Abstract
Cinnamomum camphora is a traditional aromatic plant used to produce linalool and borneol flavors in southern China; however, its leaves also contain many other unutilized essential oils. Herein, we report geographic relationships for the yield and compositional diversity of C. camphora essential oils. The essential oils of 974 individual trees from 35 populations in 13 provinces were extracted by hydrodistillation and analyzed qualitatively and quantitatively by gas chromatography-mass spectrometry and gas chromatography-flame ionization detection, respectively. Oil yields ranged from 0.01% to 3.46%, with a significantly positive correlation with latitude and a significantly negative correlation with longitude. In total, 41 compounds were identified, including 15 monoterpenoids, 24 sesquiterpenoids, and two phenylpropanoids. Essential oil compositions varied significantly among individuals and could be categorized into various chemotypes. The six main chemotypes were eucalyptol, nerolidol, camphor, linalool, selina, and mixed types. The other 17 individual plants were chemotypically rare and exhibited high levels of methyl isoeugenol, methyl eugenol, δ-selinene, or borneol. Eucalyptol-type plants had the highest average oil yield of 1.64%, followed in decreasing order by linalool-, camphor-, mixed-, selina-, and nerolidol-type plants. In addition, the five main compounds exhibited a clear geographic gradient. Eucalyptol and linalool showed a significantly positive correlation with latitude, while selina-6-en-4-ol was significantly and negatively correlated with latitude. trans-Nerolidol and selina-6-en-4-ol showed significantly positive correlations with longitude, whereas camphor was significantly and negatively correlated with longitude. Canonical correspondence analysis indicated that environmental factors could strong effect the oil yield and essential oil profile of C. camphora.
Keywords: Cinnamomum camphora, essential oil, oil yield, chemotypes, environmental factors
1. Introduction
Cinnamomum camphora, commonly known as the camphor tree, is a large evergreen tree in the Lauraceae family, which is native to China, Japan, and adjoining regions of South-East Asia [1]. It is an important commercial, timber, ornamental, and ecological tree species that is widely planted in the area south of the Yangtze River Basin in China [2]. Because of their beautiful shape, C. camphora trees have been introduced or cultivated as ornamental and street trees in many countries, including Australia, Holland, the Philippines, the USA, Cuba, and Sri Lanka [1]. In addition, many terpenoids are derived from their roots, trunks, branches, and leaves; thus, C. camphora is an important source of natural camphor and other fragrances and spices.
Linalool, camphor, and borneol derived from C. camphora are the most traded and consumed components of its essential oil. Among these compounds, borneol has various pharmaceutical functions in traditional Chinese medicine [3,4,5]. C. camphora is cultivated as an industrial crop in southern China, and its aerial parts are harvested in autumn to produce linalool or borneol. The camphor essential oil industry has become a characteristic industry, generating more than 10 billion Chinese yuan per annum, and the planting area in Jiangxi Province exceeds 10 thousand ha. C. camphora essential oil is compositionally diverse, containing nearly 100 detected compounds, including terpenes (such as myrcene, fenchol, and α-terpineol) and phenylpropenes (such as eugenol and safrole) [6,7,8].
Plant essential oils contain somewhat volatile secondary metabolites produced in response to biological and abiotic stressors. Various environmental factors affect the biosynthesis and accumulation of plant essential oils; thus, the content and composition of essential oils vary within the same species depending on location [9,10]. Chemotyping is an efficient way to describe individuals with similar essential oil compounds. C. camphora has been classified into different chemotypes based on compositional differences of the leaf essential oil, and the chemotypes vary in different regions [11,12,13]. Five chemotypes of C. camphora, named according to their dominant essential oil, are common in Jiangxi Province, including camphor, linalool, eucalyptol, isonerolidol, and borneol types [11]. The safrole and mixed types, featuring balanced oil composition, and three other chemotypes (linalool, eucalyptol, and camphor types) have been identified in Fujian Province [12]. Liu et al. [13] divided C. camphora from Guangxi Province into eucalyptol, linalool, camphor, terpene-terpineol (rich in α-pinene, sabinene, and 4-terpineol), and nerolidol types.
While the characteristics of camphor tree essential oils have been thoroughly studied, the large-scale essential oil diversity of camphor trees has been relatively overlooked. Chemotypic diversity may be related to genetic, physiological, and environmental factors, including climatic, topographic, hydrological, and edaphic factors [14,15,16,17]. The objective of this study was to characterize the chemotypic diversity of the major native camphor tree populations in China. A total of 974 individuals were studied from 35 populations ranging geographically from Sichuan to Zhejiang and from Shanxi to Guangdong. This study provides insight into the intra- and inter-population diversity of essential oils derived from camphor trees in China and a basis for the utilization and conservation of camphor tree resources.
2. Results
2.1. Variability in Essential Oil Yield
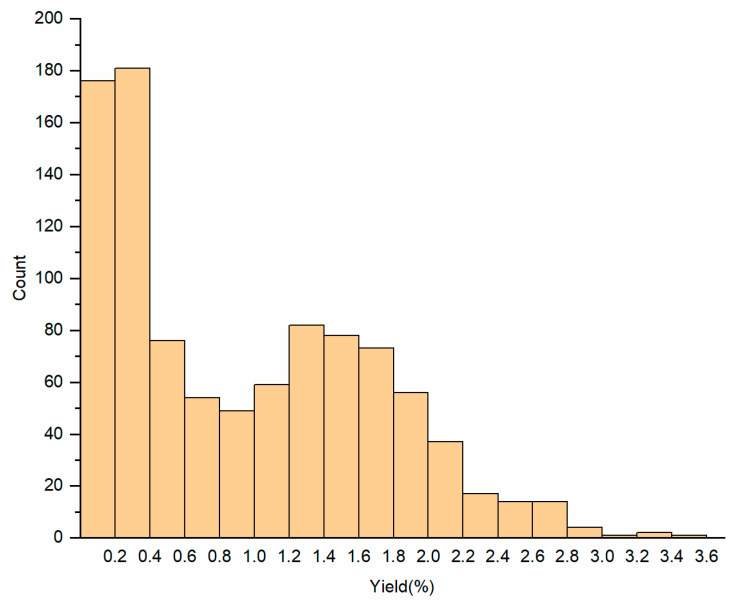
The leaf essential oils of 974 C. camphora individuals from 35 populations in 13 provinces were extracted and analyzed. A distribution interval of 0.2% was used to draw the frequency distribution map (Figure 1). The average essential oil yield of all individuals was 0.95%, and different individuals presented yields in the range of 0.01–3.46%. As shown in Figure 1, 176 individuals had essential oil yields less than 0.2%, and 181 individuals had essential oil yields between 0.2% to 0.4%. Nearly half of the individuals had relatively low essential oil yields (<0.6%), while a few individuals (53) had yields of >2.20%, accounting for 5.44% of the total samples. Additionally, several individuals with particularly high essential oil yields of >3.0%, including HB-ES-10 (3.46%), AH-QM-20 (3.28%), JX-AF-11 (3.24%), and JX-AF-38 (3.03%), were potentially excellent individuals for commercial development.
Figure 1.
Frequency distribution of the essential oil yield of C. camphora.
There was a significant difference among the essential oil yields of the different populations (p < 0.01). Camphor trees from Qimen had the highest mean essential oil yield (2.01%), followed by those from the Xinhua, Hanzhong, and Enshi populations, with mean oil yields of 1.70%, 1.54%, and 1.51%, respectively (Table 1). These four populations had high oil yields (>1.50%). Two populations from Guangdong (GD-SG and GD-ZJ), two from Fujian (FJ-FZ and FJ-HA), one from Jiangsu (JS-ZJ), one from Sichuan (SC-LX), and one from Zhejiang (ZJ-YY) exhibited low essential oil yields. Pearson’s correlation coefficient analysis revealed that essential oil yield was positively correlated with latitude. In other words, trees growing at high latitudes had higher oil yields than those growing at low latitudes. In contrast, essential oil yield was negatively correlated with longitude; higher oil yields were detected in camphor trees in western China (Table 2).
Table 1.
