Abstract
The progressive loss of skeletal muscle mass and concomitant reduction in contractile strength plays a central role in frailty syndrome. Age-related neuronal impairments are closely associated with sarcopenia in the elderly, which is characterized by severe muscular atrophy that can considerably lessen the overall quality of life at old age. Mass-spectrometry-based proteomic surveys of senescent human skeletal muscles, as well as animal models of sarcopenia, have decisively improved our understanding of the molecular and cellular consequences of muscular atrophy and associated fiber-type shifting during aging. This review outlines the mass spectrometric identification of proteome-wide changes in atrophying skeletal muscles, with a focus on contractile proteins as potential markers of changes in fiber-type distribution patterns. The observed trend of fast-to-slow transitions in individual human skeletal muscles during the aging process is most likely linked to a preferential susceptibility of fast-twitching muscle fibers to muscular atrophy. Studies with senescent animal models, including mostly aged rodent skeletal muscles, have confirmed fiber-type shifting. The proteomic analysis of fast versus slow isoforms of key contractile proteins, such as myosin heavy chains, myosin light chains, actins, troponins and tropomyosins, suggests them as suitable bioanalytical tools of fiber-type transitions during aging.
Keywords: actin, aging, atrophy, frailty, myosin, sarcomere, sarcopenia, tropomyosin, troponin
1. Introduction
The loss of skeletal muscle mass and contractile strength can be induced by the lack of suitable physical activity levels, extended periods of disuse or disease [1,2,3]. Acute forms of skeletal muscle wasting are often observed during physical trauma and sepsis [4]. Many chronic conditions are also associated with muscular atrophy, including cancer cachexia, congestive heart failure, diabetes mellitus, chronic obstructive pulmonary disease, glucocorticoid-induced Cushing syndrome, malnutrition, long-lasting infections, acquired immunodeficiency syndrome and kidney failure [5,6,7]. Chronic diseases triggering motor neuron abnormalities, such as amyotrophic lateral sclerosis, are a major clinical cause of muscular atrophy [8]. However, the most common form of contractile fiber wasting in association with muscular atrophy is represented by systemic changes during sarcopenia of old age [9,10,11].
Atrophying skeletal muscles are a major feature of the aging phenotype in humans [12], and often the degree of contractile weakness is even more pronounced than the extent of lost muscle mass [13,14,15]. Sarcopenia of old age is closely connected to frailty [16], as well as an increased frequency of falls and fractures [17,18,19], resulting in a drastically reduced quality of life in the elderly [20] that are affected by substantial skeletal muscle wasting [21]. Reduced skeletal muscle tissue mass in conjunction with low gait speed are typical indicators of sarcopenia [22], whereby the clinical definition of sarcopenia [23] relates to a significantly reduced percentage of muscle tissue quantity and/or quality as compared to the mean determined in younger and healthy adults of similar ethnic background and the same gender [24]. Variations in contractile strength due to aging can be conveniently determined by a variety of performance tests that evaluate physical parameters such as walking ability, gait speed, grip strength, standing capability and stair climbing [25,26,27]. However, the histo-morphometric characterization of the aging human musculature indicates significant differences in the degree of the structural decline in individual skeletal muscles [28].
Thus, for a deeper mechanistic understanding of the aging process and frailty syndrome, it is crucial to study aging-related changes at the level of systems biology [29,30], including the role of cellular stress, mitochondrial abnormalities, disturbed ion handling, impaired protein metabolism and epigenetic changes that may adversely affect tissue integrity and thus cause disturbed bioenergetic pathways, abnormal proteostasis, hormonal imbalances, impaired ion homeostasis and reduced neuromuscular activity [31,32,33]. The degree to which reduced fitness and higher risk for disease acquisition in association with somatic damage accumulation trigger cellular aging is intensely debated, versus the effects of adaptive processes on the development of senescence [34,35,36]. Since a variety of factors play a crucial role in promoting frailty, it is important to better understand the interplay between the dysregulation of central biochemical pathways, cellular signaling cascades and physiological systems [37]. This might lead to a more comprehensive idea of how a combination of chronic inflammation, metabolic syndrome, visceral obesity, insulin resistance, neurodegeneration and progressive skeletal muscle wasting negatively affects the general health status of the elderly [38].
Of central importance for muscle biogerontology is the determination of proteome-wide alterations in the aged organism [39,40] and the application of this biochemical knowledge to improve the treatment of frailty and muscular atrophy [41,42,43]. Proteomics is a key technology of modern biosciences [44] and crucial for advances in pharmacological research and biotechnology [45], as well as biomarker discovery [46]. Mass spectrometry is an ideal bioanalytical method for studying the molecular and cellular mechanisms that underlie normal physiological and biochemical processes, adaptive responses to changed functional demands and dysregulated mechanisms in the diseased state [47]. This includes biomolecular investigations into the multi-factorial triggering mechanisms involved in the general aging process of humans [48], and particularly frailty syndrome in the elderly [49].
This review summarizes the findings of major proteomic surveys of aged human skeletal muscles and relates them to the analysis of animal models of sarcopenia of old age. The main focus is on the mass spectrometric identification of contractile proteins as potential markers of muscle-fiber-type shifting [50,51,52]. Following an overview of proteomics as a highly useful bioanalytical tool to study skeletal muscle biology, this article discusses how biochemical and proteomic knowledge might be helpful to better understand the complexity of the neuromuscular aging process. The outline of the methodological approaches includes a description of the importance of two-dimensional gel electrophoresis for top-down proteomics, various antibody-based techniques, sample preparation for proteomic analysis, protein digestion for peptide mass spectrometry, key mass spectrometric methods, data acquisition for mass spectrometry and recent developments in single-cell proteomics and aptamer-based proteomics. The described biochemical surveys of fast versus slow isoforms of myosin, actin, troponin and tropomyosin suggest that isoform switching of these abundant muscle proteins is a suitable and robust process that can be utilized for muscle-fiber typing during age-related muscular atrophy. The final section of this review briefly summarizes the main factors that are involved in age-related muscular atrophy and associated fast-to-slow fiber-type shifting, and describes recent progress in biomarker discovery for monitoring muscle aging and the development of novel therapeutic approaches to treat sarcopenia of old age.
2. Proteomic Profiling of Skeletal Muscle Tissues
2.1. Proteomic Analysis Platforms and Associated Biochemical and Cell Biological Methodology
Following the establishment of the concept of the proteome [53] and mass-spectrometry-based proteomics as a highly useful screening tool in the modern biosciences [54], there has been a steady improvement of sample preparation, mass spectrometric instrumentation and data analysis pipelines using both bottom-up [55,56,57] and top-down proteomic techniques [58,59,60]. Importantly, modern biochemical analyses focus on the unifying concept of dynamic proteoforms being the basic units of protein activity [61,62,63]. This has given unprecedented insights into protein diversity and the role of proteins in cellular functions [64], including skeletal muscle tissues [65,66,67,68]. Advances in the field of proteomics now allow researchers to comprehensively study proteins expressed by an organism or biological system associated with physiological and pathophysiological phenotypes [44]. High-throughput technologies and more precision-based methodologies are now available to identify proteins and their modifications in complex samples [69,70,71,72]. This wide-ranging approach provides a solid platform to understand protein function in a particular biological pathway, and when perturbed, how this affects the biological system [73]. Consequently, proteomics has major applications in medicine and drug development [45,46,47]. The international HUPO Project has made enormous progress in establishing and cataloguing the highly dynamic human proteome [74,75,76], which forms the scientific basis of understanding protein homeostasis at the level of systems biology [77,78,79].
2.1.1. Two-Dimensional Gel Electrophoresis
Two-dimensional gel electrophoresis (2D-GE) is a classic and commonly used method for proteome analysis [80,81,82] and presents an ideal bioanalytical approach for optimum protein separation prior to the systematic mass spectrometric profiling of proteoforms [61]. Although current proteomic analyses use mostly gel-free systems for the initial protein separation step, 2D-GE has not been superseded by chromatographical techniques for specialized applications in top-down proteomics [80,82]. 2D-GE is still a highly useful protein separation method that plays a key role in many proteomics analysis pipelines that focus on the identification and characterization of isolated and intact proteoforms [52,61]. The 2D-GE-based separation step is especially beneficial in the field of applied myology for analyzing the highly diverse array of isoforms of contractile proteins [65,66,67]. The large-scale survey of skeletal muscle proteins can be carried out under both native or denaturing conditions [66], including the thorough separation of key contractile proteins [52]. In the most frequently employed version of the 2D-GE technique, mixtures of proteins are separated by charge (based on the isoelectric point, pI, of individual proteins) in the first dimension, and by sodium dodecyl sulfate polyacrylamide slab gel electrophoresis (SDS-PAGE), which discriminates proteins based on their molecular weight, in the second dimension [83,84,85]. This approach can be used to separate several thousand different proteins on one 2D-gel [86,87,88]. Of note, the recently described micro-needling of the first-dimension gel can be used to considerably shorten the time requirements for the initial isoelectric focusing step in 2D-GE [89].
Most 2D-GE approaches are based on the usage of high concentrations of sodium dodecyl sulfate (SDS) for optimum solubilization of proteins in the second dimension [90,91], but 2D-GE can also be carried out with combinations of alternative detergents to increase the resolution of integral membrane proteins [92]. For example, a combination of the cationic detergent named benzyldimethyl-n-hexadecylammonium chloride (BAC) in the first dimension and SDS detergent in the second dimension is used for the BAC/SDS-PAGE technique [93]. Two-dimensional blue native polyacrylamide gel electrophoresis, usually referred to as BN-PAGE [94], separates proteins under native conditions [95] and is frequently used to characterize large protein assemblies in mitochondria [96,97,98]. Natural or modified differences between skeletal muscle protein species or protein complexes can be conveniently examined by diagonal non-reducing/reducing 2D-GE following chemical cross-linking [99,100,101]. Following 2D-GE, the next steps typically involve protein spot visualization, using highly sensitive stains, such as Coomassie brilliant blue (CBB) [102], that enable femtomole detection levels of gel-separated and intact proteoforms [103]. Other routinely employed methods for protein spot visualization use silver staining or fluorescent dyes such as SYPRO Ruby or Deep Purple [104,105,106].
This is then followed by protein spot abundance analysis and, finally, protein identification by mass spectrometry [107]. In skeletal muscle proteomics, a variety of extremely large myofibrillar and cytoskeletal proteins are difficult to separate by conventional 2D-GE. This includes the giant proteins dystrophin, nebulin, obscurin and titin [108]. To overcome this technical issue, additional analyses can be carried out with a technique complementary to 2D-GE that uses 3–12% gradient 1D-GE in combination with LC-MS [109]. Findings from GeLC-MS/MS can be blended with the results from proteomic surveys employing 2D-GE and can result in more comprehensive insights into the biochemical status of skeletal muscle proteins in the 200–3500 kDa range [110]. Multidimensional protein identification technology (MudPIT) can also be used in conjunction with 2D-GE. Since MudPIT is not based on gel technology for protein separation [111], 2D liquid chromatography prior to MS analysis can add additional proteomic data than might not be as easily assessable by conventional gel electrophoresis [112].
Despite the large number of diverse 2D-GE applications, one significant disadvantage is related to the need to run large numbers of gels, each separating proteins from an individual sample. This limitation was overcome by the development of the difference gel electrophoresis (DIGE) approach [113,114,115]. This technique uses fluorescent cyanine dyes, which are covalently bound to proteins within the samples before the 2D-GE separation begins [116]. The dyes (CyDye Cy2, Cy3 and Cy5) are mass- and charge-matched, but have distinct excitation and emission spectra, allowing for independent signals from the differentially labelled protein populations to be captured [117]. Two different dyes are available: for normal applications, minimal dyes (NHS ester dyes) are used to label lysine residues, and for scarce amounts of sample, saturation dyes (maleimide dyes) are used to label cysteine residues [118,119,120]. Within the DIGE experimental set-up, an internal standard is used (conventionally CyDye Cy2 for minimal dyes and CyDye Cy3 for saturation dyes) [121]. The internal standard can be used to match and normalize the protein quantities across samples [122].
Various software packages, including DeCyder, SameSpots and Dymension 3, can be used for the determination of protein spot intensity [123,124,125]. The development of DIGE introduced several advantages for using this research platform in protein analysis and has been modified to also study native protein interactions and post-translational modifications [126,127,128]. Limitations still exist with respect to detecting/resolving low-abundant and hydrophobic proteins, proteins with a molecular mass of <10 kDa or >150 kDa and proteins with an extreme isoelectric point [129]. A significant area where protein separation is based on 2D-GE is the analysis of proteoforms [61], i.e., different molecular forms of a protein product of a single gene that are generated due to alternative mRNA splicing, the activity of more than one promoter per protein-coding gene and/or post-translational proteolysis [130]. As distinct proteoforms may increase or decrease in pathophysiological conditions, the ability to distinguish and quantitate proteoforms is an important consideration when designing an experimental approach [62].
2.1.2. Antibody-Based Methodology
Antibodies are perhaps the most frequently used and adapted detectors in biological research, including applications in studies of protein expression, protein interactions, cellular pathways and post-translational modifications (PTMs). Labelled antibodies are a fundamental component of experimental procedures, including immunoblotting [131], immunohistochemistry (IHC) [132], immunofluorescence microscopy (IFM) [133], enzyme-linked immunosorbent assays (ELISA) [134], flow cytometry (FC) [135], fluorescence-activated cell sorting (FACS) [136], mass cytometry (CyTOF) [137] and immunocapture mass spectrometry [138], due to their target specificity and high affinity for specific epitopes.
The most frequently used method to independently verify the mass spectrometric detection of abundance changes in a distinct protein is immunoblotting [139], besides using IHC/IFM methods [140] and enzyme assays [141,142] for the further characterization of proteomic hits. Considerable technological advances have emerged in Western blotting over the years [143]. This has revolved around the introduction of fluorescent-dye conjugated secondary antibodies and the associated ability to multiplex. Using imaging systems to capture the fluorescent signal, researchers can now develop chemiluminescent or fluorescent blots at the bench side. Enhancements to chemiluminescent reagents have made it possible to detect even femtogram amounts of protein, increasing the sensitivity of this approach [139,143].
Traditional IHC is commonly used as a technique that assists pathologists in making careful decisions regarding differential diagnosis, disease subtyping and designing personalized treatment plans [144], and plays a key role in the evaluation of skeletal muscle biopsy specimens [145]. IHC and IFM techniques are also used for verification studies in proteomics [133,140]. However, this methodological approach has several limitations, including a high level of inter-observer variability amongst pathologists and the ability to evaluate only one antigen per tissue section. As a result of these limitations, multiplex immunohistochemistry/immunofluorescence (mIHC/IF) technologies, which utilize chromogen-based immuno-detection and antibody stripping chemistry, are now being utilized in both research and clinical settings [146]. The benefits of this platform include increased automation, tissue sparing and cost-effective analysis, as multiple biomarkers can be evaluated on a single formalin-fixed, paraffin-embedded (FFPE) tissue slide [147].
Single-plex ELISA tests allow the sensitive and specific detection of various analytes in complex biological samples, such as serum/plasma in clinical and research laboratories, facilitating the diagnosis of diseases and identification of new therapeutic targets [134]. As with recent developments in IHC-associated technologies, there is an increasing requirement for multiplex ELISAs that are capable of obtaining large amounts of data from a limited amount of starting material [148]. Multiplex ELISAs have many advantages over single-plex ELISAs, including increased efficiency, higher throughput and an increase in the number of analytes detected and quantitated [149,150]. Typically, two types of multiplex immunoassays are routinely used; namely, planar and suspension arrays [151]. In planar microarrays, individual capture ligands are immobilized in a microarray format containing potentially several hundred spots and incubated with sample, and then subsequent fluorescent or chemiluminescent signals are detected. In suspension assays, the capture ligands are immobilized onto color-, shape-, or size-coded microspheres. These characteristics are then used to identify the specific analytes that are captured on the bead surface, with quantitation based on the detection of associated reporter molecules, including chemiluminescent or fluorescent signals. Pereira et al. [152] used multiplex ELISAs to investigate oral nutritional supplements enriched with protein, vitamin D and β-hydroxy β-methylbutyrate compared to a control group in serum samples from malnourished sarcopenic older adults. Sixteen biomarkers were found to be significantly changed in response to the supplement, including a decrease in abundance for the inflammation-related ferritin and osteopontin, and an increase in soluble receptors for cytokines, indicating decreased inflammation. To increase the sensitivity of typical multiplex ELISAs, Proximity Extension Assay (PEA) technology has been established. Specific proteins are targeted with a pair of antibodies that are labelled with DNA oligonucleotides, which are hybridized and extended by a DNA polymerase. The DNA barcode that is produced is amplified and quantified using real-time polymerase chain reaction [153].
FC and FACS analysis are partner technologies in the cell analysis process. FC is used for cell analysis and measuring protein expression or co-expression within a heterogeneous population of cells [135]. FACS is used as a cell sorter and enrichment of a subset of cells for subsequent analysis [136]. Recent improvements in FC and FACS have focused on the extension of fluorescent labels (UV and IR range), and novel tandem dyes, allowing for greater multiplexing capabilities. To determine the associations between % circulating osteoprogenitor (COP) cells and sarcopenia, Al Saedi et al. [154] used FC to quantify % COP cells by using selective gating of CD45/osteocalcin (OCN) + cells. Their finding implicates that high levels of % COP cells are associated with better skeletal muscle function when investigating debilitating muscle aging as defined using the Sarcopenia Definitions and Outcomes Consortium (SDOC) criteria [155].
CyTOF, or mass cytometry, uses molecularly tagged antibodies to detect and quantitate specific cellular antigens, allowing for highly multiplexed assays [156]. Heavy-metal isotopic tags, rather than fluorophores, are used to label antibodies, with an increasing number of panels now available for use. Cells are incubated with a mixture of tagged antibodies (non-radioactive heavy metal isotopes) and nebulized, with each droplet containing an individual single cell, and subsequent ionization of the sample [157]. The liberated cloud of ions is subjected to MS-based filtering which selects for the isotope-conjugated probes. In the Time-of-Flight (TOF) chamber, the ions are separated by their mass-to-charge ratio and converted into electrical signals, providing information on the abundance of the specific tagged analytes [137]. CyTOF does suffer from several limitations, including the reduced sensitivity of metal-isotope-tagged antibodies and the longer acquisition times needed when using TOF–MS instruments. However, with these limitations being identified, there is massive scope for advances in these areas that will contribute to increasing the research possibilities of CyTOF in the future of skeletal muscle research [158,159,160]. Recently, Porpiglia et al. [161] used CyTOF to study muscle stem cells (MuSCs) in the aged phenotype and showed high CD47 expression levels, which might be associated with dysfunctional MuSCs, and an impaired regenerative capacity.
In addition, the use of antibodies plays an important role in the affinity precipitation of post-translationally modified peptides prior to MS analysis [162]. Peptides containing a specific modification (such as phosphorylation, acetylation, methylation or ubiquitination), are enriched from protease-digested lysates using an antibody against the specific modification [163,164]. This approach facilitates the identification and quantitation of hundreds to thousands of modified peptides in a single MS run.
2.1.3. Sample Preparation for Proteomic Analysis
The proteomic analysis of skeletal muscle samples is routinely performed with both crude total extracts or subcellular fractions [165]. Subsets of organelles or enriched protein complexes can be isolated by differential centrifugation, density gradient ultracentrifugation, affinity isolation methods or chemical crosslinking approaches [166,167,168]. Optimum protein extraction for subsequent digestion and MS analysis can be carried out by a variety of standardized sample preparation methods [169,170,171]. The filter-aided sample preparation (FASP) technique is ideal for efficient buffer exchange and the removal of MS-incompatible detergents [172]. For designing an optimized proteomic analysis pipeline, it is important to take into account the biological properties of the starting material, such as individual cells, complex tissues or biofluids, and whether a top-down or bottom-up proteomic approach is needed for studying the proteins of interest [58]. Total protein extracts from tough skeletal muscle tissue samples can be conveniently prepared by the FASP method [173]. An alternative methodology for sample preparations is the In-StageTip (iST) technique [174]. In addition, single-pot solid-phase-enhanced sample preparation (SP3) [175] and its variation, named universal solid-phase protein preparation (USP3) [176], can be employed in proteomic applications. If technical complications are encountered with cell or tissue lysis prior to MS analysis [177], these issues can be addressed with recently developed pressure cycling technology (PCT) [178]. In tissue proteomics, the quantification of hydrophobic proteins by MS analysis can be particularly difficult [179,180,181]. Of note, for the proteomic evaluation of large and highly complex protein assemblies, novel high-resolution native MS techniques have been developed [182,183,184].
2.1.4. Protein Digestion for Peptide Mass Spectrometry
The controlled and highly reproducible digestion of proteins for the production of a distinct peptide population is an essential requirement for the successful proteomic identification of specific proteoforms. Protein digestion can be carried out by various approaches that differ in the presentation of the proteins of interest in solution, in a gel matrix or on a membrane. One can therefore differentiate between in-solution [185,186], in-gel [187,188] and on-membrane [189,190,191] digestion protocols. The most frequently used protease in MS-based proteomics is trypsin [192], but alternative proteases can be used alone or in combination for protein digestion [193,194,195]. A rapid in-gel digestion protocol was recently designed for GeLC-MS/MS applications [196], which suits the systematic proteomic detection of very large proteins that do not properly move into the second dimension during 2D-GE [110]. An alternative method named BAC-gel dissolution to digest PAGE-resolved objective proteins, BAC-DROP [197], uses the above-described BAC detergent in gel systems, which enables swift solubilization by chemical reduction.
2.1.5. Mass Spectrometric Analysis
The standardized detection of individual proteins in complex mixtures can be routinely performed by MS-based peptide analysis using matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) [69,198,199] or liquid chromatography tandem mass spectrometry (LC-MS/MS) [70,71,72,200]. A detailed protocol for LC-MS/MS analysis has been recently published that includes a description of all materials, chemicals, buffers, experimental steps, mass spectrometric parameters and bioinformatic software tools needed for a successful proteomic study [201]. Untargeted quantitative proteomics approaches using mass spectrometry are designed to provide a comprehensive unbiased quantitation of the global proteome using label-free and/or labelling techniques [70,71,72,73]. Label-free quantitation of proteins analyzed by MS uses either integrated peak intensity from the parent-ion mass analysis (MS1) or features from fragment-ion analysis (MS2), including the use of spectral counts. Using next-generation mass spectrometry instruments with high-resolution capabilities and enhanced sensitivity, peak intensity areas from selected parent ions in MS1 can be detected, quantitated and combined with other protein-associated peptides when comparing expression levels between samples [44,45,46]. When using spectral counting, MS2 spectra, generated by peptide fragmentation, are summed with the number of spectra matched to peptides from a specific protein and are then used as a measure of protein abundance. In the field of sarcopenia research, as outlined in more detail below, the label-free MS technique was used by Théron et al. [202] to profile the proteome from vastus lateralis muscle samples obtained during surgery from mature and older women. The comparison of protein profiling between these two cohorts identified 35 differentially expressed proteins during skeletal muscle aging, mainly associated with energy metabolism and contractile functionality [202], showing the usefulness of employing label-free MS approaches in sarcopenia research.
A critical disadvantage of using a label-free approach is that all samples must be measured independently and require significant instrument time in order to achieve a comprehensive analysis of the proteome under investigation. Alternatively, quantitation can be performed using stable heavy isotopes incorporated into proteins by metabolic or chemical labelling protocols [203]. Tandem mass tags (TMT) [204], stable isotope labelling by amino acids in cell culture (SILAC) [205], isobaric tags for relative and absolute quantitation (iTRAQ) [206] and isotope-coded affinity tags (ICAT) [207] are labelling techniques that are routinely used in research studies investigating the proteome under different conditions. TMT labelling, an example of a chemical labelling methodology, is instrumental to quantitative proteomics, especially as the multiplexing approach allows for greater throughput. This enables quantitative analyses with a comprehensive proteome coverage [208].
Each mass-tagging reagent within a set (TMTpro enables multiplexing of up to 16 samples for protein identification and quantitation) has the same nominal mass and chemical structure composed of an amine-reactive NHS-ester group, a spacer arm, and an MS/MS reporter. The intensity of the unique MS/MS reporter ions (different m/z), detected using LC-MS/MS, is used to determine the amount that each peptide from the labelled samples contributes to the selected parent mass, facilitating relative quantitation. TMT-based proteomics has the advantage of higher quantitative accuracy, fewer missing quantitative values among samples, and reduced sample run times on MS instruments. TMT probes have been used in aging research to quantitate the proteome from young versus old rats [209]. The comparative analysis of slow-twitching soleus muscles versus fast-twitch extensor digitorum longus muscles revealed 78 and 174 proteins being differentially expressed during aging, respectively, and were shown to be generally associated with energy metabolism, oxidative stress, detoxification and transport [209].
SILAC is a quantitative proteomic approach using metabolic labels, which allows the comparison of cultured cells (lysates/secretome) under different conditions [204]. Using this approach, identification and quantitation of thousands of proteins can be performed in a single experiment by combining differently labelled samples prior to analysis by LC-MS/MS [210]. A standard SILAC experiment can be used to compare two or three samples by labelling with a light label (standard media), medium label (media containing 2H4-lysine and 13C6-arginine) and a heavy label (media containing 15N213C6-lysine and 15N413C6-arginine) [211]. The complete incorporation of heavy amino acids during protein turnover, in combination with the use of trypsin as the digestive enzyme, means that peptides from the differentially labelled samples can be accurately quantified relative to each other, based on the defined mass difference between the samples [212].
In skeletal muscle proteomics, SILAC was used to study differentiation, fiber damage and fiber typing [213,214,215]. An interesting application of SILAC in combination with an immunoaffinity protocol was the investigation of muscular atrophy in mice that were fed a SILAC diet containing 13C6-lysine for 4, 7 or 11 days when comparing denervation-induced changes after sciatic nerve section in the gastrocnemius muscle as compared to control samples [216]. Ubiquitin remnant peptides (K-ε-GG) were profiled by immunoaffinity enrichment, with results showing that >2100 diglycine remnants were identified, providing an insight into the ubiquitination process during muscular atrophy [216].
Dynamic proteome profiling (DPP) with a deuterium label can be employed to determine time-dependent changes in peptide mass isotopomer abundances [217]. The DPP technique was recently applied to study the relative abundance and fractional synthesis rate of proteins in human muscle biopsy specimens [218], and during C2C12 myoblast differentiation [219] and cellular aging [220]. As listed below, a study by Murphy et al. [221] of obese and healthy men of old age, who underwent resistance training and caloric restriction, determined the amount of newly synthesized skeletal muscle proteins via deuterated water labeling.
Importantly, MS analyses combined with artificial intelligence (AI) are increasing the potential for research and analysis of proteins in the field of proteomics [222]. The MS approach has proven to be a pillar for quantitative studies in addition to the identification of PTMs. Higher-plexing labelling reagents, in combination with advanced data acquisition protocols using the next generation of instruments, provide data on hundreds of thousands of protein isoforms in large sample cohorts. As datasets are becoming more all-encompassing, the use of AI, along with Machine Learning (ML) and Deep Learning (DL) algorithms, will become common features for analyzing the complex spectral data to identify pathophysiological patterns for actionable biology.
2.1.6. Data Acquisition by Mass Spectrometry
Data acquisition by mass spectrometry can be performed using data-dependent acquisition (DDA) [223], data-independent acquisition (DIA) [224] and targeted data acquisition (TDA) [225]. The DDA analysis mode involves using the MS instrument to generate a full-scan mass spectra (MS1), where the N most intense peptide ions (i.e., top 15) are selected and MS/MS spectra acquired. This approach generates thousands of MS/MS spectra that can be used for protein identification and subsequent quantitation. However, as the most abundant peptide ions are selected in the full scan, lower abundant peptide ions are repeatedly excluded from selection, even when using filtering criteria such as dynamic exclusion.
The DIA analysis mode involves using the MS instrument to direct the analysis on a narrow mass window of precursors and acquiring MS/MS data from all precursors detected within that window [226]. By stepping across the defined mass range using specific mass windows, collected MS/MS data will be acquired from all detected precursors. This strategy then uses highly specific fragment ion maps in a spectral library for qualitatively and quantitatively analyzing DIA data sets [227]. Sequential window acquisition of all theoretical mass spectra (SWATH-MS), as described by Gillet et al. [228], is a common method to generate DIA data by dividing the mass range into small mass windows.
The verification phase of many proteomics investigations centers on confirming that the abundances of target peptides are significantly different between sample cohorts by using MS-derived quantitative measurements. Selected/Multiple-Reaction Monitoring (SRM/MRM) or Parallel-Reaction Monitoring (PRM) are examples of approaches that can be utilized, where precursor peptide ions are measured in predefined m/z and retention time [229,230,231]. Stable-isotope-labelled, synthetic peptides are often spiked into the samples of interest, a process that increases the overall accuracy of target peptide quantitation.
2.1.7. Single-Cell Proteomics
Within the last decade, single-cell RNA sequencing (scRNA-seq) has come to the fore as an informative approach to decode tissue composition at the single-cell level and to provide important mechanistic data about pathophysiological associated networks [232]. Protein abundance in single cells is often deduced from complementary analysis platforms (scRNA-seq), as the ability to quantitate the proteome at a single-cell level has remained challenging [233]. Initial approaches for quantitating proteins in single cells relied on antibodies. Hence, these methods depend heavily on the availability of high-quality antibodies, therefore limiting their impact in the analysis of many antigens [234]. However, the use of MS/MS combined with LC-based separation is gaining traction with respect to its application in the analysis of the single-cell proteome [235].
Notable breakthroughs in this area include the use of isobaric labelling for single-cell proteomics, called Single Cell ProtEomics by Mass Spectrometry (SCoPE-MS) [236], and the second-generation protocol called Single Cell ProtEomics (SCoPE2) [237]. Such protocols permit cells from heterogeneous populations to be adapted into single-cell suspensions by FACS or CellenONE [238,239]. CellenONE is a precision dispensing technology combined with advanced image processing that delivers real-time and high-accuracy single cell isolation and dispensing. The isolated single cells are lysed, proteins digested, and the resultant peptides labelled with TMTs [240]. The different steps of this protocol can be automated, allowing for reproducibility and scalability. Labelled peptides are mixed and analyzed by MS/MS combined with LC [241].
Label-free analysis of individual cells does not require the use of TMTs, but their throughput is lower than that of the labelling approach [242]. The use of TMTs and the ability to multiplex ultimately increase the amount of peptides detected and quantitated by MS, which is particularly important when analyzing small-diameter mono-nucleated cell populations. However, the analysis of skeletal muscle fibers has some advantages, given that these types of fibers are multi-nucleated single cells and relatively bulky compared to other cell types. Individual muscle fibers contain on average a few micrograms of protein, and their isolation by dissection is more straightforward than having to use FACS or CellenONE approaches. A recent manuscript by Murgia et al. [243] demonstrated the utility of single-cell proteomics when comparing the proteome of type 2X fibers to that of type 1 and 2A fibers in young individuals. Their dataset contained more than 3800 proteins detected by single-fiber proteomics, with approximately 10% of the identified proteins displaying a statistically significant difference among the fiber types investigated. This approach has the potential to increase our understanding of musculoskeletal tissue development and disease within individual muscle fibers [244]. The application of single-cell proteomics in muscle aging is discussed below. Importantly, nanotechnology is increasingly used for optimum sample preparation in single-cell proteomics, as discussed by Arias-Hidalgo et al. [245].
