Highlights
-
•
Empirically derived symptoms are related to specific changes in VS-mOFC and VS-amygdala circuits during reward processing.
-
•
Transdiagnostic symptoms (General Distress) are related to altered VS-mOFC connectivity during reward processing.
-
•
Depression related symptoms, Anhedonia-Apprehension are related to altered VS-amygdala connectivity during reward processing.
-
•
VS-mOFC findings may provide a valid target for biological interventions that innervate subcortical pathways.
-
•
VS-amygdala findings support the use of interventions that target motivation and related brain circuits.
Keywords: Reward, Functional neuroimaging, Functional connectivity, Dimensional symptoms, Mood disorders, Anxiety disorders
Abstract
Depression and anxiety are associated with abnormalities in brain regions that process rewards including the medial orbitofrontal cortex (mOFC), the ventral striatum (VS), and the amygdala. However, there are inconsistencies in these findings. This may be due to past reliance on categorical diagnoses that, while valuable, provide less precision than may be required to understand subtle neural changes associated with symptoms of depression and anxiety. In contrast, the tri-level model defines symptom dimensions that are common (General Distress) or relatively specific (Anhedonia-Apprehension, Fears) to depression and anxiety related disorders, which provide increased precision. In the current study, eligibility was assessed by quasi-orthogonal screening questionnaires measuring reward and threat sensitivity (Behavioral Activation Scale; Eysenck Personality Questionnaire-Neuroticism). These participants were assessed on tri-level symptom severity and completed the Monetary Incentive Delay task during fMRI scanning. VS-mOFC and VS-amygdala connectivity were estimated during reward anticipation and reward outcome. Heightened General Distress was associated with lower VS-mOFC connectivity during reward anticipation (b = -0.064, p = 0.021) and reward outcome (b = -0.102, p = 0.014). Heightened Anhedonia-Apprehension was associated with greater VS-amygdala connectivity during reward anticipation (b = 0.065, p = 0.004). The present work has important implications for understanding the coupling between the mOFC and vS and the amygdala and the vS during reward processing in the pathophysiology of mood and anxiety symptoms and for developing targeted behavioral, pharmacological, and neuromodulatory interventions to help manage these symptoms.
1. Introduction
Dimensional models of psychopathology provide greater clinical precision relative to categorical diagnoses and may help identify relationships between specific symptoms and specific neural circuits (Barch, 2017, Cuthbert and Insel, 2013, Kotov et al., 2017, Kramer et al., 2019). A growing body of work examines neural differences that correspond with general factors of psychopathology to identify transdiagnostic characteristics of various disorders (Barch, 2017). Equally important is the need to identify brain systems that distinguish different profiles of psychiatric symptoms. In parallel, human imaging research increasingly examines connections between brain regions as opposed to their activation in isolation from one another. This focus on integrated neural systems may provide meaningful information about underlying mechanisms that characterize specific clinical symptoms. To address this, the current study relates functional connectivity within the brain’s reward circuit to dimensional symptoms that are common and relatively specific to depression and anxiety.
Research in animals and humans highlights the fronto-striatal-amygdala neural circuit in processing reward-related stimuli (Haber, 2011, Haber and Knutson, 2010). In this circuit, the ventral striatum (VS) represents a point of convergence of the nucleus accumbens, the rostroventral putamen, and the caudate head (Haber, 2016). It is thought to assess the hedonic value of rewards and integrate reward-related information from other brain systems (Haber, 2011, Schreuders et al., 2018, Sumiya et al., 2017). The medial orbitofrontal cortex (mOFC) is believed to represent value and to regulate corresponding reward-related behavior (Rolls et al., 2020). The amygdala is linked to motivation and the salience of stimuli (LeDoux, 2007, Mahler and Berridge, 2012, Warlow et al., 2020, Warlow and Berridge, 2021) and responds to reward-relevant cues (Dhingra et al., 2020, Lichtenberg et al., 2021, Wassum and Izquierdo, 2015). Further, the amygdala may modulate reward-related behavior via excitatory connections with the vS (Haber and Knutson, 2010). Coordinated activity in the fronto-striatal-amygdala circuit is linked to positive affect and value-based decision making in clinical and non-clinical samples (Berridge and Kringelbach, 2015, Haber, 2017, Haber and Knutson, 2010, Lichtenberg et al., 2021, Rangel et al., 2008).
Depression and anxiety are associated with altered activation in the fronto-striatal-amygdala circuit (Auerbach et al., 2022, Der-Avakian and Markou, 2012, Ng et al., 2019). Depression is consistently associated with reduced vS activation to rewarding stimuli (Borsini et al., 2020, Gaffrey et al., 2018, Ng et al., 2019). Anxiety related findings are less consistent, but similar reductions in vS responses to rewarding stimuli have been noted (Auerbach et al., 2022, Robin and Martin, 2010). With respect to the mOFC, heightened or reduced activation appears in individuals with depression (Borsini et al., 2020, Ng et al., 2019, Rolls et al., 2020). In both cases, this is thought to reflect maladaptive regulation of reward processing in the subcortex and corresponding behavior in depression (Höflich et al., 2019, Ng et al., 2019, Rolls, 2000). In contrast to depression, anxiety does not appear to be associated with changes in the mOFC during reward processing (Robin and Martin, 2010). Altered amygdala activation is also believed to impact vS responses to reward (Dillon et al., 2014, Fareri and Tottenham, 2016, Robin and Martin, 2010, Tottenham and Galván, 2016). In the case of anxiety, increased amygdala activation is thought to enhance salience and motivational processing, which impacts approach and/or avoidance tendencies (Evans et al., 2008, Lira Yoon et al., 2007, Wendt et al., 2008). Importantly, these changes appear during both fear and reward processing (Robin and Martin, 2010), which highlights the relevance of the amygdala in several emotion related processes. Finally, work suggests a combination of reduced vS and heightened amygdala activation is central to increasingly severe symptoms of comorbid depression and anxiety (Andreescu and Lenze, 2012, Dillon et al., 2014).
Mental health problems are increasingly associated with abnormalities in structural and functional connectivity between regions in the brain (Cisler et al., 2014, Damme et al., 2017, Furman et al., 2011). With respect to fronto-striatal-amygdala circuits, previous work has focused largely on resting state and structural measures of connectivity (Damme et al., 2017, Furman et al., 2011, Kaiser et al., 2015). However, task-specific changes in connectivity are hypothesized to provide useful information about the underlying functions of brain networks (Damme et al., 2019). Increased connectivity between the prefrontal cortex and the vS has been observed in depressed samples when experiencing monetary losses compared with rewards (Quevedo et al., 2017). This relative increase in connectivity, or increase in communication between the prefrontal cortex and the vS is thought to reflect greater attention to losses and pessimistic thinking (Quevedo et al., 2017). In a separate study, depressed participants showed decreased connectivity between the ventromedial prefrontal cortex and several clusters in the striatum during reward anticipation (Walsh et al., 2017). Here, attenuated connectivity is thought to reflect reduced reward-related responses in the vS following down regulation by the prefrontal cortex (Walsh et al., 2017).
Dimensional symptom measures may partially address problems with comorbidity and biological overlap between categorical disorders (Barch, 2017, Cuthbert and Insel, 2013, Kotov et al., 2017, Kramer et al., 2019). This overlap has received growing attention in the context of mood and anxiety disorders (Craske et al., 2011, Kramer et al., 2019, Prenoveau et al., 2011, Shankman et al., 2013), and several taxonomies of dimensional clinical symptoms provide potential solutions (Kotov et al., 2017, Kramer et al., 2019, Naragon-Gainey et al., 2016, Prenoveau et al., 2011). The tri-level model is a well-established dimensional model that examines symptoms that are shared (General Distress) and relatively specific to depression (Anhedonia-Apprehension) and anxiety (Fears) (Kramer et al., 2019, Naragon-Gainey et al., 2016, Prenoveau et al., 2011, Williams et al., 2021). General Distress characterizes the intensity of negative emotions such as anxiety, irritability, and hopelessness (Kramer et al., 2019). Anhedonia-Apprehension corresponds with a lack of positive affect (Kramer et al., 2019). Fears describes agoraphobic, interoceptive, social and specific phobia symptoms (Kramer et al., 2019). This model is reliable (Kramer et al., 2019, Naragon-Gainey et al., 2016, Prenoveau et al., 2011, Williams et al., 2021) and is similar to other models of internalizing disorders (Kotov et al., 2017). In the context of neuroimaging, the tri-level model is well suited to reveal shared and unique patterns of disrupted brain circuitry underlying depression and anxiety. In two studies, trilevel symptoms have been related to emotion related brain processing during fMRI scanning in the context of a fear task (Peng et al., 2023, Young et al., 2021). In one case, anxiety symptoms, Fears, were associated with greater neural responsivity during a fear acquisition paradigm (Peng et al., 2023). In the second, Anhedonia-Apprehension was associated with altered activation in the amygdala, insula, and dorsal anterior cingulate cortex during fear extinction (Young et al., 2021). Both studies highlight neural changes that relate to specific dimensions of mood and anxiety symptoms. However, very little research has examined the relationship between trilevel symptoms and neural activity in the context of reward.
