Abstract
Objectives
To end tuberculosis (TB), the vast reservoir of 1.7–2.3 billion TB infections (TBIs) must be addressed, but achieving global TB preventive therapy (TPT) targets seems unlikely. This study assessed the feasibility of using interferon-γ release assays (IGRAs) at lower healthcare levels and the comparative performance of 3-month and 9-month daily TPT regimens (3HR/9H).
Design, setting, participants and intervention
This cohort study was implemented in two provinces of Viet Nam from May 2019 to September 2020. Participants included household contacts (HHCs), vulnerable community members and healthcare workers (HCWs) recruited at community-based TB screening events or HHC investigations at primary care centres, who were followed up throughout TPT.
Primary and secondary outcomes
We constructed TBI care cascades describing indeterminate and positivity rates to assess feasibility, and initiation and completion rates to assess performance. We fitted mixed-effects logistic and stratified Cox models to identify factors associated with IGRA positivity and loss to follow-up (LTFU).
Results
Among 5837 participants, the indeterminate rate was 0.8%, and 30.7% were IGRA positive. TPT initiation and completion rates were 63.3% (3HR=61.2% vs 9H=63.6%; p=0.147) and 80.6% (3HR=85.7% vs 9H=80.0%; p=0.522), respectively. Being male (adjusted OR=1.51; 95% CI: 1.28 to 1.78; p<0.001), aged 45–59 years (1.30; 1.05 to 1.60; p=0.018) and exhibiting TB-related abnormalities on X-ray (2.23; 1.38 to 3.61; p=0.001) were associated with positive IGRA results. Risk of IGRA positivity was lower in periurban districts (0.55; 0.36 to 0.85; p=0.007), aged <15 years (0.18; 0.13 to 0.26; p<0.001), aged 15–29 years (0.56; 0.42 to 0.75; p<0.001) and HCWs (0.34; 0.24 to 0.48; p<0.001). The 3HR regimen (adjusted HR=3.83; 1.49 to 9.84; p=0.005) and HCWs (1.38; 1.25 to 1.53; p<0.001) showed higher hazards of LTFU.
Conclusion
Providing IGRAs at lower healthcare levels is feasible and along with shorter regimens may expand access and uptake towards meeting TPT targets, but scale-up may require complementary advocacy and education for beneficiaries and providers.
Keywords: tuberculosis, preventive medicine, public health
STRENGTHS AND LIMITATIONS OF THIS STUDY.
A strength of the study was the large sample size of persons tested by interferon-γ release assay across two sites with varying characteristics in background tuberculosis infection as well as demographic and clinical characteristics, which enabled comparative analyses of subsegments of the sample.
The community setting in which participants were recruited and tested using sophisticated diagnostics decentralised to lower care levels further contributes to the evidence base for scale-up of tuberculosis prevention, especially given the size of the sample.
Embedding the study in routine tuberculosis programme activities exposed it to common limitations such as heterogeneity in supply chain as well as health worker knowledge, attitudes and practices commonly experienced by the programme.
Introduction
After a brief relegation due to the COVID-19 pandemic, tuberculosis (TB) is once again the world’s leading killer infectious disease.1 One of the key reasons is the estimated 1.7–2.3 billion people infected with TB without suffering from active disease, whose activation continues to fuel incidence.2 3 An estimated 5%–15% of people with TB infection (TBI) develop active TB disease in their lifetimes, serving as a vast reservoir for future TB disease, even if new TB transmission were completely eliminated today.4 5 This was also observed by a study in London at the height of the pandemic which showed that social distancing mitigated incidence of several respiratory diseases, but not of TB.6 Thus, research and modelling suggest that increased emphasis on TBI is needed in order to reduce worldwide TB incidence.7 However, while efforts to find and treat people with TB who are missed by existing TB care programmes have been launched in most high TB-burden countries, relatively few are addressing the burden of TBI at scale.8–11
This muted response was historically linked to WHO guidelines recommending TB preventive therapy (TPT) in high TB-burden settings only for people living with HIV (PLHIV), under-5 household contacts (HHCs) of persons with bacteriologically confirmed pulmonary TB and persons with occupational risk factors for infection and progression to active TB.12 Beyond conservative guidelines, other commonly cited bottlenecks have included shortages in commodities and particularly diagnostic consumables such as tuberculin, high health system costs of diagnosis, treatment and follow-up depressing TPT uptake, and lack of patient-friendly treatment regimen negatively affecting adherence.13 14
In recent years, the WHO has issued updated technical and operational guidelines with expanded TPT eligibility criteria, such as HIV-negative HHCs of all ages.15 16 However, a key recommendation for this expanded eligibility was the inclusion of an appropriate clinical and laboratory evaluation, which in select settings translated to the prerequisite of immunological confirmation of TBI by tuberculin skin test (TST) or interferon-γ release assay (IGRA) for TPT within national guidelines.14 17 The updated WHO guidelines also introduced new short-course TPT regimens with better tolerability and safety profiles, which high TB-burden countries have eagerly integrated into national TBI guidelines and national strategic plans.18 19
One of these countries is Viet Nam, which ranks 11th among the 30 high TB-burden countries. During the first prevalence survey, the annual rate of TBI was measured to be 1.7% with a TBI prevalence of 16.7% in children aged 6–14 years using TST with a threshold of 10 mm.20 A subsequent study in rural Ca Mau province measured a TBI rate of 36.8% using IGRA.21 In 2014, Viet Nam passed legislation codifying its goals to drastically reduce TB prevalence in alignment with the WHO End TB Strategy.22 On World TB Day 2020, the Ministry of Health introduced the country’s inaugural guidelines on diagnosis and treatment of TBI. These guidelines expanded TPT eligibility to all adults with TBI confirmed by recommended diagnostic tools and excluding active TB, permitted the use of various shortened regimen, and described contact investigation and follow-up requirements. Viet Nam further demonstrated its focus on TB prevention by committing at the United Nations High-Level Meeting on Ending TB to scale up provision of TPT to 343 390 people by 2022.23
However, the country has experienced many of the challenges related to the scale-up of TPT as described above. Specifically, Viet Nam requires TBI confirmation within the expanded eligibility criteria prior to treatment, but has experienced tuberculin supply chain shortages and batch variance in the positivity threshold. While WHO-recommended IGRAs are commercially available, the National TB Control Programme (NTP) has consigned this assay class to tertiary care facilities due to the delicate specimen handling and sophistical laboratory requirements,24 25 which is underscored by the lack of published evidence of the assay’s deployment at the point of care domestically and worldwide. In addition, the prohibitively high costs per test have precluded serious consideration for routine TB programme activities.
Nevertheless, the NTP remains committed to the scale-up of TPT through the optimal use of available and new diagnostic tools and treatment regimens.26 Given tuberculin supply and staff capacity challenges, and lack of evidence on the impact of recently introduced shorter TPT regimen on uptake and completion, this study assessed the use of the QuantiFERON-TB Gold Plus assay (QFT-Plus; Qiagen, Hilden, Germany) at the community level and the performance of shorter TPT regimen under programmatic conditions. The goal was to inform NTP of Viet Nam and other high TB-burden countries in their ambitions to meet their TPT goals.