2.1.8. Aptamer-Based Proteomics
Other proteomic-based platforms that are growing in popularity and number include aptamer-based approaches [246]. Aptamers are single strands of oligonucleotides (either ssDNA or ssRNA) that bind with high specificity and high affinity to preselected proteins [247]. The range of the preselected protein panels is ever increasing, with one of the leading aptamer-based proteomics platforms, SomaLogic, offering different protein panels ranging from 1300 to over 7000 targets in as little as 55 mL of plasma or serum. Hathout et al. [248] recently used the SomaLogic platform to identify 108 elevated and 70 decreased proteins in dystrophic patients who were not yet treated with glucocorticoids compared to age-matched healthy controls. High-throughput multiplexing techniques can be combined with TMT technology to detect serum biomarkers that have been released from damaged skeletal muscle fibers [249].
2.2. Proteomic Profiling of Fiber-Type Specification in Skeletal Muscles
Most individual skeletal muscles consist of a distinct mixture of fast-twitching, slow-twitching and hybrid fibers [250,251,252], and this fiber-type composition can undergo substantial alterations during progressive muscle wasting [50,51,253]. Fiber-type specification has traditionally been determined by histological, histochemical and immunohistological staining procedures [132,254,255]. Recently, Kallabis et al. [215] described a novel high-throughput proteomic workflow for myosin isoform profiling in single muscle fibers based on the usage of a capillary LC-MS gradient in a 96-well format. This is an excellent improvement of the fiber-type-specific screening of the skeletal muscle proteome. Over the last two decades, the steady improvement of protein separation methodology and mass spectrometric detection efficiency, in combination with enormous advances in bioinformatics, has resulted in the greatly enhanced coverage of the skeletal muscle protein constituents [65,66,67,256].
A large number of proteomic markers are now available for the comprehensive profiling of subcellular fractions from skeletal muscles [257]. Over 10,000 protein species belonging to the core proteome of human and animal skeletal muscles have been identified and characterized by mass spectrometry [258,259,260,261,262,263]. The proteomic profiling of differing skeletal muscles with specific fiber-type distribution patterns has especially focused on human vastus lateralis, deltoideus and trapezius muscles [264,265,266] and mouse gastrocnemius, soleus and diaphragm muscles [267,268,269]. Comparative MS-based studies of mouse extensor digitorum longus and soleus muscles [270,271,272], and tissue extracts from rodent gastrocnemius, extensor digitorum longus, tibialis anterior and soleus muscles [273,274,275,276], have given comprehensive insights into the biochemical complexity of fiber-type-specific protein expression patterns using single-fiber proteomics [277]. The study by Eggers et al. [276] utilized immunolabeling of individual skeletal muscle fibers with antibodies to specific myosin heavy chain isoforms followed by laser micro-dissection and MS analysis. The detailed biochemical characterization of mouse muscle fibers by single-cell proteomics revealed an in-depth profile of fiber-type-specific protein expression levels [276].
2.3. Composition of the Acto-Myosin Apparatus and Its Proteomic Profile
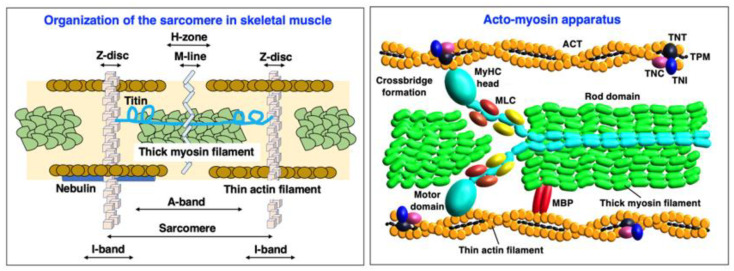
Skeletal muscle fibers are highly specialized cellular structures for the generation of force and movement [278]. The sarcomeric components of the acto-myosin apparatus [279] provide the molecular machinery for coordinated filament sliding during skeletal muscle contractions [280]. Contractile proteins exist in a large number of isoforms [281] and can be divided into groups of proteins that are mostly located in the thick myosin-containing filament [282], the thin actin-containing filament [283], the M-line [284] and the Z-disk [285], as well as auxiliary filamentous structures [286]. The sarcomere units have extensive intrinsic connections [287] and are embedded in the overall muscle structure by an extensive cytoskeletal system linking them to organelles for energy supply and signaling mechanisms, and to the costamers for force transmission [288]. Figure 1 provides an overview of the contractile acto-myosin apparatus within the sarcomeric structure of skeletal muscles.
Figure 1.
Overview of the sarcomeric structure and the contractile acto-myosin apparatus of skeletal muscle. The diagram to the left outlines the arrangements of the thick myosin-containing filament, the half-sarcomere spanning titin filament and the actin/nebulin-containing thin filament in relation to their positions within the A-band, the I-band, the H-zone, the M-line and the Z-disc structures of the sarcomere. The diagram to the right shows the interactions between the head structure of myosins and filamentous actin molecules that are involved in the crossbridge formation during skeletal muscle contractions. Details of the subunit composition and isoform diversity of myosin heavy chains, myosin light chains, myosin binding proteins, actins, tropomyosins and troponins are given in Figure 2 below. Abbreviations used: ACT, actin; MLC, myosin light chain; MYBP, myosin binding protein; MyHC, myosin heavy chain; TNC; troponin-C; TNI; troponin-I; TNT, troponin-T; TPM, tropomyosin.
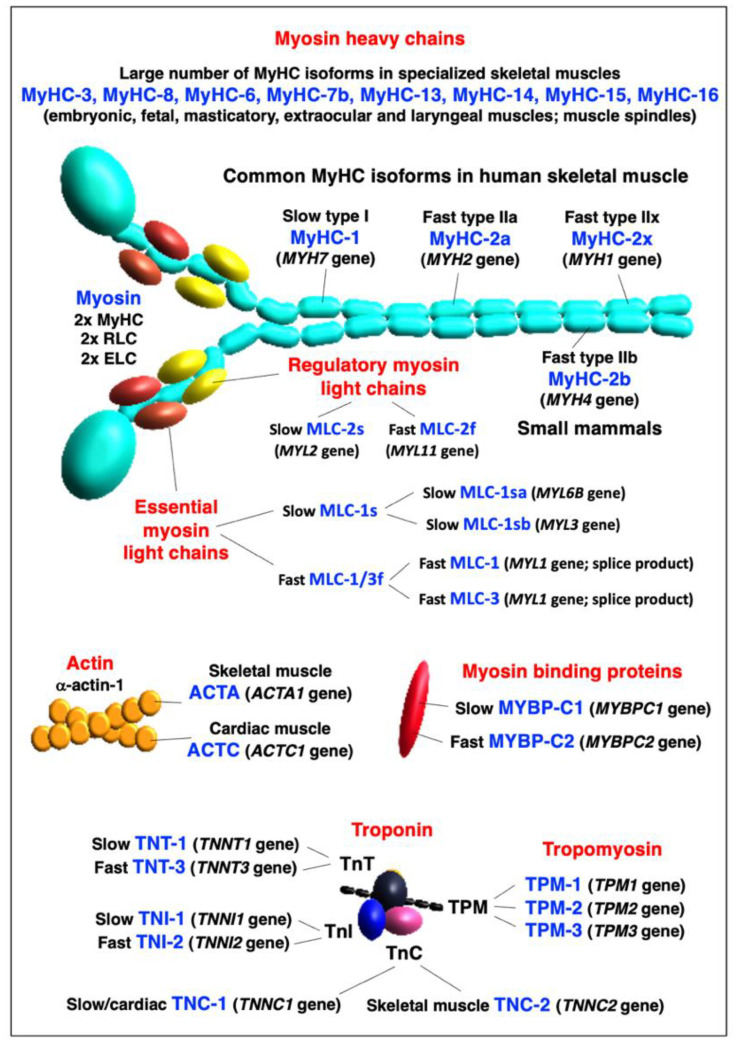
Slow versus fast isoforms of key sarcomeric proteins are displayed in Figure 2 below. The abbreviations of specific muscle protein isoforms are listed at the end of the manuscript, and are used throughout the text, Tables and Figures. The names of genes are exclusively listed in italics to avoid confusion with abbreviated protein names.
Figure 2.
Summary of slow versus fast isoforms of key sarcomeric proteins. The diagrammatic presentation and color coding of individual contractile proteins is identical to the descriptions given in the overview of the sarcomeric structure and the contractile acto-myosin apparatus of skeletal muscle in Figure 1 above. Abbreviations used: ACT, actin; ELC, essential light chain; MYBP, myosin-binding protein; MyHC, myosin heavy chain; MLC, myosin light chain; RLC, regulatory light chain; TNC; troponin-C; TNI; troponin-I; TNT, troponin-T; TPM, tropomyosin.
In the thick filaments of skeletal muscles [282,287], the hexameric composition of the major motor protein myosin consists of two myosin heavy chains (MyHCs) [289] and four myosin light chains (MLCs) [290], which can be further subdivided into two phosphorylatable regulatory light chains and two non-phosphorylatable alkali light chains [291]. The main MyHC isoforms in human skeletal muscle are the slow type I isoform MyHC-1 (MYH7 gene), the fast type IIa isoform MyHC-2a (MYH2 gene) and the fast type IIx isoform MyHC-2x (MYH1 gene) [250,252,292]. Another fast isoform of type IIb is named MyHC-2b (MYH4 gene), and is present at high concentration only in small mammals, such as mice, rats and rabbits [110]. Type IIb fibers with high levels of MyHC-2b are extremely fast-contracting and quickly fatigable units that are usually not found in mature human skeletal muscles [251]. In addition, MyHC-emb (MYH3 gene), MyHC-neo (MYH8 gene) and six other MyHC isoforms, encoded by the genes MYH6, MYH7B, MYH13, MYH14, MYH15 and MYH16, respectively, exist in embryonic/fetal muscles [293] and specialized adult muscles, including masticatory, extraocular and laryngeal muscles, as well as muscle spindles [294,295,296]. The recent proteomic profiling of extraocular muscles has also detected, besides the long-established MyHC-13 isoform, MyHC-14 and MyHC-15 being present in these highly specialized and mostly fast-twitching muscles [297]. The slow and fast isoforms of MLC proteins are represented by slow/cardiac regulatory light chain MLC-2s (MYL2 gene), fast regulatory light chain MLC-2f (MYL11 gene; with the previous HGNC gene symbol MYLPF), slow essential light chain MLC-1s (with isoform MLC-1sb encoded by the MYL3 gene; and MLC-1sa encoded by the MYL6B gene) and fast essential light chain MLC-1/3f (with MLC-1 and MLC-3 being splicing products of the MYL1 gene) [289,291,298]. Myosin-binding proteins (MYBP) [299] are located at the thick filament interface and are present as slow and fast isoforms, i.e., MYBP-C1 (slow myosin-binding protein C1; encoded by the MYBPC1 gene) and MYBP-C2 (fast myosin-binding protein C2; encoded by the MYBPC2 gene) [300,301,302].
In the thin filament [303], the basic units that form helical actin (ACT) filaments are alpha-actin-1 monomers of the skeletal muscle ACTA type (ACTA1 gene) or the cardiac muscle ACTC type (ACTC1 gene) [304,305]. The Ca2+-dependent process of regulating interactions between the MyHC heads and ACT filaments is provided by tropomyosin (TPM) and the troponin (TN) complex [306,307]. Sarcomeric TPM molecules are alpha-1-tropomyosin (TPM-1; encoded by the TPM1 gene), slow beta-tropomyosin (TPM-2; encoded by the TPM2 gene) and muscle-type alpha-3-tropomyosin (TPM-3; encoded by the TPM3 gene) [308,309]. The alpha-4-tropomyosin isoform named TPM-4 (TPM4 gene) is a non-sarcomeric cytoskeletal component [310]. The TN complex consists of the Ca2+-binding subunit TNC, the TPM-interaction subunit TNT and the inhibitory subunit TNI [311]. All three subunits exist in fast and slow isoforms and exist in various combinations in matured skeletal muscles [312]. This includes TNC-1, the slow/cardiac troponin TnC isoform (TNNC1 gene), TNC-2, the skeletal muscle troponin TnC isoform (TNNC2 gene), TNT-1, the slow muscle troponin TnT isoform (TNNT1 gene), TNT-3, the fast muscle troponin TnT isoform (TNNT3 gene), TNI-1, the slow muscle troponin TnI isoform (TNNI1 gene) and TNI-2, the fast muscle troponin TnI isoform (TNNI2 gene) [313]. In addition, the cardiac isoforms TNNI3 and TNNT2 have been found in aged and denervated skeletal muscles [314].
The Z-disk contains a large number of proteins, including filamin-C (FLNC; FLNC gene), telethonin/titin-cap protein (TCAP; TCAP gene) and alpha-actinin (ACTN) with its closely associated binding protein myozenin (MYOZ) [315,316]. They are excellent subcellular markers of this crucial sarcomeric structure [285]. The major ACTN proteins found in the Z-disc are the alpha-actinin-2 isoform ACTN-2 (ACTN2 gene) and the alpha-actinin-3 isoform ACTN-3 (ACTN3 gene) [317]. Interestingly, the ACTN3 genotype appears to be linked to the maintenance of bone and skeletal muscle mass during aging [318]. MYOZ isoforms that are present in skeletal muscles are MYOZ-1 (myozenin-1; previously named FATZ-1; MYOZ1 gene), MYOZ-2 (myozenin-2; MYOZ2 gene) and MYOZ-3 (myozenin-3; MYOZ3 gene) [319,320]. Excellent marker proteins of the M-line structure of the sarcomere [321] are the myomesin (MYOM) proteins MYOM1 (myomesin-1; MYOM1 gene) and MYOM-2 (myomesin-2; MYOM2 gene) [322,323], as well as obscurin (OBSCN; OBSCN genes) [324,325]. The M-line-associated obscurin molecule belongs to the class of giant muscle proteins [108]. Two other major sarcomeric components are also characterized by extremely high molecular masses, i.e., the actin-binding protein nebulin (NEB; NEB gene) of the thin filament [326,327] and the half-sarcomere spanning component titin (TNN; TTN gene) [328,329] with multifunctional roles in lattice order, filament interactions and the excitation–contraction–relaxation cycle [330,331]. Closely linked to titin is the muscle ankyrin repeat protein MARP (ANKRD2 gene) [332].
As a representative example of how proteomics can be employed to routinely detect and characterize a large number of specific isoforms of contractile proteins, Table 1 lists the mass spectrometric identification of major sarcomeric proteins that are associated with the thick myosin filament, thin actin filament, the titin filament, the Z-disc and the M-line in diaphragm muscle [269,333]. The information presented includes the protein names and abbreviations of particular isoforms, their accession number, the name of the coding gene, percentage of sequence coverage, number of peptides and calculated molecular mass. As listed in Table 1, diaphragm muscles are characterized by the presence of MyHC-1, MyHC-2x, MyHC-2b, MyHC-8, MLC-1/3, MLC-2 and MLC-3 in the thick filament, and muscle-type ACTA and various slow and fast isoforms of TPM, TNC, TNI and TNT in the thin filament. Abundant components in the Z-disc were established to include FLNC, TCAP, ACTN isoforms and MYOZ, and the M-line was shown to contain MYOM and OBSCN. The muscle protein that was recognized by the highest number of peptides is represented by the giant sarcomeric protein TTN [108]. A closely linked component of the titin filament was identified as the muscle ankyrin repeat protein MARP.
Table 1.
Proteomic profiling of key components involved in the contraction–relaxation cycle of mouse diaphragm muscle *.
| Contractile Protein | Accession Number/Gene | Coverage/Peptides | Molecular Mass |
|---|---|---|---|
| Myosin heavy chains (MyHC) | |||
| MyHC-1, slow muscle (Myosin-7) | Q91Z83/Myh7 | 57.7/139 | 222.7 |
| MyHC-2x, fast muscle (Myosin-1) | Q5SX40/MyH1 | 69.8/192 | 223.2 |
| MyHC-2b, fast muscle (Myosin-4) | Q5SX39/MyH4 | 66.0/174 | 222.7 |
| MyHC-8, perinatal muscle (Myosin-8) | P13542/MyH8 | 46.7/124 | 222.6 |
| Myosin light chains (MLC) | |||
| MLC-1/3, skeletal muscle | P05977/Myl1 | 84.0/19 | 20.6 |
| MLC-2, skeletal muscle | P97457/Mylpf | 88.8 /19 | 18.9 |
| MLC-2, cardiac muscle | P51667/Myl2 | 75.3/12 | 18.9 |
| MLC-3, skeletal muscle | P09542/Myl3 | 78.4 /16 | 22.4 |
| Myosin-binding proteins (MYBP) | |||
| MYBP-C2, fast-type | Q5XKE0/Mybpc2 | 59.5/51 | 127.3 |
| MYBP-H | P70402/Mybph | 25.3/7 | 52.6 |
| Actin (ACT) filament | |||
| Alpha-Actin ACTA, skeletal muscle | P68134/Acta1 | 68.2/25 | 42.0 |
| F-ACT capping protein, subunit a-2 | P47754/Capza2 | 54.9/10 | 32.9 |
| F-ACT capping protein, subunit b | P47757-2/Capzab | 33.8/8 | 30.6 |
| Tropomyosin (TPM) complex | |||
| TPM, alpha-1 chain | P58771/Tpm1 | 77.8/37 | 32.7 |
| TPM, beta chain | P58774/Tpm2 | 76.1/38 | 32.8 |
| TPM, alpha-3 chain | P21107/Tpm 3 | 68.1/25 | 33.0 |
| TPM, alpha-4 chain | Q6IRU2/Tpm 4 | 37.9/9 | 28.5 |
| Troponin (TN) complex | |||
| TNI-1, slow skeletal muscle | Q9WUZ5/Tnni1 | 28.9/7 | 21.7 |
| TNI-2, fast skeletal muscle | P13412/Tnni2 | 44.0/9 | 21.3 |
| TNT-1, slow skeletal muscle | O88346-3/Tnnt1 | 28.4/8 | 30.0 |
| TNT-3, fast skeletal muscle | Q9QZ47-12/Tnnt3 | 40.2/13 | 28.3 |
| TNC-1, slow/cardiac muscle | P19123/Tnnc1 | 47.8/6 | 18.4 |
| TNC-2, skeletal muscle | P20801/Tnnc2 | 79.4/11 | 18.1 |
| Z-disc complex | |||
| Filamin FLNC | Q8VHX6-2/Flnc | 42.0/71 | 287.2 |
| Alpha-Actinin ACTN-2 | Q9JI91/Actn2 | 68.3/50 | 103.8 |
| Alpha-Actinin ACTN-3 | O88990/Actn3 | 65.2/48 | 103.0 |
| Telethonin TCAP | O70548/Tcap | 36.5/5 | 19.1 |
| Myozenin MYOZ-1 | Q9JK37/Myoz1 | 53.7/7 | 31.4 |
| Myozenin MYOZ-2 | Q9JJW5/Myoz2 | 58.3/12 | 29.7 |
| Myozenin MYOZ-3 | Q8R4E4/Myoz3 | 29.4/5 | 27.0 |
| M-line complex | |||
| Myomesin MYOM-1 | Q62234-2/Myom1 | 57.4/70 | 175.3 |
| Myomesin MYOM-3 | A2ABU4/Myom3 | 52.2/47 | 161.7 |
| Obscurin OBSCN | A2AAJ9/Obscn | 31.5/135 | 965.8 |
| Half-sarcomere-spanning titin filament | |||
| Titin TNN | A2ASS6/Ttn | 51.4/1284 | 3904.1 |
| Muscle ankyrin repeat protein MARP | Q9WV06/Ankrd2 | 26.2/7 | 36.7 |
3. Proteomics of Age-Related Muscle Wasting
3.1. Pathobiological Hallmarks of Sarcopenia of Old Age
Skeletal muscle aging can be considered a fundamental biological process that occurs in all humans of advanced age [334]. However, individual muscles in the same body age differently [28,335] and considerable inter-individual differences exist in the extent and time course of muscle tissue loss and decline in contractile strength [9,336]. Importantly, skeletal muscle degeneration can be accompanied by progressive deterioration of myocardial functions in the elderly, causing serious medical complications due to cardio-sarcopenia syndrome [337]. Although sarcopenia of old age is due to multi-factorial mechanisms, it is most likely that neurological changes during aging play a key role in the initiation of muscular atrophy. The loss of spinal motor neurons appears to be associated with the initial decline in the proper innervation of voluntary muscles. The damage of the neuronal systems is exacerbated by a diminished capacity for reinnervation or patterns of faulty reinnervation [338]. The tendency of fast-to-slow muscle fiber-type transitions in a large number of aged human muscles was shown to be linked to a higher susceptibility of faster-contracting fibers to muscular atrophy [50,51,339]. This higher vulnerability of faster-twitching and mostly glycolytic fibers under atrophic conditions is closely related to specific signaling pathways involving peroxisome proliferator-activated receptor gamma coactivator PGC1-alpha and transforming growth factor TGF-beta [340].
Epidemiological studies of sarcopenia, assessed by both cross-sectional and longitudinal investigations, indicate that everyday life of a large proportion of the population over 75 years of age is impaired by a certain degree of physical frailty and impaired skeletal muscle functioning [341,342,343,344,345,346,347]. Worsening cofactors of age-related muscle wasting include sarcopenia-independent chronic diseases and their extensive pharmacological therapy, as well as chronic low-grade inflammation, insulin resistance, poor nutrition, extended bedrest and the lack of appropriate physical activity levels [9,10,14,348,349]. Thus, to counteract the age-dependent decline in skeletal muscle performance, optimized rehabilitation [350] and appropriate physical exercise regimes, such as moderate resistance exercises [351,352,353], are crucial to minimize oxidative stress and inflammation in sarcopenia [354,355]. Since older adults exhibit a higher rate of protein turnover [356], and an apparent imbalance between accelerated muscle protein breakdown and impaired levels of protein re-synthesis exists in aged muscles [9,10,11], the resulting reduced levels of contractile components in older individuals should be addressed by avoiding a poor diet quality [357,358,359] and instead provide an adequate intake of high-quality protein in the elderly [360,361,362,363].
Recent publications have critically examined the diverse and multi-factorial aspects of aging and sarcopenia, including senescence-related changes linked to abnormal metabolic pathways [364], mitochondrial dysfunctions [365,366,367,368,369,370,371], the role of reactive oxygen species and disrupted redox signaling [372,373,374,375], abnormal calcium handling [376], functional changes in neuromuscular transmission [377], altered myokine and myomitokine signaling [369,378], the role of miRNAs in the decline of proteostasis [379,380], anabolic resistance and impaired muscle protein metabolism [381,382,383], adipocyte crosstalk in aged skeletal muscle and sarcopenic obesity [384,385], immune system alterations, chronic inflammation and immune–metabolic dysfunction associated with oxidative stress [33,366,386,387,388], the role of telomere length during aged fiber regeneration [389], the interplay between sarcopenia, frailty and cognitive impairments in the elderly [390], cardio-sarcopenia syndrome [337] and the influence of nutrition on the aging phenotype [391]. The finding that the satellite cell pool is preferentially affected in fast type II fibers in the elderly [392] has established the idea that stem cell exhaustion is majorly involved in sarcopenia and possibly even facilitates age-associated fast-to-slow transitions [393]. Thus, the reduction in muscle-specific stem cells appears to play a key role in the impaired regenerative capacity of aged fibers [394,395,396]. This phenomenon underlines the enormous complexity of the molecular and cellular mechanisms that are associated with skeletal muscle aging.
3.2. Proteomics of Aged Skeletal Muscle
Biomarker discoveries using omics-type surveys are crucial to improve the monitoring of impaired physiological functioning, altered energy metabolism and chronic inflammation in aged muscle [397], and to advance the diagnosis, prognosis and therapeutic monitoring of frailty syndrome and sarcopenia in the aging population [398,399,400], whereby proteomics plays a key role in detecting and characterizing novel marker candidates [48,49]. In the context of aging and alterations in contractile proteins, human skeletal muscles were extensively studied using both top-down/gel-based approaches versus bottom-up/peptide-centric analyses [202,221,401,402,403,404,405,406,407,408,409,410,411,412,413,414]. Changes in particular isoforms of skeletal muscle proteins during the aging process can give detailed insights into molecular and cellular mechanisms that underlie sarcopenia of old age. Although individual skeletal muscles exhibit differing degrees of susceptibility to aging-induced muscular atrophy [28], proteomics has confirmed the previous findings from biochemical, cell-biological and histological studies that suggest a general trend of fast-to-slow transitions in senescent muscles [50,415,416] and concomitant alterations in glycolytic and mitochondrial pathways [39,417]. This includes a stepwise transition from faster isoforms of MyHC, MLC, ACT, TPM, TNC, TNI and TNT to their slower counterparts. Of note, the recent proteomic profiling of single fibers from human vastus lateralis muscle of young adults has given a comprehensive overview of fiber-related differences in protein isoform expression patterns [243]. These types of proteomic catalogs can be highly useful as reference databanks for studying proteome-wide changes during aging.
Table 2 lists major MS-based investigations with a bioanalytical focus on protein changes in contractile proteins during human skeletal muscle aging [202,221,401,402,403,404,405,406,407,408,409,410,411,412,413,414]. The listings of individual proteomic investigations summarize the analyzed muscle specimens, the age range of samples, the bioanalytical approach and the detected proteome-wide alterations with a focus on the contractile apparatus. Since considerable physiological and biochemical differences exist between untrained versus trained skeletal muscles [68,418,419,420], contractile fiber aging has also been studied in select master athletes [421,422] in addition to the below-listed studies on neuromuscular changes in the general and mostly untrained population. Major proteomics surveys of aged human muscles that did not focus on the contractile apparatus include investigations into the role of mitochondrial abnormalities [423] and molecular chaperones [424], as well as metabolic changes due to oxidatively modified proteins in satellite cells [425].
Table 2.
List of major mass-spectrometry-based proteomic profiling studies focusing on contractile proteins in aged human skeletal muscle tissue.
| Specimens | Bioanalytical Approach | Proteomic Changes | References |
|---|---|---|---|
|
Vastus lateralis (20–25 years versus 70–76 years) |
2D-DIGE, ESI-MS/MS, Pro-Q Diamond, PAGE analysis of MyHC isoforms | Increase in MLC-2s, ACTC and MyHC-I; decrease in MLC2f, TNT-3, TPM-3 and MyHC-2x; shift in phosphorylated MLC-2f to MLC-2s isoforms | Gelfi et al. [401] |
|
Vastus lateralis (47–62 years versus 76–82 years) |
2D-DIGE, MALDI-TOF, IB | Increase in ACTC; decrease in ACTA, MLC-2, TNT-1 and TNC-1 | Staunton et al. [402], Ohlendieck [403] |
|
Vastus lateralis (53 years mean age versus 78 years mean age) |
Soluble proteins, LC-MS/MS, IB | Increase in MARP/ANKRD2; decreases in MLC-1/3, MyHC-2x and TTN |
Théron et al. [202] |
|
Vastus lateralis (48–61 years versus 76–82 years post-menopausal women) |
2D-GE (CBB), LC-MS/MS, IB | Increase in MARP/ANKRD2, MLC-1/3f, ACTA, TNT-3 and MYOZ-1; decreases in MLC2s and TNN | Gueugneau et al. [404] |
|
Rectus abdominis (0–12 years versus 52–76 years) |
Oxi-proteome analysis, 2D-GE, protein carbonyl immuno detection | Detection of age-related carbonylation of MyHC-1, MYBP-C1 and TNT-1 | Dos Santos et al. [405] |
|
Vastus lateralis (18–30 years versus >55 years; trained and untrained) |
LC-MS/MS, SRM, PAGE analysis of MyHC isoforms | Increase in MyHC-1; decrease in MyHC-2a; establishment of quantitative differences in myosin light chain composition | Cobley et al. [406] |
|
Vastus lateralis (22–27 years versus 65–75 years) |
Single-muscle-fiber proteomics, LC-MS/MS | Differential effects on fast versus slow fibers based on MyHC-1, MyHC-2a and MyHC-2x distribution analysis; increase in chaperones of MyHC and ACTA | Murgia et al. [407] |
| Quadriceps muscle (66–80 years) of healthy versus cancer patients | LC-ESI-MS/MS, SWATH MS, IFM, IB | Differential expression of MyHC-1, MyHC-2a and MyHC-2x in healthy elderly versus cancer patients with or without weight loss | Ebhardt et al. [408] |
|
Vastus lateralis (23 years mean age versus 71 years mean age) |
2D-GE (CBB), Pro-Q Diamond, MALDI-TOF MS, PAGE analysis of MyHC isoforms, IB | Increase in MyHC-1; decrease in MyHC-2a and MyHC-2x; myosin/actin ratio not affected; differential effects on expression of TNT-3, ACTA and ACTC proteoforms | Brocca et al. [409] |
|
Vastus lateralis (Obese and healthy older men of average age 66 undergoing resistance training and energy restriction) |
LC-MS/MS, deuterated water labeling of newly synthesized skeletal muscle proteins | Determination of synthesis rate of myofibrillar proteins (MyHC, MLC, ACTA, TPM, TNC, TNT, TNI) | Murphy et al. [221] |
|
Vastus lateralis (range of individuals from 20 to 87 years of age) |
TMT, LC-MS/MS | Decrease in MYBP-H; switch from MyHC-2x/MyHC-2a to MyHC-1; differential effects on TNT-3, TPM-1 and MYOZ-2 expression | Ubaida-Mohien et al. [410,411] |
|
Vastus lateralis (25 years mean age versus 62 years mean age) |
LC-MS/MS, PAGE analysis of MyHC isoforms | Reduced acto-myosin abundance; decrease in ACTA and MYBP-H; increase in ACTC and TNT-1 | Vann et al. [412] |
|
Vastus lateralis (21 years mean age versus 73 years mean age) |
2D-GE (CBB), LC-MS/MS, IB | Increase in TNT-1 and MARP; decrease in ACTA, TNT-3 and MYOZ-1 |
Gueugneau et al. [413] |
|
Vastus lateralis (25 years mean age versus 67 years mean age) |
iTRAQ, LC-MS/MS | Decrease in ACTA and FLNC | Deane et al. [414] |
Top-down proteomics using routine 2D-GE or fluorescent 2D-DIGE is an ideal bioanalytical approach for the efficient separation of contractile proteins below 150 kDa [122], such as fast and slow isoforms of MLC, TPM, TNC, TNI, TNT and ACT [52,66]. Human skeletal muscles usually contain a mixture of slow-twitching fibers, which are characterized by high levels of oxidative metabolism, and faster-twitching fibers with glycolytic-oxidative or mostly glycolytic metabolism [250], in addition to various hybrid fibers [426]. Mass spectrometric analyses of separated 2D spots clearly confirmed shifts from fast protein isoforms to their slower protein counterparts [401,402,403,404,405,409,413], which agrees with the general tendency of fast-to-slow transitions during skeletal muscle aging [50,51,253,339]. These findings could be complemented with bottom-up strategies and LC-MS/MS analyses to study contractile proteins of higher molecular mass, such as MyHC and TNN [202,221,406,407,408,410,411,412]. In analogy to shifts towards faster isoforms of TPM and subunits of the TN complex, LC-based studies confirmed transitions from MyHC-2 isoforms towards MyHC-1. In addition, the application of iTRAQ demonstrated decreases in ACTA and FLNC [414]. Single-muscle-fiber proteomics showed differential effects on fast versus slow fibers based on the mass spectrometric detection of MyHC-1, MyHC-2a and MyHC-2x distribution patterns [407]. Overall, the findings from the proteomic analysis of aged human muscles, focusing mostly on the vastus lateralis muscle, agree with the higher susceptibility of fast fibers to atrophic changes [202,221,401,402,403,404,405,406,407,408,409,410,411,412,413,414] and support the cell biological concept of fast-twitching fibers being affected prior to slower fiber population during skeletal muscle aging [416].
