Abstract
Management of hospital wastewater is a challenging task, particularly during the situations like coronavirus 2019 (COVID-19) pandemic. The hospital effluent streams are likely to contain many known and unknown contaminants including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) along with a variety of pollutants arising from pharmaceuticals, life-style chemicals, drugs, radioactive species, and human excreta from the patients. The effluents are a mixed bag of contaminants with some of them capable of infecting through contact. Hence, it is essential to identify appropriate treatment strategies for hospital waste streams. In this work, various pollutants emerging in the context of COVID-19 are examined. A methodical review is conducted on the occurrence and disinfection methods of SARS-CoV-2 in wastewater. An emphasis is given to the necessity of addressing the challenges of handling hospital effluents dynamically involved during the pandemic scenario to ensure human and environmental safety. A comparative evaluation of disinfection strategies makes it evident that the non-contact methods like ultraviolet irradiation, hydrogen peroxide vapor, and preventive approaches such as the usage of antimicrobial surface coating offer promise in reducing the chance of disease transmission. These methods are also highly efficient in comparison with other strategies. Chemical disinfection strategies such as chlorination may lead to further disinfection byproducts, complicating the treatment processes. An overall analysis of various disinfection methods is presented here, including developing methods such as membrane technologies, highlighting the merits and demerits of each of these processes. Finally, the wastewater surveillance adopted during the COVID-19 outbreak is discussed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13762-023-04803-1.
Keywords: COVID-19, Disinfection, Hospital effluent, Wastewater, Wastewater surveillance
Introduction
The world has been facing phenomenal crisis due to the global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The novel coronavirus disease 2019 (COVID-19) was reported during December 2019 in Wuhan, China (Di Maria et al. 2020). Subsequently, public health emergency of international concern was announced by the World Health Organization (WHO). The spread of the virus continued at an unprecedented rate, where it crossed all geographical boundaries, spreading the infection to many countries across the world. Later as reported COVID-19 cases drastically increased and later in month of March 2020, COVID was declared by WHO as a global pandemic (Usman et al. 2020). The novel coronavirus has significantly affected the whole world causing a high number of infections and fatalities all over the world. In this crisis, the safety considerations of human beings are prior concern for the authorities at all stages.
The adequate availability of safe water is necessary to ensure the well-being of the public in this era of COVID-19. Cleanliness, sanitation and self-hygiene are key factors to keep the society safe from global pandemic. In this situation, the tracking of wastewater is considered as a potent strategy to fight against this virus (Street et al. 2020). Research investigations have revealed the occurrence of SARS-CoV-2 ribonucleic acid (RNA) in the samples collected from wastewater streams and other water bodies in various locations in countries like Australia, France, Netherlands, Finland, Germany, and USA (Mandal et al. 2020). With respect to COVID-19, immediate tracking, screening, testing, and isolation is the common strategy adopted in most of the countries (Street et al. 2020).
The major sources of toxic contaminants in the context of hospital wastewater are from diagnostics, laboratories and from research activities as well as through excreta of patients, pharmaceutical compounds, metabolites and other radioactive components in hospitals (Heller et al. 2020; Mandal et al. 2020; Wang et al. 2020a, b). The toxic effluents may cause several health issues like cancer, skin disorders, neurotoxicity, nausea, headache etc. (Sarizadeh et al. 2021). The regulatory bodies have also considered these pollutants as emerging organic pollutants, defined as non-regulated organic trace pollutants that are newly added or seen in the biosphere (Khan et al. 2020). The typical mean concentration of many of these pollutants in hospital wastewater is 4 to 150 times greater than domestic wastewater (Khan et al. 2020). Further, hospital wastewater may also contain many pathogens such as bacteria, viruses and fungi. The emission of hospital wastewater, without proper treatment technology, exposes the community to severe health risks. Therefore, it is evident that proper waste disposal units at hospitals are necessary to provide safe health and environment to public.
As an effect of COVID-19, a dramatic growth in the healthcare sectors is seen along with the research on new vaccines and drugs. It is highly evident that SARS-CoV-2 and emerging variants have the capability to persist in natural ecosystem. Literature also states that novel coronavirus and its genetic fragments are detected in different aqueous systems (Zhang et al. 2021). Hence, disinfection of these contaminated resources is critical. Hospital wastewater must be subjected to an appropriate treatment procedure before it is discharged and transported. Researchers have been investigating various modes of SARS-CoV-2 transmission and its survival against environmental factors (Achak et al. 2021). The fecal–oral route of SARS-CoV-2 transmission still remains a valid concern and necessitates safe hospital wastewater management for public health protection.
In this context, the current article aims to examine the reported data regarding the presence of SARS-CoV-2 and its genetic fragments in wastewater including hospital effluents and critically evaluate various disinfection strategies for the treatment of hospital wastewater. A systematic literature review was performed to achieve the objective. The literature was collected and analyzed following the guidelines of preferred reporting items for systematic reviews and meta-analyses (PRISMA) (https://prisma-statement.org/). Briefly, the following stage-wise methodology was pursued. In the first stage, articles were identified based on search strategies with an aim to examine the strategies adopted for hospital wastewater management during the pandemic. The search was performed using Google Scholar and Scopus databases using keywords such as hospital wastewater, hospital effluents, wastewater treatment, disinfection, COVID-19, SARS-CoV-2 and pandemic. These keywords helped to set the boundaries of the research areas. In the screening stage, titles and abstracts of the articles were checked to determine their relevance for inclusion in the review. Subsequently, the full-texts of the screened articles were read to ascertain the eligibility of articles in addressing the main objective of the study regarding treating and managing hospital wastewater. Finally, the articles to be included in the review were determined to be 111 and a critical review was performed based on the selected articles. The quality of the studies was selected in accordance with the PRISMA checklist. A summary of the procedure used for literature selection is presented in the PRISMA flow diagram (Figure S1).
Hospital wastewater characteristics
Hospitals generate pollutants resulting from various activities involving diagnostics and treatment of patients apart from medicinal excretion by patients (Wang et al. 2020a, b). The treatment of any type of waste originating from hospital, whether hazardous or non-hazardous. The healthcare-related waste materials such as discarded diagnostic samples, unused drugs, sterilants, swabs, etc. are collected separately and disposed.
The hospitals require large quantities of water to meet its daily needs. The consumption of water in a hospital is dependent on the size of the hospital, number of beds, number of inpatients, services provided, and their maintenance (Achak et al. 2021). The guidelines given by WHO suggest that almost 40–60 L/day of water is needed for each inpatient in hospitals for proper functioning of healthcare facilities. In case of specific requirements such as operation theaters and isolation wards, the water requirement varies from 100 to 400 L per patient per day depending on the intensity and nature of the disease and health of a person (Majumder et al. 2020). The high consumption of water in turn reflects in high wastewater production. Literature reports indicate that typically 400–1200 L of the wastewater streams are generated from hospitals per bed per day in developed countries and 200–400 L per capita per day in developing countries (Kumari et al. 2020).
The regulatory bodies usually classify wastewater into domestic wastewater, comprising of polluted water streams discharged from the households, residential areas; and industrial wastewater, denoting wastewater generated from the facilities in which business or production of good occurs (Verlicchi 2018). Different countries have their policies and regulations for the treatment of hospital wastewater streams (Kumari et al. 2020). The USA follows Effluent Guidelines and Standards in which specific treatment for hospital wastewater is recommended. In Italy, the waste generated from the hospitals with less than 50 beds is considered domestic wastewater and it can be discharged without prior pretreatment (Verlicchi 2018). In India, the characteristics of the effluent produced by the hospital are described in the waste management regulations (Ilyas et al. 2020) and treatment can be specific, direct disposal or cotreatment. It is observed that often the hospital wastewater streams are considered similar to urban wastewaters and mixed together in public sewer networks prior to treatment.
In addition to the above classification, pollutants existing in hospital wastewater are broadly categorized as micro-pollutants and macro-pollutants. Micro-pollutant is an anthropogenic chemical that exists in the water system above the natural level because of human actions and its concentration remains at trace levels. The main categories of micro-pollutants existent in hospital wastewater and their typical average concentrations include adsorbable organic compounds (1371 µg/L), iodized contrast media (1008 µg/L), analgesics (100 µg/L), gadolinium (32 µg/L), cytostatics (24 µg/L), antibiotics (11 µg/L), etc. While macro-pollutants include contaminants such as bacteria, viruses and contributors to physio-chemical aspects like chemical oxygen demand (COD), biological oxygen demand (BOD), suspended solids (SS), pH, etc. (Achak et al. 2021; Castillo Meza et al. 2020; Lee et al. 2014; Luo et al. 2014; Verlicchi et al. 2010). Both types of pollutants are of significant concern to humans and environment.
Table 1 represents the typical physio-chemical and microbiological characteristics for hospital wastewater that may vary depending on the operational conditions and working environment. The important parameters like COD, BOD, suspended solids, and pathogens are key in understanding hospital wastewater characteristics (Asfaw 2018). The toxicity of effluent stream described by the COD and BOD. Along with that, the E. coli load seen in municipal wastewater is typically higher than in hospital wastewater due to the higher dilution of wastewater in hospital (Carraro et al. 2016). The microbiological characteristics are generally reported in terms of population density of microorganisms expressed as most probable number per 100 ml (MPN/100 mL).
Table 1.
