Abstract
Healthy individuals exhibit blood pressure variation over a 24-hour period with higher blood pressure during wakefulness and lower blood pressure during sleep. Loss or disruption of the blood pressure circadian rhythm has been linked to adverse health outcomes, e.g., cardiovascular disease, dementia, and chronic kidney disease. However, the current diagnostic and therapeutic approaches lack sufficient attention to the circadian rhythmicity of blood pressure. Sleep patterns, hormone release, eating habits, digestion, body temperature, renal and cardiovascular function and other important host functions as well as gut microbiota exhibit circadian rhythms, and influence circadian rhythms of blood pressure. Potential benefits of non-pharmacologic interventions such as meal timing, and pharmacologic chronotherapeutic interventions, such as the bedtime administration of antihypertensive medications, have recently been suggested in some studies. However, the mechanisms underlying circadian rhythm-mediated blood pressure regulation and the efficacy of chronotherapy in hypertension remain unclear. This review summarizes the results of an NHLBI workshop convened on October 27-29, 2021 to assess knowledge gaps and research opportunities in the study of circadian rhythm of blood pressure and chronotherapy for hypertension.
Keywords: circadian clock, kidney, vasculature, microbiota, sleep
Introduction.
Circadian rhythm and blood pressure (BP)
Cardiovascular disease (CVD) is the leading cause of death globally, and a profound economic burden.1,2 Hypertension (HTN) or high blood pressure (BP) is among the most important modifiable risk factors contributing to CVD. Compared with other risk factors, HTN is associated with more severe target organ damage, CVD events, and disability-adjusted life years lost in the US. New preventive measures and therapies for HTN and CVD are urgently needed. One promising emerging area is the translation of circadian biology to BP management for preventing CVD.3-9 Increased interest in this area has led to a pioneering new field of medicine: Circadian Medicine; the goal of this field is to leverage the power of circadian biology to improve human health.10-12 Circadian rhythms — the biological processes that recur naturally on approximately a 24-hour cycle, coordinated primarily by daily rhythms of light and dark — are evident in most bodily functions, including BP, sleep patterns, hormone release, eating habits, digestion, body temperature, and renal and cardiovascular function.13,14 The strictest definition of a circadian rhythm is a process exhibiting a 24 hr rhythm that persists in the absence of time cues such as light or food. In humans, this is tested using specific protocols under highly controlled conditions (reviewed in15). Because our goal is to improve blood pressure control in humans living their daily lives in their regular environment, herein we use the term circadian to encompass physiological functions that exhibit a 24 hr rhythm in rodents or humans living under normal environmental conditions with regular food and light cues.
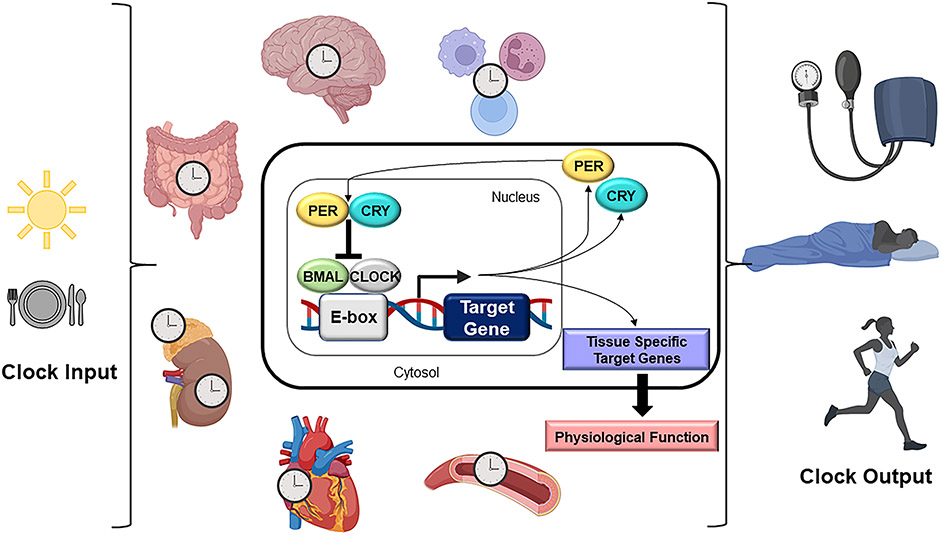
The molecular structure that governs the circadian clock in mammals is a transcriptional translation feedback loop that takes about 24 hours to complete and involves the core clock genes BMAL1, CLOCK, PER1/2, and CRY1/2. The molecular clock is present in virtually all major organs, tissues, and cells of an organism, and controls physiological outputs relevant to health and disease (Figure 1). The central clock is located in the suprachiasmatic nucleus in the hypothalamus of the brain, which provides signals to synchronize peripheral clocks in the rest of the body as the timing cue for a 24-hour day. For detailed review of the mechanisms governing circadian synchrony and communication between the central and peripheral clocks please see the following references.16-18
Figure 1. Physiology and molecular structure of circadian rhythms.
The molecular circadian clock is present in nearly every cell and tissue type (tissues relevant to this review are pictured). Clock inputs include light and food. The clock controls various physiological outputs, such as blood pressure, body temperature, hormone release, sleep/wake patterns and metabolism. The molecular structure (cell inset) of the circadian clock in mammals is a transcriptional translational feedback loop that takes about 24 hours to complete and involves the core clock genes BMAL1, CLOCK, PER1/2, and CRY1/2.
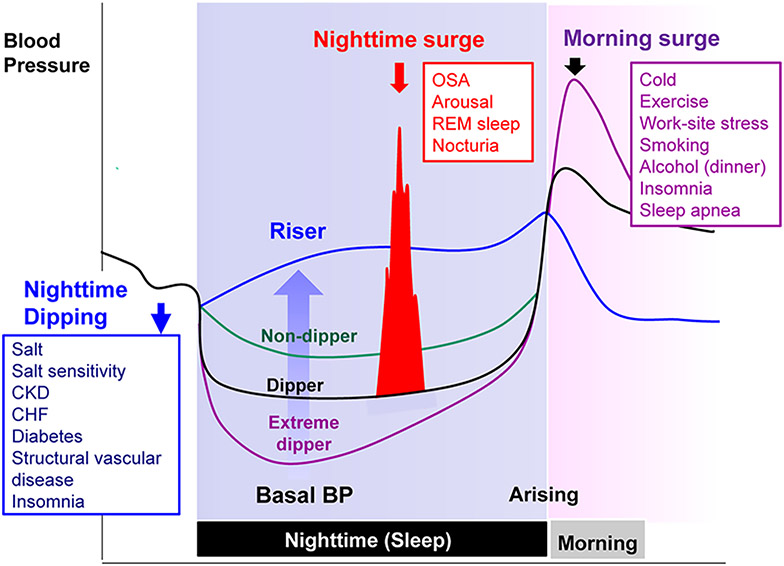
BP typically follows a day–night pattern characterized by an increase in BP after awakening and following by dip in BP at night during sleep. In the published literature, historically, phenotypes for nocturnal decline in BP in humans have included normal “dipping,” defined as an asleep phase drop in BP of 10% or more, and “non-dipping,” defined as a drop in BP of less than 10%. Phenotypes for BP dipping can also be categorized into four groups for which the dipping category (asleep phase drop in BP of 10% or more) can be further subdivided into dippers (asleep BP drop ≥10% to <20%) and extreme dippers (≥20%); and the non-dipping category (drop in BP of less than 10%) can be subdivided into non-dippers (≥0% to <10%) and risers or reverse dippers (<0%, BP during sleep).19 (Figure 2). In defining these categories, the thresholds for the drop in BP are arbitrary. Most of the evidence linking dipping categories to CVD outcomes have focused on systolic BP (SBP) rather than diastolic BP (DBP).20 It should be noted that whether human BP rhythms persist in the absence of timed food or light cues remains an open question.21-23 However, circadian BP rhythms in freely living healthy humans have been documented using intraarterial as well as non-invasive, cuff-based measures since at least the 1960s.24-29
Figure 2. Components of nocturnal hypertension and determinants—night-time dipping status and surge in blood pressure.
BP indicates blood pressure; CHF, chronic heart failure; CKD, chronic kidney disease; OSA, obstructive sleep apnea; and REM, rapid-eye-movement. Figure from Kario, Hypertension 2018, open access.19 Intense physical activity during the day and job strain can increase daytime BP and the day-night BP difference. Sedentary lifestyle during the day and fragmented nighttime sleep (elderly) can reduce the day-night BP difference.
Analysis of data from the International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcome (IDACO) revealed that non-dipping BP is associated with increased mortality and cardiovascular event risk.30 The Coronary Artery Risk Development in Young Adults (CARDIA) study reported that non-dipping BP is a heterogeneous phenotype, encompassing both those with high awake and high asleep BP and those with low awake and low asleep BP.31 However, focusing on non-dipping BP in a binary way (i.e., dipping or non-dipping) underestimates the complexities involved, including individual contributions of awake BP and asleep BP levels to outcomes. People with high nighttime systolic blood pressure (SBP) are more likely to be given a dementia diagnosis than those without high nighttime SBP.32 Lack of nocturnal BP dip and high nighttime BP are associated with higher risk of adverse renal outcomes. Likewise, isolated nocturnal HTN is associated with higher mortality and risk of cardiovascular events.33
The National Heart, Lung, and Blood Institute (NHLBI) convened a virtual workshop of multidisciplinary experts in the fields of HTN, circadian biology, and sleep science on October 27-29, 2021. The goals of this workshop were: (i) to review the current state of science and knowledge about circadian rhythm in BP regulation and chronotherapy for HTN and (ii) to identify research gaps and opportunities to bolster progress in understanding these two important connections. Five plenary sessions were followed by small group sessions and a final plenary session on the last day. Five session topics included: (i) Phenotypic Manifestations of Circadian Rhythm of BP; (ii) Abnormal Circadian Rhythm of BP, Target Organ Damage, and Disease; (iii) Mechanisms Influencing Circadian Rhythm of BP; (iv) Additional Factors Associated with Circadian Rhythm of BP; and (v) Interventions. In this report, we provide a summary of the workshop discussion and recent progress in this area.
Altered BP rhythm and hypertension (HTN)-related health risks
Based on ambulatory BP monitoring (ABPM) an abnormal BP rhythm, defined by reduced BP dipping or secondarily elevated nighttime BP, is associated with an increased risk of CVD events.30,34 For example, a meta-analysis demonstrated that among individuals from population-based samples, the adjusted hazard ratio of CVD events associated with a non-dipping BP pattern was 1.29 (95% confidence intervals [CI], 1.12-1.48).34 The association of reduced BP dipping with CVD events was independent of office BP and mean 24-hour BP on ABPM.30,34 A non-dipping BP pattern and nighttime hypertension (nighttime BP ≥120/70 mmHg) are common with a prevalence of 32-64% for non-dipping BP pattern and 27-40% for nighttime HTN35-39. Non-Hispanic Black adults experience the highest rates of HTN and CVD among all racial/ethnic groups, and there are known health disparities in treatment and CVD outcomes.40 There is evidence to suggest that African-American women and men have a high prevalence of non-dipping BP.41
Loss or dampening of rhythms, which is one example of circadian disruption,42 is common in chronic kidney disease (CKD) including loss of the normal circadian rhythm of BP43 and sleep disturbances.44 In the Chronic Renal Insufficiency Cohort Study (CRIC), ABPM revealed that 47% of CKD patients exhibited the non-dipping BP pattern and 16% the reverse dipper phenotype.45 Disruption in the BP circadian rhythm is associated with worsened kidney outcomes. Subsequent studies in CRIC demonstrated that the reverse dipper pattern was associated with a hazard ratio of 2.03 (95% CI, 1.44 to 2.85) and non-dipper pattern with hazard ratio of 1.28 (95% CI, 0.98 to 1.69) for risk of a 50% decrease in eGFR or end-stage kidney disease.43 Given the relative lack of effective treatments for CKD, these patients represent a population that could receive tremendous benefit from circadian-based therapeutic interventions to restore the normal BP rhythm.
Abnormal patterns of BP dipping have also been identified as risk factor for dementia. In the Maracaibo Aging Study, individuals with hypertension who have reverse BP dipping exhibited lower memory scores and more brain white matter lesions than dippers.46 Analysis of data from the Swedish Uppsala Longitudinal Study of Adult Men demonstrated that reverse BP dipping is associated with a 1.67-fold greater risk of being diagnosed with Alzheimer’s Disease.47
Studies have shown that potentially accumulating neuro-damaging waste products in the brain tissue during wakefulness are cleared from the brain during the first hours of sleep 48,49, i.e., a time during which BP usually dips.21 Intriguingly, as suggested by results from an animal study, the ability of the brain to flush out metabolic waste is reduced by arterial HTN.50 Thus, nocturnal BP dipping during sleep may be crucial to maintain brain health. These effects of HTN on the brain are further complicated by diet and behaviour factors that are contributing to HTN in the first place.
Measuring Circadian BP Rhythm
Measuring circadian BP rhythm in humans
ABPM provides intermittent BP sampling usually over the course of 24 hours. At present, it is considered the best method for diagnosing HTN and can assess the daily BP variation by obtaining readings across multiple settings (home, work) and activities (rest, activity) during the day, and during sleep.51 Unfortunately, ABPM is not widely available, reimbursement for ABPM is low or highly variable, is expensive compared to office- or home-based measurement, may be inconvenient and burdensome for some users due to cuff inflation (particularly at work and during sleep52), and exhibits intraindividual variability since BP is typically collected only during a single 24-hour period of monitoring53-57. Although the reproducibility of ABPM is superior to that of office BP readings for mean BP58, there is inconsistency in dipping status in about one-third of people with repeated ABPM.59
Low-cost self-home BP monitoring devices equipped with software that can take automated BP readings during nighttime sleep are a useful alternative for evaluating nocturnal HTN and the dipping pattern.60,61 Cuffless wearable BP monitors have the potential for providing a complete picture of 24-hour BP profile/behavior for days, weeks, or months, and can thereby reflect more accurately the burden of elevated BP on the cardiovascular system.62 However, the accuracy and clinical usefulness of these devices remain uncertain, and they are not currently recommended for clinical use.55
Extended period monitoring (i.e. greater than 24-hours) has been suggested to improve reproducibility of circadian patterns and estimation of overall BP control. For example, 48-hour ABPM with one-hourly BP measurement has been described as superior to conventional 24-hour ABPM with more frequent measurements.63 Given the high economic burden and life years lost due to high BP, any attempts to improve diagnostic accuracy, even if slightly more burdensome for the patient, may be highly warranted and the potential for 48-hour ABPM should continue to be explored.
