Abstract
Background
The epidemiology and treatment of acute promyelocytic leukaemia (APL) are changing. We have incorporated oral arsenic trioxide (oral-ATO) into induction/maintenance.
Methods
Newly-diagnosed APL from 1991 to 2021 divided into three 10-year periods were studied to define its epidemiology and how oral-ATO impacted on its outcome. Primary endpoints included APL incidence, early deaths (ED, first 30 days), and overall survival (OS). Secondary endpoints included post-30-day OS, relapse-free survival (RFS), and incidence of second cancers.
Results
APL occurred in 374 males and 387 females at a median age of 44 (1–97) years. Annual incidences increased progressively, averaging 0.32 per 100,000 people. All-trans retinoic acid (ATRA)-based and oral-ATO-based regimens were used in 469 and 282 patients. There were 144 EDs, occurring almost exclusively in ATRA-based inductions (N = 139), being more with males, age > 50 years, leucocyte > 10 × 109/L, diagnosis during 1991–2009 and fewer with oral-ATO-based regimens. After a median of 75 (interquartile range: 14–161) months, 5-year and 10-year OS were 68.1% and 63.3%, inferior with males, age > 50 years, leucocyte > 10 × 109/L, high-risk Sanz score and superior with oral-ATO-based regimens. Factoring out EDs, 5-year and 10-year post-30-day OS were 84.0% and 78.1%, inferior with males and superior with oral-ATO-based regimens. In 607 CR1 patients, the 5-year RFS was 83.8%, superior with diagnosis in 2010–2021 and oral-ATO-based regimens. Second cancers developed in 21 patients, unrelated to oral-ATO-based regimens.
Conclusions
There was an increasing incidence of APL, and all survivals were superior with the use of oral-ATO-based regimens. This study formed part of the Acute Promyelocytic Leukaemia Asian Consortium Project (ClinicalTrials.gov identifier: NCT04251754).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-10612-z.
Keywords: Acute promyelocytic leukaemia, Epidemiology, Oral arsenic trioxide, Early deaths, Survivals, Second primary cancers
Introduction
Acute promyelocytic leukaemia (APL) arises from t(15;17)(q24;21) and PML::RARA gene fusion [1]. On presentation, patients are at risk of life-threatening bleeding, due to thrombocytopenia and a characteristic coagulopathy. The introduction of all-trans retinoic acid (ATRA), together with vigorous supportive care, has much improved the outlook. First complete remission (CR1) rates of > 90%, and long-term survivals of > 85%, could be achieved in patients treated with ATRA and chemotherapy [2–5]. The advent of arsenic trioxide (ATO) has significantly changed frontline protocols, with most current induction regimens incorporating intravenous ATO (i.v.-ATO) with ATRA ± chemotherapy, which have resulted in CR rates of 90–100% and long-term survivals of 86–97% [6–10].
We formulated an oral preparation of ATO (oral-ATO), [11] and showed that it was efficacious for APL in first relapse (R1), inducing CR2 in more than 90% of patients [12, 13]. To prevent relapses, we moved oral-ATO forward to CR1 maintenance, showing that it was safe and resulted in favorable survivals [14]. From 2013, oral-ATO has been advanced into frontline protocols [15, 16].
While therapeutic strategies are evolving, the demographics and epidemiology of APL are also changing. Ethnic differences in the incidences of APL are emerging, together with a shift in the peak age at diagnosis to the elderly [17–19]. Furthermore, the curability of APL brings into focus the long-term safety of treatment, especially the development of second primary cancers [20, 21]. Hence, a critical appraisal of the management approach of APL is warranted, so that resources can be appropriately re-deployed in light of its changing epidemiology, and the most efficacious and least toxic regimens can be offered.
To address these issues, we evaluated the epidemiological landscape and treatment results of APL in the past three decades, with a view to improving further the service for our patients, and examining the impact of oral-ATO on treatment outcome and long-term safety.
Patients and methods
Patients. In Hong Kong, over 95% of the population of 7.5 million people receive treatment in government hospitals. There are eighteen government hospitals with haematology specialist care. Patients with newly-diagnosed APL presenting to these hospitals in a 30-year period (January 1, 1991 to March 31, 2021) were identified with the Clinical Data Analysis and Reporting System (CDARS), which computerized all patient information. Data retrieved included the diagnosis according to International Classification of Diseases coding, Ninth Revision (ICD-9), date of first admission, sex, date of birth, date of death if applicable, and causes of death according to ICD-9 coding. The Laboratory Information System within CDARS was used to retrieve the presentation blood count. Specific information on APL, including the morphology, karyotype, and reverse transcription polymerase chain reaction for PML-RARA, was collected from individual patient records. The pathology reports were scrutinized for confirmation of the diagnosis of APL according to standard morphologic/karyotypic/molecular criteria [1]. The study was approved by the Institutional Review Board of the University of Hong Kong/Hong Kong West Cluster (UW 19–873), and formed part of the Acute Promyelocytic Leukaemia Asian Consortium Project (ClinicalTrials.gov identifier: NCT04251754).
Treatment for newly-diagnosed patients. Therapeutic strategies for patients ≥ 18 years old varied according to the time periods of treatment (Supplemental file 1). From 1991 to 2012, standard induction comprised ATRA (45 mg/m2/day for 42 days) with daunorubicin (50 mg/m2/day for 3 days) (ATRA-based). Daunorubicin was omitted in patients aged 70 or above or those with severe co-morbidities. In CR1 patients, two maintenance strategies, ATRA/methotrexate/6-mercaptopurine and ATRA/oral-ATO/ascorbic acid (AAA was available from 2001 onwards), were adopted at the discretion of the attending physicians. Patients given AAA maintenance were referred to Queen Mary Hospital, the only hospital where any form of ATO was available (oral-ATO was used exclusively). From 2013 onwards, induction regimens with oral-ATO (10 mg/day for 42 days), ATRA (45 mg/m2/day for 42 days) with daunorubicin (50 mg/m2/day for 3 days) were administered at Queen Mary Hospital in clinical trials (oral-ATO-based) (Supplemental file 1) [15]. Again, daunorubicin was omitted in patients aged 70 or above or those with severe co-morbidities. In patients < 18 years old, the International Consortium for Childhood Acute Promyelocytic Leukaemia Study 01 protocol [22] was used throughout the study period (Supplemental file 1). Induction regimen comprised ATRA (25 mg/m2/day for at least 28 days) and Idarubicin (12 mg/m2/day on days 3, 5 and 7).
Treatment for relapsed patients. Re-induction with ATRA, oral-ATO and idarubicin was available since 2002 in clinical trials, and patients were referred to and treated at Queen Mary Hospital [23]. Autologous or allogeneic haematopoietic stem cell transplantation was not routinely performed in CR2, and performed for selected patients at CR3 [23]. Patients < 18 years old were treated with re-induction with i.v.-ATO or oral-ATO, together with ATRA and chemotherapy [13].
Endpoints and definitions. Primary endpoints were the incidence of APL, early deaths (ED, defined as death within the first 30 days of presentation), 30-day survival (defined as time from presentation to ED, censoring at day 30), and the overall survival (OS, defined as time from presentation to death or last follow-up). Secondary endpoints included post-30-day OS (defined as time from day 31 to death or last follow-up), relapse-free survival (RFS, defined as time from CR1 to R1, death or last follow-up) and the incidence of second primary cancers. Data for the analysis of OS, post-30 day OS and RFS were censored on November 30, 2021.
Statistical analyses. Categorical variables were analyzed with chi-square test and continuous variables with non-parametric tests. Survivals (30-day survival, OS, post-30-day survival, RFS) were analyzed with the Kaplan-Meier method, difference between groups determined with the log-rank test and Cox proportional hazard model. Prognostic impacts on survivals were evaluated for the following parameters: sex (male versus female); age groups (≤ 50 versus > 50 years); periods of diagnosis (1991–1999 versus 2000–2009 versus 2010–2021); presentation leucocyte counts (≤ 10 versus > 10 × 109/L); presentation platelet counts (≤ 40 versus > 40 × 109/L); Sanz risk scores (low versus intermediate versus high); and treatment regimens (ATRA-based versus oral-ATO-based). For 30-day survival, difference between treatment centres (academic versus non-academic) was also evaluated. Prognostic factors with P < 0.10 on univariate analysis were further examined by multivariate analyses. Two-tailed P values of < 0.05 were regarded as significant. Statistical analyses were performed with the SPSS version 26.0 (Chicago, IL, USA).
