Abstract
Background and aim
Minocycline, a tetracycline derivative, has been found to exert neuroprotective properties. The current project aimed to assess the antioxidant status and cholinergic function in the amnesia induced by scopolamine.
Methods
We evaluated the passive avoidance performance, acetylcholine esterase (AChE) enzyme activity, and the oxidative stress indicators in the following groups: Normal control, scopolamine, and the treatment groups (the animals were given minocycline (10–30 mg/kg)).
Results
Scopolamine (intraperitoneal) injection was associated with impairment of passive avoidance performance and neurotoxicity. Minocycline pronouncedly ameliorated scopolamine injury as presented by the increased latency time to darkness and stay time in lightness along with the decreased darkness entry. Moreover, minocycline decreased lipid peroxidation, while it elevated the levels of superoxide dismutase, AChE enzymes, and thiol groups in both the cortex and hippocampus.
Conclusion
Our data suggested that minocycline modulated the antioxidant status and AChE in the brains, which may contribute to its protective effects against scopolamine-induced amnesia.
Keywords: Acetylcholinesterase, Minocycline, Neuronal injury, Oxidative stress, Passive avoidance performance
1. Introduction
Dementia, characterized by impaired cognitive and memory capacity, can occurs in patients with Alzheimer’s disease (AD). The prevalence of this debilitating disease is growing, especially in modern industrial societies [1]. Various pathological pathways including cholinergic deficiency, amyloid-beta (Aβ) toxicity, tau protein hyperphosphorylation, synaptic dysfunction, oxidative stress, and neuroinflammation have been suggested to be implicated in the neurodegenerative process [1,2]. Due to the complexity of the disease, a multifunctional strategy would be helpful for its management [1].
Acetylcholinesterase (AChE), an important enzyme hydrolyzing acetylcholine, regulates the function of the cholinergic system. Indeed, AChE inhibitors are widely prescribed to improve the cognitive status of AD patients. However, these drugs have been failed to retard disease progression. In recent years, numerous studies have investigated therapeutic agents that do not have side effects and are used in the management of neurodegenerative disorders [3].
Minocycline is a tetracycline derivative with anti-inflammatory, immunomodulatory, and antioxidant potential while being independent of its anti-bacterial activity [4]. Neuro-protective effects of minocycline have also been observed in different animal models of neurodegeneration such as traumatic brain injury [5,6], AD, Parkinson’s, Huntington’s disease, amyotrophic lateral sclerosis, and multiple sclerosis. Evidence from clinical studies has also reported the beneficial effects of minocycline on neurodegenerative and psychiatric diseases [4]. In the previous studies, minocycline was found to attenuate cognitive impairment, which is linked to its anti-neuro-inflammatory and anti-apoptotic activities [[7], [8], [9]]. For instance, a previous study indicated that minocycline alleviated passive avoidance performance and long-term potentiation in a rat model of cerebral ischemia-reperfusion, possibly by modifying synaptic plasticity and oxidative stress state [10]. Minocycline was also found to induce beneficial effects against streptozotocin-induced brain injury [11]. A recent investigation reported the impact of minocycline on cognitive parameters in diabetic rats, using passive avoidance and elevated plus maze tests. Moreover, It also revealed that minocycline modified AChE, glutathione, pro-inflammatory cytokines, and histopathological changes [[12], [13]]. Administration of scopolamine, an antagonist of the muscarinic cholinergic receptor, has been associated with memory and learning impairment; therefore, the scopolamine-induced animal model is widely used as a pharmacological model for inducing dementia [14]. Oxidative stress has been reported to have an important role in learning and memory impairment induced by scopolamine [[15], [16], [17]]. In addition, the administration of scopolamine resulted in neuroinflammation [18]. Evidence suggests multiple neuroprotective mechanisms of minocycline in different pathological conditions [12]. Noticeably, scopolamine attenuates the activity of the cholinergic system in the brain tissue [19,20]. Hence, in the present study, we investigated whether treatment with minocycline can reverse scopolamine-mediated memory deficits via AChE and oxidative stress markers in the brain.
2. Material and methods
2.1. Treatment groups and experimental protocol
The current experiment was conducted in compliance with the guidelines of National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1978). Furthermore, the Research Ethics Committees of the National Institute for Medical Research Development approved the experimental procedures (ethic number: IR.NIMAD.REC.1400.098).
Group 1 (control) in which the healthy animals (n = 10) were treated with saline (intraperitoneal injection (IP)). The scopolamine group in which the animals (n = 10) were treated (IP) with saline and scopolamine (Sigma, USA), respectively [21]. The minocycline-treated groups (groups 3–6, n = 10 per group) including scopolamine-minocycline 10, scopolamine-minocycline 15, scopolamine-minocycline 20, and scopolamine-minocycline 30 groups received minocycline (Sigma Aldrich, Darmstadt, Germany) at doses of 10, 15, 20, and 30 mg/kg (IP), respectively and then the administration of scopolamine was performed [21]. During the project, the adult male Wistar rats (weighing 180–220 g) were kept under the standard conditions (temperature: 22–24 °C and a 12 h light/dark cycle).
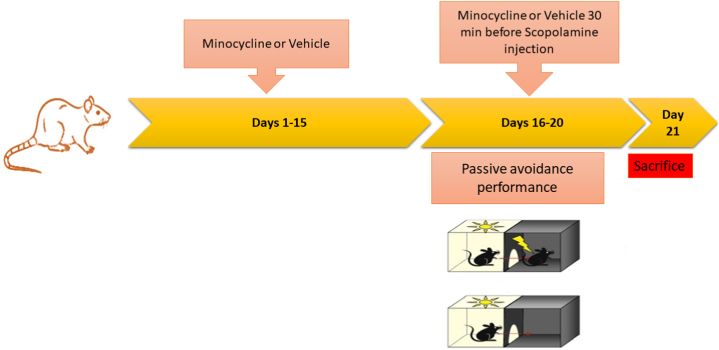
Fig. 1 presents a schematic summary of our experiment. An amnesic rat model was created by the administration of scopolamine (2 mg/kg in saline; IP). Duration and dose of scopolamine injection were determined according to recent investigations [19,22,21]. The treatment groups (groups 3–6) received 10, 15, 20, or 30 mg/kg of minocycline, whereas the scopolamine group and group 1 received the vehicle for two weeks [23,10]. During the third week, the treatment groups received minocycline (10, 15, 20, and 30 mg/kg), whereas groups 1 and 2 received vehicle 1 h before performing the behavior tests. After 30 min, scopolamine was given (except for the control group). The present research was designed according to recent investigations [[22], [24]]. All treatments were administered once per day (at 10:00 a.m.).
Fig. 1.
Timeline diagram of the experimental protocol.
Immediately after performing the behavioral tasks, the hippocampus and prefrontal cortex were extracted from the rats euthanized under deep anesthesia [using IP injection of ketamine (100 mg/kg) and xylazine (10 mg/kg)]. In order to perform biochemical analysis, the dissected cortex and hippocampus were homogenized (10% w/v) using an ice-cold phosphate-buffered saline solution (PBS; Sigma Aldrich, Darmstadt, Germany) [14].