Measuring circadian BP rhythm in animal models
Radiotelemetry is the gold standard for accurate BP measurement and is the only method that allows the longitudinal study of circadian rhythms of BP in pre-clinical models. In this method, telemetry devices connected to intra-arterial catheters are implanted within the animals under anaesthesia permitting the direct sensing of BP via the aorta or common carotid artery. Data acquisition occurs remotely, can be viewed in real-time, and processed offline as the experiment progresses. The radiotelemetry method results in minimal disruption to the behaviour of animals which are conscious, unrestrained, and untethered for data acquisition. One rather minor limitation in mice is that the transmitter is typically placed under the skin in the back or side of the animal that can provide some problems with specific feeding or restraint systems. This is not an issue with rats since the transmitter is much smaller compared to the animal and is more “hidden” within the abdominal cavity.
Typical battery life of a radiotelemetry device for mice is ~1.5 months which allows for longitudinal experimental design. This permits multiple replications of a 24-hour day to assess the reproducibility of daily rhythms and gives the potential to assess the impact of circadian disruptors or free running in the same mouse. This is a statistically powerful multiplexed experimental approach because SBP and DBP, heart rate, and activity can all be sampled simultaneously at a frequency and duration set by the researcher. This can generate dense multivariate data whereby post-acquisition analysis is not trivial. A widely-used practice is to condense data collected at intervals throughout the 24-hour day to a single mean. For circadian research, this data compression is a significant roadblock to realising the full potential of a rich cardiovascular data set. Analysis should consider time of day as an important concept with direct relevance to clinical management of hypertension in terms of translating preclinical nocturnal rodent data to diurnal humans. Ideally, 24-hour means should be avoided and instead data should be plotted as a function of time to visualise rhythms. Statistical analysis should include a temporal component. Further discussion of analyses for circadian rhythms is beyond the scope of this paper but is available elsewhere.64,65
Animal Model Studies Assessing Circadian BP Rhythms
Similar to humans, laboratory rodents exhibit clear circadian BP rhythms, providing preclinical models for understanding the role of circadian rhythms in cardiovascular physiology and pathophysiology. It should be noted that humans are mostly diurnal and lab rodents are nocturnal, so these rhythms are inverted in rodents relative to humans. One example is the use of altered light cycles to mimic shift work in humans. Spontaneously hypertensive rats subjected to environmental circadian disruption exhibited dampened rhythms in renal excretory function.66 Increased use of models of environmental and behavioral circadian disruption are needed to better approximate pathophysiology in humans who are subjected to chronic circadian disruption through shift work or excessive nighttime light exposure. Perhaps somewhat surprisingly, many animal models of HTN (i.e. chronic angiotensin II infusion or chronic high salt diet) maintain circadian BP rhythms although often at a wide range of amplitudes. In contrast, rodent models of diabetes and obesity such as the db/db mouse or chronic high fat diet have reduced day-night amplitude, without having an overall higher BP.67,68 These models are critical given that loss of the BP rhythm even in normotensive humans is still associated with adverse health outcomes.69 Of significance, global knockout of any of the core clock genes in mice is associated with a BP phenotype whether it be complete loss of BP rhythms, reduced BP with maintained rhythms, or isolated change in either day and night BP (reviewed in 70). Little is known about whether clock gene deletion in other species can recapitulate the same BP phenotype in mice. In fact, a recent report in the BMAL1 knockout rat revealed a different BP rhythm phenotype compared to the mouse equivalent.71,72 A number of other cell-specific clock gene knockout models have revealed that the canonical clock mechanism may function differently across different tissues and cell types; much more work is needed to provide clarity. Specific mechanisms examined in the various models can be found in several recent reviews.73,74 In the section below, we discuss circadian mechanisms in tissues that contribute to BP regulation.
Kidney
Kidney function is inextricably linked to cardiovascular outcomes. The kidney maintains homeostasis through the tight regulation of electrolyte and fluid balance in the body. Circadian rhythms in renal function, including electrolyte excretion and glomerular filtration rate, are well-established.75 The use of kidney-specific knockout of core clock components in mice has recently yielded critical information about the role of the circadian mechanism in regulating kidney function76. Knockout of BMAL1 using tubule-specific Cre drivers results in lower BP in male77-80 but not female mice.79,80 Interestingly, the circadian rhythm of BP remained intact in these kidney-specific BMAL1 KO models, indicating the importance of BMAL1 in the kidney for regulating BP that is surprisingly independent of circadian rhythms. These findings highlight the need for additional mechanistic exploration of how the molecular clock mechanism integrates with known regulatory pathways for BP and kidney function and how this integration works across the many different tissues that contribute to BP homeostasis. The challenge to this effort is exacerbated by the numerous and interdependent regulatory systems that include an enormous range of neuro-humoral, paracrine/autocrine, dietary and genetic factors involved in this complex physiology.
Vasculature
Circadian rhythms in the vasculature have an important role in the regulation of BP in health but also play a role in HTN and its resultant vascular complications, such as age-dependent vascular stiffening and vascular remodeling.72,81,82 In mice, circadian gene profiles in the vasculature have been observed in vascular smooth muscle cells83 and the aorta84, identifying potential circadian-targets that could reveal possible new mechanisms involved in HTN-related vascular disease. However, in studying the ‘whole’ arteries, the contribution of the endothelial layer has not been fully elucidated or at least highly underestimated, as the endothelium comprises of only a single inner layer of cells, out-massed by the other vascular cells. Despite being this single circumferential layer, its function is crucial. The endothelium is ideally situated to face the blood stream on the luminal side, with endothelial cells aligned side-by-side with each other laterally, and facing the smooth muscle cells abluminally. The endothelium thus serves as a relay of mechanical, temporal (circadian), and molecular blood-borne signals to modulate vascular remodeling, by modifying its own plight and those of its underlying layers.85 Single cell sequencing approaches, cell sorting, and purification methods to dissect vascular cell populations are robust methods, but there are limitations in translation to the intact organ and organism and are particularly difficult when applied to circadian studies.
Arterial stiffness
Arterial stiffening, an important determinant of vascular aging, independently predicts cardiovascular events, including HTN, myocardial infarction, atherosclerosis, and stroke86-89 90,91; while metabolic syndromes, diabetes mellitus, obesity, atherosclerosis and HTN further exacerbate arterial stiffening.92 It is well established that arterial stiffening is linked to the structural and functional changes of the large elastic arteries, including remodeling of extracellular matrix-elastin degradation and excessive collagen deposition93, endothelial cell dysfunction, and phenotypic modulation of vascular smooth muscle cells (VSMC).89 In particular, trans-differentiation of VSMC into bone-like cells promotes vascular calcification that increases arterial stiffness.94-97 Stiffening is the result of long term changes in the structure of the blood vessel, and may be a byproduct of long term effects of behavioral dysfunction, related to mistimed eating and sleeping (reviewed in98). Identifying the pathway of signals that connect the circadian clock and the signals that confer the stiffening of the vasculature will be an interesting area of study in the future.
VSMC calcification is regulated by multifaceted mechanisms underlying intracellular osteogenic differentiation and remodeling of extracellular matrix, involving genetic, cellular signaling and (micro)environmental regulators99. Peripheral circadian clocks modulate cell homeostasis by coupling the autonomous intracellular rhythms with changes in extracellular environment.100 The regulation of VSMC calcification by circadian clocks is unknown. Therefore, understanding the regulation of circadian rhythms in VSMC and the crosstalk between VSMC, other residential vascular cells and circulating cells that modulates vascular calcification and stiffness, as well as BP and vascular aging represents an exciting scientific research area.
Microbiota
Following the initial discovery of an association between gut microbiota composition and BP101,102, studies using germ-free rats have demonstrated a causal role for microbiota in maintaining BP homeostasis.103 Interestingly, both BP and gut microbiota composition exhibit circadian rhythms. While alterations of BP rhythms and reshaping of gut microbiota are both independently associated with HTN104,105, the relationship, if any, between the circadian rhythms of gut microbiota and BP remain unknown in humans. A recent study in Dahl salt-sensitive rats reported that major shifts in night and day patterns of specific groups of microbiota occurring between the dark (active) and light (rest) phases correlated with rhythmicity of BP.106 Unique bacterial taxa correlated independently or interactively with (i) BP rhythm, (ii) dietary salt, and (iii) nocturnal BP dip106. Phylogenetic investigation of communities revealed time-of-day-dependent effects on microbial pathways, characterized by upregulated biosynthetic processes during the active phase of the host, and upregulated degradation pathways of metabolites in the resting phase. These data provide evidence linking BP rhythm, renal damage, and select microbial communities.106 Similar associations were also revealed between sleep fragmentation, mean arterial pressure, and the gut microbiome/fecal metabolome which provide insights to links between disrupted sleep and cardiovascular pathology.107 It has also been demonstrated that circadian regulation of the gut microbiome is important for cardiac repair post-myocardial infarction, and that time-restricted feeding in mice is a feasible strategy for improving outcomes when the gut microbiome is disrupted, and also prevents blood pressure changes associated with adverse heart failure outcomes.108 These limited, albeit important studies identify a definitive synchronization between rhythms of gut microbiota composition and BP but are limited in that they do not address cause-effect relationships. Viewed from the context that a reduction in BP dipping leads to increased mortality and that the efficacy of BP medications could be altered by gut microbiota, it would be interesting to delineate whether certain communities of microbiota expand in composition at discrete times of the day to then cause elevation in BP. Identification of such causal influences by microbiota on BP rhythms will be important for targeted chronotherapy to address not only microbiota dysbiosis in hypertensive subjects but also their time-dependent responses to BP medications.
Factors That Disrupt Circadian Rhythm
Conditions known to disrupt circadian rhythm and hence increase the likelihood of HTN and its associated health risks include shift work and sleep disorders. Another important factor, meal timing, will be discussed later under “Therapies to address circadian rhythm-related health risks.”
Shift work
Epidemiological studies have implicated circadian rhythm dysregulation due to shift work as a risk factor for both CVD and cerebrovascular disease. In the Nurses’ Health Study, a higher risk of CVD was reported in women who had worked rotating night shifts relative to those with no shift work experience.1 Similarly, a Danish cohort study found that the relative risk for CVD was much greater among non-day workers than day workers.2 Furthermore, the susceptibility of the vascular system to circadian rhythm disturbances has been similarly endorsed in a study showing that stroke- and cardiovascular-related mortality, but not all-cause mortality was increased among male shift workers compared to day workers in Swedish paper mills.3
A systematic review and meta-analysis of 45 observational studies on night work and HTN risk involving 117,252 workers concluded that there was a significant increase in both SBP and DBP among permanent night workers (2.52 mmHg, 95% CI 0.75–4.29 and 1.76 mmHg, 95% CI 0.41–3.12, respectively)109. Similarly, for rotational shift workers both with and without night work, a significant increase was observed, but only for SBP (0.65 mmHg, 95% CI 0.07–1.22 and 1.28 mmHg, 95% CI 0.18–2.39, respectively), and no differences were found for HTN.
Because cross-sectional studies preclude addressing the healthy worker effect (i.e., dropping out of shift work by those with poorer health), stronger evidence is derived from longitudinal studies. Six prospective studies and one retrospective study have been reported.110-114,115 Most of these studies were conducted in men. The individual studies often included night work occupations associated with low socio-economic status, a risk factor for CVD, or higher physical strain, a risk factor for HTN. Five of the six prospective studies support a significantly increased risk of HTN in night workers.110-114 Of these, Lieu et al. reported a higher risk of HTN only among Black nurses participating in the Nurses’ Health Study.114 Only one study, the smallest of all in 488 workers with a mix of day and night work did not show an association.115 Additional indirect evidence for an association between night work and HTN comes from a study demonstrating that HTN control rates for patients taking anti-HTN meds were lower among night shift workers (OR, 0.74, 95%CI, 0.68-0.80) compared to day workers.116
There is ample evidence linking risk factors for HTN, such as metabolic disorders including type 2 diabetes and obesity, with night work. A recent Mendelian randomization study, using data from the UK Biobank, showed that higher body mass index increased the odds of reporting participation in frequent shift work.117 Night work has previously been linked to diseases in target organs of HTN such as CVD118 or the brain (stroke119, dementia120); studies linking night work with kidney function are still sparse but one study from Korea showed increased risk for CKD in female shift workers.121
One way to better understand the link between circadian disruption such as occurs in shift workers with increased CVD risk is the use of circadian misalignment protocols. Human studies have demonstrated that even short-term circadian disruption results in increased BP and markers for CVD risk, including C reactive protein.122,123 Development of animal models using chronic shifts of the light/dark (LD) cycle or circadian mis-aligned protocols are also needed to determine whether and how persistent modulation of circadian rhythms by shift work-like paradigms exacerbates the outcomes of CVD and stroke.
Although basic research in this area is limited, several studies have made remarkable progress in establishing the link between shift work-related circadian dysregulation and vascular disease. The initial proof of concept for the role of circadian rhythm disturbances in cardiovascular pathology was provided by a study demonstrating that periodic reversal of the LD cycle to simulate rotating shift work schedules accelerates death in cardiomyopathic hamsters.124 Indeed, it was found that genetic disruption of the circadian system in the Tau mutant hamster resulted in profound cardiovascular and renal disease that was ameliorated by shifting the light cycle to match the mutant rodent’s internal circadian period.125 In a similar fashion, this LD cycle shifting paradigm has been recently extended to study the impact of circadian rhythm dysregulation on ischemic stroke outcomes in rats. Long-term exposure to 12hr advances of a LD 12:12 cycle every 5 days disrupted the pattern of circadian entrainment, exacerbated stroke outcomes following middle cerebral artery occlusion, and further amplified sex differences in stroke impairments.126 Circadian rhythm dysregulation was marked by high rates of stroke-related mortality in male rats but was accompanied by significant increases in stroke-induced infarct size and sensorimotor deficits in surviving female rats. Taken together, these animal studies corroborate that in the absence of other risk factors, rotating shift work schedules alone (as simulated by reversal of LD cycles) contribute to the pathophysiology of cardio- and cerebro-vascular disease during aging.
