Abstract
Background:
Cognitive reserve (CR) has been postulated to contribute to the variation observed between neuropathology and clinical outcomes in Alzheimer’s disease (AD).
Objective:
We investigated the effect of an education-occupation derived CR proxy on biological properties of white matter tracts in patients with amnestic mild cognitive impairment (aMCI) and healthy elders (HC).
Methods:
Educational attainment and occupational complexity ratings (complexity with data, people, and things) from thirty-five patients with aMCI and twenty-eight HC were used to generate composite CR scores. Quantitative magnetic resonance imaging (qMRI) and multi-shell diffusion MRI were used to extract macromolecular tissue volume (MTV) across major white matter tracts.
Results:
We observed significant differences in the association between CR and white matter tract MTV in aMCI versus HC when age, gender, intracranial volume, and memory ability were held constant. Particularly, in aMCI, higher CR was associated with worse tract pathology (lower MTV) in the left and right dorsal cingulum, callosum forceps major, right inferior fronto-occipital fasciculus, and right superior longitudinal fasciculus (SLF) tracts. Conversely higher CR was associated with higher MTV in the right parahippocampal cingulum and left SLF in HC.
Conclusion:
Our results support compensatory CR mechanisms in aMCI and neuroprotective mechanisms in HC and suggest differential roles for CR on white matter macromolecular properties in healthy elders versus prodromal AD patients.
Keywords: Alzheimer’s disease, brain reserve, cognitive reserve, mild cognitive impairment, quantitative magnetic resonance imaging (qMRI), white matter tracts
INTRODUCTION
Alzheimer’s disease (AD) is a progressive, neurodegenerative disorder characterized by the presence of amyloid plaques and neurofibrillary tangles in the brain and accompanied by cognitive symptoms of progressive memory loss and cognitive decline, eventually resulting in death [1]. There is currently no cure for AD, and the etiology of the disease remains unknown and is likely due to multifactorial genetic and environmental causes.
Notably, a high level of variability exists between neuropathology and clinical outcomes in AD [2, 3]. A pilot epidemiological study [2] initially observed this discrepancy between pathology and clinical severity. Among 137 nursing home residents, 11 patients clinically diagnosed with AD just prior to death did not display histopathological AD signatures postmortem. Additionally, ten patients determined to be cognitively normal before death presented characteristic neurotic plaques and tangles at levels sufficient for AD diagnosis upon autopsy [2]. Further, it has been posited that between 10–40% of individuals with AD pathology assessed at autopsy do not meet the criteria for AD associated cognitive impairment [4, 5]. While part of this variation is attributed to the heterogeneity of AD and related risk factors [6], variations in levels of cognitive reserve (CR) have been suggested to play an important role in moderating the relationship between the level of pathology and clinical outcomes [7].
CR is a construct used to explain why some individuals retain cognitive function albeit increasing brain pathology and is postulated as an explanation for why certain individuals maintain cognitive abilities with age despite significant neurological insult while others presenting with similar brain pathologies go on to develop further cognitive decline in the form of AD. Participation in certain enriching and modifiable life experiences that are either cognitively, socially, or physically stimulating are believed to contribute to CR and have all been shown to be protective against AD [5, 8]. These experiences influence both the age of clinical onset of AD and affect the rate of cognitive decline following AD diagnosis, where higher levels of CR confer a decreased risk of incident dementia but an increased rate of cognitive decline once AD is diagnosed [5, 8–10].
While consensus on operational definitions of CR has not yet been established, educational attainment and occupational complexity are commonly used and widely accepted proxy measures of CR [8, 11]. Particularly, previous studies suggest that individuals with lower educational attainment or occupational complexity are at higher risk of cognitive decline [12, 13]. For example, a longitudinal study [11] demonstrated that among 593 non-demented elders observed over four years, those with more years of education completed or higher occupational complexity had a significantly lower risk of developing dementia compared to individuals with lower educational attainment or occupational complexity, but once AD was diagnosed showed an increased rate of cognitive decline. These observations suggest that higher CR may allow some individuals to resist the harmful effects of pathology on cognition until pathology levels reach a given threshold, at which point the brain’s cognitive processes can no longer cope with increasing pathology, resulting in a sharper decline in cognitive function following clinical diagnosis [14, 15].
Mechanistically, active CR models posit that current neural activity shaped through participation in enriching life experiences increases the efficiency, capacity, and/or plasticity of cognitive processes and increases resistance to the harmful effects of pathology on cognition [8]. Active CR models underlie compensatory mechanistic roles for CR, where CR increases the ability of the brain’s cognitive processes to adapt to pathological changes and buffer against cognitive decline in the presence of pathology. Alternatively, passive models of reserve (brain reserve models) posit that pre-existing individual differences in brain structure, including in measures such as regional gray matter volumes or total intracranial volume (ICV), contribute to the preservation of cognitive function despite increasing brain burden with age, either through minimizing the development of neuropathology or through an increased capacity to withstand pathological changes [16].
On the other hand, brain maintenance models posit CR may play a role in increasing or maintaining the structural integrity of the brain and helping to resist developing pathology [8]. While mechanisms attributed to active and passive reserve models may not be mutually exclusive, amounting neuroimaging and clinical evidence supports compensatory roles for CR in AD patients [17]. For example, positive associations have been observed between CR and level of brain pathology in AD patients matched for cognition across the disease spectrum [18–20].
The association between CR and gray matter atrophy has been extensively studied, yet only a few studies have looked at the relationship between CR and white matter [21–24]. Brain white matter consists of neuronal axons and their supporting macromolecular counterparts, the latter of which are composed of various tissue types, namely, myelin, astrocytes, microglia, and other lipid macromolecules such as cholesterol that support neuronal function [25]. Myelin sheaths envelop the lengths of neuronal axons in white matter and aide in the propagation of electrical signals within neuronal circuits that give rise to large scale cortical networks. Accumulating evidence suggests that AD pathology affects biological properties of white matter beginning from pre-symptomatic stages of AD, where AD disrupts integral white matter properties involving axonal transport and packaging, axonal density, axonal tract myelination, and macromolecular lipid composition [26, 27]. While MRI measures probing gray matter accurately capture neurodegeneration due to AD at the macrostructural level, MRI measures of white matter microstructure may be more sensitive to AD pathology at earlier stages of the disease, where early disruptions to white matter integrity may reflect an initial breakdown of axonal myelin which precedes cognitive decline and gray matter atrophy in AD [28].
White matter hyperintensity (WMH) measured using T2-weighted MRI is a common marker of white matter pathology in aging [29]. However, accumulating data suggest that the primary driver of WMH is ischemic and the underlying histopathology is heterogenous [30, 31]. Diffusion-weighted imaging is the most widely used method to measure biophysical changes in brain white matter in AD and can detect fine-grained microstructural damage to white matter tracts before they can be seen in conventional MRI (e.g., WMH derived from T2) [30, 32]. However, the diffusion tensor models that are used to quantify the conventional diffusion measures (i.e., fractional anisotropy (FA)) are highly influenced by the axonal orientation and density of white matter. Therefore, they provide a limited interpretation of white matter properties, particularly in the brain volumes with many crossing fibers. Additionally, previous studies of CR have suggested that structural measures such as volumetric MRI measures of regional brain atrophy may not closely reflect structurally induced changes due to CR, and that measures of white matter integrity may be more sensitive neural correlates [33].
Recent advancements in quantitative MRI (qMRI) acquisition and post-processing have led to an increased sensitivity of these measures in probing biological properties of white matter in vivo [25, 27, 34]. Measures derived from qMRI have been designed to be robust to wide range of scan parameters and field strengths [35] and were shown to distinguish patients with amnestic mild cognitive impairment (aMCI) from healthy elders [27]. Particularly, macromolecular tissue volume (MTV) is a measure derived from qMRI that quantifies the macromolecular tissue content and lipid membranes and is not confounded by the crossing fibers. MTV is higher in well-myelinated white matter that is composed of a very dense structure and contains more macromolecules than water molecules [25, 35]. For these reasons, MTV measures of white matter will provide a unique contribution to our understanding of white matter degeneration in AD beyond what can be detected by the conventional diffusion measures. MTV measures in white matter tracts provide sensitive information pertaining to subtle changes in the biological properties of white matter, namely the macromolecular content, that cannot be detected by conventional diffusion measures such as FA [36, 37].
