Abstract
The gastrointestinal tract greatly contributes to global cancer burden and cancer-related deaths. The microbiota represents the population of microorganisms that live in and around the body, located primarily in the gastrointestinal tract. The microbiota has been implicated in colorectal cancer development and progression, but its role in cancer therapy for the gastrointestinal tract is less defined, especially for extra-intestinal cancers. In this review, we discuss the past 5 years of research into microbial involvement in immune-related therapies for colorectal, pancreatic, hepatic, and gastric cancers, with the goal of highlighting recent advances and new areas for investigation in this field.
Keywords: antitumor immunity, colorectal cancer, liver cancer, microbiota, pancreatic cancer
Introduction
Cancers of the gastrointestinal (GI) tract make up 26% of the global cancer burden and 35% of cancer-related deaths.1 Recent advances in immunotherapies have led to unprecedented survival rates among select cancer types, like melanoma and non-small-cell lung cancer, but less so in GI cancers.2 The Food and Drug Administration has granted approval for the use of immune checkpoint inhibition (ICI) in colorectal cancer (CRC) with both mismatch repair deficiency and high microsatellite instability (MSI-H), but these cases represent only 15% of CRCs.2 For those with low microsatellite instability, there has been no demonstrated clinical benefit for ICI intervention. And although ICI has also been approved for hepatic and gastric cancers (GC),3 there is no clinical benefit for pancreatic cancer, even with MSI-H status.4 There is a significant need to advance immunotherapy response in the clinic for these cancers, by improving biomarkers of responsiveness and improving existing immunotherapies. Of recent interest to human health and disease is the microbiota, which is the population of microorganisms including bacteria, viruses, fungi, and archaea that populate our body. The composition and interactions among this microbial network are complex and influenced by several intrinsic and extrinsic factors such as genetics, inflammation, stress, and diet to name a few.5 Remarkably, these microorganisms have been associated with cancer prevention or progression through a number of mechanisms including production of toxins, metabolites, structural molecules (adhesins, flagellin, and membrane derivatives), and nucleic acids.6 Although great progress has been made in deciphering mechanisms by which microorganisms, especially bacteria modulate cancer development, limited progress has been made on the impact of microorganisms on immune-based therapeutics for GI cancers. The importance of microbiota in ICI responsiveness has been elegantly demonstrated in a trio of paper published in 2018.7–9 These studies demonstrated that responsiveness to PD-1 immune checkpoint blockade is associated with enrichment of three nonoverlapping bacterial strains Faecalibacterium prausnitzii, Bifidobacterium longum, and Akkermansia muciniphila in the intestinal microbiota of patients with either advanced melanoma and non-small-cell lung cancer.7–9 These studies provided the rationale for two recent clinical trials that showed that fecal microbiota transplant from melanoma patients that responded to anti-PD-1 therapy into nonresponding patients can overcome their therapeutic resistance.10,11 However, in a meta-analysis of the three landmark studies, it was found that microbial gene content was a more effective predictor of patient response than relative abundance alone, indicating that the mechanisms behind bacterial-driven response are more complex than previously thought.12 These fascinating findings generated high interest in investigating the relationship between microbiota and immunotherapy for GI cancer management. This review focuses on recent advance (past 5 years) in this field of research by discussing literature covering colorectal, pancreatic, hepatic, and GC.
Colorectal cancer
Colorectal cancers (CRC) are globally the third most common cancer and the second leading cause of death by cancer.1 Interestingly, although rates of CRC in older adults in the United States have declined in recent years due to improved screening and awareness, rates in younger adults have begun to rise, which may be attributed to the higher incidence of obesity and other diet and lifestyle trends in the western hemisphere.13 Current standard-of-care treatments for CRC include chemotherapy (oxaliplatin, 5-FU, cyclophosphamide, and irinotecan),14 although immunotherapies targeting the host immune system to mount a targeted anticancer response are emerging. In this section, we will discuss new associations between the human gut microbiome, immunity, and CRC, including findings about drug interactions and how microbes modulate anticancer therapies.
New links between gut microbiome, immunity and colorectal cancer therapies.
Combination therapy with either two immunotherapies or immune-related therapy and chemotherapy has made progress in the clinic with improvement of CRC patient survival.15,16 However, these therapeutic modalities associate with greater frequency of side effects such as fatigue, diarrhea, colitis, and other adverse immune-related events.16,17 Therefore, identifying therapeutic responders versus nonresponders has the potential to alleviate toxicity and improve treatment. A retrospective cohort study of 39 CRC patients treated with either chemotherapy alone (n = 19) or chemotherapy and adoptive cellular therapy (n = 20) found that responders to combined therapy had greater diversity of plasma circulating microbiota, and a higher relative abundance of Bifidobacterium, Lactobacillus, and Enterococcus at baseline (pretreatment) as measured by 16S rDNA sequencing.18 Higher overall survival was also associated with an increased relative abundance of Lactobacillus.18 In another study, ileal crypt apoptosis was found to be associated with better prognosis in patients with CRC who had been treated with oxaliplatin (OXA), and that this was accompanied by an increase in Erysipelotrichaceae relative abundance, and a decrease in Fusobacteriaceae.19 Using an MC38 mouse allograft model, the authors found that antibiotic depletion reduced the antitumor effect of OXA and that fecal microbiota transplant from a subset of patients with CRC also induced treatment resistance.19 The nonresponding patient biota was associated with the presence of Paraprevotella clara, while responding biota was enriched in Bacteroides fragilis.19 The ileal microbiota was also found to act as an adjuvant in helping efficacy of PD-1 blockade in models using MC38 or CT26 allografts, where B. fragilis and Erysipelatoclostridium ramosum improved OXA + anti-PD-1 efficacy while P. clara and Fusobacterium nucleatum reduced efficacy.19 Although these studies have generated interesting findings, the lack of a common bacterial abundance signal across studies (Table 1) indicates the complexity of these interactions and the need for larger cohorts and validation studies.
Table 1.