Epidemiological analyses. The age-adjusted annual incidence rates of APL per 100,000 people were calculated using R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria), [24] with the World Health Organization (WHO) (2000–2025) standard population and the Hong Kong population as reference. The incidence rate ratios (IRR) of second primary cancers between different age groups, sex, treatment periods and exposure to oral-ATO were obtained with the rateratio() function from the fmsb package within the R software [25]. Hong Kong mid-year population by sex and age from 1991 to 2020 was extracted from The Human Mortality Database (1991–2017) [26] and the Hong Kong Census and Statistics Department (2018–2020) [27]. The World Health Organization standard population data (2000–2025) were downloaded from the Surveillance, Epidemiology and End Result (SEER) website [28].
Second primary cancers. As the number of APL patients was relatively small, the standardized incidence ratio (SIR) was used to determine if the occurrence of second primary cancers was increased in APL. SIR was calculated by indirect standardization (Supplemental file 2).
Results
Patient characteristics. There were 751 patients (364 males, 387 females) at a median age of 44 (range: 1–97; interquartile range: 31–57) years (Table 1). Patients were treated in three time periods (January 1, 1991–December 31, 1999, N = 146; January 1, 2000–December 31, 2009, N = 257; January 1, 2020–March 31, 2021, N = 348). Conventional high-risk features of platelet ≤ 40 × 109/L and leucocyte > 10 × 109/L were present in 29.4% and 34.5% of cases, with no significant changes during the study periods (Table 1). High-risk Sanz score was present in 34.5% of cases, being comparable in the three time periods (32.3%, 31.3%, 37.7% respectively).
Table 1.
Clinicopathologic features, treatment and outcome of 751 patients with acute promyelocytic leukaemia in three decades
| Features | Total | 1990–1999 | 2000–2009 | 2010–2021 | P valueA |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 364 (48.5%) | 72 (49.3%) | 128 (49.8%) | 164 (47.1%) | 0.788 |
| Female | 387 (51.5%) | 74 (50.7%) | 129 (50.2%) | 184 (52.9%) | |
| Presentation leucocyte B | |||||
| ≤ 10 × 109/L | 481 (65.5%) | 90 (67.7%) | 176 (68.7%) | 215 (62.3%) | 0.22 |
| > 10 × 109/L | 253 (34.5%) | 43 (32.3%) | 81 (31.3%) | 131 (37.7%) | |
| Presentation platelet B | |||||
| ≤ 40 × 109/L | 519 (70.6%) | 89 (66.9%) | 182 (70.8%) | 248 (71.9%) | 0.56 |
| > 40 × 109/L | 216 (29.4%) | 44 (33.1%) | 75 (29.2%) | 97 (28.1%) | |
| Sanz risk score B | |||||
| Low | 142 (18.9%) | 31 (23.3%) | 52 (20.2%) | 59 (17.1%) | 0.328 |
| Intermediate | 340 (45.3%) | 59 (44.4%) | 125 (48.6%) | 156 (45.2%) | |
| High | 253 (34.5%) | 43 (32.3%) | 81 (31.3%) | 131 (37.7%) | |
| Induction regimen | |||||
| ATRA-based | 613 (81.6%) | 146 (100%) | 254 (98.8%) | 213 (61.2%) | < 0.001 |
| Oral-ATO-based | 138 (18.4%) | 0 | 3 (1.2%)C | 138 (38.8%) | |
| CR1 maintenance | |||||
| ATRA ± MTX/6-MP | 477 (63.5%) | 146 (100%) | 186 (72.4%) | 145 (41.7%) | < 0.001 |
| Oral-ATO/ATRA based | 274 (36.5%) | 0 | 71 (27.6%) | 203 (58.3%) | |
| First relapse | 97/607 (16%) | 26/107(24.3%) | 55/203 (27.1%) | 16/297(5.4%) | < 0.001 |
| HSCT | |||||
| Autologous | 13 (1.7%) | 6 (4.1%) | 5 (1.9%) | 2 (0.6%) | |
| Allogeneic | 10 (1.3%) | 9 (6.2%) | 1 (0.4%) | 0 | |
| Second cancers | 21 (2.8%) | 8 (5.5%) | 12 (4.7%) | 1 (0.3%) | |
| Deaths | |||||
| All causes | 271 | 75 (51.4%) | 109 (42.4%) | 87 (25%) | < 0.001 |
| Early deaths | 144 (53.1%) | 39 (52%) | 54 (49.5%) | 51 (58.6%) | < 0.001 |
| Vascular events | 7 (2.6%) | 0 | 3 (2.8%) | 4 (4.6%) | |
| Sepsis | 18 (6.6%) | 3 (4%) | 11 (10.1%) | 4 (4.6%) | |
| Second cancers | 12 (4.4%) | 4 (5.3%) | 8 (7.3%) | 0 | |
| HSCT-related | 7 (2.6%) | 7 (9.3%) | 0 | 0 | |
| Refractory leukaemia | 49 (18.1%) | 19 (25.3%) | 26 (23.9%) | 4 (4.6%) | |
| Others | 6 (2.2%) | 2 (2.7%) | 1 (0.9%) | 3 (3.4%) | |
| Not available | 28 (10.3%) | 1 (1.3%) | 6 (5.5%) | 21 (24.1%) |
ATRA: all trans retinoic acid; ATO: arsenic trioxide; CR1: first complete remission; MTX: methotrexate; 6-MP: 6-mercaptopurine; HSCT: haematopoietic stem cell transplantation;
A: statistical evaluation of parameters in the three time periods was performed with Chi Square test
B: Seventeen patients (2.3%) had missing presentation leucocyte counts and sixteen patients (2.1%) had missing presentation platelet counts, so that the Sanz risk scores could not be determined for these cases
C: These three patients were unfit for chemotherapy and hence were treated with oral-ATO during this period when ATRA-based chemotherapy was the standard
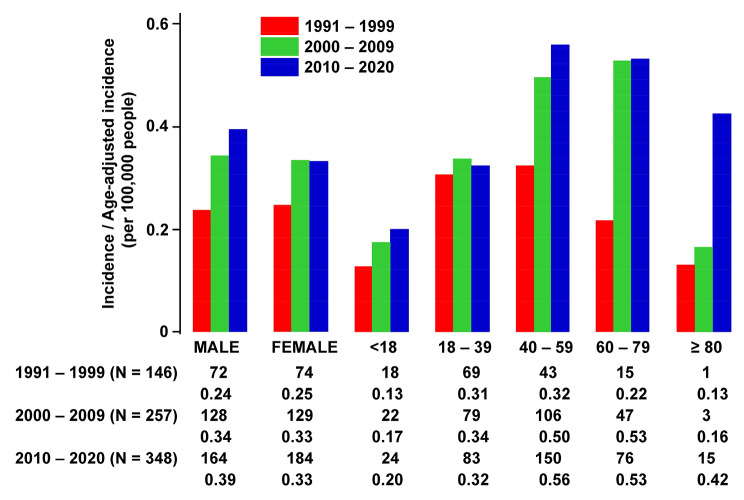
Epidemiological changes. There was a progressive increase in annual incidence rates (per 100,000 people) with time (0.24 in 1991–1999, 0.34 in 2000–2009, 0.36 in 2010–2021) (Fig. 1), giving an overall incidence of 0.32 (see Supplemental file 3 A for incidence in each calendar year). In the three time periods, two changes were observed. Firstly, there was a gradual shift of occurrence of APL to the older age groups. In 1991–1999, the highest age-adjusted incidences occurred in the groups 18–39 years and 40–59 years. However, in 2000–2009 and 2010–2020, the highest age-adjusted incidences had shifted to the groups 40–59 years and 60–79 years. Secondly, the age-adjusted annual incidences also increased with time. The increase was only very modest in the groups < 18 years and 18–39 years, but was more obvious in the groups 40–59 years (0.32 in 1991–1999; 0.50 in 2000–2009; 0.56 in 2010–2021) and 60–79 years (0.22 in 1991–1999; 0.53 in 2000–2009; 0.53 in 2010–2021). The most notable increase was in the group > 80 years (0.13 in 1991–1999; 0.26 in 2000–2009; 0.42 in 2010–2021).