The present study examined the relationship between the tri-level symptom dimensions and functional connectivity in the fronto-striatal-amygdala circuit during reward anticipation and reward outcome using the monetary incentive delay (MID) task. We examined these relationships in a sample of 18–19 year old participants who were recruited to maximize variation in threat and reward sensitivity, as well as risk for symptoms of depression and anxiety. As in prior reports, we examined the functional relationships between the vS and the mOFC (Höflich et al., 2019, Quevedo et al., 2017, Walsh et al., 2017, Young et al., 2016) and the vS and the amygdala (Fareri and Tottenham, 2016, Warlow et al., 2020). In this context, increased connectivity suggests heightened communication between two regions during reward processing. Decreased connectivity reflects reduced communication between two specified brain regions during reward processing. Drawing on previous literature we make two predictions. First, we predict that heightened General Distress will be associated with decreased VS-amygdala connectivity during reward anticipation and outcome. We base this prediction on past work that links hyperactive amygdala responses and hypoactive vS responses to heightened severity of depression and anxiety (Dillon et al., 2014). This pattern of activation may reflect reduced communication in the VS-amygdala as rewards are judged to be less salient in those with heightened General Distress. Such a finding would also support past work that notes how maladaptive changes in the VS-amygdala circuit lead to ineffective salience detection and corresponding behavior (Tottenham and Galván, 2016). Second, we predict that heightened Anhedonia-Apprehension will be associated with decreased VS-mOFC connectivity during reward anticipation and outcome. We base this prediction on meta-analytic work that documents heightened prefrontal cortical activation and lowered vS activation to rewards (Ng et al., 2019). We suggest this pattern of activation may be the result of depressed participants who engage the prefrontal cortex to downregulate vS during reward processing. The present work also performs exploratory analyses that assess the relationships between Fears and connectivity in the VS-mOFC and VS-amygdala circuits. Given the exploratory nature of these analyses, we do not make any formal predictions about associations with the Fears symptom dimension.
2. Methods
2.1. Participants
Participants were recruited for the Brain, Motivation, and Personality Development (BrainMAPD) study at the University of California, Los Angeles and Northwestern University. This study investigated risk for depression and anxiety in emerging adulthood. Data from the BrainMAPD dataset has been published on previously in the context of threat processing (Peng et al., 2023, Rosenberg et al., 2021, Young et al., 2022, 2021). The present work is the first published study that focuses on reward processing in this sample.
Participants were aged 18–19 years and were screened from an initial sample of 2,461 individuals. Initial eligibility was assessed by quasi-orthogonal screening questionnaires measuring reward sensitivity and threat sensitivity (Behavioral Activation Scale, BAS; Eysenck Personality Questionnaire-Neuroticism, EPQ-R-N). Recruitment ensured sampling from high/mid/low ranges (tertiles) on both scales, with oversampling from the two diagonals of their bivariate space (i.e., high EPQ-R-N/high BAS, low EPQ-R-N/low BAS, mid EPQ-R-N/mid BAS, high EPQ-R-N/low BAS and low EPQ-R-N/high BAS).
Participants were excluded based on the following criteria: lack of right-handed dominance, not fluent in English, traumatic brain injury, MRI contraindications, pregnancy, color blindness, lifetime psychotic symptoms, bipolar I disorder, clinically significant substance use disorder in the past 6 months, and antipsychotic medication usage. Importantly, to improve ecological validity, participants were not excluded who were currently taking other types of psychoactive medication (N = 21). Participants provided written, informed consent and all procedures were approved by the IRB at each institution.
This resulted in a sample of 256 participants (182 female, mean age = 19.16 years, SD = 0.52), some of whom were excluded for problems with data collection (e.g. participant fell asleep during MRI), if greater than 10 % of a participant’s brain images were considered motion outliers (3 mm difference between frames), or if the participant received negative winnings at the end of the task (total N = 36). We also used the generalized extreme studentized deviate test (Rosner, 1983) to identify and remove outliers based on connectivity estimates (N = 7) and trilevel symptoms (N = 1). Following exclusion, a total of 212 (148 female) participants were included in the final analytic sample (see Table 1 for demographics). There were no significant differences in any demographic variables between the final analytic sample and those who were excluded from this sample.
Table 1.
Demographic details of the current sample (n = 212).
| Demographic Information | |
|---|---|
| Biological Sex (count) | |
| Male | 64 |
| Female | 148 |
| Total | 214 |
| Medication total (count) | 21 |
| Bupropion/Wellbutrin | 0 |
| Anti-depressant | 11 |
| Anti-anxiety | 0 |
| Mood Stabilizer | 3 |
| Attention | 7 |
| Hispanic (count) | 58 |
| Race (count) | |
| White | 111 |
| Asian | 60 |
| Black | 19 |
| Native American | 3 |
| Multiracial | 18 |
| Declined to respond | 1 |
| Employment (count) | |
| Full time student | 118 |
| Part time work, full time student | 84 |
| Full time work, full time student | 5 |
| Full time work only | 0 |
| Keeping house | 1 |
| Declined to respond | 4 |
| Total Gross Family Income (count) | |
| Less than $5000 | 1 |
| $5,000-$19,999 | 5 |
| $20,000-$34,999 | 14 |
| $35,000-$49,999 | 19 |
| $50,000-$74,999 | 25 |
| $75,000-$99,999 | 18 |
| $100,000-$149,999 | 39 |
| $150,000-$199,999 | 26 |
| $200,000 and greater | 37 |
| Declined to respond | 28 |
The Structured Clinical Interview for DSM-5 (First, 2015) was used to assess for psychiatric diagnoses. All interviewers had at least a bachelor’s degree and underwent extensive training and supervision. Interviewers presented completed SCID cases at a supervision meeting led by a doctoral-level supervisor to reach consensus. Previously, our research team has achieved good inter-rater agreement for DSM diagnoses (Prenoveau et al., 2011, Zinbarg et al., in press). In the final analytic sample, 31 % of participants (N = 65) met criteria for a past diagnosis of a unipolar depressive episode and 5 % of participants (N = 10) met criteria for a current diagnosis of a unipolar depressive disorder. Thirty-three percent of participants (N = 70) met criteria for a past diagnosis of an anxiety disorder and 18 % of participants (N = 39) met criteria for a current diagnosis of an anxiety disorder.
2.2. Symptom assessment and factor analysis
Immediately prior to the MRI scan, participants completed questionnaires related to mood and anxiety disorders. Responses on these questionnaires were used to generate the tri-level model symptoms. Questionnaires included the following: Fear Survey Schedule-II (Geer, 1965), Albany Panic and Phobia Questionnaire (Rapee et al., 1994), Self-Consciousness subscale of the Social Phobia (Herbert et al., 2014, Zinbarg et al., 2016), Inventory to Diagnose Depression (Zimmerman and Coryell, 1987), Mood and Anxiety Symptom Questionnaire (Watson et al., 1995), Penn State Worry Questionnaire (Meyer et al., 1990), and Obsessive Compulsive-Inventory Revised (Foa et al., 2002).
As in prior work (Naragon-Gainey et al., 2016, Prenoveau et al., 2011), we employed a hierarchical model with three levels. This model includes a broad general factor (General Distress), two intermediate factors (Fears and Anhedonia-Apprehension), and several narrow factors. These factors are arranged in a hierarchical structure and were estimated using a bifactor model, which allows each item to load directly on multiple uncorrelated factors (Naragon-Gainey et al., 2016). Model fit for this tri-level model factor structure was tested in our sample using Mplus version 8.2 (Muthén and Muthén, 1998). Three fit indices were used: CFI, RMSEA, and WRMR. As was suggested in prior work (Hu and Bentler, 1999), cutoffs for adequate fit were CFI values greater than or equal to 0.9 (with good fit being indicated by values greater than or equal to 0.95), RMSEA values less than or equal to 0.06 and SRMR values less than or equal to 0.08. Based on the above criteria, confirmatory factor analyses demonstrated goodness of fit of the tri-level hierarchical model to the data collected in the present study (Kramer et al., 2019, Naragon-Gainey et al., 2016, Prenoveau et al., 2011, Young et al., 2021). Factor estimates from this model were saved and used to represent symptom dimensions of General Distress, Fears, and Anhedonia-Apprehension.
We defined an approximate clinical cutoff for this sample. This cutoff was meant to characterize those with a formal DSM diagnosis and to define clinical severity for those who hadn’t received a formal diagnosis in the sample, but still experience clinically elevated symptoms. As in prior work (Zinbarg et al., in press), the examination of the relative frequency distributions of General Distress factor scores suggested that a score of approximately 0.5 was where the distributions for those with a current diagnosis of an anxiety disorder or unipolar depressive disorder and those without such a diagnosis crossed and therefore is a reasonable cutoff for clinical levels (Jacobson and Truax, 1991). This is further supported by findings that showed a cutoff of 0.5 General Distress discriminated among groups of participants with no diagnosis, with either a current anxiety or unipolar depression diagnosis, and those with both current diagnoses (Zinbarg et al., in press). Based on this cutoff, 29.1 % of participants in the BrainMAPD sample scored within clinical range.
