Abstract
Background
Laboratory and clinical research on breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) is rapidly evolving. Changes in standard of care and insights into best practice were recently presented at the 3rd World Consensus Conference on BIA-ALCL.
Objectives
The authors sought to provide practice recommendations from a consensus of experts, supplemented with a literature review regarding epidemiology, etiology, pathogenesis, diagnosis, treatment, socio-psychological aspects, and international authority guidance.
Methods
A literature search of all manuscripts between 1997 and August 2021 for the above areas of BIA-ALCL was conducted with the PubMed database. Manuscripts in different languages, on non-human subjects, and/or discussing conditions separate from BIA-ALCL were excluded. The study was conducted employing the Delphi process, gathering 18 experts panelists and utilizing email-based questionnaires to record the level of agreement with each statement by applying a 5-point Likert Scale. Median response, interquartile range, and comments were employed to accept, reject, or revise each statement.
Results
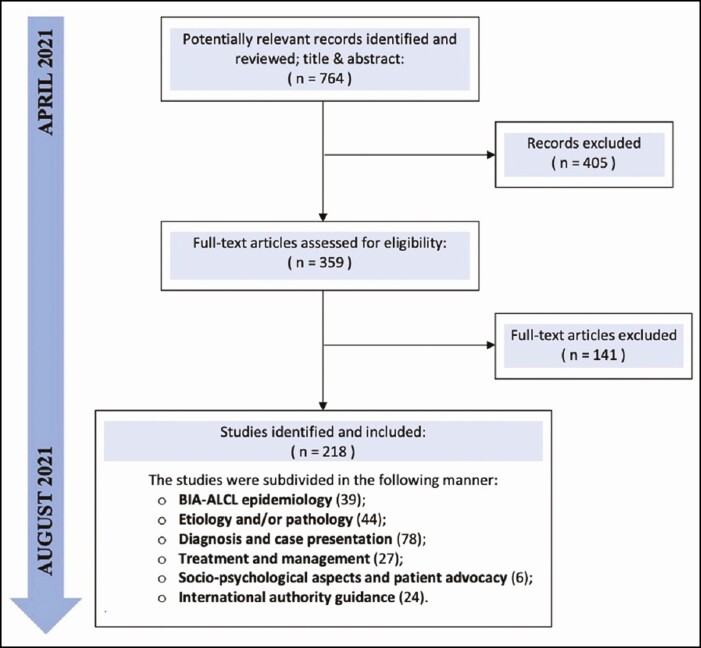
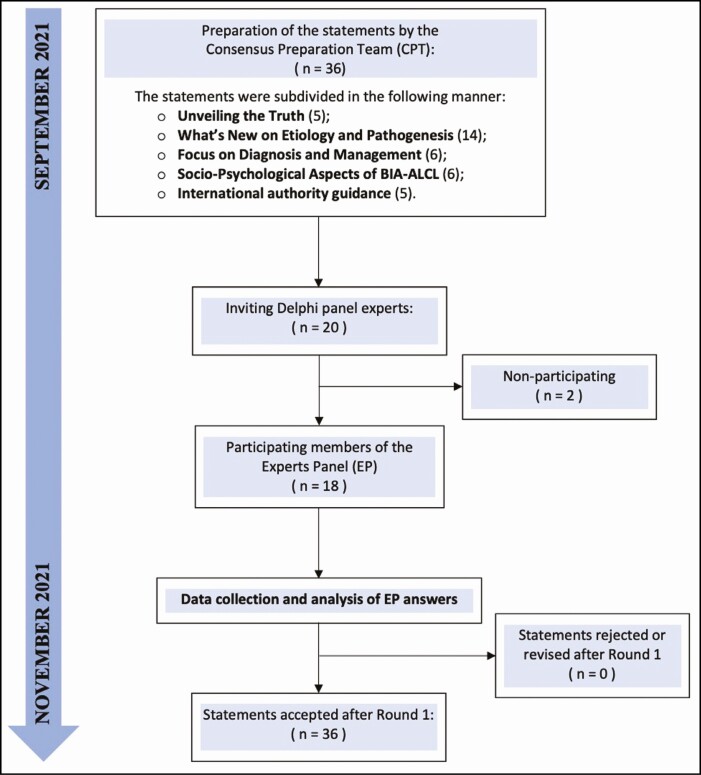
The literature search initially yielded 764 manuscripts, of which 405 were discarded. From the remaining 359, only 218 were included in the review and utilized to prepare 36 statements subdivided into 5 sections. After 1 round, panelists agreed on all criteria.
Conclusions
BIA-ALCL is uncommon and still largely underreported. Mandatory implant registries and actions by regulatory authorities are needed to better understand disease epidemiology and address initial lymphomagenesis and progression. Deviation from current diagnosis and treatment protocols can lead to disease recurrence, and research on breast implant risk factors provide insight to etiology.
Level of Evidence: 4

Breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) has been recognized as a possible complication of procedures utilizing breast implants or other devices with a textured outer surface.1,2 In 2021, the 3rd World Consensus Conference (WCC) on BIA-ALCL was held in Rome, Italy, gathering many of the world’s leading experts to address both innovations and controversies, raise awareness, and combat misinformation to share and stimulate research in the field. Evidence has accumulated, and understanding of risk and progression has significantly evolved since the first case report in 1997, or what is now identified to be an earlier initial report in 1996.3-5 The FDA’s first safety communication in 2011 and the World Health Organization’s acknowledgment of BIA-ALCL as a separate oncologic entity in 2016 are key milestones that contributed to improved global recognition and awareness.6,7
The Scientific Committee on Health, Emerging and Environmental Risks (SCHEER) of the European Health Commission recently issued a final opinion in 2021, first recognizing “sufficient evidence” for existence of a causal relationship between all types of textured breast implants and BIA-ALCL.8 Although many aspects have been unveiled, there are still facets that remain incompletely understood. Epidemiology, etiology, pathogenesis, diagnosis, treatment, and socio-psychological aspects as well as international authority guidance have been separately addressed in prior literature, with considerable discrepancies. However, there are currently no sources, to our knowledge, that provide comprehensive summary information on all these topics. This study aimed to develop and obtain expert consensus on key statements combined with a current review of the literature.
METHODS
This consensus paper was conducted employing the Delphi process, which is an established method to obtain consensus from expert opinions in areas of research where limited information is available.9
Review of the Literature and Preparation of Consensus Statements
A literature review was conducted from April to August 2021 on BIA-ALCL epidemiology, etiology and pathology, diagnosis, management, socio-psychological aspects, and international authority guidance, utilizing PubMed (National Library of Medicine, Bethesda, MD). The following search term strategy was employed: ((Lymphoma, T-Cell OR lymphoma*[tiab]) AND (breast implants/adverse effects[MeSH] OR silicone gels/adverse effects[MeSH] OR silicones/adverse effects[MeSH])) OR ((breast AND (implant or implants or prostheses* or endoprosthes*)) AND lymphoma* AND (epidemiology* or etiology* or pathogenesis* or diagnosis* or treatment* or management* or psychology* or guidance*). Only English-based manuscripts written between January 1997 and August 2021 were considered. Any non–English-based manuscript; research on non-human subjects; or manuscript discussing breast cancer and lymphomas different from BIA-ALCL (ie, primary breast lymphoma), silicone granulomatous lymphadenopathy, or other unrelated concerns to silicone were excluded from the analysis. The literature search and the independent review of titles and abstracts were conducted by 3 reviewers from a consensus preparation team (CPT). All findings were later utilized by the CPT to write, edit, and compile statements across the above-mentioned fields of expertise in BIA-ALCL.
Selection of the Delphi Panel Experts
An expert panel (EP) was selected by inviting all faculty members attending the 3rd WCC on BIA-ALCL. Eighteen members agreed to participate, and this was deemed sufficient for studies of this type employing a Delphi process.10,11 All participants were physicians and/or doctoral scientists with recognized expertise in their surgical, clinical, or research area, representing various geographical regions from across Europe, the United States, and Latin America. Details of the faculty members in the EP group are listed in Table 1.
Table 1.
Professional Background and Geographical Location of the Experts Panel Members Participating in the Delphi Process
| Demographic | Panel no. |
|---|---|
| Profession | |
| Breast surgeon | 1 |
| Oncologist | 1 |
| Pathologist | 5 |
| Plastic surgeon | 11 |
| Total | 18 |
| Geographical location | |
| Brazil | 1 |
| Canada | 1 |
| France | 1 |
| Italy | 4 |
| Netherlands | 1 |
| United Kingdom | 2 |
| United States | 8 |
| Total | 18 |
Data Collection and Analysis
The Delphi process involved an email-based questionnaire, which was piloted by the CPT, and distributed between September and November 2021. All participants were asked to evaluate each statement with a 5-point Likert scale, ranging from 1 point for “strong disagreement” to 5 for “strong agreement,” 12,13 with any additional comments given in a dedicated section (Supplemental Material 1). The level of agreement for each statement was assessed employing the median response and interquartile range (lower and upper quartile). Namely, an upper quartile value <3 led to the statement’s rejection because this indicated that a consensus agreement had not been reached. Conversely, a lower quartile value >3 led to the statement’s acceptance. An interquartile range that included 3 indicated an insufficient consensus on the statement, suggesting that further consideration was necessary. The accepted statements were distributed to the EP group members, who received their own individual and group responses. Additional comments were considered by the EP group to improve on the explanation of each statement, as described in the Discussion section of this manuscript.
