Abstract
AIM
To study the effect of palmitoylethanolamide (PEA) on apoptosis of retinal pigment epithelial (RPE) cells induced by all-trans retinal (atRAL) and to explore the possible molecular mechanism.
METHODS
CellTiter 96® Aqueous One Solution Cell Proliferation Assay (MTS) was used to detect the effect of PEA on human-derived retinal epithelial cells (ARPE-19) viability induced by atRAL. A Leica DMi8 inverted microscope was used to observe cell morphology. Reactive oxygen species (ROS) production was evaluated with 2′,7′-dichlorodihydrof-luorescein diacetate (H2DCFDA) staining and fluorescence microscopy. Expression of c-Jun N-terminal kinase (JNK), phosphorylated JNK (p-JNK), c-Jun, phosphorylated c-Jun (p-c-Jun), Bak, cleaved caspase-3, C/EBP homologous protein (CHOP), and binding (Bip) protein levels were tested by Western blot. Abca4-/-Rdh8-/- mice, mouse models of atRAL clearance defects which displays some symbolic characteristics of dry age-related macular degeneration (AMD) and Stargardt disease (STGD1). In the animal models, PEA was injected intraperitoneally. The full-field electroretinogram was used to detect visual function under scotopic conditions traced from mice. Optical coherence tomography showed reconstitution or thickening of the retinal pigment epithelium layer. Effect of PEA on fundus injury induced by light in Abca4-/-Rdh8-/- mice was observed by fundus photography.
RESULTS
PEA ameliorated ARPE-19 cells apoptosis and inhibited ROS (including mitochondrial ROS) production induced by atRAL. PEA improved the retinal functional, prohibited both RPE and photoreceptor from death, ameliorates light-induced fundus impairment in Abca4-/-Rdh8-/- mice. In vitro and in vivo, PEA inhibited JNK, p-JNK, c-Jun, p-c-Jun, Bak, cleaved caspase-3, CHOP, and Bip protein levels induced by all-trans retinal in ARPE-19 cells.
CONCLUSION
PEA has effect on treating RPE cells apoptosis in retinopathy caused by atRAL accumulation. PEA is a potential treatment strategy for dry AMD and STGD1. The molecular mechanism is affecting the ROS-JNK-CHOP signaling pathway partly.
Keywords: palmitoethanolamide, ARPE-19, fundus, all-trans retinal, apoptosis
INTRODUCTION
Age-related macular degeneration (AMD), is the main reason leading to irreversible and severe vision damage over 60 years old all over the world[1]. AMD is a multivariate fundus disease affecting the maculae, photoreceptors and retinal pigment epithelium (RPE) deprive the function owing to the late-onset progressive neurodegeneration. Many factors are relevant to AMD pathogenesis, including immunity, metabolic disorders, oxidative stress, inflammation, and so on. AMD divides two types including dry AMD and wet AMD[2]. The more general form is dry AMD about 88% in AMD patients.
In recent years, people used the aggressive and combined therapy methods, such as laser coagulation, vascular endothelial growth factor (VEGF) receptors, anti-oxidants, gene therapy, etc. However, there is no effective cure to treat dry AMD, the blindness rate continue rises[3]–[5]. Stargardt's disease (STGD) is an inherited eye disease of adolescent macular dystrophy[6]. STGD caused by Abca4 gene mutation, which is called STGD1[7]. STGD1 children may be blindness when they are adults, however there is no effective treatment. So, it's necessary to find new therapeutic agents with less toxicity. To study its molecular mechanisms to cure dry AMD and STGD1.
Palmitoylethanolamide (PEA), an endocannabinoid mimetic amide, is used in the anti-inflammatory, analgesic characteristic specifically in humans[8]–[9]. PEA has been demonstrated safety and tolerability[9]–[11]. PEA is indicated hopefully to use in many therapeutic fields both in preclinical and clinical studies as an endogenous cell protective lipid, such as eczema, pain, and neurodegeneration[12]–[17]. In the 70s of the 20th century, PEA was evaluated as a preventive and therapeutic agent for the treatment of influenza and colds[18]–[19]. Besides its anti-inflammatory and analgesic characteristic, PEA is a natural retinoprotectant[20]–[25]. PEA has been evaluated for glaucoma, diabetic retinopathy, and uveitis, pathological states based on chronic inflammation, respiratory disorders, and various pain syndromes in a number of clinical trials[26]. But by now, the role of PEA on all-trans retinal (atRAL) induced retinal denaturation has not been reported.
In the current study, we investigated effects of PEA on atRAL caused retinal denaturation. We used a mouse model of Abca4-/-Rdh8-/-, an animal model with photoreceptor loss and RPE dystrophy[27]–[29]. PEA improved the retinal functional, prohibited both RPE and photoreceptor from death, ameliorates light-induced fundus impairment in Abca4-/-Rdh8-/- mice. PEA inhibits RPE c-Jun N-terminal kinase (JNK), phosphorylated JNK (p-JNK), c-Jun, phosphorylated c-Jun (p-c-Jun), Bak, cleaved caspase-3, the transcription factor C/EBP homologous protein (CHOP) and binding (Bip) protein levels in vitro (induced by atRAL) and in vivo (Abca4-/-Rdh8-/- mice) experiments. PEA may favor for RPE cell apoptosis therapy in the presence of retinopathies caused by atRAL accumulation. It has potential as a therapeutic strategy for dry AMD and STGD1. The molecular mechanism may affect reactive oxygen species (ROS)-JNK-CHOP signaling.
MATERIALS AND METHODS
Ethical Approval
The Animal Care and Utilization Committee of School Medicine Xiamen University approved all the animal experiments. All animal operations strictly according to Chinese Association for Research in Vision and Ophthalmology (CARVO). The Institutional Review Board approved the study is “The occurrence and mechanism of photoreceptor iron death in dry age-related macular degeneration” and the approval number is XMULAC 20200072.
