Abstract
OBJECTIVE:
We aimed to examine trends in timing of diagnosis of critical congenital heart defects (CCHDs) and factors associated with delayed diagnosis (diagnosis after discharge home following delivery).
METHODS:
We examined a population-based retrospective cohort of CCHD cases among live births identified through the Massachusetts Birth Defects Monitoring Program. Congenital heart defects were considered critical if the infant received corrective surgery, interventional catheterization, palliative care, or died as a result of the defect within 12 months of birth. Timing of initial diagnosis was classified as prenatal, postnatal before discharge home, or delayed. Demographic, perinatal, and mortality information was obtained from the Registry of Vital Records and Statistics. Prevalence ratios (PRs) were used to examine associations with delayed diagnosis.
RESULTS:
Among 460 467 live births to Massachusetts residents between 2004 and 2009, we identified 916 CCHD cases, of which 126 (13.8%) had delayed diagnosis. Rates of prenatal CCHD diagnosis increased from 44.9% in 2004 to 63.8% in 2009, whereas rates of delayed diagnosis decreased from 17.1% to 10.6% over the same time period. Among cases with delayed diagnosis, the most common defects were coarctation, pulmonary valve stenosis, and tetralogy of Fallot. Delayed diagnosis was associated with delivery outside a tertiary hospital (adjusted PR: 3.6 [95% confidence interval: 2.5–5.2]) and isolated CCHD (adjusted PR: 1.7 [95% confidence interval: 1.1–2.7]).
CONCLUSIONS:
Despite increasing prenatal diagnosis of CCHDs, delayed diagnosis still occurs in over 10% of cases. Understanding factors associated with delayed diagnosis could help to improve prenatal and postnatal screening efforts, including pulse oximetry testing.
Keywords: critical congenital heart defects, delayed diagnosis, neonatal, prenatal diagnosis, infant, newborn, neonatal screening
What’s Known on This Subject:
Delayed diagnosis of critical congenital heart defects (CCHDs) is associated with increased morbidity and mortality.
What This Study Adds:
Despite increasing prenatal diagnosis rates, delayed diagnosis of CCHDs continues to occur, with rates highest among isolated cases and those delivered at nontertiary care hospitals. Better understanding of delayed diagnosis could help to improve screening efforts.
Congenital heart defects (CHDs) affect almost 90 per 10 000 births and are the leading cause of infant mortality from birth defects. 1 , 2 Critical congenital heart defects (CCHDs) are defined in various ways in the literature, based on some combination of cardiac anatomy, hypoxemia or hypoperfusion, and treatment, but all will cause life-threatening complications or death without intervention. Most CCHD definitions include the 7 defects that are considered primary targets for pulse oximetry screening, based on their tendency to result in hypoxemia: hypoplastic left heart syndrome, pulmonary atresia, tetralogy of Fallot, total anomalous pulmonary venous return, transposition of the great arteries, tricuspid atresia, and truncus arteriosus. 3 – 5 Many definitions also include some CHDs that do not consistently present with hypoxemia, such as coarctation of the aorta. 3 , 5 , 6 Several also incorporate a measure of severity, including a requirement that surgery, catheterization, or death occur within a specified time period, ranging from the first few weeks to the first year of life. 7 – 9
Although delayed diagnosis of CCHDs has been associated with serious complications, including seizure, cardiac arrest, and death, 7 , 10 , 11 few studies have examined temporal trends or factors associated with delayed diagnosis. Previous studies of late-detected CCHD differ in case definition and study methodology, but most have reported prevalence rates of ∼20% to 30%. 6 , 11 – 13 Factors that have been associated with late diagnosis include certain CCHD types, 6 , 8 , 9 , 12 nontertiary hospital nursery, 12 and absence of extracardiac defects. 6
The Secretary of Health and Human Services has recommended routine screening of newborns for CCHD with pulse oximetry, because earlier diagnosis may lead to better outcomes. The American Heart Association and the American Academy of Pediatrics have endorsed such screening, 14 and recent studies in US populations suggest that pulse oximetry screening is cost-effective and results in lower hospital costs during infancy. 3 , 11 , 15 In response, many states, including Massachusetts, have enacted legislation requiring pulse oximetry screening of newborns.
We examined a population-based retrospective cohort of CCHD cases among 2004–2009 live births to evaluate temporal trends in timing of diagnosis and factors associated with delayed diagnosis (diagnosis after discharge home following delivery).
Methods
Data Sources
Cases were ascertained through the Birth Defects Monitoring Program (BDMP) at the Massachusetts Department of Public Health, with demographic, perinatal, and mortality data obtained from the Massachusetts Registry of Vital Records and Statistics.
Since 1999, the BDMP has conducted statewide, population-based active surveillance of birth defects among Massachusetts residents. The BDMP identifies cases with structural birth defects diagnosed through 1 year of age from multiple sources, including delivery and specialty care hospitals, birthing centers, and vital records. Potential birth defect cases are assigned to trained abstractors who review maternal and infant medical records. All cases are coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification, modified British Pediatric Association (ICD-9-CM/BPA) system. Complex cases, cases with syndromes, and cases in which the infant died are reviewed by a clinical geneticist trained in pediatric cardiology. During the study period, the coverage area included Massachusetts and 2 large Rhode Island tertiary care hospitals near the Massachusetts border.