2.3. Monetary Incentive Delay task
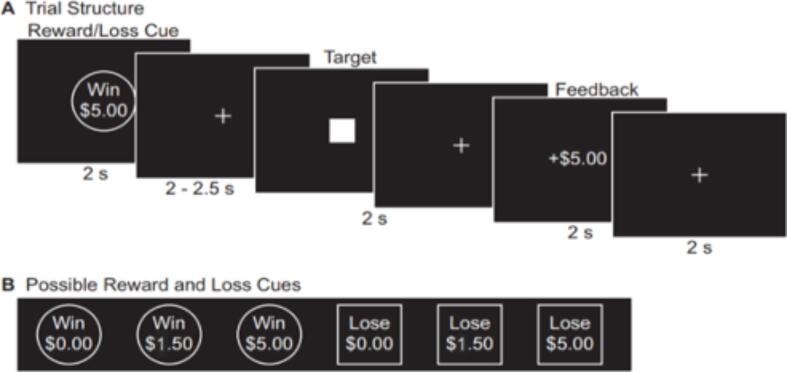
Participants completed two runs of the MID task depicted in Fig. 1 (Samanez-Larkin et al., 2014). First, a circle cue signaling a reward trial (the participant has the opportunity to Win $0.00, Win $1.50, or Win $5.00) or a square cue indicating a loss trial (the participant might Lose $0.00, Lose $1.50, or Lose $5.00) was presented for 2 s. Then, a jittered (2–2.5 s) fixation was presented followed by a solid white square. Participants were instructed to make a button response when the solid white square was still on the screen to either win money (reward trials) or avoid losing money (loss trials). After a 2 s fixation, feedback was presented to participants for 2 s that detailed the amount of money won or lost on the present trial. A jittered fixation cross was then presented for 2 s, 4 s, or 6 s as an intertrial interval. The initial target duration was calculated from each participant’s mean hit reaction time on a MID practice run completed before entering the scanner. The target duration dynamically updated during the MID task to maintain 66 % accuracy across all trials. This accuracy was calculated for each trial type separately (i.e., Win $0.00, Win $1.50, Win $5.00, Lose $0.00, Lose $1.50, Lose $5.00). The six trial types each were presented 8 times in random order, totaling 96 trials, across two runs of the task.
Fig. 1.
Monetary Incentive Delay Task.Note: A) Trial structure and timing for an example Win $5 trial B) All possible trial types presented to participants.
The current work focused on two epochs within this task. The anticipation period was defined by the start of the cue with a 4 s duration. The outcome period was defined by the start of the feedback with a 4 s duration. The motor period, which was controlled for in subsequent models, was defined by the onset of the solid white square with a 2 s duration.
2.4. fMRI acquisition and preprocessing
Structural and functional brain images were collected on Siemens 3 T scanners located at the University of California Los Angeles and Northwestern University. Structural 3D axial MPRAGE images were acquired (0.8 mm thick; TR = 2300 ms; TE = 3.03 ms; FOV = 256x256; Matrix = 160x160; Flip Angle = 7°; 192 slices). Functional runs utilized a gradient echo EPI sequence covering 64 axial slices (2.0 mm thick; TR = 2050 ms; TE = 25 ms; FOV = 208x208mm; Matrix = 104x104; Flip Angle = 76°; Multi-band acceleration Factor = 2). Data were preprocessed using fMRIPrep 20.2.3 (RRID:SCR_016216; (Esteban et al., 2019), which is based on Nipype 1.3.1 (RRID:SCR_002502; (Gorgolewski et al., 2011). Full details of this pipeline can be found in supplemental material (S1).
2.5. Region of interest selection
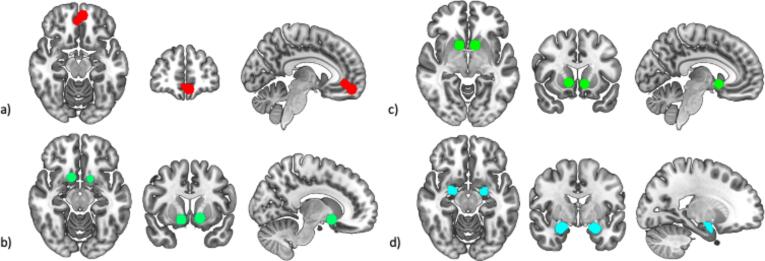
Similar to prior research (Haber, 2016), we define the vS as the nucleus accumbens, rostroventral putamen, and caudate head. Previous meta-analytic work (Oldham et al., 2018) identified two separate pairs of peak bilateral coordinates in this functionally defined vS which were specific to gain anticipation and gain outcome during the MID task. We drew 8 mm spheres around each of these peak coordinates, which served as seed regions in subsequent analysis. We extracted average signal from these spheres to be used in first level models. The same meta-analysis identified three peak coordinates in the mOFC that related to both gain anticipation and outcome (Oldham et al., 2018). Signal from these three spheres were averaged to create a single mOFC region-of-interest (ROI). A functional ROI was not available for the amygdala in the above meta-analysis so an anatomically defined amygdala mask from the Harvard Oxford brain atlas was used (Bakker et al., 2015). A 50 % probabilistic threshold was used to define this ROI. Average signal from this bilateral amygdala seed were used in subsequent analyses for the anticipation and outcome epochs. See Fig. 2 for images of all regions.
Fig. 2.
Regions of interest used for seed-to-seed analyses. All images were generated using the mricroGL software package (Rorden and Brett, 2000) a) medial orbitofrontal cortex b) ventral striatum (reward anticipation) c) ventral striatum (reward outcome) d) amygdala.
2.6. Psychophysiological interaction (PPI) models
Software from the CanlabCore toolbox (https://github.com/canlab/CanlabCore.git) and the SPM12 software suite (Ashburner et al., 2021) were used to generate PPI models. Reward anticipation and outcome were modeled separately. Whole brain first level models involved a voxelwise estimation of connectivity with the chosen vS seed. In PPI, connectivity estimates are generated in individual brains that reflect task specific changes in connectivity. Presently, these connectivity estimates reflect differences in communication during gain anticipation versus neutral gain anticipation as well as successful gain outcomes versus unsuccessful gain outcomes. Positive estimates from these models suggest heightened communication between regions noted above (VS-mOFC, VS-amygdala) during respective events in the MID task. Conversely, negative estimates from these models indicate reduced communication between regions during respective events in the MID task. Importantly, these PPI estimates are thought to reflect changes in communication above and beyond correlations between time series data between two regions of interest (O’Reilly et al., 2012). This is achieved as each model includes standard task regressors as variables of no interest as well as a single regressor to represent average activation within the seed region. This adjusts for task related activation and spontaneous activation of the seed. Connectivity estimates reflect the interaction of these signals, generated by convolving a design matrix, average time series of the vS and the hemodynamic response function to provide information above and beyond activation. PPI is considered a conservative test and there is often concern of Type II error (O’Reilly et al., 2012). However, when this approach yields significant effects, they are believed to be quite reliable.
The anticipation model included five task regressors that corresponded with Win $5/Win $1.50 trials, Lose $5/Lose $1.50 trials, Win $0 trials, Lose $0 trials, and the motor period across all trials. These regressors were convolved with the canonical hemodynamic response function and input into the PPI model as regressors of no interest. Average time series data from the anticipation vS seed were extracted using the CanlabCore toolbox and input into the model as a single regressor of no interest. Each task regressor was multiplied by the average time series data of the vS to provide four interaction terms that corresponded with the four task conditions, excluding the motor period. These four regressors, derived from the interaction between task related activation in response to anticipation trials and average activation in the vS were used for two subsequent contrasts. Gain anticipation trials were contrasted with neutral-gain anticipation trials. Loss anticipation trials were contrasted with neutral-loss anticipation trials. Masks for the mOFC and amygdala were applied to these contrasts, which resulted in estimates of VS-mOFC and VS-amygdala connectivity for the anticipation phase. Nuisance covariates included 12 motion regressors for motion in the ×, y, and z directions as well as the first derivative of each motion term. One regressor was included to account for cerebral spinal fluid and one regressor was included for average white matter signal. Finally, spike regression (Han et al., 2022) was used to identify outlier images in each participant’s time series data and flag them in first level models.
The outcome model included five task regressors that included trials where the participant successfully gained $5/$1.50, trials where the participant failed to gain $5/$1.50, trials where the participant successfully avoided losing $5/$1.50, trials where the participant lost $5/$1.50, and the motor period across all trials. These regressors were convolved with the canonical hemodynamic response function and input into the PPI model as regressors of no interest. Average time series data from the outcome vS seed were extracted using the CanlabCore toolbox and input into the model as a single regressor of no interest. Each task regressor was multiplied by the average time series data of the vS to provide four interaction terms that corresponded with the four task conditions, excluding the motor period. These four regressors used for two subsequent contrasts. Successful gain trials of $5/$1.50 were contrasted with unsuccessful gain trials of $5/$1.50. Trials where participants avoided a loss of $5/$1.50 were contrasted with trials where participants did not avoid a loss of $5/$1.50. Masks for the mOFC and amygdala were applied to these contrasts, which resulted in estimates of VS-mOFC and VS-amygdala connectivity for the outcome phase. Nuisance covariates in this model were identical to those described for the anticipation models.
