isomerase [45]. Glycolysis inhibition with 2-DG can improve the therapeutic efficacy of trastuzumab in treating HER2+ BC [46]. Similarly, the therapeutic efficacy of paclitaxel was enhanced when treated in combination with 2-DG in in vivo studies in NSCLC and osteosarcoma models [47]. A recent study showed that 2-DG could sensitize glioblastoma cells to chloroethyl nitrosourea by regulating glycolysis, intracellular ROS generation, and endoplasmic reticulum stress induction [48]. Another study reported that combining 2-DG with autophagy inhibiting drug hydroxychloroquine enhances apoptosis in BC cells. The inhibition of autophagy combined with 2-DG induced the accumulation of misfolded proteins in the endoplasmic reticulum and resulted in sustained endoplasmic reticulum stress, induced through the pERK-eIF2α-ATF4-CHOP axis to enhance apoptosis in BC cells [49]. Although a few clinical trials (NCT05314933, NCT00096707) are investigating the toxicity, tolerability, pharmacokinetics, and recommended dose of 2-DG in advanced tumors [50], additional studies and trials are required to characterize the mechanism of action and treatment benefits of 2-DG in cancer.
Curcumin, another compound with a proven anti-tumor effect, is also known to inhibit HK expression by inhibiting transcriptional repressor SLUG. Combination therapy of curcumin with docetaxel demonstrated a high response rate, low-toxicity, and improved patient tolerance in prostate cancer [51].
3-Bromopyruvate acid (3BrPA) is another classic glycolytic inhibitor, which inhibits several enzymes in the glycolytic pathway, including HK and LDH, and is a potent inhibitor of cancer cell growth [52,53,54,55]. Combining 3BrPA with rapamycin enhanced the anti-tumor efficacy through the dual inhibition of mTOR signaling and glycolysis in LC and neuroblastoma [56,57]. In BC cells, 3BrPA enhanced the expression of thioredoxin interacting protein (TXNIP) and inhibited HK2 expression via c-Myc downregulation [58], and enhanced tamoxifen-induced cytotoxicity in vitro [59]. The combination regimen with tamoxifen also enhanced oxidative stress and reduced glutathione levels in cells, and affected tumor angiogenesis and metastasis in animal models [59]. Further, the intra-cranial delivery of 3BrPA with temozolomide showed synergistic effects and increased survival in animal models of glioma [60]. Moreover, enhanced therapeutic efficacy was demonstrated when 3BrPA was combined with sorafenib in murine models of liver cancer [61]. 3BrPA can also enhance the anti-tumor effect of low-dose radiation via the reprogramming of mitochondrial metabolism and hindering of ATP generation [62]. 3BrPA also inhibits monocarboxylate transporter 1 (MCT1) expression, which mediates the bidirectional transport of lactate in cancer cells, and sensitizes cancer cells to ionizing radiation [63].
Elevated glycolysis in cancer cells results in the conversion of pyruvate to lactate, even under aerobic conditions. Lactate is excreted at high levels from tumor cells and acts as a metabolic fuel and oncometabolite with signaling properties. Lactate utilization by tumor cells depends on the expression of monocarboxylic transporters (MCTs), which are upregulated in cancer cells [64,65]. MCTs facilitate the shuttle of lactate from cancer cells to neighboring cells, tumor stroma, and tumor-associated endothelial cells and induce metabolic rewiring. Lactate is involved in several tumorigenic functions of cancer cells, including tumor microenvironment modulation and tumor angiogenesis [66]. Targeting MCTs has been shown to suppress tumor growth in cancer cells [67]. MCT1 and MCT4 inhibitors impair leukemia cell proliferation and enhance their sensitivity toward chemotherapy [68]. Currently, a phase 1 clinical trial is evaluating the toxicity and pharmacokinetic profile of AZD3965, an MCT inhibitor in cancer therapy for B-cell lymphoma [69,70].
LDH is a key glycolytic enzyme that is elevated in aggressive cancers and is essential for tumor maintenance [71,72,73]. LDH-A is regulated by numerous oncogenic transcription factors, including c-Myc and HIF-1, and is closely associated with malignant phenotypes of cancer cells [74]. LDH-A overexpression upregulated AKT phosphorylation and PI3K, which upregulated cyclin D1 and c-Myc expression in LC cells [75,76,77,78]. LDH overexpression is also associated with epithelial–mesenchymal transition (EMT)-related genes, SNAIL and SLUG, and is thus involved in regulating the metastatic progression of cancer cells [79]. LDH-A levels could thus serve as a biomarker for cancer diagnosis and prognosis [80,81,82]. LDH inhibition induces oxidative stress, impacting cancer stem cells’ renewable capacity. The overburden of mitochondrial complex II is speculated to account for the increased ROS production in LDH-A-inhibited cancer stem cells [83,84]. The widely studied LDH-A inhibitors include pyruvate analog, oxamate (OXM), and the NADH competitive inhibitor, gossypol. LDH-A inhibition with OXM triggers a specific tumor reduction in brain tumors by reducing ATP levels, increasing ROS production, and inducing apoptosis [85]. OXM also induces autophagy via the AKT-mTOR signaling pathway in certain cancer cell types [86]. Recent pre-clinical and clinical studies have supported the combined use of LDH inhibitors with concurrent treatments as a promising strategy in cancer therapy [85]. Combination therapy of OXM with other chemotherapeutic drugs, an including mTORC1 inhibitor and phenformin (phenethylbiguanidine; an anti-diabetic agent), have shown synergistic effects suggesting their implications in combination therapies [87,88]. A triple combination therapy of doxorubicin with metformin and OXM induced autophagy and apoptosis in colorectal cancers by downregulating hypoxia-induced HIF-1 expression [85].
Enolase (ENO-1), which converts 2-phosphoglycerate (2PG) to phosphoenol pyruvate (PEP), is emerging as a promising target for cancer therapy, partially owing to its diverse functions apart from being a major enzyme in glycolysis [89,90,91]. The overexpression of ENO-1 is associated with disease progression, metastasis-free survival, and overall survival in colorectal cancer, BC, gastric cancer, gliomas, head and neck cancer, and leukemia. ENO-1 promotes tumor cell progression via a plethora of mechanisms, including inducing angiogenesis, evading immune suppression and growth suppressors, and resisting cell death [92,93,94]. Small molecule inhibitors of ENO-1 have been shown to inhibit cancer cell growth [95,96,97]. POMHEX, a selective enolase inhibitor, has been shown to selectively inhibit the tumor cell progression of glioma cells in vivo by triggering apoptosis, showing a favorable safety profile and tolerance in non-human primates [98]. A previous study identified a potent inhibitor of ENO1, macrosphelide A, which demonstrates anti-cancer effects by simultaneously inactivating ENO1, aldolase, and fumarase [95].
6-phosphofructokinase/fructose-2,6-bisphophatase (PFKFBs) catalyzes the first irreversible step in glycolysis, which is the conversion of fructose-6-phosphate to fructose-1,6-bis-phosphate. As with most other glycolytic enzymes, PFKFB activity and expression are enhanced in many cancers. The selective inhibition of PFKFB3 displays broad anti-tumor activity in syngenic pre-clinical models and early human studies by inducing necroptotic cell death, apoptosis, cell cycle arrest, and inhibiting invasion [99,100]. A phase-1 dose escalation study for PFK-158, a first-in-human, first-in-class, small molecule inhibitor of PFKFB3, showed commendable tolerance and tumor burden reduction in pancreatic cancer, renal cell carcinoma, and adenocystic carcinoma patients [101,102,103]. The synergistic effect of PFK-158 with other FDA-approved targeted-chemotherapy agents can potentially improve their chemotherapy efficacy and is being validated. In gynecologic cancers, it was shown that PFK-158 improves lipophagy and sensitizes platinum therapy-resistant cells to carboplatin/oxaliplatin therapy [104]. Combining PFKFB3 inhibition with standard chemotherapy can thus be a novel strategy to improve the outcome in gynecologic and endometrial cancer patients who are resistant to therapy or have advanced, recurrent diseases [89].
Besides its glycolytic function, PFKFB3 is a crucial player in regulating endothelial cells, tumor angiogenesis, and tumor vascularization [105,106]. A transient inhibition of PFKFB3 in endothelial cells using 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) induced tumor vessel normalization, impaired metastasis, and improved chemotherapy [107,108]. The PFKFB3 inhibitor, AZ67, inhibited angiogenesis in vivo, independent of glycolysis regulation [109]. A combination therapy with PFKFB3 inhibitor and VEGF inhibitor, bevacizumab, improved tumor vasculature, alleviated tumor hypoxia, normalized lactate production, and improved the efficacy and delivery of doxorubicin in glioblastoma [110].
Pyruvate kinase M2 (PKM2), which catalyzes the conversion of PEP to pyruvate, is upregulated in numerous cancers and has emerged as a critical regulator of cancer cell metabolism [111,112]. Apart from being a key enzyme in glycolysis, nuclear PKM2 regulates the expression of GLUT1 and LDH-A through positive feedback to further support glycolytic metabolism [113]. PKM2 expression is upregulated under hypoxic conditions and induces tumor angiogenesis and metastasis [114]. The association of PKM2 expression with poor prognosis and overall survival indicates that PKM2 level could serve as a prognostic or diagnostic marker for cancers [115,116,117]. PKM2 inhibition induces apoptosis and tumor regression in xenograft models of different cancer types [89] and plays a role in maintaining redox homeostasis and glutathione turnover. PKM2 inhibition also increases the efficacy of docetaxel treatment in vitro and xenograft models of LC [118,119]. In NSCLC patients who received platinum therapy in a first-line setting, tumors with low PKM2 expression showed significantly longer progression-free survival and overall survival [120]. In tumor xenograft models of NSCLC, combination therapy with PKM2 siRNA and chemotherapeutic agents increased apoptosis and inhibited tumor growth [121].
Targeting Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is being explored as an alternative approach for inhibiting glycolysis [122]. GAPDH catalyzes the first step in which energy is derived from NADH in the ‘pay-off-phase’ of glycolysis. NADH, the first molecule generated in this phase, is critical for regulating intracellular ROS and redox balance. Targeting GAPDH triggers the accumulation of glucotrioses such as glyceraldehyde-3-phosphate and dihydroxy acetone phosphate in the cells, the partial degradation of which results in the formation of cytotoxic methylglyoxal [123]. Thus, inhibiting GAPDH not only depletes ATP but also triggers cytotoxicity through the upregulation of ROS and the accumulation of methylglyoxal [122]. 3-BrPA, discussed above, is a potent inhibitor of GAPDH and was shown to deplete intracellular ATP. Additionally, 3-BrPA showed high specificity and selectivity for GAPDH both in vitro and in vivo [122,124,125].
Although targeting glycolytic enzymes can improve the efficacy of chemotherapy and radiotherapy, the ubiquitous nature of glycolysis and glycolytic enzymes presents the challenge of the systemic toxicity of glycolysis inhibition. The selective targeting of cancer-specific enzymes or enzyme isoforms and the targeted delivery of therapeutic agents could circumvent this challenge.
2.3. Modulating Glycolysis to Overcome Drug Resistance
Aberrant glycolysis is a major contributor to drug resistance in cancer [126,127]. The mechanism underlying glucose metabolism reprogramming-induced drug resistance is not clearly understood. Increased glucose uptake induces gemcitabine resistance in pancreatic cancer, doxorubicin resistance in BC, and cisplatin resistance in genitourinary cancers [6,128]. It is thought that glucose metabolism reprogramming in cancer cells induces DNA repair and immune suppression in the tumor microenvironment, contributing to drug resistance. Anabolic alterations could account for the increased nucleotide demand required for the efficient repair of chemotherapy/radiation-induced DNA damage. The DNA repair pathways in reprogrammed cancer cells induces the activation of several pro-tumorigenic signaling pathways, including Wnt, PI3K/AKT, NF-κB, and MAPK, triggering prolonged cancer cell survival and apoptosis resistance [89,129]. Aberrant glycolysis can also promote DNA repair by increasing nucleotide turn over by enhancing the hexosamine biosynthetic pathway (HBP) and pentose phosphate pathway (PPP) [130,131]. By limiting pyruvate flux into OXPHOS, upregulated glycolysis also enables cancer cells to reduce the ROS accumulation in cells, another mechanism by which metabolic reprogramming contributes to resistance to therapy. Increasing evidence also suggests that the activation of Wnt, PI3K/AKT, and Notch pathways activate autophagy which also contributes to cancer cell survival and resistance to therapy, whereas inhibiting autophagy sensitizes cancer cells to therapy [89,132,133]. Autophagy, thus, downregulates cell metabolism leading to cancer cell quiescence and survival, inducing radio-resistance [134].
Metabolic changes in the tumor cells happen hand-in-hand with similar reprogramming of the tumor microenvironment. This metabolic reprogramming induces immunosuppression and immune escape of cancer cells and contributes to the development of resistance to chemotherapy and radiotherapy [135,136]. The upregulation of glycolytic enzyme HK2 suppresses the mTOR-S6K signaling pathway and blocks chemotherapy-induced apoptosis by binding to voltage-dependent anion channels, and suppresses the formation of mitochondrial permeability transition pores, contributing to chemoresistance. Aberrant glycolytic pathways in cancer stem cells also play critical roles in contributing to resistance to therapy via enhancing cancer cell stemness by activating the PI3K/AKT pathway and upregulating the stem cell-like properties [89]. Enhanced exosomal secretion from cancer stem cells also activates neighboring cancer cells toward stemness and promotes chemo/radio-resistance [137,138]. The overexpression of ENO-1 in cancer cells can also contribute to cisplatin resistance in different cancer types [139] and is considered a biomarker to predict prognosis and drug resistance in cancers [85,140].
Glycolytic inhibitors are reported to sensitize cancer cells to chemotherapy and radiotherapy, thereby overcoming resistance to therapy. 3BrPA aids in dissociating HK2 from the mitochondrial complex and improves therapy response to daunorubicin [15]. A combination therapy of curcumin and docetaxel has demonstrated improved drug response and tolerance in a clinical study in prostate cancer patients [141]. 2-DG, a glycolytic inhibitor that modulates several glycolytic enzymes, restores sensitivity to adriamycin in ER+ BC cells. In HER2+ BC, trastuzumab inhibits tumor growth by downregulating heat shock factor 1 (HSF1) and LDH-A, thereby inhibiting glycolysis. A combination therapy of trastuzumab with LDH-A siRNA-mediated glycolysis inhibition synergistically inhibited tumor growth in trastuzumab-resistant breast cancer cells, suggesting their usefulness in overcoming drug resistance. Further, the combination therapy of trastuzumab with glycolytic inhibitor 2-DG and oxamate increased the sensitivity of ErbB2-positive cancer cells to therapy. An allosteric inhibitor of phosphoglycerate mutase (PGAM), a glycolytic enzyme that converts 3-phosphoglycerate to 2-phosphoglycerate, has been shown to overcome erlotinib resistance in NSCLC. PGAM inhibition alters the ERK and AKT signaling pathways and induces oxidative stress and ROS production to overcome erlotinib resistance [142].
ENO-1 overexpression has been associated with chemoresistance in prostate and pancreatic cancer cells [102,143,144,145]. Cisplatin-resistant gastric cancer cells also exhibit enhanced glycolysis by upregulating ENO-1. ENO1 inhibition in cisplatin-resistant cells increased sensitivity to the therapy by activating apoptotic pathways or inducing autophagy [96]. In ovarian cancer cells, inhibiting ENO-1 expression increased cell senescence and improved cisplatin resistance [146]. Hypoxia-induced resistance to gemcitabine is a critical issue in PDAC treatment. A recent study demonstrated that the shRNA-based downregulation of ENO-1 modulated redox homeostasis, increased intracellular ROS concentration, and sensitized resistant PDAC cells to gemcitabine treatment. In ovarian cancer models, PFKFB3 inhibitors, 3-PO and PFK-158, impaired metabolic reprogramming-induced stemness and chemoresistance, possibly by modulating apoptosis via the NF-κB pathway [147,148].
PKM2 can contribute to chemoresistance against cisplatin and gemcitabine treatment in different cancer types. PKM2 overexpression has been reported to be a biomarker for cancer resistance. PKM2 regulates the DNA repair mechanism in addition to glucose metabolism and induces resistance to genotoxic damage, driving treatment resistance [149,150]. Targeting PKM2 sensitizes cancer cells to treatment. In NSCLC, shRNA-based silencing of PKM2 enhanced radiation-induced autophagy in vitro and in vivo [149] and increased the sensitivity to docetaxel treatment [119]. PKM2 expression also correlated with a resistance to platinum-based therapy in colorectal cancer [151].
A few studies, however, have reported contradicting results, where PKM2 activation was shown to act as a chemosensitizer in some cancer types. In a study by Anastasiou et al., an increase in intracellular ROS concentration in response to therapy was shown to inhibit PKM2, which in turn diverted the glucose flux into PPP generating redox potential for the detoxification of ROS. These regulatory properties of PKM2 confer an additional advantage to cancer cells to tolerate therapy-induced oxidative stress. The endogenous expression of oxidation-resistant-PKM2 mutants increased oxidative stress and impaired tumor progression [152]. PKM2 activation could thus be an attractive strategy in cancer therapy. High levels of PKM2 activate the mTOR-HIF1α pathway and are associated with a positive chemotherapy response in cervical cancer patients treated with cisplatin-neoadjuvant chemotherapy [153,154]. The high expression of PKM2 has been shown to enhance drug response to epirubicin and 5-fluorouracil in BC [155], whereas a decrease in PKM2 levels/activity contributes to cisplatin/oxaliplatin resistance in cervical cancer, colorectal cancer, and gastric cancer cells [153,156,157].
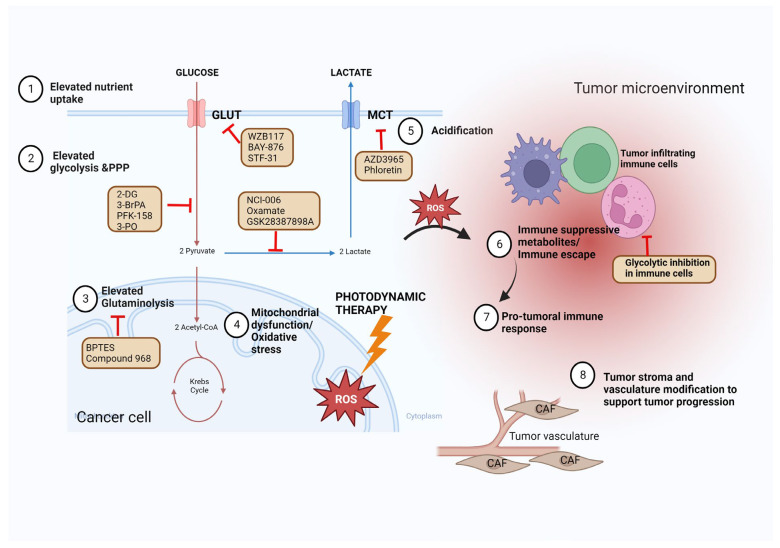
The development of resistance is frequently encountered in cancer treatment, and the link between cancer cell metabolism and the development of resistance is becoming more apparent. Targeting metabolic enzymes is an efficient strategy to re-sensitize the resistant cells to chemotherapy and radiotherapy. However, the clinical application of glycolysis inhibition to overcome drug resistance has remained limited. Future studies should identify the key metabolic shifts that contribute to the development of drug resistance and explore their potential as drug targets to improve the sensitivity of cancers to chemotherapeutic agents and radiotherapy and to overcome the development of resistance. Figure 1 summarizes how targeting glycolysis can be used to modulate cancer therapy (Figure 1).
Figure 1.
Targeting glycolysis to improve cancer therapy. The cancer cells show enhanced dependency on glycolysis that may be targeted to improve the treatment efficacy of conventional cancer therapy modalities, including chemotherapy, radiotherapy, immunotherapy, hormonal therapy, and photodynamic therapy. Glycolysis metabolism can be potentially targeted by limiting glucose uptake (targeting glucose transporters), targeting glycolysis enzymes, targeting glutaminolysis, targeting lactate synthesis, targeting MCT, or targeting mitochondrial complexes. The increased glycolysis in the cancer cells increase the release of lactic acid to the tumor microenvironment, acidifying it and making it pro-cancer and immunosuppressive. The tumor-infiltrating immune cells also show a similar shift toward glucose metabolism increasing the competition for glucose in the tumor microenvironment. Modulating glycolysis in the immune cells can potentially improve immune therapy. This figure was created using the Biorender app.
