Abstract
Early life stress is commonly experienced by infants, especially preterm infants, and may impact their neurodevelopmental outcomes in their early and later lives. Mitochondrial function/dysfunction may play an important role underlying the linkage of prenatal and postnatal stress and neurodevelopmental outcomes in infants. This review aimed to provide insights on the relationship between early life stress and neurodevelopment and the mechanisms of mitochondrial function/dysfunction that contribute to the neuropathology of stress. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used to develop this systematic review. PubMed, Scopus, PsycINFO, and Biosis databases were searched for primary research articles published between 2010 and 2021 that examined the relationships among mitochondrial function/dysfunction, infant stress, and neurodevelopment. Thirty studies were identified. There is evidence to support that mitochondrial function/dysfunction mediates the relationship between prenatal and postnatal stress and neurodevelopmental outcomes in infants. Maternal transgenerational transmission of mitochondrial bioenergetic patterns influenced prenatal stress induced neurodevelopmental outcomes and behavioral changes in infants. Multiple functionally relevant mitochondrial proteins, genes, and polymorphisms were associated with stress exposure. This is the first review of the role that mitochondrial function/dysfunction plays in the association between stress and neurodevelopmental outcomes in full-term and preterm infants. Although multiple limitations were found based on the lack of data on the influence of biological sex, and due to invasive sampling, and lack of longitudinal data, many genes and proteins associated with mitochondrial function/dysfunction were found to influence neurodevelopmental outcomes in the early life of infants.
Keywords: Stress, Mitochondrial function/dysfunction, Neurodevelopmental outcomes, Infant
Introduction
Neurodevelopmental deficits are characterized by limitations in intellectual functioning and adaptive behavior, e.g., lack of competence in social, conceptual, and practical skills [1]. Increased preterm birth rates and improved survival rates of these infants (<37 weeks’ gestational age [GA]) come with increased complications and risk for neurodevelopmental deficits in these populations [2, 3]. Through the transition from the intrauterine to the extrauterine world, newborn infants are exposed to multiple prenatal stress, e.g., maternal childhood maltreatment (MCM) and intrauterine growth restriction (IUGR), as well as postnatal stress, e.g., prematurity, toxic postnatal environment, and painful/stressful experiences during neonatal intensive care hospitalization. Mitochondrial function/dysfunction has been found to be associated with multiple neurodegenerative diseases such as Parkinson [4], Alzheimer’s disease [5], and neuropathic pain [6]. It may also influence infant neurodevelopmental outcomes, especially in preterm infants who experience excessive pain/stress in early life [7, 8].
Preterm infants may face multiple challenges in both the prenatal and postnatal periods. Mother of infants may experience high rate of MCM, defined as maternal childhood abuse (physical, sexual, emotional) or neglect (physical, emotional) [9, 10]. 18% of preterm infants have IUGR [11], defined as fetal growth repression and failure to reach full growth potential [12]. Both MCM and IUGR were reportedly associated with early-life adversity in infants, although the evidence is still limited and requires further investigation. Infant born preterm lose the protection of the placenta, which modulates homeostasis in the intrauterine environment and epigenetic effects on neurodevelopment in infants [13]. The very preterm infant is at high risk of neurodevelopmental deficits due to impaired myelination of the posterior limb of internal capsule (PLIC) which is normally initiated in the uterus by 23–29 weeks of GA [14–18]. In addition, during the neonatal intensive care unit (NICU) hospitalization, preterm infants are exposed to multiple stress events associated with adverse neurodevelopmental outcomes [19]. It has been reported that intermittent hypoxic stress during the first 2–3 months after birth was associated with motor impairment, cognitive, or language delay at 18 months in preterm infants [20]. Although the negative impacts of early life pain/stress on physiological and behavioral changes are known after birth [21] and persist in childhood and later life [22, 23], less is known regarding the biological mechanisms of stress and neurodevelopmental deficits in preterm infants.
Accumulating evidence suggests that acute and chronic stressors influence mitochondrial biology and function [24]. Mitochondrial function is the organelle’s ability to generate the chemical energy required to support the cell’s biochemical reactions and regulate communication between cells and tissues to sustain the metabolic homeostasis [25]. Besides being the powerhouse of each cell, generating adenosine triphosphate (ATP), mitochondria play a central role in reactive oxygen species (ROS) generation, calcium buffering, ion homeostasis, biosynthetic pathways, signaling, and programmed cell death [24, 26]. Prolonged and excessive stress exposure may damage mitochondrial structure and function. Mitochondrial dysfunction is defined as the inability to generate the chemical energy required to support the cell’s biochemical reaction and can occur due to mitochondrial structure fragmentation, mitochondrial DNA (mtDNA) damage, decreased ATP production, and increased ROS generation, which all pose a high risk for neuronal loss [13, 27–30]. Although mitochondrial dysfunction has been found to be related to neurological disorders such as Huntington’s disease [31], multiple sclerosis [32], and amyotrophic lateral sclerosis [33], the mechanism of mitochondrial dysfunction in stress-associated neurodevelopmental deficits in preterm infants is still poorly understood.
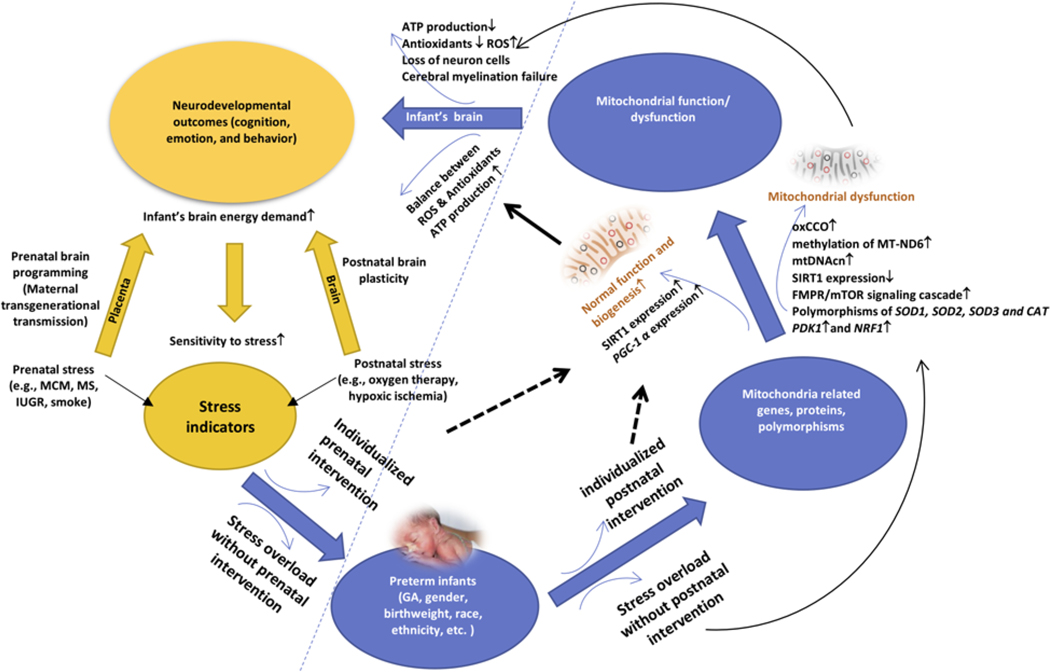
As mitochondrial function/dysfunction has been shown to be an important mechanism underlying neurodevelopmental outcomes in preterm infants, it is of interest to incorporate this mechanism into a conceptual model for the purpose of research in the field. Therefore, we developed a conceptual model to address mitochondrial function/dysfunction related to stress which impacts the neurodevelopmental outcomes in preterm infants. The model proposes that prenatal and postnatal stress-related high energy demand request increased mitochondrial function to adapt to the external changes. However, prenatal/postnatal stress overload will damage the mitochondrial function due to its limited adaptive capacity and induce the neurodevelopmental deficits (see Fig. 1). By guiding with this conceptual model, a systematic review of the literature was undertaken to provide a synthesis of the current research. In addition, this review provided insights into advances in understanding the association between infants’ early life stress and neurodevelopment and identified specific biomechanism and the significance of mitochondrial function/dysfunction in predicting neurodevelopmental outcomes in preterm infants. The research questions addressed in the review were as follows: (1) Is prenatal/postnatal stress associated with mitochondrial function/dysfunction in mothers and infants? and (2) Is mitochondrial function/dysfunction associated with neurodevelopmental outcomes in full-term and preterm infants?
Fig. 1.
A conceptual framework for the association of stress, mitochondrial function/dysfunction, and neurodevelopmental outcomes.
Methods
Review Design
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [34] statement was used in the development of this systematic review [35] to examine the role of mitochondrial function/dysfunction in the association between prenatal and postnatal stress and neurodevelopmental deficits in full-term and preterm infants. Inclusion criteria were (a) quantitative studies using any quantitative research design and methods; (b) human subject participants born prematurely (less than 37 weeks of gestational age [GA]) or full-term with early life stress, and/or women who experienced stress/adversities during pregnancy and/or the postpartum period; (c) studies focused on neurodevelopmental outcomes and mitochondrial function/dysfunction; and (d) studies published in English between 2010 and 2021. Exclusion criteria were (a) studies focused on topics other than stress, neurodevelopmental outcomes, and mitochondrial function/dysfunction; (b) studies with enrolled infants having congenital neurological deficits; and (c) qualitative research studies.
Literature Search and Data Synthesis Procedures
Articles were searched in PubMed, Scopus, PsycINFO, and Biosis Database. Four key terms were searched: stress, neurodevelopment, mitochondria, and infant. The categories were searched as follows, stress: MCM, oxidative stress, stress, pain, and fetal growth restriction (FGR); infant: preemie, preterm, young gestational age, neonatal, premature babies; genomic/proteomic of mitochondria; neurodevelopment: brain, white matter, brain injury, encephalopathy. Identified studies were imported to EndNote to remove the duplicates. The target outcomes were grouped in three main parts: (a) stress in infants including preterm infants; (b) the impact of stress in mitochondrial function/dysfunction; and (c) mitochondrial function and neurodevelopmental outcomes.
