Abstract
The ability to regulate emotions is indispensable for maintaining psychological health. It heavily relies on frontal lobe functions which are disrupted in frontal lobe epilepsy. Accordingly, emotional dysregulation and use of maladaptive emotion regulation strategies have been reported in frontal lobe epilepsy patients. Therefore, it is of clinical and scientific interest to investigate emotion regulation in frontal lobe epilepsy. We studied neural correlates of upregulating and downregulating emotions toward aversive pictures through reappraisal in 18 frontal lobe epilepsy patients and 17 healthy controls using functional magnetic resonance imaging. Patients tended to report more difficulties with impulse control than controls. On the neural level, patients had diminished activity during upregulation in distributed left‐sided regions, including ventrolateral and dorsomedial prefrontal cortex, angular gyrus and anterior temporal gyrus. Patients also showed less activity than controls in the left precuneus for upregulation compared to downregulation. Unlike controls, they displayed no task‐related activity changes in the left amygdala, whereas the right amygdala showed task‐related modulations in both groups. Upregulation‐related activity changes in the left inferior frontal gyrus, insula, orbitofrontal cortex, anterior and posterior cingulate cortex, and precuneus were correlated with questionnaire data on habitual emotion regulation. Our results show that structural or functional impairments in the frontal lobes disrupt neural mechanisms underlying emotion regulation through reappraisal throughout the brain, including posterior regions involved in semantic control. Findings on the amygdala as a major target of emotion regulation are in line with the view that specifically the left amygdala is connected with semantic processing networks.
Keywords: amygdala, emotion regulation, fMRI, frontal lobe epilepsy, reappraisal, top‐down
We investigated hemodynamic correlates of upregulation and downregulation of emotions toward negative images in frontal lobe epilepsy patients and healthy controls. Group comparisons revealed diminished activations in patients during upregulation of emotions in distributed left‐sided regions, implicated in semantic control. These group differences were related to habitual emotion regulation as upregulation‐related activity changes in the left inferior frontal gyrus, insula, orbitofrontal cortex, anterior and posterior cingulate cortex, and precuneus correlated with questionnaire data on habitual emotion regulation.
1. INTRODUCTION
Emotion regulation, the ability to exert top‐down cognitive control over emotions to reduce or increase their meaning, duration or intensity, or how they are experienced and expressed (Gross, 2015), is an important capacity for us humans. It helps us to adapt to difficult situations, to cope with aversive events from the past, to react adequately in social situations, and to maintain psychological health (Ehring et al., 2010; Gross & John, 2003; Hu et al., 2014; Kanske et al., 2012). In functional magnetic resonance imaging (fMRI) research, the most widely studied emotion regulation strategy is reappraisal. Gross and John defined reappraisal as “a form of cognitive change that involves construing a potentially emotion‐eliciting situation in a way that changes its emotional impact” (Gross & John, 2003, p. 349). Most studies on emotion regulation investigated downregulation of negative feelings (Morawetz, Bode, Derntl, et al., 2017). This seems reasonable as avoiding negative feelings follows hedonic motives and deficient abilities to downregulate such feelings is associated with a broad range of psychological disorders (Ehring et al., 2010; Jazaieri et al., 2015; Kanske et al., 2015; van der Meer et al., 2009). However, upregulating negative feelings may sometimes be important as well, as it might motivate us to take action against what has caused these feelings in the first place. Also, when interacting with somebody who is in bad shape, we may deliberately increase negative feelings in order to empathize with that person. Furthermore, upregulation of negative emotions could also be advantageous, if, for example, negative feelings toward a threatening event are exaggerated, which might help us remember and better avoid a threat in the future (Buchanan, 2007; Tyng et al., 2017).
In general, reappraisal relies on different aspects of cognitive control, that is, on planning which regulation strategy to use, working memory to keep in mind that strategy, inhibiting or increasing a prepotent emotional response, monitoring discrepancies between current and intended emotional states, directing attention toward or away from relevant stimulus features as well as the flexibility to switch attention or to switch between regulation strategies and sustained attention (Ochsner et al., 2002; Ochsner et al., 2012). Thus, it has early been predicted and shown that brain regions that subserve emotion regulation, largely overlap with those that are recruited during cognitive control tasks (Ochsner et al., 2002). These include ventrolateral (vl) and dorsolateral (dl) prefrontal cortex (PFC), dorsomedial (dm) PFC, superior frontal gyrus (SFG), supplementary motor area (SMA), and inferior parietal and lateral temporal regions (Morawetz, Bode, Derntl, et al., 2017; Ochsner et al., 2012). Furthermore, it has been found that during downregulation of emotions, activity within regions of this network correlates with activity in the amygdala both on a between‐subject (Ochsner et al., 2002; Urry et al., 2006) and within‐subject level (Banks et al., 2007; Morawetz, Bode, Baudewig, et al., 2017), indicating that these regions can modulate bottom‐up generated emotional responses. The amygdala is generally viewed as a key structure for bottom‐up processing of emotions (Davis & Whalen, 2001), guiding visual processing of emotional stimuli (Vuilleumier et al., 2004), and also helping to mobilize organismic responses in emotional situations (Davis & Whalen, 2001). Therefore, the amygdala is also a key target‐site for emotion regulation.
Other cognitive and affective skills that are commonly required for reappraisal are mentalizing and empathic concern, which can be understood as the ability to recognize and adopt the perspective of another person and to vicariously experience the inferred emotion (Cerniglia et al., 2019). Functional imaging studies suggest that mentalizing relies largely on subregions of the default mode network (DMN), including the dmPFC, the temporoparietal junction, the lateral temporal cortex and temporal pole, and also the posterior cingulate cortex (PCC) and precuneus (Andrews‐Hanna et al., 2014; Ochsner et al., 2004). The ability to empathize with another person can be particularly relevant for upregulation of negative emotions. Accordingly, studies found increased dmPFC (Morawetz, Bode, Derntl, et al., 2017; Ochsner et al., 2004; Urry et al., 2009) and PCC activation (Ochsner et al., 2004) for upregulating more than for downregulating negative emotions.
In patients with frontal lobe epilepsy (FLE) cognitive control and mentalizing networks are structurally and/or functionally disrupted. This is reflected by deficits in a number of executive control tasks that measure attention, planning, flexibility, response inhibition, verbal and spatial memory, working memory, and perceptual speed (Exner et al., 2002; Giovagnoli et al., 2020; Helmstaedter et al., 1996; Upton & Thompson, 1996; Wang et al., 2011), even in patients without identifiable frontal lesion (Wang et al., 2011). Some studies found such deficits for FLE patients in comparison to temporal lobe epilepsy (TLE) patients, underlining their specificity for frontal lobe damage (Helmstaedter et al., 1996; Patrikelis et al., 2009; Upton & Thompson, 1996). Also, in theory of mind (ToM) tasks FLE patients have been found to perform worse than controls (Farrant et al., 2005; Giovagnoli et al., 2011, 2013). Another aspect of social cognition that is related to mentalizing is facial or vocal emotion recognition (Mier et al., 2010; Morningstar et al., 2022). There is some evidence that, as in TLE, emotion recognition is impaired in FLE (Edwards et al., 2017; Morningstar et al., 2022), although the underlying mechanisms supposedly differ. Whereas in TLE such impairments are thought to reflect altered amygdala functioning and bottom‐up processing (e.g., Meletti et al., 2003), frontal contributions to emotion recognition are suggested to reflect cognitive, top‐down related task demands like emotion labeling (Adolphs, 2002). However, possible alterations in emotion perception in FLE still cannot be fully ruled out.
Few studies examined neural correlates of disrupted executive functions in FLE (Braakman et al., 2013; Swartz et al., 1996). Lower activations in bilateral vlPFC, dlPFC and anterior cingulate cortex (ACC) for FLE patients compared to controls were found in a delayed match to sample task (Swartz et al., 1996). These differences were more pronounced on the epileptogenic side. In addition, the authors reported decreased activity in other task‐relevant regions outside the frontal lobe, such as angular and supramarginal gyri. They interpreted their findings as evidence for a disrupted larger brain circuitry in FLE patients. In contrast, Braakman et al. (2013) did not find significant activation differences between pediatric FLE patients and age‐matched controls in a Sternberg Task.
Taken together, findings of impaired cognitive control and ToM in FLE patients, as well as findings about relevant functional brain alterations in this patient group, imply that emotion regulation abilities might be affected by FLE. To date, research on emotion regulation in epilepsy in general is sparse. A recent questionnaire study assessed the regular use of emotion regulation and thought control strategies in 35 FLE patients and 35 healthy controls (HC; Gul & Ahmad, 2015). They showed that controls distract themselves from negative thoughts by thinking about something positive or increasing their work load, whereas FLE patients tend to increase their ruminations about less negative things instead. Moreover, controls were more likely to share their thoughts with other people and to reappraise them. Conversely, patients were more likely to punish themselves for their unwanted thoughts. A recent study also showed that difficulties in emotion regulation partly predict depressive symptoms in epilepsy patients in general (Tombini et al., 2020), highlighting the relevance of this capability.
Regarding other frontal lobe pathologies, Falquez et al. (2015) found patients with acquired frontal brain injury to be more prone to using maladaptive emotion regulation strategies than HC. Adding to this, other studies have shown different aspects of emotion regulation to be impaired in patients with frontal lobe damage (Bechara, 2004), such as suppression of an emotional reaction (Salas et al., 2016) or decreasing subjective fear toward fear‐conditioned stimuli using reappraisal (Kroes et al., 2019). In summary, these studies underscore that emotion regulation might indeed be more generally altered in frontal lobe pathologies, including FLE. On the other hand, whereas circumscribed frontal lesions can produce specific deficits (Alexander & Stuss, 2000), the effects of epilepsy, as a network disorder, on emotion regulation may be less dependent on its exact focus location (e.g., Centeno et al., 2012).
In light of such results, the high prevalence of psychological disorders (Brandt et al., 2010; Rai et al., 2012) in epilepsy patients, and stable findings of neuropsychological impairments in FLE patients, emotion regulation in this group is a highly relevant issue that needs investigation. Additionally, studying the neural correlates of emotion regulation in FLE can inform models of top‐down control over emotions and provide further causal evidence on the cerebral mechanisms underlying emotion regulation. It can also provide important information regarding potentially clinically relevant functional changes in FLE and improve our understanding of behavioral, emotional and neural alterations in this disorder. To the best of our knowledge, there is so far no study that has experimentally investigated emotion regulation in FLE either on the behavioral or on the neural level. Therefore, we studied emotion regulation in FLE patients using questionnaires, experimental task performance and fMRI. Given its apparent relevance and yet comparatively limited investigation in former fMRI research, we also included an upregulation condition in our paradigm.
We predicted that FLE patients would report using more maladaptive strategies than HC and have more difficulties to regulate their emotions. On the neural level, we hypothesized that during both upregulation and downregulation of negative feelings, FLE patients would show less activity in frontal regions critical for emotion regulation than HC and that activity in the amygdala would be modulated by the emotion regulation condition to a lower degree than in the control group. Finally, we explored the relationship between hemodynamic activation and questionnaire measures of emotion regulation.
2. METHODS
2.1. Participants
The FLE group included 19 patients without any additional established neurological disorder or intellectual disability. Patients were recruited during an inpatient stay at Mara Hospital, Bielefeld University, Medical School, Department of Epileptology. To be assigned to the FLE group patients had to fulfill two of three criteria, namely a structural lesion within the frontal lobes, epileptiform discharges within the frontal lobes, and typical frontal seizure semiology.
We included 18 HC in this study who did not report any psychiatric or neurological disorder. Age and gender distributions were comparable between groups. Controls were monetarily compensated (25 Euros) for participation. All participants provided informed consent. One healthy participant was later excluded due to excessive head motion during scanning and one FLE patient was excluded due to incorrect task performance. All participants were fluent in German and completed questionnaires assessing current psychopathological symptoms and habitual emotion regulation. Patient and HC group characteristics regarding age, gender, handedness, years of schooling, state anxiety and Beck's Depression Inventory (BDI; Beck et al., 1996) score are listed in Table 1. Patients left school about 1 year earlier than controls (p = .011). There were no other significant group differences (all p > .280; see Table 1).
TABLE 1.