Reagents
Hoechst 33342, atRAL bought in Sigma-Aldrich (St. Louis, MO, USA). The 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) reagent was bought in Thermo Fisher Scientific (Eugene, OR). Antibodies including cleaved caspase-3 (9664S and 9661S), p-JNK (9255S), JNK (9252S), p-c-Jun (9261S), c-Jun (9165S), Bak (12105S), CHOP (D46F1; 5554S), BiP (C50B12; 3177) and GAPDH (5174S) were purchased in Cell Signaling Technology (Danvers, MA). p-JNK (4821) was bought in Abcam. Horseradish peroxidase–conjugated goat anti-rabbit IgG (H L; 31460) and donkey anti-rabbit IgG (H L; A21207) secondary antibodies were provided by Thermo Fisher Scientific (Rockford, IL).
Animals
In this experiment, we used Abca4-/-Rdh8-/- mice model which had been described before[29]. Four weeks old of Abca4-/-Rdh8-/- mice, being 48h dark-adapted, 1% tropicamide dilated the pupils, then exposed to10 000 lx light emitting diode (LED) light with 450–460 wavelength range for 1h. The control mice of Abca4-/-Rdh8-/-, feed at normal darkness with 5d without 10 000 lx LED exposed. Additionally, after 48h dark adaptation, 4 weeks old mice of Abca4-/-Rdh8-/- were intraperitoneally injected with 4 mg/kg body weight PEA or vehicle dimethyl sulfoxide (DMSO). An hour later, 1% tropicamide dilated the mice pupils and then PEA or vehicle DMSO-treated mice layed bare in 10 000 lx LED 1h. PEA or vehicle DMSO intraperitoneally injected the control Abca4-/-Rdh8-/- mice which were without light exposure. After treatment PEA or vehicle DMSO for 4d once-daily, the mice were euthanized and the eyeballs collected for follow-up study at 5d after illumination.
As previously mentioned[30], dissolved PEA in solution: DMSO with 0.01%, Tween 80 with 10% (SigmaAldrich, St. Louis, MO, USA), 20% polyethylene glycol 400, 69.9% physiological saline. To study PEA roles on Abca4-/-Rdh8-/- mice RPE, PEA (30 mg/kg body weight) was injected intraperitoneally in Abca4-/-Rdh8-/- mice.
Fundus Imaging
The mice fundus images were performed as what mentioned before[29]. All mice were induced sufficient pupil dilation by 1% tropicamide and were anesthetized deeply during testing. The mice placed on the lifting platform, applied carbomer to the cornea. A fundus imaging system (OptoProbe; OPIMG-L, UK) used to collect data. The position of the eye was adjusted to ensure the fundus image was clear. After data collection, normal saline and levofloxacin eye drops were administered to prevent infection.
Optical Coherence Tomography
Before optical coherence tomography (OCT) imaging was performed, each animal was anesthetized and the pupils were dilated with a drop of 1% tropicamide. Then, a drop of 0.2% carbomer was applied to each cornea to keep the tissue moist. An adjustable custom-made platform was used for maintaining appropriate positioning of the mice. After altering the position and angle of the mice, the retinal OCT images of each eye were acquired with the optic nerve head centered on the corresponding box by using a Small Animal Retinal Imaging System (OptoProbe, OPIMG, UK). The neural retinal thickness and thickness of the outer nuclear layer (ONL) were analyzed with using OCT Image Analysis software (Version 2.0, Optoprobe, UK).
Electroretinography
Before the electroretinogram (ERG) recordings, mice were dark-adapted at least 12h, and all operation processes were performed under dim red-light conditions. The pupils were dilated with a drop of 1% tropicamide, next, animals were anesthetized with inhalation (0.6 L/min) of 2% isoflurane for induction phase and then 1.5% isoflurane for maintenance phase. The gold electrode was placed at the center of the cornea. Scotopic ERG recordings were obtained at the following increasing light intensities: 0.01, 0.1, 1, 10 cd·s/m2. Flash ERGs responses were recorded using the Diagnosys Espion E2 ERG system (Diagnosys, LLC, Lowell, USA) and acquired data were analyzed using Espion software. Immediately after ERG recording, imaging of the fundus was performed as previously described above.
Cell Culture
Human-derived retinal epithelial cells (ARPE-19) bought in Fudan IBS Cell Center (Shanghai, China) and cultured as what mentioned before[28].
Treat with Palmitoylethanolamide and All-trans Retinal
The 1.5×104 cells per well of ARPE-19 cells inoculated in 96-well plates or 3×105 cells per well cells in 6-well plates, cultured them overnight. PEA (2.5, 5, 10, and 20 µmol/L) pretreated ARPE-19 cells for 2h. Then, atRAL (2.5, 5, 15, and 20 µmol/L) or blank control treated ARPE-19 cells for 6h.
Cell Viability
As mentioned earlier, the MTS method was used to evaluate cytotoxicity[29].
Measurement of Intracellular Reactive Oxygen Species
atRAL with 15 µmol/L treated ARPE-19 cells for 6h. After incubation with 10 µmol/L H2DCFDA or 5 µmol/L MitoSOX Red at 37°C for 10min, Hochest 33342 labeled the nuclei. After PBS washed, used confocal microscope to examine the cells.
Western Blot
Cell and tissue extraction manipulate by Western blot analysis as previously described[29].
Statistical Analysis
The software of GraphPad Prism software (Version 5.0; La Jolla, CA, USA) analyzed all data. The data with three separate tests were averaged from to compute mean and standard deviation (mean±SD). Single-factor or two-factor analysis of variance (ANOVA) was used for statistical analysis, using Tukey's test as shown in legends. Significance marked by P<0.05.
RESULTS
Palmitoylethanolamide Ameliorates ARPE-19 Cells Apoptosis Induced by All-trans Retinal
To examined the health ARPE-19 cells viability which layed bare to atRAL 6h. ARPE-19 cells viability decreased in a concentration dependent way causing by atRAL (Figure 1A). atRAL cultured with ARPE-19 cells, its IC50 value was 18 µmol/L for 6h. Besides, ARPE-19 cells culturing under (2.5, 5, 15, and 20 µmol/L) atRAL for 6h, reduced cell survival rate significantly about 10%, 28%, 45%, 65%, respectively. According to the cell viability tests results, 15 µmol/L atRAL induced ARPE-19 cells with following tests. MTS results indicated that PEA protected ARPE-19 cells from apoptosis at 2.5, 5, 10, and 20 µmol/L concentration dependent and effectively induced by 15 µmol/L atRAL (Figure 1B). When exposed to atRAL for 6h at concentrations 15 µmol/L, ARPE-19 cells morphology changed significantly assuming roundness, contraction, and cytoplasmic rupture. PEA ameliorates ARPE-19 cells morphology caused by atRAL (Figure 1C).