Summary of recent association studies in GI cancer cohorts
| Paper | Cancer type | Sample type | ICI type | Diversity association | Bacterial association | Immune phenotype | Geographic location |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Yang et al., 2021 | CRC (n = 39) | Plasma | Chemotherapy (n = 19) Chemo/Adoptive cellular therapy (n = 20) |
Increase in plasma diversity in responders | R: Enriched Bifidobacterium, Lactobacillus and Enterococcus at baseline OS: Enriched Lactobacillus |
UD | China |
| Roberti et al., 2020 | pCC (n = 97) | Ileal mucosa | Oxaliplatin | R: Enriched Erysipelotrichaceae, Decreased Fusobacteriaceae | Increased ilea cCasp3 with favorable prognosis | France | |
| Peng et al., 2020 | GI, n = 74 | Feces | anti-PD-1/PD-L1/CTLA-4 | No difference | R: Enriched Prevotellaceae, Akkermansia, Decreased Bacteroidaceae | UD | China |
| Nomura, et al., 2020 | n = 52: melanoma n = 24, head and neck n = 10, GI n = 9, Uro n = 6, lung n = 2, sarcoma n = 1 | Feces and plasma | Anti-PD-1 | N/A | N/A | Increased SCFA (fecal and plasma) in R and PFS, esp. propionic acid and butyric acid | Japan |
| Yu et al., 2017 | Recurrent CRC (n = 16) and nonrecurrent CRC (n = 15) | Feces and mucosa | UD | N/A | Recurrent CRC: more Fusobacterium, Prevotella intermedia, Parvimonas micra, and Peptostreptococcus anaerobius | Reduced autophagy signaling in recurrent CRC | China |
| Zheng et al., 2019 | HCC (n = 8) | Feces | Anti-PD-1 | Higher diversity (species numbers) in responders | 20 species including Bifidobacterium dentium, Akkermansia muciniphila, Lactobacillus species were enriched in responders. 15 species including Bacteroides nordii, Fusobacterium varium, and Bacteroides eggerthii were enriched in nonresponders |
UD | China |
| Li and Ye, 2020 | HCC (n = 65) | Feces and ora swabs | UD | Higher Shannon diversity of oral and fecal microbiotas in responder than nonresponders | Faecalibacterium associated with long progression-free survival. Bacteroidales associated with short progression-free survival | UD | China |
| Shen et al., 2021 | HCC (n = 36) | feces | Anti-PD-1/anti-PD-L1 | No difference between responders and nonresponders | No specific taxa were associated with response | UD | Taiwan |
Summary of recent association studies in GI cancer cohorts. The table presents a summary of the major findings in association studies between the microbiome and cancer therapeutic efficacy in human studies.
cCasp3, cleaved caspase-3; CRC, colorectal cancer; GI, gastrointestinal; HCC, hepatocellular carcinoma; NR, nonresponder; OS, overall survival; pCC, proximal colon cancer; PFS, progression-free survival; R, responder; SCFA, short-chain fatty acids; UD, undefined.
A novel class of drugs that may offer benefit in CRC therapy are anti-inflammatories like aspirin and immunotherapies targeting inflammatory pathways like tumor necrosis factor blockade (anti-TNF). Aspirin administration has been found to reduce tumor load in ApcMin/+ mice and in AOM/DSS mice with a depleted microbiota.20 The bacterium Lysinibacillus sphaericus was shown to have the ability to degrade aspirin, and supplementing germ-free aspirin-treated mice with this bacterium increased the number of tumors formed.20 Aspirin-treated mice were enriched in Bifidobacterium and Lactobacillus genera and had reduced abundance of Alistipes finegoldi and Bacteroides fragilis.20 Another study found that ApcMin/+ mice have reduced tumor formation and colitis following pathogenic Escherichia coli colonization when they are administered neutralizing anti-TNF antibody.21 E. coli colonization levels did not change, but instead the anti-TNF therapy shifted the gut-microbial composition of the mice towards a less inflammatory state, including an enrichment in Clostridiaceae.21 Untreated mice, on the other hand, had increased Erysipelotrichaceae and Lachnospiraceae.21 These initial findings indicate that anti-inflammatories may make promising co-therapies or adjuvant treatments to improve response in CRCs. Taken together, these studies suggest that bacterial signature may associate with treatment response, a phenomenon that could be linked to immune interaction or drug metabolism.
Microbiota and colorectal cancer immunotherapy.
Colorectal cancer has a very poor response rate to ICI, and also has a lower mutational burden, which can interfere with ICI’s mode of action.2 A study of 74 patients with advanced stage GI cancer, 26 of which were MSI-H, found that responders to therapy are enriched in Prevotellaceae and that patients with a higher relative abundance of Akkermansia were more likely to benefit from ICI therapy.22 However, there was no difference in microbial diversity between responders and nonresponders.22 Machine learning model based on the microbial composition of responders versus nonresponders identified families Ruminococcaceae, Lachnospiraceae, Bacteroides, and Catenibacterium as most predictive features of responsiveness.22
Understanding the impact of microbiota on immune response in CRC-bearing hosts could have profound impact on therapeutics. For instance, mice bearing MC38 tumors showed reduced CD8+ T-cell proliferation and increased tumor growth when colonized with Helicobacter pylori following anti-CTLA-4, anti-PD-1, or oncolytic viral therapies.23 In another study, MC38-bearing B-cell deficient mice had increased antitumor immunity via improved CD8+ INFγ+ T-cell circulation due to microbial dysbiosis induced by their immune deficiency.24 Antibiotic-depleted MC38-bearing mice have also been found to have reduced macrophages and dendritic cells and that Akkermansia supplementation rescues their response to anti-PD-1 therapy.25 Lastly, in the non-PD-1-responsive tumor model CT26, it was found that mice with high microbial diversity had a better response to anti-PD-1 treatment and increased IL-2 and IFNγ secretion than mice treated with antibiotics, which worsened response.26
Recently, much effort has been devoted to characterizing the mechanisms responsible for bacterial-mediated response to immunotherapy. The modes by which the microbiota could drive response include live bacteria acting as antigens for cancer immune response, either through cross-reactive T-cell priming or release of pathogen-associated molecular patterns. The genus Enterococcus has been highlighted in several studies as influencing CRC-specific response to immunotherapy. First, Daillère et al. found that mice bearing MC38 tumors and treated with cyclophosphamide showed enriched Enterococcus hirae relative abundance in their gut microbiota, which associated with reduced tumor burden through an increase in intratumoral CD8+ T-cell infiltration.27 In addition, Fluckiger et al. identified a prophage tape measure protein that is specific to E. hirae that binds MHC class I and improves immune response to both anti-PD-1 alone and anti-PD-1 combined with cyclophosphamide in mice bearing MC38 tumors.28 This response was shown to be also mediated by an increase presence of antitumor CD8+ T cells. Most recently, Griffin et al. found that Enterococcus faecium peptidoglycan digestion by SagA releases peptide fragments that stimulate NOD2 signaling and synergize with anti-PD-1/anti-CTLA-4 immunotherapies in the MC38 tumor model to increase antitumor response.29 These findings illustrate the complexity by which a given bacterial genera could interact with therapeutics to modulate outcomes.