2.7. Group level analysis
For each seed-to-seed estimate, three group level models were run that tested for relationships between connectivity and each symptom dimension separately (General Distress, Anhedonia-Apprehension, Fears). This analysis was done separately for both reward anticipation and reward outcome. Covariates of no interest included study site, participant gender, and psychoactive medication status. Connectivity estimates for the respective loss condition were also included in group level models, which makes effects relatively specific to reward trials. If these single symptom models yielded significant results, we assessed for specificity by running an additional model that simultaneously included all symptoms dimensions. These follow-up specificity analyses tested whether each effect is significant above and beyond the impact of other symptom dimensions. In total, this series of tests generate four models for gain anticipation and four models for gain outcome. The first three models estimate the relationship between individual symptoms and connectivity. The fourth model provides an estimate of this relationship while adjusting for other symptoms and yields an estimate for all three tri-level symptoms simultaneously.
Positive estimates from these models correspond with relative increases in connectivity that are associated with heightened clinical symptoms. Negative estimates correspond with relative decreases in connectivity that are associated with heightened clinical symptoms.
2.8. Permutation testing to assess for false discovery rate
To adjust for multiple comparisons, we used a permutation-based approach. In the case of a significant finding from one of the single symptom models described above, a null distribution of contrast estimates was generated by randomly shuffling connectivity estimates across participants and then refitting group level models to this permuted data. This test was performed 10,000 times. Using the beta estimate from each respective group level model as a threshold, the number of estimates in the null distribution that are above threshold are summed and divided by the total number of permutations. Permutation analyses result in a value (q) that is interpreted similarly to a p-value that incorporates false discovery rate multiple testing correction (Noble, 2009). Values below 0.05 are considered unlikely to be due to chance.
3. Results
3.1. Demographics
Descriptive statistics for demographic and clinical variables are presented in Table 1. Before testing for associations between clinical symptoms and brain connectivity, a series of t-tests evaluated associations between symptom dimensions (General Distress, Anhedonia-Apprehension, Fears) and other nuisance regressors used in the group level models. None of the symptom dimensions differed by study site (p’s greater than 0.056). Female participants showed significantly higher ratings on General Distress (t = 9.44, p < 0.0001), Anhedonia-Apprehension (t = 10.22, p < 0.0001), and Fears (t = 9.97, p < 0.0001). At the time of data collection, 21 participants were taking psychoactive medication (Table 1). Participants taking medication did not significantly differ from those not taking medication on ratings of General Distress (t = 0.86, p = 0.388), Anhedonia-Apprehension (t = 1.92, p = 0.056), or Fears (t = 1.14., p = 0.257).
3.2. Relationship between tri-level symptoms and neural connectivity during reward anticipation
3.2.1. General Distress
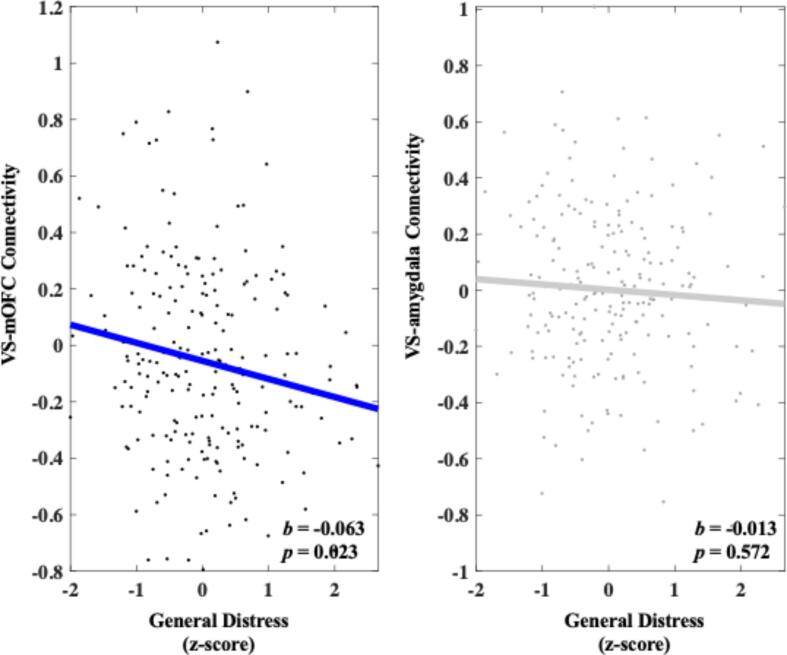
Heightened General Distress was associated with decreased VS-mOFC connectivity during reward anticipation compared with neutral trials (b = -0.063, 95 % C.I. [-0.117, −0.009], p = 0.023). A follow-up permutation test indicated that this effect was unlikely due to chance (q = 0.011). A follow-up specificity analysis showed that heightened General Distress remained significantly associated with decreased VS-mOFC connectivity above and beyond the other symptom dimensions (b = -0.064, 95 % C.I. [-0.119, −0.010], p = 0.021). Heightened General Distress was not significantly associated with VS-amygdala connectivity during reward anticipation compared with neutral trials (b = -0.013, 95 % C.I. [-0.059, 0.033], p = 0.572). These results can be seen in Fig. 3.
Fig. 3.
Relationship between General Distress and connectivity in both the VS-mOFC and VS-amygdala circuits during reward anticipation. Values on the y-axis reflect estimated differences in connectivity during gain anticipation versus neutral gain anticipation extracted from whole brain contrast maps. Values come from single symptom models that adjust for sex, site, medication status, and connectivity calculated during the corresponding loss contrast. Symptom scores are presented as z-scores and reflect variation around average symptom severity in the sample.
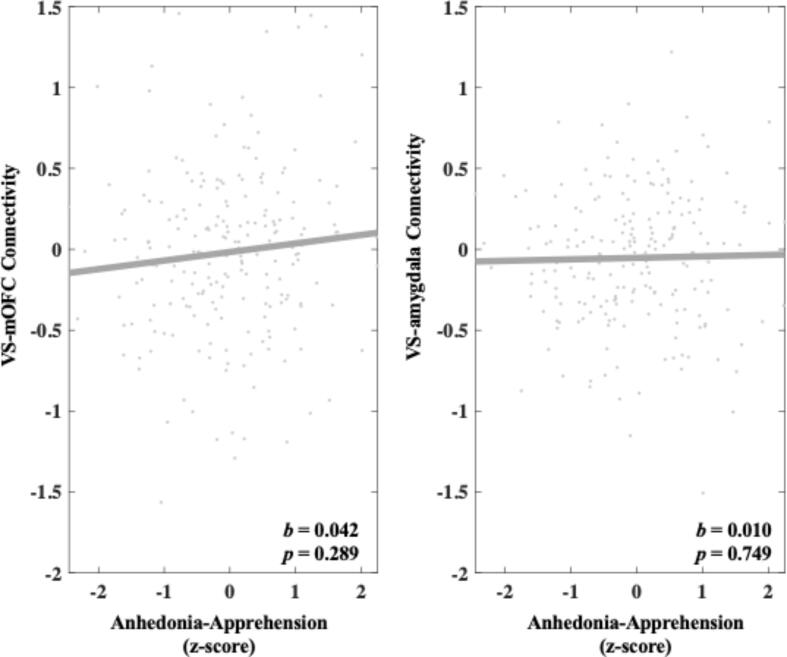
3.2.2. Anhedonia-Apprehension
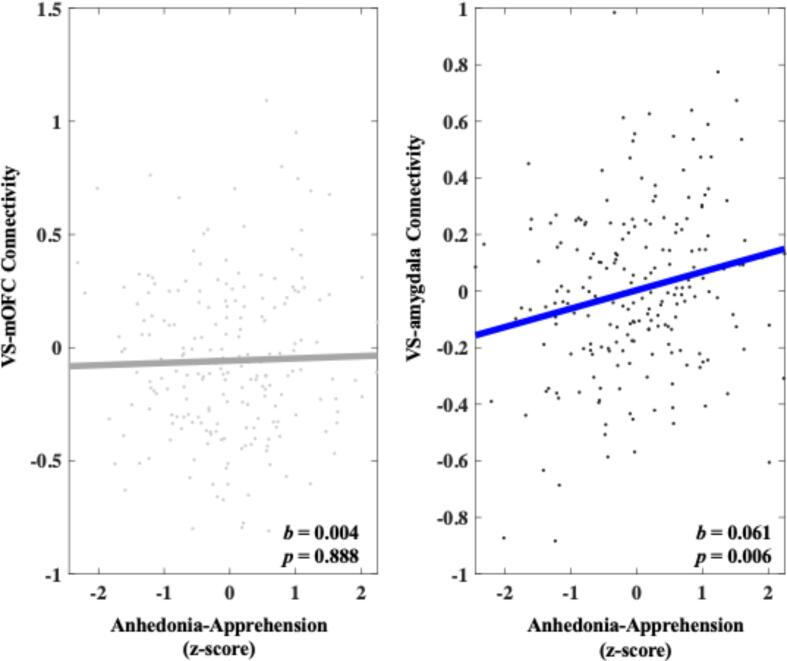
Heightened Anhedonia-Apprehension was not significantly associated with increased VS-mOFC connectivity during reward anticipation compared with neutral trials (b = 0.004, 95 % C.I. [-0.049, 0.056], p = 0.888). Heightened Anhedonia-Apprehension was significantly associated with increased VS-amygdala connectivity during reward anticipation compared with neutral trials (b = 0.061, 95 % C.I. [0.018, 0.104], p = 0.006). A follow-up permutation test indicated that this effect was unlikely due to chance (q = 0.003). A specificity analysis showed that heightened Anhedonia-Apprehension remained significantly associated with increased VS-amygdala connectivity (b = 0.065, 95 % C.I. [0.022, 0.109], p = 0.004) above and beyond other symptom dimensions. These results can be seen in Fig. 4.
