Abstract
Human epidermal growth factor receptor 3 (HER3) is a member of the human epidermal growth factor receptors family, having as its main ligands neuregulins 1 and 2. Although its poor tyrosine kinase activity entails a weak oncogenic power on its own, HER3 can heterodimerize with HER2 and/or epidermal growth factor receptor (EGFR), leading to a drastic enhancement of transphosphorylation and activation of downstream signaling pathways, ultimately promoting oncogenesis, metastatic dissemination, and drug resistance. Given its ubiquitous expression across solid tumors, multiple efforts have been done to therapeutically target HER3 by blocking either the ligand binding domain or its dimerization with other receptors. Treatment with anti-HER3 monoclonal antibodies or bispecific antibodies, both as single agents and in combination with other compounds, unfortunately led to unsatisfactory results across several tumor types. The HER3-directed delivery of cytotoxic payloads through antibody-drug conjugates has recently demonstrated encouraging activity in several tumor types, however, suggesting a potential role for the therapeutic targeting of HER3 in cancer treatment.
Key words: HER3, breast cancer, lung cancer, ADCs, patritumab deruxtecan
Highlights
-
•
HER3 is a tyrosine kinase receptor belonging to the HER family, ubiquitously expressed across different solid tumors.
-
•
HER3 has a role in tumorigenesis, disease progression, metastatic dissemination and anticancer drugs’ resistance.
-
•
Monoclonal antibodies and bispecific antibodies targeting HER3 have led to unsatisfactory results.
-
•
More promising results have been observed with novel ADCs, although burdened with hematologic and serious pulmonary toxicity
-
•
Biomarkers of response to HER3-directed agents need to be explored and prospectively validated.
Biological background
Human epidermal growth factor receptor 3 (HER3/ErbB3) is a tyrosine kinase receptor belonging to the HER family alongside epidermal growth factor receptor (EGFR; ErbB1 or HER1), HER2 (ErbB2), and HER4 (ErbB4).1 HER3 is a 180 kDa protein encoded by the ERBB3 gene on 12q13.2 Among the HER family members, HER3 is unique for different reasons. First, whereas EGFR binds several ligands and HER2 has none,3 the preferential activators of HER3 are neuregulins (NRGs) 1-2,4 also known as heregulins (HRG). Most importantly, HER3 has poor if no intracellular tyrosine kinase activity due to a divergence in critical residues in the intracellular kinase domain, which is locked in an inactive-like conformation, leading to a 1000-fold weaker kinase activity compared with EGFR.5 Nonetheless, HER3 is able to form heterodimers, preferentially with HER2 and/or EGFR, which dramatically enhance transphosphorylation and the consequent activation of mitogenic downstream pathways.6 Additionally, unlike other HER family members, HER3 dimerizes also with some non-HER receptors, namely mesenchymal-epithelial transition factor (MET) receptor and fibroblast growth factor receptor 2 (FGFR2).7,8 Furthermore, through a direct binding with the phosphoinositide-3 kinase (PI3K) p85 subunit, HER3 is a strong activator for PI3K/protein kinase B (AKT) signaling, pivotal for cancer survival.9 HER3 also activates mitogen-activated protein kinase (MAPK) cascade, janus kinase (JAK) and proto-oncogene c-Src (SRC) signaling pathways, all involved in cancer proliferation10,11 (Figure 1).
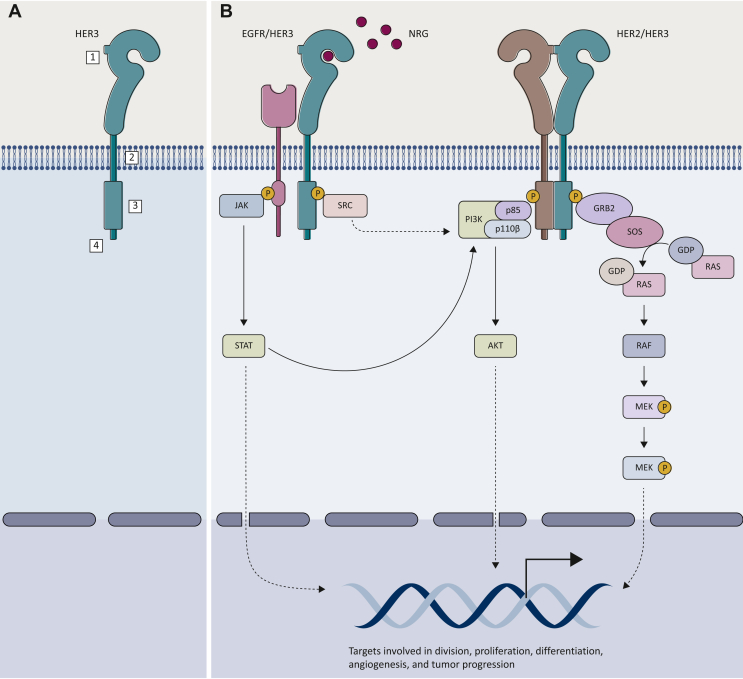
Figure 1.
HER3 and its signaling pathway. (A) In its monomeric inactivated form, HER3, as the other highly homologous HER family proteins, consists of four structural components playing different key roles: (i) a ligand-binding extracellular domain, (ii) a transmembrane domain, (iii) an intracellular kinase domain, and (iv) a C-terminal tail. (B) The binding between NRG1-2 and HER3 induces a conformational change resulting in its heterodimerization preferentially with EGFR and HER2. This causes a further conformational modification in the intracellular domain, leading to the transphosphorylation of the C-terminal tails, which eventually activates downstream intracellular signaling pathways, such as PI3K/AKT, MAPK, JAK/STAT and SRC, regulating several cellular processes including cell division, proliferation, differentiation, as well as angiogenesis and tumor progression.