A PRISMA flow diagram was developed (see online suppl. Fig. S1; see www.karger.com/doi/10.1159/000526491 for all online suppl. material) based on inclusion and exclusion criteria for study selection [34]. The quality appraisal was conducted using the Critical Appraisal Skills Programme [36]. The 11 items on the checklist evaluated the research aim, research method, subjects’ recruitment, control selection, and (exposure) measurement, etc. (see online suppl. Table S1). Due to the early level of research in the field, it was of interest to include all research studies, thus no studies were restricted based on the quality appraisal if they failed to match the requirement of one item. However, studies were excluded from the review if they did not match any requirement of 11 items. Data were extracted for study purpose, study design, sample size and characteristics, measurements (stress indicators; mitochondrial related gene, protein, function measurements, neurodevelopmental and behavioral outcomes and assessment), and main findings. The first and corresponding authors validated the literature search and extraction.
Results
A total of 317 papers were identified in the initial phase by searching the databases (see online suppl. Fig. S1). After removing duplications, reviews, mitochondrial disease and mutation publications, and preclinical studies, total 30 studies remained for the final systematic review (N = 30). Of these publications, studies were from 12 countries including the USA, Germany, Italy, Spain, France, the UK, Serbia, India, Belgium, Iran, the Czech Republic, and Japan (see Table 1).
Table 1.
Included studies that examine association of stress, mitochondrial function/dysfunction, and neurodevelopmental outcomes
| Author, year, country | Study aim | Study design | Sample (size, gender) | Intervention | Measurement | Main findings | Limitation (L) and recommendation for future study (R) | ||
|---|---|---|---|---|---|---|---|---|---|
| stress indicators | MT related gene, protein, function measures | neurodevelopmental and behavioral (outcomes and assessment) | |||||||
| Gumpp et al. [53] 2020, Germany | To explore the intergenerational transmission of maternal MCM exposure induced mt bioenergetic alternation from mother to child | Observational study | N = 209 (mothers, n = 105; newborns, n = 104); maternal PBMC and neonatal umbilical cord blood | N/A | MCM | Mitochondrial respiration and density | CTQ was used to assess MCM | 1. Maternal and neonatal citrate synthase activity was positively correlated. 2. Female infants had higher mt respiration than male cohorts which was independent of maternal CM exposure. 3. Maternal CM load is only associated with mt respiration and density in mothers, but not in infants | L: no neurodevelopmental outcomes were evaluated R: Need to be confirmed in larger sample size |
| Mitra et al. [67] 2019, UK | To explore the role of oxCCO and MABP in HIE associated brain metabolic activity | Observational study | N = 23, stable term infants | N/A | Therapeutic hypothermia following hypoxic ischemic encephalopathy | oxCCO-MA BP semblance at 48 h after birth, and Lac/NAA (day 5–7) to predict the neurodevelopment outcomes | Bayley III at 1 year | oxCCO-MABP semblance at 48 h can be used as a biomarker to (1) predict early and 1 year of age neurodevelopmental outcome after HIE; (2) differentiate between the infants with good or poor neurodevelopmental outcomes after HIE | L: no data within 24 h; no gender difference; small sample size; did not measure mitochondrial function R: Enlarge sample size |
| Lapp et al. [51] 2019, USA | To explore the association between adverse childhood experience (ACE) and mitochondrial genetic variation | Observational study | N = 90,48.4% female, age = 32.12±15.1; buccal swabs; two groups (ACE = 0, ACE ≥4) | N/A | Adverse childhood experience, lifetime discrimination, daily discrimination, perceived stress, and city stress | Polymorphisms of gene 5-HTTLPR, NR3C1, FKBP5; MT-ND6 methylation and mtDNAcn | Current stress test | (1) mitochondrially encoded NADH dehydrogenase 6 (MT-ND6) methylation level was higher in ACE ≥4 group compared with ACE = 0 group; (2) No mtDNAcn difference between these two groups | L: No gender difference was assessed; small sample size R: Future study should explore the biological mechanisms between early life stress and mitochondrial DNA methylation |
| Brunst et al. [59] 2018, USA | To assess the combined effect of utero PM2.5 and maternal stress exposure on mtDNAcn | Observational study | N = 167, first time pregnant women, placenta samples, infants’ cord blood samples | N/A | Maternal stress and PM2.5 exposure | mtDNAcn | N/A | Increased PM2.5 and maternal trauma were associated with decreased mtDNAcn; 2. PM2.5 exposure level between 25 and 40 GW was positively associated with placental mtDNAcn in boys with high maternal trauma level, while negatively associated with mtDNAcn in girls with low maternal trauma level | L: No neurodevelopmental outcomes assessed in infants R: Longitudinal study should be conducted to explore the association between mt involved genetic variations and neurobehavioral changes |
| Yang et al. [39] 2018, USA | To explore the variation of SIRT1 in preterm infants exposed to hyperoxia and the protective effect of resveratrol | 1st experimental design (n = 10: ctrl no O2; n = 30 O2) 2nd experimental design (n = 40, no O2, divided in 4 groups) | Total N = 80 (40 control, 40 neonatal respiratory distress syndrome [NRDS]), premature infants, gestational age <32 weeks; PBMC | Resveratrol | Oxidative stress | Protein levels of SIRT1; ROS level; MDA level | N/A | Hyperoxia or ROS injury induced SIRT1 transportation from nuclei of PBMC to cytoplasm and decreased SIRT1 expression and activity can be protected by Resveratrol | L: No neurodevelopmental outcomes assessed; no gender difference analyzed; no mitochondrial function detected R: Future study will focus on SIRTI’s pharmacological regulation |
| Boecket al. [54] 2017, Germany | To explore the association between MCM and cortisol, oxytocin, and mitochondrial oxygen consumption | Cohort (baseline, 3 months postpartum follow up) | N = 49 (mothers, 22–44 years old); PBMC blood sample | N/A | MCM: maltreatment load using CTQ | Mitochondrial oxygen consumption; Gene expression of PGC-1 α (qPCR) | N/A | (1) Mother with higher CTQ had higher level of anxiety; (2) The maximum respiratory capacity was only modestly increased, and no change was found in PGC-1α expression in mothers with MCM | L: small sample size and no gender difference was assessed R: Future study should focus on larger sample size |
| Brunst et al. [58] 2017, USA | To examine the associations among maternal stress, psychological functioning, and placental mt function | Prospective cohort study | N = 147, first time pregnant women, placenta samples | N/A | Maternal stress (prenatal stress, postnatal depression, PTSD, and cumulative lifetime stress) | Mitochondrial DNA copy number (mtDNAcn) | N/A | Higher level of prenatal stress and psychological symptom scores and lower mtDNAcn were found in non-white compared with white women; prenatal stress was negatively associated with mtDNAcn (marker of mt dysfunction) | L: no gender difference; no infants’ neurodevelopmental outcomes R: Future study should focus on the stress intervention in pregnant women which will benefit maternal-infant health |
| Tyrka et al. [37] 2016, USA | To explore the association between the variation of mtDNAcn and the early life adversity and psychiatric disorder | Observational study | N = 290 adults, blood sample; 4 groups by combining adverse (+/−) and diagnose (+/−) | N/A | Parental loss (death and separation) and childhood trauma | mtDNAcn | Psychiatric diagnosis using SCID, IDS-SR, STAI, PSS, CD-RISC, CTQ scales and/or inventory | The early life adversity was associated with mtDNAcn while (−/−) group had lower mtDNAcn compared with other three groups; participants who had anxiety and past substance, lifetime depressive disorder had higher mtDNAcn | L: Not preterm infants; no gender difference was assessed R: Future study should further analyze larger sample size and mitochondrial genetic variations |
| Lechpammer et al. [40] 2016, USA | To explore the role of FMRP/mTOR signaling cascade in the pathology of encephalopathy in premature infants | Pilot study | N = 30 (newborns’ normal brain, n = 15; premature hypoxic-ischemic injured brain, n = 11; adult normal control brain, n = 4) | N/A | Hypoxic-ischemic brain injury | Proteins of mTOR, total FMRP, phospho-FMRP(Ser499) | Encephalopathy of prematurity | (1) During 36–39 GW, overexpression of FMRP was confirmed in the cortical of normal brain, although down regulated total FMRP, phospho-FMRP, and overexpressed mTOR was found in cortical in preterm infants with encephalopathy; (2) During 23–37 GW, FMRP level was predominantly increased in premature white matter compared with normal brain; (3) Co-expression of mGluR5 with FMRP has been observed in infants with encephalopathy at 36–39 GW. | L: Did not consider gender; did not measure mitochondrial function; no history of neurodevelopmental outcomes R: Future study needs to focus on gender difference, and larger sample size |
| Boeck et al. [55] 2016, Germany | To explore whether mitochondrial activity and ROS are involved in the CM-induced inflammation | Cross-sectional design of the exploratory study | N = 67, women 6 days following parturition (>18 years); PBMC blood sample | N/A | CM; maltreatment load using chCTQ | Mitochondrial oxygen consumption (cellular oxygen consumption [ATP]; maximal capacity of the respiratory chain; residual oxygen consumption; flux control ratios) | Stress, anxiety and depressive symptoms | Dose-response relationship of increasing CM load with higher level of pro-inflammatory cytokines and ROS in PBMC | L: small sample size; no gender difference; no neurodevelopmental assessment; no infants assessment; not longitudinal design; no genetic evaluation R: Should have a lifespan study from childhood until adulthood to explore the association between the mitochondrial function and CM induced inflammation |
| Lambertini et al. [60] 2015, USA | To explore whether there is an association between the MT gene expression, MPSP and fetal temperament development | Cohort | Placenta samples, N = 108, mot her-infant dyads (274.26±17.16 days of gestational age) | N/A | MPSP | All 13 protein-coding mitochondrial-encoded genes were analyzed, gene MT-ND2 | Infant temperament at 6 months of age is assessed by the Infant Behavior Questionnaire Revised (IBQ-R) administered to mothers | Mitochondrial function mediated negatively impact of MPSP on infants’ tempera ment development | L: No gender difference assessed; no mitochondrial function detected R: Increased sample size of the cohort in the future |