Methods
Study design and objectives
This was a cohort study to measure the feasibility of employing IGRA at the community and primary care levels for the diagnosis of TBI. Feasibility was defined by comparing indeterminate and positivity rates with those demonstrated in facility-based studies (primary endpoints). Secondary objectives included measuring the rate of TPT initiation and completion (secondary endpoints) in cohorts provided with two different TPT regimens, and to identify participant covariates associated with IGRA positivity and loss to follow-up (LTFU). The study followed the Strengthening the Reporting of Observational Studies in Epidemiology guideline for reporting observational studies (online supplemental material 1).
bmjopen-2022-071537supp001.pdf (32.6KB, pdf)
Study setting
The study was conducted in six districts of Ho Chi Minh City (HCMC) and Hai Phong municipal provinces. In HCMC, study sites included districts 6, 8, 12, Binh Chanh, Go Vap and Tan Binh with a cumulative population of 2 387 052 and 3598 TB notifications in 2019. In Hai Phong, the study took place in Do Son with a population of 49 029 and 52 persons with drug-susceptible TB notified in 2019.
Study population and recruitment
The study was embedded into routine contact investigations at primary care commune health posts and community-based active TB case finding (ACF) events. Details of the ACF events are provided elsewhere.27 The study population included HHCs and close contacts, and vulnerable community members at elevated risk of active TB, such as the elderly, urban poor and economic migrants. Briefly, elderly persons were ≥55 years, urban poor were based on national poverty definitions and economic migrants were categorised based on residency registration in rural provinces outside of the intervention districts.28–30 The HCMC site also included a subgroup of primary-level and secondary-level healthcare workers (HCWs) based on the request from local authorities. Recruitment and follow-up occurred from May 2019 to September 2020. All individuals presenting for screening provided routine demographic and clinical information including age, sex, residency status, history of TB, comorbidities and symptomatic presentation. Following intake, persons belonging to the study population with residency in the study districts were invited to participate in the study. Persons living outside of or intending to relocate away from the study sites, or who declined to consent were excluded. Eligible, consenting participants were recruited consecutively until the quota of available QFT-Plus tests was reached (n=5000 in HCMC and n=1000 in Hai Phong). Parents consented on behalf of their children under 18 years.
Sample size
We calculated the sample size to power a one-sample Z-test of proportions for non-inferiority between a literature-based indeterminate rate of p=2.9% and a null hypothesis of p0=3.5% with a non-inferiority margin δ=0.1%. With a confidence level of α=95% and a power of β=90%, the estimated sample size was n=4915. We included a 15% contingency for attrition, data losses and post-hoc exclusion for a final sample size of n=5653.
Specimen collection and processing
Provincial lung hospital (PLH) laboratory staff hosted training sessions on specimen collection and processing for the District TB Unit (DTU) and district-level laboratory staff. The District Health Center (DHC) mobilised participants to attend ACF events or to present at commune health posts. All attendants were systematically screened for TB symptoms and directed to undergo chest radiography (CXR) to rule out active TB. Persons with parenchymal abnormalities suggestive of TB on CXR or strong clinical suspicion of TB were referred for molecular or microscopy-based sputum testing, as per contemporary national TB treatment guidelines.31 Attendants were counselled on TBI and invited to participate. Study staff collected blood specimens from consenting, eligible individuals as per manufacturer-recommended procedures. Each participant provided 4 mL of venous whole blood in four separate tubes. Blood specimens were processed and analysed per manufacturer recommendations. Briefly, all four tubes were immediately shaken ~10 times to dissolve all antigens on the tube’s wall coating. Tubes were stored inside dry ice coolers at 17°C–25°C, which were transported to the PLH biochemistry–haematology departments within 6 hours, two times per day. Samples were incubated at 37°C for 20 hours (±1 hour) and centrifuged within 1 hour of completing the incubation stage at 2000–3000 g for 14 min at room temperature. The 12-step ELISA was conducted within 16–24 hours. Results were analysed by using proprietary QuantiFERON software V.2.7.1.
TPT initiation and participant follow-up
QFT-Plus test results were returned to the DHC 2 days after receipt of the blood specimens. Individuals with negative results were informed via phone by DHC staff. Those with positive results and eligible for preventive treatment (ie, with confirmed TBI and active TB ruled out by CXR and symptomatic presentation) were invited to present at their respective DTU for pretreatment counselling and TPT initiation as per national guidelines.17 TPT regimen varied by province. In HCMC, TPT consisted of 9 months of daily isoniazid (9H), while in Hai Phong, eligible persons received 3 months of daily isoniazid and rifampicin (3HR). Individuals on TPT received in-person follow-up during monthly drug pick-up at the DTU. Community TB officers conducted phone or in-person follow-up in regular intervals or as needed, as recommended in national guidelines. Participants experiencing adverse events were asked to present at the DTU for check-up.
Statistical analyses
The primary measures of interest were QFT-Plus positivity and indeterminate rates. Secondary variables of interest included TPT initiation and completion rates within the study population. Missing data were retrieved through post-event follow-up of participants or excluded from individual analyses. We constructed TBI care cascades in aggregate and segmented by site ranging from persons recruited to participants with a successful TPT completion. We documented losses along the cascade and reported median and IQRs of diagnostic delay, that is, time from testing to TPT initiation. We calculated descriptive statistics for key sample characteristics by QFT-Plus result and TPT completion and fitted a saturated, mixed-effects logistic regression to assess associations between positivity and participant covariates to adjust for confounding and inherent bias. Study district was the random effect to account for intracluster correlation. The survival analysis designated LTFU a failure and censored adherent participants on 3HR and 9H at 3 and 9 months, respectively. We constructed Kaplan-Meier survival curves and conducted log-rank tests to assess the equality of survival between the two TPT regimens. We fitted a saturated Cox model and assessed validity of the proportionality assumption using log-log plots and Schoenfeld residuals. Violations were addressed via stratification or modelling of time variance for parameters of interest. The final model passed both the global post-estimation proportional hazards test and tests of individual parameters. P values of validation tests were provided in the online supplemental material 2. Hypothesis tests were two tailed. A threshold of p<0.05 was considered significant. Analyses were conducted using STATA V.17 (Stata Corp; College Station, Texas, USA).
bmjopen-2022-071537supp002.pdf (95.4KB, pdf)
Patient and public involvement
While patients with TB and their families were not involved in setting the research question, a consensus building meeting was held at the beginning of the study for government stakeholders and community members to provide feedback and recommendations and reach a consensus about the study design and implementation. Patients, their families and public stakeholders were also central to dissemination of study information, which helped to motivate community involvement during and beyond the study.
