Abstract
Background
Glioma incidence is 25% lower in Hispanics than White non-Hispanics. The US Hispanic population is diverse, and registry-based analyses may mask incidence differences associated with geographic/ancestral origins.
Methods
County-level glioma incidence data in Hispanics were retrieved from the Central Brain Tumor Registry of the United States. American Community Survey data were used to determine the county-level proportion of the Hispanic population of Mexican/Central American and Caribbean origins. Age-adjusted incidence rate ratios and incidence rate ratios (IRRs) quantified the glioma incidence differences across groups. State-level estimates of admixture in Hispanics were obtained from published 23andMe data.
Results
Compared to predominantly Caribbean-origin counties, predominantly Mexican/Central American-origin counties had lower age-adjusted risks of glioma (IRR = 0.83; P < 0.0001), glioblastoma (IRR = 0.86; P < 0.0001), diffuse/anaplastic astrocytoma (IRR = 0.78; P < 0.0001), oligodendroglioma (IRR = 0.82; P < 0.0001), ependymoma (IRR = 0.88; P = 0.012), and pilocytic astrocytoma (IRR = 0.76; P < 0.0001). Associations were consistent in children and adults and using more granular geographic regions. Despite having lower glioma incidence, Hispanic glioblastoma patients from predominantly Mexican/Central American-origin counties had poorer survival than Hispanics living in predominantly Caribbean-origin counties. Incidence and survival differences could be partially explained by state-level estimates of European admixture in Hispanics with European admixture associated with higher incidence and improved survival.
Conclusions
Glioma incidence and outcomes differ in association with the geographic origins of Hispanic communities, with counties of predominantly Mexican/Central American origin at significantly reduced risk and those of Caribbean origin at comparatively greater risk. Although typically classified as a single ethnic group, appreciating the cultural, socioeconomic, and genetic diversity of Hispanics can advance cancer disparities research.
Keywords: epidemiology, Continental Population Group, glioma, Hispanic Americans, incidence
Key Points.
The US Hispanic population is extremely diverse, but is frequently assessed as a single group in cancer research.
Predominant county-level country of origin is significantly associated with both incidence and survival in glioma.
Importance of the Study.
Glioma incidence has been previously shown to be significantly decreased in Hispanics as compared to White non-Hispanics. Due to the significant diversity in the US Hispanic population, we sought to evaluate differences in glioma incidence and survival associated with predominant country or region of origin.
Predominately Caribbean-origin Hispanic counties had a higher incidence of glioma as compared to Mexican or Central American Hispanic counties. Residence in a predominantly Mexican/Central American-origin Hispanic county was associated with decreased survival in glioblastoma, which appears to be partially attributable to an increased proportion of European admixture. These findings reveal previously unexplored disparities in glioma risk and patient outcomes within the US Hispanic population and complement existing knowledge about glioblastoma survival.
Glioma is the most common primary malignant brain tumor.1 Approximately 20,000 new cases are diagnosed annually in the United States, although incidence varies by sex, race/ethnicity, and age.1 Compared to White non-Hispanics (WNH), the US Hispanic/Latinx population has ~25% lower incidence of all glioma histologies.2 These individuals are pooled within cancer registry data as “Hispanic,” and we use this nomenclature throughout this manuscript to be consistent with the underlying data. However, Hispanics can trace their heritage to distinct geographical regions and are culturally and genetically diverse.3 This diversity arose within geographically distinct populations in the Americas prior to European colonization, as well as through varied patterns of contact with European- and African-ancestry populations. As a result, “Hispanics” are not only diverse in country of origin, but are genetically diverse in ways that correlate with geography.4 In general, Hispanics with Caribbean heritage have higher proportions of European and African ancestries, while those with Mexican or Central American heritage have higher proportions of indigenous American ancestry.3,5
With statistical techniques, it is possible to discern the approximate proportions of an individual’s genome that can be traced to specific continental origins, usually in a 3-continental (Europe, Asia, and Africa) or 4-continental model (Europe, Asia, Africa, and the Americas). These estimated proportions have previously been found to correlate with differential susceptibility to multiple complex traits in Hispanics, including cancer risk.6,7 In particular, multiple studies have identified that Hispanic glioma patients have higher estimated levels of European ancestry as compared to controls.8,9 These ancestry measures likely summarize population-level differences in the frequency of specific genetic variants that affect glioma risk, many of which have not yet been identified in prior genome-wide association studies in WNHs. Due to the rarity of glioma in US minority populations (~15% of all diagnoses), prior analyses have pooled Hispanics into a single group despite their substantial diversity.
We sought to quantify the extent to which registry-based analyses may mask differences in incidence and outcomes associated with underlying diversity in Hispanics. Using a dataset covering all reported US glioma cases, we examine whether heterogeneity exists in Hispanic glioma risk and outcomes geographically, and the extent to which heterogeneity in incidence and outcomes correlate with predominant geographic origins.
