ABSTRACT
Bats host a large variety of viruses, including some that may infect other vertebrates and humans. Research on bat-borne viruses attracted significant attention in recent years mainly due to epizootics caused by viruses having bats as hosts. The characterization of the viral communities of bats was then prioritized, but despite increasing efforts, there are large disparities in the geographical ranges covered and the methodologies employed around the world. As a result, large gaps remain in our current understanding of bat viromes and their role in disease emergence. This is particularly true for megadiverse regions in Latin America. This review aims to summarize the current understanding about bat viruses that inhabit Brazilian biomes, one of the most bat species-rich and diverse regions of the globe. Taking into account all known bat-associated viral families studied in Brazilian biomes, we found that almost half of all bat species (86/181 species) were not investigated for viruses at all. Moreover, only a small fraction of viral lineages or families have been studied more in depth, usually employing targeted methods with limited power to characterize a broad virus diversity. Additionally, these studies relied on limited spatiotemporal sampling and small sample sizes. Therefore, our current understanding of bat viral communities in the Brazilian biomes is limited and biased at different levels, limiting zoonotic risk assessments of bat-borne viruses. Considering these limitations, we propose strategies to bridge the existing gaps in the near future.
IMPORTANCE Bat-borne viruses have attracted much attention due to zoonotic outbreaks with large consequences to humans. Because of that, virus characterization in bats has been prioritized in tropical regions of the globe. However, bat-virus research in Latin America and particularly in Brazil, which are among the most bat species-rich regions of the world, are highly biased toward zoonotic viruses and known bat reservoir species. These results have direct implication for virus studies in general but also for new zoonotic virus and spillover events characterization. The limited knowledge we currently have about the virome of Brazilian bats drastically limits any broad assessment of zoonotic viruses they carry and calls for coordinated and large-scale studies to fill this crucial knowledge gap.
KEYWORDS: RNA virus, microbiome, surveillance studies, virus-host interactions, zoonotic infections
INTRODUCTION
Bats (order Chiroptera) compose the second most diverse mammalian order with 1,456 known species from 21 families (https://batnames.org) that participate in diverse ecological processes such as plant pollination, seed dispersion, and soil renewal up to habitat modifications (1). Bats are also reservoirs of a large diversity of zoonotic and nonzoonotic viruses (2 to 4). The identification of specific bat species as reservoirs of human pathogenic viruses (Nipah paramyxoviruses and SARS-coronaviruses) propelled increasing efforts to characterize zoonotic viruses with epidemic potential that infect and are transmitted among bats and other species (2). Many studies have underscored that chiropterans are more prone to carry and transmit zoonotic viruses to humans (5 to 8). On the other hand, nonbat intermediate amplifying hosts and the characterization of zoonotic viruses hosted by other mammalian species suggest that viruses undergoing transmission in different animal hosts ultimately lead to spillover to humans (2, 9, 10). Where there is evidence of direct transmission of bat viruses to humans, human activities had a prominent role promoting contact with bats (11 to 15). A recent study showed that the number of zoonotic viruses found in mammalian orders is proportional to the number of species of each respective order, suggesting that bats are not special pathogen reservoirs and that they host a large diversity of viruses because of their high species diversity (16). There is still no consensus if bats do carry and spread more human-pathogenic viruses than other mammalian orders (17).
Definitive answers to the role of bats as reservoirs of zoonotic viruses, and associated risks, ultimately lie in the full understanding of bats’ viromes, bat ecology, and their interaction with other vertebrates. Several studies sampled wild bat populations to more broadly characterize their virus diversity. However, there are large disparities in study efforts in different regions of the world, since the vast majority of virus studies were largely performed in Asia, Africa, and Europe (Old World), and fewer studies were conducted in Oceania and North America (3, 18, 19). Moreover, Central and South America, among the most chiropteran-rich regions of the globe, are comparatively poorly studied (20). Another hardly addressed issue in viral studies of bats is the limited representative sampling, especially considering the large habitat range, population size variations and temporal fluctuations of infection rates (21). Therefore, our understanding of bats' viromes is severely biased at different scales.
Bat virome characterization is also biased when it comes to molecular methodologies employed for viral detection, once the majority of virus detection methods were developed to target single viral lineages of zoonotic concern and not to capture the full diversity of bat viruses (20). These methodologies rely on previous information about the pathogen molecules (22), which contrasts with our scant knowledge of the animal's virome (23). Nowadays, high-throughput methodologies (HTS) are one of the most promising approaches for unbiased viral nucleic acid detection, allowing a broad description of viral genomes associated with any sample and requiring no previous knowledge about the infecting viral pathogens (24). HTS virome studies generated major breakthroughs, revealing thousands of new viral lineages and families (25), but also brought to light that the large majority of animal viruses still awaits to be formally characterized (26 to 28).
In this review, we addressed the current understanding of viruses detected in bats inhabiting Brazilian biomes (181 known species; https://www.sbeq.net/lista-de-especies). We highlight the currently limited and biased character of research on the Brazilian bat virome. Additionally, we summarize the methodologies used for characterization of bat viruses, showing that most studies used targeted low-throughput assays. Finally, we propose methodological approaches to address existing gaps.
RESULTS AND DISCUSSION
Studies of bat viruses.
Studies of bat viruses in Brazilian biomes have been mostly performed from 1990 to the present (Fig. S1 in the supplemental material). From the 81 published studies analyzed, there is a clear focus on Rhabdoviridae and Coronaviridae, with 43 and 13 studies, respectively. The majority of studies focusing on Rhabdoviridae, and more specifically on the Rabies virus (RABV), were likely motivated by concerns about fatal spillover events to humans in Brazil once different bat species were described as important RABV reservoirs (29 to 31). Comparatively few studies (1 to 3) investigated viruses from the Orthomyxoviridae, Paramyxoviridae, Hantaviridae, Herpesviridae, and Adenoviridae families (Fig. S1).
Bats and their viruses in Brazilian biomes.
Of the 181 bat species known to exist in Brazilian biomes, 95 were included in at least one study, while 86 species (47.5%) have not been screened for viruses so far (Fig. 1A). The sampling biases are also reflected at the family level: Phyllostomidae, the most diverse family in Brazil with 92 species, is represented by 51 studied species, while 41 species (44.1%) were not studied (Fig. 1A). Some less diverse bat families such as Thyropteridae (5 species) were not investigated at all (Fig. 1A).
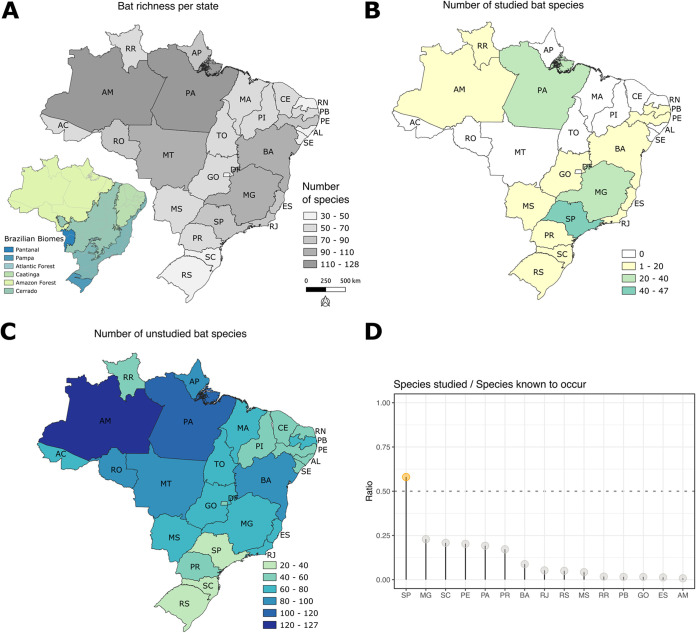
FIG 1.
