Highlights
-
•
Regular exercise confers multiple health benefits and better survival.
-
•
Regular moderate-intensity of exercise sustains the major hallmarks of health.
-
•
The health benefits of exercise lies in the remarkable integrative adaptation of multiple tissues and organs.
-
•
Exercise appears as a protective strategy to maintain health in response to stress.
-
•
Regular exercise is considered a non-pharmacological polypill for patients with certain comorbidities.
Keywords: Beneficial effects of exercise, Exercise-related physiological adaptations, Hallmarks of health, Moderate-intensity exercise, Therapeutic exercise
Abstract
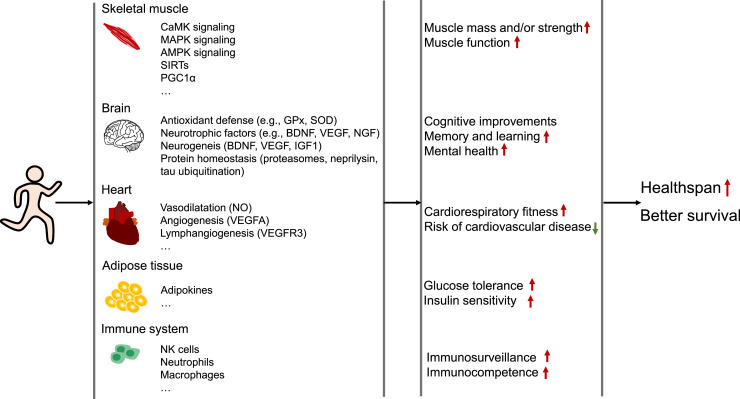
Exercise has long been known for its active role in improving physical fitness and sustaining health. Regular moderate-intensity exercise improves all aspects of human health and is widely accepted as a preventative and therapeutic strategy for various diseases. It is well-documented that exercise maintains and restores homeostasis at the organismal, tissue, cellular, and molecular levels to stimulate positive physiological adaptations that consequently protect against various pathological conditions. Here we mainly summarize how moderate-intensity exercise affects the major hallmarks of health, including the integrity of barriers, containment of local perturbations, recycling and turnover, integration of circuitries, rhythmic oscillations, homeostatic resilience, hormetic regulation, as well as repair and regeneration. Furthermore, we summarize the current understanding of the mechanisms responsible for beneficial adaptations in response to exercise. This review aimed at providing a comprehensive summary of the vital biological mechanisms through which moderate-intensity exercise maintains health and opens a window for its application in other health interventions. We hope that continuing investigation in this field will further increase our understanding of the processes involved in the positive role of moderate-intensity exercise and thus get us closer to the identification of new therapeutics that improve quality of life.
Graphical abstract
1. Introduction
While modernization has contributed to increased population longevity, it has also witnessed a continuous rise in non-communicable diseases such as obesity, hypertension, type 2 diabetes, cancer, etc. Non-communicable diseases are now considered “the number one killer” globally, as they lead to more than 80% of deaths in certain countries.1,2 The prevalence of non-communicable diseases can be at least partly attributed to insufficient physical activity or exercise.3 According to data from the World Health Organization, in 2016, more than one-quarter of adults worldwide were physically inactive.4 The worldwide pandemic of physical inactivity should be a public health priority. It is well-known that a healthy lifestyle is associated with a significantly lower risk of total mortality and a longer life expectancy.5 Together with a healthy and adequate dietary pattern, exercise represents a promising strategy for reducing the risk of chronic metabolic and inflammatory diseases, such as those related to obesity. In general, physical activity is defined as any movement requiring energy, such as walking, manual labor, or housework. By contrast, exercise refers to a routine of physical activity that is planned and structured with the aim of improving physical fitness.6 In this review, we use the term “regular exercise” to refer to regular moderate-intensity exercise at a <70% maximal oxygen uptake (VO2max).
It has been strongly recommended that children and adults should limit the amount of sedentary time, which is associated with adverse health outcomes, including all-cause mortality and the incidence of cardiovascular disease, cancer, and type 2 diabetes. Replacing sedentary behavior with physical activity of any intensity (including light intensity) is beneficial for health. For substantial health benefits, World Health Organization recommends adults should undertake at least 150–300 min of moderate-intensity or 75–150 min of vigorous-intensity aerobic physical activity throughout the week, or an equivalent combination of moderate-intensity and vigorous-intensity aerobic physical activity.7 Adults are recommended to increase moderate-intensity aerobic physical activity to more than 300 min, or perform more than 150 min of vigorous-intensity aerobic physical activity throughout the week or an equivalent combination of moderate-intensity and vigorous-intensity aerobic physical activity for additional health benefits. Adults are also recommended to do muscle-strengthening activities that involve all major muscle groups for 2 or more days a week at a moderate or greater intensity.7 Next to aerobic activities, adults aged 65 years and older are recommended to do multicomponent physical activity that includes functional balance and strength training at a moderate or higher intensity for at least 3 days a week.7
There is evidence showing that 30 metabolic equivalent task-h of pre-pregnancy is associated with a 12% reduction in the relative risk of gestational diabetes mellitus, and 7 h of physical activity per week before and during pregnancy is associated with a 30% reduction and 37% reduction of risk, respectively.8 Additionally, 500 metabolic equivalent task-min/week of aerobic physical activity, which is equivalent to 150 min of moderate-intensity or 75 min of vigorous-intensity physical activity weekly, led to a 14% risk reduction in all-cause mortality in patients with cardiovascular disease and a 7% risk reduction in healthy adults.9 And children and adolescents aged 5–17 years performed an average of 60 min of moderate-to-vigorous intensity physical activity per day, which was is associated with multiple beneficial health outcomes including cardiorespiratory fitness, muscular fitness, bone health, and cardiometabolic health.10
A large body of studies supports the notion that regular exercise plays an important role in reducing the risk of cardiovascular disease11,12 and improving the health outcomes of patients with multiple other pathologies, including obesity, type 2 diabetes, multiple sclerosis, stroke, age-related sarcopenia, and some types of cancer.13, 14, 15, 16, 17, 18 The epidemiological studies report that a higher level of physical activity is associated with lower mortality risk in individuals with or without cardiovascular disease, yet the benefits of physical activity with respect to mortality appear to be greater in people with cardiovascular disease as compared to those without cardiovascular disease.9 Moreover, regular physical activity or exercise is associated with a reduced risk of adverse health outcomes generally (e.g., disability levels, mortality) and with better survival.19, 20, 21 Exercise could partially reverse chronic conditions and multimorbidity related to low physical activity and can be employed as a preventive approach to improve quality of life.22,23 Furthermore, greater amounts and higher intensities of physical activity, as well as distinct types of physical activity (e.g., aerobic, muscle and bone-strengthening activities), are associated with multiple beneficial health outcomes (e.g., cardiorespiratory fitness, muscular fitness, bone health, and cardiometabolic health) for children and adolescents aged 5–17 years.10
Regular moderate-intensity types of exercise, such as strength, endurance, balance, flexibility, and coordination, benefit all aspects of human health and are widely accepted as a therapeutic and preventative strategy for various diseases, including cardiovascular diseases (e.g., cardiomyopathy, cardiac ischemia/reperfusion injury, heart failure), metabolic diseases (e.g., hyperlipidemia, metabolic syndrome, type 2 diabetes), neurological diseases (e.g., Parkinson's disease, multiple sclerosis), and pulmonary diseases, etc.24,25 However, if physical exercise undercuts a minimum intensity level, no significant changes in body homeostasis are to be expected. In untrained individuals, sudden bouts of vigorous-intensity exercise can lead to adverse cardiovascular events and prolonged intense exercise may increase the incidence of infarction.26 Therefore, the intensity and mode of exercise are of critical importance for producing positive effects on health.
The biology of exercise is complex and involves adaptive responses in multiple organ systems. Movement and physical activity is an evolutionary survival advantage that turns skeletal muscle into a systemic modulator to meet energy demand.27 Exercise is a dynamic energy-demanding activity that not only involves the cardiovascular, respiratory, and musculoskeletal systems but also affects the immunological and endocrine systems. Despite broad investigation into the efficacy of exercise, the underlying mechanisms of its benefits remain elusive. Although quite a few papers have summarized the psychosocial and biological responses to exercise, most of them are focused on specific diseases or organ systems. To our knowledge, few studies report exercise-induced changes from the perspective of the overall “organization” of organisms as well as the underlying mechanisms.
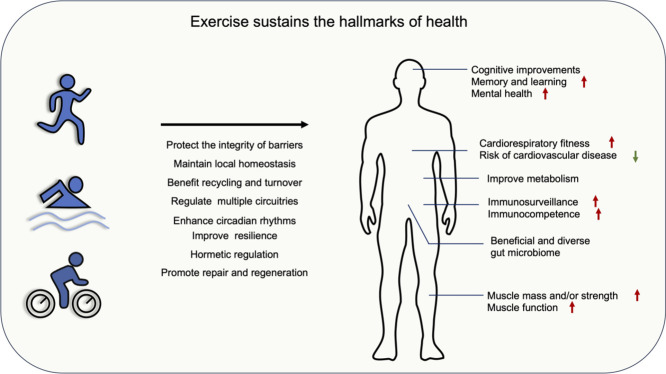
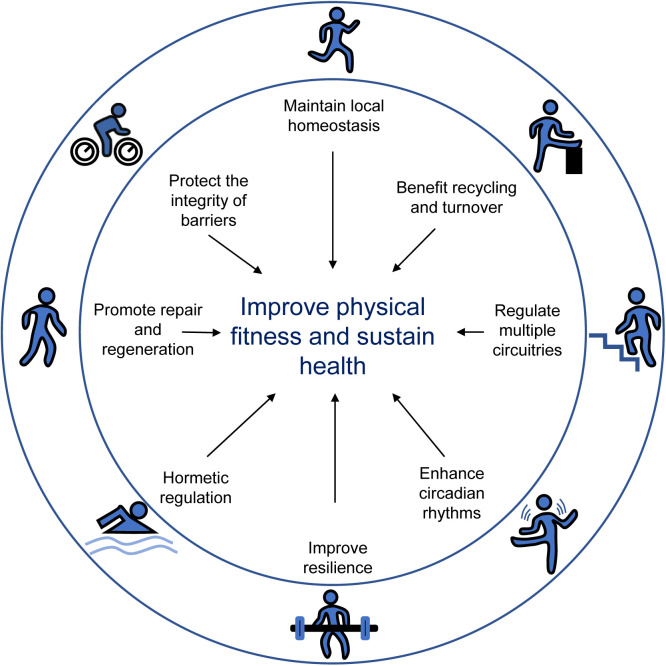
In a recent paper, two of the co-authors of this study proposed 8 hallmarks of health: integrity of barriers, containment of local perturbations, recycling and turnover, integration of circuitries, rhythmic oscillations, homeostatic resilience, hormetic regulation, as well as repair and regeneration.28 Although more investigations are needed, a wealth of evidence indicates that exercise can affect most of these hallmarks (Fig. 1). This review aimed to provide a comprehensive summary of the vital biological changes through which exercise produces beneficial effects on health. In addition, this review summarizes the mechanisms through which exercise interventions are effective in preventing diseases, specifically age-related diseases (Alzheimer's disease, Parkinson's disease), type 2 diabetes, cardiovascular diseases (cardiomyopathy, cardiac ischemia/reperfusion injury, and heart failure), and certain cancers (breast and colorectal cancer). For better reading comprehension, we have included Supplementary Table 1, which details exercise protocols in animal studies, Table 1, which details exercise protocols in human studies, and Table 2, which summarizes the evidence based on the randomized control trials cited in this review.
Fig. 1.
Summary of the main regulatory mechanisms by which exercise sustains health. Exercise impacts the major hallmarks of health, including the integrity of barriers, containment of local perturbations, recycling and turnover, integration of circuitries, rhythmic oscillations, homeostatic resilience, hormetic regulation, as well as repair and regeneration.
Table 1.
The detailed exercise protocols in human studies cited in this review.
| Reference | Exercise protocol | Subject | Effect | Mechanism |
|---|---|---|---|---|
| 51 | 120 min cycling at 60% VO2max, which is determined by an incremental ride to exhaustion on a cycle ergometer | Human | Increase fatty acid transport | Increase FAT/CD36 and FABPpm |
| 61 | 1 h of treadmill running at 80% VO2max, 0% grade | Human | Increase small intestinal permeability | / |
| 62 | A standard marathon (42.16 km) | Human | Increase intestinal permeability | / |
| 64 | Two 1-h-long bouts of submaximal treadmill exercise at 65% VO2max | Human | Increase gut barrier permeability | / |
| 95 | 45 min cycling (start with 30 min cycling at 65% of HRmax and progressed by 5% every other week until intensity reached 75% of HRmax), twice per week for 12 weeks | Human (65–86 years old) | Attenuate skin aging and enhance mitochondrial biogenesis | Increase IL-15 level |
| 116 | 12 weeks combined endurance (20 min treadmill walking at 70%–80% heart rate reserve) and resistance exercise (8 sets of exercises, 2 sets at 70%–80% of one-repetition maximum, 30 min), 3 days/week | Human (65–80 years old) | Reduced systemic inflammation | Reductions in CD14+CD16+ monocytes |
| 126 | 1-h cycling at 70% of VO2max | Human | Increased levels of inflammatory gene | / |
| 138 | Three 30-min bouts of cycling exercise at –5%, +5%, or +15% of lactate threshold | Human | Augment cytotoxicity against tumor cells | Enhanced NK cell activity |
| 188 | Marathon (a 42.2-km running race) | Athlete | Increased plasma level of GDF15 | Changes in circulating immune cells levels |
| 190 | 1 h cycling exercise at 67% of VO2max | Human | Increased plasma level of GDF15 | / |
| 234 | Maximal exercise test, start cycling with a 75 W load for 3 min (warm-up stage) followed by increments of 25 W every 3 min until volitional exhaustion | Human | Significant modulation of clock genes in effector-memory CD4+ T cells | / |
| 322 | 3 months of moderate exercise involving a combination of treadmill or cycling, each exercise session included 10 min warm-up and cool-down steps at 50%–60% of HRmax, along with 40 min of the prescribed exercise program at 65%–80% of HRmax, 3 times/week | Obese human subject (30 kg/m2 ≤ BMI < 40 kg/m2) | Alleviate obesity-related ER stress | Downregulate GRP78 signaling |
Note: / stands for unknown.
Abbreviations: BMI = body mass index; ER = endoplasmic reticulum; FABPpm = plasma membrane fatty acid-binding protein; FAT = fatty acid translocase; GDF15 = growth differentiation factor-15; GRP78 = glucose-regulated protein 78; HRmax = maximal heart rate; IL-15 = interleukin-15; NK cell = natural killer cell; VO2max = maximal oxygen uptake; W = watt.
Table 2.
Effects of exercise on health outcomes in humans with different conditions.
| Reference | Design | Participant condition | No. of participants | Age (year; mean ± SD) | Intervention | Outcome |
|---|---|---|---|---|---|---|
| 57 | RCT | Woman living in a nursing home support center; capacity to practice exercise without causing harm to themselves with approval of the medical department | 25 (C =12; I =13) | C = 82.0 ± 7.5; I = 83.5 ± 7.3 | 14 weeks of combined resistance (e.g., front squat, bench over row, standing reverse fly, etc.) and aerobic exercise training (e.g., stimulated walking, hip marching, standing rear leg extension, etc.) twice a week | Maintained concentrations of peripheral markers for BBB integrity and improved cytokine balance |
| 77 | RCT | Patients with moderate-to-severe COPD | 30 (C = 15; I = 15) | >40a | 30 min of respiratory muscle-stretching exercise (intervention group) or upper and lower limb muscle-stretching (control group), followed by 30 min of aerobic training (treadmill running), twice a week for 12 weeks | Aerobic training combined with respiratory muscle-stretching increases the functional exercise capacity with decreased dyspnea in patients with COPD |
| 89 | RCT | Healthy young adults | 16 | 24 ± 5 | A 30-min CE and a 60-min IE (1-min work and 1-min rest) at 70% of their maximum work rate on separate occasions in a random order | The 30-min CE caused mild airway damage, while a time- or work-matched IE did not |
| 97 | RCT | Patients with venous leg ulcers | 59 (C = 30; I = 29) | 71.5 ± 14.6 | 12 weeks of home-based progressive resistance exercise | A trend toward healing at a faster rate and better functional outcomes |
| 98 | RCT | Healthy adults | 28 (C = 15; I = 13) | 61.0 ± 5.5 | 1 h exercise per day (start with 10 min of warm-up floor exercises and stretching, followed by 30 min cycling, 15 min of brisk walking and/or jogging and arm-strengthening exercise, 5 min of cool-down exercise), 3 days per week for 3 months | Enhance rates of wound healing |
| 132 | RCT | Healthy adults | 144 (Cardio = 74; Flex = 70) | 69.9 ± 0.4 | 10-month cardio intervention (began at 45%–55% of VO2max and progressed to 60%–70% of VO2max within 3 months, 3 days/week, the duration of each session increased from 10–15 min to approximately 45–60 min by the 4th month) was compared to Flex intervention (2 days/week for approximately 75 min/session) | Cardio intervention resulted in a longer-lasting seroprotective response to influenza vaccination |
a The data are not mean ± SD.
Abbreviations: BBB = blood–brain barrier; C = control; Cardio = cardiovascular exercise group; CE = continuous exercise; COPD = chronic obstructive pulmonary disease; Flex = flexibility and balance group; I = intervention; IE = intermittent exercise; RCT = randomized controlled trial; VO2max = maximal oxygen uptake.
2. Exercise protects the integrity of barriers
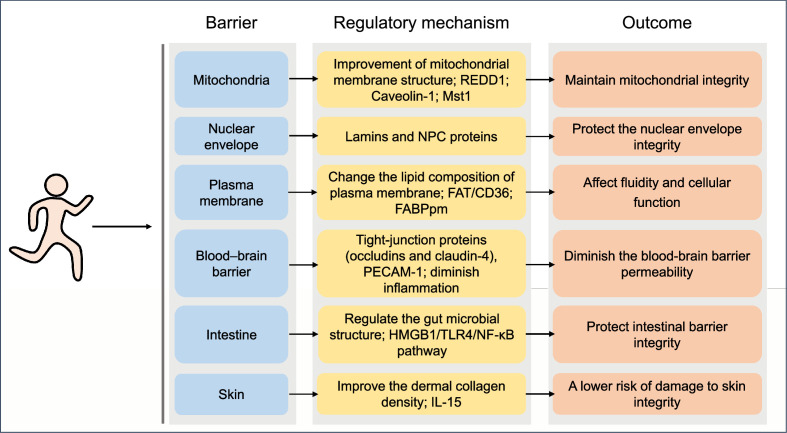
Human health is linked to the integrity of barriers at the level of organelles (mitochondria and nuclei), cell membranes, internal barriers (blood–brain), and barriers against the external (intestinal, respiratory, and cutaneous) environment. Exercise confers health benefits partly by regulating the integrity of these barriers, as explained below (Fig. 2).
Fig. 2.
Effects of exercise on the integrity of barriers and the underlying mechanisms. The biological changes of barriers through which exercise sustains health. FABPpm = plasma membrane fatty acid-binding protein; FAT = fatty acid translocase; HMGB1 = high mobility group box 1; IL-15 = interleukin-15; Mst1 = mammalian sterile 20-like kinase 1; NF-κB = nuclear factor-kappa B; NPC = nuclear pore complex; PECAM-1 = platelet and endothelial cell adhesion molecule 1; REDD1 = regulated in development and DNA damage 1; TLR4 = toll-like receptor 4.
2.1. Exercise maintains mitochondrial integrity
Mitochondrial health is of vital importance for cellular function in various tissues and, as a result, contributes to overall vitality in health and disease. Mitochondria generate cellular ATP and, thus, are key actors in whole-body energy regulation. During exercise, there is a greater demand for ATP, which is mediated by increasing mitochondrial biogenesis and enhancing mitochondrial function.29 The effects of exercise on mitochondria have been intensively studied, with muscle mitochondria being the most well-studied. Exercise training upregulates skeletal muscle sirtuins (SIRTs), which contribute to mitochondrial biogenesis and maintenance of the antioxidant system. Specifically, acute exercise (a single bout of exercise) activates SIRT1 in skeletal muscle, while several sessions of high-intensity interval training (HIIT) and aerobic or resistance exercise training activate SIRT1 and SIRT3.30
Importantly, aerobic exercise causes significant changes in mitochondrial content and quality that are good for metabolic health.31 For example, the number of myonuclei is increased in rats in response to resistance training, and this increase can be retained for a long-term detraining period. When re-subjected to resistance training, the newly acquired myonuclei contribute to a greater degree of muscle hypertrophy and mitochondrial biogenesis.32 Taken together, these results provide further support for the assertion that exposing young muscles to resistance exercise training may help to restore age-related muscle loss associated with mitochondrial dysfunction in later life.32
Mitochondria are also involved in calcium metabolism, contribute to the formation of intracellular reactive oxygen species (ROS), and play a leading role in the initiation of apoptosis; therefore, they are essential to maintaining cellular homeostasis and act as important signaling organelles in different tissues. Exercise may decisively influence all these mitochondrial functions.