Fig. 1.
Annual incidences / Age-adjusted annual incidences of acute promyelocytic leukaemia over a 30-year period. The Y axis represented annual incidences for males and females, and age-adjusted annual incidences for different age groups. The actual numbers of patients and the annual incidences / age-adjusted annual incidences for each time period were given under the different analysed groups. Annual incidence was studied until end of 2020, because only three months had elapsed for the year 2021, which was not included in the analysis
Treatment outcome. For induction, all patients received ATRA-based regimens during 1991–1999 and 2000–2009 (except three patients unfit for chemotherapy who received oral-ATO-based regimens); with significantly more patients (38.8%) receiving oral-ATO-based regimens from 2010 to 2021 (P < 0.001) (Table 1). For CR1 maintenance, all cases received ATRA-based regimens during 1991–1999, whereas afterwards significantly more patients received oral-ATO-based regimens, increasing from 27.6% in 2000–2009 to 58.3% in 2010–2021 (P < 0.001). The increasing use of oral-ATO-based regimens in induction and CR1 maintenance significantly decreased the incidence of R1 from 24.3% in 1990–1999 and 27.1% in 2000–2009 to merely 5.4% in 2010–2021 (P < 0.001) (Table 1).
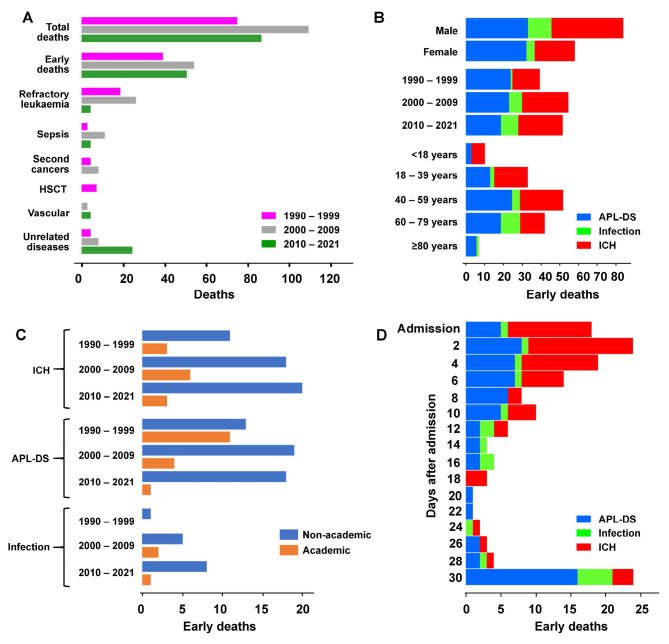
Mortalities. After a median follow-up of 75 (interquartile range: 14–161) months, there were 271 deaths (36.1%) (Fig. 2A). The two most important causes were ED and refractory leukaemia (Supplemental file 3B). In the three time periods, there was no improvement in ED (1991–1999, N = 39; 2000–2009: N = 54; 2010–2021, N = 51) (Fig. 2A). However, there was a significant decrease in death due to refractory leukaemia (1991–1999, N = 19; 2000–2009: N = 26; 2010–2021, N = 4) (Fig. 2A). Hence, in 2010–2021, with the decrease in refractory leukaemia and increase in older patients, systemic diseases unrelated to APL became the second most frequent cause of death after ED.
Fig. 2.
Mortalities of patients with acute promyelocytic leukaemia
A. Total number of deaths due to different causes according to the time period studied
B. Early deaths and their respective proportions due to acute promyelocytic leukaemia differentiation syndrome (APL-DS), infections and intracerebral haemorrhage (ICH) of the various parameters studied
C. Early deaths due to ICH, APL-DS and infection in the different time periods studied, according to treatment in academic or non-academic centres
D. Early deaths due to ICH, APL-DS and infections during the first 30 days of presentation
ED. During the study period, ED occurred in 144 patients (19.2% of all patients), accounting for 53% of all deaths. Remarkably, EDs happened almost exclusively in cases receiving ATRA-based induction (139/144, 96.5%), resulting in a 30-day mortality of 22.7% (139/613) in these patients. On the contrary, only 3.5% (5/144) of EDs happened in patients receiving oral-ATO-based induction, resulting in a 30-day mortality of only 3.6% (5/138). The three most important causes of ED were APL differentiation syndrome (APL-DS; N = 66; with 61 due to pulmonary complications and 5 due to renal failure), intracerebral haemorrhage (ICH; N = 61) and infection (N = 11). EDs were more frequent in men, owing mainly to increased ICH (Fig. 2B). Although ED due to APL-DS decreased during the study periods, ED due to ICH did not improve. The proportion of EDs due to APL-DS and ICH remained similar in different age groups (Fig. 2B). However, EDs due to ICH, APL-DS and infections differed according to whether the treatment centres were academic (Queen Mary Hospital) or non-academic (all other hospitals) (Fig. 2C). In the three study periods, ICH were 3–6 times more frequent in non-academic centres. For APL-DS, mortalities in 1991–1999 were comparable between academic and non-academic centres. In 2000–2009 and 2010–2021, mortality due to APL-DS continued to drop in the academic centre, but remained high in non-academic centres. Similarly, mortalities due to infection were also fewer in the academic centre during the study periods. In the first week post-admission, ICH was the predominant cause of death, followed by APL-DS (Fig. 2D). APL-DS became the predominant cause of death towards the end of the first 30 days.
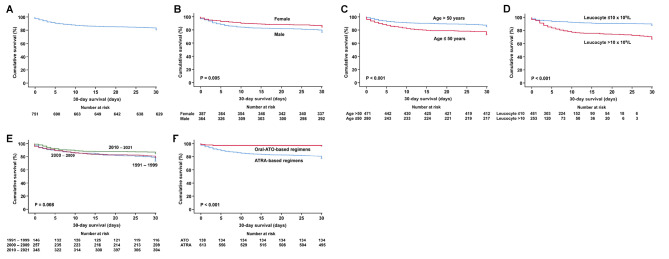
30-day survival. The 30-day survival was 80.8% (Fig. 3A). On univariate analysis, 30-day survival was inferior with male sex (P = 0.005), age > 50 years (P < 0.001), leucocyte > 10 × 109/L (P < 0.001), diagnosis in 1991–1999 and 2000–2009 (P = 0.008) (Fig. 3B–E), high-risk Sanz score (P < 0.001) (Supplemental file 4); and superior with the use of oral-ATO-based induction regimens (P < 0.001) (Fig. 3F). On multivariate analysis, 30-day survival remained significantly inferior with male sex, age > 50 years, leucocyte > 10 × 109/L, diagnosis in 1991–1999 and 2000–2009; and superior with use of oral-ATO-based induction regimens (Table 2).
Fig. 3.
30-day survivals in newly-diagnosed APL patients. Only prognostic parameters with statistical significance on multivariate analyses were shown
Table 2.