Subjects
We identified a cohort of CCHD cases among live births between January 1, 2004 and December 31, 2009. A CHD was considered critical if corrective surgery or interventional catheterization was performed, if palliative care was administered, and/or if the infant died of defect-related causes within the first year of life. Palliative care included comfort measures or do-not-resuscitate order, with documentation that treatment was required but would not be performed. Cases with ICD-9-CM/BPA codes for 1 or more of the following defects were included if determined to be critical: aortic atresia or hypoplasia, aortic stenosis (valvar), coarctation of the aorta, complete atrioventricular septal defect, dextro-transposition of the great arteries, double-outlet right ventricle, Ebstein anomaly, hypoplastic left heart syndrome, interrupted aortic arch, pulmonary atresia, pulmonary valve stenosis, single ventricle, tetralogy of Fallot, total anomalous pulmonary venous return, tricuspid atresia, and truncus arteriosus. Our CCHD definition includes the 7 primary target defects for pulse oximetry screening, as well as several additional defects that may also present with hypoxemia or hypoperfusion. 5 The ICD-9-CM/BPA codes for the defects included are shown in Supplemental Table 7.
Exposures and Outcomes
Surveillance data obtained on each CCHD case include presence of major extracardiac defects or syndromes, and date, place, and method of diagnosis. Cases without an extracardiac defect or recognizable syndrome were classified as isolated, even if multiple CHDs were present. Cases were classified into 3 groups by timing of initial diagnosis: prenatal diagnosis, diagnosis at the birth or transfer hospital before discharge home, or delayed. Home births were classified as delayed, unless diagnosed prenatally.
Demographic and perinatal information included the following maternal and infant characteristics: age, race/ethnicity, education, residence in or outside of Boston, prenatal care insurance type, prenatal care initiation month, delivery hospital level, gestational age, plurality, birth weight, and date and cause of any infant death. Each delivery hospital in Massachusetts is assigned a level based on the type of neonatal care available, and these designations were used in our analysis. Level 1 hospitals are able to provide care to low-risk mothers and infants; level 2 hospitals have special care nurseries and are able to provide care to those at moderate risk; and level 3 (tertiary) hospitals are able to provide the most advanced level of care to high-risk mothers and newborns. 16 Home and birth center deliveries were assigned to a separate category. Rhode Island hospitals were classified according to Massachusetts hospital level definitions.
The Massachusetts Department of Public Health Institutional Review Board determined that this study does not constitute human subjects research.
Statistical Analyses
We examined temporal trends in timing of initial CCHD diagnosis, as well as the distribution of demographic and clinical characteristics by timing of diagnosis. Frequency distributions were examined, and Pearson χ2 tests were used to examine relationships between covariates and to evaluate associations with timing of diagnosis. Prevalence rates and 95% confidence limits were constructed using the Poisson method. The Cochran Armitage test and linear regression were used to evaluate linear trends. Log-binomial regression models were used to estimate crude prevalence ratios (PRs) and 95% confidence intervals (CIs) for associations between demographic and clinical characteristics and delayed CCHD diagnosis. A fully adjusted multivariate model was constructed by using Poisson regression with robust error variance. 17 The fully adjusted model includes all demographic and clinical factors examined, with adjusted PR (aPR) estimates representing each individual factor while controlling for all other factors in the model.
To examine the impact of varying our CCHD definition, sensitivity analyses were performed examining risk factors for delay among CCHD cases diagnosed at ≤30 days after birth and among those with one of the 7 primary pulse oximetry screening target defects. To evaluate an alternative definition of delay, we compared those diagnosed >3 days after birth with those diagnosed earlier. To assess the potential effect of pulse oximetry screening, we examined factors associated with delay among the subset of cases diagnosed after birth.
In addition, we performed sensitivity analyses to test the effect of including cases with conditions associated with mortality: trisomy 13, trisomy 18, birth weight <1000 g, and/or gestational age <24 weeks. We also performed a subanalysis excluding cases born in Rhode Island.
All analyses were performed in SAS version 9.3 (SAS Institute, Inc, Cary, NC).
Results
Maternal and Infant Characteristics
Among 460 467 live births to Massachusetts residents, we identified 916 CCHD cases, for a birth prevalence of 19.9 per 10 000, which remained stable between 2004 and 2009 (Table 1). Four hundred seventy (51%) of these cases had one of the 7 primary target defects for pulse oximetry screening, with a birth prevalence of 10.2 per 10 000 among this subset (Table 2).
TABLE 1.
CCHD Cases by Birth Year and Timing of Diagnosis Among Massachusetts Live Births
| Birth Year | Number of Live Births a | CCHD Cases b | Prenatal, c N (%) | In hospital, d N (%) | Delayed, e N (%) | Overall CCHD Birth Prevalence f (95% CI) |
|---|---|---|---|---|---|---|
| 2004 | 78 062 | 158 | 71 (44.9) | 60 (38.0) | 27 (17.1) | 20.2 (17.2–23.6) |
| 2005 | 76 455 | 174 | 79 (45.4) | 62 (35.6) | 33 (19.0) | 22.8 (19.5–26.4) |
| 2006 | 77 237 | 164 | 87 (53.0) | 58 (35.4) | 19 (11.6) | 21.2 (18.1–24.7) |
| 2007 | 77 544 | 141 | 76 (53.9) | 49 (34.8) | 16 (11.4) | 18.2 (15.3–21.4) |
| 2008 | 76 572 | 138 | 77 (55.8) | 45 (32.6) | 16 (11.6) | 18.0 (15.1–21.3) |
| 2009 | 74 597 | 141 | 90 (63.8) | 36 (25.5) | 15 (10.6) | 18.9 (15.9–22.3) |
| Total | 460 467 | 916 | 480 (52.4) | 310 (33.8) | 126 (13.8) | 19.9 (18.6–21.2) |
Row percentages are presented. Percentages may not sum to 100 due to rounding.
