Abstract
We have recently demonstrated in a double-blind randomized trial the beneficial effects of L-Arginine in patients hospitalized for COVID-19. We hypothesize that one of the mechanisms underlying the favorable effects of L-Arginine is its action on inflammatory cytokines. To verify our hypothesis, we measured longitudinal plasma levels of pro-inflammatory and anti-inflammatory cytokines implied in the pathophysiology of COVID-19 in patients randomized to receive oral L-Arginine or placebo. The study was successfully completed by 169 patients. Patients in the L-Arginine arm had a reduced respiratory support evaluated at 10 and 20 days; moreover, the time to hospital discharge was significantly shorter in the L-Arginine group. The assessment of circulating cytokines revealed that L-Arginine significantly reduced the circulating levels of pro-inflammatory IL-2, IL-6, and IFN-γ and increased the levels of the anti-inflammatory IL-10. Taken together, these findings indicate that adding L-Arginine to standard therapy in COVID-19 patients markedly reduces the need of respiratory support and the duration of in-hospital stay; moreover, L-Arginine significantly regulates circulating levels of pro-inflammatory and anti-inflammatory cytokines.
Keywords: Arginine, COVID-19, Critical Care, Cytokine storm, Inflammation, IL-6, IL-2, IFN-γ, IL-10, Lymphocytes
Graphical Abstract
1. Introduction
We and others have demonstrated that endothelial dysfunction contributes to coronavirus disease (COVID-19)-associated acute respiratory distress syndrome [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15].
The amino acid L-Arginine has been previously shown to improve endothelial function [16], [17], [18], [19], [20]. Specifically, in a double-blind randomized, placebo-controlled trial, we have recently established the beneficial effects of adding oral L-Arginine to hospital standard-therapy in patients hospitalized for COVID-19 ( NCT04637906 ) [21]; however, the exact mechanisms have not been explored.
Based on reports showing anti-inflammatory effects of L-Arginine in vitro [22], [23] and in preclinical models [24], we hypothesized that one of the mechanisms underlying the favorable effects of L-Arginine, alongside the known improvement of endothelial dysfunction [25], could be its action on the circulating levels of inflammatory cytokines.
2. Methods
2.1. Study design and procedures
Details on the study design, inclusion and exclusion criteria, procedures, outcomes to be assessed, and statistical analyses have been reported in the published protocol [21].
2.2. Ethical approval
The Study was approved by the institutional Ethical Committee (A.O.R.N. “Ospedali dei Colli”, Naples, Italy); written informed consent was collected from all patients, or their legal representative if they were unable to provide consent. The trial was performed in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization-Good Clinical Practice guidelines.
2.3. Cytokine measurements
We assessed longitudinal plasma levels of IFN-γ, IL-2, IL-6, and IL-10, which have been implied in the pathophysiology of COVID-19 [26], [27], [28], [29], [30]. Circulating levels of cytokines were measured on hospital admission and after 2 weeks, through ELISA, as previously described [29]. Details on the study design, inclusion and exclusion criteria, procedures, outcomes to be assessed, and statistical analyses have been reported in the published trial [21].
2.4. Statistical analyses
Data were analyzed by SPSS (version 26.0; SPSS, IBM, Armonk, USA) and by the open software jamovi (version 2.3.16.0) and expressed as mean±SD or numbers and percentage, as appropriate. Differences in cytokine expression were assessed via ANOVA followed by Tukey post hoc correction. The null hypothesis was rejected at a two-tailed p < 0.05.
3. Results
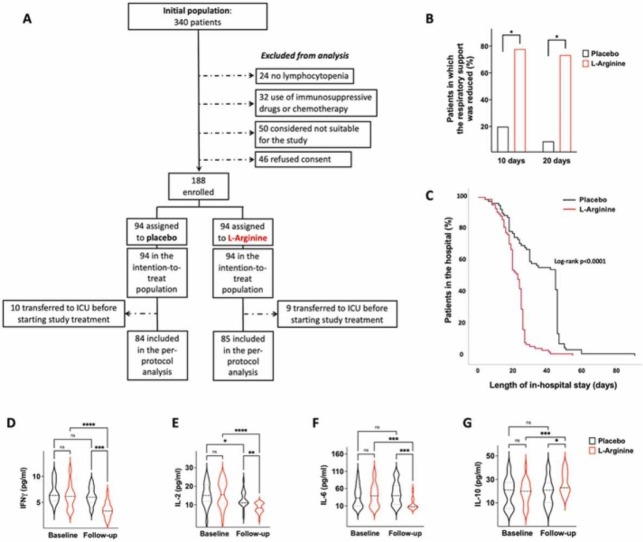
A total of 169 patients successfully completed the study (please see the flowchart shown in Fig. 1): 84 randomized to receive placebo, 85 to L-Arginine. A 100 % protocol adherence was achieved. All patients were randomized and received the assigned treatments with a mean of 7.8 days after symptoms onset. The main characteristics of the patients are shown in Table 1.
