Abstract
Emerging bio-contaminants such as viruses have affected health and environment settings of every country. Viruses are the minuscule entities resulting in severe contagious diseases like SARS, MERS, Ebola, and avian influenza. Recent epidemic like the SARS-CoV-2, the virus has undergone mutations strengthen them and allowing to escape from the remedies. Comprehensive knowledge of viruses is essential for the development of targeted therapeutic and vaccination treatments. Animal models mimicking human biology like non-human primates, rats, mice, and rabbits offer competitive advantage to assess risk of viral infections, chemical toxins, nanoparticles, and microbes. However, their economic maintenance has always been an issue. Furthermore, the redundancy of experimental results due to aforementioned aspects is also in examine. Hence, exploration for the alternative animal models is crucial for risk assessments. The current review examines zebrafish traits and explores the possibilities to monitor emerging bio-contaminants. Additionally, a comprehensive picture of the bio contaminant and virus particle invasion and abatement mechanisms in zebrafish and human cells is presented. Moreover, a zebrafish model to investigate the emerging viruses such as coronaviridae and poxviridae has been suggested.
Keywords: Zebrafish, Bio-contaminants, Risk assessments, Virus inactivation, Alternative infection model, Poxviridae
Graphical abstract
1. Introduction
Contagious infections are a major hazard to public health, causing more years of life to be lost due to early death than any other disease process (WHO, 2009, WHO, 2008). Until the nineteenth century, artists and authors typically did a better job of describing infectious syndromes than scientists. This began to change as bacteria became increasingly understood, and many ancient infections such as polio, measles, and smallpox have been explained and handled during the last 150 years. During the last three decades, however, there has been a continual stream of newly discovered illnesses that have drawn increasing interest. In 1992, emerging infections were defined as “new, re-emerging, or drug-resistant illnesses whose incidence in humans has grown during the last 20 years or whose incidence threatens to increase in the near future” by the expert committee that produced the Institute of Medicine report on emerging infections (Watkins, 2018). Changes in human demographics, technological advances, changes in industry practices, economic development, increase in international tourism, microbial adaptation, and breakdown in public health capacity were presented as some major key contributors to the diseases (Hughes, 2001). Furthermore, most of the emerging contaminants like chemical toxins, plastic components, flame retardants, surfactants, fragrances, pesticides and dissemination of nanoparticles in natural surroundings thorough cosmetic and daily products have always been a topic of concern. However, recent years 2020 and 2021 demonstrated that biological contaminants (virus) possess immediate threat that could wipe out huge population within few weeks. SARS-CoV, Zika, MARS-CoV, Rift Valley fever virus, human coronaviruses, avian influenza A, Heartland and Bourbon viruses, Nipah virus, Hendra virus, Ebola virus, MarV disease, Crimean-Congo hemorrhagic fever, and Lassa fever (Dabbu Kumar et al., 2018; Israel Nii-Trebi, 2017; Wigginton et al., 2015), are some WHO highlighted emerging biological contaminants that has the potential to cause epidemics in the future (LP and V, 2021; Watkins, 2018).
Viruses are ubiquitous entities and are the most diverse microbes on the planet. For billions of years, they have been a component of life's evolution (Nasir and Caetano-Anollés, 2015). They are found in all ecosystems and can infect a wide variety of living organisms, including animals, people, plants, and microorganisms (Couch, 1981). Viruses have aided in the evolution of life on Earth and can help preserve ecosystems and crucial natural cycles, such as the carbon cycle in the oceans (Wilhelm and Suttle, 1999). However, pathogenic viruses cause a great deal of hospitalization, animal sickness, animal fatalities, and agricultural losses. Thereby creating tremendous social, economic, and biological burden (Filipić et al., 2020). Due to their adaptability, they currently exist in all environmental conditions. They can also be passed directly from one sick person to another by contaminated intermediaries, such as surfaces, objects, air, food, and water. According to several epidemiological studies, the most important vectors of viral infections are water and air (Martínez-Montelongo et al., 2020; Mehle and Ravnikar, 2012; Shrestha et al., 2018; World Economic Forum, 2021).
Viruses comprise of two main components; a nucleic acid genome made up of double-helix DNA, or single-helix RNA, and a capsid. The capsid, which encases and protects the viral genome, is a highly symmetrical structure composed of many copies of a few proteins expressed by the viral genome (Domingo, 2020). However, it is renowned for being multifunctional, involved in cell entrance, genome uncoating, and intracellular trafficking (Freire et al., 2015; Mateu, 2013). Some viruses have an additional layer of protection, known as an envelope, in addition to the capsid, commonly comprising a lipid or glycoprotein covering. Lipid envelopes originate from the cell membrane of the infected host, whereas glycoprotein envelopes are usually programmed by the viral genome. Which finally assembles to form a virion (Banerjee and Mukhopadhyay, 2016). The viral genome also contains proteins that aid viral particle multiplication and assembly. Although most viruses have common structural features, such as symmetric capsid proteins, they are genetically heterogeneous.
Evidence suggests that viruses present in animal pools have now spread to humans and are responsible for serious illnesses. Cases such as 2019 novel coronavirus (Li et al., 2020), yellow fever in 1901, Spanish flu in 1918, Ebola outbreak in 2014 caused by flavivirus (Frierson, 2010; Taubenberger, 2006) and Ebola virus (Cdc.gov, 2022). As such, there is rekindled interest in creating methods for quicker prevention, treatment, and management of newly emerging and re-emerging viruses with significant outbreak capability.
One of the major aspects of virology is understanding how viruses multiply and cause symptoms in individuals. Understanding the processes underlying in vivo viral characteristics is vital for virologists who research viruses that are hazardous to humans. To do this, investigations using animal models are crucial for generating both accurate in vivo analyses and effective viral infection prevention strategies. The manner in which a virus behaves in its natural host frequently differs from its behavior in humans. Viruses that cause major illnesses in humans frequently show no symptoms in their native hosts. Consequently, the use of natural hosts as animal models has been limited. Studies on these viruses have been conducted using animal models by examining the host features of the viruses—their ability to reproduce, cellular tropisms, pathogenicity, and transmission—and by creating vaccines and antivirals. No animal can be an exact clone of a human. Certain animal models may be better able to depict viral infection but still have drawbacks in other areas. As a result, the optimal animal model may be determined by experimental questions, and studies should be designed such that the results acquired from animal models can be transferred to humans.
Animals that serve as natural reservoirs for influenza A viruses include swine and bird species, whereas bats and dromedary camels serve as reservoirs for MERS-CoVs (Cui et al., 2013; Mohd et al., 2016; Peck et al., 2015). Flu, Heartland, and Bourbon viruses have been studied using animal models including ferrets, mice, guinea pigs, cats, Syrian hamsters, rabbits, raccoons, chickens, and non-human primates (Bosco-Lauth et al., 2016; Bouvier, 2015; Godsey et al., 2021; Kwon et al., 2009; Lowen et al., 2006; O'Donnell and Subbarao, 2011; Van Der Laan et al., 2008; Van Hoeven et al., 2009). Mouse models, guinea pig models, and non-human primate models (marmoset, rhesus monkey, and cynomolgus macaque) have been used to study Lassa fever (Bell et al., 2017; Carrion et al., 2007; Cashman et al., 2017, Cashman et al., 2011; Flatz et al., 2010; H et al., 1975; Jahrling et al., 1982; Oestereich et al., 2016; NE et al., 2016; Rieger et al., 2013; Sattler et al., 2020; Uckun et al., 2004; Walker et al., 1982, Walker et al., 1975; Yun et al., 2013, Yun et al., 2012). In addition, hDPP4-mice and non-human primates have been used to study MERS-CoV viruses (Agrawal et al., 2015; Cockrell et al., 2016; J et al., 2014). Large animals, such as rhesus macaques and, to a lesser extent, cynomolgus macaques, are species of non-human primate that are predominantly used as Zika virus research models (Code and Beaverton, 2008; Lindsey, 2016). A variety of immunocompetent mice, immunodeficient mice, hamsters, strain 13 guinea pigs, outbred guinea pigs, macaques, African green monkeys, marmosets, baboons, rhesus macaques, and cynomolgus macaques (Bente et al., 2009; Bird et al., 2015; Patterson and Carrion, 2005; Perry et al., 2012; V et al., 2012) have been used for EboV research. Moreover, immunocompetent mice, immunocompromised mice, guinea pigs, and cynomolgus macaques (Cross et al., 2015; DA et al., 2010; LE et al., 2011; Marzi et al., 2016; Warfield et al., 2009) have been used to study the Marburg virus. Hendra virus research employs mice, syrian golden hamsters, ferrets, squirrel monkeys and African green monkeys (B et al., 2011, B et al., 2010; J. et al., 2012; Pallister et al., 2011; V et al., 2009). Research on Rift Valley fever virus includes animal models of mice, rats, sheep, claves (<1-month-old), and primates (Anderson et al., 1991; Anderson and Smith, 1987; Davenport et al., 1953; Ikegami and Makino, 2009; Jensen and Francis, 1953; Peters et al., 1988; Peters and Slone, 1982; Rippy et al., 1992; Ross et al., 2012; Smith et al., 2010). Crimean-Congo hemorrhagic fever virus models include mice and immunocompetent non-human primates (Bente et al., 2010; Bereczky et al., 2010; Farzani et al., 2019; Garrison et al., 2017; Golden et al., 2019; Haddock et al., 2018; Lindquist et al., 2018; Smith et al., 2019; Spengler et al., 2017; Tignor and Hanham, 1993). The Nipah virus models include pigs, Syrian hamsters, ferrets, African green monkeys, cats, and mice (BA et al., 2006; de Wit et al., 2011; Geisbert et al., 2010; KN et al., 2009; KP et al., 2013; Middleton et al., 2002). Finally, recent models used for research on SARS-CoV-2 include human Angiotensin-converting enzyme 2 (hACE2) mice, Golden Syrian hamsters, ferrets, cats, rhesus monkeys, cynomolgus macaques, marmosets, African green monkeys, and tree shrew (Johansen et al., 2020; Zhang et al., 2021).
Animal models of viral infection are essential for exploring viral pathogenesis and host responses. Important traits for viral studies include support for viral replication, susceptibility to viral infection, and a summary of clinical symptoms of human sickness. There are numerous viral models, as has already been indicated, and each has advantages and shortcomings that should be considered. Scientists investigating infectious diseases utilize small animals including rats, mice, and guinea pigs. The mouse model is beneficial for establishing immunological response, signal-transduction, inflammatory pathway, and effectiveness research; however, it has its limitations as a rodent model. Due to various host-range restrictions, mice are frequently difficult to infect with human viruses. If the circumstances are right, it is possible to study immune responses but not viral multiplication and dissemination. Additionally, all strains of mice lack the Mx protein, which is essential for viral replication and is caused by a faulty Mx1 gene (Zhou et al., 1999). Which further causes aberration in IFN response. Moreover, the pathogenesis and immunological responses of different inbred strains can also differ from those of humans, which may affect experimental results and restrict their predictive usefulness for research of pharmacological intervention techniques (Guénet, 2005; Rivera and Tessarollo, 2008). While transgenic mice are incredibly useful for researching pathophysiology, antivirals, and developing vaccines. Nevertheless, due to variations in immunopathology and disease severity caused by cell or tissue selectivity and/or degrees of transgenic expression among various transgenic mice lines, effectively replicating viral illness in humans has remained a challenge (Koike et al., 1994). An investigation revealed that human poliovirus receptor CD155 did not effectively invade when delivered orally, but did so when administered intravenously and intraperitoneally (Zhang and Racaniello, 1997). Post entry inhibition of HIV-1 replication exhibited, when the virus was administered in transgenic mice resulted in infecting limited number of cells (Mariani et al., 2000). Aforementioned studies explain the lack of permittivity or difference in pathophysiology could be due to absence of co-receptors or co-factors, or due to host-range restriction factor. Due to a mutation in the DNA repair enzyme prkdc (protein kinase, DNA activated, catalytic polypeptide), SCID (severe combined immunodeficient) suffer from a continuous de novo multilineage hematopoiesis defect and a total absence of the human immune system, making them unsuitable for studying the human immune response to virus infection and the effectiveness of vaccines (GC et al., 1983; Shultz et al., 2007). Although numerous factors (such as HIS functioning and engraftment variability) in humanized immunodeficient mice continue to hinder the investigation of immunopathogenesis of human virus, progress is still being made. Guinea pig has always been intermediary model (secondary) between mice and NHPs. The establishment of deadly disease models in guinea pigs frequently necessitates host adaptation of infections, such as guinea pig-adapted Ebola. The drawback of this model is that it doesn't have readily available commercial reagents, which makes it difficult to describe illnesses in these models (Nguyen et al., 2021). Hamsters offer an intriguing option for small animal disease models and their use in the field of emerging viral pathogens research. Infected hamster by paramyxoviruses Nipah and Hendra, could result in neurological or respiratory symptoms that are comparable to those in humans (B et al., 2012, B et al., 2011). The pathological presentations seen in hamster models seem to more accurately mimic the human situation as compared to mice and guinea pigs. Syrian hamsters are outbred, despite the fact that little is known about their genetic makeup and immune systems. It's possible that hamsters lack some host defensive systems and have suppressed inducible nitric oxide (Saldarriaga et al., 2012), both of which might boost the virulence of infection by specific viruses infection or human viruses. To assess host immune response, antiviral drug and vaccine efficacy against influenza virus, ferrets has been a pivotal animal model. However, lack of reagents such as ferret-specific monoclonal antibody for analysis of immune response, high cost, size and husbandry requirements are few reasons making this model in assessable to researcher. Furthermore, this inaccessibility might lead to misinterpretation of the results (Nguyen et al., 2021). Small animal models are less similar to humans than large animal model such as non-human primates (NHPs) and pigs in terms of morphology, specialized lymphoid tissues at mucosal sites, and peripheral immune system development (Hein and Griebel, 2003). Even though the use of NHPs is clearly supported by our phylogenetic and evolutionary connections to humans (F. et al., 2005). However, the intricacy of animal husbandry and scientific investigation, ethical issues, expensive and the accessibility of immunoreagents is a challenge. Due to aforementioned limitation their usage drastically reduced or limited to researches with hepatitis B, C, E virus (Bettauer, 2010). Macaques being 93–99 % similarities in protein level for several cytokine with human and relatively small in size, is the most used NHPs in virus research (F. et al., 2005). Despite having similitude with humans, there are limitations to its usage. In contrast to humans, changes in pathophysiology, MHC-restricted immune responses, and disease manifestation might result from species differences (such as MHC polymorphisms), even if they are minimal (Bontrop and Watkins, 2005; Kaiser et al., 2007). It should be emphasized that there are variations in the susceptibility and pathogenicity of NHPs among species or subspecies (Ten Haaft et al., 2001). NHPs are rigorously controlled and numbered in their experimental uses. Owing to the outbreeding of NHPs, genetic variability might cause confounding and skewed results in (statistical) studies. Pigs are good animal models for studying influenza virus, however they are not the best choice due to their high cost, challenges with housing, handling, and waste management. Aside from the major category of animals, many other animals, including cats, rabbits, chickens, goats, and raccoons, are used as animal models for viral research. However, these creatures are not suitable for high-throughput drug screening or the visualization of host-pathogen interactions due to their high maintenance requirements, complex animal husbandry procedures, ethical considerations, or experimental limitations (Dycke et al., 2019; Gabor et al., 2014). Therefore, a simpler, more robust, and widely accessible viral replication model is urgently needed. It is critical to create a paradigm that allows large-scale chemical and genetic screening to corroborate bio-contaminants studies.
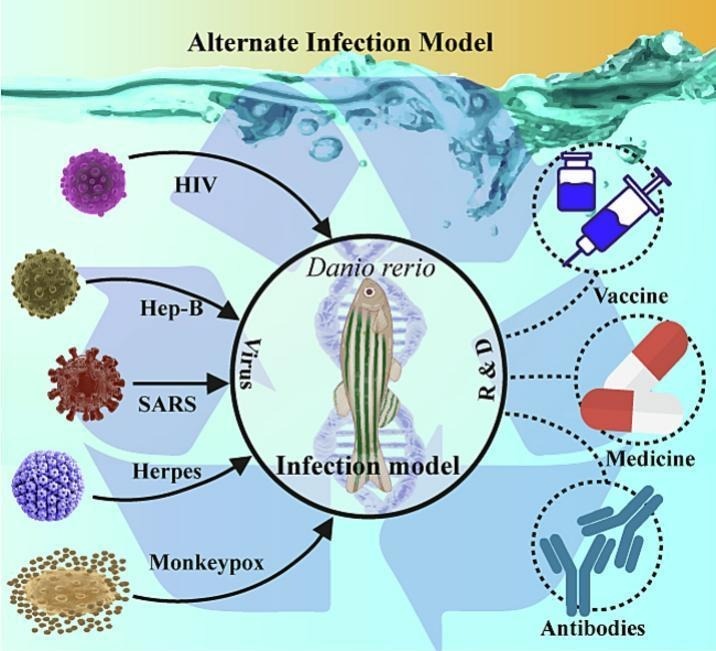
Zebrafish are superior to small and large animal models in various ways for studying emerging contaminants as well as bio-contaminants. Due to which our knowledge of the interactions between the host and pathogen has improved considerably. The ability of this species' embryos to grow outside the mother's body and their transparency, which is one of the most frequent reasons for choosing this animal model, make it more desirable to researchers even if it is generally not the first option. Despite the widespread use of small animal models by researchers, SARS-CoV-2 susceptibility in models like rats and mice is negligible due to intrinsic characteristics (variation in the amino acid sequence encoding ACE2 receptor) (Chan et al., 2020). Transgenic mice overexpressing human ACE2 have been constructed to address this issue, however they have some limitations, such as the inability to conduct long-term studies due to the scarcity of breeding and the high mortality rate, which reported 7 days after infection, and distribution facilities. Conducting experimentation on animal models such as mice, ferret, golden hamster and large animals possess limitation such as high maintenance costs, several weeks to produce offsprings sophisticated animal husbandry protocols, and many more which has been mentioned above (Chan et al., 2020; Johansen et al., 2020; Kim et al., 2020; Munnink et al., 2020). Non-human primates (NHPs), for instance, are the nearest animal species to the pathophysiology of humans, and their research has FDA permission. However, not all non-human primates exhibit complete susceptibility to infection or exhibit all COVID-19 symptoms of humans (Johansen et al., 2020). Zebrafish possess several inherent characteristics that make them excellent biological model system include a high fecundity rate, clear embryos, cheap maintenance cost, easy husbandry routine (Choi et al., 2021; Dycke et al., 2019; Gabor et al., 2014; Laghi et al., 2022; Lama et al., 2022); in some cases, no ethical permission is necessary for zebrafish embryos/larvae up to 120 h postfertilization (Strähle et al., 2012). The presence of alpha2,3- and alpha2-6-linked sialic acid receptor in zebrafish, makes them susceptible to H1N1 and influenza A virus. As human virus can be infected in zebrafish which possible give opportunity to examine viral infection and host inflammatory response in human (Gabor et al., 2014). In case of SARS-CoV-2, zebrafish has shown infectivity when injected in swim bladder, without overexpressing human ACE2 receptors (Laghi et al., 2022). In another study, when exploring SARS-CoV-2 host cell entry, ACE2 and TPC2 founded to be the major player in translocation of virus through endolysosomal when entered through peripheral sense organs (Choi et al., 2022). However, SARS-CoV-2-infected to dog, pig, tree shrew, and chicken animal models, respectively, had very few effects and no animals showed symptoms (Johansen et al., 2020). Zebrafish model may be used to perform toxicity and immunological tests, including embryotoxicity, hepatotoxicity, endocrine toxicity, genotoxicity, neurotoxicity, hematological analysis, differential white cell count, and immunological studies (Fukushima et al., 2020, Fukushima et al., 2017; McFetridge et al., 2015). Small animal models like rodents have been used for these tests till date, but recent studies show zebrafish model has proved to be an important tool in the studies of infection and immunological responses. In addition, due to their transparent embryo and larvae observation can be made in real-time allowing for the monitoring of embryogenesis. Similarly, the zebrafish model has helped in explaining bacterial infection in humans, as well as freshwater pathogens in fishes. Various freshwater pathogens (bacteria, viruses, and parasites) associated with fish diseases have already been investigated using the Danio rerio model (von Jørgensen and G., 2020; Rakus et al., 2019). Bacterial pathogens such as Mycobacterium marinum, Vibrio anguillarum, Aeromonas salmonicida, Yersinia ruckeri, and Flavobacterium psychrophilum; enteric bacteria such as Vibrio, Listeria, Shigella Salmonella, and E. coli; viruses such as infectious hematopoietic necrosis virus (IHNV), viral hemorrhagic septicemia virus (VHSV), spring viremia carp virus (SVCV), and infectious pancreatic necrosis virus (IPNV), Chikungunya virus (CHIKV), human norovirus (HuNoV), influenza A virus, and herpes simplex virus type-1 (HSV-1); and parasites such as I. multifiliis and Trypanosoma carassii have all been demonstrated to infect zebrafish larvae (Burgos et al., 2008; Dycke et al., 2019; Gabor et al., 2014; Palha et al., 2013; Kanther and Rawls, 2010). This paper discusses in detail about benefits of utilizing zebrafish in research as well as their potential applications in developing infection models. We mostly focused on the disadvantages as well as advantages of using current animal models for investigations and emphasized how zebrafish could offer several benefits over other models at the moment. Secondarily, this paper discusses how numerous zebrafish aspects are applied to the development of disease models that might closely resemble human disease, toxicity testing, and models to research bio-contaminants. We also went into further detail on evaluations of entry routes and mechanism of bio-contamination in zebrafish and how it pertains to human cells. This paper also discusses several cell mechanisms and strategies for preventing viral replication. The use of Danio rerio as a model organism for studies on the Sars-CoV-2 virus has been reviewed. Finally, we discuss the potential application of this model system to study the recently reported monkeypox virus. In our opinion, this review has the opportunity to educate researchers about the utilization of zebrafish models, encourage them to switch to using zebrafish model systems for research involving infectious diseases, environmental pollutants, and toxicological studies of contaminants, and minimize the use of certain existing models, which are typically time-consuming and difficult. This review's main argument is that zebrafish models can be used to study various bio-contaminants that might cause pandemics, and that doing so could be analogous to conducting so on humans.
2. Methodology
The systematic literature review that formed the basis of this study was conducted utilizing resources including Google Scholar, Science Direct, the Zebrafish Information Network database, and PubMed. The focus was on obtaining literature by utilizing terms like zebrafish model, bio-contaminants, toxicity, infection model, SARS-CoV-2, monkeypox virus, viral entrance mechanism, and contemporary animal models. The relevance of the zebrafish model's applicability for evaluating viruses and different emerging bio-contaminants studies was taken into consideration while choosing the papers referenced here. The papers in this list include original research articles, critical essays review and perspectives that have been published in prestigious worldwide scientific publications. Lastly, the citation was included with Mendeley program.
3. Relevance of zebrafish attributes
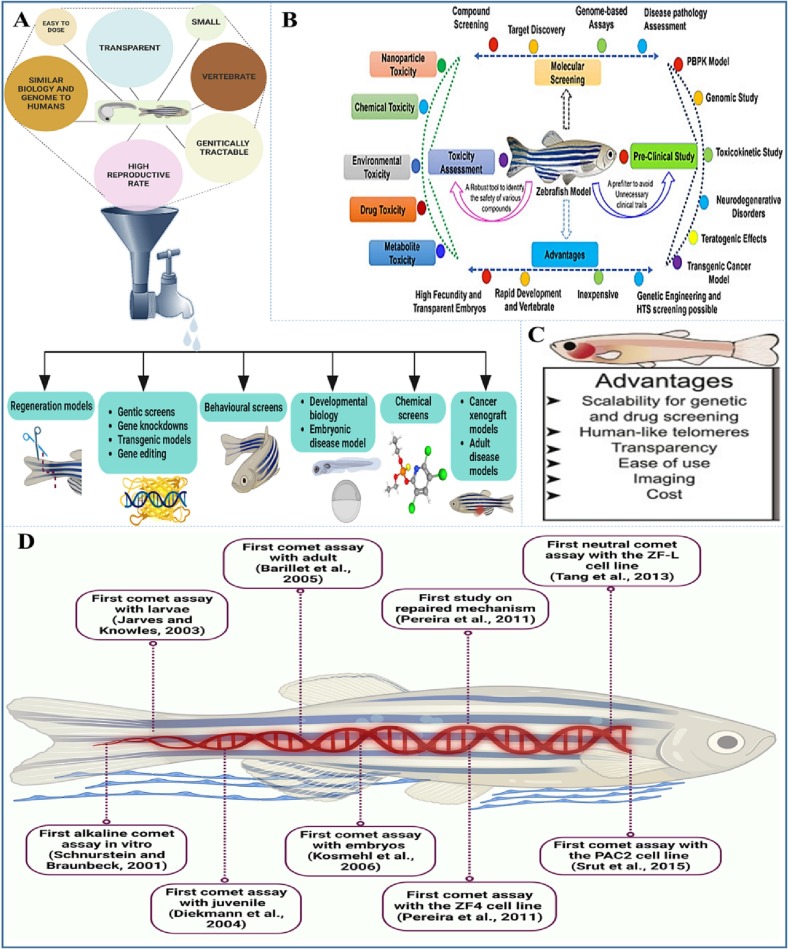
The selection criteria for animal models are intimately connected to the study's ultimate objective. Zebrafish model use within the scientific community has increased dramatically, despite small and big animal models being the most often used research animal models globally. Thanks to the Streisinger's work, Zebrafish rose to the top of the list of NIH-funded biological models (Driever et al., 1996; Eisen and Grunwald, 2002; Haffter et al., 1996; Kinth et al., 2013). And are favored above other species and are used frequently as animal models due of these benefits (Fig. 1 ). Primarily, zebrafish was widely accessible, simple to produce, and, they are less expensive to maintain than mice (Burke, 2016a). These tiny cyprinids, which reach adulthood at a length of 2–4 cm, are found in warm, slowly flowing streams, ponds, and flooded rice fields in Southern and South-Eastern Asia (Parichy, 2015). Under controlled environment zebrafish can produce approximately 200 eggs at a time and spawn daily throughout the year in a laboratory setting. Daylight period is the main activity hours for the fishes (Miller and Gerlai, 2011). In the lab, larvae hatch 72 h after fertilization and mature after 3 months, however in the wild they may take longer than six months to fertilize (Khan and Alhewairini, 2019). During mating and spawning seasons, violent behavior associated to dominance may only occur, or at least be significantly more prevalent (generally during early morning) (Khan and Alhewairini, 2019; Miller and Gerlai, 2011).
Fig. 1.
Importance of Danio rerio model in scientific arenas.
Secondly, this model is a vertebrate, therefore it has structural and physiological similarities to humans (Burke, 2016a). In comparison to the human genome, which has 25,000 protein coding regions spread across 23 pairs of chromosomes, the zebrafish genome has 26,000 protein coding regions when it was evaluated (Costa et al., 2021), and also shows that zebrafish and humans have been shown to share 70 % of the same genes (orthologous proteins are 70 % similar in terms of their amino acid residue sequence) (Animals Facts, 2015). Zebrafish contain equivalents to about 80–84 % of the genes linked to human illness (Animals Facts, 2015; Galindo-Villegas, 2020). When compared to mouse editing, zebrafish gene editing is less complicated. Another study revealed that the zebrafish homebox gene and the mouse hox-2.1 domain have a multitude of similarity amino acid sequences (Costa et al., 2021). Moreover, many other genes similarities were found between both the organisms. Study conducted to examine HSV-1 entry through 3-O sulfated heparan sulfate demonstrated the presence of human homologs receptors in zebrafish (Hubbard et al., 2010; Yakoub et al., 2014). Most commonly mutation in BRAF gene in human melanoma has been successfully created in zebrafish to make a knock-in model. Due to which other potential cancer-causing mutations have been able to identify such as SETDB1 (Burke, 2016b). Dystrophin, a muscular weakness in humans, the loss of dystrophin gene gradually leads to necrotic muscle fibers in zebrafish as well (Burke, 2016b).
The capacity to expose fish embryos to various drug/chemicals to develop or relieve specific trait can be undertaken on a big scale, as zebrafish produces hundreds of offspring at a regular interval, giving scientist a plentiful supply of embryos to analyze when studying diseases associated with human. Ex situ development enables the exposure of zebrafish embryos to drugs by putting them in an embryo support medium (Burke, 2016a; Khan and Alhewairini, 2019). The drugs do not undergo through the mother's metabolism before reaching the growing embryo, unlike placental animals. Additionally, because there are many zebrafish embryos accessible, it is easy to arrange them in microtitre dishes for exposure to various drugs, dosages, and treatment combinations. A different approach to comprehend the biology of drug response is to utilize genetic screening to identify mutants that are specifically sensitive or resilient to the effects of a certain compounds (Lardelli, 2008). When Growth rate is compared with human embryos zebrafish embryos grow incredibly quickly; they develop as much in one day as an embryonic human does in one month. Zebrafish embryos are transparent, making it simple for scientists to study the growth of interior components. Contrary to C. elegans, zebrafish have most of the major vertebrate organ systems, including the heart, eyes, and kidneys, allowing the study of organ development (Gehrig et al., 2018; Teame et al., 2019). Zebrafish have a special capacity for heart muscle healing. For instance, if a portion of their heart is removed, they may quickly regenerate it again (Beffagna, 2019; Karra et al., 2015; Sande-Melón et al., 2019). Zebrafish are incredibly useful for recent day researches because of their well-characterized genes, which allow for the targeting of individual genes, their ability to be turned “OFF”/“ON”, or the ability to introduce exogenous gene into the organism (Avcı, 2014). Concerning bacterial pathogenesis investigations, a recent work by Cao et al., demonstrated the functional properties of the anti-apoptotic protein BIRC2 (cIAP1) in response to Edwardsiella piscicida (a significant bacterial pathogen) in zebrafish (Cao et al., 2021). This study claims that piscine BIRC2 inhibits caspases and accumulates the p53 gene in a p53 transcription-dependent and -independent way in response to the E. piscicida infection, acting as a negative regulator for the antibacterial immune response. A significant family of host pattern recognition receptors (PRRs) called toll-like receptors (TLRs) may detect a wide range of molecular patterns associated with pathogens (PAMP). Currently, the zebrafish genome has 24 TLRs (Sullivan and Kim, 2008). Zebrafish TLRs have been investigated in response to viral and bacterial stimuli. Tlr3, Tlr7, Tlr8a/b, Tlr9, and Tlr22, which are specific to fish, have been linked to the response to viruses in zebrafish. According to Pietretti and Wiegertjes (2014), Tlr3 and Tlr22 exhibit striking similarities in their cellular location and molecular functions and have the ability to detect viral replication by binding to double-stranded RNA (dsRNA) or its counterpart poly I:C (a synthetic polyinosinic-polycytidylic acid dsRNA). In response to infection with the ssRNA virus snakehead rhabdovirus (SHRV), zebrafish tlr3 transcripts were up-regulated (Phelan et al., 2005a). Additionally, Tlr3 was up-regulated following infections with the viral hemorrhagic septicemia virus (VHSV) (Novoa et al., 2006), and the single-stranded RNA (ssRNA) virus popularly known as spring viremia of carp virus (SVCV), was found to infect zebrafish larvae systemically (López-Muñoz et al., 2010; Varela et al., 2014). Furthermore, a group of researchers demonstrated large similarities of chromosome between mammalians and zebrafish when mapped 144 gene of zebrafish (Postlethwait et al., 1998). Researchers have used zebrafish to study a variety of human ailments, including bio-contamination, infectious diseases, diabetes, cancer, cardiovascular disease, and renal disease. This is due to the fact that zebrafish and humans share high physiological and morphological similarity in terms of their central nervous system, adipose tissue, kidneys, skeletal muscles, and white blood cells (T-lymphocytes, erythrocytes, and myeloid cells) (Kandasamy et al., 2022). It is reasonable to anticipate that more human viral disease models will manifest in zebrafish over the next few years given the potential of zebrafish for the study of viral diseases in humans. This will result in the advancement of research into new vaccines and antiviral agents with potential biomedical applications, as well as the comprehension of viral diseases and host-pathogen interactions will also get more prominent.
4. Myriad of scientific arenas embracing zebrafish model
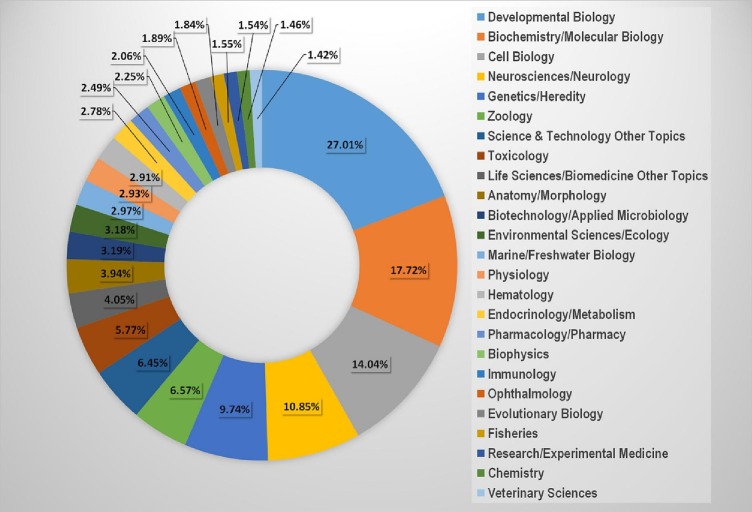
Early on, scientists understood the significance of zebrafish and a huge, thriving community of researchers employing zebrafish and other aquarium fish models because of the work done at 1970s by George Streisinger and fellow colleagues (Collodi, 2004). There are 101 different topic areas covered by zebrafish research. Developmental biology, biochemistry/molecular biology, cell biology, and neurology/neuroscience were the four subject categories with the highest percentages, according to a publication on zebrafish survey (2013). Anatomy, zoology, science technology, toxicology, genetics, life sciences and biomedicine were the next four most popular subject categories (Fig. 2 ) (Kinth et al., 2013).
Fig. 2.
Major research area where zebrafish are being used (Kinth et al., 2013).
4.1. Implementation in various scientific arenas
4.1.1. Developmental biology
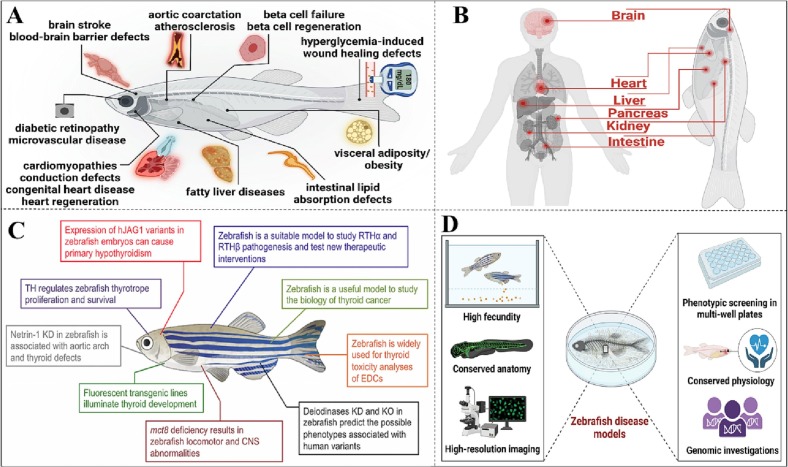
Zebrafish, having high genomic similarities with humans, are useful model to understand the pathways that control development and complex processes of development. It allows for the typical invertebrate genetic screening to be performed in a vertebrate model. The percentage of genetic similarity between humans and zebrafish is as high as 70 %. The first vertebrate to undergo ongoing genetic testing to detect developmental mutations (Driever et al., 1996). Numerous developmental genes have been found since the full-scale genetic screening (Amsterdam et al., 2004a; Golling et al., 2002; Haffter et al., 1996). Understanding how these genes are expressed and operate in space is being revealed using transgenic reporter lines, gene regulation, and precision gene editing. In addition to the desirable phenotypes, the model's genetic tractability is useful for studying developmental biology. In light of the zebrafish model's significance, the following systems and processes have been utilized to monitor their development: development of the brain, the enteric nervous system, the processes of angiogenesis, gastrulation, neurogenesis, neurulation, eye development, organogenesis, and regeneration (Fig. 3 ) (Khan and Alhewairini, 2019; Roper and Tanguay, 2018).
Fig. 3.
(A) Similarities in anatomy between higher vertebrates zebrafish could be potential model to investigate wide range of cardiovascular and metabolic diseases (Gut et al., 2017). (B) Corresponding organs in human and zebrafish. (C) Recent discoveries on thyroid hormone action and development that were acquired utilizing zebrafish as a model system shown schematically (Persani and Marelli, 2017) (KO: knock out, EDC: endocrine disruptor chemical, CNS: central nervous system, RTH: resistance to thyroid hormone, MCT8: monocarboxylate transporter 8 and KD: knock down). (D) The diagram demonstrates ways zebrafish are utilized for a range of genomic, physiological, image analysis, and small-molecule forms of assessment (Gut et al., 2017).
