Abstract
Introduction
This prospective study was conducted to investigate the prevalence and predictors of postoperative delirium (POD) in a cohort of patients aged ≥65 years who were scheduled to undergo elective spine surgery.
Methods
Patients aged ≥65 years who were scheduled to undergo elective spine surgery from February 2018 to May 2019 were prospectively recruited for this study. Delirium was diagnosed according to the Confusion Assessment Method algorithm. Candidate predictors included patient characteristics, comorbidities, surgical time, blood loss, preoperative laboratory parameters, and preoperative cognitive function, as assessed by the Mini-Cog test. These variables were compared between patients with and without POD. Multivariate logistic regression was performed to identify the independent predictors of POD. For the continuous variables, a receiver operating characteristic curve was used to determine the optimal cutoff value for predicting POD.
Results
Of the 106 patients included in the study, 12 (11.3%) patients developed POD, with a median time to onset of 3 d and median duration of 2 d. After adjusting for confounders, the occurrence of POD was independently associated with older age, a higher blood urea nitrogen (BUN) concentration, and a lower Mini-Cog score. The optimal cutoff point of the Mini-Cog score for predicting the occurrence of POD was ≤3.
Conclusions
POD was a common complication after spine surgery, showing an incidence of 11.3% in this study. Older age, a higher BUN concentration, and impaired cognition, as defined by the Mini-Cog, were independent predictors of POD. The current results may be useful for early identification of patients at risk of POD and facilitation of targeted interventions for preventing POD or mitigating its severity.
Keywords: spine surgery, delirium, cognitive impairment, renal dysfunction
Introduction
Many developing countries are currently facing the problem of an aging population because of the declining birth rate and rising life expectancy. According to the World Population Prospects 2019 published by the United Nations, one in six people worldwide will be older than 65 years by 2050, which has increased, from one in 11 people in 2019. This increase in the older population is associated with increases in the incidence of musculoskeletal degenerative conditions such as osteoarthritis, osteoporosis, spinal canal stenosis, and spinal deformity. Because musculoskeletal disorders have a markedly deleterious effect on health-related quality of life1), the number of older patients who seek musculoskeletal surgery, including spine surgery, is dramatically increasing.
Postoperative delirium (POD) is defined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), as an acute onset fluctuating change in the mental status, which is characterized by reduced awareness of the environment and a disturbance in attention2,3). POD is one of the most common complications in older patients, particularly after hip fracture and vascular surgery4). POD is associated with adverse outcomes, including impaired physical function, cognitive decline, a longer hospital stay, increased mortality, and increased healthcare costs5,6). Early identification of patients at risk for POD is important because timely interventions could prevent the occurrence of POD and its detrimental outcomes7). Furthermore, identification of the risk factors for POD may ultimately lead to a better understanding of the pathophysiology of POD.
Several studies have identified the risk factors for POD following spine surgery, such as older age, comorbidities, preexisting cognitive impairment, malnutrition, and greater blood loss8). However, the incidence of POD after spine surgery differs greatly, and the risk factors for POD are inconsistent. Thus, this study was performed to investigate the prevalence and predictors of POD in a prospective cohort of older patients undergoing elective spine surgery.
Materials and Methods
This study prospectively evaluated the risk factors for POD in patients undergoing elective spine surgery from February 2018 to May 2019. Patients aged ≥65 years who were scheduled to undergo spine surgery were eligible for inclusion. An institutional review board approval was obtained before study commencement. The protocol for this study followed the guidelines outlined in the Declaration of Helsinki and all of its later amendments. All participants provided a written informed consent at the time of enrollment.
The patients' symptoms and signs of delirium were observed and recorded by the nursing staff and attending doctors twice a day during their usual rounds in the ward. If POD was suspected based on the presence of symptoms, the diagnosis of POD was confirmed using the Confusion Assessment Method (CAM), which comprises four criteria: (1) acute onset and fluctuating course, (2) inattention, (3) disorganized thinking, and (4) altered level of consciousness9). The CAM algorithm for the diagnosis of delirium requires the presence of both the first and second criteria and either the third or fourth criteria. We also recorded the time of onset and duration of POD.