The mitochondrial membranes represent the battleground on which opposing signals fight to determine the cell's fate. The unique lipid composition and structure of mitochondrial membranes are critical for the proper functioning of mitochondria. Quality control of mitochondrial protein synthesis plays an important role in maintaining mitochondrial integrity.33 In particular, mitochondrial membrane permeabilization, a tightly regulated process, has been described as an essential feature of cell death.34 Acute exercise, as an allostatic stimulus, causes a temporary alteration of the interaction between mitochondria and other organelles, such as the endoplasmic reticulum (ER), due to the upregulation of regulated in development and DNA damage responses 1.35
The beneficial effects of exercise on health are at least partly due to the improvement of mitochondrial membrane structure and, thus, mitochondrial function. It is reported that endurance-trained athletes (e.g., cross-country and soccer players) show a higher density of mitochondrial cristae, which is related to oxygen consumption and oxidative capacity.36 Both 17-week voluntary wheel running exercise and 8-week endurance training (treadmill running) confer protection against non-alcoholic steatohepatitis-induced alterations in rat mitochondrial membrane composition, as revealed by the increased ratio of phosphatidylcholine to phosphatidylethanolamine,37 because the ratio is a key regulator of membrane integrity.38 More recent studies indicate that 8-week endurance training could inhibit non-alcoholic steatohepatitis-induced mitochondrial permeability transition pore opening, indicating decreased mitochondrial membrane permeability.39 Seven days of treadmill exercise can prevent mitochondrial outer membrane permeabilization in the rat model of cerebral ischemic injury by increasing caveolin-1 expression, thus inhibiting excessive cytochrome C release from mitochondria,40 reducing mitochondrial damage, and protecting mitochondrial integrity. In addition, 12 weeks of moderate-intensity treadmill exercise exerts cardioprotection in diabetic mice by down-regulating mammalian sterile 20-like kinase 1, which enhances mitochondrial membrane potential.41
2.2. Exercise and the sarcoplasmic reticulum (SR)
The SR is a structure involved in Ca2+ mobilization for muscle contraction. Disruption of the SR is implicated in muscular dystrophy, cardiomyopathy, sarcopenia due to aging, and neurodegenerative diseases.42 Exercise decreases lipid peroxidation and calpain activity in the SR, thereby preventing heat stress.43
2.3. Exercise and nuclear envelope (NE) integrity
The double membrane structure of the NE serves as a barrier that separates the genome from the cytoplasm in eukaryotic cells. Disruption of the NE and exposure of chromatin endanger cell viability and induce genome instability. Thus, the integrity of NE is critical for cell survival. The NE homeostasis is maintained through a balance between the various forces imposed upon the nuclei, which can be disrupted under certain conditions (e.g., alterations in the expression of lamins, migration and invasion, micronuclei, telomere fusion, heterochromatin modulation, virus infection, the loss of ATR, retinoblastoma protein or p53) resulting in transient NE collapse.44
Few studies have focused on the role of exercise on NE integrity. The NE provides a semi-permeable barrier, with its structural integrity being maintained partly by the intermediate filament proteins called lamins (A, B, and C), which are encoded by the LMNA gene. Six weeks of moderate exercise attenuates dilated cardiomyopathy development in heterozygous LMNA knockout (LMNA+/–) mice whose cardiomyocytes show altered nuclear shape.45 The selectivity of the transit channel is controlled by the proteins of the nuclear pore complex.46 It has been reported that 4 months of running wheel exercise is capable of preventing the decline of Lamin B1 and nuclear pore complex protein in older sedentary mice,46 indicating that exercise can help sustain the integrity of the NE.
2.4. Exercise and plasma membrane (PM) integrity
PM integrity is essential for cellular function and survival. Besides serving as a physical barrier between the extra- and intra-cellular environment, the PM bridges intracellular signaling and extracellular signals (e.g., ions, hormones, cytokines, enzymes), thus allowing for cellular communication with the surrounding environment. Rupture of the PM can have deleterious consequences on a cell's fate. Cells are equipped with repair mechanisms to ensure membrane integrity, including membrane fusion and replacement strategies, means for removal of the damaged membrane, and protein-driven membrane remodeling and wound closure methods.47 Exercise can induce changes in the lipid composition of membranes that affect fluidity and cellular function.48 For example, a 2-week course of treadmill exercise (13.5 m/min, 10% grade, 50 min/day) administered to high-fat diet (HFD)-fed mice prevents cholesterol accumulation in the PM of skeletal muscle cells.49 The excess of PM cholesterol may contribute to insulin resistance in the skeletal muscle of mice who are fed an HFD.50 Moreover, acute endurance exercise (120 min) is capable of increasing the expression of fatty acid translocase/CD36 and PM fatty acid-binding protein in human and rat muscle, indicating increased fatty acid transport in exercised muscle.51
2.5. Exercise supports the integrity of the blood–brain barrier (BBB)
The BBB is an active interface that separates systemic circulation from the central nervous system with a dual function: the barrier limits the entry of blood-derived potentially toxic or harmful substances into the brain, and the carrier function is responsible for the transport of molecules and cells into and out of the central nervous system as well as for the removal of metabolites. Every cell type (e.g., endothelial cells, astrocytes, pericytes) and extracellular matrix makes an indispensable contribution to the BBB's integrity.52 Specifically, pathways like Wnt/β-catenin, sonic hedgehog, retinoic acid, angiopoietins, and others are involved in the regulation of barrier maintenance.53
Exercise has been shown to maintain BBB selective permeability in health and disease.54 For example, a previous study showed that both strength training and endurance training exercises could prevent BBB disruption in a mouse model of multiple sclerosis by increasing the expression of tight-junction proteins (occludins and claudin-4) and decreasing the expression of platelet and endothelial cell adhesion molecule 1.55 Moreover, exercise can protect against BBB disruption occurring in the context of substance use disorders.56 Of note, in a clinical trial, a 14-week-long combined resistance and aerobic exercise training intervention is capable of maintaining BBB integrity and decreasing chronic inflammation in older women.57 Mechanistically, regular exercise may diminish BBB permeability by enhancing antioxidative capacity, reducing oxidative stress, diminishing inflammation, enhancing neurotrophic factors such as brain-derived neurotrophic factor (BDNF), and improving tight junction.54,55,58 On the other hand, the BBB has a selective permeability in order to receive systemic signals. Acute exercise increases phosphoinositide 3-kinase (PI3K)-p110α protein in the hypothalamus, enhancing the regulation of food intake.59
2.6. Exercise effects on intestinal barrier integrity
The intestine acts as a barrier to the external environment that defends against the invasion of pathogens and harmful food ingredients. The intestinal epithelial tight junction proteins (e.g., occluding, claudins, zonula occludens) and gut microbiome play a pivotal role in the maintenance of barrier integrity.60 Accumulating evidence suggests that exercise affects the integrity of the intestinal barrier. For instance, small intestinal permeability is increased in healthy individuals after 1 h of treadmill running at 80% VO2max.61 Moreover, a significant increase in intestinal permeability has been observed in participants immediately after completing a standard marathon (42.16 km).62 Gut barrier permeability was elevated in runners who completed two 1-h-long bouts of submaximal treadmill exercise (65% VO2max, determined by a graded treadmill test63) under normoxic and hypoxic conditions.64 Similarly, endurance exercise (1100 miles in 10 days) is associated with increased intestinal permeability in racing dogs.65 Another study reports that mice subjected to acute treadmill running at 80% VO2max (determined by a progressive exercise test on a small rodent treadmill) until exhaustion (running for at least 30 min) exhibit increased permeability in the small intestinal.66
Intestinal barrier permeability is impaired under heat stress67 and with strenuous exercise68 due, in part, to visceral hypoperfusion during exercise. Of note, glutamine supplementation appears to attenuate intestinal permeability and maintain intestinal integrity in response to a 60-min treadmill run at 70% of VO2max in an environmental chamber set at 30 °C with 40%–45% humidity.69 It has also been shown that 5 weeks of combined aerobic and resistance training counteracts HFD-induced rat microbial dysbiosis, thus protecting intestinal barrier integrity.70 Furthermore, 6 weeks of aerobic exercise (10 m/min for 30 min/day, 6 days/week) can protect against obstructive jaundice-induced intestinal mucosal barrier damage in mice.71 Mechanistically, exercise increases the level of endogenous H2S and inhibits the high mobility group box 1–toll-like receptor 4–nuclear factor-kappa B pathway, thereby protecting the intestinal mucosal barrier.71 Also, 7 weeks of swimming exercise (1 or 1.5 h/day for 5 days/week) attenuates the effect of dextran sulfate sodium–induced rat intestinal aggression by improving crypt status through regulating inflammation, oxidative stress, and apoptosis.72 Moreover, training improves zonula occludens 1, occludin, claudin-2, and the intestinal peptide transporter-1 messenger RNA expression.68 Thus, physical exercise has an ambiguous, context-dependent effect on intestinal barrier integrity.
2.7. The effects of exercise on the barrier integrity in the respiratory tract
The respiratory system, roughly divided into the upper (nasal passages, pharynx, and larynx) and the lower (trachea, bronchial tree, and lungs) respiratory tract, provides a physical, secretory, and immunologic barrier to exclude harmful substances such as ozone, particulate matter, pathogens, etc.73 As a mucosal organ system in direct contact with the external environment, protection of the respiratory barrier is vital to human health. The mechanisms contributing to increased epithelial permeability involve internal (type 2 cytokines such as interleukin-4 (IL-4) and IL-13), external (environmental molecules such as allergens, pathogens, and pollutants), genetic (e.g., protocadherin-1, cadherin-related family member 3, orosomucoid-like 3), and epigenetic (e.g., DNA methylation, histone deacetylases) factors.74
Although exercise can trigger bronchial hyperresponsiveness in cold and dry environments,75 it also has numerous health benefits, including its positive effects on individuals with chronic respiratory disease.76 For example, 12 weeks of aerobic exercise (30 min of treadmill running) combined with respiratory muscle stretching improves the functional exercise capacity and reduced dyspnea in patients with chronic obstructive pulmonary disease.77 Even low levels of physical activity in older patients with cardiovascular disease are associated with decreased mortality and hospitalization from pneumonia.78 Thus, exercise training has been recommended to support pulmonary rehabilitation in chronic disease management.79
Exercise exerts beneficial effects on cardiovascular and respiratory diseases partly via endothelial protection80,81 and also by decreasing the Th2 inflammatory response of the airways.82 During exercise, fitness level could modulate the concentration and secretion of salivary antimicrobial proteins, which function as the first line of defense against invading microorganisms.83 However, the effects of exercise on the integrity of the respiratory tract, including epithelial and endothelial barriers, has not been investigated in detail. In fact, it is well-known that athletes have an increased risk of developing upper respiratory tract infections.84 It seems that prolonged intensive exercise compromises the barrier function of respiratory epithelium85 and increases the incidence of upper respiratory tract infections.86,87 However, this topic remains the subject of debate.88 Otherwise, compared to continuous exercise, the matched-intensity and volume intermittent exercise leads to lesser respiratory epithelial cell damage,89 indicating that intermittent exercise may benefit respiratory tract health.
2.8. Exercise and skin integrity
Skin is the body's primary defense structure and is composed of the epidermis and the dermis. In general, the epidermis consists of epithelial cells that are responsible for the skin barrier and thus protect from the harmful effects of the external environment, while the dermis contains the extracellular matrix that includes collagen, elastin, and glycosaminoglycans.90 Skin integrity is restored by a complex multi-step process that includes blood clot formation, inflammation, re-epithelialization, granulation tissue formation, neovascularization, and remodeling. Skin repair requires the activation of a variety of modulators, such as growth factors, cytokines, matrix metalloproteinases, cellular receptors, and extracellular matrix components.91 Research evidence reveals that regular exercise confers advantageous effects on skin integrity. For example, a epidemiology study reports a clear correlation between skin integrity and exercise in older adults. Individuals that do not practice regular exercise have a higher risk of damage to skin integrity (27.02%) compared to those who exercise regularly (8.77%).92 Moreover, endurance running exercise (15 m/min for 45 min, 3 times/week for 5 months) prevents the age-associated thinning of the dermis in mitochondrial DNA mutator mice (PolG; PolgAD257A/D257A), a model of progeroid aging.93 In obese mice, 8 weeks of voluntary exercise improves the dermal collagen density and decreases the amount of subcutaneous tissue and adipocyte size.94 A later study demonstrates that endurance exercise protects against age-associated structural deterioration of skin that compromises its barrier function in both humans and mice, and it identifies exercise-induced IL-15 as a novel mediator of skin health.95 Functional studies indicate that daily IL-15 administration mimics some of the anti-aging effects of exercise on the skin in mice.95
3. Exercise benefits the maintenance of local homeostasis
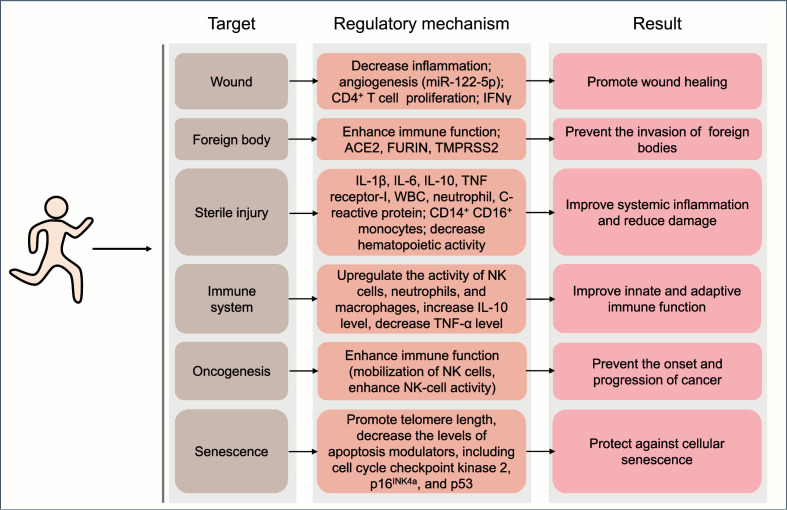
While a single bout of exercise may cause local perturbation, long-term moderate-intensity exercise generally contributes to the maintenance of systemic homeostasis and the improvement of health (Fig. 3).
Fig. 3.
Benefits of exercise on the maintenance of local homeostasis and the involved mechanisms. Regular exercise at moderate intensity generally contributes to the maintenance of systemic homeostasis. ACE2 = angiotensin-converting enzyme 2; IFNγ = interferon-γ; IL = interleukin; miR = microRNA; NK = natural killer; TMPRSS2 = transmembrane protease serine 2; TNF-α = tumor necrosis factor-α; WBC = white blood cell counts.
3.1. Exercise promotes barrier healing
Skin wound healing is a major health concern. Exercise is a relatively cheap and effective strategy that could be adopted clinically to prevent or treat damage to the skin.96,97 Moderate-intensity aerobic exercises have been shown to promote skin wound healing in both humans and mice.98,99 In addition, 4 weeks of voluntary wheel exercise decreases skin graft rejection and increases graft survival through CD4+ T cell proliferation and interferon-γ production.100 A recent study reports that exercise-associated microRNA-122-5p (miR-122-5p) is capable of promoting wound healing via increasing capillary density in the perilesional skin tissues of mice.101 However, the molecular and cellular mechanisms through which exercise stimulates healing require further investigation.
Of note, the intensity of exercise could influence the healing process. For example, in a mouse study, compared to high (80% VO2max) and strenuous intensity (90% VO2max), moderate-intensity physical training (70% VO2max) is particularly efficient in improving wound healing.102 Moreover, in diabetic mice, low-intensity treadmill exercise (12 m/min for 30 min, 5% incline, 5 days/week for 3 weeks) benefits the wound healing process, while high-intensity treadmill exercise (18 m/min for 30 min, 5% incline, 5 days/week for 3 weeks) does not.103
3.2. Exercise prevents the invasion of foreign bodies
The invasion of foreign bodies (e.g., pathogens) represents a threat to health. Lifestyle interventions, including exercise, have been identified as an effective approach to controlling certain conditions associated with viral infection. The antiviral role of exercise has received a great amount of attention. Several scientific findings indicate that exercise training contributes to the improvement of physiological and functional parameters in patients with human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS).104 Exercise could help to prevent viral infection and/or promote viral clearance. For example, regular physical activity is associated with decreased risk of infection.105,106 In mice, chronic exercise at moderate intensity may have an attenuating effect on influenza infection by decreasing viral load and illness severity.107 Indeed, exercise is considered to be supportive of the medical care and treatment of HIV-infected patients.108
A large body of investigations has highlighted the indispensable role of the immune system in combating viral infection. Exercise is well-known to have a major effect on the functioning of the immune system. In particular, regular moderate exercise is considered to be a safe and cost-effective strategy to enhance the immune system and provide protection against pathogenic viral infection.108,109 Recent studies reveal that a regular program of aerobic exercise with an intensity of 55%–80% VO2max is associated with increased immunological biomarkers, such as circulating leukocytes, lymphocytes, monocytes, and neutrophils, and thus helps to reduce the risk of coronavirus disease 2019.110,111 Aerobic exercise affects several molecules in various tissues that are involved in severe acute respiratory syndrome coronavirus 2 virus entry angiotensin-converting enzyme 2, FURIN, and transmembrane protease serine 2. However, the overall effect on the disease is not yet known.112
3.3. Exercise improves systemic inflammation
Exercise training exerts anti-inflammatory effects on the periphery and the brain.113 For example, in mice, moderate voluntary wheel running (30 days of access to wheels) attenuates colitis symptoms and reduces inflammation in response to oral administration of dextran sulfate sodium.114 In addition, regular training in mice attenuates sepsis-induced lung damage; the effect is mediated by a reduced inflammatory response, modulated by decreased IL-1β and increased IL-10.115
Moreover, the percentage of inflammatory CD14+CD16+ monocytes is reduced in older adults (65–80 years old) after completing a 12-week-long (3 days/week) combined endurance (20 min at 70%–80% heart rate reserve) and resistance exercise (8 sets of exercises, 2 sets at 70%–80% of one-repetition maximum) program,116 suggesting reduced systemic inflammation in exercised older individuals. Similarly, studies focusing on healthy adults who are aged 40 years or older show that more frequent physical activity is associated with lower systemic cellular and molecular inflammation, as evidenced by a lower level of white blood cell counts and neutrophil counts, as well as lower levels of C-reactive protein, IL-6, IL-10, IL-1 receptor antagonists, and soluble tumor necrosis factor receptor-1.117,118
Furthermore, mice subjected to regular voluntary running show decreased hematopoietic activity and reduced circulating leukocyte numbers compared to sedentary mice, indicating that regular physical activity confers health benefits partially via decreased systemic inflammation.119 Mechanistic studies indicate that voluntary running exercise regulates hematopoietic activity via lowering leptin production in adipose tissue, augmenting hematopoietic stem and progenitor cell quiescence via modulation of their niche, and reducing the hematopoietic output of inflammatory leukocytes.119
3.4. Exercise and the innate and acquired immune responses
Proper immune function is fundamental for maintaining health in most chronic conditions. In recent years, the role of exercise training in regulating the immune system has received considerable attention.120,121 Most of the current published literature suggests that regular moderate exercise exerts a positive effect on the immune system, thus reducing the incidence of disease.122 Exercise modulates both innate and adaptive immune responses, particularly upregulating the activity of natural killer (NK) cells at rest123 but also of neutrophils and macrophages following moderate exercise (about 60% of VO2max).120,121,124 In addition, regular exercise is associated in healthy individuals with decreased tumor necrosis factor-α expression levels, reduced pro-inflammatory adipokine levels, downregulated Toll-like receptor levels on monocytes and macrophages, and increased circulating levels of anti-inflammatory cytokines.125 Moreover, in healthy young men, 1 h of cycling at 70% of VO2max is sufficient to induce the number of circulating peripheral blood mononuclear cells,126 which are composed of monocytes and lymphocytes and have a critical role in immune function.127, 128, 129
Of note, moderate-to-vigorous intensity endurance exercise bouts may also exert positive immunological effects.88 For example, master athletes with lifelong regular exercise exhibit signs of anti-inflammatory regulation, as revealed by higher plasma IL-10 levels compared to the age-matched untrained control group.130 Further investigation demonstrates that the circulating IL-10 levels in lifelong trained athletes are similar to those in young adults.131 Moreover, clinical trials show that regular moderate exercise (60%–70% of VO2max) significantly improves the immune response to vaccination in adults over age 65,132,133 indicating an improved adaptive immune function in older adults.
3.5. Exercise benefits the anti-cancer immunosurveillance
It is well-accepted that the immune system has the ability to suppress certain types of cancer, and immunotherapy is recognized as a promising strategy in the treatment of cancer. A sedentary lifestyle has recently emerged as a potential independent risk factor for cancer. Conversely, exercise is associated with a reduced risk of certain cancers, including colon cancer, endometrial cancer, breast cancer, etc.134 Moreover, the Mendelian randomization analysis also found that genetically elevated physical activity levels were associated with lower risks of breast and colorectal cancer,135,136 highlighting the promotion of physical activity as a critical intervention to reduce the incidence and prevalence of these cancers. Mechanistically, exercise reduces tumor size partly via enhancing immune function. For example, tumor-bearing mice subjected to voluntary exercise (wheel running) shows a more than 60% decrease in tumor volume across different tumor models, an effect that has been attributed to the exercise-associated mobilization of NK cells, which is necessary and sufficient for this anti-tumor effect.137 Moreover, evidence indicates acute exercise could induce a preferential redeployment of NK cell subsets with a highly-differentiated phenotype and confer protection against tumor cells.138 Thus, exercise could play a role in the prevention and treatment of cancer.