When looking at host immunity, cytokine production can be an important mediator of gut-microbial crosstalk as well, especially in the context of innate lymphoid cells (ILCs) activity. Goc et al. found that CRC patient tumor tissues have a reduced number of ILC3s, which produce IL-22 that promotes epithelial homeostasis, and increased pro-inflammatory IFNγ-producing ILC1s than healthy tissue.30 The authors found that the existing ILC3s are also transcriptionally different, showing an ‘exhaustion’ phenotype, with markers indicating active transition into ILC1s.30 Loss of gut-protective ILC3s was associated with altered microbial composition, including increased abundance of Clostridiales, and lead to resistance to ICIs in the MC38 mouse model.30 Whether increased abundance of Clostridiales functionally impacts resistance to ICI treatment is unknown. Cytokines themselves can also be used as immunotherapy, like in the case of IL-2, and it has been shown that combining IL-2 therapy with administration of A. muciniphilia can increase immune-driven tumor apoptosis.31 In mice bearing CT26 tumors, a synergistic antitumor effect of IL-2/A. muciniphilia was reported, a phenomenon dependent on the TLR2 signaling pathway.31 Another hypothesis explaining microbially driven response to therapy is that microbial metabolites act as messengers, either impacting host immunity or through a direct-to-tumor signaling pathway. For example, Bifidobacterium pseudolongum producing the metabolite inosine acts as a potent modulator of Th1 effector cells, which in turn improves anti-CTLA-4 efficacy in several mouse models of CRC.32
On the other hand, microbial products can also promote resistance to therapy in CRC, such as in the cases of colibactin-producing E. coli (CoPEC) and F. nucleatum. Lopès et al. found that in 40 CRC tumor biopsies, CoPEC positive patients had a decrease level of intratumoral CD3+ cells.33 In the ApcMin/+ mouse model, mice colonized with CoPEC had more tumors and inflammation, which was accompanied by decreased colonic CD3+ and CD8+ T-cell frequency.33 In the MC38 tumor model, mice colonized with CoPEC had increased resistance to anti-PD-1 therapy, illustrating factors that may promote resistance in human patients.33 In the case of F. nucleatum, Yu et al. compared recurrent and nonrecurrent CRC biopsies and found that F. nucleatum was enriched in recurrent CRC tissues and that this bacterium’s abundance associated with shorter recurrence-free survival.34 In xenograft models of HCT116, HT29, and SW480 tumors, F. nucleatum induced resistance to chemotherapy and reduced CRC apoptosis by selectively reducing micro-RNAs responsible for autophagy signaling in tumors.34 Recurrent CRC patients were also found to have lower levels of these miRNAs than nonrecurrent patients.34 F. nucleatum is also able to produce a variety of other bacterial products that promote tumor resistance to immune attack. For instance, Fap2 and FadA are surface adhesion proteins that contribute to F. nucleatum invasion in CRCs. Fap2 expressed by F. nucleatum on colonized colon cancer cells interacts with human immune receptor TIGIT, inhibiting NK cells-mediated killing and promoting tumor immunosuppression.35,36 FadA expressed by tumor-colonizing F. nucleatum binds to E-cadherin, which promotes downstream inflammation and oncogenic signaling, and has been shown to promote chemotherapy resistance.36,37 However, novel approaches that engineer F. nucleatum to produce antitumor products are in development which may enable future tumor-localized therapies.36 Overall, these findings support the concept that intestinal bacteria exerting positive and negative effects likely coexist in the intestine and that treatment outcomes depend on not just the presence but the balance of these forces.
Fecal microbiota transplant and bacterial consortia.
Targeted modulation of the gut microbiota is one approach that has garnered interest in the field of cancer therapy. Microbiome-centered approaches may have less side effects than traditional pharmacologic drugs and can offer great benefit depending on the disease context. One such therapy is fecal microbiota transfer (FMT) where feces from a healthy donor is used to repopulate the gut of a diseased recipient. Although this therapeutic approach has been used with great success for the treatment of patients with recurrent Clostridium difficile infection,38,39 evidence that such an intervention is applicable to cancer is slow to emerge. In a case study using two patients, FMT from a healthy donor abrogated refractory ICI-associated colitis, and that recovery was associated with increased relative abundance of Blautia and Bifidobacterium.40 In another study, mice bearing MC38 tumors supplemented with Bifidobacterium bifidum responded to combined oxaliplatin and anti-PD-1 therapy.41 A more refined form of FMT is the supplementation of a defined consortium of bacteria that could synergize with host immune system to improve therapeutic response. For example, Tanoue et al. identified an 11-member consortium that enhanced anti-CTLA-4 efficacy in the MC38 tumor mice model, via increased tumoral CD8+ IFNγ + infiltration.42 More recently, Montalban-Arques et al. selected four Clostridiales strains (Roseburia intestinalis, Eubacterium hallii, F. prausnitzii, and Anaerostipes caccae) based on their ability to express butyryl-CoA transferase and showed that supplementation of this bacterial cocktail reduced tumor growth in the MC38 mouse model and was also more effective than anti-PD-1 therapy alone.43 Although much work is needed to establish therapeutic efficacy of microbial transfer for cancer management, it is likely that well-defined designer cocktails will supersede the use of FMT due to safety and regulation concerns.44 This is clearly exemplified with the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the feces of some infected patients even following clearance of the virus in the upper airway.45
Dietary interventions for colorectal cancer.
Diet-centered approaches may represent a more efficacious means by which to modify the intestinal microbiota.46 Changes in diet can be made to incorporate certain foods and eliminate others in an effort to improve microbial health. High soluble fiber intake has been associated with improved response to anti-PD-1 therapy in mice bearing MC38 tumors, which correlated with an enrichment in Akkermansia, a mucosal-associated commensal bacterium.25 Additionally, FMT from these high fiber fed-mice was enough to improve therapeutic response in a recipient MC38 tumor-bearing mouse.25 Another study found that pectin supplementation, a soluble fiber found in fruits and vegetables, could improve response to anti-PD-1 therapy in MC38-bearing mice colonized with feces from human CRC patients.47 Dietary pectin increased the abundance of several short-chain fatty acid (SCFA)-producing bacteria such as Erysipelotrichaceae, Ruminococcaceae, and Bifidobacteriaceae, as well as butyrate levels, illustrating how diet supplementation could rescue dysbiotic microbiota-induced resistance.47 Inulin, or chicory root fiber, has also been investigated as a prebiotic to improve ICI therapy.48 In the microsatellite stable CT26 tumor model, mice supplemented with inulin had improved response, and increased relative abundance of Akkermansia, Lactobacillus, and Roseburia.48 An engineered inulin gel to improve colonic retention of the fiber also improved response to anti-PD-1 and anti-CTLA-4 therapies.48 Metabolites from fiber digestion by the microbiota include SCFAs, which includes butyrate, but the benefit is unclear for CRC therapy. On one hand, Coutzac et al. supplemented mice bearing MC38 or CT26 tumors with sodium butyrate, which worsened response to anti-CTLA-4 therapy.49 Additionally, in an associative study of the fecal microbiota of 384 healthy and 390 CRC patients, Porphyromonas asaccharolytica and Porphyromonas gingivalis were found to be rare but more abundant in CRC patients than healthy subjects.50 Porphyromonas species produce butyrate, and it was shown that cell senescence was induced by these species in invaded tissue, which may be driving tumor mutation development.50 But contrary to these findings, there is evidence for a beneficial role of SCFAs in therapeutic response. An associative study found that in 52 patients with solid cancer, including CRC, responders to anti-PD-1 and PD-L1 had higher fecal and plasma SCFA levels.51 However, the SCFA identified to be associated with progression-free survival was propionic acid, associated with high mushroom intake.51 Additionally, another study of 74 patients with advanced GI cancers found that SCFA fermentation and SCFA-producing bacteria Prevotella, Ruminococcaceae, and Lachnospiraceae were enriched in responders to anti-PD-1/PD-L1 responders.22 Lastly, in an MC38 tumor model treated with oxaliplatin, butyrate was found to improve response efficacy by promoting IL-12 signaling and CD8+ T-cell stimulation.52 The conflicting evidence for and against SCFA, especially butyrate, may be due to differences in concentration and location. Luu et al. found that gut commensal microbe-derived butyrate and pentanoate were able to enhance CD8+ effector T-cell function in xenograft models of CRC, whereas Coutzac et al. measured SCFA levels in the circulating blood and found that higher levels were associated with therapeutic resistance.49,53 Further studies are needed to establish effective dosing and identify proper sources for these SCFAs like butyrate to achieve beneficial outcomes in patients.