Significant prognostic factors for survivals in a cohort of newly-diagnosed patients with acute promyelocytic leukaemia
| Univariate analysis | Multivariate analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Number | HR | 95% C.I. | P value | H.R. | 95% C.I. | P value | |||||
| 30-day survival (N = 751) | ||||||||||||
| Sex | ||||||||||||
| Male | 364 | 1.60 | 1.15–2.24 | 0.005 | 1.48 | 1.05–2.09 | 0.03 | |||||
| Female | 387 | 0.62 | 0.45–0.87 | 0.68 | 0.48–0.95 | |||||||
| Age | ||||||||||||
| ≤ 50 years | 471 | 0.51 | 0.37–0.70 | < 0.001 | 0.37 | 0.26–0.53 | < 0.001 | |||||
| > 50 years | 280 | 1.96 | 1.42–2.72 | 2.71 | 1.91–3.86 | |||||||
| Period of diagnosis | ||||||||||||
| 1991–1999 | 146 | 1.90 | 1.25–2.89 | 0.008 | 1.96 | 1.23–3.12 | 0.02 | |||||
| 2000–2009 | 257 | 1.49 | 1.02–2.18 | 1.27 | 0.85–1.89 | |||||||
| 2010–2021 | 348 | 0.53 | 0.35–0.80 | 0.51 | 0.32–0.81 | |||||||
| Leucocyte count | ||||||||||||
| ≤ 10 × 109/L | 481 | 0.33 | 0.23–0.46 | < 0.001 | 0.32 | 0.23–0.45 | < 0.001 | |||||
| > 10 × 109/L | 253 | 3.09 | 2.20–4.32 | 3.14 | 2.23–4.43 | |||||||
| Induction regimens | ||||||||||||
| ATRA-based | 613 | 6.86 | 2.81–16.73 | < 0.001 | 6.14 | 2.44–15.43 | < 0.001 | |||||
| Oral-ATO-based | 138 | 0.15 | 0.06–0.36 | 0.16 | 0.07–0.41 | |||||||
| Overall survival (N = 751) | ||||||||||||
| Sex | ||||||||||||
| Male | 364 | 1.47 | 1.16–1.89 | 0.001 | 1.35 | 1.06–1.72 | 0.015 | |||||
| Female | 387 | 0.68 | 0.53–0.86 | 0.74 | 0.58–0.94 | |||||||
| Age | ||||||||||||
| ≤ 50 years | 471 | 0.49 | 0.39–0.62 | < 0.001 | 0.35 | 0.27–0.45 | < 0.001 | |||||
| > 50 years | 280 | 2.04 | 1.61–2.60 | 2.88 | 2.23–3.73 | |||||||
| Leucocyte count | ||||||||||||
| ≤ 10 × 109/L | 481 | 0.51 | 0.40–0.65 | < 0.001 | 0.51 | 0.39–0.65 | < 0.001 | |||||
| > 10 × 109/L | 253 | 1.98 | 1.55–2.52 | 1.98 | 1.54–2.54 | |||||||
| Sanz score | ||||||||||||
| Low-risk | 142 | 0.66 | 0.47–0.91 | < 0.001 | 1.59 | 1.12–2.24 | 0.01 | |||||
| Intermediate-risk | 340 | 0.44 | 0.33–0.57 | 0.63 | 0.45–0.89 | |||||||
| High-risk | 253 | 1.53 | 1.10–2.11 | 1.98 | 1.54–2.54 | |||||||
| Treatment regimens | ||||||||||||
| ATRA-based | 469 | 5.26 | 3.57–7.69 | < 0.001 | 5.56 | 3.84–8.33 | < 0.001 | |||||
| Oral-ATO-based | 282 | 0.19 | 0.13–0.28 | 0.18 | 0.12–0.26 | |||||||
| Post-30-day survival (N = 607) | ||||||||||||
| Age | ||||||||||||
| ≤ 50 years | 402 | 0.45 | 0.31–0.64 | < 0.001 | 0.29 | 0.20–0.43 | < 0.001 | |||||
| > 50 years | 205 | 2.23 | 1.57–3.17 | 3.40 | 2.33–4.98 | |||||||
| Treatment regimens | ||||||||||||
| ATRA-based | 331 | 2.94 | 1.92–4.55 | < 0.001 | 3.03 | 1.82–5.26 | < 0.001 | |||||
| Oral-ATO-based | 276 | 0.34 | 0.22–0.52 | 0.33 | 0.19–0.55 | |||||||
| Relapse free survival (N = 607) | ||||||||||||
| Period of diagnosis | ||||||||||||
| 1991–1999 | 107 | 4.38 | 2.34–8.16 | < 0.001 | 2.51 | 1.27–4.99 | < 0.001 | |||||
| 2000–2009 | 203 | 4.52 | 2.58–7.90 | 3.40 | 1.90–6.09 | |||||||
| 2010–2021 | 297 | 0.23 | 0.12–0.43 | 0.40 | 0.25–0.73 | |||||||
| Treatment regimens | ||||||||||||
| ATRA-based | 331 | 3.45 | 2.13–5.56 | < 0.001 | 2.33 | 1.37–4.00 | 0.002 | |||||
| Oral-ATO-based | 276 | 0.29 | 0.18–0.47 | 0.43 | 0.25–0.73 | |||||||
h: hazard ratio; C.I.: confidence interval; ATRA: all trans retinoic acid; ATO: arsenic trioxide
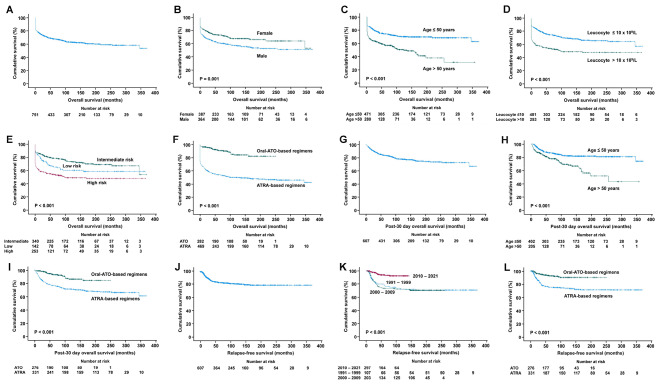
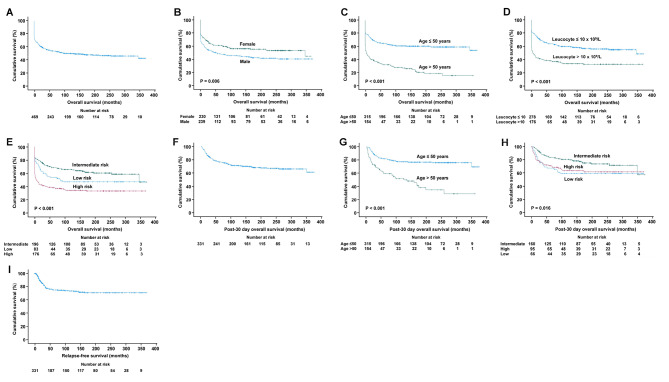
OS of the entire cohort. The 5-year and 10-year OS were 68.1% and 63.3% (Fig. 4A). On univariate analysis, OS was inferior with male sex (P = 0.001), age > 50 years (P < 0.001), leucocyte > 10 × 109/L (P < 0.001), high-risk Sanz score (P < 0.001) (Fig. 4B–E); and superior with the use of oral-ATO-based induction/maintenance regimens (P < 0.001) (Fig. 4F) and diagnosis in 2010–2021 (P < 0.001) (Supplemental file 5). On multivariate analysis, OS remained significantly inferior with male sex, age > 50 years, leucocyte > 10 × 109/L, high-risk Sanz score; and superior with oral-ATO-based induction/maintenance regimens (Table 2).
Fig. 4.
Kaplan Meier survival curves of the entire cohort of patients with acute promyelocytic leukaemia. Only prognostic parameters with statistical significance on multivariate analyses were shown
Post-30-day OS of the entire cohort. The 5-year and 10-year post-30-day OS were 84.0% and 78.1% (Fig. 4G). On univariate analysis, post-30-day OS was inferior with age > 50 years (P < 0.001) (Fig. 4H), platelet > 40 × 109/L (P = 0.003) (Supplemental file 5); and superior with intermediate Sanz score (P = 0.005) (supplemental file 5), diagnosis in 2010–2021 (P = 0.023) (Supplemental file 5), and oral-ATO-based induction/maintenance regimens (P < 0.001) (Fig. 4I). On multivariate analysis, post-30-day survival remained significantly inferior with age > 50 years and superior with oral-ATO-based induction/maintenance regimens (Table 2).