AKT, protein kinase B; EGFR, epidermal growth factor receptor; GDP, guanosine diphosphate; GTP, guanosine triphosphate; GRB2, growth factor receptor bound protein 2; HER, human epidermal growth factor receptor; JAK, janus kinase; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated extracellular signal-regulated kinase; NRG, neuregulin; PI3K, phosphoinositide-3 kinase; SOS, son of sevenless; STAT, signal transducer and activator of transcription.
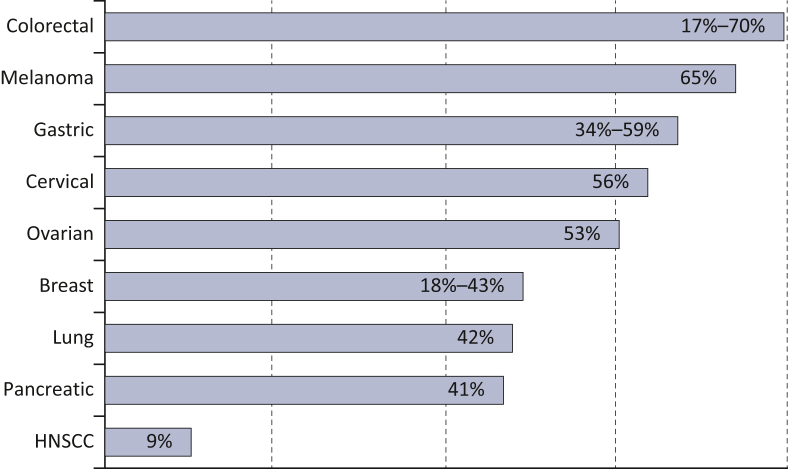
The role of HER3 in cancer biology is multifaceted. Ubiquitous HER3 expression is detected in various solid tumor types12 (Figure 2), with a proven role in disease progression. Two systematic analyses across multiple solid tumor types showed that HER3 expression was associated with worse overall survival, with a risk of death 1.60-fold higher compared with HER3-negative patients.12,13
Figure 2.
Rate of HER3 overexpression across different tumor types.
HNSCC, head and neck squamous cell carcinoma.
HER3 cooperates with other receptors not only to promote tumorigenesis and metastatic dissemination, but also to confer resistance to anticancer drugs.
Preclinical data showed that HER3 contributes to HER2-mediated resistance to tamoxifen, and, consequently, reduction of expression of HER3 can reverse this resistance in breast cancer (BC) cell lines.14 HER3 overexpression is also involved in resistance to fulvestrant: in BC cell lines, exposure to fulvestrant induced the expression and activity of HER3, significantly increasing cell proliferation compared with unexposed ones.15 HER3 confers resistance to HER2-targeted therapy too. Indeed, HER3 activates PI3K/AKT and SRC signaling pathways, two of the major molecular mechanisms involved in trastuzumab and lapatinib resistance, also through heterotrimers with HER2 and insulin-like growth factor-1 receptor (IGF-1R)11,16,17 and HRG-driven HER3-EGFR-PI3K-PDK1 signaling axis.18 Lastly, HER3 is linked to resistance to chemotherapy: indeed, combined HER3 and EGFR overexpression worsen BC-specific and distant metastasis-free survival after adjuvant chemotherapy in triple-negative BC (TNBC).19 Also, HER3 overexpression mediates paclitaxel resistance in HER2-overexpressing BC cell lines in a PI3K/AKT/mTOR signaling pathway-dependent fashion; once again, knocking down ErB3 expression can reverse this resistance mechanism.20 Up-regulated HER3 signaling is involved in resistance to several other targeted therapies used for treating several tumor types, including anti-EGFR drugs gefitinib and cetuximab.21,22 One of the multiple genomic alterations known to be involved in acquired resistance to anti-EGFR tyrosine kinase inhibitors (TKIs) in patients with EGFR-mutated advanced non-small-cell lung cancer (aNSCLC) is HER3 up-regulation induced by osimertinib. Thus blocking HER3/EGFR dimerization is hypothesized to prevent or delay both acquired and primary resistance to EGFR inhibitors.23 Moreover, MET amplification leads to gefitinib and erlotinib resistance via increased HER3/PI3K signaling.7 NRG1 and transcriptional HER3 activation are also involved in resistance to anaplastic lymphoma kinase (ALK) inhibitors and BRAF inhibitors, not only in NSCLC, but also in melanoma and thyroid cancer.24, 25, 26
Overall, there is a strong rationale behind the therapeutic targeting of HER3.
HER3-targeting with monoclonal antibodies
Monoclonal antibodies (MoAbs) target HER3 by blocking the ligand binding or the heterodimerization of the receptors (Table 1; Figure 3A).27 Several anti-HER3 MoAbs have been tested for therapeutic use in oncology and, despite a favorable toxicity profile in early clinical trials, objective responses were rarely observed, underscoring limited activity as single agents. Only three molecules demonstrated promising preliminary activity and progressed up to phase II and III clinical trials: patritumab (U3-1287), a fully human HER3-directed MoAb that binds to HRG and induces reduction of expression of HER3;28 seribantumab (MM-121), a fully human immunoglobulin G2 that inhibits HRG-mediated and downstream PI3K/AKT signaling;29 lumretuzumab (RO5479599), an immunoconjugate containing a humanized HER3-directed MoAb that binds to HER3 extracellular domain, inhibiting HER3 dimerization and EGFR-dependent signaling, and activates the immune system to exert antibody-dependent cellular cytotoxicity.30
Table 1.