Results
Sample characteristics
Of the 5837 participants in the sample, 59.3% (n=3463) were female (table 1). Children under 15 years constituted 19.5% (1136 of 5834) of the sample and the median participant age was 40 years (IQR: 20–55). Overall, most participants were recruited at community-based ACF events (55.8%; n=3257), lived in urban areas (65.6%; n=3827), were permanent residents (90.5%; 3116 of 3444) and were enrolled on social health insurance (90.4%; 5269 of 5832). About 2.9% (n=167) were diabetics and 1.1% (n=62) reported a history of TB. Moreover, 39.5% (n=2306) reported experiencing at least one of the four core TB symptoms (cough, weight loss, fever and/or night sweats) during recruitment, while 2.3% (n=134) participants exhibited TB-related CXR abnormalities.
Table 1.
Participant characteristics and adjusted ORs associated with IGRA positivity
| Total (N=5837) N (%)* |
IGRA(+) (N=1792) N (%)** |
IGRA(−) (N=4000) N (%)** |
Indeterminate (N=45) N (%)** |
aOR (95% CI) |
P value† | |
| Sex | ||||||
| Female | 3463 (59.3) | 1048 (30.3) | 2392 (69.1) | 23 (0.7) | Ref | |
| Male | 2374 (40.7) | 744 (31.3) | 1608 (67.7) | 22 (0.9) | 1.51 (1.28 to 1.78) | <0.001 |
| Age‡ | ||||||
| <15 years | 1136/5834 (19.5) | 134/1792 (11.8) | 997/3997 (87.8) | 5/45 (0.4) | 0.18 (0.13 to 0.26) | <0.001 |
| 15–29 years | 891/5834 (15.3) | 195/1792 (21.9) | 687/3997 (77.1) | 9/45 (1.0) | 0.56 (0.42 to 0.75) | <0.001 |
| 30–44 years | 1290/5834 (22.1) | 418/1792 (32.4) | 864/3997 (67.0) | 8/45 (0.6) | Ref | |
| 45–59 years | 1679/5834 (28.8) | 704/1792 (41.9) | 957/3997 (57.0) | 18/45 (1.1) | 1.30 (1.05 to 1.60) | 0.018 |
| ≥60 years | 838/5834 (14.4) | 341/1792 (40.7) | 492/3997 (58.7) | 5/45 (0.6) | 1.06 (0.80 to 1.40) | 0.673 |
| Median age (IQR) | 40 (20–55) | 49 (35–58) | 35 (15–52) | 45 (24–54) | ||
| Study site | ||||||
| Ho Chi Minh City | 4840 (82.9) | 1603 (33.1) | 3200 (66.1) | 37 (0.8) | Ref | |
| Hai Phong | 997 (17.1) | 189 (19.0) | 800 (80.2) | 8 (0.8) | 0.69 (0.40 to 1.20) | 0.186 |
| Screening location | ||||||
| Community screening event | 3257 (55.8) | 993 (30.5) | 2244 (68.9) | 20 (0.6) | Ref | |
| Primary care centre | 2580 (44.2) | 799 (31.0) | 1756 (68.1) | 25 (1.0) | 0.88 (0.69 to 1.13) | 0.325 |
| Target group | ||||||
| Household and close contacts | 2431 (41.7) | 897 (36.9) | 1495 (61.5) | 39 (1.6) | 1.11 (0.67 to 1.82) | 0.690 |
| Vulnerable community members | 2995 (51.3) | 821 (27.4) | 2168 (72.4) | 6 (0.2) | Ref | |
| Healthcare workers | 411 (7.0) | 74 (18.0) | 337 (82.0) | 0 (0.0) | 0.34 (0.24 to 0.48) | <0.001 |
| Urbanisation | ||||||
| Urban | 3827 (65.6) | 1135 (29.7) | 2669 (69.7) | 23 (0.6) | Ref | |
| Periurban | 2010 (34.4) | 657 (32.7) | 1331 (66.2) | 22 (1.1) | 0.55 (0.36 to 0.85) | 0.007 |
| Residency status§ | ||||||
| Grade 1 | 3116/3444 (90.5) | 799/907 (25.6) | 2294/2511 (73.6) | 23/26 (0.7) | Ref | |
| Grade 2 | 91/3444 (2.6) | 27/907 (29.7) | 62/2511 (68.1) | 2/26 (2.2) | 1.08 (0.66 to 1.74) | 0.765 |
| Grade 3 | 202/3444 (5.9) | 68/907 (33.7) | 134/2511 (66.3) | 0/26 (0.0) | 1.36 (0.96 to 1.92) | 0.083 |
| Grade 4 | 35/3444 (1.0) | 13/907 (37.1) | 21/2511 (60.0) | 1/26 (2.9) | 1.54 (0.73 to 3.26) | 0.260 |
| Social health insurance‡ | ||||||
| No | 563/5832 (9.7) | 180/1790 (32.0) | 376/3997 (66.8) | 7/45 (1.2) | Ref | |
| Yes | 5269/5832 (90.4) | 1610/1790 (30.6) | 3621/3997 (68.7) | 38/45 (0.7) | 1.11 (0.84 to 1.46) | 0.473 |
| Diabetes mellitus | ||||||
| No/unknown | 5670 (97.1) | 1721 (30.4) | 3906 (68.9) | 43 (0.8) | Ref | |
| Yes | 167 (2.9) | 71 (42.5) | 94 (56.3) | 2 (1.2) | 1.15 (0.75 to 1.76) | 0.516 |
| History of TB | ||||||
| No/unknown | 5775 (98.9) | 1764 (30.6) | 3967 (68.7) | 44 (0.8) | Ref | |
| Yes | 62 (1.1) | 28 (45.2) | 33 (53.2) | 1 (1.6) | 1.93 (0.96 to 3.86) | 0.063 |
| Any TB symptoms¶‡ | ||||||
| No | 3531 (60.5) | 1012 (28.7) | 2499 (70.8) | 20 (0.6) | Ref | |
| Yes | 2306 (39.5) | 780 (33.8) | 1501 (65.1) | 25 (1.1) | 0.96 (0.80 to 1.15) | 0.635 |
| Chest X-ray result | ||||||
| Normal | 5502 (94.3) | 1693 (30.8) | 3768 (68.5) | 41 (0.8) | Ref | |
| Abnormal | 134 (2.3) | 78 (58.2) | 56 (41.8) | 0 (0.0) | 2.23 (1.38 to 3.61) | 0.001 |
| No chest X-ray | 201 (3.4) | 21 (10.5) | 176 (87.6) | 4 (2.0) | 0.28 (0.15 to 0.51) | <0.001 |
*Per cent of total.
†Wald test.
‡N sizes listed due to missing values.
§Residency grade definitions: 1=permanent resident; 2=long-term intraprovince temporary resident; 3=short-term, intraprovince temporary resident; 4=short-term, interprovince temporary resident.
¶Includes cough of any duration, fever, night sweats and weight loss.
**Per cent of row total.
aOR, adjusted OR; IGRA, interferon-γ release assay; TB, tuberculosis.