Typical parameters for the hospital wastewater (Achak et al. 2021; Amouei et al. 2015; Daouk et al. 2016; Nour-eddine and Lahcen 2014; Verlicchi 2018)
| Parameter | Unit | Concentration |
|---|---|---|
| Electrical conductivity | μS/cm | 300–2700 |
| pH | 6–9 | |
| Chlorides | mg/L | 80–400 |
| Redox potential | mV | 850–950 |
| Nitrite | mg/L | 0.1–0.6 |
| Nitrate | mg/L | 1–2 |
| Total suspended solids | mg/L | 116–32,600 |
| COD | mg/L | 39–7776 |
| Dissolved organic carbon | mg/L | 120–130 |
| Total organic carbon | mg/L | 31–180 |
| BOD | mg/L | 16–2575 |
| BOD/COD | 0.3–0.4 | |
| Total disinfectants | mg/L | 2–200 |
| Total surfactants | mg/L | 4–8 |
| E. coli | MPN/100 mL | 103–106 |
| Total coliforms | MPN/100 mL | 106–109 |
| Fecal coliforms | MPN/100 mL | 103–107 |
Coronavirus in wastewater
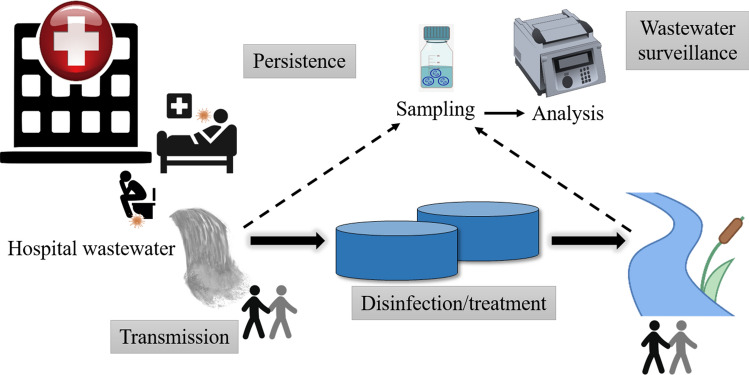
The occurrence of coronavirus has been observed in the water environment across the globe and its survival and infectivity under different conditions has been a matter of investigation. Figure 1 gives a schematic overview of various aspects that should be considered regarding SARS-CoV-2 in hospital wastewater. These include presence and persistence of virus in hospital wastewater, its potential routes of transmission (direct and indirect), methods for disinfection of wastewater and wastewater surveillance. The presence of SARS-CoV-2 and its genetic fragments in the aqueous medium has raised grave concerns with respect to human health (Mandal et al. 2020). The main transmission pathways of SARS-CoV-2 include person to person contact, inhalation of droplets and aerosols, excretory substances such as urine and feces (Daughton 2020; Foladori et al. 2020; Hart and Halden 2020). The possibility of viral transmission mediated by contact with contaminated wastewater cannot be discarded, particularly in the light of emerging newer and more infectious and resistant mutations of SARS-CoV-2 (Giacobbo et al. 2021). The presence of coronavirus has been reported in different forms in various research investigations conducted worldwide that makes it necessary to deploy suitable disinfection strategies (Choi et al. 2021). Further, the wastewater surveillance can play a significant role in limiting the community spread of disease.
Fig. 1.
Various aspects of consideration regarding SARS-CoV-2 in hospital wastewater including persistence of virus, potential routes of transmission, disinfection and wastewater surveillance
In a recent study, researchers observed the existence of SARS-CoV-2 in wastewaters in Australia. The samples were sourced from couple of separate locations namely a suburban pumping station and from a wastewater treatment plant (8 samples). Among these nine samples, two samples exhibited positive test results and pointed to the occurrence of SARS-CoV-2 in wastewater (Ahmed et al. 2020). The occurrence of SARS-CoV-2 RNA fragments in the sewage water in Italy was identified using nested reverse transcription-polymerase chain reaction (RT-PCR) and quantitative polymerase chain reaction (qPCR). The suitability of the protocol by the WHO in sewage wastewater treatment toward enveloped viruses after suitable modifications was highlighted (La Rosa et al. 2020). Another study emphasized the presence of SARS-CoV-2 RNA in untreated water collected from 3 effluent treatment units, and 3 river water samples collected from the Milano region of Italy (Rimoldi et al. 2020).
Another study was undertaken in February 2020 to investigate the presence of SARS-CoV-2 in samples procured from a hospital in Zheziang, China where confirmed infected individuals were hospitalized (Wang et al. 2020a, b). The samples were collected from solid surfaces in isolation wards, personal protective equipment of staffs and sewage. A preprocessing disinfection equipment was provided before sewage was drained from isolation wards into the final disinfection pool. Three sewage samples collected at the entry point of preprocessing disinfection pool showed positive outcome for SARS-CoV-2 RNA with cycle threshold values of 29.37, 30.58, and 32.42. After preprocessing step, the result of sewage sample was weakly positive with cycle threshold value of 33.55. The analysis of sample collected at the final outlet of the sewage disinfection pool tested negative, suggesting the efficacy of disinfection. Further, all of the 5 sewage samples collected at various locations were negative by viral culture of SARS-CoV-2. Another recent study conducted in Niterói, Rio de Janeiro, Brazil involved monitoring of wastewater samples collected over a period of 20 weeks between April 15 and August 25, 2020 (Prado et al. 2021). More than 84% samples showed positive results for SARS-CoV-2 RNA with about 42% samples showing positive results in the first week of study and 100% testing positive during the peak. However, none of the raw sewage samples collected from the two hospitals showed any positive results for detection of SARS-CoV-2 RNA. The observation could be possibly attributed to the usage of diapers in patients along with hospital sanitary measures.
In another investigation, the occurrence of SARS-CoV-2 was observed in an effluent treatment plant in Gujarat, India. The existence of open reading frame 1ab (ORF1ab), N gene and S genes of coronavirus were detected in aqueous streams using quantitative reverse transcription-polymerase chain reaction (RT-qPCR) methodology. The gradual increase in the genetic loading of virus is expected with an increment in the number of active species (Kumar et al. 2020). Researchers identified the virus in six water treatment units in the Iberian peninsula of Spain having relatively low COVID-19 pervasiveness. The RT-qPCR results showed that 35 out of 42 influent samples and 2 out of 18 samples that had undergone secondary treatment showed positive outcomes for coronavirus (Randazzo et al. 2020). Table 2 provides a glimpse of worldwide reports on the occurrence of coronavirus and its genetic fragments in wastewater. A suitable treatment technology is necessary to eliminate the presence of these viruses from water to establish a safe water distribution system.
Table 2.
Studies conducted to investigate the presence of coronavirus in wastewater
| Sample | Country | Detection method | References |
|---|---|---|---|
| Untreated wastewater | Australia | RT-qPCR | Ahmed et al. (2020) |
| Untreated wastewater | France | RT-qPCR | Wurtzer et al. (2020) |
| Untreated wastewater | Italy | RT-qPCR | La Rosa et al. (2020) |
| Untreated, biologically treated, and disinfected wastewater | India | RT-qPCR | Arora et al., (2020) |
| Sewage | Israel | RT-qPCR | Bar-Or et al. (2020) |
| Influent wastewater | Japan | RT-qPCR | Hata et al. (2021) |
| Primary and secondary wastewater and sludge | Spain | RT-qPCR | Balboa et al. (2021) |
| Activated sludge | Turkey | RT-qPCR | Kocamemi et al. (2020) |
| Human wastewater | Netherlands | RT-qPCR | Lodder and de Roda Husman (2020) |
| Untreated wastewater | USA | RT-qPCR | Sherchan et al. (2020) |
| Primary sludge | RT-qPCR | Peccia et al. (2020) | |
| Untreated hospital wastewater | China | RT-qPCR | Zhang et al. (2021) |
| Untreated hospital wastewater | Slovenia | RT-qPCR | Gonçalves et al. (2021) |
The survival of coronavirus in wastewater constitutes a crucial aspect of research investigations. The survival of virus in aquatic environments depends on a number of factors including temperature, characteristics of water, concentration of suspended solids and organic matter and alkalinity levels and requires a comprehensive study with respect to SARS-CoV-2 (Tran et al. 2021). A recent study investigated the persistent of infectious SARS-CoV-2 and SARS-CoV-2 RNA in water and wastewater.
Nevertheless, based on prior knowledge about the corona virus and improved understanding of SARS-CoV-2, wastewater-based transmission of COVID-19 has been given a milder concern so far. However, the rapid emergence of SARS-CoV-2 variants with enhanced transmissibility and varying levels of virulence emphasizes the need of a more systematic investigation on the persistence of these variants and preservation of their viral activity in aquatic environments, especially wastewater. Analysis of wastewater for virus variants is far more complicated than analysis of clinically collected samples. Since wastewater is not patient specific, rather it may contain mixture of mutants from multiple subjects, the assessment should be able to discriminate between various mutations and their apt combinations to identify specific variants. The field is relatively new and making right and justified inferences about viral lineages dominating in the population is essential. In a recent study, 122 wastewater samples from three sites in Switzerland were subjected to genomic sequencing for analysis of B.1.1.7, B.1.351, and P.1 SARS-CoV-2 variants. The studies revealed local outbreak of B.1.1.7 in two cities based on wastewater analysis upto 8 days before it was detected in clinical samples (Jahn et al. 2021). A full genome sequencing is, however, time consuming, expensive and needs sophisticated tools. The development of novel approaches such as nested RT-PCR assays that target key mutations of the spike protein in virus can aid in initial rapid screening for variants of concern in wastewater samples (La Rosa et al. 2021).
COVID-19 and changing health ecosystem
COVID-19 pandemic situation has driven discernible changes in the healthcare sector, ranging from changes in pattern of administering drugs to the use of non-therapeutic products. A significant increment in the usage of various personal hygiene products has been observed across the globe during the pandemic (Abtahi-Naeini 2020; Bs and Wambier 2020). The market reports indicated an enormous annual growth in sales of hand sanitizers by 470% in March 2020 when compared to the earlier year (Berardi et al. 2020). The proper self-hygiene is very crucial against the fight of the COVID-19 pandemic as it easily spreads through respiratory droplets. A few mechanisms for plausible action of soaps against coronaviruses have been proposed. Studies have observed that the soap has the capacity to dissolve the lipid bilayer that envelopes the virus. Once the protective lipid membrane is solubilized, the virus breaks down into fragments and is inactivated. Later the fragmented components are dissolved by surfactant molecules and washed away (Usman et al. 2020). Another possible mechanism involves entrapment of virus by surfactant micelles (Chirani et al. 2021). Although antimicrobial activity of soaps hardly has any effect on viruses, their surfactant action along with thorough hand scrubbing and water rinsing can remove coronaviruses from hands. The high residual contents of soaps and detergents with persistent chemicals, though, add a concern from environmental perspective.
