Abstract
Articular cartilage presents a mechanically sensitive tissue. Chondrocytes, the sole cell type residing in the tissue, perceive and react to physical cues as signals that significantly modulate their behavior. Hyaline cartilage is a connective tissue with high dissipative capabilities, able to increase its temperature during daily activities, thus providing a dynamic thermal milieu for the residing chondrocytes. This condition, self-heating, which is still chiefly ignored among the scientific community, adds a new thermal dimension in cartilage mechanobiology. Motivated by the lack of studies exploring this dynamic temperature increase as a potential stimulus in cartilage-engineered constructs, we aimed to elucidate whether loading-induced evolved temperature serves as an independent or complementary regulatory cue for chondrocyte function. In particular, we evaluated the chondrocytes’ response to thermal and/or mechanical stimulation in two types of scaffolds exhibiting dissipation levels close to healthy and degenerated articular cartilage. It was found, in both scaffold groups, that the combination of dynamic thermal and mechanical stimuli induced superior effects in the expression of major chondrogenic genes, such as SOX9 and LOXL2, compared to either signal alone. Similar effects were also observed in proteoglycan accumulation over time, along with increased mRNA transcription and synthesis of TRPV4, and for the first time demonstrated in chondrocytes, TREK1 ion channels. Conversely, the chondrogenic response of cells to isolated thermal or mechanical cues was generally scaffold-type dependent. Nonetheless, the significance of thermal stimulus as a chondro-inductive signal was better supported in both studied groups. Our data indicates that the temperature evolution is necessary for chondrocytes to more effectively perceive and translate applied mechanical loading.
Keywords: cartilage self-heating, mechanobiology, tissue engineering, bioreactors, extracellular matrix
1. Introduction
Hyaline cartilage develops within a biomechanical milieu which allows the formation of a unique structural tissue architecture, befitted to its biomechanical function.1,2 The sensitivity of chondrocytes to external biophysical stimuli and the subsequent adaptive responses to the extracellular matrix (ECM) present critical features of articular cartilage.3 In this way, and since mechanical cues regulate cell responses, dynamic mechanical stimulation has been deemed highly attractive during in vitro engineering of articular cartilage.4 Indeed, over the past decade, a strong correlation has been established among dynamic biophysical cues, such as dynamic compression, shear or hydrostatic pressure, and chondrocyte biological responses.4−6 Nonetheless, up to now, studies utilizing such cues during tissue culture normally consider a constant culture temperature, that is, however, far from reflecting the normal intra-articular knee temperature.
Different types of cells have been shown to respond to environmental temperature.7 However, only recently an expanding body of work has been addressing the potential role of this cue, specifically, on chondrocyte fate (phenotype and anabolism).8,9 Indeed, given that cell metabolism is temperature dependent, potential thermal changes in the microenvironment surrounding chondrocytes could constitute a signal that could significantly modulate their behavior. Chondrocytes thrive within a highly viscoelastic environment.10 Viscoelastic materials in turn can dissipate part of the mechanical energy through heat production, under dynamic loading, a phenomenon known as self-heating.11 It is therefore possible that a natural heat source could be developed, resulting in heat accumulation over time within healthy cartilage tissue. Besides cartilage dissipation, ambient temperature or heat transfer from surrounding tissues could contribute to the temperature evolution in the joint, to an extent, as well. Notably, in 2008 Becher and associates convincingly demonstrated that cartilage temperature can progressively increase from ∼32 °C (normal intra-articular knee temperature at rest) to ∼39 °C after 1 h of jogging, confirming the hypothesis that an internal heat source can be generated within the tissue, during cyclic loading.12
Accordingly, another study produced strong evidence that cartilage viscoelastic properties could induce a temperature rise in the tissue, optimal for proteoglycan production.13 Strikingly, osteoarthritic tissue was not capable of inducing the aforementioned temperature increase due to its reduced dissipative capacity. Recent in vivo studies also demonstrate a strict correlation among cartilage degeneration and cartilage dissipative capabilities. In 2019, for instance, Maier and colleagues showed that even early stage degenerated cartilage tissue samples with an ostensible normal appearance in structure and composition, can dissipate up to 50% less energy compared to healthy tissue.14
The inherent incapacity of degenerated cartilage to dissipate energy should be considered. This inability essentially denotes that the already proven capacity of cells to generate the proper extracellular components may not be enough to cope with degeneration, since chondrocytes can no longer perform under an appropriate “dynamic thermal environment”. This insight conceptually reveals a new distinct route that could be explored. Acknowledging the fact that the intra-articular cartilage temperature could significantly evolve during daily activities, might lead to the concept of using this natural thermal increase as an additional biophysical cue/signal, to more accurately mimic the dynamic environment of the tissue in vitro, thus improving chondrocyte functionality.
Currently, none of the conducted studies pertaining to chondrocytes is centered upon a biomimetic temperature evolution regime. Hence, the overarching purpose of the current research was to investigate the role of dynamic thermal signal in regulating the function of human chondrocytes, in the presence of mechanical cues. Building upon our prior work that showed positive effects of coupled thermomechanical cues on human progenitor cells at transcriptional level only,15 this study sought to promote matrix/protein accumulation within the engineered constructs (Figure 1). In particular, we aimed to answer whether the observed temperature evolution, as an indirect effect of mechanical loading in healthy cartilage, is necessary for effective mechanotransduction and subsequent chondrogenesis for adult chondrocytes as well. Toward mimicking the natural self-heating phenomenon in vitro, we utilized a bioreactor apparatus that we have recently designed, where both the evolution of temperature and mechanical load could be independently controlled.15 We then considered two poro-viscoelastic poly(2-hydroxyethyl methacrylate-co-ethylene glycol dimethacrylate)-based hydrogels, that we designed to exhibit comparable viscoelastic properties (energy dissipation level) to healthy and degenerated cartilage respectively, seeded with adult chondrocytes. We selected this type of hydrogel as no other formulation allows us to reach mechanical properties comparable to those of healthy tissue. Next, we evaluated the chondrocytes’ response following external application of isolated thermal or mechanical cues, or the combination of thereof. We hypothesized that a synergistic, biomimetic thermomechanical stimulation will augment the accumulation of hyaline cartilage-specific extracellular matrix, following enhanced chondrogenic expression of cells, regardless of the construct’s mechanical characteristics. We consistently found that chondrocytes can positively transduce the externally applied mechanical loading and maintain their biosynthetic capacity only in the presence of thermal stimulus. Conversely, the effect of dynamic mechanical loading alone on cells could vary depending on the hydrogel’s level of dissipation. Overall, our study shows that combined thermal and mechanical cues in the chondrocytes’ microenvironment contextualize each other to elicit a broad range of responses.