Fig. 4.
Relationship between Anhedonia-Apprehension and connectivity in both the VS-mOFC and VS-amygdala circuits during reward anticipation. Values on the y-axis reflect estimated differences in connectivity during gain anticipation versus neutral gain anticipation extracted from whole brain contrast maps. Values come from single symptom models that adjust for sex, site, medication status, and connectivity calculated during the corresponding loss contrast. Symptom scores are presented as z-scores and reflect variation around average symptom severity in the sample.
3.2.3. Fears
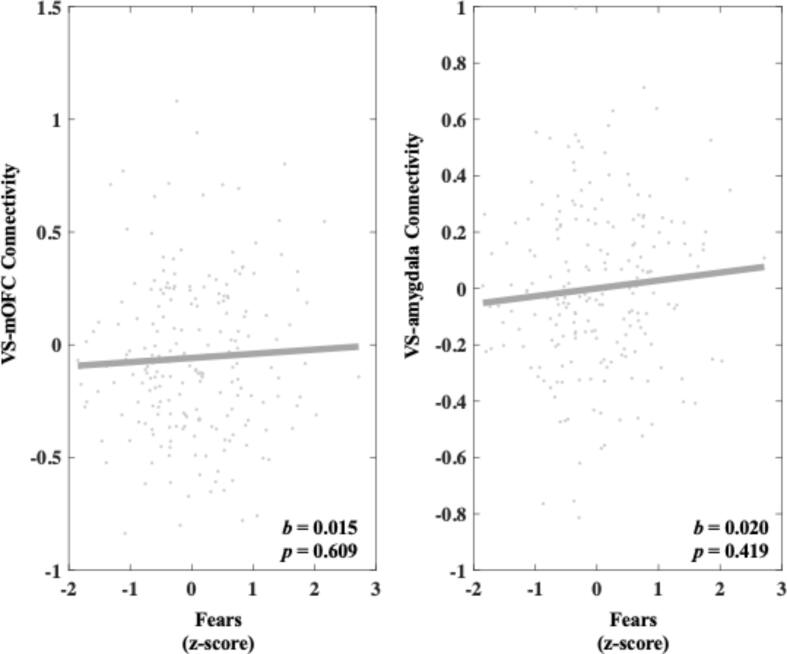
Heightened Fears was not significantly associated with VS-mOFC connectivity (b = 0.015, 95 % C.I. [-0.042, 0.072], p = 0.609) or VS-amygdala connectivity during reward anticipation compared to neutral trials (b = 0.020, 95 % C.I. [-0.028, 0.067], p = 0.419). These results can be seen in Fig. 5.
Fig. 5.
Relationship between Fears and connectivity in both the VS-mOFC and VS-amygdala circuits during reward anticipation. Values on the y-axis reflect estimated differences in connectivity during gain anticipation versus neutral gain anticipation extracted from whole brain contrast maps. Values come from single symptom models that adjust for sex, site, medication status, and connectivity calculated during the corresponding loss contrast. Symptom scores are presented as z-scores and reflect variation around average symptom severity in the sample.
3.3. Relationship between tri-level symptoms and neural connectivity during reward outcome
3.3.1. General Distress
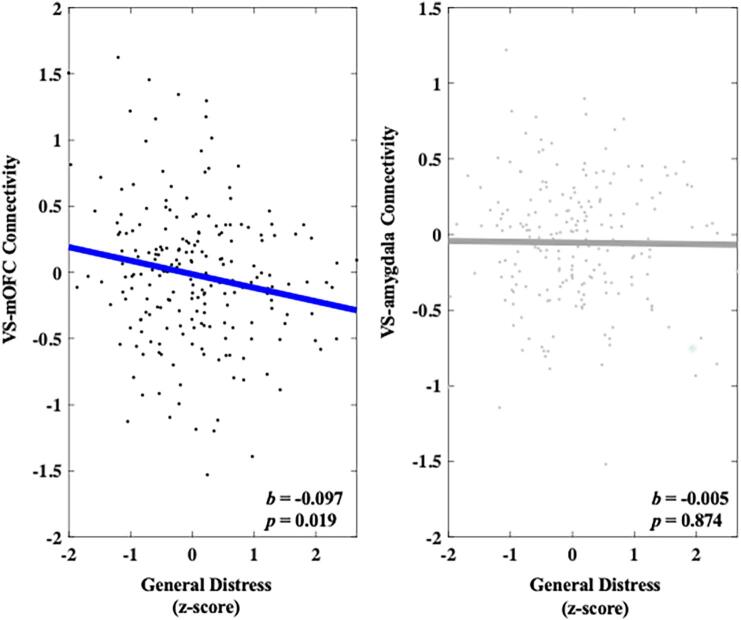
Heightened General Distress was significantly associated with decreased VS-mOFC connectivity (b = -0.097, 95 % C.I. [-0.179, −0.016], p = 0.019) during successful reward outcomes compared with unsuccessful reward outcomes. A follow-up permutation test indicated that this effect was unlikely due to chance (q = 0.010). A follow-up specificity analysis showed that heightened General Distress remained significantly associated with decreased VS-mOFC connectivity above and beyond the other symptom dimensions (b = -0.102, 95 % C.I. [-0.184, −0.021], p = 0.014). Heightened General Distress was not associated with VS-amygdala connectivity during successful reward outcomes compared with unsuccessful reward outcomes (b = -0.005, 95 % C.I. [-0.069, 0.058], p = 0.874). These results can be seen in Fig. 6.
Fig. 6.
Relationship between General Distress and connectivity in both the VS-mOFC and VS-amygdala circuits during reward outcome. Values on the y-axis reflect estimated differences in connectivity during successful gain outcomes versus unsuccessful gain outcomes extracted from whole brain contrast maps. Values come from single symptom models that adjust for sex, site, medication status, and connectivity calculated during the corresponding loss contrast. Symptom scores are presented as z-scores and reflect variation around average symptom severity in the sample.
3.3.2. Anhedonia-Apprehension
Heightened Anhedonia-Apprehension was not associated with VS-mOFC connectivity (b = 0.042, 95 % C.I. [-0.036, 0.121], p = 0.289) or VS-amygdala connectivity during successful reward outcomes compared with unsuccessful reward outcomes (b = 0.010, 95 % C.I. [-0.051, 0.071], p = 0.749). These results can be seen in Fig. 7.
Fig. 7.
Relationship between Anhedonia-Apprehension and connectivity in both the VS-mOFC and VS-amygdala circuits during reward outcome. Values on the y-axis reflect estimated differences in connectivity during successful gain outcomes versus unsuccessful gain outcomes extracted from whole brain contrast maps. Values come from single symptom models that adjust for sex, site, medication status, and connectivity calculated during the corresponding loss contrast. Symptom scores are presented as z-scores and reflect variation around average symptom severity in the sample.
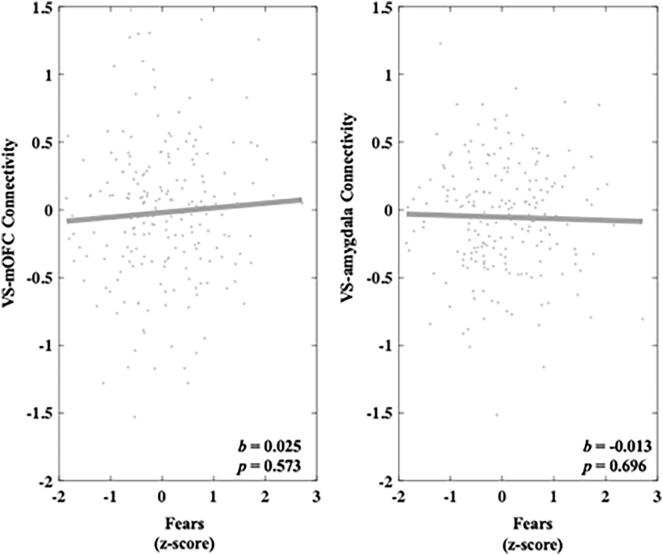
3.3.3. Fears
Heightened Fears was not associated with VS-mOFC connectivity during successful reward outcomes compared with unsuccessful reward outcomes (b = 0.025, 95 % C.I. [-0.061, 0.111], p = 0.573) or VS-amygdala connectivity during successful reward outcomes compared with unsuccessful reward outcomes (b = -0.013, 95 % C.I. [-0.079, 0.053], p = 0.696). These results can be seen in Fig. 8.