4.1.2. Cancer biology
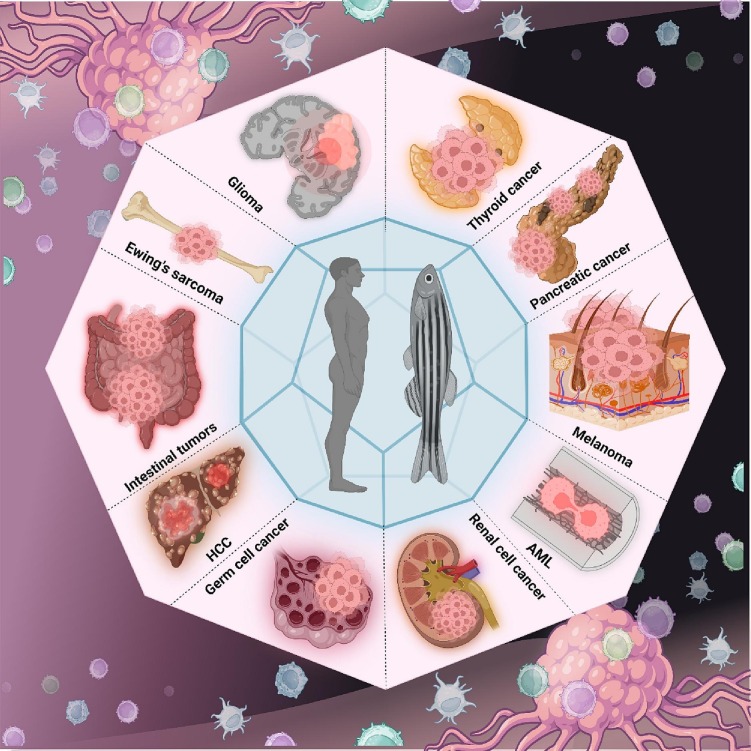
Cancer is a general word encompassing a variety of genetically heterogeneous ailments that have similar cellular and molecular characteristics. Considering that intratumor heterogeneity amplifies inter-individual variations, tumor diversity is regarded as one of the major complications in treating cancer patients. Clinically and pathologically every vertebrate species, including humans, fish, and other mammals, is affected by this dreadful ailment. Though humans and fish are not related by blood, the biochemistry of cancer is analogous in both groups, making the zebrafish an excellent model to research cancer (Fig. 4 ). Zebrafish are particularly well-suited to observe tumor formation, metastasis, and microenvironmental interactions because of their optical transparency, which has dramatically improved the capacity to see internal cell biology using fluorescent reporters. Chemical carcinogenesis, transgenic lines, mutant lines, and xenotransplantation are all methods for inducing cancer in fish (Table 1, Table 2 ). Skin, testicles, GI tract, liver, pancreas, muscles, and vasculature are some organs that could be used to develop tumor (Basten et al., 2013; Mizgireuv and Revskoy, 2006; Siew et al., 2006; Spitsbergen et al., 2000a, Spitsbergen et al., 2000b). stat3ia23, Bymb, mria32, Tg(tg:mCherry)ulb1, gria30, Tg(tg:nlsEGFP)ulb4, pigmentless Casper strain (Facchinello et al., 2017; Opitz et al., 2012; Peron et al., 2020; S et al., 2005; Shepard et al., 2005; White et al., 2008), kRASG12D solid tumor models, TEL-AML1 leukemia models, BRAFV600E melanoma models, Myc, kRASG12D embryonic rhabdomyosarcoma models and MYCN are few examples of zebrafish mutant lines and transgenic lines (Stoletov and Klemke, 2008). The most common technique for modeling cancer in zebrafish is via xenotransplanting human cancer cells. Additionally, fish are known to be susceptible to and spontaneously acquire cancer (White et al., 2013). Yolk, hindbrain, duct of cuvier and caudal vein are few most common sites for xenotransplantation for generating cancer model example breast (MDA-MB-231, MDA-MB435), prostate (PC3), Sarcoma (U20S, TC32), pancreatic (patient-derived pancreatic tumor tissue, PaTu, Panc-1), neuroblastoma (U87-L), melanoma (WM-266-4), jurkat, NB-4, colorectal (SW620), leukemia (Kt562, patient-derived leukemia samples) and ovarian (OVCA-433) (Veinotte et al., 2014).
Fig. 4.
Several cancers that have been studied using zebrafish model.
Table 1.
Various transgenic model of cancer using zebrafish (McConnell et al., 2020).
| Cancer type | Gene mutated | References |
|---|---|---|
| MPNST | brca2Q658X/Q658X; tp53M214K/+ | (Amsterdam et al., 2004b; Ki et al., 2017; Lee et al., 2016; Oppel et al., 2019; Shive et al., 2014; Zhang et al., 2013) |
| rp+/− (rps8a, rps15a, rpl7, rpl35, rpl36, rpl36a, rpl13, rpl23a, rps7, rps18, rps29) | ||
| rp+/− or p53M214K | ||
| sox10:mCherry); nf1a+/−; nf1b−/−; p53m/m | ||
| Tg(sox10:PDGFRA; sox10:mCherry); | ||
| Tg(mitfa:atg5K130R); p53M214K/+ | ||
| Tg(sox10:PDGFRAmut; | ||
| nf1a+/−; nf1b−/−; p53m/m | ||
| Thyroid cancer | Tg(tg:BRAFV600E-TOM) | (Anelli et al., 2017) |
| Ewing's sarcoma | Tg(β-actin:EWS-FLI1:IRES-GFP) +/− p53− | (Leacock et al., 2012; Park et al., 2016) |
| Tg(hsp70:EWS-FLI1) +/− p53− | ||
| Tg(hsp70:EWS-FLI1:IRES-GFP) +/− p53− | ||
| AML | Tg(spi1::loxP-EGFP-loxP::NUP98-HOXA9) | (Forrester et al., 2011; Kalev-Zylinska et al., 2002; Le et al., 2007; Lewis et al., 2006; Lu et al., 2016; Shi et al., 2014; Zhao et al., 2018; Zhuravleva et al., 2008) |
| IDH1-R132H, idh1−/− | ||
| Tg(ß-actin-LoxP-EGFP-LoxP-KRASG12D; hsp70-Cre) | ||
| stat5.1N646H or stat5.1H298R/N714F | ||
| irf8−/− | ||
| Tg(spi1:MYST3/NCOA2-EGFP) | ||
| RUNX1-CBF2T1 | ||
| Tg(spi1:FLT3-ITD-2A-EGFP/CG2) +/− spi1:NPM1-Mut-PA/CG2 | ||
| Germ cell | ns1402 | (Basten et al., 2013; Gill et al., 2010; Litchfield et al., 2016; Neumann et al., 2009; Shimizu and Matsuda, 2019) |
| Tg(flck:TAg) | ||
| dnaaf1hu255h | ||
| ENU mutagenesis | ||
| lrrc50Hu255h | ||
| Rhabdomyosarcoma | Tg(fli1:GFP2A-PAX3FOXO1) | (Ignatius et al., 2018; Kendall et al., 2018; Langenau et al., 2007; Le et al., 2007; Storer et al., 2013) |
| Tg(β-actin:LoxP-EGFP-LoxP-KRASG12D) | ||
| Tg(cdh15:KRASG12D) | ||
| Tg(mitfa:GFP2A-PAX3FOXO1) | ||
| Tg(rag2:KRASG12D) +/− p53− | ||
| Tg(ubi:GFP2A-PAX3FOXO1) | ||
| Tg(CMV:GFP2A-PAX3FOXO1) | ||
| Tg(mylz2:KRASG12D) | ||
| Glioma | Tg(gfap:Gal4VP16; UAS:mCherry-KRASG12V) | (Ju et al., 2015; Jung et al., 2013; M et al., 2017; Modzelewska et al., 2016) |
| Tg(zic4:Gal4TA4; UAS:mCherry; UAS:Xmrk) | ||
| Tg(zic4:Gal4TA4; UAS:mCherry; UAS:AKT-BFP) | ||
| Tg(sox10:mCherry-NRASwt) +/− p53M214K | ||
| Tg(ptf1:Gal4; UAS:GFP; UAS:DA-RAC1) | ||
| Tg(zic4:Gal4TA4; UAS:mCherry; UAS:YAPS5A) | ||
| Tg(sox10:mCherry-NRASS17N) +/− p53M214K | ||
| Tg(krt5:Gal4VP16; UAS:mCherry-KRASG12V) | ||
| Melanoma | Tg(kita:HRASG12V) | (Ceol et al., 2011; Dovey et al., 2009; McConnell et al., 2019; Patton et al., 2005; Santoriello et al., 2010) |
| Tg(mitfa:BRAFV600E); p53−/− | ||
| Tg(mitfa:EGFP:NRASQ61K); p53−/− | ||
| Tg(mitfa:NRASQ61R) | ||
| Tg(mitfa:BRAFV600E); p53−/−; mitfa−/− + MiniCoopR | ||
| CML | Tg(hsp70:p210BCR/ABL1) | (Xu et al., 2020) |
| Liver cancer | Tg(fabp10a:tTA; pT2-TRE-gankyrin-HcRed) | (Haramis et al., 2006; Huang et al., 2017; Li et al., 2013; Z. Li et al., 2012; Nguyen et al., 2016, Nguyen et al., 2011; Rekha et al., 2008; Wang et al., 2017) |
| Tg(fabp10:TA; TRE:Myc) | ||
| Tg(pLF2.8-HCV-core) | ||
| Tg(fabp10:LexPR; LexA:Cre; fabp10:loxP-mCherry-loxP-EGFP-KRASG12V) | ||
| Tg(fabp10:TA; TRE:xmrk) | ||
| apc+/− | ||
| Tg(fabp10:NRASQ61K) | ||
| Tg(fabp10:EGFP-KRASG12V) +/− p53M214K | ||
| Renal cell | vhl−/− | (Van Rooijen et al., 2009) |
| Neuroblastoma | Tg(dβh:ptpn11E69K-EGFP; dβh:EGFP-MYCN) | (He et al., 2016; Tao et al., 2017; Zhang et al., 2017; S. Zhu et al., 2012; Zhu et al., 2017; Zimmerman et al., 2018) |
| Tg(dβh:MYCN; dβh:EGFP) | ||
| Tg(dbh:Gab2wt; dbh:EGFP; dβh:EGFP-MYCN) | ||
| Tg(dβh:EGFP-MYCN) | ||
| Tg(dβh:MYC) | ||
| Tg(dβh:EGFP-MYCN); nf1a−/−; nf1b+/− | ||
| Tg(dβh:EGFP; dβh:ALKF1174L) | ||
| Tg(dβh:EGFP-MYCN; dβh:LMO1; dβh:mCherry) | ||
| Liposarcoma | Tg(krt4:Has.myrAkt1)cy18 | (Chu et al., 2012; Gutierrez et al., 2011b) |
| Tg(rag2:myr-mAkt2) +/− p53M214K | ||
| ALL | TEL-AML1 | (Arceci, 2008; Chen et al., 2007; Gutierrez et al., 2011a; Langenau et al., 2005; Langenau et al., 2003) |
| Tg(rag2:EGFP-mMyc) | ||
| Tg(rag2:cMyc) | ||
| Tg(rag2-ICN1-EGFP) | ||
| Tg(rag2:MYC-ER) | ||
| Pancreatic cancer | Tg(ubb:Lox-Nuc-mCherry-stop-Lox-GFP::KRASG12D) | (Hong et al., 2004; Oh and Park, 2019; Park et al., 2015, Park et al., 2008; Provost et al., 2014) |
| z-myod:MYCN, core-z-myod:MYCN | ||
| rpl36+/−; Tg(ptf1a:gal4VP16; UAS:GFP-KRASG12V) | ||
| Tg(ptf1a:eGFP-KRASG12V) | ||
| Tg(ptf1a:gal4VP16; UAS:GFP-KRASmut) |
Footnote: ALL-acute lymphoblastic leukemia; AML-acute myeloid leukemia; DA-dominant active; CML-chronic myeloid leukemia; MPNST-malignant peripheral nerve sheath tumor.
Table 2.
Various zebrafish cancer genetic model (Hason and Bartůnĕk, 2019).
| Cancer | Genotype | Zebrafish background | Reference |
|---|---|---|---|
| Testicular tumor | brca2Q658X | Wild type | (Shive et al., 2010) |
| RMS | rag2:dsRed2 | tp53M214K | (Langenau et al., 2008, Langenau et al., 2007) |
| rag2:KRASG12D | α-actin:GFP, Wild type | ||
| Intestinal tumors | + various Gal4 lines | cyp7a1−5 or wild type | (Enya et al., 2018) |
| fabp10a:mCherry | cyp7a1−5 or wild type | ||
| 5×UAS:EGFP-P2A-krasG12D | cyp7a1−5 or wild type | ||
| fabp10a:mCherry-P2A-cyp7a1 | cyp7a1−5 or wild type | ||
| MDS | tet2−/− | cd41:eGFP, cmyb:eGFP | (Gjini et al., 2015) |
| Melanoma | UAS:eGFP-HRASGV12 | tp53M214K, wild type | (Anelli et al., 2018; Kaufman et al., 2016; Lister et al., 2014; Patton et al., 2005; Santoriello et al., 2010, Santoriello et al., 2009) |
| BRAFV600Emitfavc7 | mitfavc7 | ||
| UAS:eGFP-HRASGV12 | – | ||
| UAS:eGFP-jmjd6 | tp53M214K, wild type | ||
| BRAFV600Etp53M214K | tp53M214K | ||
| hsp70I:GFP-HRASG12V | – | ||
| kita:Gal4TA, UAS:mCherry | tp53M214K, wild type | ||
| BRAFV600Etp53M214K | tp53M214K, crestin:EGFP | ||
| kita:GalTA4,UAS:mCherry | – | ||
| AML | pHsFLT3-ITD-T2a-eGFP | Wild type variant | (Dayyani et al., 2008; He et al., 2014; Yeh et al., 2008; Zhuravleva et al., 2008) |
| hsp70:AML1-ETO | Wild type variant | ||
| FLT3-ITD-T2a-mRFP | Wild type variant | ||
| spi1:MYST3/NCOA2-eGFP | – | ||
| pHsFLT3-WT-T2a-eGFP | Wild type variant | ||
| Thyroid cancer | tg:BRAFV600E-pA | Wild type variant | (Anelli et al., 2017) |
| tg:TdTomato-pA | Wild type variant | ||
| PNST | tp53M214K | tp53M214K, wild type variant | (Mensah et al., 2019; S et al., 2005) |
| tp53M214K | Wild type variant | ||
| brca2Q658X | tp53M214K, wild type variant | ||
| CML | spi1:tel-jak2a | Wild type variant | (Onnebo et al., 2012, Onnebo et al., 2005) |
| HCC | krt4:GFP | Wild type variant | (Chou et al., 2019; Li et al., 2019; Yang et al., 2019) |
| TRE:Myc | Wild type variant | ||
| fabp10:TA | Wild type variant | ||
| myl7:GFP | – | ||
| fabp10a: RPIA | – | ||
| fabp10:TA | Wild type variant | ||
| krt4:GFP | Wild type variant | ||
| TRE:xmrk | Wild type variant | ||
| TRE2:eGFP-krasG12V | lepr+/−, wild type variant | ||
| fabp10:rtTA2s-M2 | lepr+/−, wild type variant | ||
| T-ALL | spi1:tel-jak2a | Wild type variant | (Langenau et al., 2008, Langenau et al., 2005; Langenau et al., 2003; Onnebo et al., 2012) |
| rag2:GFP | Wild type variant | ||
| rag2:loxP-dsRED2-loxP-eGFP-mMyc | Wild type variant | ||
| rag2:dsRed2 | Wild type variant | ||
| rag2:mMyc | Wild type variant | ||
| Pancreatic cancer | ptf1a:CREERT2 | – | (Park and Leach, 2018; Park et al., 2008) |
| ubb:lox-Nuc-eCFP-stop-lox-GAL4-VP16 | – | ||
| ptf1a:eGFP-KRASG12V | Wild type variant | ||
| UAS:eGFP-KRASG12V | – |
Footnote: MDS-myelodysplastic syndrome, RMS-rhabdomyosarcoma, AML-acute lymphoid leukemia, PNST-peripheral nerve sheath tumor, CML-chronic myeloid leukemia, HCC-hepatocellular cancer, T-ALL-T-cell acute lymphoid leukemia.
4.1.3. Toxicology model
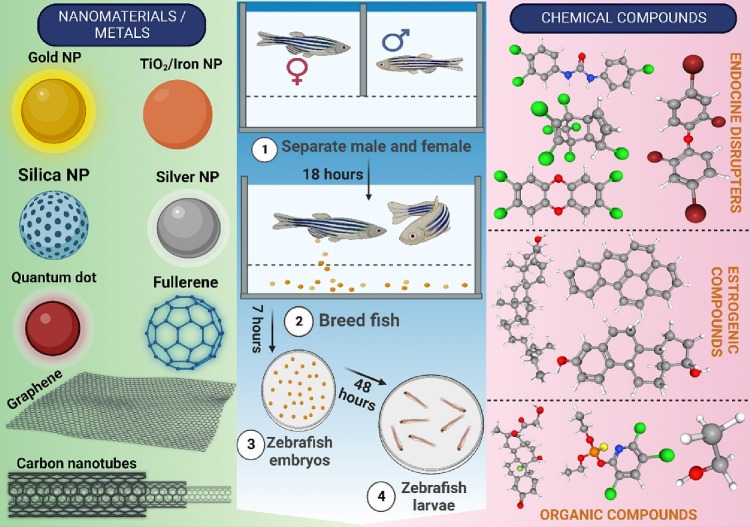
More than 200 different cell types work together in the body's intricate structure to create highly specialized organ systems, including the immune system. However, no in vitro or artificial model can precisely depict how a human internal system would react to the introduction of a medicine, chemical, nanomaterial, or any other foreign material because of the infinite number of biological processes that occur naturally in a human body. Zebrafish might therefore be used to investigate toxicity, evaluate the eco-environment, and assess various contaminants (i.e. Nano materials, toxic heavy metals, endocrine disruptors and organic pollutants) (Fig. 5 ) (Table 3 ) (García-Cambero et al., 2019; Khezri et al., 2017; Verma et al., 2021; Zhang et al., 2003).
Fig. 5.
Diagrammatic representation of different chemical compounds and metals/nanomaterials-induced toxicity profiling using the Danio rerio model (NP: nanoparticles).
Table 3.
Different types of metals and chemicals evaluated for toxicity in zebrafish.
| References | |
|---|---|
| Nanomaterial | |
| Silver (Ag) NP | (Asharani et al., 2011; Boran and Şaffak, 2020; Chao et al., 2021; Cheng and Cheng, 2012; Duan et al., 2013; Ghobadian et al., 2015; Gu et al., 2021; Ispas et al., 2009; King-Heiden et al., 2009; Lee et al., 2021; Madhubala et al., 2019; Patsiou et al., 2020; Shankar et al., 2021; Usenko et al., 2007; Van Aerle et al., 2013; Villacis et al., 2017; Wang et al., 2016; X. Zhu et al., 2012) |
| Gold (Au) NP | |
| Silicon dioxide (SiO2) NP | |
| Titanium dioxide (TiO2) NP | |
| Platinum (Pt) NP | |
| Nickel (Ni) NP | |
| Copper (Cu) NP | |
| Copper oxide (CuO) NP | |
| Nickel oxide (NiO) NP | |
| Zinc (Zn) NP | |
| Lead (Pb) NP | |
| Uranium (U) NP | |
| Magnesium (Mg) NP | |
| Magnesium oxide (MgO) NP | |
| Iron (Fe) NP | |
| αFe2O3 NP | |
| γ-Fe2O3 NP | |
| Al2O3 NP | |
| Fullerenes | |
| Carbon nanotubes | |
| Quantum dot (CdSe core/Zn shell) | |
| Estrogenics compounds | |
| Phytosterols | (Elfawy et al., 2021; Kazeto et al., 2004; Kishida et al., 2001; Nakari and Erkomaa, 2003; Weber et al., 2003; Willey and Krone, 2001) |
| Nonylphenol | |
| 17Alpha-ethinylestradiol | |
| Ethinylestradiol | |
| Benzo[a]pyrene | |
| 17-Beta estradiol | |
| Diethylstilbestrol | |
| Metals | |
| Cadmium | (Chan et al., 2006; Chan and Cheng, 2003; Dave, 1985; Dave and Xiu, 1991; Labrot et al., 1999; X. Li et al., 2012; Richetti et al., 2011; Samson et al., 2001; Wu et al., 2012) |
| Methylmercury | |
| Cobalt | |
| Nickel | |
| Lead | |
| Copper | |
| Iron | |
| Aluminum | |
| Uranium | |
| Zinc chloride | |
| Cadmium acetate | |
| Mercury chloride | |
| Lead acetate | |
| Arsenic | |
| Cd2+ | |
| Cu2+ | |
| Hg2+ | |
| Cd2+ | |
| Zn2+ | |
| Endocrine disruptors | |
| DE-71 | (Chen et al., 2012; Chow et al., 2013; Chunga et al., 2011; Heiden et al., 2008; Yu et al., 2010) |
| Bisphenol A | |
| BDE-47 | |
| Tetrabromobisphenol A | |
| Endosulfan | |
| TCDD | |
| Methoxychlor | |
| Heptachlor | |
| Triclocarban | |
| Organic compounds | |
| Dexamethasone | (Abnet et al., 1999; Collier et al., 2004; Fåhræus-Van Ree and Payne, 1997; Huang and Huang, 2012; Levin et al., 2003; Oulmi and Braunbeck, 1996; Todd and Van Leeuwen, 2002; Wiegand et al., 2001; Zhang et al., 2003) |
| Doxorubicin | |
| Epirubicin | |
| Flavopiridol | |
| Acetaminophen | |
| DCA | |
| Didemnin B | |
| Ethanol | |
| Fujisawa peptide | |
| 5-FU | |
| Vinblastine sulfate | |
| TCDD | |
| Trithiophene | |
| Naproxen | |
| Staurosporine | |
| SU5416 | |
| Ibuprofen | |
| Methotrexate | |
| Chlorpyrifos | |
| Methyl parathion | |
| PCBs | |
| PAHs | |
| Sevin | |
| Toxaphene | |
| 4-Chloroaniline | |
Footnote: Cd-cadmium, Cu-copper, Hg-mercury, Zn-zinc, DCA-dichloroacetic acid, 5-FU-5-fluorouracil, SU5416-, PCBs-polychlorinated biphenyls, DE-71-polybrominated diphenyl ethers, PAHs-polycyclic aromatic hydrocarbons, BDE-47-2,2,4,4-tetrabromodiphenyl ether, TCDD-2,3,7,8-tetrachlorodibenzo-p-dioxin.
Nevertheless, zebrafish have also been employed in several other types of toxicology studies, including those examining the effects of toxins on the reproductive system, nervous system, circulatory system, eyes, brain, and behavior, as well as cardiotoxic effects.
4.1.4. Human disease model
For the advancement of biomedical research, several animal species play crucial roles as experimental models. Research outcomes are reliable and consistent when using animal models. The fully sequenced genome, accessibility of genetic manipulation, quick growth, high fertility, and practically transparent embryo of the zebrafish have made it a popular animal model. As a result of these traits, it is a special model organism for the investigation of numerous biological and human ailments. Zebrafish are perfect subjects to study various human ailments such as dyslipidemia, hematopoietic disorder, type 2 diabetes mellitus, cardiovascular disorder (CVD), non-alcoholic fatty liver disease (NAFLD), atherosclerosis, kidney disorder, Obesity, and holoprosencephaly (HPE), as they have all the same major organs as humans (Gongal and Waskiewicz, 2008; Konantz et al., 2019; Morales and Wingert, 2017; Teame et al., 2019).
5. Zebrafish: a versatile infection paradigm to study host-pathogen interaction in vivo
Why zebrafish should be utilized as a model for infection in research? Developmental biologists have used this paradigm to bridge the gap between mammalian evolution and that of lesser living forms. Zebrafish are vertebrates, making them more similar to humans in terms of evolution than other models like fruit flies or nematodes. They are also simpler to work with and research than small animal models. Zebrafish are 21st century animal model (in-vivo) for a myriad of infections such as bacteria, viruses, fungus, protozoan, metazoan and parasites, due to advantageous traits such as ex utero, genetic accessibility, optical accessibility, whole sequence genome, and high genomic similarity with humans. Zebrafish infection models have real-time visualization and genetic screening over other vertebrate infection models like rats and mice. Forward genetic screening, which is only achievable in vertebrates, can be used in conjunction with reverse genetics.
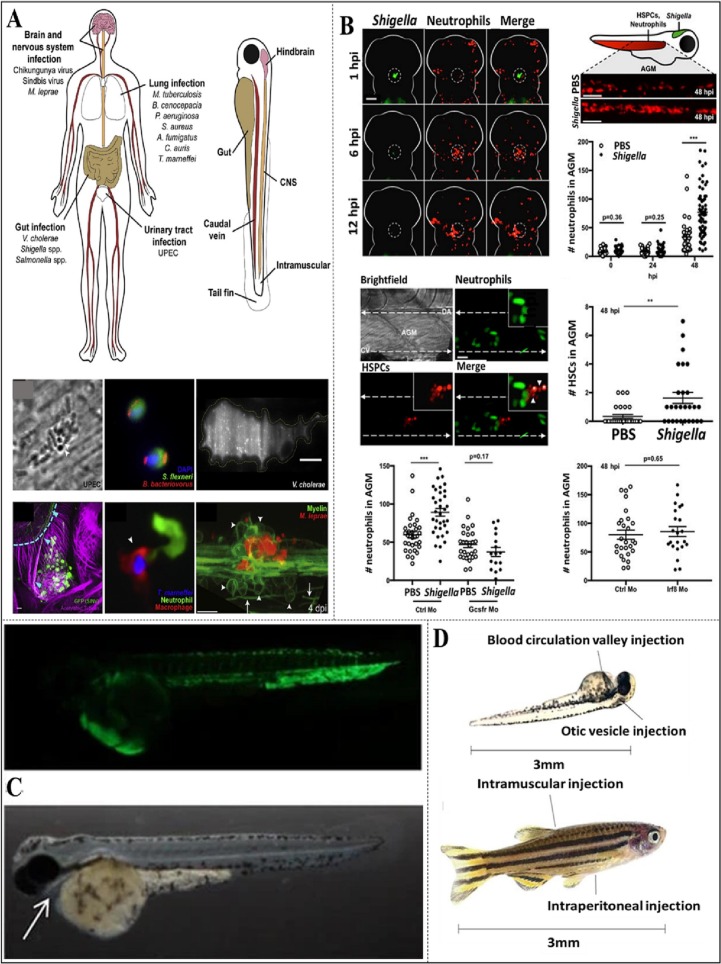
When conditions are natural, pathogens are thought to enter zebrafish through the gills, the digestive tract, or a damaged surface (O'Toole et al., 2004). However, parasites can occasionally act as an infection source. Co-incubation or incubation in medium containing the pathogen, is a method for experimentally infecting zebrafish with diverse pathogens (Chen et al., 2015; Laghi et al., 2022; Liew et al., 2017; Rendueles et al., 2012). Adult fish and its embryos have both been treated using this technique. However, experimental infections are often administered into sedated fish by injection (Akle et al., 2017; Benard et al., 2012). Both the embryonic and adult phases of development are appropriate for administering the injection (Fig. 6, Fig. 7 ). A 29-gauge needle is used together with a syringe to inject a bacterial solution into an adult fish after it has been given tricane (anesthesia) in order to make it unconscious (Phelps et al., 2009). The bacterial solution is then loaded in the embryo using a glass capillary under micromanipulator (Davis et al., 2002) or microscope (Fig. 6).
Fig. 6.
(A, left) Zebrafish are being used to study the brain, lungs, bladder, nerve system, and gut, which are the four main areas of the human body where infections occur. (A, right) Central nervous system, rear brain, tail (muscles, fin), and caudal vein are among the zebrafish injection sites that are often used. (A, bottom) represents bacterial injections in different parts of zebrafish: UroPathogenic Escherichia coli (UPEC) administered into the tail fin, Vibrio cholerae populating the gut, Sindbis virus in the central nervous system, Talaromyces marneffei conidia in the muscle tissue and Mycobacterium leprae in the CNS region (Gomes and Mostowy, 2020). (B) Zebrafish model of Shigella infection used to examine granulopoiesis (Willis et al., 2018) (AGM: aorta gonad mesonephros, HSPCs: hematopoietic stem and progenitor cells). (C) Fluorescence image of zebrafish inoculated with E. coli K46/pGEN-GFP strain (CK et al., 2017). (D) Various injection route for infecting streptococcal infection (blood circulation in larvae and intramuscular, intraperitoneal in adult) (Saralahti and Rämet, 2015).
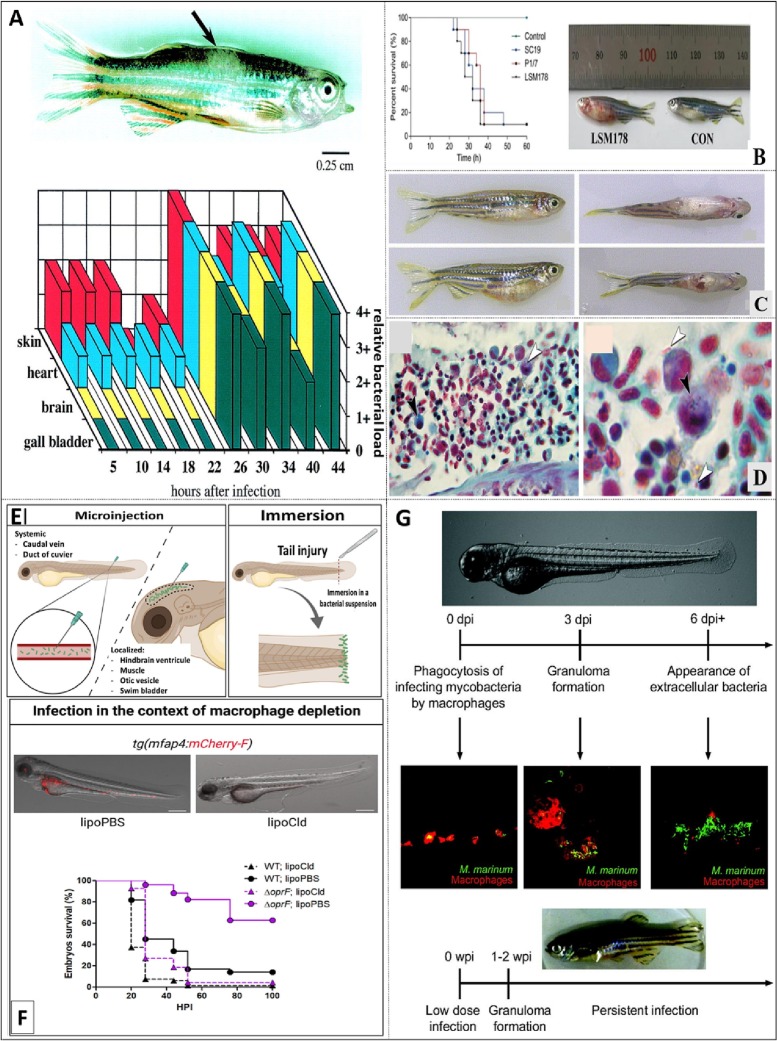
Fig. 7.
(A, top) Hypopigmented lesion at the intramuscular site of zebrafish caused due to S. iniae. (A, bottom) Relative bacterial load of S. iniae in various organ of zebrafish (Neely et al., 2002). (B) Survival rate of fish infected with Streptococcus suis LSM178, SC19 and P1/7 strain (Y. Hu et al., 2021). (C) Infection of zebrafish with E. tarda (Pressley et al., 2005). (D) Histopathology investigation (Brown and Brenn Gram staining) of adult zebrafish infected with E. tarda (Pressley et al., 2005). (E) Various injection techniques to introduce Pseudomonas aeruginosa in the larvae of zebrafish (Pont and Blanc-Potard, 2021). (F) Confocal imaging representing fluorescent labelled macrophages, P. aeruginosa (Pont and Blanc-Potard, 2021). (G) Progression of infection of Mycobacterium marinum in larvae and adult zebrafish (Cronan and Tobin, 2014).
Several different infections that are known to impact fish and mammals have previously been exploited, despite the fact that the zebrafish infection model is still relatively young. The benefit of a naturally occurring fish pathogen is that it has evolved with its host, leading to a dynamic interplay. The temperature of incubation may be a challenge for the utilization of mammalian pathogens. In order to preserve viability, zebrafish are typically kept between 26 °C and 29 °C. Mammalian pathogens, on the other hand, are adapted to their host's temperature (27 °C), and many of them, such as Salmonella arizonae and S. typhimurium, only express virulence factors at this temperature.
5.1. Bacteria
A wide range of human ailments causative bacteria, including Listeria, Flavobacterium, Aeromonas, Salmonella, Pseudomonas, Streptococci, Listonella Burkholderia, Edwardsiella, Shigella, Staphylococcus, and Mycobacteria, have been established as zebrafish infection models (Fig. 6, Fig. 7).
5.1.1. Edwardsiella tarda
One day post fertilized zebrafish embryos were susceptible to Edwardsiella tarda infection using static immersion, showing an average mortality of 31 %, when compared to control fish 11 % (Pressley et al., 2005). Adult zebrafish treated to the bacteria by static immersion after being scraped revealed a death rate between 45 and 95 %, depending on the infection dose (Pressley et al., 2005). Adults had a mortality rate of between 30 and 60 % when infected by intraperitoneal injection. They even displayed lesions, perianal edemas, and abdominal distention at the site of injection (Pressley et al., 2005). Infected embryos' histopathological analyses revealed the presence of bacteria along with significant nerve tissue deterioration, abundant black cell nuclei in the retina and brain, indication of cell death by necrosis or apoptosis, and disorganized fin axis (Pressley et al., 2005). Adults fishes injected with bacteria showed deteriorated secondary oocytes and ruptured yolk sacs, according to further investigation of their ovaries (Pressley et al., 2005). Exposed fishes showed high level of TNFα and IL-1β mRNA level through cytokine analysis (Pressley et al., 2005).
5.1.2. Streptococcus
5.1.2.1. Streptococcus iniae
Meningoencephalitis is the predominant symptom of a systemic, invasive sickness caused by the naturally occurring bacterial fish pathogen streptococcus iniae in fish. Streptococcus iniae infections in fish are similar to those caused by many streptococcal species in humans. S. iniae has a relatively low LD50 (lethal dosage) for adult zebrafish following intraperitoneal injection (103) (Neely et al., 2002). After 26 h post-infection, bacteria spread widely throughout the body and typically result in death within a few days. However, S. pyogenes, a human pathogen, has a comparable LD50 in zebrafish, demonstrating that fish are also susceptible to the virulence of these bacteria (Neely et al., 2002). Additionally investigated in adult zebrafish were two S. pyogenes mutants that showed signs of attenuation in a mouse infection model. In contrast to one of these mutants with a damaged RopB regulatory gene, the glutathione peroxidase mutant did not show decreased pathogenicity (Brenot et al., 2004; Neely et al., 2002). This finding suggests that while the zebrafish infected model may be utilized to identify virulence mutations, the outcomes need to be confirmed in other infection models. Additionally, two other potential virulence factors for S. pyogenes—ATP-binding cassette transporter and peptidoglycan-modifying enzyme—were discovered using zebrafish (Neely et al., 2002).
5.1.2.2. Streptococcus agalactiae
The gram-positive bacterium “Streptococcus agalactiae” (also known as GBS: group B streptococcus) infects a wide range of species, including both aquatic and terrestrial organisms (Delannoy et al., 2013). The primary source of infection in the newborn is due to GBS (Le Doare and Heath, 2013). Some of the typical clinical effects of a GBS infection include pneumonia, sepsis, and meningitis (Le Doare and Heath, 2013). A systemic GBS infection model using zebrafish has recently been established (Kim et al., 2015; Patterson et al., 2012). Researchers found that adult zebrafish exposed to intraperitoneal (IP) and intramuscular (IM) injections of the LD50 levels of 102 and 104 cfu, respectively, were susceptible to GBS infection (Patterson et al., 2012). The multiplication of bacteria in the blood, a high death rate, and meningoencephalitis symptoms have all been linked to intraperitoneal injections of bacteria. When the brain's bacterial burden was examined, it became clear that the bacteria had breached the blood-brain barrier (BBB) and was responsible for the model's meningitis. The GBS infection also stimulated the host's inflammatory response in adult fish, as has been observed in mice and people, as evidenced by elevation in the proinflammatory cytokines IL-1β and IL-6 (Patterson et al., 2012).
5.1.2.3. Streptococcus pneumonia
Streptococcus pneumonia (pneumococcus), a member of the Streptococcus genus, is frequently linked to adverse clinical outcomes and an invasive illness in humans (Krzyściak et al., 2013). Although pneumococcal vaccinations have improved, this pathogen still accounts for most infectious disease-related fatalities. Zebrafish larval and adult models for pneumococcal infection have been developed to explore the condition more thoroughly (Rounioja et al., 2012; Saralahti et al., 2014). The introduction of pneumococci into the bloodstream of zebrafish larvae at 2dpf demonstrated their susceptibility to pneumococcal infection, this resulted in a dose-dependent infection that was fatal within 48 h (Rounioja et al., 2012). Pneumococcus was able to multiply in this host as evidenced by the rising bacterial counts in the infected larvae, and the high bacterial levels were linked to the larvae's demise. Studies using the zebrafish embryo model for pneumococcal infection also applicable to adult zebrafish (Saralahti et al., 2014). Pneumococcus appears to be less virulent than other streptococci in adult zebrafish since an intraperitoneal infection with just 2.5 × 106 cfu was enough to kill half the people (Saralahti et al., 2014). Pneumolysin, autolysin, and pilus defective mutants infected adult fish led to a comparatively mild illness. The wild-type pneumococcus, on the other hand, induced a severe systemic infection that spread to the fish's brain when it was injected intraperitoneally into adult fish (Saralahti et al., 2014). This paradigm has a considerable advantage over the larval one because adult zebrafish exhibit both innate and adaptive responses. However, investigating the role of the adaptive immunity in a pneumococcal infection utilizing mutant zebrafish line Rag1hu1999, which is devoid of activated lymphocytes, revealed that the adaptive immune system plays no part in a severe pneumococcal infection in adult zebrafish (Saralahti et al., 2014).