RESULTS
The initial search yielded 764 manuscripts, 218 of which were included in the review according to the inclusion criteria: 39 addressed epidemiology, 44 etiology and/or pathology, 78 diagnosis, 27 management, 6 socio-psychological aspects, and 24 international authority guidance (Figure 1). The results from the literature review were utilized by the CPT to prepare 36 statements, which were grouped according to their content and divided into 5 categories: “unveiling the truth” (n = 5), “what’s new on etiology and pathogenesis” (n = 14), “focus on diagnosis and management” (n = 6), “socio-psychological aspects of BIA-ALCL” (n = 6), and “international authority guidance” (n = 5). The statements were submitted to the 18 participants, which included 5 females and 13 males. The faculty members had a mean age of 55.7 years (range, 38-76 years), and consensus was reached after a single round. Raw data from the Delphi questionnaire (Round 1) are provided in Supplemental Table 1 (Figure 2). After 1 round, panelists agreed on all the statements as follows. BIA-ALCL should not be considered “rare” but instead as an “uncommon” outcome of breast implant surgery. Mandatory reporting of cases through national breast implant registries is essential, though prevalence of the disease is likely underreported. Prophylactic capsulectomy and implant removal may not necessarily reduce the risk of developing BIA-ALCL, although it would seem that it is not associated with a higher risk of fatality compared with therapeutic capsulectomy after breast implant removal or exchange due to complications (ie, implant rupture, capsular contracture, infection, malposition, asymmetry, etc). Current breast implant classification systems are based on physical properties, with the most widespread and accepted one being the International Organization for Standardization (ISO) 14607:2018 classification that utilizes outer surface roughness to categorize implants. There is a need for a more comprehensive classification system based on biological properties that takes into consideration implant/host interactions. A causal relationship has been established between BIA-ALCL and textured implants (“micro” and “macro” according to ISO 14607:2018), although most cases have occurred in patients with specific macrotextured devices (Allergan BIOCELL [Irvine, CA] and Silimed polyurethane [Rio de Janeiro, Brazil]). Etiology for BIA-ALCL could be multifactorial and possibly includes shell shedding of particulates, shell surface characteristics leading to friction, bacterial contamination, or potential exposure to implant-associated reactive compounds. Genetic predisposition contributes at least in part, as suggested by the higher incidence of cases in Li-Fraumeni syndrome, cancer patients, and carriers of BRCA1/2 or TP53 mutations. Diagnosis of BIA-ALCL is mandatory prior to surgical intervention and is determined following fluid aspiration by morphological assessment as well as immunohistochemistry. Surgical management of BIA-ALCL by complete capsulectomy with en bloc resection of any associated masses, and excisional biopsy of lymph node involvement, is the best available treatment for the management of localized disease. In patients with unilateral disease but bilateral implant placement, prophylactic contralateral implant removal and total capsulectomy are indicated due to the possibility of incidental disease and bilateral onset and should be discussed with the patient for the known risk of bilateral disease. In patients with advanced/disseminated disease, first-line treatment should focus on traditional adjuvant options (ie, chemotherapy and radiotherapy) based on the guidelines for systemic ALCL. Immunotherapy (brentuximab vedotin) should be considered and is administered either as monotherapy or in combination as first-line or as second-line treatment. A discussion with patients should take place before surgery to state that breast implants are not lifetime devices and BIA-ALCL is a possible sequelae of textured implants, and to advise that patients will often need at least 1 or more revisional surgeries or implant removal in their lifetime. These accepted criteria are presented in detail in Table 2.
Figure 1.
Flow diagram representation of the search strategy with included and excluded articles. BIA-ALCL, breast implant-associated anaplastic large cell lymphoma.
Figure 2.
Flow diagram representation of the Delphi process, which was employed to develop the statements for the consensus paper from the World Consensus Conference on breast implant-associated anaplastic large cell lymphoma (BIA-ALCL).
Table 2.
Accepted Statements Regarding General Epidemiologic Facts, Etio-Pathogenesis, Diagnosis, Management, Socio-Psychological Aspects, and International Authority Guidance on BIA-ALCL
| Statement no. | Statement |
|---|---|
| Section 1: unveiling the truth | |
| 1 | BIA-ALCL should no longer be considered a rare complication, but rather an uncommon outcome of breast implant surgery. |
| 2 | The risk of reoperation for prophylactic capsulectomy and breast implant removal in women with high-risk textured devices is not higher than the risk of reoperation for therapeutic capsulectomy after breast implant removal or exchange due to complications (ie, implant rupture, capsular contracture, infection, malposition, asymmetry, etc). |
| 3 | Prophylactic capsulectomy is not necessarily a risk-reducing procedure for the prevention of BIA-ALCL in women with high-risk textured devices. |
| 4 | Reporting of new BIA-ALCL cases by the national breast implant registries is critically important to produce a clear picture of the epidemiology of this disease. A worthwhile implant registry should be mandatory or “opt-out” type. |
| 5 | Historical evidence suggests that older generations of smooth implants used to be associated with a higher risk of capsular contracture. Sixth-generation smooth implants are not associated with a higher risk of capsular contracture. |
| Section 2: what’s new on etiology and pathogenesis | |
| 6 | There is a moderate weight of evidence for a causal relationship between textured breast implants and BIA-ALCL, particularly in relation to implants with an intermediate to high surface roughness. |
| 7 | It is possible to demonstrate a strong weight of evidence to confirm a causal relationship between textured breast implants and BIA-ALCL. |
| 8 | To date, there are no confirmed BIA-ALCL cases in patients with a known history of only smooth breast implants. |
| 9 | Characterization of breast implant surface should be evaluated with a standardized system. Currently, the most widespread and accepted classification system is ISO 14607:2018. |
| 10 | Breast implants are Class III medical devices. Textured surfaces of these devices are currently classified by physical properties alone (ie, surface area, surface roughness, average roughness area). There is a need for a standardized classification of implants based on biological properties (ie, host reaction to the device). |
| 11 | A host inflammatory reaction–based classification of implants is beneficial for the assessment of breast implant characteristics as stratified by their biological properties. |
| 12 | All BIA-ALCL cases are associated with high-risk textured breast implants, where texturing was mostly obtained through salt loss technique or with certain types of polyurethane coating (“macro” according to ISO 14607:2018). Fewer cases occurred from devices classified as “micro” according to ISO 14607:2018. |
| 13 | Current proposed etiology pathways for BIA-ALCL include bacterial contamination, shell shedding of particulates, shell surface characteristics leading to friction, or potential exposure to implant-associated reactive compounds. |
| 14 | All proposed hypothetical pathways for BIA-ALCL etiology result in a well-established pathogenic pathway of chronic inflammation, which leads to lymphomagenesis. |
| 15 | Genetic predisposition for cancer appears to be a contributing factor for developing BIA-ALCL. In fact, the disease has an increased prevalence in women with germline mutations for TP53 and BRCA 1/2 genes. |
| 16 | The JAK-STAT3 pathway is constitutively activated in BIA-ALCL, and mutations of JAK1 and/or STAT3 are recurrent somatic mutations associated in BIA-ALCL. |
| 17 | Periprosthetic capsule biofilm, Ralstonia, and bacterial contamination are mostly abandoned as plausible etiologic hypotheses for BIA-ALCL, because initial findings from biofilm studies have not been replicated, and subsequent studies have demonstrated no distinct patient-specific microbiome. |
| 18 | Regarding possible biomarkers for BIA-ALCL, hypoxia-associated biomarker CA9 is a molecular signature that distinguishes BIA-ALCL from other ALCLs. |
| 19 | High levels of IL-10, IL-13, and eotaxin, as well as IL-10 to IL-6 ratio differentiates BIA-ALCL from all types of reactive seromas. |
| Section 3: focus on diagnosis and management | |
| 20 | In patients with symptoms of BIA-ALCL and a known history of textured implants, diagnosis by fluid aspiration and CD30 immunohistochemistry is mandatory, with imaging performed prior to surgical intervention. However, CD30+ALK− cells may not be sufficient to reach diagnosis of BIA-ALCL. |
| 21 | Surgical management of BIA-ALCL with en-bloc excision by including healthy tissue margins allows complete removal of localized disease. This approach prolongs overall survival and event-free survival compared with all other therapeutic interventions. All attempts should be made to gain complete surgical resection because retained or metastatic disease likely indicates the need for adjuvant treatments. |
| 22 | Surgical specimens should be oriented and inked to allow for the anatomic location of the disease. Capsules should be evaluated with strategic regional biopsies in a standardized approach. This is important for tumor site surveillance and in cases of recurrence requiring re-excision. |
| 23 | Approximately 2%-4% of BIA-ALCL patients develop bilateral disease. Therefore, a confirmed monolateral BIA-ALCL case in a patient with bilateral breast implants should receive prophylactic removal of the contralateral implant and capsule as well. |
| 24 | There are no prospective trials to guide the management of patients with advanced/disseminated BIA-ALCL, because their treatment options have been extrapolated from recommendations for cutaneous and systemic ALCL. Therefore, standardized protocols of adjuvant treatments (radiotherapy, chemotherapy, immunotherapy) has not yet been established. |
| 25 | Immunotherapy (brentuximab vedotin) should be employed as first-line treatment for non-surgically treatable BIA-ALCL cases in place of traditional adjuvant options (ie, CHOEP chemotherapy and radiotherapy). |
| Section 4: socio-psychological aspects of BIA-ALCL | |
| 26 | Breast implants are not lifetime devices. |
| 27 | Not all breast implants are the same: several degrees of texturization exist. However, the terms “macro,” “micro,” “mid-texture,” “nano,” “aggressive,” and “rough” have traditionally been employed in a relatively arbitrary fashion by implant manufacturing marketers to differentiate products. |
| 28 | Women with breast implants will often need at least 1 or more revisional surgeries or undergo explant surgery at some point throughout their lifetime. |
| 29 | Patients with implant-based breast reconstruction are more likely to undergo revisional surgery for correction of breast implant complications compared with breast augmentation patients. |
| 30 | Complications are relevant for both cosmetic and reconstructive procedures, and they should be adequately discussed when submitting informed consent. |
| 31 | Breast implant manufacturers that sell breast implant devices often provide a warranty for their devices, guaranteeing them for the patient’s lifetime. Patients with breast implants should undergo appropriate monitoring, as per FDA recommendations, to make use of the provided warranties. |
| Section 5: international authority guidance | |
| 32 | Prevalence of BIA-ALCL is still underestimated due to underreporting, which may be due to lack of specific measures, including: • Having a national regulatory board for breast implants and other medically implanted devices; • Having a national breast implant registry that records any reported implant-related complication; • Having national recommendations for early diagnosis and management; • Making BIA-ALCL case reporting mandatory; • Making informed consent for BIA-ALCL mandatory for any patient wishing to undergo breast implant surgery (regardless of whether for cosmetic or reconstructive purposes). |
| 33 | High-risk textured breast implants (“macro” according to ISO 14607:2018) should be recalled or banned, similar to authority actions already taken in certain countries (ie, France, Australia, Canada). |
| 34 | Other textured breast implants (“micro” according to ISO 14607:2018) should have specific measures put in place through legislation, including black box–labeled health warnings on device packages, a ban on advertising and promotion, and health education campaigns. |
| 35 | Significant differences exist in terms of BIA-ALCL reporting from country to country. BIA-ALCL requires a systematic and common international reporting system. |
| 36 | Despite the reduction in reported numbers of BIA-ALCL cases due to the COVID-19 pandemic, we can still expect a future increase in confirmed cases, with a peak at approximately 10 years from the peak in high-risk textured implant utilization, as suggested by market trends (with variations from country to country). |
BIA-ALCL, breast implant-associated anaplastic large cell lymphoma; CA9, carbonic anyhydrase-9; CHOEP, cyclophosphamide, doxorubicin, vincristine, etoposide and prednisone; IL, interleukin; ISO, International Organization for Standardization.