Sleep and BP
The importance of sleep, particularly sleep duration, to overall cardiovascular health was recently highlighted with its addition to the American Heart Association’s “Life’s Essential 8”.127 It is estimated that more than one-third of US adults do not get the recommended hours of daily sleep.128 Furthermore, racial/ethnic disparities exist: Black adults, for example, are more than twice as likely to report short sleep duration compared to white adults and have a higher likelihood of HTN than white adults.129,130
There is an association between insufficient sleep duration and elevated BP.131 Short sleep duration increases the risk of HTN in all age groups, and this association may be particularly strong among women. In a recent study of 729 participants without a prior diagnosis of HTN, shorter sleep duration was associated with higher 24-hour SBP and DBP on 24-hour ABPM132. Evidence suggests that earlier midsleep time, a proxy for sleep timing, and long sleep duration may each be associated with a higher prevalence of non-dipping systolic BP.133,134 Few findings have been published on the experimental impact of sleep restriction on BP. One study showed that repeated bouts of highly restrictive sleep (4 hour time in bed/night) increases BP relative to constant adequate sleep (8 hour time in bed/night) and that sleep restriction reduces BP dipping relative to baseline.135 In an ecological model of sleep restriction, 6 weeks of maintaining a 1.5 hour reduction in sleep resulted in higher 24-hour systolic and mean arterial pressures compared to 6 weeks of maintained adequate sleep (at least 7 hours/night) with no difference in BP dipping.136 Interestingly, in-office BP improved to a greater extent while maintaining adequate sleep compared to sleep restriction, indicating a potential benefit of stable sleep duration and timing on BP. Maintaining stable sleep patterns could mitigate the adverse effects of insufficient sleep on BP137,138, but no published clinical studies are available that support this hypothesis. Limited studies have been conducted to determine whether improving sleep duration through sleep extension could lead to reductions in BP. Two pilot studies have shown that increasing sleep duration by approximately 34 minutes139 and 1 hour140 reduces BP. However, sample sizes were small and study duration relatively short (6 weeks).
Sleep duration is only one of several domains of sleep that impact health and is often closely inter-related to other sleep exposures, including poor sleep quality, altered sleep architecture (notably reduced stage N3 and REM sleep), sleep fragmentation, periodic limb movements, and sleep disordered breathing (SDB).141 Poor sleep quality - due to one or more of these factors- is associated with altered 24-hour parasympathetic-sympathetic nervous system balance and BP rhythms and non-dipping BP.142 A form of SDB, obstructive sleep apnea is associated with hypertension and disrupted circadian rhythms in BP.143 Severe obstructive sleep apnea has been linked to non-dipping BP.144 Meta-analyses report that individuals with SDB have a 1.47 (95% CI: 1.21, 2.28) increased odds of nocturnal HTN.145 Treatment of SDB with continuous positive airway pressure (CPAP) lowers nocturnal BP.146,147 The effects of CPAP on endogenous circadian rhythms have not been extensively studied, but available evidence suggests that CPAP may restore normal clock gene expression when mRNA levels were measured at multiple times of the day148,149 but not at a single time point.150
Further, data on BP dipping from actigraphy-assessed and EEG-based sleep, coupled with objective measures of SDB (e.g., with oximetry) are limited, representing a major gap in the literature. Although out-of-office BP monitoring using ABPM and home BP monitoring have a stronger association with CVD outcomes than office BP measurement151, few data exist on the associations of actigraphy-assessed sleep duration, fragmentation, and timing with out-of-office BP.152
There appears to be close coupling of the pulsatility of cerebral spine fluid flow, waste clearance, slow EEG rhythms and hemodynamic oscillations during sleep,49 also emphasizing the importance of neurophysiological assessment of sleep in studies of 24-hour BP-related physiological processes, and studies that address the inter-relationships between sleep, disturbances of sleep such as SDB, and BP rhythms.
Interventions to Address Circadian Rhythm-related Health Risks
Potential therapeutic approaches to addressing HTN by harnessing the power of circadian rhythm include timing of food intake and chronotherapy.
Timing of food intake
A compelling scientific area for discovery about circadian BP rhythm is elucidating mechanisms of how timing of food intake regulates BP, and more specifically, nocturnal HTN and non-dipping BP. Indeed, available evidence suggests that nighttime eating is associated with increased risk for elevated BP and disrupted circadian rhythms.98 Several studies have already pointed to cardiometabolic benefit from time restricted feeding in humans.153 One emerging theory is that decreased urinary sodium excretion during the day leads to elevated nighttime BP. This is supported by a small population study showing higher nighttime BP is related to a greater proportion of sodium being excreted at night.154 Studies in older individuals also demonstrated that lower daytime sodium excretion was associated with increased nighttime BP.155 Along these lines, it is also important to understand the role of specific dietary components (e.g., salt, fat, etc.) in contributing to nocturnal BP. For example, dietary sodium restriction restored the dipping pattern in salt-sensitive, hypertensive patients.156 Finally, given that circadian disruption is common in a wide range of populations such as rotating shift workers, understanding these BP and renal control mechanisms will be important for combating the high disease risk in these individuals.157-159
It is important to recognize that circadian dysfunction occurs in other conditions such as obesity.160,161 Recently, a small study in individuals with obesity162 was published, leading to headlines in the lay press that time restricted feeding, sometimes referred to as intermittent fasting, is of no benefit, which can be largely misleading. The study showed that there was no benefit when added to a calorie restricted diet in terms of losing weight. However, it is unclear whether time restricted feeding can be used as a partial substitute to calorie restriction. It is important to note that preclinical studies report cardiovascular and end-organ benefits independent of weight loss. Indeed, Acosta-Rodriguez et al. recently showed that circadian alignment of feeding with the active phase promoted longevity in male C57BL/6 mice, independent of body weight.163
In animal studies, mice with food access restricted to the light (or rest) phase have metabolic impairments164, inverted BP rhythms165, and disrupted microbiome that impairs cardiac repair108. Interestingly, restricting food to the night (or active) phase reinstates 24-hour locomotor rhythms and improves phase synchrony in liver and kidney in brain-specific Bmal1 knockout mice in DD166. These results highlight the importance of the feeding/fasting cycle on BP control that has only become evident in recent years. Given that non-dipping BP patterns significantly increase cardiovascular morbidity and mortality risk even after accounting for daytime BP levels30,34,38,69,167-171, a multi-organ and inter-disciplinary approach is necessary for future discovery of treatments and interventions for HTN.
Accumulating evidence demonstrates that BP circadian rhythm disruption is more common in patients with diabetes than those without. For example, the prevalence of non-dipping BP in type 2 diabetes patients is reported to be as high as 55 to 73%.172-175 While multiple mechanisms are likely linking diabetes to BP circadian rhythm disruption, the precise pathways remain poorly understood.
The db/db mouse is an extensively used monogenic type 2 diabetes model in which a spontaneous inactivation mutation in the leptin receptor leads to obesity, insulin resistance, and marked hyperglycemia.176 db/db mice develop moderate HTN and non-dipping BP by 11-12 weeks of age.177-180 Several mechanisms have been demonstrated that potentially contribute to BP circadian rhythm disruption in diabetes by using the db/db mouse model. These include loss of rhythms in vascular smooth muscle contraction181, disrupted intrinsic clock oscillations181,182, and desynchrony among the clocks in multiple systems that control BP.177 Interestingly, a small molecule nobiletin targets the molecular oscillator was reported to enhance circadian rhythms and protect against metabolic syndrome in db/db mice.183 Importantly, restricting food availability to the biological active dark phase (time-restricted feeding; i.e., equivalent to humans to not eating at night either during night work or if they woke up at night) corrects liver clock Bmal1 oscillation184, prevents the development of non-dipping BP, and restores normal BP circadian rhythm in the mice that already developed non-dipping BP.67
Chronotherapy
While clinical and epidemiological studies have indicated that bedtime dosing of antihypertensive medication may help improve nighttime BP dipping profile and attenuate early morning BP surge, data demonstrating that chronotherapy--antihypertensives dosed at bedtime instead of the morning-- improves clinical outcomes have been conflicting. In placebo-controlled trials such as the Heart Outcomes Prevention and Evaluation (HOPE), Systolic Hypertension in the Elderly (Syst-Eur), and Systolic Hypertension in China (Syst-China), antihypertensive medication initiated with evening dosing (but with morning drugs added in most patients to achieve BP control) decreased the risk of cardiovascular outcomes.185-187
The Monitorización Ambulatoria para Predicción de Eventos Cardiovasculares (Ambulatory BP Monitoring for Prediction of Cardiovascular Events [MAPEC]) trial reported that dosing antihypertensive at bedtime vs. after awakening significantly reduced the composite outcome of total CVD events by 61% in 2,156 Spanish patients with high BP.188 It should be noted that about half of individuals randomized to bedtime dosing also received morning treatment, and treatment choices were not fixed in the two groups potentially resulting in treatment differences. In addition, the authors reported that there was a significant decrease in major individual events including total death, CVD events (myocardial infarction, angina pectoris, and coronary revascularization), cerebral vascular events (transient ischemic attack, ischemic stroke, hemorrhagic stroke) and heart failure. These benefits were attributed to a higher prevalence of controlled BP on ABPM (62% vs. 53%) and improved BP dipping pattern (34% vs. 62%) in the bedtime arm of the trial. The dramatic findings in MAPEC, as well as two small sub-studies in diabetes and chronic kidney disease, were provocative considering the numbers of patients enrolled and the notable large differences in outcomes despite modest differences in BP.189
The Hygia Chronotherapy Trial was a multicenter, controlled, prospective trial of 19,084 hypertensive patients in Spain conducted by the same investigators of MAPEC.190 Patients were assigned to receive either their entire daily dose of ≥1 antihypertensive medication at bedtime or all antihypertensive medications in the morning. 48-hour ABPM was performed at each clinic visit and at least annually. After a median follow-up of 6.3 years, the authors reported a 45% decrease in the primary composite outcome of CVD when comparing bedtime to morning dosing of antihypertensive medication. They also reported a decrease in total CVD events (43%) and decreases in secondary outcomes including stroke (49%), coronary events (44%), and total death (45%) when comparing bedtime to morning dosing of antihypertensive medication. The authors attributed the improvement in outcomes to improved 48-hour SBP control (−1.3 mm Hg), asleep BP (−3.3 mm Hg), and improved dipping patterns (50.3% vs. 37.5%) between the arms and favoring bedtime dosing. They also reported low rates of adverse effects (6.7% vs. 6.0%), though specific adverse effects were not listed, as well as low rates of night-time hypotension (0.41% vs. 0.27%) and poor adherence (2.8% vs. 2.9%) in the morning dosing and bedtime dosing arms, respectively.
There have been numerous questions regarding the study design, conduction, reporting, and findings of the HYGIA trial. These include but are not limited to the biological plausibility of a dramatic decrease in CV outcomes and total mortality despite a 1.3 mm Hg difference in BP with bedtime dosing, the rationale for not stopping the study early, whether the trial was truly randomized, number of study protocol discontinuations, loss to follow-up, and consent withdrawals, very high medication adherence rates, the feasibility of 48-hour ABPM monitoring, complete analysis and reporting of adverse effects and safety, and specific medications that were dosed at bedtime versus in the morning.191-193 Such concerns led to a journal investigation and independent statistical analysis, which concluded "no indications to suggest the trial or data were fraudulent in nature.”193 However, the journal could not verify the source data.
At present, most experts do not recommend bedtime administration of antihypertensive drugs, mainly because the vast majority of outcome trials have used morning dosing of drugs and there is no clear strong evidence of additional outcome benefits with bedtime dosing.192,194 Due to questions regarding the HYGIA trial, the findings should be replicated in well-designed, prospective controlled, multi-center outcomes trials. Three such trials, the Treatment in Morning versus Evening (TIME)195 and the BedMed196 and BedMed-Frail197 trials are currently ongoing.
The United Kingdom-based TIME study uses a prospective, randomized, open-label, blinded endpoint design to follow more than 20,000 participants assigned to take their usual antihypertensive medications in the morning or the evening. The primary endpoint is a composite of non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death. The secondary endpoints will include the components of the composite primary endpoint, all-cause mortality, and hospitalized heart failure. Published results are expected prior to end-2022. Sub-studies within TIME address home BP measurements, sleep quality, mood, chronotype, and cognitive function. Preliminary results of the TIME study were presented at the 2022 European Society of Cardiology. The primary endpoint event occurred among 3.4% of participants in the evening dosing group (0.69 events per 100 person-years) and 3.7% of the morning dosing group (0.72 events per 100 person-years), with an unadjusted hazard ratio (HR) of 0.95 (95% CI, 0.83-1.10; P=.53).198
BedMed is a randomized antihypertensive timing trial in a primary care population being conducted in five Canadian provinces with most recruitment coming from participating family physicians, who send letters of invitation to all their community-dwelling patients with hypertension. The only significant exclusion criterion is a personal history of glaucoma. To date 3,154 participants have been randomized to morning or bedtime antihypertensive medications. If not stopped early, final analysis should take place around February 2024. The primary outcome is first occurrence of all-cause death, or hospitalization for stroke, myocardial infarction, or congestive heart failure. Select secondary outcomes include nursing home admission, all-cause hospitalization, new glaucoma diagnosis or treatment, and a new physician diagnosis of dementia or impairment consistent with dementia (as measured by the Short Blessed Test at 18-months).
BedMed-Frail is a similar trial among the frail long-term care population being conducted in 16 Alberta nursing homes; residents with hypertension using at least one once-daily antihypertensive medication are individually randomized to either the facility’s default of morning antihypertensive medications or to switch those medications to bedtime. The median age of participants is 88 years, and 75% are female. BedMed-Frail has the same primary outcome as BedMed, and additionally examines deterioration in cognition, full thickness skin ulceration (which might be promoted by lower overnight BP), and challenging behaviours (such behaviours have a circadian rhythm referred to as “sundowning”).
These studies may confirm or exclude any additional benefit of bedtime versus morning dosing of medication for HTN. However, they will not address the practical question of tailoring treatment according to the individual’s circadian BP variation and administering bedtime treatment specifically to individuals with non-dipping or those with nocturnal HTN.
One final consideration with chronotherapy is related to basic pharmacology. A large number of drugs for the treatment of HTN, including most angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers, have half-lives that allow dosing once a day. Therefore, at steady state most of these drugs are likely staying well above the therapeutic threshold for most individuals over 24 hours and so the timing of dosing, in theory, should not have any impact on efficacy. However, very few studies examine these factors once the drugs are on the market. Interestingly, the gene encoding ACE exhibits circadian expression in human lung.199 Given individual variations in gene expression for anti-HTN drug targets as well as the enzymes that function in drug turnover, time-of-day is relevant to personalized, circadian medicine: to give the right drug, at the right time, to the right person.
