Significance
Practicing mindfulness helps individuals regulate attention, thoughts, feelings, and behavior. In recognizing these benefits, various schools, workplaces, and clinics are increasingly teaching mindfulness. How does mindful attention change brain function to support self-regulation? Addressing this question could inform how we teach mindfulness and whom we expect to benefit. We modeled the defining components of mindful experience using tools that probe the structure and function of the brain’s network. In a randomized controlled study of alcohol consumption, we found that a brain network’s dynamic shape predicts individuals’ future alcohol consumption and explains otherwise elusive components of mindful experience, such as being present. Our results provide new understanding of how mindful attention affects brain function.
Keywords: mindfulness, network control theory, neural intrinsic timescale, brain network dynamics
Abstract
Mindful attention is characterized by acknowledging the present experience as a transient mental event. Early stages of mindfulness practice may require greater neural effort for later efficiency. Early effort may self-regulate behavior and focalize the present, but this understanding lacks a computational explanation. Here we used network control theory as a model of how external control inputs—operationalizing effort—distribute changes in neural activity evoked during mindful attention across the white matter network. We hypothesized that individuals with greater network controllability, thereby efficiently distributing control inputs, effectively self-regulate behavior. We further hypothesized that brain regions that utilize greater control input exhibit shorter intrinsic timescales of neural activity. Shorter timescales characterize quickly discontinuing past processing to focalize the present. We tested these hypotheses in a randomized controlled study that primed participants to either mindfully respond or naturally react to alcohol cues during fMRI and administered text reminders and measurements of alcohol consumption during 4 wk postscan. We found that participants with greater network controllability moderated alcohol consumption. Mindful regulation of alcohol cues, compared to one’s own natural reactions, reduced craving, but craving did not differ from the baseline group. Mindful regulation of alcohol cues, compared to the natural reactions of the baseline group, involved more-effortful control of neural dynamics across cognitive control and attention subnetworks. This effort persisted in the natural reactions of the mindful group compared to the baseline group. More-effortful neural states had shorter timescales than less effortful states, offering an explanation for how mindful attention promotes being present.
When people direct efforts toward achieving their goals, they engage in self-regulation (1–3). Mindfulness is an increasingly popular strategy to direct efforts toward educational, work, and health goals (4–12). Key to many forms of mindfulness is redirecting efforts by observing mental events as transient, or “being present” (13–27). When people focus their attention on the present, they increase psychological distance from sensations, thoughts, and emotions by recognizing them as passing mental events (28–30). Mental events are interpretations or appraisals of external stimuli that are not veridical representations of reality. In contrast to mindful attention, people often habitually react to events and prolong their influence through mind wandering and self-referential thoughts, expectations, and emotions (31, 32).
Enhancing mindful attention through practice is thought to help one notice and discontinue moments of mind wandering and self-referential thoughts (32–39). Discontinuing self-referential thoughts relates to psychological self-distancing from the socioemotional content of past experiences and imagined futures, ceasing to automatically associate them with the present experience (29, 34, 40–45). Reduced self-referential processes are associated with suspending neural activity in the default mode network, including the posterior cingulate cortex (PCC), precuneus, and medial prefrontal cortex. One putative mechanism by which people transition from novice to expert meditators is by decreasing activity in their precuneus and PCC (36, 42, 46, 47), regions associated with how people perceive time’s passage and experience their sense of self (48–51).
The brain may reduce default-mode activity during tasks, such as mindful attention, by biasing activity with a system of regions associated with cognitive control, attention, and emotion (12, 52–55). These regions include frontoparietal, amygdalar, thalamic, insular, dorsolateral prefrontal, and anterior cingulate regions (18, 56–62). They tend to coactivate when people exercise executive function, a capacity to shift or sustain their attention and working memory to achieve goals by way of selecting relevant actions, monitoring ongoing activity, and reviewing outcomes. Mindfulness also involves a capacity to shift or sustain attention, here directed to salient information on the internal sensations from the body and on the external world (55, 63–68). This engagement and disengagement of attention allows a mental stance characterized by nonreaction, sometimes called nonelaborative or nonjudgmental processing. Prior work suggests that this nonreactive mental stance is related to psychological distancing through “defusion” or “decentering” from one’s emotional experience, ultimately supporting emotion regulation (28, 29). For example, mindfully attending to interfering sources of anxiety and craving may help one down-regulate those affective states (44, 69–71). Taken together, these regional (de)activations and their functions suggest a putative neural process. As people practice mindfulness, the brain may redirect neural activity and effort from default-mode regions to cooperating attention and frontoparietal regions as a nonlinear function of the amount of practice (16, 25, 72–74), similar to other types of learning (75–78). Redirecting cognitive resources to attention and executive function is consistent with deautomatizing emotional and cognitive reactions that have become habitual and spontaneous (29, 79).
We build upon this progress by developing a dynamical systems perspective of how mindful attention may function to redirect effort, discontinue transient events, and evolve nonlinearly with expertise. To explain these functions, we seek a dynamical systems model that produces them based on how system-wide neural activity changes spontaneously and during mindful attention. Neural activity changes spontaneously yet predictably, with constraints from the brain’s white matter structure and a person’s behavior. Network control theory offers a dynamical systems model of the time-dependent control of changes in system activity (80). When applied to neural systems, brain networks are modeled as nodes that represent regions with a given level of activity and edges that represent structural white matter connections with a given level of activity flow. As in prior work, we use this theory to determine the control input needed for a brain network to direct and sustain the system-wide patterns of activity evoked by behavior, such as mindful attention (81–86). Prior work has demonstrated that control inputs relate to the effects of external stimulation and metabolic energy (82, 87, 88). Here, by contrast, we use network control theory to posit dynamical measures of the effort and transience of neural states.
We operationalize effort in terms of the amount of control input required to execute a neural transition (84, 89, 90). To protect the transition from the interference of spontaneous activity, the brain requires control input to change neural activity (86). Control inputs characterize effort in that they are positively correlated with working memory load and modulated by dopaminergic signaling (90), a neurotransmitter that encodes information about a task’s cognitive demand and expected benefit (91, 92). Since the diversity of possible state transitions depends on structural connectivity (93, 94), here we complement our study of control inputs with a study of average controllability, which quantifies the capacity of a unit of control input into each brain region to drive activity to new hypothetical states (89). This statistic is also relevant to the study of effort, as it is positively correlated with activity during tasks that probe executive function (95). Hence, we operationalize effort as increased control input, and we assess the efficient utilization of control input using average controllability.
In addition to effort and efficient utilization, we are also interested in the stability versus transience of neural events. By estimating the amount of control input needed to persist in a given neural state (86), we suggest that more “energetic” or costly states are more likely to change to different states (90), consistent with constraints on the expenditure of limited resources (96, 97). A region’s activity spontaneously changes to a different neural state when it discontinues past activity by updating it to the present state. Accordingly, when regions are quicker to alter activity to different neural states, we call that region more present focused. We measure how quickly a region’s activity updates to a different (uncorrelated) state as the intrinsic neural timescale (32, 98, 99). Following prior work, the intrinsic neural timescale is a function of ongoing, spontaneous brain activity not restricted to only task-responsive regions (99), necessitating resting-state fMRI. Using these calculations, we operationalize the transience of neural events as decreased control stability and faster intrinsic neural timescales.
Here we investigate the neural dynamics of mindful attention in a sample of 76 college students, using fMRI acquired during resting state and in response to an alcohol cue reactivity task. The inclusion of the cue reactivity task was motivated by recent work demonstrating that paying mindful attention to daily cravings moderated drinking behavior (30). College students consume more alcohol than peers who do not attend college—leading to negative academic, social, and legal outcomes (100). Practicing mindfulness may help an individual to self-regulate drinking behavior (2, 101–104). In our study, participants were randomized into either an experimental group assigned to practice mindful attention or a baseline group assigned to react naturally. For the mindful attention group, we briefly trained participants to induce mindful attention in an fMRI task that included alcohol cues as well as text reminders over the subsequent 4 wk (SI Appendix, Supplementary Methods, adapted from ref. 30). Using task fMRI, we measure the effort and stability of neural responses evoked by mindful attention compared to natural reactions. In the fMRI task, the mindful attention group was instructed to react to images of alcohol either mindfully or naturally, whereas the baseline group was only instructed to react naturally (Fig. 1). Using these data, we simulate a putative neural mechanism that involves suspending the influence of the default-mode network’s precuneus and PCC activity on ongoing network dynamics (36, 42, 46). Using ecological momentary assessments and text reminders, we measured self-regulated drinking behavior over the 4 wk postscan, without explicitly instructing participants to moderate alcohol consumption. Finally, we evaluate how the effort and stability of brain activity over time relates to its intrinsic neural timescale.
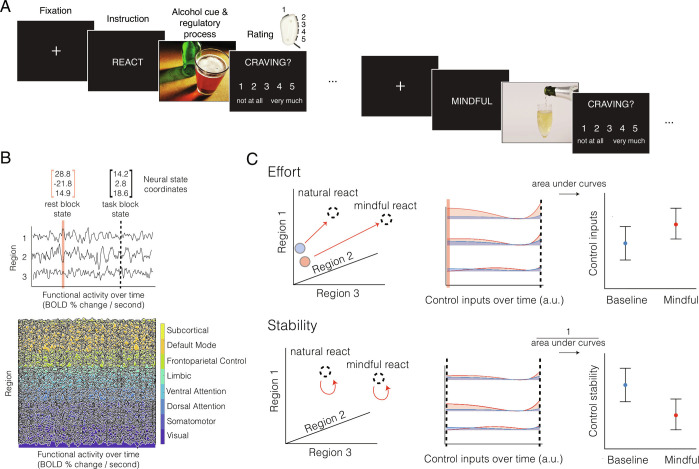
Fig. 1.
Control of brain network dynamics for regulatory strategies and behavior. (A) A schematic of the fMRI task. During mindful trials, participants were instructed to “mentally distance themselves by observing the situation and their response to it with a more impartial, nonjudgmental, or curious mindset, and without getting caught up in the situation or response.” During natural reaction trials, participants were instructed to “simply look and respond according to initial gut reactions, without thinking of anything in particular.” The task included 96 total trials arranged in blocks of four with the same regulatory strategy. We were interested in the effort and stability of neural states during the mindful regulatory process compared to the natural reaction. Images were used from the Galician Beverage Picture Set (105). (B) The conceptual schematic depicts a simplified brain with only three regions to explain the intuition underpinning the control metrics of effort and stability. The measured fMRI activity time course of these three regions defines only one of many possible activity trajectories that those regions could have produced. We use network control theory to calculate the control inputs required to steer functional states from an initial state (pink line) propagating according to simulated linear dynamics atop the structural connectome toward a target state (dashed black line). We apply the same framework to analyze the full time course of 400 cortical and 14 subcortical regions. A neural state is defined as a single instance of activity across all 414 brain regions, measured by repeated scan acquisitions. All neural states measured across time compose the trajectory of the neural dynamics. (C) Here we depict two of the main hypotheses regarding the effort and stability of mindful states. In keeping with the simplified three-region system, the system’s state can be visualized as a coordinate in three-dimensional space. To determine the effort of neural dynamics, we calculated the optimal control inputs required to transition 1) from the baseline to the natural reaction state, and 2) from the baseline to the mindful reaction state. To determine the stability of neural dynamics, we calculated the optimal control inputs required to sustain the natural reaction state and sustain the mindful reaction state. We then compared the control input and stability between the baseline and mindful conditions.