Engineered bacteria and viruses.
Another rapidly developing therapeutic is engineered bacteria and viruses that generate antitumor response in otherwise non-immunogenic cancers. The fusogenic oncolytic vaccinia virus is one such engineered phage, and coupled with anti-PD-1 mounted an effective antitumor response in the microsatellite stable CT26 mouse tumor model, where the ICI alone had no effect.54 There is also an engineered strain of Salmonella typhimurium, which produces flagellin B (FlaB) from Vibrio vulnificus, and when administered to mice accumulates in tumor tissue and suppresses tumor growth in the MC38 model.55 In the orthotopic HCT116 model of CRC, the S. typhimurium strain is also able to reduce metastatic growth, in a manner dependent on TLR5-induced macrophage polarization.55 These engineered strains and phages can also be combined with ICI. For example, intratumoral injection of an oncolytic virus expressing IL-7 and IL-12 increases CD8+ and NKT cells, and decreases tumor volume in the CT26 model.56 When combined with ICIs, response was greater in CT26, HCT116, and Detroit 562 mouse tumor models than either therapy alone.56 Another study showed that the probiotic E. coli Nissle strain engineered to convert tumoral ammonia waste to L-arginine, synergized with anti-PD-1 therapy to improve response in the MC38 mouse tumor model.57 The next step for applying therapeutically engineered microbes to CRC appears to be safety and efficacy clinical trials, which will determine future of this approach.
Immunotherapy is not commonly applied in the clinic for CRC due to the microsatellite stable phenotype of most cases.2 However, these recent findings indicate that the microbiota may be a source of biomarkers for response or a therapeutic in and of itself. However, the link between therapy and microbiota becomes even more tenuous when one considers extra-intestinal cancers along the GI tract such as pancreatic, hepatic, and gastric. Next, we cover recent findings from these cancer sites to highlight this emerging field and identify the gaps in knowledge that may inform future studies.
Pancreatic cancer
New links between the microbiota, immunity, and pancreatic cancer.
Bacterial DNA and 16S rRNA signal can be detected in human pancreatic adenocarcinoma (PDAC) samples and less prevalently in normal pancreas tissue samples,58 suggesting the presence of a resident pancreatic microbiota that dynamically changes with carcinogenesis. This has been supported by other groups surveying the transcriptome datasets from the TCGA cohort.59–61 Human PDAC tumor contains a microbiota that is distinct from that in the normal tissue62,63 and matched fecal samples.62 The pancreatic microbiota likely originates from the duodenum where the pancreatic duct opens, as Proteobacteria, which are abundant in the duodenum, are also the most common phyla found in PDAC tumors.58 In line with the gut-to-pancreas migration route, orally introduced E. coli are found to colonize the mouse pancreas.62 Emerging evidence suggests that both the local and distant (gut) microbiotas can influence pancreatic carcinogenesis and response to therapies.
Germ-free status or antibiotics-mediated microbiota depletion slows down the development of spontaneous and orthotopically implanted pancreatic tumors in mouse models,62,64 suggesting that the microbiota overall promotes pancreatic carcinogenesis. However, these models do not distinguish the contribution from the pancreatic and intestinal microbiota, as the microbiota in both compartments is affected by germ-free derivation or broad-spectrum antibiotics. In contrast, in a subcutaneous PDAC xenograft tumor model where no intratumoral bacteria can be detected, gut microbiota depletion by antibiotics still reduces tumor growth,64 clearly demonstrating a long-distance pro-tumor effect of the gut microbiota. Microbiota depletion results in increased intratumoral CD45+ cells, CD8+:CD4+ T-cell ratio, M1 macrophages, and decreased myeloid-derived suppressor cells (MDSC), suggesting the microbiota contributes to an immunosuppressive environment in pancreatic cancer.62,64 The cancer immune tolerance is at least partially induced by microbial activation of pattern recognition receptors such as TLR2 and TLR5.62
Pancreatic adenocarcinoma development is associated with changes in the gut microbiota, which could further impact pancreatic carcinogenesis. The initial evidence comes from the observation that FMT from mice with spontaneous PDAC accelerates PDAC development in recipient mice as compared with FMT from healthy wild type mice.62 Importantly, recent data suggest that having PDAC does not necessarily entail a more carcinogenic gut microbiota. FMT to mice with orthotopic pancreatic tumors showed that the gut microbiota from PDAC patients who were clinically predicted to be ‘short-term survivors’ (survive less than 5 years after surgery) significantly enhanced tumor growth, whereas the gut microbiota from patients classified as ‘long-term survivors’ (LTS, survive more than 5 years) with no evidence of disease reduced tumor growth.63 CD8+ T cells play an essential role in microbiota-mediated tumor regulation, as antibiotic treatment or CD8+ T-cell depletion abrogated the tumor-preventive effect of LTS with no evidence of disease microbiota in mouse models.