| Wisnowski et al. [41] 2013, USA | To explore whether the altered glutamate and glutamine are associated with WMI | Observational study | N = 218, born <37 GA, not older than 60 weeks post concept ion a 1 age at the time of MRI, WMI diagnosed | N/A | Glutamate induced excitotoxicity | Glutamate and N-acetyl aspartate (NAA) (both released from MT of neurons) | WMI | (1) MRI/MRS can be used to as biomarker to identify WMI in preterm infants; (2) Increased Glutamine and decreased NAA were found in parietal WM in infants with WMI | L: No demographic data were presented; no neurobehavioral outcomes reported R: Future study should focus on mitochondrial function and gender difference |
| Giusti et al. [42] 2012, Italy | To identify the association between SNPs of SOD 1,SOD2, SOD3, and CAT and the severity of RDS (MV, BPD, IVH, and ROP) in preterm infants | Retrospective study | N = 152, preterm infants, ≤28 GW, blood DNA, polymorphisms | N/A | ROS of preterm birth | SNPs of SOD 1,SOD2, SOD3, and CAT | N/A | (1) rs8192287 SOD3 was found associated with decreased risk of developing IVH; (2) The association between homozygous infants for rs4880, rs5746136 for SOD2 polymorphism and lower gestational age; (3) haplotypes of SOD1, SOD3 and CAT genes were risk predictors of RDS complications | L: No gender difference was evaluated; 2. No neurodevelopment assessed; 3. No longitudinal genetic variation was evaluated R: Larger sample size need to be assessed in the future |
| Vasiljevic et al. [52] 2011, Serbia | To examine the role of neuroinflammation, ROS, and deficient or overproduced growth factors in hypoxic-ischemic brain damage | Prospective study | N = 90; neonates gestational age >32 weeks with hypoxic-ischemic encephalopathy | N/A | Hypoxic-ischemic brain damage | IL-6, GPX, NSF, VEGF | Cerebrospinal fluid (CSF) and Denver Developmental Screening Test at 12 months | (1) NSE in CSF could be used as a biomarker of severity of HIE; (2) Remarkably increased GPX activity in CSF (indicate overproduced ROS) was associated with the severity of HIE; (3) Decreased VEGF and increased IL-6 in CSF were associated with the severity of H IE and brain damage | L: Did not clarify the number of preterm and term infants; no gender difference was discussed; no mitochondrial function was measured R: Future study focus on the diagnostic and prognostic value of these markers |
| Thomas et al. [38] 2019, USA | To testify whether the placenta mitochondria dysfunction mediates the impact of acute ROS on decreased FAO and increased esterification | Experimental study | N = 12, women age 21–41 years, term maternal face of the placenta were extracted | H2O2 incubation for 4 h (200, 400, and 800 µm); incubated for 18 h with [3H]-pa Imitate | H202 | Genes and protein expression: CPT-1b and PPAR-α ATP production; FAO | N/A | ROS decreased placental FAO and ATP production can explain damaged placenta effectiveness which cause fetal growth restriction (FGR); However, acute ROS did not change the gene and protein expression | L: no gender difference, small sample size, no neurodevelopmental outcomes were assessed R: Future study focus on the larger sample size, mitochondrial function, and neurodevelopmental outcomes |
| Jones et al. [61] 2020, USA | To determine the association between the placental expression of all 13 protein-coding mitochondrial-encoded genes and seven key nuclear glycolysis- regulatory genes with IUGR | Case-control study | 50 IUGR and 100 control pregnancies, placenta samples | N/A | IUGR | 13 protein-coding mitochondrial-encoded genes and seven key nuclear glycolysis-regulatory genes, PDK1, 2, 3, 4, PKLR, PKM, OGT | N/A | MT-ND5, MT-ND6, and MT-ATP6 were negatively associated with IUGR, while one glycolysis-regulatory gene, PDK1 was positively associated with IUGR. mtDNA abundance and OS were positively associated with IUGR. | L: no direct mt function assessed; no gender difference R: Future study should focus on the gender difference and mitochondrial function assessment |
| Naha et al. [62] 2020, India | To examine the association between the placental mtDNA and IUGR condition | Case-control study | N = 67 pregnant women, n = 29 control, n = 18 IUGR, n = 20 SGA, Placenta samples | N/A | IUGR | mtDNAcn, Whole mt genome sequencing, proteins of NRF1 and SIRT3. Succinate dehydrogenase (SDH) staining | N/A | The increased mtDNAcn, decreased SIRT3 expression, decreased SDH activity were found in IUGR placenta tissue, which indicate the association between mt dysfunction and the fetal growth restriction | L: No gender, no neurodevelopmental outcomes were assessed R: Future study will focus on the intervention strategy based on the findings |
| Bart ha et al. [63] 2012, Spain | To investigate the role of fatty acid oxidation (FAO) in women with preeclampsia | Observational study | N = 28 (ctrl = 14 and preeclapsia = 14), pregnant women, placenta samples | N/A | FAO | Gene expression of LCHAD and MC AD; mt fatty acid oxidation rate; mitochondrial citrate synthase activity | N/A | Decreased LCHAD expression but not mitochondrial content related to the FAO deficits in women with preeclampsia, which support the role of FAO in the pathology of preeclampsia | L: No gender, small sample size, no neurodevelopmental outcomes measures R: Large sample size should be considered in the future |
| Garrabou et al. [56] 2016, Spain | To explore the role of CO-induced mt dysfunction in reduced birth weight in newborns tobacco exposure | Observational study | N = 51, mother-infant dyads (non-smoking Ctrl = 21, smoking subjects = 30), pregnant women, PBMCs and CBMCs | N/A | Carbon monoxide (CO) | Protein expression of COX; citrate synthase activity (mt content); VDAC protein expression (mt apoptotic analysis); TUN EL (apoptosis in placenta) | N/A | The correlation was found between the decreased birth weight, COX activity, increased mtDNA, ROS, apoptosis and the hx of smoking in pregnant women. Which suggests mitochondrial dysfunction and apoptosis may be the key in the reduced birth weight in smoking pregnant women | L: small sample size, no gender difference, no neurodevelopment outcomes was assessed R: Explore the relationship between mt mass and citrate synthase activity; enlarge the sample size to confirm the findings |
| Díaz et al. [64] 2014, Belgium | To assess the association between fetal growth restriction (FGR) and mt dysfunction | Observational study | N = 48 (n = 24 AGA and n = 24 SGA); mother-infant dyads, placenta samples | N/A | ROS | mtDNA content, SOD activity, citrate synthase activity | N/A | Decreased mtDNAcn and increased SOD activity were associated with FGR in placenta in SGA pregnancy | L: No genetic analysis, small sample size, no gender difference, no neurodevelopment outcomes assessment R: Larger sample size should be considered in the future |
| Mandò et al. [65] 2014, Italy | To compare the different mt content, function and respiration between IUGR and preeclampsia pregnancy | Observational study | N = 22 (8 IUGR, 6 preeclampsia, 8 Ctrl); pregnant women; placenta; cytotrop ho blast cells | N/A | ROS | mtDNA content; NRF1 and respiratory chain complexes (RCC) gene expression; RCC protein expression; RCC efficiency; O2 consump-tion | N/A | Mt content and NRF1 gene expression level were higher in placenta but reversed in the isolated cytotrophoblast cells in IUGR group compared with control group. Which suggested the placental cell linage dependent mt features. Increased placental O2 consumption may explain the limitation of O2 delivery to fetus. No gender difference was found in the findings | L: No gender difference, small sample size, no neurodevelopmental outcomes R: Larger sample size should be considered in the future |
| Minghetti, et al. [44] 2013, Italy | To evaluate ROS, antioxidant capacity in newborn twins | Observational study | 80 twin preterm infant (38 F, 42 M), >28 GW, umbilical cord blood sample | N/A | Premature and twins birth caused ROS | ROS bio marker 15-F2t-isoprostane and antioxidant capacity (tAOC) | N/A | Males was higher than female cohorts in 15-F2t-isoprostane but not in tAOC. Gender difference in vulnerability of ROS injury will explain the developmental gender disparity in later life | No neurodevelopmental outcomes, which should be considered in the future study |
| Negi et al. [45] 2012, India | To analyze the association between ROS and low birth weight (LBW) and prematurity | Observational study | 55 preterm infants (<37 GW, birth-weight <2,500g) and 24 full term controls (≥37 GW, birth-weight >2,500g), umbilical cord blood | N/A | ROS caused during pregnancy stress exposure | Oxidative DNA damage marker: 8-Hyd roxy-2-deoxy guanosine (8-OHdG) and antioxidant status marker: Malondialdehyde (MDA) | N/A | Increased level of 8-OHdG, MDA, and negative association between 8-OHdG, birthweight and GA were found in preterm infants | No neurodevelopmental outcomes, which should be considered in the future study |
| Bandyopadhyay et al. [57] 2017, India | To analyze the relationship between MSAF and ROS, birth weight (BW), gestational age and prematurity | Observational study | N = 109 [59 meconium- stained amniotic fluid (MSAF) and 50 without MSAF] | N/A | MSAF induced ROS | 8-OHdG, MDA | N/A | Infants with MSAF had higher level of ROS (increased 8-OHdG, MDA), which was significantly correlated with birth weight | No neurodevelopmental outcomes, gender difference, which should be considered in the future study |
| Negi et al. [45] 2012, India | To investigate the association between ROS | Observational study | N = 240 124 preterm infants (<37 weeks, birth weight <2,500 g), 116 full term infants, umbilical cord blood | N/A | Premature caused ROS | MDA, carbonyl proteins, total antioxidant capacity and vit A, E, and C | N/A | Increased ROS and decreased antioxidant capacity were observed in the low birth weight preterm infants | No neurodevelopmental outcomes, gender difference, which should be considered in the future study |
| Shoji et al. [43] 2014, Japan | To assess the association between ROS and neurodevelopment in preterm infants | Observational study | N = 35 preterm infants (F 19, M 16), birth weight <1,500 g, urine | N/A | Premature caused ROS | 8-OHdG, and 8-isoprostaglandin F2a (8-isoPGF) marker of lipid peroxidation (ELISA) | At 18 months’ corrected age, (1) Mental and psychomotor: Bayley Scale of Infant Development (BSID)-II; (2) Mental Developmental Index (MDI); (3) Psychomotor Development Index (PDI) | Significant association had been observed between urinary 8-OHdG and mental development rather than psycho motor development at 18 months’ corrected age in preterm infants | Larger sample size and gender difference should be considered in the future study |