From Massachusetts Registry of Vital Records and Statistics. Includes live births to Massachusetts residents that occurred in Massachusetts or Rhode Island. Rhode Island accounts for ~900 live births per year.
From Massachusetts BDMP. Rhode Island contributed 2 to 5 cases per year (24 total).
Cochran Armitage test for trend P < .002, 2-sided.
Includes diagnosis at delivery hospital or transfer hospital before discharge home. Cochran Armitage test for trend P < .03, 2-sided.
Diagnosed after discharge home. Cochran Armitage test for trend P < .02, 2-sided.
Per 10 000 live births. There was no significant linear trend.
TABLE 2.
CCHD Cases With One of the 7 Primary Screening Target Defects by Birth Year and Timing of Diagnosis Among Massachusetts Live Births
| Birth Year | Number of Live Births a | Primary Target CCHD Cases | Prenatal, b N (%) | In hospital, b N (%) | Delayed, N (%) | Primary Target CCHD Birth Prevalence c (95% CI) |
|---|---|---|---|---|---|---|
| 2004 | 78 062 | 81 | 38 (46.9) | 35 (43.2) | 8 (9.9) | 10.4 (8.2–12.9) |
| 2005 | 76 455 | 84 | 49 (58.3) | 28 (33.3) | 7 (8.3) | 11.0 (8.8–13.6) |
| 2006 | 77 237 | 84 | 52 (61.9) | 27 (32.1) | 5 (6.0) | 10.9 (8.7–13.5) |
| 2007 | 77 544 | 75 | 47 (62.7) | 25 (33.3) | 3 (4.0) | 9.7 (7.6–12.1) |
| 2008 | 76 572 | 69 | 50 (72.5) | 18 (26.1) | 1 (1.4) | 9.0 (7.0–11.4) |
| 2009 | 74 597 | 77 | 55 (71.4) | 15 (19.5) | 7 (9.1) | 10.3 (8.2–12.9) |
| Total | 460 467 | 470 | 291 (61.9) | 148 (31.5) | 31 (6.6) | 10.2 (9.3–11.2) |
Row percentages are presented. Percentages may not sum to 100 due to rounding. Primary screening target defects include dextro-transposition of the great arteries, hypoplastic left heart syndrome, pulmonary atresia, tetralogy of Fallot, total anomalous pulmonary venous return, tricuspid atresia, and truncus arteriosus.
From Massachusetts Registry of Vital Records and Statistics. Includes live births to Massachusetts residents that occurred in Massachusetts or Rhode Island. Rhode Island accounts for ~900 live births per year.
Cochran Armitage test for trend P < .002, 2-sided.
Per 10 000 live births. There was no significant linear trend.
The most common defects among cases were tetralogy of Fallot, coarctation, complete atrioventricular septal defect, and pulmonary valve stenosis (Table 3). Of the 916 cases, 651 (71%) had isolated CCHD, 83 (9%) had a major extracardiac defect(s), and 182 (20%) had a syndrome (Table 4). Of those with syndromes, 13 cases had trisomy 13 or 18, and 95 cases had trisomy 21 (data not shown).
TABLE 3.
Timing of Diagnosis by CCHD Type
| CCHD Type | Total N | Prenatal, N (%) | In-hospital, a N (%) | Delayed, N (%) |
|---|---|---|---|---|
| Tetralogy of Fallot b | 191 | 110 (57.6) | 61 (31.9) | 20 (10.5) |
| Coarctation | 179 | 67 (37.4) | 58 (32.4) | 54 (30.2) |
| Complete atrioventricular septal defect | 134 | 94 (70.2) | 34 (25.4) | 6 (4.5) |
| Pulmonary valve stenosis | 134 | 42 (31.3) | 63 (47.0) | 29 (21.6) |
| dextro-Transposition of the great arteries b | 108 | 65 (60.2) | 43 (39.8) | 0 |
| Hypoplastic left heart syndrome b | 66 | 58 (87.9) | 8 (12.1) | 0 |
| Double outlet right ventricle | 57 | 47 (82.5) | 9 (15.8) | 1 (1.8) |
| Aortic stenosis, valvar | 50 | 19 (38.0) | 18 (36.0) | 13 (26.0) |
| Pulmonary atresia b | 44 | 34 (77.3) | 10 (22.7) | 0 |
| Total anomalous pulmonary venous return b | 44 | 11 (25.0) | 23 (52.3) | 10 (22.7) |
| Tricuspid atresia b | 26 | 23 (88.5) | 3 (11.5) | 0 |
| Single ventricle | 25 | 23 (92.0) | 2 (8.0) | 0 |
| Interrupted aortic arch | 22 | 13 (59.1) | 8 (36.4) | 1 (4.6) |
| Truncus arteriosus b | 13 | 7 (53.8) | 5 (38.5) | 1 (7.7) |
| Ebstein anomaly | 10 | 8 (80.0) | 2 (20.0) | 0 |
| Aortic arch atresia or hypoplasia | 1 | 1 (100) | 0 | 0 |
| Total | 1104 | 622 | 347 | 135 |
Row percentages are presented. Table values reflect number of defects (N = 1104), not number of cases (N = 916). Percentages may not sum to 100 due to rounding.