DISCUSSION
Although a consensus was achieved on all 36 statements, all members of the EP made comments suggesting valuable additions to the original statements to improve understanding of the reasoning behind some of the conveyed notions.
Section 1: Unveiling the Truth
This section addresses some of the most controversial aspects of BIA-ALCL, including prevalence and incidence. The first misconception is that BIA-ALCL is a “rare” side effect of textured surface implants. The European Medicines Agency specifically defines frequency of side effects: very common, affecting >1 in 10 people (ie, risk ≥10%); common, between 1 in 100 and 1 in 10 people (risk 1%-10%); uncommon, between 1 in 1000 and 1 in 100 people (risk 0.1%-1%); rare, between 1 in 10,000 and 1 in 1000 people (risk 0.01%-0.1%); and finally, very rare, affecting <1 in 10,000 people (risk <0.01%). Similar to inappropriate risk descriptions such as “like being struck by lightning” or “an asteroid”, the term “rare” for BIA-ALCL is a vestige from when the disease was first identified through post-market safety surveillance, and the frequency was not possible to estimate. Although still infrequently encountered, as of September 2020, the European Association of Plastic Surgeons (EURAPS) Scientific Committee on Device Safety and Development reported 420 confirmed cases in the European Union of 28 member states, with an overall prevalence estimated at 1:13,745 cases among patients with breast implants.14 Now the figures can be updated to 511 confirmed cases and a prevalence of 1:11,297. Incidence varies across study cohorts, ranging between 1:300 and 1:30,000.15-18 With these figures, careful consideration should be given towards addressing BIA-ALCL as an “uncommon” or “emerging” condition rather than an “unlikely” or “rare” occurrence.19 It has been suggested that national registries are critically important for producing a clearer picture of the epidemiology.20 Mandatory reporting of cases through an “opt-out” registry, where entries are recorded without the need for patient’s permission, has been proposed as a standard to increase the capture rate.21 This form of registry has been implemented by the Australian Breast Device Registry as well as the Dutch Breast Implant Registry. The main arguments against “opt-out” registries are that they collect personal data from women without their permission, they are not necessarily institutional review board–approved (as is the case for the US National Breast Implant Registry), and they were not designed for recording data specific to BIA-ALCL.22,23
A peculiar aspect of BIA-ALCL epidemiology is that although this malignancy is linked to all types of textured devices, specific manufacturers have produced devices associated with most cases (ie, Allergan BIOCELL followed by Silimed polyurethane).24,25 Another point of contention is whether the pre-emptive removal of such high-risk devices should be deemed safe or whether the risks outweigh the benefits. The main argument against prophylactic macrotextured implant removal with capsulectomy is the added surgical risk (ie, mortality and morbidity) for a procedure that has not yet been proven to be risk-reducing toward the onset of BIA-ALCL. Evidence presented at the 3rd WCC suggested that the specific breast implant surgery mortality rate is 0 fatalities over nearly 100,000 procedures between 2012 and 2019 in Italy.26 Morbidity associated with capsulectomy should not be dismissed, because the procedure can be associated with complications, including bleeding, hematomas, and even pneumothorax, largely for patients with a subpectoral pocket, where resection of the posterior aspect of the capsule can be more difficult.27-30 It remains unclear whether capsulectomy might reduce the risk of BIA-ALCL if conducted systematically in those with a high-risk device. However, it is certain that complete capsulectomy has proven to be an effective treatment for confirmed BIA-ALCL.31 In the presence of a diagnosis or missed diagnosis of BIA-ALCL, types of capsulectomies that are not well defined have not been proven to avoid the persistence or progression of disease.32 At this time, a total capsulectomy is not recommended for all textured breast implant exchanges or removals, even when high-risk macrotextured devices were placed, because risk mitigation has yet to be established and additional morbidity may be incurred by radical resections.33
Since the FDA’s class I device recall for Allergan BIOCELL devices, the world breast implant market has experienced a shift, with a resurgence of smooth devices.34-37 Some of the concerns that surgeons might have in converting to smooth breast implants is the risk of capsular contracture, which has historically been proven to be higher with older generations of smooth devices.38 Although it is true that first- and second-generation implants were plagued with higher rates of capsular contracture due to their manufacturing characteristics, evidence utilizing newer generation implants has proved that smooth-surface implants do not significantly increase capsular contracture risk compared with textured-surface implants.39-44
Section 2: Etiology and Pathogenesis
As stated in the SCHEER’s final opinion on the safety of breast implants, a causal relationship between BIA-ALCL and textured implants has been established due to the consistent findings of epidemiological studies.8 However, the etio-pathogenic mechanisms have not been fully characterized within knock-out animal models and/or by prospective randomized controlled trials of textured vs smooth-surface implants, the latter being currently impossible due to ethical implications. Despite the etio-pathogenesis remaining poorly understood, implant texturization, time, and genetics combined with other factors are all considered to contribute.1 According to the “bacteria theory,” an endotoxin from a subclinical Gram-negative colonization of the implant capsule may trigger BIA-ALCL.45 The theory was originally proposed as a Ralstonia spp. contamination hypothesis this likely represented a laboratory contaminant or coincidental across a small sample size. Subsequent studies have demonstrated no consistent differences of microbiota composition between capsules from BIA-ALCL–affected and contralateral control breasts.46 Additionally, results from the original study could not be replicated in subsequent studies.47 However, specific bacterial agents are likely not involved in the manner previously suggested by Hu et al but may still be a contributing factor.45 According to particulate theory, breast implants shed fragments that, through perpetual exhaustive phagocytosis, induce a cytokine storm sustaining chronic inflammation; in a hypoxic and immunocompromised interface, this leads to lymphoproliferation and ultimately lymphomagenesis.48 Furthermore, the “tribology theory” posits that a shear stress >100 Pa of the textured surface at the surrounding capsule may induce cell apoptosis sustaining chronic inflammation and bio-tribological adaptation mechanism of synovial metaplasia, with recruitment and proliferation of T lymphocytes, which may accumulate somatic mutations leading to monoclonal proliferation.49,50 The “leachables theory” proposes that toxins that have been detected in implant gels and exudates may ooze from the implant driving lymphomagenesis.51 Finally, carcinogens on the surface of breast implants, specifically aryl hydrocarbons, have been suggested as drivers of aryl hydrocarbon receptor expression in incipient tumor T cells, which in turn are converted into DNA damage-inducing epoxide derivatives that cause the accumulation of somatic mutations and lead to lymphomagenesis.52-54 Ultimately, chronic inflammation plays a prominent role in the proposed etiological pathways; it is therefore highly likely that this process is central to the development of BIA-ALCL, to which genetic predisposition to cancer may also contribute.55 In fact, a study from de Boer et al showed that BRCA1/2 mutation carriers with implants have an increased risk of BIA-ALCL.18 Moreover, Ionescu et al found that the absolute risk of developing BIA-ALCL in women with BRCA 1/2 mutations with breast implants was 1/1551 at 75 years of age compared with 1/7507 in women from the general population.56 Li-Fraumeni syndrome is another cancer-predisposing condition, caused by germline pathogenic mutations in TP53. Li-Fraumeni women have been reported to develop BIA-ALCL, thus suggesting that they may also be at greater risk.57-59 Nevertheless, evidence is still anecdotal, and more confirmatory data are needed to strengthen current evidence and to exclude the possibility that a higher incidence of BIA-ALCL in BRCA or TP53 carriers could be the result of a stricter follow-up in this category of patients.
BIA-ALCL has been linked to textured implants alone, with no confirmed BIA-ALCL cases in patients with a known history of only smooth breast implants to date. Nevertheless, only one-half of all cases of BIA-ALCL have a known and accurate history of single or multiple implant placements, and some reports of BIA-ALCL have been associated with smooth breast implants at the time of diagnosis but the previous implant histories were unknown. According to the FDA’s August 2020 report, there has been only 1 possible case with a known implant history in which only smooth implants were utilized.60 Nevertheless, the case could not be confirmed by the FDA Director of the Division of Surgical Devices, Dr Binita Ashar, during the WCC on BIA-ALCL.61 Recently, a case of BIA-ALCL developing in a patient with a history of only smooth implants was reported, but with prolonged indwelling of a textured tissue expander beforehand.62 Another case addressed the onset of right-sided BIA-ALCL in a patient who received bilateral reconstruction utilizing a textured implant on the right and smooth implant on the left.63 It is therefore reasonable to assume that no pure smooth breast implant cases can be considered associated with BIA-ALCL to date. The same cannot be said for macrotextured and microtextured devices, although it is not yet possible to determine the relative risk for BIA-ALCL according to surface characteristics. The ISO 14607:2018 is a classification system widely employed to characterize breast implants, but it only accounts for average surface roughness through scanning electron microscopy (SEM).64 There are several other proposed classification systems, including Barr/Bayat’s, which evaluates implant roughness with SEM and laser confocal microscopy; Atlan’s, which evaluates surface area with X-ray computed tomography imaging on top of SEM; Jones/Deva’s, which evaluates SEM and surface area to roughness ratio by micro-computed tomography; and James/Kinney’s, which evaluates bacterial adhesion and surface area to roughness ratio by profilometry.65-69 Despite the fact these classifications were peer reviewed in scientific publications, they focus on only the device’s physical properties without addressing biological properties, and none of them have been clinically validated.