Potential Circadian Rhythm-Based Therapeutics
There are many exciting new opportunities to translate circadian discovery to medical application for HTN, including areas of basic cardiovascular-circadian research; these areas include: (i) Lighting Chronotherapy. Studies in rodents show that maintaining sleep and circadian rhythms can benefit cardiovascular growth, renewal, remodeling, and kidney health125 and are especially relevant to cardiac repair in clinical intensive and coronary care settings.200 (ii) Drugging the Clock. Novel circadian pharmacology targeting the circadian mechanism reduces reperfusion inflammasome activity to help heal heart attacks and prevent heart failure.5,201 (iii) Time Restricted Feeding significantly improves outcomes post-myocardial infarction; both what and when you eat are important for heart health108. (iv) Chronotherapy with ACE inhibitors shows that sleep time administration better reduces cardiac remodeling as compared to wake-time therapy. Indeed, sleep time is a critical period for cardiac repair.202 Additional potential interventions not discussed at the workshop that merit mention include exercise, which has been shown in at least one study to improve dipping status in hypertensive patients203, and melatonin, which may have BP lowering effects.204
Toward Precision Medicine
Genetics and epigenetics of circadian BP rhythm in humans
The circadian clock is a fundamental component of the genome and drives transcription of clock-controlled genes and downstream molecular pathways involved in cardiovascular and metabolic regulation. The opportunity to delineate the interface of circadian rhythm and BP regulation at the molecular/genetic level offers a potentially novel landscape for strategies to improve the diagnosis and treatment of HTN. Genome-wide association studies (GWAS) provide one opportunity to investigate the genetic and molecular underpinnings of the circadian BP rhythm. For example, GWAS studies have identified >1,477 genetic variants being associated with office BP phenotype.205 Previous family and twin studies have shown that BP measured in different settings (clinical vs. 24h in real life) or different times of day (day vs. night) are influenced by different genes206,207, requiring separate GWASs on these specific BP rhythmicity parameters to fully understand the underlying genetic mechanisms of BP regulation. Small scale GWASs have been conducted on BP rhythmicity measures.208,209 The role of clock genes on BP rhythmicity has also been explored.210,211 Large scale GWAS meta-analyses of cohorts with ambulatory BP data will have the potential to move this field forward.
The BP rhythmicity parameters observed in real-life setting entail an interaction between endogenous circadian BP rhythm and rhythmic behavioral factors such as sleep-wake pattern and rest-activity rhythm.14 Well-designed family or twin studies in combination with detailed measurements on both rhythmic behavior factors and ambulatory BP are needed to examine whether the effects of momentary behavior factors on ambulatory BP can be explained by underlying and shared genetic or environmental factors. Furthermore, recent progress in statistical methodology of GWAS allows the detection of pleiotropy at both the level of individual loci and the overall genetic architecture.212 However, the prerequisite for these statistical approaches is the availability of GWAS data for ambulatory BP and relevant behavioral and sleep phenotypes.
Epigenetics refers to stable changes in DNA function that are not determined directly by the sequence of the DNA but may be heritable through mitosis or even germ cell reprogramming.213 DNA methylation, histone modifications, non-coding RNA, and chromatin conformation are potential epigenetic mediators. Epigenetic features can respond dynamically to developmental cues and environmental and lifestyle factors and may be involved in the regulation of complex traits such as BP.213,214 For example, DNA methylation is associated with BP in humans and functionally contributes to the development of HTN in animal models.215-218
A study of 281 African Americans identified several methylation regions in whole blood DNA that were significantly associated with 24-hour BP phenotypes, but not with office BP.219 Methylation regions associated with daytime, nighttime, and 24-hour average BP overlapped but were not identical. These methylation regions together explained up to 16.5% of the variance of 24-hour average BP. The association of one methylation region with 24-hour BP was validated in a separate cohort using a newly developed targeted methylation sequencing method.219 Dietary sodium intake, which may influence 24-hour BP, has been shown to influence DNA methylation in T lymphocytes and arterioles in humans.220 Sleepiness (a marker of chronic sleep disturbances) is associated with changes in methylation patterns;221 interestingly, those associations were strongest in African Americans, a group also at highest risk for nocturnal HTN. These findings suggest DNA methylation might be excellent markers of the cumulative effect of many factors that influence 24-hour BP.
Research Gaps, Barriers, and Opportunities
Workshop participants identified several important and high priority issues as well as opportunities for future research (Table 1). The role of circadian rhythms on BP has been shown in humans and animals primarily through studies focused on association, rather than cause-and-effect. Both circadian clock and non-clock mechanisms need to be examined in connection with the following three factors: endogenous, environmental, and host-microbial interactive factors. Sex as a biological variable must also be considered. Women’s cardiovascular health is under-researched, under-supported, under-diagnosed, and under-treated.222 For rationale translation of our most promising circadian medicine therapies to clinical cardiology for treatment of HTN, it is imperative to understand pathophysiology in both biological sexes and the importance of gender.223
Table 1.
| Important and High Priority Issues | Opportunities |
|---|---|
| • Time of day and night is a key biological variable as well as awake and asleep times. • However, these variables are not routinely reported in the literature, impairing efforts toward rigor and reproducibility. • This key issue impacts translation given that humans are diurnal and rodents are nocturnal. |
• Journals and funding agencies should encourage investigators to report the time of day at which samples are collected and procedures are performed in both basic and clinical studies. |
| • Sex differences and health disparities are both important factors affecting circadian rhythm of BP. | • Studies should be sufficiently powered to analyze differences between sexes and racial and ethnic subpopulations whenever possible. |
| • ABPM is a useful tool for assessing the diurnal BP variation in humans but has limitations. • Novel BP monitoring methods are emerging but not ready for widespread use. |
• Develop a reliable, low-burdensome assessment method or device to monitor BP continuously over days in populations • Include out of office BP measurements whenever possible |
| Several factors contribute to BP circadian rhythms but the mechanisms are not understood • Endogenous or genetic factors. • Environmental or behavioral factors: diet (composition and timing), ambient temperature, light, stress, sleep, and exercise/physical activity. • Host-microbial interactive factors: microbial profiles in response to various environmental factors/anti-hypertensive medications and microbial metabolites. |
• Diverse animal models for circadian rhythms as well as for chronotherapy • Cause-effect relationships of microbiota and circadian rhythms of BP • Circadian rhythms of gut microbiota and circadian effects of antihypertensive medications • Gut microbiome targeted studies • Time restricted feeding and meal timing studies • Collection of objective measurements of sleep timing, quality and sleep disturbances in population studies • Interplay of sleep dimensions, circadian rhythm, and BP • Epigenetic changes as a mechanism for the cumulative effect of genetic and environmental factors |
| • The power of circadian biology has not been leveraged for improving human health. • Lack of scalable biomarkers and measurements to provide cost-effective phenotyping of human circadian rhythms at scale. • Unknown benefits of therapies that consider circadian variation of BP and other parameters. |
• Targeted human studies based on established circadian mechanisms of BP control • High quality randomized clinical trials of antihypertensive chronotherapy • Sleep-targeted intervention studies • Implication of 24-hour BP pattern and level on health outcomes • Genetic and epigenetic assessments of circadian timing and chronotype • Scalable biomarkers for circadian biology that can be integrated into clinical and epidemiological research |
| • Lack of communication and collaboration across the fields of circadian biology, physiology of key organ systems (neuro, vascular, kidney, etc), HTN, sleep medicine, and clinical practice. | • Facilitate team science including sleep, circadian biology, and vascular disease and break down silos • Standardized “circadian” definitions, nomenclature and methods across studies (e.g., day versus night, active versus inactive, awake versus asleep, circadian misalignment) are needed to avoid confusion. • Use of well-defined and validated metrics of circadian rhythms using wearable technologies (e.g., inter-day and intra-day stability) |
Further Considerations
The basic science research presented in the workshop, mainly from rodent models, suggests that the deepening understanding of human circadian biology can be applied to improve the diagnosis and management of HTN. However, this has yet to be convincingly translated into human, patient-important outcomes at either a population or individual-patient level.224 A deeper understanding of how to leverage circadian biology to develop circadian medicine will require considerably more fundamental research that can better guide clinical studies towards better therapies.
Precision medicine is a new frontier for the prevention, diagnosis, and management of CVD and HTN. The application of circadian science to precision medicine is compelling. Research is needed to improve the understanding of mechanisms, application of technologies and development of research methods to more completely delineate the clinically relevant interface between circadian rhythm and BP.225 This includes learning more about the nature and extent of BP and circadian rhythm variation within and between individuals. Promising technological improvements in BP recording, such as at-home night-time BP measurement and cuffless ABPM, should provide much richer longitudinal data that can be used to explore associations and test hypotheses. However, epidemiology and data-driven machine learning approaches will not be possible until the big-data sources they rely on contain all the variables that would allow us to phenotype individuals and their exposures reliably, and a strong commitment for large-scale data sharing. This will require agreement on what those variables are and how they should be recorded, e.g., how should the timing of daytime activities, sleep behaviours, and circadian rhythm be recorded?
Clinical trials remain the cornerstone of assessing the efficacy and safety of human health interventions. Trials must be large enough to allow meaningful sub-group analysis and enable experimental confirmation of promising interventions. Additionally, once sufficient mechanistic understanding of any chronotherapy intervention is reached, clinical trial outcomes need to be relevant to health and patient-centered, ideally avoiding surrogate outcomes in favour of clinical outcomes such as mortality and morbidity and quality of life measures. Furthermore, as all health interventions carry a degree of risk, trials must also collect adequate safety data to allow the assessment of intervention risk. Developments in decentralised and healthcare-embedded point of care trial methods can facilitate such large pragmatic clinical trials.226-229 However, for individual patients, alternative trial designs, like n-of-1 trials230, may offer the closest approximation of genuinely personalised medicine for physiologically complex conditions like hypertension.231,232
Moving the Field Forward
There are many opportunities to move the field forward; and some examples are listed in Table 2. Workshop participants agreed that it is time to encourage team science that spans the fields of HTN, sleep, circadian biology, and CVD with the goal of breaking down silos and building bridges between clinical and basic research. The ultimate goal is to move circadian medicine into the clinic in order to realize the full potential of precision medicine. With this goal in mind, the National Heart, Lung, and Blood Institute recently released a Notice of Special Interest (NOSI) on Basic and Translational Research on Circadian Regulation of Heart, Lung, Blood, and Sleep Disorders: NOT-HL-22-043. This NOSI aims to stimulate research on understanding how circadian rhythms regulate cell function and metabolism of peripheral tissues, and to find new avenues for the investigation of heart, lung, and blood disease risk, pathogenesis, diagnosis, treatment, and prevention.233
Table 2:
Moving the Field Forward
| Needs | Goals |
|---|---|
| Limited public databases are available that contain BP, sleep times, and circadian rhythm data (including raw telemetry data) since most large cohort studies do not assess 24-hour BP or endogenous circadian rhythms. | Online repositories for storage and analyses of circadian data widely accessible to all researchers |
| Leverage wearables and artificial intelligence (AI) to scale and improve evaluation of 24-hour BP, circadian rhythm, sleep and their inter-relationships. | |
| Develop standardized approaches for annotating sleep timing in clinical and population studies. | Move circadian medicine into the clinic based on adequate evidence |
| Support sufficiently powered prospective studies and strategic ancillary studies. |
Acknowledgments:
The authors wish to thank Drs. Mercedes Carnethon, Paul Muntner, Jennifer Pollock, Mahboob Rahman, Steven Shea, David Stepp, Joseph Takahashi, and Phyllis Zee for their participation and discussion in this workshop. As well, we thank Dr. Hannah Costello and Dr. Lauren Douma for helpful discussions. We also thank Donna Lloyd-Jones for editing assistance.
Sources of Funding:
The proceedings of the workshop, “Toward Precision Medicine: Circadian Rhythm of Blood Pressure and Chronotherapy for Hypertension,” were supported through funds provided by the NHLBI.
Footnotes
Conflict of Interest: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Disclosures:
C.B. was part of the scientific advisory board for Repha GmBH, Germany between 2020 and 2021.
S.R. has received consulting fees from Eli Lilly Inc, ApniMed Inc, and Jazz Pharam between 2020-2022.