Fig. 8.
Relationship between Fears and connectivity in both the VS-mOFC and VS-amygdala circuits during reward outcome. Values on the y-axis reflect estimated differences in connectivity during successful gain outcomes versus unsuccessful gain outcomes extracted from whole brain contrast maps. Values come from single symptom models that adjust for sex, site, medication status, and connectivity calculated during the corresponding loss contrast. Symptom scores are presented as z-scores and reflect variation around average symptom severity in the sample.
4. Discussion
The current study examined associations between functional connectivity within the brain’s reward circuits and dimensional symptoms of depression and anxiety. Heightened General Distress was associated with decreased VS-mOFC connectivity during reward anticipation and reward outcome. Heightened Anhedonia-Apprehension was associated with increased VS-amygdala connectivity during reward anticipation. These effects were observed after adjusting for other symptom dimensions, which suggests they are relatively unique to each respective symptom. All models adjusted for site, sex, medication status, and connectivity during respective loss trials.
Heightened General Distress was associated with decreased VS-mOFC connectivity during reward anticipation and reward outcome. As noted above, we predicted this profile for Anhedonia-Apprehension, but we find it for General Distress instead. This suggests that individuals with high levels of negative affect, as measured by General Distress, are characterized by reduced communication between the mOFC and vS during both the anticipation and receipt of rewards. This may reflect heightened mOFC and blunted vS responses to reward, which has been linked to both anxiety and depression (Borsini et al., 2020, Gaffrey et al., 2018, Ng et al., 2019, Robin and Martin, 2010). Our results are also in line with a growing literature that links transdiagnostic measures of anxiety and depression to alterations in the VS-mOFC circuit (Auerbach et al., 2022, Dillon et al., 2014). Taken together with current results, we suggest that heightened negative emotionality may partially result from ineffective communication between the mOFC and the vS More precisely, those with high levels of distress may engage the mOFC in a manner to blunt subcortical reward responses and positive affect. This would help explain altered approach and avoidance behavior that has been noted in anxiety and depression (Struijs et al., 2017). We believe these findings highlight an important target for interventions, such as transcranial magnetic stimulation, that seek to innervate underlying subcortical reward pathways via pathways through the cortex (Ryan et al., 2022). Considering current findings, such interventions may be efficacious in treating reward dysregulation across a variety of anxiety and depression related disorders.
In addition, we found that heightened Anhedonia-Apprehension was associated with increased VS-amygdala connectivity during reward anticipation. Prior research links the amygdala to motivation and reward processing (Costa and Averbeck, 2021, Murray, 2007). Additional work notes reduced motivation and blunted brain responses to reward in depression (Gaffrey et al., 2018). Present findings bridge these assertions and suggest that heightened Anhedonia-Apprehension relates to greater involvement of the amygdala in reward processing. This may occur via excitatory projections from the amygdala to the vS (Haber and Knutson, 2010), which are thought to shape emotionally salient behavior in response to rewarding or dangerous stimuli (Fareri and Tottenham, 2016). As such, we suggest that Anhedonia-Apprehension may be partially characterized by altered judgment regarding the salience of reward related stimuli, which is thought to occur during the anticipation phase of the MID (Oldham et al., 2018). As Anhedonia-Apprehension is partially associated with reduced positive affect (Kramer et al., 2019), we expect this reflects decreased attention to reward related cues. This would potentially result in blunted reward responses and reduced motivation.
Limitations of the present work include the narrow age range of the sample, thus representing a snapshot of development that may not generalize to other age groups. Second, recruitment in the current study did not recruit specifically for mental illness. While the dataset contains those with current or past depression and anxiety disorders, future work should extend into more clinically severe samples. Third, our sample was primarily composed of white college students. Future work should strive to recruit a more diverse sample. Fourth, PPI is a useful method, but interpretation of contrasted connectivity estimates can be difficult (O’Reilly et al., 2012). Future work might combine estimates of connectivity from varying modalities, including resting state fMRI, to better contextualize present findings. Fourth, to maximize coverage of reward related signal in the vS and mOFC, we used functionally defined ROIs with peak coordinates identified in recent meta-analytic work (Oldham et al., 2018). Future work may wish to employ anatomically defined ROIs to more precisely map clinically relevant changes in reward processing to more specific brain regions. Finally, recent work highlights poor reliability in studies that link clinically relevant behavior to measures of brain function (Marek et al., 2022). This underscores the need for renewed effort to uncover more reliable measures of brain function that align with precise dimensions of clinical experience.
4.1. Conclusions
The present study suggests that empirically derived symptoms can be linked to specific changes in VS-mOFC and VS-amygdala circuits during reward processing. Effects associated with General Distress can be thought of as transdiagnostic. As such, VS-mOFC findings may provide a valid target for biological interventions that innervate subcortical pathways via cortical brain regions in an effort to treat a range of symptoms related to depression and anxiety (Ryan et al., 2022). Current results also indicate heightened communication within VS-amygdala circuit may result in blunted positive affect that is associated with Anhedonia-Apprehension. This second set of findings highlight the potential efficacy of psychotherapeutic interventions, such as cognitive behavioral therapy, that may improve normative regulation of regions like the amygdala (Shou et al., 2017). Results presented are cross-sectional, however, these results provide a framework for longitudinal studies to build on the current work. Such work could provide greater understanding of the role that VS-mOFC and VS-amygdala pathways play in the development of depression and anxiety.
CRediT authorship contribution statement
Zachary Anderson: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing, Visualization. Katherine S.F. Damme: Funding acquisition, Project administration, Supervision, Writing – review & editing. Ann L. Carroll: Funding acquisition, Project administration, Supervision, Writing – review & editing. Iris Ka-Yi Chat: Funding acquisition, Project administration, Supervision, Writing – review & editing. Katherine S. Young: Project administration, Writing – review & editing, Data curation. Michelle G. Craske: Funding acquisition, Project administration, Supervision, Writing – review & editing. Susan Bookheimer: Funding acquisition, Project administration, Supervision, Writing – review & editing. Richard Zinbarg: Funding acquisition, Project administration, Supervision, Writing – review & editing. Robin Nusslock: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Funding for Z.A. was provided by the T32 Training Program in the Neuroscience of Human Cognition (T32 NS047987). Funding for K.S.F. Damme was supported by the T32 Training Program Mental Health Earlier (T32MH126368). Additional funding for data collection and publication were provided by a R01 grant awarded by the National Institute of Mental Health (R01 MH100117). We would also like to acknowledge the following researchers who played a vital role in the collection of data or conceptualization of the original project: Marcelina Perez, Kelly Chen, Aileen Echiverri Cohen, and Meghan Vinograd.
Data availability
Data will be made available on request.
References
- Andreescu C., Lenze E.J. Comorbid anxiety and depression: bête noire or quick fix? Br. J. Psychiatry J. Ment. Sci. 2012;200:179–181. doi: 10.1192/bjp.bp.111.097022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, J., Barnes, G., Chen, C.-C., Daunizeau, J., Flandin, G., Friston, K., Gitelman, D., Glauche, V., Henson, R., Hutton, C., Jafarian, A., Kiebel, S., Kilner, J., Litvak, V., Mattout, J., Moran, R., Penny, W., Phillips, C., Razi, A., Zeidman, P., 2021. SPM12 Manual.