The patients were characterized at baseline according to sex, age, body mass index, comorbidities, number of medications, use of psychoactive medication, classification of anesthetic risk according to the American Association of Anesthesiologists (ASA) scoring system, lesion treated by spine surgery, operation time, amount of bleeding, and length of hospital stay. The severity of comorbidities was evaluated using the 14-system version of the Cumulative Illness Rating Scale (CIRS)10). Baseline cognitive function was assessed using the Mini-Cog test11,12), which combines two simple cognitive tasks (three-item word memory and clock drawing) with an empirical algorithm for scoring. It takes about 3 min to complete; tests visuospatial representation, recall, and executive function; and detects cognitive impairment or dementia with reasonable sensitivity and specificity12). The Mini-Cog score ranges from 0 (severe cognitive impairment) to 5 (normal cognition). A cutoff of ≤2 points on the Mini-Cog has been validated for dementia screening, and impaired cognition has been defined as a Mini-Cog score of ≤312).
We also collected data on the preoperative routine laboratory blood tests. These included the white blood cell count, red blood cell count, hemoglobin concentration, hematocrit, platelet count, total protein concentration, albumin concentration, aspartate transaminase concentration, alanine aminotransferase concentration, blood urea nitrogen (BUN) concentration, creatinine concentration, estimated glomerular filtration rate, sodium concentration, potassium concentration, chloride concentration, and C-reactive protein concentration.
Statistical analysis
The patients were divided into two groups according to the presence or absence of POD. Between-group comparisons were performed using the unpaired t-test for mean values, Pearson's chi-square test or Fisher's exact test for proportions, and the Wilcoxon rank-sum test for median values. To reveal the risk factors for POD, candidate risk factors, including patient characteristics, baseline functions, and laboratory blood test results, were compared between patients with and without POD. Variables that were found to be statistically significant in the univariate analyses were applied to multiple logistic regression models (forward stepwise selection, P<0.05) to adjust for confounders. Receiver operating characteristic (ROC) curves, which depict the relationship between true-positive (sensitivity) and false-positive (1 − specificity) cases, were constructed for the variables in the final model. The area under the ROC curve (AUC), which depicts the accuracy of the test, was calculated for each variable. In general, the AUC is interpreted as follows: 0.5-0.7 (low accuracy), 0.7-0.9 (moderate accuracy), and 0.9-1.0 (high accuracy)13). The optimum cutoff point that maximized the sensitivity and specificity of predicting POD was determined based on the ROC curve. All statistical analyses were performed using SPSS version 25 (IBM Corp., Armonk, NY, USA).
Results
In total, 106 patients were included in the study. Of the 106 patients, 12 (11.3%) patients developed POD, with a median time to onset of 3 d and median duration of 2 d. Table 1 compares the clinical characteristics, baseline functions, and surgical parameters between patients with and without POD. Patients with POD were significantly older, had a higher CIRS comorbidity score, were taking a larger number of medications, and showed a lower Mini-Cog score than those without POD. The length of hospital stay was also significantly longer in patients with than without POD. Comparisons of preoperative laboratory parameters between patients with and without POD are shown in Table 2. Patients with POD had a significantly lower hemoglobin concentration, lower albumin concentration, higher BUN concentration, higher creatinine concentration, and higher CRP concentration than patients without POD.
Table 1.
Comparisons of Patient Characteristics, Baseline Functions, and Surgical Parameters between Patients with and without Postoperative Delirium.
