Abstract
Phytochelatins (PCs) are small cysteine-rich peptides capable of binding metal(loid)s via SH-groups. Although the biosynthesis of PCs can be induced in vivo by various metal(loid)s, PCs are mainly involved in the detoxification of cadmium and arsenic (III), as well as mercury, zinc, lead, and copper ions, which have high affinities for S-containing ligands. The present review provides a comprehensive account of the recent data on PC biosynthesis, structure, and role in metal(loid) transport and sequestration in the vacuoles of plant cells. A comparative analysis of PC accumulation in hyperaccumulator plants, which accumulate metal(loid)s in their shoots, and in the excluders, which accumulate metal(loid)s in their roots, investigates the question of whether the endogenous PC concentration determines a plant’s tolerance to metal(loid)s. Summarizing the available data, it can be concluded that PCs are not involved in metal(loid) hyperaccumulation machinery, though they play a key role in metal(loid) homeostasis. Unraveling the physiological role of metal(loid)-binding ligands is a fundamental problem of modern molecular biology, plant physiology, ionomics, and toxicology, and is important for the development of technologies used in phytoremediation, biofortification, and phytomining.
Keywords: metal and metalloid accumulation in plants, metal and metalloid detoxification, metal and metalloid transport, phytochelatins, phytochelatin synthase, stress
1. Introduction
Metals and metalloids are natural components of the earth’s crust. Some metals, such as copper (Cu), manganese (Mn), nickel (Ni), zinc (Zn), and iron (Fe), are essential for most living organisms, while the biological roles of cadmium (Cd), mercury (Hg), lead (Pb), and arsenic (As), with rare exceptions, are unknown and they are toxic even at fairly low concentrations in the environment [1,2,3]. When essential elements are supplied in supraoptimal quantities, multiple toxic effects on a large number of physiological processes can be observed, as was shown for Cd, Pb [4,5], Ni [6,7], As [8], Zn [9] and other metals and metalloids [2,3], which is often accompanied by impaired growth and morphogenesis [10]. Due to human activity, the release of metal(loid)s into the environment has increased significantly in recent decades, including contamination resulting from mining, the intensive use of fertilizers, the combustion of liquid and solid fuels, and the development of metal smelting production [1,11]. Unlike organic compounds which can be decomposed by microorganisms, metals and metalloids do not decompose and, therefore, accumulate in the environment and are absorbed by plants, which are the main source of their entry into food chains [12]. The ability of plants to detoxify metals and metalloids, which are naturally accumulated in the soil due to the processes of rock weathering, is an ancient and widespread trait in plants [13]. They use various strategies to detoxify metals and metalloids: sequestration, exclusion, and chelation [3].
In addition to transporters, that mediate metal(loid) translocation across biological membranes [14,15,16,17], a key role in metal(loid) detoxification, transport, and homeostasis belongs to low-molecular-weight ligands capable of forming stable complexes with metal(loid)s. These include sulfur-containing ligands (for example, glutathione and phytochelatins), nitrogen-/oxygen-containing ligands (S-adenosyl-L-methionine derivatives, histidine, and other amino acids), and oxygen-containing ligands (e.g., phenols and organic acids) [18,19]. The metal fraction that can be exchanged between different ligand molecules is termed the labile pool, the maintenance of which is regarded as a key function of low-molecular-weight ligands [18,20,21].
Different plant species, as well as populations, can differ significantly in their ability to accumulate metals and metalloids in roots and shoots. Unlike excluders, that accumulate metal(loid)s primarily in their roots, hyperaccumulators are plant species in which the metal(loid) concentration in their shoots (per gram of dry weight) exceeds 100 μg of Cd, thallium (Tl), or selenium (Se); 300 µg of Cu, cobalt (Co), or chromium (Cr); 1000 µg of Ni, As, Pb, or rare-earth elements; 3000 µg of Zn; or 10,000 µg of Mn under natural growth conditions, which is much higher than in non-accumulating species [22]. Plants from different populations of the hyperaccumulators Noccaea caerulescens [23,24,25,26] and Arabidopsis halleri [27,28,29] vary in their tolerance to, and capacity to hyperaccumulate, Zn, Cd, Ni, or Zn, Cd, Pb, respectively.
The mechanisms of metal(loid) hyperaccumulation can potentially be controlled at four levels: (1) at the level of metal(loid) uptake from soil by plant root systems, (2) at the level of radial transport of metal(loid)s in roots, (3) at the level of their translocation to the aboveground organs via the xylem, and (4) at the level of accumulation in leaves in a non-toxic form [17,19,30]. Studying the molecular mechanisms of metal(loid) detoxification, as well as the mechanisms that determine the selective accumulation of metal(loid)s in various plant organs, is an important task of modern molecular biology, plant physiology, ionomics, and toxicology.
The phytochelatin-mediated detoxification of metal(loid) ions has been firmly established as a fundamental detoxification mechanism in plants. For the first time, low-molecular-weight, cysteine-rich polypeptides, capable of binding metal(loid) ions via the SH groups of cysteine residues, were found independently in Schizosaccharomyces pombe [31] and in Rauvolfia serpentina cell cultures [32]. Such peptides were called cadistins A and B [31] or phytochelatins (PCs) [32]. To date, PCs have been found not only in angiosperms and gymnosperms, but also in algae, liverworts, fungi, microorganisms, and some animals [14,33,34,35,36,37], which indicates their appearance at the early stages of evolution [38]. Phytochelatins were found in various foods of plant origin, which makes it important to study their impact on the human body [39].
Based on the available literature data, this review summarizes the modern concepts of various PC families in different plant species, PC biosynthesis, and PC participation in metal(loid) uptake and sequestration in the vacuole, as well as in the long-distance transport of metal(loid)s. In addition, we will try to answer the intriguing question of whether PCs are involved in the mechanisms of hyperaccumulation. For convenience, “metals” and “metalloids” will be referred to as “metals” throughout the text of the review. We apologize in advance to all authors whose papers were not cited in our review due to its limited scope.
2. Structure and Accumulation of Phytochelatins in Plants
2.1. Metal(loid)-Induced Phytochelatin Production in Plants
The structure of PCs has been determined for a wide range of plant species from different families (Table 1 and Table 2).
Table 1.
Classification of phytochelatins.
| PC Family | Peptide Structure | Identification |
|---|---|---|
| Phytochelatins | ||
| PCn-Gly | (γGlu-Cys)n-Gly | PCn |
| Iso-phytochelatins | ||
| PCn-Ser | (γGlu-Cys)n-Ser | iso-PCn(Ser) |
| PCn-Ala | (γGlu-Cys)n-Ala | iso-PCn(Ala) |
| PCn-βAla | (γGlu-Cys)n-βAla | iso-PCn(βAla) |
| PCn-Glu | (γGlu-Cys)n-Glu | iso-PCn(Glu) |
| PCn-Gln | (γGlu-Cys)n-Gln | iso-PCn(Gln) |
| PCn-Asn | (γGlu-Cys)n-Asn | iso-PCn(Asn) |
| PCn-Cys | (γGlu-Cys)n-Cys | iso-PCn(Cys) |
| des-Gly-PCn | (γGlu-Cys)n | des-Gly-PCn |
| des-γGlu-PCn-Gly | Cys-(γGlu-Cys)n−1-Gly | des-γGlu-iso-PCn(Gly) |
| des-γGlu-PCn-Ser | Cys-(γGlu-Cys)n−1-Ser | des-γGlu-iso-PCn(Ser) |
| des-Cys-PCn-Glu | Glu-(γGlu-Cys)n−1-Glu | des-Cys-iso-PCn(Glu) |
Table 2.
The structure of phytochelatins identified in different plant species depending on the metal(loid) concentration in the medium and the duration of exposure.
| Species | Plant Material | Metal(loid) Concentration |
Duration of Exposure |
PC Structure | Ref. |
|---|---|---|---|---|---|
| Amaranthaceae | |||||
| Amaranthus hypochondriacus | Leaves | 100 mg/kg Cd | 3 months | PC2–4 | [40] |
| Pfaffia glomerata | Roots | 25, 50, 100 µM As | 28 days | PC2–4 | [41] |
| Spinacia oleraceae | Roots, leaves | 1, 3, 5, 9 mg/L Cd | 1, 3, 5, 7, 9, 14 days | PC2–4 | [42] |
| Apiaceae | |||||
| Datura innoxia | Cell culture | 250 µM Cd | up to 2 h | PC2–5 | [43] |
| Apocynaceae | |||||
| Rauvolfia serpentina | Cell culture | 200 µM Cd | 1–9 h | PC2–5 | [44] |
| Asteraceae | |||||
| Dittrichia viscosa | Roots, shoots | 5, 10, 15 mg/L Cd | 10 days | PC2–4, iso-PC2–3(Cys), des-γGlu-iso-PC2–3(Gly), des-Gly-PC2–3 |
[45] |
| Eupatorium cannabinum | Roots | 11 mg/L As | 20 days | PC2–4, des-Gly-PC2–4, γGlu-iso-PC3(Gly) |
[46] |
| Leaves | PC3–4, des-Gly-PC2, des-γGlu-iso-PC2(Gly) |
||||
| Helianthus annuus | Roots, stems, leaves | 66 µmol/L As | 1–96 h | PC2–3 | [47] |
| Brassicaceae | |||||
| Arabidopsis halleri | Roots | 25 µM Cd 100 µM Cd |
7 days | PC2–4 PC2–5 |
[48] |
| Shoots | 25 µM Cd 100 µM Cd |
n.d. * PC2–4 |
|||
| Arabidopsis thaliana | Seedlings | 30 µM Cd | 1 day | PC2–4 | [49] |
| 30, 90 µM Cd | 3, 9 days | PC2–4 | [50] | ||
| 20 µM Cd | 3 days | PC2–4 | [51] | ||
| 10 µM Cd | 5 days | PC2–4 | [52] | ||
| Roots, leaves | 5 µM Cd | 1, 3, 7, 14, 21 days | PC2–5 | [53] | |
| Roots, shoots | 10, 25 µM Cd | 7 days | PC2–5 | [48] | |
| Leaves | 7.5 mg/kg Cd 970 mg/kg Zn |
24 days | PC2–3 | [54] | |
| Roots, shoots | 10 µM Cd 5 µM Cu 150 µM Zn |
12 days | PC2–5 | [55] | |
| Roots, shoots | 20 µM Cd | 72 h | PC2–4 | [56] | |
| Roots, shoots | 1 µM Hg, 0.1 µM phenylmercury |
4 days | PC2 | [57] | |
| Cell culture | 200 µM Cd | 1, 4, 8, 11, 24, 48 h |
PC2–4(5), iso-PC3–4(Ser), iso- PC3(Glu), iso-PC3–4(βAla), iso-PC3–4(Gln) |
[58] | |
| 50 µM Cd | 1 day | PC2–5, iso-PC3–4(βAla) | |||
| 400 µM Cd | 1 day | PC2–5, iso-PC3–4(Ser), iso- PC3(Glu), iso-PC3–4(βAla), iso-PC3–4(Gln) |
|||
| Armoracia rusticana | Roots | 1000 µM Cd | 3 days | PC3–4 | [59] |
| Brassica chinensis | Roots | 200 µM Hg | 3 days | PC2–4 | [60] |
| Brassica juncea | Roots, shoots | 50, 200 µM Cd | 7 days | PC2–4 | [61] |
| Roots, leaves | 500, 1000, 2000 mg/kg Pb | 45 days | PC2–3 | [62] | |
| Brassica oleracea | Seedlings | 90 µM Cd | 21 days | PC2–6 | [44] |
|
Noccaea caerulescens
(Thlaspi caerulescens) |
Roots, shoots | 1–50 µM Cd | 4 days | PC2–3 | [63] |
| Roots shoots |
25, 100 µM Cd 25, 100 µM Cd |
7 days 7 days |
PC2–4 n.d. |
[48] | |
| Roots, shoots | 5–500 µM Cd | 14 days | PC2–4 | [64] | |
| Sinapis alba | Leaves | 0.5, 1 mg/L Pd | 2 weeks | PC2–4 | [65] |
| Thlaspi arvense | Roots, shoots | 1–50 µM Cd | 4 days | PC2–3 | [63] |
| Caryophyllaceae | |||||
| Silene vulgaris | Cell culture | 20 µM Cd | 3 days | PC2–4 | [44] |
| Roots | 0.3, 1, 45, 135, 180 µM Cd |
3 days | PC2–4 | [66] | |
| 40 mmol m−3 Cd | 21 days | PC2–3 | [67] | ||
| Crassulaceae | |||||
| Sedum alfredii | Shoots | 500 µM Cd | 8 days | PC2–4 | [68] |
| Cucurbitaceae | |||||
| Cucumis sativus | Roots | 10–250 µM Sb | 28 days | PC2, 3 | [69] |
| Fabaceae | |||||
| Arachis hypogaea | Roots | 10 µM Cd | 30 days | PC2–4 | [70] |
| Glycine max | Roots | 20 µM Cd | 4 days | iso-PC2–7(βAla) | [71] |
| Pisum sativum | Roots | 20 µM Cd | 3 days | PC2–3, iso-PC3(βAla) | [72] |
| 1–120 µM Cd | 1–9 days | PC2–4, iso-PC2–4(βAla) | [73] | ||
| Lamiaceae | |||||
| Clinopodium vulgare | Roots | 20 mg/kg Cd 250 mg/kg Pb 400 mg/kg Cu 400 mg/kg Zn |
95, 105 days | All metals: PC2–5, iso-PC2(Ala); Cu: iso-PC2(Glu), iso-PC2–4(Cys); Cu, Cd, Pb: des-γGlu-iso-PC2–3(Gly); Cu, Cd, Pb: des-Gly-PC2 | [74] |
| Shoots | 20 mg/kg Cd 250 mg/kg Pb 400 mg/kg Cu 400 mg/kg Zn |
95, 105 days | All metals: PC2–5Cu, Cd, Pb: des-γGlu-iso-PC2–3(Gly) Cu, Cd, Pb: des-Gly-PC2 |
||
| Perilla frutescens | Roots, stems, leaves | 2, 5, 10 mg/L Cd | 14 days | PC2–4 | [75] |
| 21 days | PC2–3 | ||||
| Marchantiaceae | |||||
| Marchantia polymorpha | Gametophyte | 10–36 µM Cd | 6–120 h | PC2–4 | [76] |
| 10, 20, 36 µM Cd | 72 h | PC2–4 | [77] | ||
| Poaceae | |||||
| Agrostis tenuis | Cell culture | 20 µM Cd | 3 days | PC2–4 | [44] |
| Avena sativa | Roots | 10 µM Cd | 4 days | PC2–3 | [78] |
| Holcus lanatus | Roots | 5, 15, 40, 800 µM As | 7 days | PC2–4 | [79] |
| Lolium perenne | Roots, shoots | 20, 80 µM Cd | 9 days | PC2–6 | [80] |
| 20, 80 µM Cd | 216 h | PC2–6 | [81] | ||
| Oryza sativa | Roots | 10 µM Cd 20 µM As |
7 days | PC2–3, iso-PC2(Ser), iso-PC(Glu) | [82] |
| Roots, stems, leaves |
50, 100 µM Cd | 7 days | PC2–4, iso-PC2–3(Gln), iso-PC2–3(Asn), iso-PC2(Cys), des-γGlu-iso-PC3(Ser), des-Cys-iso-PC2(Glu), des-Gly-PC2–4, iso-PC2–4(Ser), iso-PC2–4(Glu) | [83] | |
| Roots, shoots | 100 µM As | 10 days | PC2–4 | [84] | |
| Roots, shoots | 50 µM Cd | 14 days | PC2–4 | [85] | |
| Panicum maximum | Roots | 100 µM Cd | 9 days | PC2–4, des-Gly-PC2–4, iso-PC2–3(βAla), des-γGlu-iso-PC2(Gly) |
[86] |
| Stems | PC3, 5, 6, iso-PC2–4(βAla), des-Gly-PC4, des-γGlu-iso-PC2–3(Gly) |
||||
| Leaves | PC6, iso-PC4(βAla) | ||||
| Phragmites australis | Roots | 100 µM Cd | 21 days | PC2–4 | [87] |
| Secale cereale | Roots | 50, 250 µM Cd | 3, 6, 12, 24 h, 3, 7, 14 days |
PC2–3, iso-PC2–3(Ser), des-Gly-PC2–3 | [88] |
| Shoots | 50 µM Cd 250 µM Cd |
7,14 days 7 days |
PC2–3, iso-PC2–3(Ser), des-Gly-PC2–3 | ||
| Triticum aestivum | Roots | 1 µM Cd | 12 days | PC2–3, iso-PC2–3(Ser) | [89] |
| 30 µM Cd | PC2–4, iso-PC2–4(Ser) | ||||
| Roots | 1 mM Cd | 10, 20 days | PC2–4, des-Gly-PC2–3 | [90] | |
| Shoots | PC2–4 | ||||
| Triticum turgidum var. durum | Roots | 1 µM Cd | 12 days | PC2–3, iso-PC2–3(Ser) | [89] |
| 30 µM Cd | PC2–4, iso-PC2–4(Ser) | ||||
| Triticum vulgare | Roots | 50, 250 µM Cd | 3, 6, 12, 24 h, 3, 7, 14 days |
PC2–3, iso-PC2–3(Ser), des-Gly-PC2–3 | [88] |
| Shoots | 7,14 days | ||||
| Zea mays | Roots | 50 µM Cd | 3, 6, 12, 24 h, 3, 7, 14 days |
PC2–4, iso-PC2(Glu), des-Gly-PC2–3 | [88] |
| 0.01 µM Cd 0.05, 0.1 µM Cd 0.5, 1, 10 µM Cd 3 µM Cd |
1 day 1 day 1 day 2, 4, 6 h; 1, 2 days |
PC2 PC2–3 PC2–4 PC2–4 |
[91] | ||
| 3 µM Cd | 1–7 days | PC2–4, des-Gly-PC2–4, iso-PC2–4(Glu) |
[92,93] | ||
| Roots | 38 µM Cd | 5 days | PC2–5, iso-PC2–5(Ser) | [94] | |
| Shoots | PC2–3, iso-PC2–3(Ser) | ||||
| Roots | 10, 15, 25 µM Cd | 14 days | PC2–3, PC7, 8, PC10, PC4, 9 (10 and 15 µM Cd), PC5 (25 µM Cd), PC6 (10 and 25 µM Cd) |
[95] | |
| Leaves | PC2,3,6,8,10 PC4 (25 µM Cd) |
||||
| Seedlings | 20 µM Cd | 3 days | PC2–4 | [44] | |
| Pontederiaceae | |||||
| Pontederia crassipes (Eichhornia crassipes) | Seedlings | 20 µM Cd | 3 days | PC2–4 | [44] |
| Roots | 1, 2.5, 3.5 ppm Cd | 45 days | PC2–4 | [96] | |
| Proteaceae | |||||
| Banksia seminuda | Roots | 10–250 µM Sb | 120 days | PC2, 3 | [69] |
| Hakea prostrata | Roots | 10–250 µM Sb | 120 days | PC2, 3 | [69] |
| Pteridaceae | |||||
| Pteris cretica | Fronds | 100 mg/kg As | 1 year | PC2 | [97] |
| Rubiaceae | |||||
| Rubia tinctorum | Cell culture | 100 µM Cd | 3 days | PC2–4, des-Gly-PC2–4 | [98] |
| Salicaceae | |||||
| Salix atrocinerea | Roots | 18 mg/L As | 1, 3, 10, 30 days |
PC2–3, des-Gly-PC3, des-γGlu-iso-PC2–3(Gly) |
[99] |
| Leaves | des-Gly-PC2–4 | ||||
| Solanaceae | |||||
| Nicotiana rustica | Leaves | 20 µM Cd | 7 days | PC3–4 | [100] |
| Nicotiana tabacum | Cell culture | 250 µM Cd | 3 days | PC4–5 | [101] |
| Seedlings | 30, 90 µM Cd | 3, 9 days | PC2–4 | [50] | |
| Solanum lycopersicum (Lycopersicon esculentum) | Cell culture | 100 µM Cd | 2 h | PC3–5 | [102] |
| 100, 300 µM Cd | 1–12 days | PC2 | [103] | ||
| 600 µM Cd | 36 h | PC3–4 | [104] | ||
| 10, 50, 100 µM Cd |
4 days | PC2–4 | [105] | ||
| 100 µM Cd | 7 days | PC2–4 | [106] | ||
| Roots | 3 µM Cd | 7 days | PC3 | [107] | |
| Seedlings | 90 µM Cd | 21 days | PC2–6 | [44] | |
| Roots, leaves | 25, 100 µM Cd | 14 days | PC2–4 | [108] | |
| Vitaceae | |||||
| Vitis vinifera | Roots | 100 mg/L Hg | 3 days | PC2–4 | [109] |
* n.d.—not determined.
The basic structure of PCs is (γ-Glu-Cys)n-Gly, where n = 2–11 but usually does not exceed 4–5 (Table 1) [14,33,110,111,112,113,114,115]. Other possible structures of PCs will be discussed below. The degree of PC polymerization, as well as PC accumulation in plants, largely depends on the physicochemical properties of the metal ions, the duration of exposure, and the metal concentration [44,53,75,76,92,116,117,118,119]. Phytochelatins with a longer chain were usually synthesized only after a lag period [44,75,76,120].
The kinetics of Cd uptake and PC induction during the first 4 h of incubation, and at different levels of N and P, was recently studied on the diatom Thalassiosira weissflogii [121] and traced for 14 and 21 days, respectively, in the roots and shoots of Spinacia oleracea [42] and Arabidopsis thaliana [53]. For example, when S. oleracea plants were grown in hydroponics at 3–9 mg/L Cd, glutathione and PC3 were predominant on most of the days, and the concentrations of PC2, PC3 and PC4 in the leaves usually reached a peak after 7 or 9 days of exposure, but subsequently decreased during the following days [42]. The concentration of PCs in the roots and leaves of A. thaliana plants significantly increased after 3 days of exposure to 5 µM Cd in a nutrient solution, compared to that after 1 day of exposure. After 7, 14, and 21 days of exposure, PC concentration in the roots of Cd-treated A. thaliana slightly increased and then remained at a similar level, whereas PC concentration in the leaves reached a peak after 7 days of exposure and subsequently decreased [53]. The kinetics of PC accumulation was also traced for the roots, stems, and leaves of As-treated Helianthus annuus [47], roots and leaves of As-treated Salix atrocinerea [99], roots of Cd-treated Pisum sativum [73] and Zea mays [88,91,92,93], roots and shoots of Cd-treated Secale cereale and Triticum vulgare [88], and gametophytes of Cd-treated Marchantia polymorpha [76] (Table 2).
Various metal(loid) ions can induce PC biosynthesis. When different metal(loid)s were added to R. serpentina cell cultures at high, but non-lethal, concentrations, PC biosynthesis was induced in the presence of Pb2+, Zn2+ (1 mM); Cd2+, Ni2+, tin (Sn2+), SeO32−, bismuth (Bi3+) (100 μM); silver (Ag+), Cu2+, gold (Au+) (50 μM); AsO43- (20 μM); antimony (Sb3+) and tellurium (Te4+) (10 μM) [44]. Similar results were obtained for cell cultures of Rubia tinctorum [98]. Recently, the induction of PC biosynthesis was shown in Sinapis alba under treatment with platinum (Pt), rhodium (Rh), and palladium (Pd) [65], and in Z. mays seedlings in the presence of vanadium (V) [122]. Although PC biosynthesis can be induced in vivo by various elements, they are mainly involved in the detoxification of Cd and As (III) [14,54,99,123,124,125,126,127] and, to a lesser extent, Hg [57,60,109,128,129], Pb [62,74,130,131,132,133], Zn [54,55,74,133,134], Cu [55,74,119,135,136], and Sb(V) [69], which is partly determined by the stability of the metal complexes with S-containing ligands. Boron (B) [137], magnesium (Mg), calcium (Ca), and sodium (Na) [138] did not induce the biosynthesis of PCs. The possible role of PCs in response to drought, low temperatures, salinity, and other stress factors is also discussed, which, however, requires additional studies [139,140,141]. Since PCs are S-containing compounds, the amount of PCs decreases under sulfur (S) deficiency [142].
Different metals induce the biosynthesis of PCs to a different degree in various species. Ahner and Morel [143], comparing the accumulation of PCs in the algae T. weissflogii, Tetraselmis maculate, and Emiliania huxleyi under the treatment with Cd, Cu, Zn, and Pb, showed that the induction of PC production can be not only metal-specific, but also taxon-dependent. The concentration of PCs in Emiliania huxleyi was similar in the presence of Cd and Cu [143], while in Dunaliella tertiolecta, PC induction was observed at a higher concentration of Zn compared to Cd [144]. In Sedum alfredii, PC production was induced in vivo in the presence of Cd and Pb, but not Zn [145], whereas in Pfaffia glomerata, it was induced in the presence of As, but not Hg or Pb [41]. In Clinopodium vulgare, the biosynthesis of PCs was induced by Cd, Cu, Zn, and, to a lesser extent, Pb [74], and in A. thaliana, it was induced by Cd, Zn, and to a lesser extent, Cu [55,134].