References
- 1.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. Journal of the American College of Cardiology. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 3.Martino TA, Sole MJ. Molecular time: an often overlooked dimension to cardiovascular disease. Circ Res. 2009;105:1047–1061. doi: 10.1161/CIRCRESAHA.109.206201 [DOI] [PubMed] [Google Scholar]
- 4.Alibhai FJ, Tsimakouridze EV, Reitz CJ, Pyle WG, Martino TA. Consequences of Circadian and Sleep Disturbances for the Cardiovascular System. Can J Cardiol. 2015;31:860–872. doi: 10.1016/j.cjca.2015.01.015 [DOI] [PubMed] [Google Scholar]
- 5.Aziz IS, McMahon AM, Friedman D, Rabinovich-Nikitin I, Kirshenbaum LA, Martino TA. Circadian influence on inflammatory response during cardiovascular disease. Current opinion in pharmacology. 2021;57:60–70. doi: 10.1016/j.coph.2020.11.007 [DOI] [PubMed] [Google Scholar]
- 6.Martino TA, Young ME. Influence of the cardiomyocyte circadian clock on cardiac physiology and pathophysiology. Journal of biological rhythms. 2015;30:183–205. doi: 10.1177/0748730415575246 [DOI] [PubMed] [Google Scholar]
- 7.Mistry P, Duong A, Kirshenbaum L, Martino TA. Cardiac Clocks and Preclinical Translation. Heart Fail Clin. 2017;13:657–672. doi: 10.1016/j.hfc.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 8.Reitz CJ, Martino TA. Disruption of Circadian Rhythms and Sleep on Critical Illness and the Impact on Cardiovascular Events. Curr Pharm Des. 2015;21:3505–3511. doi: 10.2174/1381612821666150706105926 [DOI] [PubMed] [Google Scholar]
- 9.Sole MJ, Martino TA. Diurnal physiology: core principles with application to the pathogenesis, diagnosis, prevention, and treatment of myocardial hypertrophy and failure. Journal of applied physiology. 2009;107:1318–1327. doi: 10.1152/japplphysiol.00426.2009 [DOI] [PubMed] [Google Scholar]
- 10.Turek FW. Circadian clocks: Not your grandfather's clock. Science. 2016;354:992–993. doi: 10.1126/science.aal2613 [DOI] [PubMed] [Google Scholar]
- 11.Martino TA, Harrington ME. The Time for Circadian Medicine. Journal of biological rhythms. 2020;35:419–420. doi: 10.1177/0748730420946501 [DOI] [PubMed] [Google Scholar]
- 12.Cederroth CR, Albrecht U, Bass J, Brown SA, Dyhrfjeld-Johnsen J, Gachon F, Green CB, Hastings MH, Helfrich-Forster C, Hogenesch JB, et al. Medicine in the Fourth Dimension. Cell Metab. 2019;30:238–250. doi: 10.1016/j.cmet.2019.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane JM, Qian J, Mignot E, Redline S, Scheer F, Saxena R. Genetics of circadian rhythms and sleep in human health and disease. Nature reviews Genetics. 2022. doi: 10.1038/s41576-022-00519-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer N, Harvey AG, Lockley SW, Dijk DJ. Circadian rhythms and disorders of the timing of sleep. Lancet. 2022. doi: 10.1016/S0140-6736(22)00877-7 [DOI] [PubMed] [Google Scholar]
- 15.Rhoads MK, Balagee V, Thomas SJ. Circadian Regulation of Blood Pressure: of Mice and Men. Current hypertension reports. 2020;22:40. doi: 10.1007/s11906-020-01043-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Astiz M, Heyde I, Oster H. Mechanisms of Communication in the Mammalian Circadian Timing System. International journal of molecular sciences. 2019;20. doi: 10.3390/ijms20020343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healy KL, Morris AR, Liu AC. Circadian Synchrony: Sleep, Nutrition, and Physical Activity. Front Netw Physiol. 2021;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nature reviews Molecular cell biology. 2020;21:67–84. doi: 10.1038/s41580-019-0179-2 [DOI] [PubMed] [Google Scholar]
- 19.Kario K. Nocturnal Hypertension: New Technology and Evidence. Hypertension. 2018;71:997–1009. doi: 10.1161/HYPERTENSIONAHA.118.10971 [DOI] [PubMed] [Google Scholar]
- 20.Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR, et al. Measurement of Blood Pressure in Humans: A Scientific Statement From the American Heart Association. Hypertension. 2019;73:e35–e66. doi: 10.1161/HYP.0000000000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shea SA, Hilton MF, Hu K, Scheer FA. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. 2011;108:980–984. doi: 10.1161/CIRCRESAHA.110.233668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerkhof GA, Van Dongen HP, Bobbert AC. Absence of endogenous circadian rhythmicity in blood pressure? Am J Hypertens. 1998;11:373–377. doi: 10.1016/s0895-7061(97)00461-5 [DOI] [PubMed] [Google Scholar]
- 23.Van Dongen HP, Maislin G, Kerkhof GA. Repeated assessment of the endogenous 24-hour profile of blood pressure under constant routine. Chronobiol Int. 2001;18:85–98. doi: 10.1081/cbi-100001178 [DOI] [PubMed] [Google Scholar]
- 24.Menzel W. [Biorhythmics and blood pressure regulation]. Z Gesamte Inn Med. 1967;22:Suppl:201–206. [PubMed] [Google Scholar]
- 25.Bevan AT, Honour AJ, Stott FH. Direct arterial pressure recording in unrestricted man. Clin Sci. 1969;36:329–344. [PubMed] [Google Scholar]
- 26.Bartter FC, Delea CS, Baker W, Halberg F, Lee JK. Chronobiology in the diagnosis and treatment of mesor-hypertension. Chronobiologia. 1976;3:199–213. [PubMed] [Google Scholar]
- 27.Millar-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood-pressure. Lancet. 1978;1:795–797. doi: 10.1016/s0140-6736(78)92998-7 [DOI] [PubMed] [Google Scholar]
- 28.Imai Y, Abe K, Munakata M, Sakuma H, Hashimoto J, Imai K, Sekino H, Yoshinaga K. Circadian blood pressure variations under different pathophysiological conditions. J Hypertens Suppl. 1990;8:S125–132. [PubMed] [Google Scholar]
- 29.Skarke C, Lahens NF, Rhoades SD, Campbell A, Bittinger K, Bailey A, Hoffmann C, Olson RS, Chen L, Yang G, et al. A Pilot Characterization of the Human Chronobiome. Scientific reports. 2017;7:17141. doi: 10.1038/s41598-017-17362-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Bjorklund-Bodegard K, Richart T, Ohkubo T, Kuznetsova T, Torp-Pedersen C, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219–1229. doi: 10.1016/S0140-6736(07)61538-4 [DOI] [PubMed] [Google Scholar]
- 31.Bello NA, Jaeger BC, Booth JN 3rd, Abdalla M, Anstey DE, Pugliese DN, Lewis CE, Gidding SS, Lloyd-Jones D, Shah SJ, et al. Associations of awake and asleep blood pressure and blood pressure dipping with abnormalities of cardiac structure: the Coronary Artery Risk Development in Young Adults study. Journal of hypertension. 2020;38:102–110. doi: 10.1097/HJH.0000000000002221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paganini-Hill A, Bryant N, Corrada MM, Greenia DE, Fletcher E, Singh B, Floriolli D, Kawas CH, Fisher MJ. Blood Pressure Circadian Variation, Cognition and Brain Imaging in 90+ Year-Olds. Front Aging Neurosci. 2019;11:54. doi: 10.3389/fnagi.2019.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Wang JG. Isolated nocturnal hypertension: a disease masked in the dark. Hypertension. 2013;61:278–283. doi: 10.1161/HYPERTENSIONAHA.111.00217 [DOI] [PubMed] [Google Scholar]
- 34.Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10. doi: HYPERTENSIONAHA.109.133900 [pii] 10.1161/HYPERTENSIONAHA.109.133900 [DOI] [PubMed] [Google Scholar]
- 35.Abdalla M, Goldsmith J, Muntner P, Diaz KM, Reynolds K, Schwartz JE, Shimbo D. Is Isolated Nocturnal Hypertension A Reproducible Phenotype? Am J Hypertens. 2016;29:33–38. doi: 10.1093/ajh/hpv058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Blunted sleep-time relative blood pressure decline increases cardiovascular risk independent of blood pressure level--the "normotensive non-dipper" paradox. Chronobiol Int. 2013;30:87–98. doi: 10.3109/07420528.2012.701127 [DOI] [PubMed] [Google Scholar]
- 37.O'Flynn AM, Dolan E, Curtin RJ, O'Brien E, Perry IJ, Kearney PM. Night-time blood pressure and target organ damage: a comparative analysis of absolute blood pressure and dipping status. Journal of hypertension. 2015;33:2257–2264. doi: 10.1097/HJH.0000000000000690 [DOI] [PubMed] [Google Scholar]
- 38.Fan HQ, Li Y, Thijs L, Hansen TW, Boggia J, Kikuya M, Bjorklund-Bodegard K, Richart T, Ohkubo T, Jeppesen J, et al. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. Journal of hypertension. 2010;28:2036–2045. doi: 10.1097/HJH.0b013e32833b49fe [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Staessen JA, Lu L, Li LH, Wang GL, Wang JG. Is isolated nocturnal hypertension a novel clinical entity? Findings from a Chinese population study. Hypertension. 2007;50:333–339. doi: 10.1161/HYPERTENSIONAHA.107.087767 [DOI] [PubMed] [Google Scholar]
- 40.Vaughan AS, Coronado F, Casper M, Loustalot F, Wright JS. County-Level Trends in Hypertension-Related Cardiovascular Disease Mortality-United States, 2000 to 2019. J Am Heart Assoc. 2022;11:e024785. doi: 10.1161/JAHA.121.024785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Booth JN, Anstey DE, Bello NA, Jaeger BC, Pugliese DN, Thomas SJ, Deng L, Shikany JM, Lloyd-Jones D, Schwartz JE, et al. Race and sex differences in asleep blood pressure: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Journal of clinical hypertension. 2019;21:184–192. doi: 10.1111/jch.13474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vetter C. Circadian disruption: What do we actually mean? Eur J Neurosci. 2020;51:531–550. doi: 10.1111/ejn.14255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahman M, Wang X, Bundy JD, Charleston J, Cohen D, Cohen J, Drawz PE, Ghazi L, Horowitz E, Lash JP, et al. Prognostic Significance of Ambulatory BP Monitoring in CKD: A Report from the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2020;31:2609–2621. doi: 10.1681/ASN.2020030236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knutson KL, Lash J, Ricardo AC, Herdegen J, Thornton JD, Rahman M, Turek N, Cohan J, Appel LJ, Bazzano LA, et al. Habitual sleep and kidney function in chronic kidney disease: the Chronic Renal Insufficiency Cohort study. Journal of sleep research. 2018;27:281–289. doi: 10.1111/jsr.12573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drawz PE, Alper AB, Anderson AH, Brecklin CS, Charleston J, Chen J, Deo R, Fischer MJ, He J, Hsu CY, et al. Masked Hypertension and Elevated Nighttime Blood Pressure in CKD: Prevalence and Association with Target Organ Damage. Clin J Am Soc Nephrol. 2016;11:642–652. doi: 10.2215/CJN.08530815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chesebro AG, Melgarejo JD, Leendertz R, Igwe KC, Lao PJ, Laing KK, Rizvi B, Budge M, Meier IB, Calmon G, et al. White matter hyperintensities mediate the association of nocturnal blood pressure with cognition. Neurology. 2020;94:e1803–e1810. doi: 10.1212/WNL.0000000000009316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan X, Sundstrom J, Lind L, Franzon K, Kilander L, Benedict C. Reverse Dipping of Systolic Blood Pressure Is Associated With Increased Dementia Risk in Older Men: A Longitudinal Study Over 24 Years. Hypertension. 2021;77:1383–1390. doi: 10.1161/HYPERTENSIONAHA.120.16711 [DOI] [PubMed] [Google Scholar]
- 48.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fultz NE, Bonmassar G, Setsompop K, Stickgold RA, Rosen BR, Polimeni JR, Lewis LD. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 2019;366:628–631. doi: 10.1126/science.aax5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, Olveda G, Thomas JH, Nedergaard M, Kelley DH. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 2018;9:4878. doi: 10.1038/s41467-018-07318-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
- 52.Verdecchia P, Angeli F, Borgioni C, Gattobigio R, Reboldi G. Ambulatory blood pressure and cardiovascular outcome in relation to perceived sleep deprivation. Hypertension. 2007;49:777–783. doi: 10.1161/01.HYP.0000258215.26755.20 [DOI] [PubMed] [Google Scholar]
- 53.Muntner P, Einhorn PT, Cushman WC, Whelton PK, Bello NA, Drawz PE, Green BB, Jones DW, Juraschek SP, Margolis KL, et al. Blood Pressure Assessment in Adults in Clinical Practice and Clinic-Based Research: JACC Scientific Expert Panel. Journal of the American College of Cardiology. 2019;73:317–335. doi: 10.1016/j.jacc.2018.10.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. Journal of hypertension. 2013;31:1731–1768. doi: 10.1097/HJH.0b013e328363e964 [DOI] [PubMed] [Google Scholar]
- 55.Stergiou GS, Palatini P, Parati G, O'Brien E, Januszewicz A, Lurbe E, Persu A, Mancia G, Kreutz R, European Society of Hypertension C, et al. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. Journal of hypertension. 2021;39:1293–1302. doi: 10.1097/HJH.0000000000002843 [DOI] [PubMed] [Google Scholar]
- 56.Dietrich E, Desai R, Garg M, Park H, Smith SM. Reimbursement of ambulatory blood pressure monitoring in the US commercial insurance marketplace. Journal of clinical hypertension. 2020;22:6–15. doi: 10.1111/jch.13772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kent ST, Shimbo D, Huang L, Diaz KM, Viera AJ, Kilgore M, Oparil S, Muntner P. Rates, amounts, and determinants of ambulatory blood pressure monitoring claim reimbursements among Medicare beneficiaries. Journal of the American Society of Hypertension : JASH. 2014;8:898–908. doi: 10.1016/j.jash.2014.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stergiou GS, Baibas NM, Gantzarou AP, Skeva II, Kalkana CB, Roussias LG, Mountokalakis TD. Reproducibility of home, ambulatory, and clinic blood pressure: implications for the design of trials for the assessment of antihypertensive drug efficacy. Am J Hypertens. 2002;15:101–104. doi: 10.1016/s0895-7061(01)02324-x [DOI] [PubMed] [Google Scholar]
- 59.Bo Y, Kwok KO, Chung VC, Yu CP, Tsoi KK, Wong SY, Lee EK. Short-term reproducibility of ambulatory blood pressure measurements: a systematic review and meta-analysis of 35 observational studies. Journal of hypertension. 2020;38:2095–2109. doi: 10.1097/HJH.0000000000002522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kollias A, Ntineri A, Stergiou GS. Association of night-time home blood pressure with night-time ambulatory blood pressure and target-organ damage: a systematic review and meta-analysis. Journal of hypertension. 2017;35:442–452. doi: 10.1097/HJH.0000000000001189 [DOI] [PubMed] [Google Scholar]
- 61.Stergiou GS, Nasothimiou EG, Destounis A, Poulidakis E, Evagelou I, Tzamouranis D. Assessment of the diurnal blood pressure profile and detection of non-dippers based on home or ambulatory monitoring. Am J Hypertens. 2012;25:974–978. doi: 10.1038/ajh.2012.82 [DOI] [PubMed] [Google Scholar]
- 62.Stergiou GS, Mukkamala R, Avolio A, Kyriakoulis KG, Mieke S, Murray A, Parati G, Schutte AE, Sharman JE, Asmar R, et al. Cuffless blood pressure measuring devices: review and statement by the European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. Journal of hypertension. 2022;40:1449–1460. doi: 10.1097/HJH.0000000000003224 [DOI] [PubMed] [Google Scholar]