To date, few studies have looked at the association between CR and tract-specific MRI measures [19, 21, 24, 38]. Additionally, no studies to our knowledge have assessed the association of CR with white matter tract myelination outcomes derived from qMRI. Prior studies assessing the effect of CR proxies on white matter outcomes in AD have demonstrated that higher CR was associated with worse white matter microstructural outcomes, as evidenced by lower white matter FA in AD patients matched for clinical status [19, 21]. Given the importance of in vivo qMRI white matter measures in detecting early biological changes among prodromal AD patients as well as amassing evidence highlighting the potential protective effects of CR on cognitive decline in aging and AD, the current study aims to confirm previous findings of positive associations between CR and brain pathology in prodromal AD patients as well as test if a similar pattern is observed between CR and tract specific white matter MTV in healthy elders. To achieve this goal, we studied a sample of healthy elders (HC) as well as patients with aMCI, who are at higher risk for developing AD. We have already described group differences in MTV values between aMCI and HC in our recent publication [27]. These data showed significant MTV loss along major white matter tracts in patients with amnestic MCI and further confirmed the advantage of qMRI measures over conventional diffusion measures in detecting subtle changes in white matter tissue in patients with aMCI. In this study, we will particularly focus on the association between CR and MTV measures.
We quantified a CR proxy composite score in our sample derived from measures of educational attainment and occupational complexity classifications as outlined by the US Dictionary of Occupational Titles (DOT) based off each subject’s reported years of education completed and/or degrees obtained and main occupation. We then tested differences in the associations between CR and white matter tract MTV—derived from qMRI—between aMCI and HC groups, after accounting for differences in memory ability. We expected that aMCI participants would display negative associations between CR and MTV outcomes, indicating lower levels of white matter MTV at similar levels of memory performance, supporting compensatory models of CR. Among HC, we would expect to see positive associations between CR and MTV outcomes, suggesting potential neuroprotective or brain maintenance roles for CR [19].
MATERIALS AND METHODS
Participant characteristics
Sixty-three older adults (40 female, 65–85 years old, mean (SD) age = 73.3 (5.85)) were recruited from the local community by means of digital and newspaper advertisements and included in the current analysis. Potential subjects completed an electronic eligibility screening form through RedCap and were excluded on the basis of any of the following criteria: presence of dementia, presence of suicidality, presence of any significant psychiatric or neurological disorder, history of alcohol or substance abuse or dependence, presence of certain medical conditions, use of medications with significant anti-cholinergic effects, MRI incompatible materials in the body, claustrophobia, and left handedness. All subjects underwent a comprehensive battery of clinical and neuropsychological assessments and questionnaires to further determine study eligibility, for group operationalization, and for cognitive assessment. Eligibility criteria for both groups were as follows: Mini-Mental State Examination (MMSE) total score ≥24, intact Independent Activities of Daily Living (IADL), Geriatric Depression Scale (GDS) score ≤5, and no indication of the presence of any current axis I psychiatric condition as determined by the Mini International Neuropsychiatric Interview [39].
Average scores adjusted for education from stories A and B of the Weschler Logical Memory II (LM-II) Delayed Recall subtest were used as the basis for categorizing participants as either aMCI (N = 35; diagnostic criteria: LM-II stories A & B average score ≤8.5, ≤4.5, ≤2.5 for ≥16, 8–15, and 0–7 years of education, respectively) or HC (N = 28; LM-II average score >8.5,>4.5,>2.5 for ≥16, 8–15, and 0–7 years of education). LM2 is a widely accepted clinical test of verbal episodic memory functioning that is sensitive in distinguishing aMCI from HC participants, since verbal episodic memory is the pre-dominant cognitive domain affected in aMCI [16]. The study was approved by Stanford Institutional Review Board and all participants provided written informed consent. Demographic characteristics and neuropsychological assessment scores are summarized in Table 1.
Table 1.
Demographic and neuropsychological sample characteristics
| Mean (SD) HC | Range HC | Mean (SD) aMCI | Range aMCI | p | |
|---|---|---|---|---|---|
| Demographics | |||||
| Sample size (n) | 28 | – | 35 | – | – |
| Age (y) | 72.89 (6.17) | 65–88 | 73.63 (5.66) | 65–89 | 0.624 |
| Female (%) | 75% | – | 54% | – | 0.092 |
| Educational Attainment | |||||
| Education (categorical) | 2.93 (0.26) | 2–3 | 2.94 (0.24) | 2–3 | 0.821 |
| Occupational Complexity | |||||
| Complexity with data | 4.68 (1.02) | 3–6 | 4.63 (0.81) | 3–6 | 0.829 |
| Complexity with people | 3.32 (2.39) | 0–8 | 4.34 (2.44) | 2–8 | 0.101 |
| Complexity with things | 1.89 (2.64) | 0–6 | 1.51 (2.62) | 0–6 | 0.572 |
| CR Composite | |||||
| CR composite score | 0.21 (1.20) | −1.39–2.36 | −0.17 (1.22) | −1.39–2.97 | 0.223 |
| Memory Composite | |||||
| Memory composite score | −2.48 (5.56) | −11.71–7.25 | 1.99 (5.33) | −7.88–16.54 | 0.002 * |
| Cognitive Measures | |||||
| MMSE total score | 29.36 (0.87) | 27–30 | 28.03 (1.64) | 24–30 | <0.001* |
| LM-II average score | 11.61 (3.30) | 6–19.5 | 5.94 (2.77) | 0–15 | <0.001* |
| NIH Toolbox RAVLT score | 22.54 (5.53) | 13–33 | 20.29 (5.33) | 7–30 | 0.107 |
| NIH Toolbox Fluid Composite USS | 91.37 (12.25) | 62–115 | 89.17 (11.24) | 69–109 | 0.465 |
| MRI Measures | |||||
| ICV (cm3) | 1383.50 (110.06) | 1134.95–1603.49 | 1438.25 (131.74) | 1227.34–1820.80 | 0.083 |
| WMHV (mL) | 6.26 (7.43) | 0–29.11 | 2.96 (2.63) | 0–9.3 | 0.017 * |
HC, healthy elder; aMCI, amnestic mild cognitive impairment; CR, cognitive reserve; MMSE, Mini-Mental State Examination (max score = 30); LM-II, Wechsler Memory Scale Logical Memory II (delayed recall raw score); RAVLT, Rey’s Auditory Verbal Learning Test raw score; USS, Uncorrected Standard Score; ICV, intracranial volume; WMHV, white matter hyperintensity volume; Reported between group statistics represent the results of a two-sample t-test for HC versus aMCI groups;
indicates measures that were significantly different between groups.
Cognitive measures
Additional cognitive measures included performance scores NIH Toolbox – an iPad-based, multi-domain cognitive assessment battery developed by the National Institutes of Health (NIH). Raw scores on the Rey’s Auditory Verbal Learning Test (RAVLT), assessing immediate free recall of an auditorily presented list of words over 5 trials, and Cognitive Fluid Composite standard scores, measuring fluid reasoning ability, were included in the analysis.
Cognitive reserve proxy
Data on educational and occupational attainment were collected electronically through the administration of a self-report questionnaire [40] where subjects indicated their main lifetime occupation as well as total number of years of education completed.
Educational attainment was operationalized categorically (range 0–3) based on the total years of education reported by each participant using the following classifications: 0 = no formal education, 1 = primary school, 2 = secondary school, 3 = university or professional degree [16, 20, 33]. Additionally, all analyses were conducted substituting the categorical definition for educational attainment with a continuous measure (years of education), which yielded essentially similar results. Occupational complexity was determined by matching each subject’s main reported occupation with a unique occupational code derived from the 1970 US Census Dictionary of Occupational Titles (DOT), Fourth Edition [41]. This approach for defining occupational complexity variables has been previously described in CR research [18, 42, 43]. Briefly, one researcher assigned a DOT code to each subject. The resultant DOT code yielded occupational complexity ratings along three domains developed by Roose & Treimen (1980) [44] and included occupational complexity scores for the complexity of work with data (range 0–6), complexity of work with people (range 0–8), and complexity of work with things (range 0–7) [41]. Lower occupational scores indicated higher occupational complexity along each domain. For the purpose of interpretability, occupational complexity scores for data, people, and things were reverse coded so that higher occupational complexity with data, people, or things corresponded to higher complexity for each domain (Table 2) [18, 42]. One aMCI subject was excluded from the analysis due to the fact that their reported occupation of a homemaker was not listed in the DOT index [43].
