Abstract
Polypharmacy may cause adverse health outcomes in the elderly. This study examined the prevalence of continuous polypharmacy and hyper‐polypharmacy, factors associated with polypharmacy, and the most frequently prescribed medications among older adults in South Korea. This was a retrospective observational study using National Health Insurance claims data. In total, 7,358,953 Korean elderly patients aged 65 years and older were included. Continuous polypharmacy and hyper‐polypharmacy were defined as the use of ≥5 and ≥10 medications, respectively, for both ≥90 days and ≥180 days within 1 year. A multivariate logistic regression analysis was conducted with adjustment for general characteristics (sex, age, insurance type), comorbidities (12 diseases, number of comorbidities, and Elixhauser Comorbidity Index [ECI] classification), and healthcare service utilization. Among 7.36 million elderly patients, 47.8% and 36.9% had polypharmacy for ≥90 and ≥180 days, and 11.9% and 7.1% of patients exhibited hyper‐polypharmacy for ≥90 and ≥180 days, respectively. Male sex, older age, insurance, comorbidities (cardio‐cerebrovascular disease, diabetes mellitus, depressive disorder, dementia, an ECI score of ≥3), and healthcare service utilization were associated with an increased probability of polypharmacy. The therapeutic class with the most prescriptions was drugs for acid‐related disorders (ATC A02). The number of outpatient visit days more strongly influenced polypharmacy than hospitalizations and ED visits. This study provides health policymakers with important evidence about the critical need to reduce polypharmacy among older adults.
Study Highlights
WHAT IS CURRENT KNOWLEDGE ON THE TOPIC?
With population aging, the use of multiple medications in the elderly has increased, and polypharmacy might negatively affect health.
WHAT QUESTION DID THIS STUDY ADDRESS?
There is a need to investigate the factors associated with high polypharmacy among elderly adults.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Polypharmacy was quite common among elderly adults in South Korea, and the number of outpatient visit days more strongly influenced polypharmacy than hospitalizations and ED visits.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Policies should be put in place to coordinate pharmacy care between different doctors' healthcare services to reduce polypharmacy in the elderly.
INTRODUCTION
Polypharmacy is defined as the administration of many drugs at the same time 1 and often as the routine use of five or more medications. 2 The definitions of polypharmacy differ according to the healthcare setting, study population, and medical field. 3 , 4 However, most studies have used the number of medications in a certain time window, and a systematic review of definitions on polypharmacy classified them as numerical‐only (using the number of medications to define polypharmacy), and numerical with an associated duration of therapy or healthcare setting (such as during a hospital stay).
Polypharmacy has been associated with various adverse health outcomes, such as functional decline, cognitive impairment, risk of hospitalization, increased healthcare expenses, and mortality. 5 , 6 , 7 , 8 A meta‐analysis of the adverse outcomes of polypharmacy in terms of healthcare utilization reported an association between polypharmacy and hospitalization. 5 Nevertheless, polypharmacy may be unavoidable in patients receiving treatment for most chronic diseases. In that sense, several factors have been found to influence polypharmacy. The determinants of polypharmacy include patient factors, disease‐related factors, and healthcare factors. 3 Aging and the presence of conditions such as cardiovascular disease or multimorbidity are major factors that influence polypharmacy, and having multiple prescribers can also lead to polypharmacy. 3 , 6 , 9 , 10 , 11 , 12 , 13 , 14 , 15 Additional causes of polypharmacy include the complexity and diversity of drug therapy, other psychosocial factors, and adverse reactions to drug therapy. 16 , 17 In view of the importance of ensuring patient safety and financial sustainability of the health insurance system, it is important to establish a strategy to use medicines appropriately.
In South Korea (hereafter, Korea), the average life expectancy has increased from 62.3 years in 1970 to 83.5 years in 2020, and Korea has rapidly become an aging society rapidly due to its low birth rate. As of 2020, 15.7% of the population consisted of seniors aged 65 years or older in South Korea, which surpassed 14%, so it is referred to as an “aging society,” and this proportion is expected to increase to 43.9% by 2060. 18
Most research has found that multimorbidity or specific diseases contributed to increased polypharmacy. However, the determinants of polypharmacy have not been examined in Korea. Therefore, this study examined the prevalence of polypharmacy in Korea, the most frequently prescribed medications among polypharmacy patients, and the factors that influenced polypharmacy.
METHODS
Data source and study population
This study used National Health Insurance (NHI) claims data between 2017 and 2019. In Korea, the NHI covers 97% of the Korean population, and claims data are submitted to the Health Insurance Review and Assessment Service. The claims data include patients' demographic characteristics, as well as the diagnosis code of the disease, the international non‐proprietary name of each drug, the prescribed dose per day, and the number of days of therapy. 19 , 20 The study population was a cohort of elderly patients (65 years or older) who received at least one outpatient prescription between January and December 2018. We defined the index date as the first outpatient visit, the history period as 1 year before the index date, and the observation period as extending from the index date to 1 year after the index date.
Since the NHI pays for services and patients could use several medical facilities, we included overall medication use from tertiary hospitals, general hospitals, primary hospitals (including convalescent hospitals), clinics, and health centers (community health centers) in the study. Data on outpatient oral prescriptions from these institutions were included in the analysis.
The therapeutic categories of medicines were classified according to the Anatomical Therapeutic Chemical (ATC) classification system of the World Health Organization (WHO) Collaborating Center. 21 We analyzed the most frequently prescribed therapeutic subclasses according to the ATC‐2 level (therapeutic class).
Outcome measures and confounding variables
The outcome measures in the current study were polypharmacy (use of ≥5 medications) and hyper‐polypharmacy (use of ≥10 medications) for periods of both 90 days or more and 180 days or more. Non‐polypharmacy was defined as four or fewer medications. We selected these cut‐offs and periods based on an existing systematic literature review and definitions of the WHO. 1 , 2 , 4
The patient was the analytic unit, and the number of medications taken every day by each patient for 365 days was calculated. We also analyzed the number of prescription days at the patient level, as well as the number of prescribed medications per day. The number of patients using a certain drug was defined as the number of patients who were prescribed that medication in the observation period.