HER3-targeted agents under clinical development
| Drug type | Name of the compound | Mechanism of action | Phase of clinical development | Sponsor |
|---|---|---|---|---|
| Monoclonal antibodies | Patritumab (U3-1287) | HER3-directed MoAb | Phase III | Daiichi Sankyo Co., Ltd |
| Seribantumab (MM-121) | HER3-directed MoAb | Phase II | Elevation Oncology | |
| Lumretuzumab (RO5479599) | Immunoconjugate containing a glycoengineered, humanized HER3-directed MoAb; ADCC | Phase Ib/II | Hoffmann-La Roche | |
| GSK2849330 | HER3-directed MoAb | Phase I | GlaxoSmithKline | |
| CDX-3379 | A human HER3-directed MoAb | Phase II | Celldex Therapeutics | |
| Barecetamab (ISU104) | A fully human HER3-directed MoAb. | Phase I | ISU Abxis Co., Ltd | |
| AV-203 | A humanized HER3-directed MoAb. | Phase I | AVEO Pharmaceuticals, Inc. | |
| Elgemtumab (LJM716) | HER3-directed MoAb | Phase I/II | MorphoSys/Novartis | |
| HMBD-001 | Anti-HER3 MoAb | Phase I/II | Hummingbird Bioscience | |
| U3P1287/01 (AMG888) | Anti-HER3 MoAb | Phase I | U3 Pharma GmbH | |
| SIBP-03 | HER3-directed recombinant humanized MoAb | Phase Ia | Shanghai Institute Of Biological Products | |
| Bispecific antibodies | Zenocutuzumab (MCLA-128) | HER2/HER3-directed IgG bispecific antibody; ADCC | Phase II | Merus N.V. |
| Sym013 | An antibody mixture composed of six humanized IgG1 MoAbs EGFR, HER2, and HER3 directed | Phase I/II | Symphogen A/S | |
| Isitarumab (MM-141) | HER3/IGF-1R-directed bispecific antibody | Phase II | Merrimack Pharmaceuticals | |
| SI-B001 | EGFR/HER3-directed bispecific IgG | Phase I | Sichuan Baili Pharmaceutical Co., Ltd | |
| MM-111 | HER2/HER3 bispecific antibody | Phase I | Merrimack Pharmaceuticals | |
| Duligotuzumab (MEHD7954A) | EGFR/HER3-directed bispecific antibody | Phase II | Genentech/Roche | |
| ADCs | Patritumab deruxtecan (U3 1402) | HER3-directed ADC, composed of patritumab, an HER3-directed MoAb, conjugated to the topoisomerase I inhibitor DX 8951 | Phase I/II | Daiichi Sankyo Co., Ltd |
ADC, antibody–drug conjugate; ADCC, antibody-dependent cellular cytotoxicity; EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; IGF-1R, insulin-like growth factor-1 receptor; IgG, immunoglobulin G; MoAb, monoclonal antibody.
Figure 3.
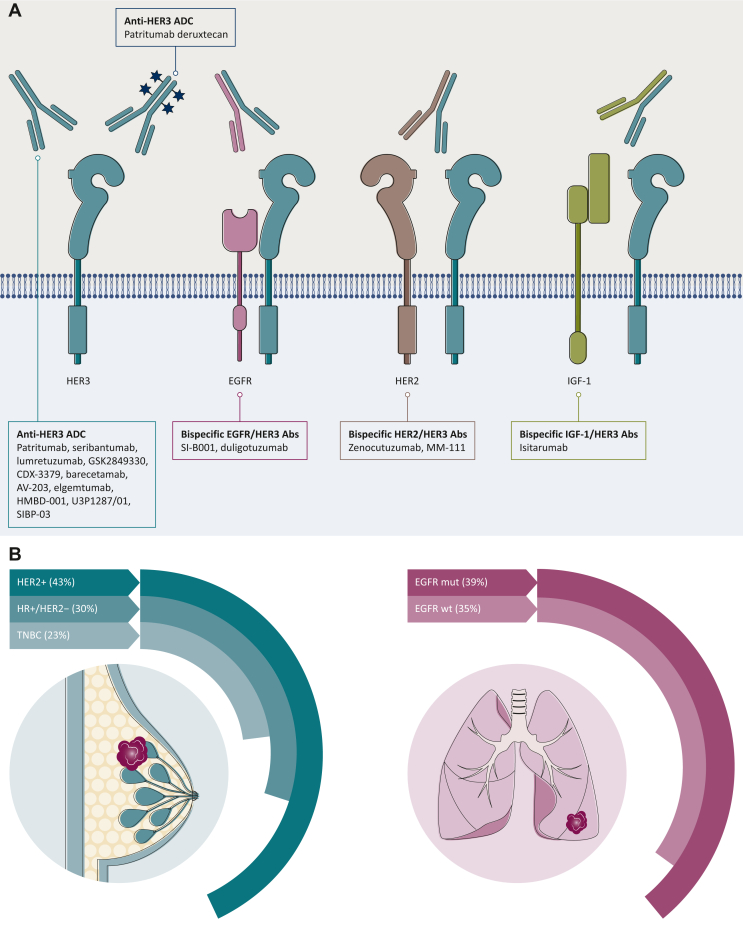
(A) Anti-HER3 therapeutic strategies. In the last decades, several anti-HER3 compounds with different mechanisms have been tested, unfortunately with disappointing results. Amongst them: MoAbs targeting HER3 preventing its heterodimerization with other receptors; bispecific Abs targeting both HER3 and EGFR or HER2 or IGF-1; the ADC patritumab-deruxtecan. (B) ORR of patritumab deruxtecan in pretreated mBC and advanced NSCLC. In BC, durable antitumor activity was observed across the range of HER3 expression in metastatic HR-positive/HER2-negative BC (ORR 30%; median DOR 7.2 months), TNBC (ORR 23%; median DOR 5.9 months) and HER2-positive BC (ORR 43%; median DOR 8.3 months) patients. In pretreated patients with EGFR-mutated and wild-type NSCLC, patritumab deruxtecan at the dose of 5.6 mg/kg achieved an ORR of 39% and 35%, respectively.