Since under field conditions, especially in polluted areas, plants are often exposed to excessive amounts of more than one metal(loid), synergism or antagonism between the elements in their effect on plants can be observed depending on metal physicochemical properties, as well as environmental factors and biological characteristics of plant species. For example, after 14 days of incubation of Triticum aestivum in the presence of Cd or Pb, an increase in PC concentrations was observed, being most prominent in the case of Cd. Under the combined treatment, the metals had a synergistic effect on PC biosynthesis, but an antagonistic effect on the biosynthesis of glutathione [146]. The concentrations of PCs, cysteine, and glutathione in the roots of Cd-treated Oryza sativa increased in the presence of silicon (Si) [147], whereas in the roots of Cr-treated plants, an increase in the concentration of PCs, but not glutathione, was observed in the presence of Ca [148]. In the roots and shoots of Nicotiana tabacum, Cr (50 µM) did not induce the production of glutathione or PCs, but their concentration increased in Cr-stressed plants when they were supplied with Se (2 µM) and molybdenum (Mo) (1 µM) [149]. The concentration of glutathione and PCs also increased in As-treated O. sativa in the presence of Si and TiO2 nanoparticles, whereas the nanoparticles alone did not affect the biosynthesis of PCs [150]. Differences between separate and combined effects of metals have also been shown for other ligands [19,151] and, in general, require more thorough studies.
2.2. The Structure of Phytochelatin Complexes with Metal(loid)s
Various analytical methods are used to identify PCs and their complexes with metals [38,56,74,83,120,152,153,154], and their advantages and disadvantages are discussed in the review by Ahmad and co-authors [155]. The most numerous studies are devoted to the role of glutathione and PCs in the detoxification of Cd, since Cd ions have the highest affinity for these ligands compared to other metals (Mn, Fe, Cu, Zn) [156]. In spectrophotometric studies, log K7.4 values for Cd complexes with ligands (1:1) were 4.8 for glutathione, 6.2 for PC2, 7.5 for PC4, and 5.5 for PC6 [157]. Potentiometric and spectroscopic studies showed that the affinity of Cd complexes increased from glutathione to PC4 almost linearly from the micromolar (log K7.4GSH = 5.93) to the femtomolar range (log K7.4PC4 = 13.39), and additional chain elongation did not significantly increase the stability [120]. The thermodynamic stability of PC complexes with other metals decreased in the following order: Zn2+ ≥ Cu2+ ≥ Fe2+ > Mg2+ > Ca2+ [158]. Hence, PCs are more efficient for metal chelation than glutathione.
The structure of PC complexes with metal(loid)s can differ both for different metals and for complexes with different degrees of polymerization [38,120,152]. For example, all peptides can form 1:1 Cd-ligand complexes, but 1:2 Cd-ligand complexes were found for glutathione, PC2, and, partially, for PC3. Moreover, binuclear species, Cdx-ligandy, were identified for the series PC3−PC6 under Cd excess [120]. In the leaves of As-tolerant Holcus lanatus, the As(III)-PC3 complex was the predominant one, although reduced glutathione, PC2, and PC3 were found in the extract, whereas the As hyperaccumulator Pteris cretica only synthesized PC2 and formed predominantly the GS-As(III)-PC2 complexes [97]. In the roots, stems, and leaves of As-treated H. annuus, complexes of arsenite with glutathione, As(III)–PC3, and GS–As(III)–PC2 complexes were detected. The roots also contained As(III)–(PC2)2 and monomethylarsonic PC2 complexes [47].
PC complexes with metals are stable and less toxic than free metal ions [14,113,114]. For example, PC–Cd complexes are approximately 1000 times less toxic to enzymes than free Cd ions [159]. Therefore, even nanomolar amounts of Cd, which can get into the nutrient solution from contaminated chemicals, can induce PC biosynthesis [160]. However, the amount of PCs as well as the stability of the resulting complexes of PCs with metals depend on the pH [46,161,162]. At the pH of the cytosol (7.2–7.5), the complexes are stable, whereas at the pH of the vacuolar sap (4.5–6.0), they break up [161], which plays an important role in PC-mediated metal detoxification. The formation of stable complexes of PCs with metals in the cytoplasm prevents metal binding to the sulfhydryl groups of proteins, and therefore, the toxic effects on metabolic processes are reduced.
2.3. Classification of Phytochelatins and Their Accumulation in Different Plant Organs
The presence of the γ-Glu bond in PCs indicates that they are not primary gene products [44] and therefore are classified as a separate third class of metallothioneins [163,164]. Instead of glycine, at the C-terminus of the polypeptide there can be glutamine (Gln), glutamic acid (Glu), serine (Ser), alanine (Ala), β-alanine (β-Ala), asparagine (Asn), or cysteine (Cys), or the C-terminal amino acid may be absent. In addition, there have been found several PC derivatives that lack either Glu or Cys residues from the γ-Glu-Cys structure [45,83,86,99]. Thus, several families of PCs have been currently identified [83,110,112,165] (Table 1). Some of them are probably specific for some families of angiosperms. Thus, homophytochelatins (homoPCs), with C-terminal β-Ala, have been currently found mainly in legumes [71,72,73,166], and hydroxymethylphytochelatins (hydroxymethylPCs), with C-terminal Ser, have been detected in cereals [82,83,88,89,90,94,116,152]. PCs with C-terminal Glu were found in the roots of Z. mays [88,92,93,167], as well as the roots and shoots of O. sativa [82,83,152]. In addition, PCs with C-terminal Gln, Asn, and Cys were found in the roots and shoots of O. sativa [83]. Phytochelatins that do not contain a C-terminal amino acid, and have the structure γ-Glu(Cys)n, have been isolated, for example, from the roots of Z. mays [88,92,93], Capsicum annuum [118], the roots and shoots of T. aestivum, S. cereale [88], O. sativa [83,152], Dettrichia viscose [45], Panicum maximum [86], Betula pubescens [168], S. atrocinerea [99], as well as from the root culture of R. tinctorum [98,169] (Table 1 and Table 2). Phytochelatins with C-terminal Ala, Glu, Gln, Ser, Asn, or Cys, and other PC derivatives, have also been proposed to be called iso-phytochelatins (iso-PCs) [83,170].
In the cell culture of A. thaliana, both PCs and all types of iso-PCs were found, which indicates the presence of iso-glutathione [58], which acts as an acceptor of γ-Glu-Cys residues during the biosynthesis of PCs [171]. However, in intact plants of A. thaliana [48,49,50,51,52,53,54,55,56], as well as in other Brassicaceae species [44,48,59,60,61,62,63], only PCs were found (Table 2). Therefore, the presence of iso-PCs in intact plants from this family remains debatable. Phytochelatins (PC2–5) and various iso-PCs were found in C. vulgare from the Lamiaceae family [74], whereas homoPCs were found in cereals [86], which suggests a wider distribution of iso-PCs in nature. However, it is still unknown why such a diverse group of PCs is synthesized in various taxa.
In addition to the differences in the terminal amino acid and the degree of polymerization, sulfur in PCs can be present in different oxidation states (thiol versus disulfide form), and metals can theoretically interact with various functional groups of peptides [165]. Since at pH values close to neutral thiol groups are predominantly in the thiolate form (-S−), it is assumed that metal binds mainly to them, although the participation of carboxyl groups cannot be excluded [60,120,130,154].
The accumulation of PCs in different plant organs can vary significantly. For example, in B. pubescens, Halimione portulacoides, and Sarcocornia perennis growing on polluted soil [168,172], in Brassica juncea under long-term treatment with Cd [173], as well as in Cd-treated Perilla frutescens [75], the concentration of PCs in the leaves was higher than in the roots. At the same time, in Spartina maritima growing on polluted soil [172], Cd-treated T. aestivum [90], Cd- and Zn-treated A. thaliana [134], C. vulgare exposed to Cd, Cu, Zn, Pb [74], As-treated Eupatorium cannabinum and S. atrocinerea [46,99], and in Pb-treated Brassica juncea [62], a higher level of PCs was observed in the roots. In O. sativa seedlings exposed to 50 or 100 µM Cd, a significant variation in the concentrations of various PC derivatives was observed in the roots, stems, and leaves [83], whereas under the exposure to As (V) (100 µM), no significant differences in the concentrations of PCs in the roots and shoots were found [84]. In addition to species-specific features, such differences can be caused by different metal concentrations, duration of exposure, and growth media, which should be taken into account in a comparative analysis of the data obtained by different authors. At the same time, the PC concentration did not always correlate with the metal(-loid) concentration, which is a reflection of the existence of other metal detoxification mechanisms that can be acting simultaneously and with different efficiency in different species, such as, for example, binding to the material of cell walls [174], metallothioneins [35,37,175], or other ligands [19]. Significant differences in the concentration of PCs may also be a reflection of plant cultivar characteristics, which, for example, was shown in a comparative analysis of the accumulation of PCs and iso-PCs in the roots of plants of twelve O. sativum cultivars exposed to As [152] and two Brassica parachinensis cultivars exposed to Cd [176].
3. Biosynthesis of Phytochelatins and Its Regulation
3.1. Glutathione as a Precursor of Phytochelatins
Biosynthesis of PCs requires L-glutamate (Glu), L-cysteine (Cys), and glycine (Gly). Metals affect various stages of PC biosynthesis, from S assimilation to glutathione biosynthesis [37,177]. PCs are synthesized from reduced glutathione, which is one of the reasons for the decrease in the pool of intracellular glutathione [44,177]. A Cd-induced decrease in the concentration of glutathione was observed, for example, in Pontederia (Eichhornia) crassipes [96], Arachis hypogaea [70], P. sativum seedlings [72], in the roots of Pistia stratiotes [178], O. sativa [83,85], and Z. mays [88,179,180], and in suspension-cultured cells of Solanum lycopersicum [102], while an As-induced decrease in the concentration of glutathione was shown in the roots of S. atrocinerea [99] and O. sativa [84]. However, in the hyperaccumulators A. halleri, N. caerulescens, and S. alfredii [48,181], the accumulator P. frutescens [75], in the roots of Phragmites australis [87] and Cajanus cajan [182], in the roots and shoots of Solanum lycopersicum [108], and in the liverwort M. polymorpha [76,77], in the presence of Cd an opposite pattern was observed. The effect of Cd, as well as other metals, on the content of glutathione may differ depending on the plant organ, the duration of exposure, and the concentration of the metal in the medium [42], which may partly explain the conflicting results obtained for B. juncea [61,183,184] and A. thaliana [48,55]. In addition, the metal’s effect may depend on the endogenous content of glutathione. Thus, the glutathione concentration in M. polymorpha gametophytes was constitutively low compared to higher plants, but a significant increase in its level was observed under the treatment with Cd [76,77].
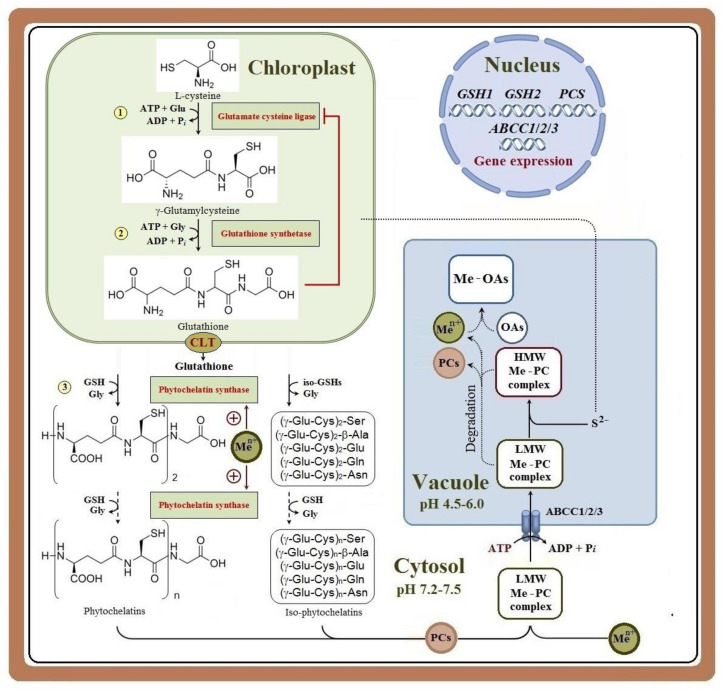
The biosynthesis of glutathione from Glu, Cys, and Gly is an ATP-dependent, two-step process (Figure 1) [177,185,186]. The first reaction for the formation of γ-Glu-Cys from Glu and Cys takes place in the chloroplasts and is catalyzed by glutamate cysteine ligase (EC 6.3.2.2), previously known as γ-glutamylcysteine synthase [177,186,187,188,189], which is encoded by the GSH1 (GCS) gene [190,191] (Figure 1). The reaction catalyzed by this enzyme is considered as the rate-limiting step in the biosynthesis of glutathione [103,126,185,186,189,191]. The second stage of the biosynthesis of glutathione from γ-Glu-Cys and Gly can occur both in the chloroplasts and in the cytosol, and is catalyzed by glutathione synthetase (EC 6.3.2.3), which is encoded by the GSH2 (GS) gene (Figure 1) [177,186,189,192]. Glutathione that is synthesized in the chloroplasts enters the cytosol, where it is involved in the biosynthesis of PCs, via the chloroquine resistance-like transporters (CLT1–3) (Figure 1) [188].
Figure 1.
Phytochelatin-mediated pathway of metal detoxification in plants.
The biosynthesis of γ-glutamylcysteine from L-cysteine and L-glutamate (Glu) (stage 1) takes place in chloroplasts and is catalyzed by glutamate cysteine ligase encoded by the GSH1 gene. The formation of glutathione (GSH) from γ-glutamylcysteine and L-glycine (Gly) (stage 2) can occur both in the chloroplasts and in the cytosol (the latter is not shown in the scheme) and is catalyzed by glutathione synthetase encoded by the GSH2 gene. Glutathione is transported from the chloroplast to the cytosol via the CLT1–3 transporters. The biosynthesis of phytochelatins (PCs) with the participation of phytochelatin synthase (PCS), the activity of which increases (+) in the presence of metal ions (Men+), takes place in the cytosol (stage 3). Phytochelatin synthase is encoded by the PCS genes, whose expression can change under plant exposure to metals. In some plant species, iso-phytochelatins were found, which are synthesized with the participation of iso-GSHs. In the cytosol, metal ions bind to phytochelatins, with the formation of various complexes that differ in the structure and degree of polymerization of phytochelatins. The ATP-dependent transport of low-molecular-weight metal complexes with phytochelatins (LMW Me-PC complexes) across the tonoplast is carried out by ABCC1/2/3 transporters. In the vacuole, high-molecular-weight complexes of metals with phytochelatins (HMW Me-PC complexes) can be formed, with the participation of acid-labile sulfide (S2-) presumably coming from chloroplasts. Due to the acidic pH values of the vacuolar sap, it is likely that these complexes can be partially destroyed, and metal ions can bind to organic acids (OAs), forming, for example, citrates and malates. The proposed processes are indicated by dotted lines.
Glutathione is present in almost all cell compartments. It is a strong reducing agent and is easily oxidized, participating in many processes, including metal binding, inactivation of reactive oxygen species, and regulation of redox homeostatic processes [33,34,53,123,126,177,185,186,193,194]. Glutathione exists in reduced and oxidized form. Glutathione reductase catalyzes the conversion of the oxidized form into the reduced one [186,192]. The ratio between these two forms is an indicator of redox balance and its maintenance at a certain level is crucial for plant survival [33]. Cadmium-induced decrease in the concentration of glutathione was accompanied by an increase in the activity of glutamate cysteine ligase, glutathione synthetase [195,196,197,198], glutathione transferase [199,200], and ATP-sulphurylase [183,201,202]. Thus, the induction of PC biosynthesis by metals can be achieved due to an increase in the activity of glutamate cysteine ligase and glutathione synthetase, involved in the biosynthesis of glutathione [203]. The increase in the activity of glutathione biosynthesis enzymes correlated with an increase in the expression of the GSH1 and GSH2 genes [99,108,150,192,204,205]. However, along with an increase in the concentration of thiol-peptides under the action of Cd, Cu, and Zn, no stimulation of the expression of the AtGSH1 and AtGSH2 genes was found in A. thaliana, which, as suggested, points to the existence of only post-transcriptional control [55]. Similar results were obtained, for example, when studying the effect of Cd on PmGSH1 expression in P. maximum [86]. At a high concentration of glutathione, its biosynthesis is regulated by a negative feedback, as well as by other mechanisms [177].
The mutants of S. pombe and A. thaliana with a reduced concentration of glutathione were characterized by PC deficiency and hypersensitivity to Cd [113,206,207,208], Hg, and As [209]. Treatment of Glycine max with L-buthionine-sulfoximine (BSO), an inhibitor of glutathione biosynthesis, led to a decrease in the PC accumulation after 5 days of incubation in the presence of As (III) and As (V), and an increase in As translocation into the leaves [194]. Transgenic B. juncea plants, overexpressing the GSH1 and GSH2 genes, contained more glutathione and PCs, were more tolerant to Cd, and accumulated more Cd than wild type plants [210,211]. However, not in all studied plant species did an increase in the level of glutathione lead to an increase in metal tolerance [177]. Nevertheless, the intracellular level of glutathione is one of the important regulators of PC biosynthesis, and the activation of glutathione biosynthesis in the presence of Cd can also occur as a result of the metal-induced production of reactive oxygen species [192]. Therefore, metal tolerance of the excluder species is determined not only by PC biosynthesis, but also by the ability to maintain the intracellular concentration of glutathione at an optimal level.
3.2. Phytochelatin Synthase Is a Key Enzyme in the Biosynthesis of Phytochelatins
PC biosynthesis from reduced glutathione is catalyzed by the key enzyme γ-glutamylcysteine dipeptidyl (trans)peptidase (phytochelatin synthase, PCS) (EC 2.3.2.15), belonging to clan CA of the papain-like cysteine proteases [14,33,212,213], whose activity is regulated at the transcriptional and post-transcriptional levels [112,214]. The enzyme is around 95,000-Mr tetramer, with a Km of 6.7 mM for glutathione [112]. The biosynthesis of PCS seems to be constitutive in plant cells and cell cultures [99,212]. Even at a low metal concentration, PCSs in prokaryotes and eukaryotes are able to provide the basic level of PCs in the cell, which indicates their role in maintaining ion homeostasis and regulating the availability of metal ions in the cell [46,76,215,216]. The reaction catalyzed by this enzyme is an autotransferase reaction, in which up to 10 dipeptidyl residues can be transferred, as was demonstrated for plant cells [72,105,124,217].
Phytochelatin synthases are evolutionarily conserved in different species of higher plants and charophytes [218] and consist of 452–545 amino acid residues with a characteristic but variable C-terminal domain called the Phytochelatin_C domain [112,219,220]. It contains numerous Cys residues involved in metal binding and determining the increased stability of the protein as well as broad metal specificity [36,52,57,138,219,220,221,222,223]. Different regions of the C-terminal domain of AtPCS1 in A. thaliana are important for its activation by Cd, Hg, Zn, and As (III) ions [54,219,221,224]. At the same time, the mechanism of AtPCS1 activation under the action of phenylmercury (PheHg) and Hg may be similar [57]. Deletion of the last 10 amino acid residues of the C-terminal domain of AtPCS1 led to an increase in As(III)-dependent PC biosynthesis, which indicates the involvement of some amino acid residues in this region in the inhibition of PCS activation by As ions [221]. The existence of a Cd- and Zn-dependent mechanism of enzyme inhibition to prevent its overactivation has recently been revealed. It involves two twin-Cys motifs in the C-terminus of MpPCS in M. polymorpha [36]. Phylogenetic analysis has shown that the N-terminal domain of PCS, known as the Phytochelatin domain [112,219], is more conservative and has catalytic activity, in which Cys-56, His-162, and Asp-180 residues play an important role [36,52,212,219,220,223,224,225,226]. The position of these specific amino acid residues may slightly vary between PCSs from different plant species. For example, the catalytic triad in BnPCS1 from Boehmeria nivea is represented by the residues Cys-58, His-164, and Asp-182 [203]. In A. thaliana, Glu-52 in AtPCS1 plays an important role in providing plant tolerance to As and Sb [227].
Despite the fact that PC biosynthesis occurs in the cytosol, PCS has also been found in some organelles. It has been shown that AtPCS1 is localized in the cytosol of root and shoot cells [228,229]. However, its distribution in plant organs is tissue-specific. Cell-type specific expression of AtPCS1-GFP in the roots of cad1-3/pAtPCS1-AtPCS1cds-GFP line was detected in the rhizodermal cells in the mature and elongation zones, and the outer-most layer of the lateral root cap cells in the meristematic zone [57]. In the shoots of A. thaliana, AtPCS1-eGFP expression was found mainly in the epidermal cells [228,229], while in Vicia sativa, VsPCS was localized in the cytoplasm of mesophyll protoplasts [52]. It has been shown that ZmPCS1 from Z. mays is also a cytoplasm-localized protein [230]. The revealed patterns may be related to the fact that rhizodermal cells are the first to contact with metals, while in shoots, metals often accumulate in the epidermal cells, which results in a decrease in their concentration in the mesophyll, and, consequently, in the manifestation of their toxic effects [19,174]. In S. pombe, SpPCS1 was localized in mitochondria [231], in Saccharum officinarum SoPCS was found in the cytosol and mitochondria [232], in O. sativum OsPCS1 and OsPCS2 were localized in the cytosol [233], whereas BnPCS1 in B. nivea [203] and AtPCS2 in A. thaliana [234] were found in the cytoplasm and nucleus. The data obtained confirm the possible involvement of PCS in the maintenance of ion homeostasis not only in the cytosol, but also in various organelles. They also indirectly indicate the participation of this enzyme in various processes in the cell.
Most plant PCSs are of plastid origin, since they are functionally similar to cyanobacterial PCSs, which, however, do not contain a C-terminal domain [212,235]. For example, a comparative analysis of PCSs from the gametophytes of M. polymorpha and the cyanobacterium Geitlerinema sp. strain PCC 7407 showed a similar pronounced transpeptidase activity under the action of Cd [76]. It is hypothesized that the mature full-length PCS in higher plants may have evolved from the cyanobacterial protein by the acquisition of more Cys residues in the N-terminal domain and by fusion with a C-terminal domain either from their own genomes or from that of another species [235]. From an evolutionary point of view, these data justify the opinion that the high metal concentration in the environment at the dawn of life could have contributed to the appearance of a metal detoxification mechanism involving PCSs in some ancient groups of organisms [236].
The molecular mechanism of PC biosynthesis was first proposed in 1989 by Grill et al. [217], who suggested that at the first stage, prior to the transpeptidase reaction, Gly is cleaved from glutathione. Then, at the second step, the remaining γ-Glu-Cys forms a peptide bond either with glutathione to form PC2 or with another PC molecule acting as an acceptor, resulting in the formation of the PCn+1 oligomer [217]. It was later confirmed that AtPCS1 is a dipeptidyltransferase, which undergoes γ-Glu-Cys acylation at two sites, with the release of Gly, during step I of the catalysis that is necessary for net PC synthesis, but the requirements for each acylation reaction are distinguishable. Kinetic studies have shown that one of the substrate binding sites has a high affinity, and the other has a low affinity, for glutathione [237]. In a medium lacking Cd ions, acylation of glutathione occurs at the first site [212,237], which contains a sequence conserved in all PCSs, including Cys, His, and Asp residues, and is metal-independent [238]. Acylation at the second site occurs only in the presence of metal ions, resulting in metal-dependent catalysis [212,238]. The proposed model of the work of eukaryotic PCS suggests that the residues Cys-56, His-162, and Asp-180 in the N-terminal domain are required for the catalytic process, and a certain sequence of the C-terminal domain is responsible for the interaction with the Metal-GS2 complex for the amplification of the catalytic process. The initial PCS reaction step (step I) at site I, when one free glutathione molecule is taken as a substrate, releasing Gly and acylating the enzyme with the remaining γ-EC residue, is catalyzed by residues localized in the N terminal domain and is metal-independent. The second reaction is supposedly carried out in site-II and is metal-dependent. This second step involves the transfer of a γ-EC residue to a second glutathione molecule (to form the Metal-GS2 complex), or PCn, resulting in a net synthesis of a PCn+1 molecule [38,212,222,235,238].
To the greatest extent, PCS is activated by Cd ions via metal binding to a specific activation site [112,225,239,240]. For other metal ions, their activating effect in vitro decreased in the following order: Cd2+ > Ag+ > Bi3+ > Pb2+ > Zn2+ > Cu2+ > Hg2+ > Au+ [112,124,217]. However, the ability of different metals to activate PCS remains an object for studies, and the results obtained are not always unambiguous. Thus, Vatamaniuk et al. [237] showed that AtPCS1 is capable of synthesizing PCs in the presence of Cu2+, Zn2+, Mg2+, Ni2+, or Co2+, whereas Oven et al. [171] did not observe the activity of AtPCS1 and GmhPCS1 after the treatment with Mg2+, Ni2+, or Co2+. Purified AtPCS1 and LjPCS1 were activated, in decreasing order, by Cd2+, Zn2+, Cu2+, and Fe3+, but not by Co2+ or Ni2+, in the presence of 5 mM glutathione and 50 mM metal ions [241]. Mo, Co, and Ni ions did not activate OsPCSs [223]. One of the reasons for such differences in the experiments with intact plants may be the heterogeneous distribution and accumulation of metals in plant tissues [174], which determines the different availability of metals for PCS [177]. In addition, PCS paralogs characterized in different species displayed functional differentiation in terms of the amount of PCs produced and the specificity of metal-mediated activation, as well as differential regulation of transcription in response to metals [82,215,242,243].