In analogy to the above-listed studies on human skeletal muscles, the analysis of various animal muscles revealed similar tendencies of fast-to-slow transitions during fiber aging. For example, the mass spectrometric profiling of the aging vastus lateralis muscle from African green vervet monkeys (Chlorocebus aethiops sabaeus) confirmed decreases in fast MyHC isoforms during age-related muscular atrophy [427]. Most aged animal studies were carried out with small rodents [39,428,429,430]. Interesting new model systems used in aging research are Drosophila, zebrafish and nematodes [431,432,433,434]. Following initial optimization experiments [435,436], several proteomic investigations of animal models focused on the analysis of mitochondria [437,438,439,440,441,442], the matrisome [443,444], the cellular stress response [445], calpain-interacting proteins [446] and key post-translational modifications [447], such as glycosylation [448], phosphorylation [449], carbonylation [450] and nitration [451,452,453,454,455] in aged muscles. General alterations in the senescent skeletal muscle proteome, including abundance changes and isoform switching of contractile proteins, were examined in a large number of MS-based surveys using both the well-established rat model of sarcopenia [209,405,456,457,458,459,460,461,462,463,464,465,466,467,468,469,470,471,472,473] and aging mouse muscles [474,475,476,477,478,479,480,481,482,483,484,485,486]. The gel-based analysis of aging rat gastrocnemius muscle clearly identified decreases in MyHC-2b, MLC-2f and TPM-1 as compared to increases in MyHC-1, MLC-2s and ACTC [456,457,458,459,460,461,462,463,464]. In particular, MLC-2s appears to be majorly affected both in its abundance and phosphorylation pattern during skeletal muscle aging [461,466], making it an excellent biomarker candidate of fiber-type switching.
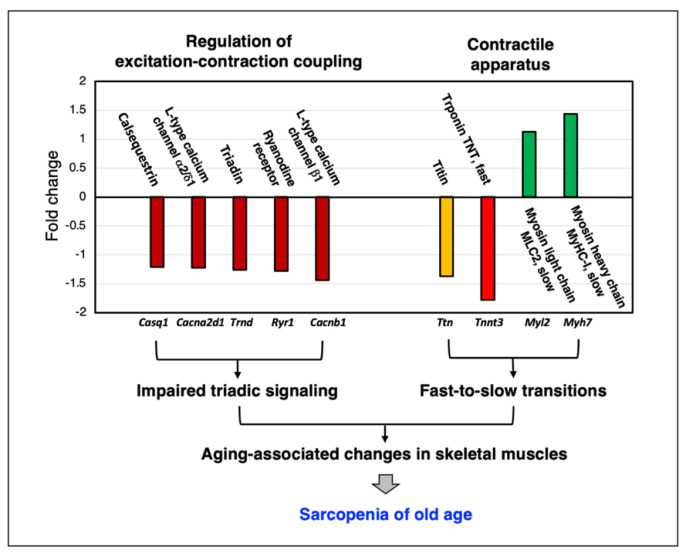
As illustrated in the representative findings on abundance changes in Ca2+-regulatory components and contractile proteins in Figure 3, MS-based proteomics is an excellent bioanalytical tool to establish decreases in important Ca2+-handling proteins that are involved in cellular signaling pathways and the regulation of excitation–contraction coupling. This includes subunits of the dihydropyridine receptor L-type Ca2+-channel of the transverse tubules, the ryanodine receptor Ca2+-release channel of the triad junction, the luminal Ca2+-binding protein calsequestrin of the terminal cisternae region within the sarcoplasmic reticulum and the structural protein triadin [487,488,489]. Thus, a key mechanism involved in skeletal muscle aging appears to be a certain degree of pathophysiological uncoupling between sarcolemmal excitation and the initiation of acto-myosin sliding that mediates fiber contraction [376,490,491,492], combined with a reduced association between Ca2+-release units and aged mitochondria [493]. Disturbed Ca2+-homeostasis may be involved in altered myocyte signaling in the context of fiber-type specification, which is supported by proteomic data that indicate a general tendency of fast-to-slow transitions at the level of isoform switching of contractile proteins [50].
Figure 3.
Representative example of the mass-spectrometry-based proteomic analysis of skeletal muscle aging. Shown are crucial regulatory proteins of excitation–contraction coupling and Ca2+- homeostasis (dihydropyridine receptor L-type Ca2+-channel, ryanodine receptor Ca2+-release channel, calsequestrin and triadin) and sarcomeric proteins (titin, troponin, myosin light chain and myosin heavy chain). Mass spectrometric analyses of young versus aged wild-type mouse diaphragm muscle specimens were carried out as previously described in detail [201,269,333].
4. Age-Related Muscular Atrophy, Biomarker Discovery and Therapeutic Approaches
4.1. Mechanisms of Age-Related Muscular Atrophy
Research over the last few decades has clearly established that the molecular and cellular mechanisms of aging are highly complex [30,31,32] and specifically affect the skeletal musculature [9,10,11]. The multi-factorial processes that are associated with age-related muscular atrophy and sarcopenia of old age include:
Progressive neurodegeneration: loss of neuromuscular junction integrity; degeneration of motor neurons and resulting denervation; faulty patterns of reinnervation; loss of entire motor units;
Excitation–contraction uncoupling at the level of the transverse tubules, triad junction and sarcoplasmic reticulum;
Impaired calcium homeostasis;
Abnormal mitochondrial functioning;
Fast-to-slow transitions due to increased susceptibility of fast fibers to atrophy;
Tendency of bioenergetic glycolytic-to-oxidative shifting;
Increased cellular stress due to proteotoxic abnormalities;
Abnormal protein turnover and synthesis causing dysregulated proteostasis;
Hormonal imbalance and disturbed cellular signaling;
Visceral obesity causing abnormal muscle-fat-axis signaling;
Metabolic syndrome and insulin resistance;
Increased levels of reactive myofibrosis triggering loss of fiber elasticity;
Chronic low-level sterile inflammation;
Reduced regenerative capacity due to satellite cell exhaustion;
Epigenetic changes.
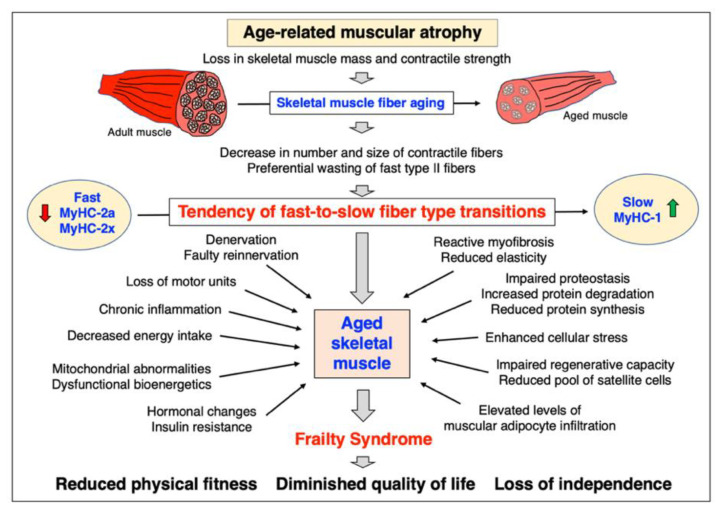
Figure 4 summarizes crucial aspects of muscle aging, including the preferential susceptibility of fast type II fibers to age-related degeneration, which causes a general shift to slower-twitching fiber populations in most senescent skeletal muscles.
Figure 4.
Overview of the multi-factorial changes during human skeletal muscle aging. The higher susceptibility of fast-twitching type II fibers causes a tendency of fast-to-slow transitions in senescent muscles. This is reflected by a switch from fast myosin heavy chain isoforms (MyHC-2a, MyHC-2x) to slower counterparts (MyHC-1) during skeletal muscle aging.
4.2. Biomarker Discovery for the Improved Evaluation of Sarcopenia of Old Age
In order to improve the differential diagnosis of pre-, mild or severe sarcopenia [9,10,11], the establishment of reliable and robust biomarkers of frailty and skeletal muscle wasting is crucial [29,49]. Suitable markers can be measured by physical performance assessments [25,26,27], imaging technology [22,28] and/or biochemical assays [494]. A novel imaging marker system is the ultrasound sarcopenic index (USI), which can determine the loss of skeletal muscle mass in association with sarcopenia in a practical and relatively inexpensive way [495]. Ideally, abundance changes in protein biomarkers of sarcopenia should be easily measurable with high levels of specificity and sensitivity [496,497], as well as not being majorly affected by gender, ethnicity, co-morbidities, exposure to pharmacological agents and unrelated therapeutic treatments [498]. To avoid potential complications due to elaborate tissue biopsy procedures, the development of non-invasive disease indicators is favorable [499]. A recent meta-analysis of proteomic studies by Stalmach et al. [500], using a gene ontology-driven approach, suggests that it is advantageous to integrate MS data sets from both muscle tissue samples and suitable biofluids to gain more comprehensive insights into atrophying changes in the human skeletal muscle proteome.
This gives non-invasive biomarker investigations of biological fluids, such as serum, saliva or urine, a central role in aging research [501,502,503]. The serum of both older humans suffering from sarcopenia [504,505,506,507] and senescent mice [508] were shown to exhibit differential changes in common markers that are associated with inflammation, remodeling of the extracellular matrix and mitochondrial functions [398]. This suggests the potential usage of pro-inflammatory cytokines, growth factors, differentiation factors and leaked mitochondrial proteins as suitable biofluid markers to evaluate the degree of skeletal muscle aging [400]. The regulatory factor myostatin and insulin growth factor IGF-1 show considerable potential to be useful as gender-specific markers of low skeletal muscle mass and frailty [509]. Ideally, proteomic findings are correlated to the results from systematic transcriptomic and metabolomic studies of sarcopenia [510,511,512].
Promising biofluid protein markers of sarcopenia are the carboxy-terminal fragment of agrin (CAF) [513,514,515,516] and the brain-derived neurotrophic factor [517,518,519]. The proteoglycan agrin is closely associated with the sarcolemmal dystrophin/utrophin-glycoprotein complex that is involved in the cytoskeletal stabilization of the neuromuscular junction [520]. The loss of neuromuscular junction integrity appears to play a key role in muscular atrophy [377,521] including sarcopenia of old age [522]. The activity of the synapse-specific protease neuro-trypsin [523], and agrin cleavage, are clearly related to the age-dependent degeneration of the neuromuscular junction [513]. The remodeling of aged motor units in turn is linked to the preferential denervation of fast-twitching and mostly glycolytic type II fibers, and faulty patterns of reinnervation by smaller motor neurons that establish slower-contracting type I motor units [524,525,526].
The age-related fiber-type shifting and accompanying changes in MyHC isoforms [527] can only generate lower maximum force levels in senescent skeletal muscles as compared to young and adult muscle systems. These alterations in the overall composition of motor units probably plays a central role in the gradual loss of skeletal muscle strength during aging [528]. This makes circulating CAF a potential biofluid biomarker of motor unit changes in sarcopenia, in conjunction with fast-to-slow fiber-type shifting in aged muscle tissues, as outlined in Figure 5.
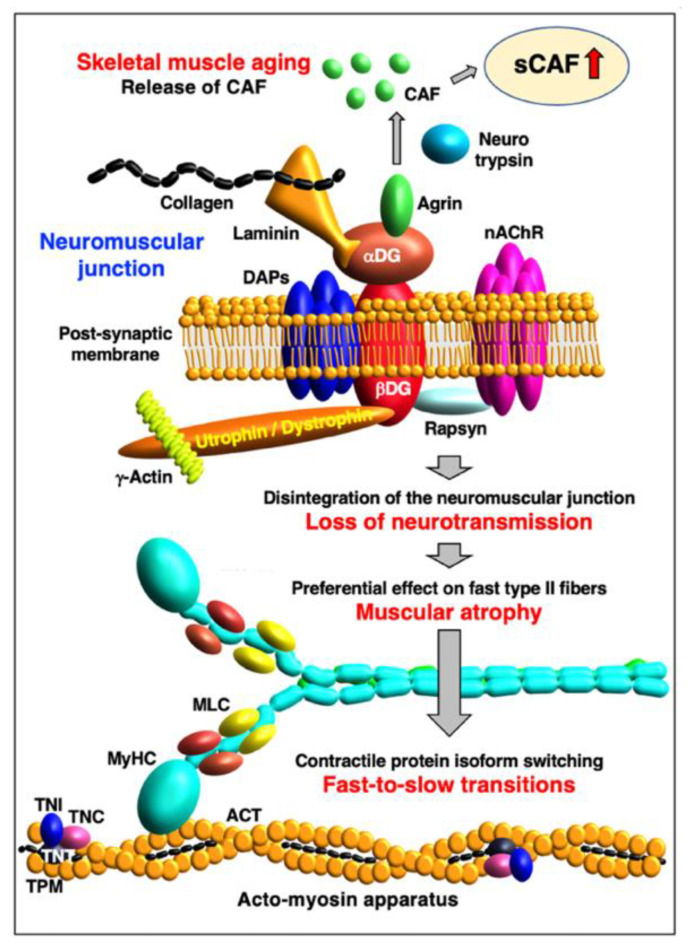
Figure 5.
Agrin as a potential serum biomarker of skeletal muscle aging. Shown is the linkage between the disintegration of the neuromuscular junction during skeletal muscle aging and resulting preferential loss of neurotransmission to fast type II fibers. A potential biomarker candidate of this process is the release of carboxy-terminal agrin fragments (CAF) that can be measured in the serum (sCAF) of patients suffering from sarcopenia of old age. At the neuromuscular junction, the proteoglycan agrin associates with the dystroglycan complex (alpha/beta-DG), which forms an integral part of the sub-sarcolemmal utrophin/dystrophin lattice and its associated proteins (DAPs) at the post-synaptic membrane. During skeletal muscle aging, the integrity of the neuromuscular junction is lost, and agrin is proteolytically cleaved by the enzyme neuro-trypsin. This results in the production of distinct agrin fragments that can be conveniently detected in a minimally invasive way in suitable biofluids.
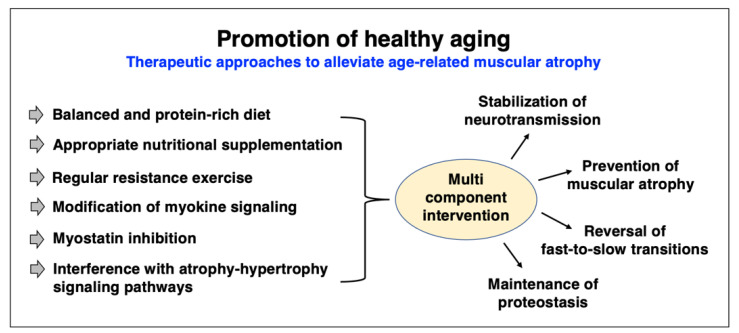
4.3. Therapeutic Approaches to Counteract Age-Related Muscular Atrophy
Aging-associated processes lead to a general decline of health status, a higher risk of disease and drastically reduced physical fitness. It is crucial to take multi-system derangements into consideration when designing novel therapeutic approaches to treat individual age-related ailments, such as sarcopenia. Frailty syndrome can result in a diminished quality of life and even loss of independence in the case of severe and chronic muscle wasting. General recommendations to support healthy aging include the positive influence of a healthy and balanced diet, sufficient sleep, regular relaxation, proper physical exercise, calm breathing patterns, regular social interactions and a positive view of life [529,530,531,532]. Thus, promoting a healthy lifestyle should include countermeasures against sarcopenia of old age to avoid the premature loss of physical strength and skeletal muscle mass. However, a crucial issue for the elderly is proper access to advanced strength training equipment and the realistic implementation of health-promoting support structures, especially during pandemics. During the current COVID-19 crisis, the aged population has only limited access to gyms, parks, recreational facilities and rehabilitation services, causing long-term negative effects on muscle health [533,534,535], and this situation has to be urgently addressed to promote healthy aging. In addition, the treatment of acute sarcopenia in patients with or without COVID-19 infection has been complicated by the restricted access to health services during the pandemic [536,537,538,539], and the increased application of mechanical ventilation and complications during ventilator weaning has caused considerable side effects, including skeletal muscle wasting [540].
Therapeutic approaches to attenuate the impact of age-related skeletal muscle degeneration include non-pharmacological interventions, such as lifestyle changes that incorporate regular and appropriate resistance training [351,352,353,541,542], and optimized dietary considerations, including a protein-rich diet and the frequent ingestion of small portions of high-quality food [358,359,360]. Nutritional combinations of Vitamin D, leucine-enriched protein supplements and whey protein were shown to have some effects on building skeletal muscle mass and improve physical functionality of the neuromuscular system [543,544,545,546]. The combination of mixed types of regular physical exercise with a balanced diet and nutritional supplementation appears to be the most suitable multi-component intervention strategy to minimize the effects of sarcopenia and avoid mobility disability in older adults [547,548]. A protein-rich diet, combined with high levels of physical activity, should both stimulate muscle protein synthesis and thus prevent impaired proteostasis in senescent fibers, and have generally positive effects on metabolism, bioenergetics and hormonal balance. At advanced age, combining a low-intensity form of home-based resistance exercise with proper nutrition and a multi-ingredient supplementation seems to be the most effective way to treat sarcopenia.
Regular exercise has a profound effect on the skeletal muscle proteome [68,354,419] and muscle fiber-type diversification [549,550,551]. In particular, resistance exercise aimed at improving the contractile strength of aged skeletal muscles is generally associated with alterations in myofiber size, muscle re-innervation, fiber-type-specific myonuclear adaptations, mitochondrial remodeling and fiber-type shifting [552,553,554,555,556,557]. Since the age-related loss of skeletal muscle mass is mostly due to a drastic reduction in the size of fast-twitching type II fibers [50,51,339,416,558], it is encouraging that resistance exercise specifically results in the hypertrophy of type II muscle fibers, although it does not appear to affect patterns of fiber-type grouping in aged muscles [559]. Distinct changes in MyHC isoform expression patterns are usually exemplified by reduced MyHC-1 and increased MyHC-2x levels [554].
Current pharmacological trials to treat sarcopenia focus on the potential suitability of various agents, including appetite stimulants, protein anabolic agents, growth hormones, anabolic steroids, androgenic steroids, androgenic receptor modulators, angiotensin-converting enzyme inhibitors, troponin activators, select receptor blockers and myostatin inhibitors [15,560,561,562,563]. Interesting therapeutic options to treat sarcopenia are also provided by interference with atrophy–hypertrophy signaling pathways [564,565,566,567,568]. The above outlined degradation of agrin by neuro-trypsin at the neuromuscular junction [513,514,515] also presents a potential therapeutic target to address abnormal innervation patterns in aged skeletal muscles by employing agrin replacement therapy [516]. One of the most interesting biomedical approaches to treat sarcopenia is myostatin therapy.
Myostatin is a secreted myogenic factor that acts as a negative regulator of skeletal muscle growth. It belongs to the transforming growth factor TGF-beta family of proteins and functions by inhibiting the phosphorylation of Akt protein kinase within the insulin-like growth factor 1–phosphatidylinositol-3-kinase–serine/threonine protein kinase PKB–mammalian target of rapamycin (IGF-1/PI3K/Akt/mTOR) signaling pathway [569]. Consequently, the inhibition of a negative regulator might result in a positive effect on skeletal muscle growth. This can be supported by (i) natural mechanisms, such as physical exercise, (ii) dietary supplements and nutraceutical agents and/or (iii) pharmacological/biotechnological intervention with myostatin inhibitors [570,571,572], including antibody-based therapy [573]. The rebalancing of muscular atrophy versus hypertrophy by a growth-promoting process could modulate the aging process and have a positive effect on physical fitness and neuromuscular function [574].
Ideally, the above-described therapeutic approaches to improve general skeletal muscle strength would especially target the fast-twitching fiber population that is mostly susceptible to muscular atrophy in the elderly [50,339,558]. Figure 6 provides a summary of current therapeutic options to treat sarcopenia of old age. For a critical assessment of current pharmacological strategies to halt or reverse age-related muscular atrophy, see the recent review articles by Cho et al. [15], Kim et al. [561] and Huang et al. [563].
Figure 6.
Overview of therapeutic approaches to counteract age-related muscular atrophy.
5. Conclusions
The proteomic analysis of muscular atrophy in association with sarcopenia has detected distinct changes in a variety of protein families. Alterations in aging skeletal muscles include proteins involved in fiber contraction and relaxation, the regulation of excitation–contraction coupling, ion homeostasis, energy metabolism, maintenance of the cytoskeleton, the extracellular matrix and the cellular stress response. Skeletal muscle aging was shown to be linked to a tendency of fast-to-slow transitions and increased oxidative bioenergetics, as well as myofibrotic changes and a drastic increase in the expression of molecular chaperones. These proteomic findings support the concept of extensive degenerative and adaptive responses in the skeletal musculature due to sarcopenia of old age. Independently verified transcriptomic and proteomic markers of fiber-type shifting and metabolic modifications can now be used as indicators of molecular and cellular changes in both aging human skeletal muscles and animal models of sarcopenia. In the future, it will be of interest to study proteome-wide differences between age-related skeletal muscle wasting and other types of muscular atrophy caused by a variety of diverse triggering factors, such as denervation following motor nerve crush or spinal cord injury, prolonged bedrest in association with chronic disease, inappropriate levels of neuromuscular loading during plaster cast immobilization or prolonged exposure to microgravity. Since skeletal muscle performance deteriorates following extended periods of microgravity [3,575,576], which has been studied by proteomics [577], it has been suggested that certain aspects of neuromuscular alterations during prolonged spaceflights resemble changes in sarcopenia [578]. This opens new possibilities to study accelerated types of muscle-related stress and the molecular and cellular factors involved in muscular atrophy by the exposure of muscle cells to microgravity [579]. The detailed comparison of proteomic and systems bioinformatic data of different forms of muscular atrophy can be helpful to dissect the signaling mechanisms and disturbed biochemical, physiological and cellular processes that lead to diverse forms of muscle wasting.
Abbreviations
| ACTA | Alpha-actin-1, skeletal muscle |
| ACTC | Alpha-actin-1, cardiac muscle |
| ACTN-2 | Alpha-actinin-2 |
| ACTN-3 | Alpha-actinin-3 |
| AI | Artificial intelligence |
| BAC | Benzyldimethyl-n-hexadecylammonium chloride |
| BAC-DROP | BAC-gel dissolution to digest PAGE-resolved objective proteins |
| BN | Blue native |
| CAF | Carboxy-terminal fragment of agrin |
| CBB | Coomassie brilliant blue |
| CyTOF | Mass cytometry |
| DAPs | Dystrophin-associated proteins |
| DDA | Data-dependent acquisition |
| DG | Dystroglycan |
| DIA | Data-independent acquisition |
| DIGE | Difference gel electrophoresis |
| DL | Deep learning |
| ELISA | Enzyme-linked immunosorbent assays |
| ESI | Electrospray ionization |
| FACS | Fluorescence-activated cell sorting |
| FASP | Filter-aided sample preparation |
| FC | Flow cytometry |
| FFPE | Formalin-fixed, paraffin-embedded |
| GE | Gel electrophoresis |
| GeLC-MS/MS | Gel electrophoresis liquid chromatography mass spectrometry |
| IB | Immunoblotting |
| ICAT | Isotope-coded affinity tags |
| IFM | Immunofluorescence microscopy |
| IHC | Immunohistochemistry |
| iST | In-StageTip |
| iTRAQ | Isobaric tags for relative and absolute quantitation |
| LC | Liquid chromatography |
| MALDI | Matrix-assisted laser desorption/ionization |
| MARP | Muscle ankyrin repeat protein (ANKRD-2) |
| mIHC/IF | Multiplex immunohistochemistry/immunofluorescence |
| ML | Machine learning |
| MLC-2f | Myosin light chain, fast, regulatory (MYL-11) |
| MLC-2s | Myosin light chain, slow/cardiac, regulatory (MYL-2) |
| MLC-1/3f | Myosin light chain, fast, essential (MLC-1 and MLC-3) |
| MLC-1s | Myosin light chain, slow, essential (MLC-1sa and MLC-1sb) |
| MS | Mass spectrometry |
| MS/MS | Tandem mass spectrometry |
| MudPIT | Multidimensional protein identification technology |
| MuSCs | Muscle stem cells |
| MYBP-C1 | Myosin-binding protein C1, slow |
| MYBP-C2 | Myosin-binding protein C2, fast |
| MyHC-1 | Myosin heavy chain, slow type-I (Myosin-7) |
| MyHC-2a | Myosin heavy chain, fast type-IIA (Myosin-2) |
| MyHC-2b | Myosin heavy chain, fast type-IIB (Myosin-4) |
| MyHC-2x | Myosin heavy chain, fast type-IIX (Myosin-1) |
| MyHC-6 | Myosin heavy chain MYH-6 (Myosin-6) |
| MyHC-7B | Myosin heavy chain MYH-7B (Myosin-7B) |
| MyHC-13 | Myosin heavy chain MYH-13, extraocular muscle (Myosin-13) |
| MyHC-14 | Myosin heavy chain MYH-14 (Myosin-14) |
| MyHC-15 | Myosin heavy chain MYH-15 (Myoisn-15) |
| MyHC-16 | Myosin heavy chain MYH-16 (Myosin-16) |
| MyHC-emb | Myosin heavy chain, embryonic muscle, MyHC-3 (Myosin-3), |
| MyHC-neo | Myosin heavy chain, perinatal muscle, MyHC-8 (Myosin-8) |
| MYOM-1 | Myomesin-1 |
| MYOM-2 | Myomesin-2 |
| MYOZ-1 | Myozenin-1 |
| MYOZ-2 | Myozenin-2 |
| MYOZ-3 | Myozenin-3 |
| nAChR | Nicotinic acetylcholine receptor |
| NEB | Nebulin |
| OBSCN | Obscurin |
| PAGE | Polyacrylamide gel electrophoresis |
| PCT | Pressure-cycling technology |
| PRM | Parallel Reaction Monitoring |
| PTM | Post-translational modification |
| sCAF | Serum carboxy-terminal fragment of agrin |
| SCoPE-MS | Single Cell ProtEomics by Mass Spectrometry |
| SCoPE2 | Second-generation protocol called Single Cell ProtEomics |
| SDOC | Sarcopenia Definitions and Outcomes Consortium |
| SDS | Sodium dodecyl sulfate |
| SILAC | Stable isotope labelling by amino acids in cell culture |
| SP3 | Single-pot solid-phase-enhanced sample preparation |
| SRM/MRM | Selected/Multiple Reaction Monitoring |
| SWATH-MS | Sequential window acquisition of all theoretical mass spectra |
| TCAP | Telethonin/titin-cap |
| TDA | Targeted data acquisition |
| TMT | Tandem mass tag |
| TNC-1 | Troponin TnC, slow/cardiac |
| TNC-2 | Troponin TnC, skeletal muscle |
| TNI-1 | Troponin TnI, slow muscle |
| TNI-2 | Troponin TnI, fast muscle |
| TNT-1 | Troponin TnT, slow muscle |
| TNT-3 | Troponin TnT, fast muscle |
| TOF | Time-of-flight |
| TPM-1 | Alpha-1-tropomyosin |
| TPM-2 | Beta-tropomyosin, slow muscle |
| TPM-3 | Alpha-3-tropomyosin, muscle |
| TPM-4 | Alpha-4-tropomyosin, cytoskeletal |
| TTN | Titin |
| USP3 | Universal solid-phase protein preparation |
Author Contributions
Conceptualization, P.D. and K.O.; writing—original draft preparation, P.D. and K.O.; writing—review and editing, P.D., S.G., D.S. and K.O. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Mass spectrometric raw data from studies of aging diaphragm muscle shown in tables and figures are available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Research was funded by a project grant from the Kathleen Lonsdale Institute for Human Health Research, Maynooth University and equipment funding under the Research Infrastructure Call 2012 by Science Foundation Ireland (SFI-12/RI/2346/3).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bonaldo P., Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013;6:25–39. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao R.Y., Li J., Dai Q., Li Q., Yang J. Muscle Atrophy: Present and Future. Adv. Exp. Med. Biol. 2018;1088:605–624. doi: 10.1007/978-981-13-1435-3_29. [DOI] [PubMed] [Google Scholar]
- 3.Capri M., Morsiani C., Santoro A., Moriggi M., Conte M., Martucci M., Bellavista E., Fabbri C., Giampieri E., Albracht K., et al. Recovery from 6-month spaceflight at the International Space Station: Muscle-related stress into a proinflammatory setting. FASEB J. 2019;33:5168–5180. doi: 10.1096/fj.201801625R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J., Huang Y., Chen Y., Shen X., Pan H., Yu W. Impact of Muscle Mass on Survival in Patients with Sepsis: A Systematic Review and Meta-Analysis. Ann. Nutr. Metab. 2021;77:330–336. doi: 10.1159/000519642. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S., Nathan J.A., Goldberg A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 6.Shum A.M.Y., Poljak A., Bentley N.L., Turner N., Tan T.C., Polly P. Proteomic profiling of skeletal and cardiac muscle in cancer cachexia: Alterations in sarcomeric and mitochondrial protein expression. Oncotarget. 2018;9:22001–22022. doi: 10.18632/oncotarget.25146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin L., Li N., Jia W., Wang N., Liang M., Yang X., Du G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021;172:105807. doi: 10.1016/j.phrs.2021.105807. [DOI] [PubMed] [Google Scholar]
- 8.Feldman E.L., Goutman S.A., Petri S., Mazzini L., Savelieff M.G., Shaw P.J., Sobue G. Amyotrophic lateral sclerosis. Lancet. 2022;400:1363–1380. doi: 10.1016/S0140-6736(22)01272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft A.J., Sayer A.A. Sarcopenia. Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 10.Larsson L., Degens H., Li M., Salviati L., Lee Y.I., Thompson W., Kirkland J.L., Sandri M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019;99:427–511. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishikawa H., Fukunishi S., Asai A., Yokohama K., Nishiguchi S., Higuchi K. Pathophysiology and mechanisms of primary sarcopenia (Review) Int. J. Mol. Med. 2021;48:156. doi: 10.3892/ijmm.2021.4989. [DOI] [PubMed] [Google Scholar]
- 12.Lippi G., Sanchis-Gomar F., Montagnana M. Biological markers in older people at risk of mobility limitations. Curr. Pharm. Des. 2014;20:3222–3244. doi: 10.2174/13816128113196660697. [DOI] [PubMed] [Google Scholar]
- 13.Dhillon R.J., Hasni S. Pathogenesis and Management of Sarcopenia. Clin. Geriatr. Med. 2017;33:17–26. doi: 10.1016/j.cger.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zullo A., Fleckenstein J., Schleip R., Hoppe K., Wearing S., Klingler W. Structural and Functional Changes in the Coupling of Fascial Tissue, Skeletal Muscle, and Nerves During Aging. Front. Physiol. 2020;11:592. doi: 10.3389/fphys.2020.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho M.R., Lee S., Song S.K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022;37:e146. doi: 10.3346/jkms.2022.37.e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch D.H., Spangler H.B., Franz J.R., Krupenevich R.L., Kim H., Nissman D., Zhang J., Li Y.Y., Sumner S., Batsis J.A. Multimodal Diagnostic Approaches to Advance Precision Medicine in Sarcopenia and Frailty. Nutrients. 2022;14:1384. doi: 10.3390/nu14071384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dos Santos L., Cyrino E.S., Antunes M., Santos D.A., Sardinha L.B. Sarcopenia and physical independence in older adults: The independent and synergic role of muscle mass and muscle function. J. Cachexia Sarcopenia Muscle. 2017;8:245–250. doi: 10.1002/jcsm.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeung S.S.Y., Reijnierse E.M., Pham V.K., Trappenburg M.C., Lim W.K., Meskers C.G.M., Maier A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle. 2019;10:485–500. doi: 10.1002/jcsm.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dent E., Martin F.C., Bergman H., Woo J., Romero-Ortunom R., Walston J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet. 2019;394:1376–1386. doi: 10.1016/S0140-6736(19)31785-4. [DOI] [PubMed] [Google Scholar]
- 20.Smith L., Sanchez G.F.L., Veronese N., Soysal P., Kostev K., Jacob L., Oh H., Tully M.A., Butler L., Parsa A.D., et al. Association between sarcopenia and quality of life among adults aged ≥ 65 years from low- and middle-income countries. Aging Clin. Exp. Res. 2022;34:2779–2787. doi: 10.1007/s40520-022-02231-8. [DOI] [PubMed] [Google Scholar]