Methods
This study was approved as part of an exempt protocol by the institutional review board of Duke University Health System. The Central Brain Tumor Registry of the United States (CBTRUS) analytic dataset 1,10 used for incidence analyses includes data from the Centers for Disease Control’s National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology, and End Results program. and covers ~100% of the US population.1,11,12 These data are derived from 51 central cancer registries (CCR) (50 states and Washington DC). The category of glioma and specific histologies were defined using the CBTRUS grouping scheme.1,2 Race/ethnicity information is abstracted from the medical record, which has been shown to have high sensitivity for race and moderate sensitivity for Hispanic ethnicity.13,14 Ethnicity is further classified using the North American Association of Cancer Registries Hispanic Identification Algorithm, which utilizes a combination of fields (including medical record, birthplace, and race) to directly and indirectly categorize patients.15 While country of birth is often collected by CCR, these variables are not publicly released to CBTRUS.
The American Community Survey (ACS) is conducted on a yearly basis as a supplement to the decennial census.16 County-level counts of the Hispanic population, reported origin, and Hispanic population born outside the United States were obtained from the ACS (2015–2019).17 These were used to generate percentages of national origin/geographic heritage groups and county foreign-born population. Three pooled categories were created based on a priori knowledge of general admixture patterns: Mexican/Central American, Caribbean (including Puerto Ricans, Cubans, and Dominicans), and all remaining origins. For counties estimated to have ≥1000 Hispanic residents, the county was categorized by predominant origin. State-level average proportions of European, African, and Indigenous American admixture among Hispanics from saliva specimens submitted to 23andMe were obtained from Bryc et al.18
All glioma cases diagnosed from 2000 to 2017 were identified in CBTRUS. Counties were pooled by predominant origin groups, and average annual age-adjusted incidence rates (AAAIR) with 95% confidence intervals (95% CIs) were generated using SEER*Stat by histology, Hispanic ethnicity, and combined race/ ethnicity (WNH, White Hispanic, and Black Hispanic), and age groups. Incidence rate ratios (IRRs) were generated using AAAIR.19,20 IRRs were considered statistically significant when P < 0.05. We applied univariate linear regression to identify significant associations with county-level incidence and proportion of national origin, county median age in Hispanics, urban/rural continuum, estimated county socioeconomic status (SES),21 and state-level genetic admixture. Counties with <10 glioma cases or <1000 total Hispanic residents were excluded. Factors where P < 0.05 were combined in multivariable linear regression models. Sensitivity analyses were performed in 25 counties with the highest Hispanic population, the 1000 smallest population counties, and all remaining counties, as well as by tertiles of county Hispanic population that was born outside of the United States. We performed regression analyses and created figures in R4.0.3.22–31
De-identified individual-level survival data were obtained from the U.S. Cancer Statistics program for 42 CCR (~82% of the United States) from 2004 to 2017, which is a subset of CBTRUS.32 Only adults from states with available admixture data residing in counties with ≥1000 Hispanic residents were included and followed until December 31, 2017. Kaplan–Meier models were used to assess survival differences, and Cox proportional hazards models were used to account for other potential prognostic factors. Each factor was tested using univariate models, after which all were assessed for violations of the proportional hazards assumption, including visual inspection of scaled Schoenfeld residuals. Analyses were repeated in a subset of glioblastoma patients that received resection and radiation to assess whether differences in healthcare access produced observed survival differences. Adjustment factors are noted in tables and figure legends.
Results
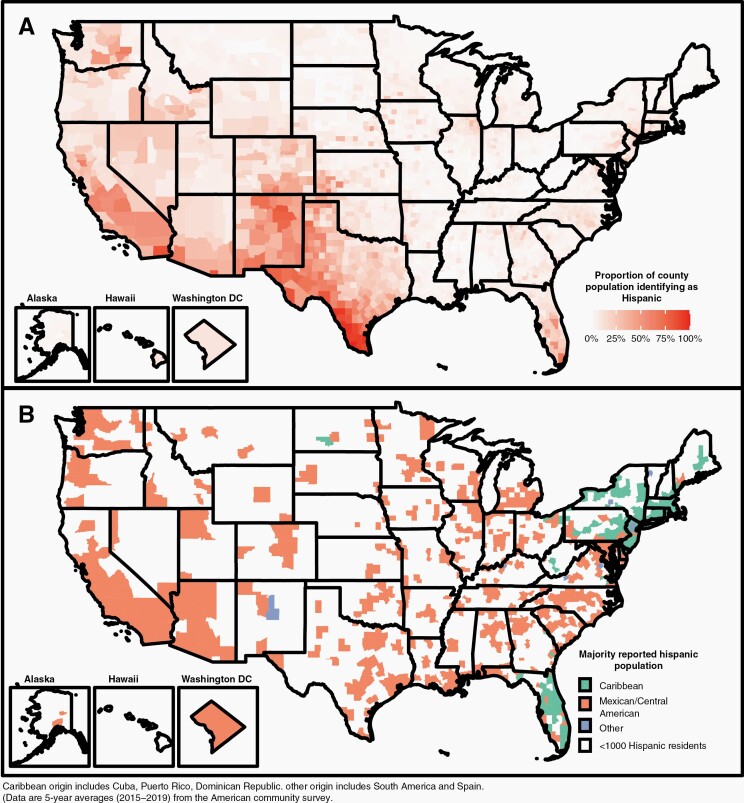
We determined the proportion of the population in each county identified as Hispanic (Figure 1A), classified each of these counties by its predominant reported origin, and observed consistent patterns: counties in the West and Midwest having a Mexican/Central American origin and Hispanic residents in Florida and the Northeast having a Caribbean origin (Figure 1B, Table S1). Median age in Mexican/Central American counties was significantly younger than in Caribbean-origin counties (45 vs. 52, P < 0.0001).