The effect of metals on the activity of PCS can differ not only between species, but also between different isoforms of the enzyme within species. For example, in Oryza sativa, OsPCS1 was activated to a greater extent by As3+ than by Cd2+, while for OsPCS2, the pattern was the opposite [82,233], and the replacement of even one amino acid residue in the C-terminal domain can affect the metal selectivity of the enzyme [223]. The involvement of various isoforms of PCS in the response to metals requires more thorough study. For example, it was shown that, in contrast to AtPCS1, AtPCS2 was not stimulated by Cd ions, leading to the assumption that only AtPCS1 determines the synthesis of PCs, metal tolerance, and plant ability to accumulate metals [215,244]. However, the results of other studies indicate that AtPCS2 may be involved in the response of plants to Cd during long-term incubation at a higher metal concentration [198]. It is also assumed that the activation of PCS may be mediated by H2O2, which is formed as a result of metal-induced oxidative stress [115,245], which in turn is consistent with the data on the possible involvement of PCs in the neutralization of H2O2 and superoxide radicals [35,235]. In addition, PCS activity can be regulated by phosphorylation and dephosphorylation of the enzyme. In vitro experiments demonstrated that PCS activity increased after its phosphorylation by casein kinase 2 (CK2) and decreased after treatment with alkaline phosphatase. Site-directed mutagenesis experiments on AtPCS1 indicate that Thr-49 near the catalytic site in the N-terminal domain is the site for phosphorylation [246]. Thus, the activity of PCS and the amount of available glutathione can be considered as important mechanisms for the regulation of PC synthesis.
The biosynthesis of iso-PCs has not been sufficiently studied yet [247]. It is possible that PCs with C-terminal Ser or Glu are synthesized through ATP-dependent ligation from γ-EC and Ser or Glu, like in glutathione biosynthesis, or through post-synthetic modifications of glutathione, like in the transpeptidation during the PC biosynthesis. It is assumed that in O. sativa seedlings, OsPCS2 can catalyze the conversion of glutathione to γ-EC in the cytosol under the action of Cd, after which γ-EC can be used as a substrate for the subsequent synthesis of hydroxymethyl-glutathione or γ-Glu-Cys-Glu [82]. Homoglutathione can be a substrate in the biosynthesis of homoPCs [72,171,241]. However, homoglutathione was found only in the leaf blades of P. maximum, while homoPCs were also found in the stems and roots [86]. It was assumed that the biosynthesis of homoPCs was carried out with the participation of homophytochelatin synthase [72,171], while later it was shown that homoPCs can be synthesized with the participation of typical PCS [241]. Therefore, the biosynthesis and transport of iso-PCs require further research. The presence in many plant species of PCs that do not contain a C-terminal amino acid (Table 2) is the result of its cleavage from PCs, homoPCs, and hydroxymethylPCs by peptidase, or due to the hydrolytic activity of PCS [72].
3.3. Phytochelatin Synthase Genes in Living Organisms: Identification and Expression
Genes encoding PCSs have been found in species from distant taxa, including bacteria [235], fungi [124,231,248], Metazoa (ciliates, flatworms and annelids, echinoderms, chordates) [124,249,250,251], algae [236], and higher plants. Over the past two decades, from the pioneering works [225,248,252], numerous PCS gene orthologues have been identified and characterized. Many plant species have more than one copy of functionally active PCS genes. PCS genes have been identified in a number of plant species (Table 3).
Table 3.
Phytochelatin synthase genes identified in plant species from different families.
| Family | Species | Genes | References |
|---|---|---|---|
| Bryophytes | |||
| Marchantiaceae | Marchantia polymorpha | MpPCS | [33] |
| Pteridophytes | |||
| Pteridaceae | Pteris vittata | PvPCS1 | [253] |
| Angiosperms | |||
| Alliaceae | Allium sativum | AsPCS1 | [254] |
| Amaranthaceae | Salicornia europaea | SePCS1 | [141] |
| Arecaceae | Phoenix dactylifera | PdPCS1 | [255] |
| Asteraceae | Helianthus annuus | HaPCS | [256] |
| Lactuca sativa | LsPCS1, LsPCS2 | [257,258] | |
| Tagetes patula | TpPCS1 | [259] | |
| Brassicaceae | Arabidopsis halleri | AhPCS1, AhPCS2 | [48] |
| Arabidopsis thaliana | AtPCS1, AtPCS2 | [215,225,244,252,260] | |
| Brassica juncea | BjPCS1 | [173] | |
| Brassica napus | BnPCS | [261] | |
| Brassica rapa | BrPCS1, BrPCS2 | [133] | |
| Noccaea caerulescens | NcPCS1, NcPCS2 | [48] | |
| Noccaea japonicum | NjPCS | [262] | |
| Ceratophyllaceae | Ceratophyllum demersum | CdPCS1 | [84] |
| Chenopodiaceae | Suaeda salsa | SsPCS | [263] |
| Convolvulaceae | Ipomoea pes-caprae | IpPCS1 | [226] |
| Fabaceae | Cajanus cajan | CcPCS1 | [182] |
| Lotus japonicus | LjPCS1, LjPCS2, LjPCS3 | [264] | |
| Medicago sativa | MsPCS1, MsPCS2 | [135,265,266] | |
| Vicia sativa | VsPCS1 | [52] | |
| Moraceae | Morus notabilis | MnPCS1, MnPCS2 | [243] |
| Nelumbonaceae | Nelumbo nucifera | NnPCS1 | [267] |
| Poaceae | Arundo donax | AdPCS1, AdPCS2, AdPCS3 | [213] |
| Cynodon dactylon | CdPCS1 | [268] | |
| Orysa sativa | OsPCS1, OsPCS2 etc. | [82,233,269,270,271] | |
| Panicum maximum | PmPCS2 | [86] | |
| Paspalum vaginatum | PvPCS1, PvPCS2 | [272] | |
| Phragmites australis | PaPCS | [273] | |
| Saccharum officinarum | SoPCS | [232,274] | |
| Triticum aestivum | TaPCS1 | [248,275] | |
| Zea mays | ZmPCS1 | [230] | |
| Rosaceae | Malus hupehensis | MhPCS | [276] |
| Salicaceae | Populus tomentosa | PtPCS | [277] |
| Populus trichocarpa | PtPCS1 | [278] | |
| Solanaceae | Nicotiana tabacum | NtPCS1 | [279] |
| Solanum lycopersicum | SlPCS | [108,280] | |
| Urticaceae | Boehmeria nivea | BnPCS1 | [203] |
PCS genes have also been identified in the yeast Schizosaccharomyces pombe (SpPCS1) [225,231], the nematodes Caenorhabditis elegans (CePSC1) [249] and Ancylostoma ceylanicum (AcePCS) [250], the tunicate Ciona intestinalis (CiPCS) [251], and other species [38], which has made it possible to carry out phylogenetic analyses [36,52,124,213,226,232,236,255,270].
From an evolutionary point of view, the absence of the PCS gene in the genome of the model moss Physcomitrium patens is of interest, which indicates the leading role of other mechanisms of metal detoxification in this species [281] and is consistent with a very low level of PCs in the Cd-tolerant moss Leptodictyum riparium [200]. However, in the liverwort M. polymorpha, not only the presence of one copy of the PCS gene was shown [36], but also the participation of MpPCS in the detoxification of Cd, but not As (III) or other divalent cations [12]. It is assumed that PCS genes probably have a bacterial origin and were subsequently inherited to different groups of organisms, in some cases multiple times. It was suggested that multiple horizontal gene transfer events from bacteria to eukaryotes occurred within the PCS gene family. The complex evolution of the PCS genes involves several gene duplications and losses, or independent insertions of the full-length PCS genes, in plants and green algae [236].
The presence of several cis-regulatory elements in the promoter regions of PCS genes, including stress-responsive elements, may explain the influence of metals and other stress factors on PCS gene expression [220]. MYB40 transcription factor was shown to regulate the expression of PCS. In A. thaliana plants, treatment with As(V) induced the expression of AtMYB40, which led to the increased expression of AtPCS1 [282]. The level of expression of PCS genes can differ in plant organs and change differently in metal-treated plants. In A. thaliana [260], Lactuca sativa [257], B. juncea [173], B. parachinensis [176], C. cajan [182], Salicornia europaea [141], and Tagetes patula [259], the PCS gene expression was higher in the roots than in the shoots, while in H. annuus [256] the opposite pattern was observed. For O. sativa, a higher level of OsPCS expression was shown in the roots [85], but the level of OsPCS1 and OsPCS2 expression in different organs can vary significantly [82,270], which can also be partly determined by the use of different varieties and plant growth conditions. The expression level of SoPCS changed differently in the roots and shoots of S. officinarum with an increase in the concentration of Cd in the medium [274]. The Cd-induced increase in the amount of BnPCS1 mRNA in the leaves of B. nivea was significantly higher than in the roots and stems [203], which is consistent with the data for NnPCS1 [267]. In Cd-treated Paspalum vaginatum, the expression of PvPCS1 and PvPCS2 in the leaves decreased within 6 h and was up-regulated after 24 h of exposure. In the roots, PvPCS1 expression showed significant up-regulation after 6 h of treatment, whereas the expression of PvPCS2 decreased after 6 h of Cd treatment and then returned to control levels [272]. In Medicago sativa, the expression level of MsPCS increased with Ni concentration to a greater extent in the roots than in the shoots [283]. An increase in the SlPCS and OsPCS expression was observed mainly in the roots of As-treated S. lycopersicum [205] and O. sativa [150]. Therefore, the differences in the PCS gene expression may be associated with the physiological characteristics of plant species, which determine their different ability to accumulate metals in different organs, as well as with different durations of exposure and metal concentrations tested, and plant varietal characteristics, which, for example, was shown in the analysis of the expression of the AtPCS1 and AtPCS2 genes [198,244]. The effect of a variable-valence metal on PCS gene expression may depend on its valence. For example, six putative PCS genes were expressed differentially in O. sativa seedlings exposed to Cr(VI) or Cr(III) [284]. In addition, the level of expression of different PCS genes in one organ can also vary, which was clearly shown by a comparative analysis of the expression of three AdPCS1-3 genes in Arundo donax roots under the treatment with Cd [213].
The expression of different PCS genes within plant species can be induced by different metals. In O. sativa, the expression of OsPCS7 was induced by Hg and Pb, the expression of OsPCS9 was induced by Cd and Zn [269], and the expression of OsPCS5/-15 was induced by Cd and As [271]. The studies of Cd, Cu, Zn, and Ni effects on different Azolla species revealed that PCS1 gene expression was species- and metal-specific, and the expression level depended on both the duration of exposure and metal concentration in the medium [285]. The relative expression of the MnPCS1 and MnPCS2 genes increased in the roots, stems, and leaves of Morus notabilis after 24 h of incubation, being significantly stronger under the action of Cd than Zn [243]. In the leaves of S. lycopersicum, the expression of the SlPCS1 gene was induced to a greater extent by Cd and Pb compared to Cu [280], while the induction of the MhPCS gene expression in M. hupehensis decreased in the series Cd ˃ Cu ˃ Pb [276], which, however, is consistent with a significant increase in PCS expression in the presence of Cd in other plant species [203,274]. Therefore, despite the constitutive expression of PCS genes, the level of expression of these genes is usually higher in the presence of Cd compared to other metals.
The expression level of PCS genes is not only metal- and organ-specific, but also depends on the level of S in the medium. The use of Na2SO4 as an additional source of S led to an increase in the level of OsPCS expression and of PC content in the roots of O. sativa [85]. Later, it was shown that under the combined treatment with Cd and S, the expression level of not only MsPCS1, but also MsGS in the roots of M. sativa increased, which was accompanied by an increase in the concentrations of glutathione and PCs [266]. The stimulating effect of S on the accumulation of glutathione and PCs was also shown in Cd-treated Fagopyrum tararicum [286] and in the roots of Pb-treated T. aestivum [275], which confirms the important role of S in metal detoxification. However, in P. maximum, no change in the expression of the PmGSH1 and PmPCS2 genes in leaves was found under the combined treatment with Cd and S, which was accompanied by multidirectional changes in the content of PCs in the roots and shoots compared with the Cd treatment [86]. The data obtained confirm that further research in this direction is necessary.
Different species of arbuscular mycorrhizal fungi can influence PC production in plants in response to Cd. For example, mycorrhizal inoculations significantly promoted the expression of the CcPCS1X1, CcPCS1X2, and CcPCS1X4 genes, more in the roots than in the leaves of C. cajan, indicating that symbiosis with arbuscular mycorrhizal fungal species could enhance Cd tolerance by modulating the expression of PCS genes in plants [182].
In most of the species studied, the increase in the level of PCS expression led to an increase in plant tolerance to Cd [50,52,203,213,230,243,252,254,261,268,273,277,287,288]. However, this effect was dependent on the concentration of Cd in the medium [50]. A significant increase in Cd tolerance in transgenic A. thaliana was observed with the overexpression of the BnPCS1 gene from B. nivea [203], the BnPCS gene from Brassica napus [261], the NnPCS1 gene from Nelumbo nucifera [267], the VsPCS1 gene from V. sativa [52], or the ZmPCS1 gene from Z. mays [230]. The elevated expression of CdPCS1 from Cynodon dactylon [268], AtPCS1 from A. thaliana [50,287], or PtPCS from Populus tomentosa [277] also increased metal tolerance of transgenic N. tabacum plants compared to the wild type plants. Transgenic N. tabacum lines overexpressing the NtPCS1 gene in the sense or antisense direction were characterized by increased tolerance to Cd and As [279]. Overexpression of MnPCS1 and MnPCS2 from M. notabilis in A. thaliana and N. tabacum enhanced the tolerance in most transgenic plants not only to Cd, but also to Zn [243]. Transgenic lines of B. juncea with an average level of AtPCS expression showed increased tolerance to Cd and As [288]. In contrast, the cad1 mutant of A. thaliana, unable to synthesize PCS, was hypersensitive to Cd [49,225,248], and its seeds did not germinate at a high Cd concentration in the medium [51], whereas the cad1-3 mutant, the AtPCS1 null mutant lacking a functional PCS1 [49], was very sensitive to Cd and As and, to a lesser extent, to Zn, Pb, Ag, Cu, and Hg [54,131,134,225,240]. The OsPCS1 mutants of O. sativa were also sensitive to Cd and As [240]. In cad1-3 transgenic mutants expressing the TaPCS1 [51] and MpPCS [36] genes, restoration of PC formation and an increase in Cd tolerance were observed. Moreover, ectopic expression of ZmPCS1 repaired the defective phenotypes in the Cd-sensitive yeast mutant Δycf1 and A. thaliana AtPCS1-deficient mutant atpcs1 under Cd stress, enhancing their Cd tolerance [230].
However, a number of studies have shown a decrease in the tolerance of transgenic plants to Cd and Zn [36,207,213,271,289,290]. For example, an increased level of AtPCS1 expression in A. thaliana and N. tabacum led to a decrease in Cd tolerance [207,289,290]. Similar results were obtained on transgenic A. thaliana plants with overexpression of the OsPCS5/-15 genes [271]. Several explanations for such discrepancies have been proposed: the supraoptimal content of PCs in relation to glutathione [207], the manifestation of metal-induced oxidative stress in transgenic plants [289], as well as experimental differences, such as the differences in the use of vectors and constructs [230]. Later, it was shown that Cd-hypersensitive N. tabacum plants expressing the AtPCS1 gene from A. thaliana had a high activity of PCS, but a significant decrease in the content of glutathione and in the cytosolic and vacuolar PC pools, whereas in Cd-tolerant N. tabacum plants expressing the CePCS gene from C. elegans, no dramatic change in the glutathione content was observed, and the PC content was significantly higher [290]. It is also assumed that with the increased expression of PCS, the rate of formation of PC complexes with Cd may exceed the capacity to transport them into the vacuole, as a result of which they are accumulated in the cytosol. This in turn can be a trigger for PC degradation, including due to the peptidase activity of PCS, which leads to an increase in the Cd2+ concentration in the cytosol and an increase in the toxic effect [12,290]. Interestingly, when three recently diverged PCS genes (AdPCS1-3) from A. donax were overexpressed in A. thaliana Col-0 wild type plants, it resulted in either enhanced (AdPCS2 and AdPCS3) or decreased (AdPCS1) sensitivity to Cd2+ [213]. It is obvious that different activity of PCS, different endogenous levels of PCs/glutathione and degrees of PC polymerization, as well as different efficiency of the translocation of metal complexes with PCs into the vacuole in transgenic plants [50,289,290,291], can significantly affect their metal tolerance. Taken together, the data obtained indicate the presence of a very fine regulation of the PC-dependent mechanism of metal detoxification in different plant species, which undoubtedly should be taken into account when creating transgenic plants.
3.4. Other Functions of Phytochelatin Synthase
The constitutive expression of PCS [292] and the presence of homologues of the PCS gene(s) in plants growing in ecosystems geographically remote from metal-contaminated sites, as well as in representatives of various kingdoms of living organisms, suggest that PCS has a wide range of different functions [124]. In addition to a response to metal-induced stress, PCS is a cysteine peptidase that regulates the catabolism of glutathione and glutathione conjugates in the cytosol [228,242,293]. As a result, the glycine residue is cleaved off from the conjugates, similarly to how it occurs during the biosynthesis of PCs. Phytochelatin synthase is also involved in maintaining Fe homeostasis in charophytes [218], AtPCS1 is involved in the control of pathogen-induced callose deposition [229,291,294], and AtPCS2 is involved in response to salinity [295]. As to the latter, it is worth mentioning that an increase in the activity of PCS, the expression of the SePCS1 gene, and the concentration of PCs was shown for the salt-tolerant halophyte species S. europaea in response to combined and separate Cd and NaCl treatments [141].
3.5. Hormonal Regulation of the Biosynthesis of Phytochelatins
There is a limited number of studies on the hormonal regulation of PC biosynthesis, which are discussed in detail in a review by Pál et al. [296]. In plants, there is hormonal regulation of glutathione biosynthesis, which, as a result, affects the biosynthesis of PCs. No direct relationship was found between the levels of glutathione/PCs and auxins [55]. However, after the treatment with an auxin inhibitor, a decrease in the content of cysteine, glutathione, and PCs was observed in the roots of O. sativa [297]. Mutant and transgenic plants of A. thaliana and N. tabacum with reduced endogenous levels of cytokinins had higher levels of glutathione and PCs, as well as a higher tolerance to As compared to the wild type [298]. Ethylene was shown to induce the expression of the genes involved in the biosynthesis of glutathione [296]. The expression of BnPCS1 in B. nivea [203] and StPCS1 in the roots of Solanum tuberosum [299,300] was induced by exogenous abscisic acid, but not by salicylic acid. However, there are very few direct studies on the effects of ethylene, abscisic and jasmonic acids, as well as gibberellins on PC biosynthesis, which is a promising direction for future research.
4. Transport and Physiological Role of Phytochelatins
4.1. Phytochelatins in Hyperaccumulators and Excluders
It is generally accepted that glutathione and PCs are involved in the mechanisms of metal detoxification and transport, but not in the mechanisms of metal hyperaccumulation [14,68,113,301,302,303,304]. In the shoots of the hyperaccumulators A. halleri, S. alfredii, and N. caerulescens, a low concentration of PCs was observed or they were completely absent [48,181,305,306]. Interestingly, when N. caerulescens plants were grown in hydroponics in the presence of Cd (5–500 μM), PC biosynthesis was induced both in roots and shoots, whereas in plants growing in their natural habitat at an old Cd/Pb/Zn mining and smelter site in Plombières (Belgium), the PCs were practically not detected [64]. In the roots and shoots of Dianthus carthusianorum plants from a non-metalliferous soil, a higher level of PCs was found in response to Cd compared to the plants from a metalliferous soil [307]. A similar phenomenon was found in S. alfredii [181], Silene vulgaris [66], and D. viscose (at 5 mg/L Cd) [45]. The treatment with BSO almost completely arrested the biosynthesis of PCs, but did not enhance the sensitivity to Cd in N. caerulescens or in D. carthusianorum plants from metallicolous populations [64,307]. Consequently, the high tolerance of hyperaccumulators and metallophytes to Cd is not associated with the increased biosynthesis of PCs [63,64,302,307,308]. It was assumed that hypertolerance may be partly determined by a constitutively high concentration of glutathione in hyperaccumulator plants [193,309]. However, in some cases, the differences in the concentration of glutathione between the plants from non-metalliferous and metalliferous soils were not detected or were ambiguous [45,307].
In general, the amount of Cd bound to S-containing ligands in the shoots of hyperaccumulators is quite low, as shown, for example, for N. caerulescens [305,310,311], Noccaea praecox [312], and A. halleri [306,313]. Zinc did not induce the biosynthesis of PCs in N. caerulescens, whereas the concentration of PCs increased with the concentration of Cd, but decreased with an increase in the duration of incubation [305]. In the leaves of S. alfredii, only 5% of the total amount of Cd was bound to PCs, which, however, does not eliminate their participation in Cd detoxification [68,145]. Although the amount of Cd complexes with S-containing ligands may depend on the duration of exposure [310], a significant amount of Cd in hyperaccumulators is often bound to O-containing ligands, possibly organic acids [306,310,311,313,314]. For Zn and Ni, histidine and nicotianamine can play a leading role in metal binding [19], while for the elements with variable valencies, the situation can be more complicated. For example, the ratio between Cu complexes with S- and O-containing ligands may not only be species- and organ-specific, but may also differ for complexes with Cu(I) and Cu(II), as was shown for the Cu accumulators Persicaria capitata, Persicaria puncata, and Conyza cordata [315].
In contrast to the hyperaccumulators, in the non-accumulator Arabidopsis lyrata, the highest amount of Cd was bound to S-containing ligands. This confirms the involvement of glutathione and PCs in Cd detoxification in non-tolerant plant species [313]. Since the post-translational activation of PCS depends on the availability of metal ions or their complexes with glutathione [171], the more efficient PC biosynthesis in the roots of excluders may be associated not only with a higher level of expression of the PCS gene, but also with higher availability of metal ions in the roots of these species as compared with hyperaccumulators [48]. Due to the highly efficient functioning of PC-independent metal detoxification pathways in the shoots of hyperaccumulators, PCS is not activated there, which, apparently, is energetically favorable considering the high energy cost of PC biosynthesis [48,63].
4.2. Phytochelatin-Mediated Transport of Metal(loid)s into the Vacuole
As mentioned above, PC biosynthesis directly depends on the activity of glutathione biosynthesis enzymes in the cytosol and chloroplasts (Figure 1) [186]. A significant amount of PCs was found in the vacuoles of Nicotiana rustica [100] and A. thaliana [290]. Hence, it was suggested that after binding Cd ions in the cytosol, PCs can be transported into the vacuole, where, due to the more acidic pH of the vacuolar sap, these complexes can dissociate, and the peptides can be degraded by vacuolar proteases and leave the vacuole, thus acting as a shuttle mechanism for Cd transfer (Figure 1) [100,113,114,117,155,316], which, however, has not yet been directly confirmed. On the contrary, As-PC complexes entering the vacuole can remain stable and prevent re-oxidation of arsenite due to the acidic pH of the vacuole, which leads to the accumulation of high concentrations of As-PC complexes there [79,114].
Three types of Cd-PC complexes, differing in molecular weight, have been identified. The low-molecular-weight (LMW) complex [100,117] and the medium-molecular-weight (MMW) complex [159] differ in the degree of polymerization, and appear to be formed immediately after PC biosynthesis in the cytosol (Figure 1). The high-molecular-weight (HMW) complex, with the highest degree of polymerization, was isolated, for example, from B. juncea [317], S. lycopersicum [318], Z. mays [93], A thaliana [49], and Canavalia lineata [197]. A distinctive feature of this complex is the presence of acid-labile sulfide (S2−), which increases its affinity for Cd ions, the number of Cd ions bound per molecule, the stability of the complex, and its resistance against proteolytic degradation [38,115,129,155]. For example, upon the formation of an HMW Cd-PC complex in Phaeodactylum tricornutum cells, the Cd/SCys ratio increased from 0.6 to 1.6 [319]. It was shown that the enzymes of purine metabolism can take part in the reactions leading to the formation of S2− in S. pombe [317,320]. It is assumed that in higher plants, S2− comes from the chloroplasts [129]. The HMW complex is probably formed in the vacuole [115,321,322] or at the tonoplast level [170] and facilitates more efficient metal binding and detoxification (Figure 1).