- 21.Veronese N., Koyanagi A., Cereda E., Maggi S., Barbagallo M., Dominguez L.J., Smith L. Sarcopenia reduces quality of life in the long-term: Longitudinal analyses from the English longitudinal study of ageing. Eur. Geriatr. Med. 2022;13:633–639. doi: 10.1007/s41999-022-00627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chianca V., Albano D., Messina C., Gitto S., Ruffo G., Guarino S., Del Grande F., Sconfienza L.M. Sarcopenia: Imaging assessment and clinical application. Abdom. Radiol. 2022;47:3205–3216. doi: 10.1007/s00261-021-03294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruz-Jentoft A.J., Landi F., Schneider S.M., Zúñiga C., Arai H., Boiriem Y., Chenm L.K., Fielding R.A., Martin F.C., Michel J.P., et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43:748–759. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morley J.E., Abbatecola A.M., Argiles J.M., Baracos V., Bauer J., Bhasin S., Cederholm T., Coats A.J., Cummings S.R., Evans W.J., et al. Society on Sarcopenia, Cachexia and Wasting Disorders Trialist Workshop. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011;12:403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Studenski S.A., Peters K.W., Alley D.E., Cawthon P.M., McLean R.R., Harris T.B., Ferrucci L., Guralnik J.M., Fragala M.S., Kenny A.M., et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ackermans L.L.G.C., Rabou J., Basrai M., Schweinlin A., Bischoff S.C., Cussenot O., Cancel-Tassin G., Renken R.J., Gómez E., Sánchez-González P., et al. Screening, diagnosis and monitoring of sarcopenia: When to use which tool? Clin. Nutr. ESPEN. 2022;48:36–44. doi: 10.1016/j.clnesp.2022.01.027. [DOI] [PubMed] [Google Scholar]
- 28.Kirkeby S., Garbarsch C. Aging affects different human muscles in various ways. An image analysis of the histomorphometric characteristics of fiber types in human masseter and vastus lateralis muscles from young adults and the very old. Histol. Histopathol. 2000;15:61–71. doi: 10.14670/HH-15.61. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman J.M., Lyu Y., Pletcher S.D., Promislow D.E.L. Proteomics and metabolomics in ageing research: From biomarkers to systems biology. Essays Biochem. 2017;61:379–388. doi: 10.1042/EBC20160083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McHugh D., Gil J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018;217:65–77. doi: 10.1083/jcb.201708092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aunan J.R., Watson M.M., Hagland H.R., Søreide K. Molecular and biological hallmarks of ageing. Br. J. Surg. 2016;103:e29–e46. doi: 10.1002/bjs.10053. [DOI] [PubMed] [Google Scholar]
- 33.Perazza L.R., Brown-Borg H.M., Thompson L.V. Physiological Systems in Promoting Frailty. Compr. Physiol. 2022;12:3575–3620. doi: 10.1002/cphy.c210034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez-Segura A., Nehme J., Demaria M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018;28:436–453. doi: 10.1016/j.tcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Omholt S.W., Kirkwood T.B.L. Aging as a consequence of selection to reduce the environmental risk of dying. Proc. Natl. Acad. Sci. USA. 2021;118:e2102088118. doi: 10.1073/pnas.2102088118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kowald A., Passos J.F., Kirkwood T.B.L. On the evolution of cellular senescence. Aging Cell. 2020;19:e13270. doi: 10.1111/acel.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmeer C., Kretz A., Wengerodt D., Stojiljkovic M., Witte O.W. Dissecting Aging and Senescence-Current Concepts and Open Lessons. Cells. 2019;8:1446. doi: 10.3390/cells8111446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campisi J., Kapahi P., Lithgow G.J., Melov S., Newman J.C., Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571:183–192. doi: 10.1038/s41586-019-1365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doran P., Donoghue P., O’Connell K., Gannon J., Ohlendieck K. Proteomics of skeletal muscle aging. Proteomics. 2009;9:989–1003. doi: 10.1002/pmic.200800365. [DOI] [PubMed] [Google Scholar]
- 40.Ohlendieck K. Proteomics of skeletal muscle differentiation, neuromuscular disorders and fiber aging. Expert Rev. Proteom. 2010;7:283–296. doi: 10.1586/epr.10.2. [DOI] [PubMed] [Google Scholar]
- 41.Baraibar M.A., Gueugneau M., Duguez S., Butler-Browne G., Bechet D., Friguet B. Expression and modification proteomics during skeletal muscle ageing. Biogerontology. 2013;14:339–352. doi: 10.1007/s10522-013-9426-7. [DOI] [PubMed] [Google Scholar]
- 42.Danese E., Montagnana M., Lippi G. Proteomics and frailty: A clinical overview. Expert Rev. Proteom. 2018;15:657–664. doi: 10.1080/14789450.2018.1505511. [DOI] [PubMed] [Google Scholar]
- 43.Moaddel R., Ubaida-Mohien C., Tanaka T., Lyashkov A., Basisty N., Schilling B., Semba R.D., Franceschi C., Gorospe M., Ferrucci L. Proteomics in aging research: A roadmap to clinical, translational research. Aging Cell. 2021;20:e13325. doi: 10.1111/acel.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aebersold R., Mann M. Mass-spectrometric exploration of proteome structure and function. Nature. 2016;537:347–355. doi: 10.1038/nature19949. [DOI] [PubMed] [Google Scholar]
- 45.Lill J.R., Mathews W.R., Rose C.M., Schirle M. Proteomics in the pharmaceutical and biotechnology industry: A look to the next decade. Expert Rev. Proteom. 2021;18:503–526. doi: 10.1080/14789450.2021.1962300. [DOI] [PubMed] [Google Scholar]
- 46.Sobsey C.A., Ibrahim S., Richard V.R., Gaspar V., Mitsa G., Lacasse V., Zahedi R.P., Batist G., Borchers C.H. Targeted and Untargeted Proteomics Approaches in Biomarker Development. Proteomics. 2020;20:e1900029. doi: 10.1002/pmic.201900029. [DOI] [PubMed] [Google Scholar]
- 47.Mann S.P., Treit P.V., Geyer P.E., Omenn G.S., Mann M. Ethical Principles, Constraints and Opportunities in Clinical Proteomics. Mol. Cell. Proteom. 2021;20:100046. doi: 10.1016/j.mcpro.2021.100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivero-Segura N.A., Bello-Chavolla O.Y., Barrera-Vázquez O.S., Gutierrez-Robledo L.M., Gomez-Verjan J.C. Promising biomarkers of human aging: In search of a multi-omics panel to understand the aging process from a multidimensional perspective. Ageing Res. Rev. 2020;64:101164. doi: 10.1016/j.arr.2020.101164. [DOI] [PubMed] [Google Scholar]
- 49.Pan Y., Ji T., Li Y., Ma L. Omics biomarkers for frailty in older adults. Clin. Chim. Acta. 2020;510:363–372. doi: 10.1016/j.cca.2020.07.057. [DOI] [PubMed] [Google Scholar]
- 50.Ohlendieck K. Proteomic Profiling of Fast-To-Slow Muscle Transitions during Aging. Front. Physiol. 2011;2:105. doi: 10.3389/fphys.2011.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dowling P., Murphy S., Ohlendieck K. Proteomic profiling of muscle fibre type shifting in neuromuscular diseases. Expert Rev. Proteom. 2016;13:783–799. doi: 10.1080/14789450.2016.1209416. [DOI] [PubMed] [Google Scholar]
- 52.Dowling P., Zweyer M., Swandulla D., Ohlendieck K. Characterization of Contractile Proteins from Skeletal Muscle Using Gel-Based Top-Down Proteomics. Proteomes. 2019;7:25. doi: 10.3390/proteomes7020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wasinger V.C., Cordwell S.J., Cerpa-Poljak A., Yan J.X., Gooley A.A., Wilkins M.R., Duncan M.W., Harris R., Williams K.L., Humphery-Smith I. Progress with gene-product mapping of the Mollicutes: Mycoplasma genitalium. Electrophoresis. 1995;16:1090–1094. doi: 10.1002/elps.11501601185. [DOI] [PubMed] [Google Scholar]
- 54.Wilkins M.R., Sanchez J.C., Gooley A.A., Appel R.D., Humphery-Smith I., Hochstrasser D.F., Williams K.L. Progress with proteome projects: Why all proteins expressed by a genome should be identified and how to do it. Biotechnol. Genet. Eng. Rev. 1996;13:19–50. doi: 10.1080/02648725.1996.10647923. [DOI] [PubMed] [Google Scholar]
- 55.Manes N.P., Nita-Lazar A. Application of targeted mass spectrometry in bottom-up proteomics for systems biology research. J. Proteom. 2018;189:75–90. doi: 10.1016/j.jprot.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dupree E.J., Jayathirtha M., Yorkey H., Mihasan M., Petre B.A., Darie C.C. A Critical Review of Bottom-Up Proteomics: The Good, the Bad, and the Future of this Field. Proteomes. 2010;8:14. doi: 10.3390/proteomes8030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Révész Á., Hevér H., Steckel A., Schlosser G., Szabó D., Vékey K., Drahos L. Collision energies: Optimization strategies for bottom-up proteomics. Mass Spectrom. Rev. 2021;2:e21763. doi: 10.1002/mas.21763. [DOI] [PubMed] [Google Scholar]
- 58.Padula M.P., Berry I.J., Rourke M.B.O., Raymond B.B., Santos J., Djordjevic S.P. A Comprehensive Guide for Performing Sample Preparation and Top-Down Protein Analysis. Proteomes. 2017;5:11. doi: 10.3390/proteomes5020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cupp-Sutton K.A., Wu S. High-throughput quantitative top-down proteomics. Mol. Omics. 2020;16:91–99. doi: 10.1039/C9MO00154A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown K.A., Melby J.A., Roberts D.S., Ge Y. Top-down proteomics: Challenges, innovations, and applications in basic and clinical research. Expert Rev. Proteom. 2020;17:719–733. doi: 10.1080/14789450.2020.1855982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carbonara K., Andonovski M., Coorssen J.R. Proteomes Are of Proteoforms: Embracing the Complexity. Proteomes. 2021;9:38. doi: 10.3390/proteomes9030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaffer L.V., Millikin R.J., Miller R.M., Anderson L.C., Fellers R.T., Ge Y., Kelleher N.L., LeDuc R.D., Liu X., Payne S.H., et al. Identification and Quantification of Proteoforms by Mass Spectrometry. Proteomics. 2019;19:e1800361. doi: 10.1002/pmic.201800361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang R., Wang Z., Lu H. Separation methods for system-wide profiling of protein terminome. Proteomics. 2022:e2100374. doi: 10.1002/pmic.202100374. in press. [DOI] [PubMed] [Google Scholar]
- 64.Bludau I., Aebersold R. Proteomic and interactomic insights into the molecular basis of cell functional diversity. Nat. Rev. Mol. Cell Biol. 2020;21:327–340. doi: 10.1038/s41580-020-0231-2. [DOI] [PubMed] [Google Scholar]
- 65.Ohlendieck K. Skeletal muscle proteomics: Current approaches, technical challenges and emerging techniques. Skelet. Muscle. 2011;1:6. doi: 10.1186/2044-5040-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murphy S., Dowling P., Ohlendieck K. Comparative Skeletal Muscle Proteomics Using Two-Dimensional Gel Electrophoresis. Proteomes. 2016;4:27. doi: 10.3390/proteomes4030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Capitanio D., Moriggi M., Gelfi C. Mapping the human skeletal muscle proteome: Progress and potential. Expert Rev. Proteom. 2017;14:825–839. doi: 10.1080/14789450.2017.1364996. [DOI] [PubMed] [Google Scholar]
- 68.Hesketh S.J., Stansfield B.N., Stead C.A., Burniston J.G. The application of proteomics in muscle exercise physiology. Expert Rev. Proteom. 2020;17:813–825. doi: 10.1080/14789450.2020.1879647. [DOI] [PubMed] [Google Scholar]
- 69.Domon B., Aebersold R. Mass spectrometry and protein analysis. Science. 2006;312:212–217. doi: 10.1126/science.1124619. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y., Fonslow B.R., Shan B., Baek M.C., Yates J.R., 3rd Protein analysis by shotgun/bottom-up proteomics. Chem. Rev. 2013;113:2343–2394. doi: 10.1021/cr3003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yates J.R., Ruse C.I., Nakorchevsky A. Proteomics by mass spectrometry: Approaches, advances, and applications. Annu. Rev. Biomed. Eng. 2009;11:49–79. doi: 10.1146/annurev-bioeng-061008-124934. [DOI] [PubMed] [Google Scholar]
- 72.Yates J.R., 3rd Recent technical advances in proteomics. F1000Res. 2019;8:F1000 Faculty Rev-351. doi: 10.12688/f1000research.16987.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sabidó E., Selevsek N., Aebersold R. Mass spectrometry-based proteomics for systems biology. Curr. Opin. Biotechnol. 2012;23:591–597. doi: 10.1016/j.copbio.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 74.Omenn G.S., Lane L., Overall C.M., Corrales F.J., Schwenk J.M., Paik Y.K., Van Eyk J.E., Liu S., Snyder M., Baker M.S., et al. Progress on Identifying and Characterizing the Human Proteome: 2018 Metrics from the HUPO Human Proteome Project. J. Proteome Res. 2018;17:4031–4041. doi: 10.1021/acs.jproteome.8b00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Omenn G.S. Reflections on the HUPO Human Proteome Project, the Flagship Project of the Human Proteome Organization, at 10 Years. Mol. Cell. Proteom. 2021;20:100062. doi: 10.1016/j.mcpro.2021.100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Omenn G.S., Lane L., Overall C.M., Pineau C., Packer N.H., Cristea I.M., Lindskog C., Weintraub S.T., Orchard S., Roehrl M.H.A., et al. The 2022 Report on the Human Proteome from the HUPO Human Proteome Project. J. Proteome Res. 2022 doi: 10.1021/acs.jproteome.2c00498. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilhelm M., Schlegl J., Hahne H., Gholami A.M., Lieberenz M., Savitski M.M., Ziegler E., Butzmann L., Gessulat S., Marx H., et al. Mass-spectrometry-based draft of the human proteome. Nature. 2014;509:582–587. doi: 10.1038/nature13319. [DOI] [PubMed] [Google Scholar]
- 78.Kim M.S., Pinto S.M., Getnet D., Nirujogi R.S., Manda S.S., Chaerkady R., Madugundu A.K., Kelkar D.S., Isserlin R., Jain S., et al. A draft map of the human proteome. Nature. 2014;509:575–581. doi: 10.1038/nature13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adhikari S., Nice E.C., Deutsch E.W., Lane L., Omenn G.S., Pennington S.R., Paik Y.K., Overall C.M., Corrales F.J., Cristea I.M., et al. A high-stringency blueprint of the human proteome. Nat. Commun. 2020;11:5301. doi: 10.1038/s41467-020-19045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Westermeier R. 2D gel-based Proteomics: There’s life in the old dog yet. Arch. Physiol. Biochem. 2016;122:236–237. doi: 10.1080/13813455.2016.1179766. [DOI] [PubMed] [Google Scholar]
- 81.Zhan X., Li B., Zhan X., Schlüter H., Jungblut P.R., Coorssen J.R. Innovating the Concept and Practice of Two-Dimensional Gel Electrophoresis in the Analysis of Proteomes at the Proteoform Level. Proteomes. 2019;7:36. doi: 10.3390/proteomes7040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marcus K., Lelong C., Rabilloud T. What Room for Two-Dimensional Gel-Based Proteomics in a Shotgun Proteomics World? Proteomes. 2020;8:17. doi: 10.3390/proteomes8030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Friedman D.B., Hoving S., Westermeier R. Isoelectric focusing and two-dimensional gel electrophoresis. Methods Enzymol. 2009;463:515–540. doi: 10.1016/S0076-6879(09)63030-5. [DOI] [PubMed] [Google Scholar]
- 84.Rabilloud T., Chevallet M., Luche S., Lelong C. Two-dimensional gel electrophoresis in proteomics: Past, present and future. J. Proteom. 2010;73:2064–2077. doi: 10.1016/j.jprot.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 85.Oliveira B.M., Coorssen J.R., Martins-de-Souza D. 2DE: The phoenix of proteomics. J. Proteom. 2014;104:140–150. doi: 10.1016/j.jprot.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 86.Westermeier R. Looking at proteins from two dimensions: A review on five decades of 2D electrophoresis. Arch. Physiol. Biochem. 2014;120:168–172. doi: 10.3109/13813455.2014.945188. [DOI] [PubMed] [Google Scholar]
- 87.Rabilloud T., Lelong C. Two-dimensional gel electrophoresis in proteomics: A tutorial. J. Proteom. 2011;74:1829–1841. doi: 10.1016/j.jprot.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 88.Lee P.Y., Saraygord-Afshari N., Low T.Y. The evolution of two-dimensional gel electrophoresis—From proteomics to emerging alternative applications. J. Chromatogr. A. 2020;1615:460763. doi: 10.1016/j.chroma.2019.460763. [DOI] [PubMed] [Google Scholar]
- 89.Carbonara K., Coorssen J.R. Sometimes faster can be better: Microneedling IPG strips enables higher throughput for integrative top-down proteomics. Proteomics. 2023;23:e2200307. doi: 10.1002/pmic.202200307. [DOI] [PubMed] [Google Scholar]
- 90.Görg A., Weiss W., Dunn M.J. Current two-dimensional electrophoresis technology for proteomics. Proteomics. 2004;4:3665–3685. doi: 10.1002/pmic.200401031. [DOI] [PubMed] [Google Scholar]
- 91.Carrette O., Burkhard P.R., Sanchez J.C., Hochstrasser D.F. State-of-the-art two-dimensional gel electrophoresis: A key tool of proteomics research. Nat. Protoc. 2006;1:812–823. doi: 10.1038/nprot.2006.104. [DOI] [PubMed] [Google Scholar]
- 92.Yoneten K.K., Kasap M., Akpinar G., Kanli A., Karaoz E. Comparative Proteomics Analysis of Four Commonly Used Methods for Identification of Novel Plasma Membrane Proteins. J. Membr. Biol. 2019;252:587–608. doi: 10.1007/s00232-019-00084-3. [DOI] [PubMed] [Google Scholar]
- 93.Zahedi R.P., Moebius J., Sickmann A. Two-dimensional BAC/SDS-PAGE for membrane proteomics. Subcell. Biochem. 2002;43:13–20. doi: 10.1007/978-1-4020-5943-8_2. [DOI] [PubMed] [Google Scholar]
- 94.Wittig I., Braun H.P., Schägger H. Blue native PAGE. Nat. Protoc. 2006;1:418–428. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- 95.Fernandez-Vizarra E., Zeviani M. Blue-Native Electrophoresis to Study the OXPHOS Complexes. Methods Mol. Biol. 2021;2192:287–311. doi: 10.1007/978-1-0716-0834-0_20. [DOI] [PubMed] [Google Scholar]
- 96.Sunderhaus S., Eubel H., Braun H.P. Two-dimensional blue native/blue native polyacrylamide gel electrophoresis for the characterization of mitochondrial protein complexes and supercomplexes. Methods Mol. Biol. 2007;372:315–324. doi: 10.1007/978-1-59745-365-3_23. [DOI] [PubMed] [Google Scholar]
- 97.Vincis Pereira Sanglard L., des Francs-Small C.C. High-Throughput BN-PAGE for Mitochondrial Respiratory Complexes. Methods Mol. Biol. 2022;2363:111–119. doi: 10.1007/978-1-0716-1653-6_10. [DOI] [PubMed] [Google Scholar]
- 98.Singh K., Duchen M.R. Analysis of Organization and Activity of Mitochondrial Respiratory Chain Complexes in Primary Fibroblasts Using Blue Native PAGE. Methods Mol. Biol. 2022;2497:339–348. doi: 10.1007/978-1-0716-2309-1_25. [DOI] [PubMed] [Google Scholar]
- 99.Maguire P.B., Briggs F.N., Lennon N.J., Ohlendieck K. Oligomerization is an intrinsic property of calsequestrin in normal and transformed skeletal muscle. Biochem. Biophys. Res. Commun. 1997;240:721–727. doi: 10.1006/bbrc.1997.7729. [DOI] [PubMed] [Google Scholar]
- 100.Froemming G.R., Murray B.E., Ohlendieck K. Self-aggregation of triadin in the sarcoplasmic reticulum of rabbit skeletal muscle. Biochim. Biophys. Acta. 1999;1418:197–205. doi: 10.1016/S0005-2736(99)00024-3. [DOI] [PubMed] [Google Scholar]
- 101.Culligan K., Banville N., Dowling P., Ohlendieck K. Drastic reduction of calsequestrin-like proteins and impaired calcium binding in dystrophic mdx muscle. J. Appl. Physiol. 2002;92:435–445. doi: 10.1152/japplphysiol.00903.2001. [DOI] [PubMed] [Google Scholar]
- 102.Noaman N., Coorssen J.R. Coomassie does it (better): A Robin Hood approach to total protein quantification. Anal. Biochem. 2018;556:53–56. doi: 10.1016/j.ab.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 103.Noaman N., Abbineni P.S., Withers M., Coorssen J.R. Coomassie staining provides routine (sub)femtomole in-gel detection of intact proteoforms: Expanding opportunities for genuine Top-down Proteomics. Electrophoresis. 2017;38:3086–3099. doi: 10.1002/elps.201700190. [DOI] [PubMed] [Google Scholar]
- 104.Chevalier F. Standard Dyes for Total Protein Staining in Gel-Based Proteomic Analysis. Materials. 2010;3:4784–4792. doi: 10.3390/ma3104784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Panfoli I., Calzia D., Santucci L., Ravera S., Bruschi M., Candiano G. A blue dive: From ‘blue fingers’ to ‘blue silver’. A comparative overview of staining methods for in-gel proteomics. Expert Rev. Proteom. 2012;9:627–634. doi: 10.1586/epr.12.63. [DOI] [PubMed] [Google Scholar]
- 106.Sundaram P. Protein Stains and Applications. Methods Mol. Biol. 2018;1853:1–14. doi: 10.1007/978-1-4939-8745-0_1. [DOI] [PubMed] [Google Scholar]
- 107.Meleady P. Two-Dimensional Gel Electrophoresis and 2D-DIGE. Methods Mol. Biol. 2023;2596:3–15. doi: 10.1007/978-1-0716-2831-7_1. [DOI] [PubMed] [Google Scholar]
- 108.Murphy S., Dowling P., Zweyer M., Swandulla D., Ohlendieck K. Proteomic profiling of giant skeletal muscle proteins. Expert Rev. Proteom. 2019;16:241–256. doi: 10.1080/14789450.2019.1575205. [DOI] [PubMed] [Google Scholar]
- 109.Murphy S., Henry M., Meleady P., Ohlendieck K. Utilization of dried and long-term stored polyacrylamide gels for the advanced proteomic profiling of mitochondrial contact sites from rat liver. Biol. Methods Protoc. 2018;3:bpy008. doi: 10.1093/biomethods/bpy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Murphy S., Ohlendieck K. Proteomic profiling of large myofibrillar proteins from dried and long-term stored polyacrylamide gels. Anal. Biochem. 2018;543:8–11. doi: 10.1016/j.ab.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 111.Banks C.A., Kong S.E., Washburn M.P. Affinity purification of protein complexes for analysis by multidimensional protein identification technology. Protein Expr. Purif. 2012;86:105–119. doi: 10.1016/j.pep.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 112.Elschenbroich S., Ignatchenko V., Sharma P., Schmitt-Ulms G., Gramolini A.O., Kislinger T. Peptide separations by on-line MudPIT compared to isoelectric focusing in an off-gel format: Application to a membrane-enriched fraction from C2C12 mouse skeletal muscle cells. J. Proteome Res. 2009;8:4860–4869. doi: 10.1021/pr900318k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Unlü M., Morgan M.E., Minden J.S. Difference gel electrophoresis: A single gel method for detecting changes in protein extracts. Electrophoresis. 1997;18:2071–2077. doi: 10.1002/elps.1150181133. [DOI] [PubMed] [Google Scholar]
- 114.Minden J.S., Dowd S.R., Meyer H.E., Stühler K. Difference gel electrophoresis. Electrophoresis. 2009;30:S156–S161. doi: 10.1002/elps.200900098. [DOI] [PubMed] [Google Scholar]
- 115.Arentz G., Weiland F., Oehler M.K., Hoffmann P. State of the art of 2D DIGE. Proteom. Clin. Appl. 2015;9:277–288. doi: 10.1002/prca.201400119. [DOI] [PubMed] [Google Scholar]
- 116.Timms J.F., Cramer R. Difference gel electrophoresis. Proteomics. 2008;8:4886–4897. doi: 10.1002/pmic.200800298. [DOI] [PubMed] [Google Scholar]
- 117.Blundon M., Ganesan V., Redler B., Van P.T., Minden J.S. Two-Dimensional Difference Gel Electrophoresis. Methods Mol. Biol. 2019;1855:229–247. doi: 10.1007/978-1-4939-8793-1_20. [DOI] [PubMed] [Google Scholar]
- 118.Gelfi C., Capitanio D. DIGE Analysis of Clinical Specimens. Methods Mol. Biol. 2023;2596:177–199. doi: 10.1007/978-1-0716-2831-7_14. [DOI] [PubMed] [Google Scholar]
- 119.Di Luca A., Hamill R., Mullen A.M., Elia G. DIGE Analysis of Animal Tissues. Methods Mol. Biol. 2023;2596:201–216. doi: 10.1007/978-1-0716-2831-7_15. [DOI] [PubMed] [Google Scholar]
- 120.Holland A. Two-Dye Versus Three-Dye DIGE for Comparative Testis Tissue Proteomic Analysis. Methods Mol. Biol. 2023;2596:245–263. doi: 10.1007/978-1-0716-2831-7_18. [DOI] [PubMed] [Google Scholar]
- 121.Ohlendieck K. Top-Down Proteomics and Comparative 2D-DIGE Analysis. Methods Mol. Biol. 2023;2596:19–38. doi: 10.1007/978-1-0716-2831-7_2. [DOI] [PubMed] [Google Scholar]
- 122.Ohlendieck K. Comparative 3-Sample 2D-DIGE Analysis of Skeletal Muscles. Methods Mol. Biol. 2023;2596:127–146. doi: 10.1007/978-1-0716-2831-7_11. [DOI] [PubMed] [Google Scholar]
- 123.Tonge R., Shaw J., Middleton B., Rowlinson R., Rayner S., Young J., Pognan F., Hawkins E., Currie I., Davison M. Validation and development of fluorescence two-dimensional differential gel electrophoresis proteomics technology. Proteomics. 2001;1:377–396. doi: 10.1002/1615-9861(200103)1:3<377::AID-PROT377>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 124.Marouga R., David S., Hawkins E. The development of the DIGE system: 2D fluorescence difference gel analysis technology. Anal. Bioanal. Chem. 2005;382:669–678. doi: 10.1007/s00216-005-3126-3. [DOI] [PubMed] [Google Scholar]
- 125.Dowling P. DIGE Analysis Software and Protein Identification Approaches. Methods Mol. Biol. 2023;2596:39–50. doi: 10.1007/978-1-0716-2831-7_3. [DOI] [PubMed] [Google Scholar]
- 126.Dani D., Dencher N.A. Native DIGE for Quantitative and Functional Analysis of Protein Interactomes. Methods Mol. Biol. 2023;2596:53–69. doi: 10.1007/978-1-0716-2831-7_4. [DOI] [PubMed] [Google Scholar]
- 127.Ackermann D., König S. Comparative Two-Dimensional Fluorescence Gel Electrophoresis. Methods Mol. Biol. 2023;2596:71–81. doi: 10.1007/978-1-0716-2831-7_5. [DOI] [PubMed] [Google Scholar]
- 128.Stasyk T., Huber L.A. DIGE-Based Phosphoproteomic Analysis. Methods Mol. Biol. 2023;2596:97–104. doi: 10.1007/978-1-0716-2831-7_7. [DOI] [PubMed] [Google Scholar]
- 129.Carberry S., Zweyer M., Swandulla D., Ohlendieck K. Application of fluorescence two-dimensional difference in-gel electrophoresis as a proteomic biomarker discovery tool in muscular dystrophy research. Biology. 2013;2:1438–1464. doi: 10.3390/biology2041438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Forgrave L.M., Wang M., Yang D., DeMarco M.L. Proteoforms and their expanding role in laboratory medicine. Pract. Lab Med. 2021;28:e00260. doi: 10.1016/j.plabm.2021.e00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Landsberger M., Brinkmeier H. Immunoblot Analysis of DIGE-Based Proteomics. Methods Mol. Biol. 2023;2596:429–443. doi: 10.1007/978-1-0716-2831-7_29. [DOI] [PubMed] [Google Scholar]
- 132.Zweyer M., Ohlendieck K., Swandulla D. Histological and Histochemical Microscopy Used to Verify 2D-DIGE Pathoproteomics. Methods Mol. Biol. 2023;2596:465–480. doi: 10.1007/978-1-0716-2831-7_31. [DOI] [PubMed] [Google Scholar]
- 133.Zweyer M., Ohlendieck K., Swandulla D. Verification of Protein Changes Determined by 2D-DIGE Based Proteomics Using Immunofluorescence Microscopy. Methods Mol. Biol. 2023;2596:445–464. doi: 10.1007/978-1-0716-2831-7_30. [DOI] [PubMed] [Google Scholar]
- 134.Tabatabaei M.S., Ahmed M. Enzyme-Linked Immunosorbent Assay (ELISA) Methods Mol. Biol. 2022;2508:115–134. doi: 10.1007/978-1-0716-2376-3_10. [DOI] [PubMed] [Google Scholar]
- 135.Siddiqui S., Livák F. Principles of Advanced Flow Cytometry: A Practical Guide. Methods Mol. Biol. 2023;2580:89–114. doi: 10.1007/978-1-0716-2740-2_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pakula A., Spinazzola J.M., Gussoni E. Purification of Myogenic Progenitors from Human Muscle Using Fluorescence-Activated Cell Sorting (FACS) Methods Mol. Biol. 2019;1889:1–15. doi: 10.1007/978-1-4939-8897-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Behbehani G.K. Immunophenotyping by Mass Cytometry. Methods Mol. Biol. 2019;2032:31–51. doi: 10.1007/978-1-4939-9650-6_2. [DOI] [PubMed] [Google Scholar]
- 138.Helali Y., Sharma S., Vandeput M., Welba D., Van Antwerpen P., Marchant A., Delporte C. Fc Glycosylation Characterization of Human Immunoglobulins G Using Immunocapture and LC-MS. Methods Mol. Biol. 2021;2271:57–71. doi: 10.1007/978-1-0716-1241-5_4. [DOI] [PubMed] [Google Scholar]
- 139.Ghosh R., Gilda J.E., Gomes A.V. The necessity of and strategies for improving confidence in the accuracy of western blots. Expert Rev. Proteom. 2014;11:549–560. doi: 10.1586/14789450.2014.939635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zweyer M., Sabir H., Dowling P., Gargan S., Murphy S., Swandulla D., Ohlendieck K. Histopathology of Duchenne muscular dystrophy in correlation with changes in proteomic biomarkers. Histol. Histopathol. 2022;37:101–116. doi: 10.14670/HH-18-403. [DOI] [PubMed] [Google Scholar]
- 141.Dowd A. Elucidating Cellular Metabolism and Protein Difference Data from DIGE Proteomics Experiments Using Enzyme Assays. Methods Mol. Biol. 2023;2596:399–419. doi: 10.1007/978-1-0716-2831-7_27. [DOI] [PubMed] [Google Scholar]
- 142.Dowd A. Enzyme Assay Methods to Validate DIGE Proteomics Data. Methods Mol. Biol. 2023;2596:421–428. doi: 10.1007/978-1-0716-2831-7_28. [DOI] [PubMed] [Google Scholar]
- 143.Mishra M., Tiwari S., Gomes A.V. Protein purification and analysis: Next generation Western blotting techniques. Expert Rev. Proteom. 2017;14:1037–1053. doi: 10.1080/14789450.2017.1388167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Meola G. Advanced microscopic and histochemical techniques: Diagnostic tools in the molecular era of myology. Eur. J. Histochem. 2005;49:93–96. [PubMed] [Google Scholar]