3.6. The protective role of exercise on cellular senescence and its clearance
Cellular senescence has been linked to multiple aging-related illnesses and risk factors. Many stimuli, such as telomere erosion, oxidants, ultraviolet B light, and DNA-damaging chemotherapies that engage the DNA damage response, can induce cellular senescence.139 Long-term endurance training upregulates telomerase activity and telomere-stabilizing proteins and decreases the levels of apoptosis modulators (including cell cycle checkpoint kinase 2, p16INK4a, and p53), pointing to the protective role exercise has on cellular senescence.140,141
One biomarker and effector of senescence is p16INK4a. The expression of p16INK4a in peripheral blood T lymphocytes increases with age.142 The level of p16INK4a is positively associated with circulating IL-6 concentrations, which is a marker of cellular senescence. One recent study reports the 4-week treadmill exercise has a significant effect on attenuating senescence in white adipose tissue, as indicated by decreased p16 level. In old mice, exercise induces changes in the immune-cell distribution (e.g., NK cells, CD8+ T cells, eosinophils, neutrophils), which may contribute to the anti-senescence effect of exercise.143 Since p16INK4a expression is strongly correlated with reduced exercise,142 this is further evidence that exercise may prevent cellular senescence by downregulating p16INK4a expression. In the aging rat model, 12 weeks of swimming training significantly reduces the senescence markers (p53 and p21) and inflammatory cytokine (IL-6) in the liver.144
However, in some settings, exercise elicits positive impacts on health via promoting senescence. As an example, the pro-senescent effect of exercise on fibro-adipogenic progenitors contributes to muscle regeneration and the improvement of muscle function.145
4. Benefits of exercise in recycling and turnover
The proper recycling and turnover of different components of the organism (e.g., cell, organelle, protein) enables healthy cell and organismal development. Each of the subcellular, cellular, and supracellular units composing the organism is replaced continually by newly synthesized ones to maintain cellular homeostasis. The health benefits of exercise may be partly due to its remarkable ability to induce cellular remodeling, including cell self-renewal and turnover of dysfunctional organelles (Fig. 4).
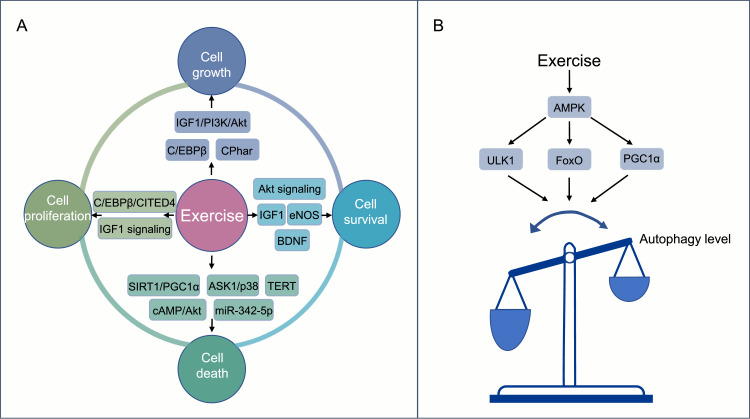
Fig. 4.
Benefits of exercise for recycling and turnover. (A) The critical regulators and signaling pathways involved in the positive effects of exercise on cellular remodeling, including cell proliferation, cell growth, cell survival, and cell death; (B) The regulation of autophagy during exercise training. The common regulatory pathways underlying exercise-induced autophagy involve the activation of the AMPK-ULK1 signaling pathway, FoxO, PGC1α, etc. AMPK = adenosine monophosphate–activated protein kinase; ASK1 = apoptosis signal-regulating kinase 1; BDNF = brain-derived neurotrophic factor; cAMP = cyclic adenosine monophosphate; C/EBPβ = CCAAT/enhancer-binding protein β; CITED4 = CBP/p300-interacting transactivators with E (glutamic acid)/D (aspartic acid)-rich-carboxyl terminal domain; CPhar = cardiac physiological hypertrophy-associated regulator; eNOS = endothelial nitric oxide synthase; FoxO = forkhead box O; IGF1 = insulin-like growth factor 1; miR = microRNA; PGC1α = peroxisome-proliferator-activated receptor coactivator 1α; PI3K = phosphoinositide 3-kinase; SIRT1 = sirtuin 1; TERT =telomerase reverse transcriptase; ULK1 = unc-51-like kinase 1.
4.1. The effects of exercise on cell death, removal, and replacement
It is reported that regular moderate exercise promotes cell survival and reduces cell death. Regular exercise is well-known to promote cardiomyocyte proliferation and growth while preventing cardiomyocyte apoptosis. Mechanistically, the CCAAT/enhancer-binding protein β–CBP/p300-interacting transactivators with E (glutamic acid)/D (aspartic acid)-rich-carboxyl terminal domain (C/EBPβ–CITED4) signaling and the insulin-like growth factor 1 (IGF1) signaling are well-established as essential regulating pathways that mediate exercise-related cardiomyocyte proliferation. Additionally, the C/EBPβ and IGF1-PI3k/Akt pathways play vital roles in exercise-induced cardiomyocyte growth. Exercise also prevents cardiomyocyte death through pathways for SIRT1 and peroxisome-proliferator-activated receptor coactivator 1α (PGC1α) as well as for apoptosis signal-regulating kinase 1/p38 signaling, etc.146 Moreover, 21 days of voluntary wheel running exercise is capable of enhancing cardiac myocyte survival and preventing cell death in the heart through the regulation of telomerase reverse transcriptase, endothelial nitric oxide synthase, and IGF1.141 Accordingly, treadmill exercise-induced myokine (Myonectin) inhibits hypoxia-induced cardiomyocyte apoptosis via activation of the cyclic adenosine monophosphate/Akt signaling pathway.147 It has been reported that long-term exercise-derived plasma exosomes are capable of attenuating hypoxia/reoxygenation-induced cardiomyocyte apoptosis via exosomal miR-342-5p.148 Mechanistically, miR-342-5p prevents cell apoptosis by targeting the mRNAs coding for caspase 9 and mitogen-activated protein kinase 9 (MAPK9, also known as JNK2), and it promotes cardiomyocyte survival by elevating Akt phosphorylation.148 Furthermore, swimming exercise-induced long non-coding RNA (lncRNA) named cardiac physiological hypertrophy-associated regulator (CPhar), reduces oxygen-glucose deprivation/reperfusion-induced apoptosis in cultured neonatal mouse cardiomyocytes.149 Finally, in rats subjected to injury of the optic nerve, 4 weeks of treadmill running (5 days per week for 30 min each day at a moderate intensity of 9 m/min) significantly increases the survival rate of retinal ganglion cells, likely due to an increase in the local expression of BDNF.150
4.2. Exercise and autophagy
Virtually all eukaryotic cells depend on autophagy to maintain cellular homeostasis. Autophagy is a highly conservative recycling mechanism that is responsible for the turnover of cellular proteins and organelles. The mechanism that regulates autophagy is complex, and its upstream signaling pathway mainly includes mammalian target of rapamycin (mToR)-dependent and mTOR-independent signaling.151,152 Mitophagy, a form of vital autophagic response for the turnover of dysfunctional or aged mitochondria, serves to maintain mitochondrial quality control. The regulatory mechanisms of mitophagy mainly involve putative kinase 1–Parkin pathway and receptor-mediated mitophagy.153 In (PINK1) mammals, 2 major types of receptors have been implicated in mitophagy. One group includes BCL2 and adenovirus E1B 19-kDa-interacting protein 3 (BNIP3) and NIP3-like protein X (NIX), and the other group contains FYN14 domain-containing protein 1 (FUNDC1).154
The regulation of autophagy during exercise training is a dynamic and complex process.155 On the one hand, exercise promotes the level of basal autophagy and enhances cell viability. For instance, endurance exercise training (long-term voluntary running) stimulates autophagy in the skeletal muscle of mice.156 This exercise-induced increase of basal autophagy is required for muscle adaptation and improvement of physical performance in endurance and resistance.157,158 The common regulatory pathways underlying exercise-induced autophagy involve the activation of adenosine monophosphate–activated protein kinase (AMPK)–unc-51-like kinase 1 signaling pathway, forkhead box O (FoxO), and PGC1α, etc.159,160 On the other hand, exercise could play a role in adjusting the level of autophagy in disease states. For example, 8 weeks of treadmill exercise improves cardiac autophagic flux in a rat model of post-myocardial infarction (MI)-induced heart failure.161
Importantly, exercise-induced autophagy seems to mediate many of its beneficial effects.162 Thus, exercise-stimulated autophagy plays an important role in muscle glucose homeostasis. Mice with a BCL2 mutation (Thr69Ala, Ser70Ala, and Ser84Ala) that prevents this exercise-induced increase in autophagy exhibit deficient exercise endurance and deficient glucose metabolism in muscle during acute exercise. Moreover, in such B-cell lymphoma-2 (BCL2)-mutated mice, long-term exercise loses its capacity to prevent type 2 diabetes induced by an HFD.163 It has also been found that exercise stimulates autophagy in the brain.164 As compared to wild-type mice, systemic autophagy-deficient (Atg4b–/–) mice exhibit reduced levels of resistance capacity after exercise training, and exercise-induced hippocampal adult neurogenesis is undetectable within them.158 Altogether, these data highlight the essential role of autophagy in exercise-induced neurogenesis.
Nonetheless, it is worth noting that excessive exercise induces excessive autophagy.165 Thus, the inhibition of autophagy reportedly alleviates heart injury induced by strenuous exercise.165 Ultimately, exercise without overtraining helps to prevent the development and progression of Alzheimer's disease via improving mitochondrial fitness, which may be accomplished by mitigating mitochondrial dysfunction with SIRT1–FoxO 1/3–putative kinase 1–Parkin-mediated mitophagy.166,167
5. The effects of exercise on the different circuitries
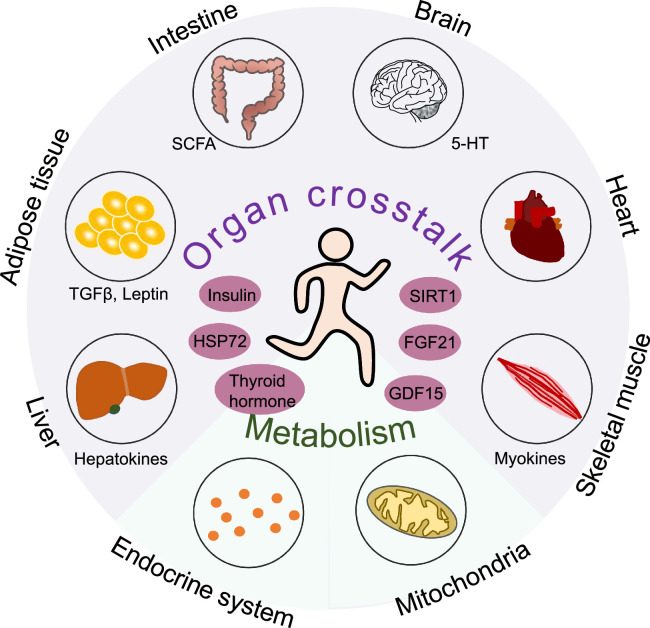
The maintenance of whole-body health involves successful crosstalk among multiple tissues and organs as well as the body microbiota at the cellular and systemic levels. Exercise evokes the coordinated and integrated adaptation of different circuitries, including the intracellular circuitries, inside–outside communication, interorgan communication, and the dialog between microbiota and the organism (Fig. 5).
Fig. 5.
Exercise has systemic effects. Exercise leads to systemic effects by evoking the coordinated and integrated adaptation of multiple organ systems. Exercise results in the secretion of myokines, adipokines (e.g., TGFβ, leptin), and hepatokines as well as by-products of gut microbiota (e.g., SCFA) that have key roles in interorgan communication and, thus, mediate many of the beneficial effects of exercise. 5-HT = serotonin; FGF21 = fibroblast growth factor-21; GDF15 = growth differentiation factor-15; HSP72 = heat shock protein 72; SCFA = short-chain fatty acids; SIRT1 = sirtuin 1; TGFβ = transforming growth factor-β.
5.1. Intracellular circuitries
The health-promoting effects of exercise involve a wide range of metabolic changes, which are mediated by numerous molecules, such as metabolites, proteins, non-coding RNAs, extracellular vesicles, etc. Firstly, exercise modifies the energy sensor AMPK.168 Moreover, ketone bodies are markedly elevated in response to exercise. In addition to serving as a bioenergetic fuel, ketone bodies function as signaling metabolites and confer a variety of benefits for performance and recovery.169 The transcription of genes is also influenced by exercise. For example, exercise can downregulate cardiac homeodomain interacting protein kinase 2, the suppression of which prevents the apoptosis of cardiomyocytes and protects against MI by reducing the activation of tumor protein p53.170 Mice subjected to 3 weeks of swimming exercise exhibit elevated adenosine deaminases acting on RNA2, which is an enzyme that deaminates adenosine to inosine nucleotide in double-stranded RNA and functions as a protective factor in MI and doxorubicin-induced cardiac injury.171 Swimming exercise also upregulates the levels of cardiac miR-486 and lncRNA-CPhar, both of which confer protection against cardiac ischemia/reperfusion injury in mice.149,172 Furthermore, exercise can induce cytokines that prevent myocardial fibrosis.173 Interestingly, circulating miRNAs could be considered biomarkers of the response to exercise.174,175
Exercise-associated health benefits may involve epigenetic changes. For example, exercise directly modulates the epigenetics of cardiac and other tissues, thereby mitigating the risk of developing cardiac disease and conferring cardioprotective effects via exerkines.176 Exercise also improves brain health by reducing DNA methylation in the mouse hippocampus.177
5.2. Inside–outside communication
The endocrine system is strongly affected by exercise. For instance, the expression of fibroblast growth factor-21 (FGF21) increases in response to experimental acute exercise.178, 179, 180, 181, 182 FGF21 is an endocrine member of the FGF superfamily responsible for the modulation of energy homeostasis, glucose and lipid metabolism, and insulin sensitivity. In adipose tissue, exercise training downregulates lipid content and inflammation, promotes browning and thermogenesis, and modulates the production of adipokines.183, 184, 185 Treadmill exercise reportedly protects mice from HFD-induced metabolic dysfunction, including hyperglycemia, hyperinsulinemia, and hyperlipidemia, by enhancing FGF21 sensitivity in adipose tissue.186,187
In addition, the plasma levels of growth differentiation factor-15 increase immediately in runners following a marathon race.188 It has also been induced in response to an experimental single bout of exercise189 and a cycling race.190 The exercise-induced levels of circulating growth differentiation factor-15 correlate with the duration of endurance exercise.191
5.3. Functional units in organs
Exercise is a complex process that involves the coordinated and integrated adaptation of numerous cells, tissues, and organs. In response to chronic exercise training, organs such as the heart and skeletal muscle undergo physiological remodeling and may grow in size, which is called hypertrophy. The plasticity of the organ allows for adaptation to multiple cell types or functional units. For example, cardiac hypertrophy requires increased cardiomyocyte growth, cardiomyocyte fate reprogramming, enhanced angiogenesis and lymphangiogenesis, mitochondrial remodeling, enhanced endothelial function, and quiescent cardiac fibroblasts.146
Exercise protects against pathogenic changes in organs or tissues. For example, a 9-week treadmill exercise program has been shown to reverse the diabetes-induced ultrastructure changes in the heart. Specifically, in diabetic rats, exercise inhibits the reduction of mitochondrial mass, decreases the percentage of dysfunctional mitochondria, and prevents the increase of collagen fiber cross-sectional surface area in the heart.192 In aging rats, a 3-week treadmill exercise program increases angiogenesis, leading to improved brain function.193 High-intensity interval exercise (5 days a week for 7 weeks)-induced increase of vascularization is mediated via the lactate receptor (hydroxycarboxylic acid receptor 1), which upregulates cerebral vascular endothelial growth factor A (VEGFA).194
5.4. Organs, tracts, and systemic circuitries
Exercise induces adaptive responses throughout the entire body. For example, exercise stimulates the upregulation of heat shock proteins (HSPs),195 which are a widespread family of molecular chaperones that play a variety of vital roles in maintaining cellular functions via regulation of chaperone activity, protein folding, and remodeling processes.196 Specifically, HSP72 is upregulated in the circulation system and multiple organs (including the liver, skeletal muscle, heart, brain, etc.) in response to various forms and types of exercise.197 Overexpression of HSP72 (induced by heat shock therapy, transgenesis, or specific drugs) protects against HFD-induced glucose intolerance and skeletal muscle insulin resistance.198,199 Moreover, induction of HSP72 in mice increases mitochondrial mass and endurance running capacity.200 Therefore, the upregulation of HSP72 might account for at least some of the beneficial effects of exercise on diabetes, obesity, and ischemic brain injury.201,202 However, the precise role of exercise-induced HSP72 expression in treating metabolic diseases warrants further investigation.
Exercise induces numerous adaptive responses in tissues and organ systems as well as with respect to interorgan communication. Many of these adaptations are observed primarily in skeletal muscle, which plays a major role in modulating whole-body functional capacity and health outcomes. In response to exercise, skeletal muscle secrets myokines, extracellular vehicles, and metabolites that facilitate crosstalk with other tissues. This crosstalk is thought to benefit the function of multiple systems and organs, such as the brain, heart, adipose tissue, gut, immune system, etc. It is noteworthy that the adaptive responses are not limited to skeletal muscle. Exercise also results in the secretion of adipokines (e.g., transforming growth factor β, leptin) and hepatokines as well as by-products of gut microbiota (e.g., short-chain fatty acids (SCFA)) that play key roles in interorgan communication and, thus, mediate many of the beneficial effects of exercise.203 Furthermore, exercise has been shown to modulate the composition of the gut microbiome, which may mediate improvements in both the gut and the brain. Exercise could also increase levels of serotonin, which is a vital neurotransmitter and hormone and is involved in the regulation of intestinal secretion and motility.204 In addition, a recent study reported that the extracellular vehicles secreted by brown adipose tissue contribute to exercise-mediated cardioprotection by delivering cardioprotective miRNAs to the heart.205
5.5. The meta-organism
It is well-known that the gut microbiota plays an essential role in many aspects of human biology, affecting metabolism, endocrine, and immune functions. Dysbiosis of the gut microbiome is associated with multiple pathological states, including systemic inflammation, obesity, cancer, mental disorders, and others.206 Beyond its local effects on the barrier function of the intestinal mucosa, the gut microbiota provides several compounds and micronutrients that mediates long-range effects on the immune and neuroendocrine systems.207,208
Endurance and resistance exercise have been shown to have a differential effect on the gut microbiota of mice.209 Moreover, a number of studies have determined that exercise influences the gut by enhancing the number of beneficial microbial species, thereby enriching microbiota diversity and functional metabolism in both humans and mice.206,210, 211, 212 Thus male elite professional rugby players have lower inflammatory and improved metabolic markers relative to healthy controls with a similar body mass index. As compared to controls, athletes carry a more diverse gut microbiota, and several taxa are significantly higher.213 In mice, antibiotic-mediated depletion of gut microbiota compromises exercise performance.214 In addition, monocolonization of germ-free mice with Bacteroides fragilis, which is a health-associated gram-negative bacterium, increases endurance exercise time.214
In sum, exercise-induced shifts in the microbial composition of the gut may contribute to the beneficial effects of physical activity on obesity, metabolic diseases, and neurological disorders.215 Indeed, exercise-induced alteration of the gut microbiome may regulate brain function.204 For example, SCFA, as microbiota-derived products, favor microglia maturation and function in the brain. Supplementation of SCFA is capable of reversing the microglial defects observed in mice lacking a healthy gut microbiota.216
6. The effects of exercise on rhythmic oscillations
The circadian rhythms represent a number of daily oscillations in physiology and behavior that take place over a 24-h period and enable the body and brain to adapt to daily changes in the environment. Virtually all cells of the body express a molecular clock that requires extremely precise regulation. Exercise is a nonphotic cue capable of restoring the transiently disturbed clock.217 Here we present examples of how exercise modulates the circadian rhythms.
6.1. Mechanics of the circadian clock
The circadian rhythms are a common trait among practically all living organisms. Accumulating evidence reveals that disruption of mammalian circadian rhythms is deleterious to health. For instance, mice with muscle-specific deletion of Bmal1, which is the core clock gene, exhibit impaired insulin-stimulated glucose uptake and metabolism, indicating that disruption of the intrinsic muscle clock system leads to muscle insulin resistance.218 In addition, night shift work-related disturbance of the circadian clock is associated with a higher risk of type 2 diabetes in humans.219,220
Several reviews have highlighted the positive effects of exercise on circadian disturbances.221, 222, 223 In particular, exercise acts as a strong zeitgeber (time cue) for the skeletal muscle clock.224 We know that exercise is capable of readjusting the molecular circadian clock and, thus, reducing the harmful effects of disrupted sleep patterns;221,225 however, exercising at different times of the day can lead to markedly different stimulation of signaling pathways and exercise performance.226,227 In a recent study, mice exhibited better performance in the maximal running test during the early daytime, and clear differences are observed in the muscle transcriptome, proteome, and phosphoproteome patterns of mice subjected to day-time exercise as compared to those subjected to night-time exercise.228 However, few studies have focused on how to apply physical exercise to achieve the restoration of circadian rhythms or on the metabolic consequences and associated health outcomes of exercising at different times of the day.