3. Targeting Glycolysis to Enhance Immunotherapy
The advances in our understanding of the remarkable potential of the immune system to fight cancer have garnered tremendous attention on immunotherapy for cancer. Arguably, immunotherapy is now being considered one of the most promising therapeutic strategies for several types of cancers, and immune checkpoint blockade (ICB)- and adoptive-cell therapy (ACT)-based therapeutic strategies have been approved for several cancers [158,159,160]. However, several reports have shown that a high percentage of patients initially fail to respond to these interventions or acquire resistance in the long run [161,162], limiting the application of these promising strategies. Several studies have reported that the metabolic reprogramming of the cancer cell that leads to the development of an immunosuppressive tumor microenvironment is one of the main contributors to the reduced efficacy of immunotherapy [163]. Further, it has been suggested that metabolic interventions can significantly enhance the efficacy of immunotherapy [164,165]. Therefore, understanding the challenges the immune cells face in the harsh immunosuppressive tumor microenvironment and identifying strategic interventions to overcome these challenges would contribute to improving immunotherapy.
3.1. Glucose Metabolism in the Immune Cells of the Tumor Microenvironment
It is known that cancer cells undergo a metabolic reprogramming called the ‘Warburg effect’ or aerobic glycolysis in response to hypoxia and oncogenic signals, such as Myc and PI3K [165]. This preference for glycolysis is also shared by other rapidly proliferating cells in the TME, including effector T-cells and M1-like macrophages, to satisfy their increased energy requirements [166]. In contrast, other cells in the TME, including memory T-cells, regulatory T-cells (Tregs), and M2-like macrophages, rely on fatty acid oxidation (FAO) to satisfy their energy needs.
The naïve T-cells utilize TCA-coupled OXPHOS as their primary energy source [167]. On MHC activation, the T-cell receptor (TCR) and CD28 activate the PI3K-AKT-mTORC1 and Myc signaling pathways and induce metabolic reprogramming [167]. The effector T-cells upregulate aerobic glycolysis and enhance their anabolic metabolism for cancer-killing and clone expansion. In addition, the glycolytic intermediates support effector T-cell activation and cytokine generation. Phosphoenolpyruvate (PEP), a glycolytic metabolite, blocks sarco/endoplasmic reticulum Ca2+-ATPase (SERCA)-mediated endoplasmic reticulum calcium uptake and the nuclear factor of activated T-cells (NFAT) signaling, enabling TCR signaling [168]. In addition, phosphoenolpyruvate carboxykinase 1 (PCK1) catalyzes the conversion of oxaloacetate (OAA) into PEP, and the overexpression of PCK1 enhances the cancer-killing functions of adoptive transferred CD4+ and CD8+T-cells [168]. However, although CD8+ T-cells experiencing continuous stimulation or hypoxia differentiated into functional effectors in vitro, it rapidly drove T-cell dysfunction and exhaustion [169].
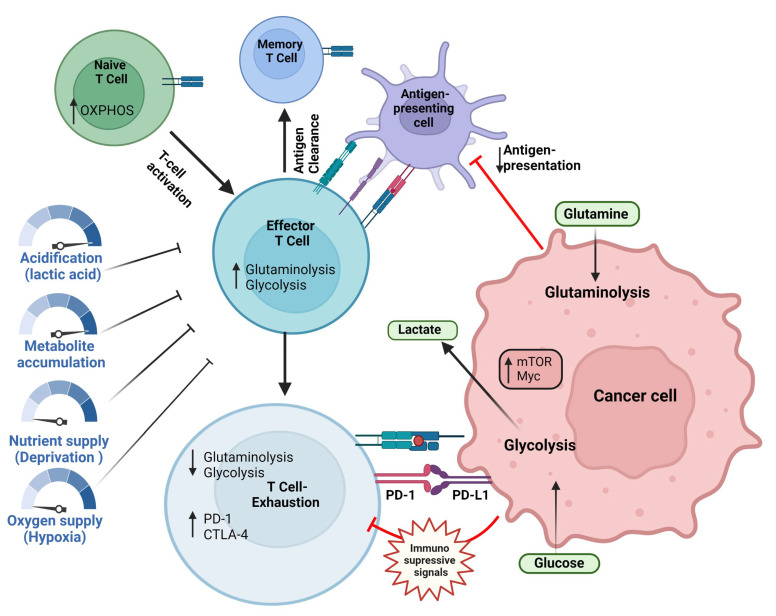
Similar to the CD8+ T-cells, the functions of the CD4+ T-cells are also affected by specific metabolic reprogramming. Increased glycolysis promotes IL-2, TNFα, and IFN secretion in the CD4+ T-cells, and the inhibition of glycolysis drives the functional and metabolic exhaustion of the CD4+ T-cells [170,171]. In line with this, the inflammatory CD4+ T-cells (Th1 and Th17) show enhanced glycolysis. The Th17 cells exclusively express the pyruvate dehydrogenase (PDH) inhibitor, pyruvate dehydrogenase kinase isozyme 1 (PDHK1), which, when downregulated, leads to the selective reduction of Th17 cells [172]. On the other hand, Tregs show upregulated OXPHOS and FAO [172], and their expression of FOXP3 inhibits Myc and attenuates PI3K-AKT-mTORC1 axis-mediated activation of glycolysis and increases oxidation and catabolic metabolism, rendering a survival advantage in the TME [64,173]. Furthermore, the Tregs utilize lactate metabolism, and culturing them in high-glucose conditions decreases their stability [167]. The mechanisms by which the low levels of oxygen, high levels of lactate, and the high competition for glucose potentially contribute to T-cell dysfunction in the tumor microenvironment is been summarized in Figure 2.
Figure 2.
Naïve T-cells relay on oxidative metabolism. After activation, the effector T-cells increase glycolysis to support their function. On antigen clearance, the effector T-cells enter a memory state. On antigen persistence, as with long-term tumor elimination, inhibitory receptors such as PD-1 and CTLA4 reprogram the T-cell metabolism leading to metabolic impairments. Exhausted T-cells show reduced glycolysis and glutaminolysis and dependence of fatty acid oxidation. The low levels of oxygen, high levels of lactate, and the high competition for glucose potentially contribute to T-cell dysfunction in the tumor microenvironment. The image was created using Biorender app.
The M1-like macrophages preferentially utilize glycolysis to sustain the inflammatory phenotype [174], while the M2-like macrophages depend on TCA and FAO to maintain an immunosuppressive phenotype [175]. The highly acidic environment of melanoma was found to induce tumor-associated macrophages (TAM) toward a cancer-promoting phenotype [176]. The tumor-associated neutrophils (TAN), on the other hand, exhibit pro- or anti-tumor effects in different cancer microenvironments. In pancreatic ductal adenocarcinoma (PDAC), it was shown that the TANs undergo an LDH-A-mediated glycolytic switch and exhibit a tumor-promoting phenotype [177]. Triple-negative BC (TNBC) cells with an accelerated glycolysis support myeloid-derived suppressor cell (MDSC) development and facilitate CD8+T-cell inhibition and cancer progression [178]. In addition, an increase in lactate generation enhances the tumor-promoting capacity of MDSCs [179].
The TME is characterized by a decrease in nutrients, insufficient vasculature, increased lactate accumulation, and hypoxia, conditions that affect the cancer-killing capacity of the T-cell. The cancer cells and the immune cells compete for glucose utilization, and the activated glycolysis of the cancer cells endows them with an advantage over the immune cells, impairing the function and survival of the effector T-cells. It has been shown that the expression of glycolysis-related genes, such as ALDOA, ALDOC, ENO2, GAPDH, GPI, and PFKM, negatively correlates with T-cell infiltration in melanoma and NSCLC [180]. The lower availability of glucose in the TME affects the glycolytic capacity of the T-cells [168], inducing T-cell exhaustion [181]. Furthermore, the lack of glucose also affects mitochondrial functions, promoting terminal CD8+ T-cell exhaustion [182]. In addition to the competition for glucose, the increased production of lactate due to the increased rate of glycolysis contributes to the immunosuppressive character of the TME. Lactate metabolism has been shown to contribute to oncogenesis [183], and LDH has been reported to be a marker for poor prognosis and immunotherapy efficacy [184,185]. The low pH in the TME triggers reduced CD25 and TCR expression in the effector CD8+ T-cells and inactivated STAT5 and ERK signaling, affecting anti-tumor immunity [186]. Lactate also affects TCR signaling [187], and exposure to large amounts of lactate makes the Tregs switch to OXPHOS for regenerating NAD+; however, the Tregs fail to maintain the NAD+/NADH balance through this pathway [64]. Moreover, lactate in the TME promotes the expression of pro-inflammatory cytokines, including IL-23 and IL-17, supporting tumorigenesis and impairing anti-tumor immunity [188]. The poor vasculature and the increased metabolism of the cancer cells contribute to the formation of a hypoxic environment, further affecting anti-tumor immunity. Hypoxia triggers epigenetic reprogramming of effector T-cells, reducing their functional capabilities [189,190]. Although hypoxia-inducible factor-1α (HIF-1α), expressed in response to hypoxia, can induce Tregs and bind to the promoter region of the FOXP3 to promote transcription [191] (22988108), it contributes to the development of an immunosuppressive TME by enhancing cancer-promoting immune cell functions [192,193].
3.2. Signaling Mechanisms Regulating Glycolysis
The signaling pathways that modulate glycolysis in the immune cells can be potentially targeted to improve their anti-tumor functions, and drugs such as metformin and phenformin have been extensively tested in the clinical setting [194,195,196]. The liver kinase B1 (LKB1)-AMPK pathway and the PI3K-AKT-mTOR axis are the two primary signaling mechanisms that modulate glycolysis in the cell. The LKB1-dependent kinases regulate metabolic pathways by targeting several effectors, including AMPK [197]. LKB1 loss upregulates GLUT1 and hexokinase 2 (HK2), increasing T-cell glycolytic transcription and flux [198].
On the other hand, the deregulation of the PI3K-AKT-mTOR axis promotes HIF-1 activation and GLUT1 expression [199,200]. PI3K activation increases AKT phosphorylation and glycolytic flux via LDH-A in T-cells [201]. AKT is the primary glycolysis regulator in both cancer and immune cells. AKT activation induces GLUT1 and LDH-A expression [202], activates HK1 by promoting HK2 and PFK2 phosphorylation [203], and inhibits PDH by activating pyruvate dehydrogenase kinase-1 (PDK1) [204], resulting in activated glycolysis. mTOR regulates the expression of HIF-1α, the major transcription factor regulating several glycolytic enzymes and GLUT1 [199]. mTOR kinases determine effector and memory CD8+ T-cell fates, and blocking mTOR promotes T-cell effector functions [205,206].
3.3. Targeting Glycolysis to Improve Immunotherapy
Adoptive-cell transfer (ACT) and immune checkpoint blockade (ICB) are the two primary strategies for immunotherapy. The interplay between anti-tumor immunity and cancer metabolism suggests that combining immunotherapy with glycolysis-targeted therapy is a promising strategy to improve treatment efficacy. ACT utilizes therapeutic-modified immune cells to directly boost anti-tumor immunity. Ex vivo-expanded T-cells and T-cells engineered to express antigen-specific TCRs or chimeric antigen receptors (CARs) are primarily used for ACT [207]. ACT has shown promising efficacies, particularly with CD19-specific CAR-T cells, in the treatment of B-cell acute lymphoblastic leukemia and B-cell lymphomas [208]. However, responses in other cancers have been poor. It has been suggested that optimizing T-cell metabolism to support robust initial and durable T-cell responses for target cells and the TME would improve and broaden their applicability.
CAR-T cells can be engineered to express specific signaling domains, and endowing them with specific properties can tailor them for effector activity and long-lasting memory. The two FDA-approved CARs carry a CD19-targeting extracellular domain coupled with an intracellular signaling domain from CD3ζ and the co-stimulatory molecules CD28 or 4-1BB [209]. Linking TCR signaling with the co-stimulatory signals enables the CARs to elicit effector functions in the absence of additional inflammatory or co-stimulatory stimuli [210]. CAR-T cells carrying the CD28 domain have increased glycolysis and show enhanced effector responses but are short-lived. On the other hand, CAR-T cells expressing the 4-1BB domain show elevated OXPHOS and FAO and display a memory phenotype [211]. This is in line with the normal physiological functions of the co-stimulatory molecules. CD28 stimulation activates PI3K/AKT/mTOR, promoting glycolysis and effector differentiation, whereas 4-1BB activates AMP-driven FAO and OXPHOS [211]. Based on this understanding, it has been proposed that introducing signaling mutations could improve CAR-T cell effector functions and survival. For example, it was shown that mutations of the YMNM signaling motif of CD28 increase CAR-T cell survival and reduce T-cell exhaustion, enabling enhanced tumor control [212]. Furthermore, additional co-stimulatory molecules modulating T-cell metabolism may be incorporated into CAR constructs to improve T-cell function. For example, ICOS, a CD28 family member, promotes glycolysis and mTORC1 activity in T follicular helper cells [213]; GITR agonists enhance cellular metabolism to support CD8+ T-cell proliferation and effector cytokine production [214]; OX40 is associated with enrichment of glycolysis and lipid metabolism transcripts; and OX40 agonists enhanced lipid uptake in Tregs [215]. Similar to the CAR-T cells, T-cells procured from tumors and expanded ex vivo, or modified to express engineered TCRs, can also be optimized through metabolic manipulations, and T-cells with low glycolysis rates can be generated or selected for longevity while retaining effector functions [216].
The in vitro stimulation and the T-cell engineering phases of the ACT strategy provides the added advantage of the opportunity to modify T-cell metabolism and mitochondria without affecting other cells and tissues and thus prevent any potential boosting of the cancer cell metabolism from the intervention. It was also shown that blocking glutamine metabolism increases T-cell function [217]. Furthermore, supplementing the culture medium with glutamine antagonist 6-Diazo-5-oxo-l-norleucine (DON) enhanced CAR-T cell FAO and reduced glycolysis, making the CAR-T cells remain in a more undifferentiated state [218]. Inhibiting glycolysis using the HK-2 inhibitor, 2-DG, before ACT induced a memory-like phenotype in the T-cells, enabling a more efficient control of tumors and prolonged survival in an animal model [219]. Similarly, CD19-CAR-T cells treated with AKT inhibitors showed reduced glycolysis and a memory-like phenotype and had robust tumor elimination potential [220]. Conversely, modulating mitochondrial OXPHOS and FAO would enable CAR-T cell longevity and the continued expression of a memory-like phenotype. Furthermore, a transient glucose restriction followed by glucose re-exposure could enhance the tumor-clearing efficacy of CD8+ T-cells [221].
Unlike ACT, which directs anti-tumor immunity through pharmaceutical interventions, ICB aims to modify inhibitory signals to activate endogenous anti-tumor-specific T-cells. Furthermore, ICB is primarily targeted against solid tumors, which show a greater influence of the TME in modulating T-cell metabolism. In addition to the initial challenge of T-cells infiltrating the tumors, ICB must overcome several obstacles, including tumor-infiltrating lymphocyte (TIL) exhaustion, the upregulation of inhibitory receptors and epigenetic modifications, metabolic adaptations resulting in nutrient deficits, impaired translocation of GLUT1 to the cell surface, the downregulation of glycolytic enzymes, GAPDH and ENO-1, and dysregulated and fragmented mitochondria with increased ROS generation [222,223,224,225]. It was reported that the TIL inflammatory function could be enhanced by rescuing TIL metabolism by expressing PCK to promote gluconeogenesis and replacing the intracellular glycolytic intermediates or by improving mitochondrial metabolism by treating with pyruvate or acetate [225]. The immunosuppressive environment of the TME and chronic antigen stress direct the T-cells to an exhausted state, characterized by the expression of immune checkpoints and decreased cytotoxicity. The checkpoint molecules, including CD28, CD40L, and cytotoxic T lymphocyte-associated protein-4 (CTLA-4), along with TCR signaling, drive TIL exhaustion and contribute to the persistence of the exhausted state. The concurrent metabolic adaptations further enhance the immunosuppressive effects of the checkpoints. Thus, targeting glycolytic regulators and metabolites that support the immune checkpoint-directed T-cell inhibition is a potential strategy to improve the efficacy of ICB.
The most well-known and commonly targeted immune checkpoints in cancer are the programmed cell death protein-1 (PD-1) and CTLA-4. CTLA-4 is expressed primarily on T-cells and plays an immunosuppressive role during the initial phase of T-cell activation and downregulates T-cell activation-triggered glycolysis. PD-1 is activated after TCR activation and impedes glucose uptake and glycolysis while promoting FAO. Thus, these checkpoint signals prevent T-cell activation and inflammation. It was shown that PD-1–deficient T-cells maintain higher metabolic activity in chronic infection [226,227]. Thus, blocking PD-1 and CTLA-4 relieves PI3K/AKT/mTORC1 signaling and allows increased T-cell stimulation and metabolism, inducing an effector-like phenotype. In addition, this metabolic shift induces epigenetic reprogramming inducing effector functions and longevity. Although PD-1 and CTLA-4 are the most extensively targeted ICB candidates, several other co-inhibitory and co-stimulatory molecules modulate T-cell metabolism. Notably, T-cell immunoglobulin mucin receptor 3 (TIM-3) downregulates glycolysis and GLUT1 expression [228], while LAG3 downregulates OXPHOS [229]. Similarly, inhibiting 4-1BB and OX40 enhances T-cell OXPHOS and promotes effector function and longevity [230]. Taken together, immune checkpoint molecules induce metabolic dysfunction and thus affect anti-tumor immunity, suggesting that combining ICB with metabolic regulators is a potential strategy to improve treatment efficacy.
3.4. Glycolysis-Targeting Therapies to Improve Immunotherapy Efficacy
mTOR is an oncogenic molecule that contributes to the regulation of metabolism in TILs. The inhibition of the mTOR signaling axis downregulates the malignant phenotype of cancer cells. Owing to their inhibitory effects, several rapamycin analogs have been approved for treating cancers [231,232]. However, it was shown that mTOR inhibition could diminish anti-tumor immunity [194] as these inhibitors directly affect the lineage differentiation-determining glycolytic activity in T-cells. It was shown that rapamycin suppresses Th17 differentiation and promotes Treg differentiation under TGFβ induction [233,234]. Further, the activation of AKT-mTORC1 signaling was associated with T-cell function restoration and the reduced expression of PD-1 and TIM-3 [235]. Additionally, the over-activation of mTORC1 affects the immunosuppressive function of Tregs, while low mTORC1 levels enhance Treg activity [236]. These suggest that an optimized inhibition of the PI3K-mTORC1 signaling axis is critical for improving the efficacy of immunotherapies.
Metformin has shown promising effects in different cancers [195] and has been shown to regulate metabolism by interacting with AMPK, the PI3K-AKT-mTOR axis, and HIF-1α [237,238]. Additionally, metformin promotes the cancer-killing capacity of CD8+ T-cells by modulating glycolysis [239,240,241] and downregulates immune checkpoint expression and glycolytic flux through HIF-1α inhibition [242,243]. Furthermore, it was shown that metformin stops the cancer cells from using the lactate and ketone bodies produced by cancer-associated fibroblasts as nutrients and thus suppresses cancer progression [244]. A recent study combined 2-DG, an HK inhibitor [245], BAY-876, a GLUT-1 inhibitor, and chloroquine and developed the nano-drug, D/B/CQ@ZIF-8@CS, which inhibited glycolysis and improved anti-CTLA-4 immunotherapy by reducing Treg metabolic fitness [246]. Tregs pretreated with 2-DG showed enhanced inhibition of T-cell proliferation in ovarian cancer [247]. Furthermore, HK upregulates PD-L1 expression in cancer cells, and combining the HK inhibitor, Lonidamine, with anti-PD-1 therapy improved cancer cell elimination in a mouse model [248].
Knocking out glucose-6-phosphate isomerase (GPI), the enzyme that catalyzes the conversion of glucose-6-phosphate (G6P) to fructose-6-phosphate (F6P), upregulated OXPHOS and sustained the survival of cancer cells [245]. Additionally, GPI inhibition selectively eliminated inflammatory encephalitogenic and colitogenic Th17 cells without affecting the homeostatic microbiota-specific Th17 cells [249]. However, it remains unknown whether GPI-targeted therapies would improve the efficacy of immunotherapy.