| Slater et al. [47] 2012, USA | To examine the relationship between pain score and behavioral and ROS changes | Observational study | N = 80 preterm infants (<37 GW) (42 with tissue damage procedures [TDP], 38 without TDP), 0.8 mL blood from central catheter 30 min before and after TDP | N/A | Pain score measured using premature infant pain profile (PIPP) | ROS marker of MDA and uric acid (UA) | N/A | Positive association between pain scores and MDA, which indicate the relationship between pain and ROS in preterm infants | Gender difference should be considered in the future study |
| Norishadkam et al. [48] 2017, Iran | To test the level of ROS and DNA damage between preterm infants and term infants | Observational study | N = 50,25 preterm infant (Ml 3, FI 2) (<34 GW, birth weight <2,000 g), 25 term infant (>37 GW, birth weight >2,500 g), umbilical cord blood | N/A | ROS | Comet assay to measure tailed DNA percent, MDA, antioxidant capacity using 1,1-diphenyl-2picrylhydrazyl (DPPH), SOD activity, plasma catalase activity | N/A | Significantly increased primary DNA damage, plasma ROS, tail DNA percent and tail moment were found in preterm infants | Gender difference and neurodevelopmental outcomes should be considered in the future study |
| Perrone et al. [49] 2016, Italy | To test the association between placental lesion (chorioamnionitis [CA] or vascular underperfusion [VU]) and ROS | Observational study (prospective cohort study) | N = 120, mother infant dyads, preterm infants (<29 GW), placenta (HCA, VU, Ctrl) and umbilical cord blood | N/A | Placental lesion caused ROS | Isoprostanes (IsoPs), non-protein bound iron (NPBI), and advanced oxidative protein products (AOPP) | N/A | IsoPs, NPBI and AOPP were increased in CA and VU groups compared with Ctrl group. Which indicated that inflammation or impaired perfusion are associated with increased ROS. | Gender difference and neurodevelopmental outcomes should be considered in the future study |
| Musilova et al. [50] 2016, Czech Republic | To evaluate the association between ROS and preterm prelabor rupture of membranes (PPROM) | Observational study (prospective cohort study) | N = 165, singleton pregnancy women with PPROM, umbilical cord vein blood | N/A | PPROM with microbial invasion of the amniotic cavity (MlAC) and/or histological chorioamnionitis (HCA) caused ROS | Total antioxidant capacity (TAC), ferric reducing antioxidant power (FRAP), thiobarbituric acidreacting substances (TBARS), advanced glycation end products (AGEs) were measured | N/A | TAC, FRAP, TBARS, and AGEs were all found in PPROM sample (MlAC and HCA); The association between ROS markers and short-term neonatal morbidity, e.g., intra ventricular hemorrhage (TBARS) | Gender difference and neurodevelopmental outcomes should be considered in the future study |
The 28 prospective observational study and two experimental studies were included in the systematic review. The total of 2,812 infants (preterm infants were 980, 34.85%), 1,346 mothers and 391 adults were enrolled in these 30 studies. Sample size was varied in individual studies, ranged from 290 subjects [37] to 12 subjects [38]. All the 30 studies met first three requirements of quality appraisal check list (see online suppl. Table S1), which worth proceeding with the remaining items [36].
Infant Subjects and Biological Sampling
Of the publications identified, 11 studies focused on preterm infants. Some studies focused on identifying the indicators and consequences of mitochondrial dysfunction by assessing: (1) the association between hyperoxia and SIRT1/resveratrol signaling [39], (2) FMRP/mTOR signaling cascade and hypoxic-ischemic brain injury [40], (3) altered glutamate and glutamine and white matter injury (WMI) [41], (4) polymorphisms of antioxidant enzymes and ROS-induced complications [42], and (5) 8-OHdG and neurodevelopment outcomes [43]. Other studies explored the association between ROS and complications of antioxidant capacity, pain, low birth weight, DNA damage, placenta lesion, and preterm prelabor rupture of the membranes (PPROM) in preterm infants [44–50].
Biological samples collected in the studies included saliva [51], buccal swabs [51], cerebrospinal fluid (CSF) [52], peripheral blood mononuclear cells (PBMC) [39, 53–55], umbilical cord blood (UBMC) [44–46, 48–50, 52–57], whole blood [37, 42, 47], placenta [38, 58–66], brain [40], cytotrophoblast cells [65], urine [43], and fibroblast [66]. Placenta samples (9 studies) and umbilical cord blood (9 studies) were the most common sampling sources among the 30 studies (60%). Only four studies of preterm infants applied noninvasive tests (e.g., saliva, buccal swabs, urine, and magnetic resonance spectroscopy [MRS]) [41, 43, 51, 67].
Intervention on the Biological Samples
Two of the 30 studies tested an intervention on the biological samples collected. Thomas et al. [38] showed that hydrogen peroxide (H2O2) could induce ROS in placenta explants and decrease ATP and placental fatty acid oxidation (FAO). Resveratrol, a plant polyphenolic compound, was used in Yang’s study as a natural agonist of silent mating-type information regulation 2 homolog 1 (SIRT1) to inhibit the hyperoxia toxicity in cultured PBMC [39].
Stress Indicators and Measurements
Stress types in these 30 studies were ROS, pain, preterm birth, placental lesion, PPROM, and meconium-stained amniotic fluid, MCM, childhood trauma, maternal stress, IUGR, hypoxic ischemic, glutamate excitotoxicity, and PM2.5.
Hyperoxia-Associated ROS
Both in vitro and in vivo ROS were examined in Yang’s study: (1) preterm infants received the oxygen supplementation during their hospitalization and (2) the extracted PBMC from preterm infants was treated with different levels of oxygen [39]. In Giusti’s study, although there was no direct ROS assessment in preterm infants, the respiratory distress syndrome (RDS) diagnosis and the history of mechanical ventilation treatment indicating ROS overload [42]. Thomas et al. [38] also reported on ROS induced by H2O2 exposure using placenta samples. Bartha et al. [63] assessed the level of FAO in placentas, which was related to ROS-induced preeclampsia. Garrabou et al. [56] considered carbon monoxide, the byproduct of smoking, as a stress factor that could impact the pregnancy. The questionnaire of Fagerstrom test was administered to assess the smoking dependence in smoking [56]. Self-reported tobacco consumption together with cotinine levels in the plasma of pregnant women and infants and urine samples of mothers were evaluated to assess the reliability of the questionnaire [56].
Pain and Other Stress Associated with ROS
Only one paper in our review focused on the association between procedure pain and ROS [47]. The pain scores were assessed using the Premature Infant Pain Profile (PIPP) which included seven items of GA, behavioral state, heart rate, oxygen saturation, brow bulge, eye squeeze, and nasolabial furrow [47]. Tissue damaging procedures such as tape removal and indwelling central arterial or venous catheter were considered as pain stress which positively associated with ROS production [47].
Díaz et al. [64] focused on the small for GA-associated ROS production in infants. The association between ROS caused by preterm birth, twin birth, maternal stress, meconium-stained amniotic fluid, placenta lesion, PPROM, and infants’ growth were also the focus in our reviewed studies [43–46, 48–50, 57].
Hypoxic Ischemic
Hypoxic-ischemic encephalopathy (HIE) as a stress in term and preterm infants was examined in three studies [40, 52, 67]. The perinatal HIE is diagnosed if four signs are present: prenatal distress, immediate depression, metabolic acidosis, and early neonatal encephalopathy [52]. HIE was then categorized into three stages (mild, moderate, and severe) based on the severity of the symptoms [52].
Maternal Childhood Maltreatment and Childhood Trauma
Three studies focused on MCM. The participants from these studies were mothers within 1 week after parturition [53–55]. They were all assessed retrospectively using the German short version of the Childhood Trauma Questionnaire (CTQ) during the mothers’ psychodiagnostics interview. CTQ includes emotional, physical, and sexual abuse, as well as emotional and physical neglect subscales. Each subscale has 5 items and a total of 25 items with scores from 25 to 125. The CTQ score was documented as MCM load. Four groups from “none” to “severe” MCM experience were classified based on CTQ sum scores.
Maternal Stress
The pregnant mothers’ lifetime stress (previous 12 months) was assessed using Life Stressor Checklist-Revised (LSC-R) [58, 59], LSC-R included 30 traumatic events (e.g., sexual assault, abortion, interpersonal violence). Mothers’ prenatal negative life events (previous 6 months) were assessed using the Crisis in Family Systems-Revised survey which has 11 domains (e.g., income, job, relationships, safety, and housing). Further, the Diagnostic and Statistical Manual of Mental Disorder-posttraumatic stress disorder (PTSD) were used to assess the PTSD symptomatology.
In another study, self-administered questionnaires were administered at the 2nd and 3rd trimesters to assess maternal psychosocial stress in pregnancy (MPSP) [60]. MPSP questionnaires included the Perceived Stress Scale (PSS-14), the Pregnancy Related Anxieties Questionnaire (PRAQ)-Revised, the Perinatal PTSD Questionnaire (PPQ), and the State-Trait Anxiety Inventory for Adults (STAI).
Intrauterine Growth Restriction
Three studies introduced IUGR as a stress indicator. IUGR was identified if the fetal growth was under 5th percentile using longitudinal sonographic measurements [65]. IUGR was also identified once the ultrasound estimation of fetal weight was <10th percentile for its gestational age [61, 62].
PM2.5 and Glutamate Excitotoxicity
One study reported maternal PM2.5 exposure as a stress indicator. Brunst et al. [59] used an exposure model assessing temporally and spatially resolved PM2.5 to estimate PM2.5 level of the pregnant women. The daily PM2.5 was estimated based on subjects’ location and time at each address. Wisnowski’s team assessed the glutamate-induced excitotoxicity in neonatal WMI, grade I or II intraventricular hemorrhage, and cerebellar hemorrhage using magnetic resonance spectroscopy [41].
Mitochondrial Function and Genetic Variations
The genetic variations assessed in the reviewed 30 studies included polymorphisms of genes 5-HTTLPR, NR3C1, FKBP5, SOD1, SOD2, SOD3, and CAT, MT-ND6 methylation, and mtDNA copy number (mtDNAcn); protein levels of SIRT1, mTOR, total FMRP, phosphor FMRP, NRF1, SIRT3, COX, VDAC, respiratory chain complexes (RCC); gene expression of PGC-1 α; and all 13 protein-coding genes encoded by mitochondria, seven key nuclear glycolysis regulatory genes (PDK1, PDK2, PDK3, PDK4, PKLR, PKM, OGT), CPT-1b, PPAR-α, LCHAD, MCAD, NRF1, RCC. The major findings are shown below.