- 63.Machado AP. The time has come to change ambulatory blood pressure monitoring from 24-hour to 48-hour for the diagnosis of hypertension and cardiovascular risk assessment. Rev Port Cardiol (Engl Ed). 2018;37:329–331. doi: 10.1016/j.repc.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 64.Refinetti R, Lissen GC, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res. 2007;38:275–325. doi: 10.1080/09291010600903692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11:16. doi: 10.1186/1742-4682-11-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hill AM, Crislip GR, Stowie A, Ellis I, Ramsey A, Castanon-Cervantes O, Gumz ML, Davidson AJ. Environmental circadian disruption suppresses rhythms in kidney function and accelerates excretion of renal injury markers in urine of male hypertensive rats. Am J Physiol Renal Physiol. 2021;320:F224–F233. doi: 10.1152/ajprenal.00421.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hou T, Su W, Duncan MJ, Olga VA, Guo Z, Gong MC. Time-restricted feeding protects the blood pressure circadian rhythm in diabetic mice. Proc Natl Acad Sci U S A. 2021;118. doi: 10.1073/pnas.2015873118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Demarco VG, Ford DA, Henriksen EJ, Aroor AR, Johnson MS, Habibi J, Ma L, Yang M, Albert CJ, Lally JW, et al. Obesity-related alterations in cardiac lipid profile and nondipping blood pressure pattern during transition to diastolic dysfunction in male db/db mice. Endocrinology. 2013;154:159–171. doi: 10.1210/en.2012-1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salles GF, Reboldi G, Fagard RH, Cardoso CR, Pierdomenico SD, Verdecchia P, Eguchi K, Kario K, Hoshide S, Polonia J, et al. Prognostic Effect of the Nocturnal Blood Pressure Fall in Hypertensive Patients: The Ambulatory Blood Pressure Collaboration in Patients With Hypertension (ABC-H) Meta-Analysis. Hypertension. 2016;67:693–700. doi: 10.1161/HYPERTENSIONAHA.115.06981 [DOI] [PubMed] [Google Scholar]
- 70.Douma LG, Gumz ML. Circadian clock-mediated regulation of blood pressure. Free Radic Biol Med. 2018;119:108–114. doi: 10.1016/j.freeradbiomed.2017.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnston JG, Speed JS, Becker BK, Kasztan M, Soliman RH, Rhoads MK, Tao B, Jin C, Geurts AM, Hyndman KA, et al. Diurnal Control of Blood Pressure Is Uncoupled From Sodium Excretion. Hypertension. 2020;75:1624–1634. doi: 10.1161/HYPERTENSIONAHA.119.13908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104:3450–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Costello HM, Gumz ML. Circadian Rhythm, Clock Genes, and Hypertension: Recent Advances in Hypertension. Hypertension. 2021;78:1185–1196. doi: 10.1161/HYPERTENSIONAHA.121.14519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang D, Pollock DM. Diurnal Regulation of Renal Electrolyte Excretion: The Role of Paracrine Factors. Annu Rev Physiol. 2020;82:343–363. doi: 10.1146/annurev-physiol-021119-034446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mohandas R, Douma L, Scindia Y, Gumz M. Circadian rhythms and renal pathophysiology. Journal of Clinical Investigation. 2022;In Press. doi: 10.1172/JCI148277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Costello HM, Johnston JG, Juffre A, Crislip GR, Gumz ML. Circadian clocks of the kidney: function, mechanism, and regulation. Physiol Rev. 2022;102:1669–1701. doi: 10.1152/physrev.00045.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tokonami N, Mordasini D, Pradervand S, Centeno G, Jouffe C, Maillard M, Bonny O, Gachon F, Gomez RA, Sequeira-Lopez ML, et al. Local renal circadian clocks control fluid-electrolyte homeostasis and BP. J Am Soc Nephrol. 2014;25:1430–1439. doi: 10.1681/ASN.2013060641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nikolaeva S, Ansermet C, Centeno G, Pradervand S, Bize V, Mordasini D, Henry H, Koesters R, Maillard M, Bonny O, et al. Nephron-Specific Deletion of Circadian Clock Gene Bmal1 Alters the Plasma and Renal Metabolome and Impairs Drug Disposition. J Am Soc Nephrol. 2016. doi: 10.1681/ASN.2015091055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang D, Jin C, Obi IE, Rhoads MK, Soliman RH, Sedaka RS, Allan JM, Tao B, Speed JS, Pollock JS, et al. Loss of circadian gene Bmal1 in the collecting duct lowers blood pressure in male, but not female, mice. Am J Physiol Renal Physiol. 2020;318:F710–F719. doi: 10.1152/ajprenal.00364.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crislip GR, Douma LG, Masten SH, Cheng KY, Lynch IJ, Johnston JG, Barral D, Glasford KB, Holzworth MR, Verlander JW, et al. Differences in renal BMAL1 contribution to Na(+) homeostasis and blood pressure control in male and female mice. Am J Physiol Renal Physiol. 2020;318:F1463–F1477. doi: 10.1152/ajprenal.00014.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119:1510–1517. doi: 10.1161/circulationaha.108.827477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anea CB, Ali MI, Osmond JM, Sullivan JC, Stepp DW, Merloiu AM, Rudic RD. Matrix metalloproteinase 2 and 9 dysfunction underlie vascular stiffness in circadian clock mutant mice. Arterioscler Thromb Vasc Biol. 2010;30:2535–2543. doi: 10.1161/ATVBAHA.110.214379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chalmers JA, Martino TA, Tata N, Ralph MR, Sole MJ, Belsham DD. Vascular circadian rhythms in a mouse vascular smooth muscle cell line (Movas-1). Am J Physiol Regul Integr Comp Physiol. 2008;295:R1529–1538. doi: 10.1152/ajpregu.90572.2008 [DOI] [PubMed] [Google Scholar]
- 84.Rudic RD, McNamara P, Reilly D, Grosser T, Curtis AM, Price TS, Panda S, Hogenesch JB, FitzGerald GA. Bioinformatic analysis of circadian gene oscillation in mouse aorta. Circulation. 2005;112:2716–2724. doi: 10.1161/CIRCULATIONAHA.105.568626 [DOI] [PubMed] [Google Scholar]
- 85.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Benetos A, Waeber B, Izzo J, Mitchell G, Resnick L, Asmar R, Safar M. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens. 2002;15:1101–1108. doi: 10.1016/s0895-7061(02)03029-7 [DOI] [PubMed] [Google Scholar]
- 87.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, et al. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62:1105–1110. doi: 10.1161/HYPERTENSIONAHA.113.01744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A, Witteman JC. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32:454–460. doi: 10.1161/01.str.32.2.454 [DOI] [PubMed] [Google Scholar]
- 89.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02 [DOI] [PubMed] [Google Scholar]
- 90.O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65 [DOI] [PubMed] [Google Scholar]
- 91.Fesler P, Safar ME, du Cailar G, Ribstein J, Mimran A. Pulse pressure is an independent determinant of renal function decline during treatment of essential hypertension. Journal of hypertension. 2007;25:1915–1920. doi: 10.1097/HJH.0b013e3281fbd15e [DOI] [PubMed] [Google Scholar]
- 92.Chirinos JA, Segers P, Hughes T, Townsend R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2019;74:1237–1263. doi: 10.1016/j.jacc.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsamis A, Krawiec JT, Vorp DA. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J R Soc Interface. 2013;10:20121004. doi: 10.1098/rsif.2012.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Monckeberg's sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168 [DOI] [PubMed] [Google Scholar]
- 95.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM, Javed A, Zhang K, Anderson PG, Chen Y. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res. 2012;111:543–552. doi: 10.1161/CIRCRESAHA.112.267237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heath JM, Sun Y, Yuan K, Bradley WE, Litovsky S, Dell'Italia LJ, Chatham JC, Wu H, Chen Y. Activation of AKT by O-linked N-acetylglucosamine induces vascular calcification in diabetes mellitus. Circ Res. 2014;114:1094–1102. doi: 10.1161/CIRCRESAHA.114.302968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kanbay M, Copur S, Demiray A, Tuttler KR. Cardiorenal Metabolic Consequences of Nighttime Snacking: Is it an Innocent Eating Behavior? Curr Nutr Rep. 2022;11:347–353. doi: 10.1007/s13668-022-00403-6 [DOI] [PubMed] [Google Scholar]
- 99.Chen Y, Zhao X, Wu H. Arterial Stiffness: A Focus on Vascular Calcification and Its Link to Bone Mineralization. Arterioscler Thromb Vasc Biol. 2020;40:1078–1093. doi: 10.1161/ATVBAHA.120.313131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47:187–197. doi: 10.1152/physiolgenomics.00136.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Joe B, McCarthy CG, Edwards JM, Cheng X, Chakraborty S, Yang T, Golonka RM, Mell B, Yeo JY, Bearss NR, et al. Microbiota Introduced to Germ-Free Rats Restores Vascular Contractility and Blood Pressure. Hypertension. 2020;76:1847–1855. doi: 10.1161/HYPERTENSIONAHA.120.15939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chakraborty S, Mandal J, Yang T, Cheng X, Yeo JY, McCarthy CG, Wenceslau CF, Koch LG, Hill JW, Vijay-Kumar M, et al. Metabolites and Hypertension: Insights into Hypertension as a Metabolic Disorder: 2019 Harriet Dustan Award. Hypertension. 2020;75:1386–1396. doi: 10.1161/HYPERTENSIONAHA.120.13896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang T, Chakraborty S, Mandal J, Mei X, Joe B. Microbiota and Metabolites as Factors Influencing Blood Pressure Regulation. Compr Physiol. 2021;11:1731–1757. doi: 10.1002/cphy.c200009 [DOI] [PubMed] [Google Scholar]
- 106.Chakraborty S, Mandal J, Cheng X, Galla S, Hindupur A, Saha P, Yeoh BS, Mell B, Yeo JY, Vijay-Kumar M, et al. Diurnal Timing Dependent Alterations in Gut Microbial Composition Are Synchronously Linked to Salt-Sensitive Hypertension and Renal Damage. Hypertension. 2020;76:59–72. doi: 10.1161/HYPERTENSIONAHA.120.14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maki KA, Burke LA, Calik MW, Watanabe-Chailland M, Sweeney D, Romick-Rosendale LE, Green SJ, Fink AM. Sleep fragmentation increases blood pressure and is associated with alterations in the gut microbiome and fecal metabolome in rats. Physiol Genomics. 2020;52:280–292. doi: 10.1152/physiolgenomics.00039.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mistry P, Reitz CJ, Khatua TN, Rasouli M, Oliphant K, Young ME, Allen-Vercoe E, Martino TA. Circadian influence on the microbiome improves heart failure outcomes. J Mol Cell Cardiol. 2020;149:54–72. doi: 10.1016/j.yjmcc.2020.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gamboa Madeira S, Fernandes C, Paiva T, Santos Moreira C, Caldeira D. The Impact of Different Types of Shift Work on Blood Pressure and Hypertension: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2021;18. doi: 10.3390/ijerph18136738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kubo T, Fujino Y, Nakamura T, Kunimoto M, Tabata H, Tsuchiya T, Kadowaki K, Odoi H, Oyama I, Matsuda S. An industry-based cohort study of the association between weight gain and hypertension risk among rotating shift workers. J Occup Environ Med. 2013;55:1041–1045. doi: 10.1097/JOM.0b013e31829731fd [DOI] [PubMed] [Google Scholar]
- 111.Sakata K, Suwazono Y, Harada H, Okubo Y, Kobayashi E, Nogawa K. The relationship between shift work and the onset of hypertension in male Japanese workers. J Occup Environ Med. 2003;45:1002–1006. doi: 10.1097/01.jom.0000085893.98441.96 [DOI] [PubMed] [Google Scholar]
- 112.Ferguson JM, Costello S, Neophytou AM, Balmes JR, Bradshaw PT, Cullen MR, Eisen EA. Night and rotational work exposure within the last 12 months and risk of incident hypertension. Scand J Work Environ Health. 2019;45:256–266. doi: 10.5271/sjweh.3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rahim A, McIsaac MA, Aronson KJ, Smith PM, Tranmer JE. The Associations of Shift Work, Sleep Quality, and Incidence of Hypertension in Ontario Adults: A Population-Based Study. Can J Cardiol. 2021;37:513–518. doi: 10.1016/j.cjca.2020.09.003 [DOI] [PubMed] [Google Scholar]
- 114.Lieu SJ, Curhan GC, Schernhammer ES, Forman JP. Rotating night shift work and disparate hypertension risk in African-Americans. Journal of hypertension. 2012;30:61–66. doi: 10.1097/HJH.0b013e32834e1ea3 [DOI] [PubMed] [Google Scholar]
- 115.Biggi N, Consonni D, Galluzzo V, Sogliani M, Costa G. Metabolic syndrome in permanent night workers. Chronobiol Int. 2008;25:443–454. [DOI] [PubMed] [Google Scholar]
- 116.Park J, Shin SY, Kang Y, Rhie J. Effect of night shift work on the control of hypertension and diabetes in workers taking medication. Ann Occup Environ Med. 2019;31:e27. doi: 10.35371/aoem.2019.31.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Daghlas I, Richmond RC, Lane JM, Dashti HS, Ollila HM, Schernhammer ES, Smith GD, Rutter MK, Saxena R, Vetter C. Selection into shift work is influenced by educational attainment and body mass index: a Mendelian randomization study in the UK Biobank. Int J Epidemiol. 2021;50:1229–1240. doi: 10.1093/ije/dyab031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vetter C, Devore EE, Wegrzyn LR, Massa J, Speizer FE, Kawachi I, Rosner B, Stampfer MJ, Schernhammer ES. Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA. 2016;315:1726–1734. doi: 10.1001/jama.2016.4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brown DL, Feskanich D, Sanchez BN, Rexrode KM, Schernhammer ES, Lisabeth LD. Rotating night shift work and the risk of ischemic stroke. Am J Epidemiol. 2009;169:1370–1377. doi: 10.1093/aje/kwp056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leso V, Caturano A, Vetrani I, Iavicoli I. Shift or night shift work and dementia risk: a systematic review. Eur Rev Med Pharmacol Sci. 2021;25:222–232. doi: 10.26355/eurrev_202101_24388 [DOI] [PubMed] [Google Scholar]
- 121.Uhm JY, Kim HR, Kang GH, Choi YG, Park TH, Kim SY, Chang SS, Choo WO. The association between shift work and chronic kidney disease in manual labor workers using data from the Korea National Health and Nutrition Examination Survey (KNHANES 2011-2014). Ann Occup Environ Med. 2018;30:69. doi: 10.1186/s40557-018-0279-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113:E1402–1411. doi: 10.1073/pnas.1516953113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Penev PD, Kolker DE, Zee PC, Turek FW. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol. 1998;275:H2334–2337. doi: 10.1152/ajpheart.1998.275.6.H2334 [DOI] [PubMed] [Google Scholar]
- 125.Martino TA, Oudit GY, Herzenberg AM, Tata N, Koletar MM, Kabir GM, Belsham DD, Backx PH, Ralph MR, Sole MJ. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1675–1683. [DOI] [PubMed] [Google Scholar]
- 126.Earnest DJ, Neuendorff N, Coffman J, Selvamani A, Sohrabji F. Sex Differences in the Impact of Shift Work Schedules on Pathological Outcomes in an Animal Model of Ischemic Stroke. Endocrinology. 2016;157:2836–2843. doi: 10.1210/en.2016-1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, et al. Life's Essential 8: Updating and Enhancing the American Heart Association's Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/CIR.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Centers for Disease Control and Prevention: Sleep and Sleep Disorders. https://www.cdc.gov/sleep/data_statistics.html. Accessed 04/26/2022.