Demographics, state anxiety, and Beck's depression inventory scores for patients and controls
| FLE patients (n = 18) | HC (n = 17) | |||
|---|---|---|---|---|
| M (SD)/count | Range | M (SD)/count | Range | |
| Age | 32.5 (9.7) | 18.8–51.5 | 31.4 (9.1) | 21.0–52.0 |
| Gender | ||||
| Male | 13 (72.2%) | – | 11 (64.7%) | – |
| Female | 5 (27.8%) | – | 6 (35.3%) | – |
| School years* | 10.1 (1.3) | 9–13 | 11.4 (1.5) | 9–13 |
| BDI score | 9.7 (9.0) | 0–29 | 8.2 (5.6) | 0–23 |
| STAI‐S mean score | 2.03 (0.59) | 1.10–2.95 | 1.84 (0.38) | 1.35–2.74 |
| Handedness | ||||
| Left | 1 (5.6%) | – | 2 (11.8%) | – |
| Right | 15 (83.3%) | – | 14 (82.4%) | – |
| Both | 1 (5.6%) | – | 1 (5.9%) | – |
| Unknown | 1 (5.6%) | – | – | – |
Abbreviations: FLE, frontal lobe epilepsy; HC, healthy controls; M, mean; SD, standard deviation of the mean; STAI‐S = state subscale from the state‐trait anxiety inventory.
p = .011, all other p > .280.
Table 2 gives an overview on epilepsy‐specific characteristics, namely age at epilepsy onset, disease duration and side of seizure focus. Detailed information on semiology, EEG and epileptogenic MRI findings for each patient can be found in Supporting Information S1. Information on anti‐epileptic drugs for each patient can be found in Supporting Information S2. About two‐thirds of patients had a left‐sided epileptic focus. Epileptogenic frontal lesions were found in seven patients, including focal cortical dysplasias, heterotopias, and tumors.
TABLE 2.
Epilepsy‐related characteristics of frontal lobe epilepsy patients
| M (SD)/count | Range | |
|---|---|---|
| Age at epilepsy onset | 20.1 (12.5) | 2–45 |
| Disease duration | 12.4 (10.4) | 1.4–37.5 |
| Side of epilepsy focus | ||
| Left | 11 (61.1%) | |
| Right | 3 (16.7%) | |
| Bilateral | 3 (16.7%) | |
| Unknown | 1 (5.6%) | |
Abbreviations: M, mean; SD, standard deviation of the mean.
2.2. Behavioral data
2.2.1. Questionnaires
To assess depressiveness, state anxiety, and habitual emotion regulation, we used the German versions of Beck's Depression Inventory II (German version: Hautzinger et al., 2006), the State Trait Anxiety Inventory (STAI; Spielberger et al., 1983; German version: Laux et al., 1981), the Emotion Regulation Questionnaire (ERQ; Gross & John, 2003; German version: Abler & Kessler, 2009) and the Difficulties in Emotion Regulation Scale (DERS; Gratz & Roemer, 2004; German Version: Ehring et al., 2008). Participants received the questionnaires a few days before further behavioral testing.
The ERQ measures habitual use of reappraisal as a preparatory emotion regulation strategy that can change the emotional experience and suppression of the emotional reaction.
The DERS consists of 36 items, from which six subscales can be computed: Non‐Acceptance of Emotional Responses (Acceptance), Difficulties Engaging in Goal‐Directed Behavior when experiencing negative emotions (Goals), Impulse Control Difficulties (Impulsivity), Emotional Awareness (Awareness), Limited Access to Emotion Regulation Strategies (Strategies), and Lack of Emotional Clarity (Clarity). We chose those questionnaires as they have been proven to be reliable and valid measures of habitual use of emotion regulation strategies and difficulties in emotion regulation (Gratz & Roemer, 2004; Preece et al., 2020).
2.2.2. Behavioral emotion regulation paradigm
One or two days before applying the emotion regulation paradigm during fMRI, the task was practiced outside of the scanner. This was part of an extended testing session during which participants also completed additional neuropsychological and affective tests, which will be reported elsewhere. Participants were instructed to either downregulate or upregulate their feelings toward negative pictures from the International Affective Picture System (IAPS; Lang et al., 2008) or to permit their feelings toward negative and neutral IAPS stimuli. To downregulate their feelings, they were asked to use distancing or reinterpretation as reappraisal strategies. For example, they could think of a crying girl to be acting in a movie or imagine that she just fell down and would be comforted by her parents in a moment. To upregulate their feelings, participants were asked to imagine themselves, a family member or a good friend in the presented situation or to reinterpret the situation. For example, they could think of the crying girl as being their daughter or imagine that the girl is crying at a funeral, because her parents have died in a car crash. In the permit condition, they were instructed to respond naturally, which might also include uninstructed automatic reappraisals. Each trial (see Figure 1 for an example trial in the corresponding fMRI paradigm) started with a fixation cross with a jittered duration of 1–3 s. Then a picture was presented for 3 s to evoke an initial emotional response before the instruction was presented for 1 s on top of the image which was followed by a 6 s regulation phase. At the end of each behavioral trial, participants were asked to rate the intensity of their negative feelings on a seven‐point Likert scale. Condition order was pseudo‐randomized in that each instruction was presented at least two times in a row. Each condition (neutral, permit, downregulate, and upregulate) was presented 12 times in two runs. To ensure that participants understood the task correctly, prior to data acquisition application of reappraisal strategies was practiced and they were asked to verbalize their reappraisals. All participants included in this study understood the instructions and were able to generate adequate reappraisals. Both the behavioral and the corresponding fMRI paradigm were programmed and presented with the software package PsychoPy2 (Version 1.84.2; Peirce et al., 2019). IAPS used in both paradigms are listed in Supporting Information S3.
FIGURE 1.

Example of one experimental trial during functional magnetic resonance imaging
2.2.3. Behavioral data analysis
Behavioral data analysis was performed using Statistical Package for Social Sciences (SPSS) 25 (IBM Corp. Released 2017). Analyses of variance (ANOVAs) were conducted for between‐group comparisons. Significance levels were set to p < .05, two‐tailed. Degrees of freedom were adjusted, using Huynh–Feldt correction, whenever indicated by Mauchly's sphericity test.
2.3. MRI Data
2.3.1. FMRI emotion regulation paradigm
To investigate neural correlates of emotion regulation, we used a paradigm very similar to the behavioral task, the only difference being that instead of the rating, an 8 s rest period was included. During rest, participants were asked to relax, try not to think about the picture any longer and let their thoughts run free. The fMRI task was split into four runs, with each run containing 24 trials and each condition being presented six times within one run adding up to 24 trials per condition. The whole task‐duration was about 35 min.
Before scanning, emotion regulation strategies were again briefly practiced to ensure that participants still understood the instructions and were able to apply them adequately. Afterward, participants were debriefed and asked to give some examples for reappraisals they used in the upregulation and downregulation conditions. Additionally, they were asked about their thoughts during the “permit” instruction. This was also done to check whether participants had performed correctly according to the instruction.
2.3.2. MRI data acquisition
MRI data were collected on a 3 T Siemens Verio at Mara Hospital, Bielefeld. For high resolution, T1‐weighted 3D images 192 sagittal slices (0.8 mm thickness, 15.36 × 24 × 24 cm field of view, 0.75 × 0.75 mm in‐plane resolution, 2.5 ms echo time, 1.9 s repetition time) were acquired using a 32‐channel head coil. For functional data, gradient‐echo planar images were obtained with a 12‐channel head coil. Thirty‐five axial slices (4 mm thickness, 4 mm gap) were collected in an interleaved order with 33 ms echo time, 3 s repetition time, 90° flip angle, and 19.2 × 19.2 × 14.0 cm field of view. Images were oriented parallel to the anterior commissure–posterior commissure line. To ensure a steady state of magnetization, three dummy scans were acquired at the beginning of each run.
2.3.3. MRI data preprocessing
Preprocessing of functional and structural imaging data was performed using fMRIPrep 1.3.0.post2 (Esteban et al., 2019), which is based on Nipype 1.1.8 (Gorgolewski et al., 2011).
The T1‐weighted (T1w) image was corrected for intensity non‐uniformity with N4BiasFieldCorrection (Tustison et al., 2010), distributed with ANTs 2.2.0 (Avants et al., 2008), and used as T1w‐reference throughout the workflow. Brain surfaces were reconstructed using recon‐all (FreeSurfer 6.0.1, Dale et al., 1999), and the brain mask estimated previously was refined with a custom variation of the method to reconcile ANTs‐derived and FreeSurfer‐derived segmentations of the cortical gray‐matter of Mindboggle (Klein et al., 2017). Spatial normalization to the International Consortium of Brain Mapping (ICBM) 152 Nonlinear Asymmetrical template version 2009c (Fonov et al., 2009) was performed through nonlinear registration with antsRegistration (ANTs 2.2.0), using brain‐extracted versions of both T1w volume and template.
For each blood oxygen level dependency (BOLD) run the following preprocessing steps were performed separately: First, a reference volume and its skull‐stripped version were generated using a custom methodology of fMRIPrep. The BOLD reference was then co‐registered to the T1w reference using bbregister (FreeSurfer). BOLD runs were slice‐time corrected using 3dTshift from Analysis of Functional Neuroimages (AFNI) 20160207 (Cox & Hyde, 1997) and resampled onto their original, native space by applying a single, composite transform, to correct for head‐motion, and susceptibility distortions (preprocessed BOLD). Automatic removal of motion artifacts using independent component analysis (ICA‐AROMA, Pruim et al., 2015) was performed on the preprocessed BOLD on Montreal Neurological Institute (MNI) space time‐series after removal of non‐steady state volumes and spatial smoothing with an isotropic, Gaussian kernel of 6 mm full‐width half‐maximum (FWHM). Corresponding “non‐aggressively” denoised runs were produced after such smoothing. Note that ICA‐AROMA specifically addresses the problem of potentially trait‐related group differences in head motion and has been demonstrated to be highly effective in removing motion‐related noise for resting‐state and task‐based fMRI while preserving signal of interest at the same time (Pruim et al., 2015). Resamplings of BOLD time‐series to MNI152NLin2009cAsym standard space were performed using antsApplyTransforms (ANTs), configured with Lanczos interpolation to minimize the smoothing effects of other kernels (Lanczos, 1964).
2.4. First and second‐level general linear models
Statistical Parametric Mapping (SPM) 12 (www.fil.ion.ucl.ac.uk/spm) was used to estimate first and second‐level models. A high‐pass filter of 128 s was used for the time‐series of each participant to remove slow signal drifts and an autoregressive model AR(1) was applied to account for serial correlations, resulting from unmodelled neural activity. Non‐steady state outliers were excluded from the time‐series. With regards to the within‐group second‐level full factorial model, on the first level, a set of eight t‐contrasts with a weight of +1 for a specific regressor of interest and a weight of zero for all remaining regressors was defined: permit, neutral, downregulate, upregulate, permit_rest, neutral_rest, downregulate_rest, and upregulate_rest. Using these first‐level contrasts, a full factorial design was specified with two factors: regulation task (four levels: permit, neutral, downregulate, and upregulate) and regulation phase (two levels: regulation and rest). Additional t‐contrasts were calculated on the first level (upregulate–permit, downregulate–permit, upregulate–downregulate, permit–neutral) and were then taken forward to perform second‐level between‐group comparisons.
If not stated otherwise, activation maps were assessed for cluster‐wise significance with a cluster‐defining threshold of p < .001, uncorrected for multiple comparisons at the whole‐brain level. The significance level for activation clusters was set to p < .05, family‐wise error (FWE) corrected. Clusters with p FWE < .1 will be reported. All coordinates for peak activation are reported in MNI space. To provide a comprehensive presentation of brain activation maps, activations are shown both at a liberal (p uncorr < .01) and a stricter cluster‐defining threshold of p uncorr < .001 and p < .05, FWE‐corrected for multiple comparisons. Liberally thresholded activation maps are plotted with higher transparency whereas clusters surviving stricter thresholding are shown in brighter colors outlined by black contours. The aim of such a combined thresholding approach was twofold: First, an explorative impression of group differences is provided which may be of particular interest as, to our best knowledge, this is the first study addressing neural correlates of emotion regulation in FLE and given the overall lack of studies addressing neural correlates of FLE. Second, it shows which subregions of these larger patterns survived rather strict thresholding, without giving a wrong impression of high specificity of comparably small circumscribed regions which can be a pitfall of insufficiently powered analysis (Yarkoni, 2009).
