ABSTRACT
Background
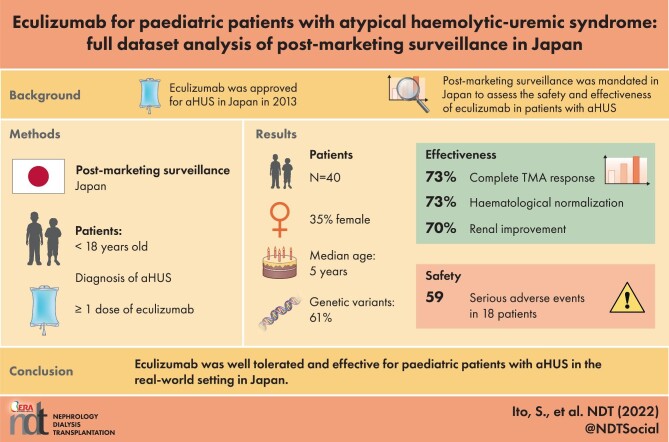
Eculizumab was approved for atypical haemolytic uraemic syndrome (aHUS) in Japan in 2013. Post-marketing surveillance (PMS) was mandated by regulatory authorities to assess the safety and effectiveness of eculizumab in patients with aHUS in a real-world setting.
Methods
Paediatric patients in the PMS cohort who were <18 years of age at the first administration of eculizumab and diagnosed with aHUS [excluding Shiga toxin–producing Escherichia coli HUS, thrombotic thrombocytopaenic purpura and secondary thrombotic microangiopathy (TMA)] were included in the effectiveness and safety analysis. Clinical endpoints of effectiveness [complete TMA response, TMA event-free status, platelet (PLT) count and lactate dehydrogenase (LDH) normalization, serum creatinine (sCr) decrease and estimated glomerular filtration rate (eGFR) improvement] were analysed in patients treated with at least one dose of eculizumab. Serious adverse events (SAEs) were also evaluated.
Results
A total of 40 paediatric patients (median age 5 years) were included. The median eculizumab treatment duration was 66 weeks. PLT count, LDH and eGFR significantly improved at 10 days post-treatment. Complete TMA response, haematologic normalization, sCr decrease, eGFR improvement and TMA event-free status were achieved by 73.3%, 73.3%, 70.0%, 78.3% and 77.5% of patients, respectively. Discontinuation criteria were met by 18 patients: 13 patients maintained treatment discontinuation at the end of observation and 5 patients, including 1 patient with aHUS relapse, continued the treatment but extended the treatment interval. During eculizumab treatment, 59 SAEs (0.66/person-year) were reported. Although four deaths were reported, none of them were related to eculizumab.
Conclusion
Eculizumab was well tolerated and effective for paediatric patients with aHUS in the real-world setting in Japan.
Keywords: atypical haemolytic uraemic syndrome, complement, eculizumab, post-marketing surveillance, thrombotic microangiopathy
Graphical Abstract
Graphical Abstract.
Introduction
Atypical haemolytic uraemic syndrome (aHUS) is a form of thrombotic microangiopathy (TMA) that is defined by a triad of non-immune microangiopathic haemolytic anaemia (MAHA), thrombocytopaenia and acute kidney injury (AKI) [1, 2]. In the USA, the annual incidence is estimated to be two cases per million and the prevalence is reported to be 3.3 per million children [1, 3]. In Japan, ∼100–200 patients are diagnosed with aHUS [1, 3].
Complement gene variants [e.g. complement factor H (CFH), complement factor I, membrane cofactor protein (MCP), thrombomodulin, complement component C3 (C3) and complement factor B] were found in 50–60% of patients with aHUS in previous reports [2, 4–6]. In patients with aHUS, TMA develops as a consequence of genetic or autoimmune abnormalities, resulting in dysregulation of the alternative complement pathway on the vascular endothelium [1, 2]. Complement-mediated TMA can occur at any age; however, 40% of patients develop the disease by 18 years of age [4]. The penetrance of aHUS is reported to be ∼20–50% (incomplete penetrance) in patients with genetic abnormalities [2, 7], which suggests the requirement for a triggering stimulus [3, 6, 8].
Mortality is higher in children than in adults (6.7% versus 0.8% at 1 year after the first aHUS episode) [4]. When plasma exchange/plasma infusion (PE/PI) was used as the standard of care for aHUS, 29–48% of paediatric patients with aHUS progressed to end-stage renal disease (ESRD) or death within 3 years following the therapy [4, 6]. Although PE/PI may stabilize haematologic outcomes, it does not yield a significant improvement in renal function [9].
The recombinant humanized monoclonal antibody eculizumab (Alexion Pharmaceuticals, Boston, MA, USA) has revolutionized the treatment and management of aHUS [10, 11]. Eculizumab binds to complement protein C5 and inhibits its cleavage by C5 convertases [10]. When aHUS is highly suspected in paediatric patients, eculizumab is recommended as the first-line treatment within 24–48 h after onset, according to general consensus [12]. When eculizumab is not available, PE is indicated; however, it is highly recommended to switch to eculizumab as soon as possible [13, 14].
In Japan, eculizumab was approved for aHUS in September 2013 [15]. For this approval, post-marketing surveillance (PMS) was mandated by regulatory authorities to assess the safety and effectiveness of eculizumab in all patients with aHUS treated in Japanese clinical practice [15]. The interim analysis demonstrated the safety and effectiveness of eculizumab in paediatric patients with aHUS treated in real-world settings in Japan [15]. The findings from the analysis of the full data set from the PMS cohort are reported here.
Materials and Methods
Study design and patients
PMS mandated by the Japanese health authority was conducted by a post-marketing study of good practice guidelines for drugs. Ethical approval by an institutional review board and informed consent from individual patients were not mandatory for PMS.