2.2. Behavioral test
In order to evaluate simple non-spatial learning ability, we performed the passive avoidance (PA) test [14,25]. The passive avoidance procedure involves placing a rat inside a shuttle box. PA apparatus consists of a light (white) compartment and a dark (black) one (20 × 20 × 30 cm) separated by an automated door. During the first stage of the experiment lasted two consecutive days, the rats were placed in the shuttle box (for 300 s) and accustomed to the apparatus. In order to perform the acquisition trial, the second stage of the experiment, the rats were placed in the light section. After 20 s of acclimatization, the rat entered the dark section was exposed to an electric foot shock (50 Hz, 2 mA for 2 s) delivered through the stainless steel rods with a constant current shock generator. Finally, the third stage was carried out after 3, 24, 48, and 72 h. In the retention trial, the same procedures were repeated without the electric shock. We noted the latency to enter the black section (sec), the time spent in the black, and light sections (sec), and the frequency of entries to the dark at 3, 24, 48, and 72 h after receiving the punishment.
2.3. Measurement of AChE enzyme level
The activity of AChE enzyme was estimated in the homogenates, as previously set [26]. For this purpose, 3 ml of cold PBS was mixed with the tissue homogenate (40 μl). Thereafter, 100 μl of 5,5′-dithiobis-2-nitrobenzoic acid (DTNB; 0.01 M) and acetylthiocholine iodide (Sigma, USA; 0.075 M, 20 μl) were added to start the reaction. Finally, the changes in absorbance were spectrophotometry (at 412 nm) measured [11,27]. In order to measure AChE activity, the following formula was used.
| R = 5.74 (10−4)ΔA/C0 |
where C0 value is the original concentration of tissue, ΔA value is the absorbance change per min, R value is the moles substrate hydrolyzed per min per g of tissue.
2.4. Evaluation of markers of oxidative injury in brain tissue
The level of SOD in brain tissue was calculated as previously explained [28]. In this experiment, SOD prevents pyrogallol autoxidation. In short, each supernatant (10 μl) was transferred to a solution containing MTT [3-(4,5-dimethyl-thiazol-2-yl) 2,5-diphenyl tetrazolium bromide] and pyrogallol (both were obtained from Sigma Aldrich, Darmstadt, Germany). Finally, the absorbance was noted at 570 nm. The amount of enzyme inhibiting 50% of formazan formation is considered one unit of SOD activity [14].
In order to estimate the non-enzymatic antioxidant capacity of brain tissue, total thiol concentration was determined using the previously described methods [[29], [30]]. For this purpose, the supernatants were transferred to a buffer solution of tris-ethylenediamine tetra acetic acid disodium salt (Na2EDTA; sigma Aldrich, Darmstadt, Germany) with a pH set at 8.6. Thereafter, the absorbance was recorded at 412 nm. Finally, DTNB reagent, at a concentration of 10 mM, was added to the mixture and the second absorbance was recorded. The difference between the optical densities was noted.
In order to estimate the extent of lipid peroxidation, malondialdehyde (MDA) concentration, was measured in the brain tissue. In brief, each supernatant (1 ml) was mixed with trichloroacetic acid (TCA), 2-thiobarbituric acid (TBA), and hydrochloric acid (HCl). Thereafter, the mixtures were kept in a hot water (100 °C) for 45 min. Finally, each sample was centrifuged (1000 g, 10 min) and the absorbance was measured using a spectrophotometer at 535 nm [25,29].
2.5. Statistical analysis
All data are expressed as mean ± standard error of the mean and a p < 0.05 was considered statistically significant. Statistical comparisons were performed using the statistical software package SPSS 23.0. The data obtained from the behavior test were analyzed by repeated-measures analysis of variance (ANOVA) (considering treatment as a between-subject factor and time point as a within-subject factor) followed by the Bonferroni multiple comparison test. Other data were analyzed by one-way ANOVA followed by Tukey’s post-hoc test.
3. Results
3.1. Minocycline restores PA performances of the amnesic animals
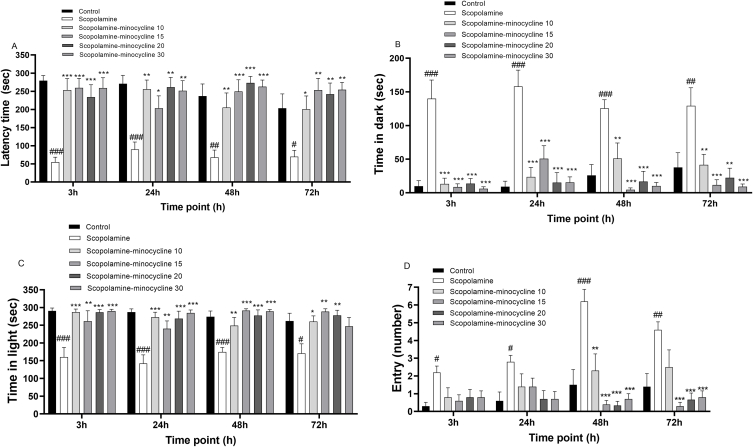
The results showed a noticeable effect of treatment (f(5, 53) = 11.53; p < 0.001) on the delay to enter the black section. A remarkable effect of treatment (f(5, 53) = 27.12; p < 0.001) on the dark stay time was also indicated. Regarding the light stay time, a noticeable difference was indicated among the treatment groups (f(5, 53) = 18.98; p < 0.001). Finally, a remarkable effect of treatment (f(5, 53) = 8.89; p < 0.001) and the time point (f(3,159) = 8.69; p < 0.001) on the entry to the black zone was observed. As illustrated in Fig. 2A–C, Bonferroni pairwise comparison revealed that, scopolamine injection was associated with a noticeable reduction in latency time (p < 0.05 to p < 0.001) and the stay time in the white zone (p < 0.05 and p < 0.001), while the dark stay time was remarkably increased (p < 0.01 and p < 0.001) at all points after receiving the punishment. Moreover, scopolamine induced a remarkable increase in the entry to the black zone at the same time points, in comparison to group 1 (p < 0.05 to p < 0.001). Accordingly, scopolamine injection impaired the PA performance which was reversed by minocycline administration. Minocycline injection, at all doses and time points, prolonged the latency (p < 0.05 to p < 0.001), the light stay time (p < 0.05 to p < 0.001), while the dark segment stay time was decreased in comparison with the group treated with scopolamine (p < 0.01 and p < 0.001). Further analysis demonstrated that minocycline treatment (at doses of 10, 15, 20, and 30) was associated with a remarkable reduction in the entry at 48 and 72 h after receiving the punishment, compared to the group treated with scopolamine (p < 0.01 and p < 0.001). Regarding all parameters recorded, no remarkable difference was indicated among the treatment groups. Accordingly, minocycline did not show any dose-dependent effect on different behavioral parameters. It is notable that the frequencies of the entry in the groups treated with the minocycline (at all doses) were comparable to group 1 (Fig. 2D).
Fig. 2.
Delay time (A), dark segment stay time (B), light segment stay time (C), and entry to dark segment (D) in the passive avoidance test. Data are expressed as mean ± SEM (n = 10). #p < 0.05, ##p < 0.01 and ###p < 0.001 compared to the normal control group and *p < 0.05, **p < 0.01 and ***p < 0.001 compared to the scopolamine group.