The possible confounders included in the analysis were sex, age, and insurance type, comorbidities, and healthcare utilization. We classified comorbidities involving chronic diseases (cerebro‐cardiovascular diseases, hypertension, hyperlipidemia, diabetes, gastric ulcers, chronic kidney disease, liver disease, respiratory disease, musculoskeletal disease, bone fracture, cancer, and dementia) based on the 10th International Classification of Diseases (ICD‐10) and previous diseases. We summed the pre‐existing conditions using the Elixhauser Comorbidity Index (ECI) score based on primary and secondary disease codes in the previous 1 year before the first outpatient visit in 2018. We calculated the ECI as scores of 0, 1, 2, and 3 or higher. Healthcare utilization included hospitalizations, emergency department (ED) visits, and the number of outpatient visits made during the observation period. We selected confounders that might influence polypharmacy based on previous studies. 3 , 6
Statistical analysis
We conducted descriptive analyses using the t‐test or chi‐square test between the polypharmacy and non‐polypharmacy groups. Due to the sufficiently large population size, normality of the continuous variables was assumed according to the central limit theorem, obviating the need to consider the normality assumption. To investigate the variables that influenced polypharmacy, a simple logistic regression analysis was conducted for each variable.
Multivariate logistic regression was performed to identify factors affecting polypharmacy and hyper‐polypharmacy. We adjusted for the subjects' general characteristics, comorbidities, and use of healthcare services in order to identify the factors that affected polypharmacy. Model calibration was assessed using the Hosmer–Lemeshow goodness‐of‐fit test and c‐statistics, and the c‐statistics were in the range 0.74–0.91. The final model included age, sex, type of health insurance, chronic diseases, ECI (0, 1, 2, 3, or higher), the presence of hospitalization, the presence of ED visits, and the number of outpatient visits (≤10, 11–30, 31–50, ≥50). Adjusted odds ratios (aORs) were reported with 95% confidence intervals (CIs).
The most frequently prescribed therapeutic categories were compared between participants with polypharmacy and hyper‐polypharmacy and those with non‐polypharmacy. SAS Enterprise version 7.1 (SAS Institute) was used for all analyses.
RESULTS
Overall medications and the prevalence of polypharmacy
Table 1 summarizes the distribution of the number of medications and duration of therapies. A total of 7,358,953 patients aged 65 years or older had prescriptions for an average of 275 days per year. In total, 91.0% of the patients received 5 or more prescriptions per day at least once, and 48.9% of the patients had been prescribed 10 or more medications per day. Continuous polypharmacy (defined as polypharmacy for ≥90 or ≥180 days) was found in 47.8% and 36.9% of patients, respectively. The prevalence of continuous hyper‐polypharmacy was 13.9% and 9.1%, respectively.
TABLE 1.
Number of simultaneous daily medications by prescription duration among the elderly
| Total patients (N), (1000 patients [%]) | 5 or more, (1000 patients [%]) | 10 or more, (1000 patients [%]) | 20 or more, (1000 patients [%]) | |||||
|---|---|---|---|---|---|---|---|---|
| Prescription duration (days) | 7359 | (100.0) | 6697 (91.0) | (100.0) | 3601 (48.9) | (100.0) | 257 (3.5) | (100.0) |
| <90 | 1055 | (14.3) | 3182 | (43.2) | 2726 | (37.0) | 235 | (3.2) |
| 90–180 | 527 | (7.2) | 796 | (10.8) | 350 | (4.8) | 12 | (0.2) |
| 180–270 | 595 | (8.1) | 652 | (8.9) | 220 | (3.0) | 6 | (0.1) |
| 270–300 | 357 | (4.9) | 311 | (4.2) | 81 | (1.1) | 2 | (0.0) |
| 300–330 | 745 | (10.1) | 500 | (6.8) | 97 | (1.3) | 1 | (0.0) |
| ≥330 | 4080 | (55.4) | 1255 | (17.1) | 127 | (1.7) | 1 | (0.0) |
| Average | 275.5 ± 117.1 | 153.3 ± 136.0 | 68.4 ± 97.5 | 27.2 ± 55.7 | ||||
General characteristics
As shown in Table 2, which presents results for study participants with at least 90 days of medication use, the mean (±standard deviation) age was 73.2 ± 6.8 years in the non‐polypharmacy group, 75.1 ± 7.0 years in the polypharmacy group, and 76.2 ± 6.6 years in the hyper‐polypharmacy group. Compared to the non‐polypharmacy group, the polypharmacy and hyper‐polypharmacy groups had a higher proportion of subjects aged 75 years or more, more female subjects, and fewer subjects with NHI coverage. The proportion of patients with the presence of diseases was higher in the polypharmacy and hyper‐polypharmacy groups than in the non‐polypharmacy group.
TABLE 2.
General characteristics of the study population