| Variable | Total
(n=106) |
With delirium
(n=12) |
Without delirium
(n=94) |
P value |
|---|---|---|---|---|
| Age (year) | 71.6±5.9 | 77.3±5.0 | 71.0±5.7 | <0.001 |
| Sex (male) | 65 (61) | 8 (67) | 57 (60) | 0.763 |
| BMI | 24.3±4.0 | 24.5±3.8 | 24.3±4.1 | 0.933 |
| CIRS Comorbidity Score | 8.2±4.0 | 10.5±4.8 | 8.0±4.0 | 0.048 |
| Number of medications | 6 (4–10) | 10 (6–12) | 6 (4–9) | 0.027 |
| Psychoactive medication use | 30 (28) | 6 (50) | 24 (26) | 0.076 |
| ASA PS classification | ||||
| ASA I | 4 (4) | 0 (0) | 4 (4) | 0.291 |
| ASA II | 76 (72) | 7 (58) | 69 (73) | |
| ASA III | 26 (24) | 5 (42) | 21 (22) | |
| Mini-Cog score | 3.8±1.1 | 2.9±0.9 | 3.9±1.1 | 0.006 |
| EQ-5D-3L | 0.56±0.18 | 0.58±0.05 | 0.56±0.19 | 0.804 |
| Surgical lesion | ||||
| Cervical spine | 35 (33) | 5 (42) | 30 (32) | 0.771 |
| Thoracic spine | 8 (8) | 1 (8) | 7 (7) | |
| Lumbar spine | 63 (59) | 6 (50) | 57 (61) | |
| Operative time (min) | 188.5±124.1 | 195.5±117.5 | 187.7±125.5 | 0.845 |
| Bleeding volume (mL) | 458.7±676.2 | 275.9±284.3 | 481.3±707.5 | 0.345 |
| Length of hospital stay (day) | 27.0±12.5 | 35.2±13.1 | 26.0±12.1 | 0.019 |
Data are shown as mean±standard deviation, number (%), or median (interquartile range). *P values were calculated using unpaired t-test for means, Fischer’s exact test for proportions, or Wilcoxon signed-rank test for medians. BMI, body mass index; CIRS, Cumulative Illness Rating Scale; ASA PS, American Society of Anesthesiologists Physical Status; EQ-5D-3L, EuroQol 5 Dimensions 3-level version
Table 2.
Comparisons of Patient Preoperative Laboratory Parameters between Patients with and without Postoperative Delirium.
| Laboratory parameter | Total
(n=106) |
With delirium
(n=12) |
Without delirium
(n=94) |
P value |
|---|---|---|---|---|
| White blood cell (103/L) | 5.8±1.8 | 6.2±1.8 | 5.8±1.8 | 0.498 |
| Red blood cell (103/L) | 407.2±62.4 | 377.0±63.4 | 411.1±61.6 | 0.075 |
| Hemoglobin (g/dL) | 12.9±1.9 | 11.7±1.9 | 13.0±1.9 | 0.025 |
| Platelet (×104/mm3) | 20.9±8.5 | 18.1±4.9 | 21.3±8.8 | 0.228 |
| Total protein (g/dL) | 7.0±0.5 | 7.0±0.8 | 7.0±0.5 | 0.759 |
| Albumin (g/dL) | 4.3±0.5 | 4.0±0.7 | 4.3±0.5 | 0.023 |
| AST (U/L) | 23.8±11.4 | 19.0±6.8 | 23.9±11.3 | 0.086 |
| ALT (U/L) | 21.1±12.7 | 16.3±6.4 | 21.3±12.9 | 0.121 |
| Blood urea nitrogen (mg/dL) | 21.4±10.3 | 30.0±17.4 | 20.4±8.5 | 0.002 |
| Creatine (mg/dL) | 1.6±1.9 | 2.7±3.0 | 1.4±1.7 | 0.032 |
| eGFR | 58.6±28.6 | 46.4±38.3 | 60.1±27.0 | 0.118 |
| Sodium | 141.2±2.6 | 140.8±2.1 | 141.3±2.7 | 0.561 |
| Potassium | 4.3±0.5 | 4.1±0.8 | 4.3±0.5 | 0.321 |
| Chlorine | 104.8±3.1 | 104.8±4.0 | 104.8±3.0 | 0.999 |
| C-reactive protein (mg/dL) | 0.4±1.3 | 1.4±3.5 | 0.3±0.7 | 0.010 |
Data are shown as mean±standard deviation, number (%), or median (interquartile range). *P values were calculated using unpaired t-test for means, Fischer’s exact test for proportions, or Wilcoxon signed-rank test for medians. AST, aspartate transaminase; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate
Multivariate logistic regression analysis identified older age (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.08-1.47), a lower Mini-Cog score (OR, 0.492; 95% CI, 0.25-0.97), and a higher BUN concentration (OR, 1.11; 95% CI, 1.03-1.21) as independent predictors of the occurrence of POD (Table 3). The adjusted R2 of the predictive model was 0.462.
Table 3.
Multivariate Logistic Regression Analysis.
| Variable | Odds ratio (95% CI) | P value |
|---|---|---|
| Age | 1.25 (1.08–1.47) | 0.004 |
| Mini-Cog | 0.49 (0.25–0.97) | 0.039 |
| BUN | 1.11 (1.03–1.21) | 0.011 |
CI, confidence interval; BUN, blood urea nitrogen. Nagelkerke’s generalized R2=0.462.