PEA ameliorates ARPE-19 cell apoptosis induced by atRAL.
A: MTS method tested ARPE-19 cell viability when ARPE-19 cells exposure to atRAL (2.5, 5, 15, and 20 µmol/L) for 6h. B: MTS assay measured ARPE-19 cell viability, incubated in 15 µmol/L atRAL with PEA (2.5, 5, 10, and 20 µmol/L) 6h. C: Leica DMi8 inverted microscope imaged cellular morphology. The scale bars, 100 µm. D: Bak immunoblot analysis ARPE-19 cells lysates with 15 µmol/L atRAL or 15 µmol/L atRAL at 10 µmol/L PEA or vehicle. E: Bak protein levels, showed folding changes relative to DMSO control. aP<0.05; bP<0.01; cP<0.001. PEA: Palmitoylethanolamide; atRAL: All-trans retinal; ARPE: human-derived retinal epithelial cells; DMSO: Dimethyl sulfoxide; MTS: CellTiter 96® Aqueous One Solution Cell Proliferation Assay.
Immunoblot analysis indicated that PEA diminished pro-apoptotic protein Bak level ARPE-19 cells induced by atRAL (Figure 1D). ARPE-19 cells were treated by 15 µmol/L atRAL or 15 µmol/L atRAL 6h with 10 µmol/L PEA or DMSO serving as a blank control for Bak immunoblot analysis. The expression of Bak decreased dramatically (Figure 1E) in atRAL with PEA-loaded ARPE-19 cells, which showed PEA suppressed Bak protein.
PEA Suppresses Reactive Oxygen Species Yield Cause by atRAL in ARPE-19 Cells
ROS leads to apoptosis, intracellular ROS production was detected by fluorescence microscopy ARPE-19 cells being atRAL-loaded which cultured in H2DCFDA. The intracellular ROS generation significantly increased in ARPE-19 cells cultured with 15 µmol/L atRAL for 6h, about 26 times as many as the control cells (Figure 2A, 2B). ARPE-19 cells ROS production effectively lessen, when treated 10 µmol/L PEA exposing 15 µmol/L atRAL after 6h (Figure 2A, 2B). Immunoblot analysis showed that 10 µmol/L PEA markedly reduced CHOP protein level in ARPE-19 cells caused by atRAL accumulation (Figure 2C, 2D).
Figure 2. PEA suppresses ROS (including mitochondrial ROS) generation in ARPE-19 cells caused by atRAL.
A: Intracellular ROS production, H2DCFDA staining tested by fluorescence microscopy. Scale bars, 50 µm. B: Ratio of H2DCFDA positive cells by fluorescence microscopy. C: Immunoblot analysis of CHOP in ARPE-19 cells cultured to atRAL 15 µmol/L or atRAL 15 µmol/L with 10 µmol/L PEA or DMSO 6h. D: Expression levels of CHOP protein relative to control. cP<0.001. PEA 10 µmol/L pretreated the cells for 2h. PEA: Palmitoylethanolamide; atRAL: All-trans retina; ROS: Reactive oxygen species; H2DCFDA: 2′,7′-dichlorodihydrofluorescein diacetate; CHOP: C/EBP homologous protein.
PEA Ameliorates ARPE-19 Cell Apoptosis by Affecting JNK Signaling Induced by atRAL
ARPE-19 cells treated by 15 µmol/L atRAL for 6h, Western blotting analyses displayed that p-JNK protein levels were significantly up-regulated. It demonstrated that atRAL actived ARPE-19 cells JNK signaling.
However, results showed that PEA down-regulated ARPE-19 cells p-JNK protein levels which treated by 15 µmol/L atRAL for 6h (Figure 3A, 3B). Since phosphorylation of C-Jun, the JNK direct substrate, which is the JNK activation indicator, we use Western blotting to test ARPE-19 cells c-Jun activated state induced by atRAL. ARPE-19 cells treatment with atRAL, protein level of p-c-Jun, which is JNK downstream transcription factor, was significantly increased. However, PEA down-regulated ARPE-19 cells p-c-Jun protein levels treated by atRAL15 µmol/L 6h (Figure 3C, 3D). Results showed PEA suppressed ARPE-19 cells apoptosis induced by atRAL through inhibition JNK signaling. Western blotting analysis indicated that 10 µmol/L PEA obviously decreased p-JNK, p-c-Jun, cleaved caspase-3 protein levels in ARPE-19 cells caused by atRAL (Figure 3E, 3F). Collectively, these findings disclose that PEA diminishes ARPE-19 apoptosis evoked by atRAL partly by affecting JNK signaling pathway.
Figure 3. PEA ameliorates RPE cell apoptosis via affecting JNK signaling induced by atRAL.
A: Western blotting to analyze JNK and p-JNK; B: p-JNK/JNK protein level ratios; C: Immunoblot to analyze c-Jun and p-c-Jun protein level; D: c-Jun and p-c-Jun protein level ratios. E: Western blotting to analyse cleaved caspase-3. F: Cleaved caspase-3 protein level ratios. cP<0.001. PEA: Palmitoylethanolamide; atRAL: All-trans retina; JNK: c-Jun N-terminal kinase; p-JNK: Phosphorylated JNK; p-c-Jun: Phosphorylated c-Jun; RPE: Retinal pigment epithelium.
