Abstract
Nowadays, the click reaction of azides with alkynes has evolved rapidly and become one of the most efficient methods to synthesize 1,2,3-triazoles, which are an important class of N-containing heterocycles. While the 1,4-selective click reaction of azides with alkynes is well established to synthesize 1,4-substituted 1,2,3-triazoles, the corresponding 1,5-selective click reaction for the generation of 1,5-substituted-1,2,3-triazoles is much less explored, and there is no systematic review for the 1,5-selective click reaction. This timely review summarizes the discovery and development of 1,5-selective click reactions of azides with alkynes for the synthesis of 1,5-substituted 1,2,3-triazoles. The 1,5-selective click reactions will be divided into three types according to the critical reactive intermediates: metallacyclic intermediates, acetylide intermediate, and formal 1,5-selective azide-alkyne cycloaddition. The related mechanistic studies will also be involved in this review.
Keywords: click reaction; azides; alkynes; cycloaddition; N-containing heterocycles; 1,2,3-triazoles
1. Introduction
Click reactions are a class of atom-economical synthetic methods discovered by a group of chemists represented by K. Barry Sharpless [1]. Since the concept was presented, click reactions have received a lot of attention and have played an important role in various fields. Click reactions are an excellent category of biocompatible reactions with high chemoselectivity, allowing only specific groups of substrates to be attached to biomolecules, and therefore they are commonly used in chemical biology splicing reactions. In 2022, Sharpless, Bertozzi, and Meldal won the Nobel Prize in chemistry for click reaction and bioorthogonal chemistry [2].
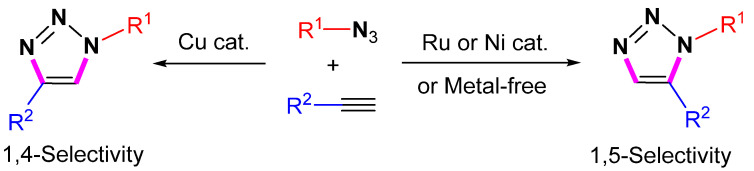
Nowadays, click reactions have evolved rapidly and extended to various types instead of being limited to a single specific reaction. Click reactions have also become an important tool for the synthesis of 1,2,3-triazoles with high selectivity. The 1,2,3-triazoles are an important class of N-containing heterocycles with diverse industrial applications as corrosion inhibitors, agrochemicals, dyes, photo stabilizers, and optical brighteners [3,4,5,6,7]. Due to their structural properties and electronic effects, they have received a lot of attention [8,9]. The Huisgen [3 + 2] cycloaddition of azides with alkynes is the most straightforward method for the synthesis of 1,2,3-triazoles. However, the reaction generally results in a mixture of 1,4-substituted and 1,5-substituted products (Scheme 1) [10,11]. The enormous synthetic potential of [3 + 2] cycloaddition reactions is limited by the obvious disadvantages, including heating requirements, prolonged reaction times, and low selectivity resulting in the formation of different isomers.
Scheme 1.
The 1,4- or 1,5-selective cycloaddition of azides with alkynes.
One of the most classical click reactions to form 1,2,3-triazoles is the Cu(I)-catalyzed azide-alkyne cycloaddition reaction. It can be well resolved for the highly regioselective synthesis of 1,4-disubstituted 1,2,3-triazoles with excellent yields. The use of click chemistry allows two molecular building blocks to react selectively under mild reaction conditions, forming the desired products with few or no by-products. The highly selective generation of 1,4-substituted 1,2,3-triazoles has been the focus of the studies on azide-alkyne cycloaddition reactions [12,13,14]. In contrast, studies on the generation of 1,5-substituted 1,2,3-triazole reactions are relatively rare.
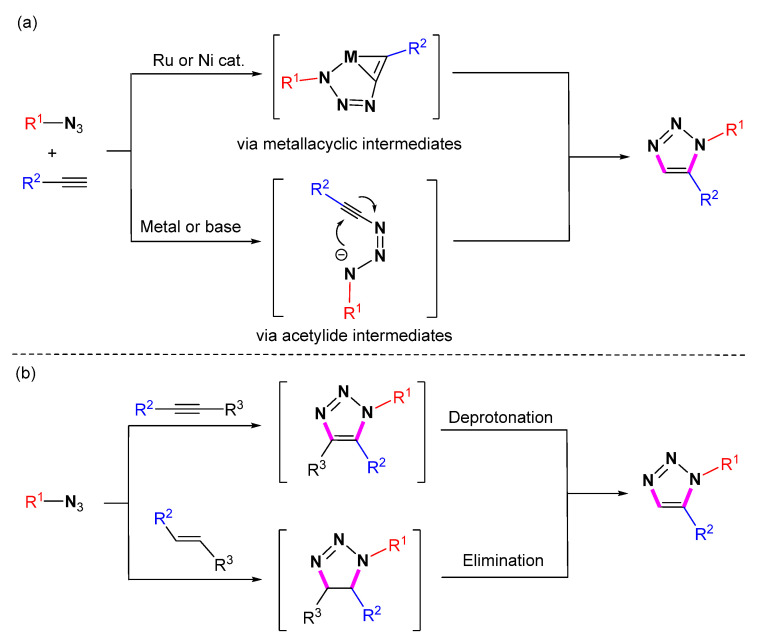
Fokin and Jia et al. discovered the synthesis of 1,5-disubstituted 1,2,3-triazoles by using ruthenium complexes to catalyze the annulation of organic azides with terminal alkynes in 2005 [15]. The full regioselective [3 + 2] cycloaddition between azides and acetylenes is possible when acetylene is activated by a strong EWG group [16]. Afterward, the syntheses of 1,5-disubstituted 1,2,3-triazoles were accomplished through many different metal-mediated or metal-free catalytic click reactions [17,18,19]. Several reviews have summarized metal-catalyzed or metal-free azide-alkyne 1,4-click reactions and the application of 1,2,3-triazoles [19,20,21,22,23,24]. Some 1,5-click reactions were sporadically incorporated into these related reviews of 1,4-click reactions. To the best of our knowledge, there is no systematic review to introduce the 1,5-selective click reaction of azides with alkynes. In this review, all the methods reported for the direct synthesis of 1,5-substituted 1,2,3-triazole compounds from azides and alkynes are described. Through this process, we have subdivided the contents into several sections according to the reaction mechanism (Scheme 2) and outlined the role of catalysts. This review aims to give a comprehensive and systematic summary of the highly selective generation of 1,5-substituted 1,2,3-triazoles.
Scheme 2.
The critical intermediates in 1,5-selective cycloaddition for the synthesis of 1,5-substituted 1,2,3-triazoles. (a) Cycloaddition via metallacyclic or acetylide intermediates (b) Formal cycloaddition via metallacyclic or acetylide intermediates.
2. 1,5-Selective Click Reaction of Azide with Alkyne via Metallacyclic Intermediates
2.1. Ruthenium-Catalyzed 1,5-Selective Click Reaction of Azide with Alkyne
2.1.1. Various Reaction Conditions of Ruthenium-Catalyzed 1,5-Selective Click Reaction of Azide with Alkyne
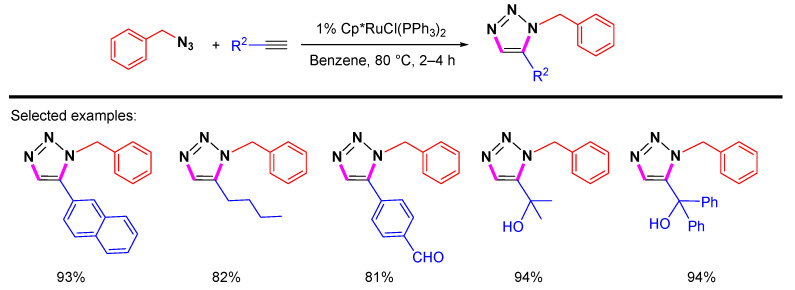
In 2005, Fokin and Jia et al. first reported the synthesis of 1,5-substituted 1,2,3-triazoles via the cycloaddition reactions of azides and alkynes catalyzed by Ru-based catalysts (Scheme 3) [15]. The results show that the regioselectivity of the reactions highly depends on the ligands in the Ru catalysts. It turns out that the Ru catalysts with Cp* (Cp* = C5Me5) and Cl give better regioselectivity than those with Cp and Cl. Both aromatic and aliphatic alkynes can react with benzyl azides to give the corresponding 1,5-disubstituted 1,2,3-triazoles.
Scheme 3.
Ru-catalyzed 1,5-selective cycloaddition of benzyl azide and terminal alkynes.
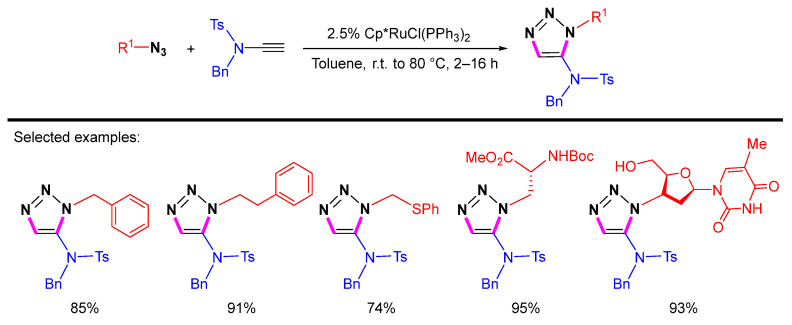
Two years later, Cintrat et al. reported that the Cp*RuCl(PPh3)2 catalyzed cycloaddition of N-benzyl N-tosyl ynamide with azides could provide 1,5-disubstituted 1,2,3-triazoles in good yields [25]. The reaction can be carried out under mild conditions to produce 1,2,3-triazoles with exclusive 1,5-regioselectivity (Scheme 4).
Scheme 4.
Ru-catalyzed 1,5-selective cycloaddition of azides and N-benzyl N-tosyl ynamide.
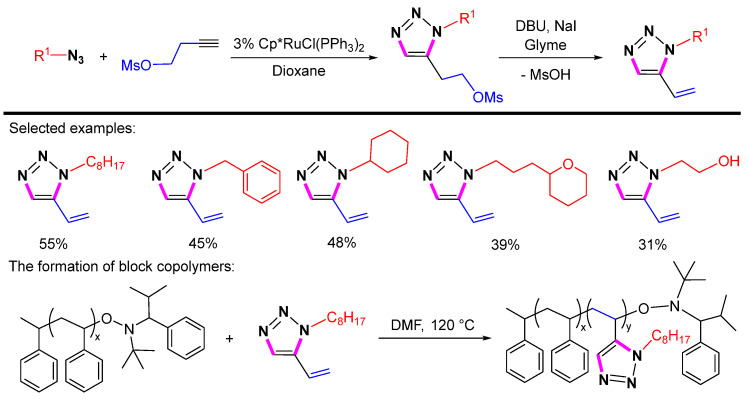
In 2009, Hawker et al. reported the synthesis of 1,5-substituted 1,2,3-triazoles using the same Cp*RuCl(PPh3)2 catalyst through the cycloaddition reaction of different azides with but-3-yn-1-yl methanesulfonate (Scheme 5). Subsequently, 1,5-substituted 1,2,3-triazoles can be converted into 1-substituted-5-vinyl-1,2,3-triazoles by the elimination of methanesulfonic acid in the presence of sodium iodide and 1,8-diazabicyclo [5.4.0] undec-7-ene (DBU). The 1-Substituted-5-vinyl 1,2,3-triazoles can act as monomers for living free radical polymerization, which has the potential for imparting tunable properties in polymeric materials [26].
Scheme 5.
Ru-catalyzed 1,5-selective cycloaddition of azides with but-3-yn-1-yl methanesulfonate.
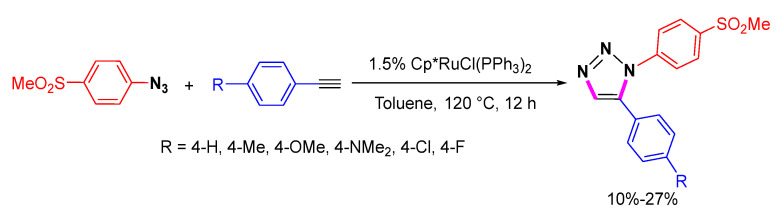
The 1,2,3-triazoles possess good properties for medicinal chemistry. Wuest et al. synthesized a series of (aryl-1,2,3-triazole-1-yl)-methanesulfonylphenyl derivatives by Cp*RuCl(PPh3)2-catalyzed cycloaddition of 1-azido-4-methane-sulfonylbenzene and para-substituted phenyl acetylenes (Scheme 6) [27]. This kind of 1,2,3-triazole can be used as in vitro cyclooxygenase (COX) inhibitors.
Scheme 6.
Ru-catalyzed 1,5-selective cycloaddition of 4-MeO2S-substituted aromatic azide with various phenyl acetylenes.