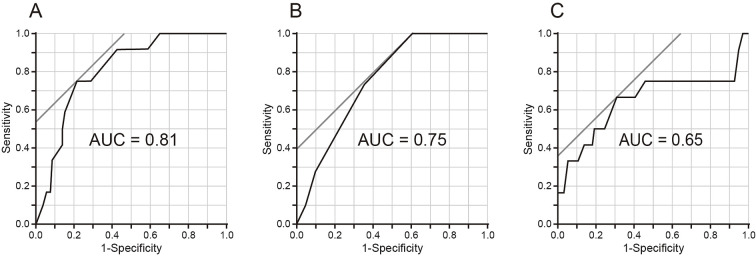
The ROC curves for age, the Mini-Cog score, and the BUN concentration are shown in Fig. 1. The optimal cutoff value for predicting the occurrence of POD was ≥75 years for age, with a sensitivity of 0.75 and specificity of 0.79; ≤3 points for the Mini-Cog score, with a sensitivity of 1.00 and specificity of 0.40; and ≥24 mg/dL for the BUN concentration, with a sensitivity of 0.67 and specificity of 0.69. The diagnostic accuracies defined by the AUCs were 0.81 for age, 0.75 for the Mini-Cog score, and 0.65 for the BUN concentration.
Figure 1.
Receiver operating characteristic (ROC) analysis. ROC curves were constructed to determine the optimal cutoff values of (A) age, (B) the Mini-Cog score, and (C) the blood urea nitrogen (BUN) concentration for predicting the occurrence of postoperative delirium (POD).
Discussion
In the present study, we investigated the predictors of POD in a prospective cohort of patients undergoing elective spine surgery. The three key findings of this study are as follows. First, 12 of the 106 patients (11.3%) developed POD, with a median time to onset of 3 d and median duration of 2 d. Second, after adjusting for numerous confounders, the occurrence of POD was independently associated with older age, a higher BUN concentration, and a lower Mini-Cog score. Third, the optimal cutoff value of the Mini-Cog score for identifying patients with an increased risk of POD was ≤3 points.
The incidence of POD following spine surgery ranges widely from 0.49% to 21.00% because of the heterogeneity across studies, such as the heterogeneity caused by differences in patients' age, comorbidities, surgical lesions, degree of surgical stress, and diagnostic criteria for POD14). A recent meta-analysis of 40 studies on spine surgery showed that the pooled incidence of POD was estimated at 8.0%, which was lower than that after knee and hip arthroplasty (17.6%)15) and hip fracture surgery (28.0%)16). The lower incidence could be partly explained by the missed diagnosis of POD in the retrospective studies included. Of the 40 studies included in the meta-analysis, 31 studies were conducted retrospectively, and the retrospective studies showed a lower incidence of POD than that in the prospective studies8). The slightly higher incidence of POD (11.3%) in the present study may also reflect the prospective identification of POD in this study. These findings highlight the importance of a prospective study design in assessing the incidence of POD.
Consistent with our results, older age has been shown to be a strong risk factor for POD regardless of the type of surgery7,17,18). POD occurs through the complex inter-relationship between multiple predisposing factors in a vulnerable patient and exposure to noxious insults or precipitating factors7). Older age is inherently associated with the accumulation of medical comorbidities and the decline of functional reserve18,19). Therefore, a relatively mild surgical insult may be enough to induce POD in older patients with multiple comorbidities and limited physiological reserve, whereas a younger patient in better health will be able to handle the stress of surgery.
In this study, coexistence of reduced renal function, as measured by the BUN concentration, was an independent predictor of POD. In line with our findings, several studies have demonstrated that patients with coexisting renal dysfunction have an increased risk of POD20-22). In a study of 293 patients undergoing lumbar decompression and fusion, Adogwa et al.21) showed that patients with chronic kidney disease (CKD) had a three-fold higher rate of delirium than those without CKD (27.8% vs. 8.4%, respectively). In a prospective cohort of 627 patients undergoing elective major abdominal surgery, Janssen et al.20) demonstrated that risk factors for POD included renal impairment, cognitive impairment, and an ASA score of ≥3 after adjusting for cofounding factors. Similarly, Sasajima et al.22) showed that POD was independently associated with an age of ≥72 years, end-stage renal failure, and cognitive impairment in patients who underwent bypass surgery for lower limb ischemia. One proposed mechanism underlying the development of POD in patients with CKD is the vascular hypothesis23). CKD-induced metabolic derangement causes a generalized, chronic proinflammatory state, leading to vascular remodeling processes and subsequent systemic atherosclerosis24). Cerebrovascular disease is an important risk factor for POD in patients undergoing cardiac surgery25). Furthermore, multiple uremic toxins have been shown to induce toxic metabolic encephalopathy, which is clinically diagnosed as “delirium” or an “acute confusion state26).”