RFS of the entire cohort. In 607 CR1 patients, 97 patients (16%) relapsed after a median of 70 (interquartile range: 27–155) months. The 5-year RFS was 83.8% (Fig. 4J). On univariate analysis, RFS was significantly superior with age > 50 years (P = 0.031) (Supplemental file 5), diagnosis in 2010–2021 (P < 0.001), and the use of oral-ATO-based induction/maintenance regimens (P < 0.001) (Fig. 4K,L). On multivariate analysis, RFS remained significantly superior with diagnosis in 2010–2021 and use of oral-ATO-based induction/maintenance regimens (Table 2).
Survivals and prognostic factors in the ATRA-based cohort. In 469 patients receiving ATRA-based regimens without exposure to oral-ATO (ATRA-based cohort), the 5-year and 10-year OS were 54.5% and 50.5% (Fig. 5A). On both univariate and multivariate analyses, OS was significantly inferior with male sex (P = 0.006), age > 50 years (P < 0.001), leucocyte > 10 × 109/L (P < 0.001) and high-risk Sanz score (P < 0.001) (Fig. 5B–E) (Table 3). The 5-year and 10-year post-30-day OS were 76.5% and 71.3% (Fig. 5F). On univariate analysis, post-30-day OS was inferior with age > 50 years (P < 0.001) (Fig. 5G), platelet > 40 × 109/L (P = 0.01) (Supplemental file 6); and superior with intermediate-risk Sanz score (P = 0.016) (Fig. 5H). On multivariate analysis, post-30-day OS remained significantly inferior with age > 50 years and superior with intermediate Sanz score (Table 3). The 5-year RFS in this cohort was 76.5% (Fig. 5I). No parameters impacted significantly on RFS (Supplemental file 6).
Fig. 5.
Kaplan Meier survival curves of patients with acute promyelocytic leukaemia treated with all-trans retinoic acid based regimens. Only prognostic parameters with statistical significance on multivariate analyses were shown
Table 3.
Significant prognostic factors for survivals in newly-diagnosed patients with acute promyelocytic leukaemia treated with ATRA-based and oral-ATO-based regimens
| Univariate analysis | Multivariate analysis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Number | HR | 95% C.I. | P value | H.R. | 95% C.I. | P value | |||||||||
| ATRA-based regimens | ||||||||||||||||
| Overall survival (N = 469) | ||||||||||||||||
| Sex | ||||||||||||||||
| Male | 239 | 1.39 | 1.08–1.79 | 0.01 | 1.41 | 1.08–1.82 | 0.012 | |||||||||
| Female | 230 | 0.72 | 0.56–0.93 | 0.71 | 0.55–0.93 | |||||||||||
| Age | ||||||||||||||||
| ≤ 50 years | 315 | 0.38 | 0.29–0.49 | < 0.001 | 0.36 | 0.27–0.46 | < 0.001 | |||||||||
| > 50 years | 154 | 2.62 | 2.03–3.39 | 2.81 | 2.16–3.66 | |||||||||||
| Leucocyte count | ||||||||||||||||
| ≤ 10 × 109/L | 278 | 0.50 | 0.39–0.65 | < 0.001 | 0.42 | 0.31–0.57 | < 0.001 | |||||||||
| > 10 × 109/L | 176 | 2.00 | 1.54–2.59 | 1.53 | 1.07–2.18 | |||||||||||
| Sanz score | ||||||||||||||||
| Low-risk | 83 | 0.65 | 0.46–0.92 | < 0.001 | 0.65 | 0.46–9.30 | < 0.001 | |||||||||
| Intermediate-risk | 196 | 0.44 | 0.33–0.59 | 0.41 | 0.31–0.56 | |||||||||||
| High-risk | 176 | 1.55 | 1.09–2.20 | 1.53 | 1.08–2.18 | |||||||||||
| Post-30-day survival (N = 331) | ||||||||||||||||
| Age | ||||||||||||||||
| ≤ 50 years | 247 | 0.31 | 0.21–0.45 | < 0.001 | 0.26 | 0.17–0.39 | < 0.001 | |||||||||
| > 50 years | 84 | 3.26 | 2.20–4.83 | 3.90 | 2.59–5.89 | |||||||||||
| Sanz score | ||||||||||||||||
| Low-risk | 83 | 1.06 | 0.64–1.17 | 0.02 | 0.92 | 0.55–1.54 | 0.001 | |||||||||
| Intermediate-risk | 196 | 0.57 | 0.36–0.91 | 0.45 | 0.28–0.72 | |||||||||||
| High-risk | 176 | 0.94 | 0.57–1.57 | 1.09 | 0.65–1.82 | |||||||||||
| Oral-ATO-based regimens | ||||||||||||||||
| Overall survival (N = 282) | ||||||||||||||||
| Age | ||||||||||||||||
| ≤ 50 years | 156 | 0.44 | 0.21–0.92 | 0.03 | -* | - | - | |||||||||
| > 50 years | 256 | 3.26 | 1.09–4.66 | - | - | |||||||||||
HR: hazard ratio; C.I.: confidence interval; ATRA: all trans retinoic acid; ATO: arsenic trioxide;*: only factor significant on univariate analysis, hence multivariate analysis not performed
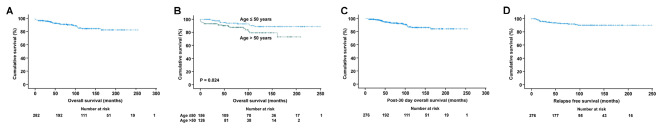
Prognostic indicators in the oral-ATO-based cohort. In 282 patients receiving oral-ATO-based induction/maintenance (oral-ATO-based cohort), the 5-year and 10-year OS were 91.5% and 84.6% (Fig. 6A). On univariate analysis, only age > 50 years was associated with inferior OS (P = 0.03) (Table 3) (Fig. 6B). The 5-year and 10-year post-30-day OS were 93.5% and 86.4% (Fig. 6C); and the 5-year RFS was 93.3% (Fig. 6D). No factors significantly impacted on post-30-day OS and RFS (Supplemental file 7).
Fig. 6.
Kaplan Meier survival curves of patients with acute promyelocytic leukaemia treated with oral arsenic trioxide based regimens. Only prognostic parameters with statistical significance on multivariate analyses were shown
Second primary cancers. In 607 CR1 patients, 21 patients (6 males, 15 females) developed second cancers (breast cancer, N = 5; colorectal cancer, N = 3; endometrial cancer; N = 2; myelodysplastic syndrome, N = 2; esophageal cancer, N = 1; thyroid cancer, N = 1; nasopharyngeal cancer, N = 1; parotid cancer, N = 1; transitional carcinoma of the ureters, N = 1; lung cancer, N = 1; renal cell carcinoma; cholangiocarcinoma, N = 1; tongue cancer, N = 1) at a median of 8 years (range: 1–24 years) after the diagnosis of APL (Supplemental file 8). The incidence was highest in females (N = 15) (SIR: 4.54, 95% confidence interval, C.I.: 2.66–7.55), and in patients diagnosed at the age of 40–59 years (N = 12) (SIR: 5.53, 95% confidence interval C.I.: 3.02–9.69), during 1991–1999 (N = 8) (SIR: 5.94, 95% C.I.: 2.79–11.75) and during 2000–2009 (N = 12) (SIR: 5.21, 95% C.I.: 2.85–9.16). Thirteen patients were oral-ATO-exposed (oral-ATO maintenance, N = 8; oral-ATO for re-induction of CR2, N = 3; autologous HSCT for oral-ATO-induced CR2 or beyond, N = 2); whereas eight patients were oral-ATO-naïve. There was no significant difference in second cancers in patients exposed or naïve to oral-ATO (IRR: 2.14; 95% C.I.: 0.89–5.17) (Supplemental file 9). However, there was an overall significant increase in second cancers in APL patients as compared to the general population (IRR: 14.3, P < 0.001), which applied to both sexes, whether or not oral-ATO had been used, age groups 18–39 years and 40–59 years, and treatment during 1991–1999 and 2000–2009 (Supplemental file 10). In patients of < 18 years, 60–75 years and > 80 years and those treated during 2010–2020, second cancers were not increased.