Table 2.
Explanation of occupational complexity variables
| Complexity with data | Complexity with people | Complexity with things | |
|---|---|---|---|
| 0 | Comparing | Taking Instructions-Helping | Handling |
| 1 | Copying | Serving | Feeding-Off bearing |
| 2 | Computing | Speaking-Signaling | Tending |
| 3 | Compiling | Persuading | Manipulating |
| 4 | Analyzing | Diverting | Driving-Operating |
| 5 | Coordinating | Supervising | Operating-Controlling |
| 6 | Synthesizing | Instructing | Precision Working |
| 7 | Negotiating | Setting Up | |
| 8 | Mentoring |
The educational attainment and occupational complexity scores with data, people, and things were first standardized. Next, principal component analysis (PCA) was performed on the standardized measures to generate a composite CR score for each participant, represented by the first principal component from the PCA on educational and occupational factors. This factorial approach for measuring CR has been extensively employed in previous studies [20, 33, 45].
Memory ability
Composite memory scores represented verbal episodic memory ability and were derived from a PCA comprising RAVLT and LM-II average scores, and the resulting first principal component was used as a composite measure of overall verbal memory ability in the subsequent regression models.
MRI data acquisition
MRI images were acquired for all subjects on a GE 3T scanner (General Electric Healthcare, Milwaukee, WI, USA) equipped with a 32-channel head coil (Nova Medical, Wilmington, MA, USA) at the Center for Neurobiological Imaging at Stanford University. Multi-shell dMRI were acquired with isotropic 2mm3 spatial resolution using the following parameters: repetition time (TR) = 2.8 s; echo time (TE) = 0.078 s; FOV = 22.4 cm; Matrix size = 112 × 112; 63 axial slices with a simultaneous multi-slice acceleration factor of 3. The sequence included 119 total directions comprising 80 diffusion gradient directions with b = 2855 s/mm2, 30 diffusion gradient directions with b = 710 s/mm2 and nine images without diffusion weighting (b = 0 s/mm2). To apply EPI distortion correction, an additional scan was acquired in the opposite phase encoding direction and included 6 diffusion gradient directions (b = 2855 s/mm2) and two non-diffusion-weighted images. Sagittal T1w images were acquired using the following parameters: inversion time (TI) = 450 ms; Matrix size: 256 × 256; Slice thickness: 0.9 mm; Flip angle: 12 degrees. The T2 fluid-attenuated inversion recovery (FLAIR) images were acquired with TR = 6500 ms, TE = 120 ms, TI = 1878 ms, flip angle = 900, Slice thickness: 1 mm and matrix size: 256 × 256. qMRI images were acquired using an inversion-recovery (IR) gradient-echo EPI sequence, and scan parameters are as follows: Flip angle = 77 degrees; TR = 3 s; FOV = 24 cm; Matrix size = 120 × 120; Slice thickness = 2 mm; TI = 1.
MRI data processing
Diffusion MRI (dMRI) data preprocessing was performed in FSL (fsl.fmrib.ox.ac.uk/fsl/fslwiki/) and MRTrix3 (mrtrix.org) and included denoising, geometric EPI distortion, eddy current distortion, slice-by-slice motion correction, outlier detection, and bias field correction (ANTs N4BiasField Correction). After motion correction, diffusion gradients were adjusted to account for the rotation applied to the measurements, and a diffusion kurtosis (DK) tensor model was then fit for each voxel’s data. Multi-tissue constrain spherical deconvolution was then applied to estimate the fiber orientation distributions (FODs) on aligned and distortion corrected dMRI data using the average tissue response function in MRtrix (3.0). Probabilistic tractography was performed using white matter FOD images to generate a whole-brain connectome for each subject. 19 major white matter tracts were segmented from the whole brain connectome of fibers using the Automated Fiber Quantification (AFQ, v1.1, github.com/yeatmanlab/AFQ/wiki) [46] software.
qT1 data was EPI and motion distortion corrected using FSL’S TOPUP, and an open-source python code (https://github.com/cni/t1fit/blob/master/t1fit_unwarp.py) was then used to process the NIFTI files and generate a quantitative T1 map for each participant. Each subject’s quantitative T1 map was then co-registered to their dMRI data using the ANTS software package to warp the quantitative T1 map to the non-diffusion weighted b0 image, and MTV values were estimated along each fascicle using the quantitative R1 (1/qT1) measurements validated in the previous study [35].
White matter hyperintensity volumes were generated by registering each participant’s T2-FLAIR images to their bias field corrected, and skull stripped T1-weighted image using the FSL toolbox (FLIRT function). WMH voxels were then defined based on the tissue probability maps where they had three SD above the mean white matter signal intensity in T2-FLAIR images.
Statistical analyses
CR-by-group interactions with MTV
We utilized multiple linear regression analysis to test for CR-by-group interaction effects (differences in the regression slopes) with MTV outcomes in aMCI and HC participants. Average MTV values for each and every of the following twenty tracts were introduced as dependent variables: left and right dorsal cingulum, parahippocampal cingulum, inferior fronto-occipital fasciculus (IFOF), superior longitudinal fasciculus (SLF), inferior longitudinal fasciculus (ILF), thalamic radiation, uncinate, arcuate and corticospinal tracts, as well as the callosum forceps major and callosum forceps minor tracts. Model covariates included age, gender, total intracranial volume (ICV), and the composite memory score. Educational attainment was not included as a covariate in the models due to its contribution as a factor in generating the CR composite score. The significance threshold for all regression models was set at alpha of 0.05 (FDR corrected).
Post-hoc within-group analyses
We also tested within-group associations of CR with MTV tract outcomes in aMCI and HC participants for tracts showing significant CR-by-group interactions (FDR corrected, pFDR = 0.019). MTV values for the following tracts were included in the post-hoc within-group linear regression models; left and right dorsal cingulum, right parahippocampal cingulum, callosum forceps major, right IFOF, right ILF, left and right SLF, and right thalamic radiation. Covariates included in within-group analyses were the same as those used in the primary analysis and included age, gender, ICV, and the overall composite memory score.
RESULTS
Descriptive statistics
Descriptive statistics and neuropsychological assessment scores are summarized in Table 1. The aMCI and HC groups did not differ significantly on measures of age, gender, level of education, occupational complexity scores, and CR composite scores. Expectedly, aMCI participants scored significantly lower on measures of verbal episodic memory function, obtaining lower scores on Logical Memory immediate (LM-I, p < 0.001) and delayed (LM-II, p < 0.001) recall compared to HC participants. aMCI participants also scored lower overall on the MMSE, measuring global cognition (p < 0.001).
Cognitive reserve and memory proxy scores
The resultant CR composite score from the dimensionality reduction of educational and occupational complexity variables accounted for 42% of the variance in the data across groups. Factor loadings for years of education and occupational complexity with people contributed the most to the CR composite (PC1) score. In all PCA analyses, occupational complexity with things loaded in the opposite direction as educational attainment and occupational complexity with people, consistent with results reported by Smart et al. [42], where individuals displaying higher occupational complexity with data or people generally displayed lower occupational complexity with things. The quantified composite memory scores were significantly lower in aMCI compared with HC (p = 0.002).
MRI outcome measures
Between-group differences in MRI outcomes have been described in our previous publications [26, 27]. ICV was not significantly different between groups. However, groups differed significantly on white matter hyperintensity volume (WMHV) (mL), where, unexpectedly, the HC group presented with greater WMHV (p = 0.017) compared to aMCI participants, and one aMCI participant was excluded because of excessive WMHV. Therefore, we ran all subsequent analyses both with and without including WMHV as a covariate, which yielded similar outcomes.
Group differences in associations between CR and MTV
The regression analysis indicated significant (FDR corrected) group differences in the association between CR and MTV in the left (T = 2.81, p = 0.007) and right (T = 3.65, p < 0.001) dorsal cingulum, right parahippocampal cingulum (T = 2.54, p = 0.014), callosum forceps major (T = 3.33, p = 0.002), right IFOF (T = 3.08, p = 0.003), right ILF (T = 2.41, p = 0.019), left (T = 2.93, p = 0.004) and right (T = 2.81, p = 0.007) SLF, and right (T = 3.72, p < 0.001) thalamic radiation (Fig. 1a). For all of these associations, the regression slopes were significantly lower in aMCI compared with HC (Fig. 2). We additionally ran these analyses with and without including WMHV as covariates, and these analyses yielded similar outcomes. We also ran all analyses substituting categorical education with continuous education (measured in years of education completed), and results remained essentially similar.