| Characteristic | 90 days or more of use (N = 6,303,479) (%) | 180 days or more of use (N = 5,776,354) (%) | ||||
|---|---|---|---|---|---|---|
| Non‐polypharmacy (N = 2,788,635) | Polypharmacy (N = 2,639,609) | Hyper‐polypharmacy (N = 875,235) | Non‐polypharmacy (N = 3,057,202) | Polypharmacy (N = 2,193,838) | Hyper‐polypharmacy (N = 525,314) | |
| Sex | ||||||
| Female | 57.9 | 59.1 | 55.9 | 59.0 | 58.2 | 54.0 |
| Health Insurance | ||||||
| National Health Insurance | 96.1 | 92.8 | 85.5 | 95.7 | 91.8 | 83.8 |
| Medical Aid | 3.8 | 7.1 | 14.3 | 4.3 | 8.1 | 15.9 |
| Veterans' Insurance | 0.0 | 0.1 | 0.3 | 0.0 | 0.1 | 0.3 |
| Age (years), mean ± SD | 73.2 ± 6.8 | 75.1 ± 7.0 | 76.2 ± 6.6 | 73.4 ± 6.7 | 75.5 ± 6.9 | 76.3 ± 6.6 |
| 65–69 | 37.0 | 25.7 | 18.3 | 35.1 | 23.6 | 17.4 |
| 70–74 | 25.7 | 24.1 | 23.9 | 23.7 | 23.7 | 24.0 |
| 75–79 | 19.2 | 23.7 | 26.9 | 24.6 | 24.6 | 26.9 |
| 80–84 | 10.9 | 16.1 | 19.6 | 17.2 | 17.2 | 19.9 |
| ≥85 | 7.2 | 10.5 | 11.4 | 11.0 | 11.0 | 11.8 |
| Comorbidities | ||||||
| Cancer | 9.1 | 9.6 | 11.3 | 9.4 | 9.6 | 11.3 |
| Hypertension | 51.3 | 62.6 | 66.6 | 58.5 | 64.6 | 65.8 |
| Hyperlipidemia | 33.5 | 36.3 | 36.7 | 37.4 | 36.0 | 36.0 |
| Diabetes mellitus | 17.7 | 34.9 | 50.3 | 20.7 | 40.2 | 53.7 |
| Cardio‐cerebro vascular diseases | 6.6 | 18.1 | 28.4 | 8.1 | 21.3 | 30.6 |
| Gastric ulcer/GERD | 11.4 | 14.8 | 19.0 | 12.6 | 14.6 | 18.0 |
| Chronic renal disease | 0.9 | 2.8 | 7.7 | 1.1 | 3.5 | 9.2 |
| Liver disease | 10.3 | 10.8 | 13.0 | 10.9 | 10.7 | 12.8 |
| Respiratory disease | 11.0 | 16.1 | 24.8 | 12.2 | 16.7 | 25.1 |
| Musculoskeletal disease | 39.5 | 47.8 | 56.8 | 42.6 | 47.2 | 54.9 |
| Bone fracture | 6.7 | 9.0 | 12.9 | 7.1 | 9.3) | 12.9 |
| Dementia | 8.3 | 14.7 | 23.4 | 9.3 | 16.1 | 25.4 |
| Depressive disorder | 5.1 | 11.0 | 21.9 | 6.4 | 12.4 | 23.3 |
| ECI score | ||||||
| 0 | 28.9 | 13.7 | 5.9 | 22.7 | 11.2 | 5.2 |
| 1 | 36.1 | 26.4 | 14.6 | 35.6 | 23.8 | 13.1 |
| 2 | 22.8 | 30.0 | 25.1 | 25.8 | 30.2 | 23.8 |
| ≥3 | 12.2 | 30.0 | 54.3 | 16.0 | 34.9 | 57.9 |
| Healthcare utilization | ||||||
| Hospitalization | 20.9 | 31.1 | 44.6 | 22.6 | 31.9 | 44.1 |
| Nursing hospital admission | 1.7 | 2.9 | 4.4 | 1.6 | 2.6 | 3.7 |
| Emergency department visits | 11.6 | 17.9 | 28.2 | 12.4 | 18.8 | 28.4 |
| Outpatient visits (n) | ||||||
| 1–10 | 14.8 | 6.3 | 1.6 | 11.2 | 6.0 | 1.5 |
| 11–30 | 60.0 | 43.8 | 23.2 | 55.2 | 42.5 | 23.9 |
| 31–50 | 18.3 | 28.7 | 28.8 | 22.2 | 27.5 | 27.6 |
| >50 | 6.9 | 21.2 | 46.4 | 11.4 | 24.0 | 47.0 |
| Outpatient visits (days), mean ± SD | 24.9 ± 19.3 | 37.8 ± 29.7 | 60.1 ± 46.2 | 29.0 ± 22.9 | 40.1 ± 32.8 | 62.2 ± 49.8 |
| Days of therapy (days), mean ± SD | 286.2 ± 83.7 | 334.5 ± 45.9 | 350.4 ± 30.8 | 319.1 ± 48.6 | 344.8 ± 29.2 | 355.0 ± 20.5 |
| Medications per day (n), mean ± SD | 2.8 ± 1.0 | 5.8 ± 1.4 | 10.5 ± 2.5 | 3.2 ± 1.2 | 6.7 ± 1.4 | 11.7 ± 2.4 |
Note: p‐value of all values <0.05.
Abbreviations: ECI, Elixhauser Comorbidity Index; GERD, gastroesophageal reflux disease; SD, standard deviation.
The presence of hospitalization, nursing hospital admission, ED visits, and ≥30 outpatient visit days were also higher in the polypharmacy and hyper‐polypharmacy groups than in the non‐polypharmacy group. The mean number of prescription days was higher in the polypharmacy group (334.5 days) and the hyper‐polypharmacy group (350.4 days) than in the non‐polypharmacy group (286.2 days) and the number of medications prescribed per day was higher in the polypharmacy and hyper‐polypharmacy groups (5.8 and 10.5, respectively) than in the non‐polypharmacy group (2.8).
Factors associated with polypharmacy
Figures 1 and 2 summarize the results of the multivariate logistic regression analysis to explore determinants associated with the probability of being a patient with polypharmacy and hyper‐polypharmacy compared to non‐polypharmacy. After adjusting for sex, age, insurance type, comorbidities (cardio‐cerebrovascular disease, diabetes mellitus, chronic kidney disease, depressive disorder, dementia, and ECI score), the presence of hospitalization, the presence of ED visits, and the number of outpatient visit days were all associated with increased odds of polypharmacy or hyper‐polypharmacy. In contrast, cancer, gastric ulcers, and liver disease were associated with a decreased risk of polypharmacy.
FIGURE 1.
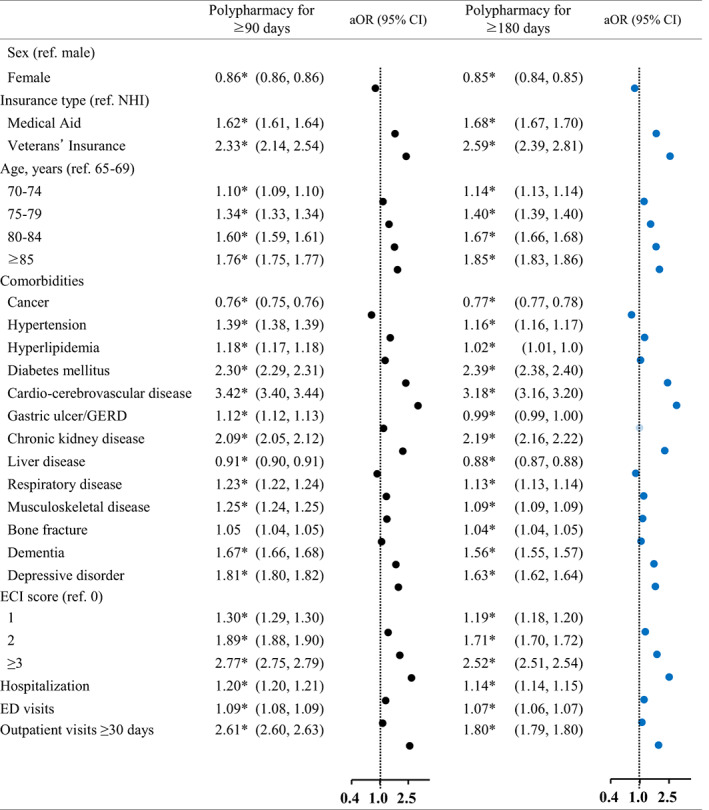
Factors associated with polypharmacy for 90 days or more and 180 days or more. aOR, adjusted odds ratio; CI, confidence interval; ECI, Elixhauser Comorbidity Index; ED, emergency department; GERD, gastroesophageal reflux disease; NHI, National Health Insurance. *p‐value <0.05
FIGURE 2.