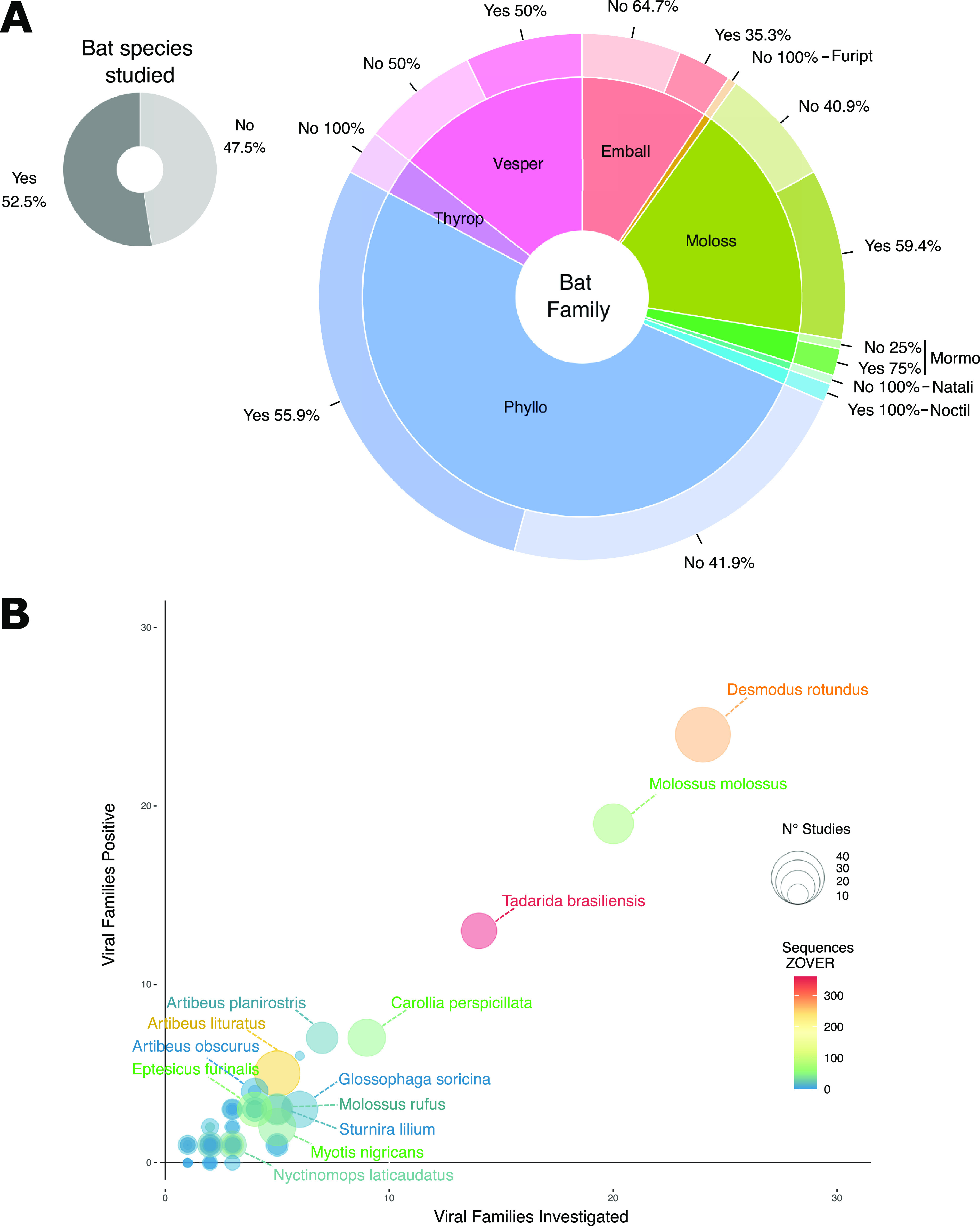
Bat species inhabiting Brazilian biomes covered by virus studies. (A) Proportion of 181 known bat species from Brazilian biomes studied (dark gray) or nonstudied (light gray) in the reviewed studies (top left donut plot). Bottom right larger donut plot shows the proportion of studied (darker colors) or nonstudied species (lighter colors) per bat family. Bat families are as follows: Phyllo, Phyllostomidae; Moloss, Molossidae; Emball, Emballonuridae; Vesper, Vespertilionidae; Thyrop, Thyropteridae; Mormo, Mormoopidae; Natali, Natalidae; Noctil, Noctilionidae. (B) Correlation between number of virus families investigated and number of virus families found in the reviewed studies. Bubble size stands for the number of studies covering each bat species, and bubble color represents the number of virus sequences of the corresponding species available in the ZOVER database.
Among the 95 studied species, there is a clear overrepresentation of some important zoonotic virus reservoirs such as the vampire bat Desmodus rotundus (Fig. 1B), which was screened for viruses in 44 of 81 studies (56.79%) reviewed (Fig. 1B). This species is known to be the main sylvatic RABV reservoir in Brazil and the Americas (32), with infections of cows, dogs, and humans traced to the D. rotundus RABV lineage (33, 34). In Brazil, rabies epidemiology shifted from a zoonotic infection mainly driven by domestic animals (dogs) to sporadic spillovers to cows and humans caused by lineages of bat origin, after effective prophylactic vaccination of rabid dogs was conducted countrywide (30, 33). Thirteen species were included in 10 or more studies (named species in Fig. 1B). All these species were detected as positive for different lineages of RABV (33, 35 to 41), indicating that sampling is biased towards certain bat species. We found a correlation between the number of viral families investigated and number of viral families found for all species herein reviewed (Fig. 1B), as well as between the number of virus families listed in this review and the sequences available from the ZOVER database (Fig. S1). Therefore, the larger the effort to sample and study viruses in a given species, the higher the likelihood of finding previously undetected virus families.
Spatial sampling bias of bats in Brazil.
The number of bat species known to occur in each Brazilian state ranges from 40 to 135 species (Fig. 2A and B). Due to multiple factors, especially geographic scale and species ecology, bat sampling with the aim of studying viruses is highly heterogeneous. The number of bat species studied varied from 0 to 47. In 12 of 27 Brazilian states, no species have been studied so far (Fig. 2C). The ratio between the number of bat species studied and the total species inhabiting the respective states also displayed a great variation. Only in the state of São Paulo more than 50% of the known bat species were assessed for virus infections (Fig. 2D). For the remaining states, a maximum of 25% of known bat taxa were investigated (Fig. 2D). Moreover, we can observe that there is a spatial bias, with several studies targeting bats from coastal states (Fig. 2C). Most of the studied species displayed large habitat ranges (covering, on average, 16 ± 6 states, out of the 27 Brazilian states). On the other hand, the nonstudied species have more restricted habitat ranges (5 ± 3 states) (Fig. S2). However, it is important to note that, having a larger habitat range per se does not necessarily mean that a given species is more important as a potential virus reservoir and virus spreader to other species, including humans. Some species with very large habitat range can be rarely captured due to small population size or restricted habitat to natural and preserved areas, and display limited interaction with other species; therefore, there is a small chance to play a significant role in spillovers events, while more endemic ones can be locally abundant and have more frequent habitat overlap and contact with other vertebrate species, including human populations.
FIG 2.
Brazilian biomes and bat species sampling effort on virus studies. (A) Bat species richness across Brazilian states. (B) Number of bat species investigated per state. (C) Bat richness of unstudied species per Brazilian state. (D) Ratio of number of bat species investigated divided by the number of known species per state. Dotted line represents the ratio in which half of the species from a state are studied for viruses. States acronyms are as follows: AC, Acre; AL, Alagoas; AM, Amazonas; AP, Amapá; BA, Bahia; CE, Ceará; DF, Distrito Federal; ES, Espírito Santo; GO, Goiás; MA, Maranhão; MT, Mato Grosso; MS, Mato Grosso do Sul; MG, Minas Gerais; PA, Pará; PB, Paraíba; PE. Pernambuco; PI, Piauí; RJ, Rio de Janeiro; RN, Rio Grande do Norte; RS, Rio Grande do Sul; RO, Rondônia; RR, Roraima; SC, Santa Catarina; SP, São Paulo; SE, Sergipe; TO, Tocantins.
Virus families studied.
Altogether, 17 RNA virus families and 14 DNA virus families were detected in our review and the ZOVER database (Fig. 3A and B). Four families of DNA virus and four of RNA viruses were detected only in the review database, while two RNA virus families were found only in the ZOVER database. We have also found differences in the number of species recorded as positive for a viral infection/exposure. In the compiled review database, we found 31 families in 62 of the 95 bat species studied, representing 5 of 9 chiropteran families living in Brazil: Phyllostomidae (42), Molossidae (14), Vespertilionidae (9), Mormoopidae (2), and Emballonuridae (1). In the ZOVER database, evidence of infection was recorded for 22 virus families in 58 of 95 bat species. Here, the bats represent 6 of the 9 bat families recorded in Brazil: Phyllostomidae (24), Molossidae (15), Vespertilionidae (11), Emballonuridae (3), Mormoopidae (3), and Natalidae (1) (Supplemental File 3).
FIG 3.