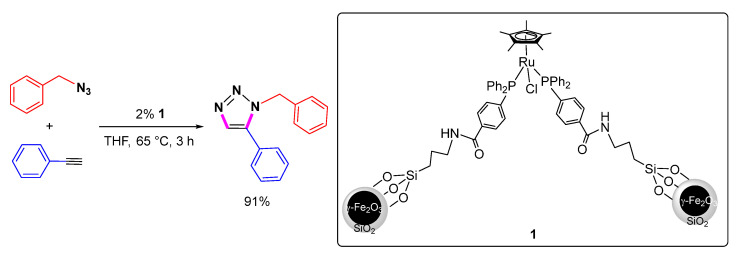
In addition to homogeneous Cp*RuCl(PPh3)2 catalyzed 1,5-selective cycloaddition of azides with alkynes, the corresponding heterogeneous 1,5-selective cycloaddition was also investigated. In 2013, Astruc et al. designed and synthesized Si(OMe)3-functionalized triarylphosphine and immobilized Si(OMe)3-functionalized triarylphosphine coordinated Ru(II) complexes into oxide magnetic nanoparticles. They found that the immobilized heterogeneous Ru(II) catalyst 1 can efficiently realize the 1,5-selective cycloaddition of benzyl azide with phenyl acetylene (Scheme 7) [28]. Catalyst 1 can be recovered by simply applying an external magnetic field using a magnetic carrier. It can be recycled at least five times with only a slight decrease in catalytic activity and selectivity, making it the first recyclable catalyst for the Ru-catalyzed 1,5-selective cycloaddition.
Scheme 7.
Heterogeneous Ru-catalyzed 1,5-selective cycloaddition of benzyl azide with phenyl acetylene.
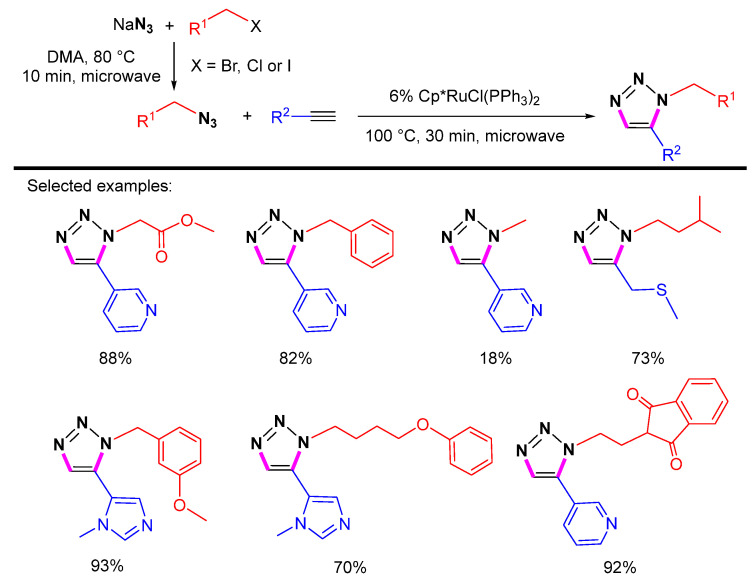
Organic azides with low molecular weight are considered to be highly energetic and pose an explosive risk. To avoid handling the dangerous alkyl azides, Kann et al. reported a one-pot method of alkynes with alkyl azides generated in situ from primary alkyl halides and sodium azide (Scheme 8) [29]. Under microwave irradiation, the Cp*RuCl(PPh3)2-catalyzed 1,5-selective cycloaddition can work smoothly.
Scheme 8.
Sequential one-pot Ru-catalyzed 1,5-selective cycloaddition of alkyl halides, sodium azide, and terminal alkynes.
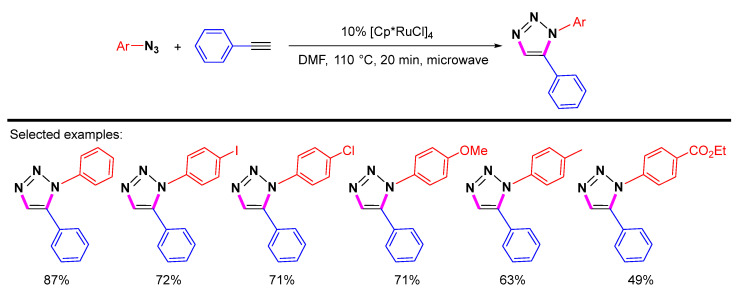
Interestingly, Fokin et al. found that the catalytic activity of ruthenium(II) tetramer [Cp*RuCl]4 for 1,5-selective cycloaddition was superior to that of Cp*RuCl(PPh3)2 [30]. The cycloaddition reaction of most aryl azides with different substituents results in the formation of corresponding 1,5-disubstituted 1,2,3-triazoles in good yields (Scheme 9). The electron-rich and moderately electron-deficient aryl azides appear to be relatively favorable for the reaction. A shorter reaction time is required when the reaction is carried out under microwave irradiation than under normal conditions.
Scheme 9.
[Cp*RuCl]4-catalyzed 1,5-selective cycloaddition of azides with terminal alkynes.
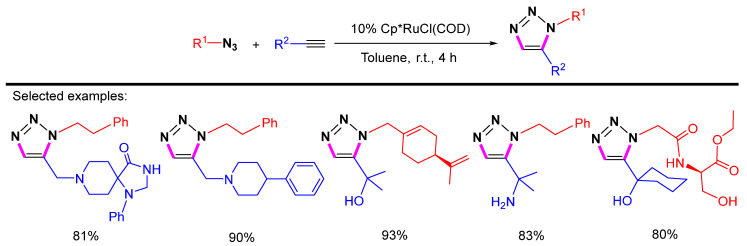
A series of ruthenium(II) complexes, such as [Cp*RuCl]4 and [Cp*RuCl(PPh3)2], had been reported as catalysts in azide-alkyne 1,5-selective cycloadditions. Due to synthetic availability and stability, the [Cp*RuCl(COD)] catalyst was further studied. The 1,5-cyclooctadiene (COD) ligand is more labile than other ligands, and the catalyst [Cp*RuCl(COD)] can work smoothly at room temperature [31]. Organic azides react with terminal alkynes containing various functionalities to give selectively 1,5-disubstituted 1,2,3-triazole products in the presence of a [Cp*RuCl(COD)] catalyst (Scheme 10).
Scheme 10.
Cp*RuCl(COD)-catalyzed 1,5-selective cycloaddition of azides with terminal alkynes.
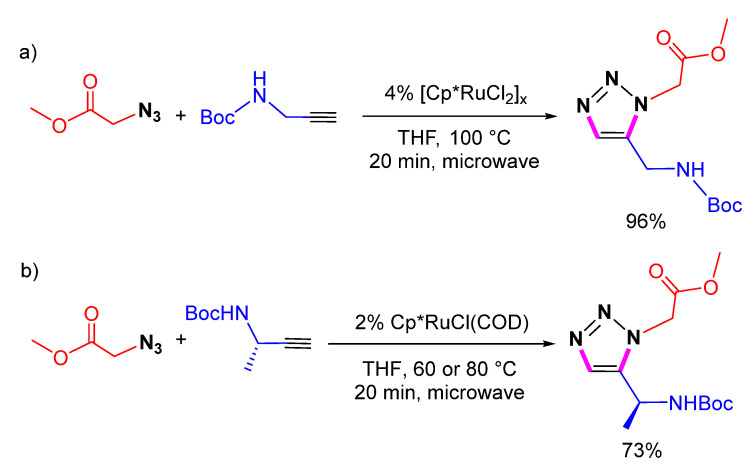
Tamas et al. reported the synthesis of 1,5-disubstituted 1,2,3-triazole amino acids by [Cp*RuCl2]x-catalyzed 1,5-selective cycloaddition of methyl 2-azidoacetate and N-Boc-propargylamine (Scheme 11a) [32]. Recently, the [Cp*RuCl(COD)]-catalyzed 1,5-selective cycloaddition of the chiral N-Boc-propargylamine with azide has been reported to produce the chiral 1,5-disubstituted 1,2,3-triazole amino acid (Scheme 11b) efficiently [33]. These 1,5-disubstituted 1,2,3-triazole scaffolds can be utilized to synthesize peptidic foldamers.
Scheme 11.
Ru-catalyzed 1,5-selective cycloaddition of methyl 2-azidoacetate (a) and N-Boc-propargylamines (b).
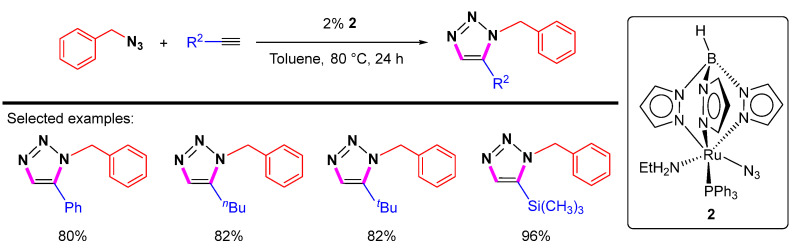
In addition to the above-mentioned “CpRu” catalytic systems, non-Cp Ru complexes were also investigated. A series of ruthenium azide complexes containing Tp ligands were prepared. Lo et al. found the complex Tp(PPh3)(EtNH2)RuN3 (2, Tp = HB(pz)3, pz = pyrazolyl) is an effective catalyst for the 1,5-selective cycloaddition of benzyl azide with terminal alkynes (Scheme 12) [34]. The synthesis of 1,5-disubstituted 1,2,3-triazoles catalyzed by 2 can tolerate a number of functional groups, and the reactions can undergo in either organic or aqueous media.
Scheme 12.
Non-Cp Ru-catalyzed 1,5-selective cycloaddition of benzyl azide with terminal alkynes.
2.1.2. Mechanism of Ruthenium-Catalyzed 1,5-Selective Click Reaction of Azide with Alkyne
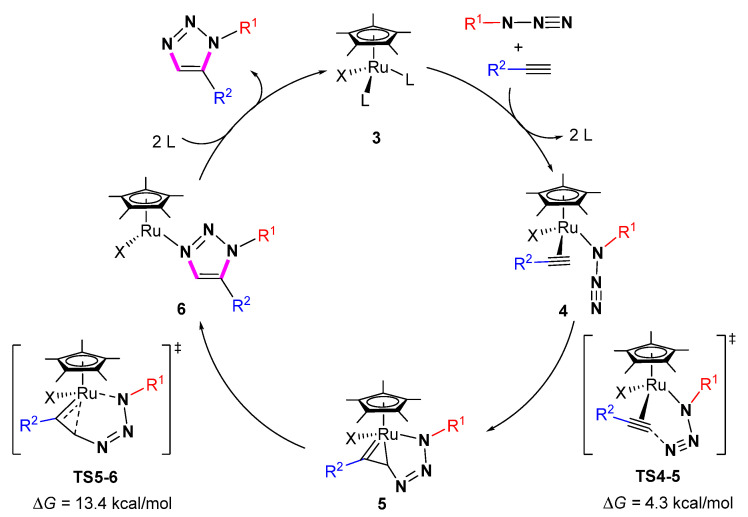
The reaction mechanism of ruthenium-catalyzed 1,5-selective cycloaddition of azides with alkynes was summarized (Scheme 13). Firstly, the spectator ligands in 3 are replaced by azides and terminal alkynes to form the activated complex 4. This is followed by the oxidative coupling to produce the ruthenacycle 5. Then 5 undergoes reductive elimination to give the intermediate 6. Finally, the dissociation of the 1,5-substituted 1,2,3 triazole and the coordination of spectator ligands in the Ru center of 6 can provide the starting 3 and complete the catalytic cycle. In 2008, Fokin and Jia et al. used DFT calculations to investigate the catalytic mechanism [31]. Computational studies indicated that the [Cp*RuCl]-catalyzed reactions of azides with alkynes involve an irreversible oxidative coupling for the nucleophilic attack of ligand alkyl groups on the terminal electrophilic nitrogen of ligand azides. The oxidative coupling step is the regioselectivity-determining step of the whole process with an energy barrier of 4.3 kcal/mol. The rate-determining step is the reductive elimination via the transition state TS5-6 with an energy barrier of 13.4 kcal/mol.
Scheme 13.
The Ru catalyzed mechanism of azide-alkyne cycloaddition.
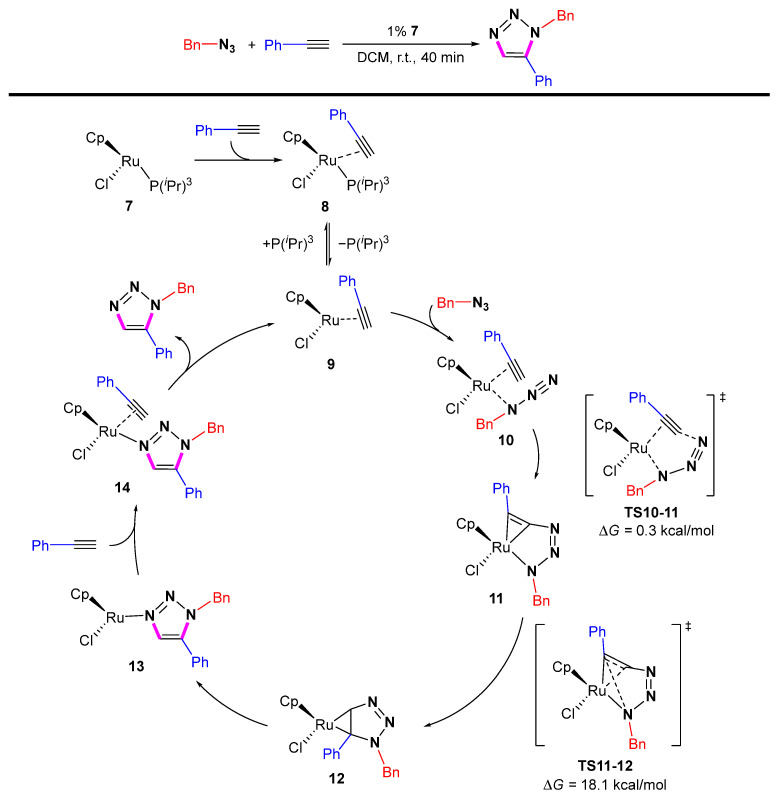
In 2012, Nolan et al. described detailed DFT studies which were in agreement with the experimental results and suggested that acetylene binding precedes azide coordination (Scheme 14) [35]. They identified previously unidentified intermediates 12 in which the formed triazole is bound to the Ru metal center in a C-Ru-C metal cyclopropane manner. Complex 12 eventually isomerizes to an N-bound triazole Ru species 13. Cp*Ru(PiPr3)Cl exhibits better performance than 18-electron ruthenium catalysts, allowing the production of 1,5-disubstituted 1,2,3-triazoles under mild conditions.