ADC, antibody–drug conjugate; BC, breast cancer; DOR, duration of response; EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; HR, hormone receptor; IGF-1, insulin-like growth factor-1; mBC, metastatic breast cancer; MoAb, monoclonal antibody; NSCLC, non-small-cell lung cancer; ORR, overall response rate; TNBC, triple-negative breast cancer.
Breast cancer
In patients with pretreated HER2-positive metastatic BC (mBC), a phase I study of patritumab, paclitaxel, and trastuzumab demonstrated manageable toxicities and encouraging preliminary activity.31
In a phase Ib trial, the addition of lumretuzumab to pertuzumab and paclitaxel in 35 patients with HER3-positive HER2-low (immunohistochemistry 1+ to 2+ and in situ hybridization negative) BC was instead burdened by a high incidence of diarrhea and the narrow therapeutic window limited further clinical development.32 As for the biomarker analysis, a phase II study of seribantumab plus exemestane in patients with hormone receptor (HR)-positive HER2-negative mBC highlighted a clinical benefit in the HRG-high subgroup.33 The company, however, prematurely terminated the phase II SHERBOC trial (NCT03241810) of seribantumab and fulvestrant in such population.
NSCLC
In the phase II HERALD study, patritumab added to erlotinib did not prolong progression-free survival (PFS) in an unselected population of 215 patients with aNSCLC and increased risk of gastrointestinal toxicity.34 Notably, PFS was improved for those tumors highly expressing HRG mRNA, although a cut-off point was not prospectively determined. The phase III HER-3Lung trial did not confirm the efficacy of patritumab and erlotinib in the subgroup of EGFR wild-type NSCLC patients with high HRG expression, however, leading to the premature termination of the trial.35
In EGFR wild-type NSCLC patients, the addition of seribantumab to erlotinib failed to improve PFS. Similarly to patritumab, a predefined retrospective analysis highlighted a PFS benefit in patients with detectable HRG mRNA,36 therefore, seribantumab received fast track designation for patients with HRG-positive aNSCLC. Nonetheless, the role or HRG as a biomarker of response to seribantumab remains controversial, as the company terminated the phase II SHERLOC trial (NCT02387216) of seribantumab and docetaxel in HRG-positive NSCLC patients, after it failed to meet its primary endpoint of improved PFS at the interim analysis.
Other tumors
A phase II trial evaluated the combination of the anti-HER3 MoAb CDX-3379 and cetuximab in a population of heavily pretreated patients with head and neck squamous cell carcinoma (HNSCC). Preliminary antitumor activity was observed with an acceptable safety profile, with diarrhea representing the most common treatment-related adverse event (TRAE).37 In a first-in-human phase I study of ISU104 in patients with advanced solid tumors, mucositis and diarrhea represented the most frequent TRAEs and among the 15 patients enrolled, seven stable disease and one partial response were observed.38
Beyond her3-directed moab: bispecific antibodies
To overcome the limits and resistance mechanisms to single-agent HER3-directed MoAb, bispecific antibodies have been tested in clinical trials. The most promising bispecific agent is the HER2/HER3-directed zenocutuzumab (MCLA-128), that inhibits HRG-stimulated HER3-dependent tumor growth and recruits natural killer (NK) cells into the tumor bed. Zenocutuzumab demonstrated clinical activity in patients with NRG1 fusion-positive solid tumors, including aNSCLC, BC, and pancreatic cancer,39,40 and it is currently under evaluation in the phase I/II eNRGy trial (NCT02912949).
Conversely, duligotuzumab (MEHD7954A), an EGFR/HER3-directed bispecific MoAb, failed to improve clinical outcomes compared with cetuximab in HNSCC in the phase II MEHGAN study, regardless of NRG1 expression, and was found to be burdened by a high incidence of gastrointestinal TRAEs.41 Moreover, the addition of duligotuzumab to FOLFIRI did not improve clinical outcomes compared with FOLFIRI/cetuximab in patients with KRAS wild-type metastatic colorectal cancer.42
The development of the bispecific HER3/IGF-1R-directed MoAb istiratumab (MM-141) was discontinued by the Sponsor following the negative results of the phase II CARRIE trial, as its addition to first-line nab-paclitaxel and gemcitabine did not show a clinical benefit in patients with metastatic pancreatic cancer with high IGF-1 serum levels.43
New horizon: the antibody-drug conjugates era
The most promising results have been observed with antibody–drug conjugates (ADCs), which allow for HER3-directed delivery of highly cytotoxic molecules to tumor cells. Patritumab deruxtecan (U3 1402; HER3-DXd) consists of patritumab linked, via a tetrapeptide-based cleavable linker, to a topoisomerase I inhibitor payload (DX-8951), that inhibits DNA replication and triggers apoptotic cell death. In addition, through DXd-induced cell damage and immune activation, HER3-DXd might elicit antitumor immune response.44 In vitro and in vivo, HER3-DXd sensitizes HER3-expressing cells for anti-programmed cell death protein 1 inhibition, therefore warranting the investigation of combinations with immune checkpoint inhibitors (ICIs).45 Table 2 summarizes current ongoing clinical trials on novel anti-HER3 strategies.