Fig. 1.
(A) Proportion of US county population identifying as Hispanic. (B) Predominant reported Hispanic origin in US counties with ≥1000 reported Hispanic residents. Caribbean origin includes Cuba, Puerto Rico, and Dominican Republic. Other origin includes South America and Spain (data are 5-year averages [2015–2019] from the American Community Survey).
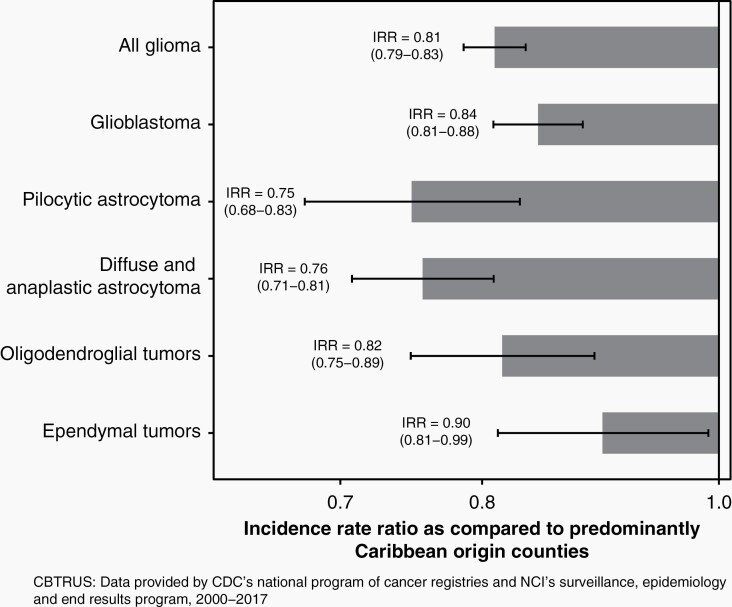
Using data from CBTRUS, we calculated the AAAIR of glioma in Hispanic individuals living in by predominant county origin (Mexican/Central American, Caribbean, or Other Hispanic). Compared to Caribbean-origin counties, Mexican/Central American-origin counties had a significantly lower incidence of glioma (IRR = 0.83; P < 0.0001). Similar results were observed across histologies with Mexican/Centra American-origin counties having significantly reduced risk of glioblastoma (IRR = 0.86; P < 0.0001), diffuse/anaplastic astrocytoma (IRR = 0.78; P < 0.0001), oligodendroglioma (IRR = 0.82; P < 0.0001), ependymoma (IRR = 0.88; P = 0.012), and pilocytic astrocytoma (PA) (IRR = 0.76; P < 0.0001) (Figure 2, Table S2). Counties with a predominant origin of Other Hispanic also had significantly lower incidence (IRR = 0.91; P < 0.0001), although the number of counties included in this group was small and histology-stratified comparisons did not reach statistical significance. In order to assess whether these associations may be driven primarily by large counties, we stratified analyses by the 25 counties with the largest Hispanic populations, 1000 counties with the lowest Hispanic population, and all remaining counties (Table S3).17 Incidence rates were consistently lower in Mexican/Central American-origin counties, irrespective of population size (Table S4). To determine whether a higher proportion of foreign-born population could be driving incidence differences, we divided counties into tertiles of proportion of Hispanics born outside of the United States (1.2%–22.4%, 22.5%–34.1%, and 34.2%–96.1%) (Table S5). Incidence rates were consistently lower in Mexican/Central American-origin counties, irrespective of proportion of the population that reported being born outside of the United States.
Fig. 2.
Incidence rate ratios and 95% CI of all glioma and specific glioma histologies in US Hispanics living in predominantly Mexican/Central American counties, as compared to Hispanics living in predominantly Caribbean-origin counties (CBTRUS: data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2000–2017).