Discussion
We observed a progressive increase in the incidence (per 100,000 people) of APL over the past three decades, with a 50% increase from 0.24 in 1991–2000 to 0.36 in 2010–2021. Furthermore, we found a shift of the peak age incidence from 18–39/40–59 years in 1991–2000 to 40–59/60–79 years in 2000–2009 and 2010–2021. Finally, from 1991 to 2000 to 2010–2021, there was a > 3-fold increase in age-adjusted incidence in patients > 80 years. Although epidemiologic studies of APL are limited, such trends were also observed in three SEER-based studies covering the periods 1992–2001, 1975–2008 and 2000–2014 [17, 18, 29, 30]. In these studies, the mean annual incidences increased from 0.20 (in 1992–2001) to 0.27 (in 2000–2008) and to 0.31 (in 2000–2014) [17, 18, 29, 30]. All studies observed increasing incidences with age, the highest incidence observed in people aged ≥ 60–65 years. This was different from much earlier studies, where the incidence of APL was regarded to be stable with age, [30, 31] leading to the hypothesis of a single rate-limiting mutation to explain the apparent constant incidence [31]. On the contrary, our observations and those of others support a more conventional multi-hit model, with accumulation and interaction of mutations throughout life, resulting in rising incidences with age. Biologically, mice transgenic for the fusion gene PML::RARA manifested a pre-leukaemic state of deranged myeloid maturation, with an APL-like leukaemia only developing if collaborating mutations such as internal tandem duplication of FLT3 were present [32–34]. Hence, although doubtlessly PML::RARA fusion is the key driver event, APL might still evolve through a multistep process of leukaemogenesis.
In contrast to other types of acute myeloid leukaemia (AML) where the male:female incidence rate is about 1.5:1, we observed a similar incidence rate of APL in men (0.33) and women (0.32). The relatively higher frequency of APL in women has also been reported in population-based studies and clinical trials [30]. It has been postulated that estrogen, which belongs to the same superfamily as retinoid receptors, might act synergistically to affect the binding of the aberrant PML/RARA protein to the RXR receptor [30].
In this study, EDs accounted for more than half of the total mortalities. In multicenter clinical trials of patients treated with ATRA, ATO and anthracyclines, early death rates of only 3–10% were reported [7, 8, 35–37]. However, population-based studies in unselected patients reported otherwise, with EDs varying from 9.6–61.5% [17, 18, 38–43]. Our ED rate of 19.2% was within this range. Efforts in the development of international recommendations have led to a gradual improvement of ED, falling from 28% in the 1990s to approximately 15% in the past two decades [17, 44, 45]. Reported risks for EDs included older age, high-risk disease, poor performance, co-existing infections; [46] and factors that increased fatal haemorrhages, including high leucocyte count, elevated lactate dehydrogenase, low fibrinogen, impaired coagulation parameters and APL-DS [47–51]. Delays in ATRA administration contributed significantly to EDs, especially at the community care level where ATRA might not be immediately available [51–53]. In addition to confirming some of these observations, there were two additional findings in this study. Firstly, use of oral-ATO-based inductions almost completely abrogated EDs. Secondly, EDs did not improve in non-academic centres, but progressively decreased in the academic centre, mainly related to reduction of mortality due to APL-DS.
The impact of EDs on survivals was further shown in the analysis of prognostic factors. Male sex, age > 50 years, leucocyte count > 10 × 109/L and use of ATRA-based instead of ATO-based induction regimens significantly increased EDs. These negative prognostic factors were precisely those that led to significantly inferior OS for the entire cohort. When EDs were factored out, survivals of 68.1% and 63.3% (OS) were improved to 84% and 78.1% (post-30-day OS) at 5 and 10 years.
Refractory leukaemia was the other main cause of mortality in this study. However, with the increasing use of oral-ATO-based regimens, refractory leukaemia was largely prevented during 2010–2020. Consequently, post-30-day OS was significantly improved with oral-ATO-based regimens, and RFS was significantly improved with oral-ATO-based regimens and diagnosis in 2010–2020, when oral-ATO-based regimens were predominantly used.
In the above analyses, use of oral-ATO-based regimens, resulted in significantly superior results in all survivals (30-day survival, OS, post-30-day OS and RFS) by decreasing EDs and refractory leukaemia. To fully evaluate the importance of treatment, we separately analysed our patients according to treatment with ATRA-based and oral-ATO-based regimens. ATRA-based regimens gave 5-year and 10-year OS of merely 54.5% and 50.5%; 5-year and 10-year post-30-day OS of 76.5% and 71.3%, and 5-year RFS of 76.5%. Conventional prognostic factors, including male sex, age > 50 years and Sanz score, expectedly portended inferior survivals. On the other hand, oral-ATO-based regimens gave much better survivals. The 5-year and 10-year OS were 91.5% and 84.6%, comparable with the 5-year and 10-year post-30-day OS of 93.5% and 86.4%; reflecting the importance of reduction of EDs on OS. The 5-year RFS was also excellent at 93.3%, due to significantly decreased relapses. With oral-ATO-based regimens, conventional prognostic indicators (leucocyte count, platelet count, Sanz score) were no longer relevant. The only factor negatively impacting on OS was age > 50 years, reflecting deaths from non-leukaemic age-related diseases.
With APL potentially curable, risks of second primary cancers have become pertinent. In a recent population-based study in the United States, the absolute incidence of second cancers per 1,000 person-months was increased from 1.4 in i.v.-ATO-naïve patients to 3.4 in i.v.-ATO-exposed patients [21]. However, in another population-based study, second cancers in i.v.-ATO-exposed patients were not significantly increased compared with the general population [17]. In APL patients treated with ATRA ± anthracycline, there were increased risks of developing liver cancers, salivary gland cancers and soft tissue malignancies compared with other subtypes of AML, [20] suggesting a potentially carcinogenic role of ATRA. In our study, we observed an increased risk of second cancers in APL patients compared to the general population. The increased risk was not associated with the use of oral-ATO, implying that chemotherapy might be involved. In subgroup analysis, second cancers were not increased in patients aged < 18 years and > 60 years; probably reflecting the low and high risks of cancers in any case in these age groups. Interestingly, second cancers were also not increased in patients treated during 2010–2018, a period when oral-ATO-based regimens with omission of daunorubicin were increasingly used. Whether the use of a chemotherapy-free strategy in APL [8] may reduce second cancers warrants further investigations. In addition, this population-based study on the epidemiology and outcome was entirely based in Hong Kong and its applicability in other patient populations such as Hispanics, Caucasians and Africans has to be carefully examined.
Conclusion
Results of our study have important implications on how to further improve the service to APL patients. EDs account for the majority of deaths and are clearly the biggest hurdle. Education of frontline healthcare workers in diagnosing APL and vigorously treating the associated coagulopathy is key to preventing ICH. It is imperative that ATRA should be available at the community care level [53]. Referral to academic centres is advantageous. Otherwise, early recognition of APL-DS leading to its timely treatment should be inculcated in physicians at non-academic centres. Oral-ATO-based regimens significantly improved all survivals, so that ATO (i.v. or oral) should be incorporated into all phases of treatment. With the gradual shift of APL to older patients, more resources and attention should be devoted to the treatment of other comorbidities to reduce non-leukaemic mortalities. In this respect, the use of an entirely non-chemotherapy approach in elderly patients to avoid toxicities should be explored. Finally, with APL now largely curable, decreasing the risks of second primary cancers by reducing or omitting chemotherapy should be a therapeutic target.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author Contribution
Contribution: H.G: conceived the study, treated the patients, analyzed the data, wrote and approved the manuscript; R.R., C.Y-Y.L.: analyzed the data, wrote and approved the manuscript; Y.Y.,H-T.C., M.Y.N., X.X., F.P.F., D.M.: analyzed the data and approved the manuscript; R.Y., P.L., L.C., V.W.K.L., L.A.: performed the laboratory studies, analyzed the data and approved the manuscript; W-Y.A.: treated the patients and approved the manuscript; E.S.K.M.: reviewed the pathologic diagnoses and approved the manuscript C. R. K.: invented oral arsenic trioxide, and approved the manuscript; Y-L.K.: conceived the study, treated the patients, wrote and approved the manuscript.