We test four main hypotheses connecting the effort and stability of neural activity to self-regulation and mindful attention. First, we hypothesize that the average controllability of the whole structural network positively correlates with later moderation of alcohol consumption. Increased average controllability suggests more efficient usage of control inputs. Second, we hypothesize that mindful attention demands greater control input than exclusively naturally reacting, and that natural reactions interspersed with mindful attention demand more control input than exclusively naturally reacting. Increased control input is characteristic of effortfully deautomatizing habitual reactions, a process that may gain efficiency by requiring reduced control input with expertise. Third, we hypothesize that the neural states of the mindful group would exhibit more control instability than those of the baseline group. Control instability suggests a drive to cease costly neural states of mindful attention. Fourth, we hypothesize that brain regions with greater average controllability, control input, and instability would have faster intrinsic neural timescales. Faster intrinsic timescales suggest present-focused activity that quickly updates past states to the present. In testing these hypotheses, we develop a dynamical systems theory of how mindful attention redirects effort and discontinues transient neural events (14, 21, 25).
Results
Average Controllability Predicts Later Behavior Change in Alcohol Consumption.
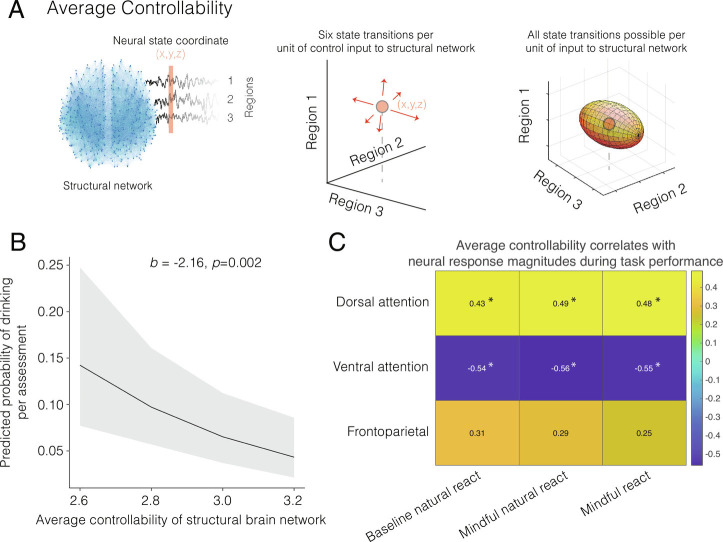
We sought to apply network control theory to investigate how mindful attention influences brain dynamics to support self-regulation (Fig. 1). Prior findings in this dataset indicate that individuals randomly assigned to the mindful attention or baseline conditions differed in their self-regulation of alcohol consumption. Specifically, mindful attention promoted moderation of alcohol consumption (30), consistent with other reports of mindful attention improving executive control and self-regulation (106). In addition to this finding of differences between groups, we first hypothesized that individuals across groups would moderate alcohol consumption by reducing drinking probability, if their structural networks afforded greater average controllability. Average controllability is a measure that characterizes the capacity of brain regions to efficiently drive activity across structural connections to any hypothetically reachable state (Fig. 2A). To model the logistic drinking probability of each individual at a given occasion, we built upon a recent report that used a multilevel model with zero inflation to account for repeated measures, individuals nested within social groups, and the nonnormally distributed drinking data with the predominance of assessments that reported zero drinking (30, 107). In this recent work, covariates were included in the model to control for the number of assessments, the number of responses to alcohol surveys, whether the day of assessment was a social week day (108), and whether individuals were exposed to mindful attention prompts that week. In testing for the effects of average controllability of the structural brain network across all participants, we additionally controlled for the baseline drinking amount or frequency in the past 6 mo, demographic variables, and the personality trait of attentional impulsivity (SI Appendix, Supplementary Methods and Fig. S1). Across both the mindful and baseline conditions, we found—with the zero-inflated model of drinking probability—that individuals with greater average controllability across all regions tended to have a lower probability of drinking per assessment beyond preexisting differences in their baseline drinking frequency (Fig. 2B; , p = 0.002). Following prior work, we found consistent results for the effect of average controllability in a sensitivity analysis that used a parsimonious model including minimal covariates (30) (SI Appendix, Fig. S2). This observation is consistent with our hypothesis and with previously reported associations between average controllability and executive function (95). The estimated neural response magnitude of mindful attention was positively correlated with the structural network’s average controllability of regions in a “dorsal attention network” ( to 0.49, Bonferroni-corrected p < 0.05) and was negatively correlated with the average controllability of regions in a “ventral attention network” ( to –0.56, Bonferroni-corrected p < 0.05; Fig. 2C). See SI Appendix, Supplementary Methods for a list of regions in these networks. There were no detected differences in average activity between groups or conditions within these networks and across the whole brain (SI Appendix, Fig. S3). Average controllability relates to the neural response magnitude evoked during states of both reacting naturally and attending mindfully to alcohol cues. In sum, the short-term induction of mindful attention and average controllability each support later behavior change in the moderation of alcohol consumption.
Fig. 2.
Average controllability predicts later behavior change in the moderation of alcohol consumption. (A) Here we visualize six possible changes in neural activity (red arrows depict change to a target state) for three brain regions. Average controllability is a measure of the capacity of the structural connectome to drive all new brain states (all target states on the surface of the ellipsoid). (B) For each individual structural brain network, we obtained an individual measure of average controllability as the mean average controllability across all brain regions. Among all individuals, structural connectivity that supported farther hypothetical neural trajectories predicted later reductions in the observed probability of consuming alcohol on a given occasion. The plot displays predicted values of the marginal effects of average controllability, using a multilevel regression model, following prior work (30). (C) The average controllability of the structural network correlates with the neural response magnitude evoked by mindful attention. Dorsal attention regions with greater average controllability tended to activate more, while ventral attention regions with greater average controllability tended to activate less. Asterisks denote significant correlations at a Bonferroni-corrected .
Mindful Attention Deautomatizes and Discontinues Neural States of Alcohol Cue Reaction.
The practice of mindfulness is typically thought to be effortful for novices (25). Here, in addition to considering all possible hypothetical trajectories by calculating the average controllability, we also sought to understand how mindful attention supports self-regulation, by comparing the specific empirical neural trajectories of the mindful group to the neural trajectories of the baseline group during the fMRI task. To investigate these neural trajectories, we calculated the control inputs that the frontoparietal control and dorsal/ventral attention subnetworks (see SI Appendix, Supplementary Methods for list of regions) should exert to drive brain function from baseline states to task states. Baseline states were defined as a neural state with zero task-related activity. Task states corresponded to the neural states of task-related activity across trials which instructed individuals in the mindful condition to react to alcohol cues mindfully, or trials which instructed them to react naturally. Task states for individuals in the baseline condition corresponded only to trials instructing them to react naturally to alcohol cues. We found that mindful attention reduced craving compared to natural reactions for the mindful attention group (, p = 0.003), but did not find a difference in average craving between groups (SI Appendix, Fig. S4). To understand how control inputs evolve with practice, we simulated an increasing down-regulation of the control inputs into the precuneus and PCC, a putative neural mechanism for the practice of mindful attention (42).
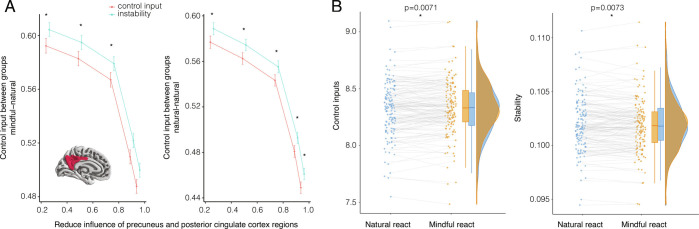
Our second hypothesis was that the deautomatizing function of mindful practice would evoke neural states that require more effort, and this effort would also persist into conditions requiring the individual to react naturally, suggesting effort related to the deautomatization of habitual reactions to alcohol. Consistent with our hypothesis, when we simulate transitions that occur with reduced influence of the precuneus and PCC, we observe that more control input is required to transition to states of mindful attention than to states of naturally reacting (Fig. 3). As people gain expertise in reducing the influence of the precuneus and PCC, they are able to transition to mindful attention and sustain it with nonlinearly decreasing control inputs. This result suggests that, as people gain expertise in this putative neural mechanism, they can exercise mindful attention with greater efficiency. Individuals who mindfully attended to cues required greater control input on trials instructing them to react naturally than individuals in the baseline condition who were instructed to exclusively react naturally (Fig. 3 A, Right). These results suggest that mindful attention is characterized by increased effort and instability compared to baseline natural reactions, an increase that can lessen with practice for more efficient and stable neural dynamics.
Fig. 3.
Mindful attention deautomatizes and discontinues neural states of reacting to alcohol cues. (A) Inset: Here we simulate a putative process by which individuals practice mindful attention by suspending the capacity of the default-mode network’s precuneus and PCC regions to influence other regions’ activity (42). Suspending default-mode network activity biases activity toward regions associated with executive control. Executive control brain circuitry is thought to comprise the frontoparietal control, dorsal attention, and ventral attention subnetworks with 145 regions. Left: While reducing the influence of precuneus and PCC regions, we found that mindful attention required more effort and was less stable for these 145 regions between baseline and mindful groups. Right: Moreover, when the mindful group interspersed mindful attention with naturally reacting, their natural reactions required more control input and were less stable than the natural reactions of the baseline group. As people transition from little to substantial reduction of the precuneus and PCC’s influence on system dynamics, differences in effort and instability decreased nonlinearly with expertise in reducing precuneus and PCC influence. Error bars depict SE of 1,000 permutations. Asterisks indicate significant differences of between-group Wilcoxon tests at a Bonferroni-corrected . (B) Within the mindful attention group, we compared mindful reactions to natural reactions when only allowing control inputs to the 145 regions using paired Wilcoxon tests. Within the mindful attention group, the neural states corresponding to mindful attention required more control input and were less stable than the neural states corresponding to natural reactions. Gray lines connect the same brain region between the trial types. Together, greater control inputs suggest greater effort, consistent with the hypothesis that mindful attention effectively supports self-regulation by deautomatizing reactions. Reduced stability of the neural states of mindful and natural reactions in the mindful condition suggest brain function that discontinues and dwells less on states of reaction.