2.5. Time course analysis of regions of interest
Time course analysis was performed on left and right amygdala regions of interest (ROI) as defined by the Harvard‐Oxford atlas. Additionally, clusters in which groups differed significantly were extracted from second‐level between‐group t‐maps using MarsBar (Brett et al., 2002). This was done to further characterize and validate task effects on the time course of the hemodynamic response in each group. First, for each participant and each run, region‐wise average time courses were extracted from preprocessed images using the nilearn module (Abraham et al., 2014) in python 3 (Van Rossum & Drake, 2009). The signal was detrended, standardized with respect to time, high‐pass filtered (128 s) and upsampled to have a time resolution of 0.1 s, thus matching the onset time resolution. Second, for each participant, all trials of each experimental condition separately were averaged and then baseline‐corrected using the first and last second. To perform group‐wise analyses, time courses of all participants within one group were averaged. Bootstrapped standard errors were calculated with 10,000 iterations and one‐sample t‐tests for the difference between two conditions were calculated for each time‐point.
2.6. Whole‐brain regression analysis for emotion regulation questionnaires
In order to explore how differences in brain activation between groups relate to habitual emotion regulation success and use of strategies, we correlated individual contrast images with questionnaire data acquired prior to fMRI scanning. To increase statistical power, we calculated correlations over both groups combined. We used three subscales from the DERS, namely Goals, Impulsivity and Strategies that measure whether someone actually experiences difficulties in emotion regulation, for example, by losing control over his or her actions, cognitions, and medium‐term emotional state instead of continuing to pursue current goals and inhibit unwanted behaviors. Thus, these measures are closely related to the executive task‐demands of this study's emotion regulation paradigm. The other three subscales, Awareness, Clarity and Acceptance were not used as they rather measure whether emotions are recognized and accepted, which, although somewhat related, does not directly reflect emotion regulation. As participants were asked to use reappraisal as a regulation strategy in this study, we additionally correlated the ERQ's Reappraisal subscale with fMRI data. As it is not reasonable to expect very high correlations with external measures and to increase statistical power (Yarkoni, 2009) a cluster‐defining threshold of p < .005, uncorrected was applied which corresponds to t = 2.733 and r = .43. Again, the significance level for activation clusters was set to p FWE < .05. In order to visualize correlations and data scattering, significant clusters were extracted with MarsBar and used as ROI masks on individual contrast images to extract a mean value per cluster and participant. The correlation heights will not be reported or interpreted due to potential inflation (Yarkoni, 2009).
3. RESULTS
3.1. Behavioral results
3.1.1. Emotion regulation paradigm
There were no significant between‐group differences in self‐rated affect for any condition (see Figure 2; F Group(1,33) = 0.076, p = .784; F Group×Condition(2.33, 76.85) = 0.785, p = .477). In both groups, all pairwise comparisons between conditions were significant, indicating adequate self‐reported task performance (F Condition(2.33, 76.85) = 96.687, p < .001; for post hoc comparisons, all p uncorr < .05).
FIGURE 2.
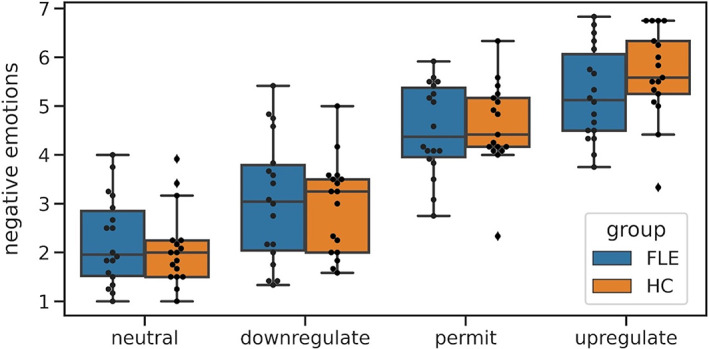
Task performance: Self‐ratings of negative affect in healthy controls (HC) and frontal lobe epilepsy (FLE) patients after regulating or permitting emotions. Black dots represent individual data points
3.1.2. Questionnaires
For the DERS the ANOVA yielded a trending Group × DERS scale interaction (F Group×Scale(4.44, 146.61) = 2.020, p = .087; F Group(1,33) = 1.096, p = .303). For the ERQ the interaction with Group, but not the main effect was significant (F Group×Scale(1,33) = 4.892, p = .034, F Group(1,33) = 0.515, p = .478), indicating a different pattern of strategy use. Post hoc comparisons revealed that HC experienced themselves as less impulsive than FLE patients (p uncorr = .032; see Table 3). STAI and BDI scores did not differ between groups (Table 1).
TABLE 3.
Emotion regulation questionnaire results
| HC | FLE patients | ||
| M (SE) | M (SE) | p uncorr | |
| DERS | |||
| Awareness | 2.55 (0.18) | 2.79 (0.18) | .358 |
| Acceptance | 2.11 (0.17) | 2.10 (0.21) | .936 |
| Clarity | 2.05 (0.22) | 2.00 (0.17) | .866 |
| Goals | 2.31 (0.22) | 2.78 (0.24) | .160 |
| Impulsivity* | 1.64 (0.16) | 2.19 (0.19) | .032 |
| Strategies | 1.92 (0.17) | 2.13 (0.20) | .428 |
| ERQ | |||
| Suppression | 3.22 (0.37) | 4.08 (0.34) | .094 |
| Reappraisal | 4.54 (0.22) | 4.15 (0.27) | .274 |
Note: Two‐tailed p‐values are reported.
Abbreviations: DERS, difficulties in emotion regulation scale; ERQ, emotion regulation questionnaire; FLE, frontal lobe epilepsy; HC, healthy controls; M, mean; SE, standard error of the mean.
p uncorr < .05.
3.2. FMRI results
3.2.1. Within‐groups results
Whole‐brain analysis
Whole‐brain activations in the individual groups are shown in Figure 3 and further detailed in Supporting Information S4, S5, and S6. In summary, in HC reappraisal‐related activation patterns were highly consistent with former fMRI studies on emotion regulation. In contrast, FLE patients showed no significant regulation‐related activity modulations in any brain region, even at a very liberal threshold of p uncorr < .01 (see blue areas in Figure 3). However, exploring the unthresholded t‐maps for upregulate and downregulate > permit revealed that patients seem to recruit similar networks as HC, albeit to a much lesser extent.
FIGURE 3.
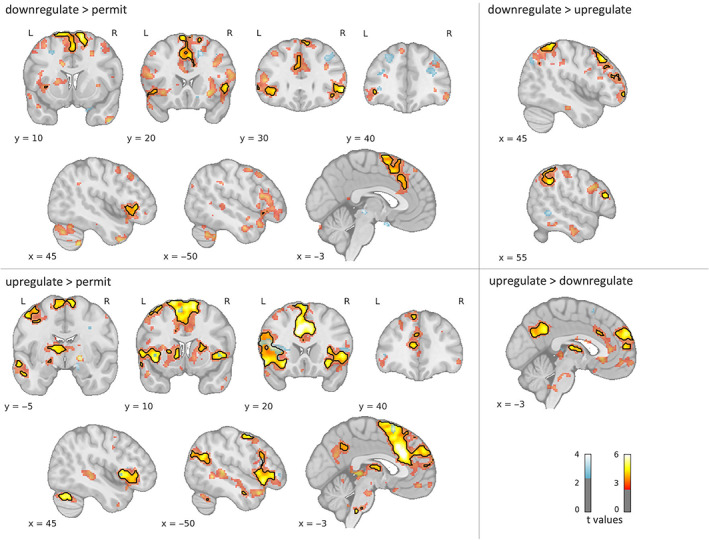
Whole‐brain activations for controls (HC: Red‐yellow) and frontal lobe epilepsy (FLE) patients (blue). Black contours delineate significant clusters (p FWE < .05). To illustrate the larger pattern, color‐coded activation maps are thresholded with p uncorr < .01 and a cluster threshold of 30 voxels for display purposes. L, left; R, right
3.2.2. Amygdala time course analysis
In both groups, we found lower amygdala activation for downregulating than for upregulating emotions as early as about 2 s after task onset. While this effect was present bilaterally in the HC group, in the FLE group we did only find a difference in the right amygdala. There was also a significant effect for upregulate > permit during the regulation phase in the right amygdala in both groups. Additionally, FLE patients showed more right‐sided activity for permit than for downregulate at the very beginning of the task, higher left‐sided activity for permit > neutral at the beginning of the relaxation phase and no undershoot in the hemodynamic response for permit at the end of the relaxation phase. Figure 4 provides an overview of significant task‐related left and right amygdala modulations in FLE patients and controls during affect induction, regulation and relaxation phase.
FIGURE 4.
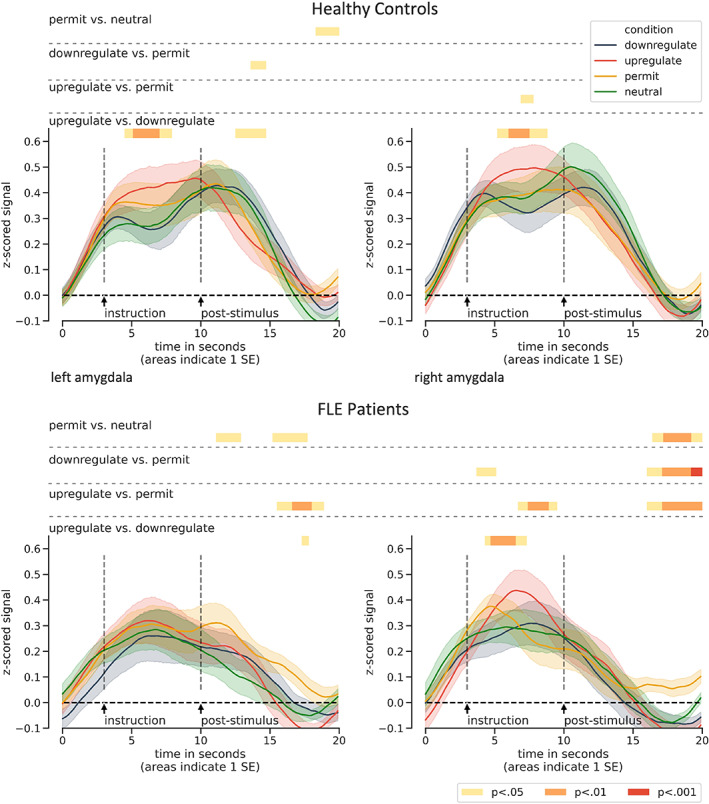
Time courses for the left and right amygdala in both groups. In the emotion regulation phases (4–10 s), healthy controls (upper panel) displayed higher activity for upregulate > downregulate bilaterally and for upregulate > permit in the right amygdala. Patients (lower panel) displayed no task‐related activity modulations in the left amygdala, whereas they displayed similar task‐related modulations as controls in the right amygdala and additionally for permit > downregulate at the very beginning of the regulation phase. Note that group differences between conditions at the onset of the instruction were not significant (all p > .15). FLE, frontal lobe epilepsy; SE, standard error of the mean
3.2.3. Between‐group comparison
Whole‐brain analysis
For the downregulate > permit contrast, we did not find any significant group differences, despite the visual impression of more activity in HC (Figure 3). For upregulate > permit, the between‐group comparison revealed significantly larger activations in HC than in FLE patients in distributed left‐sided regions, including two clusters in the dmPFC, a vlPFC cluster with peaks in the inferior frontal gyrus (IFG), pars triangularis, and orbitofrontal cortex (OFC), a parieto‐occipital cluster spanning the superior lateral occipital cortex (SLOC) and angular gyrus and a cluster in the middle temporal gyrus (MTG; see Table 4, Figures 5 and 6). In the same contrast, FLE patients showed stronger activity than HC in the right SLOC. This result was not consistent with the time course analysis, however (Supporting Information S8). We also compared task‐related activations between groups that specifically differentiate between upregulation and downregulation. These analyses revealed that patients showed less activity than controls in the left precuneus for upregulation compared to downregulation and in the left cerebellum for downregulation compared to upregulation. Time courses for all clusters can be found in Supporting Information S7 and S8. Additionally, group comparisons for permit > neutral revealed reduced deactivation for patients in the left inferior precuneus (Supporting Information S5).