Hand sanitizers constitute another category of chemical compounds essentially utilized in preventing the transmission of infectious diseases among public and health workers. The hand-antiseptic and alcohol-based hand rub are the most regularly used forms of sanitizers after the upsurge of COVID-19 infected cases (Berardi et al. 2020). Sometimes, they may cause side effects like hand dermatitis or eczema, skin irritations, hormone disorders, may weaken human immune functions etc. Studies have also reported that excessive usages of hand sanitizers may lead to antimicrobial resistance (Mitsuboshi and Tsugita 2018). Thus, it should be addressed with utmost care to minimize environmental hazards and health concerns.
A comprehensive study conducted by researchers during the first wave of pandemic examined the patterns of various pharmaceuticals in wastewater, that reflected their consumption during the pandemic (Galani et al. 2021). The analysis of wastewater samples collected from wastewater treatment plant of Athens, Greece revealed increase in the consumption of hydroxychloroquine by 387%, azithromycin by 36.3%, and paracetamol by 198%. Overall, antiviral and antibiotic drugs registered an increase in the consumption by 170% and 57%, respectively. It was also noticed that for many drugs, the consumption increased substantially even in the absence of data supporting their efficacy in treating COVID-19 infection. Such instances were primarily driven by public perception.
Therapeutics like antibiotics are supposed to play an important role in the treatment of confirmed bacterial co-infections during the COVID-19 scenario (Usman et al. 2020). Different antimicrobials like chloroquine, hydroxychloroquine, remdesivir, sarilumab, tocilizumab, lopinavir/ritonavir and ribavirin have been explored by medical researchers for COVID-19 infection (Sanders et al. 2020; Vellingiri et al. 2020). The guidelines issued by Center for Disease Control and Prevention for the clinical treatment of COVID-19 specifically stated that no treatment method was yet available for the handling of the COVID-19 (Sanders et al. 2020). In early March 2020, US Food and Drugs Administration (FDA) allowed Emergency Use Authorizations (EUA) for chloroquine, hydroxychloroquine, and remdesivir. Later in June 2020, FDA withdrew the EUA as these therapeutics like hydroxychloroquine were largely ineffective against the COVID-19 and there were possible health risks linked with the drug (Zhang et al. 2020a, b).
Here, it must be noted that an excessive usage of antimicrobial agents may lead to the development of antimicrobial resistance and pose new problems. The discharge of effluents containing these agents contributes to induction of antimicrobial resistance in microorganisms, thereby resulting in the multiplication of the growth rate of the undesirable microorganisms. Literature reports have indicated the presence of various antibiotic components in the aquatic streams (Usman et al. 2020). Therefore, this issue needs to be addressed very seriously to minimize the additional risks associated with health and environment. Nevertheless, an increased usage of medicines has opened a pathway for various harmful persistent components to enter the ecological cycle and cause environmental contamination.
Wastewater disinfection strategies
Disinfection plays a crucial role in hospital wastewater treatment. It is defined as a process of eliminating all the pathogens including viruses, bacteria or any other microorganisms (Dandie et al. 2019). Different methods like ozone, ultraviolet (UV) irradiation, chlorination are commonly used methods for disinfection of hospital wastewater (Dandie et al. 2019; Huo et al. 2020). Various factors such as the amount of discharge stream, safety and hazard considerations, stock and availability of disinfectants, potential investment, economic and potential feasibility of the process play a role in selection of a disinfection approach (Wang et al. 2020a, b).
Thermal disinfection
Thermal disinfection, used even as a benign disinfection method in household, is possibly one of the oldest disinfection treatment methods. The process works on the principle of pathogen inactivation using heat, e.g., the usage of autoclaves for sterilization purposes. The pathogen inactivation is based on three mechanisms which include (1) loss of functionality of the cell due to the denaturing of enzymes, (2) cell wall damage due to the damage of the structure of proteins and fatty acids, and (3) rupturing of the cell walls and leaking of the cell components by expansion of fluids within the cell walls.
Chemical disinfection
Chlorination is a disinfection method through the addition of chlorine or chlorine compounds in minor quantities for the destruction of microorganisms (Pichel et al. 2019). Bleaching powder, sodium hypochlorite, sulfuryl chloride and liquid chlorine are commonly used chlorination agents (Wang et al. 2020a, b). This process involves the addition of chlorine or chlorine byproducts to wastewater, which leads to the formation of hypochlorous acid and hypochlorite ion known to be free chlorine which is responsible for the destruction of the virus (Pichel et al. 2019). Generally, the chlorine-based disinfectants possess extremely high oxidizing capacity, which results in the elimination of microbes (Collivignarelli et al. 2018). The guidelines given by Chinese authorities for hospital wastewater containing SARS-CoV-2 prescribe a minimum free chlorine dosage of 6.5 mg/L for 1.5 h (Achak et al. 2021).
An interesting observation was reported by researchers investigating the presence of SARS-CoV-2 viral RNA in septic tanks of Wuchang Fangcang Hospital (Zhang et al. 2020a, b). In spite of the recommended dosage of 800 g/m3 of sodium hypochlorite, a considerable high level of viral load of approximately (0.5–18.7) × 103 copies/L was found in the effluents. The complete inactivation was observed at higher dosage of 6700 g/m3 of sodium hypochlorite, although it resulted in high concentration of disinfection byproducts, at least 15 times higher than ordinary hospital wastewater. The incomplete inactivation at lower dosage levels could be attributed to the fact that SARS-CoV-2 embedded in stool particles escaped disinfection and continued prolonged release into the aqueous environment.
Ozone, prepared by on-site passage of dry oxygen or air over the high voltage electrodes, is also employed as a water disinfectant in addition to chlorine (Wang et al. 2020a, b). It is reported that ozone is relatively more powerful disinfection agent than chlorine and chlorine dioxide (Pichel et al. 2019). However, the excessive usage of ozone leads to the development of bad odor and secondary pollution (Wang et al. 2020a, b). Therefore, this technique is generally adopted for the small-scale wastewater treatment plants.
Peracetic acid is also considered as the alternative toward classical disinfectants. The peracetic acid is synthesized as the product of reaction between hydrogen peroxide and acetic acid. The disinfecting capability of peracetic acid is ascribed to the release of intensely reactive OH radicals, which targets bacterial cells (Collivignarelli et al. 2018). This also results in rupturing of cellular wall and membrane belonging to the pathogen. While many disinfectants have been shown to be effective against SARS-CoV (Rabenau et al. 2005), a systematic study comparing efficacy of various disinfectants against SARS-CoV-2 and its variants in hospital wastewater has not been reported.
Non-contact methodologies
Ultraviolet irradiation
Ultraviolet (UV) irradiations correspond to wavelengths shorter than visible light and longer than X-rays in the electromagnetic spectrum. The UV spectrum is categorized into four wavebands: UV-A (range between 315 and 400 nm), UV-B (range between 280 and 315 nm), UV-C (range between 200 and 280 nm), and vacuum UV (range between 100 and 200 nm) (Dale Wilson et al. 2012; Yap et al. 2019). The UV band within a wavelength of 200–300 nm is completely responsible for the destruction of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) of microorganisms. So, the UV-B and UV-C corresponding to the wavelength of 200–300 nm is the suitable light source having the microbicidal effect (Wang et al. 2020a, b).
UV disinfection is usually performed by utilizing different light sources such as mercury-based sources, pulsed-xenon bulb sources, etc. for producing ultraviolet radiation (Hadi et al. 2020). Recently, a study demonstrated the utilization of Xe-based light source for the inactivation of viruses on both the hard surfaces and in N95 respirators within 300 s of irradiation (Simmons et al. 2020). A recent report claimed that the UV-C light displayed almost 100% deactivation of βHCoV-OC43 strain within 25 min at 207–222 nm (Buonanno et al. 2020). Another study investigated the impact of ultraviolet germicidal irradiation (UVGI) doses in range of 120–950 J/cm2 on the performance and integrity of N95 filtering facepiece respirators. The findings revealed that the irradiation exposure resulted in a minor increment in the particulate penetration within 1.25% with minimum flow hindrance (Lindsley et al. 2015).
However, problems related to traditional mercury lamp (health and safety considerations) can be overcome by using ultraviolet light-emitting diodes (UV-LEDs) (Pichel et al. 2019). The commonly used materials in this regard are aluminum gallium nitride, gallium nitride and aluminum nitride corresponding to wavelengths of 210–365 nm. The appropriate wavelength corresponding to deep UV or near UV region is an important parameter influencing the disinfection efficacy (Song et al. 2016). The light-emitting diode (LED) lamp is found to be environmentally friendly, compact and durable with less energy consumption and long life (Würtele et al. 2011). These specialties make UV-LED lamp more attractive for disinfection applications. The effectiveness of the ultraviolet irradiation-based disinfection for various types of microorganisms is emphasized in Table 3. Log inactivation is a mathematical term used to express the relative number of microorganisms inactivated by means of disinfection.
Table 3.
Action of ultraviolet (UV) irradiation against microorganisms
In a study conducted during the period April 04-May 02, 2020 in Iranian cities Tehran, Qom and Anzali, 28 raw and treated wastewater samples were collected from three wastewater treatment plants and analyzed for the presence of SARS-CoV-2 RNA (Nasseri et al. 2021). All the inlet samples showed positive results for the presence of SARS-CoV-2 RNA. A comparative was made between chlorination and disinfection methods used at all three sites. The results from two sites in Qom and Anzali showed absence of SARS-CoV-2 RNA irrespective of the disinfection method used. However, 2 out of 4 samples collected from the outlet of chlorine-based disinfection modules showed positive results for the presence of SARS-CoV-2 RNA whereas none of the samples from UV disinfection modules showed any traces of SARS-CoV-2 RNA. The data pointed out that UV disinfection was more effective than chlorination.