5.1.2.4. Streptococcus pyogenes
S. pyogenes (also known as GAS: group A streptococcus), which causes over than 700 million infections yearly, is the most common source of human illness (Krzyściak et al., 2013). Although S. pyogenes generally does not infect fish, it has been shown that it may infect adult zebrafish via intramuscular (IM) and intraperitoneal (IP) injections (Neely et al., 2002). S. pyogenes when injected intramuscularly, the outcome closely resembles human necrotizing fasciitis because to its constrained nature and severe muscle tissue destruction at the site of infection (Miller, 2004; Phelps and Neely, 2007). Demise of zebrafish has been observed in 36–48 h after being intramuscularly injected with S. pyogenes at a dose equal to its lethal dose (LD50) (3 × 104), which causes the fish to acquire broad hypopigmented lesions where the injection was performed (Neely et al., 2002). According to reports, the dorsal muscle is the only location where S. pyogenes infection persists in adult zebrafish, which is aligned with results from murine models of infections (Miller, 2004). In a study by Montanez et al. (Montañez et al., 2005), the adult zebrafish model was used to explore the role of iron absorption channels, which are surprisingly preserved across various species of streptococcal. Researchers used S. pyogenes mutant with an inactive Siu transporter gene to demonstrate the significance of iron uptake for the pathogenicity of S. pyogenes (Montañez et al., 2005). Another study by Brenot et al. (2004) using a zebrafish model, Brenot et al. and his group indicated that the inflammatory response is required for S. pyogenes to survive in the host in order for it to produce the enzyme glutathione peroxidase.
5.1.3. Mycobacteria
Numerous mycobacterial pathogens, including Mycobacterium fortuitum, Mycobacterium chelonae, and Mycobacterium abscessus, can infect zebrafish (Astrofsky et al., 2000). Furthermore, M. marinum, a closely related species of M. tuberculosis, is the most potent agent of fish tuberculosis (Stinear et al., 2008). M. marinum can sporadically infect humans and spreads through water, although most often, the illness just affects the skin (Linell and Norden, 1954). Compared to M. tuberculosis, M. marinum is less dangerous to handle and replicates more quickly. Since M. marinum infection in zebrafish serves as a model for TB, these facts have motivated a number of researchers to explore it (Decostere et al., 2004; Tobin and Ramakrishnan, 2008). M. marinum can live and proliferate in adult zebrafish, as predicted, and depending on the inoculum employed, can cause an acute or chronic illness. Zebrafish are a beneficial choice for M. marinum's host organism in a laboratory environment for a number of reasons: Zebrafish embryos and adults may both be infected using the multiple infection approach (Benard et al., 2012). Due to the embryos' transparency, advanced in vivo real-time imaging methods, such as the use of many leukocyte and macrophage fluorescent reporter lines, are possible (Renshaw et al., 2006; Wittamer et al., 2011). All the embryonic defenses in zebrafish are innate. Post one or two day fertilization appearance of functional neutrophils and macrophages occurs, whereas, Lymphocyte development begins four days after conception, and adaptive immunity is fully developed four weeks after fertilization (Langenau et al., 2004). Based on genetic fingerprinting, there are two groups of M. marinum strains, and the group of M. marinum that encompasses the most lethal strains simultaneously holds the bulk of the mycobacteria found in individuals with M. marinum-caused skin infections (Van Der Sar et al., 2004), However, the zebrafish embryo model has shown the most intriguing results with zebrafish M. marinum infections. According to research by Davis et al. (Davis et al., 2002), M. marinum infected embryonic macrophages extravasate and begin to congregate. This results in the activation of certain genes that are exclusively activated in the granuloma's confined environment and not in isolated infected macrophages.
5.1.4. Shigella
A clinical process known as shigellosis is caused by the human pathovar of E. coli, Shigella flexneri, which causes bacillary dysentery and gastroenteritis. Our capacity to comprehend the Shigella infection process in vivo has, as of now, been constrained. Shigellosis in humans cannot be accurately simulated by any non-primate animal model. Animal model such as rabbit (Schnupf and Sansonetti, 2012), mouse model (Li et al., 2017) and guinea pig (Shim et al., 2007) are few animals where the numerous researches have been carried out. Utilizing Danio rerio larvae to research Shigella infection has several benefits. When injected into zebrafish, Shigella has the potential to infiltrate epithelial cells and result in symptoms similar to those of human Shigellosis, including inflammation and macrophage cell death (Mazon-Moya et al., 2017; Mostowy et al., 2013). Zebrafish study has demonstrated that the type-3 secretion system (T3SS), a crucial component in the progression of illness in humans, is absolutely necessary for Shigella absorption into nonphagocytic cells (Cossart and Sansonetti, 2004). Additionally, studies have shown that Shigella can escape to the cytoplasm and be picked up by the autophagy process (Mostowy et al., 2013, Mostowy et al., 2010). Study by Cossart et al. revealed that actin and septins are attracted to the phagocytic cup during the Shigella infection, where they form rings around the invasive bacteria (Mostowy et al., 2009). Although Depletion of SEPT2 by small interfering (si)RNA greatly decreases Shigella access into host cells, despite the fact that the specific function of septins during bacterial entry is unclear (Mostowy et al., 2009). Septin caging, which is the process of recruiting septins to actin-polymerizing bacteria after bacterial escape from the phagosome to the cytosol, creates cage-like structures surrounding Shigella that prevent cell-to-cell dissemination (Mostowy et al., 2010). SEPT2, SEPT9, or nonmuscle myosin II depletion decreases septin caging and increases the proportion of bacteria with actin tails, whereas boosting SEPT2-nonmuscle myosin II interactions with tumor necrosis factor promotes septin caging and reduces the occurrence of actin tails in bacteria (Mostowy et al., 2010). Significantly, results from studies of septin cages in zebrafish cells both in vivo and in vitro support their role as an evolutionarily conserved host defense component (Mostowy et al., 2013).
5.1.5. Salmonella
The most widespread pathogen that affects several host species is Salmonella enterica serovar Typhimurium, which causes gastroenteritis in humans and other animals and symptoms that resemble fever in mice (Thiennimitr et al., 2012). The majority of studies on bacterial virulence have used traditional animal models including mice, primates, and pigs (Verma and Srikanth, 2015). Nevertheless, the utilization of mammal hosts is constrained by the limited real-time analytic options and the demands for intrusive sample collection. The development of vertebrates has been widely studied using zebrafish as a model in biological research. When S. Typhimurium is injected into zebrafish, a typhoid-like sickness similar to that caused by S. Typhimurium in rodents and S. enterica serovar Typhi in humans is induced. According to a research, guanylate-binding protein 4 (Gbp4) activates the neutrophil inflammasome, which is essential for the removal of S. Typhimurium (Tyrkalska et al., 2016). Leukotriene B4 activation, an inflammatory lipid mediator, is observed to cause neutrophil chemotaxis, which results in neutrophil recruitment. Salmonella infection is eradicated as a result of the recruited neutrophils engulfing the pathogen and activating the Gbp4 inflammasome. These results highlight the critical role neutrophils play in mediating this response in vivo. S. Typhimurium strains with poor translation fidelity are rendered ineffective in vivo by the wild type strain and attract fewer neutrophils after colonization via the gastrointestinal route (Fan et al., 2019). Zebrafish larvae exposed to S. Typhimurium develop intestinal and cloacal inflammation, according to a research by Howlader et al. (2016) and Varas et al. (2017). It has been demonstrated that hematopoietic stem and progenitor cell (HSPC) proliferation during infection is a direct result of enhanced neutrophil production using a Salmonella-zebrafish infection model (Hall et al., 2012). Salmonella infection of zebrafish has contributed significantly to the development of novel concepts in immunometabolism, emergency granulopoiesis, and cell-autonomous immunity.
5.1.6. Pseudomonas
It has been suggested that zebrafish embryos are an effective model for studying Pseudomonas aeruginosa infection (Brannon et al., 2009; Clatworthy et al., 2009; Llamas et al., 2009). Patients with cystic fibrosis (CF) frequently have lung infections caused by the opportunistic bacteria P. aeruginosa. Embryos that are one or two days post-fertilization are often used to inject P. aeruginosa bacteria into the caudal vein to cause an infection. Pseudomonas is relatively resistant to the zebrafish, and a substantial inoculum is needed to establish infection and cause host death (Brannon et al., 2009; Clatworthy et al., 2009). Researchers may evaluate the directed migration of neutrophils and macrophages towards specific infections by administering bacteria into the hindbrain, ventricle, tail muscle and otic vesicle. The notochord is also injected with bacteria to prevent the infection from reaching the phagocytic cells (Alibaud et al., 2011). Bacteria supplied systemically can be quickly engulfed and killed by neutrophils and macrophages. P. aeruginosa produces elastase, phospholipase C, and exotoxin A as a few type III secretion systems as a virulence strategy to oppose host defense (Cao et al., 2001; Yahr and Wolfgang, 2006). An investigation using P. aeruginosa mutant T3SS revealed T3SS mutant being virulent or suppressed in host with or without phagocytes depletion. This demonstrates how interactions between phagocytes and T3SS contribute to Pseudomonas pathogenicity in immunocompetent hosts. Additionally, P. aeruginosa T3SS mutant are fatal at early stages when only macrophages have begun to form, but are diminished in the subsequent stages when both neutrophils and macrophages are involved (Clatworthy et al., 2009). When compared to healthy embryos, increased bacterial multiplication in the embryos is associated with slower blood flow, a slower heartbeat, and significant tissue damage (in the tail and brain area).
5.1.7. Escherichia
Enterohemorrhagic Escherichia coli (EHEC), an enteric pathogen that colonizes in the gut and causes bloody diarrhea in humans, has been effectively mimicked in zebrafish (Danio rerio). Following foodborne delivery by Paramecium caudatum, Stones et al. and his colleagues discovered that EHEC colonizes the middle intestine of zebrafish larvae, and the survival rate drops by 40 % by 4 days post infection (Stones et al., 2017). Both the colonization of the gut by EHEC in humans and cattle, as well as the emergence of adhering and effacing lesions on enterocytes, are regulated by a set of virulence genes produced by the locus of enterocyte effacement (LEE) (Elliott et al., 2000; Phillips et al., 2000). In initial gastrointestinal tract colonization in larval zebrafish, EHEC promotes the LEE, and LEE induction is necessary for successful colonization and pathogenicity. Pathogenic E. coli is not the only bacteria that colonizes the zebrafish intestine; nevertheless, amicable E. coli from healthy human gut have been utilized to colonize zebrafish and can inhibit V. cholera colonization by reducing intestinal pH due to glucose metabolism (Nag et al., 2018).
5.1.8. Burkholderia
A common opportunistic pathogen of individuals suffering from cystic fibrosis and chronic granulomatous disease, Burkholderia cenocepacia complex (BCC) bacteria may also be detected in the rhizosphere of plants (Drevinek and Mahenthiralingam, 2010; Mahenthiralingam et al., 2008). The 17 types of BCC bacteria have all been identified in CF patients (Holden et al., 2009), Burkholderia cenocepacia is a significant health concern for CF sufferers, just like Pseudomonas aeruginosa. The main colonization of Burkholderia cenocepacia causes progressive lung function loss, septicemia (also known as cepacia syndrome), deadly necrotizing pneumonia, abscesses and bacteremia (Saiman and Siegel, 2004). Even though BCC has a number of virulence factors, including siderophores, type 3 secretion, flagella, quorum sensing, superoxide dismutase, cable pili, and protease (Mahenthiralingam et al., 2005), the disease's molecular causes are still mostly unclear. To investigate zebrafish infection with Burkholderia cenocepacia has been used as a model to study Burkholderia pathogenesis in vivo (Vergunst et al., 2010). Zebrafish larvae have been shown to be very susceptible to the B. cenocepacia ET12 strain, despite other, less virulent members of the B. cepacia complex, such as (B. vietnamiensis and B. stabilis). In a zebrafish infection model, it has recently been demonstrated that macrophages are necessary for the replication of B. cenocepacia (Vergunst et al., 2010). This is supported by the finding that bacterial replication is limited when macrophages are reduced in larvae (Mesureur et al., 2017). IL-1beta is produced by macrophages during infection, which attracts uninfected cells to create cellular aggregates (Mesureur et al., 2017; Vergunst et al., 2010). It has been observed that Burkholderia administered into zebrafish interacts with neutrophils and macrophages, and that macrophages are essential for survivability and multiplication of B. cenocepacia (Mesureur et al., 2017; Vergunst et al., 2010). Neutrophils, in contrast, have no impact on bacterial replication or the course of a disease. A critical function of macrophages in Burkholderia infection was evidenced by the chemical suppression of macrophages, which greatly decreased bacterial growth and the host's vulnerability to infection (Mesureur et al., 2017). Burkholderia intramacrophage multiplication is necessary for the transition of a chronic condition into an acute one that is characterized by inflammation and zebrafish death. The fact that macrophages are a substantial contributor of IL-1 during Burkholderia infection gives credibility to this. Although decrease of IL-1 is probacterial and anakinra's regulation of IL-1 signaling is antibacterial, it is yet unknown how exactly inflammation affects Burkholderia susceptibility or resistance. To determine the specific involvement of inflammation in Burkholderia pathogenesis in the in vivo system, further zebrafish model application would be crucial.
5.1.9. Vibrio
Researchers now have a more complete grasp of the Vibrio infection process owing to the zebrafish model. Bacteria including V. vulnificus, V. alginolyticus, V. cholera, V. parahaemolyticus, and V. anguillarum have been the subject of important scientific discoveries.
5.1.9.1. Vibrio anguillarum and Vibrio alginolyticus
Hemorrhagic septicemia, often known as vibriosis, is a serious disease that affects a wide variety of fish species. Vibrio anguillarum is the primary cause of this disease. Through facilitating chemotactic motility, the flagellum contributes to the pathogenicity of water-borne V. anguillarum. However, after the pathogen is injected beyond the fish epithelial layer, the flagellum and chemotaxis are no longer necessary (O'Toole et al., 1996; Ormonde et al., 2000). According to research by Randazzo et al., zebrafish exhibit substantial histopathological alterations in the gut and renal hematopoietic tissue within 48 h after receiving an intraperitoneal injection of V. anguillarum serotype O1. Within 7 days, the fish perished (Randazzo et al., 2015). Zebrafish were used by Zhang et al. to simulate vaccination effectiveness against V. anguillarum. On day 28, following immunization, wild-type pathogenic V. anguillarum (1 × 106 cfu/ml) were exposed to adult zebrafish by bath inoculation. When live attenuated V. anguillarum with 1 × 108 cfu/ml used in the vaccination, it offered around 90 % of high relative protection (Zhang et al., 2012).
Waterborne bacteria like Vibrio alginolyticus can affect a wide variety of creatures, including humans and fish. Additionally, zebrafish have been employed as an infection model system for this bacterium. Another research used a zebrafish model to demonstrate the innate antibacterial action of the antimicrobial protein hepcidin (conserved in humans) against V. alginolyticus (Jiang et al., 2017).
5.1.9.2. Vibrio cholera
Circumventing laborious and expensive infection processes required for other animal models, V. cholerae inoculum can be pipetted direct into beakers containing four or five zebrafish to infect them, or zebrafish can be infected via immersion (Mitchell and Withey, 2018). Following the addition of the inoculum, the zebrafish devour the bacteria orally. Similar to how the human infection cycle works, infected fish can transfer the bacterium to other fish through the fecal-oral channel. The fish subsequently display the fluid, mucus-filled diarrhea that is typical of human sickness (Mitchell and Withey, 2018; Runft et al., 2014). This allows researchers to directly compare this infection's mechanism of transmission to human routes of transmission. This infection route bypasses the neutralization of stomach acid, sedation, and antibiotics, modifies the physiological environment through a pH shift, which can have an impact on the dynamics of the infection, effects on intestinal motility, or a deficiency in intact microbiota.
5.1.9.3. Vibrio parahaemolyticus
Naturally occurring as well as clinically obtained strains of V. parahaemolyticus may infect experimental zebrafish intraperitoneally (i.p.) in a dose-dependent form. The tdh + trh mutant clinical strain with lethal dose (LD50) of 5.7 × 105 cfu was intraperitoneally injected into zebrafish were fatal (Paranjpye et al., 2013). Regardless of whether the recognized hemolysins TDH and TRH were present or not, differences in zebrafish survival following infection were seen. This model may also be helpful in determining whether additional, unidentified virulence factors are present in V. parahaemolyticus strains and/or whether they contribute to the pathogenicity of those strains. Since abnormalities in erythrocytes were noticed in various strains of V. parahaemolyticus independent of the status of TDH and TRH, this model may be utilized to find the unknown variables that impact erythrocytes. The zebrafish paradigm is helpful for illustrating variations in the mortality of fish afflicted with an isogenic mutant or wild-type strain. Additionally, It also serves as a successful platform to show the pathogenicity between wild-type and isogenic mutants (Frischkorn et al., 2013).
5.1.9.4. Vibrio vulnificus
Zebrafish studies enable researchers to comprehend how infectious the bacteria are when the infected zebrafish are fed with or without protecting or stimulating substances, making them a useful tool for comprehending the V. vulnificus infection. An investigation by a scientist and his colleague demonstrated the antimicrobial peptide epinecidin-1 from grouper (Epinephelus coioides) had a protective impact on acute V. vulnificus infection in zebrafish. Epinecidin-1 and zebrafish infection therapy were chosen for intraperitoneal injection. In contrast to treatment with epinecidin-1, which had poor survival rate scores of 57 % and 60 %, respectively, zebrafish co-treated with epinecidin-1 and V. vulnificus showed survival rates score of 78 % to 97 %. Furthermore, survival rates of zebrafish after co-injection of epinecidin-1 and V. vulnificus ranged from 22 % to 47 %. The researchers demonstrated that epinecidin-1 altered the production of immune-responsive genes such as interferon γ, IL-10, TNF-α, and IL-1β, which abetted defense (Pan et al., 2011). In another research, V. vulnificus was administered intraperitoneally to two mutant zebrafish strains generated employing the Tol2 system. Fadsd6 and Elvol5a are two highly expressed Atlantic salmon enzymes present in this this strain which catalyzes the formation of docosahexaenoic acid and eicosapentaenoic acid, respectively. When compared to wild-type (WT) fish, these strains produced 2.5 times as many n-3 polyunsaturated fatty acids (PUFAs). The transgenic fish had a survival rate of 70 % compared to 20 % in WT fish 24 h after being challenged with V. vulnificus. Around 9 and 12 h after exposure, the production of proinflammatory genes including NF-κB, IL-1β, and TNF-α was decreased, which improved the transgenic fish's survival (Cheng et al., 2015).
5.1.10. Yersinia
Freshwater bacteria called Y. ruckeri affect salmonids (Kumar et al., 2015). Fish are frequently immunized against it, although there is opportunity for improvement in terms of management practices or vaccinations. Zebrafish have been used in a few investigations using Y. ruckeri. A GFP-tagged strain of Y. ruckeri was used to create an inactivated vaccine, and transparent zebrafish of various life stages were used to observe antigen absorption after bath immunization (Korbut et al., 2016). Researchers have also looked into the biology of Y. ruckeri infection, and they discovered that the toxic effector antifeeding prophage 18 of the translocation machinery of prophage tail-like proteins interferes with the behavior of blastomere cells in zebrafish embryos (Jank et al., 2015).
5.1.11. Listeria
Food-borne bacterium called L. monocytogenes may infect people and leave them with a variety of symptoms, from fever to septicemia, which can be fatal. To spread to internal organs like the liver and spleen, L. monocytogenes can breach the human gut epithelium. The L. monocytogenes model was initially utilized in investigations on the adaptive immune system's reaction to facultative intracellular infections (Pamer, 2004). Significant bacterial virulence factors including actA and listeriolysin (Gaillard et al., 1987; Kocks et al., 1992) have been discovered as a result of significant research into the virulence mechanisms of L. monocytogenes. Which is involved in actin-based intracellular mobility and cell-to-cell adherence of bacteria, as well as their involvement in vacuolar escape. According to a study provided by the researcher, L. welshimeri isolates had the highest percentage of zebrafish larval survivorship (83.0 %), preceded by L. innocua isolates, and L. monocytogenes had the least percentage (46.5 %). Selected virulence genes, the majority of which were present in L. monocytogenes, were detected using multiplex PCRs (iap, sigB, plcB, actA, prfA, hlyA, luxS, actA2, inlB, and rrn) (Zakrzewski et al., 2020). Additionally, 2 proteins 1) internalin (InlA) 2) InlB expressed in L. monocytogenes facilitating adhesion and invasion of non-phagocytic cells. The functions of both proteins are: Internalin (InlA) stimulates entrance into human epithelial cells in culture after its interaction with E-cadherin (Mengaud et al., 1996), whereas InlB promotes entry into many other cell types when Met is engaged (Shen et al., 2000). According to a study by Ying Shan et al., zebrafish are vulnerable and may be employed in a variety of ways to create microscopic infection models for L. monocytogenes, making them useful for future study on the interaction between bacterial pathogenicity and host immune mechanisms (Shan et al., 2015). The author demonstrated Listeria monocytogenes infection models in zebrafish using injection via the blood island, brain ventricle, and yolk sac and oral ingestion. Even when exposed to egg water containing 1010 cfu/ml of L. monocytogenes EGDe strain, zebrafish larvae were not killed, but frozen sections of the animals showed GFP-expressing bacteria in the gut lumen. Several genes that are involved in the development of the innate immune system, such as irg1l, mmp9, cyp1a, and il1b, were drastically expressed when the strains M7, innocua, and EGDe were administered orally to zebrafish (Shan et al., 2015). The major virulence factors of Listeria are Lysteriolisin O (LLO) and Act A, which aid in the creation of pores, vacuolar escape, and actin tail polymerization to weaken cellular protection. Zebrafish injections have demonstrated that blood-borne Listeria is quickly absorbed by neutrophils and macrophages (Levraud et al., 2009). ActA-dependent actin tail generation and LLO-dependent vacuole escape could both be exhibited in zebrafish, where both LLO and ActA mutations are repressed.
6. Paucity of knowledge towards viral defilement
The zebrafish has acted as a scientific model on the genetics and development of vertebrates' research. Adult zebrafish have typically only been retained as breeding stock to create embryos for scientific study as most studies are carried out within the first few days of fertilization. To abet the investigation of cancer, immunology, infection, and hematopoiesis, scientists have recently created immunocompromised zebrafish. Compared to wild-type fish, immune-compromised zebrafish are presumably more prone to infectious viruses. Increased susceptibility is demonstrated by an increase in the mortality rate, morbidity frequency, number of clinical manifestations, and severity of histopathologic lesions. Therefore, the variability in results caused by confounding factors like inflammation and other host reactions to infection will be considerably reduced by utilizing virus-free fish. Additionally, it will also lessen the number of fish required in experimental models that use zebrafish that have compromised immune system in order to attain enough statistical power. The truth that chronic suffering and fatality in a zebrafish colony have often not hindered investigators from assembling an appropriate number of zebrafish embryos to conduct their experiments could be partially blamed for the scarcity of understanding regarding viral infections in zebrafish. As an outcome, a wide range of scientists now back the concept of tolerating a certain degree of mortality rates. Given its huge benefits as a model organism, zebrafish use has dramatically upped in biomedical research sectors, where factors caused on by unknown pathogenic organisms are a major worry, even though previously the zebrafish scientific community has not demonstrated the very same degree of importance for eradicating infectious illnesses as is now in the rats and mice scientific community. It is usually expected to maintain zebrafish alive for a significantly larger percentage of their lifespan in order to study the histopathologic changes that take place in adult animals while performing research in disciplines like cancer, infection, ageing, toxicity, and immunity. The presence of ectopic cytokines, tissue damage, a high death rate, and persistent inflammation are all indications of a natural infection. In research like this, each of these factors is likely to be a significant confounding factor (Baker, 1998; CM et al., 2002; Kent et al., 2012; Wade and Daly, 2005).
Since virions are dispersed throughout the water column, the fact that many aquatic viruses are not host-specific may indicate that they have evolved access to a greater variety of possible hosts. For instance, some freshwater and marine fish species are affected by the aquatic virus known as viral hemorrhagic septicemia virus (VHSV1). Other aquatic viruses have the capacity to spontaneously infect aquatic invertebrates, fish, and amphibians. Given how several aquatic viruses potentially infect multiple fish species, zebrafish could be prone to viral infections which have already been recognized in other marine species. In both aspects of size and variety, a large number of virus infections have been observed in innumerable teleost fishes. In order to uncover naturally occurring viruses in zebrafish, current studies should incorporate both novel viruses and viruses that have already been recognized from other teleost fishes.
Studies on viral infection that were carried out with the goal of using zebrafish as an infection model, showed that various viral family have the potential to infect zebrafish. These viral families include the Iridoviridae, Birnaviridae, Rhabdoviridae, SVCV: spring viremia of carp virus, SHRV, and VHSV. In addition to implying that zebrafish are frequently infected with viruses spontaneously, the likelihood of the fragility of zebrafish to experimental infections with such a diverse array of viruses increases the chance that other viral family members are present among unreported zebrafish viruses (Crim and Riley, 2012; La Patra et al., 2000; López-Muñoz et al., 2010; Lu et al., 2008; Ludwig et al., 2011; Novoa et al., 2006; Phelan et al., 2005b; Seeley et al., 1977; Xu et al., 2008).
7. Ins and outs of viral invasion and abatement
7.1. Invasion mechanism involving mammalian cells
All three of the major subcategories of living things bacteria, archaea, and eukaryotic organisms have gained the capacity for viral infection. More than 3600 viruses have been identified, hundreds of which infect human cells, causing associated illnesses. Animal viruses bind to host cell receptors and enter host cell nuclei. Recent understanding on viral entry proteins interaction with host cell receptors and the conformational changes it undergoes has opened opportunities for the development of novel therapies and vaccines (Dimitrov, 2004). Viruses, due to their high transmission rate, have been an intriguing system for delivering genes, vaccines, drugs, and peptides to cells and between cells. Furthermore, virus-based vectors and subviral systems are already being used in biotechnology and medicine. Understanding the cellular and molecular processes underlying viral entry has advanced the identification of virus receptors. Furthermore, understanding how these components assist entry, fusion of enveloped viruses with cells or breaching of cell membrane by non-enveloped viruses and what transpires after penetration is still necessary because receptor binding is only the first step (Klasse et al., 1998).
Enveloped viruses have genomes and proteins enclosed in one or more membranes. The virus receives the necessary membranes from the host cell during the assembly and budding processes. Numerous enveloped viruses, such as human immunodeficiency virus (HIV), Semliki Forest virus (alphaviruses), vesicular stomatitis virus (rhabdoviruses), influenza (orthomyxoviruses), and Sendai (paramyxoviruses), have a single membrane. Other viruses, such as the herpes virus, may undergo several steps of budding and fusing with several internal membrane compartments before eventually acquiring a single membrane via the exocytic route. As a member of the Poxvirus family, the vaccinia virus can gain numerous membranes through interactions with different membrane compartments within an infected cell. Virally encoded membrane or envelope proteins, and the lipid and protein contents of the host cell, dictate the make-up of the viral membrane(s), which differs across viruses. The Golgi apparatus, intermediate compartment, endoplasmic reticulum, and plasma membrane are just a few of the cellular locations where viruses congregate and bud during the budding mechanism, endoplasmic reticulum being the most frequent sites of viral assembly and bud development. (Bron et al., 1994; Klasse et al., 1998; Stegmann et al., 1987; Vainstein et al., 1984; Wagner et al., 1992). However, rotaviruses may briefly acquire a membrane by budding into the endoplasmic reticulum, only to lose the membrane during future maturation (Wagner et al., 1992). Once the viral genome is packed in a protein shell in the nucleoplasm of infected cells, it is then expelled from the cell either by cell lysis (Fig. 8A) or, in the case of rotavirus, through secretion.
Fig. 8.
(A) Viral entry and inhibition mechanism in humans and zebrafish model. (B) Mechanism of virus inhibition by Remdesivir drug. (C) Working mechanism of Darunavir: A potential drug for the inhibition of COVID-19.
Similar to enveloped viruses, non-enveloped viruses need to have the necessary molecular machinery in their outer protein shells to infiltrate cells. However, non-enveloped viruses rely on strategies other than fusion to reproduce, in contrast to enveloped viruses. In general, proteins and protein complexes cannot migrate across membranes independently; rather, sophisticated molecular mechanisms are required to enable their mobility. There is no one concept that is analogous to fusion, and the exact mechanism by which non-enveloped viral protein shells interact with cell membranes is unknown. To dock into depressions that resemble “canyons” on the surface of the virion, picornaviruses must first make connections with cell-surface components (Rossmann, 1996). Virions appear to generate holes in the endosome membrane after endocytosis. Without fully disintegrating the capsid or rupturing the endosomal membrane, these holes enable viral RNA to exit the virion and reach the cytoplasm (Prchla et al., 1995). It is plausible that receptor engagement initiates a series of processes that alter the structure of the capsid and form pores. On the other hand, adenoviruses employ fibers that protrude from the surface of the virion to bind to cellular receptors. After adhering to the cell surface, adenoviruses connect to integrins that bind vitronectin before entering the cell via coated vesicles, completing the endocytosis cascade. Low pH levels inside endosomes activate viral lysin, allowing the membrane of the endosome to be breached. This permits entry of the viral capsid and other endosomal components into the cytoplasm, where they are subsequently disassembles (Greber et al., 1994, Greber et al., 1993; Klasse et al., 1998).
Orthoreoviruses are responsible for the initiation of minor gastrointestinal diseases in a variety of mammalian species, including humans, in accordance with the original definition of reoviruses as “respiratory enteric orphan viruses”. The strain designations type 1 Lang (T1L), type 2 Jones (T2J), and type 3 Dearing (T3D) are the three primary serotypes of orthoreovirus (Chappell et al., 2002). The sequence of the spike protein, which dictates the viral tropism and illness pattern, serves as the major marker for the differentiation between the three strains (Duncan et al., 1990). Mechanistic studies on orthoreovirus entrance, proliferation, and escape from cells have been greatly aided by the availability of primary and immortalized cell lines that permit infection in vitro (Roth et al., 2021). Clathrin-mediated endocytosis is thought to be the most significant uptake mechanism in both polarized and non-polarized cells, despite the observation of other entry channels, such as caveolar endocytosis (Schulz et al., 2012) and micropinocytosis (Aravamudhan et al., 2020; Boulant et al., 2013; Maginnis et al., 2008). Viral infection is identified through a series of successive interactions with the host cell. Binding to the cell surface, intracellular trafficking, membrane penetration, activation of cell signaling, and apoptosis are processes involved in these interactions. When the virions latch to the primary attachment factors, they concentrate on the cell surface, and the process begins. Prior to viral entry into the cell, secondary receptors are activated (Grove and Marsh, 2011). A core particle is delivered to the cytoplasm at the end of this procedure in the case of reoviruses. A whole double-stranded RNA genome is carried by this core particle, which is transcriptionally capable (Roth et al., 2021; Swevers et al., 2021). SARS-CoV-2 cell infection is mediated by the virus' surface spike protein. Prior to activation by human proteases through proteolysis, the SARS-CoV-2 spike protein establishes a connection with its receptor hACE2 via its receptor-binding domain (RBD).
7.2. Assessing virus abatement in mammalian cells
Progress in the search for viral replication inhibitors requires an understanding of the molecular mechanisms that operate during viral infections. Any of the required stages that occur throughout a virus's life cycle may be the target of an antiviral drug. Herpes virus replication is typically inhibited by medications that cause the replication chain to break. Similarly, anti-HIV medications work largely by inhibiting the HIV reverse transcriptase. Ribavirin, an antiviral drug, inhibits viral nucleic acid replication. Protease, which oversees the breakdown of polyproteins has been a point of interest in diminishing viruses such as hepatitis C virus, rhinovirus and HIV. The complicated interaction of viral entry makes it a viable target for development of virus inhibitors. Certain stages of the viral life cycle are still unclear, resulting in partial usage of antiviral strategies. For instance, RNA capping processes and membrane attachment of replication complexes (Fig. 8A), which all positive-strand RNA viruses have in common (Magden et al., 2005). The interactions between viruses and their receptors control important regulatory processes, such as the viral host range, tissue tropism, and the establishment of viral illness. Engaging with the “lock” the receptor on the cell surface, and the viral attachment protein as the “key” to unlock host cells; these “lock-and-key” relationships are necessary to effectively infiltrate host cells and gain control of the cell's vital machinery. To mediate these functions, viruses frequently target certain classes of molecules, such as sialylated glycans, immunoglobulin superfamily members, integrins, and phosphatidylserine receptors, in formations of numerous comparable motifs in the usage of virus-receptor interactions throughout viral groups. The redundancy in receptor utilization suggests that viruses target certain receptors or “common locks” to take advantage of their biological function, which is consistent with evolutionary conservation. The importance of virus interactions with host cells in viral pathogenesis, as well as the redundant usage of viral receptors, makes the utilization of these strategies an enticing target for the development of innovative antiviral medicines (Maginnis, 2018).
The microRNA (miRNA) family is a significant post-transcriptional regulator of gene expression in mammalian cells. miRNAs have been shown to play crucial roles in viral replication and may be used by host cells to control viral infections, such as control of both lytic and latent viral replication, limitation of antiviral responses, suppression of apoptosis, and encouragement of cellular growth. A wide variety of viruses, including retroviruses, DNA viruses, and herpes viruses, express miRNAs (Grassmann and Jeang, 2008). Our immune system needs to be guided by proteins, similar to those found in mammals, which have been widely studied, to survive in virus-filled environments. Small RNA-driven antiviral immune systems can inhibit the invasion on cells by genetic material that is not native to these cells through complementary base pairing with target sequences. These RNA silencing-dependent systems are active in numerous animals. There is strong evidence that endogenous genes essential for mammalian antiviral immunity are regulated by miRNAs. Recent advances in the delivery of RNA for antiviral uses, suggests the potential for producing small non-coding RNA-directed antiviral treatments and prophylactics (Takahashi et al., 2021). The human virus adeno-associated virus (often abbreviated as AAV) has two modes of replication: an integrated provirus or a lytic infection (Atchison et al., 1965; Hoggan et al., 1966). This implies that a crucial part of AAV's life cycle is its capacity to cause latent infections. AAV frequently requires a helper virus, with a few noteworthy exceptions, to create a productive viral infection (Schlehofer et al., 1986; Yalklnoglu et al., 1988). The necessary assistance tasks can be provided by either herpes or adenovirus family members (Atchison et al., 1965; Buller et al., 1981; Hoggan et al., 1966; Melnick et al., 1965). AAV integrates into the host chromosome rather than producing progeny viruses when there is no companion virus present. AAV proviruses are stable with few variations. However, when a cell line carrying an AAV provirus is superinfected with a helper virus, the AAV genome is eradicated, and the process proceeds as if it were a typical productive infection; this process is called a “helper virus superinfection” and is a defense mechanism that enables AAV to continue living without a helper virus. This procedure enables AAV to establish a latent infection that may subsequently become active (Muzyczka, 1992).
The antiviral immunological mechanism, known as RNA interference (RNAi), has been conserved in eukaryotes. Several viruses have been shown to encode viral suppressors of RNAi (VSRs) to block the effects of antiviral RNAi. Semliki Forest virus is known to prevent RNAi in cells by entangling double-stranded RNA and short interfering RNA with the help of capsid proteins. Reverse genetics was used to inactivate VSR activity of the SFV capsid, and the resulting VSR-deficient SFV mutant showed significant replication irregularities in mammalian cells. However, blocking RNAi could reverse these abnormalities (Qian et al., 2020). The B2 protein found in short RNA viruses, such as the Nodamura virus (NoV), has been shown to function as an inhibitor of RNAi, which is triggered by either short hairpin RNAs or small interfering RNAs. NoV B2 binds to RNAs that have undergone RISC-RNA-induced silencing complex processing, as well as pre-Dicer substrate RNA. As a result, Dicer cleavage is inhibited. NoV B2 primarily binds to double-stranded RNAs that structurally match the Dicer substrates and products. Therefore, it is able to inhibit Dicer-mediated RNA cleavage in vitro, even in the absence of any other host factors (Sullivan and Ganem, 2005).
7.3. Invasion mechanism involving zebrafish
In recent years, the zebrafish has emerged as a crucial model system for the investigation of infectious disorders, notably viral infections. The outcomes of experiments utilizing adult zebrafish or embryo models as hosts for HSV-1 are intriguing because they support the theory of viral-host tropism and might test potential antiviral medications. Additionally, the presence of viral entry receptors in zebrafish, such as 3-O-sulfated heparan sulfate (HS), nectins, and the TNF receptor superfamily member 14-like receptor (with homology to its human counterpart), enables virologists to compare the structure-function basis of the virus and zebrafish receptor interaction in vivo, assessing their importance in the viral entry in a zebrafish model. The primary benefits of employing zebrafish in viral infection examination, are their completely developed immune system, opacity of the body during the early stages of life, wide accessibility to a variety of transgenic lines, and their potential for receptor-specific knockouts (Fig. 8A) (Antoine et al., 2014).