The GAPDH inhibitor, dimethyl fumarate (DMF), promotes the oxidative PPP and inhibits glycolysis and OXPHOS in cancer cells. This reduces the competition between cancer cells and T-cells for glucose consumption and promotes the efficacy of ICB and IL-2 therapy [250]. Low-dose osimertinib was shown to inhibit GAPDH and tumor endothelial glycolysis and promote vascularization and immune cell infiltration and thus improve the efficacy of anti-PD-1 therapy [251]. Inhibiting fructose-2,6-bisphosphatase 3 (PFKFB3), which is upregulated in several cancers, repressed glycolysis and upregulated PD-L1 expression [193]. On the contrary, glucose deficiency upregulated PD-L1 through the EGFR/ERK/c-Jun pathway, leading to the upregulation of PFKFB3, and promoted glycolysis [252,253]. This suggests the existence of a positive feedback loop between metabolism and checkpoint molecules [254]. A dual-target drug comprising paclitaxel and the PFKFB3 inhibitor, PFK15, blocked cancer-associated fibroblast-mediated cancer cell growth and reduced the lactate concentration in the TME [193]. PFK15 was also shown to upregulate PD-1 and LAG-3 expression in the context of type 1 diabetes [171]. Pyruvate kinase isoform M2 (PKM2), the final rate-limiting enzyme in glycolysis, promotes PD-L1 expression in macrophages, DCs, and tumor cells and contributes toward accelerated tumor progression [255]. High PKM2 expression was associated with a poor prognosis of pancreatic ductal adenocarcinoma, and the knocking down of PKM2 improved the efficacy of anti-PD-1/PD-L1 therapy [256].
ENO-1, which catalyzes the conversion of 2-phosphoglycerate to PEP and also acts as a plasminogen receptor and a DNA-binding protein, was shown to be overexpressed in several cancers. [64]. A pan-cancer analysis showed that ENO-1 expression correlated with immune cell infiltration, including B cells, CD8+ and CD4+ T-cells, macrophages, neutrophils, and dendritic cells [257]. The presence of autoantibodies against ENO-1 correlated with a better prognosis in PDA, suggesting that ENO-1 was a good molecular candidate for improving immune cell response to cancers [258]. Antibodies against ENO-1 were detected in approximately 60% of patients with PDAC, and ENO-1-specific T-cell responses are observed in patients who have the anti-ENO-1 antibodies. In line with this, an ENO-1 DNA vaccine induced an antibody and cellular response and increased the median survival in mouse models of PDA [259]. ENO-1-targeting DNA vaccines have shown prophylactic and therapeutic potential in PDAC mouse models by inducing complement-dependent cytotoxicity and immune cell response [144,259,260]. In a spontaneous mouse model of PDAC, co-treatment with gemcitabine and ENO-1 DNA vaccine enhanced CD4 anti-tumor activity and impaired tumor progression [261,262]. Additionally, a recent study showed that targeting ENO-1 using specific antibodies targets multiple TME niches involved in prostate cancer (PC) progression and bone metastasis via a plasmin-related mechanism [263]. These show the potential of ENO-1 targeted therapies in improving the efficacy of immunotherapies.
Optimizing T-cell metabolism is a promising strategy for improving cancer immunotherapy. Metabolic modification can potentially increase stemness and long-term memory, enhance effector functions, and reduce T-cell exhaustion. Future studies should identify key metabolic transitions and regulatory steps that are differently regulated in cancer cells and immune cells and develop effective targeting strategies to enhance the synergistic effects of metabolic modulation and cancer immunotherapy [264].
4. Targeting Glycolysis to Enhance Hormonal Therapy
Hormonal therapy has shown remarkable advancement as a therapeutic strategy for cancers dependent on hormones, especially in breast, prostate, and other gynecological cancers. Aromatase inhibitors (AI), estrogen receptor (ER) antagonists, ER modulators, anti-estrogens, and GnRH agonists are effective therapeutic drugs and have shown high success rates in patients with hormone-sensitive recurring or metastatic gynecologic malignancies [265]. Hormone therapy interferes with the hormone-dependency of cancer cells by limiting hormone production in the body [266]. While hormonal therapy has improved survival and reduced recurrence in different cancer types [266], de novo or acquired resistance to hormonal therapy is a major clinical problem that requires the development of innovative strategies [264]. Resistance to hormonal therapy invariably occurs in most patients with ER+ metastatic BC and castration-resistance PC (CRPC) [267]. Metabolic reprogramming is an inherent feature of endocrine-resistant cancer cells, implicating that combination therapy with metabolic regulators and conventional hormonal therapy might be beneficial in overcoming resistance [268]. However, it is unclear whether metabolic rewiring is a cause or consequence of endocrine resistance, and several studies are investigating the cross-talk between hormone signaling and cancer cell metabolism [269]. Somatic mutation in estrogen receptors is related to the clinical development of the resistance to hormone therapies [268,270,271,272]. The Y537S mutation in ER-α enhanced mitochondrial metabolism and glycolysis in BC cells. The Y537S mutation is also associated with poor clinical outcomes, suggesting that enhanced glucose metabolism is a highly conserved mechanism of endocrine resistance [268].
Elevated glucose levels resembling hyperglycemia in BC cells have been attributed to a reduced response to tamoxifen therapy and could act as a marker for responses to hormonal therapy [273,274]. An increased glycolytic rate is a characteristic feature of tamoxifen-resistant cells, and inhibiting glycolysis is expected to restore tamoxifen sensitivity [273,275]. Elevated glycolysis in BC cells is also associated with mitochondrial malfunction and upregulated AKT/mTOR and HIF-1α signaling pathways. Tamoxifen-resistant BC cells escape cell death by increasing autophagy through the inactivation of TOR-S6K via the HK2 pathway [276]. Glycolytic inhibition by the knockdown of HK2 or 3BrPA treatment downregulated AKT/mTOR signaling and could be a therapeutic strategy to overcome tamoxifen resistance in BC [277]. A recent study investigated the potency of a combination therapy employing low-dose tamoxifen (ERα antagonist) and metabolism inhibitors, 2-DG and CB-839 (glutaminolysis inhibitor), in improving the anti-proliferation effect in tamoxifen-resistant ERα-positive BC cells. The triple combination showed superior cell growth inhibition by inducing apoptosis and c-Myc downregulation; however, a combination of tamoxifen with 2-DG did not show significantly strong inhibition of cell viability [278,279]. The pharmacological inhibition of glycolysis with PFK-158, a PFKFB3 inhibitor, with tamoxifen or fulvestrant has been explored as a potential therapeutic intervention to overcome endocrine resistance. PFKFB3 upregulation, with an elevated basal expression of PFKFB3 mRNA, is observed in endocrine-therapy-resistant BC cells and is associated with adverse recurrence-free survival in BC patients. The anti-tumor effect of PFK-158 is exacerbated when combined with tamoxifen and fulvestrant treatment [280]. PFKFB3 inhibition activated necroptotic markers receptor-interacting kinase 1 (RIPK1) and mixed lineage kinase domain-like pseudokinase (MLKL), implicating the possible mechanism of PFK-158-induced cell death [281]. In a long-term estrogen deprivation model (LTED) of AI resistance, cancer cells were demonstrated to have increased glycolysis dependency. The inhibition of glycolysis with HK2 inhibitors, along with AI, and letrozole, reduced cell viability [282]. Dietary interventions that target metabolic rewiring have also been shown to improve the efficacy of endocrine therapy in liver metastatic BC patients. Metastatic burden in the liver increases with increasing carbohydrate percentage in the diet. A fasting-mimicking diet increased the efficacy of fulvestrant treatment and reduced the metastatic burden in BC liver metastatic models, providing a proof-of-concept for a more straightforward strategy to circumvent drug resistance, with potential applicability in other cancer types as well [283].
Metabolic reprogramming is emerging as a crucial mechanism contributing to resistance to endocrine therapy in PC [284]. The expression of key glycolytic enzymes, including LDH-A, MCT-4, and GLUT1, is elevated in mCRPC patients [285]. Glycolysis inhibition by targeting GLUT1 plays an important role in drug response in prostate PC [286]. In PC, elevated androgen levels increase glucose uptake and upregulate the expression of GLUTs, implying a cross-talk between androgen signaling and glycolytic pathway, a mechanism that protects PC cells from glucose-deprivation-induced oxidative stress [287,288]. NF-κB-mediated GLUT1 overexpression and upregulated glucose metabolism are associated with enzalutamide resistance in PC [289,290]. In xenograft models of CRPC and enzalutamide-resistant PC patients, GLUT1 inhibition significantly reduced tumor volume and displayed synergistic effects with androgen receptor (AR)-targeted therapy [286]. Glycolytic inhibitors, gossypol (LDH-A inhibitor), and AZD3965 (MCT-1 inhibitor) are currently in clinical trials as potential glycolysis-targeting agents in mCRPC. Progesterone treatment was reported to have an anti-tumor effect in glioblastoma multiform (GBM) in vitro and in vivo and improved the efficacy of temozolomide [291]. Recently it was shown that high-dose progesterone treatment inhibits GBM growth by inhibiting the key modulators of glycolytic metabolism. This early observation highlighted the potential of progesterone in metabolic reprogramming; however, more direct evidence is essential to validate this, and future studies should determine the synergistic effect of direct glycolytic inhibitors and progesterone in GBM treatment [291].
Endocrine resistance remains a major clinical barrier that requires the development of novel strategies to circumvent the resistance. Several mechanisms that contribute to endocrine resistance have been identified. Metabolic rewiring is frequently observed in most cancer cells that exhibit resistance, and targeting glucose metabolism with well-established glycolytic inhibitors has shown to enhance the sensitivity to endocrine therapy in breast and PC models. The mutual interplay between glucose metabolism and androgen receptor/ER signaling implies that combination approaches of endocrine therapy with metabolic modulators could be a standard-of-care to overcome resistance. Dietary interventions that modulate glucose metabolism have also been demonstrated to be an interesting strategy for evading resistance to therapy. Well-designed clinical trials are urgently needed to elucidate the clinical utility of the strategies mentioned above and to develop metabolic drugs as routine standard-of-care in endocrine-resistant cancer patients in clinical settings.
5. Targeting Glycolysis to Improve Photodynamic Therapy
Photodynamic therapy (PDT) is a relatively new, minimally invasive therapeutic procedure that relies on the selective accumulation of a photosensitive compound in the cancer cells, which, on excitation with light of an appropriate wavelength, would generate ROS, predominantly singlet oxygen, within the cancer cells, and eventually kill the cancer cell, with minimal damage to the surrounding tissue [292,293,294]. Although PDT is widely used to treat several cancers, its efficacy is limited by several factors, including the effective irradiation of deep tissue. Therefore, several studies have attempted to improve the efficacy of PDT by combining it with other chemotherapeutic agents. It has been shown that glycolytic inhibitors disrupt cancer cell metabolism, elevate the cellular ROS level, and disrupt the mitochondria, resulting in cell death [295]. Therefore, when combined with PDT, glycolytic inhibitors could, in theory, enhance the levels of cellular ROS and thus trigger increased cancer cell death.
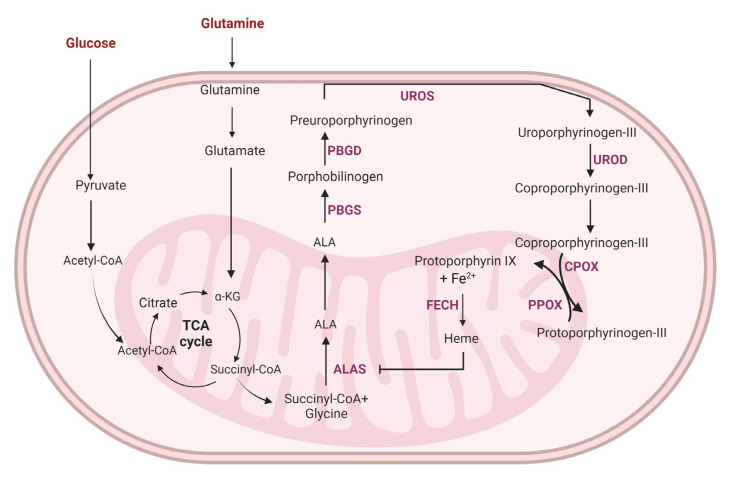
5-aminolevulinic acid (5-ALA) is one of the most commonly used photosensitizers for photodynamic therapy. 5-ALA is a naturally occurring non-proteinogenic δ-amino acid synthesized in the mitochondria by the condensation of glycine and succinyl-CoA by mitochondrial 5-ALA synthase (ALAS). This is the first committed step toward heme biosynthesis. The final precursor of heme is Protoporphyrin IX (PpIX), which is a highly potent photosensitizer. The exogenous supplementation of 5-ALA overrides the normal feedback inhibition of ALAS and results in the accumulation of PpIX selectively in cancer cells, owing to the differences in the heme biosynthesis pathway enzyme activities between the cancer cells and normal cells. This cancer-specific accumulation of PpIX is exploited for selectively purging cancer cells by PDT and for the visualization of tumor tissue by photodynamic diagnosis (PD). 5-ALA was approved by the U.S FDA in 2017 as an adjunct for the visualization of malignant tissue in grade III and IV glioma (NDA 208630/SN0014) and is currently used in the clinic to guide the resection of malignant glioma and glioblastoma. In addition to its role as a precursor of PpIX, 5-ALA has been reported to enhance aerobic bioenergetics [296], promote mitochondrial protein expression, and stimulate heme-oxygenase-1, triggering heme degradation [297]. Figure 3 describes the heme biosynthetic pathway, and how it is correlated with glycolysis.
Figure 3.
The heme biosynthesis is connected with glucose and glutamine metabolism. Enhanced glycolysis and glutaminolysis in the cancer cell might contribute to the elevated rate of heme synthesis and the upregulation of several heme biosynthesis pathway enzymes in the cancers. Addition of exogenous 5-ALA bypasses the feedback inhibition of ALAS and increase the accumulation of protoporphyrin IX (PpIX) in the cancer cells. The elevated accumulation of PpIX is exploited for fluorescence-guided detection (photodynamic diagnosis) of cancers. Further, irradiating PpIX-accumulating cancer cells with light generated singlet oxygen and ROS that kills the cancer cell, with minimal damage to the surrounding tissue (photodynamic therapy; PDT). The interdependency of glucose metabolism and the heme synthesis pathways suggest that targeting glycolysis could enable the modulation of PpIX accumulation in the cancer cells. The image was created using Biorender app.
A recent study showed that 5-ALA is a potent competitive inhibitor of LDH with efficacies comparable to oxamate (OXM) and tartronate (TART) [298]. They showed that treatment with 5-ALA induced glycolysis inhibition and triggered cell death in glioblastoma cell lines. Further, it was shown that up to 25% of the 5-ALA was used for glycolysis inhibition in these cells, leaving a lower amount of 5-ALA for conversion to PpIX and subsequent use as a photosensitizer for PDT and PD. Treating the glioblastoma cells with an LDH inhibitor before 5-ALA treatment enhanced the efficacy of PDT by 15%. Precise delineation of the tumor–normal tissue is of critical importance, especially in brain cancer surgeries, and a 15% increase in PD efficacy is a significant improvement.
Another study showed that exogenous 5-ALA suppressed oxidative metabolism and glycolysis and reduced cell proliferation in ovarian and BC cells [299]. Further, 5-ALA also destabilized Bach1 and inhibited cancer cell migration. The study also showed that 5-ALA-induced suppression of oxidative metabolism and glycolysis was mediated through different mechanisms in BC and ovarian cancer but involved Bach1 destabilization, AMPK activation, and the induction of oxidative stress. Additionally, an inverse relationship between oxidative metabolism and ALA sensitivity was revealed.
It was also shown that the administration of 5-ALA for 6 weeks reduced the plasma glucose levels in rats without affecting their plasm insulin levels and induced HO-1 expression in the liver and white adipose tissue [297]. An increase in HO-1 indicates an increase in heme in the liver, which promotes the formation of nuclear receptor subfamily 1 (Rev-Erbα) with its corepressor nuclear receptor co-repressor 1 (NCOR), which in turn downregulates the enzymes involved in gluconeogenesis such as PEPCK and G6Pase, resulting in reduced glucose production in the liver [300]. On the other hand, 5-ALA administration enhances glucose metabolism in the adipocytes by decreasing the total amount of adipose tissue or decreasing the number of mitochondria in the adipose tissue [301]. It has been shown that the induction of peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α), a master transcription coactivator, increases heme synthesis by upregulating ALAS [302]. Glucose intake repressed PGC-1α mediated ALAS in this study, suggesting that nutrient stress could trigger increased heme synthesis. Nutrient stress is a characteristic feature of the tumor microenvironment, and earlier studies have shown that BC cells (MCF7) grown under glucose deprivation produced higher levels of PpIX than those cultured under standard conditions [66,303]. Furthermore, co-treatment with glycolytic inhibitors and 5-ALA reduced intracellular PpIX levels [304]. This has been attributed to the inactivation of ABC transporters induced by ATP depletion, which in turn decreased the flux of precursors into the cell [303,305]. Interestingly, other studies have reported an increase in cellular PpIX accumulation with the inhibition of ABC transporters [306,307]. On the other hand, a combined treatment with 5-ALA-PDT and dichloroacetate, an inhibitor of pyruvate dehydrogenase, showed improved efficacy in BC (MCF 7) cells [308].
In another study, an 18 h glucose deprivation prior to PDT reduced intracellular glutathione levels and increased the cytotoxicity of PDT [309]. A similar increase in PDT efficacy was observed when inhibitors of glutathione synthesis (buthionine-sulfoximine) or its regeneration (1,3-bis-(2-chlorethyl)-1-nitrosourea) were used for co-treatment with PDT [310]. Changes in the availability of glycolytic substrates affect NADPH availability in the cells. NADPH is a critical agent involved in the anti-oxidative defense mechanisms of the cell. As mentioned, PDT relies on increased ROS in the cancer cells to kill them. PDT-based ROS generation under conditions of impaired glucose and glutathione metabolism results in much higher intracellular ROS levels, contributing to increased efficacy. In line with this, ROS scavengers were shown to protect the cells from ALA-PDT-induced damage [311].
A recent study showed that the metabolic reprogramming toward aerobic glycolysis in cancer cells contributes to the development of resistance to 5-ALA-PDT. Further, they showed that treatment with metformin reduced aerobic glycolysis and increased OXPHOS in squamous cell carcinoma cells, and improved the cytotoxic effect of PDT by increasing PpIX production, ROS generation, and AMPK expression, and inhibiting the AKT/mTOR pathway [312]. In another study, combining glycolysis inhibitor 2-DG with 5-ALA induced an enhanced accumulation of PpIX in HepG2 liver cancer cells [313], contributing to improved treatment efficacy. Further, treatment with 2-DG and 3-bromopyruvate (3-BP) synergistically improved the efficacy of PDT in BC cells [314].
Oncogenic transformation has been reported to upregulate glycolytic enzymes and contribute to the increased exogenous ALA-treatment-induced PpIX accumulation in cells [315]. The increased PpIX accumulation in cancer cells is often attributed to their lower ferrochelatase (FECH) levels. FECH is the terminal enzyme in the heme synthesis pathway that catalyzes the chelation of PpIX with the ferrous ion to generate heme. Lower FECH levels have shown a significant association with the cancer grade, the TNM stage, unfavorable prognosis, and impaired immune cell infiltration in clear cell renal cell carcinoma [316]. However, oncogenic Ras/MEK has been shown to increase the conversion of PpIX to heme by increasing HIF-1α expression and thereby promoting FECH activity [317]. Inhibiting MEK decreased HIF-1α expression and FECH activity and increased 5-ALA-induced PpIX accumulation in cancers [317,318]. Oncogenic Ras/MEK signaling-induced HIF-1α expression has also been implicated in increasing glycolytic flux and driving cancer progression [319,320]. Although 5-ALA-induced PpIX accumulation in cancer cells is a well-documented and well-studied concept, our knowledge of the underlying mechanisms remains largely vague. The inter-dependencies between oncogenic transformation, glycolytic flux, and heme metabolism should be further studied to identify the optimal targeting strategy to enhance 5-ALA-induced PpIX accumulation in cancer cells and to improve the efficacies of PDT and PD.
6. Conclusions
The metabolic signatures of tumor cells are different from normal cells, which allows the tumor cells to adapt to the increased energy and metabolite demands [321,322]. Several signaling mechanisms that regulate or hijack the canonical reactions in glucose metabolism have been identified; however, there is no universal mechanism underlying the reprogramming of glucose metabolism in all cancers. Elevated glucose metabolism, hypoxia-induced GLUT, LDH-A, and PFKFB3 overexpression, and the AKT- and c-Myc-mediated transcriptional activation of HK2 are observed in most cancer cells, and these metabolic changes may be exploited for developing effective therapeutic approaches. The tumor microenvironmental regulation and immune suppressive effects of metabolic rewiring are also crucial players in cancer cell progression, survival, and resistance. Apart from glycolytic modulation, mitochondrial dysfunction, elevated ROS production, and dysregulated TCA, cycle enzymes also participate in oncogenic signaling and tumor progression, which were not discussed in this review, and are elegantly reviewed elsewhere [323,324].