Hydrogen peroxide vapor
Hydrogen peroxide vapor (HPV) is vaporized form of hydrogen peroxide employed for the inactivation of viruses (Boyce 2016; Choi et al. 2021). Researchers also claimed that hydrogen peroxide has a higher oxidizing capability compared to chlorine dioxide and chlorine and their working principle relies upon the presence of free oxygen radicals that causes disruption of the microbial cell (Totaro et al. 2020).
Various literature reports suggest that HPV can be used as a disinfection agent against Clostridium difficile, vancomycin-resistant Enterococcus, spore-forming bacteria, gram positive and gram negative bacteria, transmittable gastroenteritis coronavirus of pigs, avian influenza virus and swine influenza virus (Blazejewski et al. 2015; Holmdahl et al. 2019; Saini et al. 2020). In a recent study, researchers demonstrated development of a very efficient and cost-effective vaporized H2O2-dependent approach for personal protective equipment (PPE) decontamination during the period of epidemic. The results also indicated that the HPV was able to disinfect PPEs and PPE room of 10 × 10 × 12 feet within 10 min (Saini et al. 2020). The usage of HPV as a room disinfectant for the elimination of norovirus was also examined. The studies revealed that the average impact of human norovirus qPCRs was 0.40 log10 (Holmdahl et al. 2019).
On comparison of UV light and H2O2-based systems, a few distinguishing aspects have been noticed (Weber et al. 2020). H2O2-based systems are less sensitive to parameters like dosage, room configuration than UV-based counterparts. The set up for disinfection system based on UV irradiation needs sophisticated equipment to ensure effective decontamination. Also, H2O2-based systems are capable to attain higher levels of sporicidal kill.
Membrane-based technologies
Pressure driven membrane processes have average pore-sizes lower than the physical sizes of virus and bacteria. Ultrafiltration which has higher average pore-size compared to the microorganisms and are well suited for disinfection for point of use applications. These units operate at lower pressures and can be backwashed to minimize effects of membrane fouling. Membrane technology illustrated on the basis of particle size enables retention of macromolecules, colloidal and suspended matters along with bacteria, pathogens, and virus. This makes it more suitable for water disinfection technologies. The main advantages of membrane-based system over conventional systems are: minimization of the requirement of disinfectants, compactness, ease of operation and maintenance, less production of sludge and high efficiency. The chief features of membrane-based technologies applicable for disinfection such as microfiltration and ultrafiltration are described in Table 4.
Table 4.
Features of membrane-based methods useful for disinfection
| Method | Microfiltration | Ultrafiltration |
|---|---|---|
| Porosity (µm) | 0.1–10 | 0.01–0.1 |
| Pressure (bar) | 1–5 | 1–7 |
| Target removal | Suspended solids, macromolecules and bacteria | Macromolecules, viruses, humic acids |
Hybrid strategies
Adopting multiple disinfection barriers has also received a major research attention with the objectives to ensure and improve the efficiency of disinfection process to a greater extent. The efficacy of combining different disinfection strategies must be examined for the complete elimination of SARS-CoV-2 from the wastewater.
Another investigative finding explained the combined effect of ozone and ultraviolet irradiation for the decontamination of Bacillus subtilis spores. The study highlighted the role of hydroxyl radicals and ozone as completely responsible for higher deactivation efficiency (Jung et al. 2008). The usage of solar and UV/TiO2 photocatalytic ozonation process was employed for the deactivation of pathogens like E. coli, Salmonella species, Shigella species and Vibrio cholera in artificially prepared and real municipal water samples. The hybrid disinfection system exhibited a 50–75% higher disinfection than the individual unit processes. Synergy indices of up to 1.86 were observed during photocatalytic ozonation. The integrated approach also overcame the limitations of the individual processes by suppressing the bacterial regrowth resulting from irreversible damage to the microbial cells (Mecha et al. 2017). In another study, researchers developed a combined pilot scale unit including anaerobic reactor, hybrid constructed wetlands and ozonation for the treatment and reusage of municipal wastewater. The hybrid wetlands comprised of floating treatment wetland anaerobic-anoxic baffled constructed wetland and a saturated vertical flow. The experimental studies exhibited a removal of 99.1% turbidity, 91% of nitrogen, 78.9% of organic matter. The system demonstrated a very simple construction with a longer lifespan (Colares et al. 2019).
The wastewater treatment plants employ a series of steps for decontamination of pollutants (Ahmed et al. 2021). The effectiveness of wastewater treatment plants in removing SARS-CoV-2 viral load was examined in the United Arab Emirates (UAE) in May and June 2020 (Hasan et al. 2021). The effluent treatment plants employed preliminary, primary, and secondary steps followed by tertiary treatment including sand filtration and chlorination. The viral load in incoming streams of treatment plants was determined using RT-qPCR to be in the range of 7.50 × 102 to 3.40 × 104 gene copies/L. The outgoing streams from none of the 11 treatment plants showed any detectable presence of covid-19, suggesting the efficacy of treatment plants.
The literature survey suggests that the biological treatment approaches have been relatively underexplored so far in the context of removal of SARS-CoV-2 from wastewater (Bhatt et al. 2020). The processes involving the use of algae have shown promising results in wastewater treatment for removal of heavy metals, organic contaminants and pathogens (Chai et al. 2021; Cheng et al. 2019; Rambabu et al. 2020). Thus, systematic research must be undertaken to explore the potential of algae-mediated approach as an alternative to energy-intensive disinfection technologies. The principal merits and limitations of different disinfection strategies are reported in Table 5. Future studies must aim at developing approaches that can tap the advantages of individual methods to deliver sustainable technologies for decontamination (Show et al. 2021).
Table 5.
Advantages and disadvantages of various disinfection methods
| Method | Advantages | Disadvantages |
|---|---|---|
| Chlorination |
Efficient against bacteria and virus Better protection against recontamination |
Ineffective against spores and cysts Issues concerned with taste and odor Possibilities for disinfection byproducts (DBPs) formation |
| Chloramination |
Minimum problems associated with taste and odor Highly effective against biofilms |
Poor disinfection capability Requirement of trained personnel |
| Chlorine dioxide |
High efficiency and low costs Independent of pH |
Problems with storage and transport Expensive Issues concerned with taste and odor |
| Ozonation | Highly efficient against virus, bacterial |
Requirement of proper maintenance Expensive Requirement of high energy input Formation of hazardous byproducts |
| UV irradiation |
High efficiency against viruses, spores, cysts No byproducts formation Minimum chemical requirements |
Cost associated with replacement and maintenance of lamp |
| UV-LEDs |
Better life than UV lamps Environmentally friendly Minimum energy consumption No byproducts formation |
High cost High energy demand |
| Boiling | Ease of operation |
Environmental issues Requirement of large quantities of fuel |
| Solar disinfection |
Simple and inexpensive No electricity requirements No byproducts formation Minimum chemical requirements |
Long time requirements Dependent on solar light intensity Necessity of pretreatment |
Preventive strategies
Several modes of transmission of SARS-CoV-2 are likely involved in the spread of infection. Some of the emerging variants like SARS-CoV-2 B.1.1.529 (Omicron) have exhibited high transmissibility. Even though the major modes of viral transmission are considered to be personal close contact and through aerosol respiratory droplets, the possibility of indirect transmission of coronavirus cannot be ruled out (Marquès and Domingo 2021). The contamination of inanimate surfaces form a prominent source of indirect transmission and poses a critical challenge. Antimicrobial surfaces have attained significant research interest in the past decade to counter microbial contamination. The usage of different materials and coatings with antimicrobial characteristics is considered as one of the best strategies to control the transmission of viruses in hospital construction, storage, covering and piping materials (Goel et al. 2020). Researchers have reported application of various materials like copper, silver nanoparticles and chitosan-based materials as antimicrobial coatings (Choi et al. 2021).
The action of an antimicrobial coating or an antimicrobial surface occurs in three modes, namely: antifouling mechanism, release-kill mechanism, and contact-kill mechanism as shown in Fig. 2. The antifouling action repels different microorganisms and restricts their adhesion to the coating surface. Further, the microorganisms get terminated in the near-surface environment through the release of antimicrobial agents. In contact-killing action, microorganisms are adhered and killed on the antimicrobial surface (Goel et al. 2020).
Fig. 2.
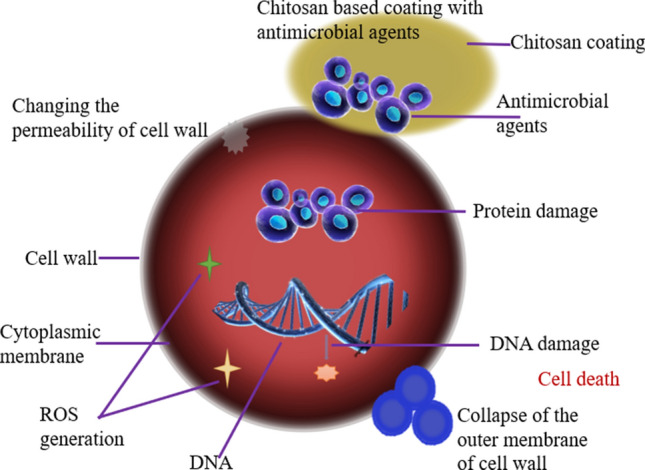
Mechanism of antimicrobial coating toward disinfection
Different researchers described the need for a long-life antimicrobial surface to restrict the transport of different agents through different surfaces. Several researchers proved the capability of a Cu-based surface against bacterial disinfection (Salgado et al. 2013). A study highlighted immediate action of copper and brass alloys in the deactivation of coronaviruses. It was also noticed that the disinfection efficiency increased with a higher composition of brass or copper in the material (Warnes et al. 2015). Another study reported that the microbes like E. coli, S. aureus and P. aeruginosa were eliminated within 15 min upon the irradiation of wire-arc sprayed with a copper coating (Kocaman and Keles 2019). Studies have also been reported regarding the utilization of cuprous oxide and polyurethane coating adhered on glass and stainless steel. These coatings exhibited very high efficacy and inactivated 99.9% of coronaviruses (Behzadinasab et al. 2020).