Table 2.
Current ongoing clinical trials investigating novel anti-HER3 strategies
| NCT number | Conditions | Setting | Phase | Treatment arms | Status | Population |
|---|---|---|---|---|---|---|
| NCT04610528 (TOT-HER3) | BC | Early | I | Patritumab deruxtecan | Recruiting | Enrollment: 80 |
| NCT05057013 | Solid tumors | Advanced or metastatic | I/II | HMBD-001 | Recruiting | Enrollment: 135 |
| NCT02980341 | BC | Advanced or metastatic | I/II | Patritumab deruxtecan | Active, not recruiting | Enrollment: 184 |
| NCT05504707 (DecipHER) | TNBC; HER2-negative BC | Early | I | HER2 and HER3 - primed dendritic cells | Recruiting | Enrollment: 30 |
| NCT04348747 | TNBC; HER2-positive BC | Advanced with brain metastasis | II | Anti-HER2/HER3 Dendritic cell Vaccine/pembrolizumab | Recruiting | Enrollment: 23 |
| NCT03065387 | Solid tumors | Advanced or metastatic | I | Neratinib/everolimus Neratinib/palbociclib Neratinib/trametinib |
Recruiting | Enrollment: 120 |
| NCT04603287 | Epithelial tumors | Advanced or metastatic | I | SI-B001 | Active, not recruiting | Enrollment: 60 |
| NCT03552406 | Solid tumors | Advanced | I | ISU104/cetuximab | Active, not recruiting | Enrollment: 33 |
| NCT04965766 (ICARUS-BREAST) | Breast cancer | Advanced or metastatic (second line) | II | Patritumab deruxtecan | Recruiting | Enrollment: 100 |
| NCT02912949 (eNRGy) | Solid tumors | Advanced or metastatic | II | Zenocutuzumab | Recruiting | Enrollment: 250 |
| NCT05338970 (HERTHENA-Lung02) | Non-squamous NSCLC | Advanced or metastatic | III | Patritumab deruxtecan versus platinum-based chemotherapy | Recruiting | Enrollment: 560 |
| NCT05203601 | Solid tumors | Advanced or metastatic | I | SIBP-03 | Recruiting | Enrollment: 68 |
| NCT04619004 (HERTHENA-Lung01) | EGFR-mutated NSCLC | Advanced or metastatic (>2 lines) | II | Patritumab deruxtecan fixed dose (group 1) Patritumab deruxtecan up-titration (group 2) |
Recruiting | Enrollment: 420 |
| NCT04676477 | EGFR-mutated NSCLC | Advanced or metastatic | I | Patritumab Deruxtecan/osimertinib | Recruiting | Enrollment: 252 |
| NCT04383210 | Solid tumors | Advanced or metastatic | II | Seribantumab | Recruiting | Enrollment: 75 |
| NCT05044897 | HNSCC | Recurrent | II | SI-B001 | Recruiting | Enrollment: 30 |
| NCT04699630 | BC | Advanced or metastatic | II | Patritumab deruxtecan | Recruiting | Enrollment: 120 |
| NCT03260491 | NSCLC | Advanced or metastatic | I | Patritumab deruxtecan | Recruiting | Enrollment: 264 |
| NCT04209465 | Solid tumors | Advanced or metastatic | I/II | BDTX-189 | Active, not recruiting | Enrollment: 91 |
BC, breast cancer; EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small-cell lung cancer; TNBC, triple-negative breast cancer.
Breast cancer
The phase I/II, first-in-human, U31402-A-J101 study evaluated HER3-DXd in 182 heavily pretreated patients with HER3-expressing mBC, showing promising and durable antitumor activity across all BC subtypes (Figure 3B). Overall, at both doses of 4.8 mg/kg and 6.4 mg/kg every 3 weeks, HER3-DXd showed a manageable toxicity profile and a low rate of discontinuation (9.9%) due to treatment-emergent adverse events (TEAEs), most commonly gastrointestinal and hematologic. Nonetheless, interstitial lung disease (ILD) was observed in 6.6% of patients, mostly grade 1 and 2 although one grade 5 event was observed. Grade 3 or higher hematologic toxicities were observed more frequently at the dose of 6.4 mg/kg and they were managed by dose delay or reduction. Thrombocytopenia occurred frequently (60.4% at 4.8 mg/kg and 71.4% at 6.4 mg/kg), but none resulted in a grade ≥3 bleeding event.46
Based on these encouraging results, HER3-DXd was investigated in the early setting. In the window-of-opportunity SOLTI TOT-HER3 study, patients with untreated HR-positive/HER2-negative resectable BC received a single preoperative dose of HER3-DXd (6.4 mg/kg). The primary endpoint was a variation in a combined score based on tumor cellularity and tumor-infiltrating lymphocytes (CelTIL score), that correlates with pathological complete response.47 Preoperative HER3-DXd led to clinically meaningful responses, with an ORR of 45% at C1D21 in the first 30 patients enrolled, alongside increased immune infiltration, suppressed proliferation, and increased CelTIL score in responders. No cases of ILD were reported and most frequent grade ≥3 TRAEs included diarrhea, neutropenia, and increased alanine aminotransferase levels.48 The TOT-HER3 substudy will continue enrollment in patients with TNBC and the SOLTI-VALENTINE will evaluate neoadjuvant HER3-DXd alone or in combination with endocrine therapy in HR-positive/HER2-negative BC. Furthermore, the phase II ICARUS-BREAST trial (NCT04965766) will investigate HER3-DXd in HR-positive BC patients with high HER3 expression progressing after cyclin-dependent kinase 4/6 inhibitors.