Next, we tested the third hypothesis, that the mindful states and the natural states of the mindful group would be more unstable than the natural reactions of the baseline group. Evidence supporting this hypothesis would suggest that practice of mindful attention elicits instability, the cessation of the current costly neural state, and approach to new neural states. To test this hypothesis, we calculated the stability of the neural state as the reciprocal of the control input required to transition from the state to itself. Consistent with our hypothesis, we found that the neural states of regions in the frontoparietal control and dorsal/ventral attention subnetworks were less stable for mindful reactions than for natural reactions in the baseline condition (Fig. 3 A, Left). Reduced stability of the neural states of mindful attention suggests future discontinuation of the costly state of processing. If natural reactions of the mindful group were more unstable than natural reactions of the baseline group, then the effort of mindful regulation that persisted to natural reactions may promote cessation of attending to the alcohol cues, due to increased cost. We found that natural reactions of individuals who practiced mindful reactions were less stable than the natural reactions of individuals in the baseline condition (Fig. 3 A, Right), suggesting greater cessation of the neural states associated with attending to the alcohol cues.
We next assessed neural transitions within only the mindful group, finding further evidence consistent with our second and third hypotheses. Mindful attention required more control input than reacting naturally (Fig. 3B; paired Wilcoxon signed rank , p = 0.007), and mindful attention was more unstable than reacting naturally (, p = 0.007). While mindful attention required, on average, more control input than reacting naturally, individuals over the course of the task needed increasingly more control input to react naturally (SI Appendix, Fig. S5). This pattern of findings suggests that natural dynamics are deautomatized using control inputs during mindful regulation. The neural states associated with mindful reactions to alcohol required greater control input than the natural reactions to alcohol of the baseline condition. This effort persisted into conditions that required individuals to react naturally, suggesting a marked deautomaticity of neural dynamics. Taken together, the greater effort and deautomaticity of reactions accompany more-unstable neural states, suggestive of discontinued attendance to alcohol cues.
Deautomatizing and Discontinuing Neural States Promote the Clarity of the Past, Present, and Future during the Resting State.
In this final section, we sought to understand the functional relevance of neural dynamics that are characterized by effort and instability more broadly by investigating resting-state neural dynamics. A key goal of mindfulness is to be present with nonelaborative awareness. Similar to prior work reporting changes in baseline functional connectivity in expert meditators (34), the consistency of effortful and unstable neural processes across both mindful and natural reactions in the mindful condition suggests that mindful attention alters brain function beyond the immediate practice. This persistence of the effects of meditative training has been proposed to transform the resting-state experience to one that is more present focused (34). Here we sought to understand whether effortful and unstable neural states during resting-state fMRI across all participants were more present focused. The resting-state scan was administered prior to exposure to the mindful attention task. We also assessed whether effortful and unstable neural states during the mindful attention task tended to focalize the present.
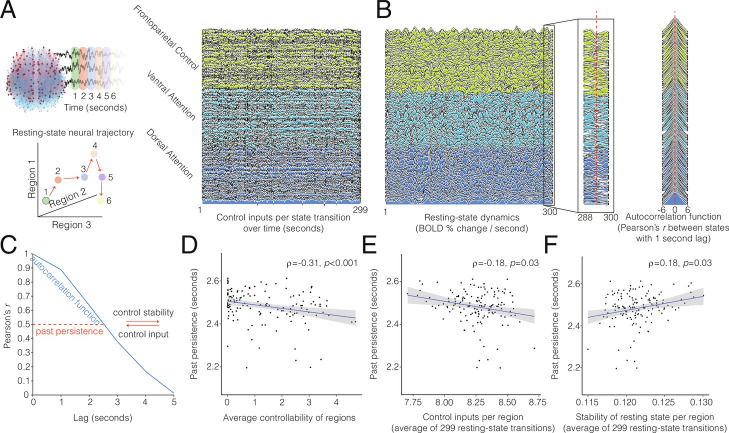
We tested our fourth hypothesis, that brain regions with greater average controllability and resting-state trajectories with greater effort and instability would have quicker intrinsic timescales consistent with present-focused activity. For each individual, we calculated the regional average controllability to assess the ease of transitioning to any hypothetical new state, the optimal control inputs to assess the cost of the empirical resting-state trajectory (Fig. 4A), and the intrinsic timescale to assess the resting-state signal persistence over time (Fig. 4B). The intrinsic timescale is the time that must elapse before the similarity between a current signal and past signal decays by half (Fig. 4C). Differences in the intrinsic timescales across brain regions form a temporal hierarchy of neural processing whereby abstract information like goals can persist over longer durations than newly attended sensorimotor and contextual information (98, 99). Here, we use the intrinsic timescale to measure the persistence of past state signals in order to operationalize “present-focused activity.” Shorter intrinsic timescales suggest present-focused activity that discontinues the past and updates the present (6, 14).
Fig. 4.
Deautomatization and discontinuation of resting-state trajectories are associated with more distinct past, present, and future states. (A) This schematic depicts the control inputs to cognitive control and attention regions that are required to transition from state to state (red arrows) as measured by the repetition time of each image in the resting-state time course. A state’s activity across all regions was defined using the blood-oxygen-level-dependent (BOLD) activity magnitude. We plot the control input values—to the executive systems per transition over time—that are theoretically required to steer all regions to a new state of activity. The resting-state is thought to characterize spontaneous brain dynamics of internally directed mind wandering and past or future prospection. These spontaneous dynamics are less constrained by external stimuli. (B and C) Although neural dynamics display substantial variability, a brain region’s neural states that occur in closer succession tend to be more similar. Conversely, states that occur farther apart tend to be more dissimilar. In addition to time between states influencing the (dis)similarity of neural states, control inputs could discontinue past states to update the present state. A primary aim of mindfulness is to cultivate a nonelaborative awareness of present experience and perceptual clarity of this awareness. We hypothesized that transitioning to different states, in general, may be related to discontinuing past states and updating the present state. More precisely, we hypothesized that brain regions with greater average controllability and resting-state trajectories with greater effort and instability would have quicker intrinsic timescales, reflecting present-focused activity. The intrinsic timescale of a neural signal quantifies how long it takes for the signal at any given state to decay, regardless of particular content or mental object of the signal. The quicker a signal decays according to calculated temporal autocorrelation functions per region, the less similar one state is from the next, and the less the past state persists into the present. (D) Regions with greater average controllability for farther trajectories tended to exhibit fast-decaying and dissimilar states. (E) Regions exerting greater control on brain function tended to have fast-decaying and dissimilar dynamics. (F) Regions with less stable and more-discontinuing states tended to exhibit fast-decaying and dissimilar dynamics. Together, neural dynamics of cognitive control and attention networks that are characterized by deautomatization and discontinuation relate to neural trajectories with more-distinct past, present, and future neural states. Analyzing task activity, we found similar results that relate faster intrinsic neural timescales to the effort and instability of mindful attention (SI Appendix, Fig. S5).
Using these metrics, we first evaluated whether the ease of transitioning to any hypothetical state promotes neural dynamics that are more likely to evolve across states which are dissimilar. We found that regions of the frontoparietal control and dorsal/ventral attention subnetworks with higher average controllability tended to have dynamics with more-dissimilar states and faster decay (Fig. 4D; , p < 0.001). This observation suggests that average controllability and executive function might support regulatory processing through present-focused resting-state dynamics. Next, we assessed whether regions with resting-state trajectories that demand the structural network to exert greater control input per state transition will drive dynamics to more-dissimilar and quickly decaying states. Consistent with this hypothesis, we found that regions which required greater control input to execute resting-state trajectories tended to have more-dissimilar dynamics with quickly decaying states (Fig. 4E; , p = 0.03), suggesting greater control inputs tended to drive quicker transitions to farther states. Lastly, we evaluated whether regions with greater control stability for sustained neural states would have slower decay and more-similar dynamics than regions with lesser control stability. Consistent with this prediction, we found that regions with greater stability tended to have more-similar dynamics with slower decaying states (Fig. 4F; , p = 0.03). This finding provides evidence that the reduced control inputs required to sustain the same state are related to the temporal stability of that state (90, 109). Analyzing task activity, we found consonant results relating faster intrinsic neural timescales to effort and instability of mindful attention (SI Appendix, Fig. S5). These results suggest that present-focused activity is related to the structural controllability and functional control associated with executive function and mindful regulation.
Discussion
In summary, our findings suggest that mindful attention and executive control resources support the regulation of alcohol consumption by biasing the network control of neural dynamics that are more deautomatized, discontinuing, and present focused. Consistent with our first hypothesis, average controllability predicted later moderation of alcohol consumption regardless of whether the participant was randomized into the mindful or baseline condition. This result parallels prior work reporting associations between average controllability and both executive function (95) and emotion regulation (110). Regions with high average controllability can more efficiently utilize control inputs for network dynamics across multiple cognitive domains (86), are heritable (111), and are overrepresented in the default-mode network (89). Consistent with our second and third hypotheses, mindful attention tended to require greater control input and was more unstable compared to naturally reacting. Compared to baseline participants who exclusively reacted naturally, this increased control input and instability persisted for the mindful group’s participants whose natural reactions were interspersed with mindful attention. This increased control input suggests a learned effort to deautomatize and discontinue habitual reactions over time (SI Appendix, Fig. S5). This pattern of findings may also suggest that participants responded more indiscriminately between instructed moments of mindful attention and reacting naturally, consistent with previous work on psychological distancing and reappraisal of emotions becoming more automatic and less effortful (60, 112). Finally, consistent with our fourth hypothesis, brain regions that could (greater average controllability) or did (greater control input) steer the neural trajectory to new and farther states were associated with faster intrinsic timescales (SI Appendix, Fig. S5). These findings are consistent with the existence of neural dynamics that discontinue and update past neural states to put the present in focus, regardless of the content or mental object of those states (34, 72).
Our network control theory simulations accord with a putative mechanism of practicing mindful attention (46, 55, 102) by redirecting the influence of the default-mode network’s precuneus and PCC activity to regions associated with executive function. Increased practice resulted in more-efficient transitions to mindful attention, suggesting the possibility for a more reflexive and less effortfully controlled mindset as in prior work on reappraisal of negative affect (60). Taken together, our results suggest a dynamical and self-regulatory function of the effort and instability evoked by mindful attention that relates to faster intrinsic neural timescales. Faster intrinsic neural timescales are consistent with a nonelaborative attention to the present moment and increased cognitive distance from transient events. Together, the theoretical approach offers a dynamical systems framework for the effort and stability of meditative states (23) and a stimulation framework for future research on neural mechanisms and behavioral outcomes of training (42, 55, 82, 87, 113, 114).