Funding
This study was supported by the Health and Medical Research Fund from the Government of the Hong Kong Special Administrative Region (Project code: 08191946).
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the University of Hong Kong/Hong Kong West Cluster (UW 19–873) and the study was carried out in accordance to the Declaration of Helsinki. Informed consent was obtained from all subjects and/or their legal guardian(s).
Consent for publication
Not applicable.
Competing interests
Conflict-of-interest disclosure: The University of Hong Kong holds two Unites States of America patents (US 7,521,071 B2) (US patent 8,906,422 B2), one Japan patent (4786341) and one European patent (EP 1562616 B1) for the use of oral arsenic trioxide in the treatment of leukemias and lymphomas. H. Gill, C.R. Kumana and Y.L. Kwong are associated with the University of Hong Kong. None of the other authors have any conflicts of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tallman MS, Altman JK. How I treat acute promyelocytic leukemia. Blood. 2009;114(25):5126–35. doi: 10.1182/blood-2009-07-216457. [DOI] [PubMed] [Google Scholar]
- 2.Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Woods WG, et al. All-trans retinoic acid in acute promyelocytic leukemia: long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood. 2002;100(13):4298–302. doi: 10.1182/blood-2002-02-0632. [DOI] [PubMed] [Google Scholar]
- 3.Lengfelder E, Haferlach C, Saussele S, Haferlach T, Schultheis B, Schnittger S, et al. High dose ara-C in the treatment of newly diagnosed acute promyelocytic leukemia: long-term results of the german AMLCG. Leukemia. 2009;23(12):2248–58. doi: 10.1038/leu.2009.183. [DOI] [PubMed] [Google Scholar]
- 4.Lo-Coco F, Avvisati G, Vignetti M, Breccia M, Gallo E, Rambaldi A, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010;116(17):3171–9. doi: 10.1182/blood-2010-03-276196. [DOI] [PubMed] [Google Scholar]
- 5.Kelaidi C, Chevret S, De Botton S, Raffoux E, Guerci A, Thomas X, et al. Improved outcome of acute promyelocytic leukemia with high WBC counts over the last 15 years: the european APL Group experience. J Clin Oncol. 2009;27(16):2668–76. doi: 10.1200/JCO.2008.18.4119. [DOI] [PubMed] [Google Scholar]
- 6.Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu YM, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2009;106(9):3342–7. doi: 10.1073/pnas.0813280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iland HJ, Bradstock K, Supple SG, Catalano A, Collins M, Hertzberg M, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4) Blood. 2012;120(8):1570–80. doi: 10.1182/blood-2012-02-410746. [DOI] [PubMed] [Google Scholar]
- 8.Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–21. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 9.Abaza Y, Kantarjian H, Garcia-Manero G, Estey E, Borthakur G, Jabbour E, et al. Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood. 2017;129(10):1275–83. doi: 10.1182/blood-2016-09-736686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(13):1295–305. doi: 10.1016/S1470-2045(15)00193-X. [DOI] [PubMed] [Google Scholar]
- 11.Kumana CR, Mak R, Kwong YL, Gill H. Resurrection of oral Arsenic Trioxide for treating Acute promyelocytic leukaemia: a historical account from Bedside to Bench to Bedside. Front Oncol. 2020;10:1294. doi: 10.3389/fonc.2020.01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Au WY, Kumana CR, Kou M, Mak R, Chan GC, Lam CW, et al. Oral arsenic trioxide in the treatment of relapsed acute promyelocytic leukemia. Blood. 2003;102(1):407–8. doi: 10.1182/blood-2003-01-0298. [DOI] [PubMed] [Google Scholar]
- 13.Au WY, Li CK, Lee V, Yuen HL, Yau J, Chan GC, et al. Oral arsenic trioxide for relapsed acute promyelocytic leukemia in pediatric patients. Pediatr Blood Cancer. 2012;58(4):630–2. doi: 10.1002/pbc.23306. [DOI] [PubMed] [Google Scholar]
- 14.Au WY, Kumana CR, Lee HK, Lin SY, Liu H, Yeung DY, et al. Oral arsenic trioxide-based maintenance regimens for first complete remission of acute promyelocytic leukemia: a 10-year follow-up study. Blood. 2011;118(25):6535–43. doi: 10.1182/blood-2011-05-354530. [DOI] [PubMed] [Google Scholar]
- 15.Gill H, Kumana CR, Yim R, Hwang YY, Chan TSY, Yip SF, et al. Oral arsenic trioxide incorporation into frontline treatment with all-trans retinoic acid and chemotherapy in newly diagnosed acute promyelocytic leukemia: a 5-year prospective study. Cancer. 2019;125(17):3001–12. doi: 10.1002/cncr.32180. [DOI] [PubMed] [Google Scholar]
- 16.Gill HS, Yim R, Kumana CR, Tse E, Kwong YL. Oral arsenic trioxide, all-trans retinoic acid, and ascorbic acid maintenance after first complete remission in acute promyelocytic leukemia: long-term results and unique prognostic indicators. Cancer. 2020;126(14):3244–54. doi: 10.1002/cncr.32937. [DOI] [PubMed] [Google Scholar]
- 17.Guru Murthy GS, Szabo A, Michaelis L, Carlson KS, Runaas L, Abedin S, et al. Improving outcomes of Acute promyelocytic leukemia in the current era: analysis of the SEER database. J Natl Compr Canc Netw. 2020;18(2):169–75. doi: 10.6004/jnccn.2019.7351. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Kantarjian H, Wang H, Cortes J, Ravandi F. Acute promyelocytic leukemia: a population-based study on incidence and survival in the United States, 1975–2008. Cancer. 2012;118(23):5811–8. doi: 10.1002/cncr.27623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinmohamed AG, Visser O. Incidence of acute promyelocytic leukemia across Europe: results of RARECAREnet-a population-based study. Stem Cell Investig. 2019;6:37. doi: 10.21037/sci.2019.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenzi L, Lee-Jones L, Mostofa MA, de Andrade DP, Ribeiro RC, Figueiredo BC. Second Primary Malignancy after Acute Promyelocytic Leukemia: A Population-Based Study. Cancers (Basel). 2020;12(12). [DOI] [PMC free article] [PubMed]
- 21.Norsworthy KJ, Avagyan A, Bird ST, Li Y, Akhtar S, Liao J, et al. Second cancers in adults with acute promyelocytic leukemia treated with or without arsenic trioxide: a SEER-medicare analysis. Leukemia. 2020;34(11):3082–4. doi: 10.1038/s41375-020-0905-y. [DOI] [PubMed] [Google Scholar]
- 22.Testi AM, Pession A, Diverio D, Grimwade D, Gibson B, de Azevedo AC, et al. Risk-adapted treatment of acute promyelocytic leukemia: results from the International Consortium for Childhood APL. Blood. 2018;132(4):405–12. doi: 10.1182/blood-2018-03-836528. [DOI] [PubMed] [Google Scholar]
- 23.Gill H, Yim R, Lee HKK, Mak V, Lin SY, Kho B, et al. Long-term outcome of relapsed acute promyelocytic leukemia treated with oral arsenic trioxide-based reinduction and maintenance regimens: a 15-year prospective study. Cancer. 2018;124(11):2316–26. doi: 10.1002/cncr.31327. [DOI] [PubMed] [Google Scholar]
- 24.https://.www.R-project.org/. Accessed 15 November 2021
- 25.https://cran.r-project.org/web/packages/fmsb/fmsb.pdf. Accessed 15 November 2021.