NSCLC
In the dose expansion cohort of a phase I trial, HER3-DXd (5.6 mg/Kg) achieved an ORR of 39% (24.4%-54.5%) in 44 patients with EGFR-mutated NSCLC progressed after osimertinib and platinum-based chemotherapy (PBC). At a median follow-up of 10.2 months, median duration of response was 6.9 months with a PFS of 8.2 months. The most common grade ≥3 TEAE was thrombocytopenia. ILD occurred in 5% of patients with one case of grade 5 event, although it was reported to be not related to study treatment.49 Based on these results, the phase II HERTENA-Lung01 (NCT04619004) is currently evaluating HER3-DXd in this population whereas the phase III HERTENA-Lung02 (NCT05338970) will compare HER3-DXd with PBC in EGFR-mutated NSCLC after failure of third-generation TKIs. The combination of HER3-DXd and osimertinib is also under evaluation in a phase I clinical trial (NCT04676477), both in first and second line. Interestingly, HER3-DXd (5.6 mg/kg) also achieved an ORR of 35% in a cohort of 47 patients with EGFR wild-type aNSCLC pretreated with PBC and ICIs (Figure 3B). Notably, HER3-DXd was active regardless of the presence of several other oncogenic alterations, including KRAS/NRAS mutations and ALK fusions. Myelotoxicity was the most common grade ≥3 TRAE and ILD was observed in 9% of the patients, without any drug-related deaths.49
Conclusion
Owing to its intrinsic impaired kinase activity, HER3 has been historically overlooked as a therapeutic target. Only recently, a deeper knowledge of its role and the interplay with other ErbB receptors has driven an interest in the development of HER3-directed agents. Anti-HER3 MoAbs showed limited activity as single agents across several solid tumors. Nonetheless, far more promising results have been observed with the targeted delivery of cytotoxic payloads through the ADC patritumab deruxtecan, although hematologic and potentially life-threatening pulmonary toxicities require prompt diagnosis and management. Biomarkers of response to HER3-directed agents need to be explored and prospectively validated, as the role of HRG mRNA expression remains controversial.
Funding
None declared.
Disclosure
GC honoraria for speaker’s engagement: Roche, Seattle Genetics, Novartis, Lilly, Pfizer, Foundation Medicine, NanoString, Samsung, Celltrion, Bristol Myers Squibb (BMS), Merck Sharp & Dohme (MSD); honoraria for providing consultancy: Roche, Seattle Genetics, NanoString; honoraria for participating in advisory board: Roche, Lilly, Pfizer, Foundation Medicine, Samsung, Celltrion, Mylan; honoraria for writing engagement: Novartis, BMS; honoraria for participation in Ellipsis Scientific Affairs Group; institutional research funding for conducting phase I and II clinical trials: Pfizer, Roche, Novartis, Sanofi, Celgene, Servier, Orion, AstraZeneca, Seattle Genetics, AbbVie, Tesaro, BMS, Merck Serono, MSD, Janssen-Cilag, Philogen, Bayer, Medivation, Medimmune. PT consulting role for AstraZeneca, Daiichi Sankyo, Lilly. All other authors have declared no conflicts of interest.
References
- 1.Hynes N.E., MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21(2):177–184. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Kraus MH, Issing W, Miki T, Popescu NC, Aaronson SA. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc Natl Acad Sci U S A. 86(23):9193-9197. [DOI] [PMC free article] [PubMed]
- 3.Burgess A.W., Cho H.-S., Eigenbrot C., et al. An open-and-shut case? recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12(3):541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 4.Montero J.C., Rodríguez-Barrueco R., Ocaña A., Díaz-Rodríguez E., Esparís-Ogando A., Pandiella A. Neuregulins and cancer. Clin Cancer Res. 2008;14(11):3237–3241. doi: 10.1158/1078-0432.CCR-07-5133. [DOI] [PubMed] [Google Scholar]
- 5.Jura N., Shan Y., Cao X., Shaw D.E., Kuriyan J. Structural analysis of the catalytically inactive kinase domain of the human EGF receptor 3. Proc Natl Acad Sci USA. 2009;106(51):21608–21613. doi: 10.1073/pnas.0912101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alimandi M., Romano A., Curia M.C., et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10(9):1813–1821. [PubMed] [Google Scholar]
- 7.Engelman J.A., Zejnullahu K., Mitsudomi T., et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 8.Kunii K., Davis L., Gorenstein J., et al. FGFR2 -amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res. 2008;68(7):2340–2348. doi: 10.1158/0008-5472.CAN-07-5229. [DOI] [PubMed] [Google Scholar]
- 9.Suenaga A., Takada N., Hatakeyama M., et al. Novel mechanism of interaction of p85 subunit of phosphatidylinositol 3-kinase and ErbB3 receptor-derived phosphotyrosyl peptides. J Biol Chem. 2005;280(2):1321–1326. doi: 10.1074/jbc.M410436200. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Kern JA. Neuregulin-1 activates the JAK-STAT pathway and regulates lung epithelial cell proliferation. Am J Respir Cell Mol Biol. 27(3):306-313. [DOI] [PubMed]