TB infection care cascade
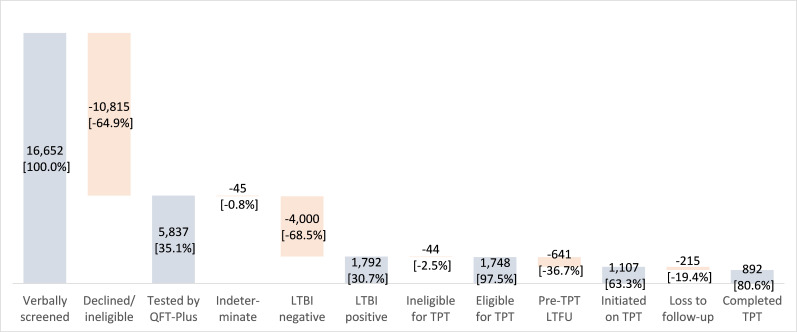
Of the 16 652 individuals verbally screened in both provinces, 35.1% (n=5837) agreed to be tested by QFT-Plus for the study (figure 1). The overall indeterminate rate was 0.8% (n=45), and 30.7% (n=1792) of participants were QFT-Plus positive, of whom 97.5% (n=1748) were eligible for TPT. About 63.3% (1107 of 1748) of eligible participants initiated TPT and 80.6% (892 of 1107) completed therapy. The sample included 4840 participants in HCMC and 997 in Hai Phong (table 2). The indeterminate rate was 0.8% in both sites, while positivity rates were 33.1% (1603 of 4840) in HCMC and 19.0% (189 of 997) in Hai Phong. The respective TPT initiation and completion rates in the 9H cohort in HCMC were 63.6% (995 of 1565) and 80.0% (796 of 995) compared with 61.2% (112 of 183) and 85.7% (96 of 112) in the 3HR cohort in Hai Phong. Neither initiation nor completion rates were significantly different between the two regimens (p=0.522 and p=0.147, respectively).
Figure 1.
Aggregate TB infection care cascade. LTBI, latent TB infection; LTFU, loss to follow-up; QFT-Plus, QuantiFERON-TB Gold Plus assay; TB, tuberculosis; TPT, TB preventive therapy.
Table 2.
TB infection care cascade by TPT cohort
| Total (N=5837) N (%) |
HCMC (N=4840) N (%) |
Hai Phong (N=997) N (%) |
|
| IGRA result and TPT | |||
| Indeterminate | 45 (0.8) | 37 (0.8) | 8 (0.8) |
| Negative | 4000 (68.5) | 3200 (66.1) | 800 (80.2) |
| Positive | 1791 (30.7) | 1603 (33.1) | 189 (19.0) |
| Ineligible for TPT (% of positive) | 44 (0.8) | 38 (0.8) | 6 (0.6) |
| No CXR | 21 (0.4) | 16 (0.3) | 5 (0.5) |
| CXR(+), no MTB test | 6 (0.1) | 5 (0.1) | 1 (0.1) |
| MTB(+) | 17 (0.3) | 17 (0.4) | 0 (0.0) |
| Eligible for TPT (% of positive) | 1748 (97.6) | 1565 (97.6) | 183 (97.3) |
| CXR(−) | 1702 (95.0) | 1524 (95.1) | 178 (94.7) |
| CXR(+), MTB(−) | 46 (2.6) | 41 (2.6) | 5 (2.7) |
| Initiated on TPT* (% of eligible) | 1107 (63.3) | 995 (63.6) | 112 (61.2) |
| Completed TPT* (% of initiated) | 892 (80.6) | 796 (80.0) | 96 (85.7) |
*TPT consisted of 9H in HCMC and of 3HR in Hai Phong.
CXR, chest X-ray; 9H, 9 months of daily isoniazid; HCMC, Ho Chi Minh City; 3HR, 3 months of daily isoniazid and rifampicin; IGRA, interferon-γ release assay; MTB, Mycobacterium tuberculosis; TB, tuberculosis; TPT, TB preventive therapy.
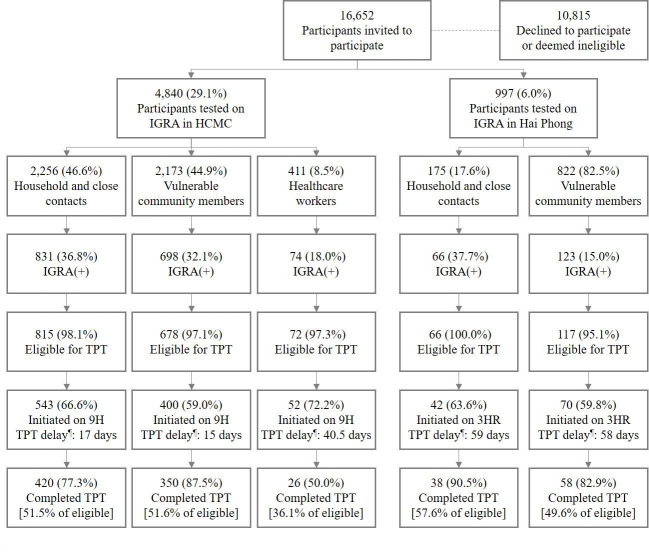
The sample included 46.6% (n=2256) HHCs, 44.9% (n=2173) vulnerable community members and 8.5% (n=411) HCWs in HCMC (figure 2). In Hai Phong, the sample consisted of 17.6% (n=175) HHCs and 82.5% (n=822) community members. IGRA positivity among HHCs was similar in both cities, but lower in community members in Hai Phong (123 of 822=15.0%) compared with HCMC (698 of 2173=32.1%). Similarly, positivity in HCWs was also comparatively lower (74 of 411=18.0%). TPT initiation rates in HHCs and community members were similar across sites ranging from 59.0% to 66.6%, and higher among HCWs (52 of 72=72.2%). Diagnostic delays in HCMC were shorter than in Hai Phong for both HHCs (17 vs 59 days) and community members (15 vs 58 days), except among HCWs (40.5). Similarly, TPT completion rates were high among HHCs and community members in both sites ranging from 77.3% to 90.5%, but only half of HCWs completed TPT.
Figure 2.
TB infection care cascade by site and target group. ¶Median number of days between QFT-Plus testing and treatment initiation. 3HR, 3 months of daily isoniazid and rifampicin; 9H, 9 months of daily isoniazid; HCMC, Ho Chi Minh City; IGRA, interferon-γ release assay; QFT-Plus, QuantiFERON-TB Gold Plus assay; TB, tuberculosis; TPT, TB preventive therapy.
Risk factors of IGRA positivity
Being male (adjusted OR=1.51; 95% CI: 1.28 to 1.78; p<0.001), aged 45–59 years (1.30; 1.05 to 1.60; p=0.018) and exhibiting CXR abnormalities suggestive of TB (2.23; 1.38 to 3.61; p=0.001) were associated with higher QFT-Plus positivity (table 1). Conversely, compared with the reference group (30–44 years), the risk of QFT-Plus positivity was significantly lower among children under 15 years (0.18; 0.13 to 0.26; p<0.001) and persons aged 15–29 years (0.56; 0.42 to 0.75; p<0.001), as well as among HCWs (0.34; 0.24 to 0.48; p<0.001) and individuals living in periurban areas (0.55; 0.36 to 0.85; p=0.007).