The unique features exhibited by metallic nanoparticles like large surface area to volume ratio, localized surface plasmon resonance, and enhanced Raman scattering can be employed for the inactivation of the virus. Different materials like FeO nanoparticles, silver nanoparticles, gold nanoparticles and TiO2 nanoparticles were found to be promising for these applications (Choi et al. 2021; Goel et al. 2020). The deactivation of influenza A and feline calicivirus using the silver nanoparticles immobilized on textile material was also proposed in another study. Researchers emphasized that the interaction of viral envelope is a key mechanism for this behavior (Huy et al. 2017). Table 6 summarizes various metal nanoparticles employed for deactivation of different viruses.
Table 6.
Various metal nanoparticles employed for disinfection
| Nanoparticle | Virus | References |
|---|---|---|
| Ag | Coxsackie virus B3 Nancy strain | Salem et al. (2012) |
| Ag | Poliovirus type-1 | Huy et al. (2017) |
| Ag | Feline calicivirus | Pangestika and Ernawati (2017) |
| TiO2-DNA nanocomposites | H1N1, H5N1 and H3N2 | Levina et al. (2016) |
| Si | Hepatitis B | Skrastina et al. (2014) |
| Ag, CuO | SARS-CoV-2 | Merkl et al. (2021) |
The interaction of water/wastewater with solid surfaces takes place during storage, physical treatment (such as filtration) and transportation. Typically, by design, the solid surfaces do not affect the quality of water during these operations. However, the characteristics of aqueous medium and surface properties of solid can alter the scenario. Some of the microbes present in wastewater can attack the surface and deteriorate it, which can subsequently impact water quality and cause cross contamination, etc. The specifically tailored and modified surfaces can counter this by facilitating disruption of vital physiological processes and biological activities of contaminating microbes. Hence, the growth and reproduction of microbes on the modified surfaces can be prevented. The approach deserves further systematic investigation, especially with respect to coronaviruses. The technologies must be developed that can actually destroy the harmful viruses rather than merely removing them (Goel et al. 2020). This could possibly be achieved by covering the membranes used in filters with antiviral coatings, for instance (Alayande et al. 2021; Sinclair et al. 2019). Such technologies have potential to check the viral transmission and reduce the spread of disease.
Wastewater surveillance
The continuous monitoring of coronavirus in effluent stream is useful for decision-makers to analyze and prepare different strategies to overcome the risks associated with COVID-19 (Sharma et al. 2021). Appropriate preventive and control measures for checking the spread of the pandemic can be taken by timely monitoring and tracking the virus. Therefore, the wastewater epidemiology is very essential in estimating the exposure of a community to coronavirus. Studies have emphasized that various factors and conditions should be taken into account to optimize methodology of the entire surveillance procedure comprising of sampling, concentration, extraction of RNA, detection and analysis (Michael-Kordatou et al. 2020). The key steps involved are briefly mentioned here. Sampling is referred to the collection of wastewater from selected points. The sampling is usually performed using grab sampling or composite sampling. Grab sampling is the individual sample procured without the addition of any other samples. On the whole, composite samples are mixtures of individual samples collected over a fixed period. The sampling is affected by the sampling time and storage temperature. Similarly, the presence of organic matter, suspended solids, dissolved solids, pretreatment temperature and virus concentration also interfere with the analysis. In the next step, the samples of a particular volume are concentrated by concentration method and the viral recovery yield is calculated.
The extraction of RNA without any destruction toward molecular structure is relatively tough in real environmental samples due to complex nature of wastewater matrices. The RNA is extracted using an extraction kit and thereby final concentration of RNA is quantified. The subsequent step involves the use of quantitative reverse transcription-polymerase chain reaction analysis (Sharma et al. 2021). The appropriate evaluation must be made regarding use of single or dual step RT-qPCR, type of qPCR equipment, thermal cycling parameters, limit of detection, limit of quantitation, etc. (Michael-Kordatou et al. 2020). Finally, the concentration of SARS-CoV-2 per volume of sample or SARS-CoV-2 per ng of RNA is determined, and the overall efficiency of the process is evaluated. A systematic assessment at each step is very essential in maintaining quality control and accuracy.
Raw wastewater sampling and monitoring corresponding to a particular region serves as an indicator for community health for the region. However, a few recent studies have suggested that hospital wastewater monitoring can provide additional critical insights that can aid in public health protection (Achak et al. 2021; Gonçalves et al. 2021). In a multicenter study performed on monitoring hospital wastewater, while it was expected that the presence of SARS-CoV-2 in wastewater would increase with increase in admission of more infected patients, the observations provided further insights (Acosta et al. 2021). The wastewater was analyzed for SARS-CoV-2 gene-targets N1, N2 and E, among which N1 exhibited the best sensitivity. The viral burden data showed distinct spikes when hospital-acquired infections happened. A positive correlation was observed between wastewater N1 signal and nosocomial cases (Pearson’s r = 0.389, p value < 0.001). The outbreak was also detectable using hospital wastewater sample analysis that showed significant differences in median SARS-CoV-2 N1-RNA for outbreak vs outbreak-free periods (112 genomic copies/ml vs 0 genomic copies/ml; p value < 0.0001). Further, the detection in hospital wastewater corresponded to infections immediately before or at the onset of symptoms, suggesting the usefulness of hospital wastewater surveillance. Such monitoring would help in better and timely understanding of nosocomial infections and outbreaks that affect patients and healthcare workers.
Conclusion
SARS-CoV-2 pandemic has created numerous potential challenges in various sectors on a global scale. Several problems have been encountered from environmental perspective due to the improper management of different hospital effluents. The research investigations have revealed the existence of SARS-CoV-2 and its genetic fragments in different wastewater sources, including hospital wastewater. Although the persistence of SARS-CoV-2 in hospital wastewater is presumed to be low, the possibility of infection of humans from liquid effluents through the fecal–oral transmission route cannot be ignored. Considering the variety of environmental factors governing the survival of coronavirus and emergence of new variants, more comprehensive studies are needed to ascertain the potential for fecal–oral transmission of SARS-CoV-2. The concern also necessitates deployment of suitable strategies for disinfection of hospital wastewater before discharging. A variety of disinfection strategies are available for dealing with presence of coronavirus in wastewater. The selection of a suitable approach or combination of approaches is likely to be governed by process effectiveness as well as site-specific factors. A techno-economic analysis should be performed with health as primary concern for a comprehensive evaluation of various technological approaches.
The studies with actual wastewater containing SARS-CoV-2, especially hospital wastewater have been limited and caution must be taken while extrapolating prior knowledge of disinfection with other similar contaminants to COVID-19 and its emerging variants. The continuously evolving nature of SARS-CoV-2 poses serious challenges in the formulation of pandemic management strategies from societal health and environmental perspectives. The mutations in virus that allow it to escape immune response in human beings even after vaccination has already raised global concern among medical experts. In the same way, the efficacy of disinfection strategies in changing scenarios must be evaluated. The survival of various strains of SARS-CoV-2 in wastewater streams must be comprehensively explored in the absence and presence of disinfection protocols. The persistence of SARS-CoV-2 on inanimate surfaces in hospital effluent handling facilities should be investigated considering the ability of virus to last on various surfaces on the timescales of hours to days. The scientific and industrial opportunities also exist to explore and develop portable and online water quality assessment tools that can be deployed for in situ sampling and monitoring. The implementation of hybrid strategies along with wastewater surveillance can prove to be effective in timely monitoring and elimination of coronavirus in wastewater streams.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the support from their institutes for access to resources.
Declarations
Conflict of interest
The authors declare no conflicts of interest for this research article. This research work is not funded by any funding agencies.