PEA Ameliorates Photoreceptor and RPE Degeneration Effectively in Light-exposed Abca4-/- Rdh8-/- Mice
PEA (4 mg/kg body weight) treated Abca4-/-Rdh8-/- mice by intraperitoneal injection. PEA effectively relieved photoreceptor atrophy, prevented the ONL and the whole neural retina thickness reduction from light-exposed of Abca4-/-Rdh8-/- mice using histological assessment with OCT (Figure 4A-4B). In addition, in vivo, fundus retinal imaging showed that intraperitoneal injection of PEA eliminated RPE degeneration in Abca4-/-Rdh8-/- mice on day 5 after light exposure (Figure 4C). The ultimate goal of retinal degeneration is to preserve visual function. Full-field electroretinal imaging (ERG) was used to measure the response of rod photoreceptors to light stimulation under dark patch conditions (Figure 4D). The retinal function of DMSO-treated and the absence of light exposure groups showed the same strong scotopic ERG curve as the normal control group, while the retinal function ERG curve of Abca4-/-Rdh8-/- mice upon light exposure group significantly decreased, indicating retinal damage. Fortunately, PEA treatment effectively prevented light-induced drop in a- and b-waves under scotopic conditions, reflecting improved retinal function after PEA treatment. The quantification of a- and b-wave amplitudes under scotopic condition further clearly demonstrated PEA improved the retinal functional (Figure 4E, 4F). Together, these datas suggest that PEA has a strong protective effect on light-induced photoreceptor and RPE atrophy in Abca4-/-Rdh8-/- mice, suggesting its promising therapeutic strategy.
Figure 4. Effects of PEA on photoreceptor and RPE degeneration in light-exposed Abca4-/-Rdh8-/- mice.
A: Examination of mouse retina morphology by using OCT. PEA or vehicle (DMSO; 4 mg/kg body weight) were intraperitoneally injected into 48h dark-adapted Abca4-/-Rdh8-/- mice at 4 weeks of age. One hour later, the mice were irradiated by 10 000 lx LED light for 1h after their pupils were dilated with 1% tropicamide, followed by once-daily administration of PEA or vehicle for 4d. Control Abca4-/-Rdh8-/- mice were intraperitoneally injected with PEA or vehicle in the absence of light exposure. Representative OCT images taken at day 5 upon light exposure. B: Thickness of ONL, and whole retina was quantified by OCT image analysis software. Data are presented as mean±SD (n=6). Statistical analyses were conducted by one-way ANOVA with Tukey's multiple comparison test. cP<0.001. C: Typical fundus image. D: Representative dark-adapted ERG wave forms at 10 cd·s/m2 in mice. E: Implicit times for scotopic a-wave were calculated at flash intensities of 0.01, 0.1, 1, and 10 cd·s/m2. F: Implicit times for scotopic b-wave were calculated at flash intensities of 0.01, 0.1, 1, and 10 cd·s/m2. Data are expressed as mean±SEM (n=6). Statistical analyses were performed with one-way ANOVA with Tukey's multiple comparison test. cP<0.001, (DMSO+light) vs (PEA+light), cP<0.001, DMSO vs (DMSO+light). PEA: Palmitoylethanolamide; atRAL: All-trans retina; OCT: Optical coherence tomography; DMSO: Dimethyl sulfoxide; ONL: Outer nuclear layer; RPE: Retinal pigment epithelium.
PEA Ameliorates RPE Cell Apoptosis by Affecting ROS-JNK-CHOP Signaling in Light-induced Abca4-/-Rdh8-/- Mice
Intraperitoneally injection of PEA ameliorated Abca4-/-Rdh8-/- mice RPE degeneration after day 5 irradiation imaging by in vivo retinal fundus (Figure 4). To verify PEA suppressed JNK signaling pathway and its relative proteins in RPE with illumination of Abca4-/-Rdh8-/- mice, p-c-Jun, p-c-Jun, Bak, CHOP, and Bip protein levels were used to detect by immunoblot. Western blotting analysis displayed p-JNK protein levels significantly increased in RPE illumination of Abca4-/-Rdh8-/- mice (Figure 5A, 5B). Additionally, p-c-Jun, Bak, CHOP, and Bip protein levels, which function relative to the JNK signaling, markably increased in Abca4-/-Rdh8-/- mice neural retina with illumination (Figure 5C–5J). The study showed that PEA administration obviously reduced p-c-Jun, Bak, CHOP, and Bip protein levels in Abca4-/-Rdh8-/- mice RPE with light exposure (Figure 5C–5J).
Figure 5. PEA ameliorates RPE cell apoptosis by affecting ROS-JNK-CHOP signaling in Light-induced Abca4-/-Rdh8-/- mice.
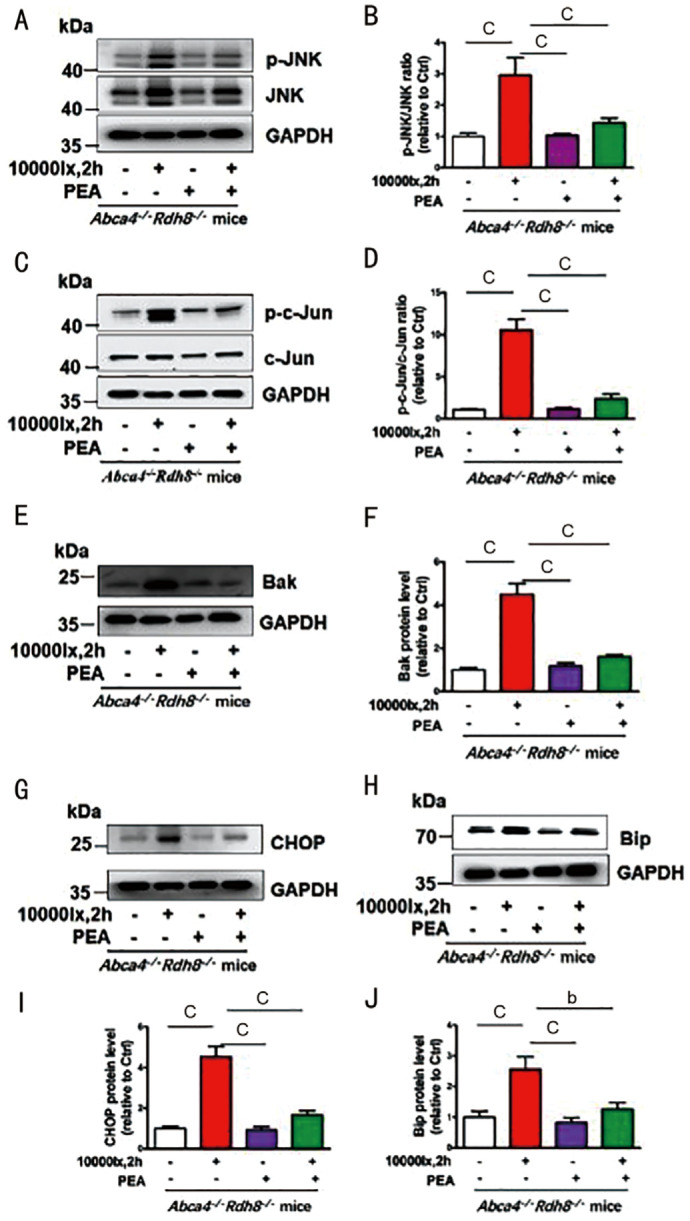
A: p-JNK and JNK Western blotting analysis. B: p-JNK/JNK protein level ratios. C: Immunoblot analysis p-c-Jun/c-Jun protein level. D: p-c-Jun/c-Jun ratio. E: Western blotting analysis of Bak protein level. F: Bak protein level ratios. G: Western blotting analysis of CHOP protein level. I: CHOP protein level ratios. H: Western blotting analysis of Bip protein level. J: Bip protein level ratios. bP<0.01; cP<0.001. PEA: Palmitoylethanolamide; atRAL: All-trans retina; ROS: Reactive oxygen species; JNK: c-Jun N-terminal kinase; p-JNK: Phosphorylated JNK; p-c-Jun: Phosphorylated c-Jun; RPE: Retinal pigment epithelium; CHOP: C/EBP homologous protein; Bip: Binding protein.