Mild cognitive impairment or dementia is a risk factor for POD and is associated with increased morbidity and mortality in geriatric patients undergoing major elective surgeries2). In this study, we used the Mini-Cog, a brief screening tool designed to detect cognitive impairment or dementia. It takes only 3 min to complete; tests visuospatial representation, recall, and executive function; and detects dementia with reasonable sensitivity and specificity11). We found that 41 of 106 patients (39%) in the present study had cognitive impairment, as defined by a Mini-Cog score of ≤3, and the diagnosis of cognitive impairment was independently associated with the occurrence of POD. Consistent with our findings, Robinson et al.12) showed that the incidence of cognitive impairment, defined as a Mini-Cog score of ≤3, was 42% in older patients undergoing elective major surgery and that cognitive impairment was associated with an increased incidence of POD, longer hospital stay, higher rate of discharge institutionalization, and higher 6-month mortality. These results suggest that a relatively high rate of older patients undergoing elective surgery have previously undiagnosed cognitive impairment at baseline and that preoperative cognitive screening can help identify those at risk of postoperative cognitive and medical complications.
The current results may be useful for early identification of patients at risk of POD and facilitation of targeted interventions for preventing POD. Among the risk factors identified in this study, older age and cognitive dysfunction are non-modifiable risk factors, whereas an increased BUN concentration can be partly modifiable27). Treatment of a high BUN concentration by renal replacement therapy is associated with a significantly decreased risk of delirium and coma in patients with acute kidney injury28). An increased BUN concentration may also be associated with dehydration, which is a typical modifiable risk factor for delirium27). Therefore, preoperative control of uremia and appropriate hydration may decrease the risk of POD in patients with a high BUN concentration. Furthermore, it is important to minimize other modifiable risk factors such as opioid use, benzodiazepine use, uncontrolled pain, hypoxia, anemia, postoperative infection, and respiratory complications27).
This study has several limitations. First, because of the small sample size, we may have missed significant relationships between baseline variables and the occurrence of POD. Second, although our analysis was limited to spine surgeries, the degree of surgical stress as indicated by the blood loss volume and operation time varied widely. The heterogeneity in surgical stress may have affected the results of our analysis. Third, we did not assess the patients' history of alcohol abuse, which is an established risk factor for POD. Adding the history of alcohol abuse and the amount of alcohol consumption into the investigation may improve the accuracy of our predictive model. Finally, surgical postoperative outcomes were not analyzed in this study. POD can lead to poorer surgical outcomes such as increased postoperative complications, impaired physical function, and poorer long-term cognitive function. A further large-scale study is warranted to reveal the impact of POD on surgical outcomes.
In conclusion, POD was a common complication after elective spine surgery, showing an incidence of 11.3% in this study. Older age, a higher BUN concentration, and impaired cognition, as defined by the Mini-Cog, were independent predictors of POD. Adding a brief cognitive screening test such as the Mini-Cog into routine preoperative assessments may provide important information on the risk of POD in older patients undergoing spine surgery.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Sources of Funding: None.
Author Contributions: Atsushi Kimura designed the study; Atsushi Kimura, Yasuyuki Shiraishi, Hideaki Sawamura, Ryo Sugawara, and Hirokazu Inoue collected and analyzed the data; Katsushi Takeshita supervised the study; Atsushi Kimura wrote the manuscript.
Ethical Approval: This study was approved by the Institutional Review Board of Jichi Medical University (Approval code: A17-092).
Informed Consent: Informed consent for publication was obtained by all participants in this study.