- 145.Nix J.S., Moore S.A. What Every Neuropathologist Needs to Know: The Muscle Biopsy. J. Neuropathol. Exp. Neurol. 2020;79:719–733. doi: 10.1093/jnen/nlaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Su T., Wang S., Huang S., Cai H., McKinley E.T., Beeghly-Fadiel A., Zheng W., Shu X.O., Cai Q. Multiplex immunohistochemistry and high-throughput image analysis for evaluation of spatial tumor immune cell markers in human breast cancer. Cancer Biomark. 2022;35:193–206. doi: 10.3233/CBM-220071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tan W.C.C., Nerurkar S.N., Cai H.Y., Ng H.H.M., Wu D., Wee Y.T.F., Lim C.T., Yeong J., Lim T.K.H. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun. 2020;40:135–153. doi: 10.1002/cac2.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alcazar J., Frandsen U., Prokhorova T., Kamper R.S., Haddock B., Aagaard P., Suetta C. Changes in systemic GDF15 across the adult lifespan and their impact on maximal muscle power: The Copenhagen Sarcopenia Study. J. Cachexia Sarcopenia Muscle. 2021;12:1418–1427. doi: 10.1002/jcsm.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mengeste A.M., Nikolić N., Fernandez A.D., Feng Y.Z., Nyman T.A., Kersten S., Haugen F., Kase E.T., Aas V., Rustan A.C., et al. Insight Into the Metabolic Adaptations of Electrically Pulse-Stimulated Human Myotubes Using Global Analysis of the Transcriptome and Proteome. Front. Physiol. 2022;13:928195. doi: 10.3389/fphys.2022.928195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Krüger K., Seimetz M., Ringseis R., Wilhelm J., Pichl A., Couturier A., Eder K., Weissmann N., Mooren F.C. Exercise training reverses inflammation and muscle wasting after tobacco smoke exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;314:R366–R376. doi: 10.1152/ajpregu.00316.2017. [DOI] [PubMed] [Google Scholar]
- 151.San Segundo-Acosta P., Montero-Calle A., Jernbom-Falk A., Alonso-Navarro M., Pin E., Andersson E., Hellström C., Sánchez-Martínez M., Rábano A., Solís-Fernández G., et al. Multiomics Profiling of Alzheimer’s Disease Serum for the Identification of Autoantibody Biomarkers. J. Proteome Res. 2021;20:5115–5130. doi: 10.1021/acs.jproteome.1c00630. [DOI] [PubMed] [Google Scholar]
- 152.Pereira S.L., Shoemaker M.E., Gawel S., Davis G.J., Luo M., Mustad V.A., Cramer J.T. Biomarker Changes in Response to a 12-Week Supplementation of an Oral Nutritional Supplement Enriched with Protein, Vitamin D and HMB in Malnourished Community Dwelling Older Adults with Sarcopenia. Nutrients. 2022;14:1196. doi: 10.3390/nu14061196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Assarsson E., Lundberg M., Holmquist G., Björkesten J., Thorsen S.B., Ekman D., Eriksson A., Rennel Dickens E., Ohlsson S., Edfeldt G., et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9:e95192. doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Al Saedi A., Phu S., Vogrin S., Gunawardene P., Duque G. Association between Circulating Osteoprogenitor Cells and Sarcopenia. Gerontology. 2022;68:1038–1043. doi: 10.1159/000520488. [DOI] [PubMed] [Google Scholar]
- 155.Kirk B., Zanker J., Bani Hassan E., Bird S., Brennan-Olsen S., Duque G. Sarcopenia Definitions and Outcomes Consortium (SDOC) Criteria are Strongly Associated With Malnutrition, Depression, Falls, and Fractures in High-Risk Older Persons. J. Am. Med. Dir. Assoc. 2021;22:741–745. doi: 10.1016/j.jamda.2020.06.050. [DOI] [PubMed] [Google Scholar]
- 156.Bandura D.R., Baranov V.I., Ornatsky O.I., Antonov A., Kinach R., Lou X., Pavlov S., Vorobiev S., Dick J.E., Tanner S.D. Mass cytometry: Technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal. Chem. 2009;81:6813–6822. doi: 10.1021/ac901049w. [DOI] [PubMed] [Google Scholar]
- 157.Tan T., Gray D.H.D., The C.E. Single-Cell Profiling of the Intrinsic Apoptotic Pathway by Mass Cytometry (CyTOF) Methods Mol. Biol. 2022;2543:83–97. doi: 10.1007/978-1-0716-2553-8_8. [DOI] [PubMed] [Google Scholar]
- 158.Porpiglia E., Samusik N., Ho A.T.V., Cosgrove B.D., Mai T., Davis K.L., Jager A., Nolan G.P., Bendall S.C., Fantl W.J., et al. High-resolution myogenic lineage mapping by single-cell mass cytometry. Nat. Cell Biol. 2017;19:558–567. doi: 10.1038/ncb3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Petrilli L.L., Riccio F., Giuliani G., Palma A., Gargioli C., Vumbaca S., Faron M., Palmieri G., Pasquini L., Sacco F., et al. Skeletal Muscle Subpopulation Rearrangements upon Rhabdomyosarcoma Development through Single-Cell Mass Cytometry. J. Clin. Med. 2021;10:823. doi: 10.3390/jcm10040823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Borok M., Didier N., Gattazzo F., Ozturk T., Corneau A., Rouard H., Relaix F. Progressive and Coordinated Mobilization of the Skeletal Muscle Niche throughout Tissue Repair Revealed by Single-Cell Proteomic Analysis. Cells. 2021;10:744. doi: 10.3390/cells10040744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Porpiglia E., Mai T., Kraft P., Holbrook C.A., de Morree A., Gonzalez V.D., Hilgendorf K.I., Frésard L., Trejo A., Bhimaraju S., et al. Elevated CD47 is a hallmark of dysfunctional aged muscle stem cells that can be targeted to augment regeneration. Cell Stem Cell. 2022;29:1653–1668.e8. doi: 10.1016/j.stem.2022.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Swaney D.L., Villén J. Enrichment of Modified Peptides via Immunoaffinity Precipitation with Modification-Specific Antibodies. Cold Spring Harb. Protoc. 2016;2016:pdb-prot088013. doi: 10.1101/pdb.prot088013. [DOI] [PubMed] [Google Scholar]
- 163.Kumar A., Baycin-Hizal D., Shiloach J., Bowen M.A., Betenbaugh M.J. Coupling enrichment methods with proteomics for understanding and treating disease. Proteom. Clin. Appl. 2015;9:33–47. doi: 10.1002/prca.201400097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Beltran L., Cutillas P.R. Advances in phosphopeptide enrichment techniques for phosphoproteomics. Amino Acids. 2012;43:1009–1024. doi: 10.1007/s00726-012-1288-9. [DOI] [PubMed] [Google Scholar]
- 165.Gargan S., Ohlendieck K. Sample Preparation and Protein Determination for 2D-DIGE Proteomics. Methods Mol. Biol. 2023;2596:325–337. doi: 10.1007/978-1-0716-2831-7_22. [DOI] [PubMed] [Google Scholar]
- 166.Murphy S. Subcellular Fractionation for DIGE-Based Proteomics. Methods Mol. Biol. 2023;2596:351–362. doi: 10.1007/978-1-0716-2831-7_24. [DOI] [PubMed] [Google Scholar]
- 167.Murphy S., Zweyer M., Henry M., Meleady P., Mundegar R.R., Swandulla D., Ohlendieck K. Proteomic analysis of the sarcolemma-enriched fraction from dystrophic mdx-4cv skeletal muscle. J. Proteom. 2019;191:212–227. doi: 10.1016/j.jprot.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 168.Murphy S., Zweyer M., Mundegar R.R., Swandulla D., Ohlendieck K. Comparative gel-based proteomic analysis of chemically crosslinked complexes in dystrophic skeletal muscle. Electrophoresis. 2018;39:1735–1744. doi: 10.1002/elps.201800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sielaff M., Kuharev J., Bohn T., Hahlbrock J., Bopp T., Tenzer S., Distler U. Evaluation of FASP, SP3, and iST Protocols for Proteomic Sample Preparation in the Low Microgram Range. J. Proteome Res. 2017;16:4060–4072. doi: 10.1021/acs.jproteome.7b00433. [DOI] [PubMed] [Google Scholar]
- 170.Wiśniewski J.R., Zougman A., Mann M. Combination of FASP and StageTip-based fractionation allows in-depth analysis of the hippocampal membrane proteome. J. Proteome Res. 2009;8:5674–5678. doi: 10.1021/pr900748n. [DOI] [PubMed] [Google Scholar]
- 171.Xie Z., Feng Q., Zhang S., Yan Y., Deng C., Ding C.F. Advances in proteomics sample preparation and enrichment for phosphorylation and glycosylation analysis. Proteomics. 2022;22:e2200070. doi: 10.1002/pmic.202200070. [DOI] [PubMed] [Google Scholar]
- 172.Wiśniewski J.R., Zougman A., Nagaraj N., Mann M. Universal sample preparation method for proteome analysis. Nat. Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 173.Wiśniewski J.R. Filter Aided Sample Preparation—A tutorial. Anal. Chim. Acta. 2019;1090:23–30. doi: 10.1016/j.aca.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 174.Kulak N.A., Pichler G., Paron I., Nagaraj N., Mann M. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods. 2014;11:319–324. doi: 10.1038/nmeth.2834. [DOI] [PubMed] [Google Scholar]
- 175.Hughes C.S., Moggridge S., Müller T., Sorensen P.H., Morin G.B., Krijgsveld J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019;14:68–85. doi: 10.1038/s41596-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 176.Dagley L.F., Infusini G., Larsen R.H., Sandow J.J., Webb A.I. Universal Solid-Phase Protein Preparation (USP3) for Bottom-up and Top-down Proteomics. J. Proteome Res. 2019;18:2915–2924. doi: 10.1021/acs.jproteome.9b00217. [DOI] [PubMed] [Google Scholar]
- 177.Dapic I., Baljeu-Neuman L., Uwugiaren N., Kers J., Goodlett D.R., Corthals G.L. Proteome analysis of tissues by mass spectrometry. Mass Spectrom. Rev. 2019;38:403–441. doi: 10.1002/mas.21598. [DOI] [PubMed] [Google Scholar]
- 178.Cai X., Xue Z., Wu C., Sun R., Qian L., Yue L., Ge W., Yi X., Liu W., Chen C., et al. High-throughput proteomic sample preparation using pressure cycling technology. Nat. Protoc. 2022;17:2307–2325. doi: 10.1038/s41596-022-00727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vit O., Petrak J. Integral membrane proteins in proteomics. How to break open the black box? J. Proteom. 2017;153:8–20. doi: 10.1016/j.jprot.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 180.Kar U.K., Simonian M., Whitelegge J.P. Integral membrane proteins: Bottom-up, top-down and structural proteomics. Expert Rev. Proteom. 2017;14:715–723. doi: 10.1080/14789450.2017.1359545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dafun A.S., Marcoux J. Structural mass spectrometry of membrane proteins. Biochim. Biophys. Acta Proteins Proteom. 2022;1870:140813. doi: 10.1016/j.bbapap.2022.140813. [DOI] [PubMed] [Google Scholar]
- 182.Boeri Erba E., Signor L., Petosa C. Exploring the structure and dynamics of macromolecular complexes by native mass spectrometry. J. Proteom. 2020;222:103799. doi: 10.1016/j.jprot.2020.103799. [DOI] [PubMed] [Google Scholar]
- 183.Tamara S., den Boer M.A., Heck A.J.R. High-Resolution Native Mass Spectrometry. Chem. Rev. 2022;122:7269–7326. doi: 10.1021/acs.chemrev.1c00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Santambrogio C., Ponzini E., Grandori R. Native mass spectrometry for the investigation of protein structural (dis)order. Biochim. Biophys. Acta Proteins Proteom. 2022;1870:140828. doi: 10.1016/j.bbapap.2022.140828. [DOI] [PubMed] [Google Scholar]
- 185.Choksawangkarn W., Edwards N., Wang Y., Gutierrez P., Fenselau C. Comparative study of workflows optimized for in-gel, in-solution, and on-filter proteolysis in the analysis of plasma membrane proteins. J. Proteome Res. 2012;11:3030–3034. doi: 10.1021/pr300188b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Glatter T., Ludwig C., Ahrné E., Aebersold R., Heck A.J., Schmidt A. Large-scale quantitative assessment of different in-solution protein digestion protocols reveals superior cleavage efficiency of tandem Lys-C/trypsin proteolysis over trypsin digestion. J. Proteome Res. 2012;11:5145–5156. doi: 10.1021/pr300273g. [DOI] [PubMed] [Google Scholar]
- 187.Goodman J.K., Zampronio C.G., Jones A.M.E., Hernandez-Fernaud J.R. Updates of the In-Gel Digestion Method for Protein Analysis by Mass Spectrometry. Proteomics. 2018;18:e1800236. doi: 10.1002/pmic.201800236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gauci V.J., Wright E.P., Coorssen J.R. Quantitative proteomics: Assessing the spectrum of in-gel protein detection methods. J. Chem. Biol. 2011;4:3–29. doi: 10.1007/s12154-010-0043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lewis C., Ohlendieck K. Mass spectrometric identification of dystrophin isoform Dp427 by on-membrane digestion of sarcolemma from skeletal muscle. Anal. Biochem. 2010;404:197–203. doi: 10.1016/j.ab.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 190.Staunton L., Ohlendieck K. Mass spectrometric characterization of the sarcoplasmic reticulum from rabbit skeletal muscle by on-membrane digestion. Protein Pept. Lett. 2012;19:252–263. doi: 10.2174/092986612799363208. [DOI] [PubMed] [Google Scholar]
- 191.Murphy S., Brinkmeier H., Krautwald M., Henry M., Meleady P., Ohlendieck K. Proteomic profiling of the dystrophin complex and membrane fraction from dystrophic mdx muscle reveals decreases in the cytolinker desmoglein and increases in the extracellular matrix stabilizers biglycan and fibronectin. J. Muscle Res. Cell. Motil. 2017;38:251–268. doi: 10.1007/s10974-017-9478-4. [DOI] [PubMed] [Google Scholar]
- 192.Murphy S., Ohlendieck K. Protein Digestion for 2D-DIGE Analysis. Methods Mol. Biol. 2023;2596:339–349. doi: 10.1007/978-1-0716-2831-7_23. [DOI] [PubMed] [Google Scholar]
- 193.Tsiatsiani L., Heck A.J. Proteomics beyond trypsin. FEBS J. 2015;282:2612–2626. doi: 10.1111/febs.13287. [DOI] [PubMed] [Google Scholar]
- 194.Giansanti P., Tsiatsiani L., Low T.Y., Heck A.J. Six alternative proteases for mass spectrometry-based proteomics beyond trypsin. Nat. Protoc. 2016;11:993–1006. doi: 10.1038/nprot.2016.057. [DOI] [PubMed] [Google Scholar]
- 195.Zhang X. Less is More: Membrane Protein Digestion Beyond Urea-Trypsin Solution for Next-level Proteomics. Mol. Cell. Proteom. 2015;14:2441–2453. doi: 10.1074/mcp.R114.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Goldman A.R., Beer L.A., Tang H.Y., Hembach P., Zayas-Bazan D., Speicher D.W. Proteome Analysis Using Gel-LC-MS/MS. Curr. Protoc. Protein Sci. 2019;96:e93. doi: 10.1002/cpps.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Takemori A., Ishizaki J., Nakashima K., Shibata T., Kato H., Kodera Y., Suzuki T., Hasegawa H., Takemori N. BAC-DROP: Rapid Digestion of Proteome Fractionated via Dissolvable Polyacrylamide Gel Electrophoresis and Its Application to Bottom-Up Proteomics Workflow. J. Proteome Res. 2021;20:1535–1543. doi: 10.1021/acs.jproteome.0c00749. [DOI] [PubMed] [Google Scholar]
- 198.Rešetar Maslov D., Svirkova A., Allmaier G., Marchetti-Deschamann M., Kraljević Pavelić S. Optimization of MALDI-TOF mass spectrometry imaging for the visualization and comparison of peptide distributions in dry-cured ham muscle fibers. Food Chem. 2019;283:275–286. doi: 10.1016/j.foodchem.2018.12.126. [DOI] [PubMed] [Google Scholar]
- 199.Evangelista A.J., Ferreira T.L. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry in the diagnosis of microorganisms. Future Microbiol. 2022;17:1409–1419. doi: 10.2217/fmb-2022-0067. [DOI] [PubMed] [Google Scholar]
- 200.Nesvizhskii A.I., Vitek O., Aebersold R. Analysis and validation of proteomic data generated by tandem mass spectrometry. Nat. Methods. 2007;4:787–797. doi: 10.1038/nmeth1088. [DOI] [PubMed] [Google Scholar]
- 201.Dowling P., Gargan S., Zweyer M., Henry M., Meleady P., Swandulla D., Ohlendieck K. Protocol for the Bottom-Up Proteomic Analysis of Mouse Spleen. STAR Protoc. 2020;1:100196. doi: 10.1016/j.xpro.2020.100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Théron L., Gueugneau M., Coudy C., Viala D., Bijlsma A., Butler-Browne G., Maier A., Béchet D., Chambon C. Label-free quantitative protein profiling of vastus lateralis muscle during human aging. Mol. Cell. Proteom. 2014;13:283–294. doi: 10.1074/mcp.M113.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rauniyar N., Yates J.R., 3rd Isobaric labeling-based relative quantification in shotgun proteomics. J. Proteome Res. 2014;13:5293–5309. doi: 10.1021/pr500880b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen X., Sun Y., Zhang T., Shu L., Roepstorff P., Yang F. Quantitative Proteomics Using Isobaric Labeling: A Practical Guide. Genom. Proteom. Bioinform. 2021;19:689–706. doi: 10.1016/j.gpb.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Beller N.C., Hummon A.B. Advances in stable isotope labeling: Dynamic labeling for spatial and temporal proteomic analysis. Mol. Omics. 2022;18:579–590. doi: 10.1039/D2MO00077F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Xing T., Wang C., Zhao X., Dai C., Zhou G., Xu X. Proteome Analysis Using Isobaric Tags for Relative and Absolute Analysis Quantitation (iTRAQ) Reveals Alterations in Stress-Induced Dysfunctional Chicken Muscle. J. Agric. Food Chem. 2017;65:2913–2922. doi: 10.1021/acs.jafc.6b05835. [DOI] [PubMed] [Google Scholar]
- 207.Chahrour O., Cobice D., Malone J. Stable isotope labelling methods in mass spectrometry-based quantitative proteomics. J. Pharm. Biomed. Anal. 2015;113:2–20. doi: 10.1016/j.jpba.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 208.Westbrook J.A., Noirel J., Brown J.E., Wright P.C., Evans C.A. Quantitation with chemical tagging reagents in biomarker studies. Proteom. Clin. Appl. 2015;9:295–300. doi: 10.1002/prca.201400120. [DOI] [PubMed] [Google Scholar]
- 209.Chaves D.F., Carvalho P.C., Lima D.B., Nicastro H., Lorenzeti F.M., Siqueira-Filho M., Hirabara S.M., Alves P.H., Moresco J.J., Yates J.R., 3rd, et al. Comparative proteomic analysis of the aging soleus and extensor digitorum longus rat muscles using TMT labeling and mass spectrometry. J. Proteome Res. 2013;12:4532–4546. doi: 10.1021/pr400644x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hoedt E., Zhang G., Neubert T.A. Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC) for Quantitative Proteomics. Adv. Exp. Med. Biol. 2019;1140:531–539. doi: 10.1007/978-3-030-15950-4_31. [DOI] [PubMed] [Google Scholar]
- 211.Shenoy A., Geiger T. Super-SILAC: Current trends and future perspectives. Expert Rev. Proteom. 2015;12:13–19. doi: 10.1586/14789450.2015.982538. [DOI] [PubMed] [Google Scholar]
- 212.Geiger T., Wisniewski J.R., Cox J., Zanivan S., Kruger M., Ishihama Y., Mann M. Use of stable isotope labeling by amino acids in cell culture as a spike-in standard in quantitative proteomics. Nat. Protoc. 2011;6:147–157. doi: 10.1038/nprot.2010.192. [DOI] [PubMed] [Google Scholar]
- 213.Rayavarapu S., Coley W., Cakir E., Jahnke V., Takeda S., Aoki Y., Grodish-Dressman H., Jaiswal J.K., Hoffman E.P., Brown K.J., et al. Identification of disease specific pathways using in vivo SILAC proteomics in dystrophin deficient mdx mouse. Mol. Cell. Proteom. 2013;12:1061–1073. doi: 10.1074/mcp.M112.023127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Goswami M.V., Tawalbeh S.M., Canessa E.H., Hathout Y. Temporal Proteomic Profiling During Differentiation of Normal and Dystrophin-Deficient Human Muscle Cells. J. Neuromuscul. Dis. 2021;8:S205–S222. doi: 10.3233/JND-210713. [DOI] [PubMed] [Google Scholar]
- 215.Kallabis S., Abraham L., Müller S., Dzialas V., Türk C., Wiederstein J.L., Bock T., Nolte H., Nogara L., Blaauw B., et al. High-throughput proteomics fiber typing (ProFiT) for comprehensive characterization of single skeletal muscle fibers. Skelet. Muscle. 2020;10:7. doi: 10.1186/s13395-020-00226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lang F., Aravamudhan S., Nolte H., Türk C., Hölper S., Müller S., Günther S., Blaauw B., Braun T., Krüger M. Dynamic changes in the mouse skeletal muscle proteome during denervation-induced atrophy. Dis. Model Mech. 2017;10:881–896. doi: 10.1242/dmm.028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sadygov R.G. Using Heavy Mass Isotopomers for Protein Turnover in Heavy Water Metabolic Labeling. J. Proteome Res. 2021;20:2035–2041. doi: 10.1021/acs.jproteome.0c00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Srisawat K., Hesketh K., Cocks M., Strauss J., Edwards B.J., Lisboa P.J., Shepherd S., Burniston J.G. Reliability of Protein Abundance and Synthesis Measurements in Human Skeletal Muscle. Proteomics. 2020;20:e1900194. doi: 10.1002/pmic.201900194. [DOI] [PubMed] [Google Scholar]
- 219.Stansfield B.N., Brown A.D., Stewart C.E., Burniston J.G. Dynamic Profiling of Protein Mole Synthesis Rates during C2C12 Myoblast Differentiation. Proteomics. 2021;21:e2000071. doi: 10.1002/pmic.202000071. [DOI] [PubMed] [Google Scholar]
- 220.Brown A.D., Stewart C.E., Burniston J.G. Degradation of ribosomal and chaperone proteins is attenuated during the differentiation of replicatively aged C2C12 myoblasts. J. Cachexia Sarcopenia Muscle. 2022;13:2562–2575. doi: 10.1002/jcsm.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Murphy C.H., Shankaran M., Churchward-Venne T.A., Mitchell C.J., Kolar N.M., Burke L.M., Hawley J.A., Kassis A., Karagounis L.G., Li K., et al. Effect of resistance training and protein intake pattern on myofibrillar protein synthesis and proteome kinetics in older men in energy restriction. J. Physiol. 2018;596:2091–2120. doi: 10.1113/JP275246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mann M., Kumar C., Zeng W.F., Strauss M.T. Artificial intelligence for proteomics and biomarker discovery. Cell Syst. 2021;12:759–770. doi: 10.1016/j.cels.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 223.Fenaille F., Barbier Saint-Hilaire P., Rousseau K., Junot C. Data acquisition workflows in liquid chromatography coupled to high resolution mass spectrometry-based metabolomics: Where do we stand? J. Chromatogr. A. 2017;1526:1–12. doi: 10.1016/j.chroma.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 224.Kitata R.B., Yang J.C., Chen Y.J. Advances in data-independent acquisition mass spectrometry towards comprehensive digital proteome landscape. Mass Spectrom. Rev. 2022:e21781. doi: 10.1002/mas.21781. in press. [DOI] [PubMed] [Google Scholar]
- 225.Abdollahi M., Segura P.A., Beaudry F. Is nontargeted data acquisition for target analysis (nDATA) in mass spectrometry a forward-thinking analytical approach? Biomed. Chromatogr. 2022:e5531. doi: 10.1002/bmc.5531. in press. [DOI] [PubMed] [Google Scholar]
- 226.Krasny L., Huang P.H. Data-independent acquisition mass spectrometry (DIA-MS) for proteomic applications in oncology. Mol. Omics. 2021;17:29–42. doi: 10.1039/D0MO00072H. [DOI] [PubMed] [Google Scholar]
- 227.Kawashima Y., Watanabe E., Umeyama T., Nakajima D., Hattori M., Honda K., Ohara O. Optimization of Data-Independent Acquisition Mass Spectrometry for Deep and Highly Sensitive Proteomic Analysis. Int. J. Mol. Sci. 2019;20:5932. doi: 10.3390/ijms20235932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gillet L.C., Navarro P., Tate S., Röst H., Selevsek N., Reiter L., Bonner R., Aebersold R. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: A new concept for consistent and accurate proteome analysis. Mol. Cell. Proteom. 2012;11:O111.016717. doi: 10.1074/mcp.O111.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kontostathi G., Makridakis M., Bitsika V., Tsolakos N., Vlahou A., Zoidakis J. Development and Validation of Multiple Reaction Monitoring (MRM) Assays for Clinical Applications. Methods Mol. Biol. 2019;1959:205–223. doi: 10.1007/978-1-4939-9164-8_14. [DOI] [PubMed] [Google Scholar]
- 230.Cho B.G., Gutierrez Reyes C.D., Goli M., Gautam S., Banazadeh A., Mechref Y. Targeted N-Glycan Analysis with Parallel Reaction Monitoring Using a Quadrupole-Orbitrap Hybrid Mass Spectrometer. Anal. Chem. 2022;94:15215–15222. doi: 10.1021/acs.analchem.2c01975. [DOI] [PubMed] [Google Scholar]
- 231.Ives A.N., Dunn H.A., Afsari H.S., Seckler H.D.S., Foroutan M.J., Chavez E., Melani R.D., Fellers R.T., LeDuc R.D., Thomas P.M., et al. Middle-Down Mass Spectrometry Reveals Activity-Modifying Phosphorylation Barcode in a Class C G Protein-Coupled Receptor. J. Am. Chem. Soc. 2022;144:23104–23114. doi: 10.1021/jacs.2c10697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Shapiro E., Biezuner T., Linnarsson S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat. Rev. Genet. 2013;14:618–630. doi: 10.1038/nrg3542. [DOI] [PubMed] [Google Scholar]
- 233.Vogel C., Marcotte E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Levy E., Slavov N. Single cell protein analysis for systems biology. Essays Biochem. 2018;62:595–605. doi: 10.1042/EBC20180014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Marx V. A dream of single-cell proteomics. Nat. Methods. 2019;16:809–812. doi: 10.1038/s41592-019-0540-6. [DOI] [PubMed] [Google Scholar]
- 236.Budnik B., Levy E., Harmange G., Slavov N. SCoPE-MS: Mass spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome Biol. 2018;19:161. doi: 10.1186/s13059-018-1547-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Specht H., Emmott E., Petelski A.A., Huffman R.G., Perlman D.H., Serra M., Kharchenko P., Koller A., Slavov N. Single-cell proteomic and transcriptomic analysis of macrophage heterogeneity using SCoPE2. Genome Biol. 2021;22:50. doi: 10.1186/s13059-021-02267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Brunner A.D., Thielert M., Vasilopoulou C., Ammar C., Coscia F., Mund A., Hoerning O.B., Bache N., Apalategui A., Lubeck M., et al. Ultra-high sensitivity mass spectrometry quantifies single-cell proteome changes upon perturbation. Mol. Syst. Biol. 2022;18:e10798. doi: 10.15252/msb.202110798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Petelski A.A., Emmott E., Leduc A., Huffman R.G., Specht H., Perlman D.H., Slavov N. Multiplexed single-cell proteomics using SCoPE2. Nat. Protoc. 2021;16:5398–5425. doi: 10.1038/s41596-021-00616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cheung T.K., Lee C.Y., Bayer F.P., McCoy A., Kuster B., Rose C.M. Defining the carrier proteome limit for single-cell proteomics. Nat. Methods. 2021;18:76–83. doi: 10.1038/s41592-020-01002-5. [DOI] [PubMed] [Google Scholar]
- 241.Huffman R.G., Chen A., Specht H., Slavov N. DO-MS: Data-Driven Optimization of Mass Spectrometry Methods. J. Proteome Res. 2019;18:2493–2500. doi: 10.1021/acs.jproteome.9b00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Cong Y., Motamedchaboki K., Misal S.A., Liang Y., Guise A.J., Truong T., Huguet R., Plowey E.D., Zhu Y., Lopez-Ferrer D., et al. Ultrasensitive single-cell proteomics workflow identifies >1000 protein groups per mammalian cell. Chem. Sci. 2020;12:1001–1006. doi: 10.1039/D0SC03636F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Murgia M., Nogara L., Baraldo M., Reggiani C., Mann M., Schiaffino S. Protein profile of fiber types in human skeletal muscle: A single-fiber proteomics study. Skelet. Muscle. 2021;11:24. doi: 10.1186/s13395-021-00279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Rai M.F., Wu C.L., Capellini T.D., Guilak F., Dicks A.R., Muthuirulan P., Grandi F., Bhutani N., Westendorf J.J. Single Cell Omics for Musculoskeletal Research. Curr. Osteoporos. Rep. 2021;19:131–140. doi: 10.1007/s11914-021-00662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Arias-Hidalgo C., Juanes-Velasco P., Landeira-Viñuela A., García-Vaquero M.L., Montalvillo E., Góngora R., Hernández Á.P., Fuentes M. Single-Cell Proteomics: The Critical Role of Nanotechnology. Int. J. Mol. Sci. 2022;23:6707. doi: 10.3390/ijms23126707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Huang J., Chen X., Fu X., Li Z., Huang Y., Liang C. Advances in Aptamer-Based Biomarker Discovery. Front. Cell Dev. Biol. 2021;9:659760. doi: 10.3389/fcell.2021.659760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ellington A.D., Szostak J.W. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 248.Hathout Y., Liang C., Ogundele M., Xu G., Tawalbeh S.M., Dang U.J., Hoffman E.P., Gordish-Dressman H., Conklin L.S., van den Anker J.N., et al. Disease-specific and glucocorticoid-responsive serum biomarkers for Duchenne Muscular Dystrophy. Sci. Rep. 2019;9:12167. doi: 10.1038/s41598-019-48548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ogundele M., Zhang J.S., Goswami M.V., Barbieri M.L., Dang U.J., Novak J.S., Hoffman E.P., Nagaraju K., Cinrg-Dnhs Investigators. Hathout Y. Validation of Chemokine Biomarkers in Duchenne Muscular Dystrophy. Life. 2021;11:827. doi: 10.3390/life11080827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bottinelli R., Reggiani C. Human skeletal muscle fibres: Molecular and functional diversity. Prog. Biophys. Mol. Biol. 2000;73:195–262. doi: 10.1016/S0079-6107(00)00006-7. [DOI] [PubMed] [Google Scholar]
- 251.Schiaffino S. Fibre types in skeletal muscle: A personal account. Acta Physiol. 2010;199:451–463. doi: 10.1111/j.1748-1716.2010.02130.x. [DOI] [PubMed] [Google Scholar]
- 252.Schiaffino S., Reggiani C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 253.Ciciliot S., Rossi A.C., Dyar K.A., Blaauw B., Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013;45:2191–2199. doi: 10.1016/j.biocel.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 254.Sawano S., Mizunoya W. History and development of staining methods for skeletal muscle fiber types. Histol. Histopathol. 2022;37:493–503. doi: 10.14670/HH-18-422. [DOI] [PubMed] [Google Scholar]
- 255.Murach K.A., Dungan C.M., Kosmac K., Voigt T.B., Tourville T.W., Miller M.S., Bamman M.M., Peterson C.A., Toth M.J. Fiber typing human skeletal muscle with fluorescent immunohistochemistry. J. Appl. Physiol. 2019;127:1632–1639. doi: 10.1152/japplphysiol.00624.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Gonzalez-Freire M., Semba R.D., Ubaida-Mohien C., Fabbri E., Scalzo P., Højlund K., Dufresne C., Lyashkov A., Ferrucci L. The human skeletal muscle proteome project: A reappraisal of the current literature. J. Cachexia Sarcopenia Muscle. 2017;8:5–18. doi: 10.1002/jcsm.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Dowling P., Gargan S., Swandulla D., Ohlendieck K. Identification of Subproteomic Markers for Skeletal Muscle Profiling. Methods Mol. Biol. 2023;2596:291–302. doi: 10.1007/978-1-0716-2831-7_20. [DOI] [PubMed] [Google Scholar]