Thus remains the need for an unambiguous, clinically validated classification system that includes parameters beyond “surface roughness.” 64 This is further validated by some authors claiming that the manufacturing process should be deemed more relevant than surface roughness itself, because the incidence per manufacturer not always corresponds to surface roughness or area.70 Nagor’s Nagotex devices have been shown to have a surface area even higher than Allergan BIOCELL’s but have fewer reported cases and a calculated BIA-ALCL risk of 1:45,454 women with these implants.65,67,71,72 Additionally, as of October 2019, POLYTECH (Dieburg, Germany) polyurethane, known as “microthane,” has been associated with 3 BIA-ALCL cases on a background of approximately 550,000 implants placed (BIA-ALCL risk of 1:180,000 implants).73 Detailed sales data has not yet been made available from the manufacturer, and it is unclear over what time period is represented by device sales. This is considerably lower than Silimed polyurethane device’s rate of 1:2832 cases.24 Based on SCHEER’s final opinion on breast implants and their relation to BIA-ALCL, there is a need to evaluate and objectively assess biological properties of the host’s reaction to the devices.8 The role of an implant surface in driving chronic inflammation (which is linked to lymphomagenesis; see above) is well-known in animal models and suggests that a roughness of 4 μm provokes the least amount of inflammation and foreign body response, although there is a need to standardize the variables and assess the immune reaction in human patients in both quantitative and qualitative terms before these findings can be of clinical utilization.74
Current evidence from BIA-ALCL research has focused on identifying features of the malignancy as possible diagnostic tools or therapeutic targets. The genomic landscape of BIA-ALCL has been characterized by JAK/STAT activating mutations. In a study from Laurent et al, the most common alterations observed in BIA-ALCL samples involved members of the JAK/STAT pathway, found in 64% (n = 14/22) of analyzed cases (employing whole-exome sequencing). Specifically, STAT3 and JAK1 were the most frequently mutated genes, accounting for 41% (n = 9/22) and 18% (n = 4/22) of cases, respectively. 75,76 Regarding possible biomarkers, BIA-ALCL has been associated with upregulation of hypoxia signaling genes, including the expression of hypoxia-associated biomarker carbonic anyhydrase-9 (CA9).77 Its potential clinical utilization is significant, although these findings may be related to the tumor microenvironment rather than the tumor cells themselves. However, investigations in paired primary and metastatic lymph nodes should be conducted to assess the diagnostic value of CA9 and whether its expression is specific to BIA-ALCL compared with other types of ALCL, such as systemic disease not related to implants. Indeed, although gene expression profiling studies highlight both similarities and differences between BIA-ALCL and other types of ALCL, additional studies are needed to determine the most consistent genetic signature of BIA-ALCL for diagnostic purposes.78,79 Other distinguishing features include its characterization by a T-helper 2-associated cytokine milieu (high levels of interleukin [IL]-10, IL-13, and eotaxin), which discriminates BIA-ALCL from other types of reactive seroma.80,81 An IL10 to IL-6 ratio >0.104 was associated with a specificity of 100% and a sensitivity of 83% in recognizing BIA-ALCL effusions. However, more studies are warranted to confirm the consistency of these findings.
Section 3: Focus on Diagnosis and Management
National Comprehensive Cancer Network (NCCN) guidelines focus on parameters for achieving a reliable diagnosis and highlight the importance of morphology and immunohistochemistry testing. In particular, CD30 is fundamental but not pathognomonic by itself, because CD30 expression is non-specific and can also be expressed by cytologically non-atypical benign inflammatory cells.31,82-84 Additionally, careful clinicopathologic correlation is paramount for reaching a correct diagnosis besides proper handling of fluid for cytology and cell block production.85 It is recommended that at least 20 mL (and as much as possible) of a seroma is sent for cytologic examination of smears and for the production of a cell block.86 Repeat aspirations may dilute cellular burden, impairing the ability to make a reliable diagnosis.
Whenever a case of localized disease is confirmed and surgical treatment is possible, en-bloc capsulectomy is indicated. Patients with complete surgical control of disease have a favorable long-term overall and event-free survival.31,87 For correct staging and tumor site surveillance, the orientation and proper processing of specimens (ie, no unintentional separation of fibrinoid material, where most tumor cells lie, from the capsule and incorrect orientation of the sections) are crucial. False-negative results could ultimately hamper the ability of the pathologist to microscopically identify scarce lymphoma cells and thus delay diagnosis.88 Incomplete resections, partial capsulectomies, and positive margins are all associated with high rates of disease recurrence and can lead to progression.33,34 Highly effective therapies, including local radiotherapy and immunotherapy (brentuximab vedotin), should be considered for specimens with positive margins when complete resection becomes increasingly morbid.31,89,90 A strongly disputed topic is the management of patients with localized unilateral disease but bilateral breast implant placement. In a series of 39 BIA-ALCL patients, Collins et al identified 7 cases with bilateral disease.91 Data from the NCCN suggest bilateral cases may represent as many as 2%-4% of total BIA-ALCL diagnoses.31 Consensus was found in favor of patients receiving removal of the contralateral implant and capsule as well. Immediate or delayed reconstruction with smooth-surface implants or autologous tissue may be offered to patients.
In cases of local residual disease, positive margins, or unresectable disease with chest wall invasion, local or involved site radiation therapy with 24 to 36 Gray has been suggested. Systemic therapy is warranted for patients with Lugano stage II-IV (ie, involvement of or beyond a single lymph node region or structure) or MD Anderson stage IIB-IV disease (ie, in cases of any lymph node involvement and/or presence of distant metastases).31,92,93 Oncologists can consider either a standard approach as is employed for systemic ALCL, such as combination anthracycline-based chemotherapy, or, alternatively, in combination with brentuximab vedotin; the efficacy of these treatments, however, has only been demonstrated through case reports and anecdotal evidence thus far.31,81,94 Protocols for adjuvant treatments in BIA-ALCL have not yet been established or validated through prospective trials because none exist to date.95 In fact, the current line of treatment for patients with disseminated disease according to NCCN guidelines should be as is applied in systemic ALCL treatment; standard first-line chemotherapy with cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone and second-line treatment with brentuximab vedotin.31 To date, the efficacy of immunotherapy for BIA-ALCL treatment has only been demonstrated through case reports, although evidence has been encouraging so far, demonstrating in some cases complete remission after the utilization of brentuximab vedotin for the treatment of BIA ALCL in the context of refractory disease.96-98 However, it does come at great financial cost, which must be considered according to national healthcare policies.
Section 4: Socio-Psychological Aspects of BIA-ALCL
Before receiving a breast implant, patients need to be aware of common misconceptions, which have now emerged thanks to advocacy groups.99 Breast implants are not lifetime devices, and several studies estimate implant lifespan to be 10 to 15 years, but the rupture rate of silicone gel-filled implants is underestimated due to the number of asymptomatic or unreported cases.100-104 Manufacturers often provide a patient’s lifetime warranty for their devices, with similar warranty plans; if a confirmed rupture or deflation occurs, the patient will receive a replacement breast implant free of charge but with no cost coverage for surgery.105
Women bearing breast implants will need at least 1 or more revisional surgeries if the implant is not explanted at some point in their lifetime. According to Forster et al, 25% of patients from their cohort of 230 breast augmentations required more than 1 revision procedure in a 10-year span, and 24.5% of patients who underwent a first revision needed a second one within 12 months.106 Reoperation rates are reported at 10-year follow-up in industry-sponsored core studies, varying from 22.3% to 59.7% according to the indication for surgery.107-109 Current evidence suggests that patients receiving breast implants for reconstruction are more likely to undergo revisional surgery, possibly because either they have received a more thorough follow-up due to cancer concerns, or because insurance covers the revisional surgery costs for reconstruction but not augmentation.71,108-110 Complications are relevant as well, affecting both cosmetic and reconstructive patients, and include capsular contraction, hematoma, infection, breast pain, ptosis, rupture, and nipple complications, which are extensively reported in core studies as well.71,108-111 These events highlight the importance of patients being properly counseled about the potential risks and benefits of the implant-based procedure before surgery.
Section 5: International Authority Guidance
On July 24, 2019, after the FDA requested a class I device recall for textured Allergan BIOCELL tissue expanders and breast implants in the United States, the company voluntarily recalled these devices worldwide following the FDA’s action. Australia, Canada, and France went 1 step further by banning certain types of textured breast implants as a precautionary measure, whereas other countries, particularly those in Europe, still market textured devices.111-112 Banning textured devices altogether has been proposed, though the matter sparks heated debates and discussion among the medical community and ultimately should be considered by legislators.113-115 Meanwhile, to help patients make an informed decision, the FDA has introduced black box–labeled health warnings on device packages and have mandated standard informed consent for textured devices.116,117
Significant geographical differences in BIA-ALCL prevalence have emerged; recorded incidences in certain countries are affected by underreporting due to a lack of oversight and information provided by bodies such as national regulatory boards for breast implants and national breast implant registries. Provision of national recommendations for early diagnosis and management and the mandating of informed consent for BIA-ALCL risks for any patient wishing to undergo breast implant surgery is also lacking in some countries. The notion of BIA-ALCL underreporting emerged from a European study which found that countries where specific measures had been implemented to tackle BIA-ALCL accounted for 61% of the European Union’s 28 member states population, which reported 91% of all cases. That case prevalence of 1:9121 women with implants is considerably higher than the European average (1:13,745).14 Other nations should adopt projections based on these measures to avoid underestimating BIA-ALCL prevalence in the future.