- 129.Kingsbury JH, Buxton OM, Emmons KM. Sleep and its Relationship to Racial and Ethnic Disparities in Cardiovascular Disease. Curr Cardiovasc Risk Rep. 2013;7. doi: 10.1007/s12170-013-0330-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pandey A, Williams N, Donat M, Ceide M, Brimah P, Ogedegbe G, McFarlane SI, Jean-Louis G. Linking sleep to hypertension: greater risk for blacks. Int J Hypertens. 2013;2013:436502. doi: 10.1155/2013/436502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0 [DOI] [PubMed] [Google Scholar]
- 132.Abdalla M, Schwartz JE, Cornelius T, Chang BP, Alcantara C, Shechter A. Objective short sleep duration and 24-hour blood pressure. Int J Cardiol Hypertens. 2020;7:100062. doi: 10.1016/j.ijchy.2020.100062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thomas SJ, Booth JN 3rd, Jaeger BC, Hubbard D, Sakhuja S, Abdalla M, Lloyd-Jones DM, Buysse DJ, Lewis CE, Shikany JM, et al. Association of Sleep Characteristics With Nocturnal Hypertension and Nondipping Blood Pressure in the CARDIA Study. J Am Heart Assoc. 2020;9:e015062. doi: 10.1161/JAHA.119.015062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Abdalla M, Sakhuja S, Akinyelure OP, Thomas SJ, Schwartz JE, Lewis CE, Shikany JM, Lloyd-Jones D, Booth JN 3rd, Shimbo D, et al. The association of actigraphy-assessed sleep duration with sleep blood pressure, nocturnal hypertension, and nondipping blood pressure: the coronary artery risk development in young adults (CARDIA) study. Journal of hypertension. 2021;39:2478–2487. doi: 10.1097/HJH.0000000000002956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang H, Haack M, Gautam S, Meier-Ewert HK, Mullington JM. Repetitive exposure to shortened sleep leads to blunted sleep-associated blood pressure dipping. Journal of hypertension. 2017;35:1187–1194. doi: 10.1097/HJH.0000000000001284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Makarem N, Zuraikat FM, Scaccia SE, RoyChoudhury A, St-Onge MP. Sustained Mild Sleep Restriction Increases Blood Pressure in Women: An Update From the American Heart Association Go Red for Women Strategically Focused Research Network. Hypertension. 2021;77:e50–e52. doi: 10.1161/HYPERTENSIONAHA.120.16370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Abbott SM, Weng J, Reid KJ, Daviglus ML, Gallo LC, Loredo JS, Nyenhuis SM, Ramos AR, Shah NA, Sotres-Alvarez D, et al. Sleep Timing, Stability, and BP in the Sueno Ancillary Study of the Hispanic Community Health Study/Study of Latinos. Chest. 2019;155:60–68. doi: 10.1016/j.chest.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sohail S, Yu L, Bennett DA, Buchman AS, Lim AS. Irregular 24-hour activity rhythms and the metabolic syndrome in older adults. Chronobiol Int. 2015;32:802–813. doi: 10.3109/07420528.2015.1041597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baron KG, Duffecy J, Richardson D, Avery E, Rothschild S, Lane J. Technology Assisted Behavior Intervention to Extend Sleep Among Adults With Short Sleep Duration and Prehypertension/Stage 1 Hypertension: A Randomized Pilot Feasibility Study. J Clin Sleep Med. 2019;15:1587–1597. doi: 10.5664/jcsm.8018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Haack M, Serrador J, Cohen D, Simpson N, Meier-Ewert H, Mullington JM. Increasing sleep duration to lower beat-to-beat blood pressure: a pilot study. Journal of sleep research. 2013;22:295–304. doi: 10.1111/jsr.12011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chung J, Goodman M, Huang T, Wallace ML, Johnson DA, Bertisch S, Redline S. Racial-ethnic Differences in Actigraphy, Questionnaire, and Polysomnography Indicators of Healthy Sleep: The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2021. doi: 10.1093/aje/kwab232 [DOI] [PubMed] [Google Scholar]
- 142.Zhao S, Fu S, Ren J, Luo L. Poor sleep is responsible for the impaired nocturnal blood pressure dipping in elderly hypertensive: A cross-sectional study of elderly. Clinical and experimental hypertension. 2018;40:582–588. doi: 10.1080/10641963.2017.1411495 [DOI] [PubMed] [Google Scholar]
- 143.Butler MP, Thosar SS, Smales C, DeYoung PN, Wu H, Hussain MV, Morimoto M, Hu K, Scheer F, Shea SA. Effects of obstructive sleep apnea on endogenous circadian rhythms assessed during relaxed wakefulness; an exploratory analysis. Chronobiol Int. 2020;37:856–866. doi: 10.1080/07420528.2020.1740723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.BaHammam AS, Alshahrani M, Aleissi SA, Olaish AH, Alhassoon MH, Shukr A. Blood pressure dipping during REM and non-REM sleep in patients with moderate to severe obstructive sleep apnea. Scientific reports. 2021;11:7990. doi: 10.1038/s41598-021-87200-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cuspidi C, Tadic M, Sala C, Gherbesi E, Grassi G, Mancia G. Blood Pressure Non-Dipping and Obstructive Sleep Apnea Syndrome: A Meta-Analysis. J Clin Med. 2019;8. doi: 10.3390/jcm8091367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hu X, Fan J, Chen S, Yin Y, Zrenner B. The role of continuous positive airway pressure in blood pressure control for patients with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. Journal of clinical hypertension. 2015;17:215–222. doi: 10.1111/jch.12472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gottlieb DJ, Punjabi NM, Mehra R, Patel SR, Quan SF, Babineau DC, Tracy RP, Rueschman M, Blumenthal RS, Lewis EF, et al. CPAP versus oxygen in obstructive sleep apnea. The New England journal of medicine. 2014;370:2276–2285. doi: 10.1056/NEJMoa1306766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Burioka N, Koyanagi S, Endo M, Takata M, Fukuoka Y, Miyata M, Takeda K, Chikumi H, Ohdo S, Shimizu E. Clock gene dysfunction in patients with obstructive sleep apnoea syndrome. The European respiratory journal. 2008;32:105–112. doi: 10.1183/09031936.00138207 [DOI] [PubMed] [Google Scholar]
- 149.Gaspar LS, Hesse J, Yalcin M, Santos B, Carvalhas-Almeida C, Ferreira M, Moita J, Relogio A, Cavadas C, Alvaro AR. Long-term continuous positive airway pressure treatment ameliorates biological clock disruptions in obstructive sleep apnea. EBioMedicine. 2021;65:103248. doi: 10.1016/j.ebiom.2021.103248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Moreira S, Rodrigues R, Barros AB, Pejanovic N, Neves-Costa A, Pedroso D, Pereira C, Fernandes D, Rodrigues JV, Barbara C, et al. Changes in Expression of the CLOCK Gene in Obstructive Sleep Apnea Syndrome Patients Are Not Reverted by Continuous Positive Airway Pressure Treatment. Front Med (Lausanne). 2017;4:187. doi: 10.3389/fmed.2017.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163:691–700. doi: 10.7326/M15-1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Makarem N, Alcantara C, Williams N, Bello NA, Abdalla M. Effect of Sleep Disturbances on Blood Pressure. Hypertension. 2021;77:1036–1046. doi: 10.1161/HYPERTENSIONAHA.120.14479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018;27:1212–1221 e1213. doi: 10.1016/j.cmet.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bankir L, Bochud M, Maillard M, Bovet P, Gabriel A, Burnier M. Nighttime blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in African subjects. Hypertension. 2008;51:891–898. doi: HYPERTENSIONAHA.107.105510 [pii] 10.1161/HYPERTENSIONAHA.107.105510 [DOI] [PubMed] [Google Scholar]
- 155.Del Giorno R, Troiani C, Gabutti S, Stefanelli K, Puggelli S, Gabutti L. Impaired Daytime Urinary Sodium Excretion Impacts Nighttime Blood Pressure and Nocturnal Dipping at Older Ages in the General Population. Nutrients. 2020;12. doi: 10.3390/nu12072013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Uzu T, Ishikawa K, Fujii T, Nakamura S, Inenaga T, Kimura G. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1997;96:1859–1862. doi: 10.1161/01.cir.96.6.1859 [DOI] [PubMed] [Google Scholar]
- 157.Resuehr D, Wu G, Johnson RL Jr., Young ME, Hogenesch JB, Gamble KL. Shift Work Disrupts Circadian Regulation of the Transcriptome in Hospital Nurses. Journal of biological rhythms. 2019;34:167–177. doi: 10.1177/0748730419826694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Solymanzadeh F, Rokhafroz D, Asadizaker M, Dastoorpoor M. The relationship between rotating shift work and blood pressure among nurses working in hospitals of Abadan, Iran. Chronobiol Int. 2021;38:1569–1574. doi: 10.1080/07420528.2021.1936542 [DOI] [PubMed] [Google Scholar]
- 159.Molzof HE, Wirth MD, Burch JB, Shivappa N, Hebert JR, Johnson RL, Gamble KL. The impact of meal timing on cardiometabolic syndrome indicators in shift workers. Chronobiol Int. 2017;34:337–348. doi: 10.1080/07420528.2016.1259242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pan X, Mota S, Zhang B. Circadian Clock Regulation on Lipid Metabolism and Metabolic Diseases. Adv Exp Med Biol. 2020;1276:53–66. doi: 10.1007/978-981-15-6082-8_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Engin A. Circadian Rhythms in Diet-Induced Obesity. Adv Exp Med Biol. 2017;960:19–52. doi: 10.1007/978-3-319-48382-5_2 [DOI] [PubMed] [Google Scholar]
- 162.Liu D, Huang Y, Huang C, Yang S, Wei X, Zhang P, Guo D, Lin J, Xu B, Li C, et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. The New England journal of medicine. 2022;386:1495–1504. doi: 10.1056/NEJMoa2114833 [DOI] [PubMed] [Google Scholar]
- 163.Acosta-Rodriguez V, Rijo-Ferreira F, Izumo M, Xu P, Wight-Carter M, Green CB, Takahashi JS. Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science. 2022:e. doi: 10.1126/science.abk0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bray MS, Ratcliffe WF, Grenett MH, Brewer RA, Gamble KL, Young ME. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int J Obes (Lond). 2013;37:843–852. doi: 10.1038/ijo.2012.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang D, Colson JC, Jin C, Becker BK, Rhoads MK, Pati P, Neder TH, King MA, Valcin JA, Tao B, et al. Timing of Food Intake Drives the Circadian Rhythm of Blood Pressure. Function (Oxf). 2021;2:zqaa034. doi: 10.1093/function/zqaa034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Izumo M, Pejchal M, Schook AC, Lange RP, Walisser JA, Sato TR, Wang X, Bradfield CA, Takahashi JS. Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain Bmal1 mutant. Elife. 2014;3. doi: 10.7554/eLife.04617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Blunted heart rate dip during sleep and all-cause mortality. Archives of internal medicine. 2007;167:2116–2121. doi: 10.1001/archinte.167.19.2116 [DOI] [PubMed] [Google Scholar]
- 168.Conen D, Bamberg F. Noninvasive 24-h ambulatory blood pressure and cardiovascular disease: a systematic review and meta-analysis. Journal of hypertension. 2008;26:1290–1299. doi: 10.1097/HJH.0b013e3282f97854 [DOI] [PubMed] [Google Scholar]
- 169.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O'Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. doi: 10.1161/01.HYP.0000170138.56903.7a [DOI] [PubMed] [Google Scholar]
- 170.Diaz KM, Veerabhadrappa P, Brown MD, Whited MC, Dubbert PM, Hickson DA. Prevalence, Determinants, and Clinical Significance of Masked Hypertension in a Population-Based Sample of African Americans: The Jackson Heart Study. Am J Hypertens. 2015;28:900–908. doi: 10.1093/ajh/hpu241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. The New England journal of medicine. 2006;354:2368–2374. doi: 10.1056/NEJMra060433 [DOI] [PubMed] [Google Scholar]
- 172.Equiluz-Bruck S, Schnack C, Kopp HP, Schernthaner G. Nondipping of nocturnal blood pressure is related to urinary albumin excretion rate in patients with type 2 diabetes mellitus. Am J Hypertens. 1996;9:1139–1143. doi: 10.1016/0895-7061(96)00302-0 [DOI] [PubMed] [Google Scholar]
- 173.Pistrosch F, Reissmann E, Wildbrett J, Koehler C, Hanefeld M. Relationship between diurnal blood pressure variation and diurnal blood glucose levels in type 2 diabetic patients. Am J Hypertens. 2007;20:541–545. doi: 10.1016/j.amjhyper.2006.10.010 [DOI] [PubMed] [Google Scholar]
- 174.Ayala DE, Moya A, Crespo JJ, Castineira C, Dominguez-Sardina M, Gomara S, Sineiro E, Mojon A, Fontao MJ, Hermida RC, et al. Circadian pattern of ambulatory blood pressure in hypertensive patients with and without type 2 diabetes. Chronobiol Int. 2013;30:99–115. doi: 10.3109/07420528.2012.701489 [DOI] [PubMed] [Google Scholar]
- 175.Oh SW, Han SY, Han KH, Cha RH, Kim S, Yoon SA, Rhu DR, Oh J, Lee EY, Kim DK, et al. Morning hypertension and night non-dipping in patients with diabetes and chronic kidney disease. Hypertension research : official journal of the Japanese Society of Hypertension. 2015;38:889–894. doi: 10.1038/hr.2015.89 [DOI] [PubMed] [Google Scholar]
- 176.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5 [DOI] [PubMed] [Google Scholar]
- 177.Hou T, Su W, Guo Z, Gong MC. A Novel Diabetic Mouse Model for Real-Time Monitoring of Clock Gene Oscillation and Blood Pressure Circadian Rhythm. Journal of biological rhythms. 2019;34:51–68. doi: 10.1177/0748730418803719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Park S, Bivona BJ, Feng Y, Lazartigues E, Harrison-Bernard LM. Intact renal afferent arteriolar autoregulatory responsiveness in db/db mice. Am J Physiol Renal Physiol. 2008;295:F1504–1511. doi: 10.1152/ajprenal.90417.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Senador D, Kanakamedala K, Irigoyen MC, Morris M, Elased KM. Cardiovascular and autonomic phenotype of db/db diabetic mice. Experimental physiology. 2009;94:648–658. doi: 10.1113/expphysiol.2008.046474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Su W, Guo Z, Randall DC, Cassis L, Brown DR, Gong MC. Hypertension and disrupted blood pressure circadian rhythm in type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol. 2008;295:H1634–1641. doi: 10.1152/ajpheart.00257.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Su W, Xie Z, Guo Z, Duncan MJ, Lutshumba J, Gong MC. Altered clock gene expression and vascular smooth muscle diurnal contractile variations in type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol. 2012;302:H621–633. doi: 10.1152/ajpheart.00825.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nernpermpisooth N, Qiu S, Mintz JD, Suvitayavat W, Thirawarapan S, Rudic DR, Fulton DJ, Stepp DW. Obesity alters the peripheral circadian clock in the aorta and microcirculation. Microcirculation. 2015;22:257–266. doi: 10.1111/micc.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, Garcia JM, Koike N, Lee CC, Takahashi JS, et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016;23:610–621. doi: 10.1016/j.cmet.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kudo T, Akiyama M, Kuriyama K, Sudo M, Moriya T, Shibata S. Night-time restricted feeding normalises clock genes and Pai-1 gene expression in the db/db mouse liver. Diabetologia. 2004;47:1425–1436. doi: 10.1007/s00125-004-1461-0 [DOI] [PubMed] [Google Scholar]
- 185.Heart Outcomes Prevention Evaluation Study I, Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The New England journal of medicine. 2000;342:145–153. doi: 10.1056/NEJM200001203420301 [DOI] [PubMed] [Google Scholar]
- 186.Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. Journal of hypertension. 1998;16:1823–1829. doi: 10.1097/00004872-199816120-00016 [DOI] [PubMed] [Google Scholar]
- 187.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhager WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–764. doi: 10.1016/s0140-6736(97)05381-6 [DOI] [PubMed] [Google Scholar]
- 188.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629–1651. doi: 10.3109/07420528.2010.510230 [DOI] [PubMed] [Google Scholar]
- 189.Carter BL, Chrischilles EA, Rosenthal G, Gryzlak BM, Eisenstein EL, Vander Weg MW. Efficacy and safety of nighttime dosing of antihypertensives: review of the literature and design of a pragmatic clinical trial. Journal of clinical hypertension. 2014;16:115–121. doi: 10.1111/jch.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hermida RC, Crespo JJ, Dominguez-Sardina M, Otero A, Moya A, Rios MT, Sineiro E, Castineira MC, Callejas PA, Pousa L, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J. 2020;41:4565–4576. doi: 10.1093/eurheartj/ehz754 [DOI] [PubMed] [Google Scholar]
- 191.Brunstrom M, Kjeldsen SE, Kreutz R, Gjesdal K, Narkiewicz K, Burnier M, Oparil S, Mancia G. Missing Verification of Source Data in Hypertension Research: The HYGIA PROJECT in Perspective. Hypertension. 2021;78:555–558. doi: 10.1161/HYPERTENSIONAHA.121.17356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kreutz R, Kjeldsen SE, Burnier M, Narkiewicz K, Oparil S, Mancia G. Disregard the reported data from the HYGIA project: blood pressure medication not to be routinely dosed at bedtime. Journal of hypertension. 2020;38:2144–2145. doi: 10.1097/HJH.0000000000002671 [DOI] [PubMed] [Google Scholar]
- 193.Luscher TF, Fox K, Hamm C, Carter RE, Taddei S, Simoons M, Crea F. Scientific integrity: what a journal can and cannot do. Eur Heart J. 2020;41:4552–4555. doi: 10.1093/eurheartj/ehaa963 [DOI] [PubMed] [Google Scholar]
- 194.Stergiou G, Brunstrom M, MacDonald T, Kyriakoulis K, Bursztyn M, Khan N, Bakris G, Kollias A, Menti A, Muntner P, et al. International Society of Hypertension Position Paper. Journal of hypertension. 2022; January;40(10):1847–1858. doi: 10.1097/HJH.0000000000003240. [DOI] [PubMed] [Google Scholar]
- 195.Rorie DA, Rogers A, Mackenzie IS, Ford I, Webb DJ, Willams B, Brown M, Poulter N, Findlay E, Saywood W, et al. Methods of a large prospective, randomised, open-label, blinded end-point study comparing morning versus evening dosing in hypertensive patients: the Treatment In Morning versus Evening (TIME) study. BMJ Open. 2016;6:e010313. doi: 10.1136/bmjopen-2015-010313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Garrison SR, Kolber MR, Allan GM, Bakal J, Green L, Singer A, Trueman DR, McAlister FA, Padwal RS, Hill MD, et al. Bedtime versus morning use of antihypertensives for cardiovascular risk reduction (BedMed): protocol for a prospective, randomised, open-label, blinded end-point pragmatic trial. BMJ Open. 2022;12:e059711. doi: 10.1136/bmjopen-2021-059711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.BedMed-Frail: does the potential benefit of bedtime antihypertensive prescribing extend to frail populations? ClinicalTrials.gov. 2022. Accessed 09/25/2022.