Just as thoughts, emotions, and experiences inevitably arise and dissipate naturally, each region has unique time windows in which their neural activity integrates information (32, 98, 99, 115). Default-mode regions tend to have slower intrinsic timescales of activity related to reacting to the mental contents of mind wandering with desire, aversion, or rumination, relative to faster intrinsic timescales of sensorimotor brain regions related to sensing and doing (32). We set out to investigate two opposing hypotheses about the time signatures of neural activity elicited by mindful awareness. First, neural activity can quickly change to new states, updating the past to a new present. To recognize thoughts and experiences as transient moments, mindful attention may elicit transient neural events. Faster timescales are consistent with more-frequent switching between neural states of task preparation and idling in people with high trait mindfulness (39, 116). Second, in contrast to neural activity updating quickly to a new present, neural activity can slowly change to new states. Slowly changing states exhibit more stable activity over time, not ushering in the future, with a more conjoined and nonseparated past, present, and future. To recognize that events are merely passing, mindful attention may involve recognizing the illusory status that privileges past, present, or future mental states unevenly, since they strongly interrelate. We found evidence for the first hypothesis of faster timescales. This result is consistent with prior work showing task activity magnitude correlates with faster intrinsic timescales (117). When people practice mindful attention, the effects of practice on neural activity are thought to transform the resting-state experience to be more present focused (34), consistent with our dynamical systems theory of transient neural events that update the present at a faster clip. However, faster neural events do not necessarily imply perception of faster mental events. To advance understanding of the link between neural and mental timescales, a promising direction of work is to investigate how nonlinear effects of reducing the influence of default-mode network regions like the PCC during mindful attention relates to nonlinear effects of mindfulness on psychological states such as well-being (36, 42, 46, 118). Just as there may be an optimal range of meditative practice for well-being, there may be an optimal range for reducing the influence of default-mode network regions like the PCC, whose activity can be perturbed to create psychological distance, short of evoking dissociative experiences of the sense of self (48–51).
Our application of network control theory revealed neural processes distinctive of mindful attention that prime the expenditure of control resources by regions in the frontoparietal control and attention networks. Our results build upon progress made by region-of-interest studies which identified neural correlates of attention, cognitive control, and self-relevant future-oriented processing in support of personal goals (119–121). The explanations offered by elucidating how pathways of activity flow are related to characteristics of neural processes align closely with the notion that reduced constraints by the brain networks associated with cognitive control and attention are likely to result in the absence of awareness of one’s present mental state (122–124). Our operationalizations of effort and stability offer richer characterizations of mindfulness that accord with defining features, including the nonelaborative awareness of the present (13). The current findings add to prior literature that early stages of mindfulness practice may improve the ability to orient attention by selecting specific information from multiple sensory stimuli and attending to conflicts between different regional activities (25).
In contrast, the inability to regulate attention is related to mind wandering (14). Mindfulness is negatively correlated with mind wandering, consistent with the understanding of mindfulness as the awareness of the present experience and of mind wandering as perceptual decoupling of attention and the external environment (19). Instead of effortfully deautomatizing responses, people spend almost half of their mental lives on spontaneous and unconstrained mind wandering (31). When people mind wander, they focus less on specific goals and more on processing social and emotional information relevant to oneself. To dissociate thoughts about oneself from the present experience, brain activity is thought to accrue information over longer periods of integration in the default-mode network compared to shorter periods in sensory and motor networks (32, 98, 99). Both mindfulness and mind wandering appear useful in different contexts and recruit brain networks associated with executive and default-mode function (14, 123).
Differing recruitment of brain regions according to context may generate constraints on automatic and deautomatized thoughts, feelings, and behaviors that vary in psychological components of intentionality, awareness, efficiency, and controllability (125). Our work further suggests a functional role of altered effort and stability during neural dynamics. The results described here suggest that the efficiency of structural controllability, previously linked to executive function (95), is positively correlated with neural dynamics that focalize the present. Future work could build upon a link between executive function and the regulation of emotions (126) to understand the relationship between attentional states of flow—defined as being effortlessly and fully involved in the present moment—when executing goal-directed tasks and well-being, mood, and self-regulation (127).
Three issues have limited our ability to explain the neural processes relating mindful attention to self-regulation. First, it is challenging to disentangle the functional significance of efficiency and that of effort, because a key goal of mindfulness practice is to more efficiently engender less automatic reactions to one’s experiences (16, 21, 29, 122, 128). Baseline natural reactions are not exclusively automatic, because they also involve controlled processes (79, 123). Our operationalization of effort allows us to detect the relative deautomaticity and controlled processing of mindful attention compared to the baseline. Our results are consistent with a putative neural process of practicing mindful attention by downregulating the influence of the precuneus and PCC (36, 46, 47). In the process of reducing the influence of these regions, network dynamics change to elicit effortful deautomatization of spontaneous activity and habitual reactions. As people gain expertise in reducing the influence of these default-mode network regions, they can efficiently utilize less control input. Second, current theoretical definitions of neural effort and efficiency require further validation. It is unfounded to interpret activity magnitude measurements conceptually as effort if the link between activity magnitude and effort has not been empirically validated (129, 130). With both increased and decreased activity within brain regions associated with different categories of meditation (131), high activity levels need not correspond to effort, and low levels need not correspond to efficiency. In fact, decreasing activity can require neural effort for inhibitory signaling (132, 133). In the present work, we utilize a theoretical framework of network control theory to operationalize effort as control input. This operationalization is supported by prior research showing that control inputs positively correlate with cognitive load, are modulated by dopaminergic signaling of cognitive effort, predict the effects of direct electrical stimulation, and relate to metabolic energy (82, 87, 88, 90–92). Third, a core element of the definition of mindfulness relates to momentary timescales—present-centered awareness—but evidence based on functional connectivity obscures time. Previous work addressed this issue by analyzing real-time neural activity evoked by meditative practice (46, 55, 102). By contrast, we model the time-dependent control of the brain network as a dynamical system (134, 135). This modeling approach allowed us to formalize dynamical notions of effort and stability during mindful attention (23) and relate them to the intrinsic neural timescale (32, 99).
Our analyses highlight the value of investigating network dynamics to provide unique insight into neural processes of meditative practices (38, 55, 136). In addition to effortful expenditure of control input to deautomatize and discontinue the processing of reactions to stimuli, other components beyond deautomatization remain relevant avenues of future study. Two such promising components are socially oriented regulatory strategies and cognitive flexibility. First, social influences serve an important role in self-regulatory processes such as moderation of alcohol consumption (3). Future research could study how socially oriented mindsets, such as loving-kindness meditation and perspective taking, elicit mindsets characterized by care for others’ well-being and openness to learning new information (121, 137). Prior hypotheses suggest that social processing is an efficient baseline for self-regulation due to cooperation, shared resources, risk mitigation, and common goals (138). Our findings naturally lead to the hypothesis that socially oriented mindsets may prime more-efficient neural dynamics that bias neural dynamics to focalize recently experienced past messages that linger into the future. Indeed, average controllability is greatest in default-mode network regions whose activity is commonly associated with social processes and thinking about the past or future (89). Second, cognitive flexibility has been hypothesized to be a key component of how mindfulness supports self-regulation (29). Prior work has shown that the time spent in a state, the frequency of state occurrence, and transitions among brain states are associated with flexibility (139) and learning (134). These approaches are well suited for research on the neural dynamics of meditative practices, because dynamics between the default mode and frontoparietal networks have been associated with cognitive flexibility. As a major aim of mindfulness is nonelaborative and nonnarrative processing of past experience, future work could apply event segmentation to assess how the narrative structure of resting-state dynamics becomes more or less elaborated in response to meditative practices and messaging (140, 141). Understanding the neural processes by which meditative practices enhance self-regulation could inform models of health behavior change, addiction, and implicit bias that theorize a critical role for executive function to change automatic or habitual behavior; such models are crucial for the design of individualized interventions that support self-regulatory goals (9, 142, 143).
As a self-regulatory strategy, mindful attention can help elicit psychological distancing and reduce craving (30). While all participants reported craving, our sample represents nondependent social drinkers. As such, these findings run parallel to research on heavy drinkers or drinkers with alcohol use disorder (144). We would not expect findings of nondependent social drinkers to directly generalize to other studies of attentional biases, motivation, and emotional salience in alcohol use disorders, or vice versa (145, 146). Although we found reduced craving during mindful attention compared to one’s own natural reaction in the experimental group, their average craving did not differ from the baseline group. Hence our findings should be considered in the context of potential effects of placebo, distraction, conditioning, and expectations. Other motivators might drive behavior in our sample of nondependent social drinkers. For example, in a separate report from the dataset we used here, feelings of purpose in life influenced whether greater alcohol cue reactivity within the ventral striatum was associated with increased or decreased alcohol use following craving in daily life (147). We encourage future work that could bridge this gap, and note that the present work suggests that network control is a promising candidate approach.
Methodological Considerations.
These findings should be considered within the context of the strengths and limitations of our approach. Strengths of the study included randomized controlled experiments; follow-up longitudinal measurements of the self-regulation of real-world behavior; multimodal analyses using diffusion, task-based, and resting-state neuroimaging; and statistical modeling tailored to the nonnormal distribution of drinking behavior beyond baseline drinking and personality differences. Moreover, network control theory was well positioned to overcome limitations of the theoretical definition of neural effort and efficiency that have prevented previous research from drawing conclusive inferences about the role of neural dynamics in meditative practices. Our use of the intrinsic timescale was also notable for its relevance to the definition of mindfulness (13), and for the fact that it provides empirical justification for the temporal stability that is typically inferred from the theoretical assumptions of control stability (90, 109, 148).