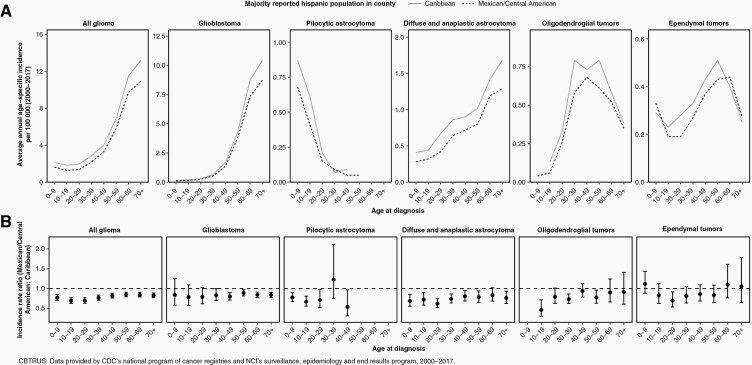
We next calculated age-stratified incidence rates overall and for specific glioma histologies. IRRs was generally tracked together across strata of age, with Caribbean-origin counties consistently having higher incidence across all ages (Figure 3). However, the incidence of pediatric glioblastoma did not vary substantially by geographic origin, and rates of oligodendroglioma and ependymoma were comparable in elderly populations across strata of geographic origin.
Fig. 3.
(A) Age-stratified AAAIR and 95% CI of all glioma and specific glioma histologies in Hispanics, by predominant county-level region of origin. (B) Age-stratified IRR for Hispanics living in predominantly Mexican/Central American-origin counties compared to predominantly Caribbean-origin counties as the reference (CBTRUS: data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2000–2017).
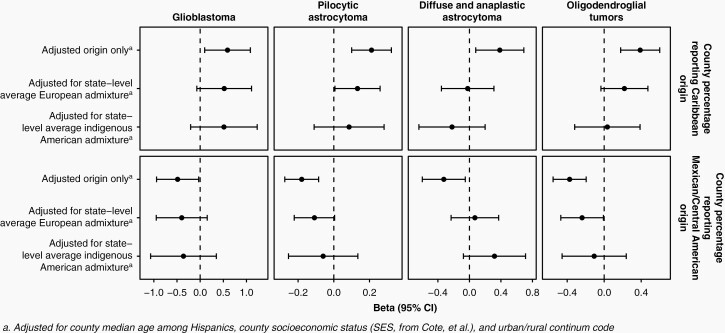
Because the above calculations necessitated discretizing a county by region of origin, we next performed univariate regression analyses of incidence as a function of the proportion of Hispanic individuals reporting Caribbean or Mexican/Central American-origin. Increased proportion of Caribbean origin was associated with a significantly higher incidence of glioma, glioblastoma, diffuse/anaplastic astrocytoma, oligodendroglioma, and PA. Similarly, an increased proportion of Mexican/Central American-origin was associated with a significantly lower incidence of glioma, oligodendroglioma, and PA (Figure 4, Table S6). When regression models were run using more granular regions of origin, associations were generally consistent with those from the broader classification (Table S7). However, the magnitude of the association with Cuban origin was substantially stronger than with Puerto Rican origin. Mexican origin was associated with a decreased incidence across all histologies, but Central American origin was associated with modestly increased incidence of several lower grade histologies, although only the association with ependymoma reached statistical significance.
Fig. 4.
Beta and 95% CIs from multivariate regression analyses of the association between Hispanic glioma incidence and county-level proportions of Hispanic individuals reporting Caribbean or Mexican/Central American origin, adjusted for county median age, urban/rural continuum code, and county socioeconomic status (CBTRUS: data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2000-2017).
Cancer registration procedures vary by state. To assess whether completeness of registry data associate with the residential composition of counties and confound results, we evaluated whether the proportion of Caribbean or Mexican/Central American origin was associated with incidence among WNHs. We observed little association between county-level incidence among WNHs and the geographic origin (Tables S8 and S9), suggesting that associations between county-level differences in incidence and geographic origin are likely unrelated to registry quality. We repeated these analyses stratified by county size, and found they were again consistent within the top 25 Hispanic population counties and smaller counties, although P-values were slightly attenuated due to reduced sample size (Table S10).
Although county-level estimates of genetic admixture have not been published, averages of European, African, and indigenous American admixture among self-identified Hispanics are available at the state level.3 We observed a significant positive correlation between the county-level percentage of Mexican/Central American origin and state-level estimates of indigenous American admixture, and a negative correlation with state-level estimates of African and European admixture. Conversely, we observed a significant positive correlation between the county-level percentage of Caribbean origin and state-level estimates of African and European admixture, and a negative correlation with state-level estimates of indigenous American admixture (Figure S1). We also observed that state-level indigenous American admixture was associated with significantly lower county-level incidence, while state-level African and European admixture were associated with significantly higher county-level incidence (Figure S2). In multivariate models, the proportion of Caribbean or Mexican/Central American origin remained positively and negatively associated with county-level incidence, respectively, although associations with geographic origin were attenuated when accounting for state-level admixture estimates (Figure 4, Tables S5 and S6).