Scheme 14.
The detailed Ru catalyzed mechanism of azide-alkyne cycloaddition.
2.2. Nickel-Catalyzed 1,5-Selective Click Reaction of Azide with Alkyne
The azide-alkyne cycloadditions catalyzed by [Cp*RuCl]-based catalysts usually need to be carried out at elevated temperatures and are sensitive to water and air. Therefore, it remains a challenge to find catalysts that are compatible with water under mild conditions.
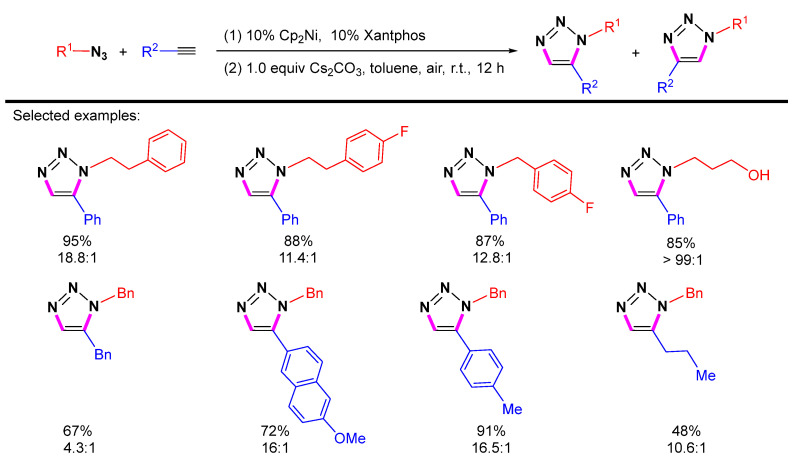
A strategy to obtain 1,5-disubstituted 1,2,3-triazoles from available substrates and inexpensive reagents via nickel catalysis in water and organic solvents at room temperature was reported by Sung et al. (Scheme 15) [36]. This nickel-catalyzed azide-alkyne cycloaddition is highly compatible with water as the only solvent and can be carried out in air at room temperature. All the substrate ranges of azides, including fluorinated aromatics and fused cyclic groups, are compatible with the reaction conditions. Both the hydroxyl and ester functional groups remain intact, and for the substrate range of alkynes, aliphatic and aromatic alkynes with different functional groups, including methoxy, amine, nitro, chlorine, and methyl, are well tolerated.
Scheme 15.
Ni-catalyzed 1,5-selective cycloaddition of azides and alkynes.
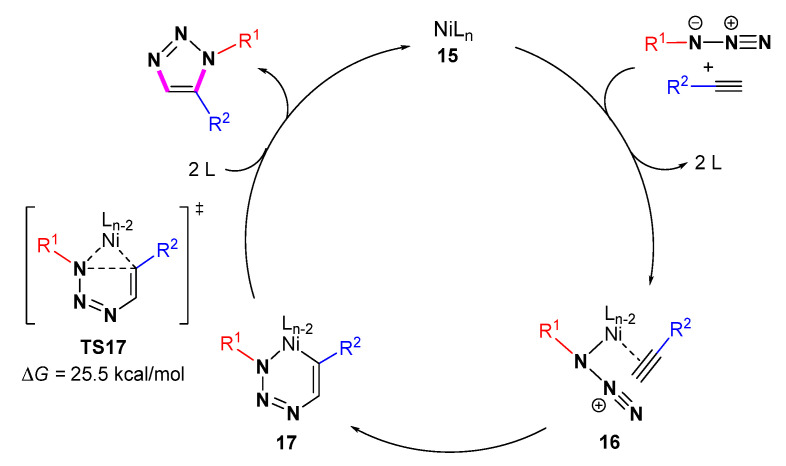
They concluded the reaction involves the process of Cp2Ni→CpNi(Xantphos)→Ni(Xantphos)2 and CpNi(Xantphos)→CpNi(Xantphos)+. Initially, both alkynes and azides are coordinated to the Ni center to form intermediate 16. The C-N bond formed in complex 17 between the alkyne and azide determines 1,5 regioselectivity. Subsequent reductive elimination leads to the formation of the target cyclization product and regenerates NiLn species (Scheme 16). In 2020, the cycloaddition reactions of azides and asymmetric alkynes were completed under nickel catalysis [37]. DFT calculations indicate that the cyclization step via TS17 is the rate-determining step with an energy barrier of 25.5 kcal/mol.
Scheme 16.
The Ni-catalyzed mechanism of cycloaddition of azides with alkynes.
3. 1,5-Selective Click Reaction of Azide with Alkyne via Acetylide Intermediates
3.1. Transition-Metal-Free 1,5-Selective Click Reaction of Azide with Alkyne
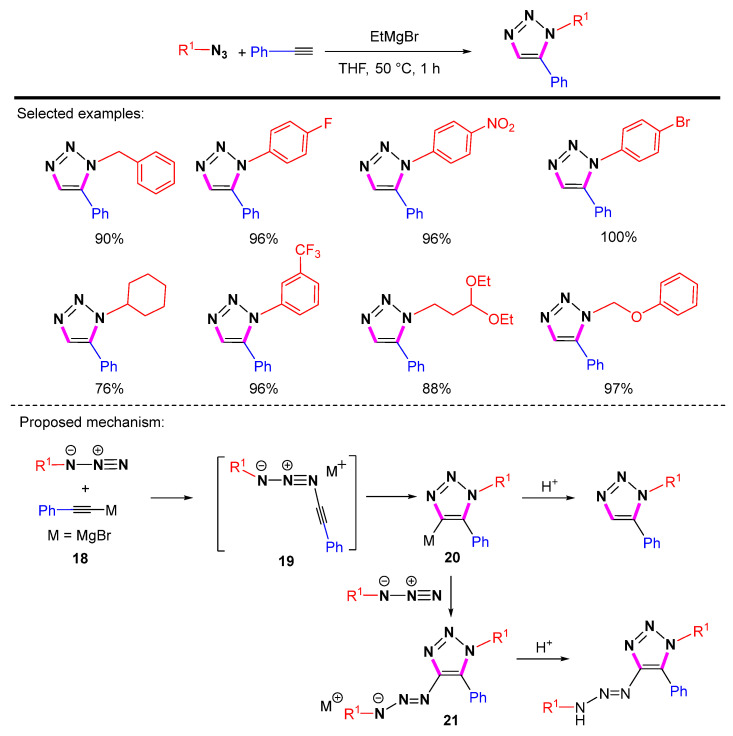
In order to control the regioselectivity of the [3 + 2] cycloaddition reaction of azides with terminal alkynes containing highly acidic C-H, a common idea is to form the corresponding alkynyl carbanions, which then selectively undergo nucleophilic attack toward the electrophilic end of the azides. The pioneer studies started in 1967 by Akimova et al., who used equivalent amounts of lithium reagents or magnesium acetylene reagents to react with terminal alkynes [38]. The reaction had limitations in terms of functional group compatibility and atomic economy. After the results had been dormant for 30 years, Sharpless et al. optimized the process to give a good yield and elaborated the mechanism of this reaction and by-product generation (Scheme 17) [39]. The mechanism begins with the nucleophilic attack of acetylide 18 on the terminal nitrogen atom of the azide to provide intermediate 19. Then the cyclization process in 19 occurs to generate intermediate 20. The 1,5-disubstituted 1,2,3-triazoles can be obtained by hydrolysis. Sharpless et al. concluded that, after the formation of cyclic intermediate 20, it could be captured with electrophilic reagents to obtain the substituted 1,2,3-triazoles.
Scheme 17.
Synthesis of 1,5-disubstituted1,2,3-triazoles using magnesium acetylene reagents and proposed mechanism.
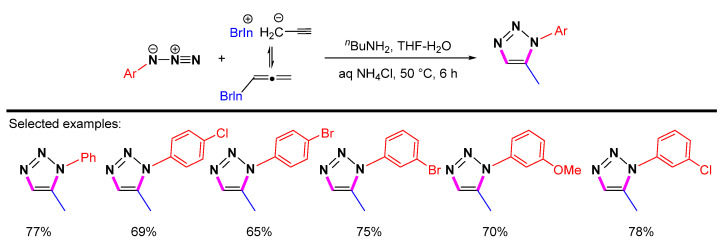
As shown in Scheme 18, the regioselective synthesis of 1-aryl-5-methyl-1,2,3-triazoles can be achieved through N/C-heterocyclization of allenylindium bromide across aryl azides [40]. The reaction can take place in an aqueous medium, and the synthesis of 1,5-disubstituted 1,2,3-triazoles can be carried out under mild reaction conditions with moderate to good yields and very high regioselectivity. However, the alkyne is limited to propargyl bromide, and only methyl-substituted 1,2,3-triazoles can be obtained.
Scheme 18.
Synthesis of 1,5-disubstituted1,2,3-triazoles from allenylindium bromides with azides.
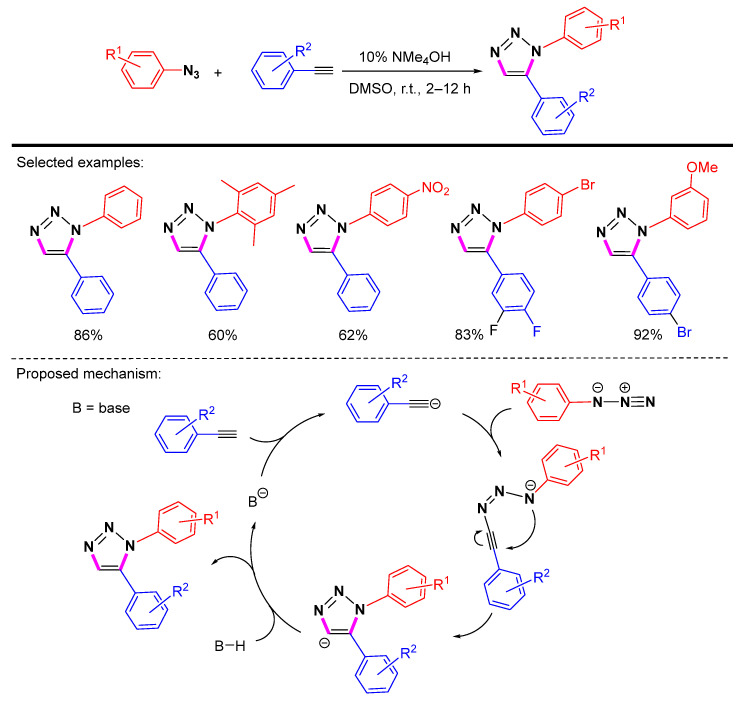
In 2010, Fokin et al. developed a base-catalyzed cycloaddition that was insensitive to both oxygen and water and did not require the involvement of metal reagents (Scheme 19) [41]. These bases, including anhydrous sodium, potassium, cesium hydroxides, aqueous tetramethylammonium, and benzyl-trimethylammonium hydroxides, can catalyze the formation of 1,5-diaryl-substituted 1H-1,2,3-triazoles. The catalytic amounts of tetramethylammonium hydroxide were used in the deprotonation of aryl acetylene to initiate the cyclic reaction. Nevertheless, the substrates for this condition are restricted to aryl alkynes, and the conversion efficiency is not very good for alkyl-substituted alkynes. The proposed mechanism shows that the reversible deprotonation of the terminal alkyne by the base produces an aryl acetylate, which then undergoes cyclization. The catalytic cycle is completed by the protonation affording the final product.
Scheme 19.
Base-catalyzed synthesis of 1,5-disubstituted 1,2,3-triazoles and proposed mechanism.
3.2. Zinc-Mediated 1,5-Selective Click Reaction of Azide with Alkyne
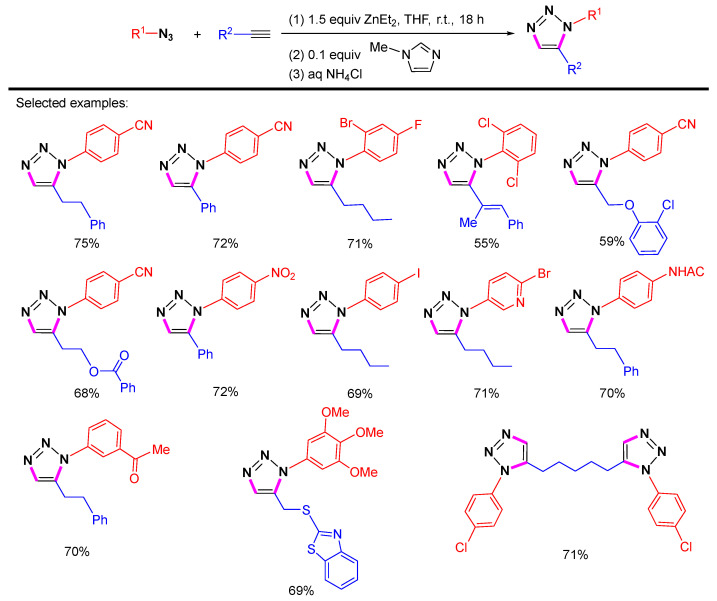
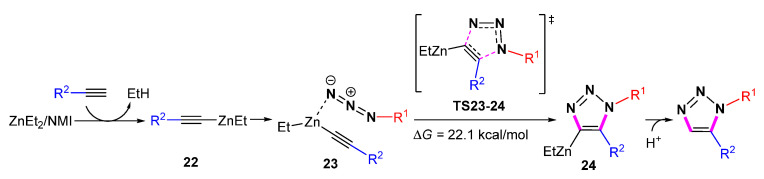
In 2013, Greaney et al. reported the regioselective synthesis of 1,5-substituted 1,2,3-triazoles through zinc-mediated cycloaddition at room temperature (Scheme 20) [42]. The range of alkynyl substrates includes alkyl and aryl terminal alkynes. A number of azides with different functional groups, including esters, amides, ketones, nitriles, nitros, aryl iodides, heterocycles, and ortho-ligands, can tolerate the zinc-mediated conditions. In addition, diacetylene is also suitable.