Patients were enrolled if aHUS was diagnosed based on the contemporaneous Japanese aHUS clinical guides [1, 16] and eculizumab treatment was initiated between September 2013 and January 2018. During this period, the diagnostic criteria for aHUS evolved in Japan, which led to an alteration of the inclusion criteria [1, 16]. In the 2013 clinical guide, aHUS was diagnosed if MAHA, thrombocytopenia and AKI were present, after excluding Shiga toxin–producing Escherichia coli (STEC) HUS and thrombotic thrombocytopaenic purpura (TTP) [16]. A haemoglobin level <10 g/dL was indicative of MAHA and a platelet (PLT) count <150 × 109/L was indicative of thrombocytopaenia. A serum creatinine (sCr) level ≥1.5 times the upper limit of the age- and sex-specific paediatric reference range was indicative of AKI in children [1, 16]. TTP was excluded if a disintegrin-like and metalloproteinase with thrombospondin type 1 motifs 13 (ADAMTS13) activity was ≥5–10% [1, 16]. The terminology in the 2013 clinical guide was similar to that in reports by Noris and Remuzzi [17] and from the Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference [18]. In the 2015 clinical guide, aHUS was designated ‘complement-mediated HUS’, in which secondary TMAs were excluded, i.e. those associated with autoimmune diseases, drugs, infection, malignant tumours, metabolic disorders or transplantation [1]. The terminology in the 2015 clinical guide was similar to that in the reports by Campistol et al. [19] and Scully and Goodship [20].
In this study, paediatric patients with complement-mediated HUS who did not have secondary TMA and were <18 years old at the first dose of eculizumab were included, according to the 2015 clinical guide by the review of individual haematologic and renal variables and underlying diseases reported by an attending physician. Patient demographics and disease characteristics were recorded at treatment initiation (baseline). Clinical data, including results of complement gene testing, were collected at 6 and 12 months and during annual follow-up thereafter. The values of sCr and estimated glomerular filtration rate (eGFR) in patients on dialysis were included in the analysis.
Treatment
The approved eculizumab dosing is based on the patient’s weight (Supplementary data, Table S1) [21]. Actual dosing and administration intervals were determined by the attending physician. An administration interval >30 days was defined as the criterion of treatment discontinuation. Anti-meningococcal vaccine administration was mandatory before the first dose of eculizumab unless urgent treatment was required [21].
Assessments of safety and effectiveness
The effectiveness and safety of eculizumab was assessed as defined in Table 1. Adverse events (AEs) were classified based on the Japanese translation of the Medical Dictionary for Regulatory Activities. The severity of serious adverse events (SAEs) was classified into six categories according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use E2D guidelines.
Table 1.
Definition of clinical endpoints and adverse events
| Clinical endpoints of effectiveness | Definition |
|---|---|
| Complete TMA response | Haematologic normalization and a ≥25% decrease in serum creatinine persisting for ≥4 weeks |
| Haematologic outcome | |
| Haematologic normalization | PLT count normalization (≥150×109/L) and LDH normalization (˂1.5 times the age-matched ULN). Each normalization should be maintained for ≥4 weeks |
| PLT count normalization | ≥150×109/L, maintained for ≥4 weeks |
| LDH normalization | ˂1.5 times the age-matched ULN, which should be maintained for ≥4 weeks |
| Renal outcome | |
| sCr level improvement | A decrease of ≥25% in sCr should be maintained for ≥4 weeks |
| eGFR improvement | eGFR improvement of ≥15 mL/min/1.73 m2 should be maintained for ≥4 weeks |
| TMA event-free status | No decrease in PLT count >25% after TMA remission (increase in PLT count to ≥150×109/L maintained for ≥2 weeks), no plasma exchange or fresh frozen plasma infusion and no initiation of dialysis for ≥12 weeks |
| Adverse event | Definition |
| AE and SAE | Defined as any untoward symptoms, disease or abnormal test values through the observational period irrespective of the causal relationship with eculizumab Causal relationship between AEs and eculizumab was evaluated as ‘unrelated’, ‘probably related’, or ‘related’ by an attending physician An event was categorized as serious if it resulted in hospitalization, prolonged hospitalization, disability, permanent injury or death or was life-threatening |
Statistical analysis
The analysis of effectiveness endpoints was summarized by the number and proportion of patients with a two-sided 95% confidence interval (CI). Absolute values and changes from baseline in PLT count, LDH, sCr and eGFR levels were summarized using descriptive statistics. The time to achieve effectiveness endpoints was estimated by Kaplan–Meier analysis. Missing data were not imputed except for body weight. Body weight was imputed by using the most recent data prior to eculizumab administration. In the safety analysis, the number of patients and incidence rates (patient-years) for each event were calculated. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for statistical analyses. In all analyses, two-sided P-values (significance level <.05) were used.
Results
A total of 72 paediatric patients who were treated with at least one dose of eculizumab were enrolled in the PMS. These patients were diagnosed with aHUS as per the Japanese 2013 and 2015 clinical guides. In all, 40 paediatric patients were further evaluated after exclusion of the following patients: 2 patients without a record at diagnosis, 3 patients with STEC-HUS, 2 patients who did not meet TMA criteria at diagnosis and 25 patients with secondary TMA according to the Japanese 2015 clinical guide (Figure 1).
FIGURE 1:
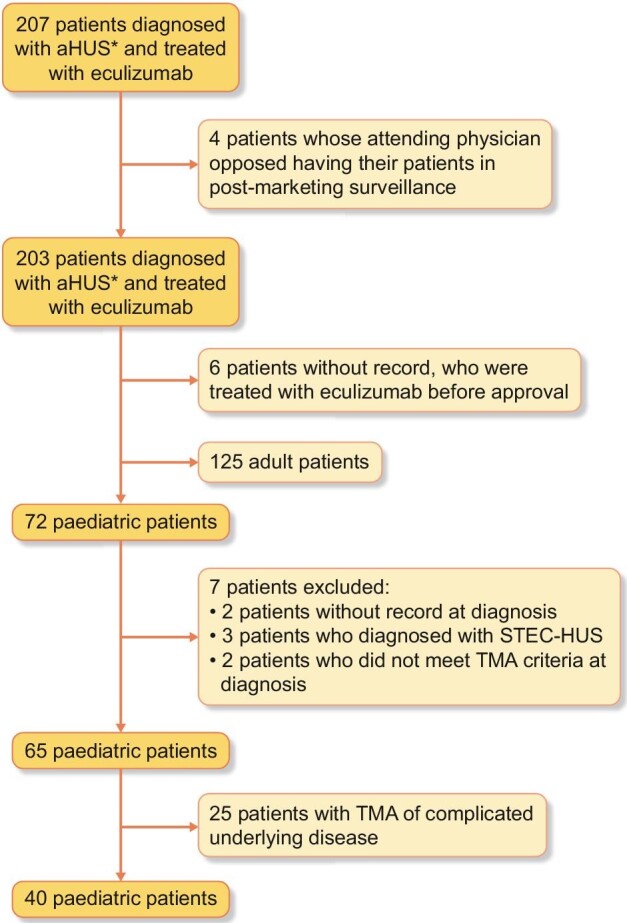
Patient disposition. A total of 203 patients were diagnosed with aHUS based on the Japanese aHUS clinical guide 2013 or 2015 and enrolled in post-marketing surveillance (*). In all, 40 paediatric patients were subjects of this analysis according to the Japanese aHUS clinical guide 2015.