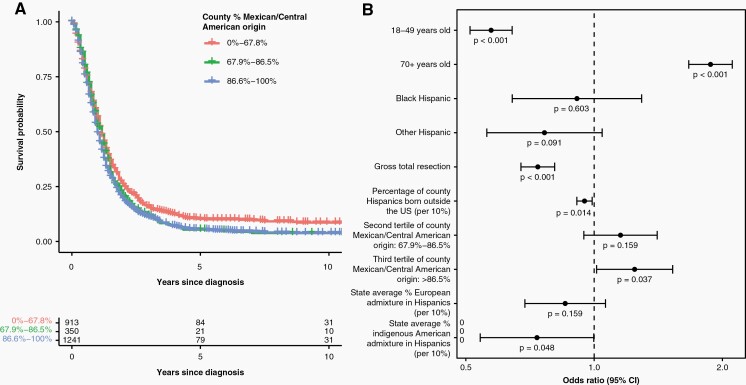
Individual-level survival data were available for a subset of 7829 Hispanic patients (56% glioblastoma) (Table S10). Median survival was shorter in Mexican/Central American-origin counties than Caribbean-origin counties (12 vs. 13 months), and a proportion of Mexican/Central American origin was associated with poorer overall survival (Figure 5A). Multivariable Cox proportional hazards models revealed significant associations between poorer glioblastoma survival and elevated county-level proportion Mexican/Central American origin (Table S11). When analyses were limited to glioblastoma patients receiving resection and radiotherapy, associations with Mexican/Central American origin persisted after adjustment for European and indigenous American admixture among Hispanics (Figure 5B, Table S12). Because these associations may be attributable to structural barriers to neuro-oncology care not captured by SES or receipt of surgery/radiotherapy, we assessed whether Mexican/Central American origin was associated with glioblastoma survival among WNHs. Although WNHs from Mexican/Central American-origin counties also experienced poorer survival, the magnitude of the association was more modest than in Hispanics (Table S12). Survival associations in lower grade gliomas complemented these results, with individuals from counties with higher Caribbean origin having better survival in univariate analyses, but was only significant in multivariable Cox models in diffuse astrocytoma and oligodendroglial tumors (Tables S13 and S14).
Fig. 5.
(A) Adjusted survival estimates of Hispanic glioblastoma patient survival in individuals receiving both resection and radiation, stratified by tertiles of the county-level percent of residents of Mexican/Central American origin. (B) Hazards ratios and 95% confidence limits for factors associated with survival among Hispanic glioblastoma patients receiving both resection and radiation (N = 2504) in multivariable Cox proportional hazards regression analysis (CBTRUS: data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2000–2017).
Discussion
Glioma incidence varies globally, with the highest rates observed in the United States, Canada, Australia, and Northern Europe.33 Within the United States, glioma incidence varies substantially by race/ethnicity, with a consistent elevated incidence in WNH observed in all ages and across primary histologic groupings.2,34 Despite this, diversity among Hispanics has frequently been neglected in glioma research.35 Cancer registry data typically pool Hispanics and do not enable more granular analysis by geographic origin, as appropriate population data are not available to calculate incidence rates. Aggregating heterogeneous populations may obscure meaningful differences in incidence and outcomes. This has previously been observed in colorectal cancer, where Americans of Cuban and Puerto Rican descent have a higher incidence than Americans of Mexican descent.36 We investigated incidence differences associated with the underlying Hispanic geographic origin of glioma patients using data from CBTRUS (~100% of reported cases in the United States) and observed that Hispanic glioma incidence differs significantly in association with the geographic origins, as did glioblastoma survival. Specifically, the incidence was lower but survival poorer in Mexican/Central American-origin counties, and incidence was higher but survival better in Cuban, Puerto Rican, and/or Dominican counties. These results were generally consistent across counties with larger and smaller populations, as well as across age and histologies.
Hispanics share many common cultural attributes that may differ from those of WNH and Black non-Hispanics.37 Hispanics are genetically admixed with European, African, and indigenous American genomic contributions that differ in proportion across populations.3 Although often classified as a single ethnic group, Hispanics are not a monolithic group. Cultural, SES, and genetic heterogeneity underscores a varied array of American experiences and merits more granular approaches. While Hispanics generally have a lower cancer incidence than WNHs for common cancer sites, they experience a higher incidence of leukemia, cervical, liver, and stomach cancers.38,39 Reported cancer statistics may mask variation, specifically those related to geographic origin and associated with underlying cultural/ancestral differences that can influence health behaviors and genetic predisposition.