Limitations of the study included the simplifying assumption of linear neural dynamics, although this simplification is common and justifiable in macroscale networks (149, 150). Another limitation was the use of an experimental baseline task that was not expected to provide similar benefits as the interventions (25). Although our primary aim was to understand the relations between mindfulness states and neural effort, efficiency, or stability, it will be crucial that future studies incorporate an active comparator in their experimental design, to evaluate mindfulness interventions in clinical or educational settings. A separate report from the dataset we used here found that behavioral outcomes were specific to mindful attention compared to a socially oriented regulation strategy (30). Prior studies have used sham mindfulness or other active controls to determine whether belief or awareness that one is meditating was a significant driver of the health and cognitive outcomes of mindfulness (114, 151, 152). Such study designs will be important to address the possibilities that placebo, distraction, conditioning, and expectations may be associated with these results. Moreover, other features of a neural signal can produce shorter intrinsic timescales, such as greater power of higher-frequency oscillations. Other contributions of the signal to the stability of neural dynamics remain important avenues of investigation, preferably using neuroimaging methods like EEG with better temporal resolution. In addition, the short-term induction of mindful attention in our fMRI task differs from meditation-oriented mindfulness developed through meditation practice (153, 154), such as a standardized 8-wk program on mindfulness-based stress reduction including meditation practice. Although short-term induction of mindful attention has been previously effective, and the instructions and behavioral effects were replicated in a separate sample (30), we encourage extensions of our work to probe mindfulness performance ratings and self-report surveys on the subjective passage of time during the fMRI task and with different forms of meditation (55). Finally, since a prevalent issue among college students is binge drinking, we studied how college students belonging to social clubs responded to alcohol cues in the fMRI scanner and in their daily lives. Although we include demographic variables in our model, our study of this particular group limits the generalizability of the findings. Future work could extend our dynamical systems framework and short-term training to a broader sample. Further extensions to different forms of meditative practices represent an important direction to assess the generalizability of our dynamical systems theory of mindful attention and its outcomes (155, 156).
Conclusion.
Taken together, we described evidence that mindful attention was associated with effortful and unstable neural states with discontinuing neural dynamics. More-effortful and unstable neural states tend to more quickly update and discontinue the past in order to focalize the present experience. Our results provide a dynamical systems framework with the potential to model various meditative practices, regulatory strategies, and mental states that differently maximize the functional value and cost of thinking about the past, planning for the future, and staying in the present, according to personal goals and contextual demands (19, 122, 157).
Materials and Methods
Participants were undergraduate students recruited from social groups (e.g., sororities, sports clubs, performance groups) at the University of Pennsylvania and Columbia University. In our study, all of the participants reported drinking at least three times per year and had past experiences drinking alcohol. Given the focus of the current study on alcohol use outcomes, participants were excluded if they consumed less than one drink in a typical drinking occasion, had a history of substance abuse, or had any history of major physical or mental health disorders. Interested participants (; 63% of invited participants) consented to participate and completed an hour-long baseline survey, as described below. The full study protocol was approved by the Institutional Review Board at the University of Pennsylvania and Columbia University, in addition to the Army Research Office.
MRI Acquisition, Preprocessing, and Modeling.
Cue-reactivity fMRI task.
Consistent with past work on regulation of alcohol craving (3), we used images of alcohol (beer, wine, and liquor) to elicit craving. Before the task, participants were randomized to one of three groups (mindful, perspective taking, or control) and were trained how to do the task, based on their group. During the task, participants saw images of alcoholic (e.g., bottle of beer) and control images of nonalcoholic beverages (e.g., water bottle) selected from the Galician Beverage Picture Set (105). This normalized stimulus set contains images that are compositionally similar to one another and without beverage brands, and balances social contexts (e.g., alone versus in a social setting). While viewing the images, participants were either instructed to react naturally (“React” trials) or regulate their responses to the images (“Regulate” trials). After each image, they rated their craving on a five-point scale (1 = not at all, 5 = very much). On half of the React trials, participants saw images of alcoholic beverages; on the other half, they saw control, nonalcoholic beverages. Participants in the control group completed the React trials only, whereas participants in the mindful and perspective-taking groups completed both React and Regulate trials. On Regulate trials, participants in the mindful group were instructed to attend mindfully to their experience, accepting their thoughts and feelings in a nonjudgmental way. Detailed instructions for the task are available at https://osf.io/gkahy/ (158).
Participants completed 96 trials across four task runs. This task used a mixed design in which trials were blocked per condition to reduce the burden associated with task switching. We based our task design on prior work that established the block design methodology (3, 159). Each block consisted of four trials, and each task run consisted of six blocks. Each block began with a condition cue (3 s) followed by four trials, each consisting of an image presentation (6 s) and a craving rating (3 s); each event was separated by a jittered fixation cross (mean 4.0 s ± 2.6 s). Jittered fixation periods between blocks and between trial elements facilitate recovery of the hemodynamic response function, to improve modeling (160–162). The duration of blocks exceeded the overall cycle of the hemodynamic response. Block order was counterbalanced across participants within each group. After the scan session, participants answered questions about the cognitive strategies they used during the task and their level of confidence using the strategies, in the postscan survey.
MRI data acquisition.
Participants completed a prescan survey, a 90-min MRI scan that included structural, diffusion-weighted, resting-state, and task fMRI scans, and a postscan survey related to the fMRI tasks. Participants were also prepared to complete the experience sampling component. See SI Appendix, Supplementary Methods, MRI data acquisition for details.
Ecological Momentary Assessments and Mindful Attention Text Reminders.
Ecological momentary assessment.
After completing the MRI session, participants () began a 28-d experience sampling assessment that measured daily drinking behavior, mood, craving, and emotion regulation, among other factors. For participants in the mindful attention and baseline groups, the experience sampling procedure also instructed participants to regulate their responses to alcohol, by reminding them with texted instructions. These instructions reminded participants to employ the cognitive strategies that they learned during training in the cue-reactivity task while undergoing fMRI, as well as in the 28-d experience sampling component. The mindful attention text reminders were delivered on alternating weeks during the experience sampling component. On these “on” weeks, participants received two prompts a day (at 2 PM and 9 PM) reminding them to use the cognitive strategy when they encountered alcohol. On “off” weeks, participants were instructed to react naturally to alcohol cues (“If you are around alcohol today, REACT NATURALLY—have whatever thoughts and feelings you would normally have.”). This approach was adopted in order to assess within-person effects of the text reminders; text reminder delivery week order (on/off/on/off or off/on/off/on) was counterbalanced across participants.
Short-term induction of mindful attention.
The short training on mindful attention was based on instructions that were iteratively refined across 14 pilot studies conducted online via Amazon’s Mechanical Turk. Participants in the mindful group were trained to approach alcohol cues mindfully, “by mentally taking a step back in order to observe the situation and [their] responses in an impartial and non-judgmental manner.” They were also trained to pay attention to their reactions without getting caught up in them. On active weeks where participants received text reminders (“on” weeks) in the experience sampling component, participants were reminded to respond mindfully to alcohol cues twice a day (“If you are around alcohol today, REACT MINDFULLY—notice, acknowledge, and accept the thoughts and feelings you have.”). Participants in the control group were not trained to use any cognitive strategy to change their responses to alcohol. Instead, they were instructed to approach alcohol cues naturally, without regulating their responses (“If you are around alcohol today, REACT NATURALLY—have whatever thoughts and feelings you would normally have.”) throughout the whole sampling period. See SI Appendix, Supplementary Methods, Task Instructions and piloting and a separate publication of the same data for description of how we developed the text reminders (30).
Network control theory.
We applied network control theory metrics to investigate the effect of mindful attention in driving brain state trajectories associated with processing alcohol cues. The network control theory framework has been used to determine how underlying white matter architecture constrains transitions between different brain states inferred from neuroimaging data (81, 84, 87, 90). The control inputs required to execute these transitions between brain states can be thought of as a way of operationalizing cognitive effort, following prior work (89, 90). In our model, we used parameter estimates (β weights from a general linear model) to specify brain states from the cue-reactivity task. The baseline state was defined by β weights equal to zero (no relationship between neural activity and task). Each participant’s structural matrix was used as A. We defined B to allow control inputs into the 145 brain regions of the dorsal attention, ventral attention, and frontoparietal networks, following prior cognitive neuroscience literature implicating these networks in exerting executive control resources (89, 122, 163). We also sought to model the control inputs required to sustain the same neural state, which allows us to determine the control stability. While the control input operationalizes cognitive effort of neural dynamics, the control stability operationalizes the temporal stability of neural states. Lastly, to simulate the down-regulation of precuneus and PCC regions’ influence on system activity, we identified the 20 regions labeled precuneus/PCC using our selected brain parcellation atlas (see SI Appendix, Supplementary Methods, Anatomical data preprocessing). This analysis follows prior work conducting control impact analyses to assess how control input and stability change as one reduces the ability of regions to propagate control inputs (90). We apply this analysis to assess a previously hypothesized model involving the deactivation of default-mode network regions (36, 42, 46, 47). See SI Appendix, Supplementary Methods, Network control theory for more details.
Intrinsic timescale.
We sought to also investigate the stability of neural states using existing metrics that estimate the similarity of neural states over time from the regional time course data. To determine the temporal stability and similarity of neural states, we calculated the intrinsic timescale per brain region using the denoised time series after removal of motion confounds. The intrinsic timescale operationalizes the temporal windows by which regional computations are thought to unfold (98). To determine the intrinsic timescale, we used a model-free estimate from a previously published method (99). Briefly, the method determines the duration required for a signal to decay by half, and does so by estimating the full width at half maximum of an autocorrelation function. We used this previously published method and corresponding MATLAB code available at https://github.com/ryraut/intrinsic-timescales (164).
Citation Diversity Statement.
Recent work in several fields of science has identified a bias in citation practices such that papers from women and other minority scholars are undercited relative to the number of such papers in the field (165–173). We used prior methods (169, 174) to measure that our references contain 19.66% woman(first)/woman(last), 9.39% man/woman, 29.13% woman/man, and 41.81% man/man authors; we used additional methods (175, 176) to measure that our references contain 9.23% author of color (first)/author of color(last), 11.44% white author/author of color, 21.53% author of color/white author, and 57.8% white author/white author. These methods have several limitations (174), and we look forward to future work that could help us to better understand how to support equitable practices in science.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We acknowledge helpful discussions with Dr. Isaac V. Kauvar. Research was sponsored by the Army Research Office and was accomplished under Grant W911NF-18-1-0244. D.Z. acknowledges support from the National Institute of Mental Health (Award F31MH126569). D.M.L.-S. acknowledges support from the National Institute on Drug Abuse (Award K01 DA047417) and the Brain & Behavior Research Foundation. D.S.B. acknowledges support from the John D. and Catherine T. MacArthur Foundation, the Swartz Foundation, the Paul G. Allen Family Foundation, the Alfred P. Sloan Foundation, and the NSF (Grants PHY-1554488 and IIS-1926757). D.S.B. and L.P. acknowledge support from the National Institute of Mental Health (Grant R01MH113550). L.P. was also supported by the National Institute of Mental Health (Award K99MH127296) and a 2020 National Alliance for Research on Schizophrenia & Depression Young Investigator Grant from the Brain & Behavior Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Army Research Office or the US government. The US government is authorized to reproduce and distribute reprints for government purposes notwithstanding any copyright notation herein.