Zebrafish models have been developed for several human viruses. Both adult and embryonic zebrafish, have been used as platform for the HSV-1 infection and to discover putative receptors enabling viral entry (Antoine et al., 2014). It is important to highlight that although live imaging was not feasible, the virus's capacity to spread to the brain was observed. Recently, CHIKV a human virus causing neurological defects in newborns and acute illness in adults, have been able to propagate CHIKV in larvae of zebrafish (Palha et al., 2013). The virus was microinjected to simulate the infection caused by a typical mosquito bite. Infection in zebrafish is controlled by the host interferon (IFN) response, which is mediated by neutrophils, after a robust initial replication phase in many distinct cell types. Most of the CHIKV-infected cells disappeared, with the remarkable exception of the brain cells. Research on the tropism and transmission of viruses has also employed fish viruses, which are frequently collected from aquaculture species. For instance, when infected with infectious hematopoietic necrosis virus and rhabdovirus, zebrafish larvae died after a short period of time (3–4 days) (Ludwig et al., 2011). The main target of the infection was the endothelium of the veins, which contributed to the noticeable hemorrhagic condition. Viral damage became irreversible at a temperature of 28.8 °C, which prevented viral multiplication. The infected fish were then subjected to this temperature. Snakehead rhabdovirus (SHRV), a different fish rhabdovirus, has well-characterized pathophysiology but not tropism (Phelan et al., 2005b). Zebrafish have been observed to contract the SVCV by the immersion technique, which can cause acute illness in goldfish or carp. The SVCV statistical approach was used to examine the unique characteristics of several type I IFNs (López-Muñoz et al., 2010, López-Muñoz et al., 2009). Furthermore, by employing loss-of-function tests with morpholino antagonists targeting putative cytokine receptors in the context of infections produced by SVCV or IHNV, zebrafish IFN receptors could be discovered. The variety of IFN-stimulated genes (ISGs) in zebrafish has been investigated using fish viruses, as well as the more potent IFN inducer, CHIKV. The antiviral effects of zebrafish IFNs have been established in vivo, whereas those of zebrafish ISGs—including ISG15, IRF7, and the fish-specific dreI/Gig2—have only been confirmed in vitro thus far (Langevin et al., 2013; S. Li et al., 2012; Xiang et al., 2010). ISGs respond less favorably to morpholino antagonist-mediated in vivo suppression than non-inducible, genes, such as those encoding IFN receptors or mitochondrial antiviral signaling protein. The viability of antiviral drug screening has been demonstrated in a system where a vector harboring hepatitis C virus (HCV) sub-replicon was injected into zebrafish zygotes, resulting in the production of HCV core RNA and protein and triggering transcriptome alterations comparable to those in human hepatocytes. Oxymatrine and ribavirin, two well-known anti-HCV drugs, drastically reduced the production of HCV core RNA and protein during drug testing in fluorescent zebrafish larvae (Ding et al., 2011). Zebrafish have endogenous retroviruses in addition to exogenous retroviruses, which is helpful in research on how these elements affect genome changes in tumors and the relevance of their function in the regulation of antiviral immunity (Frazer et al., 2012; Levraud et al., 2014; Shen and Steiner, 2004). The mechanism of zebrafish c-reactive proteins (CRP1–7) and their antiviral effectiveness against SVCV were recently studied by Perez et al. These results did not directly demonstrate either activation of the host's interferon system, reduction of G-protein fusion activity or binding steps of attachment or blockage of the viral replication cycle. Further investigation revealed that the antiviral defense provided by CRP1–7 was mostly due to the protein's capacity to obstruct autophagy-related mechanisms. To further describe the action of CRP, methyl-cyclodextrin, a cholesterol-complexing agent, and 25-hydroxycholesterol, a cholesterol molecule with antiviral characteristics, were used. This was accomplished owing to the strong affinity of CRPs for cholesterol and the recently discovered impact of the lipid raft cholesterol balance on autophagy. The results of additional research indicate that CRP prevents autophagy by disrupting cholesterol ratios in the host cell membranes. Moreover, reactive oxygen species (ROS) are negatively regulated inside the cell, which increases the pH of lysosomes. It was hypothesized that these pH variations prevent SVCV replication directly by interfering with the capacity of viral glycoprotein G to fuse membranes in response to changes in pH. This trait enables the virus to escape from endosomes and enter the cytoplasm during the entrance phase of its life cycle (Bello-Perez et al., 2020).
7.4. Assessing virus abatement using the zebrafish model
RIG-I-like receptors, commonly referred to as RLRs, control the innate immune system signaling pathway, hence play a vital role in the host's response to viral infections. To achieve an immunological balance between antiviral responses and virus survival, both the host and virus tightly regulate the RLR signaling pathway. Zebrafish TRIM25 (zbTRIM25) as a positive regulator of the RLR signaling pathway when infected with the red spotted grouper neurological necrosis virus (RGNNV). zbTRIM25 expression diminished after infection with RGNNV, and its ectopic expression increased the expression of genes associated with the RLR signaling pathway. zbTRIM25 increased zebrafish RIG-I (zbRIG-I)-mediated IFN signaling, which inhibited RGNNV replication, according to overexpression and knockdown analyses. zbTRIM25 bound to zbRIG-I, and the SPRY domain of zbTRIM25 interacted with the tandem caspase activation and recruitment domains (2CARD) and repressor domain (RD) sections of zbRIG-I. zbTRIM25 assisted K63 polyubiquitination of zbRIG-I, 2CARD and RD regions. Additionally, zbTRIM25-mediated zbRIG-I polyubiquitination was significant for RIG-I-triggered IFN production, as shown by the enhancement of zbRIG-I-mediated IFN synthesis activation by K63-linked ubiquitin. These findings indicate an unidentified mechanism through which zbTRIM25 favorably affects the innate immune response and the process involved in targeting and enhancing K63-linked polyubiquitination of zbRIG-I (Lu et al., 2021).
Various forms of IFNs, including virus-induced IFNs, as well as IL-10 and its related cytokines (IL-20, IL-22, and IL-26), are all included in fish type II helical cytokines. These cytokines may bind to various receptors. Three genes have been found in zebrafish that, when triggered by a virus, generate IFNs. A fourth IFN gene was discovered on chromosome 12, in addition to genes that are clustered together on chromosome 3. Each of these genes possesses the exons and introns found in mammalian IFN genes, where IFN-1 and IFN-2 offer protection. All of these compounds increased the expression of reporter antiviral genes in zebrafish larvae. Not all zebrafish IFNs bind the same receptors. Two subgroups of fish virus induced IFNs have been identified based on the conservation of cysteines, a common short-chain receptor (CRFB5) and a long-chain receptor specific to each receptor complex are present in both receptor complexes (CRFB1 or CRFB2) (Hamming et al., 2011). Based on the receptors they engage, IFNs in mammals can be divided into three types: type I (consisting of one exon), type II and type III IFN genes consisting of four and five exons, respectively. Furthermore, IFNs of types I and III collectively known as “virus-induced IFNs” (Haller et al., 2007). They differ from INF type II because they can be triggered by viral infection (Zhang et al., 2008).
Type I and type III IFNs both stimulate the same transcription factor, IFN-stimulated gene factor 3, as well as activates same sets of genes (Doyle et al., 2006; Marcello et al., 2006). Interestingly, the majority of organisms have a wide variety of IFNs that are generated in response to viral infections (13 beta, 1 beta, and 3 beta in humans). Mammalian type I IFN systems must be active for them to be able to withstand viral infections. The more recently discovered type III IFNs are crucial for preventing influenza infection through the nasal passageway (Mordstein et al., 2008), and some viral proteins also target this subtype, even though their exact role is not fully understood. Even though IFN-like behaviors have been observed in fish for many years, it was not until recently that fish IFNs could be identified due to developments in genome sequencing. IFNs cloned from fish were shown to have antiviral effects in 2003 (Lutfalla et al., 2003; Robertsen et al., 2003). Most teleost species include several genes that create IFNs through viral infection (Sun et al., 2009). Stein et al. argued, as fish virus-induced IFNs display traits common to both type I and type III mammalian IFNs, they should be assigned the IFN designation. IFNs serve critical functions at the nexus of innate and acquired immunity, making it challenging to distinguish their particular contributions to either kind of immunity (Stein et al., 2007). Zebrafish embryos or larvae are an appealing model for the study of innate immunity in vertebrates, as acquired immunity does not fully develop until 4–6 weeks of development (Fig. 8A). As young zebrafish allow simple genetic alterations, gain- and loss-of-function studies can be used to evaluate how certain cytokines contribute to particular innate immune pathways. Diversity of IFNs caused by fish viruses, is greater than previously believed, as demonstrated by Zou et al., where researchers were able to cloned three IFNs in rainbow trout in addition to discovering two more zebrafish genes in silico, IFN2 and IFN3 (Zou et al., 2007). They emphasized the division of these genes into two distinct subgroups (groups I and II), each of which could be distinguished by the presence of two or four conserved cysteines, respectively, in the protein sequence. IFN-1 belongs to group I of zebrafish, whereas IFN-2 and IFN-3 belong to group II. All three zebrafish IFNs have antiviral action in adult fish (López-Muñoz et al., 2010). Study by researchers examined four IFN cDNAs, that were cloned to look for potential new IFNs gene in the current zebrafish genome. Then, IFNs were administered or overexpressed using a variety of techniques in growing zebrafish embryos to examine in vivo biological functions, such as viral infection resistance. Depending on the subgroup to which they belong, zebrafish IFNs have been shown to bind to one of two different receptor complexes (Aggad et al., 2009). The antiviral defenses in mammals include ISG15, a protein that is activated by IFNs, is highly conserved among vertebrates. Viral infection and IFN treatment induce ISG15 expression in fish, similar to that in mammals. Fish also have ISG15's two ubiquitin-like domains and a consensus LRLRGG sequence, which is necessary for covalent conjugation to a substrate protein in the C-terminal region. When EPC cells (epithelioma papulosum cyprini cells) overexpressed with zebrafish ISG15 (zf-ISG15) were exposed to RNA and DNA viruses genus (Novirhabdovirus, Birnavirus and Iridovirus), a declining/inhibition in viral infection was observed. ISGylation of both phosphoproteins and non-virion proteins has been proven by co-expression experiments with IHNV proteins. Mutation of glycine residues in the consensus LRLRGG motif establishes a link between ISGylation and ISG15-dependent viral restriction. This affects both cellular defense against viral infection and the removal of zf-ISG15 conjugation to these proteins. The expression of RIG-I, viperin, and, to a lesser extent, IFN genes, also increases in response to an increase in zf-ISG15 expression levels. These findings demonstrate the antiviral properties of ISG15, and vertebrates, have a similar ISGylation mechanism that was presumably created to combat viral infections. Research further revealed that zf-ISG15 affects the IFN system at several levels and complex processes that regulate the innate antiviral response in vertebrate cells (Langevin et al., 2013).
8. Comprehending the SARS-CoV-2 mechanism of hijacking and its arrest utilizing zebrafish
SARS-CoV-2 virus is anticipated to last for years; even though vaccination is now underway (WHO, 2022), as its variants possess an unpredictability concern. To understand its diverse pathophysiology and provide novel medications and vaccines, research efforts must continue. It is a highly pathogenic bio-contaminant (virus) belonging to Coronaviridae family, causing respiratory tract infection (Singh and Yi, 2021). Respiratory tract infections are also caused by severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) (B. Hu et al., 2021). The surface of the SARS-CoV-2 virus consists of spike (S) protein, which gives it the appearance of a “crown” (Wu et al., 2022). This protein is essential for the infection process because it causes the cell membrane to fuse (Yang and Rao, 2021). Spike protein, a trimeric protein belonging to class I fusion protein, consists of S1 and S2 components which assist during entry of the virus into the cell. The S1 component facilitates the adherence of the host cell's angiotensin-converting enzyme 2 (ACE2) receptor with the virus, while the S2 component facilitates the fusion of the viral and cellular membranes (Tai et al., 2020). Additionally, the spike protein has been regarded as a potential antigen for the development of COVID-19 vaccines (Fig. 8B and C) (Albanese et al., 2022; Tai et al., 2020).
Expediting the development of medication and vaccine testing requires the establishment of laboratory animal models that accurately depict the molecular mechanisms. Rhesus macaques, cynomolgus macaques, ferrets, cats, and Syrian hamsters are few lab-compatible animal species that are biologically vulnerable to SARS-CoV-2 (Bertzbach et al., 2021; Blair et al., 2021; Browne et al., 2020; Driouich et al., 2021; Qiao et al., 2021; Wang et al., 2020; Yuan et al., 2020). However, mice that express the human ACE2 receptor serve as an effective animal model because they are not naturally receptive to the virus (Muhl et al., 2022; Piplani et al., 2021). To assess the immune responses to interactions between SARS-CoV-2 and other hosts and pathogens, each of these mammalian models has certain benefits and drawbacks, but none of them offer prompt, whole-organismal, maximum throughput. Furthermore, are expensive for preliminary testing of drugs and immunotherapies.
Zebrafish due to its experimental friendly attributes, for instances compatible for human disease model studies, comparable adaptive and innate immune systems with human, makes them perfect candidate to study the signaling cascades, interaction with chemical mediators, and host-virus interaction on mucosal surfaces. Furthermore, the ACE2 protein, which the virus employs as a receptor and authorizes to enter human cells, was shown to exist in developing zebrafish (Kim et al., 2021; Laghi et al., 2022; Postlethwait et al., 2021). Intraperitoneal (IP) injection of the rSpike protein has negative effects on the liver, kidney, ovary, and brain tissue, as well as the survival rate (Ventura Fernandes et al., 2022). Researchers have shown that intramuscular injection of SARS-CoV-2 spike protein into zebrafish might cause behavioral abnormalities, skin hemorrhages, kidney necrosis, and inflammation in the swim bladder. Additionally, they investigated Withania somnifera extract on reduction of clinically significant pathogenic characteristics (Balkrishna et al., 2021b). In a different study, the SARS-CoV-2 spike protein was injected into human alveolar epithelial cells (A549) to create humanized zebrafish. The spike protein has been shown to cause cytokine release, morphological and cellular abnormalities, apoptosis of macrophages and granulocytes in the swim bladder, and behavioral fever in fish. Furthermore, they evaluate a tri-herbal drug “Coronil” effects on infected zebrafish, where they observed reduction of pathogenic characteristic brought on by SARS-CoV-2 spike protein (Balkrishna et al., 2020). Additionally, studies have revealed that Coronil efficiently prevents the interaction of ACE2 with the S-protein, including its variation (SD614G), which is more widespread and contagious, as well as another mutant (SW436R), which has a substantially greater affinity for ACE2. Treatment with Coronil substantially and dose-dependently decreased the elevated levels of TNF-α, IL-6, and IL-β in A549 cells exposed to various S-protein variations. Another study used human lung epithelial cells transplanted into a swim bladder that had received spike-protein injections to induce pro-inflammatory as well as macrophage and granulocyte invasion into the swim bladder. This resulted in heightened systemic impairment, as determined by kidney tissue damage and behavioral fever. When Tinospora cordifolia was used to treat these pathological characteristics, it was less pronounced (Balkrishna et al., 2021a). Another study suggested that spike RBD binds to the non-sensory epithelium of the olfactory organ when administered intranasally and causes considerable histopathology, as indicated by cilia loss, edema, and hemorrhages. Electrophysiological recordings showed deterioration of smelling ability towards bile and food odorants when spike RBD was administered intranasally in animals. Furthermore, single-cell RNA-seq revealed significant olfactory receptor loss, myeloid cell cluster, and inflammatory effects in sustentacular adult zebrafish, as well as decreased numbers of Tregs (Kraus et al., 2022). Reduction in S-protein binding to ACE2, 3CL protease activity, and SARS-CoV-2 pseudovirus entrance into the cell has been observed, when exposed to an extract of Polygoni Multiflori Radix (PMR). With IC50 values ranging from 25 to 500 g/ml (Wang et al., 2022). Zheng et al. studied the connection between the SARS-COV-2 spike protein and blood coagulation and found that the S protein can competitively prevent antithrombin and heparin cofactor II from binding to heparin/HS, leading to an unexpected rise in thrombin activity. Zebrafish embryos can directly experience blood coagulation and thrombosis when exposed to SARS-CoV-2 S protein at a concentration (10 g/ml) comparable to the viral load in severely ill patients. In contrast to the injection of S protein alone, S protein when co-injection with heparin/HS considerably lengthened the bleeding duration in zebrafish embryos (Zheng et al., 2021). In a study by Laghi et al., wild-type zebrafish embryo and larvae were used to microinject SARS-CoV-2 into the coelom, pericardium, brain ventricle, and blood stream. The wild-type larvae, exhibited a sharp decline in SARS-CoV-2 RNA levels. However, after 24 h, the viral RNA stabilized when it was injected into the swim bladder. According to immunohistochemistry, epithelial cells in the swim bladder wall area were found to possess the SARS-CoV-2 nucleoprotein. The results of this investigation showed that wild-type zebrafish larvae had an abortive infection of the swim bladder, an aerial organ that is similar to the mammalian lung, and appeared non-permissive to SARS-CoV-2 (Laghi et al., 2022).
9. Transportation routes of bio-contaminants and contaminants
Aquatic ecosystem is gravely affected due to number bio-contaminants as well as contaminants caused by rapid advancement and industrialization. There is a considerable danger of biomagnification due to the fact that water pollution has affected the fish population and that these contaminants and bio-contaminants may aggregate or colonize fish internal organs where they may then go on to higher food chains (humans). Around 1400 types of microbes, including as bacteria, protozoa, protozoan parasites, parasitic worms, fungus, and viruses, have been discovered by scientists to be potentially harmful (Bashir et al., 2020). In addition, sewage treatment plant effluents include certain harmful microorganisms, and such litters and pollutants are found to have the potential harmful ecotoxicological impacts. As a result of such adverse effects, toxin surveillance, identification of adverse effects of exposure, and comprehension of the transportation routes have become imperative. In the current scientific era, experiments where the fish acting as the vector itself can provide the knowledge to improve investigations which may further allow to study target infection more effectively. Primary organs such as ocular and oral system, nasal, anal, auricle, and the epidermal layer of the skin are the possible route of these pathogen through which it enters fishes. Tenor et al., performed live imaging of the cranial blood arteries of zebrafish larvae infected with a fungal pathogen known as Cryptococcus neoformans (Tenor et al., 2015). Interestingly, post intravenous infection, it was found that C. neoformans can enter the zebrafish brain. In another study by Wen et al., the zebrafish model was used to investigate sepsis-associated acute kidney damage (Wen et al., 2018). In this investigation, Edwardsiella tarda was microinjected into the Cuvier duct of the zebrafish larvae, which further led to dose-dependent death and long-lasting bacterial infection. Mortality rate from moderate E. tarda infection was over 50 % in larvae and 20 % in adults. Larval pericardial edema and renal failure were also observed in both larval and adult zebrafish. Studies have also revealed that the ocular system of the zebrafish model may also assist in the investigation of routes for bacterial pathogenesis. For example, Takaki et al., developed the Mycobacterium marinum-infected zebrafish larva as a model to examine the early pathophysiology of ocular tuberculosis (Takaki et al., 2018). In this investigation, tricaine was used as a medium for anesthesia, and the larvae at specific developmental stages (2 or 3 dpf) were injected with M. marinum. This study concluded that, despite a functioning blood retinal barrier, hematogenous bacteria can seed the zebrafish eye. Also, M. marinum infected eye led to the formation of prototypical early granulomas. Virology based investigations for exploring the routes of infection is a major concern, several experiments have supported such circumstances. Using the zebrafish model, Widziolek et al. assessed the pathogenicity of tilapia lake (TiL) virus infection (Widziolek et al., 2021). The duct of Cuvier was selected as the route for injecting TiL Virus, further this study revealed that TiLV infection led to an increase in the expression of immune-related genes that code for pathogen recognition receptors for viral dsRNA (rig-I (ddx58), tlr3, tlr22), transcription factors (irf3, irf7), type I interferon (infϕ1), antiviral protein (mxa), and pro-inflammatory cytokine (il-1β). Another study by Passoni et al. discussed the various methods through which alphaviruses, such as the chikungunya virus (CHIKV) and the Sindbis virus (SINV), could infiltrate the central nervous system (CNS) (Passoni et al., 2017). It was found that the endothelial cells of the brain's vasculature are always infected by CHIKV but not by SINV. In contrast, axonal transport between neural tissues and from the periphery to the CNS was significantly more effective with SINV than CHIKV. Along with the bio-contaminants, the pathway followed by the transport routes of the industrial contaminants must be investigated. Recent reports discussed the prevalence of COVID-19-related litter in various ecosystems, wildlife contact with such objects, and the pollutants that can be discharged from such protective gear (Patrício Silva et al., 2021). Sendra et al., used RNA-Sequencing technique in the zebrafish larvae to analyze the toxicogenomic effects of face masks at various phases of water degradation (Sendra et al., 2022). Larvae were given three treatments over the course of ten days: 1) early stage fragmented face mask (poorly degraded masks, or PDM); 2) advanced stage degraded face mask (highly degraded masks, or HDM); and 3) water obtained from HDM (W-HDM). According to transcriptome analyses, the three treatments caused the downregulation of genes involved in reproduction, particularly the HDM products, which raises the possibility that breakdown products from face masks may have endocrine disrupting properties. The afflicted genes have a role in a number of reproductive processes, including gametogenesis, sperm-egg binding and recognition, or fertilization. The treatments also had varying effects on genes involved in metabolism and the immune system. Furthermore, developmental toxicity of TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) has been thoroughly researched; TCDD poisoning typically comprises pericardial and yolk-sac edemas in the zebrafish model (Antkiewicz et al., 2006; Teraoka et al., 2003). Studies on phenotypic mechanisms in zebrafish have made it possible to analyze the AHR (aryl hydrocarbon receptor) pathway's role in developmental toxicity (Goodale et al., 2012; Prasch et al., 2003). Studies of complex mixtures like those found in oil spills (such as Mississippi Canyon 252 oil from the Deep Water Horizon and Alaska North Slope crude oil from the Exxon Valdez) identified polycyclic aromatic cardio toxicants in zebrafish that caused cardiac edema, defective heart looping, hemorrhage, and reduction in arteriovenous circulation, which were consistent with observations by others that dioxins and dioxin-like substances activate the aryl hydrocarbon pathway. Further research may pave a path for comprehensible conclusions on the mobility route for the bio-contaminants and contaminants in the aquatic animals like fishes.
10. Leveraging the zebrafish model to understand the holistic invasion mechanism and possible deterrent of monkeypox virus: future prospective
In 1958, two occurrences of a disease that resembled the pox spread among colonies of monkeys kept for research were reported. As the disease was first identified in monkeys, hence the name “monkeypox”. During a period of increased efforts to eradicate smallpox, the first incidence of monkeypox in humans was discovered in the Democratic Republic of Congo in 1970. Several additional central and western African nations, including the Central African Republic, Liberia, Nigeria, Sierra Leone, Democratic Republic of Congo, Republic of Congo, Gabon, Cote d'Ivoire, and Cameroon, have reported cases of monkeypox among their citizens. These cases have been related to foreign travel or the importation of animals.
The WHO declared a public health emergency of international concern (PHEIC) on July 23, 2022, due to the worldwide monkeypox outbreak. Monkeypox is an uncommon illness caused by exposure to the monkeypox virus. Viruses with double-stranded DNA belong to the genus Orthopoxvirus and the family Poxviridae. This genus also includes viruses such as variola (smallpox), vaccinia (used in smallpox vaccines), and cowpox (Fig. 9 ). The monkeypox virus has been shown to affect several animal species. These include dormice, non-human primates, tree squirrels, Gambian pouched rats, rope squirrels, and other species. Although rodents are most likely the source of monkeypox, the natural source has not yet been discovered.
Fig. 9.
Representation of phylogenetic tree of the Poxviridae family (Motesi, 2021).
When an individual comes into contact with the virus from an infected animal, an afflicted human, or virus-contaminated items, the virus that causes monkeypox may be disseminated. The virus can potentially pass from the mother's placenta to the fetus. It may also be transferred from animals to humans through an infected animal bite or scratch. Direct contact with bodily fluids, sores on an infected person, or objects that come into contact with bodily fluids or sores, such as clothing or linens, can also spread the virus. Direct contact with bodily fluids is the main mechanism by which diseases spread between individuals. When there is lengthy face-to-face contact, they can also be transferred through respiratory secretions. Kissing, snuggling, or touching areas of the body with monkeypox symptoms might help to transmit the virus. Intimate contact between individuals, particularly during coitus, may also contribute to this phenomenon.
780 laboratory-confirmed cases of monkeypox had been reported to or recognized by WHO as of 13 May and 2 June 2022 from 27 Member States in four WHO zones where the monkeypox virus is not infrequent. Up to this point, no deaths related have been documented. The West African clade was determined to be the source of infection in every instance, whose samples were verified by PCR. The monkeypox virus driving the epidemic is a close match to cases that were transferred from Nigeria to Singapore, the UK, and Israel in 2018 and 2019. This information was obtained from genome sequencing of a swab sample from a confirmed case in Portugal.
According to a recent study, on September 16, 2022, there were 61,282 verified cases of monkeypox from approximately 104 areas worldwide (Fig. 10 ), and 20 deaths were recorded.
Fig. 10.
Monkeypox (MPVX) outbreak global map till 16th September 2022 (“2022 Monkeypox Outbreak Global Map _ Monkeypox _ Poxvirus _ CDC,” 2022). (Pink and green circle depicting countries with MPVX cases.) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Humans and other species routinely exchange viruses with each other. For instance, SARS-CoV-2 virus is commonly believed to have jumped from a bat or pangolins. While entering host cells, poxviruses employ 11 viral proteins that form entrance fusion complexes, in addition to four attachment proteins (Fig. 11 ). These proteins are conserved across all poxviruses. This is in contrast to SARS-CoV-2, which uses a single transmembrane protein (spike protein) (Moss, 2012). To transport their genetic material into host cells, the entry fusion complex (EFC) proteins form complexes and interact with the host cell. Infectious virions can be divided into two fundamental categories: mature virions (MV) (Fig. 11), which have a single membrane, and extracellular virions (EV) (Fig. 11), which have a second outer membrane that is ruptured before fusion (Moss, 2012). Unique sets of transmembrane proteins are found in MVs and EVs, which help the virus affix to the host cell. The outer transmembrane proteins found in EVs include A33R, B5R, A34R, F13L, A56R, A36R, and F12L (Benhnia et al., 2013; Law et al., 2006; Pickup, 2015; Wagenaar and Moss, 2009), while the MV contains just a few transmembrane proteins, including D8, H3, A26, and A27 (Chung et al., 1998; Hsiao et al., 1999, Hsiao et al., 1998; Lin et al., 2000; Singh et al., 2016). Nonetheless, the entry-fusion complex, which is mainly composed of proteins A16, A21, A28, F9, G3, G9, H2, J5, L1, L5, and O3, is used in both virions to carry out viral fusion with the host cell (Fig. 12 ) (Foo et al., 2009; Moss, 2016; Senkevich et al., 2005). The four attachment proteins, A26, A27, D8, and H3, present in the MV have been found to interact with laminin, glycosaminoglycan (GAG), chondroitin sulfate proteoglycan (CSPG), and heparin, similar to the spike protein and its interaction with the ACE2 receptor (Chung et al., 1998; Hsiao et al., 1999, Hsiao et al., 1998; Lin et al., 2000). It has been observed that EV outer transmembrane proteins (A33R, B5R, A34R, F13L, A56R, A36R, and F12L) interact with GAG before the EV membrane separates (Moss, 2016; Roberts et al., 2009). Utilizing zebrafish model system, it may be possible to study the interaction and suppression of poxviruses using these transmembrane proteins. Studies have shown that zebrafish also have the receptors that the monkeypox virus needs in order to enter cells (Farwell et al., 2017; Lee et al., 2020; Sztal et al., 2011; Zhang et al., 2009).
Fig. 11.
Picture above illustrating the complete membrane fusion of Orthopoxvirus (monkeypox virus) with mammalian cells. (1, left) representing IEV with a single lipoprotein bilayer consisting of 4 receptors interacting with the mammalian cell counterpart. (1, right) representing EEV with double lipoprotein bilayer and (5) receptors containing in the outer layer of the virion interacting with mammalian counterparts. (2, 3 & 4) Exposure of the 11 types of protein to the inner membrane layer of mammalian cells, which forms a complex which further assists in the membrane fusion. 5 illustrating a successful membrane fusion between virus and mammalian cells resulting in the transfer of the genetic material. Panels with (red asterix) showing crucial steps where the virus could potentially be checked and could be stopped from fusing with mammalian cells. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 12.
Image representing the active form of entry fusion complex (EFC).
The first draft genome of the monkeypox virus has already been published by a group of experts (Isidro et al., 2022) and could be used to identify more suitable protein targets. Further work must be done before any firm conclusions can be made since this is still a very early draft. A variety of animal models have been employed to investigate the effects of viruses from the orthopoxvirus family (monkey virus, variola virus, Ectromelia virus, Vaccinia virus, rabbitpox virus, and cowpox virus), such as macaques (species/strain: cynomolgus), macaques (species/strain: cynomolgus, rhesus), ground squirrels (species/strain:13-lined), prairie dogs (species/strain: black-tailed), dormice (species/strain: Kellner's), rabbits, rats (cotton rats), and mice (C57BL/6, A.J, BALB/c, DBA/2, and C3H). Orthopoxvirus models have benefits in terms of anatomy and immunology that are comparable to those of humans; however, utilizing non-human primates, rabbits, mice, and rats is more expensive, requires a larger living area, and can be physically demanding and risky to manage. Under biocontainment circumstances, when space is at a premium and people must use specific protective gear that makes even the simplest activity more challenging to complete, housing and handling concerns take on particular significance. As a workaround for these problems, we suggest using a zebrafish model for viral infection research. Zebrafish are a particularly useful and desirable model for the study of infectious diseases and immunity because of the similarities between their immune systems and those of mammals, their ability to study specific immune system components at various stages of immunologic development, and the availability of molecular, genetic, and imaging tools for this species. As zebrafish are vertebrates and the human reference genome can be compared, it has been shown that over 70 % of human genes have at least one clear zebrafish orthologous gene. The recent transmission of the monkeypox virus in humans (from animal to human) is a growing threat. Thus, zebrafish are a useful model for checking virus-host interactions.
11. Conclusions
In this study, we emphasized the novel use of zebrafish model as a platform to study various chemical toxins, nanoparticles toxicity as well as bio-contaminants such as fungus, protozoan, metazoan, parasites, bacteria and viruses, viruses being the most important of. We have also evaluated the benefits and drawbacks of the current model organisms, and how zebrafish outperform these models in this review. This evaluation also addresses numerous gene manipulation of zebrafish model which allows researchers to replicate or establish human disease model, infectious disease model/bio-contaminants model, and investigate its mechanisms. Furthermore, we have highlighted various routes and processes by which viruses infect zebrafish and how they are analogous to human systems, as well as how these viruses may be eliminated by employing this model. A thorough explanation of how this model been used to research the recent pandemic COVIOD-19 caused by SARS-CoV-2, as well as how it could potentially be applied to research other possible pandemic-causing viruses like MPVX (monkeypox virus), is also provided. As a potential tool, zebrafish are affordable, simple to use, less difficult to handle, and capable of having a large sample size. Furthermore, unique characteristics (transparent embryos, ex situ development, and rapid embryo development) give the researcher an advantage to see how viruses interact with various organs under a microscope and comprehend how the body reacts to a specific viral infection, including the phenotypic characteristics that result. In conclusion, the zebrafish model has not yet reached its full potential for studying human viruses. Zebrafish are not meant to replace other vertebrate models, such as mice; however, they avoid the issues that arise when working with vertebrate models and may reveal important concepts in viral pathogenesis and host defense, which may help in the creation of ground-breaking cures for recently emerging viruses.
Abbreviations
- SARS
Severe acute respiratory syndrome
- MERS
Middle East respiratory syndrome
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- MARS-CoV
Middle East respiratory syndrome coronavirus
- 229E
Human coronavirus 229E
- OC43
Human coronavirus OC43
- NL63
Human coronavirus NL63
- HKU1
Human coronavirus HKU1
- H7N9
Asian lineage avian influenza A
- DNA
Deoxyribonucleic acid
- RNA
Ribonucleic acid
- hDPP4
Human dipeptidyl peptidase 4
- SCID
Severe combined immunodeficient
- prkdc
Protein kinase, DNA activated, catalytic polypeptide
- EboV
Zaire ebolavirus
- ACI
August Copenhagen Irish Rat
- MAXX
Inbred stain of rats
- hACE2
Human angiotensin-converting enzyme 2
- IHNV
Infectious hematopoietic necrosis virus
- VHSV
Viral hemorrhagic septicemia virus
- SVCV
Spring viremia carp virus
- IPNV
Infectious pancreatic necrosis virus
- E. coli
Escherichia coli
- CHIKV
Chikungunya virus
- HuNoV
Human norovirus
- HSV-1
Herpes simplex virus type-1
- I. multifiliis
Ichthyophthirius multifiliis
- NIH
National Institutes of Health
- KO
Knock out
- EDC
Endocrine disruptor chemical
- CNS
Central nervous system
- RTH
Resistance to thyroid hormone
- MCT8
Monocarboxylate transporter 8
- KD
Knock down
- GI
Gastrointestinal
- PRRs
Pattern recognition receptors
- C. elegans
Caenorhabditis elegans
- kRASG12D solid tumor models
Kirsten rat sarcoma viral oncogene homolog G12D
- MDA-MB-231
M.D. Anderson - Metastatic Breast 231
- MDA-MB435
M.D. Anderson - Metastatic Breast 435
- PC3
Human prostate cancer
- ALL
Acute lymphoblastic leukemia
- AML
Acute myeloid leukemia
- DA
Dominant active
- CML
Chronic myeloid leukemia
- MPNST
Malignant peripheral nerve sheath tumor
- MDS
Myelodysplastic syndrome
- RMS
Rhabdomyosarcoma
- PNST
Peripheral nerve sheath tumor
- HCC
Hepatocellular cancer
- T-ALL
T-cell acute lymphoid leukemia
- Cd
Cadmium
- Cu
Copper
- Hg
Mercury
- Zn
Zinc
- DCA
Dichloroacetic acid
- 5-FU
5-Fluorouracil
- PCBs
Polychlorinated biphenyls
- DE-71
Polybrominated diphenyl ethers
- PAHs
Polycyclic aromatic hydrocarbons
- BDE-47
2,2,4,4-Tetrabromodiphenyl ether
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- CVD
Cardiovascular disorder
- NAFLD
Non-alcoholic fatty liver disease
- HPE
Holoprosencephaly
- UPEC
UroPathogenic Escherichia coli
- AGM
Aorta gonad mesonephros
- HSPCs
Hematopoietic stem and progenitor cells
- S. typhimurium
Salmonella typhimurium
- TNFα
Tumor necrosis factor alpha
- IL-1β
Interleukin 1 beta
- mRNA
Messenger RNA
- LD50
Lethal dosage
- S. pyogenes
Streptococcus pyogenes
- ATP
Adenosine triphosphate
- GBS
Group B streptococcus
- IP
Intraperitoneal
- IM
Intramuscular
- cfu
Colony-forming unit
- BBB
Blood-brain barrier
- IL
Interleukins
- dpf
Days post fertilization
- GAS
Group A streptococcus
- M. marinum
Mycobacterium marinum
- M. tuberculosis
Mycobacterium tuberculosis
- TB
Tuberculosis
- T3SS
Type-3 secretion system
- (si)RNA
Small interfering RNA
- SEPT
Septin
- S. Typhimurium
Salmonella typhimurium
- S. enterica
Salmonella enterica
- Gbp4
Guanylate-binding protein 4
- HSPC
Hematopoietic stem and progenitor cell
- CF
Cystic fibrosis
- P. aeruginosa
Pseudomonas aeruginosa
- HEC
Enterohemorrhagic Escherichia coli
- LEE
Locus of enterocyte effacement
- V. cholera
Vibrio cholerae
- pH
Potential of hydrogen
- BCC
Burkholderia cenocepacia complex
- B. cenocepacia
Burkholderia cenocepacia
- B. vietnamiensis
Burkholderia vietnamiensis
- B. stabilis
Burkholderia stabilis
- V. vulnificus
Vibrio vulnificus
- V. alginolyticus
Vibrio alginolyticus
- V. parahaemolyticus
Vibrio parahaemolyticus
- V. anguillarum
Vibrio anguillarum
- TDH
Thermostable direct hemolysin
- TRH
TDH-related hemolysin
- Fadsd6
∆-6 fatty acyl desaturase
- Elvol5a
Elongase
- WT
Wild type
- PUFAs
Polyunsaturated fatty acids
- Y. ruckeri
Yersinia ruckeri
- GFP
Green fluorescent protein
- L. monocytogenes
Listeria monocytogenes
- L. welshimeri
Listeria welshimeri
- L. innocua
Listeria innocua
- InlA
Internalin A
- InlB
Internalin B
- S. iniae
Streptococcus iniae
- E. tarda
Edwardsiella tarda
- LLO
Lysteriolisin O
- SHRV
Viral hemorrhagic septicemia Virus
- HIV
Human immunodeficiency virus
- T1L
Type 1 Lang
- T2J
Type 2 Jones
- T3D
Type 3 Dearing
- RBD
Receptor-binding domain
- miRNA
MicroRNA
- AAV
Adeno-associated viruses
- RNAi
RNA interference
- VSRs
Viral suppressors of RNAi
- SFV
Semliki Forest virus
- NoV
Nodamura virus
- RISC
RNA-induced silencing complex
- HSV
Herpes simplex virus
- HS
Heparan sulfate
- ISGs
IFN-stimulated genes
- HCV
Hepatitis C virus
- CRP
C-reactive proteins
- ROS
Reactive oxygen species
- RLRs
RIG-I-like receptors
- zbTRIM25
Zebrafish tripartite motif containing 25
- RGNNV
Red spotted grouper neurological necrosis virus
- zbRIG-I
Zebrafish retinoic acid-inducible gene I
- SPRY
SPla and the RYanodine Receptor
- CARD
Caspase activation and recruitment domains
- RD
Repressor domain
- CRFB
Cytokine receptor family B
- kDa
Kilodaltons
- EPC
Epithelioma papulosum cyprini
- S protein
Spike protein
- rSpike
Recombinant SARS-CoV-2 spike proteins
- VSVppSARS-2S
SARS-CoV-2 S-protein pseudotyped vesicular stomatitis virus
- Tregs
Regulatory T cells
- PMR
Polygoni Multiflori Radix
- IC50
Half maximal inhibitory concentration
- g/ml
Grams per milliliters
- h
Hours
- PHEIC
Public health emergency of international concern
- WHO
World health organization
- PCR
Polymerase chain reaction
- MPVX
Monkeypox virus
- EFC
Entry fusion complex
- MV
Mature virions
- EV
Extracellular virions
- GAG
Glycosaminoglycan
- CSPG
Chondroitin sulfate proteoglycan
- IEV
Intracellular enveloped virus
- EEV
Extracellular enveloped virus
- TLRs
Toll-like receptors
- PAMP
Patterns associated with
CRediT authorship contribution statement
P.P., N.K.K., N.K. and E.H.C. conceived the manuscript with inputs from all authors. P.P., N.K. and A.N. contributed to drawing diagrams on biorender and adobe illustrator software. S.K.V., A.N., N.K. and M.S. contributed substantially to writing and review of the manuscript. N.K.K., N.K., S.K.V., E.H.C. and M.S. revised the paper. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
This research was funded by the National Research Foundation of Korea (NRF), funded by the Korean government (2021R1A6A1A03038785, 2021R1F1A1055694, 2021R1C1C1013875), and by Kwangwoon University in 2022. SKV acknowledges infrastructure support available through the DBT-BUILDER program (BT/INF/22/SP42155/2021) at KIIT UNIVERSITY.