Patient demographics and baseline characteristics
At baseline, the median age of patients was 5.0 years (range 0–17); 12 (30%) patients were <12 months (1 month being the youngest). Complement gene variants or autoantibodies were reported in 20/33 patients (61%) with data available (Table 2). Rare variants with an allele frequency of <0.005 are summarized in Supplementary data, Table S2. At baseline, a PLT count <150×109/L was seen in 34 (85%) patients and LDH >1.5 times the ULN were seen in 33 (83%) patients. The median duration of eculizumab treatment was 66.0 weeks (range 0–268), and 77.5% of the patients received eculizumab treatment for ≥26 weeks (Table 2).
Table 2.
Baseline demographics and disease characteristics
| Baseline demographics | Value |
|---|---|
| Age at first eculizumab administration (years), mean (range), n = 40 | 5.0 (0–17) |
| 1–<23 months, n (%) | 12 (30.0) |
| ≥23–<5 years, n (%) | 6 (15.0) |
| ≥5–<12 years, n (%) | 15 (37.5) |
| ≥12–<18 years, n (%) | 7 (17.5) |
| Weight (kg), median (range), n = 39 | 16.50 (3.4–63.8) |
| Female, n (%)/total | 14 (35.0)/40 |
| Patient-reported family history of aHUS, n (%)/total | 6 (15.0)/40 |
| Patient-reported complement gene mutation or polymorphism, n (%)/tested | 20 (60.6)/33 |
| One mutation/polymorphism, n (%)/positive | 16 (80.0) |
| Two or more mutations/polymorphisms, n (%)/positive | 4 (20.0) |
| C3, n (%)/positive | 7 (35.0) |
| CFB, n (%)/positive | 1 (5.0) |
| CFH, n (%)/positive | 4 (20.0) |
| CFHR1/3 deletion, n (%)/positive | 8 (40.0) |
| CFHR5, n (%)/positive | 1 (5.0) |
| CFI, n (%)/positive | 0 (0.0) |
| MCP, n (%)/positive | 5 (25.0) |
| DGKE, n (%)/positive | 0 (0.0) |
| Anti-CFH antibody, n (%)a/tested | 6 (18.2)/33 |
| Clinical time course and pretreatment | Value |
| Time from first TMA symptom to the first administration of eculizumab (days), median (range), n = 40 | 15.5 (2–4485) |
| Time from the nearest TMA to the first administration of eculizumab (days), median (range), n = 40 | 13.5 (2–1445) |
| Time from the day of diagnosis to the first administration of eculizumab (days), median (range), n = 40 | 2.0 (1–1445) |
| Days of plasma therapy between the nearest TMA and the first administration of eculizumab (days), median (range), n = 40 | 3.0 (0–63) |
| Plasma therapy (past 1 year before diagnosis), n (%)/total | 26 (65.0)/40 |
| Dialysis (during 8 weeks before first dose of eculizumab), n (%)/total | 19 (47.5)/40 |
| Previous renal transplant, n (%)/total | 0 (0.0)/40 |
| Laboratory test values at baseline | Value |
| PLT count (×109/L), median (range), n = 40 | 51 (2–425) |
| PLT count <150 ×109/L, n (%)/total | 34 (85.0)/40 |
| LDH level (U/L), median (range), n = 40 | 912.0 (227–4060) |
| LDH >1.5 times the ULN, n (%)/total | 33 (82.5)/40 |
| Haemoglobin concentration (g/dL), median (range), n = 40 | 8.00 (4.4–14.7) |
| Haemoglobin concentration <10 g/dL, n (%)/total | 36 (90.0)/40 |
| Schistocytes positive, n (%)/examined | 9 (90.0)/10 |
| sCr level (mg/dL), median (range), n = 40 | 1.24 (0.2–6.1) |
| eGFR (mL/min/1.73 m2) DCC (range), n = 23 | 31.90 (13.8–134.8) |
| eGFR (mL/min/1.73 m2), n = 23 | |
| <15, n (%) | 3 (13.0) |
| ≥15–29, n (%) | 8 (34.8) |
| 30–44, n (%) | 3 (13.0) |
| 45–59, n (%) | 3 (13.0) |
| 60–89, n (%) | 2 (8.7) |
| ≥90, n (%) | 4 (17.4) |
| Eculizumab treatment | Value |
| Duration of eculizumab treatment (weeks), median (range), n = 40 | 66.0 (0–268) |
| <1 week, n (%) | 5 (12.5) |
| ≥1–<4 weeks, n (%) | 1 (2.5) |
| ≥4–<26 weeks, n (%) | 3 (7.5) |
| ≥26 weeks, n (%) | 31 (77.5) |
CFHR1/3 denotes the locus of CFHR3 to CFHR1 genes. a Four of six patients with anti-CFH antibodies (one patient did not undergo genetic testing) showed variants or deletions in CFHR genes (see also Table 4).
Effectiveness of eculizumab in paediatric patients with aHUS
Responses of PLT count, LDH and eGFR levels in patients treated with eculizumab are shown in Figure 2 and Supplementary data, Table S3. The mean PLT level at baseline was 85.4×109/L [standard deviation (SD) 86.7], which increased to 270.2 ×109/L (SD 147.5) at 10 days after the first eculizumab administration. The mean change in PLT count from baseline to 10 days was 182.9 ×109/L (SD 155.6; P < .001] (Figure 2A). The mean LDH level at baseline was 1515.1 U/L (SD 1236.5), which decreased to 601.5 U/L (SD 360.4) at 10 days after the first eculizumab administration. The mean change in LDH from baseline was statistically significant at 10 days after the first dose of eculizumab [930 U/L (SD 1086.9); P < .001] (Figure 2B). The mean eGFR at baseline was 45.5 mL/min/1.73 m2 (SD 34.9) and had improved to 69.5 mL/min/1.73 m2 (SD 41.2) at 10 days after the first eculizumab administration. The mean change in eGFR from baseline to 10 days was 20.4 mL/min/1.73 m2 (SD 31.1; P < .01) (Figure 2C). A total of 15/19 patients who were on dialysis at baseline discontinued dialysis by the end of the observation period. The median time to dialysis discontinuation was 4 days (range 1–1290).