6.2. Exercise contributes to the maintenance of immune rhythmicity
The immune system exhibits rhythmicity, and the rhythmic expression of clock genes has been implicated in both innate and adaptive immune cells.229,230 Moreover, the impairment of clock gene signaling causes chronic inflammation or aggravates the symptoms in older adults.231 Evidence indicates that lifelong regular exercise (no less than 20 years) decelerates the aging-related decline of immune system function and contributes to the maintenance of circadian rhythms throughout life.131,232,233 In addition, clock gene expression patterns following acute exhaustive exercise are different in lifelong athletes compared to those in the sedentary group. Specifically, the acute bout of exercise modulates the clock machinery mainly in effector-memory CD4+ T cells.234
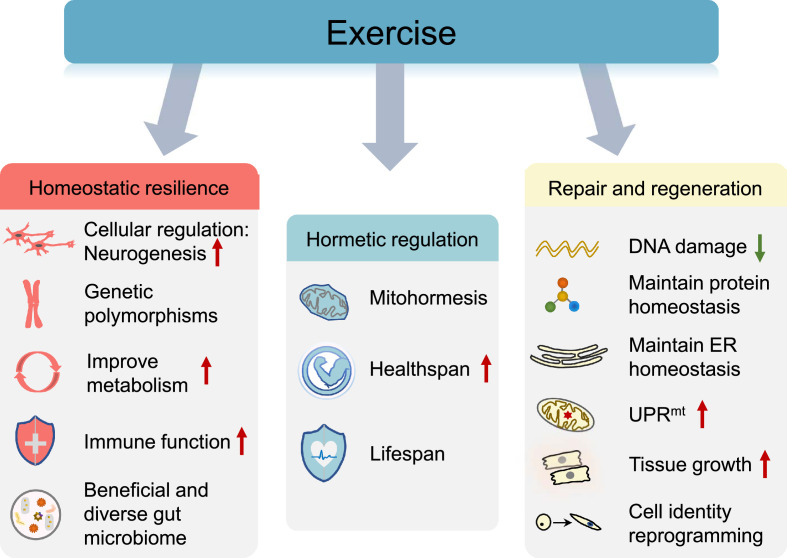
7. Benefits of exercise in homeostatic resilience
Physiological homeostasis is a major requisite for the survival of any organism. However, exposure to stressful life events is common. Hence, the maintenance of positive adaptation by individuals in the face of stress is important. Resistance to environmental risk experience or adaptation to stress/adversity is known as “resilience”,235 and resilience can be regulated via neural, genetic, metabolic, immunological, and microbiome-based mechanisms. Exercise appears to be a protective strategy for improving homeostatic resilience and preserving human health and a good quality of life. Engaging in exercise that leads to improved cardiorespiratory fitness can reduce the risk of various diseases, and these beneficial effects involve the widespread adaptations of multiple tissues (Fig. 6). Here we present examples of how exercise modulates homeostatic resilience.
Fig. 6.
Exercise-associated beneficial adaptations in response to stress. Exercise confers multiple health benefits via different strategies, including improving resilience, hormetic regulation, and modulating the process of repair and regeneration in response to damage. ER = endoplasmic reticulum; UPRmt = mitochondrial unfolded protein response.
7.1. Neural mechanisms
The brain is the key organ of stress response. Stress and stress hormones cause both adaptive and maladaptive effects on the brain throughout life.236 Moderate-intensity exercise improves brain function and helps to maintain brain health.237,238 Exercise elicits a wide range of biological changes in the nervous system. In addition to alleviating clinical symptoms, the advantageous effects of exercise include improved afferent signals, autonomic balance, mitochondrial function, increased neurogenesis, and synaptic plasticity, as well as improved metabolism and angiogenesis.238, 239, 240, 241, 242
Adult hippocampal neurogenesis is a complex and highly dynamic process that can be regulated by exercise.243 Mice subjected to endurance training (voluntary wheel running and treadmill running) and resistance training (climb the ladder) are associated with a significantly increased rate of proliferation of neural progenitor cells, indicating enhanced neurogenesis.158,244 Several receptor signaling cascades have been implicated in the exercise-modulated enhancement of adult hippocampal neurogenesis, including neurotrophic factors, such as BDNF and neurotransmitters.245,246 In addition, dexamethasone-induced Ras-related protein 1 (Dexras1), a small GTPase, modulates exercise-induced neurogenesis in the adult hippocampus. Dexras1 deletion leads to a reduced proliferation of neural progenitors, suppressed pro-mitogenic pathways, and an increased number of mature newborn granule neurons in exercised mice. At the molecular level, the deletion of dexamethasone-induced Ras-related protein 1 abolishes exercise-triggered activation of extracellular signal-regulated kinase and cyclic adenosine monophosphate response element binding protein, and it prevents the increase of N-methyl-D-aspartate receptor subunit NR2A, Bdnf, Vegf-a, and tropomyosin receptor kinase B in the dentate gyrus.247
7.2. Genetic factors
Genetic factors have been found to influence an individual's level of resilience. Results from adult twin studies show that between 31% and 52% of observed individual differences in resilience to stressful life events can be explained by genetic factors.248, 249, 250 Research suggests that the BDNF and neurotrophic tyrosine kinase receptor type 2 gene polymorphisms are associated with stress tolerance.251,252 In addition, the BDNF polymorphism appears to modulate exercise-induced cognitive benefits. For example, exercise-induced cognitive improvement has been observed to be more profound in terms of the mental status and word recall of individuals carrying the BDNF Val66Met variant than of individuals with the BDNF Val66Val genotype (wild type).253
Interestingly, genetic factors appear to influence exercise participation. For example, a study based on the exercise participation of 37,051 pairs of twins from 7 countries (Australia, Denmark, Finland, the Netherlands, Norway, Sweden, and the United Kingdom) shows that the resemblance in exercise participation during leisure time of monozygotic (genetically identical) twins is higher than that for dizygotic twins, who share about half of their segregating genes on average.254 Moreover, genetic factors may confound the observed association between low levels of physical activity and higher disease and mortality risk.255, 256, 257
In addition, several genetic polymorphisms related to elite athletes seem to be associated with exercise performance.258 For example, elite-level athletes show higher frequency of the IGF1 promoter T allele polymorphism.259 Notably, the effect of these gene variations seems to be influenced by type of exercise. The prevalence of carrying both IL-6 G174C and insulin-like growth factor-binding protein 3 (IGFBP3) A202C mutations is obviously higher among long-distance swimmers (major event: 400–1500 m) compared to long-distance runners (major event: 5000 m marathon), and among short-distance swimmers (major event: 50–100 m) compared to power athletes (major event 100–200 m sprinters and jumpers). The IGFBP3 A202C polymorphism may compensate for the potential genetically non-beneficial effect of the higher IL-6 G174C.260 In addition, the IGF1 C1245T (rs35767) single nucleotide polymorphism with the minor T allele is significantly associated with higher circulating IGF1 concentrations.261 In summary, exercise-related genetic polymorphisms at least partly contribute to the maintenance of body health.
Of note, current Mendelian randomization studies have reported findings that conflict with those of traditional observational studies. For example, observational studies show that the level of physical activity is inversely correlated with the risk of several diseases, including type 2 diabetes and cardiovascular disease. However, the two-sample Mendelian randomization study reveals that physical (in)activity is unrelated to the risk of type 2 diabetes262 and cardiovascular disease, including coronary artery disease, MI, and ischemic stroke.263 Thus, future work should pay more attention to the biological processes influenced by exercise as well as to the underlying mechanisms, which may explain the confusing results.
7.3. Hormones and metabolism
Exercise is a powerful regulator of metabolism. Generally, aerobic and resistance exercise training is associated with improved metabolic health. Organs such as skeletal muscle, the liver, the brain, and adipose tissue are important metabolic communicators, and interorgan communication during exercise contributes to whole-body homeostasis.264 Exercise-induced increase of energy demand is associated with increased mobilization of substrates, including carbohydrate, fat, and glucose, during and after exercise.265 The utilization of extracellular substrates is partly mediated by rapid and coordinated changes in the endocrine system. The hormones associated with the endocrine system exhibit autocrine, paracrine, or endocrine actions on the tissues of the body that regulate a variety of processes, such as growth and development, metabolism, and immune response.266,267 Exercise causes strong effects on the release of these hormone-like substances and thus the processes these substances modulate.6 Two major hormones whose functions are influenced by exercise are insulin and thyroid hormone. Exercise increases insulin sensitivity through several molecular pathways, including upregulation of glucose transporter protein 4 in the cell membrane of insulin-dependent cells, improvement of β cell function, modulation of insulin receptor substrate 1 phosphorylation, induction of angiogenesis, reduction in adipokines, improvement of the redox state, and downregulation of ceramide plasma levels.268 Therefore, exercise has been recommended as the management strategy for patients diagnosed with type 2 diabetes.269 Changes in thyroid hormone levels (e.g., T3, T4) are observed following exercise. However, it is hard to draw any conclusions concerning the effects of exercise on thyroid hormone.270 These controversial results could be due to the physical status of subjects, the type, intensity, and duration of exercise, or the differences in age and gender among subjects. In general, the effects of exercise on the endocrine system are of a positive nature and help to improve functional aspects of tissue and organs, resulting in improved health. For instance, exercise prevents the loss of activity of the endocrine system that is associated with aging.271
Metabolic adaptation is related to the specific type and mode of exercise. For example, regular endurance exercise enhances muscle capillarity, increases mitochondrial biogenesis, and promotes the fiber-type shift toward the type 1 phenotype, but it has no significant effect on the fiber cross-sectional area. On the contrary, strength training induces muscle hypertrophy, mainly due to the greater muscle fiber cross-sectional area.265
7.4. Immune system
The immune system responds to any type of insult that challenges cellular homeostasis. While a single bout of exercise may represent a perturbation to homeostasis, long-term moderate-intensity exercise can benefit the immune system by enhancing immunosurveillance and immunocompetence.272 Regular exercise leads to an elevated anti-inflammatory capacity, which is a critical factor in maintaining health, particularly in the context of chronic diseases.273 For example, the number of inflammatory CD14+CD16+ monocytes decreases after 12 weeks of combined moderate-strength and endurance training, indicating a reduction of more proinflammatory monocyte subtypes.116 In addition, acute bouts of exercise also elicit beneficial effects on immunological health partly through transiently mobilizing immune cells to peripheral tissues to conduct immune surveillance.274
Long-term exercise can prevent the age-associated degradation of immune system function (known as immunosenescence), which is characterized by considerable shifts in the balance of leukocyte subsets and a decline in various immune cell functions.275 As an example, long-term exercise from adulthood to old age (training time = 49 ± 8 years (mean ± SD)) induces a healthy profile of inflammatory-related cytokines, as indicated by a decline in inflammatory biomarkers, including decreased levels of white blood cell counts, neutrophil counts, IL-6, IL-10, IL-1 receptor antagonist, and soluble tumor necrosis factor receptor-1.118 Additionally, participation in long-term (training time = 45.1 ± 11.0 years) easy- to moderate-intensity exercise is associated with a more differentiated adaptive immune response.123 Furthermore, athletes with lifelong aerobic training have a lower percentage of senescent T cells when compared to healthy controls,232 suggesting that exercise has a beneficial effect on immune function.
7.5. Gut microbiota
The human gut microbiota contains numerous microorganisms essential to maintaining homeostasis and normal gut physiology. Gut microbiota composition is highly variable. Each individual is linked to a unique gut microbiota profile that plays an important role in their metabolism and in the maintenance of a healthy host microorganism. Sometimes, the gut microbiota composition may vary within a single individual276 because the gut's ecosystem development and stability can be affected by intrinsic and extrinsic factors, including exercise and diet, which in turn can influence health.277
Exercise-induced alterations in the diversity, composition, and functional profiles of the intestinal microbiome have been widely documented.278,279 Moderate endurance exercise has been shown to expand the number of beneficial microbial species, enrich microflora diversity, and promote the development of commensal bacteria.215,278 All these effects are beneficial for the host and contribute to the improvement of their health status. Indeed, elite athletes elicit uniquely beneficial and diverse gut microbiome characteristics, including a higher gut microbial diversity and a shift toward bacterial species associated with increased production of the SCFA implicated in the maintenance of intestinal barrier integrity and regulation of host immune function.280 Furthermore, exercise-induced gut microbial changes confer protection against certain diseases. For example, treadmill running exercise ameliorates cardiac dysfunction in MI mice by altering the diversity and community structure of the gut microbiome. Mechanistically, exercise increases the metabolites 3-hydroxyphenylacetic acid (3-HA) and 4-HA, which are thought to protect against cardiac dysfunction by activating nuclear factor E2-related factor 2 (Nrf2).281
Interestingly, the composition of the gut microbiota can be differently modulated by distinct types and modes of exercise. For instance, 4 weeks of resistance and endurance exercise training leads to significant differences in the gut microbiota composition in mice.209 Accordingly, mice subjected to endurance training exhibit higher diversity and evenness of gut microbiota. Specifically, endurance training is associated with a greater relative abundance of Desulfovibrio and Desulfovibrio sp., while Clostridium and C.cocleatum are enriched in mice that undergo resistance training.209
8. Benefits of exercise in hormetic regulation
There is some evidence that mild stressors inducing hormesis can contribute to the extension of lifespan and healthspan.282 A single bout of exercise represents a challenge to cellular homeostasis. However, regular exercise confers multiple health benefits and better survival (Fig. 7). Therefore, it is possible that the beneficial outcomes of exercise may be achieved partly by hormetic regulation (Fig. 6).
Fig. 7.
Regular exercise confers multiple health benefits and better survival. The adaptations in multiple tissues and organ systems contribute to the maintenance of whole-body health. AMPK = adenosine monophosphate–activated protein kinase; BDNF = brain-derived neurotrophic factor; CaMK = calcium/calmodulin-dependent protein kinase; GPx = glutathione peroxidase; IGF1 = insulin-like growth factor 1; MAPK = mitogen-activated protein kinase; NGF = nerve growth factor; NK cells = natural killer cells; NO = nitric oxide; PGC1α = peroxisome-proliferator-activated receptor coactivator 1α; SIRTs = sirtuins; SOD = superoxide dismutase; VEGF = vascular endothelial growth factor; VEGFA = vascular endothelial growth factor A; VEGFR3 = vascular endothelial growth factor receptor 3.
8.1. Exercise and mitohormesis
Mitohormesis is characterized as hormesis in mitochondria and is associated with the adaptive response processes that maintain cellular homeostasis. In response to a disturbance, mitochondria can generate and transmit a signal to the nucleus that triggers a transcriptional response leading to mitochondrial and non-mitochondrial adaptations. Although direct evidence linking mitohormesis to the exercise response is limited, mounting studies suggest that mitohormesis plays a role in mediating exercise-induced adaptions. Various signals are released from the mitochondria in response to exercise, with ROS being the primary candidate for inducing mitohormetic response.283 Mitochondrial ROS are vital signaling molecules necessary for health-promoting and lifespan-extending effects.284 The benefits of exercise are at least partly regulated by ROS signaling,285 and Nrf2 is one of the important regulators that mediates redox homeostasis during exercise. Exercise induces the activation of Nrf2, which modulates the gene level of endogenous antioxidant synthesis and ROS-eliminating enzymes.286 However, low-level and high-level mitochondrial stress could lead to opposing metabolic effects. In a recent study, severe mitochondrial stress due to mitoribosomal protein CR6-interacting factor 1 (Crif1) homo deficiency in hypothalamic proopiomelanocortin neurons of mice appeared to cause adult-onset obesity while mild mitochondrial stress caused by Crif1 heterodeficiency in proopiomelanocortin neurons was associated with higher metabolic turnover and resistance to diet-induced obesity in mice. The beneficial metabolic effects induced by mild stress are mediated by enhanced thermogenesis and mitochondrial unfolded protein responses (UPRmt) in distal adipose tissue. Interestingly, regular exercise with moderate intensity resembles the metabolic phenotype of Crif1 heterodeficiency, indicating the important regulatory role of mitohormesis in moderate-intensity regular exercise.287
8.2. The benefits of exercise on healthspan
Healthspan is characterized as healthy life expectancy or the period of life spent free of illness and disability.288 In addition to a healthy diet and mental well-being, the positive effects of regular moderate exercise on the prevention, treatment, and management of chronic disease affecting healthspan are well-established. For example, in a landmark 21-year longitudinal study, it is reported that participation in long-term running and other vigorous exercises among older adults is associated with less disability and lower mortality in later life.19 Regular exercise induces systemic adaptations in virtually all organ systems, producing multifaceted benefits for health. In particular, exercise can attenuate multi-system age-related declines and help maintain physical fitness generally, including cardiorespiratory fitness and muscle function as well as flexibility and balance. The mechanisms through which exercise improves cardiorespiratory fitness include nitric oxide-induced vasodilatation, VEGFA-mediated angiogenesis, and VEGFR3-mediated lymphangiogenesis, etc.146,289 The main signaling pathways involved in the effects of exercise on muscle include calcium/calmodulin-dependent protein kinase signaling, MAPK signaling, AMPK signaling, SIRTs, PGC1α, etc.290,291
Moreover, exercise benefits brain health via antioxidant defense (e.g., glutathione peroxidase, superoxide dismutase), neurotrophic factors (e.g., BDNF, VEGF, nerve growth factor), neurogenesis (e.g., BDNF, VEGF, IGF1), and protein homeostasis (e.g., proteasomes, neprilysin, tau ubiquitination), etc.291 Exercise also reduces adipocyte size and lipid content, increases mitochondrial activity, and alters adipokines secretion in white adipose tissue.292 In mice, the exercise-induced adaptations in white adipose tissue improve glucose tolerance and insulin sensitivity, indicating improved whole-body metabolic health.293,294 These positive effects of exercise are supposed to be mediated by exercise training-induced adipokines.294 Furthermore, exercise may enhance immune function and prevent immunosenescence, as explained in Section 7.2. Altogether, adaptations in multiple tissues and organ systems contribute to the maintenance of whole-body health. Thus, exercise has been proposed as medicine to delay dependence and prolong healthspan.295
8.3. The effects of exercise on lifespan
A large body of studies suggest that exercise is a promising intervention for extending a healthy lifespan.296,297 The positive effects of exercise on lifespan are mainly achieved through the attenuation of aging phenotypes or by delaying the process of aging. One of the essential regulators of the antiaging effects of exercise is AMPK.298 Activating AMPK is sufficient to promote longevity in multiple organisms.299,300 In muscle specifically, AMPK is an important regulator of exercise effects that are activated by increased AMP/ATP and (nicotinamide adenine dinucleotide/ nicotinamide adenine dinucleotide hydride) ratios.301
Generally, regular exercise is considered to be fundamental to overall health and longevity. However, some of the commonly ascribed beneficial effects of exercise seem to be unsupported by current randomized control trial studies. For example, current randomized control trial evidence shows that exercise does not prevent premature mortality or cardiovascular disease in older adults or in people with chronic conditions.302 Thus, additional large-scale investigations would be of value, especially high-quality studies with insights on molecular mechanisms.
9. The effects of exercise on repair and regeneration
The prompt repair and regeneration response to damage is a hallmark of health.28 Here we present examples of how exercise modulates the process of repair and regeneration in response to damage (Fig. 6).
9.1. DNA damage and repair
Oxidative DNA damage is associated with increased risk of chronic diseases, such as cancer, neurodegenerative disease, atherosclerosis, and diabetes.303,304 Therefore, interventions aimed at repairing DNA strand breaks or lesions could be useful for inhibiting or delaying the progression of disease. Regular moderate exercise confers substantial health benefits, including a lower risk of the above-mentioned diseases.24
Exercise has been directly implicated in the enhancement of DNA damage repair. As an example, 8 weeks of treadmill running leads to a decreased accumulation of oxidative damage to DNA and proteins in rat skeletal muscle.305 Moreover, exercise may promote the inherent DNA repair abilities of neurons through upregulation of BDNF.306 Several mechanisms have been implicated in the decrease of exercise-associated oxidative damage in the brain, including upregulation of antioxidant capacity, reduced production of oxidants, and consequently reduced nuclear DNA damage.307 However, acute and strenuous exercise could induce oxidative stress via increased accumulation of reactive oxygen and nitrogen species, which may have negative effects on DNA stability.308 Additionally, high-intensity normoxic exercise induces DNA double-strand breaks, as revealed by increased detection of gamma-H2A histone family member X and p53-binding protein 1. Furthermore, levels of these oxidative stress biomarkers are significantly increased following exercise in hypoxia.309
Therefore, a better understanding of the effects of different types of exercise on DNA damage and repair is necessary. Future studies focusing on exercise-induced mechanisms are critical for developing better approaches to improving health.
9.2. Protein damage and proteostasis
Proteostasis refers to the dynamic processes contributing to the maintenance of protein homeostasis, which depends on adjustments to protein synthesis and degradation. Disturbance of proteostasis contributes to the pathogenesis of many degenerative diseases through the aggregation of damaged proteins.310,311 Regular exercise induces substantial positive effects on health, with muscle being its predominant effector.312 For example, aerobic exercise increases the capacity for maintaining mitochondrial proteostasis in skeletal and heart muscle.313 Regular voluntary wheel running reduces the accumulation of cytotoxic misfolded proteins in the heart of αB-crystallin mutated (CryABR120G) mice, a model of desmin-related cardiomyopathy, by increasing autophagy.314 One week of exercise training could reverse angiotensin II–induced muscle atrophy by activating peroxisome proliferator-activated receptor gamma and inhibiting miR-29b.315 Exercise also causes protein degradation followed by a similar scale rebuilding process, thus maintaining protein homeostasis.316
Moreover, exercise activates autophagy and re-establishes proteostasis in cardiac diseases. For example, 4 weeks of treadmill running improves skeletal muscle autophagic flux and proteostasis in the rat model of neurogenic myopathy.317
9.3. The ER stress response
The ER is a versatile organelle that involves the synthesis and folding of proteins as well as calcium homeostasis and signaling. Perturbation of ER function causes ER stress and subsequently activates the unfolded protein response (UPR), which is conducive to cellular homeostasis. However, if the ER stress remains unmitigated or is too strong and the UPR fails to restore ER function, the cells may degrade part of the ER via autophagy in order to maintain homeostasis in the ER. Defects in ER homeostasis have been implicated in the pathogenesis of metabolic diseases.318,319 Exercise training represents a promising strategy for reestablishing ER homeostasis in cardiovascular diseases and obesity. For instance, 8 weeks of treadmill exercise can reestablish cardiac protein quality control and reduce ER stress, which is associated with improved cardiac function in the failed hearts of rats.320 Moreover, 8 weeks of swimming exercise is capable of suppressing aging-induced ER stress and ameliorating cardiac dysfunction in aged mice by enhancing cyclic guanosine monophosphate (cGMP)–protein kinase G signaling. Likewise, the inhibition of cGMP-specific phosphodiesterase type 5 leads to reduced ER stress and improved cardiac function in aged mice while activation of ER stress eliminates the protective effect of exercise in the same population.321 Furthermore, in humans, 3 months of moderate exercise alleviates obesity-related ER stress by downregulating glucose-regulated protein 78 signaling.322 In a mouse model of Alzheimer's disease, exercise improves vascular function and attenuates ER stress.323
Additionally, in a recent study, acute exercise is reported to activate ER-phagy, which is a selective ER autophagy regulated by ER-localized receptors, in order to ensure proper cellular homeostasis. Mechanistically, ER-phagy receptor named family with sequence similarity 134, member B anchors and fragments ER and then binds with microtubule-associated protein light-chain-3 to promote ER membrane degradation.324 This study suggests that acute exercise-induced ER-phagy may serve as an important adaptive stress response to maintain ER homeostasis.