Includes diagnosis at delivery hospital or transfer hospital before discharge home.
Primary pulse oximetry screening target defect.
TABLE 4.
Demographic and Clinical Characteristics by Timing of Initial CCHD Diagnosis Among Massachusetts Live Births 2004–2009
| N | Prenatal, N (%) | In-hospital a , N (%) | Delayed, N (%) | |
|---|---|---|---|---|
| Maternal age, y | ||||
| 15–24 | 192 | 104 (21.7) | 62 (20.0) | 26 (20.6) |
| 25–34 | 473 | 239 (49.8) | 164 (52.9) | 70 (55.6) |
| ≥35 | 251 | 137 (28.5) | 84 (27.1) | 30 (23.8) |
| Maternal race/ethnicity b | ||||
| Non-Hispanic white | 640 | 327 (68.1) | 223 (71.9) | 90 (72.0) |
| Non-Hispanic black | 81 | 51 (10.6) | 20 (6.4) | 10 (8.0) |
| Hispanic | 118 | 68 (14.2) | 37 (11.9) | 13 (10.4) |
| Asian | 49 | 19 (4.0) | 22 (7.1) | 8 (6.4) |
| Other | 27 | 15 (3.1) | 8 (2.6) | 4 (3.2) |
| Maternal education, y c | ||||
| 0–11 | 88 | 36 (7.5) | 40 (12.9) | 12 (9.6) d |
| ≥12 | 825 | 442 (92.5) | 270 (87.1) | 113 (90.4) |
| Region of residence | ||||
| Boston | 114 | 82 (17.1) | 25 (8.1) | 7 (5.6) d |
| Outside Boston | 802 | 398 (82.9) | 285 (91.9) | 119 (94.4) |
| Delivery hospital type e | ||||
| Home birth/birth center | 6 | 0 | 1 (0.3) | 5 (4.0) d |
| Level 1 | 99 | 3 (0.6) | 59 (19.0) | 37 (29.4) |
| Level 2 | 163 | 10 (2.1) | 116 (37.4) | 37 (29.4) |
| Level 3 | 648 | 467 (97.3) | 134 (43.2) | 47 (37.3) |
| Prenatal care insurance type f | ||||
| Private | 602 | 323 (67.4) | 200 (64.7) | 79 (63.2) |
| Government/other | 311 | 156 (32.6) | 109 (35.3) | 46 (36.8) |
| Prenatal care initiation g | ||||
| First trimester | 784 | 427 (90.5) | 259 (84.9) | 98 (79.0) d |
| After first trimester | 117 | 45 (9.5) | 46 (15.1) | 26 (21.0) |
| CCHD type | ||||
| Isolated h | 651 | 326 (67.9) | 221 (71.3) | 104 (82.5) d |
| Nonsyndromic CCHD with extracardiac defect | 83 | 46 (9.6) | 29 (9.4) | 8 (6.4) |
| CCHD with syndrome | 182 | 108 (22.5) | 60 (19.4) | 14 (11.1) |
| Gestational age, wk i | ||||
| 20–36 | 174 | 97 (20.2) | 63 (20.3) | 14 (11.1) |
| ≥37 | 742 | 383 (79.8) | 247 (79.7) | 112 (88.9) |
| Plurality | ||||
| Singleton | 845 | 446 (92.9) | 282 (91.0) | 117 (92.9) |
| Multiple | 71 | 34 (7.1) | 28 (9.0) | 9 (7.1) |
| Birth weight, g b | ||||
| 355–1499 | 42 | 21 (4.4) | 19 (6.2) | 2 (1.6) |
| ≥1500 | 873 | 459 (95.6) | 290 (93.8) | 124 (98.4) |
Column percentages were based on nonmissing data. Percentages may not sum to 100 due to rounding.
Includes diagnosis at delivery hospital or transfer hospital before discharge home.
One missing.
Three missing.
Pearson χ2 P < .05.
One infant born en-route to the hospital was coded to the destination hospital level.
Three missing information on prenatal care insurance. Twenty-six reported “Other” insurance: 13 diagnosed prenatally, 9 diagnosed in-hospital, and 4 with delayed diagnosis.
Fifteen missing information on prenatal care initiation. Of these, 8 were diagnosed prenatally, 5 were diagnosed in-hospital, and 2 had delayed diagnosis.
Includes cases with single CCHDs and cases with multiple cardiac defects.
Based on clinical estimate.
One hundred seven (11.7%) of the study cases died within 12 months of birth. Of these, 81 (75.7%) were diagnosed prenatally, 22 (20.6%) were diagnosed in the hospital, and 4 (3.7%) had delayed diagnosis. Among infants who died, the most common CCHDs were complete atrioventricular septal defect, hypoplastic left heart syndrome, and coarctation of the aorta. Thirty-four (31.8%) of the cases who died had a syndrome (data not shown).