Fig. 1.
Flowchart of the study.
Table 1.
Main characteristics of the two study groups. Data are presented as means ± SD for continuous variables; percentages are reported for categorical variables. ALT: Alanine aminotransferase; AST: aspartate aminotransferase; BUN: blood urea nitrogen; CPAP: continuous positive airway pressure; CRP: C Reactive Protein; HFNC: high-flow nasal cannula; LMWH: low molecular weight heparin; LTOT: long-term oxygen therapy; NIV: non-invasive ventilation.
| Placebo (n = 84) |
L-Arginine (n = 85) |
p | |
|---|---|---|---|
| Gender (M/F, %) | 75.3/34.7 | 64.7/35.3 | 0.137 |
| Age (years) | 62.0 ± 11.9 | 61.1 ± 13.2 | 0.634 |
| Hypertension (%) | 46.9 | 37.6 | 0.227 |
| Coronary artery disease (%) | 14.8 | 17.6 | 0.621 |
| Smokers (%) | 13.6 | 11.8 | 0.725 |
| Obesity (% | 9.9 | 10.6 | 0.880 |
| Diabetes (%) | 11.1 | 11.8 | 0.895 |
| Time between onset of symptoms and admission (days) | 7.9 ± 3.1 | 7.9 ± 3.3 | 0.982 |
| White blood cells (n/mL) | 8521.4 ± 5103.6 | 7709.4 ± 4222.2 | 0.265 |
| Lymphocytes (n/mL) | 796.8 ± 362.4 | 782.1 ± 329.4 | 0.788 |
| CRP (mg/L) | 8.0 ± 6.7 | 11.1 ± 10.4 | 0.029 |
| D-dimer (ng/mL) | 945.5 ± 1660.6 | 709.7 ± 1157.2 | 0.295 |
| ALT (U/L | 62.9 ± 82.7 | 53.2 ± 44.5 | 0.351 |
| AST (U/L) | 46.3 ± 40.1 | 40.8 ± 29.0 | 0.312 |
| Creatinine (mg/dL) | 0.89 ± 0.7 | 0.78 ± 0.2 | 0.100 |
| Sodium (mmol/L) | 138.5 ± 4.1 | 138.5 ± 4.6 | 0.946 |
| Potassium (mmol/L) | 4.44 ± 0.7 | 4.40 ± 0.5 | 0.690 |
| Asthenia (%) | 63.0 | 63.5 | 0.940 |
| Dyspnea (%) | 91.4 | 89.4 | 0.671 |
| Cough (%) | 37.0 | 32.9 | 0.580 |
| Fever (%) | 67.9 | 75.3 | 0.291 |
| Sputum (%) | 8.6 | 2.4 | 0.074 |
| Remdesivir (%) | 39.5 | 44.7 | 0.498 |
| LMWH (%) | 95.1 | 92.9 | 0.566 |
| Steroids (%) | 86.4 | 92.9 | 0.166 |
| Monoclonal antibodies (%) | 15.4 | 10.0 | 0.472 |
| Anti-COVID vaccine (%) | 45.7 | 47.1 | 0.859 |
| P/F (PaO2/FiO2- baseline) | 132.0 ± 57.5 | 148.6 ± 66.1 | 0.086 |
| P/F (PaO2/FiO2- day 10) | 190.5 ± 116.3 | 216.8 ± 96.0 | 0.281 |
| P/F (PaO2/FiO2- day 20) | 255.5 ± 103.4 | 258.8 ± 89.0 | 0.857 |
| Hospitalization (days) | 35.9 ± 15.4 | 21.9 ± 7.7 | 0.0001 |
| Death | 13 (13.8%) | 3 (3.4%) | 0.013 |
| Respiratory support (baseline) | |||
| None (%) | 1.2 | 0 | 0.500 |
| LTOT (%) | 3.7 | 9.4 | |