9.4. Mitochondrial stress responses
Mitochondria are essential semi-autonomous cellular organelles that are maintained through complex remodeling processes, including biogenesis, dynamic fission and fusion, and mitophagy. These remodeling processes collectively contribute to mitochondria quality control. Mitochondrial stress induced by aggravated misfolded mitochondria proteins in the mitochondrial matrix, an impaired protein quality control system, mitonuclear imbalance, or inhibition of the electron transport chain can trigger the UPRmt.325
Regular endurance exercise is a powerful stimulus that promotes mitochondrial remodeling. Activation of the UPRmt may mediate some of the exercise-induced mitochondrial adaptations.326 For example, the activation of UPRmt is observed in skeletal muscle during the acute phase of the contractile period and chronic contractile activity.327
Exercise also increases levels of the mitochondrial encoded peptide mitochondrial open-reading-frame of the 12S rRNA-c, which can directly mediate mitonuclear communication by translocating to the nucleus and regulating adaptive nuclear gene expression to promote cellular homeostasis. Treatment of mice with mitochondrial open-reading-frame of the 12S rRNA-c is associated with metabolic benefits, such as weight loss, enhanced antioxidant capacity, and increased insulin sensitivity.328,329
9.5. Lysosomal damage response
Lysosomes are the main organelles that account for proteolytic degradation and recycling.330 Exercise influences the release of lysosomal enzymes in several cell types,331,332 suggesting that physical activity could modulate lysosomal membrane permeability. Thus, 4 weeks of aerobic exercise could mitigate the HFD-associated lysosomal membrane permeabilization via the reduction of ROS.333 However, studies focused on exercise-induced changes in lysosomal adaptations are still limited.
Finally, lysosomal biogenesis is also elevated in response to exercise. The increased lysosomal biogenesis coordinates with mitophagy to enhance the breakdown of dysfunctional mitochondria, thereby contributing to the maintenance of mitochondrial quality.334
9.6. Tissue-level regeneration
The positive effects of exercise on cardiac regeneration are well-established. Regular endurance exercise is capable of promoting heart growth and inducing cardiac remodeling.335,336 An enlarged heart due to regular exercise training is associated with normal or enhanced heart function. Moreover, it is well-known that exercise confers protection against heart failure.337 Mechanistically, exercise-induced cardiac adaptation mainly includes cardiomyocyte growth, cardiomyocyte fate reprogramming, angiogenesis and lymphangiogenesis, mitochondrial remodeling, epigenetic alteration, enhanced endothelial function, quiescent cardiac fibroblast, and improved cardiac metabolism.146,338 For instance, 3 weeks of swimming exercise causes a remarkable increase in cardiac lymphangiogenesis via VEGFR3 activation. By contrast, VEGFR3 inhibition is capable of mitigating exercise-induced cardiac growth, indicating the essential role of cardiac lymphangiogenesis in exercise-associated cardiac growth.339 In particular, IGF1–PI3K signaling is one of the essential pathways responsible for exercise-related heart enlargement and protection.340
Skeletal muscle exhibits high plasticity and adaptation in response to various demands. Adult skeletal muscle has the remarkable ability to regenerate after injury due to the ability of muscle stem cells, also called satellite cells, to generate both new myofibers and new muscle stem cells.341 Exercise training is a strong stimulator of muscle plasticity, as it activates several intracellular signaling pathways that mediate muscle growth and adaptations.342 For example, 4 weeks of treadmill training improves muscle regeneration and induces muscle hypertrophy via the IGFBP7–Akt–mTOR axis in mice.343 Moderate exercise improves tendon injury healing in aging rats by increasing the number of tendon stem/progenitor cells.344
9.7. Cell identity reprogramming
Research studies demonstrate that exercise training can trigger metabolic reprogramming in multiple cell types and, thus, alter their functional phenotypes. For example, 16 weeks of treadmill exercise induces macrophage phenotypic switching from M1 to M2 in the adipose tissue of obese mice, indicating improved inflammatory outcomes in exercised obese mice.345 Additionally, 10 weeks of treadmill running could promote the differentiation of mesenchymal stem cells into osteoblasts to increase hematopoiesis.346 Moreover, 4 weeks of exercise training before surgery offers protection against hepatic injury via the metabolic reprogramming of immune cells. Specifically, exercise encourages Kupffer cells toward an anti-inflammatory phenotype with trained immunity.347
10. Integrative effects of exercise on health
Exercise maintains and restores homeostasis at the organismal, tissue, cellular, and molecular levels to stimulate positive physiological adaptations and, subsequently, to protect against various disease conditions.27 The health benefits of regular exercise training involve the acclimatization to repeated metabolic, thermoregulatory, hypoxic, oxidative, and mechanical stress. An individual exercise bout elicits a rapid but transient response while the repetition of the stimulus causes compensatory physiological changes that confer protection against greater subsequent stress.348,349
Exercise elicits systemic impacts. The preventive and therapeutic potential of exercise to combat the development and progression of various diseases lies in the remarkable integrative adaptation that takes place at the whole-organism level. Exercise-related physiological adaptations are characterized by complex interplay among multiple organ systems, including the skeletal muscle, lung, heart, brain, vascular system, liver, adipose tissue, etc.350,351 Exercise therapy has been proposed as an effective pre-operative therapy providing a series of positive effects on a patient's physical fitness prior to surgery, on their recovery period, and on the length of hospital stay.352, 353, 354 It has been suggested the protective effects of pre-operative exercise are largely due to an enhanced tolerance to systemic perturbance as well as an improved metabolism. For example, 4 weeks of aerobic pre-operative exercise is capable of significantly ameliorating liver injury and inflammation in mice with hepatic ischemia/reperfusion injury. Mechanistically, exercise promotes the production of high mobility group box 1 and upregulates the itaconate metabolism in Kupffer cells, thereby inducing anti-inflammatory trained immunity.347
11. Conclusion and prospects
The beneficial effects of exercise have been well-documented for a range of improved metabolic and cardiorespiratory fitness-related outcomes.355 Regular exercise is essentially considered a non-pharmacological polypill for patients with certain comorbidities. Therapeutic exercise can be adopted to prevent, manage, and treat diseases such as obesity, type 2 diabetes, cardiovascular disease, aging-related muscle atrophy, and certain cancers. However, caution should be taken when interpreting these discoveries, as exercise protocols may vary across studies.
Exercise is a complex, multidimensional process that is challenging to accurately assess in free-living individuals. The lack of consistency in the methods applied to quantify exercise is a limitation to data analysis and may hinder the translation from experimental findings to clinical practice. Thus, future work involving exercise should be performed based on strictly designed and well-reported trials with good reproducibility.356
In this review, we have attempted to summarize the health benefits of exercise from the view of biological causes. Due to limited space, it is hard to provide a comprehensive review for each individual topic. However, the topics and characteristics that have been discussed may provide a mechanistic view for exercise-related studies. As the biology of exercise is complex, we have listed examples to explain the effects of exercise on each topic and characteristic, which may be helpful for gaining a comprehensive understanding of exercise-induced adaptations.
Regarding the conflicting findings between current Mendelian randomization studies and traditional observational studies in Section 7.2, future researchers should perform more mechanistic studies in order to unravel the biological pathways of exercise. In addition, exercise-stimulated interorgan communication is less well-investigated. Further detailed study of exercise-related interorgan communication and its underlying mechanisms may shed light on the identification of exercise-responsive molecules. Regular exercise is undoubtedly beneficial for overall health. Yet, the mechanisms that underlie the multiple health benefits of exercise are complex, and efforts devoted to exploring the adaptation of organelles (e.g., mitochondria, nuclei, ER) may be helpful for understanding the positive effects of exercise.
Further investigation of the mechanisms underlying the beneficial effects of exercise will help to improve the design and maximize the curative effects of exercise interventions. Therefore, there is a need for prior in-depth knowledge of the scientific basis of exercise in order to better understand the effects of potential subsequent interventions oriented toward the modification of exercise methods for health promotion and to research specific molecules that may improve the health of those who are unable to exercise. We hope that continuing investigation in this field will increase our understanding of the processes involved in the beneficial role of exercise and facilitate the identification of new therapeutics to improve quality of life.
Acknowledgments
Acknowledgments
This work was supported by grants from the National Key Research and Development Project (2018YFE0113500 to JX), the National Natural Science Foundation of China (82020108002 and 82225005 to JX), the Science and Technology Commission of Shanghai Municipality (21XD1421300 and 20DZ2255400 to JX), the “Dawn” Program of Shanghai Education Commission (19SG34 to JX), and the Sailing Program from Science and Technology Commission of Shanghai (21YF1413700 to YQ). GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR)-Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); Gustave Roussy Odyssea, the European Union Horizon 2020 Projects Oncobiome and Crimson; Fondation Carrefour; Institut National du Cancer (INCa); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); a Cancer Research ASPIRE Award from the Mark Foundation; the RHU Immunolife; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001. CLO is supported by the European Union (DeAge ERC Advanced Grant), the Instituto de Salud Carlos III, Ministerio de Sanidad (Spain), and the SAF program from Ministerio de Ciencia e Innovación (Spain). BFG is supported by La Red de Investigación en Ciencias del Deporte “in motu salus”, Consejo Superior de Deportes, Spain.
Authors’ contributions
YQ and BFG drafted the manuscript; GK, CLO, and JX came up with the idea and edited the manuscript; HIL and GL reviewed and edited the manuscript. All authors have read and approved the final version of this manuscript, and agree with the order of presentation of the authors.
Competing interests
GK has held research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Sotio, Tollys, Vascage, and Vasculox/Tioma. GK has consulted for Reithera and is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics, and Therafast Bio. GK is the inventor of multiple patents covering the therapeutic targeting of aging, cancer, cystic fibrosis, and metabolic disorders. Among these, patents were licensed to Bayer (WO2014020041-A1 and WO2014020043-A1), Bristol-Myers Squibb (WO2008057863-A1), Osasuna Therapeutics (WO2019057742A1), Pharmamar (WO2022049270A1 and WO2022048775-A1), Raptor Pharmaceuticals (EP2664326-A1), Samsara Therapeutics (GB202017553D0), and Therafast Bio (EP3684471A1). The other authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2022.10.003.
Contributor Information
Guido Kroemer, Email: kroemer@orange.fr.
Carlos López-Otín, Email: clo@uniovi.es.
Junjie Xiao, Email: Junjiexiao@shu.edu.cn.
Supplementary materials
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the global burden of disease study 2015. The Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2021. Noncommunicable diseases.https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases Available at. [accessed 09.09.2022] [Google Scholar]
- 3.Yin X, Chen M, He R, et al. Association of leisure sedentary time with common chronic disease risk factors: A longitudinal study of ChinaHealth and Nutrition Surveys. Int J Health Plann Manage. 2021;36:100–112. doi: 10.1002/hpm.3070. [DOI] [PubMed] [Google Scholar]
- 4.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob Health. 2018;6:e1077–e1086. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Pan A, Wang DD, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation. 2018;138:345–355. doi: 10.1161/CIRCULATIONAHA.117.032047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kandola A, Ashdown-Franks G, Hendrikse J, Sabiston CM, Stubbs B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci Biobehav Rev. 2019;107:525–539. doi: 10.1016/j.neubiorev.2019.09.040. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization (WHO) WHO; Geneva: 2020. WHO Guidelines on physical activity and sedentary behaviour: Web annex evidence profiles. [PubMed] [Google Scholar]
- 8.Aune D, Sen A, Henriksen T, Saugstad OD, Tonstad S. Physical activity and the risk of gestational diabetes mellitus: A systematic review and dose-response meta-analysis of epidemiological studies. Eur J Epidemiol. 2016;31:967–997. doi: 10.1007/s10654-016-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong SW, Kim SH, Kang SH, et al. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur Heart J. 2019;40:3547–3555. doi: 10.1093/eurheartj/ehz564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaput JP, Willumsen J, Bull F, et al. 2020 WHO guidelines on physical activity and sedentary behaviour for children and adolescents aged 5−17 years: Summary of the evidence. Int J Behav Nutr Phys Act. 2020;17:141. doi: 10.1186/s12966-020-01037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Channon KM. Exercise and cardiovascular health: New routes to reap more rewards. Cardiovasc Res. 2020;116:e56–e58. doi: 10.1093/cvr/cvz264. [DOI] [PubMed] [Google Scholar]
- 12.Fiuza-Luces C, Santos-Lozano A, Joyner M, et al. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat Rev Cardiol. 2018;15:731–743. doi: 10.1038/s41569-018-0065-1. [DOI] [PubMed] [Google Scholar]
- 13.Mendes R, Sousa N, Almeida A, et al. Exercise prescription for patients with type 2 diabetes—A synthesis of international recommendations: Narrative review. Br J Sports Med. 2016;50:1379–1381. doi: 10.1136/bjsports-2015-094895. [DOI] [PubMed] [Google Scholar]
- 14.Halabchi F, Alizadeh Z, Sahraian MA, Abolhasani M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017;17:185. doi: 10.1186/s12883-017-0960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han P, Zhang W, Kang L, et al. Clinical evidence of exercise benefits for stroke. Adv Exp Med Biol. 2017;1000:131–151. doi: 10.1007/978-981-10-4304-8_9. [DOI] [PubMed] [Google Scholar]
- 16.Landi F, Marzetti E, Martone AM, Bernabei R, Onder G. Exercise as a remedy for sarcopenia. Curr Opin Clin Nutr Metab Care. 2014;17:25–31. doi: 10.1097/MCO.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 17.Wannamethee SG, Shaper AG, Walker M. Physical activity and risk of cancer in middle-aged men. Br J Cancer. 2001;85:1311–1316. doi: 10.1054/bjoc.2001.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matos B, Patrício D, Henriques MC, et al. Chronic exercise training attenuates prostate cancer-induced molecular remodelling in the testis. Cell Oncol (Dordr) 2021;44:311–327. doi: 10.1007/s13402-020-00567-9. [DOI] [PubMed] [Google Scholar]
- 19.Chakravarty EF, Hubert HB, Lingala VB, Fries JF. Reduced disability and mortality among aging runners: A 21-year longitudinal study. Arch Intern Med. 2008;168:1638–1646. doi: 10.1001/archinte.168.15.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stessman J, Hammerman-Rozenberg R, Cohen A, Ein-Mor E, Jacobs JM. Physical activity, function, and longevity among the very old. Arch Intern Med. 2009;169:1476–1483. doi: 10.1001/archinternmed.2009.248. [DOI] [PubMed] [Google Scholar]
- 21.Buchner DM. Physical activity and prevention of cardiovascular disease in older adults. Clin Geriatr Med. 2009;25:661–675. doi: 10.1016/j.cger.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Dhalwani NN, O'Donovan G, Zaccardi F, et al. Long terms trends of multimorbidity and association with physical activity in older English population. Int J Behav Nutr Phys Act. 2016;13:8. doi: 10.1186/s12966-016-0330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vancampfort D, Koyanagi A, Ward PB, et al. Chronic physical conditions, multimorbidity and physical activity across 46 low- and middle-income countries. Int J Behav Nutr Phys Act. 2017;14:6. doi: 10.1186/s12966-017-0463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruegsegger GN, Booth FW. Health benefits of exercise. Cold Spring Harb Perspect Med. 2018;8 doi: 10.1101/cshperspect.a029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen BK, Saltin B. Exercise as medicine−Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(Suppl. 3):S1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 26.Franklin BA, Thompson PD, Al-Zaiti SS, et al. Exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training: Placing the risks into perspective−An update: A scientific statement from the American Heart Association. Circulation. 2020;141:e705–e736. doi: 10.1161/CIR.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 27.Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell. 2014;159:738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 28.López-Otín C, Kroemer G. Hallmarks of health. Cell. 2021;184:33–63. doi: 10.1016/j.cell.2020.11.034. [DOI] [PubMed] [Google Scholar]
- 29.Hoppeler H, Fluck M. Plasticity of skeletal muscle mitochondria: Structure and function. Med Sci Sports Exerc. 2003;35:95–104. doi: 10.1249/01.MSS.0000043292.99104.12. [DOI] [PubMed] [Google Scholar]
- 30.Vargas-Ortiz K, Perez-Vazquez V, Macias-Cervantes MH. Exercise and sirtuins: A way to mitochondrial health in skeletal muscle. Int J Mol Sci. 2019;20:2717. doi: 10.3390/ijms20112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Memme JM, Erlich AT, Phukan G, Hood DA. Exercise and mitochondrial health. J Physiol. 2021;599:803–817. doi: 10.1113/JP278853. [DOI] [PubMed] [Google Scholar]
- 32.Lee H, Kim K, Kim B, et al. A cellular mechanism of muscle memory facilitates mitochondrial remodelling following resistance training. J Physiol. 2018;596:4413–4426. doi: 10.1113/JP275308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richter U, Lahtinen T, Marttinen P, Suomi F, Battersby BJ. Quality control of mitochondrial protein synthesis is required for membrane integrity and cell fitness. J Cell Biol. 2015;211:373–389. doi: 10.1083/jcb.201504062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 35.Merle A, Jollet M, Britto FA, et al. Endurance exercise decreases protein synthesis and ER-mitochondria contacts in mouse skeletal muscle. J Appl Physiol (1985) 2019;127:1297–1306. doi: 10.1152/japplphysiol.00196.2019. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen J, Gejl KD, Hey-Mogensen M, et al. Plasticity in mitochondrial cristae density allows metabolic capacity modulation in human skeletal muscle. J Physiol. 2017;595:2839–2847. doi: 10.1113/JP273040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goncalves IO, Maciel E, Passos E, et al. Exercise alters liver mitochondria phospholipidomic profile and mitochondrial activity in non-alcoholic steatohepatitis. Int J Biochem Cell Biol. 2014;54:163–173. doi: 10.1016/j.biocel.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Agellon LB, Allen TM, et al. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006;3:321–331. doi: 10.1016/j.cmet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Gonçalves IO, Passos E, Diogo CV, et al. Exercise mitigates mitochondrial permeability transition pore and quality control mechanisms alterations in nonalcoholic steatohepatitis. Appl Physiol Nutr Metab. 2016;41:298–306. doi: 10.1139/apnm-2015-0470. [DOI] [PubMed] [Google Scholar]
- 40.Pan G, Zhang H, Zhu A, et al. Treadmill exercise attenuates cerebral ischaemic injury in rats by protecting mitochondrial function via enhancement of caveolin-1. Life Sci. 2021;264 doi: 10.1016/j.lfs.2020.118634. [DOI] [PubMed] [Google Scholar]
- 41.Zhao HX, Zhang Z, Zhou HL, Hu F, Yu Y. Exercise training suppresses Mst1 activation and attenuates myocardial dysfunction in mice with type 1 diabetes. Can J Physiol Pharmacol. 2020;98:777–784. doi: 10.1139/cjpp-2020-0205. [DOI] [PubMed] [Google Scholar]
- 42.Zhang SS, Zhou S, Crowley-McHattan ZJ, Wang RY, Li JP. A review of the role of endo/sarcoplasmic reticulum-mitochondria Ca2+ transport in diseases and skeletal muscle function. Int J Environ Res Public Health. 2021;18:3874. doi: 10.3390/ijerph18083874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guarnier FA, Michelucci A, Serano M, et al. Aerobic training prevents heatstrokes in calsequestrin-1 knockout mice by reducing oxidative stress. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/4652480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gauthier BR, Comaills V. Nuclear envelope integrity in health and disease: Consequences on genome instability and inflammation. Int J Mol Sci. 2021;22:7281. doi: 10.3390/ijms22147281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chandar S, Yeo LS, Leimena C, et al. Effects of mechanical stress and carvedilol in lamin A/C-deficient dilated cardiomyopathy. Circ Res. 2010;106:573–582. doi: 10.1161/CIRCRESAHA.109.204388. [DOI] [PubMed] [Google Scholar]