Scheme 20.
Zn-mediated 1,5-selective cycloaddition of azides with alkynes.
DFT calculations have been used to clarify the regioselectivity of zinc-mediated [3 + 2] cycloaddition of azides with alkynes [43]. Computational results indicate that the catalytic cycle starts with the initial metalation of the alkyne. The regioselectivity of the cycloaddition is controlled by the nucleophilicity of the terminal alkyne. The acetylide fragment is coordinated with the Zn metal center and undergoes a cyclization reaction with an energy barrier of 22.1 kcal/mol via TS23-24 (Scheme 21).
Scheme 21.
The mechanism of Zn-mediated 1,5-selective cycloaddition of azides with alkynes.
3.3. Rare-Earth Metal Catalyzed 1,5-Selective Click Reaction of Azide with Alkyne
In 2008, Cui et al. reported the rare-earth metal-catalyzed cycloaddition of azides and alkynes to afford 1,5-disubstituted 1,2,3-triazoles within 72 h at 60 °C (Scheme 22) [44]. Different aromatic alkynes can be applied for the efficient synthesis of 1,5-disubstituted 1,2,3-triazoles. However, only small amounts of cycloaddition products can be obtained when aliphatic alkynes are utilized. The regioselectivity and conversion rate are also influenced by the nature of azides.
Scheme 22.
Rare-earth metal catalyzed 1,5-selective cycloaddition of azides with alkynes.
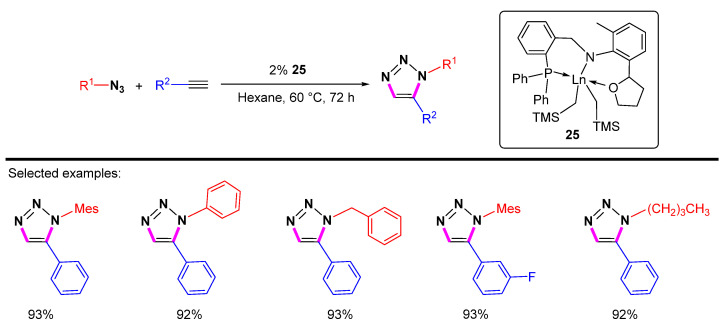
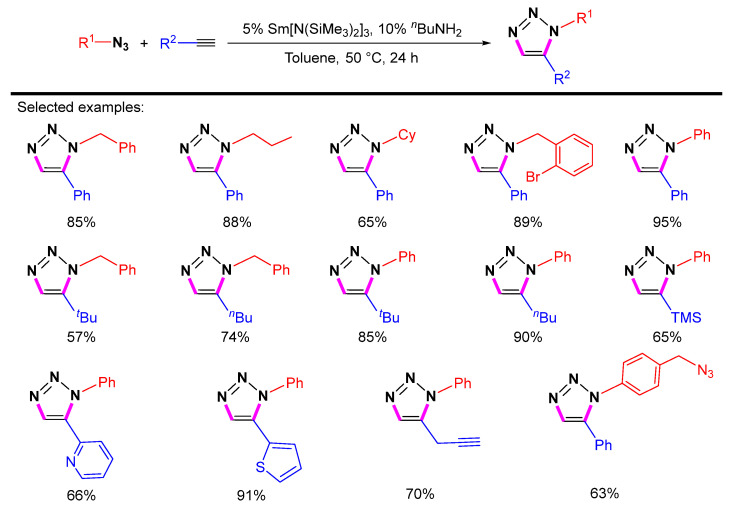
Then Zhou et al. reported the rare-earth metal-catalyzed cycloaddition of terminal alkynes with azides to provide a series of 1,5-disubstituted 1,2,3-triazoles with good to excellent yields (Scheme 23) [45]. Catalysts containing different rare-earth metals, including Sm, Nd, Y, and Gd, have been tested, and the Sm catalyst is the best choice.
Scheme 23.
Sm[N(SiMe3)2]3-catalyzed 1,5-selective cycloaddition of azides with terminal alkynes.
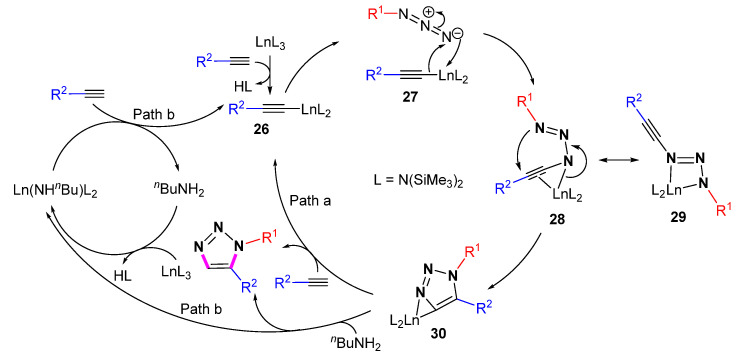
The proposed mechanism (Scheme 24) is different from the mechanism of Ru-catalyzed 1,5-selective cycloaddition. Initially, the C-H bond of the terminal alkyne can be activated to produce Ln acetylide 26. Coordination and subsequent 1,1-insertion of azide into the Ln–C bond of 27 generate intermediates 28 or 29. The intramolecular nucleophilic cyclization will form triazolate complex 30. Intermediate 30 undergoes protonation with terminal alkyne to generate the 1,5-disubstituded 1,2,3-triazloes, completing the catalytic cycle. Since the amine nBuNH2 can act as both a proton source and ligand activation catalyst, the reaction proceeding through path b cannot be excluded.
Scheme 24.
The mechanism of Sm[N(SiMe3)2]3-catalyzed 1,5-selective cycloaddition of azides with alkynes.
Li et al. performed DFT calculations to investigate the mechanism of the path, namely the samarium-catalyzed 1,5-regioselective azido-alkyne [3 + 2]-cycloaddition [46]. The rate-determining step is the insertion of azide into the samarium phenylacetylide. The calculations also infer that the addition of the samarium catalyst changes the distribution of the electrostatic potential on the surface of the alkyne, determining the direction of polarization and the formation of different intermediates, which ultimately control the regioselectivity.
4. Formal 1,5-Selective Click Reaction of Azide with Alkyne
4.1. In Situ Generation of Terminal Acetylene
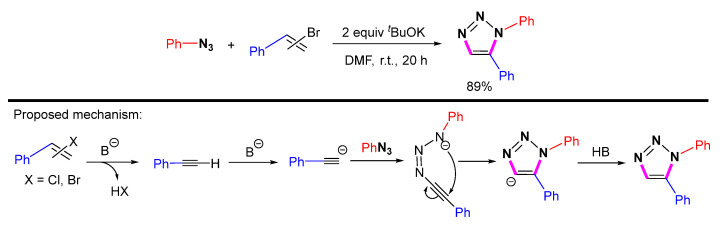
A base-mediated reaction of α- or β-vinyl bromides with azides for the synthesis of 1,5-disubstituted 1,2,3-triazoles was investigated (Scheme 25) [47]. Strong bases are necessary for the elimination of vinyl halides and the formation of alkynyl anions. Meanwhile, the reaction of aryl vinyl bromides and aryl azides tends to give high yields.
Scheme 25.
Base-mediated synthesis of 1,5-disubstituted 1,2,3-triazole from azides and vinyl bromides and proposed mechanism.
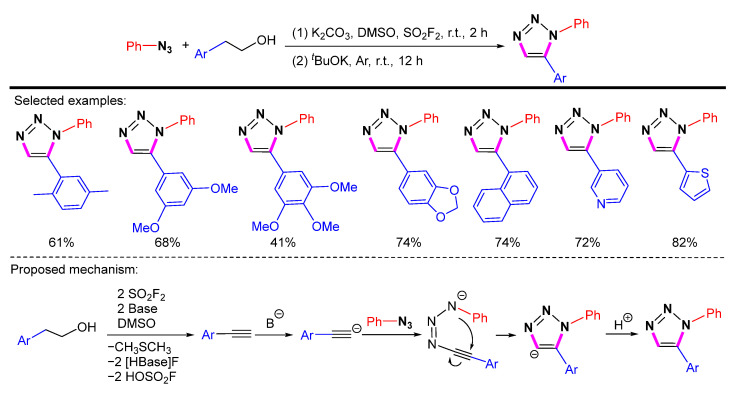
In 2019, Qin et al. found that in the presence of tBuOK, alcohols can react with azides to synthesize 1,5-disubstituted 1,2,3-triazole products in excellent yields at room temperature (Scheme 26) [48]. The mechanism of the formation of alkyne from alcohol has been reported by Qin et al. [49]. SO2F2 is used to activate DMSO for the oxidative conversion of alcohols to alkenyl sulfurofluoridates under basic conditions. Then the formation of alkynes through the elimination of HOSO2F in alkenyl sulfurofluoridates is promoted by the base. After transforming the alcohol into the corresponding terminal alkyne, deprotonation can occur in the presence of a strong base to generate acetylide, which reacts with the azide to afford the corresponding 1,2,3-triazole in situ. The reaction does not require a metal catalyst and shows good compatibility with a large number of functional groups.
Scheme 26.
Synthesis of 1,5-disubstituted 1,2,3-triazole from azides and alcohols and proposed mechanism.
4.2. Formal 1,5-Selective Click Reaction via Cycloaddition/Elimination
4.2.1. Cycloaddition/Elimination of Sulfonyl Group
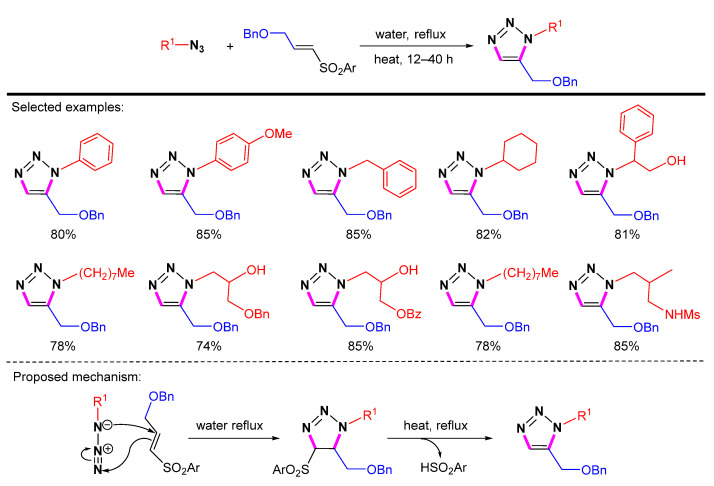
There are few reports on the use of olefins to generate 1,2,3-triazoles in the last 20 years. The main problem is that the generation of triazolines from olefins requires additional steps of elimination or oxidation to generate 1,2,3-triazoles. In 2011, Pathak et al. reported a metal-free and vinyl sulfone-based synthesis of 1,5-disubstituted 1,2,3-triazoles (Scheme 27) [50]. This convenient and versatile process eliminates the need for an inert gas atmosphere and the use of high boiling point solvents. The strategy provides a practical route for the synthesis of 1,5-disubstituted 1,2,3-triazoles using a combination of aryl/alkyl vinyl sulfones and aryl/alkyl azides. Due to the polarization of the vinyl sulfone double bond, the azide attacks the partially positively charged position, resulting in a cyclic intermediate. The sulfinic acid is eliminated, then the 1,5-disubstituted 1,2,3-triazole is selectively produced.
Scheme 27.
Synthesis of 1,5-disubstituted 1,2,3-triazoles from azides and vinyl sulfone and proposed mechanism.
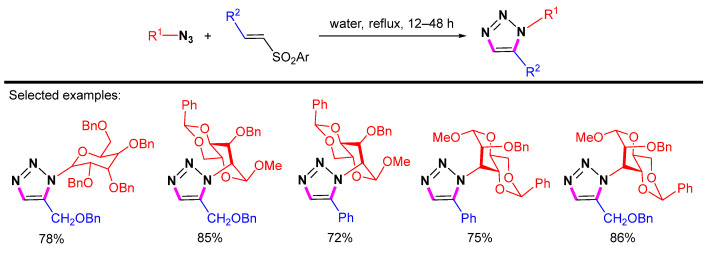
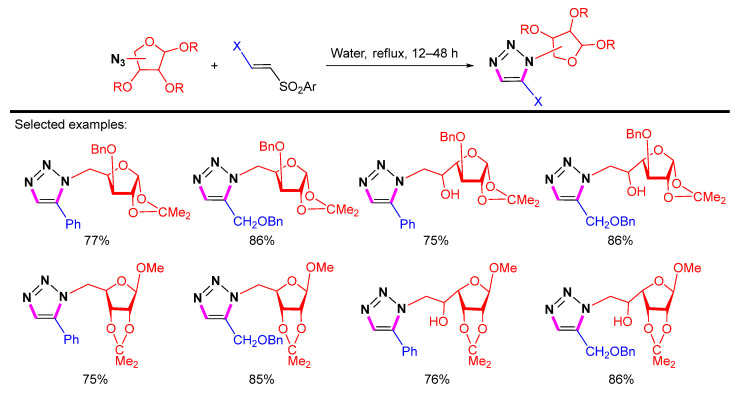
Pathak et al. showed that vinyl sulfones reacted with azidopyranosides to produce 1,5-disubstituted 1,2,3-triazoles (Scheme 28) [51]. The reaction was carried out in water at elevated temperatures without any metal catalyst to give 1,5-disubstituted triazolylated monosaccharides in high yields.