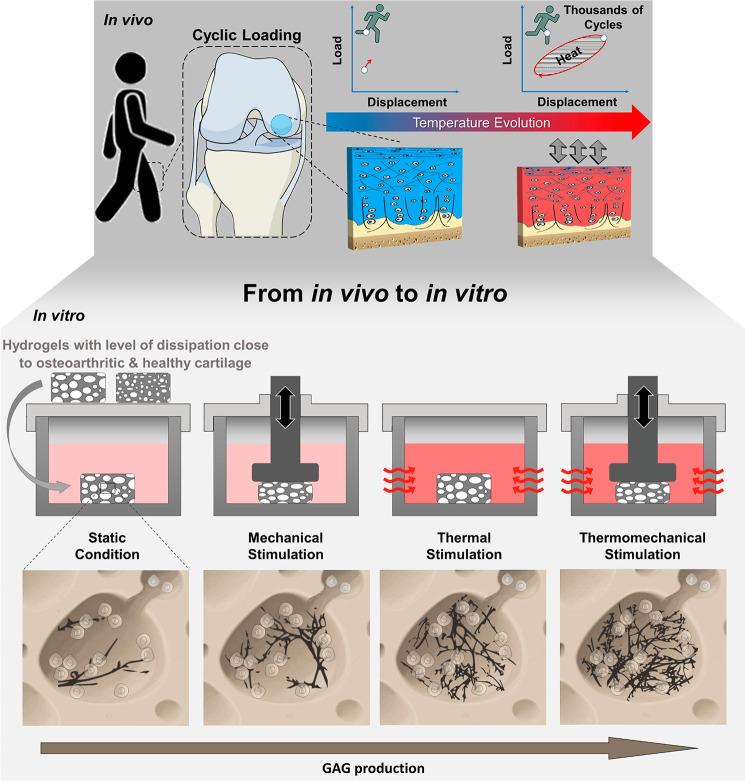
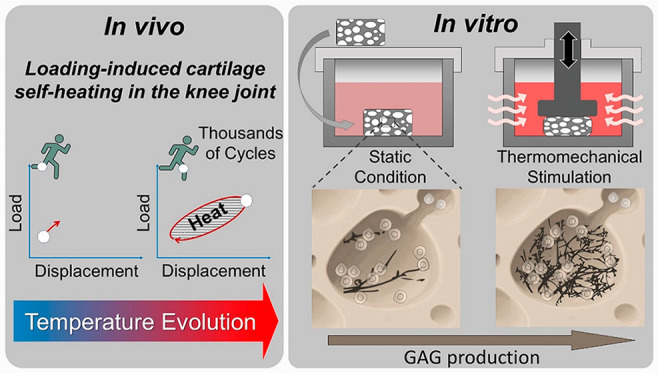
Figure 1.
Loading-induced self-heating in cartilage tissue. Energy dissipation, following joint loading, leads to a local heat accumulation inside cartilage tissue over time. Using a custom-made apparatus, cartilage self-heating can be mimicked in vitro to study its role on cartilage mechanobiology. Chondrocytes seeded in poro-viscoelastic hydrogels (mimicking both osteoarthritic and healthy cartilage tissue) produced greater amount of glycosaminoglycans (GAGs) when subjected to a combination of dynamic mechanical and thermal signal, compared to isolated forms of stimulation and control groups.
2. Materials and Methods
2.1. Fabrication of Poly(2-Hydroxyethyl Methacrylate-co-ethylene Glycol Dimethacrylate)-Based Hydrogels (p(HEMA-co-EGDMA)-Based Hydrogels) and Functionalization with RGD Peptides
Two different poly-2-hydroxyethyl methacrylate (HEMA, ≥ 99%, Sigma-Aldrich) based hydrogels were fabricated via the salt leaching method, so as to exhibit different dissipation levels when exposed to identical loading parameters. The levels of energy dissipation in turn, were chosen to span the levels of dissipation presenting in healthy and degenerated cartilage samples. This was achieved by changing the pore size among the hydrogels. Polymer solution (hydrogel precursor) was prepared as previously described,16 by mixing HEMA monomer with sodium metabisulfite/bidistilled water solution (0.526 M) (Reagent grade 97%, Sigma-Aldrich), ammonium persulfate/bidistilled water solution (0.438 M) (Biorad) and with (4.8%) ethylene glycol dimethacrylate cross-linker (EGDMA, 98%, Sigma-Aldrich). Hydrogel precursor was poured into cylindrical molds prefilled with salt crystals (150–250 μm and 300–400 μm respectively), and polymerization was achieved by heating at 65 °C, for 2 h. The resultant hydrogels were extensively rinsed with deionized water for 5 days to dissolve the trapped salt, ultimately resulting in macroporous structures. Morphological characterization of scaffolds was achieved through a Micro-CT scan (See Supporting Information page S2 for experimental details). Hydrogel RGD functionalization was performed following a two-step process described in detail elsewhere.15
2.2. Mechanical Characterization of Scaffolds
Cylindrical specimens (Ø:6, t: 2.2 mm) immersed in bidistillated water were subjected to unconfined compression experiments (10% prestrain, 1 Hz sinusoidal load of 10% amplitude) via an Electropuls Dynamic Test System (Instron E3000, Instron, Norwood, Massachusetts, USA), at room temperature. The level of energy dissipation was calculated by measuring the area embedded by the hysteresis loop, and it was further normalized to the volume of each sample.
2.3. Human Chondrocyte Expansion and Seeding
Primary human articular chondrocytes were acquired from the knee joint of a 22-year-old Caucasian, male donor, with no known musculoskeletal pathology (Innoprot, P10970). Chondrocytes were seeded and expanded on T75 plastic tissue culture flasks, up to passage 4, in chondrogenic culture medium (Alpha minimum essential medium (α-MEM)), supplemented with 10% fetal bovine serum (FBS), 1% l-glutamine, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 10 mM nonessential amino acids (NEEA), 1% penicillin, 1% streptomycin, with bioactive factors (5 ng/mL fibroblast growth factor (FGF) and 1 ng/mL transforming growth factor beta 1 (TGF-β1)).
Cell seeding inside the macroporous hydrogels (∼0.8 × 106 cells/scaffold, at passage 4) was achieved through an optimized dynamic cell-seeding method developed in our laboratory.17 Cell-hydrogel constructs were cultured at 32.5 °C and 5% CO2. The medium was refreshed every other day. After a preliminary 5-day culture period, constructs were treated with FBS-free α-MEM basal chondrocyte medium, supplemented with Insulin Transferrin Selenium (ITS-IV, 10%), l-ascorbic acid (VC, 1%), 5 ng/mL FGF, and 1 ng/mL TGF-β1.
2.4. Applied Modes of Stimulation
The cell-seeded constructs were categorized into 4 different groups; (1) samples that were mechanically conditioned under dynamic compression (termed as M), (2) samples that were subjected to dynamic thermal stimulation alone (termed as T) or (3) to a combined thermomechanical stimulus (termed as MT) and (4) control groups that remained at free swelling state at 32.5 °C (termed as C). All forms of stimulation were re-enacted via a custom-made bioreactor developed in our laboratory.15 The compressive regime was designed to mimic a normal physical activity. Dynamic mechanical stimulation involved subjecting engineered hydrogels to 20% compressive strain at 1 Hz frequency for 1.5 h. The scheme of the temperature increase following cyclic compression was modeled by a curve-fitting on reported in vivo data during jogging over a period of 1.5 h.12 When referring to combined thermomechanical stimulation, both dynamic compression and thermal stimulation were applied simultaneously. After each stimulation (a total of 3 rounds), specimens were allowed to recover. Stimulation was carried out every other day. A detailed experimental schematic can be seen in the supplementary file (Figure S1, page S2).
2.5. Assessment of Chondrocyte Growth and Viability
The PrestoBlue assay (ThermoFisher Scientific) was employed to determine chondrocyte proliferation inside the scaffolds. The PrestoBlue reagent was diluted 10 times inside normal basal medium, and samples were incubated for 1 h inside the Prestoblue measurement solution at 32.5 °C. The fluorescent signal was measured via Wallac microplate reader. Chondrocyte viability was monitored with calcein AM and ethidium homodimer (EH) staining (Biotium, Fremont, CA) and imaged by an inverted Leica SP8 confocal microscope.