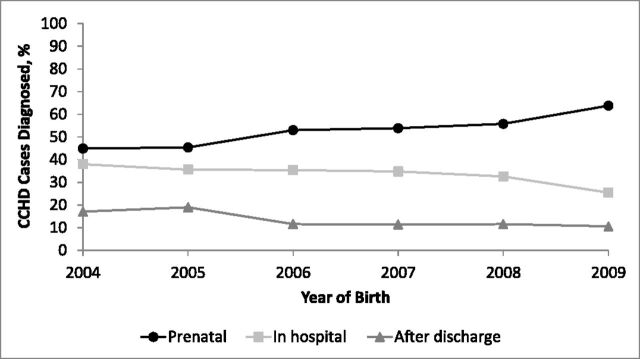
Trends in timing of diagnosis by birth year are shown in Fig 1. The prenatal diagnosis rate increased from 44.9% to 63.8% between 2004 and 2009. The delayed diagnosis rate decreased from 17.1% to 10.6% over the same time period, with the biggest decrease observed between 2005 and 2006 (Table 1). There were significant linear trends of increasing prenatal diagnosis and decreasing in-hospital and delayed diagnosis over the study period (Table 1). No significant linear trend in delayed diagnosis was observed among those with a primary screening target defect (Table 2).
FIGURE 1.
Timing of diagnosis of CCHD cases by year of birth.
Among cases with delayed diagnosis, the most common defects were coarctation, pulmonary valve stenosis, and tetralogy of Fallot (Table 3). The distributions of maternal age, race, type of insurance, and multiple births were similar by timing of diagnosis (Table 4). Residence outside of Boston, initiation of prenatal care after the first trimester, delivery in a nontertiary care setting, and isolated CCHD were more frequent among those with delayed diagnosis, and gestational age <37 weeks was less frequent. There were 5 cases among home births, all with delayed diagnosis, and 1 case born in a birth center, with an in-hospital diagnosis.
Log-Binomial and Poisson Regression Models
Crude and adjusted PRs are presented in Table 5. Delivery in a nontertiary care setting (aPR: 3.6 [95% CI: 2.5–5.2]) and having isolated CCHD (aPR: 1.7 [95% CI: 1.1–2.7]) were associated with increased risk of delayed diagnosis.
TABLE 5.
Crude and Adjusted PRs for Associations Between Demographic and Clinical Factors and Delayed CCHD Diagnosis
| Crude PR for Delayed Diagnosis (95% CI) | aPR a for Delayed Diagnosis (95% CI) | |
|---|---|---|
| Maternal age, y | ||
| 15–24 | Reference | Reference |
| 25–34 | 1.1 (0.7–1.7) | 1.2 (0.8–1.9) |
| ≥35 | 0.9 (0.5–1.4) | 1.0 (0.6–1.7) |
| Maternal race/ethnicity | ||
| Non-Hispanic white | Reference | Reference |
| Non-Hispanic black | 0.9 (0.5–1.6) | 1.2 (0.6–2.4) |
| Hispanic | 0.8 (0.4–1.3) | 0.9 (0.6–1.5) |
| Asian | 1.2 (0.6–2.2) | 1.3 (0.7–2.5) |
| Other | 1.0 (0.4–2.6) | 1.0 (0.5–2.0) |
| Maternal education, y | ||
| ≥12 | Reference | Reference |
| 0–11 | 1.0 (0.6–1.7) | 0.8 (0.4–1.5) |
| Region of residence | ||
| Boston | Reference | Reference |
| Outside Boston | 2.4 (1.2–5.0) b | 1.6 (0.7–3.7) |
| Delivery hospital type | ||
| Level 3 | Reference | Reference |
| Level 1, level 2, home or birth center | 4.1 (2.9–5.7) b | 3.6 (2.5–5.2) b |
| Prenatal care insurance type | ||
| Private | Reference | Reference |
| Government/other | 1.1 (0.8–1.6) | 1.2 (0.8–1.8) |
| Prenatal care initiation | ||
| First trimester | Reference | Reference |
| After first trimester | 1.8 (1.2–2.6) b | 1.4 (0.9–2.1) |
| CCHD type | ||
| Nonisolated | Reference | Reference |
| Isolated c | 1.9 (1.2–3.0) b | 1.7 (1.1–2.7) b |
| Gestational age, wk d | ||
| ≥37 | Reference | Reference |
| 20–36 | 0.5 (0.3–0.9) b | 0.7 (0.4–1.2) |
| Plurality | ||
| Singleton | Reference | Reference |
| Multiple | 0.9 (0.5–1.7) | 1.4 (0.8–2.6) |
| Birth weight, g | ||
| ≥1500 | Reference | Reference |
| 355–1499 | 0.3 (0.1–1.3) | 0.6 (0.2–2.5) |
Adjusted model includes all demographic and clinical factors examined. Each individual variable is presented after controlling for all other variables in the model.
P < .05.
Includes cases with single CCHDs and cases with multiple cardiac defects.
Based on clinical estimate.
Sensitivity Analyses
When we excluded those cases diagnosed >30 days after birth, we still observed an increase in delayed diagnosis with nontertiary hospital and isolated CCHD. These associations persisted when we used a different definition of delay, comparing those diagnosed >3 days after birth to those diagnosed earlier (Table 6).