Early experiments on tonoplast vesicles isolated from the roots of Avena sativa showed that Cd-PC complexes are transported by ABC (ATP-binding cassette) transporters (Figure 1) [78], one of the functions of which is to transport glutathione complexes with various secondary metabolites and xenobiotics across the tonoplast [177,323,324,325]. Two transmembrane domains (TMD) (or membrane-spanning domains, MSD) determine the substrate specificity of the transporter, and two nucleotide-binding domains (NBD) are responsible for the coupling of ATP hydrolysis and substrate transport [177,323,324,325,326]. Binding of ATP to NBD induces a conformational change in TMD, causing the substrate to enter the niche in the membrane created by the transporter. After ATP hydrolysis and phosphate release, a subsequent rearrangement of both domains occurs, which is accompanied by the release of the substrate on the other side of the membrane, as well as the release of ADP [15]. The first identified protein that carries out the ATP-dependent transport of both PCs and LMW Cd-PC complexes into the vacuole was HMT1 (heavy metal tolerance-factor 1), found in LK-100, a mutant of S. pombe that is not capable of forming HMW Cd-PC complexes. HMT1 belongs to the MRP (multi-drug resistance proteins) or ABCC (ATP-binding cassette subfamily C proteins) subfamily [326], is located at the tonoplast, consists of one TMD domain and one NBD domain, and is encoded by the HMT1 gene [321,322]. Later, HMT1 homologues were identified in C. elegans [327] and Drosophila melanogaster [328], but have not yet been found in plants. It is assumed that HMT1 has a high substrate specificity for glutathione [329], although yeast HMT1 in A. thaliana mutants was involved in the entry of metal complexes with PCs into the vacuoles of root cells, limiting metal translocation into the shoots [330].
In Saccharomyces cerevisiae, Cd is transported across the tonoplast mainly as a complex with glutathione [331,332]. The Mg-ATP-dependent transporter YCF1 (yeast cadmium factor 1) mediates the transport of Cd-GS2 [332,333], Hg-GS2 [334], and As-GS3 [335] into the vacuole. YCF1 belongs to the ABCC type and is encoded by the ScYCF1 gene, the increased expression of which leads to increased Cd tolerance in transgenic A. thaliana plants [333]. In A. thaliana, the transport of Cd-PC complexes into the vacuole is carried out by the tonoplast transporters AtABCC1/2/3 (Figure 1) [304,336,337,338]. The expression of the AtABCC3 gene is regulated by Cd, and the activity of the AtABCC3 transporter depends on metal concentration and is coordinated with the activities of AtABCC1 and AtABCC2 [338,339]. The expression of the AtABCC1 and AtABCC2 genes is positively regulated by transcriptional factor AtMYB40 [282]. In the abcc3 mutant of A. thaliana, as well as in the double mutant abcc1/abcc2, Cd accumulated in the cytosol, whereas in plants overexpressing AtABCC3, the Cd content in the vacuole was higher than in the wild type plants [304,338]. The ABCC1/2 transporters are also involved in the transport of PC complexes with As(III) and, apparently, Zn, Cu(II), Mn, and Hg, including PheHg and other compounds, into the vacuole [57,304,340,341,342]. Furthermore, in a phosphomimetic mutant study it was shown that phosphorylation of the Ser-846 residue in the linker region between NBD1 and TMD2 regulates the activity of AtABCC1, which is necessary for As sequestration in the vacuole [342]. In yeast heterologous expression analyses, OsABCC1 enhanced PC-dependent As tolerance but did not affect Cd tolerance [341], suggesting that OsABCC1 has a high selectivity for the As-PC complex but a low affinity for the Cd-PC complex. The degree of PC polymerization can probably also affect the efficiency of the transport of their complexes with metals across the tonoplast. It is assumed that the complexes of Cd with synthetic PCs with a high degree of polymerization cannot be easily transported across the tonoplast as compared to the complexes with a low degree of polymerization [343]. Comparative analysis showed that abcc1/abcc2 double mutants, as well as cad1-3 and cad1-6 mutants, which have a T-DNA insertion disrupting the C-terminal half of the Phytochelatin_C domain of AtPCS1, were hypersensitive to As(III), Hg (II), as well as to PheHg [57,221]. Therefore, both PC biosynthesis and transport of Me-PC complexes into the vacuole are important components of PC-dependent detoxification of toxic elements in plant cells (Figure 1). It is assumed that PC biosynthesis is regulated according to the principle of negative feedback: the more Me-PC complexes enter the vacuole, the more PCs are synthesized in the cytosol [340]. On the other hand, the amount of metal entering the conductive tissues and aboveground organs depends on the efficiency of metal sequestration in the vacuoles of the root cortical cells [19,341].
4.3. Participation of Phytochelatins in Long-Distance Transport of Metal(loid)s
Phytochelatins can take part not only in the detoxification, but also in the long-distance transport of metals. This is confirmed by numerous studies that assessed the changes in the concentration of metal(loid)s in transgenic plants or mutants. For example, an increase in the Cd concentration in transgenic A. thaliana plants was shown upon the expression of the BnPCS gene from B. napus [261], the NnPCS1 gene from N. nucifera [267], the AsPCS1 gene from Allium sativum [254], and the ZmPCS1 gene from Z. mays [230]; though it was not observed when the VsPCS1 gene from V. sativa was expressed in transgenic A. thaliana [52]. Overexpression of CdPCS1 from C. dactylon [268], AtPCS1 from A. thaliana [50,287], and PtPCS from P. tomentosa [277] also led to an increase in Cd accumulation in transgenic N. tabacum plants compared to the wild type, which, however, was not observed in transgenic tobacco lines with the overexpression of the NtPCS1 gene [279]. The physiological reasons for these discrepancies are not clear yet. The expression of the TaPCS1 gene in cad1-3 transgenic mutants led not only to the restoration of PC biosynthesis, but also to an increase in the Cd root-to-shoot translocation [51]. The expression of the CdPCS1 gene from Ceratophyllum demersum in transgenic O. sativa plants also led to an increase in PC concentration compared to non-transgenic plants, which was accompanied by an increase in As accumulation in the roots and shoots, while its concentration in the caryopses decreased [84]. At the same time, in the OsPCS1 mutant of O. sativa, the As concentration in the caryopses increased, while the Cd concentration decreased, which indicates the existence of different PC-dependent pathways of As and Cd transport [240]. The analysis of cad1-3 and cad1-6 mutants of A. thaliana also suggested the existence of a PC-dependent pathway of Zn root-to-shoot translocation [54].
Direct analysis showed the presence of PCs in the xylem sap of B. napus and B. juncea, as well as in the phloem sap of B. napus [344,345]. However, no As–PC complexes were found in the xylem sap of H. annuus [47]. Due to the low pH values of the xylem sap (~5.5–6.2), the stability of metal complexes with PCs may be lower there compared to the phloem sap, where, due to the neutral pH values (~7.5), the stability of the complexes is rather high. Therefore, it can be assumed that phloem is the main conducting tissue for the long-distance transport of metal complexes with PCs and glutathione. Phloem transport plays an important role in the entry of metals into generative organs and seeds. The involvement of PCs in this process was confirmed by the high expression level of the PCS1 gene in the phloem companion cells in A. thaliana [346]. Phytochelatins were also shown to be transported from shoots to roots in A. thaliana [347]. On this basis, it has been suggested that PCs are involved in metal transport from the shoots, as a result of which metal accumulation in the shoots decreases, and, consequently, the toxic effect on photosynthesis is diminished [125,345]. However, despite the presence of Me-PC complexes in the phloem and xylem sap, metals are mainly transported via conducting tissues as complexes with organic acids [19,30,348].
Glutathione is an important potential ligand for binding not only Cd, but also Cu and Zn, as a result of which there may be competition between these metal ions for binding to glutathione, as well as competition between PCs, glutathione, and other ligands for binding Cd [344,349,350] and other metal ions in the xylem vessels. Enhanced root-to-shoot translocation of Zn was shown for the transgenic lines of A. thaliana with elevated levels of glutathione [351], while mutants with reduced PC biosynthesis accumulated less Zn in the leaves compared to the wild type [54], which indicates the possible role of these compounds in the long-distance transport of not only Cd, but also Zn [18].
There are only a few studies on the mechanisms of the entry of S-containing ligands and their complexes with metals into conducting tissues. It was proposed that in A. thaliana, an oligopeptide transporter AtOPT6 can transport glutathione, PCs, and Cd complexes with these thiols into actively dividing cells around the phloem in sink organs [352]. It was also suggested that the loading of As(III)-PC2 and As(III)-GS3 complexes into the xylem vessels is mediated by the OsABCC7 transporter located on the plasma membrane of the xylem parenchyma cells in O. sativa roots [353].
4.4. Metal(loid) Detoxification in the Rhizosphere
In addition to the participation of PCs in metal entry into the vacuole and the long-distance transport through conductive tissues, their presence in root exudates has recently been shown. Under the treatment with As, (γ-Glu-Cys)2-Gly, (γ-Glu-Cys)2-Glu, (γ-Glu-Cys)2, as well as dimers linked by disulfide bridges [(PC2)2 and (PC3)2], were found in the root exudates of Lupinus albus. It is assumed that PCs can participate in As detoxification in the rhizosphere, limiting its entry into the roots, or that As(III)–(PC2)2 complexes can be exuded from the roots, possibly with the participation of ABC-type transporters [127].
The analysis of PC accumulation in plant tissues is an important biomarker for the presence of metals in the cytoplasm and the effectiveness of metal detoxification mechanisms in excluders, which is important for assessing the toxicity of metals [146,155,354], and is also an indicator of environmental pollution with metals [136,153,355].
5. Conclusions and Outlook
Having entered the cytoplasm, metal ions bind to various ligands involved in their transport and detoxification, and it is often not clear yet how the metal is transferred from the transporter to the ligand. In different plant species, various ligands can be present in the cytosol in different ratios. In addition to PCs and glutathione, an important role in metal binding is played by histidine, nicotianamine, metallothioneins, and organic acids, the affinity for which can vary significantly for different metal ions [9,19,175,356]. Therefore, there will be competition between the ligands for binding metal ions, and the amount of metal bound to one or another ligand can depend both on the strength and stability of the complexes formed, and on the amount of different ligands in the cell. In different species, the concentration of various ligands can vary significantly, which is especially evident when comparing excluders and hyperaccumulators. For example, PC concentration is low in hyperaccumulators [48,63,64,181,305,306] and, therefore, they do not play a significant role in the mechanisms of hyperaccumulation, which does not exclude a certain role of PCs in metal detoxification and maintenance of metal homeostasis. The low concentration of PCs and the high endogenous level of histidine and nicotianamine in the roots of hyperaccumulators [19] restrict metal entry into the vacuoles of root cells, facilitating their radial transport and loading into the xylem vessels. Excluders, on the contrary, have a higher level of PCs in their roots and a lower level of N-containing ligands [19], which determines the accumulation of metals in the vacuoles of root cells and their limited entry into the shoots. Obviously, the mechanisms of metal detoxification and the contribution of PCs and other metal-binding ligands to plant metal tolerance require further comparative studies.
Different metals can affect the production of low-molecular-weight ligands in cells to a different degree, and this effect may differ for different ligands, which, accordingly, will lead to a change in the buffer capacity of the cytosol [19]. There are few works that studied the combined effects of different metals on the concentration of PCs and glutathione [146,148,149,357]. However, this line of research is promising, since plants often encounter polymetallic stress in natural habitats, and PCs are considered as indicators of metal pollution [136,153,355].
Despite the extensive literature on the concentration of PCs in various plant organs summarized in this review, there is much less data on the structure of PC complexes with metals and their localization in various plant tissues. What makes it more complicated is the fact that PCs can have different degrees of polymerization and form complexes of different compositions [38,120]. The distribution and accumulation of metals can differ significantly not only in root and shoot tissues, but also in different cells of the same tissue [19,174]. Since the biosynthesis of PCs is induced by metals, it can be expected that PC concentration in plant tissues will be different. However, due to the difficulties in visualizing the ligands and their complexes with metals in plants tissues, such studies are practically absent.
The key enzyme that determines PC biosynthesis is PCS, which is present not only in the cytosol but also in various organelles [52,203,228,231,233,234], which may determine the presence of a wide range of functions. Some of them are already known [124,229,291,294,295], but we do not have a complete understanding of the role of PCS in various cell compartments. Recently, more works have appeared that testify to the biosynthesis of iso-PCs in the representatives of certain plant families. Despite the fact that significant progress has been made in deciphering the molecular mechanism of PC biosynthesis, we still know very little about the biosynthesis of iso-PCs, their functional significance, and also about the evolutionary aspects of their appearance in certain systematic groups. The information on the regulation of PC biosynthesis, including the data on hormonal regulation, is also very incomplete.
There is a certain amount of conflicting data regarding the involvement of PCs in the mechanisms of plant metal tolerance and their contribution to plant metal accumulation capacity, which is summarized in this work and in the review [214]. The resolution of the contradictions that have arisen is impossible without the elucidation of the pathways of radial and long-distance transport of metals in plants and the contribution of low-molecular-weight ligands to these processes. Recently, it has been proposed to use genetic engineering methods to create transgenic plants with enhanced metal tolerance for practical purposes [14,358,359]. However, even targeted creation of transgenic plants, for example, with overexpression of PCS genes, can lead to either an increase or a decrease in metal tolerance and plant ability to transport metals from roots to shoots (see above). Another interesting direction is the creation of plants with overexpression of synthetic genes encoding peptides similar to PCs and having the structure of Met(Glu-Cys)nGly [343] or MetHis6[α-Glu(Cys)]6Gly [360]. It is often proposed to use transgenic plants with a high metal tolerance and the capacity to accumulate metals in aboveground organs for phytoremediation; however, this approach is also limited by the existing risks [361].
Understanding the physiological mechanisms that determine metal tolerance and the ability of plants to selectively accumulate metals in aboveground or underground organs is of fundamental and practical importance. The discovery of PCs in the representatives of various kingdoms of living organisms raises the question of their origin in the process of evolution and possible reasons for their wide distribution in different taxa. The latter may be associated not only with the high concentration of metals in the environment at the dawn of life, but also with a wider range of PC functions, which, however, requires further studies and confirmation. Since PCs play an important role in the mechanisms of metal detoxification and maintenance of metal homeostasis, their study is a promising direction for further research and they have a certain potential for the use in the development of phytoremediation, biofortification, and phytomining technologies.
Acknowledgments
The authors are grateful for the free access to the academic research databases at the K.A. Timiryazev Institute of Plant Physiology, Russian Academy of Sciences, supported by the Ministry of Science and Higher Education of the Russian Federation (state assignment №122042700044-6).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This work was supported by the Russian Science Foundation grant № 21-14-00028 (https://rscf.ru/project/21-14-00028/ (accessed on 19 April 2021)).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ghori N.-H., Ghori T., Hayat M.Q., Imadi S.R., Gul A., Altay V., Ozturk M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019;16:1807–1828. doi: 10.1007/s13762-019-02215-8. [DOI] [Google Scholar]
- 2.Franić M., Galić V. As, Cd, Cr, Cu, Hg: Physiological implications and toxicity in plants. In: Sablok G., editor. Plant Metallomics and Functional Omics. Springer Nature; Cham, Switzerland: 2019. pp. 209–251. [DOI] [Google Scholar]
- 3.Angulo-Bejarano P.I., Puente-Rivera J., Cruz-Ortega R. Metal and metalloid toxicity in plants: An overview on molecular aspects. Plants. 2021;10:635. doi: 10.3390/plants10040635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seregin I.V., Ivanov V.B. Physiological aspects of cadmium and lead toxic effects on higher plants. Rus. J. Plant Physiol. 2001;48:523–544. doi: 10.1023/A:1016719901147. [DOI] [Google Scholar]
- 5.Haider F.U., Liqun C., Coulter J.A., Cheema S.A., Wu J., Zhang R., Wenjun M., Farooq M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021;211:111887. doi: 10.1016/j.ecoenv.2020.111887. [DOI] [PubMed] [Google Scholar]
- 6.Seregin I.V., Kozhevnikova A.D. Physiological role of nickel and its toxic effects on higher plants. Rus. J. Plant Physiol. 2006;53:257–277. doi: 10.1134/S1021443706020178. [DOI] [Google Scholar]
- 7.Shahzad B., Tanveer M., Rehman A., Cheema S.A., Fahad S., Rehman S., Sharma A. Nickel; Whether toxic or essential for plants and environment—A review. Plant Physiol. Biochem. 2018;132:641–651. doi: 10.1016/j.plaphy.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Abbas G., Murtaza B., Bibi I., Shahid M., Niazi N.K., Khan M.I., Amjad M., Hussain M., Tahir N. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health. 2018;15:59. doi: 10.3390/ijerph15010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur H., Garg N. Zinc toxicity in plants: A review. Planta. 2021;253:129. doi: 10.1007/s00425-021-03642-z. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov V.B., Zhukovskaya N.V. Effect of heavy metals on root growth and the use of roots as test objects. Russ. J. Plant Physiol. 2021;68:S1–S25. doi: 10.1134/S1021443721070049. [DOI] [Google Scholar]
- 11.Kubier A., Wilkin R.T., Pichler T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019;108:104388. doi: 10.1016/j.apgeochem.2019.104388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M., Leso M., Buti M., Bellini E., Bertoldi D., Saba A., Larcher R., di Toppi L.S., Varotto C. Phytochelatin synthase de-regulation in Marchantia polymorpha indicates cadmium detoxification as its primary ancestral function in land plants and provides a novel visual bioindicator for detection of this metal. J. Hazard. Mater. 2022;440:129844. doi: 10.1016/j.jhazmat.2022.129844. [DOI] [Google Scholar]
- 13.Fasani E., Li M., Varotto C., Furini A., DalCorso G. Metal detoxification in land plants: From bryophytes to vascular plants. State of the art and opportunities. Plants. 2022;11:237. doi: 10.3390/plants11030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma R., Bhardwaj R., Handa N., Gautam V., Kohli S.K., Bali S., Kaur P., Thukral A.K., Arora S., Ohri P., et al. Plant Metal Interaction: Emerging Remediation Techniques. Elsevier Inc.; Amsterdam, The Netherlands: 2016. Responses of phytochelatins and metallothioneins in alleviation of heavy metal stress in plants: An overview; pp. 263–283. [DOI] [Google Scholar]
- 15.Andresen E., Peiter E., Küpper H. Trace metal metabolism in plants. J. Exp. Bot. 2018;69:909–954. doi: 10.1093/jxb/erx465. [DOI] [PubMed] [Google Scholar]
- 16.Vigani G., Solti Á., Thomine S., Philippar K. Essential and detrimental—An update on intracellular iron trafficking and homeostasis. Plant Cell Physiol. 2019;60:1420–1439. doi: 10.1093/pcp/pcz091. [DOI] [PubMed] [Google Scholar]
- 17.Corso M., de la Torre V.S.G.G. Biomolecular approaches to understanding metal tolerance and hyperaccumulation in plants. Metallomics. 2020;12:840–859. doi: 10.1039/d0mt00043d. [DOI] [PubMed] [Google Scholar]
- 18.Clemens S. Metal ligands in micronutrient acquisition and homeostasis. Plant Cell Environ. 2019;42:2902–2912. doi: 10.1111/pce.13627. [DOI] [PubMed] [Google Scholar]
- 19.Seregin I.V., Kozhevnikova A.D. Low-molecular-weight ligands in plants: Role in metal homeostasis and hyperaccumulation. Photosynth. Res. 2021;150:51–96. doi: 10.1007/s11120-020-00768-1. [DOI] [PubMed] [Google Scholar]
- 20.Zlobin I.E., Kartashov A.V., Nosov A.V., Fomenkov A.A., Kuznetsov V.V. The labile zinc pool in plant cells. Funct. Plant Biol. 2019;46:796–805. doi: 10.1071/FP19064. [DOI] [PubMed] [Google Scholar]
- 21.Zlobin I.E. Current understanding of plant zinc homeostasis regulation mechanisms. Plant Physiol. Biochem. 2021;162:327–335. doi: 10.1016/j.plaphy.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Reeves R.D., Baker A.J., Jaffré T., Erskine P.D., Echevarria G., van der Ent A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018;218:407–411. doi: 10.1111/nph.14907. [DOI] [PubMed] [Google Scholar]
- 23.Sterckeman T., Cazes Y., Gonneau C., Sirguey C. Phenotyping 60 populations of Noccaea caerulescens provides a broader knowledge of variation in traits of interest for phytoextraction. Plant Soil. 2017;418:523–540. doi: 10.1007/s11104-017-3311-0. [DOI] [Google Scholar]
- 24.Kozhevnikova A.D., Seregin I.V., Aarts M.G.M., Schat H. Intra-specific variation in zinc, cadmium and nickel hypertolerance and hyperaccumulation capacities in Noccaea caerulescens. Plant Soil. 2020;452:479–498. doi: 10.1007/s11104-020-04572-7. [DOI] [Google Scholar]