2.6. RNA Isolation and qPCR
After the last loading cycle had ceased, each sample was placed in a 2 mL Eppendorf tube containing 300 μL of Trizol. RNA from each sample was isolated via Nucleospin RNA XS kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions. Hydrogels were homogenized in Trizol via polytron (Kinematica, Switzerland), while maintaining the tube cold on dried ice. Next, chloroform (100 μL) was added, and samples were centrifuged for 10 min at 12000 rpm at 4 °C. The aqueous phase was carefully transferred to 1.5 mL Eppendorf tubes, and the extraction was completed by adding 6.5 μL of RNA carrier and following the XS kit protocol. Total RNA amount was quantified via Nanodrop Lite Spectrophotometer (Thermo Scientific) and reversed transcribed into cDNA utilizing Taqman Reverse Transcription Reagents (Applied Biosystems) in 50 μL reaction volume containing master mix, random hexamer and RNA sample. Quantitative polymerase chain reaction (qPCR) was executed using the Fast SYBR Green PCR Master Mix (Applied Biosystems) in a final volume of 20 μL and containing 1 μL of synthesized cDNA. Respective primers were synthesized by Microsynth (Balgach, Switzerland). All sequences, as well as details pertaining to the thermal cycle condition during PCR can be seen in supplementary file (Table S1, page S4). Cycle threshold values were converted to fold expression changes (ΔΔCt method), following normalization to the housekeeping gene.
2.7. Biochemical Analysis
For the biochemical assessment of glycosaminoglycans (GAGs) and DNA content, hydrogels were digested overnight at 65 °C inside papain buffer solution (pH = 6.5), containing 100 mM Na2HPO4, 10 mM l-cysteine, 10 mM EDTA, and 6 μL ml–1 papain enzyme (Sigma-Aldrich). The GAG content was determined using the 1,9-dimethylmethylene blue assay, at pH 1.5. Sample absorbance was measured at 530 and 590 nm and compared to standard curve of bovine chondroitin sulfate (Sigma-Aldrich). DNA content was quantified using Hoechst 33258 DNA intercalating dye method (ThermoFisher Scientific), and purified Calf Thymus DNA was used as the standard.
2.8. Immunofluorescence Imaging
Immunofluorescence staining was performed to verify the expression of TREK1 and TRPV4 ion channels at protein level, and to visualize the accumulation of aggrecan following 11 days of culture. Briefly, samples were fixed in paraformaldehyde (4%), permeabilized with Triton X-100-PBS (0.25%) for 10 min and blocked in BSA (1%) within an hour. Next, samples were subjected to overnight incubation with the relevant primary antibodies at 4 °C. After washing with PBS/Tween, samples were incubated in relevant secondary antibodies for 1 h in the dark. Subsequently, cell nuclei were counterstained with DAPI (1:10000, Thermo Fisher) within 10 min. The relevant primary and secondary antibodies used can be found in supplementary file (Page S5).
Fluorescent slides of different samples were imaged using tile scan method with 20× magnification in an Olympus VS120 whole slide scanner. During the imaging process, the laser intensity and exposure duration were set consistent for all the samples. Tile scanned images were then imported into QuPath v0.3.2 before they were stitched for further analyses. Representative region of interest (ROI) were selected manually for each sample within which the nuclei of cells were detected and counted using the DAPI channel. Finally, the total intensity of the fluorescent signal of the corresponding channel (FITC) in the ROI were quantified and normalized by the number of cells detected.
2.9. Statistical Analysis
Analysis of variance (ANOVA), followed by Tukey’s post hoc tests, was performed to compare biochemical (n = 3) and gene expression (n = 3) data for multiple group comparisons. Data are presented as mean + standard deviation. (*) indicates p ≤ 0.05, (**) indicates p ≤ 0.01, (***) indicates p ≤ 0.001. Statistics were performed in Origin Pro 2021 software. Three independent experiments, considering three biological replicates for each experimental group, have been conducted to verify gene expression (PCR) and biochemical results (GAG and immunofluorescence).
3. Results
3.1. Dynamic Thermomechanical Stimulation Altered Gene Categories Encoding Various Signaling Pathways
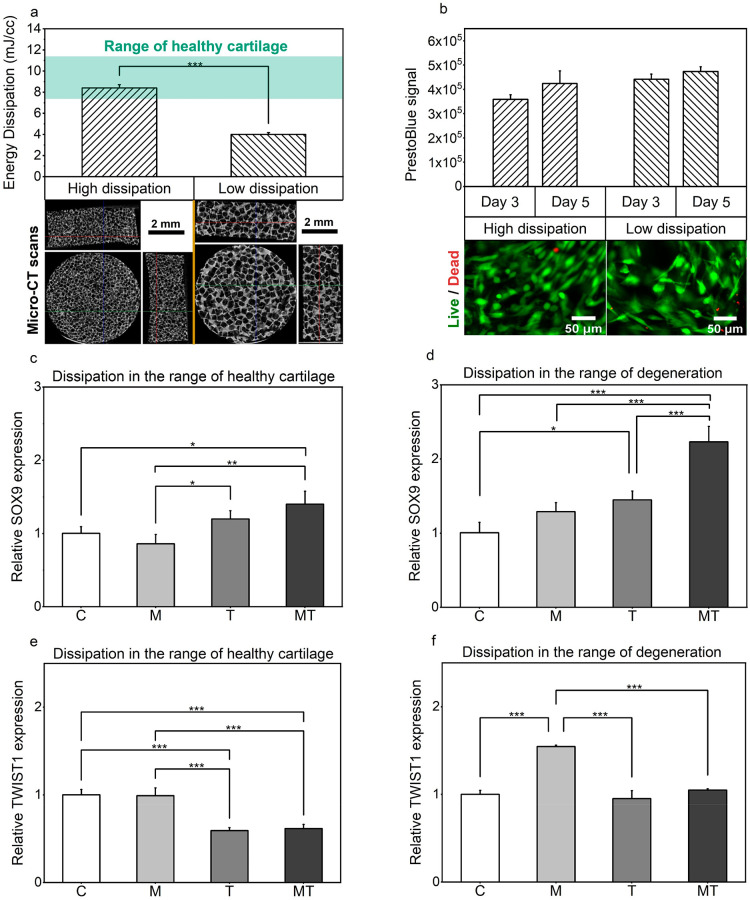
Cell-seeded constructs with different viscoelastic properties (energy dissipation level) (Figure 2a) were cultured at 32.5 °C under static conditions for 5 days, before subjecting to 1.5 h of dynamic stimulation. Prior to investigating temporal changes of gene expression following application of isolated or combined cues, we first assessed the response of human chondrocytes inside the RGD-modified hydrogels, before, as well as after stimulation. In particular, during the preculture period, cell metabolic activity was assessed using a resazurin assay. An increasing trend in the average fluorescent PrestoBlue signal was observed over time (Figure 2b), indicating a relative firm attachment and proliferation of the cells inside the porous hydrogels. Next, confocal microscopy was utilized to further assess the effects of intermittent dynamic stimulation on chondrocyte fate. Our results (Figure 2b) demonstrate a high viability and chondrocyte attachment inside the porous scaffolds following application of different stimuli.
Figure 2.
Chondrocyte behavior within RGD-modified hydrogels. (a) Energy dissipation level of the porous hydrogels together with the values reported for cartilage tissue and representative micro-CT scans of freeze-dried samples from different views.18 (b) Chondrocyte proliferation as quantified via PrestoBlue assay and typical live/dead confocal images at day 11, following thermomechanical stimulation. (c–f) Comparison of the relative expressions of genes of interest SOX9 and TWIST1 are shown for the different forms of stimulation and for the different levels of energy dissipation normalized to the control group.