- Auerbach R.P., Pagliaccio D., Hubbard N.A., Frosch I., Kremens R., Cosby E., Jones R., Siless V., Lo N., Henin A., Hofmann S.G., Gabrieli J.D.E., Yendiki A., Whitfield-Gabrieli S., Pizzagalli D.A. Reward-Related Neural Circuitry in Depressed and Anxious Adolescents: A Human Connectome Project. J. Am. Acad. Child Adolesc. Psychiatry. 2022;61:308–320. doi: 10.1016/j.jaac.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker R., Tiesinga P., Kötter R. The Scalable Brain Atlas: Instant Web-Based Access to Public Brain Atlases and Related Content. Neuroinformatics. 2015;13:353–366. doi: 10.1007/s12021-014-9258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D.M. The Neural Correlates of Transdiagnostic Dimensions of Psychopathology. Am. J. Psychiatry. 2017;174:613–615. doi: 10.1176/appi.ajp.2017.17030289. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Kringelbach M.L. Pleasure Systems in the Brain. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini A., Wallis A.S.J., Zunszain P., Pariante C.M., Kempton M.J. Characterizing anhedonia: A systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cogn. Affect. Behav. Neurosci. 2020;20:816–841. doi: 10.3758/s13415-020-00804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., Bush K., Steele J.S. A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. Neuroimage. 2014;84:1042–1052. doi: 10.1016/j.neuroimage.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa V.D., Averbeck B.B. Fluoxetine incentivizes ventral striatum encoding of reward and punishment. Neuropsychopharmacology. 2021;46:2041–2042. doi: 10.1038/s41386-021-01012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske M.G., Rauch S.L., Ursano R., Prenoveau J., Pine D.S., Zinbarg R.E. What Is an Anxiety Disorder? Focus. 2011;9:369–388. doi: 10.1176/foc.9.3.foc369. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Insel T.R. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damme K.S.F., Kelley N.J., Quinn M.E., Glazer J.E., Chat I.-K.-Y., Young K.S., Nusslock R., Zinbarg R., Bookheimer S., Craske M.G. Emotional content impacts how executive function ability relates to willingness to wait and to work for reward. Cogn. Affect. Behav. Neurosci. 2019;19:637–652. doi: 10.3758/s13415-019-00712-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damme K.S., Young C.B., Nusslock R. Elevated nucleus accumbens structural connectivity associated with proneness to hypomania: a reward hypersensitivity perspective. Soc. Cogn. Affect. Neurosci. 2017;12:928–936. doi: 10.1093/scan/nsx017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A., Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. Special Issue: Neuropsychiatric Disorders. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra I., Zhang S., Zhornitsky S., Le T.M., Wang W., Chao H.H., Levy I., Li C.-S.-R. The effects of age on reward magnitude processing in the monetary incentive delay task. Neuroimage. 2020;207 doi: 10.1016/j.neuroimage.2019.116368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon D.G., Rosso I.M., Pechtel P., Killgore W.D.S., Rauch S.L., Pizzagalli D.A. Peril and Pleasure: An Rdoc-Inspired Examination of Threat Responses and Reward Processing in Anxiety and Depression. Depress. Anxiety. 2014;31:233–249. doi: 10.1002/da.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O., Markiewicz C.J., Blair R.W., Moodie C.A., Isik A.I., Erramuzpe A., Kent J.D., Goncalves M., DuPre E., Snyder M., Oya H., Ghosh S.S., Wright J., Durnez J., Poldrack R.A., Gorgolewski K.J. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods. 2019;16:111–116. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans K.C., Wright C.I., Wedig M.M., Gold A.L., Pollack M.H., Rauch S.L. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress. Anxiety. 2008;25:496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- Fareri D.S., Tottenham N. Effects of early life stress on amygdala and striatal development. Dev. Cogn. Neurosci. 2016;19:233–247. doi: 10.1016/j.dcn.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B. In: The Encyclopedia of Clinical Psychology. Cautin R.L., Lilienfeld S.O., editors. John Wiley & Sons Inc; Hoboken, NJ, USA: 2015. Structured Clinical Interview for the DSM (SCID) pp. 1–6. [DOI] [Google Scholar]
- Foa E.B., Huppert J.D., Leiberg S., Langner R., Kichic R., Hajcak G., Salkovskis P.M. The Obsessive-Compulsive Inventory: Development and validation of a short version. Psychol. Assess. 2002;14:485–496. doi: 10.1037/1040-3590.14.4.485. [DOI] [PubMed] [Google Scholar]
- Furman D.J., Hamilton J.P., Gotlib I.H. Frontostriatal functional connectivity in major depressive disorder. Biol. Mood Anxiety Disord. 2011;1:11. doi: 10.1186/2045-5380-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey M.S., Barch D.M., Bogdan R., Farris K., Petersen S.E., Luby J.L. Amygdala Reward Reactivity Mediates the Association Between Preschool Stress Response and Depression Severity. Biol. Psychiatry. 2018;83:128–136. doi: 10.1016/j.biopsych.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geer J.H. The development of a scale to measure fear. Behav. Res. Ther. 1965;3:45–53. doi: 10.1016/0005-7967(65)90040-9. [DOI] [PubMed] [Google Scholar]
- Gorgolewski K., Burns C.D., Madison C., Clark D., Halchenko Y.O., Waskom M.L., Ghosh S.S. Nipype: A Flexible, Lightweight and Extensible Neuroimaging Data Processing Framework in Python. Front. Neuroinformatics. 2011;5 doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N. Neurobiol. Sensat; Reward: 2011. Neuroanatomy of reward: A view from the ventral striatum; p. 235. [PubMed] [Google Scholar]
- Haber S.N. Corticostriatal circuitry. Dialogues. Clin. Neurosci. 2016;18:7–21. doi: 10.31887/DCNS.2016.18.1/shaber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N. In: Decision Neuroscience. Dreher J.-.-C., Tremblay L., editors. Academic Press; San Diego: 2017. Anatomy and Connectivity of the Reward Circuit; pp. 3–19. [DOI] [Google Scholar]
- Haber S.N., Knutson B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Ashar Y.K., Kragel P., Petre B., Schelkun V., Atlas L.Y., Chang L.J., Jepma M., Koban L., Losin E.A.R., Roy M., Woo C.-W., Wager T.D. Effect sizes and test-retest reliability of the fMRI-based neurologic pain signature. Neuroimage. 2022;247 doi: 10.1016/j.neuroimage.2021.118844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert J.D., Brandsma L.L., Fischer L. Social Anxiety. Elsevier; 2014. pp. 45–94. [Google Scholar]
- Höflich A., Michenthaler P., Kasper S., Lanzenberger R. Circuit Mechanisms of Reward, Anhedonia, and Depression. Int. J. Neuropsychopharmacol. 2019;22:105–118. doi: 10.1093/ijnp/pyy081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Bentler P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. 1999;6:1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Jacobson N.S., Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J. Consult. Clin. Psychol. 1991;59:12–19. doi: 10.1037/0022-006X.59.1.12. [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiat. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R., Krueger R.F., Watson D., Achenbach T.M., Althoff R.R., Bagby R.M., Brown T.A., Carpenter W.T., Caspi A., Clark L.A., Eaton N.R., Forbes M.K., Forbush K.T., Goldberg D., Hasin D., Hyman S.E., Ivanova M.Y., Lynam D.R., Markon K., Miller J.D., Moffitt T.E., Morey L.C., Mullins-Sweatt S.N., Ormel J., Patrick C.J., Regier D.A., Rescorla L., Ruggero C.J., Samuel D.B., Sellbom M., Simms L.J., Skodol A.E., Slade T., South S.C., Tackett J.L., Waldman I.D., Waszczuk M.A., Widiger T.A., Wright A.G.C., Zimmerman M. The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J. Abnorm. Psychol. 2017;126:454–477. doi: 10.1037/abn0000258. [DOI] [PubMed] [Google Scholar]
- Kramer, A., Kelley, N.J., Chat, I.K.-Y., Young, K., Nusslock, R., Craske, M.G., Zinbarg, R., 2019. Replication of a tri-level model of anxiety and depression in a sample of young adults. https://doi.org/10.31234/osf.io/8mpd2.