- 25.Seregin I.V., Kozhevnikova A.D., Schat H. Correlated variation of the Zn accumulation and tolerance capacities among populations and ecotypes of the Zn hyperaccumulator, Noccaea caerulescens. Rus. J. Plant Physiol. 2021;68:S26–S36. doi: 10.1134/S1021443721070128. [DOI] [Google Scholar]
- 26.Seregin I.V., Kozhevnikova A.D., Schat H. Nickel tolerance and accumulation capacities in different populations of the hyperaccumulator Noccaea caerulescens. Rus. J. Plant Physiol. 2022;69:70. doi: 10.1134/S1021443722040148. [DOI] [Google Scholar]
- 27.Stein R.J., Höreth S., de Melo J.R.F., Syllwasschy L., Lee G., Garbin M.L., Clemens S., Krämer U. Relationships between soil and leaf mineral composition are element-specific, environment-dependent and geographically structured in the emerging model Arabidopsis halleri. N. Phytol. 2017;213:1274–1286. doi: 10.1111/nph.14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corso M., Schvartzman M.S., Guzzo F., Souard F., Malkowski E., Hanikenne M., Verbruggen N. Contrasting cadmium resistance strategies in two metallicolous populations of Arabidopsis halleri. N. Phytol. 2018;218:283–297. doi: 10.1111/nph.14948. [DOI] [PubMed] [Google Scholar]
- 29.Schwartzman M.S., Corso M., Fataftah N., Scheepers M., Nouet C., Bosman B., Carnol M., Motte P., Verbruggen N., Hanikenne M. Adaptation to high zinc depends on distinct mechanisms in metallicolous populations of Arabidopsis halleri. N. Phytol. 2018;218:269–282. doi: 10.1111/nph.14949. [DOI] [PubMed] [Google Scholar]
- 30.Merlot S., de la Torre V.S., Hanikenne M. Physiology and molecular biology of trace element hyperaccumulation. In: Van der Ent A., Baker A.J., Echevarria G., Simonnot M.O., Morel J.L., editors. Agromining: Farming for Metals. Mineral Resource Reviews. Springer; Cham, Switzerland: 2021. pp. 155–181. [DOI] [Google Scholar]
- 31.Kondo N., Imai K., Isobe M., Goto T., Murasugi A., Wada-Nakagawa C., Hayashi Y. Cadystin a and b, major unit peptides comprising cadmium binding peptides induced in a fission yeast—Separation, revision of structures and synthesis. Tetrahedron Lett. 1984;25:3869–3872. doi: 10.1016/S0040-4039(01)91190-6. [DOI] [Google Scholar]
- 32.Grill E., Winnacker E.-L., Zenk M.H. Phytochelatins: The principal heavy-metal complexing peptides of higher plants. Science. 1985;230:674–676. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- 33.Yadav S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010;76:167–179. doi: 10.1016/j.sajb.2009.10.007. [DOI] [Google Scholar]
- 34.Anjum N.A., Hasanuzzaman M., Hossain M.A., Thangavel P., Roychoudhury A., Gill S.S., Rodrigo M.A.M., Adam V., Fujita M., Kizek R., et al. Jacks of metal/metalloid chelation trade in plants—An overview. Front. Plant Sci. 2015;6:192. doi: 10.3389/fpls.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balzano S., Sardo A., Blasio M., Chahine T.B., Dell’Anno F., Sansone C., Brunet C. Microalgal metallothioneins and phytochelatins and their potential use in bioremediation. Front. Microbiol. 2020;11:517. doi: 10.3389/fmicb.2020.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M., Barbaro E., Bellini E., Saba A., di Toppi L.S., Varotto C. Ancestral function of the phytochelatin synthase C-terminal domain in inhibition of heavy metal-mediated enzyme overactivation. J. Exp. Bot. 2020;71:6655–6669. doi: 10.1093/jxb/eraa386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carrillo J.T., Borthakur D. Methods for metal chelation in plant homeostasis: Review. Plant Physiol. Biochem. 2021;163:95–107. doi: 10.1016/j.plaphy.2021.03.045. [DOI] [PubMed] [Google Scholar]
- 38.Pal R., Rai J.P.N. Phytochelatins: Peptides involved in heavy metal detoxification. Appl. Biochem. Biotechnol. 2010;160:945–963. doi: 10.1007/s12010-009-8565-4. [DOI] [PubMed] [Google Scholar]
- 39.Dennis K.K., Liu K.H., Uppal K., Go Y.-M., Jones D.P. Distribution of phytochelatins, metal-binding compounds, in plant foods: A survey of commonly consumed fruits, vegetables, grains and legumes. Food Chem. 2021;339:128051. doi: 10.1016/j.foodchem.2020.128051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie M., Chen W., Lai X., Dai H., Sun H., Zhou X., Chen T. Metabolic responses and their correlations with phytochelatins in Amaranthus hypochondriacus under cadmium stress. Environ. Pollut. 2019;252:1791–1800. doi: 10.1016/j.envpol.2019.06.103. [DOI] [PubMed] [Google Scholar]
- 41.Gupta D.K., Huang H.G., Nicoloso F.T., Schetinger M.R., Farias J.G., Li T.Q., Razafindrabe B.H.N., Aryal N., Inouhe M. Effect of Hg, As and Pb on biomass production, photosynthetic rate, nutrients uptake and phytochelatin induction in Pfaffia glomerata. Ecotoxicology. 2013;22:1403–1412. doi: 10.1007/s10646-013-1126-1. [DOI] [PubMed] [Google Scholar]
- 42.Gao Y., Li H., Song Y., Zhang F., Yang Z. The response of thiols to cadmium stress in spinach (Spinacia oleracea L.) Toxics. 2022;10:429. doi: 10.3390/toxics10080429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delhaize E., Jackson P.J., Lujan L.D., Robinson N.J. Poly(γ-glutamylcysteinyl)glycine synthesis in Datura innoxia and binding with cadmium. role in cadmium tolerance. Plant Physiol. 1989;89:700–706. doi: 10.1104/pp.89.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grill E., Winnacker E.-L., Zenk M.H. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc. Natl. Acad. Sci. USA. 1987;84:439–443. doi: 10.1073/pnas.84.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernández R., Fernández-Fuego D., Bertrand A., González A. Strategies for Cd accumulation in Dittrichia viscosa (L.) Greuter: Role of the cell wall, non-protein thiols and organic acids. Plant Physiol. Biochem. 2014;78:63–70. doi: 10.1016/j.plaphy.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 46.González H., Fernández-Fuego D., Bertrand A., González A. Effect of pH and citric acid on the growth, arsenic accumulation, and phytochelatin synthesis in Eupatorium cannabinum L., a promising plant for phytostabilization. Environ. Sci. Pollut. Res. 2019;26:26242–26253. doi: 10.1007/s11356-019-05657-2. [DOI] [PubMed] [Google Scholar]
- 47.Raab A., Schat H., Meharg A.A., Feldmann J. Uptake, translocation and transformation of arsenate and arsenite in sunflower (Helianthus annuus): Formation of arsenic–phytochelatin complexes during exposure to high arsenic concentrations. N. Phytol. 2005;168:551–558. doi: 10.1111/j.1469-8137.2005.01519.x. [DOI] [PubMed] [Google Scholar]
- 48.Meyer C.-L., Peisker D., Courbot M., Craciun A.R., Cazalé A.-C., Desgain D., Schat H., Clemens S., Verbruggen N. Isolation and characterization of Arabidopsis halleri and Thlaspi caerulescens phytochelatin synthases. Planta. 2011;234:83–95. doi: 10.1007/s00425-011-1378-z. [DOI] [PubMed] [Google Scholar]
- 49.Howden R., Goldsbrough P.B., Andersen C.R., Cobbett C.S. Cadmium-sensitive, cad1 Mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiol. 1995;107:1059–1066. doi: 10.1104/pp.107.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brunetti P., Zanella L., Proia A., De Paolis A., Falasca G., Altamura M.M., di Toppi L.S., Costantino P., Cardarelli M. Cadmium tolerance and phytochelatin content of Arabidopsis seedlings over-expressing the phytochelatin synthase gene AtPCS1. J. Exp. Bot. 2011;62:5509–5519. doi: 10.1093/jxb/err228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gong J.-M., Lee D.A., Schroeder J.I. Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2003;100:10118–10123. doi: 10.1073/pnas.1734072100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X., Rui H., Zhang F., Hu Z., Xia Y., Shen Z. Overexpression of a functional Vicia sativa PCS1 homolog increases cadmium tolerance and phytochelatins synthesis in arabidopsis. Front. Plant Sci. 2018;9:107. doi: 10.3389/fpls.2018.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hendrix S., Jozefczak M., Wójcik M., Deckers J., Vangronsveld J., Cuypers A. Glutathione: A key player in metal chelation, nutrient homeostasis, cell cycle regulation and the DNA damage response in cadmium-exposed Arabidopsis thaliana. Plant Physiol. Biochem. 2020;154:498–507. doi: 10.1016/j.plaphy.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Kühnlenz T., Hofmann C., Uraguchi S., Schmidt H., Schempp S., Weber M., Lahner B., Salt D.E., Clemens S. Phytochelatin synthesis promotes leaf Zn accumulation of Arabidopsis thaliana plants grown in soil with adequate Zn supply and is essential for survival on Zn-contaminated soil. Plant Cell. Physiol. 2016;57:2342–2352. doi: 10.1093/pcp/pcw148. [DOI] [PubMed] [Google Scholar]
- 55.Sofo A., Vitti A., Nuzzaci M., Tataranni G., Scopa A., Vangronsveld J., Remans T., Falasca G., Altamura M.M., Degola F., et al. Correlation between hormonal homeostasis and morphogenic responses in Arabidopsis thaliana seedlings growing in a Cd/Cu/Zn multi-pollution context. Physiol. Plant. 2013;149:487–498. doi: 10.1111/ppl.12050. [DOI] [PubMed] [Google Scholar]
- 56.Bellini E., Borsò M., Betti C., Bruno L., Andreucci A., Castiglione M.R., Saba A., di Toppi L.S. Characterization and quantification of thiol-peptides in Arabidopsis thaliana using combined dilution and high sensitivity HPLC-ESI-MS-MS. Phytochemistry. 2019;164:215–222. doi: 10.1016/j.phytochem.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Uraguchi S., Ohshiro Y., Otsuka Y., Wada E., Naruse F., Sugaya K., Nagai K., Wongkaew A., Nakamura R., Takanezawa Y., et al. Phytochelatin-mediated metal detoxification pathway is crucial for an organomercurial phenylmercury tolerance in arabidopsis. Plant Mol. Biol. 2022;109:563–577. doi: 10.1007/s11103-021-01221-0. [DOI] [PubMed] [Google Scholar]
- 58.Ducruix C., Junot C., Fiévet J.-B., Villiers F., Ezan E., Bourguignon J. New insights into the regulation of phytochelatin biosynthesis in A. thaliana cells from metabolite profiling analyses. Biochimie. 2006;88:1733–1742. doi: 10.1016/j.biochi.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 59.Kubota H., Sato K., Yamada T., Maitani T. Phytochelatin homologs induced in hairy roots of horseradish. Phytochemistry. 2000;53:239–245. doi: 10.1016/S0031-9422(99)00493-8. [DOI] [PubMed] [Google Scholar]
- 60.Chen L., Yang L., Wang Q. In vivo phytochelatins and Hg-phytochelatin complexes in Hg-stressed Brassica chinensis L. Metallomics. 2009;1:101–106. doi: 10.1039/B815477E. [DOI] [Google Scholar]
- 61.Mohamed A.A., Castagna A., Ranieri A., di Toppi L.S. Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol. Biochem. 2012;57:15–22. doi: 10.1016/j.plaphy.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Agnihotri A., Seth C.S. Does jasmonic acid regulate photosynthesis, clastogenecity, and phytochelatins in Brassica juncea L. in response to Pb-subcellular distribution? Chemosphere. 2020;243:125361. doi: 10.1016/j.chemosphere.2019.125361. [DOI] [PubMed] [Google Scholar]
- 63.Ebbs S., Lau I., Ahner B., Kochian L. Phytochelatin synthesis is not responsible for Cd tolerance in the Zn/Cd hyperaccumulator Thlaspi caerulescens (J. & C. Presl) Planta. 2002;214:635–640. doi: 10.1007/s004250100650. [DOI] [PubMed] [Google Scholar]
- 64.Wójcik M., Vangronsveld J., Tukiendorf A. Cadmium tolerance in Thlaspi caerulescens: I. growth parameters, metal accumulation and phytochelatin synthesis in response to cadmium. 2005Environ. Exp. Bot. 2005;53:151–161. doi: 10.1016/j.envexpbot.2004.03.010. [DOI] [Google Scholar]
- 65.Kińska K., Kowalska J. Comparison of platinum, rhodium, and palladium bioaccumulation by Sinapis alba and their influence on phytochelatin synthesis in plant tissues. Pol. J. Environ. Stud. 2019;28:1735–1740. doi: 10.15244/pjoes/89507. [DOI] [Google Scholar]
- 66.De Knecht J.A., van Dillen M., Koevoets P.L.M., Schat H., Verkleij J.A.C., Ernst W.H.O. Phytochelatins in cadmium-sensitive and cadmium-tolerant Silene vulgaris. Chain length distribution and sulfide incorporation. Plant Physiol. 1994;104:255–261. doi: 10.1104/pp.104.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verkleij J.A., Koevoets P., Van’T Riet J., Bank R., Nijdam Y., Ernst W.H. Poly (γ-Glutamylcysteinyl) glycines or phytochelatins and their role in cadmium tolerance of Silene vulgaris. Plant Cell Environ. 1990;13:913–921. doi: 10.1111/j.1365-3040.1990.tb01981.x. [DOI] [Google Scholar]
- 68.Zhang Z.-C., Chen B.-X., Qiu B.-S. Phytochelatin synthesis plays a similar role in shoots of the cadmium hyperaccumulator Sedum alfredii as in non-resistant plants. Plant Cell Environ. 2010;33:1248–1255. doi: 10.1111/j.1365-3040.2010.02144.x. [DOI] [PubMed] [Google Scholar]
- 69.Abbasi S., Lamb D.T., Choppala G., Burton E.D., Megharaj M. Antimony speciation, phytochelatin stimulation and toxicity in plants. Environ. Pollut. 2022;305:119305. doi: 10.1016/j.envpol.2022.119305. [DOI] [PubMed] [Google Scholar]
- 70.Bianucci E., Sobrino-Plata J., Carpena-Ruiz R.O., del Carmen Tordable M., Fabra A., Hernández L.E., Castro S. Contribution of phytochelatins to cadmium tolerance in peanut plants. Metallomics. 2012;4:1119–1124. doi: 10.1039/c2mt20146a. [DOI] [PubMed] [Google Scholar]
- 71.Grill E., Gekeler W., Winnacker E.-L., Zenk H.H. Homo-phytochelatins are heavy metal-binding peptides of homo-glutathione containing fabales. FEBS Lett. 1986;205:47–50. doi: 10.1016/0014-5793(86)80863-8. [DOI] [Google Scholar]
- 72.Klapheck S., Schlunz S., Bergmann L. Synthesis of phytochelatins and homo-phytochelatins in Pisum sativum L. Plant Physiol. 1995;107:515–521. doi: 10.1104/pp.107.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lima A.I.G., Pereira S.I.A., Figueira E.M.D.A.P., Caldeira G.C.N., de Matos Caldeira H.D.Q. Cadmium detoxification in roots of Pisum sativum seedlings: Relationship between toxicity levels, thiol pool alterations and growth. Environ. Exp. Bot. 2006;55:149–162. doi: 10.1016/j.envexpbot.2004.10.008. [DOI] [Google Scholar]
- 74.Bardarov K., Naydenov M., Djingova R. HPLC-HRMS method for fast phytochelatins determination in plants. Application to analysis of Clinopodium vulgare L. Talanta. 2015;142:20–27. doi: 10.1016/j.talanta.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 75.Xiao Q., Wang Y., Lü Q., Wen H., Han B., Chen S., Zheng X., Lin R. Responses of glutathione and phytochelatins biosysthesis in a cadmium accumulator of Perilla frutescens (L.) Britt. under cadmium contaminated conditions. Ecotoxicol. Environ. Saf. 2020;201:110805. doi: 10.1016/j.ecoenv.2020.110805. [DOI] [PubMed] [Google Scholar]
- 76.Bellini E., Varotto C., Borsò M., Rugnini L., Bruno L., di Toppi L.S. Eukaryotic and prokaryotic phytochelatin synthases differ less in functional terms than previously thought: A comparative analysis of Marchantia polymorpha and Geitlerinema sp. PCC 7407. Plants. 2020;9:914. doi: 10.3390/plants9070914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giardini S., Bellini E., Bandoni E., Saba A., di Toppi L.S. Tools for in vitro propagation/synchronization of the liverwort Marchantia polymorpha and application of a validated HPLC-ESI-MS-MS method for glutathione and phytochelatin analysis. Stresses. 2022;2:136–145. doi: 10.3390/stresses2010010. [DOI] [Google Scholar]
- 78.Salt D.E., Rauser W.E. MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiol. 1995;107:1293–1301. doi: 10.1104/pp.107.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hartley-Whitaker J., Ainsworth G., Vooijs R., Bookum W.T., Schat H., Meharg A.A. Phytochelatins are involved in differential arsenate tolerance in Holcus lanatus. Plant Physiol. 2001;126:299–306. doi: 10.1104/pp.126.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ju X.H., Tang S., Jia Y., Guo J., Ding Y., Song Z., Zhao Y. Determination and characterization of cysteine, glutathione and phytochelatins (PC2-6) in Lolium perenne L. exposed to Cd stress under ambient and elevated carbon dioxide using HPLC with fluorescence detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011;879:1717–1724. doi: 10.1016/j.jchromb.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 81.Shi Y., Liu Y., Li H., Pei H., Xu Y., Ju X. Phytochelatins formation kinetics and Cd-induced growth inhibition in Lolium perenne L. at elevated CO2 level under Cd stress. Environ. Sci. Pollut. Res. 2021;28:35751–35763. doi: 10.1007/s11356-021-12883-0. [DOI] [PubMed] [Google Scholar]
- 82.Yamazaki S., Ueda Y., Mukai A., Ochiai K., Matoh T. Rice phytochelatin synthases OsPCS1 and OsPCS2 make different contributions to cadmium and arsenic tolerance. Plant Direct. 2018;2:e00034. doi: 10.1002/pld3.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mou R.-X., Cao Z.-Y., Lin X.-Y., Wu L., Cao Z.-Z., Zhu Z.-W., Chen M.-X. Characterization of the phytochelatins and their derivatives in rice exposed to cadmium based on high-performance liquid chromatography coupled with data-dependent hybrid linear ion trap orbitrap mass spectrometry. Rapid Commun. Mass Spectrom. 2016;30:1891–1900. doi: 10.1002/rcm.7669. [DOI] [PubMed] [Google Scholar]
- 84.Shri M., Dave R., Diwedi S., Shukla D., Kesari R., Tripathi R.D., Trivedi P.K., Chakrabarty D. Heterologous expression of Ceratophyllum demersum phytochelatin synthase, CdPCS1, in rice leads to lower arsenic accumulation in grain. Sci. Rep. 2014;4:5784. doi: 10.1038/srep05784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cao Z.-Z., Qin M.-L., Lin X.-Y., Zhu Z.-W., Chen M.-X. Sulfur supply reduces cadmium uptake and translocation in rice grains (Oryza sativa L.) by enhancing iron plaque formation, cadmium chelation and vacuolar sequestration. Environ. Pollut. 2018;238:76–84. doi: 10.1016/j.envpol.2018.02.083. [DOI] [PubMed] [Google Scholar]
- 86.Rabêlo F.H.S., Fernie A.R., Navazas A., Borgo L., Keunen E., da Silva B.K.D.A., Cuypers A., Lavres J. A glimpse into the effect of sulfur supply on metabolite profiling, glutathione and phytochelatins in Panicum maximum cv. massai exposed to cadmium. Environ. Exp. Bot. 2018;151:76–88. doi: 10.1016/j.envexpbot.2018.04.003. [DOI] [Google Scholar]
- 87.Ederli L., Reale L., Ferranti F., Pasqualini S. Responses induced by high concentration of cadmium in Phragmites australis roots. Physiol. Plant. 2004;121:66–74. doi: 10.1111/j.0031-9317.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- 88.Wójcik M., Tukendorf A. Cd-tolerance of maize, rye and wheat seedlings. Acta Physiol. Plant. 1999;21:99–107. doi: 10.1007/s11738-999-0063-3. [DOI] [Google Scholar]
- 89.Stolt J.P., Sneller F.E.C., Bryngelsson T., Lundborg T., Schat H. Phytochelatin and cadmium accumulation in wheat. Environ. Exp. Bot. 2003;49:21–28. doi: 10.1016/S0098-8472(02)00045-X. [DOI] [Google Scholar]
- 90.Ranieri A., Castagna A., Scebba F., Careri M., Zagnoni I., Predieri G., Pagliari M., di Toppi L.S. Oxidative stress and phytochelatin characterisation in bread wheat exposed to cadmium excess. Plant Physiol. Biochem. 2005;43:45–54. doi: 10.1016/j.plaphy.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 91.Tukendorf A., Rauser W.E. Changes in glutathione and phytochelatins in roots of maize seedlings exposed to cadmium. Plant Sci. 1990;70:155–166. doi: 10.1016/0168-9452(90)90129-C. [DOI] [Google Scholar]
- 92.Meuwly P., Thibault P., Schwan A.L., Rauser W.E. Three families of thiol peptides are induced by cadmium in maize. Plant J. 1995;7:391–400. doi: 10.1046/j.1365-313X.1995.7030391.x. [DOI] [PubMed] [Google Scholar]
- 93.Rauser W.E., Meuwly P. Retention of cadmium in roots of maize seedlings. Role of complexation by phytochelatins and related thiol peptides. Plant Physiol. 1995;109:195–202. doi: 10.1104/pp.109.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seregin I.V., Vooijs R., Kozhevnikova A.D., Ivanov V.B., Schat H. Effects of cadmium and lead on phytochelatin accumulation in maize shoots and different root parts. Doklady Biol. Sci. 2007;415:304–306. doi: 10.1134/S0012496607040163. [DOI] [PubMed] [Google Scholar]
- 95.Szalai G., Krantev A., Yordanova R., Popova L.P., Janda T. Influence of salicylic acid on phytochelatin synthesis in Zea mays during Cd stress. Turk. J. Bot. 2013;37:708–714. doi: 10.3906/bot-1210-6. [DOI] [Google Scholar]
- 96.Pal R., Kaur R., Rajwar D., Rai J.P.N. Induction of non-protein thiols and phytochelatins by cadmium in Eichhornia crassipes. Int. J. Phytoremediat. 2019;21:790–798. doi: 10.1080/15226514.2019.1566881. [DOI] [PubMed] [Google Scholar]
- 97.Raab A., Feldmann J., Meharg A.A. The nature of arsenic-phytochelatin complexes in Holcus lanatus and Pteris cretica. Plant Physiol. 2004;134:1113–1122. doi: 10.1104/pp.103.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maitani T., Kubota H., Sato K., Yamada T. The composition of metals bound to class III metallothionein (Phytochelatin and its desglycyl peptide) induced by various metals in root cultures of Rubia tinctorum. Plant Physiol. 1996;110:1145–1150. doi: 10.1104/pp.110.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Navazas A., Hendrix S., Cuypers A., González A. Integrative response of arsenic uptake, speciation and detoxification by Salix atrocinerea. Sci. Total Environ. 2019;689:422–433. doi: 10.1016/j.scitotenv.2019.06.279. [DOI] [PubMed] [Google Scholar]
- 100.Vögeli-Lange R., Wagner G.J. Subcellular localization of cadmium and cadmium-binding peptides in tobacco leaves. Implication of a transport function for cadmium-binding peptides. Plant Physiol. 1990;92:1086–1093. doi: 10.1104/pp.92.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hirt H., Sommergruber K., Barta A. Effects of cadmium on tobacco: Synthesis and regulation of cadmium-binding peptides. Biochem. Physiol. Pflanz. 1990;186:153–163. doi: 10.1016/S0015-3796(96)80001-1. [DOI] [Google Scholar]
- 102.Scheller H.V., Huang B., Hatch E., Goldsbrough P.B. Phytochelatin synthesis and glutathione levels in response to heavy metals in tomato cells. Plant Physiol. 1987;85:1031–1035. doi: 10.1104/pp.85.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen J., Coldsbrough P.B. Increased activity of [gamma]-glutamylcysteine synthetase in tomato cells selected for cadmium tolerance. Plant Physiol. 1994;106:233–239. doi: 10.1104/pp.106.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Steffens J.C., Hunt D.F., Williams B.G. Accumulation of non-protein metal-binding polypeptides (Gamma-glutamyl-cysteinyl)n-glycine in selected cadmium-resistant tomato cells. J. Biol. Chem. 1986;261:13879–13882. doi: 10.1016/S0021-9258(18)66952-2. [DOI] [PubMed] [Google Scholar]
- 105.Chen J., Zhou J., Goldsbrough P.B. Characterization of phytochelatin synthase from tomato. Physiol. Plant. 1997;101:165–172. doi: 10.1111/j.1399-3054.1997.tb01833.x. [DOI] [Google Scholar]
- 106.Mendum M.L., Gupta S.C., Goldsbrough P.B. Effect of glutathione on phytochelatin synthesis in tomato cells. Plant Physiol. 1990;93:484–488. doi: 10.1104/pp.93.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lue-Kim H., Rauser W.E. Partial characterization of cadmium-binding protein from roots of tomato. Plant Physiol. 1986;81:896–900. doi: 10.1104/pp.81.3.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hasan M.K., Ahammed G.J., Yin L., Shi K., Xia X., Zhou Y., Yu J., Zhou J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant. Sci. 2015;6:601. doi: 10.3389/fpls.2015.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spisso A.A., Cerutti S., Silva F., Pacheco P.H., Martinez L.D. Characterization of Hg-phytochelatins complexes in vines (Vitis vinifera cv malbec) as defense mechanism against metal stress. Biometals. 2014;27:591–599. doi: 10.1007/s10534-014-9732-9. [DOI] [PubMed] [Google Scholar]