In the current study, we were interested in examining the immediate changes on gene expression and the accumulation of proteins within the constructs. To elucidate the effects of thermal and mechanical cues on biological responses, we analyzed chondrocyte gene expression with regards to different stimuli. More specifically, quantitative real-time PCR analysis, on samples that were collected immediately after the last loading cycle had ceased, revealed that dynamic thermal and thermomechanical stimulation resulted in changes in the expression of genes related to cellular signaling pathways (SRY-related HMG-box gene 9, SOX9, and Twist Family BHLH Transcription Factor 1, TWIST1), matrix remodeling enzymes (Lysyl oxidase homologue 2, LOXL2), and structural proteins (Aggrecan, ACAN). In particular, a positive combined enhancement following thermomechanical stimulation was observed, as indicated by the increased expression of SOX9, a factor considered as the master regulator for chondrogenesis (Figure 2c). This trend was evident for all constructs, despite the level of energy dissipation. Interestingly, the effect of coupled thermomechanical stimulation was significantly more pronounced in the case of scaffolds showing reduced dissipation level, leading to an almost 2.5-fold increase of SOX9 expression over the unstimulated control groups (Figure 2d).
Additionally, expression of TWIST1 that is known to hinder chondrogenesis, was more than 40% decreased following thermal and thermomechanical stimuli, relative to both the control and mechanical groups (Figure 2e). This stimulatory response, however, was highly dependent on construct’s mechanical characteristics. Indeed, decreased dissipation level was shown to somewhat hinder the effect of isolated thermal or combined thermomechanical cues, as no significant changes were observed in the mRNA levels of TWIST1 when compared to the control groups (Figure 2f). On the other hand, however, in the latter samples, dynamic thermal and thermomechanical stimulation allowed for an almost 50% lower expression of TWIST1 relative to the samples that were stimulated only with dynamic mechanical loading.
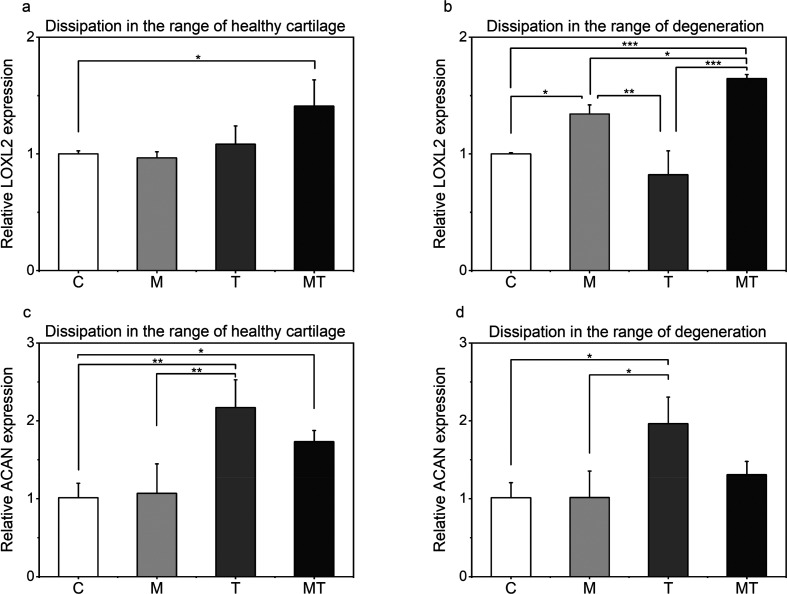
Lysyl oxidase homologue 2 (LOXL2), an enzyme that serves to cross-link collagen fibers inside the extracellular matrix of cartilage tissue, has been shown to significantly modulate chondrocyte differentiation.19 The expression of this enzyme following application of isolated forms of stimulation was scaffold-type dependent (Figure 3a and 3b). In particular, application of intermittent dynamic compression could significantly promote LOXL2 expression only in the case of low dissipative constructs, whereas no effects were observed as the dissipation level matched that of healthy tissue. It is also noticeable that the externally applied thermal increase over time could not affect the expression levels of LOXL2 when compared to the unstimulated control groups; however, a positive or negative average fold value could be observed depending on the scaffold type. Surprisingly a remarkable upstream of mRNA expression of LOXL2 (by 42% for the case of high dissipative constructs and by 60% for the case of “’degenerated”’ samples, over the control groups) can be seen when dynamic mechanical cues act concomitantly with dynamic thermal signal regardless of the scaffold’s mechanical attributes.
Figure 3.
Comparison of the relative expressions of genes of interest. (a-b) LOXL2 and (c-d) ACAN are shown for the different forms of stimulation and for the different levels of energy dissipation normalized to the control group.
Structural molecules such as aggrecan (ACAN) play a major role in the maintenance of hyaline cartilage. In line with studies exposing primary chondrocytes in different thermal environments,20 temperature evolution over time in the present study resulted in ∼90% to ∼120% more ACAN expression over the unstimulated control groups, as well as in ∼85% to ∼110% relative to the mechanical groups (Figure 3c and 3d). Additionally, unlike other genes, the level of energy dissipation did not alter the effect of each form of stimulation on ACAN expression. Nonetheless, a significant increase in ACAN expression was observed for constructs mimicking cartilage dissipative capacity under thermomechanical stimulation.
3.2. A Combination of Dynamic Compression and Thermal Stimulation Generated Synergistic Enhancements in Proteoglycan Synthesis, Along with Increased Transcription and Synthesis of TREK1 and TRPV4 Channels
Once the benefits at gene level were identified, we investigated the effects of isolated and combined cues on glycosaminoglycan (GAG) accumulation within the hydrogels. Dynamic compression has, in some cases, shown to favor proteoglycan synthesis in scaffold-based constructs. It was therefore of interest to investigate whether dynamic mechanical cues worked in tandem with dynamic thermal signal to stimulate additive improvements on chondrocytes biosynthetic responses.
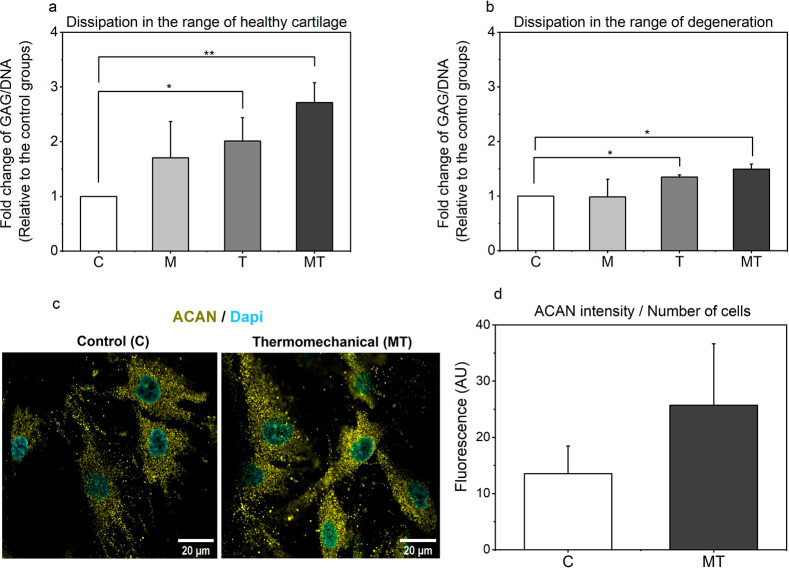
Constructs that were stimulated only with mechanical loading showed mixed results. In particular, GAG production following dynamic compression decreased in a dissipation-dependent manner and had no significant effect on the total GAG content. This trend is quite similar to what we observed at gene level. Samples exposed to isolated thermal stimulus exhibited a significantly higher amount of glycosaminoglycans compared to the free swelling controls, but not statistically different to those that were treated with dynamic mechanical loading alone. When dynamic compression was combined with dynamic thermal stimulation during culture, we observed the highest improvements in GAG content, when compared to the control groups. Even though the amount of GAG accumulated in the latter constructs is not statistically different to that of hydrogels that were treated only with dynamic thermal stimulation, these results show that by providing more biomimetic cues in vitro, we can augment the production of GAGs within the engineered constructs (Figure 4a and 4b).