TABLE 4.
Between‐group comparisons
| Side | Activation peak | Coordinates | z score | pFWE (peak) | Volume (k) | pFWE (cluster) | r | ||
| x | y | z | |||||||
| Upregulate > permit | |||||||||
| HC > FLE patients | |||||||||
| L | Anterior cingulate cortex | −4 | 24 | 30 | 4.47 | 0.377 | 144 | 0.006 | 0.631 |
| R | Paracingulate gyrus | 4 | 24 | 40 | 3.62 | 1.000 | |||
| L | IFG, triangular part | −58 | 26 | 4 | 4.40 | 0.452 | 136 | 0.008 | 0.680 |
| L | Orbitofrontal cortex | −42 | 28 | −4 | 3.51 | 1.000 | |||
| L | " | −32 | 32 | −2 | 3.48 | 1.000 | |||
| L | Paracingulate gyrus | −2 | 46 | 30 | 4.07 | 0.864 | 109 | 0.025 | 0.640 |
| L | Superior frontal gyrus | −4 | 54 | 34 | 4.01 | 0.912 | |||
| L | Frontal pole | −6 | 54 | 46 | 3.24 | 1.000 | |||
| L | Superior frontal gyrus | −10 | 8 | 66 | 3.61 | 1.000 | 82 | 0.084 | 0.637 |
| L | " | −14 | 0 | 72 | 3.55 | 1.000 | |||
| L | " | −10 | 18 | 68 | 3.54 | 1.000 | |||
| L | Angular gyrus | −46 | −60 | 24 | 3.94 | 0.950 | 98 | 0.041 | 0.620 |
| L | Superior lateral occipital cortex | −58 | −62 | 24 | 3.50 | 1.000 | |||
| L | Anterior middle temporal gyrus | −54 | 0 | −28 | 4.64 | 0.216 | 181 | 0.001 | 0.746 |
| L | Anterior superior temporal gyrus | −46 | −6 | −18 | 4.23 | 0.676 | |||
| L | Anterior middle temporal gyrus | −60 | −6 | −12 | 4.02 | 0.901 | |||
| FLE patients > HC | |||||||||
| R | Superior lateral occipital cortex | 28 | −62 | 40 | 4.04 | 0.887 | 98 | 0.041 | −0.614 |
| R | " | 34 | −66 | 32 | 3.94 | 0.951 | |||
| Upregulate > downregulate | |||||||||
| HC > FLE patients | |||||||||
| L | Precuneus | −12 | −48 | 36 | 4.24 | 0.673 | 135 | 0.008 | 0.624 |
| L | Posterior cingulate cortex | −2 | −52 | 34 | 3.65 | 0.999 | |||
| Downregulate > upregulate | |||||||||
| HC > FLE patients | |||||||||
| L | Cerebellar white matter | −36 | −54 | −40 | 3.93 | 0.956 | 107 | 0.026 | −0.689 |
| L | Cerebellar cortex | −36 | −62 | −42 | 3.86 | 0.981 | |||
Abbreviations: FLE, frontal lobe epilepsy; HC, healthy controls; IFG, inferior frontal gyrus; L, left; R, right.
FIGURE 5.
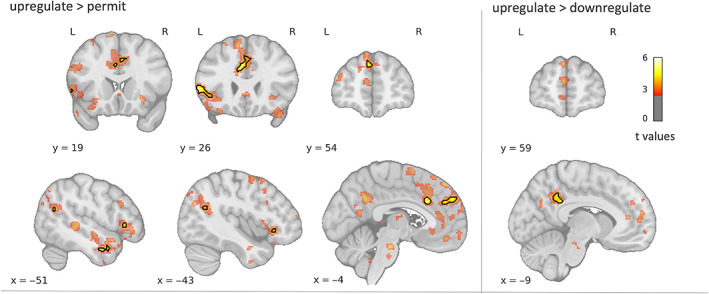
Whole‐brain activations for controls > patients. Black contours indicate significant clusters (p FWE < .05). To illustrate the full activation pattern, color‐coded maps are thresholded with p uncorr < .01 and a cluster threshold of 30 voxels. For upregulate > permit, controls displayed higher activations in task‐relevant distributed left‐sided regions, including dorsomedial and ventrolateral prefrontal cortex, angular gyrus and lateral temporal gyrus. For upregulate > downregulate, controls displayed higher activity in the left posterior medial parietal cortex. L, left; R, right
FIGURE 6.
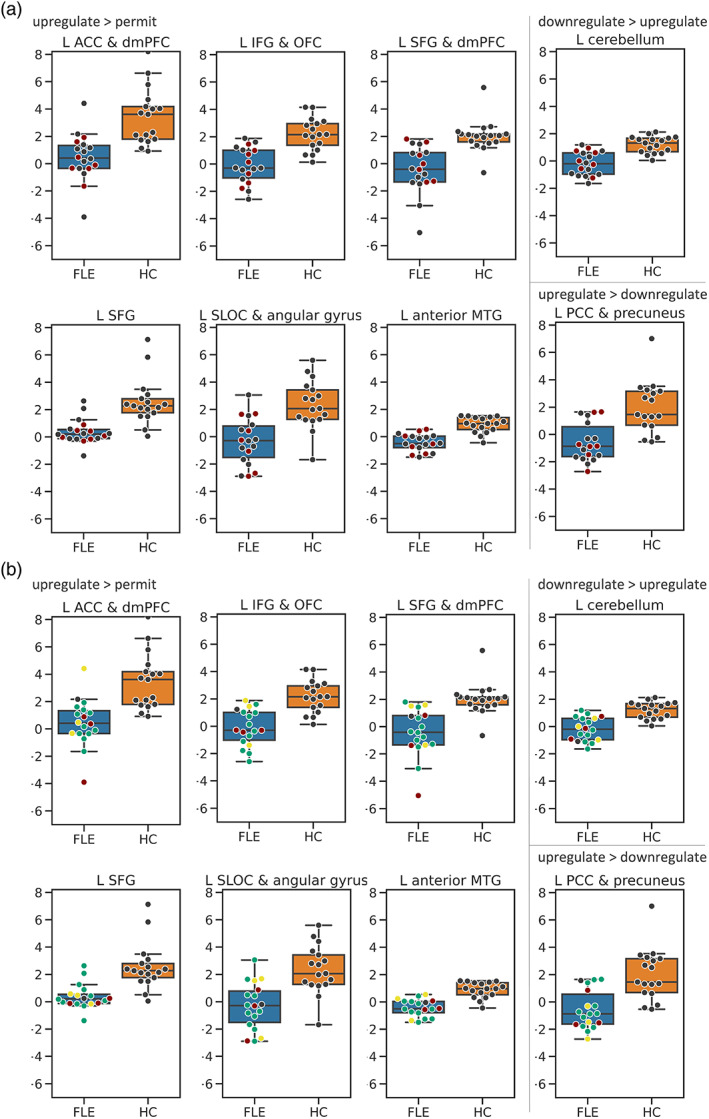
Box‐plots of controls (HC, orange) and frontal lobe epilepsy (FLE, blue) patients for clusters in which groups differed during upregulation > permit or upregulation versus downregulation (L cerebellum and L PCC & precuneus). The y‐axis shows beta estimates. Dots represent individual data points. (a) Red dots represent FLE patients with epileptogenic frontal lesions. Grey dots represent FLE patients without epileptogenic frontal lesions. (b) Red dots represent right‐sided, green dots represent left‐sided, and yellow dots represent bilateral FLE patients. A grey dot represents one patient with unknown focus lateralization. Based on visual inspection, neither the presence of an epileptogenic lesion, nor focus lateralization strongly influenced activation patterns of FLE patients. ACC, anterior cingulate cortex; dmPFC, dorsomedial prefrontal cortex; IFG, inferior frontal gyrus; L, left; MTG, middle temporal gyrus; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; SFG, superior frontal gyrus; SLOC, superior lateral occipital cortex
3.3. Regression analysis
Another aim of this study was to investigate whether the group differences that were found in regulating vs permitting emotions relate to real‐life emotion regulation abilities and habitual use of reappraisal. Thus, correlations for activity in the contrast upregulate > permit with the DERS scales Strategies, Goals, and Impulsivity and the ERQ's Reappraisal scale were computed (Table 5 and Figure 7). DERS’ Impulsivity scale was negatively correlated with activity in distributed brain regions. Bilateral frontostriatal regions played an important role in this, including putamen, caudate nucleus, insula, OFC, and vlPFC. Additionally, a right‐sided parieto‐temporal cluster with peaks in the posterior MTG and angular gyrus and a cluster within the brainstem were active. Habitual use of reappraisal was positively correlated with activity in a left vlPFC cluster, including the anterior insula and OFC, a bilateral medial prefrontal cluster and a bilateral cluster in the precuneus. As those correlations were computed over both groups to increase statistical power, it is important to note that, based on explorative visual data inspection, nearly all correlations appeared similarly strong in both groups.
TABLE 5.
Results of the regression analysis
| Regression analysis | |||||||
|---|---|---|---|---|---|---|---|
| Side | Region of activation | Coordinates | z score | Volume (k) | pFWE (cluster) | ||
| x | y | z | |||||
| Upregulate > permit | |||||||
| DERS impulsivity—Negative correlations | |||||||
| L | Putamen | −24 | 14 | 8 | 4.02 | 678 | < .001 |
| L | White matter | −20 | −4 | 12 | 3.56 | ||
| L | Precentral gyrus | −48 | 6 | 10 | 3.51 | ||
| L | IFG, triangular part | −50 | 24 | 26 | 3.80 | 216 | 0.054 |
| L | IFG, opercular part | −54 | 16 | 10 | 3.34 | ||
| L | " | −56 | 14 | 32 | 3.24 | ||
| L | Orbitofrontal cortex | −26 | 8 | −14 | 4.34 | 203 | 0.072 |
| L | Putamen | −28 | −8 | −8 | 3.92 | ||
| L | " | −30 | 0 | −4 | 2.96 | ||
| R | Middle temporal gyrus | 52 | −32 | 0 | 3.89 | 344 | 0.004 |
| R | Angular gyrus | 66 | −42 | 14 | 3.56 | ||
| R | Middle temporal gyrus | 60 | −40 | 8 | 3.14 | ||
| R | Caudate nucleus | 8 | 4 | 4 | 4.26 | 443 | 0.001 |
| R | Putamen | 20 | 6 | 4 | 4.14 | ||
| R | White matter | 24 | −16 | 14 | 3.88 | ||
| R | Cerebellum | 8 | −36 | −18 | 3.69 | 472 | < .001 |
| L | " | −6 | −56 | −8 | 3.61 | ||
| L | Brainstem | −4 | −16 | −12 | 3.58 | ||
| R | Cerebellum | 38 | −64 | −24 | 3.65 | 319 | 0.006 |
| R | " | 28 | −52 | −32 | 3.55 | ||
| R | " | 46 | −54 | −38 | 3.51 | ||
| ERQ reappraisal—Positive correlations | |||||||
| R | ACC | 4 | 32 | 12 | 3.90 | 402 | 0.001 |
| L | Paracingulate gyrus | −14 | 34 | 26 | 3.90 | ||
| L | ACC | −2 | 34 | 20 | 3.52 | ||
| L | Orbitofrontal cortex | −42 | 22 | −14 | 4.26 | 401 | 0.001 |
| L | IFG, triangular part | −44 | 22 | 8 | 3.78 | ||
| L | Orbitofrontal cortex | −34 | 28 | −16 | 3.46 | ||
| L | Precuneus | −6 | −70 | 28 | 3.23 | 219 | 0.053 |
| R | " | 6 | −64 | 34 | 2.98 | ||
| PCC | 0 | −48 | 26 | 2.92 | |||
Abbreviations: ACC, anterior cingulate cortex; IFG, inferior frontal gyrus; L, left; PCC, posterior cingulate cortex; R, right.
FIGURE 7.