Inside the pancreas, PDAC tumors contain higher loads of bacteria than normal tissue.62 16S rRNA gene sequencing on pancreatic tumor sections from two cohorts of PDAC patients revealed that LTS tumor microbiota has higher alpha diversity and distinct composition compared with short-term survivor tumor microbiota.63 Consistent with the notion of a local microbiota impact on PDAC, intratumoral microbiota diversity significantly correlates with CD8+ and GzmB+ cell infiltration, and the abundance of the top three enriched genera in LTS patients, that is, Saccharopolyspora, Pseudoxanthomonas, and Streptomyces, correlates with CD8+ T-cell tissue densities.63 Notably, these three genera, combined with Bacillus clausii, have remarkable power to predict long-term survivorship in PDAC patients.63
Microbiota effects on pancreatic cancer immunotherapy.
Given the general pro-tumorigenic role of the microbiota observed in mouse models, microbiota depletion by oral antibiotics was tested and shown to synergize with anti-PD-1 therapy to control orthotopic PDAC growth.62 However, this untargeted approach might not benefit PDAC patients because treatment with broad-spectrum antibiotics within 1 to 2 months prior or in the first 30 days of treatment has been associated with poor outcomes in patients with other cancers receiving immune-checkpoint inhibitor therapy.65 Bacterial lipopolysaccharide (LPS) accumulates in PDAC tumors compared with adjacent normal tissues, likely resultant from a leaky gut.66 In PDAC patients, serum LPS levels positively correlate with intratumoral PD-L1 expression.66 LPS induced PD-L1 expression and consequently T-cell exhaustion in subcutaneous PDAC xenografts in mice via activating the TLR4/MYD88 signaling.66 Accordingly, intraperitoneal LPS injection synergized with PD-L1 blockade to inhibit subcutaneous PDAC tumor growth.66 Although the result is promising, its clinical application is very unlikely considering the septic shock risk of systemic LPS administration.
Recently, pentanoate, a SCFA produced by human gut commensals such as Megasphaera massiliensis, is shown to enhance the antitumor activity of antigen-specific cytotoxic T lymphocytes and engineered chimeric antigen receptor T cells against subcutaneously implanted pancreatic tumors.53 Mechanistically, this effect is mediated by pentanoate inhibition of HDAC class I enzymes and subsequent increase of the mTOR activity.53 Whether strains of M. massiliensis can synergize with ICIs or adoptive chimeric antigen receptor T-cell therapy against pancreatic cancer remains to be evaluated. Nonetheless, the effect of pentanoate is likely cancer type-dependent, because in intraperitoneal injection of pentanoate abrogated the efficacy of anti-PD-1 on subcutaneous melanoma tumors in mice.53
Liver cancer
Altered gut microbiota has been reported in patients with hepatocellular carcinoma (HCC),67–71 the most common form of liver cancer (~90% of cases).72 The gut microbiota contributes to HCC development by inducing inflammation.73 FMT from patients with hepatitis C virus-related chronic liver disease (CLD) increased spontaneous and carcinogen-induced liver carcinogenesis in mice, suggesting a pro-tumorigenic role of CLD-associated gut microbiota.74 Enterococcus faecalis, a species enriched in both CLD and CLD-related HCC patients as well as in recipient mice with liver tumors, can promote mouse liver carcinogenesis via expression of the metallopeptidase GelE.74 GelE-positive E. faecalis via its gelatinase activity increases gut permeability, leading to elevated plasma LPS and activation of TLR4/MYD88 signaling in hepatocytes.74 This results in expression of proliferative genes, facilitating tumor development.74
Recent studies suggest that the gut microbiota can also enhance liver carcinogenesis by dampening tumor immunosurveillance. Consistent with the pro-tumorigenic role of the gut microbiota, treatment with broad-spectrum antibiotics prevented spontaneous HCC development and inhibited liver metastasis of tumors of other origins in mice.75 This was associated with a liver-specific increase of NKT cells, depletion of which nullified the effect of antibiotics on liver tumor growth.75 NKT cell accumulation is driven by primary bile acid-mediated CXCL16/CXCR6 chemokine signaling.75 By converting primary bile acids to secondary bile acids, the gut microbiota, particularly vancomycin-sensitive Gram-positive bacteria, reduces NKT cell liver infiltration to allow liver tumor growth.75 Aside from NKT cells, the gut microbiota could modulate other T-cell responses to establish an immunosuppressive environment. Fecal bacterial extracts from non-alcoholic fatty liver disease-related HCC patients induced the expansion of effector IL-10+ Treg cells while reducing cytotoxic CD8+ T cells ex vivo, as compared with extracts from non-alcoholic fatty liver disease controls.71 Whether the differential effects of gut microbiota on Tregs and cytotoxic T cells play a role in liver carcinogenesis in vivo, and the underlying mechanism, remain to be determined.
Several studies have explored the gut microbiota features associated with ICI therapy outcome in HCC patients, although no consistent signal (i.e. alpha diversity, composition, or specific taxa) has been identified (Table 1).76–78 This could be due to the small cohort sizes investigated in these studies as well as the complexity of HCC etiologies. Nonetheless, manipulating the gut microbiota could be an approach to potentiate immunotherapy against HCC. In a subcutaneous HCC xenograft tumor model, periodical antibiotic treatment (penicillin, chloramphenicol, and streptomycin) enhanced the antitumor efficacy of γδT cell immunotherapy.79 3-Indolepropionic acid, a bacterial metabolite from tryptophan, was found to be substantially elevated in the cecal contents of antibiotics-treated mice.79 3-Indolepropionic acid administration, in vitro and in vivo, enhanced cytotoxicity of γδT cells against HCC cells.79