Figure 4.
(a-b) Fold change of [Glycosaminoglycan/DNA] over the culture period of 11 days for the different dissipative hydrogel constructs following different forms of stimulation. (c) Typical confocal images of human chondrocytes seeded in dissipative in control and stimulated groups, (d) Integrated fluorescent intensities for aggrecan at 63× objective. Scale bar at 20 μm.
Furthermore, the average biosynthetic content accumulated in the hydrogels following application of isolated form of stimuli was generally affected by the level of construct’s dissipation. Interestingly, a higher level of dissipation allowed for enhanced average values of glycosaminoglycan accumulation. A similar trend was also observed when mechanical loading was applied concomitantly with dynamic thermal stimulus, resulting in more pronounced differences between the different hydrogels. Ultimately, despite the different mechanical characteristics of the hydrogels, application of mechanical loading could lead to significant changes in GAG content, only when applied together with dynamic thermal stimulation.
Deposition of aggrecan, a high molecular weight proteoglycan in cartilage tissue, comprising a protein core bearing many unbranched, negatively charged sulfated GAGs, was monitored with immunofluorescence staining. In line with the calorimetric data, aggrecan synthesis of chondrocytes was strongly enhanced by thermomechanical stimulation in 3D hydrogels, over the free swelling controls (Figure 4c and 4d).
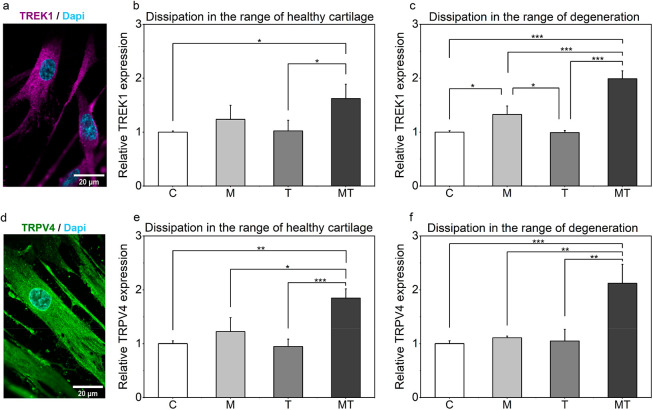
Toward examining the mechanism behind the enhanced GAG secretion following thermomechanical stimulus, we evaluated expression of the candidate signal mediators of thermomechanical cues. Our data imply that the “background K+ channel” (TREK1) and/or the transient receptor potential vanilloid 4 (TRPV4) channel may be involved in the transduction of self-heating. TREK1 channel has been widely studied in neurons and research has shown that it can be activated by heat.21−23 Our immunostaining results showed for the first time that this specific channel is expressed in primary human chondrocytes as well (Figure 5a). Transient receptor potential vanilloid (TRPV4) is also known to play a mechanosensory role in various musculoskeletal tissues.24,25 Expression of this channel was also verified in our studied system (Figure 4d). Thermomechanical stimulation significantly upregulated TREK1 and TRPV4 expression as illustrated in (Figure 5b and 5c) and (Figure 5e and 5f), suggesting that these ion channels are very likely involved in thermo-mechanotransduction.
Figure 5.
(a) Typical confocal image of TREK1 channel in RGD-modified hydrogels seeded with primary human chondrocytes. (b-c) Comparison of the relative expressions of genes of interest TREK1 are shown for the different forms of stimulation and for the different levels of energy dissipation. (d) Typical confocal image of TRPV4 channel in RGD-modified hydrogels seeded with primary human chondrocytes. (e-f) Comparison of the relative expressions of genes of interest TRPV4 are shown for the different forms of stimulation and for the different levels of energy dissipation. Scale bar at 20 μm at 63× objective.
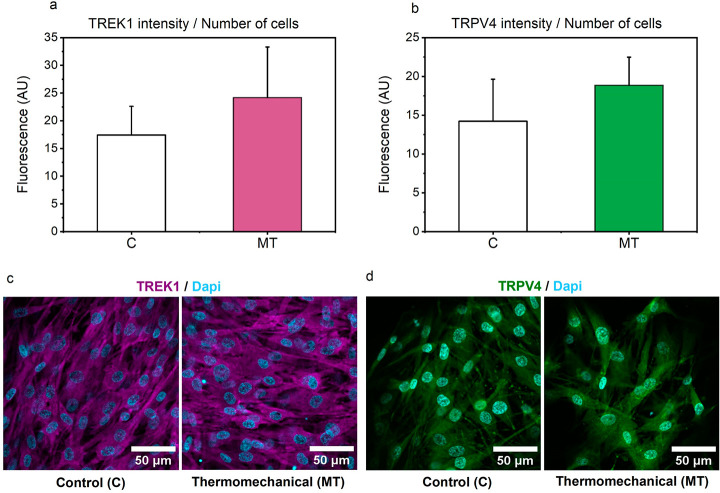
To gain a better understanding on these observations, we further analyzed these effects at the protein level. In line with the PCR outcomes, our results clearly demonstrate an upward trend in the average fluorescence intensity of both ion channels (Figure 6a–d) when constructs are subjected to biomimetic thermomechanical cues, strengthening the hypothesis that both ion channels are very likely participating in thermo-mechanotransduction process.
Figure 6.
(a-b) Integrated fluorescent intensities for TREK1 and TRPV4 ion channels. (c-d) Representative confocal images of human articular chondrocytes seeded in pHEMA-based hydrogels for TREK1 and TRPV4 channels after 12 days of culture for Control and Thermomechanically stimulated groups at 20× objective. No differences were observed based on the level of energy dissipation.
4. Discussion
Even though an appropriate strategy to engineering cartilage tissue analogues incorporates a broad array of variables that might be decisive, the goal of this study has been to examine one of them. More specifically, the already established influence of mechanical stimulation on chondrocyte metabolic response has been investigated in synergy with a naturally occurring dynamic temperature rise, measured in vivo during jogging and referred to as self-heating. Multiple research studies have previously described how different biochemical and biomechanical cues have been used to promote or even enhance chondrocyte metabolism.26−28 However, to the best of our knowledge, this inherent ability of the native cartilage to increase its temperature following a physical activity, is still largely ignored by the scientific community. Our group is the first to pioneer and introduce this latter concept.11,12,15 Urged by the central role of the biophysical interactions between chondrocytes and their environment in tissue formation and maintenance, this study was directed at elucidating the role of this new thermal signal (transient temperature increase from ∼32 °C to ∼39 °C) on cartilage-mimicking constructs. Previous studies have already considered the effect of static culture temperature on chondrocytes’ anabolism. Culturing adult chondrocytes under 32 °C for instance, allowed for a significant increase in the expression of major metabolic markers when compared to the standard culture conditions (37 °C).9 Similarly, another study also showed that the expression of matrix metalloproteinase-13 (MMP-13), a factor related to cartilage degradation, is significantly inhibited as the culture temperature is reduced to physiological values.8
Nonetheless, despite tantalizing hints that temperature regulates chondrocyte biological functions, not a single study is based on a biomimetic dynamic temperature regime. Temperature evolution following joint loading can be ascribed to different factors. Energy dissipation in avascular cartilage following a physical activity generates heat, resulting in temperature evolution over time. The absolute temperature increase, within the cartilage milieu, however, could also be affected by the ambient temperature and heat flux from surroundings tissues. Despite the actual heat source inside the joint, such varied thermal environment might affect chondrocyte biological functions. To reproduce such conditions in vitro, we seeded primary human chondrocytes into porous hydrogels that were placed inside a bioreactor apparatus designed for thermomechanical stimulation of cell-laden hydrogels.15 The hydrogels in turn, were engineered to capture the viscoelastic nature of healthy as well as degenerated cartilage, in terms of the energy dissipation level. It was of interest to understand whether the application of dynamic thermal signal in constructs with viscoelastic properties close to those of degenerated tissue, would also respond to the externally applied cues.