- 46.Gillon A, Nielsen K, Steel C, Cornwall J, Sheard P. Exercise attenuates age-associated changes in motoneuron number, nucleocytoplasmic transport proteins and neuromuscular health. Geroscience. 2018;40:177–192. doi: 10.1007/s11357-018-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dias C, Nylandsted J. Plasma membrane integrity in health and disease: Significance and therapeutic potential. Cell Discov. 2021;7:4. doi: 10.1038/s41421-020-00233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noland RC. Exercise and regulation of lipid metabolism. Prog Mol Biol Transl Sci. 2015;135:39–74. doi: 10.1016/bs.pmbts.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 49.Ambery AG, Tackett L, Penque BA, Brozinick JT, Elmendorf JS. Exercise training prevents skeletal muscle plasma membrane cholesterol accumulation, cortical actin filament loss, and insulin resistance in c57bl/6j mice fed a western-style high-fat diet. Physiol Rep. 2017;5:e13363. doi: 10.14814/phy2.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grice BA, Barton KJ, Covert JD, et al. Excess membrane cholesterol is an early contributing reversible aspect of skeletal muscle insulin resistance in C57BL/6NJ mice fed a Western-style high-fat diet. Am J Physiol Endocrinol Metab. 2019;317:E362–E373. doi: 10.1152/ajpendo.00396.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bradley NS, Snook LA, Jain SS, Heigenhauser GJ, Bonen A, Spriet LL. Acute endurance exercise increases plasma membrane fatty acid transport proteins in rat and human skeletal muscle. Am J Physiol Endocrinol Metab. 2012;302:E183–E189. doi: 10.1152/ajpendo.00254.2011. [DOI] [PubMed] [Google Scholar]
- 52.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood–brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G. Functional morphology of the blood−brain barrier in health and disease. Acta Neuropathol. 2018;135:311–336. doi: 10.1007/s00401-018-1815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Małkiewicz MA, Szarmach A, Sabisz A, Cubala WJ, Szurowska E, Winklewski PJ. Blood−brain barrier permeability and physical exercise. J Neuroinflammation. 2019;16:15. doi: 10.1186/s12974-019-1403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Souza PS, Gonçalves ED, Pedroso GS, et al. Physical exercise attenuates experimental autoimmune encephalomyelitis by inhibiting peripheral immune response and blood−brain barrier disruption. Mol Neurobiol. 2017;54:4723–4737. doi: 10.1007/s12035-016-0014-0. [DOI] [PubMed] [Google Scholar]
- 56.Małkiewicz MA, Małecki A, Toborek M, Szarmach A, Winklewski PJ. Substances of abuse and the blood brain barrier: Interactions with physical exercise. Neurosci Biobehav Rev. 2020;119:204–216. doi: 10.1016/j.neubiorev.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 57.Chupel MU, Minuzzi LG, Furtado G, et al. Exercise and taurine in inflammation, cognition, and peripheral markers of blood−brain barrier integrity in older women. Appl Physiol Nutr Metab. 2018;43:733–741. doi: 10.1139/apnm-2017-0775. [DOI] [PubMed] [Google Scholar]
- 58.Roh HT, So WY. The effects of aerobic exercise training on oxidant-antioxidant balance, neurotrophic factor levels, and blood-brain barrier function in obese and non-obese men. J Sport Health Sci. 2017;6:447–453. doi: 10.1016/j.jshs.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaspar RC, Nakandakari SCBR, Muñoz VR, et al. Acute physical exercise increases PI3K-p110α protein content in the hypothalamus of obese mice. J Anat. 2021;238:743–750. doi: 10.1111/joa.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50:1–9. doi: 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pals KL, Chang RT, Ryan AJ, Gisolfi CV. Effect of running intensity on intestinal permeability. J Appl Physiol (1985) 1997;82:571–576. doi: 10.1152/jappl.1997.82.2.571. [DOI] [PubMed] [Google Scholar]
- 62.Walter E, Gibson OR, Stacey M, Hill N, Parsons IT, Woods D. Changes in gastrointestinal cell integrity after marathon running and exercise-associated collapse. Eur J Appl Physiol. 2021;121:1179–1187. doi: 10.1007/s00421-021-04603-w. [DOI] [PubMed] [Google Scholar]
- 63.Falgiano PA, Gillum TL, Schall ZJ, Strag HR, Kuennen MR. Dietary curcumin supplementation does not alter peripheral blood mononuclear cell responses to exertional heat stress. Eur J Appl Physiol. 2018;118:2707–2717. doi: 10.1007/s00421-018-3998-5. [DOI] [PubMed] [Google Scholar]
- 64.Hill GW, Gillum TL, Lee BJ, et al. Prolonged treadmill running in normobaric hypoxia causes gastrointestinal barrier permeability and elevates circulating levels of pro- and anti-inflammatory cytokines. Appl Physiol Nutr Metab. 2020;45:376–386. doi: 10.1139/apnm-2019-0378. [DOI] [PubMed] [Google Scholar]
- 65.Davis MS, Willard MD, Williamson KK, Steiner JM, Williams DA. Sustained strenuous exercise increases intestinal permeability in racing Alaskan sled dogs. J Vet Intern Med. 2005;19:34–39. doi: 10.1892/0891-6640(2005)19<34:sseiip>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 66.Gutekunst K, Krüger K, August C, Diener M, Mooren FC. Acute exercises induce disorders of the gastrointestinal integrity in a murine model. Eur J Appl Physiol. 2014;114:609–617. doi: 10.1007/s00421-013-2791-8. [DOI] [PubMed] [Google Scholar]
- 67.Pires W, Veneroso CE, Wanner SP, et al. Association between exercise-induced hyperthermia and intestinal permeability: A systematic review. Sports Med. 2017;47:1389–1403. doi: 10.1007/s40279-016-0654-2. [DOI] [PubMed] [Google Scholar]
- 68.Freitas AKL, Silva MTB, Silva CMS, et al. Alanyl-glutamine protects the intestinal barrier function in trained rats against the impact of acute exhaustive exercise. Braz J Med Biol Res. 2020;53:e9211. doi: 10.1590/1414-431X20209211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pugh JN, Sage S, Hutson M, et al. Glutamine supplementation reduces markers of intestinal permeability during running in the heat in a dose-dependent manner. Eur J Appl Physiol. 2017;117:2569–2577. doi: 10.1007/s00421-017-3744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carbajo-Pescador S, Porras D, Garcia-Mediavilla MV, et al. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. Dis Model Mech. 2019;12 doi: 10.1242/dmm.039206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shao C, Li Y, Chen J, et al. Physical exercise repairs obstructive jaundice-induced damage to intestinal mucosal barrier function via H2S-mediated regulation of the HMBG1/toll like receptors 4/nuclear factor kappa B pathway. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.732780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qin L, Yao ZQ, Chang Q, et al. Swimming attenuates inflammation, oxidative stress, and apoptosis in a rat model of dextran sulfate sodium-induced chronic colitis. Oncotarget. 2017;8:7391–7404. doi: 10.18632/oncotarget.14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.LeMessurier KS, Tiwary M, Morin NP, Samarasinghe AE. Respiratory barrier as a safeguard and regulator of defense against influenza a virus and Streptococcus pneumoniae. Front Immunol. 2020;11:3. doi: 10.3389/fimmu.2020.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hellings PW, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol. 2020;145:1499–1509. doi: 10.1016/j.jaci.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Enhorning G, Hohlfeld J, Krug N, Lema G, Welliver RC. Surfactant function affected by airway inflammation and cooling: Possible impact on exercise-induced asthma. Eur Respir J. 2000;15:532–538. doi: 10.1034/j.1399-3003.2000.15.17.x. [DOI] [PubMed] [Google Scholar]
- 76.Spruit MA, Rochester CL, Pitta F, et al. Pulmonary rehabilitation, physical activity, respiratory failure and palliative respiratory care. Thorax. 2019;74:693–699. doi: 10.1136/thoraxjnl-2018-212044. [DOI] [PubMed] [Google Scholar]
- 77.Wada JT, Borges-Santos E, Porras DC, et al. Effects of aerobic training combined with respiratory muscle stretching on the functional exercise capacity and thoracoabdominal kinematics in patients with COPD: A randomized and controlled trial. Int J Chron Obstruct Pulmon Dis. 2016;11:2691–2700. doi: 10.2147/COPD.S114548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jung MH, Yi SW, An SJ, et al. Association of physical activity and lower respiratory tract infection outcomes in patients with cardiovascular disease. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.023775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 80.Gao J, Pan X, Li G, Chatterjee E, Xiao J. Physical exercise protects against endothelial dysfunction in cardiovascular and metabolic diseases. J Cardiovasc Transl Res. 2022;15:604–620. doi: 10.1007/s12265-021-10171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Good RB, Gilbane AJ, Trinder SL, et al. Endothelial to mesenchymal transition contributes to endothelial dysfunction in pulmonary arterial hypertension. Am J Pathol. 2015;185:1850–1858. doi: 10.1016/j.ajpath.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 82.Vieira RP, Silva RA, Oliveira-Junior MC, et al. Exercise deactivates leukocytes in asthma. Int J Sports Med. 2014;35:629–635. doi: 10.1055/s-0033-1358477. [DOI] [PubMed] [Google Scholar]
- 83.Kunz H, Bishop NC, Spielmann G, et al. Fitness level impacts salivary antimicrobial protein responses to a single bout of cycling exercise. Eur J Appl Physiol. 2015;115:1015–1027. doi: 10.1007/s00421-014-3082-8. [DOI] [PubMed] [Google Scholar]
- 84.Sivamaruthi BS, Kesika P, Chaiyasut C. Effect of probiotics supplementations on health status of athletes. Int J Environ Res Public Health. 2019;16:4469. doi: 10.3390/ijerph16224469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chimenti L, Morici G, Paternò A, et al. Bronchial epithelial damage after a half-marathon in nonasthmatic amateur runners. Am J Physiol Lung Cell Mol Physiol. 2010;298:L857–L862. doi: 10.1152/ajplung.00053.2010. [DOI] [PubMed] [Google Scholar]
- 86.Peters EM. Exercise, immunology and upper respiratory tract infections. Int J Sports Med. 1997;18(Suppl. 1):S69–S77. doi: 10.1055/s-2007-972702. [DOI] [PubMed] [Google Scholar]
- 87.Peters EM, Bateman ED. Ultramarathon running and upper respiratory tract infections. An epidemiological survey. S Afr Med J. 1983;64:582–584. [PubMed] [Google Scholar]
- 88.Campbell JP, Turner JE. There is limited existing evidence to support the common assumption that strenuous endurance exercise bouts impair immune competency. Expert Rev Clin Immunol. 2019;15:105–109. doi: 10.1080/1744666X.2019.1548933. [DOI] [PubMed] [Google Scholar]
- 89.Combes A, Dekerle J, Dumont X, et al. Continuous exercise induces airway epithelium damage while a matched-intensity and volume intermittent exercise does not. Respir Res. 2019;20:12. doi: 10.1186/s12931-019-0978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bonifant H, Holloway S. A review of the effects of ageing on skin integrity and wound healing. Br J Community Nurs. 2019;24(Suppl. 3):S28–S33. doi: 10.12968/bjcn.2019.24.Sup3.S28. [DOI] [PubMed] [Google Scholar]
- 91.Rousselle P, Montmasson M, Garnier C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019;75-76:12–26. doi: 10.1016/j.matbio.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 92.Chen CT, Tung HH, Tung TH, Denq JC. Nutrition, exercise, and skin integrity among frail older adults in Taiwan. Adv Skin Wound Care. 2017;30:364–371. doi: 10.1097/01.ASW.0000516309.92029.4e. [DOI] [PubMed] [Google Scholar]
- 93.Safdar A, Bourgeois JM, Ogborn DI, et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A. 2011;108:4135–4140. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lorincz K, Haluszka D, Kiss N, et al. Voluntary exercise improves murine dermal connective tissue status in high-fat diet-induced obesity. Arch Dermatol Res. 2017;309:209–215. doi: 10.1007/s00403-017-1715-6. [DOI] [PubMed] [Google Scholar]
- 95.Crane JD, MacNeil LG, Lally JS, et al. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell. 2015;14:625–634. doi: 10.1111/acel.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pence BD, Woods JA. Exercise, obesity, and cutaneous wound healing: Evidence from rodent and human studies. Adv Wound Care (New Rochelle) 2014;3:71–79. doi: 10.1089/wound.2012.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O'Brien J, Finlayson K, Kerr G, Edwards H. Evaluating the effectiveness of a self-management exercise intervention on wound healing, functional ability and health-related quality of life outcomes in adults with venous leg ulcers: A randomised controlled trial. Int Wound J. 2017;14:130–137. doi: 10.1111/iwj.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Emery CF, Kiecolt-Glaser JK, Glaser R, Malarkey WB, Frid DJ. Exercise accelerates wound healing among healthy older adults: A preliminary investigation. J Gerontol A Biol Sci Med Sci. 2005;60:1432–1436. doi: 10.1093/gerona/60.11.1432. [DOI] [PubMed] [Google Scholar]
- 99.Keylock KT, Vieira VJ, Wallig MA, DiPietro LA, Schrementi M, Woods JA. Exercise accelerates cutaneous wound healing and decreases wound inflammation in aged mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R179–R184. doi: 10.1152/ajpregu.00177.2007. [DOI] [PubMed] [Google Scholar]
- 100.Rael VE, Chen L, McIntosh CM, Alegre ML. Exercise increases skin graft resistance to rejection. Am J Transplant. 2019;19:1560–1567. doi: 10.1111/ajt.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lou J, Wu J, Feng M, et al. Exercise promotes angiogenesis by enhancing endothelial cell fatty acid utilization via liver-derived extracellular vesicle miR-122-5p. J Sport Health Sci. 2022;11:495–508. doi: 10.1016/j.jshs.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zogaib FG, Monte-Alto-Costa A. Moderate intensity physical training accelerates healing of full-thickness wounds in mice. Braz J Med Biol Res. 2011;44:1025–1035. doi: 10.1590/s0100-879x2011007500115. [DOI] [PubMed] [Google Scholar]
- 103.Keylock T, Meserve L, Wolfe A. Low-intensity exercise accelerates wound healing in diabetic mice. Wounds. 2018;30:68–71. [PubMed] [Google Scholar]
- 104.Gomes-Neto M, Conceicao CS, Oliveira Carvalho V, Brites C. A systematic review of the effects of different types of therapeutic exercise on physiologic and functional measurements in patients with HIV/AIDS. Clinics (Sao Paulo) 2013;68:1157–1167. doi: 10.6061/clinics/2013(08)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med. 2000;160:3082–3088. doi: 10.1001/archinte.160.20.3082. [DOI] [PubMed] [Google Scholar]
- 106.Pape K, Ryttergaard L, Rotevatn TA, et al. Leisure-time physical activity and the risk of suspected bacterial infections. Med Sci Sports Exerc. 2016;48:1737–1744. doi: 10.1249/MSS.0000000000000953. [DOI] [PubMed] [Google Scholar]
- 107.Sim YJ, Yu S, Yoon KJ, Loiacono CM, Kohut ML. Chronic exercise reduces illness severity, decreases viral load, and results in greater anti-inflammatory effects than acute exercise during influenza infection. J Infect Dis. 2009;200:1434–1442. doi: 10.1086/606014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dianatinasab M, Ghahri S, Dianatinasab A, Amanat S, Fararouei M. Effects of exercise on the immune function, quality of life, and mental health in HIV/AIDS individuals. Adv Exp Med Biol. 2020;1228:411–421. doi: 10.1007/978-981-15-1792-1_28. [DOI] [PubMed] [Google Scholar]
- 109.da Silveira MP, da Silva Fagundes KK, Bizuti MR, Starck E, Rossi RC, de Resende ESDT. Physical exercise as a tool to help the immune system against COVID-19: An integrative review of the current literature. Clin Exp Med. 2021;21:15–28. doi: 10.1007/s10238-020-00650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alawna M, Amro M, Mohamed AA. Aerobic exercises recommendations and specifications for patients with COVID-19: A systematic review. Eur Rev Med Pharmacol Sci. 2020;24:13049–13055. doi: 10.26355/eurrev_202012_24211. [DOI] [PubMed] [Google Scholar]
- 111.Abdelbasset WK. Stay home: Role of physical exercise training in elderly individuals' ability to face the COVID-19 infection. J Immunol Res. 2020;2020 doi: 10.1155/2020/8375096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tamura Y, Jee E, Kouzaki K, Kotani T, Nakazato K. Effects of endurance training on the expression of host proteins involved in SARS-CoV-2 cell entry in C57BL/6J mouse. Physiol Rep. 2021;9:e15014. doi: 10.14814/phy2.15014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Valenzuela PL, Castillo-Garcia A, Morales JS, et al. Exercise benefits on Alzheimer's disease: State-of-the-science. Ageing Res Rev. 2020;62 doi: 10.1016/j.arr.2020.101108. [DOI] [PubMed] [Google Scholar]
- 114.Cook MD, Martin SA, Williams C, et al. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav Immun. 2013;33:46–56. doi: 10.1016/j.bbi.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.de Araújo CC, Silva JD, Samary CS, et al. Regular and moderate exercise before experimental sepsis reduces the risk of lung and distal organ injury. J Appl Physiol (1985) 2012;112:1206–1214. doi: 10.1152/japplphysiol.01061.2011. [DOI] [PubMed] [Google Scholar]
- 116.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: A role in the anti-inflammatory influence of exercise? J Leukoc Biol. 2008;84:1271–1278. doi: 10.1189/jlb.0408244. [DOI] [PubMed] [Google Scholar]
- 117.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 2002;162:1286–1292. doi: 10.1001/archinte.162.11.1286. [DOI] [PubMed] [Google Scholar]
- 118.de Gonzalo-Calvo D, Fernández-García B, de Luxán-Delgado B, et al. Long-term training induces a healthy inflammatory and endocrine emergent biomarker profile in elderly men. Age (Dordr) 2012;34:761–771. doi: 10.1007/s11357-011-9266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frodermann V, Rohde D, Courties G, et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med. 2019;25:1761–1771. doi: 10.1038/s41591-019-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nieman DC. Exercise immunology: Practical applications. Int J Sports Med. 1997;18(Suppl. 1):S91–100. doi: 10.1055/s-2007-972705. [DOI] [PubMed] [Google Scholar]
- 121.Oh S, Chun S, Hwang S, et al. Vitamin D and exercise are major determinants of natural killer cell activity, which is age- and gender-specific. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.594356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Simpson RJ, Kunz H, Agha N, Graff R. Exercise and the regulation of immune functions. Prog Mol Biol Transl Sci. 2015;135:355–380. doi: 10.1016/bs.pmbts.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 123.Moro-García MA, Fernández-García B, Echeverría A, et al. Frequent participation in high volume exercise throughout life is associated with a more differentiated adaptive immune response. Brain Behav Immun. 2014;39:61–74. doi: 10.1016/j.bbi.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 124.Bartlett DB, Fox O, McNulty CL, et al. Habitual physical activity is associated with the maintenance of neutrophil migratory dynamics in healthy older adults. Brain Behav Immun. 2016;56:12–20. doi: 10.1016/j.bbi.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 126.Ulven SM, Foss SS, Skjolsvik AM, et al. An acute bout of exercise modulate the inflammatory response in peripheral blood mononuclear cells in healthy young men. Arch Physiol Biochem. 2015;121:41–49. doi: 10.3109/13813455.2014.1003566. [DOI] [PubMed] [Google Scholar]
- 127.Visvikis-Siest S, Marteau JB, Samara A, Berrahmoune H, Marie B, Pfister M. Peripheral blood mononuclear cells (PBMCs): A possible model for studying cardiovascular biology systems. Clin Chem Lab Med. 2007;45:1154–1168. doi: 10.1515/CCLM.2007.255. [DOI] [PubMed] [Google Scholar]
- 128.Liuzzo G, Angiolillo DJ, Buffon A, et al. Enhanced response of blood monocytes to in vitro lipopolysaccharide-challenge in patients with recurrent unstable angina. Circulation. 2001;103:2236–2241. doi: 10.1161/01.cir.103.18.2236. [DOI] [PubMed] [Google Scholar]
- 129.Hou W, Gibbs JS, Lu X, et al. Viral infection triggers rapid differentiation of human blood monocytes into dendritic cells. Blood. 2012;119:3128–3131. doi: 10.1182/blood-2011-09-379479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Minuzzi LG, Rama L, Bishop NC, et al. Lifelong training improves anti-inflammatory environment and maintains the number of regulatory T cells in masters athletes. Eur J Appl Physiol. 2017;117:1131–1140. doi: 10.1007/s00421-017-3600-6. [DOI] [PubMed] [Google Scholar]
- 131.Minuzzi LG, Chupel MU, Rama L, et al. Lifelong exercise practice and immunosenescence: Master athletes cytokine response to acute exercise. Cytokine. 2019;115:1–7. doi: 10.1016/j.cyto.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 132.Woods JA, Keylock KT, Lowder T, et al. Cardiovascular exercise training extends influenza vaccine seroprotection in sedentary older adults: The immune function intervention trial. J Am Geriatr Soc. 2009;57:2183–2191. doi: 10.1111/j.1532-5415.2009.02563.x. [DOI] [PubMed] [Google Scholar]
- 133.Kohut ML, Arntson BA, Lee W, et al. Moderate exercise improves antibody response to influenza immunization in older adults. Vaccine. 2004;22:2298–2306. doi: 10.1016/j.vaccine.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 134.Leitzmann M, Powers H, Anderson AS, et al. European Code against Cancer 4th edition: Physical activity and cancer. Cancer Epidemiol. 2015;39(Suppl. 1):S46–S55. doi: 10.1016/j.canep.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 135.Papadimitriou N, Dimou N, Tsilidis KK, et al. Physical activity and risks of breast and colorectal cancer: A mendelian randomisation analysis. Nat Commun. 2020;11:597. doi: 10.1038/s41467-020-14389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang X, Theodoratou E, Li X, et al. Genetically predicted physical activity levels are associated with lower colorectal cancer risk: A mendelian randomisation study. Br J Cancer. 2021;124:1330–1338. doi: 10.1038/s41416-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pedersen L, Idorn M, Olofsson GH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23:554–562. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 138.Bigley AB, Rezvani K, Chew C, et al. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav Immun. 2014;39:160–171. doi: 10.1016/j.bbi.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 139.Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014;15:1139–1153. doi: 10.15252/embr.201439245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Werner C, Furster T, Widmann T, et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120:2438–2447. doi: 10.1161/CIRCULATIONAHA.109.861005. [DOI] [PubMed] [Google Scholar]