ROS and Mitochondrial Function
Findings from the reviewed studies showed that ROS overexposure-induced mitochondrial dysfunction may occur via the nitric oxide mediated injury pathway by inhibiting oxidation state of cytochrome-c-oxidase (oxCCO) activity in infants with HIE injury [67]. Decreased citrate synthase activity [68], mitochondrial intracellular density and respiration [51], FAO [38], and increased 8-Hydroxy-2-deoxy guanosine (8-OH-dG) [43, 45, 46, 57], Malondialdehyde (MDA) [47], and 15-F2t-isoprostane [44], were associated with ROS induced mitochondrial dysfunction. Increased Glutamine and decreased N-acetylaspartate (NAA) were found in parietal white matter in infants with WMI [41], Negative relationship between the activity of glutathione peroxidase (GPX) in CSF and the adverse neurological outcomes was found in preterm infants with severe HIE injuries [52]. Dose-response relationship increased the childhood maltreatment load with higher level of pro-inflammatory cytokines and ROS in mother’s PBMC [55].
Polymorphism and Mitochondrial Function
T allele in FKBP5 was significantly associated with higher cortisol level, while methylation levels of MT-ND6 was positively associated with adverse childhood experiences (ACE) [51]. rs8192287 SOD3 polymorphism is a protective factor for intraventricular hemorrhage (IVH) in preterm infants [42].
mtDNAcn and Mitochondrial Function
mtDNAcn was found to be negatively associated with the Edinburgh Postnatal Depression Scale (EPDS) and PM2.5 exposure [58, 59]. In contrast, mtDNAcn was found positively associated with early life adversity [37]. mtDNAcn, ROS and mitochondrial gene expression were all associated with pathology of IUGR [59]. The increased mtDNAcn, decreased SIRT3 protein expression, and decreased succinate dehydrogenase (SDH) activity indicated mitochondrial dysfunction and were associated with IUGR [62]. Decreased mtDNAcn and SOD activity were associated with FGR in the placenta for the small for GA pregnancy [64].
Genetic Signal Pathway and Mitochondrial Function
Hyperoxia or ROS could activate SIRT1 expression and activity and induce SIRT1 transportation from the nuclei of PBMC to the cytoplasm [39]. The maximum respiratory capacity was only modestly increased and no change was found in PGC-1α expression in mothers with MCM, which may not explain the intergenerational transmission of MCM [54]. FMRP/mTOR signal pathway was associated with hypoxic-ischemic encephalopathy [40]. Increased mitochondrial content and NRF1 gene expression level in IUGR placenta could explain fetal growth restriction caused by increased oxygen consumption in placenta and limited oxygen delivered to the fetus [65].
Biological Sex and Ethnicity Difference in Mitochondrial Function
Five studies identified differential stress levels and mitochondrial function by biological sex and ethnicity in their findings. Female infants were found to have a higher mitochondrial respiration rate than male cohorts, which was independent of MCM [53]. At 25–40 gestational weeks, PM2.5 exposure level was positively associated with placenta mtDNAcn in boys with high maternal trauma level, while negative associations were observed in girls with the low maternal trauma level [59]. Mandò also studied gender difference although no significant findings were reported [65]. High level of prenatal stress and psychological symptom scores and lower mtDNAcn as a marker of mitochondrial dysfunction, were found in non-white mothers, which may impact infants’ health outcomes [58]. Higher levels of 15-F2t-isoprostane were found in male preterm infants compared with female cohorts, which dependent of maternal stress exposure [44].
Neurodevelopmental Outcomes in Infants
Among 30 studies, 10 studies discussed the neurodevelopment outcomes in infants and adults with early life adversity. Measurements of Bayley scores, stress tests, anxiety tests, neurological symptoms, psychiatric diagnosis, encephalopathy diagnosis, temperament assessment were applied in these 10 studies.
Mitra et al. [67] assessed infants’ neurodevelopmental outcomes following HIE and found that the ratio of thalamic lactate/N-acetylasperate higher than 0.3 using the NICHD neonatal MRS brain injury scoring system between days 5 and 7 after birth was associated with poor neurodevelopmental outcomes. They also used the Bayley Scales of Infant Development-III to evaluate the neurodevelopmental outcomes in infants at 1 year of age [67]. Infants who scored less than 85 of Bayley were considered to have adverse outcomes [67]. Another study conducted neurodevelopmental assessment using the Mental Developmental Index (MDI) and the Psychomotor Development Index (PDI) of Bayley scale at 18 months’ corrected age in preterm infants with very low birth weight (<1,500 g) [43]. MDI lower than 80 was considered as subnormal outcomes [43].
Trier Social Stress Test was conducted in adult participants to assess the acute stress response to psychosocial stimulators [51]. Lifetime psychopathology was evaluated using the Structured Clinical Interview for DSM-IV (SCID), the Inventory for Depressive Symptoms-Self Report, the State-Trait Anxiety Inventory, and the Perceived Stress Scale [37]. Psychiatric symptom load was also measured using a HADS depression sum score and HADS anxiety sum score [55]. In one study, Infant Behavior Questionnaire Revised (IBQ-R) was given to mothers to rate infants’ temperament-related behaviors at 6 months of age [60]. Six constructs of activity level, smile and laughter, fear, distress to limitations, duration of orientation, and soothability were covered by IBQ-R. Denver Developmental Screening Test was used to assess the neurodevelopmental outcomes in term infants at 1-year corrected GA [52]. The infants were categorized into three groups (normal, motor developmental delay, and severe adverse outcome) based on the Denver Developmental Screening Test score [52].
Stress, Mitochondrial Function/Dysfunction, and Neurodevelopmental Outcomes
Findings from ten studies reported a significant association between prenatal and postnatal stress and neurodevelopmental outcomes, and found this relationship was mediated by mitochondrial function/dysfunction. MPSP-induced mitochondrial dysfunction was associated with infant temperament changes at 6 months of age [60]. oxCCO-MABP semblance at 48 after birth could be used to predict HIE-associated neurodevelopmental deficits at 1 year of age [67]. Neonatal hypoxic-ischemic brain damage and mitochondrial ROS overproduction were associated with neurodevelopmental outcomes at 12 months after birth [52]. Abnormal lipid peroxidation was associated with developmental deficits in preterm infants at 18 months [43]. Further, stress-induced mitochondrial genetic variations lead to WMI and encephalopathy of prematurity in preterm infants [40, 41]. In adults, mitochondrial dysfunction played a role in (1) MCM-associated stress and anxiety, (2) parental loss and childhood trauma-associated psychiatric symptoms, and (3) stress-associated adult females with ACE [37, 51, 55].
Discussion
To the best of our knowledge, this is the first systematic review to explore the relationship between prenatal/postnatal stress, mitochondrial function/dysfunction, and neurodevelopmental outcomes in infants. Findings from the review support our proposed conceptual framework which explains the association between early life stress-associated mitochondrial function/dysfunction and neurodevelopmental outcomes in term and preterm infants. The findings suggest that mitochondrial function/dysfunction and relative genetic variations might interface between the association of early life stress and neurodevelopmental outcomes in infants. This result warrants early stress intervention at birth, as this stress experience during their early life might have life-long consequences for neurodevelopmental and behavioral health.
Stress and Mitochondrial Function/Dysfunction
Accumulative evidence has shown the association between stress and mitochondrial function/structure changes. Stress at a moderate level will initiate the prosurvival process: increased energy demand, promote mitochondrial biogenesis, stabilize cellular response, and improve immune function [68]. However, stress overload will produce more ROS, damage the mitochondrial function, and decrease ATP production [69]. The imbalance between ROS and antioxidant ability caused by stress overload are more common in preterm infants due to their developmental vulnerability and unmature antioxidant system compared with term infants [70].
In this review, evidence was found to support the notion that both prenatal and postnatal stress influence infants’ mitochondrial function/dysfunction. Prenatal stress such as MCM as a stress indicator is associated with increased ROS production, and decreased mitochondrial function in mothers [55], and was intergenerationally transmitted to their infants [53, 60, 71]. These intergenerational transmission effects of MCM were also found to play a role in infants’ physical and mental health [72, 73]. Although mitochondrial bioenergetic cross-generational transmission mechanisms are still underdeveloped, MCM-related epigenetic alternations have been documented during the embryo period through genetic maternally inherit (DNA, RNA, and protein regulation) [71]. IUGR is another example of prenatal stress which induces maternal phenotype and genotype intergenerational transmission [74]. IUGR is associated with increased ROS production, mtDNA abundance, and antioxidants consumption in the placenta, which leads to the decreased oxygen and antioxidants deliver to the fetus [61], resulting in infants’ low birth weight, complications of metabolic syndrome, and pulmonary arterial hypertension.
During the postnatal period, the preterm infant is exposed to pain stimulation, hypoxic ischemic, and hyperoxia, which all lead to ROS overproduction and mitochondrial dysfunction because of an immature antioxidant system [43]. Although procedure pain is very common in preterm infants during their NICU stay, only one study reported the association between procedure pain and mitochondrial function. Mitochondrial function such as ATP production, ROS production, mitochondrial permeability transition pore (MPTP), apoptotic pathways, and intracellular calcium mobilization play a role in the pathology of procedure pain [47, 75, 76]. Normal mitochondria produce a small but stable amount of ROS leak along the electron transport chain sustaining the redox state [77]. Alan and Isha stated that the mismatch of oxygen supply and demand will damage cellular function and mitochondrial function [78]. Long episode of hypoxic ischemia increases electron leakage, increases mitochondrial ROS production, and impairs efficacy of the antioxidant system causing the mitochondrial dysfunction [79]. Hyperoxia exposure such as oxygen therapy is standard procedure in preterm infants to sustain normal heart rate and blood oxygen level [80]. However, an excess supply of oxygen to infants will cause excessive ROS and lead to multiple organs (lung, central nervous system, and retina) injury [81].