Our observation that Hispanic residents from Caribbean-origin counties have an ~20% higher incidence of glioma than those from Mexican/Central American-origin counties may be explained by population-level differences in the frequency of specific genetic or environmental risk factors. Given consistent IRRs across age groups and histologies, a single exposure that could account for such differences would necessarily be a risk factor for diverse glioma subtypes and for both pediatric and adult glioma. Although many potential risk factors have been investigated for association with glioma risk, the only confirmed modifiable factor is ionizing radiation exposure, which accounts for an extremely small proportion of overall risk.40,41
Previous case–control studies of childhood ependymoma and of adult glioma have identified an increased European genetic admixture as a risk factor in both Hispanics and African Americans.8,9 Given the strong association between Hispanic country of origin and genetic admixture, it seems plausible that genetic ancestry may contribute to observed incidence differences. Hispanics with Caribbean origin are known to have higher European and higher African admixture than those from Mexico/Central America.3–5 If European admixture confers glioma risk, this would be consistent with the direction of effect observed in our analyses, wherein Caribbean-origin counties had a higher incidence than Mexican/Central American-origin counties. Importantly, glioblastoma survival is also better in African Americans and Hispanics than WNHs, and it has been hypothesized that elevated European ancestry may negatively impact glioblastoma survival.2 However, our data show that while counties with increased Caribbean and decreased Mexican/Central American origins have higher incidence, they also have better outcomes. This poor survival association with higher Mexican/Central American origin was robust to adjustment for state-level admixture. Univariate analyses showed improvements in survival with higher state-level African and European admixture, but these were not significant in the multivariable model. While elevated European admixture appears to contribute to increased glioma risk among both WNH and Hispanics of Caribbean origin, it may not be completely driving observed survival differences.
Because counties may have fundamental underlying differences in the quality of cancer reporting and neuro-oncology care, we assessed whether WNHs living in these counties experienced significant differences in incidence and survival. We did not observe strong evidence of this as an explanation for our results, particularly for differences in incidence, although it remains possible that structural inequalities limit the precision of our effect estimates.
Prior research has recognized a “Hispanic paradox,” wherein Hispanics have lower mortality than would be expected based on reduced SES and healthcare access. A prior analysis across all cancer types identified no survival advantage in Hispanics relative to WNHs, with the poorest survival among US-born and foreign-born individuals of Mexican origin.42 In our analysis, the county-level proportion of foreign-born Hispanics was significantly associated with improved survival in both univariate and multivariate models (Tables S11 and S12). This may be attributable to follow-up limitations of cancer registries in foreign-born individuals with a greater propensity to leave the country after diagnosis, but may also suggest that unmeasured individual or environmental factors associated with immigration may also play a role in improved outcomes for individuals with glioma.
A primary limitation of our study is a reliance on cancer registry data that currently lack molecularly-stratified glioma subgroups corresponding to current WHO classification schemes. However, the consistency of associations across age and histology may minimize its impact. There is no central pathology review in the data used for this analysis, and histology reflects assignment by the diagnosing pathologist. Race is abstracted from the medical record, while Hispanic ethnicity is both collected and assigned via an algorithm. As a result, there may be some errors in the assignment of these variables. Region of origin may influence whether individuals receive their cancer diagnosis and care in the United States, which may further contribute to a lower incidence in Mexican/Central American counties. Another limitation of our study is its reliance on county- and state-level estimates as a proxy for individual-level data, which will misclassify the origin of some reported cases as a result. However, several limitations of the ecological nature of our study were minimized in the survival analyses, as these were done using individual-level data and only relied on county-level estimates for assigning the likeliest geographic origin. Combined with emerging data from other studies, our results highlight the value of collecting more granular ethnicity data to better understand cancer etiology and outcomes.
While prior research has demonstrated significantly reduced glioma incidence and improved survival in Hispanics relative to WNHs, our results substantially extend these findings and reveal clear differences based on region of origin. The lower incidence observed in predominantly Mexican/Central American-origin counties relative to predominantly Caribbean-origin counties was generally consistent across ages and histologic groups. These differences may be attributable to an unequal prevalence of underlying risk factors, but the dearth of glioma-associated environmental and lifestyle exposures makes this unlikely. It remains possible that the prevalence of rare, high-penetrance germline, or somatic driver mutations underlying gliomagenesis may differ across Hispanic populations.43,44 With increased attention given to disparities in cancer incidence and outcomes, our analyses highlight that the cultural, socioeconomic, and genetic diversity of Hispanics merits a more detailed assessment when investigating population-level cancer disparities.
Supplementary Material
Acknowledgments
CBTRUS data were provided through an agreement with the Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries. In addition, CBTRUS used data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program. CBTRUS acknowledges and appreciates these contributions to this report and to cancer surveillance in general. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the NCI.
Contributor Information
Kyle M Walsh, Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA; The Preston Robert Tisch Brain Tumor Center, Duke University School of Medicine, Durham, NC; Duke Cancer Institute, Duke University Medical Center, Durham, North Carolina, USA.
Corey Neff, Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA; Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA.
Melissa L Bondy, Department of Epidemiology and Population Health, Stanford University School of Medicine, Stanford, California, USA.
Carol Kruchko, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA.
Jason T Huse, Department of Translational Molecular Pathology and Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Christopher I Amos, Department of Medicine, Section of Epidemiology and Population Sciences, and Institute for Clinical and Translational Research, Dan L. Duncan Comprehensive Cancer Center, Baylor College of Medicine, Houston, Texas, USA.