- 258.Deshmukh A.S., Murgia M., Nagaraj N., Treebak J.T., Cox J., Mann M. Deep proteomics of mouse skeletal muscle enables quantitation of protein isoforms, metabolic pathways, and transcription factors. Mol. Cell. Proteom. 2015;14:841–853. doi: 10.1074/mcp.M114.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Højlund K., Yi Z., Hwang H., Bowen B., Lefort N., Flynn C.R., Langlais P., Weintraub S.T., Mandarino L.J. (Characterization of the human skeletal muscle proteome by one-dimensional gel electrophoresis and HPLC-ESI-MS/MS. Mol. Cell. Proteom. 2008;7:257–267. doi: 10.1074/mcp.M700304-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Parker K.C., Walsh R.J., Salajegheh M., Amato A.A., Krastins B., Sarracino D.A., Greenberg S.A. Characterization of human skeletal muscle biopsy samples using shotgun proteomics. J. Proteome Res. 2009;8:3265–3277. doi: 10.1021/pr800873q. [DOI] [PubMed] [Google Scholar]
- 261.Malik Z.A., Cobley J.N., Morton J.P., Close G.L., Edwards B.J., Koch L.G., Britton S.L., Burniston J.G. Label-Free LC-MS Profiling of Skeletal Muscle Reveals Heart-Type Fatty Acid Binding Protein as a Candidate Biomarker of Aerobic Capacity. Proteomes. 2013;1:290–308. doi: 10.3390/proteomes1030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Burniston J.G., Connolly J., Kainulainen H., Britton S.L., Koch L.G. Label-free profiling of skeletal muscle using high-definition mass spectrometry. Proteomics. 2014;14:2339–2344. doi: 10.1002/pmic.201400118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jiang L., Wang M., Lin S., Jian R., Li X., Chan J., Robinson A.E., GTEx Consortium. Snyder M.P. A quantitative proteome map of the human body. Cell. 2020;183:269–283.e19. doi: 10.1016/j.cell.2020.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Capitanio D., Viganò A., Ricci E., Cerretelli P., Wait R., Gelfi C. Comparison of protein expression in human deltoideus and vastus lateralis muscles using two-dimensional gel electrophoresis. Proteomics. 2005;5:2577–2586. doi: 10.1002/pmic.200401183. [DOI] [PubMed] [Google Scholar]
- 265.Hadrévi J., Hellström F., Kieselbach T., Malm C., Pedrosa-Domellöf F. Protein differences between human trapezius and vastus lateralis muscles determined with a proteomic approach. BMC Musculoskelet. Disord. 2011;12:181. doi: 10.1186/1471-2474-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Deshmukh A.S., Steenberg D.E., Hostrup M., Birk J.B., Larsen J.K., Santos A., Kjøbsted R., Hingst J.R., Schéele C.C., Murgia M., et al. Deep muscle-proteomic analysis of freeze-dried human muscle biopsies reveals fiber type-specific adaptations to exercise training. Nat. Commun. 2021;12:304. doi: 10.1038/s41467-020-20556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Geiger T., Velic A., Macek B., Lundberg E., Kampf C., Nagaraj N., Uhlen M., Cox J., Mann M. Initial quantitative proteomic map of 28 mouse tissues using the SILAC mouse. Mol. Cell. Proteom. 2013;12:1709–1722. doi: 10.1074/mcp.M112.024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Raddatz K., Albrecht D., Hochgräfe F., Hecker M., Gotthardt M. A proteome map of murine heart and skeletal muscle. Proteomics. 2008;8:1885–1897. doi: 10.1002/pmic.200700902. [DOI] [PubMed] [Google Scholar]
- 269.Murphy S., Zweyer M., Raucamp M., Henry M., Meleady P., Swandulla D., Ohlendieck K. Proteomic profiling of the mouse diaphragm and refined mass spectrometric analysis of the dystrophic phenotype. J. Muscle Res. Cell. Motil. 2019;40:9–28. doi: 10.1007/s10974-019-09507-z. [DOI] [PubMed] [Google Scholar]
- 270.Murgia M., Nagaraj N., Deshmukh A.S., Zeiler M., Cancellara P., Moretti I., Reggiani C., Schiaffino S., Mann M. Single muscle fiber proteomics reveals un-expected mitochondrial specialization. EMBO Rep. 2015;16:387–395. doi: 10.15252/embr.201439757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Fomchenko K.M., Walsh E.M., Yang X., Verma R.X., Lin B.L., Nieuwenhuis T.O., Patil A.H., Fox-Talbot K., McCall M.N., Kass D.A., et al. Spatial proteomic approach to characterize skeletal muscle myofibers. J. Proteome Res. 2021;20:888–894. doi: 10.1021/acs.jproteome.0c00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Okumura N., Hashida-Okumura A., Kita K., Matsubae M., Matsubara T., Takao T., Nagai K. Proteomic analysis of slow- and fast-twitch skeletal muscles. Proteomics. 2005;5:2896–2906. doi: 10.1002/pmic.200401181. [DOI] [PubMed] [Google Scholar]
- 273.Gelfi C., Viganò A., De Palma S., Ripamonti M., Begum S., Cerretelli P., Wait R. 2-D protein maps of rat gastrocnemius and soleus muscles: A tool for muscle plasticity assessment. Proteomics. 2006;6:321–340. doi: 10.1002/pmic.200501337. [DOI] [PubMed] [Google Scholar]
- 274.Vitorino R., Ferreira R., Neuparth M., Guedes S., Williams J., Tomer K.B., Domingues P.M., Appell H.J., Duarte J.A., Amado F.M. Subcellular proteomics of mice gastrocnemius and soleus muscles. Anal. Biochem. 2007;366:156–169. doi: 10.1016/j.ab.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Drexler H.C., Ruhs A., Konzer A., Mendler L., Bruckskotten M., Looso M., Günther S., Boettger T., Krüger M., Braun T. On marathons and Sprints: An integrated quantitative proteomics and transcriptomics analysis of differences between slow and fast muscle fibers. Mol. Cell. Proteom. 2012;11:M111.010801. doi: 10.1074/mcp.M111.010801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Eggers B., Schork K., Turewicz M., Barkovits K., Eisenacher M., Schröder R., Clemen C.S., Marcus K. Advanced fiber type- specific protein profiles derived from adult murine skeletal muscle. Proteomes. 2021;9:28. doi: 10.3390/proteomes9020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Schiaffino S., Reggiani C., Murgia M. Fiber type diversity in skeletal muscle explored by mass spectrometry-based single fiber proteomics. Histol. Histopathol. 2020;35:239–246. doi: 10.14670/HH-18-170. [DOI] [PubMed] [Google Scholar]
- 278.Sweeney H.L., Hammers D.W. Muscle Contraction. Cold Spring Harb. Perspect. Biol. 2018;10:a023200. doi: 10.1101/cshperspect.a023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Squire J. Special Issue: The Actin-Myosin Interaction in Muscle: Background and Overview. Int. J. Mol. Sci. 2019;20:5715. doi: 10.3390/ijms20225715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Powers J.D., Malingen S.A., Regnier M., Daniel T.L. The Sliding Filament Theory Since Andrew Huxley: Multiscale and Multidisciplinary Muscle Research. Annu. Rev. Biophys. 2021;50:373–400. doi: 10.1146/annurev-biophys-110320-062613. [DOI] [PubMed] [Google Scholar]
- 281.Lin B.L., Song T., Sadayappan S. Myofilaments: Movers and Rulers of the Sarcomere. Compr. Physiol. 2017;7:675–692. doi: 10.1002/cphy.c160026. [DOI] [PubMed] [Google Scholar]
- 282.Ojima K. Myosin: Formation and maintenance of thick filaments. Anim. Sci. J. 2019;90:801–807. doi: 10.1111/asj.13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Tobacman L.S. Troponin Revealed: Uncovering the Structure of the Thin Filament On-Off Switch in Striated Muscle. Biophys. J. 2021;120:1–9. doi: 10.1016/j.bpj.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lange S., Pinotsis N., Agarkova I., Ehler E. The M-band: The underestimated part of the sarcomere. Biochim. Biophys. Acta Mol. Cell. Res. 2020;1867:118440. doi: 10.1016/j.bbamcr.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wadmore K., Azad A.J., Gehmlich K. The Role of Z-disc Proteins in Myopathy and Cardiomyopathy. Int. J. Mol. Sci. 2021;22:3058. doi: 10.3390/ijms22063058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Gordon A.M., Homsher E., Regnier M. Regulation of contraction in striated muscle. Physiol. Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 287.Wang L., Geist J., Grogan A., Hu L.R., Kontrogianni-Konstantopoulos A. Thick Filament Protein Network, Functions, and Disease Association. Compr. Physiol. 2018;8:631–709. doi: 10.1002/cphy.c170023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Henderson C.A., Gomez C.G., Novak S.M., Mi-Mi L., Gregorio C.C. Overview of the Muscle Cytoskeleton. Compr. Physiol. 2017;7:891–944. doi: 10.1002/cphy.c160033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Reiser P.J. Current understanding of conventional and novel co-expression patterns of mammalian sarcomeric myosin heavy chains and light chains. Arch. Biochem. Biophys. 2019;662:129–133. doi: 10.1016/j.abb.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 290.Holland A., Ohlendieck K. Proteomic profiling of the contractile apparatus from skeletal muscle. Expert Rev. Proteom. 2013;10:239–257. doi: 10.1586/epr.13.20. [DOI] [PubMed] [Google Scholar]
- 291.Sitbon Y.H., Yadav S., Kazmierczak K., Szczesna-Cordary D. Insights into myosin regulatory and essential light chains: A focus on their roles in cardiac and skeletal muscle function, development and disease. J. Muscle Res. Cell. Motil. 2020;41:313–327. doi: 10.1007/s10974-019-09517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pette D., Staron R.S. Myosin isoforms, muscle fiber types, and transitions. Microsc. Res. Tech. 2000;50:500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 293.Schiaffino S., Rossi A.C., Smerdu V., Leinwand L.A., Reggiani C. Developmental myosins: Expression patterns and functional significance. Skelet. Muscle. 2015;5:22. doi: 10.1186/s13395-015-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Hoh J.F. Laryngeal muscle fibre types. Acta. Physiol. Scand. 2005;183:133–149. doi: 10.1111/j.1365-201X.2004.01402.x. [DOI] [PubMed] [Google Scholar]
- 295.Lee L.A., Karabina A., Broadwell L.J., Leinwand L.A. The ancient sarcomeric myosins found in specialized muscles. Skelet. Muscle. 2019;9:7. doi: 10.1186/s13395-019-0192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hoh J.F.Y. Myosin heavy chains in extraocular muscle fibres: Distribution, regulation and function. Acta Physiol. 2021;231:e13535. doi: 10.1111/apha.13535. [DOI] [PubMed] [Google Scholar]
- 297.Gargan S., Dowling P., Zweyer M., Reimann J., Henry M., Meleady P., Swandulla D., Ohlendieck K. Mass Spectrometric Profiling of Extraocular Muscle and Proteomic Adaptations in the mdx-4cv Model of Duchenne Muscular Dystrophy. Life. 2021;11:595. doi: 10.3390/life11070595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Bozzo C., Spolaore B., Toniolo L., Stevens L., Bastide B., Cieniewski-Bernard C., Fontana A., Mounier Y., Reggiani C. Nerve influence on myosin light chain phosphorylation in slow and fast skeletal muscles. FEBS J. 2005;272:5771–5785. doi: 10.1111/j.1742-4658.2005.04965.x. [DOI] [PubMed] [Google Scholar]
- 299.Robinett J.C., Hanft L.M., Geist J., Kontrogianni-Konstantopoulos A., McDonald K.S. Regulation of myofilament force and loaded shortening by skeletal myosin binding protein C. J. Gen. Physiol. 2019;151:645–659. doi: 10.1085/jgp.201812200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Luther P.K., Vydyanath A. Myosin binding protein-C: An essential protein in skeletal and cardiac muscle. J. Muscle Res. Cell. Motil. 2011;31:303–305. doi: 10.1007/s10974-010-9235-4. [DOI] [PubMed] [Google Scholar]
- 301.Lin B.L., Li A., Mun J.Y., Previs M.J., Previs S.B., Campbell S.G., Dos Remedios C.G., Tombe P.P., Craig R., Warshaw D.M., et al. Skeletal myosin binding protein-C isoforms regulate thin filament activity in a Ca2+-dependent manner. Sci. Rep. 2018;8:2604. doi: 10.1038/s41598-018-21053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.McNamara J.W., Sadayappan S. Skeletal myosin binding protein-C: An increasingly important regulator of striated muscle physiology. Arch. Biochem. Biophys. 2018;660:121–128. doi: 10.1016/j.abb.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Prill K., Dawson J.F. Assembly and Maintenance of Sarcomere Thin Filaments and Associated Diseases. Int. J. Mol. Sci. 2020;21:542. doi: 10.3390/ijms21020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Dominguez R., Holmes K.C. Actin structure and function. Annu. Rev. Biophys. 2011;40:169–186. doi: 10.1146/annurev-biophys-042910-155359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Nowak K.J., Ravenscroft G., Laing N.G. Skeletal muscle α-actin diseases (actinopathies): Pathology and mechanisms. Acta Neuropathol. 2013;125:19–32. doi: 10.1007/s00401-012-1019-z. [DOI] [PubMed] [Google Scholar]
- 306.Moore J.R., Campbell S.G., Lehman W. Structural determinants of muscle thin filament cooperativity. Arch. Biochem. Biophys. 2016;594:8–17. doi: 10.1016/j.abb.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Guhathakurta P., Prochniewicz E., Thomas D.D. Actin-Myosin Interaction: Structure, Function and Drug Discovery. Int. J. Mol. Sci. 2018;19:2628. doi: 10.3390/ijms19092628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.El-Mezgueldi M. Tropomyosin dynamics. J. Muscle Res. Cell. Motil. 2014;35:203–210. doi: 10.1007/s10974-014-9377-x. [DOI] [PubMed] [Google Scholar]
- 309.Hitchcock-DeGregori S.E., Barua B. Tropomyosin Structure, Function, and Interactions: A Dynamic Regulator. Subcell. Biochem. 2017;82:253–284. doi: 10.1007/978-3-319-49674-0_9. [DOI] [PubMed] [Google Scholar]
- 310.Lehman W., Rynkiewicz M.J., Moore J.R. A new twist on tropomyosin binding to actin filaments: Perspectives on thin filament function, assembly and biomechanics. J. Muscle Res. Cell. Motil. 2020;41:23–38. doi: 10.1007/s10974-019-09501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Gomes A.V., Potter J.D., Szczesna-Cordary D. The role of troponins in muscle contraction. IUBMB Life. 2002;54:323–333. doi: 10.1080/15216540216037. [DOI] [PubMed] [Google Scholar]
- 312.Swartz D.R., Yang Z., Sen A., Tikunova S.B., Davis J.P. Myofibrillar troponin exists in three states and there is signal transduction along skeletal myofibrillar thin filaments. J. Mol. Biol. 2006;361:420–435. doi: 10.1016/j.jmb.2006.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Rasmussen M., Jin J.P. Troponin Variants as Markers of Skeletal Muscle Health and Diseases. Front. Physiol. 2021;12:747214. doi: 10.3389/fphys.2021.747214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Xu Z., Feng X., Dong J., Wang Z.M., Lee J., Furdui C., Files D.C., Beavers K.M., Kritchevsky S., Milligan C., et al. Cardiac troponin T and fast skeletal muscle denervation in ageing. J. Cachexia Sarcopenia Muscle. 2017;8:808–823. doi: 10.1002/jcsm.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Frank D., Kuhn C., Katus H.A., Frey N. The sarcomeric Z-disc: A nodal point in signalling and disease. J. Mol. Med. 2006;84:446–468. doi: 10.1007/s00109-005-0033-1. [DOI] [PubMed] [Google Scholar]
- 316.Luther P.K. The vertebrate muscle Z-disc: Sarcomere anchor for structure and signalling. J. Muscle Res. Cell. Motil. 2009;30:171–185. doi: 10.1007/s10974-009-9189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Ranta-Aho J., Olive M., Vandroux M., Roticiani G., Dominguez C., Johari M., Torella A., Böhm J., Turon J., Nigro V., et al. Mutation update for the ACTN2 gene. Hum. Mutat. 2022;43:1745–1756. doi: 10.1002/humu.24470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Taniguchi Y., Makizako H., Nakai Y., Kiuchi Y., Akaida S., Tateishi M., Takenaka T., Kubozono T., Ohishi M. Associations of the Alpha-Actinin Three Genotype with Bone and Muscle Mass Loss among Middle-Aged and Older Adults. J. Clin. Med. 2022;11:6172. doi: 10.3390/jcm11206172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Gontier Y., Taivainen A., Fontao L., Sonnenberg A., van der Flier A., Carpen O., Faulkner G., Borradori L. The Z-disc proteins myotilin and FATZ-1 interact with each other and are connected to the sarcolemma via muscle-specific filamins. J. Cell Sci. 2005;118:3739–3749. doi: 10.1242/jcs.02484. [DOI] [PubMed] [Google Scholar]
- 320.Roberts M.D., Romero M.A., Mobley C.B., Mumford P.W., Roberson P.A., Haun C.T., Vann C.G., Osburn S.C., Holmes H.H., Greer R.A., et al. Skeletal muscle mitochondrial volume and myozenin-1 protein differences exist between high versus low anabolic responders to resistance training. PeerJ. 2018;6:e5338. doi: 10.7717/peerj.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Agarkova I., Perriard J.C. The M-band: An elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 2005;15:477–485. doi: 10.1016/j.tcb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 322.Van Der Ven P.F., Obermann W.M., Weber K., Fürst D.O. Myomesin, M-protein and the structure of the sarcomeric M-band. Adv. Biophys. 1996;33:91–99. doi: 10.1016/0065-227X(96)81666-2. [DOI] [PubMed] [Google Scholar]
- 323.Prill K., Carlisle C., Stannard M., Windsor Reid P.J., Pilgrim D.B. Myomesin is part of an integrity pathway that responds to sarcomere damage and disease. PLoS One. 2019;14:e0224206. doi: 10.1371/journal.pone.0224206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Manring H.R., Carter O.A., Ackermann M.A. Obscure functions: The location-function relationship of obscurins. Biophys. Rev. 2017;9:245–258. doi: 10.1007/s12551-017-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Randazzo D., Pierantozzi E., Rossi D., Sorrentino V. The potential of obscurin as a therapeutic target in muscle disorders. Expert Opin. Ther. Targets. 2017;21:897–910. doi: 10.1080/14728222.2017.1361931. [DOI] [PubMed] [Google Scholar]
- 326.Chu M., Gregorio C.C., Pappas C.T. Nebulin, a multi-functional giant. J. Exp. Biol. 2016;219:146–152. doi: 10.1242/jeb.126383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Yuen M., Ottenheijm C.A.C. Nebulin: Big protein with big responsibilities. J. Muscle Res. Cell. Motil. 2020;41:103–124. doi: 10.1007/s10974-019-09565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Freundt J.K., Linke W.A. Titin as a force-generating muscle protein under regulatory control. J. Appl. Physiol. 2019;126:1474–1482. doi: 10.1152/japplphysiol.00865.2018. [DOI] [PubMed] [Google Scholar]
- 329.Adewale A.O., Ahn Y.H. Titin N2A Domain and Its Interactions at the Sarcomere. Int. J. Mol. Sci. 2021;22:7563. doi: 10.3390/ijms22147563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Nishikawa K., Lindstedt S.L., Hessel A., Mishra D. N2A Titin: Signaling Hub and Mechanical Switch in Skeletal Muscle. Int. J. Mol. Sci. 2020;21:3974. doi: 10.3390/ijms21113974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hessel A.L., Ma W., Mazara N., Rice P.E., Nissen D., Gong H., Kuehn M., Irving T., Linke W.A. Titin force in muscle cells alters lattice order, thick and thin filament protein formation. Proc. Natl. Acad. Sci. USA. 2022;119:e2209441119. doi: 10.1073/pnas.2209441119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Wette S.G., Smith H.K., Lamb G.D., Murphy R.M. Characterization of muscle ankyrin repeat proteins in human skeletal muscle. Am. J. Physiol. Cell Physiol. 2017;313:C327–C339. doi: 10.1152/ajpcell.00077.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Gargan S., Dowling P., Zweyer M., Henry M., Meleady P., Swandulla D., Ohlendieck K. Proteomic Identification of Markers of Membrane Repair, Regeneration and Fibrosis in the Aged and Dystrophic Diaphragm. Life. 2022;12:1679. doi: 10.3390/life12111679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Miljkovic N., Lim J.Y., Miljkovic I., Frontera W.R. Aging of skeletal muscle fibers. Ann. Rehabil. Med. 2015;39:155–162. doi: 10.5535/arm.2015.39.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Grosicki G.J., Zepeda C.S., Sundberg C.W. Single muscle fibre contractile function with ageing. J. Physiol. 2022;600:5005–5026. doi: 10.1113/JP282298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Wilkinson D.J., Piasecki M., Atherton P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018;47:123–132. doi: 10.1016/j.arr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Loh R., Tan R.S., Lim W.S., Koh A.S. Cardio-sarcopenia: A syndrome of concern in aging. Front. Med. 2022;9:1027466. doi: 10.3389/fmed.2022.1027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Aare S., Spendiff S., Vuda M., Elkrief D., Perez A., Wu Q., Mayaki D., Hussain S.N., Hettwer S., Hepple R.T. Failed reinnervation in aging skeletal muscle. Skelet. Muscle. 2016;6:29. doi: 10.1186/s13395-016-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Andersen J.L. Muscle fibre type adaptation in the elderly human muscle. Scan. J. Med. Sci. Sports. 2003;13:40–47. doi: 10.1034/j.1600-0838.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- 340.Wang Y., Pessin J.E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:243–250. doi: 10.1097/MCO.0b013e328360272d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Frontera W.R., Hughes V.A., Fielding R.A., Fiatarone M.A., Evans W.J., Roubenoff R. Aging of skeletal muscle: A 12-yr longitudinal study. J. Appl. Physiol. 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 342.Frontera W.R., Reid K.F., Phillips E.M., Krivickas L.S., Hughes V.A., Roubenoff R., Fielding R.A. Muscle fiber size and function in elderly humans: A longitudinal study. J. Appl. Physiol. 2008;105:637–642. doi: 10.1152/japplphysiol.90332.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Carvalho do Nascimento P.R., Bilodeau M., Poitras S. How do we define and measure sarcopenia? A meta-analysis of observational studies. Age Ageing. 2021;50:1906–1913. doi: 10.1093/ageing/afab148. [DOI] [PubMed] [Google Scholar]
- 344.Spexoto M.C.B., Ramírez P.C., de Oliveira Máximo R., Steptoe A., de Oliveira C., Alexandre T.D.S. European Working Group on Sarcopenia in Older People 2010 (EWGSOP1) and 2019 (EWGSOP2) criteria or slowness: Which is the best predictor of mortality risk in older adults? Age Ageing. 2022;51:afac164. doi: 10.1093/ageing/afac164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Petermann-Rocha F., Balntzi V., Gray S.R., Lara J., Ho F.K., Pell J.P., Celis-Morales C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle. 2022;13:86–99. doi: 10.1002/jcsm.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Davies B., Walter S., Rodríguez-Laso A., Carnicero Carreño J.A., García-García F.J., Álvarez-Bustos A., Rodríguez-Mañas L. Differential Association of Frailty and Sarcopenia With Mortality and Disability: Insight Supporting Clinical Subtypes of Frailty. J. Am. Med. Dir. Assoc. 2022;23:1712–1716.e3. doi: 10.1016/j.jamda.2022.03.013. [DOI] [PubMed] [Google Scholar]
- 347.Wu X., Zhang T., Zhang Y., She Y., Wang L., Gao Y., Deng Y., Chen M., He Y., Chen X., et al. Natural population cohort study on long-lived adults: West China longevity and ageing procedure (WCLAP) BMJ Open. 2022;12:e055407. doi: 10.1136/bmjopen-2021-055407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Mitchell W.K., Williams J., Atherton P., Larvin M., Lund J., Narici M. Sarcopenia, Dynapenia, and the impact of advancing age on human skeletal muscle size and strength: A quantitative review. Front. Physiol. 2012;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Chiba I., Lee S., Bae S., Makino K., Shinkai Y., Katayama O., Harada K., Takayanagi N., Shimada H. Difference in sarcopenia characteristics associated with physical activity and disability incidences in older adults. J. Cachexia Sarcopenia Muscle. 2021;12:1983–1994. doi: 10.1002/jcsm.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Frontera W.R. Rehabilitation of Older Adults with Sarcopenia: From Cell to Functioning. Prog. Rehabil. Med. 2022;7:20220044. doi: 10.2490/prm.20220044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Mcleod J.C., Stokes T., Phillips S.M. Resistance Exercise Training as a Primary Countermeasure to Age-Related Chronic Disease. Front. Physiol. 2019;10:645. doi: 10.3389/fphys.2019.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Coelho-Júnior H.J., Picca A., Calvani R., Marzetti E. Prescription of resistance training for sarcopenic older adults: Does it require specific attention? Ageing Res. Rev. 2022;81:101720. doi: 10.1016/j.arr.2022.101720. [DOI] [PubMed] [Google Scholar]
- 353.de Sá Souza H., de Melo C.M., Piovezan R.D., Miranda R.E.E.P.C., Carneiro-Junior M.A., Silva B.M., Thomatieli-Santos R.V., Tufik S., Poyares D., D’Almeida V. Resistance Training Improves Sleep and Anti-Inflammatory Parameters in Sarcopenic Older Adults: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health. 2022;19:16322. doi: 10.3390/ijerph192316322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Cobley J.N., Moult P.R., Burniston J.G., Morton J.P., Close G.L. Exercise improves mitochondrial and redox-regulated stress responses in the elderly: Better late than never! Biogerontology. 2015;16:249–264. doi: 10.1007/s10522-014-9546-8. [DOI] [PubMed] [Google Scholar]
- 355.El Assar M., Álvarez-Bustos A., Sosa P., Angulo J., Rodríguez-Mañas L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int. J. Mol. Sci. 2022;23:8713. doi: 10.3390/ijms23158713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Hirsch K.R., Church D.D., Kim I.Y., Park S., Wolfe R.R., Ferrando A.A. Comparison of basal whole-body protein kinetics and muscle protein synthesis between young and older adults. Physiol. Rep. 2020;8:e14633. doi: 10.14814/phy2.14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Sieber C.C. Malnutrition and sarcopenia. Aging Clin. Exp. Res. 2019;31:793–798. doi: 10.1007/s40520-019-01170-1. [DOI] [PubMed] [Google Scholar]
- 358.Jang E.H., Han Y.J., Jang S.E., Lee S. Association between Diet Quality and Sarcopenia in Older Adults: Systematic Review of Prospective Cohort Studies. Life. 2021;11:811. doi: 10.3390/life11080811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Coelho-Junior H.J., Calvani R., Azzolino D., Picca A., Tosato M., Landi F., Cesari M., Marzetti E. Protein Intake and Sarcopenia in Older Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health. 2022;19:8718. doi: 10.3390/ijerph19148718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Bradlee M.L., Mustafa J., Singer M.R., Moore L.L. High-Protein Foods and Physical Activity Protect Against Age-Related Muscle Loss and Functional Decline. J. Gerontol. A Biol. Sci. Med. Sci. 2017;73:88–94. doi: 10.1093/gerona/glx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Coelho-Junior H.J., Marzetti E., Picca A., Cesari M., Uchida M.C., Calvani R. Protein Intake and Frailty: A Matter of Quantity, Quality, and Timing. Nutrients. 2020;12:2915. doi: 10.3390/nu12102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Kinoshita K., Otsuka R., Nishita Y., Tange C., Tomida M., Zhang S., Ando F., Shimokata H., Arai H. Breakfast Protein Quality and Muscle Strength in Japanese Older Adults: A Community-Based Longitudinal Study. J. Am. Med. Dir. Assoc. 2022;23:729–735.e2. doi: 10.1016/j.jamda.2021.11.037. [DOI] [PubMed] [Google Scholar]
- 363.Coelho-Júnior H.J., Calvani R., Tosato M., Landi F., Picca A., Marzetti E. Protein intake and physical function in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2022;81:101731. doi: 10.1016/j.arr.2022.101731. [DOI] [PubMed] [Google Scholar]
- 364.Rezuş E., Burlui A., Cardoneanu A., Rezuş C., Codreanu C., Pârvu M., Rusu Zota G., Tamba B.I. Inactivity and Skeletal Muscle Metabolism: A Vicious Cycle in Old Age. Int. J. Mol. Sci. 2020;21:592. doi: 10.3390/ijms21020592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Peterson C.M., Johannsen D.L., Ravussin E. Skeletal muscle mitochondria and aging: A review. J. Aging Res. 2012;2012:194821. doi: 10.1155/2012/194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Picca A., Lezza A.M.S., Leeuwenburgh C., Pesce V., Calvani R., Landi F., Bernabei R., Marzetti E. Fueling Inflamm-Aging through Mitochondrial Dysfunction: Mechanisms and Molecular Targets. Int. J. Mol. Sci. 2017;18:933. doi: 10.3390/ijms18050933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Casuso R.A., Huertas J.R. The emerging role of skeletal muscle mitochondrial dynamics in exercise and ageing. Ageing Res. Rev. 2020;58:101025. doi: 10.1016/j.arr.2020.101025. [DOI] [PubMed] [Google Scholar]
- 368.Ferri E., Marzetti E., Calvani R., Picca A., Cesari M., Arosio B. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int. J. Mol. Sci. 2020;21:5236. doi: 10.3390/ijms21155236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Romanello V. The Interplay between Mitochondrial Morphology and Myomitokines in Aging Sarcopenia. Int. J. Mol. Sci. 2020;22:91. doi: 10.3390/ijms22010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Leduc-Gaudet J.P., Hussain S.N.A., Barreiro E., Gouspillou G. Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Health and Aging. Int. J. Mol. Sci. 2021;22:8179. doi: 10.3390/ijms22158179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Kimoloi S., Sen A., Guenther S., Braun T., Brügmann T., Sasse P., Wiesner R.J., Pla-Martín D., Baris O.R. Combined fibre atrophy and decreased muscle regeneration capacity driven by mitochondrial DNA alterations underlie the development of sarcopenia. J. Cachexia Sarcopenia Muscle. 2022;13:2132–2145. doi: 10.1002/jcsm.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Sakellariou G.K., McDonagh B. Redox Homeostasis in Age-Related Muscle Atrophy. Adv. Exp. Med. Biol. 2018;1088:281–306. doi: 10.1007/978-981-13-1435-3_13. [DOI] [PubMed] [Google Scholar]
- 373.Cobley J.N., Sakellariou G.K., Husi H., McDonagh B. Proteomic strategies to unravel age-related redox signalling defects in skeletal muscle. Free Radic. Biol. Med. 2019;132:24–32. doi: 10.1016/j.freeradbiomed.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 374.Shally A., McDonagh B. The redox environment and mitochondrial dysfunction in age-related skeletal muscle atrophy. Biogerontology. 2020;21:461–473. doi: 10.1007/s10522-020-09879-7. [DOI] [PubMed] [Google Scholar]