Editor: Ewa Korzeniewska
Data availability
No data was used for the research described in the article.
References
- 2022.2022 Monkeypox Outbreak Global Map _ Monkeypox _ Poxvirus _ CDC . 2022. Centers Dis. Control Prev.https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html [Google Scholar]
- Abnet C.C., Tanguay R.L., Heideman W., Peterson R.E. Transactivation activity of human, zebrafish, and rainbow trout aryl hydrocarbon receptors expressed in COS-7 cells: greater insight into species differences in toxic potency of polychlorinated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners. Toxicol. Appl. Pharmacol. 1999;159:41–51. doi: 10.1006/taap.1999.8719. [DOI] [PubMed] [Google Scholar]
- Aggad D., Mazel M., Boudinot P., Mogensen K.E., Hamming O.J., Hartmann R., Kotenko S., Herbomel P., Lutfalla G., Levraud J.-P. The two groups of zebrafish virus-induced interferons signal via distinct receptors with specific and shared chains. J. Immunol. 2009;183:3924–3931. doi: 10.4049/jimmunol.0901495. [DOI] [PubMed] [Google Scholar]
- Agrawal A.S., Garron T., Tao X., Peng B.-H., Wakamiya M., Chan T.-S., Couch R.B., Tseng C.-T.K. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J. Virol. 2015;89:3659–3670. doi: 10.1128/jvi.03427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akle V., Agudelo-Dueñas N., Molina-Rodriguez M.A., Kartchner L.B., Ruth A.M., González J.M., Forero-Shelton M. Establishment of larval zebrafish as an animal model to investigate trypanosoma cruzi motility in vivo. J. Vis. Exp. 2017;2017 doi: 10.3791/56238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese M., Mejías-Pérez E., Bauernfried S., Graf N. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat. Med. 2022;28:496–503. doi: 10.1038/s41591-022-01715-4. [DOI] [PubMed] [Google Scholar]
- Alibaud L., Rombouts Y., Trivelli X., Burguière A., Cirillo S.L.G., Cirillo J.D., Dubremetz J.F., Guérardel Y., Lutfalla G., Kremer L. A Mycobacterium marinum TesA mutant defective for major cell wall-associated lipids is highly attenuated in dictyostelium discoideum and zebrafish embryos. Mol. Microbiol. 2011;80:919–934. doi: 10.1111/j.1365-2958.2011.07618.x. [DOI] [PubMed] [Google Scholar]
- Amsterdam A., Nissen R.M., Sun Z., Swindell E.C., Farrington S., Hopkins N. Identification of 315 genes essential for early zebrafish development. Proc. Natl. Acad. Sci. U. S. A. 2004;101:12792–12797. doi: 10.1073/pnas.0403929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A., Sadler K.C., Lai K., Farrington S., Bronson R.T., Lees J.A., Hopkins N. Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol. 2004;2 doi: 10.1371/journal.pbio.0020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G.W., Lee J.O., Anderson A.O., Powell N., Mangiafico J.A., Meadors G. Efficacy of a Rift Valley fever virus vaccine against an aerosol infection in rats. Vaccine. 1991;9:710–714. doi: 10.1016/0264-410X(91)90285-E. [DOI] [PubMed] [Google Scholar]
- Anderson G.W., Smith J.F. Immunoelectron microscopy of Rift Valley fever viral morphogenesis in primary rat hepatocytes. Virology. 1987;161:91–100. doi: 10.1016/0042-6822(87)90174-7. [DOI] [PubMed] [Google Scholar]
- Anelli V., Ordas A., Kneitz S., Sagredo L.M., Gourain V., Schartl M., Meijer A.H., Mione M. Ras-induced miR-146a and 193a target Jmjd6 to regulate melanoma progression. Front. Genet. 2018;9 doi: 10.3389/fgene.2018.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anelli V., Villefranc J.A., Chhangawala S., Martinez-Mcfaline R., Riva E., Nguyen A., Verma A., Bareja R., Chen Z., Scognamiglio T., Elemento O., Houvras Y. Oncogenic BRAF disrupts thyroid morphogenesis and function via twist expression. elife. 2017;6 doi: 10.7554/eLife.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Animals Facts Why use the zebrafish in research ? Your Genome. 2015:1–5. [Google Scholar]
- Antkiewicz D.S., Peterson R.E., Heideman W. Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2006;94:175–182. doi: 10.1093/toxsci/kfl093. [DOI] [PubMed] [Google Scholar]
- Antoine T.E., Jones K.S., Dale R.M., Shukla D., Tiwari V. Zebrafish: modeling for herpes simplex virus infections. Zebrafish. 2014;11:17–25. doi: 10.1089/zeb.2013.0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravamudhan P., Raghunathan K., Konopka-Anstadt J., Pathak A., Sutherland D.M., Carter B.D., Dermody T.S. Reovirus uses macropinocytosis-mediated entry and fast axonal transport to infect neurons. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arceci R.J. TEL-AML1 transgenic zebrafish model of precursor B cell acute lymphoblastic leukemia. Yearb. Oncol. 2008;2008:263–264. doi: 10.1016/s1040-1741(08)79187-5. [DOI] [Google Scholar]
- Asharani P.V., Lianwu Y., Gong Z., Valiyaveettil S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology. 2011;5:43–54. doi: 10.3109/17435390.2010.489207. [DOI] [PubMed] [Google Scholar]
- Astrofsky K.M., Schrenzel M.D., Bullis R.A., Smolowitz R.M., Fox J.G. Diagnosis and management of atypical mycobacterium spp. Infections in established laboratory zebrafish (Brachydanio rerio) facilities. Comp. Med. 2000;50:666–672. [PubMed] [Google Scholar]
- Atchison R.W., Casto B.C., Hammon W.M.D. Adenovirus-associated defective virus particles. Science (80-. ) 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Avcı E. 2014. Zebrafish Manipulation Techniques Advantages of Using Zebrafish as a Model Organism.https://andor.oxinst.com/learning/view/article/advantages-of-using-zebrafish-as-a-model-organism [Google Scholar]
- B R., D B., J K., J C., H E., K M., H F. Clinical outcome of Henipavirus infection in hamsters is determined by the route and dose of infection. J. Virol. 2011;85:7658–7671. doi: 10.1128/JVI.00473-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- B R., KN B., F F., JB G., AC H., D B., J C., D S., A M., L K., D L., CC B., H F., TW G. A novel model of lethal Hendra virus infection in African green monkeys and the effectiveness of ribavirin treatment. J. Virol. 2010;84:9831–9839. doi: 10.1128/JVI.01163-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- B R., R W., AN F. Recent progress in henipavirus research: molecular biology, genetic diversity, animal models. Antiviral Res. 2012;95:135–149. doi: 10.1016/j.antiviral.2012.05.008. [DOI] [PubMed] [Google Scholar]
- BA M., D M., G C., J B., K H., G R., D G., J M., LI P., BT E., LF W., KN B., CC B. Feline model of acute Nipah virus infection and protection with a soluble glycoprotein-based subunit vaccine. J. Virol. 2006;80:12293–12302. doi: 10.1128/JVI.01619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D.G. Natural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin. Microbiol. Rev. 1998;11:231–266. doi: 10.1128/cmr.11.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkrishna A., Khandrika L., Varshney A. Giloy ghanvati (Tinospora cordifolia (Willd.) Hook. f. And Thomson) reversed SARS-CoV-2 viral spike-protein induced disease phenotype in the xenotransplant model of humanized zebrafish. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.635510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkrishna A., Pokhrel S., Singh H., Joshi M., Mulay V.P., Haldar S., Varshney A. Withanone from withania somnifera attenuates sars-cov-2 rbd and host ace2 interactions to rescue spike protein induced pathologies in humanized zebrafish model. Drug Des. Dev. Ther. 2021;15:1111–1133. doi: 10.2147/DDDT.S292805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkrishna A., Solleti S.K., Verma S., Varshney A. Application of humanized zebrafish model in the suppression of SARS-CoV-2 spike protein induced pathology by tri-herbal medicine coronil via cytokine modulation. Molecules. 2020;25 doi: 10.3390/molecules25215091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee N., Mukhopadhyay S. Viral glycoproteins: biological role and application in diagnosis. VirusDis. 2016;27:1–11. doi: 10.1007/s13337-015-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir I., Lone F.A., Bhat R.A., Mir S.A., Dar Z.A., Dar S.A. Bioremediation Biotechnol. Sustain. Approaches to Pollut. Degrad. 2020. Concerns and threats of contamination on aquatic ecosystems; pp. 1–26. [DOI] [Google Scholar]
- Basten S.G., Davis E.E., Gillis A.J.M., van Rooijen E., Stoop H., Babala N., Logister I., Heath Z.G., Jonges T.N., Katsanis N., Voest E.E., van Eeden F.J., Medema R.H., Ketting R.F., Schulte-Merker S., Looijenga L.H.J., Giles R.H. Mutations in LRRC50 predispose zebrafish and humans to seminomas. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffagna G. Zebrafish as a smart model to understand regeneration after heart injury: how fish could help humans. Front. Cardiovasc. Med. 2019;6 doi: 10.3389/fcvm.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell T.M., Shaia C.I., Bearss J.J., Mattix M.E., Koistinen K.A., Honnold S.P., Zeng X., Blancett C.D., Donnelly G.C., Shamblin J.D., Wilkinson E.R., Cashman K.A. Temporal progression of lesions in Guinea pigs infected with Lassa virus. Vet. Pathol. 2017;54:549–562. doi: 10.1177/0300985816677153. [DOI] [PubMed] [Google Scholar]
- Bello-Perez M., Pereiro P., Coll J., Novoa B., Perez L., Falco A. Zebrafish C-reactive protein isoforms inhibit SVCV replication by blocking autophagy through interactions with cell membrane cholesterol. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-57501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard E.L., van der Sar A.M., Ellett F., Lieschke G.J., Spaink H.P., Meijer A.H. Infection of zebrafish embryos with intracellular bacterial pathogens. J. Vis. Exp. 2012 doi: 10.3791/3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhnia M.R.-E.-I., Maybeno M., Blum D., Aguilar-Sino R., Matho M., Meng X., Head S., Felgner P.L., Zajonc D.M., Koriazova L., Kato S., Burton D.R., Xiang Y., Crowe J.E., Peters B., Crotty S. Unusual features of vaccinia virus extracellular virion form neutralization resistance revealed in human antibody responses to the smallpox vaccine. J. Virol. 2013;87:1569–1585. doi: 10.1128/jvi.02152-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bente D., Gren J., Strong J.E., Feldmann H. Disease modeling for ebola and Marburg viruses. DMM Dis. Model. Mech. 2009;2:12–17. doi: 10.1242/dmm.000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bente D.A., Alimonti J.B., Shieh W.-J., Camus G., Ströher U., Zaki S., Jones S.M. Pathogenesis and immune response of Crimean-Congo hemorrhagic fever virus in a STAT-1 knockout mouse model. J. Virol. 2010;84:11089–11100. doi: 10.1128/jvi.01383-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereczky S., Lindegren G., Karlberg H., Åkerström S., Klingström J., Mirazimi A. Crimean-Congo hemorrhagic fever virus infection is lethal for adult type I interferon receptor-knockout mice. J. Gen. Virol. 2010;91:1473–1477. doi: 10.1099/vir.0.019034-0. [DOI] [PubMed] [Google Scholar]
- Bertzbach L.D., Vladimirova D., Dietert K., Abdelgawad A., Gruber A.D., Osterrieder N., Trimpert J. SARS-CoV-2 infection of chinese hamsters (Cricetulus griseus) reproduces COVID-19 pneumonia in a well-established small animal model. Transbound. Emerg. Dis. 2021;68:1075–1079. doi: 10.1111/tbed.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettauer R.H. Chimpanzees in hepatitis C virus research: 1998–2007. J. Med. Primatol. 2010;39:9–23. doi: 10.1111/j.1600-0684.2009.00390.x. [DOI] [PubMed] [Google Scholar]
- Bird B.H., Spengler J.R., Chakrabarti A.K., Khristova M.L., Sealy T.K., Coleman-McCray J.D., Martin B.E., Dodd K.A., Goldsmith C.S., Sanders J., Zaki S.R., Nichol S.T., Spiropoulou C.F. Humanized mouse model of ebola virus disease mimics the immune responses in human disease. J. Infect. Dis. 2015;212:703–711. doi: 10.1093/infdis/jiv538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.V., Vaccari M., Doyle-Meyers L.A., Roy C.J., Russell-Lodrigue K., Fahlberg M., Monjure C.J., Beddingfield B., Plante K.S., Plante J.A., Weaver S.C., Qin X., Midkiff C.C., Lehmicke G., Golden N., Threeton B., Penney T., Allers C., Barnes M.B., Pattison M., Datta P.K., Maness N.J., Birnbaum A., Fischer T., Bohm R.P., Rappaport J. Acute respiratory distress in aged, SARS-CoV-2–Infected african green monkeys but not rhesus macaques. Am. J. Pathol. 2021;191:274–282. doi: 10.1016/j.ajpath.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontrop R.E., Watkins D.I. MHC polymorphism: AIDS susceptibility in non-human primates. Trends Immunol. 2005;26:227–233. doi: 10.1016/j.it.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Boran H., Şaffak S. Transcriptome alterations and genotoxic influences in zebrafish larvae after exposure to dissolved aluminum and aluminum oxide nanoparticles. Toxicol. Mech. Methods. 2020;30:546–554. doi: 10.1080/15376516.2020.1786759. [DOI] [PubMed] [Google Scholar]
- Bosco-Lauth A.M., Calvert A.E., Root J.J., Gidlewski T., Bird B.H., Bowen R.A., Muehlenbachs A., Zaki S.R., Brault A.C. Vertebrate host susceptibility to heartland virus. Emerg. Infect. Dis. 2016;22:2070–2077. doi: 10.3201/eid2212.160472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant S., Stanifer M., Kural C., Cureton D.K., Massol R., Nibert M.L., Kirchhausen T. Similar uptake but different trafficking and escape routes of reovirus virions and infectious subvirion particles imaged in polarized madin-Darby canine kidney cells. Mol. Biol. Cell. 2013;24:1196–1207. doi: 10.1091/mbc.E12-12-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier N.M. Animal models for influenza virus transmission studies: a historical perspective. Curr. Opin. Virol. 2015;13:101–108. doi: 10.1016/j.coviro.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon M.K., Davis J.M., Mathias J.R., Hall C.J., Emerson J.C., Crosier P.S., Huttenlocher A., Ramakrishnan L., Moskowitz S.M. Pseudomonas aeruginosa type III secretion system interacts with phagocytes to modulate systemic infection of zebrafish embryos. Cell. Microbiol. 2009;11:755–768. doi: 10.1111/j.1462-5822.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenot A., King K.Y., Janowiak B., Griffith O., Caparon M.G. Contribution of glutathione peroxidase to the virulence of streptococcus pyogenes. Infect. Immun. 2004;72:408–413. doi: 10.1128/IAI.72.1.408-413.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron R., Ortiz A., Wilschut J. Cellular cytoplasmic delivery of a polypeptide toxin by reconstituted influenza virus envelopes (Virosomes) Biochemistry. 1994;33:9110–9117. doi: 10.1021/bi00197a013. [DOI] [PubMed] [Google Scholar]
- Browne S.K., Beeler J.A., Roberts J.N. Summary of the vaccines and related biological products advisory committee meeting held to consider evaluation of vaccine candidates for the prevention of respiratory syncytial virus disease in RSV-naïve infants. Vaccine. 2020;38:101–106. doi: 10.1016/j.vaccine.2019.10.048. [DOI] [PubMed] [Google Scholar]
- Buller R.M.L., Janik J.E., Sebring E.D., Rose J.A. Herpes simplex virus types 1 and 2 completely help adenovirus-associated virus replication. J. Virol. 1981;40:241–247. doi: 10.1128/jvi.40.1.241-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos J.S., Ripoll-Gomez J., Alfaro J.M., Sastre I., Valdivieso F. Zebrafish as a new model for herpes simplex virus type 1 infection. Zebrafish. 2008;5:323–333. doi: 10.1089/zeb.2008.0552. [DOI] [PubMed] [Google Scholar]
- Burke E. 2016. Why Use Zebrafish to Study Human Diseases?irp.nih.gov/blog/post/2016/08/why-use-zebrafish-to-study-human-diseases [Google Scholar]
- Burke E. NIH; 2016. Why Use Zebrafish to Study Human Diseases? irp.nih.gov/blog/post/2016/08/why-use-zebrafish-to-study-human-diseases. [Google Scholar]
- CK K., AE B., JP N., MA M., TM L.-L. Escherichia coli O78 isolated from septicemic lambs shows high pathogenicity in a zebrafish model. Vet. Res. 2017;48:3. doi: 10.1186/s13567-016-0407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Baldini R.L., Rahme L.G. Common mechanisms for pathogens of plants and animals. Annu. Rev. Phytopathol. 2001;39:259–284. doi: 10.1146/annurev.phyto.39.1.259. [DOI] [PubMed] [Google Scholar]
- Cao L., Yan D., Xiao J., Feng H., Chang M.X. The zebrafish antiapoptotic protein BIRC2 promotes Edwardsiella piscicida infection by inhibiting caspases and accumulating p53 in a p53 transcription-dependent and -independent manner. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.781680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion R., Brasky K., Mansfield K., Johnson C., Gonzales M., Ticer A., Lukashevich I., Tardif S., Patterson J. Lassa virus infection in experimentally infected marmosets: liver pathology and immunophenotypic alterations in target tissues. J. Virol. 2007;81:6482–6490. doi: 10.1128/jvi.02876-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman K.A., Smith M.A., Twenhafel N.A., Larson R.A., Jones K.F., Allen R.D., Dai D., Chinsangaram J., Bolken T.C., Hruby D.E., Amberg S.M., Hensley L.E., Guttieri M.C. Evaluation of Lassa antiviral compound ST-193 in a Guinea pig model. Antivir. Res. 2011;90:70–79. doi: 10.1016/j.antiviral.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman K.A., Wilkinson E.R., Shaia C.I., Facemire P.R., Bell T.M., Bearss J.J., Shamblin J.D., Wollen S.E., Broderick K.E., Sardesai N.Y., Schmaljohn C.S. A DNA vaccine delivered by dermal electroporation fully protects cynomolgus macaques against Lassa fever. Hum. Vaccin. Immunother. 2017;13:2902–2911. doi: 10.1080/21645515.2017.1356500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cdc.gov . Vol. 2022. CDC; 2022. History of Ebola Virus Disease | History | Ebola (Ebola Virus Disease)https://www.cdc.gov/vhf/ebola/history/summaries.html [Google Scholar]
- Ceol C.J., Houvras Y., Jane-Valbuena J., Bilodeau S., Orlando D.A., Battisti V., Fritsch L., Lin W.M., Hollmann T.J., Ferré F., Bourque C., Burke C.J., Turner L., Uong A., Johnson L.A., Beroukhim R., Mermel C.H., Loda M., Ait-Si-Ali S., Garraway L.A., Young R.A., Zon L.I. The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature. 2011;471:513–518. doi: 10.1038/nature09806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.W., Zhang A.J., Yuan S., Poon V.K.M., Chan C.C.S., Lee A.C.Y., Chan W.M., Fan Z., Tsoi H.W., Wen L., Liang R., Cao J., Chen Y., Tang K., Luo C., Cai J.P., Kok K.H., Chu H., Chan K.H., Sridhar S., Chen Z., Chen H., To K.K.W., Yuen K.Y. Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in a Golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 2020;71:2428–2446. doi: 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.M., Ku L.L., Chan P.C.Y., Cheuk W.K. Metallothionein gene expression in zebrafish embryo-larvae and ZFL cell-line exposed to heavy metal ions. Mar. Environ. Res. 2006;62 doi: 10.1016/j.marenvres.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Chan P.K., Cheng S.H. Cadmium-induced ectopic apoptosis in zebrafish embryos. Arch. Toxicol. 2003;77:69–79. doi: 10.1007/s00204-002-0411-1. [DOI] [PubMed] [Google Scholar]
- Chao S.J., Huang C.P., Lam C.C., Hua L.C., Chang S.H., Huang C. Transformation of copper oxide nanoparticles as affected by ionic strength and its effects on the toxicity and bioaccumulation of copper in zebrafish embryo. Ecotoxicol. Environ. Saf. 2021;225 doi: 10.1016/j.ecoenv.2021.112759. [DOI] [PubMed] [Google Scholar]
- Chappell J.D., Prota A.E., Dermody T.S., Stehle T. Crystal structure of reovirus attachment protein σ1 reveals evolutionary relationship to adenovirus fiber. EMBO J. 2002;21:1–11. doi: 10.1093/emboj/21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Jette C., Kanki J.P., Aster J.C., Look A.T., Griffin J.D. NOTCH1-induced T-cell leukemia in transgenic zebrafish. Leukemia. 2007;21:462–471. doi: 10.1038/sj.leu.2404546. [DOI] [PubMed] [Google Scholar]
- Chen Xiaojuan, Huang C., Wang X., Chen J., Bai C., Chen Y., Chen Xiangping, Dong Q., Yang D. BDE-47 disrupts axonal growth and motor behavior in developing zebrafish. Aquat. Toxicol. 2012;120–121:35–44. doi: 10.1016/j.aquatox.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Chen Y.Z., Yang Y.L., Chu W.L., You M.S., Lo H.J. Zebrafish egg infection model for studying Candida albicans adhesion factors. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C.L., Huang S.J., Wu C.L., Gong H.Y., Ken C.F., Hu S.Y., Wu J.L. Transgenic expression of omega-3 PUFA synthesis genes improves zebrafish survival during Vibrio vulnificus infection. J. Biomed. Sci. 2015;22 doi: 10.1186/s12929-015-0208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Cheng S.H. Influence of carbon nanotube length on toxicity to zebrafish embryos. Int. J. Nanomedicine. 2012;7:3731–3739. doi: 10.2147/IJN.S30459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.S.A., Chan H.H., Chan C.M., Wang X., Webb S.E., Leung K.W., Tsim K.W.K., Miller A.L. Neuromasts and olfactory organs of zebrafish larvae represent possible sites of SARS-CoV-2 pseudovirus host cell entry. J. Virol. 2022;96:e01418–e01422. doi: 10.1128/jvi.01418-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi T.Y., Choi T.I., Lee Y.R., Choe S.K., Kim C.H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021;53:310–317. doi: 10.1038/s12276-021-00571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y.T., Chen L.Y., Tsai S.L., Tu H.C., Lu J.W., Ciou S.C., Wang H.D., Yuh C.H. Ribose-5-phosphate isomerase a overexpression promotes liver cancer development in transgenic zebrafish via activation of ERK and β-catenin pathways. Carcinogenesis. 2019;40:461–473. doi: 10.1093/carcin/bgy155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow W.S., Chan W.K.L., Chan K.M. Toxicity assessment and vitellogenin expression in zebrafish (Danio rerio) embryos and larvae acutely exposed to bisphenol a, endosulfan, heptachlor, methoxychlor and tetrabromobisphenol a. J. Appl. Toxicol. 2013;33:670–678. doi: 10.1002/jat.2723. [DOI] [PubMed] [Google Scholar]
- Hall Christopher J., Flores Maria Vega, Oehlers Stefan H., Sanderson Leslie E., Lam Enid Y., Crosier Kathryn E., Crosier Philip S. Infection-responsive expansion of the hematopoietic stem and progenitor cell compartment in zebrafish is dependent upon inducible nitric oxide. Cell Stem Cell. 2012;10:198–209. doi: 10.1016/j.stem.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Chu C.Y., Chen C.F., Rajendran R.S., Shen C.N., Chen T.H., Yen C.C., Chuang C.K., Lin D.S., Hsiao C.Der. Overexpression of Akt1 enhances adipogenesis and leads to lipoma formation in zebrafish. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C.-S., Hsiao J.-C., Chang Y.-S., Chang W. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J. Virol. 1998;72:1577–1585. doi: 10.1128/jvi.72.2.1577-1585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunga E., Gencoa M.C., Megrelisa L., Rudermana J.V. Effects of bisphenol a and triclocarban on brain-specific expression of aromatase in early zebrafish embryos. Proc. Natl. Acad. Sci. U. S. A. 2011;108:17732–17737. doi: 10.1073/pnas.1115187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy A.E., Lee J.S.W., Leibman M., Kostun Z., Davidson A.J., Hung D.T. Pseudomonas aeruginosa infection of zebrafish involves both host and pathogen determinants. Infect. Immun. 2009;77:1293–1303. doi: 10.1128/IAI.01181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cm W., Ej K., Aw K., Mc Z., Jc M., Es L. The mosaic structure of variation in the laboratory mouse genome. Nature. 2002;420:574–578. doi: 10.1038/nature01252. [DOI] [PubMed] [Google Scholar]
- Cockrell A.S., Yount B.L., Scobey T., Jensen K., Douglas M., Beall A., Tang X.C., Marasco W.A., Heise M.T., Baric R.S. A mouse model for MERS coronavirus-induced acute respiratory distress syndrome. Nat. Microbiol. 2016;2 doi: 10.1038/nmicrobiol.2016.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Code N.M., Beaverton A. 2008. Oregon National Primate Research Center.https://www.ohsu.edu/onprc [Google Scholar]
- Collier T.K., Scholz N.L., Incardona J.P. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol. Appl. Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Collodi P. A unique journal for a unique experimental model. Zebrafish. 2004;1 doi: 10.1089/154585404774101608. 1–1. [DOI] [PubMed] [Google Scholar]
- Cossart P., Sansonetti P.J. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science (80-. ) 2004;304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- Costa K.C.M., Brigante T.A.V., Fernandes G.G., Scomparin D.S., Scarante F.F., de Oliveira D.P., Campos A.C. Zebrafish as a translational model: An experimental alternative to study the mechanisms involved in anosmia and possible neurodegenerative aspects of covid-19? eNeuro. 2021;8 doi: 10.1523/ENEURO.0027-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch R.B. Viruses and indoor air pollution. Bull. N. Y. Acad.Med. J. Urban Health. 1981;57:907–921. [PMC free article] [PubMed] [Google Scholar]
- Crim M.J., Riley L.K. Viral diseases in zebrafish: what is known and unknown. ILAR J. 2012;53:135–143. doi: 10.1093/ilar.53.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan M.R., Tobin D.M. Fit for consumption: zebrafish as a model for tuberculosis. DMM Dis. Model. Mech. 2014;7:777–784. doi: 10.1242/dmm.016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R.W., Fenton K.A., Geisbert J.B., Ebihara H., Mire C.E., Geisbert T.W. Comparison of the pathogenesis of the Angola and ravn strains of Marburg virus in the outbred Guinea pig model. J. Infect. Dis. 2015;212:S258–S270. doi: 10.1093/infdis/jiv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Eden J.S., Holmes E.C., Wang L.F. Adaptive evolution of bat dipeptidyl peptidase 4 (dpp4): implications for the origin and emergence of Middle East respiratory syndrome coronavirus. Virol. J. 2013;10 doi: 10.1186/1743-422X-10-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DA A., AR G., MG L., NL G., JG B., C M., DS R. Aerosol exposure to the angola strain of marburg virus causes lethal viral hemorrhagic fever in cynomolgus macaques. Vet. Pathol. 2010;47:831–851. doi: 10.1177/0300985810378597. [DOI] [PubMed] [Google Scholar]
- Dabbu Kumar J., Jian L., Rong H., Hua Z. Emerging and reemerging human viral diseases. Ann. Microbiol. Res. 2018;2 doi: 10.36959/958/567. [DOI] [Google Scholar]
- Dave G. The influence of pH on the toxicity of aluminum, cadmium, and iron to eggs and larvae of the zebrafish, Brachydanio rerio. Ecotoxicol. Environ. Saf. 1985;10:253–267. doi: 10.1016/0147-6513(85)90072-7. [DOI] [PubMed] [Google Scholar]
- Dave G., Xiu R. Toxicity of mercury, copper, nickel, lead, and cobalt to embryos and larvae of zebrafish, Brachydanio rerio. Arch. Environ. Contam. Toxicol. 1991;21:126–134. doi: 10.1007/BF01055567. [DOI] [PubMed] [Google Scholar]
- Davenport F.M., Hennessy A.V., Francis T. Epidemiologic and immunologic significance of age distribution of antibody to antigenic variants of influenza virus. J. Exp. Med. 1953;98:641–656. doi: 10.1084/jem.98.6.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.M., Clay H., Lewis J.L., Ghori N., Herbomel P., Ramakrishnan L. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity. 2002;17:693–702. doi: 10.1016/S1074-7613(02)00475-2. [DOI] [PubMed] [Google Scholar]
- Dayyani F., Wang J., Yeh J.R.J., Ann E.Y., Tobey E., Zhang D.E., Bernstein I.D., Peterson R.T., Sweetser D.A. Loss of TLE1 and TLE4 from the del(9q) commonly deleted region in AML cooperates with AML1-ETO to affect myeloid cell proliferation and survival. Blood. 2008;111:4338–4347. doi: 10.1182/blood-2007-07-103291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E., Bushmaker T., Scott D., Feldmann H., Munster V.J. Nipah virus transmission in a hamster model. PLoS Negl. Trop. Dis. 2011;5 doi: 10.1371/journal.pntd.0001432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decostere A., Hermans K., Haesebrouck F. Piscine mycobacteriosis: a literature review covering the agent and the disease it causes in fish and humans. Vet. Microbiol. 2004;99:159–166. doi: 10.1016/j.vetmic.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Delannoy C.M.J., Crumlish M., Fontaine M.C., Pollock J., Foster G., Dagleish M.P., Turnbull J.F., Zadoks R.N. Human Streptococcus agalactiae strains in aquatic mammals and fish. BMC Microbiol. 2013;13 doi: 10.1186/1471-2180-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D.S. Virus entry: molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2004;2:109–122. doi: 10.1038/nrmicro817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C.B., Zhang J.P., Zhao Y., Peng Z.G., Song D.Q., Jiang J.D. Zebrafish as a potential model organism for drug test against hepatitis C virus. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo E. Introduction to virus origins and their role in biological evolution. Virus as Popul. 2020;1–33 doi: 10.1016/b978-0-12-816331-3.00001-5. [DOI] [Google Scholar]
- Dovey M., White R.M., Zon L.I. Oncogenic NRAS cooperates with p53 loss to generate melanoma in zebrafish. Zebrafish. 2009;6:397–404. doi: 10.1089/zeb.2009.0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle S.E., Schreckhise H., Khuu-Duong K., Henderson K., Rosler R., Storey H., Yao L., Liu H., Barahmand-Pour F., Sivakumar P., Chan C., Birks C., Foster D., Clegg C.H., Wietzke-Braun P., Mihm S., Klucher K.M. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology. 2006;44:896–906. doi: 10.1002/hep.21312. [DOI] [PubMed] [Google Scholar]
- Drevinek P., Mahenthiralingam E. Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence. Clin. Microbiol. Infect. 2010;16:821–830. doi: 10.1111/j.1469-0691.2010.03237.x. [DOI] [PubMed] [Google Scholar]
- Driever W., Solnica-Krezel L., Schier A.F., Neuhauss S.C.F., Malicki J., Stemple D.L., Stainier D.Y.R., Zwartkruis F., Abdelilah S., Rangini Z., Belak J., Boggs C. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- Driouich J.S., Cochin M., Lingas G., Moureau G., Touret F., Petit P.R., Piorkowski G., Barthélémy K., Laprie C., Coutard B., Guedj J., de Lamballerie X., Solas C., Nougairède A. Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-21992-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J., Yu Y., Shi H., Tian L., Guo C., Huang P., Zhou X., Peng S., Sun Z. Toxic effects of silica nanoparticles on zebrafish embryos and larvae. PLoS One. 2013;8 doi: 10.1371/journal.pone.0074606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., Horne D., William Cashdollar L., Joklik W.K., Lee P.W.K. Identification of conserved domains in the cell attachment proteins of the three serotypes of reovirus. Virology. 1990;174:399–409. doi: 10.1016/0042-6822(90)90093-7. [DOI] [PubMed] [Google Scholar]
- Dycke J.Van, Ny A., Maes J., Hosmillo M., Cuvry A., Goodfellow I., Nogueira T.C., Verbeken E., Matthijnssens J., Witte P.De, Neyts J., Rocha-pereira J. A robust human norovirus replication model in zebrafish larvae. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1008009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen J.S., Grunwald D.J. Headwaters of the zebrafish — emergence of a new model vertebrate. Nat. Rev. Genet. 2002;3:711–717. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- Elfawy H.A., Anupriya S., Mohanty S., Patel P., Ghosal S., Panda P.K., Das B., Verma S.K., Patnaik S. Molecular toxicity of Benzo(a)pyrene mediated by elicited oxidative stress infer skeletal deformities and apoptosis in embryonic zebrafish. Sci. Total Environ. 2021;789 doi: 10.1016/j.scitotenv.2021.147989. [DOI] [PubMed] [Google Scholar]
- Elliott S.J., Sperandio V., Giron J.A., Shin S., Mellies J.L., Wainwright L., Hutcheson S.W., McDaniel T.K., Kaper J.B. The locus of enterocyte effacement (LEE)-encoded regulator controls expression of both LEE- and non-LEE-encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 2000;68:6115–6126. doi: 10.1128/IAI.68.11.6115-6126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enya S., Kawakami K., Suzuki Y., Kawaoka S. A novel zebrafish intestinal tumor model reveals a role for cyp7a1-dependent tumor-liver crosstalk in causing adverse effects on the host. DMM Dis. Model. Mech. 2018;11 doi: 10.1242/dmm.032383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- F. H., P T., D G., M D. Nonhuman primates are relevant models for research in hematology, immunology and virology. Eur. Cytokine Netw. 2005;16:104–116. [PubMed] [Google Scholar]
- Facchinello N., Skobo T., Meneghetti G., Colletti E., Dinarello A., Tiso N., Costa R., Gioacchini G., Carnevali O., Argenton F., Colombo L., Dalla Valle L. Nr3c1 null mutant zebrafish are viable and reveal DNA-binding-independent activities of the glucocorticoid receptor. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-04535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fåhræus-Van Ree G.E., Payne J.F. Effect of toxaphene on reproduction of fish. Chemosphere. 1997;34:855–867. doi: 10.1016/S0045-6535(97)00016-7. [DOI] [PubMed] [Google Scholar]
- Fan Y., Thompson L., Lyu Z., Cameron T.A., De Lay N.R., Krachler A.M., Ling J. Optimal translational fidelity is critical for salmonella virulence and host interactions. Nucleic Acids Res. 2019;47:5356–5367. doi: 10.1093/nar/gkz229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell S.L.N., Reylander K.G., Iovine M.K., Lowe-Krentz L.J. Novel heparin receptor transmembrane protein 184a regulates angiogenesis in the adult zebrafish caudal fin. Front. Physiol. 2017;8 doi: 10.3389/fphys.2017.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzani T.A., Földes K., Hanifehnezhad A., Ilce B.Y., Dagalp S.B., Khiabani N.A., Ergünay K., Alkan F., Karaoglu T., Bodur H., Ozkul A. Bovine herpesvirus type 4 (BoHV-4) vector delivering nucleocapsid protein of crimean-Congo hemorrhagic fever virus induces comparable protective immunity against lethal challenge in IFNα/βγγR-/-/mice models. Viruses. 2019;11 doi: 10.3390/v11030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipić A., Gutierrez-Aguirre I., Primc G., Mozetič M., Dobnik D. Cold plasma, a new Hope in the field of virus inactivation. Trends Biotechnol. 2020;38:1278–1291. doi: 10.1016/j.tibtech.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatz L., Rieger T., Merkler D., Bergthaler A., Regen T., Schedensack M., Bestmann L., Verschoor A., Kreutzfeldt M., Brück W., Hanisch U.K., Günther S., Pinschewer D.D. T cell-dependence of Lassa fever pathogenesis. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo C.H., Lou H., Whitbeck J.C., Ponce-de-León M., Atanasiu D., Eisenberg R.J., Cohen G.H. Vaccinia virus L1 binds to cell surfaces and blocks virus entry independently of glycosaminoglycans. Virology. 2009;385:368–382. doi: 10.1016/j.virol.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester A.M., Grabher C., Mcbride E.R., Boyd E.R., Vigerstad M.H., Edgar A., Kai F.B., Da’as S.I., Payne E., Look A.T., Berman J.N. NUP98-HOXA9-transgenic zebrafish develop a myeloproliferative neoplasm and provide new insight into mechanisms of myeloid leukaemogenesis. Br. J. Haematol. 2011;155:167–181. doi: 10.1111/j.1365-2141.2011.08810.x. [DOI] [PubMed] [Google Scholar]
- Frazer J.K., Batchelor L.A., Bradley D.F., Brown K.H., Dobrinski K.P., Lee C., Trede N.S. Genomic amplification of an endogenous retrovirus in zebrafish T-cell malignancies. Adv. Hematol. 2012;2012 doi: 10.1155/2012/627920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey Freida. Cynomolgus macaque model for ZIKV research - southern research. 2016. https://southernresearch.org/news/southern-research-demonstrates-zika-virus-infection-in-cynomolgus-macaques/ South. Res.