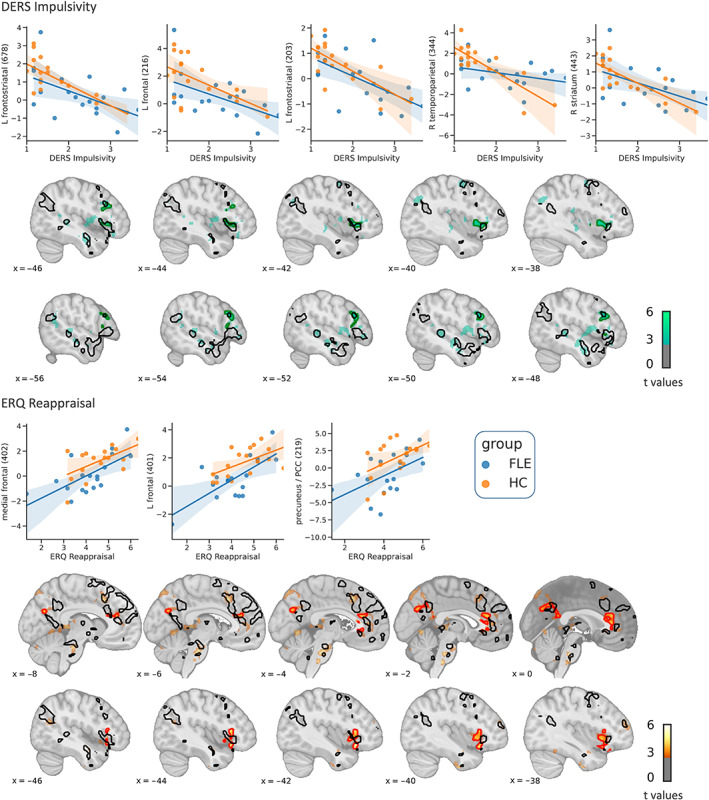
Whole‐brain results of the regression analysis. Green (negative correlations) or red (positive correlations) contours indicate significant correlations of upregulate > permit with impulsivity (upper panel) and reappraisal (lower panel; p FWE < .05). Black contours show regions in which healthy controls (HC, Orange dots) displayed higher activations than frontal lobe epilepsy (FLE, Blue dots) patients during upregulation, thresholded at p uncorr < .01 (see also Figure 5). For display purposes, activation maps are thresholded with p uncorr < .01 and a cluster threshold of 30 voxels. Scatterplots are shown only for visual inspection and not used for statistical inference. The y‐axis shows beta estimates. The number in brackets denotes the cluster size in voxels. DERS, difficulties in emotion regulation scale; ERQ, emotion regulation questionnaire. Left‐sided ventrolateral prefrontal activations that negatively correlated with DERS’ impulsivity scale (upper panel) and positively correlated with ERQ's reappraisal scale (lower panel) descriptively overlapped with higher upregulation‐related activity for controls compared to patients. Note that activity reductions in FLE patients compared to HC that are visible in the scatterplots, were significant (p uncorr < .05) in all regions but the right striatum and right temporoparietal cortex
4. DISCUSSION
To the best of our knowledge, this study is the first to investigate neural correlates of emotion regulation in FLE patients. It provides further causal evidence on the role of frontal functional brain networks in emotion regulation and on the neural and behavioral consequences of FLE pathophysiology. In general, it is of clinical value to gain deeper insight into neural correlates of disrupted cognitive and emotional functions in FLE using task‐fMRI. For example, it can reveal regions that are functionally disrupted on a given task, either linked to or irrespective of the epileptic focus localization. Moreover, task‐fMRI can provide a meaningful measure to track epilepsy‐related functional changes over time or to evaluate therapeutic outcomes.
In the present study, groups differed only marginally, albeit in the expected direction, in self‐reported emotion regulation. As it has been previously pointed out, behavioral differences between FLE patients and controls appear to be rather mild (Helmstaedter, 2001). On the other hand, FLE patients might underestimate their behavioral problems (Helmstaedter, 2001). Therefore, imaging data may provide a more objective complement to self‐reports. On the neural level, in the whole‐brain analysis, FLE patients showed no significant activations (p FWE < .05) for any regulation contrast even at a very low cluster‐forming threshold (p uncorr < .01), neither in frontal, nor in posterior brain regions. In healthy participants, by contrast, the activation patterns largely replicate former studies on emotion regulation (Morawetz, Bode, Derntl, et al., 2017; Ochsner et al., 2012). This is consistent with the notion that emotion regulation heavily relies on cognitive control networks in the PFC that are disrupted in FLE. The additional absence of posterior activations is in line with previous results on neural alterations in FLE patients during other cognitive control tasks (Braakman et al., 2013; Swartz et al., 1996) and extends them to emotion regulation skills.
Regions in which HC displayed significantly more activations than FLE patients during upregulating negative emotions were all within the left hemisphere and included anterior dmPFC and ACC, vlPFC, angular gyrus, and anterior MTG. These regions, along with the posterior MTG, are part of semantic control networks, with semantic control being defined as “task‐directed retrieval/selection of memories and integration of these internal representations with external inputs and current goals” (Noonan et al., 2013, p. 1825). As such, semantic control abilities are also needed to reinterpret negative emotional content (Buhle et al., 2014; Ochsner et al., 2012). Noonan et al. (2013) suggested an important role for the left vlPFC, angular gyrus and posterior MTG in semantic control. In a large meta‐analysis, they found a predominantly left‐sided activation pattern that showed considerable overlap with the activations we observed and included the vlPFC, posterior and anterior MTG, dmPFC, angular gyrus, ACC and paracingular gyrus (BA32, 10), thus comprising regions involved in semantic processing, executive control and mentalizing. Focal damage in any of these regions could result in reduced abilities to activate this whole network and might thus be associated with more difficulties in all these domains. Furthermore, repeated seizures may have lasting effects on brain network activity, affecting also distant regions (Englot et al., 2015; Liang et al., 2020). It has been reported that chronic epilepsy can induce neural reorganization in adult brains (Elger et al., 2004). Thus, investigating alterations throughout the brain as well as possible compensatory mechanisms is of clinical relevance. In the whole‐brain analysis, the only region in which FLE patients displayed more activity than HC for upregulating compared to permitting emotions was the right SLOC. However, inspection of the corresponding time course of the hemodynamic response (Supporting Information S8) shows that the FLE > HC difference is related to a very late segment after the time course peak, where activity is unlikely to be strongly task‐related. Therefore, this finding should be replicated before further interpretation. Thus, taken together, in the FLE group, we did not observe reliable evidence of compensatory activations in non‐frontal regions. Instead, in line with previous research (Buhle et al., 2014), our results suggest that the recruitment of frontal circuits is necessary to additionally activate posterior semantic brain regions during reappraisal. However, it cannot be ruled out that diminished posterior activations, instead of being a downstream consequence of reduced frontal recruitment, may also result from more widespread structural or functional alterations associated with FLE (Braakman et al., 2013; Campos et al., 2015).
Upon direct comparison, group differences were not significant for downregulating negative emotions. Whereas downregulation might be accomplished consistently by reminding oneself that the depicted scene is not real, during upregulation participants were asked to actively empathize, take another person's perspective, or rapidly construct a highly aversive scene and imagine oneself within it. Thus, upregulation arguably requires more internally directed cognitive effort than downregulation. This is mirrored by stronger activations in dmPFC and precuneus for upregulation compared to downregulation. Correspondingly, Ochsner et al. (2012) interpreted increased dmPFC activation during upregulation compared to downregulation as increased mentalizing efforts. Theoretically, this upregulation‐specific aspect might be particularly demanding for FLE patients, as they also displayed less activity in the left precuneus and PCC than HC during upregulation compared to downregulation. This would also dovetail with findings of reduced ToM (Farrant et al., 2005; Giovagnoli et al., 2011, 2013) and increased problems in interpersonal contact compared to controls and TLE patients (Helmstaedter & Witt, 2012; Pizzi et al., 2009). Alternatively, upregulation might have been more difficult than downregulation due to habituation effects related to the task design. This included a three‐to‐one ratio of negative to neutral pictures which may have fostered habituation effects to negative pictures throughout the brain (Breiter et al., 1996; LaBar et al., 1998; Phillips et al., 2001; Wright et al., 2001).
During downregulation compared to upregulation the left cerebellum was more active in HC than in FLE patients. Although the bilateral cerebellum was involved in upregulation and downregulation in the HC group, especially the left cerebellum was found to be more active for downregulating compared to upregulating emotions. Notably, all other cerebral cortical activations that appeared in this contrast were within the right hemisphere. This corresponds to the contralateral organization of information forwarding between cerebellum and cerebral cortex (Palesi et al., 2017). Importantly, descending pathways also connect prefrontal and temporal cortices with cerebellar cognitive regions (Palesi et al., 2017). Although other studies investigating reappraisal also found cerebellar involvement (Ochsner et al., 2004; Walter et al., 2009), it has not been consistently implicated in emotion regulation (Morawetz, Bode, Derntl, et al., 2017; Ochsner et al., 2012) and its potential role still needs to be determined.
Another aim of this study was to investigate the amygdala's response to emotion regulation as a major target of top‐down control over bottom‐up evoked emotions. We therefore performed ROI time course analysis on the left and right amygdala. Most studies on emotion regulation reported amygdala modulations consistent with the regulation goal (Morawetz, Bode, Derntl, et al., 2017; Ochsner et al., 2012). In line with this, we observed bilateral modulation of amygdala activity for HC. FLE patients, by contrast, exhibited such an effect only in the right, but not in the left amygdala.
As outlined above, FLE patients also displayed a pattern of diminished activations in left‐sided semantic control regions during upregulating emotions, including the left lateral temporal gyrus. Buhle et al. (2014) suggested that during reappraisal prefrontal executive control regions exert their effects over the amygdala through this region. Our results indicate that this may be true only for the left amygdala, whereas the right amygdala remains largely unaffected by disruptions in left lateral frontal and temporal activations. Thus, the present data suggest that during emotion regulation, disruptions in the semantic processing stream lead to reduced left, but not right amygdala modulations. Likewise, Ochsner et al. (2009) showed that bottom‐up emotional processing recruits both amygdalae more than top‐down emotion generation, whereas only the left was targeted by top‐down emotion generation, namely reappraising neutral images in a negative way. Other regions that were distinctly involved in top‐down emotion generation formed a left‐lateralized network of frontostriatal and temporal activations, including brain regions that we found to be more active in HC than in FLE patients during upregulation like the dmPFC, SFG, IFG, and MTG. Note also, that upregulation has a lot in common with Ochsner et al.'s top‐down emotion generation task, namely reappraising the meaning of images to increase or elicit negative emotions. Taken together, our data suggest that effortful top‐down regulatory emotion processing, which changes right amygdala response, also takes place in FLE patients but that semantic control, which may be more related to the left amygdala, is disrupted in this group.
Possible behavioral and emotional correlates of activation patterns in FLE patients were explored using questionnaire data on habitual emotion regulation strategies and difficulties. In order to make the regression analysis more robust and to increase statistical power, we calculated correlations over both groups combined. It is therefore important to note that all correlations discussed here were similarly strong in both groups, as post hoc visual inspections revealed.
The activation map for correlations of activity in the contrast upregulate > permit with impulsivity ratings descriptively overlapped with group differences in the left vlPFC, MTG, and angular gyrus. However, correlations were only significant for the left vlPFC. Moreover, group differences in the ACC and OFC correspond to correlations between ERQ reappraisal ratings and activity in the contrast upregulate > permit. Thus, regions, that are involved in reinterpreting situations to increase one's emotions and that correlate with self‐reported impulse control difficulties or habitual use of reappraisal strategies, are being activated by FLE patients to a lesser extent than by HC. Likewise, our data revealed that FLE patients tended to report more impulse control difficulties than HC, replicating previous findings of increased affective instability (Pizzi et al., 2009) and impulsivity (Helmstaedter & Witt, 2012) in this group compared to controls and TLE patients. This indicates that the group differences that we found in our imaging data may have implications with regard to real‐life emotion regulation difficulties. Thus, these results may inform psychotherapeutic approaches to dysfunctional emotion regulation in FLE. For instance, it might be beneficial for patients to practice using functional and adaptive reappraisals in daily life, as reappraisal success has been found to predict cognitive behavioral therapy success (Goldin et al., 2014).