DISCUSSION
There are some Food and Drug Administration (FDA) approved therapies to treat wet AMD. However, there is no approved therapies to treat dry AMD. AMD pathogenesis is very complex and isn't understood yet. Different therapeutic approaches to treat retinal damage are varied and debated in dry AMD[31]–[32], including visual cycle modulation, gene therapy, complement inhibition, neuroprotection, anti-inflammatory therapy, cell-based treatments, prosthetic devices, photobiomodulation. None of the methods described is without shortcomings. Such as, the concerns with stem cell-based therapies include immune rejection, differentiation into undesired cell types, damage to surrounding tissues, and tumor formation. Visual cycle modulators with oral route of administration are appealing to patients, but the downside of these treatments is that dark adaptation and low-light vision can be adversely affected by modulating the visual cycle. Fortunately, PEA is abioactive endogenous acyl ethanolamine lipid. Different from the secretion of other endogenous signaling molecules, PEA is synthesized and released by membrane phospholipids “on demand” only when the cell membrane is stimulated by external stimuli, and exerts physiological and pharmacological effects. PEA does not accumulate in the body after it has taken effect. It will be re-absorbed by the corresponding transporter to retrieve intracellular hydrolytic inactivation. It has been demonstrated with the high safety and tolerability. PEA is indicated hopefully to use in many therapeutic fields both in preclinical and clinical studies, such as eczema, pain, and neurodegeneration. PEA might be a natural retinoprotectant.
Central visual damaged in AMD patients partly caused by RPE cells degeneration[33], which becomes a major public health issue[34]–[35]. STGD1 and dry AMD patients had typical manifestations of retinal dystrophy which caused photoreceptors and the RPE atRAL accumulation[27]–[29]. So, vision sustaining is important to clear the released atRAL timely[2],[36]. Evidence demonstrate that transient amassing of atRAL by delayed clearance from the retina is one of the key mechanisms in light-induced retinal degeneration[27],[37]–[38]. In the visual (retinoid) cycle, ABCA4 and RDH8's responsibility are to clear up the retina atRAL[39]–[40]. And the Abca4-/-Rdh8-/- mice model exhibit the photoreceptor loss and RPE dystrophy, which are STGD1 and dry AMD primary features[27],[37]–[38]. Previous studies findings demonstrate photoreceptor/RPE dystrophy was related to atRAL toxicity[29],[31].
The stress stimuli, growth factors, and inflammatory cytokines may activate c-Jun N-terminal kinases[41]–[47]. JNK signaling activation linked to apoptosis[46], some degenerative illness, such as, Parkinson's and Alzheimer's diseases involves the development[48]–[49]. Our earlier research suggested atRAL promotes ARPE-19 cells apoptosis by mitochondrial damage[50]–[51], nucleotide oligomerization domain-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome activated lead to ARPE-19 cells death via promote caspase-3/GSDME-mediated pyroptosis caused by atRAL[28]. Recently, studies found RPE cells apoptosis relative to JNK activation caused to caspase-3/DNA injure dependent by atRAL[31].
PEA concentration is lower than normal subjects in glaucoma patients ciliary body[52]. PEA levels are lower in retina of AMD and diabetic retinopathy than healthy[24]. PEA remarkablely decreased splanchnic artery occlusion (SAO) shock cell death[53]. Nitric oxide (NO) and ROS levels decreased by PEA regulation the main cytokines in the COVID-19 infected induced by lipopolysaccharide (LPS) injure [54].
In this study, we explored PEA effects in retinal degeneration induced by atRAL. In vitro cell tests, studies indicated that 15 µmol/L atRAL induced ARPE19 cells apoptosis significantly via upregulating JNK signaling. Meanwhile, PEA downregulated JNK signaling which induced by atRAL. Previous studies showed stress and ROS production caused by atRAL in ARPE-19 cells[38],[50]–[51], activation of JNK induced RPE cells apoptosis. As expected, PEA suppressed ARPE-19 ROS (including mitochondrial ROS) generation induced by atRAL in our study. Bak plays a main role in apoptotic cell death[55]. Currently, study reviewed that NLRP3 inflammasome was activated by atRAL to induce ARPE-19 cell death and promotes caspase-3/Gasdermin domain-containing protein (GSDME)-mediated pyrodeath[28]. Bak and cleaved caspase-3 proteins expression reduced, which revealed that PEA repressed atRALR caused RPE cell apoptosis. Researches indicated stress of ER marker proteins such as Bip upregulation related to CHOP expression upregulation[56]. CHOP is downstream of JNK[57]. Our study showed in ARPE-19 cells, PEA remarkablely reduced Bip and CHOP protein levels in JNK signaling pathway by atRAL accumulation.