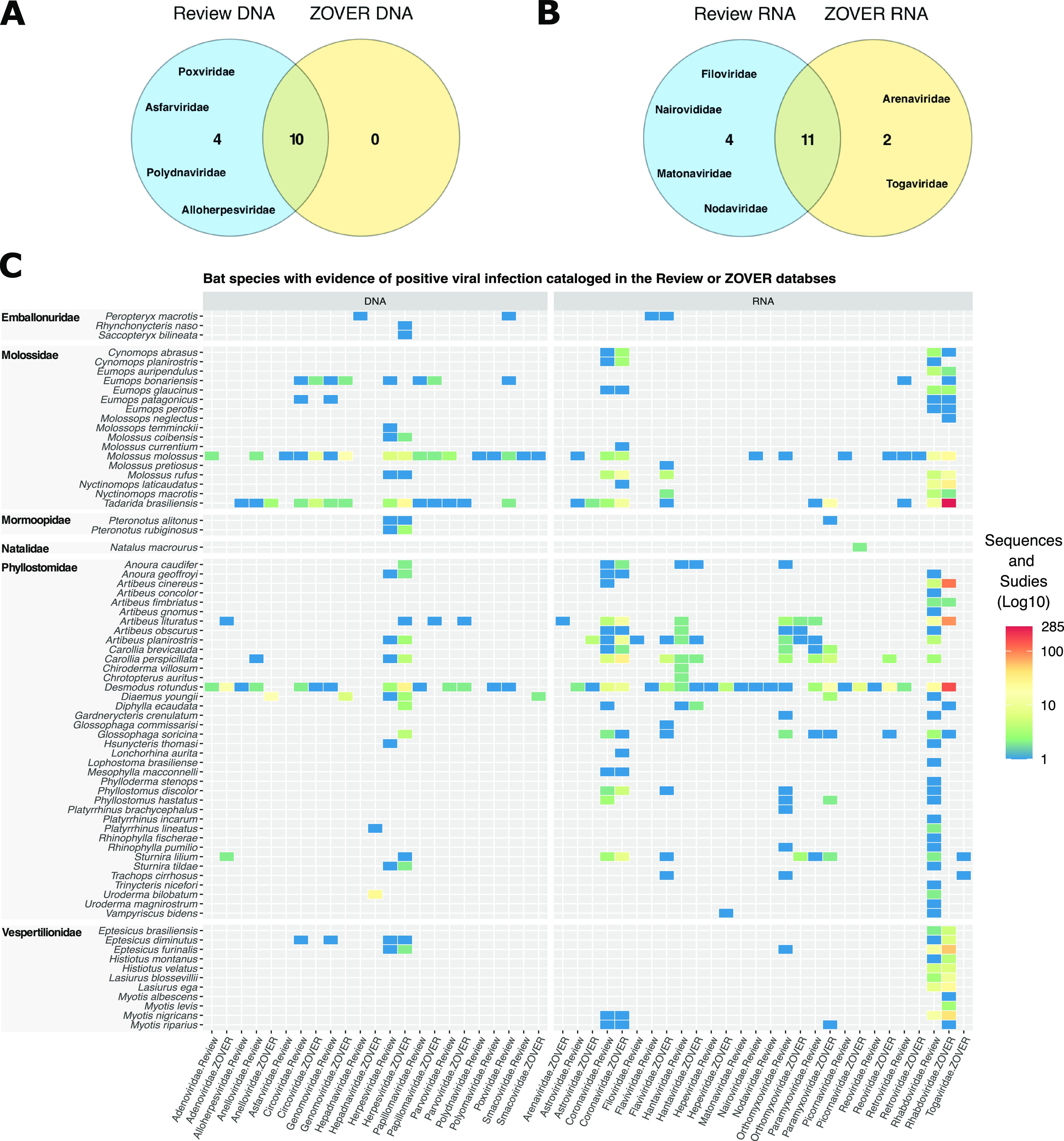
Bats inhabiting Brazilian biomes with evidence of viral infection/exposure obtained from studies cataloged in this review’s database and/or with sequences available in the ZOVER database. (A and B) Venn diagram showing the number of virus families recovered in both data sets. (C) Heatmap showing the number of studies (range: 0 to 22) where evidence of viral infection is available, and the number of virus sequences (range: 0 to 285) for each bat species per virus family (sorted by genome type).
We found a clear dominance of RNA virus data over DNA viruses available in both our review database and in the ZOVER database. The 5 most detected RNA virus families are Rhabdoviridae (47 bat species positive), Coronaviridae (21), Orthomyxoviridae (16), Paramyxoviridae (8), and Hantaviridae (9), while the remaining RNA virus families were detected in 5 or fewer bat species (Fig. 3C). The 4 most covered virus families in the ZOVER database are Rhabdoviridae (43), Coronaviridae (23), Flaviviridae (13), and Paramyxoviridae (10), while the remaining virus families were detected in fewer than four bat species. Herpesviridae was the only DNA virus family detected in more than 6 bat species, with 17 species positive in the review database and 21 species positive in the ZOVER database (Fig. 3C). The remaining DNA virus families were detected in 6 or fewer bat species.
The discrepancies in infected host diversity observed between our review database and ZOVER can be explained by two key differences. While our review cataloged studies using a large array of methodologies for viral detection, including the ones generating no genetic information (e.g., ELISA, DIAMA), the ZOVER database is based only on virus genetic information available from other public databases. This means that we were able to detect both infection (presence of RNA/DNA and antigens) and exposure (antibodies) of bat hosts in the review database, while the ZOVER database only provides information about the infection status of the host. Moreover, there are several examples of virus sequences available in the ZOVER database tagged as “unpublished” (257 entries, accession date 1 July 2022), indicating that these data were publicly released with no peer review. Nevertheless, the majority of the bat species have not been comprehensively studied since most virus families were detected only in 1 to 3 bat species (Fig. S3).
The total number of bat species sampled is an important piece of information for evaluating virus family host range. However, only the host species that tested positive were reported in a number of manuscripts focused on RABV (Supplementary File 2), and such information cannot be recovered from the ZOVER database. Therefore, we used the detection of virus families per host species as a proxy of the number of studied species from each bat family. This likely leads to an underestimation of the total host species/specimens investigated contributing to the strong correlation between studied and positive species described in Fig. 1B. Additionally, several virus families (12 of 29; DNA: Smacoviridae, Anelloviridae, Papillomaviridae, Poxviridae, Alloherpesviridae, Asfarviridae, Polydnaviridae, Papillomaviridae, and Parvoviridae, and RNA viruses: Retroviridae, Nairoviridae and Picornaviridae) were only characterized using HTS methods, screening a limited number of individuals and species (see Table 1 for methodologies for virus detection). Overall, these results highlight a very limited host range investigated for the majority of virus families studied.
TABLE 1.
Methodologies employed in 81 studies with the number of studies using each methodology and the number of virus families identifieda
| Methodology acronym | Throughput | No. studies | Virus families detected |
|---|---|---|---|
| VIIIM | LT | 26 | 1 |
| VICC | LT | 2 | 1 |
| ELANT | LT | 1 | 1 |
| WB | LT | 1 | 1 |
| DIAMA | LT | 38 | 4 |
| ELISA | LT | 5 | 5 |
| SS-PCR | LT | 39 | 5 |
| N-PCR | LT | 1 | 1 |
| FSD-PCR | LT | 17 | 9 |
| DNAVIR | HT | 4 | 13 |
| RNAVIR | HT | 3 | 3 |
| FVIR | HT | 5 | 14 |
Methodologies are presented in an ascending order from lower to higher throughput in terms of viral diversity characterization: Virus Isolation through Intracerebral Inoculation of Mice (VIIIM), the Virus Isolation in Cell Culture (VICC), Electron Microscopy and ANtigenic Profile (ELANT), Western Blot (WB), Direct Immunofluorescence Antigen Monoclonal Antibodies (DIAMA), Enzyme-Linked Immunosorbent Assay (ELISA), Strain-Specific PCR (SS-PCR), Family/Subfamily Degenerate PCR (FSD-PCR), Nested PCR (N-PCR), DNA Virome (DNAVIR), RNA Virome (RNAVIR), and Full Virome (FVIR).
Infection status and temporal sampling strategies.
The temporal sampling and the number of individuals sampled per species are heterogeneous. Longitudinal sampling was conducted only in 46 of 81 cataloged studies. Moreover, 27 of the 46 studies with longitudinal sampling focused on RABV (Supplementary File 4). A recent meta-analysis of bat coronavirus surveillance identified longitudinal repeated sampling (i.e., sampling the same site and bat populations multiple times) as the most significant determinant for viral detection (20), suggesting that the temporal variation in bat sampling is a key component to take into consideration for viral surveillance. Regarding the infection status and prevalence, we retrieved such information from only 26 of the 81 studies (Supplementary File 4). The lack of sample size information (total number of individuals collected) hindered estimates of infection prevalence for the large majority of species (Supplementary File 4). The association of the previously discussed bias toward sampling known pathogen hosts summed to the bias of reporting virus-positive species (i.e., the case of RABV surveillance studies) casts doubts about the infection prevalence estimates that can be reliably drawn from the data herein analyzed and its extrapolation to larger natural populations.