Mitochondrial Function/Dysfunction and Neurodevelopmental Outcomes
High energy demand of brain (20% of the body energy demand) requests high concentration of mitochondria [82]. In response to stress, mitochondria adjust their activity to meet the energetic demand. In our review, mitochondrial dysfunction and associated ROS have been increasingly recognized as the causes of WMI and hypoxic ischemic brain injury or abnormality although the pathological mechanisms are still unknown [41, 67]. Preclinical study showed that hypoxic ischemia induced mitochondrial fission, fragmentation, and loss of motility in myelinated axons [83]. This was consistent with our findings that mTOR signal pathway was inhibited in infants with encephalopathy which attenuate the normal mitochondrial energy production [40, 84]. Additional, mitochondrial respiration and density were increased in neonates and their mothers with MCM [53]. Mitochondrial function mediated impact of MCM in pregnancy on infant temperament development [60].
Our study found many genes, proteins, and metabolites were associated with mitochondrial function/dysfunction and could be used as biomarkers to predict neurodevelopmental outcomes in infants’ later life. However, there is a lack of longitudinal data to support these findings. Further, the signal pathways related to mitochondrial dysfunction, such as p38 MAPK, AKT/mTOR, SIRT1/Nrf2, SIRT1/p53, SIRT1/PGC-1a, AKT/SIRT3/SOD2 signaling, were not found in our reviews but were reported in the preclinical studies, which needs to be addressed in the future study design. No study in our review examined the posttranslational protein modification, such as phosphorylation, acetylation, and O-GlcNAcylation of proteins related to mitochondrial function/dysfunction in different stress levels in term and preterm infants.
Most studies in our review conducted invasive sampling using placenta, PBMC, UBMC, brain, CSF, etc. However, a few publications applied noninvasive sampling of saliva, buccal swabs, urine, and the MRS to assess the infants’ and adults’ mitochondrial function and brain development. It is urgent to verify the highly sensitive and accurate noninvasive sampling to assess mitochondrial function and predict neurodevelopmental outcomes in infants’ early and later life, avoiding any additional stress [85–87]. These findings point out the new directions for future clinical study focused on the association between stress-induced mitochondrial dysfunction and neurodevelopmental deficits in infants.
The lack of standardized infant developmental scales makes it more difficult to identify and compare neurodevelopmental deficits and behavioral changes in different age groups [88]. The time to initiate Bayley scale is another concern. In our review, two studies conducted the Bayley test; however, they applied this scale at different time points: one was at 1 year and another was at 18 months’ corrected age [43, 67]. Although the two time points described above are both within the scale range, the author should assess the infants’ stability, not just the age. Low correlation between Bayley scores and other scores were reported [89]. To confirm the validity and reliability, future study should consider choosing one standard scale at specified time range or using more than one scale to measure the neurodevelopmental outcomes [88].
CTQ was commonly used to measure childhood trauma and satisfactory validity [90]. The validity of IBQ-R was still the concern when it was applied in diverse samples [91]. For questionnaires, bias may exist in maternal recall behavior (e.g., childhood trauma) or parents-reported child temperament (e.g., IBQ-R) because of maternal psychopathology [92].
Stress, Mitochondrial Function/Dysfunction, and Neurodevelopmental Outcomes
Mitochondria play a central role at the interface between prenatal and postnatal stress and the neurodevelopmental deficits in infants’ early and later life. In our review, the stress indicators varied from prenatal stress, such as maternal stress, childhood maltreatment, IUGR to postnatal stress, to infant exposure to pain, hypo-, or hyperoxia stressors. Besides prenatal mitochondrial energetic transgenerational transmission we discussed above, during the postnatal period, impaired maternal caregiving capacity [93], poor bonding, and MCM associated depression [94] may be the major causes of developmental deficit in infants. Animal experiments showed that maternal separation in early life caused cognitive dysfunction that could persist in adolescence [95]. IUGR associated blood flow and nutrients delivery shortages further damaged fetal growth. In human IUGR and animal models, WMI is the most common neurodevelopmental deficit caused by placental insufficiency [96–98]. IUGR induced fetal hypoxia is another common clinical issue that increases the risk of neurodevelopmental deficit in the infant. Animal models pointed out that early chronic intermittent hypoxia exposure plays a role in infant WMI [99]. Although preterm infants have immature lung and antioxidant systems, they often require high oxygen concentrations (90–100%) during their NICU stay [99]. Both human and animal studies showed that hyperoxia exposure at the 2nd to 14th postnatal days put premature infants at high risk of neurodevelopmental deficit and other comorbidities such as retinopathy of prematurity and bronchopulmonary dysplasia [100–102].
Although the physiological or psychosociological difference exists between prenatal and postnatal stress factors, the mechanisms and genetic variations associated with mitochondrial function were all connected with shaping the brain structure and function. Gluckman and Hanson called this process “developmental programming” [103]. Although the mechanisms of association among stress, mitochondrial function/dysfunction, and neurodevelopmental outcomes were still unclear. The younger the GA and the smaller the birth weight are, the more sensitive the infants’ brain response to environmental changes is [87], and the more adverse consequence of neurodevelopment it is throughout the entire lifespan.
The present review included studies suggesting that mitochondrial function/dysfunction mediates the impact of prenatal and postnatal stress on neurodevelopmental outcomes in infants. Evidence from animal studies strongly demonstrates that stress impaired neurodevelopment through mitochondrial dysfunction and changed gene expression [92]. However, it remains challenging to identify the association among stress, mitochondrial dysfunction, and neurodevelopmental deficits in clinical studies due to translational failure from preclinical results to the current clinical settings [92].
Limitation of Current State of Scientific Knowledge
There has been a growing interest in studying the role played by sex difference in brain programming through regulating mitochondrial function. However, sex disparity in stress load, mitochondrial function/dysfunction, and neurodevelopmental outcomes in early life has received limited attention (5 out of 30 studies). The ethical and practical difficulties hinder the researchers from conducting the clinical trial in preterm infants. A limited number of studies were conducted on preterm infants (11 of the 30 studies). It is urgent to be aware of the challenges and the clinical needs of infants. The lack of standardized developmental scales of infants and longitudinal studies need to be addressed in future studies. Future studies are needed to identify the longitudinal impact of stress-induced mitochondrial dysfunction on neurodevelopmental outcomes in infants. Another major concern was that only five out of 30 studies conducted noninvasive sampling, which avoids extra stress. Limited early stress intervention during NICU stay and noninvasive sampling warrants more attention to prevent stress-influenced brain impairment.
Conclusions
Mitochondrial function/dysfunction was at the interface between stress and neurodevelopmental deficits in infants and understanding this association might provide alternative strategies to manage the stress-induced neurodevelopmental deficit. Prenatal intrauterine and postnatal NICU hospitalizations are critical periods for the brain development of preterm infants. Early GA and low birth weight increase the risk of neonatal neurodevelopmental deficits upon exposure to prenatal and postnatal stress. To prevent mitochondrial bioenergetic intergenerational transmission in infants, psychological assessment and social support should be provided to mothers with MCM and further research on effective interventions is warranted. Additionally, individualized care should be applied to diminish biological sex disparities in infants.
Supplementary Material
Funding Sources
This study was supported by the following grant. A grant from the NIH/NINR NR016928 (PI: Xiaomei Cong). Tingting Zhao received grant from: NIH/NINR F31NR019940 (PI); Eastern Nursing Research Society (ENRS)/Council for the Advancement of Nursing Science Dissertation Award (PI); Sigma Theta Tau International (STTI) Mu Chapter Research Grant (PI); and UConn CT Institute for the Brain and Cognitive Sciences (IBACS) Graduate Fellowship (PI).
Footnotes
Conflict of Interest Statement
There are not conflicts of interest of funding for each author. There are no declared conflicts of other interests for any of this study’s authors.
References
- 1.Lee K, Cascella M, Marwaha R. Intellectual disability. In StatPearls. StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 2.WHO. Preterm birth. 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/preterm-birth.
- 3.Aita M, De Clifford Faugère G, Lavallée A, Feeley N, Stremler R, Rioux É, et al. Effectiveness of interventions on early neurodevelopment of preterm infants: a systematic review and meta-analysis. BMC Pediatr. 2021;21(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JS, Davis RL, Sue CM. Mitochondrial dysfunction in parkinson’s disease: new mechanistic insights and therapeutic perspectives. Curr Neurol Neurosci Rep. 2018;18(5):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cenini G, Voos W. Mitochondria as potential targets in alzheimer disease therapy: an update. Front pharmacol. 2019;10:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai CQ, Guo Y, Chu XY. Neuropathic pain: the dysfunction of Drp1, mitochondria, and ROS homeostasis. Neurotox Res. 2020;38(3):553–63. [DOI] [PubMed] [Google Scholar]
- 7.Sui BD, Xu TQ, Liu JW, Wei W, Zheng CX, Guo BL, et al. Understanding the role of mitochondria in the pathogenesis of chronic pain. Postgrad Med J. 2013;89(1058):709–14. [DOI] [PubMed] [Google Scholar]
- 8.English K, Barton MC. HDAC6: a key link between mitochondria and development of peripheral neuropathy. Front Mol Neurosci. 2021;14:684714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Child maltreatment. 2016. Available from: https://www.who.int/news-room/fact-sheets/detail/child-maltreatment.