Elevated glucose metabolism and nutrient uptake have been exploited for tumor diagnosis in the clinic, and F-18 fluoro-2-deoxyglucose PET is widely used in the clinical staging of cancers [325,326]. However, glycolysis inhibition has not been exploited to its full potential in cancer therapy and it has not translated into the clinic. The inhibition of glycolysis may have undesirable consequences as normal cells also use the same glycolytic enzymes, and it is hence necessary to identify the enzymes or enzyme isoforms that are specifically upregulated or preferentially used by cancer cells. Additionally, several of the glycolysis inhibitors that have been developed for clinical use have been shown to have off-target effects, killing non-cancerous cells [327]. In addition, though the inhibition of glycolysis might inhibit cancer cell proliferation, cancer cells may adapt by upregulating OXPHOS or glutaminolysis, which could result in the development of resistance to therapy, in addition to co-morbidities such as cachexia in patients. This rewiring of metabolic pathways also poses challenges to precision therapies. In fact, previous studies have highlighted the dispensability of the Warburg effect in cancer cell growth and have shown that a complete disruption of glycolysis would require the deletion of both LDHA and LDHB genes. The study evaluated a potent LDH A/B dual inhibitor GNE-140 and demonstrated that the “glycolytic Warburg” phenotype of tumor cells depends on both LDH A and LDH B expression, and is a dispensable phenotype that can be replaced by OXPHOS [328]. This emphasizes the importance of evaluating the simultaneous inhibition of glycolysis and OXPHOS as a therapeutic strategy. In cell lines that are innately resistant to GNE-140 as they predominantly use OXPHOS, the inhibition of OXPHOS sensitized the cells to GNE-140 treatment [329]. Understanding the pathways that contribute to resistance in glycolysis targeting drugs is hence crucial [245,329,330,331].
Despite early indications, glycolytic inhibitors such as 2-DG have failed in the clinical setting due to their limited effects as a monotherapy agent. In addition, it might be necessary to combine glycolytic inhibitors with other agents that target alternate pathways that are activated in response to glycolytic inhibition. Studies have shown promising effects when metformin (that inhibits mitochondrial complex 1) was used along with glycolytic inhibitors. The simultaneous targeting of MCT1 inhibitor AZD3965 and the mitochondrial respiratory complex 1 inhibitor IACS01759 is a more clinically relevant strategy compared with targeting only one pathway in B-cell lymphomas [332]. Using glycolytic inhibitors as adjuvant therapy with already approved conventional therapies, including chemotherapy, radiotherapy, immunotherapy, and photodynamic therapy, is an attractive strategy, and several early studies have shown promising results. Dietary interventions and lifestyle changes also affect the metabolic landscape of cancer cells, and studies should address this issue in detail to emphasize their clinical implications.
Though metabolic rewiring unquestionably affects cancer cell proliferation, the translation of metabolic reprogramming to the clinic must overcome several hurdles [333]. In-depth analytical and extensive pre-clinical studies should identify targetable metabolic enzymes/enzyme isoforms that are efficacious in different tumor types with minimal toxicity to normal cells. Another major challenge in the clinical development of cancer therapeutics is the need to identify patient groups that would benefit from the therapy. Dependency on metabolic pathways is not universal for all cancer types, which makes it all the more important to identify appropriate patient groups to avoid unwanted adverse effects and toxicity. Technical hurdles in measuring metabolism in vivo also add more complexity. It is essential to integrate genetic and metabolic biomarkers and tissue information to optimize the criteria for patient selection. An interesting study published recently showed the possibility of using glycolytic enzymes as a surrogate for glycolytic activity in cancer cells, using liquid biopsy. Cells expressing high levels of HK2 were identified in both cytokeratin (CK)-positive and CK-negative CTC populations isolated from lung adenocarcinoma patients [334]. The possibility of monitoring the glycolytic metabolic rewiring in real-time using liquid biopsy samples and downstream single-cell level molecular, genomic, and metabolic studies is likely to create a paradigm shift in the clinical utility of glycolytic modulations in cancer therapy. Well-designed clinical trials that unravel the metabolic dependencies of different cancer types and their association with genetic and histopathological information might answer several questions on the complex and sophisticated nature of cancer metabolism. Metabolomic studies, with powerful technological backing, would take the lead in the early identification of cancer risk factors and aid in improving cancer therapy and cancer screening, diagnosis, and therapy monitoring in the coming years.
Author Contributions
Conceptualization, C.C. and V.S.C. resources, C.C. and V.S.C.; writing—original draft preparation, C.C., and V.S.C.; writing—review and editing, C.C., V.S.C., and K.S.; supervision, Y.S.; project administration, K.S.; funding acquisition, Y.S. and K.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 202200070001, No. 202100400001).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeBerardinis R.J., Lum J.J., Hatzivassiliou G., Thompson C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen P.L. Tumor mitochondria and the bioenergetics of cancer cells. Prog. Exp. Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- 4.Liberti M.V., Locasale J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemberg K.M., Gori S.S., Tsukamoto T., Rais R., Slusher B.S. Clinical development of metabolic inhibitors for oncology. J. Clin. Investig. 2022;132:e148550. doi: 10.1172/JCI148550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng J., Cui Y., Xu S., Wu X., Huang Y., Zhou W., Wang S., Fu Z., Xie H. Altered glycolysis results in drug-resistant in clinical tumor therapy. Oncol. Lett. 2021;21:369. doi: 10.3892/ol.2021.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand A., Singer K., Koehl G.E., Kolitzus M., Schoenhammer G., Thiel A., Matos C., Bruss C., Klobuch S., Peter K., et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016;24:657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Sonveaux P., Vegran F., Schroeder T., Wergin M.C., Verrax J., Rabbani Z.N., De Saedeleer C.J., Kennedy K.M., Diepart C., Jordan B.F., et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatenby R.A., Gillies R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 10.Pusapati R.V., Daemen A., Wilson C., Sandoval W., Gao M., Haley B., Baudy A.R., Hatzivassiliou G., Evangelista M., Settleman J. mTORC1-Dependent Metabolic Reprogramming Underlies Escape from Glycolysis Addiction in Cancer Cells. Cancer Cell. 2016;29:548–562. doi: 10.1016/j.ccell.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Fu V., Moroishi T., Guan K.L. Glycoholics Anonymous: Cancer Sobers Up with mTORC1. Cancer Cell. 2016;29:432–434. doi: 10.1016/j.ccell.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Fiorillo M., Ozsvari B., Sotgia F., Lisanti M.P. High ATP Production Fuels Cancer Drug Resistance and Metastasis: Implications for Mitochondrial ATP Depletion Therapy. Front. Oncol. 2021;11:740720. doi: 10.3389/fonc.2021.740720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang T., Ma F., Qian H.L. Defueling the cancer: ATP synthase as an emerging target in cancer therapy. Mol. Ther. Oncolytics. 2021;23:82–95. doi: 10.1016/j.omto.2021.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vultaggio-Poma V., Sarti A.C., Di Virgilio F. Extracellular ATP: A Feasible Target for Cancer Therapy. Cells. 2020;9:2496. doi: 10.3390/cells9112496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rai Y., Yadav P., Kumari N., Kalra N., Bhatt A.N. Hexokinase II inhibition by 3-bromopyruvate sensitizes myeloid leukemic cells K-562 to anti-leukemic drug, daunorubicin. Biosci. Rep. 2019;39:BSR20190880. doi: 10.1042/BSR20190880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almouhanna F., Blagojevic B., Can S., Ghanem A., Wolfl S. Pharmacological activation of pyruvate kinase M2 reprograms glycolysis leading to TXNIP depletion and AMPK activation in breast cancer cells. Cancer Metab. 2021;9:5. doi: 10.1186/s40170-021-00239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou M., Zhao Y., Ding Y., Liu H., Liu Z., Fodstad O., Riker A.I., Kamarajugadda S., Lu J., Owen L.B., et al. Warburg effect in chemosensitivity: Targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol. Cancer. 2010;9:33. doi: 10.1186/1476-4598-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carvalho K.C., Cunha I.W., Rocha R.M., Ayala F.R., Cajaiba M.M., Begnami M.D., Vilela R.S., Paiva G.R., Andrade R.G., Soares F.A. GLUT1 expression in malignant tumors and its use as an immunodiagnostic marker. Clinics. 2011;66:965–972. doi: 10.1590/S1807-59322011000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu M., Yongzhi H., Chen S., Luo X., Lin Y., Zhou Y., Jin H., Hou B., Deng Y., Tu L., et al. The prognostic value of GLUT1 in cancers: A systematic review and meta-analysis. Oncotarget. 2017;8:43356–43367. doi: 10.18632/oncotarget.17445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xintaropoulou C., Ward C., Wise A., Queckborner S., Turnbull A., Michie C.O., Williams A.R.W., Rye T., Gourley C., Langdon S.P. Expression of glycolytic enzymes in ovarian cancers and evaluation of the glycolytic pathway as a strategy for ovarian cancer treatment. BMC Cancer. 2018;18:636. doi: 10.1186/s12885-018-4521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin E., Koo J.S. Glucose Metabolism and Glucose Transporters in Breast Cancer. Front. Cell Dev. Biol. 2021;9:728759. doi: 10.3389/fcell.2021.728759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng Y., Xu X., Luan H., Li L., Dai W., Li Z., Bian J. The progress and development of GLUT1 inhibitors targeting cancer energy metabolism. Future Med. Chem. 2019;11:2333–2352. doi: 10.4155/fmc-2019-0052. [DOI] [PubMed] [Google Scholar]
- 23.Sawayama H., Ogata Y., Ishimoto T., Mima K., Hiyoshi Y., Iwatsuki M., Baba Y., Miyamoto Y., Yoshida N., Baba H. Glucose transporter 1 regulates the proliferation and cisplatin sensitivity of esophageal cancer. Cancer Sci. 2019;110:1705–1714. doi: 10.1111/cas.13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weng H.C., Sung C.J., Hsu J.L., Leu W.J., Guh J.H., Kung F.L., Hsu L.C. The Combination of a Novel GLUT1 Inhibitor and Cisplatin Synergistically Inhibits Breast Cancer Cell Growth By Enhancing the DNA Damaging Effect and Modulating the Akt/mTOR and MAPK Signaling Pathways. Front. Pharmacol. 2022;13:879748. doi: 10.3389/fphar.2022.879748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pliszka M., Szablewski L. Glucose Transporters as a Target for Anticancer Therapy. Cancers. 2021;13:4184. doi: 10.3390/cancers13164184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Q., Ba-Alawi W., Deblois G., Cruickshank J., Duan S., Lima-Fernandes E., Haight J., Tonekaboni S.A.M., Fortier A.M., Kuasne H., et al. GLUT1 inhibition blocks growth of RB1-positive triple negative breast cancer. Nat. Commun. 2020;11:4205. doi: 10.1038/s41467-020-18020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibuya K., Okada M., Suzuki S., Seino M., Seino S., Takeda H., Kitanaka C. Targeting the facilitative glucose transporter GLUT1 inhibits the self-renewal and tumor-initiating capacity of cancer stem cells. Oncotarget. 2015;6:651–661. doi: 10.18632/oncotarget.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y.L., Weng H.C., Hsu J.L., Lin S.W., Guh J.H., Hsu L.C. The Combination of MK-2206 and WZB117 Exerts a Synergistic Cytotoxic Effect Against Breast Cancer Cells. Front. Pharmacol. 2019;10:1311. doi: 10.3389/fphar.2019.01311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Q., Meng Y.Q., Xu X.F., Gu J. Blockade of GLUT1 by WZB117 resensitizes breast cancer cells to adriamycin. Anticancer Drugs. 2017;28:880–887. doi: 10.1097/CAD.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 30.Zhao F., Ming J., Zhou Y., Fan L. Inhibition of Glut1 by WZB117 sensitizes radioresistant breast cancer cells to irradiation. Cancer Chemother. Pharmacol. 2016;77:963–972. doi: 10.1007/s00280-016-3007-9. [DOI] [PubMed] [Google Scholar]
- 31.Mori Y., Yamawaki K., Ishiguro T., Yoshihara K., Ueda H., Sato A., Ohata H., Yoshida Y., Minamino T., Okamoto K., et al. ALDH-Dependent Glycolytic Activation Mediates Stemness and Paclitaxel Resistance in Patient-Derived Spheroid Models of Uterine Endometrial Cancer. Stem Cell Rep. 2019;13:730–746. doi: 10.1016/j.stemcr.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee C.K., Choi J.S. Effects of silibinin, inhibitor of CYP3A4 and P-glycoprotein in vitro, on the pharmacokinetics of paclitaxel after oral and intravenous administration in rats. Pharmacology. 2010;85:350–356. doi: 10.1159/000312690. [DOI] [PubMed] [Google Scholar]
- 33.Pashaei-Asl F., Pashaei-Asl R., Khodadadi K., Akbarzadeh A., Ebrahimie E., Pashaiasl M. Enhancement of anticancer activity by silibinin and paclitaxel combination on the ovarian cancer. Artif. Cells Nanomed. Biotechnol. 2018;46:1483–1487. doi: 10.1080/21691401.2017.1374281. [DOI] [PubMed] [Google Scholar]
- 34.Li X., Jiang C., Wang Q., Yang S., Cao Y., Hao J.N., Niu D., Chen Y., Han B., Jia X., et al. A “Valve-Closing” Starvation Strategy for Amplification of Tumor-Specific Chemotherapy. Adv. Sci. 2022;9:e2104671. doi: 10.1002/advs.202104671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tilekar K., Upadhyay N., Iancu C.V., Pokrovsky V., Choe J.Y., Ramaa C.S. Power of two: Combination of therapeutic approaches involving glucose transporter (GLUT) inhibitors to combat cancer. Biochim. Biophys. Acta Rev. Cancer. 2020;1874:188457. doi: 10.1016/j.bbcan.2020.188457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Icard P., Teboul B., El Baze P. A Simple Method to Optimize the Effectiveness of Chemotherapy: Modulation of Glucose Intake During Chemotherapy. Anticancer Res. 2017;37:6199–6202. doi: 10.21873/anticanres.12069. [DOI] [PubMed] [Google Scholar]
- 37.Omar E.M., Omran G.A., Mustafa M.F., El-Khodary N.M. Intermittent fasting during adjuvant chemotherapy may promote differential stress resistance in breast cancer patients. J. Egypt. Natl. Cancer Inst. 2022;34:38. doi: 10.1186/s43046-022-00141-4. [DOI] [PubMed] [Google Scholar]
- 38.Vaziri-Gohar A., Hue J.J., Graor H.G., Abbas A., Zarei M., Hajihassani O., Titomihelakis G., Feczko J., Rathore M., Wang R., et al. Increased glucose availability sensitizes pancreatic cancer to chemotherapy. bioRxiv. 2022 doi: 10.1101/2022.04.29.490090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y., Zhang Y., Mao X., Qi Q., Zhu M., Zhang C., Pan X., Ling Y. Palliative treatment efficacy of glucose inhibition combined with chemotherapy for non-small cell lung cancer with widespread bone and brain metastases: A case report. Biomed. Rep. 2017;7:553–557. doi: 10.3892/br.2017.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gottlob K., Majewski N., Kennedy S., Kandel E., Robey R.B., Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahn K.J., Hwang H.S., Park J.H., Bang S.H., Kang W.J., Yun M., Lee J.D. Evaluation of the role of hexokinase type II in cellular proliferation and apoptosis using human hepatocellular carcinoma cell lines. J. Nucl. Med. 2009;50:1525–1532. doi: 10.2967/jnumed.108.060780. [DOI] [PubMed] [Google Scholar]
- 42.Sato-Tadano A., Suzuki T., Amari M., Takagi K., Miki Y., Tamaki K., Watanabe M., Ishida T., Sasano H., Ohuchi N. Hexokinase II in breast carcinoma: A potent prognostic factor associated with hypoxia-inducible factor-1alpha and Ki-67. Cancer Sci. 2013;104:1380–1388. doi: 10.1111/cas.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patra K.C., Wang Q., Bhaskar P.T., Miller L., Wang Z., Wheaton W., Chandel N., Laakso M., Muller W.J., Allen E.L., et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zajicek G. The normal and the pathological. Harefuah. 1994;127:24–25. [PubMed] [Google Scholar]
- 45.Zhang X.D., Deslandes E., Villedieu M., Poulain L., Duval M., Gauduchon P., Schwartz L., Icard P. Effect of 2-deoxy-D-glucose on various malignant cell lines in vitro. Anticancer Res. 2006;26:3561–3566. [PubMed] [Google Scholar]
- 46.Zhao Y., Liu H., Liu Z., Ding Y., Ledoux S.P., Wilson G.L., Voellmy R., Lin Y., Lin W., Nahta R., et al. Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res. 2011;71:4585–4597. doi: 10.1158/0008-5472.CAN-11-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maschek G., Savaraj N., Priebe W., Braunschweiger P., Hamilton K., Tidmarsh G.F., De Young L.R., Lampidis T.J. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64:31–34. doi: 10.1158/0008-5472.CAN-03-3294. [DOI] [PubMed] [Google Scholar]
- 48.Sun X., Fan T., Sun G., Zhou Y., Huang Y., Zhang N., Zhao L., Zhong R., Peng Y. 2-Deoxy-D-glucose increases the sensitivity of glioblastoma cells to BCNU through the regulation of glycolysis, ROS and ERS pathways: In vitro and in vivo validation. Biochem. Pharmacol. 2022;199:115029. doi: 10.1016/j.bcp.2022.115029. [DOI] [PubMed] [Google Scholar]
- 49.Zhou N., Liu Q., Wang X., He L., Zhang T., Zhou H., Zhu X., Zhou T., Deng G., Qiu C. The combination of hydroxychloroquine and 2-deoxyglucose enhances apoptosis in breast cancer cells by blocking protective autophagy and sustaining endoplasmic reticulum stress. Cell Death Discov. 2022;8:286. doi: 10.1038/s41420-022-01074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raez L.E., Papadopoulos K., Ricart A.D., Chiorean E.G., Dipaola R.S., Stein M.N., Rocha Lima C.M., Schlesselman J.J., Tolba K., Langmuir V.K., et al. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2013;71:523–530. doi: 10.1007/s00280-012-2045-1. [DOI] [PubMed] [Google Scholar]
- 51.Geng C., Li J., Ding F., Wu G., Yang Q., Sun Y., Zhang Z., Dong T., Tian X. Curcumin suppresses 4-hydroxytamoxifen resistance in breast cancer cells by targeting SLUG/Hexokinase 2 pathway. Biochem. Biophys. Res. Commun. 2016;473:147–153. doi: 10.1016/j.bbrc.2016.03.067. [DOI] [PubMed] [Google Scholar]
- 52.Pedersen P.L. Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen. J. Bioenerg. Biomembr. 2007;39:211–222. doi: 10.1007/s10863-007-9094-x. [DOI] [PubMed] [Google Scholar]
- 53.Pedersen P.L., Mathupala S., Rempel A., Geschwind J.F., Ko Y.H. Mitochondrial bound type II hexokinase: A key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention. Biochim. Biophys. Acta. 2002;1555:14–20. doi: 10.1016/S0005-2728(02)00248-7. [DOI] [PubMed] [Google Scholar]
- 54.Nelson K. 3-Bromopyruvate kills cancer cells in animals. Lancet Oncol. 2002;3:524. doi: 10.1016/S1470-2045(02)00867-7. [DOI] [PubMed] [Google Scholar]