Our 3D scaffold system presents some limitations, e.g., RGD modification of synthetic scaffolds resulted in a less round morphology of chondrocytes. Nevertheless, considering that we utilized identically prepared samples during all of our experimental studies as well as growth factors to prevent chondrocyte dedifferentiation, we assume that the role of externally applied stimuli on cells’ chondrogenic response could be evaluated regardless of the morphology.
In the current mechanobiological study, cell-seeded constructs were precultured for 5 days, before subjecting them to intermittent dynamic stimulation. Dynamic stimulation has been shown to generate matrix biosynthesis much more efficiently in systems where relatively denser matrix-related components are present.29 Therefore, it was initially hypothesized that a preaccumulated extracellular matrix might better facilitate the transmission of thermomechanical stimulus in the dissipative hydrogels. Previous investigations have shown that a preculture of approximately 5 days is adequate for a significant up-regulation of cartilage-specific genes, such as cartilage oligomeric matrix protein, proteoglycan 4, collagen type II, and aggrecan following a relatively short-term stimulation, as well as for the formation of a pericellular matrix.29,30 We have confirmed these observations by showing that chondrocytes can produce functional proteins (such as collagen type 2) during 5 days of preculture (see supplementary data, page S4). Given that there is currently no available “universal” stimulating protocol and based on the fact that chondrocytes can produce collagen type 2 during 5 days preculture, we decided to proceed with the experimental protocol we used in our previous published study.15
To investigate the immediate changes on gene expression, constructs were sampled for total RNA isolation directly after (0 h) stimulation had ceased. Overall, application of a physiologically relevant compressive loading regime of 20% strain alone, could not significantly stimulate the expression of critical chondrogenic marker genes such as ACAN or SOX9. This trend was consistent, irrespective of the construct’s structural characteristics. Even though mechanical cues (dynamic compression) have been previously utilized to successfully induce chondrogenic markers expression and subsequent matrix deposition in mesenchymal stem cells,31 or in progenitor cells,18 a great corpus of evidence suggests that mature chondrocytes are less sensitive to this type of stimulation.32,33 This behavior is probably due to fast adaptations to such stimuli and subsequent desensitization.34 As for the constructs subjected to isolated thermal stimulus, our findings show that this naturally occurring temperature increase, as a byproduct of loading in healthy tissue, could possibly serve two main purposes: first and foremost, enhancing the transcription of genes that encode major structural components in the tissue, such as aggrecan; and second, suppressing the expression of factors that could potentially hamper chondrogenesis, such as in the case of TWIST1.
Major gene categories related to cartilage normal function appeared to be responsive to concomitant application of thermomechanical cues. Our results showed that the expression of SOX9 and LOXL2 peaked only when dynamic compression acted together with dynamic thermal stimulation. Additionally, with regards to LOXL2 expression, in some cases, individual forms of stimulation were ineffective or yielded even adverse effects in the expression of this enzyme. Spectacularly, when dynamic compression was combined with a biomimetic temperature increase there was always a direction toward highest expression. This trend is consistent and overall similar to the responsive pattern of TREK1 channel, encouraging us to believe that there is a link among potassium channels and lysyl oxidase enzymes. This latter observation is extremely important, and it illustrates that the obtained coupled improvements are distinct and exert a more pronounced effect on chondrocytes behavior over pure mechanical cues, confirming the complementary role of this thermal cue. Such additive enhancements were evident regardless of biomechanical construct’s differences.
Glycosaminoglycan content was also highly dependent on the type of stimulation, but also on the construct’s dissipative capacity. In agreement with the PCR outcomes, dynamic mechanical loading could not bring significant improvements in glycosaminoglycan synthesis over the control groups. In fact, dissipation level resembling cartilage degeneration resulted in decreased average GAG content following this form of stimulation. Furthermore, similar to findings with chondrocytes,20 between the stimulated groups, there was always a direction toward elevated glycosaminoglycan level in the groups containing dynamic thermal signal. Ultimately, when dynamic thermal stimulation was coupled with mechanical loading, significant GAG synthesis was observed, irrespective of the level of energy dissipation. Our results indicate that chondrocytes can perceive and translate the externally applied mechanical strain much more effectively when thriving in a dynamic thermal environment.
Herein it could be argued that it is unclear whether a difference in “absolute” temperature could not also explain the observed additive effects in the current study. With respect to the latter, we have already showed in chondroprogenitor cells, at transcriptional level, that loading-induced self-heating condition can better enhance major chondrogenic markers compared to mechanical stimulus at constant temperatures (32.5 or 37 °C).15 Additionally, culturing cells at high temperatures of approximately 39 °C have been shown to significantly prevent chondrocytes’ biosynthetic capacity; therefore, such conditions were not considered during our experimental design.8 Collectively, these results indicate that cells can sense the transient temperature rise.
Furthermore, glycosaminoglycan production following thermomechanical stimulation was higher in the hydrogels exhibiting increased dissipation levels. Energy dissipation is directly related to scaffold’s microstructure,35,36 which may affect cell behavior. Previous research has shown that polymeric scaffolds with enhanced energy dissipation levels improved expression of major chondrogenic markers.16 In agreement with the latter observations, our results show that the transmission of externally applied thermomechanical cues to cells can be significantly affected by the construct’s level of energy dissipation, resulting in different GAG contents within the constructs.
The increased GAG synthesis following combined thermal and mechanical stimuli led us to explore cellular mechanisms by which self-heating may affect the engineered constructs. The ultimate goal is to identify potential pathways through which mechanotransduction occurs to induce production of extracellular matrix components. It has already been shown that primary cilia activation in shear or Piezo channel activation during compression are key mediators for transduction of dynamic mechanical stimulation on primary chondrocytes.37,38 For the first time, we now add to this list the activation of the mechanically gated potassium channel TREK1, through thermomechanical stimulation. In peripheral sensory neurons, this polymodally gated ion channel allows for signal transmission and temperature perception.39 For chondrocytes, it has been proposed that related potassium channels allow for the influx of ions, stabilizing membrane potential.40 To the best of our knowledge, this study is the first to identify a specific type of potassium ion channel (TREK1), at protein level, that was not known to be expressed on primary human chondrocytes. Since our immunostaining data show that TREK1 actually exists in primary human chondrocytes, our results add to this body of work in connecting the TREK1 channel in transducing thermomechanical stimulation as well.