Natural variation of virus prevalence in bat populations is highly variable depending on several factors, such as the host immunological barrier acquired from previous infections, the number of susceptible newborns in a population, and specific physiological, ecological, and life history factors (15). Implementing a sampling strategy that allows a broad assessment of bat viruses is also challenging due to the diverse virus cycles and distinct “biology.” Viruses interact with their hosts in various ways, e.g., pathogenic viruses cause acute and short-term infections, and others lead to persistent/chronic infections. In each case, infectious status of individuals and populations may vary through time following virus-specific, tissue-specific replication and shedding (2). Hence, infection estimates may be affected by the sampling strategy (longitudinal versus cross-sectional). For instance, nonpathogenic viruses that develop a mutualistic interaction with their host may become highly prevalent in a population. In such cases, cross-sectional sampling schemes should still be effective to detect the virus, since a large fraction of individuals are infected at distinct time points. On the other hand, pathogenic viruses that induce acute infection and long-lasting immunological response (weeks to months) usually increase in prevalence only during short periods of time. Therefore, such viruses can be more easily detected during ongoing sporadic outbreaks, when a large fraction of the host population is infected. The virus may go undetected if sampling does not take into account host physiological traits that influence prevalence in time (e.g., antibodies, starvation, etc.). Coronaviruses, for instance, show remarkable variance in fecal shedding, ranging from 0% to 80% in Old World bats (44). Paramyxoviruses such as Henipa viruses have been shown to be discharged in a pulse-like pattern, tightly linked to bat physiology status and waning of maternal antibodies in newborns (45). Filoviruses (e.g., Marburg virus) also vary in prevalence, with infection peaking during the birthing season of Rousettus aegyptiacus (46).
Different tissue tropism and route of viral transmission are additional factors impacting the sampling strategy. Collecting oral swabs provides information about viruses that are excreted via oral/nasal fluids but not about nonexcreted viruses or those excreted through a different route, providing only a restricted view of the virome. Similarly, studies performing sampling of feces may characterize only viruses that are excreted through bats’ digestive tract along with a large diversity of viruses that are derived from food sources, but do not infect bats (47 to 49). The cataloged studies of bats from Brazilian biomes used various tissue types chosen with a priori knowledge of the target virus infectious dynamics and shedding. One illustrative example is that of RABV, it was investigated in 43 studies using euthanasia and collection of brain tissue as the gold standard methodologies for RABV detection, since it requires numerous infectious particles for intracerebral inoculation and isolation (Supplementary File 4). Four of 13 studies investigating coronaviruses employed oral/nasal/anal swabbing due to the upper respiratory and intestinal replication and known viral shedding route though oral/anal fluids (Supplementary File 4). It is important to note that three studies that screened coronaviruses in bats inhabiting Brazilian biomes used various tissues of internal organs from convenience samples obtained from RABV surveillance programs (Supplementary File 4). Convenience samples are known to be biased and generate misleading results for pathogen and disease prevalence (50).
Species populations and metapopulation size should be considered key driving factors in study design since virus prevalence is population size dependent and the host’s physiology can affect the likelihood of detecting positive individuals. However, sampling a wide range of bat sample types is only possible via euthanasia, which should be carefully considered in order to reduce the impact on the population and/or species. Some bat species have considerably low population size, and sampling too many individuals may have a drastic impact on bat populations, leading to local extinction and likely long-term impact on these species.
Overall, studies on bats in Brazil are very heterogeneous in their temporal sampling strategy and the type of analyzed material. In order to broadly characterize bat viromes, flexible sampling strategies, including systematic longitudinal sampling of representative individuals/populations and targeting different tissues, must be adopted.
Methodological approaches for virus characterization.
Various methodologies were employed for characterization of viruses in Brazilian bats. The large majority of the studies employed low-throughput (LT) methodologies (Fig. S1 and Fig. 4A) such as VIIIM, which are based on the maceration of bat tissues and experimental intracerebral inoculation of mice (51). Positive VIIIM is followed by validation using direct immunofluorescence of RABV-derived antigens with monoclonal antibodies (DIAMA, the most used methodology along with SS-PCR) (52) (Table 1, Fig. 4A). Studies focused on Coronaviridae (11 studies) used only amplicon-based methodologies varying from strain-specific PCR (SS-PCR) to family/subfamily-level degenerated RT-PCR (FSD-PCR) (Fig. 4A), while studies on other families employed a mix of different LT methods (Fig. 4A). Twelve studies employed high-throughput nucleic acid sequencing methodologies (FVIR, RNAVIR, and DNAVIR) (Fig. 4A). These studies employed complementary low-throughput methodologies such as FSD-PCR, SS-PCR, ELISA, DIAMA, and VICC (Fig. 4A and Supplementary File 4).
FIG 4.
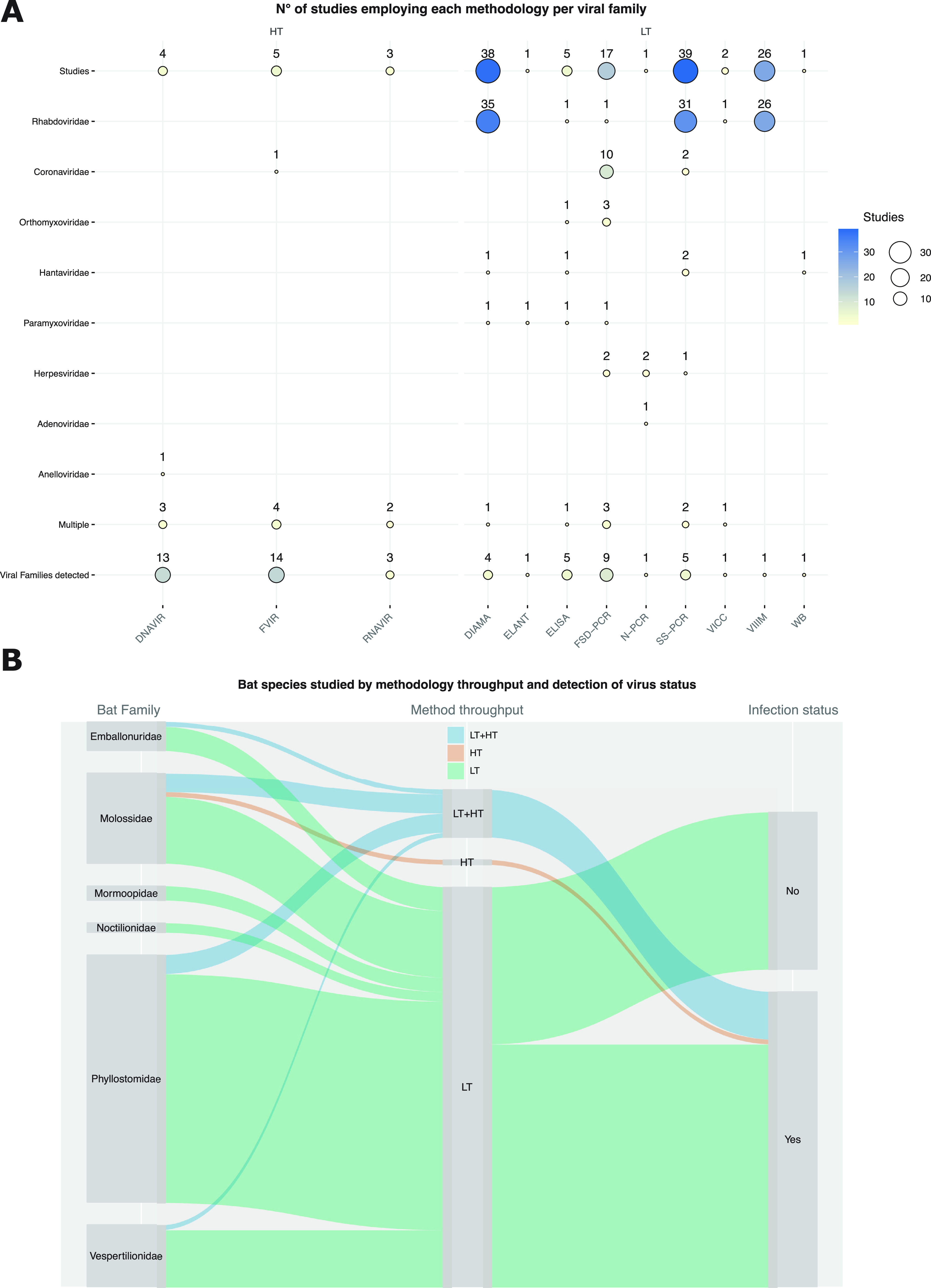
Methodological approaches used to investigate virus and bat species stratified by throughput and infection/exposure status. (A) Number of studies employing each methodology to investigate specific virus families. (B) Bat species included in at least one study connected by methodology throughput (LT, low throughput; HT, high throughput; LT+HT, low and high throughput) and virus infection/exposure status.