Gastric cancer
Gastric cancer is the classical example of cancer influenced by local bacteria, with H. pylori colonization long established as a risk factor.80 Many studies have shown that the gastric microbiota is significantly different between GC patients and noncancer individuals.6 And the gastric microbiota can modulate GC susceptibility directly or by interacting with H. pylori in mouse models.6 To our knowledge, no study has examined the gastric microbiota association with GC immunotherapy. Peng et al. investigated fecal microbiota signatures correlated with clinical response to anti-PD-1/PD-L1 immunotherapy in a cohort of patients with GI cancer (19 CRC, 23 GC, 14 esophageal carcinoma, and 18 other GI cancer types).22 In this small, mixed cohort, no difference in the alpha diversity of the fecal microbiota was observed between responders and nonresponders regardless of cancer type, although the abundance of Bacteroides appears higher in nonresponders compared with responders in each cancer type (Table 1).22
Perspective and conclusion
Immunotherapy-based treatment of esophageal cancer, pancreatic, HCC, GC, and CRC is still at an early stage compared with other immunogenic tumors such a melanoma and non-small-cell lung cancer,81 and overcoming tumor-immune evasion represents a critical challenge for this field of research. The emergence of the microbiota as an adjuvant to immunotherapy may open new possibility for GI cancer treatment, although further research is needed to formally establish this paradigm (Fig. 1). The research so far points to an associative relationship between microorganisms and immunotherapy, but whether this link could be used as predictive tool of responsiveness or even as an active therapeutic agent is still unclear. More specifically, the lack of clarity into which microbial features are associated with immunotherapy response to GI cancer and whether specific microorganisms associate with a given cancer type need to be addressed. An outstanding limitation in this field is the lack of clinical benefit immune checkpoint inhibitors have in most CRC cases, which precludes most prospective studies looking at microbial signature of response. Therefore, most findings on the role of the microbiome in immunotherapy response are preclinical, limiting the translational impact. More human studies are required to define microbial biomarkers and mechanisms driving response to these therapies. There is currently one clinical trial underway (NCT04729322) investigating the use of FMT in patients with metastatic CRC who have previously not responded to anti-PD-1 therapy, indicating that the field is moving in the direction of translation of preclinical findings to the clinic. In gastric and liver cancers, however, ICIs are Food and Drug Administration approved and do provide some clinical benefit, but there are still relatively few associative or even preclinical studies regarding the microbiome and ICI response. In this case, more effort should be devoted to clarifying the role of the microbiome in response given their close proximity to the intestinal tract and associated microbial metabolites. Also, given that in diseased states both organs are colonized by pathogenic bacteria shown to play a role in their associated malignancies, there is a greater likelihood of there also being an impact of immunotherapy response.82,83 Additionally, because intratumoral bacteria are present in almost all cancer forms,59,84 it is clear that intestinal microbiota is not the sole influencer of immune responses and consequently immunotherapy modulation. It will be important to investigate the relationship between the tumor microenvironment in GI cancer and treatment outcomes to make conclusions about potential therapeutic partnership. Immunotherapy represents an umbrella term for immune-based intervention for which ICI is only one arm. Interventions such as adoptive cell transfer (chimeric antigen receptor T cell and tumor infiltrating lymphocyte) and tumor vaccines may also have a microbiota component in responsiveness and studies dedicated to this question should be undertaken. Answers to these questions will allow for the design of novel interventions integrating microbiota with existing immunotherapeutics. These interventions could include specific microbial cocktails selective for a given treatment or cancer type, or even precision dietetics aimed at increasing metabolic output of beneficial bacteria identified as synergistic with a given drug (Fig. 1). In conclusion, although numerous unanswered questions remained, the microbiota brings a new research era for cancer treatment and integration of microorganisms with therapeutics could prove transformative for patient outcomes.
Figure 1.
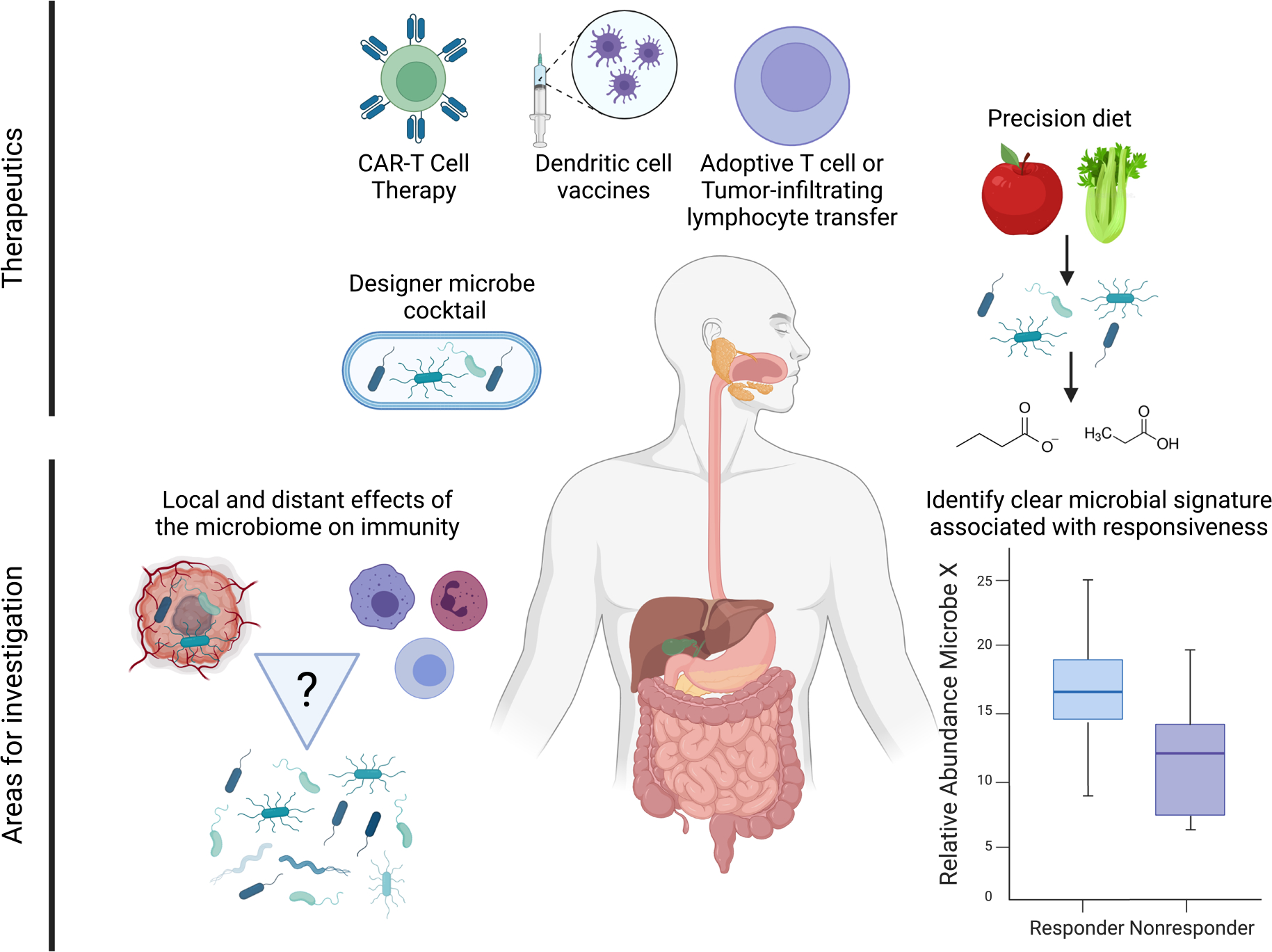
Summary of future directions for microbial-related therapies in gastrointestinal cancers. Potential future therapeutics includes non-immune checkpoint blockade immunotherapies like adoptive T-cell transfer and immune cell vaccines, which must also be investigated in the context of the microbiome. Precision dietetics and designer microbe cocktails represent future targeted and co-therapies that may improve GI cancer patient outcomes. Still unclear is the relationship between the microbiota, immunity, and cancer therapy, and how this affects immunotherapy response in these cancer types. The relationship between local and distant effects of the microbiome, especially regarding the tumor microenvironment, should also be parsed to clarify existing associations. Also necessary is the identification of a clear signal separating responders and nonresponders to immunotherapies. Created with BioRender.com.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in decision to publish or preparation of the manuscript.