FIGURE 2:
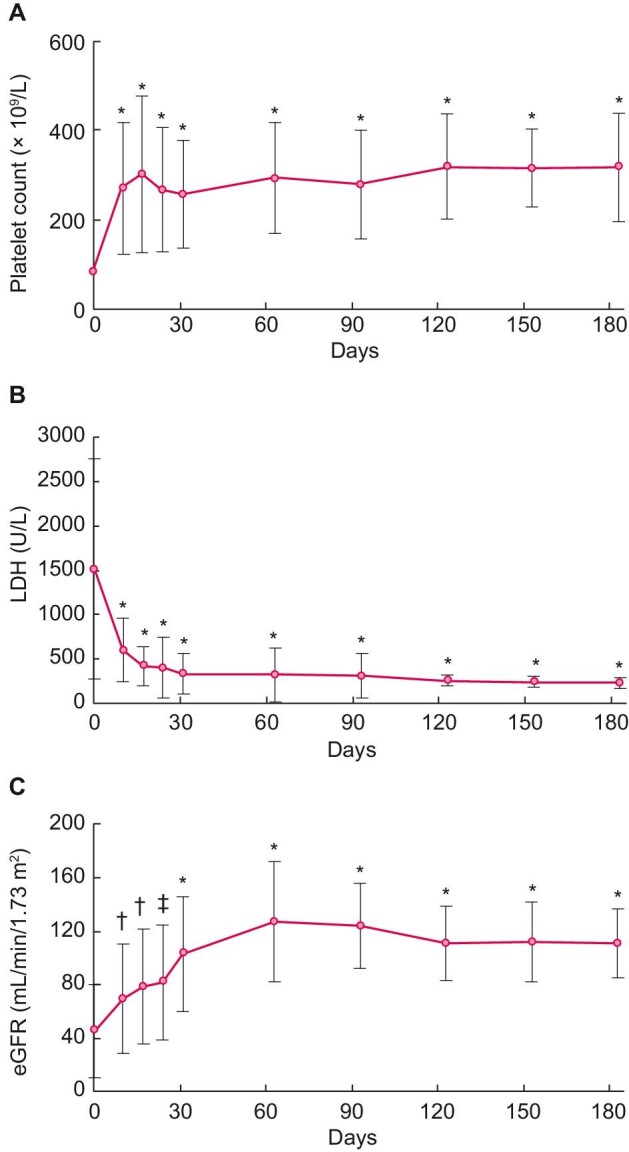
Mean observed values of haematologic and renal parameters. Mean values for (A) PLT count, (B) LDH, and (C) eGFR levels over time during eculizumab treatment. The eGFR for paediatric patients ages 2–18 years was calculated using the following formulae, with X as body height (m): eGFR = 110.2 × (−1.259 × 5 + 7.815 × 4 − 18.57 × 3 + 21.39 × 2 − 11.71X + 2.628)/(sCr) + 2.93 for boys and 110.2 × (−4.536 × 5 + 27.16 × 4 − 63.47 × 3 + 72.43 × 2 − 40.06X + 8.778)/(sCr) + 2.93 for girls [40]. Although eGFR is generally used to evaluate chronic kidney disease, it was used to assess acute kidney dysfunction in this study. Changes from baseline were compared by using the paired t-test. *P < .001, †P < .01, ‡P < .05.
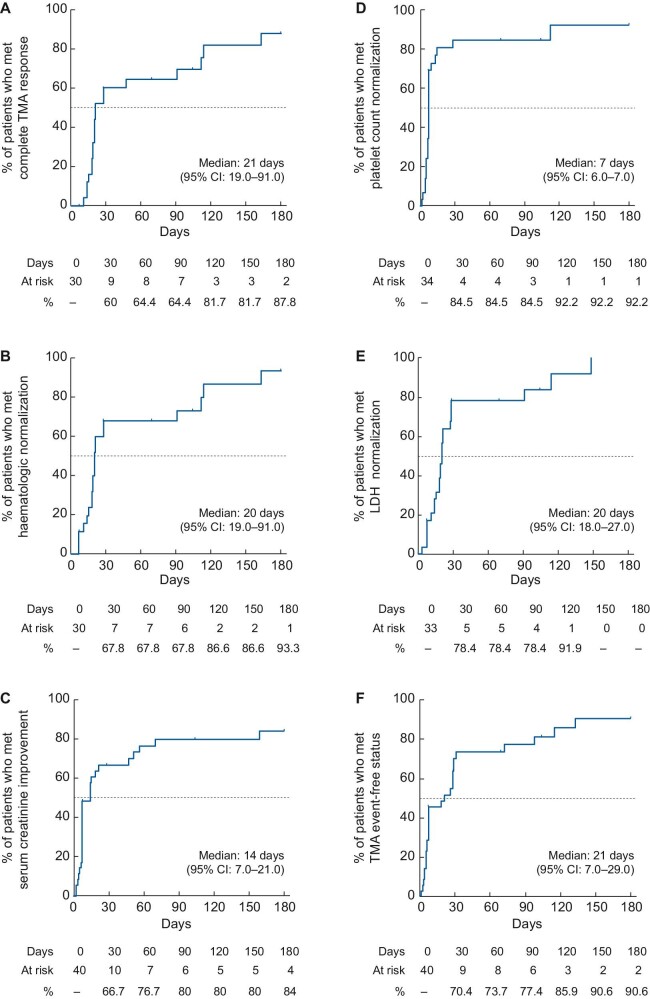
The percentages of patients who met the effectiveness endpoints are summarized in Table 3. A total of 22/30 patients (73.3%) achieved complete TMA response. The time to achieve each of the effectiveness endpoints was estimated by Kaplan–Meier analysis (Figure 3). The median times to achieve the effectiveness endpoints were 21 days (95% CI 19–91) for complete TMA response, 14 days (95% CI 7–21) for sCr level improvement, 7 days (95% CI 6–7) for PLT count normalization and 20 days (95% CI 18–27) for LDH normalization.