Fig. 1.
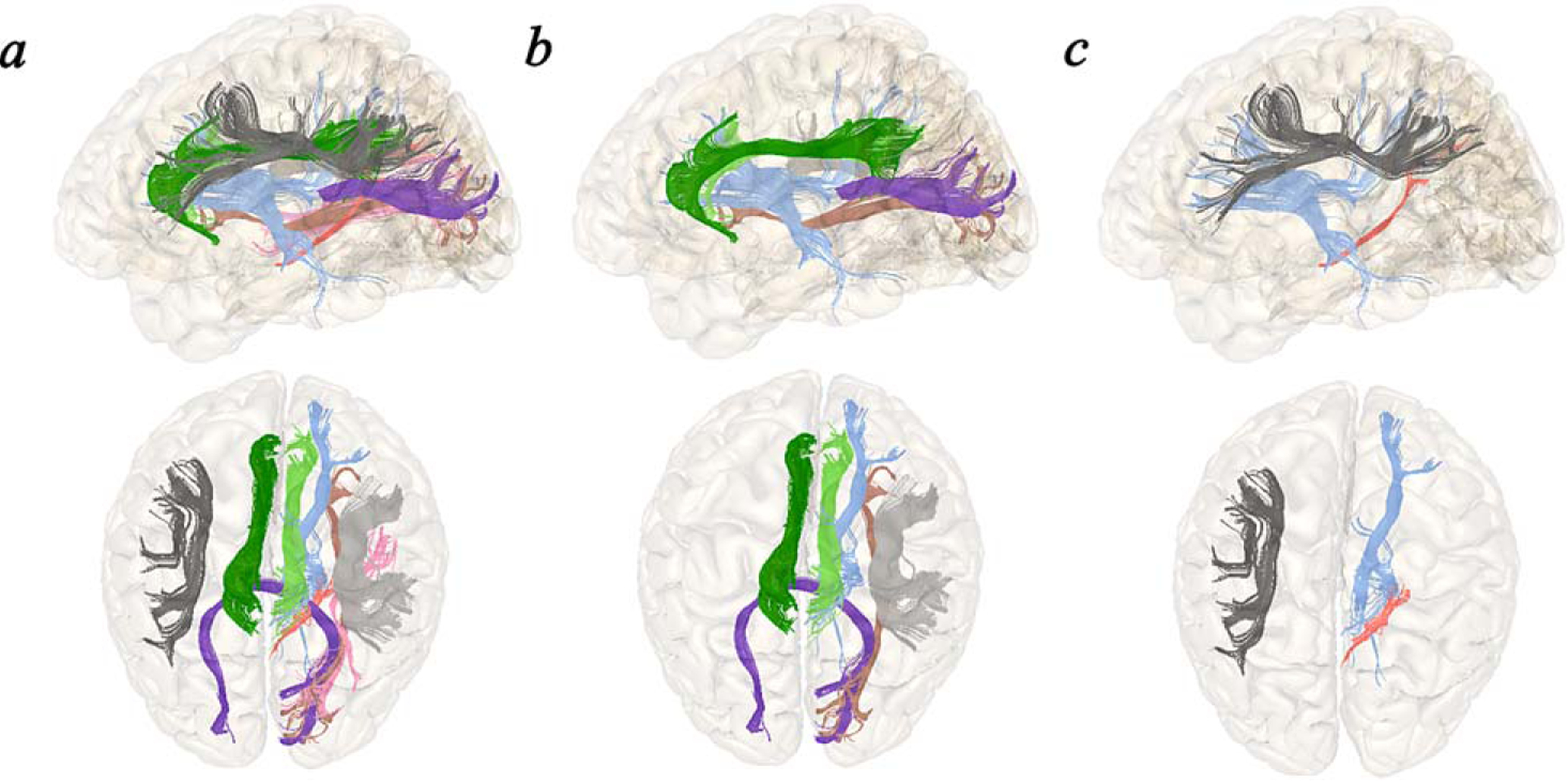
a) Tracts displaying significant (FDR corrected) between-group differences in the association between CR with MTV outcomes: left and right dorsal cingulum, right parahippocampal cingulum, callosum forceps major, right IFOF, right ILF, left and right SLF, and right thalamic radiation. b) Significant (uncorrected) within-group associations between CR and MTV in aMCI: left and right dorsal cingulum, callosum forceps major, right IFOF, right SLF, and right thalamic radiation. c) Significant (uncorrected) within-group associations between CR and MTV in HC: right parahippocampal cingulum, left SLF, and right thalamic radiation.
Fig. 2.
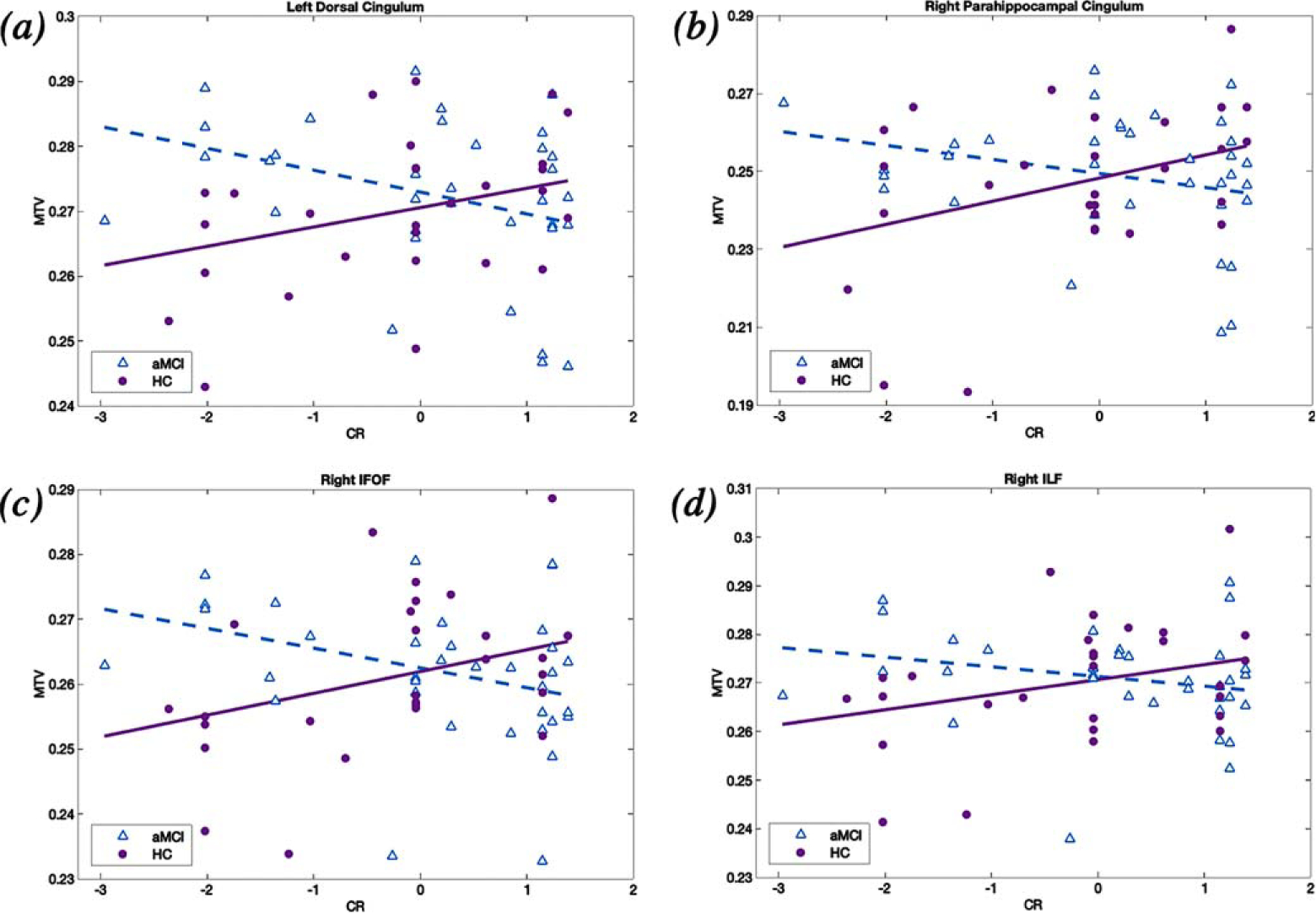
Significant group differences in the association between CR and MTV outcomes in the (a) left dorsal cingulum (b) right parahippocampal cingulum, (c) right IFOF, and (d) right ILF. MTV values were adjusted for age, gender, ICV, and memory ability. The relationship between CR and MTV was more negative in aMCI versus HC participants in all tracts showing significant CR-by-group interactions. HC, healthy elders; aMCI, amnestic mild cognitive impairment; MTV, macromolecular tissue volume; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus
Post-hoc within-group analyses in aMCI and HC
Post-hoc within-group regression analyses for the associations between CR and MTV values in the aMCI group revealed significant (FDR corrected) negative associations between CR and MTV values in the left (T = −2.73, p = 0.002) and right (T = −3.06, p = −0.005) dorsal cingulum, callosum forceps major (T = −3.4, p = 0.002), right IFOF (T = −2.37, p = 0.025) right SLF (T = −2.78, p = 0.009), and right (T = −3.35, p = 0.002) thalamic radiation when age, gender, ICV, and composite memory ability were included as covariates (Fig. 1b). Regression analyses within the HC group indicated that increasing CR was significantly associated with higher MTV values in the right parahippocampal cingulum (T = 2.15, p = 0.043), left SLF (T = 2.33, p = 0.029), and right thalamic radiation (T = 2.25, p = 0.035) (Fig. 1c).