The current research work demonstrates that TRPV4 channels can be triggered by thermomechanical stimulation as well. Previous research in our lab showed that TRPV4 channels are potent modulators for the expression of major chondrogenic genes (e.g., collagen type 2) in human chondroprogenitor cells, in response to thermomechanical cues.15 In the case of primary chondrocytes, we hypothesize that application of thermomechanical stimulation involves concurrent modulation of both thermo-mechanotransducers, TREK1 and TRPV4. Even though additional research, including antagonizing these channels to demonstrate loss or gain of function, is required to conclusively identify thermomechanical stimulation mechanism of action on chondrocytes, this research study demonstrated that dynamic thermomechanical stimulation increases expression of potential signal mediators to facilitate thermo-mechanotransduction process and augment extracellular matrix secretion.
Furthermore, since we examined chondrocyte transcriptional response following isolated and combined thermomechanical cues only in short-term studies, further long-term experiments are required to fully explore the role of dynamic thermal or thermomechanical loading on the quality of the newly formed tissue. Nonetheless, this work was far from the scope of this research study. Even though supplementary studies are required to explore rigorously the modes of action of thermomechanical loading, our preliminary gene expression and matrix deposition findings denote that the early response to thermomechanical loading is involved in alterations in cellular signaling pathways (SOX9 and TWIST1), matrix remodelling (LOXL2), and structural proteins (ACAN and GAGs) which, eventually, may result in neo-cartilage formation with enhanced biomechanical properties.
5. Conclusion
In conclusion, this study aimed to answer whether the observed temperature evolution, as an indirect effect of mechanical loading in healthy cartilage tissue could be used to promote chondrogenesis in tissue-engineered constructs in vitro. Cellular response was evaluated through varied expression of major chondrogenic genes and subsequent downstream protein synthesis, following an applied stimulus. The results shown in this work notably demonstrate that biomimetic thermomechanical cues can induce tissue maturation through enhanced mRNA transcription, and an improvement in the content of major functional proteins. Overall, the current study places self-heating into the realm as a prominent approach for stimulating the metabolic as well as biosynthetic activity of primary human chondrocytes.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsbiomaterials.2c00723.
Additional experimental details including a schematic timeline of the mechanobiological study, typical live/dead fluorescent images for all constructs subjected to different forms of stimulation, total RNA and DNA content of constructs following different forms of stimulation, fluorescent images confirming synthesis of collagen type 2 and primers data table used for qRT-PCR and relevant primary and secondary antibodies used for immunostaining experiments (PDF)
Author Contributions
‡ (T.S., Y.G.) Equal contribution.
This research project is supported by a SNF grant # CR23I3_159301.
The authors declare no competing financial interest.
Supplementary Material
References
- Stampoultzis T.; Karami P.; Pioletti D. P. Thoughts on cartilage tissue engineering: A 21st century perspective. Curr. Res. Transl. Med. 2021, 69, 103299. 10.1016/j.retram.2021.103299. [DOI] [PubMed] [Google Scholar]
- Anderson D. E.; Johnstone B. Dynamic Mechanical Compression of Chondrocytes for Tissue Engineering: A Critical Review. Front. Bioeng. Biotechnol 2017, 5, 1–20. 10.3389/fbioe.2017.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Adams J.; Leddy H. A.; McNulty A. L.; O’Conor C. J.; Guilak F. The Mechanobiology of Articular Cartilage: Bearing the Burden of Osteoarthritis. Curr. Rheumatol. Rep 2014, 16, 451. 10.1007/s11926-014-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson B. M.; Walker C. J.; Maples M. M.; Randolph M. A.; Bryant S. J.; Anseth K. S. Mechanobiological Interactions between Dynamic Mechanical Loafing and Viscoelasticity on Chondrocytes in Hydrazone Covalent Adaptable Networks for Cartilage Tissue Engineering. Adv. Healthc. Mater. 2021, 10, 2002030. 10.1002/adhm.202002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharravi A. M.; Orazizadeh M.; Hashemitabar M. Fluid-induced low shear stress improves cartilage like tissue fabrication by encapsulating chondrocytes. Cell Tissue Bank 2016, 17, 117–122. 10.1007/s10561-015-9529-2. [DOI] [PubMed] [Google Scholar]
- Savadipour A.; Nims R. J.; Katz D. B.; Guilak F. Regulation of chondrocyte biosynthetic activity by dynamic hydrostatic pressure: the role of TRP channels. Connect. Tissue Res. 2022, 63, 69. 10.1080/03008207.2020.1871475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A.; Aoyama T.; Iijima H.; Nishitani K.; Tajino J.; Kuroki H. Periodic mild heat stimuli diminish extracellular matrix synthesis in pellet cultured human chondrocytes. BMC Res. Notes 2019, 12, 1–7. 10.1186/s13104-019-4058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A.; Aoyama T.; Tajino J.; Nagai M.; Yamaguchi S.; Iijima H.; Zhang X.; Akiyama H.; Kuroki H. Effects of the thermal environment on articular chondrocyte metabolism: A fundamental study to facilitate establishment of an effective thermotherapy for osteoarthritis. J. Jpn. Phys. Ther. Assoc 2014, 17, 14–21. 10.1298/jjpta.Vol17_003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A.; Nagai M.; Tajino J.; Yamaguchi S.; Iijima H.; Zhang X.; Aoyama T.; Kuroki H. Culture temperature affects human chondrocyte messenger RNA expression in monolayer and pellet culture systems. PLoS One 2015, 10, e0128082. 10.1371/journal.pone.0128082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal P.; Lee H. P.; Smeriglio P.; Grandi F.; Goodman F.; Chaudhuri O.; Bhutani N. A dysfunctional TRPV4–GSK3β pathway prevents osteoarthritic chondrocytes from sensing changes in extracellular matrix viscoelasticity. Nat. Biomed. Eng. 2021, 5, 1472. 10.1038/s41551-021-00691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Sayed P.; Vogel A.; Moghadam M. N.; Pioletti D. P. Cartilage self-heating contributes to chondrogenic expression. Eur. Cells Mater. 2013, 26, 171–178. 10.22203/eCM.v026a12. [DOI] [Google Scholar]
- Becher C.; Springer J.; Feil S.; Cerulli G.; Paessler H. H. Intra-articular temperatures of the knee in sports - An in-vivo study of jogging and alpine skiing. BMC Musculoskelet. Disord 2008, 9, 1–7. 10.1186/1471-2474-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Sayed P.; Moghadam M. N.; Salomir R.; Tchernin D.; Pioletti D. P. Intrinsic viscoelasticity increases temperature in knee cartilage under physiological loading. J. Mech Behav Biomed Mater. 2014, 30, 123–130. 10.1016/j.jmbbm.2013.10.025. [DOI] [PubMed] [Google Scholar]
- Maier F.; Lewis C. G.; Pierce D. M. The evolving large-strain shear responses of progressively osteoarthritic human cartilage. Osteoarthr. Cartil 2019, 27, 810–822. 10.1016/j.joca.2018.12.025. [DOI] [PubMed] [Google Scholar]