Table 3.
Effectiveness of eculizumab in paediatric patients with aHUS
| Effectiveness endpoint | Total number of patients | Number of patients reporting the effectiveness outcome | Percentage of patients reporting the effectiveness outcome |
95% CI |
|---|---|---|---|---|
| Complete TMA response | 30 | 22 | 73.3 | 54.1–87.7 |
| Haematologic normalization | 30 | 22 | 73.3 | 54.1–87.7 |
| PLT count normalization | 34 | 26 | 76.5 | 58.8–89.3 |
| LDH normalization (<1.5 times the ULN) | 33 | 25 | 75.8 | 57.7–88.9 |
| sCr level improvement | 40 | 28 | 70.0 | 53.5–83.4 |
| eGFR improvement | 23 | 18 | 78.3 | 56.3–92.5 |
| TMA event-free status | 40 | 31 | 77.5 | 61.5–89.2 |
FIGURE 3:
Time to achieve effectiveness endpoints. The percentage of patients achieving (A) complete TMA response, (B) haematologic normalization, (C) sCr, (D) PLT count, (E) LDH normalization and (F) TMA event-free status was estimated using Kaplan–Meier analysis.
Patients who discontinued eculizumab
In all, 18/40 patients met the criteria for treatment discontinuation; in addition, 4 patients died and thereby also met the criteria. Among these 18 patients, 13 remained completely withdrawn from treatment until the end of observation (Table 4) and 5 patients prolonged the treatment interval but continued the treatment at the end of observation. Of the 13 patients who remained withdrawn from eculizumab treatment, 6 were positive for anti-CFH antibodies (patients 1, 2, 5, 8, 9 and 12). Patients 5 and 9 were withdrawn from treatment after a reduction in anti-CFH antibody levels and patient 12 started immunosuppressive treatment after eculizumab discontinuation. One patient (patient 7) reported a deterioration in renal function after withdrawal of eculizumab (Supplementary data, Table S4).
Table 4.
Patients who remained withdrawn from eculizumab treatment at the end of the observation period
| Patient | Agea | Sex | Number of TMA episodes prior to eculizumab | Family history of aHUS | Genetic variantb/antibody | Total days of eculizumab treatment | Recurrence of aHUS | Days after discontinuation | Treatment at last observation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 years | F | 1 | Y | CFHR1 p.Asp35fsX36, anti-CFH antibody | 1010 | N | 337 | – |
| 2 | 10 years | M | 1 | N | CFHR3 exon 6 deletion, anti-CFH antibody | 105 | N | 1681 | PSL, CsA |
| 3 | 17 years | M | 2 | Y | C3 p.Ile1157Thr | 424 | N | 308 | – |
| 4 | 17 years | M | 3 | N | MCP p.Asp170Met FsX9 (Homo) | 1 | N | 408 | – |
| 5 | 5 years | M | 1 | N | Not examined, anti-CFH antibody | 357 | N | 1438 | – |
| 6 | 16 years | F | 1 | N | Not examined | 213 | N | 754 | – |
| 7 | 16 years | M | 1 | N | Not examined (positive for haemolytic assay) | 575 | N | 126 | Peritoneal dialysis |
| 8 | 7 years | M | 1 | N | CFHR1-CFHR3 deletion, anti-CFH antibody | 595 | N | 371 | – |
| 9 | 11 years | F | 1 | N | Not identified, anti-CFH antibody | 603 | N | 128 | – |
| 10 | 7 months | F | 1 | N | Not identified | 8 | N | 727 | PSL |
| 11 | 9 months | M | 1 | N | C3 p.Ser179Pro, CFH p.Hus402Tyr (homo), p.Glu936Asp (homo) | 327 | N | 465 | – |
| 12 | 4 years | M | 1 | N | CFHR1-CFHR3 deletion, anti-CFH antibody | 385 | N | 708 | PSL, MMF |
| 13 | 6 years | F | 1 | N | CFH p.His402Tyr, p.Glu936Asp, MCP p.Thr163Ile | 29 | N | 326 | – |
F, female; M, male; Y, yes; N, no; PSL, prednisolone; CsA, cyclosporin.
aAge at first administration. bThe information on homozygosity or heterozygosity of the genetic variant is not available if there is no description in this table.
Among the five patients who prolonged the treatment interval, one patient experienced aHUS recurrence. This 13-year-old boy had a C3 p.Ile1157Thr variant, a history of a TMA episode on two prior occasions and a family history of aHUS, which is a known risk factor of recurrence [22]. He discontinued eculizumab due to difficulty visiting the hospital; however, at 120 days after discontinuation, the patient had a recurrence of aHUS and restarted treatment.
Safety of eculizumab in paediatric patients with aHUS
Safety analyses are shown in Table 5. For the 40 patients in the safety analysis set, the total exposure to eculizumab was 89.42 patient-years. A total of 59 SAEs (0.66/patient-year) were reported in 18 patients. In all, 29 infection-related SAEs were reported in 12 patients, including 1 case of meningococcal bacteraemia. Complete recovery of meningococcal bacteraemia was reported following appropriate treatment with antimicrobial agents. Acute liver failure, pulmonary haemorrhage, renal impairment and respiratory failure were reported as SAEs related to death in one patient. Cardiac failure, immunodeficiency and pulmonary haemorrhage, respectively, were reported as SAEs related to death in three patients. Most of the SAEs not related to death resolved during eculizumab treatment; however, renal impairment in two patients and bone marrow failure and thrombosis in one patient did not fully resolve. The most frequent SAE was renal impairment (5 events, 0.06/patient-year) (Table 5, Supplementary data, Table S5).
Table 5.