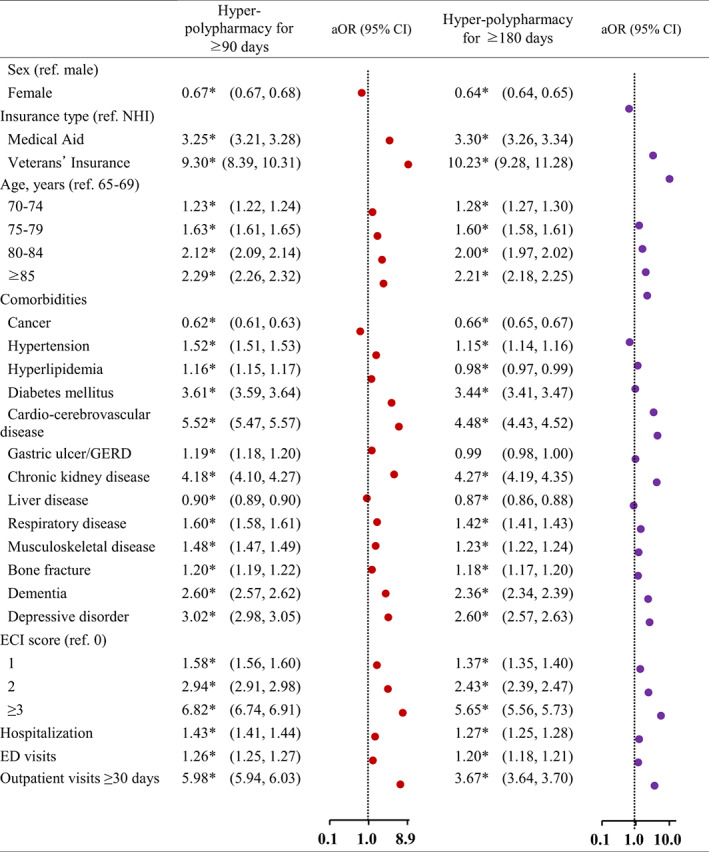
Factors associated with hyper‐polypharmacy for 90 days or more and 180 days or more. aOR, adjusted odds ratio; CI, confidence interval; ECI, Elixhauser Comorbidity Index; ED, emergency department; GERD, gastroesophageal reflux disease; NHI, National Health Insurance. *p‐value <0.05
Among elderly patients with 90 days or more of medication use, the odds ratios (ORs) of polypharmacy and hyper‐polypharmacy for women were 0.86 and 0.67. Compared to those with NHI coverage, those who were covered by Medical Aid and Veterans' Insurance were 1.62 and 2.33 times more likely to have polypharmacy (3.25 and 9.30 for hyper‐polypharmacy, respectively). When compared to those in the age group of 65–69 years, the ORs for polypharmacy and hyper‐polypharmacy increased with age (1.10 and 1.23 for those 70–74 years of age, 1.34 and 1.63 for those 75–79 years of age, and 1.60 and 2.12 for those ≥85 years of age, respectively).
Furthermore, the odds of polypharmacy and hyper‐polypharmacy increased by 3.42 and 5.52 times for elderly individuals with cardio‐cerebrovascular diseases, 2.30 and 3.61 times for those with diabetes mellitus, 2.09 and 4.18 times for those with chronic kidney disease, 1.81 and 3.02 times for those with depressive disorder, and 1.67 and 2.60 times for those with dementia, respectively. In contrast, the odds of polypharmacy and hyper‐polypharmacy decreased by 0.76 and 0.62 times for those with cancer, and by 0.91 and 0.90 times for those with liver disease. A linear increase was observed with regard to the ECI. For polypharmacy, an OR of 1.30 was found for an ECI score of 1, 1.89 for a score of 2, and 2.77 for a score of, while the corresponding ORs were 1.58, 2.94, and 6.82 for hyper‐polypharmacy, respectively. The odds of having polypharmacy and hyper‐polypharmacy were 1.20 and 1.09 higher, respectively, among those who had experienced hospitalization, and 1.09 and 1.26 higher among those who had visited the ED. Compared to patients with <30 outpatient visits, the ORs were 2.61 and 5.98 for those with ≥30 visit days.
In the analysis of elderly individuals with 180 days or more of medication use, the overall factors showed similar patterns, but lower odds for hyper‐polypharmacy were found for hyperlipidemia (OR = 0.98; 95% CI 0.97–0.98) and gastric ulcer/gastroesophageal reflux disease (GERD; OR = 0.99; 95% CI 0.99–1.00).
Most frequently prescribed drug categories
The most frequent drug categories were analyzed based on the second level of the ATC classification system (Table 3). Drugs for acid‐related disorders (ATC 02) were consistently the most frequently prescribed drug category among polypharmacy and hyper‐polypharmacy patients for 90 days or more, as well as hyper‐polypharmacy patients for 180 days or more.
TABLE 3.