- 10.Lehnig F, Nagl M, Stepan H, Wagner B, Kersting A. Associations of postpartum mother-infant bonding with maternal childhood maltreatment and postpartum mental health: a cross-sectional study. BMC Pregnancy Child-birth. 2019;19(1):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyberg R, Boos R, Babajan A, Ertan AK, Schmidt W. [Intrauterine growth retardation-perinatal mortality and postnatal morbidity in a perinatal center]. Z Geburtshilfe Neonatol. 2000;204(6):218–23. [DOI] [PubMed] [Google Scholar]
- 12.Tolcos M, Petratos S, Hirst JJ, Wong F, Spencer SJ, Azhan A, et al. Blocked, delayed, or obstructed: what causes poor white matter development in intrauterine growth restricted infants? Prog Neurobio. 2017;154:62–77. [DOI] [PubMed] [Google Scholar]
- 13.Zitkovsky EK, Daniels TE, Tyrka AR. Mitochondria and early-life adversity. Mitochondrion. 2021; 57:213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bronson SL, Bale TL. The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology. 2016; 41(1):207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lester BM, Marsit CJ. Epigenetic mechanisms in the placenta related to infant neurodevelopment. Epigenomics. 2018; 10(3):321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sosunov SA, Niatsetskaya ZV, Stepanova AA, Galkin AS, Juliano CE, Ratner VI, et al. Developmental window of vulnerability to white matter injury driven by sublethal intermittent hypoxemia. Pediatr Res. 2022; 91(6):1383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McArdle CB, Richardson CJ, Nicholas DA, Mirfakhraee M, Hayden CK, Amparo EG. Developmental features of the neonatal brain: MR imaging. Part I. Gray-white matter differentiation and myelination. Radiology. 1987; 162(1 Pt 1):223–9. [DOI] [PubMed] [Google Scholar]
- 18.Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003; 143(2):171–9. [DOI] [PubMed] [Google Scholar]
- 19.Ten VS. Mitochondrial dysfunction in alveolar and white matter developmental failure in premature infants. Pediatr Res. 2017; 81(2):286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cong X, Wu J, Vittner D, Xu W, Hussain N, Galvin S, et al. The impact of cumulative pain/stress on neurobehavioral development of preterm infants in the NICU. Early Hum Dev. 2017; 108:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranger M, Grunau RE. Early repetitive pain in preterm infants in relation to the developing brain. Pain Manag. 2014; 4(1):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arpi E, Ferrari F. Preterm birth and behaviour problems in infants and preschool-age children: a review of the recent literature. Dev Med Child Neurol. 2013; 55(9):788–96. [DOI] [PubMed] [Google Scholar]
- 23.Victoria NC, Murphy AZ. The long-term impact of early life pain on adult responses to anxiety and stress: historical perspectives and empirical evidence. Exp Neurol. 2016; 275 Pt 2(Pt 2):261–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picard M, McEwen BS. Psychological stress and mitochondria: a systematic review. Psychosom Med. 2018; 80(2):141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012; 148(6):1145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaughnessy DT, McAllister K, Worth L, Haugen AC, Meyer JN, Domann FE, et al. Mitochondria, energetics, epigenetics, and cellular responses to stress. Environ Health Perspect. 2014; 122(12):1271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann A, Spengler D. The mitochondrion as potential interface in early-life stress brain programming. Front Behav Neurosci. 2018;12: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders: a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis. 2017; 1863(5):1066–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. 2007;83(1): 84–92. [DOI] [PubMed] [Google Scholar]
- 30.Nicolson GL. Mitochondrial dysfunction and chronic disease: treatment with natural supplements. Integr Med. 2014;13(4):35–43. [PMC free article] [PubMed] [Google Scholar]
- 31.Johri A, Beal MF. Antioxidants in Huntington’s disease. Biochim Biophys Acta. 2012; 1822(5):664–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witte ME, Mahad DJ, Lassmann H, van Horssen J. Mitochondrial dysfunction contributes to neurodegeneration in multiple sclerosis. Trends Mol Med. 2014; 20(3):179–87. [DOI] [PubMed] [Google Scholar]
- 33.Antinone SE, Ghadge GD, Lam TT, Wang L, Roos RP, Green WN. Palmitoylation of superoxide dismutase 1 (SOD1) is increased for familial amyotrophic lateral sclerosis-linked SOD1 mutants. J Biol Chem. 2013;288(30):21606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris JD, Quatman CE, Manring MM, Siston RA, Flanigan DC. How to write a systematic review. Am J Sports Med. 2014;42(11):2761–8. [DOI] [PubMed] [Google Scholar]
- 36.Critical Appraisal Skills Programme. CASP (Case Control Study) Checklist. 2018. Available from: https://casp-uk.b-cdn.net/wp-content/uploads/2018/03/CASP-Case-Control-Study-Checklist-2018_fillable_form.pdf.
- 37.Tyrka AR, Parade SH, Price LH, Kao HT, Porton B, Philip NS, et al. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biol Psychiatry. 2016;79(2):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas MM, Haghiac M, Grozav C, Minium J, Calabuig-Navarro V, O’Tierney-Ginn P. Oxidative stress impairs fatty acid oxidation and mitochondrial function in the term placenta. Reprod Sci. 2019;26(7):972–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X, Dong WB, Lei XP, Li QP, Zhang LY, Zhang LP. Resveratrol suppresses hyperoxiainduced nucleocytoplasmic shuttling of SIRT1 and ROS production in PBMC from preterm infants in vitro. J Matern Fetal Neonatal Med. 2018;31(9):1142–50. [DOI] [PubMed] [Google Scholar]
- 40.Lechpammer M, Wintermark P, Merry KM, Jackson MC, Jantzie LL, Jensen FE. Dysregulation of FMRP/mTOR signaling cascade in hypoxic-ischemic injury of premature human brain. J Child Neurol. 2016;31(4):426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wisnowski JL, Blüml S, Paquette L, Zelinski E, Nelson MD Jr, Painter MJ, et al. Altered glutamatergic metabolism associated with punctate white matter lesions in preterm infants. PloS one. 2013;8(2):e56880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giusti B, Vestrini A, Poggi C, Magi A, Pasquini E, Abbate R, et al. Genetic polymorphisms of antioxidant enzymes as risk factors for oxidative stress-associated complications in preterm infants. Free Radic Res. 2012;46(9):1130–9. [DOI] [PubMed] [Google Scholar]
- 43.Shoji H, Ikeda N, Hosozawa M, Ohkawa N, Matsunaga N, Suganuma H, et al. Oxidative stress early in infancy and neurodevelopmental outcome in very low-birthweight infants. Pediatr Int. 2014;56(5):709–13. [DOI] [PubMed] [Google Scholar]
- 44.Minghetti L, Greco A, Zanardo V, Suppiej A. Early-life sex-dependent vulnerability to oxidative stress: the natural twining model. J Matern Fetal Neonatal Med. 2013;26(3):259–62. [DOI] [PubMed] [Google Scholar]
- 45.Negi R, Pande D, Kumar A, Khanna RS, Khanna HD. Evaluation of biomarkers of oxidative stress and antioxidant capacity in the cord blood of preterm low birth weight neonates. J Matern Fetal Neonatal Med. 2012;25(8):1338–41. [DOI] [PubMed] [Google Scholar]
- 46.Negi R, Pande D, Kumar A, Khanna RS, Khanna HD. In vivo oxidative DNA damage and lipid peroxidation as a biomarker of oxidative stress in preterm low-birthweight infants. J Trop Pediatr. 2012;58(4):326–8. [DOI] [PubMed] [Google Scholar]
- 47.Slater L, Asmerom Y, Boskovic DS, Bahjri K, Plank MS, Angeles KR, et al. Procedural pain and oxidative stress in premature neonates. J Pain. 2012;13(6):590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norishadkam M, Andishmand S, Zavar Reza J, Zare Sakhvidi MJ, Hachesoo VR. Oxidative stress and DNA damage in the cord blood of preterm infants. Mutat Res. 2017;824:20–4. [DOI] [PubMed] [Google Scholar]
- 49.Perrone S, Tataranno ML, Negro S, Longini M, Toti MS, Alagna MG, et al. Placental histological examination and the relationship with oxidative stress in preterm infants. Placenta. 2016;46:72–8. [DOI] [PubMed] [Google Scholar]
- 50.Musilova I, Tothova L, Menon R, Vlkova B, Celec P, Hornychova H, et al. Umbilical cord blood markers of oxidative stress in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2016;29(12):1900–10. [DOI] [PubMed] [Google Scholar]
- 51.Lapp HE, Ahmed S, Moore CL, Hunter RG. Toxic stress history and hypothalamic-pituitary-adrenal axis function in a social stress task: genetic and epigenetic factors. Neurotoxicol Teratol. 2019;71:41–9. [DOI] [PubMed] [Google Scholar]
- 52.Vasiljevic B, Maglajlic-Djukic S, Gojnic M, Stankovic S, Ignjatovic S, Lutovac D. New insights into the pathogenesis of perinatal hypoxic-ischemic brain injury. Pediatr Int. 2011;53(4):454–62. [DOI] [PubMed] [Google Scholar]
- 53.Gumpp AM, Boeck C, Behnke A, Bach AM, Ramo-Fernández L, Welz T, et al. Childhood maltreatment is associated with changes in mitochondrial bioenergetics in maternal, but not in neonatal immune cells. Proc Natl Acad Sci U S A. 2020;117(40):24778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boeck C, Gumpp AM, Calzia E, Radermacher P, Waller C, Karabatsiakis A, et al. The association between cortisol, oxytocin, and immune cell mitochondrial oxygen consumption in postpartum women with childhood maltreatment. Psychoneuroendocrinology. 2018;96:69–77. [DOI] [PubMed] [Google Scholar]
- 55.Boeck C, Koenig AM, Schury K, Geiger ML, Karabatsiakis A, Wilker S, et al. Inflammation in adult women with a history of child maltreatment: the involvement of mitochondrial alterations and oxidative stress. Mitochondrion. 2016;30:197–207. [DOI] [PubMed] [Google Scholar]
- 56.Garrabou G, Hernàndez AS, Catalán García M, Morén C, Tobías E, Córdoba S, et al. Molecular basis of reduced birth weight in smoking pregnant women: mitochondrial dysfunction and apoptosis. Addict Biol. 2016;21(1):159–70. [DOI] [PubMed] [Google Scholar]
- 57.Bandyopadhyay T, Bhatia BD, Khanna HD. A study of oxidative stress in neonates delivered through meconium-stained amniotic fluid. Eur J Pediatr. 2017;176(3):317–25. [DOI] [PubMed] [Google Scholar]