Frequency and classification of SAEs
| Total number of patients for safety analysis | 40 |
| Total exposure time (years) | 89.42 |
| Total number of SAEs, n (patient-years) | 59 (0.66) |
| Total number of patients with SAEs | 18 |
| Number of treatment-related SAEs/number of patients | 36/10 |
| Number of not treatment-related SAEs/number of patients | 23/13 |
| Number of infections (patient-years)/number of patients | 29 (0.32)/12 |
| Number of cases of meningococcal bacteraemia (patient-years) | 1 (0.01) |
| Seriousness of SAE | |
| Number of SAEs related to death (number of patients) | 7 (4) |
| Number of life-threatening SAEs (number of patients) | 8 (4) |
| Number of SAEs which required inpatient hospitalization or prolongation of existing hospitalization (number of patients) | 23 (8) |
| Number of SAEs related to persistent or significant disability/incapacity (number of patients) | 3 (2) |
| Number of congenital anomalies/birth defects (number of patients) | 0 (0) |
| Number of other IMEa (number of patients) | 4 (2) |
| SAE reported more than once, n (patient-years) | |
| Renal impairment | 5(0.06) |
| Device-related infection | 3(0.03) |
| Staphylococcal infection | 2(0.02) |
| Urinary tract infection | 2(0.02) |
| Infection | 2(0.02) |
| Respiratory syncytial virus bronchitis | 2(0.02) |
| Pulmonary haemorrhage | 2(0.02) |
| Enterocolitis | 2(0.02) |
| Pyrexia | 2(0.02) |
RS, respiratory syncytial; IME, important medical event.
aAn IME that may not result in death, be life-threatening or require hospitalization may be considered a serious adverse drug experience when, based on medical judgement, it may jeopardize the patient and may require medical or surgical intervention to prevent one of the outcomes listed in this definition.
The overall survival rate of this cohort was estimated to be 89.2% at 180 days (Supplementary data, Figure S1). Four deaths were reported, but none were related to eculizumab treatment. Three of the four deaths were described in the interim report [15]. The fourth death was reported after the interim report and occurred in a 5-year-old girl who experienced TMA complicated by immunodeficiency disease and presented with frequently recurring methicillin-resistant Staphylococcus aureus infections. The episode of TMA occurred when she was age 1 year, yet eculizumab was administered 1445 days after the onset of the TMA. A total of 60 days after this single dose of eculizumab, she died of worsening immunodeficiency disease. Complement gene testing was not performed on this patient.
DISCUSSION
This is the final analysis of the safety and effectiveness of eculizumab for the treatment of aHUS in paediatric patients for 4.5 years in Japan. This study presents the added value of the real-world situation, which illustrates the clinical practice of paediatric aHUS in Japan and further confirms that eculizumab is safe and effective for paediatric patients with aHUS. In this cohort, the median eculizumab treatment duration was 66 weeks; 77.5% of patients received treatment for at least 26 weeks as recommended by expert opinion [12, 14, 23].
In the current analysis we adopted the definition of <1.5 times the ULN for LDH normalization instead of less than the age-matched ULN in the clinical trial of eculizumab in paediatric patients and our previous interim analysis [11, 15]. In the interim analysis of this PMS from September 2013 to March 2017, 36 and 29% of paediatric and adult patients had met complete TMA response, respectively [15, 24]. The ratio of complete TMA response in the interim analysis of paediatric patients was higher than that of adult patients; however, the ratio was lower compared with the original clinical trial reported by Greenbaum et al. [11] which was largely attributed to the low ratio of LDH normalization when the definition of less than the age-matched ULN was used (48% versus 82%, respectively), although LDH levels were largely reduced in individual paediatric patients during eculizumab treatment in that analysis [15]. In addition, after TMA remission, 85.2% of patients had no obvious signs of TMA recurrence. Therefore the definition of LDH normalization, less than the age-matched ULN, was too stringent to evaluate the effectiveness of eculizumab in the real-world setting for paediatric patients in Japan [15]. Using the modified definition of LDH normalization as <1.5 times the ULN, LDH normalization and complete TMA response were observed in 77 and 73% of patients, respectively. A similar ratio [64% (95% CI 41–83)] of complete TMA response was reported in the clinical trial [11]. The estimated median time to complete a TMA response was 21 days; the median time for PLT count and LDH normalization and sCr improvement was 7, 20 and 14 days, respectively. PLT count, LDH and eGFR significantly improved at 10 days. An early response to eculizumab was consistently observed similar to the clinical trial [11].
In a Global aHUS Registry study to illustrate the natural history of aHUS, the most common identified cause of aHUS in paediatric patients ages 6–17 years (45% of tested patients) was anti-CFH antibodies [25]. In the current study, 18% of tested paediatric patients displayed anti-CFH antibodies, whereas 3/13 (23%) paediatric patients ages 6–17 years were positive for the antibodies. A total of four of the six patients with the antibodies (one patient did not undergo genetic testing; 80%) showed a gene variant or deletion resulting in the loss of CFHR proteins, which suggested the relationship between genetic predisposition and antibody production. In an interim analysis, adult patients with anti-CFH antibodies were not reported [24].
In this study, 18/40 patients met treatment discontinuation criteria; 13 patients remained withdrawn from eculizumab treatment and 5 patients prolonged the treatment interval. In all, 2/18 patients (11%) showed a worsening of renal function or aHUS recurrence. This proportion is lower than previous reports of 21–32% aHUS recurrence after eculizumab discontinuation, probably due to the lower proportion (20%) of patients with CFH variants in variant-positive patients of this cohort than in the European population [22, 26–31]. The largest of these studies was from the Global aHUS Registry; 21.6% (11/51) of paediatric patients in that analysis experienced a TMA recurrence after eculizumab discontinuation at a median of 5.1 months and 10 overall had to restart treatment [22]. The risk of aHUS recurrence associated with anti-CFH antibodies appears to be high (11–68%), as previously reported [32]. In 6/13 patients who remained withdrawn from treatment in this study, aHUS was associated with anti-CFH antibodies. In this study, two of six patients with anti-CFH antibodies discontinued eculizumab treatment after a reduction in anti-CFH antibody levels and the other patient started immunosuppression treatment after eculizumab discontinuation; an international consensus report recommended the association of eculizumab with glucocorticoids and an immunosuppressive drug (mycophenolate mofetil) to decrease the anti-CFH antibody titre, allowing eculizumab withdrawal [33]. In other patients who discontinued eculizumab treatment in the current study, rare variants in the MCP or C3 gene had additionally been identified. The presence of rare complement gene variants in CFH (31–55%), MCP (18–52%) and C3 (50%) seem to be associated with a higher risk of recurrence of TMA [34]. MCP variants are assumed to have a rather good outcome of renal function in the long-term, although the frequency of aHUS recurrence appeared to be high [7]. Similarly, a previous report by Fujisawa et al. [35] suggested that C3 p.I1157T, the predominant variant in the Japanese population, was related to favourable outcomes despite repeated recurrences [35]. In a recent case report, a renal biopsy indicated that substantial progression of irreversible injuries in kidneys was associated with repeated TMA in the patient with a C3 p.Ile1157Thr variant [36]. Although there is no clear description of the criteria for the discontinuation in the 2015 clinical guide, the decision for the discontinuation in the clinical practice appeared to be carefully made according to available evidence. Since evidence is still too limited to evaluate the prognosis of paediatric patients after discontinuation of eculizumab, expert consensus recommended that discontinuation of eculizumab could be considered on a case-by-case basis in patients [12, 22, 23, 33].