Most frequently prescribed medications by Anatomical Therapeutic Chemical level 2 (ATC‐2)
| 90 days or more of use | 180 days or more of use | |||||||
|---|---|---|---|---|---|---|---|---|
| ATC‐2 level | Patients (n) (1000 patients [%]) | ATC‐2 level | Patients (n) (1000 patients [%]) | |||||
| Non‐polypharmacy patients | ||||||||
| 1 | C09 | Agents acting on the renin‐angiotensin system | 908 | (32.6) | C09 | Agents acting on the renin‐angiotensin system | 1101 | (36.0) |
| 2 | C10 | Lipid‐modifying agents | 889 | (31.9) | C10 | Lipid‐modifying agents | 1053 | (34.4) |
| 3 | B01 | Antithrombotic agents | 523 | (18.8) | B01 | Antithrombotic agents | 644 | (21.1) |
| 4 | A02 | Drugs for acid‐related disorders | 501 | (18.0) | C08 | Calcium channel blockers | 582 | (19.0) |
| 5 | C08 | Calcium channel blockers | 491 | (17.6) | A10 | Drugs used in diabetes | 425 | (13.9) |
| 6 | A10 | Drugs used in diabetes | 333 | (11.9) | A02 | Drugs for acid‐related disorders | 353 | (11.5) |
| 7 | M01 | Anti‐inflammatory and antirheumatic products | 284 | (10.2) | G04 | Urologicals | 250 | (8.2) |
| 8 | G04 | Urologicals | 238 | (8.5) | N06 | Psychoanaleptics | 234 | (7.7) |
| 9 | N06 | Psychoanaleptics | 222 | (7.9) | C07 | Beta‐blocking agents | 210 | (6.9) |
| 10 | N07 | Other nervous system drugs | 193 | (6.9) | N07 | Other nervous system drugs | 189 | (6.2) |
| Polypharmacy patients | ||||||||
| 1 | A02 | Drugs for acid‐related disorders | 1547 | (58.6) | C10 | Lipid‐modifying agents | 1319 | (60.1) |
| 2 | C10 | Lipid‐modifying agents | 1498 | (56.7) | B01 | Antithrombotic agents | 1230 | (56.1) |
| 3 | B01 | Antithrombotic agents | 1323 | (50.1) | C09 | Agents acting on the renin‐angiotensin system | 1144 | (52.1) |
| 4 | C09 | Agents acting on the renin‐angiotensin system | 1319 | (50.0) | A02 | Drugs for acid‐related disorders | 1076 | (49.1) |
| 5 | A10 | Drugs used in diabetes | 827 | (31.3) | A10 | Drugs used in diabetes | 802 | (36.6) |
| 6 | M01 | Anti‐inflammatory and antirheumatic products | 755 | (28.6) | C08 | Calcium channel blockers | 612 | (27.9) |
| 7 | C08 | Calcium channel blockers | 731 | (27.7) | N06 | Psychoanaleptics | 545 | (24.9) |
| 8 | N06 | Psychoanaleptics | 646 | (24.5) | C07 | Beta‐blocking agents | 468 | (21.3) |
| 9 | A03 | Drugs for functional gastrointestinal disorders | 575 | (21.8) | M01 | Anti‐inflammatory and antirheumatic products | 393 | (17.9) |
| 10 | C07 | Beta‐blocking agents | 499 | (18.9) | N07 | Other nervous system drugs | 367 | (16.7) |
| Hyper‐polypharmacy patients | ||||||||
| 1 | A02 | Drugs for acid‐related disorders | 751 | (85.8) | A02 | Drugs for acid‐related disorders | 421 | (80.1) |
| 2 | C10 | Lipid‐modifying agents | 627 | (71.7) | B01 | Antithrombotic agents | 382 | (72.7) |
| 3 | B01 | Antithrombotic agents | 620 | (70.8) | C10 | Lipid‐modifying agents | 378 | (72.0) |
| 4 | C09 | Agents acting on the renin‐angiotensin system | 528 | (60.3) | C09 | Agents acting on the renin‐angiotensin system | 311 | (59.3) |
| 5 | N06 | Psychoanaleptics | 426 | (48.6) | A10 | Drugs used in diabetes | 269 | (51.3) |
| 6 | A10 | Drugs used in diabetes | 419 | (47.9) | N06 | Psychoanaleptics | 259 | (49.4) |
| 7 | M01 | Anti‐inflammatory and antirheumatic products | 416 | (47.5) | A03 | Drugs for functional gastrointestinal disorders | 184 | (35.0) |
| 8 | A03 | Drugs for functional gastrointestinal disorders | 382 | (43.6) | C07 | Beta‐blocking agents | 184 | (34.9) |
| 9 | C08 | Calcium channel blockers | 314 | (35.8) | C08 | Calcium channel blockers | 182 | (34.6) |
| 10 | C07 | Beta‐blocking agents | 296 | (33.8) | M01 | Anti‐inflammatory and antirheumatic products | 181 | (34.5) |
Among elderly individuals with 90 days or more of medication use, the most frequently used therapeutic class among polypharmacy patients were drugs for acid‐related disorders (ATC A02) with 58.6%, lipid‐modifying agents (ATC C10) with 56.7%, antithrombotic agents (ATC B01) with 50.5%, and agents acting on the renin‐angiotensin system (ATC C09) with 50%. Among hyper‐polypharmacy patients, the most‐prescribed therapeutic class was drugs for acid‐related disorders (ATC A02), which were prescribed to 85.8% of these patients, followed by lipid‐modifying agents (ATC C10) with 71.7%, antithrombotic agents (ATC B01) with 70.8%, and agents acting on the renin‐angiotensin system (ATC C09) with 60.3%.
Among elderly individuals with 180 days or more of medication use, the most frequently used therapeutic class among polypharmacy patients was lipid‐modifying agents (ATC C10) with 60.1%, antithrombotic agents (ATC B01) with 56.1%, and agents acting on the renin‐angiotensin system (ATC C09) with 52.1%. However, among hyper‐polypharmacy patients, the most‐prescribed therapeutic class was drugs for acid‐related disorders (ATC A02), which were prescribed to 80.1% of these patients, followed by antithrombotic agents (ATC B01) with 72.7%, and lipid‐modifying agents (ATC C10) with 72%.
DISCUSSION
Rapid population aging has been accompanied by an increase in comorbidities among the elderly. Polypharmacy frequently occurs in the treatment of patients with comorbidities, and concerns have been raised regarding adverse health outcomes due to polypharmacy. According to several studies, including clinical trials, observational studies, and systematic reviews, polypharmacy and hyper‐polypharmacy in the elderly have been found to show negative effects on their health. 5 , 6 , 7 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 In particular, several systematic reviews reported that polypharmacy and excessive polypharmacy (the use of 10 or more medications) was associated with adverse outcomes, such as all‐cause mortality, frailty, hospitalization, and 3‐month readmission. 5 , 7 , 8 , 26 , 30 , 31 , 34 , 39 , 40 , 41 , 42 , 43 , 44 , 45 Therefore, there is an increasing need to manage polypharmacy in patients with multiple conditions, both in Korea and around the world. 46 , 47 , 48
To our knowledge, this is the first study to examine the determinants of polypharmacy among outpatient prescription drugs in an overall population‐based study of more than 7.36 million individuals including the entire elderly population of South Korea. We found that the prevalence of continuous polypharmacy (≥90 days or more and ≥180 days or more) was 41.9% and 38%, while that of continuous hyper‐polypharmacy was 13.9% and 9.1%, respectively.
This study investigated factors associated with polypharmacy and hyper‐polypharmacy compared to non‐polypharmacy. We found associations for several factors (male, age, insurance type, the presence of chronic diseases, the presence of admission or ED visits, and number of physician encounters ≥30 days) with the likelihood of polypharmacy and hyper‐polypharmacy. Patients with cardio‐cerebrovascular diseases, diabetes mellitus, chronic kidney disease, depressive disorder, and dementia were more likely to have continuous polypharmacy or hyper‐polypharmacy than those without these diseases. In elderly patients with medication use for 180 days or more, while the effects of other factors (sex, age, type of health insurance, ECI score, and physician encounters) were similar, hyperlipidemia and gastric ulcers/GERD were associated with a lower likelihood of polypharmacy and hyper‐polypharmacy. The current results are consistent with previous studies reporting that polypharmacy was associated with male sex, 49 older age, the presence of a chronic health condition, 50 , 51 contact with a general practitioner, 49 , 52 , 53 , 54 and hospitalization during the study period. 49
We also examined the most frequently prescribed therapeutic categories classified according to ATC level 2 and compared frequently prescribed medicines among elderly patients with continuous polypharmacy. This study also found that the most used medications were cardiovascular drugs, antiulcer drugs, drugs acting on the nervous system, and pain‐related drugs; these findings are consistent with previous studies. Although the subject medications were limited to oral medicines, the most prescribed medicines included drugs for diabetes mellitus, unlike previous studies.