Author contributions
D.Z., Y.K., P.J.M., K.N.O., D.M.L.-S., E.B.F., and D.S.B. designed research; D.Z., Y.K., D.C., M.J., X.H., A.M., J.A., O.S., N.C., E.J.C., L.P., and D.M.L.-S. performed research; D.Z., D.C., M.J., X.H., A.M., J.A., E.J.C., and D.M.L.-S. contributed new reagents/analytic tools; D.Z., D.C., M.J., X.H., A.M., J.A., J.K.B., N.C., and D.M.L.-S. analyzed data; and D.Z., Y.K., P.J.M., K.N.O., D.M.L.-S., E.B.F., and D.S.B. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Materials, processed anonymized data, and code have been deposited in Open Science Framework [https://osf.io/gkahy/ (158)], NeuroVault (https://neurovault.org/collections/QCJQYFVZ/) (177), and GitHub (https://github.com/dalejn/contemplativeControl) (178).
Supporting Information
References
- 1.Carver C. S., Scheier M. F., On the Self-Regulation of Behavior (Cambridge University Press, 2001). [Google Scholar]
- 2.Hustad J. T., Carey K. B., Carey M. P., Maisto S. A., Self-regulation, alcohol consumption, and consequences in college student heavy drinkers: A simultaneous latent growth analysis. J. Stud. Alcohol Drugs 70, 373–382 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naqvi N. H., et al. , Cognitive regulation of craving in alcohol-dependent and social drinkers. Alcohol. Clin. Exp. Res. 39, 343–349 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann S. G., Asmundson G. J. G., Acceptance and mindfulness-based therapy: New wave or old hat? Clin. Psychol. Rev. 28, 1–16 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Hofmann S. G., Grossman P., Hinton D. E., Loving-kindness and compassion meditation: Potential for psychological interventions. Clin. Psychol. Rev. 31, 1126–1132 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubia K., The neurobiology of Meditation and its clinical effectiveness in psychiatric disorders. Biol. Psychol. 82, 1–11 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Armitage C. J., Harris P. R., Arden M. A., Evidence that self-affirmation reduces alcohol consumption: Randomized exploratory trial with a new, brief means of self-affirming. Health Psychol. 30, 633–641 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Howell J. L., Shepperd J. A., Reducing information avoidance through affirmation. Psychol. Sci. 23, 141–145 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Kang Y., Gray J. R., Dovidio J. F., The nondiscriminating heart: Lovingkindness meditation training decreases implicit intergroup bias. J. Exp. Psychol. Gen. 143, 1306–1313 (2014). [DOI] [PubMed] [Google Scholar]
- 10.MacKenzie M. B., Kocovski N. L., Mindfulness-based cognitive therapy for depression: Trends and developments. Psychol. Res. Behav. Manag. 9, 125–132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McTeague L. M., Goodkind M. S., Etkin A., Transdiagnostic impairment of cognitive control in mental illness. J. Psychiatr. Res. 83, 37–46 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creswell J. D., et al. , Alterations in resting-state functional connectivity link mindfulness meditation with reduced interleukin-6: A randomized controlled trial. Biol. Psychiatry 80, 53–61 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Thera N., The Power of Mindfulness (Wheel, 1968). [Google Scholar]
- 14.Brown K. W., Ryan R. M., The benefits of being present: Mindfulness and its role in psychological well-being. J. Pers. Soc. Psychol. 84, 822–848 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Fresco D. M., et al. , Initial psychometric properties of the experiences questionnaire: Validation of a self-report measure of decentering. Behav. Ther. 38, 234–246 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Lutz A., Slagter H. A., Dunne J. D., Davidson R. J., Attention regulation and monitoring in meditation. Trends Cogn. Sci. 12, 163–169 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabat-Zinn J., Hanh T. N., Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness (Delta, 2009). [Google Scholar]
- 18.Hölzel B. K., et al. , How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect. Psychol. Sci. 6, 537–559 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Schooler J. W., et al. , Meta-awareness, perceptual decoupling and the wandering mind. Trends Cogn. Sci. 15, 319–326 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Papies E. K., Barsalou L. W., Custers R., Mindful attention prevents mindless impulses. Soc. Psychol. Personal. Sci. 3, 291–299 (2012). [Google Scholar]
- 21.Chiesa A., Serretti A., Jakobsen J. C., Mindfulness: Top-down or bottom-up emotion regulation strategy? Clin. Psychol. Rev. 33, 82–96 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Seppala E. M., Hutcherson C. A., Nguyen D. T., Doty J. R., Gross J. J., Loving-kindness meditation: A tool to improve healthcare provider compassion, resilience, and patient care. J. Compassionate Health Care 1, 1–9 (2014). [Google Scholar]
- 23.Lutz A., Jha A. P., Dunne J. D., Saron C. D., Investigating the phenomenological matrix of mindfulness-related practices from a neurocognitive perspective. Am. Psychol. 70, 632–658 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweeney A. M., Moyer A., Self-affirmation and responses to health messages: A meta-analysis on intentions and behavior. Health Psychol. 34, 149–159 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Tang Y. Y., Hölzel B. K., Posner M. I., The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 16, 213–225 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Papies E. K., Pronk T. M., Keesman M., Barsalou L. W., The benefits of simply observing: Mindful attention modulates the link between motivation and behavior. J. Pers. Soc. Psychol. 108, 148–170 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Lebois L. A., et al. , A shift in perspective: Decentering through mindful attention to imagined stressful events. Neuropsychologia 75, 505–524 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang Y., Gruber J., Gray J. R., Mindfulness and de-automatization. Emot. Rev. 5, 192–201 (2013). [Google Scholar]
- 29.Kang Y., Gruber J., Gray J. R., “Deautomatization of cognitive and emotional life” in The Wiley Blackwell Handbook of Mindfulness, Ie A., Ngnoumen C. T., Langer E. J., Eds. (Wiley, 2014), vol. 1, pp. 168–185. [Google Scholar]
- 30.Jovanova M., et al. , Psychological distance intervention reminders reduce alcohol consumption frequency in daily life. psyArxiv [Preprint] (2022). 10.31234/osf.io/yw7s3. Accessed 23 June 2022. [DOI] [PMC free article] [PubMed]
- 31.Killingsworth M. A., Gilbert D. T., A wandering mind is an unhappy mind. Science 330, 932 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Wolff A., et al. , Intrinsic neural timescales: Temporal integration and segregation. Trends Cogn. Sci. 26, 159–173 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Mason M. F., et al. , Wandering minds: The default network and stimulus-independent thought. Science 315, 393–395 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brewer J. A., et al. , Meditation experience is associated with differences in default mode network activity and connectivity. Proc. Natl. Acad. Sci. U.S.A. 108, 20254–20259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaurya Prakash R., De Leon A. A., Klatt M., Malarkey W., Patterson B., Mindfulness disposition and default-mode network connectivity in older adults. Soc. Cogn. Affect. Neurosci. 8, 112–117 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garrison K. A., et al. , Real-time fMRI links subjective experience with brain activity during focused attention. Neuroimage 81, 110–118 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirk U., et al. , Mindfulness training increases cooperative decision making in economic exchanges: Evidence from fMRI. Neuroimage 138, 274–283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellamil M., et al. , Dynamics of neural recruitment surrounding the spontaneous arising of thoughts in experienced mindfulness practitioners. Neuroimage 136, 186–196 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Lim J., Teng J., Patanaik A., Tandi J., Massar S. A. A., Dynamic functional connectivity markers of objective trait mindfulness. Neuroimage 176, 193–202 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Goldin P. R., McRae K., Ramel W., Gross J. J., The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol. Psychiatry 63, 577–586 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ives-Deliperi V. L., Solms M., Meintjes E. M., The neural substrates of mindfulness: An fMRI investigation. Soc. Neurosci. 6, 231–242 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Brewer J. A., Garrison K. A., The posterior cingulate cortex as a plausible mechanistic target of meditation: Findings from neuroimaging. Ann. N. Y. Acad. Sci. 1307, 19–27 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Segal Z. V., Williams M., Teasdale J., Mindfulness-Based Cognitive Therapy for Depression (Guilford, 2018). [Google Scholar]
- 44.Bilevicius E., Smith S. D., Kornelsen J., Resting-state network functional connectivity patterns associated with the mindful attention awareness scale. Brain Connect. 8, 40–48 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Harrison R., Zeidan F., Kitsaras G., Ozcelik D., Salomons T. V., Trait mindfulness is associated with lower pain reactivity and connectivity of the default mode network. J. Pain 20, 645–654 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Garrison K. A., et al. , Effortless awareness: Using real time neurofeedback to investigate correlates of posterior cingulate cortex activity in meditators’ self-report. Front. Hum. Neurosci. 7, 440 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kral T. R. A., et al. , Mindfulness-Based Stress Reduction-related changes in posterior cingulate resting brain connectivity. Soc. Cogn. Affect. Neurosci. 14, 777–787 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vesuna S., et al. , Deep posteromedial cortical rhythm in dissociation. Nature 586, 87–94 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parvizi J., et al. , Altered sense of self during seizures in the posteromedial cortex. Proc. Natl. Acad. Sci. U.S.A. 118, e2100522118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peer M., Salomon R., Goldberg I., Blanke O., Arzy S., Brain system for mental orientation in space, time, and person. Proc. Natl. Acad. Sci. U.S.A. 112, 11072–11077 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brietzke S., Meyer M. L., Temporal self-compression: Behavioral and neural evidence that past and future selves are compressed as they move away from the present. Proc. Natl. Acad. Sci. U.S.A. 118, e2101403118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raichle M. E., et al. , A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98, 676–682 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasenkamp W., Barsalou L. W., Effects of meditation experience on functional connectivity of distributed brain networks. Front. Hum. Neurosci. 6, 38 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jenkins A. C., Rethinking cognitive load: A default-mode network perspective. Trends Cogn. Sci. 23, 531–533 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Kim H. C., et al. , Mediation analysis of triple networks revealed functional feature of mindfulness from real-time fMRI neurofeedback. Neuroimage 195, 409–432 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Nelson S. M., et al. , Role of the anterior insula in task-level control and focal attention. Brain Struct. Funct. 214, 669–680 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allen M., et al. , Cognitive-affective neural plasticity following active-controlled mindfulness intervention. J. Neurosci. 