Survival analysis and risk factors of TPT completion
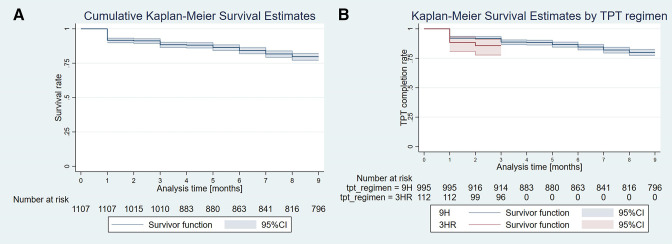
A total of 1107 participants were followed for a total of 8211 person-months with 215 recorded LTFUs (table 3). There were 7904 and 307 person-months of observations with mean follow-up times of 7.9 (7.8–8.1) months and 2.7 (2.6–2.9) months, and 199 and 16 LTFUs in the 9H and 3HR cohorts, respectively. The respective LTFU incidence rates were 25.2 and 52.1 per 1000 person-months. Most LTFUs occurred after the first month of TPT in both the 9H (79 of 199=39.7%) and 3HR (13 of 16=81.2%) cohorts (figure 3A, B). The survival analysis showed that the 3HR regimen (adjusted HR=3.83; 1.49 to 9.84; p=0.005) and HCWs (1.38; 1.25 to 1.53; p<0.001) were associated with higher risk of LTFU.
Table 3.
Participant characteristics and adjusted risk factors associated with TPT loss to follow-up*
| Total (N=1107) N (%)† |
TPT completed (N=892) N (%)‡ |
LTFU (N=215) N (%)‡ |
aHR of LTFU (95% CI) |
P value§ | |
| TPT regimen | |||||
| 9H | 995 (89.9) | 796 (80.0) | 199 (20.0) | Ref | |
| 3HR | 112 (10.1) | 96 (85.7) | 16 (14.3) | 3.83 (1.49 to 9.84) | 0.005 |
| Sex | |||||
| Female | 645 (58.3) | 512 (79.4) | 133 (20.6) | Ref | |
| Male | 462 (41.7) | 380 (82.3) | 082 (17.8) | 1.02 (0.94 to 1.11) | 0.608 |
| Age | |||||
| <15 years | 86 (7.8) | 72 (83.7) | 14 (16.3) | 0.63 (0.22 to 1.79) | 0.390 |
| 15–29 years | 116 (10.5) | 90 (77.6) | 26 (22.4) | 1.71 (0.88 to 3.35) | 0.116 |
| 30–44 years | 249 (22.5) | 195 (78.3) | 54 (21.7) | Ref | |
| 45–59 years | 426 (38.5) | 354 (83.1) | 72 (16.9) | 0.97 (0.56 to 1.69) | 0.911 |
| ≥60 years | 230 (20.8) | 181 (78.7) | 49 (21.3) | 1.14 (0.56 to 2.32) | 0.723 |
| Median age (IQR) | 50 (35–58) | 50 (35–58) | 49 (35–59) | ||
| Screening location | |||||
| Community screening event | 627 (56.6) | 523 (83.4) | 104 (16.6) | Ref | |
| Primary care centre | 480 (43.4) | 369 (76.9) | 111 (23.1) | 1.19 (0.62 to 2.30) | 0.593 |
| Target group | |||||
| Household and close contacts | 585 (52.9) | 458 (78.3) | 127 (21.7) | 1.03 (0.75 to 1.39) | 0.874 |
| Vulnerable community members | 470 (42.5) | 408 (86.8) | 62 (13.2) | Ref | |
| Healthcare workers | 52 (4.7) | 26 (50.0) | 26 (50.0) | 1.38 (1.25 to 1.53) | <0.001 |
| Urbanisation | |||||
| Urban | 729 (65.9) | 598 (82.0) | 131 (18.0) | Ref | |
| Periurban | 378 (34.2) | 294 (77.8) | 84 (22.2) | 1.00 (0.58 to 1.73) | 0.990 |
| Diabetes mellitus | |||||
| No/unknown | 1065 (96.2) | 859 (80.7) | 206 (19.3) | Ref | |
| Yes | 42 (3.8) | 33 (78.6) | 9 (21.4) | 0.74 (0.18 to 3.11) | 0.679 |
| History of TB | |||||
| No/unknown | 1096 (99.0) | 883 (80.6) | 213 (19.4) | Ref | |
| Yes | 11 (1.0) | 9 (81.8) | 2 (18.2) | 1.03 (0.14 to 7.63) | 0.980 |
*Model stratified by health insurance and residency status, so these parameters were excluded; parameters of sex and target group fitted as time-varying covariates; includes a total of 8211 person-months.
†Per cent of total.
‡Per cent of row total.
§Wald test.
aHR, adjusted HR; 9H, 9 months of daily isoniazid; 3HR, 3 months of daily isoniazid and rifampicin; LTFU, loss to follow-up; TB, tuberculosis; TPT, TB preventive therapy.
Figure 3.
Kaplan-Meier TPT survival curves (A) for all participants and (B) by TPT regimen. 3HR, 3 months of daily isoniazid and rifampicin; 9H, 9 months of daily isoniazid; TPT, tuberculosis preventive therapy.
Discussion
In the array of obstacles to scaling up TPT in Viet Nam, TBI diagnosis remains a critical step in the country’s targeted approach. To date, however, it has also represented an insuperable bottleneck. This stems from an over-reliance on TST from a single product (PPD-Bulbio), for which there is documented performance deviation compared with other TSTs and IGRA.32 These issues are in addition to the well-understood range of confounders affecting clinical performance of TSTs in comparison with IGRAs.33 Despite its shortcomings, TST remains the programmatic standard of care partly due to the perceived operational challenges in deploying IGRAs outside of hospital settings.
This evaluation builds on the evidence base that it is possible to deploy IGRAs at lower healthcare levels.21 As shown previously, fidelity to manufacturer-recommended procedures in terms of handling, timing and temperature control throughout collection, transport and processing of specimens from the community to the laboratory resulted in positivity34 and indeterminate rates35 36 that were comparable with those of facility-based studies. Our measured positivity was also aligned with previously published IGRA positivity measured in the community in Viet Nam (pooled positivity: 37.7%; n=2706).21 37 We also observed the expected dose–response pattern of rising positivity and risk of TBI in older individuals as well as the higher risk of QFT-Plus positivity in males.20 21 Concordant with these results, our study highlighted that IGRA can be used at the community level as another option for TBI diagnosis and accelerating scale-up of TPT.
However, there were patterns in the TBI care cascade indicating that scale-up of available TBI diagnostic tools and regimens requires more than simply decentralisation. Fewer than half of the individuals mobilised during these ACF campaigns agreed to or were eligible for an IGRA test and only 6 out of 10 eligible persons initiated TPT, which was concordant with prior studies in Viet Nam.34 One potential reason for the drop-off may be process related, since we embedded the study in a programmatic setting, which meant that in general, over 2 weeks elapsed from when participants were tested until eligible persons initiated TPT. Nevertheless, slow turnaround time may only partially explain the pretreatment LTFU, as TPT initiation rate was consistent across both settings despite the difference in turnaround time.