3.2. Minocycline restores AChE enzyme
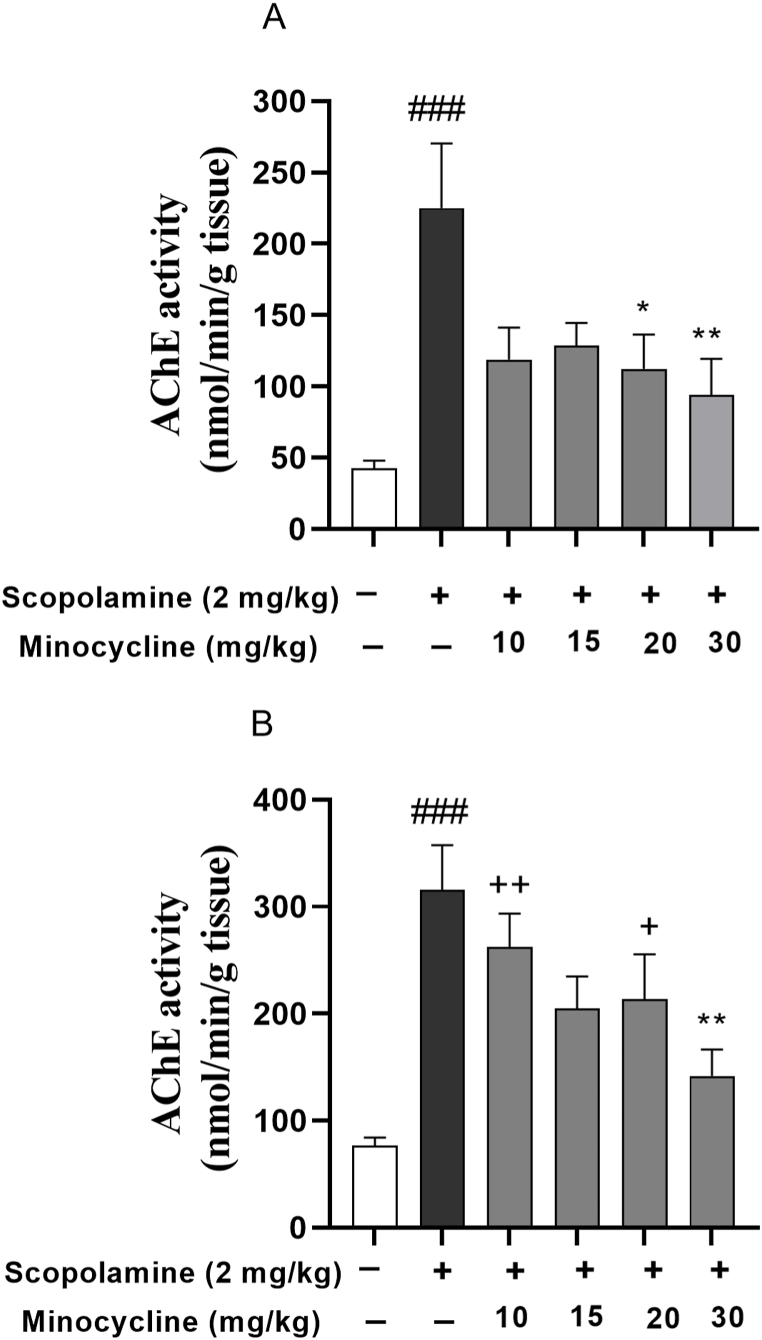
As Fig. 3A and B exhibit, scopolamine injection was accompanied by a remarkable elevation of the enzyme activity relative to group 1 (p < 0.001). However, minocycline significantly attenuated the enzyme level as presented by the lower enzyme activities in the cortex of scopolamine-minocycline 20 and scopolamine-minocycline 30 (p < 0.05 and p < 0.01) as well as the hippocampus of scopolamine-minocycline 30 group (p < 0.01). Notably, scopolamine-minocycline 10 (p < 0.01) and scopolamine-minocycline 20 groups (p < 0.05) exhibited a significant difference in term of AChE activity in the hippocampus relative to group 1. Considering the data, the effect of minocycline on AChE activity was not dose-dependent. However, the enzyme activity in the cortex of all treatment groups was similar to group 1 (Fig. 3).
Fig. 3.
AChE activity in the cortex (A) and hippocampus (B). Data are expressed as mean ± SEM (n = 10). ###p < 0.001 compared to the normal control group and *p < 0.05 and **p < 0.001 compared to the scopolamine group. +p < 0.05 and ++p < 0.01 Minocycline-treated groups compared to the normal control group.
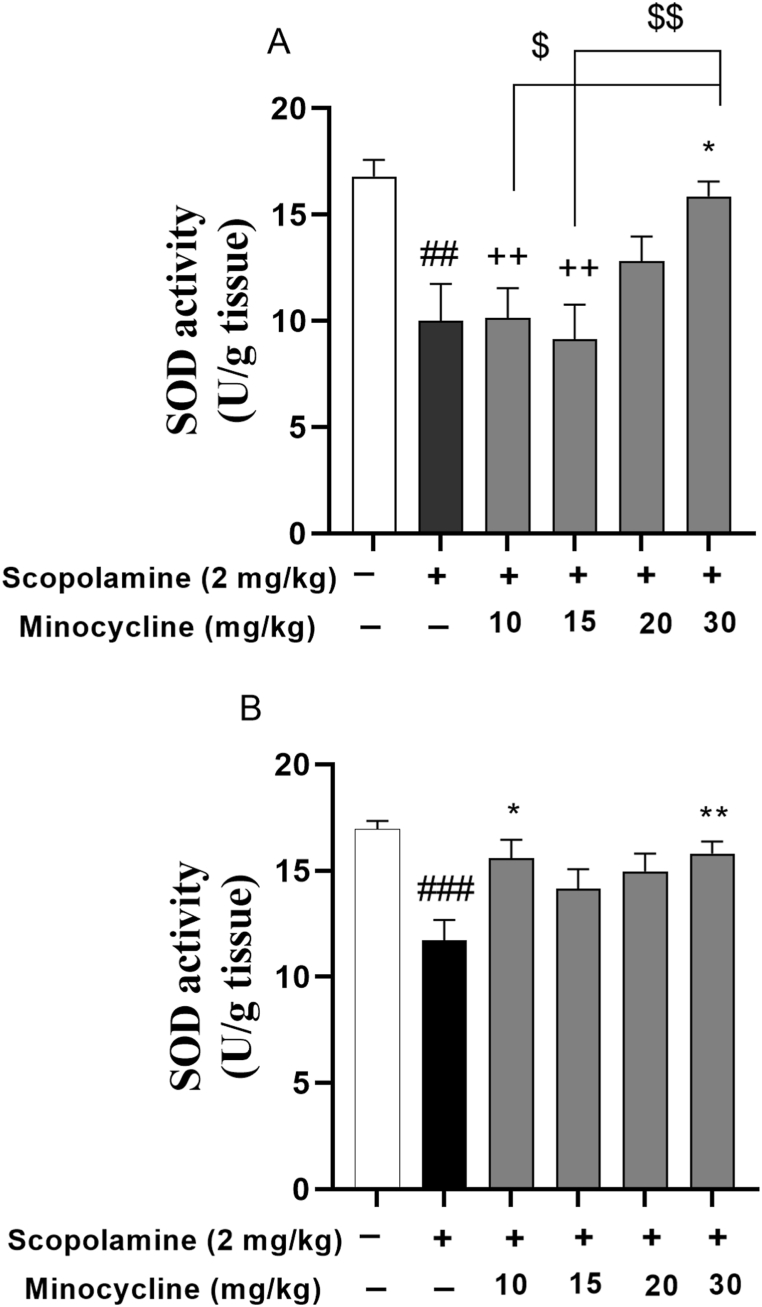
3.3. Minocycline counteracts oxidative stress
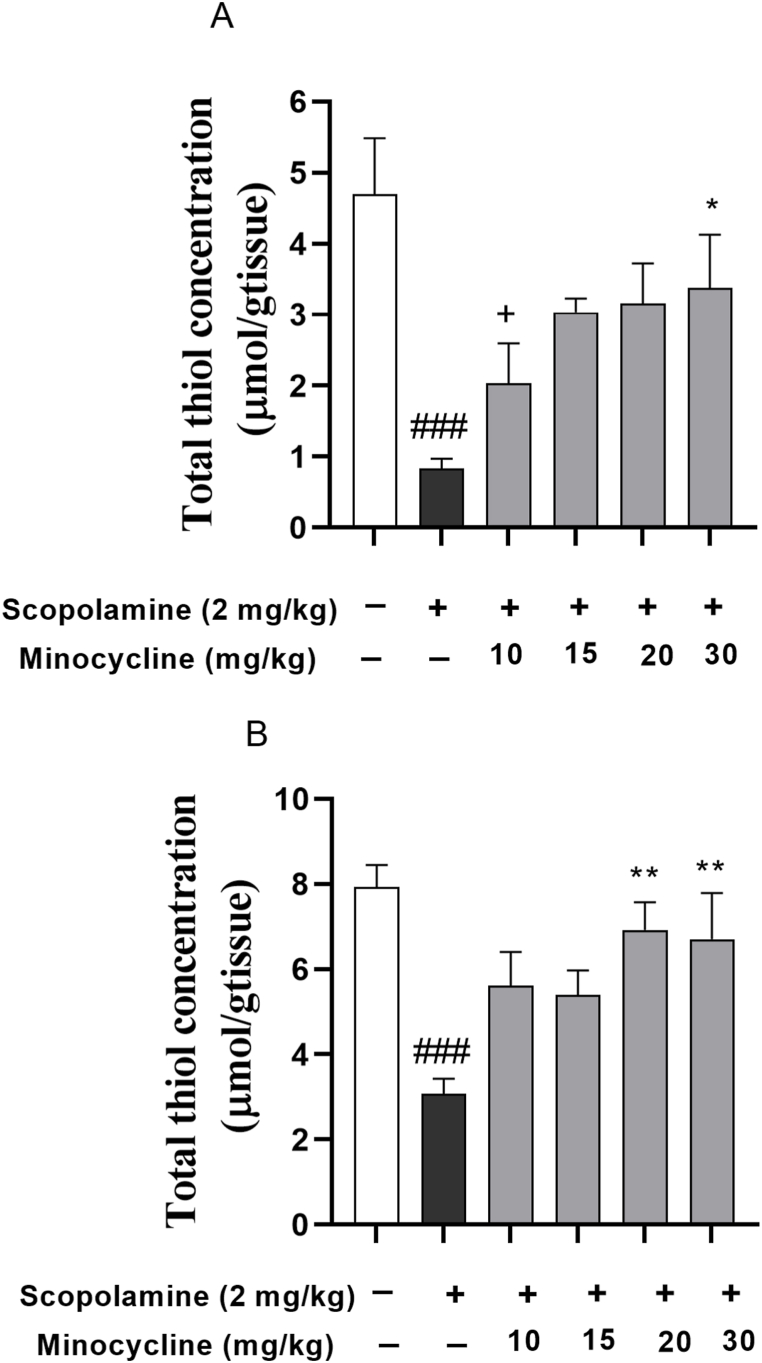
The data analysis revealed a remarkable reduction in total thiol concentration (p < 0.001) as well as SOD enzyme activity in the scopolamine-injected groups relative to group 1 (Fig. 4, Fig. 5; p < 0.01 and p < 0.001). In contrast, minocycline restored the antioxidant values (SOD and thiol level) in the cortex of the scopolamine-minocycline 30 group (Fig. 4, Fig. 5A; p < 0.05). Similarly, a significant difference in terms of hippocampal SOD activity was found in the scopolamine-minocycline 10 (p < 0.05) and scopolamine-minocycline 30 groups, compared with the scopolamine-treated animals (Fig. 5B; p < 0.05 and p < 0.01). As Fig. 4B exhibited, the thiol concentration of the hippocampus of the scopolamine-minocycline 20 and scopolamine-minocycline 30 groups was noticeably elevated when compared with the scopolamine-treated rats (p < 0.01). Meanwhile, the cortical level of antioxidant (SOD) enzyme in the scopolamine-minocycline 10 group was remarkably lower than that of the scopolamine-minocycline 15 and scopolamine-minocycline 30 groups (Fig. 5B, p < 0.01 and p < 0.05, respectively).
Fig. 4.
Thiol content in the cortex (A) and hippocampus (B). Data are expressed as mean ± SEM (n = 10). ###p < 0.001 compared to the control group and *p < 0.05 and **p < 0.001 compared to the scopolamine group. +p < 0.05 Minocycline-treated groups compared to the normal control group.
Fig. 5.
SOD activity in the cortex (A) and hippocampus (B). Data are expressed as mean ± SEM (n = 10). ##p < 0.01 and ###p < 0.001 compared to the normal control group and *p < 0.05 and **P < 0.001 compared to the scopolamine group. ++p < 0.05 Minocycline-treated groups compared to the normal control group. $p < 0.05 and $$p < 0.01 comparison among the treatment groups.
Notably, the levels of antioxidant (SOD) enzyme in the cortex of the animals treated with the low doses of minocycline (10 and 15 mg/kg) were smaller relative to group 1 (Fig. 5A; p < 0.01). Similarly, the cortical thiol level in the scopolamine-minocycline 10 group was smaller relative to the normal control rats. However, the antioxidant values (SOD and thiol level) in the hippocampus of all treatment groups were similar to group 1. Notably, the results did not show any noticeable difference among the treatment groups in terms of the thiol level in both hippocampus and cortex as well as the hippocampal level of SOD. Accordingly, minocycline showed a dose-dependent effect on the cortical level of SOD in the minocycline-treated groups (Fig. 4, Fig. 5).