DISCUSSION
In the current study, we investigated group differences in the association of CR with white matter tract MTV in aMCI versus HC participants, where MTV values were derived from qMRI measurements and reflected the level of myelination in white matter tracts [25, 27]. Results of the current study indicated significant (FDR corrected) group differences in the association between CR and white matter tract MTV in aMCI versus HC participants in a subset of major white matter tracts known to be affected in pre-clinical and prodromal AD. In all tracts displaying significant CR-by-group interactions with tract MTV outcomes, higher CR in aMCI participants was associated with lower MTV, indicating decreased tract myelination in aMCI patients with higher CR when age, gender, ICV, and overall memory ability were held constant. Results suggest that aMCI subjects with higher CR were therefore able to tolerate higher levels of white matter tract pathology given the same level of clinical severity, which is consistent with prior structural MRI evidence of CR in aMCI and AD patients which support a compensatory role for CR in these patient populations [19, 21, 47]. In HC participants, the opposite pattern was observed, and higher CR was associated with increased MTV, or higher white matter tract myelination, which is consistent with prior evidence that CR may promote neuroprotective or brain maintenance mechanisms among healthy participants which results in enhanced brain structure [17, 22–24].
Factors contributing to overall CR
We initially defined a composite CR proxy score via factor analysis of educational attainment, defined categorically, and occupational complexity variables, as outlined under the Dictionary of Occupational Titles (DOT). Of the education-occupational variables, complexity of work with people contributed most to the overall CR composite score across groups, consistent with prior evidence that involvement in complex interpersonal interactions contributes significantly to overall CR [48]. This is also consistent with findings by Boots et al. [18], where authors demonstrated that among the occupational complexity variables, complexity of work with people led to the greatest effect on brain outcomes in healthy middle-aged individuals at high risk for AD.
Early alterations to white matter tract microstructure in prodromal AD
Of the tracts displaying significant CR-by-group interactions, the dorsal cingulum, parahippocampal cingulum, callosum forceps major, and IFOF tracts have consistently been shown to display early white matter microstructural alterations in pre-clinical and prodromal AD and are also implicated in memory function [49]. The dorsal cingulum and parahippocampal cingulum tracts constitute the cingulum bundle, which is associated with episodic memory decline in AD [26]. Additionally, the cingulum bundle interconnects the posterior cingulate cortex with the entorhinal cortex and hippocampus, all of which are among the first brain regions targeted by AD pathology [49]. Further, the dorsal cingulum, callosum forceps major, and SLF tracts interconnect major hubs of the default mode network (DMN), a large-scale, distributed cognitive network that is disrupted across a variety of neurological conditions, including in AD [50]. The DMN is composed of a network of brain regions that deactivate during cognitive tasks and display increased activity during episodic memory tasks [51]. Resting-state functional connectivity of the DMN has also been shown to reflect structural connectivity within the network, assessed using diffusion tensor imaging [51], and decreased functional connectivity of the DMN in older adults has been shown to correlate with declines in white matter integrity (mean regional anisotropy) [33, 52]. Additionally, results from our previous study indicated that decreased MTV in white matter tracts, namely in tracts connecting integral DMN regions, was sensitive to macromolecular volume loss in aMCI and distinguished aMCI from HC participants [27]. The DMN has also previously been implicated in CR studies, where generally, higher CR was associated with increased DMN functional connectivity across resting-sate fMRI studies of CR [17].
Compensatory effect of CR in aMCI
Results in the aMCI group support compensatory models of reserve which posit that CR acts by increasing the brain’s ability to buffer against cognitive decline with increasing pathology, possibly by means of neural or cognitive compensation, and thus allowing individuals with higher levels of CR to sustain higher levels of brain pathology while maintaining a given level of cognitive functioning [14]. While the cross-sectional nature of the current study does not allow for direct evidence that compensation underlies the results observed in the aMCI group, previous CR research among the AD spectrum has supported compensatory CR models across neuroimaging modalities, where patients with higher CR have tolerated higher levels of brain pathology when matched for clinical severity. Matching patients for clinical severity allows for the comparison of brain pathology outcomes in a given sample assuming that all participants perform cognitively similar in regards to memory or cognitive functioning. This pattern of findings has been demonstrated across neuroimaging outcomes, including with measures of cortical volume and thickness, white matter diffusion measures, fMRI, FDG-PET, cerebral blood flow, and postmortem histopathology measures [7, 14, 17, 21, 53, 54]. For example, in a study by Boots et al. [18], higher occupational attainment was associated with decreased hippocampal volume in otherwise healthy middle-aged older adults at high risk for developing AD, as evidenced by APOE status. Higher educational attainment was also correlated with decreased whole brain and regional gray matter volumes in aMCI and AD patients [44, 55] and with increased cortical thinning in frontal and temporoparietal association cortices in AD [56]. Studies probing the relationship between CR and white matter microstructure in aMCI and AD patients have also observed similar outcomes. One study found that higher composite CR derived from education-occupational variables as well as from measures of participation in stimulating leisure and physical activities was associated with decreased white matter tract integrity in aMCI and AD patients, as evidenced by negative associations between CR and FA in the genum and callosal body, bilateral cingulum bundle, ILF, SLF, and IFOF [19]. Another recent study [21] also observed that greater educational attainment in AD patients was associated with lower FA in medial temporal and association tracts when patients were matched for cognitive ability. The ability of aMCI and AD individuals with higher CR to maintain similar cognition despite harboring higher levels of AD pathology may therefore be attributed to an increase in compensatory cognitive or neural mechanisms. The latter attribution is supported by fMRI studies showing that patients with higher CR displayed higher activations in task-related regions and greater task-induced de-activations of the DMN, indicating decreased task network efficiency which is paired with increased activation in other (compensatory) regions [17]. Despite these lines of evidence, it is noteworthy that results in the aMCI group may also reflect the fact that aMCI patients with lower MTV and higher CR were likely in a more advanced stage of disease progression while aMCI patients with higher MTV and lower CR may reflect an earlier disease stage.
Neuroprotective or brain maintenance effect of CR in HC
In contrast, results in the HC group indicated higher white matter tract MTV in response to increasing CR, supporting potential neuroprotective or brain maintenance models of reserve. While the current analysis does not allow us to determine if neuroprotective mechanisms underlie the positive associations observed between CR and tract MTV outcomes in HC, results in the HC group are consistent with prior neuroimaging evidence supporting neuroprotective effects of CR on brain structure among healthy participants from across the aging spectrum [23, 24, 47, 53]. Higher composite CR in healthy elders has been associated with increased whole brain [20] as well as regional [5, 53, 57] volumes. Higher CR in healthy participants has also been associated with preservation of white matter in inferior frontal regions [23], as well as with increased white matter integrity, as evidenced by increased FA, with increasing education in the medial temporal lobe and association fibers among healthy elders [21]. Higher CR has also been associated with increased whole brain FA (but not gray matter volumes) in response to higher occupational complexity in middle-aged, healthy adults [22], as well as with lower hippocampal mean diffusivity (MD) in response to higher educational attainment in healthy, middle-aged controls [24]. Haut et al. [58] also found that in healthy young adults, higher CR derived through factor analysis of years of education, Full Scale Intelligence Quotient (FSIQ), as well as reading scores was associated with increased FA in the right ILF tract, possibly indicating increased anterior-posterior connectivity in higher CR individuals. In a review of functional neuroimaging studies of CR, Anthony and Lin (2017) [17] concluded that among healthy participants, higher CR was associated with stronger functional connectivity of the ACC with other DMN regions, providing support for neural reserve. Additionally, in a group of healthy elders, higher CR was associated with increased gray matter volumes in the left supramarginal gyrus and left middle frontal gyrus, even after including voxel-by-voxel fMRI regional activations as covariates in the model, suggesting that CR affects neuronal structural integrity [53].