TABLE 6.
aPR for Associations Between Demographic and Clinical Factors and Delayed Diagnosis Among Subgroups of CCHD Cases
| aPR a (95% CI) for Delay b Excluding Diagnoses After 30 d, N = 848 | aPR a (95% CI) for Delay b Among Primary 7 Defect c Cases, N = 470 | aPR a (95% CI) for Delay b Excluding Prenatal, N = 436 | aPR a (95% CI) for Diagnosis > 3 d Versus ≤ 3 d, N = 916 | |
|---|---|---|---|---|
| Maternal age, y | ||||
| 15–24 | Reference | Reference | Reference | Reference |
| 25–34 | 2.2 (0.9–5.2) | 0.8 (0.3–1.9) | 1.0 (0.6–1.5) | 1.2 (0.9–1.8) |
| ≥35 | 1.8 (0.7–4.6) | 0.8 (0.3–2.3) | 0.9 (0.5–1.4) | 1.2 (0.8–1.9) |
| Maternal race/ethnicity | ||||
| Non-Hispanic white | Reference | Reference | Reference | Reference |
| Non-Hispanic black | 0.7 (0.2–2.4) | 0.8 (0.2–4.0) | 1.2 (0.7–2.2) | 1.4 (0.8–2.3) |
| Hispanic | 0.9 (0.4–2.0) | 0.7 (0.2–2.2) | 1.0 (0.6–1.7) | 1.0 (0.7–1.6) |
| Asian | 1.9 (0.9–4.0) | 3.0 (1.0–8.9) d | 1.0 (0.6–1.9) | 1.4 (0.8–2.6) |
| Other | 0.5 (0.1–2.5) | 1.9 (0.6–6.4) | 1.2 (0.6–2.4) | 1.0 (0.6–1.8) |
| Maternal education, y | ||||
| ≥12 | Reference | Reference | Reference | Reference |
| 0–11 | 1.5 (0.6–3.6) | 0.8 (0.3–2.1) | 0.7 (0.4–1.3) | 0.8 (0.5–1.3) |
| Region of residence | ||||
| Boston | Reference | Reference | Reference | Reference |
| Outside Boston | 1.5 (0.6–4.1) | 0.7 (0.2–3.0) | 1.6 (0.8–3.5) | 2.0 (0.9–4.2) |
| Delivery hospital type | ||||
| Level 3 | Reference | Reference | Reference | Reference |
| Nonlevel 3 | 3.1 (1.8–5.2) d | 4.8 (2.1–11.1) d | 1.0 (0.7–1.4) | 3.6 (2.7–4.9) d |
| Prenatal care insurance | ||||
| Private | Reference | Reference | Reference | Reference |
| Government/other | 1.3 (0.7–2.3) | 1.9 (0.8–4.6) | 1.1 (0.8–1.6) | 1.2 (0.9–1.7) |
| Prenatal care initiation | ||||
| First trimester | Reference | Reference | Reference | Reference |
| After first trimester | 1.1 (0.5–2.3) | 1.5 (0.6–3.4) | 1.2 (0.9–1.7) | 1.1 (0.8–1.6) |
| CCHD type | ||||
| Nonisolated | Reference | Reference | Reference | Reference |
| Isolated e | 2.0 (1.0–4.0) d | 0.9 (0.4–2.2) | 1.6 (1.0–2.5) d | 1.6 (1.1–2.2) d |
| Gestational age, wk f | ||||
| ≥37 | Reference | Reference | Reference | Reference |
| 20–36 | 0.4 (0.1–1.2) | 0.4 (0.1–1.8) | 0.7 (0.4–1.2) | 1.1 (0.8–1.7) |
| Plurality | ||||
| Singleton | Reference | Reference | Reference | Reference |
| Multiple | 1.0 (0.4–3.1) | 3.1 (0.9–10.4) | 1.2 (0.6–2.3) | 1.4 (0.9–2.3) |
| Birth weight, g | ||||
| ≥1500 | Reference | Reference | Reference | Reference |
| 355–1499 | 1.2 (0.2–8.8) | NA | 0.5 (0.1–2.0) | 1.1 (0.5–2.2) |
NA, not applicable.
Adjusted model includes all demographic and clinical factors examined. Each individual variable is presented after controlling for all other variables in the model.
Diagnosed after discharge home following delivery.
Includes dextro-transposition of the great arteries, hypoplastic left heart syndrome, pulmonary atresia, tetralogy of Fallot, total anomalous pulmonary venous return, tricuspid atresia, and truncus arteriosus.
P < .05.
Includes cases with single CCHDs and cases with multiple cardiac defects.
Based on clinical estimate.
When we limited our analysis to cases with one of the 7 primary screening target defects, we no longer observed an association between isolated CCHD and delayed diagnosis. When prenatally diagnosed cases were excluded, we no longer observed an association between nontertiary hospital and delayed diagnosis (Table 6).
aPRs were similar when we excluded 33 cases with trisomy 13, trisomy 18, birth weight <1000 g, and/or gestational age <24 weeks and in a subanalysis, which excluded 24 cases born in Rhode Island (data not shown).
Discussion
Consistent with other recent estimates, 12 , 13 we observed a CCHD prevalence rate of ∼20 per 10 000 live births overall and ∼10 per 10 000 live births among those with a primary 7 screening target defect, which remained constant during the 2004–2009 study period. Prenatal diagnosis increased by 42%, whereas in-hospital diagnosis decreased by 33%, and delayed diagnosis decreased by 38%.
The biggest decrease in delayed diagnosis occurred between 2005 and 2006. The temporal decline in delayed diagnosis observed in the current study and by others 9 , 12 may be explained in part by an increase in prenatal diagnosis. This increase likely results from a combination of increased utilization of prenatal ultrasound and improvements in prenatal cardiac imaging, including recently updated 2006 guidelines for obtaining 4-chamber views and evaluating outflow tracts whenever feasible. 18 – 20 In 2006, Massachusetts enacted mandatory health insurance legislation, which may have also contributed to the increase in prenatal diagnosis and the decrease in delayed diagnosis observed after 2005.