Financial support:
C. Jobin was supported by UF Health Cancer Center Funds and the University of Florida Department of Medicine Gatorade Fund. R. C. Newsome was supported by the National Cancer Institute of the National Institutes of Health under Award Number T32CA257923.
Footnotes
Declaration of conflict of interest: Christian Jobin is an Editorial Board member of JGH and a co-author of this article. To minimize bias, they were excluded from all editorial decision-making related to the acceptance of this article for publication.
References
- 1.Arnold M, Abnet CC, Neale RE et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020; 159: 335–49.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganesh K, Stadler ZK, Cercek A et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019; 16: 361–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Khoueiry AB, Sangro B, Yau T et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gou M, Zhang Y, Si H, Dai G. Efficacy and safety of nivolumab for metastatic biliary tract cancer. Onco. Targets. Ther. 2019; 25: 861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xavier JB, Young VB, Skufca J et al. The cancer microbiome: distinguishing direct and indirect effects requires a systemic view. Trends Cancer. 2020; 6: 192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smet A, Kupcinskas J, Link A, Hold GL, Bornschein J. The Role of Microbiota in Gastrointestinal Cancer and Cancer Treatment - Chance or Curse? Cell. Mol. Gastroenterol. Hepatol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Routy B, Le Chatelier E, Derosa L et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359: 91–7. [DOI] [PubMed] [Google Scholar]
- 8.Matson V, Fessler J, Bao R et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018; 359: 104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopalakrishnan V, Spencer CN, Nezi L et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018; 359: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davar D, Dzutsev AK, McCulloch JA et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021; 371: 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baruch EN, Youngster I, Ben-Betzalel G et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021; 371: 602–9. [DOI] [PubMed] [Google Scholar]
- 12.Gharaibeh RZ, Jobin C. Microbiota and cancer immunotherapy: in search of microbial signals. Gut 2019; 68: 385–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kehm RD, Lima SM, Swett K et al. Age-specific trends in colorectal cancer incidence for women and men, 1935–2017. Gastroenterology 2021; 161: 1060–2.e3. [DOI] [PubMed] [Google Scholar]
- 14.Xie Y-H, Chen Y-X, Fang J-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020; 5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z-X, Cao J-X, Liu Z-P et al. Combination of chemotherapy and immunotherapy for colon cancer in China: a meta-analysis. World J. Gastroenterol. 2014; 20: 1095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overman MJ, Lonardi S, Wong KYM et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 2018; 36: 773–9. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B, Wu Q, Zhou YL, Guo X, Ge J, Fu J. Immune-related adverse events from combination immunotherapy in cancer patients: a comprehensive meta-analysis of randomized controlled trials. Int. Immunopharmacol. 2018; 63: 292–8. [DOI] [PubMed] [Google Scholar]
- 18.Yang D, Wang X, Zhou X et al. Blood microbiota diversity determines response of advanced colorectal cancer to chemotherapy combined with adoptive T cell immunotherapy. Onco. Targets. Ther. 2021; 10: 1976953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberti MP, Yonekura S, Duong CPM et al. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat. Med. 2020; 25: 919–31. [DOI] [PubMed] [Google Scholar]
- 20.Zhao R, Coker OO, Wu J et al. Aspirin reduces colorectal tumor development in mice and gut microbes reduce its bioavailability and chemopreventive effects. Gastroenterology 2020; 159: 969–83.e4. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Gharaibeh RZ, Newsome RC, Jobin C. Amending microbiota by targeting intestinal inflammation with TNF blockade attenuates development of colorectal cancer. Nat. Cancer. 2020; 1: 723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng Z, Cheng S, Kou Y et al. The gut microbiome is associated with clinical response to anti-PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol. Res. 2020; 8: 1251–61. [DOI] [PubMed] [Google Scholar]
- 23.Oster P, Vaillant L, Riva E et al. Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies. Gut 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akrami M, Menzies R, Chamoto K et al. Circulation of gut-preactivated naïve CD8+ T cells enhances antitumor immunity in B cell-defective mice. Proc. Natl. Acad. Sci. U. S. A. 2020; 117: 23674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam KC, Araya RE, Huang A et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 2021; 184: 5338–56.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Lv J, Guo F et al. Gut microbiome influences the efficacy of PD-1 antibody immunotherapy on MSS-type colorectal cancer via metabolic pathway. Front. Microbiol. 2020; 30: 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daillère R, Vétizou M, Waldschmitt N et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 2016; 45: 931–43. [DOI] [PubMed] [Google Scholar]
- 28.Fluckiger A, Daillère R, Sassi M et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 2020; 369: 936–42. [DOI] [PubMed] [Google Scholar]
- 29.Griffin ME, Espinosa J, Becker JL et al. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science 2021; 373: 1040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goc J, Lv M, Bessman NJ et al. Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell 2021; 184: 5015–30.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi L, Sheng J, Chen G et al. Combining IL-2-based immunotherapy with commensal probiotics produces enhanced antitumor immune response and tumor clearance. J. Immunother. Cancer 2020; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mager LF, Burkhard R, Pett N et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020; 369: 1481–9. [DOI] [PubMed] [Google Scholar]
- 33.Lopès A, Billard E, Casse AH et al. Colibactin-positive Escherichia coli induce a procarcinogenic immune environment leading to immunotherapy resistance in colorectal cancer. Int. J. Cancer 2020; 146: 3147–59. [DOI] [PubMed] [Google Scholar]
- 34.Yu T, Guo F, Yu Y et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 2017; 170: 548–63.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gur C, Ibrahim Y, Isaacson B et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015; 42: 344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganesan K, Guo S, Fayyaz S, Zhang G, Xu B. Targeting programmed Fusobacterium nucleatum Fap2 for colorectal cancer therapy. Cancers (Basel) 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S, Yang Y, Weng W et al. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res. 2019; 38: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am. J. Gastroenterol. 2014; 109: 1065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuo T, Wong SH, Lam K, Lui R, Cheung K, Tang W et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut 2018; 67: 634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 2018; 24: 1804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S-H, Cho S-Y, Yoon Y, Park C, Sohn J, Jeong J-J et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat. Microbiol. 2021; 6: 277–88. [DOI] [PubMed] [Google Scholar]