Geographical information of samples of C. camphora populations with mean essential oil yield of each population.
| Site | Province | Code | N 1 | E | MAAT | MAIT | MAP | Elevation | n | Oil Yield (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Qimen | Anhui | AH-QM | 29.696716° | 117.506601° | 23 | 13 | 2396 | 90 | 30 | 2.01 ± 0.74 |
| Shapingba | Chongqing | CQ-SPB | 29.579210° | 106.417387° | 23 | 16 | 982 | 465 | 30 | 0.92 ± 0.16 |
| Fuzhou | Fujian | FJ-FZ | 26.157219° | 119.283930° | 27 | 18 | 1836 | 86 | 30 | 0.49 ± 0.50 |
| Huaan | Fujian | FJ-HA | 24.959486° | 117.534795° | 28 | 17 | 2989 | 205 | 10 | 0.48 ± 0.14 |
| Yongan | Fujian | FJ-YA | 26.011571° | 117.390753° | 27 | 16 | 2876 | 224 | 30 | 0.61 ± 0.44 |
| Youxi | Fujian | FJ-YX | 26.040294° | 118.128517° | 27 | 16 | 2769 | 176 | 30 | 0.55 ± 0.35 |
| Heyuan | Guangdong | GD-HY | 23.718087° | 115.209347° | 28 | 17 | 2648 | 343 | 28 | 0.66 ± 0.63 |
| Meizhou | Guangdong | GD-MZ | 24.146901° | 116.076344° | 28 | 18 | 2099 | 132 | 30 | 0.68 ± 0.59 |
| Shaoguan | Guangdong | GD-SG | 24.849486° | 113.562527° | 26 | 17 | 2814 | 82 | 27 | 0.28 ± 0.25 |
| Shixing | Guangdong | GD-SX | 24.855446° | 114.100072° | 27 | 16 | 2867 | 199 | 17 | 0.95 ± 0.92 |
| Zhanjiang | Guangdong | GD-ZJ | 21.126940° | 110.246939° | 28 | 21 | 1909 | 13 | 27 | 0.36 ± 0.47 |
| Gaofeng | Guangxi | GX-GF | 22.931939° | 108.357974° | 27 | 19 | 2014 | 118 | 28 | 1.10 ± 0.89 |
| Quanzhou | Guangxi | GX-QZ | 25.986144° | 110.921399° | 25 | 16 | 1979 | 184 | 30 | 0.91 ± 0.68 |
| Guian | Guizhou | GZ-GA | 26.412591° | 106.369501° | 20 | 13 | 1490 | 1226 | 29 | 1.47 ± 0.67 |
| Guiyang | GZ-GY | 26.496424° | 106.735431° | 19 | 12 | 1981 | 1124 | 32 | 1.00 ± 0.66 | |
| Enshi | Hubei | HB-ES | 30.281372° | 109.484778° | 23 | 14 | 1263 | 430 | 30 | 1.51 ± 0.86 |
| Xianning | Hubei | HB-XN | 29.569482° | 114.862824° | 23 | 13 | 1445 | 57 | 29 | 1.41 ± 0.46 |
| Changde | Hunan | HN-CD | 29.050278° | 111.592693° | 22 | 15 | 1327 | 91 | 30 | 1.35 ± 0.45 |
| Changsha | Hunan | HN-CS | 28.111381° | 113.054928° | 24 | 16 | 1591 | 90 | 30 | 0.69 ± 0.57 |
| Chenzhou | Hunan | HN-CZ | 25.789165° | 112.996943° | 23 | 15 | 2524 | 211 | 28 | 1.09 ± 0.70 |
| Xinhua | Hunan | HN-XH | 27.780197° | 111.180908° | 24 | 15 | 2204 | 181 | 30 | 1.70 ± 0.46 |
| Yongzhou | Hunan | HN-YZ | 26.391109° | 111.761389° | 24 | 16 | 822 | 176 | 30 | 1.02 ± 0.49 |
| Zhenjiang | Jiangsu | JS-ZJ | 32.175807° | 119.441861° | 21 | 12 | 1444 | 33 | 30 | 0.41 ± 0.47 |
| Anfu | Jiangxi | JX-AF | 27.406026° | 114.560550° | 25 | 15 | 2656 | 116 | 38 | 1.18 ± 1.00 |
| Dexing | Jiangxi | JX-DX | 28.844566° | 117.579117° | 25 | 15 | 2478 | 49 | 27 | 1.09 ± 0.84 |
| Jinxi | Jiangxi | JX-JX | 27.871332° | 116.584137° | 25 | 15 | 2821 | 47 | 11 | 0.89 ± 0.55 |
| Nanchang | Jiangxi | JX-NC | 28.779930° | 115.779720° | 24 | 16 | 2515 | 129 | 29 | 0.78 ± 0.69 |
| Ruijin | Jiangxi | JX-RJ | 25.864169° | 115.932219° | 26 | 16 | 2604 | 223 | 30 | 0.80 ± 0.54 |
| Wuyuan | Jiangxi | JX-WY | 29.252720° | 117.859167° | 25 | 14 | 2787 | 80 | 29 | 1.12 ± 0.73 |
| Xingguo | Jiangxi | JX-XG | 26.495143° | 115.771943° | 26 | 17 | 2341 | 265 | 29 | 1.42 ± 0.68 |
| Luxian | Sichuan | SC-LX | 29.138419° | 105.385134° | 23 | 15 | 1168 | 478 | 29 | 0.47 ± 0.35 |
| Hanzhong | Shaanxi | SX-HZ | 33.135874° | 107.301057° | 22 | 12 | 1764 | 443 | 30 | 1.54 ± 0.49 |
| Changxing | Zhejiang | ZJ-CX | 30.977313° | 119.609480° | 23 | 14 | 1614 | 115 | 30 | 0.60 ± 0.62 |
| Tonglu | Zhejiang | ZJ-TL | 29.818942° | 119.757321° | 23 | 13 | 2392 | 51 | 14 | 0.77 ± 0.65 |
| Yuyao | Zhejiang | ZJ-YY | 30.038909° | 120.992886° | 23 | 14 | 2972 | 19 | 33 | 0.44 ± 0.44 |
1 Abbreviations: N: latitude, E: longitude, MAAT: mean annual maximum temperature, MAIT: mean annual minimum temperature, MAP: mean annual precipitation.
Table 2.
Pearson correlation coefficients between essential oil and geographical position.
| Latitude | Longitude | |
|---|---|---|
| Yield | 0.162 ** | −0.174 ** |
| Eucalyptol | 0.175 ** | −0.014 |
| Linalool | 0.096 ** | −0.059 |
| Camphor | −0.014 | −0.282 ** |
| Selina-6-en-4-ol | −0.224 ** | 0.154 ** |
| trans-Nerolidol | 0.003 | 0.189 ** |
** Correlation was significant at the level of 0.01.
2.2. Essential Oil Profiles
A total of 41 compounds, including 15 monoterpenoids, 24 sesquiterpenoids, and two phenylpropanoids, were identified in the leaf essential oil of the camphor tree (Table 3). Interestingly, the vast majority of individuals in natural populations had distinct single principal components. The main component of 281 samples was eucalyptol, and its relative content in the essential oil was in the range of 11.63–65.33%. A total of 155 samples were mainly composed of linalool, and its relative content in the essential oil was in the range of 11.23–96.45%. A total of 168 and 274 samples were mainly composed of camphor and trans-nerolidol, respectively, and their relative contents in the essential oil were in the ranges of 13.89–87.69% and 15.24–88.66%, respectively. Eucalyptol, linalool, camphor, and trans-nerolidol with mean relative contents of >10% were considered major compounds. For example, the relative content of eucalyptol in GD-SX-22 essential oil was 65.33%, that of linalool in FJ-YX-29 was 93.73%, that of camphor in HN-CS-14 was 84.35%, and that of trans-nerolidol in JX-AF-14 was 60.30% (Table 3). Although the number of monoterpenoids was less than that of sesquiterpenoids, three of the four major compounds (eucalyptol, linalool, and camphor) were monoterpenoids, indicating that monoterpenoids dominated camphor tree essential oil.
Table 3.