Seventy-five bat species were screened for viruses using LT only, one (E. bonariensis) was investigated using an HT method only, and 10 species were investigated using LT and HT (P. macrotis, D. rotundus, G. soricina, C. perspicillata, A. lituratus, E. patagonicus, M. molossus, M. rufus, T. brasiliensis, E. diminutus) (Fig. 4B). Seven of these species (D. rotundus, G. soricina, C. perspicillata, A. lituratus, M. molossus, M. rufus, T. brasiliensis) are also among the most studied ones (Fig. 1B). All 12 bat species that were investigated by at least one HT methodology were found positive (Fig. 4B), while 33 species investigated using LT methodologies were found negative (Fig. 4B). The negative status of several bat species is likely a result from the methodology chosen for viral characterization, but it may also reflect a composite effect of other sources of biases discussed before, such as sampling, bias toward species and/or tissue sampled, as well as virus/host life history traits such as temporal variation in population/metapopulations infection rate.
Detection and characterization of single or related virus lineages.
RABV was extensively characterized via VIIIM and DIAMA, and to a lesser extent these methods have been used for hantaviruses and paramyxoviruses. These methods provide precise information about symptoms induced by RABV infection in mice models and the possibility to isolate abundant viral material. However, they are laborious methodologies requiring euthanasia or sampling sick/dead bats. Therefore, these methodologies are not well suited for more broad virus discovery and virome community characterization.
Only one reviewed study used ELISA to detect Henipa- and Filo-like viruses (53). Antibody and antigen detection using ELISA provide only a narrow characterization of viral diversity, restricted to a single or closely related viral lineage. Cross-antibody recognition may allow the detection of other viruses, but it only extends to viruses from the same genus and rarely to the same family (54). Moreover, cross-reactivity of serotypes provides little or no information about which other viruses infected or are infecting a given sample, limiting substantially the use of these methods for virus discovery.
Amplicon-based screenings have been applied for diagnosing several viral pathogens mainly because of low cost, high sensitivity, and speedy results, allowing high turnover rates and screening of a large set of samples. It is also possible to estimate the virus genome copy number and couple it with sequencing of amplified fragments.
One key constraint of all these methodologies is the need for previous information about viral molecules (proteins, DNA/RNA), which is lacking for the large majority of poorly studied viruses infecting animals (23). Therefore, they are not suited for viral discovery and diversity characterization, but are better suited to obtain detailed information about known viruses.
Characterization of multiple virus lineages.
Degenerated primers targeting conserved regions may allow a broader detection of divergent viruses; however, high genetic divergence precludes broad surveys. Nowadays, there are brute force methodologies that allow more unbiased detection and characterization of virus genetic material. For instance, high-throughput sequencing (HTS) approaches can be applied to characterize the complete genomes of DNA and/or RNA viruses (24). But due to the costs, HTS has been mainly used for a few individuals and species of Brazilian bats. Nevertheless, there is a clear increase in HTS usage for the study of Brazilian bats (Fig. S1 A). This reflects the global shift of the scientific community toward comprehensive characterization of viromes and microbiomes. Moreover, viral enrichment protocols coupled with HTS may provide the most comprehensive and cost-effective available methodological pipeline for unbiased assessment of viral communities, because they substantially reduce the number of sequenced reads needed to obtain viral genomes (55).
The viral diversity characterization predominantly performed using targeted low-throughput approaches imposes clear limits on our ability to understand the true diversity of viromes in Brazilian bats. Our findings are in close agreement with a recent review describing methodological biases involved in the attempts to broadly characterize coronaviruses worldwide (20).
PERSPECTIVES
The results compiled in this review bring to light the limited understanding of viromes of Brazilian bats. Several biases shape the greater picture: (i) a complete lack of studies for almost half of known bat species occurring in the country, (ii) an extensive focus on few zoonotic viruses or virus families, (iii) sampling design failing to take into account host population size and spatiotemporal population and infection dynamics, (iv) a heterogeneous use of tissue and convenience sampling, and (v) the deployment of targeted LT methods. On the other hand, the number of studies using HTS for virome exploration is growing in Brazil and South America. However, they remain focused on a few bat species and small sample sizes due to high costs. Therefore, the biases and gaps identified point to our limited basic understanding about the virome of neotropical bats. Uncovering viromes of bats and new zoonotic viruses requires hypothesis-driven experimental design that will reveal not only their core virome, but will also uncover the biotic and abiotic factors modulating infection through time and space. Additionally, it can allow the identification of risk factors pertaining to cross-species transmission events.
Call and recommendations for less biased virus diversity, prevalence information, and assessment of new viruses with zoonotic potential.
(i) Sampling strategy: the importance of bat and virus traits. Bat populations or metapopulations vary widely in size and geographical range, hence appropriate sampling of bat populations should be estimated taking into account these parameters. Infection rate through time is another key factor to take into account while planning sampling design. Prevalence and transmission may vary drastically in space and time, being correlated with the reproductive season and antibody waning in juveniles. Random longitudinal sampling may yield a more comprehensive picture of long-term infections and overall prevalence in a given population. However, this sampling strategy could miss short-lived acute infections in geographically isolated populations. A disease surveillance sampling strategy may be a useful alternative, where the sampling design is planned based on probability of sampling infected individuals (disease prevalence). But it is highly dependent on previous knowledge of disease and the etiological agent, which is largely lacking for bats in Brazil.
Comprehensive assessments of virus infections and diversity in bats should use multiple tissue/body fluid collections as much as possible, since mechanisms of infection, replication, and transmission route vary. Sampling strategies trying to answer questions about zoonotic potential can also use specific tissues/fluids that are more relevant in the transmission cycle. Coronaviruses, for example, infect respiratory and intestinal tracts in mammals (56), hence oral/nasal fluids as well as excreta are suitable material for learning about infection and shedding dynamics. Moreover, less invasive sampling such as swabbing reduces animal suffering and impact on populations, but may also limit the detection to viruses excreted by specific routes. There is no ideal sampling strategy for covering all virus/hosts aspects, but keeping in mind the complexity of the virome and its intricate interactions with their hosts is crucial for designing cost-effective, minimally invasive studies that can offer comprehensive and relevant results.
(ii) Sampling strategy: methodologies for the characterization of viromes. Understanding the sensitivity and specificity of different methodologies is of utmost importance for assessing the virome diversity. Different molecular assays can be used for complementary testing, but viral enrichment protocols preceding deep HTS are the most powerful workflow for exploring virus communities. Such methodology will set the stage for in situ viral discovery and characterization of core viromes of bats and other animals in the next few years. These HTS technologies allow us to move from questions such as “What can we afford to do for virome characterization?” to “What should we do for comprehensive virome characterization?” Such approaches may, for instance, allow the sequencing of the virome of every individual sampled, not requiring sample pooling and providing a direct measurement of infection rate and coinfection of several viruses in the same individual. Challenges of HTS should be kept in mind: growing data sets of hundreds of samples are currently amenable only using powerful computational infrastructure and trained researchers (57), substantially limiting its deployment in low-resource settings.
Standardized bat and viral sampling, viral enrichment, and bioinformatic protocols are required in order to enable cross-studies comparison. It is important to highlight that the gaps and proposed solutions described in this review also apply to other regions of the globe. One key example is the limited temporal sampling of bats, which is pervasive in the relevant literature. Therefore, our findings reflect more or less the current state of bat-borne virus research worldwide. The strategies proposed to bridge the knowledge gaps and standardize the generated knowledge can be applied to bat virome studies overall benefiting the entire scientific community working in this area.
One Health perspective.
Direct infection of humans by bat-borne viruses is only known in two specific cases: Nipah virus in Bangladesh (58, 59), and RABV in the Americas (29). Otherwise, bat-borne viruses that are able to infect humans also infect a large range of vertebrates, including several sylvatic and domestic animals as well as vector species (2). Moreover, there is evidence indicating that these viruses underwent prior adaptation in other mammal hosts before spilling over to humans (56). All these viruses have complex transmission cycles infecting a range of closely and distantly related host species, suggesting that viruses with a large host range are more prone to host switching that potentially triggers new outbreaks (10). Therefore, a system-level One Health approach is needed for understanding biotic and abiotic factors shaping transmission dynamics and emergence risk of bat-borne viruses.
Conclusion.
The characterization of Brazilian bats’ viromes is biased toward viruses of zoonotic concern. Moreover, despite the accumulated knowledge regarding these viruses, we have shown large discrepancies stemming from spatiotemporal sampling bias and the use of techniques unsuitable for comprehensive virome characterization. Altogether, our review reveals a limited understanding of viromes in bats in Brazil substantially limiting a proper assessment of the zoonotic potential of most viruses. Ongoing changes of land use in Brazilian biomes (60) represent new opportunities for cross-species virus transmission events between humans and wildlife (61, 62). Thus, spillover events from bats and other vertebrates inhabiting Brazilian biomes may lead to outbreaks and epidemics. High-throughput techniques for pathogen discovery and surveillance applied to bats from sylvatic–urban interfaces should be prioritized in high-risk contact areas.