Abca4-/-Rdh8-/- mice after light exposure revealed dry AMD and STGD1 characteristics with photoreceptor/RPE degeneration caused by rapid elevation of atRAL levels in the retina[32]. In the retina, Abca4-/-Rdh8-/- mice were exposed to fluorescent light at 10 000 lx resulting in rapid accumulation of atRAL[58], and promoted photoreceptor cell death and retinal damage[59]. Similar data were found in in vivo studies, studies showed that Abca4-/-Rdh8-/- mice which irradiated by light activated JNK signaling in the neuro-retina[29]. More importantly, intraperitoneal injection of PEA effectively ameliorated Abca4-/-Rdh8-/- mice RPE degeneration and apoptosis after light irradiation. PEA effectively relieved photoreceptor atrophy and prevented the reduction in the thickness of ONL and whole neural retina from light-exposed Abca4-/-Rdh8-/- mice. PEA treatment effectively prevented light-induced drop in a- and b-waves under scotopic conditions, reflecting an improvement in retinal function by PEA administration.
PEA also inhibits JNK, p-JNK, c-Jun, p-c-Jun, Bak, cleaved caspase-3, CHOP, and Bip protein levels in RPE partly. Thus, PEA may be favor in the therapy of RPE cell death of retinal degeneration caused by atRAL accumulation.
Our results indicated that PEA may be a therapeutic to treat dry AMD and STGD1. However, the mechanism of PEA action is not clear yet. In the future, we will continue to study the mechanism of PEA in dry AMD and STGD1.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.82171064; No.81870671; No.82274162); Natural Science Foundation of Fujian Province (No.2020J01013); Guangdong Basic and Applied Basic Research Foundation (No.2022A1515012514; No.2021A1515011391).
Conflicts of Interest: Han Y, None; Yang KH, None; He DX, None; Yu CF, None; Tao L, None; Liao CY, None; Cai BX, None; Liu ZG, None; Qiu Y, None; Wu YL, None.
REFERENCES
- 1.Ricci F, Bandello F, Navarra P, Staurenghi G, Stumpp M, Zarbin M. Neovascular age-related macular degeneration: therapeutic management and new-upcoming approaches. Int J Mol Sci. 2020;21(21):8242. doi: 10.3390/ijms21218242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X, Chen JM, Liu Z, Li J, Yao K, Wu YL. Potential therapeutic agents against retinal diseases caused by aberrant metabolism of retinoids. Invest Ophthalmol Vis Sci. 2016;57(3):1017–1030. doi: 10.1167/iovs.15-18429. [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulos Z. Recent developments in the treatment of wet age-related macular degeneration. Curr Med Sci. 2020;40(5):851–857. doi: 10.1007/s11596-020-2253-6. [DOI] [PubMed] [Google Scholar]
- 4.Rakoczy EP. Gene therapy for the long term treatment of wet AMD. Lancet. 2017;390(10089):6–7. doi: 10.1016/S0140-6736(17)31262-X. [DOI] [PubMed] [Google Scholar]
- 5.Hernández-Zimbrón LF, Zamora-Alvarado R, Ochoa-De la Paz L, et al. Age-related macular degeneration: new paradigms for treatment and management of AMD. Oxidative Med Cell Longev. 2018;2018:8374647. doi: 10.1155/2018/8374647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanna P, Strauss RW, Fujinami K, Michaelides M. Stargardt disease: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol. 2017;101(1):25–30. doi: 10.1136/bjophthalmol-2016-308823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cremers FPM, Lee W, Collin RWJ, Allikmets R. Clinical spectrum, genetic complexity and therapeutic approaches for retinal disease caused by ABCA4 mutations. Prog Retin Eye Res. 2020;79:100861. doi: 10.1016/j.preteyeres.2020.100861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrosino S, di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol. 2017;174:1349–1365. doi: 10.1111/bph.13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuboi K, Uyama T, Okamoto Y, Ueda N. Endocannabinoids and related N-acylethanolamines: biological activities and metabolism. Inflamm Regener. 2018;38(1):28. doi: 10.1186/s41232-018-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabrielsson L, Mattsson S, Fowler CJ. Palmitoylethanolamide for the treatment of pain: pharmacokinetics, safety and efficacy. Br J Clin Pharmacol. 2016;82(4):932–942. doi: 10.1111/bcp.13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nestmann ER. Safety of micronized palmitoylethanolamide (microPEA): lack of toxicity and genotoxic potential. Food Sci Nutr. 2017;5(2):292–309. doi: 10.1002/fsn3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clayton P, Hill M, Bogoda N, Subah S, Venkatesh R. Palmitoylethanolamide: a natural compound for health management. Int J Mol Sci. 2021;22(10):5305. doi: 10.3390/ijms22105305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skaper SD, Laura F, Pietro G. Mast cells, glia and neuroinflammation: partners in crime? Immunology. 2014;141(3):314–327. doi: 10.1111/imm.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iannotti FA, Marzo VD, Petrosino S. Endocannabinoids and endocannabinoid-related mediators: targets, metabolism and role in neurological disorders. Prog Lipid Res. 2016;62:107–128. doi: 10.1016/j.plipres.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Brotini S, Schievano C, Guidi L. Ultra-micronized palmitoylethanolamide: an efficacious adjuvant therapy for Parkinson's disease. CNS Neurol Disord Drug Targets. 2017;16(6):705–713. doi: 10.2174/1871527316666170321124949. [DOI] [PubMed] [Google Scholar]
- 16.D'Orio B, Fracassi A, Ceru MP, Moreno S. Targeting PPARalpha in Alzheimer's disease. Curr Alzheimer Res. 2018;15(4):345–354. doi: 10.2174/1567205014666170505094549. [DOI] [PubMed] [Google Scholar]