Relative content of 41 compounds in essential oil and samples with different chemotypes.
| Constituents | RI 1 | Mean Content 2 (%) | GD-SX -22 |
FJ-YX -29 |
HN-CS -14 |
JX-AF -14 |
GX-QZ -03 |
GX-GF -22 |
GX-GF -06 |
|---|---|---|---|---|---|---|---|---|---|
| α-Pinene | 939 | 1.20 | 2.91 | - | 0.63 | 0.06 | 1.56 | - | - |
| β-Phellandrene | 978 | 3.75 | 10.47 | - | - | 0.04 | 0.35 | 0.04 | - |
| α-Phellandrene | 1011 | 0.10 | 0.01 | - | - | 0.04 | 0.32 | 0.06 | - |
| D-limonene | 1035 | 0.40 | - | - | 1.15 | 0.17 | 1.90 | 0.05 | - |
| Eucalyptol | 1039 | 15.14 | 65.33 | 0.03 | 1.63 | 0.38 | 1.77 | 0.07 | - |
| γ-Terpinene | 1063 | 0.26 | - | - | - | - | - | 0.17 | - |
| trans-Linalool oxide | 1074 | 0.54 | 0.01 | 0.15 | 0.22 | 0.03 | 0.42 | 3.78 | - |
| Linalool | 1106 | 13.57 | 1.59 | 93.73 | - | 0.39 | 2.87 | 0.44 | 0.02 |
| β-Terpineol | 1109 | 0.28 | - | - | - | 0.04 | 0.16 | 3.58 | - |
| Camphor | 1155 | 12.77 | - | 0.23 | 84.35 | 0.75 | 23.37 | 0.01 | 0.02 |
| Borneol | 1181 | 0.47 | 0.13 | - | 0.22 | 0.09 | 52.22 | - | 0.15 |
| 4-Terpineol | 1188 | 0.95 | 2.99 | 0.04 | 0.52 | 0.03 | 0.46 | 4.49 | 0.02 |
| α-Terpineol | 1205 | 3.75 | 9.25 | 0.27 | 1.03 | 0.23 | 1.32 | 0.43 | 0.15 |
| trans-Geraniol | 1229 | 0.13 | 0.20 | - | - | 0.04 | - | 0.02 | - |
| Bornyl acetate | 1290 | 0.14 | - | - | - | 0.14 | - | 0.13 | 0.03 |
| δ-Elemene | 1342 | 0.43 | 0.05 | 0.08 | 0.30 | 0.17 | 0.58 | 0.36 | 0.53 |
| Methyl eugenol | 1401 | 0.21 | - | 0.01 | - | - | - | 7.03 | - |
| β-Caryophyllene | 1433 | 2.93 | 0.54 | 0.37 | 2.32 | 2.92 | 1.91 | 3.05 | 4.35 |
| γ-Elemene | 1439 | 0.10 | 0.01 | - | - | 0.09 | - | - | 0.01 |
| Humulene | 1469 | 1.53 | 0.01 | - | 1.14 | 0.89 | 1.28 | 2.36 | 2.46 |
| Valencene | 1485 | 0.23 | 0.01 | - | - | 0.03 | - | 0.07 | 0.62 |
| γ-Muurolene | 1483 | 0.20 | - | - | - | - | - | 0.02 | 1.00 |
| Germacrene D | 1489 | 1.04 | 0.17 | 0.31 | 1.40 | 0.93 | 0.41 | - | 0.34 |
| δ-Selinene | 1496 | 0.88 | - | - | 1.22 | - | 1.87 | - | 7.80 |
| α-Guaiene | 1503 | 0.89 | 0.06 | 0.07 | 1.27 | 0.04 | 1.33 | - | 0.29 |
| Methyl isoeugenol | 1509 | 1.73 | 0.01 | 0.43 | - | 0.32 | - | 71.99 | 0.30 |
| Copaene | 1542 | 0.36 | - | - | - | 0.03 | - | 0.01 | 0.07 |
| α-Muurolene | 1547 | 0.25 | - | - | - | - | - | 0.01 | - |
| Elemol | 1559 | 0.29 | - | 0.02 | 0.23 | 0.68 | - | 0.05 | 0.52 |
| trans-Nerolidol | 1564 | 13.93 | - | 0.02 | 0.23 | 60.30 | - | 0.04 | 0.43 |
| Germacrene B | 1574 | 0.50 | 0.05 | 0.07 | - | 0.01 | - | 0.02 | 1.13 |
| Spathulenol | 1589 | 0.85 | 0.01 | 0.10 | - | 0.46 | - | 0.02 | 1.90 |
| caryophyllene oxide | 1598 | 0.90 | 0.03 | 0.05 | - | 1.03 | - | 0.03 | 0.53 |
| Guaiol | 1609 | 0.24 | 0.04 | - | - | 2.71 | - | 0.12 | - |
| Selina-6-en-4-ol | 1637 | 5.58 | - | 0.02 | - | 0.11 | - | 0.01 | 71.20 |
| Viridiflorol | 1648 | 0.10 | - | - | - | 0.07 | - | 0.02 | 0.28 |
| τ-Muurolol | 1657 | 0.34 | 0.01 | - | - | 0.06 | - | - | 0.20 |
| α-Cadinol | 1667 | 0.18 | - | - | - | - | 0.17 | - | 0.81 |
| Juniper camphor | 1672 | 0.52 | 0.07 | 0.01 | - | 0.78 | - | 0.13 | - |
| Bulnesol | 1678 | 0.28 | 0.01 | 0.03 | - | 1.21 | - | 0.06 | 0.41 |
| 3-Methyl-but-2-enoic acid-1,7,7- trimethyl-bicyclo [2.2.1]hept-2-yl ester | 1731 | 4.39 | 0.46 | - | - | 20.88 | - | 0.03 | 0.36 |
| Monoterpene hydrocarbons | 19.28 | 13.39 | - | 1.78 | 0.31 | 4.13 | 0.32 | - | |
| Oxygenated Monoterpenes | 34.17 | 79.50 | 94.45 | 87.97 | 2.12 | 82.59 | 12.95 | 0.39 | |
| Sesquiterpenes hydrocarbons | 9.34 | 0.85 | 0.90 | 7.65 | 5.11 | 7.38 | 5.90 | 18.60 | |
| Oxygenated sesquiterpenes | 27.60 | 0.68 | 0.25 | 0.46 | 88.29 | 0.17 | 0.51 | 76.64 | |
| Phenylpropanoids | 1.94 | 0.01 | 0.44 | - | 0.32 | - | 79.02 | 0.30 |
1 Retention index (RI) relative to the homologous series of n-hexane on the SH-Rxi-5Sil MS quartz capillary column. 2 Average relative content of a constituent in all sample essential oils.
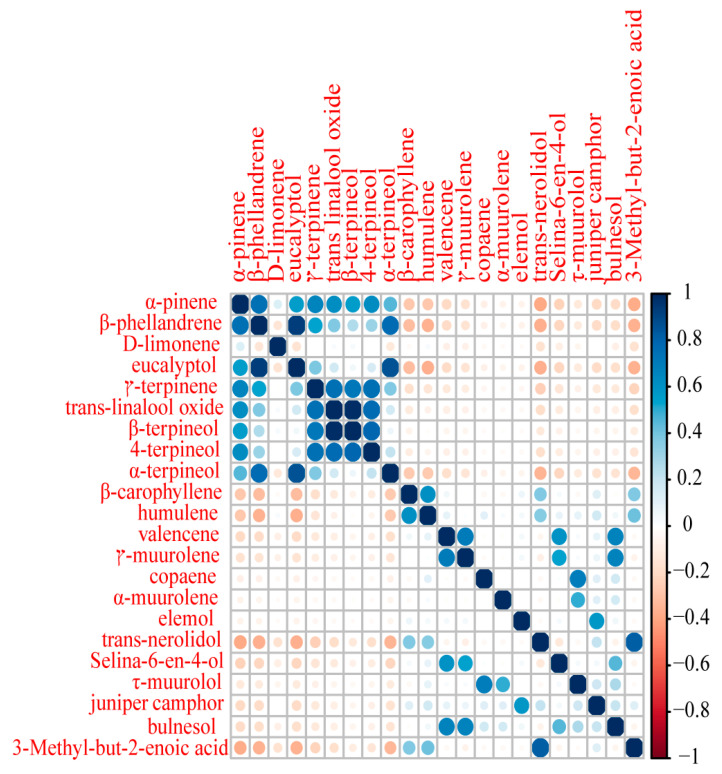
Correlation coefficients indicated significant positive or negative correlations among several sets of compounds (Figure 2). Strongly positive correlations, meaning the compounds were present as a group, were observed among α-pinene, β-phellandrene, eucalyptol, γ-terpinene, trans-linalool oxide, β-terpineol, 4-terpineol, and α-terpineol. β-caryophyllene, humulene, germacrene D, valencene, α-muurolene, germacrene B, selina-6-en-4-ol, and bulnesol formed another positively correlated group. Notably, strongly negative correlations were observed between compounds from the different groups.