Considering the potential burden of aHUS, the safety of eculizumab treatment was generally tolerable, and this was consistent with results from clinical trials and a 10-year pharmacovigilance analysis [10, 11, 37, 38]. Although four deaths were reported, none of them were related to eculizumab. Since Neisseria meningitidis is primarily cleared by terminal complement components, eculizumab-treated patients are at increased risk for developing meningococcal infections and meningococcal vaccination is recommended to minimize the risk [38]. During this PMS, only one case of meningococcal bacteraemia was observed; the patient recovered fully with appropriate antimicrobial treatment [15].
This study had some limitations. First, due to the nature of the design of PMS, there could also have been the possibility of missing, underreporting or incomplete follow-up of data. Second, patients in this cohort were not investigated for the polymorphism C5 p.Arg885His, which is present in ∼3.2% of the Japanese population; this polymorphism prevents eculizumab from binding to C5, thereby blocking its therapeutic activity [39]. This report focuses on paediatric patients with aHUS; however, a report on the adult patients in the PMS is in preparation.
In conclusion, this full dataset analysis further confirms that eculizumab is safe and effective for paediatric patients with aHUS in the real-world setting in Japan. The decision to withdraw eculizumab treatment or prolong treatment intervals was carefully made in the clinical practice according to currently available evidence. Despite this, >10% of patients showed disease worsening or recurrence after treatment discontinuation/elongation of treatment intervals. This further highlights the need to very closely monitor patients if one wants to attempt treatment discontinuation.
Supplementary Material
ACKNOWLEDGEMENTS
PMS is mandatory and was conducted by Alexion Pharma (Tokyo, Japan), the sponsor and funder of this analysis. The authors thank all participating physicians and registered patients who participated in this study and their families. We also thank Isabel Firmino, Christoph Gasteyger, Sarah Guadagno, Asa Lommele, Radha Narayan and Jonathan Mathias of Alexion Pharmaceuticals for reviewing the article. Statcom (Tokyo, Japan) provided medical writing support funded by Alexion Pharma. The data underlying this article are available in the article and in its online supplementary data.
Contributor Information
Shuichi Ito, Department of Paediatrics, Graduate School of Medicine, Yokohama City University, Yokohama, Japan.
Hiroshi Hataya, Department of General Paediatrics, Department of Nephrology, Tokyo Metropolitan Children's Medical Centre, Tokyo, Japan.
Akira Ashida, Department of Paediatrics, Osaka Medical and Pharmaceutical University, Osaka, Japan.
Riku Hamada, Department of Nephrology, Tokyo Metropolitan Children's Medical Centre, Tokyo, Japan.
Tomoaki Ishikawa, Department of Paediatrics, Nara Medical University, Nara, Japan.
Yumiko Ishikawa, Alexion Pharma GK, Tokyo, Japan.
Akihiko Shimono, Alexion Pharma GK, Tokyo, Japan.
Takao Konomoto, Division of Pediatrics, Faculty of Medicine, University of Miyazaki, Miyazaki, Japan.
Tomoki Miyazawa, Department of Paediatrics, Kindai University Faculty of Medicine, Osaka, Japan.
Masao Ogura, Division of Nephrology and Rheumatology, Department of Medical Subspecialties, National Centre for Child Health and Development, Tokyo, Japan.
Kazuki Tanaka, Department of Nephrology, Aichi Children's Health and Medical Centre, Aichi, Japan.
Shoji Kagami, Tokushima University Hospital, Tokushima, Japan.
Authors’ contributions
All authors contributed to the study conception and design. Clinical data, at diagnosis and treatment initiation for patient disposition, were reviewed by S.I. The first draft of the article was reviewed by S.I. and A.S. and all authors commented on previous versions of the article. All authors read and approved the final manuscript.
Conflict of interest statement
S.I. reports payments for lectures and participation on the advisory board from Alexion Pharma during the conduct of the study. H.H. reports consulting fees and payments for lectures and participation on the advisory board from Alexion Pharma. A.A. reports grants, consulting fees and payments for lectures from Alexion Pharma. R.H. reports consulting fees and payments for lectures from Alexion Pharma. Y.I. and A.S. were employees of Alexion Pharma and shareholders of Alexion Pharmaceutical during the conduct of the study. K.T. reported consulting fees from Alexion Pharma. All other authors have nothing to disclose.