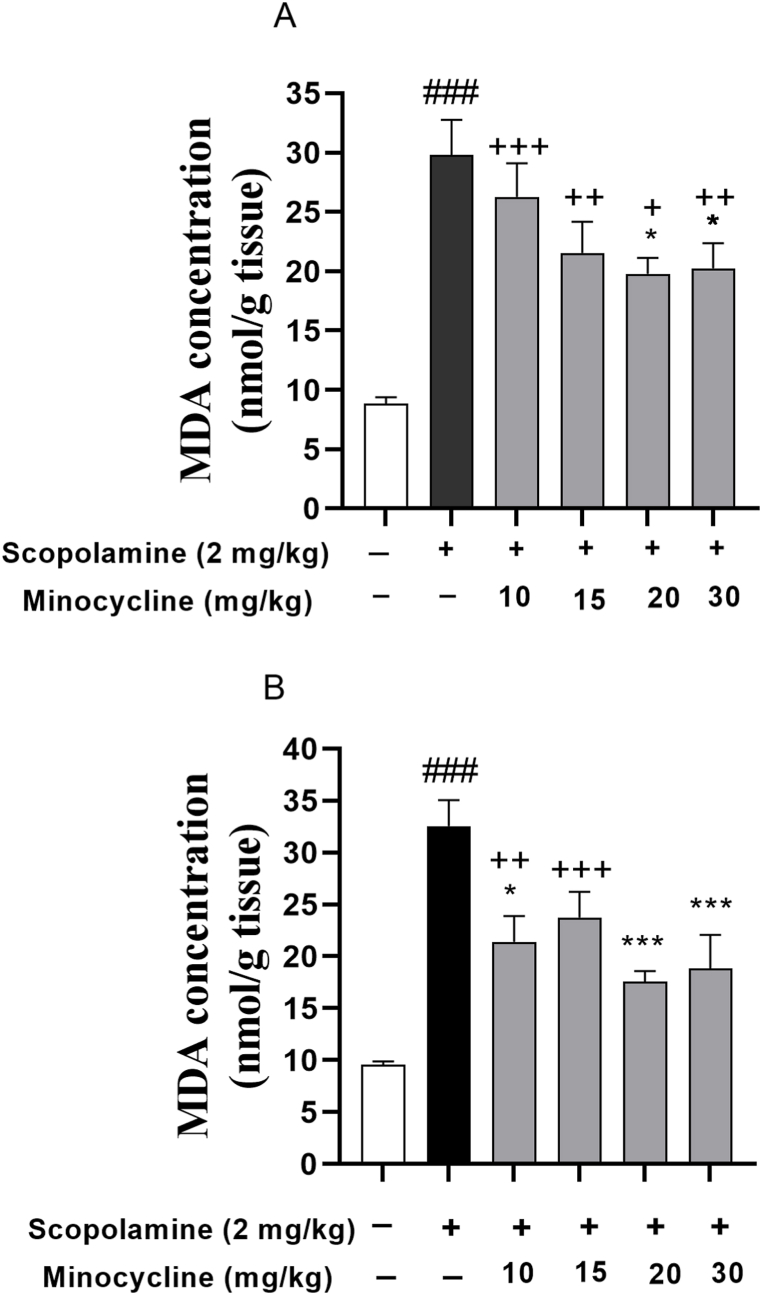
The amount of MDA in the hippocampus and cortex was noticeably elevated following scopolamine injection (Fig. 6A and B; p < 0.001). However, in comparison to the scopolamine-treated group, a decreased amount of MDA was indicated in the cortex of scopolamine-minocycline 20 (p < 0.05) and scopolamine-minocycline 30 (p < 0.05) as well as the hippocampus of scopolamine-minocycline 10 (p < 0.05), scopolamine-minocycline 20, and scopolamine-minocycline 30 groups (p < 0.001 for both). Furthermore, the MDA levels in cortical tissue of all minocycline-treated groups were remarkably greater relative to the normal control group (p < 0.05 to p < 0.001). Similarly, some noticeable differences regarding the MDA level in the hippocampus were found between the low doses of minocycline (scopolamine-minocycline 10 and scopolamine-minocycline 15) and the normal control group (Fig. 6; p < 0.01 and p < 0.001, respectively). Fig. 6A and B also revealed that the amount MDA in the hippocampus of the rats treated with minocycline (at doses of 20 and 30 mg/kg) was comparable to group 1 (p > 0.05). As the data analysis revealed, the effect of minocycline on the hippocampal and cortical levels of MDA was not dose-dependent (Fig. 6).
Fig. 6.
MDA levels in the cortex (A) and hippocampus (B). Data are expressed as mean ± SEM (n = 10). ###p < 0.001 compared to the normal control group and *p < 0.05 and ***p < 0.001 compared to the scopolamine group. +p < 0.05, ++p < 0.01, and +++p < 0.001 Minocycline-treated groups compared to the normal control group.
4. Discussion
The current study demonstrated that minocycline mitigated cognitive and memory dysfunction in scopolamine-induced amnesia based on the passive avoidance test. This behavior test links learning and memory ability to hippocampal activities [31]. Scopolamine is an acetylcholine receptor blocker agent, which can impair learning and memory performance, especially during the learning acquisition [24,32,33]. Scopolamine causes abnormalities in the cholinergic system [21,32,34]. In order to find therapeutic candidates for memory disorders, the scopolamine-injured animal model has been commonly used [24,32,33]. As our data revealed, the scopolamine injection was accompanied by a shorter latency to the black area and light stay time. In addition, scopolamine increased the entry to the dark suggesting impairment of learning functions in the rats.
Scopolamine was found to reduce acetylcholine (ACh) contents in response to enhanced acetylcholinesterase activity [32,33,35]. This enzyme is responsible for the degradation of ACh, the neurotransmitter essential for cognitive function [36]. During dementia, progressive deterioration of learning and memory performance occurs, which is linked to ACh deficiency in the brain [36]. AChE inhibitors prevent ACh hydrolysis in the brain tissue, thereby inhibiting the degeneration of cholinergic neurons [1,36]. Accordingly, inhibitions of the AChE enzyme may prove a helpful strategy in the control of dementia development [32,36]. The current study indicated that scopolamine injection enhanced AChE activity in the brain tissue. Therefore, our results support the idea that learning and memory impairment induced by scopolamine is at least in part mediated by disturbance of AChE activity.
Oxidative stress is another major risk factor for amnesia which participates in the neurodegeneration process [32]. Indeed, the overproduction of oxidative metabolites such as reactive oxygen species (ROS) contributes to the increased lipid peroxidation, which consequently mediates neurotoxicity [32]. According to different pieces of evidence, scopolamine promotes oxidative brain injury and neuronal death [32,37]. In the present study, the administration of scopolamine elevated the MDA level, while it lowered the total thiol content and SOD activity indicating the inactivation of detoxification systems and consumption of antioxidants in both the hippocampus and cortex. These results, thus, suggest increased oxidative stress in the hippocampus and cortex. Our findings were concurred with the previous studies indicating the correlation between oxidative stress and dementia following scopolamine administration [33,38]. For instance, in the year 2021, investigators demonstrated depletion of brain antioxidant capacity and elevated AChE activity in scopolamine-induced dementia.
Interestingly, attenuation of oxidative stress using antioxidants may prove a promising and effective strategy against amnesia [25,32,37]. Kowalczyk et al. [39] demonstrated that the administration of antioxidants would counteract scopolamine-induced neurodegeneration by boosting the activity of the antioxidant enzymes [39]. In the present research, minocycline noticeably restored the oxidative indicators in both the cortex and hippocampus sections. The results revealed that minocycline attenuated MDA while elevating thiol and SOD levels. The modifying effect of minocycline on MDA and thiol content was not dose-dependent. However, the activity of the antioxidant enzyme in the scopolamine-minocycline 10 and scopolamine-minocycline 15 groups was remarkably smaller than that of the scopolamine-minocycline 30 suggesting a dose-dependent impact of minocycline on the antioxidant defense.