Jill S Barnholtz-Sloan, Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA; Center for Biomedical Informatics & Information Technology and Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland, USA.
Quinn T Ostrom, Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA; The Preston Robert Tisch Brain Tumor Center, Duke University School of Medicine, Durham, NC; Duke Cancer Institute, Duke University Medical Center, Durham, North Carolina, USA; Central Brain Tumor Registry of the United States, Hinsdale, Illinois, USA.
Funding
Funding for CBTRUS was provided by the Centers for Disease Control and Prevention under Contract No. 75D30119C06056; the American Brain Tumor Association; The Sontag Foundation, Novocure; the Musella Foundation, National Brain Tumor Society; the Pediatric Brain Tumor Foundation; the Uncle Kory Foundation; the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in-kind donations. Additional funding provided by R01CA194189 to K.M.W., P30CA125123 to C.I.A., P30CA014236 to K.M.W., P50CA190991 to K.M.W. and Q.T.O., P50CA127001 to J.T.H., M.L.B, and C.I.A.
Author contributions
Conceptualization, funding acquisition, investigation, writing—original draft, writing—review and editing: Kyle M. Walsh. Formal analysis, writing—review and editing: Corey Neff. Funding acquisition, writing—review and editing: Melissa L. Bondy. Funding acquisition, writing—review and editing: Carol Kruchko. Funding acquisition, writing—review and editing: Jason T. Huse. Funding acquisition, writing—review and editing: Christopher I. Amos. Funding acquisition, writing—review and editing: Jill S. Barnholtz-Sloan. Conceptualization, investigation, data curation, formal analysis, methodology, project administration, writing—original draft, writing—review and editing: Quinn T. Ostrom.
Conflicts of interest statement. J.S.B. is a full time, paid employee of the NIH/NCI.
References
- 1. Ostrom QT, Patil N, Cioffi G, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro-Oncology 2020;22(12 Suppl 2):iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 2018;4(9):1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bryc K, Velez C, Karafet T, et al. . Genome-wide patterns of population structure and admixture among Hispanic/Latino populations. Proc Natl Acad Sci USA. 2010;107(Suppl 2):8954–8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang S, Ray N, Rojas W, et al. . Geographic patterns of genome admixture in Latin American Mestizos. PLoS Genet. 2008;4(3):e1000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salazar-Flores J, Zuñiga-Chiquette F, Rubi-Castellanos R, et al. . Admixture and genetic relationships of Mexican Mestizos regarding Latin American and Caribbean populations based on 13 CODIS-STRs. Homo 2015;66(1):44–59. [DOI] [PubMed] [Google Scholar]
- 6. Walsh KM, Chokkalingam AP, Hsu LI, et al. . Associations between genome-wide Native American ancestry, known risk alleles and B-cell ALL risk in Hispanic children. Leukemia 2013;27(12):2416–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zavala VA, Bracci PM, Carethers JM, et al. . Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer 2021;124(2):315–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang C, Ostrom QT, Hansen HM, et al. . European genetic ancestry associated with risk of childhood ependymoma. Neuro-Oncology 2020;22(11):1637–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ostrom QT, Egan KM, Nabors LB, et al. . Glioma risk associated with extent of estimated European genetic ancestry in African-Americans and Hispanics. Int J Cancer. 2020;146(3):739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Central Brain Tumor Registry of the United States SEER*Stat Database. CDC National Program of Cancer Registries and NCI Surveillance, Epidemiology and End Results Incidence Data, 2019 submission (2000–2017).2020. Accessed March 21, 2022.
- 11. Centers for Disease Control and Prevention National Center for Health Statistics. National Program of Cancer Registries and Surveillance, Epidemiology, and End Results SEER*Stat Database: U.S. Cancer Statistics Incidence Analytic Database – 2001-2018. United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Based on the November 2020 submission. 2021. Accessed March 21, 2022. [Google Scholar]
- 12. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (2000-2016) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. 2019. Accessed March 21, 2022.
- 13. Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States). Cancer Causes Control 2006;17(6):771–781. [DOI] [PubMed] [Google Scholar]
- 14. West CN, Geiger AM, Greene SM, et al. . Race and ethnicity: comparing medical records to self-reports. J Natl Cancer Inst Monogr. 2005;(35):72–74. [DOI] [PubMed] [Google Scholar]
- 15. NAACCR Race and Ethnicity Work Group. NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1]. September 2012. Accessed March 16, 2021.
- 16. United States Census Bureau. About the American Community Survey. 2021; https://www.census.gov/programs-surveys/acs/about.html. Accessed March 16, 2021.
- 17. U.S. Census Bureau. 2015-2019 American Community Survey 5-Year Estimates. 2019; https://data.census.gov/cedsci/table?q=B03001&tid=ACSDT5Y2019.B03001. Accessed January 7, 2021.