MATERIALS AND METHODS
Database construction.
We screened Pubmed, Google Scholar, and Scopus scientific literature databases for manuscripts containing the terms “bat-borne viruses,” “viruses AND bats,” “viruses AND Chiroptera,” “virome,” “South American bats”, “Brazilian bats,” “Rabies,” “Coronavirus,” and “Hantavirus” until May 2022. All the search terms were known to be represented in the published literature of viruses detected in South American bats. Additionally, we added manuscripts in Portuguese and Spanish, which could be missing in the searches based on English keywords. Although the review was focused on bats inhabiting the Brazilian biomes/territory, some species have larger distribution areas covering other South and Central American countries, and studies conducted in South American countries other than Brazil were also included. We built a reference library of 87 publications, for which 81 remained after filtering for bat sampling or full publication accessibility (Supplementary Files 1 and 2).
For each study we extracted title, year of publication, viral family/lineage studied (specific viral families or several for virome studies), country, state, bat species, number of sampled individuals per species, number of positive individuals, methodology of virus detection, sampling strategies (longitudinal or single sampling), and type of sample (tissue or body fluid). We extracted data to species level for viruses and bats, and used family-level taxonomy for visualization. The methodologies employed for virus detection and characterization were classified according to throughput: low throughput (LT), high throughput (HT), and studies using both methodologies (LT + HT). We detected the following methodologies used in the published articles: Virus Isolation through Intracerebral Inoculation of Mice (VIIIM), the Virus Isolation in Cell Culture (VICC), Electron Microscopy and Antigenic Profile (ELANT), Western Blot (WB), Direct Immunofluorescence Antigen Monoclonal Antibodies (DIAMA), Enzyme-Linked Immunosorbent Assay (ELISA), Strain-Specific PCR (SS-PCR), Family/Subfamily Degenerate PCR (FSD-PCR), Nested PCR (N-PCR), DNA Virome (DNAVIR), RNA Virome (RNAVIR), and Full Virome (FVIR) (Table 1).
A number of studies have sampled and characterized viruses from bat excrements (e.g. guano) where many virus genomes were likely derived from food sources and internal or external bat microbiota. Therefore, we only extracted information of viruses that were known to infect vertebrates, irrespective of the original sample (guano, oral/anal fluids or tissues).
Nucleotide database search and information extraction.
In order to assess the available virus sequence information, we retrieved the number of entries per bat species and virus family from the ZOVER database (http://www.mgc.ac.cn/cgi-bin/ZOVER/main.cgi; last accessed May 2022) (43) using the most up-to-date taxonomy (https://www.sbeq.net/lista-de-especies). This database was chosen because it is the only database that compiles all available viral genetic information found in bats.
Figures were rendered with R statistical language (https://www.r-project.org/) employing packages such as ggplot2 (63), webr 0.1.2 (64), dplyr 1.0.9 (65), tidyr 1.2.0 (66), ggpubr 0.4.0 (67), reshape2 1.4.4 (68), ggforce 0.3.3 (69), ggridges 0.5.3 (70), treemapify 2.5.5 (71), ggdist 3.1.1 (42), gghalves 0.1.3 (72), packcircles 0.3.4 (73), ggrepel 0.9.1 (74), patchwork 1.1.1 (75), and ggvenn 0.1.9 (76). Maps were rendered using QGIS version 3.26 (77).
ACKNOWLEDGMENTS
G.L.W. is supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) through their productivity research fellowships (303902/2019-1). In addition, G.L.W. was also supported by Coodenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Alexander von Humboldt Foundation. E.Ba. is supported by CNPq through a postdoctoral fellowship (152672/2022-2). E.Be. is supported by a CNPq productivity grant. We thank Lais Ceschini Machado for map rendering assistance and Matheus Filgueira Bezerra and the two anonymous reviewers for highly valuable suggestions that improved the manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Gabriel Luz Wallau, Email: gabriel.wallau@fiocruz.br.
Biao He, Changchun Veterinary Research Institute.
REFERENCES
- 1.Piló LB, Calux A, Scherer R, Bernard E. 2022. Bats as ecosystem engineers in iron ore caves in the Carajás National Forest, Brazilian Amazonia. bioRxiv. doi: 10.1101/2022.04.19.488750. [DOI] [PMC free article] [PubMed]
- 2.Van Brussel K, Holmes EC. 2022. Zoonotic disease and virome diversity in bats. Curr Opin Virol 52:192–202. doi: 10.1016/j.coviro.2021.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, Yang L, Ren X, He G, Zhang J, Yang J, Qian Z, Dong J, Sun L, Zhu Y, Du J, Yang F, Zhang S, Jin Q. 2016. Deciphering the bat virome catalog to better understand the ecological diversity of bat viruses and the bat origin of emerging infectious diseases. ISME J 10:609–620. doi: 10.1038/ismej.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Letko M, Seifert SN, Olival KJ, Plowright RK, Munster VJ. 2020. Bat-borne virus diversity, spillover and emergence. Nat Rev Microbiol 18:461–471. doi: 10.1038/s41579-020-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez V, Banerjee A. 2022. Molecular, ecological, and behavioural drivers of the bat–virus relationship. iScience 25:104779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith I, Wang LF. 2013. Bats and their virome: an important source of emerging viruses capable of infecting humans. Curr Opin Virol 3:84–91. doi: 10.1016/j.coviro.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. 2006. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev 19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luis AD, Hayman DTS, O’Shea TJ, Cryan PM, Gilbert AT, Pulliam JRC, Mills JN, Timonin ME, Willis CKR, Cunningham AA, Fooks AR, Rupprecht CE, Wood JLN, Webb CT. 2013. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc R Soc Lond B Biol Sci 280:20122753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moratelli R, Calisher CH. 2015. Bats and zoonotic viruses: can we confidently link bats with emerging deadly viruses? Mem Inst Oswaldo Cruz 110:1–22. doi: 10.1590/0074-02760150048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreuder Johnson C, Hitchens PL, Smiley Evans T, Goldstein T, Thomas K, Clements A, Joly DO, Wolfe ND, Daszak P, Karesh WB, Mazet JK. 2015. Spillover and pandemic properties of zoonotic viruses with high host plasticity. Sci Rep 5:14830–14838. doi: 10.1038/srep14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessler MK, Becker DJ, Peel AJ, Justice NV, Lunn T, Crowley DE, Jones DN, Eby P, Sánchez CA, Plowright RK. 2018. Changing resource landscapes and spillover of henipaviruses. Ann N Y Acad Sci 1429:78–99. doi: 10.1111/nyas.13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olivero J, Fa JE, Farfán MÁ, Márquez AL, Real R, Juste FJ, Leendertz SA, Nasi R. 2020. Human activities link fruit bat presence to Ebola virus disease outbreaks. Mam Rev 50:1–10. doi: 10.1111/mam.12173. [DOI] [Google Scholar]
- 13.Epstein JH, Anthony SJ, Islam A, Kilpatrick AM, Ali Khan S, Balkey MD, Ross N, Smith I, Zambrana-Torrelio C, Tao Y, Islam A, Quan PL, Olival KJ, Khan MSU, Gurley ES, Hossein MJ, Field HE, Fielder MD, Briese T, Rahman M, Broder CC, Crameri G, Wang L-F, Luby SP, Lipkin WI, Daszak P. 2020. Nipah virus dynamics in bats and implications for spillover to humans. Proc Natl Acad Sci USA 117:29190–29201. doi: 10.1073/pnas.2000429117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rulli MC, D’Odorico P, Galli N, Hayman DTS. 2021. Land-use change and the livestock revolution increase the risk of zoonotic coronavirus transmission from rhinolophid bats. Nat Food 2:409–416. doi: 10.1038/s43016-021-00285-x. [DOI] [PubMed] [Google Scholar]