Figure 2.
Correlation analysis of the 22 compounds identified in the essential oil of C. camphora.
Although the mean essential oil compound content ranged from 0.10% to 15.14%, the content of individual compounds varied considerably; a given individual sample often contained one or two main compounds and low amounts of other compounds. The data in Table 3 show that eucalyptol (GD-SX-22), linalool (FJ-YX-29), camphor (HN-CS-14), borneol and camphor (GX-QZ-03), methyl isoeugenol (GX-GF-22), trans-nerolidol (JX-AF-14), and selina-6-en-4-ol (GX-GF-06) were the major compounds for their respective individuals. The variety of essential oil components among individual plants reflected the variety of camphor tree chemotypes.
2.3. Chemotype Classification and Distribution
Essential oil compounds varied greatly among plants of different chemotypes. Clustering was applied to classify the essential oils of these 974 samples into different chemotypes, depending on the dominant compound in the sample. Five chemotypes were identified, including the eucalyptol type (eucalyptol), nerolidol type (trans-nerolidol), camphor type (camphor), linalool type (linalool), and selina type (selina-6-en-4-ol). Briefly, 249 eucalyptol-, 153 camphor-, 140 linalool-, 234 nerolidol-, and 97 selina-type individuals were classified. Furthermore, 84 samples with no predominant compounds were classified as mixed type, and 17 samples that contained other predominant compounds were classified as other type (Table 4).
Table 4.
The quantity and oil yield of each chemotype.
| Chemotype | n | Mean Yield (%) | Min. Yield (%) | Max. Yield (%) |
|---|---|---|---|---|
| Eucalyptol | 249 | 1.64 ± 0.04a 1 | 0.09 | 3.28 |
| Camphor | 153 | 0.95 ± 0.04b | 0.04 | 3.46 |
| Linalool | 140 | 1.55 ± 0.04a | 0.27 | 2.83 |
| trans-Nerolidol | 234 | 0.22 ± 0.01e | 0.01 | 1.41 |
| Selina-6-en-4-ol | 97 | 0.45 ± 0.02d | 0.12 | 2.07 |
| Mix | 84 | 0.66 ± 0.07c | 0.05 | 2.76 |
| Other | 17 | 0.34 ± 0.08de | 0.01 | 0.99 |
1 Different letter in the same column represented significant differences (p < 0.01).
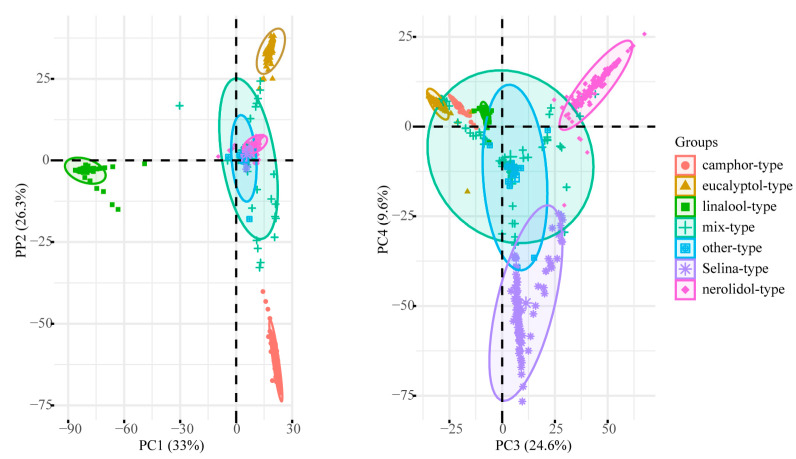
Four principal components (PCs) accounted for 93.69% of the total variance; therefore, these four PCs were used to explain the variance in essential oil (Table 5). A strongly negative correlation was observed between PC1 and linalool levels. PC2 showed strongly positive correlations with β-phellandrene, eucalyptol, and α-terpineol and a negative correlation with camphor. PC3 was strongly and positively correlated with β-caryophyllene, trans-nerolidol, caryophyllene oxide, and 3-methyl-but-2-enoic acid-1,7,7- trimethyl-bicyclo [2.2.1]hept-2-yl ester and negatively correlated with α-pinene, β-phellandrene, eucalyptol, and α-terpineol. PC4 was strongly and positively correlated with valencene, α-muurolene, germacrene B, selina-6-en-4-ol, and bulnesol. As shown in Figure 3, PC1 and PC2 accounted for 33.01% and 26.37% of the total variation, respectively, whereas PC3 and PC4 accounted for 24.72% and 9.59% of the total variation, respectively. PC1 was dominant in samples defined as the linalool type; PC2 and PC3 were dominant in samples defined as the camphor, eucalyptol, and nerolidol types; and PC4 was dominant in samples defined as the selina type. Although the four PCs clearly distinguished these chemotypes, various samples did not clearly fit a specific chemotype.
Table 5.
Four principal components of the 41 essential oil compounds of C. camphora.
| Compounds | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| α-Pinene | 0.36 | 0.18 | −0.54 | −0.14 |
| β-Phellandrene | 0.32 | 0.62 | −0.62 | −0.19 |
| α-Phellandrene | 0.034 | −0.06 | −0.04 | 0.01 |
| D-limonene | 0.13 | −0.45 | −0.15 | −0.04 |
| Eucalyptol | 0.33 | 0.65 | −0.64 | −0.21 |
| γ-Terpinene | 0.18 | 0.26 | −0.32 | −0.05 |
| trans-Linalool oxide | 0.08 | 0.13 | −0.19 | −0.00 |
| Linalool | −0.99 | −0.04 | −0.11 | −0.10 |
| β-Terpineol | 0.06 | 0.09 | −0.09 | 0.04 |
| Camphor | 0.28 | −0.89 | −0.31 | −0.14 |
| Borneol | 0.06 | −0.13 | −0.04 | −0.01 |
| 4-Terpineol | 0.10 | 0.14 | −0.16 | 0.02 |
| α-Terpineol | 0.30 | 0.56 | −0.58 | −0.18 |
| trans-Geraniol | 0.03 | 0.04 | 0.02 | 0.01 |
| Bornyl acetate | 0.11 | −0.27 | −0.05 | −0.05 |
| δ-Elemene | 0.14 | −0.10 | 0.36 | 0.11 |
| Methyl eugenol | 0.01 | 0.01 | 0.01 | 0.04 |
| β-caryophyllene | 0.15 | −0.10 | 0.56 | −0.00 |
| γ-Elemene | 0.04 | 0.02 | 0.18 | 0.08 |
| Humulene | 0.19 | −0.14 | 0.46 | 0.08 |
| Valencene | 0.05 | 0.02 | 0.15 | 0.61 |
| γ-Muurolene | 0.04 | 0.02 | 0.09 | 0.55 |
| Germacrene D | 0.18 | −0.20 | 0.46 | −0.20 |
| δ-Selinene | 0.05 | −0.06 | 0.06 | 0.40 |
| α-Guaiene | 0.21 | −0.42 | 0.10 | −0.03 |
| Methyl isoeugenol | 0.03 | 0.05 | 0.17 | 0.02 |
| Copaene | 0.02 | 0.01 | 0.04 | 0.10 |
| α-Muurolene | 0.01 | 0.01 | 0.02 | 0.06 |
| Elemol | 0.01 | −0.01 | 0.03 | 0.09 |
| trans-Nerolidol | 0.18 | 0.10 | 0.91 | −0.33 |
| Germacrene B | 0.04 | 0.04 | 0.15 | 0.60 |
| Spathulenol | 0.07 | 0.03 | 0.30 | 0.39 |
| caryophyllene oxide | 0.11 | 0.05 | 0.53 | 0.20 |
| Guaiol | 0.05 | 0.04 | 0.19 | 0.12 |
| Selina-6-en-4-ol | 0.06 | 0.02 | 0.14 | 0.95 |
| Viridiflorol | 0.04 | 0.02 | 0.18 | 0.11 |
| τ-Muurolol | 0.03 | 0.01 | 0.07 | 0.15 |
| α-Cadinol | 0.04 | 0.00 | 0.12 | 0.26 |
| Juniper camphor | 0.07 | 0.03 | 0.29 | 0.09 |
| Bulnesol | 0.05 | 0.02 | 0.12 | 0.52 |
| 3-Methyl-but-2-enoic acid-1,7,7- trimethyl-bicyclo [2.2.1]hept-2-yl ester | 0.16 | 0.10 | 0.81 | −0.21 |
| % of Variance | 33.01 | 26.37 | 24.72 | 9.59 |
Figure 3.