- 11.Huang X., Gao L., Wang S., et al. Heterotrimerization of the growth factor receptors erbB2, erbB3, and insulin-like growth factor-I receptor in breast cancer cells resistant to herceptin. Cancer Res. 2010;70(3):1204–1214. doi: 10.1158/0008-5472.CAN-09-3321. [DOI] [PubMed] [Google Scholar]
- 12.Ocana A., Vera-Badillo F., Seruga B., Templeton A., Pandiella A., Amir E. HER3 overexpression and survival in solid tumors: a meta-analysis. J Natl Cancer Inst. 2013;105(4):266–273. doi: 10.1093/jnci/djs501. [DOI] [PubMed] [Google Scholar]
- 13.Li Q., Zhang R., Yan H., et al. Prognostic significance of HER3 in patients with malignant solid tumors. Oncotarget. 2017;8(40):67140–67151. doi: 10.18632/oncotarget.18007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu B., Ordonez-Ercan D., Fan Z., Edgerton S.M., Yang X., Thor A.D. Downregulation of erbB3 abrogates erbB2-mediated tamoxifen resistance in breast cancer cells. Int J Cancer. 2007;120(9):1874–1882. doi: 10.1002/ijc.22423. [DOI] [PubMed] [Google Scholar]
- 15.Hutcheson I.R., Goddard L., Barrow D., et al. Fulvestrant-induced expression of ErbB3 and ErbB4 receptors sensitizes oestrogen receptor-positive breast cancer cells to heregulin β1. Breast Cancer Res. 2011;13(2):R29. doi: 10.1186/bcr2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S., Huang W.-C., Li P., et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17(4):461–469. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandarlapaty S., Sakr R.A., Giri D., et al. Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin Cancer Res. 2012;18(24):6784–6791. doi: 10.1158/1078-0432.CCR-12-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia W., Petricoin E.F., Zhao S., et al. An heregulin-EGFR-HER3 autocrine signaling axis can mediate acquired lapatinib resistance in HER2+ breast cancer models. Breast Cancer Res. 2013;15(5):R85. doi: 10.1186/bcr3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogden A., Bhattarai S., Sahoo B., et al. Combined HER3-EGFR score in triple-negative breast cancer provides prognostic and predictive significance superior to individual biomarkers. Sci Rep. 2020;10(1):3009. doi: 10.1038/s41598-020-59514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S., Huang X., Lee C.-K., Liu B. Elevated expression of erbB3 confers paclitaxel resistance in erbB2-overexpressing breast cancer cells via upregulation of Survivin. Oncogene. 2010;29(29):4225–4236. doi: 10.1038/onc.2010.180. [DOI] [PubMed] [Google Scholar]
- 21.Erjala K., Sundvall M., Junttila T.T., et al. Signaling via ErbB2 and ErbB3 associates with resistance and epidermal growth factor receptor (EGFR) amplification with sensitivity to EGFR inhibitor gefitinib in head and neck squamous cell carcinoma cells. Clin Cancer Res. 2006;12(13):4103–4111. doi: 10.1158/1078-0432.CCR-05-2404. [DOI] [PubMed] [Google Scholar]
- 22.Yonesaka K., Zejnullahu K., Okamoto I., et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med. 2011;3(99) doi: 10.1126/scitranslmed.3002442. 99ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romaniello D., Marrocco I., Belugali Nataraj N., et al. Targeting HER3, a catalytically defective receptor tyrosine kinase, prevents resistance of lung cancer to a third-generation EGFR kinase inhibitor. Cancers. 2020;12(9):2394. doi: 10.3390/cancers12092394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson F.H., Johannessen C.M., Piccioni F., et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell. 2015;27(3):397–408. doi: 10.1016/j.ccell.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prasetyanti P.R., Capone E., Barcaroli D., et al. ErbB-3 activation by NRG-1β sustains growth and promotes vemurafenib resistance in BRAF-V600E colon cancer stem cells (CSCs) Oncotarget. 2015;6(19):16902–16911. doi: 10.18632/oncotarget.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abel E.V., Basile K.J., Kugel C.H., et al. Melanoma adapts to RAF/MEK inhibitors through FOXD3-mediated upregulation of ERBB3. J Clin Invest. 2013;123(5):2155–2168. doi: 10.1172/JCI65780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacob W., James I., Hasmann M., Weisser M. Clinical development of HER3-targeting monoclonal antibodies: Perils and progress. Cancer Treat Rev. 2018;68:111–123. doi: 10.1016/j.ctrv.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 28.LoRusso P., Jänne P.A., Oliveira M., et al. Phase I study of U3-1287, a fully human anti-HER3 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2013;19(11):3078–3087. doi: 10.1158/1078-0432.CCR-12-3051. [DOI] [PubMed] [Google Scholar]
- 29.Denlinger C.S., Keedy V.L., Moyo V., MacBeath G., Shapiro G.I. Phase 1 dose escalation study of seribantumab (MM-121), an anti-HER3 monoclonal antibody, in patients with advanced solid tumors. Invest New Drugs. 2021;39(6):1604–1612. doi: 10.1007/s10637-021-01145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meulendijks D., Jacob W., Martinez-Garcia M., et al. First-in-human phase I study of lumretuzumab, a glycoengineered humanized anti-HER3 monoclonal antibody, in patients with metastatic or advanced HER3-positive solid tumors. Clin Cancer Res. 2016;22(4):877–885. doi: 10.1158/1078-0432.CCR-15-1683. [DOI] [PubMed] [Google Scholar]