- LeDoux J. The amygdala. Curr. Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lichtenberg N.T., Sepe-Forrest L., Pennington Z.T., Lamparelli A.C., Greenfield V.Y., Wassum K.M. The Medial Orbitofrontal Cortex-Basolateral Amygdala Circuit Regulates the Influence of Reward Cues on Adaptive Behavior and Choice. J. Neurosci. 2021;41:7267–7277. doi: 10.1523/JNEUROSCI.0901-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira Yoon K., Fitzgerald D.A., Angstadt M., McCarron R.A., Phan K.L. Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: A 4-Tesla functional MRI study. Psychiatry Res. Neuroimaging. 2007;154:93–98. doi: 10.1016/j.pscychresns.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Mahler S.V., Berridge K.C. What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology. 2012;221:407–426. doi: 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S., Tervo-Clemmens B., Calabro F.J., Montez D.F., Kay B.P., Hatoum A.S., Donohue M.R., Foran W., Miller R.L., Hendrickson T.J., Malone S.M., Kandala S., Feczko E., Miranda-Dominguez O., Graham A.M., Earl E.A., Perrone A.J., Cordova M., Doyle O., Moore L.A., Conan G.M., Uriarte J., Snider K., Lynch B.J., Wilgenbusch J.C., Pengo T., Tam A., Chen J., Newbold D.J., Zheng A., Seider N.A., Van A.N., Metoki A., Chauvin R.J., Laumann T.O., Greene D.J., Petersen S.E., Garavan H., Thompson W.K., Nichols T.E., Yeo B.T.T., Barch D.M., Luna B., Fair D.A., Dosenbach N.U.F. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603:654–660. doi: 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T.J., Miller M.L., Metzger R.L., Borkovec T.D. Development and validation of the penn state worry questionnaire. Behav. Res. Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Murray E.A. The amygdala, reward and emotion. Trends Cogn. Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Muthén L.K., Muthén B. Muthén & Muthén; Los Angeles, CA: 1998. Mplus User’s Guide. [Google Scholar]
- Naragon-Gainey K., Prenoveau J.M., Brown T.A., Zinbarg R.E. A comparison and integration of structural models of depression and anxiety in a clinical sample: Support for and validation of the tri-level model. J. Abnorm. Psychol. 2016;125:853–867. doi: 10.1037/abn0000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T.H., Alloy L.B., Smith D.V. Meta-analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Transl. Psychiatry. 2019;9:1–10. doi: 10.1038/s41398-019-0644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble W.S. How does multiple testing correction work? Nat. Biotechnol. 2009;27:1135–1137. doi: 10.1038/nbt1209-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly J.X., Woolrich M.W., Behrens T.E.J., Smith S.M., Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Soc. Cogn. Affect. Neurosci. 2012;7:604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S., Murawski C., Fornito A., Youssef G., Yücel M., Lorenzetti V. The anticipation and outcome phases of reward and loss processing: A neuroimaging meta-analysis of the monetary incentive delay task. Hum. Brain Mapp. 2018;39:3398–3418. doi: 10.1002/hbm.24184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Knotts J.D., Young K.S., Bookheimer S.Y., Nusslock R., Zinbarg R.E., Kelley N.J., Echiverri-Cohen A.M., Craske M.G. Threat Neurocircuitry Predicts the Development of Anxiety and Depression Symptoms in a Longitudinal Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2023;8(1):102–110. doi: 10.1016/j.bpsc.2021.12.013. [DOI] [PubMed] [Google Scholar]
- Prenoveau J.M., Craske M.G., Zinbarg R.E., Mineka S., Rose R.D., Griffith J.W. Are anxiety and depression just as stable as personality during late adolescence? Results from a three-year longitudinal latent variable study. J. Abnorm. Psychol. 2011;120:832–843. doi: 10.1037/a0023939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K., Ng R., Scott H., Kodavaganti S., Smyda G., Diwadkar V., Phillips M. Ventral Striatum Functional Connectivity during Rewards and Losses and Symptomatology in Depressed Patients. Biol. Psychol. 2017;123:62–73. doi: 10.1016/j.biopsycho.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A., Camerer C., Montague P.R. A framework for studying the neurobiology of value-based decision making. Nat. Rev. Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee R.M., Craske M.G., Barlow D.H. Assessment instrument for panic disorder that includes fear of sensation-producing activities: The albany panic and phobia questionnaire. Anxiety. 1994;1:114–122. doi: 10.1002/anxi.3070010303. [DOI] [PubMed] [Google Scholar]
- Robin L.A., Martin P.P. Neural systems underlying approach and avoidance in anxiety disorders. Dialogues Clin. Neurosci. 2010;12:517–531. doi: 10.31887/DCNS.2010.12.4/raupperle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T. The Orbitofrontal Cortex and Reward. Cereb. Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Cheng W., Feng J. The orbitofrontal cortex: reward, emotion and depression. Brain Commun. 2020;2:fcaa196. doi: 10.1093/braincomms/fcaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg B.M., Taschereau-Dumouchel V., Lau H., Young K.S., Nusslock R., Zinbarg R.E., Craske M.G. A Multivoxel Pattern Analysis of Anhedonia During Fear Extinction: Implications for Safety Learning. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2021;S2451902221003517 doi: 10.1016/j.bpsc.2021.12.008. [DOI] [PubMed] [Google Scholar]
- Rosner B. Percentage Points for a Generalized ESD Many-Outlier Procedure. Technometrics. 1983;25:165–172. doi: 10.2307/1268549. [DOI] [Google Scholar]
- Ryan J., Pouliot J.J., Hajcak G., Nee D.E. Manipulating Reward Sensitivity Using Reward Circuit-Targeted Transcranial Magnetic Stimulation. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2022;7:833–840. doi: 10.1016/j.bpsc.2022.02.011. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin G.R., Worthy D.A., Mata R., McClure S.M., Knutson B. Adult age differences in frontostriatal representation of prediction error but not reward outcome. Cogn. Affect. Behav. Neurosci. 2014;14:672–682. doi: 10.3758/s13415-014-0297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreuders E., Braams B.R., Blankenstein N.E., Peper J.S., Güroğlu B., Crone E.A. Contributions of Reward Sensitivity to Ventral Striatum Activity Across Adolescence and Early Adulthood. Child Dev. 2018;89:797–810. doi: 10.1111/cdev.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman S.A., Nelson B.D., Sarapas C., Robison-Andrew E.J., Campbell M.L., Altman S.E., McGowan S.K., Katz A.C., Gorka S.M. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. J. Abnorm. Psychol. 2013;122:322–338. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou H., Yang Z., Satterthwaite T.D., Cook P.A., Bruce S.E., Shinohara R.T., Rosenberg B., Sheline Y.I. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. NeuroImage Clin. 2017;14:464–470. doi: 10.1016/j.nicl.2017.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struijs S.Y., Lamers F., Vroling M.S., Roelofs K., Spinhoven P., Penninx B.W.J.H. Approach and avoidance tendencies in depression and anxiety disorders. Psychiatry Res. 2017;256:475–481. doi: 10.1016/j.psychres.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Sumiya M., Koike T., Okazaki S., Kitada R., Sadato N. Brain networks of social action-outcome contingency: The role of the ventral striatum in integrating signals from the sensory cortex and medial prefrontal cortex. Neurosci. Res. 2017;123:43–54. doi: 10.1016/j.neures.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Galván A. Stress and the adolescent brain. Neurosci. Biobehav. Rev. 2016;70:217–227. doi: 10.1016/j.neubiorev.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E., Carl H., Eisenlohr-Moul T., Minkel J., Crowther A., Moore T., Gibbs D., Petty C., Bizzell J., Smoski M.J., Dichter G.S. Attenuation of Frontostriatal Connectivity During Reward Processing Predicts Response to Psychotherapy in Major Depressive Disorder. Neuropsychopharmacology. 2017;42:831–843. doi: 10.1038/npp.2016.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warlow S.M., Berridge K.C. Incentive motivation: ‘wanting’ roles of central amygdala circuitry. Behav. Brain Res. 2021;411 doi: 10.1016/j.bbr.2021.113376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warlow S.M., Naffziger E.E., Berridge K.C. The central amygdala recruits mesocorticolimbic circuitry for pursuit of reward or pain. Nat. Commun. 2020;11:2716. doi: 10.1038/s41467-020-16407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum K.M., Izquierdo A. The basolateral amygdala in reward learning and addiction. Neurosci. Biobehav. Rev. 2015;57:271–283. doi: 10.1016/j.neubiorev.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Weber K., Assenheimer J.S., Clark L.A., Strauss M.E., McCormick R.A. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J. Abnorm. Psychol. 1995;104:3–14. doi: 10.1037/0021-843X.104.1.3. [DOI] [PubMed] [Google Scholar]
- Wendt J., Lotze M., Weike A.I., Hosten N., Hamm A.O. Brain activation and defensive response mobilization during sustained exposure to phobia-related and other affective pictures in spider phobia. Psychophysiology. 2008;45:205–215. doi: 10.1111/j.1469-8986.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- Williams A.L., Craske M.G., Mineka S., Zinbarg R.E. Neuroticism and the longitudinal trajectories of anxiety and depressive symptoms in older adolescents. J. Abnorm. Psychol. 2021;130:126–140. doi: 10.1037/abn0000638. [DOI] [PubMed] [Google Scholar]
- Young K.S., Bookheimer S.Y., Nusslock R., Zinbarg R.E., Damme K.S.F., Chat I.-K.-Y., Kelley N.J., Vinograd M., Perez M., Chen K., Cohen A.E., Craske M.G. Dysregulation of threat neurocircuitry during fear extinction: the role of anhedonia. Neuropsychopharmacology. 2021;46:1650–1657. doi: 10.1038/s41386-021-01003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C.B., Chen T., Nusslock R., Keller J., Schatzberg A.F., Menon V. Anhedonia and general distress show dissociable ventromedial prefrontal cortex connectivity in major depressive disorder. Transl. Psychiatry. 2016;6:e810–e. doi: 10.1038/tp.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K.S., Ward C., Vinograd M., Chen K., Bookheimer S.Y., Nusslock R., Zinbarg R.E., Craske M.G. Individual differences in threat and reward neural circuitry activation: Testing dimensional models of early adversity, anxiety and depression. Eur. J. Neurosci. 2022;55:2739–2753. doi: 10.1111/ejn.15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M., Coryell W. The inventory to diagnose depression, lifetime version. Acta Psychiatr. Scand. 1987;75:495–499. doi: 10.1111/j.1600-0447.1987.tb02824.x. [DOI] [PubMed] [Google Scholar]
- Zinbarg, R.E., Schmidt, M., Feinstein, B., Williams, A.L., Murillo, A., Echiverri-Cohen, A.M., Enders, C., Craske, M.G., Nusslock, R., in press. Personality predicts pre-COVID-19 to COVID-19 trajectories of transdiagnostic anxiety and epression symptoms. J. Psychopathol. Clin. Sci. [DOI] [PMC free article] [PubMed]
- Zinbarg R.E., Mineka S., Bobova L., Craske M.G., Vrshek-Schallhorn S., Griffith J.W., Wolitzky-Taylor K., Waters A.M., Sumner J.A., Anand D. Testing a Hierarchical Model of Neuroticism and Its Cognitive Facets: Latent Structure and Prospective Prediction of First Onsets of Anxiety and Unipolar Mood Disorders During 3 Years in Late Adolescence. Clin. Psychol. Sci. 2016;4:805–824. doi: 10.1177/2167702615618162. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.