Principal component analysis of the first four dimensions.
Analysis of variance (ANOVA) revealed significant differences (p < 0.01) in essential oil yield among the different chemotypes (Table 4). The yields for the eucalyptol and linalool types (1.64% and 1.55%, respectively) were significantly higher than those for the other five chemotypes (<1.00%), whereas the trans-nerolidol chemotype had the lowest yield (0.22%). Within each chemotype, the yields of essential oils showed significant variance. For the eucalyptol type, the highest yield (in the range of 0.09–3.28%) was 36 times higher than the lowest yield; 86 times higher for the camphor type, and 10 times higher for the linalool type. The extreme variability within chemotypes provided sufficient basis for selecting optimal wild individuals.
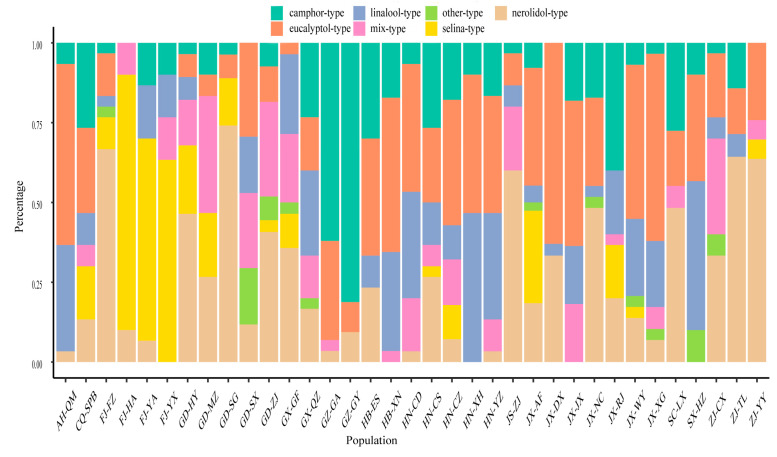
One or two chemotypes were predominant in each population (Figure 4). The eucalyptol and linalool types were predominant in populations from Qimen, Xianning, Changde, Xinhua, Yongzhou, and Hanzhong; the nerolidol type was predominant in populations from Fuzhou, Shaoguan, Zhenjiang, Tonglu, and Yuyao; the selina type was predominant in populations from Huaan, Yongan, and Youxi; and the camphor type was predominant in populations from Gaofeng and Guiyang.
Figure 4.
Distribution frequencies of chemotypes in different populations.
Pearson’s correlation coefficient analysis of longitude, latitude, and the five major compounds (eucalyptol, linalool, camphor, selina-6-en-4-ol, and trans-nerolidol) correlated the variations in essential oil with geographic location (Table 2). A significantly positive correlation was detected between eucalyptol, linalool, and latitude; the higher the latitude, the higher the eucalyptol and linalool contents. Selina-6-en-4-ol was significantly and positively correlated with longitude and significantly and negatively correlated with latitude. trans-Nerolidol was positively correlated with longitude, and camphor was negatively correlated with longitude.
2.4. Exceptional Chemotypes
Despite the predominance of the eucalyptol, camphor, linalool, nerolidol, and mixed types in camphor trees, 17 individuals were present with high amounts of other compounds (Table 3). Six (GX-GF-22, JX-AF-13, JX-XG-21, SX-HZ-22, and SX-HZ-24, SX-HZ-25) of the 17 individual plants exhibited high amounts of methyl isoeugenol (68.29% to 83.60%). Two individual plants (FJ-FZ-30 and GD-ZJ-04) exhibited high amounts of methyl eugenol (87.71% and 84.19%, respectively). Two individuals (JX-NC-23 and JX-WY-20) had high amounts of δ-selinene (74.48% and 60.54%, respectively). One individual (GX-QZ-03) had an elevated borneol content (52.22%).
2.5. Impact of Environmental Factors on Essential Oil Variability
In order to estimate the effects of environmental factors on essential oil yield and compound variability, data of four environmental factors were assessed, including mean annual maximum temperature (MAAT), mean annual minimum temperature (MAIT), mean annual precipitation (MAP), and elevation. The environmental factors were inputted as the first group, while oil yield and five major essential oil components (eucalyptol, linalool, camphor, selina-6-en-4-ol, and trans-nerolidol) were inputted as the second group for canonical correspondence analysis (CCA) in SPSS 26.0. The four first canonical variables could explain 100% of the total variation (p<0.01). The coefficient of the first CCA set explained 55% of the variation and revealed that MAAT positively affected the environmental variation, and camphor negatively affected the essential oil variation. The second canonical set explained 33.2% of the variation and disclosed that MAAT and elevation negatively influenced the coefficient variation in relation to environmental factors, whereas eucalyptol and linalool positively affected the oil yield, while selina-6-en-4-ol was negatively correlated with oil character variation. The third CCA set explained 9% of the variation and had a positive correlation with MAIT and negative correlation with MAAT in relation to environmental factors. The contribution of these coefficients to the third set showed that oil yield, eucalyptol, linalool, and selina-6-en-4-ol had negative effects on oil character variation (Table 6). Based on the first row of CCA sets, high MAAT and low elevation in areas were the major environmental factors influencing the characters of oil yield, eucalyptol, and linalool.
Table 6.
Canonical coefficients for four CCA sets between phytochemicals with environmental factors.
| Characters | Standardized Canonical Coefficients | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Environmental factors |
MAAT | 0.94 | −1.05 | −1.55 | −1.41 |
| MAIT | −0.26 | 0.34 | 1.95 | 0.68 | |
| MAP | 0.06 | −0.19 | 0.31 | 1.25 | |
| Elevation | −0.35 | −1.12 | 0.00 | −0.23 | |
| Oil characters | Oil yield | −0.19 | −0.60 | −0.59 | 0.16 |
| Eucalyptol | −0.46 | 0.75 | −0.58 | 1.21 | |
| Linalool | −0.25 | 0.58 | −0.74 | 0.30 | |
| Camphor | −0.99 | −0.25 | −0.21 | 0.77 | |
| trans-Nerolidol | −0.33 | 0.22 | −0.35 | 1.42 | |
| Selina-6-en-4-ol | 0.25 | −0.57 | −0.64 | 0.86 | |
| Eigen-value | 0.46 | 0.38 | 0.21 | 0.12 | |
| p value | 0.00 | 0.00 | 0.000 | 0.00 | |
| Cumulative variance | 55.00 | 88.20 | 97.20 | 100.00 | |
3. Discussion
Sect. Camphora (Trew) Meissn spice plants are commercially important and chemically diverse. Almost all Cinnamomum plants contain essential oils, as reported in various studies [18,19,20,21]. Dozens of essential oil compounds, including terpenes, terpenoids, and phenylpropanoids, have been identified in Cinnamomum Trew plants, enabling categorization by chemotype. The linalool type is chemotypical for Cinnamomum osmophloeum [22], Cinnamomum porrectum [23], Cinnamomum kanehirae [24], Cinnamomum champhora [25], and Cinnamomum verum [26]; the eugenol type is chemotypical for Cinnamomum impressinervium [27] and C. verum [28]; and the eucalyptol type is chemotypical for C. kanehirae [24] and C. porrectum [23]. Some other chemotypes, including the safrole, cinnamaldehyde, citral, camphor, eucalyptol, and nerolidol types, are also found in Cinnamomum Trew. [20,23,29,30]. Although the variations in the essential oil composition were minimal for some species, the variation in Cinnamomum Trew chemotypes did not depend on differences in species. The 974 samples from 35 populations in 13 provinces of C. camphora were divided into six main chemotypes (eucalyptol, camphor, linalool, nerolidol, selina, and mixed types) and four other rare chemotypes (methyl isoeugenol, methyl eugenol, selinene, and borneol types). All the main chemotypes have been reported previously [11,12,13,31], while for the rare chemotypes, only the borneol type has been previously reported in C. camphora [11].