- 31.Saeki T., Mukai H., Aogi K., et al. Phase I study of HER3 targeted antibody patritumab in combination with trastuzumab and paclitaxel in patients with HER2-overexpressing metastatic breast cancer (MBC) J Clin Oncol. 2015;33(15_suppl) 584. [Google Scholar]
- 32.Schneeweiss A., Park-Simon T.-W., Albanell J., et al. Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Invest New Drugs. 2018;36(5):848–859. doi: 10.1007/s10637-018-0562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins M.J., Doyle C., Paepke S., et al. A randomized, double-blind phase II trial of exemestane plus MM-121 (a monoclonal antibody targeting ErbB3) or placebo in postmenopausal women with locally advanced or metastatic ER+/PR+, HER2-negative breast cancer. J Clin Oncol. 2014;32(15_suppl) 587. [Google Scholar]
- 34.Von Pawel J., Tseng J., Dediu M., et al. Phase 2 HERALD study of patritumab (P) with erlotinib (E) in advanced NSCLC subjects (SBJs) J Clin Oncol. 2014;32(15_suppl) 8045. [Google Scholar]
- 35.Paz-Arez L., Serwatowski P., Szczęsna A., et al. P3.02b-045 patritumab plus erlotinib in EGFR wild-type advanced Non–Small Cell Lung Cancer (NSCLC): Part a results of HER3-lung study. J Thorac Oncol. 2017;12(1):S1214–S1215. [Google Scholar]
- 36.Sequist L.V., Gray J.E., Harb W.A., et al. Randomized phase II trial of seribantumab in combination with Erlotinib in patients with EGFR wild-type non-small cell lung cancer. Oncologist. 2019;24(8):1095–1102. doi: 10.1634/theoncologist.2018-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauman J.E., Saba N.F., Wise-Draper T.M., et al. CDX3379-04: Phase II evaluation of CDX-3379 in combination with cetuximab in patients with advanced head and neck squamous cell carcinoma (HNSCC) J Clin Oncol. 2019;37(15_suppl) 6025. [Google Scholar]
- 38.Kim S.-B., Keam B., Shin S., et al. First in human, a phase I study of ISU104, a novel ErbB3 monoclonal antibody, in patients with advanced solid tumours. Ann Oncol. 2019;30:v168. [Google Scholar]
- 39.Schram A.M., O’Reilly E.M., Somwar R., et al. Abstract PR02: Clinical proof of concept for MCLA-128, a bispecific HER2/3 antibody therapy, in NRG1 fusion-positive cancers. Mol. Cancer Ther. 2019;18(12_Supplement) PR02. [Google Scholar]
- 40.Schram A.M., O’Reilly E.M., O’Kane G.M., et al. Efficacy and safety of zenocutuzumab in advanced pancreas cancer and other solid tumors harboring NRG1 fusions. J Clin Oncol. 2021;39(15_suppl) 3003. [Google Scholar]
- 41.Fayette J., Wirth L., Oprean C., et al. Randomized phase II study of duligotuzumab (MEHD7945A) vs. cetuximab in squamous cell carcinoma of the head and neck (MEHGAN Study) Front Oncol. 2016;6:232. doi: 10.3389/fonc.2016.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill A.G., Findlay M.P., Burge M.E., et al. Phase II study of the dual EGFR/HER3 inhibitor duligotuzumab (MEHD7945A) versus cetuximab in combination with FOLFIRI in second-line RAS wild-type metastatic colorectal cancer. Clin Cancer Res. 2018;24(10):2276–2284. doi: 10.1158/1078-0432.CCR-17-0646. [DOI] [PubMed] [Google Scholar]
- 43.Ko A.H., Cubillo A., Kundranda M., et al. CARRIE: A randomized, double-blind, placebo-controlled phase II study of istiratumab (MM-141) plus nab-paclitaxel and gemcitabine versus nab-paclitaxel and gemcitabine in front-line metastatic pancreatic cancer. Ann Oncol. 2018;29:viii720. doi: 10.1016/j.annonc.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Hashimoto Y., Koyama K., Kamai Y., et al. A novel HER3-targeting antibody–drug conjugate, U3-1402, exhibits potent therapeutic efficacy through the delivery of cytotoxic payload by efficient internalization. Clin Cancer Res. 2019;25(23):7151–7161. doi: 10.1158/1078-0432.CCR-19-1745. [DOI] [PubMed] [Google Scholar]
- 45.Haratani K., Yonesaka K., Takamura S., et al. U3-1402 sensitizes HER3-expressing tumors to PD-1 blockade by immune activation. J Clin Invest. 2019;130(1):374–388. doi: 10.1172/JCI126598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krop I.E., Masuda N., Mukohara T., et al. Results from the phase 1/2 study of patritumab deruxtecan, a HER3-directed antibody-drug conjugate (ADC), in patients with HER3-expressing metastatic breast cancer (MBC) J Clin Oncol. 2022;40(16_suppl) doi: 10.1200/JCO.23.00882. 1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nuciforo P., Pascual T., Cortés J., et al. A predictive model of pathologic response based on tumor cellularity and tumor-infiltrating lymphocytes (CelTIL) in HER2-positive breast cancer treated with chemo-free dual HER2 blockade. Ann Oncol. 2018;29(1):170–177. doi: 10.1093/annonc/mdx647. [DOI] [PubMed] [Google Scholar]
- 48.Prat A., Falato C., Paré L., et al. Patritumab deruxtecan in early-stage HR+/HER2- breast cancer: final results of the SOLTI TOT-HER3 preoperative trial. Ann Oncol. 2022;33(suppl_3):S165–S174. [Google Scholar]
- 49.Steuer C.E., Hayashi H., Su W.-C., et al. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in advanced/metastatic non-small cell lung cancer (NSCLC) without EGFR -activating mutations. J Clin Oncol. 2022;40(16_suppl) 9017. [Google Scholar]