- 26.https://www.mortality.org/cgi-bin/hmd/country.php?cntr=HKG&level=1. Accessed 15 November 2021.
- 27.https://www.censtatd.gov.hk/en/web_table.html?id=1A. Accessed 15 November 2021.
- 28.https://seer.cancer.gov/stdpopulations/stdpop.singleages.html. Accessed 15 November 2021.
- 29.Matasar MJ, Ritchie EK, Consedine N, Magai C, Neugut AI. Incidence rates of acute promyelocytic leukemia among Hispanics, blacks, Asians, and non-hispanic whites in the United States. Eur J Cancer Prev. 2006;15(4):367–70. doi: 10.1097/00008469-200608000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Douer D. The epidemiology of acute promyelocytic leukaemia. Best Pract Res Clin Haematol. 2003;16(3):357–67. doi: 10.1016/s1521-6926(03)00065-3. [DOI] [PubMed] [Google Scholar]
- 31.Vickers M, Jackson G, Taylor P. The incidence of acute promyelocytic leukemia appears constant over most of a human lifespan, implying only one rate limiting mutation. Leukemia. 2000;14(4):722–6. doi: 10.1038/sj.leu.2401722. [DOI] [PubMed] [Google Scholar]
- 32.Kelly LM, Kutok JL, Williams IR, Boulton CL, Amaral SM, Curley DP, et al. PML/RARalpha and FLT3-ITD induce an APL-like disease in a mouse model. Proc Natl Acad Sci U S A. 2002;99(12):8283–8. doi: 10.1073/pnas.122233699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.David G, Terris B, Marchio A, Lavau C, Dejean A. The acute promyelocytic leukemia PML-RAR alpha protein induces hepatic preneoplastic and neoplastic lesions in transgenic mice. Oncogene. 1997;14(13):1547–54. doi: 10.1038/sj.onc.1200989. [DOI] [PubMed] [Google Scholar]
- 34.Early E, Moore MA, Kakizuka A, Nason-Burchenal K, Martin P, Evans RM, et al. Transgenic expression of PML/RARalpha impairs myelopoiesis. Proc Natl Acad Sci U S A. 1996;93(15):7900–4. doi: 10.1073/pnas.93.15.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanz MA, Martin G, Gonzalez M, Leon A, Rayon C, Rivas C, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood. 2004;103(4):1237–43. doi: 10.1182/blood-2003-07-2462. [DOI] [PubMed] [Google Scholar]
- 36.Sanz MA, Montesinos P, Rayon C, Holowiecka A, de la Serna J, Milone G, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115(25):5137–46. doi: 10.1182/blood-2010-01-266007. [DOI] [PubMed] [Google Scholar]
- 37.Powell BL, Moser B, Stock W, Gallagher RE, Willman CL, Stone RM, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: north american Leukemia Intergroup Study C9710. Blood. 2010;116(19):3751–7. doi: 10.1182/blood-2010-02-269621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacomo RH, Melo RA, Souto FR, de Mattos ER, de Oliveira CT, Fagundes EM, et al. Clinical features and outcomes of 134 brazilians with acute promyelocytic leukemia who received ATRA and anthracyclines. Haematologica. 2007;92(10):1431–2. doi: 10.3324/haematol.10874. [DOI] [PubMed] [Google Scholar]
- 39.Lehmann S, Ravn A, Carlsson L, Antunovic P, Deneberg S, Mollgard L, et al. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the swedish adult Acute Leukemia Registry. Leukemia. 2011;25(7):1128–34. doi: 10.1038/leu.2011.78. [DOI] [PubMed] [Google Scholar]
- 40.Park JH, Qiao B, Panageas KS, Schymura MJ, Jurcic JG, Rosenblat TL, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118(5):1248–54. doi: 10.1182/blood-2011-04-346437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McClellan JS, Kohrt HE, Coutre S, Gotlib JR, Majeti R, Alizadeh AA, et al. Treatment advances have not improved the early death rate in acute promyelocytic leukemia. Haematologica. 2012;97(1):133–6. doi: 10.3324/haematol.2011.046490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulson K, Serebrin A, Lambert P, Bergeron J, Everett J, Kew A, et al. Acute promyelocytic leukaemia is characterized by stable incidence and improved survival that is restricted to patients managed in leukaemia referral centres: a pan-canadian epidemiological study. Br J Haematol. 2014;166(5):660–6. doi: 10.1111/bjh.12931. [DOI] [PubMed] [Google Scholar]
- 43.Rahme R, Thomas X, Recher C, Vey N, Delaunay J, Deconinck E, et al. Early death in acute promyelocytic leukemia (APL) in french centers: a multicenter study in 399 patients. Leukemia. 2014;28(12):2422–4. doi: 10.1038/leu.2014.240. [DOI] [PubMed] [Google Scholar]
- 44.Sanz MA, Fenaux P, Tallman MS, Estey EH, Lowenberg B, Naoe T, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the european LeukemiaNet. Blood. 2019;133(15):1630–43. doi: 10.1182/blood-2019-01-894980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kayser S, Rahme R, Martinez-Cuadron D, Ghiaur G, Thomas X, Sobas M, et al. Outcome of older (>/=70 years) APL patients frontline treated with or without arsenic trioxide-an International Collaborative Study. Leukemia. 2020;34(9):2333–41. doi: 10.1038/s41375-020-0758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jillella AP, Kota VK. The global problem of early deaths in acute promyelocytic leukemia: a strategy to decrease induction mortality in the most curable leukemia. Blood Rev. 2018;32(2):89–95. doi: 10.1016/j.blre.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Naymagon L, Moshier E, Tremblay D, Mascarenhas J. Predictors of early hemorrhage in acute promyelocytic leukemia. Leuk Lymphoma. 2019;60(10):2394–403. doi: 10.1080/10428194.2019.1581187. [DOI] [PubMed] [Google Scholar]
- 48.Chang H, Kuo MC, Shih LY, Dunn P, Wang PN, Wu JH, et al. Clinical bleeding events and laboratory coagulation profiles in acute promyelocytic leukemia. Eur J Haematol. 2012;88(4):321–8. doi: 10.1111/j.1600-0609.2011.01747.x. [DOI] [PubMed] [Google Scholar]
- 49.Mantha S, Goldman DA, Devlin SM, Lee JW, Zannino D, Collins M, et al. Determinants of fatal bleeding during induction therapy for acute promyelocytic leukemia in the ATRA era. Blood. 2017;129(13):1763–7. doi: 10.1182/blood-2016-10-747170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hou W, Zhang Y, Jin B, Cao W, Lu M, Yan L, et al. Factors affecting thrombohemorrhagic early death in patients with acute promyelocytic leukemia treated with arsenic trioxide alone. Blood Cells Mol Dis. 2019;79:102351. doi: 10.1016/j.bcmd.2019.102351. [DOI] [PubMed] [Google Scholar]
- 51.Gill H, Yung Y, Chu HT, Au WY, Yip PK, Lee E, et al. Characteristics and predictors of early hospital deaths in newly diagnosed APL: a 13-year population-wide study. Blood Adv. 2021;5(14):2829–38. doi: 10.1182/bloodadvances.2021004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Altman JK, Rademaker A, Cull E, Weitner BB, Ofran Y, Rosenblat TL, et al. Administration of ATRA to newly diagnosed patients with acute promyelocytic leukemia is delayed contributing to early hemorrhagic death. Leuk Res. 2013;37(9):1004–9. doi: 10.1016/j.leukres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Geer MJ, Foucar CE, Devata S, Benitez L, Perissinotti AJ, Marini BL et al. Clinical Availability of ATRA for Patients With Suspected Acute Promyelocytic Leukemia: Why Guidelines May Not Be Followed. J Natl Compr Canc Netw. 2021. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.