- 55.Fan T., Sun G., Sun X., Zhao L., Zhong R., Peng Y. Tumor Energy Metabolism and Potential of 3-Bromopyruvate as an Inhibitor of Aerobic Glycolysis: Implications in Tumor Treatment. Cancers. 2019;11:317. doi: 10.3390/cancers11030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Q., Pan J., Lubet R.A., Komas S.M., Kalyanaraman B., Wang Y., You M. Enhanced antitumor activity of 3-bromopyruvate in combination with rapamycin in vivo and in vitro. Cancer Prev. Res. 2015;8:318–326. doi: 10.1158/1940-6207.CAPR-14-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gan L., Ren Y., Lu J., Ma J., Shen X., Zhuang Z. Synergistic Effect of 3-Bromopyruvate in Combination with Rapamycin Impacted Neuroblastoma Metabolism by Inhibiting Autophagy. Onco Targets Ther. 2020;13:11125–11137. doi: 10.2147/OTT.S273108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J., Pan J., Liu Y., Luo X., Yang C., Xiao W., Li Q., Yang L., Zhang X. 3-Bromopyruvic acid regulates glucose metabolism by targeting the c-Myc/TXNIP axis and induces mitochondria-mediated apoptosis in TNBC cells. Exp. Ther. Med. 2022;24:520. doi: 10.3892/etm.2022.11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Attia Y.M., El-Abhar H.S., Al Marzabani M.M., Shouman S.A. Targeting glycolysis by 3-bromopyruvate improves tamoxifen cytotoxicity of breast cancer cell lines. BMC Cancer. 2015;15:838. doi: 10.1186/s12885-015-1850-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wicks R.T., Azadi J., Mangraviti A., Zhang I., Hwang L., Joshi A., Bow H., Hutt-Cabezas M., Martin K.L., Rudek M.A., et al. Local delivery of cancer-cell glycolytic inhibitors in high-grade glioma. Neuro-Oncol. 2015;17:70–80. doi: 10.1093/neuonc/nou143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim Y., Na J., Youn H., Yu J.S., Kang K.W., Chung J.-K. Enhanced therapeutic efficacy of combined Sorafenib and 3-bromopyruvate in murine orthotopic liver cancer assessed by bioluminescence image. J. Nucl. Med. 2016;57:1331. [Google Scholar]
- 62.Roy S.K., Dukic T., Bhandary B., Acharya A., Tu K.J., Ko Y.H., Shukla H.D. 3-Bromopyruvate in combination with radiation inhibits pancreatic cancer growth by dismantling mitochondria and ATP generation in a preclinical mouse model. Cancer Res. 2022;82:5243–5243. doi: 10.1158/1538-7445.AM2022-5243. [DOI] [Google Scholar]
- 63.Skaripa-Koukelli I., Hauton D., Walsby-Tickle J., Thomas E., Owen J., Lakshminarayanan A., Able S., McCullagh J., Carlisle R.C., Vallis K.A. 3-Bromopyruvate-mediated MCT1-dependent metabolic perturbation sensitizes triple negative breast cancer cells to ionizing radiation. Cancer Metab. 2021;9:37. doi: 10.1186/s40170-021-00273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Angelin A., Gil-de-Gomez L., Dahiya S., Jiao J., Guo L., Levine M.H., Wang Z., Quinn W.J., 3rd, Kopinski P.K., Wang L., et al. Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metab. 2017;25:1282–1293.e1287. doi: 10.1016/j.cmet.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brooks G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018;27:757–785. doi: 10.1016/j.cmet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Involvement of physicians in capital punishment. N. Y. State J. Med. 1991;91:271–272. [PubMed] [Google Scholar]
- 67.Morais-Santos F., Granja S., Miranda-Goncalves V., Moreira A.H., Queiros S., Vilaca J.L., Schmitt F.C., Longatto-Filho A., Paredes J., Baltazar F., et al. Targeting lactate transport suppresses in vivo breast tumour growth. Oncotarget. 2015;6:19177–19189. doi: 10.18632/oncotarget.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saulle E., Spinello I., Quaranta M.T., Pasquini L., Pelosi E., Iorio E., Castelli G., Chirico M., Pisanu M.E., Ottone T., et al. Targeting Lactate Metabolism by Inhibiting MCT1 or MCT4 Impairs Leukemic Cell Proliferation, Induces Two Different Related Death-Pathways and Increases Chemotherapeutic Sensitivity of Acute Myeloid Leukemia Cells. Front. Oncol. 2020;10:621458. doi: 10.3389/fonc.2020.621458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halford S.E., Walter H., McKay P., Townsend W., Linton K., Heinzmann K., Dragoni I., Brotherton L., Veal G., Siskos A., et al. Phase I expansion study of the first-in-class monocarboxylate transporter 1 (MCT1) inhibitor AZD3965 in patients with diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma (BL) J. Clin. Oncol. 2021;39:3115. doi: 10.1200/JCO.2021.39.15_suppl.3115. [DOI] [Google Scholar]
- 70.Halford S.E.R., Jones P., Wedge S., Hirschberg S., Katugampola S., Veal G., Payne G., Bacon C., Potter S., Griffin M., et al. A first-in-human first-in-class (FIC) trial of the monocarboxylate transporter 1 (MCT1) inhibitor AZD3965 in patients with advanced solid tumours. J. Clin. Oncol. 2017;35((Suppl. 15)):2516. doi: 10.1200/JCO.2017.35.15_suppl.2516. [DOI] [Google Scholar]
- 71.Claps G., Faouzi S., Quidville V., Chehade F., Shen S., Vagner S., Robert C. The multiple roles of LDH in cancer. Nat. Rev. Clin. Oncol. 2022;19:749–762. doi: 10.1038/s41571-022-00686-2. [DOI] [PubMed] [Google Scholar]
- 72.Flores A., Sandoval-Gonzalez S., Takahashi R., Krall A., Sathe L., Wei L., Radu C., Joly J.H., Graham N.A., Christofk H.R., et al. Increased lactate dehydrogenase activity is dispensable in squamous carcinoma cells of origin. Nat. Commun. 2019;10:91. doi: 10.1038/s41467-018-07857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fantin V.R., St-Pierre J., Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 74.Lu R., Jiang M., Chen Z., Xu X., Hu H., Zhao X., Gao X., Guo L. Lactate dehydrogenase 5 expression in Non-Hodgkin lymphoma is associated with the induced hypoxia regulated protein and poor prognosis. PLoS ONE. 2013;8:e74853. doi: 10.1371/journal.pone.0074853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zha X., Wang F., Wang Y., He S., Jing Y., Wu X., Zhang H. Lactate dehydrogenase B is critical for hyperactive mTOR-mediated tumorigenesis. Cancer Res. 2011;71:13–18. doi: 10.1158/0008-5472.CAN-10-1668. [DOI] [PubMed] [Google Scholar]
- 76.Shim H., Dolde C., Lewis B.C., Wu C.S., Dang G., Jungmann R.A., Dalla-Favera R., Dang C.V. c-Myc transactivation of LDH-A: Implications for tumor metabolism and growth. Proc. Natl. Acad. Sci. USA. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He T.L., Zhang Y.J., Jiang H., Li X.H., Zhu H., Zheng K.L. The c-Myc-LDHA axis positively regulates aerobic glycolysis and promotes tumor progression in pancreatic cancer. Med. Oncol. 2015;32:187. doi: 10.1007/s12032-015-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen L., Wu Q., Xu X., Yang C., You J., Chen F., Zeng Y. Cancer/testis antigen LDHC promotes proliferation and metastasis by activating the PI3K/Akt/GSK-3beta-signaling pathway and the in lung adenocarcinoma. Exp. Cell Res. 2021;398:112414. doi: 10.1016/j.yexcr.2020.112414. [DOI] [PubMed] [Google Scholar]
- 79.Jin L., Chun J., Pan C., Alesi G.N., Li D., Magliocca K.R., Kang Y., Chen Z.G., Shin D.M., Khuri F.R., et al. Phosphorylation-mediated activation of LDHA promotes cancer cell invasion and tumour metastasis. Oncogene. 2017;36:3797–3806. doi: 10.1038/onc.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferraris A.M., Giuntini P., Gaetani G.F. Serum lactic dehydrogenase as a prognostic tool for non-Hodgkin lymphomas. Blood. 1979;54:928–932. doi: 10.1182/blood.V54.4.928.928. [DOI] [PubMed] [Google Scholar]
- 81.Mohammad G.H., Olde Damink S.W., Malago M., Dhar D.K., Pereira S.P. Pyruvate Kinase M2 and Lactate Dehydrogenase A Are Overexpressed in Pancreatic Cancer and Correlate with Poor Outcome. PLoS ONE. 2016;11:e0151635. doi: 10.1371/journal.pone.0151635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Petrelli F., Cabiddu M., Coinu A., Borgonovo K., Ghilardi M., Lonati V., Barni S. Prognostic role of lactate dehydrogenase in solid tumors: A systematic review and meta-analysis of 76 studies. Acta Oncol. 2015;54:961–970. doi: 10.3109/0284186X.2015.1043026. [DOI] [PubMed] [Google Scholar]
- 83.Xie H., Hanai J., Ren J.G., Kats L., Burgess K., Bhargava P., Signoretti S., Billiard J., Duffy K.J., Grant A., et al. Targeting lactate dehydrogenase--a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab. 2014;19:795–809. doi: 10.1016/j.cmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le A., Cooper C.R., Gouw A.M., Dinavahi R., Maitra A., Deck L.M., Royer R.E., Vander Jagt D.L., Semenza G.L., Dang C.V. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Altinoz M.A., Ozpinar A. Oxamate targeting aggressive cancers with special emphasis to brain tumors. Biomed. Pharmacother. 2022;147:112686. doi: 10.1016/j.biopha.2022.112686. [DOI] [PubMed] [Google Scholar]
- 86.Zhao Z., Han F., Yang S., Wu J., Zhan W. Oxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: Involvement of the Akt-mTOR signaling pathway. Cancer Lett. 2015;358:17–26. doi: 10.1016/j.canlet.2014.11.046. [DOI] [PubMed] [Google Scholar]
- 87.Miskimins W.K., Ahn H.J., Kim J.Y., Ryu S., Jung Y.S., Choi J.Y. Synergistic anti-cancer effect of phenformin and oxamate. PLoS ONE. 2014;9:e85576. doi: 10.1371/journal.pone.0085576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xing B.C., Wang C., Ji F.J., Zhang X.B. Synergistically suppressive effects on colorectal cancer cells by combination of mTOR inhibitor and glycolysis inhibitor, Oxamate. Int. J. Clin. Exp. Pathol. 2018;11:4439–4445. [PMC free article] [PubMed] [Google Scholar]
- 89.Almaguel F.A., Sanchez T.W., Ortiz-Hernandez G.L., Casiano C.A. Alpha-Enolase: Emerging Tumor-Associated Antigen, Cancer Biomarker, and Oncotherapeutic Target. Front. Genet. 2020;11:614726. doi: 10.3389/fgene.2020.614726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He P., Naka T., Serada S., Fujimoto M., Tanaka T., Hashimoto S., Shima Y., Yamadori T., Suzuki H., Hirashima T., et al. Proteomics-based identification of alpha-enolase as a tumor antigen in non-small lung cancer. Cancer Sci. 2007;98:1234–1240. doi: 10.1111/j.1349-7006.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hsiao K.C., Shih N.Y., Chu P.Y., Hung Y.M., Liao J.Y., Chou S.W., Yang Y.Y., Chang G.C., Liu K.J. Anti-alpha-enolase is a prognostic marker in postoperative lung cancer patients. Oncotarget. 2015;6:35073–35086. doi: 10.18632/oncotarget.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fu Q.F., Liu Y., Fan Y., Hua S.N., Qu H.Y., Dong S.W., Li R.L., Zhao M.Y., Zhen Y., Yu X.L., et al. Alpha-enolase promotes cell glycolysis, growth, migration, and invasion in non-small cell lung cancer through FAK-mediated PI3K/AKT pathway. J. Hematol. Oncol. 2015;8:22. doi: 10.1186/s13045-015-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhan P., Zhao S., Yan H., Yin C., Xiao Y., Wang Y., Ni R., Chen W., Wei G., Zhang P. alpha-enolase promotes tumorigenesis and metastasis via regulating AMPK/mTOR pathway in colorectal cancer. Mol. Carcinog. 2017;56:1427–1437. doi: 10.1002/mc.22603. [DOI] [PubMed] [Google Scholar]
- 94.Capello M., Ferri-Borgogno S., Riganti C., Chattaragada M.S., Principe M., Roux C., Zhou W., Petricoin E.F., Cappello P., Novelli F. Targeting the Warburg effect in cancer cells through ENO1 knockdown rescues oxidative phosphorylation and induces growth arrest. Oncotarget. 2016;7:5598–5612. doi: 10.18632/oncotarget.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Song K., Rajasekaran N., Chelakkot C., Lee H.S., Paek S.M., Yang H., Jia L., Park H.G., Son W.S., Kim Y.J., et al. Macrosphelide A Exhibits a Specific Anti-Cancer Effect by Simultaneously Inactivating ENO1, ALDOA, and FH. Pharmaceuticals. 2021;14:1060. doi: 10.3390/ph14101060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qian X., Xu W., Xu J., Shi Q., Li J., Weng Y., Jiang Z., Feng L., Wang X., Zhou J., et al. Enolase 1 stimulates glycolysis to promote chemoresistance in gastric cancer. Oncotarget. 2017;8:47691–47708. doi: 10.18632/oncotarget.17868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang C.K., Sun Y., Lv L., Ping Y. ENO1 and Cancer. Mol. Ther. Oncolytics. 2022;24:288–298. doi: 10.1016/j.omto.2021.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin Y.H., Satani N., Hammoudi N., Yan V.C., Barekatain Y., Khadka S., Ackroyd J.J., Georgiou D.K., Pham C.D., Arthur K., et al. An enolase inhibitor for the targeted treatment of ENO1-deleted cancers. Nat. Metab. 2020;2:1413–1426. doi: 10.1038/s42255-020-00313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jones B.C., Pohlmann P.R., Clarke R., Sengupta S. Treatment against glucose-dependent cancers through metabolic PFKFB3 targeting of glycolytic flux. Cancer Metastasis Rev. 2022;41:447–458. doi: 10.1007/s10555-022-10027-5. [DOI] [PubMed] [Google Scholar]
- 100.Zhu W., Ye L., Zhang J., Yu P., Wang H., Ye Z., Tian J. PFK15, a Small Molecule Inhibitor of PFKFB3, Induces Cell Cycle Arrest, Apoptosis and Inhibits Invasion in Gastric Cancer. PLoS ONE. 2016;11:e0163768. doi: 10.1371/journal.pone.0163768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Telang S., O’Neal J., Tapolsky G., Clem B., Kerr A., Imbert-Ferndandez Y., Chesney J. Discovery of a PFKFB3 inhibitor for phase I trial testing that synergizes with the B-Raf inhibitor vemurafenib. Cancer Metab. 2014;2:1–2. doi: 10.1186/2049-3002-2-S1-P14. [DOI] [Google Scholar]
- 102.Phase 1 Safety Study of ACT-PFK-158, 2HCl in Patients with Advanced Solid Malignancies_NCT02044861. [(accessed on 30 September 2015)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02044861.
- 103.Redman R.A., Pohlmann P.R., Kurman M.R., Tapolsky G., Chesney J. A phase I, dose-escalation, multicenter study of ACT-PFK-158, 2HCl in patients with advanced solid malignancies explores a first-in-human inhibitor of glycolysis. J. Clin. Oncol. 2015;33:TPS494. doi: 10.1200/jco.2015.33.3_suppl.tps494. [DOI] [Google Scholar]
- 104.Yan S., Zhou N., Zhang D., Zhang K., Zheng W., Bao Y., Yang W. PFKFB3 Inhibition Attenuates Oxaliplatin-Induced Autophagy and Enhances Its Cytotoxicity in Colon Cancer Cells. Int. J. Mol. Sci. 2019;20:5415. doi: 10.3390/ijms20215415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Bock K., Georgiadou M., Carmeliet P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013;18:634–647. doi: 10.1016/j.cmet.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 106.De Bock K., Georgiadou M., Schoors S., Kuchnio A., Wong B.W., Cantelmo A.R., Quaegebeur A., Ghesquiere B., Cauwenberghs S., Eelen G., et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 107.Schoors S., De Bock K., Cantelmo A.R., Georgiadou M., Ghesquiere B., Cauwenberghs S., Kuchnio A., Wong B.W., Quaegebeur A., Goveia J., et al. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 2014;19:37–48. doi: 10.1016/j.cmet.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 108.Cantelmo A.R., Conradi L.C., Brajic A., Goveia J., Kalucka J., Pircher A., Chaturvedi P., Hol J., Thienpont B., Teuwen L.A., et al. Inhibition of the Glycolytic Activator PFKFB3 in Endothelium Induces Tumor Vessel Normalization, Impairs Metastasis, and Improves Chemotherapy. Cancer Cell. 2016;30:968–985. doi: 10.1016/j.ccell.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Emini Veseli B., Van Wielendaele P., Delibegovic M., Martinet W., De Meyer G.R.Y. The PFKFB3 Inhibitor AZ67 Inhibits Angiogenesis Independently of Glycolysis Inhibition. Int. J. Mol. Sci. 2021;22:5970. doi: 10.3390/ijms22115970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang J., Xue W., Xu K., Yi L., Guo Y., Xie T., Tong H., Zhou B., Wang S., Li Q., et al. Dual inhibition of PFKFB3 and VEGF normalizes tumor vasculature, reduces lactate production, and improves chemotherapy in glioblastoma: Insights from protein expression profiling and MRI. Theranostics. 2020;10:7245–7259. doi: 10.7150/thno.44427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hsu M.C., Hung W.C. Pyruvate kinase M2 fuels multiple aspects of cancer cells: From cellular metabolism, transcriptional regulation to extracellular signaling. Mol. Cancer. 2018;17:35. doi: 10.1186/s12943-018-0791-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Allen A.E., Locasale J.W. Glucose Metabolism in Cancer: The Saga of Pyruvate Kinase Continues. Cancer Cell. 2018;33:337–339. doi: 10.1016/j.ccell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang W., Zheng Y., Xia Y., Ji H., Chen X., Guo F., Lyssiotis C.A., Aldape K., Cantley L.C., Lu Z. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat. Cell Biol. 2012;14:1295–1304. doi: 10.1038/ncb2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Azoitei N., Becher A., Steinestel K., Rouhi A., Diepold K., Genze F., Simmet T., Seufferlein T. PKM2 promotes tumor angiogenesis by regulating HIF-1alpha through NF-kappaB activation. Mol. Cancer. 2016;15:3. doi: 10.1186/s12943-015-0490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu J., Hu L., Chen M., Cao W., Chen H., He T. Pyruvate kinase M2 overexpression and poor prognosis in solid tumors of digestive system: Evidence from 16 cohort studies. Onco Targets Ther. 2016;9:4277–4288. doi: 10.2147/OTT.S106508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu H., Luo H., Zhu X., Hu X., Zheng L., Zhu X. Pyruvate kinase M2 (PKM2) expression correlates with prognosis in solid cancers: A meta-analysis. Oncotarget. 2017;8:1628–1640. doi: 10.18632/oncotarget.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tennant D.A., Duran R.V., Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat. Rev. Cancer. 2010;10:267–277. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 118.Shi H.S., Li D., Zhang J., Wang Y.S., Yang L., Zhang H.L., Wang X.H., Mu B., Wang W., Ma Y., et al. Silencing of pkm2 increases the efficacy of docetaxel in human lung cancer xenografts in mice. Cancer Sci. 2010;101:1447–1453. doi: 10.1111/j.1349-7006.2010.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yuan S., Qiao T., Zhuang X., Chen W., Xing N., Zhang Q. Knockdown of the M2 Isoform of Pyruvate Kinase (PKM2) with shRNA Enhances the Effect of Docetaxel in Human NSCLC Cell Lines In Vitro. Yonsei Med. J. 2016;57:1312–1323. doi: 10.3349/ymj.2016.57.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Papadaki C., Sfakianaki M., Lagoudaki E., Giagkas G., Ioannidis G., Trypaki M., Tsakalaki E., Voutsina A., Koutsopoulos A., Mavroudis D., et al. PKM2 as a biomarker for chemosensitivity to front-line platinum-based chemotherapy in patients with metastatic non-small-cell lung cancer. Br. J. Cancer. 2014;111:1757–1764. doi: 10.1038/bjc.2014.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guo W., Zhang Y., Chen T., Wang Y., Xue J., Zhang Y., Xiao W., Mo X., Lu Y. Efficacy of RNAi targeting of pyruvate kinase M2 combined with cisplatin in a lung cancer model. J. Cancer Res. Clin. Oncol. 2011;137:65–72. doi: 10.1007/s00432-010-0860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ganapathy-Kanniappan S., Kunjithapatham R., Geschwind J.F. Glyceraldehyde-3-phosphate dehydrogenase: A promising target for molecular therapy in hepatocellular carcinoma. Oncotarget. 2012;3:940–953. doi: 10.18632/oncotarget.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thornalley P.J., Rabbani N. Glyoxalase in tumourigenesis and multidrug resistance. Semin. Cell Dev. Biol. 2011;22:318–325. doi: 10.1016/j.semcdb.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 124.Ganapathy-Kanniappan S., Vali M., Kunjithapatham R., Buijs M., Syed L.H., Rao P.P., Ota S., Kwak B.K., Loffroy R., Geschwind J.F. 3-bromopyruvate: A new targeted antiglycolytic agent and a promise for cancer therapy. Curr. Pharm. Biotechnol. 2010;11:510–517. doi: 10.2174/138920110791591427. [DOI] [PubMed] [Google Scholar]