Also, frontostriatal activation patterns were associated with DERS’ Impulsivity scores. Striatal activation is a stable finding in emotion regulation tasks. The striatum is associated with feedback‐based learning processes (Shohamy, 2011). Therefore, its activation in emotion regulation tasks may reflect processing of one's emotional state as feedback for regulation success (Morawetz, Bode, Derntl, et al., 2017). Moreover, frontostriatal circuits are more broadly involved in the execution of goal‐directed behaviors through motivational and executive loops and are important for successful performance in a variety of tasks, including working memory (Nour et al., 2019), episodic memory (Nyberg et al., 2016), and sociocognitive tasks (Ziv et al., 2013). Ziv and colleagues showed that during reappraisal of criticism HC exhibited higher activity in the left putamen and descriptively also a stronger reduction in negative emotions than participants with social anxiety disorder. Moreover, left putamen activation has been observed for imitating happy and sad facial emotions as compared to expressive suppression (Vrticka et al., 2013), indicating that this region might also play a role in perspective taking. Adding to this, Eimontaite et al. (2019) found the left putamen to be implicated in regulation of anger during a prisoner's dilemma game. The authors suggested, that the left putamen may have helped in suppressing anger and engaging in cooperative behavior instead. In light of such results investigating the role of frontostriatal circuits in emotion‐regulation further seems promising.
4.1. Limitations
We included 17 HC and 18 FLE patients in our study which lies in the range of other publications that investigated neural correlates of FLE (e.g., Braakman et al., 2013; Centeno et al., 2012; Dong et al., 2016; Moeller et al., 2009; Swartz et al., 1996; Woodward et al., 2014). However, given the heterogeneity of the patient group (some with and some without frontal epileptogenic lesions, seizure origin in different parts of the frontal lobe, partly overlapping with neighboring cortex areas) to draw robust and generalizable conclusions our findings need to be replicated and extended in other studies. Additionally, our between‐group analyses might have been unable to find existing differences due to a lack in statistical power. Indeed, for all group differences, effect sizes were at least moderate. Also, for regression analysis, larger sample sizes are desirable as this would offer possibilities to further validate the results, for example, by performing a split‐half analysis.
Moreover, two‐thirds of patients had left‐sided epileptic foci, which may have influenced our results as all group differences that we found were in the left hemisphere. Especially upregulation, as compared to downregulation yielded a largely left lateralized activation pattern, which might be impacted more by left‐sided than by right‐sided FLE, as frontotemporal connectivity is more pronounced ipsilateral than contralateral (Lacruz et al., 2007). Unfortunately, we do not have information on language lateralization for all patients, which might also affect activation patterns, particularly in semantic control regions. In general, most epilepsy patients have left‐lateralized language, but atypical language lateralization is more common, especially in extra‐temporal lobe epilepsy (Woermann et al., 2003).
Future studies should specifically address the effect of focus lateralization and a more precise localization of the epileptogenic areas within the frontal lobes as this would, for instance, further inform models of hemispheric asymmetries or the role of midline structures compared to lateral regions for emotion processing. In our data, the presence of an epileptogenic lesion was not related to activity within regions in which group differences for upregulate vs permit or upregulate vs downregulate were significant (Figure 6). Considering the frontal lobes as highly interconnected parts of the brain and emphasizing this network character, especially with respect to a network‐disorder like epilepsy (Kramer & Cash, 2012), the exact location of the epileptogenic area might not be decisive.
We interpret our results as evidence for group differences in emotion regulation. However, one might argue that such differences are, at least in part, the consequence of altered emotion perception in FLE as previous findings may indicate impaired emotion recognition abilities in FLE patients (Edwards et al., 2017; Morningstar et al., 2022). Though this is a possibility that we cannot fully rule out, we do not believe that potentially altered emotion perception has a strong influence on our results. In contrast to facial expression rating tasks, the stimuli that were used in the present study mostly displayed complex scenes conveying much more context, helpful for interpretation. More importantly, patients and controls did not differ in their emotionality ratings during the behavioral emotion regulation task. This was mirrored by the fMRI‐task, in which FLE patients did not differ from HC, in regions commonly associated with bottom‐up emotion generating processes (Ochsner et al., 2012), while permitting negative emotions compared to neutral images.
5. CONCLUSION
Taken together, our results provide novel insights into functional correlates of emotion regulation in FLE that are of clinical value and help delineate the neural mechanisms involved in reappraisal. We found that during emotion (up‐)regulation FLE patients compared to controls show reduced activations in frontal and posterior brain regions that are linked to domain‐general cognitive control and semantic processing. Furthermore, our results support the notion that posterior brain activations in semantic control regions during emotion regulation are orchestrated by activations in frontal executive control regions, assuming brain damage associated with FLE to be largely restricted to the frontal lobes. With respect to expected frontal modulations of the amygdala, we found that the left but not the right amygdala shows no response to the regulation task in the FLE group. We interpret this finding as a consequence of diminished recruitment of left‐sided lateral frontotemporal semantic control regions, which in turn resulted from disrupted functioning in frontal executive control regions. Finally, our findings suggest that activation differences between groups may be relevant for real‐life emotional experiences and behavior in emotionally demanding situations, as they overlap with regions, which activations correlate with self‐reported habitual emotion regulation.
FUNDING INFORMATION
This research was supported by a grant from the Gerd‐Altenhof Foundation within the German Founders Foundation (Deutscher Stifterverband).
CONFLICT OF INTEREST
The authors declare no competing financial interests.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
We thank Merisa Ferati, Mirijam Behrens, Stefanie Hedtmann, Marina Hedtmann, Sebastian Heilmann, Sophia Dunkel, Justin Hachenberger, Simon Laumen, and Markus Mertens for support with data acquisition and data management. We also thank all participants for their participation. Open Access funding enabled and organized by Projekt DEAL.
Benzait, A. , Krenz, V. , Wegrzyn, M. , Doll, A. , Woermann, F. , Labudda, K. , Bien, C. G. , & Kissler, J. (2023). Hemodynamic correlates of emotion regulation in frontal lobe epilepsy patients and healthy participants. Human Brain Mapping, 44(4), 1456–1475. 10.1002/hbm.26133
Funding information Gerd‐Altenhof Foundation, Grant/Award Number: T0465/28102/2016/sm
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abler, B. , & Kessler, H. (2009). Emotion Regulation Questionnaire—Eine deutschsprachige Fassung des ERQ von Gross und John. Diagnostica, 55(3), 144–152. 10.1026/0012-1924.55.3.144 [DOI] [Google Scholar]
- Abraham, A. , Pedregosa, F. , Eickenberg, M. , Gervais, P. , Mueller, A. , Kossaifi, J. , Gramfort, A. , Thirion, B. , & Varoquaux, G. (2014). Machine learning for neuroimaging with scikit‐learn. Frontiers in Neuroinformatics, 8, 14. 10.3389/fninf.2014.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs, R. (2002). Neural systems for recognizing emotion. Current Opinion in Neurobiology, 12(2), 169–177. 10.1016/S0959-4388(02)00301-X [DOI] [PubMed] [Google Scholar]
- Alexander, M. , & Stuss, D. (2000). Disorders of frontal lobe functioning. Seminars in Neurology, 20, 427–437. 10.1055/s-2000-13175 [DOI] [PubMed] [Google Scholar]
- Andrews‐Hanna, J. R. , Saxe, R. , & Yarkoni, T. (2014). Contributions of episodic retrieval and mentalizing to autobiographical thought: Evidence from functional neuroimaging, resting‐state connectivity, and fMRI meta‐analyses. NeuroImage, 91, 324–335. 10.1016/j.neuroimage.2014.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants, B. B. , Epstein, C. L. , Grossman, M. , & Gee, J. C. (2008). Symmetric diffeomorphic image registration with cross‐correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis, 12(1), 26–41. 10.1016/j.media.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks, S. J. , Eddy, K. T. , Angstadt, M. , Nathan, P. J. , & Phan, K. L. (2007). Amygdala–frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2(4), 303–312. 10.1093/scan/nsm029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara, A. (2004). Disturbances of emotion regulation after focal brain lesions. International Review of Neurobiology, 62(159), 93. 10.1016/S0074-7742(04)62006-X [DOI] [PubMed] [Google Scholar]
- Beck, A. T. , Steer, R. A. , & Brown, G. K. (1996). BDI‐II manual. The Psychological Corporation. [Google Scholar]
- Braakman, H. M. , Vaessen, M. J. , Jansen, J. F. , Debeij‐van Hall, M. H. , de Louw, A. , Hofman, P. A. , Vles, J. S. , Aldenkamp, A. P. , & Backes, W. H. (2013). Frontal lobe connectivity and cognitive impairment in pediatric frontal lobe epilepsy. Epilepsia, 54(3), 446–454. 10.1111/epi.12044 [DOI] [PubMed] [Google Scholar]
- Brandt, C. , Schoendienst, M. , Trentowska, M. , May, T. W. , Pohlmann‐Eden, B. , Tuschen‐Caffier, B. , Schrecke, M. , Fueratsch, N. , Witte‐Boelt, K. , & Ebner, A. (2010). Prevalence of anxiety disorders in patients with refractory focal epilepsy—A prospective clinic based survey. Epilepsy & Behavior, 17(2), 259–263. 10.1016/j.yebeh.2009.12.009 [DOI] [PubMed] [Google Scholar]
- Breiter, H. C. , Etcoff, N. L. , Whalen, P. J. , Kennedy, W. A. , Rauch, S. L. , Buckner, R. L. , Strauss, M. M. , Hyman, S. E. , & Rosen, B. R. (1996). Response and habituation of the human amygdala during visual processing of facial expression. Neuron, 17(5), 875–887. 10.1016/S0896-6273(00)80219-6 [DOI] [PubMed] [Google Scholar]
- Brett, M. , Anton, J. L. , Valabregue, R. , & Poline, J. B. (2002). Region of interest analysis using an SPM toolbox. In Proceedings of the 8th International Conference on Functional Mapping of the Human Brain (Vol. 16, No. 2, p. 497).