Plant essential oils are divided into terpenoids and phenylpropanoids and are synthesized in plants through independent and compartmentally separate pathways. Sesquiterpenes are derived from the terpenoid mevalonate (MVA) pathway, whereas hemiterpenes, monoterpenes, and diterpenes are produced via the methyl-erythritol phosphate (MEP) pathway. Phenylpropanoids are biosynthesized via the shikimate pathway [32]. The monoterpenes eucalyptol, camphor, linalool, and borneol originate from the MEP pathway [33]. The sesquiterpenes trans-nerolidol, selina-6-en-4-ol, and δ-selinene are derived from the MVA pathway [32]. The shikimate pathway produces methyl isoeugenol/methyl eugenol [34]. All three biosynthetic pathways are present in the camphor tree, wherein they regulate the synthesis of essential oils depending on genotype, developmental stage, and biotic stressors.
Positively correlated compounds may be synthesized by the same enzyme or by different products in the same synthetic pathway in the camphor tree, whereas negatively correlated chemicals may have a substrate competition relationship or an upstream and downstream relationship in the same synthetic route. α-Terpineol has been proposed to be the precursor of eucalyptol; enzymes catalyze protonation and internal addition of the endocyclic double bond of α-terpineol to produce eucalyptol. Thus, α-terpineol, β-pinene, α-pinene, sabinene, and myrcene are minor products of the conversion of (2E)-geranyl diphosphate to eucalyptol [35]. In this study, a significantly positive correlation was detected between eucalyptol, α-terpineol, and α-pinene levels. Furthermore, camphor is converted from borneol by the NAD-dependent dehydrogenation of the alcohol to a ketone [36,37,38]. Borneol dehydrogenase is the key enzyme that converts borneol into camphor, and its activity plays a decisive role in essential oil camphor content. Two individual borneol-type camphor tree plants have previously been found in Jiangxi Province [11]. In the current study, one borneol type (GX-QZ-03, 52.22%) and four mixed types with high borneol content (GX-QZ-08, GX-QZ-05, SC-LX-03, and SC-LX-23; 46.61%, 36.58%, 37.71%, and 42.17%, respectively) and high camphor content (23.37%, 31.48%, 41.42%, 39.51%, and 34.35%, respectively) were found. This finding suggests that borneol-type camphor trees are rare. Borneol was present in the essential oil and was closely correlated with camphor, suggesting that the borneol type originates from a decrease in borneol dehydrogenase activity; borneol-type samples may be promising materials for studying borneol dehydrogenases.
Although camphor trees are native to subtropical monsoon climates, their essential oil contents varied according to a geographical gradient. Northern camphor trees had higher eucalyptol and linalool contents and lower selina-6-en-4-ol content. Eastern trees had higher selina-6-en-4-ol and trans-nerolidol contents and lower camphor content. These trends show that the biosynthesis and accumulation of essential oils may be affected by environmental factors, as well as genetic mutations. Rainfall and temperature are the most important environmental factors associated with various essential oil yields and compositions [39,40]. China is a suitable area for camphor trees since the annual mean temperature decreases from south to north, ranging from 23 °C (Zhanjing) to 14 °C (Hanzhong). The annual rainfall decreases from east to west, ranging from 1800 mm (Yuyao) to 750 mm (Luxian). Thus, the biosynthesis and accumulation of essential oils by camphor trees may be affected by rainfall and temperature at their locations. Individuals rich in eucalyptol and linalool inhabit regions with low annual mean temperatures. Plants rich in selina-6-en-4-ol are found in Fujian and Guangdong provinces of southeast China, which have a higher annual mean temperature and higher annual mean rainfall. Furthermore, the plant organ developmental stage can influence biosynthesis and accumulation of essential oils; thus, the yields and profiles of essential oils change seasonally. The yield of essential oil and the proportion of linalool in the essential oil in linalool-type camphor trees were highest during the rainy season from May to July [41]. To minimize the effect of organ development stage on essential oil yield and profile, we collected plant samples from May to September for this study.
Camphor trees have been cultivated as a crop for many decades in China to produce camphor, linalool, eucalyptol, and borneol, but we also found individual plants rich in methyl isoeugenol, methyl eugenol, or δ-selinene. Methyl isoeugenol has been reported in Cymbopogon nardus and Pimenta pseudocaryophyllus [42,43], while methyl eugenol has been found in Croton malambo [44], and δ-selinene has been reported in Jatropha elliptica rhizomes [45]. Although these chemotypes rarely occur in camphor trees, they are valuable for developing new essential oil products.
Camphor trees are wildly distributed and they can adapt to different environments; therefore, it is necessary to determine the influence of environmental factors on essential oil heterogeneity. The results of CCA showed a strong relationship between environmental factors and essential oil constituents, indicating that the essential oils of camphor trees are greatly affected by the environment, similar to the results reported in other studies.
4. Materials and Methods
4.1. Plant Material
Leaves of more than 10 individual plants from each population were collected and the weights were measured immediately. Then, the samples were separately packed into valve bags to take back to the laboratory for analysis. A total of 974 individual plants from 35 populations in 13 provinces of China were sampled from May to September 2019 (Table 1). The geographic location (latitude and longitude) of each plant was recorded, and one herbarium specimen was collected and taxonomically identified according to the description of the flora in China [2].
4.2. Extraction of the Essential Oils
The essential oil was obtained from each of the samples using a hydrodistillation method. The extracts of fresh leaves (~100 g) were distilled (3 h) using a modified Clevenger-type apparatus. After 3 h, the oil was collected with a centrifugal tube and approximately 1 g anhydrous sodium sulfate was added into the tube. Then, the oil was poured into another tube after shaking for approximately 10 s and weighed. Solutions of essential oil (3%) in ethanol were analyzed using gas chromatography-mass spectrometry (GC-MS) and gas chromatography-flame ionization detection (GC-FID).
4.3. Essential Oil Compound Identification
GC-MS for compound identification was performed using a SHIMADZU GC-2010 Plus apparatus (Shimadzu corporation, Japan) equipped with a GCMS-QP2020 mass spectrometer coupled with an SH-Rxi-5Sil MS quartz capillary column (30 m × 0.25 mm, film thickness 0.25 μm). Helium (1.0 mL/min) was used as the carrier gas. Samples (1 μL; split ratio, 1:20) were injected into the GC (detector temperature, 280 °C), and subjected to the following temperature ramping program: initial temperature (60 °C; 2 min), followed by ramping (5 °C/min) to the final temperature (220 °C; 20 min). Qualitative analysis used electron-impact ionization (source temperature, 200 °C; interface temperature, 250 °C; ionization energy, 70 eV; scanning speed, 1000 mm/s; scanning interval, 0.50 fragments per second; mass scan range, 40–650 amu). Essential oils were identified using a digital library of mass spectral data (NIST 8.0) and literature comparisons of retention indices calibrated against a homologous series of C8-C32 n-alkanes [46].
4.4. Compound Calculation
GC-FID quantitative analysis was performed using a SHIMADZU GC-2010 Plus instrument (Shimadzu corporation, Japan) fitted with an SH-Rxi-5Sil MS quartz capillary column under the same conditions as GC-MS, except that N2 was used as the carrier gas. The FID detector temperature was 250 °C. The relative percentage of each compound in the essential oil was estimated using the peak area normalization method, based on the ratio of the area of the respective peak to the total area of all compounds in the sample; compound response factors were considered equivalent.