Scheme 28.
Synthesis of 1,5-disubstituted 1,2,3-triazolylated monosaccharides from azidopyranosides and vinyl sulfones.
In 2015, they reported that the [3 + 2] cycloaddition reaction of vinyl sulfone derivatives with azides under reflux conditions without metal catalyst could provide 1,5-disubstituted 1,2,3-triazolylated monofuranosides and difuranosides (Scheme 29) [52]. A new possibility for linking furanosides with a stable triazole backbone is offered by the synthesis of these 1,5-disubstituted triazolylated monosaccharides as well as 1,5-disubstituted 1,2,3-triazole-linked disaccharides.
Scheme 29.
Synthesis of 1,5-disubstituted 1,2,3-triazolylated monofuranosides.
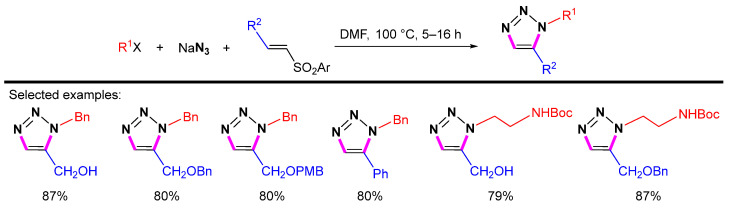
Furthermore, Pathak et al. developed the one-pot three-component cycloaddition of vinyl sulfones and sodium azide with various third components, including alkyl bromides, -tosylates, -mesylates or aryl amines, -iodides, to offer a wide variety of 1,5-disubstituted 1,2,3-triazoles (Scheme 30) [53].
Scheme 30.
One-pot three-component synthesis of 1,5-disubstituted 1,2,3-triazoles.
4.2.2. Cycloaddition/Elimination of HNO2/HOAc Group
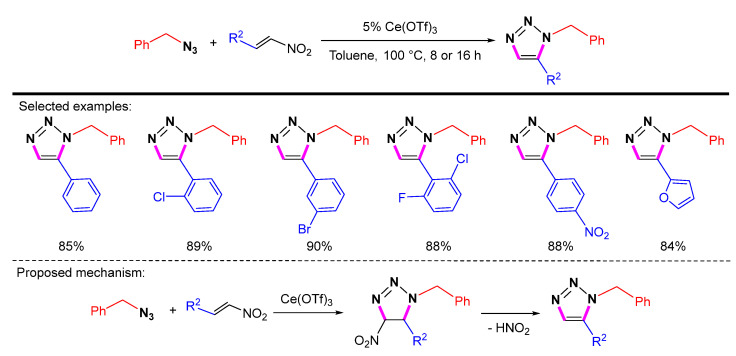
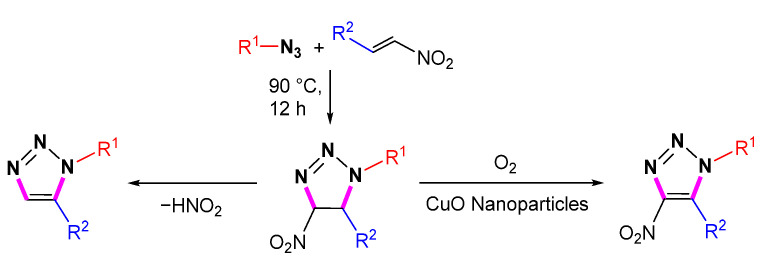
In the presence of Ce(OTf)3, organic azides undergo [3 + 2] cycloaddition with nitroalkenes. Then the elimination of nitro groups leads to the formation of 1,5-disubstituted 1,2,3-triazoles via aromatization (Scheme 31) [54]. This reaction produces 1,5-disubstituted 1,2,3-triazoles in good to excellent yields with good compatibility for tertiary amines, hydroxyls, and halogens. The advantages of this procedure are the high availability of starting materials, the convenience of the experimental procedure, and the low cost of the catalyst.
Scheme 31.
Synthesis of 1,5-disubstituted 1,2,3-triazoles from azides and nitroolefins and proposed mechanism.
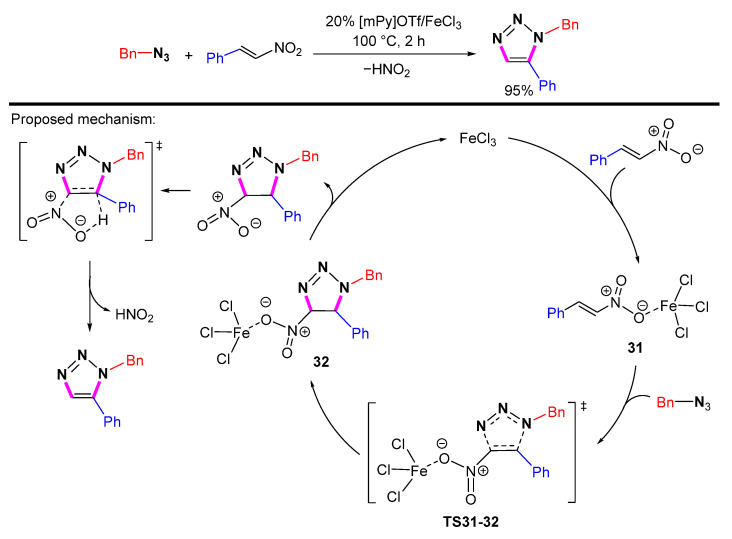
In 2018, Maiuolo et al. reported the preparation of 1,5-disubstituted 1,2,3-triazole derivatives via FeCl3-mediated azide-olefin cycloaddition in ionic liquids (Scheme 32) [55]. DFT calculations indicate that the first step of the reaction is the coordination of FeCl3 with the nitroolefin compound to form an activated intermediate 31. Intermediate 31 reacts with the azide to produce the triazoline intermediate via the transition state TS31-32. The last step eliminates HNO2 to give 1,5-disubstituted 1,2,3-triazoles.
Scheme 32.
Synthesis of 1,5-disubstituted 1,2,3-triazole by azide-olefin cycloaddition and proposed mechanism.
Elangovan et al. described the synthesis of 1,2,3-triazoles through [3 + 2] cycloaddition under solvent-free and catalyst-free conditions (Scheme 33) [56]. The triazolines formed from the cycloaddition of azides and olefins are unstable. The aromatized 1,2,3-triazoles are obtained by the elimination of HNO2 in the absence of a catalyst. The aromatic stability of the product and the good leaving ability of the NO2 group are the main driving forces.
Scheme 33.
The eliminative azide-olefin cycloaddition and oxidative azide-olefin cycloaddition of azides and nitro alkenes.
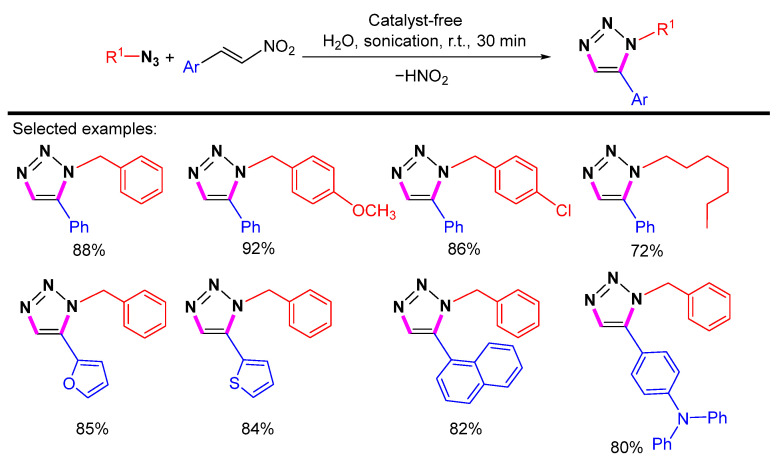
In 2021, Karthikeyan et al. reported a simple and efficient catalyst-free cycloaddition for the preparation of 1,5-disubstituted 1,2,3-triazoles from azides and nitro alkenes in an aqueous base (Scheme 34) [57]. This cycloaddition proceeds under ultrasound irradiation with the scope of broad substrates, simple work-up, and high regioselectivity.
Scheme 34.
Synthesis of 1,5-disubstituted 1,2,3-triazole from azides and nitro alkenes.
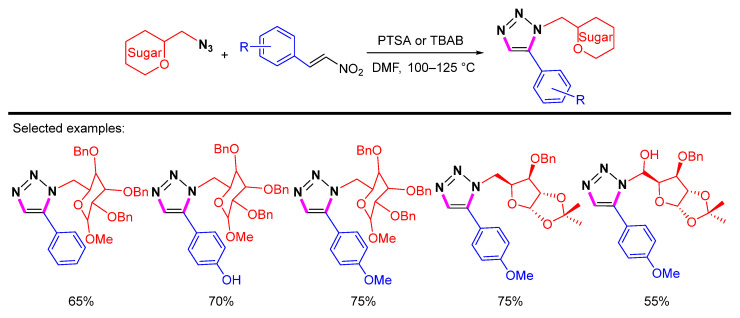
The generation of 1,5-substituted 1,2,3-triazoles through eliminating NO2 groups is an important method for the synthesis of sugar scaffolds. In 2016, Tiwari et al. presented a cycloaddition for the synthesis of 1,5-disubstituted triazolyl glycoconjugates from different glycosyl azides with nitro-olefins with phase transfer catalysts, e.g., p-toluenesulfonic acid (PTSA), tetrabutylammonium bromide (TBAB) (Scheme 35) [58].
Scheme 35.
Synthesis of 1,5-disubstituted triazolyl glycoconjugates from glycosyl azides with nitro alkenes.
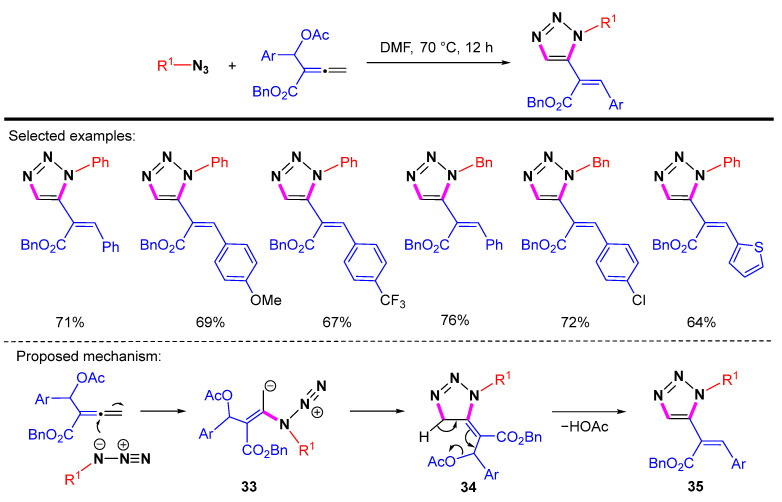
In 2021, Swamy et al. reported the cycloaddition/cyclization reactions for the synthesis of 1,5-disubstituted 1,2,3-triazoles under metal-free conditions using β-acetoxy allenoates [59]. A plausible pathway for the formation of 1,2,3-triazoles is shown in Scheme 36. The allyl/alkyl nitrogen atom first attacks the β-position of the allenoates to give the intermediate 33. Next, 33 undergoes an intramolecular addition to give the cycloaddition adduct 34. Finally, acetic acid is eliminated from intermediate 34 to form the final product 35.
Scheme 36.
[3 + 2]-Cycloaddition of β-acetoxy allenoates with azides and proposed mechanism.
4.2.3. Cycloaddition/Elimination of Protection Group
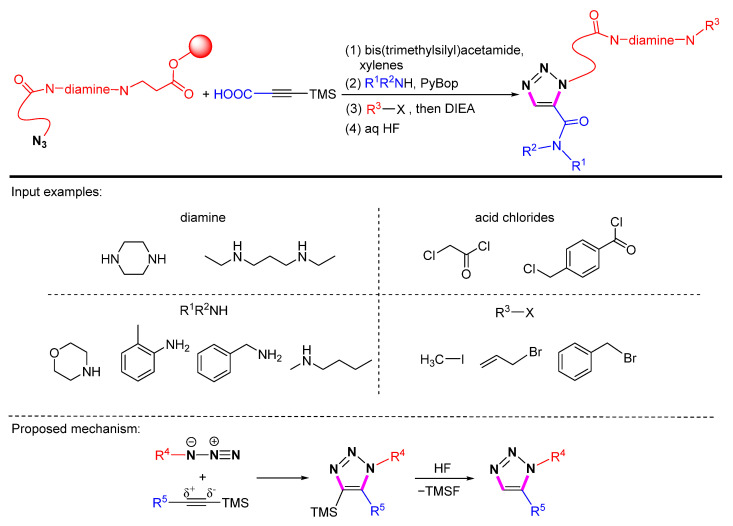
Generally, 1,5-selective cycloaddition has limited use in solid-phase synthesis and drug discovery for the synthesis of 1,2,3-triazoles. In 2004, Hlasta et al. found that 1-trimethylsilylacetylene can react with azides immobilized on REM resin in a [3+2] cycloaddition to yield the corresponding 1,4,5-substituted 1,2,3-triazoles [60]. The TMS group can be removed by contacting with 10 equiv of HF (50% aq) in THF for 4 h at room temperature to produce the 1,5-substituted 1,2,3-triazoles (Scheme 37). A small library (2 × 2 × 4 × 3) of 1,5-substituted 1,2,3-triazoles with an average purified yield of 68% was established through the cycloaddition of azides on REM resin with acetylene.