By fielding the study in two separate sites with different TPT regimens and TBI rates in the community, we recorded several noteworthy observations. Specifically, while initiation rates in both sites were similar, there was a slightly higher completion rate in the 3HR cohort. Thus, even though we did not observe a greater uptake of TPT as seen on prior studies, the shorter treatment duration of 3HR may have contributed to higher TPT completion rates.38–40 However, the survival analysis showed that more persons were lost to follow-up than expected over the shorter period of treatment. Based on informal qualitative feedback from field staff, reasons for the large drop-offs in the cascade included a lack of understanding of the risk of progression from TBI to active TB and the benefits of TPT in the general population, but also among healthcare providers, which leads to the de-prioritisation of TPT as optional prophylaxis rather than valuable intervention. Since the 3HR regimen was only used in one province which may have faced site-specific challenges, we cannot generalise these results to other areas of the country. However, they highlight the need for more education and advocacy for providers and participants to improve the acceptance and prioritisation of TPT.41 42
Moreover, advocacy and awareness building may need to be tailored to individual subgroups. Even though positivity, initiation and completion rates did not vary substantially across sites, gender or age category, there were, however, notable differences across study populations. In our study, HCWs exhibited a lower proportion and risk of positivity, higher TPT initiation and significantly higher risk of LTFU compared with HHCs and community members in either site. The low positivity rate was particularly noteworthy for its discordance with published, although dated, evidence from Viet Nam43 and WHO guidelines warranting intervention in this group due to higher occupational risk of TBI.44 A potential explanation for the discordance is that a sizeable proportion of HCWs were generalist primary care workers. The more recent EnTIC Study (NCT02073240) measured lower TBI rates among Vietnamese HCWs in general hospitals compared with HCWs in TB hospitals (27.9% (22.8%–33.6%) vs 41.7% (26.2%–58.9%)).45 However, this TBI rate in general hospital HCWs is still higher than the rate among HCWs on this study; a future comparative analyses of TBI in HCWs in tertiary/quaternary general hospitals versus primary care workers may offer further insight.
The diagnostic delay was unacceptably long among HCWs and across all groups in Hai Phong. In Hai Phong, the lower burden and more limited TB care capacity as well as greater reliance on the lung hospital in TB care and prevention activities may have contributed to the long delay in treatment initiation. Meanwhile, upon investigation, HCWs indicated a preference to wait for the new 12-dose regimen of isoniazid and rifapentine (3HP), but then agreed to initiate TPT on 9H as concerns over nitrosamine impurities delayed scale-up of 3HP in Viet Nam.46 47 Nevertheless, despite a delay of almost 6 weeks, the TPT initiation rate among HCWs was highest across all groups and also above rates measured on prior studies (39.0%–49.6%).48 49 Conversely, the low completion rate measured on this study was on par with other studies on HCWs receiving 9H for TPT. However, this low rate may have been avoided with shorter regimen as adherence in this study at month 3 was 100% and month 8 was still at 80.0%. These results were in line with previous studies that indicated health workers were significantly more likely to complete TPT on 3HR compared with 9H (91.4% vs 76.7%, p=0.02).50–52
The use of the 9H regimen in the majority of participants also highlights a key limitation of this study. By conducting it under routine programme conditions, the study was exposed to external bias and confounding, such as the variability in the available TPT regimen. HCMC historically has had a substantially larger burden of TB and TBI, as evidenced on this study. Thus, 9H was the local regimen of choice due to its greater availability and lower costs. Similarly, we relied on routine diagnostics to rule out active TB rather than more sensitive tools such as culture due to cost implications. With respect to costs, another limitation of our study was the lack of a formal assessment of the cost barrier of IGRAs in our low-resource setting with limited programme budgets. Operationally, WHO recommends integrating TPT into routine HHC investigations and ACF.16 It stands to reason that such integration may also improve value for money as has been well established for highly vulnerable PLHIV.53 There is ample evidence that HHC investigations and community-based ACF campaigns can reach those most vulnerable to active TB and thus most in need of TPT.29 54 55 Nevertheless, given the lack of an accompanying health economic evaluation, future research should conduct impact evaluations and cost-effectiveness analyses of integrated TB and TBI testing and treatment on ACF campaigns, and differences in incidence and disability-adjusted life years compared with a control cohort. Another limitation is that our cohort design did not include a post-treatment follow-up to assess incidence of TB in those with and without TPT, in part due to the social distancing measures launched in response to the COVID-19 pandemic. The study’s convenience sampling and selection of HCMC and Hai Phong as study sites likely introduced bias towards densely populated urban settings, which consequently limits the generalisability of this study. Nevertheless, the study benefited from its large sample size and integration into routine programme operations that may help to translate the findings to recommendations for densely populated, high TB-burden settings in general.
Conclusions
WHO’s End TB Strategy highlights the need for increased testing and treatment of TBI as a core intervention to reduce transmission and thus achieve incidence targets. While many high TB-burden countries have incorporated this emphasis into their national strategic plans, operationalisation of these plans is often hindered by the suboptimal application of available tools. IGRAs are the current gold standard for TBI testing, but are often underused, particularly at the lower healthcare levels. Shorter TPT regimens are recommended, but require further studies to assess their potential to support broad-scale TPT. This study elucidated the potential to decentralise and leverage these tools for wider and more cost-effective deployment towards meeting TPT targets, but also highlighted that scale-up of these tools, as well as overall TPT access and uptake, will likely require complementary, tailored advocacy and education for both beneficiaries and providers.
Supplementary Material
Acknowledgments
We would like to acknowledge Viet Nam’s National TB Programme, the HCMC and Hai Phong Provincial Departments of Health, and PLHs, the DHCs and commune health posts and participating public health staff for their support. We would also like to thank the Ho Chi Minh City Public Health Association for their support. Lastly, we feel a debt of gratitude to our patients, family members and communities for their participation and support. We would like to especially thank the site coordinators and CHWs for their tireless efforts to care for their patients and contribute to ending TB in Viet Nam.
Footnotes
Twitter: @Jacob_Creswell
Contributors: LNQV, NN, VVT, HTMD and THM contributed to the conceptualisation of the study. The methodology was developed by LNQV, NTTN, TTTD, THM, HTMD and VVT. LNQV and PTL conducted the formal analysis. The investigation was conducted by VVT, NTTN, TTTD and PTL. Resources for the study were provided by LHN, HTMD, HTT, HBN and VNN. Data were curated by LNQV, AC, PTL and KTT, and LNQV visualised the data. LNQV, NTTN and TTTD wrote the original draft, while the manuscript was reviewed and edited by LNQV, AC, JC, NN, HTT and MC. Study supervision was provided by JC, MC, LNQV, LHN, THM, HTT, HBN and VNN; while RF, AC, VVT, NTTN and TTTD were responsible for project administration. Funding acquisition was led by LNQV, RF and AC. LNQV is the guarantor. All authors have read and approved the final manuscript.