- 42.Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Yet al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019; 565: 600–5. [DOI] [PubMed] [Google Scholar]
- 43.Montalban-Arques A, Katkeviciute E, Busenhart P, Bircher A, Wirbel J, Zeller G et al. Commensal Clostridiales strains mediate effective anti-cancer immune response against solid tumors. Cell Host Microbe 2021; 29: 1573–88.e7. [DOI] [PubMed] [Google Scholar]
- 44.Merrick B, Allen L, Masirah M, Zain N, Forbes B, Shawcross DL, Goldenberg SD. Regulation, risk and safety of faecal microbiota transplant. Infect. Prev. Pract. 2020. Sep; 2: 100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology 2020; 159: 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolodziejczyk AA, Zheng D, Elinav E. Diet-microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019; 17: 742–53. [DOI] [PubMed] [Google Scholar]
- 47.Zhang S-L, Mao Y-Q, Zhang Z-Y, Li Z-M, Kong C-Y, Chen H-L et al. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics. 2021; 11: 4155–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han K, Nam J, Xu J, Sun X, Huang X, Animasahun O et al. Generation of systemic antitumour immunity via the in situ modulation of the gut microbiome by an orally administered inulin gel. Nat. Biomed. Eng. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coutzac C, Jouniaux J-M, Paci A, Schmidt J, Mallardo D, Seck A et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 2020; 11: 2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okumura S, Konishi Y, Narukawa M, Sugiura Y, Yoshimoto S, Arai Y et al. Gut bacteria identified in colorectal cancer patients promote tumourigenesis via butyrate secretion. Nat. Commun. 2021; 12: 5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nomura M, Nagatomo R, Doi K, Shimizu J, Baba K, Saito T et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw. Open 2020; 3: e202895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He Y, Fu L, Li Y, Wang W, Gong M, Zhang J et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 2021; 33: 988–1000.e7. [DOI] [PubMed] [Google Scholar]
- 53.Luu M, Riester Z, Baldrich A, Reichardt N, Yuille S, Busetti A et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021; 12: 4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakatake M, Kuwano N, Kaitsurumaru E, Kurosaki H, Nakamura T. Fusogenic oncolytic vaccinia virus enhances systemic antitumor immune response by modulating the tumor microenvironment. Mol. Ther. 2020; 29: 1782–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng JH, Nguyen VH, Jiang S-N, Park S-H, Tan W, Hong SH et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 2017; 9. [DOI] [PubMed] [Google Scholar]
- 56.Nakao S, Arai Y, Tasaki M, Yamashita M, Murakami R, Kawase T et al. Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade. Sci. Transl. Med. 2020; 12. [DOI] [PubMed] [Google Scholar]
- 57.Canale FP, Basso C, Antonini G, Perotti M, Li N, Sokolovska A et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 2021; 598: 662–6. [DOI] [PubMed] [Google Scholar]
- 58.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017; 357: 1156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poore GD, Kopylova E, Zhu Q, Carpenter C, Fraraccio S, Wandro S et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 2020; 579: 567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020; 368: 973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chakladar J, Kuo SZ, Castaneda G, Li WT, Gnanasekar A, Yu MA et al. The pancreatic microbiome is associated with carcinogenesis and worse prognosis in males and smokers. Cancers (Basel) 2020; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018; 8: 403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 2019; 178: 795–806.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas RM, Gharaibeh RZ, Gauthier J, Beveridge M, Pope JL, Guijarro MV et al. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis 2018; 39: 1068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elkrief A, Derosa L, Kroemer G, Zitvogel L, Routy B. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: a new independent prognostic factor? Ann. Oncol. 2019; 30: 1572–9. [DOI] [PubMed] [Google Scholar]
- 66.Yin H, Pu N, Chen Q, Zhang J, Zhao G, Xu X et al. Gut-derived lipopolysaccharide remodels tumoral microenvironment and synergizes with PD-L1 checkpoint blockade via TLR4/MyD88/AKT/NF-κB pathway in pancreatic cancer. Cell Death Dis. 2021; 12: 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology 2019; 69: 107–20. [DOI] [PubMed] [Google Scholar]
- 68.Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 2019; 68: 1014–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ni J, Huang R, Zhou H, Xu X, Li Y, Cao P et al. Analysis of the relationship between the degree of dysbiosis in gut microbiota and prognosis at different stages of primary hepatocellular carcinoma. Front. Microbiol. 2019; 25: 1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Piñero F, Vazquez M, Baré P, Rohr C, Mendizabal M, Sciara M et al. A different gut microbiome linked to inflammation found in cirrhotic patients with and without hepatocellular carcinoma. Ann. Hepatol. 2019; 18: 480–7. [DOI] [PubMed] [Google Scholar]
- 71.Behary J, Amorim N, Jiang X-T, Raposo A, Gong L, McGovern E et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021; 12: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2021; 7: 6. [DOI] [PubMed] [Google Scholar]
- 73.Giraud J, Chalopin D, Blanc J-F, Saleh M. Hepatocellular carcinoma immune landscape and the potential of immunotherapies. Front. Immunol. 2021; 18: 655697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iida N, Mizukoshi E, Yamashita T, Yutani M, Seishima J, Wang Z et al. Chronic liver disease enables gut Enterococcus faecalis colonization to promote liver carcinogenesis. Nat. Cancer. 2021; 2: 1039–54. [DOI] [PubMed] [Google Scholar]
- 75.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018; 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J. Immunother. Cancer 2019; 7: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li L, Ye J. Characterization of gut microbiota in patients with primary hepatocellular carcinoma received immune checkpoint inhibitors: a Chinese population-based study. Medicine (Baltimore) 2020; 99: e21788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen Y-C, Lee P-C, Kuo Y-L, Wu W-K, Chen C-C, Lei C-H et al. An exploratory study for the association of gut microbiome with efficacy of immune checkpoint inhibitor in patients with hepatocellular carcinoma. J. Hepatocell Carcinoma 2021; 24: 809–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han J, Zhang S, Xu Y, Pang Y, Zhang X, Hu Y et al. Beneficial effect of antibiotics and microbial metabolites on expanded vδ2vγ9 T cells in hepatocellular carcinoma immunotherapy. Front. Immunol. 2020; 22: 1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stewart OA, Wu F, Chen Y. The role of gastric microbiota in gastric cancer. Gut Microbes. 2020; 11: 1220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw. Open 2019; 2: e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alarcón T, Llorca L, Perez-Perez G. Impact of the microbiota and gastric disease development by Helicobacter pylori. Curr. Top. Microbiol. Immunol. 2017; 400: 253–75. [DOI] [PubMed] [Google Scholar]
- 83.Minemura M, Shimizu Y. Gut microbiota and liver diseases. World J. Gastroenterol. 2015; 21: 1691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science 2021; 371. [DOI] [PMC free article] [PubMed] [Google Scholar]