4.5. Statistical Analysis
The 41 compounds identified in the essential oil were analyzed collectively. ANOVA was performed between the chemotypes and frequency distributions. Pearson’s correlation coefficients of essential oil yields and latitude or longitude and CCA analysis were calculated using IBM SPSS Statistics software version 26.0 (IBM Corp., Armonk, NY, USA). Principal component analysis and correlation coefficient analysis between the essential oil compounds were conducted using the factoextra and corrplot packages in R, respectively (The R Foundation for Statistical Computing, Vienna, Austria).
5. Conclusions
C. camphora in China exhibited diversity in essential oil yield and profile. The Qimen, Xinhua, Hanzhong, and Enshi populations had high oil yields, with mean yields of >1.5%. Leaf essential oils contained 41 identifiable compounds and were classifiable into six major chemotypes and several other rare chemotypes. Among them, five major chemotypes were classified: eucalyptol, camphor, linalool, nerolidol, and selina types. Individuals with no predominant constituent were classified as the mixed type.
Geographic temperature and rainfall gradients could rationalize trends in essential oil yield and composition. With increasing latitude, oil yield and eucalyptol and linalool contents increased, whereas selina-6-en-4-ol content decreased. In contrast, selina-6-en-4-ol and trans-nerolidol contents increased with increasing longitude, whereas yield and camphor content decreased. Significant differences in oil yield were observed among chemotypes, with the eucalyptol type having the highest yields, followed by the linalool, camphor, nerolidol, selina, and mixed types in order of decreasing oil yield. Despite one or two chemotypes having been commercialized and several clones of camphor trees available to extract essential oils, other chemotypes or high-yield individuals could be applied as new, valuable commercial resources. Additionally, the geographical distribution of essential oils provides a valuable reference for the regional allocation of agricultural production for these oils.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28030973/s1, File S1.
Author Contributions
Conceptualization, T.Z. and Z.W.; Data curation, Z.W.; Investigation, T.Z., C.F., F.Q. and X.W.; Methodology, T.Z., H.Y. and X.L.; Project administration, T.Z.; Software, Y.Z. and C.F.; Supervision, Z.W.; Validation, X.L. and F.Q.; Visualization, Z.W.; Writing–original draft, T.Z.; Writing–review & editing, Y.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are shown in the main manuscript and in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Funding Statement
This research was funded by Major Science and Technology R & D Projects of Jiangxi Province (Project No. 20203ABC28W016), Special Project of Camphor Tree Research of Jiangxi Forestry Bureau (Project No. [2020]07), and the Key R&D Projects of Jiangxi Academy of Forestry (Project No. 2019512701).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ravindran P.N., Babu K.N., Shylaja M. Cinnamon and Cassia: The Genus Cinnamomum. CRC Press; Boca Raton, FL, USA: 2003. [Google Scholar]
- 2.Li X., Li J., Van der Werff H. Cinnamomum. In: Wu Z., Raven P.H., Hong D., editors. Flora of China. Science Press; Beijing, China: 2008. pp. 166–187. [Google Scholar]
- 3.Park T.-J., Park Y.-S., Lee T.-G., Ha H., Kim K.-T. Inhibition of acetylcholine-mediated effects by borneol. Biochem. Pharmacol. 2003;65:83–90. doi: 10.1016/S0006-2952(02)01444-2. [DOI] [PubMed] [Google Scholar]
- 4.Liu R., Zhang L., Lan X., Li L., Zhang T.T., Sun J.H., Du G.H. Protection by borneol on cortical neurons against oxygen-glucose deprivation/reperfusion: Involvement of anti-oxidation and anti-inflammation through nuclear transcription factor κappaB signaling pathway. Neuroscience. 2011;176:408–419. doi: 10.1016/j.neuroscience.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q., Wu D., Wu J., Ou Y., Mu C., Han B., Zhang Q. Improved blood–brain barrier distribution: Effect of borneol on the brain pharmacokinetics of kaempferol in rats by in vivo microdialysis sampling. J. Ethnopharmacol. 2015;162:270–277. doi: 10.1016/j.jep.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Guo S., Geng Z., Zhang W., Liang J., Wang C., Deng Z., Du S. The Chemical composition of essential oils from Cinnamomum camphora and their insecticidal activity against the stored product pests. Int. J. Mol. Sci. 2016;17:1836. doi: 10.3390/ijms17111836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi S., Wu Q., Su J., Li C., Zhao X., Xie J., Gui S., Su Z., Zeng H. Composition analysis of volatile oils from flowers, leaves and branches of Cinnamomum camphora chvar. Borneol in china. J. Essent. Oil Res. 2013;25:395–401. doi: 10.1080/10412905.2013.809323. [DOI] [Google Scholar]
- 8.Pragadheesh V.S., Saroj A., Yadav A., Chanotiya C.S., Alam M., Samad A. Chemical characterization and antifungal activity of Cinnamomum camphora essential oil. Ind. Crops Prod. 2013;49:628–633. doi: 10.1016/j.indcrop.2013.06.023. [DOI] [Google Scholar]
- 9.He X., Wang S., Shi J., Sun Z., Lei Z., Yin Z., Qian Z., Tang H., Xie H. Genotypic and Environmental Effects on the Volatile Chemotype of Valeriana jatamansi Jones. Front. Plant Sci. 2018;9:1003. doi: 10.3389/fpls.2018.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moniodis J., Renton M., Jones C.G., Barbour L., Byrne M. Genetic and environmental parameters show associations with essential oil composition in West Australian sandalwood (Santalum spicatum) Aust. J. Bot. 2018;66:48–58. doi: 10.1071/BT17116. [DOI] [Google Scholar]
- 11.Shi W., He W., Wen G. Study on chemical constituents of the essential oil and classification of types from Cinnamomum camphora. J. Integr. Plant Biol. 1989;31:209–214. [Google Scholar]
- 12.Zhang G.F., Chen C.J., Chen Z.P., Chen R.Y., Lin X.S. Analysis of principle component and chemistry type of essential oil from Cinnamomum camphora leaf in Fujian Province. J. Plant Resour. Environ. 2008;17:24–27. [Google Scholar]
- 13.Liu H., Shen M.Y., He Z.Y. Five biochemical types of Cinnamomum camphora leaf oil in Guangxi. Guangxi For. Sci. Technol. 1992;21:181–186. [Google Scholar]
- 14.Rahimmalek M., Heidari E.F., Ehtemam M.H., Mohammadi S. Essential oil variation in Iranian Ajowan (Trachyspermum ammi (L.) Sprague) populations collected from different geographical regions in relation to climatic factors. Ind. Crops Prod. 2017;95:591–598. doi: 10.1016/j.indcrop.2016.11.017. [DOI] [Google Scholar]
- 15.Martínez-Natarén D.A., Parra-Tabla V., Dzib G., Acosta-Arriola V., Canul-Puc K.A., Calvo-Irabién L.M. Essential oil yield variation within and among wild populations of Mexican oregano (Lippia graveolens H.B.K.-Verbenaceae), and its relation to climatic and edaphic conditions. J. Essent. Oil Bear. Plants. 2012;15:589–601. doi: 10.1080/0972060X.2012.10644093. [DOI] [Google Scholar]
- 16.Kumar R., Kaundal M., Sharma S., Thakur M., Kumar N., Kaur T., Vyas D., Kumar S. Effect of elevated [CO2] and temperature on growth, physiology and essential oil composition of Salvia sclarea L. in the western Himalayas. J. Appl. Res. Med. Aromat. Plants. 2017;6:22–30. [Google Scholar]