Previous literature exploring the effects of CR in healthy individuals, however, has produced mixed outcomes, where studies have observed no association, positive associations, or negative associations of CR with brain outcomes. A recent study [38] found no association between education and hippocampal MD or hippocampal volumes in a sample of healthy older adults. In white matter, Arenaza-Urquijo et al. [19] observed a significant negative correlation between CR and FA in the genu of the corpus callosum, where authors noted the possibility that some participants classified as healthy controls may have been harboring pre-clinical AD pathology, so it is possible that inconsistent outcomes across CR studies may be in part due to the heterogeneity of healthy control samples [20].
Contrasting effect of CR on brain outcomes in aMCI versus HC
Finally, contrasting effects of CR in HC and aMCI/AD participants similar to outcomes observed in the current study have also been reported in prior studies of CR that have utilized other imaging modalities. Teipel et al. [21] observed that higher education was associated with decreased white matter tract integrity (FA) in medial temporal and association fibers in AD patients but increased white matter integrity in similar tracts in healthy controls. Arenaza-Urquijo et al. [19] also reported evidence supporting brain maintenance mechanisms in healthy elders compared with an increased ability to tolerate pathological brain changes to white matter FA at similar memory performance in aMCI subjects. An fMRI study [20] demonstrated significant CR-by-group interactions in healthy elders and AD participants, where higher CR in healthy elders led to larger brain volumes and increased neural efficiency (fMRI task activation) and lower brain volumes and less efficient activation of task-related networks in aMCI and AD. Bosch et al. [33] also observed contrasting effects of composite CR on task-related fMRI signal during a speech comprehension task, where higher CR led to increased activity in task-related regions in addition to decreased DMN activity in aMCI and AD participants compared with decreased activity in task-related regions and increased DMN activity in healthy elders [17]. Furthermore, Ewers et al. [59] observed a significant interaction effect between level of education and amyloid-β (Aβ) burden in the posterior cingulate and angular gyrus when age, gender, and global cognition were included as covariates, with higher education (CR) associating with lower glucose metabolism in Aβ+ (considered to be pre-clinical AD) patients and vice versa in Aβ–(considered as healthy control) participants.
While initially the aspects of reserve affecting structural (i.e., neuroprotective or brain maintenance CR mechanisms) versus functional (i.e., compensation) components of CR were thought of as distinct conceptual frameworks, much recent literature has suggested that participation in enriching life experiences such as obtaining higher educational attainment or greater occupational complexity affects both the structure and function of the brain and therefore influences both neuroprotective/brain maintenance as well as compensatory aspects of reserve [55, 60]. Participation in enriching life experiences may influence both neural network efficiency as well as increase neuronal capacity, which is evidenced by outcomes of increased volumetric measures or increased measures of white matter integrity with increasing CR in healthy populations [53]. For example, the act of learning has been speculated to influence white matter tracts by influencing the diameter of axons, myelin thickness, the number of myelinated axons contained within white matter tracts, in addition to other features of white matter microstructure [61]. Complementary research in animals, as well as cross-sectional studies and longitudinal cognitive training studies in humans have supported the idea that participating in enriching life experiences has the potential to induce lasting structural brain morphological changes that in turn increase the efficiency, capacity, or flexibility of brain networks underlying cognition [7, 62]. Engaging in cognitively stimulating activities also promotes BDNF production, which is a known promotor of neurogenesis and has been previously associated with neuronal plasticity [8] as well as with the preservation of white matter integrity [63, 64]. Additionally, rats placed in enriched environments have displayed increases in myelination of neuronal white matter (measured using electron microscopy) in the splenium of the corpus callosum compared to rats reared in impoverished conditions [65, 66]. Finally, numerous cognitive training studies have demonstrated significant alterations to white matter microstructure (i.e., increasing FA) in response to completing cognitive training programs [67], suggesting that experienced-induced neuronal plasticity is reflected by measurable changes in neuronal white matter [68]. Together, these studies suggest that engagement in enriching life experiences may increase the structural integrity of the brain, which may also lead to an increased ability to resist developing AD pathology and result in greater cognitive efficiency with increasing CR [69]. Under the condition of developing AD pathology, such as in pre-clinical and prodromal stages of AD, the protective effects of CR on brain structure may become attenuated due to increasing pathological load [70]. Higher CR may then help in resisting cognitive decline in the face of AD pathology, as evidenced by reduced neural efficiency and increased neural compensation in functional neuroimaging studies or by worse structural pathology outcomes in aMCI and AD patients matched for cognitive ability. One theory is that the mechanistic role for CR changes as AD progresses, conferring neuroprotection and resistance to developing pathology (brain maintenance) in early disease stages until reaching a critical point where developing pathology surpasses the neuroprotective functions of CR. The mechanistic role for CR may then switch to one that is compensatory as AD pathology continues to develop [55, 71]. However, further longitudinal studies of CR in healthy and AD populations are warranted in order to both probe the neural underpinnings of CR mechanisms as well as to determine if the neural implementations of CR change as AD pathology progresses.
One limitation of the current study is that the analyses were performed on cross-sectional data, and we were therefore unable to determine the underlying causal mechanistic influences of CR on white matter tract myelination outcomes. Another limitation is that we did not acquire PET measures as part of the current study and were therefore unable to confirm amyloid or tau pathology burden within our sample. Additionally, other factors that may contribute to CR were not included in our model. Contributions of lifestyle factors to CR are likely to be quite complex, with many variables potentially influencing CR through differential mechanisms that affect both active and passive CR components. Consequently, an additional limitation of our study is that models in the current analysis did not adjust for other important AD-risk factors also known to affect brain structure and health with aging such as measures of diet, vascular health, sleep, exercise, early-life exposure to adverse or traumatic experiences, engagement in stimulating late life leisure activities, or personality measures such as resilience or self-efficacy measures [72]. Additionally, other environmental factors influencing tract measures were not included as covariates in the regression models. For example, we did not include socioeconomic status as a covariate in our regression models; however, prior research has shown that associations between education and AD risk persist, even when controlling for socioeconomic factors [10]. The current study also did not consider APOE genotype since this measure was not collected as part of the study, and possessing the APOE ε4 gene variant confers higher AD risk and additionally has important implications on cholesterol and lipid transport in the brain and has been hypothesized to affect the growth and regeneration of neurons [49]. Epigenetic factors have also been shown to influence white matter integrity [61]. A fully comprehensive CR model should therefore integrate all factors impacting reserve in order to accurately predict outcomes of CR on both brain imaging as well as on cognitive outcomes. Another challenge is in comparing brain outcomes with other CR studies, since definitions of CR are inconsistent across studies. Current education-occupation derived CR proxies such as the CR proxy utilized in the current study are additionally limited to broad definitions of educational and occupational attainment. These general educational and occupational classifications may result in variable modifications on CR in different people. With regard to occupational complexity, individuals holding the same occupational title may have variable work responsibilities as well as different workloads. Additionally, the amount of time spent in a given occupation may impact occupational complexity contributions to CR.