- 141.Werner C, Hanhoun M, Widmann T, et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52:470–482. doi: 10.1016/j.jacc.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 142.Liu Y, Sanoff HK, Cho H, et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8:439–448. doi: 10.1111/j.1474-9726.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yoon KJ, Ahn A, Park SH, et al. Exercise reduces metabolic burden while altering the immune system in aged mice. Aging (Albany NY) 2021;13:1294–1313. doi: 10.18632/aging.202312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang CC, Chiang WD, Huang WC, Huang CY, Hsu MC, Lin WT. Hepatoprotective effects of swimming exercise against d-galactose-induced senescence rat model. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/275431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saito Y, Chikenji TS, Matsumura T, Nakano M, Fujimiya M. Exercise enhances skeletal muscle regeneration by promoting senescence in fibro-adipogenic progenitors. Nat Commun. 2020;11:889. doi: 10.1038/s41467-020-14734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Qiu Y, Pan X, Chen Y, Xiao J. Hallmarks of exercised heart. J Mol Cell Cardiol. 2022;164:126–135. doi: 10.1016/j.yjmcc.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 147.Otaka N, Shibata R, Ohashi K, et al. Myonectin is an exercise-induced myokine that protects the heart from ischemia−reperfusion injury. Circ Res. 2018;123:1326–1338. doi: 10.1161/CIRCRESAHA.118.313777. [DOI] [PubMed] [Google Scholar]
- 148.Hou Z, Qin X, Hu Y, et al. Longterm exercise-derived exosomal miR-342-5p: A novel exerkine for cardioprotection. Circ Res. 2019;124:1386–1400. doi: 10.1161/CIRCRESAHA.118.314635. [DOI] [PubMed] [Google Scholar]
- 149.Gao R, Wang L, Bei Y, et al. Long noncoding RNA cardiac physiological hypertrophy-associated regulator induces cardiac physiological hypertrophy and promotes functional recovery after myocardial ischemia−reperfusion Injury. Circulation. 2021;144:303–317. doi: 10.1161/CIRCULATIONAHA.120.050446. [DOI] [PubMed] [Google Scholar]
- 150.He YY, Wang L, Zhang T, Weng SJ, Lu J, Zhong YM. Aerobic exercise delays retinal ganglion cell death after optic nerve injury. Exp Eye Res. 2020;200 doi: 10.1016/j.exer.2020.108240. [DOI] [PubMed] [Google Scholar]
- 151.Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 152.Cao W, Li J, Yang K, Cao D. An overview of autophagy: Mechanism, regulation and research progress. Bull Cancer. 2021;108:304–322. doi: 10.1016/j.bulcan.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 153.Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol. 2018;20:1013–1022. doi: 10.1038/s41556-018-0176-2. [DOI] [PubMed] [Google Scholar]
- 154.Onishi M, Yamano K, Sato M, Matsuda N, Okamoto K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021;40 doi: 10.15252/embj.2020104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang L, Wang J, Cretoiu D, Li G, Xiao J. Exercise-mediated regulation of autophagy in the cardiovascular system. J Sport Health Sci. 2020;9:203–210. doi: 10.1016/j.jshs.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grumati P, Coletto L, Schiavinato A, et al. Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles. Autophagy. 2011;7:1415–1423. doi: 10.4161/auto.7.12.17877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lira VA, Okutsu M, Zhang M, et al. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013;27:4184–4193. doi: 10.1096/fj.13-228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Codina-Martínez H, Fernández-García B, Díez-Planelles C, et al. Autophagy is required for performance adaptive response to resistance training and exercise-induced adult neurogenesis. Scand J Med Sci Sports. 2020;30:238–253. doi: 10.1111/sms.13586. [DOI] [PubMed] [Google Scholar]
- 159.Park SS, Seo YK, Kwon KS. Sarcopenia targeting with autophagy mechanism by exercise. BMB Rep. 2019;52:64–69. doi: 10.5483/BMBRep.2019.52.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Escobar KA, Cole NH, Mermier CM, VanDusseldorp TA. Autophagy and aging: Maintaining the proteome through exercise and caloric restriction. Aging Cell. 2019;18:e12876. doi: 10.1111/acel.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Campos JC, Queliconi BB, Bozi LHM, et al. Exercise reestablishes autophagic flux and mitochondrial quality control in heart failure. Autophagy. 2017;13:1304–1317. doi: 10.1080/15548627.2017.1325062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Halling JF, Pilegaard H. Autophagy-dependent beneficial effects of exercise. Cold Spring Harb Perspect Med. 2017;7 doi: 10.1101/cshperspect.a029777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.He C, Sumpter R, Jr, Levine B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy. 2012;8:1548–1551. doi: 10.4161/auto.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu H, Lei H, Shi Y, et al. Autophagy inhibitor 3-methyladenine alleviates overload-exercise-induced cardiac injury in rats. Acta Pharmacol Sin. 2017;38:990–997. doi: 10.1038/aps.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhao N, Xia J, Xu B. Physical exercise may exert its therapeutic influence on Alzheimer's disease through the reversal of mitochondrial dysfunction via SIRT1-FOXO1/3-PINK1-Parkin-mediated mitophagy. J Sport Health Sci. 2021;10:1–3. doi: 10.1016/j.jshs.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang Y, Liao B, Hu S, et al. High intensity interval training induces dysregulation of mitochondrial respiratory complex and mitophagy in the hippocampus of middle-aged mice. Behav Brain Res. 2021;412 doi: 10.1016/j.bbr.2021.113384. [DOI] [PubMed] [Google Scholar]
- 168.Ovens AJ, Scott JW, Langendorf CG, Kemp BE, Oakhill JS, Smiles WJ. Post-translational modifications of the energy guardian amp-activated protein kinase. Int J Mol Sci. 2021;22:1229. doi: 10.3390/ijms22031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. J Physiol. 2017;595:2857–2871. doi: 10.1113/JP273185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhou Q, Deng J, Yao J, et al. Exercise downregulates HIPK 2 and HIPK 2inhibition protects against myocardial infarction. EBioMedicine. 2021;74 doi: 10.1016/j.ebiom.2021.103713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wu X, Wang L, Wang K, et al. ADAR2 increases in exercised heart and protects against myocardial infarction and doxorubicin-induced cardiotoxicity. Mol Ther. 2022;30:400–414. doi: 10.1016/j.ymthe.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bei Y, Lu D, Bar C, et al. miR-486 attenuates cardiac ischemia/reperfusion injury and mediates the beneficial effect of exercise for myocardial protection. Mol Ther. 2022;30:1675–1691. doi: 10.1016/j.ymthe.2022.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu M, Jiang H, Xiao J. Exercise protects sympathetic stress-induced myocardial fibrosis by regulating cytokines. J Cardiovasc Transl Res. 2020;13:570–571. doi: 10.1007/s12265-019-09933-x. [DOI] [PubMed] [Google Scholar]
- 174.Fernández-Sanjurjo M, de Gonzalo-Calvo D, Fernández-García B, et al. Circulating microRNA as emerging biomarkers of exercise. Exerc Sport Sci Rev. 2018;46:160–171. doi: 10.1249/JES.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 175.Fernández-Sanjurjo M, Díaz-Martínez ÁE, Díez-Robles S, et al. Circulating microRNA profiling reveals specific subsignatures in response to a maximal incremental exercise test. J Strength Cond Res. 2021;35:287–291. doi: 10.1519/JSC.0000000000003930. [DOI] [PubMed] [Google Scholar]
- 176.Wu G, Zhang X, Gao F. The epigenetic landscape of exercise in cardiac health and disease. J Sport Health Sci. 2021;10:648–659. doi: 10.1016/j.jshs.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Urdinguio RG, Tejedor JR, Fernández-Sanjurjo M, et al. Physical exercise shapes the mouse brain epigenome. Mol Metab. 2021;54 doi: 10.1016/j.molmet.2021.101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cuevas-Ramos D, Almeda-Valdés P, Meza-Arana CE, et al. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One. 2012;7:e38022. doi: 10.1371/journal.pone.0038022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim KH, Kim SH, Min YK, Yang HM, Lee JB, Lee MS. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS One. 2013;8:e63517. doi: 10.1371/journal.pone.0063517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hansen JS, Clemmesen JO, Secher NH, et al. Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Mol Metab. 2015;4:551–560. doi: 10.1016/j.molmet.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hansen JS, Pedersen BK, Xu G, Lehmann R, Weigert C, Plomgaard P. Exercise-induced secretion of FGF21 and follistatin are blocked by pancreatic clamp and impaired in type 2 diabetes. J Clin Endocrinol Metab. 2016;101:2816–2825. doi: 10.1210/jc.2016-1681. [DOI] [PubMed] [Google Scholar]
- 182.Slusher AL, Whitehurst M, Zoeller RF, Mock JT, Maharaj M, Huang CJ. Attenuated fibroblast growth factor 21 response to acute aerobic exercise in obese individuals. Nutr Metab Cardiovasc Dis. 2015;25:839–845. doi: 10.1016/j.numecd.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 183.Görgens SW, Eckardt K, Jensen J, Drevon CA, Eckel J. Exercise and regulation of adipokine and myokine production. Prog Mol Biol Transl Sci. 2015;135:313–336. doi: 10.1016/bs.pmbts.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 184.Stanford KI, Middelbeek RJ, Erratum Goodyear LJ. Exercise effects on white adipose tissue: Beiging and metabolic adaptations. Diabetes. 2015;64:3334. doi: 10.2337/db15-er09. Diabetes 2015;64:2361-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Thompson D, Karpe F, Lafontan M, Frayn K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol Rev. 2012;92:157–191. doi: 10.1152/physrev.00012.2011. [DOI] [PubMed] [Google Scholar]
- 186.Geng L, Liao B, Jin L, et al. Exercise alleviates obesity-induced metabolic dysfunction via enhancing FGF21 sensitivity in adipose tissues. Cell Rep. 2019;26:2738–2752. doi: 10.1016/j.celrep.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 187.Zhang H, Fealy CE, Kirwan JP. Exercise training promotes a GDF15-associated reduction in fat mass in older adults with obesity. Am J Physiol Endocrinol Metab. 2019;316:E829–E836. doi: 10.1152/ajpendo.00439.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Campderros L, Sanchez-Infantes D, Villarroya J, et al. Altered GDF15 and fgf21 levels in response to strenuous exercise: A study in marathon runners. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.550102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kleinert M, Clemmensen C, Sjøberg KA, et al. Exercise increases circulating GDF15 in humans. Mol Metab. 2018;9:187–191. doi: 10.1016/j.molmet.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Conte M, Martucci M, Mosconi G, et al. GDF15 plasma level is inversely associated with level of physical activity and correlates with markers of inflammation and muscle weakness. Front Immunol. 2020;11:915. doi: 10.3389/fimmu.2020.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Klein AB, Nicolaisen TS, Ørtenblad N, et al. Pharmacological but not physiological GDF15 suppresses feeding and the motivation to exercise. Nat Commun. 2021;12:1041. doi: 10.1038/s41467-021-21309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Searls YM, Smirnova IV, Fegley BR, Stehno-Bittel L. Exercise attenuates diabetes-induced ultrastructural changes in rat cardiac tissue. Med Sci Sports Exerc. 2004;36:1863–1870. doi: 10.1249/01.mss.0000145461.38224.ec. [DOI] [PubMed] [Google Scholar]
- 193.Ding YH, Li J, Zhou Y, Rafols JA, Clark JC, Ding Y. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res. 2006;3:15–23. doi: 10.2174/156720206775541787. [DOI] [PubMed] [Google Scholar]
- 194.Morland C, Andersson KA, Haugen ØP, et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat Commun. 2017;8:15557. doi: 10.1038/ncomms15557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Archer AE, Von Schulze AT, Geiger PC. Exercise, heat shock proteins and insulin resistance. Philos Trans R Soc Lond B Biol Sci. 2018;373 doi: 10.1098/rstb.2016.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rosenzweig R, Nillegoda NB, Mayer MP, Bukau B. The HSP70 chaperone network. Nat Rev Mol Cell Biol. 2019;20:665–680. doi: 10.1038/s41580-019-0133-3. [DOI] [PubMed] [Google Scholar]
- 197.Henstridge DC, Febbraio MA, Hargreaves M. Heat shock proteins and exercise adaptations. Our knowledge thus far and the road still ahead. J Appl Physiol (1985) 2016;120:683–691. doi: 10.1152/japplphysiol.00811.2015. [DOI] [PubMed] [Google Scholar]
- 198.Chung J, Nguyen AK, Henstridge DC, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gupte AA, Bomhoff GL, Morris JK, Gorres BK, Geiger PC. Lipoic acid increases heat shock protein expression and inhibits stress kinase activation to improve insulin signaling in skeletal muscle from high-fat-fed rats. J Appl Physiol (1985) 2009;106:1425–1434. doi: 10.1152/japplphysiol.91210.2008. [DOI] [PubMed] [Google Scholar]
- 200.Henstridge DC, Bruce CR, Drew BG, et al. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes. 2014;63:1881–1894. doi: 10.2337/db13-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Celik O, Yildiz BO. Obesity and physical exercise. Minerva Endocrinol (Torino) 2021;46:131–144. doi: 10.23736/S2724-6507.20.03361-1. [DOI] [PubMed] [Google Scholar]
- 202.Wang YL, Lin CH, Chen CC, et al. Exercise preconditioning attenuates neurological injury by preserving old and newly formed HSP72-containing neurons in focal brain ischemia rats. Int J Med Sci. 2019;16:675–685. doi: 10.7150/ijms.32962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.McGee SL, Hargreaves M. Exercise adaptations: Molecular mechanisms and potential targets for therapeutic benefit. Nat Rev Endocrinol. 2020;16:495–505. doi: 10.1038/s41574-020-0377-1. [DOI] [PubMed] [Google Scholar]
- 204.Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10:555–568. doi: 10.1080/19490976.2018.1562268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhao H, Chen X, Hu G, et al. Small extracellular vesicles from brown adipose tissue mediate exercise cardioprotection. Circ Res. 2022;130:1490–1506. doi: 10.1161/CIRCRESAHA.121.320458. [DOI] [PubMed] [Google Scholar]
- 206.Allen JM, Mailing LJ, Niemiro GM, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50:747–757. doi: 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 207.Gomaa EZ. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek. 2020;113:2019–2040. doi: 10.1007/s10482-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 208.Wosinska L, Walsh CJ, O'Connor PM, et al. In vitro and in silico based approaches to identify potential novel bacteriocins from the athlete gut microbiome of an elite athlete cohort. Microorganisms. 2022;10:701. doi: 10.3390/microorganisms10040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fernández J, Fernández-Sanjurjo M, Iglesias-Gutiérrez E, et al. Resistance and endurance exercise training induce differential changes in gut microbiota composition in murine models. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.748854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Allen JM, Berg Miller ME, Pence BD, et al. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J Appl Physiol (1985) 2015;118:1059–1066. doi: 10.1152/japplphysiol.01077.2014. [DOI] [PubMed] [Google Scholar]
- 211.Choi JJ, Eum SY, Rampersaud E, Daunert S, Abreu MT, Toborek M. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ Health Perspect. 2013;121:725–730. doi: 10.1289/ehp.1306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kang SS, Jeraldo PR, Kurti A, et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol Neurodegener. 2014;9:36. doi: 10.1186/1750-1326-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Clarke SF, Murphy EF, O'Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 214.Hsu YJ, Chiu CC, Li YP, et al. Effect of intestinal microbiota on exercise performance in mice. J Strength Cond Res. 2015;29:552–558. doi: 10.1519/JSC.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 215.Monda V, Villano I, Messina A, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/3831972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Erny D, Hraběde Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mancilla R, Krook A, Schrauwen P, Hesselink MKC. Diurnal regulation of peripheral glucose metabolism: Potential effects of exercise timing. Obesity (Silver Spring) 2020;28(Suppl. 1):S38–S45. doi: 10.1002/oby.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dyar KA, Ciciliot S, Wright LE, et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab. 2014;3:29–41. doi: 10.1016/j.molmet.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Vetter C, Dashti HS, Lane JM, et al. Night shift work, genetic risk, and type 2 diabetes in the UK Biobank. Diabetes Care. 2018;41:762–769. doi: 10.2337/dc17-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bescos R, Boden MJ, Jackson ML, et al. Four days of simulated shift work reduces insulin sensitivity in humans. Acta Physiol (Oxf) 2018;223:e13039. doi: 10.1111/apha.13039. [DOI] [PubMed] [Google Scholar]
- 221.Gabriel BM, Zierath JR. Circadian rhythms and exercise—Re-setting the clock in metabolic disease. Nat Rev Endocrinol. 2019;15:197–206. doi: 10.1038/s41574-018-0150-x. [DOI] [PubMed] [Google Scholar]
- 222.Tahara Y, Aoyama S, Shibata S. The mammalian circadian clock and its entrainment by stress and exercise. J Physiol Sci. 2017;67:1–10. doi: 10.1007/s12576-016-0450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Youngstedt SD, Elliott JA, Kripke DF. Human circadian phase-response curves for exercise. J Physiol. 2019;597:2253–2268. doi: 10.1113/JP276943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wolff G, Esser KA. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med Sci Sports Exerc. 2012;44:1663–1670. doi: 10.1249/MSS.0b013e318255cf4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Choi Y, Cho J, No MH, et al. Re-setting the circadian clock using exercise against sarcopenia. Int J Mol Sci. 2020;21:3106. doi: 10.3390/ijms21093106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Facer-Childs E, Brandstaetter R. The impact of circadian phenotype and time since awakening on diurnal performance in athletes. Curr Biol. 2015;25:518–522. doi: 10.1016/j.cub.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 227.Facer-Childs E, Brandstaetter R. Circadian phenotype composition is a major predictor of diurnal physical performance in teams. Front Neurol. 2015;6:208. doi: 10.3389/fneur.2015.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Maier G, Delezie J, Westermark PO, Santos G, Ritz D, Handschin C. Transcriptomic, proteomic and phosphoproteomic underpinnings of daily exercise performance and zeitgeber activity of training in mouse muscle. J Physiol. 2022;600:769–796. doi: 10.1113/JP281535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Scheiermann C, Gibbs J, Ince L, Loudon A. Clocking in to immunity. Nat Rev Immunol. 2018;18:423–437. doi: 10.1038/s41577-018-0008-4. [DOI] [PubMed] [Google Scholar]
- 230.Nobis CC, Dubeau Laramée G, Kervezee L, Maurice De Sousa D, Labrecque N, Cermakian N. The circadian clock of CD8 T cells modulates their early response to vaccination and the rhythmicity of related signaling pathways. Proc Natl Acad Sci U S A. 2019;116:20077–20086. doi: 10.1073/pnas.1905080116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.de Souza Teixeira AA, Lira FS, Rosa-Neto JC. Aging with rhythmicity. Is it possible? Physical exercise as a pacemaker. Life Sci. 2020;261 doi: 10.1016/j.lfs.2020.118453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Minuzzi LG, Rama L, Chupel MU, et al. Effects of lifelong training on senescence and mobilization of T lymphocytes in response to acute exercise. Exerc Immunol Rev. 2018;24:72–84. [PubMed] [Google Scholar]
- 233.Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J Appl Physiol (1985) 2020;128:87–99. doi: 10.1152/japplphysiol.00495.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.de Souza Teixeira AA, Minuzzi LG, Lira FS, et al. Improvement in the anti-inflammatory profile with lifelong physical exercise is related to clock genes expression in effector-memory CD4+ T cells in master athletes. Exerc Immunol Rev. 2021;27:67–83. [PubMed] [Google Scholar]
- 235.Rutter M. Implications of resilience concepts for scientific understanding. Ann N Y Acad Sci. 2006;1094:1–12. doi: 10.1196/annals.1376.002. [DOI] [PubMed] [Google Scholar]
- 236.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 237.Deslandes A, Moraes H, Ferreira C, et al. Exercise and mental health: Many reasons to move. Neuropsychobiology. 2009;59:191–198. doi: 10.1159/000223730. [DOI] [PubMed] [Google Scholar]
- 238.Lee CB, Baek SS. Impact of exercise on hippocampal neurogenesis in hyperglycemic diabetes. J Exerc Rehabil. 2020;16:115–117. doi: 10.12965/jer.2040210.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Voss MW, Soto C, Yoo S, Sodoma M, Vivar C, van Praag H. Exercise and hippocampal memory systems. Trends Cogn Sci. 2019;23:318–333. doi: 10.1016/j.tics.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Teixeira AL, Fernandes IA, Vianna LC. Cardiovascular control during exercise: The connectivity of skeletal muscle afferents to the brain. Exerc Sport Sci Rev. 2020;48:83–91. doi: 10.1249/JES.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 241.Mahalakshmi B, Maurya N, Lee SD, Bharath Kumar V. Possible neuroprotective mechanisms of physical exercise in neurodegeneration. Int J Mol Sci. 2020;21:5895. doi: 10.3390/ijms21165895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Oosterwijck JV, Marusic U, De Wandele I, et al. The role of autonomic function in exercise-induced endogenous analgesia: A case-control study in myalgic encephalomyelitis/chronic fatigue syndrome and healthy people. Pain Physician. 2017;20:E389–E399. [PubMed] [Google Scholar]