Interestingly, the anti-oxidant effects of minocycline which were observed in our study, were accompanied by improvement in the PA performance of the amnesic rats. The present findings suggest that all doses of minocycline increased latencies to enter the dark area and total time spent in the light area while reducing the time spent in the dark area of the shuttle box. Considering these results, it was postulated that minocycline restored learning and memory through modulation of the cholinergic system and oxidative stress. However, the specific molecular components and mechanisms for the effects of minocycline remain to be established. These results indicated that the ameliorating effect of minocycline on cognition disturbance was not dose-dependent.
These beneficial effects of minocycline on the nervous system have already been supported by previous investigations. For instance, Dai et al. [40] noted the protective effects of minocycline (20 μM) on toxicity and death of the primary cortical neuronal cells through suppression of ROS formation and elevating antioxidant enzymes namely SOD and catalase [40]. In the same way, minocycline (30 mg/kg) alleviated the neuropathic pain in the rat hyperalgesia model associated with lowered MDA production as well as enhanced SOD and glutathione peroxidase activities [41]. In another recent study, minocycline modified the behavior performance in alcohol-induced neurodegeneration using the Morris water maze task. The authors claimed that the antioxidant activity of minocycline is implicated in the neuroprotective effects [6]. Furthermore, some reports are indicating the cognition-enhancing and neuroprotection potential of minocycline in Aβ, streptozotocin, and lipopolysaccharide (LPS)-injured animals [8,42,43]. The previous evidence was in line with our findings [6,8,42,43]. According to our data, the positive effects of minocycline on passive avoidance memory were further confirmed by biochemical assessment of AChE enzyme and anti-oxidant activities in the rat brain. The AChE inhibitory activity of minocycline has been reported in the literature [11,43]. A body of evidence has indicated that scopolamine impairs antioxidant defense and induces oxidative stress, which is accompanied by cholinergic system dysfunction [15,18,44]. In our study, an elevated level of AChE was observed under conditions of oxidative stress which was modified by minocycline. We previously showed that the mRNA expression of pro-inflammatory cytokines such as tumor necrosis factor-α and interleukin-(IL) 1β was elevated concomitant with augmented AChE levels in brain tissue of scopolamine-injected rats [44]. Further, scopolamine was found to induce oxidative injury in the hippocampus and prefrontal cortex [44]. According to previous investigations [45,46], the anti-amnesic properties of minocycline are also consistent with its protective effect against neuro-inflammation and oxidative damage. In this regard, Haj-Mirzaian et al. [46] reported that minocycline (40 and 80 mg/kg) lowered pro-inflammatory cytokines and ROS formation while elevating glutathione levels in LPS-injured rats. A previous study reported that minocycline lowered IL-6 and IL-1β as well as suppressed microglial activity [47,48]. It is well-documented that repeated administration of scopolamine mimics the accumulation of neurotoxic aggregates [49,50]. Scopolamine-induced oxidative stress can increase Aβ production while also triggering synaptic dysfunction and subsequent memory dysfunction [49,50]. Additional research should be conducted to assess the impact of minocycline on the cerebral level of Aβ, inflammatory mediators, and histopathological alteration in scopolamine-induced amnesia as well as to compare its effectiveness to those of minocycline alone.
5. Conclusion
The present data confirm the hypothesis that minocycline may improve animal memory by reversing the deleterious effects of scopolamine on brain function through modulation of AChE activity and oxidative stress state.
Production notes
Author contribution statement
Mohammad Hosein Eshaghi Ghalibaf, Mohsen Parviz, Mahsan Akbarian, Sabiheh Amirahmadi, Farzaneh Vafaee: Performed the experiments; Analyzed and interpreted the data.
Arezoo Rajabian: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mahmoud Hosseini: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Research reported in this publication was supported by Vice Chancellery for Research and Technology, Mashhad University of Medical Sciences and Elite Researcher Grant Committee under award number (4000373) from the National Institute for Medical Research Development (NIMAD), Tehran, Iran.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of interest’s statement
The authors have no declaration of interest.
Contributor Information
Arezoo Rajabian, Email: rajabianar@mums.ac.ir.
Mahmoud Hosseini, Email: Hosseinim@mums.ac.ir.
References
- 1.Akhtar A., Andleeb A., Waris T.S., Bazzar M., Moradi A.R., Awan N.R., Yar M. Neurodegenerative diseases and effective drug delivery: a review of challenges and novel therapeutics. J. Contr. Release. 2021;330:1152–1167. doi: 10.1016/j.jconrel.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Njan A.A., Adenuga F.O., Ajayi A.M., Sotunde O., Ologe M.O., Olaoye S.O., Erdogan O.N., Iwalewa O.E. Neuroprotective and memory-enhancing effects of methanolic leaf extract of Peristrophe bicalyculata in rat model of type 2 diabetes mellitus. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bortolami M., Rocco D., Messore A., Di Santo R., Costi R., Madia V.N., Scipione L., Pandolfi F. Acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease - a patent review (2016-present) Expert Opin. Ther. Pat. 2021;31:399–420. doi: 10.1080/13543776.2021.1874344. [DOI] [PubMed] [Google Scholar]
- 4.Romero-Miguel D., Lamanna-Rama N., Casquero-Veiga M., Gómez-Rangel V., Desco M., Soto-Montenegro M.L. Minocycline in neurodegenerative and psychiatric diseases: an update. Eur. J. Neurol. 2021;28:1056–1081. doi: 10.1111/ene.14642. [DOI] [PubMed] [Google Scholar]
- 5.Whitney K., Nikulina E., Rahman S.N., Alexis A., Bergold P.J. Delayed dosing of minocycline plus N-acetylcysteine reduces neurodegeneration in distal brain regions and restores spatial memory after experimental traumatic brain injury. Exp. Neurol. 2021;345 doi: 10.1016/j.expneurol.2021.113816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motaghinejad M., Mashayekh R., Motevalian M., Safari S. The possible role of CREB-BDNF signaling pathway in neuroprotective effects of minocycline against alcohol-induced neurodegeneration: molecular and behavioral evidences. Fundam. Clin. Pharmacol. 2021;35:113–130. doi: 10.1111/fcp.12584. [DOI] [PubMed] [Google Scholar]