- 375.Foreman N.A., Hesse A.S., Ji L.L. Redox Signaling and Sarcopenia: Searching for the Primary Suspect. Int. J. Mol. Sci. 2021;22:9045. doi: 10.3390/ijms22169045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Protasi F., Pietrangelo L., Boncompagni S. Improper Remodeling of Organelles Deputed to Ca2+ Handling and Aerobic ATP Production Underlies Muscle Dysfunction in Ageing. Int. J. Mol. Sci. 2021;22:6195. doi: 10.3390/ijms22126195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Iyer S.R., Shah S.B., Lovering R.M. The Neuromuscular Junction: Roles in Aging and Neuromuscular Disease. Int. J. Mol. Sci. 2021;22:8058. doi: 10.3390/ijms22158058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Mancinelli R., Checcaglini F., Coscia F., Gigliotti P., Fulle S., Fanò-Illic G. Biological Aspects of Selected Myokines in Skeletal Muscle: Focus on Aging. Int. J. Mol. Sci. 2021;22:8520. doi: 10.3390/ijms22168520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Cannataro R., Carbone L., Petro J.L., Cione E., Vargas S., Angulo H., Forero D.A., Odriozola-Martínez A., Kreider R.B., Bonilla D.A. Sarcopenia: Etiology, Nutritional Approaches, and miRNAs. Int. J. Mol. Sci. 2021;22:9724. doi: 10.3390/ijms22189724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Francisco S., Martinho V., Ferreira M., Reis A., Moura G., Soares A.R., Santos M.A.S. The Role of MicroRNAs in Proteostasis Decline and Protein Aggregation during Brain and Skeletal Muscle Aging. Int. J. Mol. Sci. 2022;23:3232. doi: 10.3390/ijms23063232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Prokopidis K., Chambers E., Ni Lochlainn M., Witard O.C. Mechanisms Linking the Gut-Muscle Axis With Muscle Protein Metabolism and Anabolic Resistance: Implications for Older Adults at Risk of Sarcopenia. Front. Physiol. 2021;12:770455. doi: 10.3389/fphys.2021.770455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Tan K.T., Ang S.J., Tsai S.Y. Sarcopenia: Tilting the Balance of Protein Homeostasis. Proteomics. 2020;20:e1800411. doi: 10.1002/pmic.201800411. [DOI] [PubMed] [Google Scholar]
- 383.Fernando R., Drescher C., Nowotny K., Grune T., Castro J.P. Impaired proteostasis during skeletal muscle aging. Free Radic. Biol. Med. 2019;132:58–66. doi: 10.1016/j.freeradbiomed.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 384.Wilhelmsen A., Tsintzas K., Jones S.W. Recent advances and future avenues in understanding the role of adipose tissue cross talk in mediating skeletal muscle mass and function with ageing. Geroscience. 2021;43:85–110. doi: 10.1007/s11357-021-00322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Campos G.C., Lourenço R.A., Molina M.D.C.B. Mortality, sarcopenic obesity, and sarcopenia: Frailty in Brazilian Older People Study-FIBRA-RJ. Rev. Saude Publica. 2021;55:75. doi: 10.11606/s1518-8787.2021055002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Zhang X., Li H., He M., Wang J., Wu Y., Li Y. Immune system and sarcopenia: Presented relationship and future perspective. Exp. Gerontol. 2022;164:111823. doi: 10.1016/j.exger.2022.111823. [DOI] [PubMed] [Google Scholar]
- 387.Nelke C., Dziewas R., Minnerup J., Meuth S.G., Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. 2019;49:381–388. doi: 10.1016/j.ebiom.2019.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Jimenez-Gutierrez G.E., Martínez-Gómez L.E., Martínez-Armenta C., Pineda C., Martínez-Nava G.A., Lopez-Reyes A. Molecular Mechanisms of Inflammation in Sarcopenia: Diagnosis and Therapeutic Update. Cells. 2022;11:2359. doi: 10.3390/cells11152359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Kadi F., Ponsot E. The biology of satellite cells and telomeres in human skeletal muscle: Effects of aging and physical activity. Scand. J. Med. Sci. Sports. 2010;20:39–48. doi: 10.1111/j.1600-0838.2009.00966.x. [DOI] [PubMed] [Google Scholar]
- 390.Sui S.X., Williams L.J., Holloway-Kew K.L., Hyde N.K., Pasco J.A. Skeletal Muscle Health and Cognitive Function: A Narrative Review. Int. J. Mol. Sci. 2020;22:255. doi: 10.3390/ijms22010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Papadopoulou S.K., Voulgaridou G., Kondyli F.S., Drakaki M., Sianidou K., Andrianopoulou R., Rodopaios N., Pritsa A. Nutritional and Nutrition-Related Biomarkers as Prognostic Factors of Sarcopenia, and Their Role in Disease Progression. Diseases. 2022;10:42. doi: 10.3390/diseases10030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Verdijk L.B., Koopman R., Schaart G., Meijer K., Savelberg H.H., van Loon L.J. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am. J. Physiol. Endocrinol. Metab. 2007;292:E151–E157. doi: 10.1152/ajpendo.00278.2006. [DOI] [PubMed] [Google Scholar]
- 393.Renault V., Thornell L.E., Eriksson P.O., Butler-Browne G., Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1:132–139. doi: 10.1046/j.1474-9728.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- 394.Joanisse S., Nederveen J.P., Snijders T., McKay B.R., Parise G. Skeletal Muscle Regeneration, Repair and Remodelling in Aging: The Importance of Muscle Stem Cells and Vascularization. Gerontology. 2017;63:91–100. doi: 10.1159/000450922. [DOI] [PubMed] [Google Scholar]
- 395.Huo F., Liu Q., Liu H. Contribution of muscle satellite cells to sarcopenia. Front. Physiol. 2022;13:892749. doi: 10.3389/fphys.2022.892749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Fernández-Lázaro D., Garrosa E., Seco-Calvo J., Garrosa M. Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia. Proteomes. 2022;10:29. doi: 10.3390/proteomes10030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Liu J.C., Dong S.S., Shen H., Yang D.Y., Chen B.B., Ma X.Y., Peng Y.R., Xiao H.M., Deng H.W. Multi-omics research in sarcopenia: Current progress and future prospects. Ageing Res. Rev. 2022;76:101576. doi: 10.1016/j.arr.2022.101576. [DOI] [PubMed] [Google Scholar]
- 398.Curcio F., Ferro G., Basile C., Liguori I., Parrella P., Pirozzi F., Della-Morte D., Gargiulo G., Testa G., Tocchetti C.G., et al. Biomarkers in sarcopenia: A multifactorial approach. Exp. Gerontol. 2016;85:1–8. doi: 10.1016/j.exger.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 399.Picca A., Calvani R., Sirago G., Coelho-Junior H.J., Marzetti E. Molecular routes to sarcopenia and biomarker development: Per aspera ad astra. Curr. Opin. Pharmacol. 2021;57:140–147. doi: 10.1016/j.coph.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 400.Picca A., Calvani R., Marzetti E. Multisystem derangements in frailty and sarcopenia: A source for biomarker discovery. Curr. Opin. Clin. Nutr. Metab. Care. 2022;25:173–177. doi: 10.1097/MCO.0000000000000828. [DOI] [PubMed] [Google Scholar]
- 401.Gelfi C., Vigano A., Ripamonti M., Pontoglio A., Begum S., Pellegrino M.A., Grassi B., Bottinelli R., Wait R., Cerretelli P. The human muscle proteome in aging. J. Proteome Res. 2006;5:1344–1353. doi: 10.1021/pr050414x. [DOI] [PubMed] [Google Scholar]
- 402.Staunton L., Zweyer M., Swandulla D., Ohlendieck K. Mass spectrometry-based proteomic analysis of middle-aged vs. aged vastus lateralis reveals increased levels of carbonic anhydrase isoform 3 in senescent human skeletal muscle. Int. J. Mol. Med. 2012;30:723–733. doi: 10.3892/ijmm.2012.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Ohlendieck K. Two-CyDye-Based 2D-DIGE Analysis of Aged Human Muscle Biopsy Specimens. Methods Mol. Biol. 2023;2596:265–289. doi: 10.1007/978-1-0716-2831-7_19. [DOI] [PubMed] [Google Scholar]
- 404.Gueugneau M., Coudy-Gandilhon C., Gourbeyre O., Chambon C., Combaret L., Polge C., Taillandier D., Attaix D., Friguet B., Maier A.B., et al. Proteomics of muscle chronological ageing in post-menopausal women. BMC Genom. 2014;15:1165. doi: 10.1186/1471-2164-15-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Dos Santos S.L., Baraibar M.A., Lundberg S., Eeg-Olofsson O., Larsson L., Friguet B. Oxidative proteome alterations during skeletal muscle ageing. Redox Biol. 2015;5:267–274. doi: 10.1016/j.redox.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Cobley J.N., Ab Malik Z., Morton J.P., Close G.L., Edwards B.J., Burniston J.G. Age- and Activity-Related Differences in the Abundance of Myosin Essential and Regulatory Light Chains in Human Muscle. Proteomes. 2016;4:15. doi: 10.3390/proteomes4020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Murgia M., Toniolo L., Nagaraj N., Ciciliot S., Vindigni V., Schiaffino S., Reggiani C., Mann M. Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging. Cell Rep. 2017;19:2396–2409. doi: 10.1016/j.celrep.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 408.Ebhardt H.A., Degen S., Tadini V., Schilb A., Johns N., Greig C.A., Fearon K.C.H., Aebersold R., Jacobi C. Comprehensive proteome analysis of human skeletal muscle in cachexia and sarcopenia: A pilot study. J. Cachexia Sarcopenia Muscle. 2017;8:567–582. doi: 10.1002/jcsm.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Brocca L., McPhee J.S., Longa E., Canepari M., Seynnes O., De Vito G., Pellegrino M.A., Narici M., Bottinelli R. Structure and function of human muscle fibres and muscle proteome in physically active older men. J. Physiol. 2017;595:4823–4844. doi: 10.1113/JP274148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Ubaida-Mohien C., Lyashkov A., Gonzalez-Freire M., Tharakan R., Shardell M., Moaddel R., Semba R.D., Chia C.W., Gorospe M., Sen R., et al. Discovery proteomics in aging human skeletal muscle finds change in spliceosome, immunity, proteostasis and mitochondria. Elife. 2019;8:e49874. doi: 10.7554/eLife.49874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Ubaida-Mohien C., Gonzalez-Freire M., Lyashkov A., Moaddel R., Chia C.W., Simonsick E.M., Sen R., Ferrucci L. Physical Activity Associated Proteomics of Skeletal Muscle: Being Physically Active in Daily Life May Protect Skeletal Muscle From Aging. Front. Physiol. 2019;10:312. doi: 10.3389/fphys.2019.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Vann C.G., Roberson P.A., Osburn S.C., Mumford P.W., Romero M.A., Fox C.D., Moore J.H., Haun C.T., Beck D.T., Moon J.R., et al. Skeletal Muscle Myofibrillar Protein Abundance Is Higher in Resistance-Trained Men, and Aging in the Absence of Training May Have an Opposite Effect. Sports. 2020;8:7. doi: 10.3390/sports8010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Gueugneau M., Coudy-Gandilhon C., Chambon C., Verney J., Taillandier D., Combaret L., Polge C., Walrand S., Roche F., Barthélémy J.C., et al. Muscle Proteomic and Transcriptomic Profiling of Healthy Aging and Metabolic Syndrome in Men. Int. J. Mol. Sci. 2021;22:4205. doi: 10.3390/ijms22084205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Deane C.S., Phillips B.E., Willis C.R.G., Wilkinson D.J., Smith K., Higashitani N., Williams J.P., Szewczyk N.J., Atherton P.J., Higashitani A., et al. Proteomic features of skeletal muscle adaptation to resistance exercise training as a function of age. Geroscience. 2022 doi: 10.1007/s11357-022-00658-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Balagopalm P., Rooyackers O.E., Adey D.B., Ades P.A., Nair K.S. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am. J. Physiol. 1997;273:E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- 416.Lexell J., Taylor C.C., Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 1988;84:275–294. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 417.Staunton L., O'Connell K., Ohlendieck K. Proteomic Profiling of Mitochondrial Enzymes during Skeletal Muscle Aging. J. Aging Res. 2011;2011:908035. doi: 10.4061/2011/908035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Egan B., Dowling P., O’Connor P.L., Henry M., Meleady P., Zierath J.R., O’Gorman D.J. 2-D DIGE analysis of the mitochondrial proteome from human skeletal muscle reveals time course-dependent remodelling in response to 14 consecutive days of endurance exercise training. Proteomics. 2011;11:1413–1428. doi: 10.1002/pmic.201000597. [DOI] [PubMed] [Google Scholar]
- 419.Burniston J.G., Hoffman E.P. Proteomic responses of skeletal and cardiac muscle to exercise. Expert Rev. Proteom. 2011;8:361–377. doi: 10.1586/epr.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Schild M., Ruhs A., Beiter T., Zügel M., Hudemann J., Reimer A., Krumholz-Wagner I., Wagner C., Keller J., Eder K., et al. Basal and exercise induced label-free quantitative protein profiling of m. vastus lateralis in trained and untrained individuals. J. Proteom. 2015;122:119–132. doi: 10.1016/j.jprot.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 421.Mckendry J., Breen L., Shad B.J., Greig C.A. Muscle morphology and performance in master athletes: A systematic review and meta-analyses. Ageing Res. Rev. 2018;45:62–82. doi: 10.1016/j.arr.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 422.Coudy-Gandilhon C., Gueugneau M., Chambon C., Taillandier D., Combaret L., Polge C., Millet G.Y., Féasson L., Béchet D. A Single Bout of Ultra-Endurance Exercise Reveals Early Signs of Muscle Aging in Master Athletes. Int. J. Mol. Sci. 2022;23:3713. doi: 10.3390/ijms23073713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Ubaida-Mohien C., Spendiff S., Lyashkov A., Moaddel R., MacMillan N.J., Filion M.E., Morais J.A., Taivassalo T., Ferrucci L., Hepple R.T. Unbiased proteomics, histochemistry, and mitochondrial DNA copy number reveal better mitochondrial health in muscle of high-functioning octogenarians. Elife. 2022;11:e74335. doi: 10.7554/eLife.74335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Poussard S., Pires-Alves A., Diallo R., Dupuy J.W., Dargelos E. A natural antioxidant pine bark extract, Oligopin®, regulates the stress chaperone HSPB1 in human skeletal muscle cells: A proteomics approach. Phytother. Res. 2013;27:1529–1535. doi: 10.1002/ptr.4895. [DOI] [PubMed] [Google Scholar]
- 425.Baraibar M.A., Hyzewicz J., Rogowska-Wrzesinska A., Bulteau A.L., Prip-Buus C., Butler-Browne G., Friguet B. Impaired energy metabolism of senescent muscle satellite cells is associated with oxidative modifications of glycolytic enzymes. Aging. 2016;8:3375–3389. doi: 10.18632/aging.101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Medler S. Mixing it up: The biological significance of hybrid skeletal muscle fibers. J. Exp. Biol. 2019;222:jeb200832. doi: 10.1242/jeb.200832. [DOI] [PubMed] [Google Scholar]
- 427.Feng X., Zhang T., Xu Z., Choi S.J., Qian J., Furdui C.M., Register T.C., Delbono O. Myosin heavy chain isoform expression in the Vastus Lateralis muscle of aging African green vervet monkeys. Exp. Gerontol. 2012;47:601–607. doi: 10.1016/j.exger.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Christian C.J., Benian G.M. Animal models of sarcopenia. Aging Cell. 2020;19:e13223. doi: 10.1111/acel.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Xie W.Q., He M., Yu D.J., Wu Y.X., Wang X.H., Lv S., Xiao W.F., Li Y.S. Mouse models of sarcopenia: Classification and evaluation. J. Cachexia Sarcopenia Muscle. 2021;12:538–554. doi: 10.1002/jcsm.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Demontis F., Piccirillo R., Goldberg A.L., Perrimon N. Mechanisms of skeletal muscle aging: Insights from Drosophila and mammalian models. Dis. Model Mech. 2013;6:1339–1352. doi: 10.1242/dmm.012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Yang L., Cao Y., Zhao J., Fang Y., Liu N., Zhang Y. Multidimensional Proteomics Identifies Declines in Protein Homeostasis and Mitochondria as Early Signals for Normal Aging and Age-associated Disease in Drosophila. Mol. Cell. Proteom. 2019;18:2078–2088. doi: 10.1074/mcp.RA119.001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Yatsenko A.S., Kucherenko M.M., Xie Y., Aweida D., Urlaub H., Scheibe R.J., Cohen S., Shcherbata H.R. Profiling of the muscle-specific dystroglycan interactome reveals the role of Hippo signaling in muscular dystrophy and age-dependent muscle atrophy. BMC Med. 2020;18:8. doi: 10.1186/s12916-019-1478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Fisher A.L. Of worms and women: Sarcopenia and its role in disability and mortality. J. Am. Geriatr. Soc. 2004;52:1185–1190. doi: 10.1111/j.1532-5415.2004.52320.x. [DOI] [PubMed] [Google Scholar]
- 434.Daya A., Donaka R., Karasik D. Zebrafish models of sarcopenia. Dis. Model. Mech. 2020;13:dmm042689. doi: 10.1242/dmm.042689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Cobon G.S., Verrills N., Papakostopoulos P., Eastwood H., Linnane A.W. The proteomics of ageing. Biogerontology. 2002;3:133–136. doi: 10.1023/A:1015240304287. [DOI] [PubMed] [Google Scholar]
- 436.Chang J., Van Remmen H., Cornell J., Richardson A., Ward W.F. Comparative proteomics: Characterization of a two-dimensional gel electrophoresis system to study the effect of aging on mitochondrial proteins. Mech. Ageing Dev. 2003;124:33–41. doi: 10.1016/S0047-6374(02)00167-7. [DOI] [PubMed] [Google Scholar]
- 437.O'Connell K., Ohlendieck K. Proteomic DIGE analysis of the mitochondria-enriched fraction from aged rat skeletal muscle. Proteomics. 2009;9:5509–5524. doi: 10.1002/pmic.200900472. [DOI] [PubMed] [Google Scholar]
- 438.Ibebunjo C., Chick J.M., Kendall T., Eash J.K., Li C., Zhang Y., Vickers C., Wu Z., Clarke B.A., Shi J., et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol. Cell Biol. 2013;33:194–212. doi: 10.1128/MCB.01036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Alves R.M., Vitorinom R., Figueiredom P., Duartem J.A., Ferreiram R., Amadom F. Lifelong physical activity modulation of the skeletal muscle mitochondrial proteome in mice. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:832–842. doi: 10.1093/gerona/glq081. [DOI] [PubMed] [Google Scholar]
- 440.Pollard A., Shephard F., Freed J., Liddell S., Chakrabarti L. Mitochondrial proteomic profiling reveals increased carbonic anhydrase II in aging and neurodegeneration. Aging. 2016;8:2425–2436. doi: 10.18632/aging.101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Stolle S., Ciapaite J., Reijne A.C., Talarovicova A., Wolters J.C., Aguirre-Gamboa R., van der Vlies P., de Lange K., Neerincx P.B., van der Vries G., et al. Running-wheel activity delays mitochondrial respiratory flux decline in aging mouse muscle via a post-transcriptional mechanism. Aging Cell. 2018;17:e12700. doi: 10.1111/acel.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Lu X., Gong Y., Hu W., Mao Y., Wang T., Sun Z., Su X., Fu G., Wang Y., Lai D. Ultrastructural and proteomic profiling of mitochondria-associated endoplasmic reticulum membranes reveal aging signatures in striated muscle. Cell Death Dis. 2022;13:296. doi: 10.1038/s41419-022-04746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Lofaro F.D., Cisterna B., Lacavalla M.A., Boschi F., Malatesta M., Quaglino D., Zancanaro C., Boraldi F. Age-Related Changes in the Matrisome of the Mouse Skeletal Muscle. Int. J. Mol. Sci. 2021;22:10564. doi: 10.3390/ijms221910564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Schüler S.C., Kirkpatrick J.M., Schmidt M., Santinha D., Koch P., Di Sanzo S., Cirri E., Hemberg M., Ori A., von Maltzahn J. Extensive remodeling of the extracellular matrix during aging contributes to age-dependent impairments of muscle stem cell functionality. Cell Rep. 2021;35:109223. doi: 10.1016/j.celrep.2021.109223. [DOI] [PubMed] [Google Scholar]
- 445.Doran P., Gannon J., O’Connell K., Ohlendieck K. Aging skeletal muscle shows a drastic increase in the small heat shock proteins alphaB-crystallin/HspB5 and cvHsp/HspB7. Eur. J. Cell Biol. 2007;86:629–640. doi: 10.1016/j.ejcb.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 446.Brulé C., Dargelos E., Diallo R., Listrat A., Béchet D., Cottin P., Poussard S. Proteomic study of calpain interacting proteins during skeletal muscle aging. Biochimie. 2010;92:1923–1933. doi: 10.1016/j.biochi.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 447.Wei L., Gregorich Z.R., Lin Z., Cai W., Jin Y., McKiernan S.H., McIlwain S., Aiken J.M., Moss R.L., Diffee G.M., et al. Novel Sarcopenia-related Alterations in Sarcomeric Protein Post-translational Modifications (PTMs) in Skeletal Muscles Identified by Top-down Proteomics. Mol. Cell. Proteom. 2018;17:134–145. doi: 10.1074/mcp.RA117.000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.O'Connell K., Doran P., Gannon J., Ohlendieck K. Lectin-based proteomic profiling of aged skeletal muscle: Decreased pyruvate kinase isozyme M1 exhibits drastically increased levels of N-glycosylation. Eur. J. Cell Biol. 2008;87:793–805. doi: 10.1016/j.ejcb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 449.Gannon J., Staunton L., O’Connell K., Doran P., Ohlendieck K. Phosphoproteomic analysis of aged skeletal muscle. Int. J. Mol. Med. 2008;22:33–42. doi: 10.3892/ijmm.22.1.33. [DOI] [PubMed] [Google Scholar]
- 450.Feng J., Xie H., Meany D.L., Thompson L.V., Arriaga E.A., Griffin T.J. Quantitative proteomic profiling of muscle type-dependent and age-dependent protein carbonylation in rat skeletal muscle mitochondria. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:1137–1152. doi: 10.1093/gerona/63.11.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Kanski J., Alterman M.A., Schöneich C. Proteomic identification of age-dependent protein nitration in rat skeletal muscle. Free Radic. Biol. Med. 2003;35:1229–1239. doi: 10.1016/S0891-5849(03)00500-8. [DOI] [PubMed] [Google Scholar]
- 452.Sharov V.S., Galeva N.A., Kanski J., Williams T.D., Schöneich C. Age-associated tyrosine nitration of rat skeletal muscle glycogen phosphorylase b: Characterization by HPLC-nanoelectrospray-tandem mass spectrometry. Exp. Gerontol. 2006;41:407–416. doi: 10.1016/j.exger.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 453.Kanski J., Schöneich C. Protein nitration in biological aging: Proteomic and tandem mass spectrometric characterization of nitrated sites. Methods Enzymol. 2005;396:160–171. doi: 10.1016/S0076-6879(05)96016-3. [DOI] [PubMed] [Google Scholar]
- 454.Kanski J., Hong S.J., Schöneich C. Proteomic analysis of protein nitration in aging skeletal muscle and identification of nitrotyrosine-containing sequences in vivo by nanoelectrospray ionization tandem mass spectrometry. J. Biol. Chem. 2005;280:24261–24266. doi: 10.1074/jbc.M501773200. [DOI] [PubMed] [Google Scholar]
- 455.Xie T., Qiao X., Sun C., Chu B., Meng J., Chen C. GAPDH S-nitrosation contributes to age-related sarcopenia through mediating apoptosis. Nitric Oxide. 2022;120:1–8. doi: 10.1016/j.niox.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 456.Piec I., Listrat A., Alliot J., Chambon C., Taylor R.G., Béchet D. Differential proteome analysis of aging in rat skeletal muscle. FASEB J. 2005;19:1143–1145. doi: 10.1096/fj.04-3084fje. [DOI] [PubMed] [Google Scholar]
- 457.O'Connell K., Gannon J., Doran P., Ohlendieck K. Proteomic profiling reveals a severely perturbed protein expression pattern in aged skeletal muscle. Int. J. Mol. Med. 2007;20:145–153. doi: 10.3892/ijmm.20.2.145. [DOI] [PubMed] [Google Scholar]
- 458.Doran P., O'Connell K., Gannon J., Kavanagh M., Ohlendieck K. Opposite pathobiochemical fate of pyruvate kinase and adenylate kinase in aged rat skeletal muscle as revealed by proteomic DIGE analysis. Proteomics. 2008;8:364–377. doi: 10.1002/pmic.200700475. [DOI] [PubMed] [Google Scholar]
- 459.Capitanio D., Vasso M., Fania C., Moriggi M., Viganò A., Procacci P., Magnaghi V., Gelfi C. Comparative proteomic profile of rat sciatic nerve and gastrocnemius muscle tissues in ageing by 2-D DIGE. Proteomics. 2009;9:2004–2020. doi: 10.1002/pmic.200701162. [DOI] [PubMed] [Google Scholar]
- 460.Lombardim A., Silvestri E., Cioffi F., Senese R., Lanni A., Goglia F., de Lange P., Moreno M. Defining the transcriptomic and proteomic profiles of rat ageing skeletal muscle by the use of a cDNA array, 2D- and Blue native-PAGE approach. J Proteom. 2009;72:708–721. doi: 10.1016/j.jprot.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 461.Gannon J., Doran P., Kirwan A., Ohlendieck K. Drastic increase of myosin light chain MLC-2 in senescent skeletal muscle indicates fast-to-slow fibre transition in sarcopenia of old age. Eur. J. Cell Biol. 2009;88:685–700. doi: 10.1016/j.ejcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 462.Donoghue P., Staunton L., Mullen E., Manning G., Ohlendieck K. DIGE analysis of rat skeletal muscle proteins using nonionic detergent phase extraction of young adult versus aged gastrocnemius tissue. J. Proteom. 2010;73:1441–1453. doi: 10.1016/j.jprot.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 463.Gannon J., Ohlendieck K. Subproteomic analysis of basic proteins in aged skeletal muscle following offgel pre-fractionation. Mol. Med. Rep. 2012;5:993–1000. doi: 10.3892/mmr.2012.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Carberry S., Ohlendieck K. Gel electrophoresis-based proteomics of senescent tissues. Methods Mol. Biol. 2013;1048:229–246. doi: 10.1007/978-1-62703-556-9_17. [DOI] [PubMed] [Google Scholar]
- 465.Faure C., Morio B., Chafey P., Le Plénier S., Noirez P., Randrianarison-Huetz V., Cynober L., Aussel C., Moinard C. Citrulline enhances myofibrillar constituents expression of skeletal muscle and induces a switch in muscle energy metabolism in malnourished aged rats. Proteomics. 2013;13:2191–2201. doi: 10.1002/pmic.201200262. [DOI] [PubMed] [Google Scholar]
- 466.Gregorich Z.R., Peng Y., Cai W., Jin Y., Wei L., Chen A.J., McKiernan S.H., Aiken J.M., Moss R.L., Diffee G.M., et al. Top-Down Targeted Proteomics Reveals Decrease in Myosin Regulatory Light-Chain Phosphorylation That Contributes to Sarcopenic Muscle Dysfunction. J. Proteome Res. 2016;15:2706–2716. doi: 10.1021/acs.jproteome.6b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Capitanio D., Vasso M., De Palma S., Fania C., Torretta E., Cammarata F.P., Magnaghi V., Procacci P., Gelfi C. Specific protein changes contribute to the differential muscle mass loss during ageing. Proteomics. 2016;16:645–656. doi: 10.1002/pmic.201500395. [DOI] [PubMed] [Google Scholar]
- 468.Li F.H., Sun L., Wu D.S., Gao H.E., Min Z. Proteomics-based identification of different training adaptations of aged skeletal muscle following long-term high-intensity interval and moderate-intensity continuous training in aged rats. Aging. 2019;11:4159–4182. doi: 10.18632/aging.102044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.de Sousa Neto I.V., Carvalho M.M., Marqueti R.C., Almeida J.A., Oliveira K.S., Barin F.R., Petriz B., de Araújo H.S.S., Franco O.L., Durigan J.L.Q. Proteomic changes in skeletal muscle of aged rats in response to resistance training. Cell. Biochem. Funct. 2020;38:500–509. doi: 10.1002/cbf.3497. [DOI] [PubMed] [Google Scholar]
- 470.Gao H.E., Wu D.S., Sun L., Yang L.D., Qiao Y.B., Ma S., Wu Z.J., Ruan L., Li F.H. Effects of lifelong exercise on age-related body composition, oxidative stress, inflammatory cytokines, and skeletal muscle proteome in rats. Mech. Ageing Dev. 2020;189:111262. doi: 10.1016/j.mad.2020.111262. [DOI] [PubMed] [Google Scholar]
- 471.Kim J.A., Vetrivel P., Kim S.M., Ha S.E., Kim H.H., Bhosale P.B., Heo J.D., Lee W.S., Senthil K., Kim G.S. Quantitative Proteomics Analysis for the Identification of Differential Protein Expression in Calf Muscles between Young and Old SD Rats Using Mass Spectrometry. ACS Omega. 2021;6:7422–7433. doi: 10.1021/acsomega.0c05821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Shembel A.C., Kanshin E., Ueberheide B., Johnson A.M. Proteomic Characterization of Senescent Laryngeal Adductor and Plantaris Hindlimb Muscles. Laryngoscope. 2022;132:148–155. doi: 10.1002/lary.29683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Barbé C., Salles J., Chambon C., Giraudet C., Sanchez P., Patrac V., Denis P., Boirie Y., Walrand S., Gueugneau M. Characterization of the Skeletal Muscle Proteome in Undernourished Old Rats. Int. J. Mol. Sci. 2022;23:4762. doi: 10.3390/ijms23094762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Nuss J.E., Amaning J.K., Bailey C.E., DeFord J.H., Dimayuga V.L., Rabek J.P., Papaconstantinou J. Oxidative modification and aggregation of creatine kinase from aged mouse skeletal muscle. Aging. 2009;1:557–572. doi: 10.18632/aging.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Hwang C.Y., Kim K., Choi J.Y., Bahn Y.J., Lee S.M., Kim Y.K., Lee C., Kwon K.S. Quantitative proteome analysis of age-related changes in mouse gastrocnemius muscle using mTRAQ. Proteomics. 2014;14:121–132. doi: 10.1002/pmic.201200497. [DOI] [PubMed] [Google Scholar]