REFERENCES
- 1. Kato H, Nangaku M, Hataya Het al. . Clinical guides for atypical hemolytic uremic syndrome in Japan. Clin Exp Nephrol 2016; 20: 536–543 [DOI] [PubMed] [Google Scholar]
- 2. Afshar-Kharghan V. Atypical hemolytic uremic syndrome. Hematology Am Soc Hematology Educ Program 2016; 2016: 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoshida Y, Kato H, Nangaku M. Atypical hemolytic uremic syndrome. Ren Replace Ther 2017; 3: 5 [Google Scholar]
- 4. Fremeaux-Bacchi V, Fakhouri F, Garnier Aet al. . Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol 2013; 8: 554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schaefer F, Ardissino G, Ariceta Get al. . Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int 2018; 94: 408–418 [DOI] [PubMed] [Google Scholar]
- 6. Noris M, Caprioli J, Bresin Eet al. . Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol 2010; 5: 1844–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ardissino G, Longhi S, Porcaro Let al. . Risk of atypical HUS among family members of patient carring complement regulatory gene abnormality. Kidney Int Rep 2021; 6: 1614–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geerdink LM, Westra D, van Wijk JAEet al. . Atypical hemolytic uremic syndrome in children: complement mutations and clinical characteristics. Pediatr Nephrol 2012; 27: 1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwartz J, Padmanabhan A, Aqui Net al. . Guidelines on the use of therapeutic apheresis in clinical practice–evidence–based approach from the Writing Committee of the American Society for Apheresis: the Seventh Special Issue. J Clin Apher 2016; 31: 149–162 [DOI] [PubMed] [Google Scholar]
- 10. Legendre CM, Licht C, Muus Pet al. . Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 2013; 368: 2169–2181 [DOI] [PubMed] [Google Scholar]
- 11. Greenbaum LA, Fila M, Ardissino Get al. . Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int 2016; 89: 701–711 [DOI] [PubMed] [Google Scholar]
- 12. Raina R, Grewal MK, Radhakrishnan Yet al. . Optimal management of atypical hemolytic uremic disease: challenges and solutions. Int J Nephrol Renovasc Dis 2019; 12: 183–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee H, Kang E, Kang HGet al. . Consensus regarding diagnosis and management of atypical hemolytic uremic syndrome. Korean J Intern Med 2020; 35: 25–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fakhouri F, Loirat C. Anticomplement treatment in atypical and typical hemolytic uremic syndrome. Semin Hematol 2018; 55: 150–158 [DOI] [PubMed] [Google Scholar]
- 15. Ito S, Hidaka Y, Inoue Net al. . Safety and effectiveness of eculizumab for pediatric patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance. Clin Exp Nephrol 2019; 23: 112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sawai T, Nangaku M, Ashida Aet al. . Diagnostic criteria for atypical hemolytic uremic syndrome proposed by the Joint Committee of the Japanese Society of Nephrology and the Japan Pediatric Society. Pediatr Int 2014; 56: 1–5 [DOI] [PubMed] [Google Scholar]
- 17. Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med 2009; 361: 1676–1687 [DOI] [PubMed] [Google Scholar]
- 18. Goodship TH, Cook HT, Fakhouri Fet al. . Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 2017; 91: 539–551 [DOI] [PubMed] [Google Scholar]
- 19. Campistol JM, Arias M, Ariceta Get al. . An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia 2015; 35: 421–447 [DOI] [PubMed] [Google Scholar]
- 20. Scully M, Goodship T. How I treat thrombotic thrombocytopenic purpura and atypical haemolytic uraemic syndrome. Br J Haematol 2014; 164: 759–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soliris package insert version 8. https://www.info.pmda.go.jp/go/pack/6399424A1023_1_16 (20 May 2021, date last accessed) [Google Scholar]
- 22. Ariceta G, Fakhouri F, Sartz Let al. . Eculizumab discontinuation in atypical hemolytic uremic syndrome: TMA recurrence risk and renal outcomes. Clin Kidney J 2021; 14: 2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ariceta G. Optimal duration of treatment with eculizumab in atypical hemolytic uremic syndrome (aHUS) — a question to be addressed in a scientific way. Pediatr Nephrol 2019; 34: 943–949 [DOI] [PubMed] [Google Scholar]
- 24. Kato H, Miyakawa Y, Hidaka Yet al. . Safety and effectiveness of eculizumab for adult patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance. Clin Exp Nephrol 2019; 23: 65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schaefer F, Ardissino G, Ariceta Get al. . Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int 2018; 94: 408–418 [DOI] [PubMed] [Google Scholar]
- 26. Ardissino G, Possenti I, Tel Fet al. . Discontinuation of eculizumab treatment in atypical hemolytic uremic syndrome: an update. Am J Kidney Dis 2015; 66: 172–173 [DOI] [PubMed] [Google Scholar]
- 27. Sheerin NS, Kavanagh D, Goodship THet al. . A national specialized service in England for atypical haemolytic uraemic syndrome–the first year's experience. QJM 2016; 109: 27–33 [DOI] [PubMed] [Google Scholar]
- 28. Fakhouri F, Fila M, Provot Fet al. . Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol 2017; 12: 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wijnsma KL, Duineveld C, Volokhina EBet al. . Safety and effectiveness of restrictive eculizumab treatment in atypical haemolytic uremic syndrome. Nephrol Dial Transplant 2018; 33: 635–645 [DOI] [PubMed] [Google Scholar]
- 30. Menne J, Delmas Y, Fakhouri Fet al. . Outcomes in patients with atypical hemolytic uremic syndrome treated with eculizumab in a longterm observational study. BMC Nephrol 2019; 20: 125–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fakhouri F, Fila M, Hummel Aet al. . Eculizumab discontinuation in children and adults with atypical haemolytic uremic syndrome: a prospective multicentric study. Blood 2021; 137: 2438–2449 [DOI] [PubMed] [Google Scholar]
- 32. Hofer J, Riedl Khursigara M, Perl Met al. . Early relapse rate determines further relapse risk: results of a 5-year follow-up study on pediatric CFH-Ab HUS. Pediatr Nephrol 2021; 36: 917–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Loirat C, Fakhouri F, Ariceta Get al. . An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol 2016; 31: 15–39 [DOI] [PubMed] [Google Scholar]
- 34. Laurence J. Defining treatment duration in atypical hemolytic uremic syndrome in adults: a clinical and pathological approach. Clin Adv Hematol Oncol 2020; 18: 221–230 [PubMed] [Google Scholar]
- 35. Fujisawa M, Kato H, Yoshida Yet al. . Clinical characteristics and genetic backgrounds of Japanese patients with atypical hemolytic uremic syndrome. Clin Exp Nephrol 2018; 22: 1088–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Okabe M, Kobayashi A, Marumoto Het al. . Renal damage in recurrent atypical hemolytic uremic syndrome associated with C3 p.Ile1157Thr gene mutation. Intern Med 2021; 60: 917–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fakhouri F, Hourmant M, Campistol JMet al. . Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis 2016; 68: 84–93 [DOI] [PubMed] [Google Scholar]
- 38. Socié G, Caby-Tosi MP, Marantz JLet al. . Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome: 10-year pharmacovigilance analysis. Br J Haematol 2019; 185: 297–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nishimura J, Yamamoto M, Hayashi Set al. . Genetic variants in C5 and poor response to eculizumab. N Engl J Med 2014; 370: 632–639 [DOI] [PubMed] [Google Scholar]
- 40. Uemura O, Nagai T, Ishikura Ket al. . Creatinine-based equations to estimate glomerular filtration rate in Japanese children and adolescents with chronic kidney disease Clin Exp Nephrol 2014; 18: 626–633 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.