References
- Abtahi-Naeini B. Frequent handwashing amidst the COVID-19 outbreak: prevention of hand irritant contact dermatitis and other considerations. Heal Sci Rep. 2020 doi: 10.1002/hsr2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achak M, Alaoui Bakri S, Chhiti Y, M’hamdi Alaoui FE, Barka N, Boumya W. SARS-CoV-2 in hospital wastewater during outbreak of COVID-19: a review on detection, survival and disinfection technologies. Sci Total Environ. 2021;761:143192. doi: 10.1016/j.scitotenv.2020.143192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta N, Bautista MA, Hollman J, McCalder J, Beaudet AB, Man L, Waddell BJ, Chen J, Li C, Kuzma D, Bhatnagar S, Leal J, Meddings J, Hu J, Cabaj JL, Ruecker NJ, Naugler C, Pillai DR, Achari G, Ryan MC, Conly JM, Frankowski K, Hubert CR, Parkins MD. A multicenter study investigating SARS-CoV-2 in tertiary-care hospital wastewater viral burden correlates with increasing hospitalized cases as well as hospital-associated transmissions and outbreaks. Water Res. 2021;201:117369. doi: 10.1016/j.watres.2021.117369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W, Angel N, Edson J, Bibby K, Bivins A, O’Brien JW, Choi PM, Kitajima M, Simpson SL, Li J, Tscharke B, Verhagen R, Smith WJM, Zaugg J, Dierens L, Hugenholtz P, Thomas KV, Mueller JF. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SF, Mofijur M, Nuzhat S, Chowdhury AT, Rafa N, Uddin MA, Inayat A, Mahlia TMI, Ong HC, Chia WY, Show PL. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J Hazard Mater. 2021;416:125912. doi: 10.1016/J.JHAZMAT.2021.125912. [DOI] [PubMed] [Google Scholar]
- Alayande AB, Kang Y, Jang J, Jee H, Lee YG, Kim IS, Yang E. Antiviral nanomaterials for designing mixed matrix membranes. Membranes (basel) 2021 doi: 10.3390/membranes11070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amouei A, Asgharnia H, Fallah H, Faraji H, Barari R, Naghipour D. Characteristics of effluent wastewater in hospitals of Babol University of Medical Sciences, Babol, Iran. Heal Scope. 2015 doi: 10.17795/jhealthscope-23222. [DOI] [Google Scholar]
- Arora S, Nag A, Rajpal A, Tiwari SB, Sethi J, Sutaria D, Rajvanshi J, Saxena S, Srivastava S, Kazmi AA, Tyagi VK. Detection of SARS-CoV-2 RNA in fourteen wastewater treatment systems in Uttarakhand and Rajasthan States of North India. medRxiv. 2020 doi: 10.1101/2020.09.18.20197178. [DOI] [Google Scholar]
- Asfaw T. Review on hospital wastewater as a source of emerging drug resistance pathogens. J Res Environ Sci Toxicol. 2018;7:47–52. doi: 10.14303/jrest.2018.020. [DOI] [Google Scholar]
- Balboa S, Mauricio-Iglesias M, Rodriguez S, Martínez-Lamas L, Vasallo FJ, Regueiro B, Lema JM. The fate of SARS-COV-2 in WWTPS points out the sludge line as a suitable spot for detection of COVID-19. Sci Total Environ. 2021;772:145268. doi: 10.1016/j.scitotenv.2021.145268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or I, Yaniv K, Shagan M, Ozer E, Erster O, Mendelson E, Mannasse B, Shirazi R, Kramarsky-Winter E, Nir O, Abu-Ali H, Ronen Z, Rinott E, Lewis YE, Friedler E, Bitkover E, Paitan Y, Berchenko Y, Kushmaro A. Regressing SARS-CoV-2 sewage measurements onto COVID-19 burden in the population: A proof-of-concept for quantitative environmental surveillance. medRxiv. 2020 doi: 10.1101/2020.04.26.20073569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadinasab S, Chin A, Hosseini M, Poon L, Ducker WA. A surface coating that rapidly inactivates SARS-CoV-2. ACS Appl Mater Interfaces. 2020;12:34723–34727. doi: 10.1021/acsami.0c11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi A, Perinelli DR, Merchant HA, Bisharat L, Basheti IA, Bonacucina G, Cespi M, Palmieri GF. Hand sanitisers amid CoViD-19: a critical review of alcohol-based products on the market and formulation approaches to respond to increasing demand. Int J Pharm. 2020;584:119431. doi: 10.1016/j.ijpharm.2020.119431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt A, Arora P, Prajapati SK. Occurrence, fates and potential treatment approaches for removal of viruses from wastewater: a review with emphasis on SARS-CoV-2. J Environ Chem Eng. 2020;8:104429. doi: 10.1016/j.jece.2020.104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazejewski C, Wallet F, Rouzé A, Le Guern R, Ponthieux S, Salleron J, Nseir S. Efficiency of hydrogen peroxide in improving disinfection of ICU rooms. Crit Care. 2015;19:1–8. doi: 10.1186/s13054-015-0752-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce JM. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob Resist Infect Control. 2016;5:1–10. doi: 10.1186/s13756-016-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bs GC, Wambier CG. Rational hand hygiene during the coronavirus 2019 (COVID-19) pandemic. J Am Dermatol. 2020;82:e211. doi: 10.1016/j.jaad.2020.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno M, Welch D, Shuryak I, Brenner DJ. Far-UVC light (222 nm) efficiently and safely inactivates airborne human coronaviruses. Sci Rep. 2020;10:1–8. doi: 10.1038/s41598-020-67211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro E, Bonetta Si, Bertino C, Lorenzi E, Bonetta Sa, Gilli G. Hospital effluents management: chemical, physical, microbiological risks and legislation in different countries. J Environ Manage. 2016;168:185–199. doi: 10.1016/j.jenvman.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Castillo Meza L, Piotrowski P, Farnan J, Tasker TL, Xiong B, Weggler B, Murrell K, Dorman FL, Vanden Heuvel JP, Burgos WD. Detection and removal of biologically active organic micropollutants from hospital wastewater. Sci Total Environ. 2020;700:134469. doi: 10.1016/j.scitotenv.2019.134469. [DOI] [PubMed] [Google Scholar]
- Chai WS, Tan WG, Halimatul Munawaroh HS, Gupta VK, Ho S-H, Show PL. Multifaceted roles of microalgae in the application of wastewater biotreatment: a review. Environ Pollut. 2021;269:116236. doi: 10.1016/j.envpol.2020.116236. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Show P-L, Lau BF, Chang J-S, Ling TC. New prospects for modified algae in heavy metal adsorption. Trends Biotechnol. 2019;37:1255–1268. doi: 10.1016/j.tibtech.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Chirani MR, Kowsari E, Teymourian T, Ramakrishna S. Environmental impact of increased soap consumption during COVID-19 pandemic: Biodegradable soap production and sustainable packaging. Sci Total Environ. 2021;796:149013. doi: 10.1016/j.scitotenv.2021.149013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Chatterjee P, Lichtfouse E, Martel JA, Hwang M, Jinadatha C, Sharma VK. Classical and alternative disinfection strategies to control the COVID-19 virus in healthcare facilities: a review. Environ Chem Lett. 2021 doi: 10.1007/s10311-021-01180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colares GS, da Silva FP, de Souza Celente G, de Loreto AC, Lutterbeck CA, Machado ÊL, Kist LT. Combined system for the treatment and reuse of urban wastewater: the efficiency of anaerobic reactors þ hybrid constructed wetlands þ ozonation. Water Sci Technol. 2019;80:254–264. doi: 10.2166/wst.2019.270. [DOI] [PubMed] [Google Scholar]
- Collivignarelli MC, Abbà A, Benigna I, Sorlini S, Torretta V. Overview of the main disinfection processes for wastewater and drinking water treatment plants. Sustain. 2018;10:1–21. doi: 10.3390/su10010086. [DOI] [Google Scholar]
- Dale Wilson B, Moon S, Armstrong F. Comprehensive review of ultraviolet radiation and the current status on sunscreens. J Clin Aesthet Dermatol. 2012;5:18–23. [PMC free article] [PubMed] [Google Scholar]
- Dandie CE, Ogunniyi AD, Ferro S, Hall B, Drigo B, Chow CWK, Venter H, Myers B, Deo P, Donner E, Lombi E. Disinfection options for irrigation water: Reducing the risk of fresh produce contamination with human pathogens. Crit Rev Environ Sci Technol. 2019 doi: 10.1080/10643389.2019.1704172. [DOI] [Google Scholar]
- Daouk S, Chèvre N, Vernaz N, Widmer C, Daali Y, Fleury-Souverain S. Dynamics of active pharmaceutical ingredients loads in a Swiss university hospital wastewaters and prediction of the related environmental risk for the aquatic ecosystems. Sci Total Environ. 2016 doi: 10.1016/j.scitotenv.2015.12.117. [DOI] [PubMed] [Google Scholar]
- Daughton CG. Wastewater surveillance for population-wide Covid-19: The present and future. Sci Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maria F, Beccaloni E, Bonadonna L, Cini C, Confalonieri E, La Rosa G, Milana MR, Testai E, Scaini F. Minimization of spreading of SARS-CoV-2 via household waste produced by subjects affected by COVID-19 or in quarantine. Sci Total Environ. 2020;743:140803. doi: 10.1016/j.scitotenv.2020.140803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foladori P, Cutrupi F, Segata N, Manara S, Pinto F, Malpei F, Bruni L, La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: What do we know? A review. Sci Total Environ. 2020;743:140444. doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani A, Alygizakis N, Aalizadeh R, Kastritis E, Dimopoulos M-A, Thomaidis NS. Patterns of pharmaceuticals use during the first wave of COVID-19 pandemic in Athens, Greece as revealed by wastewater-based epidemiology. Sci Total Environ. 2021;798:149014. doi: 10.1016/j.scitotenv.2021.149014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobbo A, Rodrigues MAS, Zoppas Ferreira J, Bernardes AM, de Pinho MN. A critical review on SARS-CoV-2 infectivity in water and wastewater. What do we know? Sci Total Environ. 2021;774:145721. doi: 10.1016/j.scitotenv.2021.145721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, Hawi S, Goel G, Thakur VK, Agrawal A, Hoskins C, Pearce O, Hussain T, Upadhyaya HM, Cross G, Barber AH. Resilient and agile engineering solutions to address societal challenges such as coronavirus pandemic. Mater Today Chem. 2020;17:100300. doi: 10.1016/j.mtchem.2020.100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves J, Koritnik T, Mioč V, Trkov M, Bolješič M, Berginc N, Prosenc K, Kotar T, Paragi M. Detection of SARS-CoV-2 RNA in hospital wastewater from a low COVID-19 disease prevalence area. Sci Total Environ. 2021;755:143226. doi: 10.1016/J.SCITOTENV.2020.143226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A, Popović V, Pierscianowski J, Biancaniello M, Warriner K, Koutchma T. Inactivation of Escherichia coli, Listeria and Salmonella by single and multiple wavelength ultraviolet-light emitting diodes. Innov Food Sci Emerg Technol. 2018;47:353–361. doi: 10.1016/j.ifset.2018.03.019. [DOI] [Google Scholar]