Scheme 37.
Solid-phase 1,5-selective cycloaddition of azides immobilized on REM resin with 1-trimethylsilylacetylene.
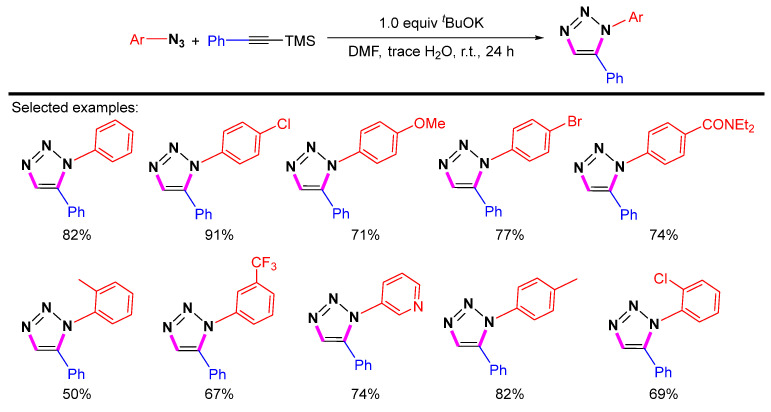
In 2012, Lin et al. studied the synthesis of 1,5-disubstituted 1,2,3-triazoles through direct desilylation of TMS-alkynes (Scheme 38) [61]. The use of tBuOK as a desilylating reagent results in the regioselective formation of 1,5-disubstituted 1,2,3-triazoles. When a trace of water is added, this cycloaddition has good yields at room temperature. In this process, desilylation and subsequent cycloaddition are necessary.
Scheme 38.
Synthesis of 1,5-disubstituted 1,2,3-triazoles from azides and TMS-alkynes.
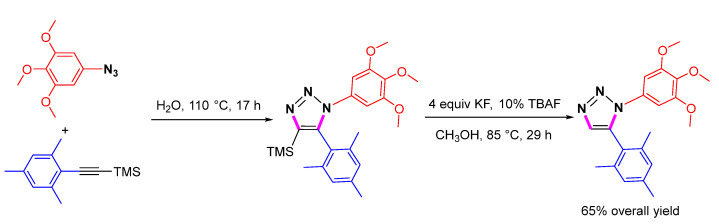
Another synthetic route to 1,5-disubstituted 1,2,3-triazoles with high efficiency and regioselectivity was developed using a thermal dipolar cycloaddition reaction between trimethylsilylacetylenes and azides (Scheme 39) [62]. The TMS-modified 1,2,3-triazoles can be desilylated using potassium fluoride and catalytic amounts of tetrabutylammonium fluoride (TBAF) in a methanol solution.
Scheme 39.
Synthesis of 1,5-disubstituted 1,2,3-triazoles by one pot cycloaddition/desilylation.
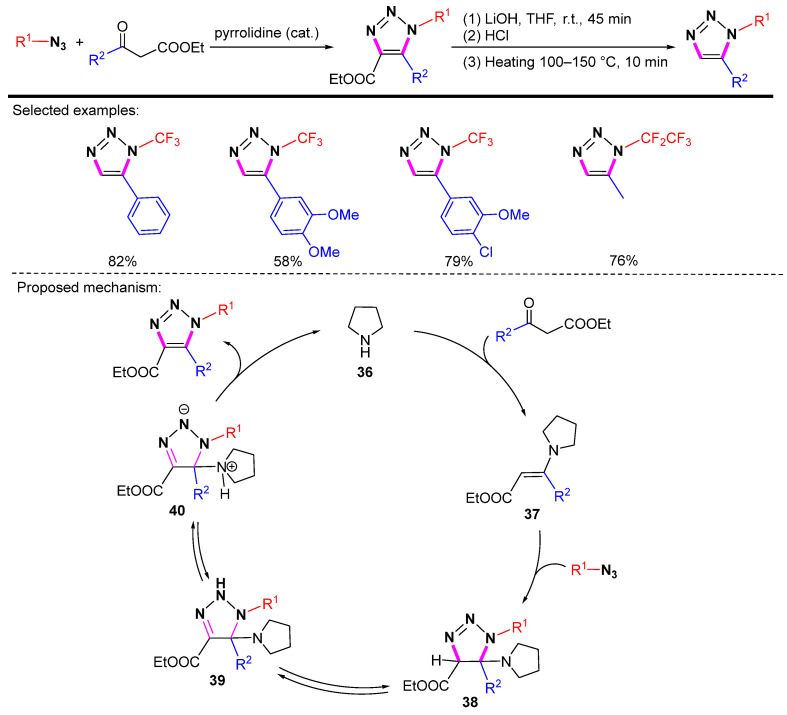
Highly functionalized N-perfluoroalkyl-1,2,3-triazoles were efficiently synthesized from azidoperfluoroalkanes [63]. Enamine 37 generated in situ could easily participate in the azidecarbonyl [3 + 2] cycloaddition reaction, providing a simple method for the synthesis of triazole frameworks with good to excellent yields. The basic hydrolysis and decarboxylation of the ethoxycarbonyl-substituted 1,2,3-triazoles can form 1,5-disubstituted 1,2,3-triazoles in high yields (Scheme 40). The proposed mechanism can be summarized as follows. Azide and enamine 37 undergo cycloaddition to form triazoline intermediate 38. After the 1,3-hydrogen shift, 38 rearranges to give 40. One molecule of amine is then eliminated to complete the catalytic cycle.
Scheme 40.
Synthesis of 1,5-disubstituted 1,2,3-triazoles via hydrolysis and decarboxylation and proposed mechanism.
4.3. Formal 1,5-Selective Click Reaction of Azide with Alkyne via Wittig Reaction
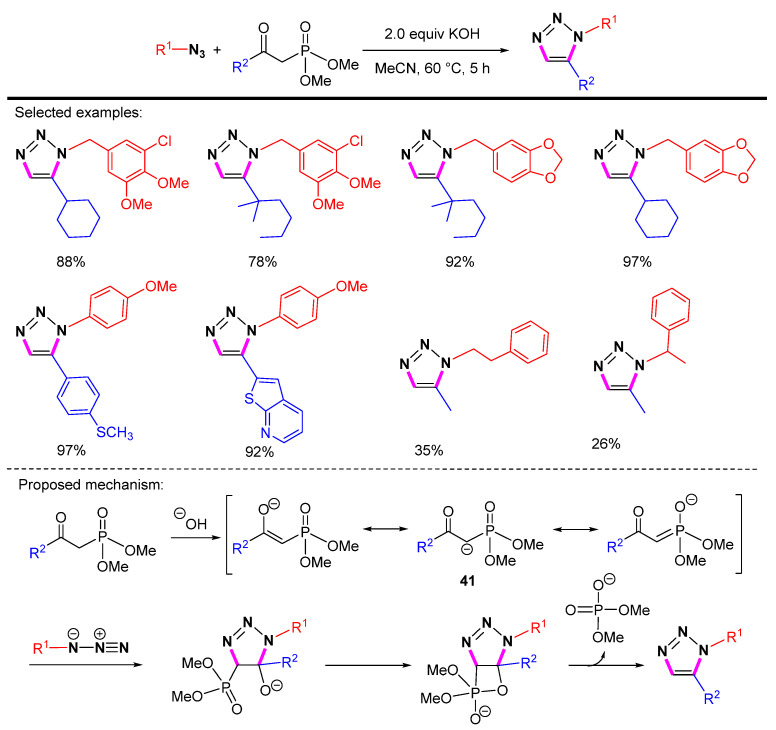
In 2016, Carlos et al. developed a simple and efficient method for the 1,5-selective cycloaddition of azides with β-ketophosphonates (Scheme 41) [64]. The scope and diversity of this protocol include the effective synthesis of 1-alkyl-substituted, 1-aryl-substituted, 5-alkyl-substituted, and 5-aryl-substituted 1,2,3-triazoles. The phosphoryl-stabilized carbanion 41 is able to couple with the azide in a highly regioselective manner to form the corresponding oxaphosphetane. Washing with water makes it easy to separate the desired products as well as a free phosphate by-product.
Scheme 41.
Azide-enolate [3 + 2] cycloaddition and proposed mechanism.
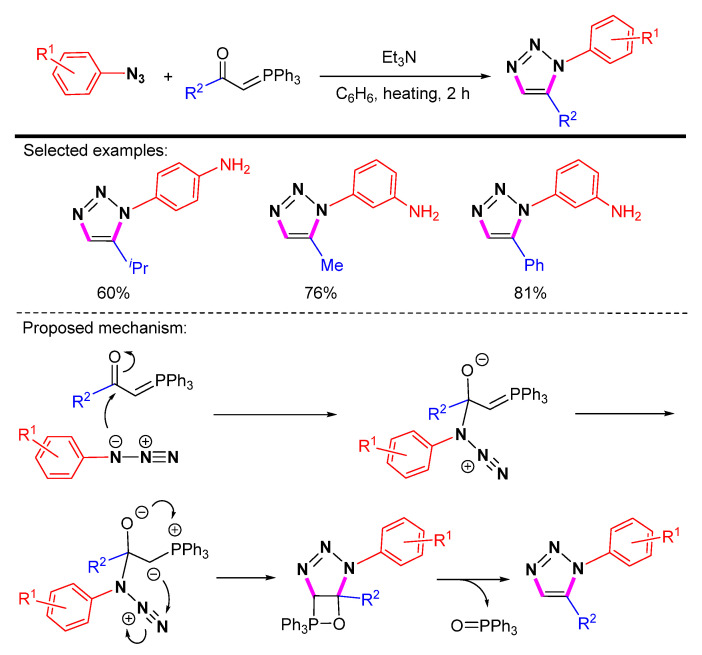
Obushak et al. found that the reaction of aryl azides with phosphorus ketoylides was a convenient method for the synthesis of 1,5-disubstituted 1,2,3-triazoles (Scheme 42) [65]. The 1,5-disubstituted 1,2,3-triazoles could be obtained by eliminating phosphorus-containing compounds in near quantitative yields.
Scheme 42.
Synthesis of 1,5-disubstituted 1,2,3-triazoles by eliminating phosphorus-containing compounds and proposed mechanism.
4.4. Other Formal 1,5-Selective Click Reaction of Azide with Alkyne
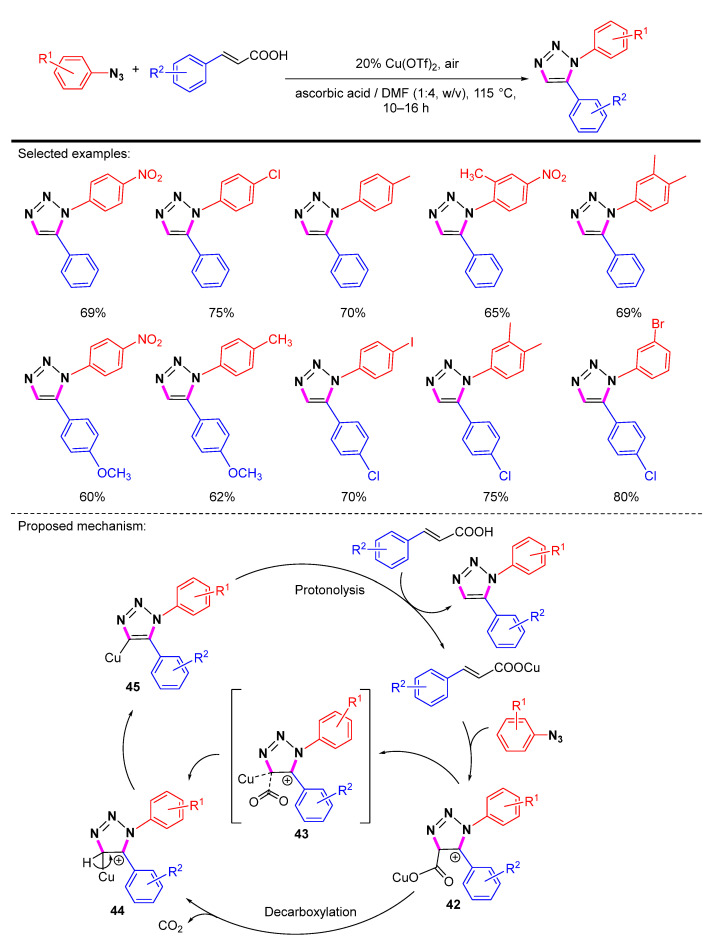
Kumar et al. presented the first evidence that Cu(II) can catalyze the synthesis of 1,5-disubstituted 1,2,3-triazoles via the coupling of benzoyl derivatives and substituted styryl carboxylic acids (Scheme 43) [66]. The first step of the reaction is the regioselective cyclization of azide with cinnamic acid to form the cationic intermediate 42. Intermediate 42 undergoes a decarboxylation reaction to produce copper triazolide 44, which subsequently loses a proton to give a copper complex of 1,4,5-trisubstituted 1,2,3-triazoles. In acidic media, the copper complex 45 readily undergoes proton decomposition to produce 1,5-disubstituted 1,2,3-triazoles and the Cu(II) species to complete the catalytic cycle. They believed that the Cu(II) species could be regenerated from the Cu(I) species with oxygen under acidic conditions. However, in the proposed mechanism, the origin of Cu(I) at the very beginning and how air was involved in the catalytic cycle as an oxidant is not mentioned and is still unclear.