- Freire J.M., Santos N.C., Veiga A.S., Da Poian A.T., Castanho M.A.R.B. Rethinking the capsid proteins of enveloped viruses: multifunctionality from genome packaging to genome transfection. FEBS J. 2015;282:2267–2278. doi: 10.1111/febs.13274. [DOI] [PubMed] [Google Scholar]
- Frierson J.G. The yellow fever vaccine: a history. Yale J. Biol. Med. 2010;83:77–85. [PMC free article] [PubMed] [Google Scholar]
- Frischkorn K.R., Stojanovski A., Paranjpye R. Vibrio parahaemolyticus type IV pili mediate interactions with diatom-derived chitin and point to an unexplored mechanism of environmental persistence. Environ. Microbiol. 2013;15:1416–1427. doi: 10.1111/1462-2920.12093. [DOI] [PubMed] [Google Scholar]
- Fukushima H., Bailone R.L., Baumgartner I., Borra R.C., Correa T., De Aguiar L.K., Janke H., Roça R., Setti P.G. Rev. Educ. Contin. em Med. Veterinária e Zootec. do CRMV-SP 18. 2020. Potenciais usos do modelo animal Zebrafish Danio rerio em pesquisas na Medicina Veterinária. [DOI] [Google Scholar]
- Fukushima H.C.S., Leal C.A.G., Cavalcante R.B., Figueiredo H.C.P., Arijo S., Moriñigo M.A., Ishikawa M., Borra R.C., Ranzani-Paiva M.J.T. Lactococcus garvieae outbreaks in brazilian farms lactococcosis in pseudoplatystoma sp. – development of an autogenous vaccine as a control strategy. J. Fish Dis. 2017;40:263–272. doi: 10.1111/jfd.12509. [DOI] [PubMed] [Google Scholar]
- Gabor K.A., Goody M.F., Mowel W.K., Breitbach M.E., Gratacap R.L., Witten P.E., Kim C.H. Influenza a virus infection in zebrafish recapitulates mammalian infection and sensitivity to anti-influenza drug treatment. DMM Dis. Model. Mech. 2014;7:1227–1237. doi: 10.1242/dmm.014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J.L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Villegas J. The zebrafish disease and drug screening model: a strong ally against Covid-19. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cambero J.P., Beltrán F.J., Encinas A., Rivas F.J., Oropesa A.L. The added value of a zebrafish embryo-larval model in the assessment of wastewater tertiary treatments. Environ. Sci. Water Res. Technol. 2019;5:2269–2279. doi: 10.1039/c9ew00411d. [DOI] [Google Scholar]
- Garrison A.R., Shoemaker C.J., Golden J.W., Fitzpatrick C.J., Suschak J.J., Richards M.J., Badger C.V., Six C.M., Martin J.D., Hannaman D., Zivcec M., Bergeron E., Koehler J.W., Schmaljohn C.S. A DNA vaccine for crimean-Congo hemorrhagic fever protects against disease and death in two lethal mouse models. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gc B., Rp C., Mj R. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Gehrig J., Pandey G., Westhoff J.H. Zebrafish as a model for drug screening in genetic kidney diseases. Front. Pediatr. 2018;6 doi: 10.3389/fped.2018.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert T.W., Daddario-Dicaprio K.M., Hickey A.C., Smith M.A., Chan Y.P., Wang L.F., Mattapallil J.J., Geisbert J.B., Bossart K.N., Broder C.C. Development of an acute and highly pathogenic nonhuman primate model of nipah virus infection. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghobadian M., Nabiuni M., Parivar K., Fathi M., Pazooki J. Toxic effects of magnesium oxide nanoparticles on early developmental and larval stages of zebrafish (Danio rerio) Ecotoxicol. Environ. Saf. 2015;122:260–267. doi: 10.1016/j.ecoenv.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Gill J.A., Lowe L., Nguyen J., Liu P.P., Blake T., Venkatesh B., Aplan P.D. Enforced expression of simian virus 40 large T-antigen leads to testicular germ cell tumors in zebrafish. Zebrafish. 2010;7:333–341. doi: 10.1089/zeb.2010.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjini E., Mansour M.R., Sander J.D., Moritz N., Nguyen A.T., Kesarsing M., Gans E., He S., Chen S., Ko M., Kuang Y.-Y., Yang S., Zhou Y., Rodig S., Zon L.I., Joung J.K., Rao A., Look A.T. A zebrafish model of myelodysplastic syndrome produced through tet2 genomic editing. Mol. Cell. Biol. 2015;35:789–804. doi: 10.1128/mcb.00971-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsey M.S., Rose D., Burkhalter K.L., Breuner N., Bosco-Lauth A.M., Kosoy O.I., Savage H.M. Experimental infection of amblyomma americanum (Acari: Ixodidae) with bourbon virus (Orthomyxoviridae: Thogotovirus) J. Med. Entomol. 2021;58:873–879. doi: 10.1093/jme/tjaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J.W., Shoemaker C.J., Lindquist M.E., Zeng X., Daye S.P., Williams J.A., Liu J., Coffin K.M., Olschner S., Flusin O., Altamura L.A., Kuehl K.A., Fitzpatrick C.J., Schmaljohn C.S., Garrison A.R. GP38-targeting monoclonal antibodies protect adult mice against lethal crimean-Congo hemorrhagic fever virus infection. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aaw9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golling G., Amsterdam A., Sun Z., Antonelli M., Maldonado E., Chen W., Burgess S., Haldi M., Artzt K., Farrington S., Lin S.Y., Nissen R.M., Hopkins N. Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat. Genet. 2002;31:135–140. doi: 10.1038/ng896. [DOI] [PubMed] [Google Scholar]
- Gomes M.C., Mostowy S. The case for modeling human infection in zebrafish. Trends Microbiol. 2020;28:10–18. doi: 10.1016/j.tim.2019.08.005. [DOI] [PubMed] [Google Scholar]
- Gongal P.A., Waskiewicz A.J. Zebrafish model of holoprosencephaly demonstrates a key role for TGIF in regulating retinoic acid metabolism. Hum. Mol. Genet. 2008;17:525–538. doi: 10.1093/hmg/ddm328. [DOI] [PubMed] [Google Scholar]
- Goodale B.C., la Du J.K., Bisson W.H., Janszen D.B., Waters K.M., Tanguay R.L. AHR2 mutant reveals functional diversity of aryl hydrocarbon receptors in zebrafish. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassmann R., Jeang K.T. The roles of microRNAs in mammalian virus infection. Biochim. Biophys. Acta - Gene Regul. Mech. 2008;1779:706–711. doi: 10.1016/j.bbagrm.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber U.F., Singh I., Helenius A. Mechanisms of virus uncoating. Trends Microbiol. 1994;2:52–56. doi: 10.1016/0966-842X(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Greber U.F., Willetts M., Webster P., Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-Z. [DOI] [PubMed] [Google Scholar]
- Grove J., Marsh M. The cell biology of receptor-mediated virus entry. J. Cell Biol. 2011;195:1071–1082. doi: 10.1083/jcb.201108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Guo M., Huang C., Wang X., Zhu Y., Wang L., Wang Z., Zhou L., Fan D., Shi L., Ji G. Titanium dioxide nanoparticle affects motor behavior, neurodevelopment and axonal growth in zebrafish (Danio rerio) larvae. Sci. Total Environ. 2021;754 doi: 10.1016/j.scitotenv.2020.142315. [DOI] [PubMed] [Google Scholar]
- Guénet J.L. Assessing the genetic component of the susceptibility of mice to viral infections. Brief. Funct. Genomic. Proteomic. 2005;4:225–240. doi: 10.1093/bfgp/4.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut P., Reischauer S., Stainier D.Y.R., Arnaout R. Little fish, big data: zebrafish as a model for cardiovascular and metabolic disease. Physiol. Rev. 2017;97:889–938. doi: 10.1152/physrev.00038.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A., Grebliunaite R., Feng H., Kozakewich E., Zhu S., Guo F., Payne E., Mansour M., Dahlberg S.E., Neuberg D.S., den Hertog J., Prochownik E.V., Testa J.R., Harris M., Kanki J.P., Look A.T. Pten mediates myc oncogene dependence in a conditional zebrafish model of T cell acute lymphoblastic leukemia. J. Cell Biol. 2011;194 doi: 10.1083/jcb1941oia4. i4–i4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A., Snyder E.L., Marino-Enriquez A., Zhang Y.X., Sioletic S., Kozakewich E., Grebliunaite R., Ou W.Bin, Sicinska E., Raut C.P., Demetri G.D., Perez-Atayde A.R., Wagner A.J., Fletcher J.A., Fletcher C.D.M., Look A.T. Aberrant AKT activation drives well-differentiated liposarcoma. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16386–16391. doi: 10.1073/pnas.1106127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- H W.D., H W., A M.F. Experimental Lassa virus infection in the squirrel monkey. Am. J. Pathol. 1975;80:261–278. [PMC free article] [PubMed] [Google Scholar]
- Haddock E., Feldmann F., Hawman D.W., Zivcec M., Hanley P.W., Saturday G., Scott D.P., Thomas T., Korva M., Avšič-Županc T., Safronetz D., Feldmann H. A cynomolgus macaque model for Crimean-Congo haemorrhagic fever. Nat. Microbiol. 2018;3:556–562. doi: 10.1038/s41564-018-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffter P., Granato M., Brand M., Mullins M.C., Hammerschmidt M., Kane D.A., Odenthal J., Van Eeden F.J.M., Jiang Y.J., Heisenberg C.P., Kelsh R.N., Furutani-Seiki M., Vogelsang E., Beuchle D., Schach U., Fabian C., Nüsslein-Volhard C. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- Haller O., Kochs G., Weber F. Interferon, mx, and viral countermeasures. Cytokine Growth Factor Rev. 2007;18:425–433. doi: 10.1016/j.cytogfr.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming O.J., Lutfalla G., Levraud J.-P., Hartmann R. Crystal structure of zebrafish interferons I and II reveals conservation of type I interferon structure in vertebrates. J. Virol. 2011;85:8181–8187. doi: 10.1128/jvi.00521-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramis A.P.G., Hurlstone A., van der Velden Y., Begthel H., van den Born M., Offerhaus G.J.A., Clevers H.C. Adenomatous polyposis coli-deficient zebrafish are susceptible to digestive tract neoplasia. EMBO Rep. 2006;7:444–449. doi: 10.1038/sj.embor.7400638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hason M., Bartůnĕk P. Zebrafish models of cancer-new insights on modeling human cancer in a non-mammalian vertebrate. Genes (Basel) 2019;10 doi: 10.3390/genes10110935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.L., Shi X., Man C.H., Ma A.C.H., Ekker S.C., Chow H.C.H., So C.W.E., Choi W.W.L., Zhang W., Zhang Y., Leung A.Y.H. Functions of flt3 in zebrafish hematopoiesis and its relevance to human acute myeloid leukemia. Blood. 2014;123:2518–2529. doi: 10.1182/blood-2013-02-486688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Mansour M.R., Zimmerman M.W., Ki D.H., Layden H.M., Akahane K., Gjini E., de Groh E.D., Perez-Atayde A.R., Zhu S., Epstein J.A., Thomas Look A. Synergy between loss of NF1 and overexpression of MYCN in neuroblastoma is mediated by the GAP-related domain. elife. 2016;5 doi: 10.7554/eLife.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiden T.C.K., Struble C.A., Rise M.L., Hessner M.J., Hutz R.J., Carvan M.J. Molecular targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) within the zebrafish ovary: insights into TCDD-induced endocrine disruption and reproductive toxicity. Reprod. Toxicol. 2008;25:47–57. doi: 10.1016/j.reprotox.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein W.R., Griebel P.J. A road less travelled: large animal models in immunological research. Nat. Rev. Immunol. 2003;3:79–84. doi: 10.1038/nri977. [DOI] [PubMed] [Google Scholar]
- Hoggan M.D., Blacklow N.R., Rowe W.P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc. Natl. Acad. Sci. U. S. A. 1966;55:1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden M.T.G., Seth-Smith H.M.B., Crossman L.C., Sebaihia M., Bentley S.D., Cerdeño-Tárraga A.M., Thomson N.R., Bason N., Quail M.A., Sharp S., Cherevach I., Churcher C., Goodhead I., Hauser H., Holroyd N., Mungall K., Scott P., Walker D., White B., Rose H., Iversen P., Mil-Homens D., Rocha E.P.C., Fialho A.M., Baldwin A., Dowson C., Barrell B.G., Govan J.R., Vandamme P., Hart C.A., Mahenthiralingam E., Parkhill J. The genome of burkholderia cenocepacia J2315, an epidemic pathogen of cystic fibrosis patients. J. Bacteriol. 2009;91:261–277. doi: 10.1128/JB.01230-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W.Y., Kutok J.L., Nam H.L., Hui Y.P., Fletcher C.D.M., Kanki J.P., Look A.T. Targeted expression of human MYCN selectively causes pancreatic neuroendocrine tumors in transgenic zebrafish. Cancer Res. 2004;64:7256–7262. doi: 10.1158/0008-5472.CAN-04-0931. [DOI] [PubMed] [Google Scholar]
- Howlader D.R., Sinha R., Nag D., Majumder N., Mukherjee P., Bhaumik U., Maiti S., Withey J.H., Koley H. Zebrafish as a novel model for non-typhoidal salmonella pathogenesis, transmission and vaccine efficacy. Vaccine. 2016;34:5099–5106. doi: 10.1016/j.vaccine.2016.08.077. [DOI] [PubMed] [Google Scholar]
- Hsiao J.-C., Chung C.-S., Chang W. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J. Virol. 1999;73:8750–8761. doi: 10.1128/jvi.73.10.8750-8761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao J.-C., Chung C.-S., Chang W. Cell surface proteoglycans are necessary for A27L protein-mediated cell fusion: identification of the N-terminal region of A27L protein as the glycosaminoglycan-binding domain. J. Virol. 1998;72:8374–8379. doi: 10.1128/jvi.72.10.8374-8379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Fu S., Zou G., Kerdsin A., Chen X., Dong X., Teng L., Li J. Genome analysis provides insight into hyper-virulence of Streptococcus suis LSM178, a human strain with a novel sequence type 1005. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-03370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q., Huang H.Q. Alterations of protein profile in zebrafish liver cells exposed to methyl parathion: a membrane proteomics approach. Chemosphere. 2012;87:68–76. doi: 10.1016/j.chemosphere.2011.11.061. [DOI] [PubMed] [Google Scholar]
- Huang S.J., Cheng C.L., Chen J.R., Gong H.Y., Liu W., Wu J.L. Inducible liver-specific overexpression of gankyrin in zebrafish results in spontaneous intrahepatic cholangiocarcinoma and hepatocellular carcinoma formation. Biochem. Biophys. Res. Commun. 2017;490:1052–1058. doi: 10.1016/j.bbrc.2017.06.164. [DOI] [PubMed] [Google Scholar]
- Hubbard S., Darmani N.A., Thrush G.R., Dey D., Burnham L., Thompson J.M., Jones K., Tiwari V. Zebrafish-encoded 3-O-sulfotransferase-3 isoform mediates herpes simplex virus type 1 entry and spread. Zebrafish. 2010;7:181–187. doi: 10.1089/zeb.2009.0621. [DOI] [PubMed] [Google Scholar]
- Hughes J.M. Emerging infectious diseases: a CDC perspective. Emerg. Infect. Dis. 2001;7:494–496. doi: 10.3201/eid0707.017702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius M.S., Hayes M.N., Moore F.E., Tang Q., Garcia S.P., Blackburn P.R., Baxi K., Wang L., Jin A., Ramakrishnan A., Reeder S., Chen Y., Nielsen G.P., Chen E.Y., Hasserjian R.P., Tirode F., Ekker S.C., Langenau D.M. Tp53 deficiency causes a wide tumor spectrum and increases embryonal rhabdomyosarcoma metastasis in zebrafish. elife. 2018;7 doi: 10.7554/eLife.37202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T., Makino S. Rift Valley fever vaccines. Vaccine. 2009;27 doi: 10.1016/j.vaccine.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidro J., Borges V., Pinto M., Ferreira R., Sobral D., Nuner A., Dourado Santos J., Borrego M.J., Nuncio S., Pelerito A., Cordeiro R., Gomes J.P. First draft genome sequence of Monkeypox virus associated with the suspected multi-country outbreak, May 2022 (confirmed case in Portugal) Virological. 2022;16 [Google Scholar]
- Ispas C., Andreescu D., Patel A., Goia D.V., Andreescu S., Wallace K.N. Toxicity and developmental defects of different sizes and shape nickel nanoparticles in zebrafish. Environ. Sci. Technol. 2009;43:6349–6356. doi: 10.1021/es9010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel Nii-Trebi N. Emerging and neglected infectious diseases: insights, advances, and challenges. Biomed Res. Int. 2017:1–15. doi: 10.1155/2017/5245021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J. D., D. M., M. Y., P. M., F. L., R. R., G.A. M., L.-F. W. A new model for Hendra virus encephalitis in the mouse. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J Z., K L., C W.-L., SS A., C F., J Z., J M., J G., RS B., L E., T G., B P., J M., S P. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc. Natl. Acad. Sci. U. S. A. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrling P.B., Smith S., Hesse R.A., Rhoderick J.B. Pathogenesis of Lassa virus infection in Guinea pigs. Infect. Immun. 1982;37:771–778. doi: 10.1128/iai.37.2.771-778.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jank T., Eckerle S., Steinemann M., Trillhaase C., Schimpl M., Wiese S., Van Aalten D.M.F., Driever W., Aktories K. Tyrosine glycosylation of rho by yersinia toxin impairs blastomere cell behaviour in zebrafish embryos. Nat. Commun. 2015;6 doi: 10.1038/ncomms8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K.E., Francis T. The antigenic composition of influenza virus measured by antibody-absorption. J. Exp. Med. 1953;98:619–639. doi: 10.1084/jem.98.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X.F., Liu Z.F., Lin A.F., Xiang L.X., Shao J.Z. Coordination of bactericidal and iron regulatory functions of hepcidin in innate antimicrobial immunity in a zebrafish model. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-04069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen M.D., Irving A., Montagutelli X., Tate M.D., Rudloff I., Nold M.F., Hansbro N.G., Kim R.Y., Donovan C., Liu G., Faiz A., Short K.R., Lyons J.G., McCaughan G.W., Gorrell M.D., Cole A., Moreno C., Couteur D., Hesselson D., Triccas J., Neely G.G., Gamble J.R., Simpson S.J., Saunders B.M., Oliver B.G., Britton W.J., Wark P.A., Nold-Petry C.A., Hansbro P.M. Animal and translational models of SARS-CoV-2 infection and COVID-19. Mucosal Immunol. 2020;13:877–891. doi: 10.1038/s41385-020-00340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Jørgensen L., G. Zebrafish as a model for fish diseases in aquaculture. Pathogens. 2020;9:1–20. doi: 10.3390/pathogens9080609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju B., Chen W., Orr B.A., Spitsbergen J.M., Jia S., Eden C.J., Henson H.E., Taylor M.R. Oncogenic KRAS promotes malignant brain tumors in zebrafish. Mol. Cancer. 2015;14 doi: 10.1186/s12943-015-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung I.H., Leem G.L., Jung D.E., Kim M.H., Kim E.Y., Kim S.H., Park H.C., Park S.W. Glioma is formed by active Akt1 alone and promoted by active Rac1 in transgenic zebrafish. Neuro-Oncology. 2013;15:290–304. doi: 10.1093/neuonc/nos387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KN B., Z Z., D M., J K., G C., J B., JA M., D G., TJ H., Y-P C., AC H., DS D., L-F W., CC B. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute Nipah virus infection. PLoS Pathog. 2009:5. doi: 10.1371/journal.ppat.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KP D., C M., M C., JM R., A V., H R., B H. Type i interferon signaling protects mice from lethal henipavirus infection. J. Infect. Dis. 2013;207:142–151. doi: 10.1093/infdis/jis653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S.M., Malik H.S., Emerman M. Restriction of an extinct retrovirus by the human TRIM5α antiviral protein. Science (80-. ) 2007;316:1756–1758. doi: 10.1126/science.1140579. [DOI] [PubMed] [Google Scholar]
- Kalev-Zylinska M.L., Horsfield J.A., Flores M.V.C., Postlethwait J.H., Vitas M.R., Baas A.M., Crosier P.S., Crosier K.E. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX-1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development. 2002;129:2015–2030. doi: 10.1242/dev.129.8.2015. [DOI] [PubMed] [Google Scholar]
- Kandasamy T., Chandrasekar S., Pichaivel M., Pachaiappan S., Muthusamy G., Sumathi L. A review of zebrafish as an alternative animal model and its benefits over other animal models in various disease conditions. Saudi J. Biomed. Res. 2022;12:355–359. [Google Scholar]
- Kanther M., Rawls J.F. Host-microbe interactions in the developing zebrafish. Curr. Opin. Immunol. 2010;22:10–19. doi: 10.1016/j.coi.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karra R., Knecht A.K., Kikuchi K., Poss K.D. Myocardial NF-κB activation is essential for zebrafish heart regeneration. Proc. Natl. Acad. Sci. U. S. A. 2015;112:13255–13260. doi: 10.1073/pnas.1511209112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman C.K., Mosimann C., Fan Z.P., Yang S., Thomas A.J., Ablain J., Tan J.L., Fogley R.D., Van Rooijen E., Hagedorn E.J., Ciarlo C., White R.M., Matos D.A., Puller A.C., Santoriello C., Liao E.C., Young R.A., Zon L.I. A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science (80-. ) 2016:351. doi: 10.1126/science.aad2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazeto Y., Place A.R., Trant J.M. Effects of endocrine disrupting chemicals on the expression of CYP19 genes in zebrafish (Danio rerio) juveniles. Aquat. Toxicol. 2004;69:25–34. doi: 10.1016/j.aquatox.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Kendall G.C., Watson S., Xu L., Lavigne C.A., Murchison W., Rakheja D., Skapek S.X., Tirode F., Delattre O., Amatruda J.F. PAX3-FOXO1 transgenic zebrafish models identify HES3 as a mediator of rhabdomyosarcoma tumorigenesis. elife. 2018;7 doi: 10.7554/eLife.33800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent M.L., Harper C., Wolf J.C. Documented and potential research impacts of subclinical diseases in zebrafish. ILAR J. 2012;53:126–134. doi: 10.1093/ilar.53.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F.R., Alhewairini S.S. Zebrafish (Danio rerio) as a model organism. Curr. Trends Cancer Manag. 2019:13. [Google Scholar]
- Khezri A., Fraser T.W.K., Nourizadeh-Lillabadi R., Kamstra J.H., Berg V., Zimmer K.E., Ropstad E. A mixture of persistent organic pollutants and perfluorooctanesulfonic acid induces similar behavioural responses, but different gene expression profiles in zebrafish larvae. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki D.H., He S., Rodig S., Look A.T. Overexpression of PDGFRA cooperates with loss of NF1 and p53 to accelerate the molecular pathogenesis of malignant peripheral nerve sheath tumors. Oncogene. 2017;36:1058–1068. doi: 10.1038/onc.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.J., Hancock B.M., Del Cid N., Bermudez A., Traver D., Doran K.S. Streptococcus agalactiae infection in zebrafish larvae. Microb. Pathog. 2015;79:57–60. doi: 10.1016/j.micpath.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G., Hyun J., Melgoza A., Jiang F., Guo S. The effect of renin–angiotensin–aldosterone system inhibitors on organ-specific ace2 expression in zebrafish and its implications for COVID-19. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-03244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Young Il, Kim S.G., Kim S.M., Kim E.H., Park S.J., Yu K.M., Chang J.H., Kim E.J., Lee S., Casel M.A.B., Um J., Song M.S., Jeong H.W., Lai V.D., Kim Yeonjae, Chin B.S., Park J.S., Chung K.H., Foo S.S., Poo H., Mo I.P., Lee O.J., Webby R.J., Jung J.U., Choi Y.K. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27:704–709.e2. doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Heiden T.C., Wiecinski P.N., Mangham A.N., Metz K.M., Nesbit D., Pedersen J.A., Hamers R.J., Heideman W., Peterson R.E. Quantum dot nanotoxicity assessment using the zebrafish embryo. Environ. Sci. Technol. 2009;43:1605–1611. doi: 10.1021/es801925c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinth P., Mahesh G., Panwar Y. Mapping of zebrafish research: a global outlook. Zebrafish. 2013;10:510–517. doi: 10.1089/zeb.2012.0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida M., McLellan M., Miranda J.A., Callard G.V. Estrogen and xenoestrogens upregulate the brain aromatase isoform (P450aromB) and perturb markers of early development in zebrafish (Danio rerio) CompBiochem. Physiol. - B Biochem. Mol. Biol. 2001;129:261–268. doi: 10.1016/S1096-4959(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Klasse P.J., Bron R., Marsh M. Mechanisms of enveloped virus entry into animal cells. Adv. Drug Deliv. Rev. 1998;34:65–91. doi: 10.1016/S0169-409X(98)00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C., Gouin E., Tabouret M., Berche P., Ohayon H., Cossart P. L. Monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-I. [DOI] [PubMed] [Google Scholar]
- Koike S., Taya C., Aoki J., Matsuda Y., Ise I., Takeda H., Matsuzaki T., Amanuma H., Yonekawa H., Nomoto A. Characterization of three different transgenic mouse lines that carry human poliovirus receptor gene-influence of the transgene expression on pathogenesis. Arch. Virol. 1994;139:351–363. doi: 10.1007/BF01310797. [DOI] [PubMed] [Google Scholar]
- Konantz M., Schürch C., Hanns P., Müller J.S., Sauteur L., Lengerke C. Modeling hematopoietic disorders in zebrafish. DMM Dis. Model. Mech. 2019;12 doi: 10.1242/dmm.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbut R., Mehrdana F., Kania P.W., Larsen M.H., Frees D., Dalsgaard I., Jørgensen L.V.G. Antigen uptake during different life stages of zebrafish (Danio rerio) using a GFP-tagged Yersinia ruckeri. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus A., Huertas M., Ellis L., Boudinot P., Levraud J.P., Salinas I. Intranasal delivery of SARS-CoV-2 spike protein is sufficient to cause olfactory damage, inflammation and olfactory dysfunction in zebrafish. Brain Behav. Immun. 2022;102:341–359. doi: 10.1016/j.bbi.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyściak W., Pluskwa K.K., Jurczak A., Kościelniak D. The pathogenicity of the streptococcus genus. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32:1361–1376. doi: 10.1007/s10096-013-1914-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G., Menanteau-Ledouble S., Saleh M., El-Matbouli M. Yersinia ruckeri, the causative agent of enteric redmouth disease in fish. Vet. Res. 2015;46 doi: 10.1186/s13567-015-0238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y.K., Lipatov A.S., Swayne D.E. Bronchointerstitial pneumonia in Guinea pigs following inoculation with H5N1 high pathogenicity avian influenza virus. Vet. Pathol. 2009;46:138–141. doi: 10.1354/vp.46-1-138. [DOI] [PubMed] [Google Scholar]
- LE H., DA A., JB G., EA F., C R., T L., TW G. Pathogenesis of marburg hemorrhagic fever in cynomolgus macaques. J. Infect. Dis. 2011;204:S1021–S1031. doi: 10.1093/infdis/jir339. [DOI] [PubMed] [Google Scholar]
- LP K., V S. A review on emerging infectious diseases prioritized underthe 2018 WHO research and development blueprint: lessons from the Indian context. Vector-Borne Zoonotic Dis. 2021;21:149–159. doi: 10.1089/vbz.2020.2661. [DOI] [PubMed] [Google Scholar]
- La Patra S.E., Barone L., Jones G.R., Zon L.I. Effects of infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus infection on hematopoietic. Precursors of the zebrafish. Blood cellsMol. Dis. 2000;26:445–452. doi: 10.1006/bcmd.2000.0320. [DOI] [PubMed] [Google Scholar]
- Labrot F., Narbonne J.F., Ville P., Saint Denis M., Ribera D. Acute toxicity, toxicokinetics, and tissue target of lead and uranium in the clam Corbicula fluminea and the worm eisenia fetida: comparison with the fish Brachydanio rerio. Arch. Environ. Contam. Toxicol. 1999;36:167–178. doi: 10.1007/s002449900457. [DOI] [PubMed] [Google Scholar]
- Laghi V., Rezelj V., Boucontet L., Frétaud M., Da Costa B., Boudinot P., Salinas I., Lutfalla G., Vignuzzi M., Levraud J.P. Exploring zebrafish larvae as a COVID-19 model: probable abortive SARS-CoV-2 replication in the swim bladder. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.790851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lama R., Pereiro P., Figueras A., Novoa B. Zebrafish as a vertebrate model for studying nodavirus infections. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.863096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenau D.M., Feng H., Berghmans S., Kanki J.P., Kutok J.L., Look A.T. Cre/lox-regulated transgenic zebrafish model with conditional myc-induced T cell acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. U. S. A. 2005;102:6068–6073. doi: 10.1073/pnas.0408708102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenau D.M., Ferrando A.A., Traver D., Kutok J.L., Hezel J.P.D., Kanki J.P., Zon L.I., Thomas Look A., Trede N.S. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc. Natl. Acad. Sci. U. S. A. 2004;101:7369–7374. doi: 10.1073/pnas.0402248101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenau D.M., Keefe M.D., Storer N.Y., Guyon J.R., Kutok J.L., Le X., Goessling W., Neuberg D.S., Kunkel L.M., Zon L.I. Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes Dev. 2007;21:1382–1395. doi: 10.1101/gad.1545007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenau D.M., Keefe M.D., Storer N.Y., Jette C.A., Smith A.C.H., Ceol C.J., Bourque C., Look A.T., Zon L.I. Co-injection strategies to modify radiation sensitivity and tumor initiation in transgenic zebrafish. Oncogene. 2008;27:4242–4248. doi: 10.1038/onc.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenau D.M., Traver D., Ferrando A.A., Kutok J.L., Aster J.C., Kanki J.P., Lin S., Prochownik E., Trede N.S., Zon L.I., Look A.T. Myc-induced T cell leukemia in transgenic zebrafish. Science (80-. ) 2003;299:887–890. doi: 10.1126/science.1080280. [DOI] [PubMed] [Google Scholar]
- Langevin C., van der Aa L.M., Houel A., Torhy C., Briolat V., Lunazzi A., Harmache A., Bremont M., Levraud J.-P., Boudinot P. Zebrafish ISG15 exerts a strong antiviral activity against RNA and DNA viruses and regulates the interferon response. J. Virol. 2013;87:10025–10036. doi: 10.1128/jvi.01294-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardelli M. Proc. 2008 ANZCCART Conf. 23–28. 2008. Using zebrafish in human disease research: some advantages, disadvantages and ethical considerations. [Google Scholar]
- Law M., Carter G.G., Roberts K.L., Hollinshead M., Smith G.L. Ligand-induced and nonfusogenic dissolution of a viral membrane. Proc. Natl. Acad. Sci. U. S. A. 2006;103:5989–5994. doi: 10.1073/pnas.0601025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Doare K., Heath P.T. An overview of global GBS epidemiology. Vaccine. 2013;31 doi: 10.1016/j.vaccine.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Le X., Langenau D.M., Keefe M.D., Kutok J.L., Neuberg D.S., Zon L.I. Heat shock-inducible Cre/Lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc. Natl. Acad. Sci. U. S. A. 2007;104:9410–9415. doi: 10.1073/pnas.0611302104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leacock S.W., Basse A.N., Chandler G.L., Kirk A.M., Rakheja D., Amatruda J.F. A zebrafish transgenic model of Ewing’s sarcoma reveals conserved mediators of EWS-FLI1 tumorigenesis. DMM Dis. Model. Mech. 2012;5:95–106. doi: 10.1242/dmm.007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E., Wei Y., Zou Z., Tucker K., Rakheja D., Levine B., Amatruda J.F. Genetic inhibition of autophagy promotes p53 loss-ofheterozygosity and tumorigenesis. Oncotarget. 2016;7:67919–67933. doi: 10.18632/ONCOTARGET.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Lee B., Kim K.T. Mechanisms and effects of zinc oxide nanoparticle transformations on toxicity to zebrafish embryos. Environ. Sci. Nano. 2021;8:1690–1700. doi: 10.1039/d1en00305d. [DOI] [Google Scholar]
- Lee Y.H., Kawakami K., HuangFu W.C., Liu I.H. Chondroitin sulfate proteoglycan 4 regulates zebrafish body axis organization via Wnt/ planar cell polarity pathway. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E.D., Chrysanthis E., Yacisin K., Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol. Teratol. 2003;25:51–57. doi: 10.1016/S0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Levraud J.P., Disson O., Kissa K., Bonne I., Cossart P., Herbomel P., Lecuit M. Real-time observation of listeria monocytogenes-phagocyte interactions in living zebrafish larvae. Infect. Immun. 2009;77:3651–3660. doi: 10.1128/IAI.00408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levraud J.P., Palha N., Langevin C., Boudinot P. Through the looking glass: witnessing host-virus interplay in zebrafish. Trends Microbiol. 2014;22:490–497. doi: 10.1016/j.tim.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Lewis R.S., Stephenson S.E.M., Ward A.C. Constitutive activation of zebrafish Stat5 expands hematopoietic cell populations in vivo. Exp. Hematol. 2006;34:179–187. doi: 10.1016/j.exphem.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Li H., Liu S.M., Yu X.H., Tang S.L., Tang C.K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Lu J.W., Huo X., Li Y., Li Z., Gong Z. Effects of sex hormones on liver tumor progression and regression in Myc/xmrk double oncogene transgenic zebrafish. Gen. Comp. Endocrinol. 2019;277:112–121. doi: 10.1016/j.ygcen.2019.03.018. [DOI] [PubMed] [Google Scholar]
- Li P., Jiang W., Yu Q., Liu W., Zhou P., Li J., Xu J., Xu B., Wang F., Shao F. Ubiquitination and degradation of GBPs by a shigella effector to suppress host defence. Nature. 2017;551:378–383. doi: 10.1038/nature24467. [DOI] [PubMed] [Google Scholar]
- Li S., Sun F., Zhang Y.B., Gui J.F., Zhang Q.Y. Identification of DreI as an antiviral factor regulated by RLR signaling pathway. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Ma Y., Li D., Gao X., Li P., Bai N., Luo M., Tan X., Lu C., Ma X. Arsenic impairs embryo development via down-regulating Dvr1 expression in zebrafish. Toxicol. Lett. 2012;212:161–168. doi: 10.1016/j.toxlet.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Li Z., Huang X., Zhan H., Zeng Z., Li C., Spitsbergen J.M., Meierjohann S., Schartl M., Gong Z. Inducible and repressable oncogene-addicted hepatocellular carcinoma in tet-on xmrk transgenic zebrafish. J. Hepatol. 2012;56:419–425. doi: 10.1016/j.jhep.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Li Z., Zheng W., Wang Z., Zeng Z., Zhan H., Li C., Zhou L., Yan C., Spitsbergen J.M., Gong Z. A transgenic zebrafish liver tumor model with inducible myc expression reveals conserved myc signatures with mammalian liver tumors. DMM Dis. Model. Mech. 2013;6:414–423. doi: 10.1242/dmm.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew N., Moya M.J.M., Wierzbicki C.J., Hollinshead M., Dillon M.J., Thornton C.R., Ellison A., Cable J., Fisher M.C., Mostowy S. Chytrid fungus infection in zebrafish demonstrates that the pathogen can parasitize non-amphibian vertebrate hosts. Nat. Commun. 2017;8 doi: 10.1038/ncomms15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.-L., Chung C.-S., Heine H.G., Chang W. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 2000;74:3353–3365. doi: 10.1128/jvi.74.7.3353-3365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist M.E., Zeng X., Altamura L.A., Daye S.P., Delp K.L., Blancett C., Coffin K.M., Koehler J.W., Coyne S., Shoemaker C.J., Garrison A.R., Golden J.W. Exploring crimean-Congo hemorrhagic fever virus-induced hepatic injury using antibody-mediated type I interferon blockade in mice. J. Virol. 2018;92 doi: 10.1128/jvi.01083-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linell F., Norden A. Mycobacterium balnei, a new acid-fast bacillus occurring in swimming pools and capable of producing skin lesions in humans. Acta Tuberc. Scand. Suppl. 1954;33:1–84. [PubMed] [Google Scholar]
- Lister J.A., Capper A., Zeng Z., Mathers M.E., Richardson J., Paranthaman K., Jackson I.J., Patton E.E. A conditional zebrafish MITF mutation reveals MITF levels are critical for melanoma promotion vs. regression in vivo. J. Invest. Dermatol. 2014;134:133–140. doi: 10.1038/jid.2013.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield K., Levy M., Dudakia D., Proszek P., Shipley C., Basten S., Rapley E., Bishop D.T., Reid A., Huddart R., Broderick P., De Castro D.G., O’Connor S., Giles R.H., Houlston R.S., Turnbull C. Rare disruptive mutations in ciliary function genes contribute to testicular cancer susceptibility. Nat. Commun. 2016;7 doi: 10.1038/ncomms13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamas M.A., Van Der Sar A., Chu B.C.H., Sparrius M., Vogel H.J., Bitter W. A novel extracytoplasmic function (ECF) sigma factor regulates virulence in Pseudomonas aeruginosa. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Muñoz A., Roca F.J., Meseguer J., Mulero V. New insights into the evolution of IFNs: zebrafish group II IFNs induce a rapid and transient expression of IFN-dependent genes and display powerful antiviral activities. J. Immunol. 2009;182:3440–3449. doi: 10.4049/jimmunol.0802528. [DOI] [PubMed] [Google Scholar]