- Hadi J, Dunowska M, Wu S, Brightwell G. Control measures for sars-cov-2: A review on light-based inactivation of single-stranded rna viruses. Pathogens. 2020;9:1–30. doi: 10.3390/pathogens9090737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart OE, Halden RU. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: Feasibility, economy, opportunities and challenges. Sci Total Environ. 2020;730:138875. doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan SW, Ibrahim Y, Daou M, Kannout H, Jan N, Lopes A, Alsafar H, Yousef AF. Detection and quantification of SARS-CoV-2 RNA in wastewater and treated effluents: Surveillance of COVID-19 epidemic in the United Arab Emirates. Sci Total Environ. 2021;764:142929. doi: 10.1016/J.SCITOTENV.2020.142929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A, Hara-Yamamura H, Meuchi Y, Imai S, Honda R. Detection of SARS-CoV-2 in wastewater in Japan during a COVID-19 outbreak. Sci Total Environ. 2021;758:143578. doi: 10.1016/j.scitotenv.2020.143578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller L, Mota CR, Greco DB. COVID-19 faecal-oral transmission: Are we asking the right questions? Sci Total Environ. 2020;729:138919. doi: 10.1016/j.scitotenv.2020.138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmdahl T, Odenholt I, Riesbeck K, Medstrand P, Widell A. Hydrogen peroxide vapour treatment inactivates norovirus but has limited effect on post-treatment viral RNA levels. Infect Dis (auckl) 2019;51:197–205. doi: 10.1080/23744235.2018.1546056. [DOI] [PubMed] [Google Scholar]
- Huo ZY, Du Y, Chen Z, Wu YH, Hu HY. Evaluation and prospects of nanomaterial-enabled innovative processes and devices for water disinfection: A state-of-the-art review. Water Res. 2020 doi: 10.1016/j.watres.2020.115581. [DOI] [PubMed] [Google Scholar]
- Huy TQ, Hien Thanh NT, Thuy NT, Chung PV, Hung PN, Le AT, Hong Hanh NT. Cytotoxicity and antiviral activity of electrochemical—synthesized silver nanoparticles against poliovirus. J Virol Methods. 2017;241:52–57. doi: 10.1016/j.jviromet.2016.12.015. [DOI] [PubMed] [Google Scholar]
- Ilyas S, Srivastava RR, Kim H. Disinfection technology and strategies for COVID-19 hospital and bio-medical waste management. Sci Total Environ. 2020;749:141652. doi: 10.1016/j.scitotenv.2020.141652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn K, Dreifuss D, Topolsky I, Kull A, Ganesanandamoorthy P, Fernandez-Cassi X, Bänziger C, Devaux AJ, Stachler E, Caduff L, Cariti F, Corzón AT, Fuhrmann L, Chen C, Jablonski KP, Nadeau S, Feldkamp M, Beisel C, Aquino C, Stadler T, Ort C, Kohn T, Julian TR, Beerenwinkel N. Detection and surveillance of SARS-CoV-2 genomic variants in wastewater. medRxiv. 2021 doi: 10.1101/2021.01.08.21249379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YJ, Oh BS, Kang JW. Synergistic effect of sequential or combined use of ozone and UV radiation for the disinfection of Bacillus subtilis spores. Water Res. 2008;42:1613–1621. doi: 10.1016/j.watres.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Khan NA, Khan SU, Ahmed S, Farooqi IH, Yousefi M, Mohammadi AA, Changani F. Recent trends in disposal and treatment technologies of emerging-pollutants—a critical review. TrAC Trends Anal Chem. 2020;122:115744. doi: 10.1016/j.trac.2019.115744. [DOI] [Google Scholar]
- Kocaman A, Keles O. Antibacterial efficacy of wire arc sprayed copper coatings against various pathogens. J Therm Spray Technol. 2019;28:504–513. doi: 10.1007/s11666-018-0824-x. [DOI] [Google Scholar]
- Kocamemi BA, Kurt H, Sait A, Sarac F, Saatci AM, Pakdemirli B. SARS-CoV-2 detection in Istanbul wastewater treatment plant sludges. medRxiv. 2020 doi: 10.1101/2020.05.12.20099358. [DOI] [Google Scholar]
- Kumar M, Patel AK, Shah AV, Raval J, Rajpara N, Joshi M, Joshi CG. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A, Maurya NS, Tiwari B. Hospital wastewater treatment scenario around the globe. Curr Dev Biotechnol Bioeng. 2020 doi: 10.1016/b978-0-12-819722-6.00015-8. [DOI] [Google Scholar]
- La Rosa G, Iaconelli M, Mancini P, Bonanno Ferraro G, Veneri C, Bonadonna L, Lucentini L, Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G, Mancini P, Bonanno Ferraro G, Veneri C, Iaconelli M, Lucentini L, Bonadonna L, Brusaferro S, Brandtner D, Fasanella A, Pace L, Parisi A, Galante D, Suffredini E. Rapid screening for SARS-CoV-2 variants of concern in clinical and environmental samples using nested RT-PCR assays targeting key mutations of the spike protein. Water Res. 2021;197:117104. doi: 10.1016/j.watres.2021.117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kovalova L, McArdell CS, von Gunten U. Prediction of micropollutant elimination during ozonation of a hospital wastewater effluent. Water Res. 2014;64:134–148. doi: 10.1016/j.watres.2014.06.027. [DOI] [PubMed] [Google Scholar]
- Levina AS, Repkova MN, Bessudnova EV, Filippova EI, Mazurkova NA, Zarytova VF. High antiviral effect of TiO2·PL-DNA nanocomposites targeted to conservative regions of (−)RNA and (+)RNA of influenza A virus in cell culture. Beilstein J Nanotechnol. 2016;7:1166–1173. doi: 10.3762/bjnano.7.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GQ, Wang WL, Huo ZY, Lu Y, Hu HY. Comparison of UV-LED and low pressure UV for water disinfection: photoreactivation and dark repair of Escherichia coli. Water Res. 2017;126:134–143. doi: 10.1016/j.watres.2017.09.030. [DOI] [PubMed] [Google Scholar]
- Li GQ, Huo ZY, Wu QY, Lu Y, Hu HY. Synergistic effect of combined UV-LED and chlorine treatment on Bacillus subtilis spore inactivation. Sci Total Environ. 2018 doi: 10.1016/j.scitotenv.2018.05.240. [DOI] [PubMed] [Google Scholar]
- Lindsley WG, Martin SB, Thewlis RE, Sarkisian K, Nwoko JO, Mead KR, Noti JD. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J Occup Environ Hyg. 2015;12:509–517. doi: 10.1080/15459624.2015.1018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W, de Roda Husman AM. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Guo W, Ngo HH, Nghiem LD, Hai FI, Zhang J, Liang S, Wang XC. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci Total Environ. 2014 doi: 10.1016/j.scitotenv.2013.12.065. [DOI] [PubMed] [Google Scholar]
- Majumder A, Gupta AK, Ghosal PS, Varma M. A review on hospital wastewater treatment: A special emphasis on occurrence and removal of pharmaceutically active compounds, resistant microorganisms, and SARS-CoV-2. J Environ Chem Eng. 2020 doi: 10.1016/j.jece.2020.104812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal P, Gupta AK, Dubey BK. A review on presence, survival, disinfection/removal methods of coronavirus in wastewater and progress of wastewater-based epidemiology. J Environ Chem Eng. 2020 doi: 10.1016/j.eplepsyres.2019.106192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquès M, Domingo JL. Contamination of inert surfaces by SARS-CoV-2: Persistence, stability and infectivity. A review. Environ Res. 2021;193:110559. doi: 10.1016/j.envres.2020.110559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecha AC, Onyango MS, Ochieng A, Momba MNB. Evaluation of synergy and bacterial regrowth in photocatalytic ozonation disinfection of municipal wastewater. Sci Total Environ. 2017;601–602:626–635. doi: 10.1016/j.scitotenv.2017.05.204. [DOI] [PubMed] [Google Scholar]
- Merkl P, Long S, McInerney GM, Sotiriou GA. Antiviral Activity of silver, copper oxide and zinc oxide nanoparticle coatings against SARS-CoV-2. Nanomaterials. 2021;11:1312. doi: 10.3390/nano11051312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael-Kordatou I, Karaolia P, Fatta-Kassinos D. Sewage analysis as a tool for the COVID-19 pandemic response and management: The urgent need for optimised protocols for SARS-CoV-2 detection and quantification. J Environ Chem Eng. 2020;8:104306. doi: 10.1016/j.jece.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuboshi S, Tsugita M. Impact of alcohol-based hand sanitizers, antibiotic consumption, and other measures on detection rates of antibiotic-resistant bacteria in rural Japanese hospitals. J Infect Chemother. 2018 doi: 10.1016/j.jiac.2018.08.013. [DOI] [PubMed] [Google Scholar]
- Nasseri S, Yavarian J, Baghani AN, Azad TM, Nejati A, Nabizadeh R, Hadi M, Jandaghi NZS, Vakili B, Vaghefi SKA, Baghban M, Yousefi S, Nazmara S, Alimohammadi M. The presence of SARS-CoV-2 in raw and treated wastewater in 3 cities of Iran: Tehran, Qom and Anzali during coronavirus disease 2019 (COVID-19) outbreak. J Environ Heal Sci Eng. 2021;19:573–584. doi: 10.1007/s40201-021-00629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour-eddine A, Lahcen B. Estimate of the metallic contamination of the urban effluents by the effluents of the Mohamed V Hospital of Meknes. Eur Sci J. 2014;10:70–78. [Google Scholar]
- Pangestika R, Ernawati R. Antiviral activity effect of silver nanoparticles (Agnps) solution against the growth of infectious bursal disease virus on embryonated chicken eggs with Elisa Test. KnE Life Sci. 2017;3:536. doi: 10.18502/kls.v3i6.1181. [DOI] [Google Scholar]