- 7.Li L., Xing X., Li Q., Zhang Q., Fu L., Liu Y. Minocycline improves learning and memory functions in ischemic stroke rats via reduction of cerebral ischemia induced neuroinflammation and apoptosis. Trop. J. Pharmaceut. Res. 2021;20:288. [Google Scholar]
- 8.Garcez M.L., Mina F., Bellettini-Santos T., Carneiro F.G., Luz A.P., Schiavo G.L., Andrighetti M.S., Scheid M.G., Bolfe R.P., Budni J. Minocycline reduces inflammatory parameters in the brain structures and serum and reverses memory impairment caused by the administration of amyloid β (1-42) in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;77:23–31. doi: 10.1016/j.pnpbp.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J., Boska M., Zheng Y., Liu J., Fox H.S., Xiong H. Minocycline attenuation of rat corpus callosum abnormality mediated by low-dose lipopolysaccharide-induced microglia activation. J. Neuroinflammation. 2021;18:100. doi: 10.1186/s12974-021-02142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parvardeh S., Sheikholeslami M.A., Ghafghazi S., Pouriran R., Mortazavi S.E. Minocycline improves memory by enhancing hippocampal synaptic plasticity and restoring antioxidant enzyme activity in a rat model of cerebral ischemia-reperfusion. Basic Clin. Neurosci. 2022;13(2):225–236. doi: 10.32598/bcn.12.6.2062.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma V.K., Goyal A., Sarma S.G. Minocycline decreases acetylcholinesterase activity in intra-cerebroventricular streptozocin infused rats. Int. J. Pharmaceut. Sci. Res. 2010;1:52–57. [Google Scholar]
- 12.Mehta B.K., Banerjee S. Minocycline reverses diabetes-associated cognitive impairment in rats. Pharmacol. Rep. 2019;71(4):713–720. doi: 10.1016/j.pharep.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Amirahmadi S., Farimani F.D., Akbarian M., Mirzavi F., Eshaghi Ghalibaf, Rajabian A., Hosseini M. Minocycline attenuates cholinergic dysfunction and neuro-inflammation-mediated cognitive impairment in scopolamine-induced Alzheimer’s rat model. Inflammopharmacology. 2022;30(6):2385–2397. doi: 10.1007/s10787-022-01071-2. [DOI] [PubMed] [Google Scholar]
- 14.Amirahmadi S., Hosseini M., Ahmadabady S., Akbarian M., Abrari K., Vafaee F., Rajabian A. Folic acid attenuated learning and memory impairment via inhibition of oxidative damage and acetylcholinesterase activity in hypothyroid rats. Metab. Brain Dis. 2021;36:2393–2403. doi: 10.1007/s11011-021-00815-3. [DOI] [PubMed] [Google Scholar]
- 15.Ghasemi S., Moradzadeh M., Hosseini M., Beheshti F., Sadeghnia H.R. Beneficial effects of Urtica dioica on scopolamine-induced memory impairment in rats: protection against acetylcholinesterase activity and neuronal oxidative damage. Drug Chem. Toxicol. 2019;42:167–175. doi: 10.1080/01480545.2018.1463238. [DOI] [PubMed] [Google Scholar]
- 16.Mohammadpour T., Hosseini M., Naderi A., Karami R., Sadeghnia H.R., Soukhtanloo M., Vafaee F. Protection against brain tissues oxidative damage as a possible mechanism for the beneficial effects of Rosa damascena hydroalcoholic extract on scopolamine-induced memory impairment in rats. Nutr. Neurosci. 2015;18:329–336. doi: 10.1179/1476830514Y.0000000137. [DOI] [PubMed] [Google Scholar]
- 17.Hosseini M., Mohammadpour T., Karami R., Rajaei Z., Sadeghnia H.R., Soukhtanloo M. Effects of the hydro-alcoholic extract of Nigella sativa on scopolamine-induced spatial memory impairment in rats and its possible mechanism. Chin. J. Integr. Med. 2015;21:438–444. doi: 10.1007/s11655-014-1742-5. [DOI] [PubMed] [Google Scholar]
- 18.Beheshti F., Akbari H.R., Baghcheghi Y., Mansouritorghabeh F., Mortazavi Sani S.S., Hosseini M. Beneficial effects of angiotensin converting enzyme inhibition on scopolamine-induced learning and memory impairment in rats, the roles of brain-derived neurotrophic factor, nitric oxide and neuroinflammation. Clin. Exp. Hypertens. 2021;43:505–515. doi: 10.1080/10641963.2021.1901112. [DOI] [PubMed] [Google Scholar]
- 19.Gunasekaran V., Avarachan J., Augustine A., Khayum A., Arivukkarasu R. 3-O-Acetyl-11-keto-β-boswellic acid ameliorates acquired, consolidated and recognitive memory deficits through the regulation of hippocampal PPAR γ, MMP9 and MMP2 genes in dementia model. Heliyon. 2021;7(12) doi: 10.1016/j.heliyon.2021.e08523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao J., Nai Y., Feng L., Chen Y., Li M., Xu H. Walnut Oil prevents scopolamine-induced memory dysfunction in a mouse model. Molecules (Basel) 2020;25(7):1630. doi: 10.3390/molecules25071630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosseini Z., Mansouritorghabeh F., Kakhki F., Hosseini M., Rakhshandeh H., Hosseini A., Hasanpour M., Iranshahi M., Rajabian A. Effect of Sanguisorba minor on scopolamine-induced memory loss in rat: involvement of oxidative stress and acetylcholinesterase. Metab. Brain Dis. 2022;37:473–488. doi: 10.1007/s11011-021-00898-y. [DOI] [PubMed] [Google Scholar]
- 22.Yang Q., Lin J., Zhang H., Liu Y., Kan M., Xiu Z., Chen X., Lan X., Li X., Shi X., Li N., Qu X. Ginsenoside compound K regulates amyloid β via the Nrf2/Keap1 signaling pathway in mice with scopolamine hydrobromide-induced memory impairments. J. Mol. Neurosci. 2019;67(1):62–71. doi: 10.1007/s12031-018-1210-3. [DOI] [PubMed] [Google Scholar]
- 23.Abdo Qaid E.Y., Abdullah Z., Zakaria R., Long I. Minocycline protects against lipopolysaccharide-induced glial cells activation and oxidative stress damage in the medial prefrontal cortex (mPFC) of the rat. Int. J. Neurosci. 2022:1–10. doi: 10.1080/00207454.2022.2084092. [DOI] [PubMed] [Google Scholar]
- 24.Park H.S., Hwang E.S., Choi G.Y., Kim H.B., Park K.S., Sul J.Y., Hwang Y., Choi G.W., Kim B.I., Park H., Maeng S., Park J.H. Sulforaphane enhances long-term potentiation and ameliorate scopolamine-induced memory impairment. Physiol. Behav. 2021;238 doi: 10.1016/j.physbeh.2021.113467. [DOI] [PubMed] [Google Scholar]
- 25.Kim M.-J., Hwang E.-S., Kim K.J., Maeng S., Heo H.J., Par k J-H., Kim D.-O. Anti-amnesic effects of epigallocatechin gallate on scopolamine-induced learning and memory dysfunction in sprague-dawley rats. Antioxidants. 2022;11:1. doi: 10.3390/antiox11010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellman G.L., Courtney K.D., Andres V., Jr., Feather-Stone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 27.Tippabathani J., Nellore J., Kathirkannan P., Nachiyar C.V. Developmental effects of three textile chemicals on locomotor activity, antioxidant markers and acetylcholine esterase activity in zebrafish. Indian J. Exp. Biol. 2020;58:212–218. [Google Scholar]
- 28.Madesh M., Balasubramanian K.A. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J. Biochem. Biophys. 1998;35:184–188. [PubMed] [Google Scholar]