- 198.No Added Benefit from Nighttime Dosing of Blood Pressure. Practical Cardiology; 2022. Accessed 09/25/2022. [Google Scholar]
- 199.Anafi RC, Francey LJ, Hogenesch JB, Kim J. CYCLOPS reveals human transcriptional rhythms in health and disease. Proc Natl Acad Sci U S A. 2017;114:5312–5317. doi: 10.1073/pnas.1619320114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Alibhai FJ, Tsimakouridze EV, Chinnappareddy N, Wright DC, Billia F, O'Sullivan ML, Pyle WG, Sole MJ, Martino TA. Short-term disruption of diurnal rhythms after murine myocardial infarction adversely affects long-term myocardial structure and function. Circ Res. 2014;114:1713–1722. doi: 10.1161/CIRCRESAHA.114.302995 [DOI] [PubMed] [Google Scholar]
- 201.Reitz CJ, Alibhai FJ, Khatua TN, Rasouli M, Bridle BW, Burris TP, Martino TA. SR9009 administered for one day after myocardial ischemia-reperfusion prevents heart failure in mice by targeting the cardiac inflammasome. Commun Biol. 2019;2:353. doi: 10.1038/s42003-019-0595-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Martino TA, Tata N, Simpson JA, Vanderlaan R, Dawood F, Kabir MG, Khaper N, Cifelli C, Podobed P, Liu PP, et al. The primary benefits of angiotensin-converting enzyme inhibition on cardiac remodeling occur during sleep time in murine pressure overload hypertrophy. Journal of the American College of Cardiology. 2011;57:2020–2028. doi: 10.1016/j.jacc.2010.11.022 [DOI] [PubMed] [Google Scholar]
- 203.Ramirez-Jimenez M, Morales-Palomo F, Moreno-Cabanas A, Alvarez-Jimenez L, Ortega JF, Mora-Rodriguez R. Aerobic exercise training improves nocturnal blood pressure dipping in medicated hypertensive individuals. Blood Press Monit. 2022;27:272–275. doi: 10.1097/MBP.0000000000000598 [DOI] [PubMed] [Google Scholar]
- 204.Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004;43:192–197. doi: 10.1161/01.HYP.0000113293.15186.3b [DOI] [PubMed] [Google Scholar]
- 205.Padmanabhan S, Dominiczak AF. Genomics of hypertension: the road to precision medicine. Nat Rev Cardiol. 2021;18:235–250. doi: 10.1038/s41569-020-00466-4 [DOI] [PubMed] [Google Scholar]
- 206.Wang X, Ding X, Su S, Harshfield G, Treiber F, Snieder H. Genetic influence on blood pressure measured in the office, under laboratory stress and during real life. Hypertension research : official journal of the Japanese Society of Hypertension. 2011;34:239–244. doi: 10.1038/hr.2010.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang X, Ding X, Su S, Yan W, Harshfield G, Treiber F, Snieder H. Genetic influences on daytime and night-time blood pressure: similarities and differences. Journal of hypertension. 2009;27:2358–2364. doi: 10.1097/HJH.0b013e328330e84d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Leu HB, Chung CM, Lin SJ, Lu TM, Yang HC, Ho HY, Ting CT, Lin TH, Sheu SH, Tsai WC, et al. A novel SNP associated with nighttime pulse pressure in young-onset hypertension patients could be a genetic prognostic factor for cardiovascular events in a general cohort in Taiwan. PLoS One. 2014;9:e97919. doi: 10.1371/journal.pone.0097919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rimpela JM, Porsti IH, Jula A, Lehtimaki T, Niiranen TJ, Oikarinen L, Porthan K, Tikkakoski A, Virolainen J, Kontula KK, et al. Genome-wide association study of nocturnal blood pressure dipping in hypertensive patients. BMC Med Genet. 2018;19:110. doi: 10.1186/s12881-018-0624-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Leu HB, Chung CM, Lin SJ, Chiang KM, Yang HC, Ho HY, Ting CT, Lin TH, Sheu SH, Tsai WC, et al. Association of circadian genes with diurnal blood pressure changes and non-dipper essential hypertension: a genetic association with young-onset hypertension. Hypertension research : official journal of the Japanese Society of Hypertension. 2015;38:155–162. doi: 10.1038/hr.2014.152 [DOI] [PubMed] [Google Scholar]
- 211.Sheng CS, Cheng YB, Wei FF, Yang WY, Guo QH, Li FK, Huang QF, Thijs L, Staessen JA, Wang JG, et al. Diurnal Blood Pressure Rhythmicity in Relation to Environmental and Genetic Cues in Untreated Referred Patients. Hypertension. 2017;69:128–135. doi: 10.1161/HYPERTENSIONAHA.116.07958 [DOI] [PubMed] [Google Scholar]
- 212.Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, ReproGen C, Psychiatric Genomics C, Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Case Control C, Duncan L, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–1241. doi: 10.1038/ng.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liang M. Epigenetic Mechanisms and Hypertension. Hypertension. 2018;72:1244–1254. doi: 10.1161/HYPERTENSIONAHA.118.11171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kotchen TA, Cowley AW Jr., Liang M. Ushering Hypertension Into a New Era of Precision Medicine. JAMA. 2016;315:343–344. doi: 10.1001/jama.2015.18359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Richard MA, Huan T, Ligthart S, Gondalia R, Jhun MA, Brody JA, Irvin MR, Marioni R, Shen J, Tsai PC, et al. DNA Methylation Analysis Identifies Loci for Blood Pressure Regulation. Am J Hum Genet. 2017;101:888–902. doi: 10.1016/j.ajhg.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Huang Y, Ollikainen M, Muniandy M, Zhang T, van Dongen J, Hao G, van der Most PJ, Pan Y, Pervjakova N, Sun YV, et al. Identification, Heritability, and Relation With Gene Expression of Novel DNA Methylation Loci for Blood Pressure. Hypertension. 2020;76:195–205. doi: 10.1161/HYPERTENSIONAHA.120.14973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liu Y, Liu P, Yang C, Cowley AW Jr., Liang M. Base-resolution maps of 5-methylcytosine and 5-hydroxymethylcytosine in Dahl S rats: effect of salt and genomic sequence. Hypertension. 2014;63:827–838. doi: 10.1161/HYPERTENSIONAHA.113.02637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Liu P, Liu Y, Liu H, Pan X, Li Y, Usa K, Mishra MK, Nie J, Liang M. Role of DNA De Novo (De)Methylation in the Kidney in Salt-Induced Hypertension. Hypertension. 2018;72:1160–1171. doi: 10.1161/HYPERTENSIONAHA.118.11650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Roberts ML, Kotchen TA, Pan X, Li Y, Yang C, Liu P, Wang T, Laud PW, Chelius TH, Munyura Y, et al. Unique Associations of DNA Methylation Regions With 24-Hour Blood Pressure Phenotypes in Black Participants. Hypertension. 2022;79:761–772. doi: 10.1161/HYPERTENSIONAHA.121.18584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kidambi S, Pan X, Yang C, Liu P, Roberts ML, Li Y, Wang T, Laud PW, Liu Y, Rubens M, et al. Dietary Sodium Restriction Results in Tissue-Specific Changes in DNA Methylation in Humans. Hypertension. 2021;78:434–446. doi: 10.1161/HYPERTENSIONAHA.120.17351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Barfield R, Wang H, Liu Y, Brody JA, Swenson B, Li R, Bartz TM, Sotoodehnia N, Chen YI, Cade BE, et al. Epigenome-wide association analysis of daytime sleepiness in the Multi-Ethnic Study of Atherosclerosis reveals African-American-specific associations. Sleep. 2019;42. doi: 10.1093/sleep/zsz101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.HSFC. Ms. Understood. Women’s hearts are victims of a system that is ill-equipped to diagnose, treat and support them. eart & Stroke 2018 Heart Report. Heart and Stroke Foundation of Canada. https://www.heartandstroke.ca/-/media/pdf-files/canada/2018-heart-month/hs_2018-heart-report_en.ashx?rev=71bed5e2bcf148b4a0bf5082e50de6c6&hash=020E4A77E9D4B8C71F66E597CCC95218. 2018. Accessed 04/28/2022. [Google Scholar]
- 223.Pyle WG, Martino TA. Circadian rhythms influence cardiovascular disease differently in males and females: role of sex and gender. Current Opinion in Physiology. 2018;5:30–37. doi: 10.1016/j.cophys.2018.05.003 [DOI] [Google Scholar]
- 224.Ho CLB, Chowdhury EK, Doust J, Nelson MR, Reid CM. The effect of taking blood pressure lowering medication at night on cardiovascular disease risk. A systematic review. Journal of human hypertension. 2021;35:308–314. doi: 10.1038/s41371-020-00469-1 [DOI] [PubMed] [Google Scholar]
- 225.Wilkinson J, Arnold KF, Murray EJ, van Smeden M, Carr K, Sippy R, de Kamps M, Beam A, Konigorski S, Lippert C, et al. Time to reality check the promises of machine learning-powered precision medicine. Lancet Digit Health. 2020;2:e677–e680. doi: 10.1016/S2589-7500(20)30200-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hirsch IB, Martinez J, Dorsey ER, Finken G, Fleming A, Gropp C, Home P, Kaufer DI, Papapetropoulos S. Incorporating Site-less Clinical Trials Into Drug Development: A Framework for Action. Clin Ther. 2017;39:1064–1076. doi: 10.1016/j.clinthera.2017.03.018 [DOI] [PubMed] [Google Scholar]
- 227.Inan OT, Tenaerts P, Prindiville SA, Reynolds HR, Dizon DS, Cooper-Arnold K, Turakhia M, Pletcher MJ, Preston KL, Krumholz HM, et al. Digitizing clinical trials. NPJ Digit Med. 2020;3:101. doi: 10.1038/s41746-020-0302-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.van Staa TP, Klungel O, Smeeth L. Use of electronic healthcare records in large-scale simple randomized trials at the point of care for the documentation of value-based medicine. J Intern Med. 2014;275:562–569. doi: 10.1111/joim.12211 [DOI] [PubMed] [Google Scholar]
- 229.Zuidgeest MGP, Goetz I, Groenwold RHH, Irving E, van Thiel G, Grobbee DE, GetReal Work P. Series: Pragmatic trials and real world evidence: Paper 1. Introduction. J Clin Epidemiol. 2017;88:7–13. doi: 10.1016/j.jclinepi.2016.12.023 [DOI] [PubMed] [Google Scholar]
- 230.Chalmers I, Smeeth L, Goldacre B. Personalised Medicine Using N-of-1 Trials: Overcoming Barriers to Delivery. Healthcare (Basel). 2019;7. doi: 10.3390/healthcare7040134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Clinical Trials Transformation Initiative. CTTI Recommendations: Decentralized Clinical Trials. https://www.ctti-clinicaltrials.org/sites/www.ctti-clinicaltrials.org/files/dct_recommendations_final.pdf. 2018. Accessed 04/26/2022.
- 232.Rogers A, Mackenzie I, Hawkins K. D1.1 First set of recommendations for RDCTs (to be implemented in the pan-EU pilot RDCT). . https://trialsathome.com/wp-content/uploads/2020/09/Trials@Home_D1.1-First-set-of-recommendations-for-RDCTs-to-be-implemented-in-the-pan-EU-pilot-RDCT.pdf. 2020. Accessed 04/26/2022. [Google Scholar]
- 233.Notice of Special Interest (NOSI): Basic and Translational Research on Circadian Regulation of Heart, Lung, Blood, and Sleep Disorders (R01). NIH.gov. 2022. Accessed 09/25/2022.