- 110.Rauser W.E. Phytochelatins and related peptides. Structure, biosynthesis, and function. Plant Physiol. 1995;109:1141–1149. doi: 10.1104/pp.109.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zenk M.H. Heavy metal detoxification in higher plants—A review. Gene. 1996;179:21–30. doi: 10.1016/S0378-1119(96)00422-2. [DOI] [PubMed] [Google Scholar]
- 112.Cobbett C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000;123:825–832. doi: 10.1104/pp.123.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cobbett C., Goldsbrough P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant. Biol. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- 114.Mirza N., Mahmood Q., Maroof Shah M., Pervez A., Sultan S. Plants as useful vectors to reduce environmental toxic arsenic content. Sci. World J. 2014;2014:921581. doi: 10.1155/2014/921581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thakur M., Praveen S., Divte P.R., Mitra R., Kumar M., Gupta C.K., Kalidindi U., Bansal R., Roy S., Anand A., et al. Metal tolerance in plants: Molecular and physicochemical interface determines the “Not so heavy effect” of heavy metals. Chemosphere. 2022;287:131957. doi: 10.1016/j.chemosphere.2021.131957. [DOI] [PubMed] [Google Scholar]
- 116.Klapheck S., Fliegner W., Zimmer I. Hydroxymethyl-phytochelatins [(γ-Glutamylcysteine)n-serine] are metal-induced peptides of the Poaceae. Plant Physiol. 1994;104:1325–1332. doi: 10.1104/pp.104.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vögeli-Lange R., Wagner G.J. Relationship between cadmium, glutathione and cadmium-binding peptides (phytochelatins) in leaves of intact tobacco seedlings. Plant Sci. 1996;114:11–18. doi: 10.1016/0168-9452(95)04299-7. [DOI] [Google Scholar]
- 118.Jemal F., Didierjean L., Ghrir R., Ghorbal M.H., Burkard G. Characterization of cadmium binding peptides from pepper (Capsicum annuum) Plant Sci. 1998;137:143–154. doi: 10.1016/S0168-9452(98)00120-4. [DOI] [Google Scholar]
- 119.Navarrete A., González A., Gómez M., Contreras R.A., Díaz P., Lobos G., Brown M.T., Sáez C.A., Moenne A. Copper excess detoxification is mediated by a coordinated and complementary induction of glutathione, phytochelatins and metallothioneins in the green seaweed Ulva compressa. Plant Physiol. Biochem. 2019;135:423–431. doi: 10.1016/j.plaphy.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 120.Wątły J., Łuczkowski M., Padjasek M., Krȩżel A. Phytochelatins as a dynamic system for Cd(II) buffering from the micro- to femtomolar range. Inorg. Chem. 2021;60:4657–4675. doi: 10.1021/acs.inorgchem.0c03639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu Y., Yuan Y., Yuan H., Zhang W., Zhang L. Predicting cadmium toxicity with the kinetics of phytochelatin induction in a marine diatom. Aquat. Toxicol. 2019;207:101–109. doi: 10.1016/j.aquatox.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 122.Hou M., Li M., Yang X., Pan R. Responses of nonprotein thiols to stress of vanadium and mercury in maize (Zea mays L.) seedlings. Bull. Environ. Contam. Toxicol. 2019;102:425–431. doi: 10.1007/s00128-019-02553-w. [DOI] [PubMed] [Google Scholar]
- 123.Verbruggen N., Hermans C., Schat H. Molecular mechanisms of metal hyperaccumulation in plants. N. Phytol. 2009;181:759–776. doi: 10.1111/j.1469-8137.2008.02748.x. [DOI] [PubMed] [Google Scholar]
- 124.Clemens S., Peršoh D. Multi-tasking phytochelatin synthases. Plant Sci. 2009;177:266–271. doi: 10.1016/j.plantsci.2009.06.008. [DOI] [Google Scholar]
- 125.Mendoza-Cózatl D.G., Jobe T.O., Hauser F., Schroeder J.I. Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr. Opin. Plant Biol. 2011;14:554–562. doi: 10.1016/j.pbi.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Singh S., Tripathi D.K., Chauhan D.K., Dubey N.K. Plant Metal Interaction: Emerging Remediation Techniques. Elsevier Inc.; Amsterdam, The Netherlands: 2016. Glutathione and phytochelatins mediated redox homeostasis and stress signal transduction in plants: An integrated overview; pp. 285–310. [DOI] [Google Scholar]
- 127.Frémont A., Sas E., Sarrazin M., Gonzalez E., Brisson J., Pitre F.E., Brereton N.J.B. Phytochelatin and coumarin enrichment in root exudates of arsenic-treated white lupin. Plant Cell Environ. 2022;45:936–954. doi: 10.1111/pce.14163. [DOI] [PubMed] [Google Scholar]
- 128.Iglesia-Turiño S., Febrero A., Jauregui O., Caldelas C., Araus J.L., Bort J. Detection and quantification of unbound phytochelatin 2 in plant extracts of Brassica napus grown with different levels of mercury. Plant Physiol. 2006;142:742–749. doi: 10.1104/pp.106.085068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gupta D.K., Vandenhove H., Inouhe M. Role of phytochelatins in heavy metal stress and detoxification mechanisms in plants. In: Gupta D.K., Corpas F.J., Palma J.M., editors. Heavy Metal Stress in Plants. Springer; Berlin/Heidelberg, Germany: 2013. pp. 73–94. [DOI] [Google Scholar]
- 130.Scheidegger C., Suter M.J.-F., Behra R., Sigg L. Characterization of lead-phytochelatin complexes by nano-electrospray ionization mass spectrometry. Front. Microbiol. 2012;3:41. doi: 10.3389/fmicb.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fischer S., Kühnlenz T., Thieme M., Schmidt H., Clemens S. Analysis of plant Pb tolerance at realistic submicromolar concentrations demonstrates the role of phytochelatin synthesis for Pb detoxification. Environ. Sci. Technol. 2014;48:7552–7559. doi: 10.1021/es405234p. [DOI] [PubMed] [Google Scholar]
- 132.Zhou C., Huang M., Ren H., Yu J., Wu J., Ma X. Bioaccumulation and detoxification mechanisms for lead uptake identified in Rhus chinensis Mill. seedlings. Ecotoxicol. Environ. Saf. 2017;142:59–68. doi: 10.1016/j.ecoenv.2017.03.052. [DOI] [PubMed] [Google Scholar]
- 133.Liu J., Zhang J., Kim S.H., Lee H.-S., Marinoia E., Song W.-Y. Characterization of Brassica rapa metallothionein and phytochelatin synthase genes potentially involved in heavy metal detoxification. PLoS ONE. 2021;16:e0252899. doi: 10.1371/journal.pone.0252899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tennstedt P., Peisker D., Böttcher C., Trampczynska A., Clemens S. Phytochelatin Synthesis is essential for the detoxification of excess zinc and contributes significantly to the accumulation of zinc. Plant Physiol. 2009;149:938–948. doi: 10.1104/pp.108.127472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen J., Liu Y.-Q., Yan X.-W., Wei G.-H., Zhang J.-H., Fang L.-C. Rhizobium inoculation enhances copper tolerance by affecting copper uptake and regulating the ascorbate-glutathione cycle and phytochelatin biosynthesis-related gene expression in Medicago sativa seedlings. Ecotoxicol. Environ. Saf. 2018;162:312–323. doi: 10.1016/j.ecoenv.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 136.Krayem M., Pinault E., Deluchat V., Labrousse P. Are cysteine, glutathione and phytochelatins responses of Myriophyllum alterniflorum to copper and arsenic stress affected by trophic conditions? BioMetals. 2022;35:729–739. doi: 10.1007/s10534-022-00396-3. [DOI] [PubMed] [Google Scholar]
- 137.Landi M., Margaritopoulou T., Papadakis I.E., Araniti F. Boron toxicity in higher plants: An update. Planta. 2019;250:1011–1032. doi: 10.1007/s00425-019-03220-4. [DOI] [PubMed] [Google Scholar]
- 138.Vestergaard M., Matsumoto S., Nishikori S., Shiraki K., Hirata K., Takagi M. Chelation of cadmium ions by phytochelatin synthase: Role of the cystein-rich C-terminal. Anal. Sci. 2008;24:277–281. doi: 10.2116/analsci.24.277. [DOI] [PubMed] [Google Scholar]
- 139.Zagorchev L., Seal C.E., Kranner I., Odjakova M. A central role for thiols in plant tolerance to abiotic stress. Int. J. Mol. Sci. 2013;14:7405–7432. doi: 10.3390/ijms14047405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Repkina N., Talanova V., Ignatenko A., Titov A. Involvement of proline and non-protein thiols in response to low temperature and cadmium stresses in wheat. Biol Plant. 2019;63:70–77. doi: 10.32615/bp.2019.009. [DOI] [Google Scholar]
- 141.Zhu T., Liu X., Zhang M., Chen M. Mechanism of cadmium tolerance in Salicornia europaea at optimum levels of NaCl. Plant Biol. 2022;24:41–51. doi: 10.1111/plb.13348. [DOI] [PubMed] [Google Scholar]
- 142.Yamaguchi C., Khamsalath S., Takimoto Y., Suyama A., Mori Y., Ohkama-Ohtsu N., Maruyama-Nakashita A. SLIM1 transcription factor promotes sulfate uptake and distribution to shoot, along with phytochelatin accumulation, under cadmium stress in Arabidopsis thaliana. Plants. 2020;9:163. doi: 10.3390/plants9020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ahner B.A., Morel F.M.M. Phytochelatin production in marine algae. 2. induction by various metals. Limnol. Oceanogr. 1995;40:658–665. doi: 10.4319/lo.1995.40.4.0658. [DOI] [Google Scholar]
- 144.Hirata K., Tsujimoto Y., Namba T., Ohta T., Hirayanagi N., Miyasaka H., Zenk M.H., Miyamoto K. Strong induction of phytochelatin synthesis by zinc in marine green alga, Dunaliella tertiolecta. J. Biosci. Bioeng. 2001;92:24–29. doi: 10.1016/S1389-1723(01)80193-6. [DOI] [PubMed] [Google Scholar]
- 145.Zhang Z., Gao X., Qiu B. Detection of phytochelatins in the hyperaccumulator Sedum alfredii exposed to cadmium and lead. Phytochemistry. 2008;69:911–918. doi: 10.1016/j.phytochem.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 146.Sun Q., Wang X.-R., Ding S.-M., Yuan X.-F. Effects of interaction between cadmium and plumbum on phytochelatins and glutathione production in wheat (Triticum aestivum L.) J. Integr. Plant Biol. 2005;47:435–442. doi: 10.1111/j.1744-7909.2005.00073.x. [DOI] [Google Scholar]
- 147.Bari M.A., Prity S.A., Das U., Akther M.S., Sajib S.A., Reza M.A., Kabir A.H. Silicon induces phytochelatin and ROS scavengers facilitating cadmium detoxification in rice. Plant Biol. 2020;22:472–479. doi: 10.1111/plb.13090. [DOI] [PubMed] [Google Scholar]
- 148.Mukta R.H., Khatun M.R., Huda A.K.M.N. Calcium induces phytochelatin accumulation to cope with chromium toxicity in rice (Oryza sativa L.) J. Plant Interact. 2019;14:295–302. doi: 10.1080/17429145.2019.1629034. [DOI] [Google Scholar]
- 149.Qu L., Jia W., Dai Z., Xu Z., Cai M., Huang W., Han D., Dang B., Ma X., Gao Y., et al. Selenium and molybdenum synergistically alleviate chromium toxicity by modulating Cr uptake and subcellular distribution in Nicotiana tabacum L. Ecotoxicol. Environ. Saf. 2022;248:114312. doi: 10.1016/j.ecoenv.2022.114312. [DOI] [PubMed] [Google Scholar]
- 150.Kiany T., Pishkar L., Sartipnia N., Iranbakhsh A., Barzin G. Effects of silicon and titanium dioxide nanoparticles on arsenic accumulation, phytochelatin metabolism, and antioxidant system by rice under arsenic toxicity. Environ. Sci. Pollut. Res. 2022;29:34725–34737. doi: 10.1007/s11356-021-17927-z. [DOI] [PubMed] [Google Scholar]
- 151.Kozhevnikova A.D., Seregin I.V., Schat H. Translocation of Ni and Zn in Odontarrhena corsica and Noccaea caerulescens: The effects of exogenous histidine and Ni/Zn interactions. Plant Soil. 2021;468:295–318. doi: 10.1007/s11104-021-05080-y. [DOI] [Google Scholar]
- 152.Batista B.L., Nigar M., Mestrot A., Rocha B.A., Júnior F.B., Price A.H., Raab A., Feldmann J. Identification and quantification of phytochelatins in roots of rice to long-term exposure: Evidence of individual role on arsenic accumulation and translocation. J. Exp. Bot. 2014;65:1467–1479. doi: 10.1093/jxb/eru018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lara-Almazán N., Zarazúa-Ortega G., Ávila-Pérez P., Barrera-Díaz C.E., Cedillo-Cruz A. Validation and uncertainty estimation of analytical method for quantification of phytochelatins in aquatic plants by UPLC-MS. Phytochemistry. 2021;183:112643. doi: 10.1016/j.phytochem.2020.112643. [DOI] [PubMed] [Google Scholar]
- 154.Zhao Y., Gouda M., Yu G., Zhang C., Lin L., Nie P., Huang W., Ye H., Ye Y., Zhou C., et al. Analyzing cadmium-phytochelatin2 complexes in plant using terahertz and circular dichroism information. Ecotoxicol. Environ. Saf. 2021;225:112800. doi: 10.1016/j.ecoenv.2021.112800. [DOI] [PubMed] [Google Scholar]
- 155.Ahmad J., Ali A.A., Baig M.A., Iqbal M., Haq I., Qureshi M.I. Role of phytochelatins in cadmium stress tolerance in plants. In: Hasanuzzaman M., Prasad M.N.V., Fujita M., editors. Cadmium Toxicity and Tolerance in Plants. Elsevier Inc.; Amsterdam, The Netherlands: 2019. pp. 185–212. [DOI] [Google Scholar]
- 156.Uraguchi S., Nagai K., Naruse F., Otsuka Y., Ohshiro Y., Nakamura R., Takanezawa Y., Kiyono M. Development of affinity bead–based in vitro metal–ligand binding assay reveals dominant cadmium affinity of thiol-rich small peptides phytochelatins beyond glutathione. Metallomics. 2021;13:mfab068. doi: 10.1093/mtomcs/mfab068. [DOI] [PubMed] [Google Scholar]
- 157.Jacquart A., Brayner R., El Hage Chahine J.-M., Ha-Duong N.-T. Cd2+ and Pb2+ complexation by glutathione and the phytochelatins. Chem. Biol. Interact. 2017;267:2–10. doi: 10.1016/j.cbi.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 158.Pochodylo A.L., Aristilde L. Molecular dynamics of stability and structures in phytochelatin complexes with Zn, Cu, Fe, Mg, and Ca: Implications for metal detoxification. Environ. Chem. Lett. 2017;15:495–500. doi: 10.1007/s10311-017-0609-3. [DOI] [Google Scholar]
- 159.Kneer R., Zenk M.H. Phytochelatins protect plant enzymes from heavy metal poisoning. Phytochemistry. 1992;31:2663–2667. doi: 10.1016/0031-9422(92)83607-Z. [DOI] [Google Scholar]
- 160.Andresen E., Mattusch J., Wellenreuther G., Thomas G., Arroyo Abad U., Küpper H. Different strategies of cadmium detoxification in the submerged macrophyte Ceratophyllum demersum L. Metallomics. 2013;5:1377–1386. doi: 10.1039/c3mt00088e. [DOI] [PubMed] [Google Scholar]
- 161.Shanab S., Essa A., Shalaby E. Bioremoval capacity of three heavy metals by some microalgae species (Egyptian Isolates) Plant Signal. Behav. 2012;7:392–399. doi: 10.4161/psb.19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Šestáková I., Skalová Š., Navrátil T. Labile lead phytochelatin complex could enhance transport of lead ions across biological membrane. J. Electroanal. Chem. 2018;821:92–96. doi: 10.1016/j.jelechem.2017.11.052. [DOI] [Google Scholar]
- 163.Robinson N.J., Tommey A.M., Kuske C., Jackson P.J. Plant metallothioneins. Biochem J. 1993;295:1–10. doi: 10.1042/bj2950001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shrivastava M., Khandelwal A., Srivastava S. Heavy metal hyperaccumulator plants: The resource to understand the extreme adaptations of plants towards heavy metals. In: Srivastava S., Srivastava A., Suprasanna P., editors. Plant-Metal Interactions. Springer; Cham, Switzerland: 2019. pp. 79–97. [DOI] [Google Scholar]
- 165.Dennis K.K., Uppal K., Liu K.H., Ma C., Liang B., Go Y.M., Jones D.P. Phytochelatin database: A resource for phytochelatin complexes of nutritional and environmental metals. Database. 2019;2019:baz083. doi: 10.1093/database/baz083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Piechalak A., Tomaszewska B., Baralkiewicz D., Malecka A. Accumulation and detoxification of lead ions in legumes. Phytochemistry. 2002;60:153–162. doi: 10.1016/S0031-9422(02)00067-5. [DOI] [PubMed] [Google Scholar]
- 167.Meuwly P., Thibault P., Rauser W.E. γ-Glutamylcysteinylglutamic acid—A new homologue of glutathione in maize seedlings exposed to cadmium. FEBS Lett. 1993;336:472–476. doi: 10.1016/0014-5793(93)80858-R. [DOI] [PubMed] [Google Scholar]
- 168.Fernández-Fuego D., Bertrand A., González A. Metal accumulation and detoxification mechanisms in mycorrhizal Betula pubescens. Environ. Pollut. 2017;231:1153–1162. doi: 10.1016/j.envpol.2017.07.072. [DOI] [PubMed] [Google Scholar]
- 169.Kubota H., Sato K., Yamada T., Maitani T. Phytochelatins (Class III metallothioneins) and their desglycyl peptides induced by cadmium in normal root cultures of Rubia tinctorum L. Plant Sci. 1995;106:157–166. doi: 10.1016/0168-9452(95)04020-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Di Toppi L.S., Gabbrielli R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999;41:105–130. doi: 10.1016/S0098-8472(98)00058-6. [DOI] [Google Scholar]
- 171.Oven M., Page J.E., Zenk M.H., Kutchan T.M. Molecular characterization of the homo-phytochelatin synthase of soybean Glycine max. Relation to phytochelatin synthase. J. Biol. Chem. 2002;277:4747–4754. doi: 10.1074/jbc.M108254200. [DOI] [PubMed] [Google Scholar]
- 172.Negrin V.L., Teixeira B., Godinho R.M., Mendes R., Vale C. Phytochelatins and monothiols in salt marsh plants and their relation with metal tolerance. Mar. Pollut. Bull. 2017;121:78–84. doi: 10.1016/j.marpolbul.2017.05.045. [DOI] [PubMed] [Google Scholar]
- 173.Heiss S., Wachter A., Bogs J., Cobbett C., Rausch T. Phytochelatin synthase (PCS) protein is induced in Brassica juncea leaves after prolonged Cd exposure. J. Exp. Bot. 2003;54:1833–1839. doi: 10.1093/jxb/erg205. [DOI] [PubMed] [Google Scholar]
- 174.Seregin I.V., Kozhevnikova A.D. Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium. Rus. J. Plant Physiol. 2008;55:1–22. doi: 10.1134/S1021443708010019. [DOI] [Google Scholar]
- 175.Ziller A., Fraissinet-Tachet L. Metallothionein diversity and distribution in the tree of life: A multifunctional protein. Metallomics. 2018;10:1549–1559. doi: 10.1039/C8MT00165K. [DOI] [PubMed] [Google Scholar]
- 176.Sun Y., Ye H., Wei Z., Kong X., Wu Q. Root cell walls and phytochelatins in low-cadmium cultivar of Brassica parachinensis. Pedosphere. 2020;30:426–432. doi: 10.1016/S1002-0160(17)60452-1. [DOI] [Google Scholar]
- 177.Ivanov A.A. Role of glutathione in enhancing metal hyperaccumulation in plants. In: Hasanuzzaman M., Prasad M.N.V., editors. Handbook of Bioremediation. Physiological, Molecular and Biotechnological Interventions. Elsevier Inc.; Amsterdam, The Netherlands: 2021. pp. 115–152. [DOI] [Google Scholar]
- 178.Rai U.N., Tripathi R.D., Gupta M., Chandra P. Induction of phytochelatins under cadmium stress in water lettuce (Pistia stratiotes L.) J. Environ. Sci. Health A. 1995;30:2007–2026. doi: 10.1080/10934529509376318. [DOI] [Google Scholar]
- 179.Rauser W.E. Changes in glutathione content of maize seedlings exposed to cadmium. Plant Sci. 1987;51:171–175. doi: 10.1016/0168-9452(87)90190-7. [DOI] [Google Scholar]
- 180.Nocito F.F., Pirovano L., Cocucci M., Sacchi G.A. Cadmium-induced sulfate uptake in maize roots. Plant Physiol. 2002;129:1872–1879. doi: 10.1104/pp.002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sun Q., Ye Z.H., Wang X.R., Wong M.H. Cadmium hyperaccumulation leads to an increase of glutathione rather than phytochelatins in the cadmium hyperaccumulator Sedum alfredii. J. Plant Physiol. 2007;164:1489–1498. doi: 10.1016/j.jplph.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 182.Bisht A., Bhalla S., Kumar A., Kaur J., Garg N. Arbuscular mycorrhizae impart Cd tolerance in Cajans cajan (L.) Millsp. by upregulating the expression of metallothionein (CcMT1) and phytochelatin synthase (CcPCS1) genes. J. Plant Growth Regul. 2022:1–20. doi: 10.1007/s00344-022-10864-2. [DOI] [Google Scholar]
- 183.Heiss S., Schäfer H.J., Haag-Kerwer A., Rausch T. Cloning sulfur assimilation genes of Brassica juncea L.: Cadmium differentially affects the expression of a putative low-affinity sulfate transporter and isoforms of ATP sulfurylase and APS reductase. Plant Mol. Biol. 1999;39:847–857. doi: 10.1023/A:1006169717355. [DOI] [PubMed] [Google Scholar]
- 184.Gadapati W.R., Macfie S.M. Phytochelatins are only partially correlated with Cd-stress in two species of Brassica. Plant Sci. 2006;170:471–480. doi: 10.1016/j.plantsci.2005.09.017. [DOI] [Google Scholar]
- 185.Mendoza-Cózatl D., Loza-Tavera H., Hernández-Navarro A., Moreno-Sánchez R. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol. Rev. 2005;29:653–671. doi: 10.1016/j.femsre.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 186.Hasanuzzaman M., Nahar K., Anee T.I., Fujita M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants. 2017;23:249–268. doi: 10.1007/s12298-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hell R., Bergmann L. γ-Glutamylcysteine synthetase in higher plants: Catalytic properties and subcellular localization. Planta. 1990;180:603–612. doi: 10.1007/BF02411460. [DOI] [PubMed] [Google Scholar]
- 188.Nouet C., Motte P., Hanikenne M. Chloroplastic and mitochondrial metal homeostasis. Trends Plant Sci. 2011;16:395–404. doi: 10.1016/j.tplants.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 189.Gayomba S.R., Zhai Z., Jung H.-I., Vatamaniuk O.K. Local and systemic signaling of iron status and its interactions with homeostasis of other essential elements. Front. Plant Sci. 2015;6:716. doi: 10.3389/fpls.2015.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.May M.J., Leaver C.J. Arabidopsis thaliana gamma-glutamylcysteine synthetase is structurally unrelated to mammalian, yeast, and Escherichia coli homologs. Proc. Natl. Acad. Sci. USA. 1994;91:10059–10063. doi: 10.1073/pnas.91.21.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.May M.J., Vernoux T., Sánchez-Fernández R., Van Montagu M., Inzé D. Evidence for posttranscriptional activation of γ-glutamylcysteine synthetase during plant stress responses. Proc. Natl. Acad. Sci. USA. 1998;95:12049–12054. doi: 10.1073/pnas.95.20.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xiang C., Oliver D.J. Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in arabidopsis. Plant Cell. 1998;10:1539–1550. doi: 10.1105/tpc.10.9.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Freeman J.L., Persans M.W., Nieman K., Albrecht C., Peer W., Pickering I.J., Salt D.E. Increased glutathione biosynthesis plays a role in nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Cell. 2004;16:2176–2191. doi: 10.1105/tpc.104.023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vezza M.E., Luna D.F., Agostini E., Talano M.A. Glutathione, a key compound for as accumulation and tolerance in soybean plants treated with AsV and AsIII. Environ. Exp. Bot. 2019;162:272–282. doi: 10.1016/j.envexpbot.2019.03.002. [DOI] [Google Scholar]
- 195.Rüegsegger A., Schmutz D., Brunold C. Regulation of glutathione synthesis by cadmium in Pisum sativum L. Plant Physiol. 1990;93:1579–1584. doi: 10.1104/pp.93.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rüegsegger A., Brunold C. Effect of cadmium on γ-glutamylcysteine synthesis in maize seedlings. Plant Physiol. 1992;99:428–433. doi: 10.1104/pp.99.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Choi H.R., Hwang I.D., Lee C.-H., Kwon Y.M. Phytochelatins in cadmium-treated seedlings of Canavalia lineata. Mol. Cells. 1996;6:451–455. [Google Scholar]
- 198.Moudouma C.F.M., Gloaguen V., Riou C., Forestier L., Saladin G. High concentration of cadmium induces AtPCS2 gene expression in Arabidopsis thaliana (L.) Heynh ecotype Wassilewskija seedlings. Acta Physiol. Plant. 2012;34:1083–1091. doi: 10.1007/s11738-011-0905-7. [DOI] [Google Scholar]
- 199.Uotila M., Aioub A.A.A., Gullner G., Komives T., Brunold C. Induction of glutathione transferase activity in wheat and pea seedlings by cadmium. Acta Biol. Hung. 1994;45:11–16. [PubMed] [Google Scholar]