- 476.McDonagh B., Sakellariou G.K., Smith N.T., Brownridge P., Jackson M.J. Differential cysteine labeling and global label-free proteomics reveals an altered metabolic state in skeletal muscle aging. J. Proteome Res. 2014;13:5008–5021. doi: 10.1021/pr5006394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Huang Y.L., Shen Z.Q., Wu C.Y., Teng Y.C., Liao C.C., Kao C.H., Chen L.K., Lin C.H., Tsai T.F. Comparative proteomic profiling reveals a role for Cisd2 in skeletal muscle aging. Aging Cell. 2018;17:e12705. doi: 10.1111/acel.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.McDonagh B., Sakellariou G.K., Smith N.T., Brownridge P., Jackson M.J. Redox proteomic analysis of the gastrocnemius muscle from adult and old mice. Data Brief. 2015;4:344–348. doi: 10.1016/j.dib.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Smith N.T., Soriano-Arroquia A., Goljanek-Whysall K., Jackson M.J., McDonagh B. Redox responses are preserved across muscle fibres with differential susceptibility to aging. J. Proteom. 2018;177:112–123. doi: 10.1016/j.jprot.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Kelley R.C., McDonagh B., Ferreira L.F. Advanced aging causes diaphragm functional abnormalities, global proteome remodeling, and loss of mitochondrial cysteine redox flexibility in mice. Exp. Gerontol. 2018;103:69–79. doi: 10.1016/j.exger.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Sataranatarajan K., Pharaoh G., Brown J.L., Ranjit R., Piekarz K.M., Street K., Wren J.D., Georgescu C., Kinter C., Kinter M., et al. Molecular changes in transcription and metabolic pathways underlying muscle atrophy in the CuZnSOD null mouse model of sarcopenia. Geroscience. 2020;42:1101–1118. doi: 10.1007/s11357-020-00189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Bareja A., Draper J.A., Katz L.H., Lee D.E., Grimsrud P.A., White J.P. Chronic caloric restriction maintains a youthful phosphoproteome in aged skeletal muscle. Mech. Ageing Dev. 2021;195:111443. doi: 10.1016/j.mad.2021.111443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Hunt L.C., Graca F.A., Pagala V., Wang Y.D., Li Y., Yuan Z.F., Fan Y., Labelle M., Peng J., Demontis F. Integrated genomic and proteomic analyses identify stimulus-dependent molecular changes associated with distinct modes of skeletal muscle atrophy. Cell Rep. 2021;37:109971. doi: 10.1016/j.celrep.2021.109971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Roberts B.M., Deemer S.E., Smith D.L., Jr., Mobley J.A., Musi N., Plaisance E.P. Effects of an exogenous ketone ester using multi-omics in skeletal muscle of aging C57BL/6J male mice. Front. Nutr. 2022;9:1041026. doi: 10.3389/fnut.2022.1041026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Campbell M.D., Martín-Pérez M., Egertson J.D., Gaffrey M.J., Wang L., Bammler T., Rabinovitch P.S., MacCoss M., Qian W.J., Villen J., et al. Elamipretide effects on the skeletal muscle phosphoproteome in aged female mice. Geroscience. 2022;44:2913–2924. doi: 10.1007/s11357-022-00679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Jessica Lo H.T., Yiu T.L., Wang Y., Feng L., Li G., Lui M.P., Lee W.Y. Fetal muscle extract improves muscle function and performance in aged mice. Front. Physiol. 2022;13:816774. doi: 10.3389/fphys.2022.816774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Ryan M., Carlson B.M., Ohlendieck K. Oligomeric status of the dihydropyridine receptor in aged skeletal muscle. Mol. Cell. Biol. Res. Commun. 2000;4:224–229. doi: 10.1006/mcbr.2001.0282. [DOI] [PubMed] [Google Scholar]
- 488.Ryan M., Butler-Browne G., Erzen I., Mouly V., Thornell L.E., Wernig A., Ohlendieck K. Persistent expression of the alpha1S-dihydropyridine receptor in aged human skeletal muscle: Implications for the excitation-contraction uncoupling hypothesis of sarcopenia. Int. J. Mol. Med. 2003;11:425–434. [PubMed] [Google Scholar]
- 489.O'Connell K., Gannon J., Doran P., Ohlendieck K. Reduced expression of sarcalumenin and related Ca2+-regulatory proteins in aged rat skeletal muscle. Exp. Gerontol. 2008;43:958–961. doi: 10.1016/j.exger.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 490.Weisleder N., Brotto M., Komazaki S., Pan Z., Zhao X., Nosek T., Parness J., Takeshima H., Ma J. Muscle aging is associated with compromised Ca2+ spark signaling and segregated intracellular Ca2+ release. J. Cell Biol. 2006;174:639–645. doi: 10.1083/jcb.200604166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Delbono O. Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle. Curr. Aging Sci. 2011;4:248–259. doi: 10.2174/1874609811104030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Qaisar R., Bhaskaran S., Premkumar P., Ranjit R., Natarajan K.S., Ahn B., Riddle K., Claflin D.R., Richardson A., Brooks S.V., et al. Oxidative stress-induced dysregulation of excitation-contraction coupling contributes to muscle weakness. J. Cachexia Sarcopenia Muscle. 2018;9:1003–1017. doi: 10.1002/jcsm.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Pietrangelo L., D'Incecco A., Ainbinder A., Michelucci A., Kern H., Dirksen R.T., Boncompagni S., Protasi F. Age-dependent uncoupling of mitochondria from Ca2⁺ release units in skeletal muscle. Oncotarget. 2015;6:35358–35371. doi: 10.18632/oncotarget.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Chen B.B., Wang J.Q., Meng X.H., Luo Z., Liu X.W., Shen H., Xiao H.M., Deng H.W. Putative Candidate Drug Targets for Sarcopenia-Related Traits Identified Through Mendelian Randomization Analysis of the Blood Proteome. Front. Genet. 2022;13:923429. doi: 10.3389/fgene.2022.923429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Narici M., McPhee J., Conte M., Franchi M.V., Mitchell K., Tagliaferri S., Monti E., Marcolin G., Atherton P.J., Smith K., et al. Age-related alterations in muscle architecture are a signature of sarcopenia: The ultrasound sarcopenia index. J. Cachexia Sarcopenia Muscle. 2021;12:973–982. doi: 10.1002/jcsm.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Calvani R., Marini F., Cesari M., Tosato M., Picca A., Anker S.D., von Haehling S., Miller R.R., Bernabei R., Landi F., et al. SPRINTT Consortium. Biomarkers for physical frailty and sarcopenia. Aging Clin. Exp. Res. 2017;29:29–34. doi: 10.1007/s40520-016-0708-1. [DOI] [PubMed] [Google Scholar]
- 497.Picca A., Coelho-Junior H.J., Calvani R., Marzetti E., Vetrano D.L. Biomarkers shared by frailty and sarcopenia in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2022;73:101530. doi: 10.1016/j.arr.2021.101530. [DOI] [PubMed] [Google Scholar]
- 498.Ohlendieck K. Proteomic identification of biomarkers of skeletal muscle disorders. Biomark. Med. 2013;7:169–186. doi: 10.2217/bmm.12.96. [DOI] [PubMed] [Google Scholar]
- 499.Murphy S., Zweyer M., Mundegar R.R., Swandulla D., Ohlendieck K. Proteomic serum biomarkers for neuromuscular diseases. Expert Rev. Proteom. 2018;15:277–291. doi: 10.1080/14789450.2018.1429923. [DOI] [PubMed] [Google Scholar]
- 500.Stalmach A., Boehm I., Fernandes M., Rutter A., Skipworth R.J.E., Husi H. Gene Ontology (GO)-Driven Inference of Candidate Proteomic Markers Associated with Muscle Atrophy Conditions. Molecules. 2022;27:5514. doi: 10.3390/molecules27175514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Lin C.H., Liao C.C., Huang C.H., Tung Y.T., Chang H.C., Hsu M.C., Huang C.C. Proteomics Analysis to Identify and Characterize the Biomarkers and Physical Activities of Non-Frail and Frail Older Adults. Int. J. Med. Sci. 2017;14:231–239. doi: 10.7150/ijms.17627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Dlamini S.N., Norris S.A., Mendham A.E., Mtintsilana A., Ward K.A., Olsson T., Goedecke J.H., Micklesfield L.K. Targeted proteomics of appendicular skeletal muscle mass and handgrip strength in black South Africans: A cross-sectional study. Sci. Rep. 2022;12:9512. doi: 10.1038/s41598-022-13548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Liu X., Pan S., Xanthakis V., Vasan R.S., Psaty B.M., Austin T.R., Newman A.B., Sanders J.L., Wu C., Tracy R.P., et al. Plasma proteomic signature of decline in gait speed and grip strength. Aging Cell. 2022;21:e13736. doi: 10.1111/acel.13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Picca A., Calvani R., Coelho-Júnior H.J., Marini F., Landi F., Marzetti E. Circulating Inflammatory, Mitochondrial Dysfunction, and Senescence-Related Markers in Older Adults with Physical Frailty and Sarcopenia: A BIOSPHERE Exploratory Study. Int. J. Mol. Sci. 2022;23:14006. doi: 10.3390/ijms232214006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Fielding R.A., Atkinson E.J., Aversa Z., White T.A., Heeren A.A., Achenbach S.J., Mielke M.M., Cummings S.R., Pahor M., Leeuwenburgh C., et al. Associations between biomarkers of cellular senescence and physical function in humans: Observations from the lifestyle interventions for elders (LIFE) study. Geroscience. 2022;44:2757–2770. doi: 10.1007/s11357-022-00685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Kwak J.Y., Hwang H., Kim S.K., Choi J.Y., Lee S.M., Bang H., Kwon E.S., Lee K.P., Chung S.G., Kwon K.S. Prediction of sarcopenia using a combination of multiple serum biomarkers. Sci. Rep. 2018;8:8574. doi: 10.1038/s41598-018-26617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Wu J., Cao L., Wang J., Wang Y., Hao H., Huang L. Characterization of serum protein expression profiles in the early sarcopenia older adults with low grip strength: A cross-sectional study. BMC Musculoskelet. Disord. 2022;23:894. doi: 10.1186/s12891-022-05844-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Van Long N., Chienm P.N., Tung T.X., Van Anh L.T., Giang N.N., Nga P.T., Linh L.T.T., Nam S.Y., Heo C.Y. Complementary combination of biomarkers for diagnosis of sarcopenia in C57BL/6J mice. Life Sci. 2022;312:121213. doi: 10.1016/j.lfs.2022.121213. [DOI] [PubMed] [Google Scholar]
- 509.Chew J., Tay L., Lim J.P., Leung B.P., Yeo A., Yew S., Ding Y.Y., Lim W.S. Serum Myostatin and IGF-1 as Gender-Specific Biomarkers of Frailty and Low Muscle Mass in Community-Dwelling Older Adults. J. Nutr. Health Aging. 2019;23:979–986. doi: 10.1007/s12603-019-1255-1. [DOI] [PubMed] [Google Scholar]
- 510.Perez K., Ciotlos S., McGirr J., Limbad C., Doi R., Nederveen J.P., Nilsson M.I., Winer D.A., Evans W., Tarnopolsky M., et al. Single nuclei profiling identifies cell specific markers of skeletal muscle aging, frailty, and senescence. Aging. 2022;14:9393–9422. doi: 10.18632/aging.204435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Zhao Q., Shen H., Liu J., Chiu C.Y., Su K.J., Tian Q., Kakhniashvili D., Qiu C., Zhao L.J., Luo Z., et al. Pathway-based metabolomics study of sarcopenia-related traits in two US cohorts. Aging. 2022;14:2101–2112. doi: 10.18632/aging.203926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Tsai J.S., Wang S.Y., Chang C.H., Chen C.Y., Wen C.J., Chen G.Y., Kuo C.H., Tseng Y.J., Chen C.Y. Identification of traumatic acid as a potential plasma biomarker for sarcopenia using a metabolomics-based approach. J. Cachexia Sarcopenia Muscle. 2022;13:276–286. doi: 10.1002/jcsm.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Drey M., Sieber C.C., Bauer J.M., Uter W., Dahinden P., Fariello R.G., Vrijbloed J.W., FiAT Intervention Group C-terminal Agrin Fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp. Gerontol. 2013;48:76–80. doi: 10.1016/j.exger.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 514.Hettwer S., Dahinden P., Kucsera S., Farina C., Ahmed S., Fariello R., Drey M., Sieber C.C., Vrijbloed J.W. Elevated levels of a C-terminal agrin fragment identifies a new subset of sarcopenia patients. Exp. Gerontol. 2013;48:69–75. doi: 10.1016/j.exger.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 515.Bütikofer L., Zurlinden A., Bolliger M.F., Kunz B., Sonderegger P. Destabilization of the neuromuscular junction by proteolytic cleavage of agrin results in precocious sarcopenia. FASEB J. 2011;25:4378–4393. doi: 10.1096/fj.11-191262. [DOI] [PubMed] [Google Scholar]
- 516.Hettwer S., Lin S., Kucsera S., Haubitz M., Oliveri F., Fariello R.G., Ruegg M.A., Vrijbloed J.W. Injection of a soluble fragment of neural agrin (NT-1654) considerably improves the muscle pathology caused by the disassembly of the neuromuscular junction. PLoS One. 2014;9:e88739. doi: 10.1371/journal.pone.0088739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Shimada H., Makizako H., Doi T., Yoshida D., Tsutsumimoto K., Anan Y., Uemura K., Lee S., Park H., Suzuki T. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front. Aging Neurosci. 2014;6:69. doi: 10.3389/fnagi.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Miyazaki S., Iino N., Koda R., Narita I., Kaneko Y. Brain-derived neurotrophic factor is associated with sarcopenia and frailty in Japanese hemodialysis patients. Geriatr. Gerontol. Int. 2021;21:27–33. doi: 10.1111/ggi.14089. [DOI] [PubMed] [Google Scholar]
- 519.Kaneko Y. Brain-derived neurotrophic factor as a potential biomarker for sarcopenia and frailty in hemodialysis patients. Geriatr. Gerontol. Int. 2021;21:874–875. doi: 10.1111/ggi.14239. [DOI] [PubMed] [Google Scholar]
- 520.Belhasan D.C., Akaaboune M. The role of the dystrophin glycoprotein complex on the neuromuscular system. Neurosci. Lett. 2020;722:134833. doi: 10.1016/j.neulet.2020.134833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Sirago G., Pellegrino M.A., Bottinelli R., Franchi M.V., Narici M.V. Loss of neuromuscular junction integrity and muscle atrophy in skeletal muscle disuse. Ageing Res. Rev. 2022;83:101810. doi: 10.1016/j.arr.2022.101810. [DOI] [PubMed] [Google Scholar]
- 522.Moreira-Pais A., Ferreira R., Oliveira P.A., Duarte J.A. A neuromuscular perspective of sarcopenia pathogenesis: Deciphering the signaling pathways involved. Geroscience. 2022;44:1199–1213. doi: 10.1007/s11357-021-00510-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Guarino S.R., Canciani A., Forneris F. Dissecting the Extracellular Complexity of Neuromuscular Junction Organizers. Front. Mol. Biosci. 2020;6:156. doi: 10.3389/fmolb.2019.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Rudolf R., Khan M.M., Labeit S., Deschenes M.R. Degeneration of neuromuscular junction in age and dystrophy. Front. Aging Neurosci. 2014;6:00099. doi: 10.3389/fnagi.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.da Orssatto L.B.R., Wiest M.J., Diefenthaeler F. Neural and musculotendinous mechanisms underpinning age-related force reductions. Mech. Ageing Dev. 2018;175:17–23. doi: 10.1016/j.mad.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 526.Kwon Y.N., Yoon S.S. Sarcopenia: Neurological point of view. J. Bone Metab. 2017;24:83–89. doi: 10.11005/jbm.2017.24.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Li M., Larsson L. Force-generating capacity of human myosin isoforms extracted from single muscle fibre segments. J. Physiol. 2010;588:5105–5114. doi: 10.1113/jphysiol.2010.199067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Verdijk L.B., Snijders T., Beelen M., Savelberg H.H., Meijer K., Kuipers H., Van Loon L.J. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J. Am. Geriatr. Soc. 2010;58:2069–2075. doi: 10.1111/j.1532-5415.2010.03150.x. [DOI] [PubMed] [Google Scholar]
- 529.Friedman S.M. Lifestyle (Medicine) and Healthy Aging. Clin. Geriatr. Med. 2020;36:645–653. doi: 10.1016/j.cger.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 530.Eckstrom E., Neukam S., Kalin L., Wright J. Physical Activity and Healthy Aging. Clin. Geriatr. Med. 2020;36:671–683. doi: 10.1016/j.cger.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 531.Roberts S.B., Silver R.E., Das S.K., Fielding R.A., Gilhooly C.H., Jacques P.F., Kelly J.M., Mason J.B., McKeown N.M., Reardon M.A., et al. Healthy Aging-Nutrition Matters: Start Early and Screen Often. Adv. Nutr. 2021;12:1438–1448. doi: 10.1093/advances/nmab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Yeung S.S.Y., Kwan M., Woo J. Healthy Diet for Healthy Aging. Nutrients. 2021;13:4310. doi: 10.3390/nu13124310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Kirwan R., McCullough D., Butler T., Perez de Heredia F., Davies I.G., Stewart C. Sarcopenia during COVID-19 lockdown restrictions: Long-term health effects of short-term muscle loss. Geroscience. 2020;42:1547–1578. doi: 10.1007/s11357-020-00272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Shur N.F., Creedon L., Skirrow S., Atherton P.J., MacDonald I.A., Lund J., Greenhaff P.L. Age-related changes in muscle architecture and metabolism in humans: The likely contribution of physical inactivity to age-related functional decline. Ageing Res. Rev. 2021;68:101344. doi: 10.1016/j.arr.2021.101344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Demonceau C., Beaudart C., Reginster J.Y., Veronese N., Bruyère O. The interconnection between Covid-19, sarcopenia and lifestyle. Maturitas. 2022:S0378-5122(22)00204-3. doi: 10.1016/j.maturitas.2022.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Welch C., Greig C., Masud T., Wilson D., Jackson T.A. COVID-19 and Acute Sarcopenia. Aging Dis. 2020;11:1345–1351. doi: 10.14336/AD.2020.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Soares M.N., Eggelbusch M., Naddaf E., Gerrits K.H.L., van der Schaaf M., van den Borst B., Wiersinga W.J., van Vugt M., Weijs P.J.M., Murray A.J., et al. Skeletal muscle alterations in patients with acute Covid-19 and post-acute sequelae of Covid-19. J. Cachexia Sarcopenia Muscle. 2022;13:11–22. doi: 10.1002/jcsm.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Piotrowicz K., Gąsowski J., Michel J.P., Veronese N. Post-COVID-19 acute sarcopenia: Physiopathology and management. Aging Clin. Exp. Res. 2021;33:2887–2898. doi: 10.1007/s40520-021-01942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Wierdsma N.J., Kruizenga H.M., Konings L.A., Krebbers D., Jorissen J.R., Joosten M.I., van Aken L.H., Tan F.M., van Bodegraven A.A., Soeters M.R., et al. Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission. Clin. Nutr. ESPEN. 2021;43:369–376. doi: 10.1016/j.clnesp.2021.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Damanti S., Cristel G., Ramirez G.A., Bozzolo E.P., Da Prat V., Gobbi A., Centurioni C., Di Gaeta E., Del Prete A., Calabrò M.G., et al. Influence of reduced muscle mass and quality on ventilator weaning and complications during intensive care unit stay in COVID-19 patients. Clin. Nutr. 2022;41:2965–2972. doi: 10.1016/j.clnu.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Lu L., Mao L., Feng Y., Ainsworth B.E., Liu Y., Chen N. Effects of different exercise training modes on muscle strength and physical performance in older people with sarcopenia: A systematic review and meta-analysis. BMC Geriatr. 2021;21:708. doi: 10.1186/s12877-021-02642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Tsekoura M., Billis E., Kastrinis A., Katsoulaki M., Fousekis K., Tsepis E., Konstantoudaki X., Gliatis J. The Effects of Exercise in Patients with Sarcopenia. Adv. Exp. Med. Biol. 2021;1337:281–290. doi: 10.1007/978-3-030-78771-4_31. [DOI] [PubMed] [Google Scholar]
- 543.Gryson C., Ratel S., Rance M., Penando S., Bonhomme C., Le Ruyet P., Duclos M., Boirie Y., Walrand S. Four-month course of soluble milk proteins interacts with exercise to improve muscle strength and delay fatigue in elderly participants. J. Am. Med. Dir. Assoc. 2014;15:958.e1-9. doi: 10.1016/j.jamda.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 544.Martínez-Arnau F.M., Fonfría-Vivas R., Buigues C., Castillo Y., Molina P., Hoogland A.J., van Doesburg F., Pruimboom L., Fernández-Garrido J., Cauli O. Effects of Leucine Administration in Sarcopenia: A Randomized and Placebo-controlled Clinical Trial. Nutrients. 2020;12:932. doi: 10.3390/nu12040932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Bauer J.M., Verlaan S., Bautmans I., Brandt K., Donini L.M., Maggio M., McMurdo M.E., Mets T., Seal C., Wijers S.L., et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015;16:740–747. doi: 10.1016/j.jamda.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 546.Yoo J.I., Chung H.J., Kim B.G., Jung Y.K., Baek K.W., Song M.G., Cho M.C. Comparative analysis of the association between various serum vitamin D biomarkers and sarcopenia. J. Clin. Lab. Anal. 2021;35:e23946. doi: 10.1002/jcla.23946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Negm A.M., Lee J., Hamidian R., Jones C.A., Khadaroo R.G. Management of Sarcopenia: A Network Meta-Analysis of Randomized Controlled Trials. J. Am. Med. Dir. Assoc. 2022;23:707–714. doi: 10.1016/j.jamda.2022.01.057. [DOI] [PubMed] [Google Scholar]
- 548.Bernabei R., Landi F., Calvani R., Cesari M., Del Signore S., Anker S.D., Bejuit R., Bordes P., Cherubini A., Cruz-Jentoft A.J., et al. Multicomponent intervention to prevent mobility disability in frail older adults: Randomised controlled trial (SPRINTT project) BMJ. 2022;377:e068788. doi: 10.1136/bmj-2021-068788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Yan Z., Okutsu M., Akhtar Y.N., Lira V.A. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J. Appl. Physiol. 2011;110:264–274. doi: 10.1152/japplphysiol.00993.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Wilson J.M., Loenneke J.P., Jo E., Wilson G.J., Zourdos M.C., Kim J.S. The effects of endurance, strength, and power training on muscle fiber type shifting. J. Strength Cond. Res. 2012;26:1724–1729. doi: 10.1519/JSC.0b013e318234eb6f. [DOI] [PubMed] [Google Scholar]
- 551.Qaisar R., Bhaskaran S., Van Remmen H. Muscle fiber type diversification during exercise and regeneration. Free Radic. Biol. Med. 2016;98:56–67. doi: 10.1016/j.freeradbiomed.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 552.Coletti C., Acosta G.F., Keslacy S., Coletti D. Exercise-mediated reinnervation of skeletal muscle in elderly people: An update. Eur. J. Transl. Myol. 2022;32:10416. doi: 10.4081/ejtm.2022.10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Blocquiaux S., Gorski T., Van Roie E., Ramaekers M., Van Thienen R., Nielens H., Delecluse C., De Bock K., Thomis M. The effect of resistance training, detraining and retraining on muscle strength and power, myofibre size, satellite cells and myonuclei in older men. Exp. Gerontol. 2020;133:110860. doi: 10.1016/j.exger.2020.110860. [DOI] [PubMed] [Google Scholar]
- 554.Miller M.S., Callahan D.M., Tourville T.W., Slauterbeck J.R., Kaplan A., Fiske B.R., Savage P.D., Ades P.A., Beynnon B.D., Toth M.J. Moderate-intensity resistance exercise alters skeletal muscle molecular and cellular structure and function in inactive older adults with knee osteoarthritis. J. Appl. Physiol. 2017;122:775–787. doi: 10.1152/japplphysiol.00830.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Mesquita P.H.C., Lamb D.A., Parry H.A., Moore J.H., Smith M.A., Vann C.G., Osburn S.C., Fox C.D., Ruple B.A., Huggins K.W., et al. Acute and chronic effects of resistance training on skeletal muscle markers of mitochondrial remodeling in older adults. Physiol. Rep. 2020;8:e14526. doi: 10.14814/phy2.14526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Moro T., Brightwell C.R., Volpi E., Rasmussen B.B., Fry C.S. Resistance exercise training promotes fiber type-specific myonuclear adaptations in older adults. J. Appl. Physiol. 2020;128:795–804. doi: 10.1152/japplphysiol.00723.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Fry C.S., Noehren B., Mula J., Ubele M.F., Westgate P.M., Kern P.A., Peterson C.A. Fibre type-specific satellite cell response to aerobic training in sedentary adults. J. Physiol. 2014;592:2625–2635. doi: 10.1113/jphysiol.2014.271288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Nilwik R., Snijders T., Leenders M., Groen B.B., van Kranenburg J., Verdijk L.B., van Loon L.J. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 2013;48:492–498. doi: 10.1016/j.exger.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 559.Kraková D., Holwerda A.M., Betz M.W., Lavin K.M., Bamman M.M., van Loon L.J.C., Verdijk L.B., Snijders T. Muscle fiber type grouping does not change in response to prolonged resistance exercise training in healthy older men. Exp. Gerontol. 2023;173:112083. doi: 10.1016/j.exger.2023.112083. [DOI] [PubMed] [Google Scholar]
- 560.Li Y., Chen M., Zhao Y., Li M., Qin Y., Cheng S., Yang Y., Yin P., Zhang L., Tang P. Advance in Drug Delivery for Ageing Skeletal Muscle. Front. Pharmacol. 2020;11:1016. doi: 10.3389/fphar.2020.01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Kim J.W., Kim R., Choi H., Lee S.J., Bae G.U. Understanding of sarcopenia: From definition to therapeutic strategies. Arch. Pharm. Res. 2021;44:876–889. doi: 10.1007/s12272-021-01349-z. [DOI] [PubMed] [Google Scholar]
- 562.Canfora I., Tarantino N., Pierno S. Metabolic Pathways and Ion Channels Involved in Skeletal Muscle Atrophy: A Starting Point for Potential Therapeutic Strategies. Cells. 2022;11:2566. doi: 10.3390/cells11162566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Huang L., Li M., Deng C., Qiu J., Wang K., Chang M., Zhou S., Gu Y., Shen Y., Wang W., et al. Potential Therapeutic Strategies for Skeletal Muscle Atrophy. Antioxidants. 2023;12:44. doi: 10.3390/antiox12010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Sartori R., Romanello V., Sandri M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021;12:330. doi: 10.1038/s41467-020-20123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Guo M., Yao J., Li J., Zhang J., Wang D., Zuo H., Zhang Y., Xu B., Zhong Y., Shen F., et al. Irisin ameliorates age-associated sarcopenia and metabolic dysfunction. J. Cachexia Sarcopenia Muscle. 2022 doi: 10.1002/jcsm.13141. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Vainshtein A., Sandri M. Signaling Pathways That Control Muscle Mass. Int. J. Mol. Sci. 2020;21:4759. doi: 10.3390/ijms21134759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Penniman C.M., Bhardwaj G., Nowers C.J., Brown C.U., Junck T.L., Boyer C.K., Jena J., Fuqua J.D., Lira V.A., O’Neill B.T. Loss of FoxOs in muscle increases strength and mitochondrial function during aging. J. Cachexia Sarcopenia Muscle. 2022 doi: 10.1002/jcsm.13124. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Sirago G., Picca A., Calvani R., Coelho-Júnior H.J., Marzetti E. Mammalian Target of Rapamycin (mTOR) Signaling at the Crossroad of Muscle Fiber Fate in Sarcopenia. Int. J. Mol. Sci. 2022;23:13823. doi: 10.3390/ijms232213823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.White T.A., LeBrasseur N.K. Myostatin and sarcopenia: Opportunities and challenges—A mini-review. Gerontology. 2014;60:289–293. doi: 10.1159/000356740. [DOI] [PubMed] [Google Scholar]
- 570.Curiel-Cervantes V., Solis-Sainz J.C., Camacho-Barrón M., Aguilar-Galarza A., Valencia M.E., Anaya-Loyola M.A. Systematic training in master swimmer athletes increases serum insulin growth factor-1 and decreases myostatin and irisin levels. Growth Factors. 2022;40:1–12. doi: 10.1080/08977194.2022.2049262. [DOI] [PubMed] [Google Scholar]
- 571.Baig M.H., Ahmad K., Moon J.S., Park S.Y., Ho Lim J., Chun H.J., Qadri A.F., Hwang Y.C., Jan A.T., Ahmad S.S., et al. Myostatin and its Regulation: A Comprehensive Review of Myostatin Inhibiting Strategies. Front. Physiol. 2022;13:876078. doi: 10.3389/fphys.2022.876078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Ahmad S.S., Ahmad K., Lee E.J., Shaikh S., Choi I. Computational Identification of Dithymoquinone as a Potential Inhibitor of Myostatin and Regulator of Muscle Mass. Molecules. 2021;26:5407. doi: 10.3390/molecules26175407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Camporez J.P., Petersen M.C., Abudukadier A., Moreira G.V., Jurczak M.J., Friedman G., Haqq C.M., Petersen K.F., Shulman G.I. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc. Natl. Acad. Sci. USA. 2016;113:2212–2217. doi: 10.1073/pnas.1525795113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Santos H.O., Cerqueira H.S., Tinsley G.M. The Effects of Dietary Supplements, Nutraceutical Agents, and Physical Exercise on Myostatin Levels: Hope or Hype? Metabolites. 2022;12:1146. doi: 10.3390/metabo12111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Winnard A., Scott J., Waters N., Vance M., Caplan N. Effect of Time on Human Muscle Outcomes During Simulated Microgravity Exposure Without Countermeasures-Systematic Review. Front. Physiol. 2019;10:1046. doi: 10.3389/fphys.2019.01046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Lee P.H.U., Chung M., Ren Z., Mair D.B., Kim D.H. Factors mediating spaceflight-induced skeletal muscle atrophy. Am. J. Physiol. Cell Physiol. 2022;322:C567–C580. doi: 10.1152/ajpcell.00203.2021. [DOI] [PubMed] [Google Scholar]
- 577.Schulz H., Strauch S.M., Richter P., Wehland M., Krüger M., Sahana J., Corydon T.J., Wise P., Baran R., Lebert M., et al. Latest knowledge about changes in the proteome in microgravity. Expert Rev. Proteom. 2022;19:43–59. doi: 10.1080/14789450.2022.2030711. [DOI] [PubMed] [Google Scholar]
- 578.Cannavo A., Carandina A., Corbi G., Tobaldini E., Montano N., Arosio B. Are Skeletal Muscle Changes during Prolonged Space Flights Similar to Those Experienced by Frail and Sarcopenic Older Adults? Life. 2022;12:2139. doi: 10.3390/life12122139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Takahashi H., Nakamura A., Shimizu T. Simulated microgravity accelerates aging of human skeletal muscle myoblasts at the single cell level. Biochem. Biophys. Res. Commun. 2021;578:115–121. doi: 10.1016/j.bbrc.2021.09.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Mass spectrometric raw data from studies of aging diaphragm muscle shown in tables and figures are available on request.