References
- 1.Roux CH, Guillemin F, Boini S, et al. Impact of musculoskeletal disorders on quality of life: an inception cohort study. Ann Rheum Dis. 2005;64(4):606-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009;103(Suppl 1):i41-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson JE, Mart MF, Cunningham C, et al. Delirium. Nat Rev Dis Primers. 2020;6(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54(10):1578-89. [DOI] [PubMed] [Google Scholar]
- 5.Moskowitz EE, Overbey DM, Jones TS, et al. Post-operative delirium is associated with increased 5-year mortality. Am J Surg. 2017;214(6):1036-8. [DOI] [PubMed] [Google Scholar]
- 6.Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-23. [DOI] [PubMed] [Google Scholar]
- 7.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X, Sun W, Tan M. Incidence and risk factors for postoperative delirium in patients undergoing spine surgery: a systematic review and meta-analysis. Biomed Res Int. 2019;2019:2139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method (CAM): a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41(3):237-48. [DOI] [PubMed] [Google Scholar]
- 11.Borson S, Scanlan JM, Chen P, et al. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451-4. [DOI] [PubMed] [Google Scholar]
- 12.Robinson TN, Wu DS, Pointer LF, et al. Preoperative cognitive dysfunction is related to adverse postoperative outcomes in the elderly. J Am Coll Surg. 2012;215(1):12-7; discussion 7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45(1-2):23-41. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi K, Imagama S, Ando K, et al. Risk factors for delirium after spine surgery in extremely elderly patients aged 80 years or older and review of the literature: Japan Association of Spine Surgeons with Ambition Multicenter Study. Glob Spine J. 2017;7(6):560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rong X, Ding ZC, Yu HD, et al. Risk factors of postoperative delirium in the knee and hip replacement patients: a systematic review and meta-analysis. J Orthop Surg Res. 2021;16(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai J, Liang Y, Zhang P, et al. Association between postoperative delirium and mortality in elderly patients undergoing hip fractures surgery: a meta-analysis. Osteoporos Int. 2020;31(2):317-26. [DOI] [PubMed] [Google Scholar]
- 17.Iamaroon A, Wongviriyawong T, Sura-Arunsumrit P, et al. Incidence of and risk factors for postoperative delirium in older adult patients undergoing noncardiac surgery: a prospective study. BMC Geriatr. 2020;20(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korc-Grodzicki B, Root JC, Alici Y. Prevention of post-operative delirium in older patients with cancer undergoing surgery. J Geriatr Oncol. 2015;6(1):60-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255-63. [DOI] [PubMed] [Google Scholar]
- 20.Janssen TL, Steyerberg EW, Faes MC, et al. Risk factors for postoperative delirium after elective major abdominal surgery in elderly patients: a cohort study. Int J Surg. 2019;71:29-35. [DOI] [PubMed] [Google Scholar]
- 21.Adogwa O, Elsamadicy AA, Sergesketter A, et al. The impact of chronic kidney disease on postoperative outcomes in patients undergoing lumbar decompression and fusion. World Neurosurg. 2018;110:e266-e70. [DOI] [PubMed] [Google Scholar]
- 22.Sasajima Y, Sasajima T, Azuma N, et al. Factors related to postoperative delirium in patients with lower limb ischaemia: a prospective cohort study. Eur J Vasc Endovasc Surg. 2012;44(4):411-5. [DOI] [PubMed] [Google Scholar]
- 23.Arnold R, Issar T, Krishnan AV, et al. Neurological complications in chronic kidney disease. JRSM Cardiovasc Dis. 2016;5:2048004016677687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jankowski J, Floege J, Fliser D, et al. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gosselt AN, Slooter AJ, Boere PR, et al. Risk factors for delirium after on-pump cardiac surgery: a systematic review. Crit Care. 2015;19(1):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnan V, Leung LY, Caplan LR. A neurologist's approach to delirium: diagnosis and management of toxic metabolic encephalopathies. Eur J Intern Med. 2014;25(2):112-6. [DOI] [PubMed] [Google Scholar]
- 27.O'Regan NA, Fitzgerald J, Timmons S, et al. Delirium: a key challenge for perioperative care. Int J Surg. 2013;11(2):136-44. [DOI] [PubMed] [Google Scholar]
- 28.Siew ED, Fissell WH, Tripp CM, et al. Acute kidney injury as a risk factor for delirium and coma during critical illness. Am J Respir Crit Care Med. 2017;195(12):1597-607. [DOI] [PMC free article] [PubMed] [Google Scholar]