- Peccia J, Zulli A, Brackney DE, Grubaugh ND, Kaplan EH, Casanovas-Massana A, Ko AI, Malik AA, Wang D, Wang M, Warren JL, Weinberger DM, Arnold W, Omer SB. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat Biotechnol. 2020;38:1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichel N, Vivar M, Fuentes M. The problem of drinking water access: a review of disinfection technologies with an emphasis on solar treatment methods. Chemosphere. 2019;218:1014–1030. doi: 10.1016/j.chemosphere.2018.11.205. [DOI] [PubMed] [Google Scholar]
- Prado T, Fumian TM, Mannarino CF, Resende PC, Motta FC, Eppinghaus ALF, Chagas do Vale VH, Braz RMS, de Andrade J da SR, Maranhão AG, Miagostovich MP (2021) Wastewater-based epidemiology as a useful tool to track SARS-CoV-2 and support public health policies at municipal level in Brazil. Water Res 191:116810. 10.1016/j.watres.2021.116810 [DOI] [PMC free article] [PubMed]
- Rabenau HF, Kampf G, Cinatl J, Doerr HW. Efficacy of various disinfectants against SARS coronavirus. J Hosp Infect. 2005;61:107–111. doi: 10.1016/j.jhin.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambabu K, Banat F, Pham QM, Ho S-H, Ren N-Q, Show PL. Biological remediation of acid mine drainage: review of past trends and current outlook. Environ Sci Ecotechnology. 2020;2:100024. doi: 10.1016/j.ese.2020.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W, Truchado P, Cuevas-Ferrando E, Simón P, Allende A, Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi SG, Stefani F, Gigantiello A, Polesello S, Comandatore F, Mileto D, Maresca M, Longobardi C, Mancon A, Romeri F, Pagani C, Cappelli F, Roscioli C, Moja L, Gismondo MR, Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini V, Sikri K, Batra SD, Kalra P, Gautam K. Development of a highly effective low-cost vaporized hydrogen peroxide-based method for disinfection of personal protective equipment for their selective reuse during pandemics. Gut Pathog. 2020;12:1–11. doi: 10.1186/s13099-020-00367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem ANB, Zyed R, Lassoued MA, Nidhal S, Sfar S, Mahjoub A. Plant-derived nanoparticles enhance antiviral activity against coxsakievirus B3 by acting on virus particles and vero cells. Dig J Nanomater Biostructures. 2012;7:737–744. [Google Scholar]
- Salgado CD, Sepkowitz KA, John JF, Cantey JR, Attaway HH, Freeman KD, Sharpe PA, Michels HT, Schmidt MG. Copper surfaces reduce the rate of healthcare-acquired infections in the Intensive Care Unit. Infect Control Hosp Epidemiol. 2013;34:479–486. doi: 10.1086/670207. [DOI] [PubMed] [Google Scholar]
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA J Am Med Assoc. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Sarizadeh G, Geravandi S, Takdastan A, Javanmaerdi P, Mohammadi MJ. Efficiency of hospital wastewater treatment system in removal of level of toxic, microbial, and organic pollutant. Toxin Rev. 2021 doi: 10.1080/15569543.2021.1922923. [DOI] [Google Scholar]
- Sharma VK, Jinadatha C, Lichtfouse E, Decroly E, van Helden J, Choi H, Chatterjee P. COVID-19 epidemiologic surveillance using wastewater. Environ Chem Lett. 2021 doi: 10.1007/s10311-021-01188-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Griffith TM, Nyangaresi PO, Qin Y, Pang X, Chen G, Li M, Lu Y, Zhang B. Efficacy of UVC-LED in water disinfection on Bacillus species with consideration of antibiotic resistance issue. J Hazard Mater. 2020;386:121968. doi: 10.1016/j.jhazmat.2019.121968. [DOI] [PubMed] [Google Scholar]
- Sherchan SP, Shahin S, Ward LM, Tandukar S, Aw TG, Schmitz B, Ahmed W, Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: A study in Louisiana, USA. Sci Total Environ. 2020;743:140621. doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Show PL, Thangalazhy-Gopakumar S, Foo DCY. Sustainable technologies for waste reduction and pollutants removals. Clean Technol Environ Policy. 2021;23:1–2. doi: 10.1007/s10098-020-02001-y. [DOI] [Google Scholar]
- Simmons S, Carrion R, Alfson K, Staples H, Jinadatha C, Jarvis W, Sampathkumar P, Chemaly RF, Khawaja F, Povroznik M, Jackson S, Kaye KS, Rodriguez RM, Stibich M. Deactivation of SARS-CoV-2 with Pulsed Xenon Ultraviolet: Implications for environmental COVID-19 control. Infect Control Hosp Epidemiol. 2020 doi: 10.1017/ice.2020.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR, Patil A, Raza BG, Reurink D, van den Hengel SK, Rutjes SA, de Roda Husman AM, Roesink HDW, de Vos WM. Cationically modified membranes using covalent layer-by-layer assembly for antiviral applications in drinking water. J Membr Sci. 2019;570–571:494–503. doi: 10.1016/j.memsci.2018.10.081. [DOI] [Google Scholar]
- Skrastina D, Petrovskis I, Lieknina I, Bogans J, Renhofa R, Ose V, Dishlers A, Dekhtyar Y, Pumpens P. Silica nanoparticles as the adjuvant for the immunisation of mice using hepatitis B core virus-like particles. PLoS ONE. 2014 doi: 10.1371/journal.pone.0114006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Mohseni M, Taghipour F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review. Water Res. 2016;94:341–349. doi: 10.1016/j.watres.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Street R, Malema S, Mahlangeni N, Mathee A. Wastewater surveillance for Covid-19: An African perspective. Sci Total Environ. 2020;743:140719. doi: 10.1016/j.scitotenv.2020.140719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totaro M, Casini B, Profeti S, Tuvo B, Privitera G, Baggiani A. Role of hydrogen peroxide vapor (HPV) for the disinfection of hospital surfaces contaminated by multiresistant bacteria. Pathogens. 2020 doi: 10.3390/pathogens9050408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HN, Le GT, Nguyen DT, Juang RS, Rinklebe J, Bhatnagar A, Lima EC, Iqbal HMN, Sarmah AK, Chao HP. SARS-CoV-2 coronavirus in water and wastewater: a critical review about presence and concern. Environ Res. 2021;193:110265. doi: 10.1016/J.ENVRES.2020.110265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usman M, Farooq M, Hanna K. Environmental side effects of the injudicious use of antimicrobials in the era of COVID-19. Sci Total Environ. 2020;745:141053. doi: 10.1016/j.scitotenv.2020.141053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellingiri B, Jayaramayya K, Iyer M, Narayanasamy A, Govindasamy V, Giridharan B, Ganesan S, Venugopal A, Venkatesan D, Ganesan H, Rajagopalan K, Rahman PKSM, Cho SG, Kumar NS, Subramaniam MD. COVID-19: A promising cure for the global panic. Sci Total Environ. 2020;725:138277. doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlicchi P. Hospital Wastewaters, The Handbook of Environmental Chemistry. Cham: Springer International Publishing; 2018. [Google Scholar]
- Verlicchi P, Galletti A, Petrovic M, BarcelÓ D. Hospital effluents as a source of emerging pollutants: An overview of micropollutants and sustainable treatment options. J Hydrol. 2010;389:416–428. doi: 10.1016/j.jhydrol.2010.06.005. [DOI] [Google Scholar]
- Wang J, Feng H, Zhang S, Ni Z, Ni L, Chen Y, Zhuo L, Zhong Z, Qu T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int J Infect Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shen J, Ye D, Yan X, Zhang Y, Yang W, Li X, Wang J, Zhang L, Pan L. Disinfection technology of hospital wastes and wastewater: Suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ Pollut. 2020;262:114665. doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes SL, Little ZR, Keevil CW. Human coronavirus 229E remains infectious on common touch surface materials. Mbio. 2015;6:1–10. doi: 10.1128/mBio.01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DJ, Kanamori H, Rutla WA. “No touch” technologies for environmental decontamination: Focus on ultraviolet devices and hydrogen peroxide systems. Curr Opin Infect Dis. 2020;82:1511–1512. doi: 10.1097/QCO.0000000000000284. [DOI] [PubMed] [Google Scholar]
- Würtele MA, Kolbe T, Lipsz M, Külberg A, Weyers M, Kneissl M, Jekel M. Application of GaN-based ultraviolet-C light emitting diodes - UV LEDs - for water disinfection. Water Res. 2011;45:1481–1489. doi: 10.1016/j.watres.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Wurtzer S, Marechal V, Mouchel JM, Maday Y, Teyssou R, Richard E, Almayrac JL, Moulin L. Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters. medRxiv. 2020 doi: 10.1101/2020.04.12.20062679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap HC, Pang YL, Lim S, Abdullah AZ, Ong HC, Wu CH. A comprehensive review on state-of-the-art photo-, sono-, and sonophotocatalytic treatments to degrade emerging contaminants. Int J Environ Sci Technol. 2019;16:601–628. doi: 10.1007/s13762-018-1961-y. [DOI] [Google Scholar]
- Zhang D, Ling H, Huang X, Li J, Li W, Yi C, Zhang T, Jiang Y, He Y, Deng S, Zhang X, Wang X, Liu Y, Li G, Qu J. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci Total Environ. 2020;741:140445. doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xie B, Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav Immun. 2020;87:59–73. doi: 10.1016/j.bbi.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang X, Yang Y, Huang X, Jiang J, Li M, Ling H, Li J, Liu Y, Li G, Li W, Yi C, Zhang T, Jiang Y, Xiong Y, He Z, Wang X, Deng S, Zhao P, Qu J. SARS-CoV-2 spillover into hospital outdoor environments. J Hazard Mater Lett. 2021;2:100027. doi: 10.1016/j.hazl.2021.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou XY, Lin YL, Xu B, Cao TC, Tang YL, Pan Y, Gao ZC, Gao NY. Enhanced inactivation of E. coli by pulsed UV-LED irradiation during water disinfection. Sci Total Environ. 2019;650:210–215. doi: 10.1016/j.scitotenv.2018.08.367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.