| HFNC (%) | 77.8 | 71.8 | |
| CPAP (%) | 11.1 | 11.8 | |
| NIV (%) | 6.2 | 7.1 | |
| Respiratory support (day 10) | |||
| None (%) | 7.4 | 49.4 | < 0.0001 |
| LTOT (%) | 14.8 | 28.2 | |
| HFNC (%) | 67.9 | 20.0 | |
| CPAP (%) | 1.2 | 1.2 | |
| NIV (%) | 8.6 | 1.2 | |
| Respiratory support (day 20) | |||
| None (%) | 22.2 | 81.0 | < 0.0001 |
| LTOT (%) | 9.9 | 9.5 | |
| HFNC (%) | 65.4 | 9.5 | |
| CPAP (%) | 2.5 | 0 | |
| NIV (%) | 0 | 0 | |
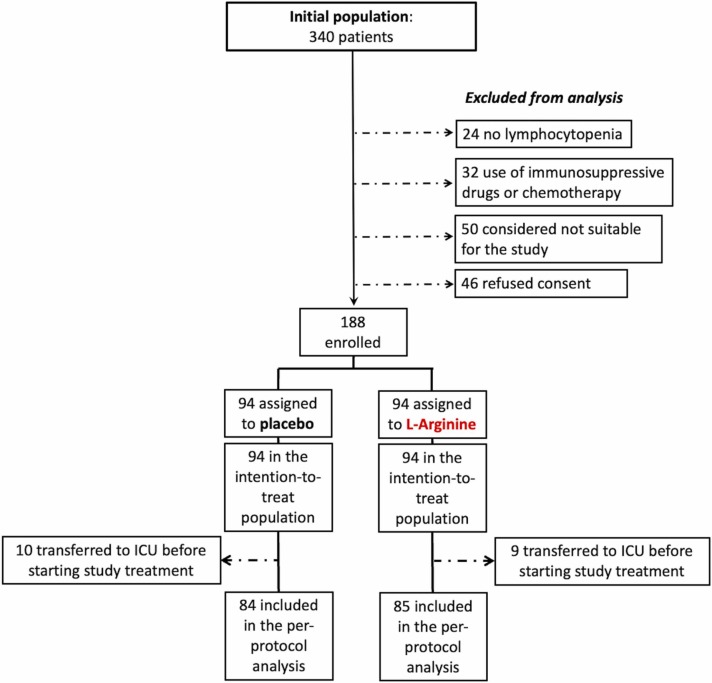
At 10-day evaluation, 31 % in the placebo group and 82 % in the L-Arginine group had reduced the respiratory support ( Fig. 2 A). A multivariable logistic regression analysis was performed in order to examine the magnitude of association between the treatment with L-Arginine and the primary outcome: when adjusting for potential confounders including age, gender, C-reactive protein, P/F ratio, and use of monoclonal antibodies, the odds of having a reduction in respiratory support were 6.27-fold higher in those who were in the L-Arginine group compared to those in the placebo group (OR:6.27; 95 %CI: 2.08–18.87; p = 0.001). Furthermore, 20 days after randomization we detected a significant difference in the primary outcome between the two study arms (Placebo group: 5 out of 56, 9 %; L-Arginine group: 11 out of 15, 73 %; p < 0.0001; Fig. 1). On the contrary, changes in P/F ratio at 10 and 20 days were not significantly different (Table 1).
Fig. 2.
Percentage of patients in which the respiratory support was reduced 10 days and 20 days after starting the therapy (A). Length of hospitalization examined in the L-Arginine and in the Placebo groups (B).
Among the secondary outcome measures, differences between active treatment group vs placebo group were significantly different only for the time to hospital discharge, which was significantly shorter in the L-Arginine arm (22 ± 8 vs 36 ± 15 days, p < 0.0001; Fig. 2 B) and was confirmed in a Cox regression analysis using a fully adjusted multivariable model including age, gender, and C-reactive protein (HR:4.06; 95 %CI: 2.62–6.32; p < 0.0001). The rate of lymphocyte number normalization and the time to obtain a negative RT-qPCR for SARS-CoV-2 on nasopharyngeal swab were similar between the 2 groups.