- 18. Bryc K, Durand EY, Macpherson JM, Reich D, Mountain JL. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet. 2015;96(1):37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat software version 8.4.0. 2022; www.seer.cancer.gov/seerstat. Accessed May 27, 2022.
- 20. Fay MP, Tiwari RC, Feuer EJ, Zou Z. Estimating average annual percent change for disease rates without assuming constant change. Biometrics 2006;62(3):847–854. [DOI] [PubMed] [Google Scholar]
- 21. Cote DJ, Ostrom QT, Gittleman H, et al. . Glioma incidence and survival variations by county-level socioeconomic measures. Cancer 2019;125(19):3390–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. R Core Team. R: A Language and Environment for Statistical Computing. 2020; http://www.R-project.org/. Accessed July 21, 2020.
- 23. Wickham H, Averick M, Bryan J, et al. . Welcome to the Tidyverse. J Open Source Softw. 2019;4(43):16861686. [Google Scholar]
- 24. Luo J. SEER2R: Reading and Writing SEER*STAT Data Files. R package Version 1.0. 2012; http://CRAN.R-project.org/package=SEER2R. [Google Scholar]
- 25. Kassambara A, Kosinski M, Biecek P, Fabian S. survminer: Drawing Survival Curves using “ggplot2”. 2020; https://rpkgs.datanovia.com/survminer/index.html. Accessed May 27, 2022.
- 26. R Core Team. R: A Language and Environment for Statistical Computing. 2021; http://www.R-project.org/. Accessed April 20, 2021.
- 27. Heinzen E, Sinnwell J, Atkinson E, et al. . arsenal: An Arsenal of “R” Functions for Large-Scale Statistical Summaries. 2020; https://github.com/mayoverse/arsenal. Accessed July 22, 2020.
- 28. Dardis C. survMisc: Miscellaneous Functions for Survival Data. 2018; https://github.com/dardisco/survMisc. Accessed July 22, 2020.
- 29. Soetaert K. Diagram: Functions for Visualising Simple Graphs (Networks), Plotting Flow Diagrams 2017; https://CRAN.R-project.org/package=diagram. Accessed July 24, 2020.
- 30. Therneau TM. A Package for Survival Analysis in R. R Package Version 3.2-7. 2020; https://CRAN.R-project.org/package=survival. Accessed March 21, 2022. [Google Scholar]
- 31. Mogensen UB, Ishwaran H, Gerds TA. Evaluating random forests for survival analysis using prediction error curves. J Stat Softw 2012;50(11):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. U.S. Cancer Statistics Working Group. NPCR Survival Analytical Database, November 2020 Submission, Diagnosis Year 2001-2017. 2021. Accessed March 21, 2022.
- 33. Leece R, Xu J, Ostrom QT, et al. . Global incidence of malignant brain and other central nervous system tumors by histology, 2003-2007. Neuro-Oncology 2017;19(11):1553–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muskens IS, Feng Q, Francis SS, et al. . Pediatric glioma and medulloblastoma risk and population demographics: a Poisson regression analysis. Neurooncol Adv. 2020;2(1):vdaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pinheiro PS, Sherman RL, Trapido EJ, et al. . Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2162–2169. [DOI] [PubMed] [Google Scholar]
- 36. Miller KD, Goding Sauer A, Ortiz AP, et al. . Cancer STatistics for Hispanics/Latinos, 2018. CA Cancer J Clin. 2018;68(6):425–445. [DOI] [PubMed] [Google Scholar]
- 37. Gonzalez Burchard E, Borrell LN, Choudhry S, et al. . Latino populations: a unique opportunity for the study of race, genetics, and social environment in epidemiological research. Am J Public Health 2005;95(12):2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O’Brien K, Cokkinides V, Jemal A, et al. . Cancer statistics for Hispanics, 2003. CA Cancer J Clin 2003;53(4):208–226. [DOI] [PubMed] [Google Scholar]
- 39. Yamamoto JF, Goodman MT. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997-2002. Cancer Causes Control 2008;19(4):379–390. [DOI] [PubMed] [Google Scholar]
- 40. Ostrom QT, Bauchet L, Davis FG, et al. . The epidemiology of glioma in adults: a “state of the science” review. Neuro-Oncology 2014;16(7):896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Walsh KM, Claus EB. Diet and risk of glioma: targets for prevention remain elusive. Neuro-Oncology 2019;21(7):832–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pinheiro PS, Williams M, Miller EA, et al. . Cancer survival among Latinos and the Hispanic paradox. Cancer Causes Control 2011;22(4):553–561. [DOI] [PubMed] [Google Scholar]
- 43. Sandoval RL, Masotti C, de Macedo MP, et al. . Identification of the TP53 p.R337H variant in tumor genomic profiling should prompt consideration of germline testing for Li-Fraumeni syndrome. JCO Glob Oncol. 2021;7:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCormack RM, Zhu P, Dono A, et al. . Role of ethnicity and geographic location on glioblastoma IDH1/IDH2 mutations. World Neurosurg. 2021;149:e894–e912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.