- 58.Brunst KJ, Sanchez Guerra M, Gennings C, Hacker M, Jara C, Bosquet Enlow M, et al. Maternal lifetime stress and prenatal psychological functioning and decreased placental mitochondrial DNA copy number in the PRISM study. Am J Epidemiol. 2017;186(11):1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brunst KJ, Sanchez-Guerra M, Chiu YHM, Wilson A, Coull BA, Kloog I, et al. Prenatal particulate matter exposure and mitochondrial dysfunction at the maternal-fetal interface: effect modification by maternal lifetime trauma and child sex. Environ Int. 2018;112:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lambertini L, Chen J, Nomura Y. Mitochondrial gene expression profiles are associated with maternal psychosocial stress in pregnancy and infant temperament. PloS one. 2015;10(9):e0138929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones R, Peña J, Mystal E, Marsit C, Lee MJ, Stone J, et al. Mitochondrial and glycolysis-regulatory gene expression profiles are associated with intrauterine growth restriction. J Matern Fetal Neonatal Med. 2020;33(8):1336–45. [DOI] [PubMed] [Google Scholar]
- 62.Naha R, Anees A, Chakrabarty S, Naik PS, Pandove M, Pandey D, et al. Placental mitochondrial DNA mutations and copy numbers in intrauterine growth restricted (IUGR) pregnancy. Mitochondrion. 2020;55:85–94. [DOI] [PubMed] [Google Scholar]
- 63.Bartha JL, Visiedo F, Fernández-Deudero A, Bugatto F, Perdomo G. Decreased mitochondrial fatty acid oxidation in placentas from women with preeclampsia. Placenta. 2012;33(2):132–4. [DOI] [PubMed] [Google Scholar]
- 64.Díaz M, Aragonés G, Sánchez-Infantes D, Bassols J, Pérez-Cruz M, de Zegher F, et al. Mitochondrial DNA in placenta: associations with fetal growth and superoxide dismutase activity. Horm Res Paediatr. 2014;82(5):303–9. [DOI] [PubMed] [Google Scholar]
- 65.Mandò C, De Palma C, Stampalija T, Anelli GM, Figus M, Novielli C, et al. Placental mitochondrial content and function in intrauterine growth restriction and preeclampsia. Am J Physiol Endocrinol Metab. 2014; 306(4):E404–13. [DOI] [PubMed] [Google Scholar]
- 66.Paquay S, Bourillon A, Pichard S, Benoist JF, de Lonlay P, Dobbelaere D, et al. Mitochondrial acetoacetyl-CoA thiolase deficiency: basal ganglia impairment may occur independently of ketoacidosis. J Inherit Metab Dis. 2017;40(3):415–22. [DOI] [PubMed] [Google Scholar]
- 67.Mitra S, Bale G, Highton D, Gunny R, Uria-Avellanal C, Bainbridge A, et al. Pressure passivity of cerebral mitochondrial metabolism is associated with poor outcome following perinatal hypoxic ischemic brain injury. J Cereb Blood Flow Metab. 2019; 39(1):118–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khaitan D, Dwarakanath BS. Endogenous and induced oxidative stress in multi-cellular tumour spheroids: implications for improving tumour therapy. Indian J Biochem Biophys. 2009;46(1):16–24. [PubMed] [Google Scholar]
- 69.Ozsurekci Y, Aykac K. Oxidative stress related diseases in newborns. Oxid Med Cell Longev. 2016;2016:2768365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee JW, Davis JM. Future applications of antioxidants in premature infants. Curr Opin Pediatr. 2011;23(2):161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buss C, Entringer S, Moog NK, Toepfer P, Fair DA, Simhan HN, et al. Intergenerational transmission of maternal childhood maltreatment exposure: implications for fetal brain development. J Am Acad Chid Adolesc Psychiatry. 2017;56(5):373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith MV, Gotman N, Yonkers KA. Early childhood adversity and pregnancy outcomes. Matern Child Health J. 2016;20(4):790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flory JD, Bierer LM, Yehuda R. Maternal exposure to the holocaust and health complaints in offspring. Dis Markers. 2011;30(2–3):133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Z, Luo X, Lv Y, Yan L, Xu S, Wang Y, et al. Intrauterine growth restriction programs intergenerational transmission of pulmonary arterial hypertension and endothelial dysfunction via sperm epigenetic modifications. Hypertension. 2019;74(5):1160–71. [DOI] [PubMed] [Google Scholar]
- 75.Sui BD, Xu TQ, Liu JW, Wei W, Zheng CX, Guo BL, et al. Understanding the role of mitochondria in the pathogenesis of chronic pain. Postgrad Med J. 2013;89(1058):709–14. [DOI] [PubMed] [Google Scholar]
- 76.Kim WS, Park JY, Kim TK, Baik SW. The changes of mitochondrial cytochrome c and GABAergic neuron in neuropathic pain model. Korean J Anesthesiol. 2012;62(4):365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014; 94(3):909–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baik AH, Jain IH. Turning the oxygen dial: balancing the highs and lows. Trends Cell Biol. 2020;30(7):516–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jassem W, Heaton ND. The role of mitochondria in ischemia/reperfusion injury in organ transplantation. Kidney Int. 2004;66(2):514–7. [DOI] [PubMed] [Google Scholar]
- 80.Torres-Cuevas I, Parra-Llorca A, Sánchez-Il-lana A, Nuñez-Ramiro A, Kuligowski J, Cháfer-Pericás C, et al. Oxygen and oxidative stress in the perinatal period. Redox Biol. 2017;12:674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao HW, Ali SS, Haddad GG. Does hyperoxia selection cause adaptive alterations of mitochondrial electron transport chain activity leading to a reduction of superoxide production? Antioxid Redox Signal. 2012;16(10):1071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86(4):883–901. [DOI] [PubMed] [Google Scholar]
- 83.Bastian C, Politano S, Day J, McCray A, Brunet S, Baltan S. Mitochondrial dynamics and preconditioning in white matter. Conditioning Med. 2018;1(2):64–72. [PMC free article] [PubMed] [Google Scholar]
- 84.Morita M, Gravel SP, Hulea L, Larsson O, Pollak M, St-Pierre J, et al. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell cycle. 2015;14(4):473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.D’Agata AL, Roberts MB, Ashmeade T, Dutra SVO, Kane B, Groer MW. Novel method of measuring chronic stress for preterm infants: skin cortisol. Psychoneuroendocrinology. 2019; 102:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wallin DJ, Zamora TG, Alexander M, Ennis KM, Tran PV, Georgieff MK. Neonatal mouse hippocampus: phlebotomy-induced anemia diminishes and treatment with erythropoietin partially rescues mammalian target of rapamycin signaling. Pediatr Res. 2017;82(3):501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peña-Bautista C, Durand T, Vigor C, Oger C, Galano JM, Cháfer-Pericás C. Non-invasive assessment of oxidative stress in preterm infants. Free Radic Biol Med. 2019;142:73–81. [DOI] [PubMed] [Google Scholar]
- 88.Bell MA, Ross AP, Goodman G. Assessing infant cognitive development after prenatal iodine supplementation. Am J Clin Nutr. 2016; 104(Suppl 3):928S–34S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Connolly BH, Dalton L, Smith JB, Lamberth NG, McCay B, Murphy W. Concurrent validity of the Bayley Scales of Infant Development II (BSID-II) Motor Scale and the Peabody Developmental Motor Scale II (PDMS-2) in 12-month-old infants. Pediatr Phys Ther. 2006;18(3):190–6. [DOI] [PubMed] [Google Scholar]
- 90.Aloba O, Opakunle T, Ogunrinu O. Childhood Trauma Questionnaire-Short Form (CTQ-SF): dimensionality, validity, reliability and gender invariance among Nigerian adolescents. Child Abuse Negl. 2020;101:104357. [DOI] [PubMed] [Google Scholar]
- 91.Peterson ER, Waldie KE, Mohal J, Reese E, Atatoa Carr PE, Grant CC, et al. Infant behavior questionnaire-revised very short form: a new factor structure’s associations with parenting perceptions and child language outcomes. J Pers Assess. 2017;99(6):561–73. [DOI] [PubMed] [Google Scholar]
- 92.Olino TM, Guerra-Guzman K, Hayden EP, Klein DN. Evaluating maternal psychopathology biases in reports of child temperament: an investigation of measurement invariance. Psycho Assess. 2020;32(11):1037–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bosquet Enlow M, Englund MM, Egeland B. Maternal childhood maltreatment history and child mental health: mechanisms in intergenerational effects. J Clin Child Adolesc Psychol. 2018;47(Suppl 1):S47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alto ME, Warmingham JM, Handley ED, Rogosch F, Cicchetti D, Toth SL. Developmental pathways from maternal history of childhood maltreatment and maternal depression to toddler attachment and early childhood behavioral outcomes. Attach Hum Dev. 2021;23(3):328–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.do Prado CH, Narahari T, Holland FH, Lee HN, Murthy SK, Brenhouse HC. Effects of early adolescent environmental enrichment on cognitive dysfunction, prefrontal cortex development, and inflammatory cytokines after early life stress. Dev Psychobiol. 2016;58(4):482–91. [DOI] [PubMed] [Google Scholar]
- 96.Chang JL, Bashir M, Santiago C, Farrow K, Fung C, Brown AS, et al. Intrauterine growth restriction and hyperoxia as a cause of white matter injury. Dev Neurosci. 2018;40(4):344–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wixey JA, Sukumar KR, Pretorius R, Lee KM, Colditz PB, Bjorkman ST, et al. Ibuprofen treatment reduces the neuroinflammatory response and associated neuronal and white matter impairment in the growth restricted newborn. Front Physiol. 2019;10:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ross MM, Cherkerzian S, Mikulis ND, Turner D, Robinson J, Inder TE, et al. A randomized controlled trial investigating the impact of maternal dietary supplementation with pomegranate juice on brain injury in infants with IUGR. Sci Rep. 2021;11(1):3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sosunov SA, Niatsetskaya ZV, Stepanova AA, Galkin AS, Juliano CE, Ratner VI, et al. Developmental window of vulnerability to white matter injury driven by sublethal intermittent hypoxemia. Pediatr Res. 2022;91(6):1383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramani M, Kumar R, Halloran B, Lal CV, Ambalavanan N, McMahon LL. Supraphysiological levels of oxygen exposure during the neonatal period impairs signaling pathways required for learning and memory. Sci Rep. 2018;8(1):9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee JW, McElrath T, Chen M, Wallace DK, Allred EN, Leviton A, et al. Pregnancy disorders appear to modify the risk for retinopathy of prematurity associated with neonatal hyperoxemia and bacteremia. J Matern Fetal Neonatal Med. 2013;26(8):811–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gentle SJ, Abman SH, Ambalavanan N. Oxygen therapy and pulmonary hypertension in preterm infants. Clin Perinatol. 2019; 46(3): 611–9. [DOI] [PubMed] [Google Scholar]
- 103.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004; 305(5691):1733–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.