- 29.Boroushaki M.T., Fanoudi S., Rajabian A., Boroumand S., Aghaee A., Hosseini A. Evaluation of Rheum turkestanicum in hexachlorobutadien-induced renal toxicity. Drug Res. (Stuttg) 2019;69:434–438. doi: 10.1055/a-0821-5653. [DOI] [PubMed] [Google Scholar]
- 30.Rastegar-Moghaddam S.H., Hosseini M., Alipour F., Rajabian A., Ebrahimzadeh Bideskan A. The effects of vitamin D on learning and memory of hypothyroid juvenile rats and brain tissue acetylcholinesterase activity and oxidative stress indicators. Naunyn-Schmiedeberg’s Arch. Pharm. 2022;395(3):337–351. doi: 10.1007/s00210-021-02195-y. [DOI] [PubMed] [Google Scholar]
- 31.Isaacson R.L., Wickelgren W.O. Hippocampal ablation and passive avoidance. Science. 1962;138:1104–1106. doi: 10.1126/science.138.3545.1104. [DOI] [PubMed] [Google Scholar]
- 32.Tang K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: a model of Alzheimer’s biomarkers. Life Sci. 2019;233 doi: 10.1016/j.lfs.2019.116695. [DOI] [PubMed] [Google Scholar]
- 33.Brinza I., Boiangiu R.S., Hancianu M., Cioanca O., Erdogan Orhan I., Hritcu L. Bay Leaf (Laurus Nobilis L.) Incense improved scopolamine-induced amnesic rats by restoring cholinergic dysfunction and brain antioxidant status. Antioxidants (Basel) 2021;10:259. doi: 10.3390/antiox10020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sohn E., Lim H.S., Kim Y.J., Kim B.Y., Kim J.H., Jeong S.J. Elaeagnus glabra f. oxyphylla attenuates scopolamine-induced learning and memory impairments in mice by improving cholinergic transmission via activation of CREB/NGF signaling. Nutrients. 2019;11(6):1205. doi: 10.3390/nu11061205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lv J., Lu C., Jiang N., Wang H., Huang H., Chen Y., Li Y., Liu X. Protective effect of ginsenoside Rh2 on scopolamine-induced memory deficits through regulation of cholinergic transmission, oxidative stress and the ERK-CREB-BDNF signaling pathway. Phytother Res. 2021;35:337–345. doi: 10.1002/ptr.6804. [DOI] [PubMed] [Google Scholar]
- 36.Hampel H., Mesulam M.M., Cuello A.C., Khachaturian A.S., Vergallo A., Farlow M.R., Snyder P.J., Giacobini E., Khachaturian Z.S. Revisiting the cholinergic hypothesis in Alzheimer’s disease: emerging evidence from translational and clinical research. J. Prev. Alzheimer’s Dis. 2019;6:2–15. doi: 10.14283/jpad.2018.43. [DOI] [PubMed] [Google Scholar]
- 37.Tripathi S., Mitra Mazumder P. Comprehensive investigations for a potential natural prophylaxis-A cellular and murine model for apple cider vinegar against hydrogen peroxide and scopolamine induced oxidative stress. Drug Dev. Res. 2021;83(1):105–118. doi: 10.1002/ddr.21849. [DOI] [PubMed] [Google Scholar]
- 38.Sharma V., Firdaus Z., Rai H., Nayak P.K., Singh T.D., Gautam D.N.S. Consumption of Ashtanga Ghrita (clarified cow butter added with herb extracts) improves cognitive dysfunction induced by scopolamine in rats via regulation of acetylcholinesterase activity and oxidative stress. Drug Metab. Pers. Ther. 2021;36:337–350. doi: 10.1515/dmpt-2021-0108. [DOI] [PubMed] [Google Scholar]
- 39.Kowalczyk J., Kurach L., Boguszewska-Czubara A., Skalicka-Woźniak K., Kruk-Słomka M., Kurzepa J., Wydrzynska-Kuźma M., Biała G., Skiba A., Budzyńska B. Bergapten improves scopolamine-induced memory impairment in mice via cholinergic and antioxidative mechanisms. Front. Neurosci. 2020;14:730. doi: 10.3389/fnins.2020.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai C., Ciccotosto G.D., Cappai R., Wang Y., Tang S., Xiao X., Velkov T. Minocycline attenuates colistin-induced neurotoxicity via suppression of apoptosis, mitochondrial dysfunction and oxidative stress. J. Antimicrob. Chemother. 2017;72:1635–1645. doi: 10.1093/jac/dkx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abbaszadeh A., Darabi S., Hasanvand A., Amini-Khoei H., Abbasnezhad A., Choghakhori R., Aaliehpour A. Minocycline through attenuation of oxidative stress and inflammatory response reduces the neuropathic pain in a rat model of chronic constriction injury. Iran J. Basic Med. Sci. 2018;21:138–144. doi: 10.22038/IJBMS.2017.24248.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hiskens M.I., Vella R.K., Schneiders A.G., Fenning A.S. Minocycline improves cognition and molecular measures of inflammation and neurodegeneration following repetitive mTBI. Brain Inj. 2021;35:831–841. doi: 10.1080/02699052.2021.1909139. [DOI] [PubMed] [Google Scholar]
- 43.Rai P., Kishor B., Bharatia R., Kumar S., Gupta S.K., Sinha A. Synergistic Neuroprotective potential of combined treatment with vinpocetine and minocycline against streptozotocin and lipopolysaccharide induced memory impaired mice. J. Pharm. Sci. Pharmacol. 2017;3:124–132. [Google Scholar]
- 44.Akbarian M., Mirzavi F., Amirahmadi S., Hosseini M., Alipour M., Feizi H., Rajabian A. Amelioration of oxidative stress, cholinergic dysfunction, and neuroinflammation in scopolamine-induced amnesic rats fed with pomegranate seed. Inflammopharmacology. 2022;30(3):1021–1035. doi: 10.1007/s10787-022-00971-7. [DOI] [PubMed] [Google Scholar]
- 45.Hou Y., Xie G., Liu X., Li G., Jia C., Xu J., Wang B. Minocycline protects against lipopolysaccharide-induced cognitive impairment in mice. Psychopharmacology. 2016;233:905–916. doi: 10.1007/s00213-015-4169-6. [DOI] [PubMed] [Google Scholar]
- 46.Haj-Mirzaian A., Ramezanzadeh K., Tafazolimoghadam A., Kazemi K., Nikbakhsh R., Nikbakhsh R., Amini-Khoei H., Afshari K., Haddadi N.S., Shakiba S., Azimirad F., Mousavi S.E., Dehpour A.R. Protective effect of minocycline on LPS-induced mitochondrial dysfunction and decreased seizure threshold through nitric oxide pathway. Eur. J. Pharmacol. 2019;858 doi: 10.1016/j.ejphar.2019.172446. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y.L., Han Q.Q., Gong W.Q., Pan D.H., Wang L.Z., Hu W., Yang M., Li B., Yu J., Liu Q. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J. Neuroinflammation. 2018;15:21. doi: 10.1186/s12974-018-1054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moini-Zanjani T., Ostad S.N., Labibi F., Ameli H., Mosaffa N., Sabetkasaei M. Minocycline effects on IL-6 concentration in macrophage and microglial cells in a rat model of neuropathic pain. Iran. Biomed. J. 2016;20:273–279. doi: 10.22045/ibj.2016.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C., Li X.H., Zhang S., Tu Y., Wang Y.M., Sun H.T. 7,8-Dihydroxyflavone ameliorates scopolamine-induced Alzheimer-like pathologic dysfunction. Rejuvenation Res. 2014;17(3):249–254. doi: 10.1089/rej.2013.1519. [DOI] [PubMed] [Google Scholar]
- 50.Bihaqi S.W., Singh A.P., Tiwari M. Supplementation of Convolvulus pluricaulis attenuates scopolamine-induced increased tau and amyloid precursor protein (AbetaPP) expression in rat brain. Indian J. Pharmacol. 2012;44(5):593–598. doi: 10.4103/0253-7613.100383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.