Finally, we should expect an increase in reported cases in the future at approximately 10 years from the peak of high-risk textured implant utilization, as suggested by national market trends.118 Based on a 10-year median time from implant placement to BIA-ALCL diagnosis, the peak number of cases in the United States is anticipated to occur in 2026 or thereafter. However, compared with a mean prevalence of 1:9338 in the United States, which has only a 12.7 % textured implant market, the mean prevalence of 1:11,386 in Europe with a 95% textured implant market share suggests that the incidence of BIA-ALCL is severely underreported.
Delphi studies are meant to debate on topics to arrive at consensus. In our study, consensus on 36 statements was achieved within a single round, which might seem like a potential limitation. However, the role of the WCC is to address the controversies but at the same time offer clear statements/guidelines that arise from research and clinical experience. A more open type of statement could be added in our next study to stimulate even more discussion and debate on BIA-ALCL and related controversial topics.
CONCLUSIONS
To produce the first, to our knowledge, consensus paper on BIA-ALCL after 3 WCCs, key themes were discussed and agreed on by experts through the Delphi process. According to consensus findings, BIA-ALCL is an uncommon condition, still largely underreported due to deficiencies in breast implant registries and a lack of sufficient actions taken by regulatory authorities. Prophylactic capsulectomy with implant removal has not yet demonstrated a reduction in the risk for development of ALCL, nor has a higher risk of fatality been shown for this procedure compared with therapeutic capsulectomy after implant replacement. There is a need for an improved classification system for implants that considers implant/host interactions and other biological factors as well as metrics of texturization. A causal relationship between BIA-ALCL and textured implants can be made without doubt, although the etiology is likely multifactorial and genetic predisposition may contribute to disease pathogenesis. A diagnosis considering cellular morphology and immunohistochemical expression of CD30 of fluid aspirates is mandatory prior to surgical intervention. En-bloc therapeutic capsulectomy with contralateral prophylactic implant removal and total capsulectomy are indicated as the best available treatment for the management of localized disease. Traditional adjuvant chemotherapy and/or brentuximab vedotin may be considered as first line treatment in collaboration with a multidisciplinary evaluation. Patients should be counseled before surgery and informed that breast implants are not lifetime devices and they will need further surgeries. Findings from this consensus paper cement state-of-the-art knowledge that has the potential to improve patient and physician education and to pave the way for future developments in the many aspects of BIA-ALCL.
Supplementary Material
Acknowledgments
The authors thank the members of the European Association of Plastic Surgeons (EURAPS) Committee on Device Safety and Development (E. Athanasopoulos, Greece; K. Arctander, Norway; B. Berenguer, Spain; K. Bozikov, Slovenia; A. Cardoso, Portugal; Å. Edsander Nord, Sweden; C. Filip, Norway; A. Georgeskou, Romania; C. Heitman, Germany; O. Kaarela, Finland; M. Kolenda, Poland; M. Hamdi, Belgium; L. Lantieri, France; D. Lumenta, Austria; N. Mercer, United Kingdom; E. Ruegg, Switzerland; F. Santanelli di Pompeo, Italy [Chair]; Z. Stanec, Croatia; R. Van Der Hulst, the Netherlands; and J.J. Vranckx [EURAPS SG elect.]) for their contribution in the form of European BIA-ALCL data collection and communication.
Contributor Information
Fabio Santanelli di Pompeo, Faculty of Medicine and Psychology, Sapienza University of Rome, Department NESMOS, Sant’Andrea Hospital, Rome, Italy.
Mark W Clemens, Department of Plastic Surgery, The University of Texas MD Anderson Cancer Center, Houston, TX, USA and is a Breast Surgery section editor for Aesthetic Surgery Journal.
Michael Atlan, Aesthetic Plastic Reconstructive Unit/CHU TENON PARIS—APHP, Université Pierre et Marie Curie, Paris, France.
Peter G Cordeiro, Plastic and Reconstructive Surgery Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, New York, NY, USA.
Daphne De Jong, Amsterdam UMC-Vrije Universiteit Amsterdam, Department of Pathology and Cancer Center Amsterdam, Amsterdam, the Netherlands.
Arianna Di Napoli, Pathology Unit, Department of Clinical and Molecular Medicine, Sapienza University, Sant’Andrea Hospital, Rome, Italy.
Cara L Haymaker, Department of Translational Molecular Pathology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Steven M Horwitz, Lymphoma Service, Memorial Sloan-Kettering Cancer Center, New York, NY, USA.
Kelly Hunt, Department of Breast Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Peter Lennox, Department of Surgery, Division of Plastic and Reconstructive Surgery, University of British Columbia, Vancouver, BC, Canada and is a clinical editor for Aesthetic Surgery Journal.
Roberto N Miranda, Department of Hematopathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Alexandre M Munhoz, Plastic Surgery Department, Hospital Moriah, Hospital Sírio-Libanês, Higienópolis, São Paulo, Brazil.
Suzanne D Turner, Division of Cellular and Molecular Pathology, Department of Pathology, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
Guido Firmani, Faculty of Medicine and Psychology, Sapienza University of Rome, Department of Plastic Surgery, Sant’Andrea Hospital, Rome, Italy.
Michail Sorotos, Faculty of Medicine and Psychology, Sapienza University of Rome, Department NESMOS, Sant’Andrea Hospital, Rome, Italy.
Disclosures
Prof Santanelli di Pompeo is affiliated with the NESMOS Department, which received research funds from Motiva (Houston, TX), Establishment Labs (Alajuela, Costa Rica) in 2017, and from GC Aesthetics (Dublin, Ireland) in 2018 and 2020. The NESMOS Department also received mini-implants from Establishment Labs, GC Aesthetics and Sebbin (Boissy l'Aillerie, France) for research purposes. Dr Santanelli di Pompeo is also a paid consultant for BellaSeno GmbH (Leipzig, Germany), received reimbursements for travel/lodgment expenses from ICEAG (Bilthoven, Utrecht, the Netherlands) in 2015 and SCHEER-WG (Luxembourg City, Luxembourg) in 2019, 2020, and 2021, and is a member of Notified Body 0373, which is part of the Superior Institute of Health, and which carried out CE Mark certification activities for the Italian Ministry of Health for the year 2021. He declared no ownerships or investments. Dr Hammond has a consulting agreement with the Mentor Corporation (Irvine, CA) and Establishment Labs, is a member of the medical advisory board for Establishment Labs, is a stockholder, owns unexcersized stock options with Establishment Labs, and holds a royalty agreement related to a medical device with Establishment Labs. Dr Turner receives research funding from Allergan (Irvine, CA). MD Anderson Cancer Center participates in clinical trials for Mentor Corporation and Establishment Labs. Drs Clemens, Haymaker, Hunt, and Miranda are faculty of MD Anderson Cancer Center. Dr. Haymaker receives speaker’s fees from the Society for Immunotherapy of Cancer (Milwaukee, WI), serves as an advisory board member for Briacell (W Vancouver, CA) and the Mesothelioma Applied Research Foundation (Washington, DC), has received personal fees from Nanobiotix (Paris, France) and receives funding to the MD Anderson Cancer Center from Iovance (San Carlos, CA) Sanofi (Paris, France), Dragonfly Therapeutics (Waltham, MA), and BTG (São Paulo, Brazil) outside the submitted work. Dr. Atlan is a speaker/consultant for GC Aesthetics (FixNip) and Renuvion (Apyx Medical Corporation, Clearwater, FL). Dr Swanson receives royalties from Springer Nature (Cham, Switzerland). Dr Horwitz discloses research Support from Affimed (Heidelberg, Germany), Millennium/Takeda (Cambridge, MA), Seattle Genetics (Bothell, WA) and consultancy relationships with Seattle Genetics, Takeda, and Tubulis (München, Germany). Dr. Hunt is on the Medical Advisory Board of Armada Health (Hunt Valley, MD) and AstraZeneca (Cambridge, UK) and discloses research funding from her Institution – Cairn Surgical (Lebanon, NH), Eli Lilly and Company (Indianapolis, IN) and Lumicell (Newton, MA). Dr. Mallucci is a temporary consultant for POLYTECH (Dieburg, Germany), Laboratoires SeBBin and BD (Franklin Lakes, NJ) and is an investor in B-Lite (Amsterdam, the Netherlands). Dr. Munhoz is a shareholder and member of the advisory board for Establishment Labs. Drs De Jong, Di Napoli, Lennox, Cordeiro, Sorotos, Firmani, and Botti declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
- 1. Clemens MW, DeCoster RC, Fairchild B, Bessonov AA, Santanelli di Pompeo F. Finding consensus after two decades of breast implant-associated anaplastic large cell lymphoma. Semin Plast Surg. 2019;33(4):270-278. doi: 10.1055/s-0039-1696998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Santanelli di Pompeo F, Paolini G, Firmani G, Sorotos M. From breast implant to rough implant associated-ALCL (RIA-ALCL). Aesthet Surg J. 2022:42(6):NP445-NP446. doi: 10.1093/asj/sjac005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keech JA, Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100(2):554-555. doi: 10.1097/00006534-199708000-00065 [DOI] [PubMed] [Google Scholar]
- 4. Said JW, Tasaka T, Takeuchi S, et al. Primary effusion lymphoma in women: report of two cases of Kaposi’s sarcoma herpes virus-associated effusion-based lymphoma in human immunodeficiency virus-negative women. Blood. 1996;88(8):3124-3128. doi: 10.1182/blood.V88.8.3124.bloodjournal8883124 [DOI] [PubMed] [Google Scholar]
- 5. Lyapichev KA, Medeiros LJ, Clemens MW, et al. Reconsideration of the first recognition of breast implant-associated anaplastic large cell lymphoma: a critical review of the literature. Ann Diagn Pathol. 2020;45:151474. doi: 10.1016/j.anndiagpath.2020.151474 [DOI] [PubMed] [Google Scholar]
- 6. Food and Drug Administration. FDA update on the safety of silicone gel-filled breast implants. Published June 18, 2011. Accessed March 1, 2022. https://www.fda.gov/media/80685/download.