- 125.Ganapathy-Kanniappan S., Kunjithapatham R., Geschwind J.F. Anticancer efficacy of the metabolic blocker 3-bromopyruvate: Specific molecular targeting. Anticancer Res. 2013;33:13–20. [PubMed] [Google Scholar]
- 126.Gao Y., Zhang T., Terai H., Ficarro S.B., Kwiatkowski N., Hao M.F., Sharma B., Christensen C.L., Chipumuro E., Wong K.K., et al. Overcoming Resistance to the THZ Series of Covalent Transcriptional CDK Inhibitors. Cell Chem. Biol. 2018;25:135–142.e5. doi: 10.1016/j.chembiol.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marcucci F., Rumio C. Glycolysis-induced drug resistance in tumors-A response to danger signals? Neoplasia. 2021;23:234–245.e5. doi: 10.1016/j.neo.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun H., Wang H., Wang X., Aoki Y., Wang X., Yang Y., Cheng X., Wang Z., Wang X. Aurora-A/SOX8/FOXK1 signaling axis promotes chemoresistance via suppression of cell senescence and induction of glucose metabolism in ovarian cancer organoids and cells. Theranostics. 2020;10:6928–6945. doi: 10.7150/thno.43811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Efimova E.V., Takahashi S., Shamsi N.A., Wu D., Labay E., Ulanovskaya O.A., Weichselbaum R.R., Kozmin S.A., Kron S.J. Linking Cancer Metabolism to DNA Repair and Accelerated Senescence. Mol. Cancer Res. 2016;14:173–184. doi: 10.1158/1541-7786.MCR-15-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kohnken R., Kodigepalli K.M., Wu L. Regulation of deoxynucleotide metabolism in cancer: Novel mechanisms and therapeutic implications. Mol. Cancer. 2015;14:176. doi: 10.1186/s12943-015-0446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Le T.M., Poddar S., Capri J.R., Abt E.R., Kim W., Wei L., Uong N.T., Cheng C.M., Braas D., Nikanjam M., et al. ATR inhibition facilitates targeting of leukemia dependence on convergent nucleotide biosynthetic pathways. Nat. Commun. 2017;8:241. doi: 10.1038/s41467-017-00221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kwon Y., Kim M., Jung H.S., Kim Y., Jeoung D. Targeting Autophagy for Overcoming Resistance to Anti-EGFR Treatments. Cancers. 2019;11:1374. doi: 10.3390/cancers11091374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lamoureux F., Zoubeidi A. Dual inhibition of autophagy and the AKT pathway in prostate cancer. Autophagy. 2013;9:1119–1120. doi: 10.4161/auto.24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Reyes-Castellanos G., Abdel Hadi N., Carrier A. Autophagy Contributes to Metabolic Reprogramming and Therapeutic Resistance in Pancreatic Tumors. Cells. 2022;11:1374. doi: 10.3390/cells11030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Senthebane D.A., Rowe A., Thomford N.E., Shipanga H., Munro D., Mazeedi M., Almazyadi H.A.M., Kallmeyer K., Dandara C., Pepper M.S., et al. The Role of Tumor Microenvironment in Chemoresistance: To Survive, Keep Your Enemies Closer. Int. J. Mol. Sci. 2017;18:1586. doi: 10.3390/ijms18071586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cerezo M., Rocchi S. Cancer cell metabolic reprogramming: A keystone for the response to immunotherapy. Cell Death Dis. 2020;11:964. doi: 10.1038/s41419-020-03175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Santos J.C., Lima N.D.S., Sarian L.O., Matheu A., Ribeiro M.L., Derchain S.F.M. Exosome-mediated breast cancer chemoresistance via miR-155 transfer. Sci. Rep. 2018;8:829. doi: 10.1038/s41598-018-19339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yoshida G.J., Saya H. Therapeutic strategies targeting cancer stem cells. Cancer Sci. 2016;107:5–11. doi: 10.1111/cas.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tsai S.T., Chien I.H., Shen W.H., Kuo Y.Z., Jin Y.T., Wong T.Y., Hsiao J.R., Wang H.P., Shih N.Y., Wu L.W. ENO1, a potential prognostic head and neck cancer marker, promotes transformation partly via chemokine CCL20 induction. Eur. J. Cancer. 2010;46:1712–1723. doi: 10.1016/j.ejca.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 140.Song Y., Luo Q., Long H., Hu Z., Que T., Zhang X., Li Z., Wang G., Yi L., Liu Z., et al. Alpha-enolase as a potential cancer prognostic marker promotes cell growth, migration, and invasion in glioma. Mol. Cancer. 2014;13:65. doi: 10.1186/1476-4598-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mahammedi H., Planchat E., Pouget M., Durando X., Cure H., Guy L., Van-Praagh I., Savareux L., Atger M., Bayet-Robert M., et al. The New Combination Docetaxel, Prednisone and Curcumin in Patients with Castration-Resistant Prostate Cancer: A Pilot Phase II Study. Oncology. 2016;90:69–78. doi: 10.1159/000441148. [DOI] [PubMed] [Google Scholar]
- 142.Huang K., Liang Q., Zhou Y., Jiang L.L., Gu W.M., Luo M.Y., Tang Y.B., Wang Y., Lu W., Huang M., et al. A Novel Allosteric Inhibitor of Phosphoglycerate Mutase 1 Suppresses Growth and Metastasis of Non-Small-Cell Lung Cancer. Cell Metab. 2019;30:1107–1119.e8. doi: 10.1016/j.cmet.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 143.Tu S.H., Chang C.C., Chen C.S., Tam K.W., Wang Y.J., Lee C.H., Lin H.W., Cheng T.C., Huang C.S., Chu J.S., et al. Increased expression of enolase alpha in human breast cancer confers tamoxifen resistance in human breast cancer cells. Breast Cancer Res. Treat. 2010;121:539–553. doi: 10.1007/s10549-009-0492-0. [DOI] [PubMed] [Google Scholar]
- 144.Principe M., Borgoni S., Cascione M., Chattaragada M.S., Ferri-Borgogno S., Capello M., Bulfamante S., Chapelle J., Di Modugno F., Defilippi P., et al. Alpha-enolase (ENO1) controls alpha v/beta 3 integrin expression and regulates pancreatic cancer adhesion, invasion, and metastasis. J. Hematol. Oncol. 2017;10:16. doi: 10.1186/s13045-016-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang L., Qu M., Huang S., Fu Y., Yang L., He S., Li L., Zhang Z., Lin Q., Zhang L. A novel alpha-enolase-targeted drug delivery system for high efficacy prostate cancer therapy. Nanoscale. 2018;10:13673–13683. doi: 10.1039/C8NR03297A. [DOI] [PubMed] [Google Scholar]
- 146.Santana-Rivera Y., Rabelo-Fernandez R.J., Quinones-Diaz B.I., Grafals-Ruiz N., Santiago-Sanchez G., Lozada-Delgado E.L., Echevarria-Vargas I.M., Apiz J., Soto D., Rosado A., et al. Reduced expression of enolase-1 correlates with high intracellular glucose levels and increased senescence in cisplatin-resistant ovarian cancer cells. Am. J. Transl. Res. 2020;12:1275–1292. [PMC free article] [PubMed] [Google Scholar]
- 147.Jiang Y.X., Siu M.K.Y., Wang J.J., Leung T.H.Y., Chan D.W., Cheung A.N.Y., Ngan H.Y.S., Chan K.K.L. PFKFB3 Regulates Chemoresistance, Metastasis and Stemness via IAP Proteins and the NF-kappaB Signaling Pathway in Ovarian Cancer. Front. Oncol. 2022;12:748403. doi: 10.3389/fonc.2022.748403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mondal S., Roy D., Sarkar Bhattacharya S., Jin L., Jung D., Zhang S., Kalogera E., Staub J., Wang Y., Xuyang W., et al. Therapeutic targeting of PFKFB3 with a novel glycolytic inhibitor PFK158 promotes lipophagy and chemosensitivity in gynecologic cancers. Int. J. Cancer. 2019;144:178–189. doi: 10.1002/ijc.31868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Meng M.B., Wang H.H., Guo W.H., Wu Z.Q., Zeng X.L., Zaorsky N.G., Shi H.S., Qian D., Niu Z.M., Jiang B., et al. Targeting pyruvate kinase M2 contributes to radiosensitivity of non-small cell lung cancer cells in vitro and in vivo. Cancer Lett. 2015;356:985–993. doi: 10.1016/j.canlet.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 150.Sizemore S.T., Zhang M., Cho J.H., Sizemore G.M., Hurwitz B., Kaur B., Lehman N.L., Ostrowski M.C., Robe P.A., Miao W., et al. Pyruvate kinase M2 regulates homologous recombination-mediated DNA double-strand break repair. Cell Res. 2018;28:1090–1102. doi: 10.1038/s41422-018-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sfakianaki M., Papadaki C., Tzardi M., Trypaki M., Manolakou S., Messaritakis I., Saridaki Z., Athanasakis E., Mavroudis D., Tsiaoussis J., et al. PKM2 Expression as Biomarker for Resistance to Oxaliplatin-Based Chemotherapy in Colorectal Cancer. Cancers. 2020;12:2058. doi: 10.3390/cancers12082058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Anastasiou D., Poulogiannis G., Asara J.M., Boxer M.B., Jiang J.K., Shen M., Bellinger G., Sasaki A.T., Locasale J.W., Auld D.S., et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhu H., Wu J., Zhang W., Luo H., Shen Z., Cheng H., Zhu X. PKM2 enhances chemosensitivity to cisplatin through interaction with the mTOR pathway in cervical cancer. Sci. Rep. 2016;6:30788. doi: 10.1038/srep30788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sun Q., Chen X., Ma J., Peng H., Wang F., Zha X., Wang Y., Jing Y., Yang H., Chen R., et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc. Natl. Acad. Sci. USA. 2011;108:4129–4134. doi: 10.1073/pnas.1014769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lin Y., Lv F., Liu F., Guo X., Fan Y., Gu F., Gu J., Fu L. High Expression of Pyruvate Kinase M2 is Associated with Chemosensitivity to Epirubicin and 5-Fluorouracil in Breast Cancer. J. Cancer. 2015;6:1130–1139. doi: 10.7150/jca.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yoo B.C., Ku J.L., Hong S.H., Shin Y.K., Park S.Y., Kim H.K., Park J.G. Decreased pyruvate kinase M2 activity linked to cisplatin resistance in human gastric carcinoma cell lines. Int. J. Cancer. 2004;108:532–539. doi: 10.1002/ijc.11604. [DOI] [PubMed] [Google Scholar]
- 157.Martinez-Balibrea E., Plasencia C., Gines A., Martinez-Cardus A., Musulen E., Aguilera R., Manzano J.L., Neamati N., Abad A. A proteomic approach links decreased pyruvate kinase M2 expression to oxaliplatin resistance in patients with colorectal cancer and in human cell lines. Mol. Cancer Ther. 2009;8:771–778. doi: 10.1158/1535-7163.MCT-08-0882. [DOI] [PubMed] [Google Scholar]
- 158.Al-Sawaf O., Bazeos A., Robrecht S., Bahlo J., Gower C., Fink A.M., Tresckow J., Cramer P., Langerbeins P., Kutsch N., et al. Mode of progression after first line treatment correlates with outcome of chronic lymphocytic leukemia (CLL) Am. J. Hematol. 2019;94:1002–1006. doi: 10.1002/ajh.25561. [DOI] [PubMed] [Google Scholar]
- 159.Cohen E.E.W., Bell R.B., Bifulco C.B., Burtness B., Gillison M.L., Harrington K.J., Le Q.T., Lee N.Y., Leidner R., Lewis R.L., et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC) J. Immunother. Cancer. 2019;7:184. doi: 10.1186/s40425-019-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Reck M., Remon J., Hellmann M.D. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022;40:586–597. doi: 10.1200/JCO.21.01497. [DOI] [PubMed] [Google Scholar]
- 161.Bagchi S., Yuan R., Engleman E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 162.Hegde P.S., Chen D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity. 2020;52:17–35. doi: 10.1016/j.immuni.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 163.DeBerardinis R.J., Chandel N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016;2:e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.DePeaux K., Delgoffe G.M. Metabolic barriers to cancer immunotherapy. Nat. Rev. Immunol. 2021;21:785–797. doi: 10.1038/s41577-021-00541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chang C.H., Qiu J., O’Sullivan D., Buck M.D., Noguchi T., Curtis J.D., Chen Q., Gindin M., Gubin M.M., van der Windt G.J., et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Andrejeva G., Rathmell J.C. Similarities and Distinctions of Cancer and Immune Metabolism in Inflammation and Tumors. Cell Metab. 2017;26:49–70. doi: 10.1016/j.cmet.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Watson M.J., Vignali P.D.A., Mullett S.J., Overacre-Delgoffe A.E., Peralta R.M., Grebinoski S., Menk A.V., Rittenhouse N.L., DePeaux K., Whetstone R.D., et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. 2021;591:645–651. doi: 10.1038/s41586-020-03045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ho P.C., Bihuniak J.D., Macintyre A.N., Staron M., Liu X., Amezquita R., Tsui Y.C., Cui G., Micevic G., Perales J.C., et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Scharping N.E., Rivadeneira D.B., Menk A.V., Vignali P.D.A., Ford B.R., Rittenhouse N.L., Peralta R., Wang Y., Wang Y., DePeaux K., et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 2021;22:205–215. doi: 10.1038/s41590-020-00834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jancewicz I., Szarkowska J., Konopinski R., Stachowiak M., Swiatek M., Blachnio K., Kubala S., Oksinska P., Cwiek P., Rusetska N., et al. PD-L1 Overexpression, SWI/SNF Complex Deregulation, and Profound Transcriptomic Changes Characterize Cancer-Dependent Exhaustion of Persistently Activated CD4(+) T Cells. Cancers. 2021;13:4148. doi: 10.3390/cancers13164148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Martins C.P., New L.A., O’Connor E.C., Previte D.M., Cargill K.R., Tse I.L., Sims-Lucas S., Piganelli J.D. Glycolysis Inhibition Induces Functional and Metabolic Exhaustion of CD4(+) T Cells in Type 1 Diabetes. Front. Immunol. 2021;12:669456. doi: 10.3389/fimmu.2021.669456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gerriets V.A., Kishton R.J., Nichols A.G., Macintyre A.N., Inoue M., Ilkayeva O., Winter P.S., Liu X., Priyadharshini B., Slawinska M.E., et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Investig. 2015;125:194–207. doi: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gerriets V.A., Kishton R.J., Johnson M.O., Cohen S., Siska P.J., Nichols A.G., Warmoes M.O., de Cubas A.A., MacIver N.J., Locasale J.W., et al. Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat. Immunol. 2016;17:1459–1466. doi: 10.1038/ni.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kelly B., O’Neill L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Netea-Maier R.T., Smit J.W.A., Netea M.G. Metabolic changes in tumor cells and tumor-associated macrophages: A mutual relationship. Cancer Lett. 2018;413:102–109. doi: 10.1016/j.canlet.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 176.Bohn T., Rapp S., Luther N., Klein M., Bruehl T.J., Kojima N., Aranda Lopez P., Hahlbrock J., Muth S., Endo S., et al. Tumor immunoevasion via acidosis-dependent induction of regulatory tumor-associated macrophages. Nat. Immunol. 2018;19:1319–1329. doi: 10.1038/s41590-018-0226-8. [DOI] [PubMed] [Google Scholar]
- 177.Wang L., Liu Y., Dai Y., Tang X., Yin T., Wang C., Wang T., Dong L., Shi M., Qin J., et al. Single-cell RNA-seq analysis reveals BHLHE40-driven pro-tumour neutrophils with hyperactivated glycolysis in pancreatic tumour microenvironment. Gut. 2022:1–14. doi: 10.1136/gutjnl-2021-326070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li W., Tanikawa T., Kryczek I., Xia H., Li G., Wu K., Wei S., Zhao L., Vatan L., Wen B., et al. Aerobic Glycolysis Controls Myeloid-Derived Suppressor Cells and Tumor Immunity via a Specific CEBPB Isoform in Triple-Negative Breast Cancer. Cell Metab. 2018;28:87–103.e6. doi: 10.1016/j.cmet.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yang X., Lu Y., Hang J., Zhang J., Zhang T., Huo Y., Liu J., Lai S., Luo D., Wang L., et al. Lactate-Modulated Immunosuppression of Myeloid-Derived Suppressor Cells Contributes to the Radioresistance of Pancreatic Cancer. Cancer Immunol. Res. 2020;8:1440–1451. doi: 10.1158/2326-6066.CIR-20-0111. [DOI] [PubMed] [Google Scholar]
- 180.Cascone T., McKenzie J.A., Mbofung R.M., Punt S., Wang Z., Xu C., Williams L.J., Wang Z., Bristow C.A., Carugo A., et al. Increased Tumor Glycolysis Characterizes Immune Resistance to Adoptive T Cell Therapy. Cell Metab. 2018;27:977–987.e974. doi: 10.1016/j.cmet.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Song B.S., Moon J.S., Tian J., Lee H.Y., Sim B.C., Kim S.H., Kang S.G., Kim J.T., Nga H.T., Benfeitas R., et al. Mitoribosomal defects aggravate liver cancer via aberrant glycolytic flux and T cell exhaustion. J. Immunother. Cancer. 2022;10:e004337. doi: 10.1136/jitc-2021-004337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yu Y.R., Imrichova H., Wang H., Chao T., Xiao Z., Gao M., Rincon-Restrepo M., Franco F., Genolet R., Cheng W.C., et al. Disturbed mitochondrial dynamics in CD8(+) TILs reinforce T cell exhaustion. Nat. Immunol. 2020;21:1540–1551. doi: 10.1038/s41590-020-0793-3. [DOI] [PubMed] [Google Scholar]
- 183.Kooshki L., Mahdavi P., Fakhri S., Akkol E.K., Khan H. Targeting lactate metabolism and glycolytic pathways in the tumor microenvironment by natural products: A promising strategy in combating cancer. Biofactors. 2022;48:359–383. doi: 10.1002/biof.1799. [DOI] [PubMed] [Google Scholar]
- 184.Fischer K., Hoffmann P., Voelkl S., Meidenbauer N., Ammer J., Edinger M., Gottfried E., Schwarz S., Rothe G., Hoves S., et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 185.Robert C., Lewis K.D., Gutzmer R., Stroyakovskiy D., Gogas H., Protsenko S., Pereira R.P., Eigentler T., Rutkowski P., Demidov L., et al. Biomarkers of treatment benefit with atezolizumab plus vemurafenib plus cobimetinib in BRAF(V600) mutation-positive melanoma. Ann. Oncol. 2022;33:544–555. doi: 10.1016/j.annonc.2022.01.076. [DOI] [PubMed] [Google Scholar]
- 186.Calcinotto A., Filipazzi P., Grioni M., Iero M., De Milito A., Ricupito A., Cova A., Canese R., Jachetti E., Rossetti M., et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012;72:2746–2756. doi: 10.1158/0008-5472.CAN-11-1272. [DOI] [PubMed] [Google Scholar]
- 187.Mendler A.N., Hu B., Prinz P.U., Kreutz M., Gottfried E., Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int. J. Cancer. 2012;131:633–640. doi: 10.1002/ijc.26410. [DOI] [PubMed] [Google Scholar]
- 188.Shime H., Yabu M., Akazawa T., Kodama K., Matsumoto M., Seya T., Inoue N. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J. Immunol. 2008;180:7175–7183. doi: 10.4049/jimmunol.180.11.7175. [DOI] [PubMed] [Google Scholar]
- 189.Ma S., Zhao Y., Lee W.C., Ong L.T., Lee P.L., Jiang Z., Oguz G., Niu Z., Liu M., Goh J.Y., et al. Hypoxia induces HIF1alpha-dependent epigenetic vulnerability in triple negative breast cancer to confer immune effector dysfunction and resistance to anti-PD-1 immunotherapy. Nat. Commun. 2022;13:4118. doi: 10.1038/s41467-022-31764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ford B.R., Vignali P.D.A., Rittenhouse N.L., Scharping N.E., Peralta R., Lontos K., Frisch A.T., Delgoffe G.M., Poholek A.C. Tumor microenvironmental signals reshape chromatin landscapes to limit the functional potential of exhausted T cells. Sci. Immunol. 2022;7:eabj9123. doi: 10.1126/sciimmunol.abj9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Clambey E.T., McNamee E.N., Westrich J.A., Glover L.E., Campbell E.L., Jedlicka P., de Zoeten E.F., Cambier J.C., Stenmark K.R., Colgan S.P., et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc. Natl. Acad. Sci. USA. 2012;109:E2784–E2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Deng J., Li J., Sarde A., Lines J.L., Lee Y.C., Qian D.C., Pechenick D.A., Manivanh R., Le Mercier I., Lowrey C.H., et al. Hypoxia-Induced VISTA Promotes the Suppressive Function of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Cancer Immunol. Res. 2019;7:1079–1090. doi: 10.1158/2326-6066.CIR-18-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zheng J.B., Wong C.W., Liu J., Luo X.J., Zhou W.Y., Chen Y.X., Luo H.Y., Zeng Z.L., Ren C., Xie X.M., et al. Glucose metabolism inhibitor PFK-015 combined with immune checkpoint inhibitor is an effective treatment regimen in cancer. Oncoimmunology. 2022;11:2079182. doi: 10.1080/2162402X.2022.2079182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bader J.E., Voss K., Rathmell J.C. Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy. Mol. Cell. 2020;78:1019–1033. doi: 10.1016/j.molcel.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chow E., Yang A., Chung C.H.L., Chan J.C.N. A Clinical Perspective of the Multifaceted Mechanism of Metformin in Diabetes, Infections, Cognitive Dysfunction, and Cancer. Pharmaceuticals. 2022;15:442. doi: 10.3390/ph15040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rubiño M.E., Carrillo E., Alcalá G., Domínguez-Martín A., Marchal J.A., Boulaiz H. Phenformin as an Anticancer Agent: Challenges and Prospects. Int. J. Mol. Sci. 2019;20:3316. doi: 10.3390/ijms20133316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shackelford D.B., Shaw R.J. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.MacIver N.J., Blagih J., Saucillo D.C., Tonelli L., Griss T., Rathmell J.C., Jones R.G. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J. Immunol. 2011;187:4187–4198. doi: 10.4049/jimmunol.1100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Denko N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 200.Icard P., Simula L., Fournel L., Leroy K., Lupo A., Damotte D., Charpentier M.C., Durdux C., Loi M., Schussler O., et al. The strategic roles of four enzymes in the interconnection between metabolism and oncogene activation in non-small cell lung cancer: Therapeutic implications. Drug Resist. Updat. 2022;63:100852. doi: 10.1016/j.drup.2022.100852. [DOI] [PubMed] [Google Scholar]