- 17.Scuderi C, Bronzuoli MR, Facchinetti R, et al. Ultramicronized palmitoylethanolamide rescues learning and memory impairments in a triple transgenic mouse model of Alzheimer's disease by exerting anti-inflammatory and neuroprotective effects. Transl Psychiatry. 2018;8:32. doi: 10.1038/s41398-017-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mašek K, Perlík F, Klíma J, Kahlich R. Prophylactic efficacy of n-2-hydroxyethyl palmitamide (Impulsin®) in acute respiratory tract infections. Eur J Clin Pharmacol. 1974;7(6):415–419. doi: 10.1007/BF00560353. [DOI] [PubMed] [Google Scholar]
- 19.Keppel Hesselink JM, de Boer T, Witkamp RF. Palmitoylethanolamide: a natural body-own anti-inflammatory agent, effective and safe against influenza and common cold. Int J Inflamm. 2013;2013:151028. doi: 10.1155/2013/151028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costagliola C, Romano MR, dell'Omo R, Russo A, Mastropasqua R, Semeraro F. Effect of palmitoylethanolamide on visual field damage progression in normal tension glaucoma patients: results of an open-label six-month follow-up. J Med Food. 2014;17(9):949–954. doi: 10.1089/jmf.2013.0165. [DOI] [PubMed] [Google Scholar]
- 21.Strobbe E, Cellini M, Campos EC. Effectiveness of palmitoylethanolamide on endothelial dysfunction in ocular hypertensive patients: a randomized, placebo-controlled cross-over study. Invest Ophthalmol Vis Sci. 2013;54(2):968–973. doi: 10.1167/iovs.12-10899. [DOI] [PubMed] [Google Scholar]
- 22.Gagliano C, Ortisi E, Pulvirenti L, Reibaldi M, Scollo D, Amato R, Avitabile T, Longo A. Ocular hypotensive effect of oral palmitoyl-ethanolamide: a clinical trial. Invest Ophthalmol Vis Sci. 2011;52(9):6096–6100. doi: 10.1167/iovs.10-7057. [DOI] [PubMed] [Google Scholar]
- 23.Pescosolido N, Librando A, Puzzono M, Nebbioso M. Palmitoylethanolamide effects on intraocular pressure after Nd: YAG laser iridotomy: an experimental clinical study. J Ocul Pharmacol Ther. 2011;27(6):629–635. doi: 10.1089/jop.2010.0191. [DOI] [PubMed] [Google Scholar]
- 24.Massengill MT, Ahmed CM, Lewin AS, Ildefonso CJ. Neuroinflammation in retinitis pigmentosa, diabetic retinopathy, and age-related macular degeneration: a minireview. Adv Exp Med Biol. 2018;1074:185–191. doi: 10.1007/978-3-319-75402-4_23. [DOI] [PubMed] [Google Scholar]
- 25.Keppel Hesselink JM, Costagliola C, Fakhry J, Kopsky DJ. Palmitoylethanolamide, a natural retinoprotectant: its putative relevance for the treatment of glaucoma and diabetic retinopathy. J Ophthalmol. 2015;2015:430596. doi: 10.1155/2015/430596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye SH, Chen Q, Jiang N, Liang X, Li JM, Zong RR, Huang CH, Qiu Y, Ma JX, Liu ZG. PPARα-dependent effects of palmitoylethanolamide against retinal neovascularization and fibrosis. Invest Ophthalmol Vis Sci. 2020;61(4):15. doi: 10.1167/iovs.61.4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waugh N, Loveman E, Colquitt J, Royle P, Yeong JL, Hoad G, Lois N. Treatments for dry age-related macular degeneration and Stargardt disease: a systematic review. Health Technol Assess. 2018;22(27):1–168. doi: 10.3310/hta22270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao Y, Zhang HJ, He DX, Wang Y, Cai BX, Chen JM, Ma JX, Liu ZG, Wu YL. Retinal pigment epithelium cell death is associated with NLRP3 inflammasome activation by all-trans retinal. Invest Ophthalmol Vis Sci. 2019;60(8):3034–3045. doi: 10.1167/iovs.18-26360. [DOI] [PubMed] [Google Scholar]
- 29.Liao C, Cai B, Feng Y, Chen J, Wu Y, Zhuang J, Liu Z, Wu Y. Activation of JNK signaling promotes all-trans-retinal-induced photoreceptor apoptosis in mice. J Biol Chem. 2020;295(20):6958–6971. doi: 10.1074/jbc.RA120.013189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li YH, Zhou P, Chen HX, Chen Q, Kuang XF, Lu CZ, Ren J, Qiu Y. Inflammation-restricted anti-inflammatory activities of a N-acylethanolamine acid amidase (NAAA) inhibitor F215. Pharmacol Res. 2018;132:7–14. doi: 10.1016/j.phrs.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Liu HL, Hu FY, Xu P, Wu JH. Regulation of mitophagy by metformin improves the structure and function of retinal ganglion cells following excitotoxicity-induced retinal injury. Exp Eye Res. 2022;217:108979. doi: 10.1016/j.exer.2022.108979. [DOI] [PubMed] [Google Scholar]
- 32.Rubner R, Li KV, Canto-Soler MV. Progress of clinical therapies for dry age-related macular degeneration. Int J Ophthalmol. 2022;15(1):157–166. doi: 10.18240/ijo.2022.01.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Somasundaran S, Constable IJ, Mellough CB, Carvalho LS. Retinal pigment epithelium and age-related macular degeneration: a review of major disease mechanisms. Clin Exp Ophthalmol. 2020;48(8):1043–1056. doi: 10.1111/ceo.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nashine S. Potential therapeutic candidates for age-related macular degeneration (AMD) Cells. 2021;10(9):2483. doi: 10.3390/cells10092483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultz NM, Bhardwaj S, Barclay C, Gaspar L, Schwartz J. Global burden of dry age-related macular degeneration: a targeted literature review. Clin Ther. 2021;43(10):1792–1818. doi: 10.1016/j.clinthera.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J, Liao Y, Chen J, Dong X, Gao Z, Zhang H, Wu X, Liu Z, Wu Y. Aberrant buildup of all-trans-retinal dimer, a nonpyridinium bisretinoid lipofuscin fluorophore, contributes to the degeneration of the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2017;58(2):1063–1075. doi: 10.1167/iovs.16-20734. [DOI] [PubMed] [Google Scholar]