A previous study conducted in Spain reported that the most commonly used medications were for blood pressure (51.6%), pain (42.8%), and cholesterol (28.2%), 55 and another study from Finland showed that the most commonly used drugs were cardiovascular drugs and analgesics, and the use of psychotropics was markedly higher in the polypharmacy group. 56 A study from Japan showed the most used drug categories were edema/heart failure/atrial fibrillation‐related drugs, insomnia/anxiety‐related drugs, pain‐related drugs, lifestyle disease‐related drugs, and dementia‐related drugs. 49 In Korea, payments are based on a fee‐for‐service system, which often results in overlapping prescriptions of digestives to prevent gastrointestinal disorders due to physicians' prescribing patterns, and a study from Colombia also found the most used drugs were antiulcer medications. 57 It is noteworthy that the prescriptions of gastrointestinal drugs also increased along with the increase in polypharmacy. However, compared to the prevalence of hyperlipidemia (36%), the prevalence of gastric ulcers was lower (14%–19%). Furthermore, when considering that treatment is prescribed for a short period, the use of gastrointestinal drugs was quite high.
This is the first research to investigate the determinants of polypharmacy and hyper‐polypharmacy in the overall elderly population from South Korea. Korea's NHI covers all healthcare utilization in all medical facilities on a fee‐for‐service basis. We used large‐population data to obtain generalizable results. Secondly, the current study is significant in that it covered the entire elderly population from the elderly living in long‐term care facilities to the healthy elderly. Previous studies investigating factors associated with polypharmacy in the elderly were limited to the elderly in home‐care patients, 58 long‐term care facilities, 59 or frail elderly subjects, 54 and used survey data 52 , 60 , 61 , 62 or sample data. 63 Thirdly, this study attempted to define continuous polypharmacy as ≥90 days or more and ≥180 days or more, and adjusted for the influence of chronic medication use as much as possible by performing subgroup analyses of patients with medication use for 90 days or more and 180 days or more. Fourthly, this study classified disease‐related factors into (1) presence or absence of medical conditions during the observation period (from the index date to 1 year after the index date) and (2) history of diseases, which was calculated with the ECI score using all previous diseases from 1 year before the index date. Lastly, we examined healthcare utilization in terms of admissions, ED visits, and outpatient physician visits.
However, our study has the following limitations. We were limited to outpatient prescriptions and oral medications in the analysis, therefore the medication use might have been underestimated because drugs administered during hospitalization were not included. Additionally, if cancer patients admitted in hospitals did not utilize outpatient care, they were not included in this study. For this reason, cancer was associated with a lower likelihood of polypharmacy, which is inconsistent with previous studies reporting that polypharmacy was higher among cancer survivors than among non‐cancer patients. 64 , 65 Secondly, although we tried to adjust for confounding variables that are measurable in the claims database, there was a limitation in terms of internal validity in that unmeasurable variables such as clinical risk could not be included. Thirdly, we used administrative NHI claims data; therefore, some of the prescriptions might not have actually been dispensed.
In conclusion, multimorbidity (cardio‐cerebrovascular diseases, chronic kidney disease, depressive disorder, and dementia) was found to be associated with higher odds of continuous polypharmacy, while cancer and liver disease were associated with a lower likelihood of polypharmacy, compared to those without these conditions. The number of outpatient visits (≥30 days) influenced polypharmacy more strongly than hospitalizations and ED visits.
Policies should be put in place to coordinate pharmacy care between different doctors' healthcare services to reduce polypharmacy in the elderly. Lastly, further research should examine the adverse outcomes associated with polypharmacy among patients with multiple morbidities. It is also necessary to develop interventions to reduce inappropriate polypharmacy, including comprehensive medication reviews and deprescribing algorithms that target factors affecting polypharmacy, such as multimorbidity.
AUTHOR CONTRIBUTIONS
H.J.C. and D.‐S.K. wrote the manuscript. D.‐S.K. designed the research. D.‐S.K., J.C., and S.‐H.Y. performed the research. H.J.C. analyzed the data.
FUNDING INFORMATION
No funding was received for this work.
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
DATA AVAILABILTY STATEMENT
The datasets presented in this article are not readily available because access to the dataset is limited. Request to access the datasets should be directed to our institution.
ETHICS STATEMENT
The studies involving human participants were reviewed and approved by the Institutional Review Board by Health Insurance Review and Assessment Service (HIRA). Written informed consent for participation was not required for this study in accordance with national legislation.