- 201.Xu K., Yin N., Peng M., Stamatiades E.G., Shyu A., Li P., Zhang X., Do M.H., Wang Z., Capistrano K.J., et al. Glycolysis fuels phosphoinositide 3-kinase signaling to bolster T cell immunity. Science. 2021;371:405–410. doi: 10.1126/science.abb2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jacobs S.R., Herman C.E., Maciver N.J., Wofford J.A., Wieman H.L., Hammen J.J., Rathmell J.C. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Miyamoto S., Murphy A.N., Brown J.H. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008;15:521–529. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- 204.Chae Y.C., Vaira V., Caino M.C., Tang H.Y., Seo J.H., Kossenkov A.V., Ottobrini L., Martelli C., Lucignani G., Bertolini I., et al. Mitochondrial Akt Regulation of Hypoxic Tumor Reprogramming. Cancer Cell. 2016;30:257–272. doi: 10.1016/j.ccell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Araki K., Turner A.P., Shaffer V.O., Gangappa S., Keller S.A., Bachmann M.F., Larsen C.P., Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rao R.R., Li Q., Odunsi K., Shrikant P.A. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Madden M.Z., Rathmell J.C. The Complex Integration of T-cell Metabolism and Immunotherapy. Cancer Discov. 2021;11:1636–1643. doi: 10.1158/2159-8290.CD-20-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Huang J., Huang X., Huang J. CAR-T cell therapy for hematological malignancies: Limitations and optimization strategies. Front. Immunol. 2022;13:1019115. doi: 10.3389/fimmu.2022.1019115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Qin X., Wu F., Chen C., Li Q. Recent advances in CAR-T cells therapy for colorectal cancer. Front. Immunol. 2022;13:904137. doi: 10.3389/fimmu.2022.904137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kawalekar O.U., O’Connor R.S., Fraietta J.A., Guo L., McGettigan S.E., Posey A.D., Jr., Patel P.R., Guedan S., Scholler J., Keith B., et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016;44:380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 212.Guedan S., Madar A., Casado-Medrano V., Shaw C., Wing A., Liu F., Young R.M., June C.H., Posey A.D., Jr. Single residue in CD28-costimulated CAR-T cells limits long-term persistence and antitumor durability. J. Clin. Investig. 2020;130:3087–3097. doi: 10.1172/JCI133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zeng H., Cohen S., Guy C., Shrestha S., Neale G., Brown S.A., Cloer C., Kishton R.J., Gao X., Youngblood B., et al. mTORC1 and mTORC2 Kinase Signaling and Glucose Metabolism Drive Follicular Helper T Cell Differentiation. Immunity. 2016;45:540–554. doi: 10.1016/j.immuni.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sabharwal S.S., Rosen D.B., Grein J., Tedesco D., Joyce-Shaikh B., Ueda R., Semana M., Bauer M., Bang K., Stevenson C., et al. GITR Agonism Enhances Cellular Metabolism to Support CD8(+) T-cell Proliferation and Effector Cytokine Production in a Mouse Tumor Model. Cancer Immunol. Res. 2018;6:1199–1211. doi: 10.1158/2326-6066.CIR-17-0632. [DOI] [PubMed] [Google Scholar]
- 215.Pacella I., Procaccini C., Focaccetti C., Miacci S., Timperi E., Faicchia D., Severa M., Rizzo F., Coccia E.M., Bonacina F., et al. Fatty acid metabolism complements glycolysis in the selective regulatory T cell expansion during tumor growth. Proc. Natl. Acad. Sci. USA. 2018;115:E6546–E6555. doi: 10.1073/pnas.1720113115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kishton R.J., Sukumar M., Restifo N.P. Metabolic Regulation of T Cell Longevity and Function in Tumor Immunotherapy. Cell Metab. 2017;26:94–109. doi: 10.1016/j.cmet.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Leone R.D., Zhao L., Englert J.M., Sun I.M., Oh M.H., Sun I.H., Arwood M.L., Bettencourt I.A., Patel C.H., Wen J., et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366:1013–1021. doi: 10.1126/science.aav2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shen L., Xiao Y., Zhang C., Li S., Teng X., Cui L., Liu T., Wu N., Lu Z. Metabolic reprogramming by ex vivo glutamine inhibition endows CAR-T cells with less-differentiated phenotype and persistent antitumor activity. Cancer Lett. 2022;538:215710. doi: 10.1016/j.canlet.2022.215710. [DOI] [PubMed] [Google Scholar]
- 219.Sukumar M., Liu J., Ji Y., Subramanian M., Crompton J.G., Yu Z., Roychoudhuri R., Palmer D.C., Muranski P., Karoly E.D., et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Investig. 2013;123:4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Klebanoff C.A., Crompton J.G., Leonardi A.J., Yamamoto T.N., Chandran S.S., Eil R.L., Sukumar M., Vodnala S.K., Hu J., Ji Y., et al. Inhibition of AKT signaling uncouples T cell differentiation from expansion for receptor-engineered adoptive immunotherapy. JCI Insight. 2017;2:e95103. doi: 10.1172/jci.insight.95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Klein Geltink R.I., Edwards-Hicks J., Apostolova P., O’Sullivan D., Sanin D.E., Patterson A.E., Puleston D.J., Ligthart N.A.M., Buescher J.M., Grzes K.M., et al. Metabolic conditioning of CD8(+) effector T cells for adoptive cell therapy. Nat. Metab. 2020;2:703–716. doi: 10.1038/s42255-020-0256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gemta L.F., Siska P.J., Nelson M.E., Gao X., Liu X., Locasale J.W., Yagita H., Slingluff C.L., Jr., Hoehn K.L., Rathmell J.C., et al. Impaired enolase 1 glycolytic activity restrains effector functions of tumor-infiltrating CD8(+) T cells. Sci. Immunol. 2019;4:eaap9520. doi: 10.1126/sciimmunol.aap9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Philip M., Fairchild L., Sun L., Horste E.L., Camara S., Shakiba M., Scott A.C., Viale A., Lauer P., Merghoub T., et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature. 2017;545:452–456. doi: 10.1038/nature22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Scharping N.E., Menk A.V., Moreci R.S., Whetstone R.D., Dadey R.E., Watkins S.C., Ferris R.L., Delgoffe G.M. The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity. 2016;45:374–388. doi: 10.1016/j.immuni.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Siska P.J., Beckermann K.E., Mason F.M., Andrejeva G., Greenplate A.R., Sendor A.B., Chiang Y.J., Corona A.L., Gemta L.F., Vincent B.G., et al. Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI Insight. 2017;2:e93411. doi: 10.1172/jci.insight.93411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bengsch B., Johnson A.L., Kurachi M., Odorizzi P.M., Pauken K.E., Attanasio J., Stelekati E., McLane L.M., Paley M.A., Delgoffe G.M., et al. Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8(+) T Cell Exhaustion. Immunity. 2016;45:358–373. doi: 10.1016/j.immuni.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Patsoukis N., Bardhan K., Chatterjee P., Sari D., Liu B., Bell L.N., Karoly E.D., Freeman G.J., Petkova V., Seth P., et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lee M.J., Yun S.J., Lee B., Jeong E., Yoon G., Kim K., Park S. Association of TIM-3 expression with glucose metabolism in Jurkat T cells. BMC Immunol. 2020;21:48. doi: 10.1186/s12865-020-00377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tsurutani N., Mittal P., St Rose M.C., Ngoi S.M., Svedova J., Menoret A., Treadway F.B., Laubenbacher R., Suarez-Ramirez J.E., Cauley L.S., et al. Costimulation Endows Immunotherapeutic CD8 T Cells with IL-36 Responsiveness during Aerobic Glycolysis. J. Immunol. 2016;196:124–134. doi: 10.4049/jimmunol.1501217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Previte D.M., Martins C.P., O’Connor E.C., Marre M.L., Coudriet G.M., Beck N.W., Menk A.V., Wright R.H., Tse H.M., Delgoffe G.M., et al. Lymphocyte Activation Gene-3 Maintains Mitochondrial and Metabolic Quiescence in Naive CD4(+) T Cells. Cell Rep. 2019;27:129–141.e4. doi: 10.1016/j.celrep.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 231.Xie J., Wang X., Proud C.G. mTOR inhibitors in cancer therapy. F1000Res. 2016;5:2078. doi: 10.12688/f1000research.9207.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Jia W., Luo S., Guo H., Kong D. Development of PI3Kalpha inhibitors for tumor therapy. J. Biomol. Struct. Dyn. 2022:1–18. doi: 10.1080/07391102.2022.2132293. [DOI] [PubMed] [Google Scholar]
- 233.Kopf H., de la Rosa G.M., Howard O.M., Chen X. Rapamycin inhibits differentiation of Th17 cells and promotes generation of FoxP3+ T regulatory cells. Int. Immunopharmacol. 2007;7:1819–1824. doi: 10.1016/j.intimp.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Shi L.Z., Wang R., Huang G., Vogel P., Neale G., Green D.R., Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Siska P.J., van der Windt G.J., Kishton R.J., Cohen S., Eisner W., MacIver N.J., Kater A.P., Weinberg J.B., Rathmell J.C. Suppression of Glut1 and Glucose Metabolism by Decreased Akt/mTORC1 Signaling Drives T Cell Impairment in B Cell Leukemia. J. Immunol. 2016;197:2532–2540. doi: 10.4049/jimmunol.1502464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chapman N.M., Zeng H., Nguyen T.M., Wang Y., Vogel P., Dhungana Y., Liu X., Neale G., Locasale J.W., Chi H. mTOR coordinates transcriptional programs and mitochondrial metabolism of activated Treg subsets to protect tissue homeostasis. Nat. Commun. 2018;9:2095. doi: 10.1038/s41467-018-04392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nozhat Z., Mohammadi-Yeganeh S., Azizi F., Zarkesh M., Hedayati M. Effects of metformin on the PI3K/AKT/FOXO1 pathway in anaplastic thyroid Cancer cell lines. Daru. 2018;26:93–103. doi: 10.1007/s40199-018-0208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shao S., Zhao L., An G., Zhang L., Jing X., Luo M., Li W., Meng D., Ning Q., Zhao X., et al. Metformin suppresses HIF-1alpha expression in cancer-associated fibroblasts to prevent tumor-stromal cross talk in breast cancer. FASEB J. 2020;34:10860–10870. doi: 10.1096/fj.202000951RR. [DOI] [PubMed] [Google Scholar]
- 239.Pearce E.L., Walsh M.C., Cejas P.J., Harms G.M., Shen H., Wang L.S., Jones R.G., Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chao R., Nishida M., Yamashita N., Tokumasu M., Zhao W., Kudo I., Udono H. Nutrient Condition in the Microenvironment Determines Essential Metabolisms of CD8(+) T Cells for Enhanced IFNgamma Production by Metformin. Front. Immunol. 2022;13:864225. doi: 10.3389/fimmu.2022.864225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chen S., Zhou X., Yang X., Li W., Li S., Hu Z., Ling C., Shi R., Liu J., Chen G., et al. Dual Blockade of Lactate/GPR81 and PD-1/PD-L1 Pathways Enhances the Anti-Tumor Effects of Metformin. Biomolecules. 2021;11:1373. doi: 10.3390/biom11091373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chung Y.M., Khan P.P., Wang H., Tsai W.B., Qiao Y., Yu B., Larrick J.W., Hu M.C. Sensitizing tumors to anti-PD-1 therapy by promoting NK and CD8+ T cells via pharmacological activation of FOXO3. J. Immunother. Cancer. 2021;9:e002772. doi: 10.1136/jitc-2021-002772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Song C.W., Kim H., Cho H., Kim M.S., Paek S.H., Park H.J., Griffin R.J., Terezakis S., Cho L.C. HIF-1alpha Inhibition Improves Anti-Tumor Immunity and Promotes the Efficacy of Stereotactic Ablative Radiotherapy (SABR) Cancers. 2022;14:3273. doi: 10.3390/cancers14133273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mostafavi S., Zalpoor H., Hassan Z.M. The promising therapeutic effects of metformin on metabolic reprogramming of cancer-associated fibroblasts in solid tumors. Cell. Mol. Biol. Lett. 2022;27:58. doi: 10.1186/s11658-022-00356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pouyssegur J., Marchiq I., Parks S.K., Durivault J., Zdralevic M., Vucetic M. ‘Warburg effect’ controls tumor growth, bacterial, viral infections and immunity—Genetic deconstruction and therapeutic perspectives. Semin. Cancer Biol. 2022;86:334–346. doi: 10.1016/j.semcancer.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 246.Luo Y., Li Y., Huang Z., Li X., Wang Y., Hou J., Zhou S. A Nanounit Strategy Disrupts Energy Metabolism and Alleviates Immunosuppression for Cancer Therapy. Nano Lett. 2022;22:6418–6427. doi: 10.1021/acs.nanolett.2c02475. [DOI] [PubMed] [Google Scholar]
- 247.Xu R., Wu M., Liu S., Shang W., Li R., Xu J., Huang L., Wang F. Glucose metabolism characteristics and TLR8-mediated metabolic control of CD4(+) Treg cells in ovarian cancer cells microenvironment. Cell Death Dis. 2021;12:22. doi: 10.1038/s41419-020-03272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Guo D., Tong Y., Jiang X., Meng Y., Jiang H., Du L., Wu Q., Li S., Luo S., Li M., et al. Aerobic glycolysis promotes tumor immune evasion by hexokinase2-mediated phosphorylation of IkappaBalpha. Cell Metab. 2022;34:1312–1324.e6. doi: 10.1016/j.cmet.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 249.Wu L., Hollinshead K.E.R., Hao Y., Au C., Kroehling L., Ng C., Lin W.Y., Li D., Silva H.M., Shin J., et al. Niche-Selective Inhibition of Pathogenic Th17 Cells by Targeting Metabolic Redundancy. Cell. 2020;182:641–654.e20. doi: 10.1016/j.cell.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lei J., Yang Y., Lu Z., Pan H., Fang J., Jing B., Chen Y., Yin L. Taming metabolic competition via glycolysis inhibition for safe and potent tumor immunotherapy. Biochem. Pharmacol. 2022;202:115153. doi: 10.1016/j.bcp.2022.115153. [DOI] [PubMed] [Google Scholar]
- 251.Shan Y., Ni Q., Zhang Q., Zhang M., Wei B., Cheng L., Zhong C., Wang X., Wang Q., Liu J., et al. Targeting tumor endothelial hyperglycolysis enhances immunotherapy through remodeling tumor microenvironment. Acta Pharm. Sin. B. 2022;12:1825–1839. doi: 10.1016/j.apsb.2022.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Chen D.P., Ning W.R., Jiang Z.Z., Peng Z.P., Zhu L.Y., Zhuang S.M., Kuang D.M., Zheng L., Wu Y. Glycolytic activation of peritumoral monocytes fosters immune privilege via the PFKFB3-PD-L1 axis in human hepatocellular carcinoma. J. Hepatol. 2019;71:333–343. doi: 10.1016/j.jhep.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 253.Liu Y., Yan H., Gu H., Zhang E., He J., Cao W., Qu J., Xu R., Cao L., He D., et al. Myeloma-derived IL-32gamma induced PD-L1 expression in macrophages facilitates immune escape via the PFKFB3-JAK1 axis. Oncoimmunology. 2022;11:2057837. doi: 10.1080/2162402X.2022.2057837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yu Y., Liang Y., Li D., Wang L., Liang Z., Chen Y., Ma G., Wu H., Jiao W., Niu H. Glucose metabolism involved in PD-L1-mediated immune escape in the malignant kidney tumour microenvironment. Cell Death Discov. 2021;7:15. doi: 10.1038/s41420-021-00401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Palsson-McDermott E.M., Dyck L., Zaslona Z., Menon D., McGettrick A.F., Mills K.H.G., O’Neill L.A. Pyruvate Kinase M2 Is Required for the Expression of the Immune Checkpoint PD-L1 in Immune Cells and Tumors. Front. Immunol. 2017;8:1300. doi: 10.3389/fimmu.2017.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Xia Q., Jia J., Hu C., Lu J., Li J., Xu H., Fang J., Feng D., Wang L., Chen Y. Tumor-associated macrophages promote PD-L1 expression in tumor cells by regulating PKM2 nuclear translocation in pancreatic ductal adenocarcinoma. Oncogene. 2022;41:865–877. doi: 10.1038/s41388-021-02133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Xu W., Yang W., Wu C., Ma X., Li H., Zheng J. Enolase 1 Correlated with Cancer Progression and Immune-Infiltrating in Multiple Cancer Types: A Pan-Cancer Analysis. Front. Oncol. 2020;10:593706. doi: 10.3389/fonc.2020.593706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Cappello P., Tomaino B., Chiarle R., Ceruti P., Novarino A., Castagnoli C., Migliorini P., Perconti G., Giallongo A., Milella M., et al. An integrated humoral and cellular response is elicited in pancreatic cancer by alpha-enolase, a novel pancreatic ductal adenocarcinoma-associated antigen. Int. J. Cancer. 2009;125:639–648. doi: 10.1002/ijc.24355. [DOI] [PubMed] [Google Scholar]
- 259.Cappello P., Rolla S., Chiarle R., Principe M., Cavallo F., Perconti G., Feo S., Giovarelli M., Novelli F. Vaccination with ENO1 DNA prolongs survival of genetically engineered mice with pancreatic cancer. Gastroenterology. 2013;144:1098–1106. doi: 10.1053/j.gastro.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 260.Cappello P., Principe M., Bulfamante S., Novelli F. Alpha-Enolase (ENO1), a potential target in novel immunotherapies. Front. Biosci. (Landmark Ed.) 2017;22:944–959. doi: 10.2741/4526. [DOI] [PubMed] [Google Scholar]
- 261.Mandili G., Curcio C., Bulfamante S., Follia L., Ferrero G., Mazza E., Principe M., Cordero F., Satolli M.A., Spadi R., et al. In pancreatic cancer, chemotherapy increases antitumor responses to tumor-associated antigens and potentiates DNA vaccination. J. Immunother. Cancer. 2020;8:e001071. doi: 10.1136/jitc-2020-001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Curcio C., Brugiapaglia S., Bulfamante S., Follia L., Cappello P., Novelli F. The Glycolytic Pathway as a Target for Novel Onco-Immunology Therapies in Pancreatic Cancer. Molecules. 2021;26:1642. doi: 10.3390/molecules26061642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Chen M.L., Yuan T.T., Chuang C.F., Huang Y.T., Chung I.C., Huang W.C. A Novel Enolase-1 Antibody Targets Multiple Interacting Players in the Tumor Microenvironment of Advanced Prostate Cancer. Mol. Cancer Ther. 2022;21:1337–1347. doi: 10.1158/1535-7163.MCT-21-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hanker A.B., Sudhan D.R., Arteaga C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell. 2020;37:496–513. doi: 10.1016/j.ccell.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Reeves G.K., Beral V., Green J., Gathani T., Bull D., Million Women Study C. Hormonal therapy for menopause and breast-cancer risk by histological type: A cohort study and meta-analysis. Lancet Oncol. 2006;7:910–918. doi: 10.1016/S1470-2045(06)70911-1. [DOI] [PubMed] [Google Scholar]