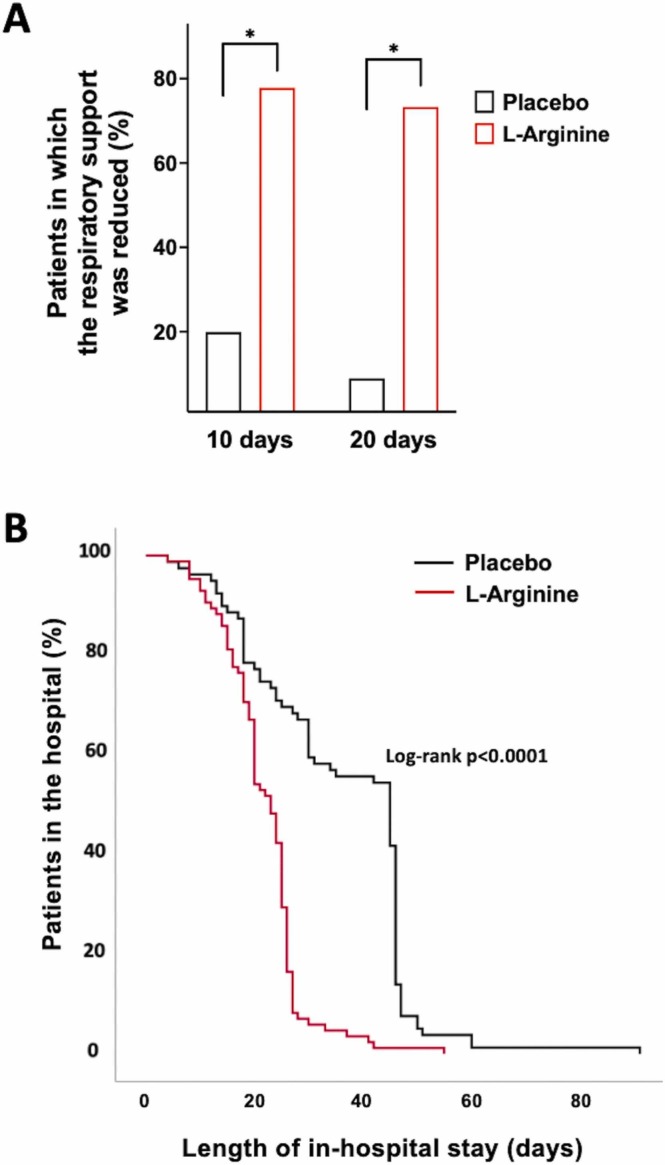
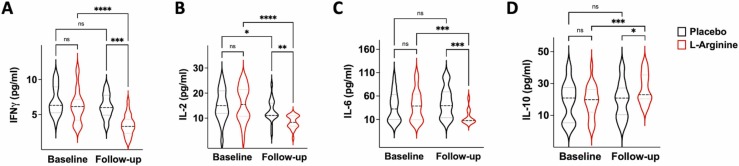
Strikingly, the evaluation of circulating cytokines revealed that L-Arginine treatment significantly reduced the pro-inflammatory IL-2, IL-6, and IFN-γ while increasing the levels of the anti-inflammatory IL-10 ( Fig. 3).
Fig. 3.
Violin plots representing the circulating levels of IFN-γ, IL-2, IL-6, and IL-10 (A–D); *:p < 0.05; **:p < 0.01; ***:p < 0.005; ****:p < 0.001.; ns: non-significant (ANOVA and Tukey post hoc correction).
4. Discussion
In addition to confirming in a larger population compared to the initial analysis [21] the beneficial effects of L-Arginine in patients hospitalized for COVID-19, we identified in the modulation of cytokines involved in the COVID-induced inflammatory response an unprecedented mechanism underlying the favorable effects of L-Arginine in COVID-19. These results are consistent with recent metabolomics reports evidencing reduced circulating levels of L-Arginine in COVID-19 patients who experienced a severe outcome [31], [32].
Of note, the peculiar modulation of both anti- and pro- inflammatory cytokines [33], [34] is remarkable and emphasizes the key role of L-Arginine metabolism in COVID-19 [20], [35], [36]. Having observed that L-Arginine significantly reduces IL-6 levels is especially significant considering the recently published results of a secondary analysis of a Bayesian adaptive randomized clinical platform trial that included 4791 COVID-19 patients, showing that there was a greater than 99.9 % probability that sarilumab and tocilizumab (IL-6 receptor antagonists) improved survival through 6 months [37].
Our study is not exempt from limitations, including having measured only 4 cytokines, having enrolled exclusively patients hospitalized (which reduces the generalizability of our findings to patients with non-severe forms of COVID-19), and not having a population of patients with pulmonary disease not caused by COVID-19.
Further studies, ideally exploring more cytokines and chemokines, are warranted to dissect how exactly L-Arginine is able to modulate their circulating levels. Potential mechanisms by which L-Arginine exerts anti-inflammatory effects may include the reconstitution of circulating L-Arginine levels, a correction of nitric oxide dysfunction, and/or a direct action on immune cells [38], [39], [40], [41], [42], [43], [44], [45].