Funding: This study and LNQV, AC, RF, NTTN and MC were supported by the European Commission’s Horizon 2020 Programme (grant number: 733174). The study and TTTD received additional financial support from the Government of Canada through the Stop TB Partnership’s TB REACH Initiative (grant number: STBP/TBREACH/GSA/W6SU-09). JC received support from the Government of Canada (grant number: CA-3-D000920001). Qiagen contributed the QFT-Plus kits to this study through an in-kind donation (grant number: N/A; clinical collaboration agreement dated 27 November 2017).
Disclaimer: None of these funding bodies had a role in the design of the study, in collection, analysis, and interpretation of data, or in writing the manuscript.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available from the Viet Nam National TB Control Programme, Hai Phong Provincial Lung Hospital and Pham Ngoc Thach Provincial TB Hospital, but restrictions apply to their availability. Data can be made available from the authors upon reasonable request and with permission of the Viet Nam National TB Control Programme, Hai Phong Provincial Lung Hospital and Pham Ngoc Thach Provincial TB Hospital.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
This study was approved by the Pham Ngoc Thach Hospital Ethics Committee for Biomedical Research (897/HDDD-PNT). In addition, QFT-Plus testing is part of national guidelines and activities were approved by the NTP (1069/BVPTW-DAPCL). Participation was voluntary and did not affect the provision or standard of care. All personal identifying information was removed from the dataset prior to analysis.
References
- 1. World Health Organization . Global tuberculosis report 2022. Geneva, Switzerland, 2022. [Google Scholar]
- 2. Houben R, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLOS Med 2016;13:e1002152. 10.1371/journal.pmed.1002152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Esmail H, Barry CE, Young DB, et al. The ongoing challenge of latent tuberculosis. Philos Trans R Soc Lond B Biol Sci 2014;369:20130437. 10.1098/rstb.2013.0437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matteelli A, Sulis G, Capone S, et al. Tuberculosis elimination and the challenge of latent tuberculosis. Presse Med 2017;46:e13–21. 10.1016/j.lpm.2017.01.015 [DOI] [PubMed] [Google Scholar]
- 5. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 2016;13:e1002152. 10.1371/journal.pmed.1002152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lewer D, Mulchandani R, Roche A, et al. Why has the incidence of tuberculosis not reduced in london during the COVID-19 pandemic? Lancet Respir Med 2022;10:231–3. 10.1016/S2213-2600(22)00012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shrestha S, Kendall EA, Chang R, et al. Achieving a “step change” in the tuberculosis epidemic through comprehensive community-wide intervention: a model-based analysis. BMC Med 2021;19:244. 10.1186/s12916-021-02110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Creswell J, Khan A, Bakker MI, et al. The TB reach initiative: supporting TB elimination efforts in the asia-pacific. Trop Med Infect Dis 2020;5:1–11. 10.3390/tropicalmed5040164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hinderaker SG, Rusen ID, Chiang C-Y, et al. The fidelis initiative: innovative strategies for increased case finding. Int J Tuberc Lung Dis 2011;15:71–6. [PubMed] [Google Scholar]
- 10. The Global Fund . 2020-2022 strategic initiatives. Available: https://www.theglobalfund.org/media/9228/fundingmodel20202022strategicinitiativeslisten.pdf [Accessed 14 Nov 2022].
- 11. Rangaka MX, Cavalcante SC, Marais BJ, et al. Controlling the seedbeds of tuberculosis: diagnosis and treatment of tuberculosis infection. Lancet 2015;386:2344–53. 10.1016/S0140-6736(15)00323-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization . Guidelines on the management of latent tuberculosis infection. 2015. [PubMed] [Google Scholar]
- 13. World Health Organization . Report of the global consultation on the programmatic management of latent tuberculosis infection. 2016. Available: http://www.who.int/tb/challenges/consultationmeetingltbi/en/
- 14. Faust L, Ruhwald M, Schumacher S, et al. How are high burden countries implementing policies and tools for latent tuberculosis infection? A survey of current practices and barriers. Health Sci Rep 2020;3. 10.1002/hsr2.158 Available: https://onlinelibrary.wiley.com/toc/23988835/3/2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization . Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. 1st edn. Geneva: World Health Organization Press, 2018. [PubMed] [Google Scholar]
- 16. World Health Organization . WHO operational handbook on tuberculosis - module 1: prevention. 2022. Available: https://apps.who.int/iris/bitstream/handle/10665/340256/9789240022614-eng.pdf
- 17. Ministry of Health . Decision on the promulgation of the guidelines on the detection and treatment of latent TB infection. Vietnamese. Viet nam. 2020. [Google Scholar]
- 18. National Tuberculosis Leprosy and Lung Disease Program . National strategic plan for tuberculosis, leprosy and lung health 2019-2023. 2019. [Google Scholar]
- 19. Revised National Tuberculosis Control Programme . National strategic plan for tuberculosis elimination 2017–2025. New delhi, India. 2017. [Google Scholar]
- 20. Hoa NB, Cobelens FGJ, Sy DN, et al. First national tuberculin survey in Viet Nam: characteristics and association with tuberculosis prevalence. Int J Tuberc Lung Dis 2013;17:738–44. 10.5588/ijtld.12.0200 [DOI] [PubMed] [Google Scholar]
- 21. Marks GB, Nhung NV, Nguyen TA, et al. Prevalence of latent tuberculous infection among adults in the general population of Ca mau, Viet Nam. Int J Tuberc Lung Dis 2018;22:246–51. 10.5588/ijtld.17.0550 [DOI] [PubMed] [Google Scholar]
- 22. Office of the Prime Minister . Approval of the national strategy for TB prevention and control until 2020 with vision to 2030. Vietnamese. Viet nam. 2014. [Google Scholar]
- 23. Viet Nam National TB Control Programme . Viet nam national strategic plan for TB 2021-2025. Ha noi, Viet nam. 2018. [Google Scholar]
- 24. Ganmaa D, Khudyakov P, Buyanjargal U, et al. Risk factors for active tuberculosis in 938 quantiferon-positive schoolchildren in mongolia: a community-based cross-sectional study. BMC Infect Dis 2019;19:532. 10.1186/s12879-019-4160-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization . Operational handbooks, module 1: prevention, annex 6. answers to frequently asked questions on igras. WHO TB knowl. shar. platf. 2021. Available: https://tbksp.org/en/node/666 [Google Scholar]
- 26. Viet Nam National TB Control Programme . Viet nam national strategic plan for TB 2021-2025. Ha noi, Viet nam. 2020. [Google Scholar]