- 266.Rugo H.S., Rumble R.B., Macrae E., Barton D.L., Connolly H.K., Dickler M.N., Fallowfield L., Fowble B., Ingle J.N., Jahanzeb M., et al. Endocrine Therapy for Hormone Receptor-Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J. Clin. Oncol. 2016;34:3069–3103. doi: 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 267.Pan H., Gray R., Braybrooke J., Davies C., Taylor C., McGale P., Peto R., Pritchard K.I., Bergh J., Dowsett M., et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N. Engl. J. Med. 2017;377:1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Fiorillo M., Sanchez-Alvarez R., Sotgia F., Lisanti M.P. The ER-alpha mutation Y537S confers Tamoxifen-resistance via enhanced mitochondrial metabolism, glycolysis and Rho-GDI/PTEN signaling: Implicating TIGAR in somatic resistance to endocrine therapy. Aging. 2018;10:4000–4023. doi: 10.18632/aging.101690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kulkoyluoglu-Cotul E., Arca A., Madak-Erdogan Z. Crosstalk between Estrogen Signaling and Breast Cancer Metabolism. Trends Endocrinol. Metab. 2019;30:25–38. doi: 10.1016/j.tem.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 270.Toy W., Shen Y., Won H., Green B., Sakr R.A., Will M., Li Z., Gala K., Fanning S., King T.A., et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 2013;45:1439–1445. doi: 10.1038/ng.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Li S., Shen D., Shao J., Crowder R., Liu W., Prat A., He X., Liu S., Hoog J., Lu C., et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep. 2013;4:1116–1130. doi: 10.1016/j.celrep.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Robinson D.R., Wu Y.M., Vats P., Su F., Lonigro R.J., Cao X., Kalyana-Sundaram S., Wang R., Ning Y., Hodges L., et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat. Genet. 2013;45:1446–1451. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ambrosio M.R., D’Esposito V., Costa V., Liguoro D., Collina F., Cantile M., Prevete N., Passaro C., Mosca G., De Laurentiis M., et al. Glucose impairs tamoxifen responsiveness modulating connective tissue growth factor in breast cancer cells. Oncotarget. 2017;8:109000–109017. doi: 10.18632/oncotarget.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Rivenzon-Segal D., Boldin-Adamsky S., Seger D., Seger R., Degani H. Glycolysis and glucose transporter 1 as markers of response to hormonal therapy in breast cancer. Int. J. Cancer. 2003;107:177–182. doi: 10.1002/ijc.11387. [DOI] [PubMed] [Google Scholar]
- 275.Zhang P., Yang Y., Qian K., Li L., Zhang C., Fu X., Zhang X., Chen H., Liu Q., Cao S., et al. A novel tumor suppressor ZBTB1 regulates tamoxifen resistance and aerobic glycolysis through suppressing HER2 expression in breast cancer. J. Biol. Chem. 2020;295:14140–14152. doi: 10.1074/jbc.RA119.010759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lv L., Yang S., Zhu Y., Zhai X., Li S., Tao X., Dong D. Relationship between metabolic reprogramming and drug resistance in breast cancer. Front. Oncol. 2022;12:942064. doi: 10.3389/fonc.2022.942064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Woo Y.M., Shin Y., Lee E.J., Lee S., Jeong S.H., Kong H.K., Park E.Y., Kim H.K., Han J., Chang M., et al. Inhibition of Aerobic Glycolysis Represses Akt/mTOR/HIF-1alpha Axis and Restores Tamoxifen Sensitivity in Antiestrogen-Resistant Breast Cancer Cells. PLoS ONE. 2015;10:e0132285. doi: 10.1371/journal.pone.0132285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Steifensand F., Gallwas J., Bauerschmitz G., Grundker C. Inhibition of Metabolism as a Therapeutic Option for Tamoxifen-Resistant Breast Cancer Cells. Cells. 2021;10:2398. doi: 10.3390/cells10092398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Chen Z., Wang Y., Warden C., Chen S. Cross-talk between ER and HER2 regulates c-MYC-mediated glutamine metabolism in aromatase inhibitor resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 2015;149:118–127. doi: 10.1016/j.jsbmb.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sengupta S., Sevigny C.M., Liu X., Jin L., Pohlmann P.R., Clarke R. Targeting glycolysis enzyme, PFKFB3, in endocrine therapy resistant breast cancers. Cancer Res. 2018;78:907. doi: 10.1158/1538-7445.AM2018-907. [DOI] [Google Scholar]
- 281.Jones B.C., Sengupta S., Sevigny C.M., Jin L., Pohlmann P.R., Shajahan-Haq A., Clarke R. Pfkfb3 inhibition significantly decreases endocrine-resistant breast cancer growth and induces necroptotic cell death; Proceedings of the San Antonio Breast Cancer Symposium; San Antonio, TX, USA. 7–10 December 2021. [Google Scholar]
- 282.Bacci M., Giannoni E., Fearns A., Ribas R., Gao Q., Taddei M.L., Pintus G., Dowsett M., Isacke C.M., Martin L.A., et al. miR-155 Drives Metabolic Reprogramming of ER+ Breast Cancer Cells Following Long-Term Estrogen Deprivation and Predicts Clinical Response to Aromatase Inhibitors. Cancer Res. 2016;76:1615–1626. doi: 10.1158/0008-5472.CAN-15-2038. [DOI] [PubMed] [Google Scholar]
- 283.Zuo Q., Mogol A.N., Liu Y.J., Santaliz Casiano A., Chien C., Drnevich J., Imir O.B., Kulkoyluoglu-Cotul E., Park N.H., Shapiro D.J., et al. Targeting Metabolic Adaptations in the Breast Cancer-Liver Metastatic Niche Using Dietary Approaches to Improve Endocrine Therapy Efficacy. Mol. Cancer Res. 2022;20:923–937. doi: 10.1158/1541-7786.MCR-21-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Blundon M.A., Dasgupta S. Metabolic Dysregulation Controls Endocrine Therapy-Resistant Cancer Recurrence and Metastasis. Endocrinology. 2019;160:1811–1820. doi: 10.1210/en.2019-00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Pertega-Gomes N., Felisbino S., Massie C.E., Vizcaino J.R., Coelho R., Sandi C., Simoes-Sousa S., Jurmeister S., Ramos-Montoya A., Asim M., et al. A glycolytic phenotype is associated with prostate cancer progression and aggressiveness: A role for monocarboxylate transporters as metabolic targets for therapy. J. Pathol. 2015;236:517–530. doi: 10.1002/path.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wang J., Xu W., Wang B., Lin G., Wei Y., Abudurexiti M., Zhu W., Liu C., Qin X., Dai B., et al. GLUT1 is an AR target contributing to tumor growth and glycolysis in castration-resistant and enzalutamide-resistant prostate cancers. Cancer Lett. 2020;485:45–55. doi: 10.1016/j.canlet.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 287.Gonzalez-Menendez P., Hevia D., Alonso-Arias R., Alvarez-Artime A., Rodriguez-Garcia A., Kinet S., Gonzalez-Pola I., Taylor N., Mayo J.C., Sainz R.M. GLUT1 protects prostate cancer cells from glucose deprivation-induced oxidative stress. Redox Biol. 2018;17:112–127. doi: 10.1016/j.redox.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Gonzalez-Menendez P., Hevia D., Mayo J.C., Sainz R.M. The dark side of glucose transporters in prostate cancer: Are they a new feature to characterize carcinomas? Int. J. Cancer. 2018;142:2414–2424. doi: 10.1002/ijc.31165. [DOI] [PubMed] [Google Scholar]
- 289.Cui Y., Nadiminty N., Liu C., Lou W., Schwartz C.T., Gao A.C. Upregulation of glucose metabolism by NF-kappaB2/p52 mediates enzalutamide resistance in castration-resistant prostate cancer cells. Endocr. Relat. Cancer. 2014;21:435–442. doi: 10.1530/ERC-14-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Efstathiou E., Titus M., Wen S., Hoang A., Karlou M., Ashe R., Tu S.M., Aparicio A., Troncoso P., Mohler J., et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur. Urol. 2015;67:53–60. doi: 10.1016/j.eururo.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Atif F., Yousuf S., Stein D.G. Anti-tumor effects of progesterone in human glioblastoma multiforme: Role of PI3K/Akt/mTOR signaling. J. Steroid Biochem. Mol. Biol. 2015;146:62–73. doi: 10.1016/j.jsbmb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 292.Agostinis P., Berg K., Cengel K.A., Foster T.H., Girotti A.W., Gollnick S.O., Hahn S.M., Hamblin M.R., Juzeniene A., Kessel D., et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Cengel K.A., Simone C.B., 2nd, Glatstein E. PDT: What’s Past Is Prologue. Cancer Res. 2016;76:2497–2499. doi: 10.1158/0008-5472.CAN-16-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Hamblin M.R. Photodynamic Therapy for Cancer: What’s Past is Prologue. Photochem. Photobiol. 2020;96:506–516. doi: 10.1111/php.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 296.Ogura S., Maruyama K., Hagiya Y., Sugiyama Y., Tsuchiya K., Takahashi K., Abe F., Tabata K., Okura I., Nakajima M., et al. The effect of 5-aminolevulinic acid on cytochrome c oxidase activity in mouse liver. BMC Res. Notes. 2011;4:66. doi: 10.1186/1756-0500-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Hara T., Koda A., Nozawa N., Ota U., Kondo H., Nakagawa H., Kamiya A., Miyashita K., Itoh H., Nakajima M., et al. Combination of 5-aminolevulinic acid and ferrous ion reduces plasma glucose and hemoglobin A1c levels in Zucker diabetic fatty rats. FEBS Open Bio. 2016;6:515–528. doi: 10.1002/2211-5463.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Grigalavicius M., Ezzatpanah S., Papakyriakou A., Raabe T.T.H., Yannakopoulou K., Theodossiou T.A. 5-ALA Is a Potent Lactate Dehydrogenase Inhibitor but Not a Substrate: Implications for Cell Glycolysis and New Avenues in 5-ALA-Mediated Anticancer Action. Cancers. 2022;14:4003. doi: 10.3390/cancers14164003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kaur P., Nagar S., Bhagwat M., Uddin M., Zhu Y., Vancurova I., Vancura A. Activated heme synthesis regulates glycolysis and oxidative metabolism in breast and ovarian cancer cells. PLoS ONE. 2021;16:e0260400. doi: 10.1371/journal.pone.0260400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Simcox J.A., Mitchell T.C., Gao Y., Just S.F., Cooksey R., Cox J., Ajioka R., Jones D., Lee S.H., King D., et al. Dietary iron controls circadian hepatic glucose metabolism through heme synthesis. Diabetes. 2015;64:1108–1119. doi: 10.2337/db14-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Sato T., Yasuzawa T., Uesaka A., Izumi Y., Kamiya A., Tsuchiya K., Kobayashi Y., Kuwahata M., Kido Y. Type 2 diabetic conditions in Otsuka Long-Evans Tokushima Fatty rats are ameliorated by 5-aminolevulinic acid. Nutr. Res. 2014;34:544–551. doi: 10.1016/j.nutres.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 302.Handschin C., Lin J., Rhee J., Peyer A.K., Chin S., Wu P.H., Meyer U.A., Spiegelman B.M. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alpha. Cell. 2005;122:505–515. doi: 10.1016/j.cell.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 303.McNicholas K., MacGregor M.N., Gleadle J.M. In order for the light to shine so brightly, the darkness must be present-why do cancers fluoresce with 5-aminolaevulinic acid? Br. J. Cancer. 2019;121:631–639. doi: 10.1038/s41416-019-0516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Golding J.P., Wardhaugh T., Patrick L., Turner M., Phillips J.B., Bruce J.I., Kimani S.G. Targeting tumour energy metabolism potentiates the cytotoxicity of 5-aminolevulinic acid photodynamic therapy. Br. J. Cancer. 2013;109:976–982. doi: 10.1038/bjc.2013.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Nakano A., Tsuji D., Miki H., Cui Q., El Sayed S.M., Ikegame A., Oda A., Amou H., Nakamura S., Harada T., et al. Glycolysis inhibition inactivates ABC transporters to restore drug sensitivity in malignant cells. PLoS ONE. 2011;6:e27222. doi: 10.1371/journal.pone.0027222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Hagiya Y., Fukuhara H., Matsumoto K., Endo Y., Nakajima M., Tanaka T., Okura I., Kurabayashi A., Furihata M., Inoue K., et al. Expression levels of PEPT1 and ABCG2 play key roles in 5-aminolevulinic acid (ALA)-induced tumor-specific protoporphyrin IX (PpIX) accumulation in bladder cancer. Photodiagnosis Photodyn. Ther. 2013;10:288–295. doi: 10.1016/j.pdpdt.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 307.Kobuchi H., Moriya K., Ogino T., Fujita H., Inoue K., Shuin T., Yasuda T., Utsumi K., Utsumi T. Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid-mediated protoporphyrin IX accumulation. PLoS ONE. 2012;7:e50082. doi: 10.1371/journal.pone.0050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Alkarakooly Z., Al-Anbaky Q.A., Kannan K., Ali N. Metabolic reprogramming by Dichloroacetic acid potentiates photodynamic therapy of human breast adenocarcinoma MCF-7 cells. PLoS ONE. 2018;13:e0206182. doi: 10.1371/journal.pone.0206182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kiesslich T., Plaetzer K., Oberdanner C.B., Berlanda J., Obermair F.J., Krammer B. Differential effects of glucose deprivation on the cellular sensitivity towards photodynamic treatment-based production of reactive oxygen species and apoptosis-induction. FEBS Lett. 2005;579:185–190. doi: 10.1016/j.febslet.2004.11.073. [DOI] [PubMed] [Google Scholar]
- 310.Jiang F., Lilge L., Belcuig M., Singh G., Grenier J., Li Y., Chopp M. Photodynamic therapy using Photofrin in combination with buthionine sulfoximine (BSO) to treat 9L gliosarcoma in rat brain. Lasers Surg. Med. 1998;23:161–166. doi: 10.1002/(SICI)1096-9101(1998)23:3<161::AID-LSM5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 311.Perotti C., Casas A., Del C.B.A.M. Scavengers protection of cells against ALA-based photodynamic therapy-induced damage. Lasers Med. Sci. 2002;17:222–229. doi: 10.1007/s101030200033. [DOI] [PubMed] [Google Scholar]
- 312.Mascaraque-Checa M., Gallego-Rentero M., Nicolas-Morala J., Portillo-Esnaola M., Cuezva J.M., Gonzalez S., Gilaberte Y., Juarranz A. Metformin overcomes metabolic reprogramming-induced resistance of skin squamous cell carcinoma to photodynamic therapy. Mol. Metab. 2022;60:101496. doi: 10.1016/j.molmet.2022.101496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Xie R., Xu T., Zhu J., Wei X., Zhu W., Li L., Wang Y., Han Y., Zhou J., Bai Y. The Combination of Glycolytic Inhibitor 2-Deoxyglucose and Microbubbles Increases the Effect of 5-Aminolevulinic Acid-Sonodynamic Therapy in Liver Cancer Cells. Ultrasound Med. Biol. 2017;43:2640–2650. doi: 10.1016/j.ultrasmedbio.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 314.Feng X., Zhang Y., Wang P., Liu Q., Wang X. Energy metabolism targeted drugs synergize with photodynamic therapy to potentiate breast cancer cell death. Photochem. Photobiol. Sci. 2014;13:1793–1803. doi: 10.1039/c4pp00288a. [DOI] [PubMed] [Google Scholar]
- 315.Yang X., Palasuberniam P., Myers K.A., Wang C., Chen B. Her2 oncogene transformation enhances 5-aminolevulinic acid-mediated protoporphyrin IX production and photodynamic therapy response. Oncotarget. 2016;7:57798–57810. doi: 10.18632/oncotarget.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zhong G., Li Q., Luo Y., Liu Y., Liu D., Li B., Wang T. FECH Expression Correlates with the Prognosis and Tumor Immune Microenvironment in Clear Cell Renal Cell Carcinoma. J. Oncol. 2022;2022:8943643. doi: 10.1155/2022/8943643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Chelakkot V.S., Liu K., Yoshioka E., Saha S., Xu D., Licursi M., Dorward A., Hirasawa K. MEK reduces cancer-specific PpIX accumulation through the RSK-ABCB1 and HIF-1alpha-FECH axes. Sci. Rep. 2020;10:22124. doi: 10.1038/s41598-020-79144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Yoshioka E., Chelakkot V.S., Licursi M., Rutihinda S.G., Som J., Derwish L., King J.J., Pongnopparat T., Mearow K., Larijani M., et al. Enhancement of Cancer-Specific Protoporphyrin IX Fluorescence by Targeting Oncogenic Ras/MEK Pathway. Theranostics. 2018;8:2134–2146. doi: 10.7150/thno.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kierans S.J., Taylor C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. Physiol. 2021;599:23–37. doi: 10.1113/JP280572. [DOI] [PubMed] [Google Scholar]
- 320.Franklin D.A., Sharick J.T., Ericsson-Gonzalez P.I., Sanchez V., Dean P.T., Opalenik S.R., Cairo S., Judde J.G., Lewis M.T., Chang J.C., et al. MEK activation modulates glycolysis and supports suppressive myeloid cells in TNBC. JCI Insight. 2020;5:e134290. doi: 10.1172/jci.insight.134290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. doi: 10.1126/science.124.3215.269. [DOI] [PubMed] [Google Scholar]
- 322.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 323.Yang M., Soga T., Pollard P.J. Oncometabolites: Linking altered metabolism with cancer. J. Clin. Investig. 2013;123:3652–3658. doi: 10.1172/JCI67228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Vasan K., Werner M., Chandel N.S. Mitochondrial Metabolism as a Target for Cancer Therapy. Cell Metab. 2020;32:341–352. doi: 10.1016/j.cmet.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Vander Heiden M.G. Targeting cancer metabolism: A therapeutic window opens. Nat. Rev. Drug Discov. 2011;10:671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 326.Groves A.M., Win T., Haim S.B., Ell P.J. Non-[18F] FDG PET in clinical oncology. Lancet Oncol. 2007;8:822–830. doi: 10.1016/S1470-2045(07)70274-7. [DOI] [PubMed] [Google Scholar]
- 327.Zhang Y., Li Q., Huang Z., Li B., Nice E.C., Huang C., Wei L., Zou B. Targeting Glucose Metabolism Enzymes in Cancer Treatment: Current and Emerging Strategies. Cancers. 2022;14:4568. doi: 10.3390/cancers14194568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Zdralevic M., Brand A., Di Ianni L., Dettmer K., Reinders J., Singer K., Peter K., Schnell A., Bruss C., Decking S.M., et al. Double genetic disruption of lactate dehydrogenases A and B is required to ablate the “Warburg effect” restricting tumor growth to oxidative metabolism. J. Biol. Chem. 2018;293:15947–15961. doi: 10.1074/jbc.RA118.004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Boudreau A., Purkey H.E., Hitz A., Robarge K., Peterson D., Labadie S., Kwong M., Hong R., Gao M., Del Nagro C., et al. Metabolic plasticity underpins innate and acquired resistance to LDHA inhibition. Nat. Chem. Biol. 2016;12:779–786. doi: 10.1038/nchembio.2143. [DOI] [PubMed] [Google Scholar]
- 330.Stine Z.E., Schug Z.T., Salvino J.M., Dang C.V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 2022;21:141–162. doi: 10.1038/s41573-021-00339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Cassim S., Vucetic M., Zdralevic M., Pouyssegur J. Warburg and Beyond: The Power of Mitochondrial Metabolism to Collaborate or Replace Fermentative Glycolysis in Cancer. Cancers. 2020;12:1119. doi: 10.3390/cancers12051119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Noble R.A., Thomas H., Zhao Y., Herendi L., Howarth R., Dragoni I., Keun H.C., Vellano C.P., Marszalek J.R., Wedge S.R. Simultaneous targeting of glycolysis and oxidative phosphorylation as a therapeutic strategy to treat diffuse large B-cell lymphoma. Br. J. Cancer. 2022;127:937–947. doi: 10.1038/s41416-022-01848-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Hay N. Reprogramming glucose metabolism in cancer: Can it be exploited for cancer therapy? Nat. Rev. Cancer. 2016;16:635–649. doi: 10.1038/nrc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Yang L., Yan X., Chen J., Zhan Q., Hua Y., Xu S., Li Z., Wang Z., Dong Y., Zuo D., et al. Hexokinase 2 discerns a novel circulating tumor cell population associated with poor prognosis in lung cancer patients. Proc. Natl. Acad. Sci. USA. 2021;118:e2012228118. doi: 10.1073/pnas.2012228118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.