Scheme 43.
Synthesis of 1,5-disubstituted 1,2,3-triazoles via Cu-catalyzed decarboxylation.
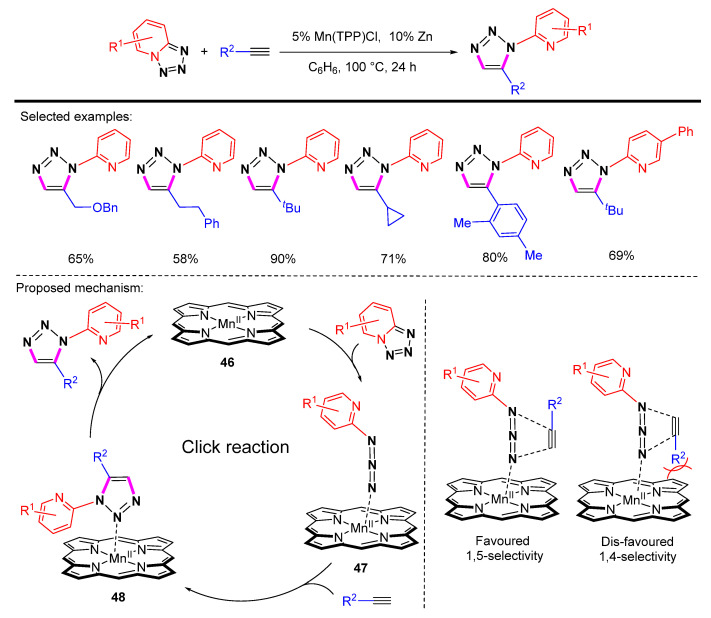
Chattopadhyay et al. reported the synthesis of pyridyl-substituted 1,5-disubstituted 1,2,3-triazoles by Mn-porphyrin catalyzed cycloaddition of tetrazole with terminal alkynes (Scheme 44) [67]. The method is compatible with a wide range of substrates for the reactions. A possible mechanism was proposed. Mn-bound complex 46 coordinates with azide to form intermediate 47. Intermediate 47 readily undergoes a cycloaddition reaction in the presence of terminal alkyne to produce intermediate 48, which then releases the click product and regenerates the active species 46. The source of the 1,5-selectivity generated in this reaction is the effect of steric hindrance.
Scheme 44.
Synthesis of pyridyl-substituted 1,5-disubstituted 1,2,3-triazoles by Mn-porphyrin catalyzed cycloaddition of tetrazoles with terminal alkynes and proposed mechanism.
5. Conclusions
In this context, we briefly described the discovery and overviews of 1,5-selective click chemistry for the synthesis of 1,5-disubstituted 1,2,3-triazoles via metallacyclic intermediate, acetylide intermediate, or the elimination of substituents. There has been growing interest in 1,5-disubstituted 1,2,3-triazoles, and chemists have attempted and developed a number of processes that can effectively control the regioselectivity of the cycloaddition reaction by utilizing metal catalysts or controlling the substituent steric hindrance and electronic effect.
The 1,5-selective [3 + 2] cycloaddition of azides with alkynes is still an area that has not yet been fully developed. Many interesting problems are still waiting to be solved. The involvement of organometallic reagents can, of course, be effective in the rapid generation of anions, but there are problems, such as insufficient compatibility of functional groups or harsh reaction conditions unsuitable for chemical biology studies as well as metal residues. With the development of DFT theoretical calculations, more and more scientists are focusing on understanding reaction mechanisms and sources of regioselectivity, but the mechanisms are still unclear for most currently reported reactions.
The impact of 1,5-selective click chemistry is increasing tremendously day by day, not only in the field of organic synthesis but also in drug discovery efforts, polymer chemistry, and in different disciplines of material science. The cycloaddition reactions forming 1,5-disubstituted 1,2,3-triazoles have found extremely successful applications in the synthesis of nanostructures, protein conjugates, and polymeric materials due to their regiospecificity and unique chemoselectivity. This provides more possibilities for building functionalized and well-defined macromolecules and nanostructures that will be used in more areas.
Author Contributions
Writing—original draft preparation, Y.Z.; writing—review and editing, Z.C.; visualization, Q.Z.; supervision, W.-X.Z.; project administration, W.-X.Z.; funding acquisition, W.-X.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Nos. 22131001, 21890721, and 21725201).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rostovtsev V.V., Green L.G., Fokin V.V., Sharpless K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Prize Announcement. [(accessed on 3 January 2023)]. Available online: https://www.nobelprize.org/prizes/chemistry/2022/summary/
- 3.Abd-Elaal A.A., Aiad I., Shaban S.M., Tawfik S.M., Sayed A. Synthesis and evaluation of some triazole derivatives as corrosion inhibitors and biocides. J. Surfactants Deterg. 2014;17:483–491. doi: 10.1007/s11743-013-1547-0. [DOI] [Google Scholar]
- 4.Duan T., Fan K., Fu Y., Zhong C., Chen X., Peng T., Qin J. Triphenylamine-based organic dyes containing a 1,2,3-triazole bridge for dye-sensitized solar cells via a ‘Click’ reaction. Dyes Pigm. 2012;94:28–33. doi: 10.1016/j.dyepig.2011.11.008. [DOI] [Google Scholar]
- 5.Abu-Orabi S.T., Atfah M.A., Jibril I., Mari’i F.M., Ali A.A.-S. Dipolar cycloaddition reactions of organic azides with some acetylenic-compounds. J. Heterocycl. Chem. 1989;26:1461–1468. doi: 10.1002/jhet.5570260541. [DOI] [Google Scholar]
- 6.Jiang Y., Li X., Li X., Sun Y., Zhao Y., Jia S., Guo N., Xu G., Zhang W. Copper(II) acetylacetonate: An efficient catalyst for Huisgen-click reaction for synthesis of 1,2,3-triazoles in water. Chin. J. Chem. 2017;35:1239–1245. doi: 10.1002/cjoc.201700007. [DOI] [Google Scholar]
- 7.Shi S., Wang Z., Deng Y., Tian F., Wu Q., Zheng P. Combination of click chemistry and enzymatic ligation for stable and efficient protein immobilization for single-molecule force spectroscopy. CCS Chem. 2022;4:598–604. doi: 10.31635/ccschem.021.202100779. [DOI] [Google Scholar]
- 8.Totobenazara J., Burke A.J. New click-chemistry methods for 1,2,3-triazoles synthesis: Recent advances and applications. Tetrahedron Lett. 2015;56:2853–2859. doi: 10.1016/j.tetlet.2015.03.136. [DOI] [Google Scholar]
- 9.Jalani H.B., Karagöz A.C., Tsogoeva S.B. Synthesis of substituted 1,2,3-triazoles via metal-free click cycloaddition reactions and alternative cyclization methods. Synthesis. 2017;49:29–41. [Google Scholar]
- 10.Huisgen R. 1,3-Dipolar cycloadditions. Past and future. Angew. Chem. Int. Ed. 1963;2:565–598. doi: 10.1002/anie.196305651. [DOI] [Google Scholar]
- 11.Ríos-Gutiérrez M., Domingo L.R. Unravelling the mysteries of the [3+2] cycloaddition reactions. Eur. J. Org. Chem. 2019;2019:267–282. doi: 10.1002/ejoc.201800916. [DOI] [Google Scholar]
- 12.Hashjin M.C., Ciyabi R., Baharloui M., Hosseini G., Tavakoli H. Copper supported on the SiO2 nanoparticle in click chemistry: An alternative catalytic system for regioselective and one-pot synthesis of 1,2,3-triazoles and β-hydroxytriazoles. Chin. J. Chem. 2012;30:223–227. doi: 10.1002/cjoc.201100231. [DOI] [Google Scholar]
- 13.Albadi J., Keshavarz M., Abedini M., Vafaie-nezhad M. Copper iodide nanoparticles on poly(4-vinyl pyridine) as new and green catalyst for multicomponent click synthesis of 1,4-disubstituted-1,2,3-triazoles in water. Chin. Chem. Lett. 2012;23:797–800. doi: 10.1016/j.cclet.2012.05.009. [DOI] [Google Scholar]
- 14.Friscourt F., Boons G.J. One-pot three-step synthesis of 1,2,3-triazoles by copper-catalyzed cycloaddition of azides with alkynes formed by a sonogashira cross-coupling and desilylation. Org. Lett. 2010;12:4936–4939. doi: 10.1021/ol1022036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L., Chen X., Xue P., Sun H.H.Y., Williams I.D., Sharpless K.B., Fokin V.V., Jia G. Ruthenium-catalyzed cycloaddition of alkynes and organic azides. J. Am. Chem. Soc. 2005;127:15998–15999. doi: 10.1021/ja054114s. [DOI] [PubMed] [Google Scholar]
- 16.Jasiński R. Nitroacetylene as dipolarophile in [2+3] cycloaddition reactions with allenyl-type three-atom components: DFT computational study. Monatsh. Chem. 2015;146:591–599. doi: 10.1007/s00706-014-1389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C., Ikhlef D., Kahlal S., Saillard J.Y., Astruc D. Metal-catalyzed azide-alkyne "click" reactions: Mechanistic overview and recent trends. Coord. Chem. Rev. 2016;316:1–20. doi: 10.1016/j.ccr.2016.02.010. [DOI] [Google Scholar]
- 18.Gomes R.S., Jardim G.A.M., de Carvalho R.L., Araujo M.H., da Silva E.N. Beyond copper-catalyzed azide-alkyne 1,3-dipolar cycloaddition: Synthesis and mechanism insights. Tetrahedron. 2019;75:3697–3712. doi: 10.1016/j.tet.2019.05.046. [DOI] [Google Scholar]
- 19.Johansson J.R., Beke-Somfai T., Stålsmeden A.S., Kann N. Ruthenium-catalyzed azide alkyne cycloaddition reaction: Scope, mechanism, and applications. Chem. Rev. 2016;116:14726–14768. doi: 10.1021/acs.chemrev.6b00466. [DOI] [PubMed] [Google Scholar]
- 20.Singh M.S., Chowdhury S., Koley S. Advances of azide-alkyne cycloaddition-click chemistry over the recent decade. Tetrahedron. 2016;72:5257–5283. doi: 10.1016/j.tet.2016.07.044. [DOI] [Google Scholar]
- 21.Lauria A., Delisi R., Mingoia F., Terenzi A., Martorana A., Barone G., Almerico A.M. 1,2,3-Triazole in heterocyclic compounds, endowed with biological activity, through 1,3-dipolar cycloadditions. Eur. J. Org. Chem. 2014;2014:3289–3306. doi: 10.1002/ejoc.201301695. [DOI] [Google Scholar]
- 22.Chen Z., Cao G., Song J., Ren H. Recent developments in azide-free synthesis of 1,2,3-triazoles. Chin. J. Chem. 2017;35:1797–1807. doi: 10.1002/cjoc.201700459. [DOI] [Google Scholar]
- 23.Agrahari A.K., Bose P., Jaiswal M.K., Rajkhowa S., Singh A.S., Hotha S., Mishra N., Tiwari V.K. Cu(I)-catalyzed click chemistry in glycoscience and their diverse applications. Chem. Rev. 2021;121:7638–7955. doi: 10.1021/acs.chemrev.0c00920. [DOI] [PubMed] [Google Scholar]
- 24.Opsomer T., Dehaen W. Metal-free syntheses of N-functionalized and NH-1,2,3-triazoles: An update on recent developments. Chem. Commun. 2021;57:1568–1590. doi: 10.1039/D0CC06654K. [DOI] [PubMed] [Google Scholar]
- 25.Oppilliart S., Mousseau G., Zhang L., Jia G., Thuery P., Rousseau B., Cintrat J.C. 1-Protected 5-amido 1,2,3-triazoles via ruthenium-catalyzed [3+2] cycloaddition of azides and ynamides. Tetrahedron. 2007;63:8094–8098. doi: 10.1016/j.tet.2007.06.008. [DOI] [Google Scholar]
- 26.Nulwala H., Takizawa K., Odukale A., Khan A., Thibault R.J., Taft B.R., Lipshutz B.H., Hawker C.J. Synthesis and characterization of isomeric vinyl-1,2,3-triazole materials by azide-alkyne click chemistry. Macromolecules. 2009;42:6068–6074. doi: 10.1021/ma900892h. [DOI] [Google Scholar]
- 27.Wuest F., Tang X., Kniess T., Pietzsch J., Suresh M. Synthesis and cyclooxygenase inhibition of various (aryl-1,2,3-triazole-1-yl)-methanesulfonylphenyl derivatives. Bioorg. Med. Chem. 2009;17:1146–1151. doi: 10.1016/j.bmc.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 28.Wang D., Salmon L., Ruiz J., Astruc D. A recyclable ruthenium(II) complex supported on magnetic nanoparticles: A regioselective catalyst for alkyne-azide cycloaddition. Chem. Commun. 2013;49:6956–6958. doi: 10.1039/c3cc43048k. [DOI] [PubMed] [Google Scholar]