Overall, the results of the current study provide unique support for the effect of CR on white matter tract macromolecular content in aMCI compared to HC subjects. In aMCI subjects, positive associations of CR with white matter tract MTV outcomes indicated that higher CR individuals could sustain worse tract pathology while controlling for memory differences, suggesting that these individuals may revert to compensatory neural or cognitive means of maintaining cognition when AD white matter tract pathology is present. In contrast, results in HC indicated that higher CR was associated with increased MTV outcomes (increased myelination), suggesting that CR may even confer some level of neuroprotection through experienced-induced tract myelination and aide in resisting pathological changes with aging. Further research delineating more sensitive and robust definitions for CR and its constituents as well as further elucidation of which neural mechanisms underlie CR in both healthy elders as well as AD participants will supplement our understanding of how experience modulates brain structure and function in both pathological and normal aging. Evidence suggesting CR may delay behavioral symptom presentations of AD or prevent its onset exhort further investigations of CR mechanisms, as even delaying the onset of AD would greatly reduce its prevalence in the population [73]. Additionally, further research on the neural underpinnings of CR will eventually reveal how we can harness this knowledge in order to develop novel, experience-based cognitive therapies for preventing or delaying AD onset and aide our understanding how these modifiable life experiences influence cognitive outcomes in healthy and pathological aging.
ACKNOWLEDGMENTS
We thank the participants for their involvement in the study as well as the researchers involved in coordinating data collection for this project. The study was partly funded by a Career Development Award from the National Institute on Aging (NIA) to SMH (K25AG050759). EG’s effort was partly supported by Stanford Maternal and Child Health Research Institute (MCHRI). SMH’s effort was supported in part by NIA (K25AG050759, R21AG064263 and R21AG073973) and National Institute of Mental Health (NIMH) (R61MH119289 and R21MH123873).
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0197r1).
REFERENCES
- [1].DeTure MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A (1988) Clinical, pathological, and neurochemical changes in dementia: A subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol 23, 138–144. [DOI] [PubMed] [Google Scholar]
- [3].Steffener J, Stern Y (2012) Exploring the neural basis of cognitive reserve in aging. Biochim Biophys Acta 1822, 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mortimer JA (1997) Brain reserve and the clinical expression of Alzheimer’s disease. Geriatrics 52, S50–S53. [PubMed] [Google Scholar]
- [5].Valenzuela MJ, Sachdev P (2006) Brain reserve and dementia: A systematic review. Psychol Med 36, 441–454. [DOI] [PubMed] [Google Scholar]
- [6].Habes M, Grothe MJ, Tunc B, McMillan C, Wolk DA, Davatzikos C (2020) Disentangling heterogeneity in Alzheimer’s disease and related dementias using data-driven methods. Biol Psychiatry 88, 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stern Y (2012) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11, 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stern Y, Barnes CA, Grady C, Jones RN, Raz N (2019) Brain reserve, cognitive reserve, compensation, and maintenance: Operationalization, validity, and mechanisms of cognitive resilience. Neurobiol Aging 83, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bruandet A, Richard F, Bombois S, Maurage C, Masse I, Amouyel P, Pasquier F (2008) Cognitive decline and survival in Alzheimer’s disease according to education level. Dement Geriatr Cogn Disord 25, 74–80. [DOI] [PubMed] [Google Scholar]
- [10].Garibotto V, Borroni B, Kalbe E, Herholz K, Salmon E, Holtoff V, Sorbi S, Cappa SF, Padovani A, Fazio F (2008) Education and occupation as proxies for reserve in aMCI converters and AD: FDG-PET evidence. Neurology 71, 1342–1349. [DOI] [PubMed] [Google Scholar]
- [11].Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R (1994) Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 271, 1004–1010. [PubMed] [Google Scholar]
- [12].Whalley LJ, Deary IJ, Appleton CL, Starr JM (2004) Cognitive reserve and the neurobiology of cognitive aging. Ageing Res Rev 3, 369–382. [DOI] [PubMed] [Google Scholar]
- [13].Zhang M, Katzman R, Salmon D, Jin H, Cai G, Wang Z, Qu G, Grant I, Yu E, Levy P (1990) The prevalence of dementia and Alzheimer’s disease in Shanghai, China: Impact of age, gender, and education. Ann Neurol 27, 428–437. [DOI] [PubMed] [Google Scholar]
- [14].Stern Y (2009) Cognitive reserve. Neuropsychologia 47, 2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zahodne LB, Mayeda ER, Hohman TJ, Fletcher E, Racine AM, Gavett B, Manly JJ, Schupf N, Mayeux R, Brickman AM (2019) The role of education in a vascular pathway to episodic memory: Brain maintenance or cognitive reserve? Neurobiol Aging 84, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Staff RT, Murray AD, Deary IJ, Whalley LJ (2004) What provides cerebral reserve? Brain 127, 1191–1199. [DOI] [PubMed] [Google Scholar]
- [17].Anthony M, Lin F (2018) A systematic review for functional neuroimaging studies of cognitive reserve across the cognitive aging spectrum. Arch Clin Neuropsychol 33, 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Boots EA, Schultz SA, Almeida RP, Oh JM, Koscik RL, Dowling MN, Gallagher CL, Carlsson CM, Rowley HA, Bendlin BB (2015) Occupational complexity and cognitive reserve in a middle-aged cohort at risk for Alzheimer’s disease. Arch Clin Neuropsychol 30, 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Arenaza-Urquijo EM, Bosch B, Sala-Llonch R, Solé-Padullés C, Junqué C, Fernandez-Espejo D, Bargallo N, Rami L, Molinuevo JL, Bartrés-Faz D (2011) Specific anatomic associations between white matter integrity and cognitive reserve in normal and cognitively impaired elders. Am J Geriatr Psychiatry 19, 33–42. [DOI] [PubMed] [Google Scholar]
- [20].Solé-Padullés C, Bartrés-Faz D, Junqué C, Vendrell P, Rami L, Clemente IC, Bosch B, Villar A, Bargalló N, Jurado MA (2009) Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 30, 1114–1124. [DOI] [PubMed] [Google Scholar]
- [21].Teipel SJ, Meindl T, Wagner M, Kohl T, Bürger K, Reiser MF, Herpertz S, Möller HJ, Hampel H (2009) White matter microstructure in relation to education in aging and Alzheimer’s disease. J Alzheimers Dis 17, 571–583. [DOI] [PubMed] [Google Scholar]
- [22].Kaup AR, Xia F, Launer LJ, Sidney S, Nasrallah I, Erus G, Allen N, Yaffe K (2018) Occupational cognitive complexity in earlier adulthood is associated with brain structure and cognitive health in midlife: The CARDIA study. Neuropsychology 32, 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gordon BA, Rykhlevskaia EI, Brumback CR, Lee Y, Elavsky S, Konopack JF, McAuley E, Kramer AF, Colcombe S, Gratton G (2008) Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology 45, 825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Piras F, Cherubini A, Caltagirone C, Spalletta G (2011) Education mediates microstructural changes in bilateral hippocampus. Hum Brain Mapp 32, 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yeatman JD, Wandell BA, Mezer AA (2014) Lifespan maturation and degeneration of human brain white matter. Nat Commun 5, 4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gozdas E, Fingerhut H, Chromik LC, O’Hara R, Reiss AL, Hosseini S (2020) Focal white matter disruptions along the cingulum tract explain cognitive decline in amnestic mild cognitive impairment (aMCI). Sci Rep 10, 10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gozdas E, Fingerhut H, Wu H, Bruno JL, Dacorro L, Jo B, O’Hara R, Reiss AL, Hosseini SH (2021) Quantitative measurement of macromolecular tissue properties in white and gray matter in healthy aging and amnestic MCI. Neuroimage 237, 118161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Delano-Wood L, Stricker NH, Sorg SF, Nation DA, Jak AJ, Woods SP, Libon DJ, Delis DC, Frank LR, Bondi MW (2012) Posterior cingulum white matter disruption and its associations with verbal memory and stroke risk in mild cognitive impairment. J Alzheimers Dis 29, 589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kim KW, MacFall JR, Payne ME (2008) Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry 64, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Prins ND, Scheltens P (2015) White matter hyperintensities, cognitive impairment and dementia: An update. Nat Rev Neurol 11, 157–165. [DOI] [PubMed] [Google Scholar]