- 7. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. doi: 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Jong WH, Panagiotakos D, Proykova A, et al. Final opinion on the safety of breast implants in relation to anaplastic large cell lymphoma: report of the Scientific Committee on Health, Emerging and Environmental Risks (SCHEER). Regul Toxicol Pharmacol. 2021;125:104982. doi: 10.1016/j.yrtph.2021.104982 [DOI] [PubMed] [Google Scholar]
- 9. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008-1015. [PubMed] [Google Scholar]
- 10. Waggoner J, Carline JD, Durning SJ. Is there a consensus on consensus methodology? Descriptions and recommendations for future consensus research. Acad Med. 2016;91(5):663-668. doi: 10.1097/ACM.0000000000001092 [DOI] [PubMed] [Google Scholar]
- 11. Weir NM, Pattison SH, Kearney P, et al. Criteria required for an acceptable point-of-care test for UTI detection: obtaining consensus using the Delphi technique. PLoS One. 2018;13(6):e0198595. doi: 10.1371/journal.pone.0198595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sullivan GM, ArtinoAR, Jr. Analyzing and interpreting data from Likert-type scales. J Grad Med Educ. 2013;5(4):541-542. doi: 10.4300/JGME-5-4-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jamieson S. Likert scales: how to (ab)use them. Med Educ. 2004;38(12):1217-1218. doi: 10.1111/j.1365-2929.2004.02012.x [DOI] [PubMed] [Google Scholar]
- 14. Santanelli di Pompeo F, Sorotos M, Clemens MW, Firmani G. European Association of Plastic Surgeons (EURAPS) Committee on Device Safety and Development. Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL): review of epidemiology and prevalence assessment in Europe. Aesthet Surg J. 2021;41(9):1014-1025. doi: 10.1093/asj/sjaa285 [DOI] [PubMed] [Google Scholar]
- 15. Cordeiro P. “Variability in incidence of BIA-ALCL in the literature: why the difference.” In: 3rd World Consensus Conference on BIA-ALCL. Accessed February 27, 2022. https://youtu.be/YYHSUPUyUJs?t=5295.
- 16. Doren EL, Miranda RN, Selber JC, et al. U.S. epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2017;139(5):1042-1050. doi: 10.1097/PRS.0000000000003282 [DOI] [PubMed] [Google Scholar]
- 17. Collett DJ, Rakhorst H, Lennox P, Magnusson M, Cooter R, Deva AK. Current risk estimate of breast implant-associated anaplastic large cell lymphoma in textured breast implants. Plast Reconstr Surg. 2019;143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):30S-40S. doi: 10.1097/PRS.0000000000005567 [DOI] [PubMed] [Google Scholar]
- 18. de Boer M, van Leeuwen FE, Hauptmann M, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol. 2018;4(3):335-341. doi: 10.1001/jamaoncol.2017.4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. U.S. Food and Drug Administration. Rare diseases at FDA. Accessed March 20, 2022. https://www.fda.gov/patients/rare-diseases-fda.
- 20. Cooter RD, Barker S, Carroll SM, et al. International importance of robust breast device registries. Plast Reconstr Surg. 2015;135(2):330-336. doi: 10.1097/PRS.0000000000000885 [DOI] [PubMed] [Google Scholar]
- 21. Rakhorst HA, Mureau MAM, Cooter RD, et al. The new opt-out Dutch National Breast Implant Registry - lessons learnt from the road to implementation. J Plast Reconstr Aesthet Surg. 2017;70(10):1354-1360. doi: 10.1016/j.bjps.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 22. Swanson E. The case against the National Breast Implant Registry. Ann Plast Surg. 2021;86(3):245-247. doi: 10.1097/SAP.0000000000002743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCarthy CM, Loyo-Berríos N, Qureshi AA, et al. Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology (PROFILE): initial report of findings, 2012-2018. Plast Reconstr Surg. 2019;143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):65S-73S. doi: 10.1097/PRS.0000000000005571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magnusson M, Beath K, Cooter R, et al. The epidemiology of breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand confirms the highest risk for grade 4 surface breast implants. Plast Reconstr Surg. 2019;143(5):1285-1292. doi: 10.1097/PRS.0000000000005500 [DOI] [PubMed] [Google Scholar]
- 25. U.S. Food and Drug Administration (FDA). Medical device reports of breast implant-associated anaplastic large cell lymphoma. Accessed February 26, 2022. https://www.fda.gov/medical-devices/breast-implants/medical-device-reports-breast-implant-associated-anaplastic-large-cell-lymphoma.
- 26. Santanelli di Pompeo F. “An insight to risk for re-operation”. In: 3rd World Consensus Conference on BIA-ALCL. Accessed February 27, 2022. https://youtu.be/YYHSUPUyUJs?t=3724.
- 27. Katsnelson JY, Spaniol JR, Buinewicz JC, Ramsey FV, Buinewicz BR. Outcomes of implant removal and capsulectomy for breast implant illness in 248 patients. Plast Reconstr Surg Glob Open. 2021;9(9):e3813. doi: 10.1097/GOX.0000000000003813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Swanson E. The case for breast implant removal or replacement without capsulectomy. Aesthetic Plast Surg. 2021;45(3):1338-1341. doi: 10.1007/s00266-020-02079-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Swanson E. Evaluating the necessity of capsulectomy in cases of textured breast implant replacement. Ann Plast Surg. 2020;85(6):691-698. doi: 10.1097/SAP.0000000000002301 [DOI] [PubMed] [Google Scholar]
- 30. Gascoigne AC, Malata CM. Pleural damage during capsulectomy and exchange of long-standing breast implants in Poland syndrome: a cautionary tale. Ann Plast Surg. 2012;69(2):148-151. doi: 10.1097/SAP.0b013e318226b4c4 [DOI] [PubMed] [Google Scholar]
- 31. Clemens MW, Jacobsen ED, Horwitz SM. 2019 NCCN consensus guidelines on the diagnosis and treatment of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Aesthet Surg J. 2019;39(Suppl_1):S3-S13. doi: 10.1093/asj/sjy331 [DOI] [PubMed] [Google Scholar]
- 32. Evans MG, Medeiros LJ, Marques-Piubelli ML, et al. Breast implant-associated anaplastic large cell lymphoma: clinical follow-up and analysis of sequential pathologic specimens of untreated patients shows persistent or progressive disease. Mod Pathol. 2021;34(12):2148-2153. doi: 10.1038/s41379-021-00842-6 [DOI] [PubMed] [Google Scholar]
- 33. Tevis SE, Hunt KK, Clemens MW. Stepwise en bloc resection of breast implant-associated anaplastic large cell lymphoma with oncologic considerations. Aesthet Surg J Open Forum. 2019;1(1):ojz005. doi: 10.1093/asjof/ojz005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Montemurro P, Tay VKS. Transitioning from conventional textured to nanotextured breast implants: our early experience and modifications for optimal breast augmentation outcomes. Aesthet Surg J. 2021;41(2):189-195. doi: 10.1093/asj/sjaa169 [DOI] [PubMed] [Google Scholar]
- 35. Roberts JM, Carr LW, Jones A, Schilling A, Mackay DR, Potochny JD. A prospective approach to inform and treat 1340 patients at risk for BIA-ALCL. Plast Reconstr Surg. 2019;144(1):46-54. doi: 10.1097/PRS.0000000000005703 [DOI] [PubMed] [Google Scholar]
- 36. Calobrace MB. Elective implant removal and replacement in asymptomatic aesthetic patients with textured devices. Plast Reconstr Surg. 2021;147(5S):14S-23S. doi: 10.1097/PRS.0000000000008041 [DOI] [PubMed] [Google Scholar]
- 37. Santanelli di Pompeo F, Paolini G, Firmani G, Sorotos M. History of breast implants: back to the future. JPRAS Open. 2022;32:166-177. doi: 10.1016/j.jpra.2022.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Malata CM, Feldberg L, Coleman DJ, Foo IT, Sharpe DT. Textured or smooth implants for breast augmentation? Three year follow-up of a prospective randomised controlled trial. Br J Plast Surg. 1997;50(2):99-105. doi: 10.1016/s0007-1226(97)91320-5 [DOI] [PubMed] [Google Scholar]
- 39. Spear SL, Jespersen MR. Breast implants: saline or silicone? Aesthet Surg J. 2010;30(4):557-570. doi: 10.1177/1090820X10380401 [DOI] [PubMed] [Google Scholar]
- 40. Maxwell GP, Gabriel A. The evolution of breast implants. Plast Reconstr Surg. 2014;134(1 Suppl):12S-17S. doi: 10.1097/PRS.0000000000000348 [DOI] [PubMed] [Google Scholar]