Considering that favorable effects of L-Arginine, in combination with ascorbic acid, have been recently shown also in the so-called Long-COVID [36], dedicated investigations exploring the modulation of inflammatory markers in patients with Long-COVID are needed as well.
CRediT authorship contribution statement
Valentina Trimarco: Writing – original draft, Data curation, Formal analysis, Visualization. Raffaele Izzo: Writing – original draft, Data curation, Software. Angela Lombardi: Investigation, Visualization, Writing – review & editing. Antonietta Coppola: Investigation, Data curation, Visualization. Giuseppe Fiorentino: Data curation, Visualization. Gaetano Santulli: Conceptualization, Writing – review & editing.
Data Availability
Data will be made available upon reasonable request to the first Author(s).
Ethical Approval
The Study was approved by the institutional Ethical Committee of the Hospital (A.O.R.N. “Ospedali dei Colli”, Naples, Italy); written informed consent was collected from all patients, or their legal representative if they were unable to provide consent. The trial was performed in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization-Good Clinical Practice guidelines.
Sources of Funding
The Santulli’s Lab is supported in part by the National Institutes of Health (NIH): National Heart, Lung, and Blood Institute (NHLBI: R01-HL159062, R01-HL164772, R01-HL146691, T32-HL144456), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: R01-DK123259, R01-DK033823), National Center for Advancing Translational Sciences (NCATS: UL1TR002556-06) to G.S., by the Diabetes Action Research and Education Foundation (to G.S.), and by the Monique Weill-Caulier and Irma T. Hirschl Trusts (to G.S.). This study was also supported by Grant 00014PRIN-2017 ID43237 to V.T.
Declaration of Competing Interest
None.
Acknowledgement
We thank Drs. Wang and Gambardella for helpful discussion.
References
- 1.Sardu C., Gambardella J., Morelli M.B., Wang X., Marfella R., Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J. Clin. Med. 2020;9(5):1417. doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trimarco V., Izzo R., Mone P., Trimarco B., Santulli G. Targeting endothelial dysfunction and oxidative stress in long-COVID. Pharm. Res. 2022;184 doi: 10.1016/j.phrs.2022.106451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trimarco V., Izzo R., Zanforlin A., Tursi F., Scarpelli F., Santus P., Pennisi A., Pelaia G., Mussi C., Mininni S., Messina N., Marazzi G., Maniscalco M., Mallardo M., Fazio G., Diana A., Capra Marzani M., Aloe T., Mone P., Trimarco B., Santulli G. Endothelial dysfunction in long-COVID: new insights from the nationwide multicenter LINCOLN Study. Pharm. Res. 2022;185 doi: 10.1016/j.phrs.2022.106486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matarese A., Gambardella J., Sardu C., Santulli G. miR-98 regulates TMPRSS2 expression in human endothelial cells: key implications for COVID-19. Biomedicines. 2020;8(11) doi: 10.3390/biomedicines8110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Salazar B., Holwerda M., Studle C., Piragyte I., Mercader N., Engelhardt B., Rieben R., Doring Y. COVID-19 and the vasculature: current aspects and long-term consequences. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.824851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castanares-Zapatero D., Chalon P., Kohn L., Dauvrin M., Detollenaere J., Maertens de Noordhout C., Primus-de Jong C., Cleemput I., Van den Heede K. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann. Med. 2022;54(1):1473–1487. doi: 10.1080/07853890.2022.2076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambrosino P., Calcaterra I.L., Mosella M., Formisano R., D'Anna S.E., Bachetti T., Marcuccio G., Galloway B., Mancini F.P., Papa A., Motta A., Di Minno M.N.D., Maniscalco M. Endothelial dysfunction in COVID-19: a unifying mechanism and a potential therapeutic target. Biomedicines. 