- López-Muñoz A., Roca F.J., Sepulcre M.P., Meseguer J., Mulero V. Zebrafish larvae are unable to mount a protective antiviral response against waterborne infection by spring viremia of carp virus. Dev. Comp. Immunol. 2010;34:546–552. doi: 10.1016/j.dci.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Lowen A.C., Mubareka S., Tumpey T.M., García-Sastre A., Palese P. The Guinea pig as a transmission model for human influenza viruses. Proc. Natl. Acad. Sci. U. S. A. 2006;103:9988–9992. doi: 10.1073/pnas.0604157103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.W., Hou H.A., Hsieh M.S., Tien H.F., Lin L.I. Overexpression of FLT3-ITD driven by spi-1 results in expanded myelopoiesis with leukemic phenotype in zebrafish. Leukemia. 2016;30:2098–2101. doi: 10.1038/leu.2016.132. [DOI] [PubMed] [Google Scholar]
- Lu L.F., Zhang C., Li Z.C., Zhou X.Y., Jiang J.Y., Chen D.D., Zhang Y.A., Xiong F., Zhou F., Li S. A novel role of zebrafish TMEM33 in negative regulation of interferon production by two distinct mechanisms. PLoS Pathog. 2021;17 doi: 10.1371/JOURNAL.PPAT.1009317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M.W., Chao Y.M., Guo T.C., Santi N., Evensen Ø., Kasani S.K., Hong J.R., Wu J.L. The interferon response is involved in nervous necrosis virus acute and persistent infection in zebrafish infection model. Mol. Immunol. 2008;45:1146–1152. doi: 10.1016/j.molimm.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Ludwig M., Palha N., Torhy C., Briolat V., Colucci-Guyon E., Brémont M., Herbomel P., Boudinot P., Levraud J.P. Whole-body analysis of a viral infection: vascular endothelium is a primary target of infectious hematopoietic necrosis virus in zebrafish larvae. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfalla G., Crollius H.R., Stange-Thomann N., Jaillon O., Mogensen K., Monneron D. Comparative genomic analysis reveals independent expansion of a lineage-specific gene family in vertebrates: the class II cytokine receptors and their ligands in mammals and fish. BMC Genomics. 2003;4 doi: 10.1186/1471-2164-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer M., V G., M R., P A., A J., J-S J., M B., F D., PL P., D S., Mione M. A novel brain tumour model in zebrafish reveals the role of YAP activation in MAPK- and PI3K-induced malignant growth. DMM Dis. Model. Mech. 2017;10:15–28. doi: 10.1242/dmm.026500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhubala V., Kalaivani T., Kirubha A., Prakash J.S., Manigandan V., Dara H.K. Study of structural and magnetic properties of hydro/solvothermally synthesized α-Fe2O3 nanoparticles and its toxicity assessment in zebrafish embryos. Appl. Surf. Sci. 2019;494:391–400. doi: 10.1016/j.apsusc.2019.07.090. [DOI] [Google Scholar]
- Magden J., Kääriäinen L., Ahola T. Inhibitors of virus replication: recent developments and prospects. Appl. Microbiol. Biotechnol. 2005;66:612–621. doi: 10.1007/s00253-004-1783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maginnis M.S. Virus-receptor interactions: the key to cellular invasion. J. Mol. Biol. 2018;430:2590–2611. doi: 10.1016/j.jmb.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maginnis M.S., Mainou B.A., Derdowski A., Johnson E.M., Zent R., Dermody T.S. NPXY motifs in the β1 integrin cytoplasmic tail are required for functional reovirus entry. J. Virol. 2008;82:3181–3191. doi: 10.1128/jvi.01612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahenthiralingam E., Baldwin A., Dowson C.G. Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J. Appl. Microbiol. 2008;104:1539–1551. doi: 10.1111/j.1365-2672.2007.03706.x. [DOI] [PubMed] [Google Scholar]
- Mahenthiralingam E., Urban T.A., Goldberg J.B. The multifarious, multireplicon burkholderia cepacia complex. Nat. Rev. Microbiol. 2005;3:144–156. doi: 10.1038/nrmicro1085. [DOI] [PubMed] [Google Scholar]
- Marcello T., Grakoui A., Barba-Spaeth G., Machlin E.S., Kotenko S.V., Macdonald M.R., Rice C.M. Interferons α and λ inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131:1887–1898. doi: 10.1053/j.gastro.2006.09.052. [DOI] [PubMed] [Google Scholar]
- Mariani R., Rutter G., Harris M.E., Hope T.J., Kräusslich H.-G., Landau N.R. A block to human immunodeficiency virus type 1 assembly in murine cells. J. Virol. 2000;74:3859–3870. doi: 10.1128/jvi.74.8.3859-3870.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Montelongo J.H., Medina-Ramírez I.E., Romo-Lozano Y., Zapien J.A. Development of a sustainable photocatalytic process for air purification. Chemosphere. 2020;257 doi: 10.1016/j.chemosphere.2020.127236. [DOI] [PubMed] [Google Scholar]
- Marzi A., Banadyga L., Haddock E., Thomas T., Shen K., Horne E.J., Scott D.P., Feldmann H., Ebihara H. A hamster model for Marburg virus infection accurately recapitulates Marburg hemorrhagic fever. Sci. Rep. 2016;6 doi: 10.1038/srep39214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateu M.G. Assembly, stability and dynamics of virus capsids. Arch. Biochem. Biophys. 2013;531:65–79. doi: 10.1016/j.abb.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Mazon-Moya M.J., Willis A.R., Torraca V., Boucontet L., Shenoy A.R., Colucci-Guyon E., Mostowy S. Septins restrict inflammation and protect zebrafish larvae from shigella infection. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell A.M., Mito J.K., Ablain J., Dang M., Formichella L., Fisher D.E., Zon L.I. Neural crest state activation in NRAS driven melanoma, but not in NRAS-driven melanocyte expansion. Dev. Biol. 2019;449:107–114. doi: 10.1016/j.ydbio.2018.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell A.M., Noonan H.R., Zon L.I. Reeling in the zebrafish cancer models. Annu. Rev. Cancer Biol. 2020;5:331–350. doi: 10.1146/annurev-cancerbio-051320-014135. [DOI] [Google Scholar]
- McFetridge R., ter Meulen A.S., Folkerth S.D., Hoekstra J.A., Dallas M., Hoover P.A., Marchese R.D., Zacholski D.M., Watson W.J., Stek J.E., Hartzel J.S., Musey L.K. Safety, tolerability, and immunogenicity of 15-valent pneumococcal conjugate vaccine in healthy adults. Vaccine. 2015;33:2793–2799. doi: 10.1016/j.vaccine.2015.04.025. [DOI] [PubMed] [Google Scholar]
- Mehle N., Ravnikar M. Plant viruses in aqueous environment - survival, water mediated transmission and detection. Water Res. 2012;46:4902–4917. doi: 10.1016/j.watres.2012.07.027. [DOI] [PubMed] [Google Scholar]
- Melnick J.L., Mayor H.D., Smith K.O., Rapp F. Association of 20-millimicron particles with adenoviruses. J. Bacteriol. 1965;90:271–274. doi: 10.1128/jb.90.1.271-274.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengaud J., Ohayon H., Gounon P., Mege R.M., Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. Monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/S0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- Mensah L., Ferguson J.L., Shive H.R. Genotypic and phenotypic variables affect meiotic cell cycle progression, tumor ploidy, and cancer-associated mortality in a brca2-mutant zebrafish model. J. Oncol. 2019;2019 doi: 10.1155/2019/9218251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesureur J., Feliciano J.R., Wagner N., Gomes M.C., Zhang L., Blanco-Gonzalez M., van der Vaart M., O’Callaghan D., Meijer A.H., Vergunst A.C. Macrophages, but not neutrophils, are critical for proliferation of burkholderia cenocepacia and ensuing host-damaging inflammation. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton D.J., Westbury H.A., Morrissy C.J., Van der Heide B.M., Russell G.M., Braun M.A., Hyatt A.D. Experimental nipah virus infection in pigs and cats. J. Comp. Pathol. 2002;126:124–136. doi: 10.1053/jcpa.2001.0532. [DOI] [PubMed] [Google Scholar]
- Miller J. Zebrafish as a model host for streptococcal pathogenesis. Acta Trop. 2004;91:53–68. doi: 10.1016/s0001-706x(04)00043-9. [DOI] [PubMed] [Google Scholar]
- Miller N.Y., Gerlai R. Shoaling in zebrafish: what we don’t know. Rev. Neurosci. 2011;22:17–25. doi: 10.1515/RNS.2011.004. [DOI] [PubMed] [Google Scholar]
- Mitchell K.C., Withey J.H. Danio rerio as a native host model for understanding pathophysiology of vibrio cholerae. Methods Mol. Biol. 2018;1839:97–102. doi: 10.1007/978-1-4939-8685-9_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizgireuv I.V., Revskoy S.Y. Transplantable tumor lines generated in clonal zebrafish. Cancer Res. 2006;66:3120–3125. doi: 10.1158/0008-5472.CAN-05-3800. [DOI] [PubMed] [Google Scholar]
- Modzelewska K., Boer E.F., Mosbruger T.L., Picard D., Anderson D., Miles R.R., Kroll M., Oslund W., Pysher T.J., Schiffman J.D., Jensen R., Jette C.A., Huang A., Stewart R.A. MEK inhibitors reverse growth of embryonal brain tumors derived from oligoneural precursor cells. Cell Rep. 2016;17:1255–1264. doi: 10.1016/j.celrep.2016.09.081. [DOI] [PubMed] [Google Scholar]
- Mohd H.A., Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV) origin and animal reservoir Susanna lau. Virol. J. 2016;13 doi: 10.1186/s12985-016-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montañez G.E., Neely M.N., Eichenbaum Z. The streptococcal iron uptake (Siu) transporter is required for iron uptake and virulence in a zebrafish infection model. Microbiology. 2005;151:3749–3757. doi: 10.1099/mic.0.28075-0. [DOI] [PubMed] [Google Scholar]
- Morales E.E., Wingert R.A. Zebrafish as a model of kidney disease. Results Probl. Cell Differ. 2017;60:55–75. doi: 10.1007/978-3-319-51436-9_3. [DOI] [PubMed] [Google Scholar]
- Mordstein M., Kochs G., Dumoutier L., Renauld J.-C., Paludan S.R., Klucher K., Staeheli P. 267 interferon-λ contributes to innate immunity of mice against influenza a virus but not against hepatotropic viruses. Cytokine. 2008;43:307. doi: 10.1016/j.cyto.2008.07.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Membrane fusion during poxvirus entry. Semin. Cell Dev. Biol. 2016;60:89–96. doi: 10.1016/j.semcdb.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Poxvirus cell entry: how many proteins does it take? Viruses. 2012;4:688–707. doi: 10.3390/v4050688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S., Bonazzi M., Hamon M.A., Tham T.N., Mallet A., Lelek M., Gouin E., Demangel C., Brosch R., Zimmer C., Sartori A., Kinoshita M., Lecuit M., Cossart P. Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microbe. 2010;8:433–444. doi: 10.1016/j.chom.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Mostowy S., Boucontet L., Mazon Moya M.J., Sirianni A., Boudinot P., Hollinshead M., Cossart P., Herbomel P., Levraud J.P., Colucci-Guyon E. The zebrafish as a new model for the in vivo study of shigella flexneri interaction with phagocytes and bacterial autophagy. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S., Tham T.N., Danckaert A., Guadagnini S., Boisson-Dupuis S., Pizarro-Cerdá J., Cossart P. Septins regulate bacterial entry into host cells. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motesi C.M.De. Poxvirus cGAMP nucleases: clues and mysteries from a stolen gene. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhl L., He L., Sun Y., Andaloussi Mäe M., Pietilä R., Liu J., Genové G., Zhang L., Xie Y., Leptidis S., Mocci G., Stritt S., Osman A., Anisimov A., Hemanthakumar K.A., Räsänen M., Hansson E.M., Björkegren J., Vanlandewijck M., Blomgren K., Mäkinen T., Peng X.R., Hu Y., Ernfors P., Arnold T.D., Alitalo K., Lendahl U., Betsholtz C. The SARS-CoV-2 receptor ACE2 is expressed in mouse pericytes but not endothelial cells: implications for COVID-19 vascular research. Stem Cell Rep. 2022;17:1089–1104. doi: 10.1016/j.stemcr.2022.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnink B.B.O., Meulder D.De, Amerongen G.Van, Brand J.Van Den, Okba N.M.A., Schipper D., Run P.Van, Leijten L., Sikkema R., Vlissingen M.F.Van, Fouchier R., Swart R.De. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science (80-. ) 2020;368:1012–1015. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr. Top. Microbiol. Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- NE Y., S R., A T., T K., AV S., KT D., M M., R C., N S., AG W., JN S., JN F., N W., B B., SH K., T M., S P. Animal model of sensorineural hearing loss associated with Lassa virus infection. J. Virol. 2016;90:2920–2927. doi: 10.1128/JVI.02948-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag D., Breen P., Raychaudhuri S., Withey J.H. Glucose metabolism by Escherichia coli inhibits vibrio cholerae intestinal colonization of zebrafish. Infect. Immun. 2018;86 doi: 10.1128/IAI.00486-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakari T., Erkomaa K. Effects of phytosterols on zebrafish reproduction in multigeneration test. Environ. Pollut. 2003;123:267–273. doi: 10.1016/S0269-7491(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Nasir A., Caetano-Anollés G. A phylogenomic data-driven exploration of viral origins and evolution. Sci. Adv. 2015;1 doi: 10.1126/sciadv.1500527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely M.N., Pfeifer J.D., Caparon M. Streptococcus-zebrafish model of bacterial pathogenesis. Infect. Immun. 2002;70:3904–3914. doi: 10.1128/IAI.70.7.3904-3914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J.C., Dovey J.S., Chandler G.L., Carbajal L., Amatruda J.F. Identification of a heritable model of testicular germ cell tumor in the zebrafish. Zebrafish. 2009;6:319–327. doi: 10.1089/zeb.2009.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A.T., Emelyanov A., Koh C.H.V., Spitsbergen J.M., Lam S.H., Mathavan S., Parinov S., Gong Z. A high level of liver-specific expression of oncogenic kras V12 drives robust liver tumorigenesis in transgenic zebrafish. DMM Dis. Model. Mech. 2011;4:801–813. doi: 10.1242/dmm.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A.T., Koh V., Spitsbergen J.M., Gong Z. Development of a conditional liver tumor model by mifepristone-inducible cre recombination to control oncogenic kras V12 expression in transgenic zebrafish. Sci. Rep. 2016;6 doi: 10.1038/srep19559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.Q., Rollon R., Choi Y.K. Animal models for influenza research: strengths and weaknesses. Viruses. 2021;13 doi: 10.3390/v13061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa B., Romero A., Mulero V., Rodríguez I., Fernández I., Figueras A. Zebrafish (Danio rerio) as a model for the study of vaccination against viral haemorrhagic septicemia virus (VHSV) Vaccine. 2006;24:5806–5816. doi: 10.1016/j.vaccine.2006.05.015. [DOI] [PubMed] [Google Scholar]
- O’Donnell C.D., Subbarao K. The contribution of animal models to the understanding of the host range and virulence of influenza a viruses. Microbes Infect. 2011;13:502–515. doi: 10.1016/j.micinf.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole R., Milton D.L., Wolf-Watz H. Chemotactic motility is required for invasion of the host by the fish pathogen vibrio anguillarum. Mol. Microbiol. 1996;19:625–637. doi: 10.1046/j.1365-2958.1996.412927.x. [DOI] [PubMed] [Google Scholar]
- O’Toole R., Von Hofsten J., Rosqvist R., Olsson P.E., Wolf-Watz H. Visualisation of zebrafish infection by GFP-labelled vibrio anguillarum. Microb. Pathog. 2004;37:41–46. doi: 10.1016/j.micpath.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Oestereich L., Lüdtke A., Ruibal P., Pallasch E., Kerber R., Rieger T., Wurr S., Bockholt S., Pérez-Girón J.V., Krasemann S., Günther S., Muñoz-Fontela C. Chimeric mice with competent hematopoietic immunity reproduce key features of severe Lassa fever. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S., Park J.T. Zebrafish model of KRAS-initiated pancreatic endocrine tumor. Animal Cells Syst. (Seoul) 2019;23:209–218. doi: 10.1080/19768354.2019.1610058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onnebo S.M.N., Condron M.M., McPhee D.O., Lieschke G.J., Ward A.C. Hematopoietic perturbation in zebrafish expressing a tel-jak2a fusion. Exp. Hematol. 2005;33:182–188. doi: 10.1016/j.exphem.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Onnebo S.M.N., Rasighaemi P., Kumar J., Liongue C., Ward A.C. Alternative TEL-JAK2 fusions associated with T-cell acute lymphoblastic leukemia and atypical chronic myelogenous leukemia dissected in zebrafish. Haematologica. 2012;97:1895–1903. doi: 10.3324/haematol.2012.064659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz R., Maquet E., Huisken J., Antonica F., Trubiroha A., Pottier G., Janssens V., Costagliola S. Transgenic zebrafish illuminate the dynamics of thyroid morphogenesis and its relationship to cardiovascular development. Dev. Biol. 2012;372:203–216. doi: 10.1016/j.ydbio.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Oppel F., Tao T., Shi H., Ross K.N., Zimmerman M.W., He S., Tong G., Aster J.C., Thomas Look A. Loss of atrx cooperates with p53-deficiency to promote the development of sarcomas and other malignancies. PLoS Genet. 2019;15 doi: 10.1371/journal.pgen.1008039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormonde P., Hörstedt P., O’Toole R., Milton D.L. Role of motility in adherence to and invasion of a fish cell line by vibrio anguillarum. J. Bacteriol. 2000;182:2326–2328. doi: 10.1128/JB.182.8.2326-2328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulmi Y., Braunbeck T. Toxicity of 4-chloroaniline in early life-stages of zebrafish ( Brachydanio rerio ):I. Cytopathology of liver and kidney after microinjection. Arch. Environ. Contam. Toxicol. 1996;30:390–402. doi: 10.1007/s002449900053. [DOI] [PubMed] [Google Scholar]
- Palha N., Guivel-Benhassine F., Briolat V., Lutfalla G., Sourisseau M., Ellett F., Wang C.H., Lieschke G.J., Herbomel P., Schwartz O., Levraud J.P. Real-time whole-body visualization of chikungunya virus infection and host interferon response in zebrafish. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallister J., Middleton D., Wang L.F., Klein R., Haining J., Robinson R., Yamada M., White J., Payne J., Feng Y.R., Chan Y.P., Broder C.C. A recombinant hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal hendra virus challenge. Vaccine. 2011;29:5623–5630. doi: 10.1016/j.vaccine.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer E.G. Immune responses to listeria monocytogenes. Nat. Rev. Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- Pan C.Y., Wu J.L., Hui C.F., Lin C.H., Chen J.Y. Insights into the antibacterial and immunomodulatory functions of the antimicrobial peptide, epinecidin-1, against Vibrio vulnificus infection in zebrafish. Fish Shellfish Immunol. 2011;31:1019–1025. doi: 10.1016/j.fsi.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Paranjpye R.N., Myers M.S., Yount E.C., Thompson J.L. Zebrafish as a model for Vibrio parahaemolyticus virulence. Microbiology (United Kingdom) 2013;159:2605–2615. doi: 10.1099/mic.0.067637-0. [DOI] [PubMed] [Google Scholar]
- Parichy D.M. The natural history of model organisms: advancing biology through a deeper understanding of zebrafish ecology and evolution. elife. 2015;2015 doi: 10.7554/eLife.05635.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Galbraith R., Turner T., Mehojah J., Azuma M. Loss of Ewing sarcoma EWS allele promotes tumorigenesis by inducing chromosomal instability in zebrafish. Sci. Rep. 2016;6 doi: 10.1038/srep32297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.T., Johnson N., Liu S., Levesque M., Wang Y.J., Ho H., Huso D., Maitra A., Parsons M.J., Prescott J.D., Leach S.D. Differential in vivo tumorigenicity of diverse KRAS mutations in vertebrate pancreas: a comprehensive survey. Oncogene. 2015;34:2801–2806. doi: 10.1038/onc.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.T., Leach S.D. Zebrafish model of KRAS-initiated pancreatic cancer. Animal Cells Syst. (Seoul) 2018;22:353–359. doi: 10.1080/19768354.2018.1530301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.W., Davison J.M., Rhee J., Hruban R.H., Maitra A., Leach S.D. Oncogenic KRAS induces progenitor cell expansion and malignant transformation in zebrafish exocrine pancreas. Gastroenterology. 2008;134:2080–2090. doi: 10.1053/j.gastro.2008.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passoni G., Langevin C., Palha N., Mounce B.C., Briolat V., Affaticati P., De Job E., Joly J.S., Vignuzzi M., Saleh M.C., Herbomel P., Boudinot P., Levraud J.P. Imaging of viral neuroinvasion in the zebrafish reveals that sindbis and chikungunya viruses favour different entry routes. DMM Dis. Model. Mech. 2017;10:847–857. doi: 10.1242/dmm.029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrício Silva A.L., Prata J.C., Mouneyrac C., Barcelò D., Duarte A.C., Rocha-Santos T. Risks of Covid-19 face masks to wildlife: present and future research needs. Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsiou D., del Rio-Cubilledo C., Catarino A.I., Summers S., Mohd Fahmi A., Boyle D., Fernandes T.F., Henry T.B. Exposure to pb-halide perovskite nanoparticles can deliver bioavailable pb but does not alter endogenous gut microbiota in zebrafish. Sci. Total Environ. 2020;715 doi: 10.1016/j.scitotenv.2020.136941. [DOI] [PubMed] [Google Scholar]
- Patterson H., Saralahti A., Parikka M., Dramsi S., Trieu-Cuot P., Poyart C., Rounioja S., Rämet M. Adult zebrafish model of bacterial meningitis in Streptococcus agalactiae infection. Dev. Comp. Immunol. 2012;38:447–455. doi: 10.1016/j.dci.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Patterson J.L., Carrion R. Demand for nonhuman primate resources in the age of biodefense. ILAR J. 2005;46:15–22. doi: 10.1093/ilar.46.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton E.E., Widlund H.R., Kutok J.L., Kopani K.R., Amatruda J.F., Murphey R.D., Berghmans S., Mayhall E.A., Traver D., Fletcher C.D.M., Aster J.C., Granter S.R., Look A.T., Lee C., Fisher D.E., Zon L.I. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr. Biol. 2005;15:249–254. doi: 10.1016/j.cub.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Peck K.M., Burch C.L., Heise M.T., Baric R.S. Coronavirus host range expansion and Middle East respiratory syndrome coronavirus emergence: biochemical mechanisms and evolutionary perspectives. Annu. Rev. Virol. 2015;2:95–117. doi: 10.1146/annurev-virology-100114-055029. [DOI] [PubMed] [Google Scholar]
- Peron M., Dinarello A., Meneghetti G., Martorano L., Facchinello N., Vettori A., Licciardello G., Tiso N., Argenton F. The stem-like Stat3-responsive cells of zebrafish intestine are Wnt/β-catenin dependent. Dev. 2020;147 doi: 10.1242/dev.188987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D.L., Bollinger L., White G.L. The baboon (Papio spp.) as a model of human ebola virus infection. Viruses. 2012;4:2400–2416. doi: 10.3390/v4102400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persani L., Marelli F. How zebrafish research has helped in understanding thyroid diseases. F1000Research. 2017;6 doi: 10.12688/f1000research.12142.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C.J., Jones D., Trotter R., Donaldson J., White J., Stephen E., Slone T.W. Experimental Rift Valley fever in rhesus macaques. Arch. Virol. 1988;99:31–44. doi: 10.1007/BF01311021. [DOI] [PubMed] [Google Scholar]
- Peters C.J., Slone T.W. Inbred rat strains mimic the disparate human response to rift valley fever virus infection. J. Med. Virol. 1982;10:45–54. doi: 10.1002/jmv.1890100107. [DOI] [PubMed] [Google Scholar]
- Phelan P.E., Mellon M.T., Kim C.H. Functional characterization of full-length TLR3, IRAK-4, and TRAF6 in zebrafish (Danio rerio) Mol. Immunol. 2005;42:1057–1071. doi: 10.1016/j.molimm.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Phelan P.E., Pressley M.E., Witten P.E., Mellon M.T., Blake S., Kim C.H. Characterization of snakehead rhabdovirus infection in zebrafish ( Danio rerio ) J. Virol. 2005;79:1842–1852. doi: 10.1128/jvi.79.3.1842-1852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps H.A., Neely M.N. SalY of the streptococcus pyogenes lantibiotic locus is required for full virulence and intracellular survival in macrophages. Infect. Immun. 2007;75:4541–4551. doi: 10.1128/IAI.00518-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps H.A., Runft D.L., Neely M.N. Adult zebrafish model of streptococcal infection. Curr. Protoc. Microbiol. 2009;13:9D.1.1–9D.1.27. doi: 10.1002/9780471729259.mc09d01s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A.D., Navabpour S., Hicks S., Dougan G., Wallis T., Frankel G. Enterohaemorrhagic Escherichia coli O157:H7 target Peyer’s patches in humans and cause attaching/effacing lesions in both human and bovine intestine. Gut. 2000;47:377–381. doi: 10.1136/gut.47.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup D.J. Extracellular virions: the advance guard of poxvirus infections. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietretti D., Wiegertjes G.F. Ligand specificities of toll-like receptors in fish: indications from infection studies. Dev. Comp. Immunol. 2014;43:205–222. doi: 10.1016/j.dci.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Piplani S., Singh P.K., Winkler D.A., Petrovsky N. In silico comparison of SARS-CoV-2 spike protein-ACE2 binding affinities across species and implications for virus origin. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-92388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pont S., Blanc-Potard A.B. Zebrafish embryo infection model to investigate Pseudomonas aeruginosa interaction with innate immunity and validate new therapeutics. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.745851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwait J.H., Yan Y.-L., Gates M.A., Horne S., Amores A., Brownlie A., Donovan A., Egan E.S., Force A., Gong Z., Goutel C., Fritz A., Kelsh R., Knapik E., Liao E., Paw B., Ransom D., S. A., Thomson M., Abduljabbar T.S., Yelick P., Beier D., Joly J.S., Larhammar D., Rosa F., Westerfield M., Zon L.I., Johnson S.L.A., Talbot W.S. Vertebrate genome evolution and the zebrafish gene map. Nat. Genet. 1998;18:345–349. doi: 10.1038/ng0498-345. [DOI] [PubMed] [Google Scholar]
- Postlethwait J.H., Massaquoi M.S., Farnsworth D.R., Yan Y.L., Guillemin K., Miller A.C. The SARS-CoV-2 receptor and other key components of the renin-angiotensin-aldosterone system related to COVID-19 are expressed in enterocytes in larval zebrafish. Biol. Open. 2021;10 doi: 10.1242/BIO.058172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasch A.L., Teraoka H., Carney S.A., Dong W., Hiraga T., Stegeman J.J., Heideman W., Peterson R.E. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 2003;76:138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- Prchla E., Plank C., Wagner E., Blaas D., Fuchs R. Virus-mediated release of endosomal content in vitro: different behavior of adenovirus and rhinovirus serotype 2. J. Cell Biol. 1995;131:111–123. doi: 10.1083/jcb.131.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressley M.E., Phelan P.E., Eckhard Witten P., Mellon M.T., Kim C.H. Pathogenesis and inflammatory response to Edwardsiella tarda infection in the zebrafish. Dev. Comp. Immunol. 2005;29:501–513. doi: 10.1016/j.dci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Provost E., Bailey J.M., Aldrugh S., Liu S., Iacobuzio-Donahue C., Leach S.D. The tumor suppressor rpl36 restrains KRASG12V-induced pancreatic cancer. Zebrafish. 2014;11:551–559. doi: 10.1089/zeb.2014.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Q., Zhou H., Shu T., Mu J., Fang Y., Xu J., Li T., Kong J., Qiu Y., Zhou X. The capsid protein of semliki Forest virus antagonizes RNA interference in mammalian cells. J. Virol. 2020;94 doi: 10.1128/jvi.01233-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J., Li Y.S., Zeng R., Liu F.L., Luo R.H., Huang C., Wang Y.F., Zhang J., Quan B., Shen C., Mao X., Liu Xinlei, Sun W., Yang W., Ni X., Wang K., Xu L., Duan Z.L., Zou Q.C., Zhang H.L., Qu W., Long Y.H.P., Li M.H., Yang R.C., Liu Xiaolong, You J., Zhou Y., Yao R., Li W.P., Liu J.M., Chen P., Liu Y., Lin G.F., Yang X., Zou J., Li L., Hu Y., Lu G.W., Li W.M., Wei Y.Q., Zheng Y.T., Lei J., Yang S. SARS-CoV-2 Mpro inhibitors with antiviral activity in a transgenic mouse model. Science (80-. ) 2021;371:1374–1378. doi: 10.1126/science.abf1611originally. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakus K., Mojżesz M., Kłak K., Chadzińska M., Adamek M., Steinhagen D., Podlasz P., Chmielewska-Krzesińska M., Naumowicz K., Kasica-Jarosz N., Rakers S., Way K. Evaluation of zebrafish (Danio rerio) as an animal model for the viral infections of fish. J. Fish Dis. 2019;42:923–934. doi: 10.1111/jfd.12994. [DOI] [PubMed] [Google Scholar]
- Randazzo B., Abbate F., Marino F., Mancuso M., Guerrera M.C., Muglia U., Navarra M., Germanà A. Induction of mild enterocolitis in zebrafish Danio rerio via ingestion of vibrio anguillarum serovar O1. Dis. Aquat. Org. 2015;115:47–55. doi: 10.3354/dao02864. [DOI] [PubMed] [Google Scholar]
- Rekha R.D., Amali A.A., Her G.M., Yeh Y.H., Gong H.Y., Hu S.Y., Lin G.H., Wu J.L. Thioacetamide accelerates steatohepatitis, cirrhosis and HCC by expressing HCV core protein in transgenic zebrafish Danio rerio. Toxicology. 2008;243:11–22. doi: 10.1016/j.tox.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Rendueles O., Ferrières L., Frétaud M., Bégaud E., Herbomel P., Levraud J.P., Ghigo J.M. A new zebrafish model of oro-intestinal pathogen colonization reveals a key role for adhesion in protection by probiotic bacteria. PLoS Pathog. 2012;8:12. doi: 10.1371/journal.ppat.1002815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw S.A., Loynes C.A., Trushell D.M.I., Elworthy S., Ingham P.W., Whyte M.K.B. A transgenic zebrafish model of neutrophilic inflammation | Blood | American Society of Hematology. Blood. 2006;108:3976–3978. doi: 10.1182/blood-2006-05-024075. [DOI] [PubMed] [Google Scholar]
- Richetti S.K., Rosemberg D.B., Ventura-Lima J., Monserrat J.M., Bogo M.R., Bonan C.D. Acetylcholinesterase activity and antioxidant capacity of zebrafish brain is altered by heavy metal exposure. Neurotoxicology. 2011;32:116–122. doi: 10.1016/j.neuro.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Rieger T., Merkler D., Günther S. Infection of type I interferon receptor-deficient mice with various Old World arenaviruses: a model for studying virulence and host species barriers. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippy M.K., Topper M.J., Mebus C.A., Morrill J.C. Rift Valley fever virus-induced encephalomyelitis and hepatitis in calves. Vet. Pathol. 1992;29:495–502. doi: 10.1177/030098589202900602. [DOI] [PubMed] [Google Scholar]
- Rivera J., Tessarollo L. Genetic background and the dilemma of translating mouse studies to humans. Immunity. 2008;28:1–4. doi: 10.1016/j.immuni.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Roberts K.L., Breiman A., Carter G.C., Ewles H.A., Hollinshead M., Law M., Smith G.L. Acidic residues in the membrane-proximal stalk region of vaccinia virus protein B5 are required for glycosaminoglycan-mediated disruption of the extracellular enveloped virus outer membrane. J. Gen. Virol. 2009;90:1582–1591. doi: 10.1099/vir.0.009092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertsen B., Bergan V., Røkenes T., Larsen R., Albuquerque A. Atlantic Salmon interferon genes: cloning, sequence analysis, expression, and biological activity. J. Interf. Cytokine Res. 2003;23:601–612. doi: 10.1089/107999003322485107. [DOI] [PubMed] [Google Scholar]
- Roper C., Tanguay R.L. Zebrafish as a model for developmental biology and toxicology. Handb. Dev. Neurotoxicology. 2018;143–151 doi: 10.1016/B978-0-12-809405-1.00012-2. [DOI] [Google Scholar]
- Ross T.M., Bhardwaj N., Bissel S.J., Hartman A.L., Smith D.R. Animal models of Rift Valley fever virus infection. Virus Res. 2012;163:417–423. doi: 10.1016/j.virusres.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Rossmann M.G. Viral cell recognition and entry. Curr. Sci. 1996;71:193–204. doi: 10.1142/9789814513357_0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A.N., Aravamudhan P., Fernández de Castro I., Tenorio R., Risco C., Dermody T.S. Ins and outs of reovirus: vesicular trafficking in viral entry and egress. Trends Microbiol. 2021;29:363–375. doi: 10.1016/j.tim.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounioja S., Saralahti A., Rantala L., Parikka M., Henriques-Normark B., Silvennoinen O., Rämet M. Defense of zebrafish embryos against Streptococcus pneumoniae infection is dependent on the phagocytic activity of leukocytes. Dev. Comp. Immunol. 2012;36:342–348. doi: 10.1016/j.dci.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Runft D.L., Mitchell K.C., Abuaita B.H., Allen J.P., Bajer S., Ginsburg K., Neely M.N., Withey J.H. Zebrafish as a natural host model for vibrio cholerae colonization and transmission. Appl. Environ. Microbiol. 2014;80:1710–1717. doi: 10.1128/AEM.03580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S B., RD M., E W., D N., JL K., CDM F., JP M., TX L., S S.-M., JP K., R P., LI Z., AT L. Tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc. Natl. Acad. Sci. U. S. A. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiman L., Siegel J. Infection control in cystic fibrosis. Clin. Microbiol. Rev. 2004;17:57–71. doi: 10.1128/CMR.17.1.57-71.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldarriaga O.A., Travi B.L., Choudhury G.G., Melby P.C. Identification of hamster inducible nitric oxide synthase ( iNOS ) promoter sequences that influence basal and inducible iNOS expression. J. Leukoc. Biol. 2012;92:205–218. doi: 10.1189/jlb.1010568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson J.C., Goodridge R., Olobatuyi F., Weis J.S. Delayed effects of embryonic exposure of zebrafish (Danio rerio) to methylmercury (MeHg) Aquat. Toxicol. 2001;51:369–376. doi: 10.1016/S0166-445X(00)00128-4. [DOI] [PubMed] [Google Scholar]
- Sande-Melón M., Marques I.J., Galardi-Castilla M., Langa X., Pérez-López M., Botos M.A., Sánchez-Iranzo H., Guzmán-Martínez G., Ferreira Francisco D.M., Pavlinic D., Benes V., Bruggmann R., Mercader N. Adult sox10+ cardiomyocytes contribute to myocardial regeneration in the zebrafish. Cell Rep. 2019;29:1041–1054.e5. doi: 10.1016/j.celrep.2019.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoriello C., Deflorian G., Pezzimenti F., Kawakami K., Lanfrancone L., Di Fagagna F.D.A., Mione M. Expression of H-RASV12 in a zebrafish model of costello syndrome causes cellular senescence in adult proliferating cells. DMM Dis. Model. Mech. 2009;2:56–67. doi: 10.1242/dmm.001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoriello C., Gennaro E., Anelli V., Distel M., Kelly A., Köster R.W., Hurlstone A., Mione M. Kita driven expression of oncogenic HRAS leads to early onset and highly penetrant melanoma in zebrafish. PLoS One. 2010;5:1–11. doi: 10.1371/journal.pone.0015170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saralahti A., Piippo H., Parikka M., Henriques-Normark B., Rämet M., Rounioja S. Adult zebrafish model for pneumococcal pathogenesis. Dev. Comp. Immunol. 2014;42:345–353. doi: 10.1016/j.dci.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Saralahti A., Rämet M. Zebrafish and streptococcal infections. Scand. J. Immunol. 2015;82:174–183. doi: 10.1111/sji.12320. [DOI] [PubMed] [Google Scholar]