- 41. Henriksen TF, Fryzek JP, Hölmich LR, et al. Surgical intervention and capsular contracture after breast augmentation: a prospective study of risk factors. Ann Plast Surg. 2005;54(4):343-351. doi: 10.1097/01.sap.0000151459.07978.fa [DOI] [PubMed] [Google Scholar]
- 42. Lista F, Austin RE, Saheb-Al-Zamani M, Ahmad J. Does implant surface texture affect the risk of capsular contracture in subglandular breast augmentation and breast augmentation-mastopexy? Aesthet Surg J. 2020;40(5):499-512. doi: 10.1093/asj/sjz241 [DOI] [PubMed] [Google Scholar]
- 43. Calobrace MB, Schwartz MR, Zeidler KR, Pittman TA, Cohen R, Stevens WG. Long-term safety of textured and smooth breast implants. Aesthet Surg J. 2017;38(1):38-48. doi: 10.1093/asj/sjx157 [DOI] [PubMed] [Google Scholar]
- 44. Coroneos CJ, Selber JC, Offodile AC 2nd, Butler CE, Clemens MW. US FDA breast implant postapproval studies: long-term outcomes in 99,993 patients. Ann Surg. 2019;269(1):30-36. doi: 10.1097/SLA.0000000000002990 [DOI] [PubMed] [Google Scholar]
- 45. Hu H, Johani K, Almatroudi A, et al. Bacterial biofilm infection detected in breast implant-associated anaplastic large-cell lymphoma. Plast Reconstr Surg. 2016;137(6):1659-1669. doi: 10.1097/PRS.0000000000002010 [DOI] [PubMed] [Google Scholar]
- 46. Walker JN, Hanson BM, Pinkner CL, et al. Insights into the microbiome of breast implants and periprosthetic tissue in breast implant-associated anaplastic large cell lymphoma. Sci Rep. 2019;9(1):10393. doi: 10.1038/s41598-019-46535-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Walker JN, Hanson BM, Myckatyn TM. Commentary on: optimizing breast pocket irrigation: the breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) era. Aesthet Surg J. 2020;40(6):626-629. doi: 10.1093/asj/sjz269 [DOI] [PubMed] [Google Scholar]
- 48. Hallab NJ, Samelko L, Hammond D. The inflammatory effects of breast implant particulate shedding: comparison with orthopedic implants. Aesthet Surg J. 2019;39(Suppl_1):S36-S48. doi: 10.1093/asj/sjy335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hall-Findlay EJ. Breast implant complication review: double capsules and late seromas. Plast Reconstr Surg. 2011;127(1):56-66. doi: 10.1097/PRS.0b013e3181fad34d [DOI] [PubMed] [Google Scholar]
- 50. Giot JP, Paek LS, Nizard N, et al. The double capsules in macro-textured breast implants. Biomaterials. 2015;67:65-72. doi: 10.1016/j.biomaterials.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 51. Deva AK, Turner SD, Kadin ME, et al. Etiology of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL): current directions in research. Cancers (Basel). 2020;12(12):3861. doi: 10.3390/cancers12123861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fitzal F, Turner SD, Kenner L. Is breast implant-associated anaplastic large cell lymphoma a hazard of breast implant surgery? Open Biol. 2019;9(4):190006. doi: 10.1098/rsob.190006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Turner SD. The cellular origins of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL): implications for immunogenesis. Aesthet Surg J. 2019;39(Suppl_1):S21-S27. doi: 10.1093/asj/sjy229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kadin ME. What cytokines can tell us about the pathogenesis of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Aesthet Surg J. 2019;39(Suppl_1):S28-S35. doi: 10.1093/asj/sjy250 [DOI] [PubMed] [Google Scholar]
- 55. George EV, Pharm J, Houston C, et al. Breast implant-associated ALK-negative anaplastic large cell lymphoma: a case report and discussion of possible pathogenesis. Int J Clin Exp Pathol. 2013;6(8):1631-1642. [PMC free article] [PubMed] [Google Scholar]
- 56. Ionescu P, Vibert F, Amé S, Mathelin C. New data on the epidemiology of breast implant-associated anaplastic large cell lymphoma. Eur J Breast Health. 2021;17(4):302-307. doi: 10.4274/ejbh.galenos.2021.2021-5-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oishi N, Brody GS, Ketterling RP, et al. Genetic subtyping of breast implant-associated anaplastic large cell lymphoma. Blood. 2018;132(5):544-547. doi: 10.1182/blood-2017-12-821868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Oishi N, Miranda RN, Feldman AL. Genetics of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Aesthet Surg J. 2019;39(Suppl_1):S14-S20. doi: 10.1093/asj/sjy311 [DOI] [PubMed] [Google Scholar]
- 59. Adlard J, Burton C, Turton P. Increasing evidence for the association of breast implant-associated anaplastic large cell lymphoma and li fraumeni syndrome. Case Rep Genet. 2019;2019:5647940. doi: 10.1155/2019/5647940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. U.S. Food and Drug Administration. Medical device reports of breast implant-associated anaplastic large cell lymphoma. Accessed March 5, 2022. https://www.fda.gov/medical-devices/breast-implants/medical-device-reports-breast-implant-associated-anaplastic-large-cell-lymphoma.
- 61. Ashar B. FDA perspective: BIA-ALCL and breast implant safety. Presented at the 2nd World Consensus Conference on BIA-ALCL at MD Anderson Cancer Center, November 7, 2020, Houston, TX.
- 62. Akhavan AA, Wirtz EC, Ollila DW, Bhatt N. An unusual case of BIA-ALCL associated with prolonged/complicated BIOCELL-textured expander, followed by smooth round breast implant exposure, and concurrent use of adalimumab. Plast Reconstr Surg. 2021;148(2):299-303. doi: 10.1097/PRS.0000000000008155 [DOI] [PubMed] [Google Scholar]
- 63. Johnson L, Lowry K, Scheel J, Mau B, Rockoff SJ. Breast implant-associated anaplastic large cell lymphoma with contralateral invasive lobular carcinoma. Radiol Case Rep. 2020;15(12):2572-2576. doi: 10.1016/j.radcr.2020.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Asaad M, Offodile AC, Santanelli Di Pompeo F, et al. Management of symptomatic patients with textured implants. Plast Reconstr Surg. 2021;147(5S):58S-68S. doi: 10.1097/PRS.0000000000008047 [DOI] [PubMed] [Google Scholar]
- 65. Munhoz AM, Clemens MW, Nahabedian MY. Breast implant surfaces and their impact on current practices: where we are now and where are we going? Plast Reconstr Surg Glob Open. 2019;7(10):e2466. doi: 10.1097/GOX.0000000000002466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Barr S, Hill EW, Bayat A. Functional biocompatibility testing of silicone breast implants and a novel classification system based on surface roughness. J Mech Behav Biomed Mater. 2017;75:75-81. doi: 10.1016/j.jmbbm.2017.06.030 [DOI] [PubMed] [Google Scholar]
- 67. Atlan M, Nuti G, Wang H, Decker S, Perry T. Breast implant surface texture impacts host tissue response. J Mech Behav Biomed Mater. 2018;88:377-385. doi: 10.1016/j.jmbbm.2018.08.035 [DOI] [PubMed] [Google Scholar]
- 68. Jones P, Mempin M, Hu H, et al. The functional influence of breast implant outer shell morphology on bacterial attachment and growth. Plast Reconstr Surg. 2018;142(4):837-849. doi: 10.1097/PRS.0000000000004801 [DOI] [PubMed] [Google Scholar]
- 69. James GA, Boegli L, Hancock J, Bowersock L, Parker A, Kinney BM. Bacterial adhesion and biofilm formation on textured breast implant shell materials. Aesthetic Plast Surg. 2019;43(2):490-497. doi: 10.1007/s00266-018-1234-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Loch-Wilkinson A, Beath KJ, Knight RJW, et al. Breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand: high-surface-area textured implants are associated with increased risk. Plast Reconstr Surg. 2017;140(4):645-654. doi: 10.1097/PRS.0000000000003654 [DOI] [PubMed] [Google Scholar]
- 71. Duteille F, Perrot P, Bacheley MH, Bell E, Stewart S. Ten-year safety data for Eurosilicone’s round and anatomical silicone gel breast implants. Aesthet Surg J Open Forum. 2019;1(2):ojz012. doi: 10.1093/asjof/ojz012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Loch-Wilkinson A, Beath KJ, Magnusson MR, et al. Breast implant-associated anaplastic large cell lymphoma in Australia: a longitudinal study of implant and other related risk factors. Aesthet Surg J. 2020;40(8):838-846. doi: 10.1093/asj/sjz333 [DOI] [PubMed] [Google Scholar]
- 73. POLYTECH Health and Aesthetics GmbH. 2019. BIA-ALCL – POLYTECH update October 2019 and clarification of misleading statements. Accessed March 5, 2022. https://aleamed.eu/wp-content/uploads/2019/11/2019-10-25-_-BIA-ALCL-Polytech-Update-and-Comments-on-Misleading-Statements.pdf.