The most common defects among cases with delayed diagnosis were tetralogy of Fallot, coarctation, pulmonary valve stenosis, and aortic stenosis. Unlike most of the primary screening target defects, which tend to present with greatly reduced pulmonary or systemic blood flow, some tetralogy of Fallot cases occur with minimal right ventricular outflow obstruction (“pink tets”), which can result in delayed diagnosis. Similarly, some CCHDs such as coarctation, aortic stenosis, and pulmonary valve stenosis may not present clinically until weeks or months after birth, depending on the severity of the obstruction. Some CCHDs can also be difficult to detect prenatally. Coarctation, in particular, is often missed, as evaluation of the aortic arch is not part of routine obstetric ultrasound screening. 18
Delivery in a nontertiary care setting and isolated CCHD were significantly associated with delayed diagnosis in a multivariate model. These findings are consistent with other recent studies. 6 , 12 The association with nontertiary hospital delivery may be partly explained by an increase in planned tertiary deliveries for infants with a prenatal CCHD diagnosis. The fact that we no longer observed this association when we excluded prenatally diagnosed cases supports this theory.
Our definition of CCHD differs from that used in several other studies. We included CHD cases where intervention or death occurred within 1 year, in contrast to other studies, which used shorter time periods. However, when we limited our analysis to those diagnosed at ≤30 days of birth the observed associations remained unchanged. Our CCHD definition also includes more than just the 7 primary screening target defects for pulse oximetry. When we limited our analysis to cases with one of these 7 defects, we no longer observed an association between delayed diagnosis and isolated defect, likely because of the high frequency of hypoxemia in these infants.
In addition, we defined delay as diagnosis after discharge home, whereas a recent study defined delay as diagnosis >3 days after birth. 6 When we analyzed our data using this alternate definition, the observed associations did not change. This is not surprising, because 124 of the 126 cases in our original delayed group were diagnosed >3 days after birth.
Strengths of our study include an active, population-based birth defects surveillance program for case ascertainment, with each case individually reviewed. All complex cases, cases with syndromes, and infant deaths were reviewed by a clinical geneticist trained in pediatric cardiology. In contrast to several previous studies, 11 – 13 our definition of CCHD required documentation of clinical severity in addition to defect code. To be considered critical, infants with a CHD diagnosis had to receive corrective surgery, interventional catheterization, palliative care, or die of CHD-related causes within the first 12 months after delivery. Our individual case review allowed us to determine whether these cases were critical or not, enabling us to include several defects that are often excluded from CCHD studies because of the inability to determine their severity.
Potential limitations of our study include the use of birth certificate data for demographic and perinatal information, with some covariates missing information and possibly misclassified for some cases. Also, we may not have identified the earliest diagnosis date for some cases, leading to the potential for misclassification of timing of diagnosis and subanalysis categories. Because our study included cases with multiple anomalies, syndromes, low birth weight, and/or prematurity, it was sometimes difficult to rule out the possibility that we included as cases infants who died of causes unrelated to CCHD. However, when we analyzed our data after excluding those cases with trisomy 13, trisomy 18, gestational age <24 weeks, and/or birth weight <1000 g, we observed similar results. We also cannot rule out the possibility that an infant had surgery or catheterization out of state, which we would not have been able to capture, thereby missing some true cases. We feel this scenario would be uncommon, however, because we only included births to Massachusetts residents, and because Massachusetts has a large number of pediatric cardiologists and a large pediatric referral center where many complex CHD surgeries are performed. Also, our results were unchanged when we excluded the Rhode Island births.
Massachusetts is a state with excellent access to prenatal care, making this an ideal place for studying delayed diagnosis. During most of the study period, Massachusetts had mandated health care coverage. This fact, as well as the large number of tertiary care hospitals in Massachusetts, may have contributed to the high rates of prenatal diagnosis we observed, which are higher than those seen in many other states. 21 , 22
Although rates declined during the 5-year study period, delayed diagnosis continues to occur, with rates highest among isolated cases and those delivered at nontertiary care hospitals. Pulse oximetry screening of asymptomatic newborns was recently mandated in Massachusetts, with the goal of reducing this rate even further. However, timely detection of certain defects that do not consistently present with hypoxemia will continue to be challenging.
Conclusions
Despite increasing rates of prenatal diagnosis, delayed diagnosis still occurs in over 10% of CCHD cases. Understanding the risk factors for delayed diagnosis of CCHD may help to reduce delayed detection rates, to better target prenatal and postnatal CCHD screening programs, and to allow for evaluation of screening program effectiveness.