- 200.Bellini E., Maresca V., Betti C., Castiglione M.R., Fontanini D., Capocchi A., Sorce C., Borsò M., Bruno L., Sorbo S., et al. The moss Leptodictyum riparium counteracts severe cadmium stress by activation of glutathione transferase and phytochelatin synthase, but slightly by phytochelatins. Int. J. Mol. Sci. 2020;21:1583. doi: 10.3390/ijms21051583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ernst W.H.O. Effects of heavy metals in plants at the cellular and organismic level. In: Schuurmann G., Markert B., editors. Ecotoxicology. Ecological Fundamentals, Chemical Exposure and Biological Effects. Wiley; Heidelberg, Germany: 1999. pp. 587–620. [Google Scholar]
- 202.Harada E., Yamaguchi Y., Koizumi N., Sano H. Cadmium stress induces production of thiol compounds and transcripts for enzymes involved in sulfur assimilation pathways in Arabidopsis. J. Plant Physiol. 2002;159:445–448. doi: 10.1078/0176-1617-00733. [DOI] [Google Scholar]
- 203.Zhu S., Shi W., Jie Y. Overexpression of BnPCS1, a novel phytochelatin synthase gene from ramie (Boehmeria nivea), enhanced Cd tolerance, accumulation, and translocation in Arabidopsis thaliana. Front. Plant Sci. 2021;12:639189. doi: 10.3389/fpls.2021.639189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Schäfer H.J., Haag-Kerwer A., Rausch T. cDNA cloning and expression analysis of genes encoding GSH synthesis in roots of the heavy-metal accumulator Brassica juncea L.: Evidence for Cd-induction of a putative mitochondrial γ-glutamylcysteine synthetase isoform. Plant Mol. Biol. 1998;37:87–97. doi: 10.1023/A:1005929022061. [DOI] [PubMed] [Google Scholar]
- 205.Ghorbani A., Pishkar L., Roodbari N., Pehlivan N., Wu C. Nitric oxide could allay arsenic phytotoxicity in tomato (Solanum lycopersicum L.) by modulating photosynthetic pigments, phytochelatin metabolism, molecular redox status and arsenic sequestration. Plant Physiol. Biochem. 2021;167:337–348. doi: 10.1016/j.plaphy.2021.08.019. [DOI] [PubMed] [Google Scholar]
- 206.Cobbett C.S., May M.J., Howden R., Rolls B. The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. Plant J. 1998;16:73–78. doi: 10.1046/j.1365-313x.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- 207.Lee S., Moon J.S., Ko T.-S., Petros D., Goldsbrough P.B., Korban S.S. Overexpression of arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiol. 2003;131:656–663. doi: 10.1104/pp.014118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jobe T.O., Sung D.-Y., Akmakjian G., Pham A., Komives E.A., Mendoza-Cózatl D.G., Schroeder J.I. Feedback inhibition by thiols outranks glutathione depletion: A luciferase-based screen reveals glutathione-deficient γ-ECS and glutathione synthetase mutants impaired in cadmium-induced sulfate assimilation. Plant J. 2012;70:783–795. doi: 10.1111/j.1365-313X.2012.04924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Li Y., Dankher O.P., Carreira L., Smith A.P., Meagher R.B. The shoot-specific expression of γ-glutamylcysteine synthetase directs the long-distance transport of thiol-peptides to roots conferring tolerance to mercury and arsenic. Plant Physiol. 2006;141:288–298. doi: 10.1104/pp.105.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhu Y.L., Pilon-Smits E.A.H., Jouanin L., Terry N. Overexpression of glutathione synthetase in Indian mustard enhances cadmium accumulation and tolerance. Plant Physiol. 1999;119:73–80. doi: 10.1104/pp.119.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhu Y.L., Pilon-Smits E.A.H., Tarun A.S., Weber S.U., Jouanin L., Terry N. Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing γ-glutamylcysteine synthetase. Plant Physiol. 1999;121:1169–1177. doi: 10.1104/pp.121.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rea P.A. Phytochelatin synthase: Of a protease a peptide polymerase made. Physiol Plant. 2012;145:154–164. doi: 10.1111/j.1399-3054.2012.01571.x. [DOI] [PubMed] [Google Scholar]
- 213.Li M., Stragliati L., Bellini E., Ricci A., Saba A., di Toppi L.S., Varotto C. Evolution and functional differentiation of recently diverged Phytochelatin Synthase genes from Arundo donax L. J. Exp. Bot. 2019;70:5391–5405. doi: 10.1093/jxb/erz266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Marques D.N., Gaziola S.A., Azevedo R.A. Phytochelatins and their relationship with modulation of cadmium tolerance in plants. In: Hasanuzzaman M., Prasad M.N.V., editors. Handbook of Bioremediation. Physiological, Molecular and Biotechnological Interventions. Elsevier Inc.; Amsterdam, The Netherlands: 2021. pp. 91–152. [DOI] [Google Scholar]
- 215.Kühnlenz T., Schmidt H., Uraguchi S., Clemens S. Arabidopsis thaliana phytochelatin synthase 2 is constitutively active in vivo and can rescue the growth defect of the PCS1-Deficient cad1-3 mutant on Cd-contaminated soil. J. Exp. Bot. 2014;65:4241–4253. doi: 10.1093/jxb/eru195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hasan M.K., Cheng Y., Kanwar M.K., Chu X.-Y., Ahammed G.J., Qi Z.-Y. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017;8:1492. doi: 10.3389/fpls.2017.01492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Grill E., Löffler S., Winnacker E.-L., Zenk M.H. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (Phytochelatin synthase) Proc. Natl. Acad. Sci. USA. 1989;86:6838–6842. doi: 10.1073/pnas.86.18.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Fontanini D., Andreucci A., Castiglione M.R., Basile A., Sorbo S., Petraglia A., Degola F., Bellini E., Bruno L., Varotto C., et al. The phytochelatin synthase from Nitella mucronata (charophyta) plays a role in the homeostatic control of iron(II)/(III) Plant Physiol. Biochem. 2018;127:88–96. doi: 10.1016/j.plaphy.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 219.Ruotolo R., Peracchi A., Bolchi A., Infusini G., Amoresano A., Ottonello S. Domain organization of phytochelatin synthase. Functional properties of truncated enzyme species identified by limited proteolysis. J. Biol. Chem. 2004;279:14686–14693. doi: 10.1074/jbc.M314325200. [DOI] [PubMed] [Google Scholar]
- 220.Filiz E., Saracoglu I.A., Ozyigit I.I., Yalcin B. Comparative analyses of phytochelatin synthase (PCS) genes in higher plants. Biotechnol. Biotechnol. Equip. 2019;33:178–194. doi: 10.1080/13102818.2018.1559096. [DOI] [Google Scholar]
- 221.Uraguchi S., Sone Y., Ohta Y., Ohkama-Ohtsu N., Hofmann C., Hess N., Nakamura R., Takanezawa Y., Clemens S., Kiyono M. Identification of C-terminal regions in Arabidopsis thaliana phytochelatin synthase 1 specifically involved in activation by arsenite. Plant Cell Physiol. 2018;59:500–509. doi: 10.1093/pcp/pcx204. [DOI] [PubMed] [Google Scholar]
- 222.García-García J.D., Sánchez-Thomas R., Saavedra E., Fernández-Velasco D.A., Romero-Romero S., Casanova-Figueroa K.I., Mendoza-Cózatl D.G., Moreno-Sánchez R. Mapping the metal-catalytic site of a zinc-activated phytochelatin synthase. Algal Res. 2020;47:101890. doi: 10.1016/j.algal.2020.101890. [DOI] [Google Scholar]
- 223.Hayashi S., Tanikawa H., Kuramata M., Abe T., Ishikawa S. Domain exchange between Oryza sativa phytochelatin synthases reveals a region that determines responsiveness to arsenic and heavy metals. Biochem. Biophys. Res. Commun. 2020;523:548–553. doi: 10.1016/j.bbrc.2019.12.093. [DOI] [PubMed] [Google Scholar]
- 224.Romanyuk N.D., Rigden D.J., Vatamaniuk O.K., Lang A., Cahoon R.E., Jez J.M., Rea P.A. Mutagenic definition of a papain-like catalytic triad, sufficiency of the N-terminal domain for single-site core catalytic enzyme acylation, and C-terminal domain for augmentative metal activation of a eukaryotic phytochelatin synthase. Plant Physiol. 2006;141:858–869. doi: 10.1104/pp.106.082131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ha S.-B., Smith A.P., Howden R., Dietrich W.M., Bugg S., O’Connell M.J., Goldsbrough P.B., Cobbett C.S. Phytochelatin synthase genes from arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell. 1999;11:1153–1163. doi: 10.1105/tpc.11.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Su H., Zou T., Lin R., Zheng J., Jian S., Zhang M. Characterization of a phytochelatin synthase gene from Ipomoea pes-caprae involved in cadmium tolerance and accumulation in yeast and plants. Plant Physiol. Biochem. 2020;155:743–755. doi: 10.1016/j.plaphy.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 227.Kamiya T., Fujiwara T. A Novel allele of the Arabidopsis phytochelatin synthase 1 gene conferring high sensitivity to arsenic and antimony. Soil Sci. Plant Nutr. 2011;57:272–278. doi: 10.1080/00380768.2011.576398. [DOI] [Google Scholar]
- 228.Blum R., Meyer K.C., Wünschmann J., Lendzian K.J., Grill E. Cytosolic action of phytochelatin synthase. Plant Physiol. 2010;153:159–169. doi: 10.1104/pp.109.149922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hématy K., Lim M., Cherk C., Piślewska-Bednarek M., Sanchez-Rodriguez C., Stein M., Fuchs R., Klapprodt C., Lipka V., Molina A., et al. Moonlighting function of phytochelatin synthase1 in extracellular defense against fungal pathogens. Plant Physiol. 2020;182:1920–1932. doi: 10.1104/pp.19.01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jin D., Zhang Q., Liu Y., Liang M., Li A., Wu J. Overexpression of the maize phytochelatin synthase gene (ZmPCS1) enhances Cd tolerance in plants. Acta Physiol. Plant. 2022;44:114. doi: 10.1007/s11738-022-03451-1. [DOI] [Google Scholar]
- 231.Shine A.M., Shakya V.P.S., Idnurm A. Phytochelatin synthase is required for tolerating metal toxicity in a basidiomycete yeast and is a conserved factor involved in metal homeostasis in fungi. Fungal Biol. Biotechnol. 2015;2:3. doi: 10.1186/s40694-015-0013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kolahi M., Yazdi M., Goldson-Barnaby A., Tabandeh M.R. In silico prediction, phylogenetic and bioinformatic analysis of SoPCS gene, survey of its protein characterization and gene expression in response to cadmium in Saccharum officinarum. Ecotoxicol. Environ. Saf. 2018;163:7–18. doi: 10.1016/j.ecoenv.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 233.Hayashi S., Kuramata M., Abe T., Takagi H., Ozawa K., Ishikawa S. Phytochelatin synthase OsPCS1 plays a crucial role in reducing arsenic levels in rice grains. Plant J. 2017;91:840–848. doi: 10.1111/tpj.13612. [DOI] [PubMed] [Google Scholar]
- 234.Ding G., Peng J., Zhang G., Yi H., Fu Y., Gong J.M. Regulation of the phytochelatin synthase gene AtPCS2 in Arabidopsis thaliana. Scientia Sinica Vitae. 2013;43:1112. doi: 10.1360/052013-309. [DOI] [Google Scholar]
- 235.Hirata K., Tsuji N., Miyamoto K. Biosynthetic regulation of phytochelatins, heavy metal-binding peptides. J. Biosci. Bioeng. 2005;100:593–599. doi: 10.1263/jbb.100.593. [DOI] [PubMed] [Google Scholar]
- 236.Olsson S., Penacho V., Puente-Sánchez F., Díaz S., Gonzalez-Pastor J.E., Aguilera A. Horizontal gene transfer of phytochelatin synthases from bacteria to extremophilic green algae. Microb. Ecol. 2017;73:50–60. doi: 10.1007/s00248-016-0848-z. [DOI] [PubMed] [Google Scholar]
- 237.Vatamaniuk O.K., Mari S., Lu Y.P., Rea P.A. Mechanism of heavy metal ion activation of phytochelatin (PC) synthase: Blocked thiols are sufficient for PC synthase-catalyzed transpeptidation of glutathione and related thiol peptides. J. Biol. Chem. 2000;275:31451–31459. doi: 10.1074/jbc.M002997200. [DOI] [PubMed] [Google Scholar]
- 238.Clemens S. Evolution and function of phytochelatin synthases. J. Plant Physiol. 2006;163:319–332. doi: 10.1016/j.jplph.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 239.Maier T., Yu C., Küllertz G., Clemens S. Localization and functional characterization of metal-binding sites in phytochelatin synthases. Planta. 2003;218:300–308. doi: 10.1007/s00425-003-1091-7. [DOI] [PubMed] [Google Scholar]
- 240.Uraguchi S., Tanaka N., Hofmann C., Abiko K., Ohkama-Ohtsu N., Weber M., Kamiya T., Sone Y., Nakamura R., Takanezawa Y., et al. Phytochelatin synthase has contrasting effects on cadmium and arsenic accumulation in rice grains. Plant Cell Physiol. 2017;58:1730–1742. doi: 10.1093/pcp/pcx114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Loscos J., Naya L., Ramos J., Clemente M.R., Matamoros M.A., Becana M. A reassessment of substrate specificity and activation of phytochelatin synthases from model plants by physiologically relevant metals. Plant Physiol. 2006;140:1213–1221. doi: 10.1104/pp.105.073635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Blum R., Beck A., Korte A., Stengel A., Letzel T., Lendzian K., Grill E. Function of phytochelatin synthase in catabolism of glutathione-conjugates. Plant J. 2007;49:740–749. doi: 10.1111/j.1365-313X.2006.02993.x. [DOI] [PubMed] [Google Scholar]
- 243.Fan W., Guo Q., Liu C.Y., Liu X., Zhang M., Long D., Xiang Z., Zhao A. Two mulberry phytochelatin synthase genes confer zinc/cadmium tolerance and accumulation in transgenic Arabidopsis and tobacco. Gene. 2018;645:95–104. doi: 10.1016/j.gene.2017.12.042. [DOI] [PubMed] [Google Scholar]
- 244.Cazalé A.-C., Clemens S. Arabidopsis thaliana expresses a second functional phytochelatin synthase. FEBS Lett. 2001;507:215–219. doi: 10.1016/S0014-5793(01)02976-3. [DOI] [PubMed] [Google Scholar]
- 245.Manara A. Plant responses to heavy metal toxicity. In: Furini A., editor. Plants and Heavy Metals. Springer; Dordrecht, The Netherlands: 2012. pp. 27–53. Springer Briefs in Molecular Science. [DOI] [Google Scholar]
- 246.Wang H.-C., Wu J.-S., Chia J.-C., Yang C.-C., Wu Y.-J., Juang R.-H. Phytochelatin synthase is regulated by protein phosphorylation at a threonine residue near its catalytic site. J. Agric. Food Chem. 2009;57:7348–7355. doi: 10.1021/jf9020152. [DOI] [PubMed] [Google Scholar]
- 247.Parihar P., Singh S., Singh R., Rajasheker G., Rathnagiri P., Srivastava R.K., Singh V.P., Suprasanna P., Prasad S.M., Kavi Kishor P.B. An integrated transcriptomic, proteomic, and metabolomic approach to unravel the molecular mechanisms of metal stress tolerance in plants. In: Srivastava S., Srivastava A., Suprasanna P., editors. Plant-Metal Interactions. Springer; Cham, Switzerland: 2019. pp. 1–28. [DOI] [Google Scholar]
- 248.Clemens S., Kim E.J., Neumann D., Schroeder J.I. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J. 1999;18:3325–3333. doi: 10.1093/emboj/18.12.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Vatamaniuk O.K., Bucher E.A., Ward J.T., Rea P.A. A new pathway for heavy metal detoxification in animals. Phytochelatin synthase is required for cadmium tolerance in Caenorhabditis elegans. J. Biol. Chem. 2001;276:20817–20820. doi: 10.1074/jbc.C100152200. [DOI] [PubMed] [Google Scholar]
- 250.Rigouin C., Vermeire J.J., Nylin E., Williams D.L. Characterization of the phytochelatin synthase from the human parasitic nematode Ancylostoma ceylanicum. Mol. Biochem. Parasitol. 2013;191:1–6. doi: 10.1016/j.molbiopara.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Franchi N., Piccinni E., Ferro D., Basso G., Spolaore B., Santovito G., Ballarin L. Characterization and transcription studies of a phytochelatin synthase gene from the solitary tunicate Ciona intestinalis exposed to cadmium. Aquat. Toxicol. 2014;152:47–56. doi: 10.1016/j.aquatox.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 252.Vatamaniuk O.K., Mari S., Lu Y.-P., Rea P.A. AtPCS1, a phytochelatin synthase from Arabidopsis: Isolation and in vitro reconstitution. Proc. Natl. Acad. Sci. USA. 1999;96:7110–7115. doi: 10.1073/pnas.96.12.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Dong R., Formentin E., Losseso C., Carimi F., Benedetti P., Terzi M., Lo Schiavo F. Molecular cloning and characterization of a phytochelatin synthase gene, PvPCS1, from Pteris vittata L. J. Ind. Microbiol. Biotechnol. 2005;32:527–533. doi: 10.1007/s10295-005-0234-1. [DOI] [PubMed] [Google Scholar]
- 254.Guo J., Dai X., Xu W., Ma M. Overexpressing GSH1 and AsPCS1 simultaneously increases the tolerance and accumulation of cadmium and arsenic in Arabidopsis thaliana. Chemosphere. 2008;72:1020–1026. doi: 10.1016/j.chemosphere.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 255.Zayneb C., Imen R.H., Walid K., Grubb C.D., Bassem K., Franck V., Hafedh M., Amine E. The phytochelatin synthase gene in date palm (Phoenix dactylifera L.): Phylogeny, evolution and expression. Ecotoxicol. Environ. Saf. 2017;140:7–17. doi: 10.1016/j.ecoenv.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 256.Azizi N., Shahpiri A. Functional characterization of Helianthus annuus phytochelatin synthase (HaPCS): Gene expression and protein profiles of HaPCS responding to arsenic and evaluation of arsenic accumulation in engineered bacteria expressing HaPCS. Environ. Exp. Bot. 2021;187:104470. doi: 10.1016/j.envexpbot.2021.104470. [DOI] [Google Scholar]
- 257.He Z., Li J., Zhang H., Ma M. Different effects of calcium and lanthanum on the expression of phytochelatin synthase gene and cadmium absorption in Lactuca sativa. Plant Sci. 2005;168:309–318. doi: 10.1016/j.plantsci.2004.07.001. [DOI] [Google Scholar]
- 258.Yazdi M., Kolahi M., Kazemi E.M., Barnaby A.G. Study of the contamination rate and change in growth features of lettuce (Lactuca sativa Linn.) in response to cadmium and a survey of its phytochelatin synthase gene. Ecotoxicol. Environ. Saf. 2019;180:295–308. doi: 10.1016/j.ecoenv.2019.04.071. [DOI] [PubMed] [Google Scholar]
- 259.Zha Y.Q., Zhang K.K., Pan F., Liu X., Han S.M., Guan P. Cloning of PCS gene (TpPCS1) from Tagetes patula L. and expression analysis under cadmium stress. Plant Biol. 2021;23:508–516. doi: 10.1111/plb.13207. [DOI] [PubMed] [Google Scholar]
- 260.Lee S., Korban S.S. Transcriptional regulation of Arabidopsis thaliana phytochelatin synthase (AtPCS1) by cadmium during early stages of plant development. Planta. 2002;215:689–693. doi: 10.1007/s00425-002-0821-6. [DOI] [PubMed] [Google Scholar]
- 261.Bai J., Wang X., Wang R., Wang J., Le S., Zhao Y. Overexpression of three duplicated BnPCS genes enhanced Cd accumulation and translocation in Arabidopsis thaliana mutant Cad1–3. Bull. Environ. Contam. Toxicol. 2019;102:146–152. doi: 10.1007/s00128-018-2487-1. [DOI] [PubMed] [Google Scholar]
- 262.Mizuno T., Sonoda T., Horie K., Senoo K., Tanaka A., Mizuno N., Obata H. Cloning and characterization of phytochelatin synthase from a nickel hyperaccumulator Thlaspi japonicum and its expression in yeast. Soil Sci. Plant Nutr. 2003;49:285–290. doi: 10.1080/00380768.2003.10410009. [DOI] [Google Scholar]
- 263.Cong M., Zhao J., Lü J., Ren Z., Wu H. Homologous cloning, characterization and expression of a new halophyte phytochelatin synthase gene in Suaeda salsa. Chin. J. Oceanol. Limnol. 2016;34:1034–1043. doi: 10.1007/s00343-016-4382-0. [DOI] [Google Scholar]
- 264.Ramos J., Clemente M.R., Naya L., Loscos J., Pérez-Rontomé C., Sato S., Tabata S., Becana M. Phytochelatin synthases of the model legume Lotus japonicus. A small multigene family with differential response to cadmium and alternatively spliced variants. Plant Physiol. 2007;143:1110–1118. doi: 10.1104/pp.106.090894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Rahman M.A., Kabir A.H., Mandal A., Roy S.K., Song Y., Ji H.C., Lee K.-W. Glutathione restores Hg-induced morpho-physiological retardations by inducing phytochelatin and oxidative defense in alfalfa. Biology. 2020;9:364. doi: 10.3390/biology9110364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Das U., Rahman M.A., Ela E.J., Lee K.-W., Kabir A.H. Sulfur triggers glutathione and phytochelatin accumulation causing excess Cd bound to the cell wall of roots in alleviating Cd-toxicity in alfalfa. Chemosphere. 2021;262:128361. doi: 10.1016/j.chemosphere.2020.128361. [DOI] [PubMed] [Google Scholar]
- 267.Liu Z., Gu C., Chen F., Yang D., Wu K., Chen S., Jiang J., Zhang Z. Heterologous expression of a Nelumbo nucifera phytochelatin synthase gene enhances cadmium tolerance in Arabidopsis thaliana. Appl. Biochem. Biotechnol. 2012;166:722–734. doi: 10.1007/s12010-011-9461-2. [DOI] [PubMed] [Google Scholar]
- 268.Li J., Guo J., Xu W., Ma M. Enhanced cadmium accumulation in transgenic tobacco expressing the phytochelatin synthase gene of Cynodon dactylon L. J. Integr. Plant Biol. 2006;48:928–937. doi: 10.1111/j.1744-7909.2006.00314.x. [DOI] [Google Scholar]
- 269.Shen G.-M., Zhu C., Du Q.-Z. Genome-wide identification of PHYTOCHELATIN and PHYTOCH_SYNTH domain-containing phytochelatin family from rice. Electron. J. Biol. 2010;6:73–79. [Google Scholar]
- 270.Das N., Bhattacharya S., Bhattacharyya S., Maiti M.K. Identification of alternatively spliced transcripts of rice phytochelatin synthase 2 gene OsPCS2 involved in mitigation of cadmium and arsenic stresses. Plant Mol. Biol. 2017;94:167–183. doi: 10.1007/s11103-017-0600-1. [DOI] [PubMed] [Google Scholar]
- 271.Park H.C., Hwang J.E., Jiang Y., Kim Y.J., Kim S.H., Nguyen X.C., Kim C.Y., Chung W.S. Functional characterisation of two phytochelatin synthases in rice (Oryza sativa cv. Milyang 117) that respond to cadmium stress. Plant Biol. 2019;21:854–861. doi: 10.1111/plb.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chen Y., Chen C., Tan Z., Liu J., Zhuang L., Yang Z., Huang B. Functional identification and characterization of genes cloned from halophyte seashore paspalum conferring salinity and cadmium tolerance. Front. Plant Sci. 2016;7:102. doi: 10.3389/fpls.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zhao C., Xu J., Li Q., Li S., Wang P., Xiang F. Cloning and characterization of a Phragmites australis phytochelatin synthase (PaPCS) and achieving Cd tolerance in tall fescue. PLoS ONE. 2014;9:103771. doi: 10.1371/journal.pone.0103771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Yousefi Z., Kolahi M., Majd A., Jonoubi P. Effect of cadmium on morphometric traits, antioxidant enzyme activity and phytochelatin synthase gene expression (SoPCS) of Saccharum officinarum Var. cp48–103 in vitro. Ecotoxicol. Environ. Saf. 2018;157:472–481. doi: 10.1016/j.ecoenv.2018.03.076. [DOI] [PubMed] [Google Scholar]
- 275.Rahman M.M., Swaraz A.M., El-Shehawi A.M., Elseehy M.M., Alam M.F., Kabir A.H. The mechanistic basis of sulfur-mediated alleviation of Pb toxicity in wheat. Gesunde Pflanz. 2022;74:571–581. doi: 10.1007/s10343-022-00632-3. [DOI] [Google Scholar]
- 276.Jiang Q.Q., Sun X.L., Cao H., Zou Y.M., Shu H.R. Isolation and expression analysis of a phytochelatin synthase gene (MhPCS) from Malus hupehensis. J. Fruit Sci. 2013;3:341–347. [Google Scholar]
- 277.Chen Y., Liu Y., Ding Y., Wang X., Xu J. Overexpression of PtPCS enhances cadmium tolerance and cadmium accumulation in tobacco. Plant Cell Tissue Organ. Cult. 2015;121:389–396. doi: 10.1007/s11240-015-0710-x. [DOI] [Google Scholar]
- 278.Adams J.P., Adeli A., Hsu C.Y., Harkess R.L., Page G.P., Depamphilis C.W., Schultz E.B., Yuceer C. Poplar maintains zinc homeostasis with heavy metal genes HMA4 and PCS1. J. Exp. Bot. 2011;62:3737–3752. doi: 10.1093/jxb/err025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Lee B.D., Hwang S. Tobacco phytochelatin synthase (NtPCS1) plays important roles in cadmium and arsenic tolerance and in early plant development in tobacco. Plant Biotechnol. Rep. 2015;9:107–114. doi: 10.1007/s11816-015-0348-5. [DOI] [Google Scholar]