- 15.Plowright RK, Eby P, Hudson PJ, Smith IL, Westcott D, Bryden WL, Middleton D, Reid PA, McFarlane RA, Martin G, Tabor GM, Skerratt LF, Anderson DL, Crameri G, Quammen D, Jordan D, Freeman P, Wang LF, Epstein JH, Marsh GA, Kung NY, McCallum H. 2014. Ecological dynamics of emerging bat virus spillover. Proc R Soc Lond B Biol Sci 282:20142124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mollentze N, Streicker DG. 2020. Viral zoonotic risk is homogenous among taxonomic orders of mammalian and avian reservoir hosts. Proc Natl Acad Sci USA 117:9423–9430. doi: 10.1073/pnas.1919176117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guth S, Mollentze N, Renault K, Streicker DG, Visher E, Boots M, Brook CE. 2022. Bats host the most virulent—but not the most dangerous—zoonotic viruses. Proc Natl Acad Sci USA 119:e2113628119. doi: 10.1073/pnas.2113628119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donaldson EF, Haskew AN, Gates JE, Huynh J, Moore CJ, Frieman MB. 2010. Metagenomic analysis of the viromes of three North American bat species: viral diversity among different bat species that share a common habitat. J Virol 84:13004–13018. doi: 10.1128/JVI.01255-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu D, Zhu C, Wang Y, Ai L, Yang L, Ye F, Ding C, Chen J, He B, Zhu J, Qian H, Xu W, Feng Y, Tan W, Wang C. 2017. Virome analysis for identification of novel mammalian viruses in bats from Southeast China. Sci Rep 7:10917. doi: 10.1038/s41598-017-11384-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen LE, Fagre AC, Chen B, Carlson CJ, Becker DJ. 2022. Sampling strategies and pre-pandemic surveillance gaps for bat coronaviruses. bioRxiv. doi: 10.1101/2022.06.15.496296. [DOI]
- 21.Páez DJ, Giles J, McCallum H, Field H, Jordan D, Peel AJ, Plowright RK. 2017. Conditions affecting the timing and magnitude of Hendra virus shedding across pteropodid bat populations in Australia. Epidemiol Infect 145:3143–3153. doi: 10.1017/S0950268817002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cassedy A, Parle-McDermott A, O’Kennedy R. 2021. Virus detection: a review of the current and emerging molecular and immunological methods. Front Mol Biosci 8:637559. doi: 10.3389/fmolb.2021.637559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey E, Holmes EC. 2022. Diversity and evolution of the animal virome. Nat Rev Microbiol 20:321–334. doi: 10.1038/s41579-021-00665-x. [DOI] [PubMed] [Google Scholar]
- 24.Smith SE, Huang W, Tiamani K, Unterer M, Khan Mirzaei M, Deng L. 2022. Emerging technologies in the study of the virome. Curr Opin Virol 54:101231. doi: 10.1016/j.coviro.2022.101231. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y-Z, Chen Y-M, Wang W, Qin X-C, Holmes EC. 2019. Expanding the RNA virosphere by unbiased metagenomics. Annu Rev Virol 6:119–139. doi: 10.1146/annurev-virology-092818-015851. [DOI] [PubMed] [Google Scholar]
- 26.Delwart E. 2012. Animal virus discovery: improving animal health, understanding zoonoses, and opportunities for vaccine development. Curr Opin Virol 2:344–352. doi: 10.1016/j.coviro.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolja VV, Koonin EV. 2018. Metagenomics reshapes the concepts of RNA virus evolution by revealing extensive horizontal virus transfer. Virus Res 244:36–52. doi: 10.1016/j.virusres.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He B, Li Z, Yang F, Zheng J, Feng Y, Guo H, Li Y, Wang Y, Su N, Zhang F, Fan Q, Tu C. 2013. Virome profiling of bats from Myanmar by metagenomic analysis of tissue samples reveals more novel mammalian viruses. PLoS One 8:e61950. doi: 10.1371/journal.pone.0061950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher CR, Streicker DG, Schnell MJ. 2018. The spread and evolution of rabies virus: conquering new frontiers. Nat Rev Microbiol 16:241–255. doi: 10.1038/nrmicro.2018.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horta MA, Ledesma LA, Moura WC, Lemos ERS. 2022. From dogs to bats: concerns regarding vampire bat-borne rabies in Brazil. PLoS Negl Trop Dis 16:e0010160. doi: 10.1371/journal.pntd.0010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Streicker DG, Turmelle AS, Vonhof MJ, Kuzmin IV, McCracken GF, Rupprecht CE. 2010. Host phylogeny constrains cross-species emergence and establishment of rabies virus in bats. Science 329:676–679. doi: 10.1126/science.1188836. [DOI] [PubMed] [Google Scholar]
- 32.Kuzmin IV, Rupprecht CE. 2007. Bat rabies, p 259–307. In Jackson AC, Wunner WH (ed), Rabies (2nd ed). Academic Press, Oxford, England. [Google Scholar]
- 33.Queiroz LH, Favoretto SR, Cunha EMS, Campos ACA, Lopes MC, de Carvalho C, Iamamoto K, Araújo DB, Venditti LLR, Ribeiro ES, Pedro WA, Durigon EL. 2012. Rabies in southeast Brazil: a change in the epidemiological pattern. Arch Virol 157:93–105. doi: 10.1007/s00705-011-1146-1. [DOI] [PubMed] [Google Scholar]
- 34.da Rosa EST, Kotait I, Barbosa TFS, Carrieri ML, Brandão PE, Pinheiro AS, Begot AL, Wada MY, de Oliveira RC, Grisard EC, Ferreira M, Lima RJDS, Montebello L, Medeiros DBA, Sousa RCM, Bensabath G, Carmo EH, Vasconcelos PFC. 2006. Bat-transmitted human rabies outbreaks, Brazilian Amazon. Emerg Infect Dis 12:1197–1202. doi: 10.3201/eid1208.050929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vieira LFP, Pereira SRFG, Carnieli P, Tavares LCB, Kotait I. 2013. Phylogeography of rabies virus isolated from herbivores and bats in the Espírito Santo State, Brazil. Virus Genes 46:330–336. doi: 10.1007/s11262-012-0866-y. [DOI] [PubMed] [Google Scholar]
- 36.Menozzi BD, de Novaes Oliveira R, Paiz LM, Richini-Pereira VB, Langoni H. 2017. Antigenic and genotypic characterization of rabies virus isolated from bats (Mammalia: Chiroptera) from municipalities in São Paulo State, Southeastern Brazil. Arch Virol 162:1201–1209. doi: 10.1007/s00705-017-3220-9. [DOI] [PubMed] [Google Scholar]
- 37.Allendorf SD, Cortez A, Heinemann MB, Harary CMA, Antunes JMAP, Peres MG, Vicente AF, Sodré MM, da Rosa AR, Megid J. 2012. Rabies virus distribution in tissues and molecular characterization of strains from naturally infected non-hematophagous bats. Virus Res 165:119–125. doi: 10.1016/j.virusres.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Oliveira R de N, de Souza SP, Lobo RSV, Castilho JG, Macedo CI, Carnieli P, Fahl WO, Achkar SM, Scheffer KC, Kotait I, Carrieri ML, Brandão PE. 2010. Rabies virus in insectivorous bats: implications of the diversity of the nucleoprotein and glycoprotein genes for molecular epidemiology. Virology 405:352–360. doi: 10.1016/j.virol.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi Y, Suzuki Y, Itou T, Carvalho AAB, Cunha EMS, Ito FH, Gojobori T, Sakai T. 2010. Low genetic diversities of rabies virus populations within different hosts in Brazil. Infect Genet Evol 10:278–283. doi: 10.1016/j.meegid.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Bernardi F, Nadin-Davis SA, Wandeler AI, Armstrong J, Gomes AaB, Lima FS, Nogueira FRB, Ito FH. 2005. Antigenic and genetic characterization of rabies viruses isolated from domestic and wild animals of Brazil identifies the hoary fox as a rabies reservoir. J Gen Virol 86:3153–3162. doi: 10.1099/vir.0.81223-0. [DOI] [PubMed] [Google Scholar]
- 41.Shoji Y, Kobayashi Y, Sato G, Itou T, Miura Y, Mikami T, Cunha EMS, Samara SI, Carvalho AAB, Nocitti DP, Ito FH, Kurane I, Sakai T. 2004. Genetic characterization of rabies viruses isolated from frugivorous bat (Artibeus spp.) in Brazil. J Vet Med Sci 66:1271–1273. doi: 10.1292/jvms.66.1271. [DOI] [PubMed] [Google Scholar]
- 42.Kay M, Wiernik BM. 2022. ggdist: visualizations of distributions and uncertainty (3.2.0). [DOI] [PubMed]