Supporting information
Table S1
Cho HJ, Chae J, Yoon S‐H, Kim D‐S. Factors related to polypharmacy and hyper‐polypharmacy for the elderly: A nationwide cohort study using National Health Insurance data in South Korea. Clin Transl Sci. 2023;16:193‐205. doi: 10.1111/cts.13438
REFERENCES
- 1. World Health Organization (WHO) . A Glossary of Terms for Community Health Care and Services for Older Persons. WHO Centre for Health Development; 2004. [Google Scholar]
- 2. World Health Organization (WHO) . Medication Safety in Polypharmacy. WHO Document Production Service; 2019. [Google Scholar]
- 3. Guillot J, Maumus‐Robert S, Bezin J. Polypharmacy: a general review of definitions, descriptions and determinants. Therapie. 2020;75:407‐416. [DOI] [PubMed] [Google Scholar]
- 4. Masnoon N, Shakib S, Kalisch‐Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davies LE, Spiers G, Kingston A, Todd A, Adamson J, Hanratty B. Adverse outcomes of polypharmacy in older people: systematic review of reviews. J Am Med Dir Assoc. 2020;21:181‐187. [DOI] [PubMed] [Google Scholar]
- 6. Khezrian M, McNeil CJ, Murray AD, Myint PK. An overview of prevalence, determinants and health outcomes of polypharmacy. Ther Adv Drug Saf. 2020;11:2042098620933741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leelakanok N, Holcombe AL, Lund BC, Gu X, Schweizer ML. Association between polypharmacy and death: a systematic review and meta‐analysis. J Am Pharm Assoc. 2017;57(729–38):e10. [DOI] [PubMed] [Google Scholar]
- 8. Masnoon N, Shakib S, Kalisch‐Ellett L, Caughey GE. Tools for assessment of the appropriateness of prescribing and association with patient‐related outcomes: a systematic review. Drugs Aging. 2018;35:43‐60. [DOI] [PubMed] [Google Scholar]
- 9. Zaninotto P, Huang YT, Di Gessa G, Abell J, Lassale C, Steptoe A. Polypharmacy is a risk factor for hospital admission due to a fall: evidence from the English longitudinal study of ageing. BMC Public Health. 2020;20:1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Menditto E, Gimeno Miguel A, Moreno Juste A, et al. Patterns of multimorbidity and polypharmacy in young and adult population: systematic associations among chronic diseases and drugs using factor analysis. PloS One. 2019;14:e0210701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rawle MJ, Richards M, Davis D, Kuh D. The prevalence and determinants of polypharmacy at age 69: a British birth cohort study. BMC Geriatr. 2018;18:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castioni J, Marques‐Vidal P, Abolhassani N, Vollenweider P, Waeber G. Prevalence and determinants of polypharmacy in Switzerland: data from the CoLaus study. BMC Health Serv Res. 2017;17:840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abolhassani N, Castioni J, Marques‐Vidal P, Vollenweider P, Waeber G. Determinants of change in polypharmacy status in Switzerland: the population‐based CoLaus study. Eur J Clin Pharmacol. 2017;73:1187‐1194. [DOI] [PubMed] [Google Scholar]
- 14. O'Dwyer M, Peklar J, McCallion P, McCarron M, Henman MC. Factors associated with polypharmacy and excessive polypharmacy in older people with intellectual disability differ from the general population: a cross‐sectional observational nationwide study. BMJ Open. 2016;6:e010505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rasu RS, Iqbal M, Hanifi S, et al. Level, pattern, and determinants of polypharmacy and inappropriate use of medications by village doctors in a rural area of Bangladesh. ClinicoEcon Outcomes Res. 2014;6:515‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin RP. Polypharmacy as a risk factor in the treatment of type 2 diabetes. Diabetes Spectr. 2006;19:13‐16. [Google Scholar]
- 17. Monane M, Monane S, Semla T. Optimal medication use in elders. Key to successful aging. West J Med. 1997;167:233‐237. [PMC free article] [PubMed] [Google Scholar]
- 18. Office KNS. Senior Citizen Statistics Data in 2020. Korea National Statistics Office; 2020. [Google Scholar]
- 19. Park D, Lee H, Kim DS. High‐cost users of prescription drugs: National Health Insurance Data from South Korea. J Gen Intern Med. 2022;37:2390‐2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cho DY, Park J, Kim DS. The impact of expanding health insurance coverage for anti‐cancer drugs on cancer survival in Korea. Cancer Med. 2021;10:4555‐4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization Collaborating Center (WHOCC) . Anatomical Therapeutic Chemical (ATC)/Defined Daily Dose (DDD) Index. WHOCC; 2020. [Google Scholar]
- 22. Wastesson JW, Morin L, Tan ECK, Johnell K. An update on the clinical consequences of polypharmacy in older adults: a narrative review. Expert Opin Drug Saf. 2018;17:1185‐1196. [DOI] [PubMed] [Google Scholar]
- 23. Proietti M, Raparelli V, Olshansky B, Lip GY. Polypharmacy and major adverse events in atrial fibrillation: observations from the AFFIRM trial. Clin Res Cardiol. 2016;105:412‐420. [DOI] [PubMed] [Google Scholar]
- 24. Sganga F, Landi F, Ruggiero C, et al. Polypharmacy and health outcomes among older adults discharged from hospital: results from the CRIME study. Geriatr Gerontol Int. 2015;15:141‐146. [DOI] [PubMed] [Google Scholar]
- 25. Khezrian M, McNeil CJ, Myint PK, Murray AD. The association between polypharmacy and late life deficits in cognitive, physical and emotional capability: a cohort study. Int J Clin Pharm. 2019;41:251‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leelakanok N, D'Cunha RR. Association between polypharmacy and dementia ‐ a systematic review and metaanalysis. Aging Ment Health. 2019;23:932‐941. [DOI] [PubMed] [Google Scholar]
- 27. Lim LM, McStea M, Chung WW, et al. Prevalence, risk factors and health outcomes associated with polypharmacy among urban community‐dwelling older adults in multi‐ethnic Malaysia. PloS One. 2017;12:e0173466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Midao L, Brochado P, Almada M, Duarte M, Paul C, Costa E. Frailty status and polypharmacy predict all‐cause mortality in community dwelling older adults in Europe. Int J Environ Res Public Health. 2021;18:3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fabbietti P, Di Stefano G, Moresi R, et al. Impact of potentially inappropriate medications and polypharmacy on 3‐month readmission among older patients discharged from acute care hospital: a prospective study. Aging Clin Exp Res. 2018;30:977‐984. [DOI] [PubMed] [Google Scholar]
- 30. Fried TR, O'Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community‐dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62:2261‐2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gallagher C, Nyfort‐Hansen K, Rowett D, et al. Polypharmacy and health outcomes in atrial fibrillation: a systematic review and meta‐analysis. Open Heart. 2020;7:e001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herr M, Robine JM, Pinot J, Arvieu JJ, Ankri J. Polypharmacy and frailty: prevalence, relationship, and impact on mortality in a French sample of 2350 old people. Pharmacoepidemiol Drug Saf. 2015;24:637‐646. [DOI] [PubMed] [Google Scholar]
- 33. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palmer K, Villani ER, Vetrano DL, et al. Association of polypharmacy and hyperpolypharmacy with frailty states: a systematic review and meta‐analysis. Eur Geriatr Med. 2019;10:9‐36. [DOI] [PubMed] [Google Scholar]