- 17.Curado M.A., Oliveira C.B.A., Jesus J.G., Santos S.C., Seraphin J.C., Ferri P.H. Environmental factors influence on chemical polymorphism of the essential oils of Lychnophora ericoides. Phytochemistry. 2006;67:2363–2369. doi: 10.1016/j.phytochem.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Mdoe F.P., Cheng S.-S., Msangi S., Nkwengulila G., Chang S.-T., Kweka E.J. Activity of Cinnamomum osmophloeum leaf essential oil against Anopheles gambiae s.s. Parasites Vectors. 2014;7:209. doi: 10.1186/1756-3305-7-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unlu M., Ergene E., Unlu G.V., Zeytinoglu H.S., Vural N. Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae) Food Chem. Toxicol. 2010;48:3274–3280. doi: 10.1016/j.fct.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhang B., Wu C., Xiao Z., Zhang H., Cao M., Liu Y., Jin Z. Chemical constituents and chemotypes of fresh leaf essential oil of wild species belonging to Sect. Camphor (Trew.) Meissn. in southeastern China. J. Essent. Oil Bear. Plants. 2019;22:1115–1122. doi: 10.1080/0972060X.2019.1662331. [DOI] [Google Scholar]
- 21.Chang C.-T., Chang W.-L., Hsu J.-C., Shih Y., Chou S.-T. Chemical composition and tyrosinase inhibitory activity of Cinnamomum cassia essential oil. Bot. Stud. 2013;54:617–623. doi: 10.1186/1999-3110-54-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang H.-T., Lin C.-Y., Hsu L.-S., Chang S.-T. Thermal degradation of linalool-chemotype Cinnamomum osmophloeum leaf essential oil and its stabilization by microencapsulation with β-cyclodextrin. Molecules. 2021;26:409. doi: 10.3390/molecules26020409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu F., Yang H., Zhang T., Wang X., Wen S., Su X. Chemical Composition of Leaf Essential Oil of Cinnamomum porrectum (Roxb.) Kosterm. J. Essent. Oil Bear. Plants. 2019;22:1313–1321. doi: 10.1080/0972060X.2019.1689178. [DOI] [Google Scholar]
- 24.Yang H., Zhang T., Wang X., Wen S., Guo Y., Jiang X. A study on the chemical components in essential oil from leaves of Cinnamomum kanehirae and chemotype divisions. Acta Agric. Univ. Jiangxiensis. 2016;38:668–673. [Google Scholar]
- 25.Frizzo C., Santos A., Paroul N., Serafini L., Dellacassa E., Lorenzo D., Moyna P. Essential oils of camphor tree (Cinnamomum camphora Nees & Eberm) cultivated in southern Brazil. Braz. Arch. Biol. Technol. 2000;43:313–316. [Google Scholar]
- 26.Rao B.R.R., Rajput D.K., Bhattacharya A.K. Essential oil composition of petiole of Cinnamomum verum Bercht. & Presl. J. Spices Aromat. Crops. 2007;16:38–41. [Google Scholar]
- 27.Nath S.C., Sarma Baruah A.K. Eugenol as the Major Component of the Leaf Oils of Cinnamomum impressinervium Meissn. J. Essent. Oil Res. 1994;6:211–212. doi: 10.1080/10412905.1994.9698360. [DOI] [Google Scholar]
- 28.Patel K., Ali S., Sotheeswaran S., Dufour J.-P. Composition of the leaf essential oil of Cinnamomum verum (Lauraceae) from Fiji islands. J. Essent. Oil Bear. Plants. 2007;10:374–377. doi: 10.1080/0972060X.2007.10643569. [DOI] [Google Scholar]
- 29.Huang C.-Y., Yeh T.-F., Hsu F.-L., Lin C.-Y., Chang S.-T., Chang H.-T. Xanthine oxidase inhibitory activity and thermostability of cinnamaldehyde-chemotype leaf oil of Cinnamomum osmophloeum microencapsulated with β-cyclodextrin. Molecules. 2018;23:1107. doi: 10.3390/molecules23051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sriramavaratharajan V., Stephan J., Sudha V., Murugan R. Leaf essential oil of Cinnamomum agasthyamalayanum from the Western Ghats, India—A new source of camphor. Ind. Crops Prod. 2016;86:259–261. doi: 10.1016/j.indcrop.2016.03.054. [DOI] [Google Scholar]
- 31.Liang Z., Li G., Qin Z., Liu H. Study on the component of a new chemical type of leaves oil from Cinnamomum camphora. J. Fujian For. Sci. Technol. 2010;37:102–104. [Google Scholar]
- 32.Dudareva N., Klempien A., Muhlemann J.K., Kaplan I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013;198:16–32. doi: 10.1111/nph.12145. [DOI] [PubMed] [Google Scholar]
- 33.Tian Z., Luo Q., Zhaojiang Z. Seasonal emission of monoterpenes from four chemotypes of Cinnamomum camphora. Ind. Crops Prod. 2021;163:113327. doi: 10.1016/j.indcrop.2021.113327. [DOI] [Google Scholar]
- 34.Yahyaa M., Berim A., Nawade B., Ibdah M., Dudareva N., Ibdah M.J.P. Biosynthesis of methyleugenol and methylisoeugenol in Daucus carota leaves: Characterization of eugenol/isoeugenol synthase and O-Methyltransferase. Phytochemistry. 2019;159:179–189. doi: 10.1016/j.phytochem.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Kampranis S., Ioannidis D., Purvis A., Mahrez W., Ninga E., Katerelos N., Anssour S., Dunwell J., Degenhardt J., Makris A., et al. Rational conversion of substrate and product specificity in a Salvia monoterpene synthase: Structural insights into the evolution of terpene synthase function. Plant Cell. 2007;19:1994–2005. doi: 10.1105/tpc.106.047779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Croteau R. Biosynthesis and catabolism of monoterpenoids. Chem. Rev. 1987;87:929–954. doi: 10.1021/cr00081a004. [DOI] [Google Scholar]
- 37.Ma R., Su P., Jin B., Guo J., Tian M., Mao L., Tang J., Chen T., Lai C., Zeng W., et al. Molecular cloning and functional identification of a high-efficiency (+)-borneol dehydrogenase from Cinnamomum camphora (L.) Presl. Plant Physiol. Biochem. 2021;158:363–371. doi: 10.1016/j.plaphy.2020.11.023. [DOI] [PubMed] [Google Scholar]
- 38.Yang Z., Xie C., Zhan T., Li L., Liu S., Huang Y., An W., Zheng X., Huang S. Genome-wide identification and functional characterization of the trans-Iisopentenyl diphosphate synthases gene family in Cinnamomum camphora. Front. Plant. Sci. 2021;12:708697. doi: 10.3389/fpls.2021.708697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jochum G., Mudge K., Thomas R. Elevated temperatures increase leaf senescence and root secondary metabolite concentrations in the understory herb Panax quinquefolius (Araliaceae) Am. J. Bot. 2007;94:819–826. doi: 10.3732/ajb.94.5.819. [DOI] [PubMed] [Google Scholar]
- 40.Mirali N., Aziz R., Nabulsi I. Genetic characterization of Rosa damascena species growing in different regions of Syria and its relationship to the quality of the essential oils. Int. J. Med. Aromat. Plants. 2012;2:41–52. [Google Scholar]
- 41.Zhang T., Yang H., Wen S., Qiu F., Liu X. Effects of harvest season and storage time on the essential oil of the linalool chemotype of Cinnamomum camphora. J. Essent. Oil Bear. Plants. 2019;22:1379–1385. doi: 10.1080/0972060X.2019.1699868. [DOI] [Google Scholar]
- 42.Herath H.M.W., Iruthayathas E.E., Ormrod D.P. Temperature effects on essential oil composition of citronella selections. Econ. Bot. 1979;33:425–430. doi: 10.1007/BF02858338. [DOI] [Google Scholar]
- 43.Paula J.A.M., Ferri P.H., Bara M.T.F., Tresvenzol L.M.F., Sá F.A.S., Paula J.R. Infraspecific chemical variability in the essential oils of Pimenta pseudocaryophyllus (Gomes) L.R. Landrum (Myrtaceae) Biochem. Syst. Ecol. 2011;39:643–650. doi: 10.1016/j.bse.2011.05.013. [DOI] [Google Scholar]
- 44.Suárez A.I., Vásquez L., Manzano M., Compagnone R. Essential oil composition of Croton cuneatus and Croton malambo growing in Venezuela. Flavour Fragr. J. 2005;20:611–614. doi: 10.1002/ffj.1498. [DOI] [Google Scholar]
- 45.Brum R., Honda N., Hess S. Jatropha elliptica Muell. Arg. a source of δ-selinene. J. Essent. Oil Res. 1997;9:477–478. doi: 10.1080/10412905.1997.9700756. [DOI] [Google Scholar]
- 46.Adams R.P. Identification of Essential Oil Compontnts by Gas Chromatography/Mass Spectrometry. Allured Publishing; Carol Stream, IL, USA: 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data are shown in the main manuscript and in the Supplementary Materials.