- Nasrollahzadeh N.; Karami P.; Wang J.; Bagheri L.; Guo Y.; Abdel-Sayed P.; Laurent-Applegate L.; Pioletti D. P. Temperature evolution following joint loading promotes chondrogenesis by synergistic cues via calcium signaling. eLife 2022, 11, e72068 10.7554/eLife.72068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Sayed P.; Darwiche S. E.; Kettenberger U.; Pioletti D. P. The role of energy dissipation of polymeric scaffolds in the mechanobiological modulation of chondrogenic expression. Biomaterials 2014, 35, 1890–1897. 10.1016/j.biomaterials.2013.11.048. [DOI] [PubMed] [Google Scholar]
- Nasrollahzadeh N.; Applegate L. A.; Pioletti D. P. Development of an Effective Cell Seeding Technique: Simulation, Implementation, and Analysis of Contributing Factors. Tissue Eng. Part C Methods 2017, 23, 485–496. 10.1089/ten.tec.2017.0108. [DOI] [PubMed] [Google Scholar]
- Nasrollahzadeh N.; Karami P.; Pioletti D. P. Control of Dissipation Sources: A Central Aspect for Enhancing the Mechanical and Mechanobiological Performances of Hydrogels. CS Appl. Mater. Interfaces 2019, 11, 39662–39671. 10.1021/acsami.9b15450. [DOI] [PubMed] [Google Scholar]
- Iftikhar M.; Hurtado P.; Bais M. V.; Wigner N.; Stephens D. N.; Gerstenfeld L. C.; Trackman P. C. Lysyl oxidase-like-2 (LOXL2) is a major isoform in chondrocytes and is critically required for differentiation. J. Biol. Chem. 2011, 286, 909–918. 10.1074/jbc.M110.155622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A.; Aoyama T.; Iijima H.; Nagai M.; Yamaguchi S.; Tajino J.; Zhang X.; Akiyama H.; Kuroki H.; et al. ‘Optimum temperature for extracellular matrix production by articular chondrocytes. Int. J. Hyperthermia 2014, 30, 96–101. 10.3109/02656736.2014.880857. [DOI] [PubMed] [Google Scholar]
- Maingret F.; Lauritzen I.; Patel A. J.; Heurteaux C.; Reyes R.; Lesage F.; Lazdunski M.; Honore E. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000, 19, 2483–2491. 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebe S.; Schellig K.; Lesage F.; Breer H.; Fleischer J. The thermosensitive potassium channel TREK-1 contributes to coolness-evoked responses of grueneberg ganglion neurons. Cell. Mol. Neurobiol 2014, 34, 113–122. 10.1007/s10571-013-9992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B. Y.; Jin Y.; Ma X. H.; Wang C. Y.; Guo Y.; Zhou D. The potential role of mechanically sensitive ion channels in the physiology, injury, and repair of articular cartilage. J. Orthop. Surg 2020, 28, 1–8. 10.1177/2309499020950262. [DOI] [PubMed] [Google Scholar]
- O’Conor C. J.; Leddy H. A.; Benefield H. C.; Liedtke W. B.; Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 1316–1321. 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C.; McCulloch C. A. TRPV4 integrates matrix mechanosensing with Ca2+ signaling to regulate extracellular matrix remodeling. FEBS J. 2021, 288, 5867. 10.1111/febs.15665. [DOI] [PubMed] [Google Scholar]
- Kwon H.; O’Leary S. A.; Hu J. C.; Athanasiou K. A. Translating the application of transforming growth factor-β1, chondroitinase-ABC, and lysyl oxidase-like 2 for mechanically robust tissue-engineered human neocartilage. J. Tissue Eng. Regen Med. 2019, 13, 283–294. 10.1002/term.2791. [DOI] [PubMed] [Google Scholar]
- Huwe L. W.; Sullan G. K.; Hu J. C.; Athanasiou K. A.; et al. ‘Using Costal Chondrocytes to Engineer Articular Cartilage with Applications of Passive Axial Compression and Bioactive Stimuli. Tissue Eng. Part A 2018, 24, 516–526. 10.1089/ten.tea.2017.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. K.; Huwe L. W.; Paschos N.; Aryaei A.; Gegg C. A.; Hu J. C.; Athanasiou K. A. Tension stimulation drives tissue formation in scaffold-free systems. Nat. Mater. 2017, 16, 864–873. 10.1038/nmat4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernike E.; Li Z.; Alini M.; Grad S. Effect of reduced oxygen tension and long-term mechanical stimulation on chondrocyte-polymer constructs. Cell Tissue Res. 2008, 331, 473–483. 10.1007/s00441-007-0500-9. [DOI] [PubMed] [Google Scholar]
- Owida H. A.; Kuiper N. L.; Yang Y. Maintenance and Acceleration of Pericellular Matrix Formation within 3D Cartilage Cell Culture Models. Cartilage 2021, 13, 847S–861S. 10.1177/1947603519870839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T.; Yu L.; Lim C. G.; Goodley A. S.; Xiao X.; Placone J. K.; Ferlin K. M.; Nguyen B.-N. B.; Hsieh A. H.; Fisher J. P. Effect of Dynamic Culture and Periodic Compression on Human Mesenchymal Stem Cell Proliferation and Chondrogenesis. Ann. Biomed. Eng. 2016, 44, 2103–2113. 10.1007/s10439-015-1510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinert C.; Schrobback K.; Hutmacher D. W.; Klein T. J. A novel bioreactor system for biaxial mechanical loading enhances the properties of tissue-engineered human cartilage. Sci. Rep 2017, 7, 16997. 10.1038/s41598-017-16523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas O.; De-Juan-Pardo E. M.; Meinert C.; D’Angella D.; Baldwin J. G.; Bray L. J.; Wellard R. M.; Kollmannsberger S.; Rank E.; Werner C.; Klein T. J.; Catelas I.; Hutmacher D. W. Biofabricated soft network composites for cartilage tissue engineering. Biofabrication 2017, 9, 025014. 10.1088/1758-5090/aa6b15. [DOI] [PubMed] [Google Scholar]
- Weber J. F.; Waldman S. D. Calcium signaling as a novel method to optimize the biosynthetic response of chondrocytes to dynamic mechanical loading. Biomech. Model. Mechanobio 2014, 13, 1387–1397. 10.1007/s10237-014-0580-x. [DOI] [PubMed] [Google Scholar]
- Karami P.; Nasrollahzadeh N.; Wyss C. S.; O’Sullivan A.; Broome M.; Procter P.; Bourban P. E.; Moser C.; Pioletti D. P. An Intrinsically-Adhesive Family of Injectable and Photo-Curable Hydrogels with Functional Physicochemical Performance for Regenerative Medicine. Macromol. Rapid Commun. 2021, 42, 2000660. 10.1002/marc.202000660. [DOI] [PubMed] [Google Scholar]
- Karami P.; Wyss C. S.; Khoushabi A.; Schmocker A.; Broome M.; Moser C.; Bourban P. E.; Pioletti D. P. Composite Double-Network Hydrogels to Improve Adhesion on Biological Surfaces. ACS Appl. Mater. Interfaces 2018, 10, 38692–38699. 10.1021/acsami.8b10735. [DOI] [PubMed] [Google Scholar]
- Salinas E. Y.; Aryaei A; Paschos N; Berson E; Kwon H; Hu J. C.; Athanasiou K. A. Athanasiou K.A. Shear stress induced by fluid flow produces improvements in tissue-engineered cartilage. Biofabrication 2020, 12, 045010. 10.1088/1758-5090/aba412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.; Leddy H. A.; Chen Y.; Lee S. H.; Zelenski N. A.; McNulty A. L.; Wu J.; Beicker K. N.; Coles J.; Zauscher S.; Grandl J.; Sachs F.; Guilak F.; Liedtke W. B. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, E5114–E5122. 10.1073/pnas.1414298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel J.; Zimmermann K.; Busserolles J.; Deval E.; Alloui A.; Diochot S.; Guy N.; Borsotto M.; Reeh P.; Eschalier A.; Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009, 28, 1308–1318. 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B.; Kondo C.; Belke D. D.; Giles W. R. Two-pore domain K+ channels regulate membrane potential of isolated human articular chondrocytes. J. Physiol 2011, 589, 5071–5089. 10.1113/jphysiol.2011.210757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.