- 35. Pan HH, Li CY, Chen TJ, Su TP, Wang KY. Association of polypharmacy with fall‐related fractures in older Taiwanese people: age‐ and gender‐specific analyses. BMJ Open. 2014;4:e004428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pazan F, Wehling M. Polypharmacy in older adults: a narrative review of definitions, epidemiology and consequences. Eur Geriatrc Med. 2021;12:443‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosted E, Schultz M, Sanders S. Frailty and polypharmacy in elderly patients are associated with a high readmission risk. Dan Med J. 2016;63:A5274. [PubMed] [Google Scholar]
- 38. Turnbull AJ, Donaghy E, Salisbury L, et al. Polypharmacy and emergency readmission to hospital after critical illness: a population‐level cohort study. Br J Anaesth. 2021;126:415‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Al‐Musawe L, Martins AP, Raposo JF, Torre C. The association between polypharmacy and adverse health consequences in elderly type 2 diabetes mellitus patients; a systematic review and meta‐analysis. Diabetes Res Clin Pract. 2019;155:107804. [DOI] [PubMed] [Google Scholar]
- 40. Anees Ur R, Ahmad Hassali MA, Muhammad SA, et al. The economic burden of chronic obstructive pulmonary disease (COPD) in the USA, Europe, and Asia: results from a systematic review of the literature. Expert Rev Pharmacoecon Outcomes Res. 2020;20:661‐672. [DOI] [PubMed] [Google Scholar]
- 41. Chen LJ, Trares K, Laetsch DC, Nguyen TNM, Brenner H, Schottker B. Systematic review and meta‐analysis on the associations of polypharmacy and potentially inappropriate medication with adverse outcomes in older cancer patients. J Gerontol A Biol Sci Med Sci. 2021;76:1044‐1052. [DOI] [PubMed] [Google Scholar]
- 42. Gutierrez‐Valencia M, Izquierdo M, Cesari M, Casas‐Herrero A, Inzitari M, Martinez‐Velilla N. The relationship between frailty and polypharmacy in older people: a systematic review. Br J Clin Pharmacol. 2018;84:1432‐1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mohamed MR, Ramsdale E, Loh KP, et al. Associations of polypharmacy and inappropriate medications with adverse outcomes in older adults with cancer: a systematic review and meta‐analysis. Oncologist. 2020;25:e94‐e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Okpechi IG, Tinwala MM, Muneer S, et al. Prevalence of polypharmacy and associated adverse health outcomes in adult patients with chronic kidney disease: protocol for a systematic review and meta‐analysis. Syst Rev. 2021;10:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rehman AU, Hassali MAA, Muhammad SA, Harun SN, Shah S, Abbas S. The economic burden of chronic obstructive pulmonary disease (COPD) in Europe: results from a systematic review of the literature. Eur J Health Econ. 2020;21:181‐194. [DOI] [PubMed] [Google Scholar]
- 46. Anderson LJ, Schnipper JL, Nuckols TK, et al. A systematic overview of systematic reviews evaluating interventions addressing polypharmacy. Am J Health Syst Pharm. 2019;76:1777‐1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rankin A, Cadogan CA, Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2018;9:CD008165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Urfer M, Elzi L, Dell‐Kuster S, Bassetti S. Intervention to improve appropriate prescribing and reduce polypharmacy in elderly patients admitted to an internal medicine unit. PloS One. 2016;11:e0166359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ishizaki T, Mitsutake S, Hamada S, et al. Drug prescription patterns and factors associated with polypharmacy in >1 million older adults in Tokyo. Geriatr Gerontol Int. 2020;20:304‐311. [DOI] [PubMed] [Google Scholar]
- 50. Slater N, White S, Venables R, Frisher M. Factors associated with polypharmacy in primary care: a cross‐sectional analysis of data from the English longitudinal study of ageing (ELSA). BMJ Open. 2018;8:e020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nobili A, Licata G, Salerno F, et al. Polypharmacy, length of hospital stay, and in‐hospital mortality among elderly patients in internal medicine wards. The REPOSI study. Eur J Clin Pharmacol. 2011;67:507‐519. [DOI] [PubMed] [Google Scholar]
- 52. Walckiers D, Van der Heyden J, Tafforeau J. Factors associated with excessive polypharmacy in older people. Arch Public Health. 2015;73:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007;5:345‐351. [DOI] [PubMed] [Google Scholar]
- 54. Chan DC, Hao YT, Wu SC. Characteristics of outpatient prescriptions for frail Taiwanese elders with long‐term care needs. Pharmacoepidemiol Drug Saf. 2009;18:327‐334. [DOI] [PubMed] [Google Scholar]
- 55. Carmona‐Torres JM, Cobo‐Cuenca AI, Recio‐Andrade B, Laredo‐Aguilera JA, Martins MM, Rodriguez‐Borrego MA. Prevalence and factors associated with polypharmacy in the older people: 2006–2014. J Clin Nurs. 2018;27:2942‐2952. [DOI] [PubMed] [Google Scholar]
- 56. Jyrkka J, Enlund H, Korhonen MJ, Sulkava R, Hartikainen S. Patterns of drug use and factors associated with polypharmacy and excessive polypharmacy in elderly persons: results of the Kuopio 75+ study: a cross‐sectional analysis. Drugs Aging. 2009;26:493‐503. [DOI] [PubMed] [Google Scholar]
- 57. Castro‐Rodriguez A, Machado‐Duque ME, Gaviria‐Mendoza A, Medina‐Morales DA, Alvarez‐Vera T, Machado‐Alba JE. Factors related to excessive polypharmacy (≥15 medications) in an outpatient population from Colombia. Int J Clin Pract. 2018;e13278. [DOI] [PubMed] [Google Scholar]
- 58. Komiya H, Umegaki H, Asai A, et al. Factors associated with polypharmacy in elderly home‐care patients. Geriatr Gerontol Int. 2018;18:33‐41. [DOI] [PubMed] [Google Scholar]
- 59. Jokanovic N, Tan EC, Dooley MJ, Kirkpatrick CM, Bell JS. Prevalence and factors associated with polypharmacy in long‐term care facilities: a systematic review. J Am Med Dir Assoc. 2015;16(535):e1‐e12. [DOI] [PubMed] [Google Scholar]
- 60. Gutierrez‐Valencia M, Aldaz Herce P, Lacalle‐Fabo E, Contreras Escamez B, Cedeno‐Veloz B, Martinez‐Velilla N. Prevalence of polypharmacy and associated factors in older adults in Spain: data from the National Health Survey 2017. Med Clin (Barc). 2019;153:141‐150. [DOI] [PubMed] [Google Scholar]
- 61. Ie K, Felton M, Springer S, Wilson SA, Albert SM. Physician factors associated with polypharmacy and potentially inappropriate medication use. J Am Board Fam Med. 2017;30:528‐536. [DOI] [PubMed] [Google Scholar]
- 62. Niclos G, Olivar T, Rodilla V. A cross‐sectional evaluation of the prevalence and detection of predictors of polypharmacy amongst adult in Spain. Int J Pharm Pract. 2018;26:242‐249. [DOI] [PubMed] [Google Scholar]
- 63. Baek YH, Shin JY. Trends in polypharmacy over 12 years and changes in its social gradients in South Korea. PloS One. 2018;13:e0204018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hsu CD, Nichols HB, Lund JL. Polypharmacy and medication use by cancer history in a nationally representative group of adults in the USA, 2003–2014. J Cancer Surviv. 2022;16:659‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Murphy CC, Fullington HM, Alvarez CA, et al. Polypharmacy and patterns of prescription medication use among cancer survivors. Cancer. 2018;124:2850‐2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