32, 15601–15610 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lutz A., McFarlin D. R., Perlman D. M., Salomons T. V., Davidson R. J., Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. Neuroimage 64, 538–546 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taren A. A., et al. , Mindfulness meditation training alters stress-related amygdala resting state functional connectivity: A randomized controlled trial. Soc. Cogn. Affect. Neurosci. 10, 1758–1768 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Denny B. T., Inhoff M. C., Zerubavel N., Davachi L., Ochsner K. N., Getting over it: Long-lasting effects of emotion regulation on amygdala response. Psychol. Sci. 26, 1377–1388 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kral T. R. A., et al. , Impact of short- and long-term mindfulness meditation training on amygdala reactivity to emotional stimuli. Neuroimage 181, 301–313 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang Z., et al. , Anterior insula regulates brain network transitions that gate conscious access. Cell Rep. 35, 109081 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menon V., Uddin L. Q., Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 214, 655–667 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dickenson J., Berkman E. T., Arch J., Lieberman M. D., Neural correlates of focused attention during a brief mindfulness induction. Soc. Cogn. Affect. Neurosci. 8, 40–47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uddin L. Q., Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 16, 55–61 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Uddin L. Q., Nomi J. S., Hébert-Seropian B., Ghaziri J., Boucher O., Structure and function of the human insula. J. Clinical Neurophysiol. 34, 300 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taren A. A., et al. , Mindfulness meditation training and executive control network resting state functional connectivity: A randomized controlled trial. Psychosom. Med. 79, 674–683 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Velden A. M., et al. , Mindfulness training changes brain dynamics during depressive rumination: A randomized controlled trial. Biol. Psychiatry, https://doi.org.10.1016/j.biopsych.2022.06.038 (2022). Accessed 22 July 2022. [DOI] [PubMed] [Google Scholar]
- 69.Ortner C. N., Kilner S. J., Zelazo P. D., Mindfulness meditation and reduced emotional interference on a cognitive task. Motiv. Emot. 31, 271–283 (2007). [Google Scholar]
- 70.Kober H., et al. , Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc. Natl. Acad. Sci. U.S.A. 107, 14811–14816 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doll A., et al. , Mindful attention to breath regulates emotions via increased amygdala-prefrontal cortex connectivity. Neuroimage 134, 305–313 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Brefczynski-Lewis J. A., Lutz A., Schaefer H. S., Levinson D. B., Davidson R. J., Neural correlates of attentional expertise in long-term meditation practitioners. Proc. Natl. Acad. Sci. U.S.A. 104, 11483–11488 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manna A., et al. , Neural correlates of focused attention and cognitive monitoring in meditation. Brain Res. Bull. 82, 46–56 (2010). [DOI] [PubMed] [Google Scholar]
- 74.Falcone G., Jerram M., Brain activity in mindfulness depends on experience: A meta-analysis of fMRI studies. Mindfulness 9, 1319–1329 (2018). [Google Scholar]
- 75.Karbach J., Kray J., How useful is executive control training? Age differences in near and far transfer of task-switching training. Dev. Sci. 12, 978–990 (2009). [DOI] [PubMed] [Google Scholar]
- 76.Karbach J., Verhaeghen P., Making working memory work: A meta-analysis of executive-control and working memory training in older adults. Psychol. Sci. 25, 2027–2037 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakai K. L., Language acquisition and brain development. Science 310, 815–819 (2005). [DOI] [PubMed] [Google Scholar]
- 78.Doyon J., et al. , Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc. Natl. Acad. Sci. U.S.A. 99, 1017–1022 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fiske S., “Examining the role of intent: Toward understanding its role in stereotyping and prejudice” in Unintended Thought, Uleman J. S., Bargh J. A., Eds. (Guilford, 1989), pp. 253–283. [Google Scholar]
- 80.Pasqualetti F., Zampieri S., Bullo F., Controllability metrics, limitations and algorithms for complex networks. IEEE Trans. Control Netw. Syst. 1, 40–52 (2014). [Google Scholar]
- 81.Betzel R. F., Gu S., Medaglia J. D., Pasqualetti F., Bassett D. S., Optimally controlling the human connectome: The role of network topology. Sci. Rep. 6, 30770 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muldoon S. F., et al. , Stimulation-based control of dynamic brain networks. PLOS Comput. Biol. 12, e1005076 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zañudo J. G. T., Yang G., Albert R., Structure-based control of complex networks with nonlinear dynamics. Proc. Natl. Acad. Sci. U.S.A. 114, 7234–7239 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gu S., et al. , Optimal trajectories of brain state transitions. Neuroimage 148, 305–317 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Z., Sarma S. V., Dynamic Neuroscience (Springer, Cham, 2018). [Google Scholar]
- 86.Karrer T. M., et al. , A practical guide to methodological considerations in the controllability of structural brain networks. J. Neural Eng. 17, 026031 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stiso J., et al. , White matter network architecture guides direct electrical stimulation through optimal state transitions. Cell Rep. 28, 2554–2566.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He X., et al. , Pathological and metabolic underpinnings of energetic inefficiency in temporal lobe epilepsy. bioRxiv [Preprint] (2021). 10.1101/2021.09.23.461495. Accessed 24 September 2021. [DOI]
- 89.Gu S., et al. , Controllability of structural brain networks. Nat. Commun. 6, 8414 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Braun U., et al. , Brain network dynamics during working memory are modulated by dopamine and diminished in schizophrenia. Nat. Commun. 12, 3478 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shenhav A., Botvinick M. M., Cohen J. D., The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron 79, 217–240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cools R., Chemistry of the adaptive mind: Lessons from dopamine. Neuron 104, 113–131 (2019). [DOI] [PubMed] [Google Scholar]
- 93.Tang E., et al. , Developmental increases in white matter network controllability support a growing diversity of brain dynamics. Nat. Commun. 8, 1252 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weninger L., et al. , The information content of brain states is explained by structural constraints on state energetics. Phys. Rev. E 106, 014401-1–014401-14 (2021). [DOI] [PubMed] [Google Scholar]
- 95.Cornblath E. J., et al. , Sex differences in network controllability as a predictor of executive function in youth. Neuroimage 188, 122–134 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bullmore E., Sporns O., The economy of brain network organization. Nat. Rev. Neurosci. 13, 336–349 (2012). [DOI] [PubMed] [Google Scholar]
- 97.Zhou D., et al. , Efficient coding in the economics of human brain connectomics. Network Neuroscience 6, 234–274 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murray J. D., et al. , A hierarchy of intrinsic timescales across primate cortex. Nat. Neurosci. 17, 1661–1663 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raut R. V., Snyder A. Z., Raichle M. E., Hierarchical dynamics as a macroscopic organizing principle of the human brain. Proc. Natl. Acad. Sci. U.S.A. 117, 20890–20897 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Slutske W. S., Alcohol use disorders among US college students and their non-college-attending peers. Arch. Gen. Psychiatry 62, 321–327 (2005). [DOI] [PubMed] [Google Scholar]
- 101.Presley C. A., Meilman P. W., Lyerla R., Development of the core alcohol and drug survey: Initial findings and future directions. J. Am. Coll. Health 42, 248–255 (1994). [DOI] [PubMed] [Google Scholar]
- 102.Weiss F., et al. , Using mind control to modify cue-reactivity in AUD: The impact of mindfulness-based relapse prevention on real-time fMRI neurofeedback to modify cue-reactivity in alcohol use disorder: A randomized controlled trial. BMC Psychiatry 20, 309 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Balconi M., Angioletti L., “Mindfulness-based interventions (MBIs) as a way for treating EFS in addiction-related disorders” in Advances in Substance and Behavioral Addiction, Balconi M., Campanella S., Eds. (Springer, 2021), pp. 149–167. [Google Scholar]
- 104.Rosenthal A., Levin M. E., Garland E. L., Romanczuk-Seiferth N., Mindfulness in treatment approaches for addiction—Underlying mechanisms and future directions. Curr. Addict. Rep. 8, 282–297 (2021). [Google Scholar]
- 105.López-Caneda E., Carbia C., The Galician Beverage Picture Set (GBPS): A standardized database of alcohol and non-alcohol images. Drug Alcohol Depend. 184, 42–47 (2018). [DOI] [PubMed] [Google Scholar]
- 106.Mrazek M. D., Smallwood J., Schooler J. W., Mindfulness and mind-wandering: Finding convergence through opposing constructs. Emotion 12, 442–448 (2012). [DOI] [PubMed] [Google Scholar]
- 107.Atkins D. C., Baldwin S. A., Zheng C., Gallop R. J., Neighbors C., A tutorial on count regression and zero-altered count models for longitudinal substance use data. Psychol. Addict. Behav. 27, 166–177 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maggs J. L., Williams L. R., Lee C. M., Ups and downs of alcohol use among first-year college students: Number of drinks, heavy drinking, and stumble and pass out drinking days. Addict. Behav. 36, 197–202 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang E., et al. , Control of brain network dynamics across diverse scales of space and time. Phys. Rev. E 101, 062301 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McGowan A. L., et al. , Controllability of structural brain networks and the waxing and waning of negative affect in daily life. Bio. Psych. Glob. Open Sci. 2, 432–439 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee W. H., Rodrigue A., Glahn D. C., Bassett D. S., Frangou S., Heritability and cognitive relevance of structural brain controllability. Cereb. Cortex 30, 3044–3054 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Denny B. T., Ochsner K. N., Behavioral effects of longitudinal training in cognitive reappraisal. Emotion 14, 425–433 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fox K. C., Christoff K., Transcranial direct current stimulation to lateral prefrontal cortex could increase meta-awareness of mind wandering. Proc. Natl. Acad. Sci. U.S.A. 112, E2414 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Witkiewitz K., et al. , Mindfulness-based relapse prevention and transcranial direct current stimulation to reduce heavy drinking: A double-blind sham-controlled randomized trial. Alcohol. Clin. Exp. Res. 43, 1296–1307 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hasson U., Yang E., Vallines I., Heeger D. J., Rubin N., A hierarchy of temporal receptive windows in human cortex. J. Neurosci. 28, 2539–2550 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marusak H. A., et al. , Mindfulness and dynamic functional neural connectivity in children and adolescents. Behav. Brain Res. 336, 211–218 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ito T., Hearne L. J., Cole M. W., A cortical hierarchy of localized and distributed processes revealed via dissociation of task activations, connectivity changes, and intrinsic timescales. Neuroimage 221, 117141 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Britton W. B., Can mindfulness be too much of a good thing? The value of a middle way. Curr. Opin. Psychol. 28, 159–165 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.D’Argembeau A., et al. , The neural basis of personal goal processing when envisioning future events. J. Cogn. Neurosci. 22, 1701–1713 (2010). [DOI] [PubMed] [Google Scholar]