2022;10(4) doi: 10.3390/biomedicines10040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urata R., Ikeda K., Yamazaki E., Ueno D., Katayama A., Shin-Ya M., Ohgitani E., Mazda O., Matoba S. Senescent endothelial cells are predisposed to SARS-CoV-2 infection and subsequent endothelial dysfunction. Sci. Rep. 2022;12(1):11855. doi: 10.1038/s41598-022-15976-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kansakar U., Gambardella J., Varzideh F., Avvisato R., Jankauskas S.S., Mone P., Matarese A., Santulli G. miR-142 targets TIM-1 in human endothelial cells: potential implications for stroke, COVID-19, Zika, Ebola, Dengue, and other viral infections. Int. J. Mol. Sci. 2022;23(18) doi: 10.3390/ijms231810242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mone P., Gambardella J., Wang X., Jankauskas S.S., Matarese A., Santulli G. miR-24 targets the transmembrane glycoprotein neuropilin-1 in human brain microvascular endothelial cells. Noncoding RNA. 2021;7(1) doi: 10.3390/ncrna7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joffre J., Rodriguez L., Matthay Z.A., Lloyd E., Fields A.T., Bainton R.J., Kurien P., Sil A., Calfee C.S., Woodruff P.G., Erle D.J., Hendrickson C., Krummel M.F., Langelier C.R., Matthay M.A., Kornblith L.Z., Hellman J., Consortium C.-M.-P.f.E.T., Covid-19 Associated Coagulopathy I., Thrombosis Study G. COVID-19-associated lung microvascular endotheliopathy: a "from the bench" perspective. Am. J. Respir. Crit. Care Med. 2022;206(8):961–972. doi: 10.1164/rccm.202107-1774OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jankauskas S.S., Kansakar U., Sardu C., Varzideh F., Avvisato R., Wang X., Matarese A., Marfella R., Ziosi M., Gambardella J., Santulli G. COVID-19 causes ferroptosis and oxidative stress in human endothelial cells. Antioxidants. 2023;12(2):326. doi: 10.3390/antiox12020326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gambardella J., Santulli G. What is linking COVID-19 and endothelial dysfunction? Updates on nanomedicine and bioengineering from the 2020 AHA scientific sessions. Eur. Heart J. Cardiovasc. Pharm. 2021;7(3):e2–e3. doi: 10.1093/ehjcvp/pvaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gambardella J., Coppola A., Izzo R., Fiorentino G., Trimarco B., Santulli G. Role of endothelial miR-24 in COVID-19 cerebrovascular events. Crit. Care. 2021;25(1):306. doi: 10.1186/s13054-021-03731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gambardella J., Kansakar U., Sardu C., Messina V., Jankauskas S.S., Marfella R., Maggi P., Wang X., Mone P., Paolisso G., Sorriento D., Santulli G. Exosomal miR-145 and miR-885 regulate thrombosis in COVID-19. J. Pharm. Exp. Ther. 2023;384(1):109–115. doi: 10.1124/jpet.122.001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gambardella J., Khondkar W., Morelli M.B., Wang X., Santulli G., Trimarco V. Arginine and endothelial function. Biomedicines. 2020;8(8) doi: 10.3390/biomedicines8080277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monti L.D., Casiraghi M.C., Setola E., Galluccio E., Pagani M.A., Quaglia L., Bosi E., Piatti P. L-arginine enriched biscuits improve endothelial function and glucose metabolism: a pilot study in healthy subjects and a cross-over study in subjects with impaired glucose tolerance and metabolic syndrome. Metabolism. 2013;62(2):255–264. doi: 10.1016/j.metabol.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Mammedova J.T., Sokolov A.V., Freidlin I.S., Starikova E.A. The mechanisms of L-arginine metabolism disorder in endothelial cells. Biochemistry. 2021;86(2):146–155. doi: 10.1134/S0006297921020036. [DOI] [PubMed] [Google Scholar]
- 19.Melik Z., Zaletel P., Virtic T., Cankar K. L-arginine as dietary supplement for improving microvascular function. Clin. Hemorheol. Microcirc. 2017;65(3):205–217. doi: 10.3233/CH-16159. [DOI] [PubMed] [Google Scholar]
- 20.Adebayo A., Varzideh F., Wilson S., Gambardella J., Eacobacci M., Jankauskas S.S., Donkor K., Kansakar U., Trimarco V., Mone P., Lombardi A., Santulli G. l-arginine and COVID-19: an update. Nutrients. 2021;13(11) doi: 10.3390/nu13113951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorentino G., Coppola A., Izzo R., Annunziata A., Bernardo M., Lombardi A., Trimarco V., Santulli G., Trimarco B. Effects of adding L-arginine orally to standard therapy in patients with COVID-19: a randomized, double-blind, placebo-controlled, parallel-group trial. Results of the first interim analysis. EClinicalMedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geiger R., Rieckmann J.C., Wolf T., Basso C., Feng Y., Fuhrer T., Kogadeeva M., Picotti P., Meissner F., Mann M., Zamboni N., Sallusto F., Lanzavecchia A. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. 2016;167(3):829–842. doi: 10.1016/j.cell.2016.09.031. (e13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu Y., Yang X., Wang L., Gao K., Jiang Z. L-arginine inhibited inflammatory response and oxidative stress induced by lipopolysaccharide via arginase-1 signaling in IPEC-J2 cells. Int. J. Mol. Sci. 2019;20(7) doi: 10.3390/ijms20071800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hnia K., Gayraud J., Hugon G., Ramonatxo M., De La Porte S., Matecki S., Mornet D. L-arginine decreases inflammation and modulates the nuclear factor-kappaB/matrix metalloproteinase cascade in mdx muscle fibers. Am. J. Pathol. 2008;172(6):1509–1519. doi: 10.2353/ajpath.2008.071009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghavan S.A., Dikshit M. Vascular regulation by the L-arginine metabolites, nitric oxide and agmatine. Pharm. Res. 2004;49(5):397–414. doi: 10.1016/j.phrs.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Yang L., Xie X., Tu Z., Fu J., Xu D., Zhou Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal. Transduct. Target Ther. 2021;6(1):255. doi: 10.1038/s41392-021-00679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhar S.K., K V., Damodar S., Gujar S., Das M. IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: results from meta-analysis and regression. Heliyon. 2021;7(2) doi: 10.1016/j.heliyon.2021.e06155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon A.C., Mouncey P.R., Al-Beidh F., Rowan K.M., Nichol A.D., Arabi Y.M., Annane D., Beane A., van Bentum-Puijk W., Berry L.R., Bhimani Z., Bonten M.J.M., Bradbury C.A., Brunkhorst F.M., Buzgau A., Cheng A.C., Detry M.A., Duffy E.J., Estcourt L.J., Fitzgerald M., Goossens H., Haniffa R., Higgins A.M., Hills T.E., Horvat C.M., Lamontagne F., Lawler P.R., Leavis H.L., Linstrum K.M., Litton E., Lorenzi E., Marshall J.C., Mayr F.B., McAuley D.F., McGlothlin A., McGuinness S.P., McVerry B.J., Montgomery S.K., Morpeth S.C., Murthy S., Orr K., Parke R.L., Parker J.C., Patanwala A.E., Pettila V., Rademaker E., Santos M.S., Saunders C.T., Seymour C.W., Shankar-Hari M., Sligl W.I., Turgeon A.F., Turner A.M., van de Veerdonk F.L., Zarychanski R., Green C., Lewis R.J., Angus D.C., McArthur C.J., Berry S., Webb S.A., Derde L.P.G. Interleukin-6 receptor antagonists in critically Ill patients with covid-19. N. Engl. J. Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gadotti A.C., de Castro Deus M., Telles J.P., Wind R., Goes M., Garcia Charello Ossoski R., de Padua A.M., de Noronha L., Moreno-Amaral A., Baena C.P., Tuon F.F. IFN-gamma is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020;289 doi: 10.1016/j.virusres.2020.198171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merad M., Blish C.A., Sallusto F., Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375(6585):1122–1127. doi: 10.1126/science.abm8108. [DOI] [PubMed] [Google Scholar]
- 31.Rees C.A., Rostad C.A., Mantus G., Anderson E.J., Chahroudi A., Jaggi P., Wrammert J., Ochoa J.B., Ochoa A., Basu R.K., Heilman S., Harris F., Lapp S.A., Hussaini L., Vos M.B., Brown L.A., Morris C.R. Altered amino acid profile in patients with SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA. 2021;118(25) doi: 10.1073/pnas.2101708118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Alessandro A., Thomas T., Akpan I.J., Reisz J.A., Cendali F.I., Gamboni F., Nemkov T., Thangaraju K., Katneni U., Tanaka K., Kahn S., Wei A.Z., Valk J.E., Hudson K.E., Roh D., Moriconi C., Zimring J.C., Hod E.A., Spitalnik S.L., Buehler P.W., Francis R.O. Biological and clinical factors contributing to the metabolic heterogeneity of hospitalized patients with and without COVID-19. Cells. 2021;10(9):2293. doi: 10.3390/cells10092293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y., Perlman S. COVID-19: inflammatory profile. Annu. Rev. Med. 2022;73:65–80. doi: 10.1146/annurev-med-042220-012417. [DOI] [PubMed] [Google Scholar]