- 74. Doloff JC, Veiseh O, de Mezerville R, et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nat Biomed Eng. 2021;5(10):1115-1130. doi: 10.1038/s41551-021-00739-4 [DOI] [PubMed] [Google Scholar]
- 75. Laurent C, Nicolae A, Laurent C, et al. Gene alterations in epigenetic modifiers and JAK-STAT signaling are frequent in breast implant-associated ALCL. Blood. 2020;135(5):360-370. doi: 10.1182/blood.2019001904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Blombery P, Thompson ER, Prince HM. Molecular drivers of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2019;143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):59S-64S. doi: 10.1097/PRS.0000000000005570 [DOI] [PubMed] [Google Scholar]
- 77. Oishi N, Hundal T, Phillips JL, et al. Molecular profiling reveals a hypoxia signature in breast implant-associated anaplastic large cell lymphoma. Haematologica. 2021;106(6):1714-1724. doi: 10.3324/haematol.2019.245860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Di Napoli A, De Cecco L, Piccaluga PP, et al. Transcriptional analysis distinguishes breast implant-associated anaplastic large cell lymphoma from other peripheral T-cell lymphomas. Mod Pathol. 2019;32(2):216-230. doi: 10.1038/s41379-018-0130-7 [DOI] [PubMed] [Google Scholar]
- 79. Di Napoli A, Vacca D, Bertolazzi G, et al. RNA sequencing of primary cutaneous and breast-implant associated anaplastic large cell lymphomas reveals infrequent fusion transcripts and upregulation of PI3K/AKT signaling via neurotrophin pathway genes. Cancers (Basel). 2021;13(24):6174. doi: 10.3390/cancers13246174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Di Napoli A, Greco D, Scafetta G, et al. IL-10, IL-13, eotaxin and IL-10/IL-6 ratio distinguish breast implant-associated anaplastic large-cell lymphoma from all types of benign late seromas. Cancer Immunol Immunother. 2021;70(5):1379-1392. doi: 10.1007/s00262-020-02778-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kadin ME, Morgan J, Kouttab N, et al. Comparative analysis of cytokines of tumor cell lines, malignant and benign effusions around breast implants. Aesthet Surg J. 2020;40(6):630-637. doi: 10.1093/asj/sjz243 [DOI] [PubMed] [Google Scholar]
- 82. Santanelli di Pompeo F, Laporta R, Sorotos M, et al. Breast implant-associated anaplastic large cell lymphoma: proposal for a monitoring protocol. Plast Reconstr Surg. 2015;136(2):144e-151e. doi: 10.1097/PRS.0000000000001416 [DOI] [PubMed] [Google Scholar]
- 83. Di Napoli A, Pepe G, Giarnieri E, et al. Cytological diagnostic features of late breast implant seromas: from reactive to anaplastic large cell lymphoma. PLoS One. 2017;12(7):e0181097. doi: 10.1371/journal.pone.0181097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Di Napoli A. Achieving reliable diagnosis in late breast implant seromas: from reactive to anaplastic large cell lymphoma. Plast Reconstr Surg. 2019;143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):15S-22S. doi: 10.1097/PRS.0000000000005565 [DOI] [PubMed] [Google Scholar]
- 85. Jones JL, Hanby AM, Wells C, et al. Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL): an overview of presentation and pathogenesis and guidelines for pathological diagnosis and management. Histopathology. 2019;75(6):787-796. doi: 10.1111/his.13932 [DOI] [PubMed] [Google Scholar]
- 86. Jaffe ES, Ashar BS, Clemens MW, et al. Best practices guideline for the pathologic diagnosis of breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol. 2020;38(10):1102-1111. doi: 10.1200/JCO.19.02778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Turton P, El-Sharkawi D, Lyburn I, et al. UK guidelines on the diagnosis and treatment of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) on behalf of the Medicines and Healthcare products Regulatory Agency (MHRA) Plastic, Reconstructive and Aesthetic Surgery Expert Advisory Group (PRASEAG). J Plast Reconstr Aesthet Surg. 2021;74(1):13-29. doi: 10.1016/j.bjps.2020.10.064 [DOI] [PubMed] [Google Scholar]
- 88. Lyapichev KA, Piña-Oviedo S, Medeiros LJ, et al. A proposal for pathologic processing of breast implant capsules in patients with suspected breast implant anaplastic large cell lymphoma. Mod Pathol. 2020;33(3):367-379. doi: 10.1038/s41379-019-0337-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Clemens MW, Horwitz SM. NCCN consensus guidelines for the diagnosis and management of breast implant-associated anaplastic large cell lymphoma. Aesthet Surg J. 2017;37(3):285-289. doi: 10.1093/asj/sjw259 [DOI] [PubMed] [Google Scholar]
- 90. Di Napoli A, Firmani G, Sorotos M, et al. Successful treatment of a patient with breast implant-associated anaplastic large cell lymphoma with local residual disease: a case report. Ann Plast Surg. 2022;88(2):152-156. doi: 10.1097/SAP.0000000000003033 [DOI] [PubMed] [Google Scholar]
- 91. Collins MS, Miranda RN, Medeiros LJ, et al. Characteristics and treatment of advanced breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2019;143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):41S-50S. doi: 10.1097/PRS.0000000000005568 [DOI] [PubMed] [Google Scholar]
- 92. Clemens MW, Medeiros LJ, Butler CE, et al. Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol. 2016;34(2):160-168. doi: 10.1200/JCO.2015.63.3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Clemens MW, Nava MB, Rocco N, Miranda RN. Understanding rare adverse sequelae of breast implants: anaplastic large-cell lymphoma, late seromas, and double capsules. Gland Surg. 2017;6(2):169-184. doi: 10.21037/gs.2016.11.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Thibodeau R, Fan KL, Wehner PB. Stage IV breast implant-associated anaplastic large-cell lymphoma with complete pathologic response to neoadjuvant chemotherapy. Plast Reconstr Surg Glob Open. 2019;7(9):e2446. doi: 10.1097/GOX.0000000000002446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mehta-Shah N, Clemens MW, Horwitz SM. How I treat breast implant-associated anaplastic large cell lymphoma. Blood. 2018;132(18):1889-1898. doi: 10.1182/blood-2018-03-785972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bartlett NL, Chen R, Fanale MA, et al. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol. 2014;7:24. doi: 10.1186/1756-8722-7-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Richardson K, Alrifai T, Grant-Szymanski K, et al. Breast implant-associated anaplastic large-cell lymphoma and the role of brentuximab vedotin (SGN-35) therapy: a case report and review of the literature. Mol Clin Oncol. 2017;6(4):539-542. doi: 10.3892/mco.2017.1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Stack A, Ali N, Khan N. Breast implant-associated anaplastic large cell lymphoma: a review with emphasis on the role of brentuximab vedotin. J Cell Immunol. 2020;2(3):80-89. doi: 10.33696/immunology.2.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Park JO, Webb CE, Temple-Oberle CF. Navigating women’s BIA-ALCL information needs: group seminars may offer an opportunity to empower the patient-surgeon team. Plast Reconstr Surg Glob Open. 2020;8(9):e3142. doi: 10.1097/GOX.0000000000003142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Goodman CM, Cohen V, Thornby J, Netscher D. The life span of silicone gel breast implants and a comparison of mammography, ultrasonography, and magnetic resonance imaging in detecting implant rupture: a meta-analysis. Ann Plast Surg. 1998;41(6):577-585; discussion 585. doi: 10.1097/00000637-199812000-00001 [DOI] [PubMed] [Google Scholar]
- 101. Benadiba L, Pétoin DS, Berlie J, Rouëssé J, Girard M. Durée de vie des implants mammaires en reconstruction mammaire. A propos de 949 implants [Survivorship of breast implants used in breast reconstruction. 949 implants]. Ann Chir Plast Esthet. 2000;45(1):31-40. [PubMed] [Google Scholar]
- 102. Dowden RV. Detection of gel implant rupture: a clinical test. Plast Reconstr Surg. 1993;91(3):548-550. doi: 10.1097/00006534-199303000-00025 [DOI] [PubMed] [Google Scholar]
- 103. Hölmich LR, Fryzek JP, Kjøller K, et al. The diagnosis of silicone breast-implant rupture: clinical findings compared with findings at magnetic resonance imaging. Ann Plast Surg. 2005;54(6):583-589. doi: 10.1097/01.sap.0000164470.76432.4f [DOI] [PubMed] [Google Scholar]
- 104. Hillard C, Fowler JD, Barta R, Cunningham B. Silicone breast implant rupture: a review. Gland Surg. 2017;6(2):163-168. doi: 10.21037/gs.2016.09.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Patel BC, Wong CS, Wright T, et al. Breast implants. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Accessed April 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441998/. [Google Scholar]
- 106. Forster NA, Künzi W, Giovanoli P. The reoperation cascade after breast augmentation with implants: what the patient needs to know. J Plast Reconstr Aesthet Surg. 2013;66(3):313-322. doi: 10.1016/j.bjps.2012.09.033 [DOI] [PubMed] [Google Scholar]
- 107. Caplin DA, Calobrace MB, Wixtrom RN, Estes MM, Canady JW. MemoryGel breast implants: final safety and efficacy results after 10 years of follow-up. Plast Reconstr Surg. 2021;147(3):556-566. doi: 10.1097/PRS.0000000000007635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hammond DC, Canady JW, Love TR, Wixtrom RN, Caplin DA. Mentor contour profile gel implants: clinical outcomes at 10 years. Plast Reconstr Surg. 2017;140(6):1142-1150. doi: 10.1097/PRS.0000000000003846 [DOI] [PubMed] [Google Scholar]
- 109. Maxwell GP, Van Natta BW, Bengtson BP, Murphy DK. Ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study. Aesthet Surg J. 2015;35(2):145-155. doi: 10.1093/asj/sju084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Stevens WG, Calobrace MB, Alizadeh K, Zeidler KR, Harrington JL, d’Incelli RC. Ten-year core study data for Sientra’s Food And Drug Administration-approved round and shaped breast implants with cohesive silicone gel. Plast Reconstr Surg. 2018;141(4S Sientra Shaped and Round Cohesive Gel Implants):7S-19S. doi: 10.1097/PRS.0000000000004350 [DOI] [PubMed] [Google Scholar]
- 111. Danino MA, Dao L, Retchkiman M, Matetsa E, Iezzoni J, Bou-Merhi JS. Analysis of Allergan’s BIOCELL implant recall in a major university breast center. Plast Reconstr Surg Glob Open. 2020;8(6):e2906. doi: 10.1097/GOX.0000000000002906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vaysse C, Laurent C, Ysebaert L, Chantalat E, Chaput B. France: the first country to ban a type of breast implant linked to anaplastic large cell lymphoma. Aesthet Surg J. 2019;39(8):NP352-NP353. doi: 10.1093/asj/sjz142 [DOI] [PubMed] [Google Scholar]
- 113. Swanson E. Plastic surgeons defend textured breast implants at 2019 U.S. Food and Drug Administration hearing: why it is time to reconsider. Plast Reconstr Surg Glob Open. 2019;7(8):e2410. doi: 10.1097/GOX.0000000000002410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Swanson E, Hall-Findlay E. Banning textured implants is a rational decision to eliminate the risk of breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL). Aesthet Surg J. 2020;40(8):NP474-NP477. doi: 10.1093/asj/sjaa053 [DOI] [PubMed] [Google Scholar]
- 115. Swanson E. The textured breast implant crisis: a call for action. Ann Plast Surg. 2019;82(6):593-594. doi: 10.1097/SAP.0000000000001963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. US Food and Drugs Administration. 2019. Breast implants - certain labeling recommendations to improve patient communication - guidance for industry and Food and Drug Administration staff. Accessed March 6, 2022. https://www.fda.gov/media/131885/download.
- 117. Parham CS, Hanson SE, Butler CE, et al. Advising patients about breast implant associated anaplastic large cell lymphoma. Gland Surg. 2021;10(1):417-429. doi: 10.21037/gs.2020.03.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Matros E, Shamsunder MG, Rubenstein RN, et al. Textured and smooth implant use reported in the tracking operations and outcomes for plastic surgeons database: epidemiologic implications for BIA-ALCL. Plast Reconstr Surg Glob Open. 2021;9(3):e3499. doi: 10.1097/GOX.0000000000003499 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.