Supplementary Material
Glossary
- aPR
adjusted prevalence ratio
- BDMP
Birth Defects Monitoring Program
- BPA
British Pediatric Association
- CCHD
critical congenital heart defect
- CHD
congenital heart defect
- CI
confidence interval
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- PR
prevalence ratio
Footnotes
Ms Liberman carried out the data analysis and drafted the initial manuscript; Dr Getz advised on data analysis and interpretation of results and reviewed and revised the manuscript; Dr Lin performed case review, assisted in the interpretation of the results, and reviewed and revised the manuscript; Ms Higgins was part of the case review team, and reviewed and revised the manuscript; Dr Sekhavat assisted in the interpretation of results, and reviewed and revised the manuscript; Dr Markenson assisted in the interpretation of results, and reviewed and revised the manuscript; Dr Anderka conceptualized and designed the study, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
FUNDING: No external funding.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1. Bjornard K , Riehle-Colarusso T , Gilboa SM , Correa A . Patterns in the prevalence of congenital heart defects, metropolitan Atlanta, 1978 to 2005. Birth Defects Res A Clin Mol Teratol. 2013;97(2):87–94 [DOI] [PubMed] [Google Scholar]
- 2. Yang Q , Chen H , Correa A , Devine O , Mathews TJ , Honein MA . Racial differences in infant mortality attributable to birth defects in the United States, 1989–2002. Birth Defects Res A Clin Mol Teratol. 2006;76(10):706–713 [DOI] [PubMed] [Google Scholar]
- 3. Garg LF , Van Naarden Braun K , Knapp MM , et al. Results from the New Jersey statewide critical congenital heart defects screening program. Pediatrics. 2013;132(2). Available at: www.pediatrics.org/cgi/content/full/132/2/e314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kemper AR , Mahle WT , Martin GR , et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011;128(5). Available at: www.pediatrics.org/cgi/content/full/128/5/e1259 [DOI] [PubMed] [Google Scholar]
- 5. Mahle WT , Newburger JW , Matherne GP , et al. American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research American Academy of Pediatrics Section on Cardiology and Cardiac Surgery, and Committee on Fetus and Newborn . Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation. 2009;120(5):447–458 [DOI] [PubMed] [Google Scholar]
- 6. Peterson C , Ailes E , Riehle-Colarusso T , et al. Late detection of critical congenital heart disease among US infants: estimation of the potential impact of proposed universal screening using pulse oximetry. JAMA Pediatr. 2014;168(4):361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schultz AH , Localio AR , Clark BJ , Ravishankar C , Videon N , Kimmel SE . Epidemiologic features of the presentation of critical congenital heart disease: implications for screening. Pediatrics. 2008;121(4):751–757 [DOI] [PubMed] [Google Scholar]
- 8. Mellander M , Sunnegårdh J . Failure to diagnose critical heart malformations in newborns before discharge—an increasing problem? Acta Paediatr. 2006;95(4):407–413 [DOI] [PubMed] [Google Scholar]
- 9. Chang RK , Gurvitz M , Rodriguez S . Missed diagnosis of critical congenital heart disease. Arch Pediatr Adolesc Med. 2008;162(10):969–974 [DOI] [PubMed] [Google Scholar]
- 10. Brown KL , Ridout DA , Hoskote A , Verhulst L , Ricci M , Bull C . Delayed diagnosis of congenital heart disease worsens preoperative condition and outcome of surgery in neonates. Heart. 2006;92(9):1298–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peterson C , Dawson A , Grosse SD , et al. Hospitalizations, costs, and mortality among infants with critical congenital heart disease: how important is timely detection? Birth Defects Res A Clin Mol Teratol. 2013;97(10):664–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dawson AL , Cassell CH , Riehle-Colarusso T , et al. Factors associated with late detection of critical congenital heart disease in newborns. Pediatrics. 2013;132(3). Available at: www.pediatrics.org/cgi/content/full/132/3/e604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131(5). Available at: www.pediatrics.org/cgi/content/full/131/5/e1502 [DOI] [PMC free article] [PubMed]
- 14. Mahle WT , Martin GR , Beekman RH III , Morrow WR Section on Cardiology and Cardiac Surgery Executive Committee . Endorsement of Health and Human Services recommendation for pulse oximetry screening for critical congenital heart disease. Pediatrics. 2012;129(1):190–192 [DOI] [PubMed] [Google Scholar]
- 15. Peterson C , Grosse SD , Oster ME , Olney RS , Cassell CH . Cost-effectiveness of routine screening for critical congenital heart disease in US newborns. Pediatrics. 2013;132(3). Available at: www.pediatrics.org/cgi/content/full/132/3/e595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massachusetts Department of Public Health Regulations & Policies. 105 CMR 130.000: Hospital Licensure. 2009; Available at: www.mass.gov/eohhs/docs/dph/regs/105cmr130.pdf. Accessed May 20, 2014
- 17. Lee J , Tan CS , Chia KS . A practical guide for multivariate analysis of dichotomous outcomes. Ann Acad Med Singapore. 2009;38(8):714–719 [PubMed] [Google Scholar]
- 18. Carvalho JS , Allan LD , Chaoui R , et al. International Society of Ultrasound in Obstetrics and Gynecology . ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol. 2013;41(3):348–359 [DOI] [PubMed] [Google Scholar]
- 19. Abuhamad AZ , ACOG Committee on Practice Bulletins-Obstetrics . ACOG Practice Bulletin, clinical management guidelines for obstetrician-gynecologists number 98, October 2008 (replaces Practice Bulletin number 58, December 2004). Ultrasonography in pregnancy. Obstet Gynecol. 2008;112(4):951–961 [DOI] [PubMed] [Google Scholar]
- 20. American Institute of Ultrasound in Medicine . AIUM practice guideline for the performance of obstetric ultrasound examinations. J Ultrasound Med. 2013;32(6):1083–1101 [DOI] [PubMed] [Google Scholar]
- 21. Israel SW , Roofe LR , Saville BR , Walsh WF . Improvement in antenatal diagnosis of critical congenital heart disease implications for postnatal care and screening. Fetal Diagn Ther. 2011;30(3):180–183 [DOI] [PubMed] [Google Scholar]
- 22. Pinto NM , Keenan HT , Minich LL , Puchalski MD , Heywood M , Botto LD . Barriers to prenatal detection of congenital heart disease: a population-based study. Ultrasound Obstet Gynecol. 2012;40(4):418–425 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.