- 43.Zhou S, Liu B, Han Y, Wang Y, Chen L, Wu Z, Yang J. 2022. ZOVER: the database of zoonotic and vector-borne viruses. Nucleic Acids Res 50:D943–D949. doi: 10.1093/nar/gkab862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drexler JF, Corman VM, Wegner T, Tateno AF, Zerbinati RM, Gloza-Rausch F, Seebens A, Müller MA, Drosten C. 2011. Amplification of emerging viruses in a bat colony. Emerg Infect Dis 17:449–456. doi: 10.3201/eid1703.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mortlock M, Geldenhuys M, Dietrich M, Epstein JH, Weyer J, Pawęska JT, Markotter W. 2021. Seasonal shedding patterns of diverse henipavirus-related paramyxoviruses in Egyptian rousette bats. Sci Rep 11:24262. doi: 10.1038/s41598-021-03641-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amman BR, Carroll SA, Reed ZD, Sealy TK, Balinandi S, Swanepoel R, Kemp A, Erickson BR, Comer JA, Campbell S, Cannon DL, Khristova ML, Atimnedi P, Paddock CD, Crockett RJK, Flietstra TD, Warfield KL, Unfer R, Katongole-Mbidde E, Downing R, Tappero JW, Zaki SR, Rollin PE, Ksiazek TG, Nichol ST, Towner JS. 2012. Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog 8:e1002877. doi: 10.1371/journal.ppat.1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ge X, Li Y, Yang X, Zhang H, Zhou P, Zhang Y, Shi Z. 2012. Metagenomic analysis of viruses from bat fecal samples reveals many novel viruses in insectivorous bats in China. J Virol 86:4620–4630. doi: 10.1128/JVI.06671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, Victoria JG, Wang C, Jones M, Fellers GM, Kunz TH, Delwart E. 2010. Bat guano virome: predominance of dietary viruses from insects and plants plus novel mammalian viruses. J Virol 84:6955–6965. doi: 10.1128/JVI.00501-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardmeier I, Aeberhard N, Qi W, Schoenbaechler K, Kraettli H, Hatt J-M, Fraefel C, Kubacki J. 2021. Metagenomic analysis of fecal and tissue samples from 18 endemic bat species in Switzerland revealed a diverse virus composition including potentially zoonotic viruses. PLoS One 16:e0252534. doi: 10.1371/journal.pone.0252534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nusser SM, Clark WR, Otis DL, Huang L. 2008. Sampling considerations for disease surveillance in wildlife populations. J Wildl Manag 72:52–60. doi: 10.2193/2007-317. [DOI] [Google Scholar]
- 51.Bones VC, Gameiro AH, Castilho JG, Molento CFM. 2015. Comparative costs of the Mouse Inoculation Test (MIT) and Virus Isolation in Cell Culture (VICC) for use in rabies diagnosis in Brazil. Altern Lab Anim 43:81–87. doi: 10.1177/026119291504300203. [DOI] [PubMed] [Google Scholar]
- 52.Dean DJ, Abelseth MK. 1973. Laboratory techniques in rabies: the fluorescent antibody test. Monogr Ser World Health Organ 23:73–84. [PubMed] [Google Scholar]
- 53.Schulz JE, Seifert SN, Thompson JT, Avanzato V, Sterling SL, Yan L, Letko MC, Matson MJ, Fischer RJ, Tremeau-Bravard A, Seetahal JFR, Ramkissoon V, Foster J, Goldstein T, Anthony SJ, Epstein JH, Laing ED, Broder CC, Carrington CVF, Schountz T, Munster VJ. 2020. Serological evidence for Henipa-like and Filo-like viruses in Trinidad bats. J Infect Dis 221:S375–S382. doi: 10.1093/infdis/jiz648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frank SA. 2002. Immunology and evolution of infectious disease. Princeton University Press, Princeton, NJ. [PubMed] [Google Scholar]
- 55.Zhang YZ, Shi M, Holmes EC. 2018. Using metagenomics to characterize an expanding virosphere. Cell 172:1168–1172. doi: 10.1016/j.cell.2018.02.043. [DOI] [PubMed] [Google Scholar]
- 56.Ruiz-Aravena M, McKee C, Gamble A, Lunn T, Morris A, Snedden CE, Yinda CK, Port JR, Buchholz DW, Yeo YY, Faust C, Jax E, Dee L, Jones DN, Kessler MK, Falvo C, Crowley D, Bharti N, Brook CE, Aguilar HC, Peel AJ, Restif O, Schountz T, Parrish CR, Gurley ES, Lloyd-Smith JO, Hudson PJ, Munster VJ, Plowright RK. 2022. Ecology, evolution and spillover of coronaviruses from bats. Nat Rev Microbiol 20:299–314. doi: 10.1038/s41579-021-00652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rose R, Constantinides B, Tapinos A, Robertson DL, Prosperi M. 2016. Challenges in the analysis of viral metagenomes. Virus Evol 2:vew022. doi: 10.1093/ve/vew022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Islam MS, Sazzad HMS, Satter SM, Sultana S, Hossain MJ, Hasan M, Rahman M, Campbell S, Cannon DL, Ströher U, Daszak P, Luby SP, Gurley ES. 2016. Nipah virus transmission from bats to humans associated with drinking traditional liquor made from date palm sap, Bangladesh, 2011–2014. Emerg Infect Dis 22:664–670. doi: 10.3201/eid2204.151747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luby SP, Rahman M, Hossain MJ, Blum LS, Husain MM, Gurley ES, Khan R, Ahmed B-N, Rahman S, Nahar N, Kenah E, Comer JA, Ksiazek TG. 2006. Foodborne transmission of Nipah virus, Bangladesh. Emerg Infect Dis 12:1888–1894. doi: 10.3201/eid1212.060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silva Junior CHL, Pessôa ACM, Carvalho NS, Reis JBC, Anderson LO, Aragão LEOC. 2021. The Brazilian Amazon deforestation rate in 2020 is the greatest of the decade. Nat Ecol Evol 5:144–145. doi: 10.1038/s41559-020-01368-x. [DOI] [PubMed] [Google Scholar]
- 61.Ellwanger JH, Kulmann-Leal B, Kaminski VL, Valverde-Villegas JM, Veiga ABGD, Spilki FR, Fearnside PM, Caesar L, Giatti LL, Wallau GL, Almeida SEM, Borba MR, Hora VPD, Chies JAB. 2020. Beyond diversity loss and climate change: impacts of Amazon deforestation on infectious diseases and public health. An Acad Bras Cienc 92:e20191375. doi: 10.1590/0001-3765202020191375. [DOI] [PubMed] [Google Scholar]
- 62.Winck GR, Raimundo RLG, Fernandes-Ferreira H, Bueno MG, D'Andrea PS, Rocha FL, Cruz GLT, Vilar EM, Brandão M, Cordeiro JLP, Andreazzi CS. 2022. Socioecological vulnerability and the risk of zoonotic disease emergence in Brazil. Sci Adv 8:eabo5774. doi: 10.1126/sciadv.abo5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wickham H. 2016. ggplot2. https://link.springer.com/book/10.1007/978-3-319-24277-4. Retrieved 8 September 2022.
- 64.Data and Functions for Web-Based Analysis. 2022. https://cardiomoon.github.io/webr/index.html. Retrieved 8 September 2022.
- 65.Wickham H, François R, Henry L, Müller K. 2022. dplyr: A Grammar of Data Manipulation. https://dplyr.tidyverse.org, https://github.com/tidyverse/dplyr.
- 66. Wickham A. 2022. tidyr: “tidyr” Tidy massy data (1.2.1).
- 67.Kassambara A. 2022. ggpubr: “ggplot2” based publication ready plots (0.5.0).
- 68.Wickham H. 2020. reshape2: flexibly reshape data: a reboot of the reshape package (1.4.4).
- 69.Pedersen TL, RStudio . 2022. ggforce: accelerating “ggplot2” (0.4.1).
- 70.Wilke CO. 2022. ggridges: ridgeline plots in “ggplot2” (0.5.4).
- 71.Wilkins D, Rudis B. 2021. treemapify: draw treemaps in “ggplot2” (2.5.5).
- 72.Tiedemann F. 2022. gghalves: compose half-half plots using your favourite geoms (0.1.4).
- 73.Bedward M. 2022. packcircles: circle packing (0.3.5).
- 74.Slowikowski K, Schep A, Hughes S, Dang TK, Lukauskas S, Irisson J-O, Kamvar ZN, Ryan T, Christophe D, Hiroaki Y, Gramme P, Abdol AM, Barrett M, Cannoodt R, Krassowski M, Chirico M, Aphalo P. 2022. ggrepel: automatically position non-overlapping text labels with “ggplot2” (0.9.2).
- 75.Pedersen TL. 2022. patchwork: the composer of plots (1.1.2).
- 76.Yan L. 2022. ggvenn 0.1.9. R package.
- 77.QGIS Development Team. 2022. QGIS Geographic Information System. Open Source Geospatial Foundation Project (3.26).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.04077-22-s0001.xlsx, XLSX file, 0.03 MB (28.6KB, xlsx)
Supplemental material. Download spectrum.04077-22-s0002.xlsx, XLSX file, 2.8 MB (2.8MB, xlsx)
Supplemental material. Download spectrum.04077-22-s0003.xlsx, XLSX file, 0.02 MB (23.5KB, xlsx)
Supplemental material. Download spectrum.04077-22-s0004.xlsx, XLSX file, 0.04 MB (42.6KB, xlsx)
Supplemental material. Download spectrum.04077-22-s0005.pdf, PDF file, 0.2 MB (161.1KB, pdf)