- [31].Wardlaw JM, Valdés Hernández MC, Muñoz-Maniega S (2015) What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc 4, e001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brickman AM, Zahodne LB, Guzman VA, Narkhede A, Meier IB, Griffith EY, Provenzano FA, Schupf N, Manly JJ, Stern Y (2015) Reconsidering harbingers of dementia: Progression of parietal lobe white matter hyperintensities predicts Alzheimer’s disease incidence. Neurobiol Aging 36, 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bosch B, Bartrés-Faz D, Rami L, Arenaza-Urquijo EM, Fernández-Espejo D, Junqué C, Solé-Padullés C, Sanchez-Valle R, Bargallo N, Falcon C (2010) Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer’s disease. Cortex 46, 451–461. [DOI] [PubMed] [Google Scholar]
- [34].Tang X, Cai F, Ding DX, Zhang LL, Cai XY, Fang Q (2018) Magnetic resonance imaging relaxation time in Alzheimer’s disease. Brain Res Bull 140, 176–189. [DOI] [PubMed] [Google Scholar]
- [35].Mezer A, Yeatman JD, Stikov N, Kay KN, Cho NJ, Dougherty RF, Perry ML, Parvizi J, Hua LH, Butts-Pauly K (2013) Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nat Med 19, 1667–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Filo S, Shtangel O, Salamon N, Kol A, Weisinger B, Shifman S, Mezer AA (2019) Disentangling molecular alterations from water-content changes in the aging human brain using quantitative MRI. Nat Commun 10, 3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Deoni SC (2010) Quantitative relaxometry of the brain. Top Magn Reson Imaging 21, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kalzendorf J, Brueggen K, Teipel S (2020) Cognitive reserve is not associated with hippocampal microstructure in older adults without dementia. Front Aging Neurosci 11, 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59, 22–33. [PubMed] [Google Scholar]
- [40].Montross LP, Depp C, Daly J, Reichstadt J, Golshan S, Moore D, Sitzer D, Jeste DV (2006) Correlates of self-rated successful aging among community-dwelling older adults. Am J Geriatr Psychiatry 14, 43–51. [DOI] [PubMed] [Google Scholar]
- [41].United States Employment Service (1991) Dictionary of occupational titles (4th ed.). U.S. Government Printing Office, Washington, DC. [Google Scholar]
- [42].Smart EL, Gow AJ, Deary IJ (2014) Occupational complexity and lifetime cognitive abilities. Neurology 83, 2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jonaitis E, La Rue A, Mueller KD, Koscik RL, Hermann B, Sager MA (2013) Cognitive activities and cognitive performance in middle-aged adults at risk for Alzheimer’s disease. Psychol Aging 28, 1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Roos PA, Treiman DJ (1980) Worker functions and worker traits for the 1970 US census classification. In Work, Jobs, and Occupations: A Critical Review of the Dictionary of Occupational Titles, Miller AR, Treiman DJ, Cain PS, Roos PA, eds. National Academy Press, Washington, DC, pp. 336–389. [Google Scholar]
- [45].Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, Flynn J, Sackeim H, Van Heertum R (2005) Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex 15, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM (2012) Tract profiles of white matter properties: Automating fiber-tract quantification. PloS One 7, e49790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Arenaza-Urquijo EM, Landeau B, La Joie R, Mevel K, Mézenge F, Perrotin A, Desgranges B, Bartrés-Faz D, Eustache F, Chételat G (2013) Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage 83, 450–457. [DOI] [PubMed] [Google Scholar]
- [48].Fratiglioni L, Paillard-Borg S, Winblad B (2004) An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol 3, 343–353. [DOI] [PubMed] [Google Scholar]
- [49].Gold BT, Johnson NF, Powell DK, Smith CD (2012) White matter integrity and vulnerability to Alzheimer’s disease: Preliminary findings and future directions. Biochim Biophys Acta 1822, 416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Carmichael O, McLaren DG, Tommet D, Mungas D, Jones RN; Alzheimer’s Disease Neuroimaging Initiative (2013) Coevolution of brain structures in amnestic mild cognitive impairment. Neuroimage 66, 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Greicius MD, Supekar K, Menon V, Dougherty RF (2009) Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL (2007) Disruption of large-scale brain systems in advanced aging. Neuron 56, 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bartrés-Faz D, Solé-Padullés C, Junqué C, Rami L, Bosch B, Bargalló N, Falcón C, Sánchez-Valle R, Molinuevo JL (2009) Interactions of cognitive reserve with regional brain anatomy and brain function during a working memory task in healthy elders. Biol Psychol 80, 256–259. [DOI] [PubMed] [Google Scholar]
- [54].Bennett DA, Wilson R, Schneider J, Evans D, De Leon CM, Arnold S, Barnes L, Bienias J (2003) Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 60, 1909–1915. [DOI] [PubMed] [Google Scholar]
- [55].Menardi A, Pascual-Leone A, Fried PJ, Santarnecchi E (2018) The role of cognitive reserve in Alzheimer’s disease and aging: A multi-modal imaging review. J Alzheimers Dis 66, 1341–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Seo SW, Im K, Lee JM, Kim ST, Ahn HJ, Go SM, Kim SH, Na DL (2011) Effects of demographic factors on cortical thickness in Alzheimer’s disease. Neurobiol Aging 32, 200–209. [DOI] [PubMed] [Google Scholar]
- [57].Wook Yoo S, Han CE, Shin JS, Won Seo S, Na DL, Kaiser M, Jeong Y, Seong JK (2015) A network flow-based analysis of cognitive reserve in normal ageing and Alzheimer’s disease. Sci Rep 5, 10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Haut MW, Moran MT, Lancaster MA, Kuwabara H, Parsons MW, Puce A (2007) White matter correlates of cognitive capacity studied with diffusion tensor imaging: Implications for cognitive reserve. Brain Imaging Behav 1, 83–92. [Google Scholar]
- [59].Ewers M, Insel PS, Stern Y, Weiner MW; Alzheimer’s Disease Neuroimaging Initiative (ADNI) (2013) Cognitive reserve associated with FDG-PET in preclinical Alzheimer disease. Neurology 80, 1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Barulli D, Stern Y (2013) Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn Sci 17, 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zatorre RJ, Fields RD, Johansen-Berg H (2012) Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat Neurosci 15, 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Draganski B, May A (2008) Training-induced structural changes in the adult human brain. Behav Brain Res 192, 137–142. [DOI] [PubMed] [Google Scholar]
- [63].Maillard P, Satizabal C, Beiser A, Himali J, Chen T, Vasan R, Seshadri S, DeCarli C (2016) Effects of brain derived neurotrophic factor on white matter integrity in middle-aged adults: A voxel-based diffusion tensor imaging study. Neurology 86, S39.006. [Google Scholar]
- [64].Anatürk M, Demnitz N, Ebmeier K, Sexton C (2018) A systematic review and meta-analysis of structural magnetic resonance imaging studies investigating cognitive and social activity levels in older adults. Neurosci Biobehav Rev 93, 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Juraska JM, Kopcik JR (1988) Sex and environmental influences on the size and ultrastructure of the rat corpus callosum. Brain Res 450, 1–8. [DOI] [PubMed] [Google Scholar]
- [66].Markham JA, Greenough WT (2004) Experience-driven brain plasticity: Beyond the synapse. Neuron Glia Biol 1, 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chapman SB, Aslan S, Spence JS, Hart JJ Jr, Bartz EK, Didehbani N, Keebler MW, Gardner CM, Strain JF, DeFina LF (2015) Neural mechanisms of brain plasticity with complex cognitive training in healthy seniors. Cereb Cortex 25, 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Long P, Corfas G (2014) Neuroscience. To learn is to myeli-nate. Science 346, 298–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Arfanakis K, Wilson RS, Barth CM, Capuano AW, Vasireddi A, Zhang S, Fleischman DA, Bennett DA (2016) Cognitive activity, cognitive function, and brain diffusion characteristics in old age. Brain Imaging Behav 10, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Soldan A, Pettigrew C, Albert M (2020) Cognitive reserve from the perspective of preclinical Alzheimer disease: 2020 update. Clin Geriatr Med 36, 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Arenaza-Urquijo EM, Wirth M, Chetélat G (2015) Cognitive reserve and lifestyle: Moving towards preclinical Alzheimer’s disease. Front Aging Neurosci 7, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bartrés-Faz D, Arenaza-Urquijo EM (2011) Structural and functional imaging correlates of cognitive and brain reserve hypotheses in healthy and pathological aging. Brain Topogr 24, 340–357. [DOI] [PubMed] [Google Scholar]
- [73].Valenzuela M, Brayne C, Sachdev P, Wilcock G, Matthews F; Medical Research Council Cognitive Function and Ageing Study (2011) Cognitive lifestyle and long-term risk of dementia and survival after diagnosis in a multicenter population-based cohort. Am J Epidemiol 173, 1004–1012. [DOI] [PubMed] [Google Scholar]