- 37.Ortega JT, Parmar T, Golczak M, Jastrzebska B. Protective effects of flavonoids in acute models of light-induced retinal degeneration. Mol Pharmacol. 2021;99(1):60–77. doi: 10.1124/molpharm.120.000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Getter T, Suh S, Hoang T, Handa JT, Dong Z, Ma X, Chen Y, Blackshaw S, Palczewski K. The selective estrogen receptor modulator raloxifene mitigates the effect of all-trans-retinal toxicity in photoreceptor degeneration. J Biol Chem. 2019;294(24):9461–9475. doi: 10.1074/jbc.RA119.008697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan M, Cremers FPM. ABCA4-associated stargardt disease. Klin Monbl Augenheilkd. 2020;237(3):267–274. doi: 10.1055/a-1057-9939. [DOI] [PubMed] [Google Scholar]
- 41.Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274(5290):1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 42.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103(2):239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 43.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 44.Lin AN, Dibling B. The true face of JNK activation in apoptosis. Aging Cell. 2002;1(2):112–116. doi: 10.1046/j.1474-9728.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 45.Sabapathy K, Hochedlinger K, Nam SY, Bauer A, Karin M, Wagner EF. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol Cell. 2004;15(5):713–725. doi: 10.1016/j.molcel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 46.Huang M, Huang M, Li X, Liu S, Fu L, Jiang X, Yang M. Bisphenol A induces apoptosis through GPER-dependent activation of the ROS/Ca2+-ASK1-JNK pathway in human granulosa cell line KGN. Ecotoxicol Environ Saf. 2021;208:111429. doi: 10.1016/j.ecoenv.2020.111429. [DOI] [PubMed] [Google Scholar]
- 47.Ahmad R, Vaali-Mohammed MA, Elwatidy M, Al-Obeed O, Al-Khayal K, Eldehna WM, Abdel-Aziz HA, Alafeefy A, Abdulla M. Induction of ROS-mediated cell death and activation of the JNK pathway by a sulfonamide derivative. Int J Mol Med. 2019;44(4):1552–1562. doi: 10.3892/ijmm.2019.4284. [DOI] [PubMed] [Google Scholar]
- 48.Hunot S, Vila M, Teismann P, Davis RJ, Hirsch EC, Przedborski S, Rakic P, Flavell RA. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A. 2004;101(2):665–670. doi: 10.1073/pnas.0307453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu X, Raina AK, Rottkamp CA, Aliev G, Perry G, Boux H, Smith MA. Activation and redistribution of c-Jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer's disease. J Neurochem. 2001;76(2):435–441. doi: 10.1046/j.1471-4159.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Cai XH, Xia QQ, Yao K, Chen JM, Zhang YL, Naranmandura H, Liu X, Wu YL. Involvement of endoplasmic reticulum stress in all-trans-retinal-induced retinal pigment epithelium degeneration. Toxicol Sci. 2014;143(1):196–208. doi: 10.1093/toxsci/kfu223. [DOI] [PubMed] [Google Scholar]
- 51.Li J. All-trans-retinal dimer formation alleviates the cytotoxicity of all-trans-retinal in human retinal pigment epithelial cells. Toxicology. 2016;371:41–48. doi: 10.1016/j.tox.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Chen JE. Finding of endocannabinoids in human eye tissues: implications for glaucoma. Biochem Biophys Res Commun. 2005;330(4):1062–1067. doi: 10.1016/j.bbrc.2005.03.095. [DOI] [PubMed] [Google Scholar]
- 53.Impellizzeri D, Siracusa R, Cordaro M, et al. N-palmitoylethanolamine-oxazoline (PEA-OXA): a new therapeutic strategy to reduce neuroinflammation, oxidative stress associated to vascular dementia in an experimental model of repeated bilateral common carotid arteries occlusion. Neurobiol Dis. 2019;125:77–91. doi: 10.1016/j.nbd.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Uberti F, Ruga S, Farghali M, Galla R, Molinari C. A Combination of α-lipoic acid (ALA) and palmitoylethanolamide (PEA) blocks endotoxin-induced oxidative stress and cytokine storm: a possible intervention for COVID-19. J Diet Suppl. 2021:1–23. doi: 10.1080/19390211.2021.1966152. [DOI] [PubMed] [Google Scholar]
- 55.Cosentino K, Hertlein V, Jenner A, et al. The interplay between BAX and BAK tunes apoptotic pore growth to control mitochondrial-DNA-mediated inflammation. Mol Cell. 2022;82(5):933–949.e9. doi: 10.1016/j.molcel.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Y, Liu Y, Zhang Y, Ji W, Wang L, and Lee SC. Periplogenin activates ROS-ER stress pathway to trigger apoptosis via BIP-eIF2alpha-CHOP and IRE1alpha-ASK1-JNK signaling routes. Anticancer Agents Med Chem. 2021;21(1):61–70. doi: 10.2174/1871520620666200708104559. [DOI] [PubMed] [Google Scholar]
- 57.Zhao C, Yu D, He Z, Bao L, Feng L, Chen L, Liu Z, Hu X, Zhang N, Wang T, Fu Y. Endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis of renal tubular epithelial cells. Free Radic Biol Med. 2021;175:236–248. doi: 10.1016/j.freeradbiomed.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Tao L, He D, Liao C, Cai B, Chen C, Wang Y, Chen J, Liu Z, Wu Y. Repressing c-Jun N-terminal kinase signaling mitigates retinal pigment epithelium degeneration in mice with failure to clear all-trans-retinal. Exp Eye Res. 2022;214:108877. doi: 10.1016/j.exer.2021.108877. [DOI] [PubMed] [Google Scholar]
- 59.Maeda A, Maeda T, Golczak M, Chou S, Desai A, Hoppel CL, Matsuyama S, Palczewski K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem. 2009;284(22):15173–15183. doi: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]