- 34.Tang L., Yin Z., Hu Y., Mei H. Controlling cytokine storm is vital in COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.570993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mone P., Izzo R., Marazzi G., Manzi M.V., Gallo P., Campolongo G., Cacciotti L., Tartaglia D., Caminiti G., Varzideh F., Santulli G., Trimarco V. L-arginine enhances the effects of cardiac rehabilitation on physical performance: new insights for managing cardiovascular patients during the COVID-19 pandemic. J. Pharm. Exp. Ther. 2022;381(3):197–203. doi: 10.1124/jpet.122.001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izzo R., Trimarco V., Mone P., Aloe T., Capra Marzani M., Diana A., Fazio G., Mallardo M., Maniscalco M., Marazzi G., Messina N., Mininni S., Mussi C., Pelaia G., Pennisi A., Santus P., Scarpelli F., Tursi F., Zanforlin A., Santulli G., Trimarco B. Combining L-arginine with vitamin C improves long-COVID symptoms: the LINCOLN survey. Pharm. Res. 2022;183 doi: 10.1016/j.phrs.2022.106360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins A.M., Berry L.R., Lorenzi E., Murthy S., McQuilten Z., Mouncey P.R., Al-Beidh F., Annane D., Arabi Y.M., Beane A., van Bentum-Puijk W., Bhimani Z., Bonten M.J.M., Bradbury C.A., Brunkhorst F.M., Burrell A., Buzgau A., Buxton M., Charles W.N., Cove M., Detry M.A., Estcourt L.J., Fagbodun E.O., Fitzgerald M., Girard T.D., Goligher E.C., Goossens H., Haniffa R., Hills T., Horvat C.M., Huang D.T., Ichihara N., Lamontagne F., Marshall J.C., McAuley D.F., McGlothlin A., McGuinness S.P., McVerry B.J., Neal M.D., Nichol A.D., Parke R.L., Parker J.C., Parry-Billings K., Peters S.E.C., Reyes L.F., Rowan K.M., Saito H., Santos M.S., Saunders C.T., Serpa-Neto A., Seymour C.W., Shankar-Hari M., Stronach L.M., Turgeon A.F., Turner A.M., van de Veerdonk F.L., Zarychanski R., Green C., Lewis R.J., Angus D.C., McArthur C.J., Berry S., Derde L.P.G., Gordon A.C., Webb S.A., Lawler P.R. Long-term (180-day) outcomes in critically Ill patients with COVID-19 in the REMAP-CAP randomized clinical trial. JAMA. 2023;329(1):39–51. doi: 10.1001/jama.2022.23257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crowther R.R., Schmidt S.M., Lange S.M., McKell M.C., Robillard M.C., Zhao J., Haffey W.D., Wyder M.A., Greis K.D., Setchell K.D.R., Qualls J.E. Cutting edge: l-arginine transfer from antigen-presenting cells sustains CD4(+) T cell viability and proliferation. J. Immunol. 2022;208(4):793–798. doi: 10.4049/jimmunol.2100652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grzywa T.M., Sosnowska A., Matryba P., Rydzynska Z., Jasinski M., Nowis D., Golab J. Myeloid cell-derived arginase in cancer immune response. Front. Immunol. 2020;11:938. doi: 10.3389/fimmu.2020.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee Y.C., Su Y.T., Liu T.Y., Tsai C.M., Chang C.H., Yu H.R. L-arginine and l-citrulline supplementation have different programming effect on regulatory T-cells function of infantile rats. Front. Immunol. 2018;9:2911. doi: 10.3389/fimmu.2018.02911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bronte V., Serafini P., Mazzoni A., Segal D.M., Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24(6):302–306. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 42.Cao Y., Wang Q., Du Y., Liu F., Zhang Y., Feng Y., Jin F. l-arginine and docetaxel synergistically enhance anti-tumor immunity by modifying the immune status of tumor-bearing mice. Int. Immunopharmacol. 2016;35:7–14. doi: 10.1016/j.intimp.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Bronte V., Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005;5(8):641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 44.Yu H.R., Hsu T.Y., Tsai C.C., Huang H.C., Cheng H.H., Lai Y.J., Lin Y.J., Chen C.C., Li S.C., Yang K. The functional DNA methylation signatures relevant to altered immune response of neonatal T cells with l-arginine supplementation. Nutrients. 2021;13(8) doi: 10.3390/nu13082780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J., Wang S., Guo X., Lu Y., Liu X., Jiang M., Li X., Qin B., Luo Z., Liu H., Li Q., Du Y.Z., Luo L., You J. Arginine supplementation targeting tumor-killing immune cells reconstructs the tumor microenvironment and enhances the antitumor immune response. ACS Nano. 2022;16(8):12964–12978. doi: 10.1021/acsnano.2c05408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request to the first Author(s).