- 243.Xu X, Fu Z, Le W. Exercise and Parkinson's disease. Int Rev Neurobiol. 2019;147:45–74. doi: 10.1016/bs.irn.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 244.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Berg DA, Belnoue L, Song H, Simon A. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development. 2013;140:2548–2561. doi: 10.1242/dev.088005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Vilar M, Mira H. Regulation of neurogenesis by neurotrophins during adulthood: Expected and unexpected roles. Front Neurosci. 2016;10:26. doi: 10.3389/fnins.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bouchard-Cannon P, Lowden C, Trinh D, Cheng HM. Dexras1 is a homeostatic regulator of exercise-dependent proliferation and cell survival in the hippocampal neurogenic niche. Sci Rep. 2018;8:5294. doi: 10.1038/s41598-018-23673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Amstadter AB, Myers JM, Kendler KS. Psychiatric resilience: Longitudinal twin study. Br J Psychiatry. 2014;205:275–280. doi: 10.1192/bjp.bp.113.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Boardman JD, Blalock CL, Button TM. Sex differences in the heritability of resilience. Twin Res Hum Genet. 2008;11:12–27. doi: 10.1375/twin.11.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Waaktaar T, Torgersen S. Genetic and environmental causes of variation in trait resilience in young people. Behav Genet. 2012;42:366–377. doi: 10.1007/s10519-011-9519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Aizawa S, Ishitobi Y, Masuda K, et al. Genetic association of the transcription of neuroplasticity-related genes and variation in stress-coping style. Brain Behav. 2015;5:e00360. doi: 10.1002/brb3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Notaras M, van den Buuse M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol Psychiatry. 2020;25:2251–2274. doi: 10.1038/s41380-019-0639-2. [DOI] [PubMed] [Google Scholar]
- 253.Liu T, Canon MD, Shen L, et al. The influence of the BDNF Val66Met polymorphism on the association of regular physical activity with cognition among individuals with diabetes. Biol Res Nurs. 2021;23:318–330. doi: 10.1177/1099800420966648. [DOI] [PubMed] [Google Scholar]
- 254.Stubbe JH, Boomsma DI, Vink JM, et al. Genetic influences on exercise participation in 37,051 twin pairs from seven countries. PLoS One. 2006;1:e22. doi: 10.1371/journal.pone.0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kujala UM, Kaprio J, Koskenvuo M. Modifiable risk factors as predictors of all-cause mortality: The roles of genetics and childhood environment. Am J Epidemiol. 2002;156:985–993. doi: 10.1093/aje/kwf151. [DOI] [PubMed] [Google Scholar]
- 256.Waller K, Kujala UM, Rantanen T, et al. Physical activity, morbidity and mortality in twins: A 24-year prospective follow-up. Eur J Epidemiol. 2010;25:731–739. doi: 10.1007/s10654-010-9493-x. [DOI] [PubMed] [Google Scholar]
- 257.Ballin M, Nordström A, Nordstrom P. Cardiovascular disease and all-cause mortality in male twins with discordant cardiorespiratory fitness: A nationwide cohort study. Am J Epidemiol. 2020;189:1114–1123. doi: 10.1093/aje/kwaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Silva HH, Silva MG, Cerqueira F, Tavares V, Medeiros R. Genomic profile in association with sport-type, sex, ethnicity, psychological traits and sport injuries of elite athletes. J Sports Med Phys Fitness. 2022;62:418–434. doi: 10.23736/S0022-4707.21.12020-1. [DOI] [PubMed] [Google Scholar]
- 259.Eliakim A. Endocrine response to exercise and training—Closing the gaps. Pediatr Exerc Sci. 2016;28:226–232. doi: 10.1123/pes.2015-0244. [DOI] [PubMed] [Google Scholar]
- 260.Ben-Zaken S, Meckel Y, Nemet D, Kassem E, Eliakim A. The combined frequencies of the IL-6 G-174C and IGFBP3 A-202C polymorphisms among swimmers and runners. Growth Horm IGF Res. 2020;51:17–21. doi: 10.1016/j.ghir.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 261.Palles C, Johnson N, Coupland B, et al. Identification of genetic variants that influence circulating IGF1 levels: A targeted search strategy. Hum Mol Genet. 2008;17:1457–1464. doi: 10.1093/hmg/ddn034. [DOI] [PubMed] [Google Scholar]
- 262.Meisinger C, Linseisen J, Leitzmann M, Baurecht H, Baumeister SE. Association of physical activity and sedentary behavior with type 2 diabetes and glycemic traits: A two-sample mendelian randomization study. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2020-001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Bahls M, Leitzmann MF, Karch A, et al. Physical activity, sedentary behavior and risk of coronary artery disease, myocardial infarction and ischemic stroke: A two-sample mendelian randomization study. Clin Res Cardiol. 2021;110:1564–1573. doi: 10.1007/s00392-021-01846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Murphy RM, Watt MJ, Febbraio MA. Metabolic communication during exercise. Nat Metab. 2020;2:805–816. doi: 10.1038/s42255-020-0258-x. [DOI] [PubMed] [Google Scholar]
- 265.Moghetti P, Bacchi E, Brangani C, DonàS Negri C. Metabolic effects of exercise. Front Horm Res. 2016;47:44–57. doi: 10.1159/000445156. [DOI] [PubMed] [Google Scholar]
- 266.Hackney AC, Lane AR. Exercise and the regulation of endocrine hormones. Prog Mol Biol Transl Sci. 2015;135:293–311. doi: 10.1016/bs.pmbts.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 267.Kraemer WJ, Ratamess NA, Hymer WC, Nindl BC, Fragala MS. Growth hormone(s), testosterone, insulin-like growth factors, and cortisol: Roles and integration for cellular development and growth with exercise. Front Endocrinol (Lausanne) 2020;11:33. doi: 10.3389/fendo.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yaribeygi H, Atkin SL, Simental-Mendía LE, Sahebkar A. Molecular mechanisms by which aerobic exercise induces insulin sensitivity. J Cell Physiol. 2019;234:12385–12392. doi: 10.1002/jcp.28066. [DOI] [PubMed] [Google Scholar]
- 269.Hamasaki H. Interval exercise therapy for type 2 diabetes. Curr Diabetes Rev. 2018;14:129–137. doi: 10.2174/1573399812666161101103655. [DOI] [PubMed] [Google Scholar]
- 270.Hackney AC, Davis HC, Lane AR. Growth hormone-insulin-like growth factor axis, thyroid axis, prolactin, and exercise. Front Horm Res. 2016;47:1–11. doi: 10.1159/000445147. [DOI] [PubMed] [Google Scholar]
- 271.Janssen JA. Impact of physical exercise on endocrine aging. Front Horm Res. 2016;47:68–81. doi: 10.1159/000445158. [DOI] [PubMed] [Google Scholar]
- 272.Nieman DC, Pence BD. Exercise immunology: Future directions. J Sport Health Sci. 2020;9:432–445. doi: 10.1016/j.jshs.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985) 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 274.Campbell JP, Turner JE. Debunking the myth of exercise-induced immune suppression: Redefining the impact of exercise on immunological health across the lifespan. Front Immunol. 2018;9:648. doi: 10.3389/fimmu.2018.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Weyh C, Kruger K, Strasser B. Physical activity and diet shape the immune system during aging. Nutrients. 2020;12:622. doi: 10.3390/nu12030622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 278.Clauss M, Gérard P, Mosca A, Leclerc M. Interplay between exercise and gut microbiome in the context of human health and performance. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.637010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Bai J, Hu Y, Bruner DW. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7−18 years old children from the American Gut Project. Pediatr Obes. 2019;14:e12480. doi: 10.1111/ijpo.12480. [DOI] [PubMed] [Google Scholar]
- 280.Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 281.Zhou Q, Deng J, Pan X, et al. Gut microbiome mediates the protective effects of exercise after myocardial infarction. Microbiome. 2022;10:82. doi: 10.1186/s40168-022-01271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Pardon MC. Hormesis is applicable as a pro-healthy aging intervention in mammals and human beings. Dose Response. 2009;8:22–27. doi: 10.2203/dose-response.09-020.Pardon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Merry TL, Ristow M. Mitohormesis in exercise training. Free Radic Biol Med. 2016;98:123–130. doi: 10.1016/j.freeradbiomed.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 284.Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis) Exp Gerontol. 2010;45:410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 285.Gomez-Cabrera MC, Salvador-Pascual A, Cabo H, Ferrando B, Viña J. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic Biol Med. 2015;86:37–46. doi: 10.1016/j.freeradbiomed.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 286.Kasai S, Shimizu S, Tatara Y, Mimura J, Itoh K. Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules. 2020;10:320. doi: 10.3390/biom10020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kang GM, Min SH, Lee CH, et al. Mitohormesis in hypothalamic POMC neurons mediates regular exercise-induced high-turnover metabolism. Cell Metab. 2021;33:334–349. doi: 10.1016/j.cmet.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: Linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Sarzynski MA, Ghosh S, Bouchard C. Genomic and transcriptomic predictors of response levels to endurance exercise training. J Physiol. 2017;595:2931–2939. doi: 10.1113/JP272559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Gremeaux V, Gayda M, Lepers R, Sosner P, Juneau M, Nigam A. Exercise and longevity. Maturitas. 2012;73:312–317. doi: 10.1016/j.maturitas.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 291.Garatachea N, Pareja-Galeano H, Sanchis-Gomar F, et al. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015;18:57–89. doi: 10.1089/rej.2014.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lehnig AC, Stanford KI. Exercise-induced adaptations to white and brown adipose tissue. J Exp Biol. 2018;221(Suppl. 1) doi: 10.1242/jeb.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Stanford KI, Middelbeek RJ, Townsend KL, et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes. 2015;64:2002–2014. doi: 10.2337/db14-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise effects on white adipose tissue: Beiging and metabolic adaptations. Diabetes. 2015;64:2361–2368. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Guan Y, Yan Z. Molecular mechanisms of exercise and healthspan. Cells. 2022;11:872. doi: 10.3390/cells11050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Moore SC, Patel AV, Matthews CE, et al. Leisure time physical activity of moderate to vigorous intensity and mortality: A large pooled cohort analysis. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: A detailed pooled analysis of the dose−response relationship. JAMA Intern Med. 2015;175:959–967. doi: 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Carapeto PV, Aguayo-Mazzucato C. Effects of exercise on cellular and tissue aging. Aging (Albany NY) 2021;13:14522–14543. doi: 10.18632/aging.203051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Su Y, Wang T, Wu N, et al. Alpha-ketoglutarate extends Drosophila lifespan by inhibiting mTOR and activating AMPK. Aging (Albany NY) 2019;11:4183–4197. doi: 10.18632/aging.102045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Liu H, Ding J, Köhnlein K, et al. The gid ubiquitin ligase complex is a regulator of AMPK activity and organismal lifespan. Autophagy. 2020;16:1618–1634. doi: 10.1080/15548627.2019.1695399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Viollet B, Lantier L, Devin-Leclerc J, et al. Targeting the AMPK pathway for the treatment of type 2 diabetes. Front Biosci (Landmark Ed) 2009;14:3380–3400. doi: 10.2741/3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ballin M, Nordstrom P. Does exercise prevent major non-communicable diseases and premature mortality? A critical review based on results from randomized controlled trials. J Intern Med. 2021;290:1112–1129. doi: 10.1111/joim.13353. [DOI] [PubMed] [Google Scholar]
- 303.Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 304.Ayala-Pena S. Role of oxidative DNA damage in mitochondrial dysfunction and Huntington's disease pathogenesis. Free Radic Biol Med. 2013;62:102–110. doi: 10.1016/j.freeradbiomed.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Radak Z, Naito H, Kaneko T, et al. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch. 2002;445:273–278. doi: 10.1007/s00424-002-0918-6. [DOI] [PubMed] [Google Scholar]
- 306.Schmidt RH, Nickerson JM, Boatright JH. Exercise as gene therapy: BDNF and DNA damage repair. Asia Pac J Ophthalmol (Phila) 2016;5:309–311. doi: 10.1097/APO.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Vilela TC, de Andrade VM, Radak Z, de Pinho RA. The role of exercise in brain DNA damage. Neural Regen Res. 2020;15:1981–1985. doi: 10.4103/1673-5374.282237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wagner KH, Reichhold S, Neubauer O. Impact of endurance and ultraendurance exercise on DNA damage. Ann N Y Acad Sci. 2011;1229:115–123. doi: 10.1111/j.1749-6632.2011.06106.x. [DOI] [PubMed] [Google Scholar]
- 309.Williamson J, Hughes CM, Burke G, Davison GW. A combined γ-H2AX and 53BP1 approach to determine the DNA damage-repair response to exercise in hypoxia. Free Radic Biol Med. 2020;154:9–17. doi: 10.1016/j.freeradbiomed.2020.04.026. [DOI] [PubMed] [Google Scholar]
- 310.Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–1995. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- 311.Davis AA, Leyns CEG, Holtzman DM. Intercellular spread of protein aggregates in neurodegenerative disease. Annu Rev Cell Dev Biol. 2018;34:545–568. doi: 10.1146/annurev-cellbio-100617-062636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology (Bethesda) 2013;28:330–358. doi: 10.1152/physiol.00019.2013. [DOI] [PubMed] [Google Scholar]
- 313.Musci RV, Hamilton KL, Miller BF. Targeting mitochondrial function and proteostasis to mitigate dynapenia. Eur J Appl Physiol. 2018;118:1–9. doi: 10.1007/s00421-017-3730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Bhuiyan MS, Pattison JS, Osinska H, et al. Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest. 2013;123:5284–5297. doi: 10.1172/JCI70877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Liu Q, Chen L, Liang X, et al. Exercise attenuates angiotensin-induced muscle atrophy by targeting PPARγ/miR-29b. J Sport Health Sci. 2022;11:696–707. doi: 10.1016/j.jshs.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Dokladny K, Myers OB, Moseley PL. Heat shock response and autophagy–cooperation and control. Autophagy. 2015;11:200–213. doi: 10.1080/15548627.2015.1009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Campos JC, Baehr LM, Gomes KMS, et al. Exercise prevents impaired autophagy and proteostasis in a model of neurogenic myopathy. Sci Rep. 2018;8:11818. doi: 10.1038/s41598-018-30365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Toth A, Nickson P, Mandl A, Bannister ML, Toth K, Erhardt P. Endoplasmic reticulum stress as a novel therapeutic target in heart diseases. Cardiovasc Hematol Disord Drug Targets. 2007;7:205–218. doi: 10.2174/187152907781745260. [DOI] [PubMed] [Google Scholar]
- 319.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Bozi LH, Jannig PR, Rolim N, et al. Aerobic exercise training rescues cardiac protein quality control and blunts endoplasmic reticulum stress in heart failure rats. J Cell Mol Med. 2016;20:2208–2212. doi: 10.1111/jcmm.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Chang P, Zhang X, Zhang M, et al. Swimming exercise inhibits myocardial ER stress in the hearts of aged mice by enhancing cGMP‑PKG signaling. Mol Med Rep. 2020;21:549–556. doi: 10.3892/mmr.2019.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Khadir A, Kavalakatt S, Abubaker J, et al. Physical exercise alleviates ER stress in obese humans through reduction in the expression and release of GRP78 chaperone. Metabolism. 2016;65:1409–1420. doi: 10.1016/j.metabol.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 323.Hong J, Hong SG, Lee J, et al. Exercise training ameliorates cerebrovascular dysfunction in a murine model of Alzheimer's disease: Role of the P2Y2 receptor and endoplasmic reticulum stress. Am J Physiol Heart Circ Physiol. 2020;318:H1559–H1569. doi: 10.1152/ajpheart.00129.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Jin S, Li L, Ren Z, Li J, Wang R, Ding H. FAM134B-mediated ER-phagy alleviates endoplasmic reticulum stress of rat soleus muscle in response to acute exercise. Gen Physiol Biophys. 2022;41:71–78. doi: 10.4149/gpb_2021046. [DOI] [PubMed] [Google Scholar]
- 325.Wang SF, Chen S, Tseng LM, Lee HC. Role of the mitochondrial stress response in human cancer progression. Exp Biol Med (Maywood) 2020;245:861–878. doi: 10.1177/1535370220920558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Zhang Y, Oliveira AN, Hood DA. The intersection of exercise and aging on mitochondrial protein quality control. Exp Gerontol. 2020;131 doi: 10.1016/j.exger.2019.110824. [DOI] [PubMed] [Google Scholar]
- 327.Memme JM, Oliveira AN, Hood DA. Chronology of UPR activation in skeletal muscle adaptations to chronic contractile activity. Am J Physiol Cell Physiol. 2016;310:C1024–C1036. doi: 10.1152/ajpcell.00009.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Kim KH, Son JM, Benayoun BA, Lee C. The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metab. 2018;28:516–524. doi: 10.1016/j.cmet.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Woodhead JST, Merry TL. Mitochondrial-derived peptides and exercise. Biochim Biophys Acta Gen Subj. 2021;1865 doi: 10.1016/j.bbagen.2021.130011. [DOI] [PubMed] [Google Scholar]
- 330.Rajawat YS, Hilioti Z, Bossis I. Aging: Central role for autophagy and the lysosomal degradative system. Ageing Res Rev. 2009;8:199–213. doi: 10.1016/j.arr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 331.Tassi C, Mancuso F, Gambelunghe C, et al. Beta-N-acetylhexosaminidase activity and isoenzyme profile in the kidney and urine of trained rats. Immunopharmacol Immunotoxicol. 2001;23:573–583. doi: 10.1081/iph-100108603. [DOI] [PubMed] [Google Scholar]
- 332.Salminen A, Hongisto K, Vihko V. Lysosomal changes related to exercise injuries and training-induced protection in mouse skeletal muscle. Acta Physiol Scand. 1984;120:15–19. doi: 10.1111/j.1748-1716.1984.tb07367.x. [DOI] [PubMed] [Google Scholar]
- 333.Guo R, Yu Q, Liong EC, Fung ML, Tipoe GL. Cathepsin-b dependent autophagy ameliorates steatoheaptitis in chronic exercise rats. Histol Histopathol. 2020;35:833–847. doi: 10.14670/HH-18-204. [DOI] [PubMed] [Google Scholar]
- 334.Triolo M, Hood DA. Mitochondrial breakdown in skeletal muscle and the emerging role of the lysosomes. Arch Biochem Biophys. 2019;661:66–73. doi: 10.1016/j.abb.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 335.Bernardo BC, Ooi JYY, Weeks KL, Patterson NL, McMullen JR. Understanding key mechanisms of exercise-induced cardiac protection to mitigate disease: Current knowledge and emerging concepts. Physiol Rev. 2018;98:419–475. doi: 10.1152/physrev.00043.2016. [DOI] [PubMed] [Google Scholar]
- 336.Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407. doi: 10.1038/s41569-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 337.Cattadori G, Segurini C, Picozzi A, Padeletti L, Anza C. Exercise and heart failure: An update. ESC Heart Fail. 2018;5:222–232. doi: 10.1002/ehf2.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Wang H, Xie Y, Guan L, Elkin K, Xiao J. Targets identified from exercised heart: Killing multiple birds with one stone. NPJ Regen Med. 2021;6:23. doi: 10.1038/s41536-021-00128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Bei Y, Huang Z, Feng X, et al. Lymphangiogenesis contributes to exercise-induced physiological cardiac growth. J Sport Health Sci. 2022;11:466–478. doi: 10.1016/j.jshs.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Bass-Stringer S, Tai CMK, McMullen JR. IGF1-PI3K-induced physiological cardiac hypertrophy: Implications for new heart failure therapies, biomarkers, and predicting cardiotoxicity. J Sport Health Sci. 2021;10:637–647. doi: 10.1016/j.jshs.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Le Moal E, Pialoux V, Juban G, et al. Redox control of skeletal muscle regeneration. Antioxid Redox Signal. 2017;27:276–310. doi: 10.1089/ars.2016.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Nader GA, Esser KA. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol (1985) 2001;90:1936–1942. doi: 10.1152/jappl.2001.90.5.1936. [DOI] [PubMed] [Google Scholar]
- 343.Chen Z, Li L, Wu W, et al. Exercise protects proliferative muscle satellite cells against exhaustion via the Igfbp7-Akt-mTOR axis. Theranostics. 2020;10:6448–6466. doi: 10.7150/thno.43577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Zhang J, Yuan T, Wang JH. Moderate treadmill running exercise prior to tendon injury enhances wound healing in aging rats. Oncotarget. 2016;7:8498–8512. doi: 10.18632/oncotarget.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. 2010;16:105–118. [PubMed] [Google Scholar]
- 346.Baker JM, De Lisio M, Parise G. Endurance exercise training promotes medullary hematopoiesis. FASEB J. 2011;25:4348–4357. doi: 10.1096/fj.11-189043. [DOI] [PubMed] [Google Scholar]
- 347.Zhang H, Chen T, Ren J, et al. Pre-operative exercise therapy triggers anti-inflammatory trained immunity of Kupffer cells through metabolic reprogramming. Nat Metab. 2021;3:843–858. doi: 10.1038/s42255-021-00402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17:162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 349.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: The evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Koelwyn GJ, Zhuang X, Tammela T, Schietinger A, Jones LW. Exercise and immunometabolic regulation in cancer. Nat Metab. 2020;2:849–857. doi: 10.1038/s42255-020-00277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Koelwyn GJ, Quail DF, Zhang X, White RM, Jones LW. Exercise-dependent regulation of the tumour microenvironment. Nat Rev Cancer. 2017;17:620–632. doi: 10.1038/nrc.2017.78. [DOI] [PubMed] [Google Scholar]
- 352.Vermillion SA, James A, Dorrell RD, et al. Preoperative exercise therapy for gastrointestinal cancer patients: A systematic review. Syst Rev. 2018;7:103. doi: 10.1186/s13643-018-0771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Nakajima H, Yokoyama Y, Inoue T, et al. Clinical benefit of preoperative exercise and nutritional therapy for patients undergoing hepato-pancreato-biliary surgeries for malignancy. Ann Surg Oncol. 2019;26:264–272. doi: 10.1245/s10434-018-6943-2. [DOI] [PubMed] [Google Scholar]
- 354.Pouwels S, Stokmans RA, Willigendael EM, et al. Preoperative exercise therapy for elective major abdominal surgery: A systematic review. Int J Surg. 2014;12:134–140. doi: 10.1016/j.ijsu.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 355.Xiao J, Rosenzweig A. Exercise and cardiovascular protection: Update and future. J Sport Health Sci. 2021;10:607–608. doi: 10.1016/j.jshs.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Bei Y, Wang L, Ding R, et al. Animal exercise studies in cardiovascular research: Current knowledge and optimal design—A position paper of the committee on cardiac rehabilitation, Chinese Medical Doctors’ Association. J Sport Health Sci. 2021;10:660–674. doi: 10.1016/j.jshs.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.