- Buchanan, T. W. (2007). Retrieval of emotional memories. Psychological Bulletin, 133(5), 761–779. 10.1037/0033-2909.133.5.761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle, J. T. , Silvers, J. A. , Wager, T. D. , Lopez, R. , Onyemekwu, C. , Kober, H. , Weber, J. , & Ochsner, K. N. (2014). Cognitive reappraisal of emotion: A meta‐analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos, B. M. , Coan, A. C. , Beltramini, G. C. , Liu, M. , Yassuda, C. L. , Ghizoni, E. , Beaulieu, C. , Gross, D. W. , & Cendes, F. (2015). White matter abnormalities associate with type and localization of focal epileptogenic lesions. Epilepsia, 56(1), 125–132. 10.1111/epi.12871 [DOI] [PubMed] [Google Scholar]
- Centeno, M. , Vollmar, C. , O'Muircheartaigh, J. , Stretton, J. , Bonelli, S. B. , Symms, M. R. , Barker, G. J. , Kumari, V. , Thompson, P. J. , Duncan, J. S. , Richardson, M. P. , & Koepp, M. J. (2012). Memory in frontal lobe epilepsy: An fMRI study. Epilepsia, 53(10), 1756–1764. 10.1111/j.1528-1167.2012.03570.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia, L. , Bartolomeo, L. , Capobianco, M. , Lo Russo, S. L. , Festucci, F. , Tambelli, R. , Adriani, W. , & Cimino, S. (2019). Intersections and divergences between empathizing and mentalizing: Development, recent advancements by neuroimaging and the future of animal modelling. Frontiers in Behavioral Neuroscience, 13, 212. 10.3389/fnbeh.2019.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, R. W. , & Hyde, J. S. (1997). Software tools for analysis and visualization of fMRI data. NMR in Biomedicine, 10(4–5), 171–178. [DOI] [PubMed] [Google Scholar]
- Dale, A. M. , Fischl, B. , & Sereno, M. I. (1999). Cortical surface‐based analysis: I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179–194. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Davis, M. , & Whalen, P. J. (2001). The amygdala: Vigilance and emotion. Molecular Psychiatry, 6(1), 13–34. 10.1038/sj.mp.4000812 [DOI] [PubMed] [Google Scholar]
- Dong, L. , Wang, P. , Peng, R. , Jiang, S. , Klugah‐Brown, B. , Luo, C. , & Yao, D. (2016). Altered basal ganglia‐cortical functional connections in frontal lobe epilepsy: A resting‐state fMRI study. Epilepsy Research, 128, 12–20. 10.1016/j.eplepsyres.2016.10.011 [DOI] [PubMed] [Google Scholar]
- Edwards, M. , Stewart, E. , Palermo, R. , & Lah, S. (2017). Facial emotion perception in patients with epilepsy: A systematic review with meta‐analysis. Neuroscience & Biobehavioral Reviews, 83, 212–225. 10.1016/j.neubiorev.2017.10.013 [DOI] [PubMed] [Google Scholar]
- Ehring, T. , Fischer, S. , Schnülle, J. , Bösterling, A. , & Tuschen‐Caffier, B. (2008). Characteristics of emotion regulation in recovered depressed versus never depressed individuals. Personality and Individual Differences, 44(7), 1574–1584. 10.1016/j.paid.2008.01.013 [DOI] [Google Scholar]
- Ehring, T. , Tuschen‐Caffier, B. , Schnülle, J. , Fischer, S. , & Gross, J. J. (2010). Emotion regulation and vulnerability to depression: Spontaneous versus instructed use of emotion suppression and reappraisal. Emotion, 10(4), 563–572. 10.1037/a0019010 [DOI] [PubMed] [Google Scholar]
- Eimontaite, I. , Schindler, I. , De Marco, M. , Duzzi, D. , Venneri, A. , & Goel, V. (2019). Left amygdala and putamen activation modulate emotion driven decisions in the iterated Prisoner's dilemma game. Frontiers in Neuroscience, 13, 741. 10.3389/fnins.2019.00741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elger, C. E. , Helmstaedter, C. , & Kurthen, M. (2004). Chronic epilepsy and cognition. The Lancet Neurology, 3(11), 663–672. 10.1016/S1474-4422(04)00906-8 [DOI] [PubMed] [Google Scholar]
- Englot, D. J. , Hinkley, L. B. , Kort, N. S. , Imber, B. S. , Mizuiri, D. , Honma, S. M. , Findlay, A. M. , Garrett, C. , Cheung, P. L. , Mantle, M. , Tarapore, P. E. , Knowlton, R. C. , Chang, E. F. , Kirsch, H. E. , & Nagarajan, S. S. (2015). Global and regional functional connectivity maps of neural oscillations in focal epilepsy. Brain, 138(8), 2249–2262. 10.1093/brain/awv130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban, O. , Markiewicz, C. J. , Blair, R. W. , Moodie, C. A. , Isik, A. I. , Erramuzpe, A. , Kent, J. D. , Goncalves, M. , DuPre, E. , Snyder, M. , Oya, H. , Ghosh, S. S. , Wright, J. , Durnez, J. , Poldrack, R. A. , & Gorgolewski, K. J. (2019). fMRIPrep: A robust preprocessing pipeline for functional MRI. Nature Methods, 16(1), 111–116. 10.1038/s41592-018-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner, C. , Boucsein, K. , Lange, C. , Winter, H. , Weniger, G. , Steinhoff, B. J. , & Irle, E. (2002). Neuropsychological performance in frontal lobe epilepsy. Seizure, 11(1), 20–32. 10.1053/seiz.2001.0572 [DOI] [PubMed] [Google Scholar]
- Falquez, R. , Dinu‐Biringer, R. , Stopsack, M. , Arens, E. A. , Wick, W. , & Barnow, S. (2015). Examining cognitive emotion regulation in frontal lobe patients: The mediating role of response inhibition. NeuroRehabilitation, 37(1), 89–98. 10.3233/NRE-151242 [DOI] [PubMed] [Google Scholar]
- Farrant, A. , Morris, R. G. , Russell, T. , Elwes, R. , Akanuma, N. , Alarcón, G. , & Koutroumanidis, M. (2005). Social cognition in frontal lobe epilepsy. Epilepsy & Behavior, 7(3), 506–516. 10.1016/j.yebeh.2005.07.018 [DOI] [PubMed] [Google Scholar]
- Fonov, V. S. , Evans, A. C. , McKinstry, R. C. , Almli, C. R. , & Collins, D. L. (2009). Unbiased nonlinear average age‐appropriate brain templates from birth to adulthood. NeuroImage, 47, S102. 10.1016/S1053-8119(09)70884-5 [DOI] [Google Scholar]
- Giovagnoli, A. R. , Franceschetti, S. , Reati, F. , Parente, A. , Maccagnano, C. , Villani, F. , & Spreafico, R. (2011). Theory of mind in frontal and temporal lobe epilepsy: Cognitive and neural aspects. Epilepsia, 52(11), 1995–2002. 10.1111/j.1528-1167.2011.03215.x [DOI] [PubMed] [Google Scholar]
- Giovagnoli, A. R. , Parente, A. , Villani, F. , Franceschetti, S. , & Spreafico, R. (2013). Theory of mind and epilepsy: What clinical implications? Epilepsia, 54(9), 1639–1646. 10.1111/epi.12255 [DOI] [PubMed] [Google Scholar]
- Giovagnoli, A. R. , Tallarita, G. M. , Parente, A. , Pastori, C. , & de Curtis, M. (2020). The understanding of mental states and the cognitive phenotype of frontal lobe epilepsy. Epilepsia, 61(4), 747–757. 10.1111/epi.16457 [DOI] [PubMed] [Google Scholar]
- Goldin, P. R. , Lee, I. , Ziv, M. , Jazaieri, H. , Heimberg, R. G. , & Gross, J. J. (2014). Trajectories of change in emotion regulation and social anxiety during cognitive‐behavioral therapy for social anxiety disorder. Behaviour Research and Therapy, 56, 7–15. 10.1016/j.brat.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski, K. , Burns, C. D. , Madison, C. , Clark, D. , Halchenko, Y. O. , Waskom, M. L. , & Ghosh, S. S. (2011). Nipype: A flexible, lightweight and extensible neuroimaging data processing framework in python. Frontiers in Neuroinformatics, 5, 13. 10.3389/fninf.2011.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz, K. L. , & Roemer, L. (2004). Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment, 26(1), 41–54. 10.1023/B:JOBA.0000007455.08539.94 [DOI] [Google Scholar]
- Gross, J. J. (2015). Emotion regulation: Current status and future prospects. Psychological Inquiry, 26(1), 1–26. 10.1080/1047840X.2014.940781 [DOI] [Google Scholar]
- Gross, J. J. , & John, O. P. (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well‐being. Journal of Personality and Social Psychology, 85(2), 348–362. 10.1037/0022-3514.85.2.348 [DOI] [PubMed] [Google Scholar]
- Gul, A. , & Ahmad, H. (2015). Thought control strategies predict emotion regulation abilities in patients with frontal lobe epilepsy. Neuroscience Discovery, 3(1), 1–7. 10.7243/2052-6946-3-1 [DOI] [Google Scholar]
- Hautzinger, M. , Keller, F. , & Kühner, C. (2006). Beck depressions‐inventar (BDI‐II). Harcourt Test Services. [Google Scholar]
- Helmstaedter, C. (2001). Behavioral aspects of frontal lobe epilepsy. Epilepsy & Behavior, 2(5), 384–395. 10.1006/ebeh.2001.0259 [DOI] [PubMed] [Google Scholar]
- Helmstaedter, C. , Kemper, B. , & Elger, C. E. (1996). Neuropsychological aspects of frontal lobe epilepsy. Neuropsychologia, 34(5), 399–406. 10.1016/0028-3932(95)00121-2 [DOI] [PubMed] [Google Scholar]
- Helmstaedter, C. , & Witt, J. A. (2012). Multifactorial etiology of interictal behavior in frontal and temporal lobe epilepsy. Epilepsia, 53(10), 1765–1773. 10.1111/j.1528-1167.2012.03602.x [DOI] [PubMed] [Google Scholar]
- Hu, T. , Zhang, D. , Wang, J. , Mistry, R. , Ran, G. , & Wang, X. (2014). Relation between emotion regulation and mental health: A meta‐analysis review. Psychological Reports, 114(2), 341–362. 10.2466/03.20.PR0.114k22w4 [DOI] [PubMed] [Google Scholar]
- Jazaieri, H. , Morrison, A. S. , Goldin, P. R. , & Gross, J. J. (2015). The role of emotion and emotion regulation in social anxiety disorder. Current Psychiatry Reports, 17(1), 531. 10.1007/s11920-014-0531-3 [DOI] [PubMed] [Google Scholar]
- Kanske, P. , Heissler, J. , Schönfelder, S. , & Wessa, M. (2012). Neural correlates of emotion regulation deficits in remitted depression: The influence of regulation strategy, habitual regulation use, and emotional valence. NeuroImage, 61(3), 686–693. 10.1016/j.neuroimage.2012.03.089 [DOI] [PubMed] [Google Scholar]
- Kanske, P. , Schönfelder, S. , Forneck, J. , & Wessa, M. (2015). Impaired regulation of emotion: Neural correlates of reappraisal and distraction in bipolar disorder and unaffected relatives. Translational Psychiatry, 5(1), e497. 10.1038/tp.2014.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, A. , Ghosh, S. S. , Bao, F. S. , Giard, J. , Häme, Y. , Stavsky, E. , Lee, N. , Rossa, B. , Reuter, M. , Chaibub Neto, E. , & Keshavan, A. (2017). Mindboggling morphometry of human brains. PLoS Computational Biology, 13(2), e1005350. 10.1371/journal.pcbi.1005350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, M. A. , & Cash, S. S. (2012). Epilepsy as a disorder of cortical network organization. The Neuroscientist, 18(4), 360–372. 10.1177/1073858411422754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes, M. C. , Dunsmoor, J. E. , Hakimi, M. , Oosterwaal, S. , NYU PROSPEC Collaboration , Meager, M. R. , & Phelps, E. A. (2019). Patients with dorsolateral prefrontal cortex lesions are capable of discriminatory threat learning but appear impaired in cognitive regulation of subjective fear. Social Cognitive and Affective Neuroscience, 14(6), 601–612. 10.1093/scan/nsz039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar, K. S. , Gatenby, J. C. , Gore, J. C. , LeDoux, J. E. , & Phelps, E. A. (1998). Human amygdala activation during conditioned fear acquisition and extinction: A mixed‐trial fMRI study. Neuron, 20(5), 937–945. 10.1016/S0896-6273(00)80475-4 [DOI] [PubMed] [Google Scholar]
- Lacruz, M. E. , Garcia Seoane, J. J. , Valentin, A. , Selway, R. , & Alarcon, G. (2007). Frontal and temporal functional connections of the living human brain. European Journal of Neuroscience, 26(5), 1357–1370. 10.1111/j.1460-9568.2007.05730.x [DOI] [PubMed] [Google Scholar]
- Lanczos, C. (1964). Evaluation of noisy data. Journal of the Society for Industrial and Applied Mathematics, Series B: Numerical Analysis, 1(1), 76–85. 10.1137/0701007 [DOI] [Google Scholar]
- Lang, P. J. , Bradley, M. M. , & Cuthbert, B. N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A‐8. University of Florida. [Google Scholar]
- Laux, L. , Glanzmann, P. , Schaffner, P. , & Spielberger, C. D. (1981). Das state–trait‐Angstinventar (STAI). Hogrefe. [Google Scholar]