- 27. Nguyen LH, Codlin AJ, Vo LNQ, et al. An evaluation of programmatic community-based chest X-ray screening for tuberculosis in Ho Chi Minh City, Vietnam. Trop Med Infect Dis 2020;5:185. 10.3390/tropicalmed5040185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vo LNQ, Codlin AJ, Forse RJ, et al. Evaluating the yield of systematic screening for tuberculosis among three priority groups in ho chi minh city, viet nam. Infect Dis Poverty 2020;9:166. 10.1186/s40249-020-00766-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mac TH, Phan TH, Nguyen VV, et al. Optimizing active tuberculosis case finding: evaluating the impact of community referral for chest X-ray screening and xpert testing on case notifications in two cities in viet nam. Trop Med Infect Dis 2020;5:1–15. 10.3390/tropicalmed5040181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vo LNQ, Codlin AJ, Forse RJ, et al. Tuberculosis among economic migrants: a cross-sectional study of the risk of poor treatment outcomes and impact of a treatment adherence intervention among temporary residents in an urban district in ho chi minh city, viet nam. BMC Infect Dis 2020;20:134. 10.1186/s12879-020-4865-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Viet Nam Ministry of Health . Decision on the promulgation of the guidelines for diagnosis, treatment and prevention of tuberculosis (vietnamese) viet nam. 2018. [Google Scholar]
- 32. Mulder C, Erkens C, Kouw P, et al. Tuberculin skin test reaction depends on type of purified protein derivative: implications for cut-off values. Int J Tuberc Lung Dis 2019;23:1327–34. 10.5588/ijtld.18.0838 [DOI] [PubMed] [Google Scholar]
- 33. Carranza C, Pedraza-Sanchez S, de Oyarzabal-Mendez E, et al. Diagnosis for latent tuberculosis infection: new alternatives. Front Immunol 2020;11:2006. 10.3389/fimmu.2020.02006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khan A, Phares CR, Phuong HL, et al. Overseas treatment of latent tuberculosis infection in US-bound immigrants. Emerg Infect Dis 2022;28:582–90. 10.3201/eid2803.212131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharninghausen JC, Shapiro AE, Koelle DM, et al. Risk factors for indeterminate outcome on interferon gamma release assay in non-US-born persons screened for latent tuberculosis infection. Open Forum Infect Dis 2018;5:ofy184. 10.1093/ofid/ofy184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Banach DB, Harris TG. Indeterminate quantiferon®-TB gold results in a public health clinic setting. Int J Tuberc Lung Dis 2011;15:1623–30. 10.5588/ijtld.11.0017 [DOI] [PubMed] [Google Scholar]
- 37. International Union Against Tuberculosis and Lung Disease . Abstract book. in: 53rd world conference on lung health of the international union against tuberculosis and lung disease (the union). 2022: S1–468. [Google Scholar]
- 38. Oxlade O, den Boon S, Menzies D, et al. Tb preventive treatment in high- and intermediate-incidence countries: research needs for scale-up. Int J Tuberc Lung Dis 2021;25:823–31. 10.5588/ijtld.21.0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McClintock AH, Eastment M, McKinney CM, et al. Treatment completion for latent tuberculosis infection: a retrospective cohort study comparing 9 months of isoniazid, 4 months of rifampin and 3 months of isoniazid and rifapentine. BMC Infect Dis 2017;17:146. 10.1186/s12879-017-2245-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. World Health Organization . Consolidated guidelines on tuberculosis: module 1: prevention. Geneva, Switzerland, 2020. [Google Scholar]
- 41. Mølhave M, Wejse C. Historical review of studies on the effect of treating latent tuberculosis. Int J Infect Dis 2020;92S:S31–6. 10.1016/j.ijid.2020.03.011 [DOI] [PubMed] [Google Scholar]
- 42. Paton NI, Borand L, Benedicto J, et al. Diagnosis and management of latent tuberculosis infection in asia: review of current status and challenges. Int J Infect Dis 2019;87:21–9. 10.1016/j.ijid.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 43. Lien LT, Hang NTL, Kobayashi N, et al. Prevalence and risk factors for tuberculosis infection among hospital workers in hanoi, viet nam. PLoS One 2009;4:e6798. 10.1371/journal.pone.0006798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ngo CQ, Manabe T, Vu GV, et al. Difficulties in tuberculosis infection control in a general hospital of vietnam: a knowledge, attitude, and practice survey and screening for latent tuberculosis infection among health professionals. BMC Infect Dis 2019;19:951. 10.1186/s12879-019-4593-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. International Union Against Tuberculosis and Lung Disease . Abstract book. in: 48th world conference on lung health of the international union against tuberculosis and lung disease (the union). 2017. Available: http://www.abstractserver.com/TheUnion2017/TheUnion2017_Abstracts_Web.pdf
- 46. U.S. Food & Drug Administration (FDA) . FDA updates and press announcements on nitrosamine in rifampin and rifapentine. Available: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updatesandpressannouncementsnitrosaminesrifampinandrifapentine [Accessed Nov 2022].
- 47. World Health Organization . Nitrosamine concerns for rifapentine and rifampicin update and faqs. Available: https://extranet.who.int/pqweb/sites/default/files/documents/FAQNitrosamine18Dec2020.pdf [Accessed 7 Nov 2022].
- 48. Arguello Perez E, Seo SK, Schneider WJ, et al. Management of latent tuberculosis infection among healthcare workers: 10-year experience at a single center. Clin Infect Dis 2017;65:2105–11. 10.1093/cid/cix725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Han S-S, Lee SJ, Yim J-J, et al. Evaluation and treatment of latent tuberculosis infection among healthcare workers in korea: a multicentre cohort analysis. PLoS One 2019;14:e0222810. 10.1371/journal.pone.0222810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park SY, Lee E, Lee EJ, et al. Screening and treatment of latent tuberculosis infection among healthcare workers at a referral hospital in Korea. Infect Chemother 2019;51:355–64. 10.3947/ic.2019.51.4.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lardizabal A, Passannante M, Kojakali F, et al. Enhancement of treatment completion for latent tuberculosis infection with 4 months of rifampin. Chest 2006;130:1712–7. 10.1378/chest.130.6.1712 [DOI] [PubMed] [Google Scholar]
- 52. Horsburgh CR, Goldberg S, Bethel J, et al. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest 2010;137:401–9. 10.1378/chest.09-0394 [DOI] [PubMed] [Google Scholar]
- 53. Uppal A, Rahman S, Campbell JR, et al. Economic and modeling evidence for tuberculosis preventive therapy among people living with HIV: a systematic review and meta-analysis. PLoS Med 2021;18:e1003712. 10.1371/journal.pmed.1003712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fox GJ, Nhung NV, Sy DN, et al. Household-contact investigation for detection of tuberculosis in Vietnam. N Engl J Med 2018;378:221–9. 10.1056/NEJMoa1700209 [DOI] [PubMed] [Google Scholar]
- 55. Morishita F, Garfin AMCG, Lew W, et al. Bringing state-of-the-art diagnostics to vulnerable populations: the use of a mobile screening unit in active case finding for tuberculosis in palawan, the philippines. PLoS One 2017;12:e0171310. 10.1371/journal.pone.0171310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-071537supp001.pdf (32.6KB, pdf)
bmjopen-2022-071537supp002.pdf (95.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data that support the findings of this study are available from the Viet Nam National TB Control Programme, Hai Phong Provincial Lung Hospital and Pham Ngoc Thach Provincial TB Hospital, but restrictions apply to their availability. Data can be made available from the authors upon reasonable request and with permission of the Viet Nam National TB Control Programme, Hai Phong Provincial Lung Hospital and Pham Ngoc Thach Provincial TB Hospital.