- 120.Cascio C. N., et al. , Self-affirmation activates brain systems associated with self-related processing and reward and is reinforced by future orientation. Soc. Cogn. Affect. Neurosci. 11, 621–629 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kang Y., et al. , Effects of self-transcendence on neural responses to persuasive messages and health behavior change. Proc. Natl. Acad. Sci. U.S.A. 115, 9974–9979 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smallwood J., Schooler J. W., The restless mind. Psychol. Bull. 132, 946–958 (2006). [DOI] [PubMed] [Google Scholar]
- 123.Christoff K., Irving Z. C., Fox K. C., Spreng R. N., Andrews-Hanna J. R., Mind-wandering as spontaneous thought: A dynamic framework. Nat. Rev. Neurosci. 17, 718–731 (2016). [DOI] [PubMed] [Google Scholar]
- 124.Ross L. N., Causal concepts in biology: How pathways differ from mechanisms and why it matters. Br. J. Philos. Sci. 72, 131–158 (2021). [Google Scholar]
- 125.Bargh J., “The four horsemen of automaticity: Awareness, intention, efficiency, and control in social cognition” in Handbook of Social Cognition: Basic Processes; Applications, Wyer R. S. Jr., Srull T. K., Eds. (Lawrence Erlbaum Associates, 1994), pp. 1–40. [Google Scholar]
- 126.Hallion L. S., Ruscio A. M., Jha A. P., Fractionating the role of executive control in control over worry: A preliminary investigation. Behav. Res. Ther. 54, 1–6 (2014). [DOI] [PubMed] [Google Scholar]
- 127.Nakamura J., Csikszentmihalyi M., “The concept of flow” in Flow and the Foundations of Positive Psychology, Csikszentmihalyi M., Ed. (Springer, 2014), pp. 239–263. [Google Scholar]
- 128.Chambers R., Gullone E., Allen N. B., Mindful emotion regulation: An integrative review. Clin. Psychol. Rev. 29, 560–572 (2009). [DOI] [PubMed] [Google Scholar]
- 129.Luna B., Padmanabhan A., O’Hearn K., What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 72, 101–113 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Poldrack R. A., Is “efficiency” a useful concept in cognitive neuroscience? Dev. Cogn. Neurosci. 11, 12–17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fox K. C., et al. , Functional neuroanatomy of meditation: A review and meta-analysis of 78 functional neuroimaging investigations. Neurosci. Biobehav. Rev. 65, 208–228 (2016). [DOI] [PubMed] [Google Scholar]
- 132.Buzsáki G., Kaila K., Raichle M., Inhibition and brain work. Neuron 56, 771–783 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cools R., D’Esposito M., Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 69, e113–e125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bassett D. S., et al. , Dynamic reconfiguration of human brain networks during learning. Proc. Natl. Acad. Sci. U.S.A. 108, 7641–7646 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sizemore A. E., Bassett D. S., Dynamic graph metrics: Tutorial, toolbox, and tale. Neuroimage 180, 417–427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weng H. Y., et al. , Focus on the breath: Brain decoding reveals internal states of attention during meditation. Front. Hum. Neurosci. 14, 336 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kang Y., Examining interpersonal self-transcendence as a potential mechanism linking meditation and social outcomes. Curr. Opin. Psychol. 28, 115–119 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Coan J. A., Brown C. L., Beckes L., “Our social baseline: The role of social proximity in economy of action” in Mechanisms of Social Connection: From Brain to Group, Mikulincer M., Shaver P. R., Eds. (American Psychological Association, 2014), pp. 89–104. [Google Scholar]
- 139.Uddin L. Q., Cognitive and behavioural flexibility: Neural mechanisms and clinical considerations. Nat. Rev. Neurosci. 22, 167–179 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen J., et al. , Shared memories reveal shared structure in neural activity across individuals. Nat. Neurosci. 20, 115–125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cohen S. S., Tottenheim N., Baldassano C., Developmental changes in story-evoked responses in the neocortex and hippocampus, vol. 11, 2022, e69430, 10.7554/eLife.69430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chua H. F., Liberzon I., Welsh R. C., Strecher V. J., Neural correlates of message tailoring and self-relatedness in smoking cessation programming. Biol. Psychiatry 65, 165–168 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Boness C. L., Watts A. L., Moeller K. N., Sher K. J., The etiologic, theory-based, ontogenetic hierarchical framework of alcohol use disorder: A translational systematic review of reviews. Psychol. Bull. 147, 1075–1123 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Krieglmeyer R., Deutsch R., Houwer J. De, Raedt R. De, Being moved: Valence activates approach-avoidance behavior independently of evaluation and approach-avoidance intentions. Psychol. Sci. 21, 607–613 (2010). [DOI] [PubMed] [Google Scholar]
- 145.Ihssen N., Cox W. M., Wiggett A., Fadardi J. S., Linden D. E., Differentiating heavy from light drinkers by neural responses to visual alcohol cues and other motivational stimuli. Cereb. Cortex 21, 1408–1415 (2011). [DOI] [PubMed] [Google Scholar]
- 146.Townshend J. M., Duka T., Attentional bias associated with alcohol cues: Differences between heavy and occasional social drinkers. Psychopharmacology (Berl.) 157, 67–74 (2001). [DOI] [PubMed] [Google Scholar]
- 147.Kang Y., et al. , Purpose in life, neural alcohol cue reactivity, and daily alcohol use in social drinkers. Addiction, 10.1111/add.16012 (2022). Accessed 1 August 2022. [DOI] [PubMed] [Google Scholar]
- 148.Cornblath E. J., et al. , Temporal sequences of brain activity at rest are constrained by white matter structure and modulated by cognitive demands. Commun. Biol. 3, 261 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Honey C. J., et al. , Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. U.S.A. 106, 2035–2040 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nozari E., et al. Is the brain macroscopically linear? a system identification of resting state dynamics. arXiv [Preprint] (2020). 10.1101/2020.12.21.423856. Accessed 11 August 2021. [DOI]
- 151.Zeidan F., et al. , Mindfulness meditation-based pain relief employs different neural mechanisms than placebo and sham mindfulness meditation-induced analgesia. J. Neurosci. 35, 15307–15325 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Johnson S., Gur R. M., David Z., Currier E., One-session mindfulness meditation: A randomized controlled study of effects on cognition and mood. Mindfulness 6, 88–98 (2015). [Google Scholar]
- 153.Kabat-Zinn J., Mindfulness -based interventions in context: Past, present, and future. Clin. Psychol. Sci. Practice 10, 144–156 (2003). [Google Scholar]
- 154.Desbordes G., Negi L. T., A new era for mind studies: Training investigators in both scientific and contemplative methods of inquiry. Front. Hum. Neurosci. 7, 741 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Van Dam N. T., et al. , Mind the hype: A critical evaluation and prescriptive agenda for research on mindfulness and meditation. Perspect. Psychol. Sci. 13, 36–61 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Britton W. B., Lindahl J. R., Cooper D. J., Canby N. K., Palitsky R., Defining and measuring meditation-related adverse effects in mindfulness-based programs. Clin. Psychol. Sci. 9, 1185–1204 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gerlach K. D., Spreng R. N., Gilmore A. W., Schacter D. L., Solving future problems: Default network and executive activity associated with goal-directed mental simulations. Neuroimage 55, 1816–1824 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cosme D., et al. , Social Health Impact of Network Effects (SHINE) Study. https://osf.io/gkahy/. Accessed 29 July 2022.
- 159.Kober H., Buhle J., Weber J., Ochsner K. N., Wager T. D., Let it be: Mindful acceptance down-regulates pain and negative emotion. Soc. Cogn. Affect. Neurosci. 14, 1147–1158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ollinger J. M., Shulman G. L., Corbetta M., Separating processes within a trial in event-related functional MRI. I. The method. Neuroimage 13, 210–217 (2001). [DOI] [PubMed] [Google Scholar]
- 161.Ollinger J. M., Corbetta M., Shulman G. L., Separating processes within a trial in event-related functional MRI. II. Analysis. Neuroimage 13, 218–229 (2001). [DOI] [PubMed] [Google Scholar]
- 162.Wager T. D., Nichols T. E., Optimization of experimental design in fMRI: A general framework using a genetic algorithm. Neuroimage 18, 293–309 (2003). [DOI] [PubMed] [Google Scholar]
- 163.Schaefer A., et al. , Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex 28, 3095–3114 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Raut R., et al. , Intrinsic-timescales. https://github.com/ryraut/intrinsic-timescales. Accessed 21 May 2021.
- 165.Mitchell S. M., Lange S., Brus H., Gendered citation patterns in international relations journals. Int. Stud. Perspect. 14, 485–492 (2013). [Google Scholar]
- 166.Dion M. L., Sumner J. L., Mitchell S. M., Gendered citation patterns across political science and social science methodology fields. Polit. Anal. 26, 312–327 (2018). [Google Scholar]
- 167.Caplar N, Tacchella S, Birrer S, Quantitative evaluation of gender bias in astronomical publications from citation counts. Nat. Astron. 1, 0141 (2017). [Google Scholar]
- 168.Maliniak D., Powers R., Walter B. F., The gender citation gap in international relations. Int. Organ. 67, 889–922 (2013). [Google Scholar]
- 169.Dworkin J. D., et al. , The extent and drivers of gender imbalance in neuroscience reference lists. bioRxiv [Preprint] (2020). 10.1101/2020.01.03.894378. Nat. Neurosci. 23, 918–926 (2020). [DOI] [PubMed]
- 170.Bertolero M. A., et al. , Racial and ethnic imbalance in neuroscience reference lists and intersections with gender. bioRxiv [Preprint] (2020). 10.1101/2020.10.12.336230. Accessed 12 October 2020. [DOI]
- 171.Wang X., et al. , Gendered citation practices in the field of communication. Ann. Int. Commun. Assoc. 45, 134–153 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chatterjee P., Werner R. M., Gender disparity in citations in high-impact journal articles. JAMA Netw. Open 4, e2114509 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fulvio J. M., Akinnola I., Postle B. R., Gender (im)balance in citation practices in cognitive neuroscience. J. Cogn. Neurosci. 33, 3–7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhou D., et al. , Gender diversity statement and code notebook v1.0 (2020). dalejn/cleanBib: v1.1.1 (1.1.2). Zenodo. 10.5281/zenodo.4104748. Accessed 27 October 2022. [DOI]
- 175.Ambekar A., Ward C., Mohammed J., Male S., Skiena S., “Name -ethnicity classification from open sources” in Proceedings of the 15th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, Elder J. F. IV, Fogelman-Soulié F., Flach P., Zaki M., Eds. (Association for Computing Machinery, 2009), pp. 49–58. [Google Scholar]
- 176.Sood G., Laohaprapanon S., Predicting race and ethnicity from the sequence of characters in a name. arXiv [Preprint] (2018). 10.48550/arXiv.1805.02109. Accessed 2 October 2020. [DOI]
- 177.Cosme D., Statistical maps. NeuroVault. https://neurovault.org/collections/QCJQYFVZ/download. Deposited 22 August 2022.
- 178.Zhou D., contemplativeControl. GitHub. https://github.com/dalejn/contemplativeControl. Deposited 8 December 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Materials, processed anonymized data, and code have been deposited in Open Science Framework [https://osf.io/gkahy/ (158)], NeuroVault (https://neurovault.org/collections/QCJQYFVZ/) (177), and GitHub (https://github.com/dalejn/contemplativeControl) (178).