- Sattler R.A., Paessler S., Ly H., Huang C. Animal models of Lassa fever. Pathogens. 2020;9 doi: 10.3390/pathogens9030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlehofer J.R., Ehrbar M., Hausen H.Zur. Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helpervirus dependent parvovirus. Virology. 1986;152:110–117. doi: 10.1016/0042-6822(86)90376-4. [DOI] [PubMed] [Google Scholar]
- Schnupf P., Sansonetti P.J. Quantitative RT-PCR profiling of the rabbit immune response: assessment of acute shigella flexneri infection. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz W.L., Haj A.K., Schiff L.A. Reovirus uses multiple endocytic pathways for cell entry. J. Virol. 2012;86:12665–12675. doi: 10.1128/jvi.01861-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley R.J., Perlmutter A., Seeley V.A. Inheritance and longevity of infectious pancreatic necrosis virus in the zebra fish, Brachydanio rerio (Hamilton Buchanan) Appl. Environ. Microbiol. 1977;34:50–55. doi: 10.1128/aem.34.1.50-55.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendra M., Pereiro P., Yeste M.P., Novoa B., Figueras A. Surgical face masks as a source of emergent pollutants in aquatic systems: analysis of their degradation product effects in Danio rerio through RNA-seq. J. Hazard. Mater. 2022;428 doi: 10.1016/j.jhazmat.2021.128186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich T.G., Ojeda S., Townsley A., Nelson G.E., Moss B. Poxvirus multiprotein entry-fusion complex. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18572–18577. doi: 10.1073/pnas.0509239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y., Fang C., Cheng C., Wang Y., Peng J., Fang W. Immersion infection of germ-free zebrafish with listeria monocytogenes induces transient expression of innate immune response genes. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar P., Dashner-Titus E.J., Truong L., Hayward K., Hudson L.G., Tanguay R.L. Developmental toxicity in zebrafish (Danio rerio) exposed to uranium: a comparison with lead, cadmium, and iron. Environ. Pollut. 2021;269 doi: 10.1016/j.envpol.2020.116097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C.-H., Steiner L.A. Genome structure and thymic expression of an endogenous retrovirus in zebrafish. J. Virol. 2004;78:899–911. doi: 10.1128/jvi.78.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Naujokas M., Park M., Ireton K. InIB-dependent internalization of listeria is mediated by the met receptor tyrosine kinase. Cell. 2000;103:501–510. doi: 10.1016/S0092-8674(00)00141-0. [DOI] [PubMed] [Google Scholar]
- Shepard J.L., Amatruda J.F., Stern H.M., Subramanian A., Finkelstein D., Ziai J., Finley K.R., Pfaff K.L., Hersey C., Zhou Y., Barut B., Freedman M., Lee C., Spitsbergen J., Neuberg D., Weber G., Golub T.R., Glickman J.N., Kutok J.L., Aster J.C., Zon L.I. A zebrafish bmyb mutation causes genome instability and increased cancer susceptibility. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13194–13199. doi: 10.1073/pnas.0506583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., He B., Ma A.C., Guo Y., Leung A.Y. Functions of IDH1 and its mutation in the regulation of developmental hematopoiesis in zebrafish. Exp. Hematol. 2014;42:S59. doi: 10.1016/j.exphem.2014.07.225. [DOI] [PubMed] [Google Scholar]
- Shim D.-H., Suzuki T., Chang S.-Y., Park S.-M., Sansonetti P.J., Sasakawa C., Kweon M.-N. New animal model of shigellosis in the Guinea pig: its usefulness for protective efficacy studies. J. Immunol. 2007;178:2476–2482. doi: 10.4049/jimmunol.178.4.2476. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Matsuda M. Identification of a novel zebrafish mutant line that develops testicular germ cell tumors. Zebrafish. 2019;16:15–28. doi: 10.1089/zeb.2018.1604. [DOI] [PubMed] [Google Scholar]
- Shive H.R., West R.R., Embree L.J., Azuma M., Sood R., Liu P., Hicksteina D.D. Brca2 in zebrafish ovarian development, spermatogenesis, and tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19350–19355. doi: 10.1073/pnas.1011630107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shive H.R., West R.R., Embree L.J., Golden C.D., Hickstein D.D. Brca2 and Tp53 collaborate in tumorigenesis in zebrafish. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha Sadhana, Shrestha Shankar, Shindo J., Sherchand J.B., Haramoto E. Virological quality of irrigation water sources and pepper mild mottle virus and tobacco mosaic virus as index of pathogenic virus contamination level. Food Environ. Virol. 2018;10:107–120. doi: 10.1007/s12560-017-9324-2. [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Ishikawa F., Greiner D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- Siew H.L., Yi L.W., Vega V.B., Miller L.D., Spitsbergen J., Tong Y., Zhan H., Govindarajan K.R., Lee S., Mathavan S., Murthy K.R.K., Buhler D.R., Liu E.T., Gong Z. Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat. Biotechnol. 2006;24:73–75. doi: 10.1038/nbt1169. [DOI] [PubMed] [Google Scholar]
- Singh D., Yi S.V. On the origin and evolution of SARS-CoV-2. Exp. Mol. Med. 2021;53:537–547. doi: 10.1038/s12276-021-00604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Gittis A.G., Gitti R.K., Ostazeski S.A., Su H.-P., Garboczi D.N. The vaccinia virus H3 envelope protein, a major target of neutralizing antibodies, exhibits a glycosyltransferase fold and binds UDP-glucose. J. Virol. 2016;90:5020–5030. doi: 10.1128/jvi.02933-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.R., Shoemaker C.J., Zeng X., Garrison A.R., Golden J.W., Schellhase C.W., Pratt W., Rossi F., Fitzpatrick C.J., Shamblin J., Kimmel A., Zelko J., Flusin O., Koehler J.W., Liu J., Coffin K.M., Ricks K.M., Voorhees M.A., Schoepp R.J., Schmaljohn C.S. Persistent crimean-Congo hemorrhagic fever virus infection in the testes and within granulomas of non-human primates with latent tuberculosis. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1008050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.R., Steele K.E., Shamblin J., Honko A., Johnson J., Reed C., Kennedy M., Chapman J.L., Hensley L.E. The pathogenesis of Rift Valley fever virus in the mouse model. Virology. 2010;407:256–267. doi: 10.1016/j.virol.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Spengler J.R., Keating M.K., McElroy A.K., Zivcec M., Coleman-McCray J.A.D., Harmon J.R., Bollweg B.C., Goldsmith C.S., Bergeron É., Keck J.G., Zaki S.R., Nichol S.T., Spiropoulou C.F. Crimean-Congo hemorrhagic fever in humanized mice reveals glial cells as primary targets of neurological infection. J. Infect. Dis. 2017;216:1386–1397. doi: 10.1093/INFDIS/JIX215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitsbergen J.M., Tsai H.W., Reddy A., Miller T., Arbogast D., Hendricks J.D., Bailey G.S. Neoplasia in zebrafish (Danio rerio) treated with N-methyl-N’-nitro-N-nitrosoguanidine by three exposure routes at different developmental stages. Toxicol. Pathol. 2000;28:716–725. doi: 10.1177/019262330002800512. [DOI] [PubMed] [Google Scholar]
- Spitsbergen J.M., Tsai H.W., Reddy A., Miller T., Arbogast D., Hendricks J.D., Bailey G.S. Neoplasia in zebrafish (Danio rerio) treated with 7,12-dimethylbenz[a]anthracene by two exposure routes at different developmental stages. Toxicol. Pathol. 2000;28:705–715. doi: 10.1177/019262330002800511. [DOI] [PubMed] [Google Scholar]
- Stegmann T., Morselt H.W., Booy F.P., van Breemen J.F., Scherphof G., Wilschut J. Functional reconstitution of influenza virus envelopes. EMBO J. 1987;6:2651–2659. doi: 10.1002/j.1460-2075.1987.tb02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C., Caccamo M., Laird G., Leptin M. Conservation and divergence of gene families encoding components of innate immune response systems in zebrafish. Genome Biol. 2007;8 doi: 10.1186/gb-2007-8-11-r251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear T.P., Seemann T., Harrison P.F., Jenkin G.A., Davies J.K., Johnson P.D.R., Abdellah Z., Arrowsmith C., Chillingworth T., Churcher C., Clarke K., Cronin A., Davis P., Goodhead I., Holroyd N., Jagels K., Lord A., Moule S., Mungall K., Norbertczak H., Quail M.A., Rabbinowitsch E., Walker D., White B., Whitehead S., Small P.L.C., Brosch R., Ramakrishnan L., Fischbach M.A., Parkhill J., Cole S.T. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of mycobacterium tuberculosis. Genome Res. 2008;18:729–741. doi: 10.1101/gr.075069.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoletov K., Klemke R. Catch of the day: zebrafish as a human cancer model. Oncogene. 2008;27:4509–4520. doi: 10.1038/onc.2008.95. [DOI] [PubMed] [Google Scholar]
- Stones D.H., Fehr A.G.J., Thompson L., Rocha J., Perez-Soto N., Madhavan V.T.P., Voelz K., Krachler A.M. Zebrafish (Danio rerio) as a vertebrate model host to study colonization, pathogenesis, and transmission of foodborne Escherichia coli O157. mSphere. 2017;2 doi: 10.1128/mspheredirect.00365-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer N.Y., White R.M., Uong A., Price E., Petur Nielsen G., Langenau D.M., Zon L.I. Zebrafish rhabdomyosarcoma reflects the developmental stage of oncogene expression during myogenesis. Development. 2013;140:3040–3050. doi: 10.1242/dev.087858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strähle U., Scholz S., Geisler R., Greiner P., Hollert H., Rastegar S., Schumacher A., Selderslaghs I., Weiss C., Witters H., Braunbeck T. Zebrafish embryos as an alternative to animal experiments-a commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. 2012;33:128–132. doi: 10.1016/j.reprotox.2011.06.121. [DOI] [PubMed] [Google Scholar]
- Sullivan C., Kim C.H. Zebrafish as a model for infectious disease and immune function. Fish Shellfish Immunol. 2008;25:341–350. doi: 10.1016/j.fsi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Sullivan C.S., Ganem D. A virus-encoded inhibitor that blocks RNA interference in mammalian cells. J. Virol. 2005;79:7371–7379. doi: 10.1128/jvi.79.12.7371-7379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Robertsen B., Wang Z., Liu B. Identification of an Atlantic salmon IFN multigene cluster encoding three IFN subtypes with very different expression properties. Dev. Comp. Immunol. 2009;33:547–558. doi: 10.1016/j.dci.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Swevers L., Kontogiannatos D., Kolliopoulou A., Ren F., Feng M., Sun J. Mechanisms of cell entry by dsRNA viruses: insights for efficient delivery of dsRNA and tools for improved RNAi-based Pest control. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.749387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztal T., Berger S., Currie P.D., Hall T.E. Characterization of the laminin gene family and evolution in zebrafish. Dev. Dyn. 2011;240:422–431. doi: 10.1002/dvdy.22537. [DOI] [PubMed] [Google Scholar]
- Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S., Zhou Y., Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020;17:613–620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Heaton S.M., Parrish N.F. Mammalian antiviral systems directed by small RNA. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki K., Ramakrishnan L., Basu S. A zebrafish model for ocular tuberculosis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0194982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T., Sondalle S.B., Shi H., Zhu S., Perez-Atayde A.R., Peng J., Baserga S.J., Look A.T. The pre-rRNA processing factor DEF is rate limiting for the pathogenesis of MYCN-driven neuroblastoma. Oncogene. 2017;36:3852–3867. doi: 10.1038/onc.2016.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger J.K. The origin and virulence of the 1918 “Spanish” influenza virus. Proc. Am. Philos. Soc. 2006;150:86–112. [PMC free article] [PubMed] [Google Scholar]
- Teame T., Zhang Z., Ran C., Zhang H., Yang Y., Ding Q., Xie M., Gao C., Ye Y., Duan M., Zhou Z. The use of zebrafish (Danio rerio) as biomedical models. Anim. Front. 2019;9:68–77. doi: 10.1093/af/vfz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Haaft P., Almond N., Biberfeld G., Cafaro A., Cranage M., Ensoli B., Hunsmann G., Polyanskaya N., Stahl-Hennig C., Thortensson R., Titti F., Heeney J. Comparison of early plasma RNA loads in different macaque species and the impact of different routes of exposure on SIV/SHIV infection. J. Med. Primatol. 2001;30:207–214. doi: 10.1034/j.1600-0684.2001.d01-54.x. [DOI] [PubMed] [Google Scholar]
- Tenor J.L., Oehlers S.H., Yang J.L., Tobin D.M., Perfect J.R. Live imaging of host-parasite interactions in a zebrafish infection model reveals cryptococcal determinants of virulence and central nervous system invasion. MBio. 2015;6 doi: 10.1128/mBio.01425-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraoka H., Dong W., Tsujimoto Y., Iwasa H., Endoh D., Ueno N., Stegeman J.J., Peterson R.E., Hiraga T. Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochem. Biophys. Res. Commun. 2003;304:223–228. doi: 10.1016/S0006-291X(03)00576-X. [DOI] [PubMed] [Google Scholar]
- Thiennimitr P., Winter S.E., Umler A.J.B. Salmonella, the host and its microbiota. Curr. Opin. Microbiol. 2012;15:108–114. doi: 10.1016/j.mib.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tignor G.H., Hanham C.A. Ribavirin efficacy in an in vivo model of crimean-Congo hemorrhagic fever virus (CCHF) infection. Antivir. Res. 1993;22:309–325. doi: 10.1016/0166-3542(93)90040-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin D.M., Ramakrishnan L. Comparative pathogenesis of Mycobacterium marinum and mycobacterium tuberculosis. Cell. Microbiol. 2008;10:1027–1039. doi: 10.1111/j.1462-5822.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- Todd N.E., Van Leeuwen M. Effects of sevin (carbaryl insecticide) on early life stages of zebrafish (Danio rerio) Ecotoxicol. Environ. Saf. 2002;53:267–272. doi: 10.1006/eesa.2002.2231. [DOI] [PubMed] [Google Scholar]
- Tyrkalska S.D., Candel S., Angosto D., Gómez-Abellán V., Martín-Sánchez F., García-Moreno D., Zapata-Pérez R., Sánchez-Ferrer Á., Sepulcre M.P., Pelegrín P., Mulero V. Neutrophils mediate salmonella typhimurium clearance through the GBP4 inflammasome-dependent production of prostaglandins. Nat. Commun. 2016;7 doi: 10.1038/ncomms12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F.M., Petkevich A.S., Vassilev A.O., Tibbles H.E., Titov L. Stampidine prevents mortality in an experimental mouse model of viral hemorrhagic fever caused by Lassa virus. BMC Infect. Dis. 2004;4 doi: 10.1186/1471-2334-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usenko C.Y., Harper S.L., Tanguay R.L. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon N. Y. 2007;45:1891–1898. doi: 10.1016/j.carbon.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- V G., KT W., RY L., M-C G.-C., L B., R B., TF W., B H. Acute Hendra virus infection: Analysis of the pathogenesis and passive antibody protection in the hamster model. Virology. 2009;387:459–465. doi: 10.1016/j.virol.2009.03.001. [DOI] [PubMed] [Google Scholar]
- V W.-J., L B., D S., F D.K.-M., DP S., H E. Use of the syrian hamster as a new model of ebola virus disease and other viral hemorrhagic fevers. Viruses. 2012;4:3754–3784. doi: 10.3390/v4123754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainstein A., Hershkovitz M., Israel S., Rabin S., Loyter A. A new method for reconstitution of highly fusogenic Sendai virus envelopes. Biochim. Biophys. Acta Biomembr. 1984;773:181–188. doi: 10.1016/0005-2736(84)90081-6. [DOI] [PubMed] [Google Scholar]
- Van Aerle R., Lange A., Moorhouse A., Paszkiewicz K., Ball K., Johnston B.D., De-Bastos E., Booth T., Tyler C.R., Santos E.M. Molecular mechanisms of toxicity of silver nanoparticles in zebrafish embryos. Environ. Sci. Technol. 2013;47:8005–8014. doi: 10.1021/es401758d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Laan J.W., Herberts C., Lambkin-Williams R., Boyers A., Mann A.J., Oxford J. Animal models in influenza vaccine testing. Expert Rev. Vaccines. 2008;7:783–793. doi: 10.1586/14760584.7.6.783. [DOI] [PubMed] [Google Scholar]
- Van Der Sar A.M., Abdallah A.M., Sparrius M., Reinders E., Vandenbroucke-Grauls C.M.J.E., Bitter W. Mycobacterium marinum strains can be divided into two distinct types based on genetic diversity and virulence. Infect. Immun. 2004;72:6306–6312. doi: 10.1128/IAI.72.11.6306-6312.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoeven N., Belser J.A., Szretter K.J., Zeng H., Staeheli P., Swayne D.E., Katz J.M., Tumpey T.M. Pathogenesis of 1918 pandemic and H5N1 influenza virus infections in a Guinea pig model: antiviral potential of exogenous alpha interferon to reduce virus shedding. J. Virol. 2009;83:2851–2861. doi: 10.1128/jvi.02174-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooijen E., Voest E.E., Logister I., Korving J., Schwerte T., Schulte-Merker S., Giles R.H., Van Eeden F.J. Zebrafish mutants in the von hippel-Lindau tumor suppressor display a hypoxic response and recapitulate key aspects of Chuvash polycythemia. Blood. 2009;113:6449–6460. doi: 10.1182/blood-2008-07-167890. [DOI] [PubMed] [Google Scholar]
- Varas M., Ortíz-Severín J., Marcoleta A.E., Díaz-Pascual F., Allende M.L., Santiviago C.A., Chávez F.P. Salmonella typhimurium induces cloacitis-like symptomsin zebrafish larvae. Microb. Pathog. 2017;107:317–320. doi: 10.1016/j.micpath.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Varela M., Romero A., Dios S., van der Vaart M., Figueras A., Meijer A.H., Novoa B. Cellular visualization of macrophage pyroptosis and interleukin-1β release in a viral hemorrhagic infection in zebrafish larvae. J. Virol. 2014;88:12026–12040. doi: 10.1128/jvi.02056-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinotte C.J., Dellaire G., Berman J.N. Hooking the big one: the potential of zebrafish xenotransplantation to reform cancer drug screening in the genomic era. DMM Dis. Model. Mech. 2014;7:745–754. doi: 10.1242/dmm.015784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura Fernandes B.H., Feitosa N.M., Barbosa A.P., Bomfim C.G., Garnique A.M.B., Rosa I.F., Rodrigues M.S., Doretto L.B., Costa D.F., Camargo-dos-Santos B., Franco G.A., Neto J.F., Lunardi J.S., Bellot M.S., Alves N.P.C., Costa C.C., Aracati M.F., Rodrigues L.F., Cirilo R.H., Colagrande R.M., Gomes F.I.F., Nakajima R.T., Belo M.A.A., Giaquinto P.C., de Oliveira S.L., Eto S.F., Fernandes D.C., Manrique W.G., Conde G., Rosales R.R.C., Todeschini I., Rivero I., Llontop E., Sgro G.G., Oka G.U., Bueno N.F., Ferraris F.K., de Magalhães M.T.Q., Medeiros R.J., Mendonça-Gomes J.M., Junqueira M.S., Conceição K., de Pontes L.G., Condino-Neto A., Perez A.C., Barcellos L.J.G., Júnior J.D.C., Dorlass E.G., Camara N.O.S., Durigon E.L., Cunha F.Q., Nóbrega R.H., Machado-Santelli G.M., Farah C.S., Veras F.P., Galindo-Villegas J., Costa-Lotufo L.V., Cunha T.M., Chammas R., Carvalho L.R., Guzzo C.R., Malafaia G., Charlie-Silva I. Toxicity of spike fragments SARS-CoV-2 S protein for zebrafish: a tool to study its hazardous for human health? Sci. Total Environ. 2022;813 doi: 10.1016/j.scitotenv.2021.152345. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Vergunst A.C., Meijer A.H., Renshaw S.A., O’Callaghan D. Burkholderia cenocepacia creates an intramacrophage replication niche in zebrafish embryos, followed by bacterial dissemination and establishment of systemic infection. Infect. Immun. 2010;78:1495–1508. doi: 10.1128/IAI.00743-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S., Srikanth C.V. Understanding the complexities of salmonella-host crosstalk as revealed by in vivo model organisms. IUBMB Life. 2015;67:482–497. doi: 10.1002/iub.1393. [DOI] [PubMed] [Google Scholar]
- Verma S.K., Nandi A., Sinha A., Patel P., Jha E., Mohanty S., Panda P.K., Ahuja R., Mishra Y.K., Suar M. Zebrafish (Danio rerio) as an ecotoxicological model for nanomaterial induced toxicity profiling. Precis. Nanomed. 2021;4 doi: 10.33218/001c.21978. [DOI] [Google Scholar]
- Villacis R.A.R., Filho J.S., Piña B., Azevedo R.B., Pic-Taylor A., Mazzeu J.F., Grisolia C.K. Integrated assessment of toxic effects of maghemite (Γ-Fe2O3) nanoparticles in zebrafish. Aquat. Toxicol. 2017;191:219–225. doi: 10.1016/j.aquatox.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Wade C.M., Daly M.J. Genetic variation in laboratory mice. Nat. Genet. 2005;37:1175–1180. doi: 10.1038/ng1666. [DOI] [PubMed] [Google Scholar]
- Wagenaar T.R., Moss B. Expression of the A56 and K2 proteins is sufficient to inhibit vaccinia virus entry and cell fusion. J. Virol. 2009;83:1546–1554. doi: 10.1128/jvi.01684-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Plank C., Zatloukal K., Cotten M., Birnstiel M.L. Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes: toward a synthetic virus-like gene-transfer vehicle. Proc. Natl. Acad. Sci. U. S. A. 1992;89:7934–7938. doi: 10.1073/pnas.89.17.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D.H., Johnson K.M., Lange J.V., Gardner J.J., Kiley M.P., McCormick J.B. Experimental infection of rhesus monkeys with Lassa virus and a closely related arenavirus, Mozambique virus. J. Infect. Dis. 1982;146:360–368. doi: 10.1093/infdis/146.3.360. [DOI] [PubMed] [Google Scholar]
- Walker D.H., Wulff H., Lange J.V., Murphy F.A. Comparative pathology of Lassa virus infection in monkeys, Guinea pigs, and Mastomys natalensis. Bull. World Health Organ. 1975;52:523–534. [PMC free article] [PubMed] [Google Scholar]
- Wang J., Leng X., Wang G., Wan X., Cao H. The construction of intrahepatic cholangiocarcinoma model in zebrafish. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-13815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Qihui, Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.Y., Wang Qisheng, Zhou H., Yan J., Qi J. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904.e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Lin S., Tang R.W.L., Lee H.C., Chan H.H., Choi S.S.A., Leung K.W., Webb S.E., Miller A.L., Tsim K.W.K. Polygoni multiflori radix extracts inhibit SARS-CoV-2 pseudovirus entry in HEK293T cells and zebrafish larvae. Phytomedicine. 2022;102 doi: 10.1016/j.phymed.2022.154154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Xie D., Liu H., Bao Z., Wang Y. Toxicity assessment of precise engineered gold nanoparticles with different shapes in zebrafish embryos. RSC Adv. 2016;6:33009–33013. doi: 10.1039/c6ra00632a. [DOI] [Google Scholar]
- Warfield K.L., Bradfute S.B., Wells J., Lofts L., Cooper M.T., Alves D.A., Reed D.K., VanTongeren S.A., Mech C.A., Bavari S. Development and characterization of a mouse model for Marburg hemorrhagic fever. J. Virol. 2009;83:6404–6415. doi: 10.1128/jvi.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins K. Emerging infectious diseases: a review. Curr. Emerg. Hosp. Med. Rep. 2018;6:86–93. doi: 10.1007/s40138-018-0162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber L.P., Hill R.L., Janz D.M. Developmental estrogenic exposure in zebrafish (Danio rerio): II. Histological evaluation of gametogenesis and organ toxicity. Aquat. Toxicol. 2003;63:431–446. doi: 10.1016/S0166-445X(02)00208-4. [DOI] [PubMed] [Google Scholar]
- Wen X., Cui L., Morrisroe S., Maberry D., Emlet D., Watkins S., Hukriede N.A., Kellum J.A. A zebrafish model of infection-associated acute kidney injury. Am. J. Physiol. - Ren. Physiol. 2018;315:F291–F299. doi: 10.1152/ajprenal.00328.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R., Rose K., Zon L. Zebrafish cancer: the state of the art and the path forward. Nat. Rev. Cancer. 2013;13:624–636. doi: 10.1038/nrc3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.M., Sessa A., Burke C., Bowman T., LeBlanc J., Ceol C., Bourque C., Dovey M., Goessling W., Burns C.E., Zon L.I. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2:183–189. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Heal. Organ; 2022. 14.9 Million Excess Deaths Associated With the COVID-19 Pandemic in 2020 and 2021.https://www.who.int/news/item/05-05-2022-14.9-million-excess-deaths-were-associated-with-the-covid-19-pandemic-in-2020-and-2021 [Google Scholar]
- WHO . 2009. World Health Statistics 2009 - World Health Organisation. [Google Scholar]
- WHO . Vol. 146. 2008. The Global Burden of Disease: 2004 Update. Update 2010. [Google Scholar]
- Widziolek M., Janik K., Mojzesz M., Pooranachandran N., Adamek M., Pecio A., Surachetpong W., Levraud J.P., Boudinot P., Chadzinska M., Rakus K. Type I interferon-dependent response of zebrafish larvae during tilapia lake virus (TiLV) infection. Dev. Comp. Immunol. 2021;116 doi: 10.1016/j.dci.2020.103936. [DOI] [PubMed] [Google Scholar]
- Wiegand C., Krause E., Steinberg C., Pflugmacher S. Toxicokinetics of atrazine in embryos of the zebrafish (Danio rerio) Ecotoxicol. Environ. Saf. 2001;49:199–205. doi: 10.1006/eesa.2001.2073. [DOI] [PubMed] [Google Scholar]
- Wigginton K.R., Ye Y., Ellenberg R.M. Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle. Environ. Sci. Water Res. Technol. 2015;1:735–746. doi: 10.1039/c5ew00125k. [DOI] [Google Scholar]
- Wilhelm S.W., Suttle C.A. Viruses and nutrient cycles in the sea; viruses play critical roles in the structure and function of aquatic food webs. Bioscience. 1999;49:781–788. [Google Scholar]
- Willey J.B., Krone P.H. Effects of endosulfan and nonylphenol on the primordial germ cell population in pre-larval zebrafish embryos. Aquat. Toxicol. 2001;54:113–123. doi: 10.1016/S0166-445X(00)00178-8. [DOI] [PubMed] [Google Scholar]
- Willis A.R., Torraca V., Gomes M.C., Shelley J., Mazon-Moya M., Filloux A., Lo Celso C., Mostowy S. Shigella-induced emergency granulopoiesis protects zebrafish larvae from secondary infection. MBio. 2018;9 doi: 10.1128/mBio.00933-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittamer V., Bertrand J.Y., Gutschow P.W., Traver D. Characterization of the mononuclear phagocyte system in zebrafish. Blood. 2011;117:7126–7135. doi: 10.1182/blood-2010-11-321448. [DOI] [PubMed] [Google Scholar]
- World Economic Forum . The Global Risks Report 2021. 16th edition. Weforum.Org; 2021. p. 96. [Google Scholar]
- Wu C.Rong, Yin W.Chao, Jiang Y., Xu H.E. Structure genomics of SARS-CoV-2 and its Omicron variant: drug design templates for COVID-19. Acta Pharmacol. Sin. 2022 doi: 10.1038/s41401-021-00851-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.M., Tsai P.R., Yan C.J. Maternal cadmium exposure induces mt2 and smtB mRNA expression in zebrafish (Danio rerio) females and their offspring. Comp. Biochem. Physiol. - C Toxicol. Pharmacol. 2012;156:1–6. doi: 10.1016/j.cbpc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Xiang Z., Dong C., Qi L., Chen W., Huang L., Li Z., Xia Q., Liu D., Huang M., Weng S., He J. Characteristics of the interferon regulatory factor pairs zfIRF5/7 and their stimulation expression by ISKNV infection in zebrafish (Danio rerio) Dev. Comp. Immunol. 2010;34:1263–1273. doi: 10.1016/j.dci.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Xu M., Ye Y., Ye Z., Xu S., Liu W., Xu J., Zhang Y., Liu Q., Huang Z., Zhang W. Human BCR/ABL1 induces chronic myeloid leukemia-like disease in zebrafish. Haematologica. 2020;105:674–686. doi: 10.3324/haematol.2019.215939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Zhang L., Weng S., Huang Z., Lu J., Lan D., Zhong X., Yu X., Xu A., He J. A zebrafish (Danio rerio) model of infectious spleen and kidney necrosis virus (ISKNV) infection. Virology. 2008;376:1–12. doi: 10.1016/j.virol.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Yahr T.L., Wolfgang M.C. Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system. Mol. Microbiol. 2006;62:631–640. doi: 10.1111/j.1365-2958.2006.05412.x. [DOI] [PubMed] [Google Scholar]
- Yakoub A.M., Rawal N., Maus E., Baldwin J., Shukla D., Tiwari V. Comprehensive analysis of herpes simplex virus 1 (HSV-1) entry mediated by zebrafish 3- O -sulfotransferase isoforms: implications for the development of a zebrafish model of HSV-1 infection. J. Virol. 2014;88:12915–12922. doi: 10.1128/jvi.02071-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalklnoglu A.Ö., Heilbronn R., Bürkle A., Schlehofer J.R., Hausen H.Zur. Dna amplification of adeno-associated virus as a response to cellular genotoxic stress. Cancer Res. 1988;48:3123–3129. [PubMed] [Google Scholar]
- Yang H., Rao Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat. Rev. Microbiol. 2021;19:685–700. doi: 10.1038/s41579-021-00630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Yan C., Wang X., Gong Z. Leptin induces muscle wasting in a zebrafish kras-driven hepatocellular carcinoma (HCC) model. DMM Dis. Model. Mech. 2019;12 doi: 10.1242/dmm.038240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J.R.J., Munson K.M., Chao Y.L., Peterson Q.P., MacRae C.A., Peterson R.T. AML1-ETO reprograms hematopoietic cell fate by downregulating scl expression. Development. 2008;135:401–410. doi: 10.1242/dev.008904. [DOI] [PubMed] [Google Scholar]
- Yu L., Deng J., Shi X., Liu C., Yu K., Zhou B. Exposure to DE-71 alters thyroid hormone levels and gene transcription in the hypothalamic-pituitary-thyroid axis of zebrafish larvae. Aquat. Toxicol. 2010;97:226–233. doi: 10.1016/j.aquatox.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Yuan L., Tang Q., Cheng T., Xia N. Animal models for emerging coronavirus: progress and new insights. Emerg. Microbes Infect. 2020;9:949–961. doi: 10.1080/22221751.2020.1764871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun N.E., Poussard A.L., Seregin A.V., Walker A.G., Smith J.K., Aronson J.F., Smith J.N., Soong L., Paessler S. Functional interferon system is required for clearance of Lassa virus. J. Virol. 2012;86:3389–3392. doi: 10.1128/jvi.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun N.E., Seregin A.V., Walker D.H., Popov V.L., Walker A.G., Smith J.N., Miller M., de la Torre J.C., Smith J.K., Borisevich V., Fair J.N., Wauquier N., Grant D.S., Bockarie B., Bente D., Paessler S. Mice lacking functional STAT1 are highly susceptible to lethal infection with Lassa virus. J. Virol. 2013;87:10908–10911. doi: 10.1128/jvi.01433-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewski A.J., Chajęcka-Wierzchowska W., Zadernowska A., Podlasz P. Virulence characterization of listeria monocytogenes, listeria innocua, and listeria welshimeri isolated from fish and shrimp using in vivo early zebrafish larvae models and molecular study. Pathogens. 2020;9:1–10. doi: 10.3390/pathogens9121028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Willett C., Fremgen T. Zebrafish: an animal model for toxicological studies. Curr. Protoc. Toxicol. Chapter. 2003;1 doi: 10.1002/0471140856.tx0107s17. [DOI] [PubMed] [Google Scholar]
- Zhang F., Zhang Z., Thistle R., McKeen L., Hosoyama S., Toida T., Linhardt R.J., Page-Mccaw P. Structural characterization of glycosaminoglycans from zebrafish in different ages. Glycoconj. J. 2009;26:211–218. doi: 10.1007/s10719-008-9177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.J., Hoersch S., Amsterdam A., Whittaker C.A., Beert E., Catchen J.M., Farrington S., Postlethwait J.H., Legius E., Hopkins N., Lees J.A. Comparative oncogenomic analysis of copy number alterations in human and zebrafish tumors enables cancer driver discovery. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Chen S., Zhang W., Yang H., Jin Y., Duan G. An update on animal models for severe acute respiratory syndrome coronavirus 2 infection and countermeasure development. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.770935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Chapgier A., Yang K., Bustamante J., Puel A., Picard C., Abel L., Jouanguy E., Casanova J. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-α/β, IFN-γ and IFN-λ in host defense. Immunol. Rev. 2008;226:29–40. doi: 10.1111/j.1600-065X.2008.00698.x. [DOI] [PubMed] [Google Scholar]
- Zhang S., Racaniello V.R. Expression of the poliovirus receptor in intestinal epithelial cells is not sufficient to permit poliovirus replication in the mouse gut. J. Virol. 1997;71:4915–4920. doi: 10.1128/jvi.71.7.4915-4920.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Dong Z., Zhang C., Ung C.Y., He S., Tao T., Oliveira A.M., Meves A., Ji B., Look A.T., Li H., Neel B.G., Zhu S. Critical role for GAB2 in neuroblastoma pathogenesis through the promotion of SHP2/MYCN cooperation. Cell Rep. 2017;18:2932–2942. doi: 10.1016/j.celrep.2017.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Wu H., Xiao J., Wang Q., Liu Q., Zhang Y. Immune responses of zebrafish (Danio rerio) induced by bath-vaccination with a live attenuated vibrio anguillarum vaccine candidate. Fish Shellfish Immunol. 2012;33:36–41. doi: 10.1016/j.fsi.2012.03.031. [DOI] [PubMed] [Google Scholar]
- Zhao F., Shi Y., Huang Y., Zhan Y., Zhou L., Li Y., Wan Y., Li H., Huang H., Ruan H., Luo L., Li L. Irf8 regulates the progression of myeloproliferative neoplasm-like syndrome via mertk signaling in zebrafish. Leukemia. 2018;32:149–158. doi: 10.1038/leu.2017.189. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Zhao J., Li J., Guo Z., Sheng J., Ye X., Jin G., Wang C., Chai W., Yan J., Liu D., Liang X. SARS-CoV-2 spike protein causes blood coagulation and thrombosis by competitive binding to heparan sulfate. Int. J. Biol. Macromol. 2021;193:1124–1129. doi: 10.1016/j.ijbiomac.2021.10.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A., Paranjape J.M., Der S.D., Williams B.R.G., Silverman R.H. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology. 1999;258:435–440. doi: 10.1006/viro.1999.9738. [DOI] [PubMed] [Google Scholar]
- Zhu S., Lee J.S., Guo F., Shin J., Perez-Atayde A.R., Kutok J.L., Rodig S.J., Neuberg D.S., Helman D., Feng H., Stewart R.A., Wang W., George R.E., Kanki J.P., Look A.T. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell. 2012;21:362–373. doi: 10.1016/j.ccr.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Zhang X., Weichert-Leahey N., Dong Z., Zhang C., Lopez G., Tao T., He S., Wood A.C., Oldridge D., Ung C.Y., van Ree J.H., Khan A., Salazar B.M., Lummertz da Rocha E., Zimmerman M.W., Guo F., Cao H., Hou X., Weroha S.J., Perez-Atayde A.R., Neuberg D.S., Meves A., McNiven M.A., van Deursen J.M., Li H., Maris J.M., Look A.T. LMO1 synergizes with MYCN to promote neuroblastoma initiation and metastasis. Cancer Cell. 2017;32:310–323.e5. doi: 10.1016/j.ccell.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Tian S., Cai Z. Toxicity assessment of iron oxide nanoparticles in zebrafish (Danio rerio) early life stages. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuravleva J., Paggetti J., Martin L., Hammann A., Solary E., Bastie J.N., Delva L. MOZ/TIF2-induced acute myeloid leukaemia in transgenic fish. Br. J. Haematol. 2008;143:378–382. doi: 10.1111/j.1365-2141.2008.07362.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman M.W., Liu Y., He S., Durbin A.D., Abraham B.J., Easton J., Shao Y., Xu B., Zhu S., Zhang X., Li Z., Weichert-Leahey N., Young R.A., Zhang J., Look A.T. MYC drives a subset of high-risk pediatric neuroblastomas and is activated through mechanisms including enhancer hijacking and focal enhancer amplification. Cancer Discov. 2018;8:320–335. doi: 10.1158/2159-8290.CD-17-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Tafalla C., Truckle J., Secombes C.J. Identification of a second Group of Type I IFNs in fish sheds light on IFN evolution in vertebrates. J. Immunol. 2007;179:3859–3871. doi: 10.4049/jimmunol.179.6.3859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.