- 280.Klsa D. Responses of phytochelatin and proline-related genes expression associated with heavy metal stress in Solanum lycopersicum. Acta Bot. Croat. 2019;78:9–16. doi: 10.2478/botcro-2018-0023. [DOI] [Google Scholar]
- 281.Bellini E., Betti C., di Toppi L.S. Responses to cadmium in early-diverging streptophytes (Charophytes and bryophytes): Current views and potential applications. Plants. 2021;10:770. doi: 10.3390/plants10040770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Chen Y., Wang H.Y., Chen Y.F. The transcription factor MYB40 is a central regulator in arsenic resistance in Arabidopsis. Plant Commun. 2021;2:100234. doi: 10.1016/j.xplc.2021.100234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Helaoui S., Boughattas I., Hattab S., Mkhinini M., Alphonse V., Livet A., Bousserrhine N., Banni M. Physiological, biochemical and transcriptomic responses of Medicago sativa to nickel exposure. Chemosphere. 2020;249:126121. doi: 10.1016/j.chemosphere.2020.126121. [DOI] [PubMed] [Google Scholar]
- 284.Yu X.-Z., Ling Q.-L., Li Y.-H., Lin Y.-J. mRNA analysis of genes encoded with phytochelatin synthase (PCS) in rice seedlings exposed to chromium: The role of phytochelatins in Cr detoxification. Bull. Environ. Contam. Toxicol. 2018;101:257–261. doi: 10.1007/s00128-018-2362-0. [DOI] [PubMed] [Google Scholar]
- 285.Talebi M., Tabatabaei B.E.S., Akbarzadeh H. Hyperaccumulation of Cu, Zn, Ni, and Cd in Azolla species inducing expression of methallothionein and phytochelatin synthase genes. Chemosphere. 2019;230:488–497. doi: 10.1016/j.chemosphere.2019.05.098. [DOI] [PubMed] [Google Scholar]
- 286.Lu Y., Wang Q.-F., Li J., Xiong J., Zhou L.-N., He S.-L., Zhang J.-Q., Chen Z.-A., He S.-G., Liu H. Effects of exogenous sulfur on alleviating cadmium stress in tartary buckwheat. Sci. Rep. 2019;9:7397. doi: 10.1038/s41598-019-43901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Pomponi M., Censi V., Di Girolamo V., De Paolis A., di Toppi L.S., Aromolo R., Costantino P., Cardarelli M. Overexpression of arabidopsis phytochelatin synthase in tobacco plants enhances Cd2+ tolerance and accumulation but not translocation to the shoot. Planta. 2006;223:180–190. doi: 10.1007/s00425-005-0073-3. [DOI] [PubMed] [Google Scholar]
- 288.Gasic K., Korban S.S. Transgenic Indian mustard (Brassica juncea) plants expressing an Arabidopsis phytochelatin synthase (AtPCS1) Exhibit enhanced as and Cd tolerance. Plant Mol. Biol. 2007;64:361–369. doi: 10.1007/s11103-007-9158-7. [DOI] [PubMed] [Google Scholar]
- 289.Wojas S., Clemens S., Hennig J., Skłodowska A., Kopera E., Schat H., Bal W., Antosiewicz D.M. Overexpression of phytochelatin synthase in tobacco: Distinctive effects of AtPCS1 and CePCS genes on plant response to cadmium. J. Exp. Bot. 2008;59:2205–2219. doi: 10.1093/jxb/ern092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wojas S., Ruszczyńska A., Ewa B., Clemens S., Antosiewicz D.M. The role of subcellular distribution of cadmium and phytochelatins in the generation of distinct phenotypes of AtPCS1- and CePCS3-expressing tobacco. J. Plant Physiol. 2010;167:981–988. doi: 10.1016/j.jplph.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 291.de Benedictis M., Brunetti C., Brauer E.K., Andreucci A., Popescu S.C., Commisso M., Guzzo F., Sofo A., Castiglione M.R., Vatamaniuk O.K., et al. The Arabidopsis thaliana knockout mutant for Phytochelatin Synthase1 (cad1-3) is defective in callose deposition, bacterial pathogen defense and auxin content, but shows an increased stem lignification. Front. Plant Sci. 2018;9:19. doi: 10.3389/fpls.2018.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Vatamaniuk O.K., Mari S., Lang A., Chalasani S., Demkiv L.O., Rea P.A. Phytochelatin synthase, a dipeptidyltransferase that undergoes multisite acylation with γ-glutamylcysteine during catalysis. Stoichiometric and site-directed mutagenic analysis of Arabidopsis thaliana PCS1-catalyzed phytochelatin synthesis. J. Biol. Chem. 2004;279:22449–22460. doi: 10.1074/jbc.M313142200. [DOI] [PubMed] [Google Scholar]
- 293.Inoue R., Nakamura N., Matsumoto C., Takase H., Sekiya J., Prieto R. Characterization of γ-glutamyltransferase- and phytochelatin synthase-mediated catabolism of glutathione and glutathione S-conjugates in Arabidopsis thaliana. Plant Biotechnol. 2022;39:381–389. doi: 10.5511/plantbiotechnology.22.1003a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Clay N.K., Adio A.M., Denoux C., Jander G., Ausubel F.M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science. 2009;323:95–101. doi: 10.1126/science.1164627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kim Y.-O., Kang H., Ahn S.-J. Overexpression of phytochelatin synthase AtPCS2 enhances salt tolerance in Arabidopsis thaliana. J. Plant Physiol. 2019;240:153011. doi: 10.1016/j.jplph.2019.153011. [DOI] [PubMed] [Google Scholar]
- 296.Pál M., Janda T., Szalai G. Interactions between plant hormones and thiol-related heavy metal chelators. Plant Growth Regul. 2018;85:173–185. doi: 10.1007/s10725-018-0391-7. [DOI] [Google Scholar]
- 297.Begum M.C., Islam M., Sarkar M.R., Azad M.A.S., Huda A.K.M.N., Kabir A.H. Auxin signaling is closely associated with Zn-efficiency in rice (Oryza sativa L.) J. Plant Interact. 2016;11:124–129. doi: 10.1080/17429145.2016.1220026. [DOI] [Google Scholar]
- 298.Mohan T.C., Castrillo G., Navarro C., Zarco-Fernández S., Ramireddy E., Mateo C., Zamarreño A.M., Paz-Ares J., Muñoz R., García-Mina J.M., et al. Cytokinin determines thiol-mediated arsenic tolerance and accumulation. Plant Physiol. 2016;171:1418–1426. doi: 10.1104/pp.16.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Stroiński A., Chadzinikolau T., Giżewska K., Zielezińska M. ABA or cadmium induced phytochelatin synthesis in potato tubers. Biol. Plant. 2010;54:117–120. doi: 10.1007/s10535-010-0017-z. [DOI] [Google Scholar]
- 300.Stroiński A., Giżewska K., Zielezińska M. Abscisic acid is required in transduction of cadmium signal to potato roots. Biol. Plant. 2013;57:121–127. doi: 10.1007/s10535-012-0135-x. [DOI] [Google Scholar]
- 301.Clemens S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta. 2001;212:475–486. doi: 10.1007/s004250000458. [DOI] [PubMed] [Google Scholar]
- 302.Schat H., Llugany M., Vooijs R., Hartley-Whitaker J., Bleeker P.M. The role of phytochelatins in constitutive and adaptive heavy metal tolerances in hyperaccumulator and non-hyperaccumulator metallophytes. J. Exp. Bot. 2002;53:2381–2392. doi: 10.1093/jxb/erf107. [DOI] [PubMed] [Google Scholar]
- 303.Clemens S., Simm C. Schizosaccharomyces pombe as a model for metal homeostasis in plant cells: The phytochelatin-dependent pathway is the main cadmium detoxification mechanism. N. Phytol. 2003;159:323–330. doi: 10.1046/j.1469-8137.2003.00811.x. [DOI] [PubMed] [Google Scholar]
- 304.Park J., Song W.-Y., Ko D., Eom Y., Hansen T.H., Schiller M., Lee T.G., Martinoia E., Lee Y. The Phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012;69:278–288. doi: 10.1111/j.1365-313X.2011.04789.x. [DOI] [PubMed] [Google Scholar]
- 305.Wójcik M., Skórzyńska-Polit E., Tukiendorf A. Organic acids accumulation and antioxidant enzyme activities in Thlaspi caerulescens under Zn and Cd stress. Plant Growth Regul. 2006;48:145–155. doi: 10.1007/s10725-005-5816-4. [DOI] [Google Scholar]
- 306.Huguet S., Bert V., Laboudigue A., Barthès V., Isaure M.-P., Llorens I., Schat H., Sarret G. Cd speciation and localization in the hyperaccumulator Arabidopsis halleri. Environ. Exp. Bot. 2012;82:54–65. doi: 10.1016/j.envexpbot.2012.03.011. [DOI] [Google Scholar]
- 307.Wójcik M., Dresler S., Plak A., Tukiendorf A. Naturally evolved enhanced Cd tolerance of Dianthus carthusianorum L. is not related to accumulation of thiol peptides and organic acids. Environ. Sci. Pollut. Res. 2015;22:7906–7917. doi: 10.1007/s11356-014-3963-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Meyer C.-L., Verbruggen N. The use of the model species Arabidopsis halleri towards phytoextraction of cadmium polluted soils. N. Biotechnol. 2012;30:9–14. doi: 10.1016/j.nbt.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 309.Ernst W.H.O., Krauss G.-J., Verkleij J.A.C., Wesenberg D. Interaction of heavy metals with the sulphur metabolism in angiosperms from an ecological point of view. Plant Cell Environ. 2008;31:123–143. doi: 10.1111/j.1365-3040.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 310.Küpper H., Mijovilovich A., Meyer-Klaucke W., Kroneck P.M.H. Tissue- and age-dependent differences in the complexation of cadmium and zinc in the cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges ecotype) revealed by X-ray absorption spectroscopy. Plant Physiol. 2004;134:748–757. doi: 10.1104/pp.103.032953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Ueno D., Ma J.F., Iwashita T., Zhao F.-J., McGrath S.P. Identification of the form of Cd in the leaves of a superior Cd-accumulating ecotype of Thlaspi caerulescens using 113Cd-NMR. Planta. 2005;221:928–936. doi: 10.1007/s00425-005-1491-y. [DOI] [PubMed] [Google Scholar]
- 312.Vogel-Mikuš K., Arčon I., Kodre A. Complexation of cadmium in seeds and vegetative tissues of the cadmium hyperaccumulator Thlaspi praecox as studied by X-ray absorption spectroscopy. Plant Soil. 2010;331:439–451. doi: 10.1007/s11104-009-0264-y. [DOI] [Google Scholar]
- 313.Isaure M.-P., Huguet S., Meyer C.-L., Castillo-Michel H., Testemale D., Vantelon D., Saumitou-Laprade P., Verbruggen N., Sarret G. Evidence of various mechanisms of Cd sequestration in the hyperaccumulator Arabidopsis halleri, the non-accumulator Arabidopsis lyrata, and their progenies by combined synchrotron-based techniques. J. Exp. Bot. 2015;66:3201–3214. doi: 10.1093/jxb/erv131. [DOI] [PubMed] [Google Scholar]
- 314.Tian S., Lu L., Labavitch J., Yang X., He Z., Hu H., Sarangi R., Newville M., Commisso J., Brown P. Cellular sequestration of cadmium in the hyperaccumulator plant species Sedum alfredii. Plant Physiol. 2011;157:1914–1925. doi: 10.1104/pp.111.183947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Van der Ent A., Vinya R., Erskine P.D., Malaisse F., Przybyłowicz W.J., Barnabas A.D., Harris H.H., Mesjasz-Przybyłowicz J. Elemental distribution and chemical speciation of copper and cobalt in three metallophytes from the copper-cobalt belt in Northern Zambia. Metallomics. 2020;12:682–701. doi: 10.1039/c9mt00263d. [DOI] [PubMed] [Google Scholar]
- 316.Raza A., Habib M., Kakavand S.N., Zahid Z., Zahra N., Sharif R., Hasanuzzaman M. Phytoremediation of cadmium: Physiological, biochemical, and molecular mechanisms. Biology. 2020;9:177. doi: 10.3390/biology9070177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Speiser D.M., Ortiz D.F., Kreppel L., Scheel G., Mcdonald G., Ow D.W. Purine biosynthetic genes are required for cadmium tolerance in Schizosaccharomyces pombe. Mol. Cell Biol. 1992;12:5301–5310. doi: 10.1128/mcb.12.12.5301-5310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Reese R.N., White C.A., Winge D.R. Cadmium-sulfide crystallites in Cd-(γEC)nG peptide complexes from tomato. Plant Physiol. 1992;98:225–229. doi: 10.1104/pp.98.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Morelli E., Cruz B.H., Somovigo S., Scarano G. Speciation of cadmium—γ-glutamyl peptides complexes in cells of the marine microalga Phaeodactylum tricornutum. Plant Sci. 2002;163:807–813. doi: 10.1016/S0168-9452(02)00216-9. [DOI] [Google Scholar]
- 320.Juang R.H., McCue K.F., Ow D.W. Two purine biosynthetic enzymes that are required for cadmium tolerance in Schizosaccharomyces pombe utilize cysteine sulfinate in vitro. Arch. Biochem. Biophys. 1993;304:392–401. doi: 10.1006/abbi.1993.1367. [DOI] [PubMed] [Google Scholar]
- 321.Ortiz D.F., Kreppel L., Speiser D.M., Scheel G., McDonald G., Ow D.W. Heavy metal tolerance in the fission yeast requires an ATP-binding cassette-type vacuolar membrane transporter. EMBO J. 1992;11:3491–3499. doi: 10.1002/j.1460-2075.1992.tb05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Ortiz D.F., Ruscitti T., McCue K.F., Ow D.W. Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein. J. Biol. Chem. 1995;270:4721–4728. doi: 10.1074/jbc.270.9.4721. [DOI] [PubMed] [Google Scholar]
- 323.Rea P.A., Li Z.S., Lu Y.P., Drozdowicz Y.M., Martinoia E. From vacuolar GS-X pumps to multispecific ABC transporters. Annu. Rev. Plant Biol. 1998;49:727–760. doi: 10.1146/annurev.arplant.49.1.727. [DOI] [PubMed] [Google Scholar]
- 324.Wanke D., Üner Kolukisaoglu H. An update on the ABCC transporter family in plants: Many genes, many proteins, but how many functions? Plant Biol. 2010;12:15–25. doi: 10.1111/j.1438-8677.2010.00380.x. [DOI] [PubMed] [Google Scholar]
- 325.Jalmi S.K. The role of ABC transporters in metal transport in plants. In: Kumar K., Srivastava S., editors. Plant Metal and Metalloid Transporters. Springer Nature; Berlin/Heidelberg, Germany: 2022. pp. 55–71. [DOI] [Google Scholar]
- 326.Thounaojam T.C., Khan Z., Meetei T.T., Srivastava S., Panda S.K., Upadhyaya H. Transporters: The molecular drivers of arsenic stress tolerance in plants. J. Plant Biochem. Biotechnol. 2021;30:730–743. doi: 10.1007/s13562-021-00748-z. [DOI] [Google Scholar]
- 327.Vatamaniuk O.K., Bucher E.A., Sundaram M.V., Rea P.A. CeHMT-1, a putative phytochelatin transporter, is required for cadmium tolerance in Caenorhabditis elegans. J. Biol. Chem. 2005;280:23684–23690. doi: 10.1074/jbc.M503362200. [DOI] [PubMed] [Google Scholar]
- 328.Sooksa-nguan T., Yakubov B., Kozlovskyy V.I., Barkume C.M., Howe K.J., Thannhauser T.W., Rutzke M.A., Hart J.J., Kochian L.V., Rea P.A., et al. Drosophila ABC transporter, DmHMT-1, confers tolerance to cadmium. DmHMT-1 and its yeast homolog, SpHMT-1, are not essential for vacuolar phytochelatin sequestration. J. Biol. Chem. 2009;284:354–362. doi: 10.1074/jbc.M806501200. [DOI] [PubMed] [Google Scholar]
- 329.Prévéral S., Gayet L., Moldes C., Hoffmann J., Mounicou S., Gruet A., Reynaud F., Lobinski R., Verbavatz J.-M., Vavasseur A., et al. A common highly conserved cadmium detoxification mechanism from bacteria to humans. heavy metal tolerance conferred by the ATP-binding cassette (ABC) transporter SpHMT1 requires glutathione but not metal-chelating phytochelatin peptides. J. Biol. Chem. 2009;284:4936–4943. doi: 10.1074/jbc.M808130200. [DOI] [PubMed] [Google Scholar]
- 330.Huang J., Zhang Y., Peng J.-S., Zhong C., Yi H.-Y., Ow D.W., Gong J.-M. Fission yeast HMT1 lowers seed cadmium through phytochelatin-dependent vacuolar sequestration in arabidopsis. Plant Physiol. 2012;158:1779–1788. doi: 10.1104/pp.111.192872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Li Z.-S., Szczypka M., Lu Y.-P., Thiele D.J., Rea P.A. The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J. Biol. Chem. 1996;271:6509–6517. doi: 10.1074/jbc.271.11.6509. [DOI] [PubMed] [Google Scholar]
- 332.Li Z.-S., Lu Y.-P., Zhen R.-G., Szczypka M., Thiele D.J., Rea P.A. A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc. Natl. Acad. Sci. USA. 1997;94:42–47. doi: 10.1073/pnas.94.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Song W.-Y., Sohn E.J., Martinoia E., Lee Y.J., Yang Y.-Y., Jasinski M., Forestier C., Hwang I., Lee Y. Engineering tolerance and accumulation of lead and cadmium in transgenic plants. Nat. Biotechnol. 2003;21:914–919. doi: 10.1038/nbt850. [DOI] [PubMed] [Google Scholar]
- 334.Gueldry O., Lazard M., Delort F., Dauplais M., Grigoras I., Blanquet S., Plateau P. Ycf1p-dependent Hg(II) detoxification in Saccharomyces cerevisiae. Eur. J. Biochem. 2003;270:2486–2496. doi: 10.1046/j.1432-1033.2003.03620.x. [DOI] [PubMed] [Google Scholar]
- 335.Ghosh M., Shen J., Rosen B.P. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1999;96:5001–5006. doi: 10.1073/pnas.96.9.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Bovet L., Feller U., Martinoia E. Possible involvement of plant ABC transporters in cadmium detoxification: A cDNA sub-microarray approach. Environ. Int. 2005;31:263–267. doi: 10.1016/j.envint.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 337.Dunkley T.P.J., Hester S., Shadforth I.P., Runions J., Weimar T., Hanton S.L., Griffin J.L., Bessant C., Brandizzi F., Hawes C., et al. Mapping the Arabidopsis organelle proteome. Proc. Natl. Acad. Sci. USA. 2006;103:6518–6523. doi: 10.1073/pnas.0506958103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Brunetti P., Zanella L., De Paolis A., Di Litta D., Cecchetti V., Falasca G., Barbieri M., Altamura M.M., Costantino P., Cardarelli M. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J. Exp. Bot. 2015;66:3815–3829. doi: 10.1093/jxb/erv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Bovet L., Eggmann T., Meylan-Bettex M., Polier J., Kammer P., Marin E., Feller U., Martinoia E. Transcript levels of AtMRPs after cadmium treatment: Induction of AtMRP3. Plant Cell Environ. 2003;26:371–381. doi: 10.1046/j.1365-3040.2003.00968.x. [DOI] [Google Scholar]
- 340.Song W.-Y., Park J., Mendoza-Cózatl D.G., Suter-Grotemeyer M., Shima D., Hörtensteiner S., Geisler M., Weder B., Rea P.A., Rentsch D., et al. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc. Natl. Acad. Sci. USA. 2010;107:21187–21192. doi: 10.1073/pnas.1013964107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Song W.-Y., Yamaki T., Yamaji N., Ko D., Jung K.H., Fujii-Kashino M., An G., Martinoia E., Lee Y., Ma J.F. A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc. Natl. Acad. Sci. USA. 2014;111:15699–15704. doi: 10.1073/pnas.1414968111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Zhang J., Hwang J.-U., Song W.-Y., Martinoia E., Lee Y. Identification of amino acid residues important for the arsenic resistance function of Arabidopsis ABCC1. FEBS Lett. 2017;591:656–666. doi: 10.1002/1873-3468.12576. [DOI] [PubMed] [Google Scholar]
- 343.Shukla D., Tiwari M., Tripathi R.D., Nath P., Trivedi P.K. Synthetic phytochelatins complement a phytochelatin-deficient Arabidopsis mutant and enhance the accumulation of heavy metal(loid)s. Biochem. Biophys. Res. Commun. 2013;434:664–669. doi: 10.1016/j.bbrc.2013.03.138. [DOI] [PubMed] [Google Scholar]
- 344.Wei Z.G., Wong J.W.C., Zhao H.Y., Zhang H.J., Li H.X., Hu F. Separation and determination of heavy metals associated with low molecular weight chelators in xylem saps of Indian mustard (Brassica juncea) by size exclusion chromatography and atomic absorption spectrometry. Biol. Trace Elem. Res. 2007;118:146–158. doi: 10.1007/s12011-007-0022-z. [DOI] [PubMed] [Google Scholar]
- 345.Mendoza-Cózatl D.G., Butko E., Springer F., Torpey J.W., Komives E.A., Kehr J., Schroeder J.I. Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J. 2008;54:249–259. doi: 10.1111/j.1365-313X.2008.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Mustroph A., Zanetti M.E., Jang C.J.H., Holtan H.E., Repetti P.P., Galbraith D.W., Girke T., Bailey-Serres J. Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2009;106:18843–18848. doi: 10.1073/pnas.0906131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Chen A., Komives E.A., Schroeder J.I. An improved grafting technique for mature arabidopsis plants demonstrates long-distance shoot-to-root transport of phytochelatins in arabidopsis. Plant Physiol. 2006;141:108–120. doi: 10.1104/pp.105.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Flis P., Ouerdane L., Grillet L., Curie C., Mari S., Lobinski R. Inventory of metal complexes circulating in plant fluids: A reliable method based on HPLC coupled with dual elemental and high-resolution molecular mass spectrometric detection. N. Phytol. 2016;211:1129–1141. doi: 10.1111/nph.13964. [DOI] [PubMed] [Google Scholar]
- 349.Deinlein U., Weber M., Schmidt H., Rensch S., Trampczynska A., Hansen T.H., Husted S., Schjoerring J.K., Talke I.N., Krämer U., et al. Elevated nicotianamine levels in Arabidopsis halleri roots play a key role in zinc hyperaccumulation. Plant Cell. 2012;24:708–723. doi: 10.1105/tpc.111.095000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Krężel A., Maret W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016;611:3–19. doi: 10.1016/j.abb.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Wongkaew A., Nakamura S.-I., Suzui N., Yin Y.-G., Ishii S., Kawachi N., Kojima K., Sekimoto H., Yokoyama T., Ohkama-Ohtsu N. Elevated glutathione synthesis in leaves contributes to zinc transport from roots to shoots in arabidopsis. Plant Sci. 2019;283:416–423. doi: 10.1016/j.plantsci.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 352.Wongkaew A., Asayama K., Kitaiwa T., Nakamura S.-I., Kojima K., Stacey G., Sekimoto H., Yokoyama T., Ohkama-Ohtsu N. AtOPT6 protein functions in long-distance transport of glutathione in Arabidopsis thaliana. Plant Cell Physiol. 2018;59:1443–1451. doi: 10.1093/pcp/pcy074. [DOI] [PubMed] [Google Scholar]
- 353.Tang Z., Chen Y., Miller A.J., Zhao F.-J. The C-type ATP-binding cassette transporter OsABCC7 is involved in the root-to-shoot translocation of arsenic in rice. Plant Cell Physiol. 2019;60:1525–1535. doi: 10.1093/pcp/pcz054. [DOI] [PubMed] [Google Scholar]
- 354.Keltjens W.G., van Beusichem M.L. Phytochelatins as biomarkers for heavy metal stress in maize (Zea mays L.) and wheat (Triticum aestivum L.): Combined effects of copper and cadmium. Plant Soil. 1998;203:119–126. doi: 10.1023/A:1004373700581. [DOI] [Google Scholar]
- 355.Dago À., González I., Ariño C., Díaz-Cruz J.M., Esteban M. Chemometrics applied to the analysis of induced phytochelatins in Hordeum vulgare plants stressed with various toxic non-essential metals and metalloids. Talanta. 2014;118:201–209. doi: 10.1016/j.talanta.2013.09.058. [DOI] [PubMed] [Google Scholar]
- 356.Sterckeman T., Thomine S. Mechanisms of cadmium accumulation in plants. Crit. Rev. Plant Sci. 2020;39:322–359. doi: 10.1080/07352689.2020.1792179. [DOI] [Google Scholar]
- 357.Sun Q., Wang X.R., Ding S.M., Yuan X.F. Effects of interactions between cadmium and zinc on phytochelatin and glutathione production in wheat (Triticum aestivum L.) Environ. Toxicol. 2005;20:195–201. doi: 10.1002/tox.20095. [DOI] [PubMed] [Google Scholar]
- 358.Ozyigit I.I., Can H., Dogan I. Phytoremediation using genetically engineered plants to remove metals: A review. Environ. Chem. Lett. 2021;19:669–698. doi: 10.1007/s10311-020-01095-6. [DOI] [Google Scholar]
- 359.Sharma I., Pandey H., Thakur K., Pandey D. Phytochelatins and their application in bioremediation. In: Malik J.A., editor. Microbial and Biotechnological Interventions in Bioremediation and Phytoremediation. Springer International Publishing; Midtown Manhattan, NY, USA: 2022. pp. 81–109. [DOI] [Google Scholar]
- 360.Vershinina Z.R., Maslennikova D.R., Chubukova O.V., Khakimova L.R., Fedyaev V.V. Contribution of artificially synthetized phytochelatin encoded by the gene PPH6HIS to increase the phytoremediative qualities of tobacco plants. Russ. J. Plant Physiol. 2022;69:71. doi: 10.1134/S1021443722040185. [DOI] [Google Scholar]
- 361.Gunarathne V., Mayakaduwa S., Ashiq A., Weerakoon S.R., Biswas J.K., Vithanage M. Transgenic Plant Technology for Remediation of Toxic Metals and Metalloids. Elsevier; Amsterdam, The Netherlands: 2018. Transgenic Plants: Benefits, Applications, and Potential Risks in Phytoremediation; pp. 89–102. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.