- 29.Johansson J.R., Lincoln P., Nordén B., Kann N. Sequential one-pot ruthenium-catalyzed azide-alkyne cycloaddition from primary alkyl halides and sodium azide. J. Org. Chem. 2011;76:2355–2359. doi: 10.1021/jo200134a. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen L.K., Boren B.C., Fokin V.V. Ruthenium-catalyzed cycloaddition of aryl azides and alkynes. Org. Lett. 2007;9:5337–5339. doi: 10.1021/ol701912s. [DOI] [PubMed] [Google Scholar]
- 31.Boren B.C., Narayan S., Rasmussen L.K., Zhang L., Zhao H., Lin Z., Jia G., Fokin V.V. Ruthenium-catalyzed azide-alkyne cycloaddition: Scope and mechanism. J. Am. Chem. Soc. 2008;130:8923–8930. doi: 10.1021/ja0749993. [DOI] [PubMed] [Google Scholar]
- 32.Johansson J.R., Hermansson E., Nordén B., Kann N., Beke-Somfai T. Peptides from RuAAC-derived 1,5-disubstituted triazole units. Eur. J. Org. Chem. 2014;2014:2703–2713. doi: 10.1002/ejoc.201400018. [DOI] [Google Scholar]
- 33.Stålsmeden A.S., Paterson A.J., Szigyártó I.C., Thunberg L., Johansson J.R., Beke-Somfai T., Kann N. Chiral 1,5-disubstituted 1,2,3-triazoles—Versatile tools for foldamers and peptidomimetic applications. Org. Biomol. Chem. 2020;18:1957–1967. doi: 10.1039/D0OB00168F. [DOI] [PubMed] [Google Scholar]
- 34.Wang T.-H., Wu F.-L., Chiang G.-R., He S.-T., Lo Y.-H. Preparation of ruthenium azido complex containing a Tp ligand and ruthenium-catalyzed cycloaddition of organic azides with alkynes in organic and aqueous media: Experimental and computational studies. J. Organomet. Chem. 2014;774:57–60. doi: 10.1016/j.jorganchem.2014.09.038. [DOI] [Google Scholar]
- 35.Lamberti M., Fortman G.C., Poater A., Broggi J., Slawin A.M.Z., Cavallo L., Nolan S.P. Coordinatively unsaturated ruthenium complexes as efficient alkyne-azide cycloaddition catalysts. Organometallics. 2012;31:756–767. doi: 10.1021/om2012425. [DOI] [Google Scholar]
- 36.Kim W.G., Kang M.E., Lee J.B., Jeon M.H., Lee S., Lee J., Cho B., Cal P., Kang S., Kee J.M., et al. Nickel-catalyzed azide-alkyne cycloaddition to access 1,5-disubstituted 1,2,3-triazoles in air and water. J. Am. Chem. Soc. 2017;139:12121–12124. doi: 10.1021/jacs.7b06338. [DOI] [PubMed] [Google Scholar]
- 37.Kim W.G., Baek S.Y., Jeong S.Y., Nam D., Jeon J.H., Choe W., Baik M.H., Hong S.Y. Chemo- and regioselective click reactions through nickel-catalyzed azide-alkyne cycloaddition. Org. Biomol. Chem. 2020;18:3374–3381. doi: 10.1039/D0OB00579G. [DOI] [PubMed] [Google Scholar]
- 38.Akimova G., Chistokletov V., Petrov A. 1,3-dipolar addition to unsaturated compounds XVII. The reaction of azides with Iotsich complexes obtained from phenyl- and alkenylacetylenes. Zh. Obshch. Khim. 1967;3:968–974. [Google Scholar]
- 39.Krasinski A., Fokin V.V., Sharpless K.B. Direct synthesis of 1,5-disubstituted-4-magnesio-1,2,3-triazoles, revisited. Org. Lett. 2004;6:1237–1240. doi: 10.1021/ol0499203. [DOI] [PubMed] [Google Scholar]
- 40.Banday A., Hruby V. Regioselective N/C-heterocyclization of allenylindium bromide across aryl azides: One-pot synthesis of 5-methyl-1,2,3-triazoles. Synlett. 2014;25:1859–1862. doi: 10.1055/s-0034-1378327. [DOI] [Google Scholar]
- 41.Kwok S.W., Fotsing J.R., Fraser R.J., Rodionov V.O., Fokin V.V. Transition-metal-free catalytic synthesis of 1,5-diaryl-1,2,3-triazoles. Org. Lett. 2010;12:4217–4219. doi: 10.1021/ol101568d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith C.D., Greaney M.F. Zinc mediated azide-alkyne ligation to 1,5-and 1,4,5-substituted 1,2,3-triazoles. Org. Lett. 2013;15:4826–4829. doi: 10.1021/ol402225d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y., Qi X., Lei Y., Lan Y. Mechanism and selectivity for zinc-mediated cycloaddition of azides with alkynes: A computational study. RSC Adv. 2015;5:49802–49808. doi: 10.1039/C5RA02703A. [DOI] [Google Scholar]
- 44.Liu B., Cui D. Rare-earth metal complexes stabilized by amino-phosphine ligand. Reaction with mesityl azide and catalysis of the cycloaddition of organic azides and aromatic alkynes. Dalton Trans. 2009;3:550–556. doi: 10.1039/B811363G. [DOI] [PubMed] [Google Scholar]
- 45.Hong L., Lin W., Zhang F., Liu R., Zhou X. Ln[N(SiMe3)2]3-catalyzed cycloaddition of terminal alkynes to azides leading to 1,5-disubstituted 1,2,3-triazoles: New mechanistic features. Chem. Commun. 2013;49:5589–5591. doi: 10.1039/c3cc42534g. [DOI] [PubMed] [Google Scholar]
- 46.Wang J.-M., Yu S.-B., Li Z.-M., Wang Q.-R., Li Z.-T. Mechanism of samarium-catalyzed 1,5-regioselective azide-alkyne [3+2]-cycloaddition: A quantum mechanical investigation. J. Phys. Chem. A. 2015;119:1359–1368. doi: 10.1021/jp5104615. [DOI] [PubMed] [Google Scholar]
- 47.Wu L., Chen Y., Luo J., Sun Q., Peng M., Lin Q. Base-mediated reaction of vinyl bromides with aryl azides: One-pot synthesis of 1,5-disubstituted 1,2,3-triazoles. Tetrahedron Lett. 2014;55:3847–3850. doi: 10.1016/j.tetlet.2014.03.029. [DOI] [Google Scholar]
- 48.Zhang X., Rakesh K.P., Qin H. Transition-metal-free regioselective construction of 1,5-diaryl-1,2,3-triazoles through dehydrative cycloaddition of alcohols with aryl azides mediated by SO2F2. Chem. Commun. 2019;55:2845–2848. doi: 10.1039/C8CC09693G. [DOI] [PubMed] [Google Scholar]
- 49.Zha G., Fang W., Li Y., Leng J., Chen X., Qin H.L. SO2F2-mediated oxidative dehydrogenation and dehydration of alcohols to alkynes. J. Am. Chem. Soc. 2018;140:17666–17673. doi: 10.1021/jacs.8b10069. [DOI] [PubMed] [Google Scholar]
- 50.Pathak T., Dey S., Datta D. A metal-free, aqueous and general route to 1,5-disubstituted-1,2,3-triazoles: Reversed regioisomeric 1,3-dipolar cycloaddition of azides and vinyl sulfones. Synlett. 2011;2011:2521–2524. doi: 10.1055/s-0030-1260306. [DOI] [Google Scholar]
- 51.Kayet A., Pathak T. 1,5-Disubstituted 1,2,3-Triazolylation at C1, C2, C3, C4, and C6 of pyranosides: A metal-free route to triazolylated monosaccharides and triazole-linked disaccharides. J. Org. Chem. 2013;78:9865–9875. doi: 10.1021/jo401576n. [DOI] [PubMed] [Google Scholar]
- 52.Kayet A., Dey S., Pathak T. A metal free aqueous route to 1,5-disubstituted 1,2,3-triazolylated monofuranosides and difuranosides. Tetrahedron Lett. 2015;56:5521–5524. doi: 10.1016/j.tetlet.2015.08.030. [DOI] [Google Scholar]
- 53.Dey S., Pathak T. A general route to 1,5-disubstituted 1,2,3-triazoles with alkyl/alkyl, alkyl/aryl, aryl/aryl combinations: A metal-free, regioselective, one-pot three component approach. RSC Adv. 2014;4:9275–9278. doi: 10.1039/c3ra47062h. [DOI] [Google Scholar]
- 54.Wang Y.-C., Xie Y.-Y., Qu H.-E., Wang H.-S., Pan Y.-M., Huang F.-P. Ce(OTf)3-catalyzed [3+2] cycloaddition of azides with nitroolefins: Regioselective synthesis of 1,5-disubstituted 1,2,3-triazoles. J. Org. Chem. 2014;79:4463–4469. doi: 10.1021/jo5004339. [DOI] [PubMed] [Google Scholar]
- 55.De Nino A., Merino P., Algieri V., Nardi M., Di Gioia M.L., Russo B., Tallarida M.A., Maiuolo L. Synthesis of 1,5-functionalized 1,2,3-triazoles using ionic liquid/Iron(III) chloride as an efficient and reusable homogeneous catalyst. Catalysts. 2018;8:364. doi: 10.3390/catal8090364. [DOI] [Google Scholar]
- 56.Gangaprasad D., Raj J.P., Kiranmye T., Sasikala R., Karthikeyan K., Rani S.K., Elangovan J. A tunable route to oxidative and eliminative [3+2] cycloadditions of organic azides with nitroolefins: CuO nanoparticles catalyzed synthesis of 1,2,3-triazoles under solvent-free condition. Tetrahedron Lett. 2016;57:3105–3108. doi: 10.1016/j.tetlet.2016.06.004. [DOI] [Google Scholar]
- 57.Kiranmye T., Vadivelu M., Sampath S., Muthu K., Karthikeyan K. Ultrasound-assisted catalyst free synthesis of 1,4-/1,5-disubstituted-1,2,3-triazoles in aqueous medium. Sustain. Chem. Pharm. 2021;19:100358. doi: 10.1016/j.scp.2020.100358. [DOI] [Google Scholar]
- 58.Mishra K.B., Tiwari V.K. One-pot facile synthesis of 1,5-disubstituted triazolyl glycoconjugates from nitrostyrenes. Chemistryselect. 2016;1:3693–3698. doi: 10.1002/slct.201600994. [DOI] [Google Scholar]
- 59.Qureshi A.A., Kumar A.S., Chauhan S., Swamy K.C.K. Stereo- and regioselective [3+2] cycloaddition of acetoxy allenoates with azides: Metal-free synthesis of multisubstituted triazoles. Synthesis. 2022;54:965–974. doi: 10.1055/s-0040-1719838. [DOI] [Google Scholar]
- 60.Coats S.J., Link J.S., Gauthier D., Hlasta D.J. Trimethylsilyl-directed 1,3-dipolar cycloaddition reactions in the solid-phase synthesis of 1,2,3-triazoles. Org. Lett. 2005;7:1469–1472. doi: 10.1021/ol047637y. [DOI] [PubMed] [Google Scholar]
- 61.Wu L., Chen Y., Tang M., Song X., Chen G., Song X., Lin Q. Potassium tert-butoxide promoted cycloaddition reaction for the synthesis of 1,5-disubstituted 1,2,3-triazoles from aromatic azides and trimethylsilyl-protected alkynes. Synlett. 2012;23:1529–1533. doi: 10.1055/s-0031-1291042. [DOI] [Google Scholar]
- 62.Kloss F., Köhn U., Jahn B.O., Hager M.D., Görls H., Schubert U.S. Metal-free 1,5-regioselective azide-alkyne [3+2]-cycloaddition. Chem. Asian. J. 2011;6:2816–2824. doi: 10.1002/asia.201100404. [DOI] [PubMed] [Google Scholar]
- 63.Blastik Z.E., Klepetářová B., Beier P. Enamine-mediated azide-ketone [3+2] cycloaddition of azidoperfluoroalkanes. Chemistryselect. 2018;3:7045–7048. doi: 10.1002/slct.201801344. [DOI] [Google Scholar]
- 64.González-Calderón D., Fuentes-Benítes A., Díaz-Torres E., González-González C.A., González-Romero C. Azide-enolate 1,3-dipolar cycloaddition as an efficient approach for the synthesis of 1,5-disubstituted 1,2,3-triazoles from alkyl/aryl azides and β-ketophosphonates. Eur. J. Org. Chem. 2016;2016:668–672. doi: 10.1002/ejoc.201501465. [DOI] [Google Scholar]
- 65.Pokhodylo N.T., Tupychak M.A., Obushak M.D. Metal-free synthesis of 1,5-disubstituted 1,2,3-triazoles. Russ. J. Org. Chem. 2022;58:209–218. doi: 10.1134/S1070428022020087. [DOI] [Google Scholar]
- 66.Kumar N., Ansari M.Y., Kant R., Kumar A. Copper-catalyzed decarboxylative regioselective synthesis of 1,5-disubstituted 1,2,3-triazoles. Chem. Commun. 2018;54:2627–2630. doi: 10.1039/C7CC09934G. [DOI] [PubMed] [Google Scholar]
- 67.Khatua H., Das S.K., Roy S., Chattopadhyay B. Dual reactivity of 1,2,3,4-tetrazole: Manganese-catalyzed click reaction and denitrogenative annulation. Angew. Chem. Int. Ed. 2021;60:304–312. doi: 10.1002/anie.202009078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.