- Liang, Y. , Chen, C. , Li, F. , Yao, D. , Xu, P. , & Yu, L. (2020). Altered functional connectivity after epileptic seizure revealed by scalp EEG. Neural Plasticity, 2020, 1–8. 10.1155/2020/8851415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletti, S. , Benuzzi, F. , Rubboli, G. , Cantalupo, G. , Maserati, M. S. , Nichelli, P. , & Tassinari, C. (2003). Impaired facial emotion recognition in early‐onset right mesial temporal lobe epilepsy. Neurology, 60(3), 426–431. 10.1212/WNL.60.3.426 [DOI] [PubMed] [Google Scholar]
- Mier, D. , Lis, S. , Neuthe, K. , Sauer, C. , Esslinger, C. , Gallhofer, B. , & Kirsch, P. (2010). The involvement of emotion recognition in affective theory of mind. Psychophysiology, 47(6), 1028–1039. 10.1111/j.1469-8986.2010.01031.x [DOI] [PubMed] [Google Scholar]
- Moeller, F. , Tyvaert, L. , Nguyen, D. K. , LeVan, P. , Bouthillier, A. , Kobayashi, E. , Tampieri, D. , Dubeau, F. , & Gotman, J. (2009). EEG‐fMRI: Adding to standard evaluations of patients with nonlesional frontal lobe epilepsy. Neurology, 73(23), 2023–2030. 10.1212/WNL.0b013e3181c55d17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz, C. , Bode, S. , Baudewig, J. , & Heekeren, H. R. (2017). Effective amygdala‐prefrontal connectivity predicts individual differences in successful emotion regulation. Social Cognitive and Affective Neuroscience, 12(4), 569–585. 10.1093/scan/nsw169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz, C. , Bode, S. , Derntl, B. , & Heekeren, H. R. (2017). The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta‐analysis of fMRI studies. Neuroscience & Biobehavioral Reviews, 72, 111–128. 10.1016/j.neubiorev.2016.11.014 [DOI] [PubMed] [Google Scholar]
- Morningstar, M. , Grannis, C. , Mattson, W. I. , & Nelson, E. E. (2022). Functional patterns of neural activation during vocal emotion recognition in youth with and without refractory epilepsy. NeuroImage: Clinical, 34, 102966. 10.1016/j.nicl.2022.102966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan, K. A. , Jefferies, E. , Visser, M. , & Lambon Ralph, M. A. (2013). Going beyond inferior prefrontal involvement in semantic control: Evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. Journal of Cognitive Neuroscience, 25(11), 1824–1850. 10.1162/jocn_a_00442 [DOI] [PubMed] [Google Scholar]
- Nour, M. M. , Dahoun, T. , McCutcheon, R. A. , Adams, R. A. , Wall, M. B. , & Howes, O. D. (2019). Task‐induced functional brain connectivity mediates the relationship between striatal D2/3 receptors and working memory. eLife, 8, e45045. 10.7554/eLife.45045.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg, L. , Karalija, N. , Salami, A. , Andersson, M. , Wåhlin, A. , Kaboovand, N. , Köhncke, Y. , Axelsson, J. , Rieckmann, A. , Papenberg, G. , Garrett, D. D. , Riklund, K. , Lövdén, M. , Lindenberger, U. , & Bäckman, L. (2016). Dopamine D2 receptor availability is linked to hippocampal–caudate functional connectivity and episodic memory. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7918–7923. 10.1073/pnas.1606309113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner, K. N. , Bunge, S. A. , Gross, J. J. , & Gabrieli, J. D. (2002). Rethinking feelings: An FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14(8), 1215–1229. 10.1162/089892902760807212 [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , Knierim, K. , Ludlow, D. H. , Hanelin, J. , Ramachandran, T. , Glover, G. , & Mackey, S. C. (2004). Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience, 16(10), 1746–1772. [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , Ray, R. R. , Hughes, B. , McRae, K. , Cooper, J. C. , Weber, J. , Gabrieli, J. D. , & Gross, J. J. (2009). Bottom‐up and top‐down processes in emotion generation: Common and distinct neural mechanisms. Psychological Science, 20(11), 1322–1331. 10.1162/0898929042947829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner, K. N. , Silvers, J. A. , & Buhle, J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251(1), E1–E24. 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palesi, F. , De Rinaldis, A. , Castellazzi, G. , Calamante, F. , Muhlert, N. , Chard, D. , Tournier, J. D. , Magenes, G. , D'Angelo, E. , & Wheeler‐Kingshott, C. A. G. (2017). Contralateral cortico‐ponto‐cerebellar pathways reconstruction in humans in vivo: Implications for reciprocal cerebro‐cerebellar structural connectivity in motor and non‐motor areas. Scientific Reports, 7(1), 1–13. 10.1038/s41598-017-13079-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrikelis, P. , Angelakis, E. , & Gatzonis, S. (2009). Neurocognitive and behavioral functioning in frontal lobe epilepsy: A review. Epilepsy & Behavior, 14(1), 19–26. 10.1016/j.yebeh.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Peirce, J. , Gray, J. R. , Simpson, S. , MacAskill, M. , Höchenberger, R. , Sogo, H. , Kastman, E. , & Lindeløv, J. K. (2019). PsychoPy2: Experiments in behavior made easy. Behavior Research Methods, 51(1), 195–203. 10.3758/s13428-018-01193-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, M. L. , Medford, N. , Young, A. W. , Williams, L. , Williams, S. C. , Bullmore, E. T. , Gray, J. A. , & Brammer, M. J. (2001). Time courses of left and right amygdalar responses to fearful facial expressions. Human Brain Mapping, 12(4), 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzi, A. M. , Chapin, J. S. , Tesar, G. E. , & Busch, R. M. (2009). Comparison of personality traits in patients with frontal and temporal lobe epilepsies. Epilepsy & Behavior, 15(2), 225–229. 10.1016/j.yebeh.2009.03.028 [DOI] [PubMed] [Google Scholar]
- Preece, D. A. , Becerra, R. , Robinson, K. , & Gross, J. J. (2020). The emotion regulation questionnaire: Psychometric properties in general community samples. Journal of Personality Assessment, 102(3), 348–356. 10.1080/00223891.2018.1564319 [DOI] [PubMed] [Google Scholar]
- Pruim, R. H. , Mennes, M. , van Rooij, D. , Llera, A. , Buitelaar, J. K. , & Beckmann, C. F. (2015). ICA‐AROMA: A robust ICA‐based strategy for removing motion artifacts from fMRI data. NeuroImage, 112, 267–277. 10.1016/j.neuroimage.2015.02.064 [DOI] [PubMed] [Google Scholar]
- Rai, D. , Kerr, M. P. , McManus, S. , Jordanova, V. , Lewis, G. , & Brugha, T. S. (2012). Epilepsy and psychiatric comorbidity: A nationally representative population‐based study. Epilepsia, 53(6), 1095–1103. 10.1111/j.1528-1167.2012.03500.x [DOI] [PubMed] [Google Scholar]
- Salas, C. E. , Castro, O. , Yuen, K. S. , Radovic, D. , d'Avossa, G. , & Turnbull, O. H. (2016). “Just can't hide it”: A behavioral and lesion study on emotional response modulation after right prefrontal damage. Social Cognitive and Affective Neuroscience, 11(10), 1528–1540. 10.1093/scan/nsw075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy, D. (2011). Learning and motivation in the human striatum. Current Opinion in Neurobiology, 21(3), 408–414. 10.1016/j.conb.2011.05.009 [DOI] [PubMed] [Google Scholar]
- Spielberger, C. D. , Gorsuch, R. L. , Lushene, R. , Vagg, P. R. , & Jacobs, G. A. (1983). Manual for the state–trait anxiety inventory. Consulting Psychologists Press. [Google Scholar]
- Swartz, B. E. , Halgren, E. , Simpkins, F. , Fuster, J. , Mandelkern, M. , Krisdakumtorn, T. , Gee, M. , Brown, C. , Ropchan, J. R. , & Blahd, W. H. (1996). Primary or working memory in frontal lobe epilepsy: An18 FDG‐PET study of dysfunctional zones. Neurology, 46(3), 737–747. 10.1212/WNL.46.3.737 [DOI] [PubMed] [Google Scholar]
- Tombini, M. , Assenza, G. , Quintiliani, L. , Ricci, L. , Lanzone, J. , Ulivi, M. , & Di Lazzaro, V. (2020). Depressive symptoms and difficulties in emotion regulation in adult patients with epilepsy: Association with quality of life and stigma. Epilepsy & Behavior, 107, 107073. 10.1016/j.yebeh.2020.107073 [DOI] [PubMed] [Google Scholar]
- Tustison, N. J. , Avants, B. B. , Cook, P. A. , Zheng, Y. , Egan, A. , Yushkevich, P. A. , & Gee, J. C. (2010). N4ITK: Improved N3 bias correction. IEEE Transactions on Medical Imaging, 29(6), 1310–1320. 10.1109/tmi.2010.2046908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyng, C. M. , Amin, H. U. , Saad, M. N. , & Malik, A. S. (2017). The influences of emotion on learning and memory. Frontiers in Psychology, 8, 1454. 10.3389/fpsyg.2017.01454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton, D. , & Thompson, P. J. (1996). General neuropsychological characteristics of frontal lobe epilepsy. Epilepsy Research, 23(2), 169–177. 10.1016/0920-1211(95)00096-8 [DOI] [PubMed] [Google Scholar]
- Urry, H. L. , van Reekum, C. M. , Johnstone, T. , & Davidson, R. J. (2009). Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. NeuroImage, 47(3), 852–863. 10.1016/j.neuroimage.2009.05.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry, H. L. , Van Reekum, C. M. , Johnstone, T. , Kalin, N. H. , Thurow, M. E. , Schaefer, H. S. , Jackson, C. A. , Frye, C. J. , Greischar, L. L. , Alexander, A. L. , & Davidson, R. J. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience, 26(16), 4415–4425. 10.1523/JNEUROSCI.3215-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer, L. , van't Wout, M. , & Aleman, A. (2009). Emotion regulation strategies in patients with schizophrenia. Psychiatry Research, 170(2–3), 108–113. 10.1016/j.psychres.2009.07.010 [DOI] [PubMed] [Google Scholar]
- Van Rossum, G. , & Drake, F. L. (2009). Python 3 reference manual. CreateSpace. [Google Scholar]
- Vrticka, P. , Simioni, S. , Fornari, E. , Schluep, M. , Vuilleumier, P. , & Sander, D. (2013). Neural substrates of social emotion regulation: A fMRI study on imitation and expressive suppression to dynamic facial signals. Frontiers in Psychology, 4, 95. 10.3389/fpsyg.2013.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier, P. , Richardson, M. P. , Armony, J. L. , Driver, J. , & Dolan, R. J. (2004). Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience, 7(11), 1271–1278. 10.1038/nn1341 [DOI] [PubMed] [Google Scholar]
- Walter, H. , von Kalckreuth, A. , Schardt, D. , Stephan, A. , Goschke, T. , & Erk, S. (2009). The temporal dynamics of voluntary emotion regulation. PLoS One, 4(8), e6726. 10.1371/journal.pone.0006726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. Q. , Lang, S. Y. , Hong, L. U. , Lin, M. A. , Yan‐Ling, M. A. O. , & Yang, F. (2011). Changes in extrafrontal integrity and cognition in frontal lobe epilepsy: A diffusion tensor imaging study. Epilepsy & Behavior, 20(3), 471–477. 10.1016/j.yebeh.2010.12.039 [DOI] [PubMed] [Google Scholar]
- Woermann, F. G. , Jokeit, H. , Luerding, R. , Freitag, H. , Schulz, R. , Guertler, S. , Okujava, M. , Wolf, P. , Tuxhorn, I. , & Ebner, A. (2003). Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology, 61(5), 699–701. 10.1212/01.WNL.0000078815.03224.57 [DOI] [PubMed] [Google Scholar]
- Woodward, K. E. , Gaxiola‐Valdez, I. , Goodyear, B. G. , & Federico, P. (2014). Frontal lobe epilepsy alters functional connections within the brain's motor network: A resting‐state fMRI study. Brain Connectivity, 4(2), 91–99. 10.1089/brain.2013.0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, C. I. , Fischer, H. , Whalen, P. J. , McInerney, S. C. , Shin, L. M. , & Rauch, S. L. (2001). Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. NeuroReport, 12(2), 379–383. 10.1097/00001756-200102120-00039 [DOI] [PubMed] [Google Scholar]
- Yarkoni, T. (2009). Big correlations in little studies: Inflated fMRI correlations reflect low statistical power—Commentary on Vul et al. (2009). Perspectives on Psychological Science, 4(3), 294–298. 10.1111/j.1745-6924.2009.01127.x [DOI] [PubMed] [Google Scholar]
- Ziv, M. , Goldin, P. R. , Jazaieri, H. , Hahn, K. S. , & Gross, J. J. (2013). Emotion regulation in social anxiety disorder: Behavioral and neural responses to three socio‐emotional tasks. Biology of Mood & Anxiety Disorders, 3(1), 1–17. 10.1186/2045-5380-3-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


